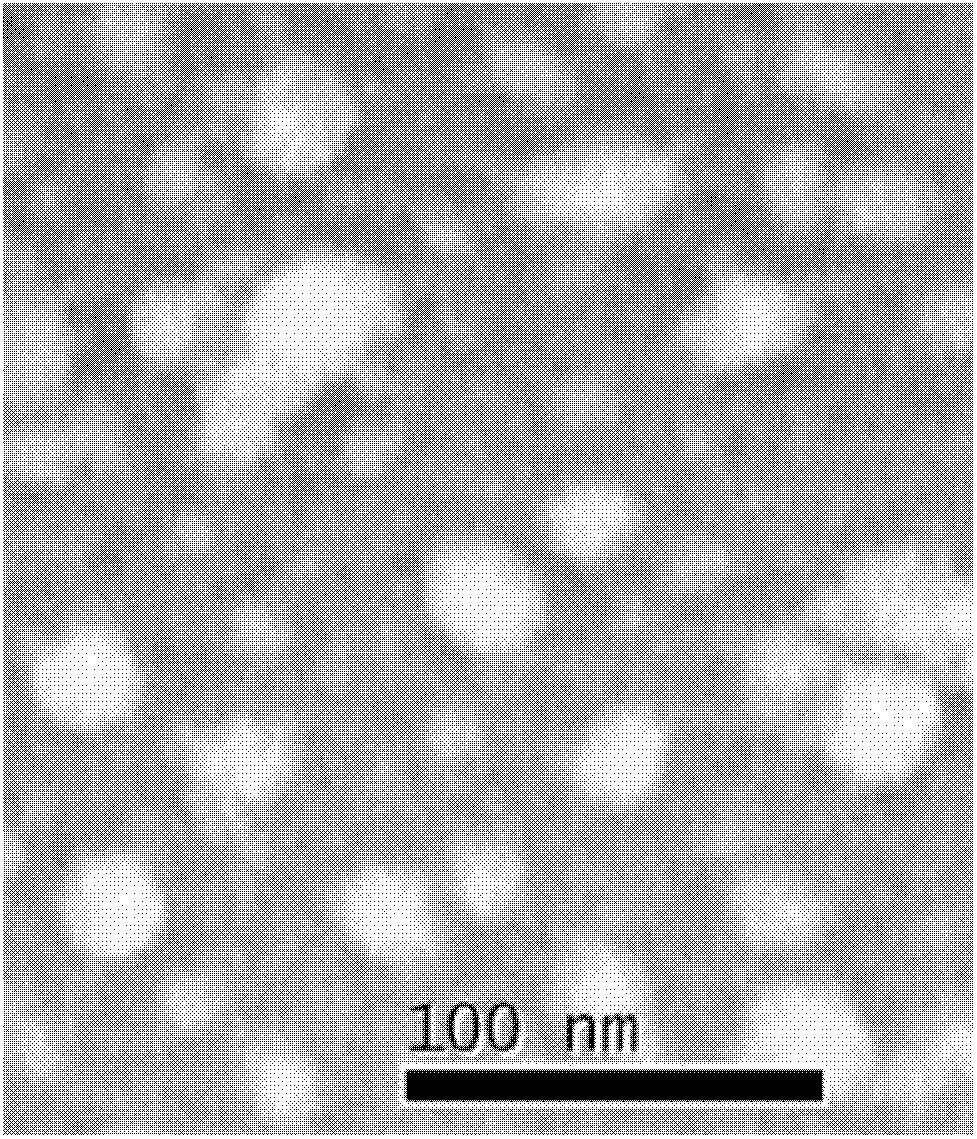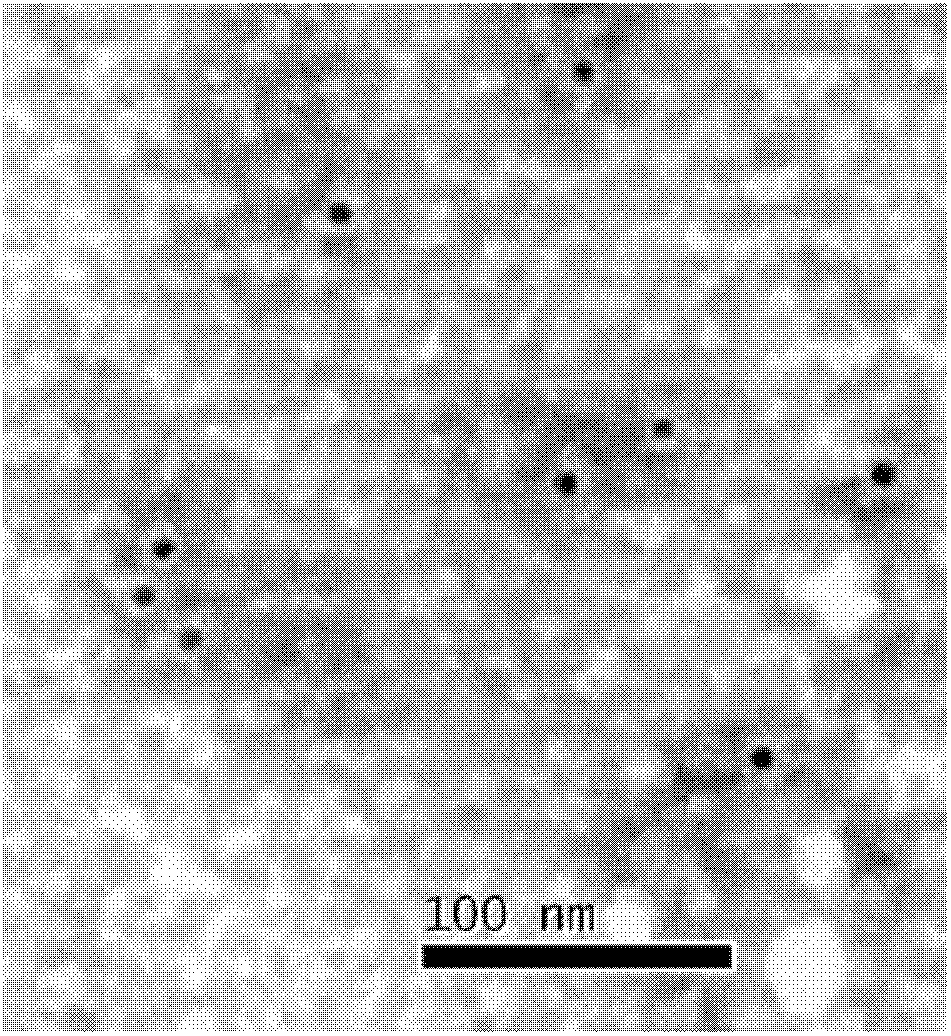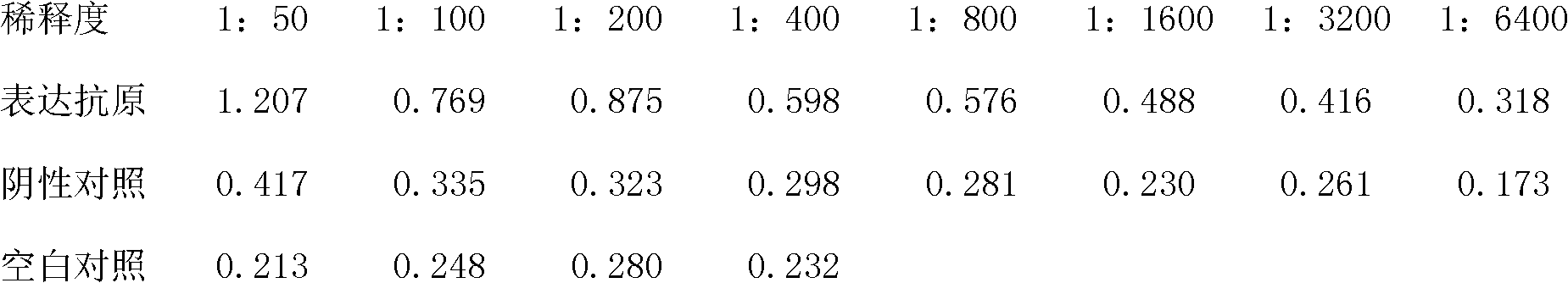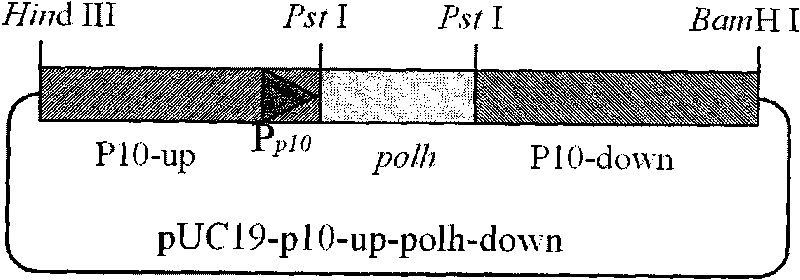Patents
Literature
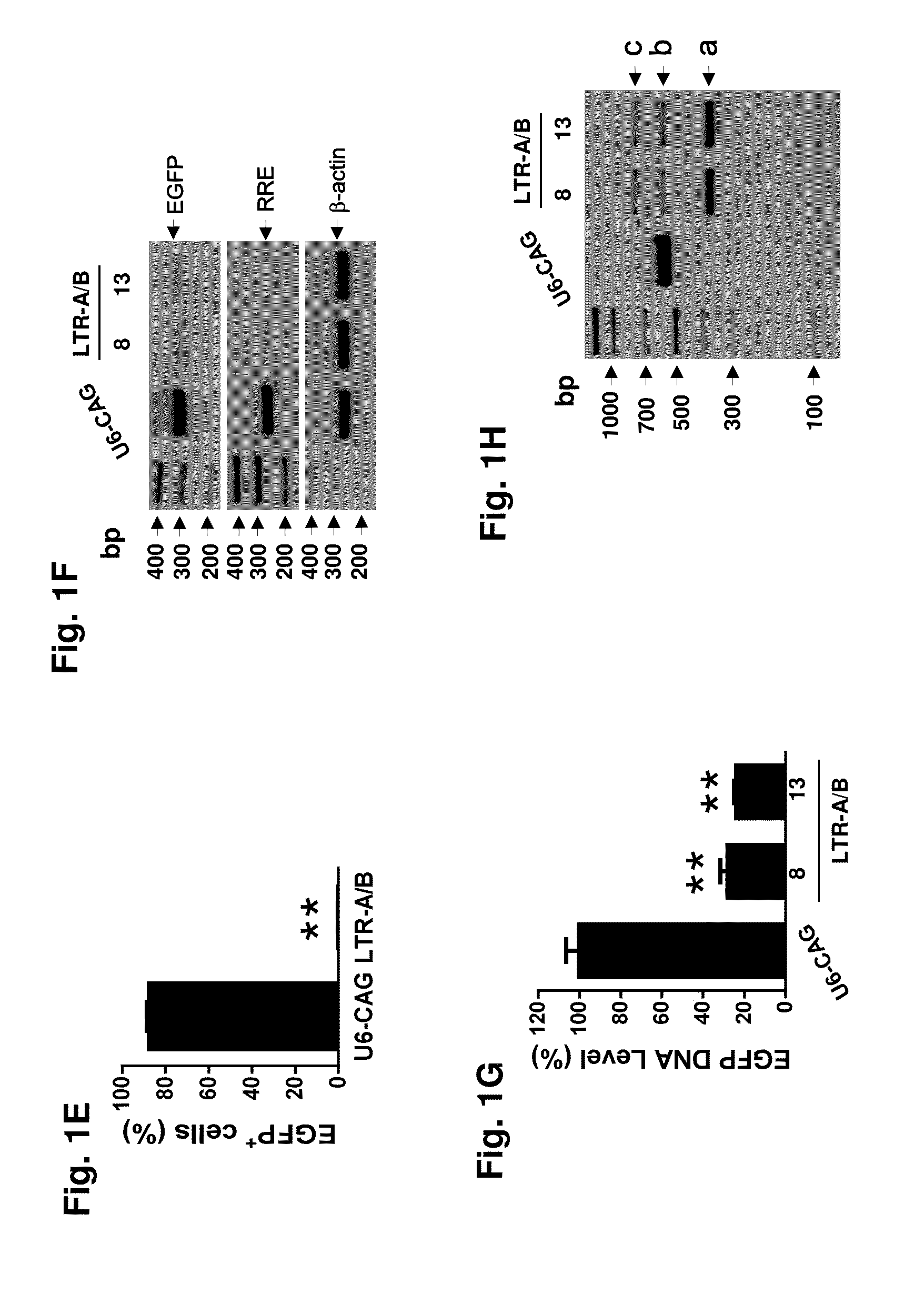
217 results about "Viral dna" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viruses can be classified based on proteins encoded within the viral genetic material or genome . Viruses with deoxyribonucleic acid (DNA) genomes are called DNA viruses. Like all viruses, DNA viruses are small when compared to the cells they infect and as such are obligate intracellular parasites (parasites that can only replicate within cells).
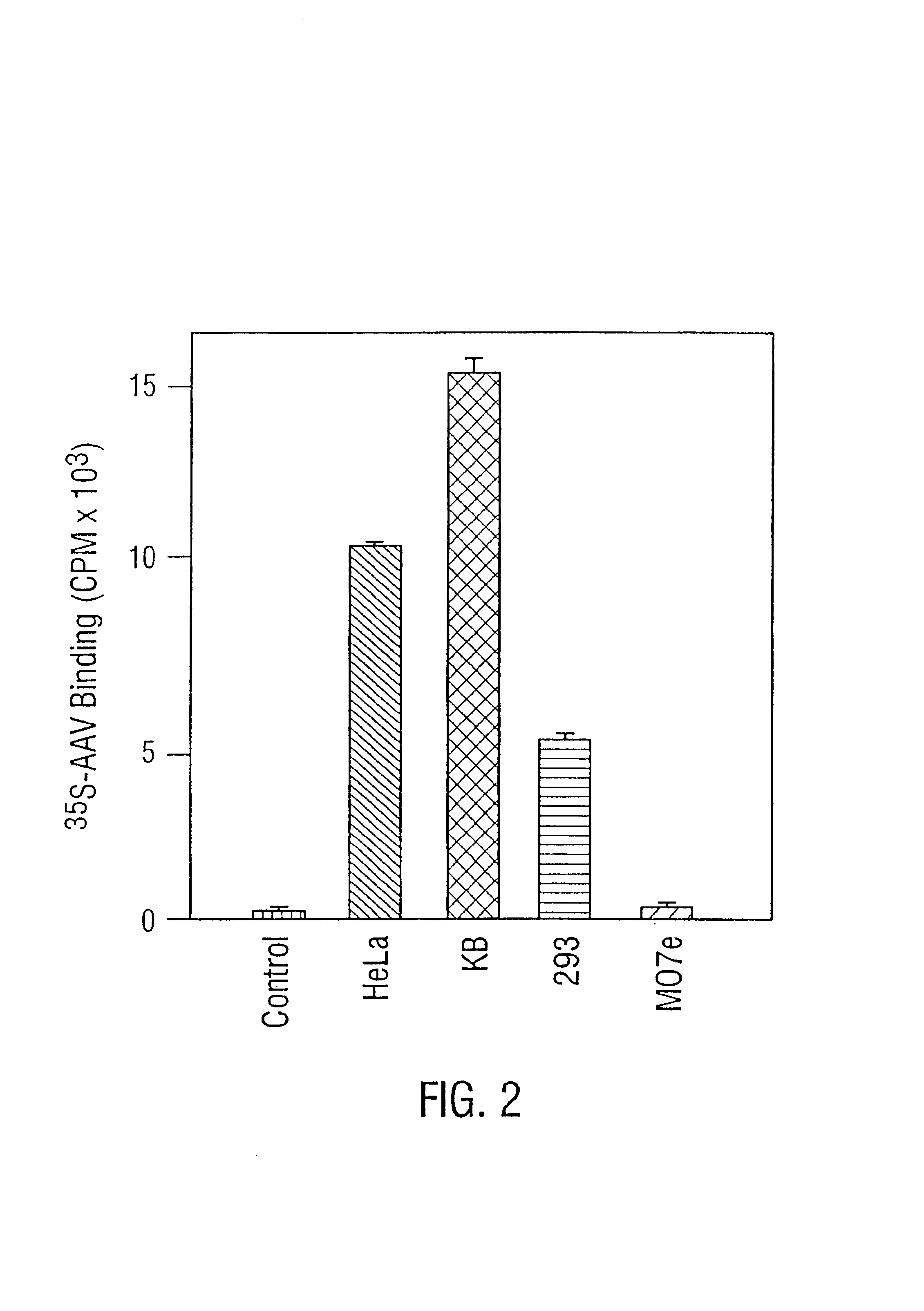
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
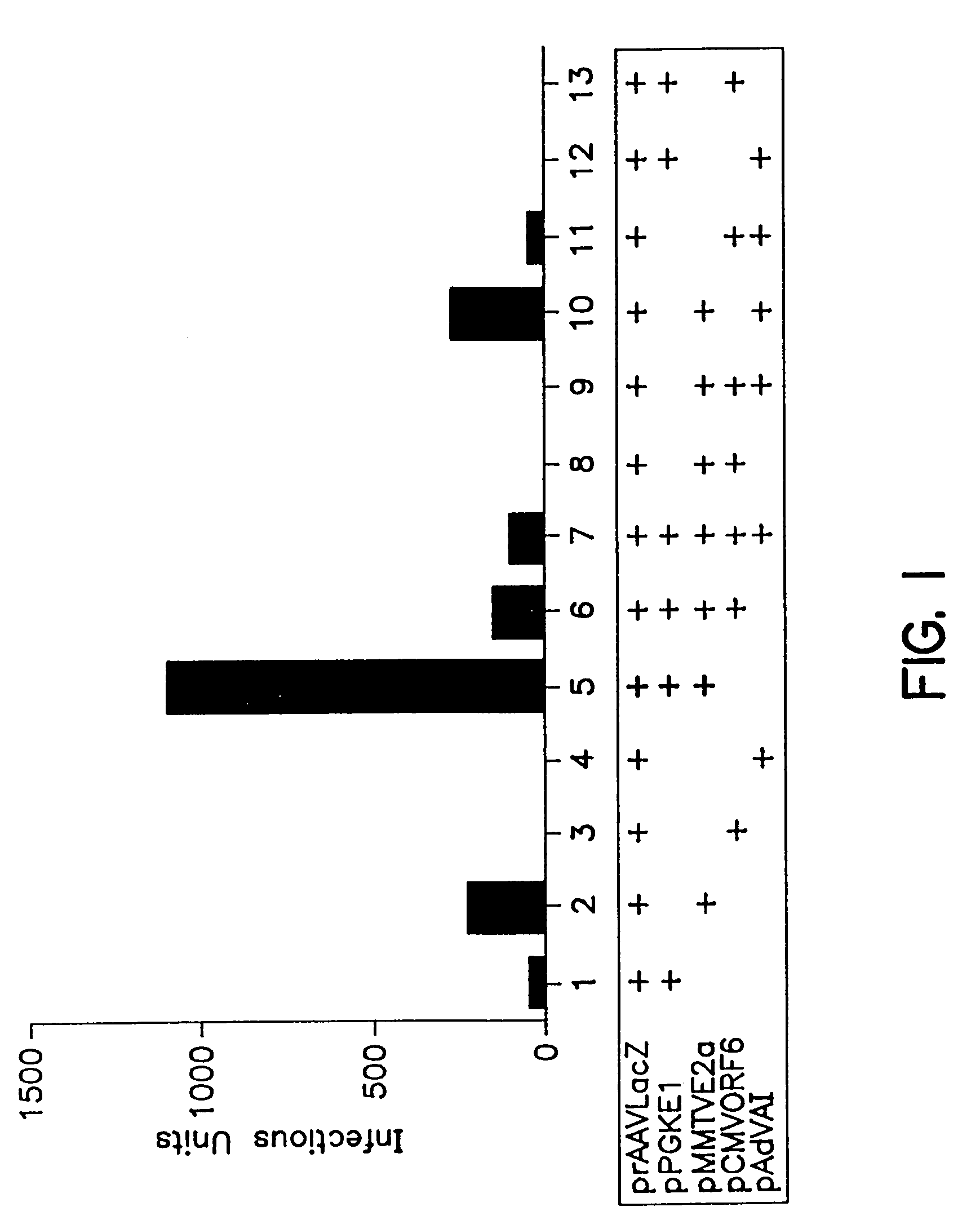
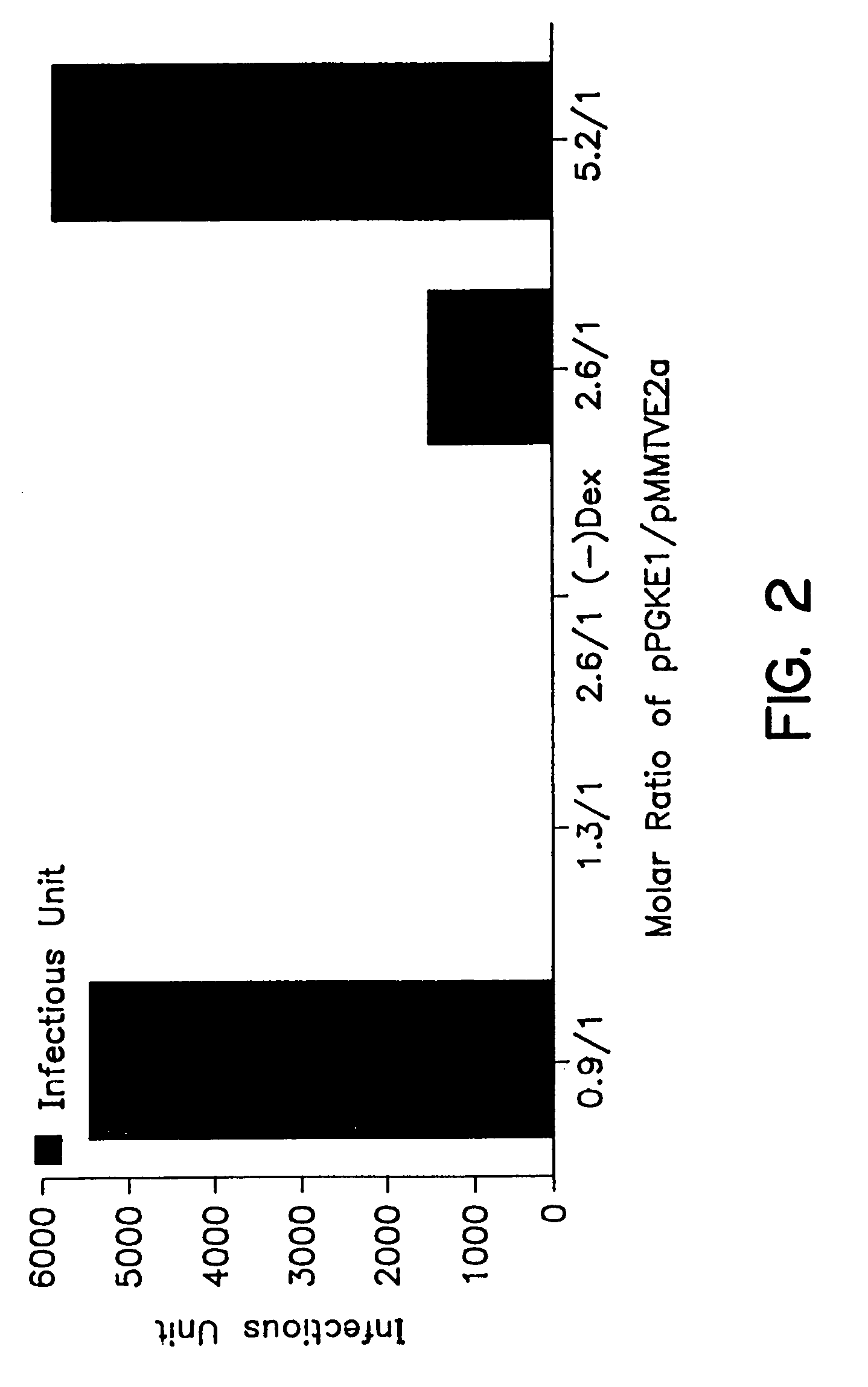
