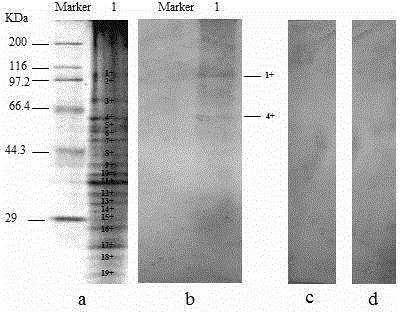
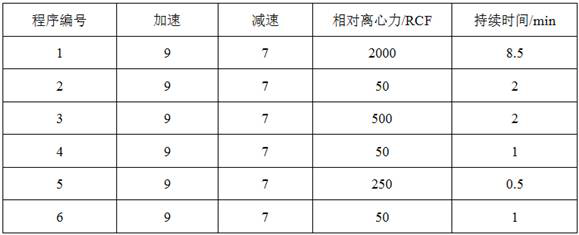
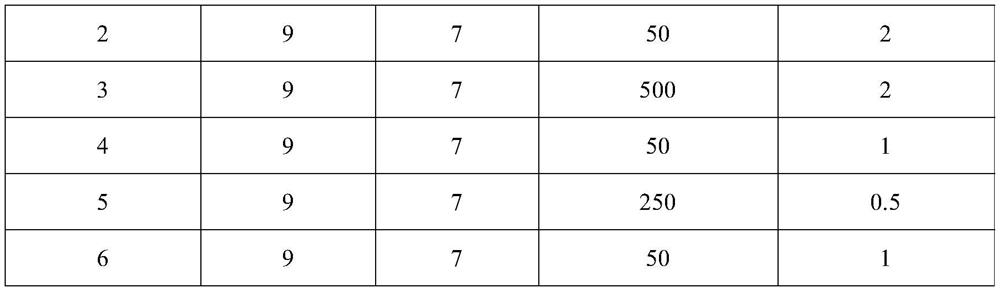
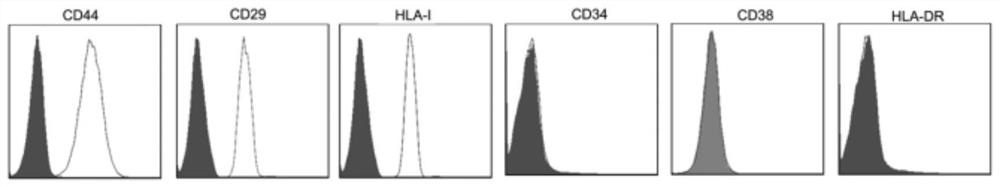
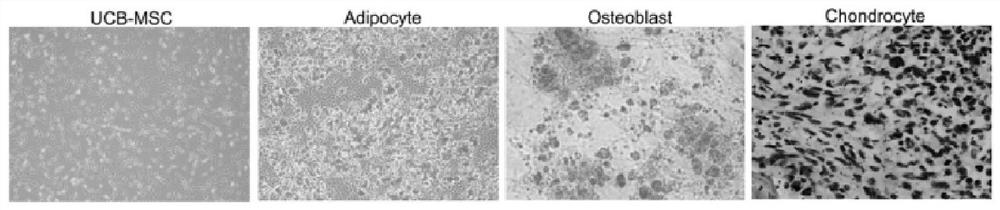
Patents
Literature
66 results about "Intravenous IG" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intravenous immune globulin (IVIG) is made of antibodies that have been extracted from blood donations from 3,000-10,000 healthy donors. IVIG is used to treat many autoimmune disorders, idiopathic diseases (disease of unknown cause), and infections.
Systemic administration of NAC as an adjunct in the treatment of bioterror exposures such as anthrax, smallpox or radiation and for vaccination prophylaxis, and use in combination with DHEA for the treatment of smallpox and other viruses
InactiveUS20040022873A1No toxicityWithout fear of compromisingBiocideTripeptide ingredientsWhole bodyPoisonous effects
The invention is for the combination and related methods of N-acetyl-cysteine oral, inhaled, or intravenous, or glutathione inhaled or intravenous, generally in combination with antibiotic and / or antiviral therapy to ameliorate the toxic effects of infection with materials used in Bioterror incidents such as Bacillus anthracis and smallpox virus, and alternatively, upon exposure to radiation, during testing, and vaccination, as treatment prior to treatment with antibiotic or antiviral therapy to ameliorate the toxic effects of infection and exposure with these organisms.
