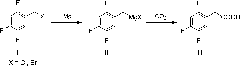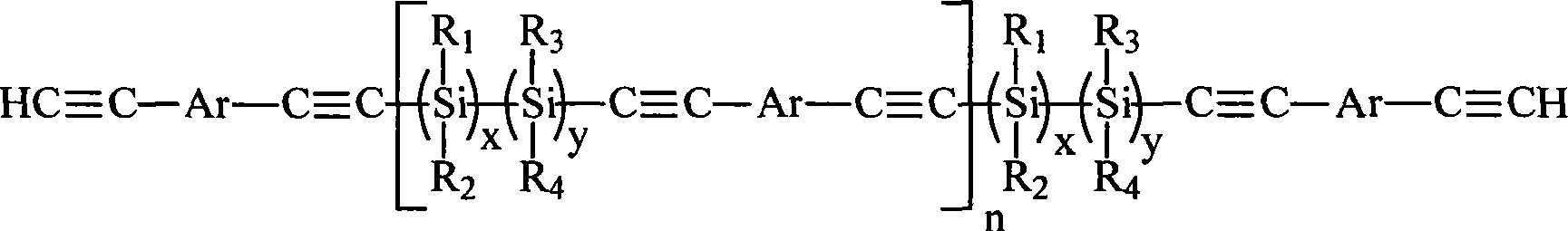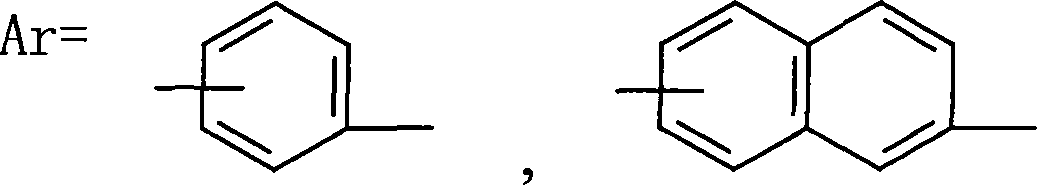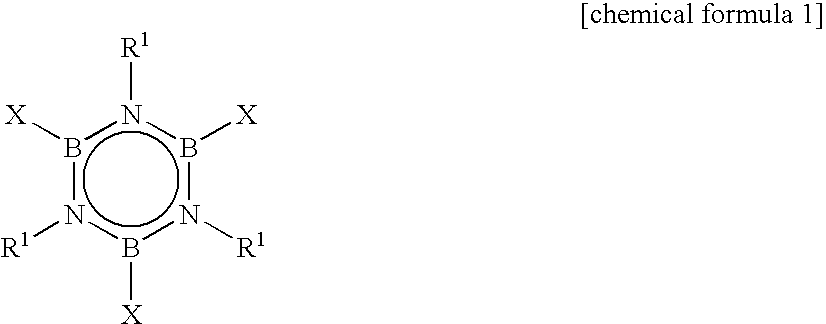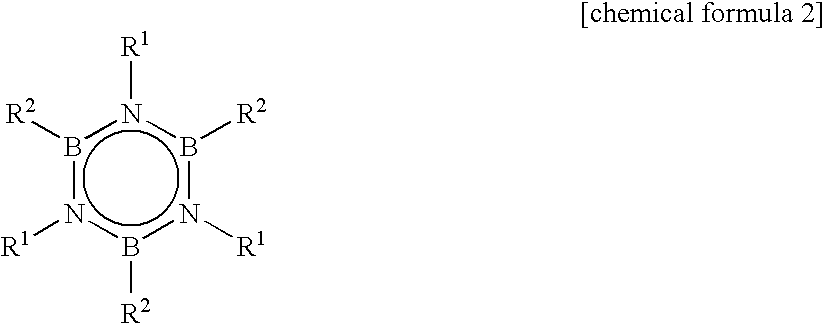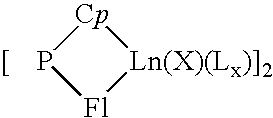Patents
Literature
1544 results about "Grignard reagent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
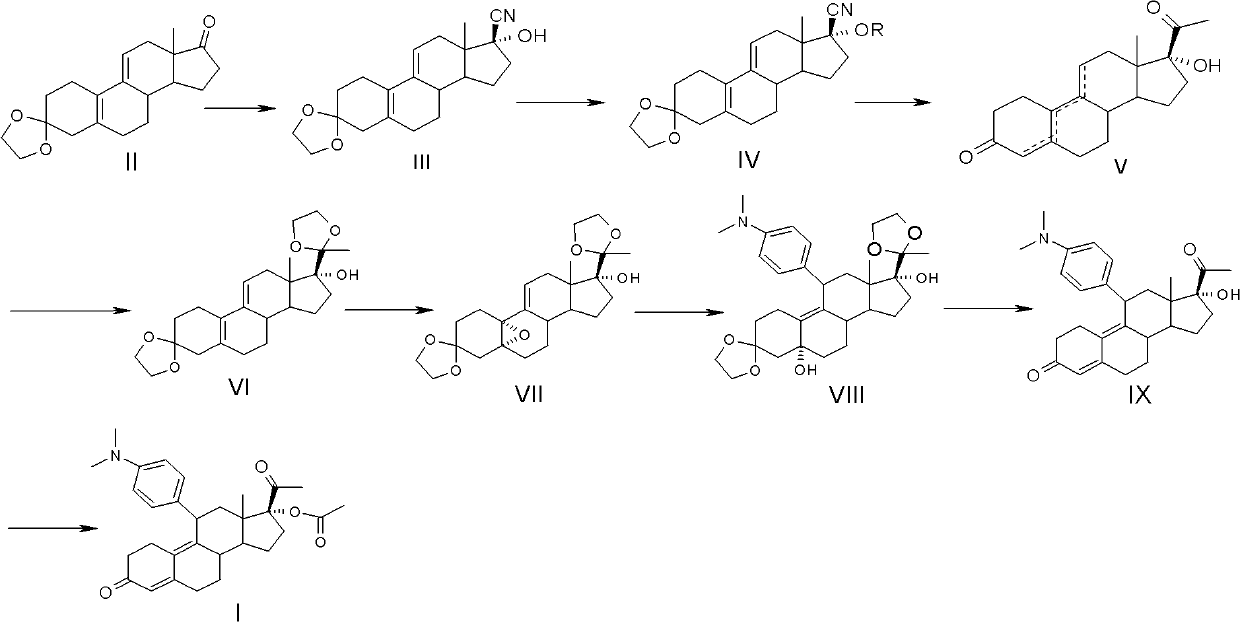
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
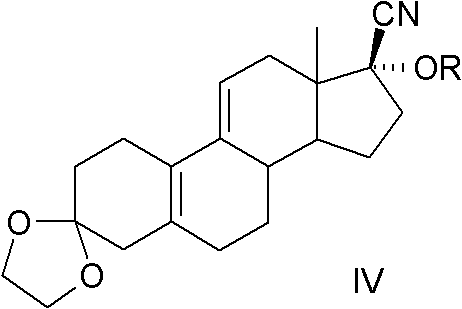
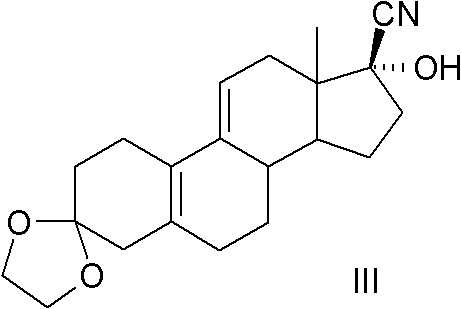
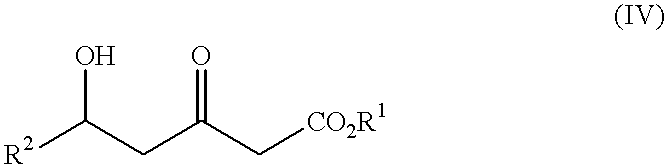
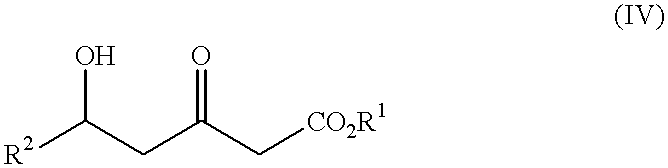
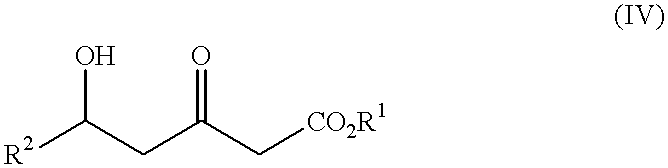
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Electrolyte for magnesium battery
InactiveUS20130034780A1Safe handlingReduce environmental impactCell electrodesOrganic electrolyte cellsMagnesium saltGrignard reagent
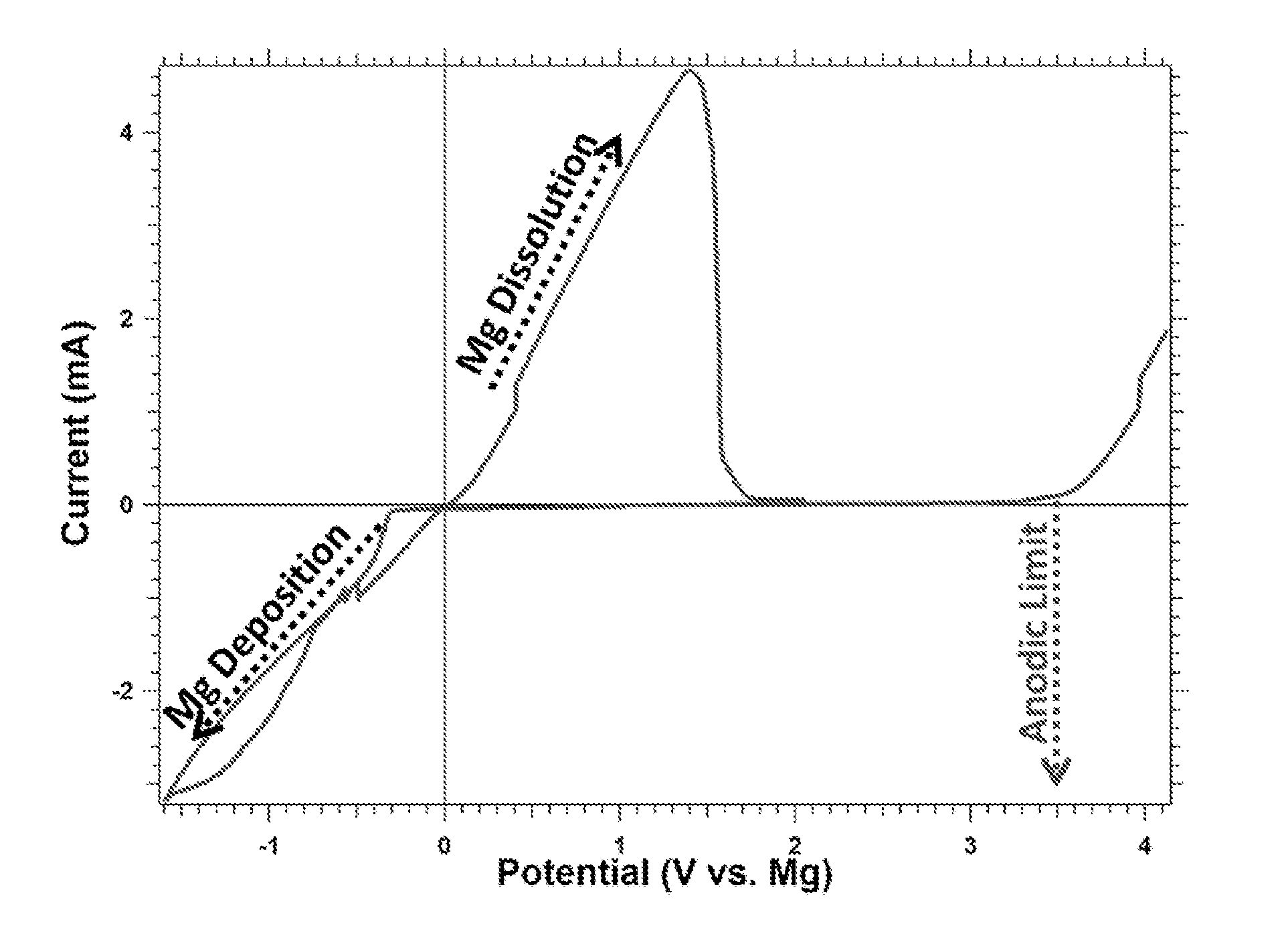
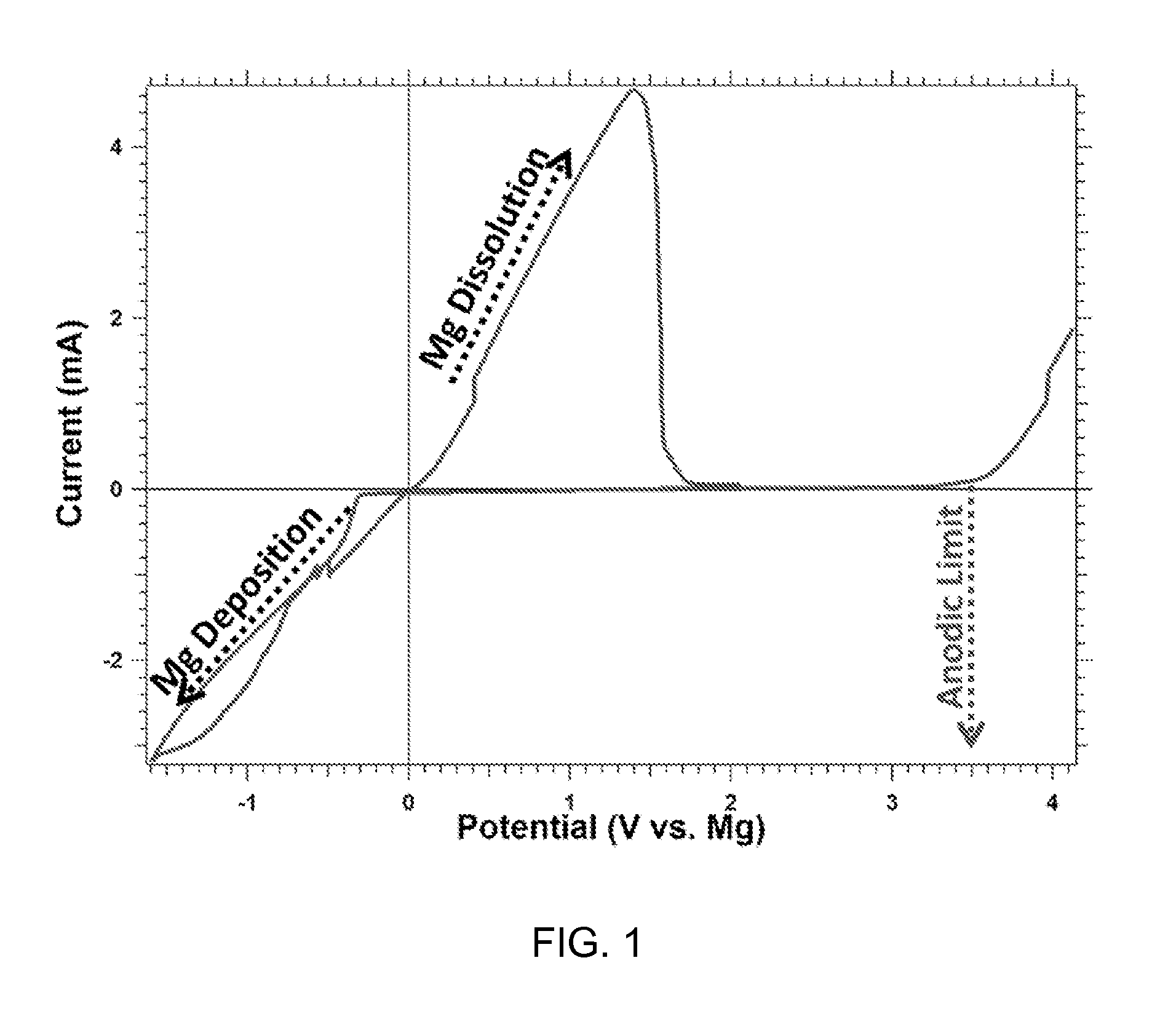
A magnesium battery, having an anode containing magnesium; a cathode stable to a voltage of at least 2.6 V relative to a magnesium reference; and an electrolyte containing an electrochemically active magnesium salt obtained by reaction of a Grignard reagent or Hauser base with a boron compound of formula BR3 is provided. The electrolyte is stable to 2.6 E.V. vs. Mg in the presence of stainless steel.
