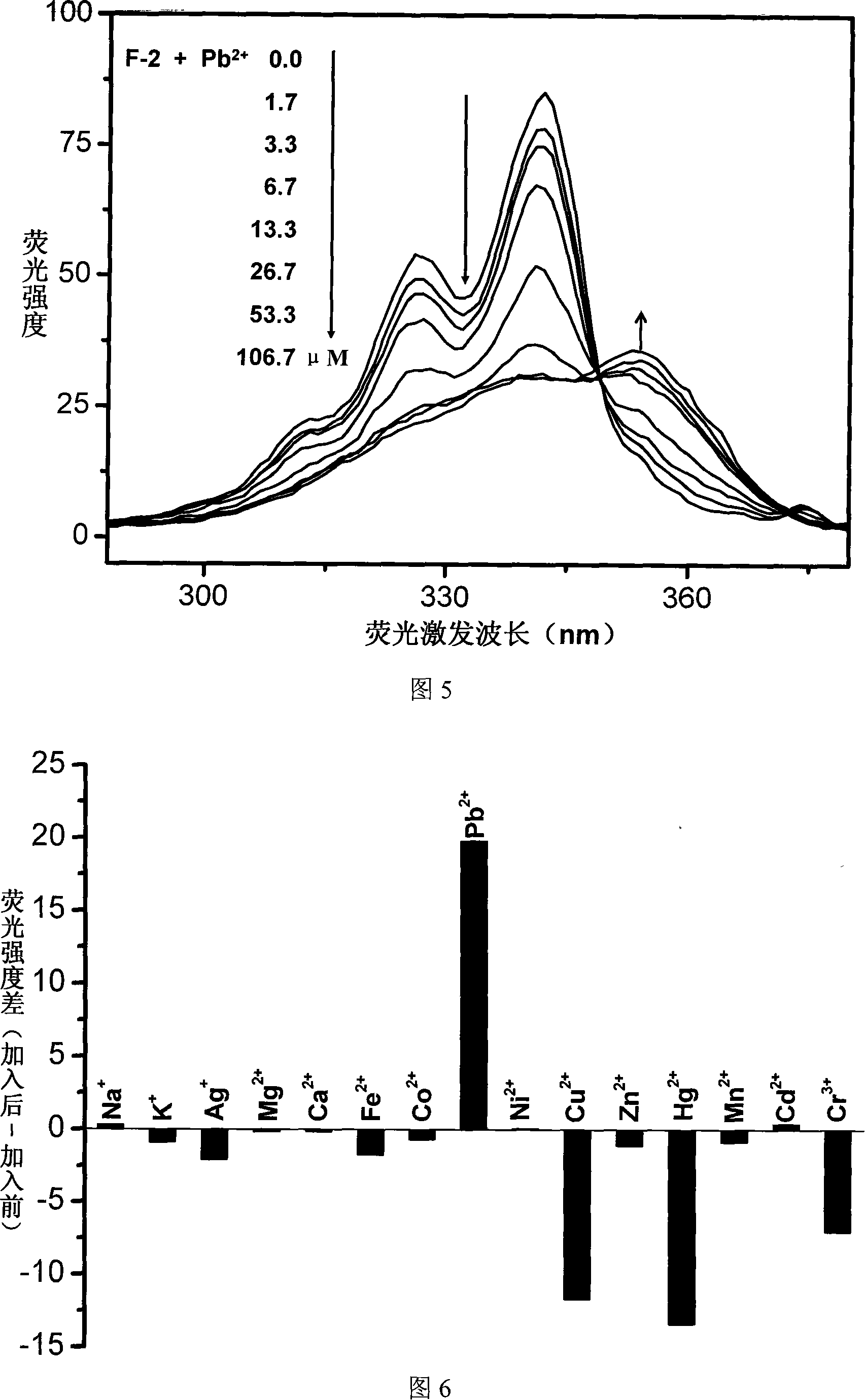
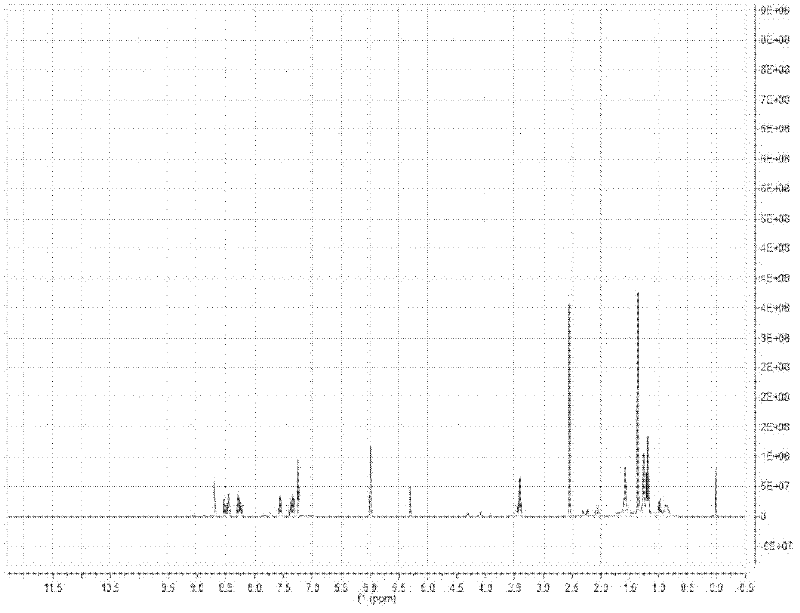
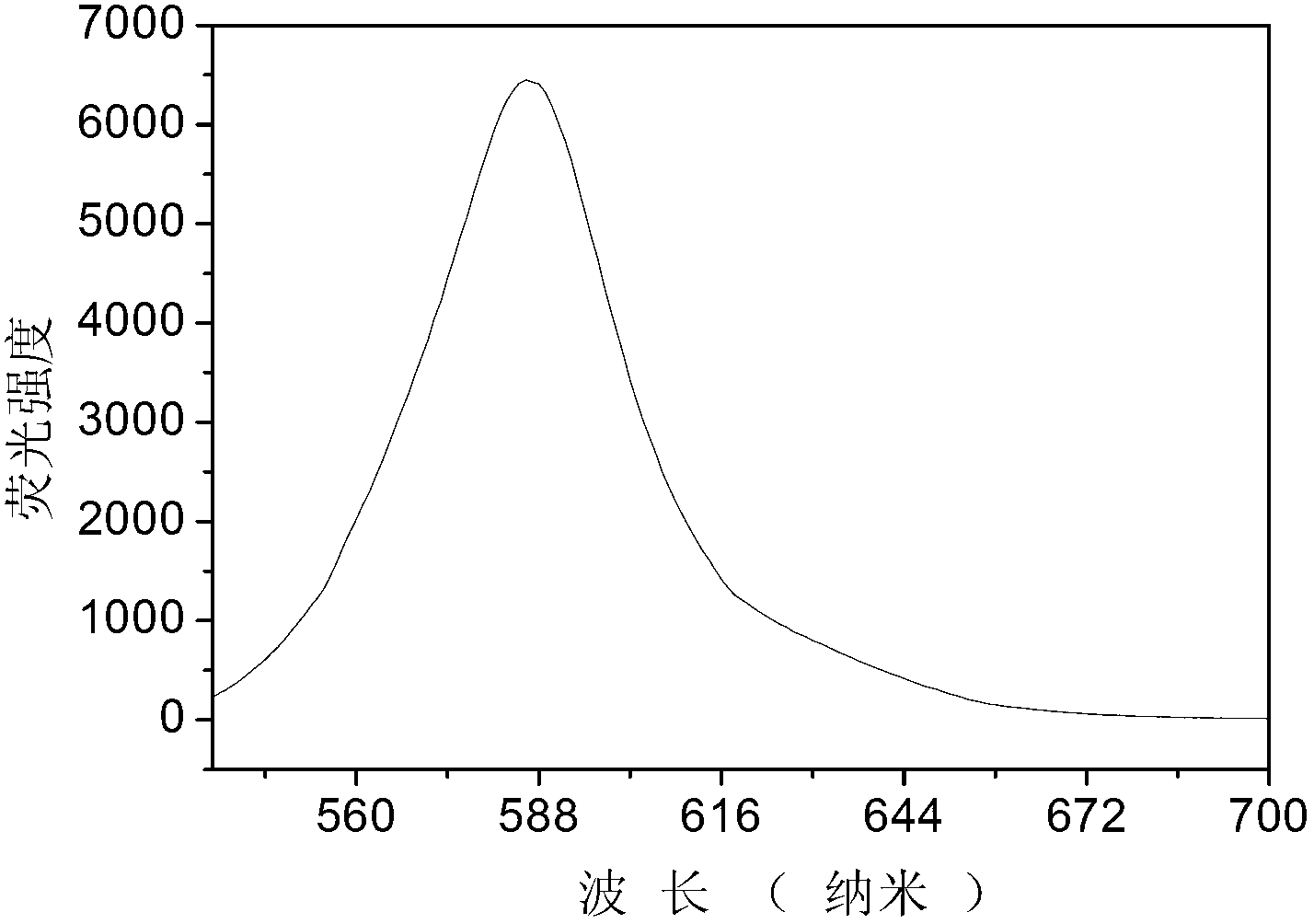
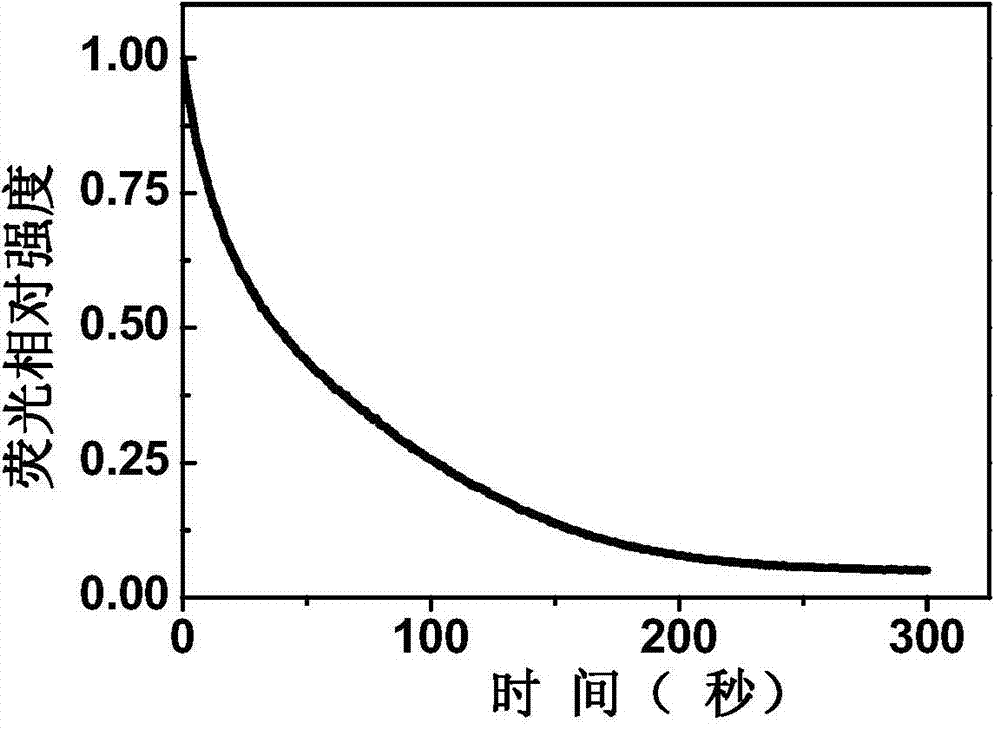
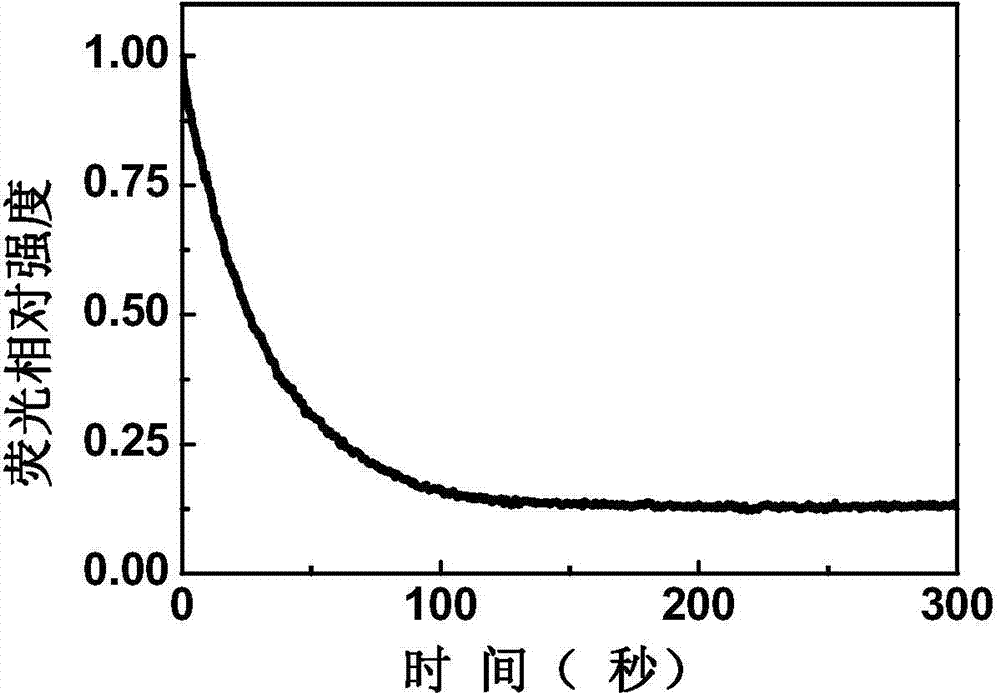
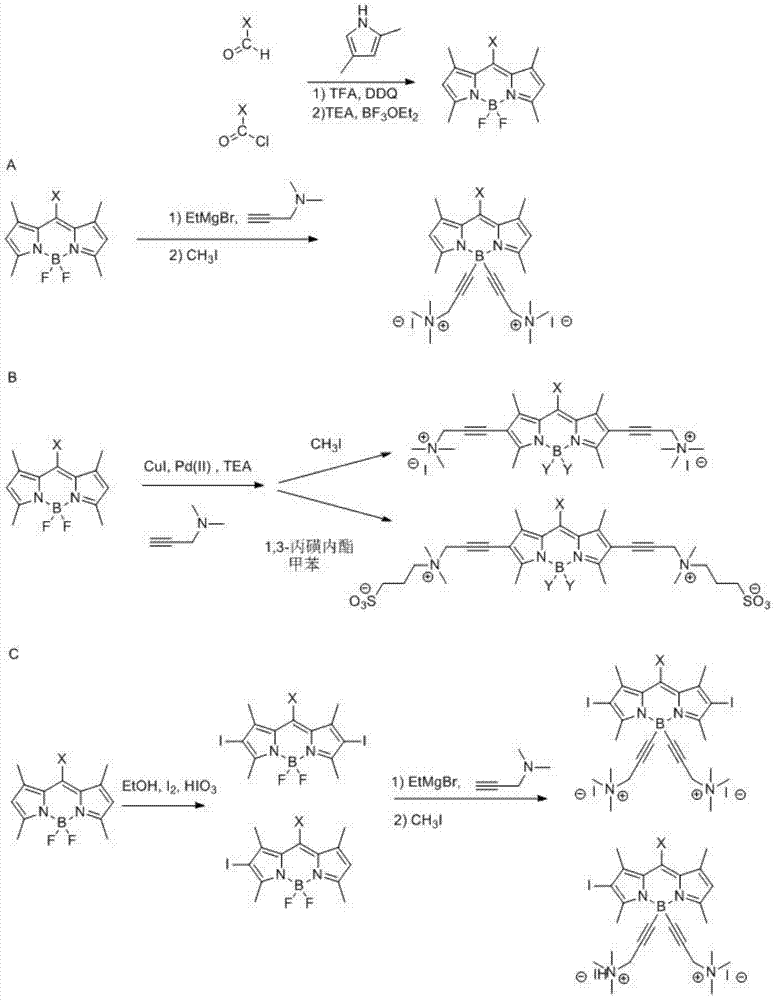
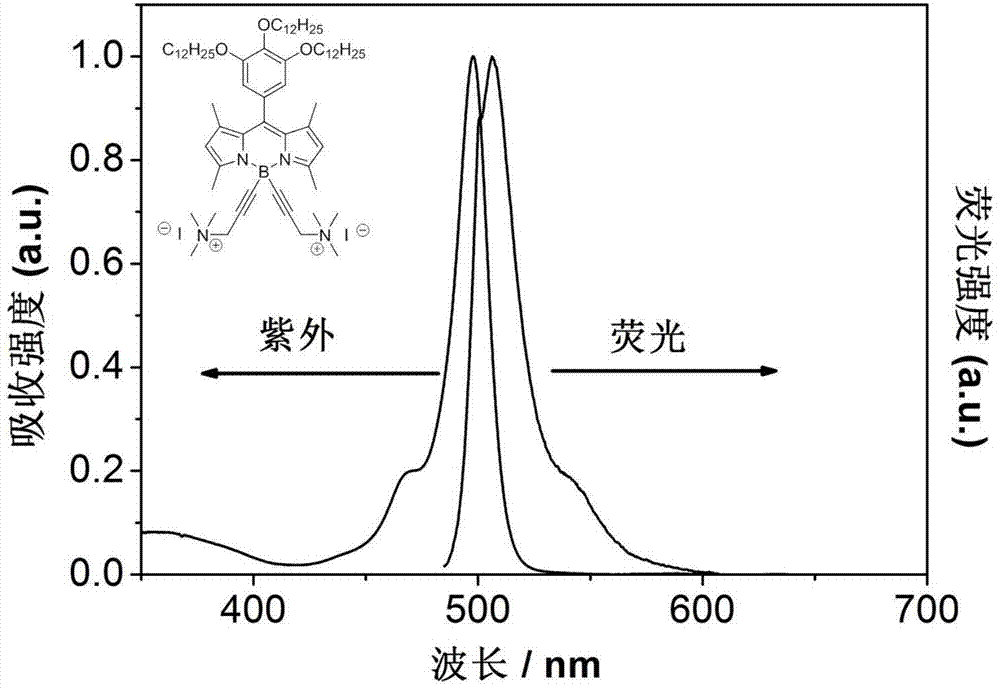
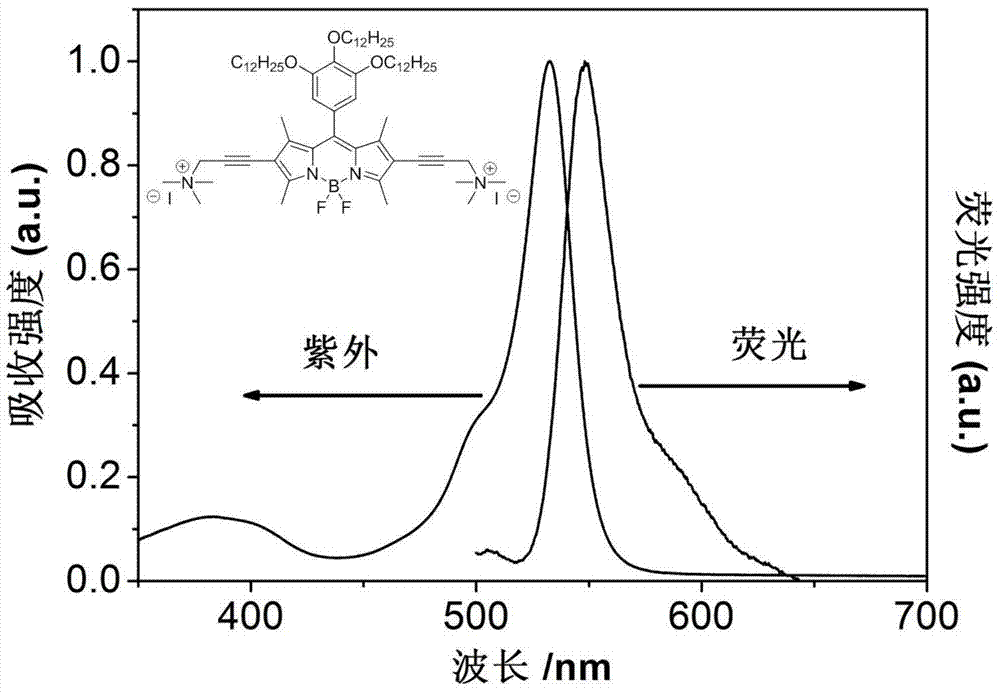
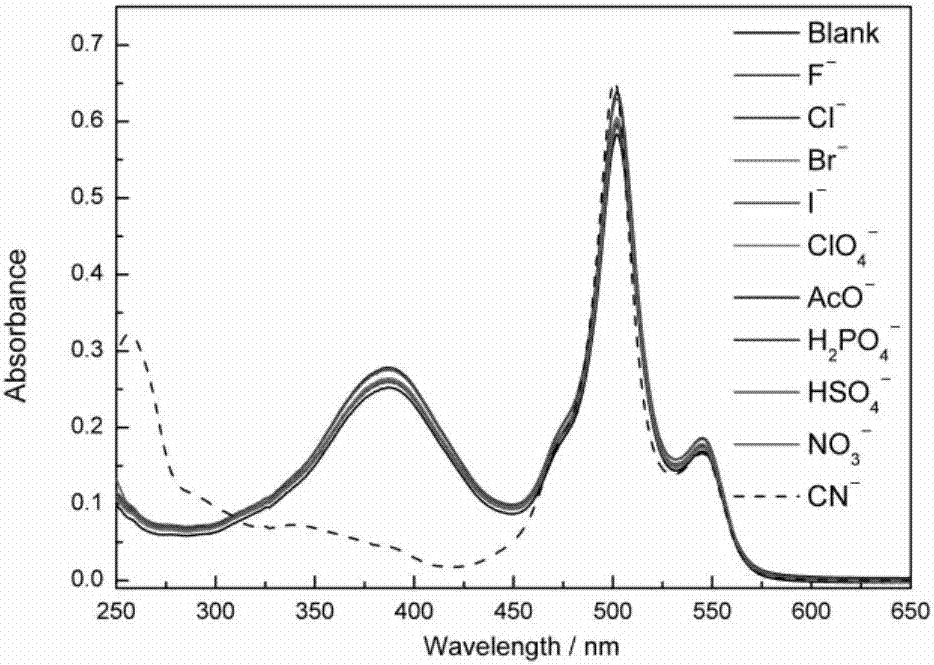
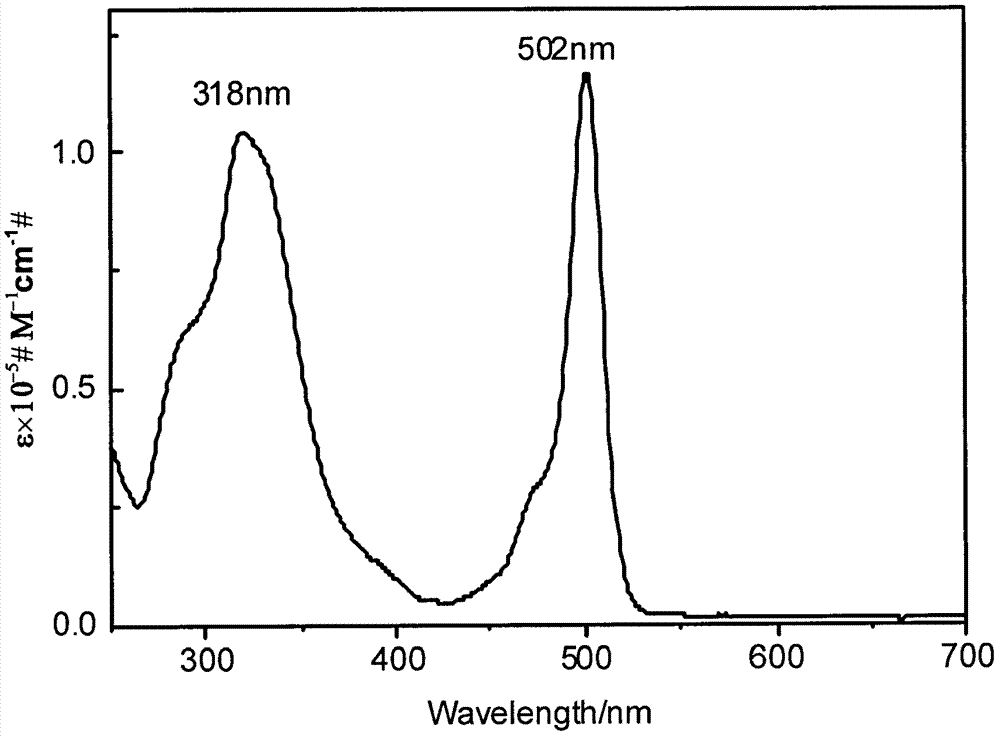
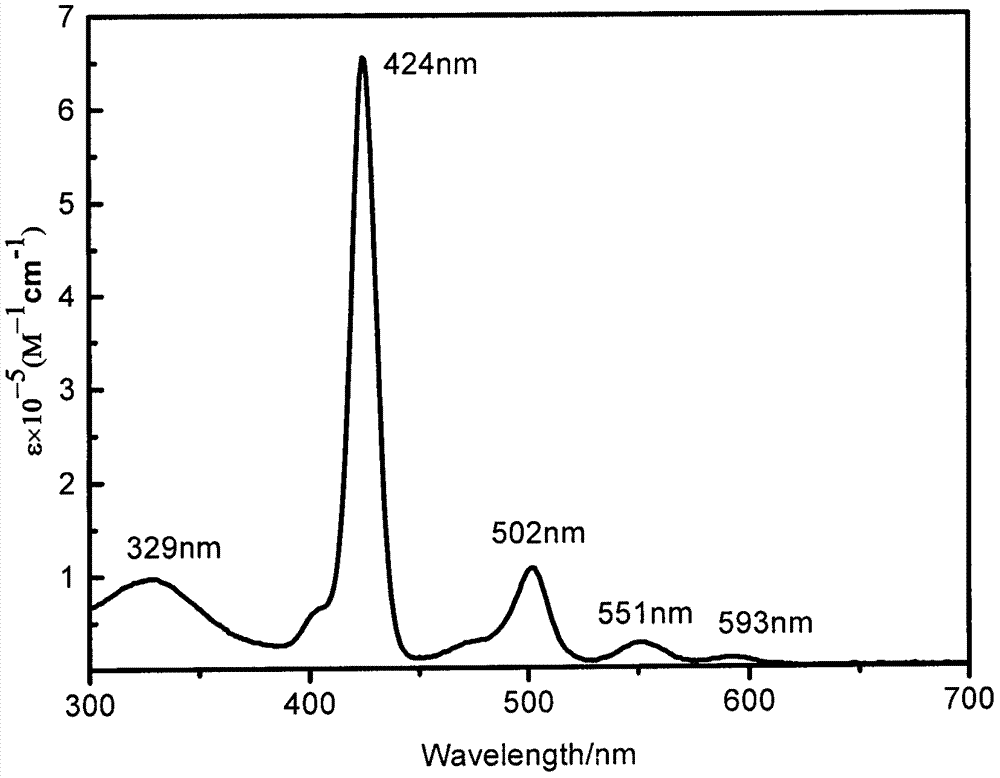
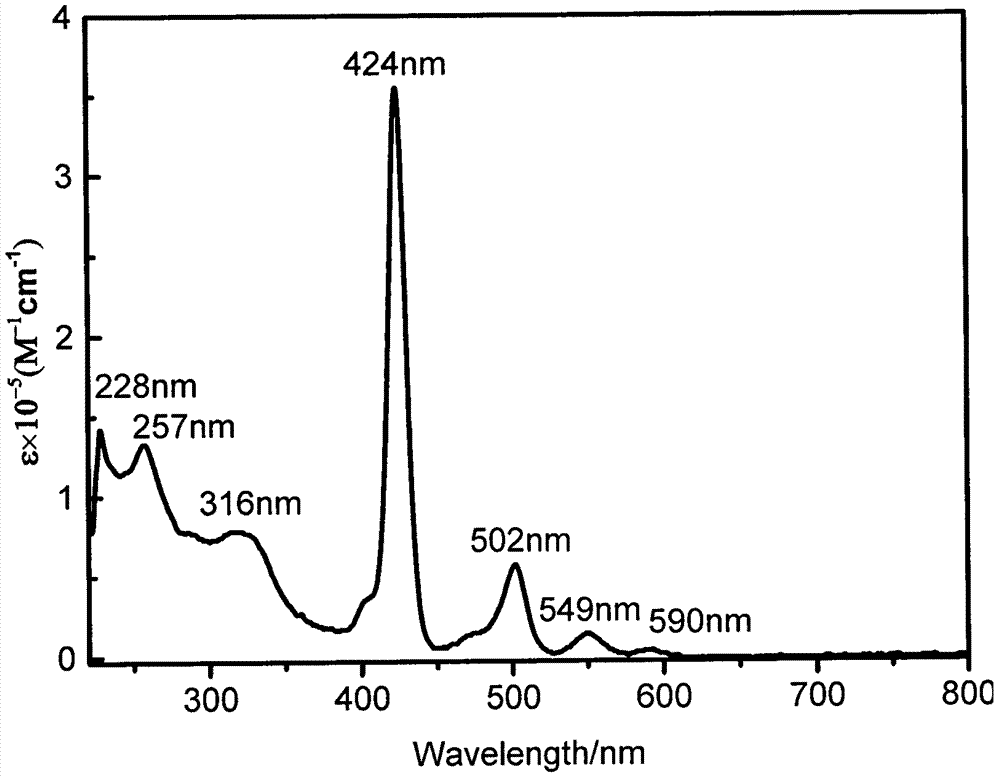
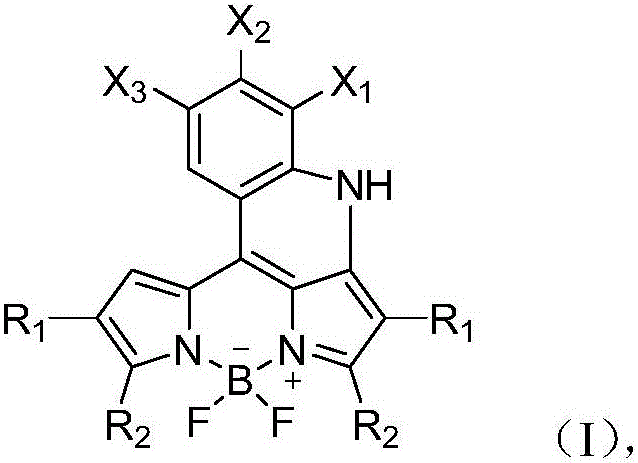
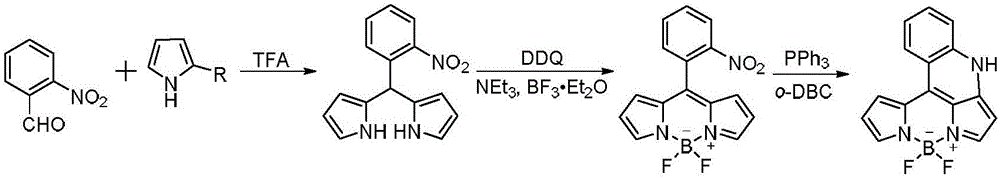Patents
Literature
371 results about "BODIPY" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
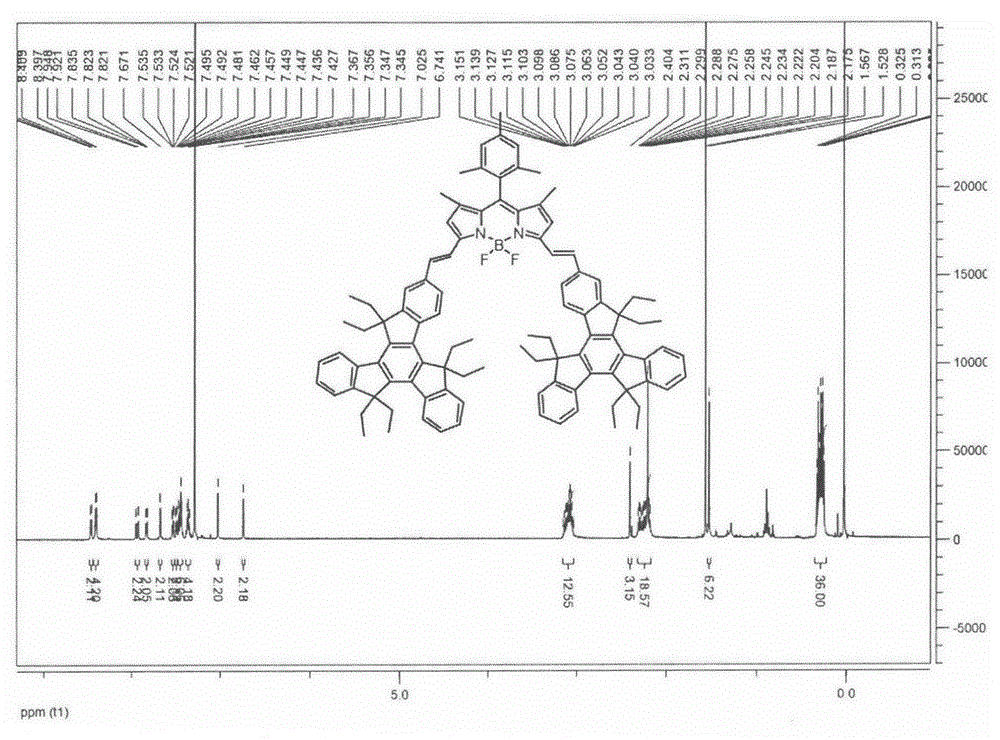
