Patents
Literature
47 results about "Chloranil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
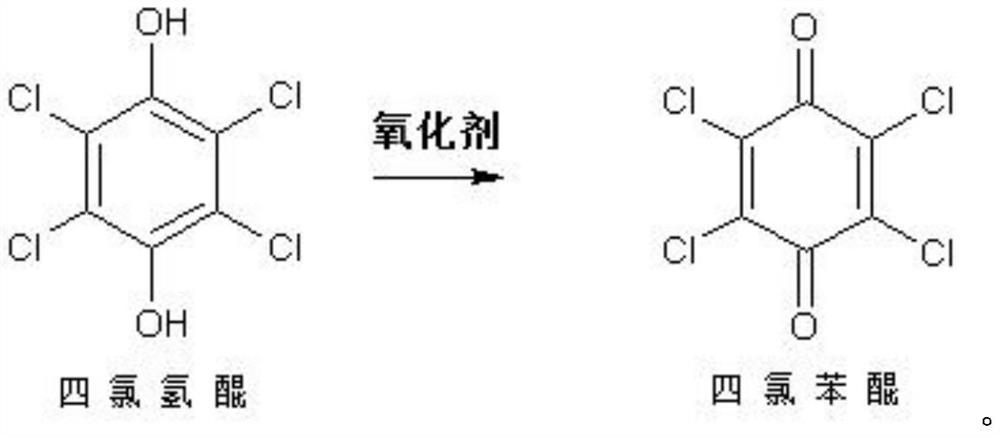
Chloranil is a quinone with the molecular formula C₆Cl₄O₂. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule that functions as a mild oxidant.
Process and systems for peptide synthesis
ActiveUS7439222B2Reduce in quantityReduce the amount requiredBiocidePeptide/protein ingredientsCouplingCombinatorial chemistry
Owner:ROCHE PALO ALTO LLC
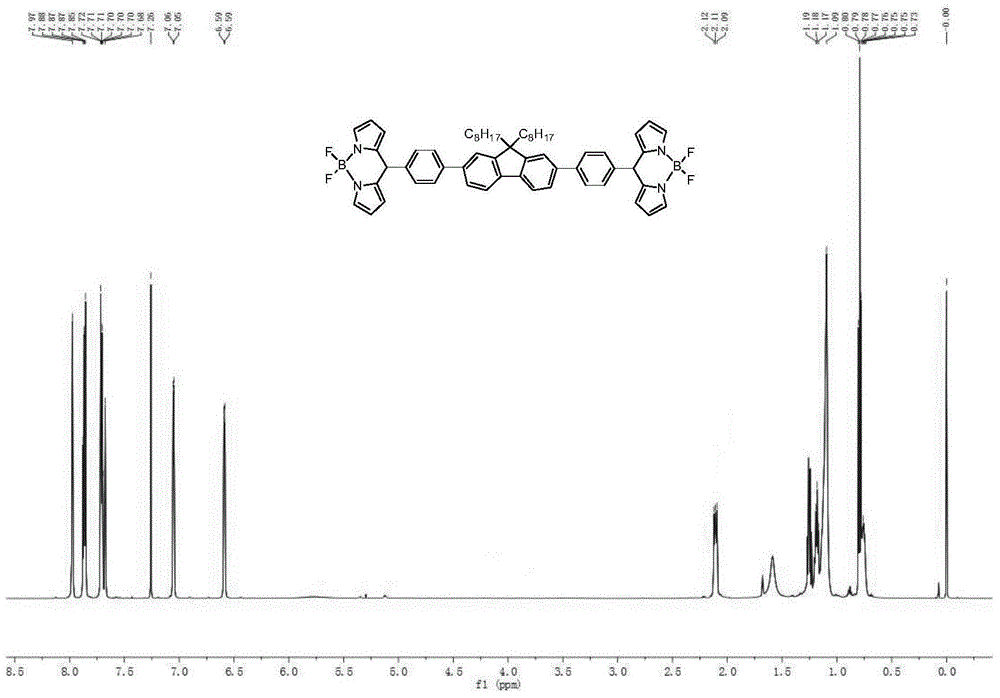
Pyrrole dimethine fluorescent dyes as well as synthetic method and use thereof
InactiveCN101205416ASuitable for excitationSuitable emission wavelengthMethine/polymethine dyesReactive dyesFluorescenceSynthesis methods
The invention provides a pyrrole di-methine fluorescent dye and a synthesis method and application; the synthesis method is as follows: a. preparing 2-phenylaziridine from styrene; b. adding 2-phenylaziridine into acetophenone, and reacting to produce 2, 4-diphenylpyrrole in the presence of sodium hydride under room temperature; c. adding 2, 4-diphenylpyrrole into p-hydroxybenzaldehyde, then adding chloranil according to the weight proportion 2, 4-diphenylpyrrole : chloranil =1:3-7; reacting under room temperature with stirring, removing excessive chloranil after reaction, and spinning dry to get blue solid pyrromethene; d. adding blue solid pyrromethene into dichloromethane for dissolution, then adding boron trifluoride diethyl etherate under the protection of inert gas, and reacting under room temperature; after reaction, adding chloroform and extracting 2 to 3 times; combining extract liquids, water washing, drying by anhydrous magnesium sulfate, spinning dry, and column separation to yield products.
Owner:SHANDONG NORMAL UNIV
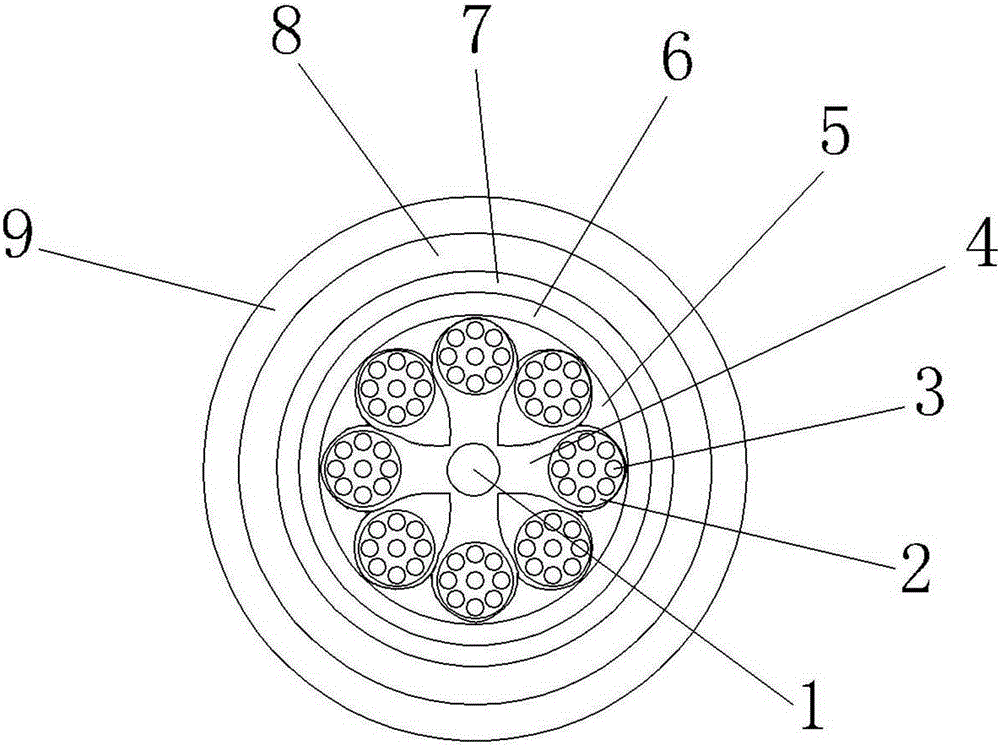
High-intensity wear resistant cable used for construction
InactiveCN105244109AImprove stabilityImprove extrusion resistancePower cables with screens/conductive layersInsulated cablesCalcium silicateWear resistant
The invention discloses a high-intensity wear resistant cable used for construction. A raw material of a sheath layer comprises, by weight, 60-90 parts of terpolymer EP rubber, 30-60 parts of W type chloroprene rubber, 40-80 parts of nitrile rubber, 30-60 parts of polyvinyl fluoride resin, 20-50 parts of EVA resin, 30-60 parts of modified epoxy resin, 12-22 parts of fluorinated polyethylene, 5-12 parts of zinc oxide, 3-8 parts of magnesium oxide, 2-9 parts of calcium stearate, 3-9 parts of nylon, 2-12 parts of active white carbon black, 2-8 parts of nanometer white carbon black, 2-6 parts of active calcium silicate, 3-8 parts of nanometer silica, 1-5 parts of a vulcanizing agent DCP, 2-8 parts of chloranil, 3-9 parts of a promoter DM, 3-9 parts of polyethylene glycol, 2-8 parts of a dispersant CNF, 1-5 parts of an antiager RD, 2-6 parts of an antiager AW, and 1-6 parts of N-cyclohexylthiophthalimide. The cable is high in intensity and highly resistant to wear.
Owner:安徽华峰电缆集团有限公司
Process and systems for peptide synthesis
ActiveUS20050165217A1Reduce reagent usageLow costPeptide/protein ingredientsPeptide preparation methodsCouplingCombinatorial chemistry
The invention provides methods of synthesizing peptides, involving the steps of providing a composition including a peptide fragment, wherein the peptide fragment has at least one amino acid residue and includes a base-sensitive, N-terminal protecting group; removing the base-sensitive, N-terminal protecting group from the peptide fragment using a deprotection reagent that includes a base, whereby an N-terminal functionality on the peptide fragment is deprotected; removing the base from the composition to provide a residual base content of more than 100 ppm; causing a reactive peptide fragment having a reactive C-terminus and a base-sensitive N-terminal protecting group to react with the deprotected N-terminal functionality of the peptide fragment under conditions such that the reactive peptide fragment is added to the peptide fragment; and optionally repeating the deprotection and coupling steps until a desired peptide is obtained. Also provided are methods of synthesizing peptides, wherein base is removed from the composition to a point where the composition would provide a positive chloranil test. Also provided are methods of synthesizing peptides, wherein coupling is performed in basic reaction mixtures.
Owner:ROCHE PALO ALTO LLC
Modified epoxy resin-containing high-strength and wear-resistant cable material
InactiveCN105778301AImprove mechanical propertiesHigh strengthRubber insulatorsCalcium silicateEpoxy
The invention discloses a modified epoxy resin-containing high-strength and wear-resistant cable material, prepared from the following raw materials: ethylene propylene diene monomer, W type neoprene, buna-n rubber, polyvinyl fluoride resin, ethylene-vinyl acetate copolymer (EVA) resin, modified epoxy resin, fluorinated polyethylene, zinc oxide, magnesium oxide, calcium stearate, nylon, active white carbon black, nano white carbon black, active calcium silicate, nano silicon dioxide, a vulcanizing agent DCP, chloranil, an accelerant DM, polyethylene glycol, a dispersing agent CNF, an anti-aging agent RD, an anti-aging agent AW and N-cyclohexyl sulfo phthalimide. The cable material has high strength and high wear resistance.
Owner:ANHUI DUJIANG CABLE GROUP
Preparation method of chloranil
The invention discloses a preparation method of chloranil. The preparation method includes steps of firstly, charging chlorine in a tank, and stirring and dissolving p-aminophenol in acetate solution, wherein the mass ratio of acetic acid in the acetate solution is 20-80%; the mass ratio of p-aminophenol and acetate solution is 10: 25-100; then charging chorine, and reacting at 35-90 DEG C; stopping chorine charging when it is detected that chloranil in the material has yellow crystal obviously, acquiring the chloranil crystal body and ammonia chloride crystal body; the ammonia chloride gas generated in the reaction is absorbed to be hydrochloric acid by water.
