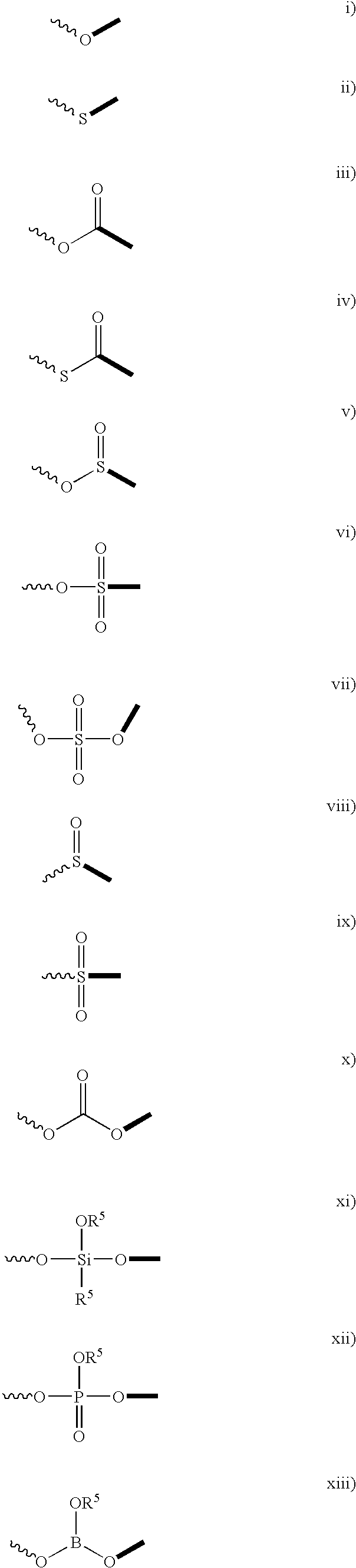
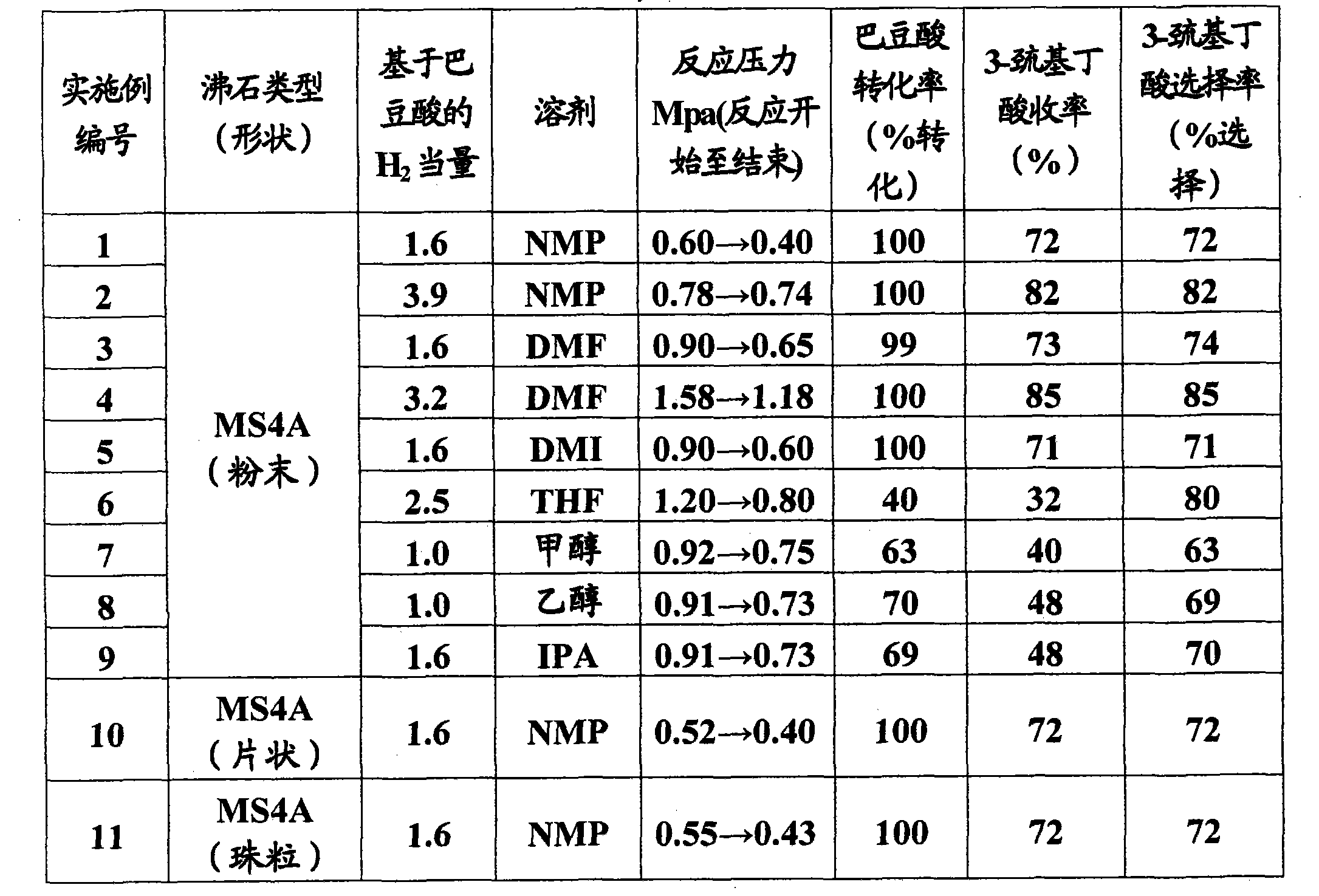
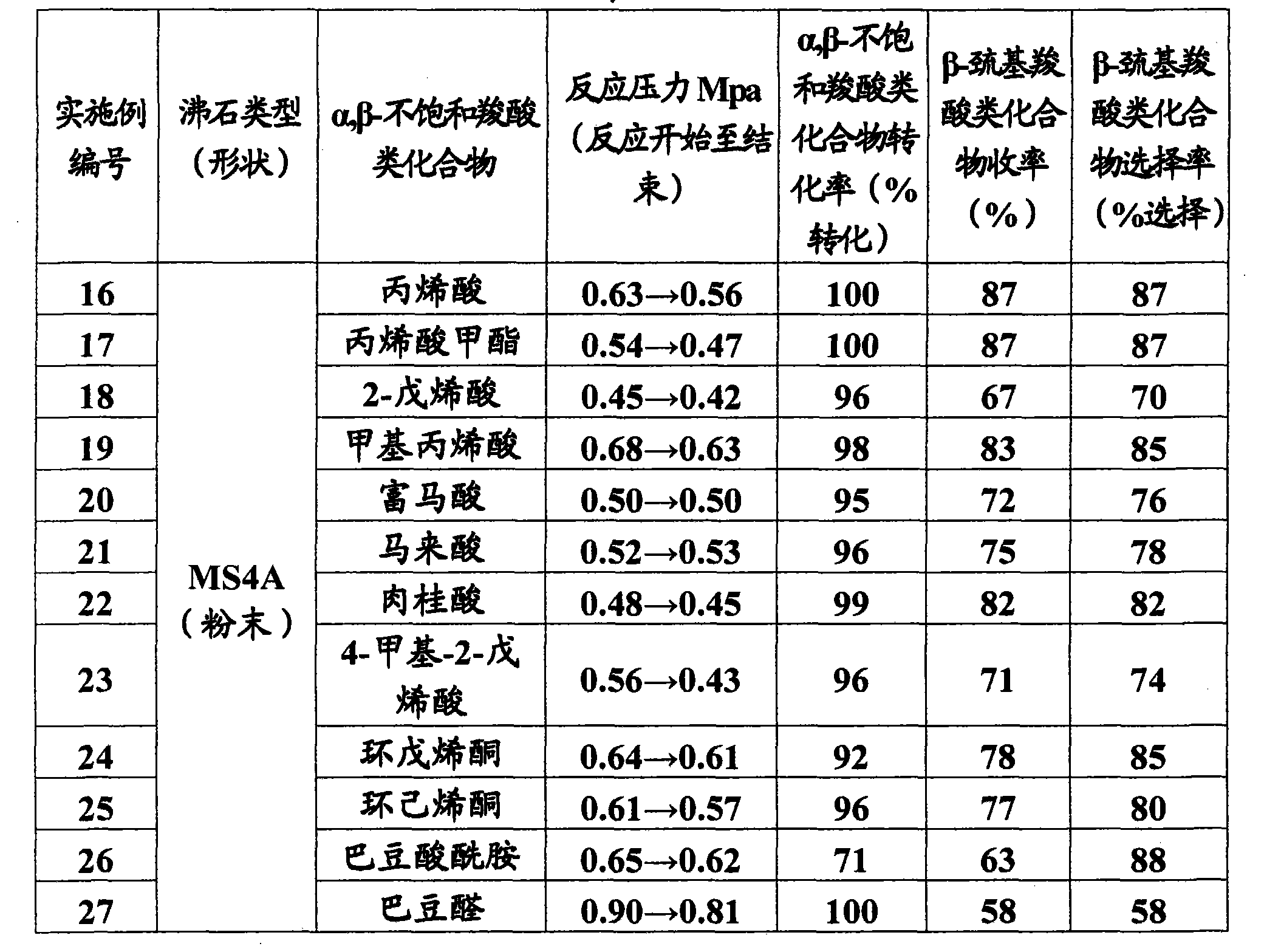
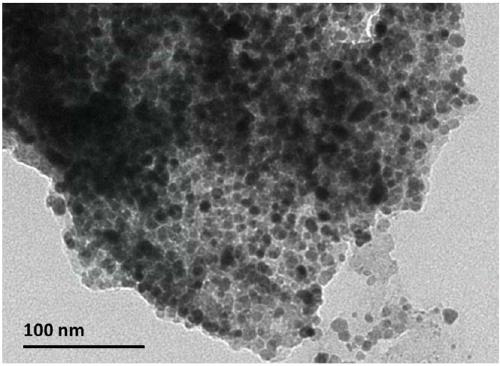
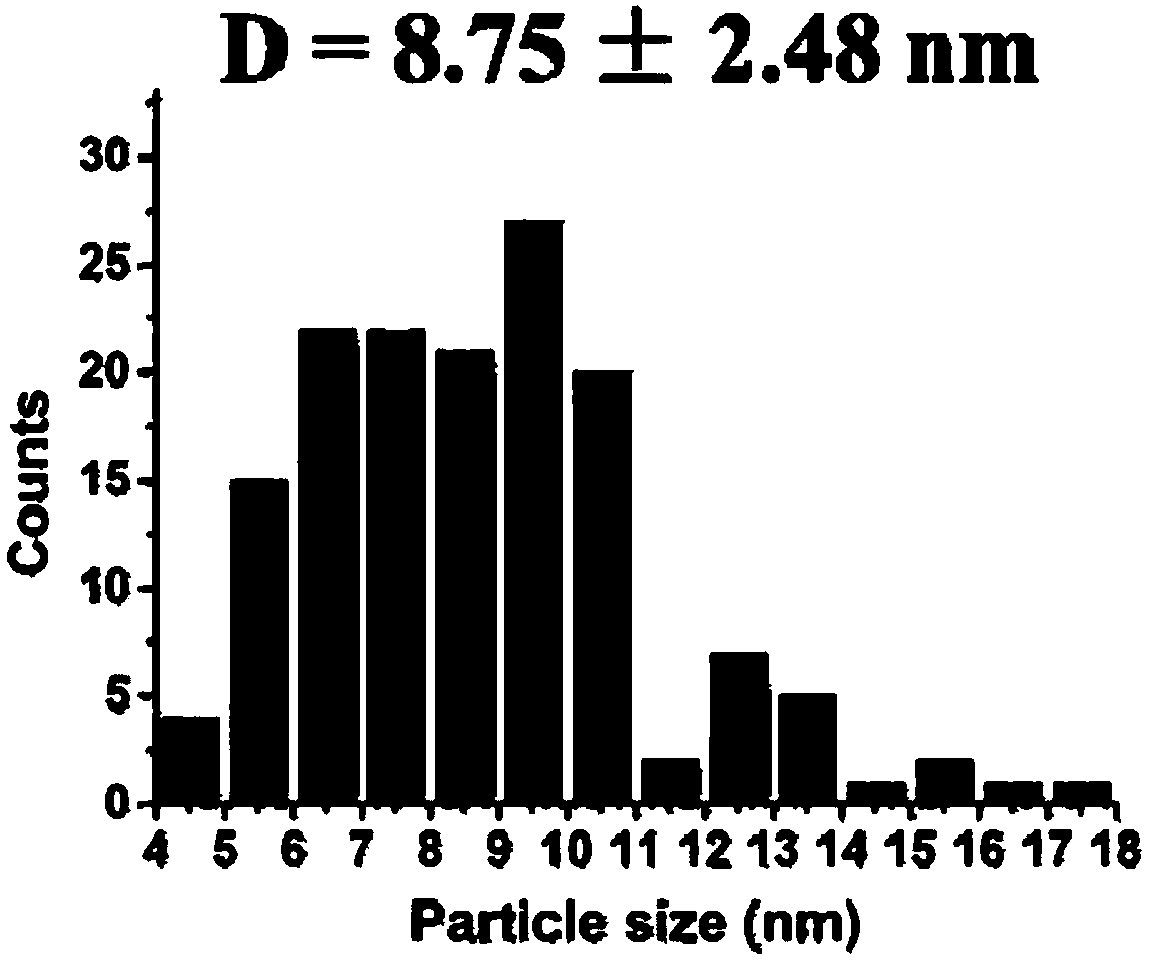
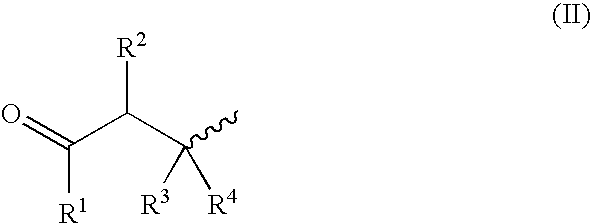
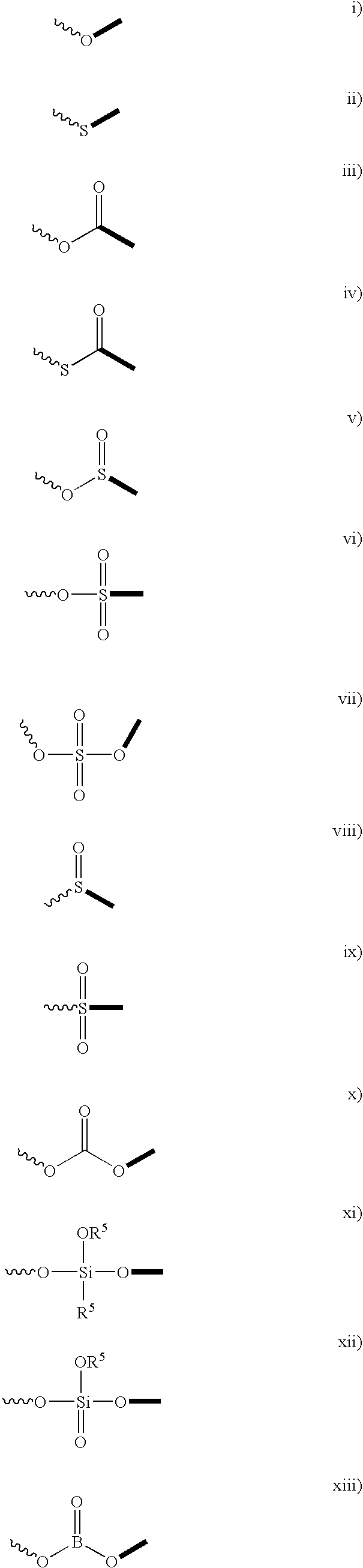
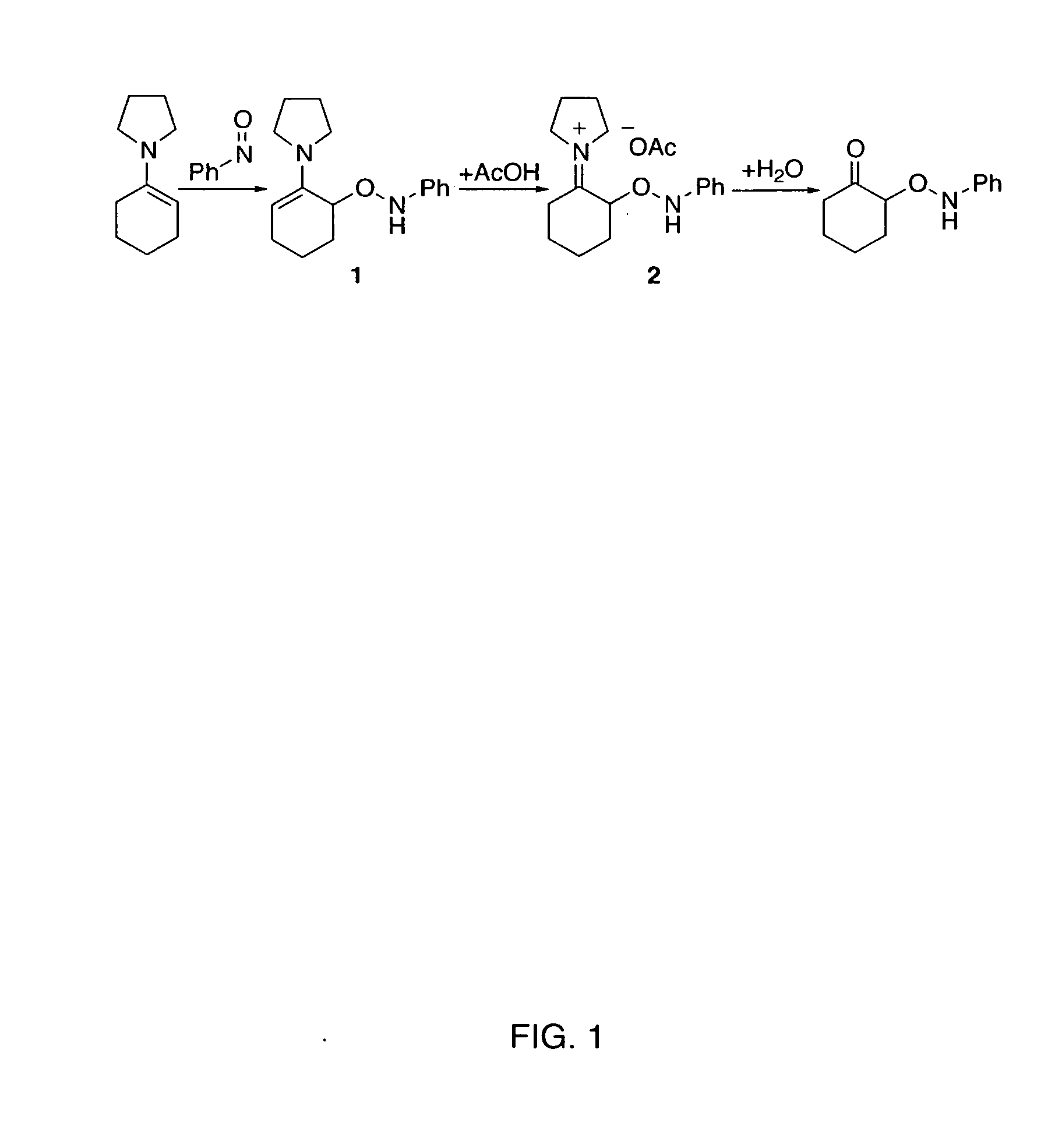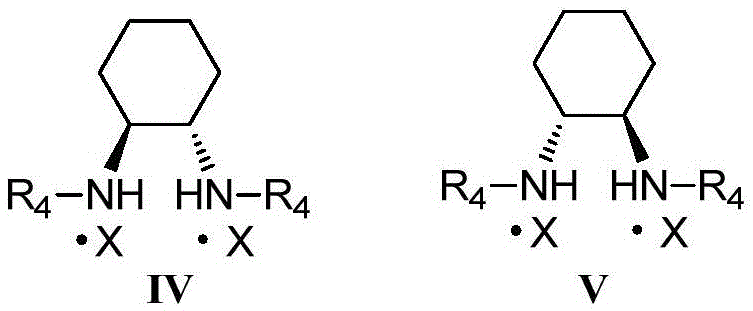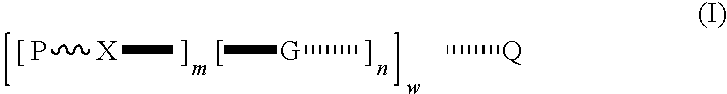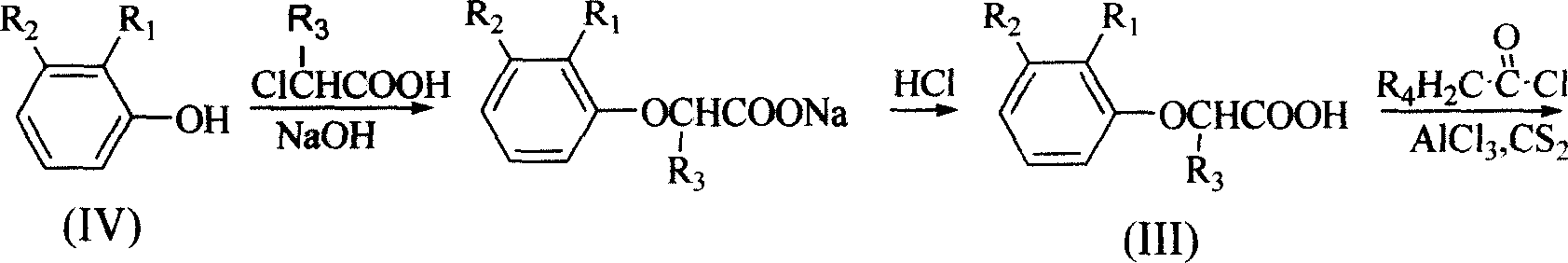Patents
Literature
412 results about "Unsaturated ketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Unsaturated implies presence of a c=c or a c-triple-c bond. Ketones are just a branch of organic compounds that have a c=o non terminal bond for sure. Now if any other c atom is bonded with another c atom via double or triple bond, it is unsaturated else, saturated. CH3-CH=CH-CO-CH3 is an unsaturated ketone.
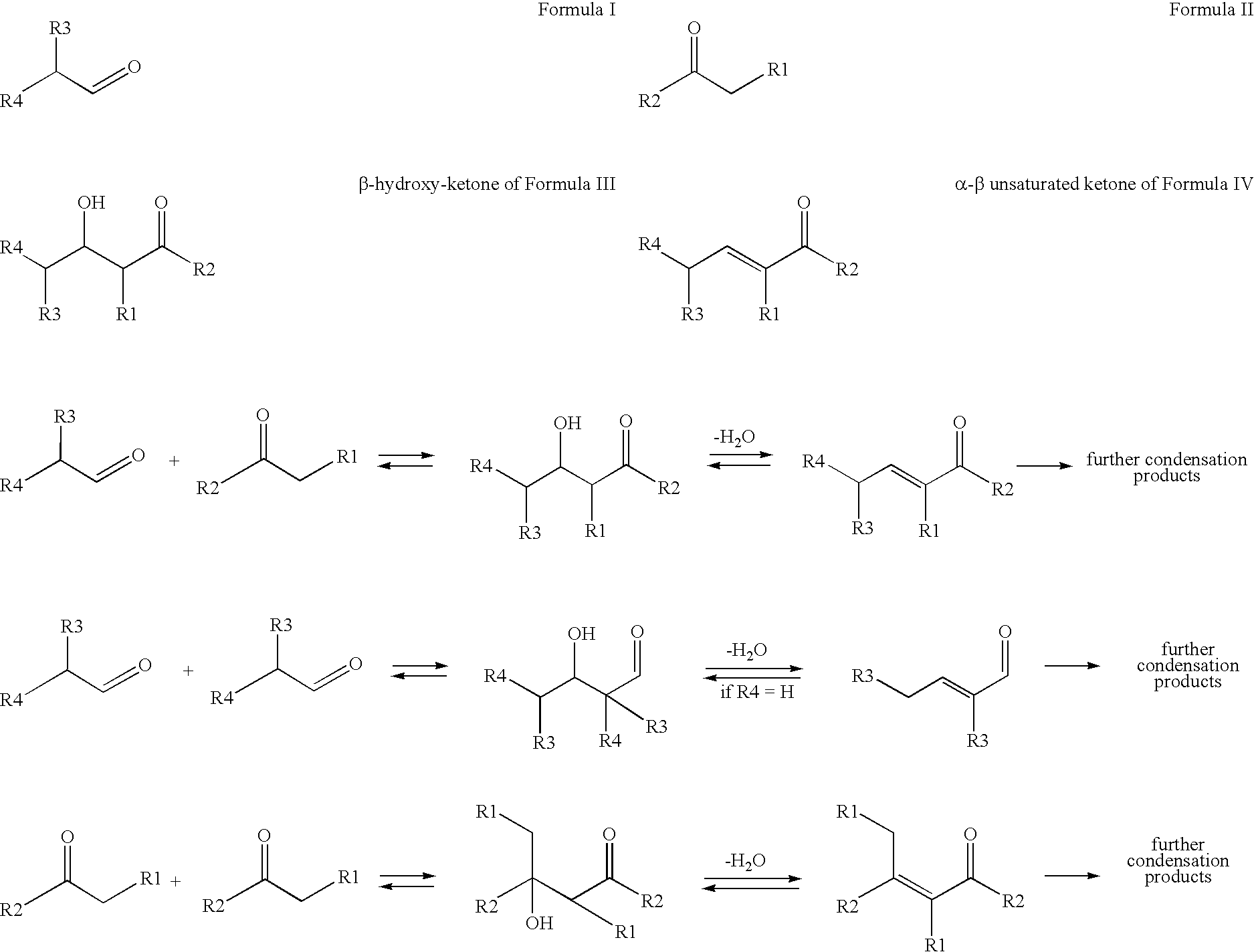
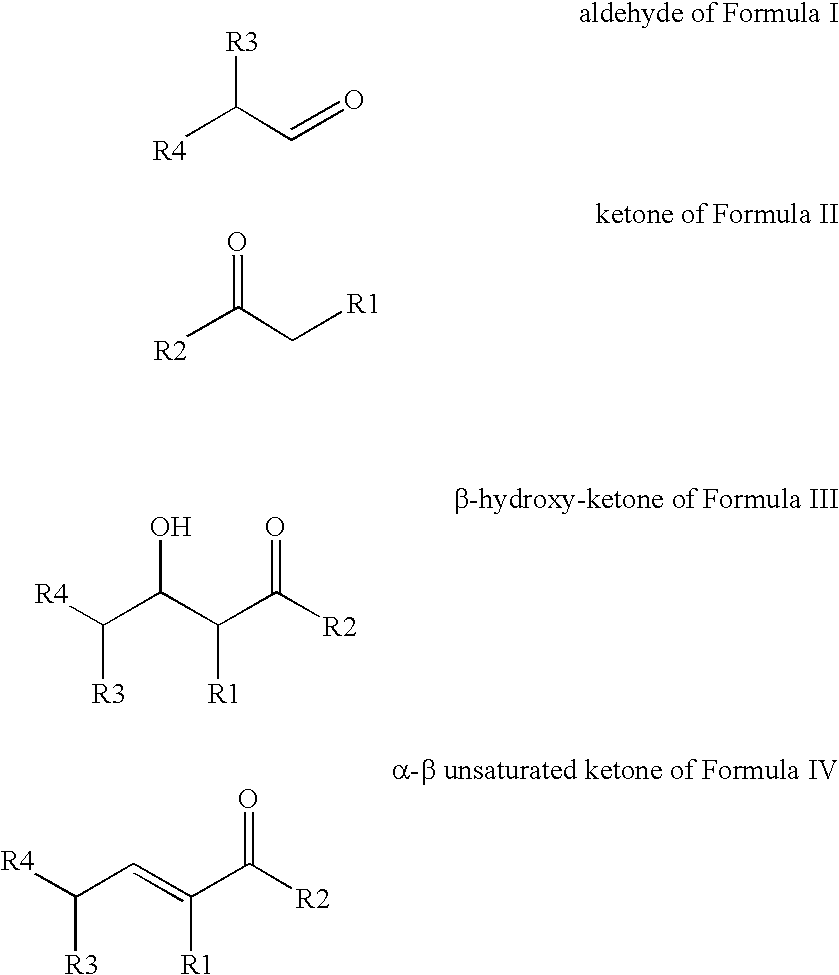
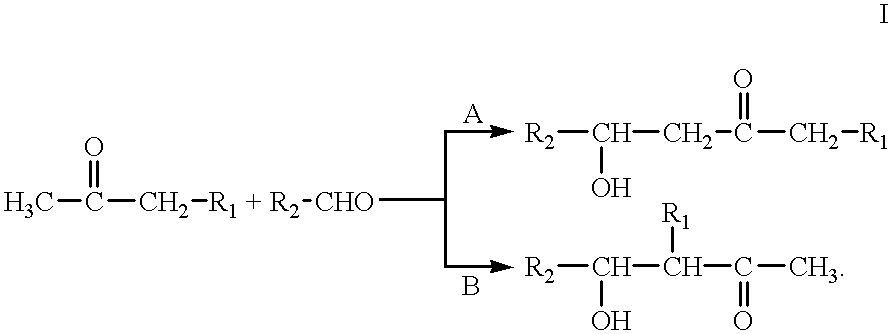
Processes for preparing beta-hydroxy-ketones and alpha,beta-unsaturated ketones
InactiveUS20050004401A1Delayed reaction timeOrganic compound preparationCarbonyl compound preparationAlkaline earth metalSolvent
Processes for producing β-hydroxy-ketones and α,β-unsaturated ketones are disclosed which comprise the crossed condensation of an aldehyde with a ketone in the presence of a hydroxide or alkoxide of alkali metal or an alkaline earth metal as catalyst. The products of the