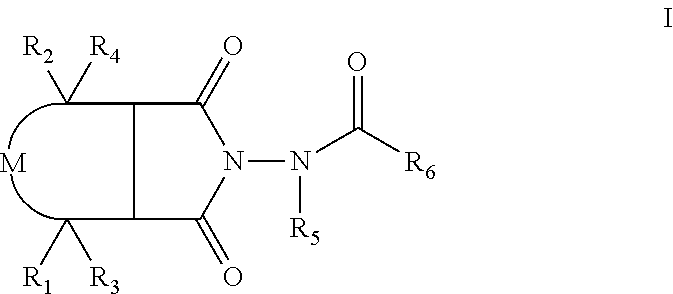
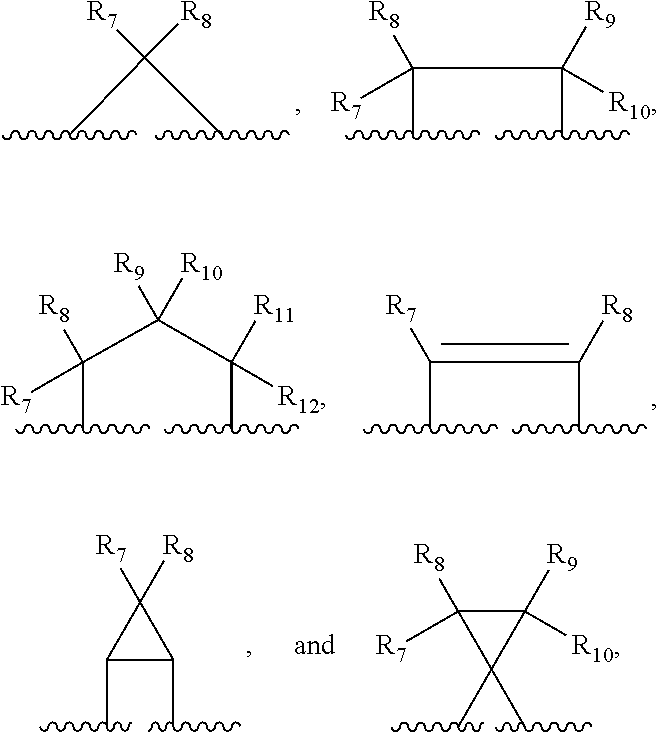
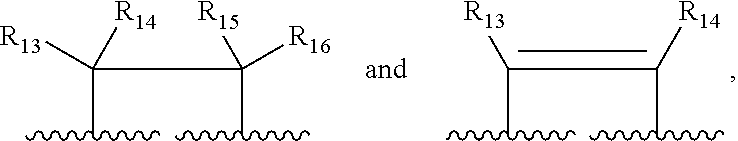Patents
Literature
42 results about "Poxvirus Infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
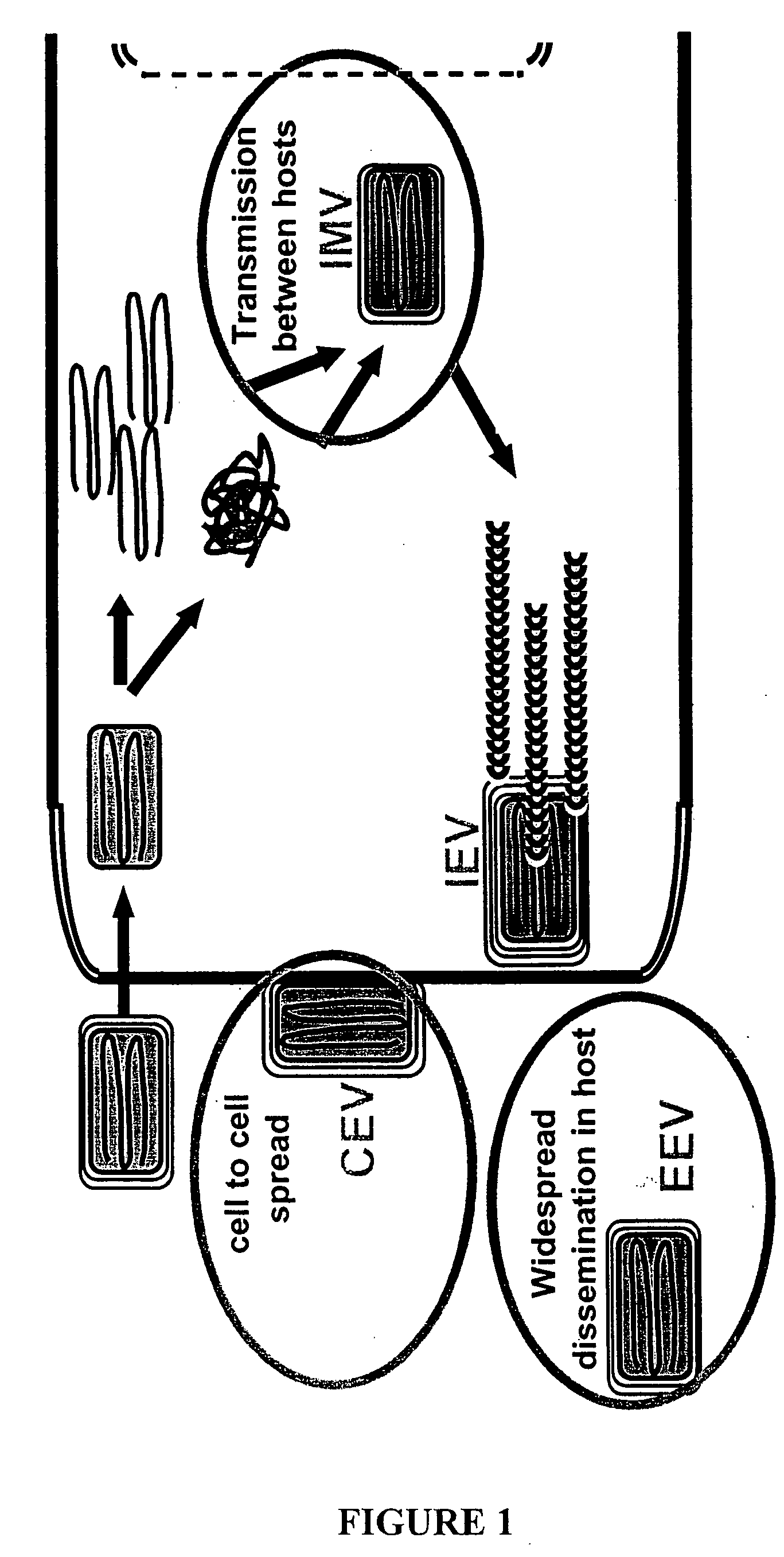
Poxvirus infections typically result in the formation of lesions, skin nodules, or disseminated rash. Infection in humans usually occurs due to contact with contaminated animals, people, or materials.
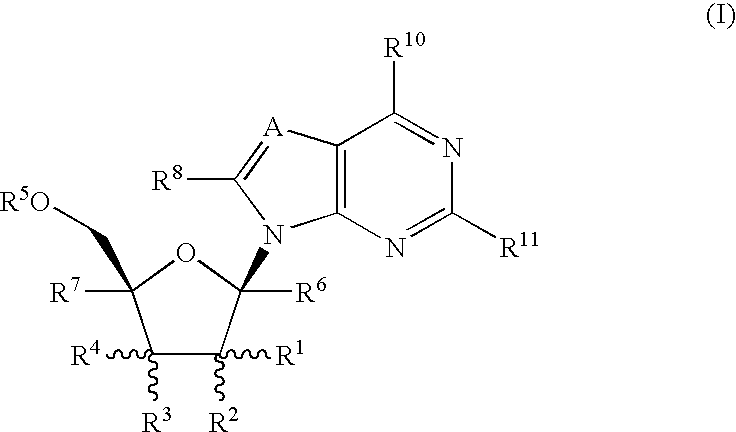
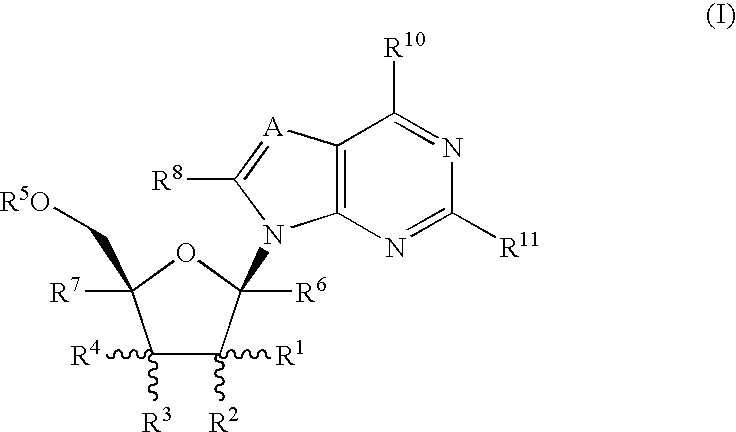
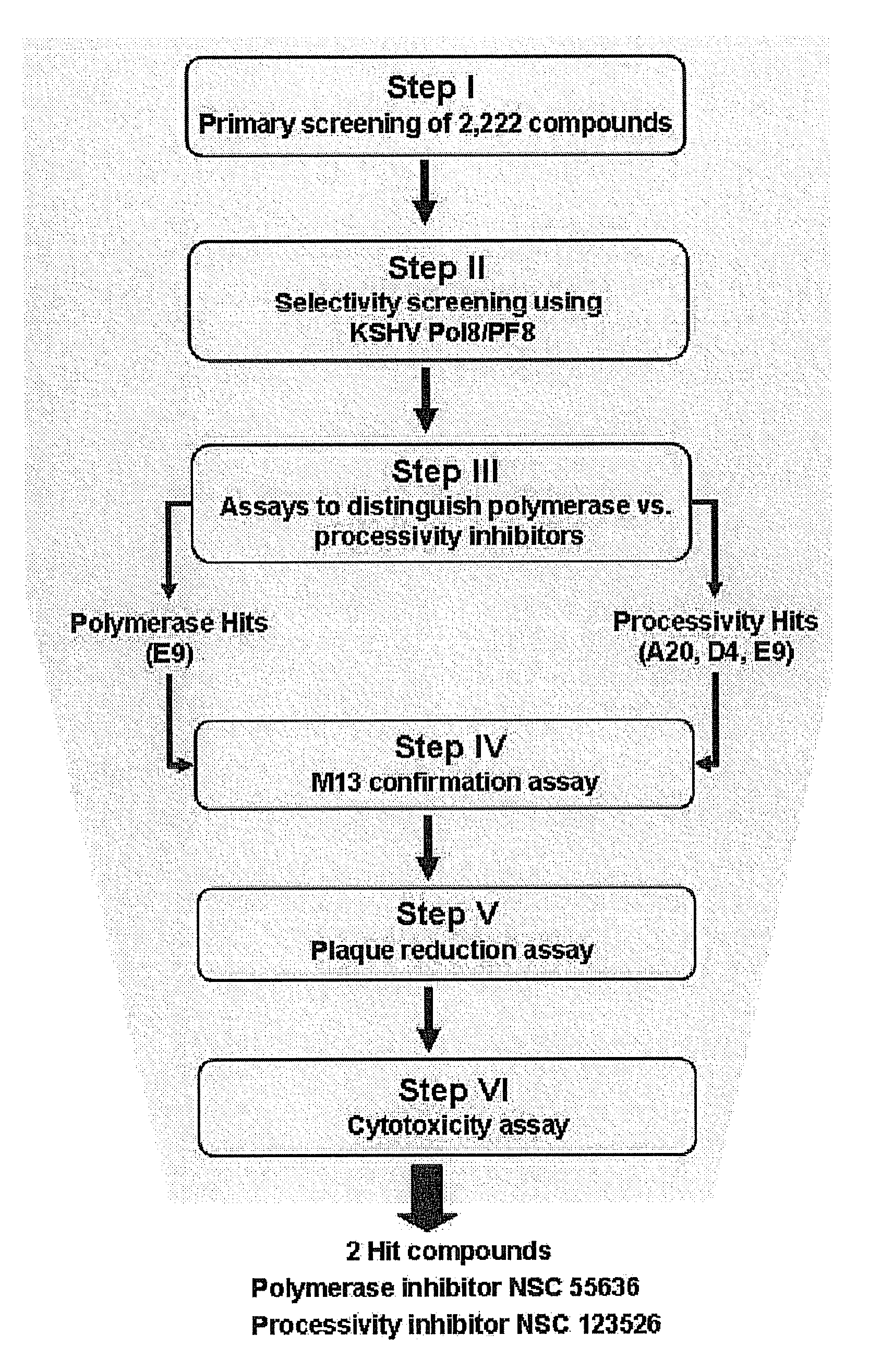
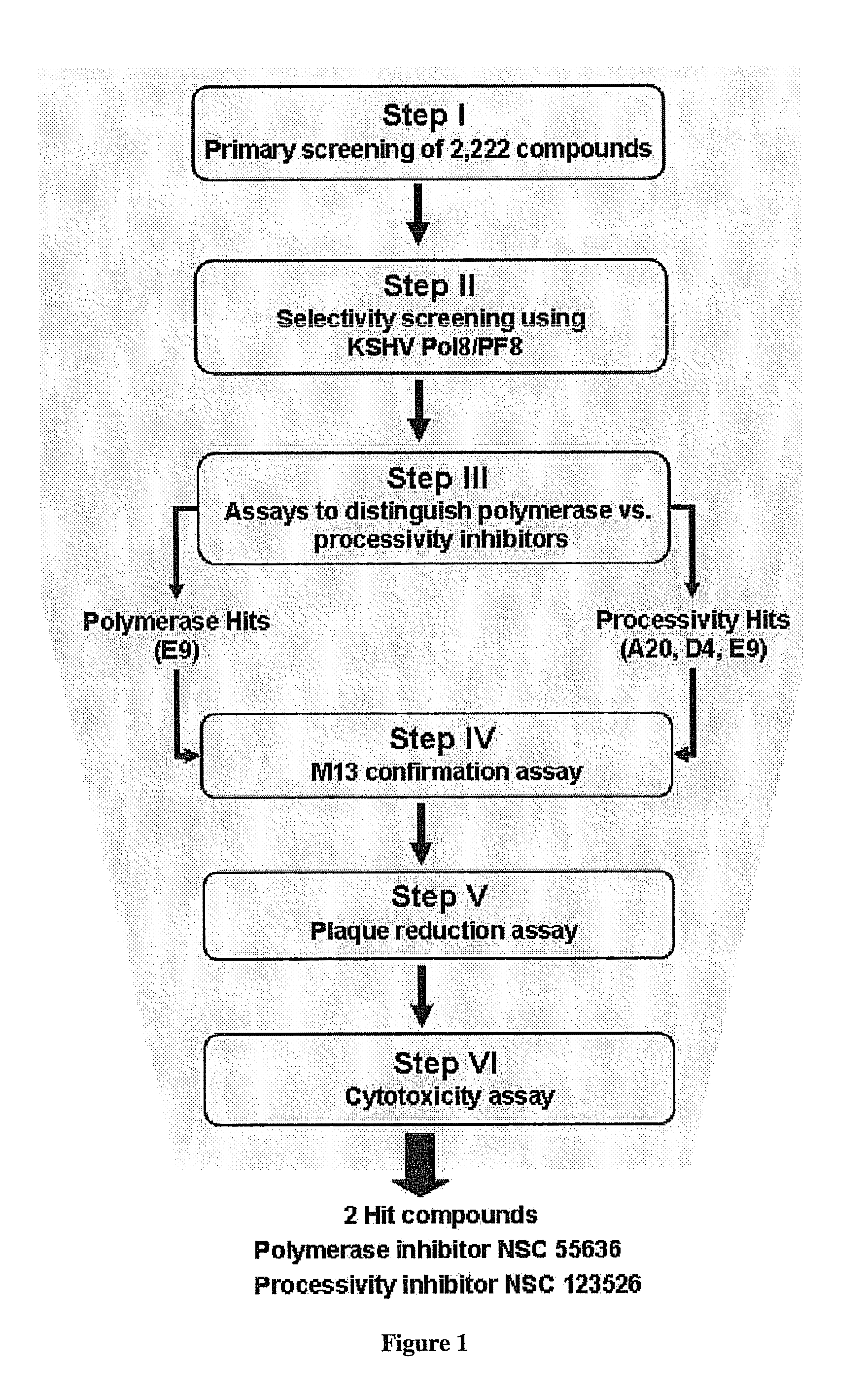
Methods of inhibiting orthopoxvirus replication with nucleoside compounds
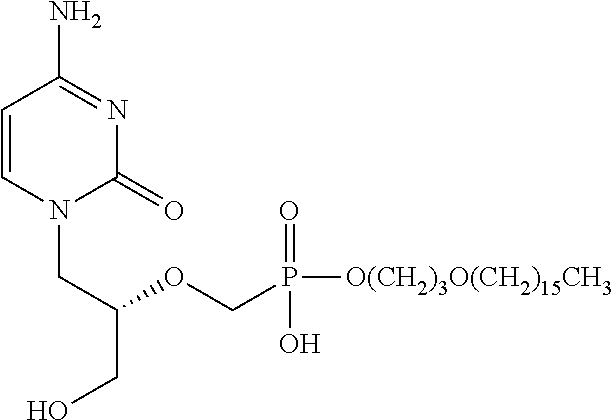
The present invention provides methods of inhibiting orthopoxvirus replication and / or treating orthopoxvirus infection with certain nucleoside compounds and derivatives thereof. These compounds are particularly useful as inhibitors of vaccinia virus and variola virus replication and / or for the treatment of vaccinia virus and variola virus infection. The nucleoside compounds may be administered alone or in combination with other agents active against orthopoxvirus infection, in particular against vaccinia virus or variola virus infection. Another aspect of the present invention provides for the use of such nucleoside compounds in the manufacture of a medicament for the inhibition of orthopoxvirus replication and / or for the treatment of orthopoxvirus infection. Yet a further aspect of the present invention provides such nucleoside compounds for use as a medicament for the inhibition of orthopoxvirus replication and / or for the treatment of orthopoxvirus infection.
Owner:MERCK SHARP & DOHME CORP +1
Therapeutic compounds for blocking DNA synthesis of pox viruses
ActiveUS20100035887A1Inhibition of replicationInhibit and reduce activityBiocideTetracycline active ingredientsMedicinePoxvirus Infections
