Strong fluorescence fluoro-boron dipyrrole compound containing triphenylamine structure as well as preparation method and application thereof
A fluorescent fluoroboron dipyrrole and triphenylamine technology, which is applied in the fields of compounds containing elements of Group 3/13 of the periodic table, chemical instruments and methods, organic chemistry, etc. Fluorescence quantum efficiency, effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the strong fluorescence boron dipyrrole compound containing triphenylamine structure comprises the following steps:
[0041] (1) Under the protection of an inert atmosphere, weigh formyl-substituted triphenylamine and 2,4-dimethylpyrrole and dissolve them in a dry solvent, add a catalyst, stir and react at room temperature for 6-10 hours in the dark, and obtain a purple-red solution ;
[0042] The formyl-substituted triphenylamine is bis(4-formylphenyl)aniline or tris-(4-formylphenyl)amine;
[0043] (2) Weigh the oxidant and dissolve it in a dry solvent, add it to the purple-red solution obtained in step (1), stir and react at room temperature for 20 to 40 minutes to obtain a black solution;
[0044] (3) Add triethylamine to the black solution, stir at room temperature for 5 to 10 minutes, add boron trifluoride complex, stir and react in an ice bath for 3 to 8 hours, and obtain a reaction solution, which is separated by extraction and reduced ...
Embodiment 1
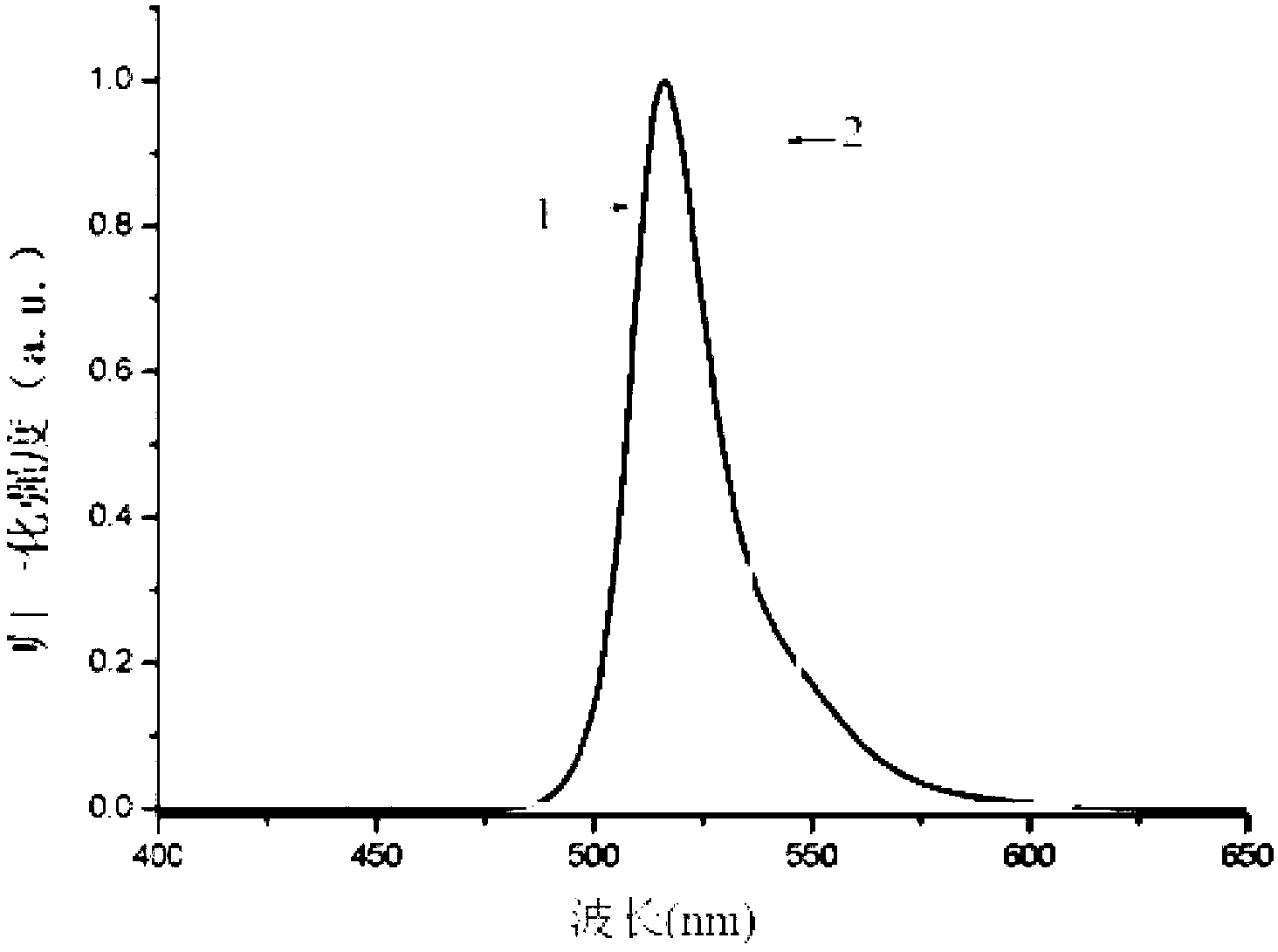
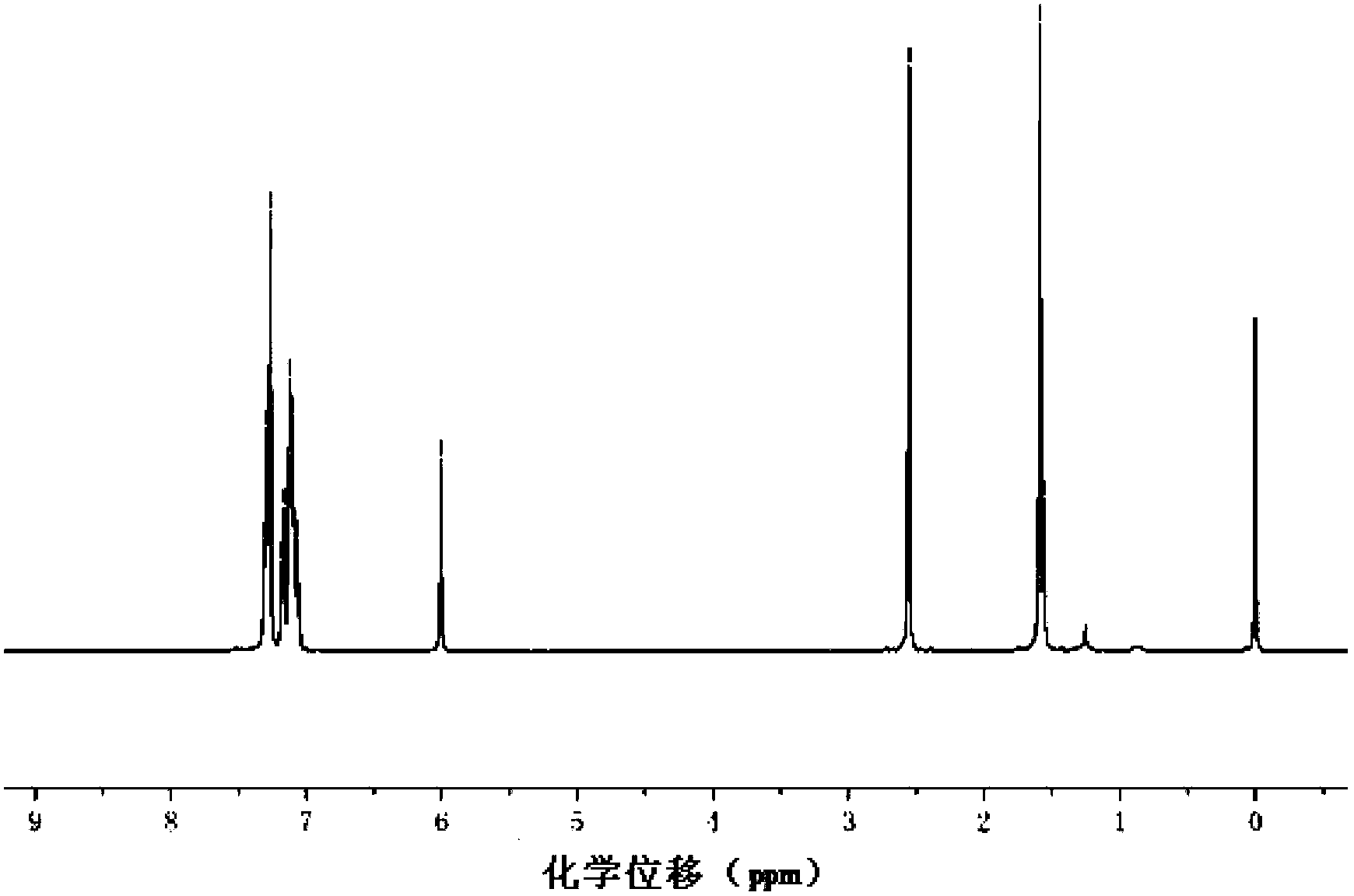
[0074] Preparation of strong fluorescent fluoroboron dipyrrole compound containing triphenylamine structure of formula (I):
[0075] (1) Weigh 0.274 grams of 4-dianilinobenzaldehyde, and dissolve 0.19 grams of 2,4-dimethylpyrrole in 20 milliliters of anhydrous dichloromethane, add 2 drops (10ul) of trifluoroacetic acid, under nitrogen protection, Magnetically stirred at room temperature for 6 hours to obtain a purple solution;
[0076] (2) 0.3 g of chloranil was dissolved in 10 ml of dichloromethane, added to the above-mentioned purple-red solution, and stirred at room temperature for 30 minutes to obtain a black solution;
[0077] (3) Add 3 milliliters of triethylamine and 3 milliliters of boron trifluoride ether solution to the above black solution, stir at room temperature for 6 hours, add 100 milliliters of water to quench the reaction, extract with dichloromethane, dry the organic phase with anhydrous magnesium sulfate, Concentrated under reduced pressure, the crude prod...
