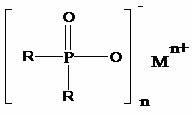
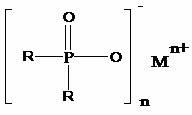
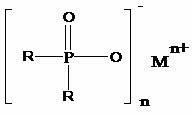
Preparation method of dialkyl hypophosphite
A technology of dialkylphosphinate and dialkylphosphinic acid, applied in the field of preparation of dialkylphosphinate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of diethylphosphine oxide
[0040] Under the protection of argon, add 22g (0.9mol) magnesium powder and 250ml dry THF into a 2000ml three-necked flask, first add 5ml bromoethane, after the reaction is triggered, start stirring, dropwise add 71ml bromoethane and 400ml dry The mixture of THF (1.0 mol of ethyl bromide in total) was kept in a slightly boiling state. After about 2.5-3 hours of dripping, the stirring reaction was continued at room temperature for 30 minutes.
[0041] Under cooling in an ice-water bath, add dropwise a mixture of 40ml (0.3mol) diethyl phosphite and 150ml dry THF to the above-prepared ethylmagnesium bromide. React in a water bath for about 4 hours. Under cooling in an ice-water bath, ice-cold potassium carbonate solution (126.5g (0.9mol) potassium carbonate dissolved in 150ml water) was added dropwise for hydrolysis. Filtrate, remove the solvent from the filtrate on a rotary evaporator (50°C, vacuum degree of the circulating wa...
Embodiment 2
[0048] Prepare diethylphosphorus oxide as in Example 1, add the obtained diethylphosphorus oxide into a 500ml three-necked flask, add 120ml of water, stir and add NaOH solution (40gNaOH dissolved in 80ml of water), heat to 50°C, drop it in 1 hour 50ml of 30% hydrogen peroxide, keep warm at 50~70℃ for 2 hours. After cooling, dilute hydrochloric acid (60ml of concentrated hydrochloric acid dissolved in 60ml of water) was added dropwise to neutralize to pH=2 to obtain an aqueous solution of diethylphosphinic acid. The HPLC result shows that the purity is 99.2%, and its content is 7.5% according to titration analysis, and the yield is 87%. 95 g of aluminum diethylphosphinate was obtained in the same manner as in Example 1, and the cumulative total yield was 81.5%. Infrared and TG are the same as Example 1.
Embodiment 3
[0050] Under the protection of argon, add 22g (0.9mol) magnesium powder and 250ml dry THF into a 2000ml three-necked flask, first add 10ml of ether solution filled with methyl chloride through the steel cylinder, after the reaction is triggered, start stirring, and add dropwise under cooling in a water bath 350ml ether solution of methyl chloride (total 1.0 mol of methyl chloride), keep in a slightly boiling state, after about 2.5-3 hours, continue to stir and react at room temperature for 30 minutes.
[0051] Under ice-water bath cooling, in the above-mentioned prepared methylmagnesium chloride, add dropwise the mixed solution of 40ml (0.3mol) diethyl phosphite and 160ml dry ether, operation method obtains dimethyl phosphorus oxide 18.7g with embodiment 2 afterwards, Yield 80%; Obtain dimethylphosphinic acid 22g, accumulative yield 78%, 31 The P-NMR spectral shift value δ was -48 ppm.
[0052] Heat the aqueous solution of dimethylphosphinic acid to about 85°C, drop the aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com