Owner:TOYOTA JIDOSHA KK
Method for preparing medetomidine and its salts.
ActiveUS20100048915A1Increase productionHigh yieldOrganic active ingredientsOrganic chemistryGrignard reagentMedetomidine
The invention provides an improved, highly efficient method for preparing Medetomidine, and its salts, in particular its pharmaceutically acceptable salts. The method utilizes the high reactivity of halogenated imidazoles towards transmetalation with Grignard reagents and the subsequent reaction with 2,3-dimethylbenzaldehyde.
Owner:GRINDEKS
Preparation method of ulipristal acetate and key intermediate thereof
ActiveCN102516345AHigh puritySimple methodKetal steroidsSteroids preparationEthylenedioxyGrignard reagent
Owner:UTOPHARM SHANGHAI +1
High voltage rechargeable magnesium batteries having a non-aqueous electrolyte
InactiveUS20130252112A1Improve conductivityImprove solubilityOrganic electrolyte cellsSecondary cellsGrignard reagentMetallic materials
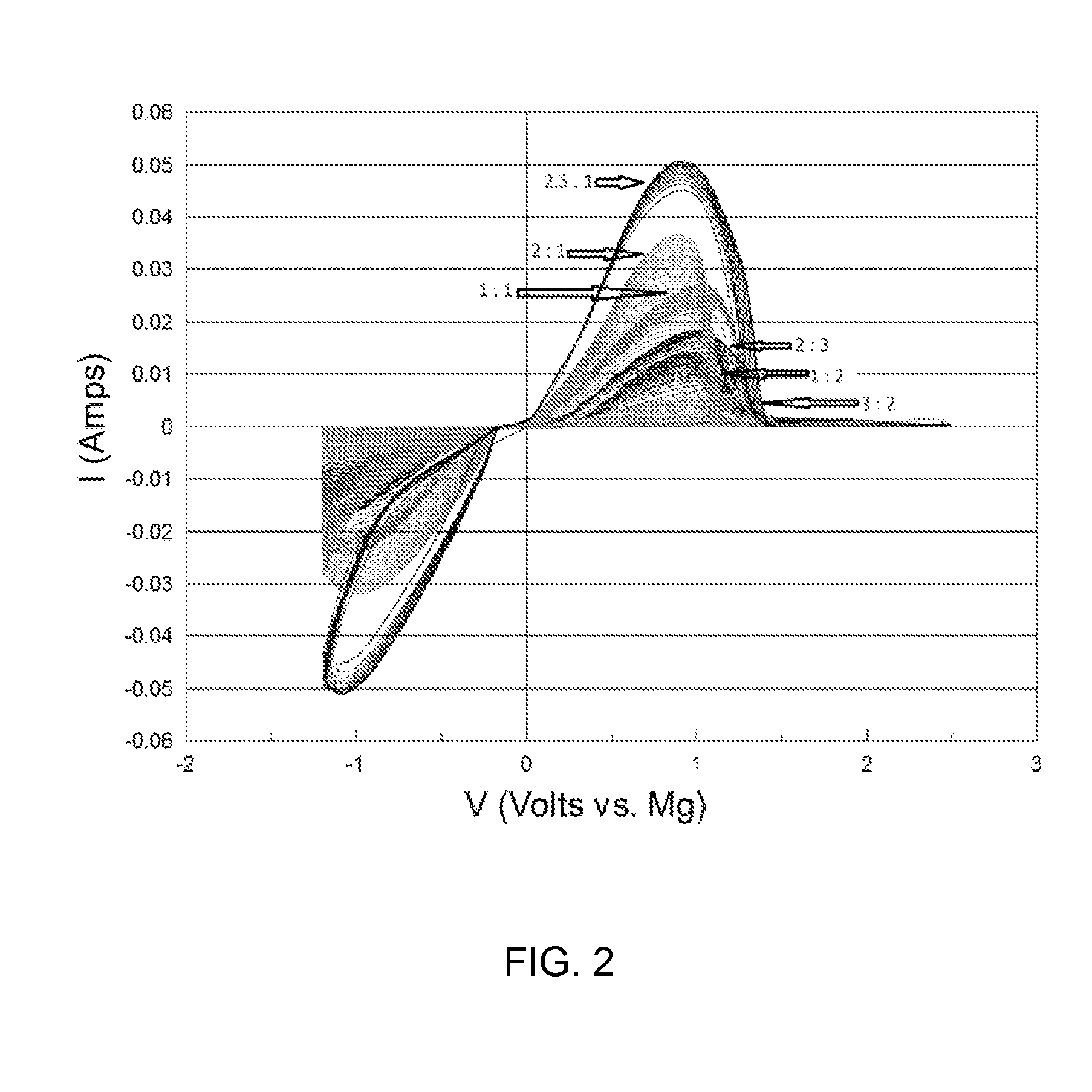
A rechargable magnesium battery having an non-aqueous electrolyte is provided. The properties of the electrolyte include high conductivity, high Coulombic efficiency, and an electrochemical window that can exceed 3.5 V vs. Mg / Mg+2. The use of the electrolyte promotes the electrochemical deposition and dissolution of Mg without the use of any Grignard reagents, other organometallic materials, tetraphenyl borate, or tetrachloroaluminate derived anions. Other Mg-containing electrolyte systems that are expected to be suitable for use in secondary batteries are also described.
Owner:PELLION TECH
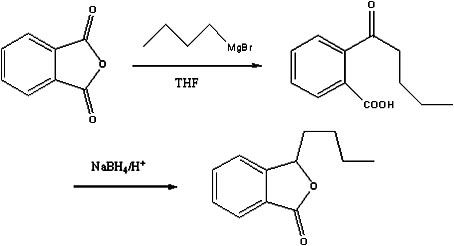
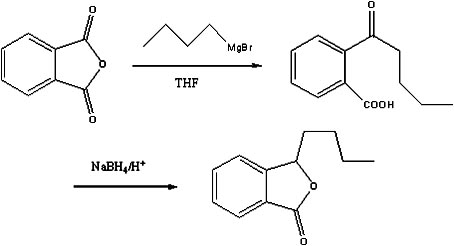
Preparation method of butylphthalide
InactiveCN101962374AReaction raw materials are readily availableFew reaction stepsOrganic chemistryBenzoic acidGrignard reagent
The invention discloses a preparation method of butylphthalide, which comprises the following steps: taking phthalic anhydride as a raw material; enabling the phthalic anhydride to carry out addition reaction with the Grignard reagent of butyl halide to obtain an intermediate of o-valeryl benzoic acid; and then, reducing by sodium borohydride, and carrying out acidic cyclization to obtain the butylphthalide. The phthalic anhydride and the butyl halide which are used as raw materials in the preparation method of the butylphthalide of the invention are commercial products, and the reaction raw materials can be obtained easily. Because the Grignard reaction, the sodium borohydride reduction and the acidic cyclization are classical reactions, the operation is simple, the industrialized production can be realized easily, the yield of the butylphthalide reaches 50-60%, and the purity of the butylphthalide reaches 97-98%.
Owner:SHANGHAI INST OF TECH
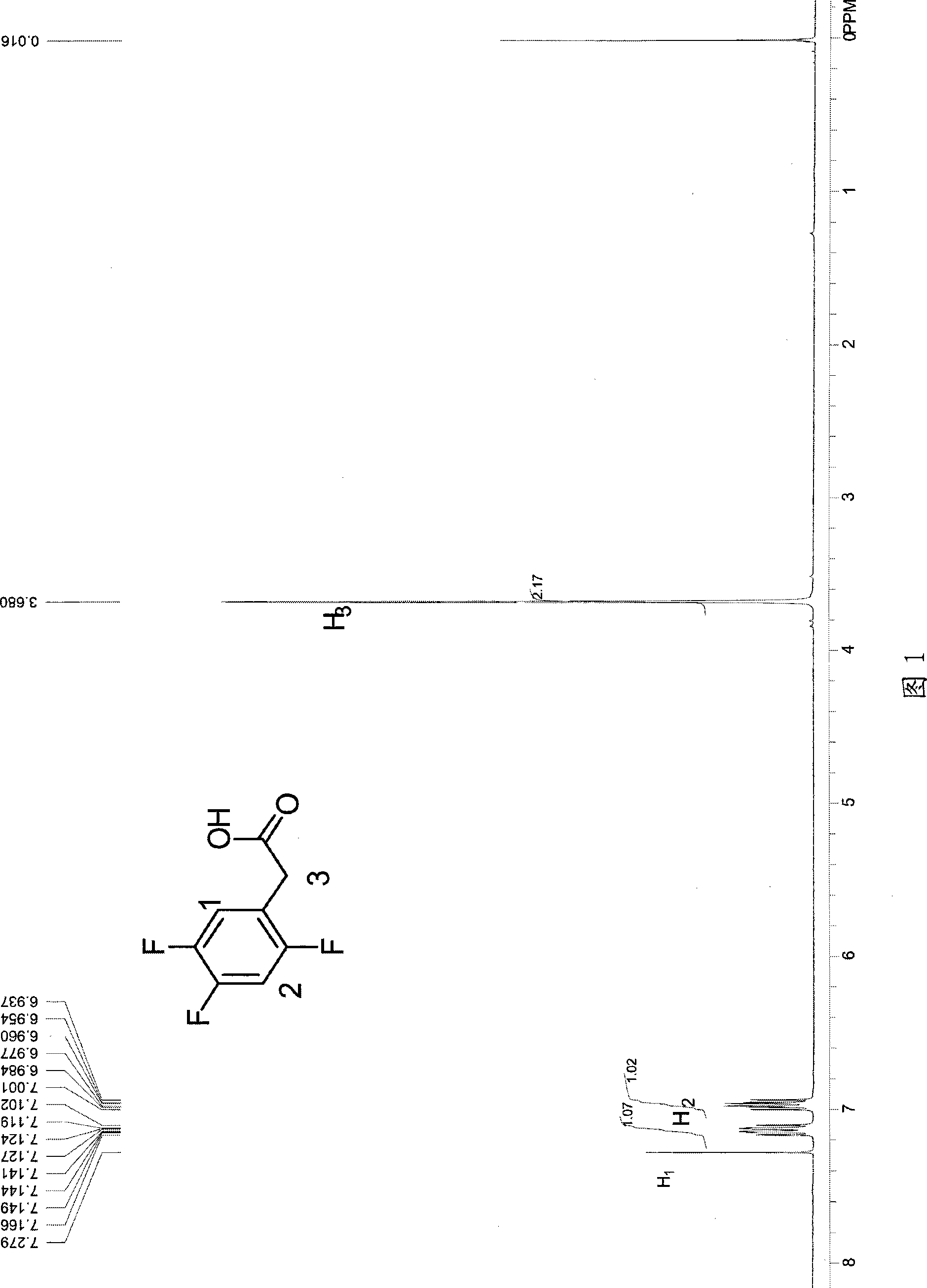
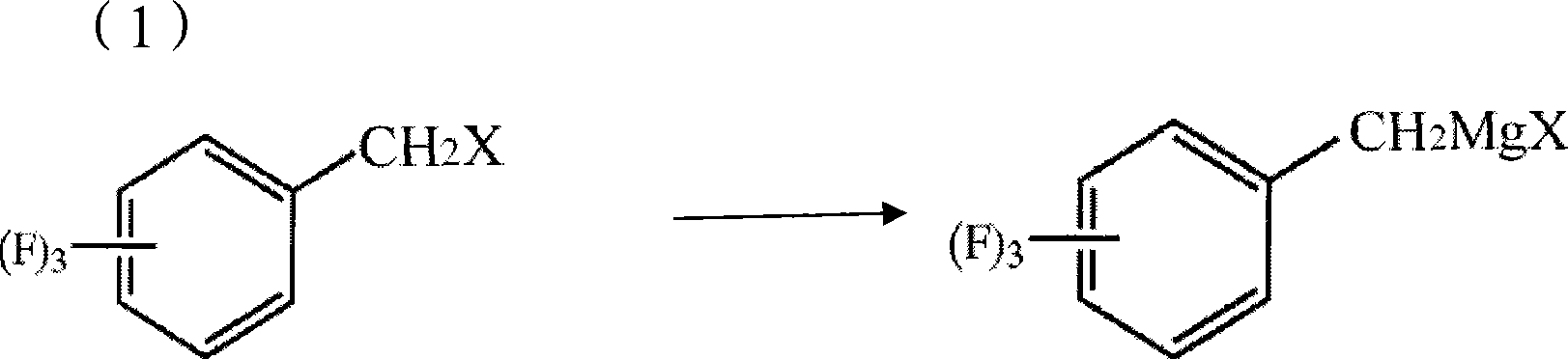
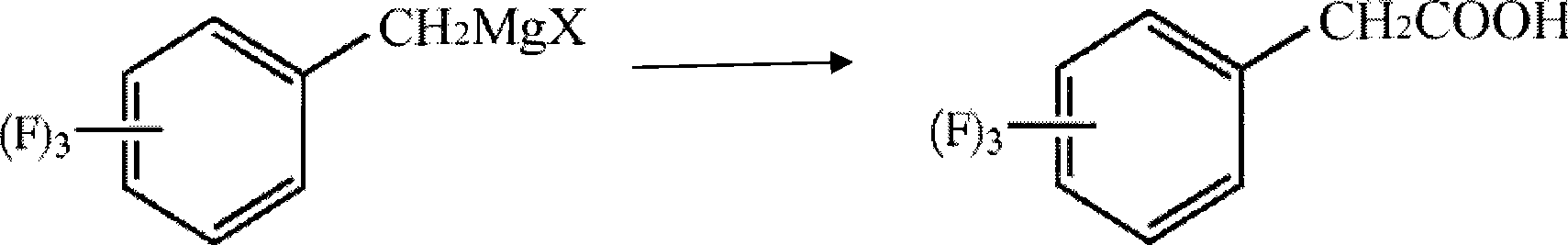
Process for producing trifluoro benzene acetic acid and sitagliptin
ActiveCN101429115AHigh process yieldHigh purityOrganic compound preparationCarboxylic compound preparationSitagliptinBenzene
The invention discloses a method for preparing trifluoro-phenylacetic acid. The method comprises the following steps: (1) in the presence of an evocating agent, trifluoro-benzyl halides and magnesium in an organic solvent react to obtain a Grignard reagent; (2) carbon dioxide gas is introduced into the Grignard reagent for reaction; and (3) a product obtained in the step (2) is hydrolyzed to obtain the trifluoro-phenylacetic acid. The invention also discloses a method for preparing sitagliptin. The method has the characteristics of high yield, good purity, low cost, simple process, mild condition, few three wastes and good safety, and is suitable for industrialized production.
