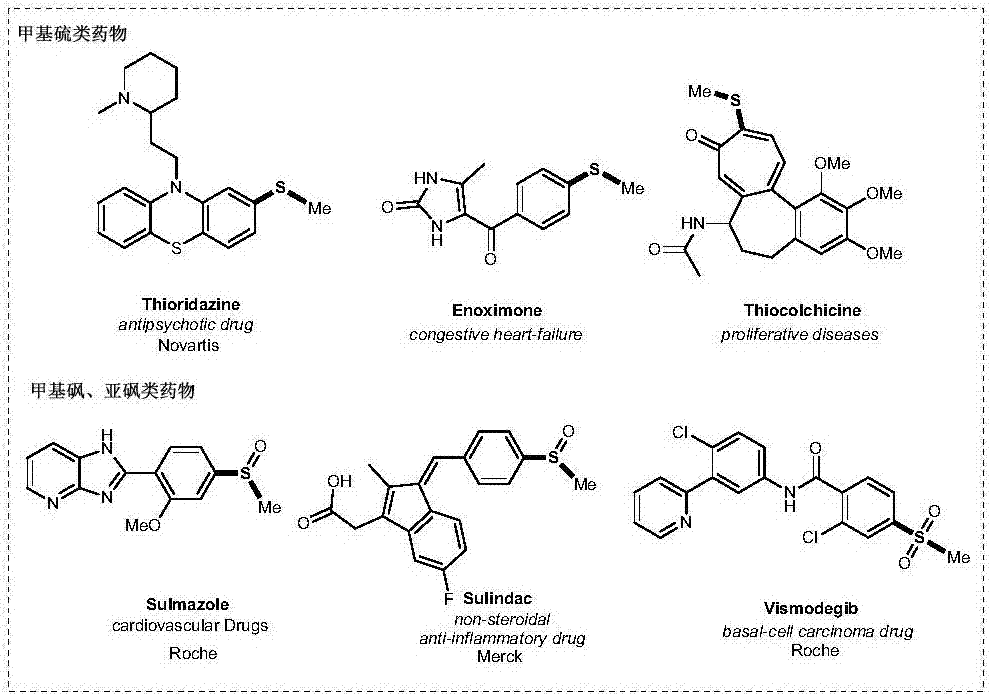
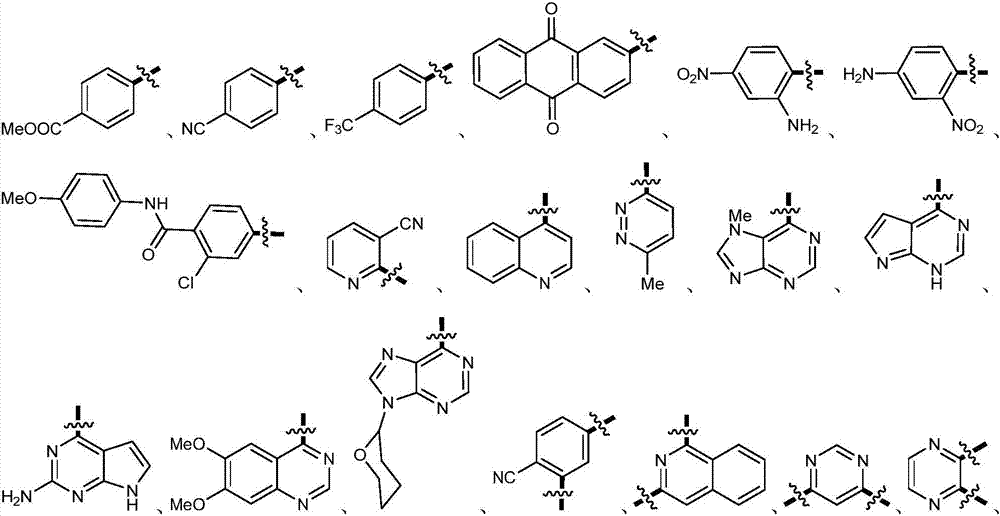
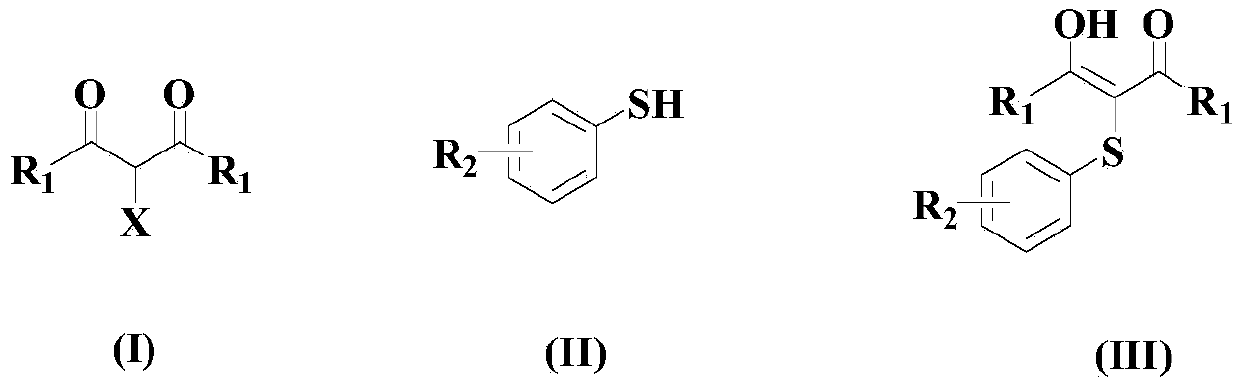
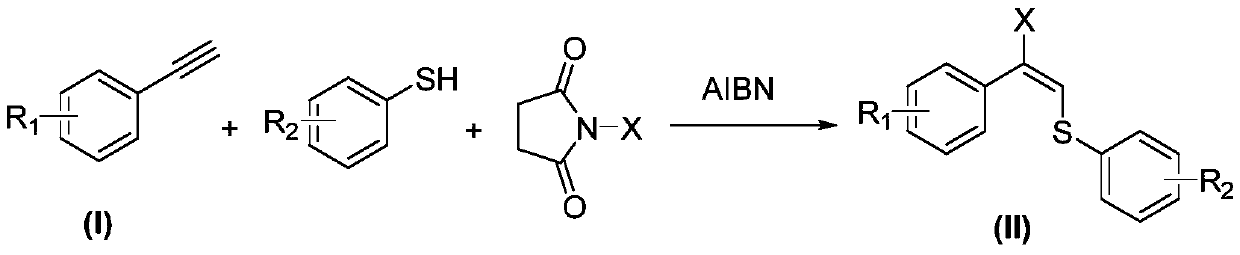
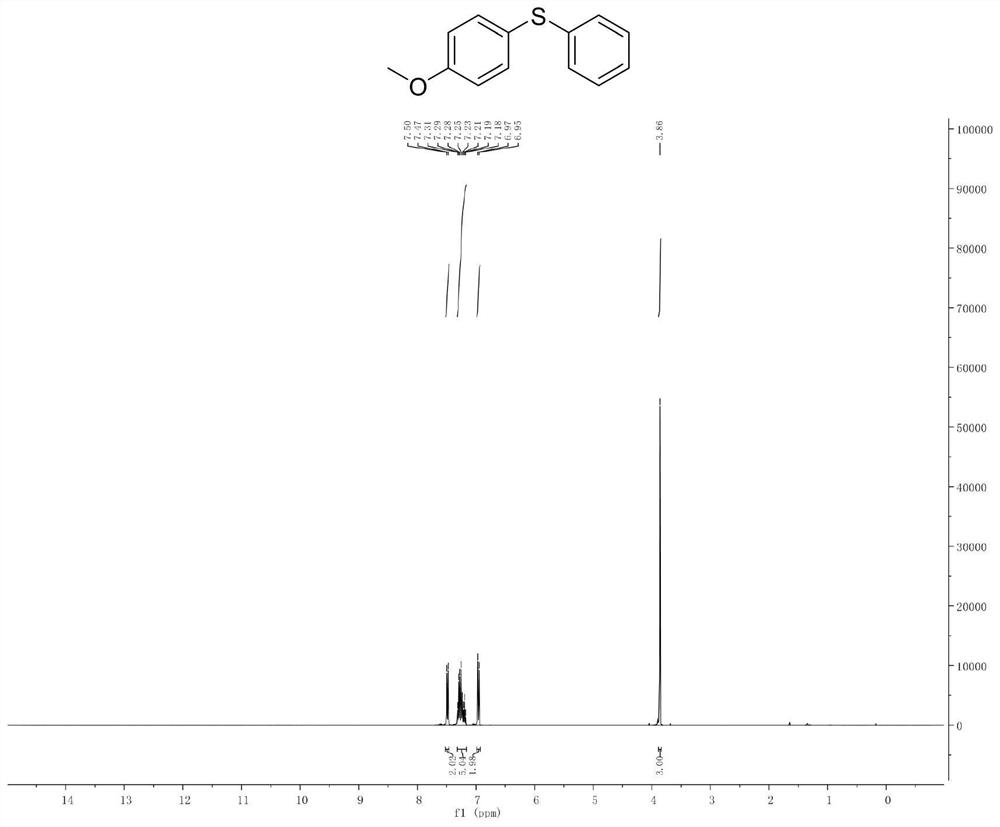
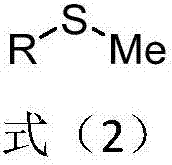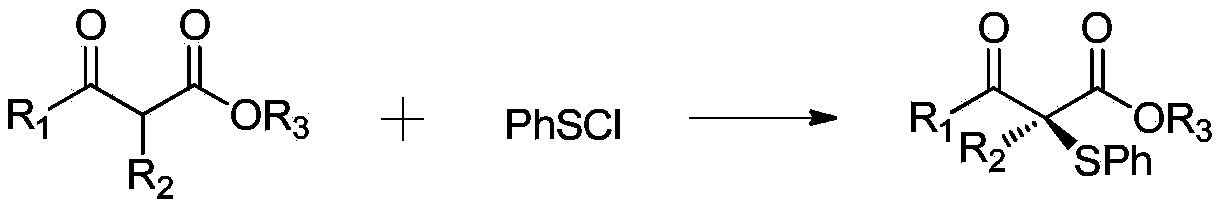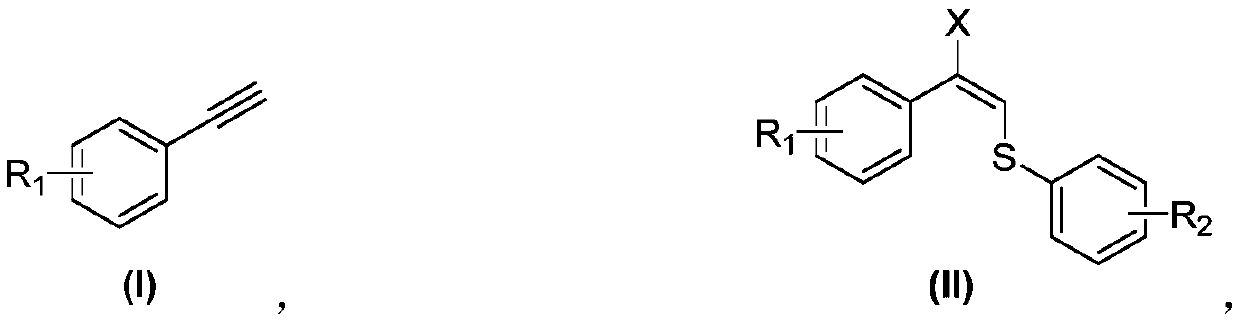Patents
Literature
92results about "Mercapto/sulfide group formation/introduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
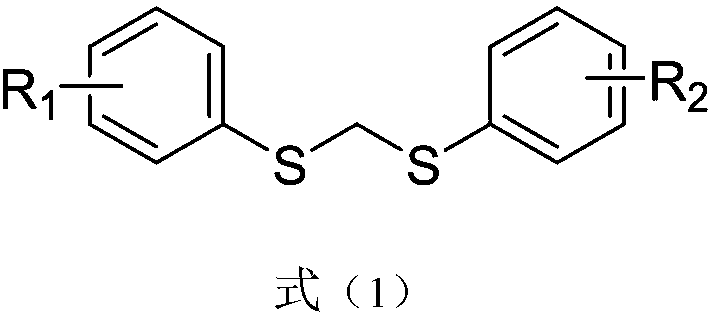
Aryl alkyl thioether compounds and synthesis method thereof
ActiveCN103787802AAchieve conversionLow costMercapto/sulfide group formation/introductionSugar derivativesVulcanizationSynthesis methods
The invention discloses aryl alkyl thioether compounds and a synthesis method thereof. The synthesis method comprises the steps: in a reaction solvent, with an arylamine derivative and halogenated hydrocarbon as reaction raw materials, and Na2S2O3 as a vulcanization reagent, in the presence of a copper reagent catalyst, and reacting to obtain the aryl alkyl thioether compounds. According to the synthesis method disclosed by the invention, raw materials are easy to obtain and low in price, the reaction operation is simple, the reaction conditions are mild, the yield is higher, the tolerance of a functional group is excellent, later-phase modification of compounds of medicines, sugars, amino acids and the like is successfully realized, and an efficient method for constructing a carbon-sulfur bond is provided for researches of medicinal chemistry and biology orthogonal chemistry.