process, β-hydroxy-ketones and α,β-unsaturated ketones, are useful for the preparation of many commercially important products in the chemical process industries including solvents, drug intermediates, flavors and fragrances, other specialty chemical intermediates.
Owner:EASTMAN CHEM CO
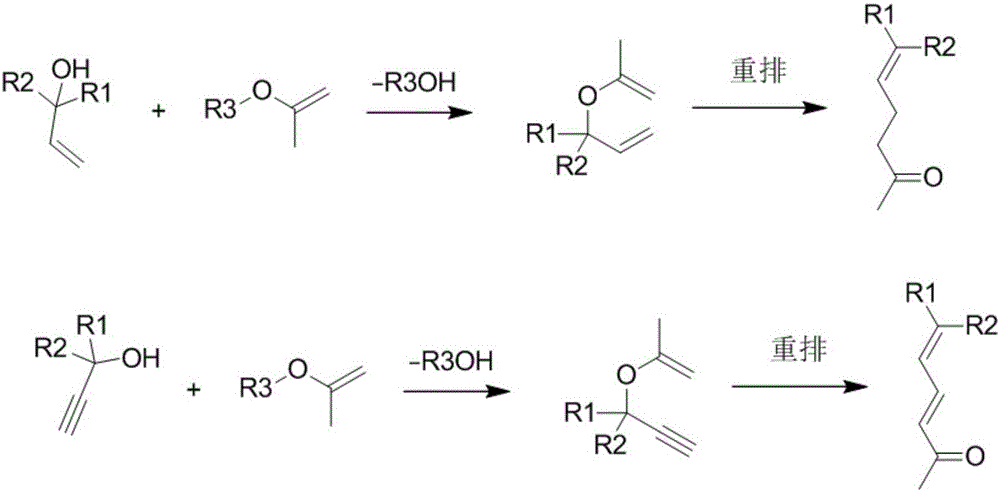
Method for the production of gamma , delta -unsaturated ketones by reacting tertiary allyl alcohols with alkenyl alkyl ethers
InactiveUS6034279AHigh yieldShort reaction timeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsArylDiol
PCT No. PCT / EP97 / 06425 Sec. 371 Date Jan. 29, 1999 Sec. 102(e) Date Jan. 29, 1999 PCT Filed Nov. 18, 1997 PCT Pub. No. WO98 / 23570 PCT Pub. Date Jun. 4, 1998An improved process is described for preparing gamma , delta -unsaturated ke-tones, which are in demand as aroma substances and intermediates for vitamins and carotenoids, by reacting tertiary allyl alcohols and alkenyl alkyl ethers in the presence of acid catalysts at elevated temperature, which comprises carrying out the reaction in the presence of a phosphorus derivative of the formula IV where A and B are each a branched or unbranched alkyl or alkoxy having from 1 to 10 carbons, a substituted or unsubstituted aryl, cycloalkyl, aryloxy or cycloaryloxy; A can additionally be -H or -OH or A and B together are a substituted or unsubstituted tetramethylene or pentamethylene or substituted or unsubstituted phenyl-1,2-diol or 1,1'-binaphthyl-2,2'-diol.