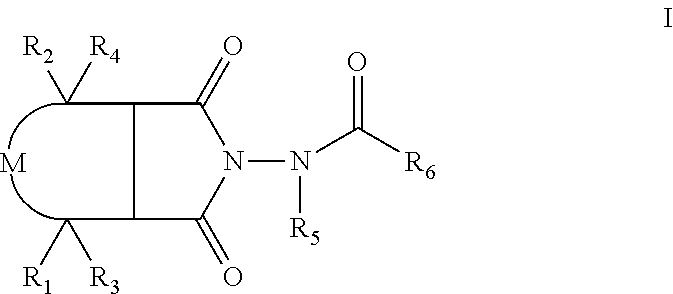
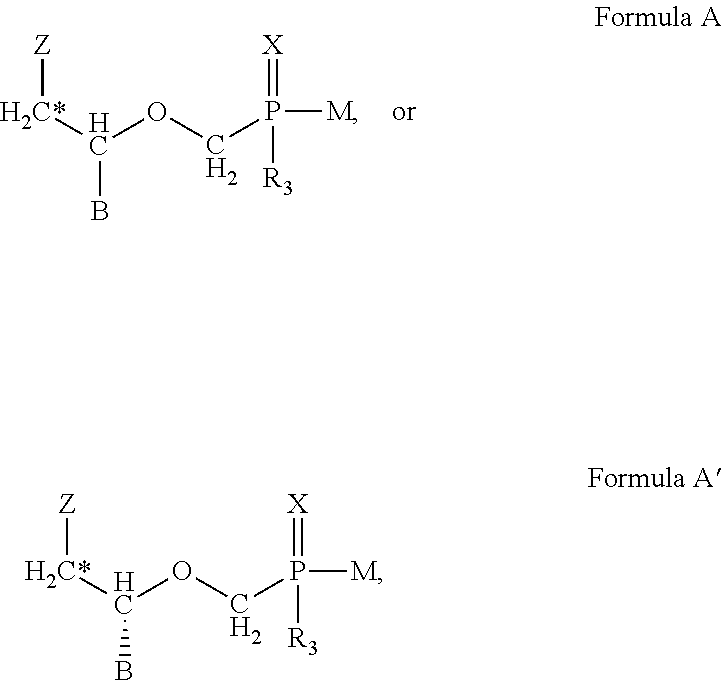
This invention provides methods of inhibiting replication of a poxvirus by contacting a poxvirus with a compound having formula I, formula XXI, formula XXXII, or formula XLI which in turn reduce, inhibit, or abrogate poxvirus DNA polymerase activity and / or its interaction with its processivity factor. Formula I, formula XXI, formula XXXII, or formula XLI can be utilized to treat humans and animals suffering from a poxvirus infection. Pharmaceutical compositions for treating poxvirus infected subjects are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Immune globulin formulations for the treatment and prevention of an orthopoxvirus infection
InactiveUS20060110407A1Serum immunoglobulinsMicrobiological testing/measurementGenus OrthopoxvirusIntravenous IG
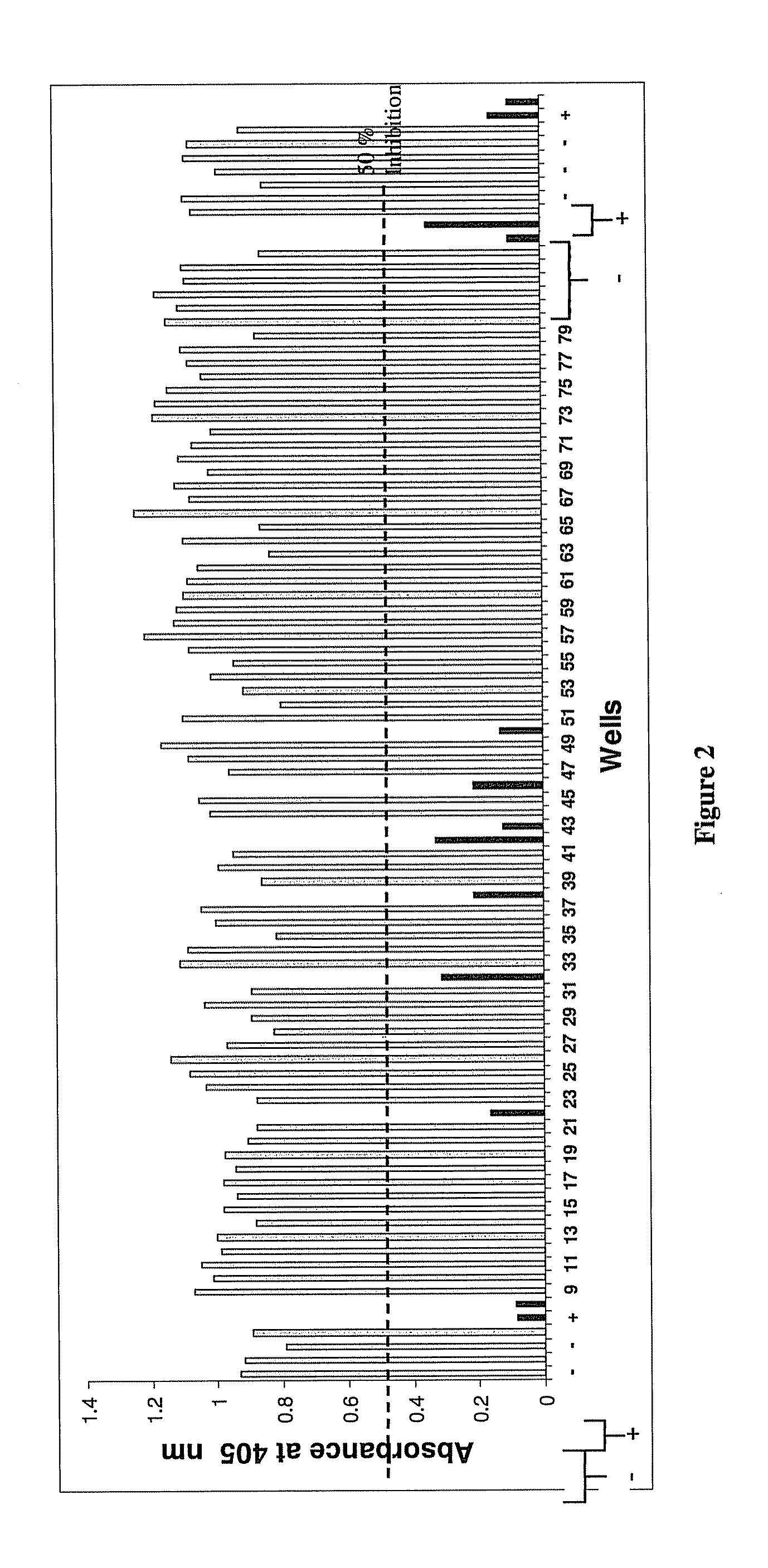
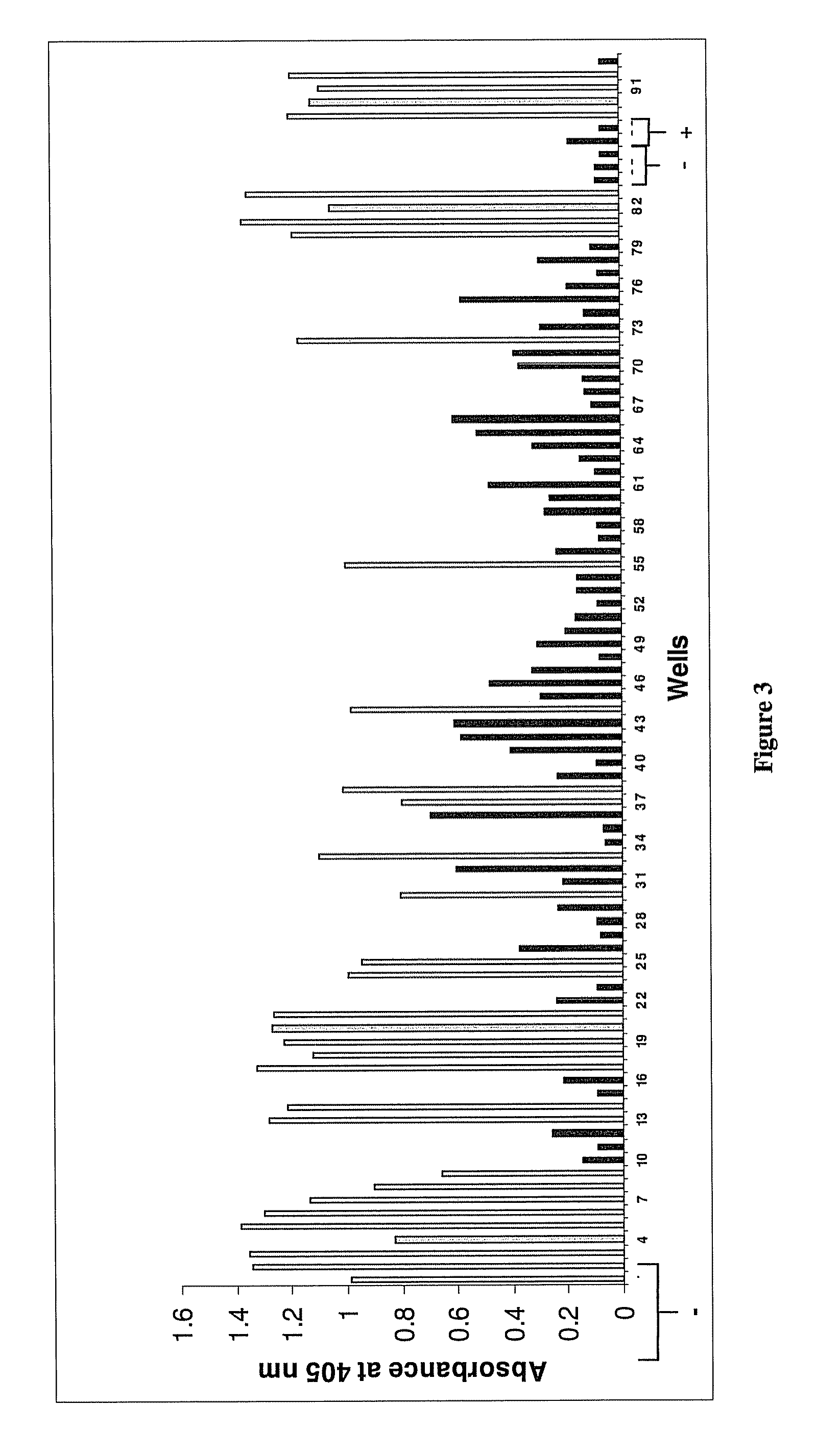
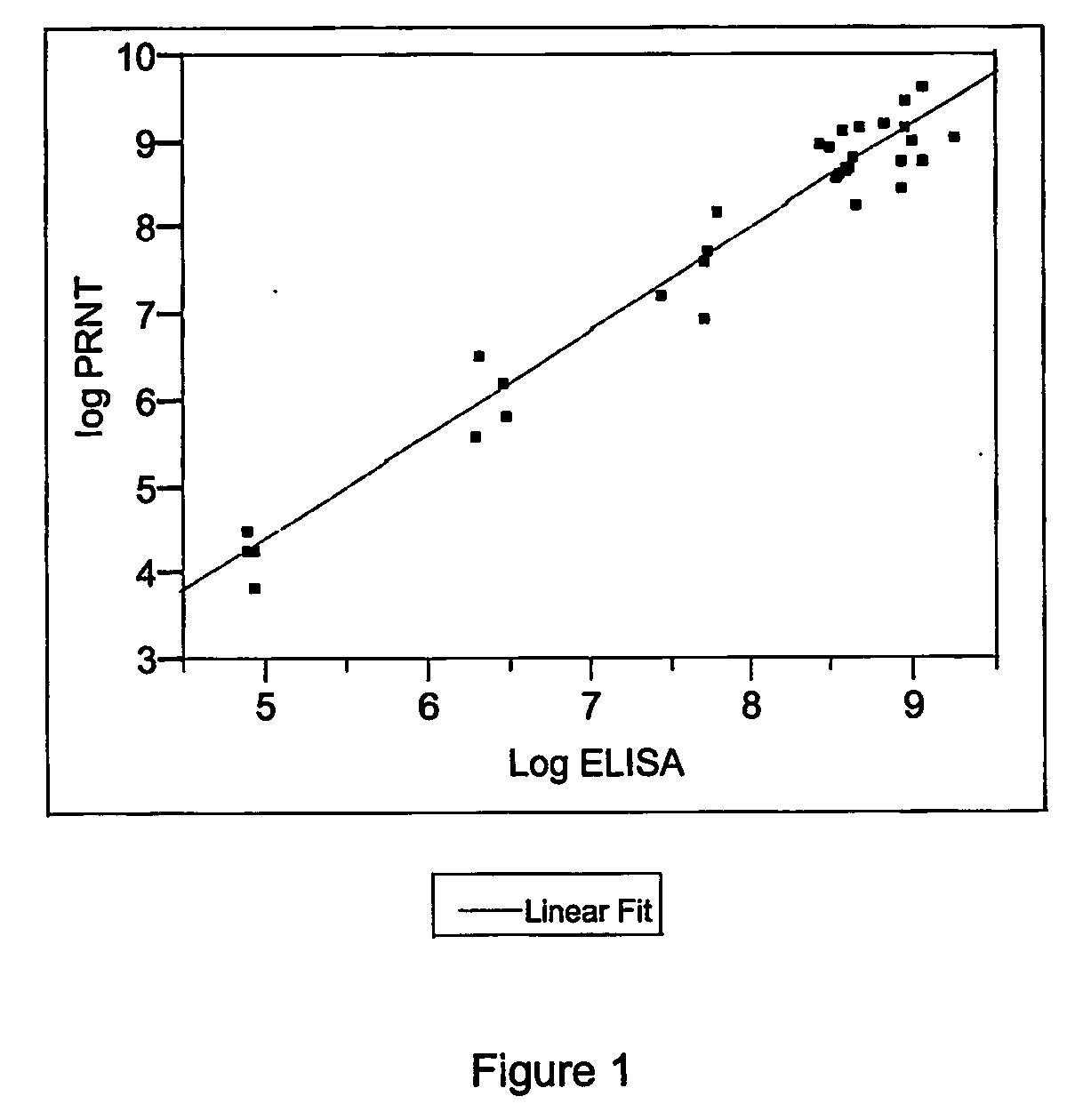
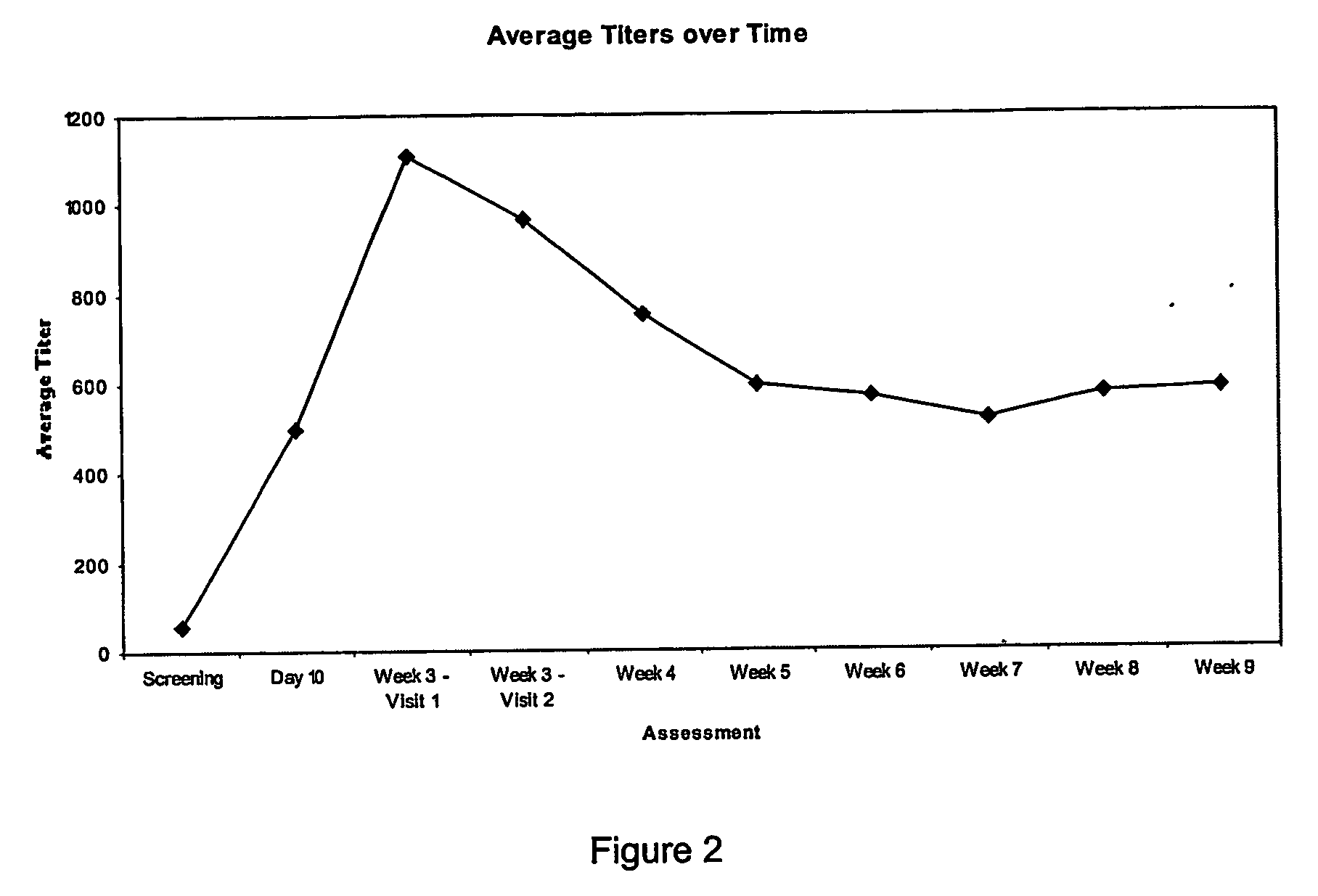
The invention provides an immune globulin having a high titre of antibody to Orthopoxvirus, pharmaceutical compositions comprising the immune globulin and methods for making same. In one embodiment the immune globulin is intravenously injectable. The invention also provides a colorimetric assay to detect neutralizing antibodies to vaccinia virus. The invention has a number of uses including detection of neutralizing antibodies to vaccinia virus and the immunization of persons against the Orthopoxvirus and in the treatment of related conditions.
Owner:CANGENE CORP
Compounds, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases
Owner:SIGA TECH INC
Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases
Owner:SIGA TECH INC
Compositions, methods and kits relating to poxvirus subunit vaccines