Owner:ZHEJIANG HISOAR PHARMA
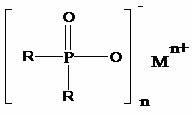
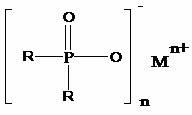
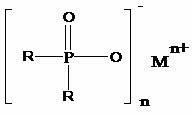
Preparation method of dialkyl hypophosphite
InactiveCN102050835AHigh purityAvoid it happening againGroup 5/15 element organic compoundsPhosphorous acidOrganic solvent
The invention discloses a preparation method of dialkyl hypophosphite. The structure of the dialkyl hypophosphite is disclosed as a Formula (I). The preparation method of the dialkyl hypophosphite comprises the following steps: preparing a Grignard reagent from alkylogen, magnesium powder and organic solvent, preparing dialkyl phosphorus oxide from the Grignard reagent and diethylester phosphite,carrying out reaction on the dialkyl phosphorus oxide and an oxidant, acidifying to obtain a dialkyl phosphinic acid solution, and carrying out reaction on the dialkyl phosphinic acid solution and a metal salt to obtain the corresponding dialkyl hypophosphite. The preparation method of dialkyl hypophosphite has the advantages of high yield and simple synthesis technique, is convenient to operate,greatly lowers the production cost, simplifies the production equipment and enhances the safety of the production process.(I).
Owner:GUANGZHOU KINGSKY MATERIAL
Processes for the preparation of 5-hydroxy-3-oxopentanoic acid derivatives
InactiveUS6340767B1Increase productionGreat advantageCarboxylic acid nitrile preparationOrganic compound preparationAcetic acid3-Hydroxypropionic acid
This invention provides a process for producing a 5-hydroxy-3-oxopentanoic acid, a useful pharmaceutical intermediate, easily from a readily available, inexpensive starting material without using any extraordinary production equipment such as a very-low-temperature reactor.Thus, this invention provides a process for producing a 5-hydroxy-3-oxopentanoic acidwhich comprises permitting a lithium amide to act upon a mixture of an acetic acid ester and a 3-hydroxypropionic acid derivative at not below -20° C.Further, this invention also provides a process for producing a 5-hydroxy-3-oxopentanoic acidwhich comprises treating a mixture of an acetic acid ester and a 3-hydroxypropionic acid derivative with a Grignard reagent to prepare a mixture of a compound and an acetic acid ester of the above formula (I),and permitting a lithium amide to act upon the mixture at a temperature not below -20° C.
Owner:KANEKA CORP
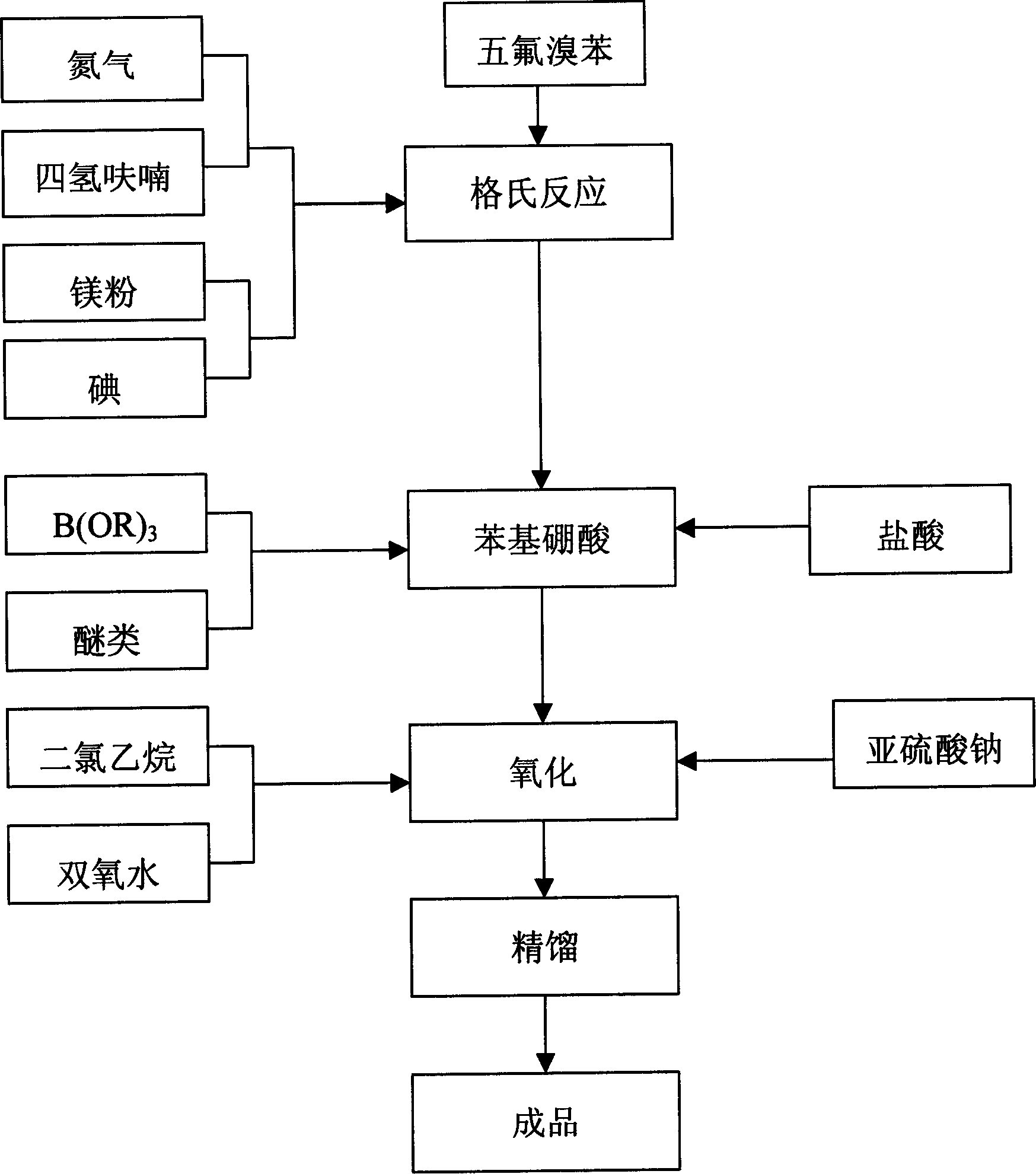
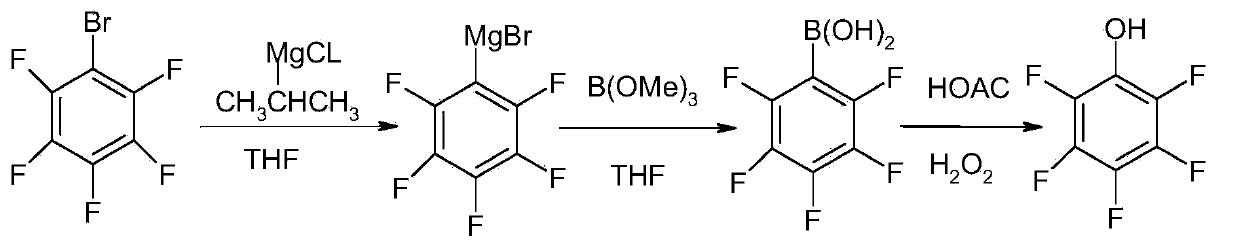
Process of producing pentafluorophenol
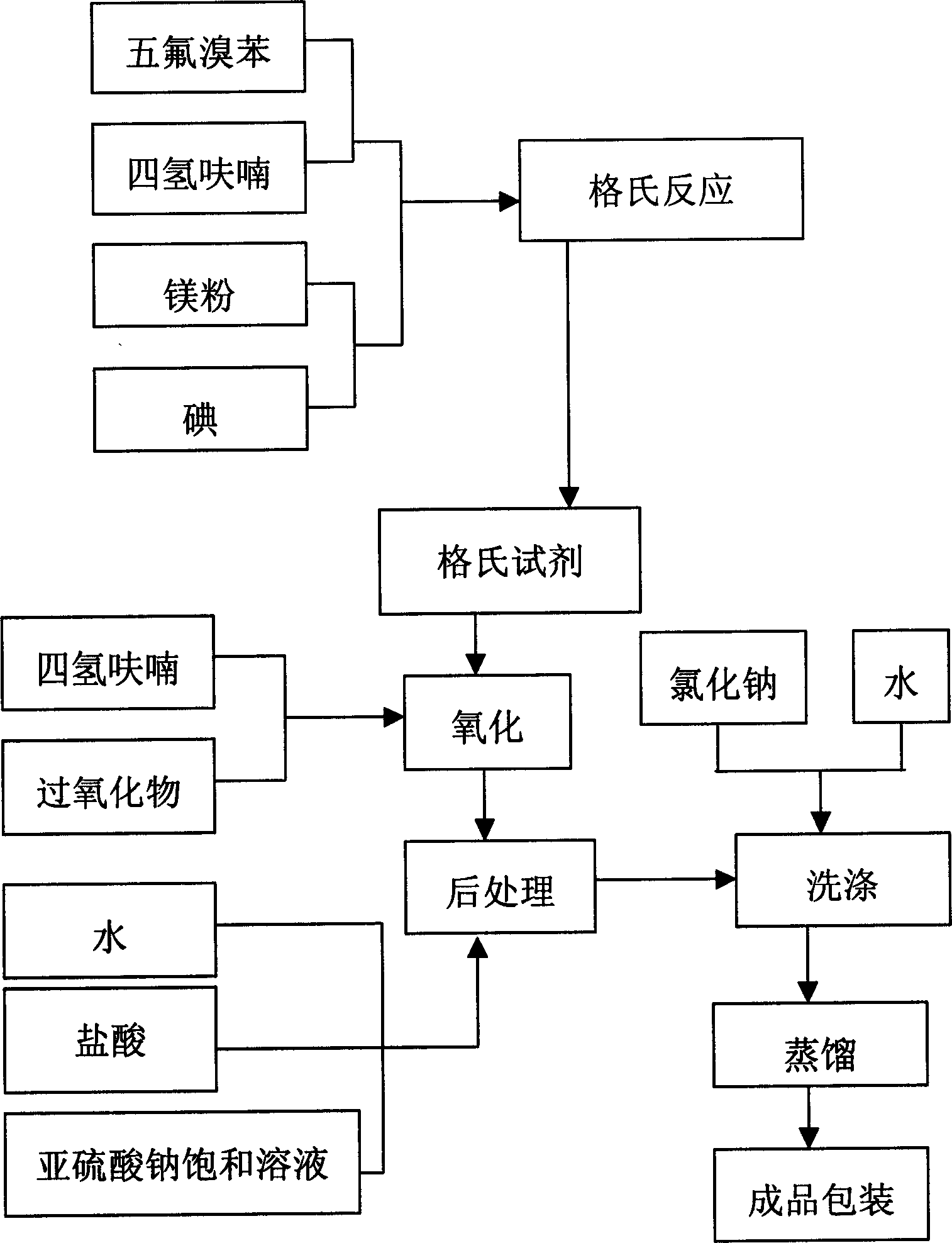
ActiveCN1847210ASimple production processHigh purityOrganic chemistryOrganic compound preparationGrignard reagentGrignard reaction
The present invention provides process of preparing pentafluoropheol. Pentafluoro bromobenzene as initial material is first made to produce Grignard reaction to result in Grignard reagent, and the Grignard reagent is then oxidized with peroxide to prepare pentafluoropheol. The process of the present invention has simple technological process, no need of special apparatus, high product yield, high product purity and low production cost.