Owner:EAST CHINA NORMAL UNIVERSITY
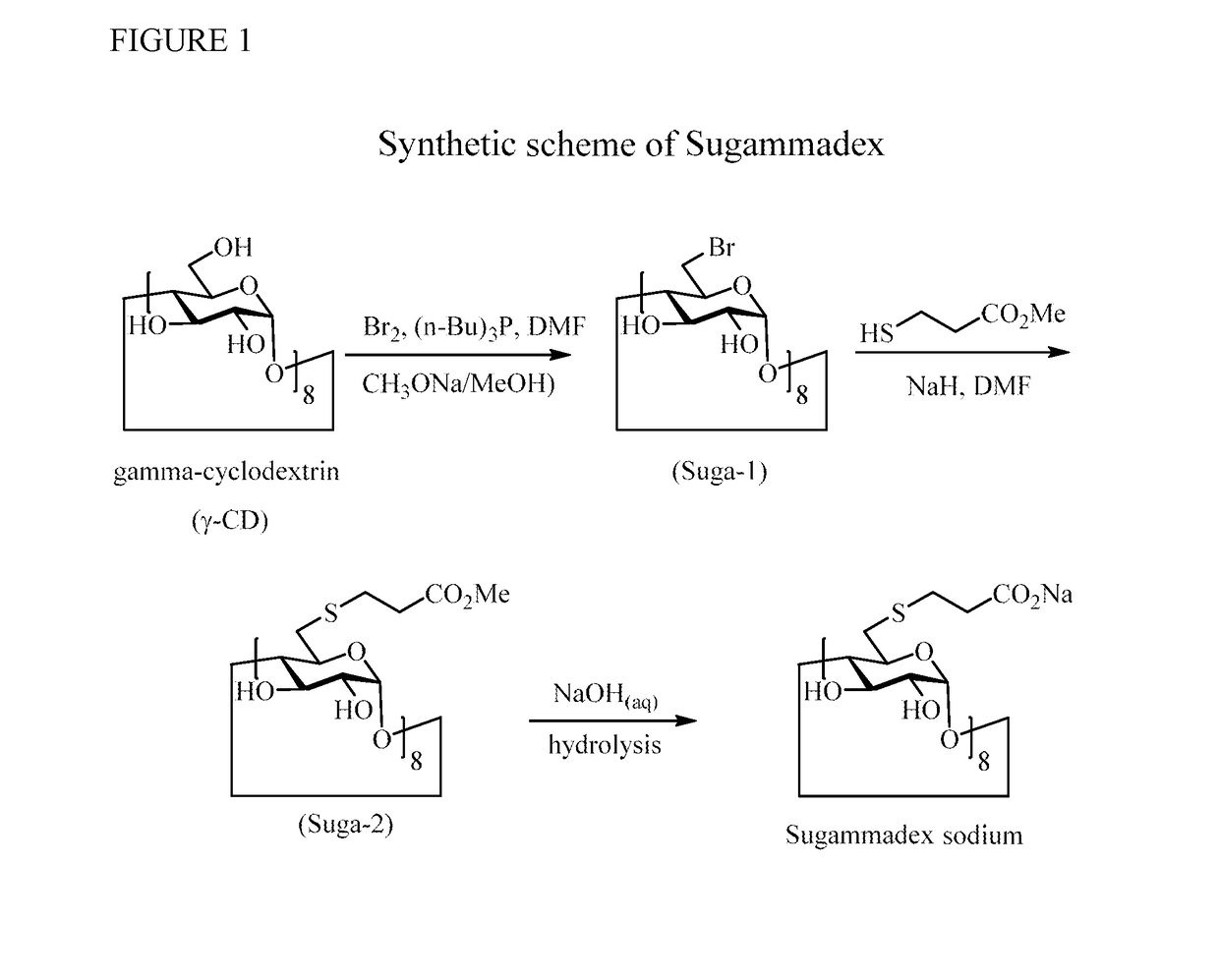
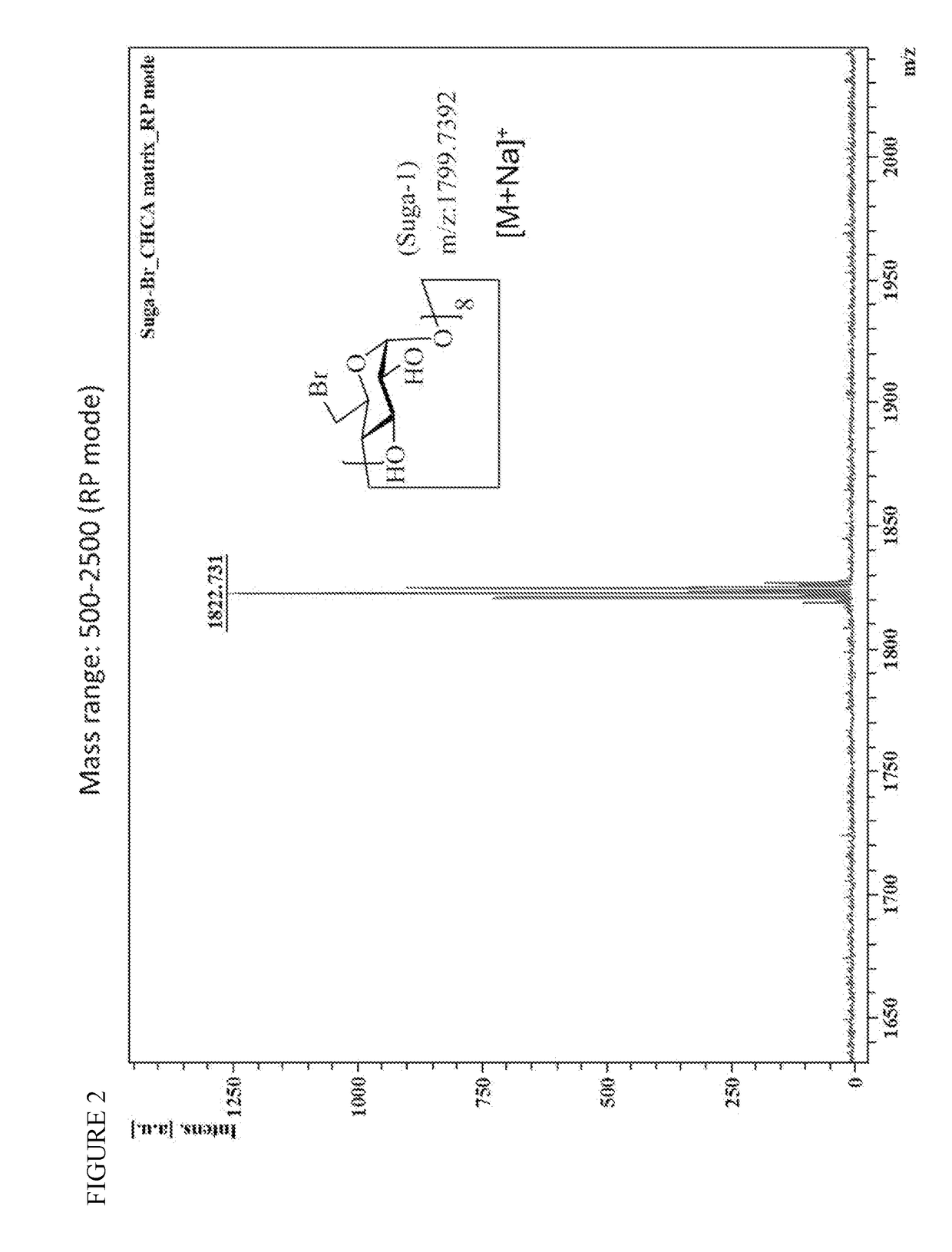
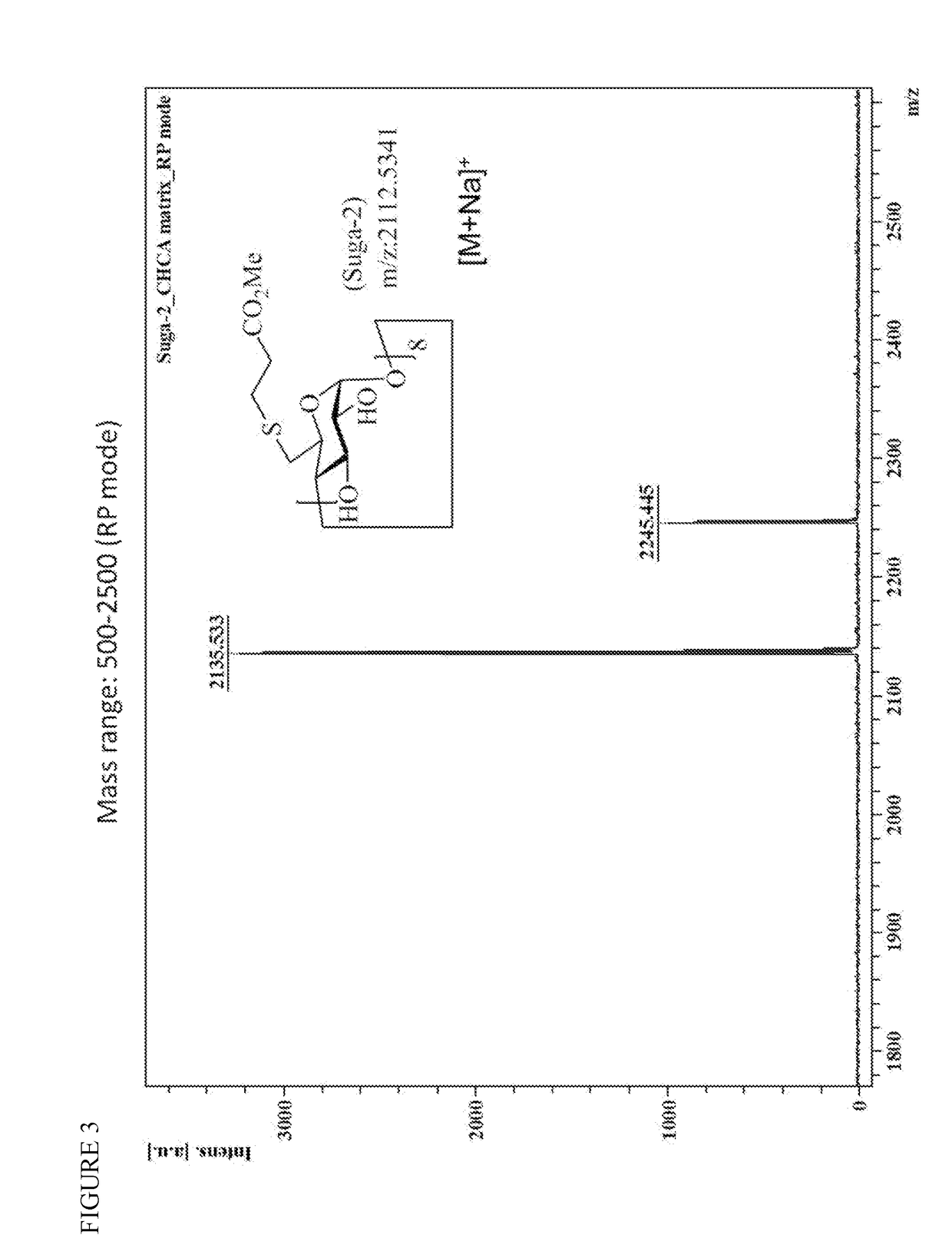
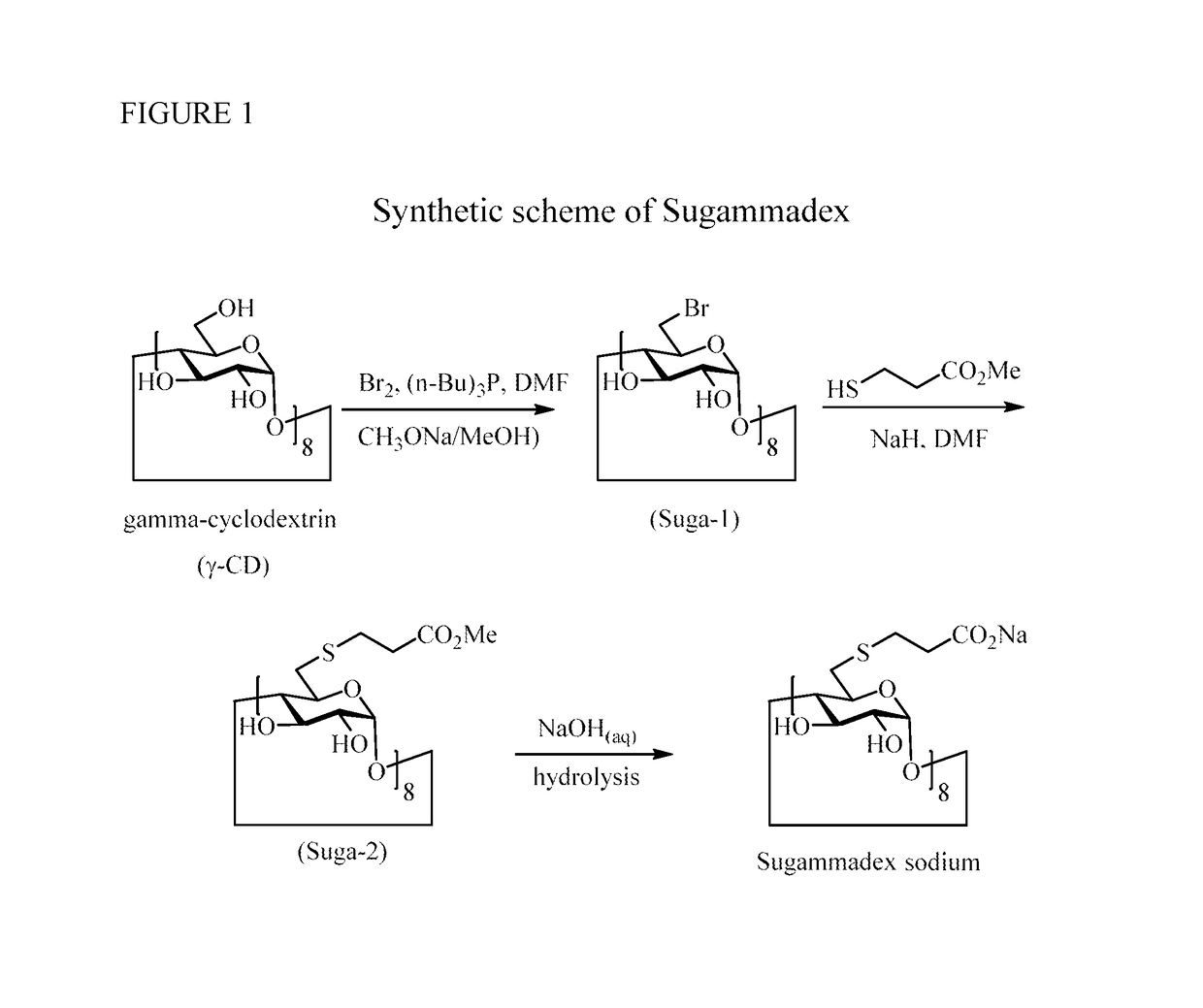
Method for preparation of sugammadex sodium
InactiveUS20190062459A1Mercapto/sulfide group formation/introductionOrganic active ingredientsSugammadex SodiumCombinatorial chemistry
Owner:FORMOSA LAB
Method for preparing symmetrical disulfide compound
InactiveCN101928193AReduce dosageLow impurity contentMercapto/sulfide group formation/introductionOrganic-compounds/hydrides/coordination-complexes catalystsState of artAfter treatment
The invention discloses a method for preparing a symmetrical disulfide compound. In the method, a compound shown as a formula (III) is taken as a raw material, and a CoSalen complex shown as a formula (I) or (II) is taken as a catalyst; and the method comprises the following steps of: in an organic solvent, introducing air or oxygen serving as an oxidant, reacting at the temperature of between 20 and 100 DEG C, tracking and monitoring until the reaction is completed, and after the reaction is finished, performing after treatment on reacting fluid to prepare the symmetrical disulfide compound shown as a formula (IV). Compared with the prior art, the method for preparing the symmetrical disulfide compound has the advantages of simple and convenient operation, mild reaction condition, low cost, easy preparation and small dosage of the adopted catalyst, low cost, easy acquirement and cleanness of the adopted oxidant gas, low content of the impurity of disulfide as the finished product, higher yield, commercial prospect and remarkable application value and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH +1
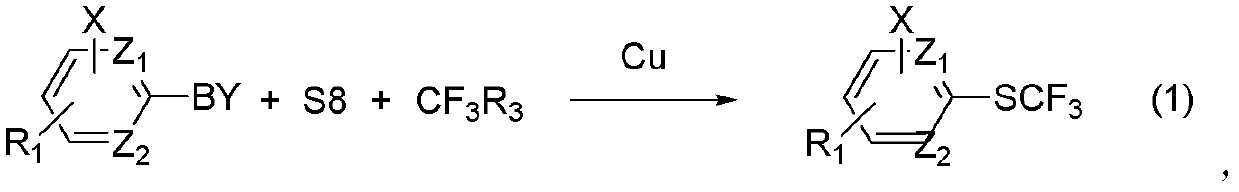
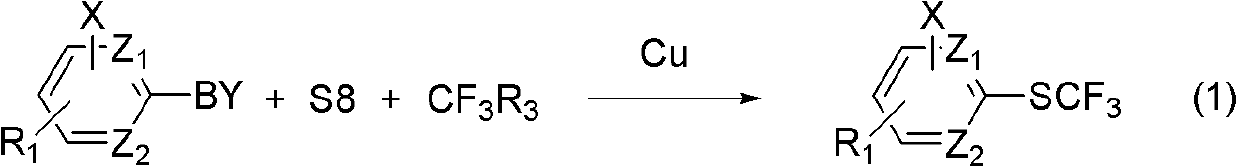
Method for synthesizing aryl trifluoromethyl sulphydryl compound
The invention relates to a method for synthesizing an aryl trifluoromethyl sulphydryl compound. According to the invention, cheap industrial raw materials organic boric acid, elemental sulfur and fluorine-containing building block are used as raw materials to react under the catalysis of monovalent copper salt at room temperature by one kettle way so as to prepare the fluoro-substituted alkyl sulfhydryl ether compound with good yield being about 90%.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
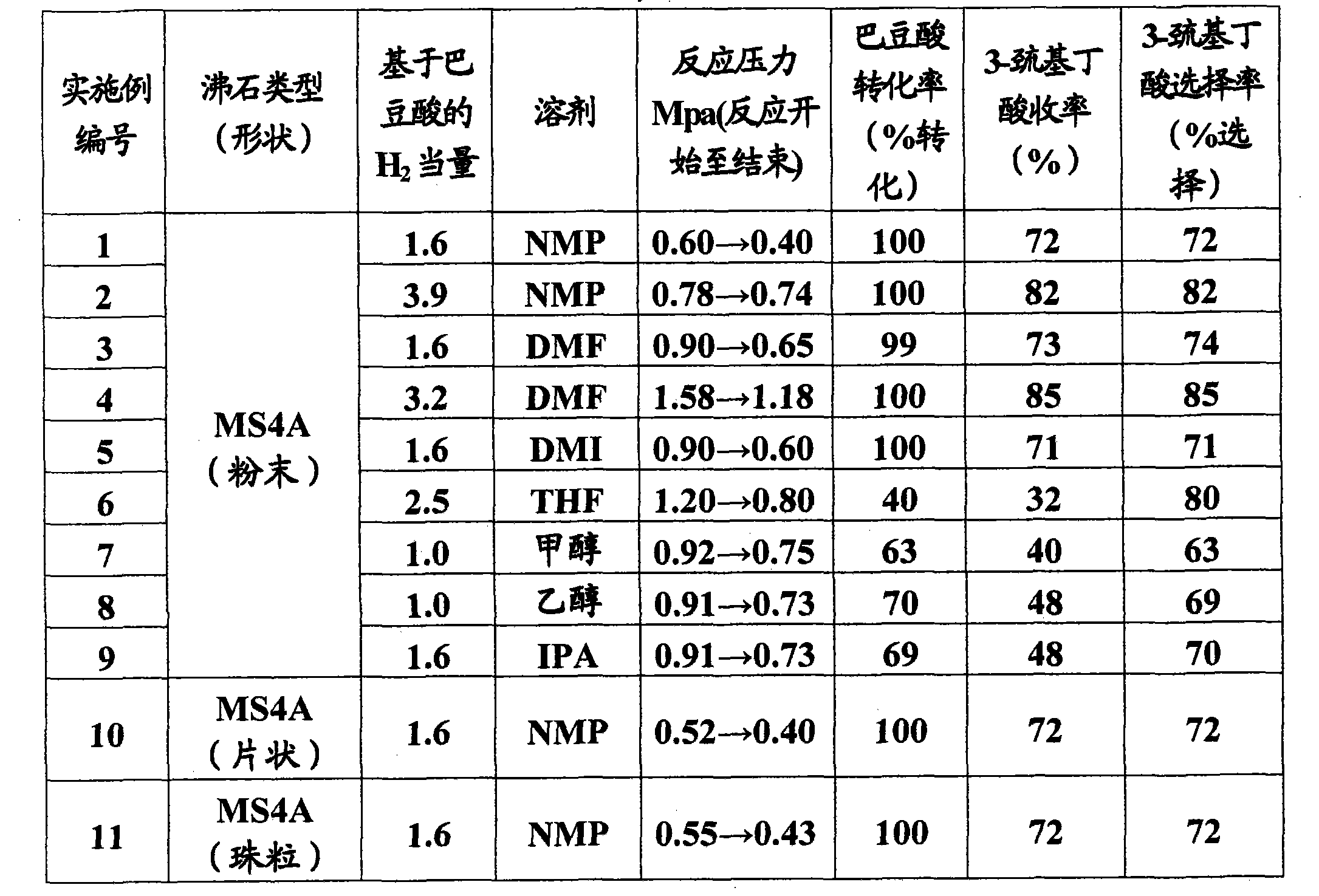
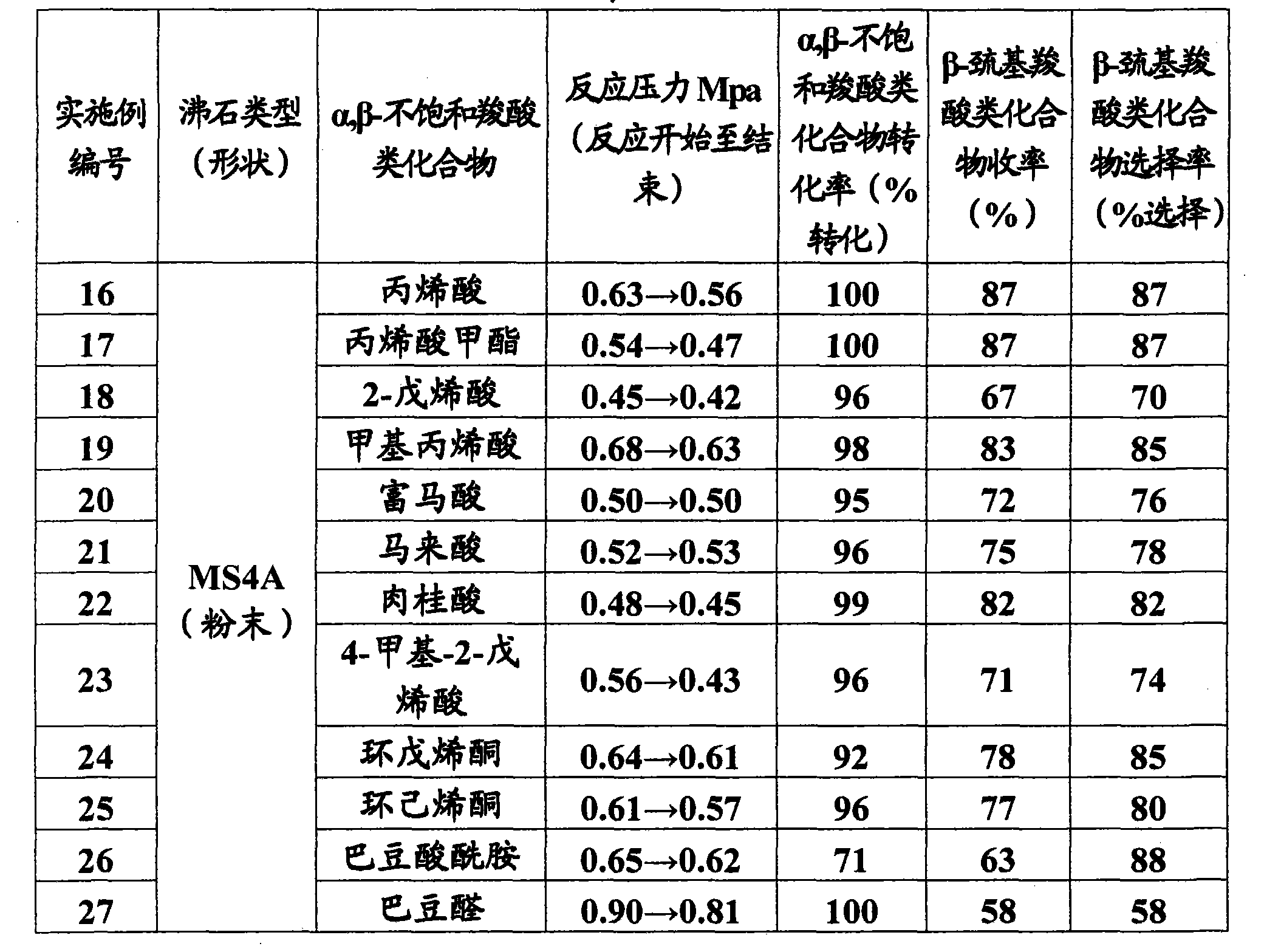
Method of producing beta-mercaptocarboxylic acids
InactiveCN101801922AMercapto/sulfide group formation/introductionThiol preparationHydrogenSynthetic materials
The invention relates to a method for efficiently producing beta-mercaptocarboxylic acids using a solid acid catalyst such as zeolite, which product corresponds to respective starting materials selected from alpha, beta-unsaturated carboxylic acids (alpha, beta-unsaturated carboxylic acid, alpha, beta-unsaturated carboxylic acid ester, alpha, beta-unsaturated amide, alpha, beta-unsaturated aldehide and alpha, beta-unsaturated ketone) and hydrogen sulfides (hydrogen sulfide, sulfide salt and hydrosulfide salt), wherein a solvent compatible with water is used in the reaction. According to the invention, beta-mercaptocarboxylic acids which are useful as additives in synthetic materials for pharmaceutical or agricultural agents and in polymer compounds can be industrially produced efficiently by using easily available alpha, beta-unsaturated carboxylic acid (such as crotonic acid) at high yield.