ActiveUS20060062800A1Organic active ingredientsGenetic material ingredientsPoxvirus InfectionsAntibody
The invention is directed to a poxvirus vaccine comprising a soluble truncated poxvirus envelope protein. The invention is also directed to a vaccine comprising a nucleic acid encoding such proteins. Also included is an antibody which specifically binds to the proteins and nucleic acid encoding the same, as well as methods of preventing and treating a poxvirus infection using the afore-mentioned vaccine, antibody, protein, and nucleic acid encoding them.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH +2
Compounds, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases
Owner:SIGA TECH INC
Antivirals
InactiveUS20100272706A1Inhibit and decrease viral infectionBiocideOrganic active ingredientsSelect agentGene silencing
This invention provides methods for inhibiting or treating infection by viruses, in particular pox viruses by modulating a kinase, in particular by inhibiting a host cell kinase, involved in mediating viral infection. Methods to identify, validate, and classify the cellular proteins required by viruses during infection of host cells in order to select agents which can inhibit viral infection are described herein. Using a systems biology approach the virus / host cell interaction is studied from initial attachment of the incoming virus to the cell surface, to entry, transcription, replication, biosynthesis, and assembly of progeny particles. The method employs a siRNA screening platform and uses gene silencing to map the ‘viral infectome’—a compilation of cellular proteins that the virus needs to establish infection and drive the infectious cycle. Charting the infectome provides information on the viral biology by the identification of host cell proteins involved in viral infection and allows the development of novel anti-viral drugs that prevent the viruses from establishing productive infection in cells.