Owner:内蒙古永太化学有限公司
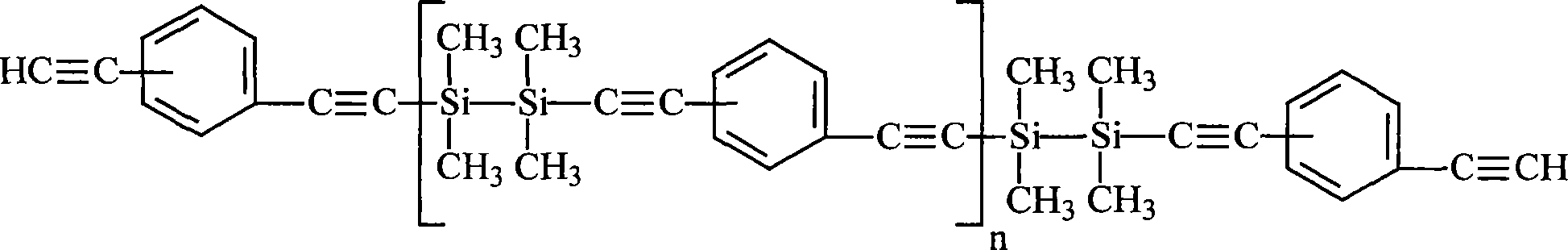
Novel polycarbosilane and preparation method thereof
The invention relates to novel polycarbosilane and a preparation method thereof. A polycarbosilane ceramic precursor is prepared by adopting a Grignard reagent coupling method; a molecular structure contains unsaturated groups such as a Si-H bond, C=C and the like; the precursor can be crosslinked and cured at a certain temperature, and has low curing weight loss and high manufacturability; the Si / C ratio in the precursor and the process performance of a precursor product can be adjusted effectively by adjusting the functionality and feed ratio of a chlorosilane monomer serving as a reactant and optimizing reaction conditions; and the obtained product has excellent heat resistance, high ceramic yield, low free carbon content in ceramic and high SiC ceramic phase purity, is suitable for serving as a high-performance SiC ceramic precursor, and can be used for preparing an ultrahigh-temperature ceramic-based composite material submerged substrate as well as high-performance materials such as SiC ceramic coatings, fibers and the like.
Owner:AEROSPACE RES INST OF MATERIAL & PROCESSING TECH +1
High-performance rechargeable magnesium battery and manufacturing method thereof
ActiveCN102024996AMild conditions for material preparationHigh specific capacityFinal product manufactureCell electrodesGrignard reagentPolypropylene
The invention discloses a high-performance rechargeable magnesium battery consisting of a positive plate, a negative plate, a diaphragm and an electrolyte. The positive plate is made from highly stripped nano-supramoly which is of highly erosive structure, wherein the average number of the layers of the nano-supramoly is not more than 4, and the average thickness is not more than 3nm. The negative plate is made from grain-shaped nanometer-level magnesium or nanometer-micrometer level composite magnesium, and the average grain diameter is 1-10nm. The diaphragm is a three-layer film made from polythene, polypropylene and polyethylene. The electrolyte is made from a tetrahydrofuran solution of Grignard reagent derivate. The invention has the advantages that: the rechargeable battery has gentler material preparation conditions (from the room temperature to 150 DEG C), larger specific capacity (170mAhg<-1>), higher operating voltage (1.8V), better circulation performance (still keeping 95%of initial capacity after circulating for 50 periods) and the like compared with the reported magnesium secondary battery system, and the rechargeable battery can be applied to the next generation large-scale energy storage batteries.
Owner:NANKAI UNIV
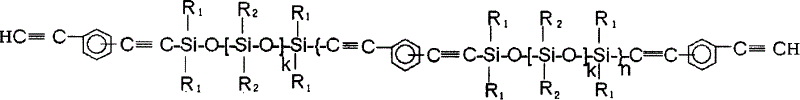
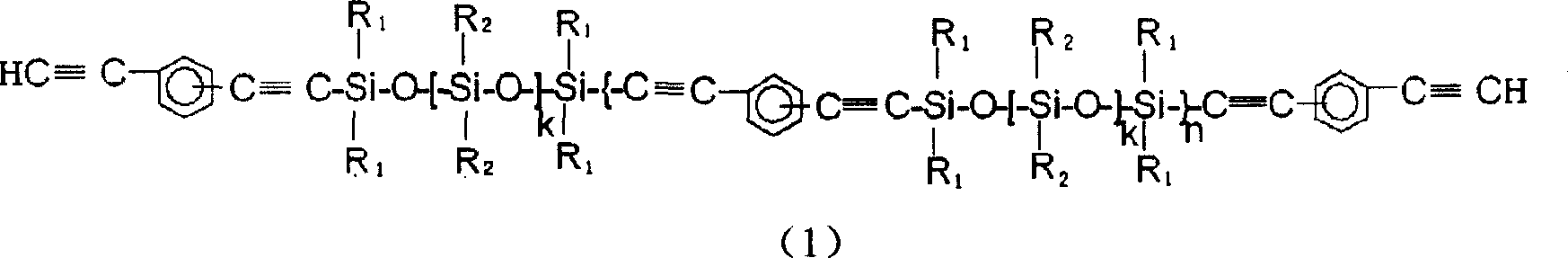
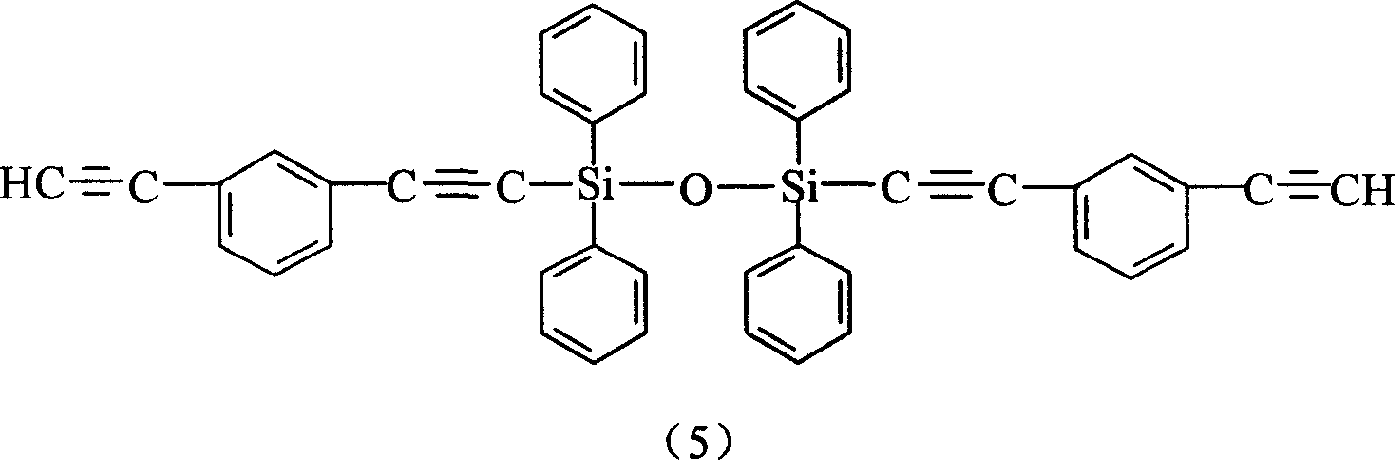
Aryne resin containing silicone
InactiveCN1709928AImprove flexibilityGood high temperature ceramic performanceSilanesGrignard reagent
The invention relates to a kind of arylenealkyne resin containing siloxanes. The said resin makes diethynylene benzene (DEB) and silicon alkyl chloride as raw materials, firstly make use of the reaction of magnesium and alkyl bromide to produce Grignard reagentú¼then react with DEB to produce alkyne Grignard reagent; then react with silicon alkyl chloride, finally hydrolyze and condense in alkaline water liquor, or extend chain with siloxanes chloride, to form a kind of arylenealkyne resin containing siloxanes of new-style structure. The resin designed and synthesized by the invention has good processing property, and can be solidified into crosslinked resin of good thermostability under heat, light or radiative condition. Besides it has good machine capability, electric insulation capability and ceramic performance. So it can be used as the base material of high performance compound material resin, insulation material, the ceramic precursors and so on, and also has wide application prospect in such high technology fields as spaceflight, aviation, navigation and so on.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing 2,4,5-trifluorophenylacetic acid
InactiveCN101823952AMild conditionsEasy to industrializeOrganic compound preparationCarboxylic compound preparationHalogenOrganic solvent
The invention belongs to the field of fine chemical engineering, and relates to a method for preparing 2,4,5-trifluorophenylacetic acid. The method is characterized by comprising the following steps of: reacting 2,4,5-trifluorobenzyl halogen serving as a raw material with magnesium metal in an anhydrous organic solvent to obtain a 2,4,5-trifluorobenzyl Grignard reagent, wherein an initiator is used or not used during the preparation of the Grignard reagent; reacting the 2,4,5-trifluorobenzyl Grignard reagent with carbon dioxide to obtain 2,4,5-trifluorophenylacetate; and finally, acidifying and extracting the 2,4,5-trifluorophenylacetate to obtain a coarse 2,4,5-trifluorophenylacetic acid product, and re-crystallizing the coarse product to obtain the 2,4,5-trifluorophenylacetic acid. The method has the advantages of short synthetic route, mild condition, environmental friendliness and easy implementation of industrialization; the obtained product has high purity; and the method uses carbon dioxide greenhouse gas as a carbon source, so the raw material is easily obtained and cheap.
Owner:DALIAN UNIV OF TECH
Industrialization production process of entecavir-monohydrate
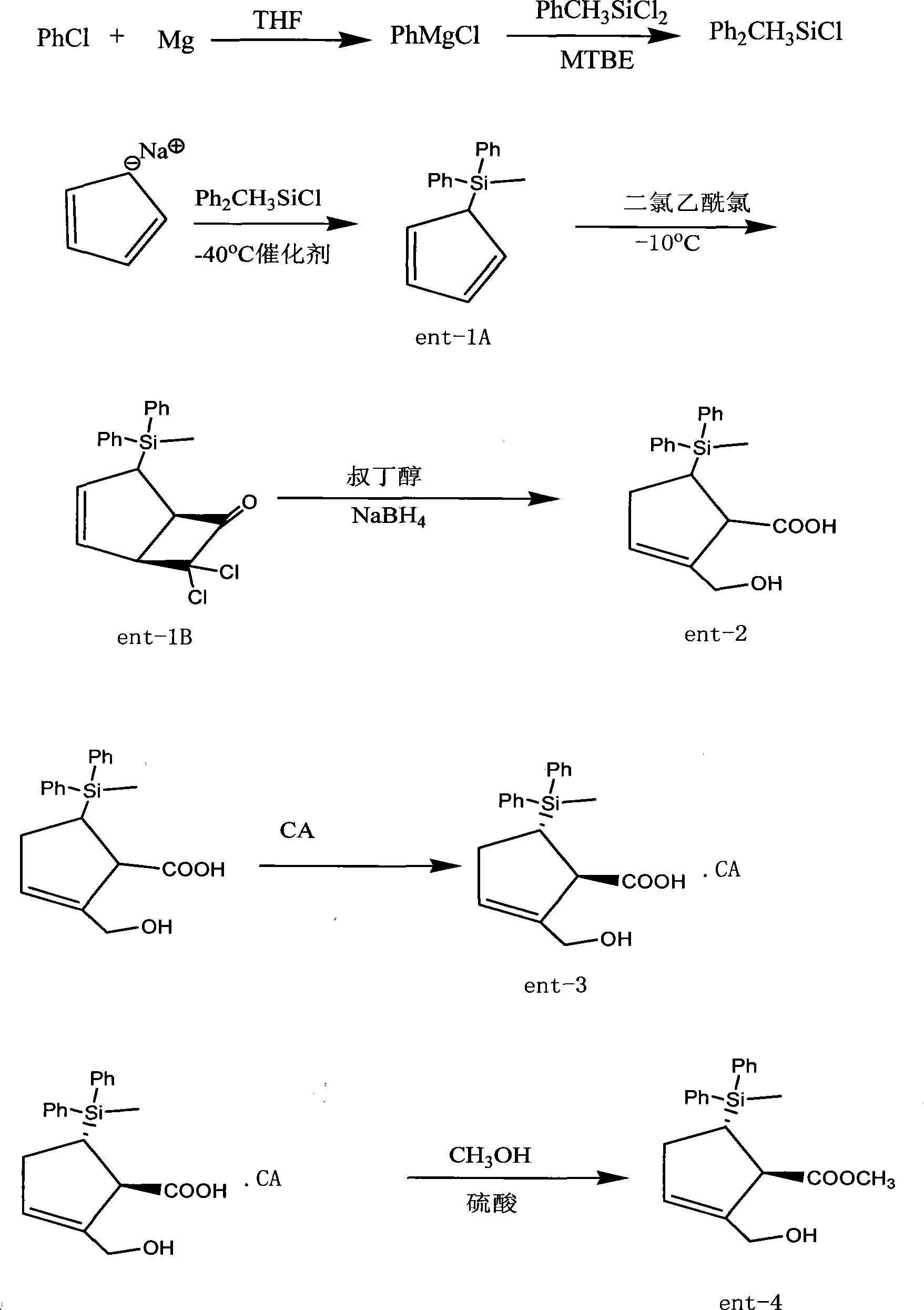
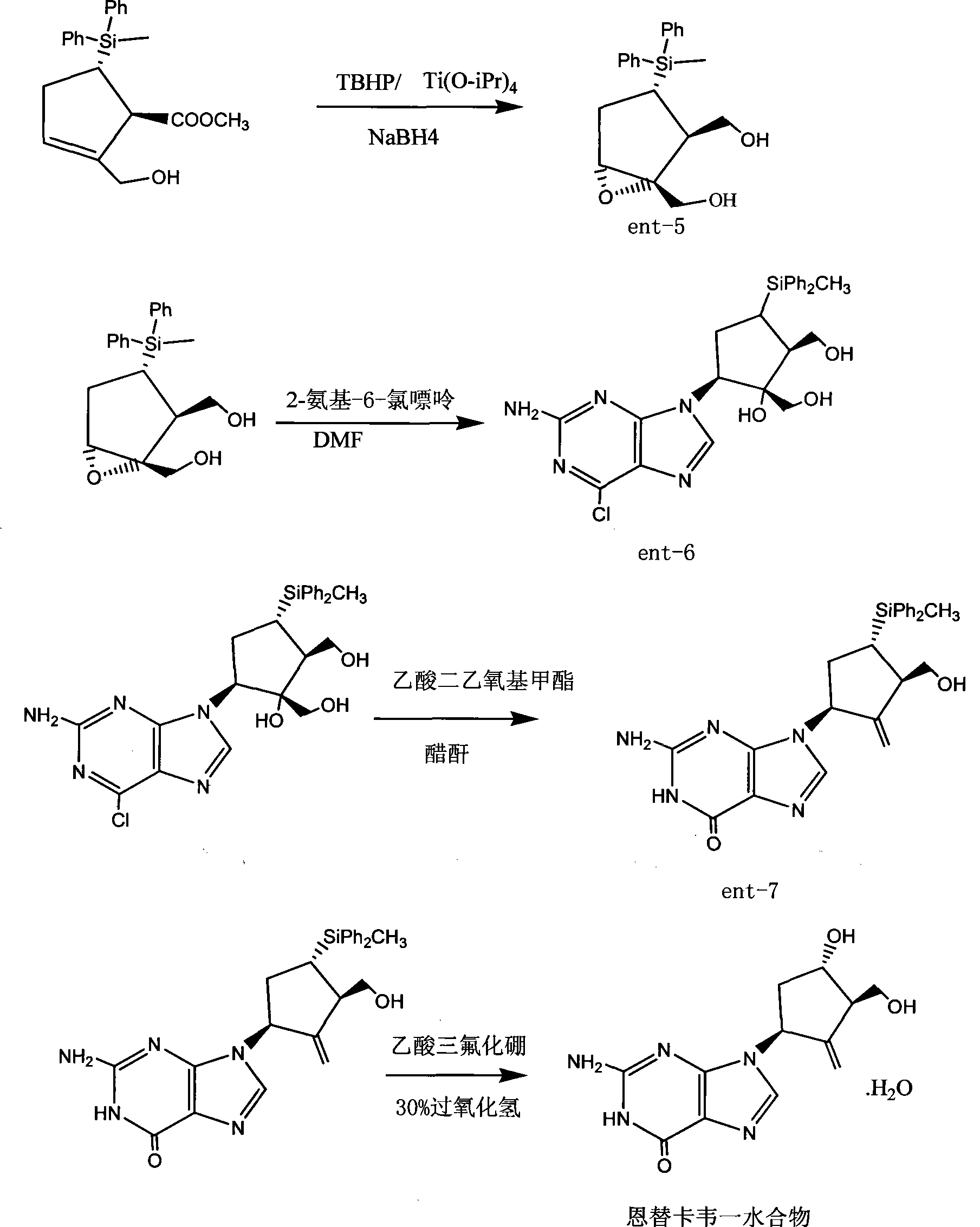
ActiveCN101531660ALower synthesis costRaw materials are cheap and easy to getGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsChlorobenzeneGrignard reagent
The invention provides an industrialization production process of entecavir-monohydrate. The special features of the process different from the other process are that: methyldiphenylchlorosilane is used as the silanization protector and special catalyst, thus the reaction temperature is -40 DEG C and the object product is smoothly produced (the reaction temperature is -78 DEG C in the prior process). Thus the production process is more suitable for industry production and because the silanization protector is cheaper and easily obtained, the production cost is greatly reduced. The invention comprises the preparation of methyldiphenylchlorosilane as the silanization protector which is obtained by directly synthesizing the methylphenyldichlorosilane and Grignard reagent of chlorobenzene under the action of special catalyst.