Owner:RESONAC HOLDINGS CORPORATION
Synthetic method of aryl sulfide type compound
ActiveCN104725172AEasy to operateWide variety of sourcesMercapto/sulfide group formation/introductionMetallocenesArylNatural product
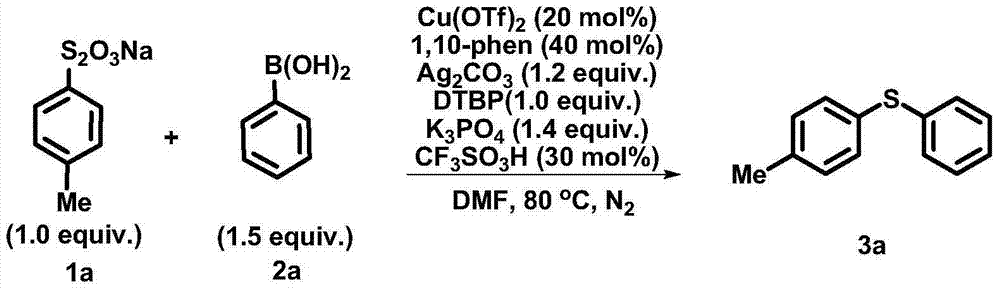
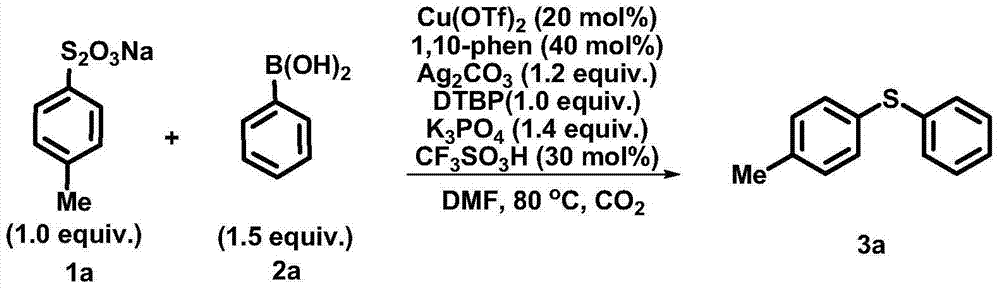
The invention discloses a synthetic method of an aryl sulfide type compound shown by a formula (3). In a reaction solvent, an aryl or alkenyl thiosulfate type derivative and a boric acid, boric acid grease or borate type derivative are used as reaction raw materials and react with each other to obtain a polysubstituted aryl sulfide type compound under the action of a metal copper catalyst. According to the synthetic method disclosed by the invention, reaction conditions are mild, raw materials are easily-available and low in price, the reaction operation is simple, the yield is relatively high, and a key skeleton structure is provided for the synthesis of many natural products and medicaments, so that the method can be widely applicable to industrial scale production. A formula is shown in the specification.
Owner:EAST CHINA NORMAL UNIV
Alkyl trifluoromethyl thioether compound and preparation method thereof
ActiveCN104557358AEasy to introduceMild reaction conditionsMercapto/sulfide group formation/introductionSulfide preparationSolventOxidizing agent
The invention discloses an alkyl trifluoromethyl thioether compound and a preparation method thereof. The alkyl trifluoromethyl thioether compound comprises the following step: under protection of inertial gas, carrying out trifluoromethylthiolation reaction on an alkyl primer and a trifluoromethylthio reagent to obtain the alkyl trifluoromethyl thioether compound. The trifluoromethylthio reagent comprises the following components: a metal salt containing trifluoromethylthio, an oxidizing agent and a nitrile solvent. The trifluoromethylthio reagent disclosed by the invention is low in price and easily available. When the trifluoromethylthio reagent is used for synthesizing the alkyl trifluoromethyl thioether compound, the trifluoromethylthio can be quickly and conveniently introduced to the alkyl primer which is not pre-functionalized. Moreover, the synthetic method is wide in range of application of the primer, mild in reaction condition, high in reaction efficiency, mostly direct in reaction, simple to operate, low in cost and more suitable for industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
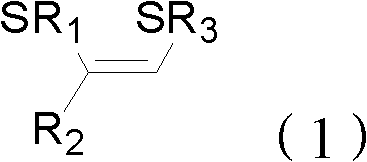
Method for preparing (Z)-1,2-disulfide-1-olefin by catalysis of metal copper salt
InactiveCN102351622ARaw materials are easy to getEasy to prepareMercapto/sulfide group formation/introductionGroup 8/9/10/18 element organic compoundsOrganic solventThiol
The invention discloses a method for preparing (Z)-1,2-disulfide-1-olefin by the catalysis of metal copper salt, comprising the following steps of: using metal copper salt as a catalyst and using (Z)-1-bromine-2-thioether-1-olefin or mercaptan as a substrate under the action of alkaline in an organic solvent to synthesize (Z)-1,2-disulfide-1-olefin. The invention has the following advantages: the raw materials in the method are easily available; the preparation method is simple; as the catalyst, the metal copper salt is cheap and easily available; (Z)-1,2-disulfide-1-olefin prepared by the method has an extensive application prospect in the fields of coordination chemistry, material science, organic synthesis and the like.