Owner:BASF AG
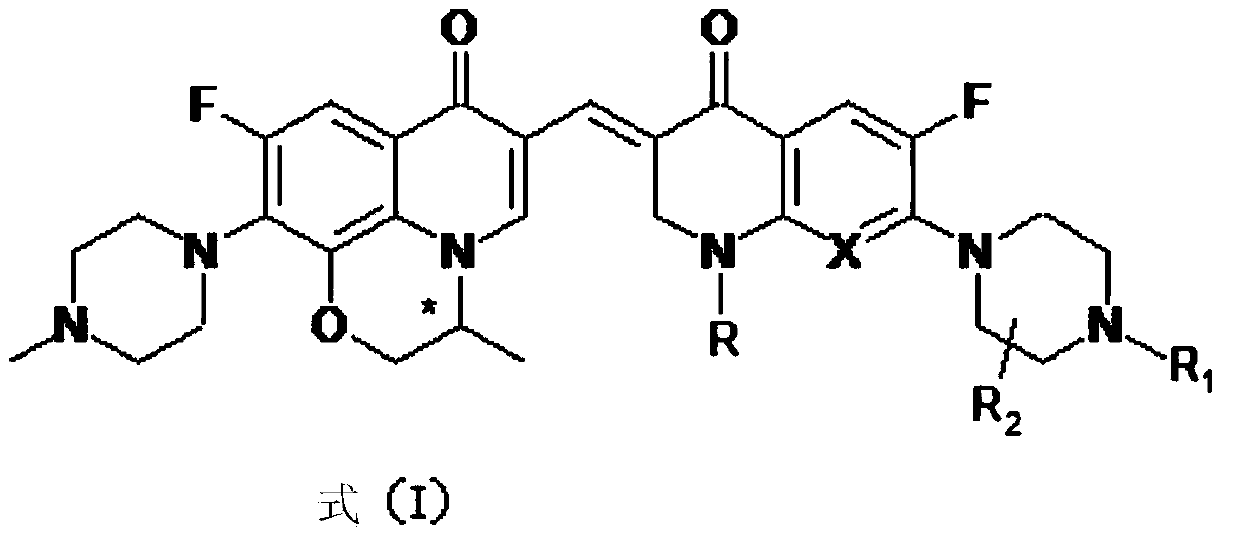
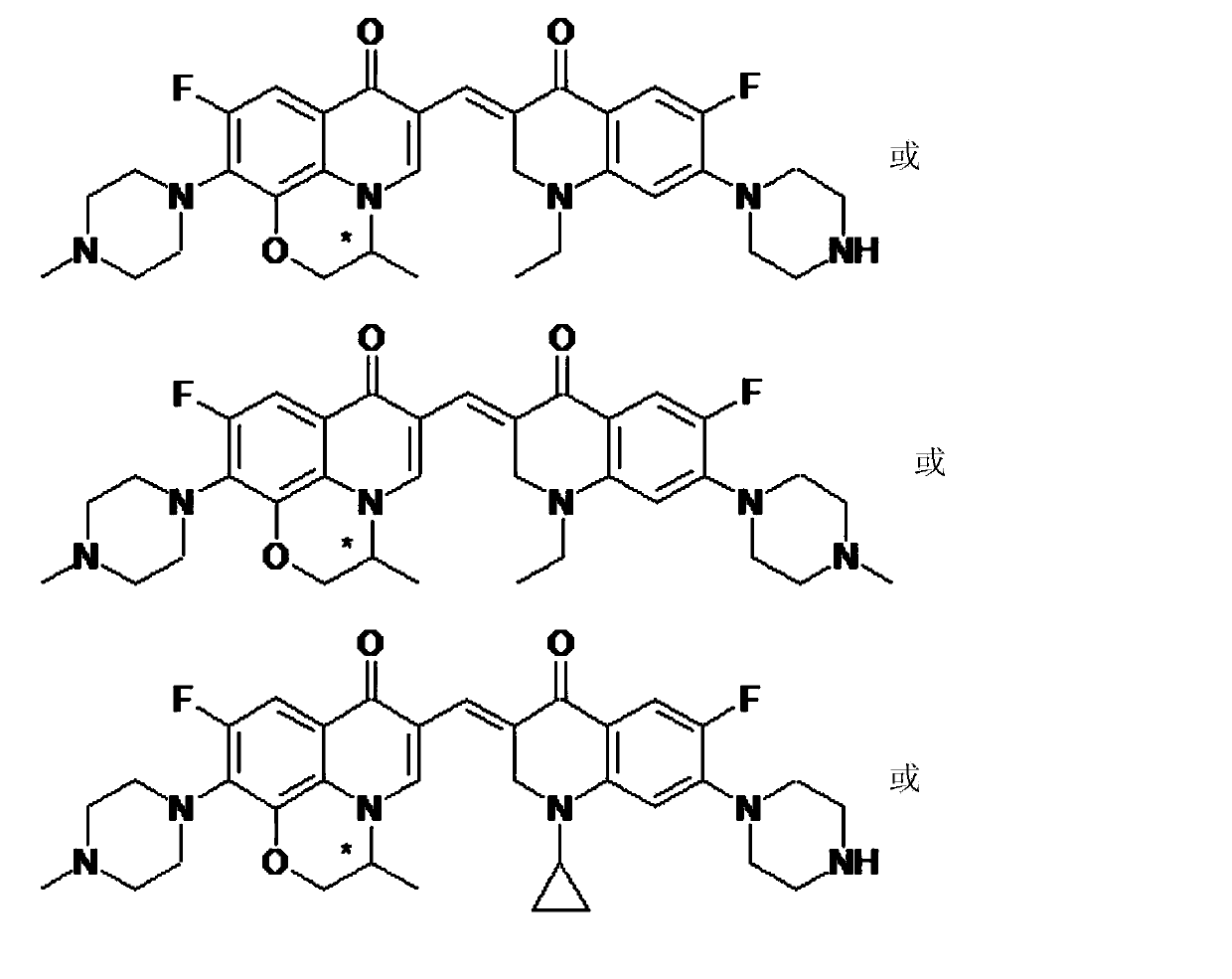
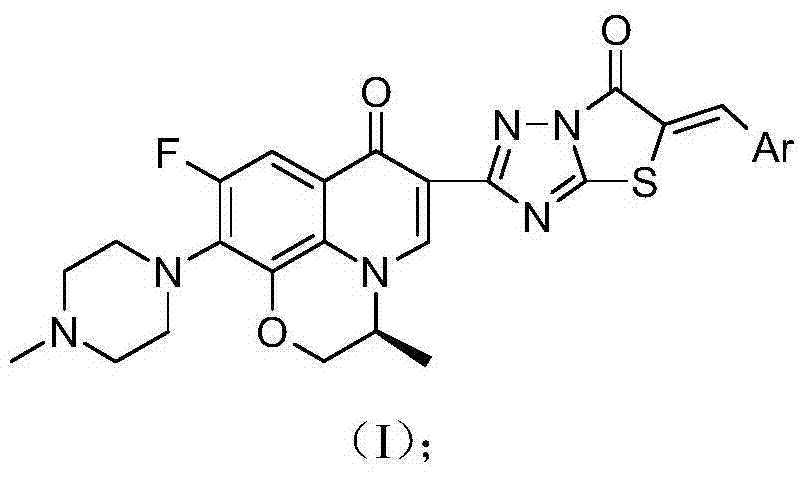
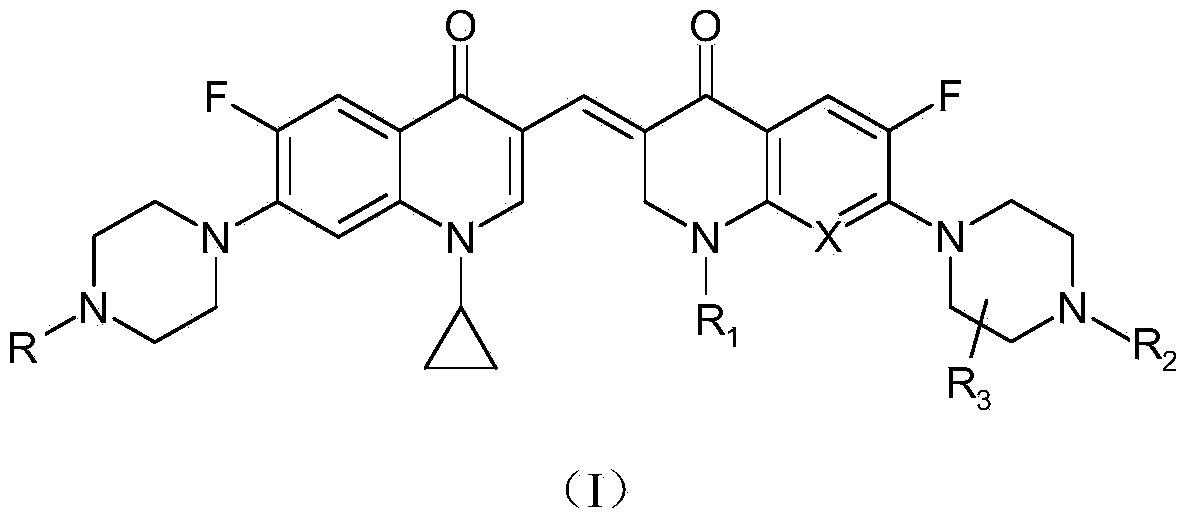
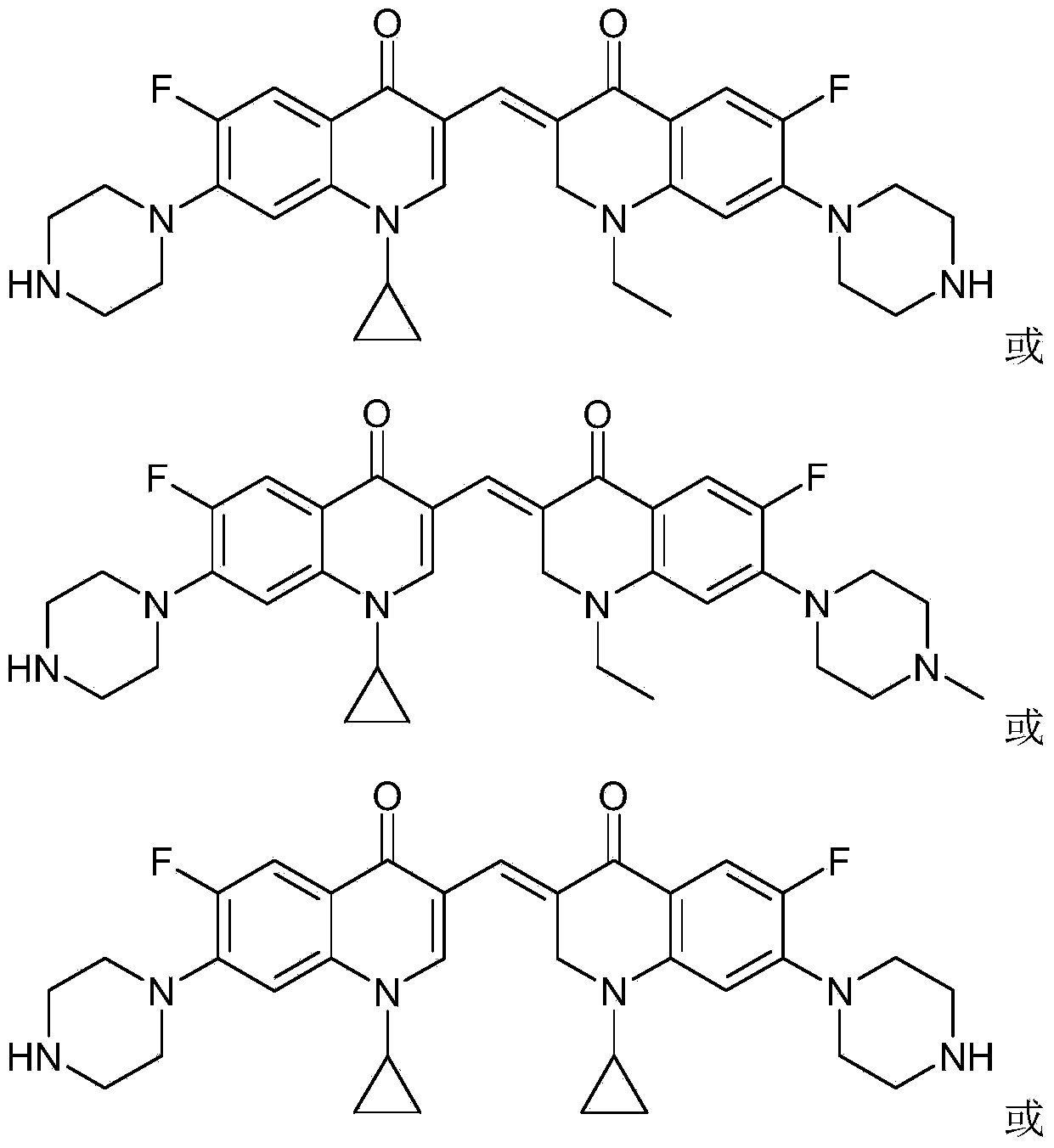
3,3'-methene-difluoroquinolone derivative of chiral oxazine quinoline ring as well as preparation method and application of 3,3'-methene-difluoroquinolone derivative
InactiveCN104557973AStrong complementarityTo achieve the effect of increasing efficiency and reducing toxicityOrganic active ingredientsOrganic chemistrySide effectPharmacophore
The invention discloses a 3,3'-methene-difluoroquinolone derivative of a chiral oxazine quinoline ring as well as a preparation method and application of the 3,3'-methene-difluoroquinolone derivative. The 3,3'-methene-difluoroquinolone derivative has a chemical general structural formula I shown in the specification, wherein R represents cyclopropyl or ethyl or fluoroethyl; R1 represents a hydrogen atom or methyl or ethyl; R2 represents a hydrogen atom or methyl; X represents a nitrogen atom or a hydrocarbon (CH) group or a fluoro-substituted carbon atom (F-C) or a methoxyl-substituted carbon atom (CH3O-C). The 3,3'-methene-difluoroquinolone derivative of the chiral oxazine quinoline ring, disclosed by the invention, can be used for realizing the superposition of a difluoroquinolone framework and alpha, beta-unsaturated ketone pharmacophores, so that the antitumor activity of a new compound is improved, the toxic and side effects on normal cells can be reduced, and the 3,3'-methene-difluoroquinolone derivative can be used as an antitumor active substance for developing an antitumor drug of a totally new structure.