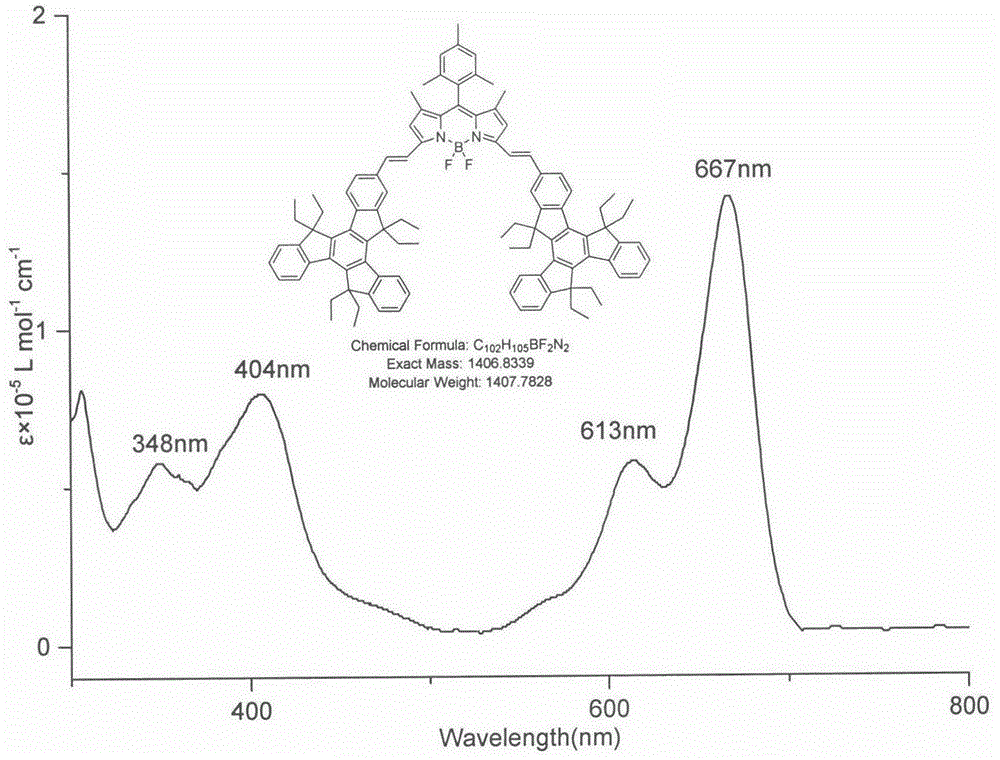
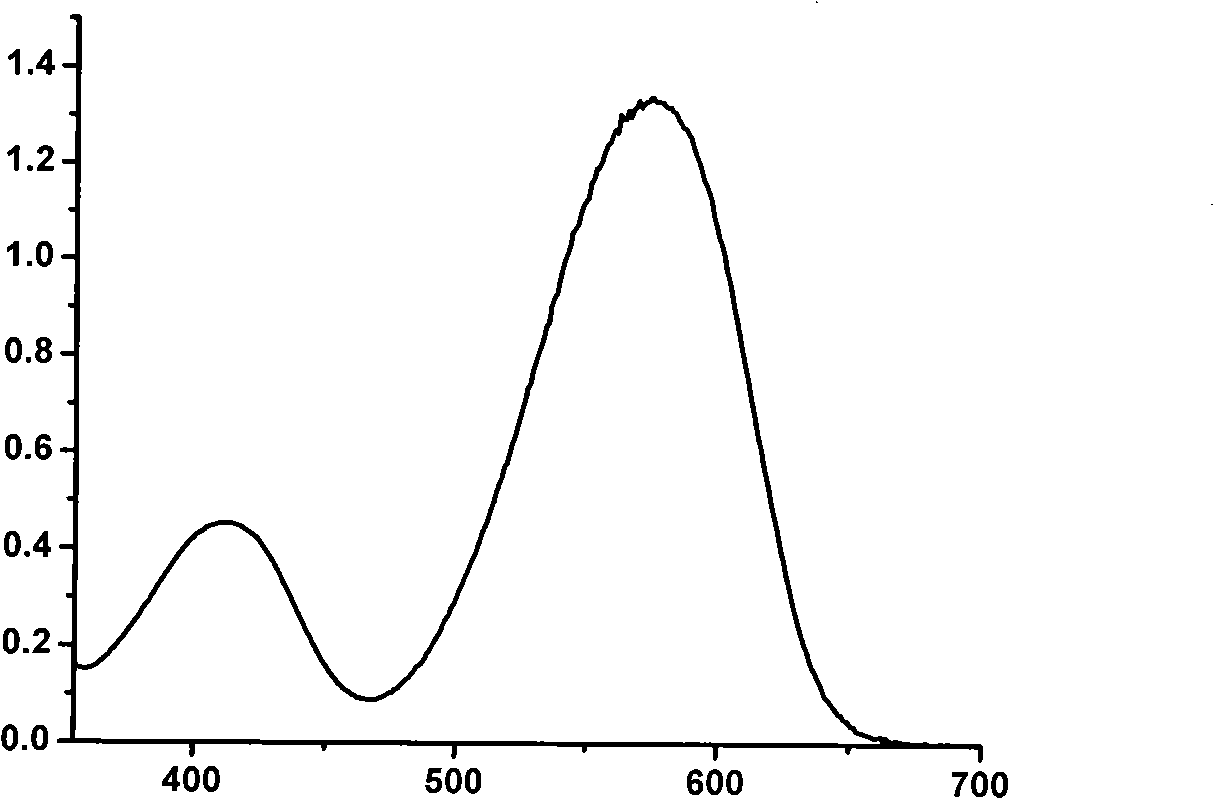
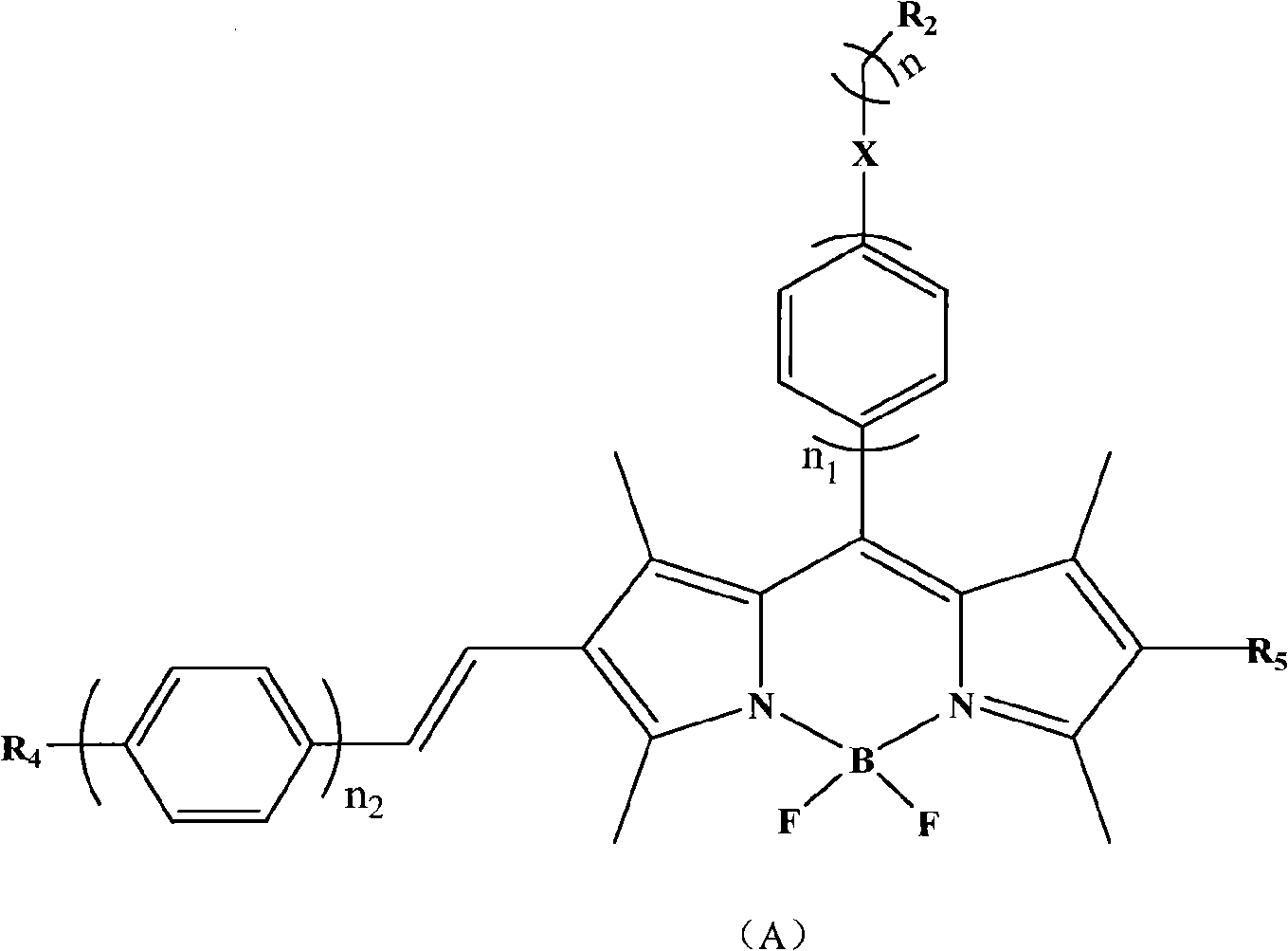
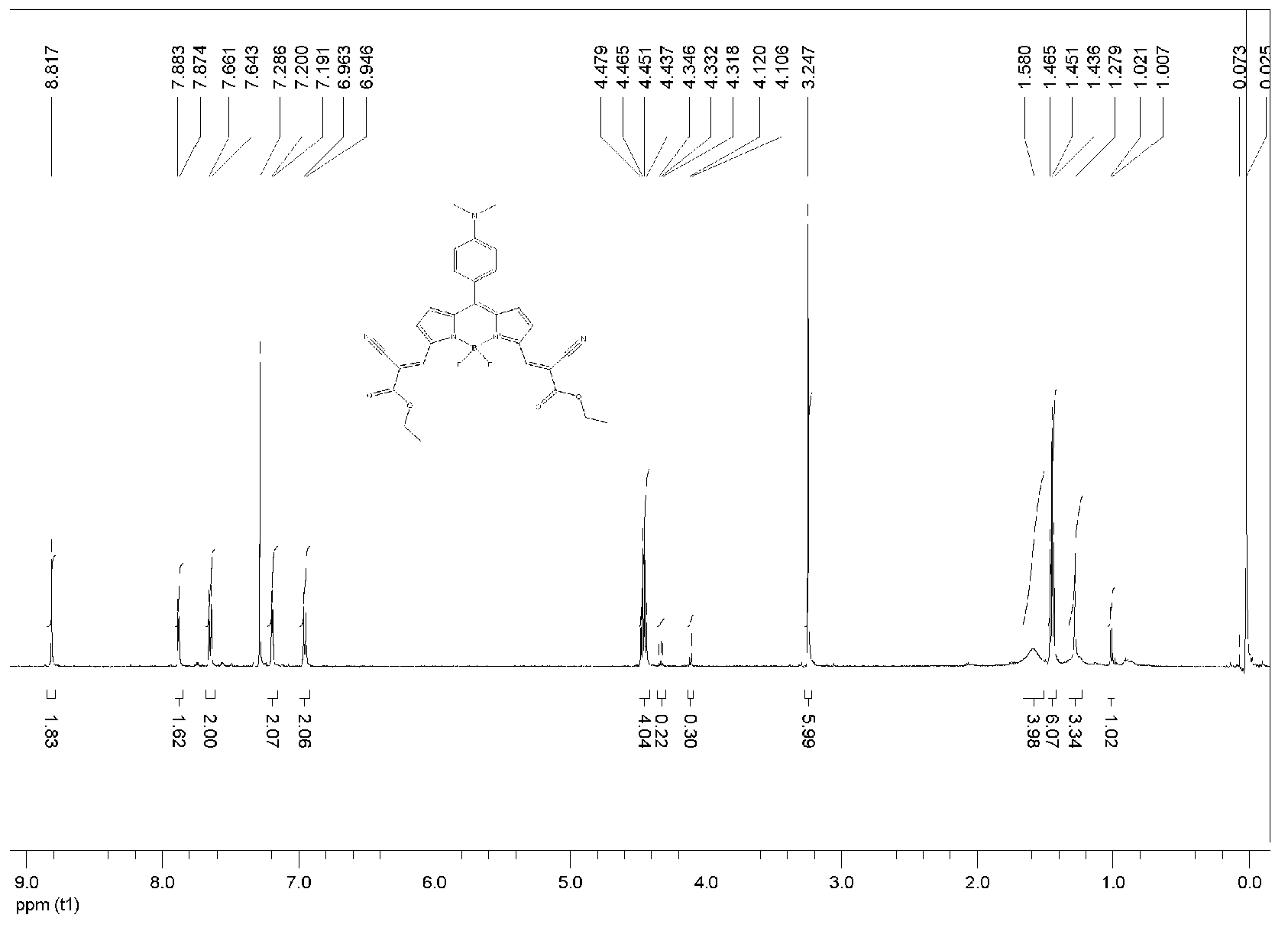
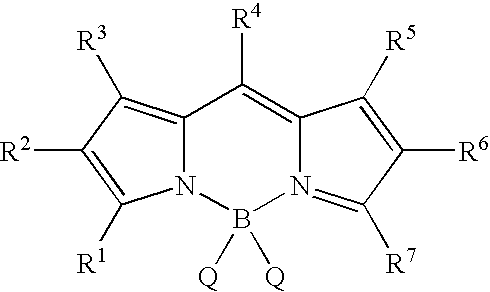
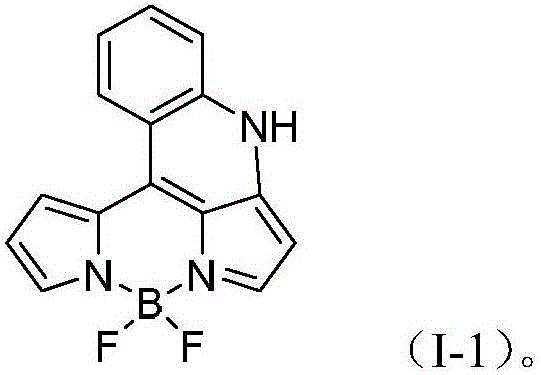
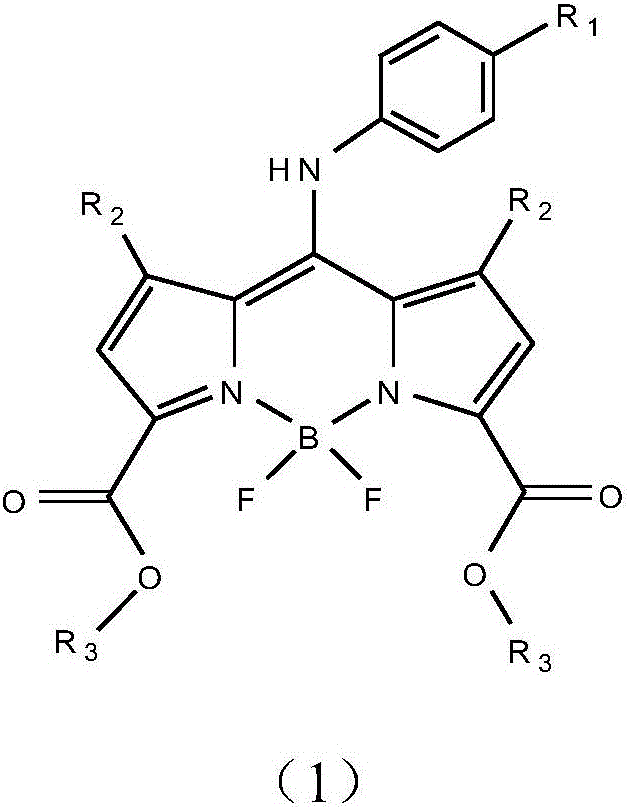
BODIPY, an abbreviation for boron-dipyrromethene, is a family Organoboron compounds of interest as fluorescent dyes. BODIPY is composed of dipyrromethene complexed with a disubstituted boron center, typically BF₂. The IUPAC name for the BODIPY core is 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene. Reflecting its instability, the unsubstituted BODIPY dye had not been prepared until 2009.