Embodiment 2
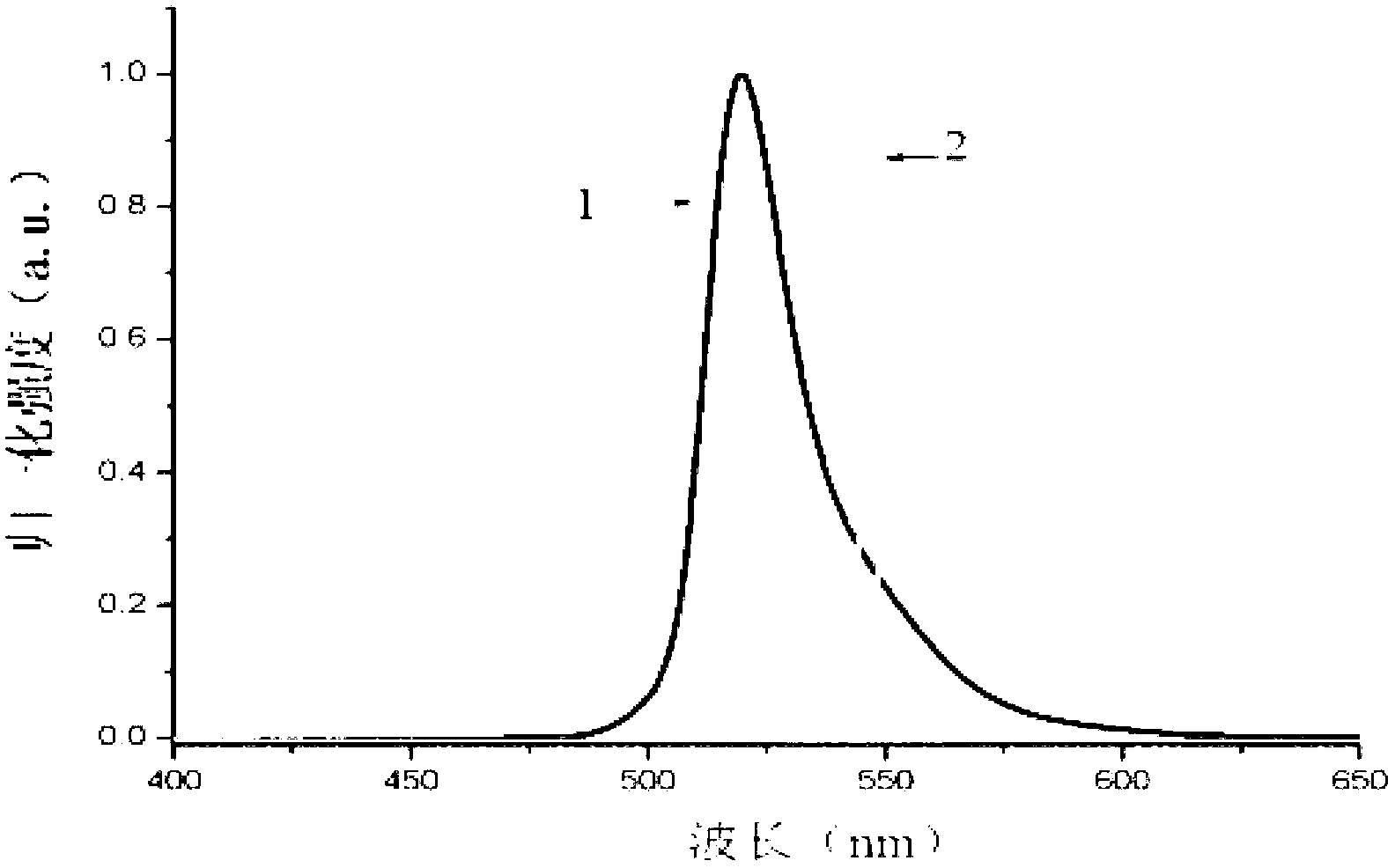
[0081] Preparation of strong fluorescent fluoroboron dipyrrole compound containing triphenylamine structure of formula (П) structure:
[0082] (1) Weigh 0.301 g of bis(4-formylphenyl) aniline, dissolve 0.38 g of 2,4-dimethylpyrrole in 40 ml of anhydrous dichloromethane, add 2 drops (10ul) of trifluoroacetic acid as a catalyst , under nitrogen protection, magnetically stirred at room temperature for 8 hours to obtain a purple-red solution;
[0083] (2) 0.455 g of chloranil was dissolved in 20 ml of dichloromethane, added to the above purple-red solution, and stirred at room temperature for 30 minutes to obtain a black solution;
[0084] (3) Add 5 milliliters of triethylamine and 5 milliliters of boron trifluoride ether solution to the above-mentioned black solution, stir at room temperature for 8 hours, add 200 milliliters of water to quench the reaction, extract with dichloromethane, dry the organic phase over anhydrous magnesium sulfate, subtract Concentrate under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com