Method of producing beta-mercaptocarboxylic acids
A technology of mercaptocarboxylic acid and compound, which is applied in the field of preparation of β-mercaptocarboxylic acid compounds, can solve the problems of cheap preparation process, low productivity, long reaction time, etc., and achieve the effects of simplified purification process, high productivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
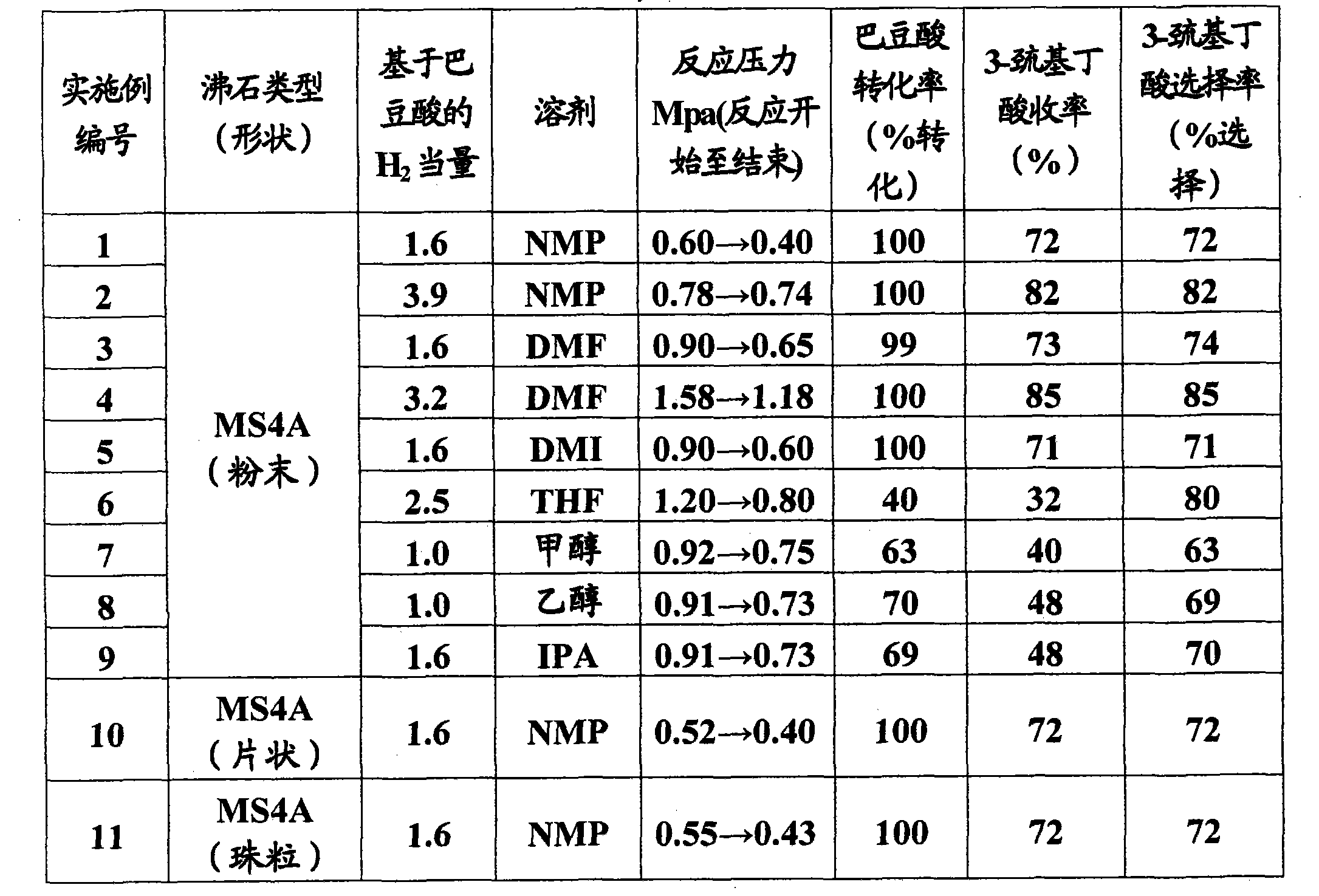
[0125] N-Methylpyrrolidone (NMP) (313 g, produced by Junsei Chemical Co., Ltd.) was charged in a 500 ml volume autoclave made of HASTELLOY C (registered trademark), and crotonic acid (25.5 g, 0.3 mol, Tokyo Chemical Industry Co., Ltd.) and 4A molecular sieve (MS4A) (24 g, powder, produced by Junsei Chemical Co., Ltd.) as a solid acid catalyst. Hydrogen sulfide (16.1 g, 0.5 mol, 1.6 equivalents based on crotonic acid, manufactured by Sumitomo Seika Chemicals Co., Ltd.) was absorbed into the mixture while maintaining the temperature in the range of 2 to 7°C. The temperature was then raised to 100°C and held at this temperature for 5 hours. The autoclave was cooled to 25°C and the solution therein was sampled. The sample was analyzed by HPLC, and it was confirmed that the generated reaction product was 3-mercaptobutyric acid (25.6 g, 0.2 mol, yield: 72%). The conversion of crotonic acid was 100%. The reaction pressure at the beginning of the reaction was 0.6Mpa, and the reacti...