Owner:MERCER JASON +4
Pigeon pox resisting Chinese medicinal composition and method for preparing oral liquid thereof
ActiveCN101530481AReduce or eliminate residueConducive to pollution-free productionPharmaceutical delivery mechanismAntiviralsForsythiaPigeon pox
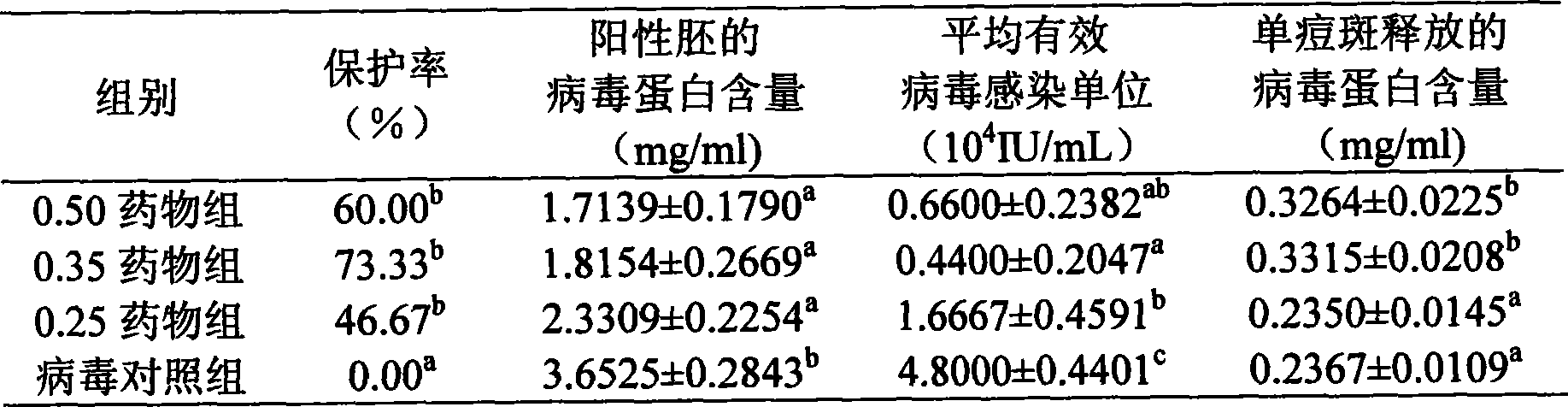
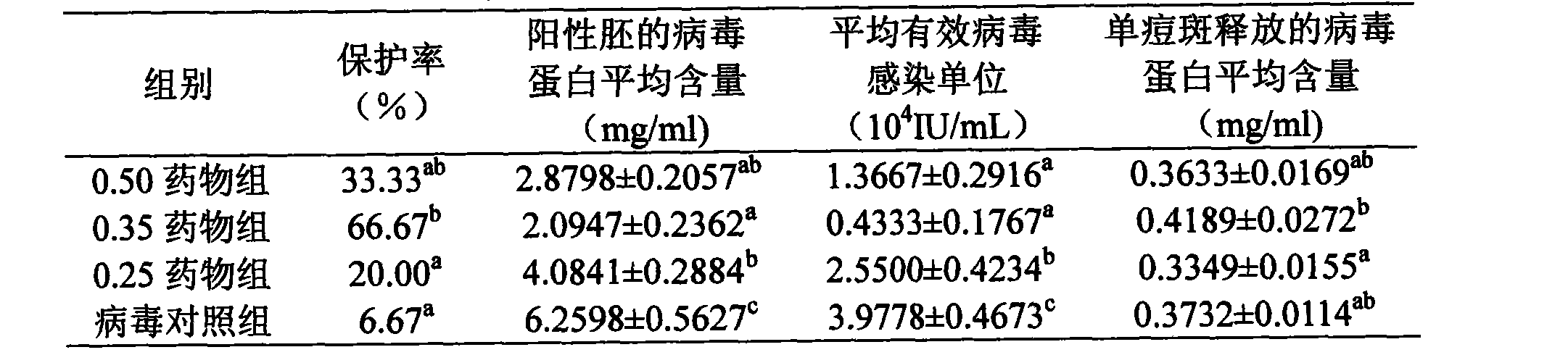
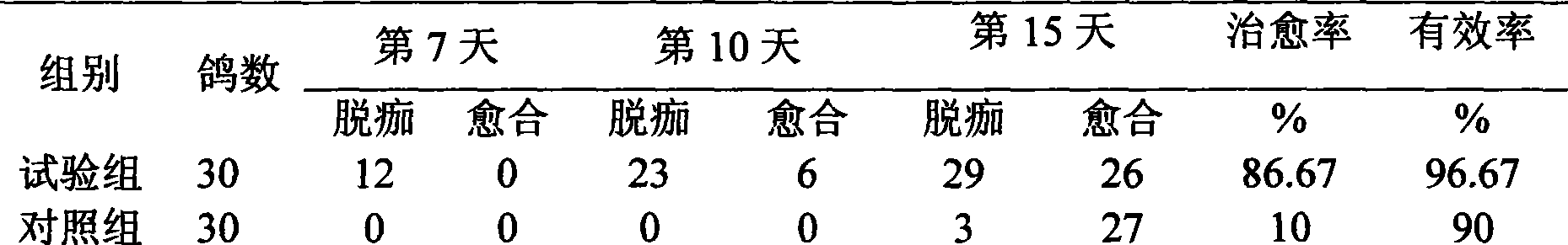
The invention discloses a pigeon pox resisting Chinese medicinal composition, which comprises the following components in portion by weight: 25 to 45 portions of root of kudzuvine, 25 to 45 portions of forsythia, 20 to 35 portions of cimicifuga foetida, 20 to 35 portions of glabrous greenbrier rhizome, 20 to 35 portions of dianthus superbus, 15 to 30 portions of honeysuckle, and 15 to 30 portions of liquorice. The invention also discloses a method for preparing oral liquid of the Chinese medicinal composition for resisting the pigeon pox. The Chinese medicament replaced for western medicine is used for treating virus infection of the pigeon pox, and can reduce or eliminate medicament residues in poultry and eggs so that the Chinese medicinal composition is favorable for pollution-free production of animal products, and can be widely applied to pox virus infection of various birds comprising pigeons; after the pigeons suffer from the pox virus infection, the product can treat, so remarkable curative effect can be obtained, and the healing rate reaches more than 95 percent; and in particular for the produced smallpox scabs, the smallpox scabs can fall and heal after medication for one week and do not relapse.
Owner:江苏欧克动物药业有限公司
Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases
Owner:SIGA TECH INC
Muscovy duck parvovirus VP3 genetic recombination fowl pox virus transfer vector and building method thereof
ActiveCN105132462AImprove build efficiencyPromote stable expressionFermentationGenetic engineeringTransfer vectorTGE VACCINE
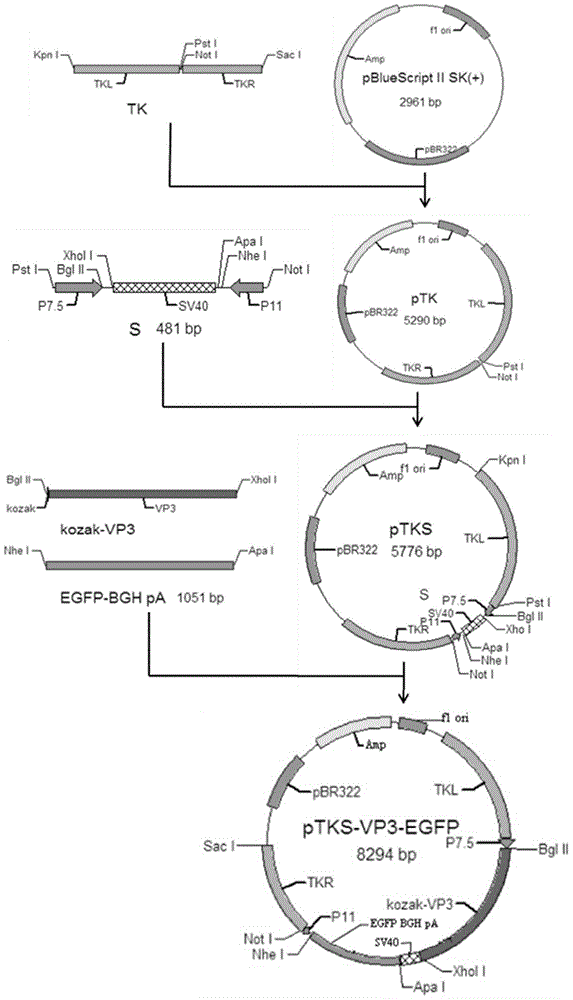
The invention discloses a Muscovy duck parvovirus VP3 genetic recombination fowl pox virus transfer vector and a building method thereof. The method includes: fowl pox virus genome is used as the template to amplify the homologous recombination arms TKL and TKR of TK genes, the TKL and TKR are connected into a TK fragment with multiple cloning sites (MCS), and the TK fragment is connected into a framework plasmid to obtain the plasmid pTK; a reverse serial connection expression box S is synthesized and inserted into the plasmid pTK to obtain the plasmid pTKS; VP3 genes are inserted into an MSC1 position behind an early promotor P7.5, and EGFP BGH pA is inserted into an MCS2 position behind a late promotor P11 to obtain the target transfer vector pTKS-VP3-EGFP. After the pTKS-VP3-EGFP transfects chicken embryonic fibroblast infected by the fowl pox virus, RT-PCR and fluorescent protein detection show that VP3 can be expressed normally. By the Muscovy duck parvovirus VP3 genetic recombination fowl pox virus transfer vector and the building method thereof, a foundation is laid on the further development of safe and efficient MDPV recombinant fowl pox virus vaccines.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Method for preparing chicken pox virus antibody
InactiveCN102757493AHigh purityStrong specificitySerum immunoglobulinsImmunoglobulins against virusesPoxvirus InfectionsChicken Pox
The invention discloses a preparation method of a chicken pox virus antibody, belonging to the technical field of biology. The method comprises the following steps: (1) culturing the chicken pox virus, and preparing a coarse antigen; (2) preparing the concentrated and purified antigen; (3) preparing the animal immune and antiserum; and (4) purifying the antibody. The antibody prepared by the method disclosed by the invention has the advantages of high purity and favorable specificity, and can be widely used for preparing reagents for detecting chicken pox virus infection.
Owner:北京健翔和牧生物科技有限公司
Interferon alpha and interferon kappa fusion protein
InactiveUS7888475B2Sugar derivativesPeptide preparation methodsInterferon alphaDrug biological activity
Herein is described a system to combat poxvirus infection wherein antagonists are developed that bind the soluble cytokine receptor but have no significant biological activity in the host, effectively blocking the virus-mediated suppressor of interferon function, thereby permitting the host's own cytokines to stimulate an antiviral response. Alternatively, interferon molecules can be developed that retain biological activity on their native receptors but fail to bind the viral cytokine binding protein, thereby circumventing this virus immune modulation mechanism.