Imaging of protease activity in live cells using activity based probes
ActiveUS20070036725A1Improve resolutionHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsSurgeryProteinase activityFluorophore
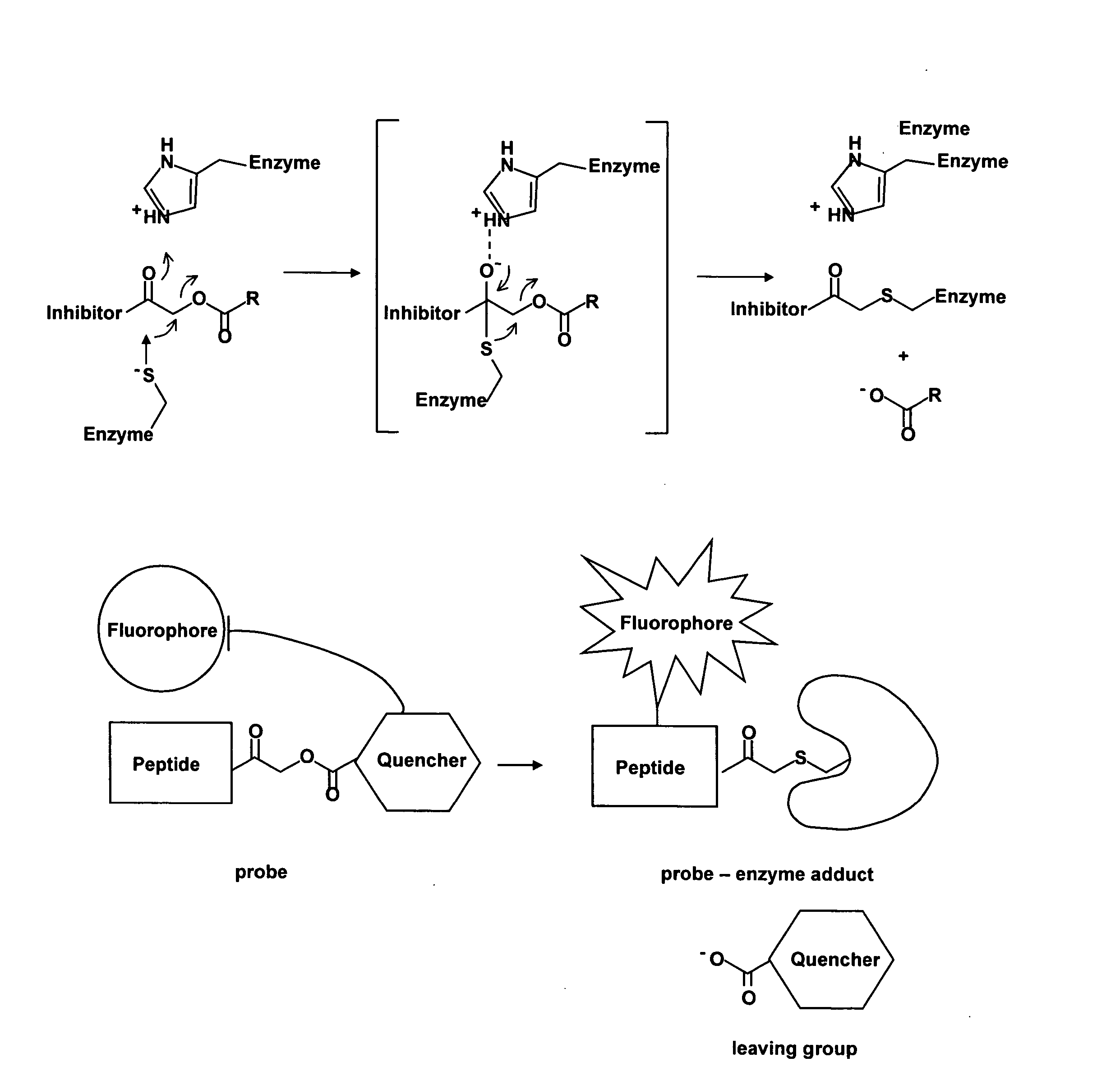
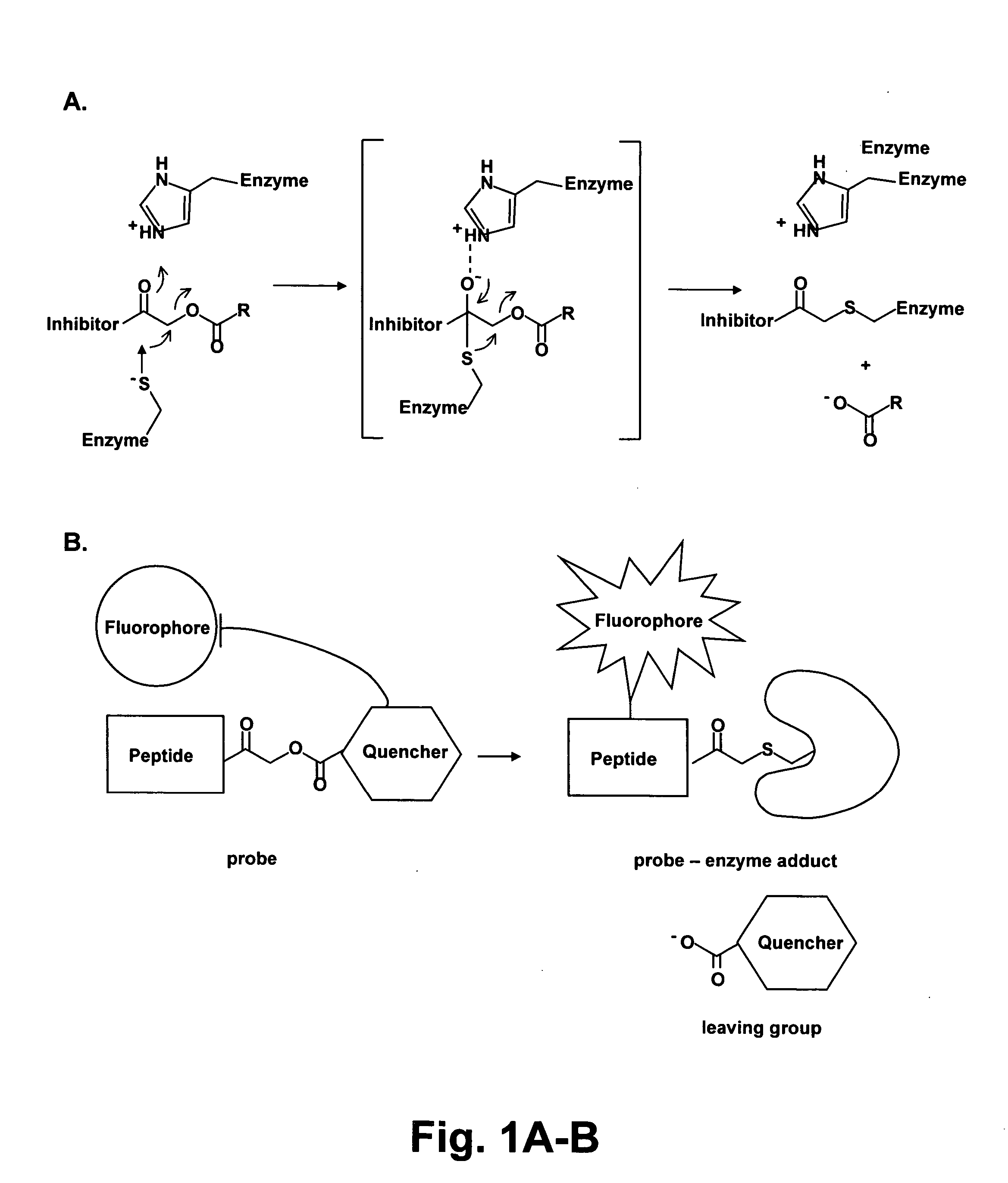
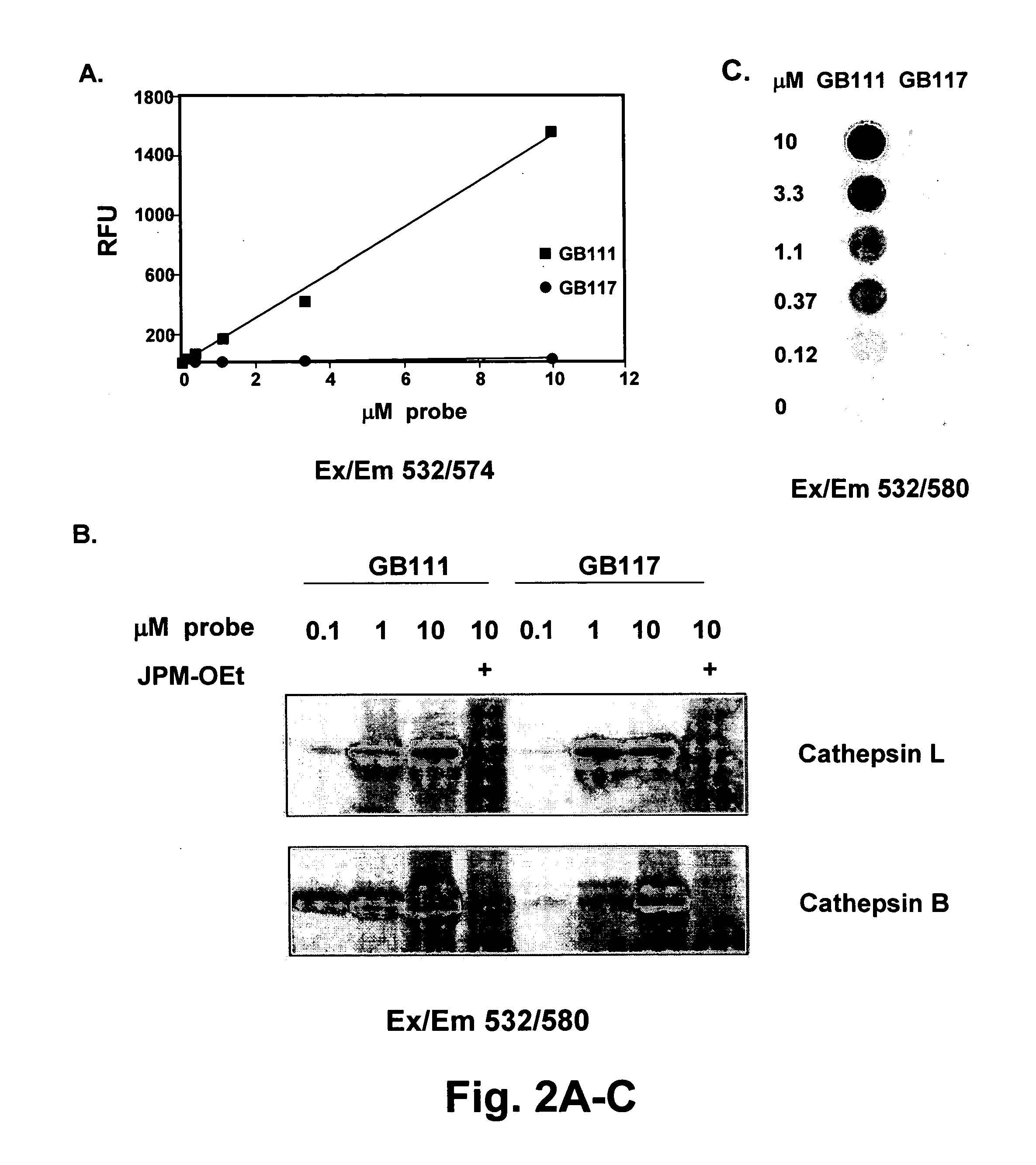
Methods and materials for the imaging of cells containing active proteases such as cathepsin are disclosed. The present materials include activity based probes that bind to an enzyme and are subsequently cleaved. Cleavage results in a fluorescent signal due to removal of a quenching group which, when present on the probe causes altered or no fluorescence. The probes employ an acyloxymethyl ketone reactive group, one or more amino acids for determining specificity, a fluorophore and a quencher. The probes are cell permeable and may use, for example, a QSY7 (diarylyrhodamine) quencher and a BODIPY (bora-diaza-indecene) dye.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Fluorine-boron fluorescent dye as well as preparation method and application thereof
InactiveCN103865290ANarrow absorbencyNarrow emission peakAzo dyesGroup 3/13 element organic compoundsQuantum yieldHalogen
The invention discloses a fluorine-boron fluorescent dye as well as a preparation method and application thereof, wherein the structure of the fluorine-boron fluorescent dye is shown as a formula (I) or a formula (II), in the formula (I) and the formula (II), R1 is H or halogen; R2 is CN; R3, R4, R5 and R6 are independently selected from H, halogen, C1-C6 alkyl or C1-C6 alkoxy; V, W, X, Y and Z are independently CH or N, and when V, W, X, Y or Z is N, N has no substituent group. According to the fluorine-boron fluorescent dye and the preparation method thereof, the maximal fluorescence emission wavelength of the fluorine-boron fluorescent dye is 518-600nm, and the fluorine-boron fluorescent dye also has excellent fluorescence quantum yield and Stokes shift, which shows that the fluorine-boron fluorescent dye has good application prospect in the bioanalysis fields of fluorescence labeling, bioimaging and so on; meanwhile, the preparation method is simple in steps, and raw materials can be obtained easily.
Owner:ANHUI NORMAL UNIV
Fluorescent ion probe and its application in ion detecting
InactiveCN101153848AEasy to synthesizeSimple structureChemiluminescene/bioluminescenceLuminescent compositionsBenzoxazoleFluorophore
The invention relates to a fluorescent ion probe (I) with high selectivity and high sensitivity in detection and the application of the fluorescent ion probe in identifying and detecting heavy metal ion and transition metal ion, wherein, the Y is an organic conjugate group with fluorescence transmitting function such as pyrene, naphthalene, 4-amidogen-1, 8-naphthyl imide, Dan sulfonamide, anthracene, carbazole, benzimidazoins, benzoxazoles, boron fluoride bipyrrole (BODIPY), fluorescein, 3, 4, 9, 10-perylenetetracarboxylic diimide or rhodamine B; the X is acylamino group, sulfoamino group or ester group. The fluorescent ion probe uses the fluorescence peak of fluorescence chromophore aggregate as the response signal to identify metal ion, thereby effectively avoiding the quenching effect of transition metal ion and heavy metal ion on fluorophore; moreover, the fluorescent ion probe realizes selective identification of heavy metal ion and transition metal ion in various solvents and aqueous solution in particular.
Owner:JILIN UNIV
Fluorescent probe for detecting biologic thiol and preparation method and usage method thereof
InactiveCN102532178AAvoid interferenceHigh sulfhydryl detection sensitivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceThiolNitrobenzene
The invention discloses a fluorescent probe for detecting a biologic thiol and a preparation method and a usage method thereof. The fluorescent probe for detecting the biologic thiol consists of two parts: namely, a 2, 4-bi-nitrobenzene sulfonyl group which is a recognizing group and a boron difluoride-dipyrryl methane (BODIPY) derivative which is an information reporting functional group. The molecule of the probe can simply and quickly enter a living cell, generates a specificity reaction with the thiol in the cell, and causes to obviously enhance fluorescence intensity, so that the fluorescent probe further can be used for the fluorescence detection and the imaging of the active thiol in the living cell. The fluorescent probe has good stability, can be stored and used for a long time, is applicable to various environments in which the living cell grows, and has higher detection sensitivity for the thiol, strong anti-interference capability, excellent selectivity and no action on other common biologic interfering molecules. The fluorescent probe can simply enter the living cell and a living tissue; the single recognition of the thiol in a biologic system can be achieved effectively; and therefore, the fluorescent probe can be used for the fluorescence imaging of the living cell.