Owner:YOUR ENERGY SYST
Immune globulin formulations for the treatment and prevention of an orthopoxvirus infection
InactiveUS20060110407A1Serum immunoglobulinsMicrobiological testing/measurementGenus OrthopoxvirusIntravenous IG
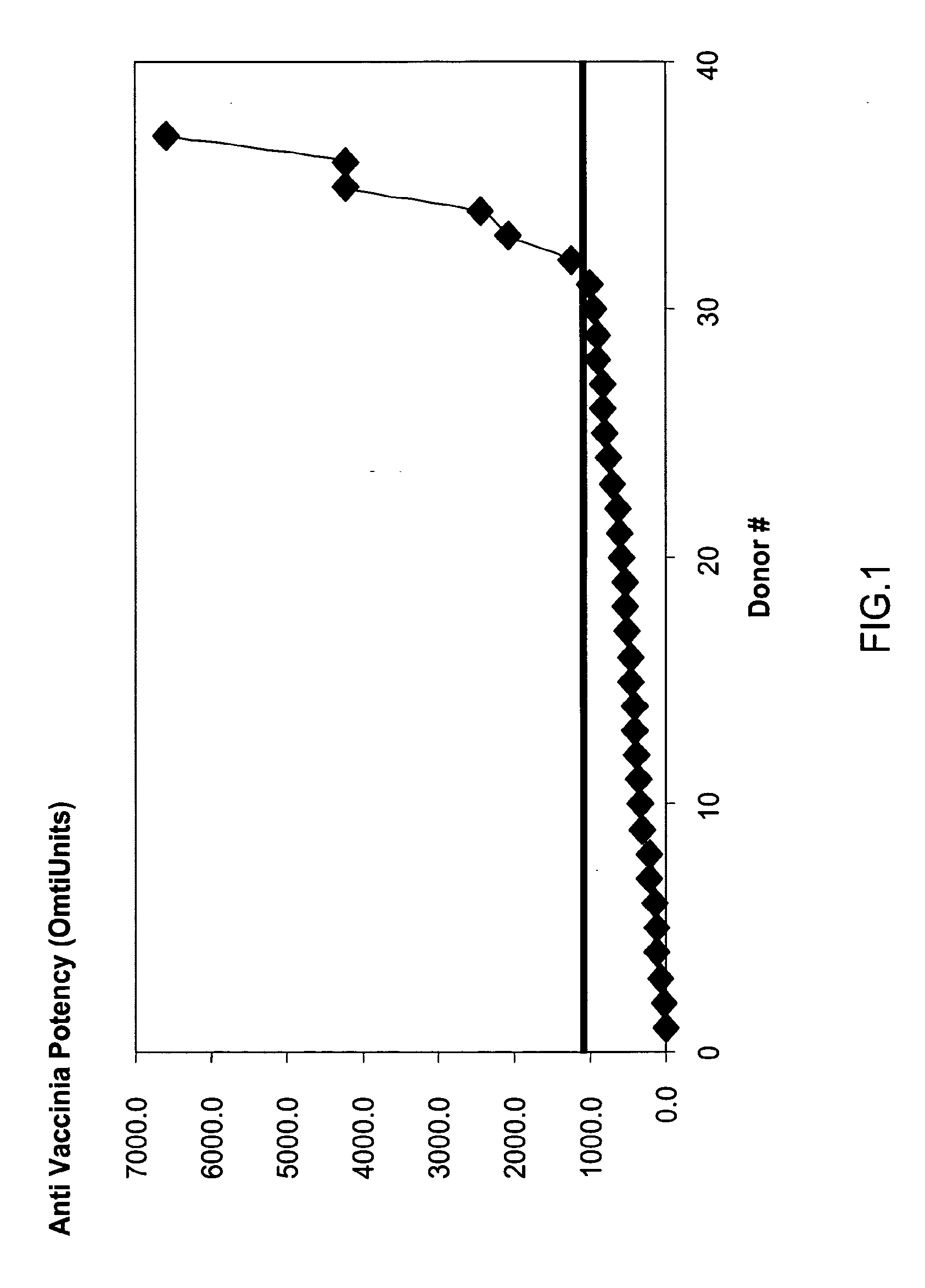
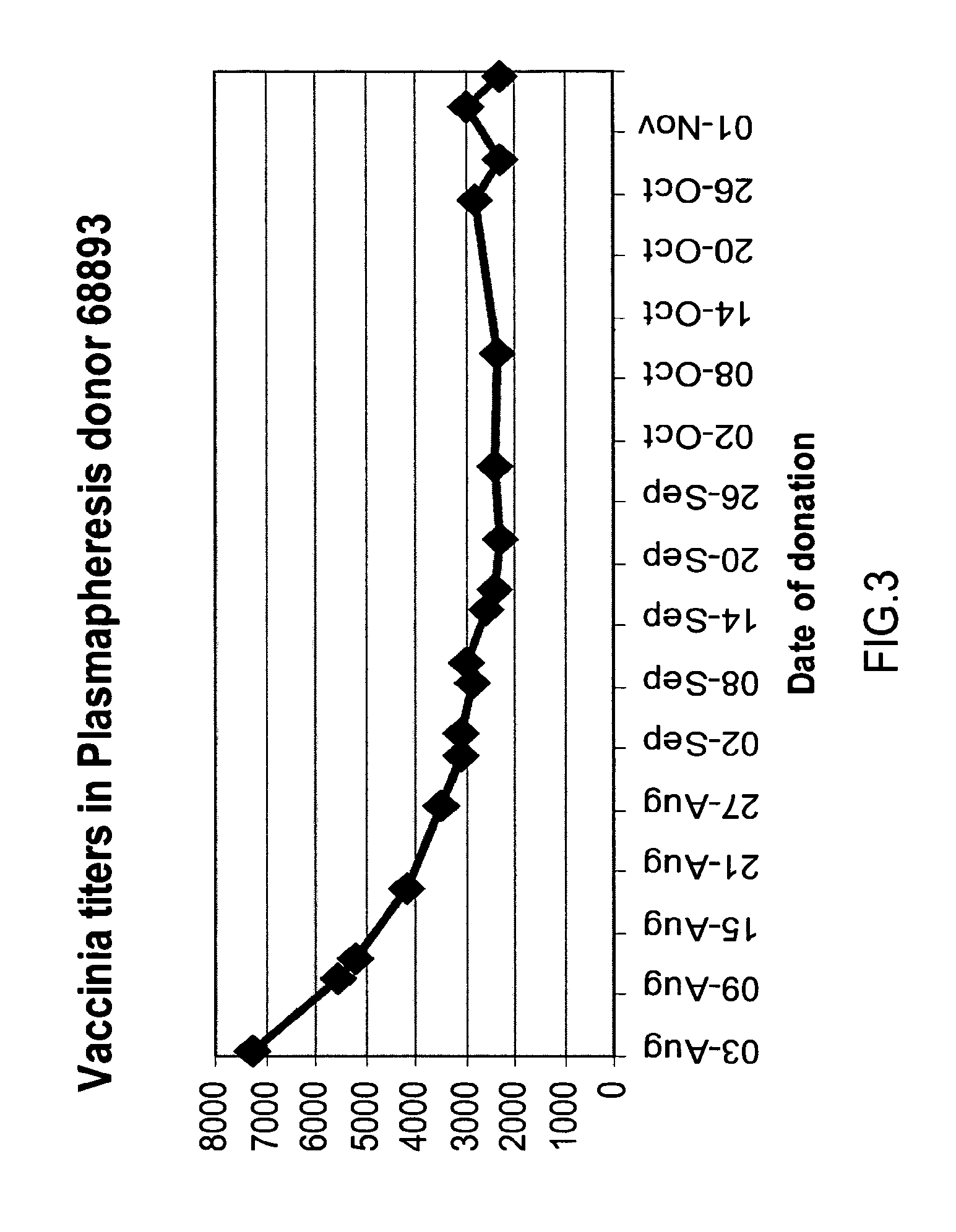
The invention provides an immune globulin having a high titre of antibody to Orthopoxvirus, pharmaceutical compositions comprising the immune globulin and methods for making same. In one embodiment the immune globulin is intravenously injectable. The invention also provides a colorimetric assay to detect neutralizing antibodies to vaccinia virus. The invention has a number of uses including detection of neutralizing antibodies to vaccinia virus and the immunization of persons against the Orthopoxvirus and in the treatment of related conditions.
Owner:CANGENE CORP
