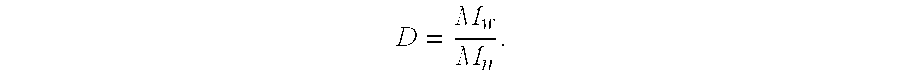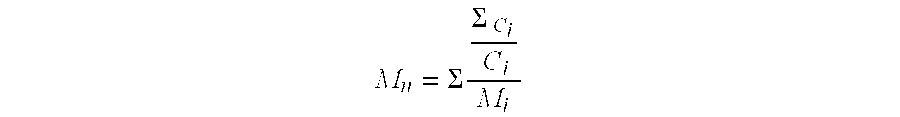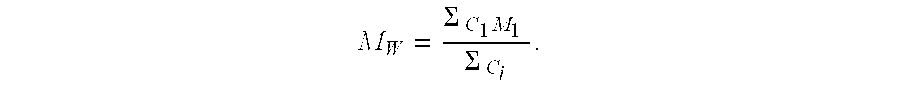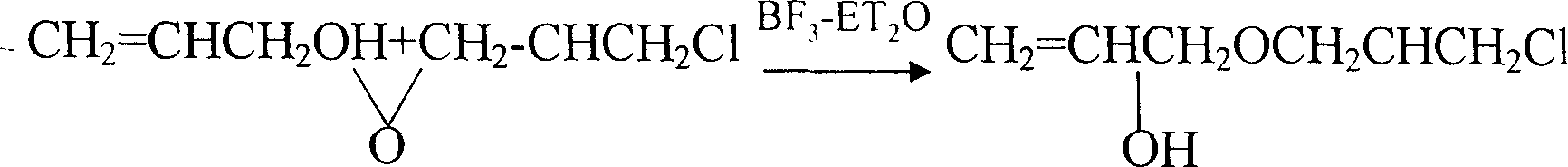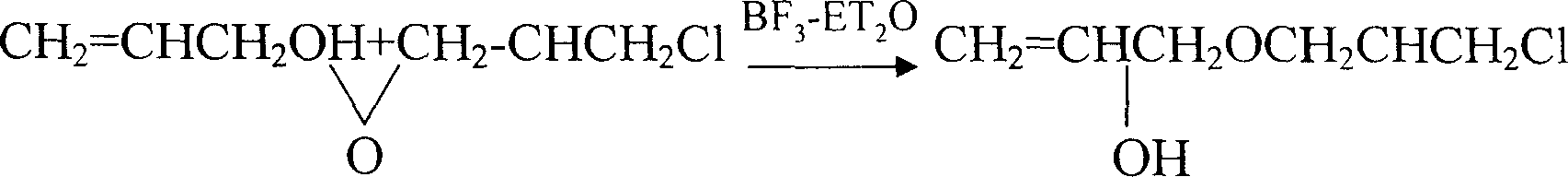Patents
Literature
1324 results about "Boron trifluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Boron trifluoride is the inorganic compound with the formula BF₃. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
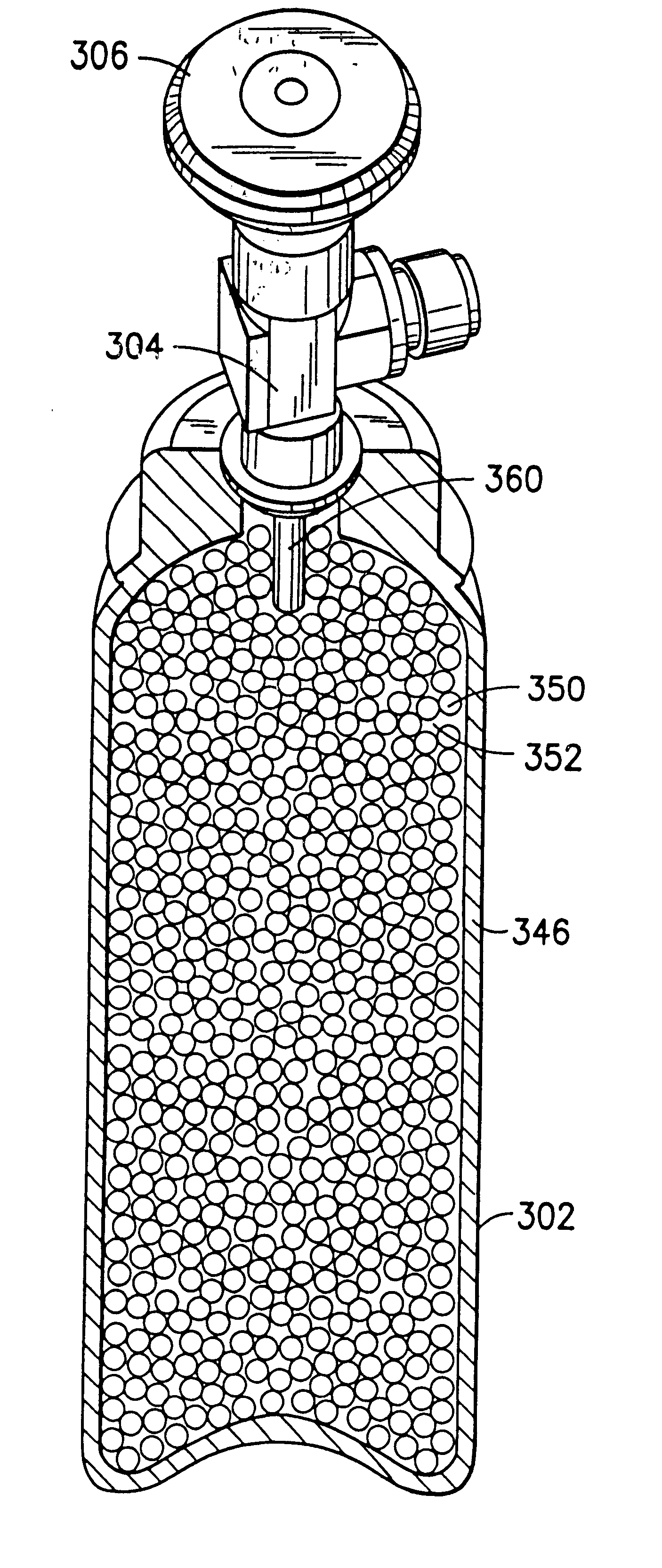
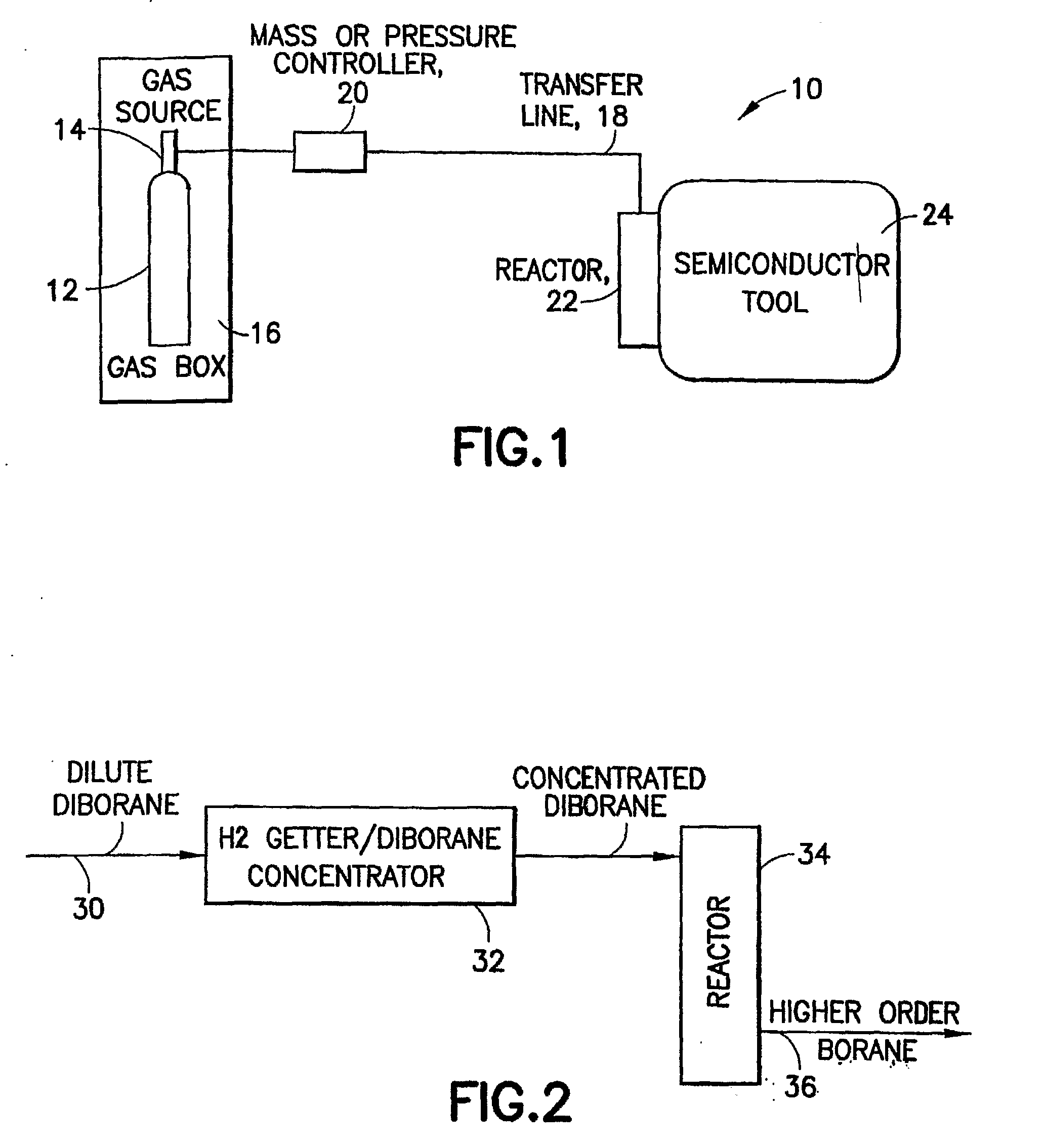
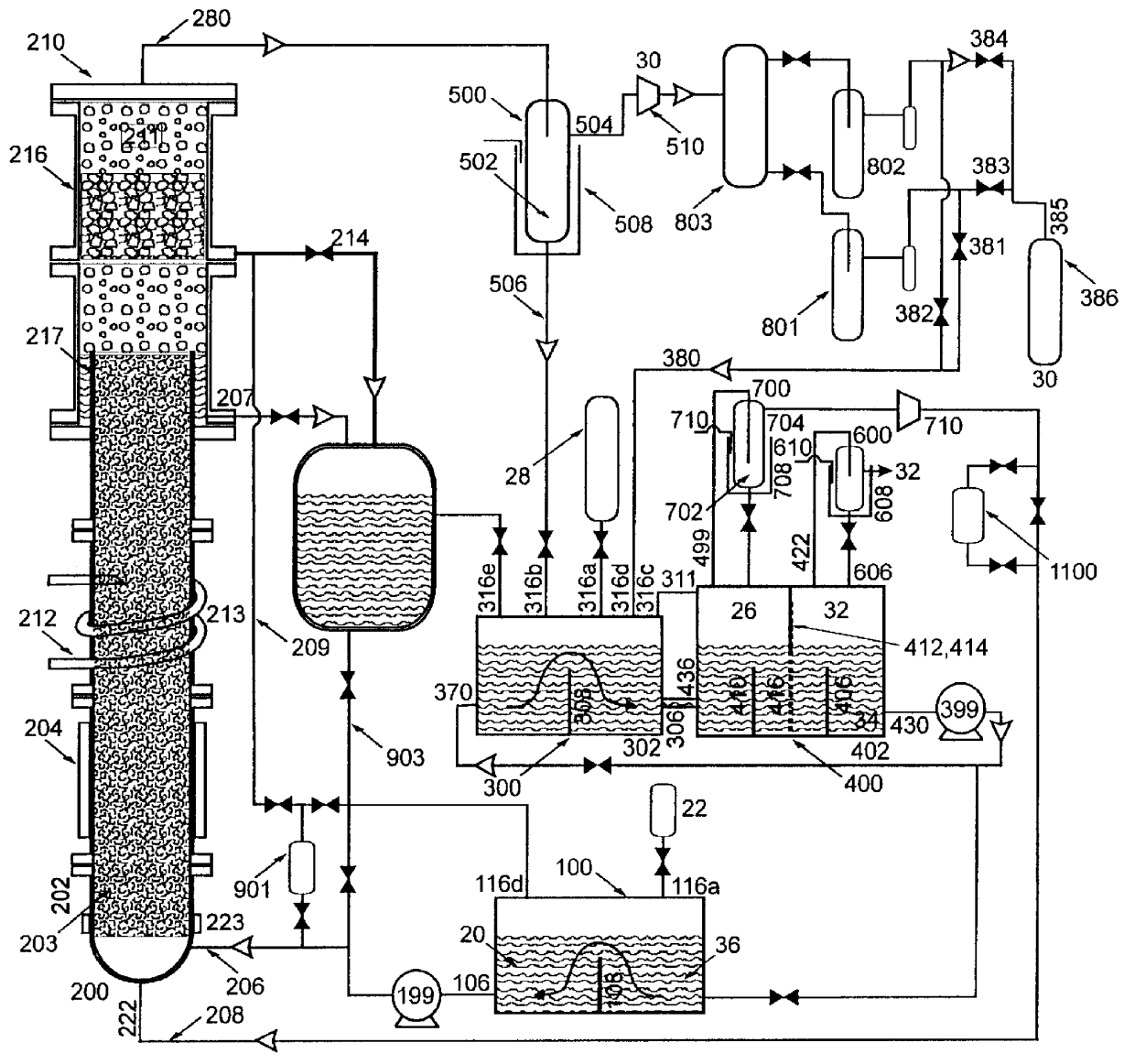
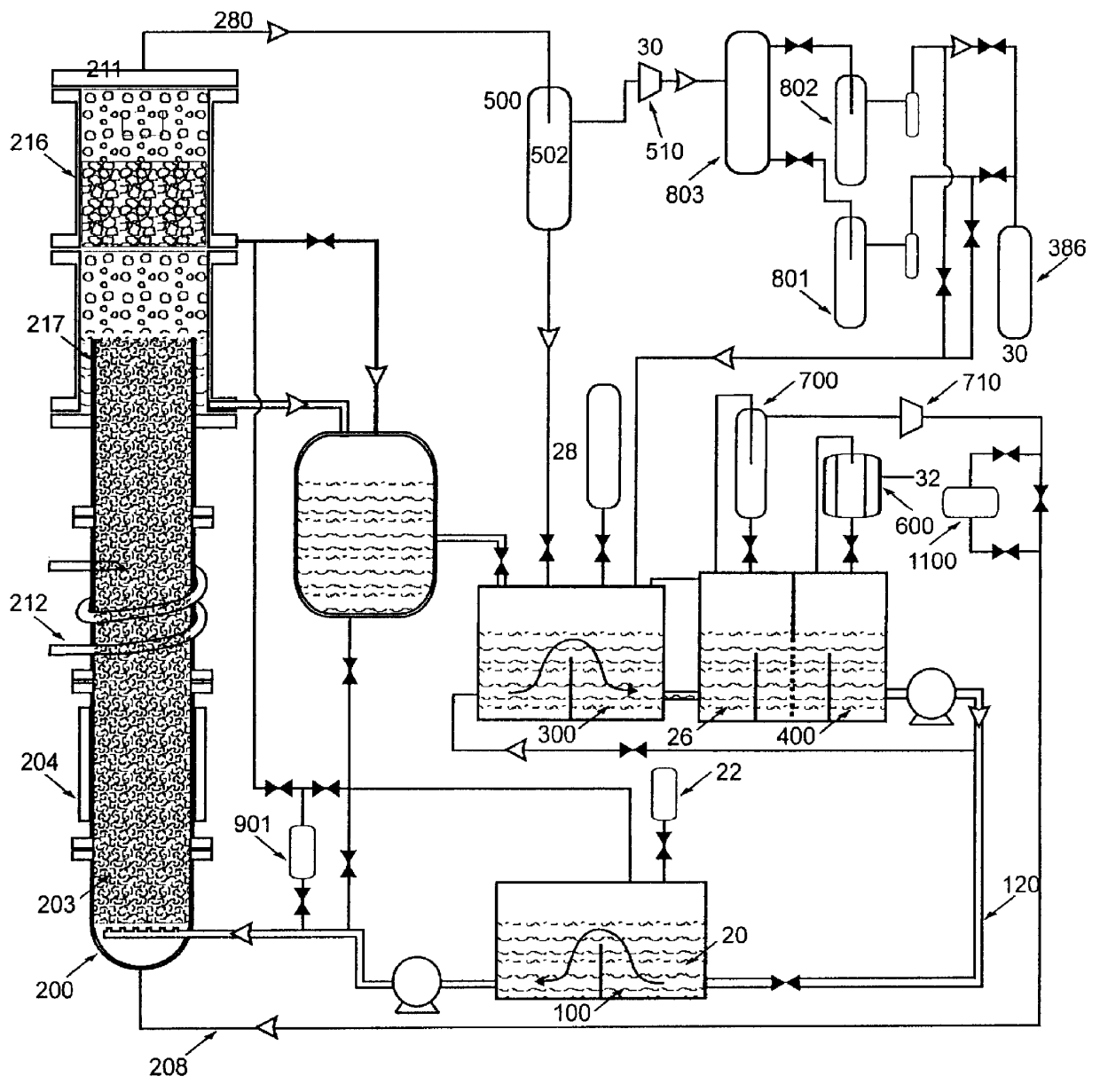
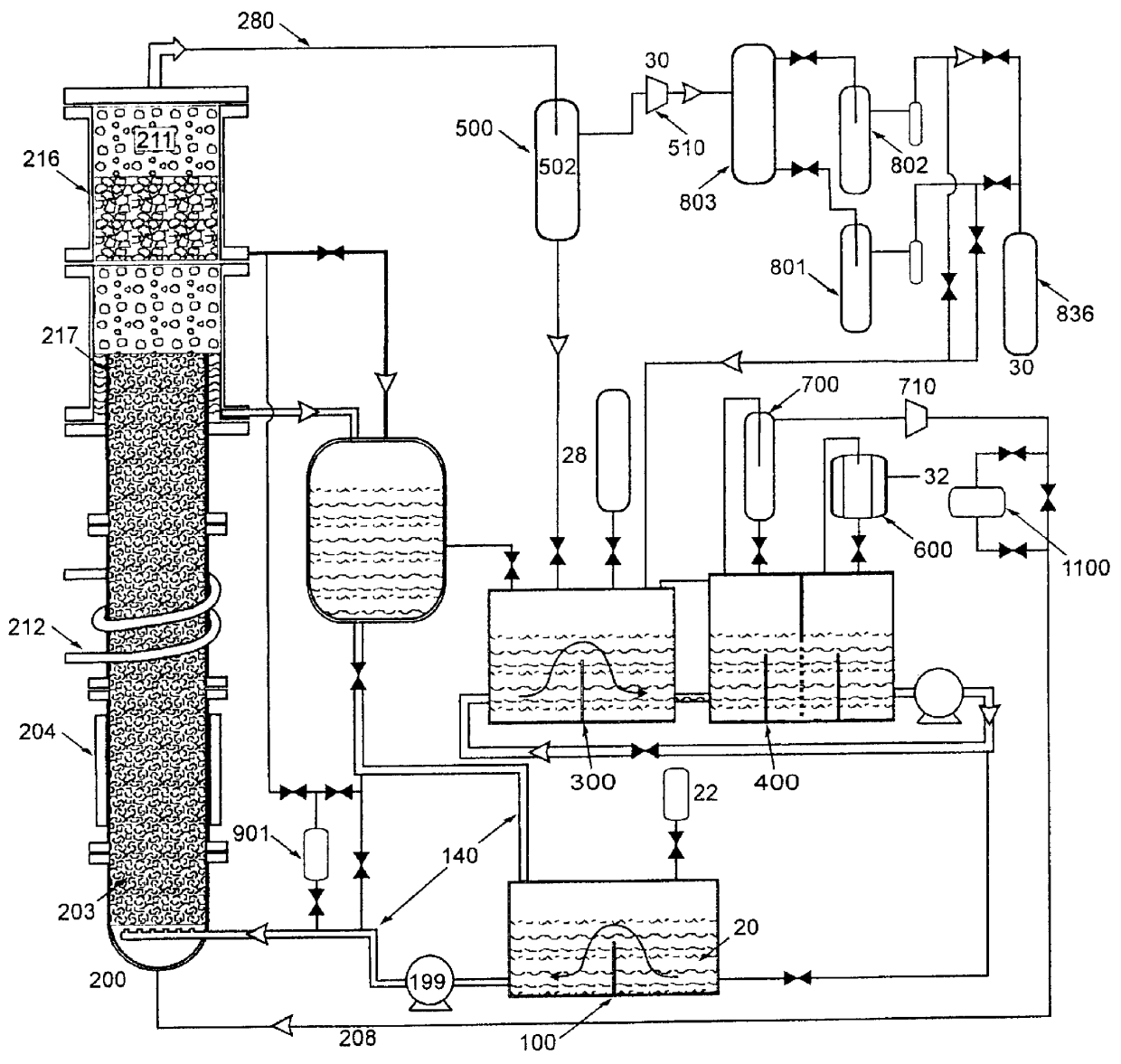
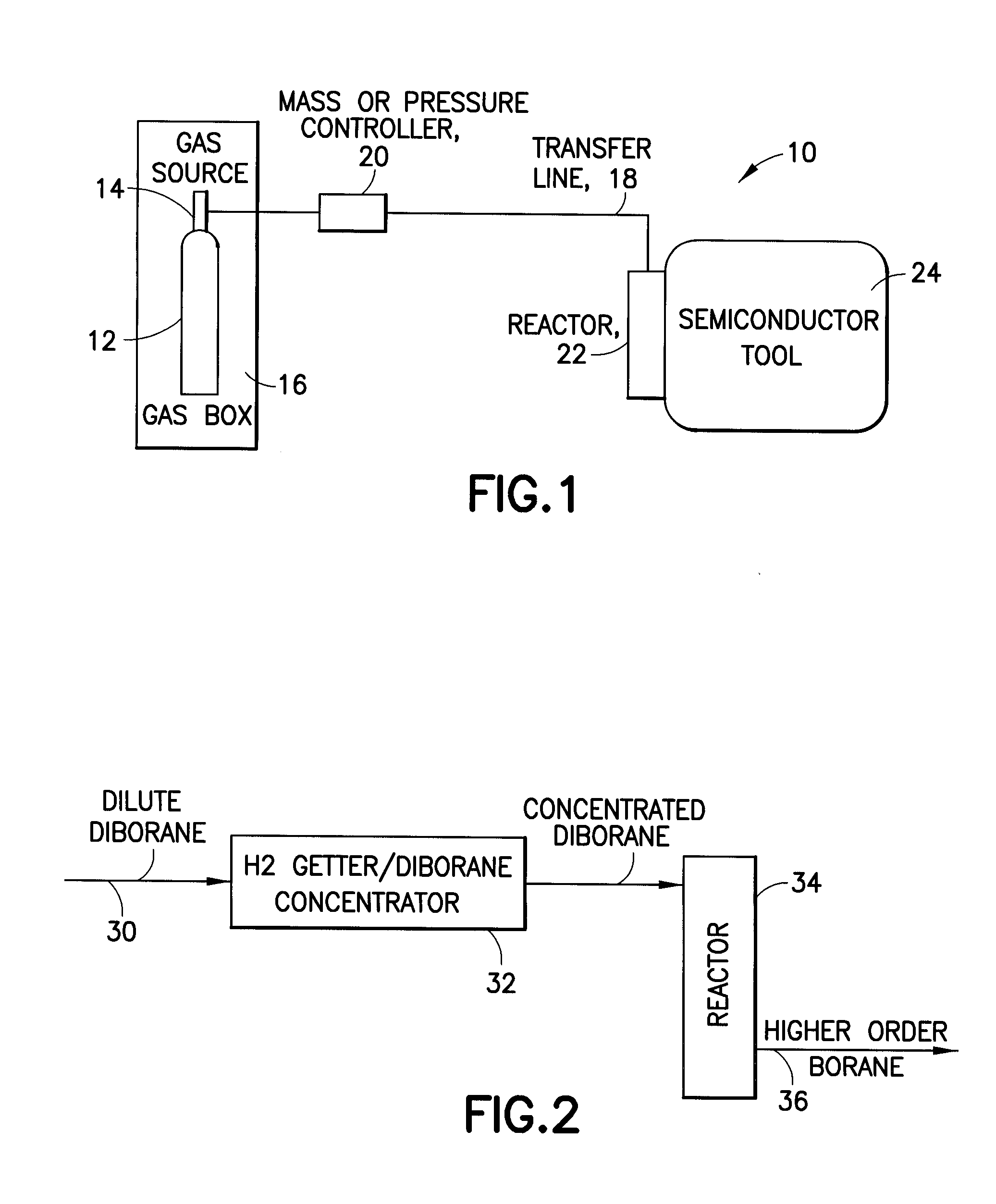
Fluid storage and delivery system utilizing low heels carbon sorbent medium
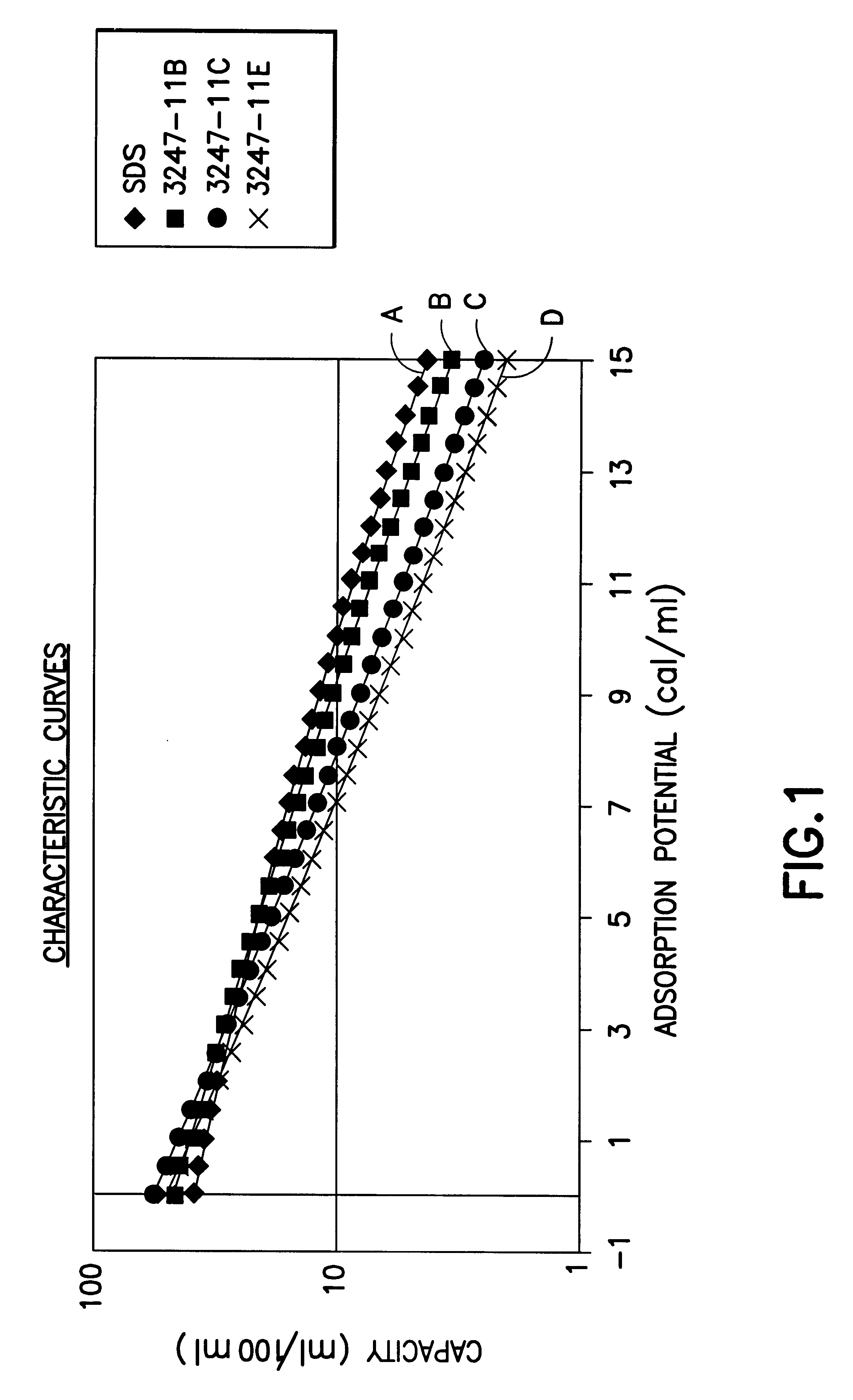
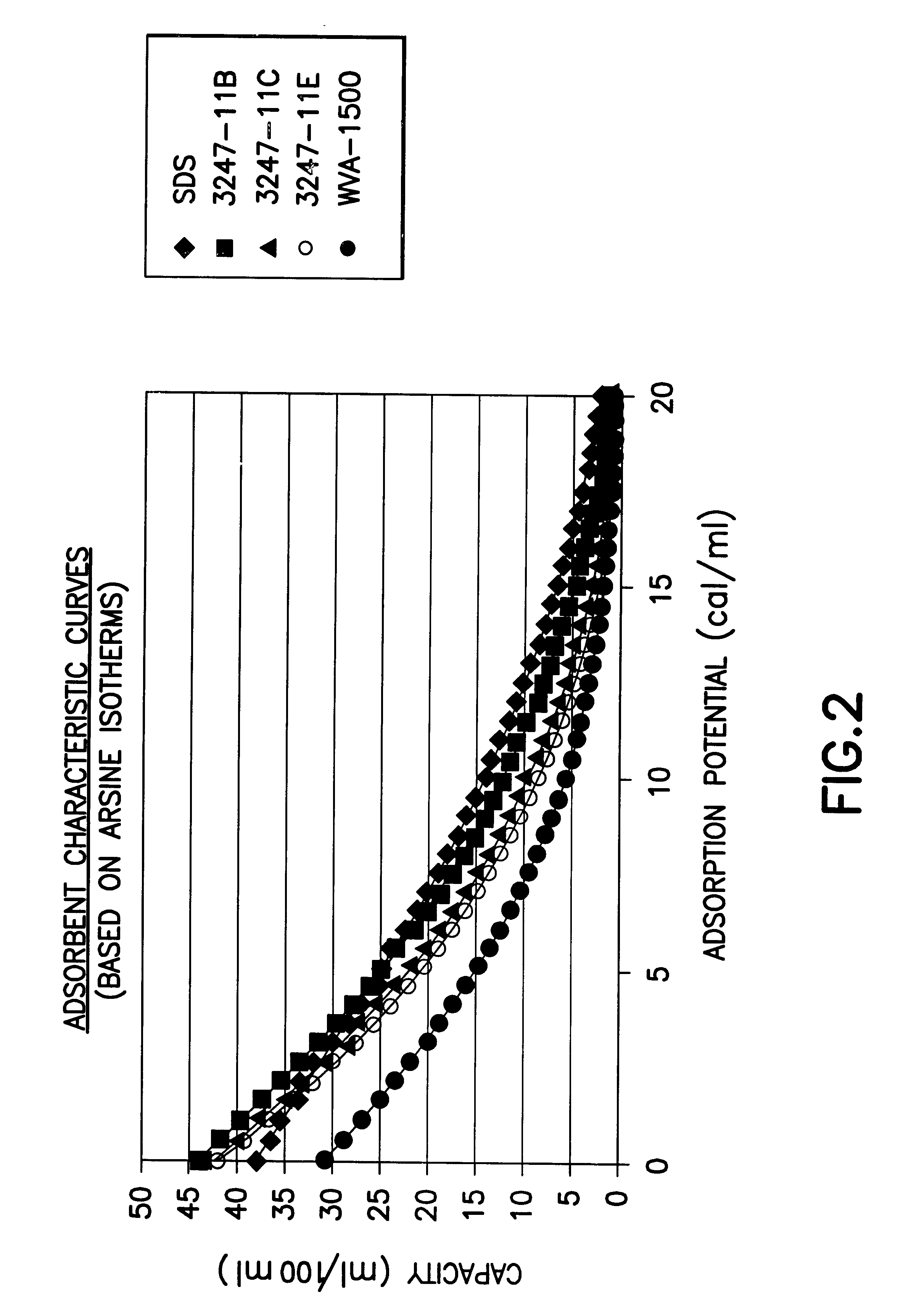
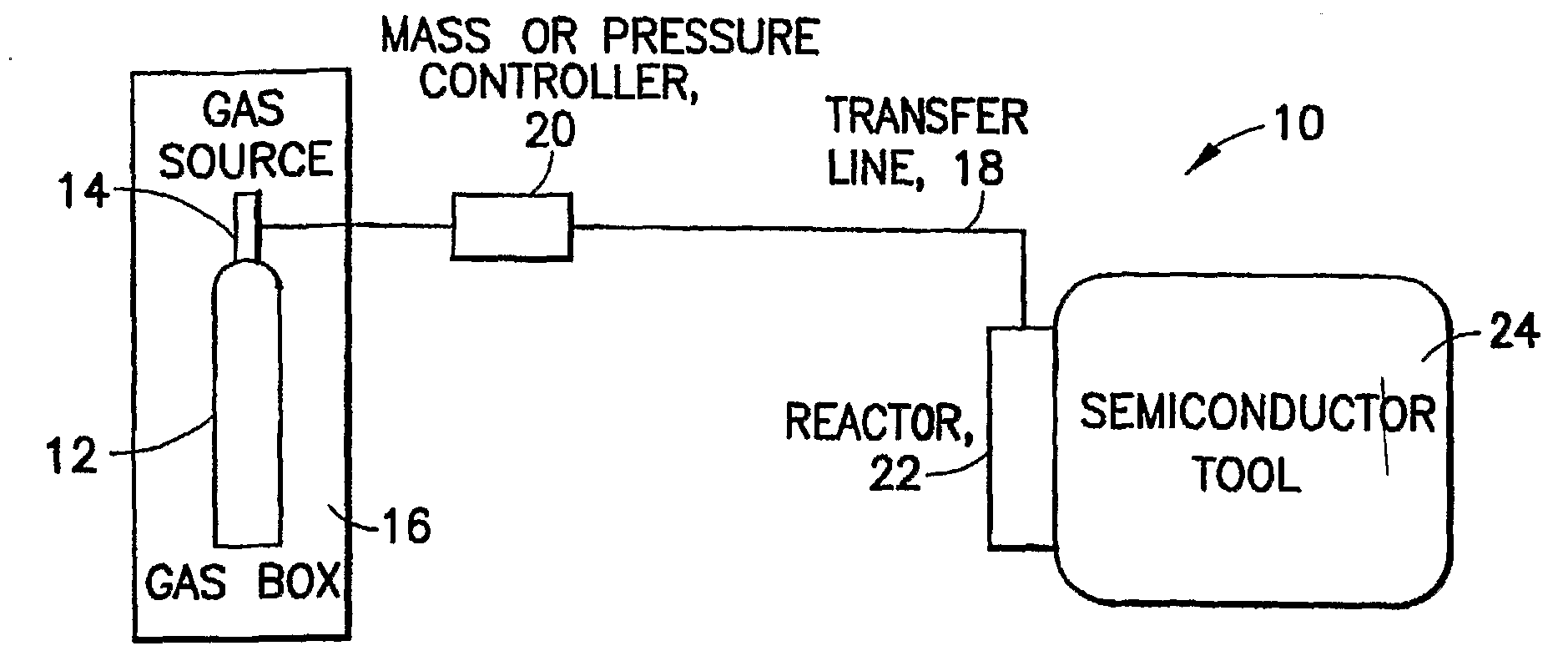
InactiveUS6592653B2Reduces sufficiencyImprove adsorption capacityGas treatmentOther chemical processesDesorptionSorbent
