Fluorine-boron fluorescent dye as well as preparation method and application thereof
A fluorescent dye and fluoroboron technology, applied in the field of fluorescent dyes, can solve the problems of unavailable raw materials, small Stokes shift, complicated steps of fluoroboron fluorescent dyes, etc., and achieve the effect of easily available raw materials, simple steps, and excellent fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
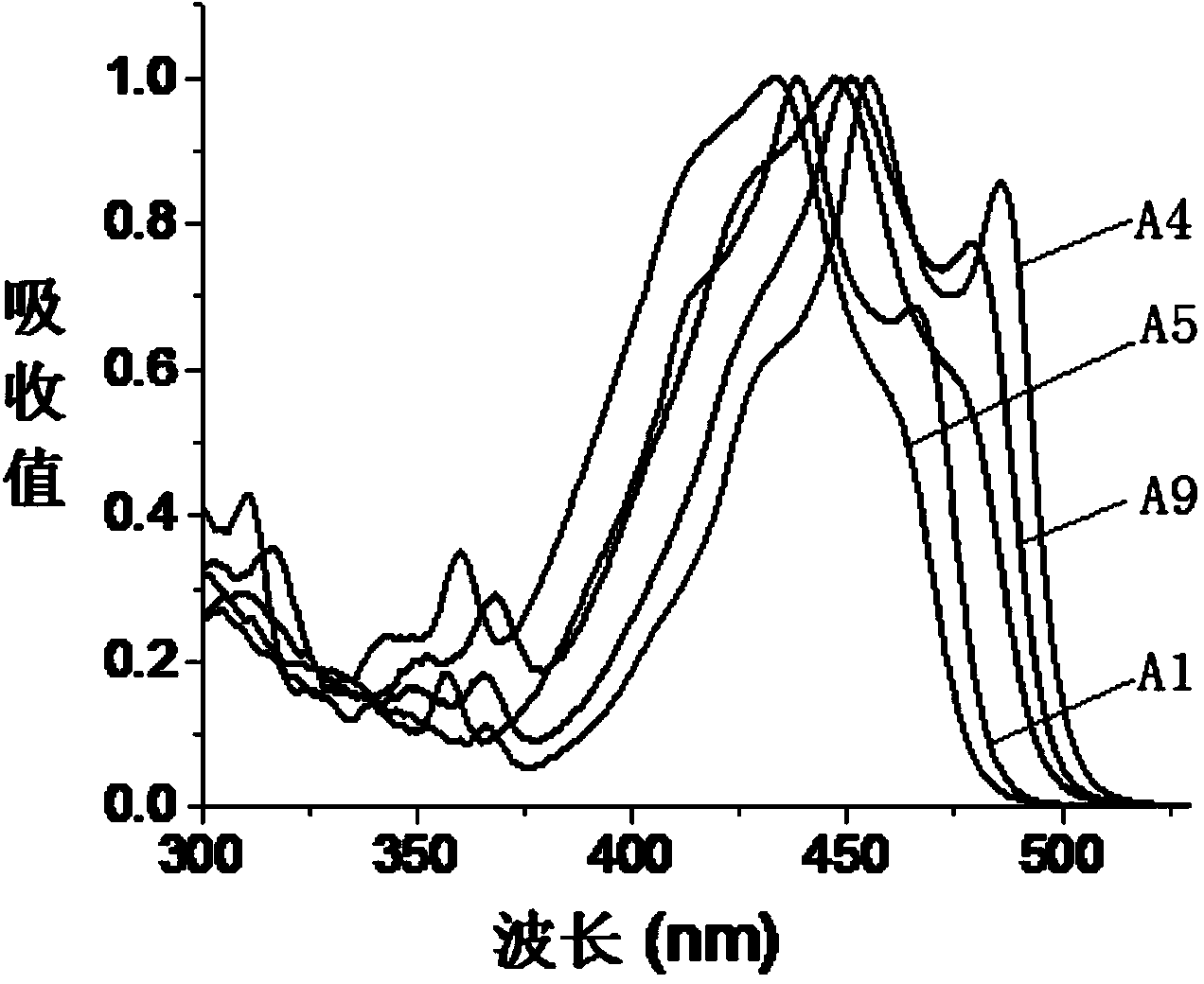
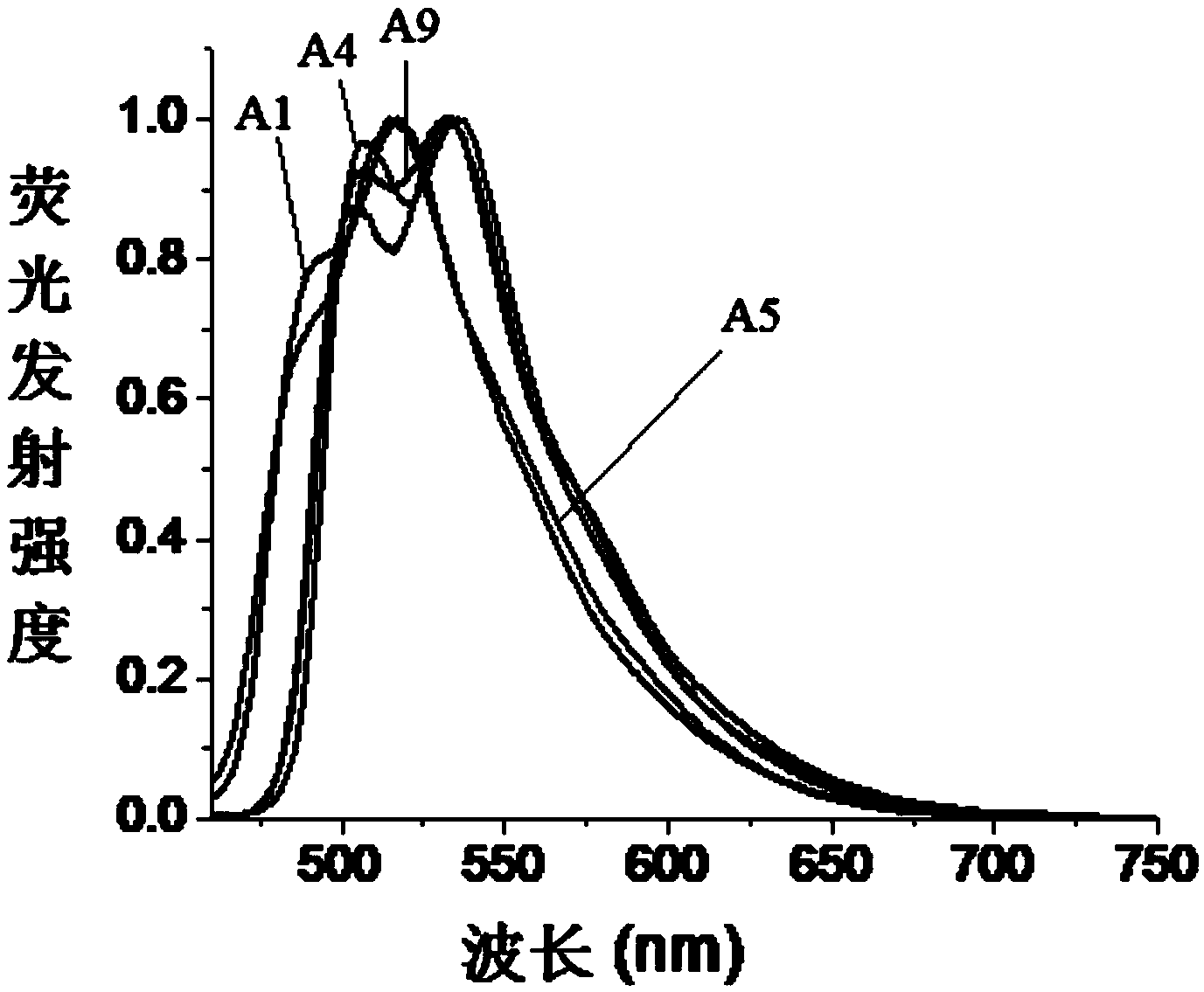
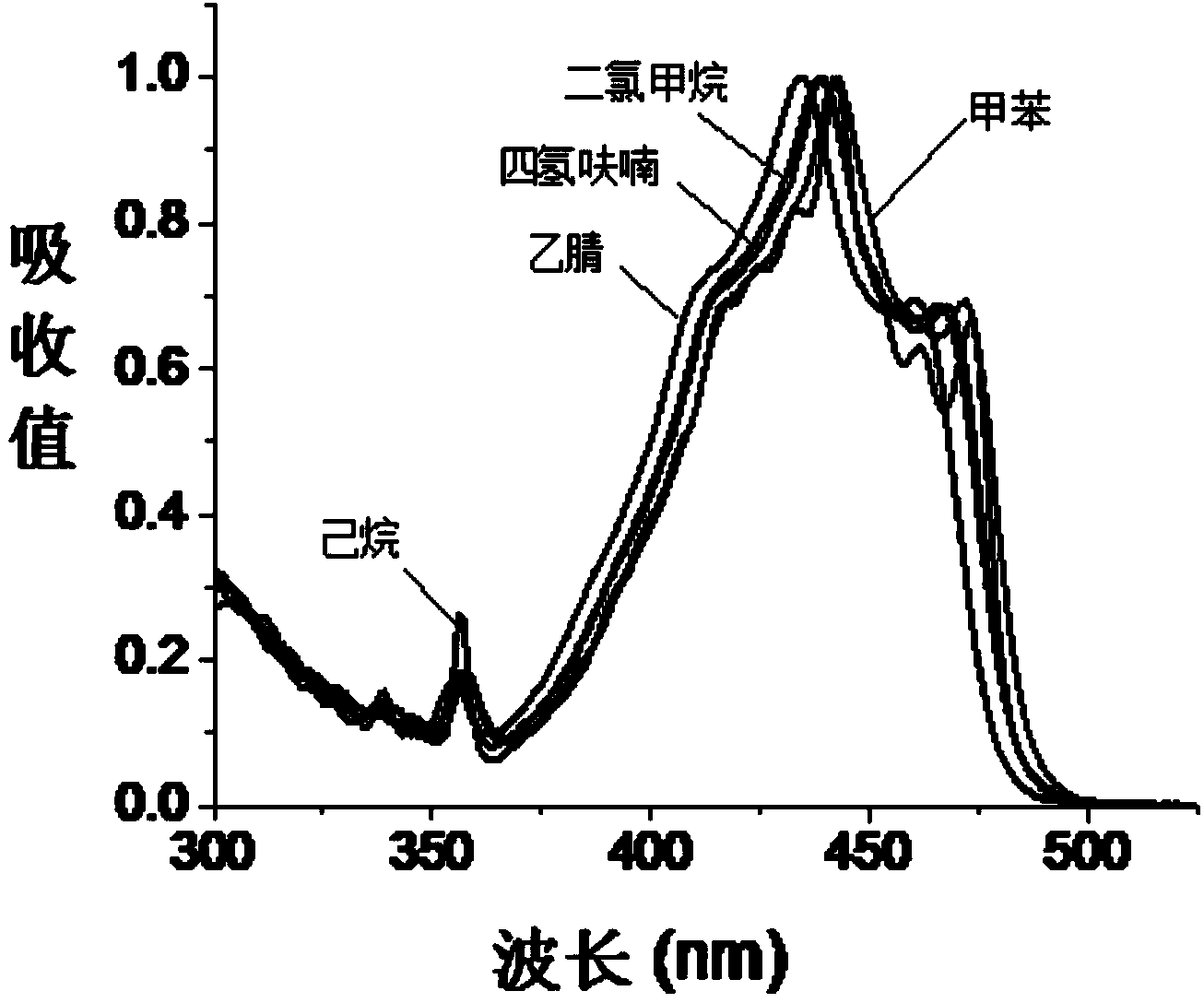
[0034] The present invention also provides a preparation method of fluoroboron fluorescent dye, the structure of which is shown in formula (I) or formula (II), wherein the preparation method includes the following steps:
[0035] In the presence of a Lewis acid, after the first contact reaction between the compound represented by the formula (X) and the reactant C, the product after the first contact reaction is obtained, and trifluoro is added to the obtained product after the first contact reaction Carry out the second contact reaction with boron ether;