Intravenous immunoglobulin composition
ActiveUS20070037170A1High potencyLow volumePeptide/protein ingredientsAntibody mimetics/scaffoldsPopulationSmallpox virus
A method for preparing a concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, comprising: (a) selecting a population of individuals previously vaccinated against one or more antigens associated with the pathogenic microorganism; (b) determining the level of specific antibodies immunoreactive with the pathogenic microorganism in a blood or blood component of the individuals to identify very high titre individuals having a very high titre of the specific antibodies; (c) combining blood or blood components comprising immunoglobulins from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c), thereby obtaining a concentrated immunoglobulin composition. Also disclosed is a concentrated immunoglobulin composition comprising specific antibodies immunoreactive with a pathogenic microorganism, characterized in that the titre of specific antibodies of the composition is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against one or more antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates, and is therefore suitable for both iv and im administration. In a preferred embodiment, the pathogenic microorganism is smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
Intravenous immunoglobulin composition
ActiveUS20130011388A1Reduce riskEasy to managePeptide/protein ingredientsAntibody mimetics/scaffoldsSpecific antibodyHigh titre
A concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, is made by (a) selecting a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism; (b) identifying very high titre individuals by determining the level of specific antibodies immunoreactive with the pathogenic microorganism in the blood of the individuals; (c) combining blood from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c). A concentrated immunoglobulin composition can include specific antibodies immunoreactive with a pathogenic microorganism, wherein the titre of specific antibodies is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates. The pathogenic microorganism is preferably smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
Enterotoxin gene cluster (egc) superantigens to treat malignant disease
InactiveUS20090162315A1Good effectPrevent morbidityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseSystemic chemotherapy
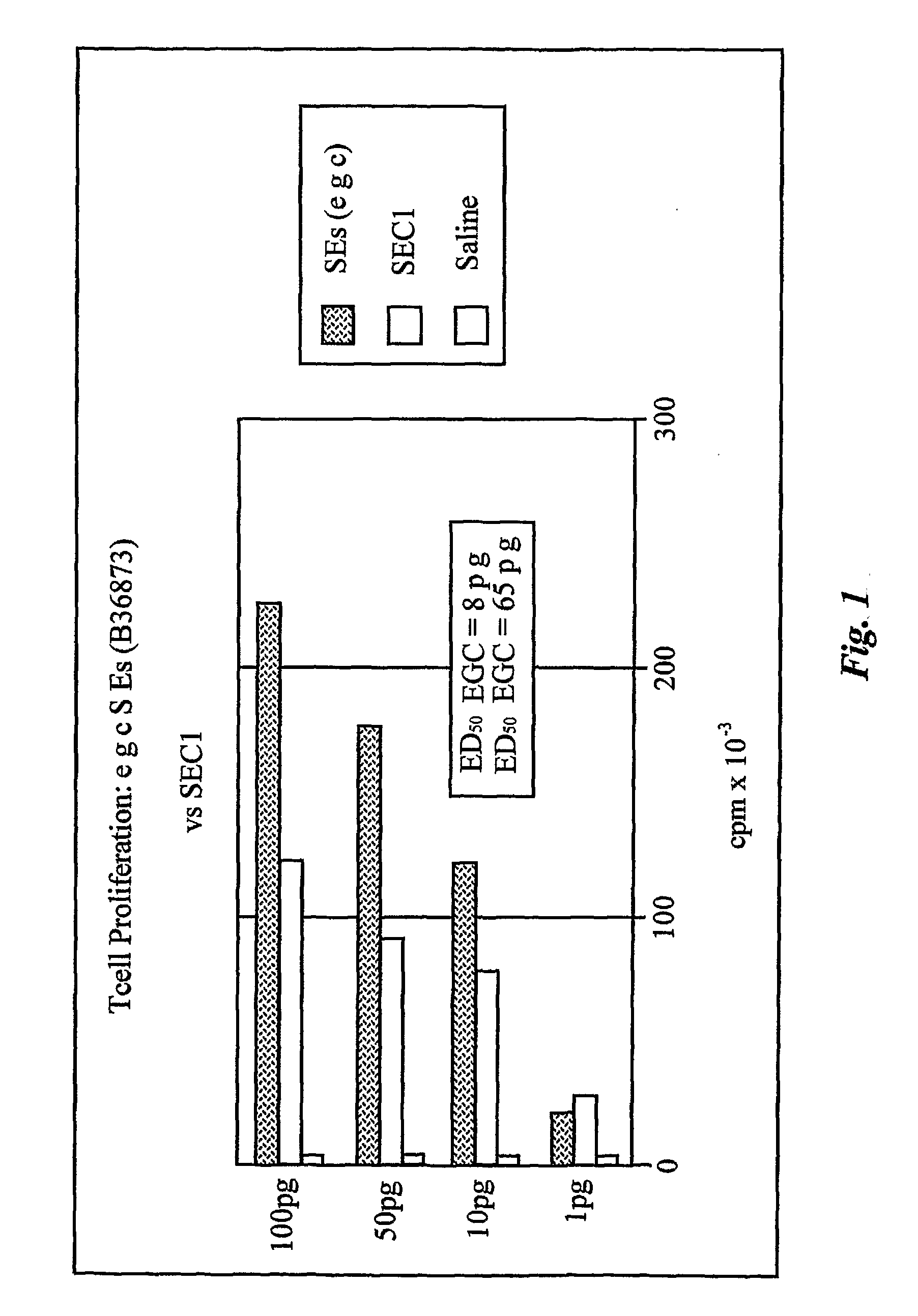
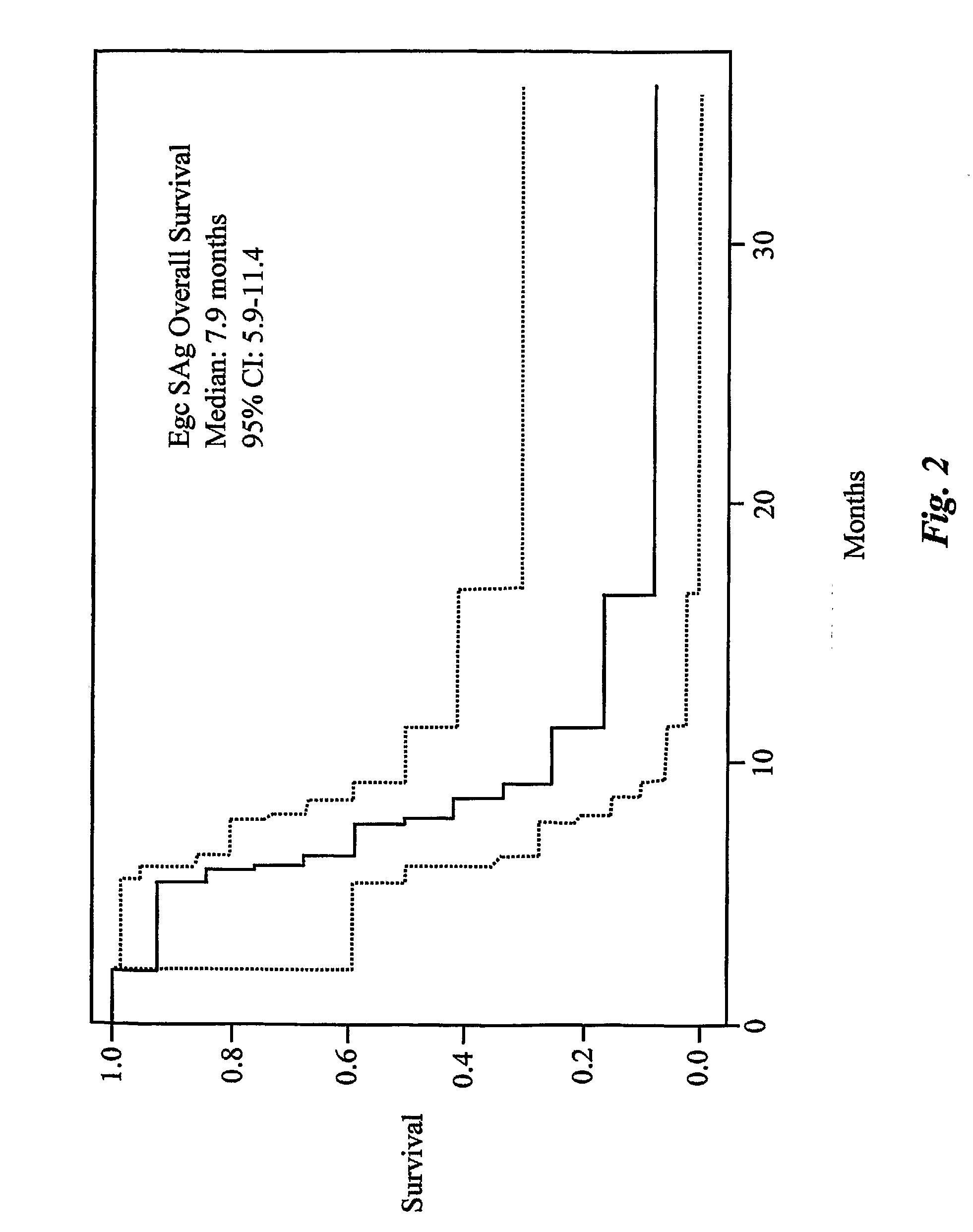
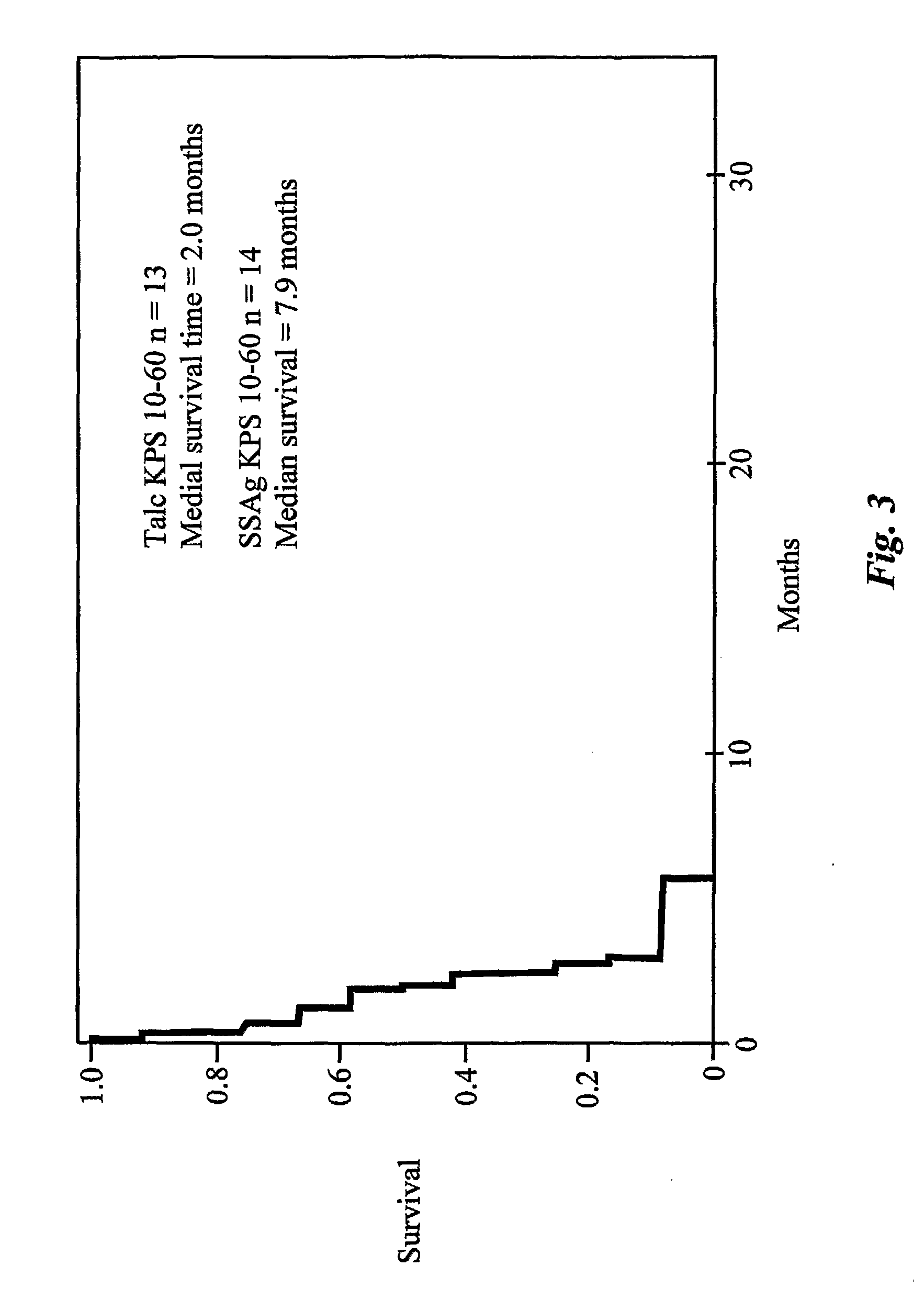
