Patents
Literature
42463results about How to "Improve solubility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
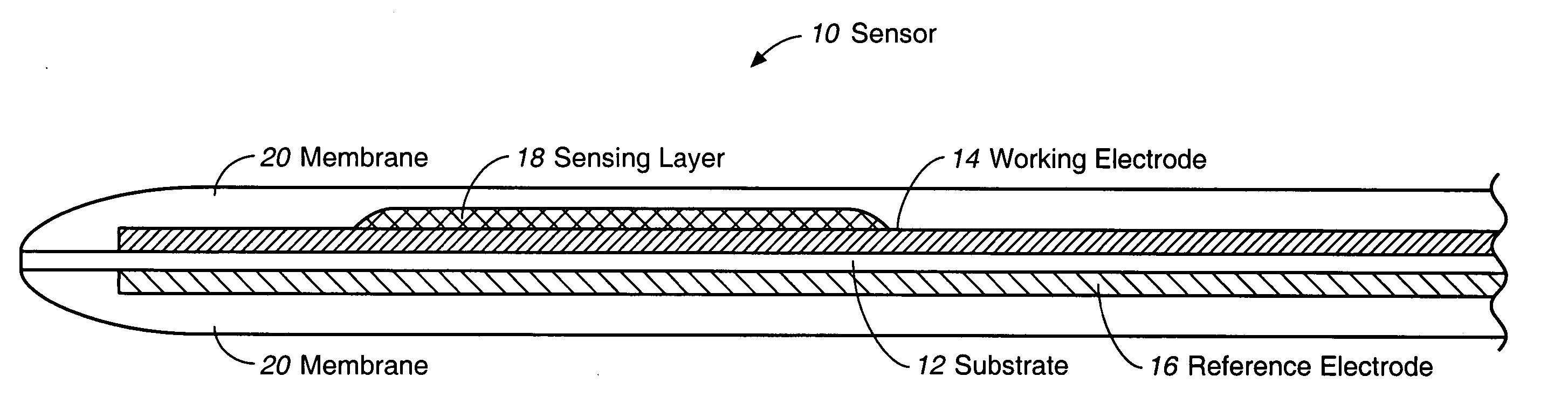
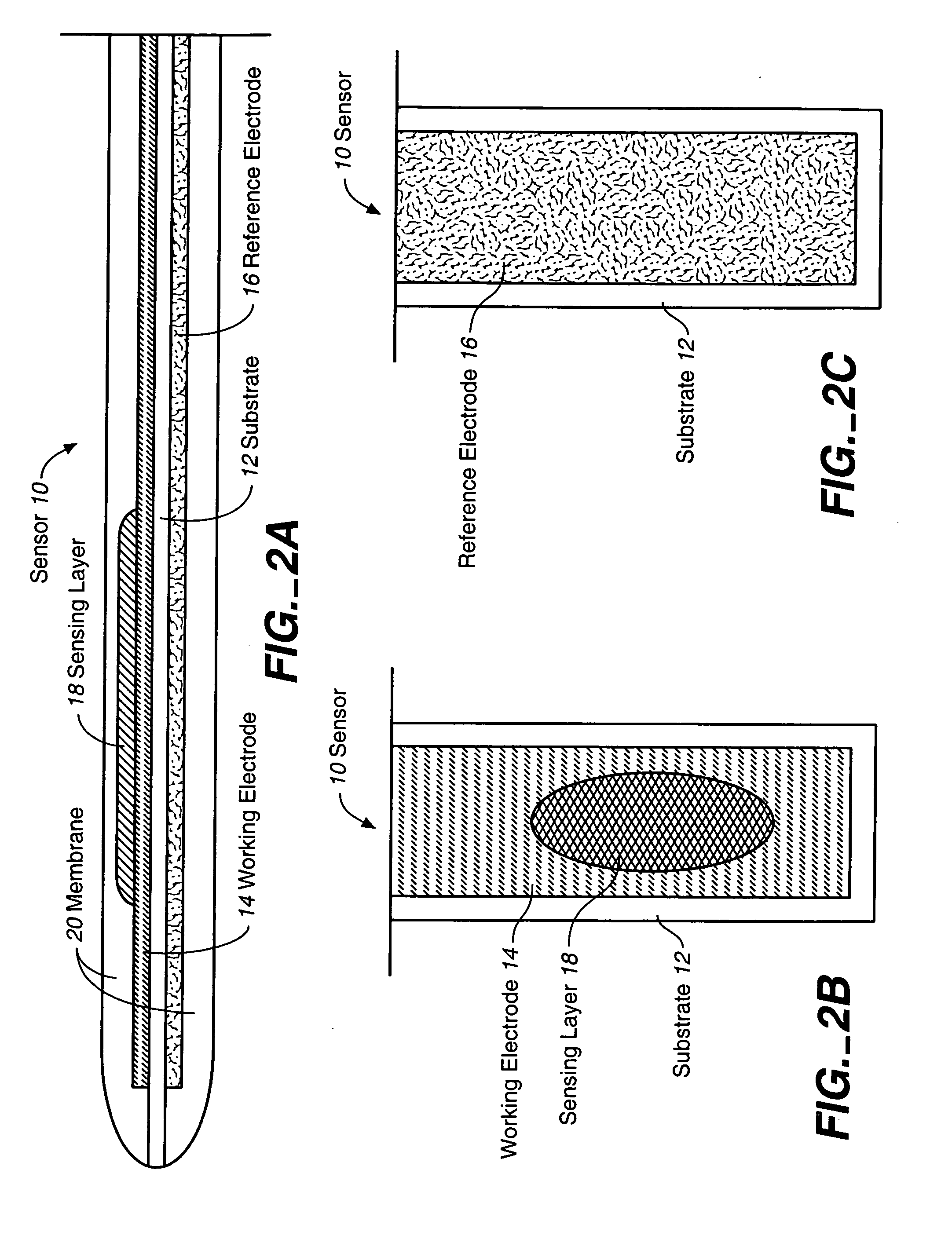
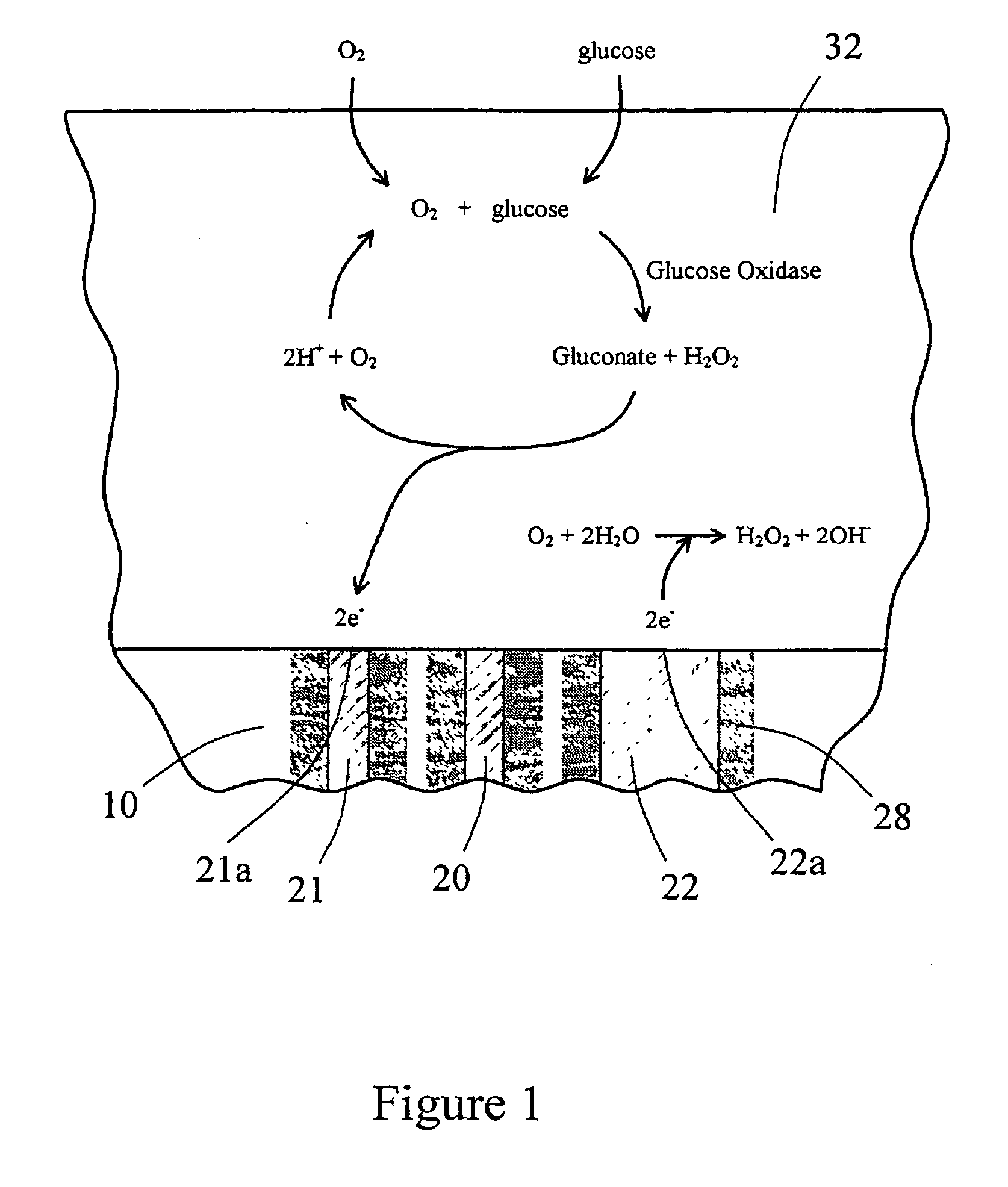
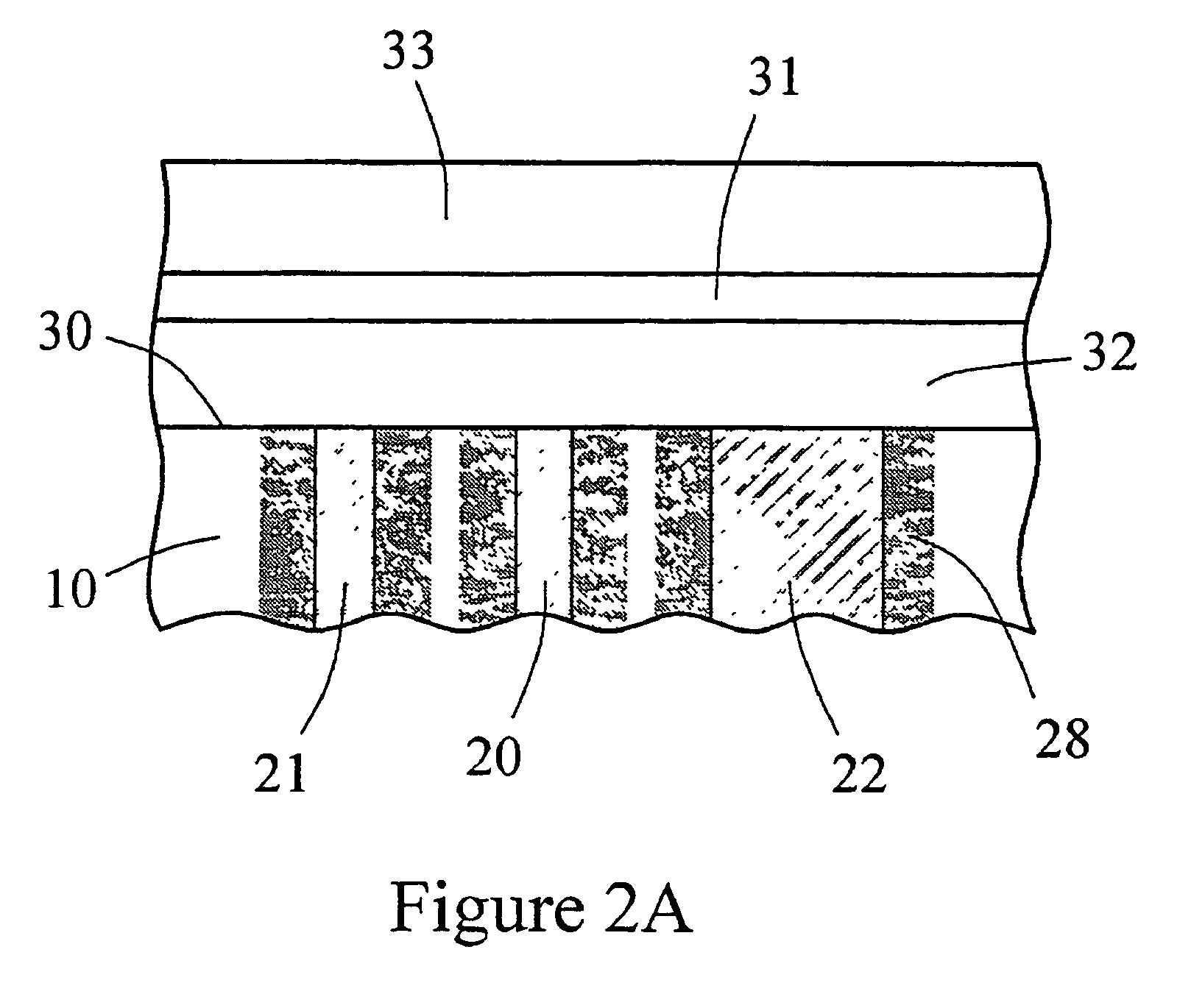
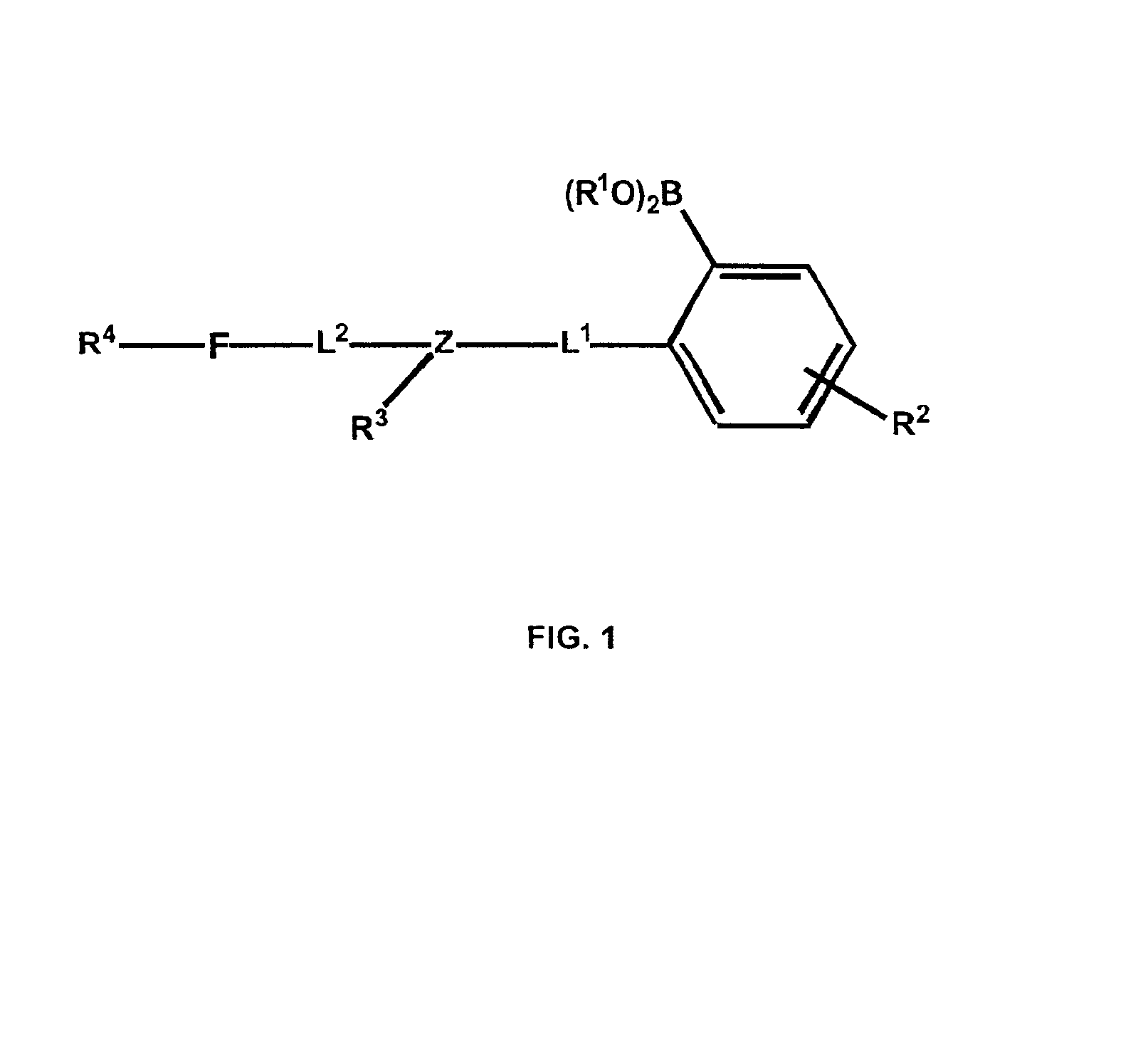
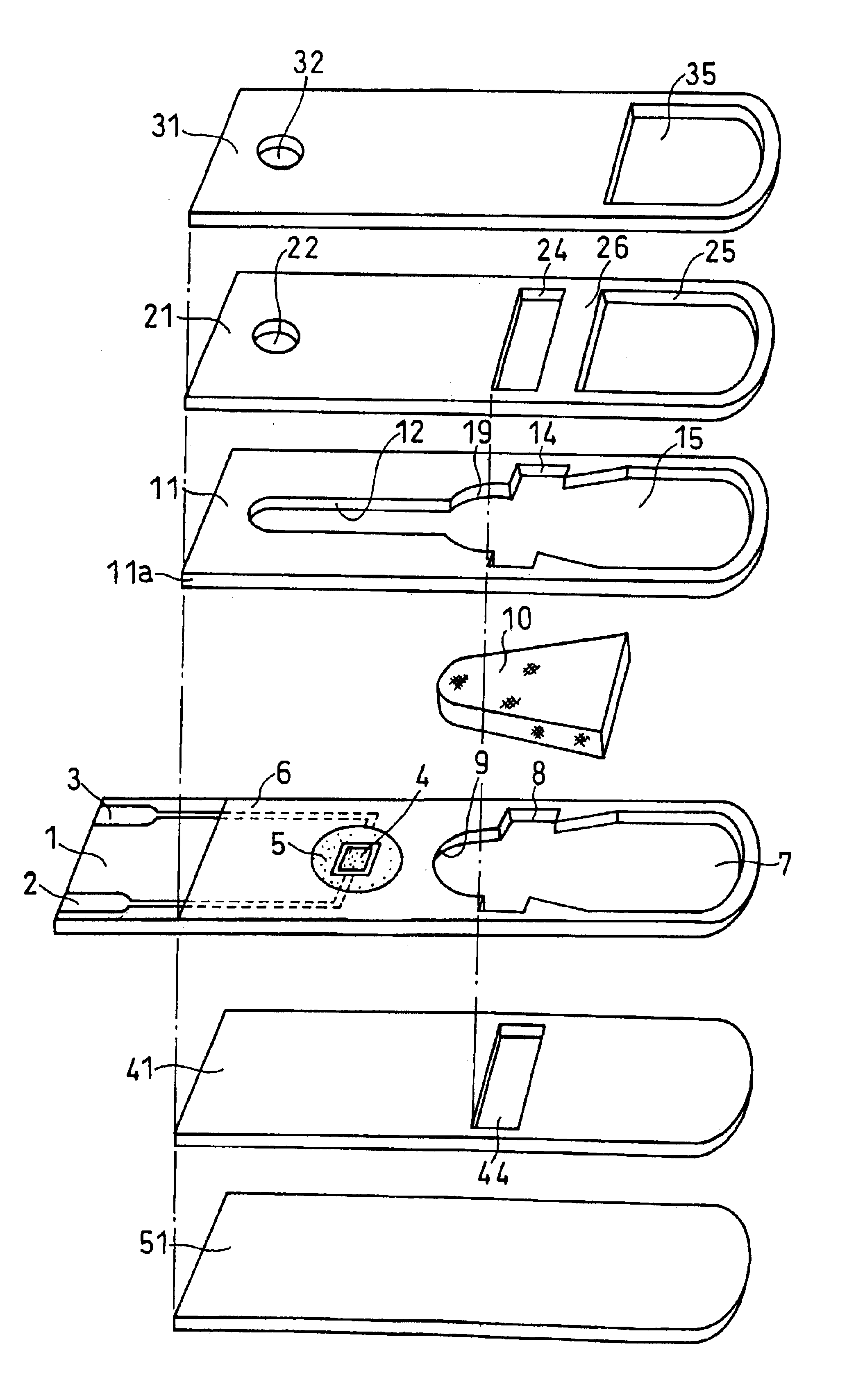
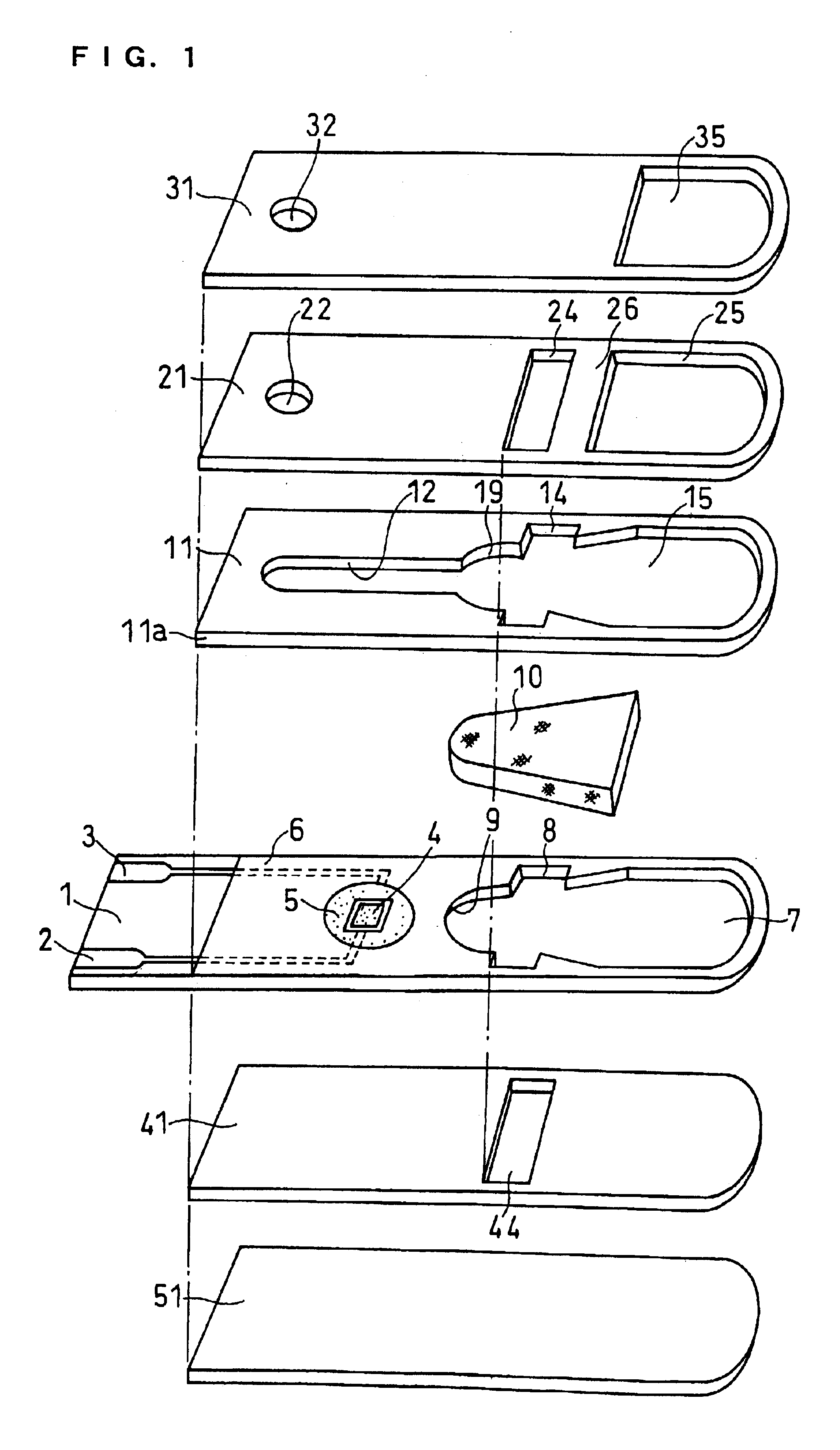
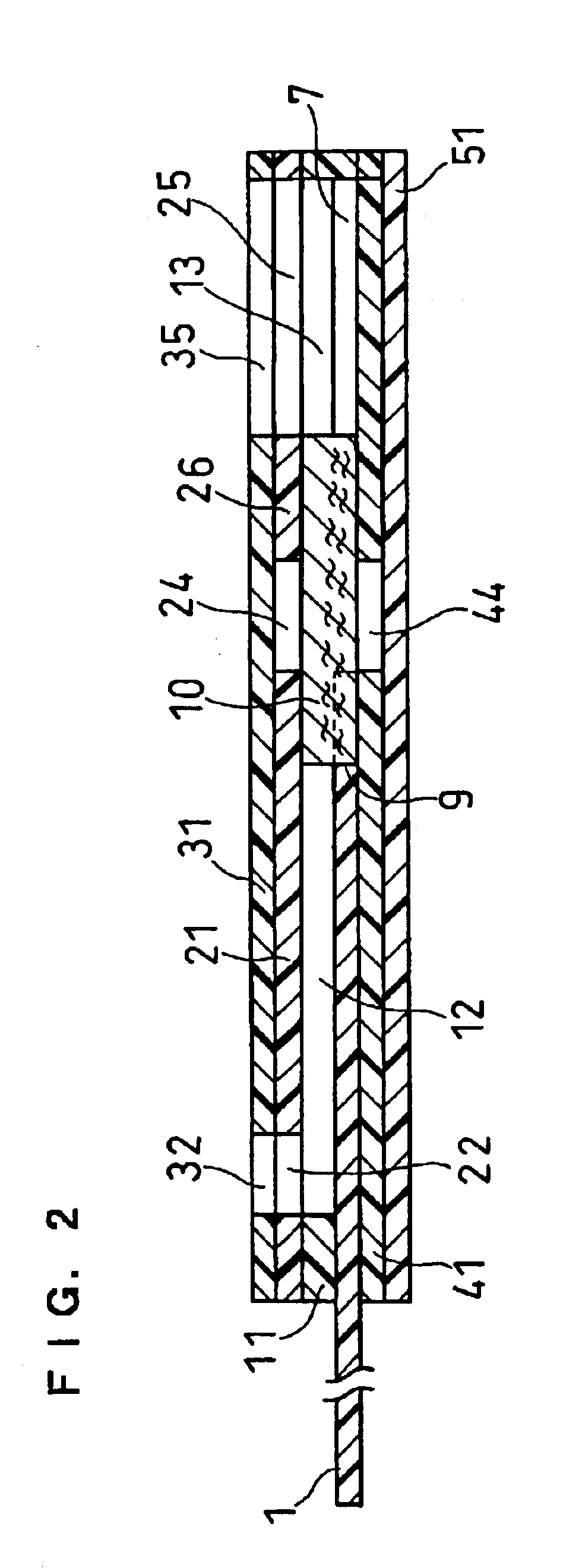
Sensor head for use with implantable devices
InactiveUS7471972B2Minimizing negative potential extremesImprove solubilityMicrobiological testing/measurementMaterial analysis by electric/magnetic meansElectrochemical responseAnalyte
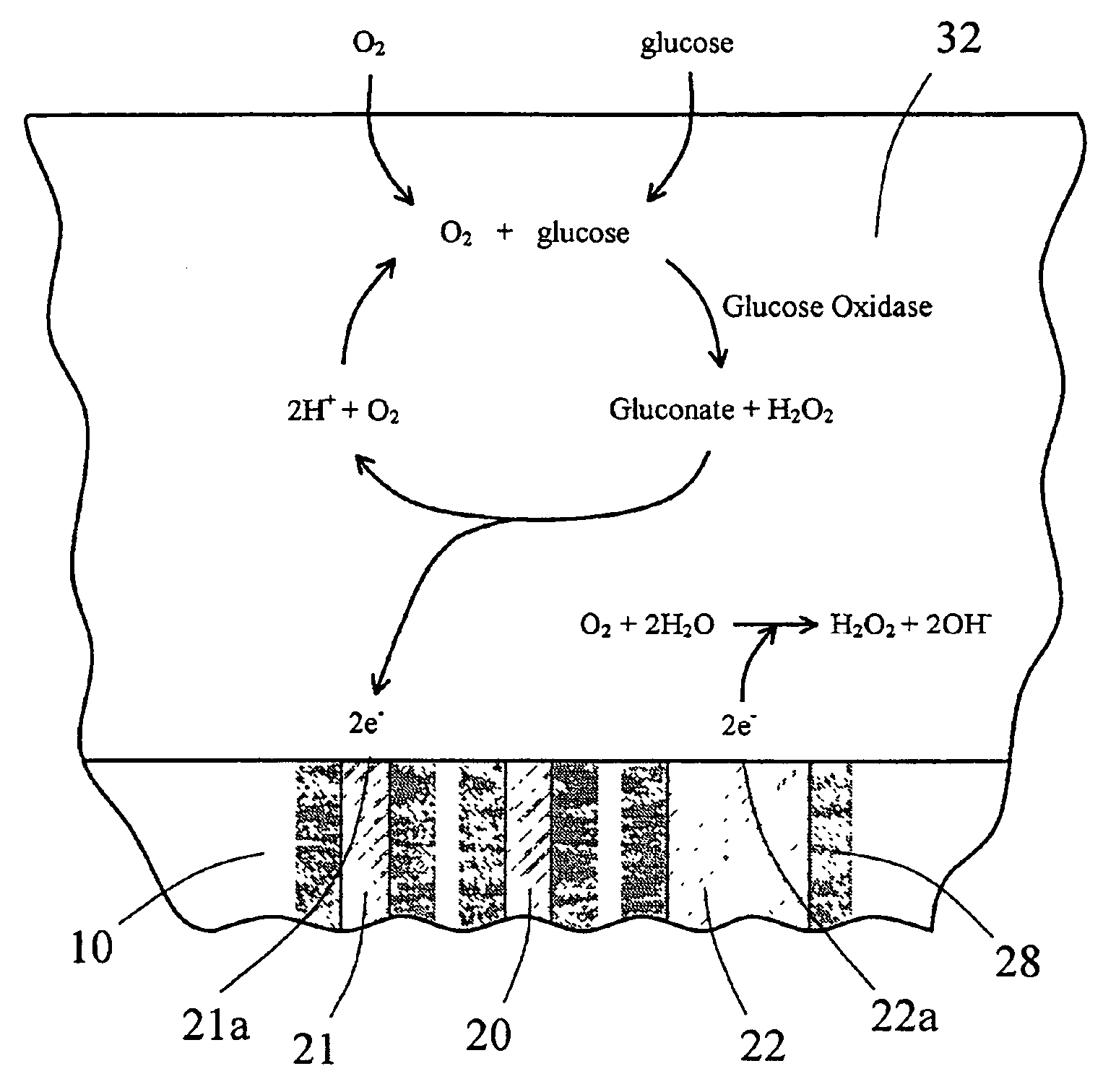
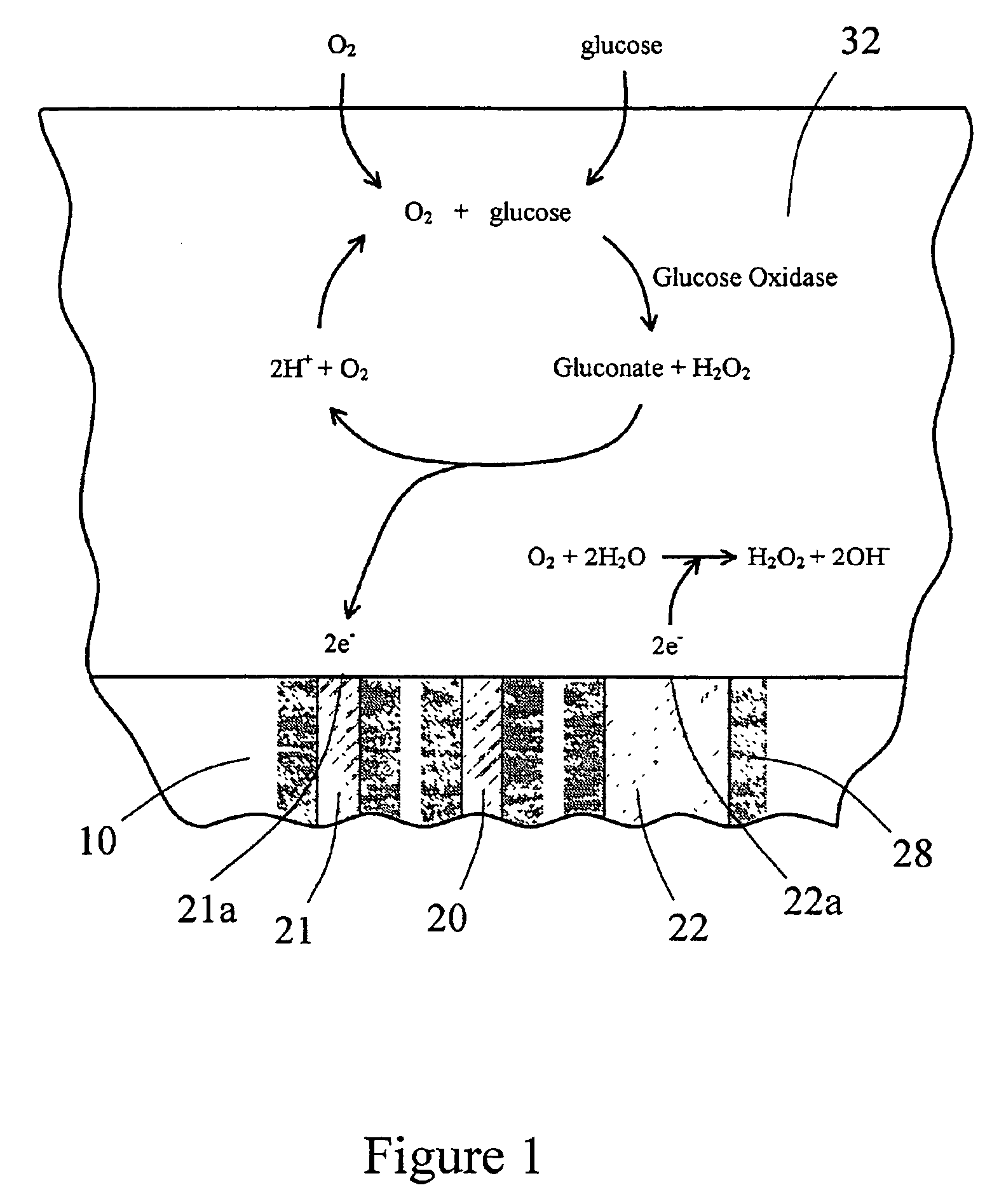
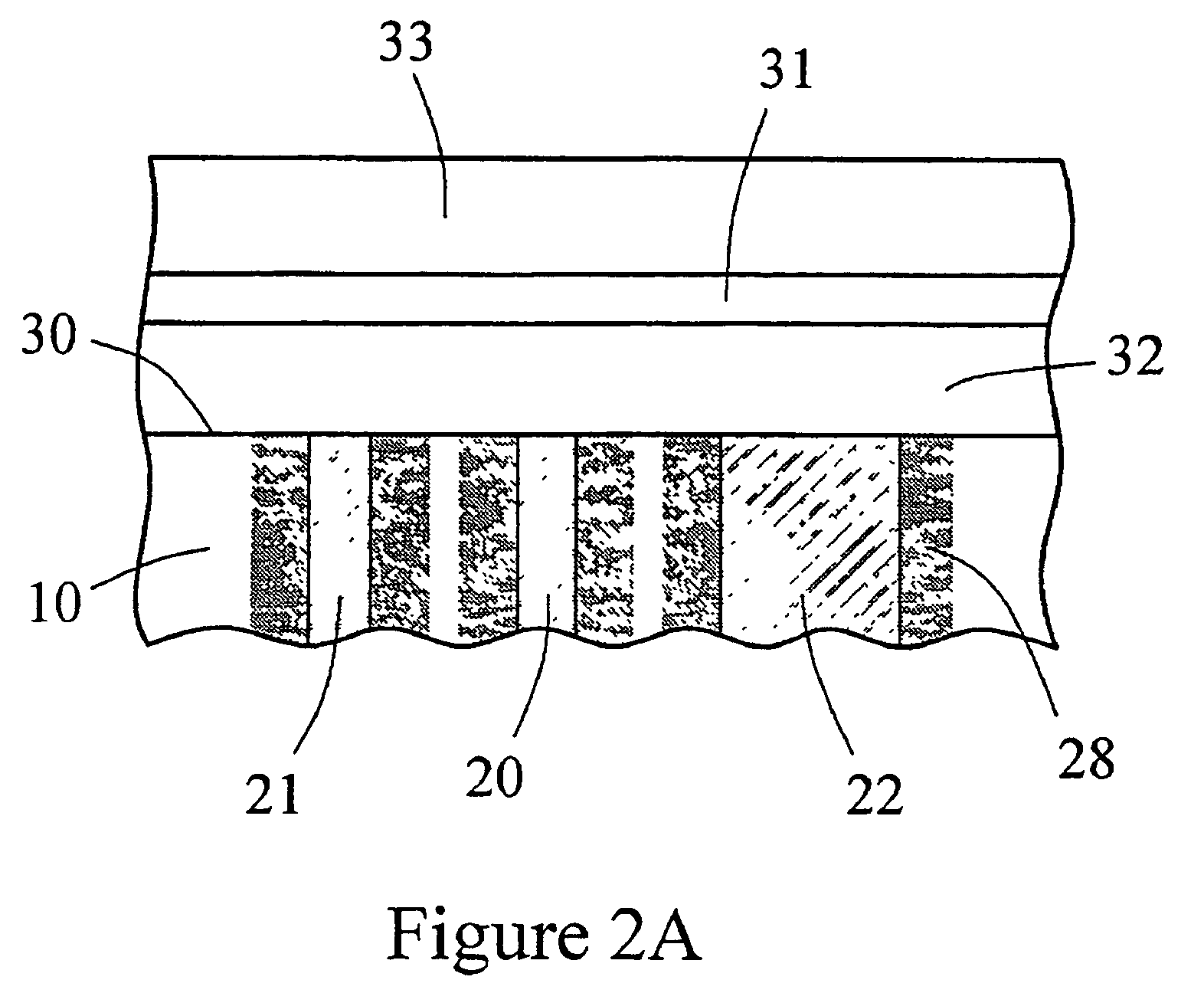
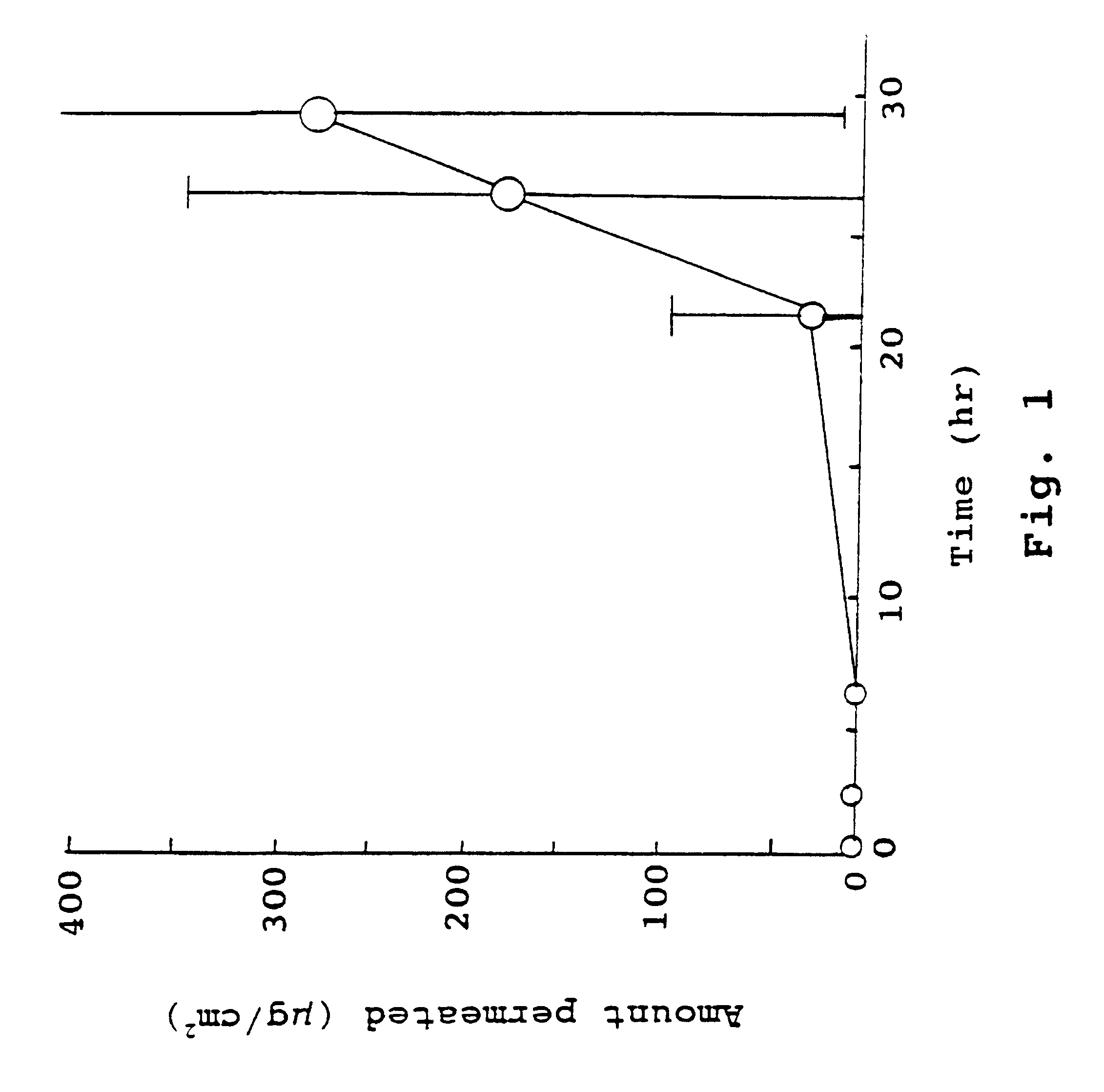
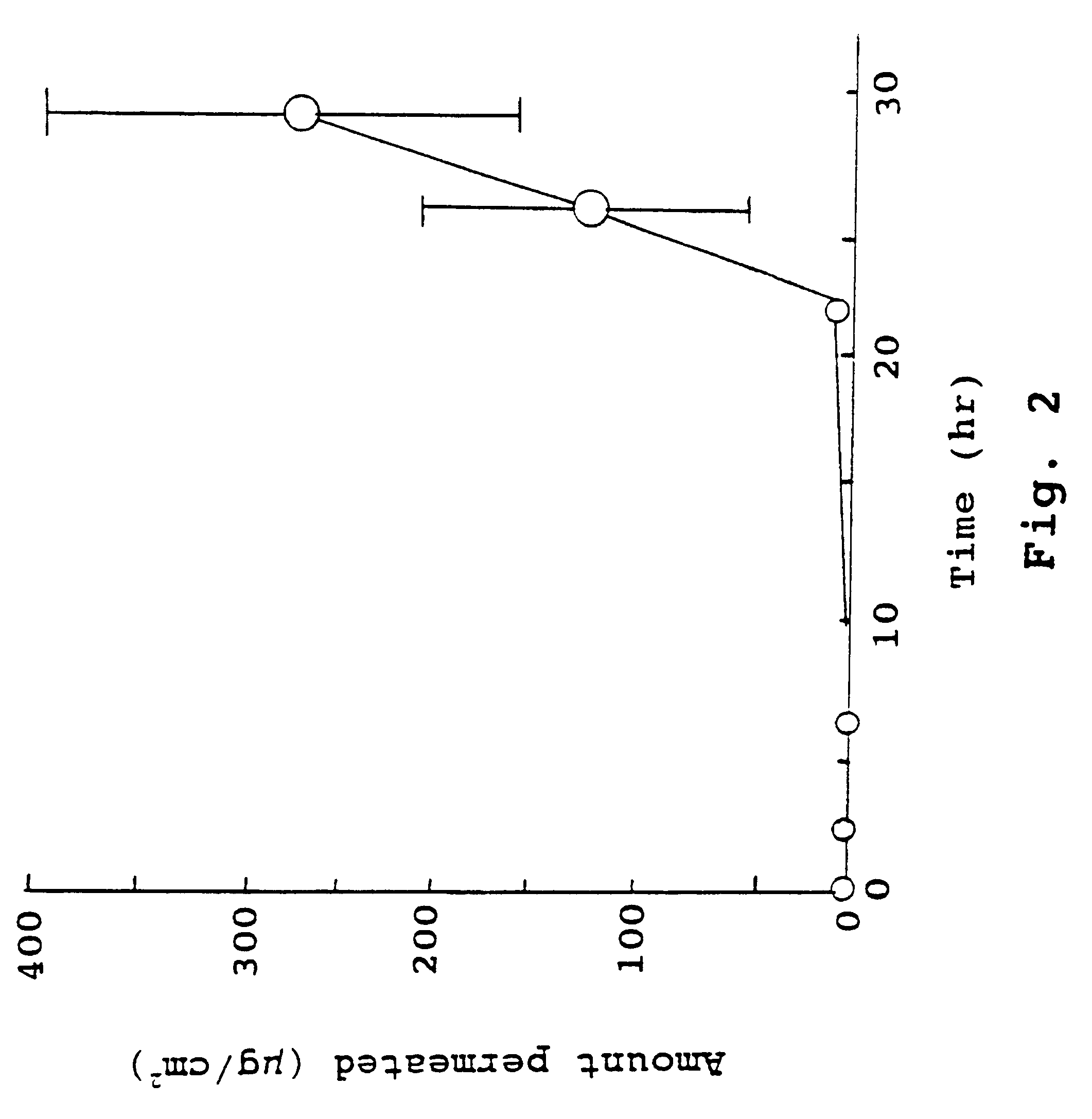
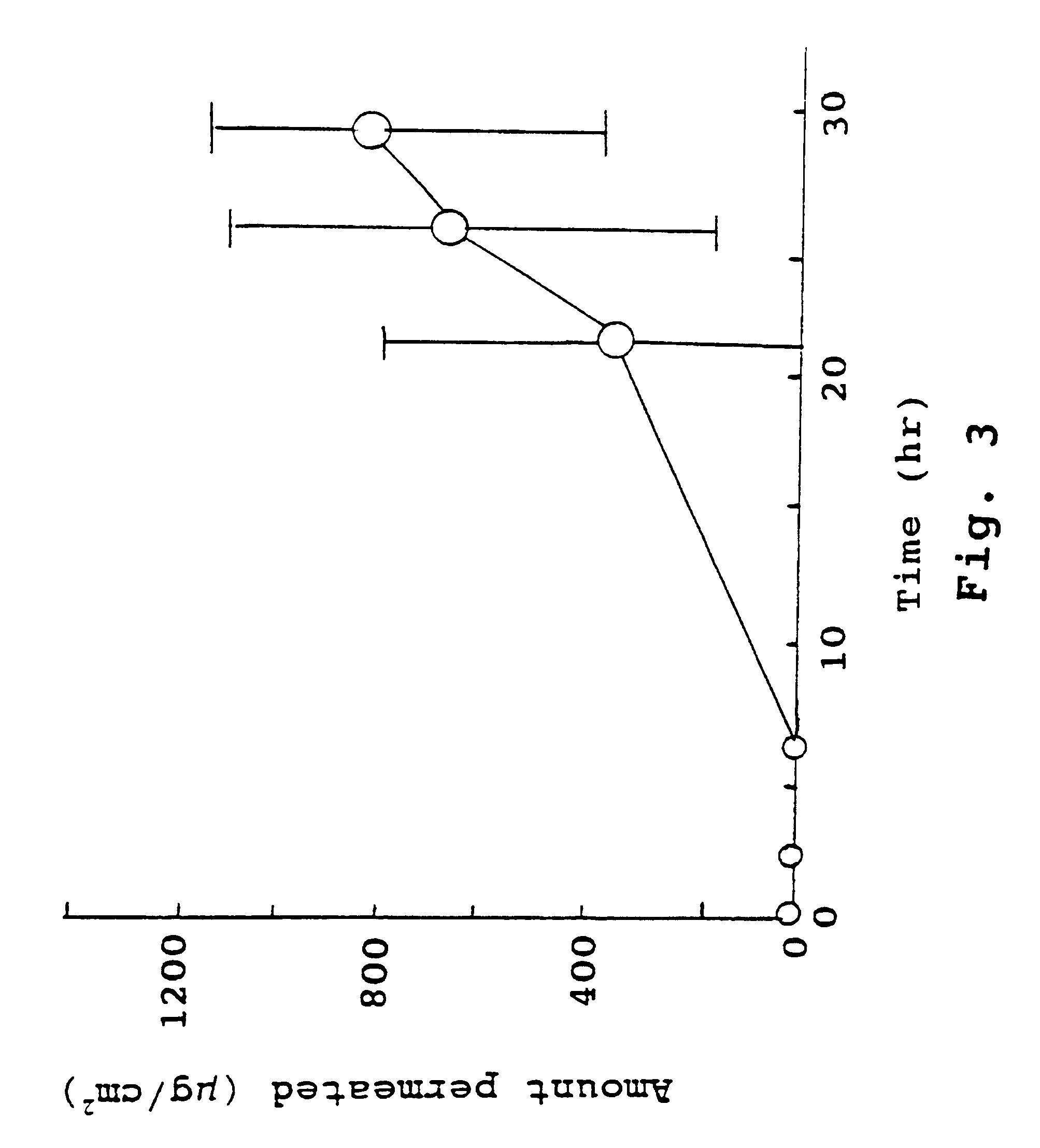
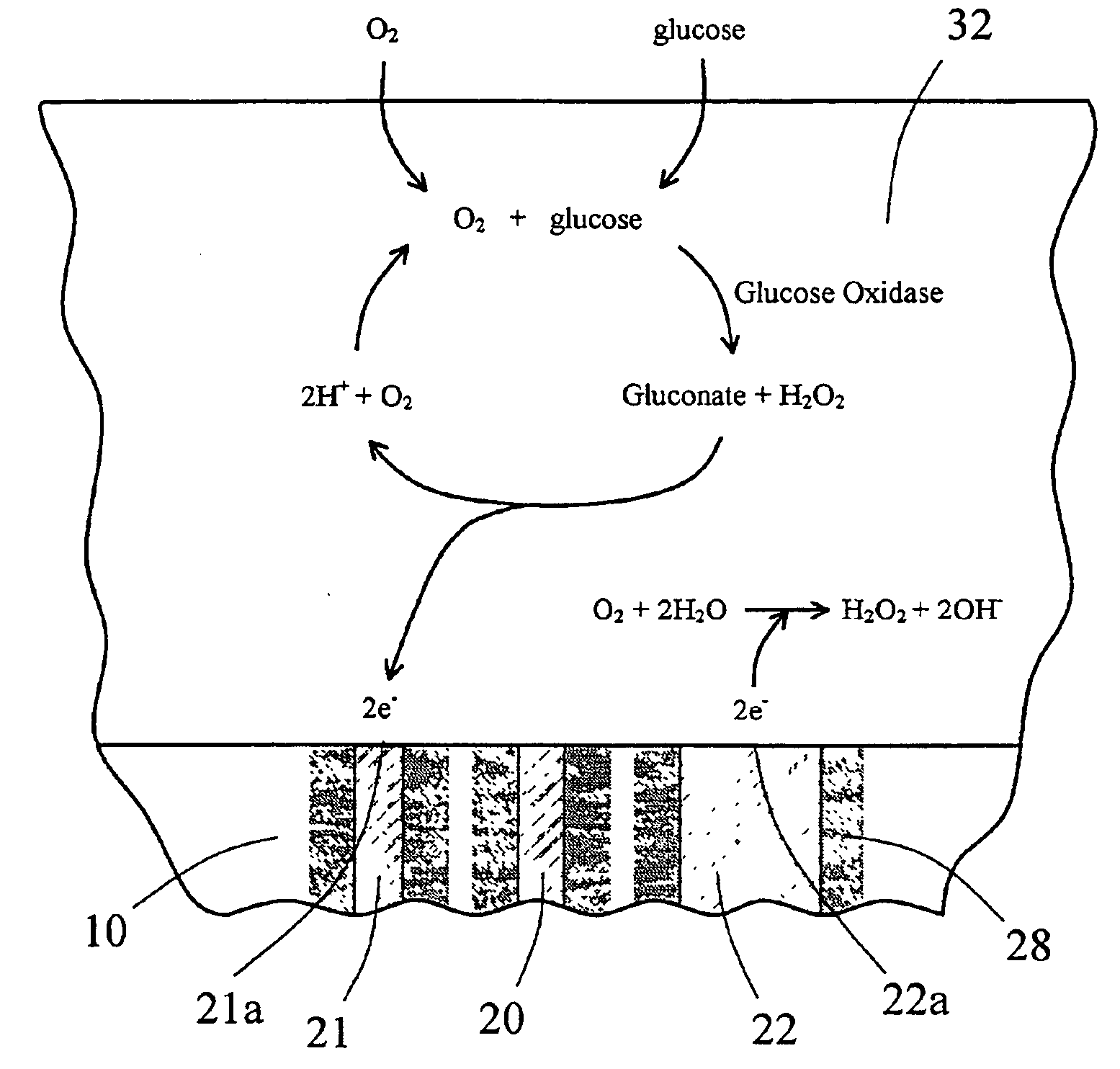
The present invention provides a sensor head for use in an implantable device that measures the concentration of an analyte in a biological fluid which includes: a non-conductive body; a working electrode, a reference electrode and a counter electrode, wherein the electrodes pass through the non-conductive body forming an electrochemically reactive surface at one location on the body and forming an electronic connection at another location on the body, further wherein the electrochemically reactive surface of the counter electrode is greater than the surface area of the working electrode; and a multi-region membrane affixed to the nonconductive body and covering the working electrode, reference electrode and counter electrode. In addition, the present invention provides an implantable device including at least one of the sensor heads of the invention and methods of monitoring glucose levels in a host utilizing the implantable device of the invention.