Owner:许克宇
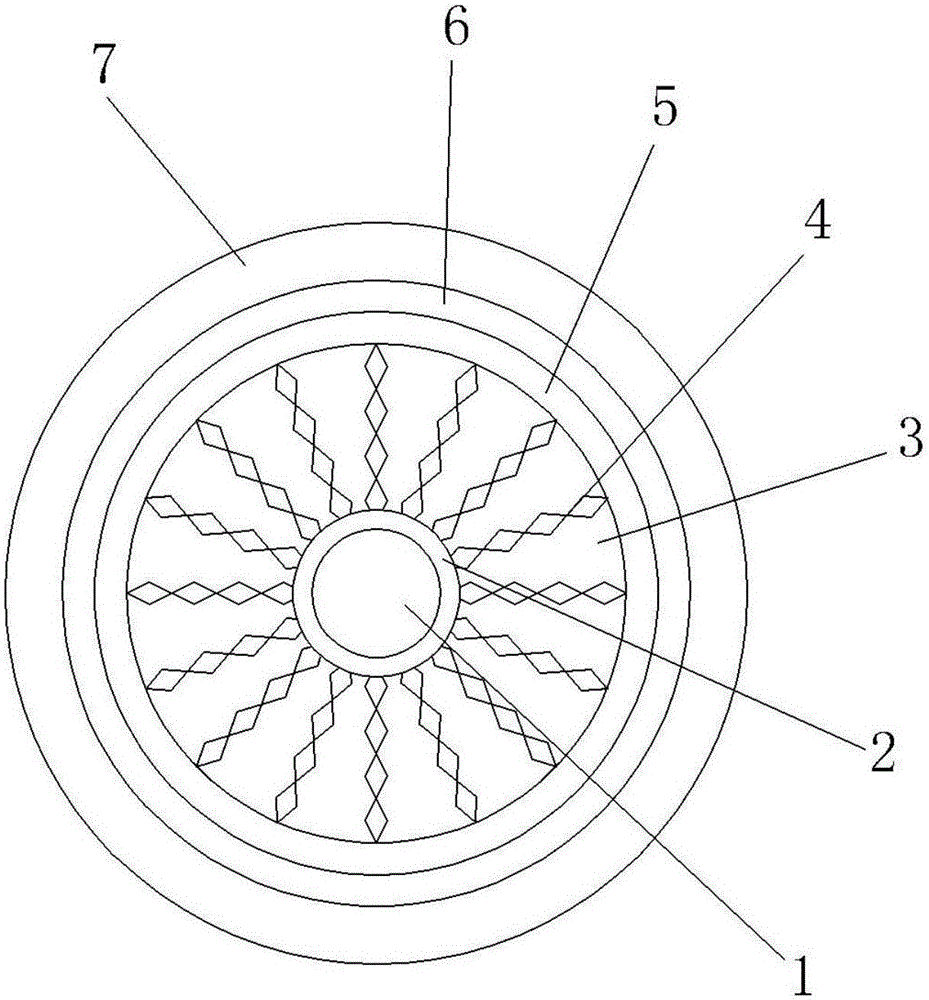
Anti-extrusion cable
The invention discloses an anti-extrusion cable, which comprises a cable core, an insulation layer, a filling layer, a plurality of buffer plates, a wrapping layer, a buffer layer and an armor layer, wherein each buffer plate comprises the following ingredients in parts by weight: 100 parts of natural rubber, 70 parts of chloroprene rubber, 55 parts of silicone rubber, 45 parts of butadiene styrene rubber, 35 parts of butadiene rubber, 40 parts of nitrile rubber, 5.5 parts of zinc oxide, 3 parts of stearic acid, 8.5 parts of polyolefin elastomers, 3 parts of KH550 silane coupling agents, 4 parts of KH570 silane coupling agents, 8 parts of quinone alcohol ester, 4 parts of dicumyl peroxides, 3 parts of vulcanizing agents DCP, 5 parts of chloranil, 6 parts of accelerants DM, 7 parts of active white carbon black, 6 parts of polyethylene glycol, 5 parts of dispersants CNF, 3 parts of anti-aging agents RD, 4 parts of anti-aging agents AW and 4 parts of N-cyclohexylthiophthalimide. The anti-extrusion cable has excellent anti-extrusion performance.
Owner:安徽华峰电缆集团有限公司
Preparation method of permanent violet pigment
The invention discloses a preparation method of a permanent violet pigment. The method comprises the following steps: dissolving 3-amino-N-ethyl carbazole in an organic solvent and carrying out a condensation reaction by adding chloranil in the presence of an acid-binding agent so as to obtain an organic solution containing 2,5-dichloro-3,6-di(9-ethyl-3-carbazole amino)-1,4-benzoquinone after the reaction; adding a metal salt catalyst into the organic solution obtained in the step a or simultaneously adding the metal salt catalyst and a quinone compound cocatalyst into the organic solution; feeding air or oxygen into a reaction kettle to reach a certain pressure; carrying out heat preservation at 120-180 DEG C for 2-10 hours; cooling to 80-120 DEG C after the reaction; washing a filter cake obtained after filtering with a small amount of organic solvent; then, respectively washing with methyl alcohol and water and drying, thereby obtaining a crude permanent violet pigment product. According to the method, a traditional poisonous and harmful closed-loop agent is not adopted, so that the cost of raw materials is saved; the generation of byproducts is reduced, so that the selectivity and total yield of the reaction are improved. Thus, less environmental pollution is generated.
Owner:NANTONG HAIDI CHEM
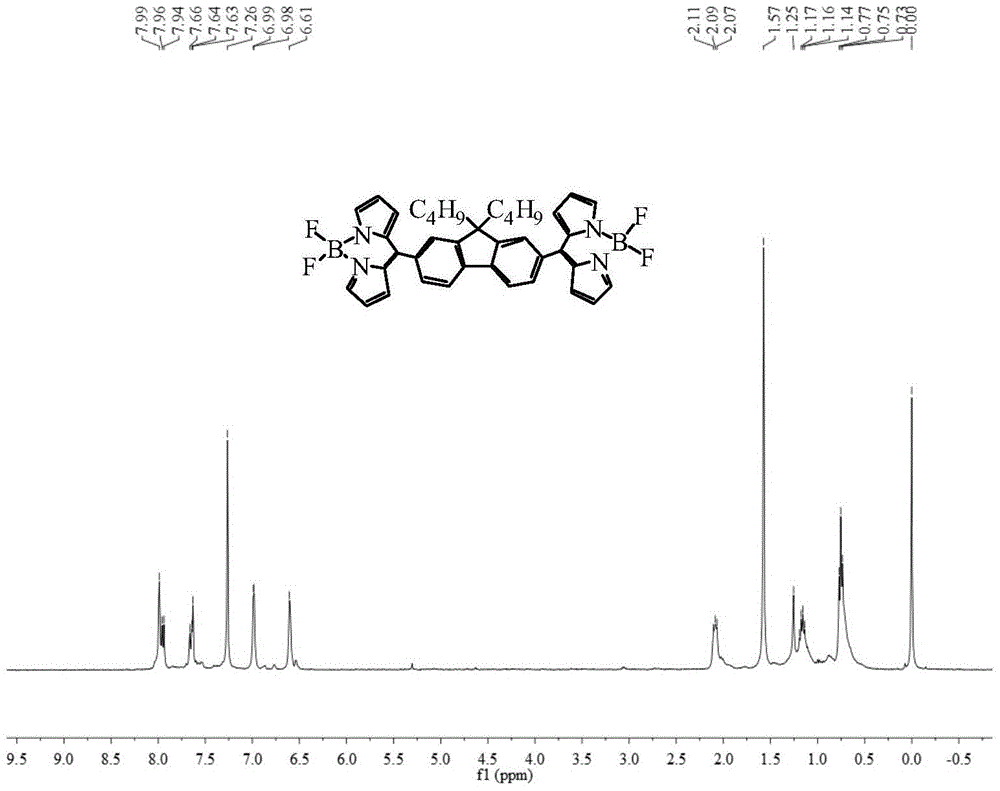
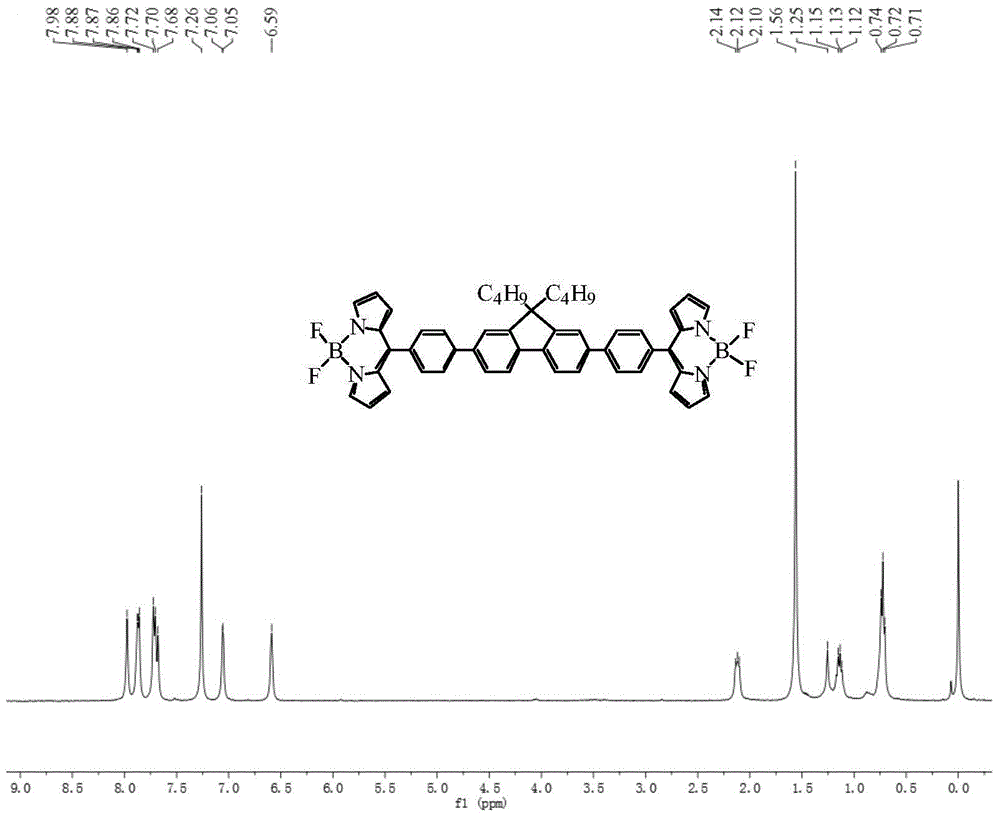
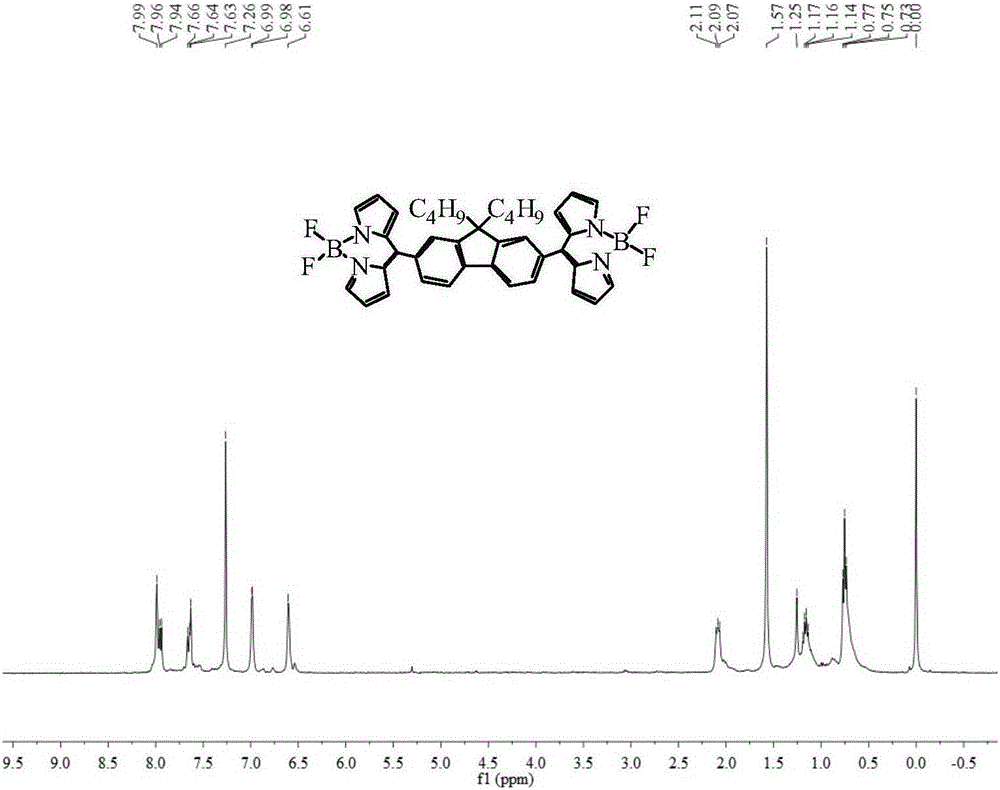
Bridged bis-boron-dipyrromethene (BODIPY) derivative containing fluorene at meso-position and preparation method thereof
ActiveCN105348308ALow costHigh yieldAzo dyesGroup 3/13 element organic compoundsIndium TrichlorideEnvironmental energy
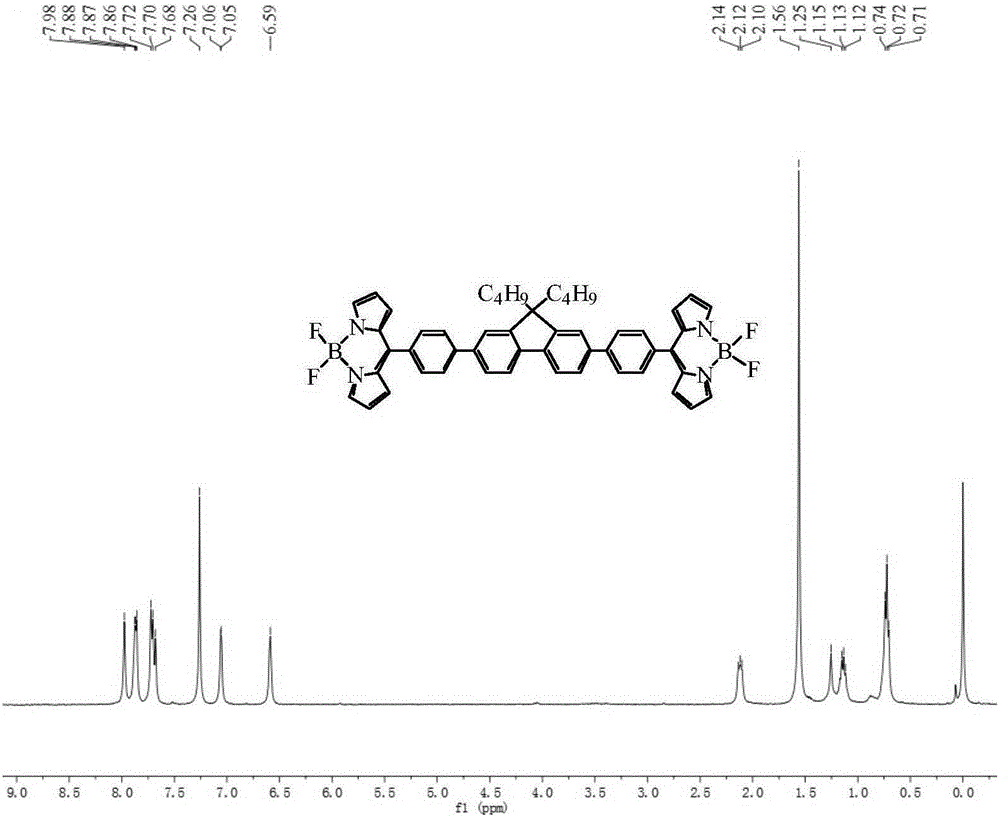
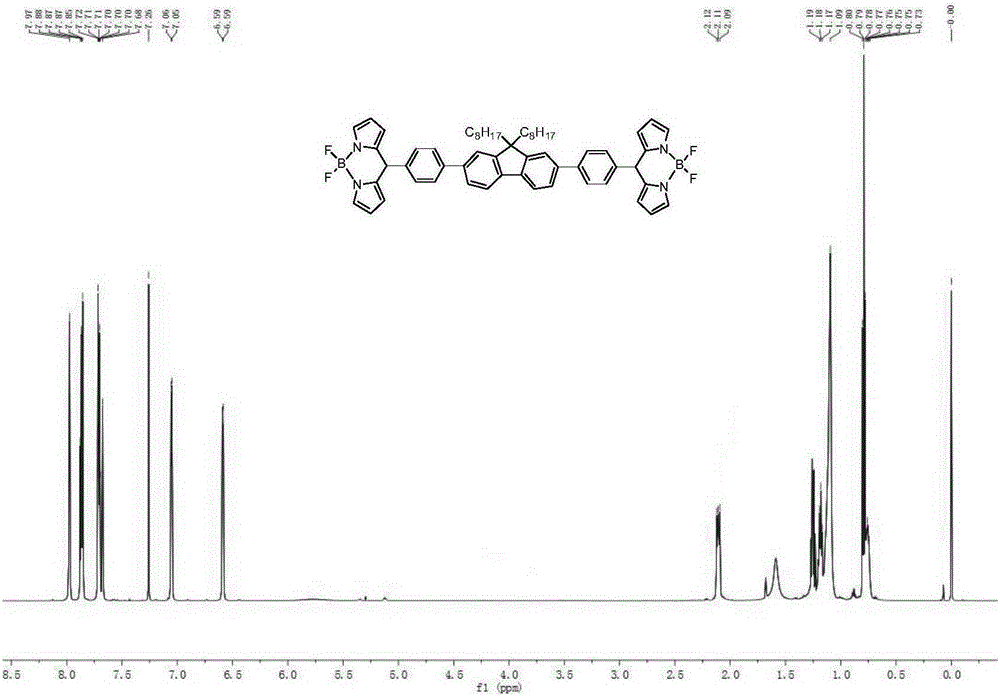