A fluid storage and dispensing system including a vessel containing a low heel carbon sorbent having fluid adsorbed thereon, with the system arranged to effect desorption of the fluid from the sorbent for dispensing of fluid on demand. The low heel carbon sorbent preferably is characterized by at least one of the following characteristics: (i) Heel, measured for gaseous arsine (AsH3) at 20° C. at 20 Torr, of not more than 50 grams AsH3 per liter of bed of the sorbent material; (ii) Heel, measured for gaseous boron trifluoride (BF3) at 20° C. at 20 Torr, of not more than 20 grams boron trifloride per liter of bed of the sorbent material; (iii) Heel, measured for gaseous germanium tetrafluoride (GeF4) at 20° C. at 20 Torr, of not more than 250 grams AsH3 per liter of bed of the sorbent material; (iv) Heel, measured for gaseous arsenic pentafluoride (AsF5) at 20° C. at 20 Torr, of not more than 700 grams AsF5 per liter of bed of the sorbent material; (v) Heel, measured for gaseous trimethyl silane (3MS) at 20° C. at 20 Torr, of not more than 160 grams 3MS per liter of bed of the sorbent material; and (vi) Heel, measured for gaseous ethane (C2H4) at 21° C. at 25 Torr, of not more than 10 grams ethane per liter of bed of the sorbent material.