Owner:DEXCOM INC
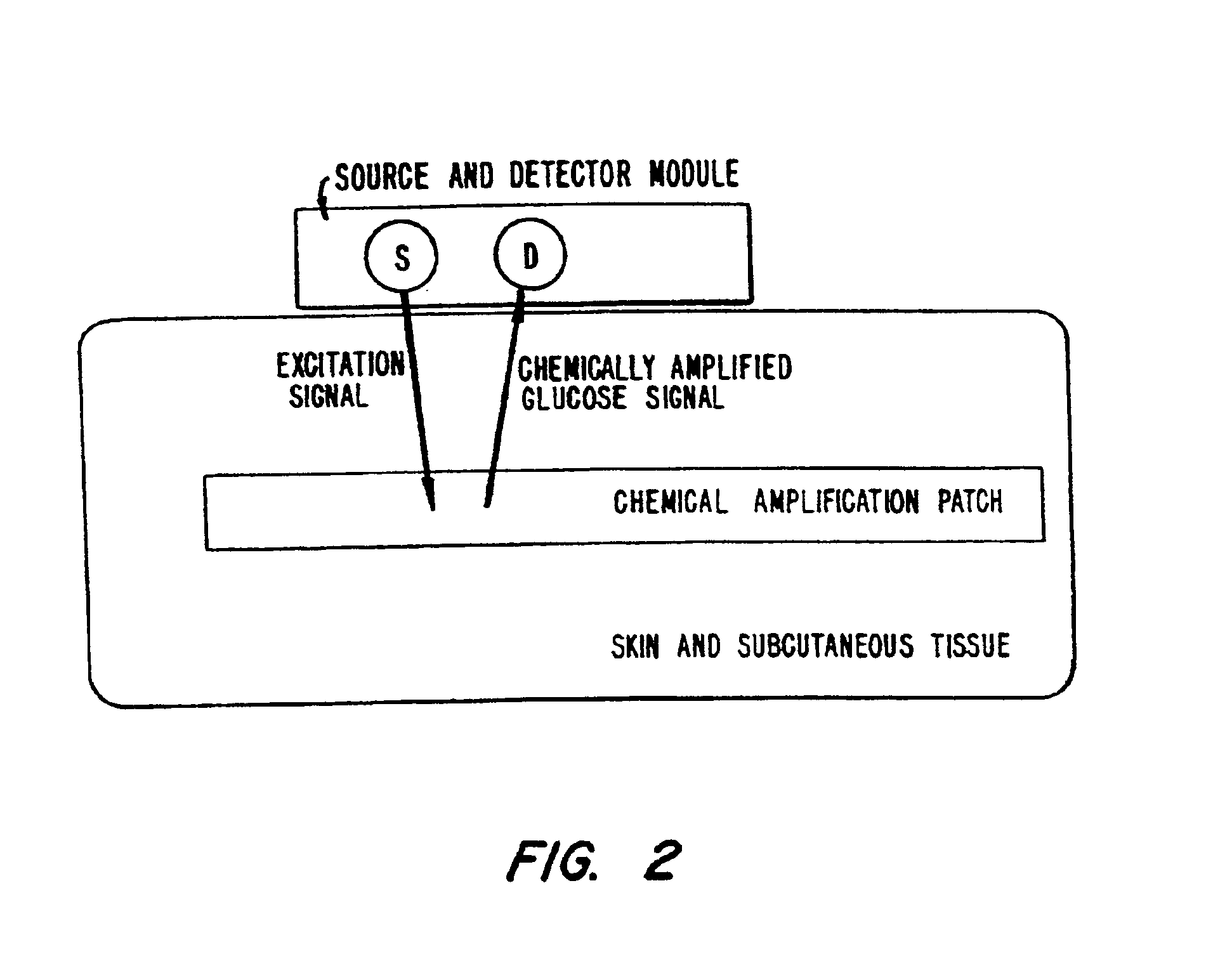
Membrane suitable for use in an analyte sensor, analyte sensor, and associated method
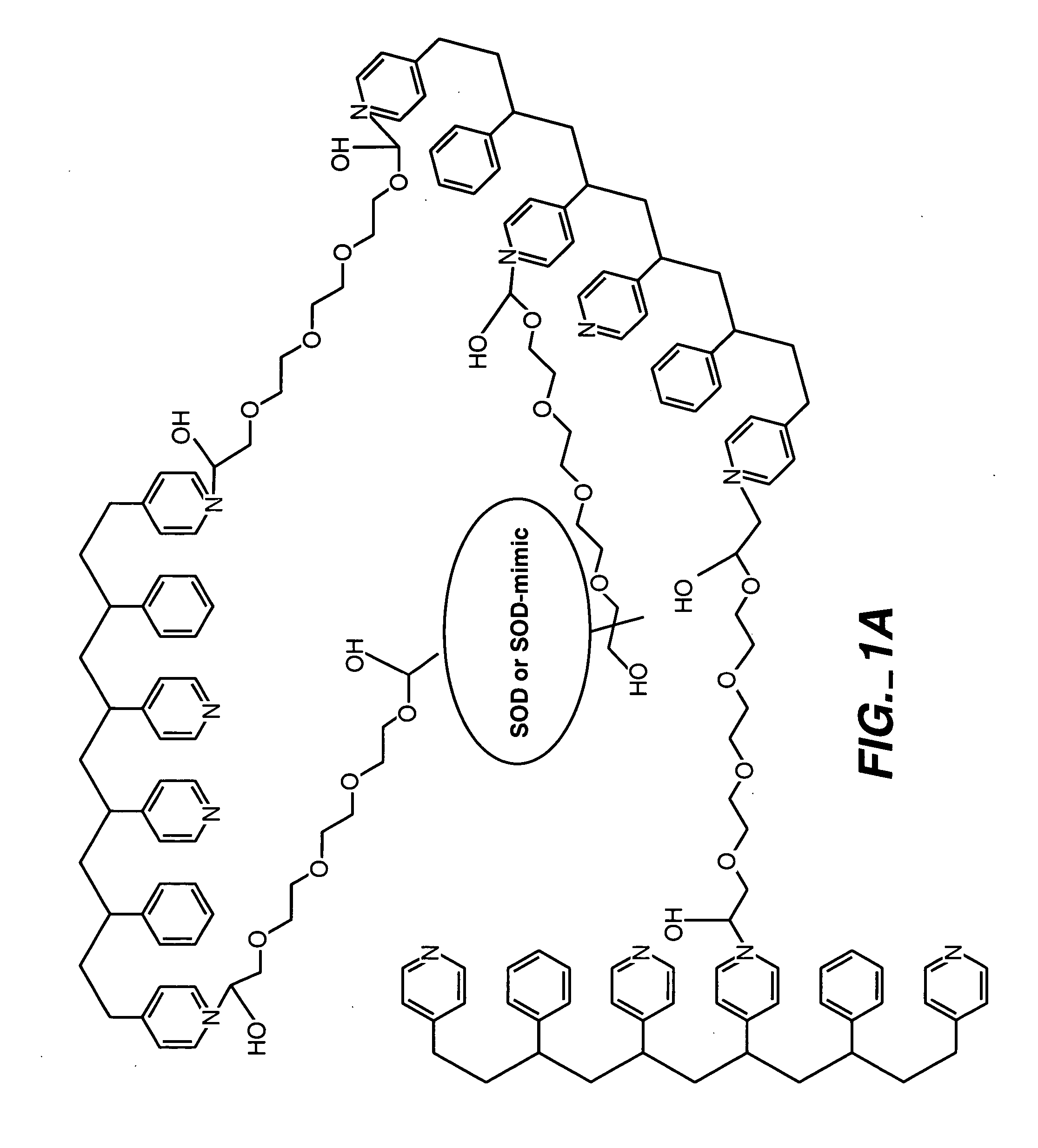
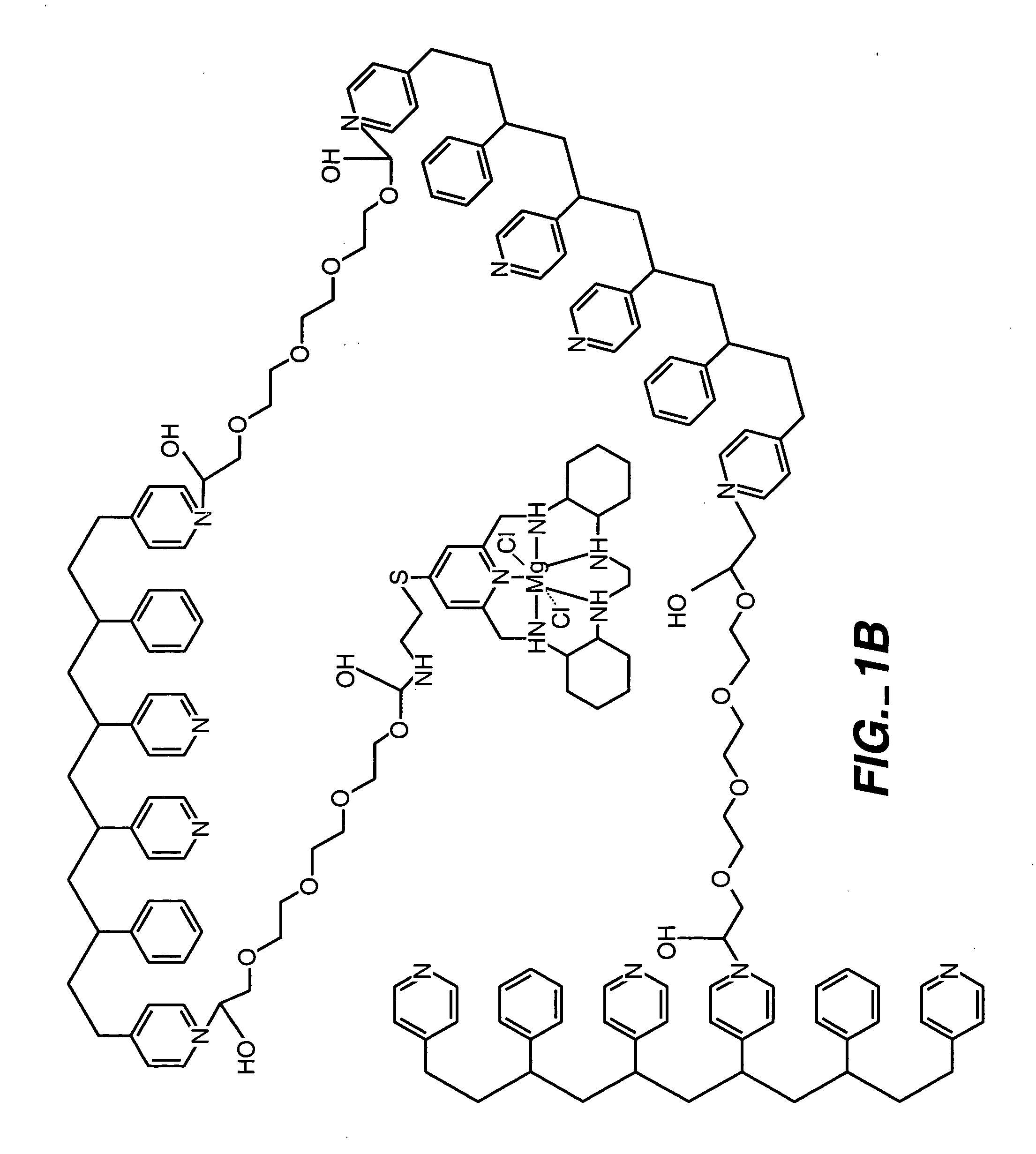
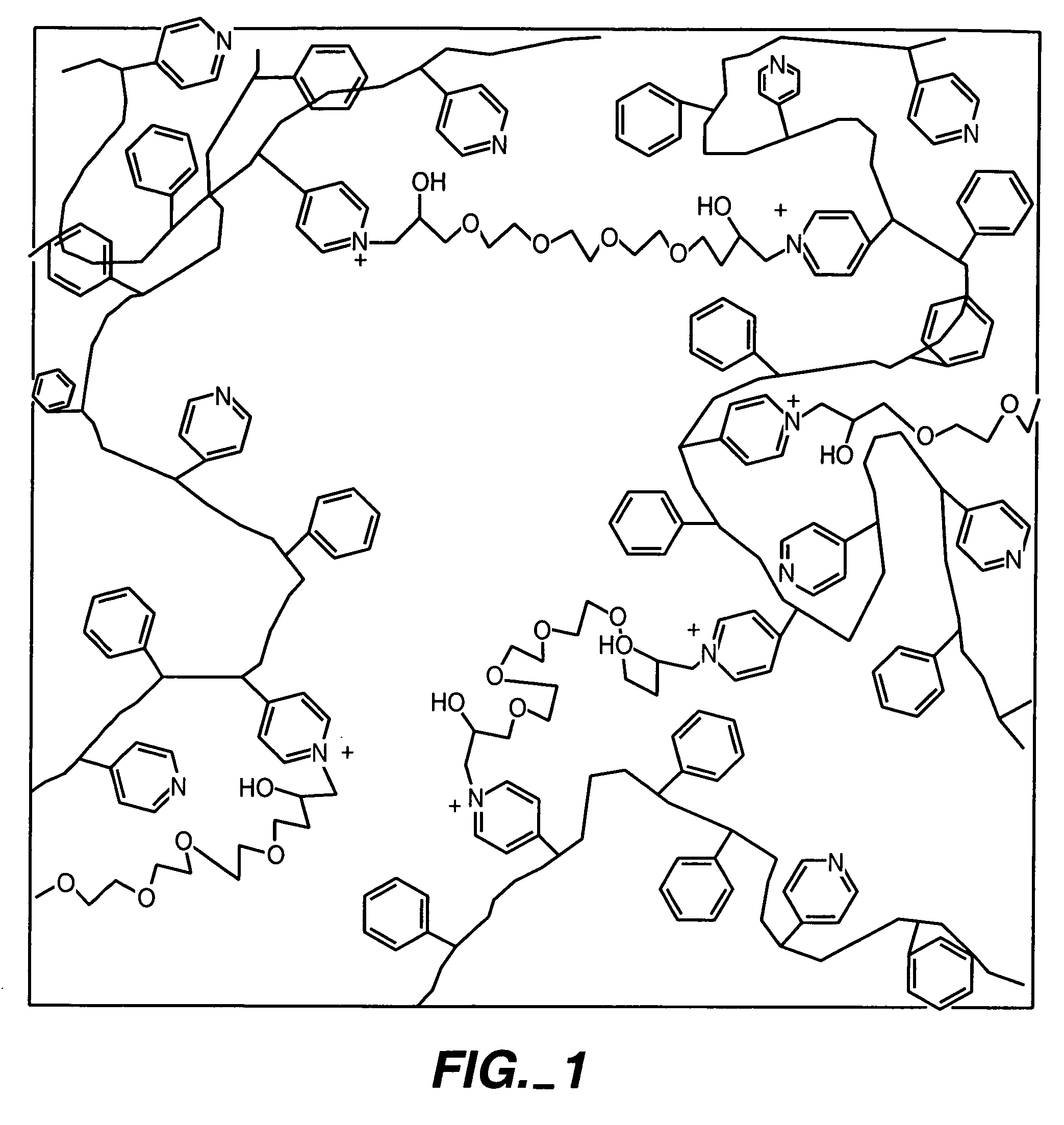
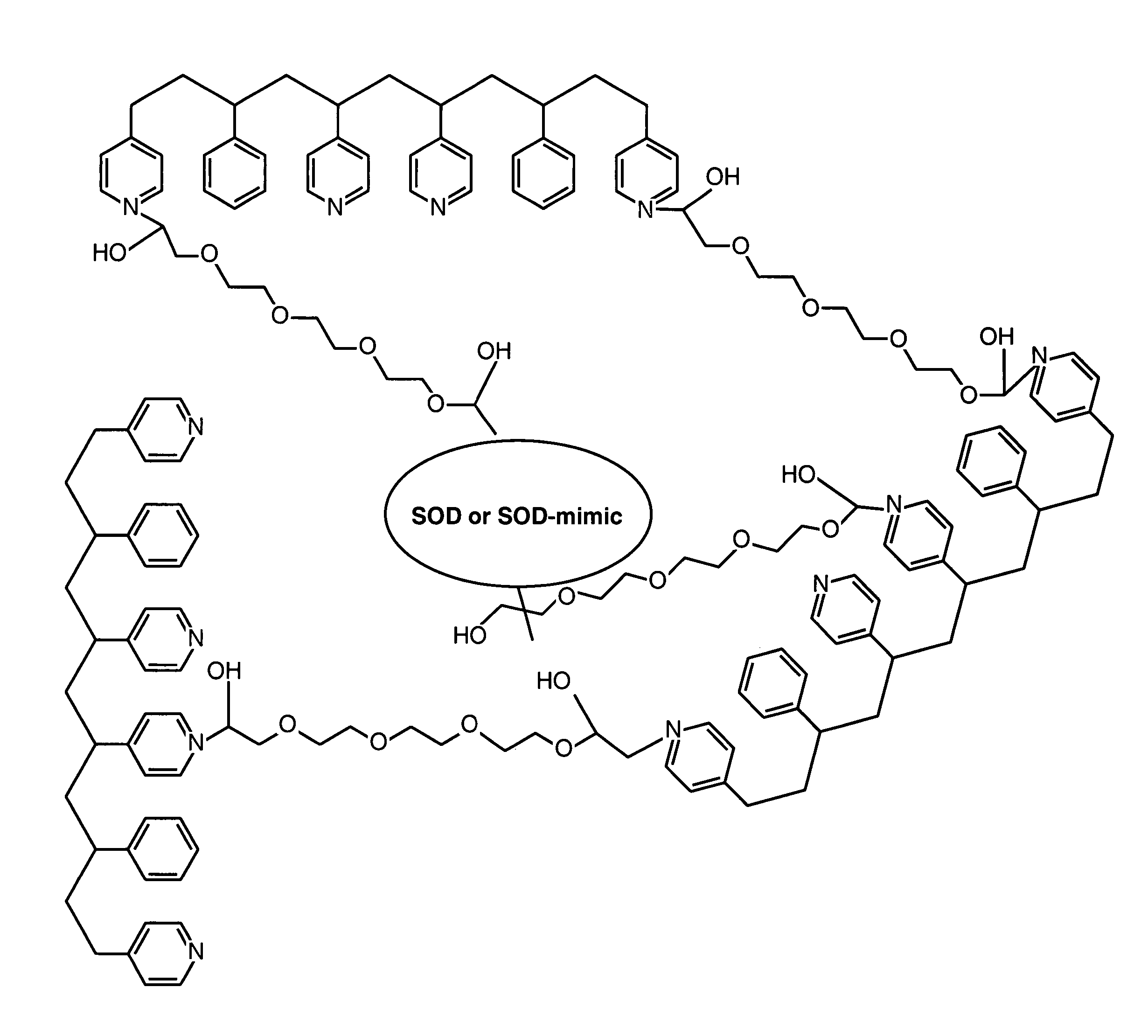
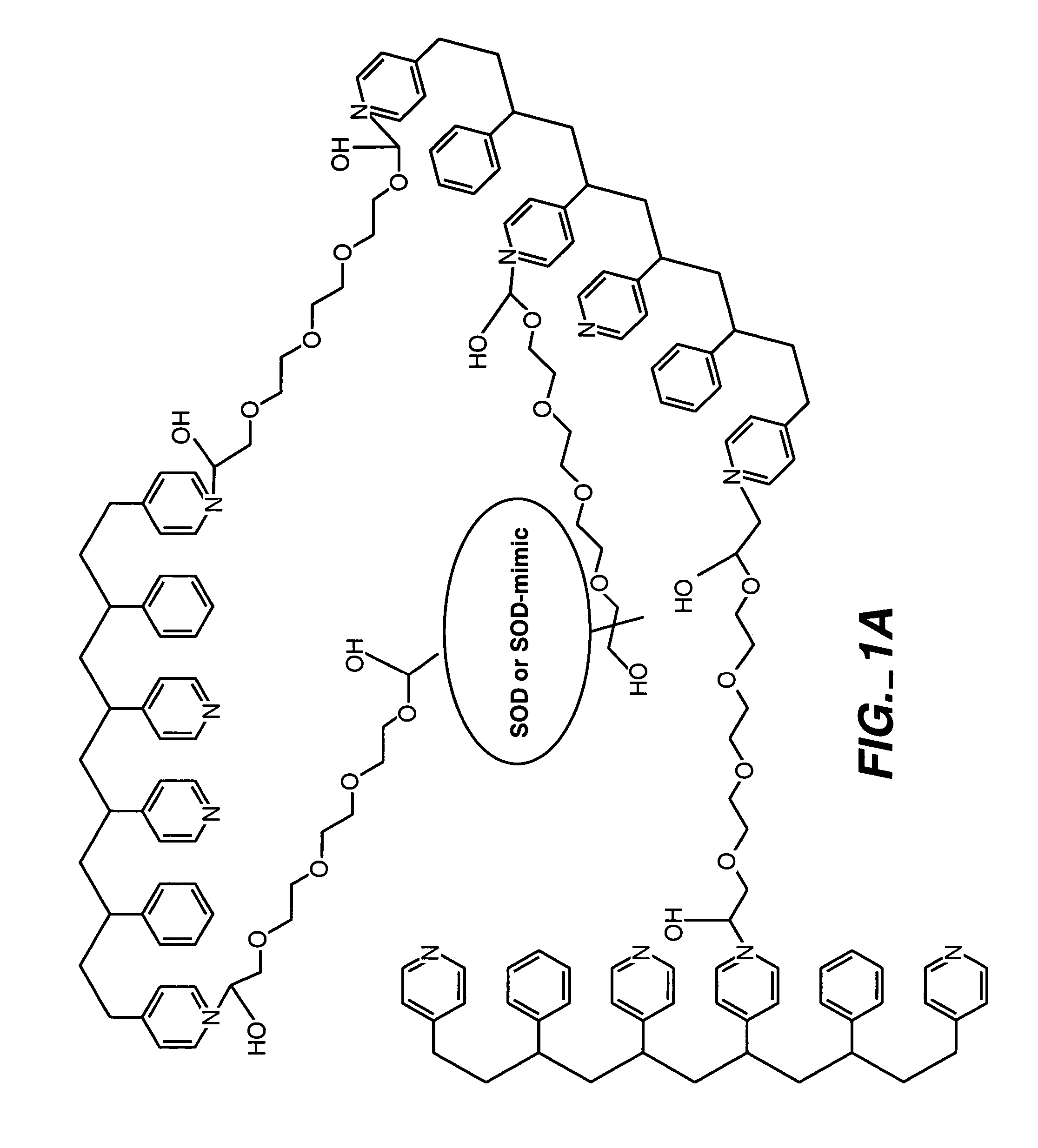
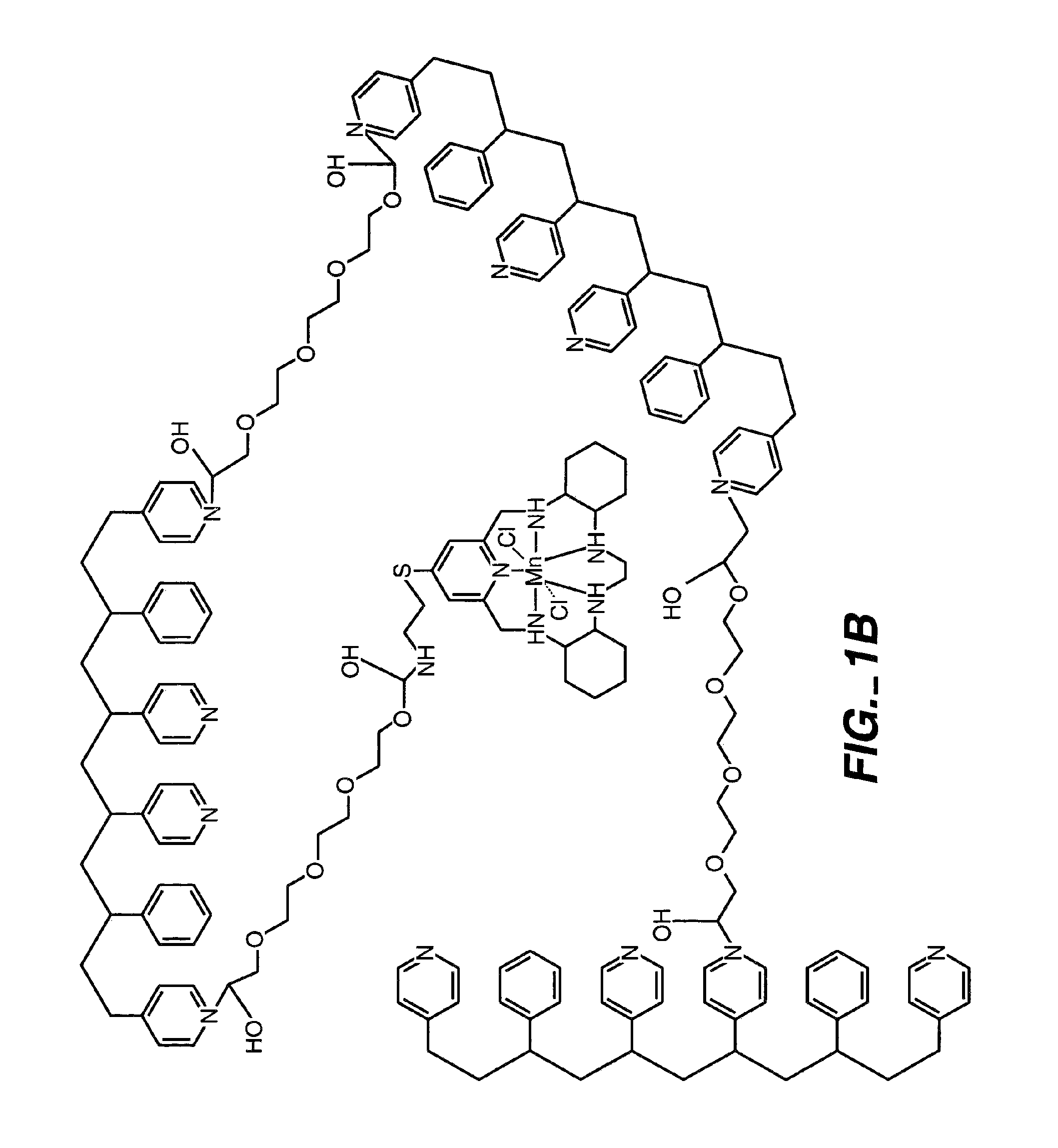
ActiveUS20050173245A1Facilitate linear responsivenessEasy CalibrationImmobilised enzymesBioreactor/fermenter combinationsMetaboliteSuperoxide
A multifunctional membrane is provided. The multifunctional membrane is suitable for use in an analyte sensor. In a particular application, the multifunctional membrane may be used in connection with an amperometric biosensor, such as a transcutaneous amperometric biosensor. Some functions of the membrane are associated with properties of membrane itself, which is comprised of crosslinked polymers containing heterocyclic nitrogen groups. For example, the membrane, by virtue of its polymeric composition, may regulate the flux of an analyte to a sensor. Such regulation generally improves the kinetic performance of the sensor over a broad range of analyte concentration. Other functions of the membrane are associated with functional components, such as a superoxide-dismutating / catalase catalyst, either in the form of an enzyme or an enzyme mimic, that can be bound to the scaffold provided by the membrane. The effect of any such enzyme or enzyme mimic is to lower the concentration of a metabolite, such as superoxide and / or hydrogen peroxide, in the immediate vicinity of the sensing layer of the biosensor. Lowering the concentrations of such metabolites, which are generally deleterious to the function of the sensor, generally protects or enhances biosensor integrity and performance. The membrane is thus an important tool for use in connection with analyte sensors, amperometric sensors, biosensors, and particularly, transcutaneous biosensors. A membrane-covered sensor and a method for making same are also provided.
Owner:ABBOTT DIABETES CARE INC
Biosensor membranes composed of polymers containing heterocyclic nitrogens
InactiveUS20050241957A1Easy CalibrationConsider sensitivityMicrobiological testing/measurementVolume/mass flow measurementAnalyteNitrogen
Owner:ABBOTT DIABETES CARE INC
Protein scaffolds for antibody mimics and other binding proteins
InactiveUS7115396B2Easy to foldImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsWAS PROTEINAntibody
Disclosed herein are proteins that include an immunoglobulin fold and that can be used as scaffolds. Also disclosed herein are nucleic acids encoding such proteins and the use of such proteins in diagnostic methods and in methods for evolving novel compound-binding species and their ligands.
Owner:BRISTOL MYERS SQUIBB CO
Compositions for rapid and non-irritating transdermal delivery of pharmaceutically active agents and methods for formulating such compositions and delivery thereof
InactiveUS6444234B1Effective therapeutic doseReduced barrier effectBiocideCosmetic preparationsSolventActive agent
Pharmaceutical compositions for the transdermal administration of a medicament or other active agent by topical application of the composition to the skin of humans or other animals are described. Methodology for formulating such compositions which provide for very rapid uptake of the medicament and transmigration into and through the skin to either fatty tissues or the vascular system, while minimizing irritation to the skin and / or immunological response, is based on a transdermal delivery system (TDS) wherein the medicament is modified to form a true solution in a complex formed from particular solvents and solvent and solute modifiers in combination with skin stabilizers. Uptake of the medicament is further facilitated and made more rapid by including Forskolin or other source of cellular energy, namely induction of cAMP or cGMP. Selection of specific solvents and solvent and solute modifiers and other functional ingredients and the amounts thereof are chosen such that there is a balance between the sum of the mole-moments [(molar amount of each individual ingredient)x(dipole moment of that ingredient)] of the delivery system and the sum of the moler moments of the composition in which the medicament is dissolved. Preferably, the van der Waals forces of the delivery system is also similarly matched to the van der Waals forces of the total composition, namely, delivery system plus active agent.