Owner:ZHEJIANG SCI-TECH UNIV
Reactive labelling compounds and uses thereof
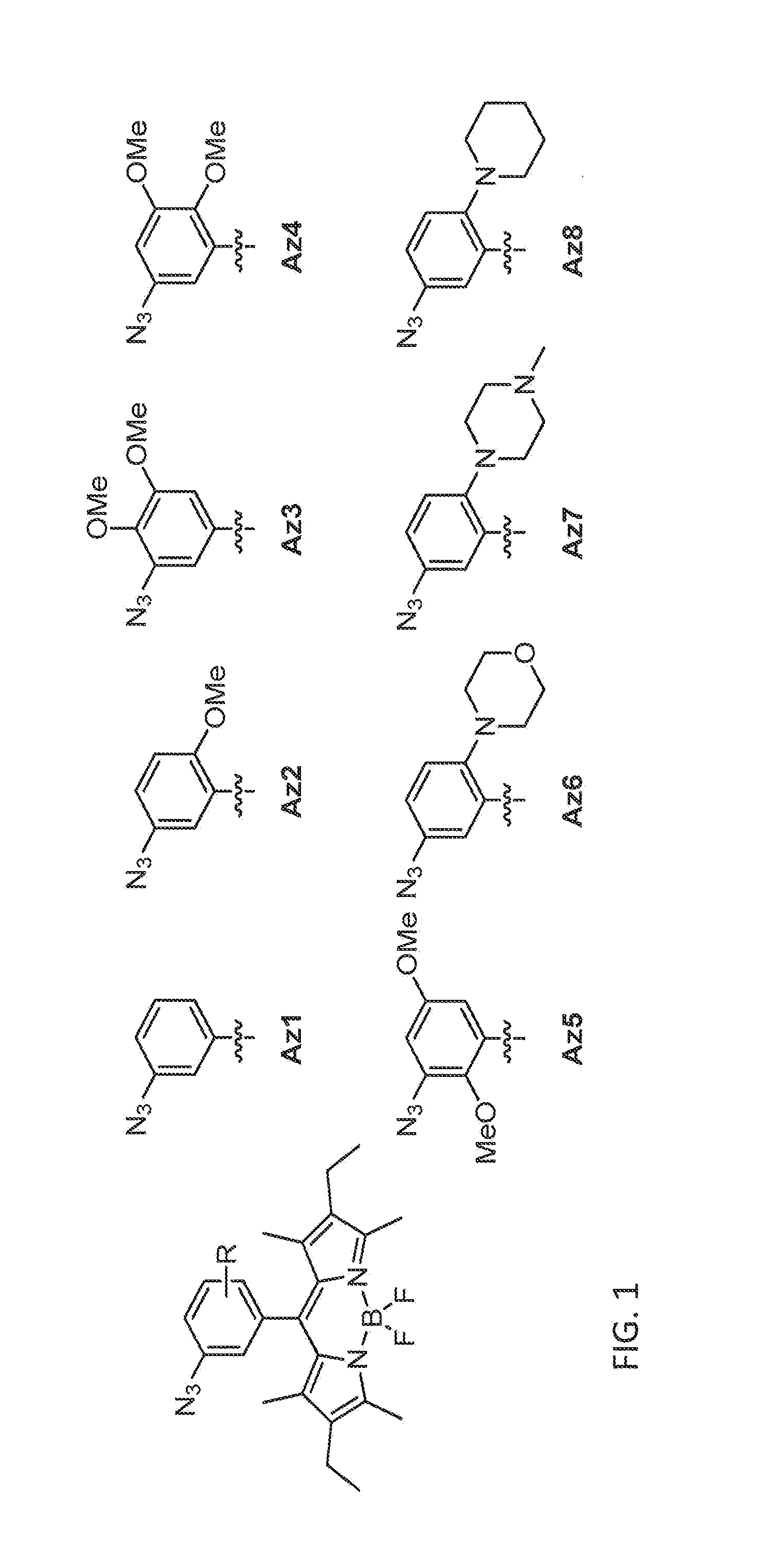
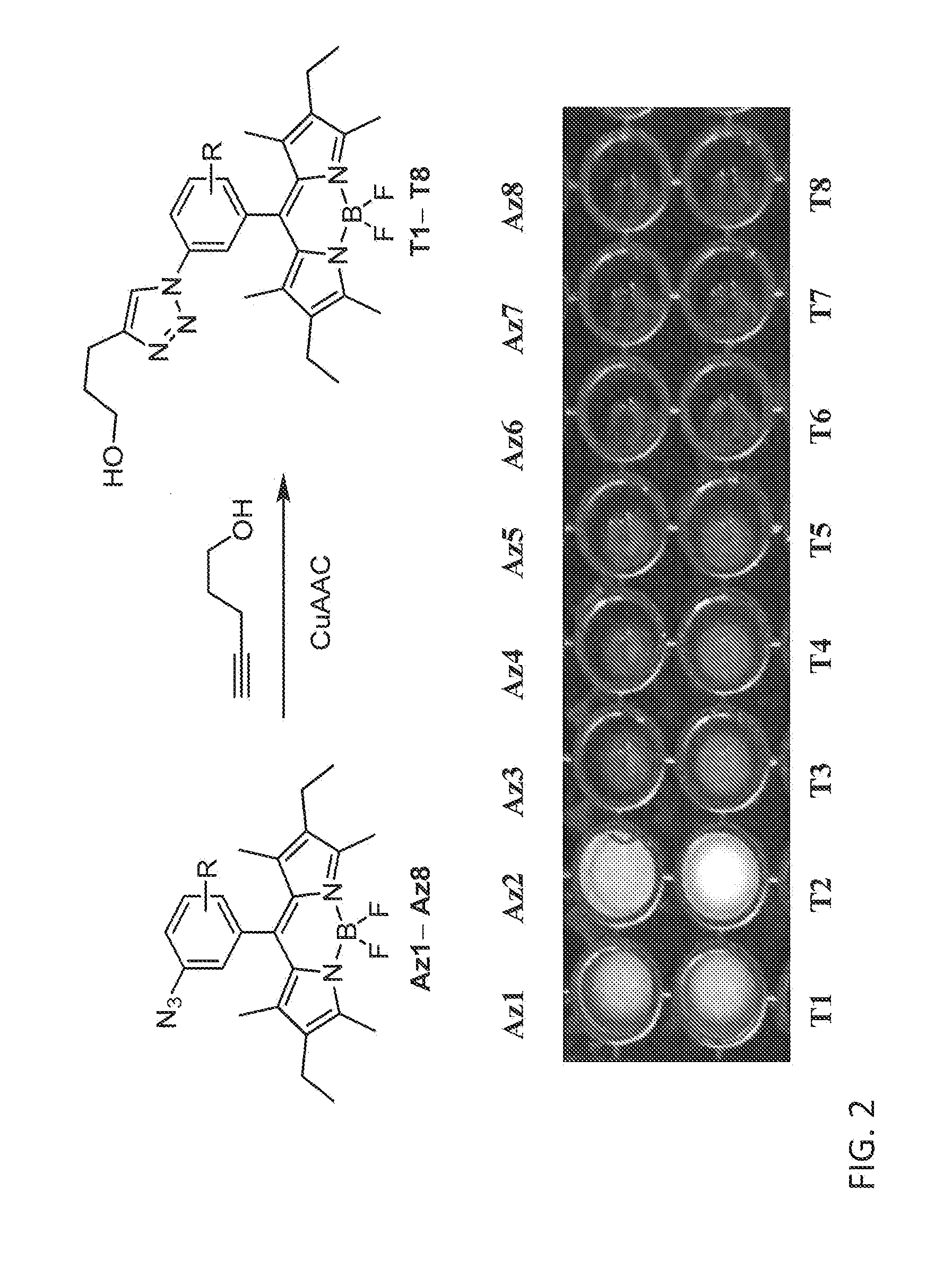
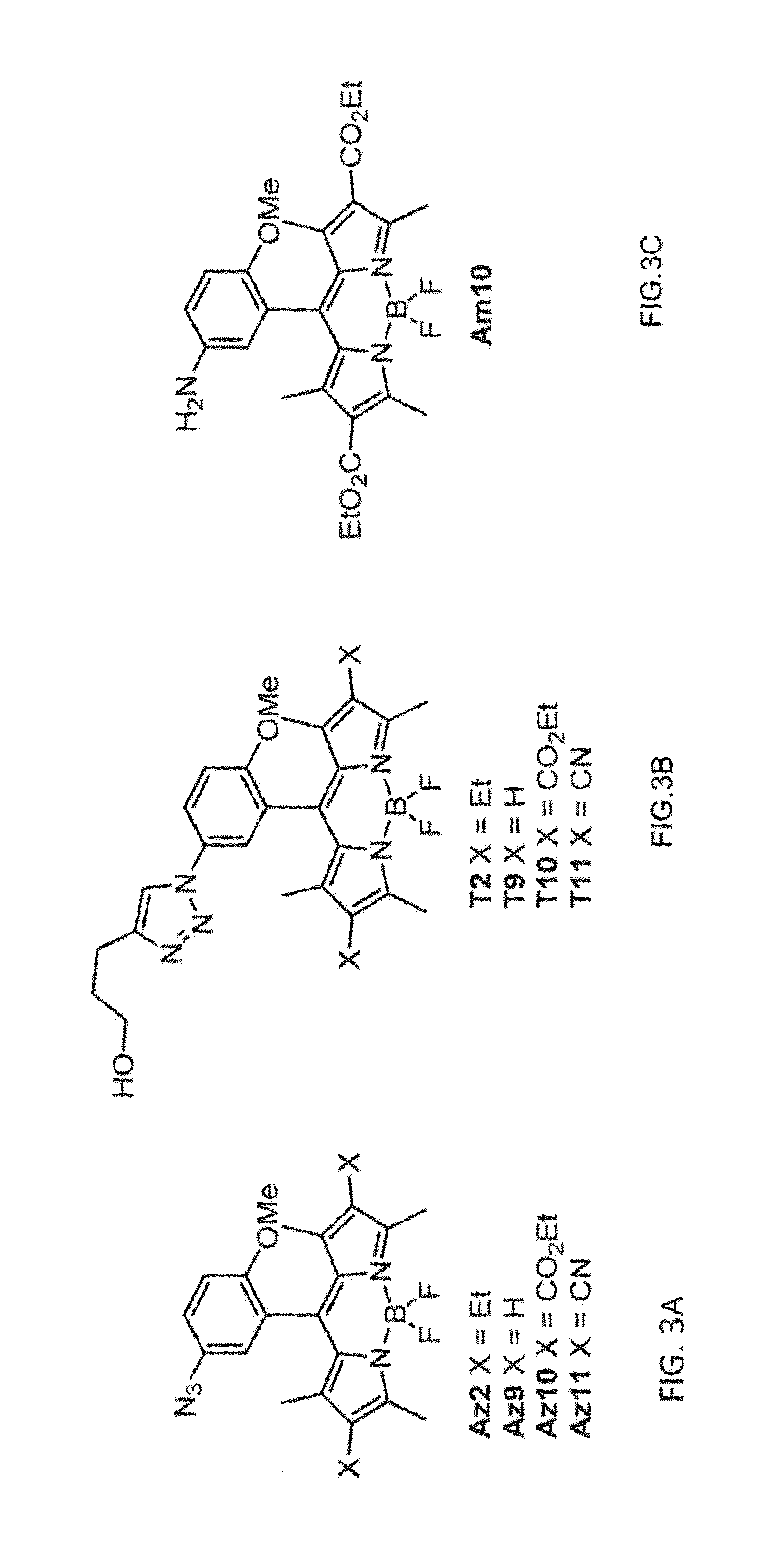
ActiveUS20150309041A1Fluorescence enhancementEasy to detectSilicon organic compoundsMicrobiological testing/measurementCombinatorial chemistryAlkyne
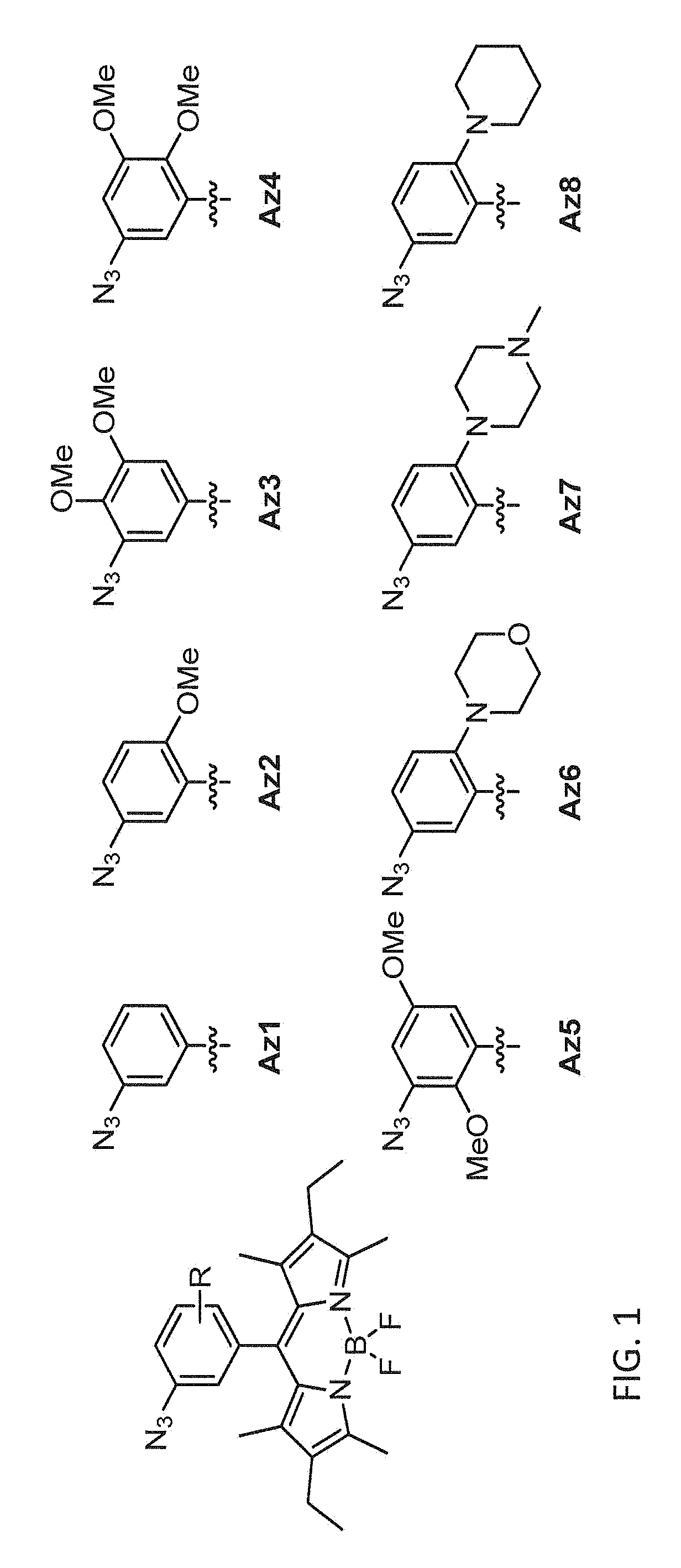
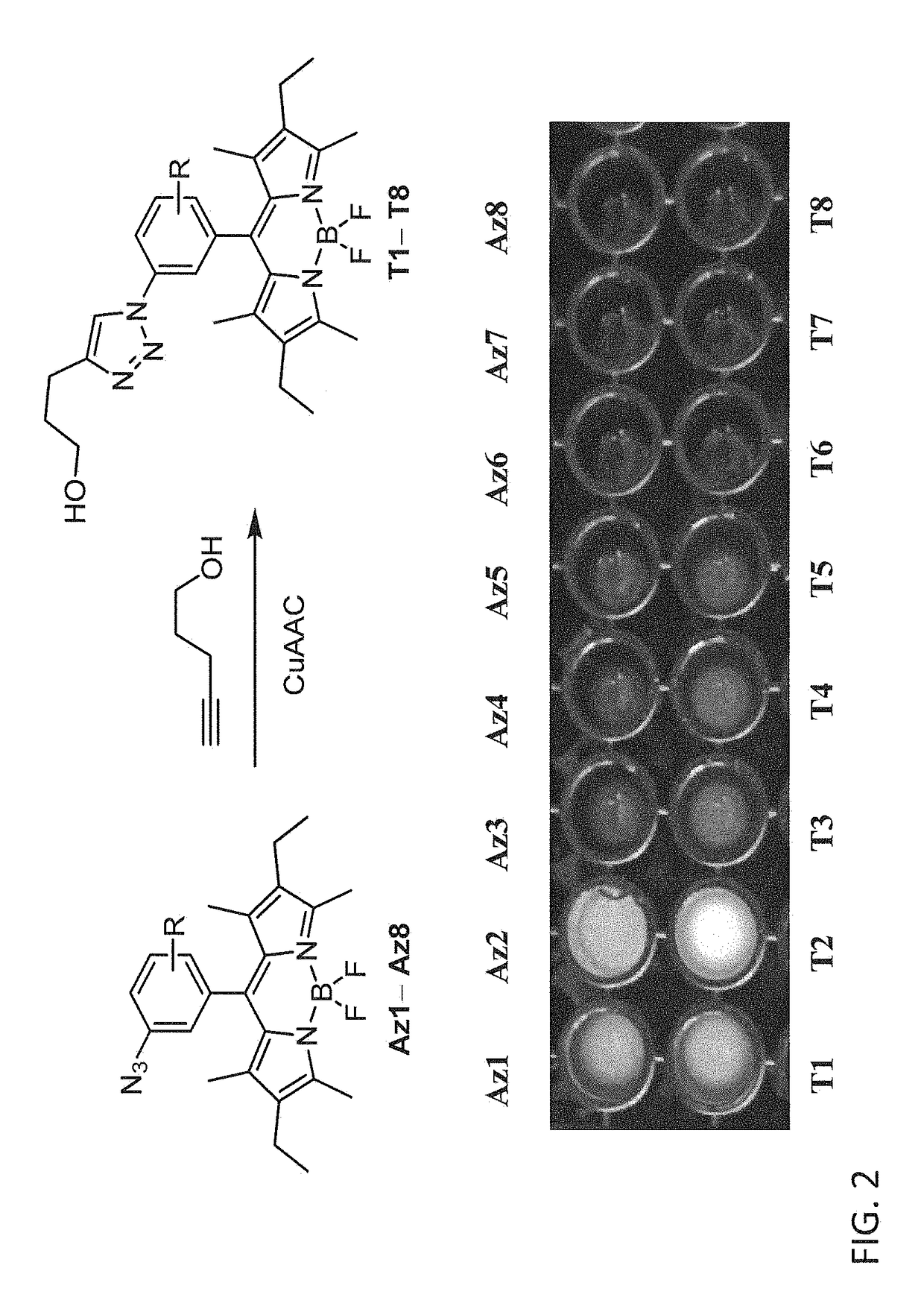
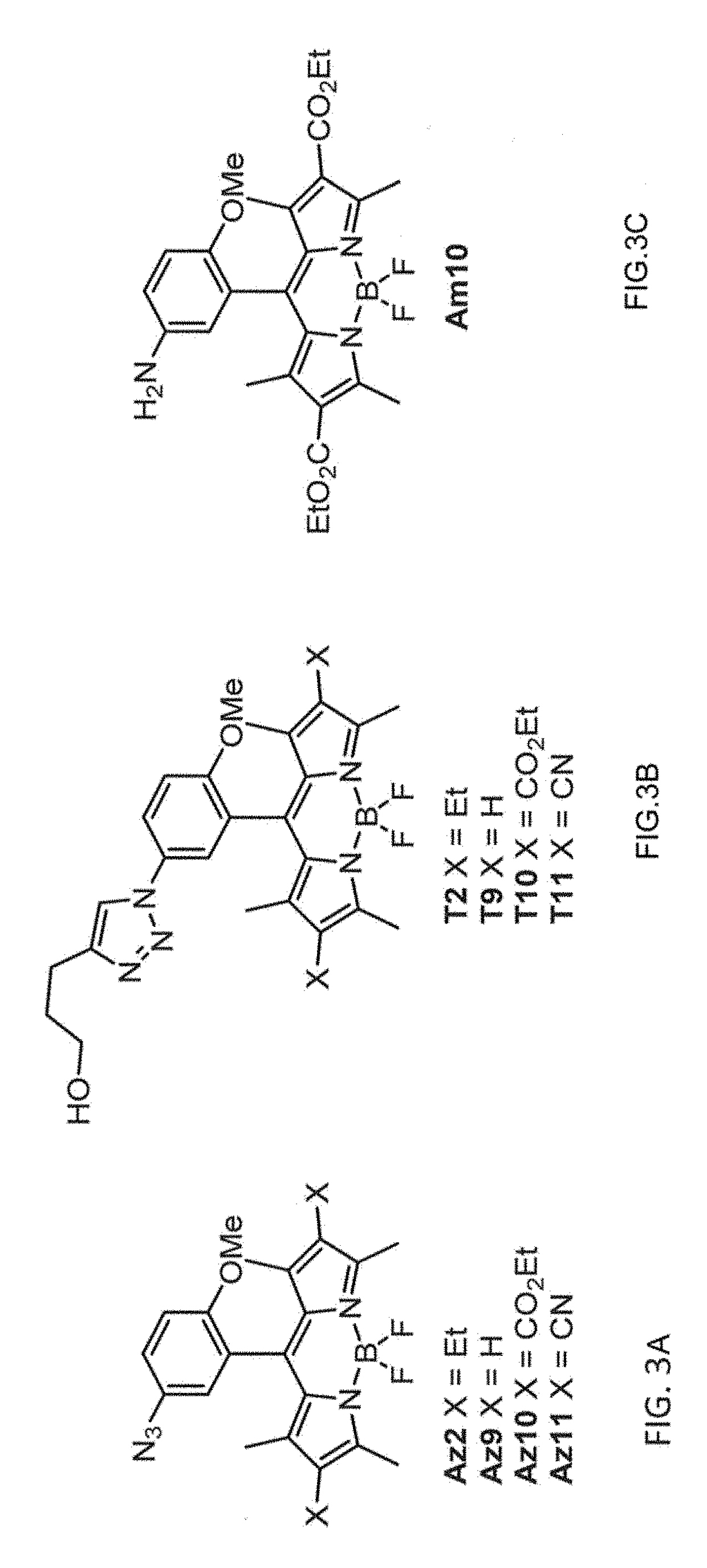
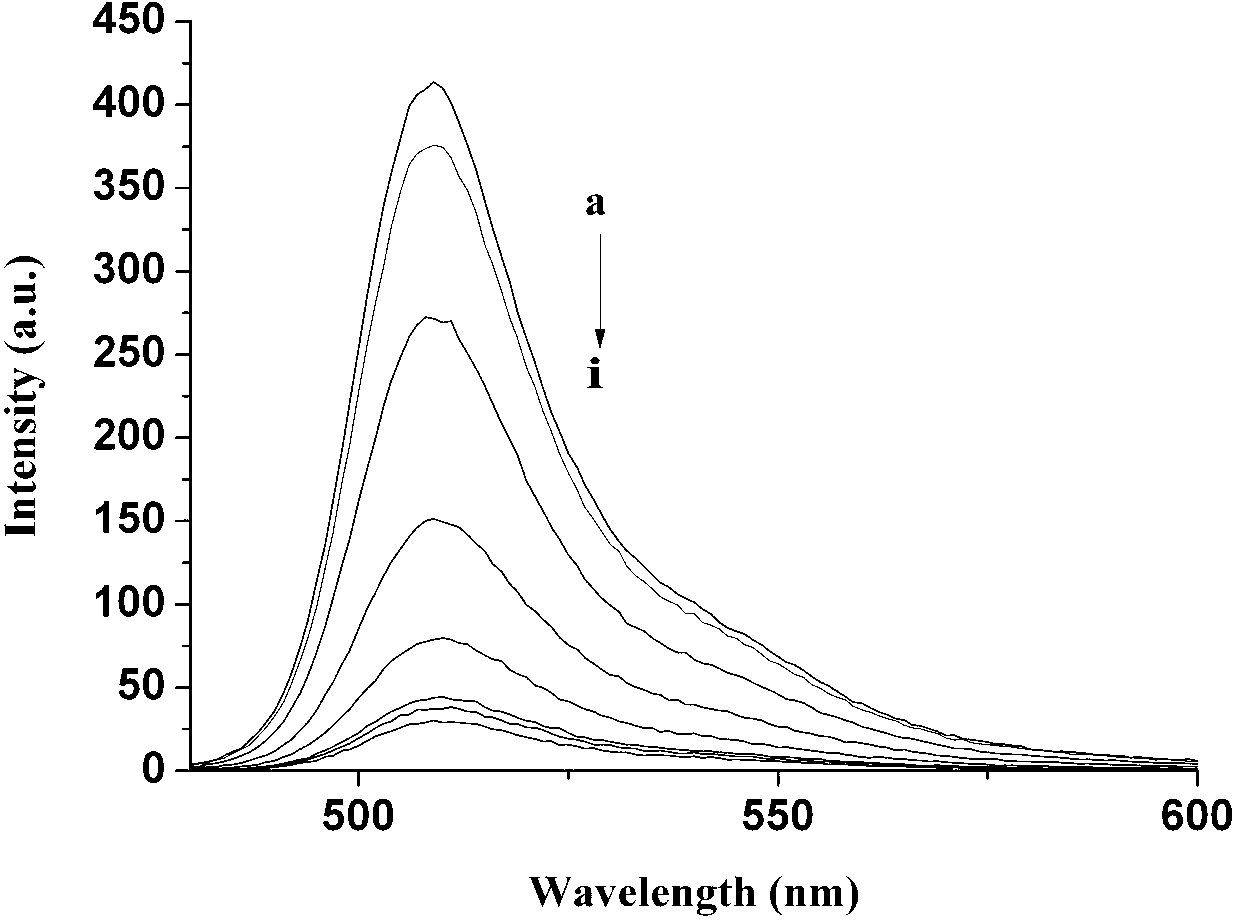
Provided are azido-BODIPY compounds of formula (I), cyclooctyne-based fluorogenic probes of formula (IV), and activity-based probes of formula (VI). These compounds undergo azide alkyne cycloadditions (AAC) with to form triazolyl products. The provided compounds are useful for detection and imaging of alkyne-, or azide-containing molecules. Methods for detection and imaging biomolecules using compounds of the present disclosure are disclosed.