Owner:ANHUI BIOCHEM UNITED PHARMA CO LTD
Method for preparing pentafluorophenol
ActiveCN103420801AEasy format responseFormat response stableOrganic chemistryOrganic compound preparationOrganic solventGrignard reagent
The invention provides a method for preparing pentafluorophenol. The method is characterized by comprising the following steps of 1, dropping alkyl magnesium halide into pentafluorophenol organic solvent to carry out format exchange at 30 DEG C to 60 DEG C, 2, dropping the grignard reagent obtained in the step 1 to borate B (OR) 3 organic solvent of -20 DEG C to 30 DEG C to carry out esterification reaction, and then acidifying and distilling to recycle the organic solvent to obtain penta fluoro benzeneboronic acid, and 3, reacting the penta fluoro benzeneboronic acid obtained in the step 2 with the organic solvent and peroxide at 10 DEG C to 50 DEG C to generate the pentafluorophenol and finally rectifying the pentafluorophenol to obtain accepted products. The raw materials of the method are easy to obtain. The method is stable in process, convenient to operate, high in purity (larger than or equal to 99%) of products, high in yield (more than 65%), low in cost, good in safety and suitable for industrial large-scale production.
Owner:DALIAN QIKAI MEDICAL TECH
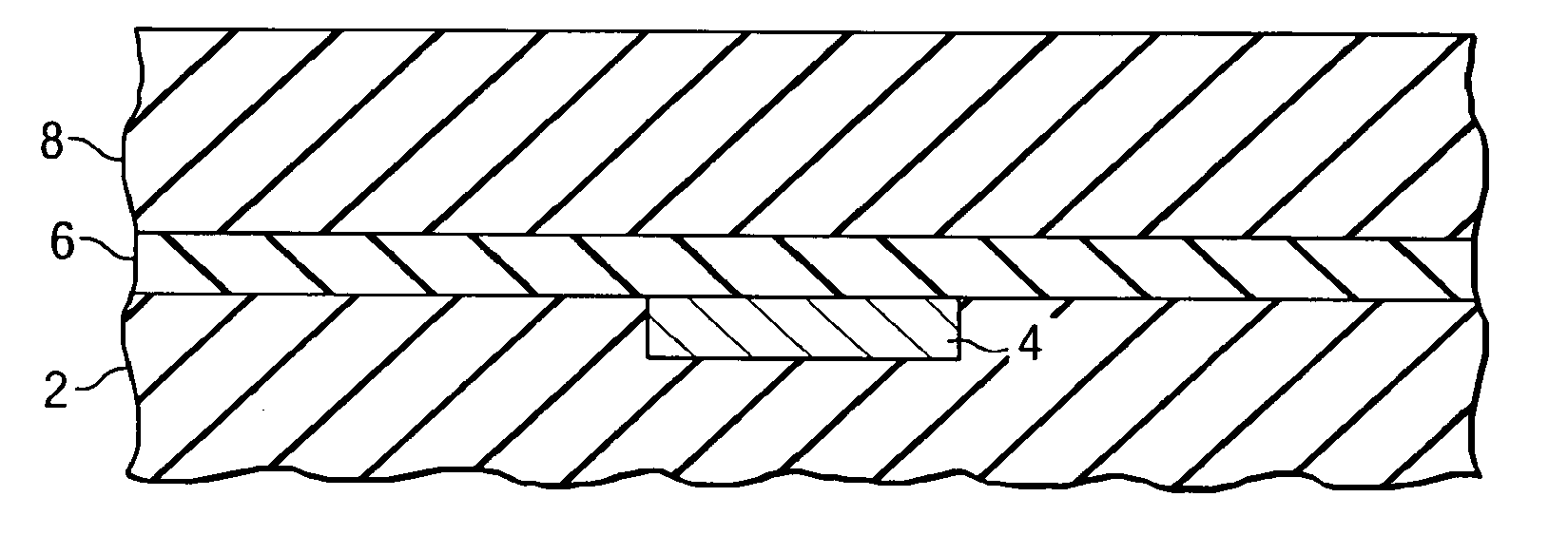
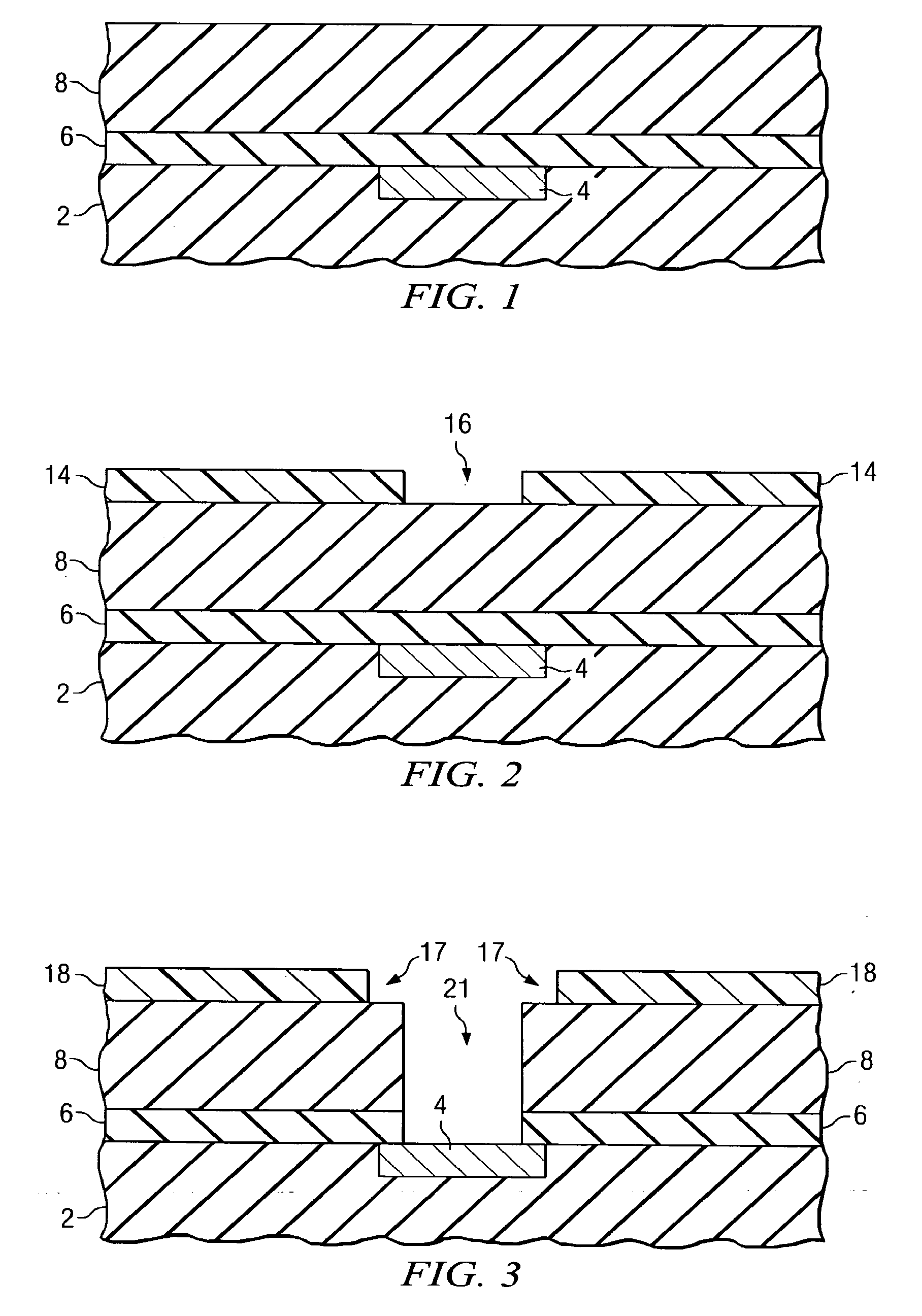
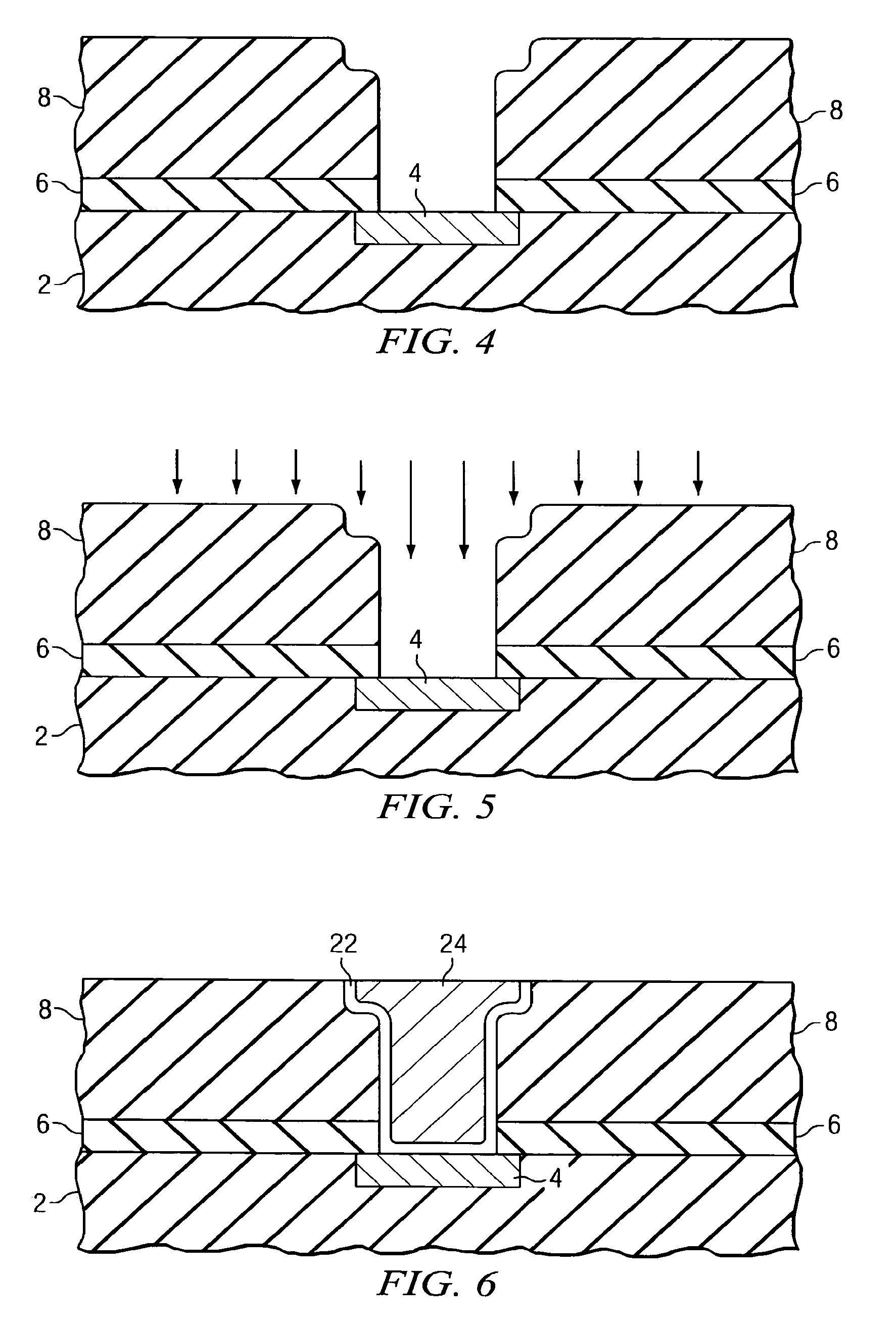
Repair of carbon depletion in low-k dielectric films
InactiveUS20060046516A1Solution value is not highEasy to useSemiconductor/solid-state device manufacturingDevelopersGrignard reagentDevice material
A method of repairing damaged low-k dielectric materials is disclosed. Plasma-based processes, which are commonly used in semiconductor device manufacturing, frequently damage carbon-containing, low-k dielectric materials. Upon exposure to moisture, the damaged dielectric material may form silanol groups. In preferred embodiments, a two-step approach converts the silanol to a suitable organic group. The first step includes using a halogenating reagent to convert the silanol to a silicon halide. The second step includes using a derivatization reagent, preferably an organometallic compound, to replace the halide with the suitable organic group. In a preferred embodiment, the halogenating agent includes thionyl chloride and the organometallic compound includes an alkyllithium, preferably methyllithium. In another preferred embodiment, the organometallic compound comprises a Grignard reagent. Embodiments disclosed herein advantageously enable the manufacturer to engineer the density, polarization, and ionization properties of the low-k dielectric material by selective incorporation of the organic group.