Owner:TRANSDERMAL DELIVERY SOLUTIONS
Sensor head for use with implantable devices
ActiveUS20050103625A1Improve functioningHigh oxygen solubilityImmobilised enzymesBioreactor/fermenter combinationsElectrochemical responseMonitoring glucose
The present invention provides a sensor head for use in an implantable device that measures the concentration of an analyte in a biological fluid which includes: a non-conductive body; a working electrode, a reference electrode and a counter electrode, wherein the electrodes pass through the non-conductive body forming an electrochemically reactive surface at one location on the body and forming an electronic connection at another location on the body, further wherein the electrochemically reactive surface of the counter electrode is greater than the surface area of the working electrode; and a multi-region membrane affixed to the nonconductive body and covering the working electrode, reference electrode and counter electrode. In addition, the present invention provides an implantable device including at least one of the sensor heads of the invention and methods of monitoring glucose levels in a host utilizing the implantable device of the invention.
Owner:DEXCOM
Novel dosage form
ActiveUS20060024365A1Effectively control release rateReduce sizePill deliveryEster active ingredientsHigh dosesSolubility
A dosage form comprising of a high dose, high solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of modified release active ingredient per unit is from 500 mg to 1500 mg; a process for preparing the dosage form.
Owner:TORRENT PHARMA LTD
Film forming foamable composition
InactiveUS20060193789A1Improve solubilityReduce deliveryCosmetic preparationsBiocideAlcohol freeFilm-forming agent
A foamable composition, includes (1) about 6% to about 70% by weight of at least one organic carrier; (2) about 0.1% to about 5% by weight of at least one surface-active agent; (3) about 0.01% to about 5% by weight of at least one film forming agent; (4) water; and (5) about 3% to about 25% by weight of the total composition of at least one liquefied or compressed gas propellant. The composition is substantially alcohol free and is used in treating, alleviating or preventing a disorder.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
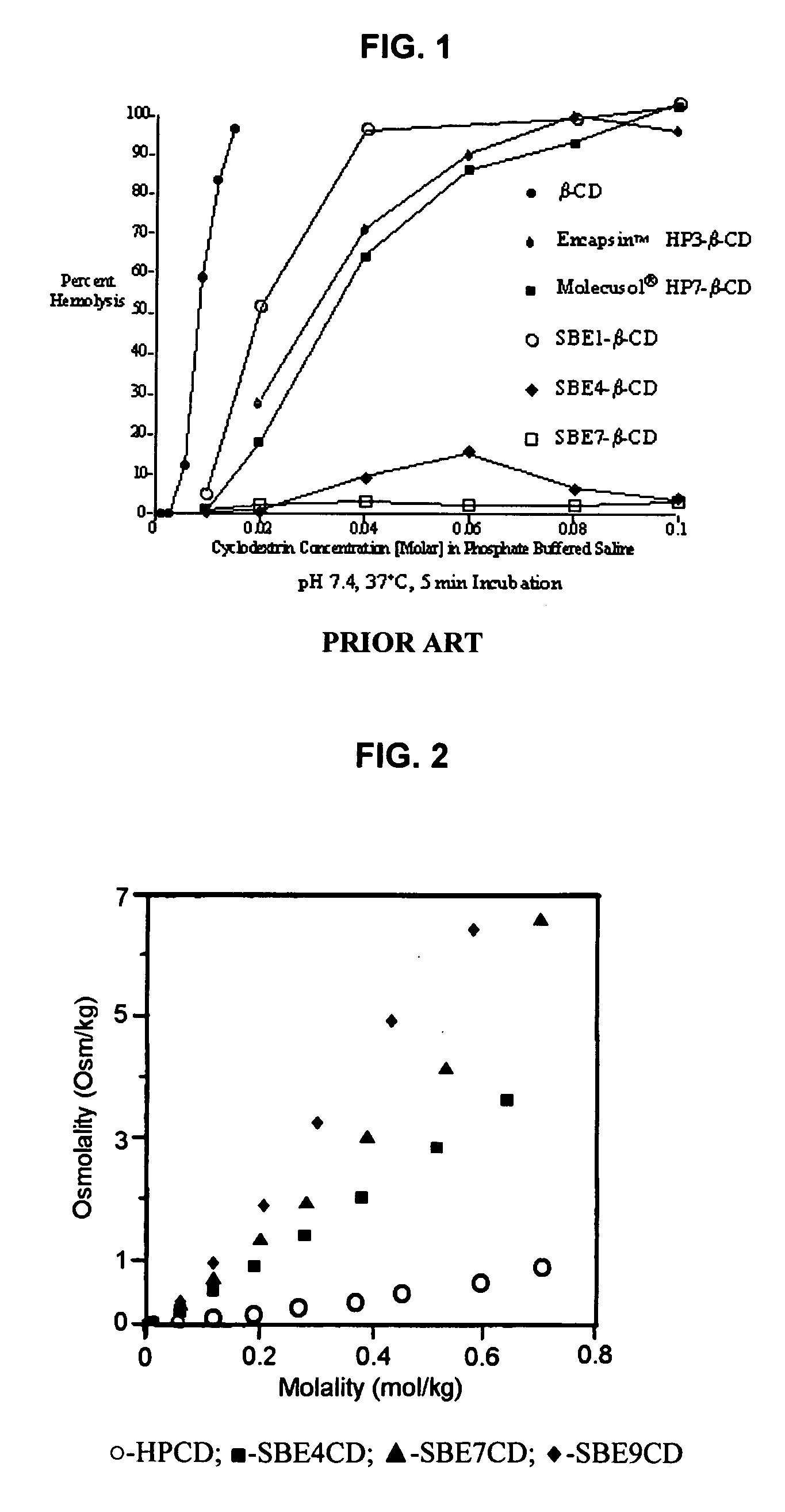
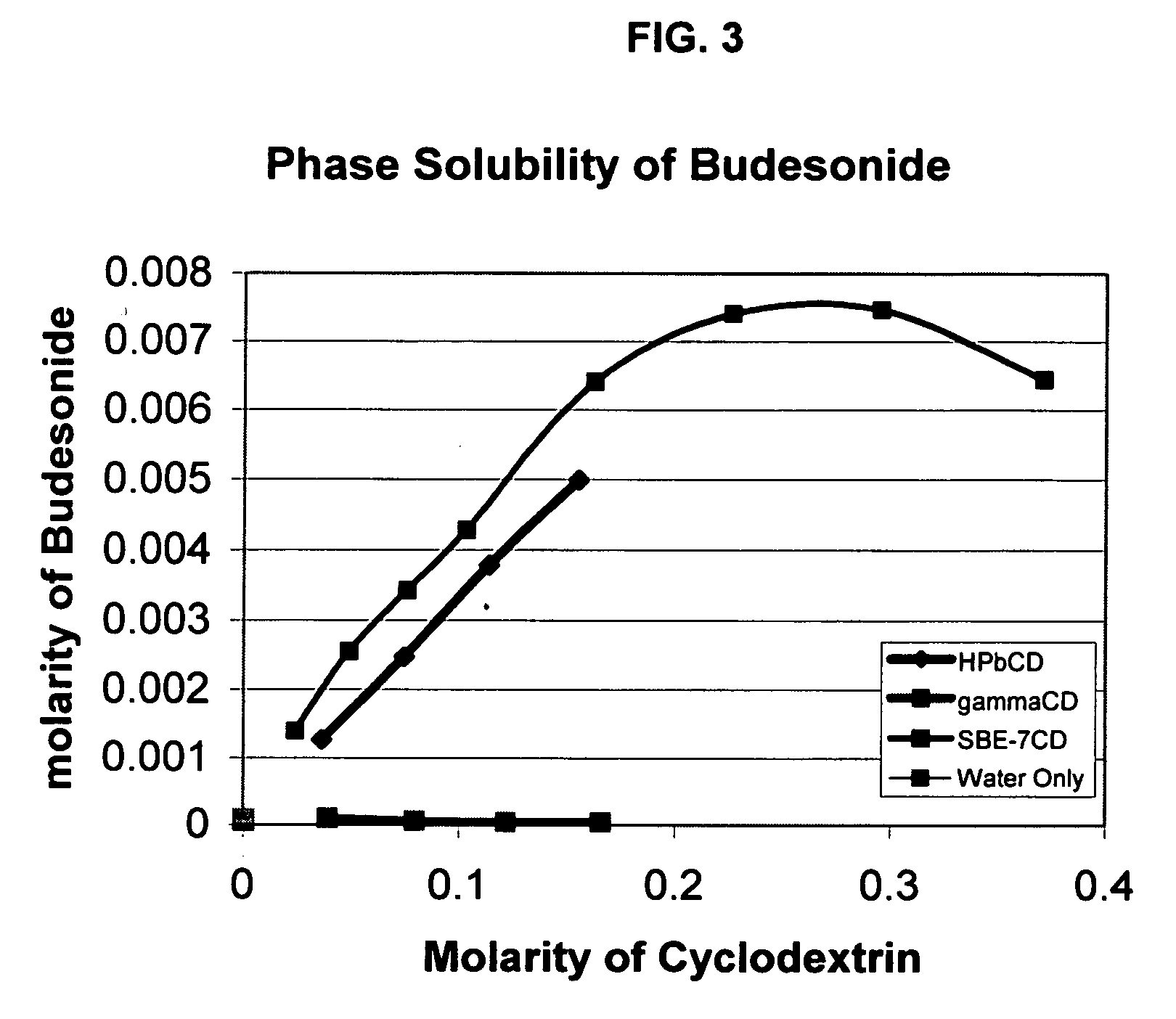
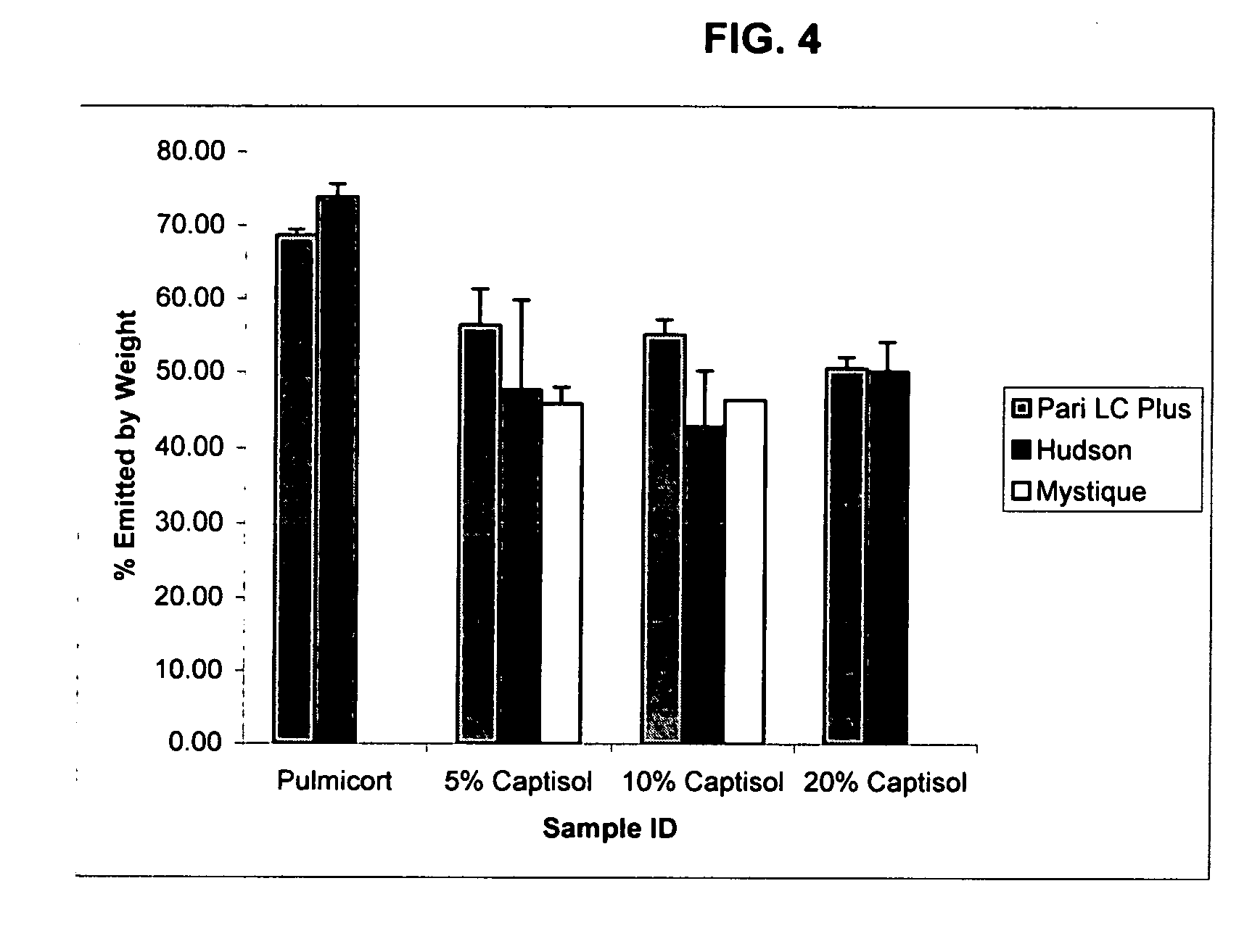
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
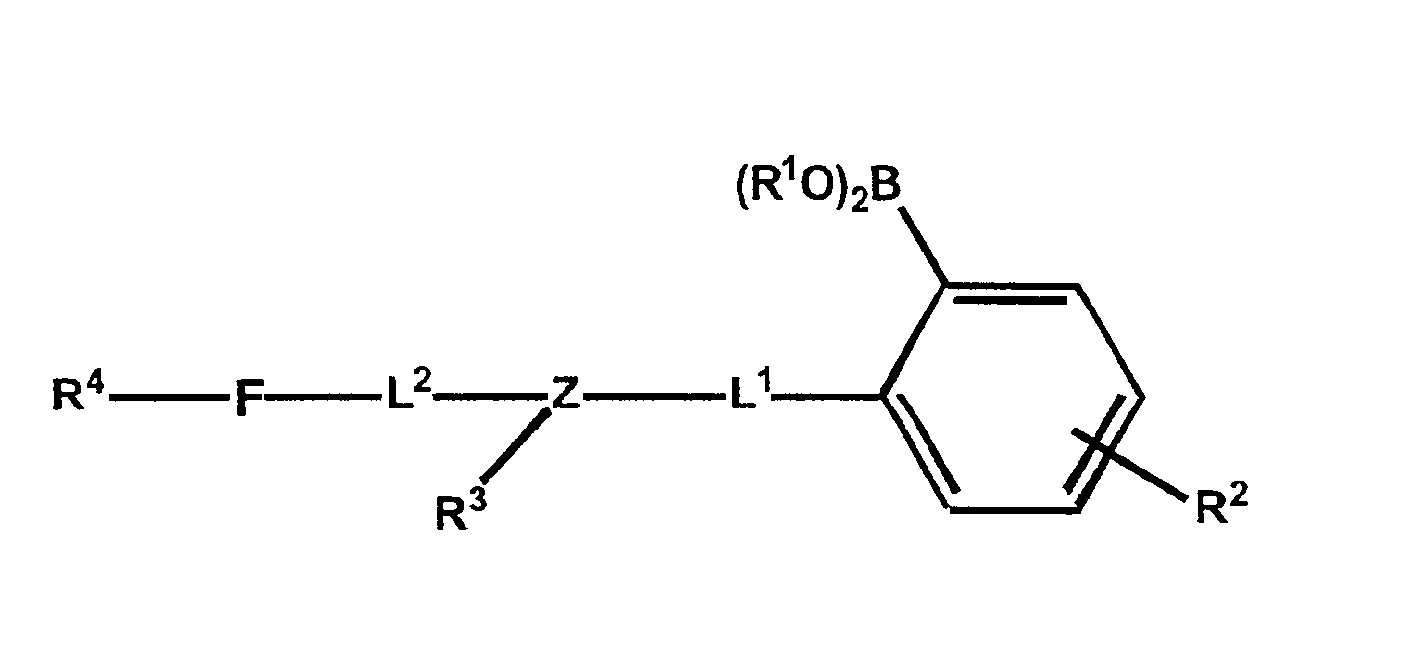
Polymers functionalized with fluorescent boronate motifs and methods for making them
InactiveUS6927246B2Enhance swellabilityGood biocompatibilityGroup 3/13 element organic compoundsBiological testingAnalyteConcentrations glucose
Improved polymer matrices which incorporate fluorescent biosensor molecules as well as methods of making and using these polymer matrices are described. Such matrices can be used in fluorescent biosensors and biosensor systems, including those which are used in the detection of polyhydroxylated analytes such as glucose. The properties of the polymer matrices of the invention renders biosensors utilizing such matrices particularly well-suited for detecting and measuring in-vivo glucose concentrations.
Owner:MEDTRONIC MIMIMED INC
Membrane suitable for use in an analyte sensor, analyte sensor, and associated method
ActiveUS7699964B2Facilitate linear responsiveness and calibration and stabilityPreserve and improve performance of sensorImmobilised enzymesBioreactor/fermenter combinationsMetaboliteAmperometric biosensor