Owner:ENTEGRIS INC
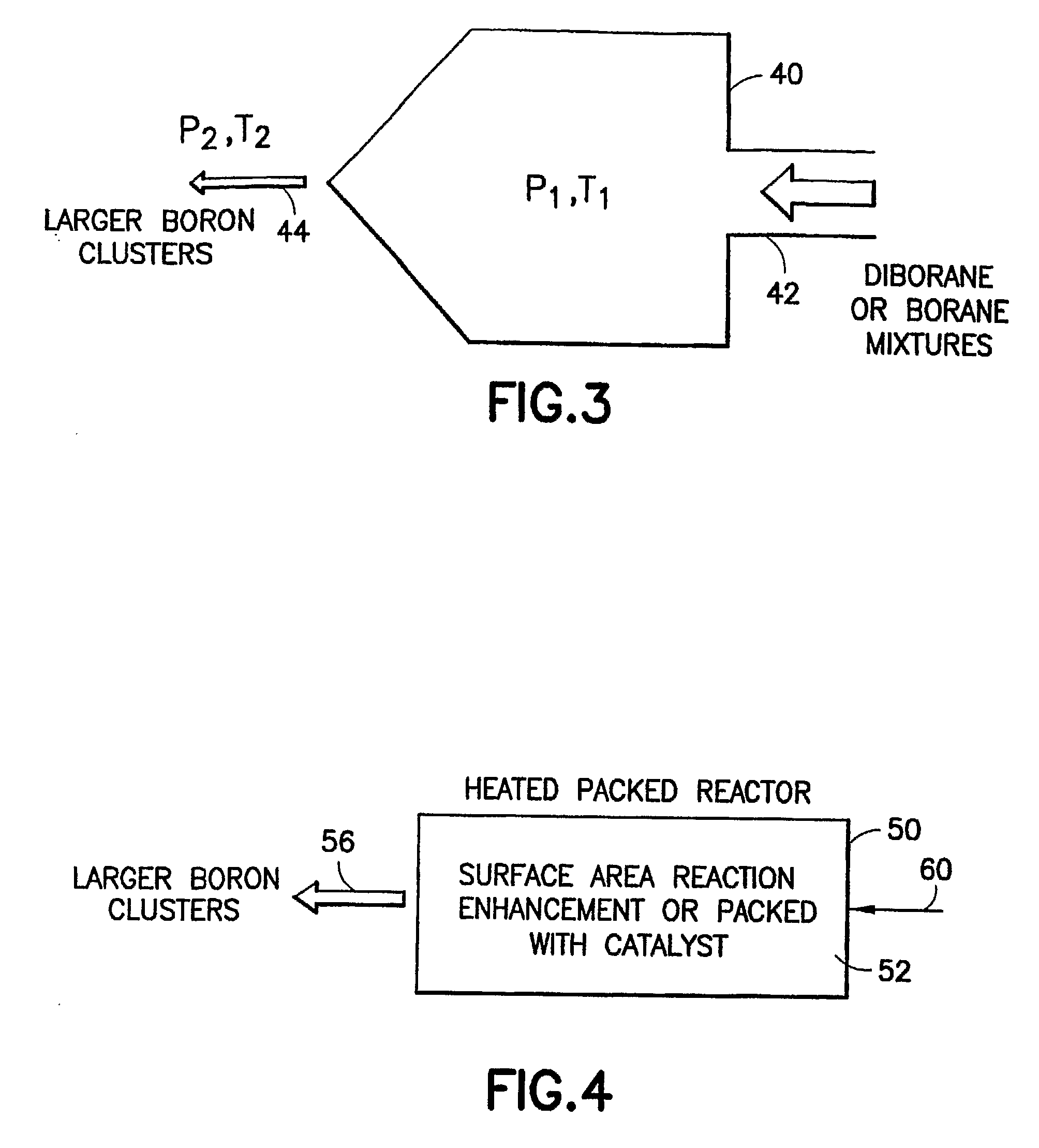
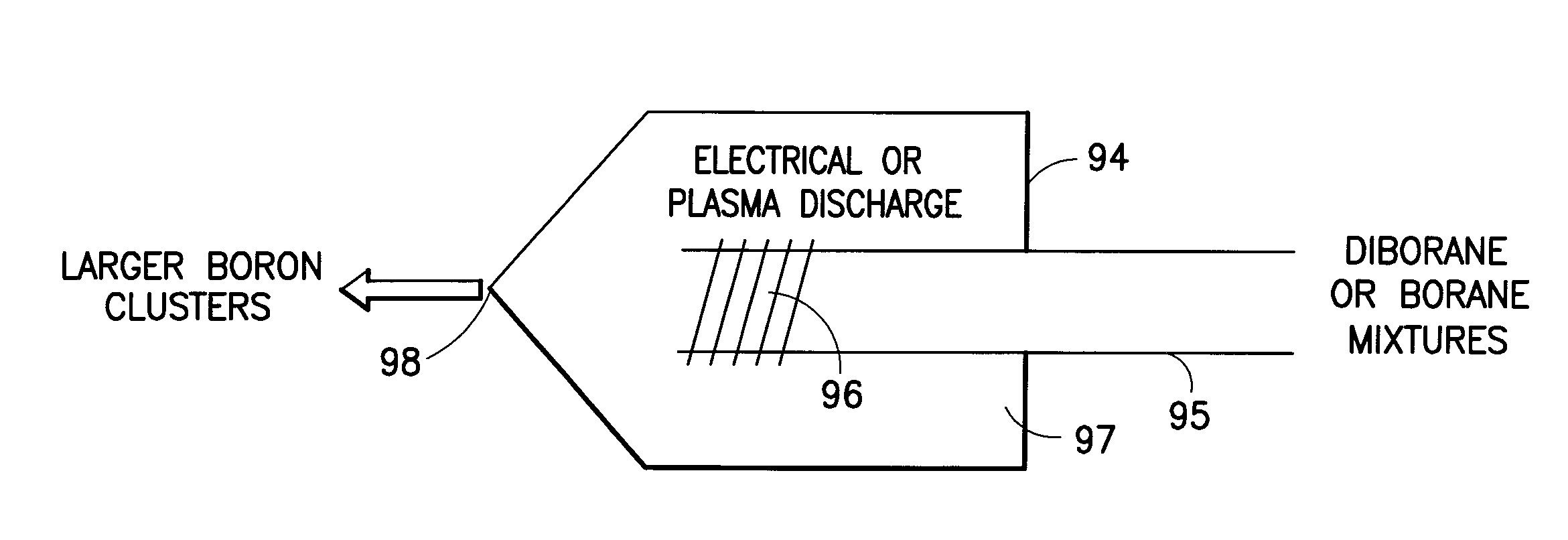
Boron Ion Implantation Using Alternative Fluorinated Boron Precursors, and Formation of Large Boron Hydrides for Implanation
ActiveUS20080248636A1Easy to cutImprove efficiencyOther chemical processesElectric discharge tubesBoron trifluorideIon implantation
Methods of implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. A method of manufacturing a semiconductor device including implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. Also disclosed are a system for supplying a boron hydride precursor, and methods of forming a boron hydride precursor and methods for supplying a boron hydride precursor. In one implementation of the invention, the boron hydride precursors are generated for cluster boron implantation, for manufacturing semiconductor products such as integrated circuitry.
Owner:ENTEGRIS INC
Treatment fluids containing a boron trifluoride complex and methods for use thereof
Treatment fluids for use in subterranean formations, particularly sandstone and other siliceous formations, may contain a source of fluoride ions to aid in mineral dissolution. In some cases, it may be desirable to generate the fluoride ions from a fluoride ion precursor, particularly a hydrofluoric acid precursor, such as a boron trifluoride complex. Methods described herein can comprise providing a treatment fluid that comprises an aqueous base fluid, a boron trifluoride complex, and a chelating agent composition, and introducing the treatment fluid into a subterranean formation,
Owner:HALLIBURTON ENERGY SERVICES INC
Method for producing polyisobutene
ActiveUS7217773B2Reduce molecular weightReduce the amount requiredHydrocarbons from unsaturated hydrocarbon additionHydrocarbonsHydrocarbon mixturesBoron trifluoride
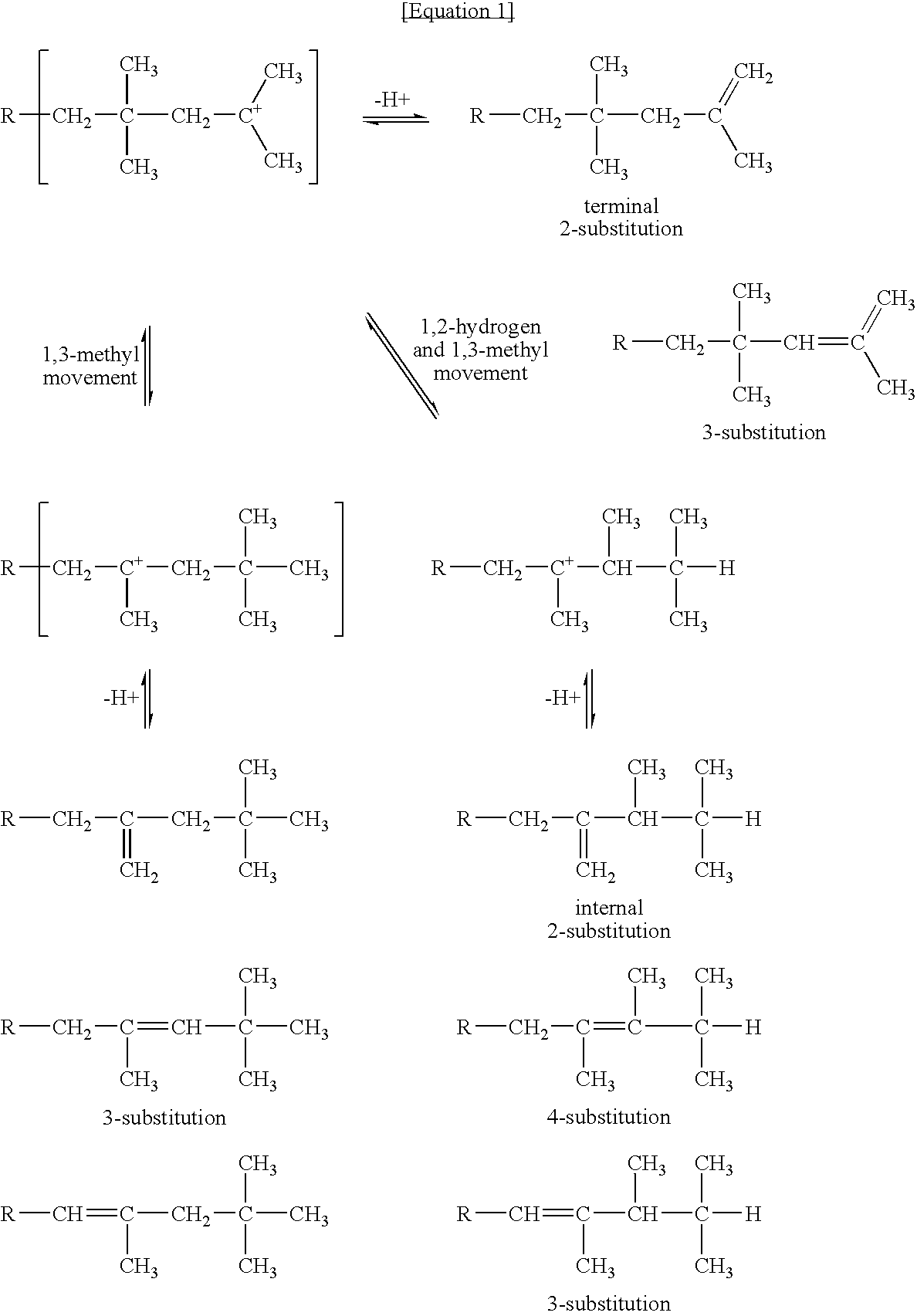
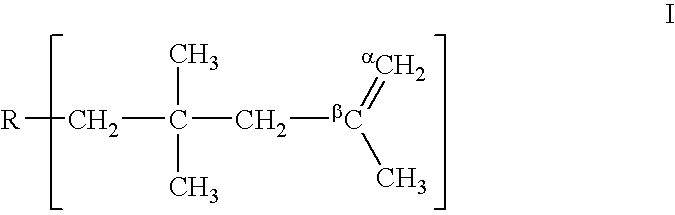
A process is described for preparing polyisobutene having a content of terminal vinylidene groups of at least 75 mol % by polymerizing isobutene or isobutenic hydrocarbon mixtures in the liquid phase in the presence of a boron trifluoride complex catalyst of the composition(BF3)a.L1b.L2c.L3dwhere L1 is water, a primary C1–C5-alkanol and / or a secondary C3–C5-alkanol, L2 is at least one aldehyde and / or one ketone, L3 is an ether having at least 5 carbon atoms, a secondary alkanol having at least 6 carbon atoms, a primary alkanol having at least 6 carbon atoms and / or a tertiary alkanol, the b:a ratio is in the range from 0.9 to 3.0, the c:a ratio is in the range from 0.01 to 0.5, and the d:a ratio is in the range from 0 to 1.0.