Owner:HENAN UNIVERSITY
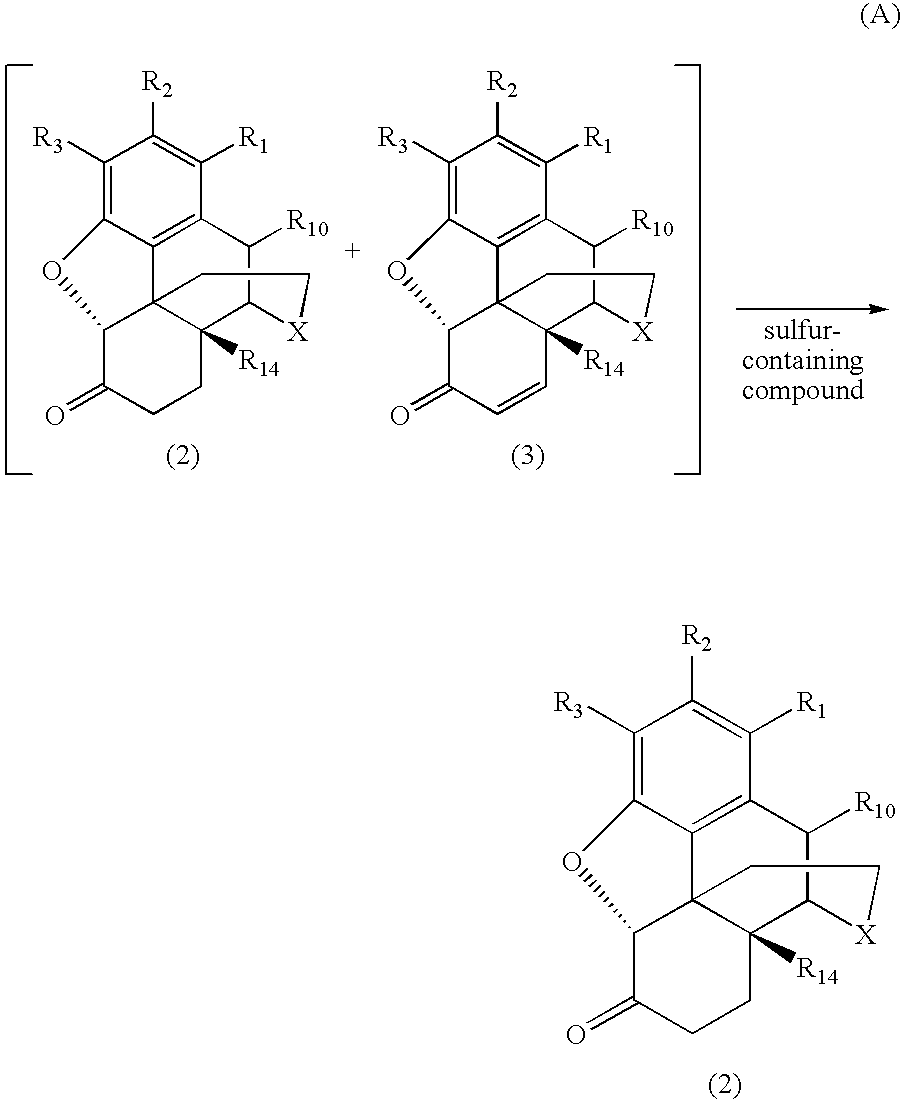
Process for Preparing Morphinan-6-One Products with Low Levels of Alpha, Beta-Unsaturated Ketone Compounds
The present invention generally relates to processes for preparing highly pure morphinan-6-one products. The processes involve reducing the concentration of α,β-unsaturated ketone compounds present as impurities in morphinan 6 one products or reaction mixtures including morphinan 6 one compounds by treatment with a sulfur-containing compound. (A)
Owner:SPECGX LLC
Condensation of aldehydes with ketones by multiphase reaction
InactiveUS6603047B2Improve responseHigh selectivityOrganic compound preparationPreparation by hydrogenationPhotochemistryUnsaturated ketone
Owner:OXENO OLEFINCHEMIE GMBH
Synthesis method for betaine-type amphoteric ion compound containing reactive group
InactiveCN103274955AEasy to synthesizeEasy to implementOrganic compound preparationSulfonic acids salts preparationBetaineCarboxylic acid
The invention discloses a synthesis method for a betaine-type amphoteric ion compound containing a reactive group. The synthesis method comprises two synthesis methods, namely, a synthesis method for a betaine-type amphoteric ion compound containing carbon-carbon double bonds, and a synthesis method for a betaine-type amphoteric ion compound containing hydroxyl group, wherein the synthesis method for the betaine-type amphoteric ion compound containing carbon-carbon double bonds comprises the step of reacting tertiary amine containing carbon-carbon double bonds and having a molar ratio of 1: 0.2 to 1: 1.5 with a carboxylic acid compound or a sulfonic acid compound containing an alpha, beta-unsaturated ketone structure for 1-120 hours at 0-100 DEG C in a condition of the presence of a first solvent and a polymerization inhibitor; and the synthesis method for the betaine-type amphoteric ion compound containing hydroxyl group comprises the step of reacting tertiary amine containing hydroxyl group and having a molar ratio of 1:0.5 to 1:2 with a carboxylic acid compound or a sulfonic acid compound containing an alpha, beta-unsaturated ketone structure for 1-120 hours at 0-100 DEG C in a condition of the presence of a second solvent. The synthesis method is simple, moderate in conditions, few in side reactions, high in product purity, simple in purification process, and high in yield, thus being capable of reducing the synthesis cost for the product.
Owner:SOUTH CHINA UNIV OF TECH
Method for synthesizing gamma, delta-nonsaturated ketones compound
ActiveCN106478514ANot easy to loseExtend your lifeOrganic compound preparationGroup 5/15 element organic compoundsAlcoholBoiling point
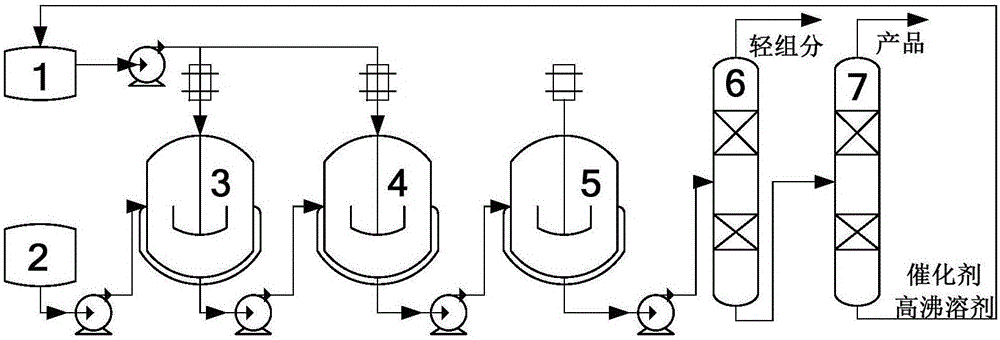
The invention discloses a method for synthesizing a gamma, delta-nonsaturated ketones compound. The method comprises the following steps: under protection of inert gas, nonsaturated alcohol and 2-alkyloxy propylene are taken as the raw materials, Bronsted acid functional ionic liquid is dissolved in a high boiling solvent as a catalyst, a rearrangement reaction is carried out, after the reaction is complete, the materials is subjected to post-treatment to obtain the gamma, delta-nonsaturated ketones compound and a catalyst solution. The used Bronsted acid functional ionic liquid catalyst has a plurality of physical and chemical properties which are similar with the conventional ionic liquid, the acid functional group is introduced, so that the catalyst has the advantages of high acid site density, adjustable acidity, uniform acidity distribution and difficult loss of acidity. An autoclave series mode is used for realizing the continuous synthesis of nonsaturated ketone and continuous recycling of the catalyst.
Owner:ZHEJIANG NHU CO LTD +2
Compounds for a controlled release of active molecules
Owner:FIRMENICH SA
Chiral fluoroquinolone C-3 fused heterocycle alpha, beta-unsaturated ketone derivative as well as preparation method and application thereof
InactiveCN104497014AImprove anti-tumor activitySmall toxicityOrganic active ingredientsOrganic chemistrySide effectThiazole
The invention discloses a chiral fluoroquinolone C-3 fused heterocycle alpha, beta-unsaturated ketone derivative as well as a preparation method and application thereof. The chiral fluoroquinolone C-3 fused heterocycle alpha, beta-unsaturated ketone derivative is a compound having the general structural formula (I), wherein Ar represents phenyl, substituted phenyl or pyridyl. According to the chiral fluoroquinolone C-3 fused heterocycle alpha, beta-unsaturated ketone derivative disclosed by the invention, three medicinal groups with different structural characteristics, including fluoroquinolone, a fused heterocycle of thiadiazole and s-triazole, and alpha, beta-unsaturated ketone are spliced to build a chiral fluoroquinolone-fused heterocycle-unsaturated ketone derivative and realize structural compensation and overlapping of the three medicinal groups, including the fluoroquinolone, thiazole [3,2-b][1,2,4] triazole and the alpha, beta-unsaturated ketone, so that the antitumor activity of a new compound is improved, toxic and side effects on normal cells are reduced, and the chiral fluoroquinolone C-3 fused heterocycle alpha, beta-unsaturated ketone derivative can be used as an antitumor active substance for developing an antitumor drug with a totally new structure.
Owner:HENAN UNIVERSITY
Production of unsaturated ketone
ActiveCN1817841AReduce usageNo recyclingOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholOrganic solvent
Production of unsaturated ketone is carried out by taking unsaturated alcohol and 2-alkoxy-propene as raw materials, taking acid ion as catalyst and reactive solvent, Saucy-Marbet reacting and synthesizing unsaturated ketone. It has better selectivity and recovery rate, non-volatility and no environmental pollution.
Owner:ZHEJIANG UNIV +1
Process for preparing saturated alcohols
A process for preparing saturated alcohols comprising effecting an aldol condensation of alkyl methyl ketones of 6 to 8 carbon atoms which are branched at the beta-carbon atom with aldehydes of 4 to 15 carbon atoms which are branched at the alpha-carbon atom to form alpha,beta-unsaturated ketones and subsequent hydrogenation of the alpha,beta-unsaturated ketones to obtain alcohols, wherein the aldol condensation is carried out at a temperature of 60 to 130° C. in the presence of a 30-55% strength aqueous solution of an alkali metal hydroxide resulting in very low by-product formation.
Owner:CELANESE CHEM EURO GMBH
Titanium-rich lamellar Ti-Si molecular sieve and compound method thereof
InactiveCN102689909AHigh titanium contentHigh-chain olefin epoxidation catalytic activityCrystalline aluminosilicate zeolitesMolecular sieveCycloalkene
The invention relates to a titanium-rich lamellar Ti-Si molecular sieve and a compound method thereof. The titanium-rich lamellar Ti-Si molecular sieve employs the main elements of silicon, titanium and oxygen, and the mol component of the sieve is xTiO2:SiO2 (x ranges from 0.001 to 0.02) in the form of oxide. The titanium-rich lamellar Ti-Si molecular sieve and the compound method have the advantages that the titanium content is large, and the titanium content can be increased selectively in a 10-membered ring channel in theTi-YNU-1 molecular sieve layer, thereby realizing not only favorable macrocycloalkene and unsaturated ketone ring oxidation catalysis function, but also higher chain-type cycloalkene oxidation catalysis activity.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
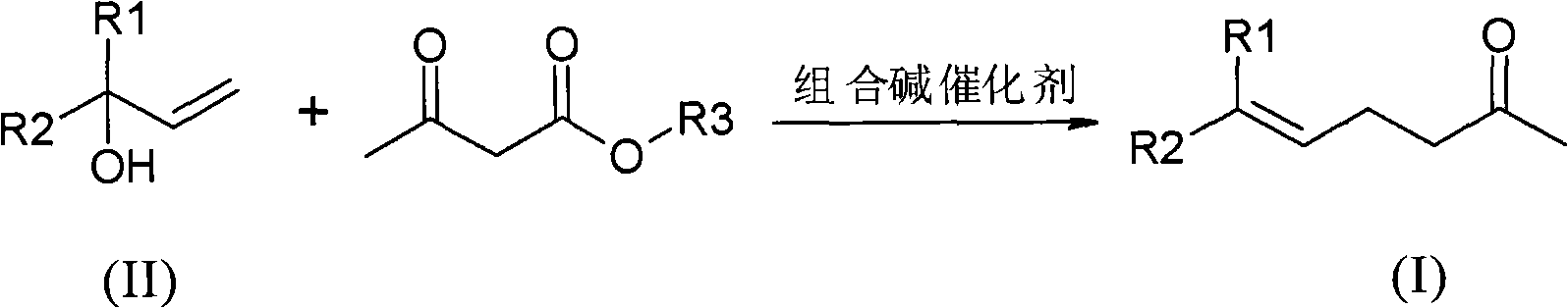
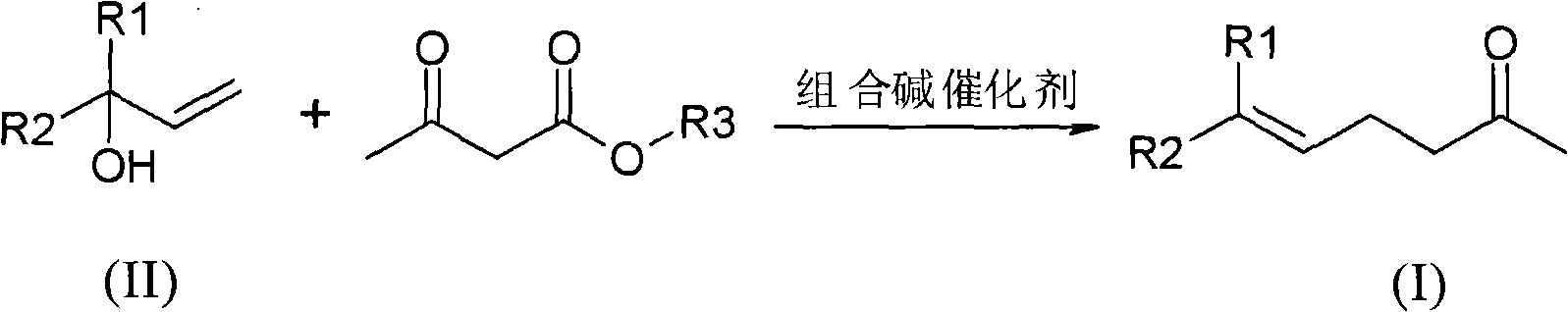
Method for preparing gamma and delta unsaturated ketone
ActiveCN102115437AEasy to recycleHigh yieldOrganic compound preparationCarbonyl compound preparationAlcoholAcetoacetic acid
The invention discloses a method for preparing gamma and delta unsaturated ketone, which comprises the steps of: leading unsaturated alcohol to have alkyl ester reaction with acetoacetic acid under the action of combined alkali catalyst, collecting products and obtaining the target product gamma and delta unsaturated ketone with high purity and high yield. The combined alkali catalyst adopted in the method is easily recovered and applied mechanically, the reaction selectivity is high, and the cost of raw materials is greatly reduced, so that the method is suitable for industrialized production.