Owner:INFINEON TECH AG
Aryne resin containing polysilicone and preparation method thereof
The invention relates to a polysilane-containing aryne resin represented as right, wherein n is 0-20, x and y are 0-20, R1, R2, R3 and R4 are CH3 and C6H5, or R1 is H, R2, R3 and R4 are CH3 and C6H5, or R1 and R3 are H, R2 and R4 are CH3 and C6H5. The preparation method of polysilane-containing aryne resin uses aryne Grignard reagent to be reacted with terminated chlorine polysilane, while terminated chlorine polysilane is prepared by chlorination process and sodium condensation method. The inventive polysilane-containing aryne resin has better processing character, mechnical character, heat endurance, oxidation resistance and fire-retardancy, which can form ceramic material in high temperature and own better cementation with reinforcing fiber, with wide application in resin substrate and ceramic boving precursor.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of ribofuranose phosphate derivative
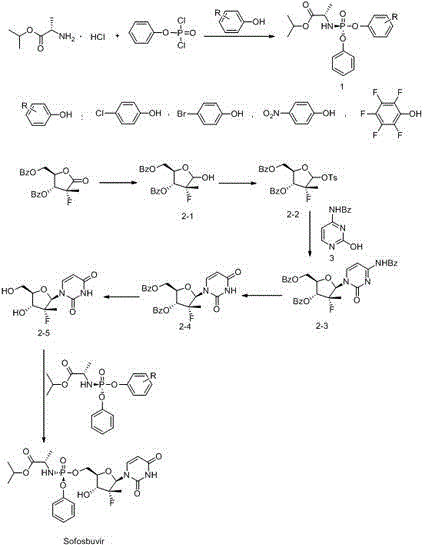
ActiveCN104610404APurity is easy to controlHigh yield of docking reactionSugar derivativesSugar derivatives preparationPhosphateGrignard reagent
The invention discloses a preparation method of a ribofuranose phosphate derivative. The preparation method comprises preparation steps as follows: L-alanine isopropyl ester hydrochloride, phenol dichlorophosphate and substituted phenol are taken as starting materials and have a docking reaction under the action of alkali; (2R)-2-deoxy-2-difluoro-2-methyl-D-erythropentonic acid GAMMA-lactone and 3,5-dibenzoate reduce carbonyl in a dichloromethane or ether solvent into an alcoholic hydroxyl group under the action of a strong reducing agent; an intermediate with a formula 2-1 has a reaction with paratoluensulfonyl chloride under the action of alkali to obtain p-toluenesulfonates; an intermediate with a formula 2-2 and a benzoyl cytosine derivative have a docking reaction under the action of a condensing agent; an intermediate with a formula 2-3 converts cytosine into uracil under the action of organic acid; benzoyl protection for an intermediate with a formula 2-4 is released under the action of an alkaline agent; an intermediate with a formula 2-5 and an intermediate with a formula 1 are docked under the action of a Grignard reagent to obtain Sofosbuvir.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
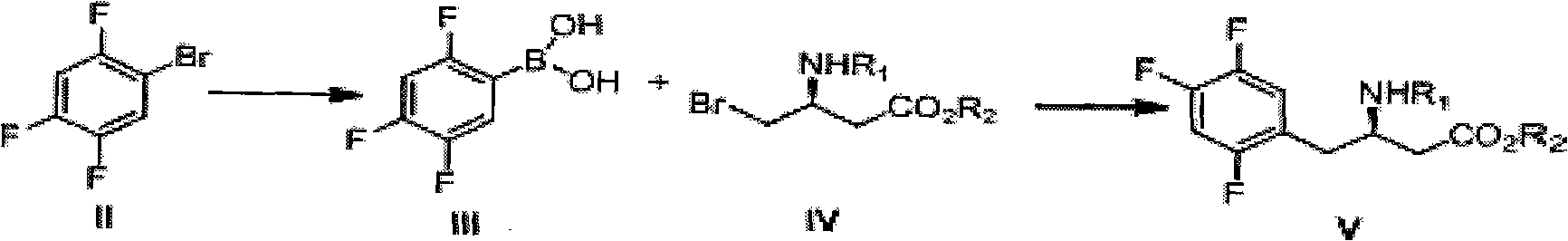
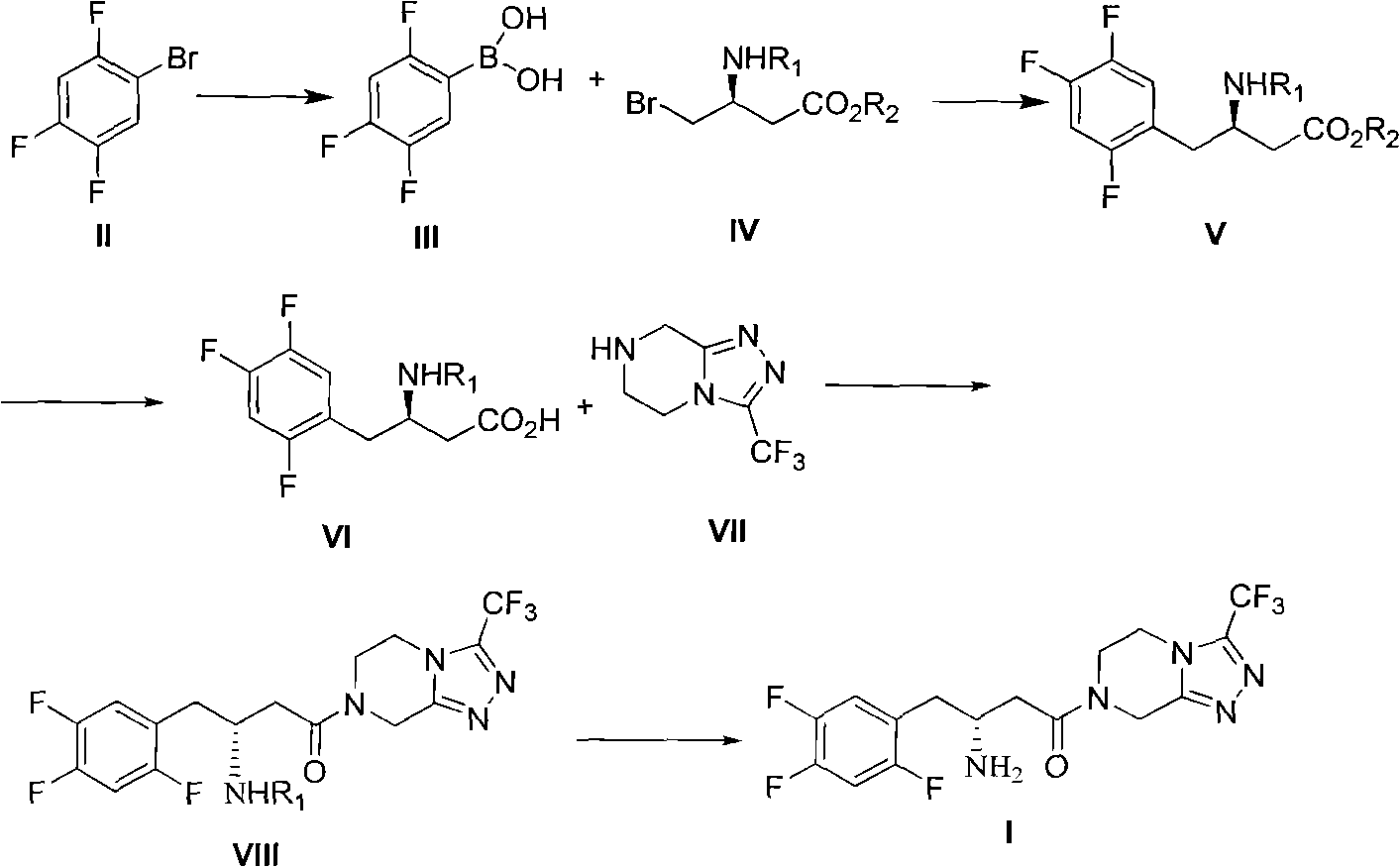
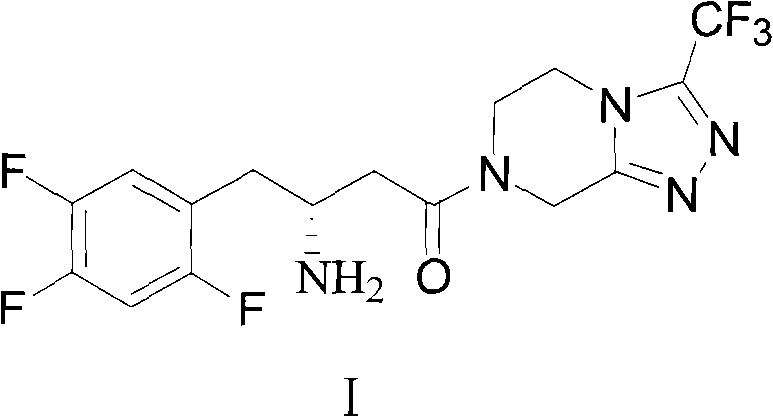
Preparation method of sitagliptin intermediate, sitagliptin or salts thereof
ActiveCN102093245AAvoid low synthesis efficiency and high cost technical defectsOrganic compound preparationCarboxylic acid amides preparationSitagliptinOrganic solvent
The invention discloses a preparation method of sitagliptin intermediate, sitagliptin or salts thereof. The method comprises the following steps of: (1) preparing a compound, namely 2,4,5-trifluorobromobenzene shown as a formula (II) into a Grignard reagent; (2) reacting the Grignard reagent with boric acid trimester in an organic solvent under the action of a catalyst to obtain a compound, namely 2,4,5-trifluorobenzolc acid shown as a formula (III); and (3) preparing a compound, namely (3R)-3-substituted amido-4-bromine-butyric ester shown as a formula (IV) and the 2,4,5-trifluorobenzolc acid shown as the formula (II) into a compound, namely (3R)-3-substituted amido-4-(2,4,5-trifluorophenyl)-butyric ester shown as a formula (V) under the action of a transition metal catalyst and alkali. By using the method, the synthesis efficiency is increased, and the cost is lowered.
Owner:ZHEJIANG HISOAR PHARMA
Butylphthalide synthesis method and purification technology
The invention relates to a butylphthalide synthesis method. The method comprises the steps that methyl 2-formyl benzoic acid is adopted as a starting material, THF is adopted as a solvent to react with an n-butyl magnesium chloride Grignard reagent, and acid regulation is performed to prepare a butylphthalide product. The invention further relates to a technology for preparing high-purity butylphthalide. The obtained crude butylphthalide product is subjected to hydrolysis treatment by an alkaline substance, acid regulation is performed to separate out solids, and filtering is performed to obtain a butylphthalide midbody; the acid regulation and alkali regulation processes are executed repeatedly, and finally ring closure and decompression desolvation are performed to obtain high-purity butylphthalide. According to the synthesis method, low-flash diethyl ether is prevented from being adopted as a solvent, the purification technology is easy to implement, the reagent can be purchased in bulk easily, column chromatography product purification and reduced pressure distillation under high temperature and high vacuum degree are not needed, and industrial enlarged production is easy.
Owner:福建省宝诺医药研发有限公司
Asymmetric synthesis method of (S)-rivastigmine
InactiveCN101134738AFew reaction stepsHigh reaction yieldCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsGrignard reagent
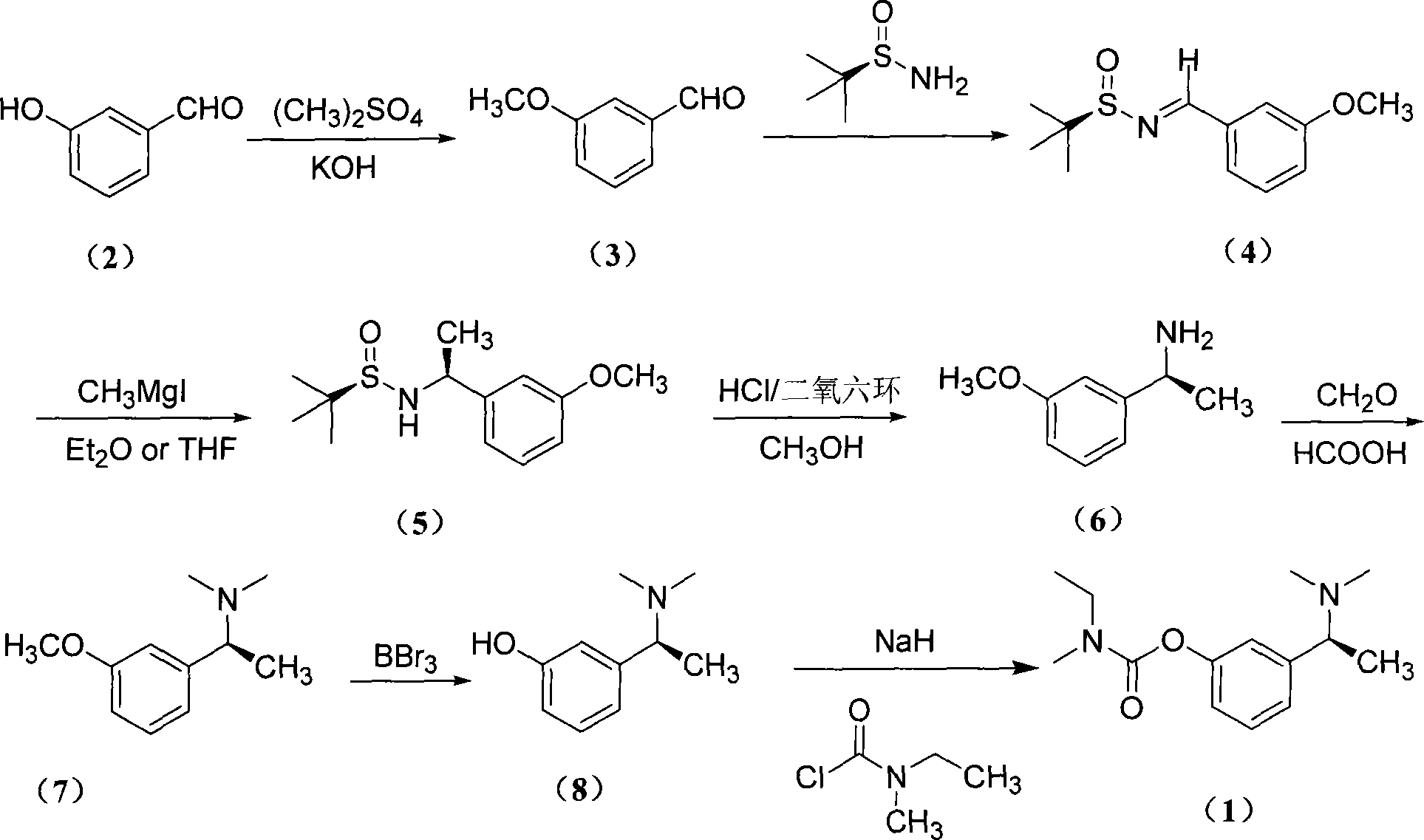
The present invention is asymmetrical (S)-carbalatine synthesizing process including the following steps: protecting the phenolic hydroxyl group of m-hydroxy benzaldehyde, reacting with chiral tert-butyl sulfonamide to produce (R, E)-3-methoxyl-phenyl methylene tert-butyl sulfonamide, addition reacting with methyl Grignard reagent, hydrolyzing, Eschweiler-Clarke methylation reaction, demethylating BBr3 to synthesize important intermediate (S)-1-(3-hydroxy phenyl)-N, N-dimethyl ethyl amine, and esterification with methyl ethyl carbamyl chloride to obtain (S)-carbalatine. The present invention has less reaction steps, high yield, total yield up to 21.85 %, and low cost.