The invention discloses a bridged double-center BODIPY derivative containing fluorene at a meso-position and a preparation method thereof. The preparation method comprises the following steps: synthesizing a symmetric dialdehyde compound containing bridging groups consisting of benzene, thiophene and furan and different substituent groups like a butyl group and an octyl group; then subjecting the symmetric dialdehyde compound and pyrrole to a reaction under the catalysis of indium trichloride so as to synthesize a bis(dipyrrolidine) compound; and successively carrying out oxidation by chloranil and fluoroboronation by boron trifluoride ether so as to obtain a fluorene-containing different bridging group-substituted BODIPY dye which has a structural general formula as shown in a formula I that is described in the specification. According to the invention, fluorene and the bridging groups consisting of benzene, thiophene and furan are introduced for the first time and conventional synthetic methods are improved, so the obtained double-center BODIPY dye has stable spectral absorption; and introduction of thiophene, furan and fluorene enables the fluorescence emission peak of the derivative presents obvious red shift, and fluorescence effect is enhanced with enhancement of system conjugative effect. The organic dye can be efficiently synthesized and widely used in fields like life science, analytical chemistry and environmental energy.
Owner:江苏博凡科精密五金科技有限公司
Electrochemical sensor
InactiveUS20130256133A1Improve reaction speedLower activation energyWeather/light/corrosion resistanceVolume/mass flow measurementElectrochemical gas sensorReaction rate
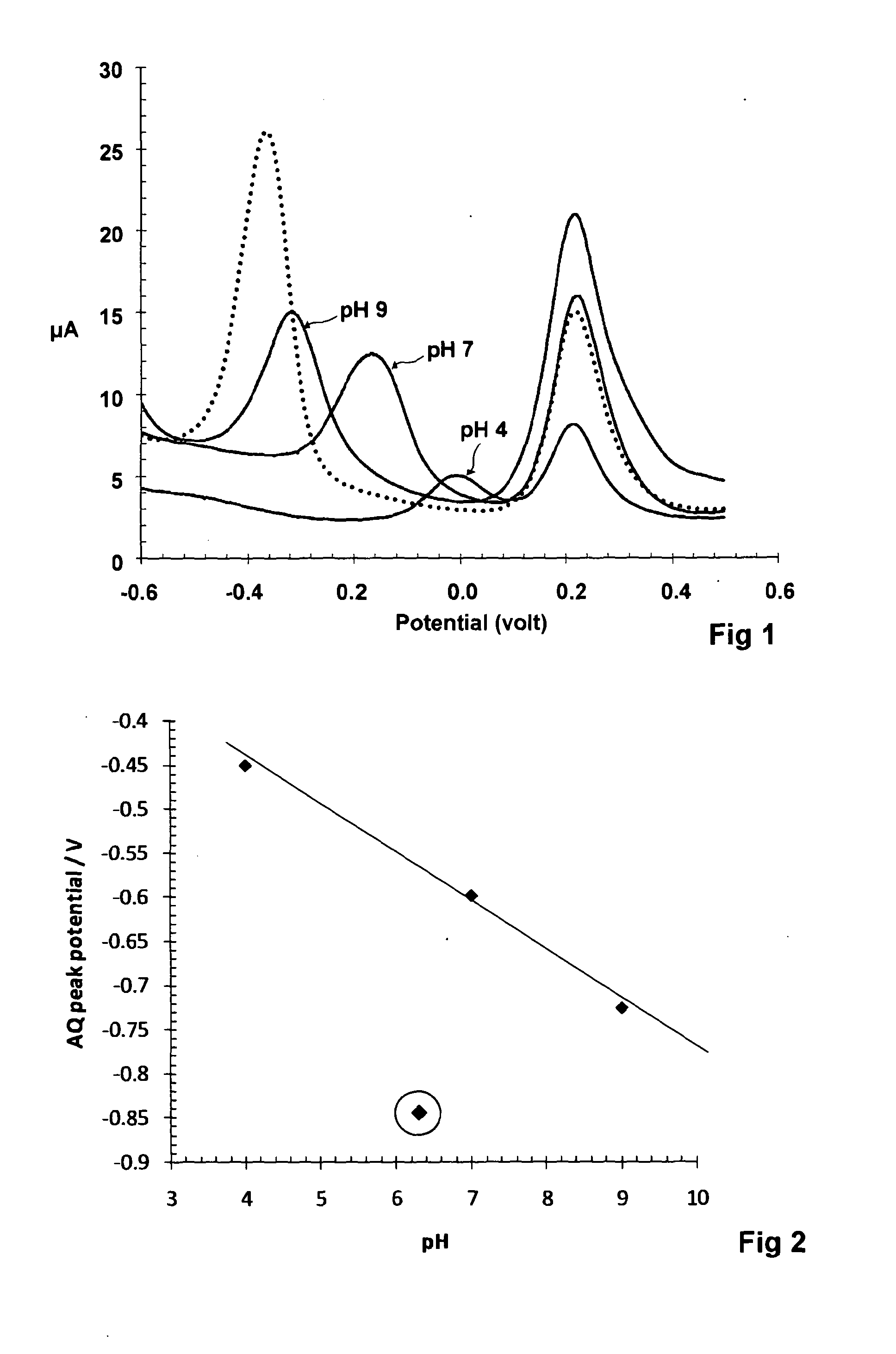
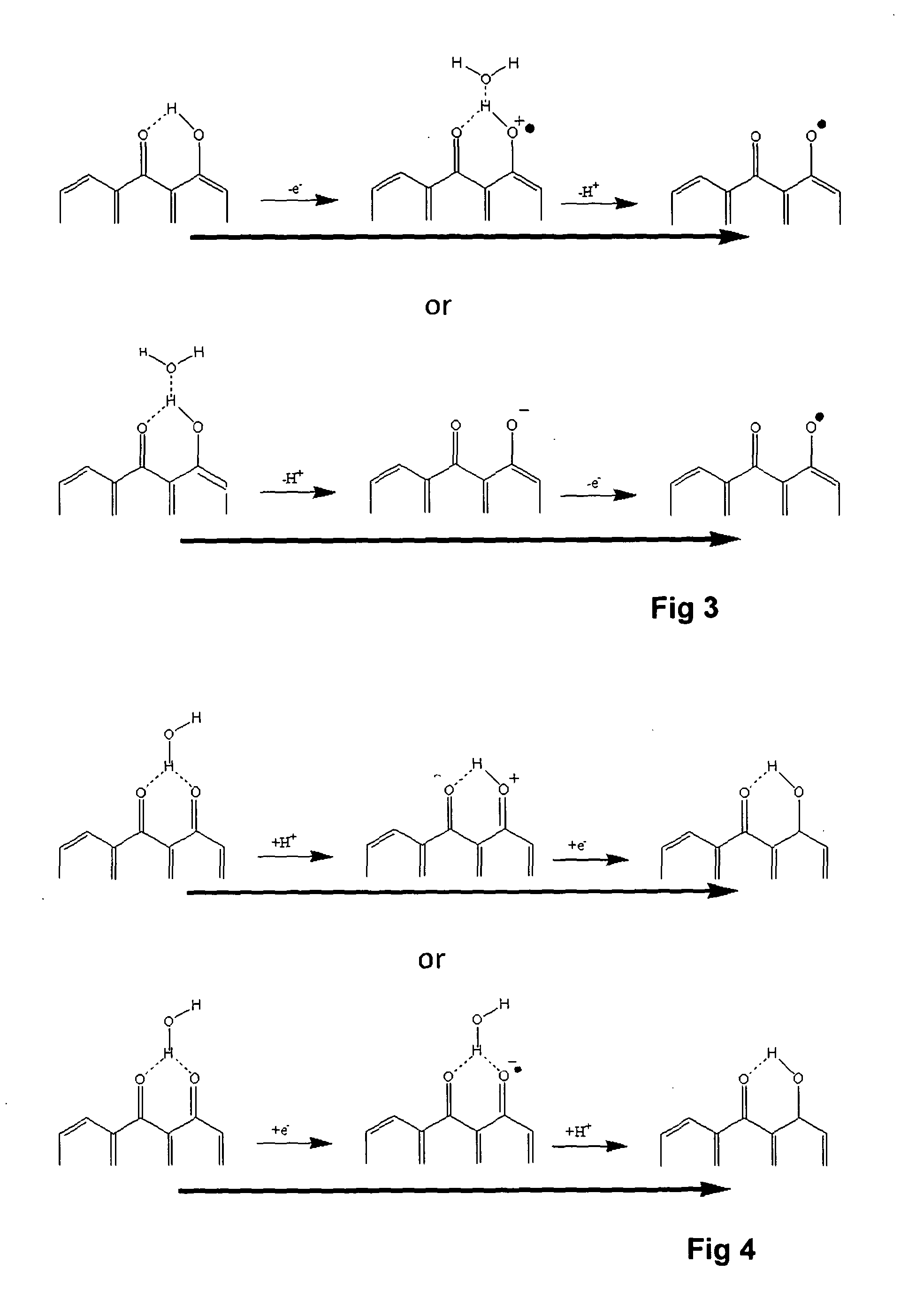
A voltammetric pH sensor, especially for characterising wellbore fluids, comprises a plurality of electrodes with a redox active organic compound attached to an electrode and having at least one functional group convertible electrochemically between reduced and oxidized forms with transfer of at least one proton between the compound and surrounding aqueous phase, wherein the compound has at least one substituent group which promotes hydrogen bonding at a said functional group and thereby increases the reaction rate of proton transfer. The substituent group may form an internal hydrogen bond with a redox-convertible group or may enhance polarity to promote electrostatic interaction with water molecules and reduce activation energy. Typical examples include alizarin or 1,2-dihydroxy-anthraquinone (RH=72-48-0), quinizarin or 1,4-dihydroxy-anthraquinone (RN=81-64-1), 2-acetoxy-benzoquinone (RN=1125-55-9), chloranil or 2,3,4,5-tetrachloro-benzoquinone (RN=118-75-2) and 1,4-diamino-2,3-dichloro-anthraquinone (RN=81-42-5) deposited on a glassy carbon electrode. In this way, anomalous measurements at low ionic strength and low concentrations of pH buffering species can be overcome.