Owner:PADGETT HAL S +2
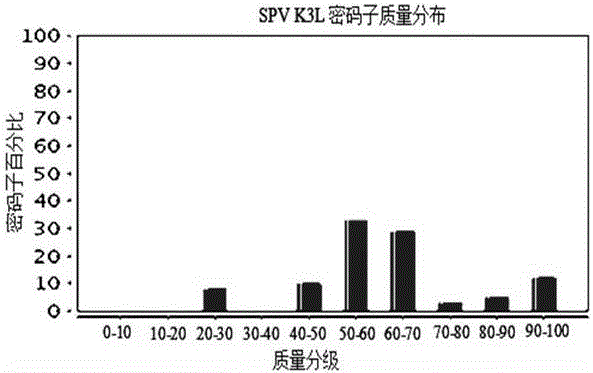
Anti-sheeppox virus K3L protein monoclonal antibody and application thereof
ActiveCN105754951AReduce transmissionEasy to formulateImmunoglobulins against virusesMicroorganism based processesProtein.monoclonalPoxvirus Infections
The invention discloses a hybridoma cell line K3L25. The preservation number of the hybridoma cell line K3L25 in China Center for Type Culture Collection (CCTCC) is CCTCC NO: C2015222. The hybridoma cell line K3L25 and the monoclonal antibody secreted by the hybridoma cell line K3L25 show good immunogenicity, and can be applied in preparation of an early diagnosis reagent or a reagent for early diagnosing sheeppox virus infection or a reagent for basic tests.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