Owner:BASF AG
Process for producing butene polymer
InactiveUS6300444B1Reduce fluorine contentReduce residual organic fluorine contentChemical/physical/physico-chemical stationary reactorsBuilding insulationsPolymer scienceAlcohol
It is possible to produce butene polymer at high yield, which polymer contains 80 mol % or more of polymer molecules having terminal vinylidene structure and being low in the content of residual organic fluorine by a process comprising Step (I) to polymerize in liquid phase by adding complex catalyst composed of boron trifluoride, ether and alcohol and / or water in specified ratios to C4 fractions and Step (II) to reduce the content of trimer and lighter components contained in the obtained polymer to 0.2% by weight or less by distillation.
Owner:NIPPON PETROCHEMICAL CO LTD
Method for deactivating and recovering boron trifluoride when producing polyisobutenes
InactiveUS6939943B2Speed up the processCatalyst regeneration/reactivationAlcoholCationic polymerization
The invention relates to a method for deactivating and recovering boron trifluoride when producing polyisobutenes by means of cationic polymerization of isobutene or hydrocarbon streams containing isobutene in the liquid phase in the presence of boron trifluoride or in the form of a boron trifluoride catalyst complex. The catalyst complex is separated, essentially in the liquid phase, from the reactor discharge. The method comprises the following steps: a) removing from the polymerization reactor at −60 to 020 C., methanol, ethanol or a mixture of methanol and ethanol in such a quantity that an alcohol phase rich in boron trifluoride is formed; b) separating the alcohol phase according to (a) and, (c) optionally recycling the boron trifluoride of the alcohol phase obtained from (b) to the method in a suitable manner.
Owner:BASF AG
Method for producing polybutene
InactiveUS7411104B2Easy to getHydrocarbons from unsaturated hydrocarbon additionHydrocarbonsAlcoholBoron trifluoride
A method for producing high reactive polybutene (HRPB), in which carbon-carbon double bond is positioned at an end of polybutene, is disclosed. The high reactive polybutene having 300˜5000 of number average molecular weight (Mn) can be produced from a raw material containing isobutene, wherein a polymerization reaction of the isobutene is carried out in the presence of a catalyst system including secondary alkylether, tertiary alcohol, and boron trifluoride, the amount of boron trifluoride is 0.05˜1.0 weight part per 100 weight part of isobutene, the mole ratio of a co-catalyst including secondary alkylether and tertiary alcohol:boron trifluoride is 1.0˜2.0:1, and the mole ratio of secondary alkylether:tertiary alcohol is 0.5˜1.2:1.
Owner:DL CHEM CO LTD
Preparation method for non-ionic reactive water-borne epoxy resin emulsion
InactiveCN104558524AImprove water-based effectGood dispersionEpoxy resin coatingsFreeze thawingEpoxy
The invention provides a preparation method for non-ionic reactive water-borne epoxy resin emulsion. A preparation process for the non-ionic reactive water-borne epoxy resin emulsion comprises the following steps: simultaneously adding a certain amount of non-ionic hydrophilic segment polyethylene glycol and boron trifluoride diethyl etherate serving as a catalyst into epoxy resin, performing stable reaction for 3-8 hours to obtain non-ionic reactive epoxy resin, then strongly stirring at the temperature of 50 DEG C, and uniformly and dropwise adding distilled water to form water-dispersed epoxy resin emulsion by a phase inversion technology. The preparation method has the benefits that the particle size of the dispersion phase of the prepared non-ionic reactive water-borne epoxy resin emulsion is dozens to hundreds of nanometer; both under high-speed centrifugation and long-time storage, the non-ionic reactive water-borne epoxy resin emulsion is high in stability; furthermore, the emulsion is higher in dilutability, salt resistance, alkali resistance, high temperature resistance, and freeze-thaw stability. The raw materials used in the method are simple, efficient, environment-friendly, safe and non-toxic and can be widely applied to the fields of coatings, composite materials and the like.
Owner:BEIJING UNIV OF CHEM TECH
Method for producing polyisobutylenes
InactiveUS6846903B2Hydrocarbons from unsaturated hydrocarbon additionHydrocarbonsCationic polymerizationBoron trifluoride
Owner:BASF AG
Method of preparing waterless lithium terafluoroborate
InactiveCN101318664AThe product quality is quiteEfficient removalTretrafluoboric acidLithium hydroxideX-ray
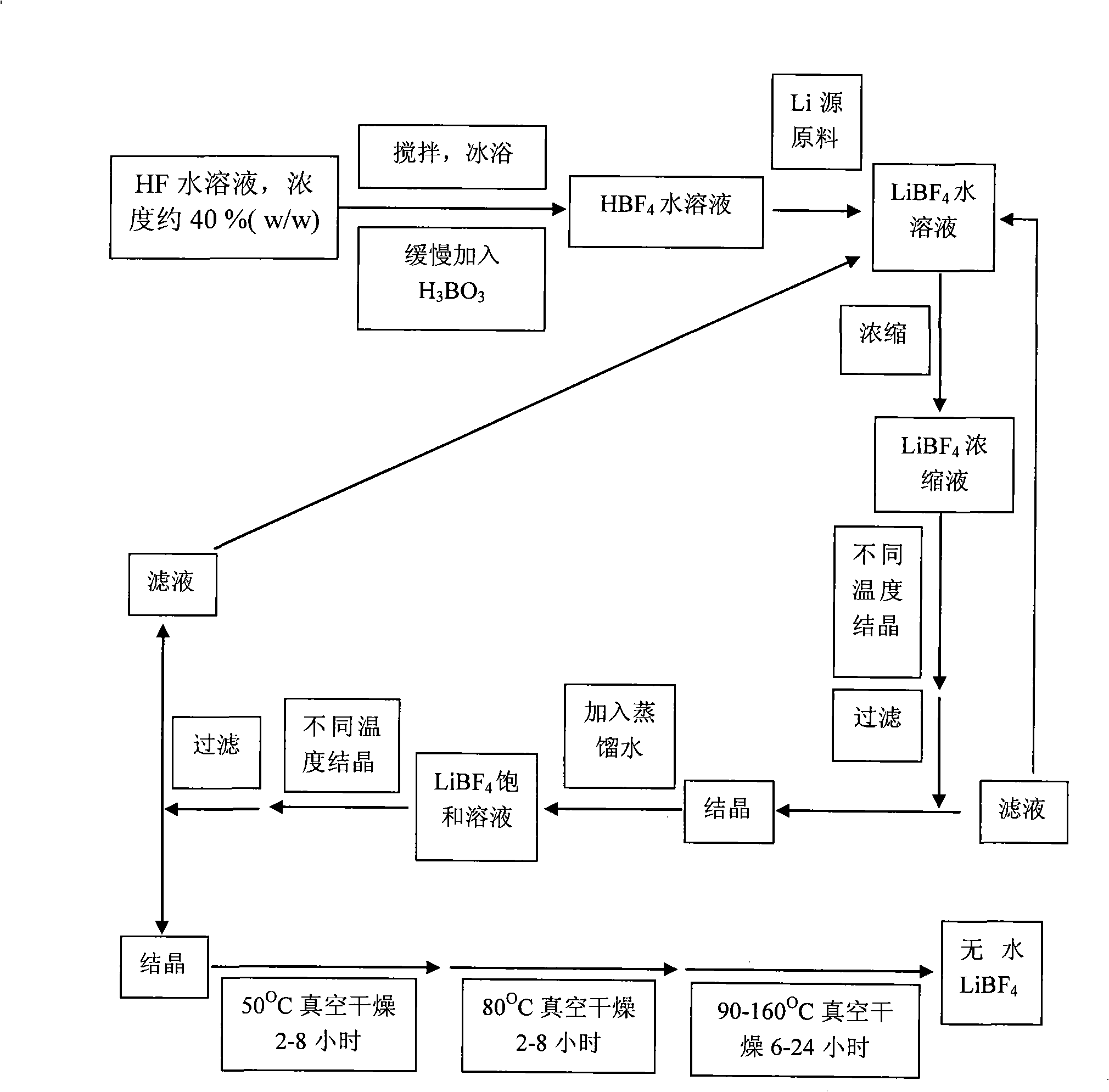
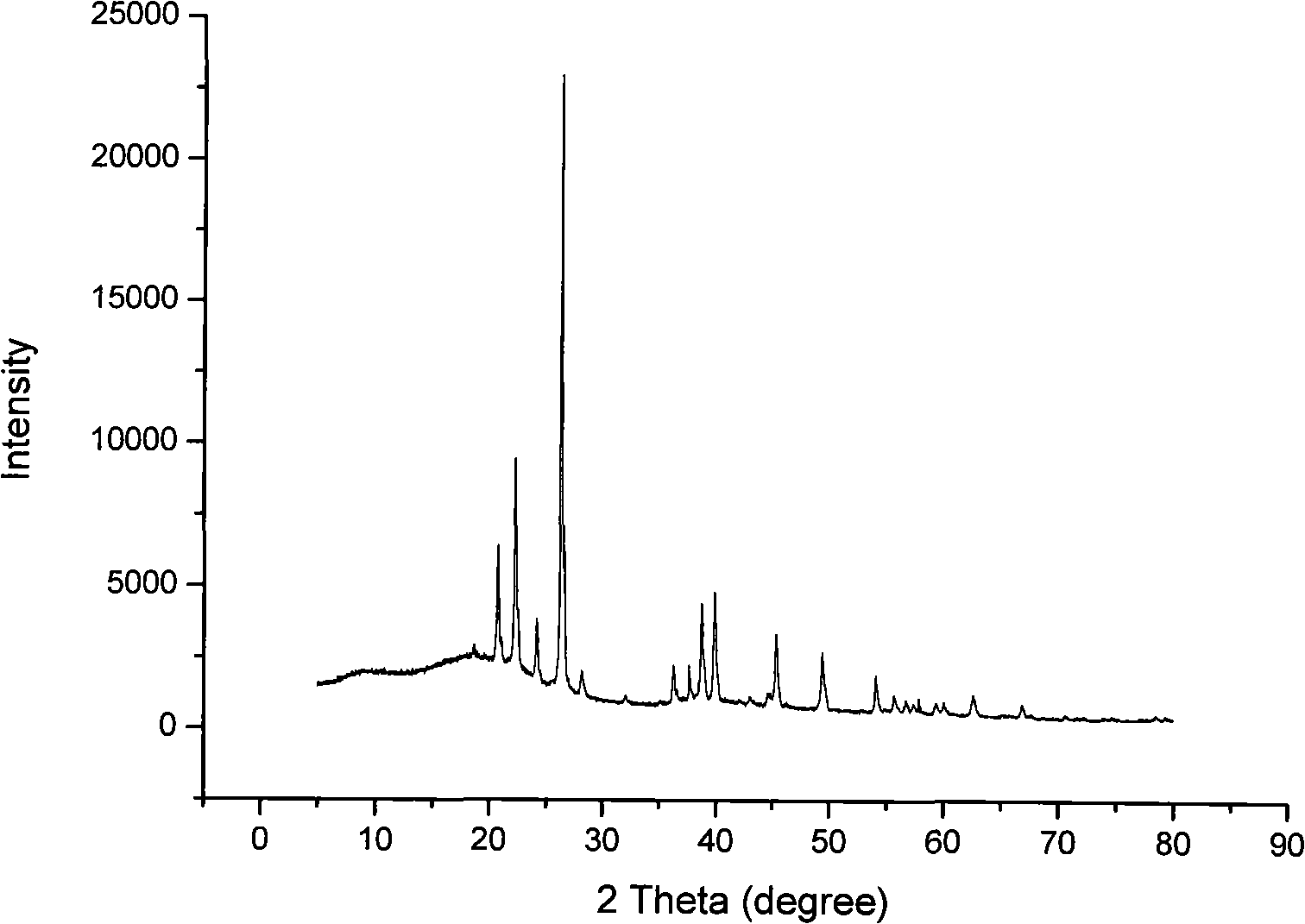
The invention relates to a method for preparing an anhydrous lithium tetrafluoroborate which comprises the following steps: a lithium source including lithium hydroxide, lithium carbonate, and the like, reacts with fluorine hydride and boric acid to obtain lithium tetrafluoroborate solution, then the lithium tetrafluoroborate solution is condensed, crystallized and recrystallized, ground, and vacuum dried to obtain the anhydrous lithium tetrafluoroborate. The method of the invention adopts a staged temperature rise control, the process of the preparation is simple, raw materials are cheap and the preparation cost is low, no organic solvent is used during the synthetic process, no poison is produced, therefore, the method accords with the concept of green environmental protection, the anhydrous lithium tetrafluoroborate prepared by the method is determined by a X-ray diffraction map, the diffraction peak is clear and sharp and completely matches the standard card, which shows that the product prepared by the method of the invention is anhydrous LiBF4 with complete crystal form, the quality of the product is equal to the quality of the anhydrous lithium tetrafluoroborate prepared by the reaction of lithium fluoride and boron trifluoride.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Nitrogen trifluoride production apparatus
InactiveUS6010605AHigh purityReduce impurityElectrolysis componentsNitrogen trifluorideHydrogen fluorideBoron trifluoride
Apparatus is disclosed for the production of nitrogen trifluoride (NF3), starting with an anhydrous molten flux including ammonia (NH3), a metal fluoride (MF), and hydrogen fluoride (HF). The apparatus includes an electrolyzer, an ammonia solubilizer, a hydrogen fluoride solubilizer, a nitrogen trifluoride reactor, two compressors, two pumps, three condensers a gas recycle loop, and, two flux loops of the same component ternary flux, but each loop with different concentration.
Owner:FLORIDA SCI LAB
Process for preparation of high 1,4-CIS polybutadiene
InactiveUS6451934B1Hydrocarbon by isomerisationOrganic-compounds/hydrides/coordination-complexes catalystsNickel saltPolymer science
This invention relates to a process for preparation of high 1,4-cis polybutadiene and more particularly, to the process for preparing polybutadiene by polymerizing 1,3-butadiene monomer in the presence of a catalyst prepared by aging a mixture of a neodymium salt compound, a nickel salt compound, an organoaluminium compound and a borontrifluoride complex compound in the presence or absence of a conjugated diene compound. With much remarked catalytic activity, polybutadiene with a very high 1,4-cis content can be prepared in a high yield using a small amount of catalyst.