Owner:SCHLUMBERGER TECH CORP
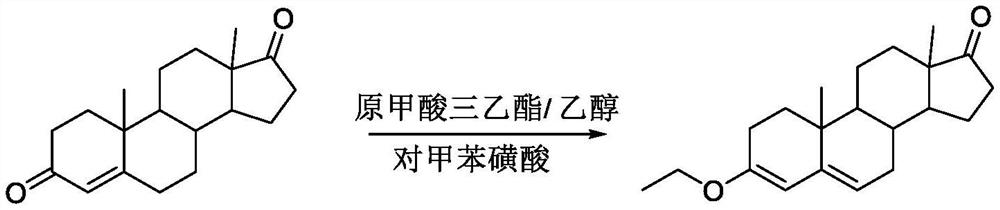
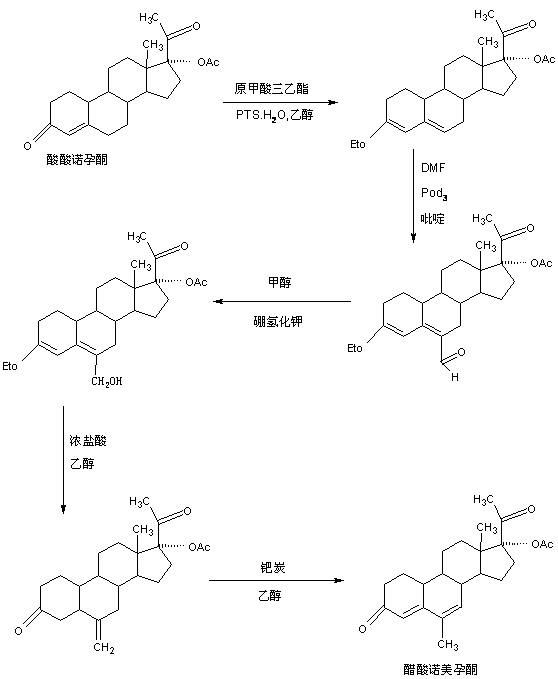
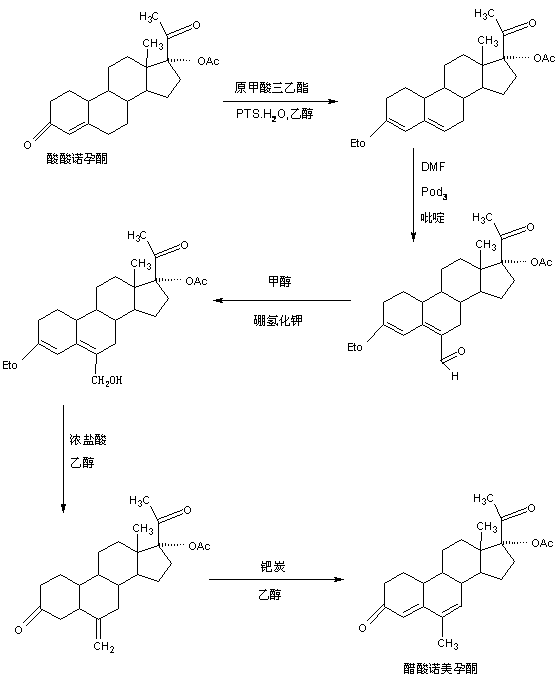
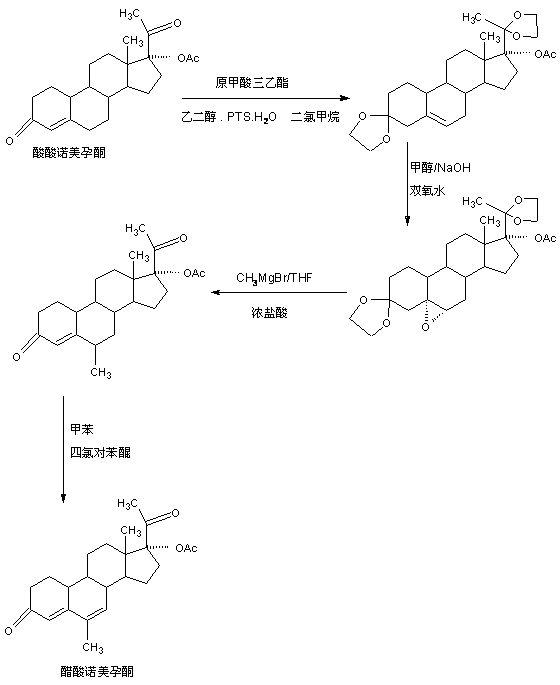
Preparation method of nomegestrol acetate
InactiveCN107629102AShort synthetic routeEasy to operateSteroidsCarboxylic acid salt preparationEpoxyStrong acids
The invention discloses a preparation method of nomegestrol acetate. The method comprises that gestonorone acetate as a raw material is dissolved in an organic solvent and then undergoes a reaction with ethylene glycol under acid catalysis in the presence of triethyl orthoformate to produce diketal, the diketal is dissolved in an organic solvent and undergoes a reaction with hydrogen peroxide under alkali catalysis to produce an epoxy compound, the epoxy compound is dissolved in an organic solvent and undergoes a Grignard addition reaction with methylmagnesium halide, the reaction product is hydrolyzed in a strong acid solution and is subjected to dehydration deprotection so that a methyl compound is obtained, and the methyl compound is dissolved in an organic solvent and undergoes a dehydrogenation reaction with tetrachloro-p-benzoquinone to produce nomegestrol acetate. The nomegestrol acetate has HPLC content of 99.0-99. 5% and a four-step synthesis total yield of 60-62%. Compared with the traditional method, the method has the advantages of simple and convenient operation, economy, environmental friendliness, high total synthesis yield and good product quality and reduces a costby 35-40%. The solvent used by the method can be recovered and recycled and is conducive to industrial production.
Owner:HUNAN KEREY BIOTECH
Method for preparing canrenone
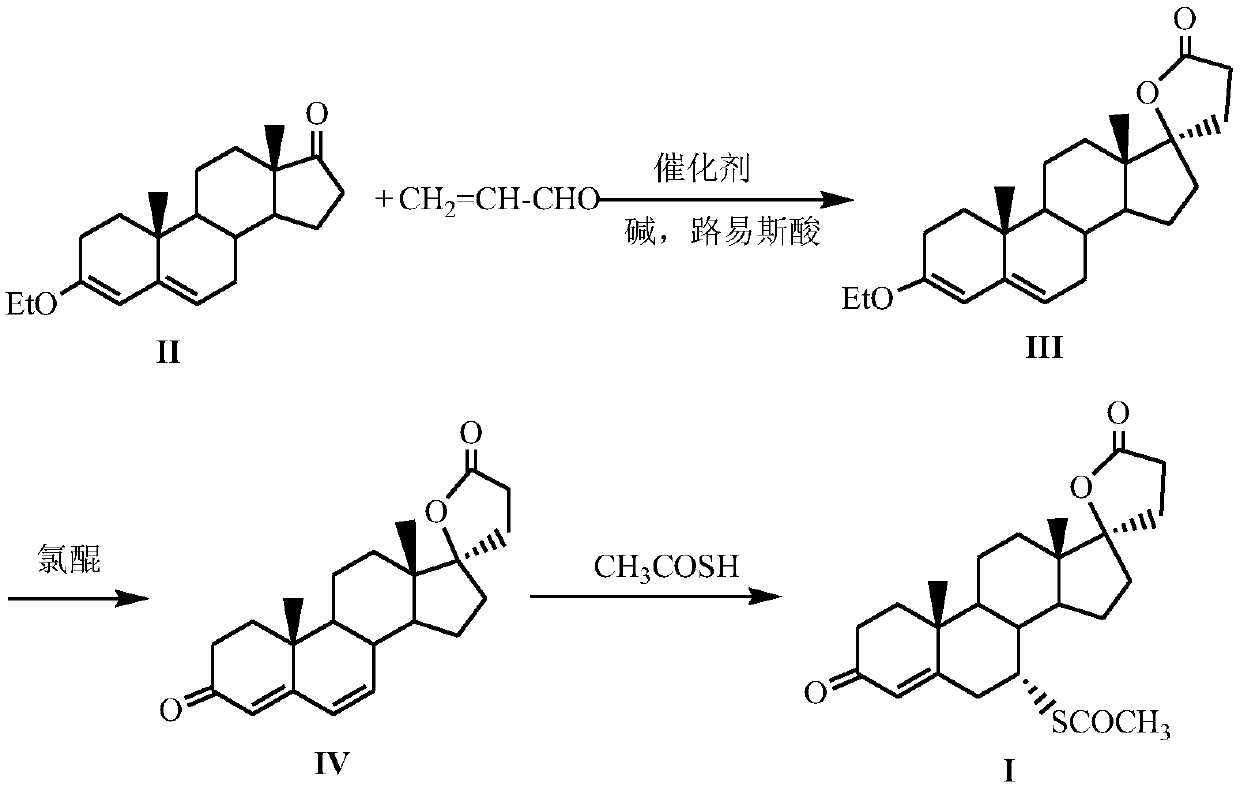
The invention discloses a method for preparing canrenone. The method comprises the following steps of step one, reacting a compound shown in formula (II) with acraldehyde under the action of a catalyst to obtain a compound shown in formula (III); step two, reacting the compound shown in the formula (III) with chloranil to dehydrogenize, thus obtaining the canrenone. The method for preparing the canrenone, provided by the invention, has the advantages of short steps, simplicity and convenience in operation, high synthesis efficiency and suitability for industrial production; a new path is provided for preparing the canrenone.