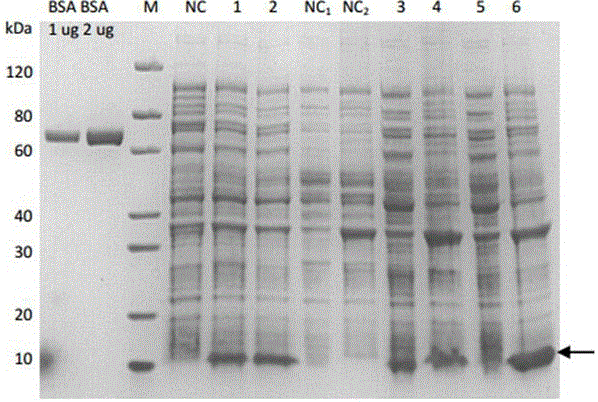
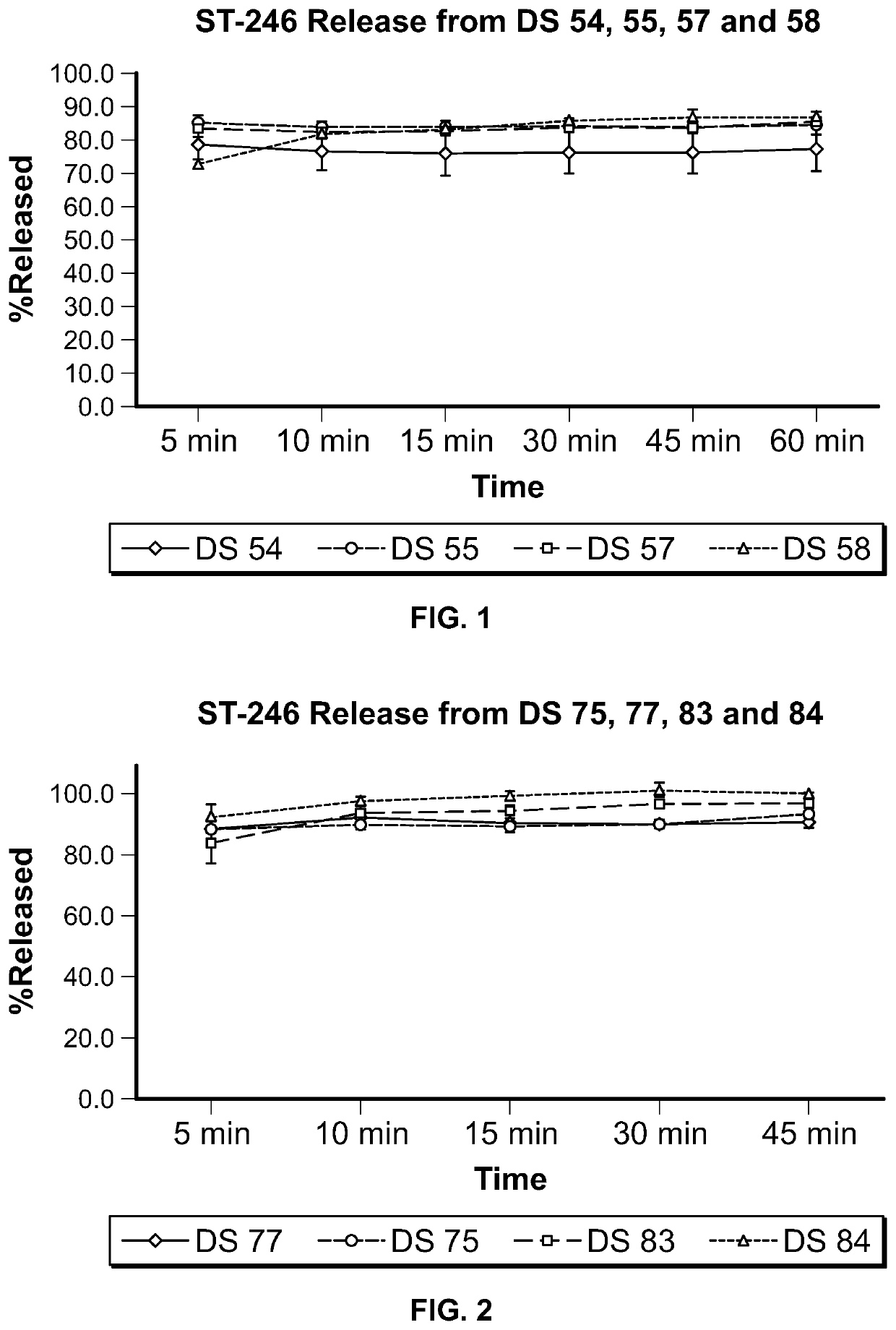
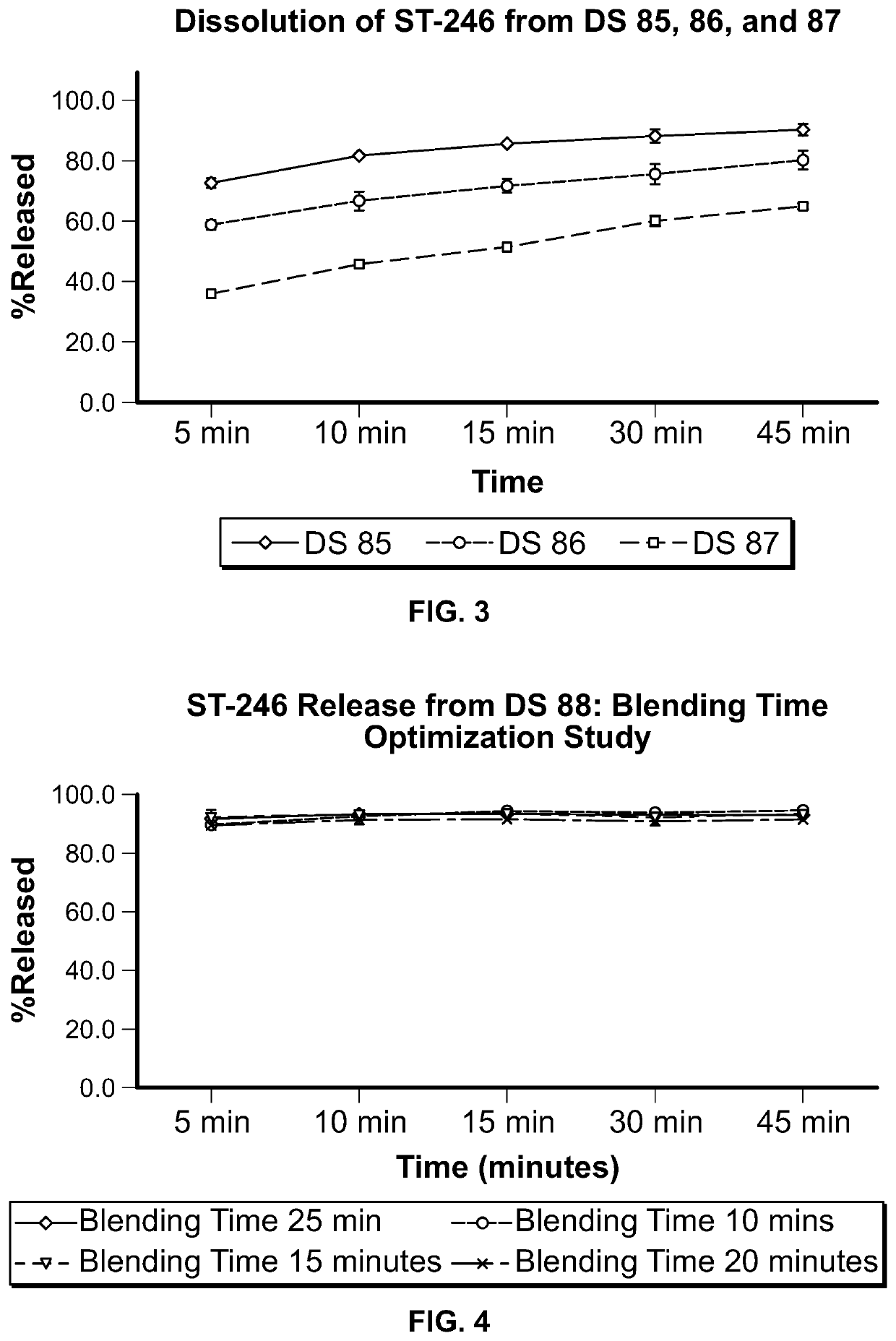
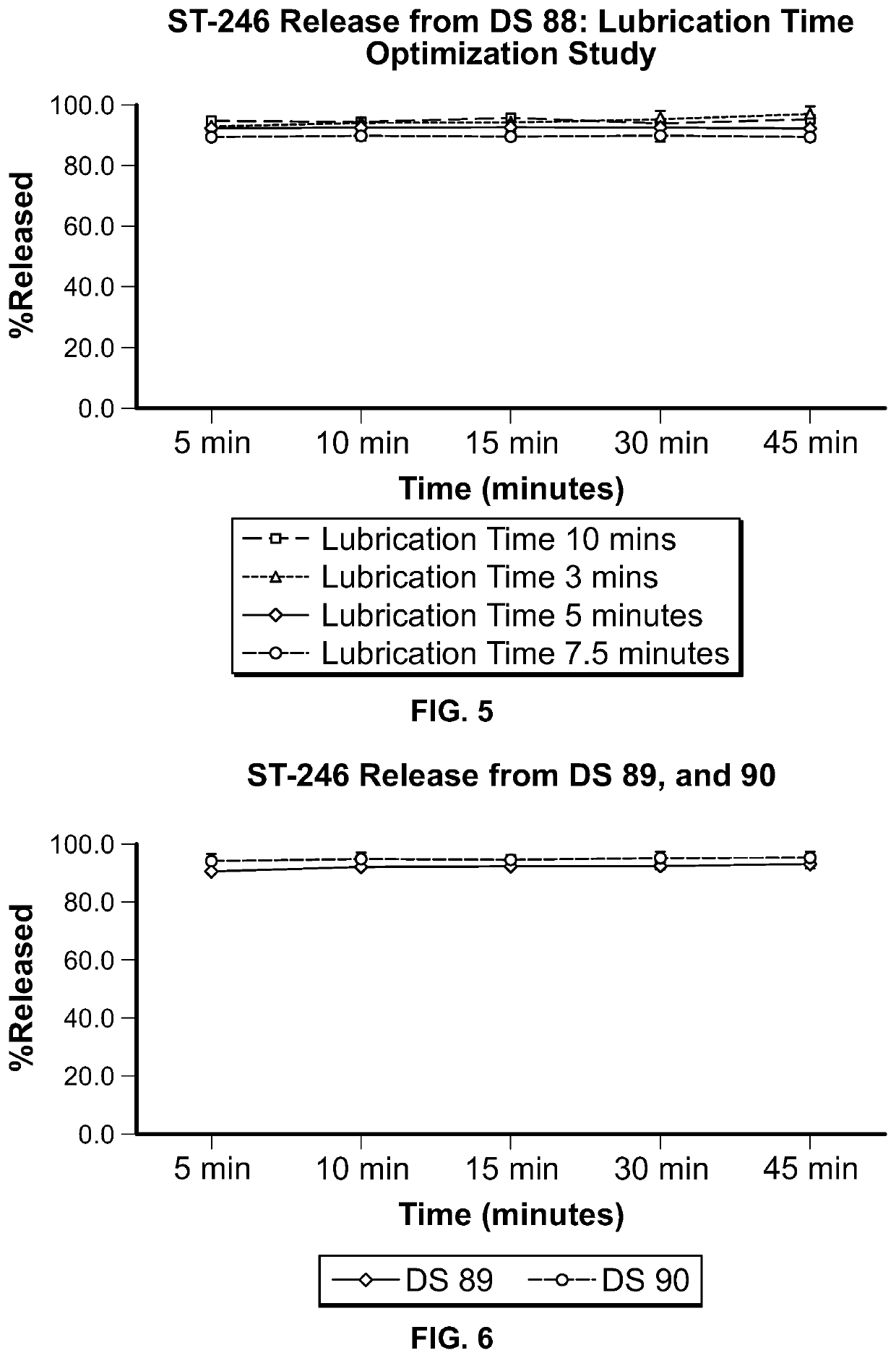
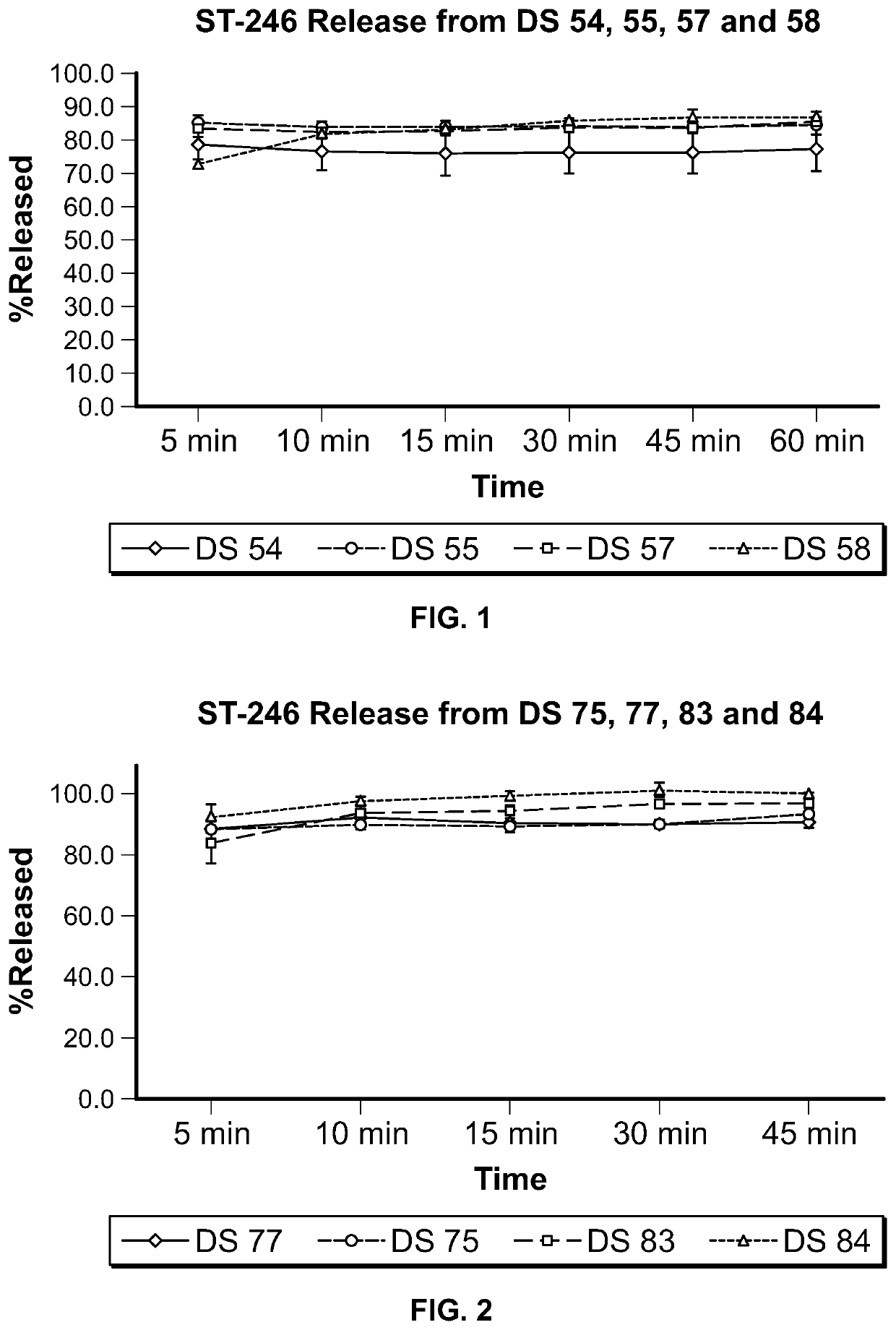
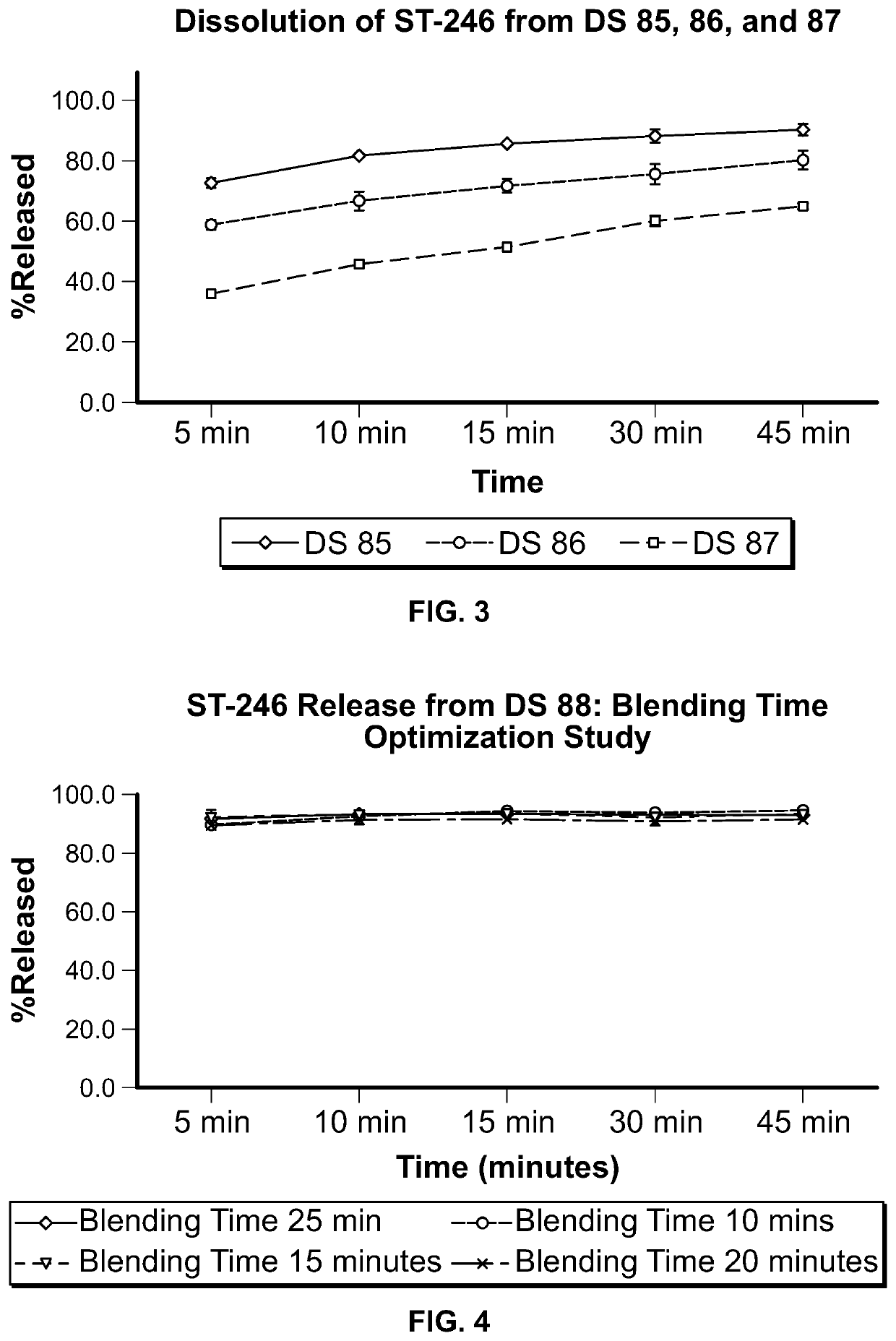
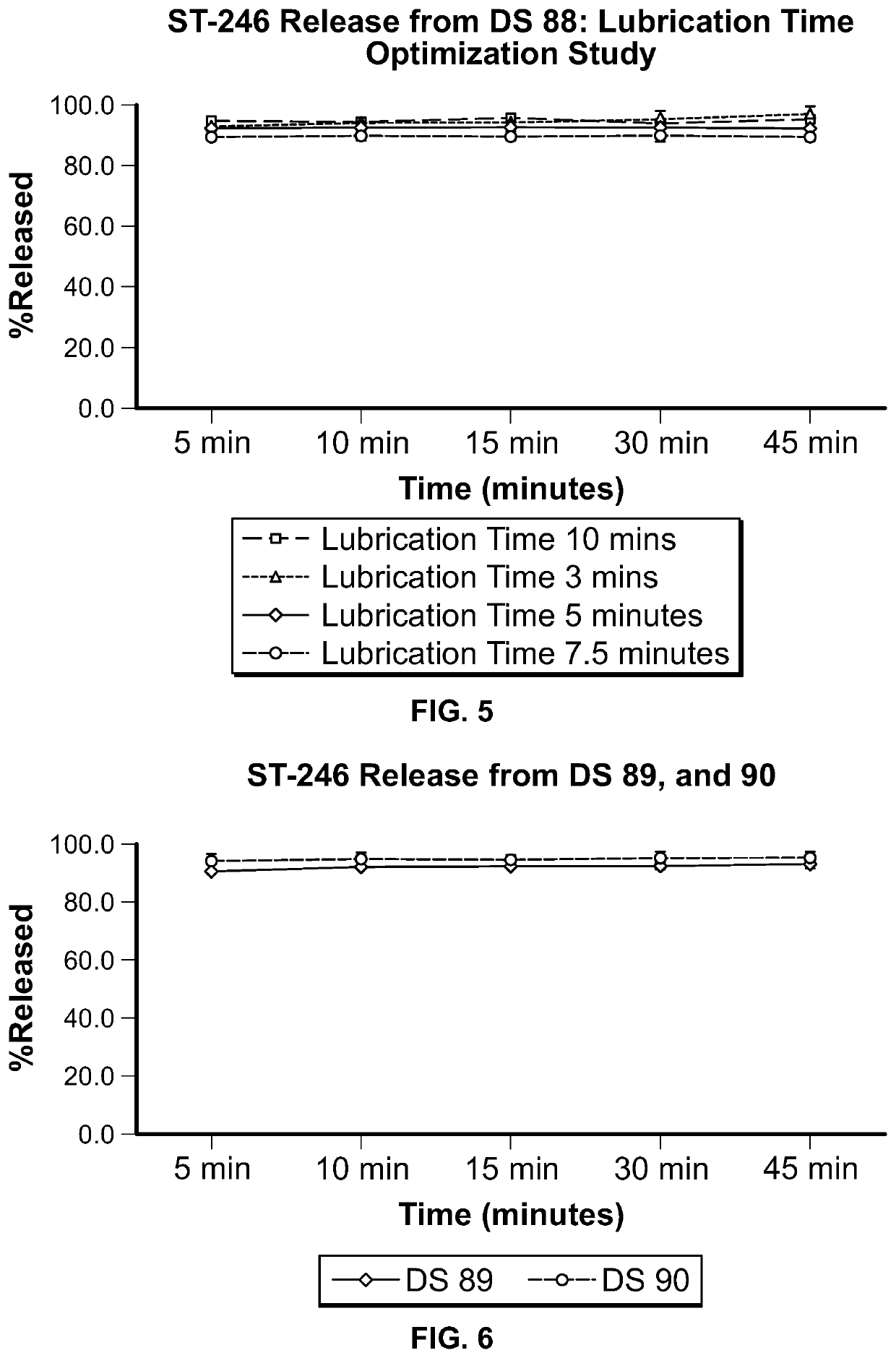
ST-246 (tecovirimat monohydrate) SUSPENSION FORMULATIONS
The present invention is directed to a dry suspension for reconstitution containing Tecovirimat (ST-246) powder and simethicone. The dry suspension is dispersed in water to provide an aqueous pharmaceutical suspension formulation for oral administration for treating orthopoxvirus infections and / or eczema vaccinatum. The suspension formulation exhibits excellent stability and good dissolution and has an improved taste and texture.