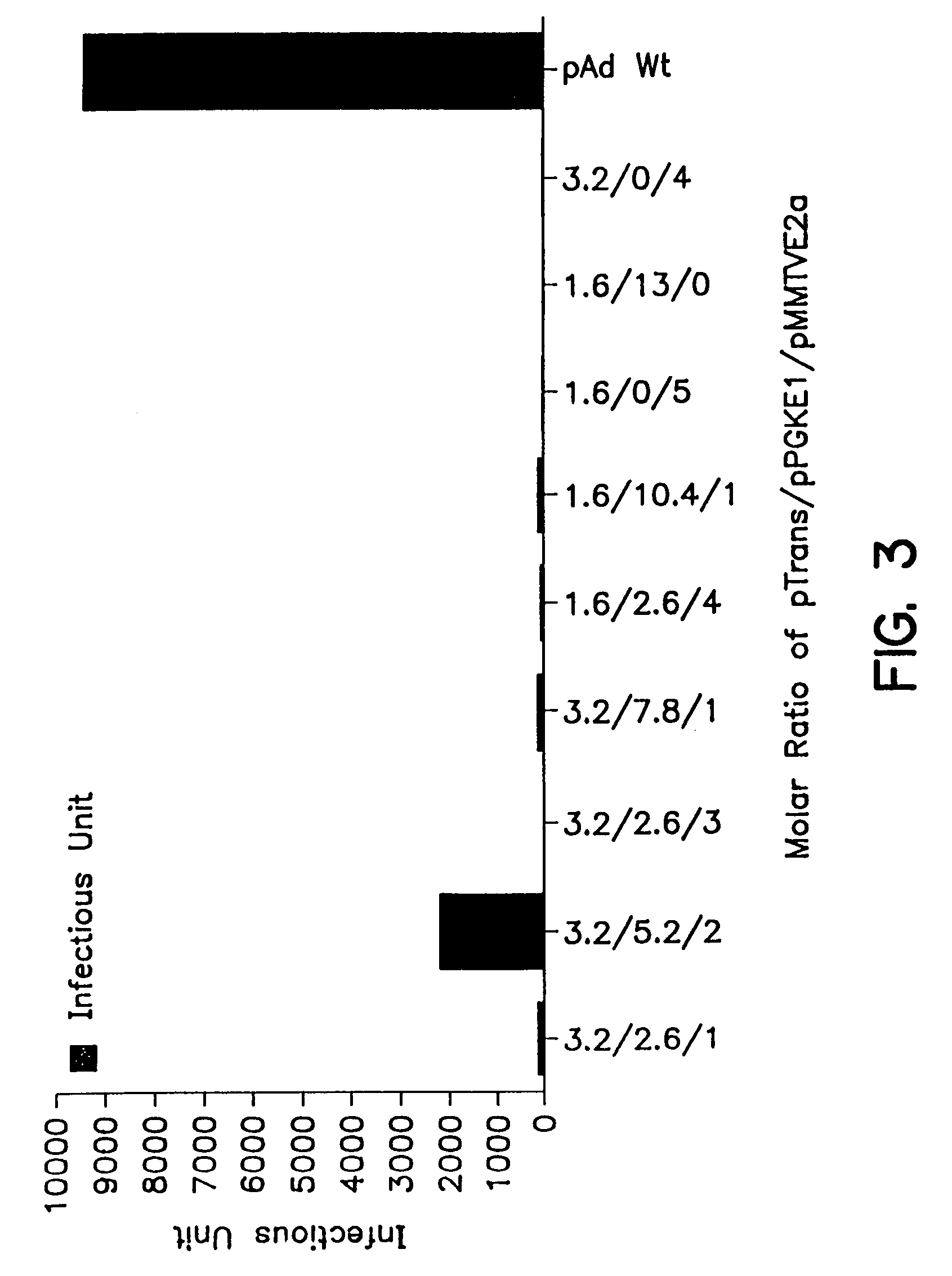
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
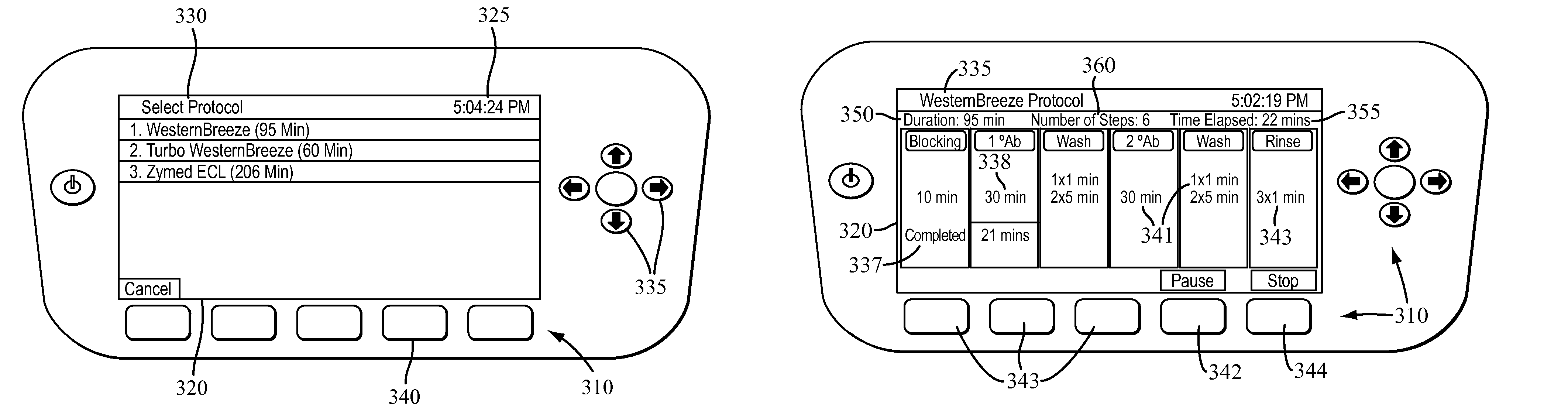
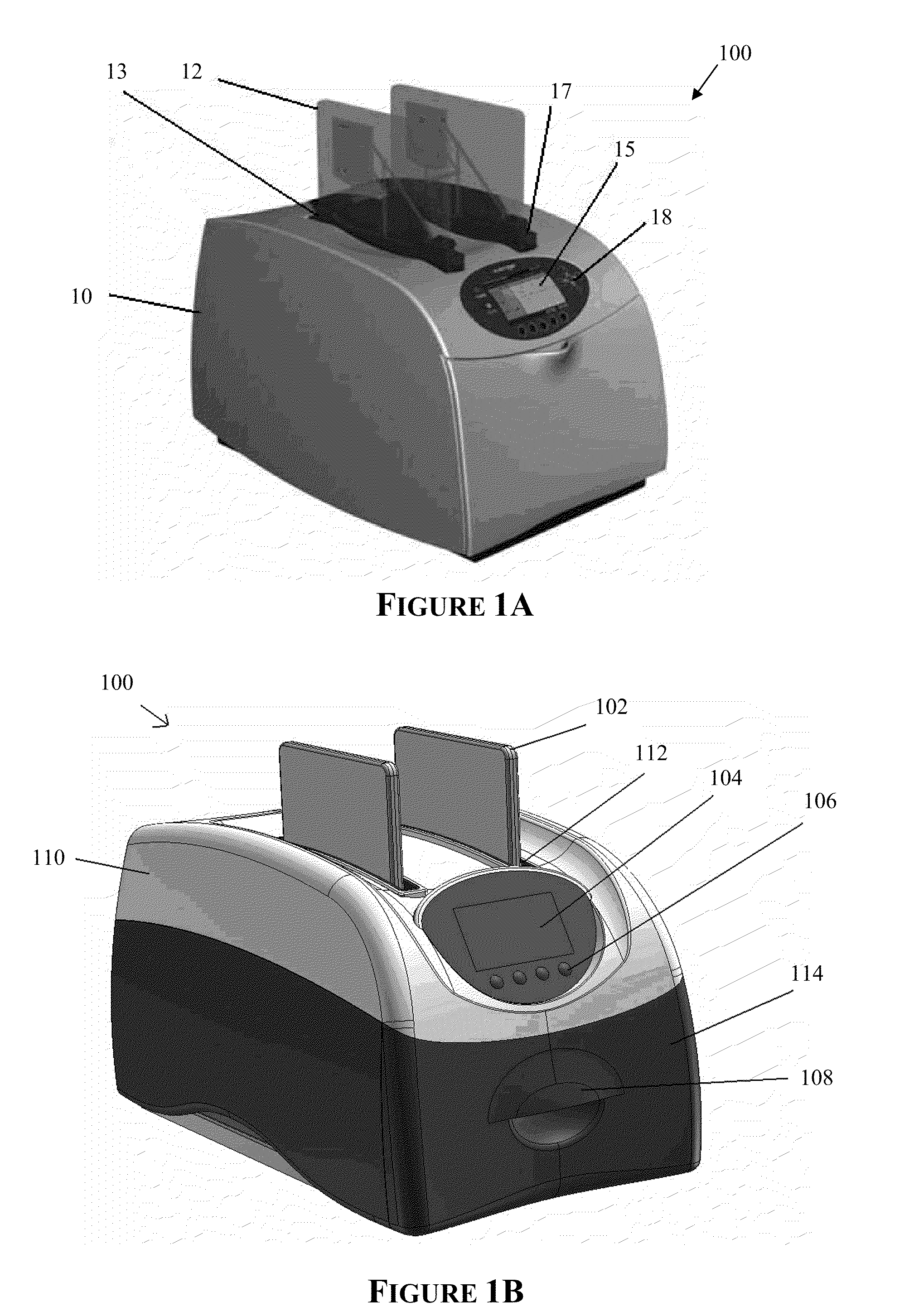
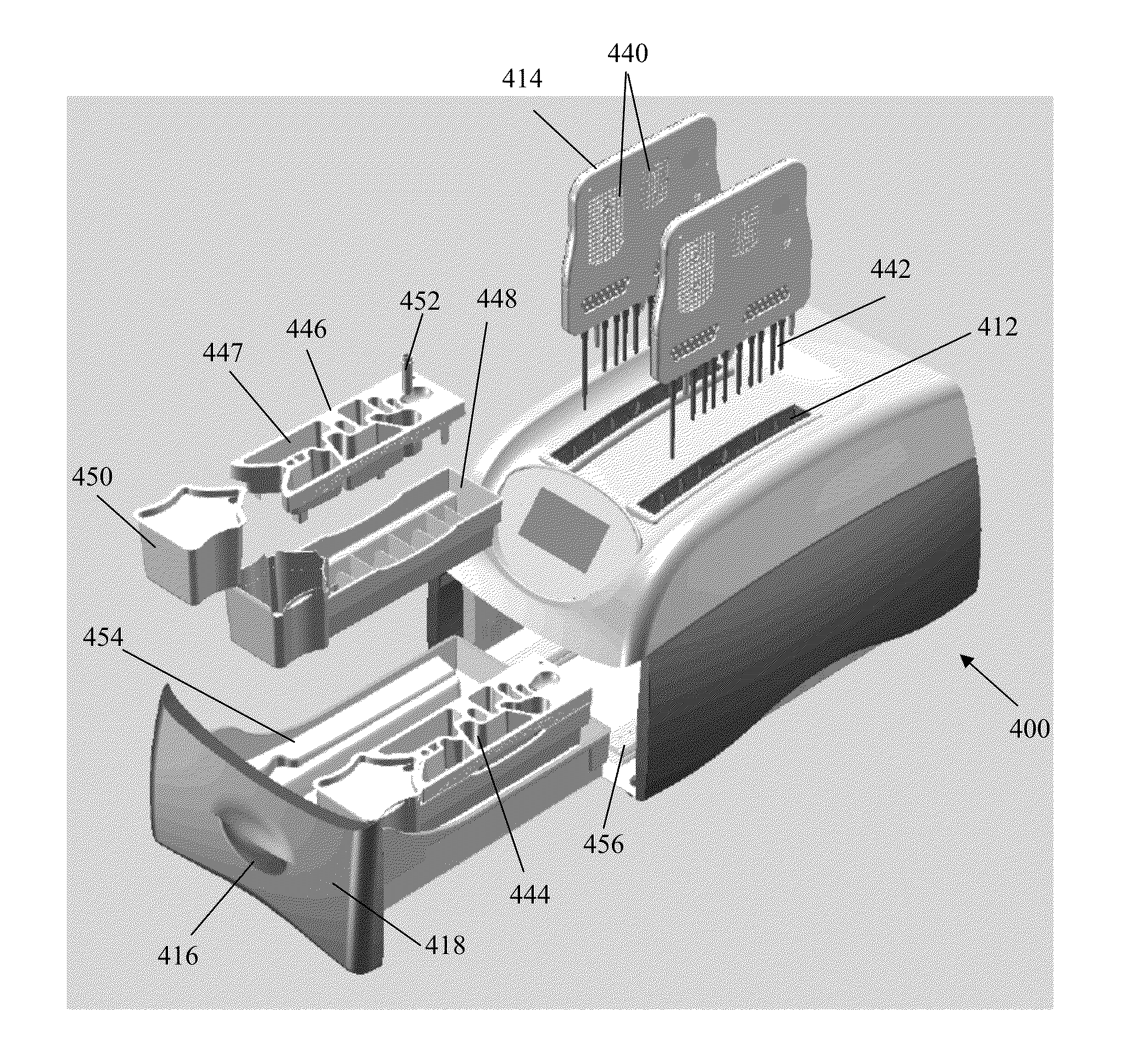
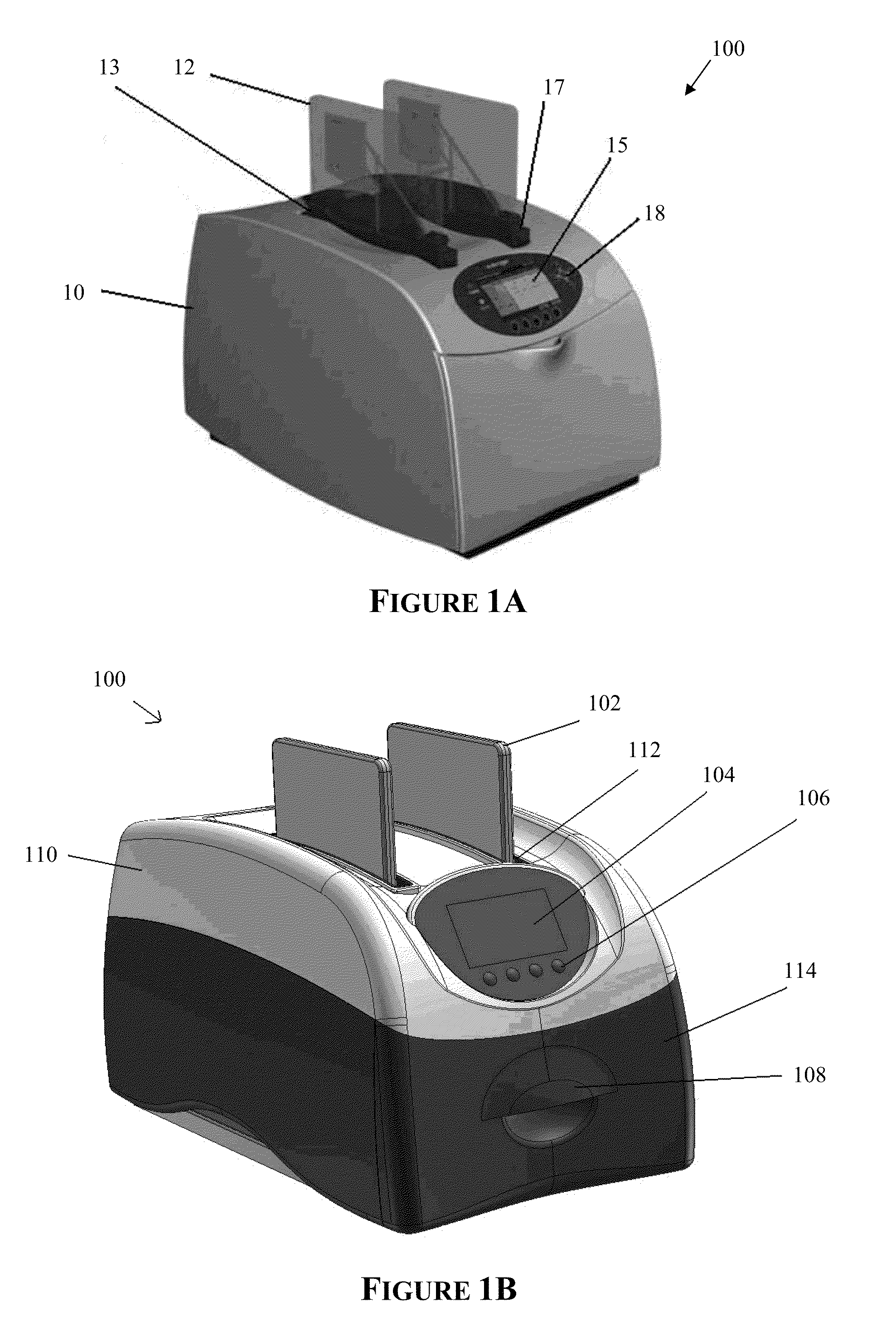
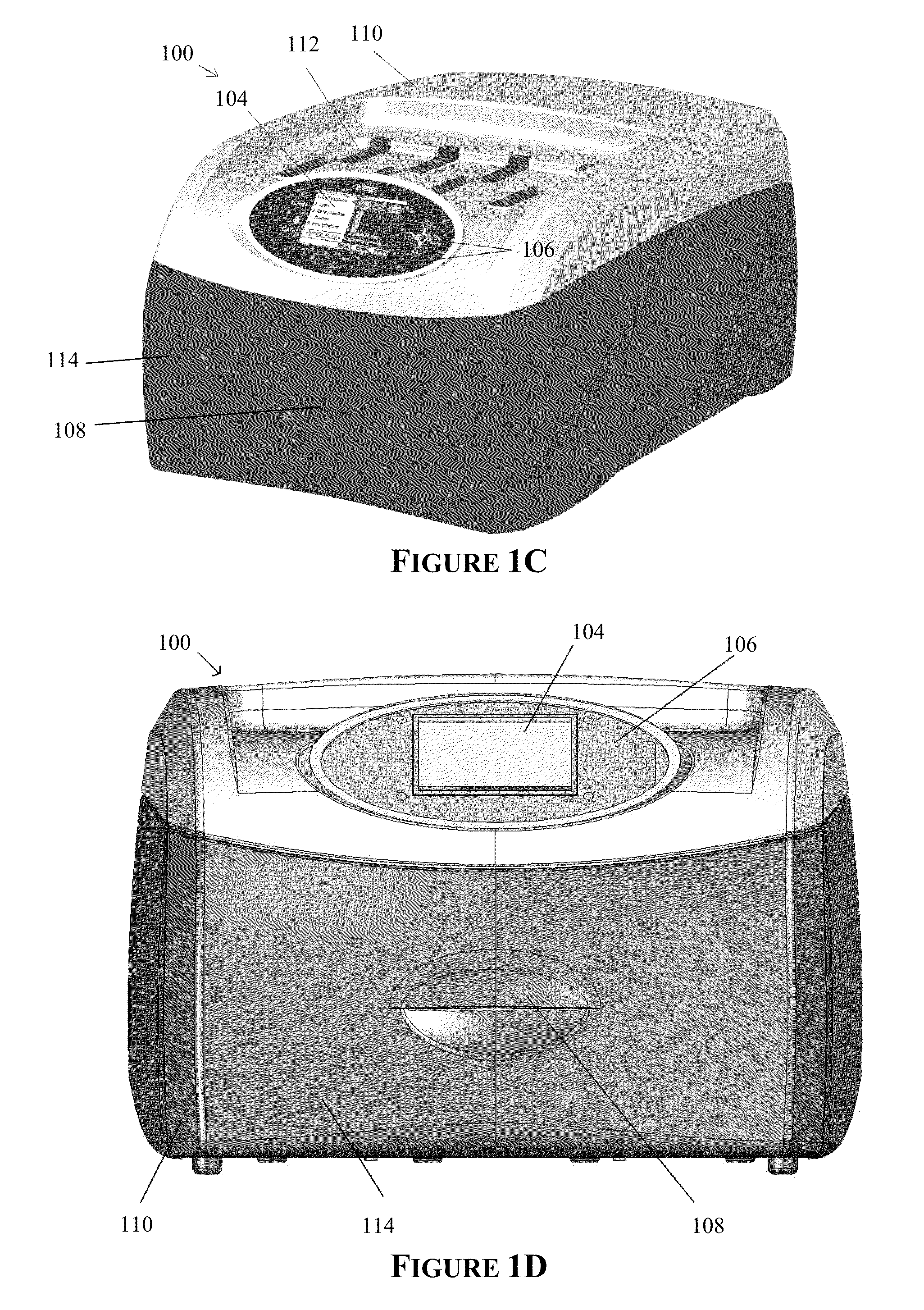
Apparatus for and method of processing biological samples
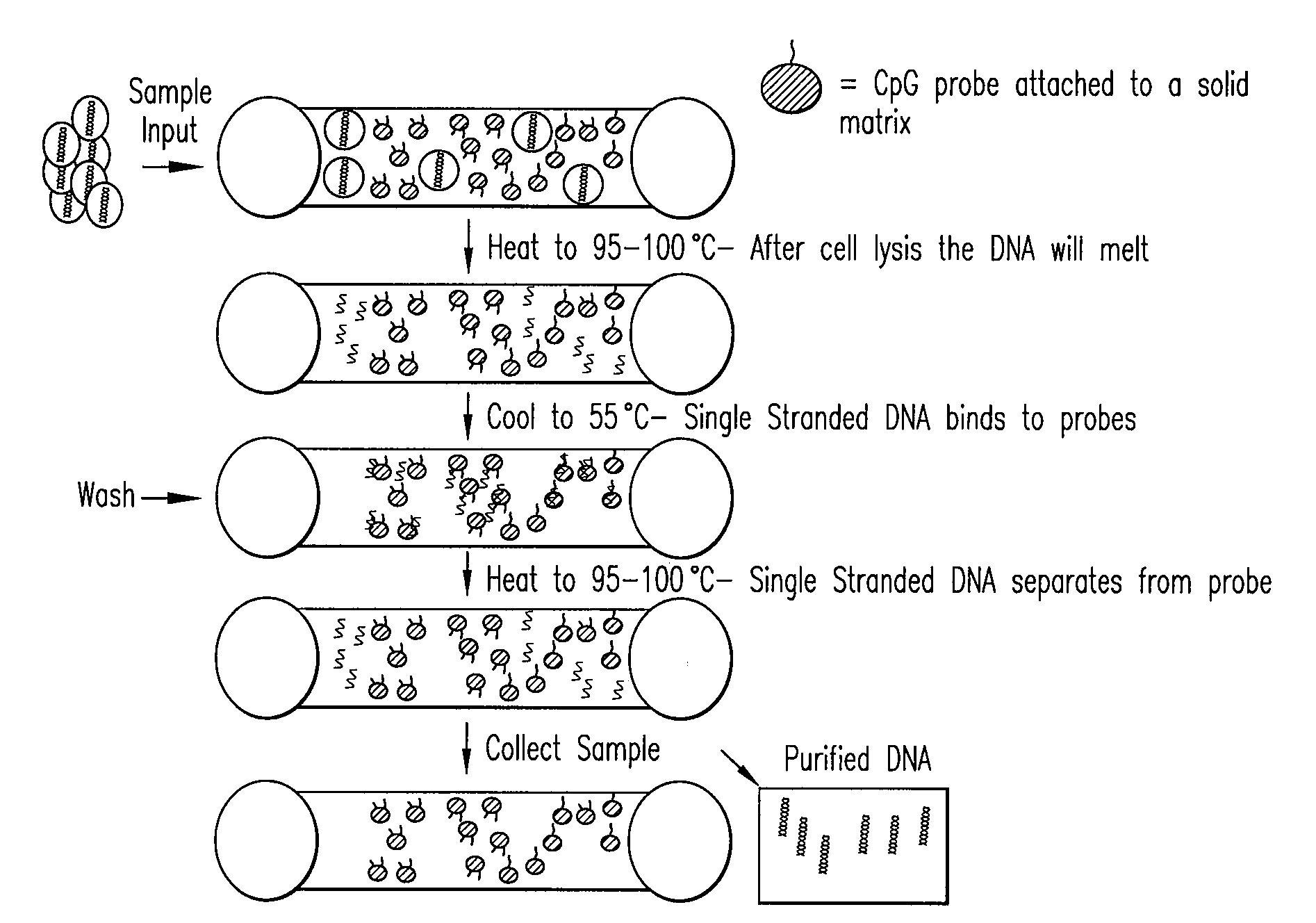
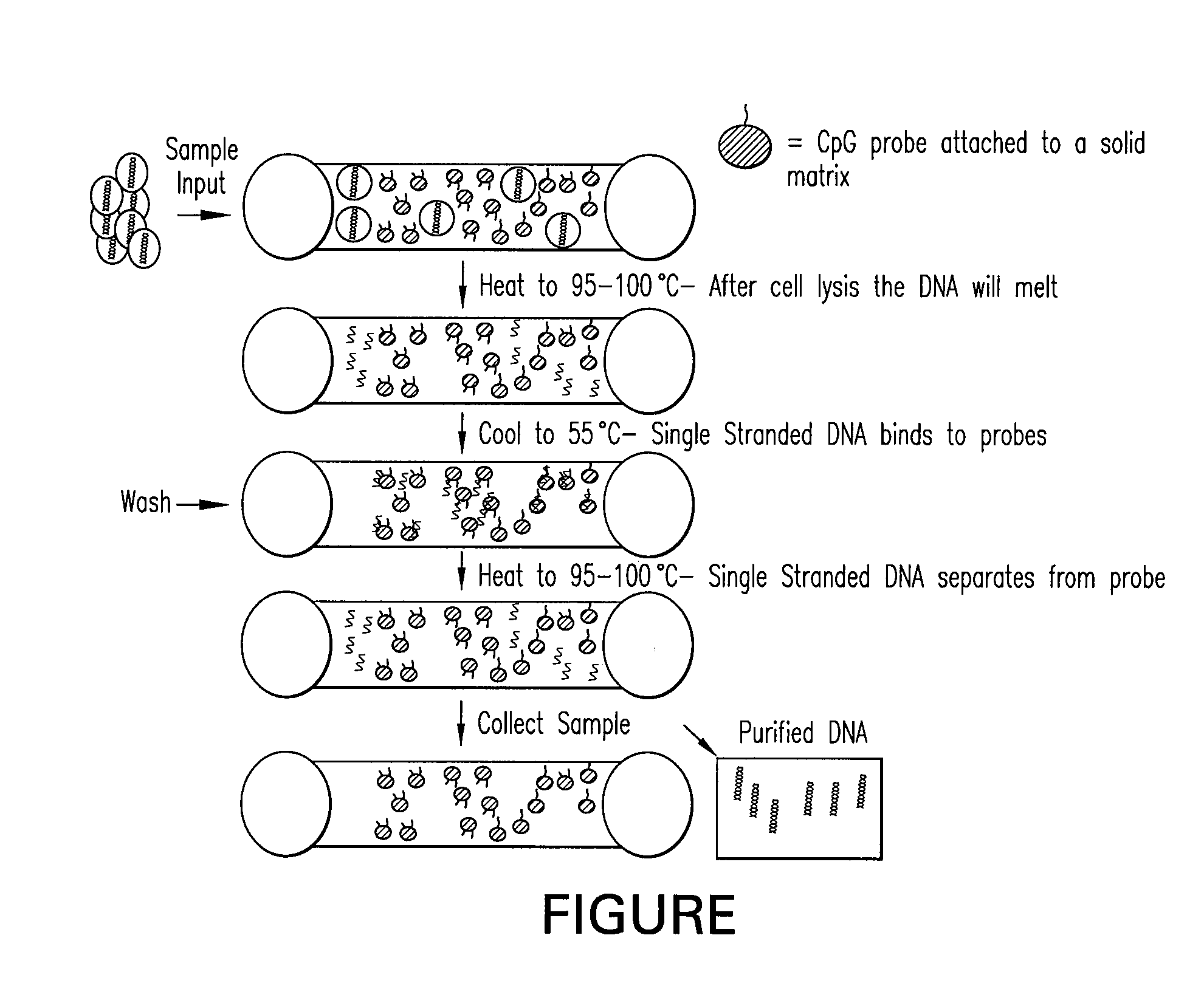
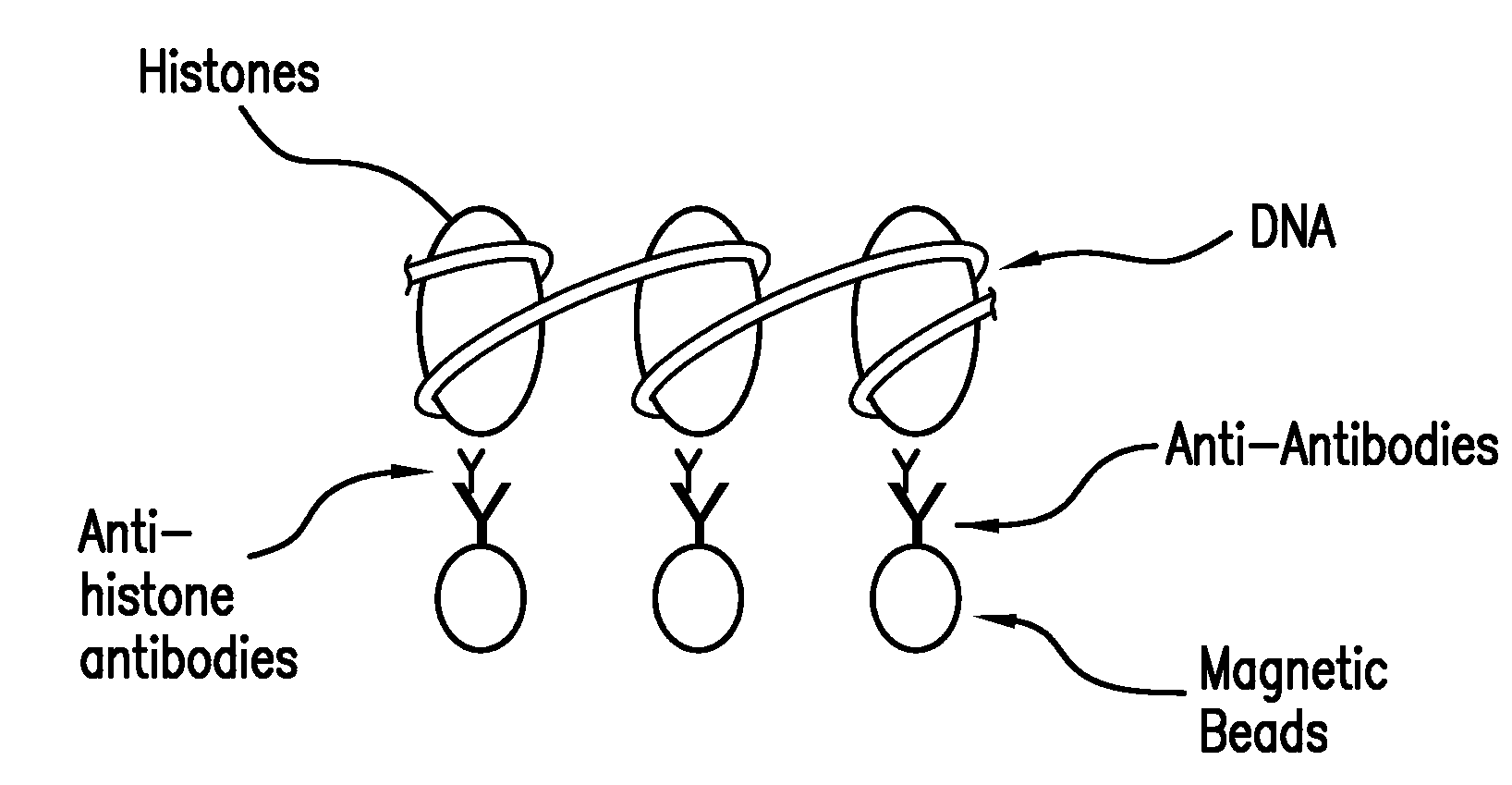
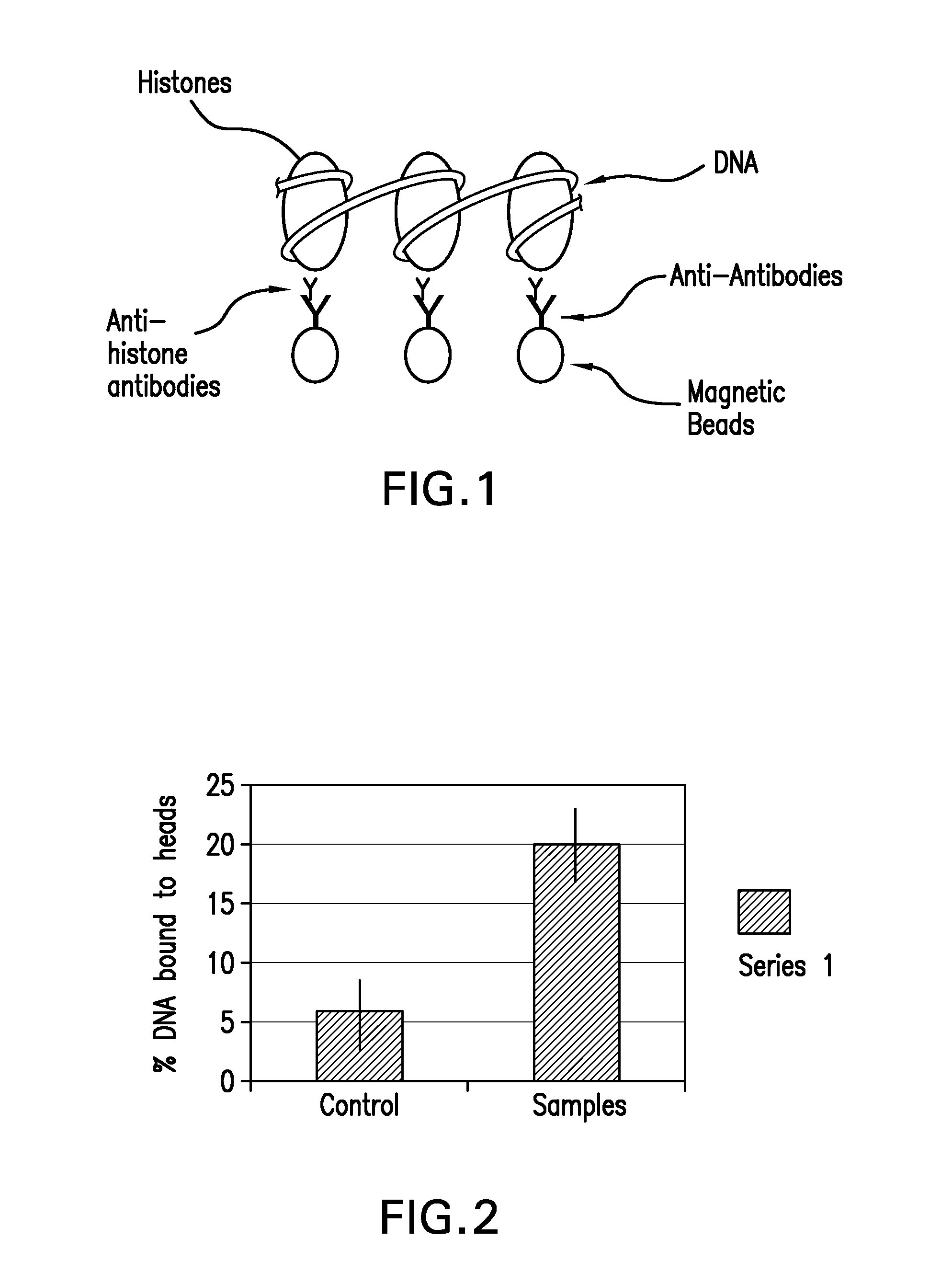
The present invention provides systems, devices, apparatuses and methods for automated bioprocessing. Examples of protocols and bioprocessing procedures suitable for the present invention include but are not limited to: immunoprecipitation, chromatin immunoprecipitation, recombinant protein isolation, nucleic acid separation and isolation, protein labeling, separation and isolation, cell separation and isolation, food safety analysis and automatic bead based separation. In some embodiments, the invention provides automated systems, automated devices, automated cartridges and automated methods of western blot processing. Other embodiments include automated systems, automated devices, automated cartridges and automated methods for separation, preparation and purification of nucleic acids, such as DNA or RNA or fragments thereof, including plasmid DNA, genomic DNA, bacterial DNA, viral DNA and any other DNA, and for automated systems, automated devices, automated cartridges and automated methods for processing, separation and purification of proteins, peptides and the like.