Owner:ZHEJIANG UNIV
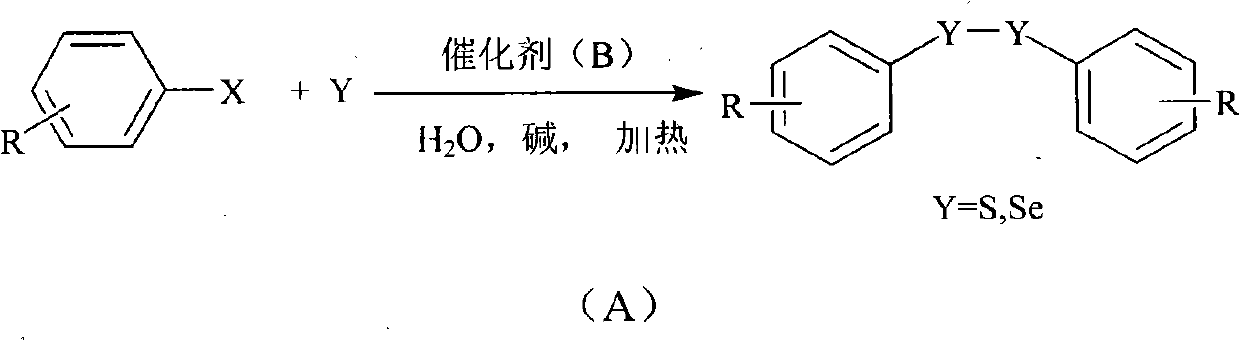
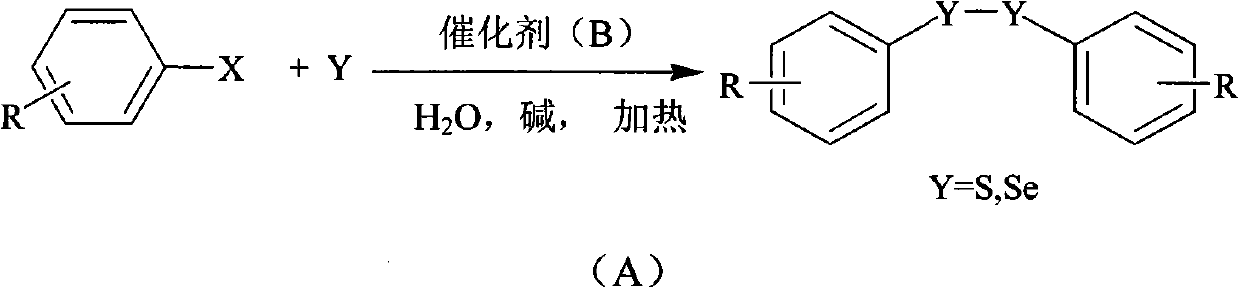
Method for preparing diaryl disulfide and diaryl diselenide under catalysis of aqueous phase
InactiveCN102010282AMercapto/sulfide group formation/introductionHydropoly/poly sulfide preparationSulfurWater soluble
The invention discloses a method for preparing diaryl disulfide and diaryl diselenide under the catalysis of an aqueous phase. A water-soluble catalyst is used for efficiently catalyzing the reaction of sulfur powder or selenium powder with aryl halide in the pure aqueous phase, so the method for preparing disulfide-bond-containing and diselenide-bond-containing aromatic compounds is environmentally-friendly, simple and convenient to operate, safe, low in cost and efficient. Compared with the prior art, the method can be suitable for a large number of functional groups, ensures high yield and few side products, and is simple and convenient to operate, safe, low in cost and environmentally-friendly.
Owner:SICHUAN UNIV
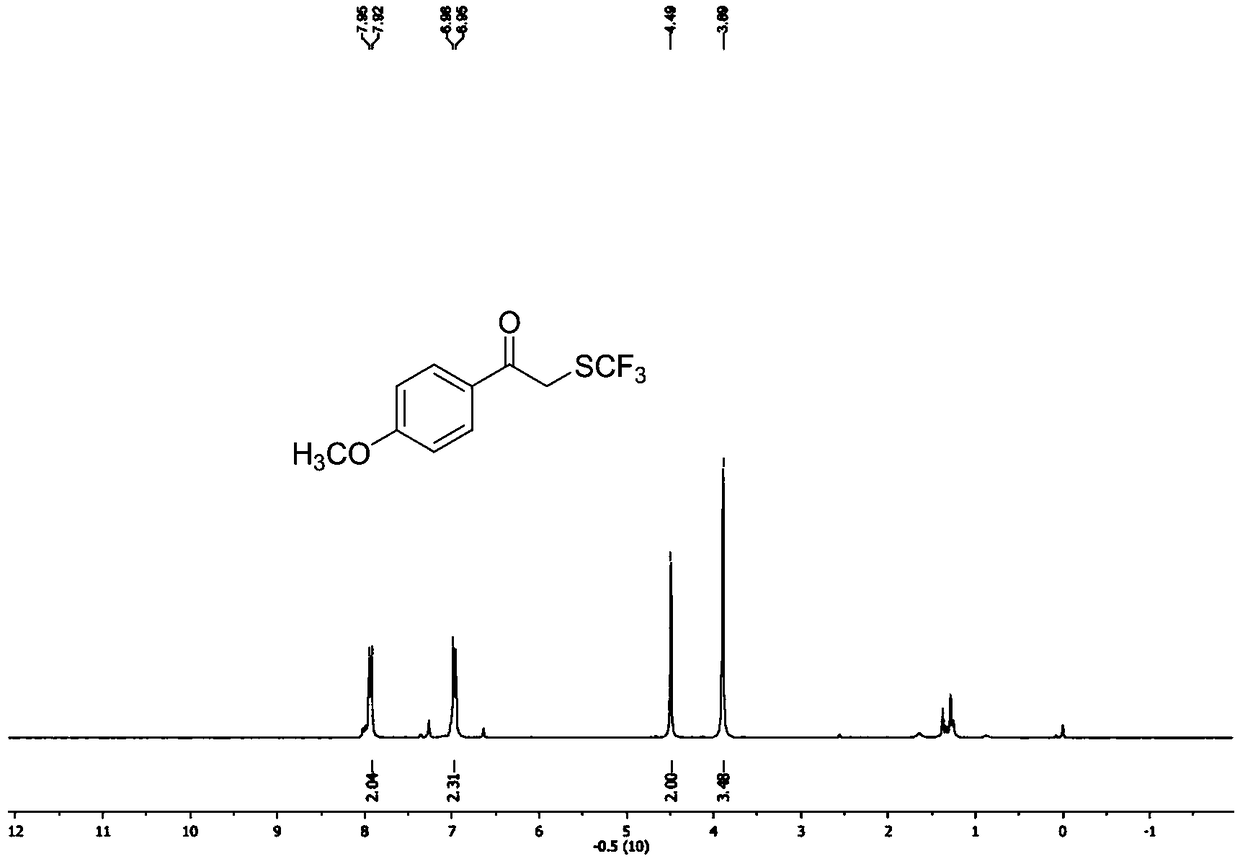
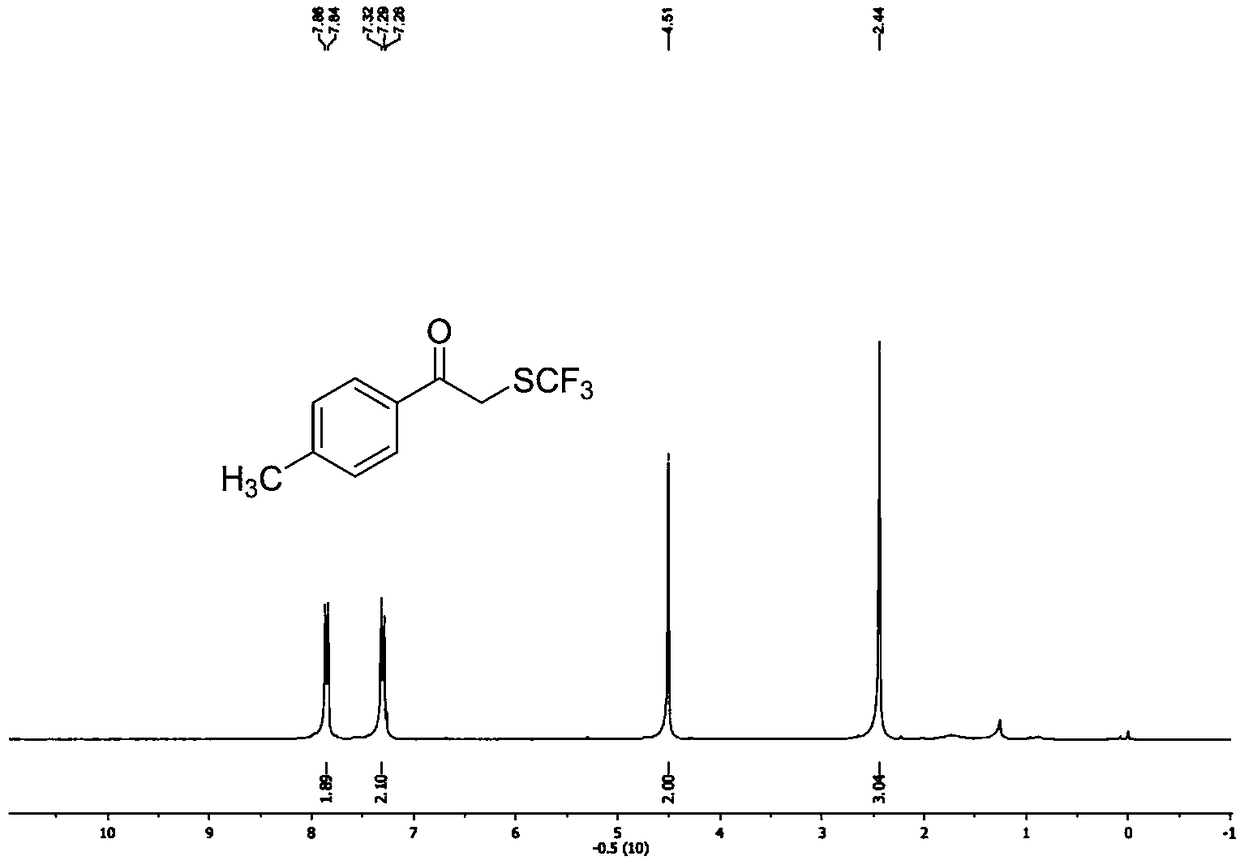
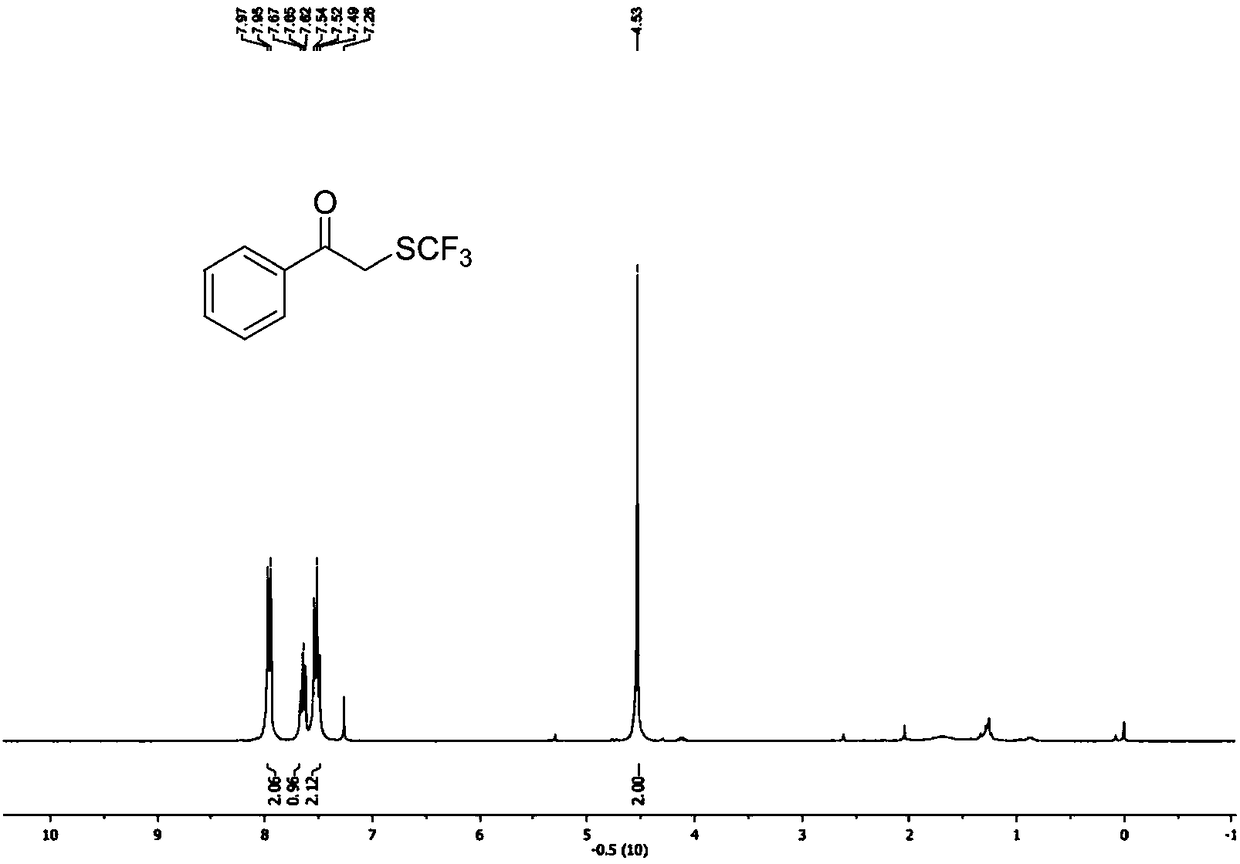
Preparation method of alpha-trifluoromethylthio substituted acetophenone compound
ActiveCN108569942AHigh yieldEasy to prepareMercapto/sulfide group formation/introductionSulfide preparationAcetophenoneEthane Dichloride
The invention discloses a preparation method of an alpha-trifluoromethylthio substituted acetophenone compound. The preparation method comprises the following steps: by taking aryl ketone as a substrate, sodium trifluoromethanesulfinate as a trifluoromethylthio reagent, triphosgene as an additive, pyridine as a catalyst and dichloroethane as a solvent, in the nitrogen protection mode, stirring for12 hours at the temperature of 60 DEG C, performing a TLC tracking reaction, and conducting column chromatography isolation after sufficiently completing the reaction, thereby obtaining the alpha-trifluoromethylthio substituted acetophenone compound. According to the preparation method, the triphosgene is used as the reaction additive, so that using an expensive trifluoromethylthio reagent is avoided, but so far, a method for introducing trifluomethylthio has not been reported, the preparation method is simple and convenient, is low in cost, and is high in yield, a product is directly obtained, without any transition metal catalysis, and therefore, the preparation method is extremely high in practical popularization value.