Owner:SIGA TECH INC
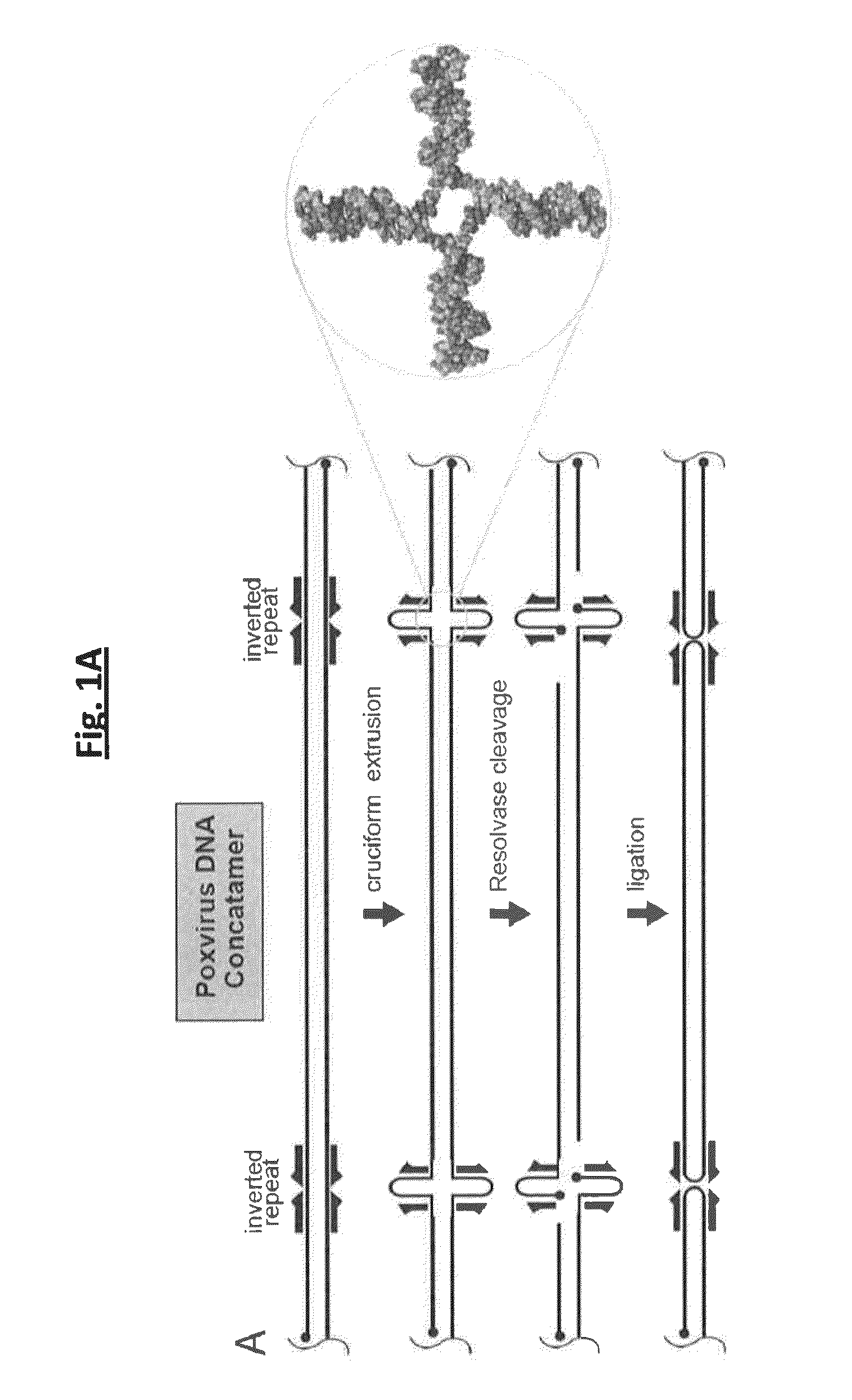
Compositions and methods for inhibiting resolvases
The invention provides a fluorescence polarization (FP)-based assay to identify inhibitors of resolvase's DNA cleavage activity. The invention also provides resolvase inhibitors identified by the assay, as well as derivatives and analogs thereof. In certain embodiments, the compounds of the invention are useful to treat a poxvirus infection in an infected subject.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Therapeutic compounds for blocking DNA synthesis of pox viruses
ActiveUS8278342B2Inhibit and reduce activityInhibition of replicationBiocideTetracycline active ingredientsPoxvirus InfectionsViral replication
This invention provides methods of inhibiting replication of a poxvirus by contacting a poxvirus with a compound having formula XVII which in turn reduce, inhibit, or abrogate poxvirus DNA polymerase activity and / or its interaction with its processivity factor. Formula XVII can be utilized to treat humans and animals suffering from a poxvirus infection. Pharmaceutical compositions for treating poxvirus infected subjects are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
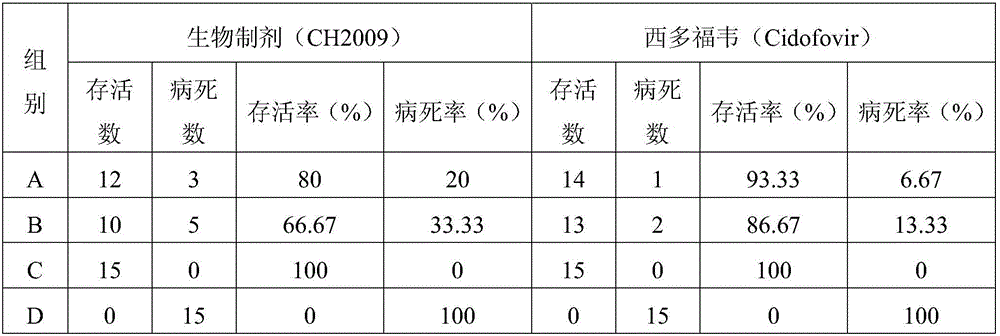
Bioactive preparation of specific anti-variola virus infection model strain and application of bioactive preparation
ActiveCN105663166AImprove absorption rateLow toxicityViral antigen ingredientsViral/bacteriophage medical ingredientsInflammationInoculation
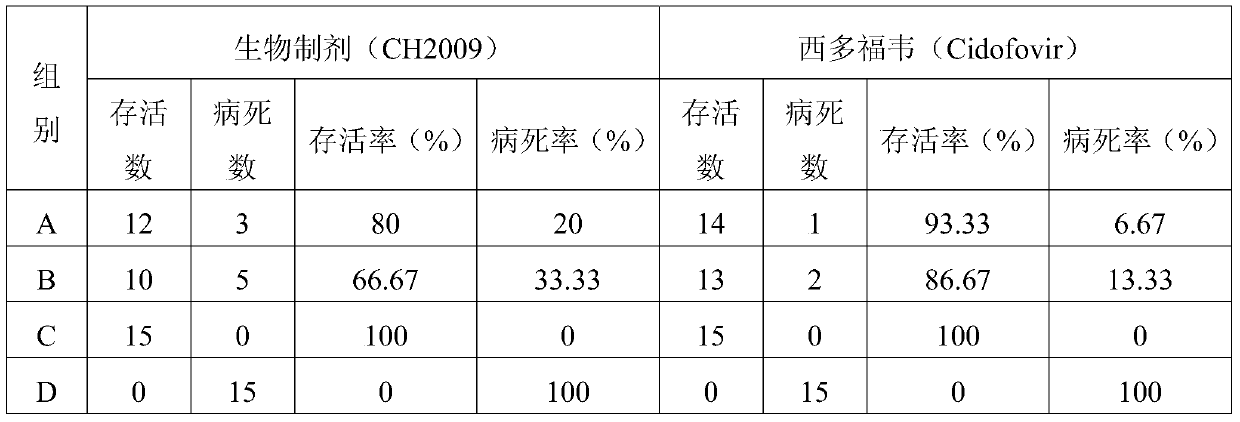
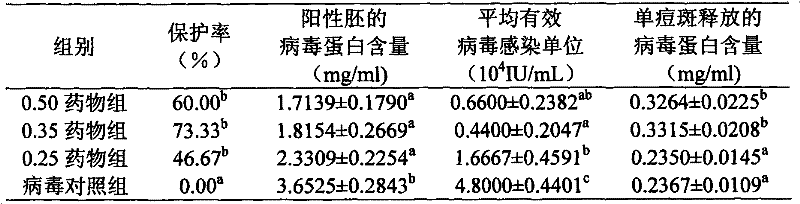
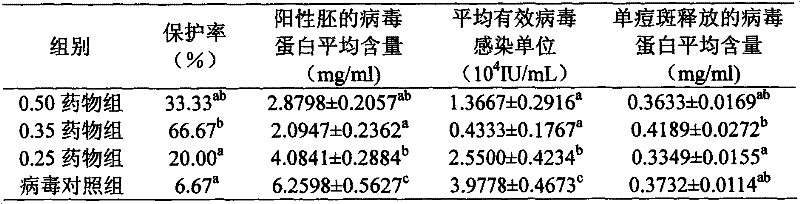
The invention discloses a bioactive preparation of a specific anti-variola virus infection model strain and application of the bioactive preparation. The bioactive preparation CH2009 is specifically applied to (1) preparation of products for inhibiting poxvirus infection or (2) inhibition of poxvirus infection. The bioactive preparation CH2009 with a specific anti-poxvirus infection function is prepared from inflammatory skin tissues of a New Zealand white rabbit through the steps of high temperature and high pressure treatment, solvent extraction, acid and alkali treatment, adsorption, elution, centrifugal concentration and the like by utilizing intracutaneous inoculation of poxviruses on the New Zealand white rabbit. The invention provides a brand-new design strategy for research and development of new-generation drugs for resisting infection of poxviruses, such as variola; and the obtained antiviral drug certainly has wide application prospects and huge commercial values.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
ST-246 (tecovirimat monohydrate) suspension formulations
The present invention is directed to a dry suspension for reconstitution containing Tecovirimat (ST-246) powder and simethicone. The dry suspension is dispersed in water to provide an aqueous pharmaceutical suspension formulation for oral administration for treating orthopoxvirus infections and / or eczema vaccinatum. The suspension formulation exhibits excellent stability and good dissolution and has an improved taste and texture.
Owner:SIGA TECH INC
Anti-sheeppox virus K3L protein C-terminal monoclonal antibody and application thereof
ActiveCN105754952AReduce transmissionConvenience in formulation of prevention and control measuresMicroorganism based processesImmunoglobulins against virusesPoxvirus InfectionsSheeppox virus
The invention discloses a hybridoma cell line capable of secreting an anti-sheeppox virus K3L protein C-terminal monoclonal antibody, the anti-sheeppox virus K3L holoprotein monoclonal antibody secreted by the hybridoma cell line as well as application of the hybridoma cell line and the antibody. The preservation number of a hybridoma cell line K3L35 capable of secreting the anti-sheeppox virus K3L protein C-terminal monoclonal antibody in China Center for Type Culture Collection (CCTCC) is CCTCC NO: C2015223. The K3L protein C-terminal monoclonal antibody can be secreted by the hybridoma cell line K3L35. The hybridoma cell line K3L35 and the monoclonal antibody secreted by the hybridoma cell line K3L35 can be applied in preparation of a reagent for diagnosing or detecting sheeppox virus infection or a reagent for experiments.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
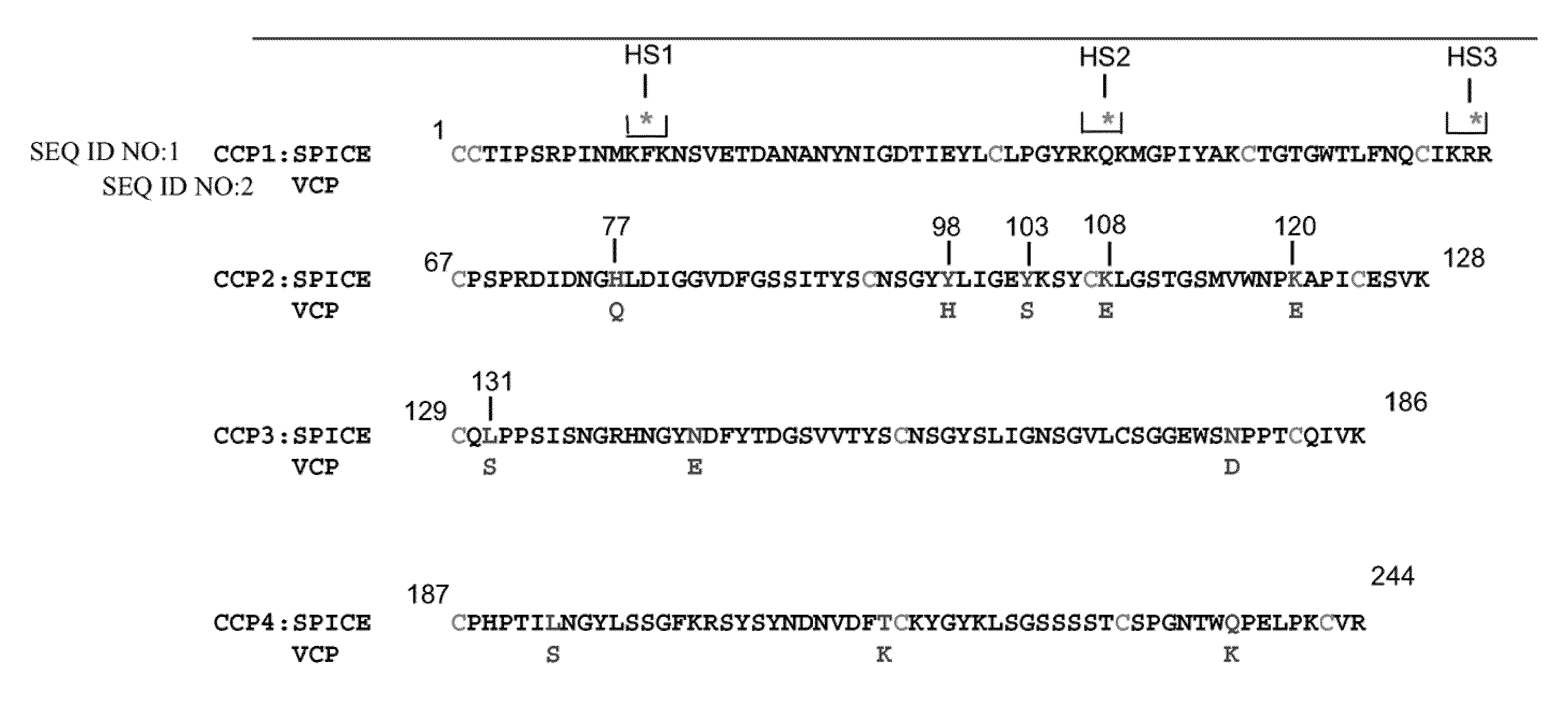
Compositions and methods for the detection and treatment of poxviral infections
InactiveUS20090291428A1Microbiological testing/measurementInactivation/attenuationPoxvirus InfectionsVirus
The invention encompasses an antibody that binds to and substantially inhibits the activity of at least one poxvirus complement inhibitor. Additionally, the application encompasses methods of detecting a poxvirus complement inhibitor and methods of decreasing the activity of a poxvirus complement inhibitor.