Owner:LIFE TECH CORP
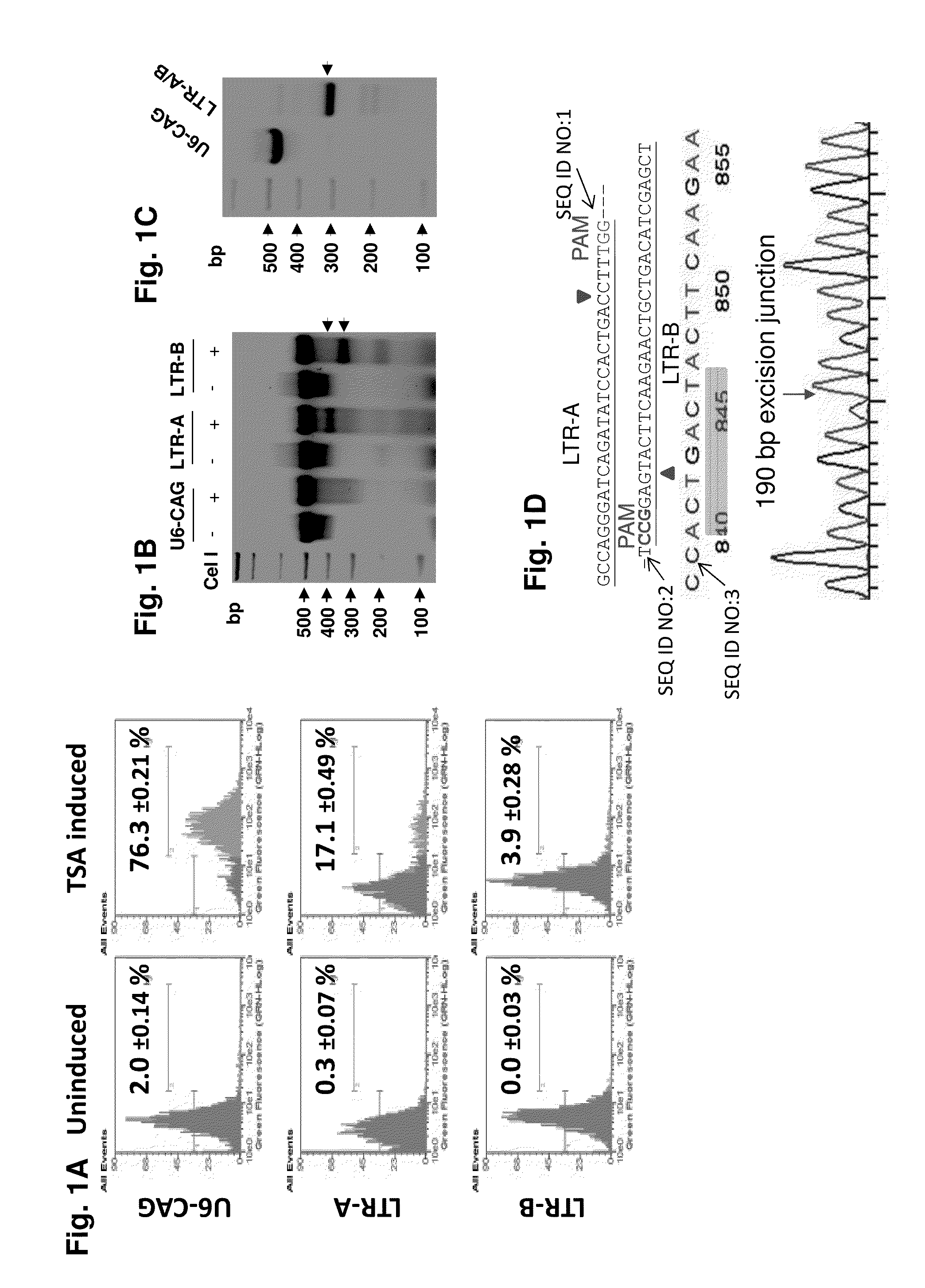
Methods and compositions for rna-guided treatment of HIV infection
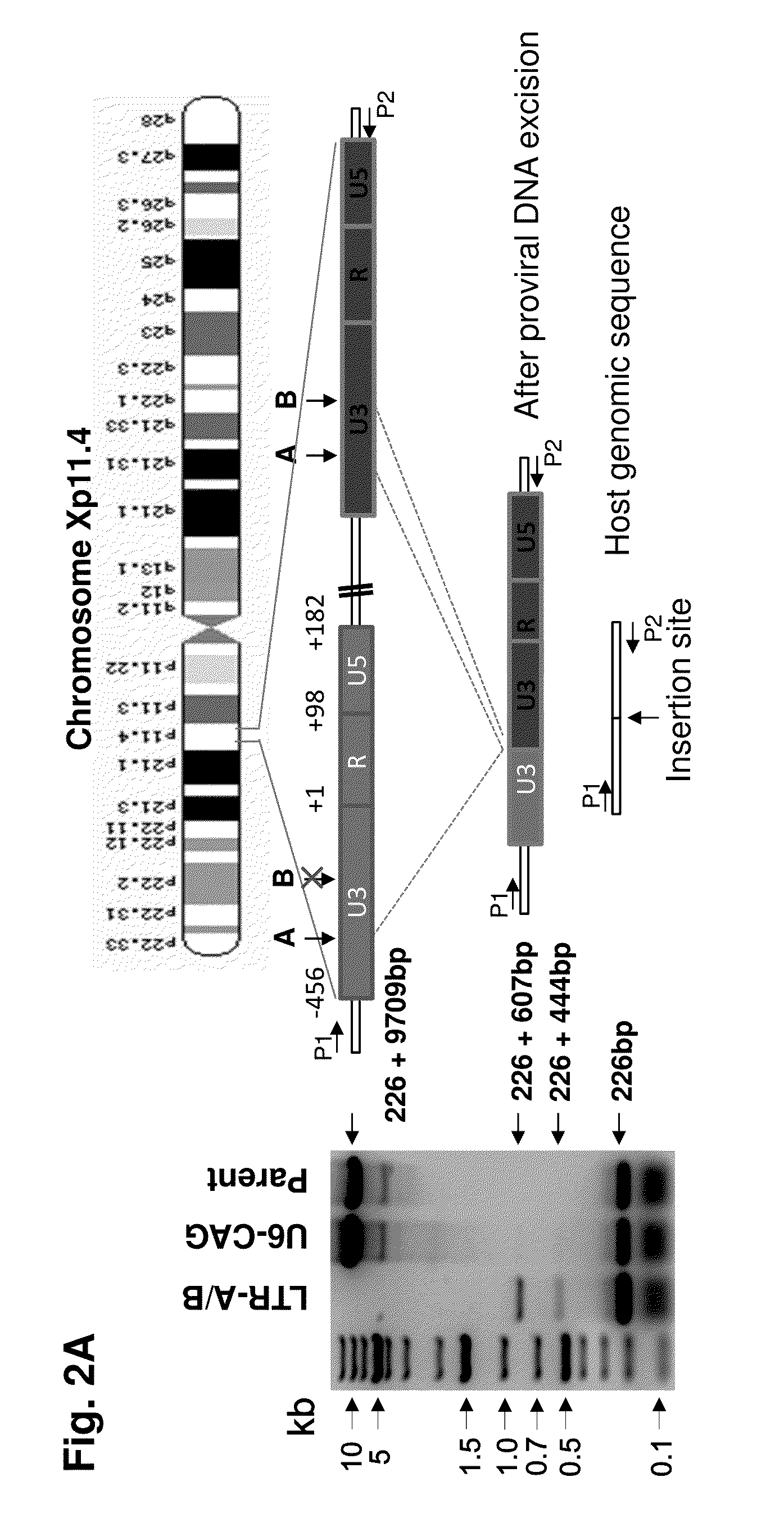
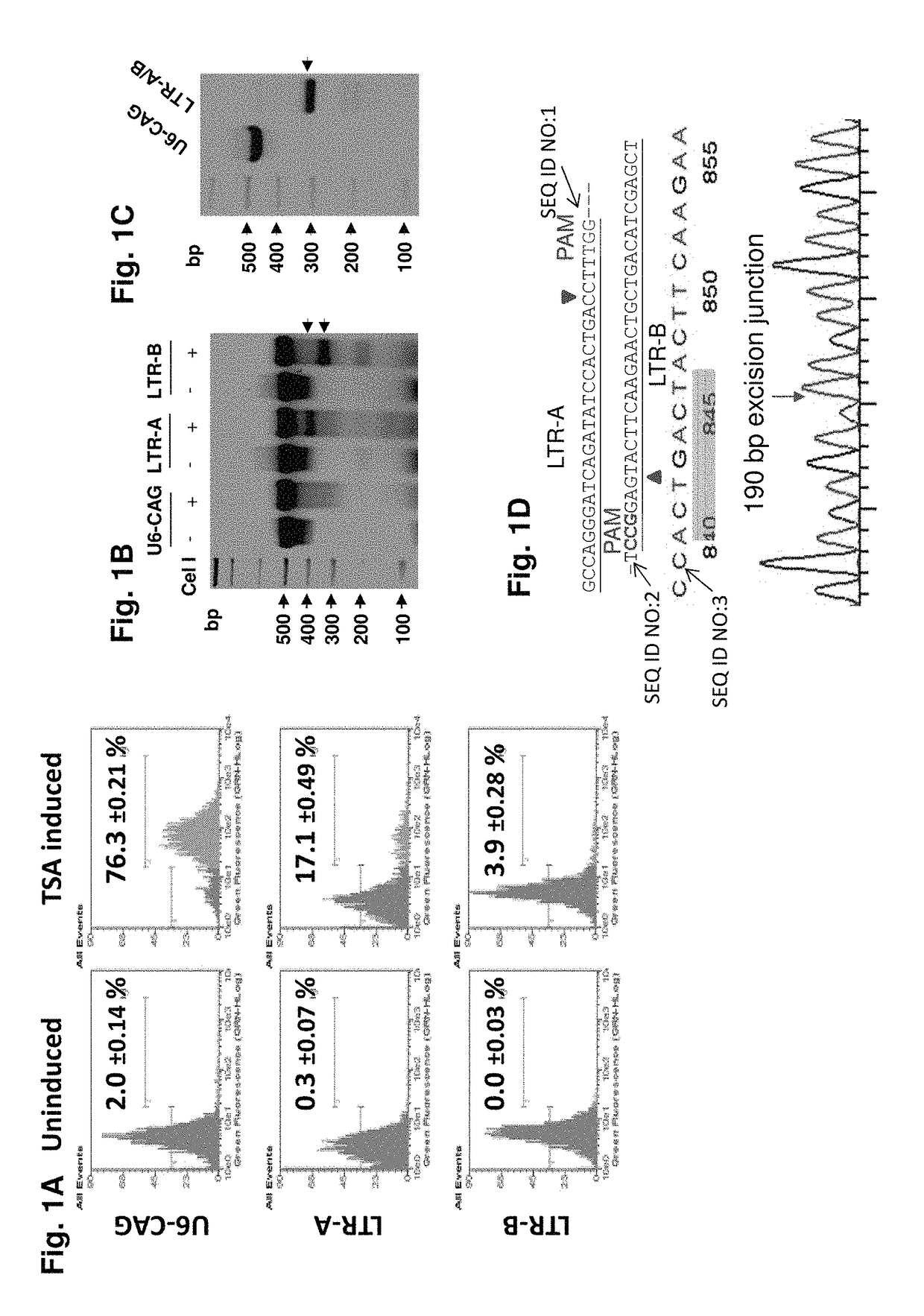
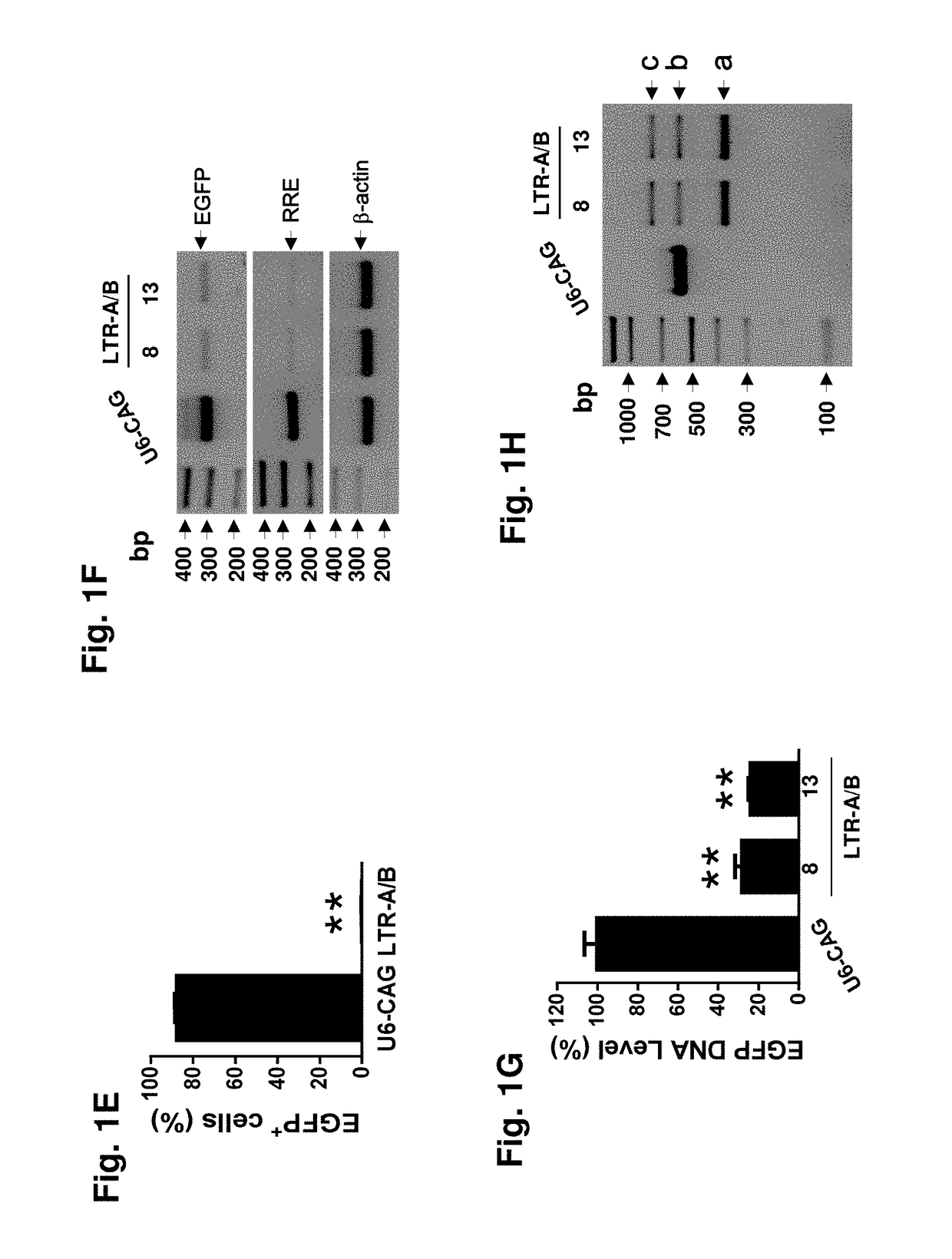
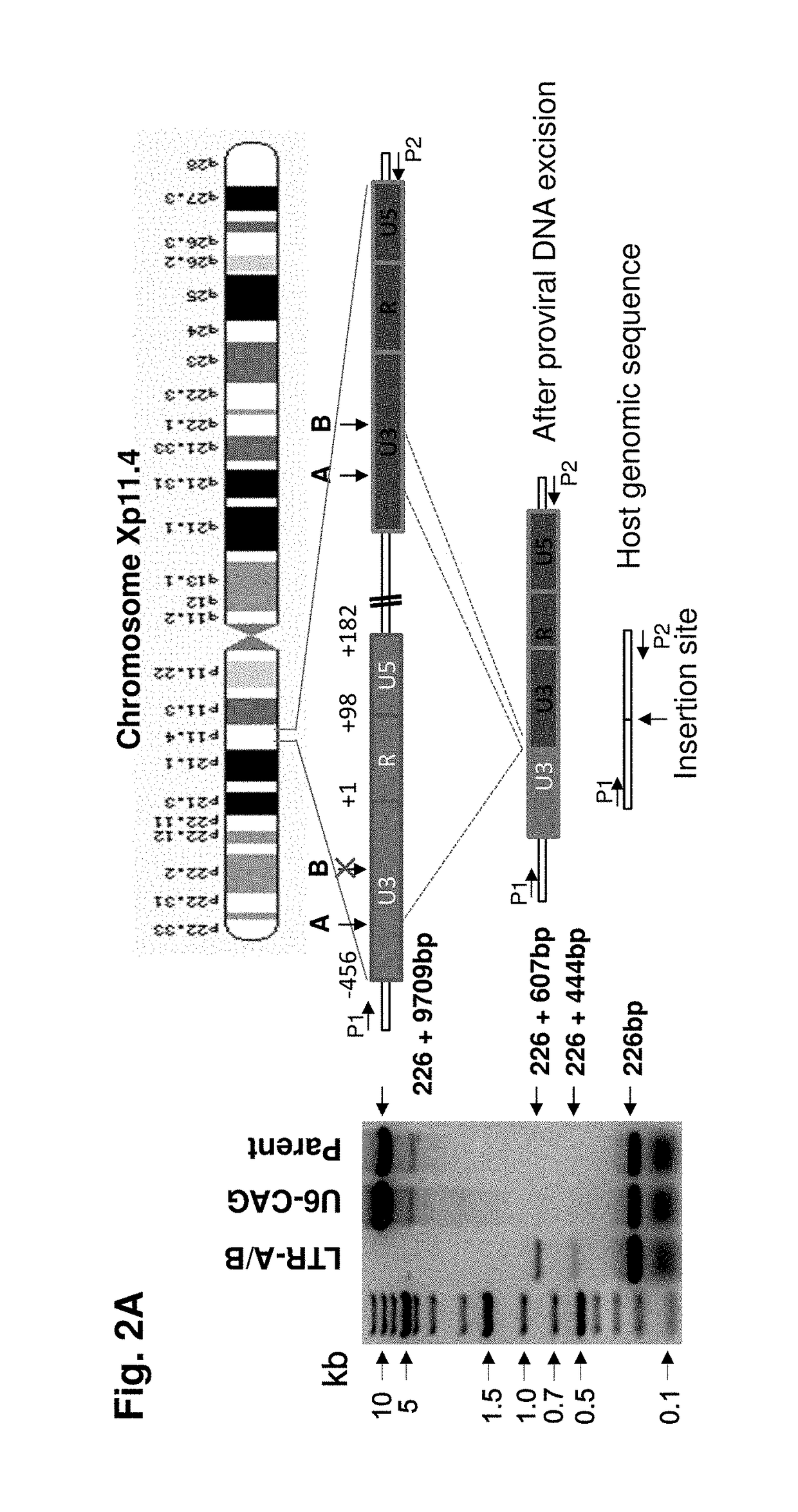
A method of inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus by treating the host cell with a composition comprising a Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)-associated endonuclease, and two or more different guide RNAs (gRNAs), wherein each of the at least two gRNAs is complementary to a different target nucleic acid sequence in a long terminal repeat (LTR) in the proviral DNA, and inactivating the proviral DNA. A composition for use in inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus including isolated nucleic acid sequences comprising a CRISPR-associated endonuclease and a guide RNA, wherein the guide RNA is complementary to a target sequence in a human immunodeficiency virus.
Owner:TEMPLE UNIVERSITY
RNA respiratory syncytial virus vaccines
InactiveUS6060308AFaster replicationImprove efficiencySsRNA viruses negative-senseBiocideF proteinViral Vaccine
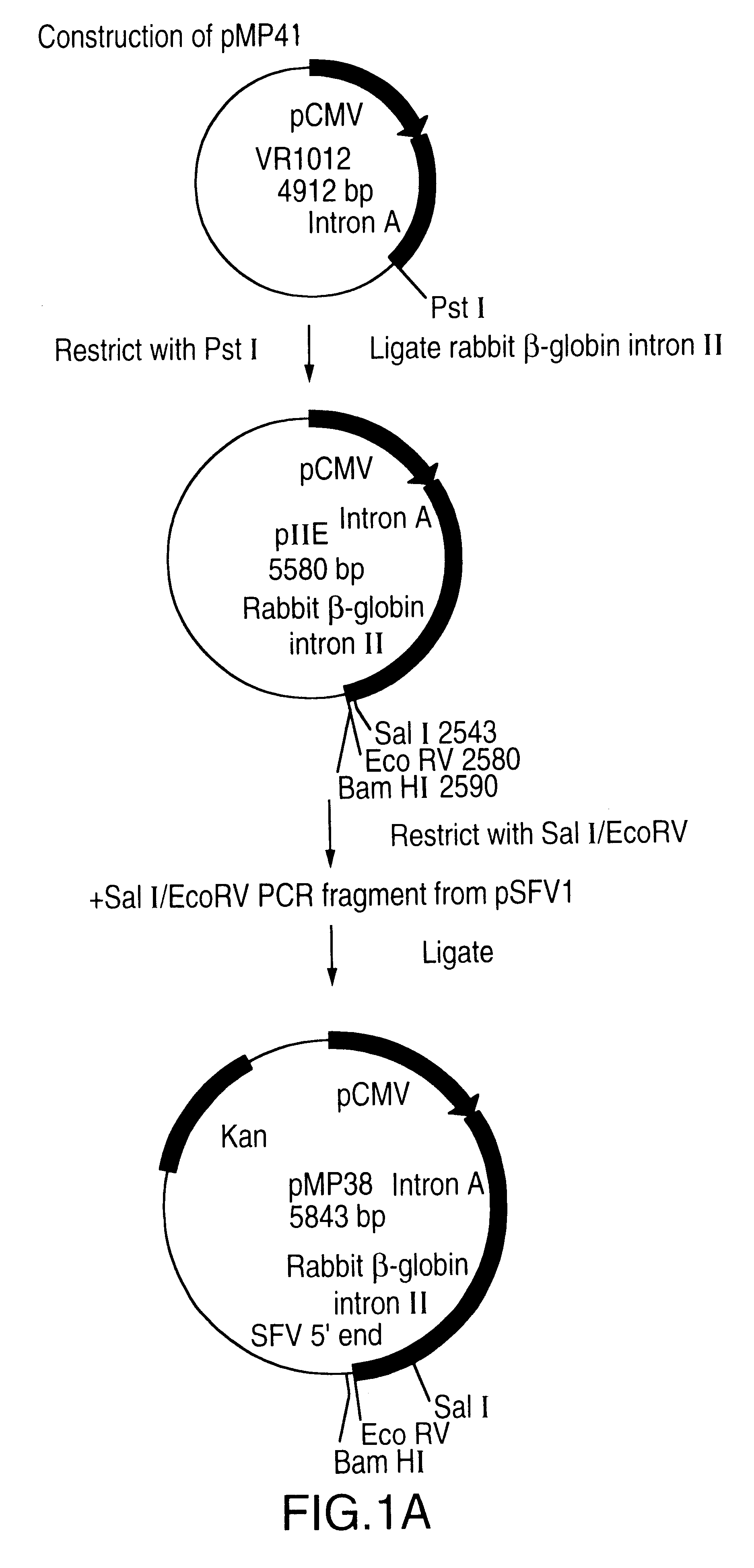
A vector comprising a first DNA sequence which is complementary to at least part of an alphavirus RNA genome and having the complement of complete alphavirus DNA genome replication regions, a second DNA sequence encoding a paramyxovirus protein, particularly a respiratory syncytial virus fusion (RSV F) protein or a RSV F protein fragment that generates antibodies that specifically react with RSV F protein, the first and second DNA sequences being under the transcriptional control of a promoter is described. Such vector may be used to produce an RNA transcript which may be used to immunize a host, including a human host, to protect the host against disease caused by paramyxovirus, particularly respiratory syncytial virus, by administration to the host.