A multifunctional membrane is provided. The multifunctional membrane is suitable for use in an analyte sensor. In a particular application, the multifunctional membrane may be used in connection with an amperometric biosensor, such as a transcutaneous amperometric biosensor. Some functions of the membrane are associated with properties of membrane itself, which is comprised of crosslinked polymers containing heterocyclic nitrogen groups. For example, the membrane, by virtue of its polymeric composition, may regulate the flux of an analyte to a sensor. Such regulation generally improves the kinetic performance of the sensor over a broad range of analyte concentration. Other functions of the membrane are associated with functional components, such as a superoxide-dismutating / catalase catalyst, either in the form of an enzyme or an enzyme mimic, that can be bound to the scaffold provided by the membrane. The effect of any such enzyme or enzyme mimic is to lower the concentration of a metabolite, such as superoxide and / or hydrogen peroxide, in the immediate vicinity of the sensing layer of the biosensor. Lowering the concentrations of such metabolites, which are generally deleterious to the function of the sensor, generally protects or enhances biosensor integrity and performance. The membrane is thus an important tool for use in connection with analyte sensors, amperometric sensors, biosensors, and particularly, transcutaneous biosensors. A membrane-covered sensor and a method for making same are also provided.
Owner:ABBOTT DIABETES CARE INC
Novel drug delivery system
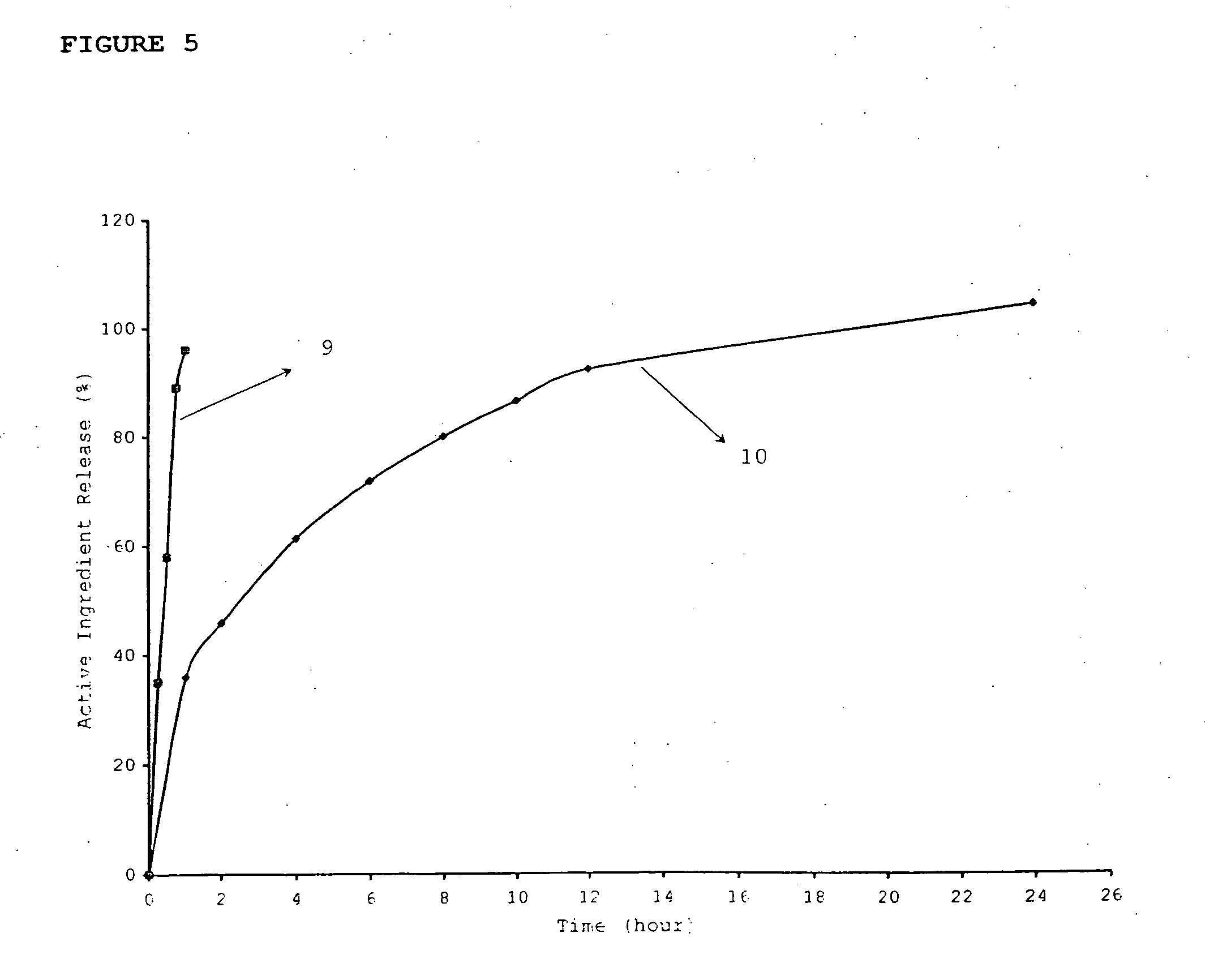
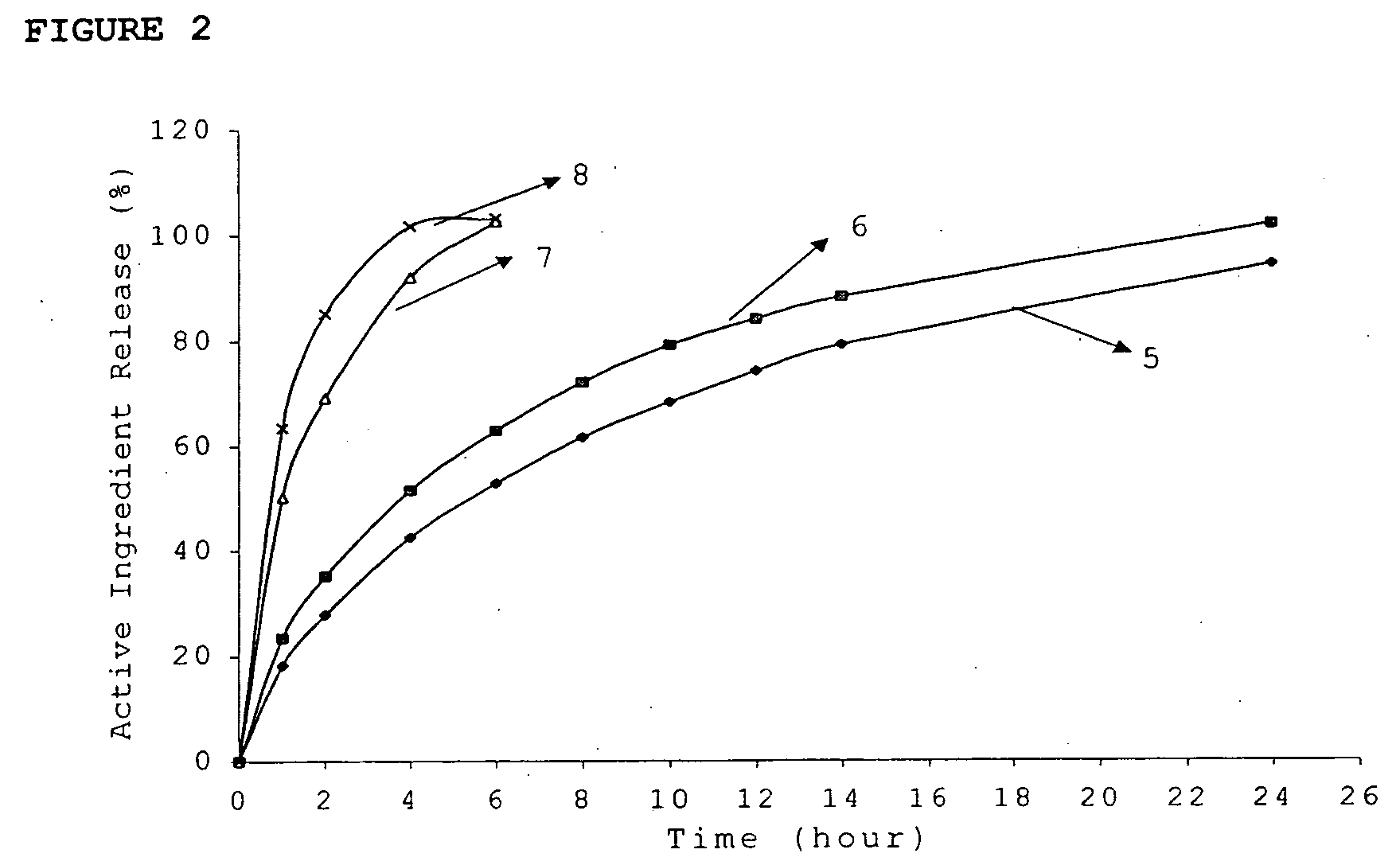
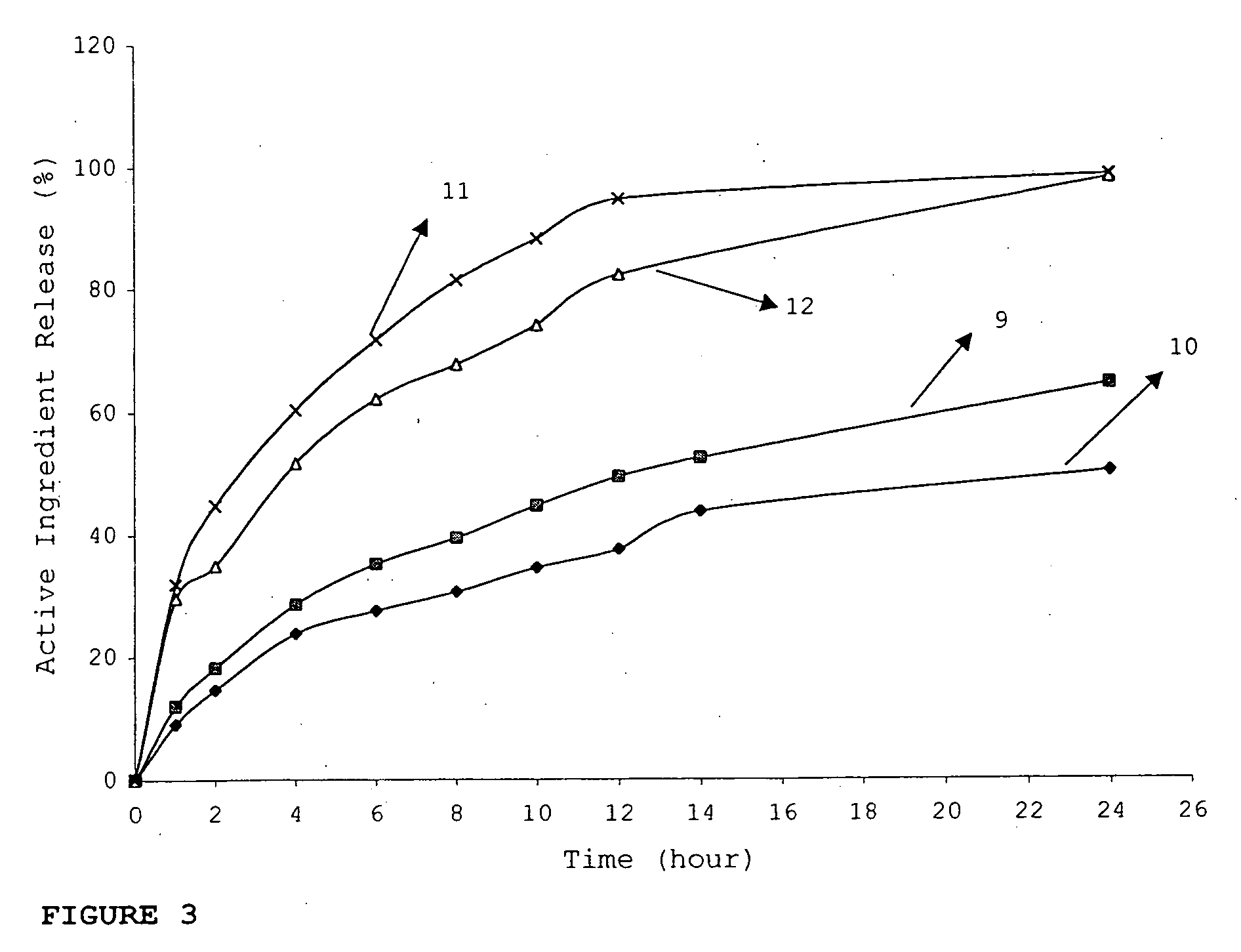
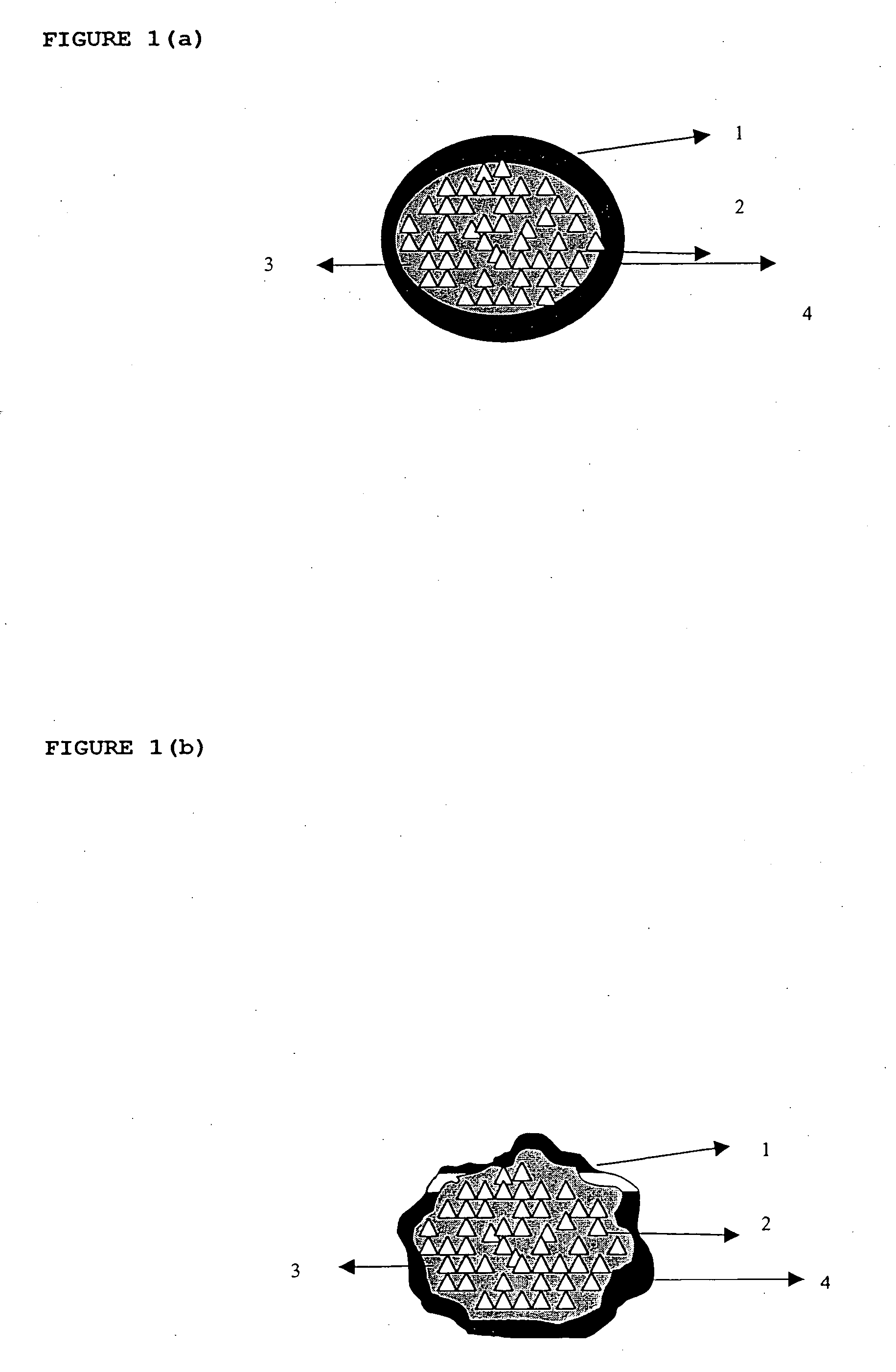
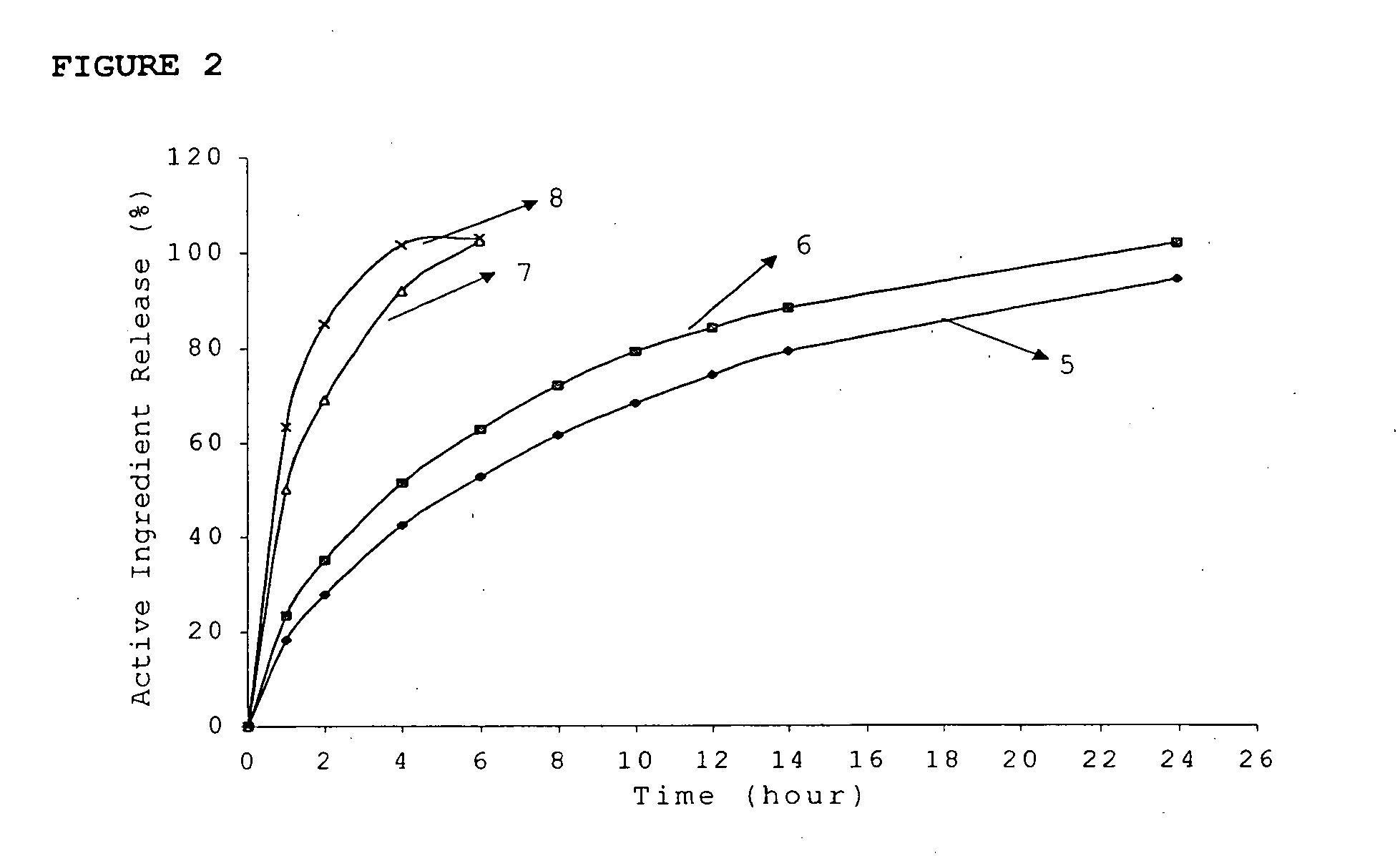
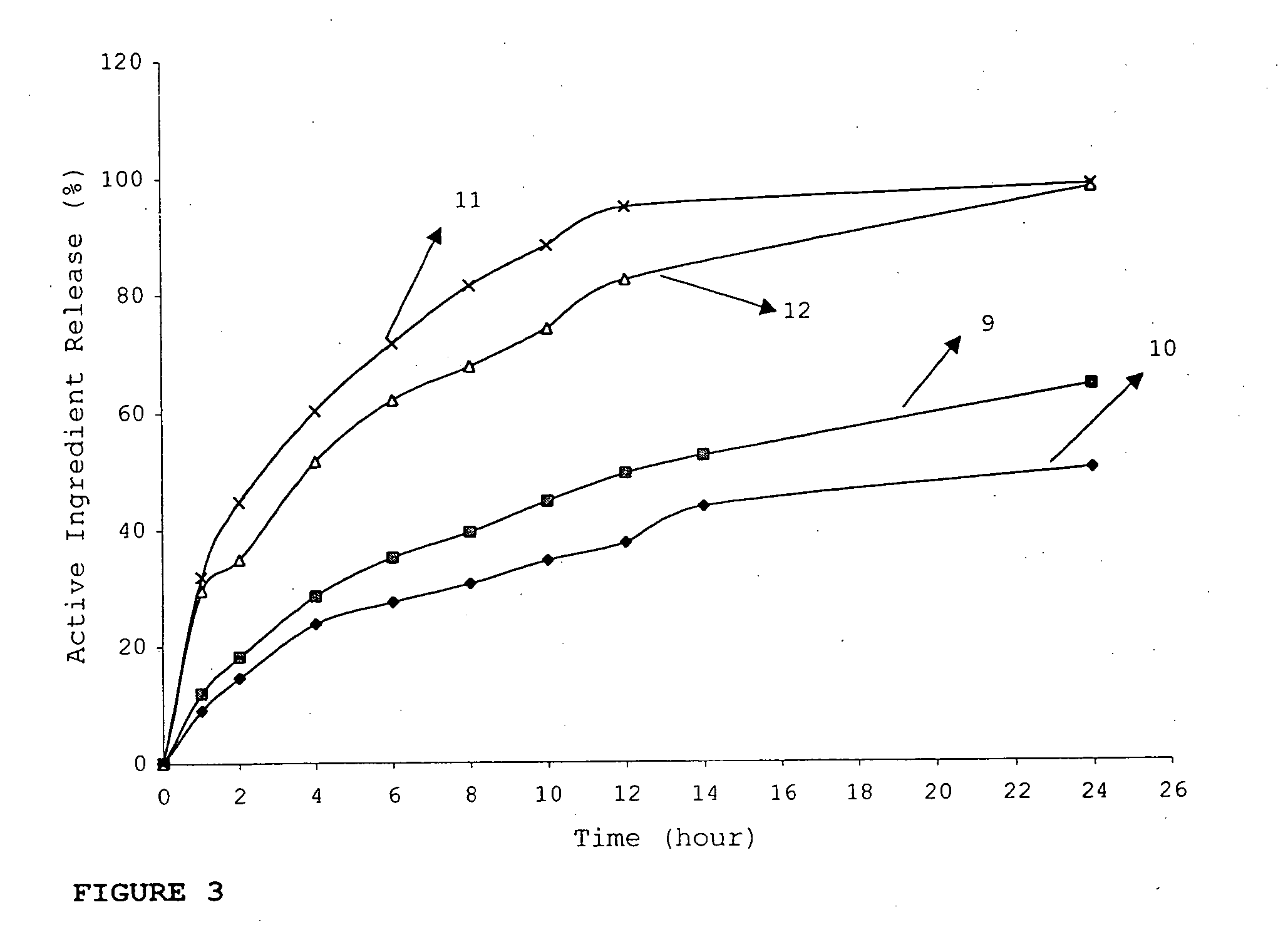
InactiveUS20060018933A1Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
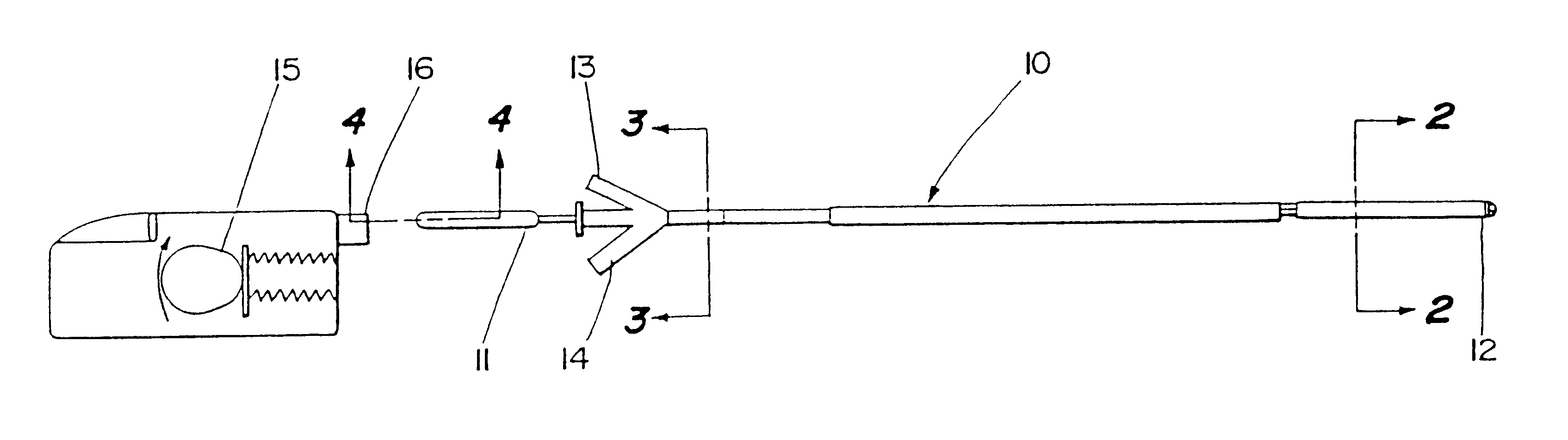
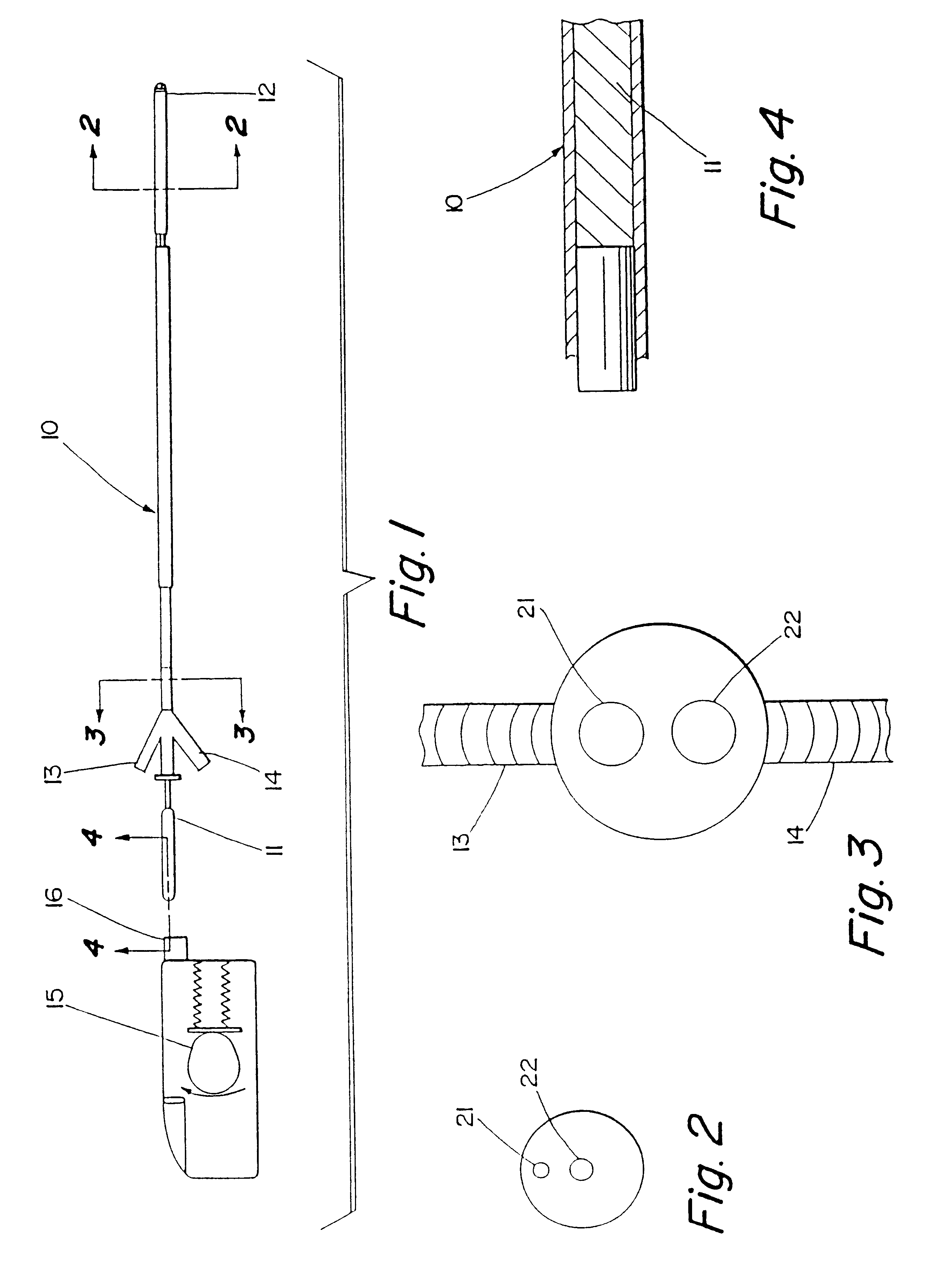
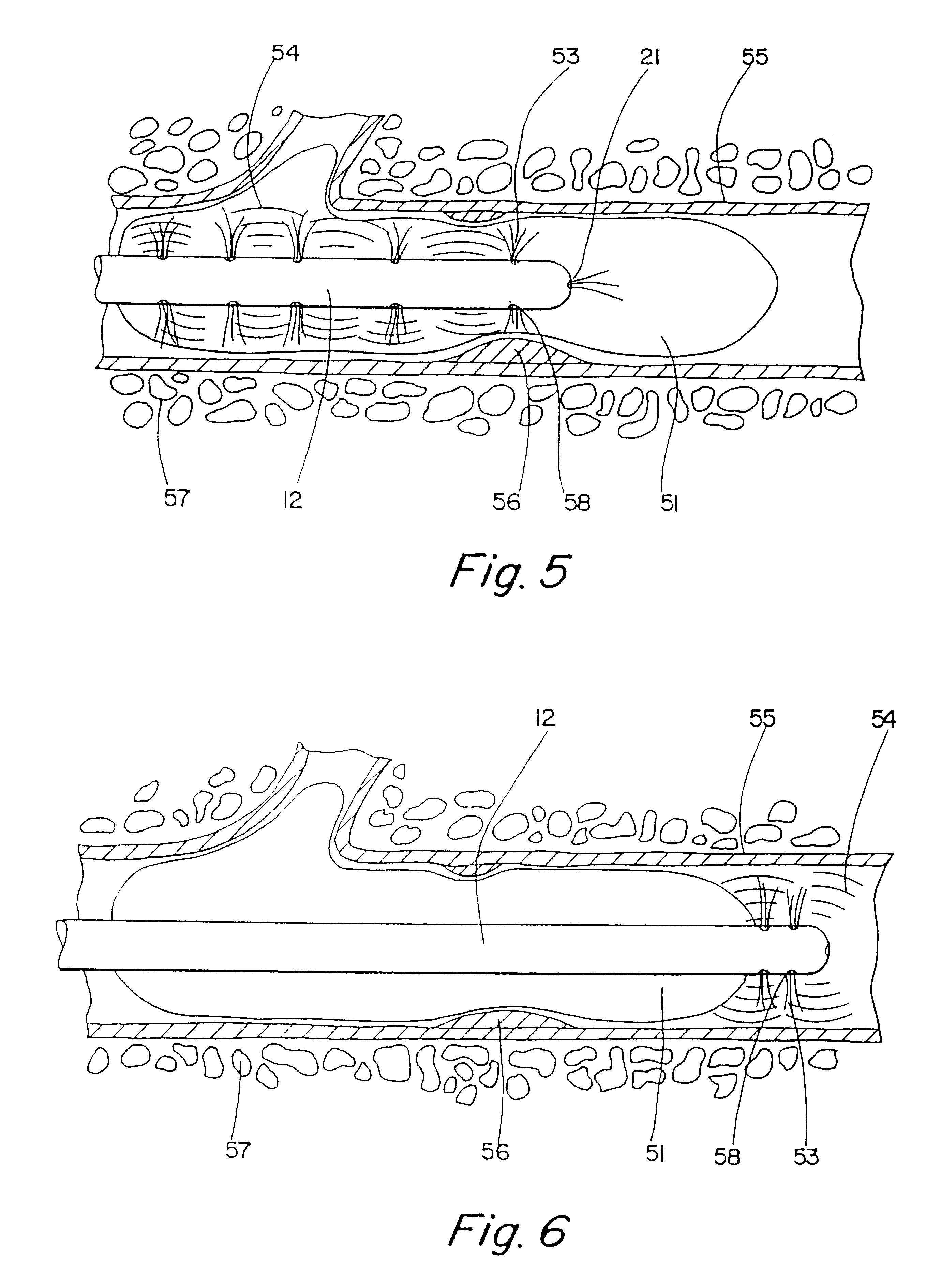
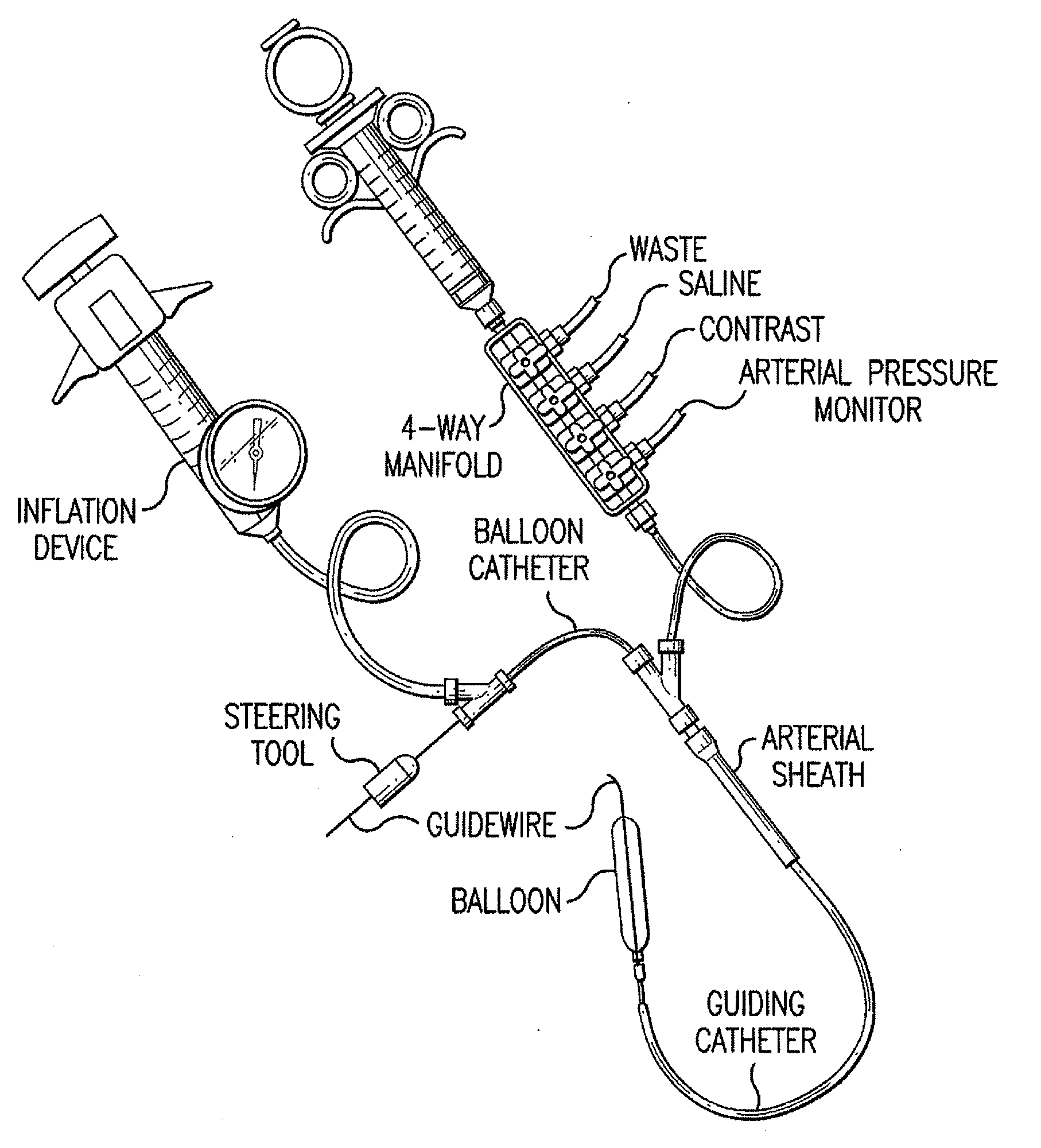
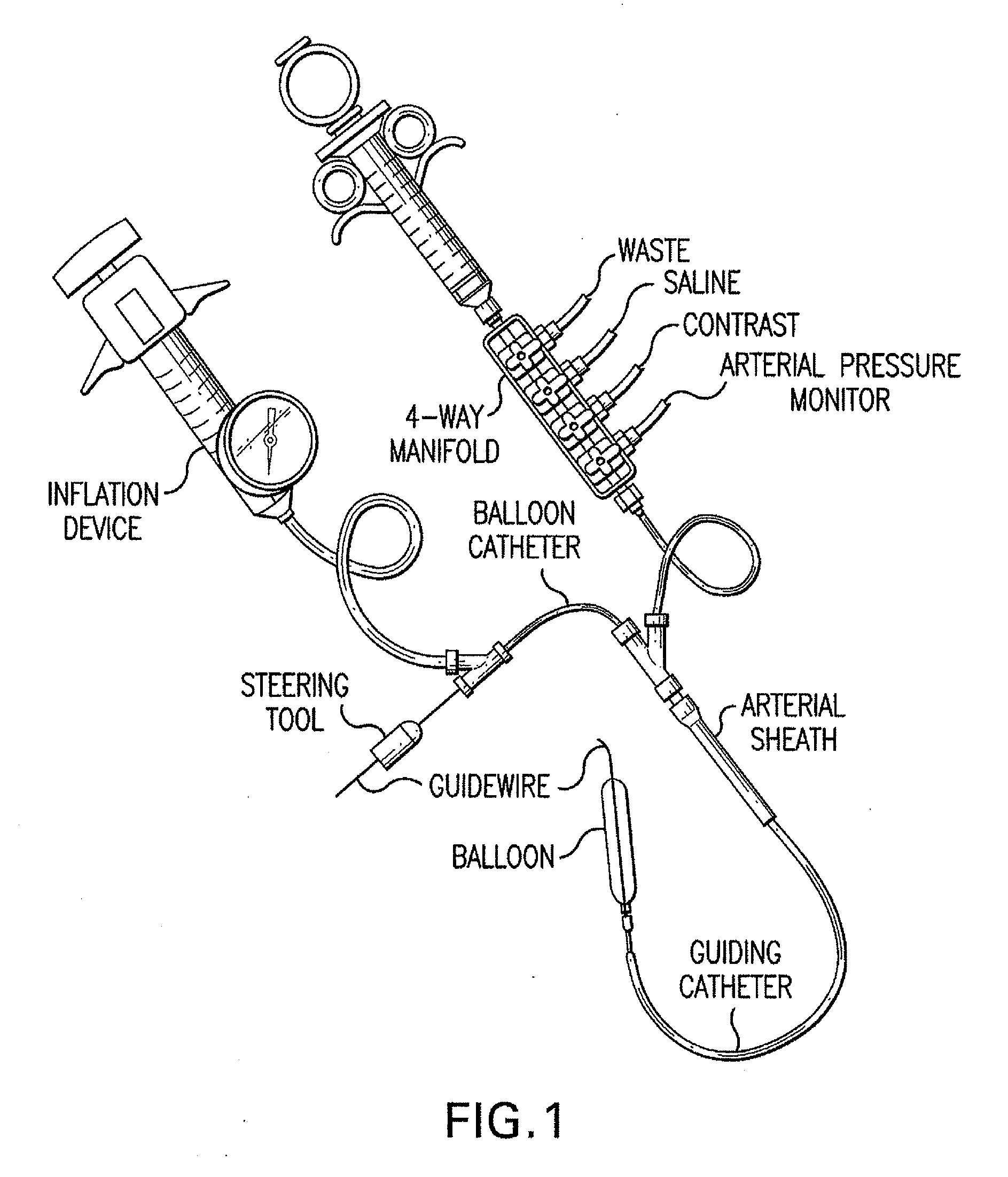
Motion catheter
The present invention describes a catheter suitable for introduction into a tubular tissue for dissolving blockages in such tissue. The catheter is particularly useful for removing thrombi within blood vessels. In accordance with the preferred embodiments, a combination of vibrating motion and injection of a lysing agent is utilized to break up blockages in vessels. The vessels may be veins, arteries, ducts, intestines, or any lumen within the body that may become blocked from the material that flows through it. As a particular example, dissolution of vascular thrombi is facilitated by advancing a catheter through the occluded vessel, the catheter causing a vibrating, stirring action in and around the thrombus usually in combination with the dispensing of a thrombolytic agent such as urokinase into the thrombus. The catheter has an inflatable or expandable member near the distal tip which, when inflated or expanded, prevents the passage of dislodged thrombus around the catheter. The dislodged portions of thrombus are directed through a perfusion channel in the catheter, where they are removed by filtration means housed within the perfusion channel before the blood exits the tip of the catheter. Catheters that allow both frequency (1-1000 Hz) vibratory motion and delivery of such agents to a blockage and a method for using such catheters are disclosed.