Owner:JINAN UNIVERSITY
Cyclobutyl silanes for catalysts useful for making highly isotactic olefin polymers
InactiveUSH2060H1High catalytic efficiencyError toleranceOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationGrignard reagentSilanes
One aspect of the present invention relates to a catalyst system for use in olefinic polymerization, containing a solid titanium catalyst component prepared by contacting a titanium compound and a magnesium compound; an organoaluminum compound having at least one aluminum-carbon bond; and an organosilicon compound comprising at least one of cyclobutyl group. Another aspect of the present invention relates to a method of making a catalyst for use in olefinic polymerization, involving the steps of reacting a Grignard reagent having a cyclobutyl group with an orthosilicate to provide an organosilicon compound having a cyclobutyl moiety; and combining the organosilicon compound with an organoaluminum compound having at least one aluminum-carbon bond and a solid titanium catalyst component prepared by contacting a titanium compound and a magnesium compound.
Owner:ENGELHARD CORP
Process for Producing alpha Substituted Ester
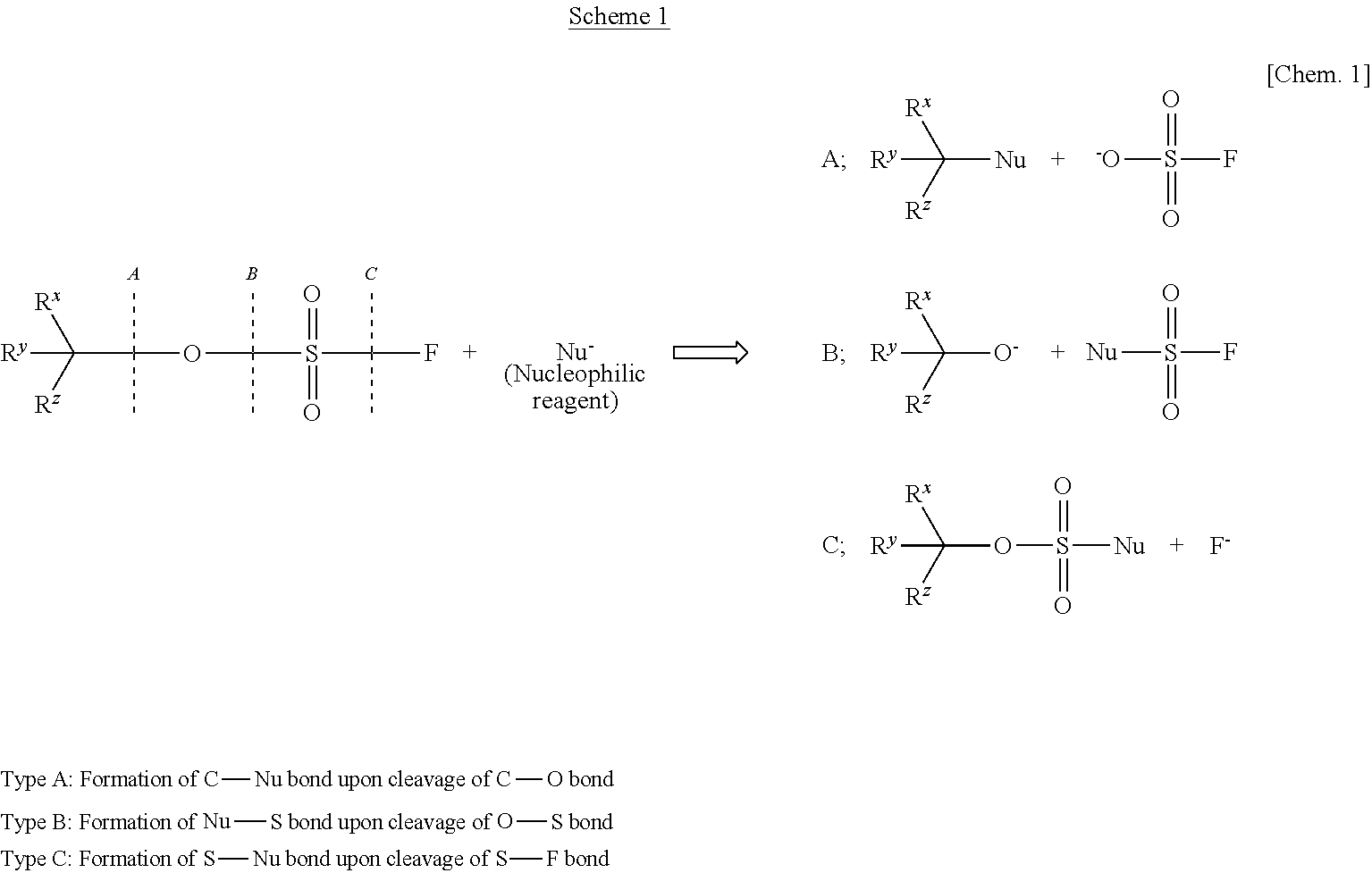
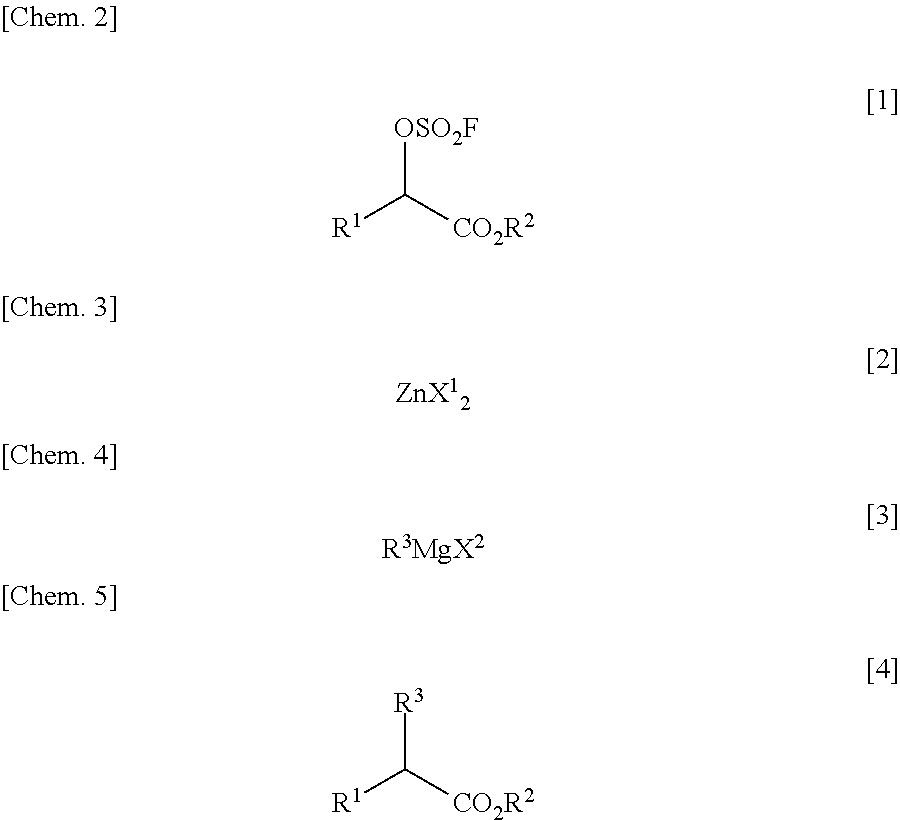
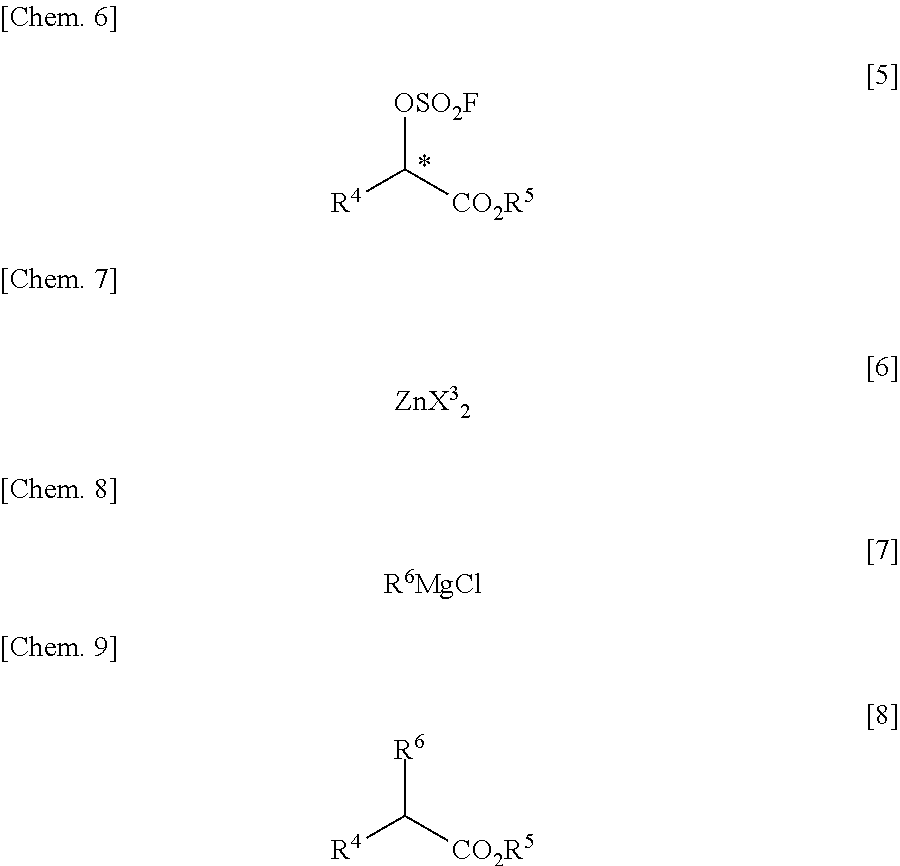
InactiveUS20110213176A1Low costEffective combinationOrganic compound preparationSulfuric acid esters preparationGrignard reagentTriflic acid
There is provided a process for producing an α-substituted ester by reaction of a fluorosulfuric acid ester of α-hydroxyester with a Grignard reagent in the presence of a zinc catalyst. It is newly found that the reaction for production of α-substituted esters, in which the raw reaction substrate is limited to expensive trifluoromethanesulfonic acid esters, can proceed favorably with the use of fluorosulfuric acid esters suitable for mass-production uses. By the use of the fluorosulfuric acid ester high in optical purity, it is possible to obtain the α-substituted ester with high optical purity upon inversion of the asymmetric carbon configuration. The process of the present invention can solve all of the prior art problems and can be applied for industrial uses.
Owner:CENT GLASS CO LTD
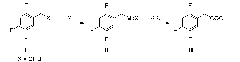
Method for preparing fluorophenol
ActiveCN101445431ALow costMild reaction conditionsOrganic chemistryOrganic compound preparationGrignard reagentChloride
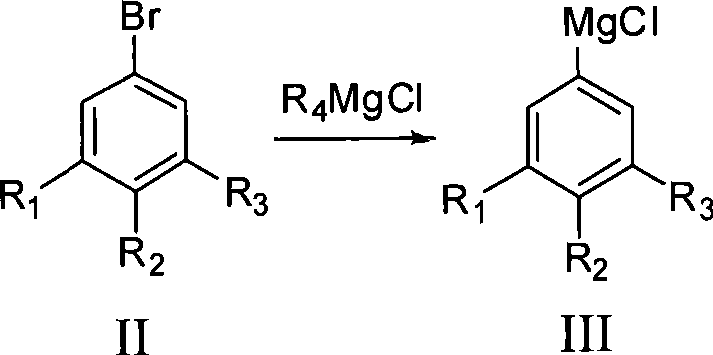
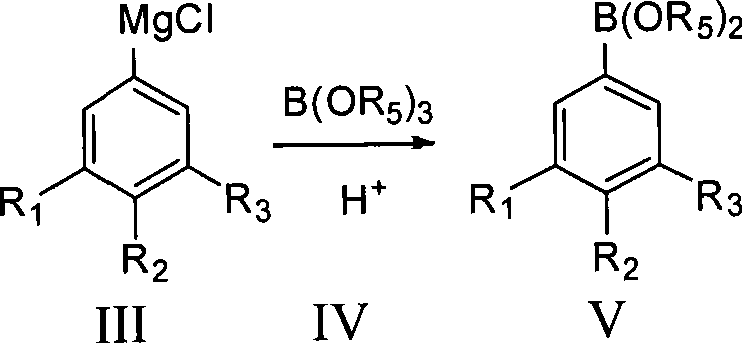
The invention provides a method for preparing fluorophenol. Fluorobromobenzene is used as raw material to take Grignard exchange reaction with R4MgCl to generate difluorophenyl magnesium chloride and then react with B(OR5)3 to generate fluorophenol boric acid ester, and the fluorophenol boric acid ester is hydrolyzed under an acid condition to generate fluorophenol boric acid. The fluorophenol boric acid is oxidized to generate crude fluorophenol to be carried out post treatment to get a product of the fluorophenol. The invention prepares Grignard reagent of the fluorobromobenzene by the Grignard exchange reaction, thereby improving the purity and the yield of the final product greatly.