Owner:UPCHEM CHINA
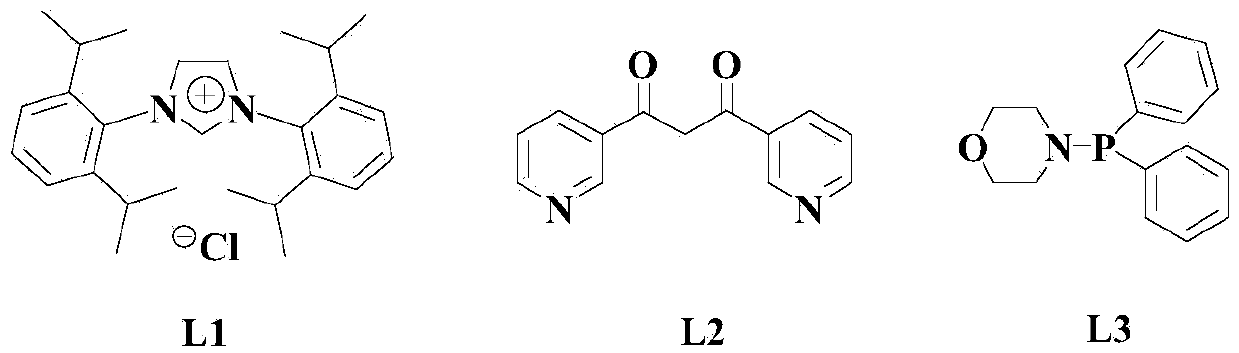
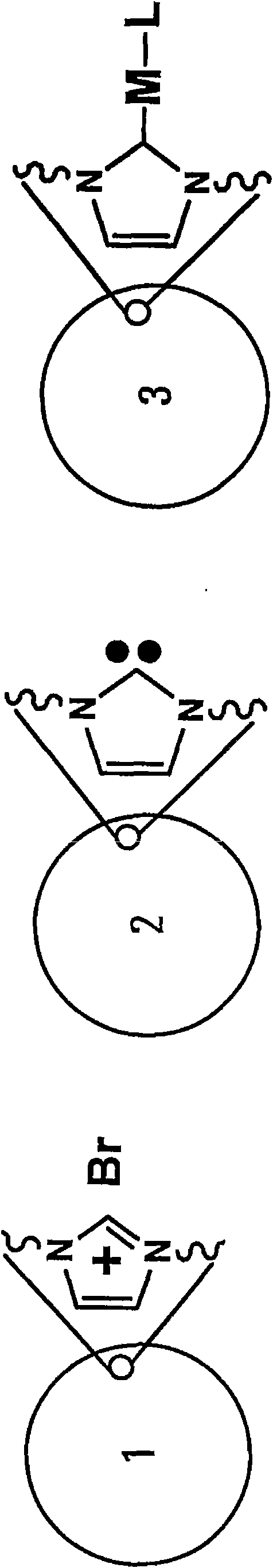
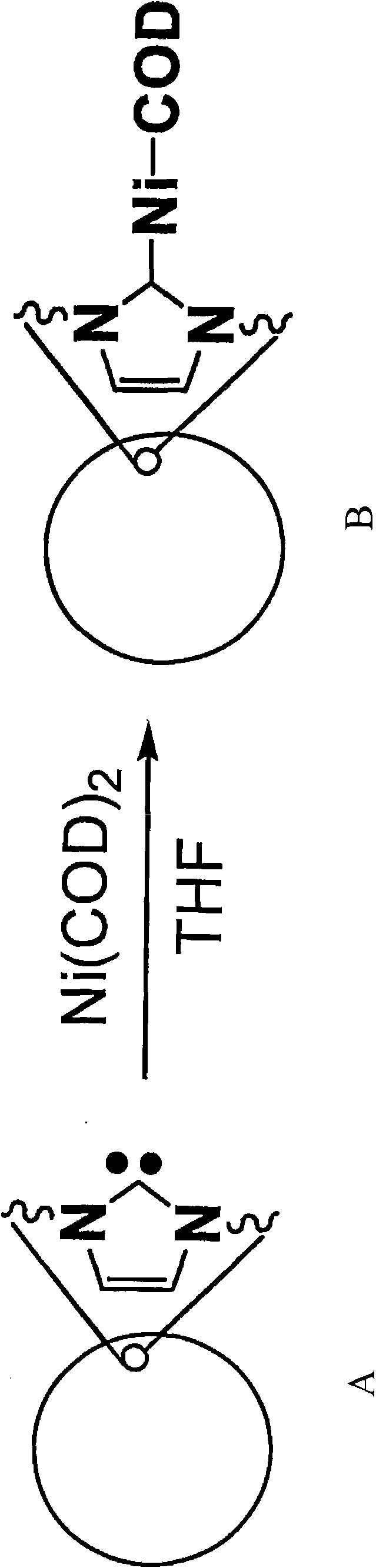
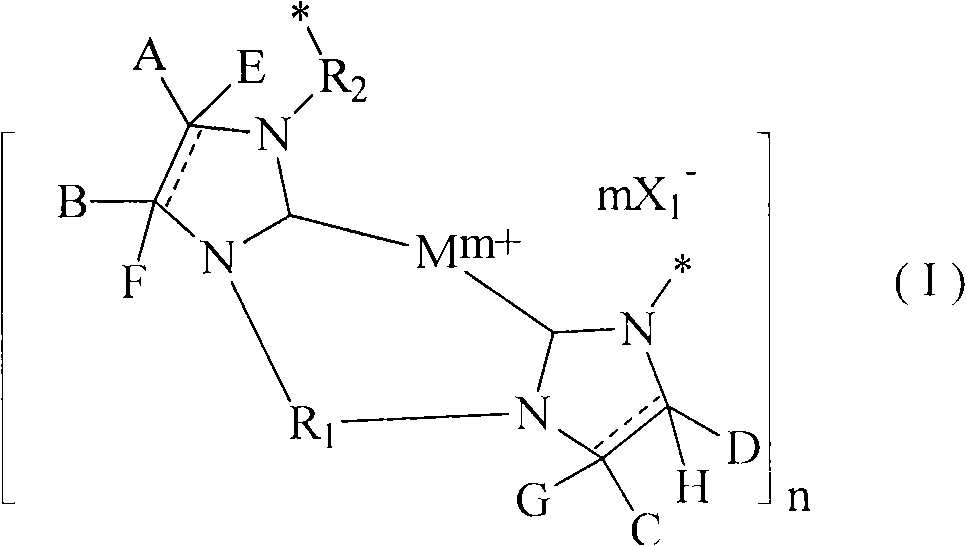
Poly-N-heterocyclic carbene transition metal complexes and N-heterocyclic carbene transition metal complexes for carbon-sulfur and carbon-oxygen coupling reactions
InactiveCN101687723AMercapto/sulfide group formation/introductionNickel organic compoundsSulfurCoupling
Methods for carbon-sulfur (C S) or carbon-oxygen (C-O) coupling reactions are provided. The methods involve the use of a transition metal complex comprising a heterocyclic carbene ligand complexed with a transition metal. Transition metal complexes comprising a heterocyclic carbene ligand complexed with nickel are also provided. The nickel heterocylic carbene complexes may be used for C-S or C-O coupling reactions.
Owner:AGENCY FOR SCI TECH & RES
Method for synthesizing benzyl alkyl sulfur ether
InactiveCN103755505AEase of mass productionReduce usageMercapto/sulfide group formation/introductionSulfide preparationBenzoyl bromideThiol
The invention relates to a method for synthesizing benzyl alkyl sulfur ether. With thiourea as a sulfur source, benzyl bromide or benzyl halide reacts with alkyl alkyl halide under the catalysis effect of inorganic alkali to obtain an asymmetric benzyl alkyl sulfur ether compound. The method avoids the use of mercaptan with a pungent smell as the sulfur source, and adopts the one-pot method, so that the purpose of environmental protection can be achieved, and the operational program is simplified to further reduce the cost of synthesis and large-scale production.
Owner:中国人民解放军63975部队
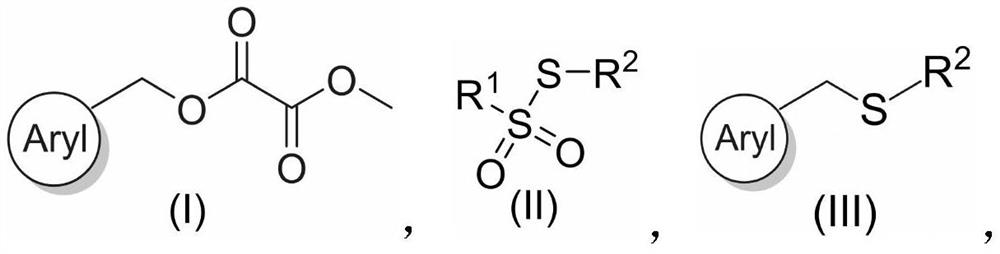
Benzyl thioether compound and preparation method thereof
PendingCN114181122ARaw materials are easy to obtainMild reaction conditionsMercapto/sulfide group formation/introductionSulfide preparationSimple Organic CompoundsPtru catalyst
The invention relates to a benzyl thioether compound and a preparation method thereof, and belongs to the technical field of organic compounds. The preparation method provided by the invention comprises the following step: in a protective atmosphere, oxalate and a sulfur source react in a solvent under the action of a catalyst, a reducing agent and a ligand to obtain the benzyl thioether compound. According to the preparation process of the benzyl thioether compound, oxalate and a sulfur source are used as raw materials, the raw materials are simple and easy to obtain, the reaction condition is mild and environment-friendly, nickel halide is used as a catalyst in the reaction, the obtained yield is high, and the method can be suitable for amplification reaction and lays a foundation for industrial production.
Owner:苏州照固新材料科技有限公司
Method for preparation of sugammadex sodium
InactiveUS10233263B1Mercapto/sulfide group formation/introductionOrganic active ingredientsSugammadex SodiumEngineering
Owner:FORMOSA LAB
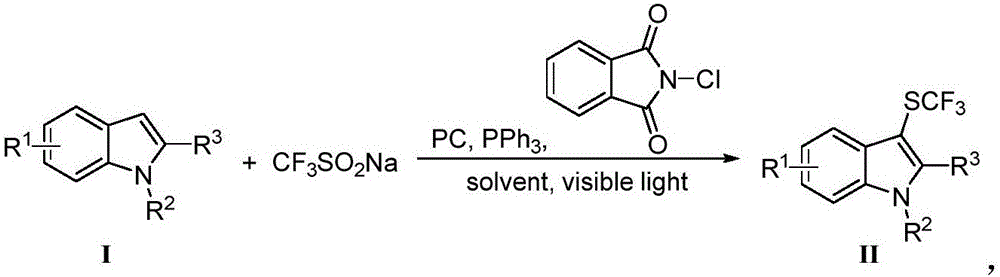
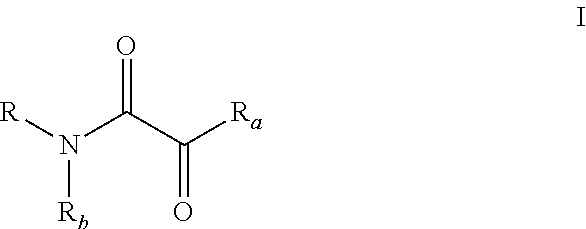
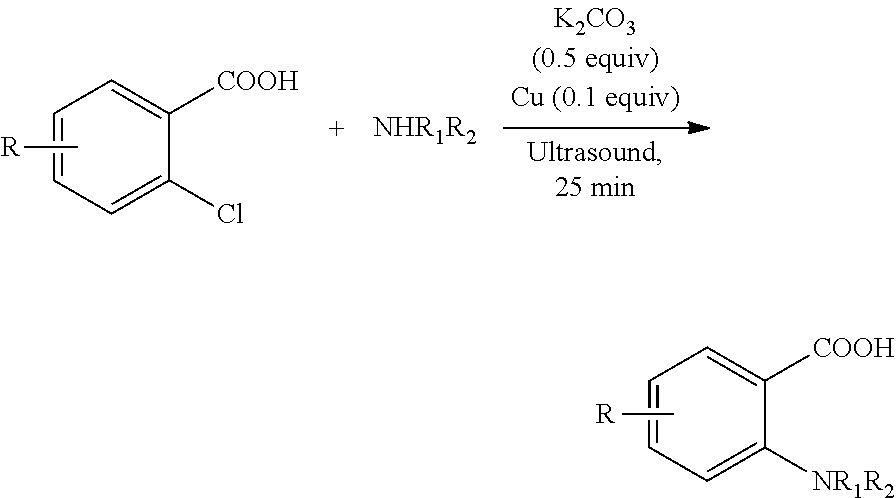
Oxalic acid monoamide ligand, and uses thereof in coupling reaction of copper-catalyzed aryl halogen substitute
ActiveUS20180207628A1Efficient executionMercapto/sulfide group formation/introductionCarboxylic acid nitrile preparationOXALIC ACID DIHYDRATEHalogen
The present invention provides oxalic amide ligands and uses thereof in copper-catalyzed coupling reaction of aryl halides. Specifically, the present invention provides a use of a compound represented by formula I, wherein definitions of each group are described in the specification. The compound represented by formula I can be used as a ligand in copper-catalyzed coupling reaction of aryl halides for the formation of C—N, C—O and C—S bonds.