Owner:KOREA KUMHO PETROCHEMICAL CO LTD
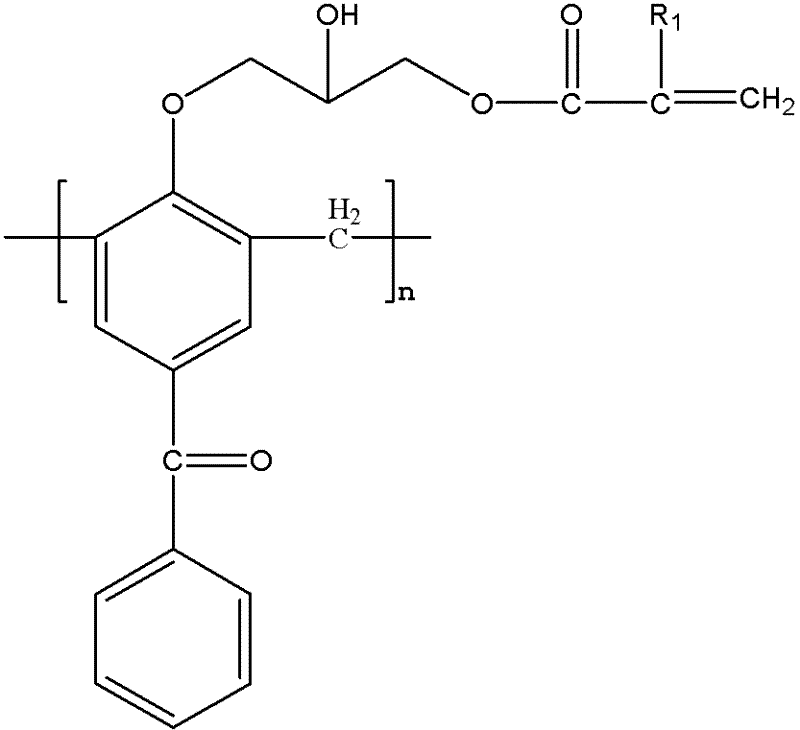
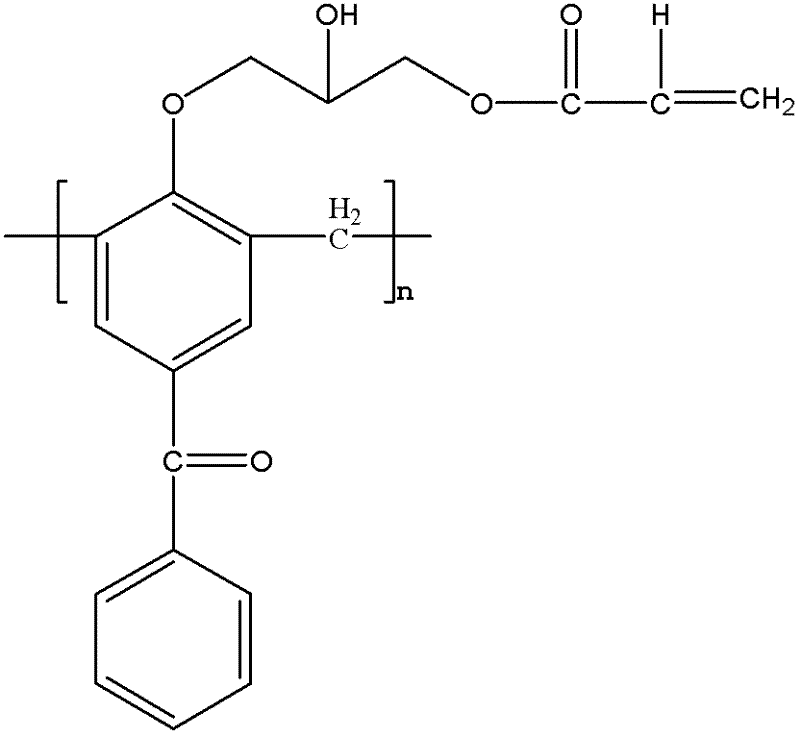
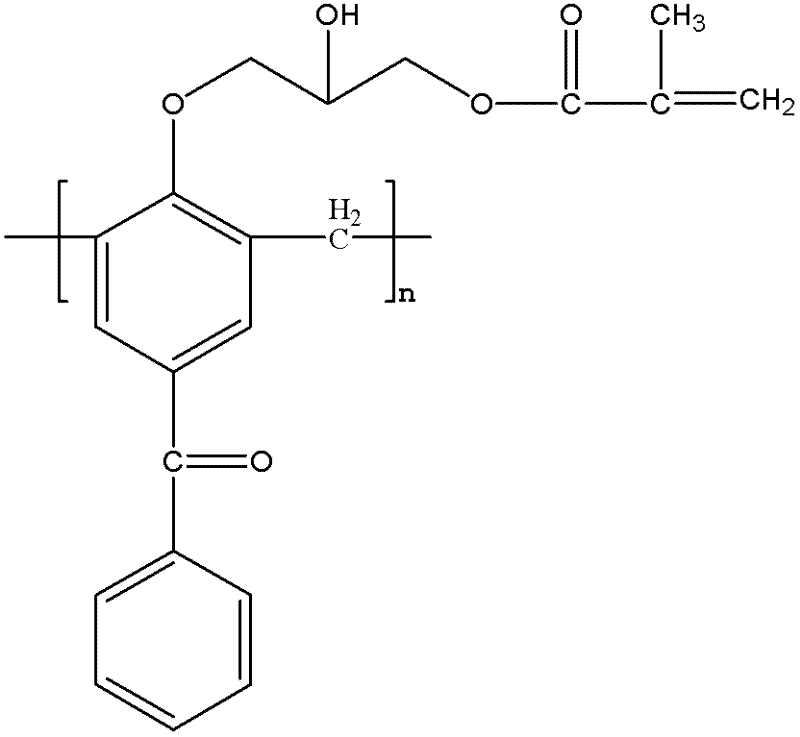
Macromolecular polymerizable photoinitiator and preparation thereof
InactiveCN102585045AAvoid reaction stepSimple operating conditionsHydroxybenzoate EthersOrganic solvent
The invention discloses a macromolecular polymerizable photoinitiator and a preparation thereof. The method comprises the following steps of: adding 4-hydroxybenzophenone and methanal to a three-necked flask, heating to 95 DEG C, reacting for 2 hours, heating to 150DEG C, leaching under at reduced pressure for 20 minutes, cooling to 105 DEG C, collecting products, pouring products into water, leaching to obtain a macromolecular photoinitiator, dissolving the macromolecular photoinitiator in an organic solvent, dissolving epoxy chloropropane in the solvent under catalysis of boron trifluoride,dropwise adding mixed liquid, reacting at a temperature of 0-5 DEG C, then stirring for 3 hours at a temperature of 70 DEG C, removing epoxy chloropropane and solvent, dissolving residues with a solvent, adding alkali and reacting for 0.5 hour at a temperature of 40 DEG C, removing salt and the solvent, re-dissolving residues with a solvent, washing with water and drying, adding crylic acid, p-hydroxyanisole and tetrabutylammonium bromide, heating for reacting for 6 hours, and then removing the solvent so as to obtain macromolecular polymerizable photoinitiator. The macromolecular polymerizable photoinitiator prepared by the method has the advantages of large molecular weight, weak mobility and good polymerizability.
Owner:HANGZHOU INST OF ADVANCED MATERIAL BEIJING UNIV OF CHEM TECH
Boron ion implantation using alternative fluorinated boron precursors, and formation of large boron hydrides for implantation
ActiveUS20110065268A1Easy to cutImprove efficiencyElectric discharge tubesVacuum evaporation coatingBoron trifluorideBoron containing
Methods of implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. A method of manufacturing a semiconductor device including implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. Also disclosed are a system for supplying a boron hydride precursor, and methods of forming a boron hydride precursor and methods for supplying a boron hydride precursor. In one implementation of the invention, the boron hydride precursors are generated for cluster boron implantation, for manufacturing semiconductor products such as integrated circuitry.
Owner:ENTEGRIS INC
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
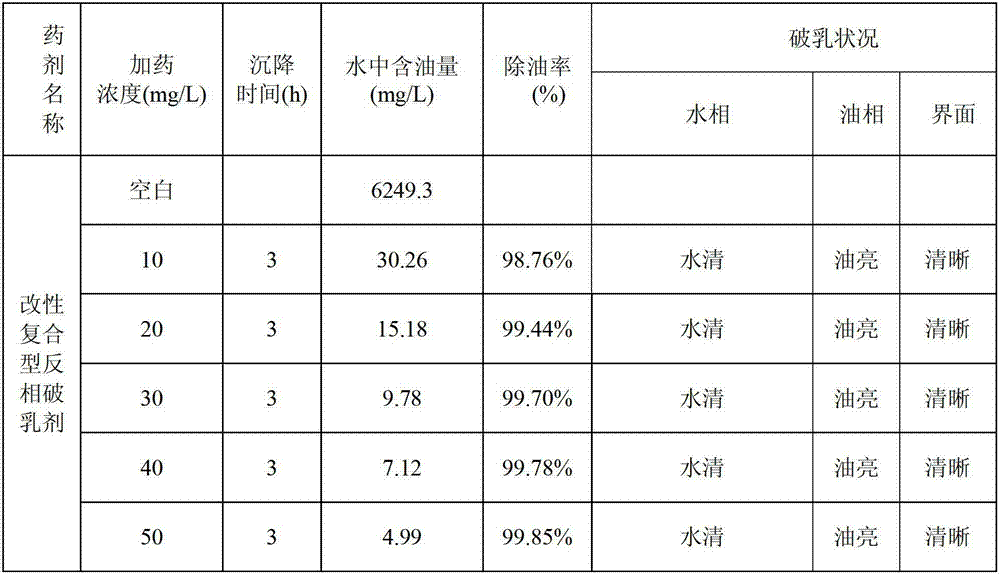
High-temperature sewage treatment reverse demulsifier of ultra-heavy oil
InactiveCN103086472ASimple technical routeMild reaction conditionsFatty/oily/floating substances removal devicesNon-miscible liquid separationCross-linkPotassium hydroxide
The invention discloses a high-temperature sewage treatment reverse demulsifier of ultra-heavy oil. The method comprises the following steps of preparing chlorinated polyether by taking propanetriol and epichlorohydrin as materials and boron trifluoride ether solution as an initiator; preparing cationic poly ether by placing the chlorinated polyether and trimethylamine in a high-pressure reactor; by taking carbinol as a solvent, and boron trifluoride diethyl etherate as the initiator, repeatedly and alternatively carrying out Michael addition reaction and amidation by ethanediamine, methyl acrylate and the like to prepare 3.0-generation dendritic macromolecule polyamide-amine; by taking polyethylene glycol and poly propylene glycol as materials, and potassium hydroxide powder as a catalyst, adding polypropylene glycol to prepare non-ionic polyether after the polyethylene glycol reacts a period of time; finally by taking 3.0-generation polyamide-amine as a cross-linking agent, preparing the modified composite polyamide-amine-polyether high-temperature sewage treatment reverse demulsifier of the ultra-heavy oil by compounding cationic polyether and non-ionic polyether.
Owner:KARAMAY SANDA NEW TECH +1
Electrolyte and lithium ion battery comprising same
ActiveCN105789701AImprove cycle performanceEasy to storeSecondary cellsOrganic electrolytesOrganic solventBoron trifluoride
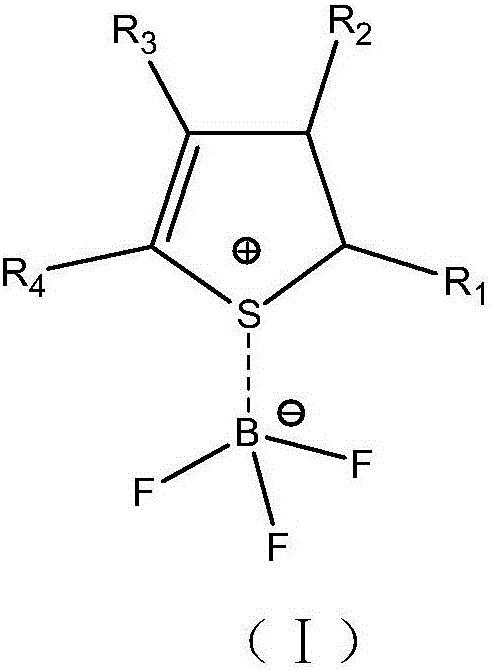
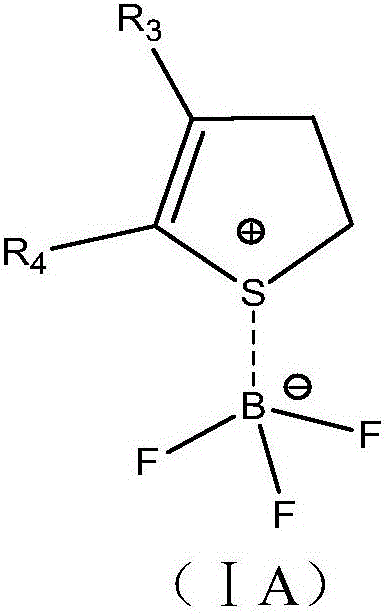
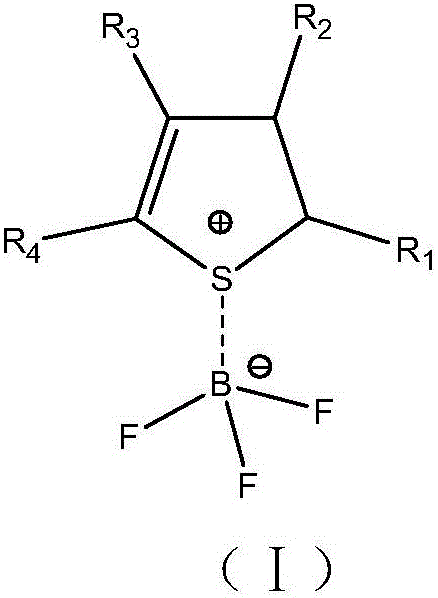
The invention relates to the field of batteries and particularly relates to an electrolyte and a lithium ion battery comprising the same. The electrolyte comprises an organic solvent, lithium salt and additives, wherein the additives include thiophene hydride-boron trifluoride coordination compound and lithium fluorophosphate. According to the electrolyte provided by the invention, under the joint synergistic effect between the thiophene hydride-boron trifluoride coordination compound and the lithium fluorophosphate, an SEI film capable of inhibiting electrolyte decomposition is formed on the surfaces of the positive and negative plates of the lithium ion battery respectively, and acidic materials generated in the electrolyte also can be neutralized, thereby greatly improving the cycle performance and storage performance of the lithium ion battery.
Owner:JIANGSU CONTEMPORARY AMPEREX TECHNOLOGY LIMITED
Electrolyte and lithium ion battery comprising same
ActiveCN105655639AImprove cycle performanceIncrease storage capacitySecondary cellsOrganic electrolytesOrganic solventBoron trifluoride
The invention relates to electrolyte and a lithium ion battery comprising the same. The electrolyte comprises an organic solvent, a lithium salt and an additive, wherein the additive comprises a nitrogen-containing heterocyclic ring-boron trifluoride coordination compound and a sultone compound, and a nitrogen-containing heterocyclic ring is selected from at least one of a pyridyl-containing heterocyclic ring, a pyridazinyl-containing heterocyclic ring, a pyrimidinyl-containing heterocyclic ring, a pyrazinyl-containing heterocyclic ring, a pyrryl-containing heterocyclic ring, a pyrazolyl-containing heterocyclic ring and an imidazolyl-containing heterocyclic ring. The electrolyte simultaneously comprises the nitrogen-containing heterocyclic ring-boron trifluoride coordination compound and the sultone compound, so that the cycling performance of the lithium ion battery at a normal temperature and a high temperature and the storage performance of the lithium ion battery at a high temperature can be improved.
Owner:DONGGUAN AMPEREX TECH
Method for synthesizing isomerous tridecanol polyoxyethylene ether
The invention relates to a synthetic method of isomeric tridecanol polyoxyethylene ether, belonging to the organic compound synthesis technical field, which uses the materials of isometric tridecanol and ethylene oxide. The synthetic method is characterized in that: firstly, the polymerization reaction is carried out with the boron trifluoride catalyst, and then the isomeric tridecanol polyoxyethylene ether is obtained by the polymerization reaction carried out with the strong alkali catalyst; wherein, the addition of the boron trifluoride adopted in the reaction is 0.1 to 0.6 percent of the weight of the isometric tridecanol, the strong alkali catalyst is one of or the mixture of solid sodium methylate, methanol solution of sodium methylate, KOH and NaOH, the addition of the strong alkali catalyst is 0.05 to 0.2 percent of the weight of isomeric tridecanol polyoxyethylene ether. The synthetic method of isomeric tridecanol polyoxyethylene ether has the advantages that: the synthetic method adopts a two-step reaction technical proposal that: the polymerization reaction is carried out with the boron trifluoride catalyst at first, and then the polymerization reaction is carried out with the strong alkali catalyst, so the isomeric tridecanol polyoxyethylene ether has mild reaction conditions, high yield, no formation of impurities and environment friendly performance.
Owner:ZHEJIANG HUANGMA TECH
Method for producing polyisobutenes
A process for the preparation of polyisobutylenes by cationic polymerization of isobutylene or isobutylene-containing hydrocarbon streams in the liquid phase in the presence of boron trifluoride acting as catalyst, the catalytic activity of boron trifluoride being partially or completely stopped by means of a solid deactivator following a given timelapse, which deactivator is an inorganic, anhydrous or hydrous oxygen compound of aluminum which is insoluble in the reaction mixture.
Owner:BASF AG
Mesophase pitch raw material as well as preparation method and application of mesophase pitch raw material in preparing high-performance carbon fiber
InactiveCN104087331ASimple preparation processImprove controllabilityWorking-up pitch/asphalt/bitumen by chemical meansFibre chemical featuresCarbon fibersElemental analysis
The invention relates to a mesophase pitch raw material as well as a preparation method and an application of the mesophase pitch raw material in preparing a high-performance carbon fiber. By virtue of measurement based on an elemental analysis method for the mesophase pitch raw material, the molar ratio H / C of hydrogen atoms to carbon atoms in the molecular structure of the mesophase pitch raw material is 0.55-1.0; the molar ratio of the naphthenic carbon content to the total carbon content in the molecular structure measured by virtue of a nuclear magnetic resonance method is greater than 9%; the softening point temperature, measured by an insertion method, of the mesophase pitch raw material is 200-240 DEG C; the content, measured by a polarizing microscope method, of the mesophase is 100%. The preparation method comprises the following step: polymerizing naphthalene-based compounds such as methylnaphthalene used as raw materials in the presence of a mixture of hydrogen fluoride and boron trifluoride as a catalyst by virtue of controlling the polymerization conditions. Compared with the prior art, the mesophase pitch raw material has the advantages of good spinning stability and high pre-oxidation activity, and is conductive to the control and operation of the carbon fiber spinning and pre-oxidation processes, the operation process is simple and the mesophase pitch raw material is conducive to preparing a high-performance pitch-based carbon fiber.