Owner:JIANGSU MARINE RESOURCES DEV RES INST LIAN YUNGANG
Preparation method for disperse red
InactiveCN102924961AEasy to makeStrong industrial operabilityAzo dyesPhosphoric acidHydroquinone Compound
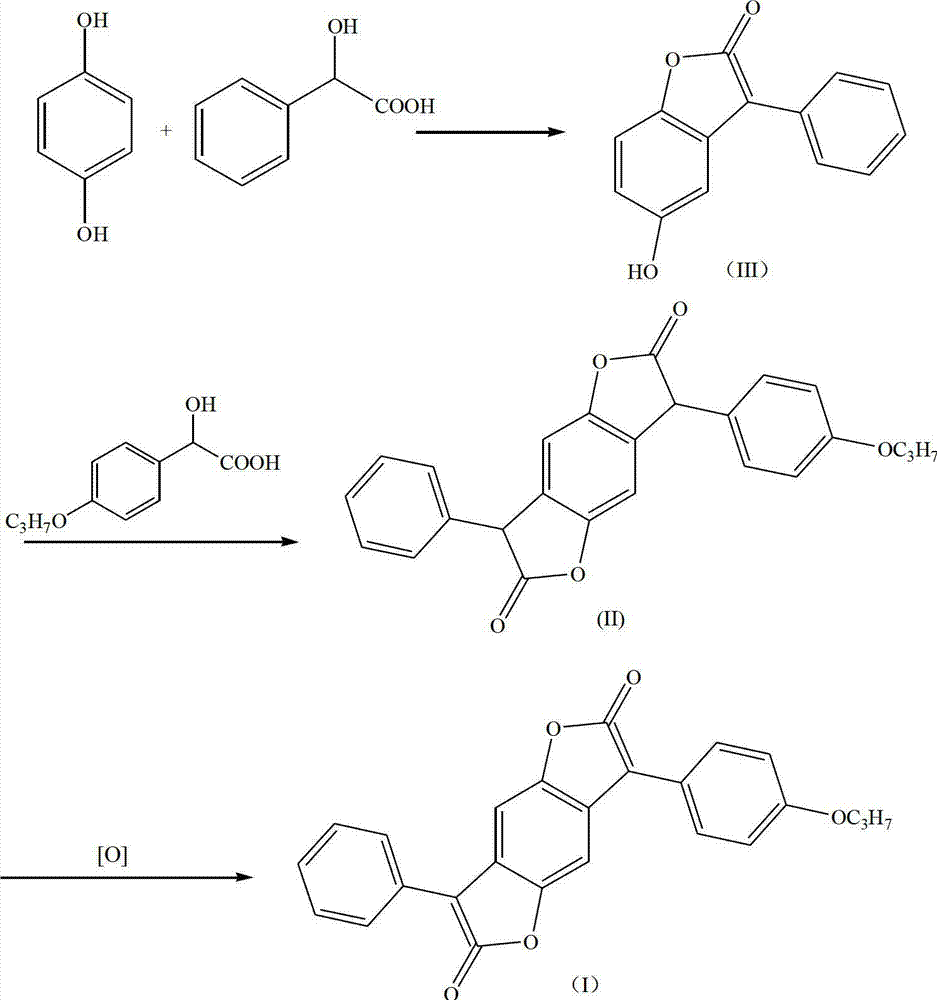
The invention discloses a preparation method for disperse red, which comprises the following steps: hydroquinone is reacted with mandelic acid to obtain an intermediate compound (III); the compound (III) is further reacted with propoxy mandelic acid in presence of phosphoric acid to obtain an intermediate compound (II); and the intermediate compound (II) is oxidized by chloranil to obtain the target product. The process route disclosed by the invention is simple in preparation and strong in industrial operability; the yield of the compound (III) preparation step is over 91%; the yield of the compound (II) preparation step is over 71%; the yield in the preparation process of the target product compound (I) is over 98%; and the final product purity is high and is above 99.8%.
Owner:江苏德旺数码科技有限公司
Tetra-(0,0-dialkyl phosphonyl) p-benzoquinone and preparation method thereof
InactiveCN101941989ADoes not change mechanical propertiesImprove flame retardant performanceGroup 5/15 element organic compoundsEpoxyPolyester
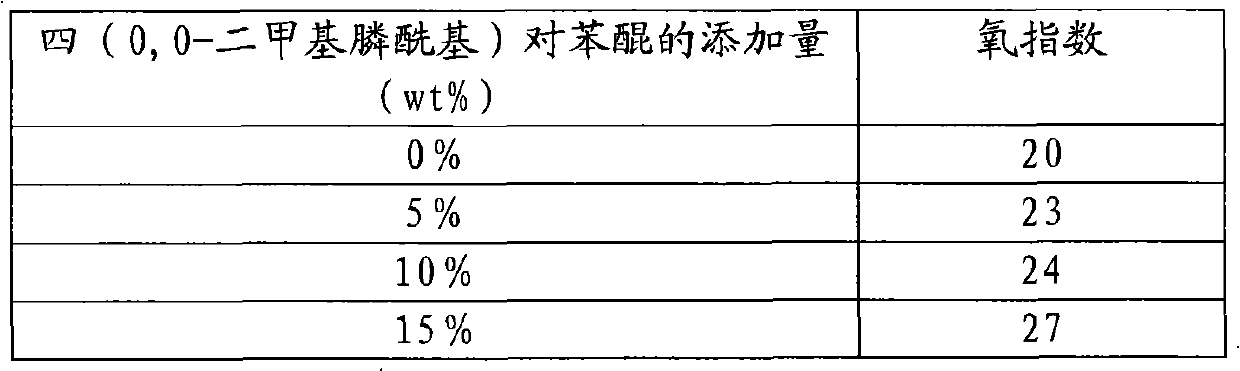
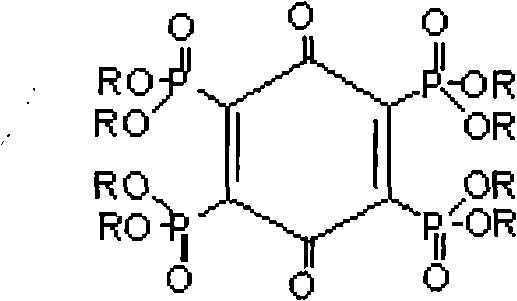
The invention relates to tetra-(0,0-dialkyl phosphonyl) p-benzoquinone and a preparation method thereof. The structure of the compound is shown in the specification, wherein R is straight chain or branched chain alkyl containing 1-8 C atoms. The preparation method comprises the following steps: adding a certain molar ratio of chloranil and tris phosphite (P(OR)3) into an organic solvent under the heating condition for carrying out sustained reaction for a period of time, and then, purifying and drying the reactants to prepare the tetra-(0,0-dialkyl phosphonyl) p-benzoquinone. The tetra-(0,0-dialkyl phosphonyl) p-benzoquinone of the invention has high flame resistance, stable physical and chemical properties and good compatibility with high molecular materials, can be used as the flame retardant of polyester, polyamide, epoxy resin, glass reinforced plastic resin, paint and the like, is favorable for environment protection, has simple process, low equipment investment, low cost and high phosphorus content in products, and can realize scale production easily.
Owner:李留新
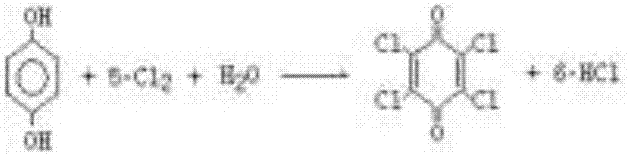
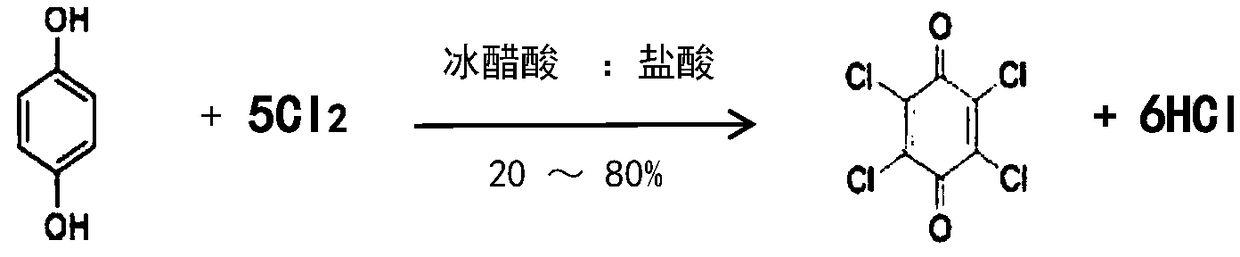
Chloranil and preparation method thereof
ActiveCN106866399AStable supplyPrice stabilityOrganic compound preparationQuinone preparation by oxidationSimple Organic CompoundsOrganic solvent
The invention provides chloranil and a preparation method thereof and relates to the field of organic compounds. The method for preparing the chloranil comprises the following steps: a) enabling phenol to react with chlorine so as obtain polychlorophenol; and b) adding oxidation reagents to enable the polychlorophenol to react with chlorine in the presence of an organic solvent, thereby obtaining the chloranil. According to the method, synthesis is performed by taking phenol as a raw material, and the technical problem in the prior art that the chloranil cannot be normally produced because p-diphenol and p-aminophenol are high in price and unstable is solved. According to the method, a two-step synthesis process is adopted, the problems that multiple preparation steps are needed formerly and more wastewater is produced in the process can be solved. The preparation method has the advantages that the raw material cost is low, the process flow is simple, the auxiliary raw materials are readily available, the reacted solvent can be repeatedly used, much wastewater is not produced in the process flow, and the like.
Owner:NANTONG SHUCHUANG PHARMA
A kind of method for preparing spironolactone
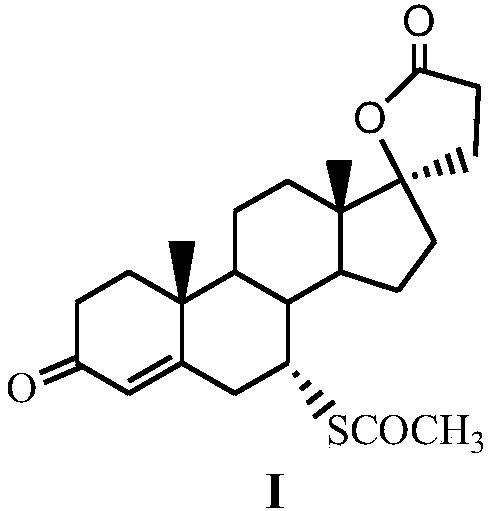
The invention discloses a method for preparing spirolactone. The method comprises the following steps: 1, performing a reaction on a compound shown as a formula (II) and acrolein under the action of a catalyst, alkali and Lewis acid to obtain a compound shown as a formula (III); 2, performing a reaction on the compound shown as the formula (III) and chloranil to obtain a compound shown as a formula (IV); 3, performing an addition reaction on the compound shown as the formula (IV) and thioacetic acid to obtain the spirolactone. The preparation method of the spirolactone, provided by the invention, has the advantages of a short synthetic route, cheapness and easy obtainment of used reagents, simple operation, high total yield rate, and suitability for industrial production; a novel way for preparing the spirolactone is provided.
