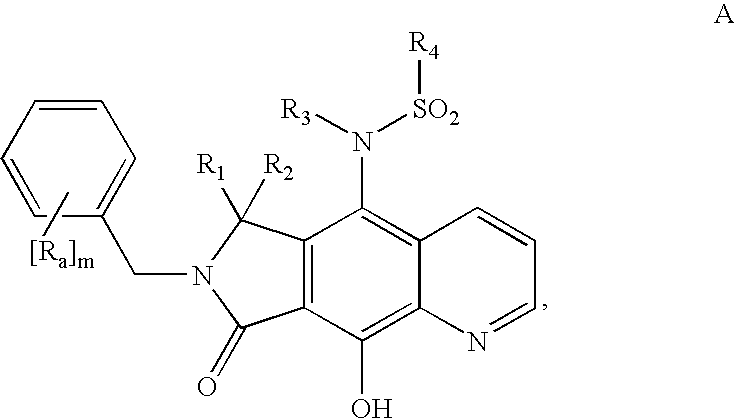
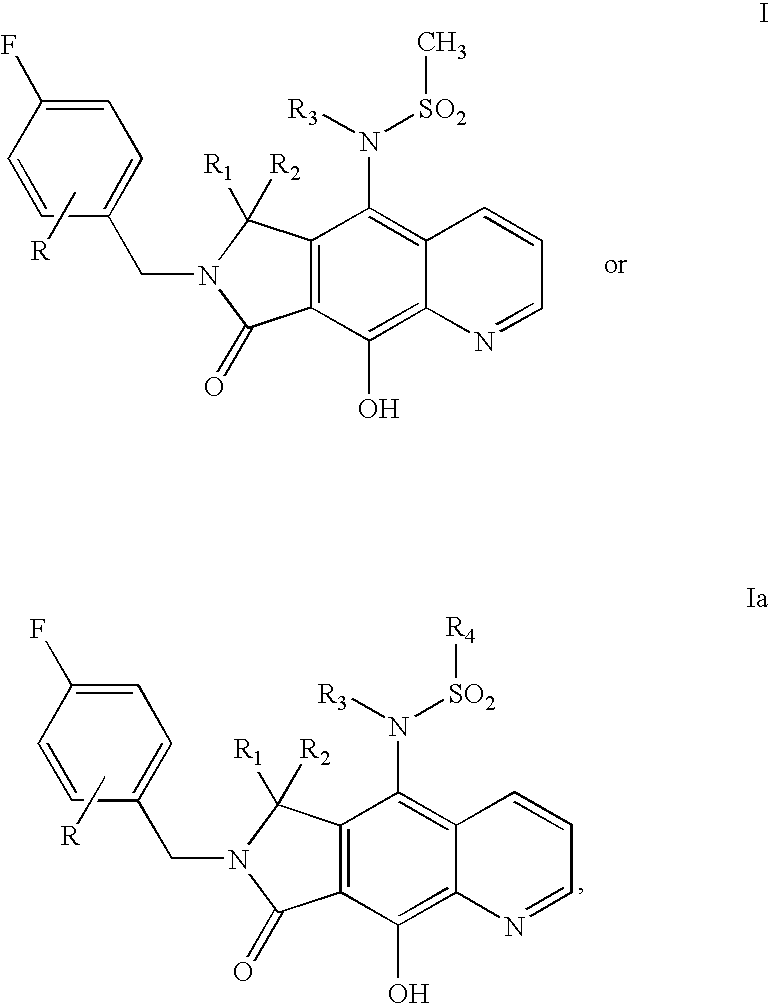
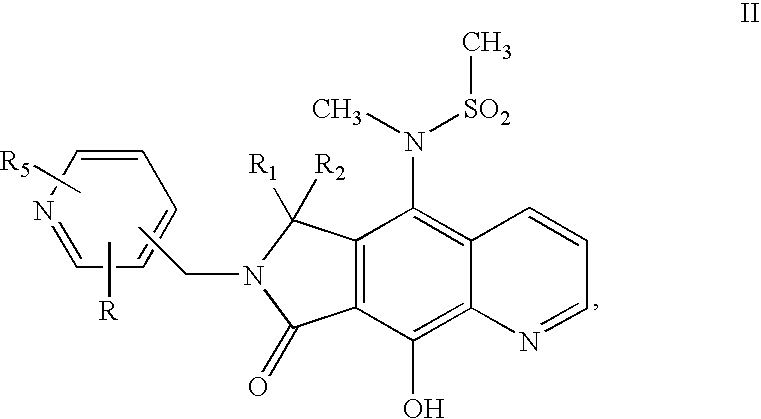
Integrase inhibitor compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0519] Intermediates useful in synthesizing compounds of the invention can be prepared by the following methodology. It should be noted that after every step, the product may be recovered and optionally purified by conventional methods such as precipitation, filtration, evaporation, crystallization, chromatography and the like. Alternatively, the products can be used directly in the next step without purification and / or isolation.