Owner:CONNAUGHT LAB
Therapeutic compounds for blocking DNA synthesis of pox viruses
ActiveUS20100035887A1Inhibition of replicationInhibit and reduce activityBiocideTetracycline active ingredientsMedicinePoxvirus Infections
This invention provides methods of inhibiting replication of a poxvirus by contacting a poxvirus with a compound having formula I, formula XXI, formula XXXII, or formula XLI which in turn reduce, inhibit, or abrogate poxvirus DNA polymerase activity and / or its interaction with its processivity factor. Formula I, formula XXI, formula XXXII, or formula XLI can be utilized to treat humans and animals suffering from a poxvirus infection. Pharmaceutical compositions for treating poxvirus infected subjects are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
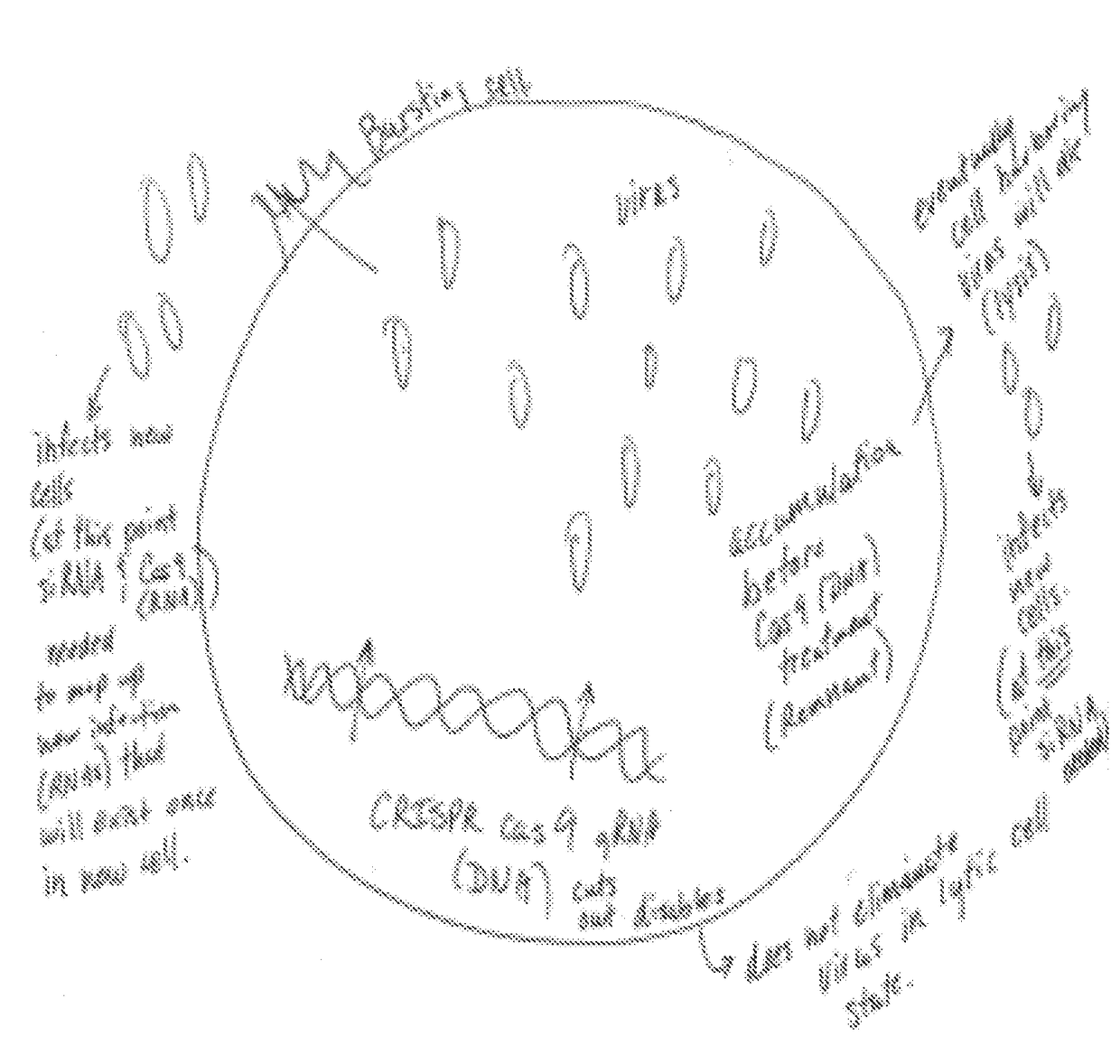
CRISPRs
A composition for treating a lysogenic virus, including a vector encoding isolated nucleic acid encoding two or more gene editors chosen from gene editors that target viral DNA, gene editors that target viral RNA, and combinations thereof. A composition for treating a lytic virus, including a vector encoding isolated nucleic acid encoding at least one gene editor that targets viral DNA and a viral RNA targeting composition. A composition for treating both lysogenic and lytic viruses, including a vector encoding isolated nucleic acid encoding two or more gene editors that target viral RNA. A composition for treating lytic viruses. Methods of treating a lysogenic virus or a lytic virus, by administering the above compositions to an individual having a virus and inactivating the virus.
Owner:EXCISION BIOTHERAPEUTICS INC
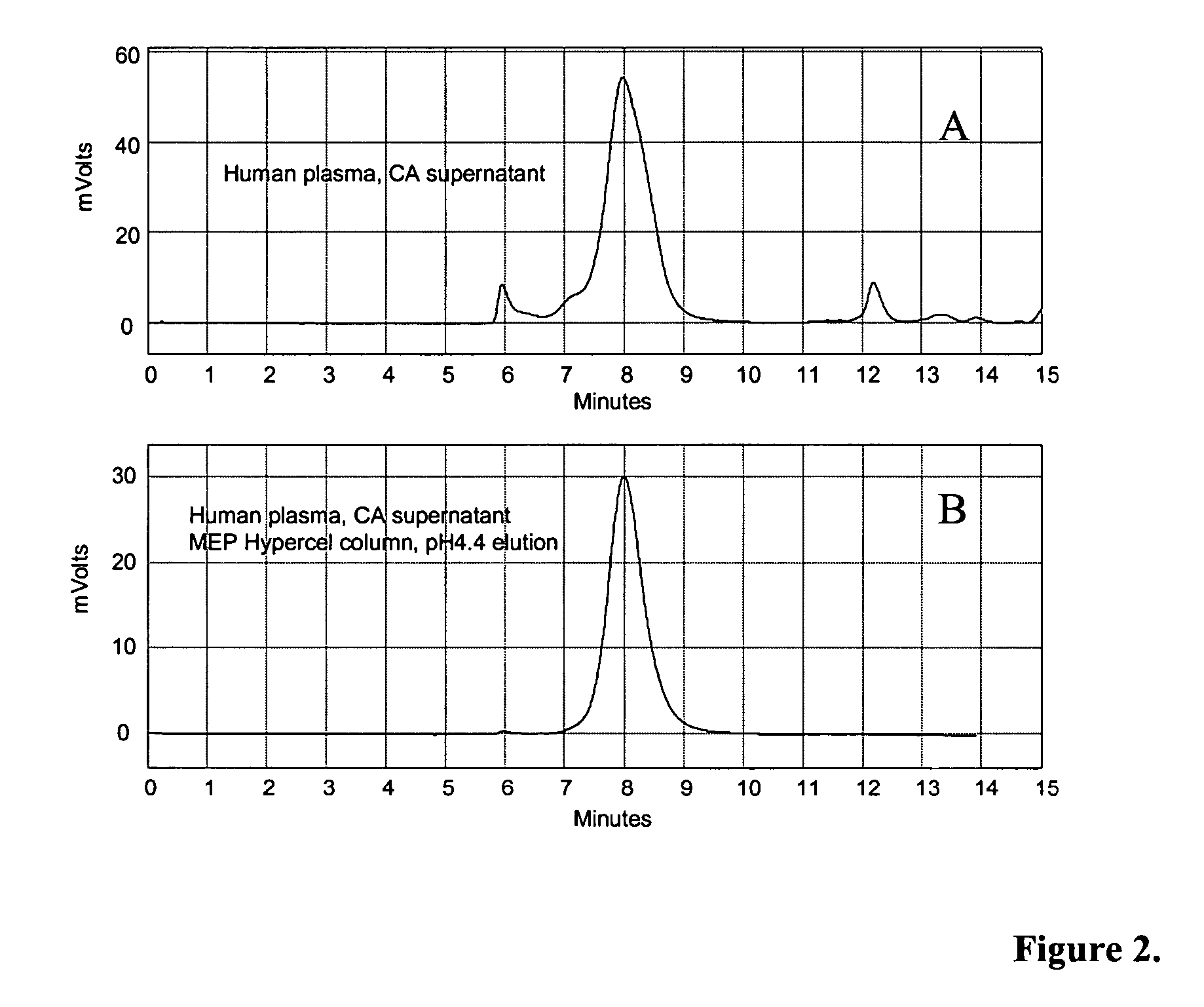
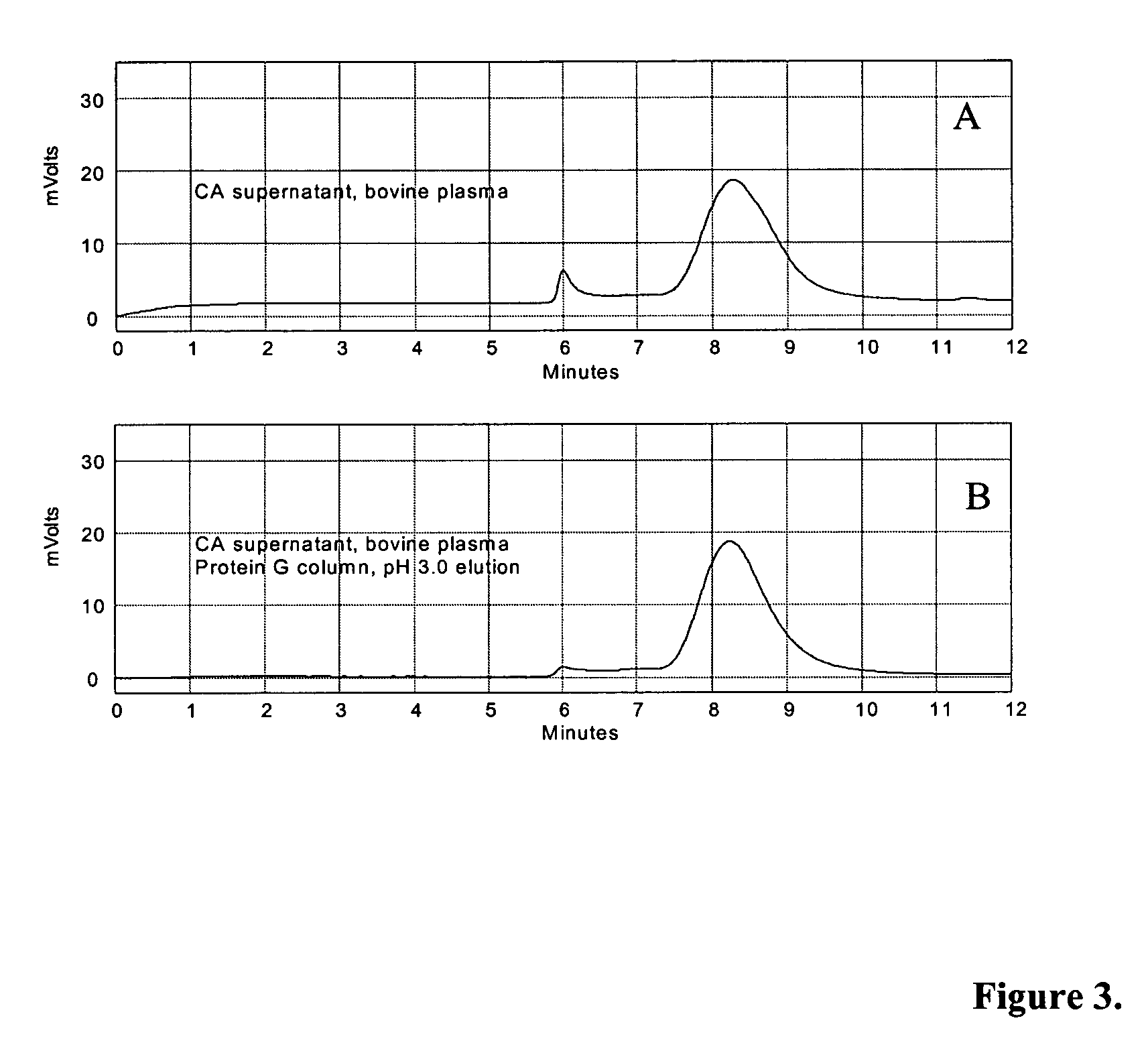
Methods for immunoglobulin purification
InactiveUS20050272917A1Serum immunoglobulinsImmunoglobulins against animals/humansImmunglobulin eBovine serum albumin
Disclosed herein are methods for purifying immunoglobulin G (IgG). The methods feature the use of particular buffers and reagents to isolate and purify human IgG or to remove host contaminating proteins, non-human or chimeric IgG, IgG dimers, IgG aggregates, bovine serum albumin, transmissible spongiform encephalopathy, DNA, viral DNA, or viral particles from a feedstock. IgG purified by the methods described herein can be used for research, diagnostic, or therapeutic purposes.
Owner:KYOWA HAKKO KIRIN CO LTD
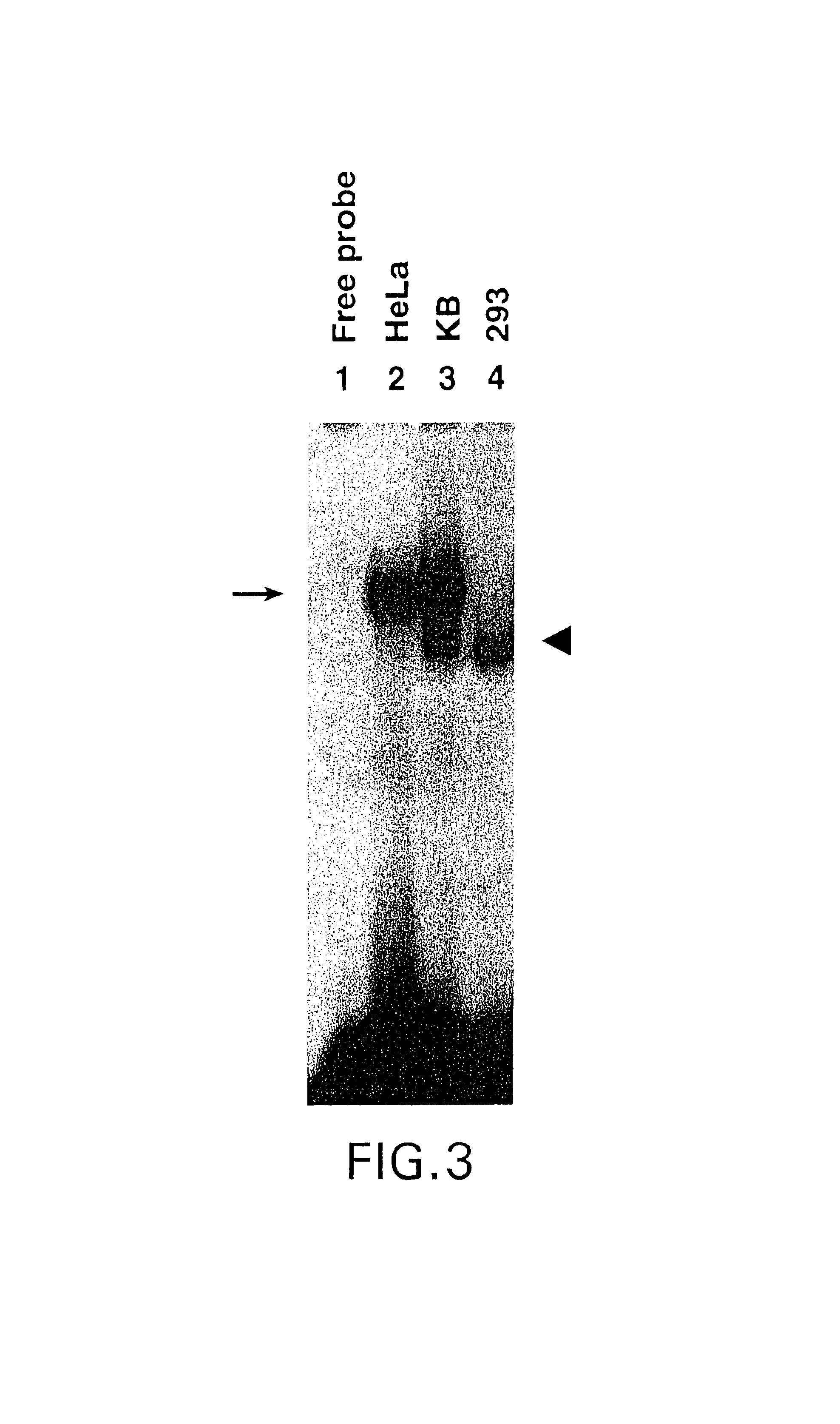
Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression
The present invention identifies a protein, designated the D-sequence-binding protein (D-BP), is phosphorylated at tyrosine residues and blocks AAV-mediated transgene expression in infected cells by inhibiting the leading strand viral DNA synthesis. More particularly, the present invention demonstrates that D-BP is phosphorylated by EGF-R protein tyrosine kinase. Methods of increasing transcription and promoting replication of transgenes exploiting this information are disclosed herein.
Owner:ADVANCED RES TECH INST
Kit for extracting DNA/RNA of virus through magnetic bead method and using method
The invention discloses a kit for extracting DNA / RNA of a virus by utilizing magnetic beads and a using method. The kit comprises the following eight components: an extracting solution I (cracking), an extracting solution II (binding), an extracting solution III (scrubbing), an extracting solution IV (scrubbing), an extracting solution V (eluting), magnetic bead suspension, a protease K working solution and a nucleic acid settling agent. The kit extraction method comprises the following steps: cracking, binding, scrubbing and eluting. The kit and the extraction method can be used for extracting the DNA / RNA of the viruses of different types of samples, the impurities such as proteins and lipids in the samples are effectively removed, the extracted nucleic acid is high in purity and complete in fragments, and the extraction process is safe and non-toxic.