Owner:HUAIHAI INST OF TECH
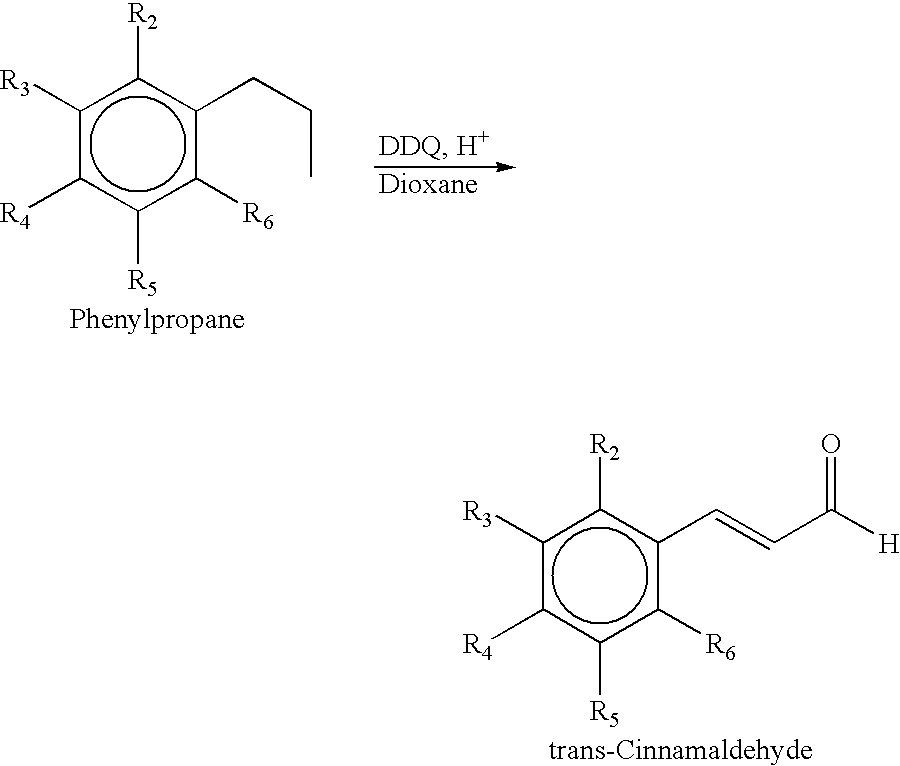
Process for the preparation of substituted trans-cinnamaldehyde, a natural yellow dye, from phenylpropane derivatives
The present invention relates to the preparation of substituted trans-cinnamaldehyde, a natural yellow dye from Phenylpropane derivatives having R2-R3-R4-R5-R6 substitution, wherein R2 to R6 equal or different, being hydrogen or hydroxy or acyl or halogen or alkyl or heterocyclic or aryl or dioxymethylene or alkoxy groups, etc., by oxidizing the said phenylpropane derivatives using a oxidising agent such as 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) or p-chloranil or pyridinium chlorochromate (PCC) or tBuOOH or CrO3 with a catalytic amount of inorganic / organic acid or alumina, celite, and silica gel as a solid support for microwave irradiation and thus substituted trans-cinnamaldehydes, a natural yellow dye, are obtained in high yield ranging from 68-82%.
Owner:COUNCIL OF SCI & IND RES
Method for reducing dioxin in preparation of chloranil
ActiveCN108623442AHigh speedReduce the chance of occurrenceQuinone preparation by oxidationFiltrationReaction temperature
The invention relates to a method for reducing dioxin in preparation of chloranil. The preparation method of the chloranil comprises the steps of stirring a mixed raw material containing hydroquinoneand an appropriate amount of p-aminophenol for enabling the material to be dissolved in an acetic acid solution, wherein the mass ratio of acetic acid in the acetic acid solution is 20-80wt%, and themass ratio of the mixed raw material to the acetic acid solution is less than or equal to 15 wt%; then, introducing chlorine gas while stirring, carrying out a reaction at 50-90 DEG C, sampling in different periods, and stopping the introduction of the chlorine gas when the melting point of a target material is detected to be higher than or equal to 292 DEG C so as to obtain crystals of the chloranil and crystals of ammonia chloride; adding an appropriate amount of ammonium chloride during first kettle production, wherein the adding amount of the ammonium chloride and the controlling amount ofammonium chloride are the presence of ammonia chloride crystals in mother liquid at the chlorination reaction temperature; after a chlorination reaction is finished, heating up to 60-105 DEG C, wherein the temperature of the chlorination reaction is increased by 10-15 DEG C; filtering the chloranil material when the ammonium chloride salt crystals do not exist, and recovering the mother liquor obtained after filtration for the following use.
Owner:许克宇
Preparation method of 7-chloro-6H-benzopyran[4,3-b]quinoline and derivative thereof
The invention belongs to the technical fields of medicine chemical intermediate and related chemical technologies and relates to a preparation method of a 7-chloro-6H-benzopyran[4,3-b]quinoline compound. The 7-chloro-6H-benzopyran[4,3-b]quinoline compound is widely applied in various fields of chemistry and has a wide market prospect. In the invention, a series of 7-chloro-6H-benzopyran[4,3-b]quinoline compounds are synthesized through a Diels-Alder reaction with a Schiff base being a raw material, tetrachloro-p-benzoquinone being a chlorine source and a copper salt being a catalyst. The method is short in synthetic route, is gentle in conditions, is simple in operation and is expected to achieve industrialized production. The method is short in synthetic route, is gentle in conditions, is simple in operation, is high in yield and has large use value and socially economic benefit.
Owner:DALIAN UNIV OF TECH
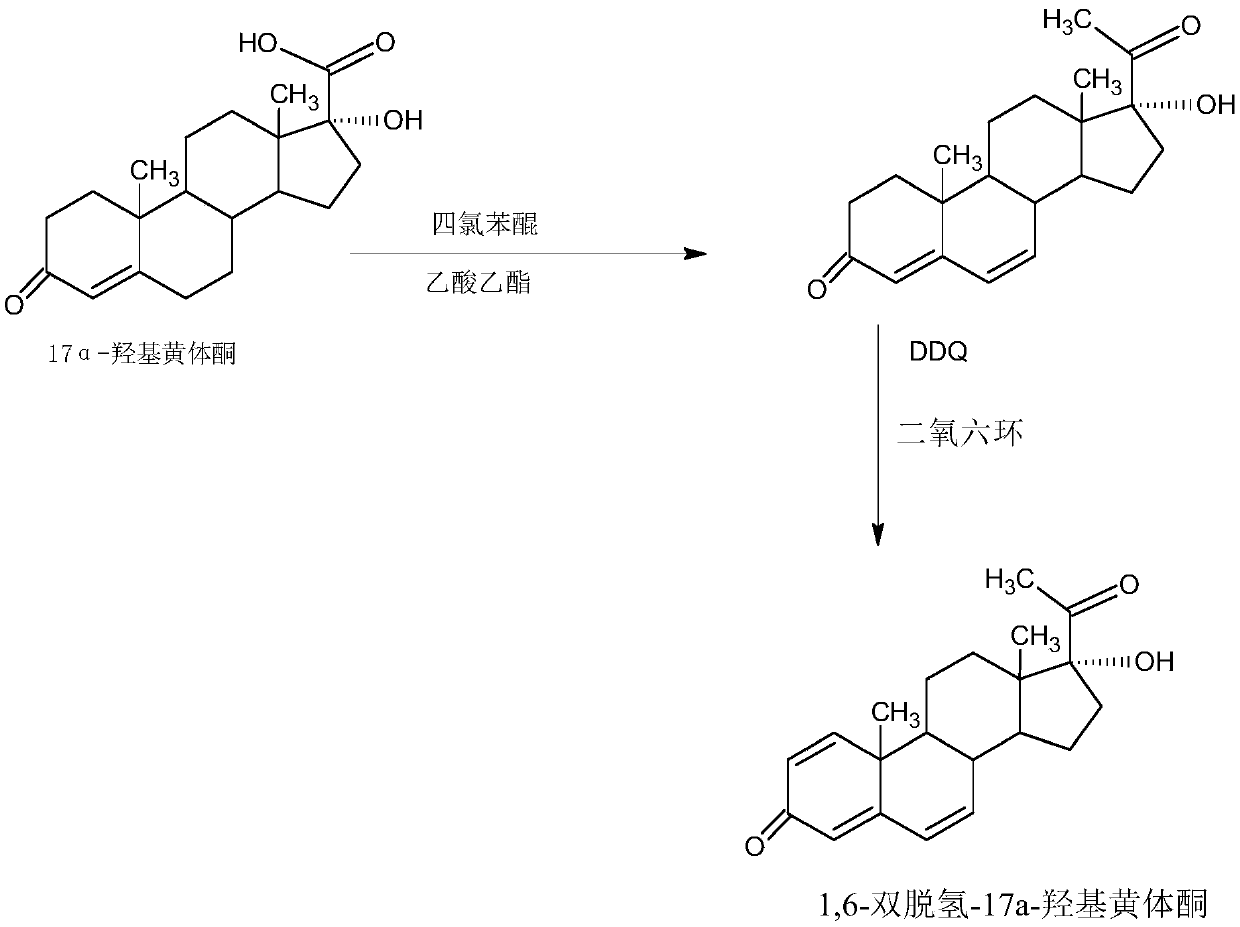
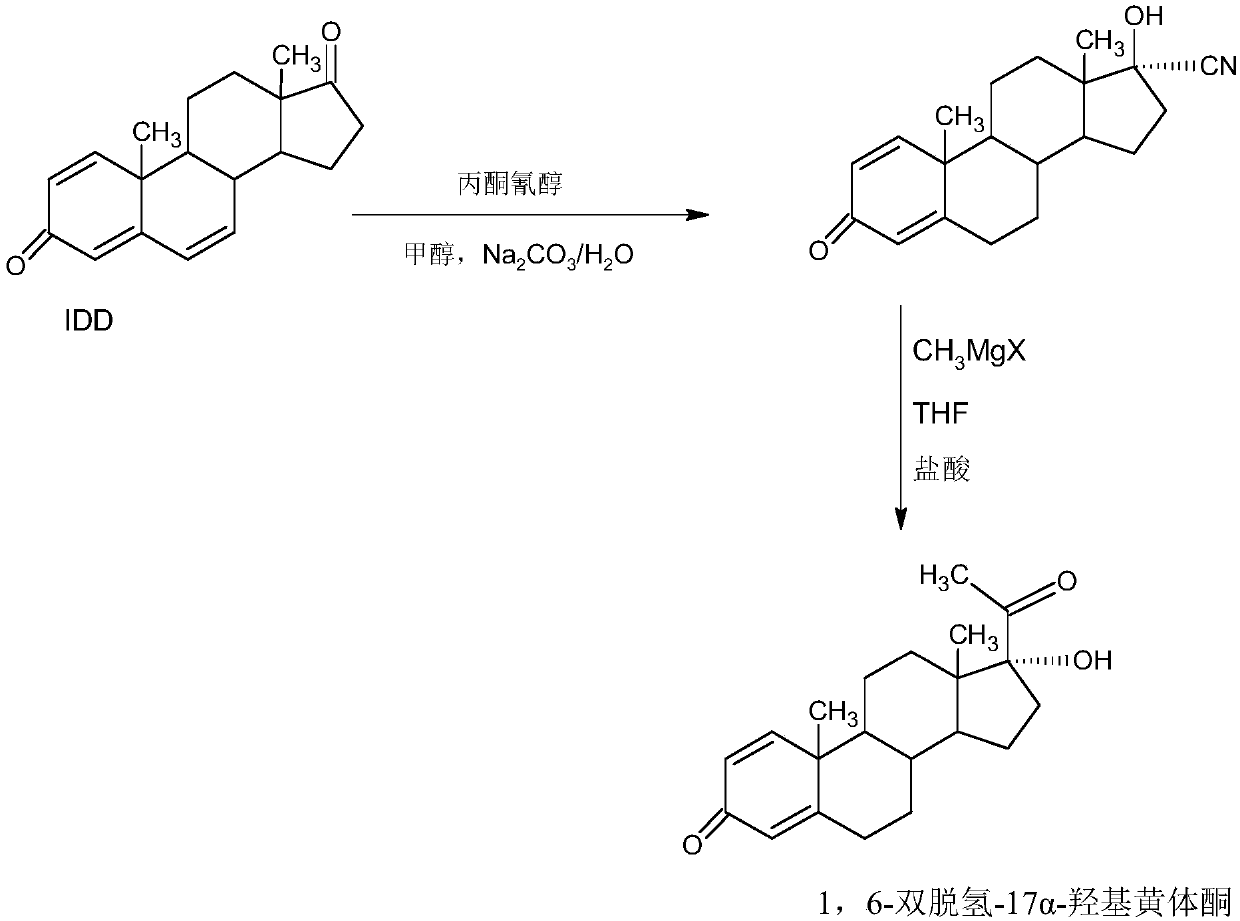
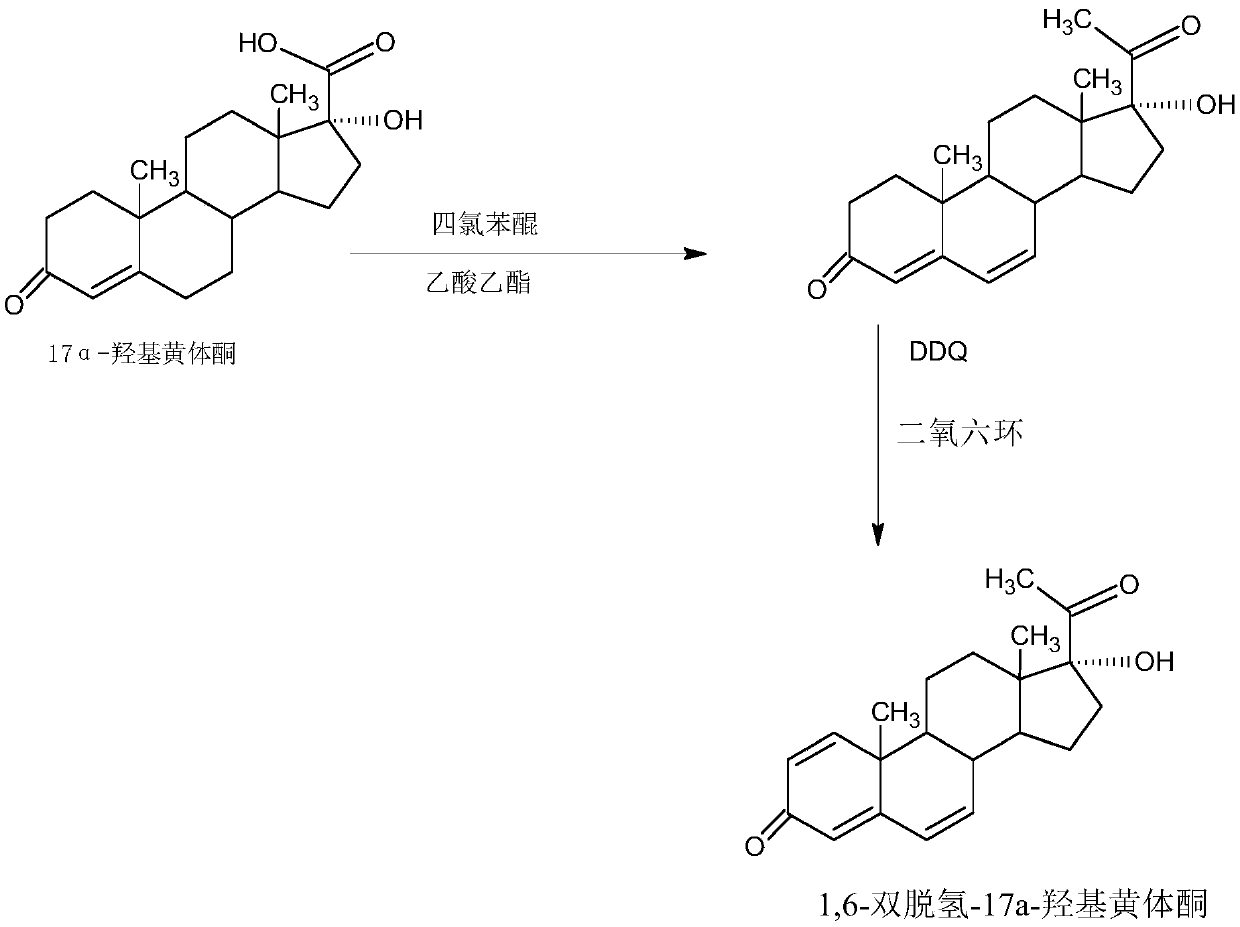
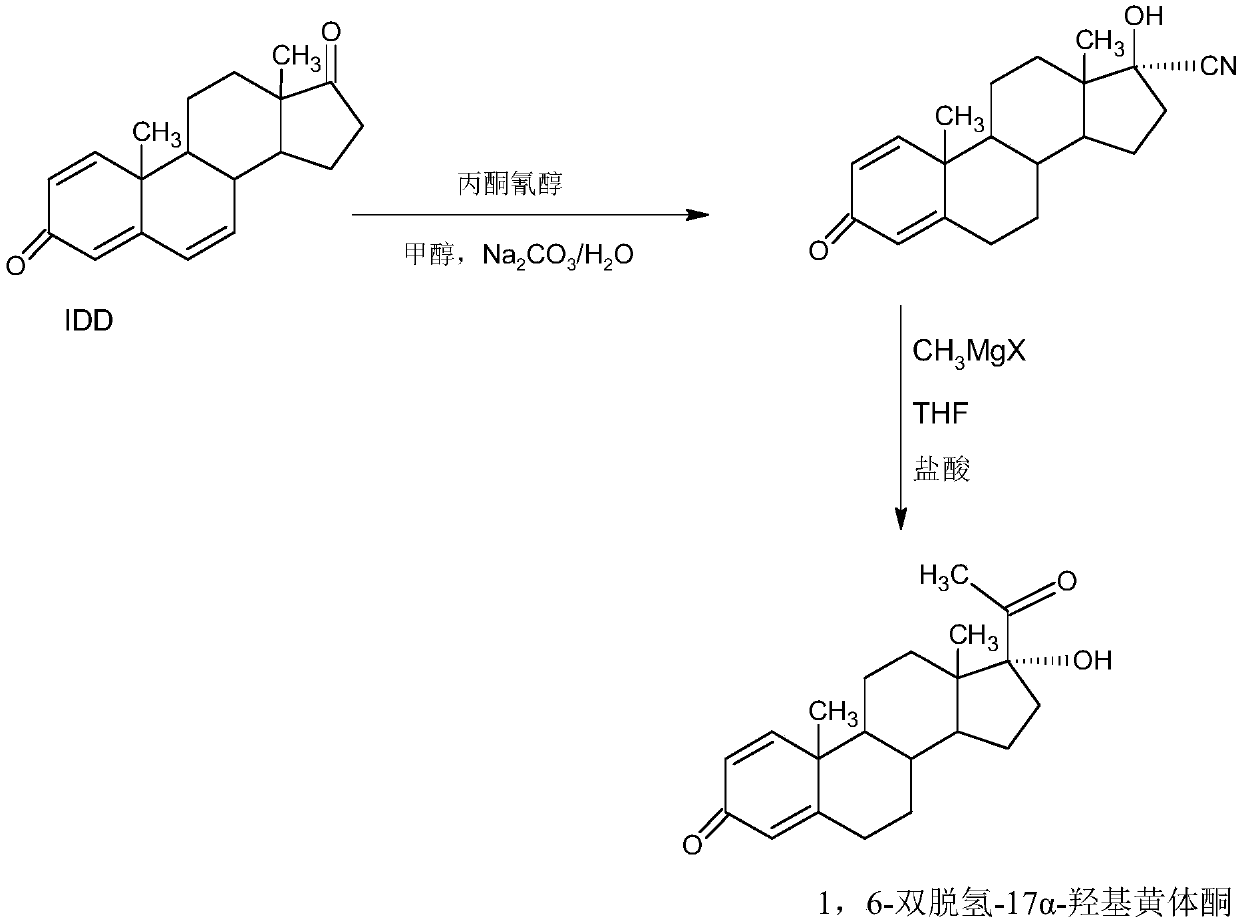
Preparation method of 1,6-didehydrogenation-17a-hydroxyl progesterone product