Owner:QUZHOU CHEMSPEC CORP +1
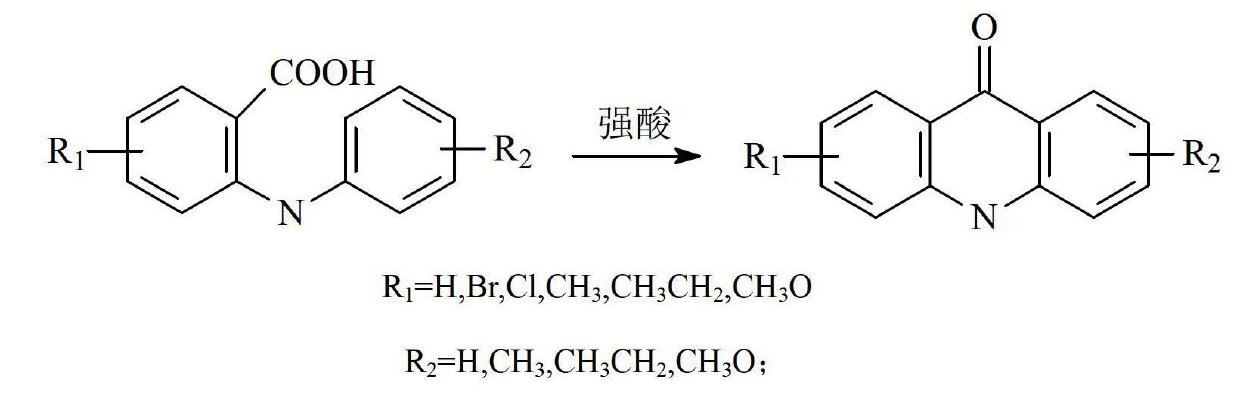
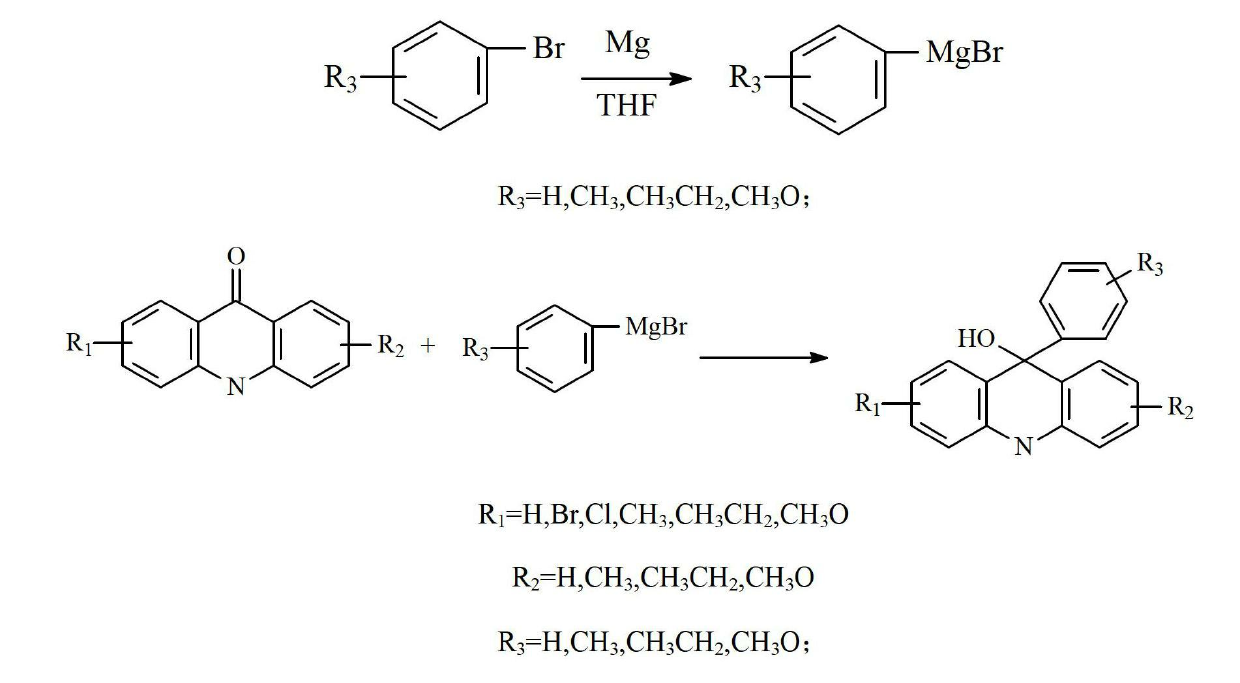
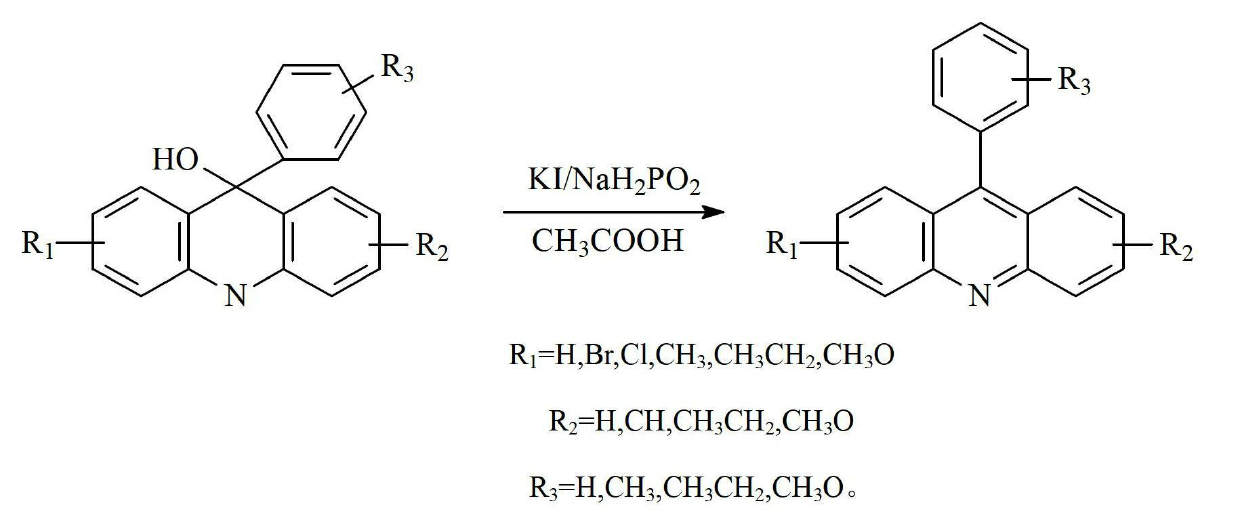
Preparation method of acridine compounds
ActiveCN102675203AReduce usageMild preparation conditionsOrganic chemistryGrignard reagent9-phenylacridine
The invention provides a preparation method of acridine compounds, and relates to the technical field of photoinitiator. The preparation method comprises the following steps of: taking substituted N-methyl anthranilic acid as initial raw material, generating substituted acridone under the condition of acid, carrying out reaction on substituted bromobenzene and magnesium to generate grignard reagent, carrying out reaction on substituted acridone and the grignard reagent to generate substituted 9-oxhydryl-9-phenylacridine, and reducing to obtain the substituted 9-phenylacridine compounds. Different from the conventional synthesis route, the preparation method of the acridine compounds provided by the invention has more operation steps compared with the conventional one-step method, thus being slightly lower in yield compared with the conventional synthesis route, however, the preparation condition is mild relatively, and the high-temperature and high-pressure reaction and the use of expensive heavy metal catalyst can be avoided, so that the preparation method is good in environment protection.
Owner:芜湖启博知识产权运营有限公司
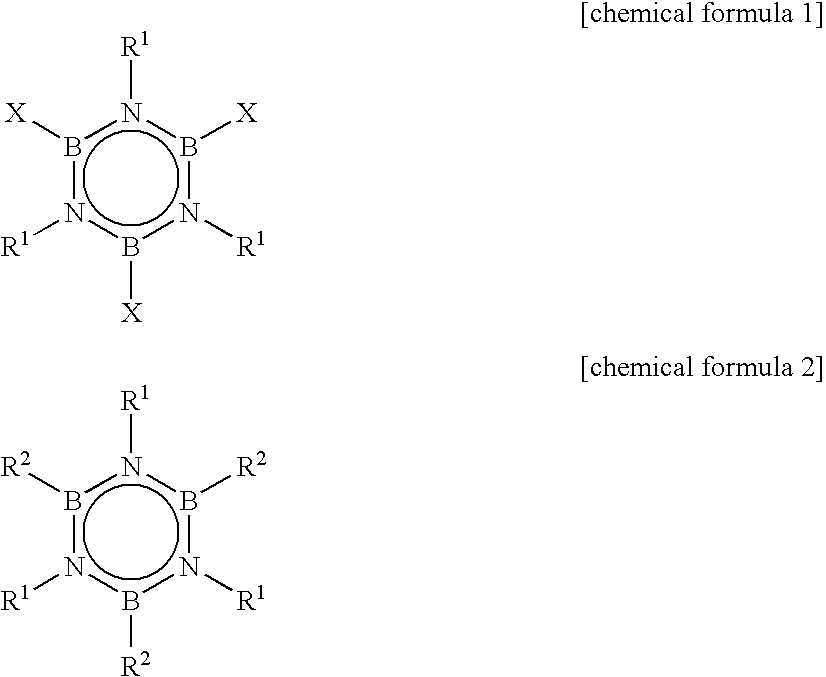
Alkylborazine compound and production method for the same
InactiveUS20050177002A1Boiling pointImprove flammabilitySilicon organic compoundsGroup 3/13 element organic compoundsHalogenDistillation
In the process of synthesizing alkylborazine compound represented by the chemical formula 2, by a reaction of a halogenated borazine compound represented by the chemical formula 1 with a Grignard reagent, thus synthesized alkylborazine compound is washed with water, or subjected to sublimation purification or distillation purification at least three times, and / or subjected to distillation purification at least twice. In the formulas, R1 independently represents alkyl group; R2 independently represents alkyl group; and X represents halogen atom.
Owner:NIPPON SHOKUBAI CO LTD
Clean generation of a perfluoroaryl grignard reagent
InactiveUS6129863ASilicon organic compoundsGroup 3/13 element organic compoundsGrignard reagentBoron
Perfluoroaryl Grignard reagents are produced from a hydrocarbyl Grignard reagent and polyhaloaromatic compounds via separate additions of different polyhaloaromatic compounds, such that the conversion of hydrocarbyl Grignard reagent to the desired perfluoroaryl Grignard reagent is essentially complete, and thus the reaction product is free or essentially free of agents that may negatively affect subsequent reactions. The perfluoroaryl Grignard reagents may be further reacted with boron trihalides in order to obtain tris(perfluoroaryl)boranes or tetrakis(perfluoroaryl)borates.
Owner:ALBEMARLE CORP
Catalytic system for obtaining conjugated diene/monoolefin copolymers and these copolymers
InactiveUS20060160969A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationGrignard reagentLanthanide
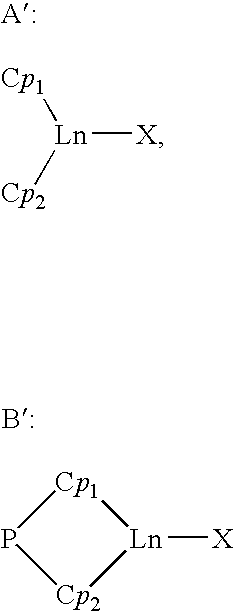
A catalytic system usable for the copolymerization of at least one conjugated diene and at least one monoolefin, a process for preparing this catalytic system, a process for preparing a copolymer of a conjugated diene and at least one monoolefin using said catalytic system, and said copolymer are described. This catalytic system includes: (i) an organometallic complex represented by the following formula: {[P(Cp)(Fl)Ln(X)(Lx)}p (1) where Ln represents a lanthanide atom to which is attached a ligand molecule comprising cyclopentadienyl Cp and fluorenyl Fl groups linked to one another by a bridge P of the formula MR1R2, M is an element from column IVa of Mendeleev's periodic table and R1 and R2 each represent alkyl groups of 1 to 20 carbon atoms or cycloalkyl or phenyl groups of 6 to 20 carbon atoms, X represents a halogen atom, L represents an optional complexing molecule, such as an ether, and optionally a substantially less complexing molecule, such as toluene, p is a natural integer greater than or equal to 1 and x is greater than or equal to 0, and (ii) a co-catalyst selected from alkylmagnesiums, alkyllithiums, alkylaluminums, Grignard reagents and mixtures of these constituents.
Owner:MICHELIN RECH & TECH SA +1
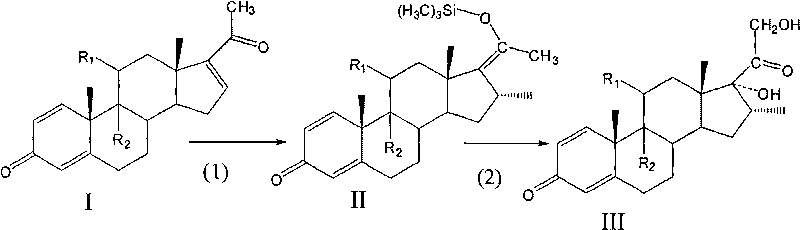
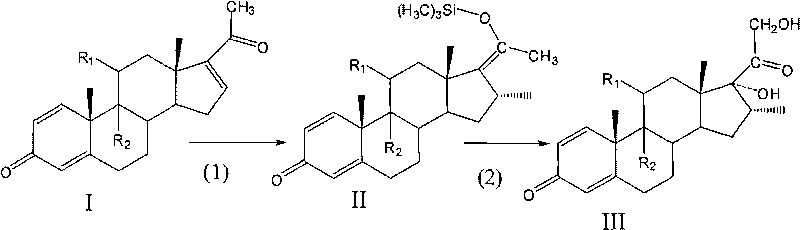
Preparation method of 16 Alpha-methyl steroidal compound
A preparation method of a 16 Alpha-methyl steroidal compound comprises: taking a formula (I) compound as a substrate, reacting with 0.2 to 0.1 time catalyst, 1 to 1.5 times trimethyl chlorosilane, 1 to 1.5 times Grignard reagent and 2 to 4 times triamine phosphate in organic solvent to obtain a formula (I) compound, and taking the formula (I) compound to react with 1 to 5 times alkali and 1 to 3 times meta-chloroperoxybenzoic acid in organic solvent to obtain a formula (III) compound.
Owner:TIANJIN JINYAO GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)