The use of classical superantigens for treatment of cancer has resulted in a low response rates and serious toxicity in humans which is attributable, in part, to the presence of preformed superantigen specific antibodies in the plasma of treated patients. The present invention addresses this problem by providing a method for treating tumors comprising the administration of one or a plurality of egc (enterotoxin gene cluster) staphylococcal enterotoxins comprising staphylococcal enterotoxins G, I, M, N, O. These superantigens in native unmodified form can be administered intrathecally, intratumorally, intravenously to humans with advanced lung cancer while resolving pleural effusions and prolonging survival to 300% above control patients treated with talc pleurodesis. Intratumoral egc superantigens induces a significant and sustained reduction of the tumor size. In contrast to classic Sags, the egc superantigens induced minimal toxicity, are rarely associated with the presence of preformed antibodies and are used as a plurality with a broad T cell Vβ profile. Useful egc superantigen compositions for parenteral administration native egc enterotoxins, homologues, fragments and fusion proteins of native egc enterotoxins capable of activating a broad spectrum of T cells expressing T cell receptor / α motifs. T cell survival-enhancing cytokines IL-7, Il-15, Il-23 are used. together with parenteral egc SE therapy. Also disclosed is combined therapy that includes parenteral, intratumoral or intrathecal superantigen compositions in combination with (i) intratumoral low, non-toxic doses of one or more chemotherapeutic drugs or (ii) systemic chemotherapy at reduced and non-toxic doses of chemotherapeutic drugs or (iii) radiation therapy or (iv) anti-angiogenic and tyrosine kinase inhibitors.