Owner:CE PHARM CO LTD
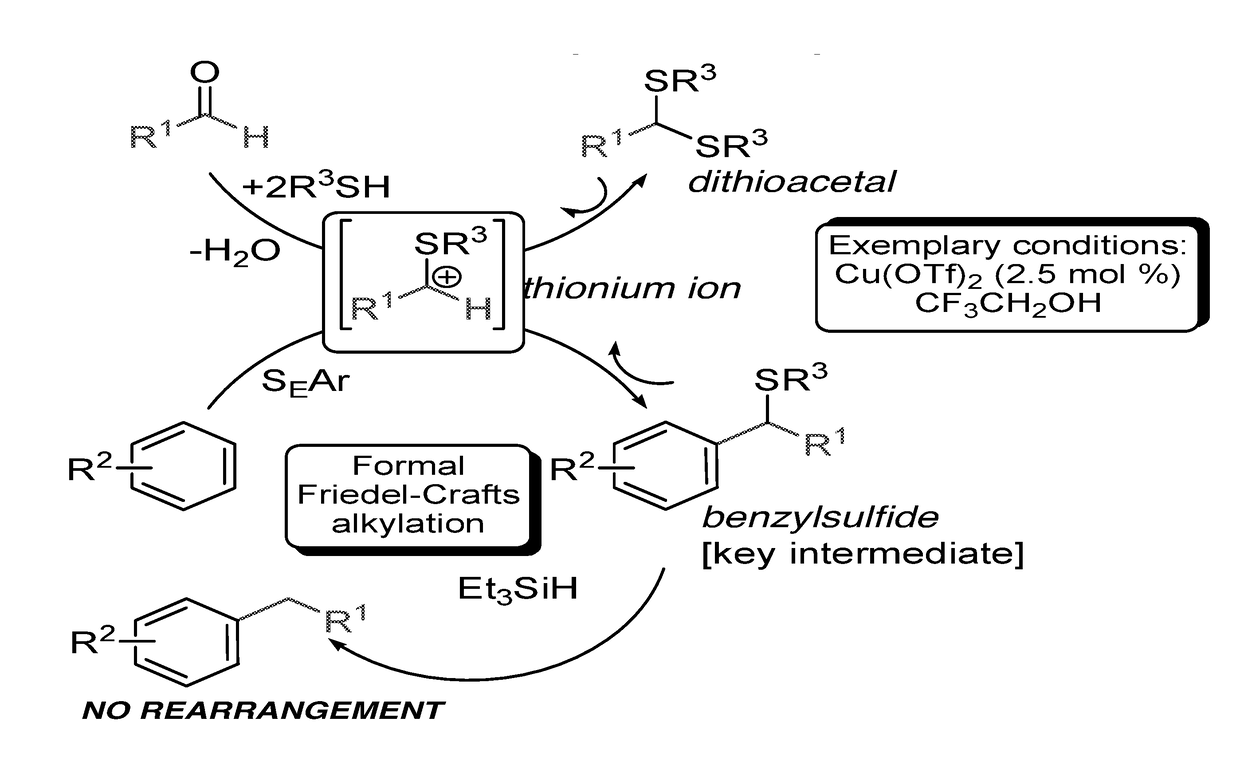
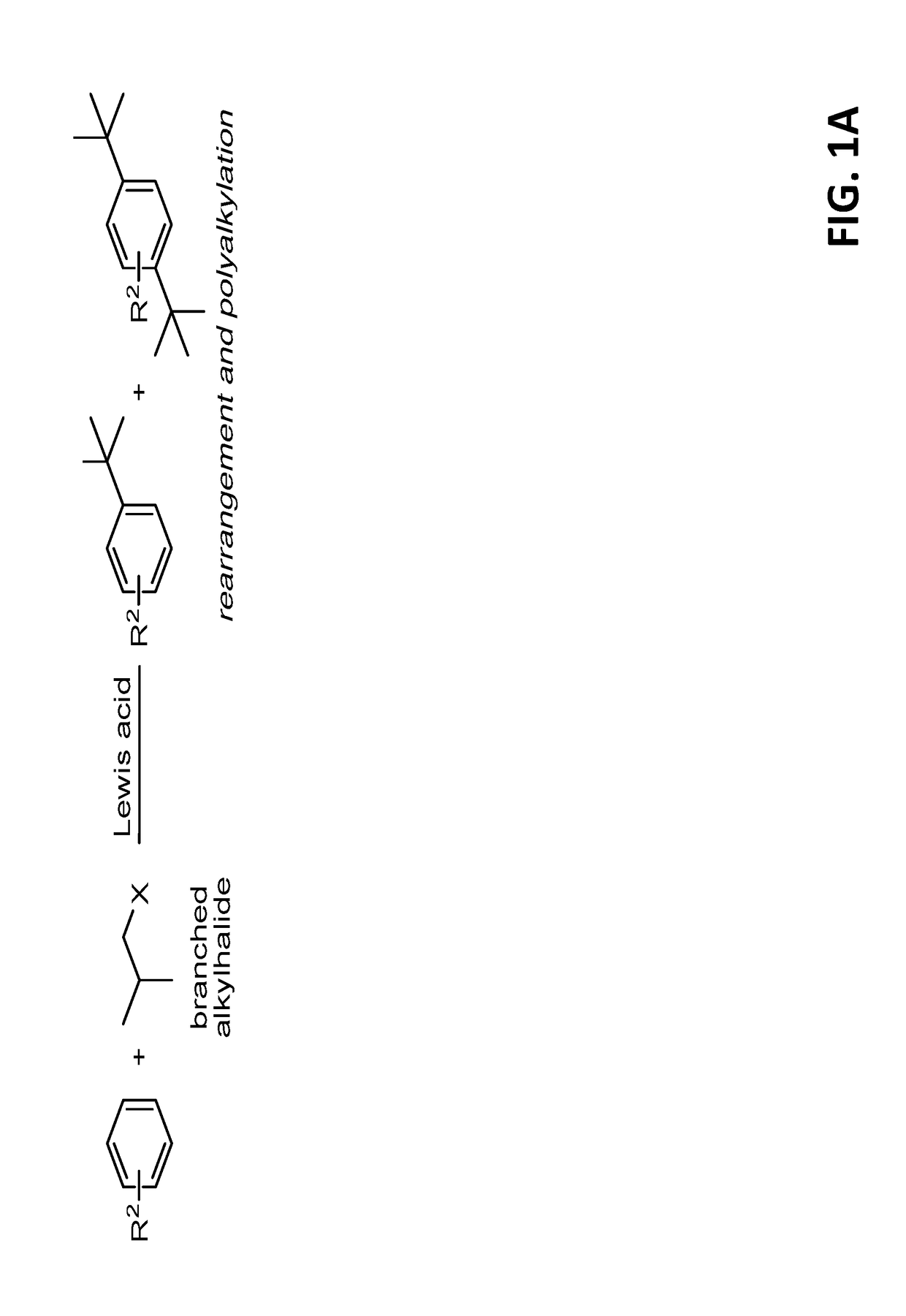
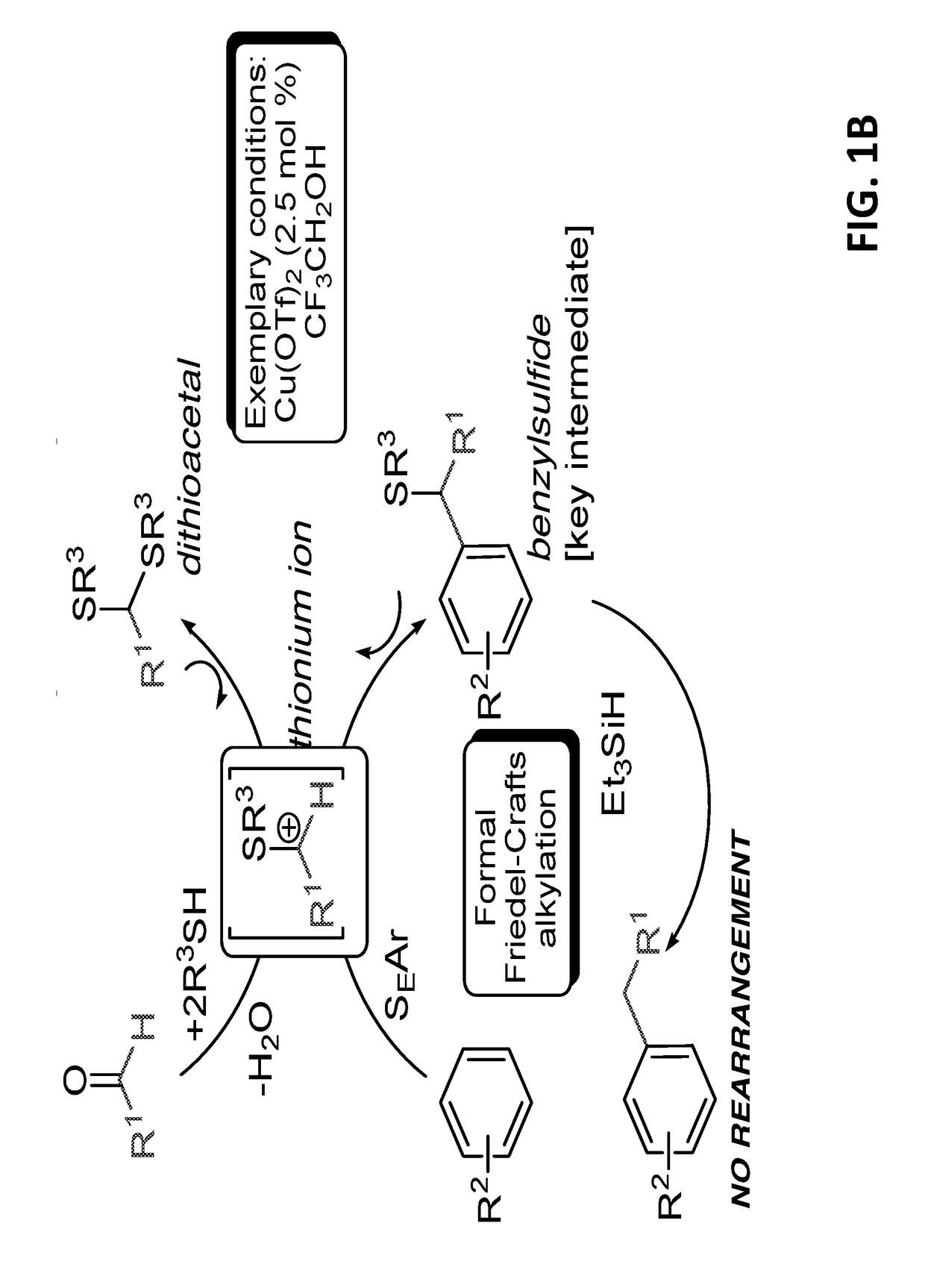
Introduction of alkyl substituents to aromatic compounds
InactiveUS20180065904A1Mercapto/sulfide group formation/introductionOrganic reductionThioenolElectrophilic aromatic substitution
Novel selective synthesis route to introduce primary alkyl groups on aromatic compounds is disclosed. The synthesis route is based on electrophilic aromatic substitutions of thionium ion species that are generated in-situ from aldehydes and thiols, affording benzyl sulfide that can be reduced with triethylsilane.
Owner:B G NEGEV TECH & APPL LTD
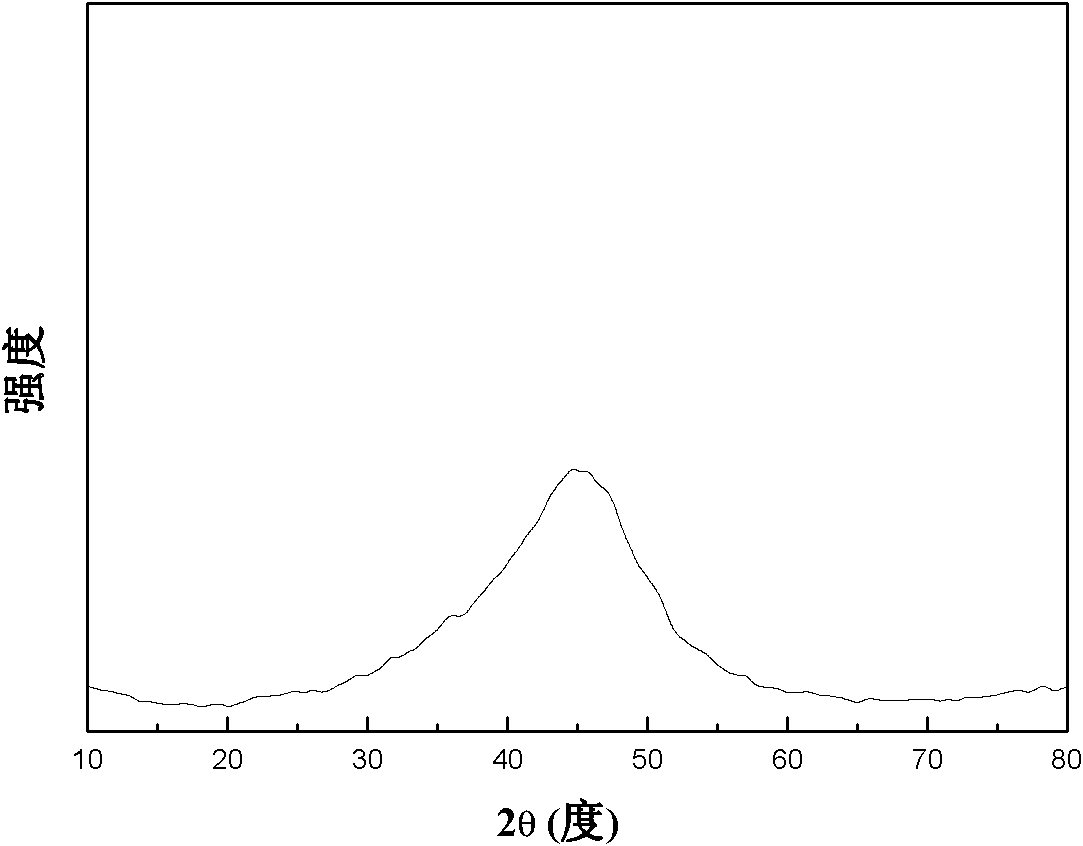
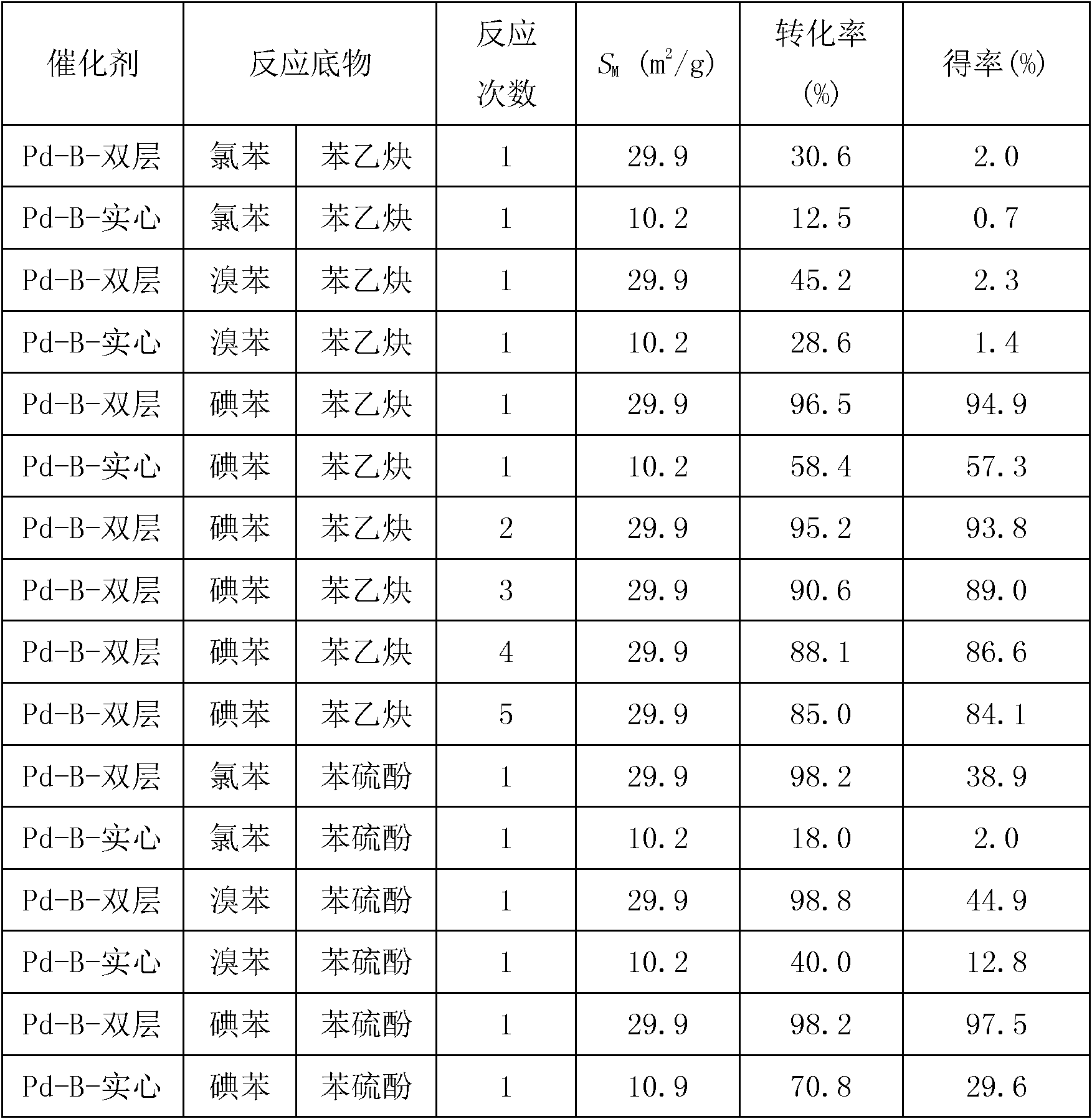
Double-layer hollow amorphous alloy nanometer ball as well as preparation method and application thereof
InactiveCN102179248AGood catalyticLong catalyst lifeMercapto/sulfide group formation/introductionOrganic reductionActivity ratiosPhenol
The invention discloses a double-layer hollow amorphous alloy nanometer ball which is characterized in that the activity ratio surface area is 20-40 square meters per gram; the diameter of an inner cavity is 240-260 nanometers; the distance between two layers is 40-60 nanometers; the thickness of a ball shell is 10-20 nanometers; the ball shell of the double-layer hollow nanometer ball is made from M-B amorphous alloy nanometer particles with the particle size of 4-6 nanometers; M is a metallic element; and the active specific surface area of the ball is 2-3 times of the active specific surface area of a solid amorphous alloy catalyst. The double-layer hollow amorphous alloy nanometer ball has long catalysis life, can be repeatedly used, has the almost constant catalysis efficiency, can be used as hydrogenation catalyst for the compounds containing unsaturated functional groups such as olefin, alkyne, phenols, nitryl and carbonyl compounds, and also can be used as the catalyst for coupling reaction, such as C-C, C-S, and the like. The double-layer hollow amorphous alloy nanometer ball is prepared by a hard mould plate reverse copying method in combination with a chemical reduction method. The preparation method is simple, the condition is easily controlled and the cost is low.