Owner:WASHINGTON UNIV IN SAINT LOUIS
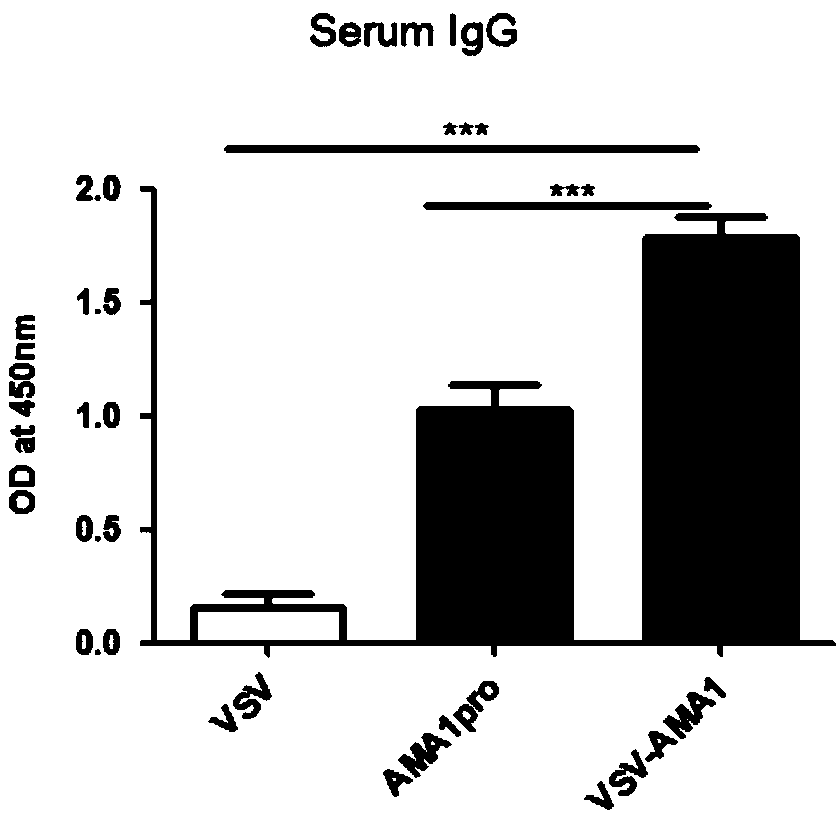
Preparation method of viral vaccine expressing plasmodium ovale AMA1 protein
ActiveCN109022456AStrong immune responseEffective immune protectionSsRNA viruses negative-sensePeptidesImmune cycleStructural protein
Belonging to the technical field of virology and genetic engineering, the invention discloses a preparation method of a viral vaccine expressing plasmodium ovale AMA1 protein. According to the invention, a gene expressing plasmodium ovale apical membrane protein-1 is inserted into a vesicular stomatitis virus VSV genome vector to construct the recombinant virus vector skeleton plasmid XN2-AMA1, apoxvirus containing T7RNA polymerase is employed to infect the baby hamster kidney cell (BHK-21), then the plasmid XN2-AMA1 and the structural protein plasmids pN, pP, pL are utilized for cotransfection of BHK-21 cells to realize rescue of recombinant virus in cells, virus-like particles expressing AMA1 are packaged, and the recombinant virus rVSV-AMA1, i.e. the vaccine can be formed. The vaccineprovided by the invention has the advantages of simple operation, high production titer and short immune cycle, can induce strong humoral and cellular immune response, and has the great potential of clinical application.
Owner:JIANGNAN UNIV
Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases
Owner:SIGA TECH INC
Potent poxvirus inhibitor
ActiveUS20140343114A1Reduce and inhibit and activity of DNA polymeraseBiocideOrganic chemistryPoxvirus InfectionsStereochemistry
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Preparation for inhibiting poxvirus infections
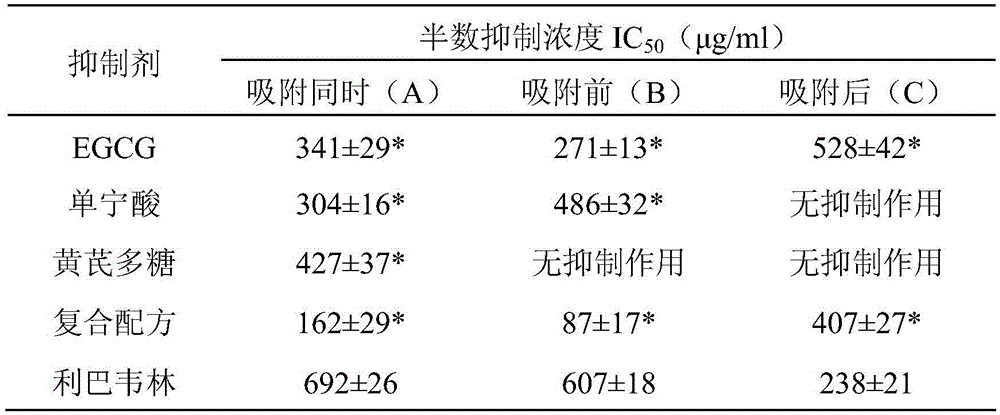
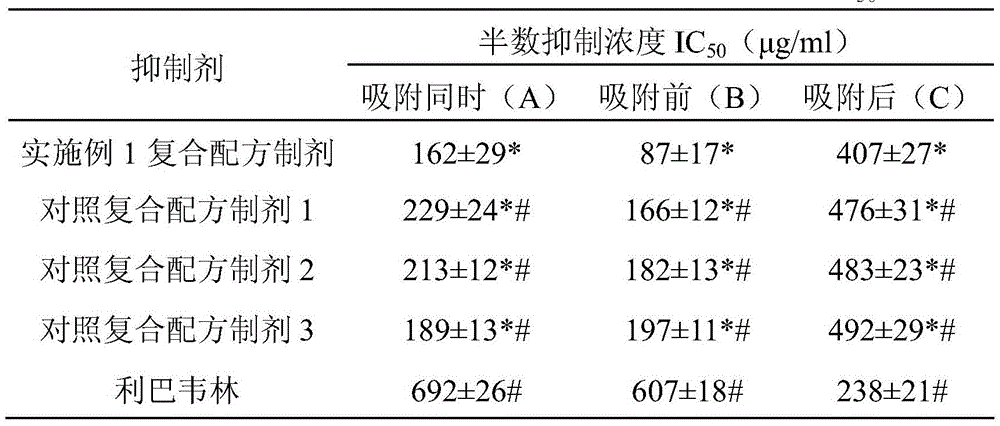
ActiveCN105663218AAvoid infectionLow cytotoxicityOrganic active ingredientsAntiviralsAstragalus polysaccharideCytotoxicity
The invention discloses a preparation for inhibiting poxvirus infections and provides an application of the preparation to the following example (1) or (2): (1) preparation of a product for inhibiting the poxvirus infections; (2) inhibition of the poxvirus infections; the preparation is mainly prepared by mixing epigallocatechin gallate, tannin and astragalus polysaccharides in the mass ratio being (0.5-1.0):(0.5-1.0):(0.5-1.5). The cytotoxicity of the compound formula preparation is not higher than that of control sample ribavirin already having the security permission, and the preparation is safer. Under the condition of safe use concentration, the poxvirus infections can be effectively inhibited by preventively using the preparation, and the preparation has the commercial value in being further developed into a poxvirus infection inhibitor.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Methods of treating orthopox virus infections and associated diseases
The present invention provides methods of treating diseases associated with at least one virus. The methods include administering a compound described in the invention in a therapeutically effective amount. According to some aspects of the present invention, the methods provide treatment of an orthopox virus infection or a disease related to orthopox virus.
Owner:CHIMERIX INC
Bioactive preparations specific against variola virus infection model strains and applications thereof
InactiveUS20200237901A1Inhibiting poxvirus infectionAvoid infectionViral antigen ingredientsViral/bacteriophage medical ingredientsPoxviridae InfectionsPharmaceutical Substances
The present invention discloses a bioactive preparation specific against variola virus infection model strains and applications thereof. The application provided by the present invention specifically is the use of bioactive preparation CH2009 in following (1) or (2): (1) preparing the products for inhibiting poxvirus infection; or (2) using for inhibiting poxvirus infection. Bioactive preparation CH2009 is a bioactive preparation specific against poxvirus infection prepared and obtained from inflammatory skin tissues by intradermal vaccination of New Zealand white rabbit with vaccinia virus, and through the steps such as high temperature and pressure, solvent extraction, acid-base treatment, adsorption, elution, concentration by centrifugation and others. The present invention provides a completely new design strategy for the development of new generation of drugs against poxviridae infection such as smallpox and others, and it is no doubt that the resultant antiviral drugs have a wide application prospect and a huge commercial value.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Poxvirus Host Range Protein K3 as a Positive Selection Marker for Generation of Recombinant Poxviruses, a Therapeutic Target for Poxvirus Infection and a Therapeutic Agent for PKR Related Diseases
PendingUS20210244739A1Organic active ingredientsPeptide/protein ingredientsHost cell lineRecombinant virus
Described herein is a novel method for generation of recombinant poxviruses using an E3 and K3 double deletion mutant virus as the parental virus for generation of recombinant viruses. Following allowing for crossing over between the parental virus and an insertion cassette including an orthopox K3 peptide and the gene of interest, recombinant viruses are selected by infecting a host cell line permissive for the orthopox K3 peptide but not for the E3 and K3 double mutant parental virus. It is also demonstrated that a specific small molecule inhibitor of NEDD8 activating enzyme, MLN4924, can completely block poxvirus K3 family protein mediated PKR degradation and virus replication.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Biologically active preparation and application of specific anti-variola virus infection model strain
InactiveCN105663166BImprove absorption rateLow toxicityViral antigen ingredientsViral/bacteriophage medical ingredientsVariola virusAntiviral drug
The invention discloses a bioactive preparation and application of a specific anti-smallpox virus infection model strain. The application provided by the present invention is specifically the application of the bioactive preparation CH2009 in the following (1) or (2): (1) preparing a product for inhibiting poxvirus infection; (2) for inhibiting poxvirus infection; The biologically active preparation CH2009 is prepared from the inflammatory skin tissue of New Zealand white rabbits by intradermally inoculating vaccinia virus, high temperature and high pressure, solvent extraction, acid-base treatment, adsorption, elution, and centrifugal concentration. Bioactive agents specific for poxvirus infection. The present invention provides a new design strategy for the research and development of a new generation of anti-pox virus infection drugs such as smallpox, and the obtained anti-viral drugs undoubtedly have broad application prospects and huge commercial value.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
A kind of anti-pigeon pox traditional Chinese medicine composition and preparation method of oral liquid thereof
ActiveCN101530481BReduce or eliminate residueConducive to pollution-free productionPharmaceutical delivery mechanismAntiviralsAdditive ingredientPigeon pox
The invention discloses an anti-pigeon pox traditional Chinese medicine composition, which comprises the following components in parts by weight: 25-45 parts of kudzu root, 25-45 parts of forsythia, 20-35 parts of Cimicifuga, 20-35 parts of Smilax cocos, Qu Mai 20-35 parts, 15-30 parts of honeysuckle, 15-30 parts of licorice. The invention also discloses a preparation method of the oral liquid of the anti-pox traditional Chinese medicine composition. In the present invention, the use of traditional Chinese medicines instead of western medicines for pigeon pox virus infection can reduce or eliminate drug residues in poultry meat and eggs, thereby facilitating the pollution-free production of livestock products; and can be widely used in the treatment of various poultry including pigeons Pox virus infection: Pigeons suffering from pox virus infection can obtain significant curative effect by using this product, and the cure rate is over 95%. Especially for the pox scabs, they will fall off and heal within one week after medication, and will not recur.
Owner:江苏欧克动物药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com