Owner:ACAD SINIC
Near infrared BODIPY fluorescence dye and preparation method thereof
ActiveCN105462576AHigh molar absorptivityHigh fluorescence quantum efficiencyMethine/polymethine dyesGroup 3/13 element organic compoundsInfraredSolubility
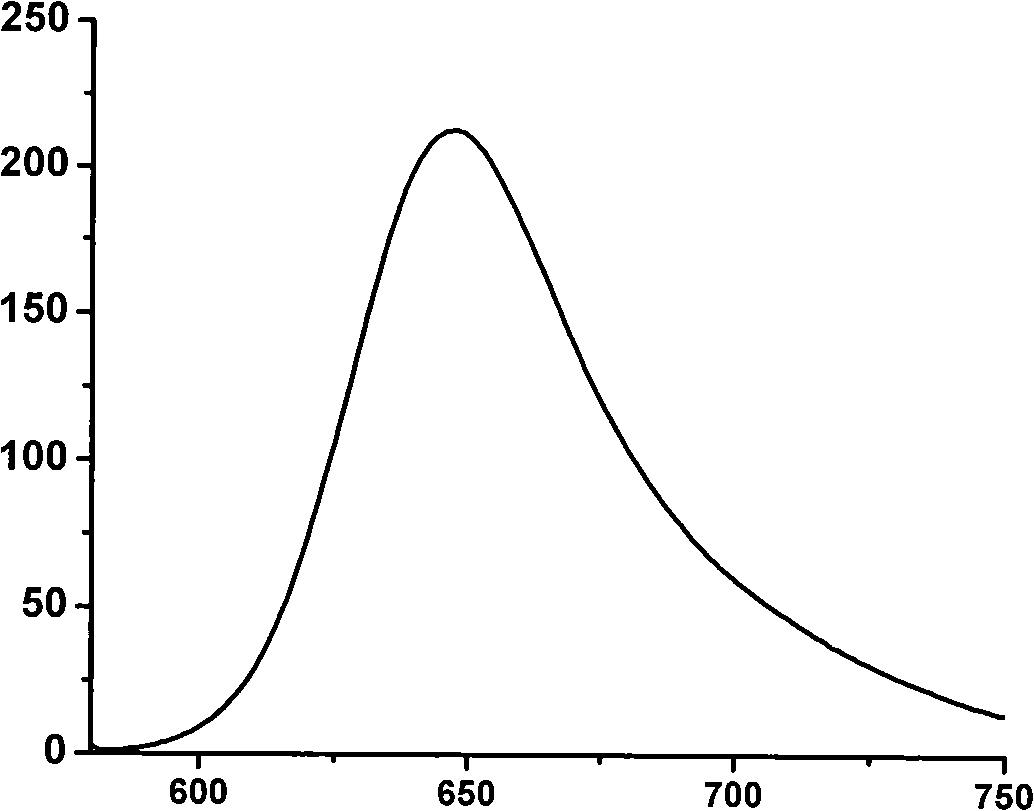
The invention relates to a near infrared BODIPY fluorescence dye and a preparation method thereof. A BODIPY derivative and 2-aldotruxene undergo a Knoevenagel condensation reaction under the catalysis action of p-toluenesulfonic acid and piperidine to synthesize the dye, and the maximal absorption wavelength and the maximal emission wavelength of the dye in an organic solvent are 650nm or above respectively. The preparation method has the advantages of simple reaction steps, mild reaction conditions and good selectivity. Like fluorescence dyes have the advantages of high molar extinction coefficient, good solubility and light stability, excellent photophysical performances, and good application prospect in cell imaging and biological marking.
Owner:南京颐维环保科技有限公司
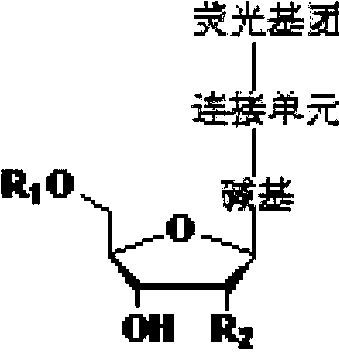
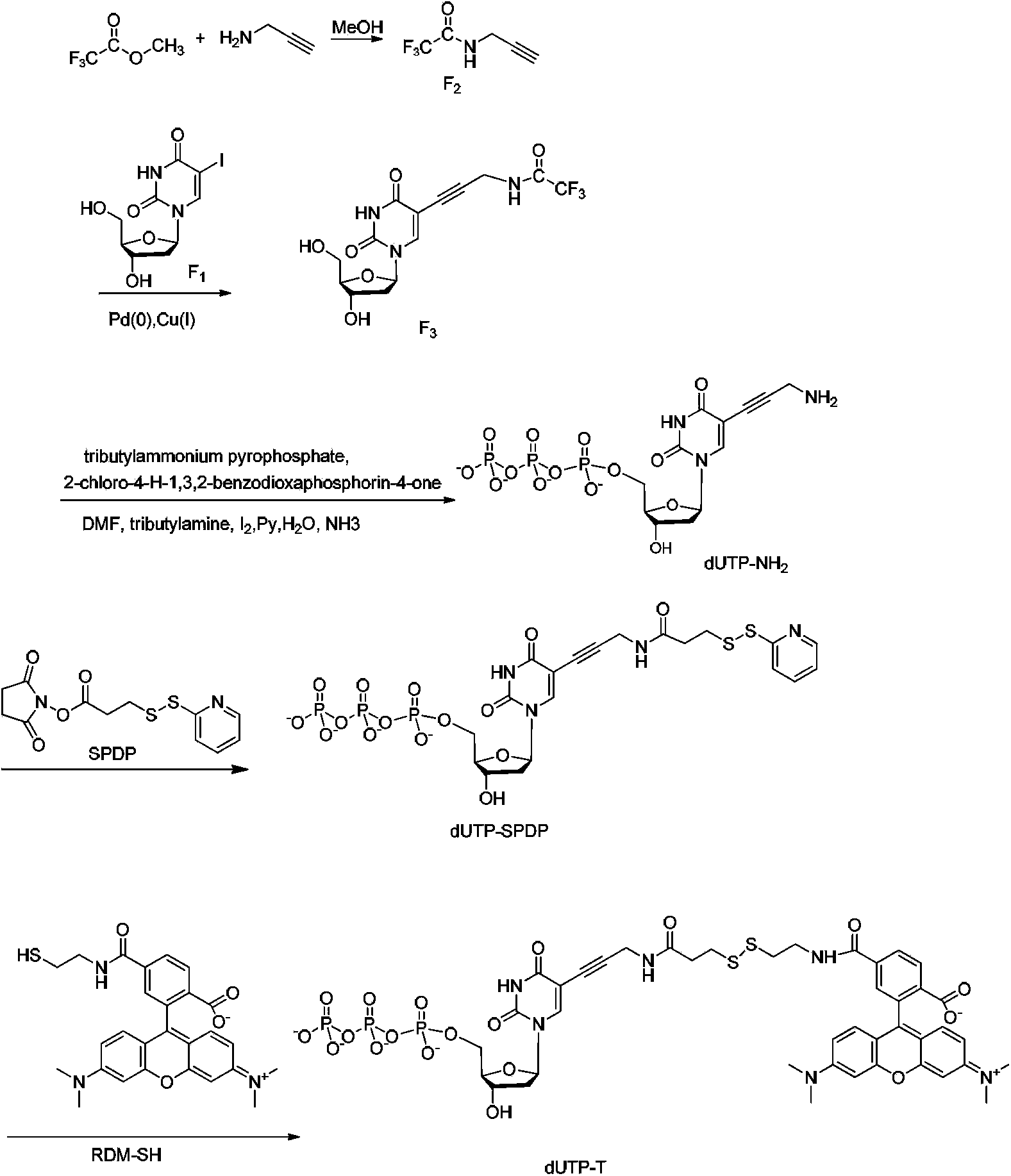
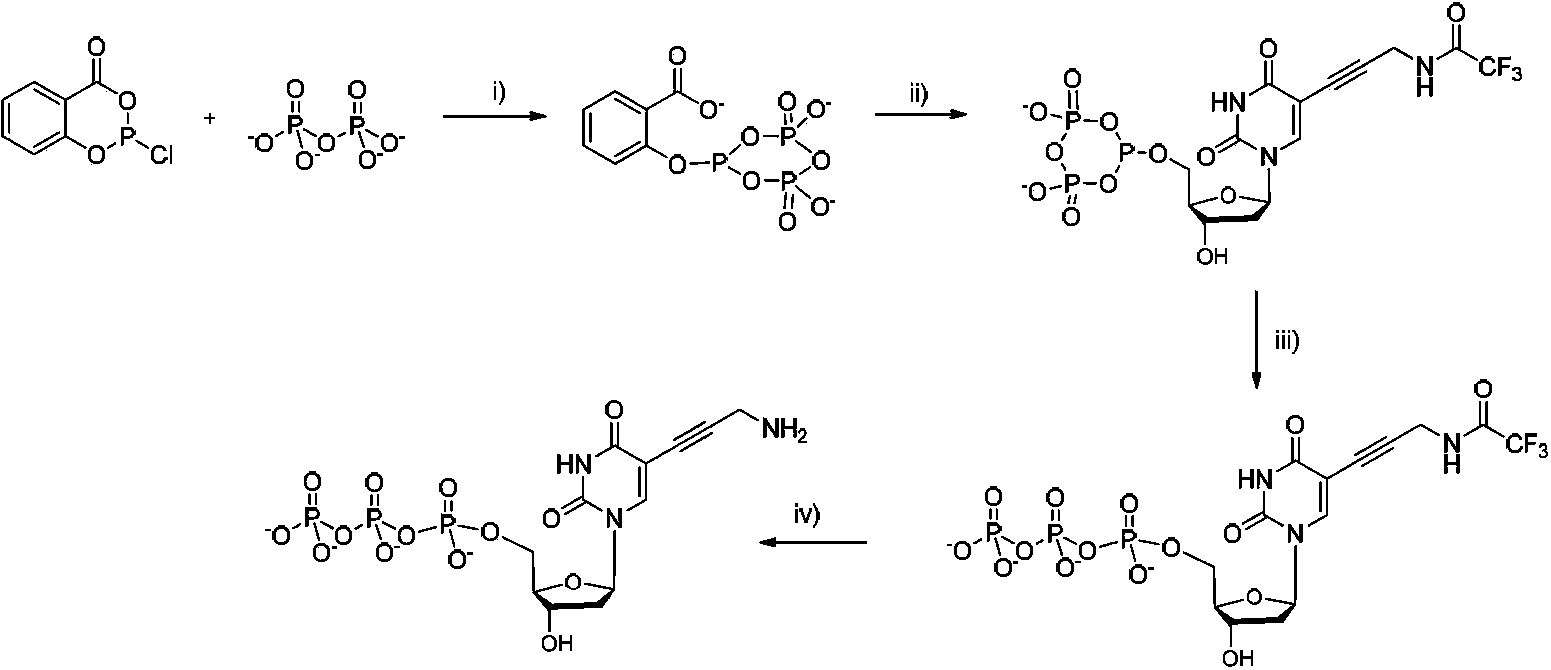
Four-color fluorescence labeling reversible terminal and use thereof in DNA (Deoxyribonucleic Acid) sequencing
ActiveCN103484106ARaw materials are easy to obtainSugar derivativesMicrobiological testing/measurementChemical reactionBifunctional
The invention discloses a four-color fluorescence labeling reversible terminal and a use thereof in DNA (Deoxyribonucleic Acid) sequencing. The structural formula of the reversible terminal is as shown in a formula (I) in the specification, wherein R1 is triphosphate; R2 is H or OH; the basic group is U, C, A, G or derivatives thereof; the connecting unit is a bifunctional compound which is breakable under a mild condition; the fluorescence group is one selected from a combination of BODIPY, fluorescein, rhodamine, coumarin, xanthene, cyanin, pyrene, phthalocyanine, alexa, squarene dye and an energy transferring dye, and derivatives thereof. The reversible terminal provided by the invention can be used for DNA single-molecule sequencing; simultaneously, raw materials required by the synthesis of the reversible terminal provided by the invention are simple and easy to get and the synthesis process of the reversible terminal is completely involved with conventional chemical reactions, so that the four-color fluorescence labeling reversible terminal can be popularized and utilized to a large scale; biological evaluation results indicate that the reversible terminal is capable of completely meeting the biochemical reaction requirements of high-flux sequencing and has a good practical prospect.
Owner:SHANGHAI JIAO TONG UNIV
Dipyrrol borane (BODIPY) and preparation method and application thereof
ActiveCN103232483ARestore fluorescenceWide range of absorption spectraAnalysis by subjecting material to chemical reactionGroup 3/13 element organic compoundsQuantum efficiencyReversible reaction
The invention discloses a formaldehyde detection probe compound and a detection method. The compound is a high fluorescence quantum efficiency fluorescence dye based on dipyrrol borane BODIPY. The compound and alkali substances used in the invention generate a reversible reaction for forming a compound. The detection of formaldehyde is based on alkali reaction in the compound, and the reaction result causes the BODIPY to regenerate and develop colors, and simultaneously recover fluorescence. The detection method is a novel detection reagent which can be recycled. On the other hand, based on the high fluorescence quantum efficiency characteristics of the BODIPY compounds, the detection reagent has high sensitivity and lower detection limit.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Long wavelength boron dipyrromethene dye and preparation thereof
The invention provides a preparation method of a long wavelength BODIPY dye and a derivative thereof, belonging to the organic chemical industry and the fine chemical industry technology field. Bromine or iodo-BODIPY and substituted alenyle boric acid are used as the raw material with the mol ratio around 1 to between 2 and 10 in the preparation method of the long wavelength BODIPY dye and the derivative thereof, palladium carbon or zero-valent palladium is used as a catalyst, the mol ratio of bromine or iodo-BODIPY to the catalyst is 1 to between 0.01 and 0.20. Allowing the bromine or iodo-BODIPY and the substituted alenyle boric as well as the catalyst to react in an organic solvent for one to seventy-two hours at a temperature of between forty and one hundred and sixty DEG C with the protection and stirring of argon or nitrogen, then the bromine or iodine on the raw material is substituted, conjugated double bond BODIPY derivative is generated and the boric acid and conjugated double bond BODIPY derivative is substituted, and after the introduction of conjugated double bond groups in different numbers, the optical property of the BODIPY is changed to absorb and emit spectrum and Einstein shift occurs.