Owner:SHANGHAI JIAO TONG UNIV
Preparation method for waterborne polyurethane modified epoxy resin curing agent
The invention relates to a preparation method for a waterborne polyurethane modified epoxy resin curing agent. The preparation method comprises the following steps: adding catalyst boron trifluoride ether to a polyethylene glycol, then adding epoxy resin, stirring and heating the mixture for some time to prepare modified epoxy resin with epoxy groups at both ends; dripping monoepoxy compound 501 to diethylenetriamine according to an equal molar ratio to prepare a blocking product; reacting the obtained modified epoxy resin with bisepoxy compound 128 and polyurethane prepolymer by mixing to prepare polyurethane modified diepoxide; dripping the prepared polyurethane modified diepoxide to the prepared blocking product to obtain an addition product; and neutralizing the addition product into a salt by dripping glacial acetic acid, and then adding water to prepare the waterborne polyurethane modified epoxy resin curing agent. By introducing the epoxy resin, the invention enables the paint film obtained after curing to have superior water resistance, solvent resistance, chemical resistance and flexibility, thereby achieving the fine characteristics of the polyurethane, and overcoming the disadvantages of the epoxy resin.
Owner:TIANJIN UNIV
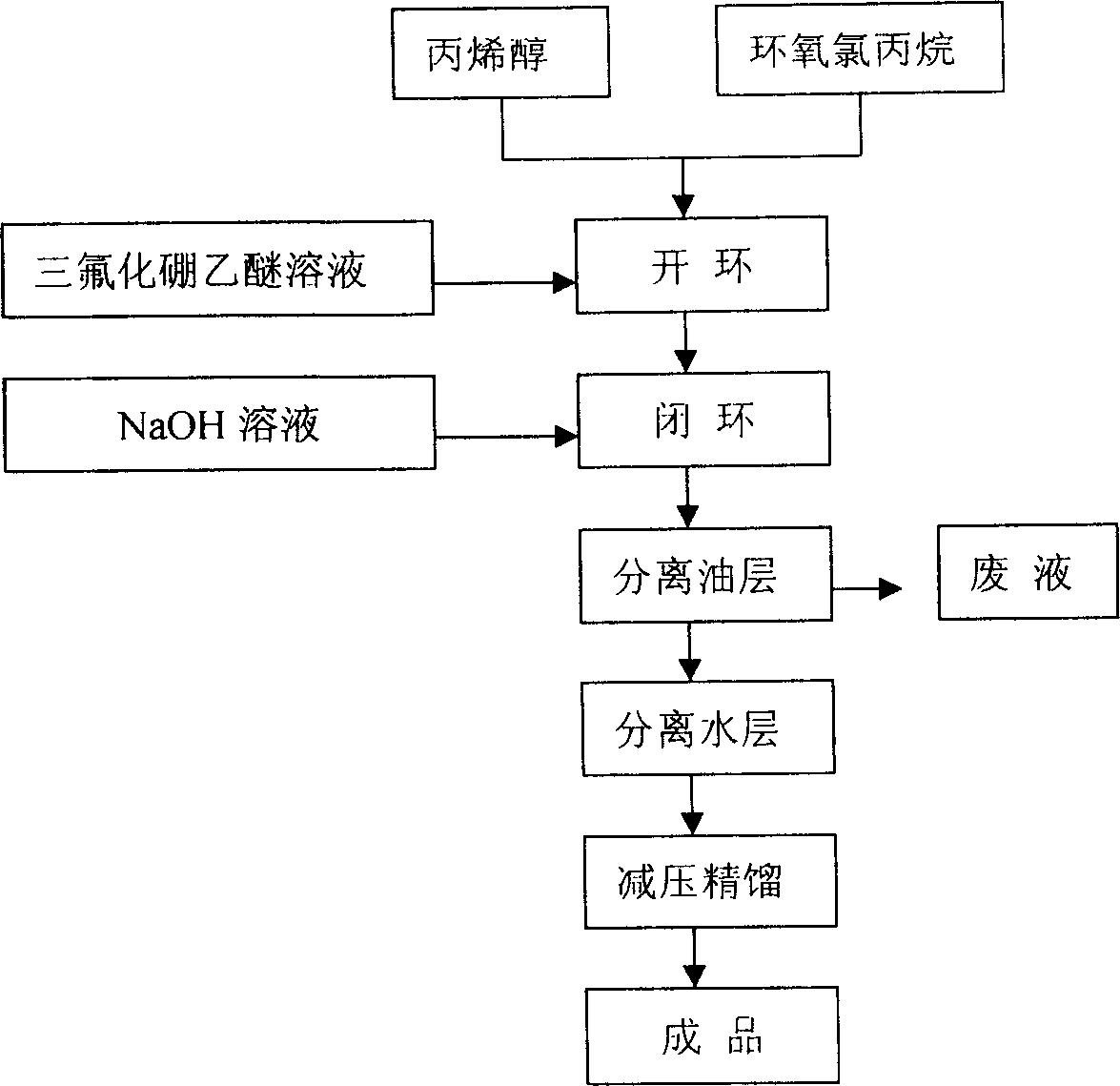
Process of industrialized preparing allyl glycidol ether
This invention provides an allyl glycidyl ether industrialized preparation method. It uses Boron trifluoride diethyl ether comoles compound as catalyst, propylene alcohol and epichlorohydrin as raw material, pass through the condensation ring-opening reactions generate 1-chloro-2-hydroxy-3- allyloxy propane, then under alkaline conditions through close-loop to obtain allyl glycidyl ether; the product yield rate is 78%, the content of 98%.
Owner:樊福定
Process for producing polymerization type hindered phenol antioxidant 616
The invention discloses a method for preparing a polymeric hindered phenol antioxidant 616, which pertains to the chemical technology field. Under normal pressure in an acid and alkali resistant container at a temperature of 60 DEG C to 120 DEG C and under the catalysis of a boron trifluoride etherate complex, dicyclopentadiene is dripped into cresol and dicyclopentadiene to carry out a condensation reaction to generate a petroleum resin; then at the temperature of 60 DEG C to 100 DEG C, a benzene solvent is added and under the catalysis of concentrated sulphuric acid, isobutene is added to carry out an alkylation so as to generate an antioxidant 616; then at the temperature of 70 DEG C to 80 DEG C, activated clay is added and stirred for 1h to 2h, decatalyzation and decoloration are carried out, a filter cake is filtered and recovered, and decompression is conducted and the benzene solvent is recovered so as to obtain a solid; and the solid is ground into a white powder, which is the product. The method can reduce equipment corrosion and environmental protection and the product obtained has the advantages of being light in color and having high quality and wide application range and the like.
Owner:SANMENXIA XIAWEI CHEM