Embodiment 2-11
[0127] The reaction is carried out in the same manner as in Example 1, except that, as shown in Table 1, the solvent used is N-methyl-2-pyrrolidone (NMP), dimethylformamide (DMF), 1,3-bis Methyl-2-imidazolidinone (DMI), tetrahydrofuran (THF), methanol, ethanol or isopropanol (IPA), and 4A molecular sieves as solid acid catalysts vary in shape and amount of hydrogen sulfide. The results are shown in Table 1.
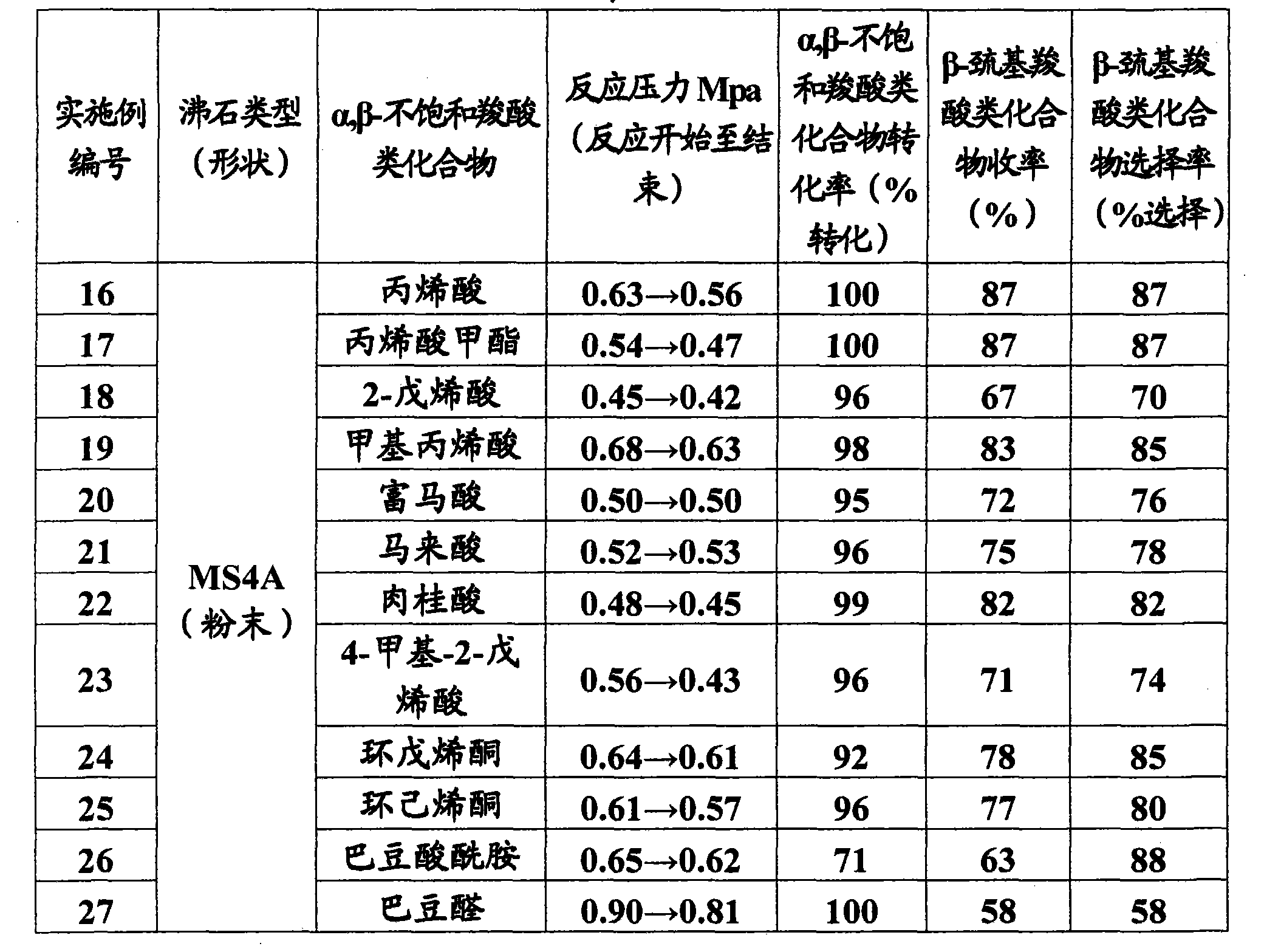
[0128] Table 1
[0129]
Embodiment 12
[0131] The reaction was carried out in the same manner as in Example 1, except that Y-54 molecular sieves (24 g, powder, produced by Union Showa K.K.) were used, and a solvent of NMP (266 g, produced by Junsei Chemical Co., Ltd.) and distilled water (47 g) was used. The mixture was used as solvent, and the reaction temperature was 115°C. It was confirmed that the generated reaction product was 3-mercaptobutyric acid (27.0 g, 0.2 mol, yield: 76%). The conversion rate of crotonic acid was 100%. The reaction pressure at the beginning of the reaction is 0.5Mpa, and the reaction pressure at the end of the reaction is 0.4Mpa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com