Owner:TYCO HEALTHCARE GRP LP
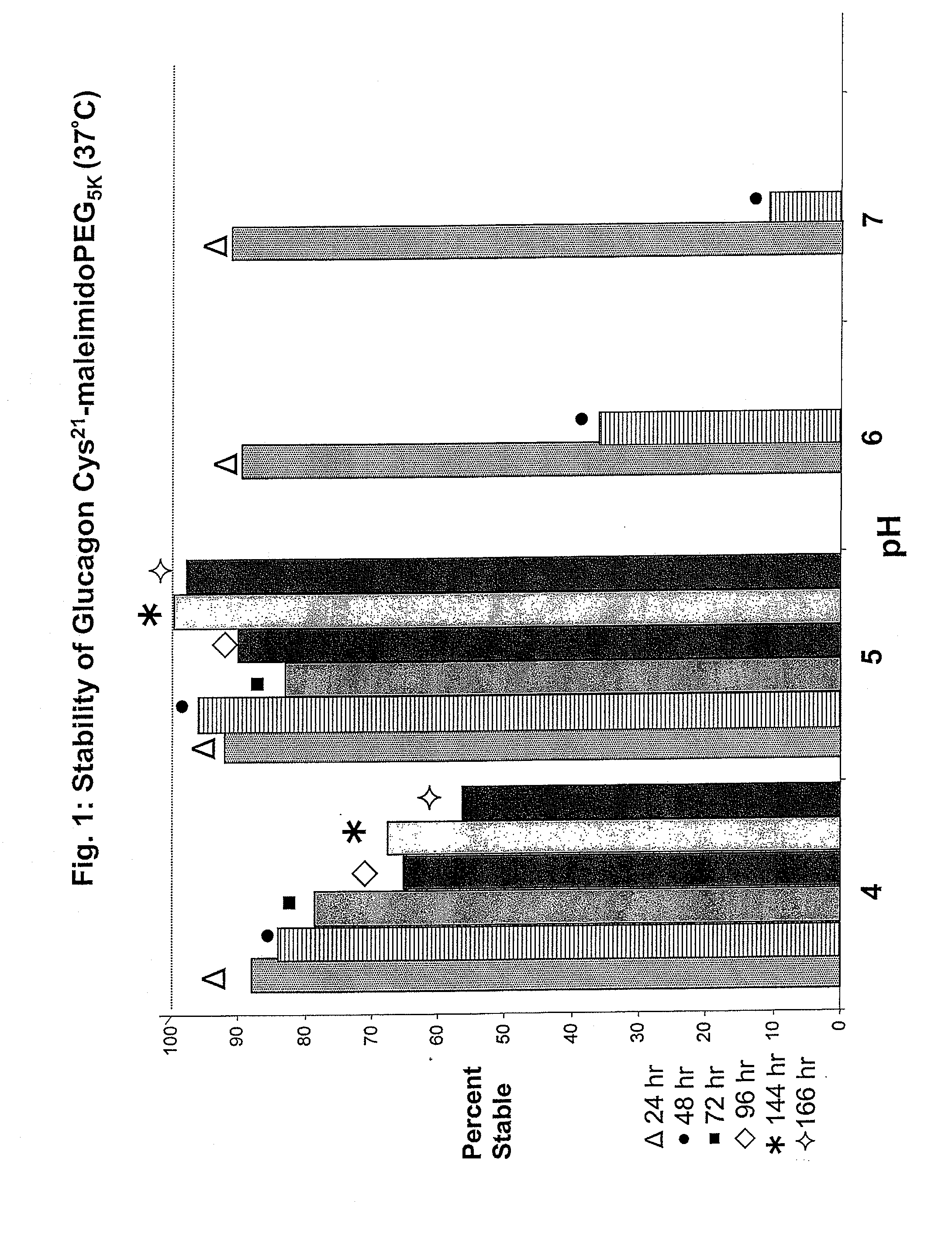
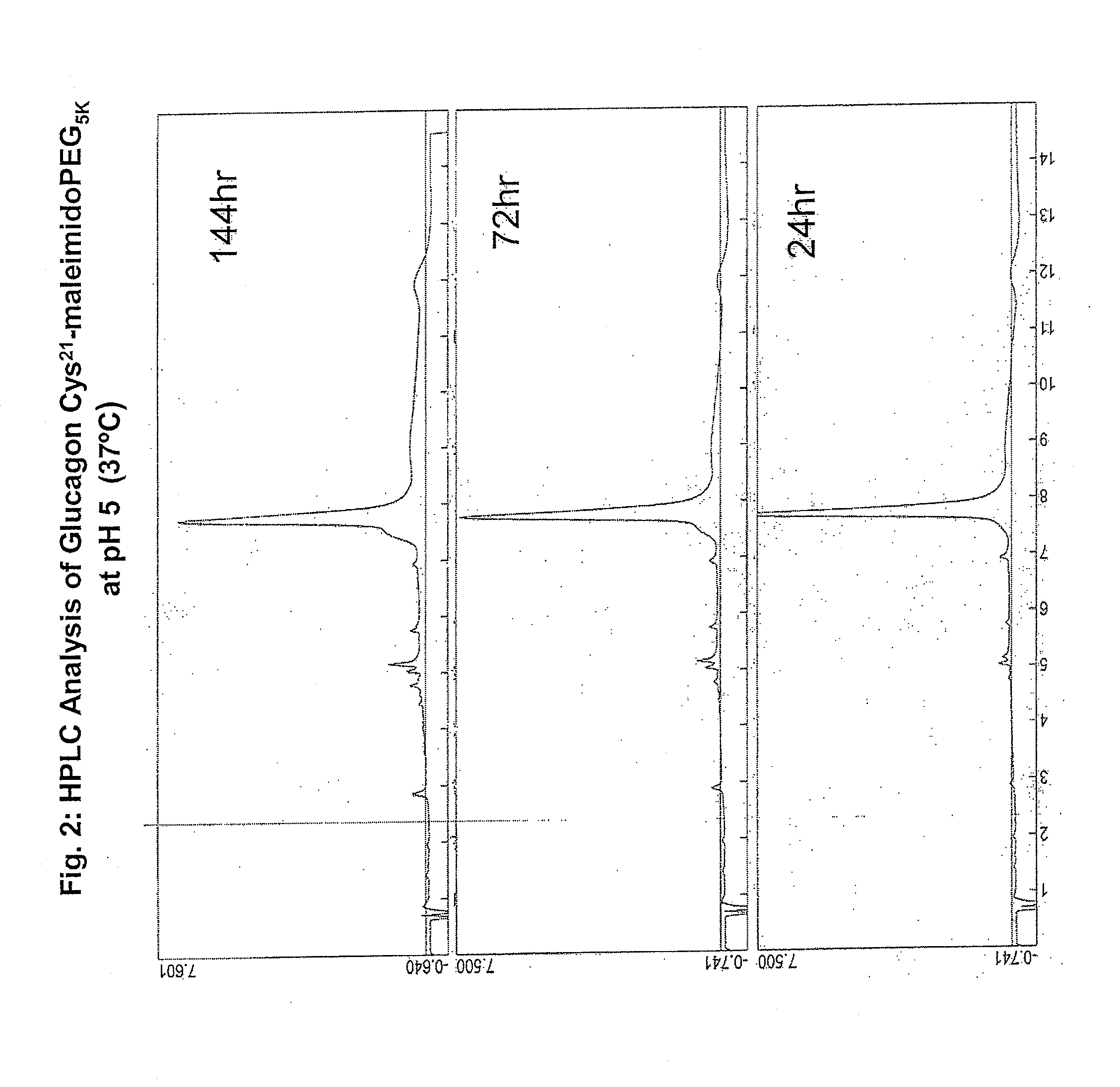
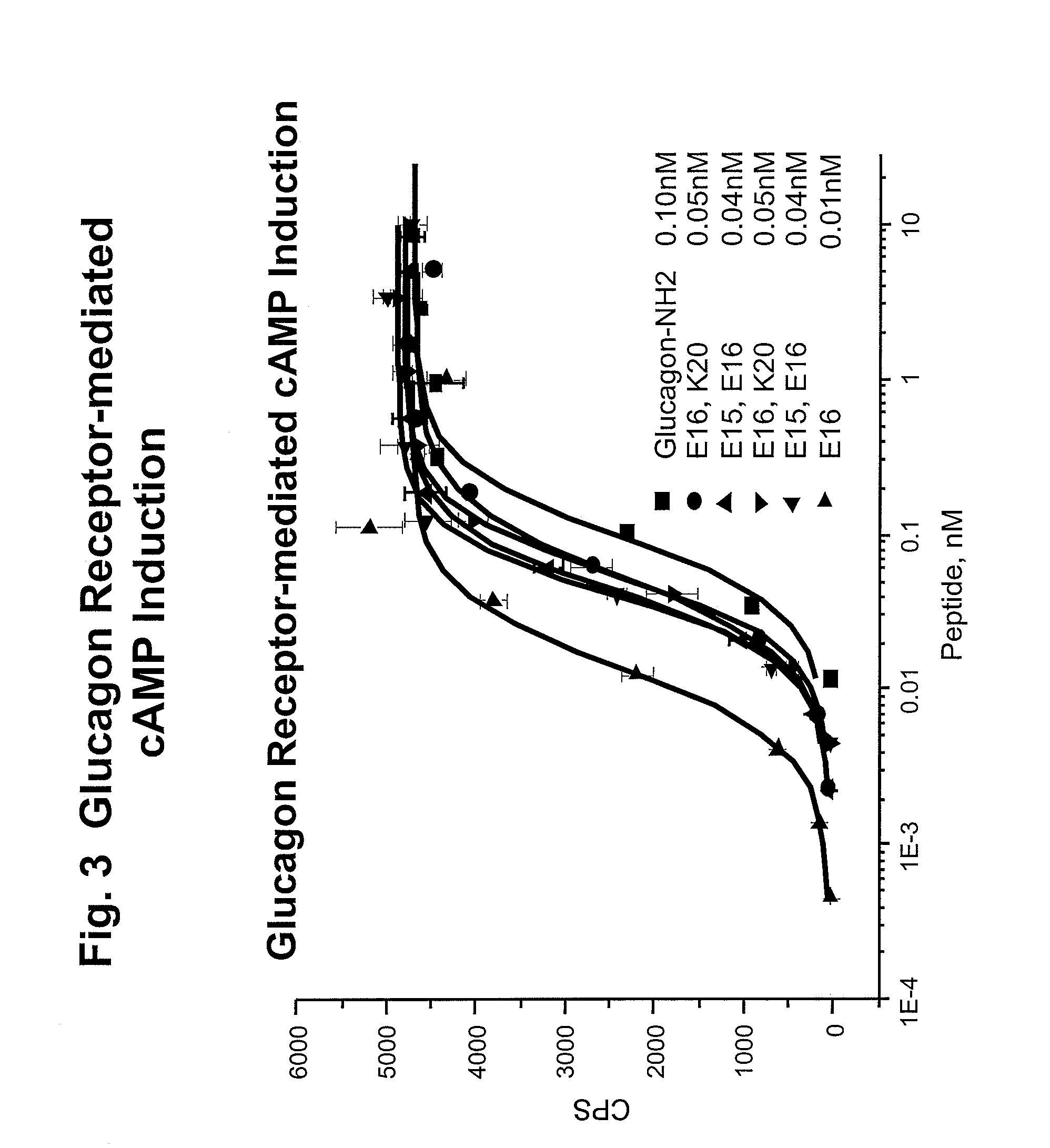
Glucagon/glp-1 receptor co-agonists
InactiveUS20100190701A1High activityEnhanced biophysical stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityCarboxylic acid
Modified glucagon peptides are disclosed having enhanced potency at the glucagon receptor relative to native glucagon. Further modification of the glucagon peptides by forming lactam bridges or the substitution of the terminal carboxylic acid with an amide group produces peptides exhibiting glucagon / GLP-1 receptor co-agonist activity. The solubility and stability of these high potency glucagon analogs can be further improved by modification of the polypeptides by pegylation, substitution of carboxy terminal amino acids, or the addition of a carboxy terminal peptide selected from the group consisting of SEQ ID NO: 26 (GPSSGAPPPS), SEQ ID NO: 27 (K-RNRNNIA) and SEQ ID NO: 28 (KRNR).
Owner:INDIANA UNIV RES & TECH CORP
Methods for direct synthesis of compounds having complementary structure to a desired molecular entity and use thereof
InactiveUS6127154AConstrain versatilityReduce in quantityMaterial nanotechnologyIon-exchanger regenerationHormoneEnzyme
Compounds which possess a complementary structure to a desired molecule, such as a biomolecule, in particular polymeric or oligomeric compounds, which are useful as in vivo or in vitro diagnostic and therapeutic agents are provided. Also, various methods for producing such compounds are provided. These polymeric or oligomeric compounds are useful in particular as antimicrobial agents, receptor, hormone or enzyme agonists and antagonists.
Owner:KLAUS MOSBACH CENT FOR CHEM & CHEM ENG PURE & APPLIED BIOCHEM
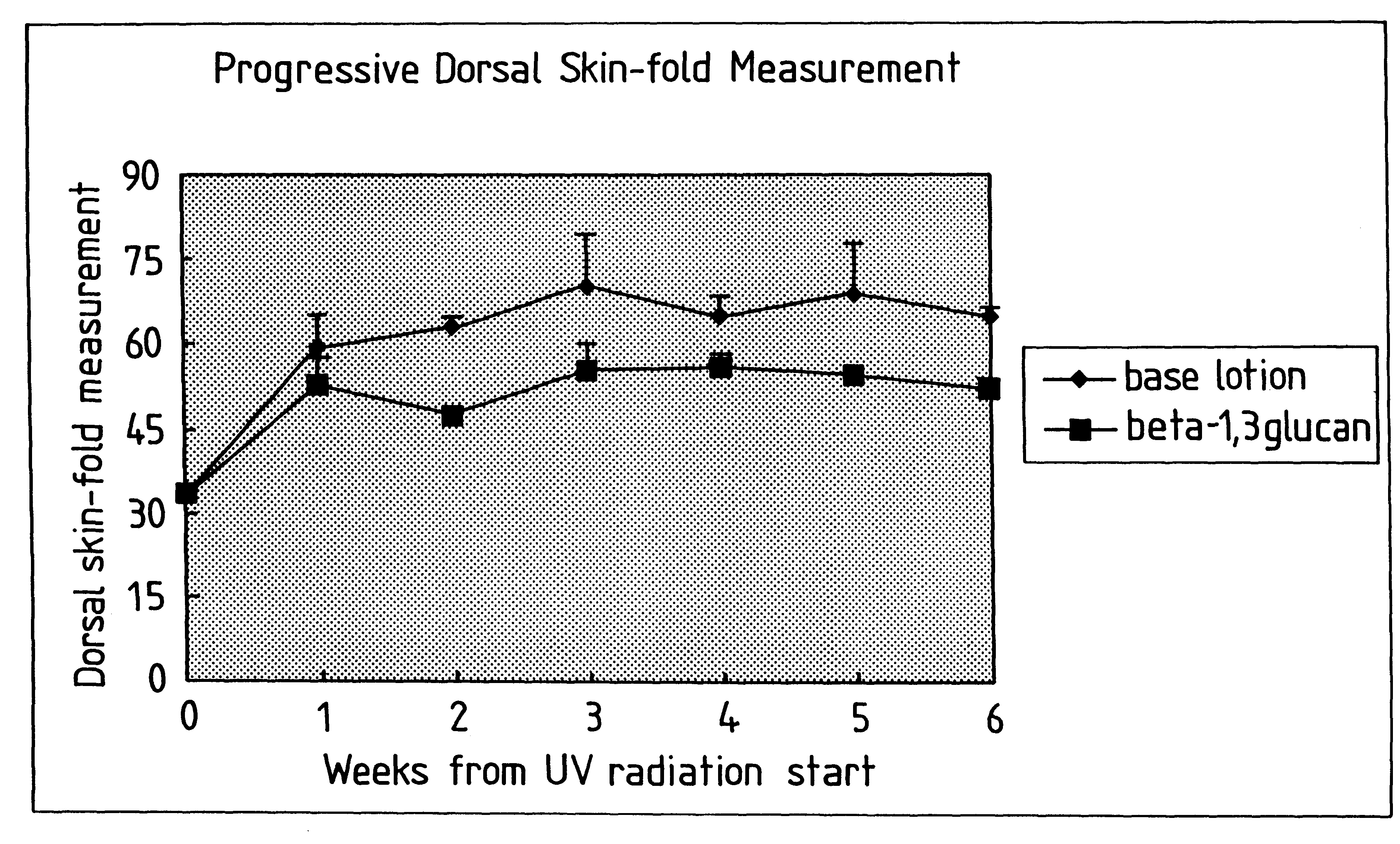
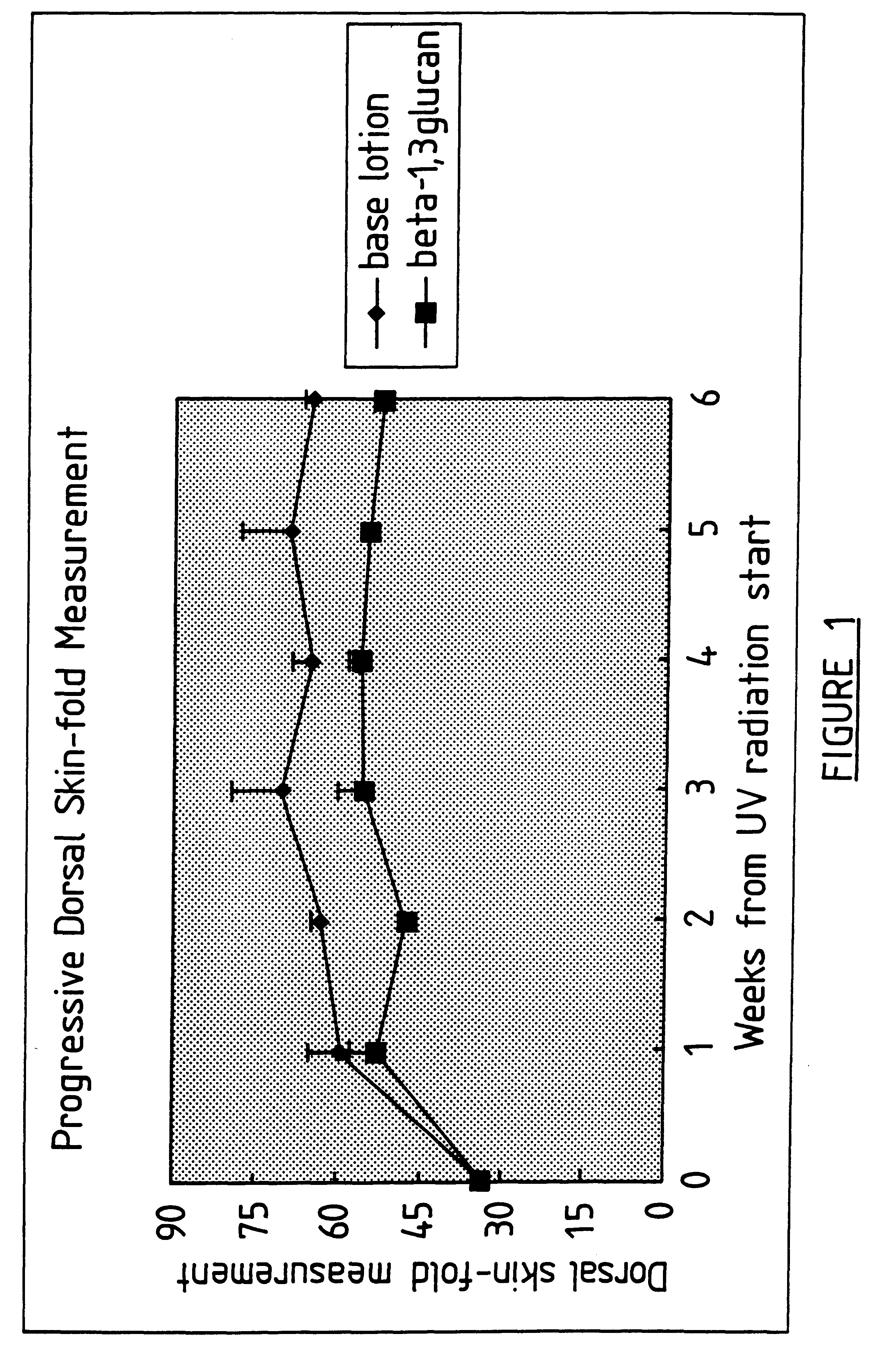
Process for glucan preparation and therapeutic uses of glucan
InactiveUS6242594B1Low costSuitable solubility characteristicAntibacterial agentsOrganic active ingredientsOrganic solventMicroparticle
A process for the production of beta-3-(1,3)(1,6) glucan from a glucan containing cellular source is described, together with compositions and uses / methods of treatment involving glucan. The process of the invention comprises the steps of: (a) extracting glucan containing cells with alkali and heat, in order to remove alkali soluble components; (b) acid extracting the cells of step (a) with an acid and heat to form a suspension; (c) extracting the suspension obtained of step (b) or recovered hydrolyzed cells with an organic solvent which is non-miscible with water and which has a density greater than that of water separating the resultant aqueous phase, solvent containing phase and interface so that substantially only the aqueous phase comprising beta-(1,3)(1,6) glucan particulate material remains; wherein the extraction with said organic solvent provides separation of glucan subgroups comprising branched beta-(1,3)(1,6)-glucan, and essentially unbranched beta-(1,3) glucan which is associated with residual non-glucan contaminents; and (d) drying the glucan material from step (c) to give microparticulate glucan.