Owner:宝瑞源生物技术(北京)有限公司
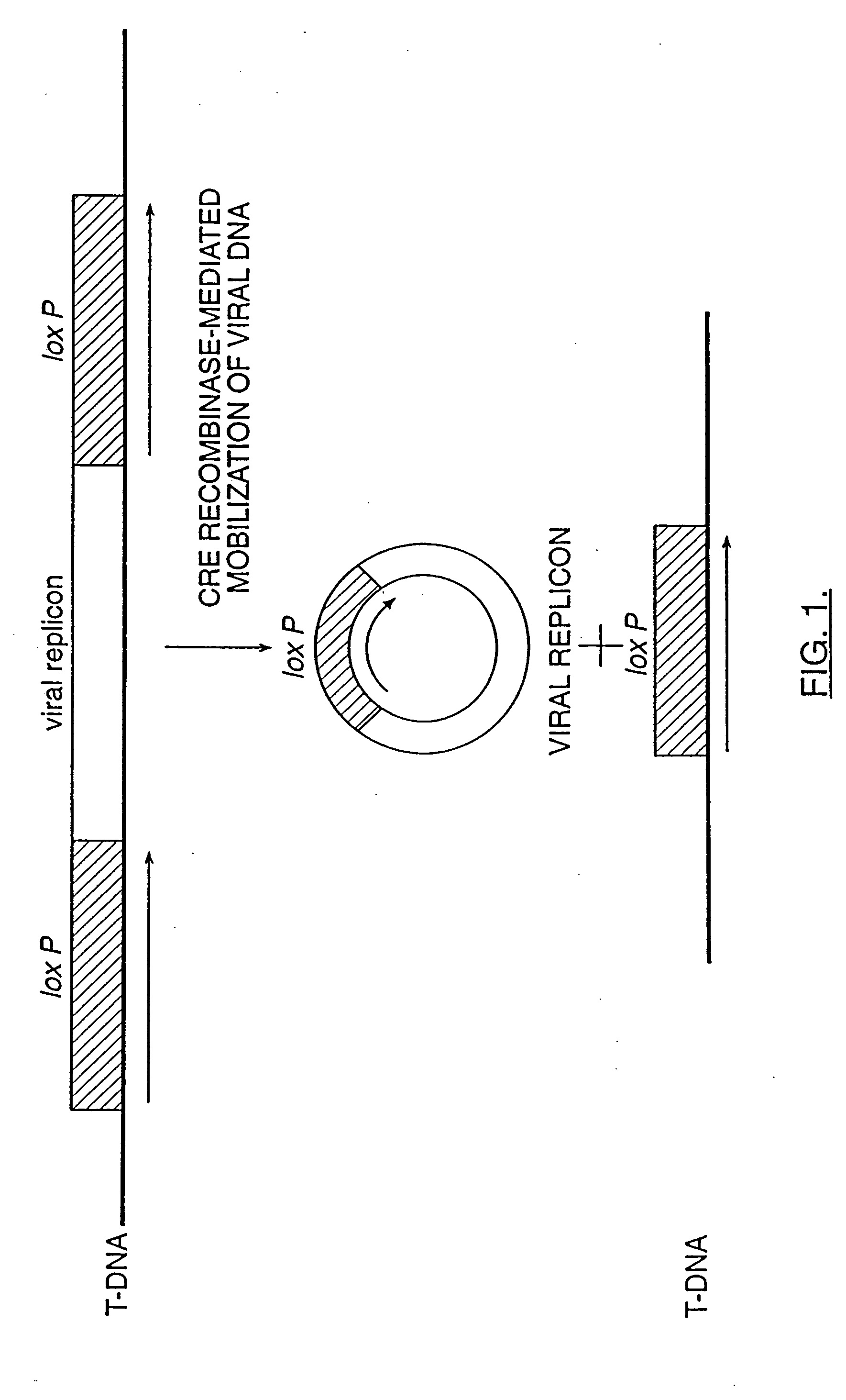
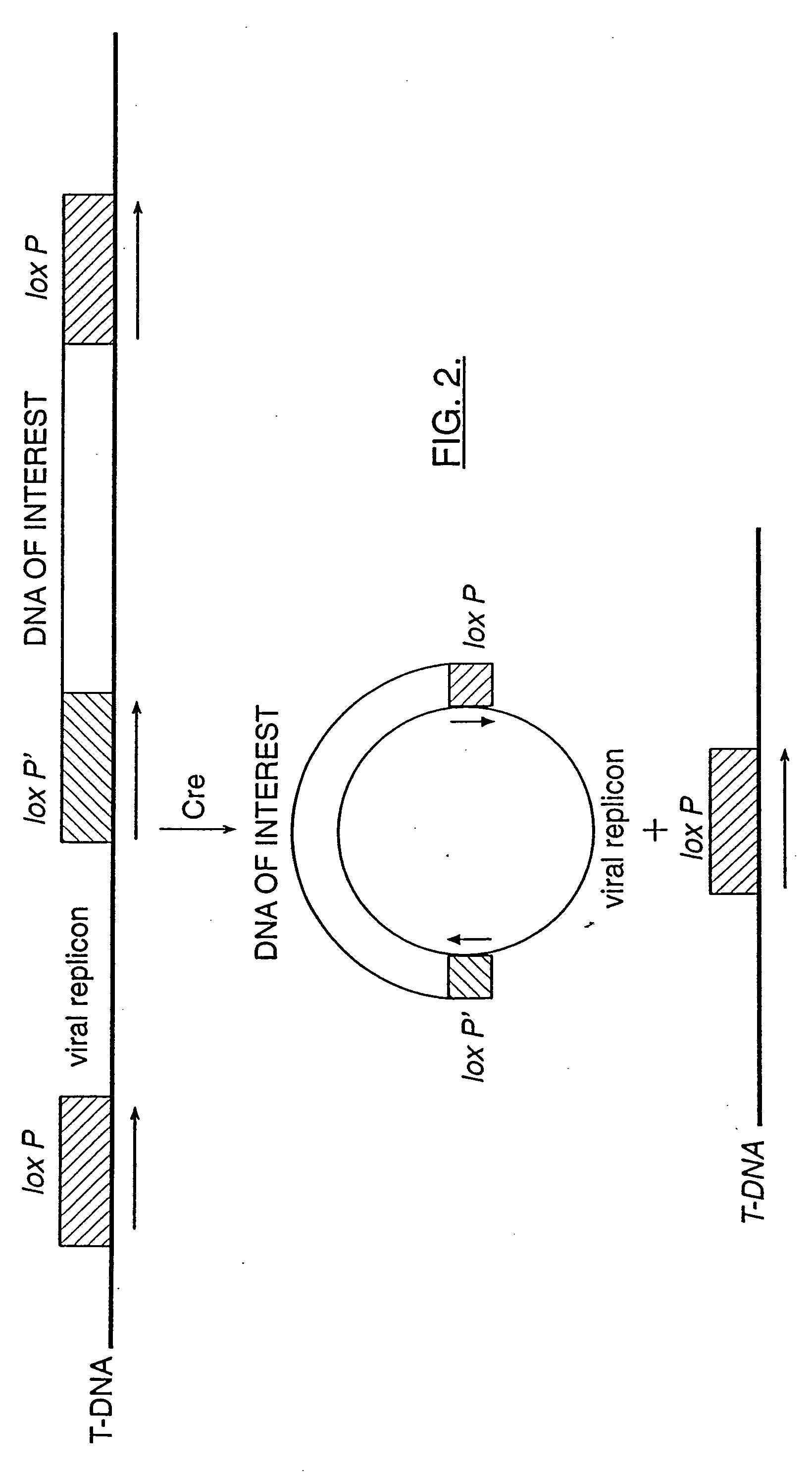
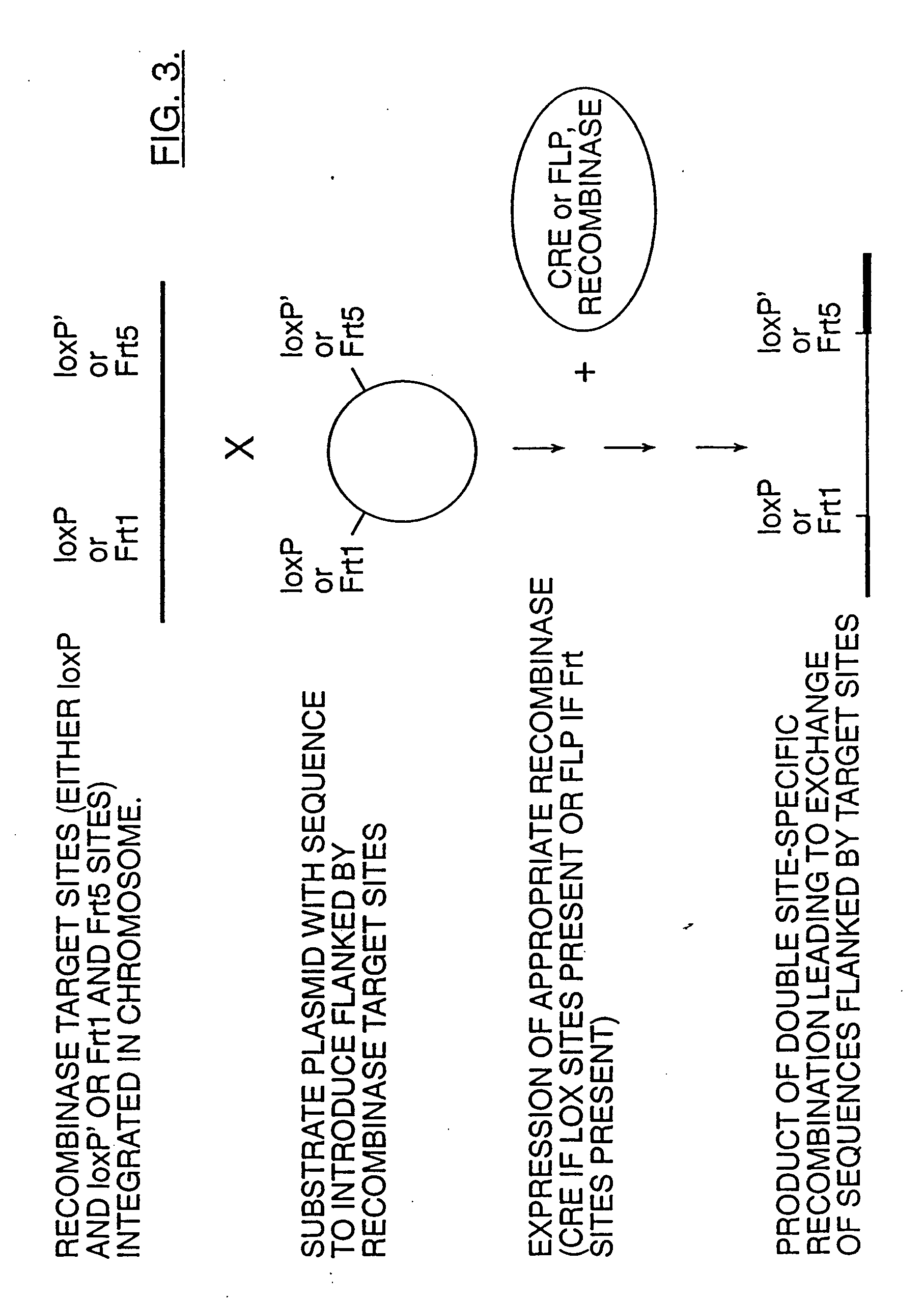
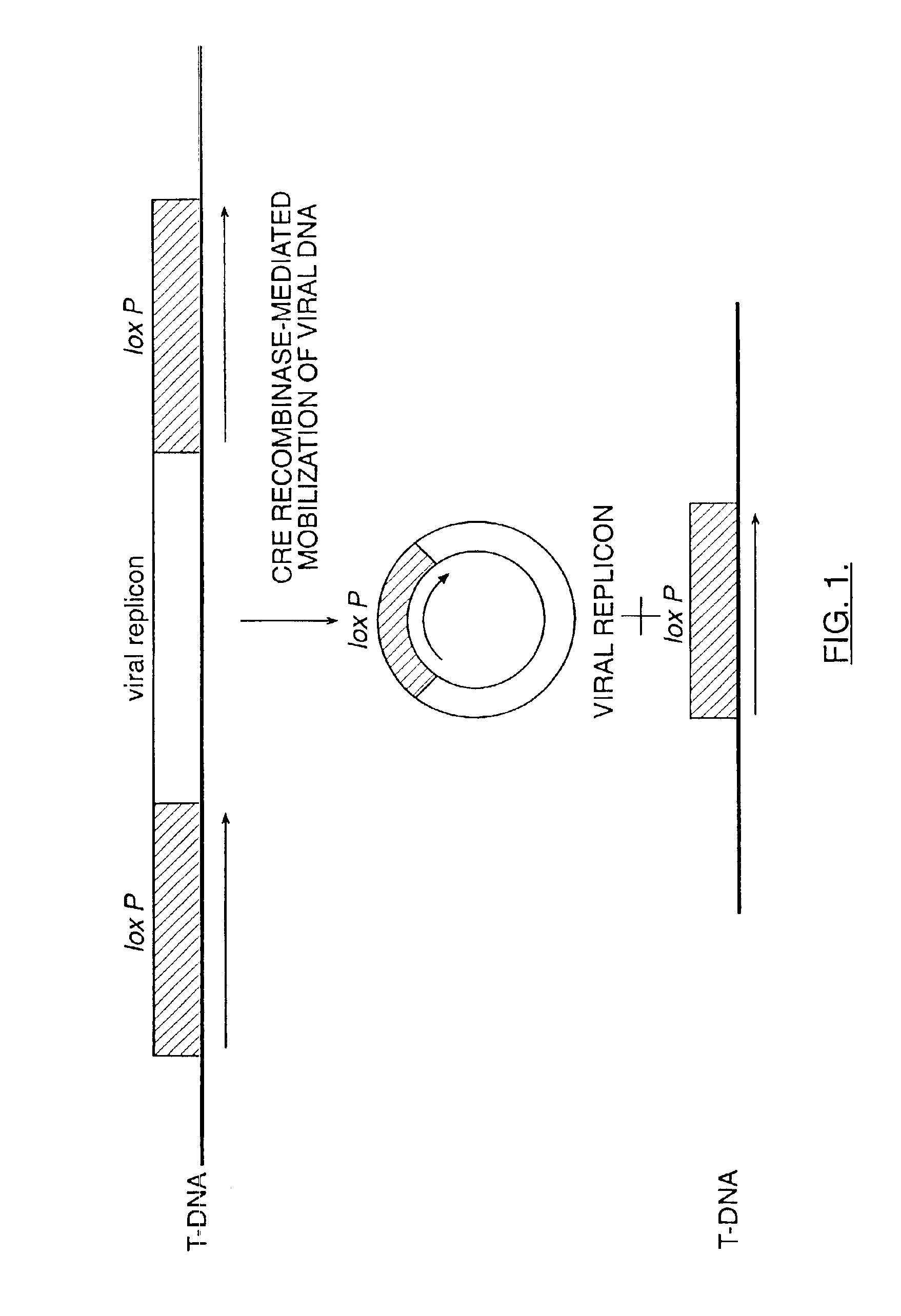
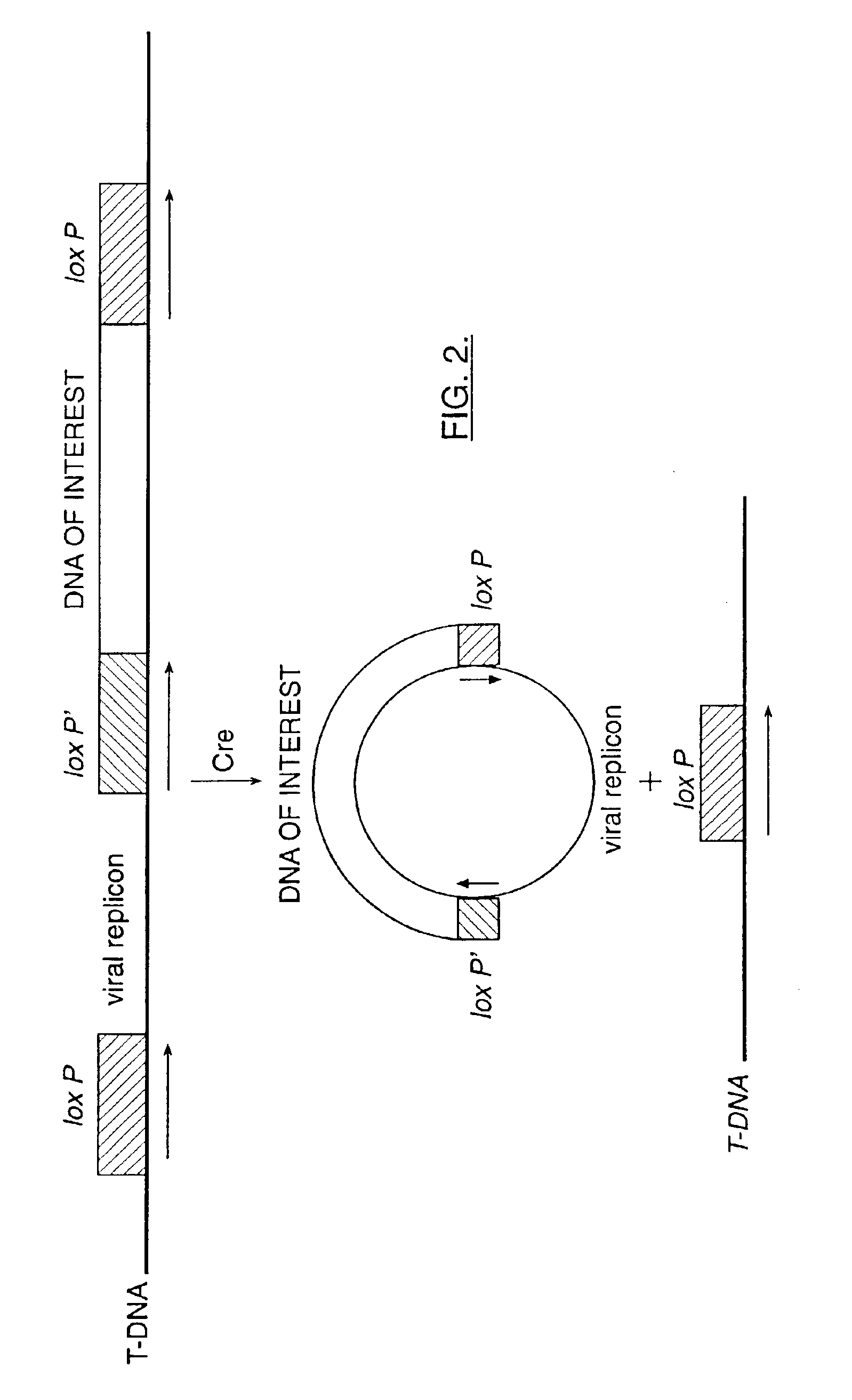
Mobilization of viral genomes from T-DNA using site-specific recombination systems
InactiveUS20060253939A1Easy constructionSimplifying stable maintenanceBacteriaSugar derivativesSite-specific recombinationPlant cell
The invention relates to methods and compositions for site-specific recombinase-mediated mobilization of viral replicons and associated DNAs of interest from T-DNA. The methods of the invention comprise Agrobacterium-mediated transfer of T-DNA to a plant cell, wherein the T-DNA contains a viral replicon flanked by directly repeated target sites for a site-specific recombinase and optionally a DNA of interest linked to the viral replicon. The DNA of interest may also contain a non-identical target site for the recombinase. An expression cassette for the site-specific recombinase is present on the T-DNA or the plant genome, or is transiently introduced into the plant cell. Expression of the site-specific recombinase in the plant cell results in excision of the viral replicon and the associated DNA of interest. The viral replicon and DNA of interest are then replicated to high copy number in the host plant cell. The compositions of the invention comprise nucleic acids, such as T-DNAs containing a viral DNA flanked by directly repeated target sites for a site-specific recombinase. The nucleic acids of the invention may additionally contain expression cassettes encoding the cognate site-specific recombinase for the target sites flanking the viral genome. The compositions of the invention further comprise Agrobacterium containing the nucleic acids of the invention. The compositions and methods of the invention have use in increasing the efficiency of agroinfection, providing high copy numbers of a DNA of interest for transient expression or for integration into a plant chromosome, and in simplifying the construction and stable maintenance of vectors for agroinfection and transformation.