Owner:SHANGHAI HEGNO PHARMA HLDG +1
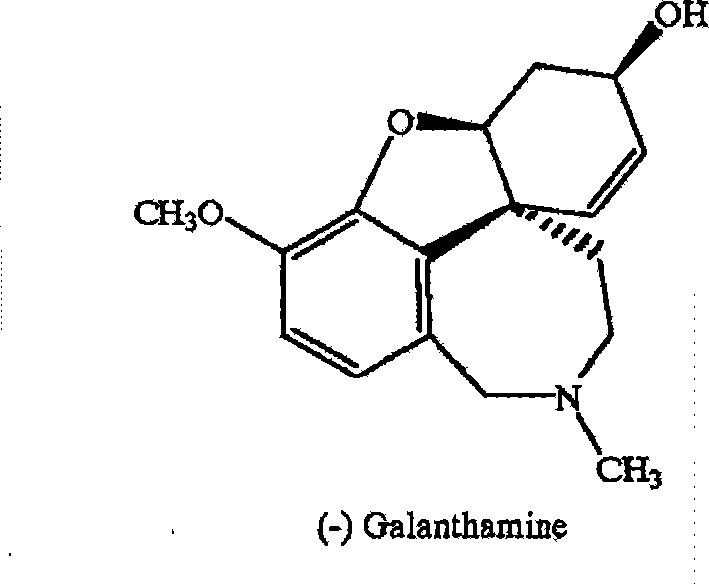
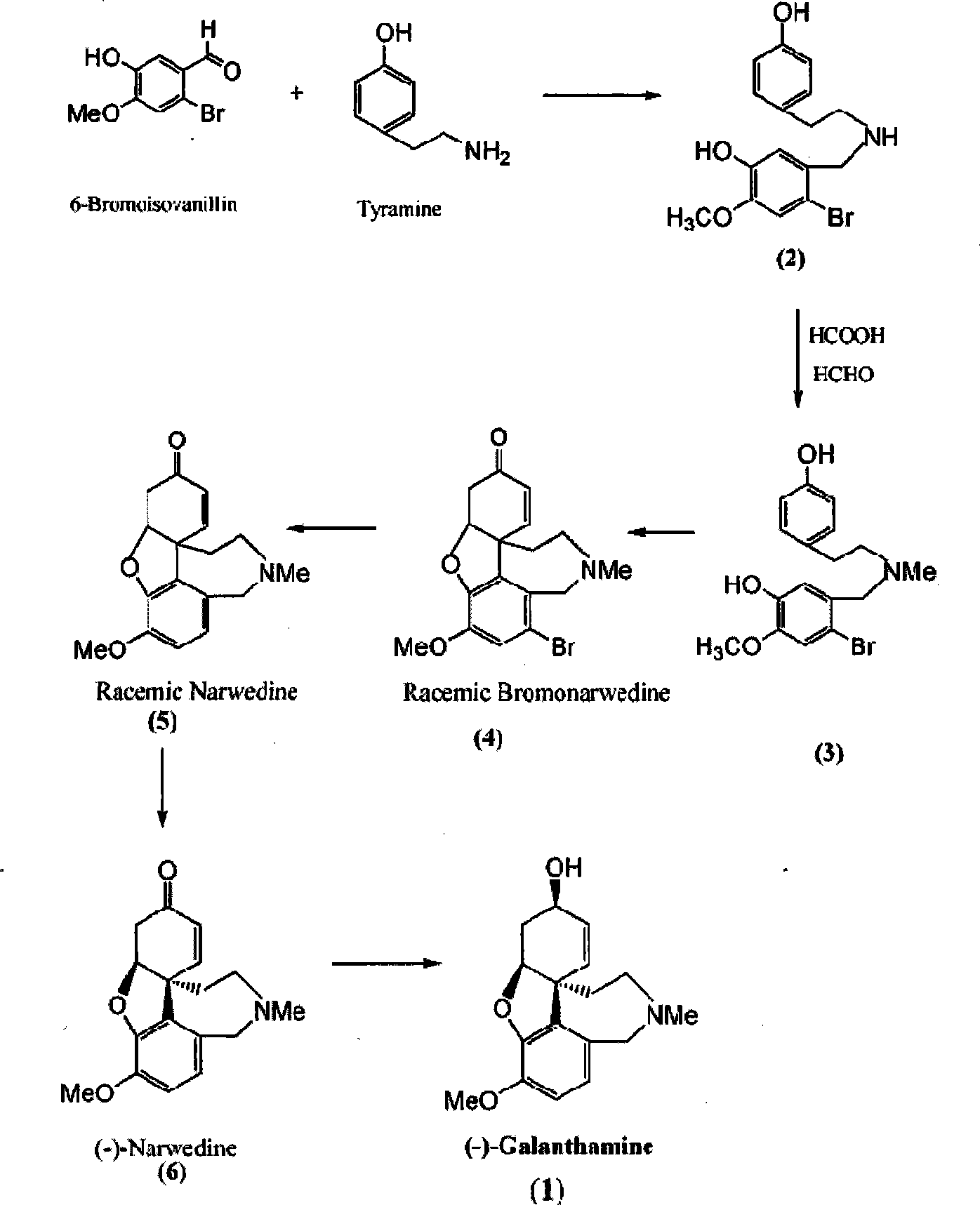
Chiral synthesis method for (-)-galantamin hydrobromide
InactiveCN101239983AFavorable manufacturing methodNervous disorderAsymmetric synthesesHydrobromideEphedrine
Ethamine compound is obtained by condensing starting materials of 6-bromoisovanillin and tyramine under catalysis of NaBH4 in carbinol. In formylation of ethamine compound, aminic acid and formaldehyde of same amount are used to produce formyl compound, with temperature between 90 DEG C. and 110 DEG C., 8h. Racemic bromonarwedine is obtain by oxidation and cyclization of the formyl compound. Racemic narwedine is obtained by reduction reaction under catalysis of NaCO2H, PPh3, Pd(OAc)2. Racemic narwedine is transformed into (-)-narwedine by a process of crystal inoculation. (-)-narwedine is reduced into unsaturated ketone by (-)-N-methyl ephedrine as chiral reagent, whereby optically active alcohol is obtained, and (-)-galanthamine is also obtained.
Owner:泰州市宝嵘新材料有限公司
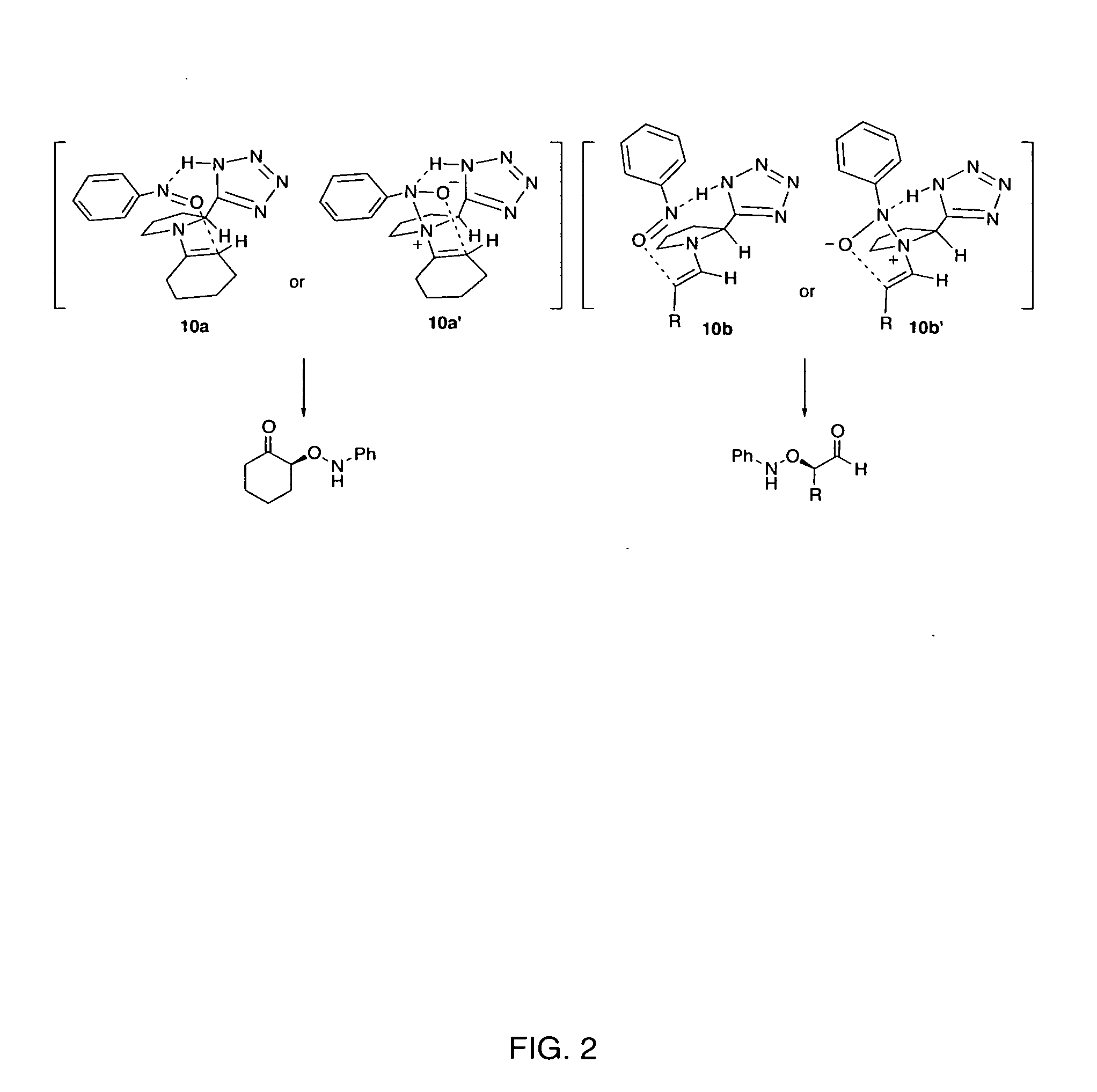
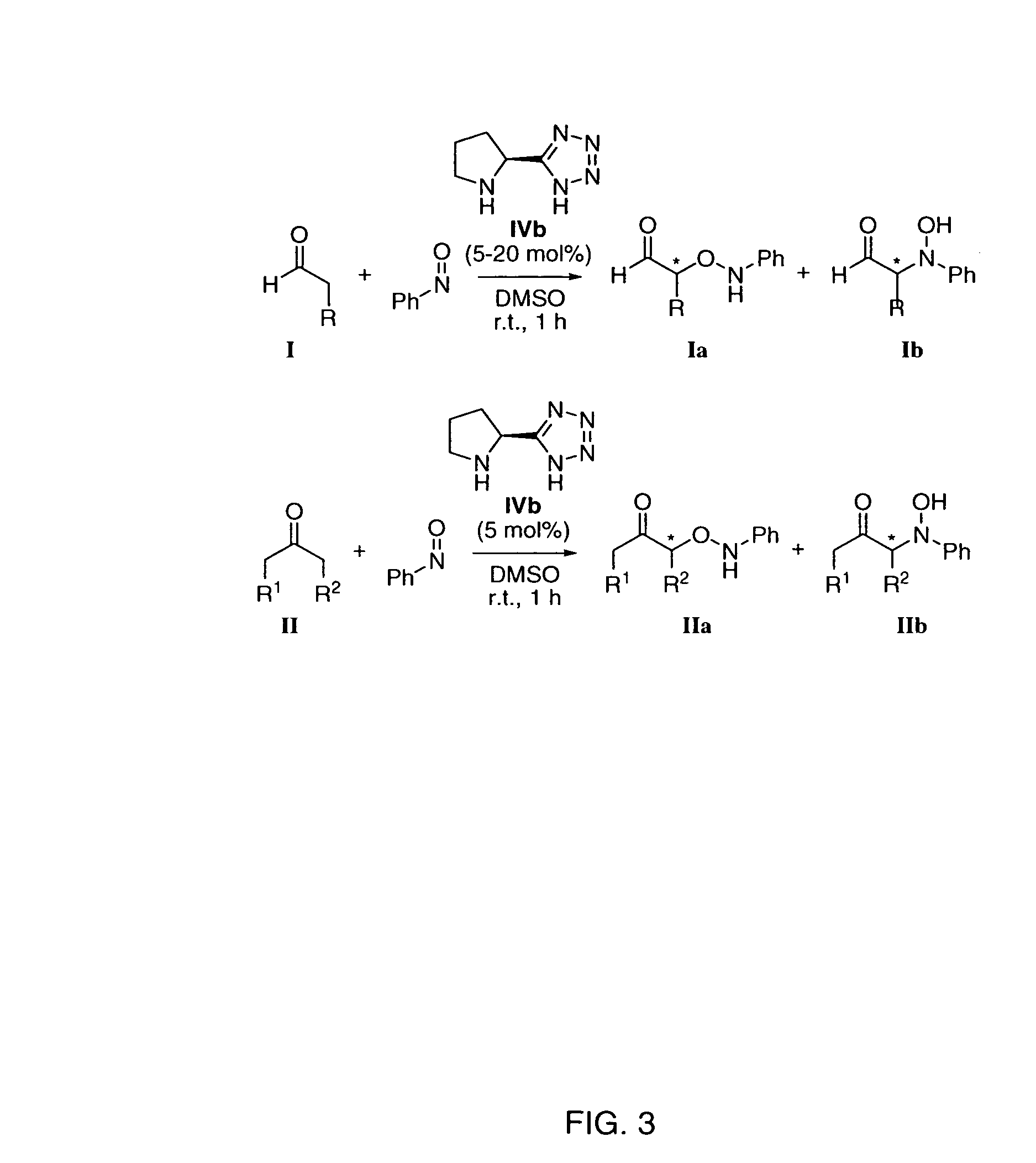
Process of making alpha-aminooxyketone/alpha-aminooxyaldehyde and alpha-hydroxyketone/alpha-hydroxyaldehyde compounds and a process making reaction products from cyclic alpha,beta-unsaturated ketone substrates and nitroso substrates
The present invention is directed to a process of making α-aminooxyketone and α-hydroxyketone compounds. The synthetic pathway generally involves reacting an aldehyde or ketone substrate and a nitroso substrate in the presence of a catalyst of the formula (IV): wherein Xa—Xc represent independently nitrogen, carbon, oxygen or sulfur and Z represents a 4 to 10-membered ring with or without a substituent and optionally a further step to convert the α-aminooxyketone compound formed to the α-hydroxyketone compound. The present invention results in α-aminooxyketone and α-hydroxyketone compounds with high enantioselectivity and high purity. The present invention is also directed to a catalytic asymmetric O-nitroso Aldol / Michael reaction. The substrates of this reaction are generally cyclic α,β-unsaturated ketone substrate and a nitroso substrate. This methodology generally involves reacting the cyclic α,β-unsaturated ketone substrate and the nitroso substrate in the presence of a proline-based catalyst, to provide a heterocyclic product.
Owner:JAPAN SCI & TECH CORP
Method for preparing optically active carbonyl compound
ActiveCN105541579AEasy to separate and purifyMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenDihydropyridine
The invention discloses a method for preparing an optically active carbonyl compound, which comprises the following step: under catalysis of a chiral amine salt and a transition metal catalyst, carrying out asymmetric catalytic hydrogenation reaction on alpha, beta-unsaturated aldehyde or alpha, beta-unsaturated ketone compound by taking hydrogen gas and a catalysis amount of dihydropyridine compound as a hydrogen source, so as to obtain the optically active carbonyl compound. The method has mild reaction conditions and simple operation, and the use amount of the dihydropyridine compound is the catalysis amount, so that a target product is easily separated and purified from a reaction system; and at the same time, the metal catalyst can be recycled, thereby meeting the economy requirement.
Owner:ZHEJIANG NHU CO LTD +2
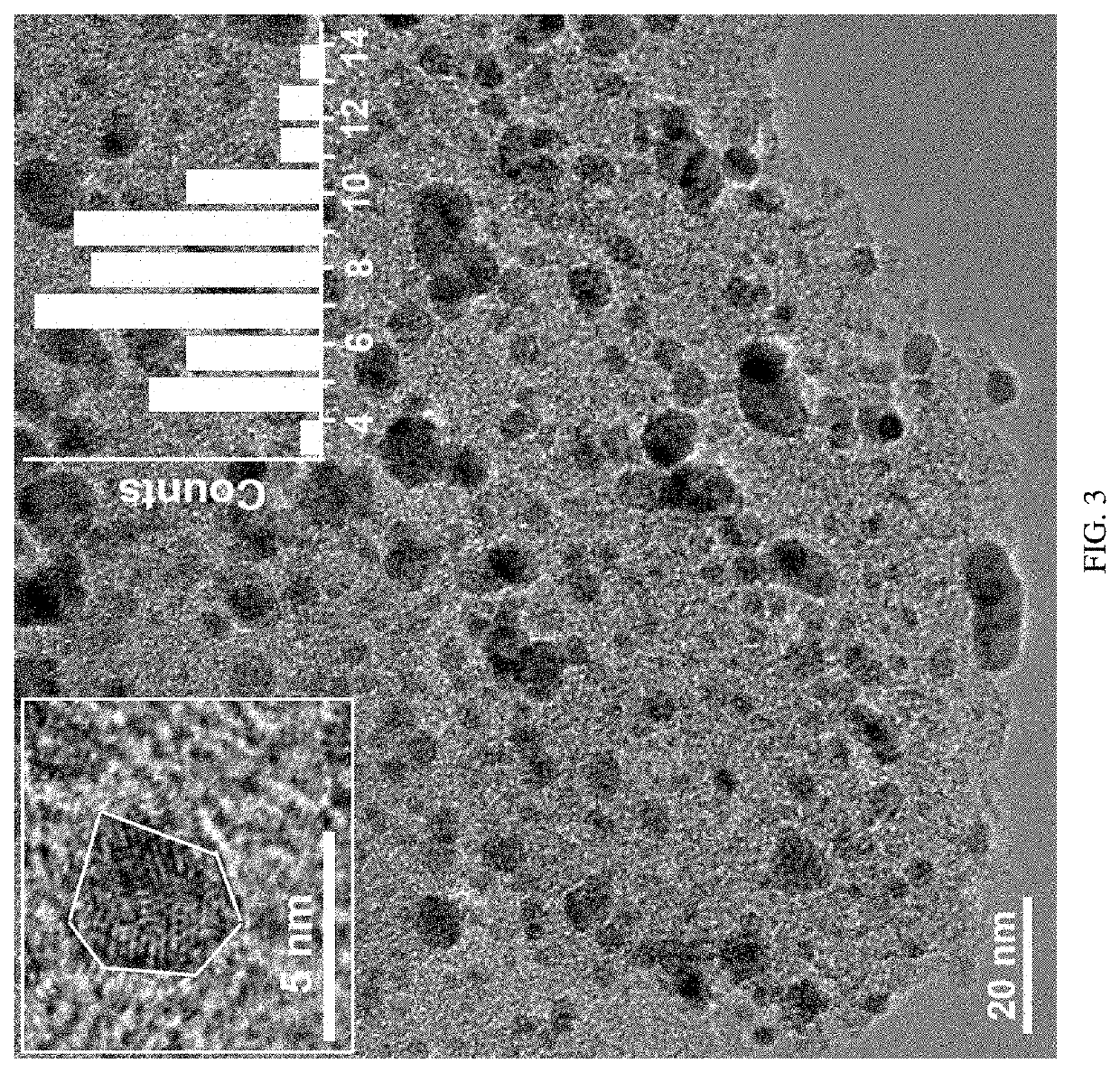
Preparation method of nitrogen-doped hierarchical-porous carbon-loaded nanometer pd catalyst and product and application thereof
ActiveUS20210121855A1Improve catalytic performanceStable rateOrganic compound preparationPreparation by dehydrogenationNano catalystPtru catalyst
Disclosed are a nitrogen-doped hierarchical-porous carbon-loaded nano-Pd catalyst and a preparation method thereof. The preparation method includes preparing nitrogen-doped hierarchical-porous carbon, mixing the nitrogen-doped hierarchical-porous carbon with water, adjusting a pH value of the mixed solution to be alkaline, mixing the mixed solution with a Pd metal precursor aqueous solution, and then adding a reducing agent to obtain the nitrogen-doped hierarchical-porous carbon-loaded nano-Pd catalyst after reduction. The prepared nitrogen-doped hierarchical-porous carbon-loaded nano-Pd catalyst includes a nitrogen-doped porous carbon material carrier with hierarchical pores and Pd metal nanoparticles loaded in the hierarchical pores of the carrier. The Pd metal nanoparticles have a size of 2˜14 nm and a regular polyhedron shape. The nitrogen-doped hierarchical-porous carbon-loaded nano-Pd catalyst has excellent catalytic performance, especially has ultra-high conversion rate, selectivity and cycle stability in the selective hydrogenation reaction of unsaturated ketones, and is a key to open a new synthetic route of vitamin E.