Owner:TR THERAPEUTICS
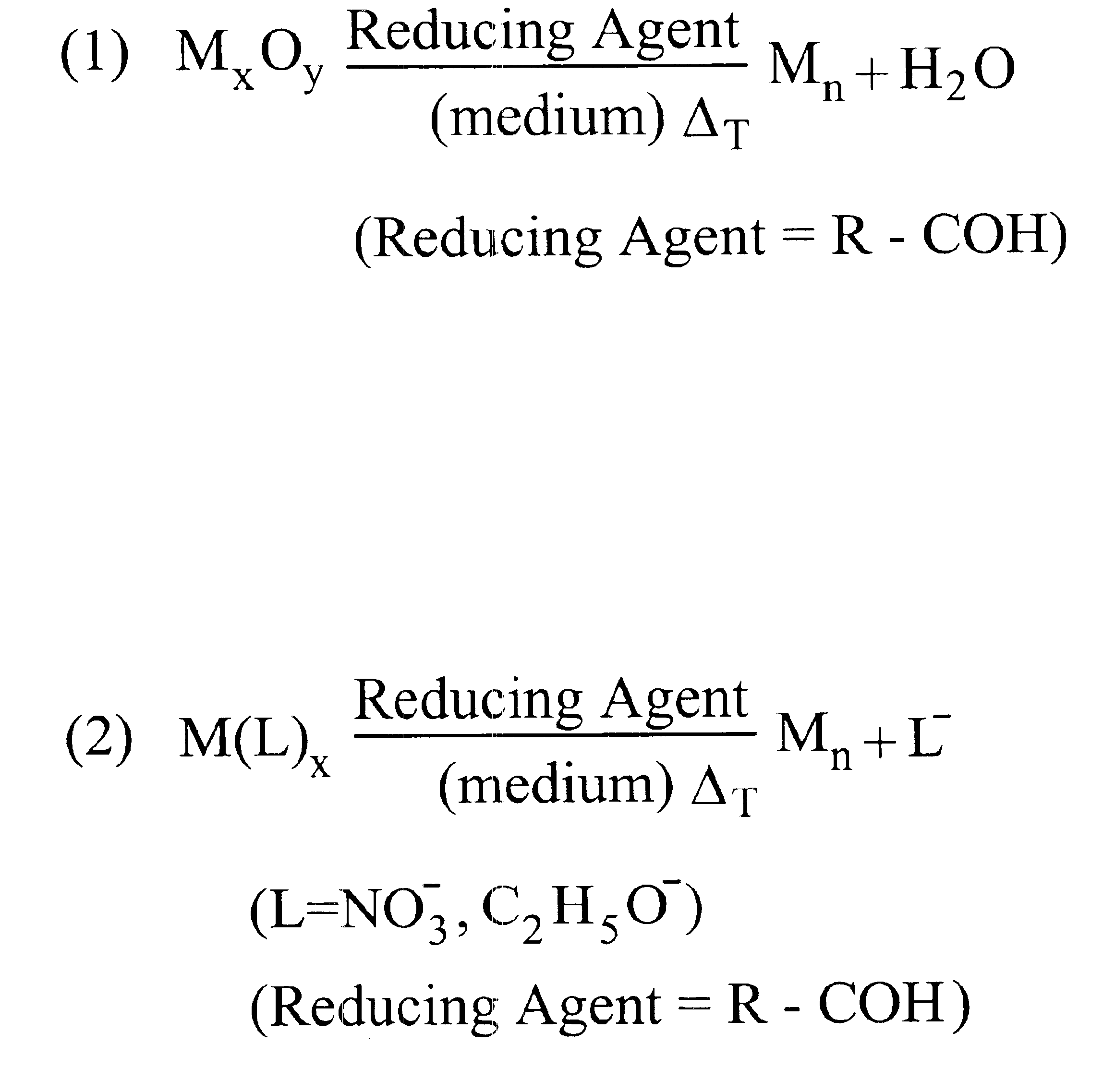
Nanoparticle synthesis and the formation of inks therefrom
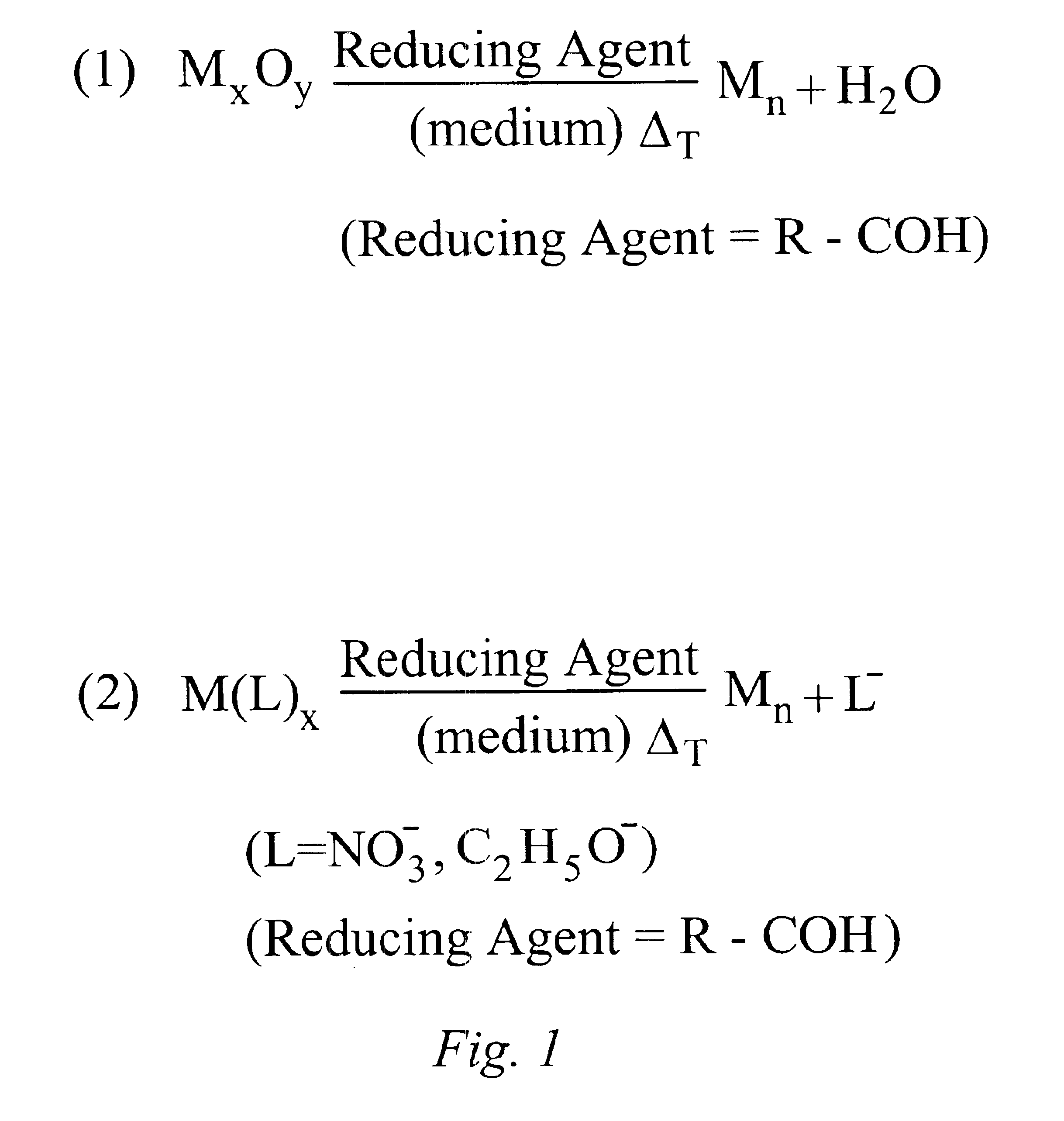
InactiveUS6878184B1High yieldImprove solubilityMaterial nanotechnologyTransportation and packagingAlloy nanoparticleAmount of substance
Methods for making metal-based nanoparticles and inks are disclosed. In accordance with the method of the present invention, molecular metal precursors are reduced in the presence of a reaction medium to form the nanoparticles. The molecular metal precursors are preferably reduced by heating the metal precursor in the medium, by adding a reducing agent, such an aldehyde or a combination thereof. Metal precursor are preferably metal oxides, transition metal complexes or combination thereof. The method of the present invention is used to make high yield nanoparticles with a range of particle size distributions. Nanoparticle formed by the present invention include mixtures of nanoparticle, alloy nanoparticles, metal core shell nanoparticles or nanoparticle comprising a single metal species.
Owner:THIN FILM ELECTRONICS ASA
Hydrogel-forming sustained-release preparation
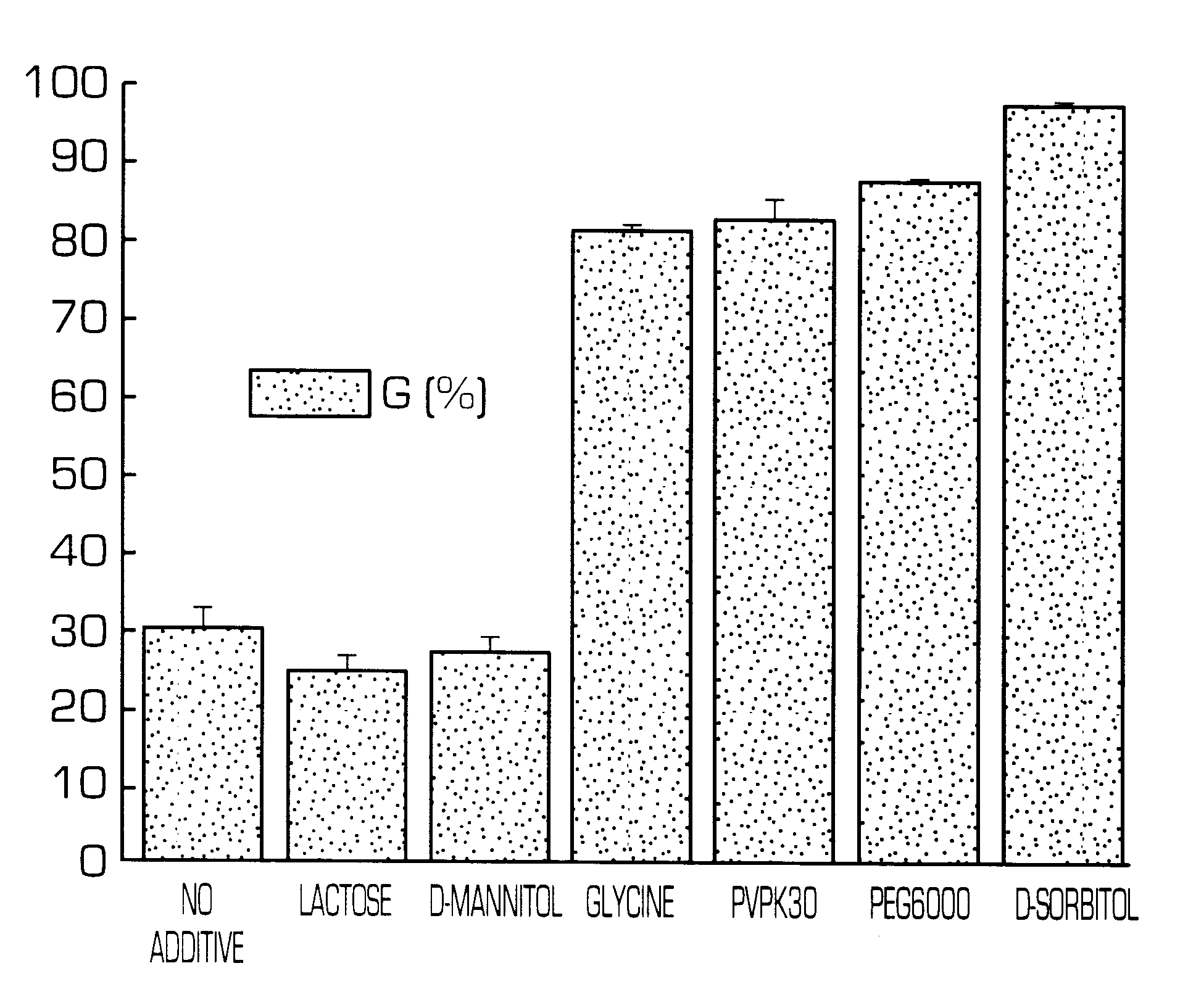
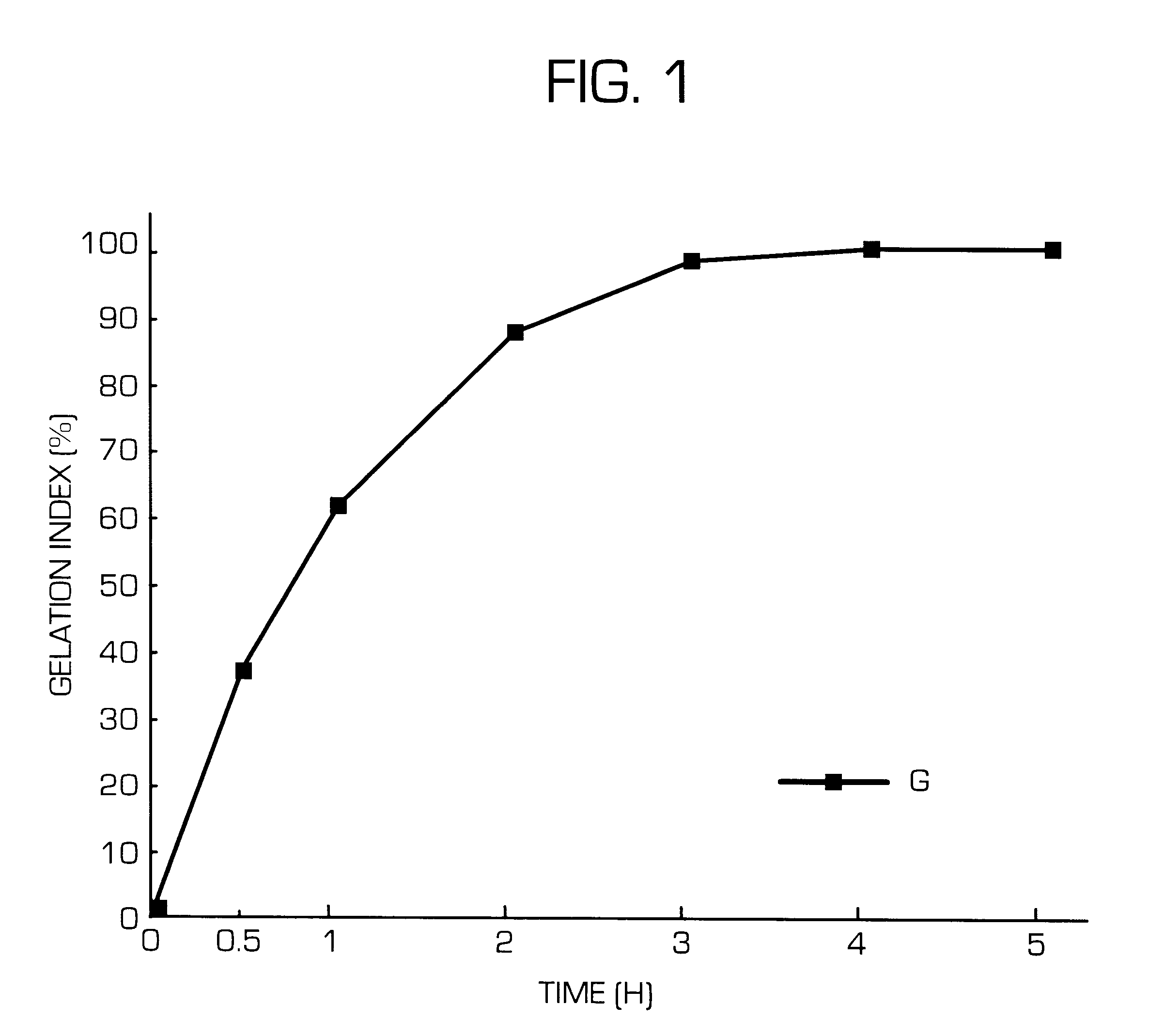
InactiveUS6436441B1Rapid drug releaseReduce the amount of solutionPowder deliveryPill deliverySmall intestinePolymer
The invention provides a hydrogel-type sustained-release preparation comprising (1) at least one drug, (2) an additive which insures a penetration of water into the core of the preparation and (3) a hydrogel-forming polymer, wherein said preparation is capable of undergoing substantially complete gelation during its stay in the upper digestive tract such as stomach and small intestine and is capable of releasing the drug in the lower digestive tract including colon.By the preparation of the invention, the drug is efficiently released and absorbed even in the colon so that a steady and sustained release effect can be achieved.
Owner:ASTELLAS PHARMA INC
Antibody producing non-human mammals
ActiveUS20100146647A1Low variabilityLow immunogenicityAnimal cellsAntibody mimetics/scaffoldsHuman animalDNA rearrangement
Owner:MERUS NV
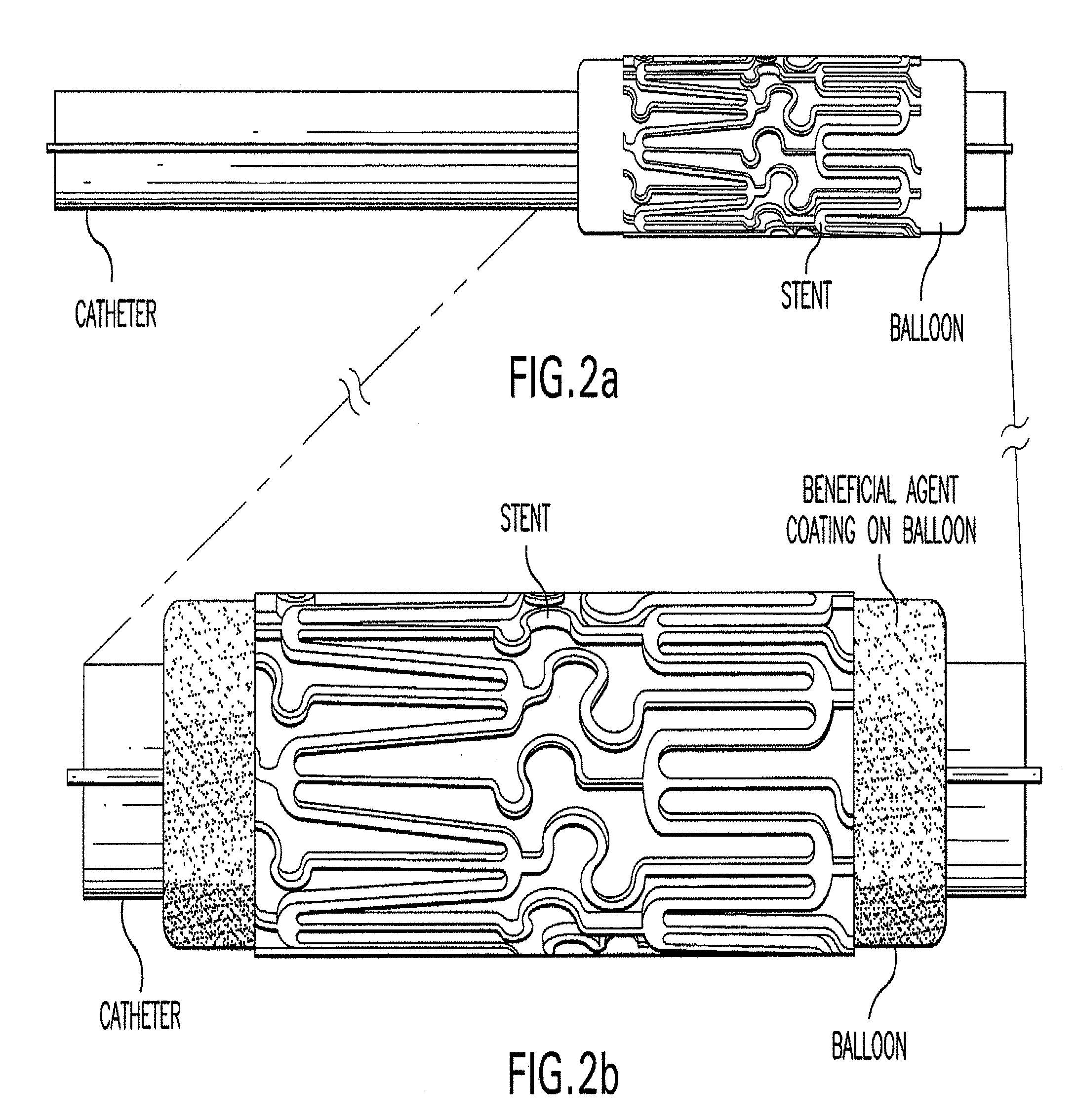
Method of treating vascular disease at a bifurcated vessel using a coated balloon
InactiveUS20100030183A1Low water solubilityGood film-forming propertiesOrganic active ingredientsStentsVascular diseaseVascular problem
Disclosed is a method for delivery of at least one therapeutic agent from an angioplasty balloon for treating vascular disease at a bifurcated vessel. The invention also relates to the method of loading the beneficial agents onto the balloon and the device, as well as the method of delivery of the agents from separate surfaces. The invention also relates to a method of loading multiple beneficial agents onto the balloon surfaces
Owner:ABBOTT LAB INC
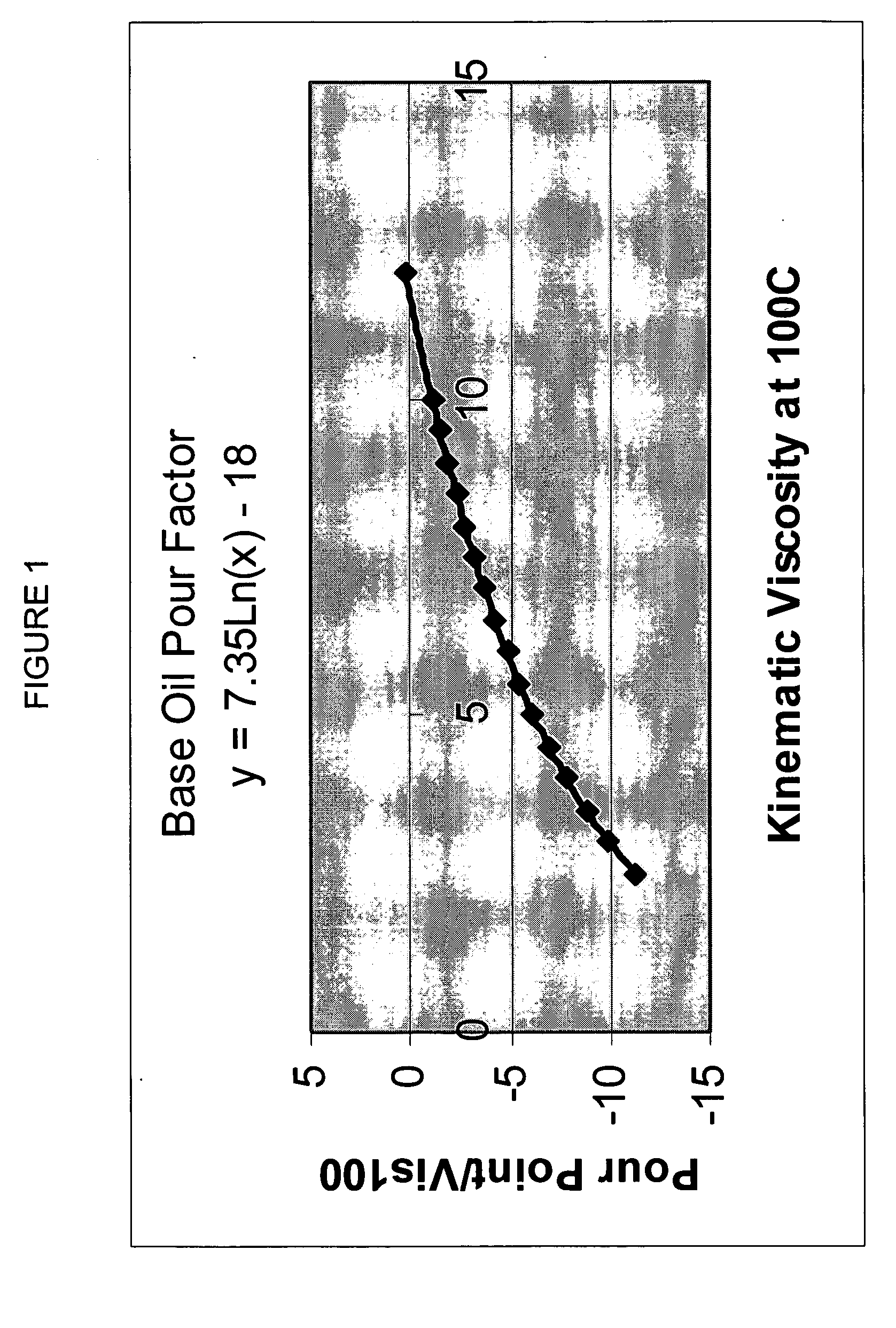
Process for manufacturing lubricating base oil with high monocycloparaffins and low multicycloparaffins
InactiveUS20050133409A1Improve Oxidation StabilityHigh viscosity indexTreatment with hydrotreatment processesAdditivesSyngasMolecular sieve
A process for manufacturing a lubricating base oil by: a) performing Fischer-Tropsch synthesis on syngas to provide a product stream; b) isolating from said product stream a substantially paraffinic wax feed having less than about 30 ppm total nitrogen and sulfur, and less than about 1 wt % oxygen; c) dewaxing said feed by hydroisomerization dewaxing using a shape selective intermediate pore size molecular sieve comprising a noble metal hydrogenation component, wherein the hydroisomerization temperature is between about 600° F. (315° C.) and about 750° F. (399° C.), to produce an is dimerized oil; and d) hydrofinishing said isomerized oil to produce a lubricating base oil having specific desired properties.