[0520] Compound 1 is converted under conventional conditions to the corresponding anhydride 2. Specifically, compound 1 is refluxed in a suitable solvent, such as acetone, methyl ethyl ketone in the presence of an excess of 15 acetic anhydride to provide the anhydride 2. Compound 2 is then refluxed in the presence of an approximately single equivalent of isopropanol for about 2 to about 20 hours to provide for the mono-carboxy, mono-isopropoxy derivative, compound 3. Compound 3 is then condensed under conventional conditions with methylsulfonyl chloride i...
example 2
Preparation of N-(7-(4-fluorobenzyl)-9-hydroxy-8-oxo-7,8-di hydro-6H-pyrrolo[3,4-g]quinolin-5-yl)-N-methylmethanesulfonamide
[0522] Procedure:
[0523] Freshly ground K2CO3 (31 g, 225 mmol) was added to dry acetone (200 mL) in a 3-necked flask equipped with drying tube, condenser, and mechanical stirrer. To this was added succinimide (7.43 g, 75 mmol) and 4-fluorobenzylbromide (11.21 mL, 90 mmol). Refluxed for 19 hours. Mixture filtered through Celite, then acetone removed under vacuum, diluted with EtOAc, washed with saturated aqueous sodium bicarbonate and also with brine, dried (MgSO4), filtered and concentrated to give crude. Crude product chromatographed (EtOAc / hexane) on silica gel to give 6 as white solid (13.22 g, 85%). 1H NMR (CDCI3) δ 7.4 (dd, 2H), 7.0 (t, 2H), 4.6 (s, 1H), 2.7 (s, 4 H).
[0524] Compound 6 (8 g, 38.6 mmol) and 2,3-pyridine carboxylic acid dimethyl ester (7.9 g, 40.6 mmol) were dissolved in dry THF (78 mL) and dry MeOH (1.17 mL) in a 3-necked flask with mecha...
example 5
Preparation of N-(7-(4-fluorobenzyl)-9-hydroxy-8-oxo-7,8-dihydro-6H-pyrrolo[3,4-g]quinolin-5-yl)-N,N′,N′-trimethylsulfamide
[0568]
[0569] Intermediate 37 was synthesized from 25 in a manner similar to intermediate 28. Intermediate 37 ( 22 mg, 38 μmol) was dissolved in 200 μL of DCM and treated with TFA (22 mg, 190 μmol) and triethylsilane (9.0 mg, 76 μmol ). After stirring for 15 minutes at room temperature, the reaction mixture was azeotroped with toluene three times. The residue was then triturated with 3:1 hexane:ether to provide 38. 300 MHz 1H NMR (CDCl3) δ (ppm): 9.11 (d, 1H); 8.65 (d, 1H); 7.71 (m, 1H); 7.30 (t, 2H); 7.02 (t, 2H); 4.90 (d, 2H); 4.72 (d, 1H); 4.58 (d, 1H); 4.35 (d, 1H); 3.1 (s, 3H); 2.9 (s, 3H). MS=445.5 ( M +1). Example 6
Alternate Preparation of N-(7-(4-fluorobenzyl)-9-hydroxy-8-oxo-7,8-dihydro-6H-pyrrolo[3,4-g]quinolin-5-yl)-N,N′,N′-trimethylsulfamide
[0570]
[0571] To the amine 27 (50 g, 0.099 mmol, 1 eq) contained in a microwave vial was added acetonitrile (2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Magnetic field | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com