[0036] The reactant C is selected from compounds of formula (V) or formula (VI),
[0037]
[0038] In formula (X), R 1 is H or halogen; preferably, in formula (X), R 1 For H or Br.
[0039] In formula (V) and formula (VI), R 2 is CN; R 3 , R 4 , R 5 and R 6 Each independently selected from H, halogen, C1-C6 alkyl or C1-C6 alkoxy; V, W, X, Y and Z are each independently CH or N, and in V, W, X, Y Or when Z is N, ...
preparation example 1
[0061] Preparation of raw material 4-bromo-1,8-naphthoimide: Put 0.52g (3.1mmol) of 1,8-naphthoimide in a 50ml round-bottomed flask, add 15ml of chloroform, cool to 0°C, and stir Add 0.16ml (3.1mmol) of bromine dropwise to the solution, drop it within 1min, then place the reaction at 20°C and stir for 24 hours. After the reaction is complete, extract with chloroform and dry to obtain a light yellow solid. The pale yellow solid was detected by H NMR spectroscopy to obtain: 1 H NMR (300MHz, CDCl 3 )δ8.21(d,J=8.1Hz,1H),8.13(d,J=8.1Hz,1H),7.80-7.87(m,2H),7.67(d,J=7.5Hz,1H),6.85( d, J=7.5Hz, 1H).