Owner:CHEVROU USA INC
High-throughput formation, identification, and analysis of diverse solid-forms
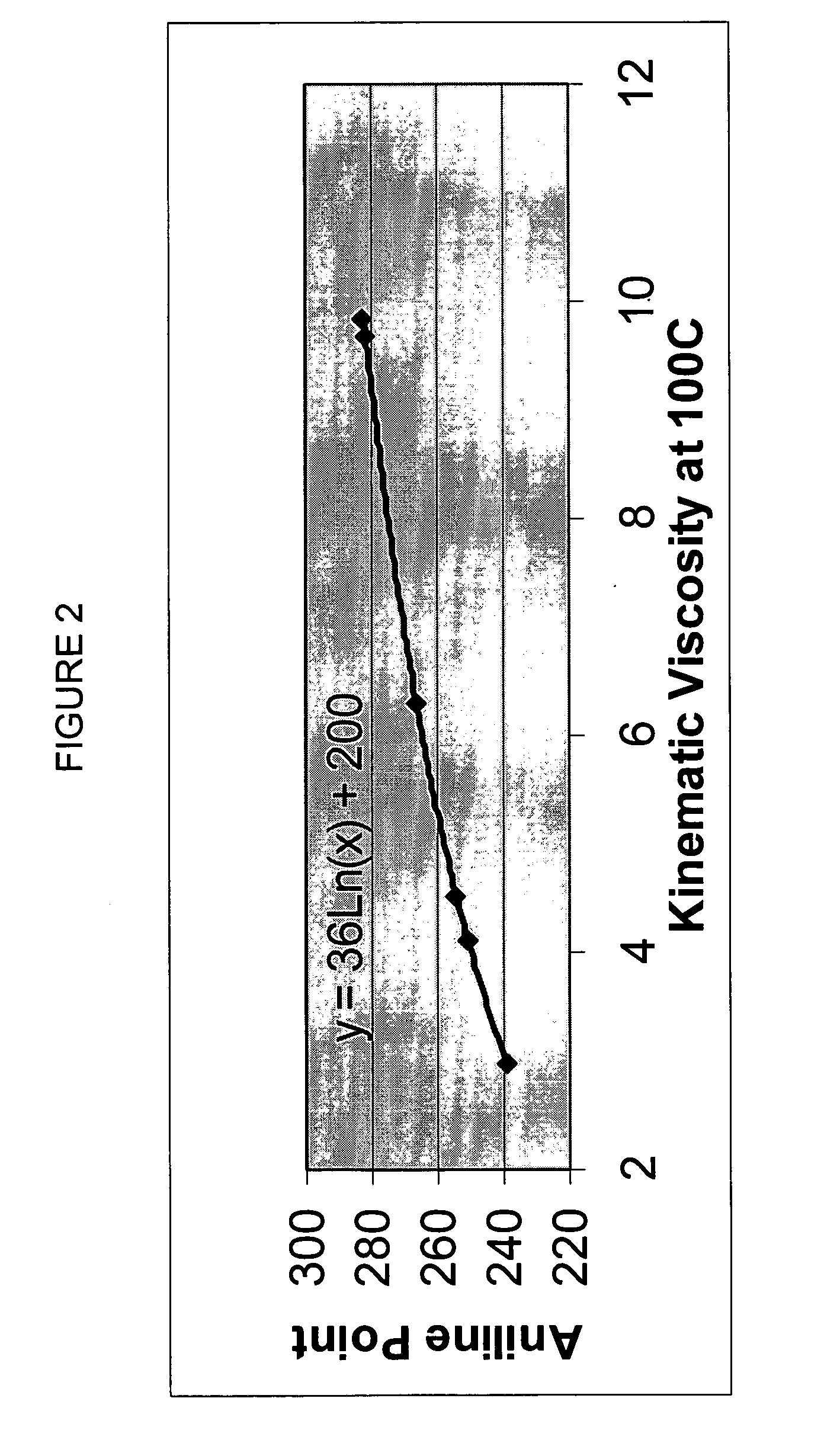
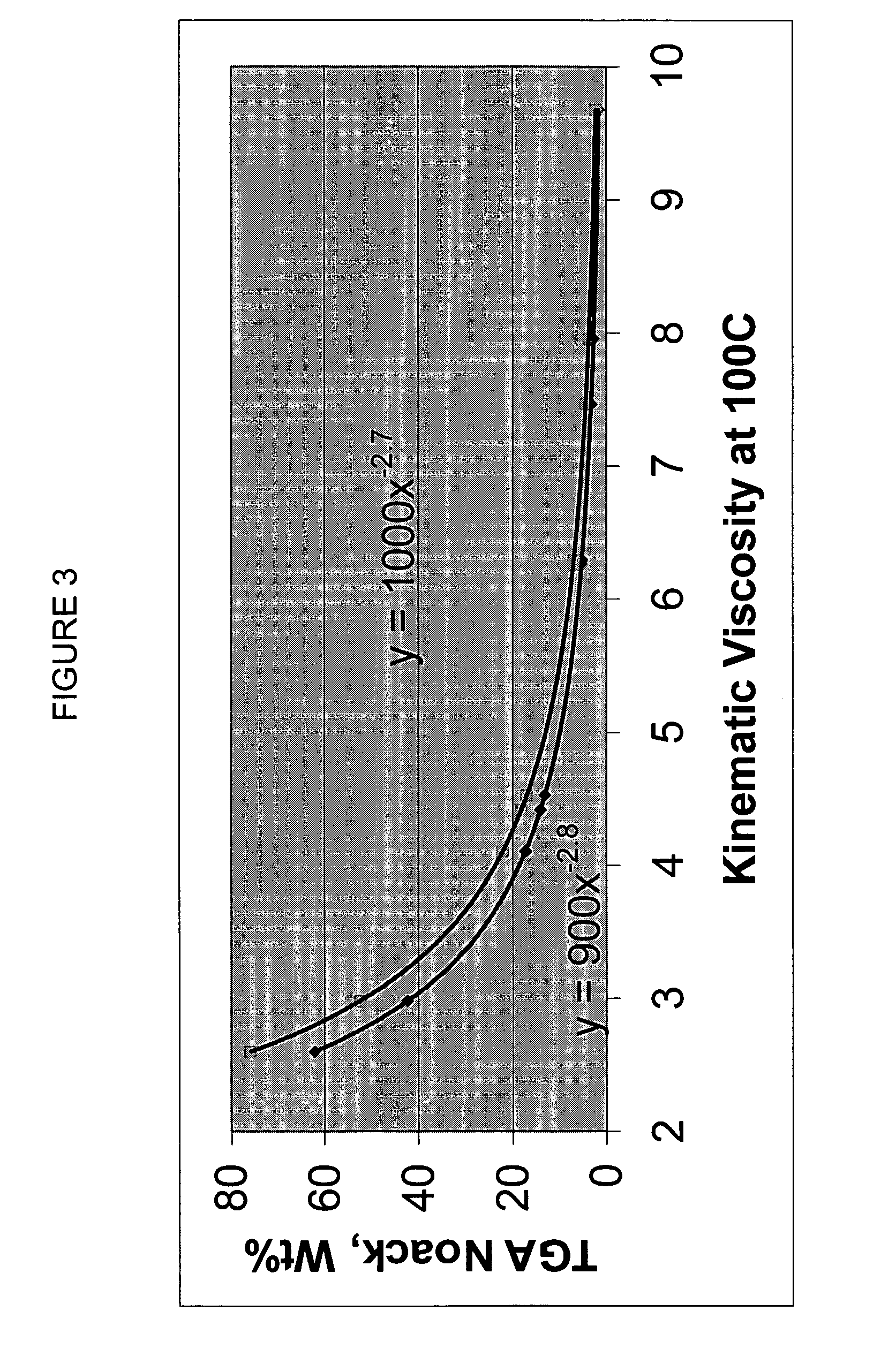
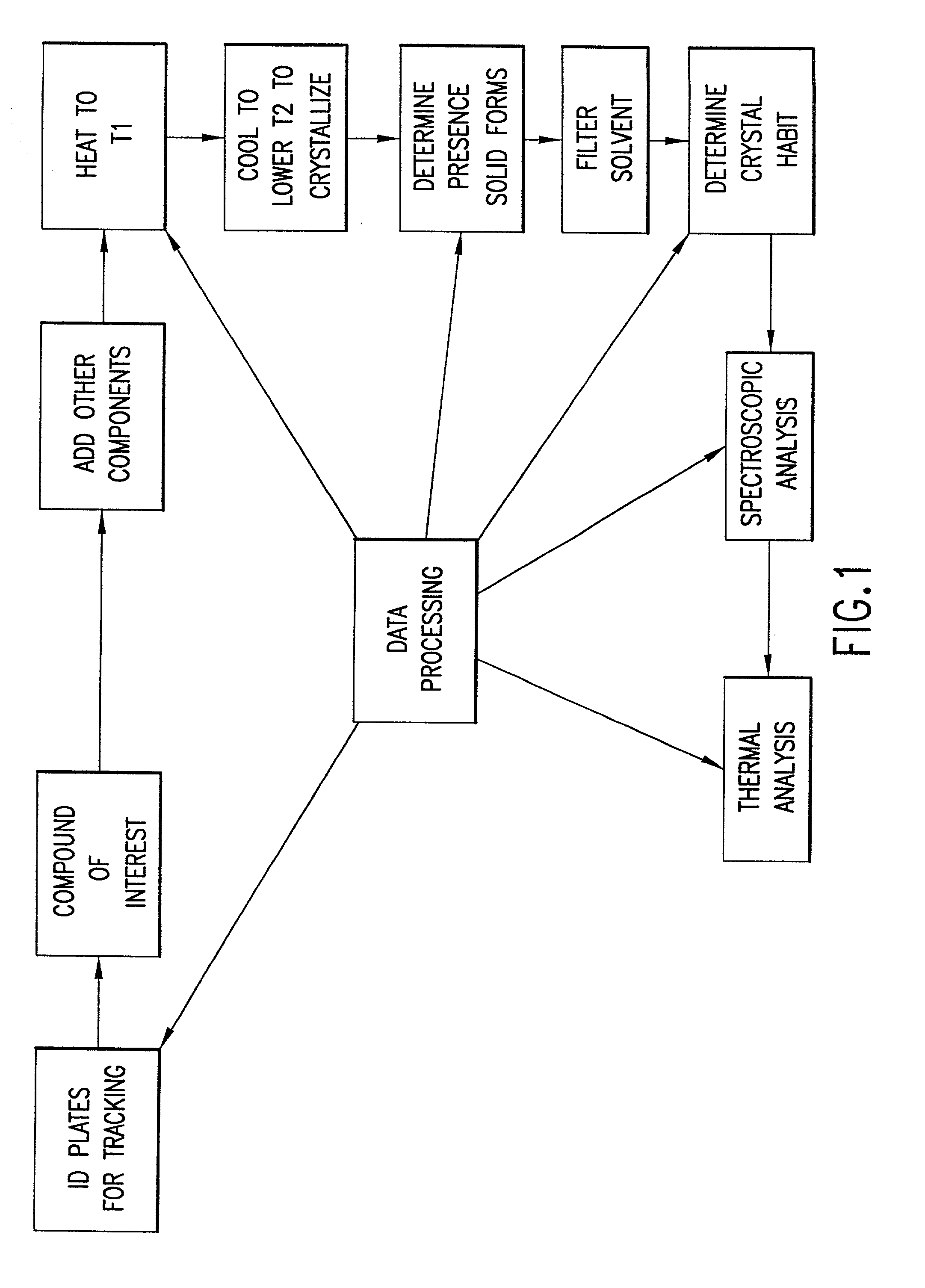
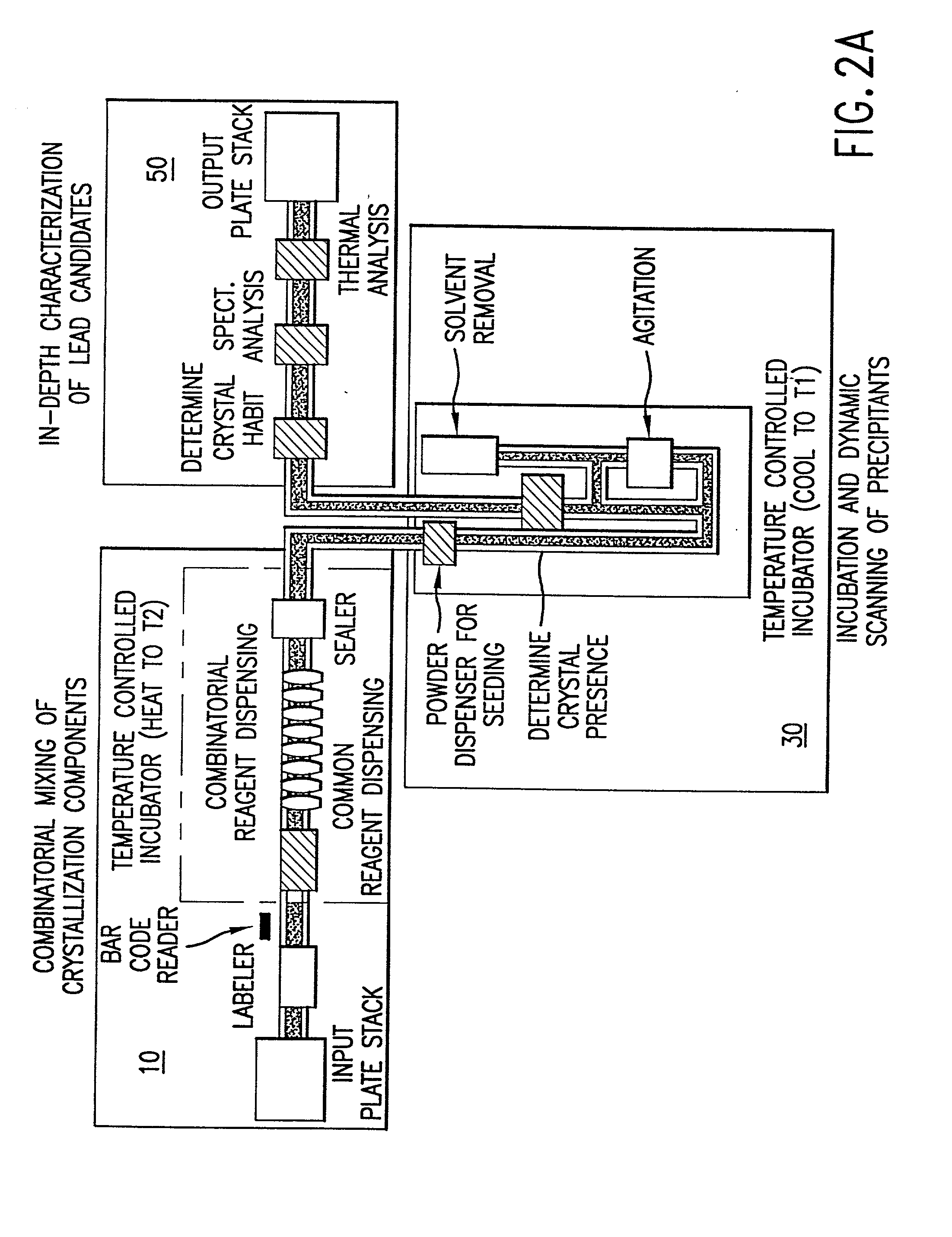
InactiveUS20020048610A1Cost-effectiveImprove bioavailabilitySequential/parallel process reactionsFrom normal temperature solutionsSolubilitySolid mass
The invention concerns arrays of solid-forms of substances, such as compounds and rapid-screening methods therefor to identify solid-forms, particularly of pharmaceuticals, with enhanced properties. Such properties include improved bioavailability, solubility, stability, delivery, and processing and manufacturing characteristics. The invention relates to a practical and cost-effective method to rapidly screen hundreds to thousands of samples in parallel. The invention further provides methods for determining the conditions and / or ranges of conditions required to produce crystals with desired compositions, particle sizes, habits, or polymorphic forms. In a further aspect, the invention provides high-throughput methods to identify sets of conditions and / or combinations of components compatible with particular solid-forms, for example, conditions and / or components that are compatible with advantageous polymorphs of a particular pharmaceutical.
Owner:MILLENNIUM PHARMA INC +1
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Pharmaceutical composition
InactiveUS6946120B2Improve solubilityImprove abilitiesCosmetic preparationsOrganic active ingredientsAlcoholPolyol
A pharmaceutical composition for topical administration, including, as the pharmaceutically active component, at least 5% by weight, based on the total weight of the composition of a piperidinopyrimidine derivative or a pharmaceutically acceptable salt thereof; an acid in an amount to completely solubilise the piperidinopyrimidine derivative or a pharmaceutically acceptable salt thereof; a solvent composition including at least two of water, a lower alcohol and a co-solvent selected from one or more of the group consisting of aromatic and polyhydric alcohols; wherein when the co-solvent includes propylene glycol, it is present in an amount of less than approximately 10% by weight.
Owner:STIEFEL RESEARCH AUSTRALIA PTY LTD
Biosensor
InactiveUS6719887B2Improve solubilityNot easy to dissolveImmobilised enzymesBioreactor/fermenter combinationsReaction layerEngineering
Owner:PHC HLDG CORP
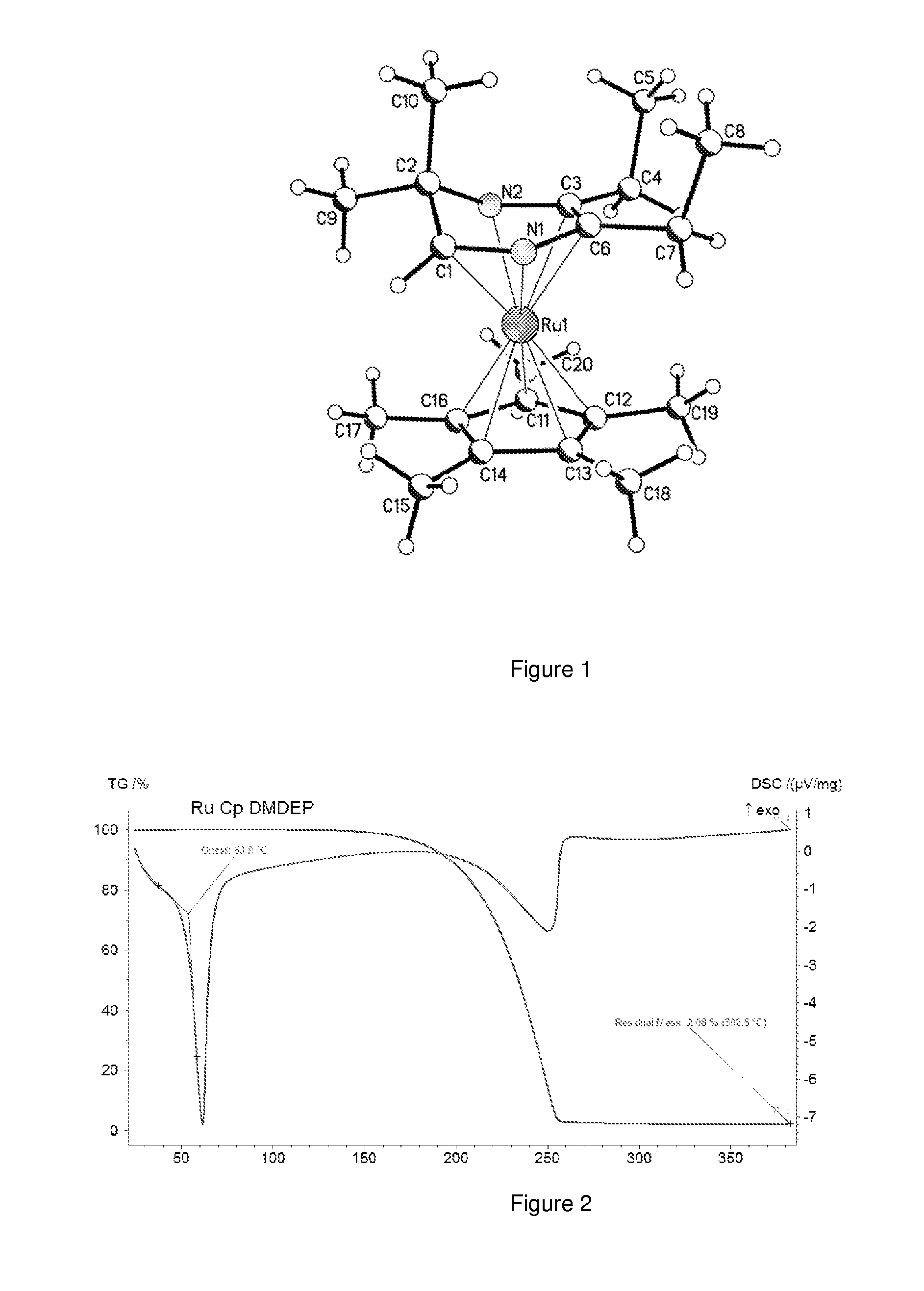
Volatile dihydropyrazinly and dihydropyrazine metal complexes
ActiveUS20150030782A1Improve responseHighly effectiveGroup 5/15 element organic compoundsVacuum evaporation coatingProtonationRuthenium
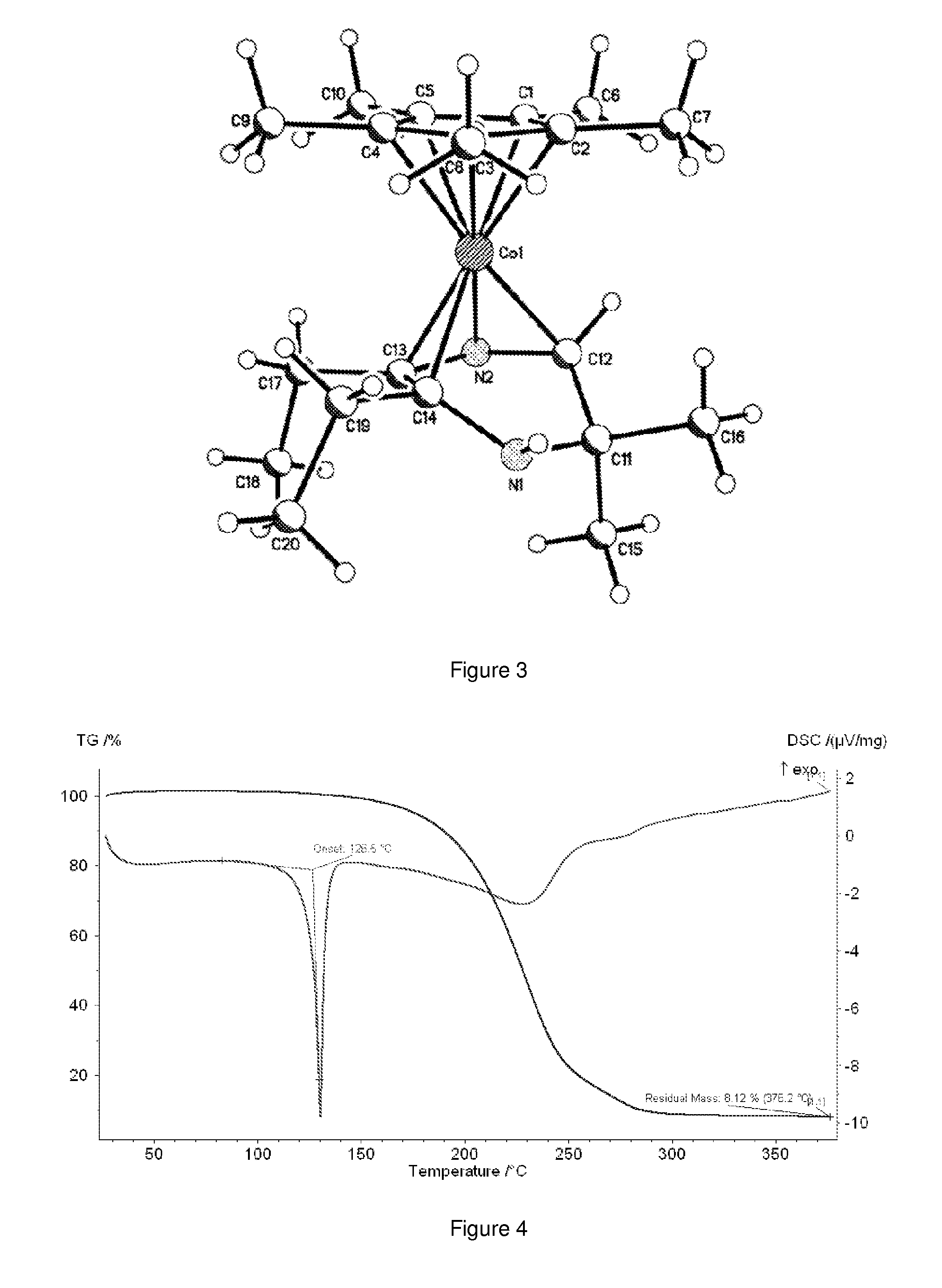
A composition comprising dihydropyrazinyl anions that can be coordinated as 6 electron ligands to a broad range of different metals to yield volatile metal complexes for ALD and CVD depositions are described herein. Also described herein are undeprotonated dihydropyrazines that can coordinate to metals as stabilizing neutral ligands. In one embodiment, the composition is used for the direct liquid injection delivery of the metal dihydropyrazinyl complex precursor to the chamber of an ALD or CVD chamber for the deposition of metal-containing thin films such as, for example, ruthenium or cobalt metal films.
Owner:VERSUM MATERIALS US LLC
Integrase inhibitor compounds
InactiveUS20070072831A1Increasing cellular accumulationImprove bioavailabilityBiocideAntiviralsBiochemistryIntegrase inhibitor
Owner:GILEAD SCI INC
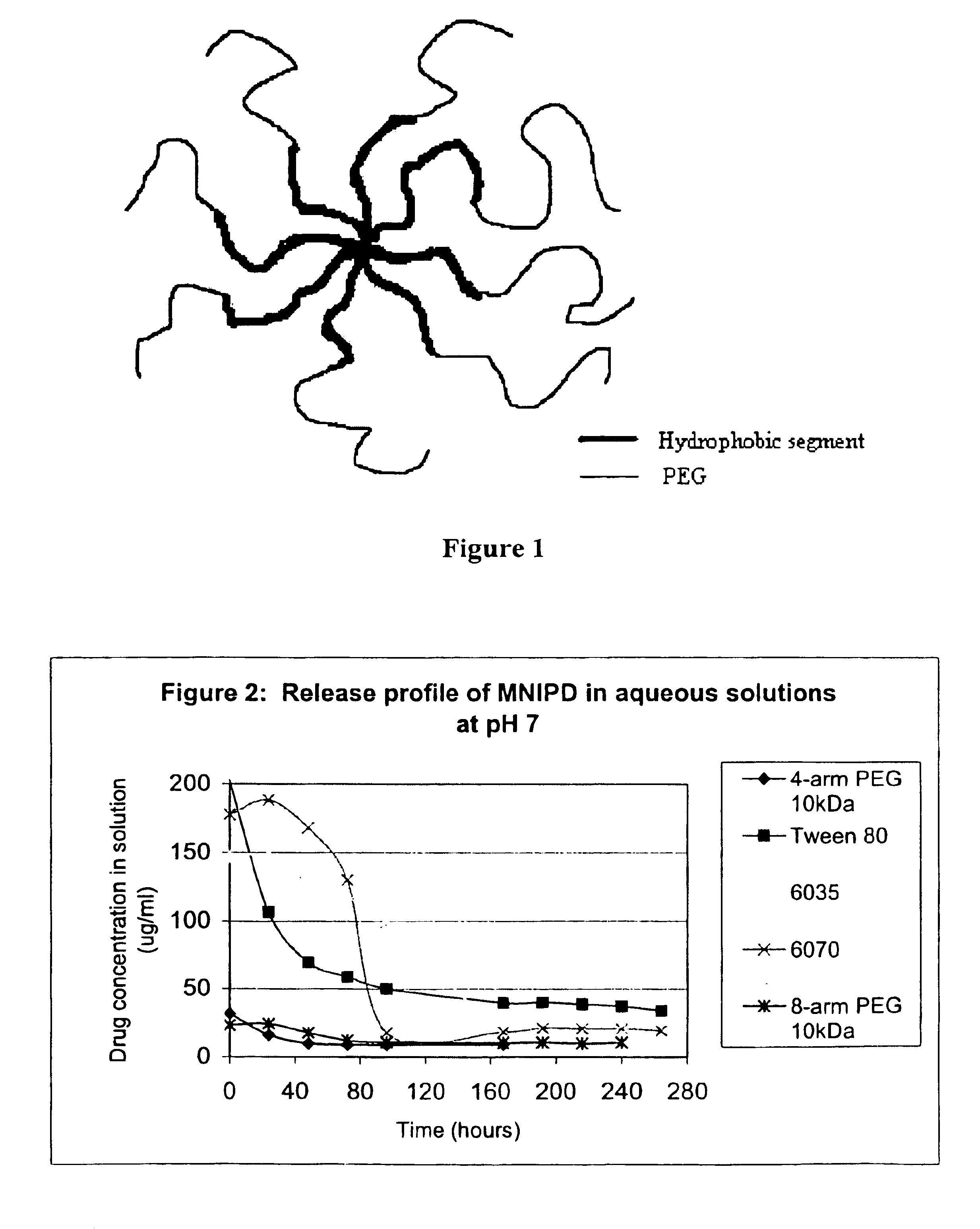
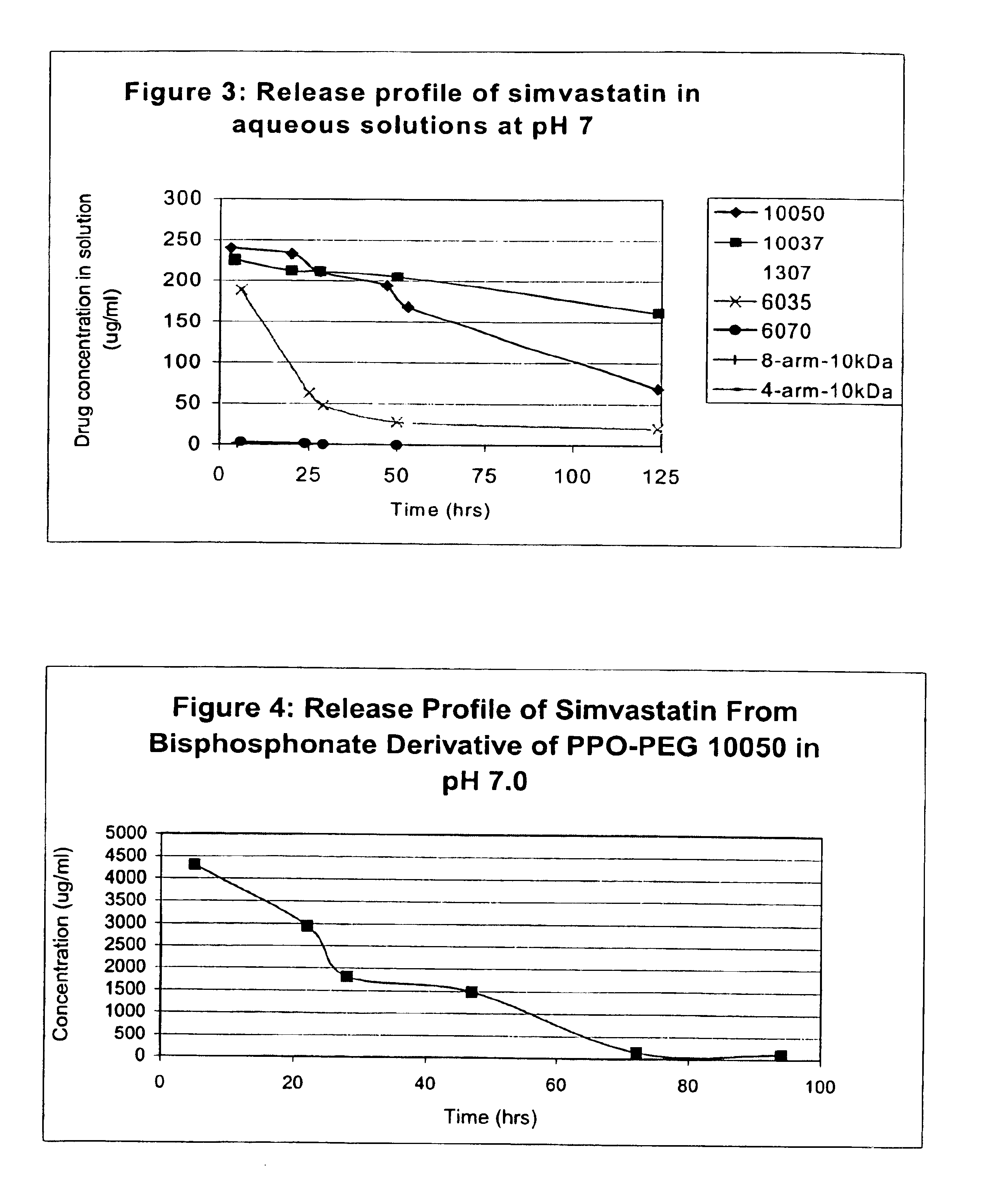
Multi-arm block copolymers as drug delivery vehicles
InactiveUS6838528B2Improve solubilityReduce deliveryPeptide/protein ingredientsNanomedicineSolubilityHydrophilic polymers
The invention provides multi-arm block copolymers useful as drug delivery vehicles comprising a central core molecule, such as a residue of a polyol, and at least three copolymer arms covalently attached to the central core molecule, each copolymer arm comprising an inner hydrophobic polymer segment covalently attached to the central core molecule and an outer hydrophilic polymer segment covalently attached to the hydrophobic polymer segment, wherein the central core molecule and the hydrophobic polymer segment define a hydrophobic core region. The solubility of hydrophobic biologically active agents can be improved by entrapment within the hydrophobic core region of the block copolymer. The invention further includes pharmaceutical compositions including such block copolymers, methods of making such copolymers and pharmaceutical compositions, and methods of using the block copolymers as drug delivery vehicles.
Owner:NEKTAR THERAPEUTICS INC
Catalyst and process of paraffin hydrocarbon conversion
InactiveUS20040077914A1Improve solubilityEasy to useHydrocarbon by isomerisationHydrocarbon by hydrogenationAlkanePtru catalyst
A catalyst composition and process for the conversion of linear and / or branched paraffin hydrocarbons based on the use of an ionic liquid catalyst in combination with a Brønsted Acid, which provides a catalytic composition with an increased activity compared with said ionic liquid. Under suitable reaction conditions this conversion is leading to paraffin hydrocarbon fraction with higher octane number.
Owner:HALDOR TOPSOE AS
Novel drug delivery system
InactiveUS20060018934A1Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com