Owner:DALIAN UNIV OF TECH
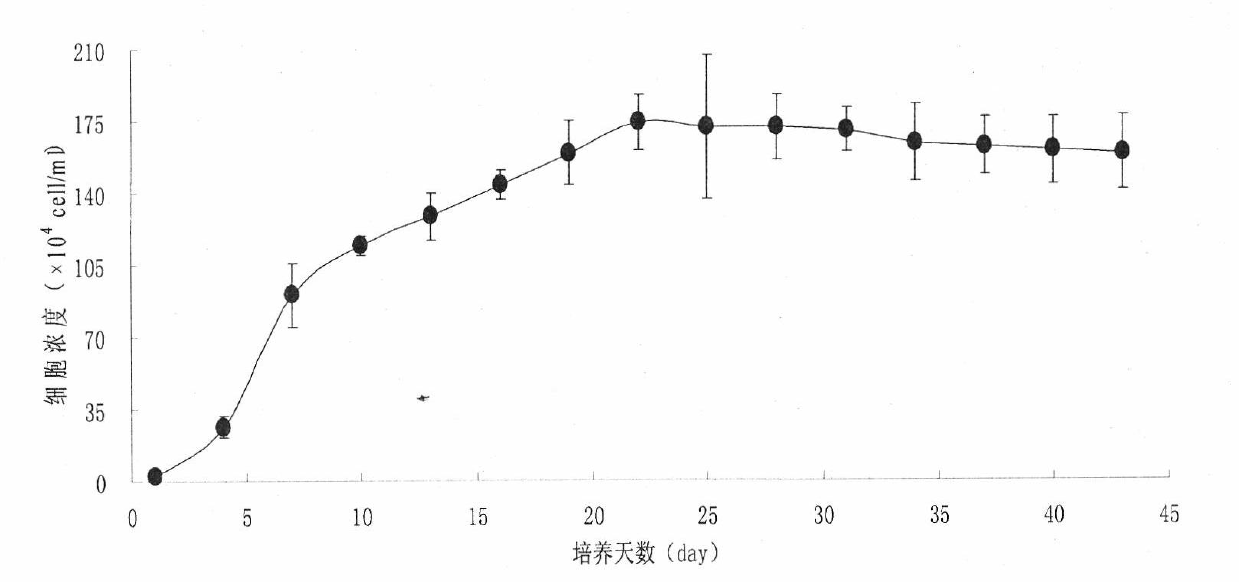
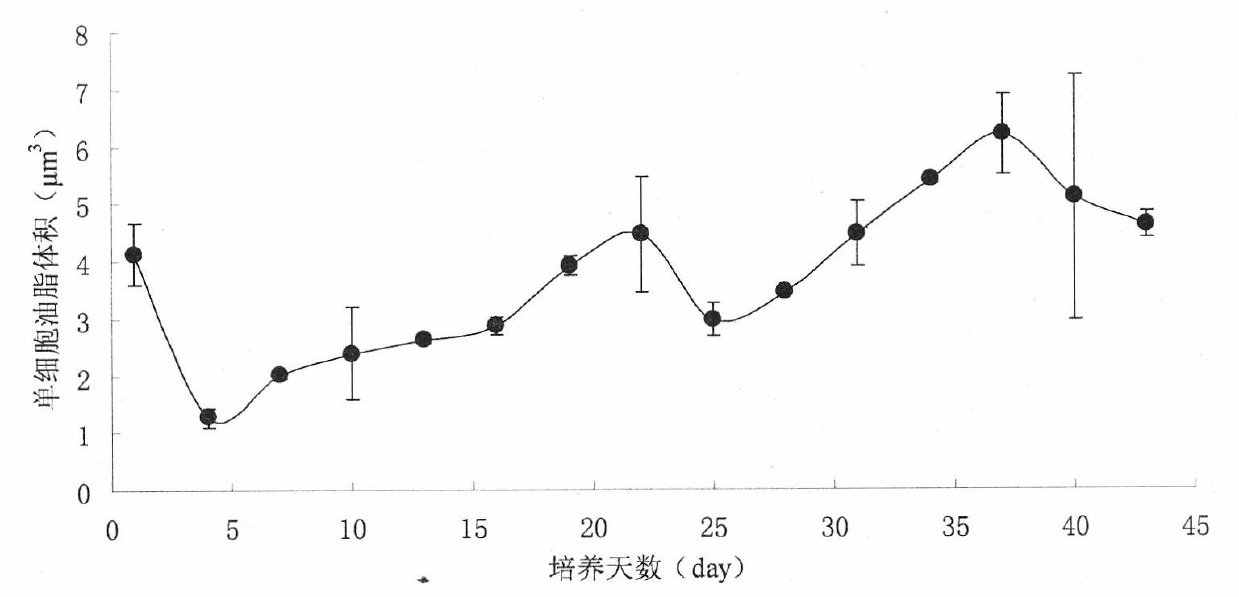
Method for detecting oil content of microalgae
InactiveCN102565012AEasy to detectThe process is simple and convenientUsing optical meansIndividual particle analysisLipid formationMicrobial oil
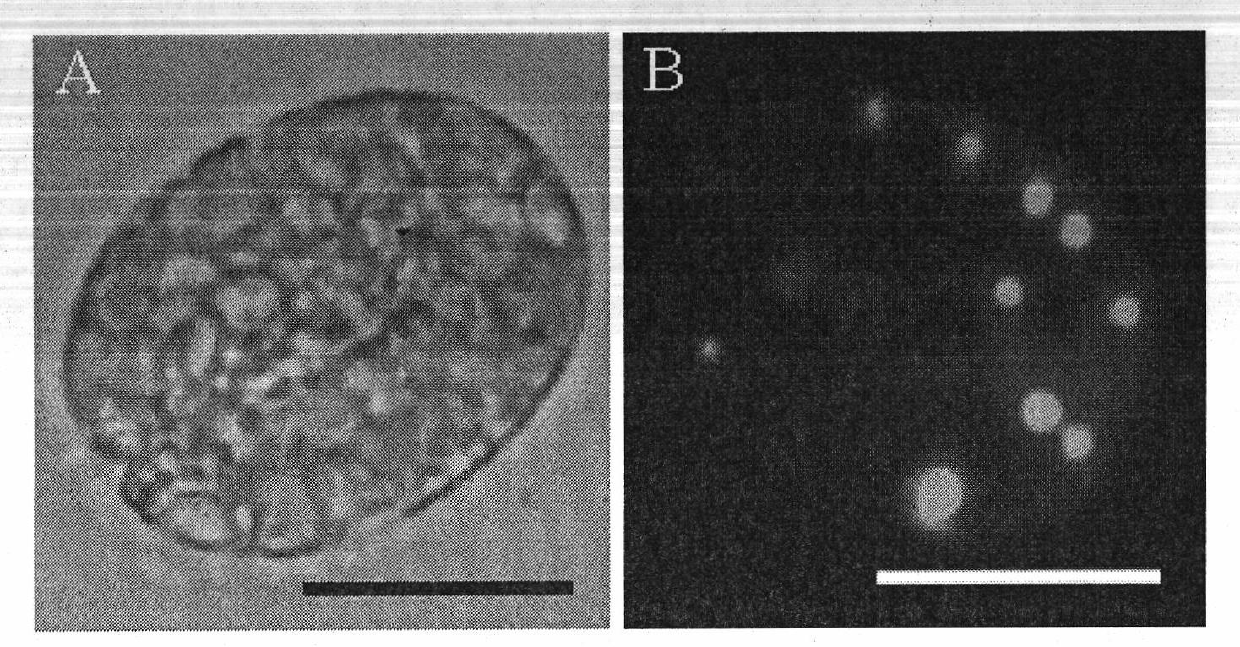
The invention relates to lipid producing microalgae, in particular relates to a method for detecting the oil content of microalgae, comprising the following steps of: 1) taking microalgae-cultivated algae liquid, and computing the quantity concentration C of microalgae cells; 2) observing the lipid of the microalgae: adopting a BODIPY (boron-dipyrromethene) 505 / 515 staining fluorescent recording method; staining for 1-2min by adopting dye use liquid with concentration of 1-2mM, and observing and recording accumulation change conditions of single-celled lipid in a photographing way by using excitation light with wave length of 488nm under a fluorescence microscope; recording the number (n) of single-celled oil drop grains according to a photograph, detecting the diameter (d) of each oil drop, and computing the volume of each oil drop according to a ball volume formula, wherein the content of each cell lipid is the sum of the volume of all the oil drops; and computing the lipid content of the algae liquid in unit volume according to the quantity concentration C of the microalgae and the lipid content of a single cell: Vt=CV, wherein C is the concentration of tetraselmis cells, V is the lipid volume of the single cell, and Vt is the lipid content in the unit volume.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
BODIPY (Boron Dipyrromethene) compound-based lysosome fluorescence probe as well as preparation method and applications thereof
InactiveCN103242355AAvoid damageImproving Imaging (Treatment) EfficiencyMethine/polymethine dyesGroup 3/13 element organic compoundsQuantum yieldExtinction
The invention provides a near infrared BODIPY (Boron Dipyrromethene) compound-based molecular fluorescence probe which has the following structure, wherein the substituent group Ar is one of the following four substituent groups described in the specification. The BODIPY probe with an alpha bit containing a strong electrondrawing group is synthesized for the first time, the absorption spectrum red shift of the fluorescence probe can achieve 606-633nm through the introduced substituent group, and the emission peak can achieve 616-646nm. The BODIPY molecule is a favorable fluorescence, can be used for specifically marking lysosome in biological cells, and can emit light through the self molecules, and the absorption and emission of the BODIPY molecule are close to the near-infrared zone; and in addition, the fluorescence probe has the advantages of high mole extinction coefficient, acute emission peak, high fluorescence quantum yield and the like, and has attractive application prospects on the lysosome marking application.
Owner:NANJING UNIV
BODIPY-based high-sensitivity fluorescent probe and synthesis method and application thereof
InactiveCN107417714AMicrobiological testing/measurementGroup 5/15 element organic compoundsSynthesis methodsBiocompatibility Testing
The invention relates to a BODIPY-based high-sensitivity fluorescent probe and a synthesis method and application thereof. A structural general formula of the probe is shown as (I), wherein Trigger is stimulant triggering groups, R1 and R2 are groups for regulating and controlling fluorescent transmission wavelength of the probe and introducing organelle targeting, and R1 and R2 are defined in the description. By the probe, detection of different substrates can be realized without changing mother nucleus structure of the probe by only changing different Trigger groups. In addition, according to different needs on wavelength and targeting, the mother nucleus structure of the probe can be quickly modified. By changing R1 and R2, maximum fluorescent emission wavelength of the probe can be changed, and the probe can target mitochondrion to realize detection of active substances in the mitochondrion. The probe has good biocompatibility, thereby being applicable to detecting biological systems. Application value of the fluorescent probe in the aspect of detecting bioactive molecules and protease over-expressed in inflammatory or tumor tissue has potential social benefit and economic benefit.
Owner:NANKAI UNIV
Reactive labelling compounds and uses thereof
ActiveUS9759726B2Fluorescence enhancementEasy to detectPeptidesGroup 3/13 element organic compoundsAlkyneCombinatorial chemistry
Provided are azido-BODIPY compounds of formula (I), cyclooctyne-based fluorogenic probes of formula (IV), and activity-based probes of formula (VI). These compounds undergo azide-alkyne cycloadditions (AAC) with to form triazolyl products. The provided compounds are useful for detection and imaging of alkyne-, or azide-containing molecules. Methods for detection and imaging biomolecules using compounds of the present disclosure are disclosed.
Owner:ACAD SINIC
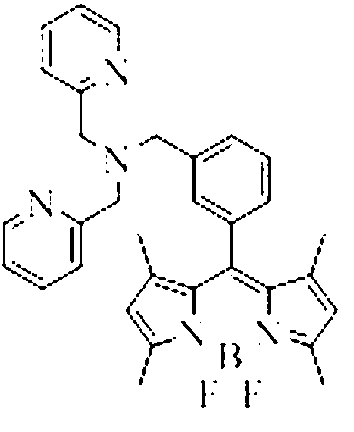
Copper ion fluorescence probe and synthetic method thereof
InactiveCN103013495ALow toxicitySmall inhibition rateGroup 3/13 element organic compoundsFluorescence/phosphorescenceSynthesis methodsMethyl group
The invention belongs to the technical field of analysis chemistry and relates to a copper ion fluorescence probe and a synthetic method of the copper ion fluorescence probe. The copper ion fluorescence probe disclosed by the invention has a chemical name of 8-[di(2-picolyl)amine-3-benzyl]-4, 4-difluoro-1,3,5,7-tetramethyl-4-boron-3a, 4a-dipyrrole (called BODIPY-DPA for short). The synthetic method of the copper ion fluorescence probe comprises the steps of: mixing 2, 4-dimethylpyrrole with 3-chloromethyl benzoyl chloride, then adding CH2Cl2 into the mixture, then adding boron trifluoride for reaction, and then orderly adding di(2-picoly)amine and triethylamine for reaction, at last, obtaining the copper ion fluorescence probe. The BODIPY-DPA shows light yellow in the solution, has high fluorescence emission at 590 nm, can enter HepG-2 to show green fluorescence imaging, and has a lowest limit of detection of 2.78 muM on copper ions in water solution. The copper ion fluorescence probe prepared by the synthetic method is characterized in low toxicity and the inhibition rate of 100 muM of HepG-2 is smaller than 10%, so the copper ion fluorescence probe can be used for detection of living cell imaging and copper irons in the cell, and has a very good application prospect in environment monitoring and detection of copper irons in a biologic system.
Owner:JIANGSU UNIV
Novel red BODIPY fluorescent dye and preparation method and application thereof
InactiveCN102702768ANarrow absorbencyNarrow fluorescence emission spectrumMethine/polymethine dyesMicrobiological testing/measurementChemical reaction1,4-Benzoquinone
The invention relates to a novel red BODIPY fluorescent dye with the chemical formula of C10+mH7+nBF2N2+xOy, wherein m, n, x and y are integers from 0 to 100. The preparation method comprises the following steps: dissolving pyrrole with substituent groups R1, R2 and R3 in an organic solution; adding ethyl glyoxylate together with nitrogen to the organic solution for a chemical reaction by using trifluoroacetic acid or toluenesulfonic acid as a catalyst; adding 2,3-dichloro-5,6-dicyano-1,4-benzoquinone oxidative dehydrogenation; and adding organic amine and a boron trifluoride diethyl ether solution for another reaction. After the reaction solution is concentrated, chromatography is performed with a silicagel column to obtain the fluorescent dye. The fluorescent dye can be used for cell imaging, fluorescent probe or laser dye. The fluorescent dye has the advantages that the ultraviolet-visible absorption spectrum and the fluorescence emission spectrum of the fluorescent dye are narrow; fluorescent quanta has high efficiency and good light stability; and the fluorescent dye has simple molecular structure and can be synthesized easily, so as to facilitate popularization and application.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Compound for detecting secondary amine, and preparation and application thereof
ActiveCN103694269AHigh fluorescence quantum yieldThe synthesis method is simpleMaterial analysis by observing effect on chemical indicatorGroup 3/13 element organic compoundsAnthraceneBenzene
The invention relates to a compound for detecting secondary amine, and preparation and application thereof. The compound contains boron ester and a BODIPY structure unit in conjugate connection with the boron ester. The compound has a structural general formula shown as in (I), wherein R1 and R2 are independently selected from the group consisting of benzene, naphthalene, anthracene, pyrene, carbazole, triphenylamine, binaphthalene, thiophene, quinoline, imidazole and rhodamine. The preparation method has the advantages of simpleness, high yield, easy separation and high purity. The compound provided by the invention has a novel structure and rapid and sensitive detection of secondary amine; and when the compound reacts with secondary amine, the film turns into purple from red, and the fluorescence is quenched. The test paper and film prepared from the compound react with secondary amine, and significant changes of the color and fluorescence take place in a few minutes and are visible to the naked eyes, so as to conveniently identify secondary amine.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Boron fluoride dipyrrole fluorescent dye containing hydrophilic groups and preparation method thereof
InactiveCN103865289ASimple reaction conditionsHigh yieldMethine/polymethine dyesLuminescent compositionsFluorescent spectraQuinone
Owner:TIANJIN UNIV
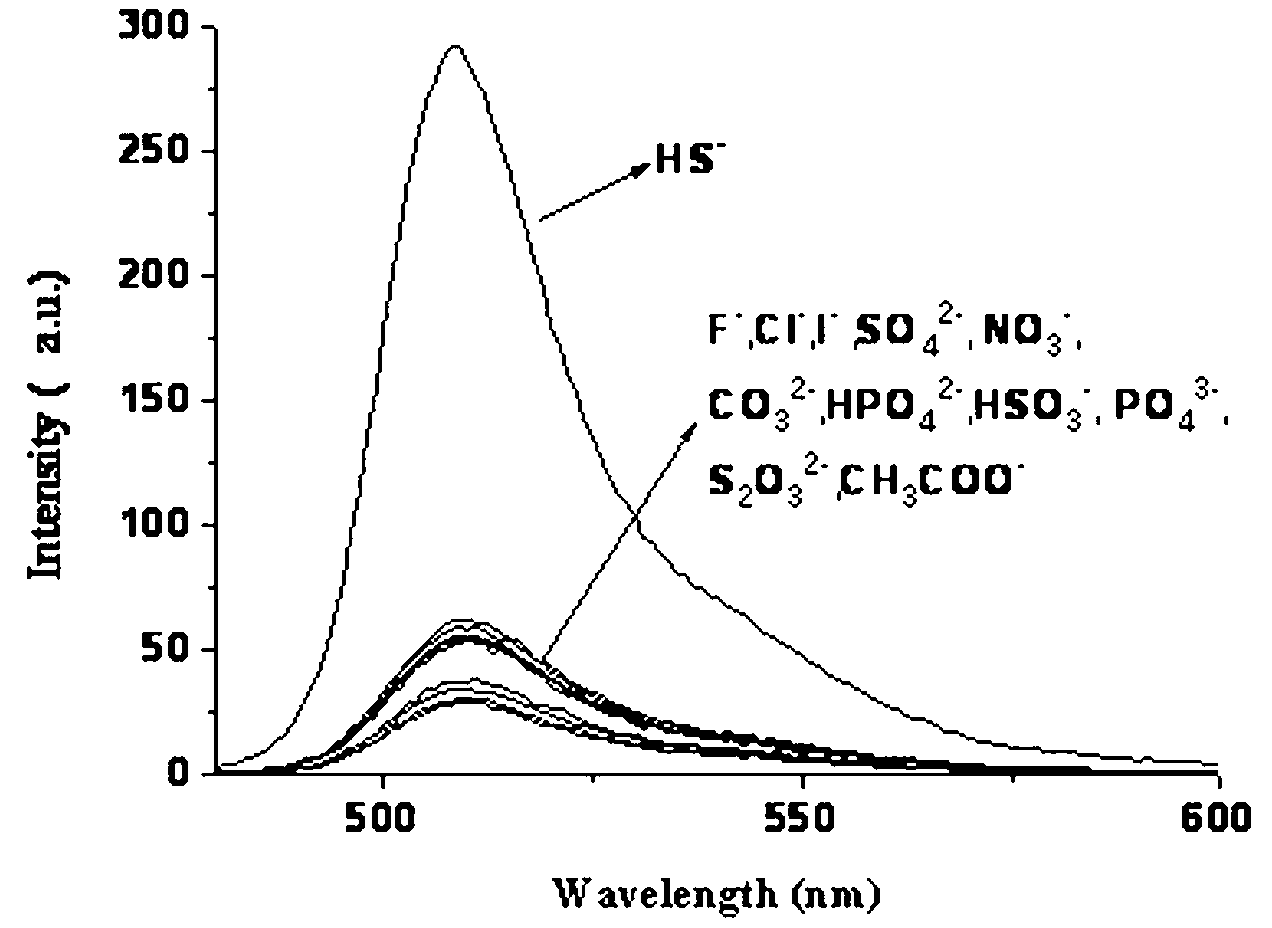
Fluorescent probe for detecting cyanide ion and synthesis and application method thereof
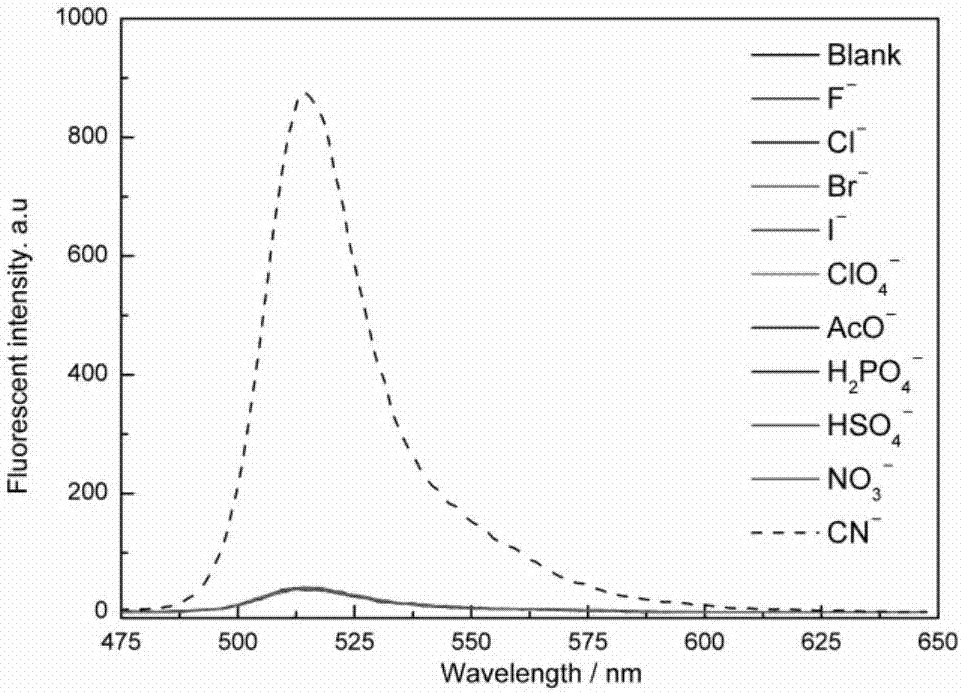
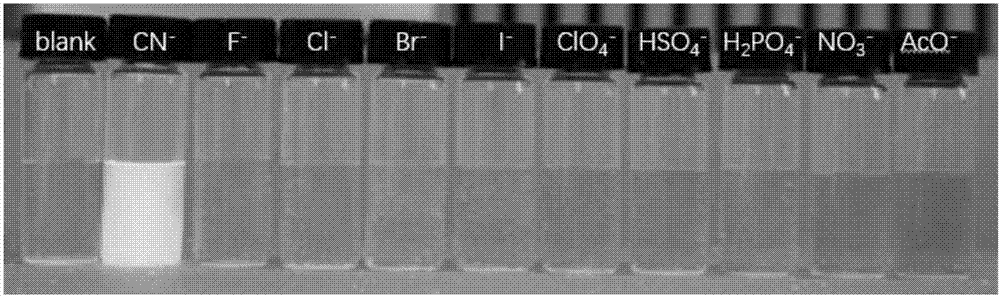
ActiveCN107082785AThe synthesis method is simpleLow costGroup 3/13 element organic compoundsFluorescence/phosphorescenceIodideRelative standard deviation
The invention discloses a fluorescent probe for detecting a cyanide ion and a synthesis and application method thereof. A cyanide ion fluorescent probe takes boron fluoride dipyrrole (BODIPY) as a fluorescent signal group and hemicyanine as a recognition site, and the probe is obtained by carrying out Knoevenagel condensation reaction on 1,3,5,6-tetramethyl 8-(4(formylphenyl)) boron fluoride dipyrrole and 1,2,3,3-tetramethyl-3H-indolium iodide. Ultraviolet absorption and fluorescence emission spectroscopy research finds that the probe can solely identify the cyanide ion, the lowest detection limit can reach 59 nM, the identification process is not interfered by other negative ions, and response speed is high. The fluorescent probe disclosed by the invention can be used for detection of the actual water sample, and relative standard deviation is less than 5%. Besides, the fluorescent probe is simple in synthesis method and relatively low in cost and has a good application prospect in cyanide ion detection.
Owner:JIANGHAN UNIVERSITY
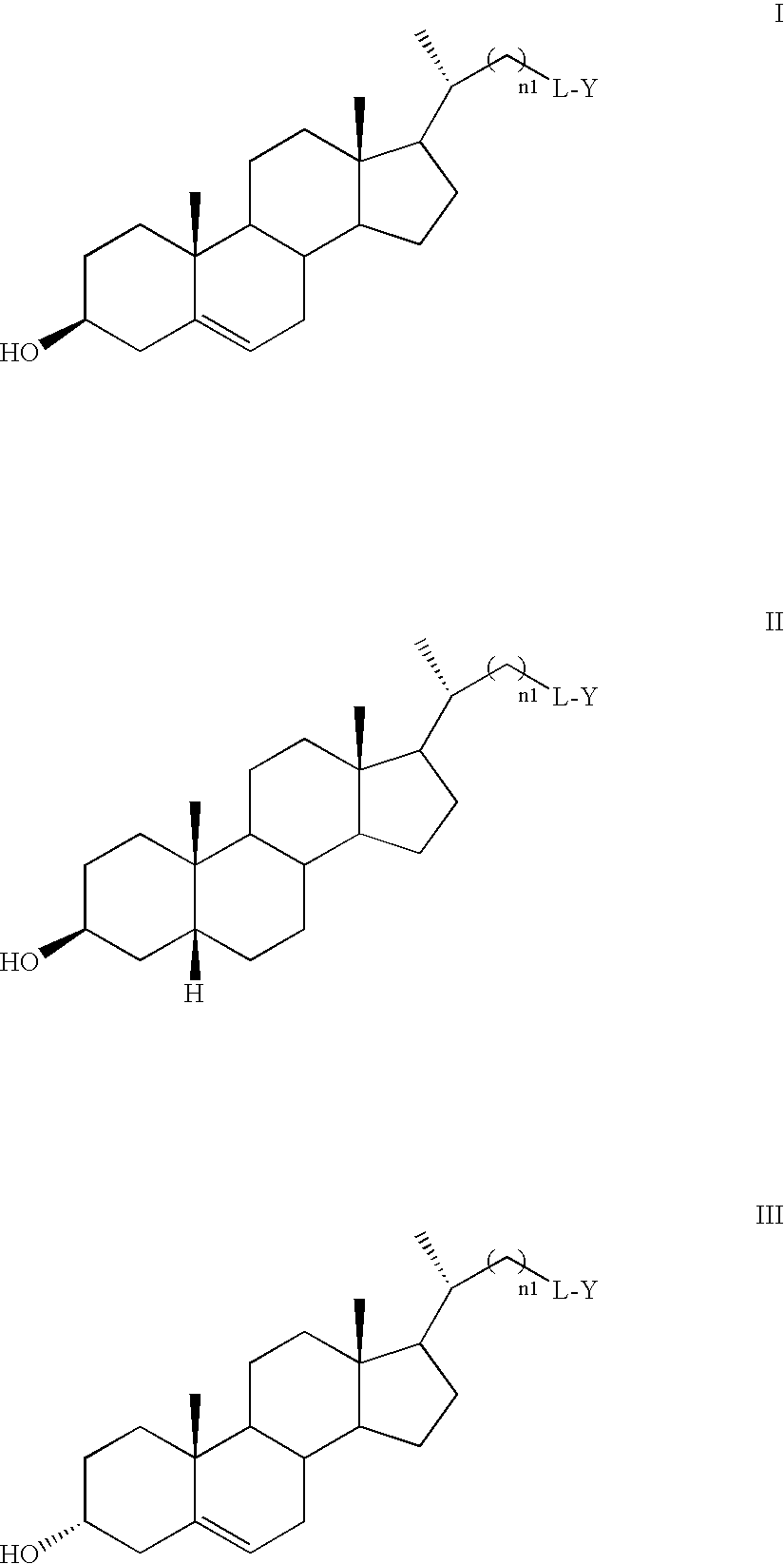
Free cholesterol analogs bearing a boron dipyrromethene difluoro (bodipy) fluorophore in the side chain and method of preparation and use thereof
The present invention relates to free cholesterol analogs bearing a boron dipyrromethene difluoro (BODIPY) fluorophore in the side chain and methods of preparation. The compounds of the present invention can be used in fluorescence spectroscopy and fluorescence microscopy to visualize the exchange, distribution, and trafficking of free cholesterol between living cells and to monitor the movement of free cholesterol between ordered and disordered lipid domains in membranes.