Owner:SHANGHAI NORMAL UNIVERSITY
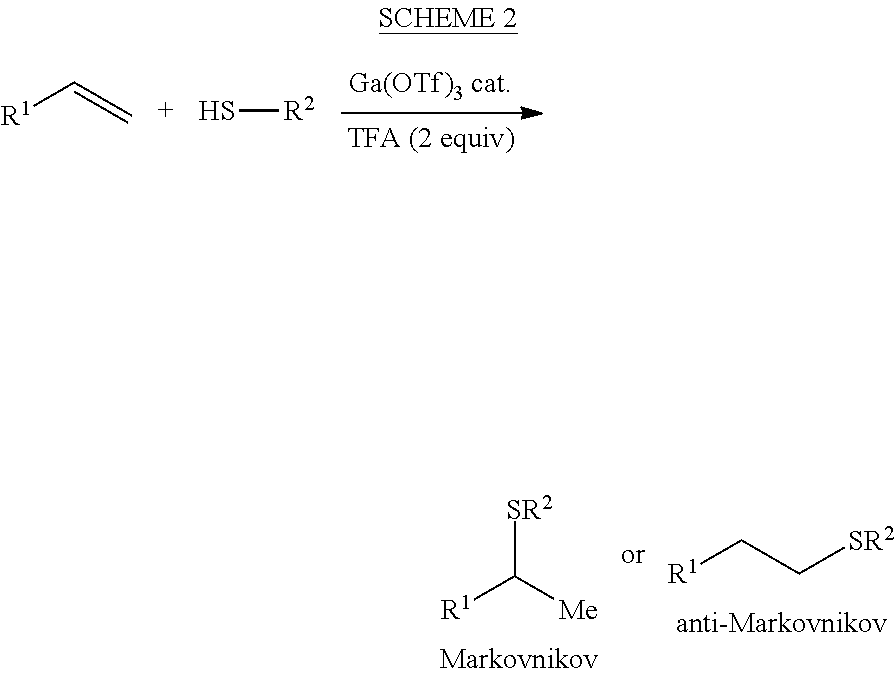
Hydrothiolation of Unactivated Alkenes
InactiveUS20130190505A1Mercapto/sulfide group formation/introductionPhysical/chemical process catalystsThiolGallium triflate
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
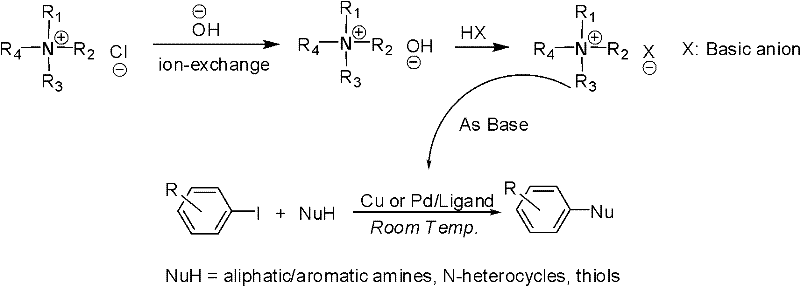
Method for performing metal catalytic coupling reaction by utilizing organic anion-cation pair
InactiveCN102603448ALow costImprove solubilityMercapto/sulfide group formation/introductionOrganic compound preparationQuaternary ammonium cationFreeze-drying
The invention discloses a method for performing metal catalytic coupling reaction by utilizing an organic anion-cation pair, and belongs to the field of preparation of a high polymer material. The method for preparing the organic anion-cation pair comprises the following steps of: (a) mixing tetra-alkyl ammonium hydroxide or phosphine hydroxide with acid in water, and after dissolving, obtaining a reaction mixture; (b) stirring the obtained reaction mixture for 8-12 hours under the protection of nitrogen N2, thereby obtaining an ion exchange product; (c) cooling and drying the obtained ion exchange product for 24-48 hours, and after freeze-drying, obtaining a solid ion exchange product; and (d) vacuum-drying the obtained solid ion exchange product for 24-36 hours under the 30-50 DEG C temperature condition, thereby obtaining the organic anion-cation pair. The method for preparing the organic anion-cation pair is simple in process and convenient in preparation; the quaternary ammonium base is low in cost; the cost of base in the coupling reaction is efficiently reduced; the quaternary ammonium cation or quaternary phosphine cation is easy to dissolve in an organic solvent; and the problem that inorganic base is difficult to dissolve in the organic solvent is efficiently solved.
Owner:UNIV OF SCI & TECH OF CHINA
Synthetic method of asymmetrical thioether
ActiveCN106117096ACatalytic conditions are simpleCatalytic conditions are mildMercapto/sulfide group formation/introductionSugar derivativesHydrogenSulfur
The invention belongs to the field of chemistry, and relates to a synthetic method of asymmetrical thioether. The synthetic method comprises the following steps of (a) under the condition of catalysis of tetrabutyl ammonium halide, enabling a structural compound as shown in a formula (I), a structural compound as shown in a formula (II) and sulfur oxonium salt to react in a solvent so as to obtain the asymmetrical thioether with a structure as shown in formula (III), wherein R1 is selected from phenyl, substituted phenyl, naphtyl, substituted naphtyl, thienyl, or substituted thienyl; R2 is selected from hydrogen, phenyl, substituted phenyl, naphtyl, substituted naphtyl, thienyl or substituted thienyl; or R1, R2 and C connected with R1 and R2 form fluorene or thioxanthene; R3 is selected from hydrogen or an alkyl group; R4 is selected from hydrogen, phenyl, substituted phenyl, naphtyl, substituted naphtyl, thienyl or substituted thienyl; R5 is selected from hydrogen; or R4, R5 and C connected with R4 and R5 form the fluorene or the thioxanthene; R6 is selected form the alkyl group or the substituted alkyl group; and X is selected from C1, Br or I.
Owner:SUZHOU UNIV
Synthetic method of asymmetric thioether
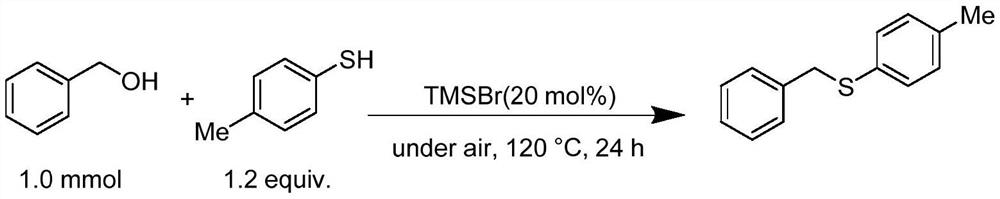
PendingCN112521240AWide variety of sourcesEasy to operateMercapto/sulfide group formation/introductionSulfide preparationThioetherSide product
The invention discloses a synthetic method of asymmetric thioether. According to the method, alcohol which is cheap, easy to obtain, wide in source, stable and low in toxicity is used as an alkylationreagent, trimethyl halosilane is used as a non-transition metal catalyst, a solvent is not needed, and an asymmetric thioether compound is directly synthesized through a high-selectivity dehydrationSalkylation reaction. According to the method, the use of a transition metal catalyst and alkali is avoided, the catalytic method is simple, the condition is simple, the operation is easy, the byproduct is water, the synthesis efficiency is high, and the selectivity is good.
Owner:YANGZHOU UNIV
Thioether compound and synthetic method and application thereof
InactiveCN108774098AReaction raw materials are readily availableEmission reductionMercapto/sulfide group formation/introductionSulfide preparationHalohydrocarbonOrganic synthesis
The invention belongs to the technical field of organic synthesis of sulfur-containing compounds and discloses a thioether compound and a synthetic method and application thereof. The thioether compound is prepared by the following steps: mixing acetone, a disulfide compound, inorganic base and 18-crown ether-6, heating and stirring, reacting under the action of a catalyst of inorganic base in theair atmosphere, concentrating and purifying to prepare the thioether compound. The molecular structural formula of the thioether compound is as shown in the formula (1) in the specification, whereinR1 and R2 are selected from hydrogen atom, alkyl group, alkoxy group, nitro group, amino group, hydroxyl group, halogeno, acyl group, aryl group or heterocyclic group. The use of high-toxicity halohydrocarbon, such as dichloromethane and diiodomethane is avoided by the method. The synthetic method has simple steps, is simple to operate and is feasible, is environmentally-friendly and has high conversion rate under mild conditions. Yield of the product reaches up to 96%. The thioether compound can be applied in the field of preparation of organic synthetic intermediates.
Owner:GUANGDONG UNIV OF TECH
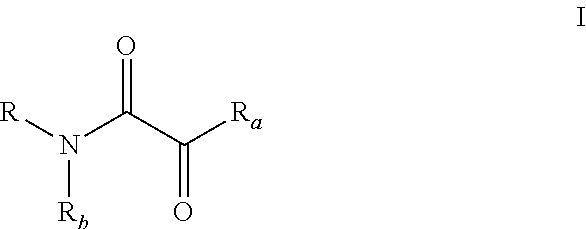
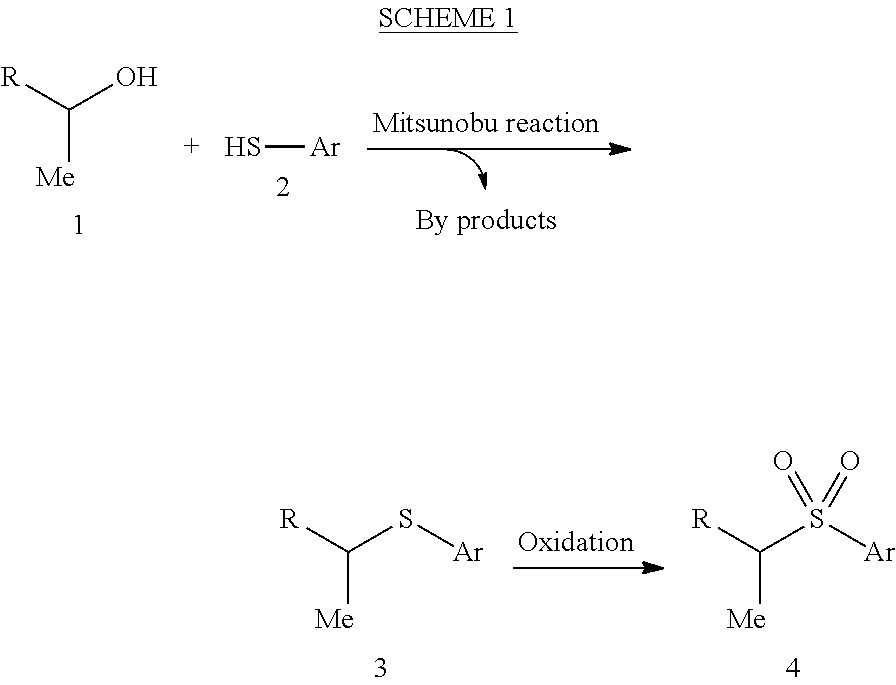
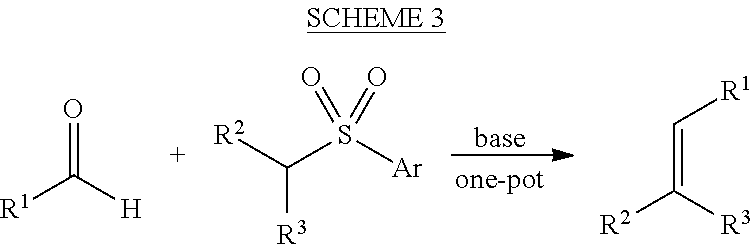
Preparation of [alfa]-sulfenylated carbonyl compounds from propargylic alcohols in one step
InactiveUS20150011796A1% atom efficiencyHigh yieldMercapto/sulfide group formation/introductionOrganic compound preparationArylThiol
The present relates to a method and a kit to produce an optically pure α-sulfenylated carbonyl compound comprising a primary or a secondary propargylic alcohol and an aryl thiol, a transition metal catalyst and a solvent.
Owner:KAT2BIZ INTERPARES KONSULT
Methyl aryl thioether compound, and synthetic method and applications thereof
InactiveCN106866327AEasy to operateWide variety of sourcesAntibacterial agentsMercapto/sulfide group formation/introductionNatural productPalladium catalyst
The invention discloses a methyl aryl thioether compound represented by formula 2, and a synthetic method and applications thereof. According to the synthetic method, in a reaction solvent, an aryl halide or an aromatic halide, dimethyl carbonate, and potassium thioacetate are taken as reaction raw materials, reaction is carried out in the presence of metal palladium catalyst under the action of a ligand and an alkali so as to obtain the methyl aryl thioether compound. The reaction conditions of the synthetic method are mild; the raw materials are cheap and easily available; reaction operation is simple; yield is relatively high. The methyl aryl thioether compound can be used for providing skeleton structures for the synthesis of a plurality of natural products and medicines, and can be widely applied in industrialized large-scale production.