The invention provides a preparation method of a 1,6-didehydrogenation-17a-hydroxyl progesterone product. The preparation method includes the steps that 1,4-androstenedione (IDD) is adopted as a raw material, firstly, IDD molecules and acetone cyanohydrin react in a first organic solvent under the catalyzation of alkali, so that a hydroxyl-cyanogen product is obtained; then the hydroxyl-cyanogen product is prepared into 1,6-didehydrogenation-17a-hydroxyl progesterone under the existence of methyl magnesium halide, a second organic solvent and acid; then 1,6-didehydrogenation-17a-hydroxyl progesterone is subjected to heating reflux and decoloration in methylbenzene, acetone or lower alcohol of C4 or below and recrystallized, so that the1,6-didehydrogenation-17a-hydroxyl progesterone productis obtained. With the IDD being the raw material, compared with the traditional production method that dioscin is used as a raw material, the preparation method has the advantages of being wide in source of the raw material, making the technology economic and environmentally friendly and lowering production costs substantially. In the preparation method, expensive and toxic DDQ and a chloranil dehydrogenating agent do not need to be used; the solvent used in the technology can be recycled and applied mechanically, economy and environmental protection are realized, and industrialized production is quite easy.
Owner:HUNAN KEREY BIOTECH
Preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and derivative thereof
ActiveCN104262357AGood anti-HBV effectShort synthetic routeOrganic chemistryOrganic synthesisQuinoline
The invention belongs to the pharmaceutical chemical engineering intermediates and relative chemical technical field and relates to preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and a derivative thereof. Benzothiapyran [4,3-b] quinoline is an important bioactive molecule, has good curative efficacy of resisting hepatitis B virus and has an important application in the fields of organic synthesis, medicinal chemistry and the like. The 7-chlorine-6H-benzothiapyran [4,3-b] quinoline is one of derivatives of benzothiapyran [4,3-b] quinoline, and a synthetic method of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline is not reported yet so far. The 7-chlorine-6H-benzothiapyran [4,3-b] quinoline is prepared by the following steps: with a schiff base derivative as a raw material, carrying out Diels-Alder reaction under the actions of salt, chloranil and a metal copper compound catalyst to generate the 7-chlorine-6H-benzothiapyran [4,3-b] quinoline. The preparation methods have the advantages of short synthesis rout, mild conditions, high yield and the like, is simple to operate and have large use value and social and economic benefits.
Owner:DALIAN UNIV OF TECH
Preparation method of chloranil
ActiveCN112608223AReduce solubilityImprove solubilityQuinone preparation by oxidationSolubilityAcetic acid
The invention provides a preparation method of chloranil, and belongs to the technical field of chemical production. According to the invention, glacial acetic acid is used as a solvent, and glacial acetic acid has certain solubility to tetrachlorohydroquinone, but has very low solubility to the product tetrachlorobenzoquinone, thus being beneficial to improving the product yield; the glacial acetic acid is acidic, so that the oxidability of nitric acid can be improved when the nitric acid is used as an oxidizing agent. Nitric acid or chlorine is used as an oxidizing agent to oxidize tetrachlorohydroquinone to prepare tetrachlorobenzoquinone, the raw materials are cheap and easy to obtain, the operation method is simple, the reaction route is short, the equipment investment is low, the market competitiveness in the production cost is extremely high, the product yield is higher than 98%, special purification treatment is not needed, and the final product purity is higher than 99.0%.
Owner:ZHEJIANG SHENZHOU PHARMA
Dehydrogenation method for preparing canrenone
The invention discloses a dehydrogenation method for preparing canrenone, which comprises the following steps: by taking an intermediate A as a raw material, brominating to obtain a brominated intermediate, then debrominating the brominated intermediate, and adopting calcium bromide and calcium carbonate as debrominating reagents, thereby realizing a dehydrogenation process for clean production of canrenone with high yield and high content. The problem that a large amount of phenol-containing wastewater is generated by dehydrogenation of chloranil is solved.