Owner:RES FOUND THE CITY UNIV OF NEW YORK
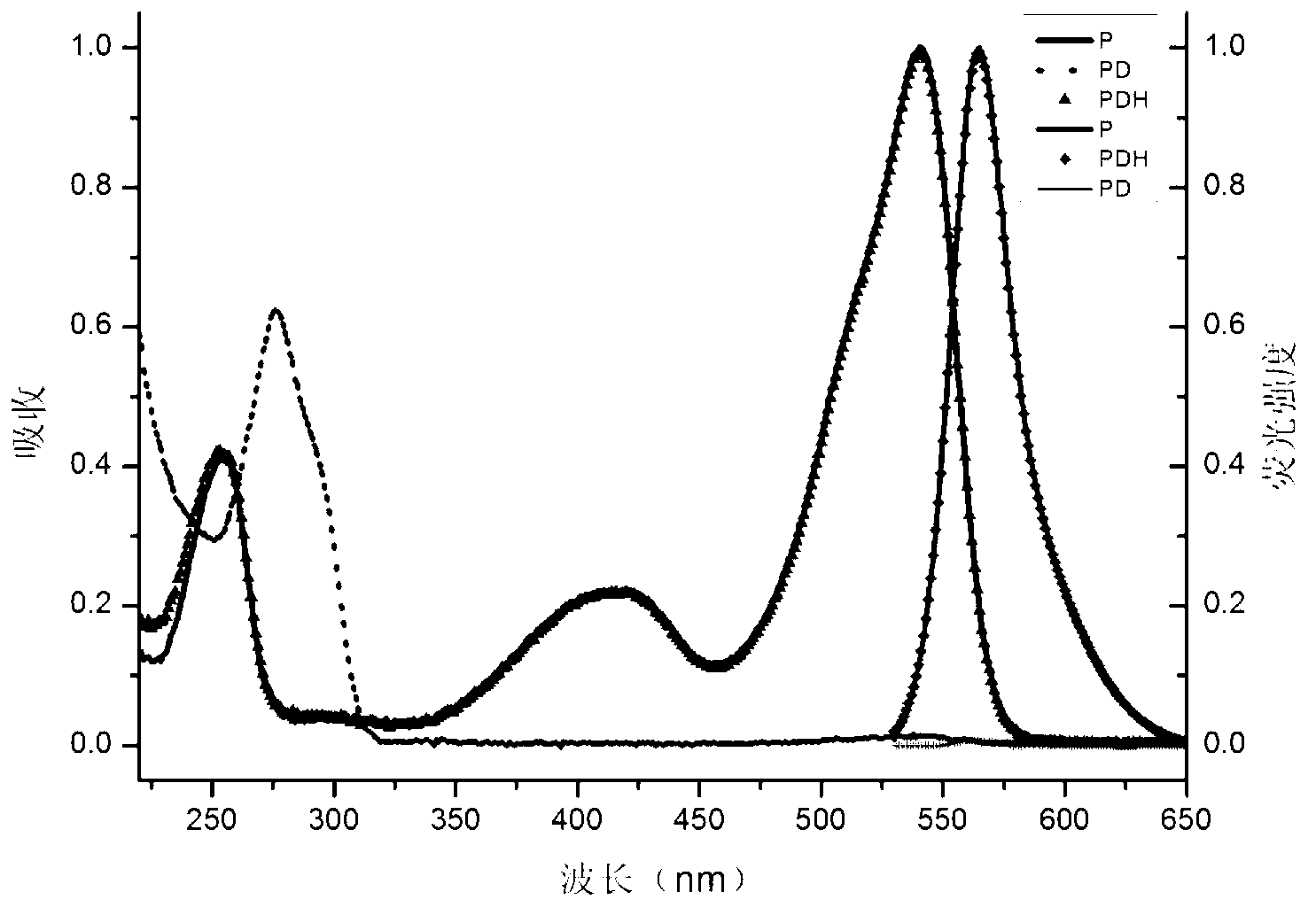
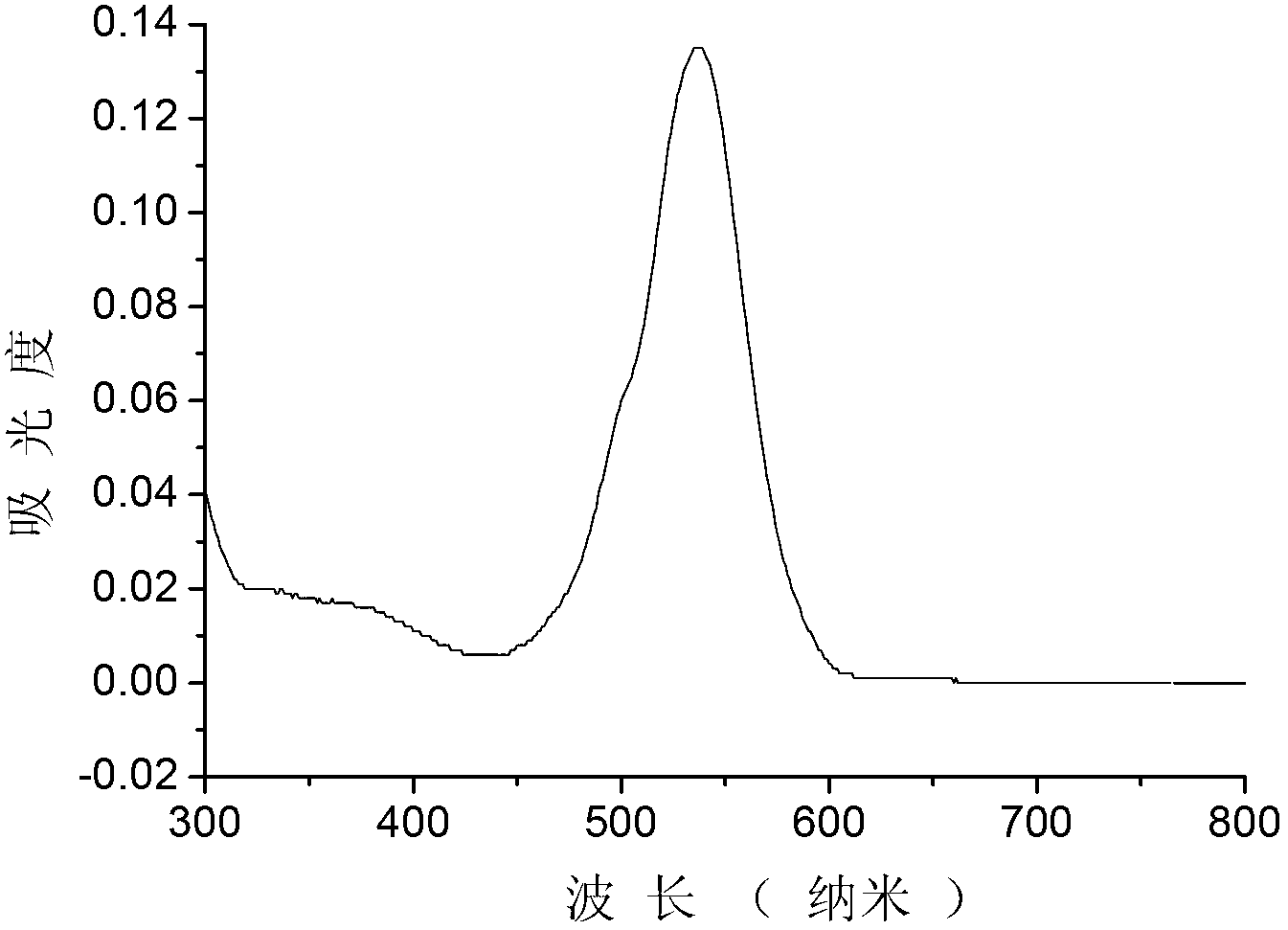
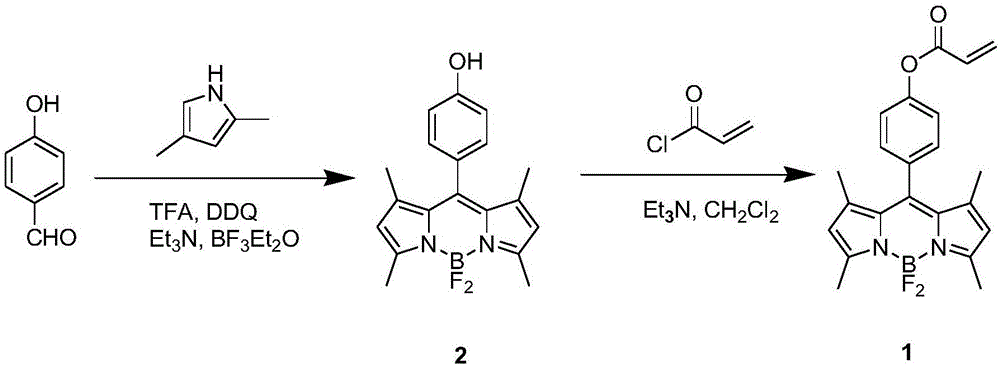
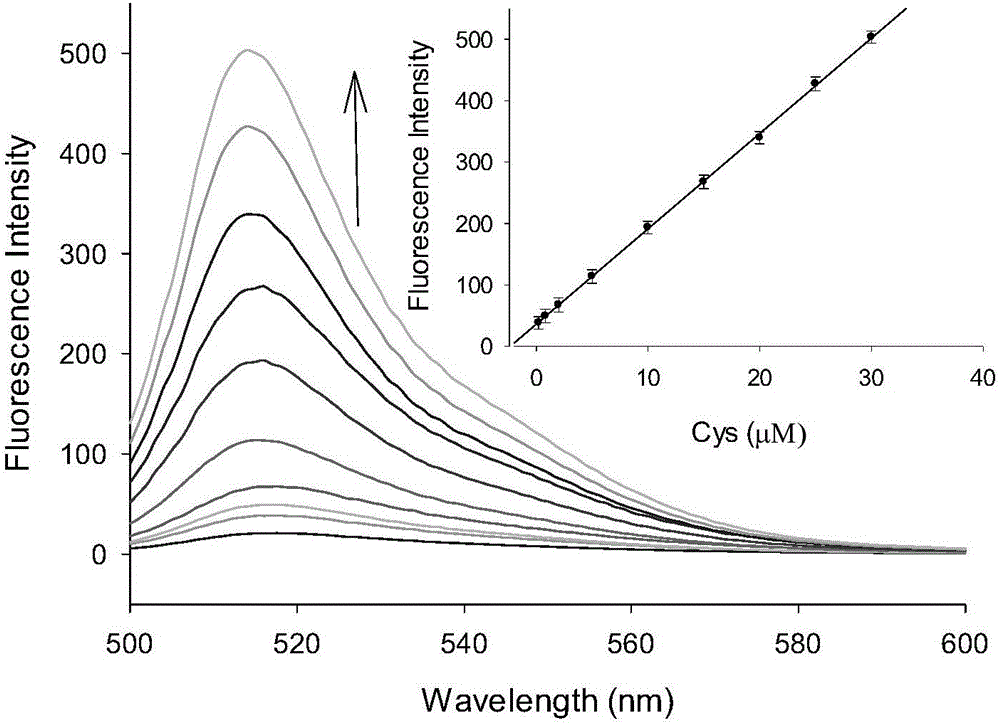
Preparation method and application of BODIPY (boron-dipyrromethene) and Cys (cysteine) fluorescent probe
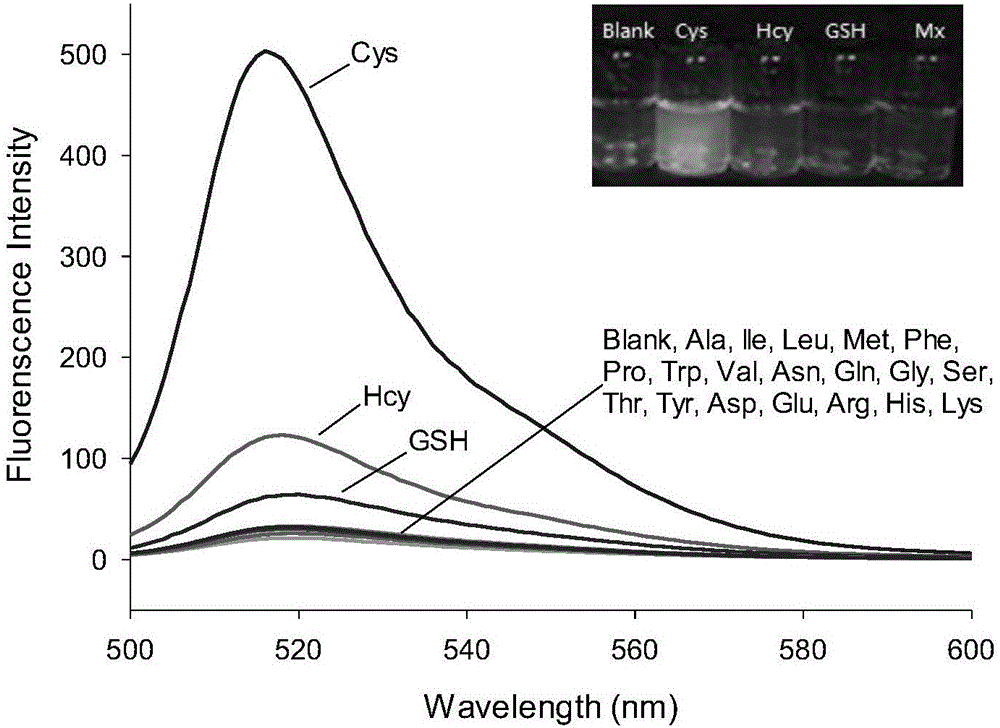
InactiveCN106432315AEnhanced fluorescence emission peakGood choiceGroup 3/13 element organic compoundsFluorescence/phosphorescenceThiolStructural formula
The invention discloses a preparation method and an application of a boron-dipyrromethene (BODIPY) based fluorescent probe for determining cysteine (Cys). The structural formula of the probe is shown in the specification. The preparation method of the BODIPY derivative based Cys fluorescent probe with a simple structure is provided, and the BODIPY derivative based Cys fluorescent probe is used for directly measuring Cys concentration. In the system, the fluorescent probe shows good selectivity and is not interfered with other biological thiols (Hcy and GSH) and other 19 amino acids. The probe shows quite high sensitivity to Cys, and the fluorescence intensity is enhanced by 23 times when 3 equivalents of Cys are added. When the pH is between 6.0 and 8.0, determination of Cys by the fluorescent probe is not affected by pH. The fluorescence probe and Cys act quickly, and the response time is within 6 minutes. In addition, the probe can also be used in cell imaging to detect intracellular Cys.
Owner:XIANGTAN UNIV
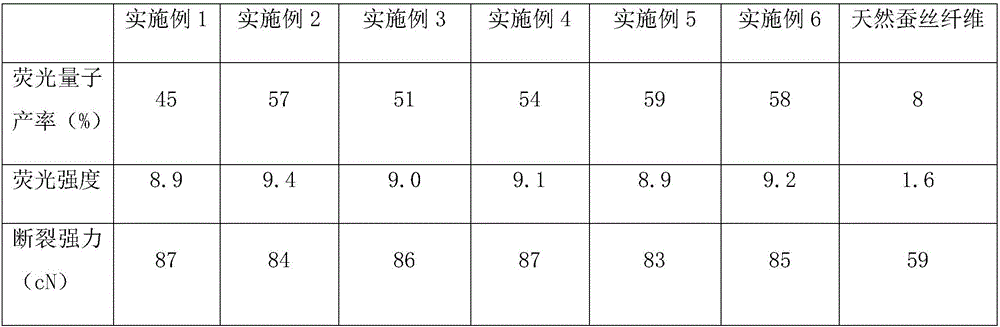
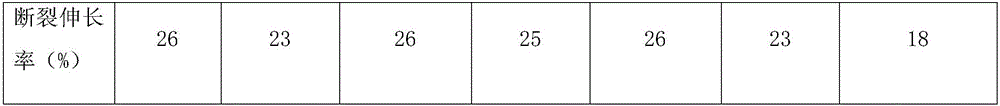
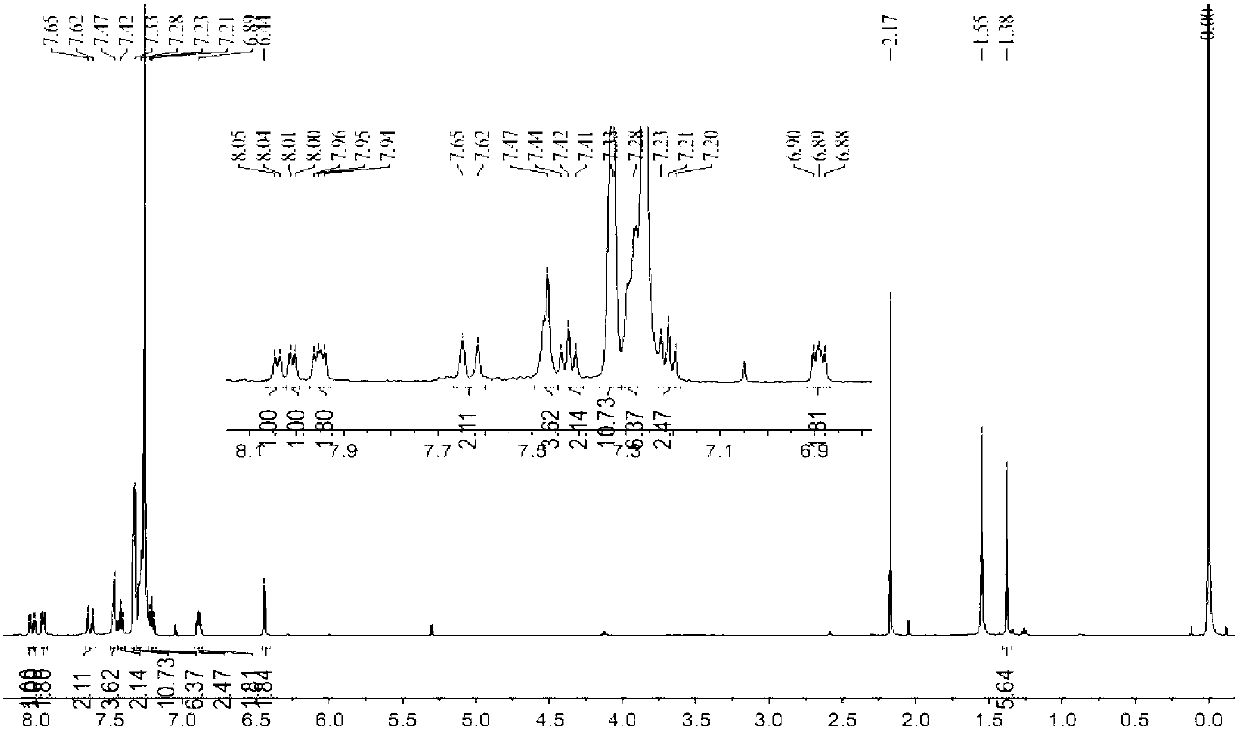
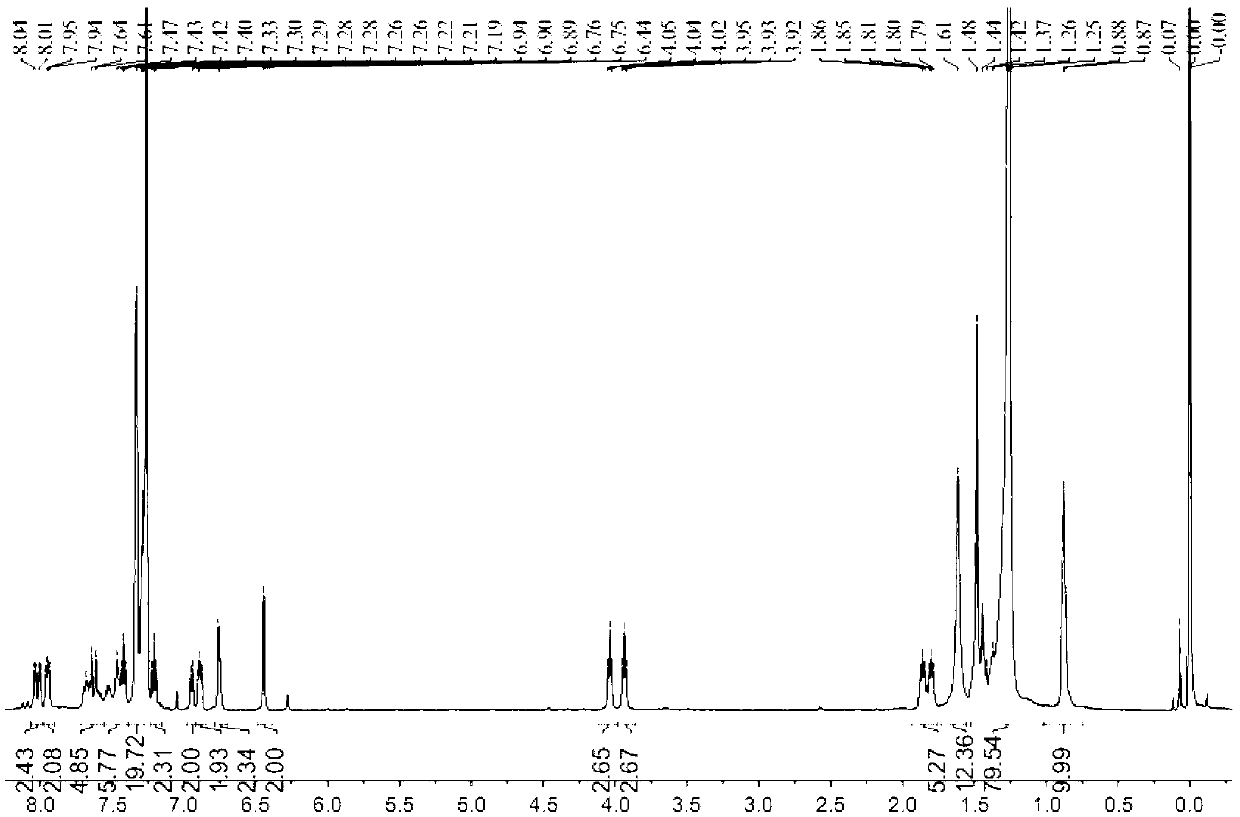
Fluorescent silk fiber based on BODIPY dyes and preparation method of fluorescent silk fiber
ActiveCN105862451AImprove adsorption capacityHigh fluorescence quantum yieldDyeing processPolymer sciencePre treatment
The invention provides a fluorescent silk fiber based on BODIPY dyes and a preparation method of the fluorescent silk fiber. The preparation method comprises the following steps: swollen silk fibers are immersed in a zeolite-containing aqueous solution and are pretreated, then are repeatedly immersed in hydrophilic BODIPY fluorescent dyes and a low molecular chitosan solution, and finally are steamed, heated and cured to obtain the fluorescent silk fiber. The fluorescent silk fiber based on BODIPY dyes prepared according to the method is stable in fluorescence, high in strength and uniform in color, can be produced in large scale, has excellent mechanical properties and high flexibility, and is applicable to the textile, bio-medical treatment, anti-counterfeiting and many other fields.