Owner:ZHEJIANG UNIV
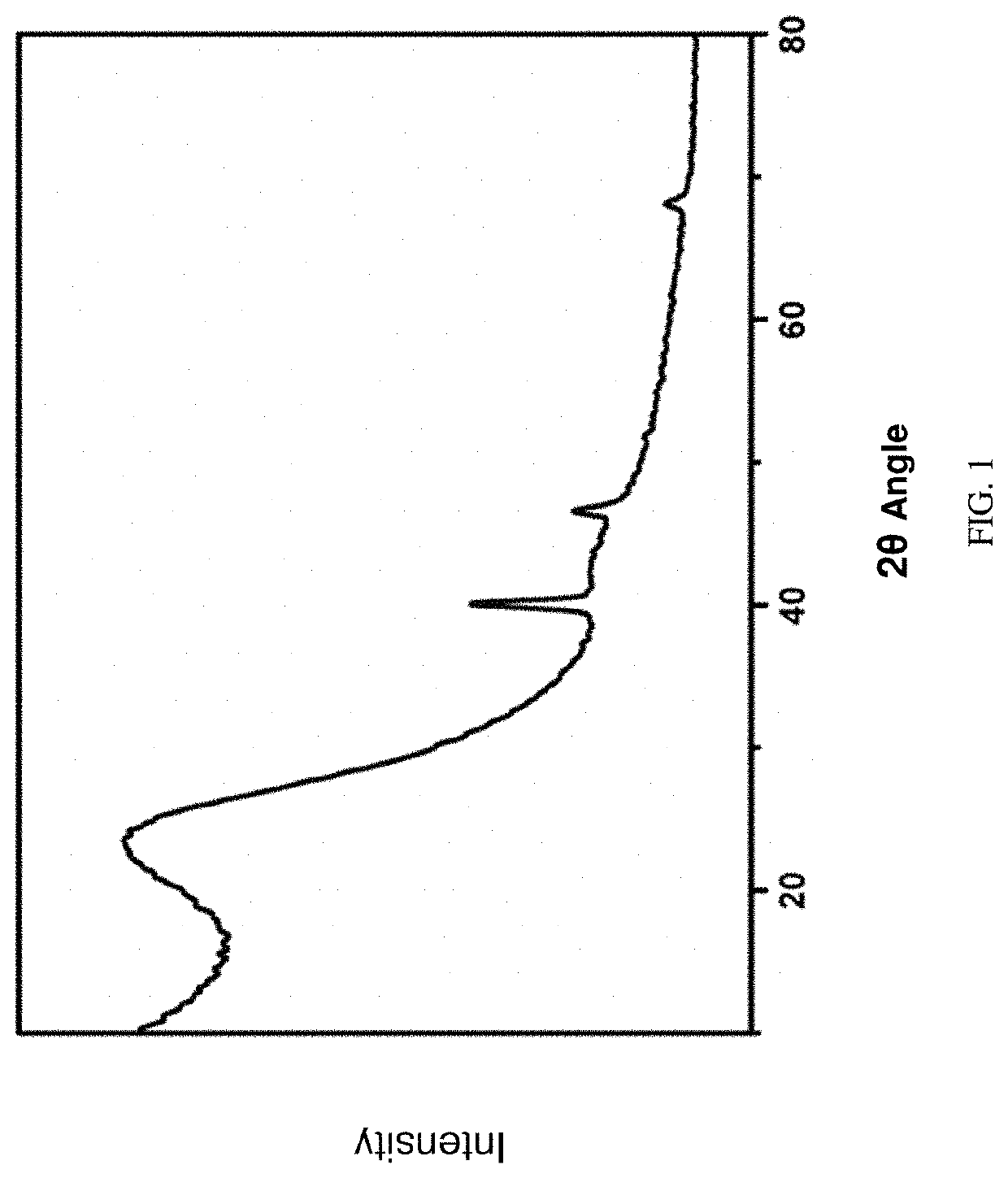
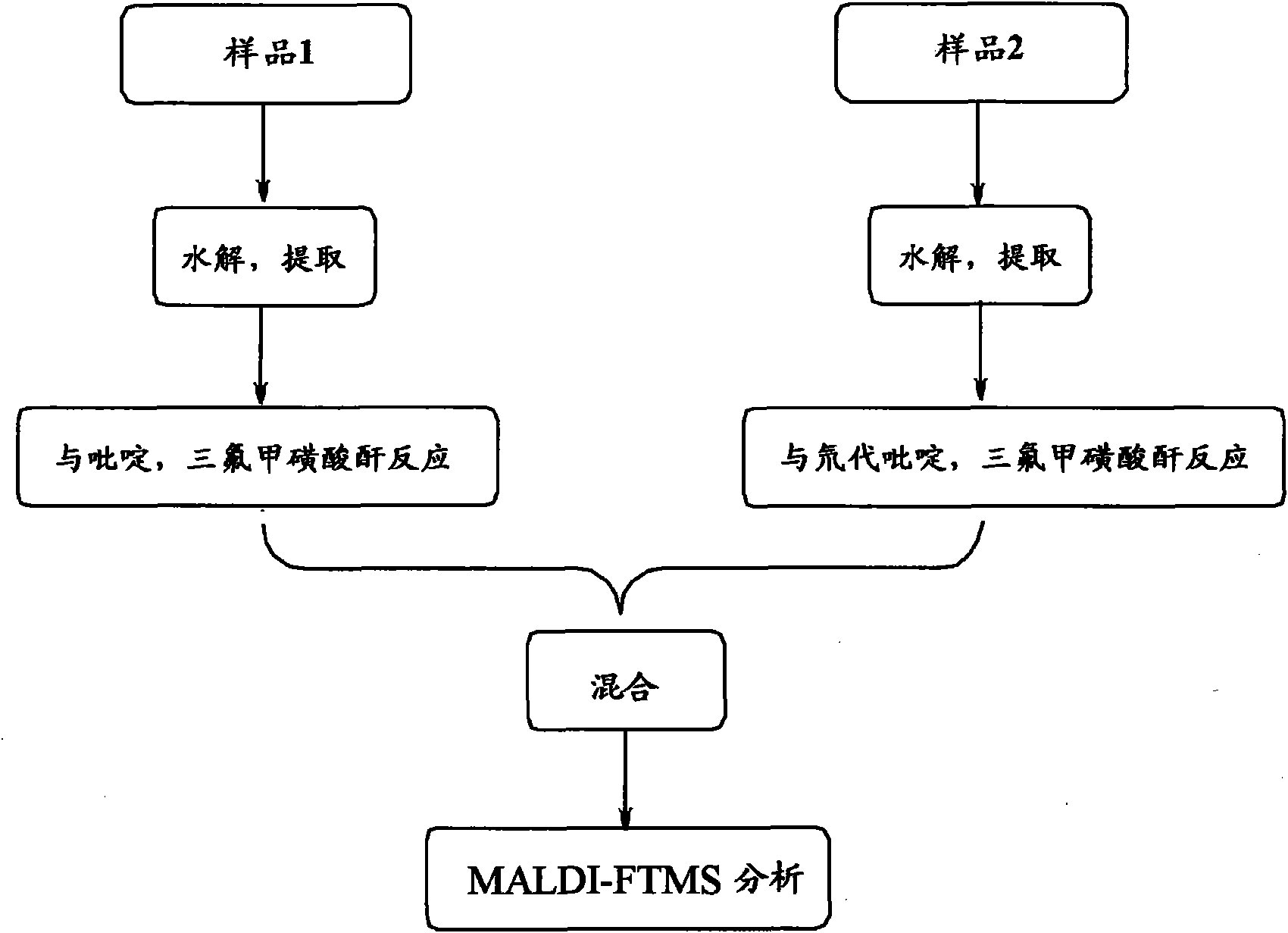
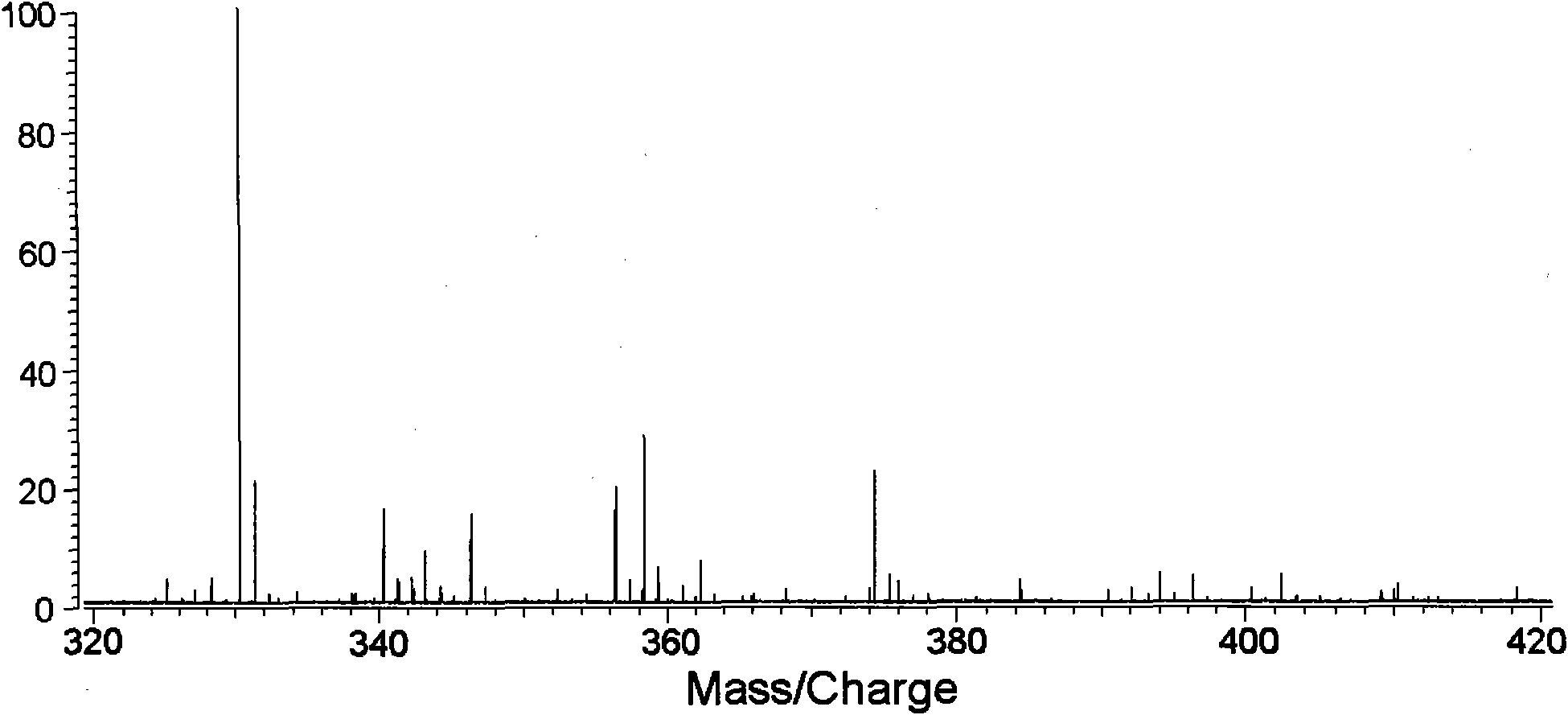
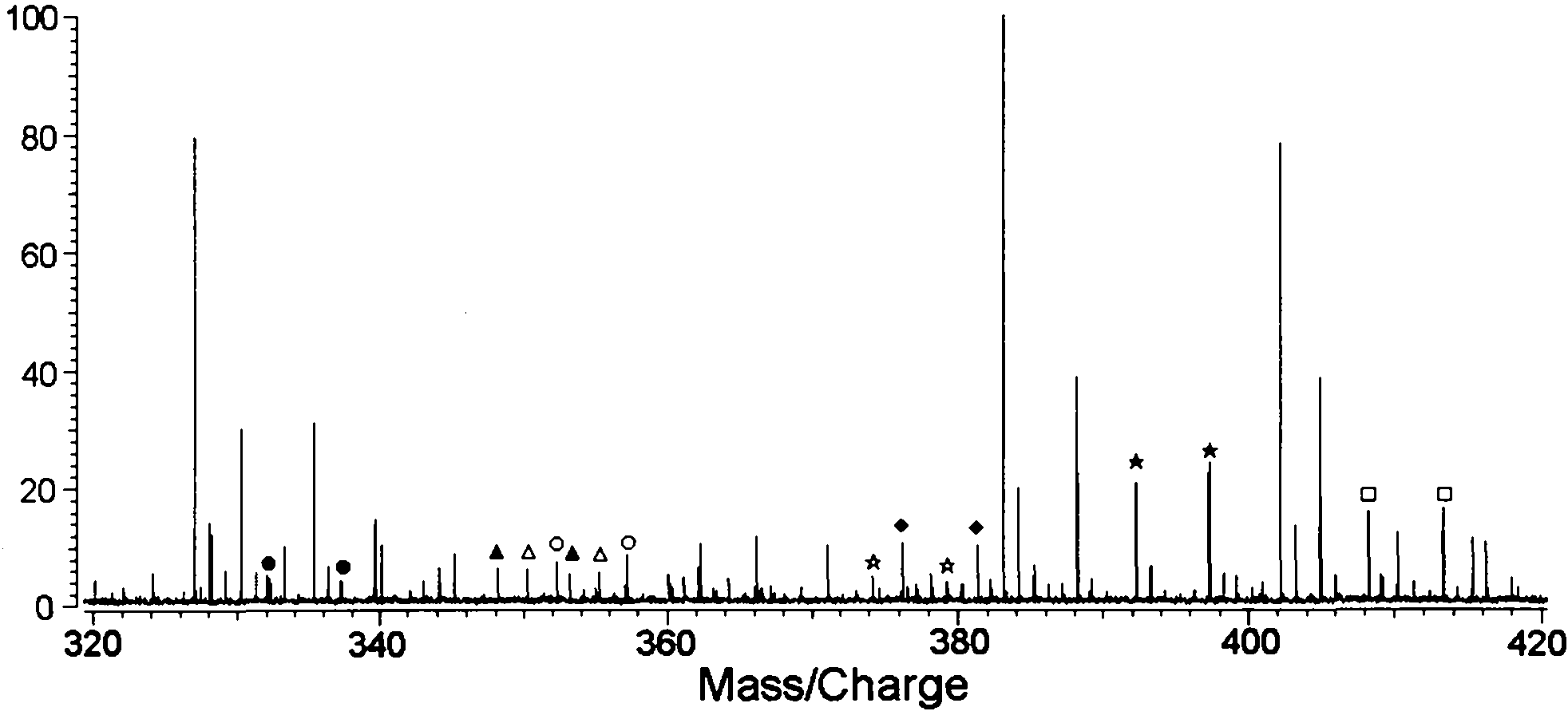
Method for analyzing biological samples by using matrix assisted laser desorption ionization-Fourier transform ion cyclotron resonance mass spectra
InactiveCN102175750AImprove MS signalReduce complexityPreparing sample for investigationMaterial analysis by electric/magnetic meansIsotopeDerivatization
The invention relates to a method for analyzing biological samples by using matrix assisted laser desorption ionization-Fourier transform ion cyclotron resonance mass spectra (MALDI-FTMS), which is a method for analyzing alcohol compounds or / and alpha,beta-unsaturated ketone compounds in hair and urine samples qualitatively and quantitatively by combining a N-alkyl pyridine isotope derivatization method with the MALDI-FTMS. The method comprises the following steps of: performing sample preprocessing on a target analyte; reacting alcoholic hydroxyl or alpha,beta-unsaturated ketone on the target analyte with pyridine or deuterated pyridine; and performing MALDI-FTMS analysis on the marked sample solution directly. The analytical method is quick, efficient and sensitive.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
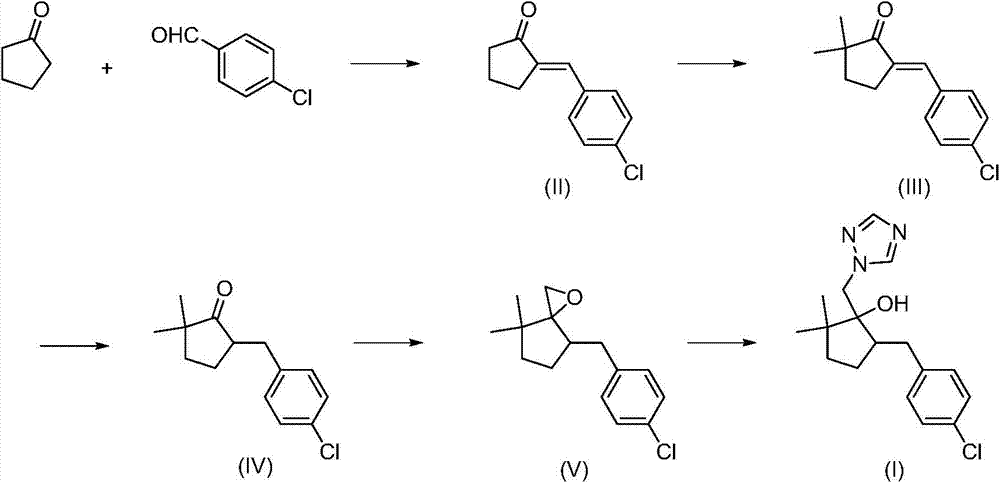
High purity metconazole and preparation method thereof
The invention discloses high purity metconazole and a preparation method thereof; the preparation is as follows: exocyclic double bond alpha, beta-unsaturated ketone (II) can be obtained by condensation reaction of cyclopentanone and p-chlorobenzaldehyde; then the exocyclic double bond alpha, beta-unsaturated ketone (II) is reacted with a methylation reagent to obtain alpha', alpha'-dimethyl substituted exocyclic double bond alpha, beta unsaturated ketone (III); then in the presence of a catalyst, the alpha', alpha'-dimethyl substituted exocyclic double bond alpha, beta unsaturated ketone (III) is reacted with hydrogen to obtain 2, 2-dimethyl-5-(4-chloro-benzyl) cyclopentanone (IV) with the double bond being reduced; an epoxypropane compound (V) is obtained by Johnson-Corey-Chaykovsky of the reduced product (IV); finally the epoxypropane compound (V) is reacted with 1, 2, 4 - triazole, and then the high purity metconazole (I) can be obtained by recrystallization. The preparing method has the advantages of chip and easy available raw materials, short route, good selectivity, high overall yield and good atom economy, and is very suitable for industrial production.