Owner:PIONEER HI BRED INT INC
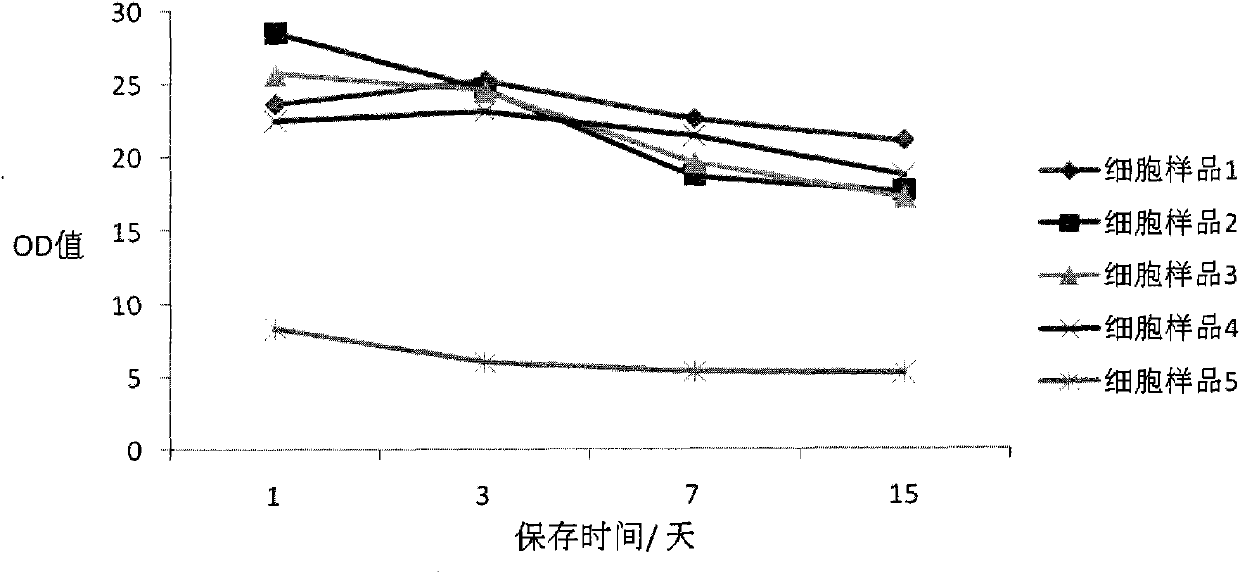
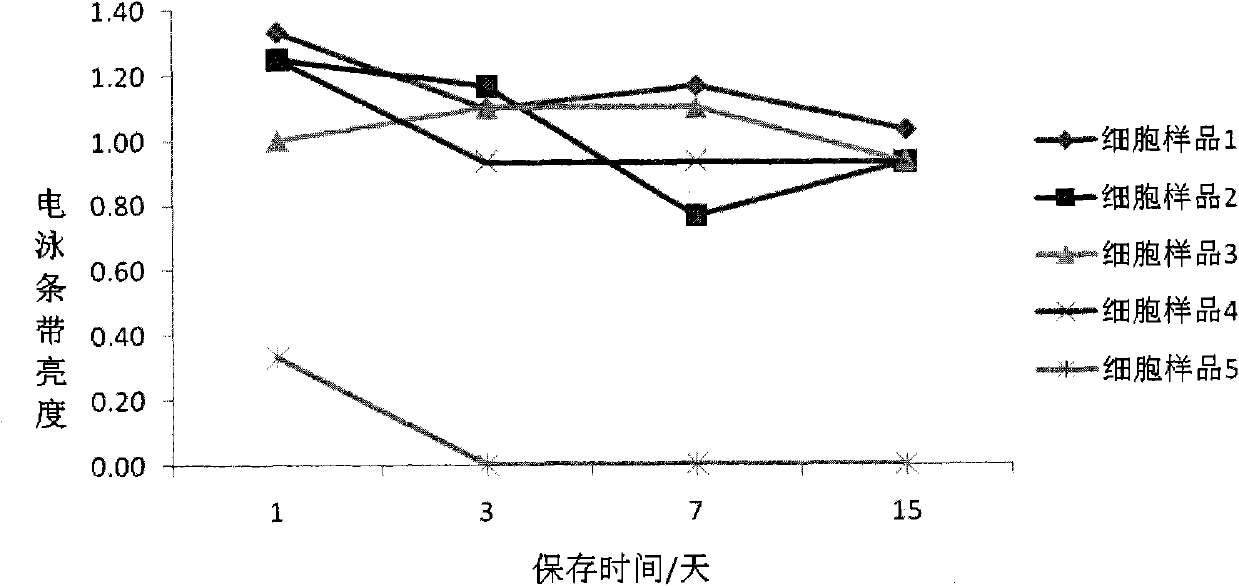
Cell preserving fluid and preparation method and use thereof
ActiveCN101999343AAvoid degradationConducive to follow-up detectionMicrobiological testing/measurementDead animal preservationDisulfide bondChemistry
The invention belongs to the field of cell biology, relating to a cell preserving fluid and a preparation method and use thereof. Concretely, the cell preserving fluid comprises a fixing agent, a fixing agent assistant, an anticoagulant, a cushion fluid and an ionic strength maintenance agent. The cell preserving fluid is characterized in that the content of the anticoagulant is 0.01% to 1.5%(w / w); the content of the ionic strength maintenance agent is 0.01% to 1%(w / w); and the cell preserving fluid does not contain a disulfide bond open reagent. The cell preserving fluid can effectively preserve cell DNA (deoxyribonucleic acid) and virus DNA infecting the cell, is convenient to collect the cast-off cells and extract DNA and can give attention to storage of the cell structure. The invention also relates to the preparation method and the use of the cell preserving fluid. The invention also relates to a kit and a method for detecting HPV (Human Papilloma Virus) or HPV DNA.
Owner:BGI GENOMICS CO LTD
Using method for extracting virus DNA by using micro-nucleic acid releasing agent
InactiveCN104962553AGuaranteed cracking efficiencySubsequent experiment impactDNA preparationBiologyPollution
The invention discloses a method for extracting virus DNA contained in a biological sample by utilizing a micro-nucleic acid releasing agent, belongs to the technical field of molecular biology, and particularly relates to a reagent for extracting virus DNA by utilizing the micro-nucleic acid releasing reagent and a using method thereof. The micro-nucleic acid releasing agent cracks the virus DNA contained in the biological sample (a serum or a plasma) and can more effectively ensure the cracking and release efficiency through the assistance of a release promoting agent; the micro-nucleic acid releasing agent completely releases nucleic acid and also has the function of closing various factor substances, namely protein, drugs, hemolyzed blood and the like which are contained in a sample and have interference effects on PCR amplification, so that false negative in a detection process is prevented. The method disclosed by the invention is simple, convenient, flexible, fast and accurate in sample extraction, prevents the pollution and nucleic acid loss which are caused by repeated centrifugation and elution uncapping, and realizes the 'one-step' and 'one-room' operation of PCR extraction.
Owner:宝瑞源生物技术(北京)有限公司
Mobilization of viral genomes from T-DNA using site-specific recombination systems
InactiveUS7179599B2Simplifying construction and stable maintenanceImprove efficiencyBacteriaAntibody mimetics/scaffoldsSite-specific recombinationPlant cell
The invention relates to methods and compositions for site-specific recombinase-mediated mobilization of viral replicons and associated DNAs of interest from T-DNA. The methods of the invention comprise Agrobacterium-mediated transfer of T-DNA to a plant cell, wherein the T-DNA contains a viral replicon flanked by directly repeated target sites for a site-specific recombinase and optionally a DNA of interest linked to the viral replicon. The DNA of interest may also contain a non-identical target site for the recombinase. An expression cassette for the site-specific recombinase is present on the T-DNA or the plant genome, or is transiently introduced into the plant cell. Expression of the site-specific recombinase in the plant cell results in excision of the viral replicon and the associated DNA of interest. The viral replicon and DNA of interest are then replicated to high copy number in the host plant cell. The compositions of the invention comprise nucleic acids, such as T-DNAs containing a viral DNA flanked by directly repeated target sites for a site-specific recombinase. The nucleic acids of the invention may additionally contain expression cassettes encoding the cognate site-specific recombinase for the target sites flanking the viral genome. The compositions of the invention further comprise Agrobacterium containing the nucleic acids of the invention. The compositions and methods of the invention have use in increasing the efficiency of agroinfection, providing high copy numbers of a DNA of interest for transient expression or for integration into a plant chromosome, and in simplifying the construction and stable maintenance of vectors for agroinfection and transformation.
Owner:PIONEER HI BRED INT INC
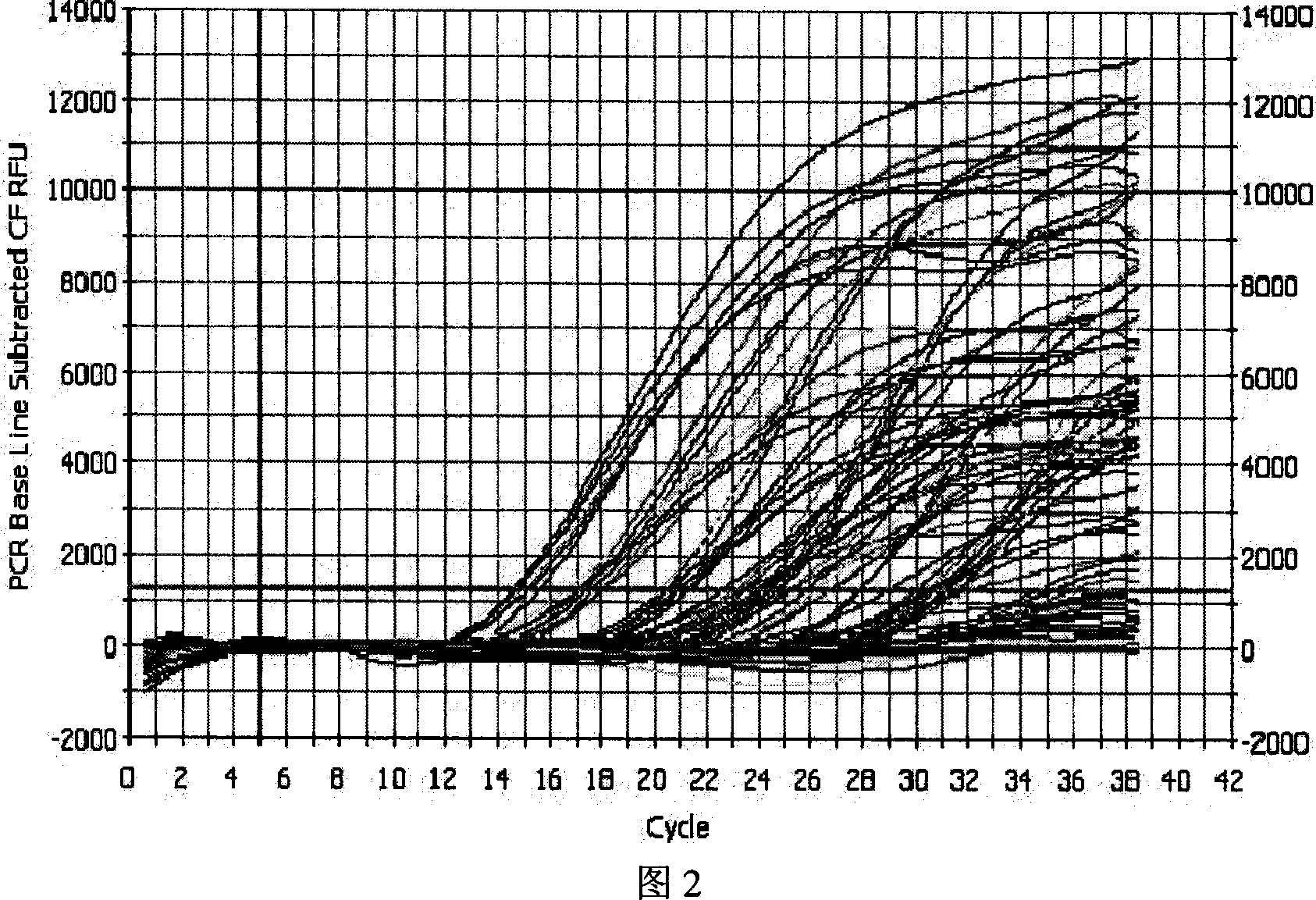
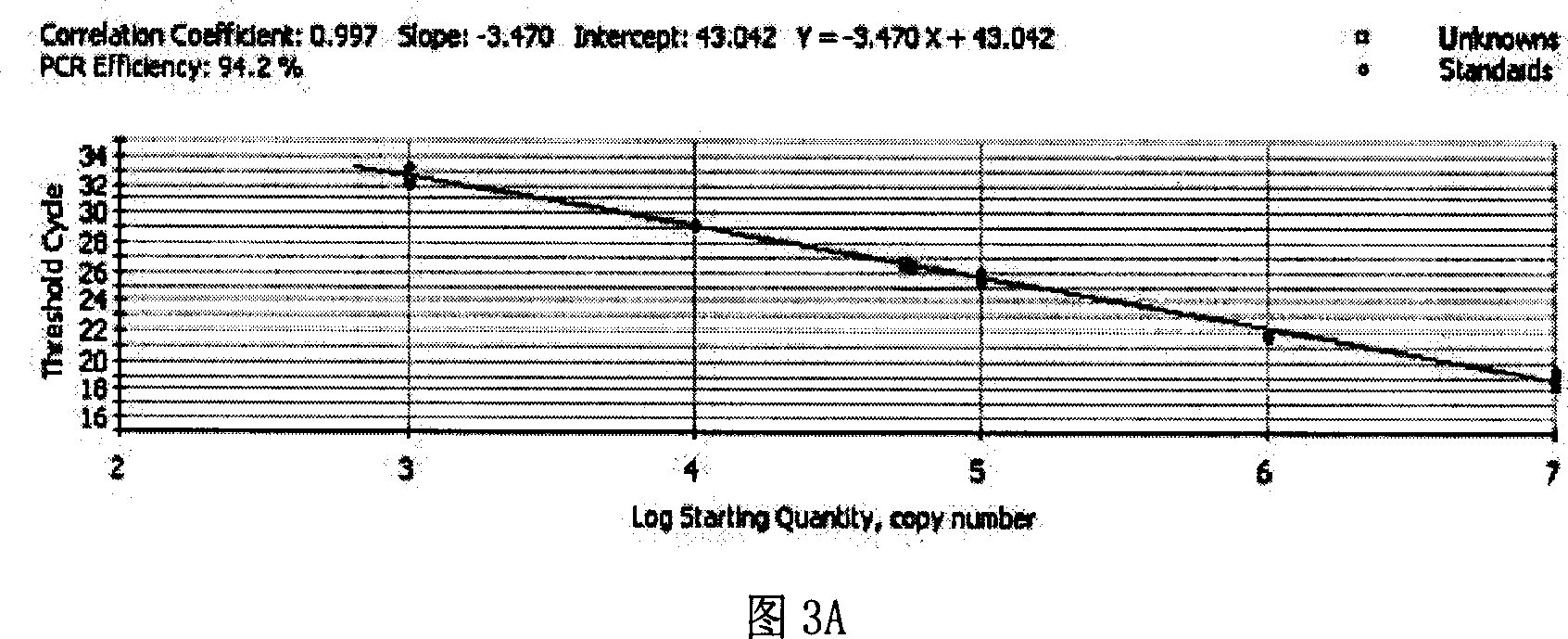
Fluorescent quantitative PCR detecting method for hepatitis B virus and special reagent kit
ActiveCN1944673AReduce extraction timeEasy to operateMicrobiological testing/measurementBiological testingBiotechnologyAntigen
The present invention is fluorescent quantitative HBV PCR detecting method and special reagent kit, and belongs to the field of biotechnology. The method includes comparing three pairs of primer and probe of HBV surface antigen, kernel antigen and E antigen region to screen out one pair of primer and probe with best proliferation effect; comparing the variation in HBV copy number during fluorescent quantitative PCR proliferation of the serum DNA samples extracted in different methods to optimize the HBV extracting method, and performing quantitative PCR detection in optimized primer and probe and in simple virus DNA extracting method. The present invention has high sensitivity, high specificity, low cost, short time, accurate detection and other advantages. The present invention may be used clinically and in scientific research.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Method for producing porcine parvovirus antigen and its product
ActiveCN102382845AImprove immune activityReduce manufacturing costGenetic material ingredientsAntiviralsAntigenBaculovirus expression
The invention discloses a method for producing a porcine parvovirus antigen and its product. The method comprises the following steps: porcine parvovirus capsid protein VP2 gene or optimized VP2 gene is cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the constructed transfer expression carrier and baculovirus DNA are carried out cotransfection to obtain recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding porcine parvovirus capsid protein; and the expressed antigen is ingathered and purified so as to obtain the porcine parvovirus antigen. The method adopts a baculovirus expression system to make safe and efficient porcine parvovirus antigen capsid particles in a domestic silkworm bioreactor; the prepared purified antigen by the method has high safety, and can be directly produced to vaccines for animal immunity. The method for producing porcine parvovirus antigen has the advantages of high expression efficiency, high immunization activity of the expressed antigen, low production cost, large scale production realization and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Methods and compositions for RNA-guided treatment of HIV infection
A method of inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus by treating the host cell with a composition comprising a Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)-associated endonuclease, and two or more different guide RNAs (gRNAs), wherein each of the at least two gRNAs is complementary to a different target nucleic acid sequence in a long terminal repeat (LTR) in the proviral DNA, and inactivating the proviral DNA. A composition for use in inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus including isolated nucleic acid sequences comprising a CRISPR-associated endonuclease and a guide RNA, wherein the guide RNA is complementary to a target sequence in a human immunodeficiency virus.
Owner:TEMPLE UNIVERSITY
Primer, probe and kit for detecting infectious bovine rhinotracheitis viruses
ActiveCN104862419AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesForward primerInfectious bovine rhinotracheitis virus
The invention relates to a primer, a probe and a kit for detecting infectious bovine rhinotracheitis viruses, and discloses a primer and probe combination used for detecting the infectious bovine rhinotracheitis viruses according to an RPA technology, the forward primer sequence of the primer and probe combination is shown as SEQ ID No.1; the reverse primer sequence is shown as SEQ ID No.2; the probe sequence is shown as SEQ ID No.3. The invention further discloses the kit for detecting the infectious bovine rhinotracheitis viruses. The primer and the probe are adopted for detection, a clinical sample is only subjected to virus DNA crude extraction and RPA isothermal amplification, the result can be displayed on a lateral flow chromatography test strip, a heat circular reaction is not needed, and amplification needs not to be performed in a PCR instrument; the primer, the probe and the kit have the advantages of being high in sensitivity, strong in specificity, simple in reaction procedure, short in detection time, and suitable for clinical field detection in a non-lab environment, and have a wide application prospect.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Construction method of recombination double expressions cultivated silkworm polyhedrosis baculovirus containing SOD gene
A method for constructing a recombinant double-expression silkworm polyhedral baculiform virus which contains the SOD gene comprises that the general RNA of the silkworm is extracted and an MnSOD primer is designed; a single-stranded cDNA is synthesized and an MnSOD gene is synthesized; the MnSOD gene is connected with a p FastBacHTa to transform XL-Blue cells; and the recombinant donor plasmid p FastBacHTa / MnSOD can be extracted; the baculiform virus DNA of the silkworm is taken as the template to synthesize the silkworm polyhedral gene and the gene is cloned to the p FastBac Dual plasmid; the MnSOD gene is inserted under the P10 promoter of the double-expression plasmid to construct the double-expression vector: Bm polyhedrin pFB dual / MnSOD; after the transinfection of the Bm polyhedrin pFB dual / MnSOD, the double-expression virus DNA which contains the MnSOD gene and the polyhedral gene is distilled; the DNA is transduced into the silkworm culture cell of transinfection with the mediation of transinfection reagent lipidosome to obtain the recombinant virus. Virus particles which can feed and infect the silkworm is provided and the object gene MnSOD is expressed by the silkworm and large-scale and industrial production is realized.
Owner:ZHEJIANG UNIV
Apparatus for and method of processing biological samples
ActiveUS20130040376A1Bioreactor/fermenter combinationsHeating or cooling apparatusWestern blotGenomic DNA
The present invention provides systems, devices, apparatuses and methods for automated bioprocessing. Examples of protocols and bioprocessing procedures suitable for the present invention include but are not limited to: immunoprecipitation, chromatin immunoprecipitation, recombinant protein isolation, nucleic acid separation and isolation, protein labeling, separation and isolation, cell separation and isolation, food safety analysis and automatic bead based separation. In some embodiments, the invention provides automated systems, automated devices, automated cartridges and automated methods of western blot processing. Other embodiments include automated systems, automated devices, automated cartridges and automated methods for separation, preparation and purification of nucleic acids, such as DNA or RNA or fragments thereof, including plasmid DNA, genomic DNA, bacterial DNA, viral DNA and any other DNA, and for automated systems, automated devices, automated cartridges and automated methods for processing, separation and purification of proteins, peptides and the like.
Owner:LIFE TECH CORP
Method of Separating Target DNA from Mixed DNA
The present invention relates to methods of separating target DNA from mixed DNA in a sample. In some embodiments, the target DNA may be viral DNA, prokaryotic DNA, fungal DNA or combinations thereof. In some embodiments the mixed DNA includes target DNA and non-target DNA.