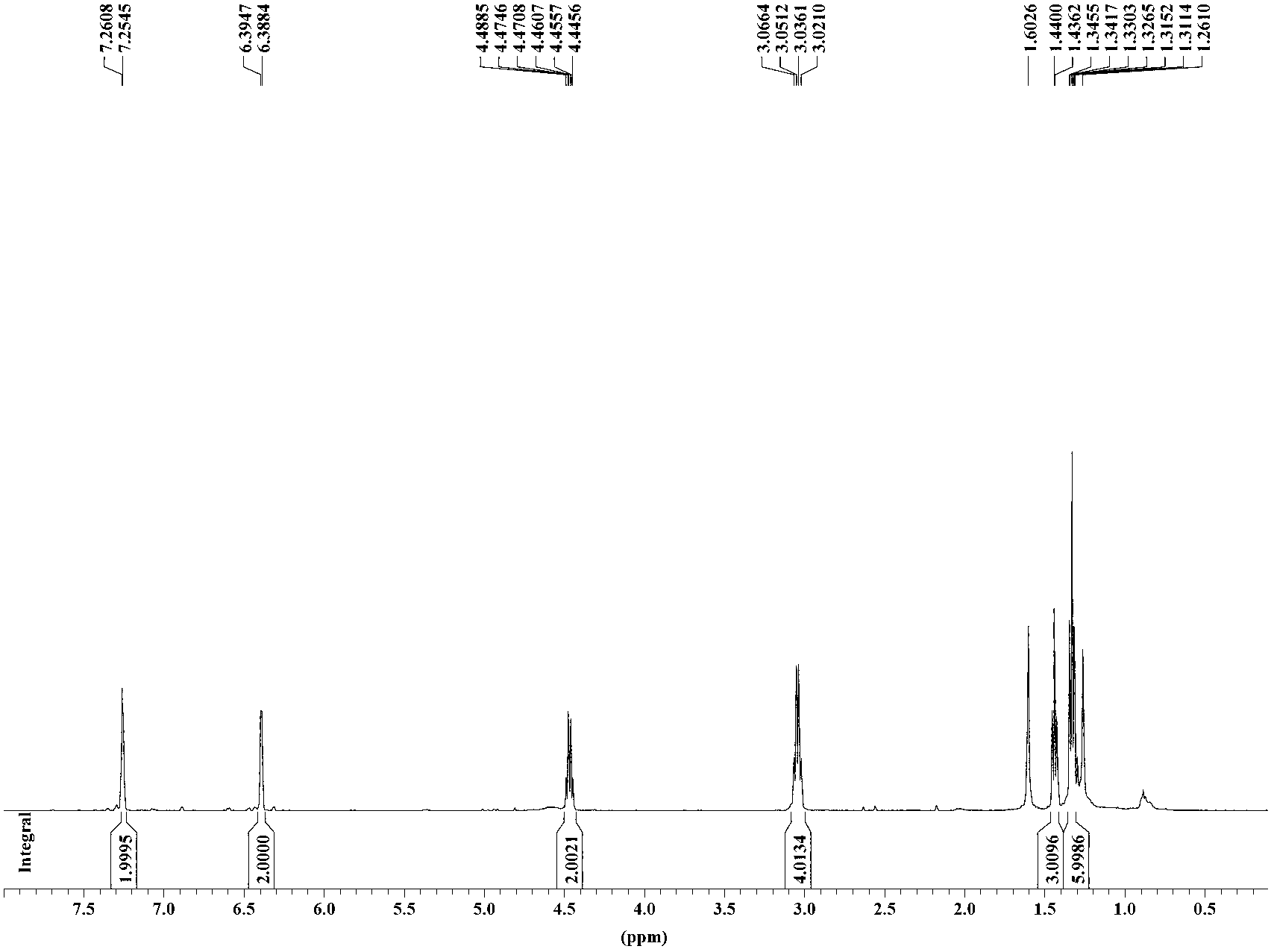
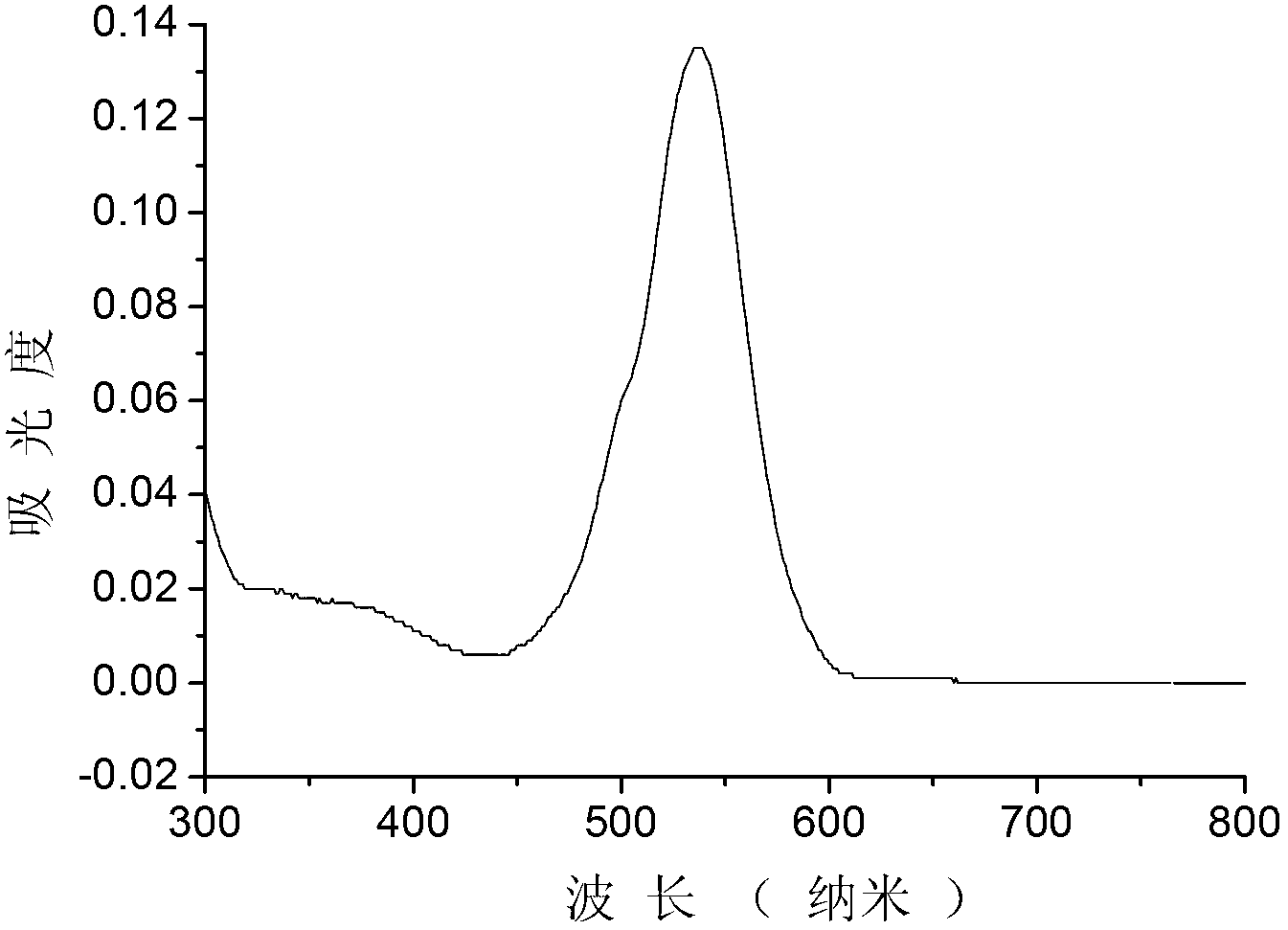
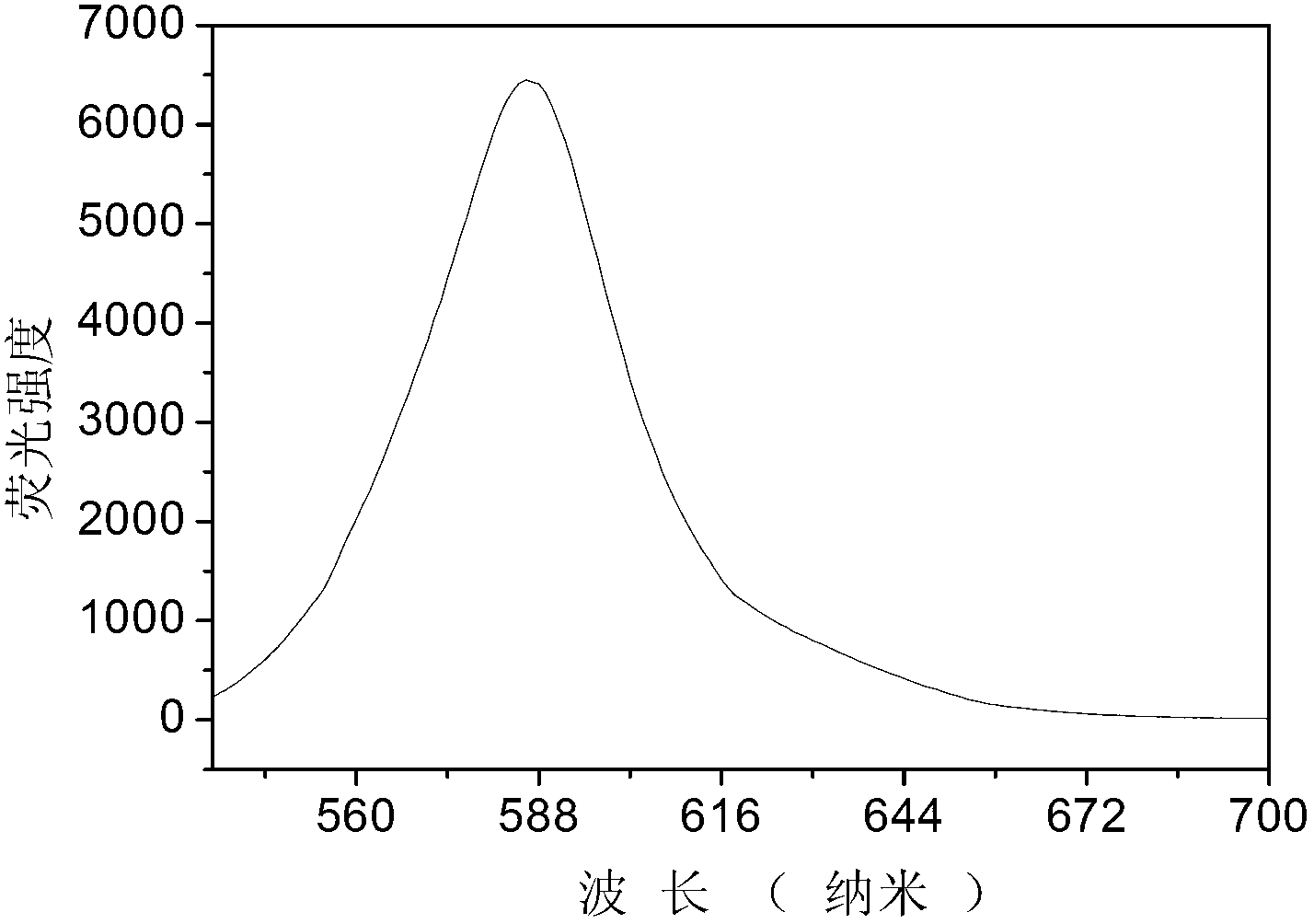
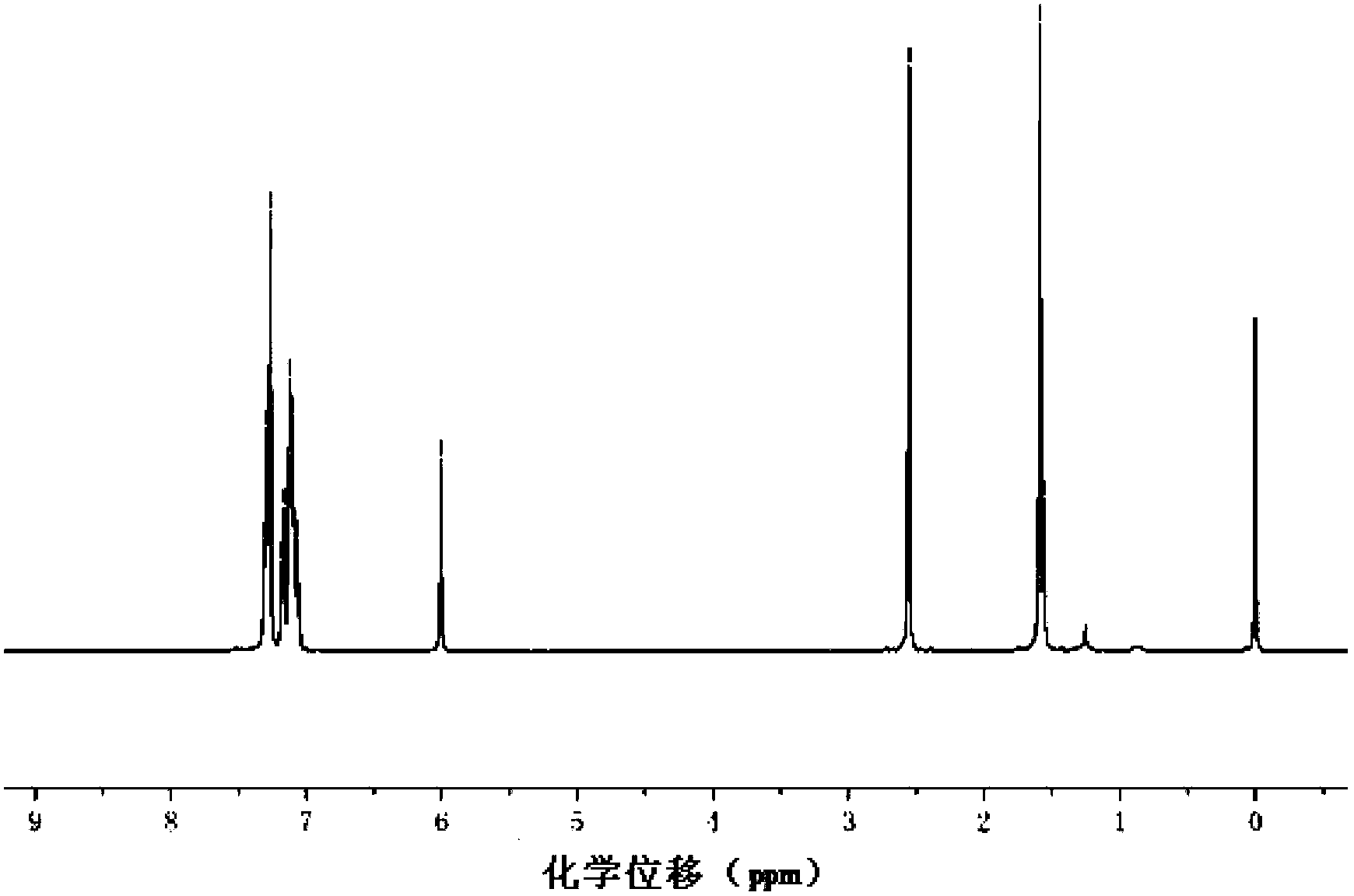
Copper ion fluorescence probe and synthetic method thereof
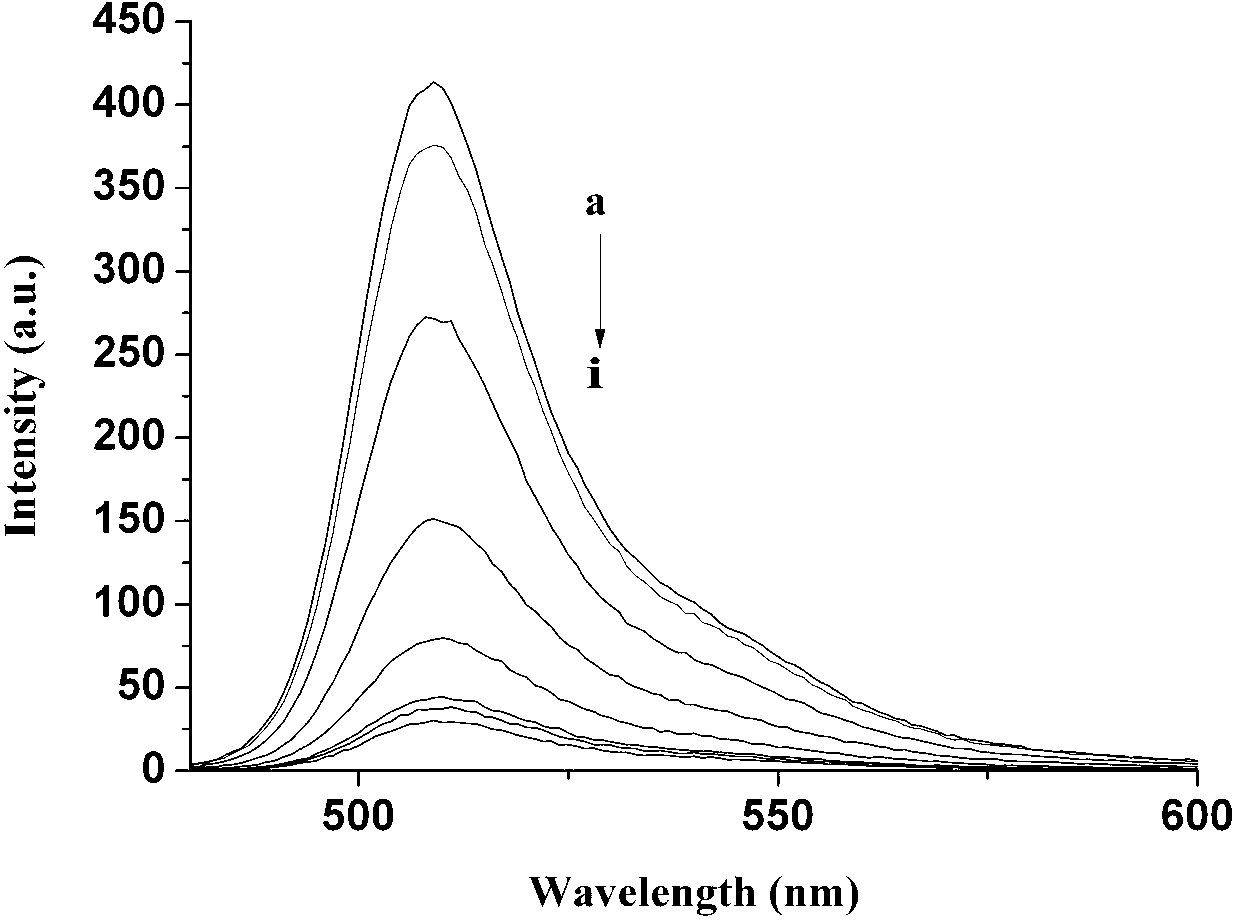
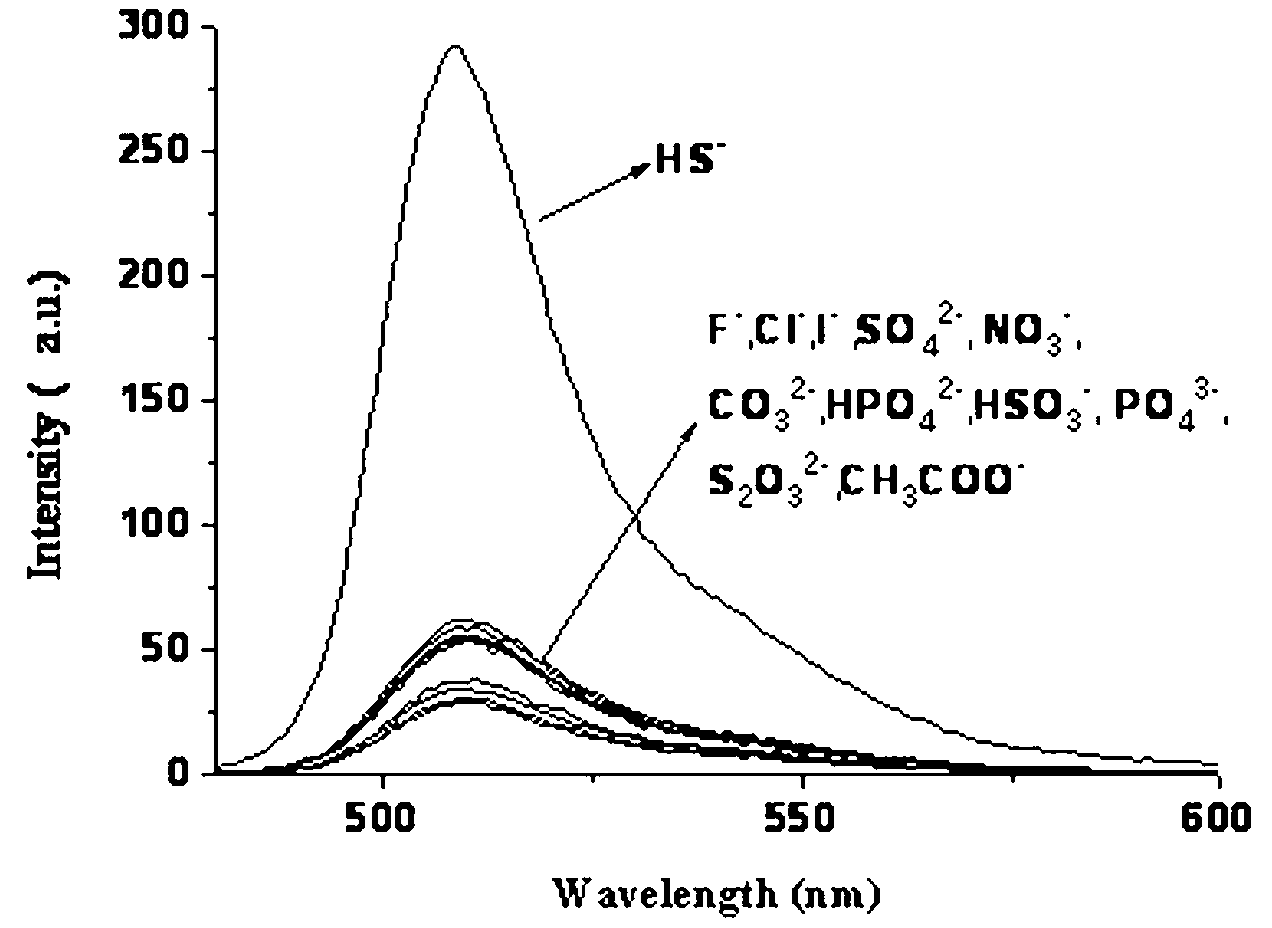
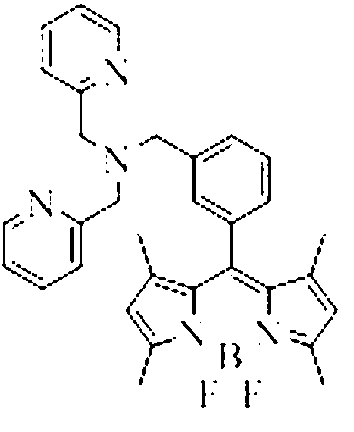
InactiveCN103013495ALow toxicitySmall inhibition rateGroup 3/13 element organic compoundsFluorescence/phosphorescenceSynthesis methodsMethyl group
The invention belongs to the technical field of analysis chemistry and relates to a copper ion fluorescence probe and a synthetic method of the copper ion fluorescence probe. The copper ion fluorescence probe disclosed by the invention has a chemical name of 8-[di(2-picolyl)amine-3-benzyl]-4, 4-difluoro-1,3,5,7-tetramethyl-4-boron-3a, 4a-dipyrrole (called BODIPY-DPA for short). The synthetic method of the copper ion fluorescence probe comprises the steps of: mixing 2, 4-dimethylpyrrole with 3-chloromethyl benzoyl chloride, then adding CH2Cl2 into the mixture, then adding boron trifluoride for reaction, and then orderly adding di(2-picoly)amine and triethylamine for reaction, at last, obtaining the copper ion fluorescence probe. The BODIPY-DPA shows light yellow in the solution, has high fluorescence emission at 590 nm, can enter HepG-2 to show green fluorescence imaging, and has a lowest limit of detection of 2.78 muM on copper ions in water solution. The copper ion fluorescence probe prepared by the synthetic method is characterized in low toxicity and the inhibition rate of 100 muM of HepG-2 is smaller than 10%, so the copper ion fluorescence probe can be used for detection of living cell imaging and copper irons in the cell, and has a very good application prospect in environment monitoring and detection of copper irons in a biologic system.
Owner:JIANGSU UNIV
Preparation method of lithium ion battery electrolyte salt LiODFB (lithium oxalyldifluroborate)
InactiveCN104628754AAvoid wastingIncrease profitGroup 3/13 element organic compoundsFiltrationSolvent
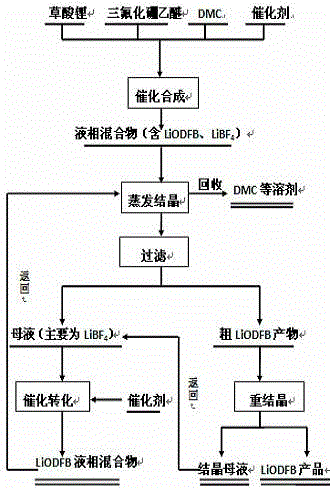
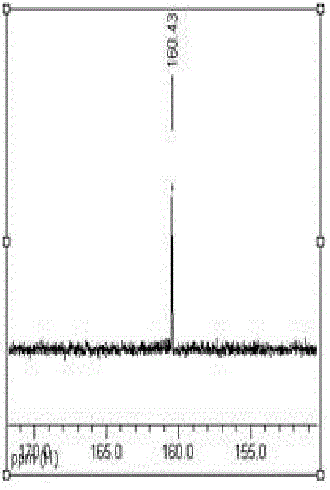
The invention relates to a preparation method of lithium ion battery electrolyte salt LiODFB (lithium oxalyldifluroborate). The method comprises the following steps: performing catalytic synthesis of lithium oxalate and boron trifluoride diethyl ether in a solvent of DMC (dimethyl carbonate) and the like to obtain a liquid-phase mixture and a little unreacted lithium oxalate solid, performing filtration, and then performing evaporative crystallization to obtain crude LiODFB; recrystallizing the crude LiODFB to meet the requirement of lithium ion battery electrolyte salt; and collecting filtered mother liquid and crystallization mother liquid, adding oxalate and a catalyst, performing catalytic conversion to obtain a liquid-phase mixture which mainly contains LiODFB, and performing evaporative crystallization again. The technological process is low in cost, the purity of the prepared LiODFB is above 99.9%, the yield is above 90%, and the method is convenient to operate, has favorable economic benefits and environmental benefits and is suitable for industrial production.