Owner:SHANGHAI JIAO TONG UNIV +1
Allylic oxidation method for cyclohexene derivative
ActiveCN101143810AReduce consumptionMetal/metal-oxides/metal-hydroxide catalystsKetenes preparationFood additiveOrganic solvent
The invention discloses a method of the allylic oxidation of a cyclohexenyl derivative. In organic solvent, the cyclohexenyl derivative reacts with vanadium compound and alkyl hydroperoxide at a temperature lower than 80 DEG C, so that Alpha, Beta-unsaturated carbonyl compound is composed. The invention has the advantages of simple method and high product yield, and the produced Alpha, Beta-unsaturated ketone can be widely applied to food additives, cosmetics, medicines, etc.
Owner:CHINA TOBACCO HUNAN INDAL CORP
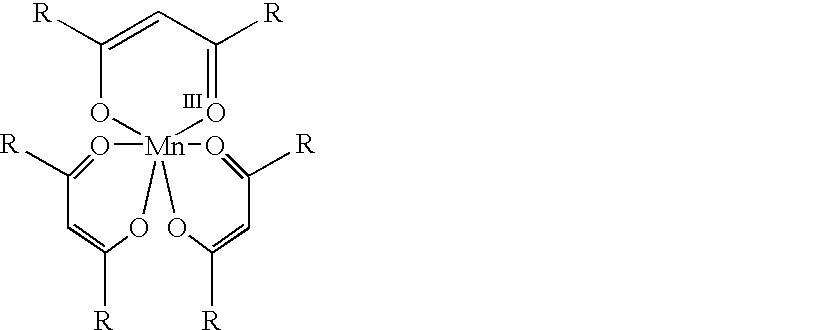
Conversion of alpha,beta-unsaturated ketones and alpha,beta-unsaturated esters into alpha-hydroxy ketones and alpha-hydroxy esters using Mn(III) catalyst, phenylsilane and dioxygen
InactiveUS20020120170A1Group 4/14 element organic compoundsOrganic compound preparationOxygenSide reaction
The present invention provides a novel process for the conversion of alpha,beta-unsaturated ketones. This invention is an improvement over existing processes in that it operates at neutral reaction conditions that prevent the formation of side reactions and that it is a single step, which proceeds with complete selectivity and gives a yield that is approximately 30% higher than the currently used processes. An example of this process is the conversion of 16-dehydroprogesterone into 17 alpha-hydroxyprogesterone.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
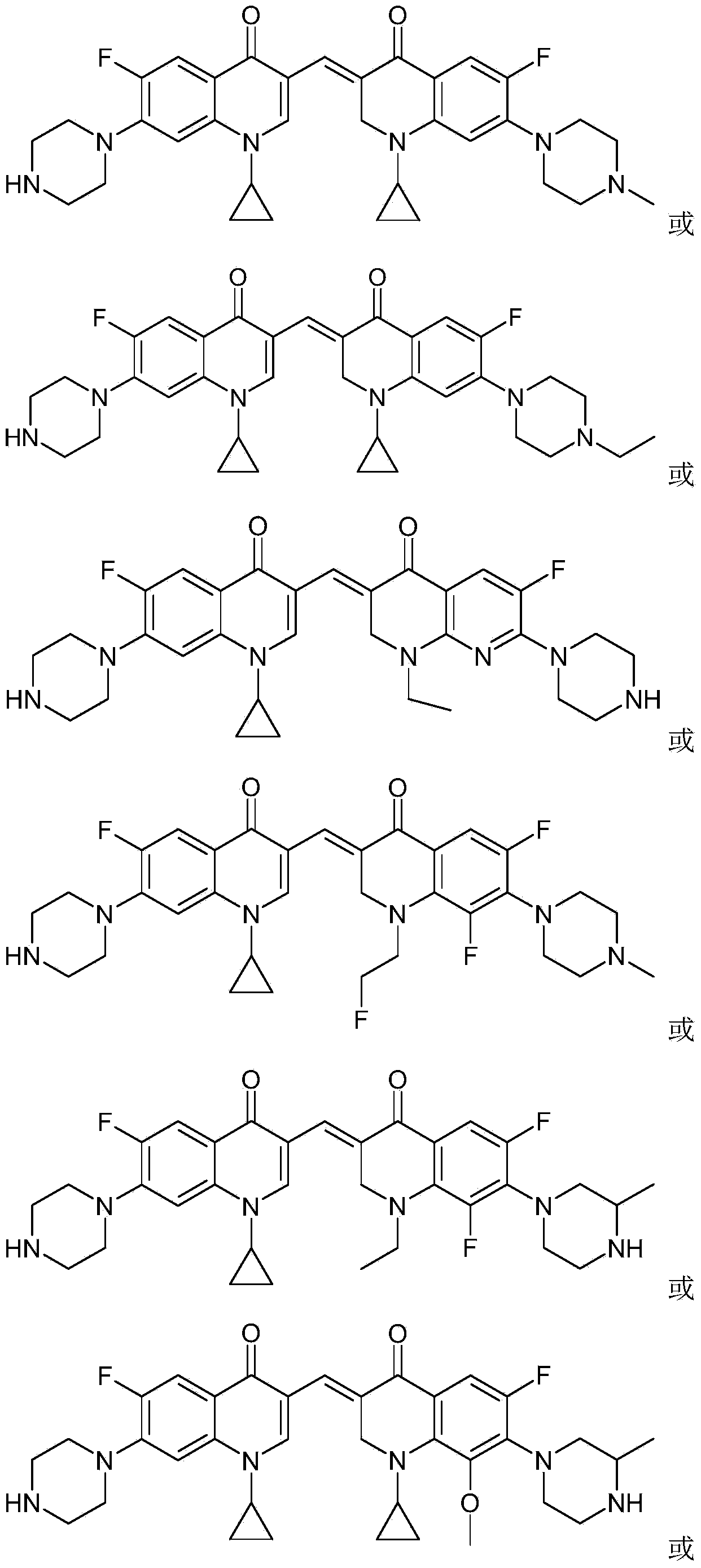
3,3'-methylene-bisfluoroquinolone derivative containing cyclopropylquinoline ring as well as preparation method and application of 3,3'-methylene-bisfluoroquinolone derivative
InactiveCN104370812ASmall toxicityAchieve overlayOrganic chemistryAntineoplastic agentsFluoroethylNormal cell
The invention discloses a 3,3'-methylene-bisfluoroquinolone derivative containing a cyclopropylquinoline ring as well as a preparation method and application of the 3,3'-methylene-bisfluoroquinolone derivative. The 3,3'-methylene-bisfluoroquinolone derivative containing the cyclopropylquinoline ring is a compound having a structural general formula as shown in the specification, wherein R is H, methyl or ethyl; R1 is ethyl, cyclopropyl or fluoroethyl; R2 is H, methyl or ethyl; R3 is H or methyl; and X is CH, N, F-C or CH3O-C. According to the 3,3'-methylene-bisfluoroquinolone derivative containing the cyclopropylquinoline ring, based on the combination principle of pharmacophores, the superposition of bisfluoroquinolone pharmacophore and alpha, beta-unsaturated ketone is achieved, and a 'fluoroquinolone chalcone' derivative is designed and synthesized; by the structural complementation, the anti-tumor activity is increased, the toxic or side effect on normal cells is decreased so as to achieve synergistic and toxicity-attenuation effects and the 3,3'-methylene-bisfluoroquinolone derivative can be used as an anti-tumor active substance to develop an anti-tumor drug having a new structure.
Owner:HENAN UNIVERSITY
Method for artificially synthesizing galanthamine
The invention discloses a method for artificially synthesizing galanthamine, which uses isovanillin and bromine as the raw materials. After the raw materials are subjected to substitution reaction, the reaction and industrial chemicla tyramine are subjected to amination and formylation to obtain formamide, the formamide is subjected to reaction to obtain a derivative of racemic narwedine, the derivative of racemic narwedine is reduced into racemic narwedine, N-methylephedrine is used to reduce unsaturated ketone and tartaric acid is used to carry out resolution to obtain levogyrate galanthamine. The method not only can conveniently and rapidly prepare the galanthamine, but also adopts artificial preparation so as to have little limination in the preparation process.
Owner:泰州市宝嵘新材料有限公司
Method of producing beta-mercaptocarboxylic acids
InactiveCN101801922AMercapto/sulfide group formation/introductionThiol preparationHydrogenSynthetic materials
The invention relates to a method for efficiently producing beta-mercaptocarboxylic acids using a solid acid catalyst such as zeolite, which product corresponds to respective starting materials selected from alpha, beta-unsaturated carboxylic acids (alpha, beta-unsaturated carboxylic acid, alpha, beta-unsaturated carboxylic acid ester, alpha, beta-unsaturated amide, alpha, beta-unsaturated aldehide and alpha, beta-unsaturated ketone) and hydrogen sulfides (hydrogen sulfide, sulfide salt and hydrosulfide salt), wherein a solvent compatible with water is used in the reaction. According to the invention, beta-mercaptocarboxylic acids which are useful as additives in synthetic materials for pharmaceutical or agricultural agents and in polymer compounds can be industrially produced efficiently by using easily available alpha, beta-unsaturated carboxylic acid (such as crotonic acid) at high yield.
Owner:RESONAC HOLDINGS CORPORATION
Selective hydrogenation catalyst for unsaturated ketone and preparation method and application thereof
InactiveCN108940346AAgglomeration formation preventionLarge specific surface areaPhysical/chemical process catalystsOrganic compound preparationExperimental methodsPorous carbon
The invention discloses a selective hydrogenation catalyst for unsaturated ketone. By taking a highly dispersed metal oxide compounded nitrogen doped porous carbon material as a carrier, noble metal particles are attached to the nitrogen doped porous carbon material, wherein the noble metal particles is 0.1-20% with respect to the mass content of the nitrogen doped porous carbon material. The invention also discloses a preparation method and an application of the catalyst. The metal oxide in the catalyst is uniformly distributed in and compactly combined with the nitrogen doped porous carbon material. The nano noble metal particles are dispersed highly. The experimental method is simple. The raw materials are very wide in source and high in sustainability, so that scaled production can beachieved. The catalyst shows excellent catalytic activity and stability in selective hydrogenation reaction of carbon-carbon double bonds of a key intermediate in a vitamin industrial chain.
Owner:ZHEJIANG UNIV
Compounds for a controlled release of active molecules
InactiveUS20090181878A1Desired utilityImprove and enhance and modify odorInorganic/elemental detergent compounding agentsCosmetic preparationsControl releaseThio-
The present invention relates to the field of perfumery. More particularly, it concerns compounds comprising at least one β-oxy or β-thio carbonyl moiety capable of liberating a perfuming molecule such as, for example, an α,β-unsaturated ketone, aldehyde or carboxylic ester. The present invention concerns also the use of the compounds in perfumery as well as the perfuming compositions or perfumed articles comprising the invention's compounds.
