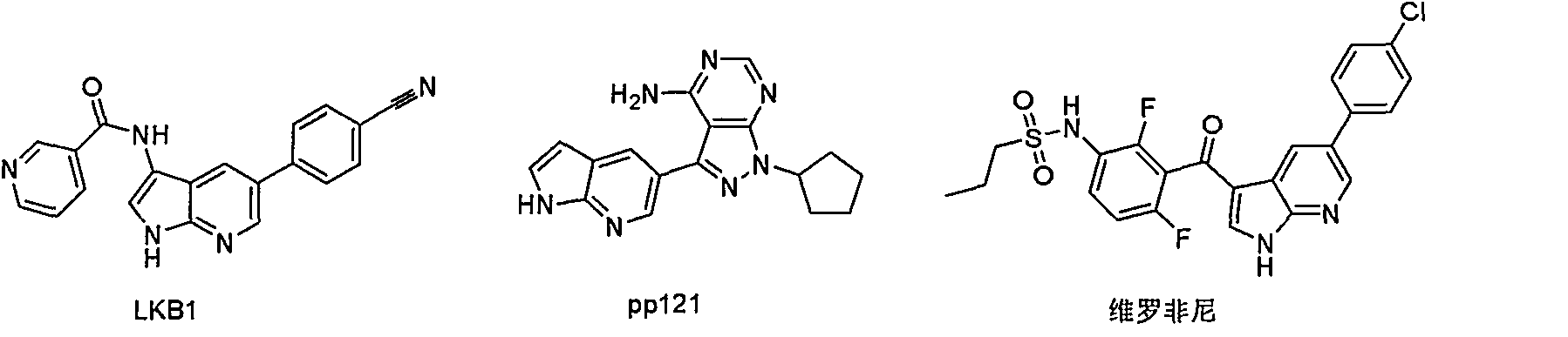
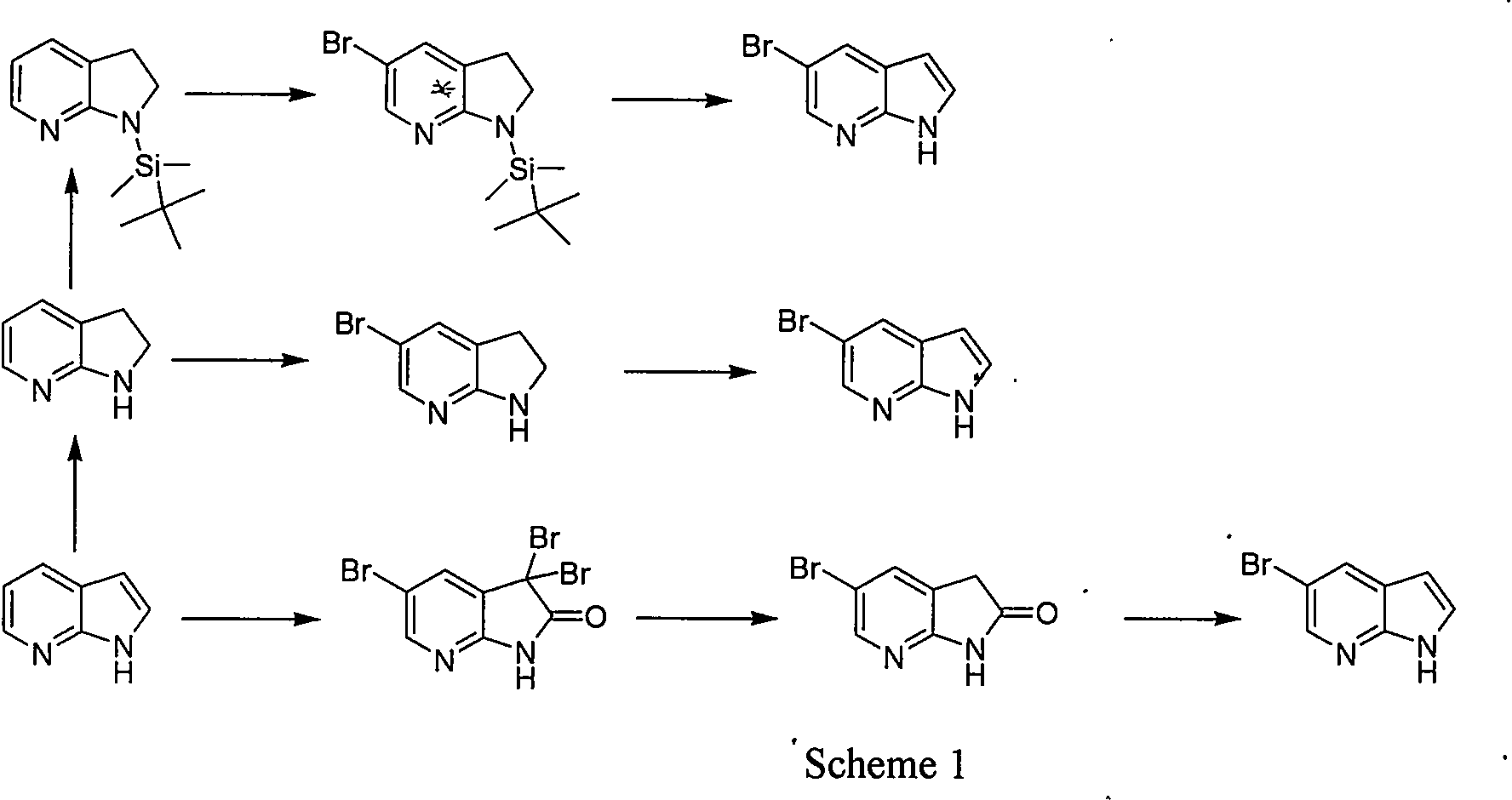
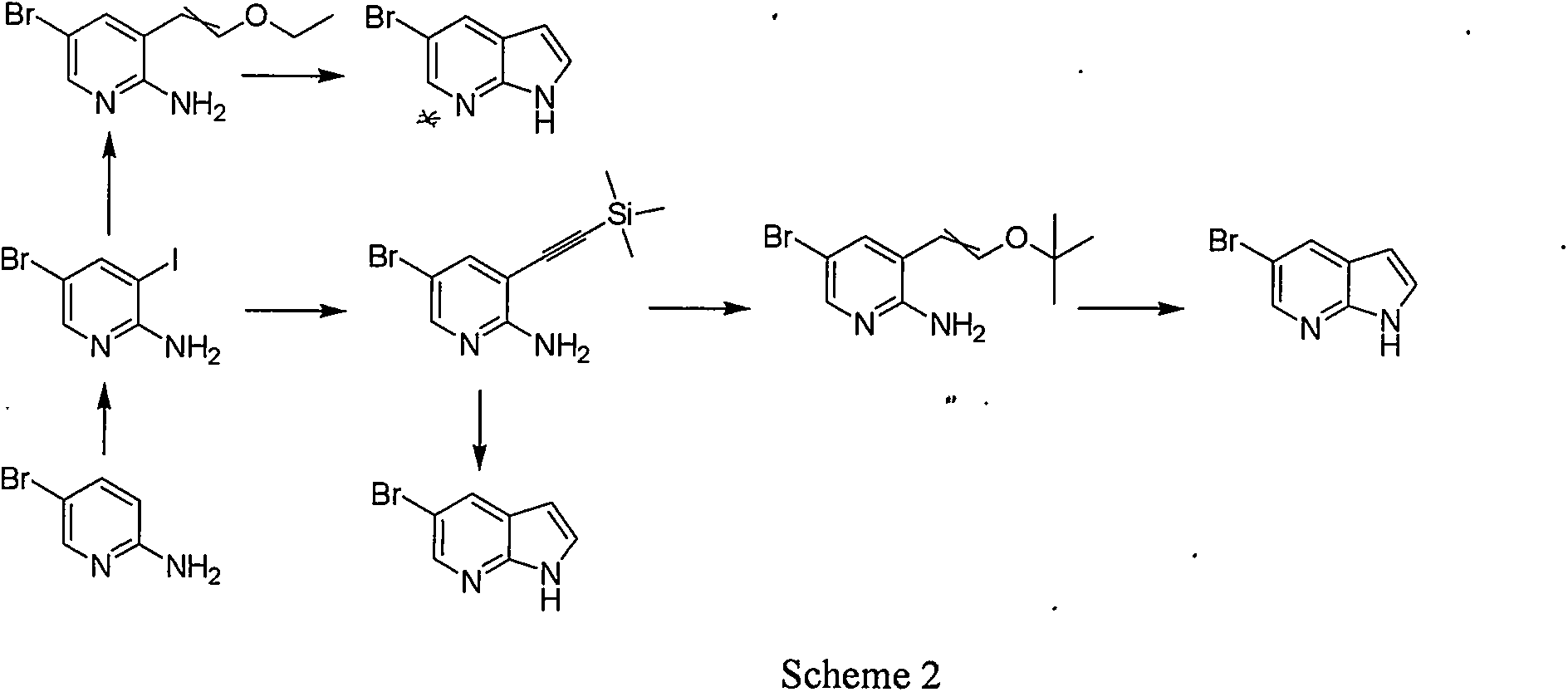
Synthetic process of 5-bromo-7-azaindole
A technology for the synthesis of azaindole and azaindole, which is applied in the field of synthesis of 5-bromo-7-azaindole, an anticancer drug intermediate, can solve the problems of high cost, high solid waste, and high total cost, and achieve Low cost, simple operation, and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The first step reaction: preparation of intermediate 2
[0026] In an ice-water bath, 2-amino-3-methyl-5-bromopyridine (10.8 g, 57.8 mmol) was sequentially added into the concentrated sulfuric acid solution (60 mL) solution at one time under mechanical stirring, keeping the temperature below 5°C, Add dropwise pre-cooled caro'sacid (a mixed solution of 86.4mL hydrogen peroxide and 179.2mL concentrated sulfuric acid). After the dropwise addition, remove the ice bath, naturally warm to room temperature, and continue stirring overnight. TLC shows that the raw materials react After completion, the reaction solution was carefully poured into a mixture of ice and water, and sodium hydroxide was added to adjust the alkalinity. A large amount of solids precipitated out and filtered. The filter cake was washed with cold water and dried under vacuum to obtain 9.8g of compound 2, HPLC: 98.3%, yield 78%. 1 H-NMR (400MHz, CDCl 3 ): δ8.620(s, 1H), 8.091(s, 1H), 2.320(s, 3H).
[002...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com