Owner:HUNAN ZHENGYUAN ENERGY STORAGE MATERIALS & DEVICE INST +1
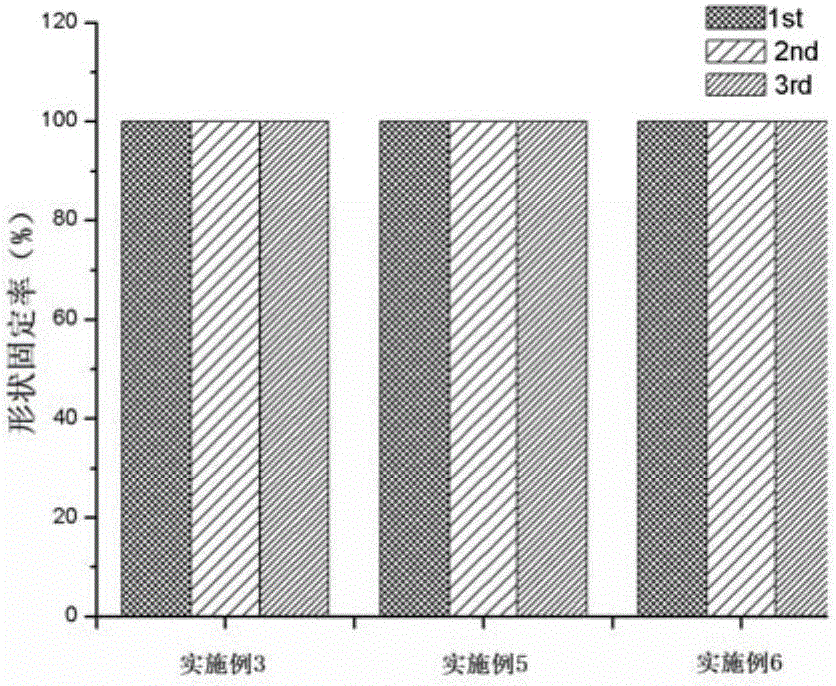
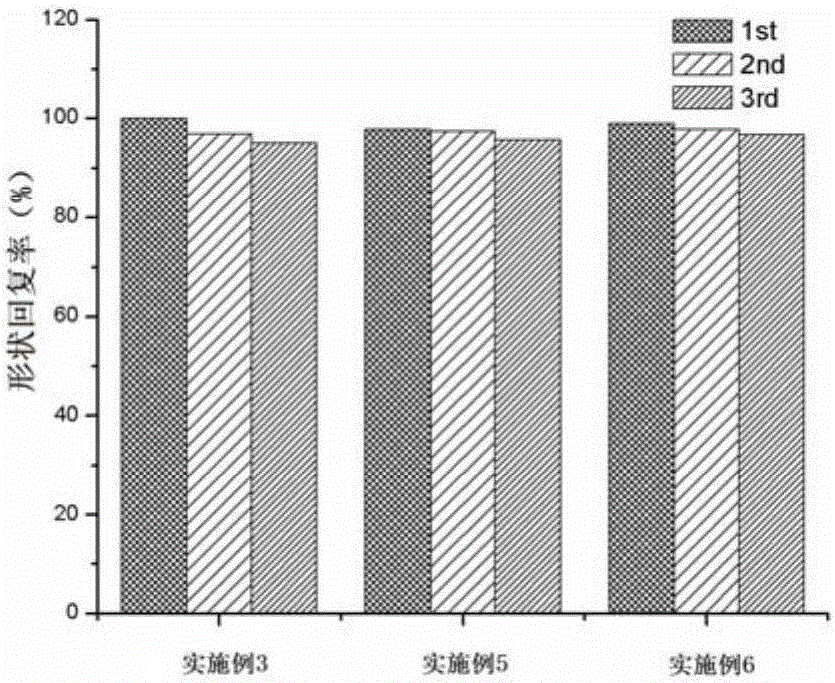
Bio-based thermoplastic elastomer with shape memory function and preparation method thereof
The invention relates to a bio-based thermoplastic elastomer with shape memory function and a preparation method thereof. The bio-based thermoplastic elastomer with shape memory function is prepared from the following raw materials in parts by mass: 30-80 parts of polylactic acid, 20-70 parts of epoxidized natural rubber, 0.2-2 parts of antioxidant, 0.02-2 parts of boron trifluoride etherate and 0.1-3 parts of crosslinking agent. The preparation method comprises the following steps: uniformly mixing the polylactic acid, antioxidant, boron trifluoride etherate and epoxidized natural rubber at 150-190 DEG C, and adding the crosslinking agent under high-speed shearing actions to vulcanize the rubber in situ, thereby obtaining the bio-based thermoplastic elastomer with shape memory function. The rubber phase and plastic phase of the bio-based thermoplastic elastomer respectively appear a continuous phase structure. The structure endows the material with excellent shape memory function and mechanical properties, so that the shape fixation rate is up to 100%, and the shape recovery rate is up to 90% or above.
Owner:SOUTH CHINA UNIV OF TECH
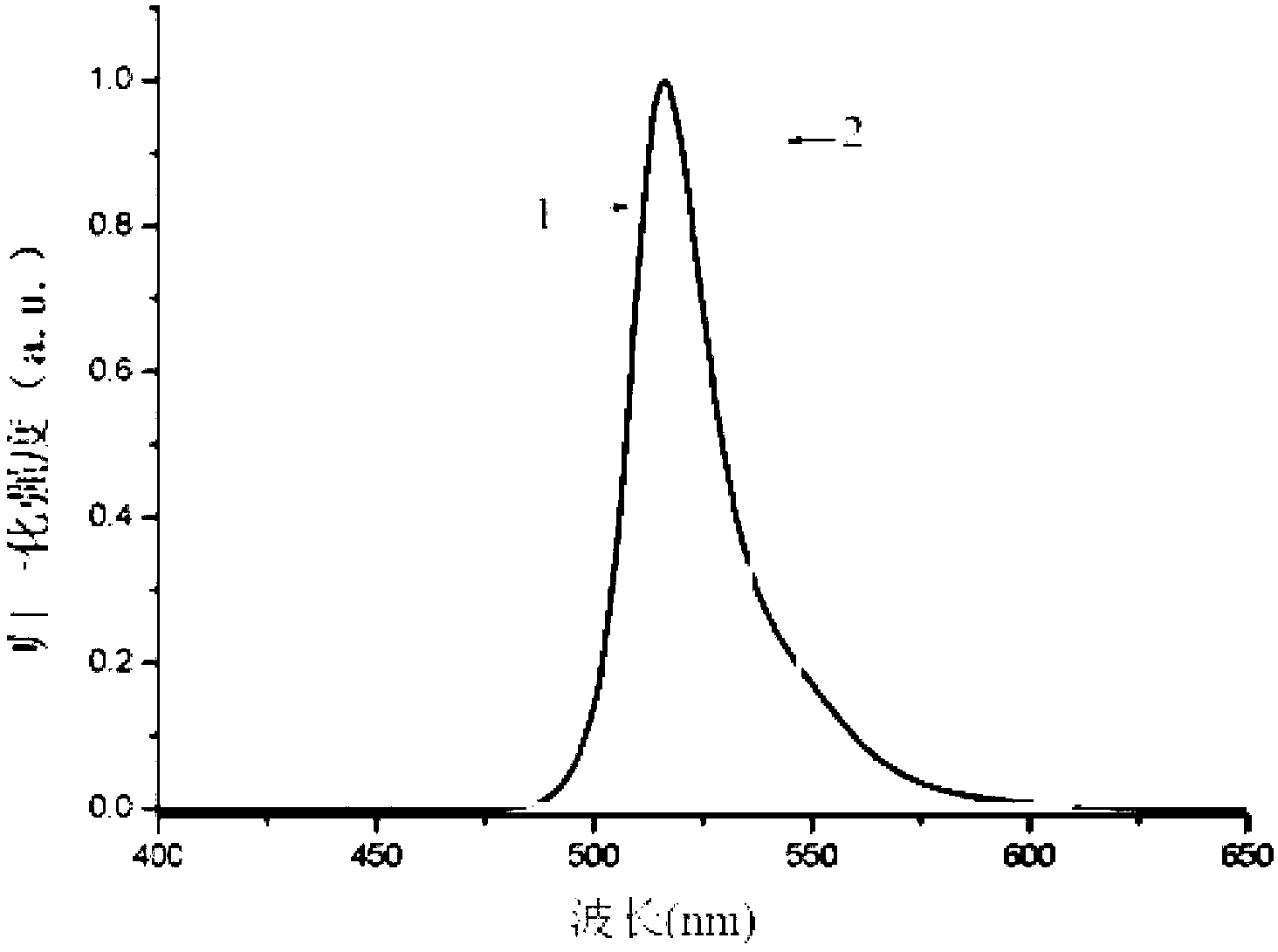
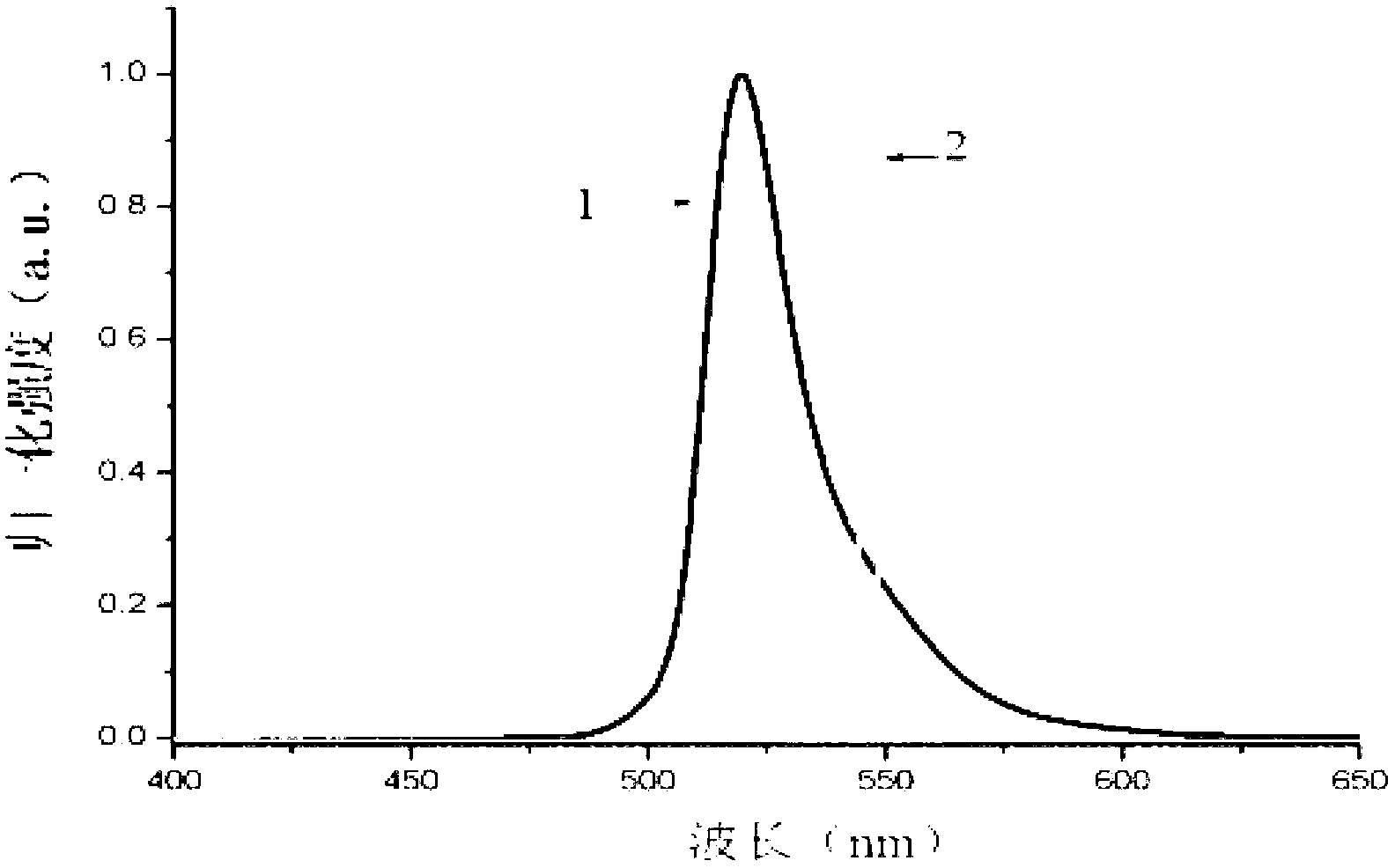
Novel red BODIPY fluorescent dye and preparation method and application thereof
InactiveCN102702768ANarrow absorbencyNarrow fluorescence emission spectrumMethine/polymethine dyesMicrobiological testing/measurementChemical reaction1,4-Benzoquinone
The invention relates to a novel red BODIPY fluorescent dye with the chemical formula of C10+mH7+nBF2N2+xOy, wherein m, n, x and y are integers from 0 to 100. The preparation method comprises the following steps: dissolving pyrrole with substituent groups R1, R2 and R3 in an organic solution; adding ethyl glyoxylate together with nitrogen to the organic solution for a chemical reaction by using trifluoroacetic acid or toluenesulfonic acid as a catalyst; adding 2,3-dichloro-5,6-dicyano-1,4-benzoquinone oxidative dehydrogenation; and adding organic amine and a boron trifluoride diethyl ether solution for another reaction. After the reaction solution is concentrated, chromatography is performed with a silicagel column to obtain the fluorescent dye. The fluorescent dye can be used for cell imaging, fluorescent probe or laser dye. The fluorescent dye has the advantages that the ultraviolet-visible absorption spectrum and the fluorescence emission spectrum of the fluorescent dye are narrow; fluorescent quanta has high efficiency and good light stability; and the fluorescent dye has simple molecular structure and can be synthesized easily, so as to facilitate popularization and application.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Strong fluorescence fluoro-boron dipyrrole compound containing triphenylamine structure as well as preparation method and application thereof
InactiveCN103172650AGood symmetryImprove laser efficiencyAzo dyesGroup 3/13 element organic compoundsFluorescenceUv vis absorbance
The invention relates to a strong fluorescence fluoro-boron dipyrrole compound containing a triphenylamine structure as well as a preparation method and an application thereof, belonging to the technical fields of organic chemical industry and fine chemical industry. The preparation method comprises the following steps of: weighing and dissolving formyl substituted triphenylamine and 2,4-dimethyl pyrrolo in a dry dissolvent under the protection of inert atmosphere; adding a catalyst; reacting at a room temperature in a dark place for 6-10 hours; then adding an oxidant; reacting at room temperature for 20-40 minutes; and finally adding organic amine and a boron triflouride complex compound and reacting for 3-8 hours, thus obtaining the strong fluorescence fluoro-boron dipyrrole compound containing a triphenylamine structure. The synthetic method is simple and convenient, and the synthetic compound serves as a laser dye and has relatively high solution pumping laser efficiency, narrowed ultraviolet and visible absorption spectrum and fluorescence emission spectrum, high fluorescence quantum efficiency and good light stability.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
Method of preparing poly-pyrrole/polythiofuran derivative composite conductive macromolecule material for super electrical condenser
InactiveCN101492545AIncrease specific volumeImprove cycle stabilityCapacitor electrodesPolymer sciencePolypyrrole
The invention relates to a preparation method for polypyrrole / polythiophene derivative compound conductive high polymer material, which comprises the following steps: firstly, boron trifluoride diethyl ether and aether are blended, and then added with ionic liquid to obtain A solution; pyrrole and thiofuran derivative monomer are added into the A solution to prepare B solution; the B solution is added into an electrolytic cell equipped with three electrodes; and electrochemical polymerization is carried out under the protection of nitrogen; polymerized current density is 1-10 mA / cm2; a layer of uniform polypyrrole / polythiophene derivative complex film can be obtained on a working electrode after polymerization is finished; the working electrode is taken out for washing and dried in the temperature of 60 DEG C. The preparation method has the beneficial effects that the composite electrode material prepared by the invention has about 200 F / g of bulking value and an electrical potential widow with about 0-2.8 width in organic solvent, and still has higher specific capacity and better circulation stability when discharging in high power.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com