Owner:FIRMENICH SA
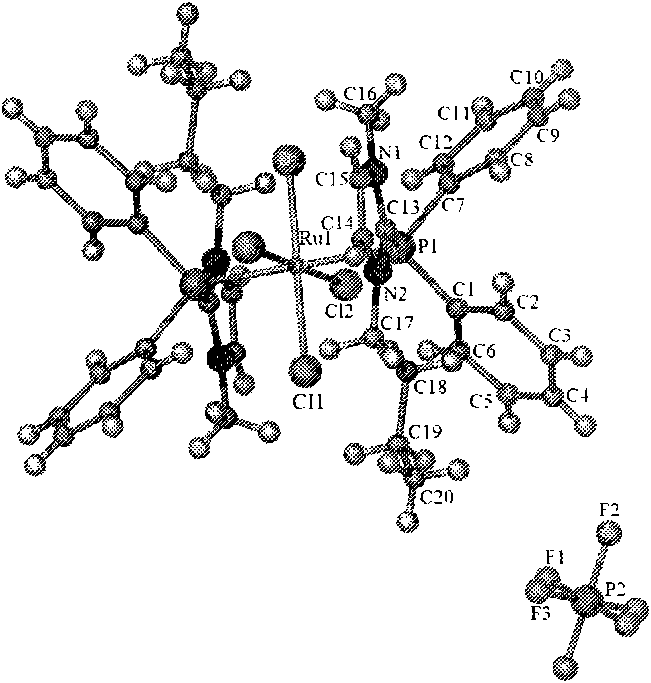
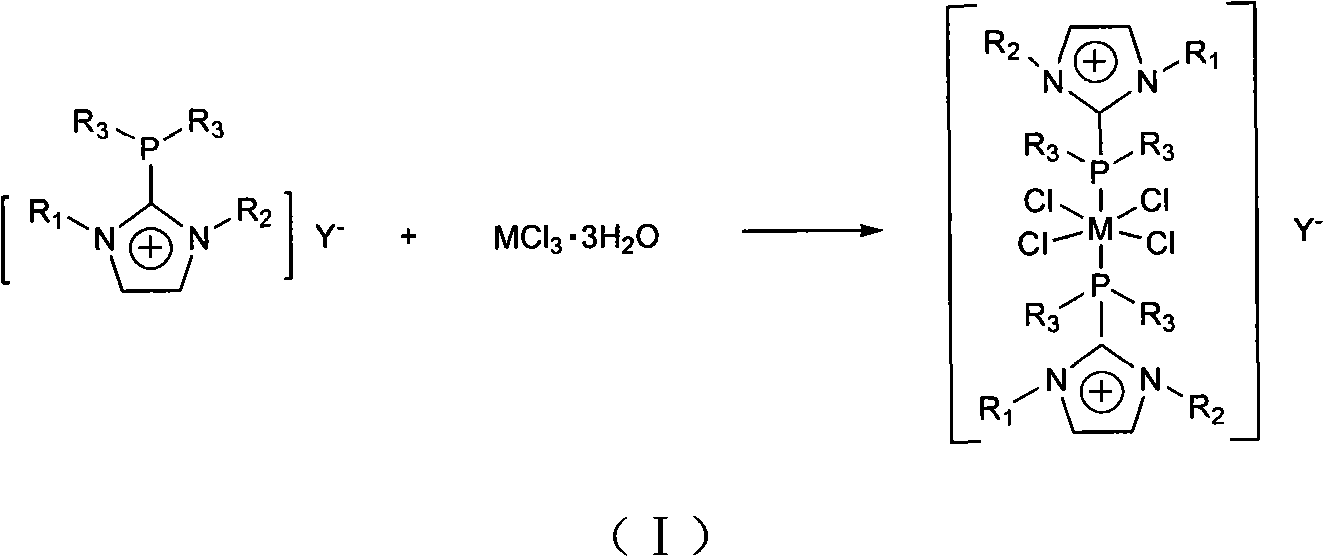
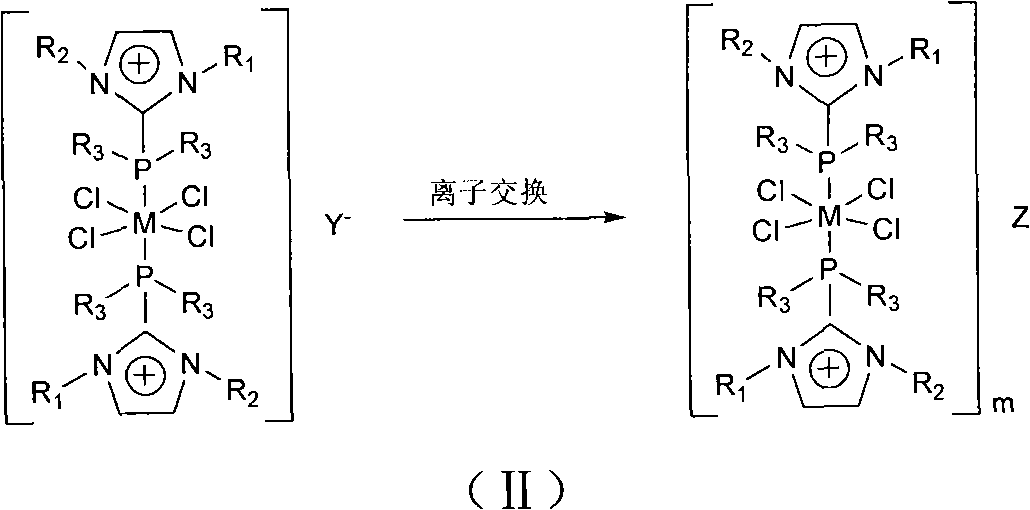
Preparation method and application of ruthenium and rhodium transition metal complex functional ionic liquid
The invention discloses a preparation method and application of ruthenium and rhodium transition metal complex functional ionic liquid. In the method, phosphine ligand functional ionic liquid and chloride hydrate of ruthenium or rhodium are taken as raw materials to be synthesized into corresponding ruthenium or rhodium complex functional ionic liquid. The prepared ruthenium or rhodium complex functional ionic liquid is an ionic compound and comprises two parts, namely a cation part and an anion part; ruthenium or rhodium central coordination atoms are positioned in a cation area; the anion can be replaced by other types of anions through an ionic liquid exchange reaction according to the need, while the structure of the cation is kept invariable; and the prepared ruthenium or rhodium complex functional ionic liquid is high in stability and insensitive to air and moisture. The prepared ruthenium or rhodium complex functional ionic liquid serves as a catalyst to be applied to homogeneous-phase hydrogenation reaction of simple olefins, aromatic hydrocarbons, aryl-substituted olefins, acrylate, aldehydes, ketones, unsaturated aldehydes, unsaturated ketones or ketones or aldehydes with different substituents so as to prepare corresponding high-value-added chemicals.
Owner:EAST CHINA NORMAL UNIV
Lysosomal targeting fluorescent probe and preparation method thereof
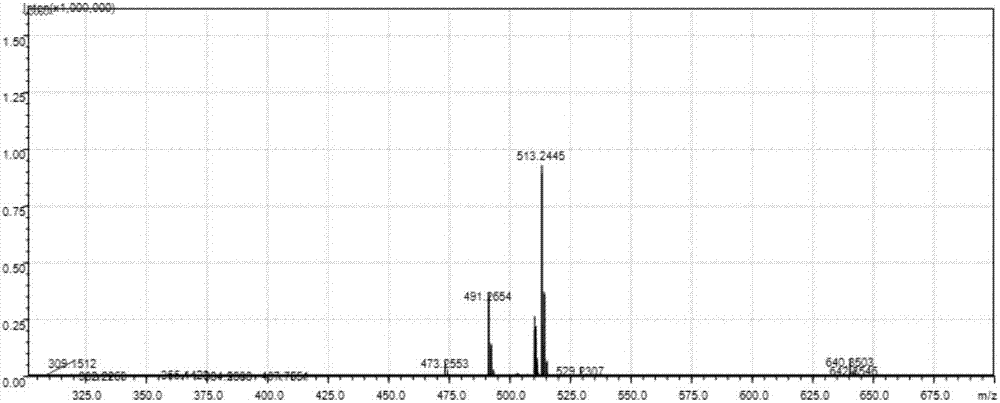
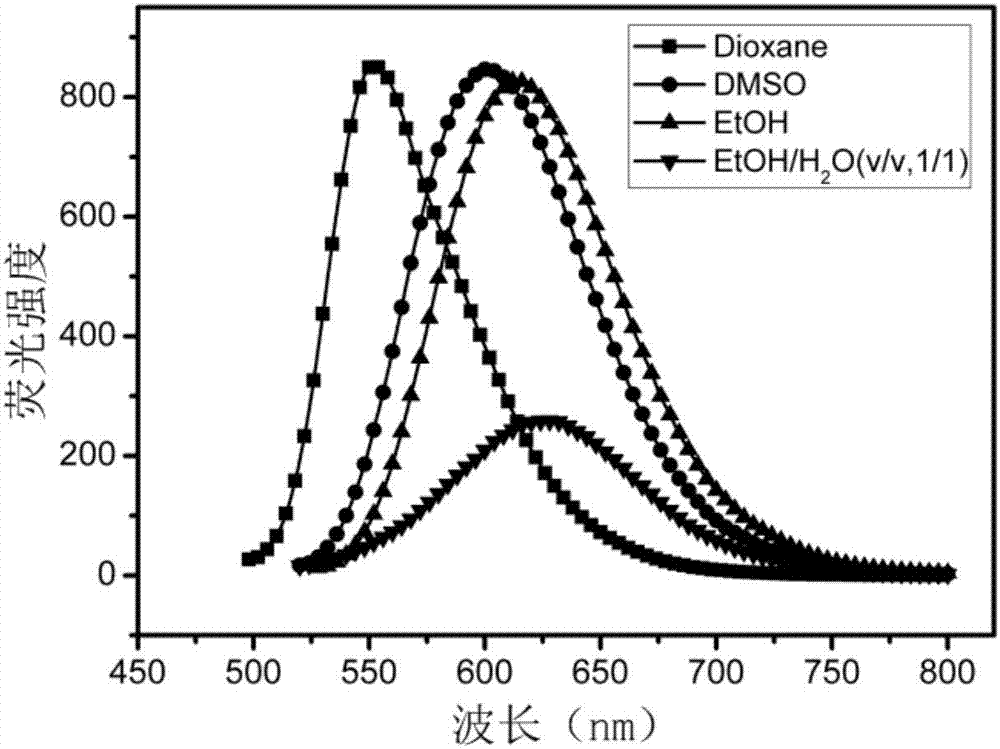
ActiveCN107226783AAchieve targeted markersReduce distractionsOrganic compound preparationCarboxylic acid amides preparationLysosomal targetingRed fluorescence
The invention discloses a lysosomal targeting fluorescent probe and a preparation method thereof. A D-Pi-A-Pi-D (donor-Pi-receptor-Pi-donor)-type large Pi conjugated system is constructed by alpha, beta-unsaturated ketone as a receptor. Through intramolecular charge transfer, compared with the existing many fluorescent probes for lysosome, lysosomal targeting fluorescent probe can realize large red shift of the excitation wavelength and emission wavelength of the molecule produce so that background signal interference is effectively reduced. The compound provided by the invention is used for fluorescence imaging of lysosome. In the cell environment, red fluorescence can be emitted under acidic conditions, and the maximum emission wavelength is about 625nm so that the lysosome targeting marker is obtained and background interference is small. The preparation method is sensitive, simple and quick.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Alpha,beta-unsaturated ketone or arone environment-friendly synthesis method
ActiveCN1970523AReduce dosageStop pollutionOrganic compound preparationCarbonyl group formation/introductionImideBenzoyl peroxide
The invention discloses a green synthesizing method of alpha, beta-unsaturated ketone or aromatic ketone, which is characterized by the following: adopting relative unsaturated hydrocarbons or aromatic hydrocarbons as raw material; setting imide as catalyst; making azobisisobutyronitrile (AIBN) or benzoyl peroxide (BPO) as initiator; oxidizing in the organic solvent; dehydrating through dehydrant; separating and purifying to obtain the alpha, beta-unsaturated ketone; improving reacting receiving rate; reducing manufacturing cost; possessing mild reacting condition; using little catalyst; avoiding pollution.
Owner:ZHEJIANG UNIV OF TECH +1
Alpha, beta-unsaturated ketone compound and its prepn process and GST-Pi inhibiting activity
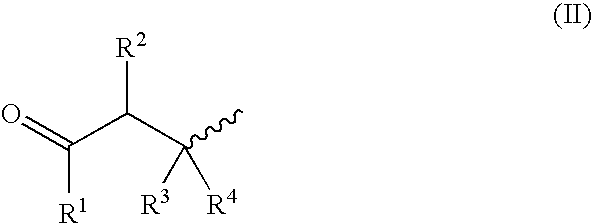
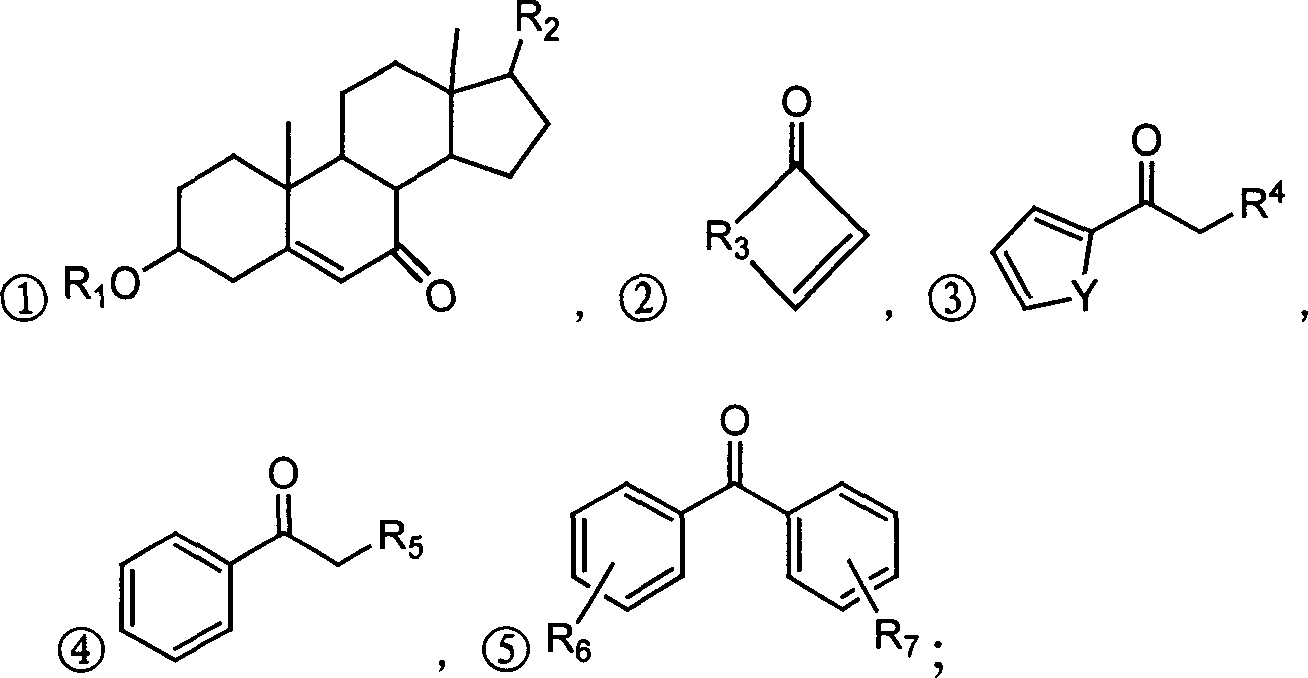
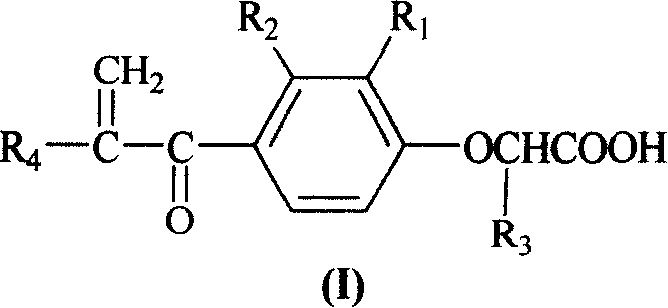
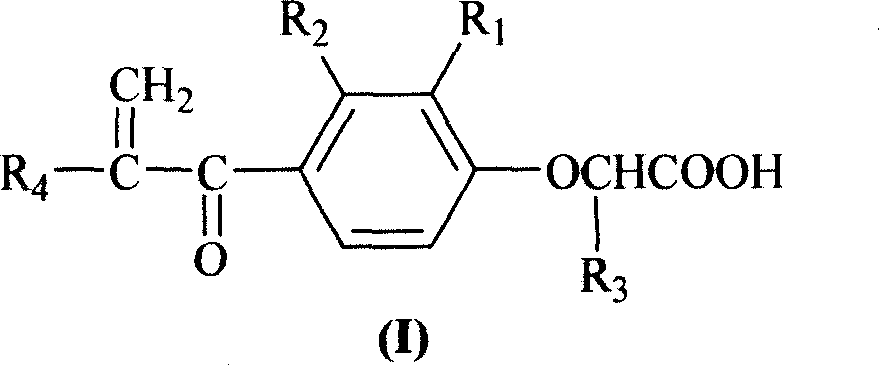
InactiveCN1706789AEasy to prepareRaw materials are easy to getOrganic chemistryCarboxylic acidDrug resistance
The present invention relates to alpha, beta-unsaturated ketone compound shown in general expression (I) and its preparation process. The preparation process includes the reaction between substituted phenol as material and 2-chlorocarboxylic acid to obtain 2-(2, 3-substituent phenoxy) carboxylic acid; the subsequent Friedel-Crafts reaction with acyl chloride to obtain 2-[2, 3-substituent-4-(1-oxy-substituent)phenoxy] carboxylic acid; and further reaction with formaldehyde to obtain 2-[2, 3-substituent-4-(2-methylene-1-oxy-substituent)phenoxy] carboxylic acid. The present invention also relates to the application of the compound as tumor drug resistance reversing agent.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com