Owner:EAST CHINA NORMAL UNIV
Synthetic method of medical intermediate carbonyl-replacement aryl thioether compound
InactiveCN105367465AHigh yieldMild responseMercapto/sulfide group formation/introductionOrganic compound preparationArylAfter treatment
The invention relates to a synthetic method of a carbonyl-replacement aryl thioether compound as shown in the formula (III). The method comprises the steps of making a compound in the formula (I) react with a compound in the formula (II) under the nitrogen atmosphere in organic solvent with the existence of a catalyst, organic ligand, an activating agent and alkali, conducting after-treatment after the reaction is ended, obtaining the compound as shown in the formula (III), wherein each R1 is independent C1-C6 alkyl; R2 is C1-C6 alkyl, C1-C6 alkoxy, halogen or nitryl; X is halogen. In conclusion, according to the synthetic method of the carbonyl-replacement aryl thioether compound, a novel composite reaction system is adopted, a target product high in yield can be obtained through comprehensive selection of the catalyst, the organic ligand, the activating agent and the alkali, the reaction process is mild, requirements of such fields as chemical engineering and medicine are satisfied, and the market prospect is broad.
Owner:张道敬
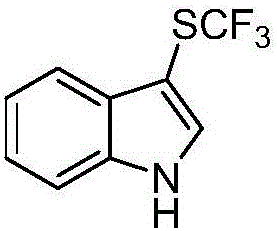
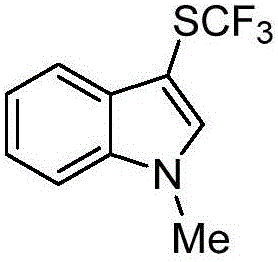
Method for preparing trifluoro-methylmercapto-substituted indole compound
InactiveCN106748608AChemically stableLow toxicityMercapto/sulfide group formation/introductionSulfonateEosin Y
The invention discloses a method for preparing a trifluoro-methylmercapto-substituted indole compound. The method comprises the following step: by taking trifluoro-methyl sodium sulfonate as a trifluoro-methylmercapto source and triphenylphosphine as a reducing agent, under the action of a visible light catalyst eosin Y, and enabling a substituted indole compound, the trifluoro-methyl sodium sulfonate and triphenylphosphine to react with N-chloro-o-phthalimide, thereby obtaining the trifluoro-methylmercapto-substituted indole compound. The method disclosed by the invention is simple, a trifluoro-methylmercapto reagent used in the method is low in toxicity, and no heating is needed in the preparation process, so that the energy can be saved, the environment can be protected, and direct trifluoro-methylmercapto reaction of a 3-position C (sp2)-H bond of an indole compound is achieved.
Owner:NANJING UNIV OF SCI & TECH
Modified cyclodextrins for the selective sequestration of fentanyl related compounds and uses thereof
ActiveUS20180371110A1Mercapto/sulfide group formation/introductionOrganic active ingredientsMedicineCyclodextrin
Novel thioalkylcarboxylate-modified CDs and pharmaceutical compositions comprising these thioalkylcarboxylate-modified CDs are disclosed, as well as methods of using the disclosed thioalkylcarboxylate-modified CDs and pharmaceutical compositions thereof to neutralize or reduce undesired effects or symptoms associated with one or more fentanyl related compounds in a subject in need thereof. The use of the disclosed thioalkylcarboxylate-modified CDs to detect the presence of one or more fentanyl related compounds in a sample is also disclosed, which comprises contacting the sample with said thioalkylcarboxylate-modified CDs or a composition comprising these CDs.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Vulcanizing agent for hydrogenation catalyst and preparation method of vulcanizing agent
ActiveCN108097333AHigh sulfur contentModerate decomposition temperatureMercapto/sulfide group formation/introductionCatalyst activation/preparationSolubilityDecomposition
The invention provides a vulcanizing agent for a hydrogenation catalyst and a preparation method of the vulcanizing agent. Sulfur content of the vulcanizing agent is 40wt%-80wt%, and the vulcanizing agent contains straight-chain polysulfide and cyclic polysulfide. The preparation method comprises steps as follows: elemental sulfur and a catalyst are weighed proportionally and added to a reactor, the reactor is closed and heated, and then olefins are added to the reactor; pressure in the reactor is increased continuously, when pressure in the reactor reaches a certain value, an air release valve of the reactor is opened, gas in the reaction system is released, pressure in the reactor is kept in a constant-value range, the reaction is performed for a period of time in the reaction state, andafter separation, the vulcanizing agent is obtained. The problems of higher toxicity, high production cost, low safety performance, difficulty in transportation and the like of an existing vulcanizing agent are solved, and the organic polysulfide vulcanizing agent which contains simple components and is high in sulfur content, wide in decomposition temperature range and good in oil solubility isprovided.
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthetic method of allyl halosulfide
ActiveCN107746381BEasy to useEasy to operateMercapto/sulfide group formation/introductionSulfide preparationSynthesis methodsSulfide
The invention discloses an allyl halogenated sulfide synthesis method, wherein a phenylacetylene derivative is used as raw material and completely reacts at a temperature of 60-80 DEG C in the presence of a free radical initiator azoisobutyronitrile by using acetonitrile as a solvent and using mercaptan and N-halogenated succinimide as addition reagents, and after the reaction is completely performed, the reaction liquid is subjected to separation purification to obtain the allyl halogenated sulfide. According to the present invention, the synthesis method has advantages of reasonable processconditions, simple operation, stable conversion rate, stable yield and broad substrate range, uses the simple and stable halogenated reagent so as to convenient treat, does not require metal catalysts, and is the efficient method for constructing the allyl C-S bond and C-X bond.
Owner:上海贝通色彩科技有限公司 +1
Preparation method of aryl monothioether compound
ActiveCN111635343AReduce manufacturing costQuick responseMercapto/sulfide group formation/introductionSulfide preparationHalohydrocarbonGrignard reagent
The invention discloses a preparation method of an aryl monothioether compound. The method comprises the following steps: reacting halogenated hydrocarbon with an isopropyl magnesium halide Grignard reagent at -20 DEG C for 30 minutes until a halogen-magnesium exchange reaction is completely completed; cooling a reaction solution to -78 DEG C, slowly dropwise adding a tetrahydrofuran solution of asubstituted diphenyl disulfide compound into the newly prepared Grignard reagent, maintaining the concentration of the reactants to be 0.5-1 mmol / mL, carrying out a stirring reaction for 1 hour in anorganic solvent at -78 DEG C, and then slowly heating the reaction solution to room temperature; and carrying out a quenching reaction by using a saturated ammonium chloride solution, extracting an organic phase by using ethyl acetate or diethyl ether, drying the organic phase by using anhydrous magnesium sulfate, and concentrating the organic phase to obtain the aryl thioether compound. The method is simple in preparation process, low in cost, high in speed, easy to operate and small in environmental pollution, groups sensitive to Grignard reagents can also be tolerated, and high yield is obtained.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Popular searches
Sugar derivatives preparation Reverse osmosis Ether/acetal/ketal group formation/introduction Chemical recycling Carboxylic acid amides preparation Amino-carboxyl compound preparation Amino group formation/introduction Amino-hyroxy compound preparation Hydroxy group formation/introduction Catalytic reactions
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
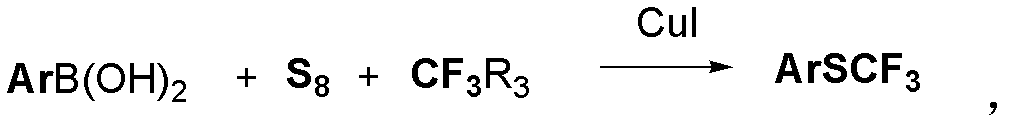
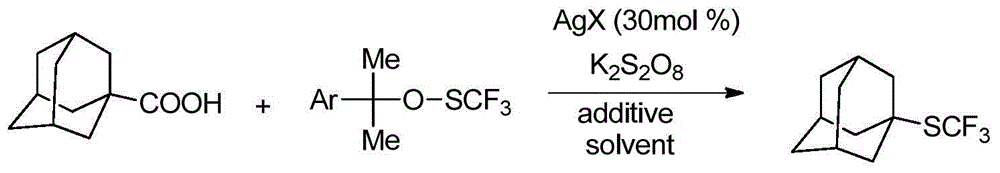
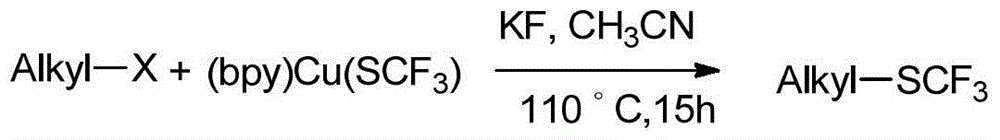
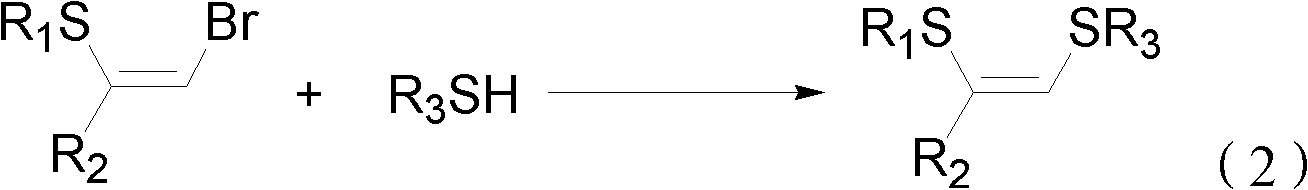
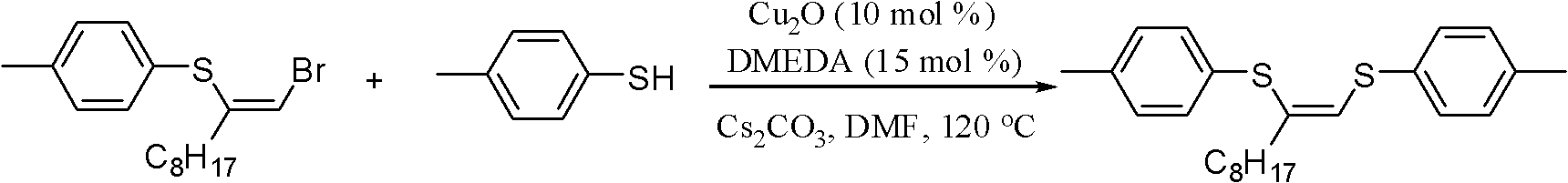
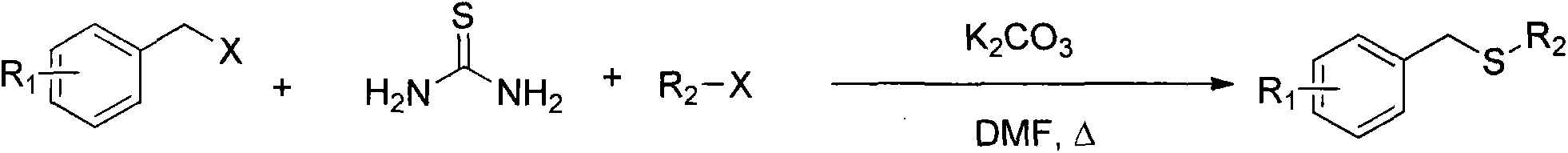
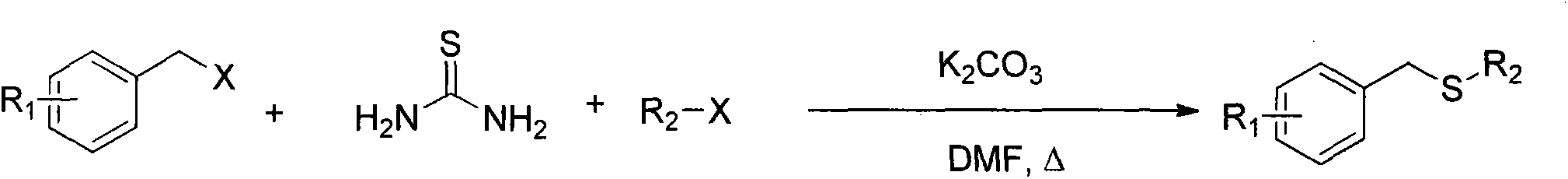

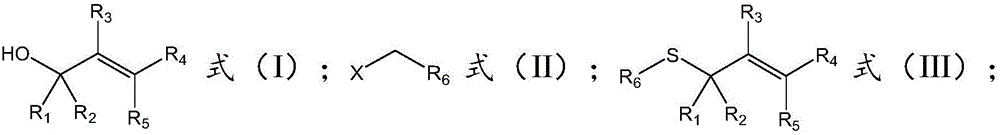
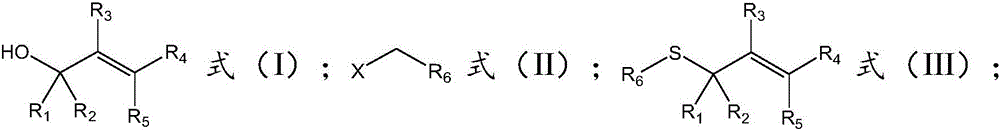
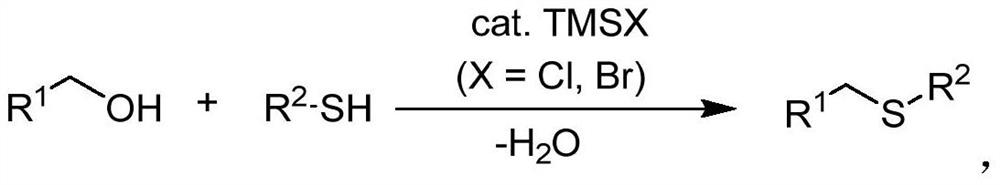
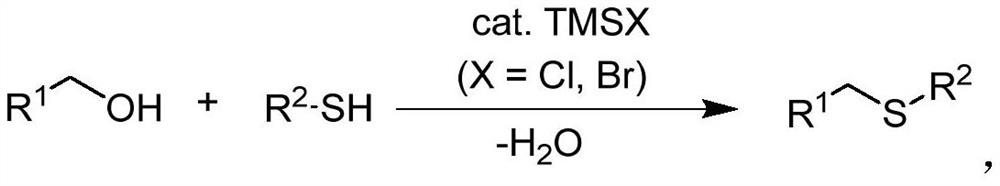
![Preparation of [alfa]-sulfenylated carbonyl compounds from propargylic alcohols in one step Preparation of [alfa]-sulfenylated carbonyl compounds from propargylic alcohols in one step](https://images-eureka.patsnap.com/patent_img/5fa8abe1-56ac-4d7f-86f9-138044016e9c/US20150011796A1-20150108-C00001.PNG)
![Preparation of [alfa]-sulfenylated carbonyl compounds from propargylic alcohols in one step Preparation of [alfa]-sulfenylated carbonyl compounds from propargylic alcohols in one step](https://images-eureka.patsnap.com/patent_img/5fa8abe1-56ac-4d7f-86f9-138044016e9c/US20150011796A1-20150108-C00002.PNG)