Owner:CANON US LIFE SCIENCES INC
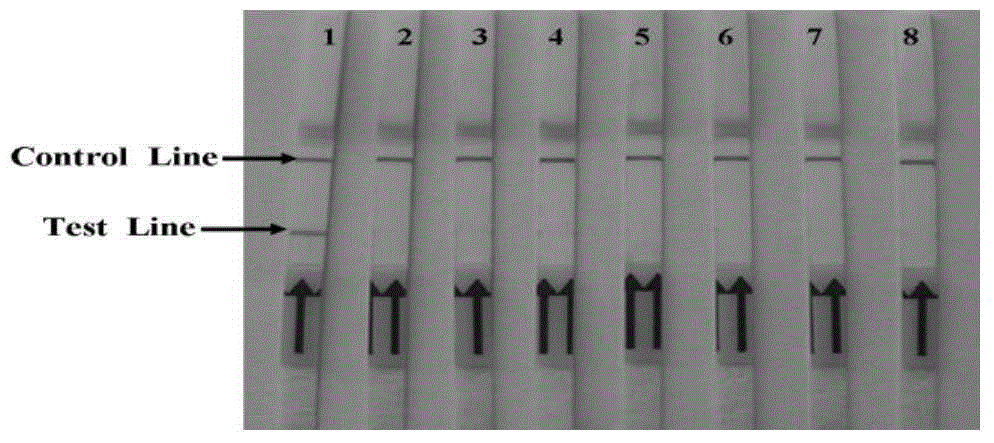
Porcine circovirus 2 LAMP detection kit and detecting method
InactiveCN101586169AMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementSensitivity testPorcine circovirus
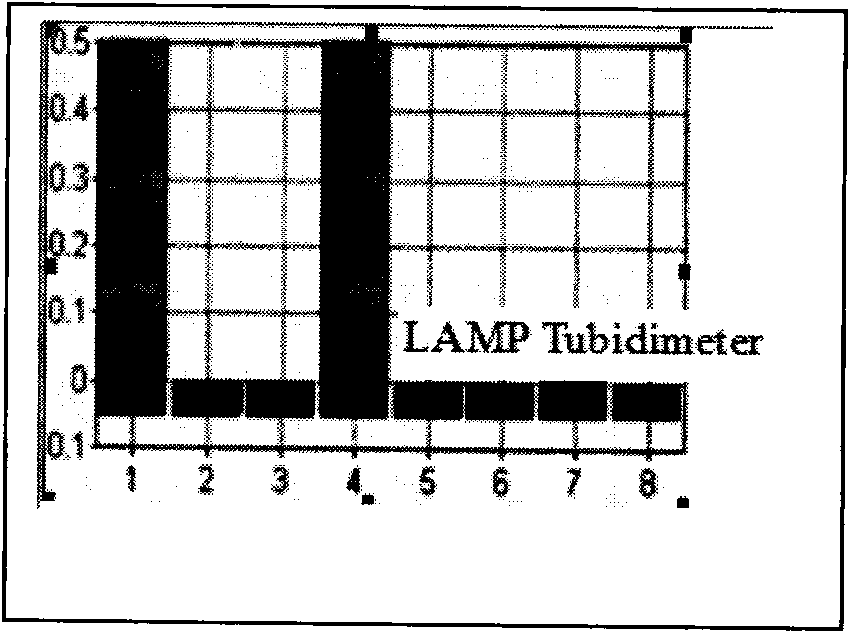
The invention relates to a porcine circovirus 2 LAMP detection kit and detecting method. Based on the PCV2 gene sequence disclosed by GenBank, four PCV2 LAMP primers are designed in the sequence conservative region; the set PCV2 LAMPreaction system is adopted to conduct the LAMP reaction by taking the PCV2 HuB strain, PCV2LN strain virus DNA as a formwork. the LA-320 LAMP Tubidimeter is used for analyzing the added SYBRgreenI developer in the reaction process and after the reaction to judge the result, the result shows that the PCV2DNA has high-efficiency specificity amplification in LA-320 LAMP Tubidmeter at 63 DEG for 30 min, the SYBRgreenI developer is added to judge the result is consistent with the result displayed by the instrumental analysis. the sensitivity test proves that in the method, the 50ngPCV2 DNA formwork is diluted by 10, the efficient amplification still can be conducted, thus the method has high susceptibility. It shows that the method is special, simple and rapid, and is suitable for the PCV2 detecting work by the specificity test and the LAMP detecting of PVC2nucleic acid clinical sample.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
CONSTRUCTION OF ONCOLYTIC HERPES SIMPLEX VIRUSES (oHSV) OBLIGATE VECTOR AND CONSTRUCTS FOR CANCER THERAPY
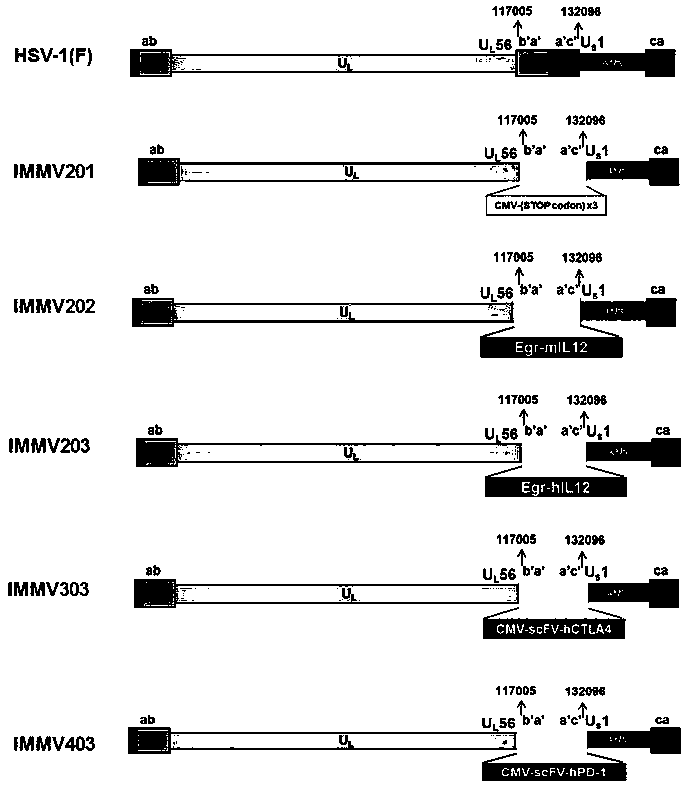
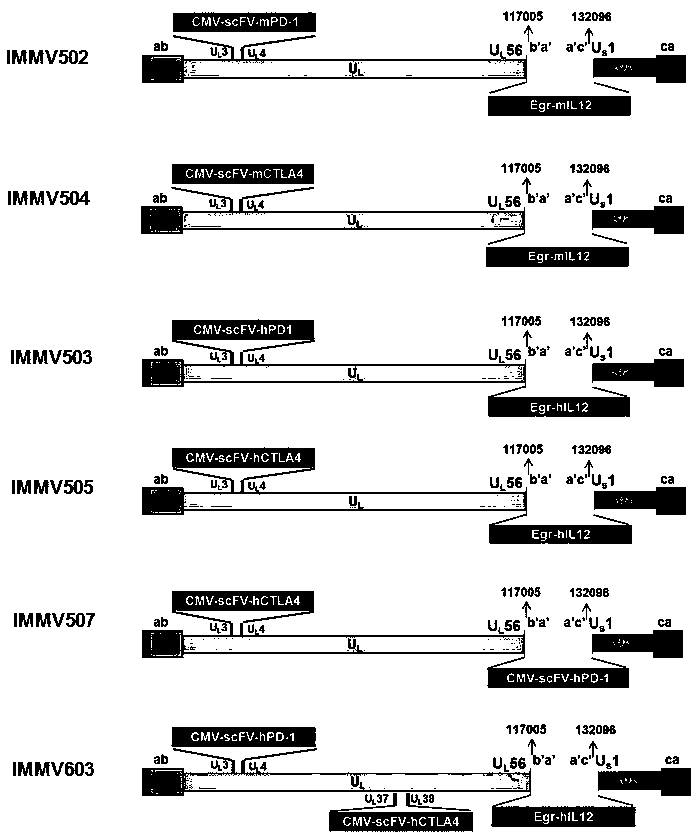
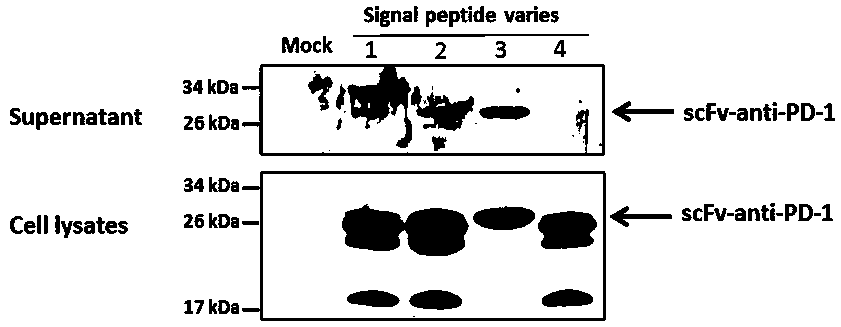
ActiveCN108350468AGenetic material ingredientsViral/bacteriophage medical ingredientsImmunotherapeutic agentNucleic acid sequencing
An obligate oHSV vector comprising modified viral DNA genome is provided. A recombinant oHSV-1 construct comprising the obligate oHSV vector and a heterologous nucleic acid sequence encoding an immunostimulatory and / or immunotherapeutic agent is also provided. Compositions comprising the recombinant oHSV-1 construct can be used for treating cancers.
Owner:IMMVIRA CO LTD
Alphavirus vectors for paramyxovirus vaccines
InactiveUS6475780B1Reduce doseLess timeSsRNA viruses negative-senseOrganic active ingredientsDiseaseF protein
A DNA vector comprises a first DNA sequence which is complementary to at least part of an alphavirus RNA genome and having the complement of complete alphavirus DNA genome replication regions, and a second DNA sequence encoding a paramyxovirus protein, particularly a respiratory syncytial virus fusion (RSV F) protein or a RSV F protein fragment that generates antibodies that specifically react with RSV F protein, the first and second DNA sequences being under the transcriptional control of a promoter, preferably a cytomegalovirus promoter, which may include Intron A. Such vectors also contain a further nucleotide sequence located between the promoter sequence and the alphavirus sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such DNA vectors may be used to immunize a host against disease caused by infection with RSV or other paramyxovirus, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purposes. Such vectors also may be used to produce antibodies for detection of RSV or other paramyxovirus infection in a sample.
Owner:AVENTIS PASTUER LTD
Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors
The invention encompasses a series of bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Preparation method of rabbit hemorrhagic fever virus empty capsid antigen
ActiveCN102304529APromote safe productionReduce consumptionViral antigen ingredientsVirus peptidesAntigenBombyx mori
The invention relates to the field of genetic engineering and particularly relates to a preparation method of a rabbit hemorrhagic fever virus empty capsid antigen. The method provided by the invention comprises the following steps: 1) constructing a rhabdovirus transfer carrier containing a rabbit hemorrhagic fever virus capsid protein VP60 gene or an optimized gene, wherein codon optimization is performed according to the codon frequency of bombyx mori; 2) performing cotransfection on the constructed transfer expression carrier and DNA (deoxyribonucleic acid) of rhabdovirus so as to carry out homologous recombination or transposition to further obtain the recombinant rhabdovirus; 3) infecting the recombinant rhabdovirus with the host cells of an insect; and 4) culturing the infected host of the insect to express the corresponding rabbit hemorrhagic fever virus empty capsid antigen, and harvesting and purifying the expressed antigen. By adopting the method provided by the invention, the production cost of the rabbit hemorrhagic fever virus empty capsid antigen can be greatly reduced, and the method has a plurality of advantages of safety, high efficiency, low energy consumption, low cost and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Building method of silkworm BmNPV Polh+Bac-to-Bac rhabdovirus expression system
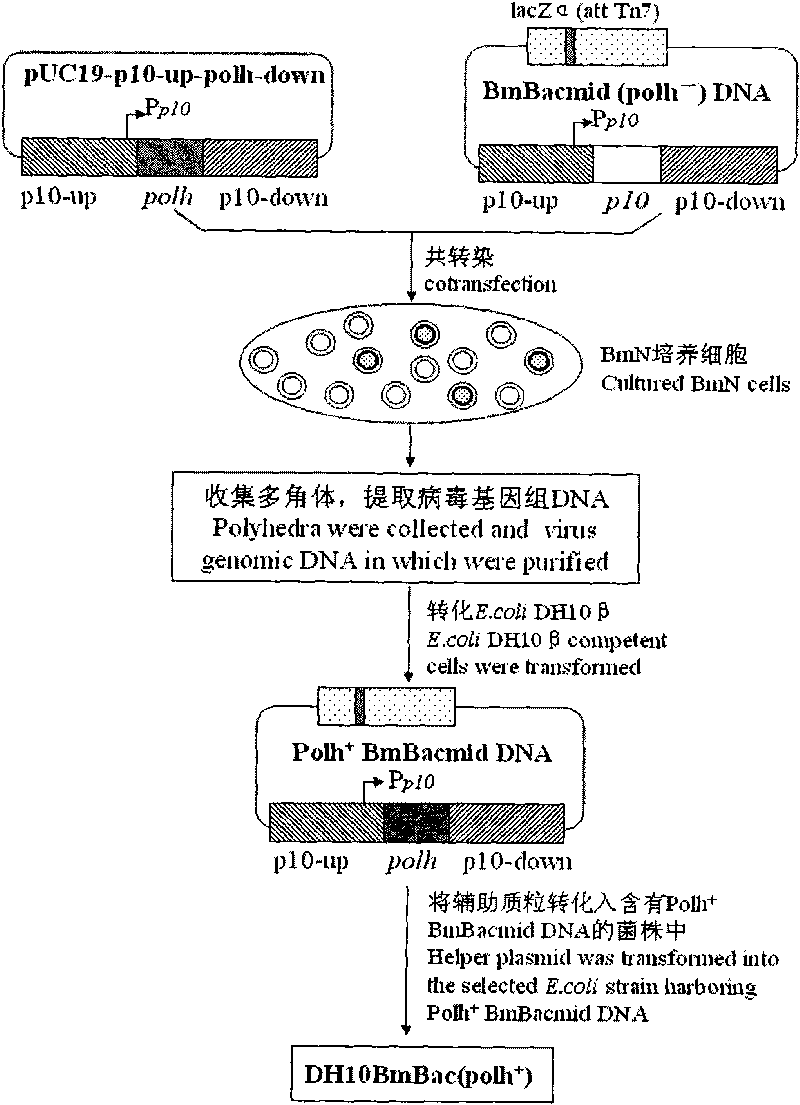
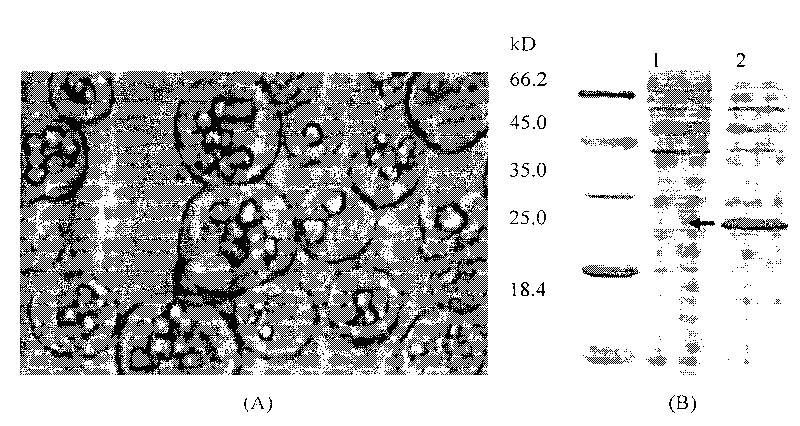
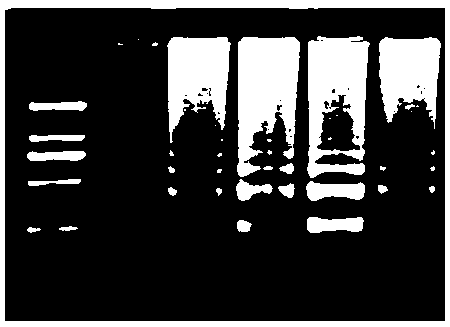
The invention discloses a building method of a silkworm BmNPV Polh+Bac-to-Bac rhabdovirus expression system, comprising: taking silkworm wild type BmNPV genome DNA as the template; respectively taking P10-upF / P10-upB and P10-downF / P10-down B as a primer PCR for amplification to obtain p10-up and p10-down; after processed, inserting into BamHI-PstI-HindIII locus in pUC 19 to obtain recombinant plasmid pUC19-p10-up-down; using pUC-19-p10-up-polh-down, liposome lipofectin and MilliQ H2O to prepare DNA-Lipofectin mixed liquor; adding the mixed liquor into BmN cells for cultivating; collecting transfection cell supernatant; inoculating BmN cells, and recovering a polyhedral body; extracting virus DNA from the polyhedral body and electrically converting DH10 beta competent cells; screening locus ceruleus and cultivating; after cultivating PCR positive bacterial plaque, extracting macro-molecular DNA to transfect to the BmN cells; separating to obtain helper plasmids from DH10Bac culture bacteria of AcMNPV Bac-to-Bac; converting the helper plasmids into E.coli DH10 beta containg Ploh+BmBacmid; and screening the DH10 beta bacterial strain of the helper plasmid containg Ploh+BmBacmid. The invention can produce recombinant virus capable of infecting by eating with mouth, and recombinant virus does not need to infect by intracutaneous inoculation, thus improving the production efficiency of the silkworm rhabdovirus expression system.
Owner:ZHEJIANG UNIV
Kit for detecting proviral DNA (Deoxyribonucleic Acid) of HIV (Human Immunodeficiency Virus)
The invention discloses a kit for detecting the proviral DNA (Deoxyribonucleic Acid) of an HIV (Human Immunodeficiency Virus). An isothermal amplification reagent is placed in the kit and contains an aqueous solution of primers LTR-F3, LTR-B3, LTR-FIP and LTR-BIP, an aqueous solution of MgCl2, an aqueous solution of dNTP, a 10*betaine buffer, a 20*SYBR dye, a BstDNA polymerase and 3.9 microliters of sterile water for injection. According to the kit, after a loop-mediated isothermal amplification reaction system is successfully synthesized, the system is combined with a fluorescent dye, the SYBR Green I fluorescent dye is combined with amplified fragments along with the amplification, the fluorescence intensity is recorded by a fluorescent quantitative PCR (Polymerase Chain Reaction) instrument, and finally, the virus content is given according to the fluorescence intensity.
Owner:杭州市儿童医院
Reagent kit and method for extracting viral genome deoxyribonucleic acid (DNA) of human whole blood
InactiveCN102676498AEasy to operateImprove work efficiencyMicrobiological testing/measurementFermentationWhite Blood Cell LysisRed blood cell
The invention relates to a reagent kit and a method for extracting viral genome deoxyribonucleic acid (DNA) of human whole blood. The reagent kit and a method for extracting the viral genome DNA of the human whole blood are characterized in that the reagent kit comprises red blood cell lysis buffer, white blood cell lysis buffer, digestive juice, purifying liquid and DNA preserving liquid, adopt two types of lysis buffer to respectively process a blood sample containing virus cells, and can efficiently and thoroughly lyse various virus cells while lysing blood cells, enable virus DNA to be released and enable the virus DNA to mutually wounded with blood genome DNA to be extracted. When the reagent kit is used for extracting the virus DNA, blood plasma and blood serum of blood are not required to be separated in advance, traces of high-purity viral DNA can be extracted only by taking fresh or frozen 0.1-1ml of anti-coagulating whole blood and can be completely unlinked and amplified efficiently. The reagent kit and a method for extracting the viral genome DNA of the human whole blood is suitable for scientific research units, laboratories for molecular genetic studies and medical treatment units, blood stations and disease control centers carrying out clinical genetic diagnosis technologies.
Owner:缪林 +1
Method of Separating Target DNA from Mixed DNA
The present invention relates to methods of separating target DNA from mixed DNA in a sample. In some embodiments, the target DNA may be viral DNA, bacterial DNA, fungal DNA or combinations thereof. In some embodiments the mixed DNA includes target DNA and non-target DNA.
Owner:CANON US LIFE SCIENCES INC
Fluorescence real-time quantitative PCR (Polymerase Chain Reaction) detection method of micropterus salmoides ulcer syndrome viruses
InactiveCN101928785AThe detection process is fastThe test result is accurateMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceDNA extraction
The invention provides a fluorescence real-time quantitative PCR (Polymerase Chain Reaction) detection method of micropterus salmoides ulcer syndrome viruses, comprising the following steps of: designing and synthesizing primers according to noncoding region sequences of DNA methyltransferase 3' sides of the micropterus salmoides ulcer syndrome viruses, previously amplifying 167 bp snippets: an upstream primer PF: 5'-GCTCGTTCGGTTGTGCTGAC-3', a downstream primer PR: 5'-GTGTCTCCCTGGTAGTCTTTCAAAC-3'; also synthesizing a probe PB: 5'-(FAM)ATCTGTGTAACCGCCCGCCGCAAAG(Eclipse)-3' used for fluorescence detection; and judging the content of virus particles contained in a sample according to detected CT (Computed Tomography) value through template DNA extraction and PCR amplified reaction. The invention has the advantages of fast detection and high detection accuracy.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka.patsnap.com/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00001.png)
![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka.patsnap.com/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00002.png)
![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka.patsnap.com/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00003.png)