Owner:陈赛飞
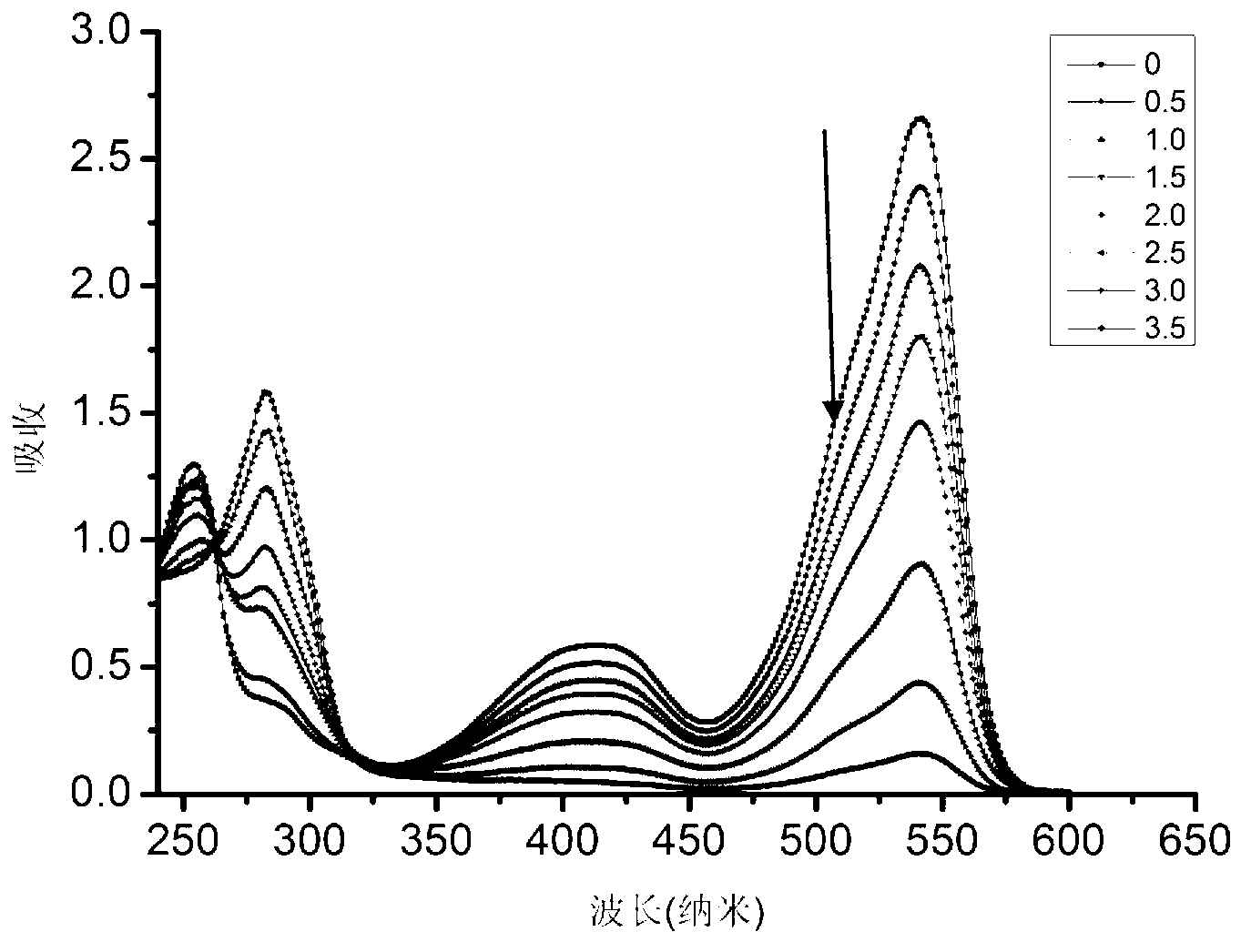
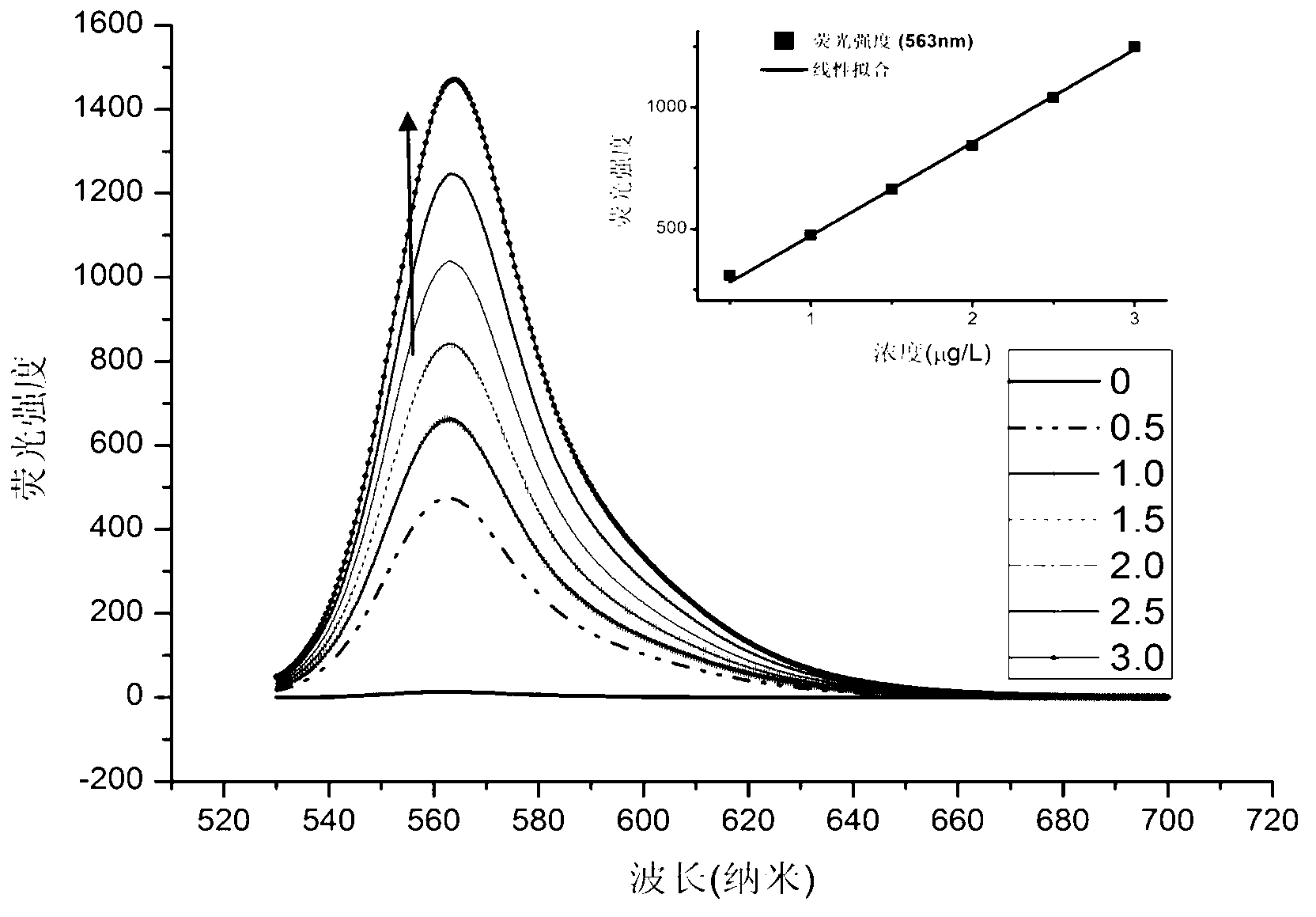
Near infrared BODIPY (Boron Dipyrromethene Compounds) hydroxyl radical probes and synthesis method and usage thereof
InactiveCN103342720AEfficient detectionEfficient detection and detectionGroup 5/15 element organic compoundsBiological testingInfraredDiphenyl phosphate
The invention discloses a kind of near infrared boron dipyrromethene compounds (BODIPY) based on duplex thiophene pyrrole groups. The near infrared boron dipyrromethene compounds have the following structure: FORMULA, wherein the group at meso site is changed to obtain a series of hydroxyl radical probes. The BODIPY hydroxyl radical probes based on 3,5-(diphenyl phosphate styryl) are synthesized for the first time in the invention, the 3,5 site groups of the BODIPY are introduced into the group, so that the absorption spectrum red shift of the probe reaches 630nm, the emission peak reaches 650nm and is located within the range (650-900nm) of a biological window, and meanwhile, the fluorescence quantum efficiency of the probe is high. Moreover, the kind of probes can be used for efficiently and quickly detecting hydroxyl radicals and can be successfully applied in biological imaging. Therefore, the probes have a brilliant application prospect of detecting the hydroxyl radicals in biological systems.
Owner:NANJING UNIV
Platinum-based bodipy compound, preparation method and application
ActiveCN106519213AIncreased conjugated systemGood water solubilityPhotodynamic therapyAntineoplastic agentsTherapeutic effectBODIPY
The invention provides synthesis of a platinum-based bodipy compound and a new application thereof in pharmacy, and particularly relates to the synthesis of platinum-based bodipy molecules and derivatives thereof, preparation of a nano-scale medicament, and an application to anti-tumor photosensitive medicament in photodynamics and photothermal therapy. Self-assembling nano-particles with photodynamic and photothermal dual activities are constructed by taking bodipy dye molecules as parent nuclei, introducing divalent platinum ions in a covalent coordination mode, and performing modifying through a polymer hydrophilic chain. Under light conditions, a self-assembling nano-scale medicament shows good photodynamic and photothermal therapeutic effects. The platinum-based bodipy compound confirms dual effects of the photodynamics and photothermal therapy at cell and animal levels, and the light induction of tumor ablation is realized.
Owner:SUZHOU UNIV
Preparation method of truxene-based star-like compound
ActiveCN107056828AUniversalThe synthetic route is simpleGroup 3/13 element organic compoundsPorphyrinCycloaddition
The invention discloses a structure and a preparation method of a truxene-based star-like compound (TBP-C60). The preparation method is implemented by the following steps: by taking 7,12-dibromo-truxene-aldehyde derivative as a raw material and taking tetrahydrofuran as a solvent, performing Suzuki reaction on the raw material, the solvent and a BODIPY borate derivative under the catalysis action of tetra(triphenylphosphine) palladium, thus obtaining a truxene-BODIPY binary system compound TB; then by taking the TB and a porphyrin borate derivative as raw materials, performing reaction under the catalysis action of the tetra(triphenylphosphine) palladium, thus obtaining a BODIPY-truxene-porphyrin ternary system compound TBP; then performing cycloaddition reaction on the TBP, sarcosine and fullerene through 1,3-dipole, thus obtaining the star-like compound TBP-C60 in which the truxene is used as a core and the porphyrin, the BODIPY and the fullerene are used as arms. The preparation method of the star-like compound is simple, mild in reaction condition and easy and convenient to operate, shows excellent energy transfer efficiency and high heat stability and can be used for a light absorption antenna, a solar panel, light-simulated biological photosynthesis and other aspects.
Owner:江苏盛叶欣化工新材料有限公司
BODIPY fluorescent dye, and preparation method and application thereof
InactiveCN106478702AHigh fluorescence quantum yieldGood light stabilityAzo dyesGroup 3/13 element organic compoundsQuantum yieldBODIPY
The invention relates to development of a BODIPY fluorescent dye with a novel structure and excellent photochemical properties and provides a BODIPY fluorescent dye, and a preparation method and application thereof, belonging to the field of organic chemistry. The BODIPY fluorescent dye has high fluorescence quantum yield (0.99), good photostability and other excellent photochemical properties; and the preparation method is simple, uses easily available raw materials, has low requirements on equipment and is easy for industrial production.
Owner:杭州博派生物技术有限公司
Near-infrared aza-BODIPY dye and microwave synthesis method thereof
ActiveCN106479216AShort reaction timeHigh reaction yieldAzo dyesGroup 3/13 element organic compoundsInfraredMicrowave method
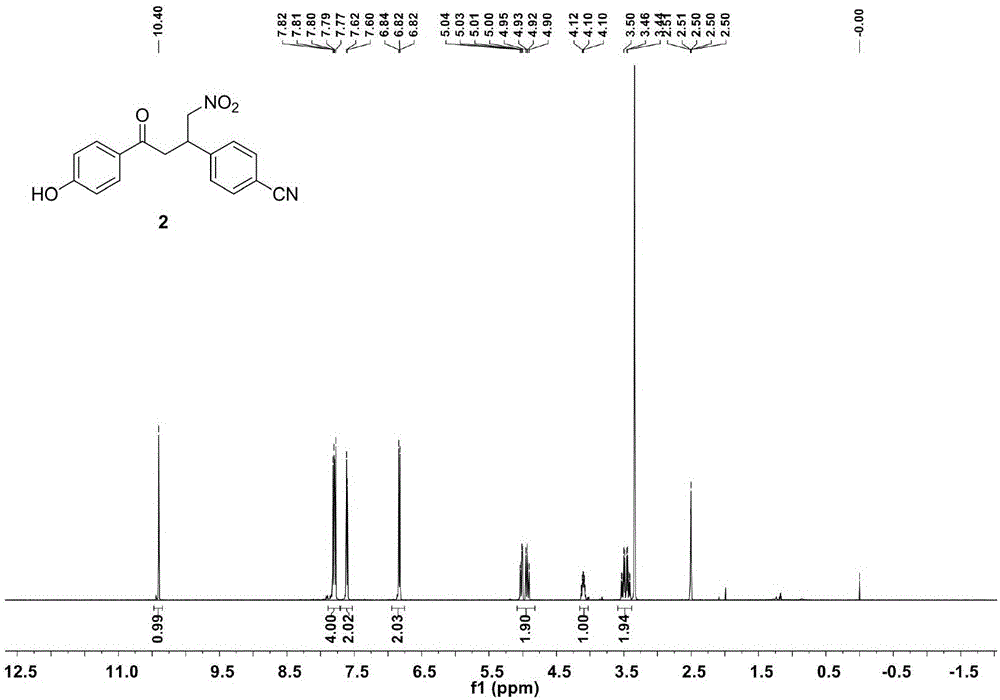
The invention belongs to the technical field of organic synthesis and particularly discloses near-infrared aza-BODIPY dye and a microwave synthesis method thereof. The compound structural formula of the aza-BODIPY fluorescent dye is shown in the abstract figure. The synthesis method comprises synthesis steps as follows: p-hydroxyacetophenone and p-cyanobenzaldehyde are taken as raw materials, and an alpha, beta-unsaturated ketone compound 1 containing conjugated double bonds is obtained; the compound 1 is subjected to heating reflux and Michael addition with nitromethane for nitrosation under the alkaline condition, and a compound 2 is obtained; the compound 2 reacts with ammonium acetate with a microwave method at the reaction temperature of 95 DEG C for 6 h, a tetraaryl-aza-dipyrromethene compound 3 is obtained; the compound 3 reacts with triethylamine and boron trifluoride to produce the near-infrared aza-BODIPY dye. The near-infrared aza-BODIPY dye and the synthesis method have the advantages that compared with a conventional heating method for synthesizing the fluorescent dye, the microwave method can greatly shorten the reaction time, reduce by-products and increase the reaction yield to a certain extent.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Preparation and application of fluorescent molecular probe for detecting hypochlorite ions
ActiveCN106749364AAchieve fluorescence-enhanced recognitionGood effectGroup 3/13 element organic compoundsFluorescence/phosphorescenceHypochloriteEthyl acetate
The invention relates to preparation and an application of a fluorescent molecular probe for detecting hypochlorite ions, and belongs to the technical field of chemical fluorescent materials. The probe is a BODIPY derivative with the molecular formula of C26H23BF2N6O4. Firstly, an intermediate compound 2 is synthesized. Secondly, a certain quantity of 2,4-dinitrophenylhydrazine is added mixed with the intermediate compound, a certain quantity of ethanol and ethyl acetate mixed solvents are added and dissolved, and stirring reaction is performed at room temperature after acetic acid serving as a catalyst is dropped in. A crude product is recrystallized by ethanol and then purified by column chromatography to finally obtain a purple pure probe. The probe is used for detecting the hypochlorite ions under various conditions, ClO-fluorescent enhanced recognition is realized by the synthesized novel fluorescent probe, the probe is remarkable and good in effect, high in sensitivity and low in measuring detection limit, and hypochlorite can be effectively recognized even under interference of some other ions.
Owner:JIANGSU UNIV
Triphenylamine-BODIPY derivative organic dye and preparation method thereof
InactiveCN104559286AHigh yieldReaction conditions are easy to controlMethine/polymethine dyesGroup 3/13 element organic compoundsEnvironmental energyOrganic solar cell
The invention discloses a triphenylamine-BODIPY derivative organic dye and a preparation method thereof. The triphenylamine-BODIPY derivative organic dye is prepared by that a triphenylamine-aldehyde compound is in reaction with pyrrole under the catalytic action of a catalyst, so as to synthetize dipyrromethane, and then a triphenylamine-BODIPY derivative is obtained through further oxidation and fluorine boronizing, and the general formula is shown in a structural formula I. The stokes displacement value of the triphenylamine-BODIPY derivative can reach 180 to 238 nm, and the fluorescence emission peak thereof locates in the near infrared region of 700 to 800 nm. The synthetic method of the compound is simple, the reaction is easy to control, the yield is high, common applicability is achieved, so that the BODIPY dye can be composited efficiently, and can be widely applied to the field of bioscience, analytic chemistry, environmental energy science and the like, and is particularly used as a near-infrared fluorescent dye and an organic solar cell material (refer to the description).
Owner:DONGGUAN UNIV OF TECH
Infrared BODIPY fluorochrome as well as preparation method and application thereof
InactiveCN106543213ANarrow absorbencyNarrow fluorescence emission spectrumAzo dyesGroup 3/13 element organic compoundsInfraredUv vis absorbance
The invention relates to the field of optical functional materials, and more specifically relates to an infrared BODIPY fluorochrome. The fluorochrome has a structure shown in a formula (1), wherein R1 and R2 are mutually independent and selected from C1-C10 alkyl, aromatic bases or heterocyclic aromatic bases; R3 is one of H atom, C1-C10 alkyl or aromatic bases. The fluorochrome has the advantages of narrow ultraviolet visible absorption spectroscopy and fluorescence emission spectrum, high molar absorption coefficient, high fluorescence quantum efficiency, good light stability, trace detection, and high sensitivity; and the fluorochrome can be applied to cell imaging, fluorescence probe, laser dye, fluorescence sensor and near infrared dynamics, and different application fields with good practicality.
Owner:SUZHOU BAIYUAN GENT CO LTD
Near-infrared two-window fluorescence probe based on Aza-BODIPY, as well as preparation and application thereof
ActiveCN109320536AGood light stabilityNo side effectsGroup 3/13 element organic compoundsFluorescence/phosphorescenceChemistryImage resolution
The invention relates to design synthesis and application of a near-infrared two-window fluorescence probe based on Aza-BODIPY, and belongs to the field of organic fluorescence probes. A near-infraredtwo-area dyestuff (NIR-II, 1000-1700nm) has a long emission wavelength and less light scattering and tissue autofluorescence interference so as to obtain better resolution ratio and imaging depth inbioimaging. An Aza-BODIPY fluorescent dye has a longer absorption emission wavelength, a narrower peak width at half height and a larger molar extinction coefficient, and is widely applied to the fields such as photodynamic therapy. The structural formula of the near-infrared two-window fluorescence probe designed and synthesized by the invention is as shown in the figure (I). According to the probe, electron-donating group julolidine is introduced onto the classic Aza-BODIPY structure, and the molecular rigidity is improved. The probe has excellent light stability and a capacity of resistingdisturbance. The probe successfully realizes near-infrared two-window imaging during the mice imaging experiment.
Owner:NANJING UNIV OF TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com