Owner:SHANDONG SITO BIO TECHNOLOGY CO LTD +1
Chlorobenzoquinone and its preparation method
ActiveCN106866399BStable supplyPrice stabilityOrganic compound preparationQuinone preparation by oxidationP-AminophenolPhenol
The invention provides chloranil and a preparation method thereof and relates to the field of organic compounds. The method for preparing the chloranil comprises the following steps: a) enabling phenol to react with chlorine so as obtain polychlorophenol; and b) adding oxidation reagents to enable the polychlorophenol to react with chlorine in the presence of an organic solvent, thereby obtaining the chloranil. According to the method, synthesis is performed by taking phenol as a raw material, and the technical problem in the prior art that the chloranil cannot be normally produced because p-diphenol and p-aminophenol are high in price and unstable is solved. According to the method, a two-step synthesis process is adopted, the problems that multiple preparation steps are needed formerly and more wastewater is produced in the process can be solved. The preparation method has the advantages that the raw material cost is low, the process flow is simple, the auxiliary raw materials are readily available, the reacted solvent can be repeatedly used, much wastewater is not produced in the process flow, and the like.
Owner:NANTONG SHUCHUANG PHARMA
Method for preparing 1,6-bi-dehydrogenized-17a-hydroxy progesterone
The invention provides a method for preparing 1,6-bi-dehydrogenized-17a-hydroxy progesterone. The method comprises the following steps: firstly, adopting a nutrient medium and one or more microbial strain for performing microbial fermentation on phytosterol, thereby acquiring 1,4-androstenedione, namely, IDD; taking IDD as a raw material and reacting with acetone cyanohydrins in a first organic solvent under the condition of base catalysis, thereby acquiring hydroxyl cyanide; preparing the 1,6-bi-dehydrogenized-17a-hydroxy progesterone by using the hydroxyl cyanide under the existence of methyl magnesium halide, second organic solvent and acid. Compared with the traditional production method which uses diosgenin as the raw material, the method using IDD as the raw material according to theinvention has the advantages of extensive source of raw material, economical and environment-friendly process, greatly reduced production cost, and the like; in the invention, high-cost and toxic DDQand chloranil dehydrogenating agent are no longer used; solvents used in the process are recyclable, economical, environment-friendly and beneficial to industrial production.
Owner:HUNAN KEREY BIOTECH
Colored flame retardant charring agent tetraPEPA oxy-p-benzoquinone compound and preparation method thereof
InactiveCN109021016AAdapt to high temperature processingGood symmetryGroup 5/15 element organic compoundsPolyesterColor effect
The invention relates to a colored flame retardant charring agent tetraPEPA oxy-p-benzoquinone compound and a preparation method thereof. The structure of the compound is shown as the following formula in the specification. The preparation method includes: in a reactor equipped with a stirrer, a thermometer and a high-efficiency reflux condensation tube, adding a proper amount of an organic solvent and tetrachloro-p-benzoquinone, then adding a PEPA sodium salt that is 4-5 times the mole of tetrachloro-p-benzoquinone, performing heating to 100-160DEG C, carrying out heat preservation reaction for 6-9h, performing pumping filtration, removing sodium chloride, cooling and crystallizing the filtrate, and conducting filtering and drying, thus obtaining the reddish brown colored flame retardantcharring agent tetraPEPA oxy-p-benzoquinone. The compound provided by the invention has excellent flame retardant and charring properties, also has coloring effect, and is suitable for use as a flameretardant charring agent and coloring agent for nylon, polyester, polyurethane, rubber, polyolefin, unsaturated resin and other materials. Also the preparation method has the characteristics of one-step reaction, simple process and low equipment investment, and is easy to realize industrial production.
Owner:SUZHOU UNIV OF SCI & TECH
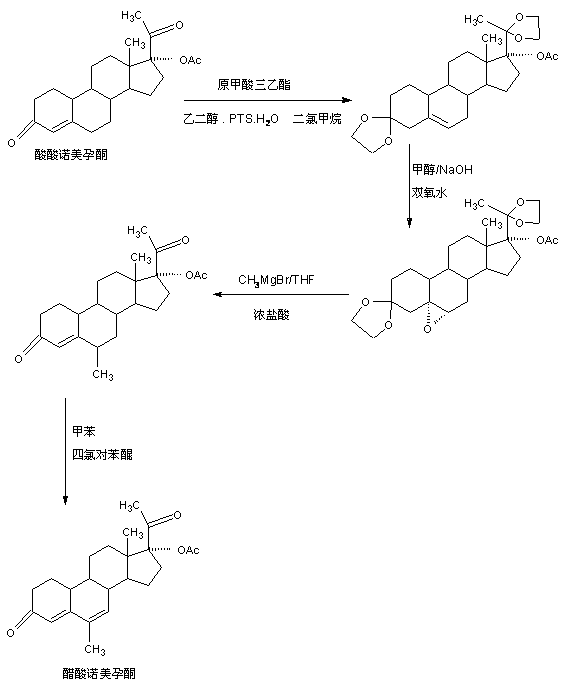
The preparation method of nomegestrol acetate
InactiveCN107629102BShort synthetic routeEasy to operateSteroidsCarboxylic acid salt preparationEpoxyDehydrogenation
The invention discloses a preparation method of nomegestrol acetate. The method comprises that gestonorone acetate as a raw material is dissolved in an organic solvent and then undergoes a reaction with ethylene glycol under acid catalysis in the presence of triethyl orthoformate to produce diketal, the diketal is dissolved in an organic solvent and undergoes a reaction with hydrogen peroxide under alkali catalysis to produce an epoxy compound, the epoxy compound is dissolved in an organic solvent and undergoes a Grignard addition reaction with methylmagnesium halide, the reaction product is hydrolyzed in a strong acid solution and is subjected to dehydration deprotection so that a methyl compound is obtained, and the methyl compound is dissolved in an organic solvent and undergoes a dehydrogenation reaction with tetrachloro-p-benzoquinone to produce nomegestrol acetate. The nomegestrol acetate has HPLC content of 99.0-99. 5% and a four-step synthesis total yield of 60-62%. Compared with the traditional method, the method has the advantages of simple and convenient operation, economy, environmental friendliness, high total synthesis yield and good product quality and reduces a costby 35-40%. The solvent used by the method can be recovered and recycled and is conducive to industrial production.
Owner:HUNAN KEREY BIOTECH
A kind of preparation method of permanent violet pigment
The invention discloses a preparation method of a permanent violet pigment. The method comprises the following steps: dissolving 3-amino-N-ethyl carbazole in an organic solvent and carrying out a condensation reaction by adding chloranil in the presence of an acid-binding agent so as to obtain an organic solution containing 2,5-dichloro-3,6-di(9-ethyl-3-carbazole amino)-1,4-benzoquinone after the reaction; adding a metal salt catalyst into the organic solution obtained in the step a or simultaneously adding the metal salt catalyst and a quinone compound cocatalyst into the organic solution; feeding air or oxygen into a reaction kettle to reach a certain pressure; carrying out heat preservation at 120-180 DEG C for 2-10 hours; cooling to 80-120 DEG C after the reaction; washing a filter cake obtained after filtering with a small amount of organic solvent; then, respectively washing with methyl alcohol and water and drying, thereby obtaining a crude permanent violet pigment product. According to the method, a traditional poisonous and harmful closed-loop agent is not adopted, so that the cost of raw materials is saved; the generation of byproducts is reduced, so that the selectivity and total yield of the reaction are improved. Thus, less environmental pollution is generated.
Owner:NANTONG HAIDI CHEM
Method for recovering 7-chloroquinaldine mother liquor
PendingCN114634409ANothing producedAvoid pollutionQuinone preparation by oxidationOrganic chemistry methodsPtru catalystEngineering
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a method for recovering 7-chloroquinaldine mother liquor. The method mainly comprises the following steps: reacting tetrachloroquinone serving as a raw material and hydrogen peroxide serving as an oxidizing agent in the presence of a copper catalyst under an acidic condition, and performing simple filtering operation and separation to obtain a product chloranil, so as to realize regeneration of chloranil. The method for regenerating the chloranil is relatively low in cost, simple and convenient in post-treatment, relatively high in yield of the chloranil, capable of recycling the solvent for multiple times, simple to operate, less in generated sewage, green and environment-friendly, and suitable for industrial large-scale production.
Owner:JIANGSU ALPHA PHARM CO LTD
A boron bisfluoride complexed dipyrromethene derivative containing a fluorene bridge in the middle and its preparation method
The invention discloses a bridged double-center BODIPY derivative containing fluorene at a meso-position and a preparation method thereof. The preparation method comprises the following steps: synthesizing a symmetric dialdehyde compound containing bridging groups consisting of benzene, thiophene and furan and different substituent groups like a butyl group and an octyl group; then subjecting the symmetric dialdehyde compound and pyrrole to a reaction under the catalysis of indium trichloride so as to synthesize a bis(dipyrrolidine) compound; and successively carrying out oxidation by chloranil and fluoroboronation by boron trifluoride ether so as to obtain a fluorene-containing different bridging group-substituted BODIPY dye which has a structural general formula as shown in a formula I that is described in the specification. According to the invention, fluorene and the bridging groups consisting of benzene, thiophene and furan are introduced for the first time and conventional synthetic methods are improved, so the obtained double-center BODIPY dye has stable spectral absorption; and introduction of thiophene, furan and fluorene enables the fluorescence emission peak of the derivative presents obvious red shift, and fluorescence effect is enhanced with enhancement of system conjugative effect. The organic dye can be efficiently synthesized and widely used in fields like life science, analytical chemistry and environmental energy.
Owner:江苏博凡科精密五金科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
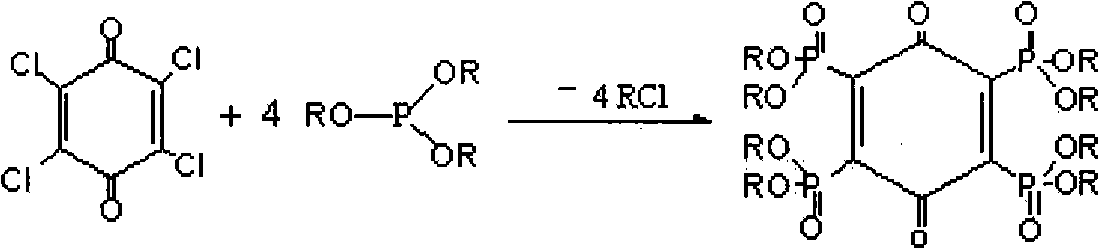
![Preparation method of 7-chloro-6H-benzopyran[4,3-b]quinoline and derivative thereof Preparation method of 7-chloro-6H-benzopyran[4,3-b]quinoline and derivative thereof](https://images-eureka.patsnap.com/patent_img/28c6c5dd-b402-4bd9-a7df-f1faef6e53b6/HDA0000576354600000011.PNG)
![Preparation method of 7-chloro-6H-benzopyran[4,3-b]quinoline and derivative thereof Preparation method of 7-chloro-6H-benzopyran[4,3-b]quinoline and derivative thereof](https://images-eureka.patsnap.com/patent_img/28c6c5dd-b402-4bd9-a7df-f1faef6e53b6/HDA0000576354600000021.PNG)
![Preparation method of 7-chloro-6H-benzopyran[4,3-b]quinoline and derivative thereof Preparation method of 7-chloro-6H-benzopyran[4,3-b]quinoline and derivative thereof](https://images-eureka.patsnap.com/patent_img/28c6c5dd-b402-4bd9-a7df-f1faef6e53b6/HDA0000576354600000031.PNG)
![Preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and derivative thereof Preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and derivative thereof](https://images-eureka.patsnap.com/patent_img/42bbfcc4-2fe5-4253-ae2c-b46937f42851/HDA0000576354430000011.PNG)
![Preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and derivative thereof Preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and derivative thereof](https://images-eureka.patsnap.com/patent_img/42bbfcc4-2fe5-4253-ae2c-b46937f42851/HDA0000576354430000021.PNG)
![Preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and derivative thereof Preparation methods of 7-chlorine-6H-benzothiapyran [4,3-b] quinoline and derivative thereof](https://images-eureka.patsnap.com/patent_img/42bbfcc4-2fe5-4253-ae2c-b46937f42851/HDA0000576354430000031.PNG)