Method for preparing pentafluorophenol
A technology of pentafluorophenol and pentafluorobromobenzene, which is applied in the field of preparation of TFT liquid crystals and pharmaceutical intermediates pentafluorophenol, can solve the problems of hydrolysis of pentafluorobenzene boric acid into pentafluorobenzene, increased probability of coupling reaction, electronic Low cloud density and other issues, achieve the effect of reduced response time, improved security performance, and good security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
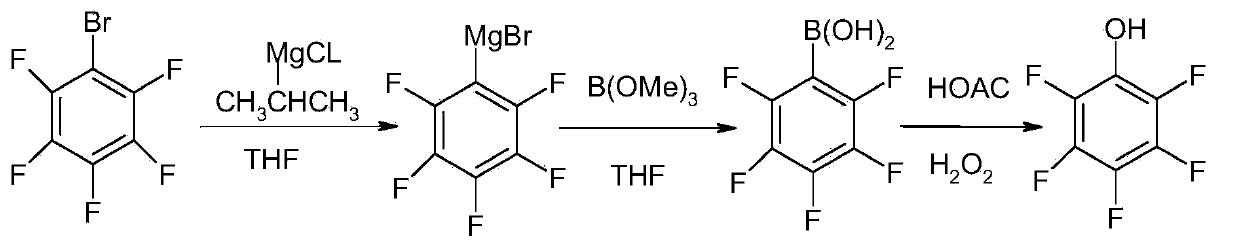
[0036] Step (1): Dry the 1000ml four-necked bottle, replace it with nitrogen, start stirring to maintain good nitrogen protection, add 365ml THF, 200g (0.81mol) pentafluorobromobenzene 415ml (0.83mol) of isopropylmagnesium chloride / THF solution was added dropwise at ℃, and then kept at 40-50℃ for 2 hours.
[0037] Step (2): Add 500ml THF and 97g (0.94mol) trimethyl borate into a 2000ml four-necked bottle, cool down to -10~0°C, add the Grignard reagent prepared in the above step (1) dropwise, and then heat After reaching room temperature and keeping it warm for 2 hours, after acidifying with hydrochloric acid, washing and separating the layers, the organic layer was distilled under reduced pressure, and the residue was crystallized and filtered to obtain 126 g of pentafluorophenylboronic acid solid, with a yield of 74%.
[0038] Step (3): Add 450ml of glacial acetic acid into a 1000ml four-necked bottle, start stirring, add 124g of the above-mentioned pentafluorophenylboronic a...
Embodiment 2
[0042] Step (1): Dry the 1000ml four-necked bottle, replace it with nitrogen, start stirring to maintain good nitrogen protection, add 365ml THF, 200g (0.81mol) pentafluorobromobenzene 470ml (0.94mol) of isopropylmagnesium chloride / THF solution was added dropwise at ℃, and then kept at 40-50℃ for 2 hours.
[0043] Step (2): Add 500ml THF and 113g (1.09mol) trimethyl borate into a 2000ml four-neck bottle, cool down to -10~0°C, add the Grignard reagent prepared in the above step (1) dropwise, and then heat After reaching room temperature and keeping warm for 2 hours, then acidified with hydrochloric acid, washed and separated, the organic layer was distilled under reduced pressure, and the residue was crystallized and filtered to obtain 129 g of pentafluorophenylboronic acid solid, with a yield of 75%.
[0044] Step (3): Add 400ml of glacial acetic acid into a 1000ml four-neck bottle, start stirring, add 124g of the above-mentioned pentafluorophenylboronic acid solid, add 81g (0...
Embodiment 3
[0046] Step (1): Same as Example 2.
[0047]Step (2): Add 500ml THF and 129.6g (1.25mol) trimethyl borate into a 2000ml four-necked bottle, cool down to -20~-10℃, add the Grignard reagent prepared in the above step (1) dropwise, Then heated to room temperature and kept warm for 2 hours, then acidified with hydrochloric acid, washed and separated, the organic layer was distilled under reduced pressure, and the residue was crystallized and filtered to obtain 120 g of pentafluorophenylboronic acid solid, with a yield of 70%.
[0048] Step (3): Add 300ml of glacial acetic acid into a 1000ml four-necked bottle, start stirring, add 120g of the above-mentioned pentafluorophenylboronic acid solid, add 75g (0.66mol) of hydrogen peroxide dropwise into the bottle at 25-35°C, and the dropwise addition is completed Insulate for 2 hours, then add sodium bisulfite solution into the bottle to destroy excess hydrogen peroxide, and obtain 95 g of white crystals with a main content of ≥99.5% thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com