Embodiment 1
[0063] Preparation of boron fluorescent dye represented by formula (A1):
[0064]
[0065] In a 50ml round-bottomed flask, add 1,8 naphthalimide (60mg, 0.36mmol) and 2-aminopyridine (171mg, 1.8mmol), add 20ml of toluene, put it in an oil bath at 110°C and stir to reflux, then Triethylamine (1ml, 7.17mmol) and titanium tetrachloride (0.3ml, 2.7mmol) were added, and after 12 hours of reaction, boron trifluoride ether (1ml, 8.10mmol) was added, and the reaction was continued for 4 hours. After the reaction, it was cooled, extracted three times with 50 mL of dichloromethane, dried over anhydrous sodium sulfate, distilled under reduced pressure, and silica gel column chromatography was used to obtain a yellow solid with a yield of 42%.
[0066] 1 H NMR (300MHz, CDCl 3 )δ8.45(d,J=6.3Hz,1H),8.30(d,J=7.2Hz,1H),8.10((d,J=5.1Hz,1H),7.93-7.99(m,1H),7.75 -7.80(m,1H),7.54-7.69(m,4H),7.22-7.24(m,1H); 13 C NMR (75MHz, CDCl 3 ) 160.9, 155.3, 141.7, 140.4, 138.6, 131.4, 130.1, 129.5, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com