Owner:TERMAN DAVID S +4
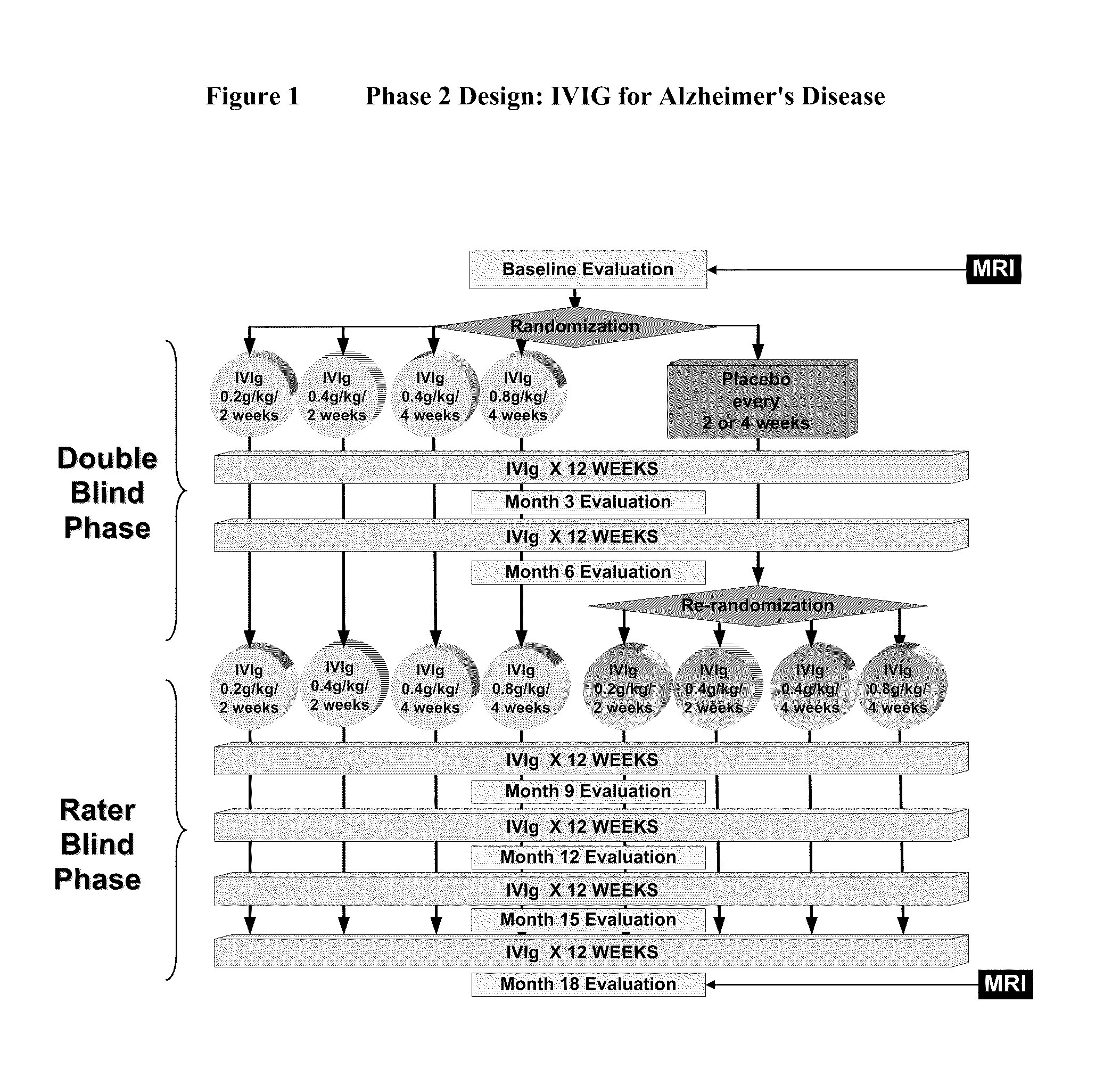
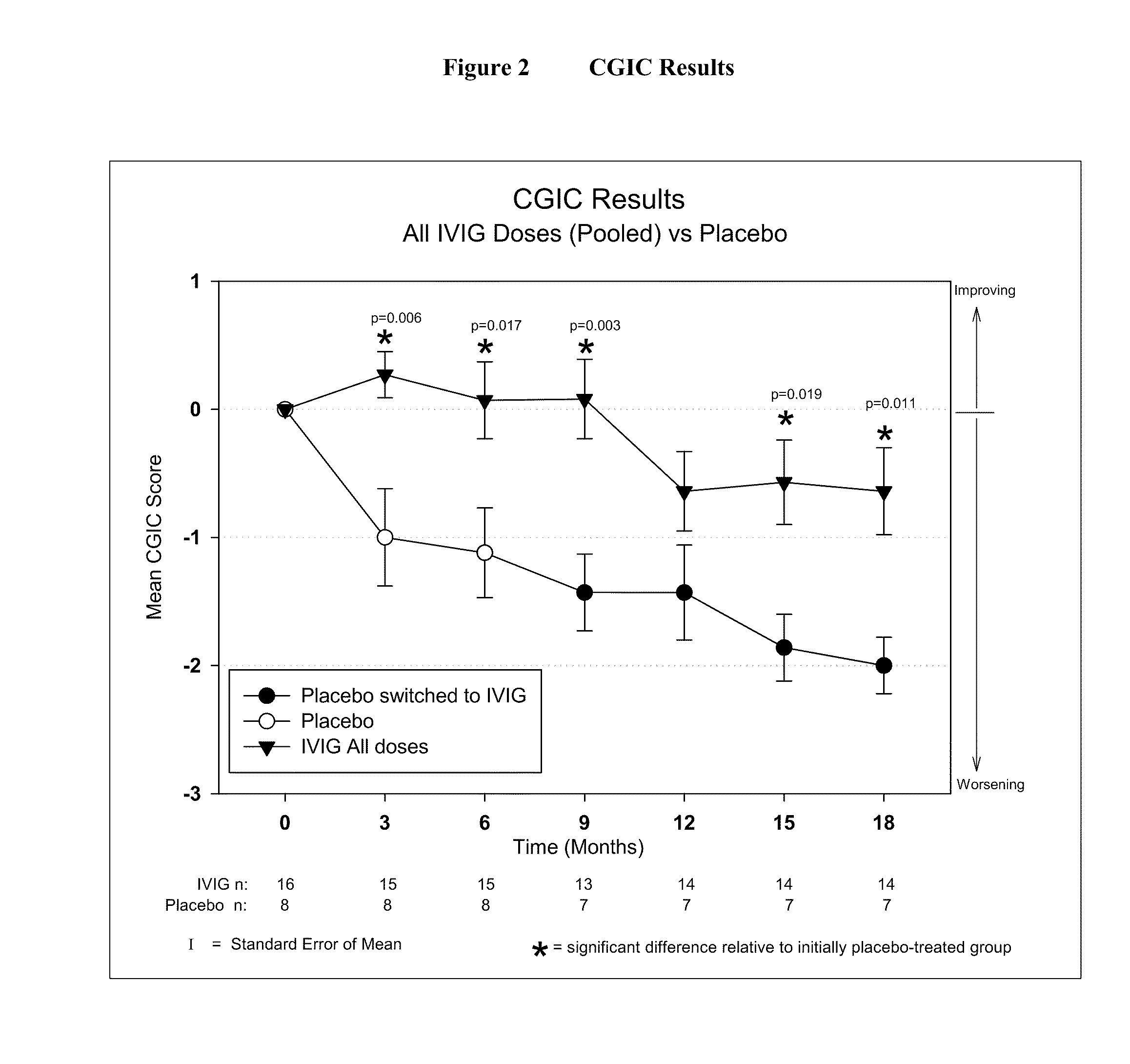
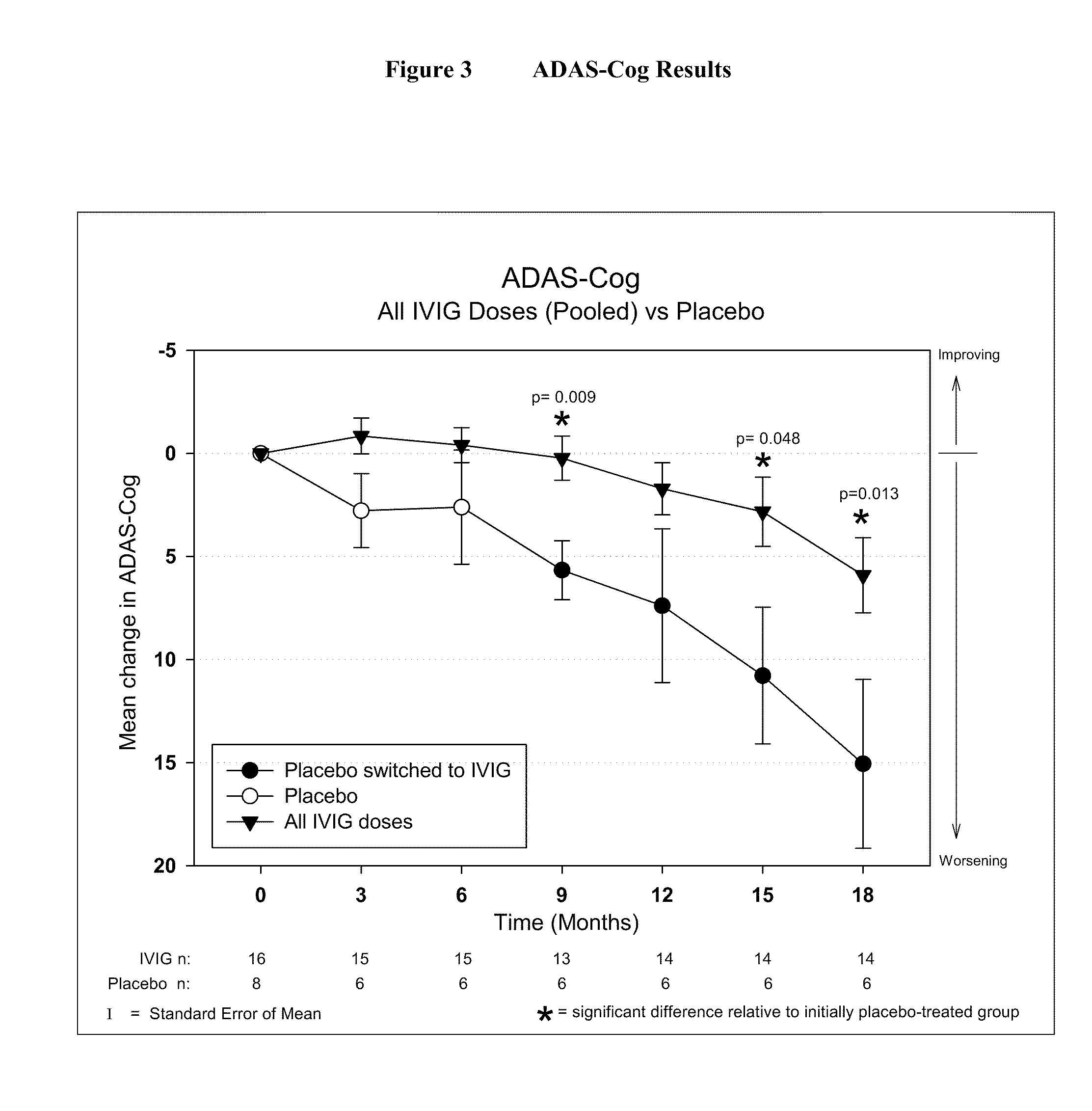
Use of ventricular enlargement rate in intravenous immunoglobulin treatment of alzheimer's disease
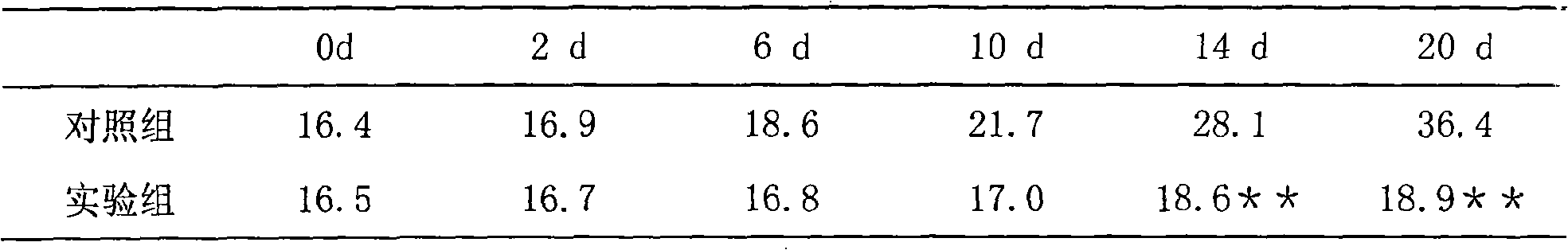
ActiveUS20110251479A1Guaranteed monitoring effectNervous disorderPeptide/protein ingredientsIntravenous IGDisease progression
The present invention relates to the use of MRI monitoring of ventricular enlargement rate as an objective measure for the purpose of assessing disease progression in patients suffering from Alzheimer's disease and for the purpose of determining therapeutic effectiveness of a treatment regimen for Alzheimer's patients. Methods for treating Alzheimer's Disease and monitoring therapeutic effectiveness are provided.
Owner:BAXALTA GMBH
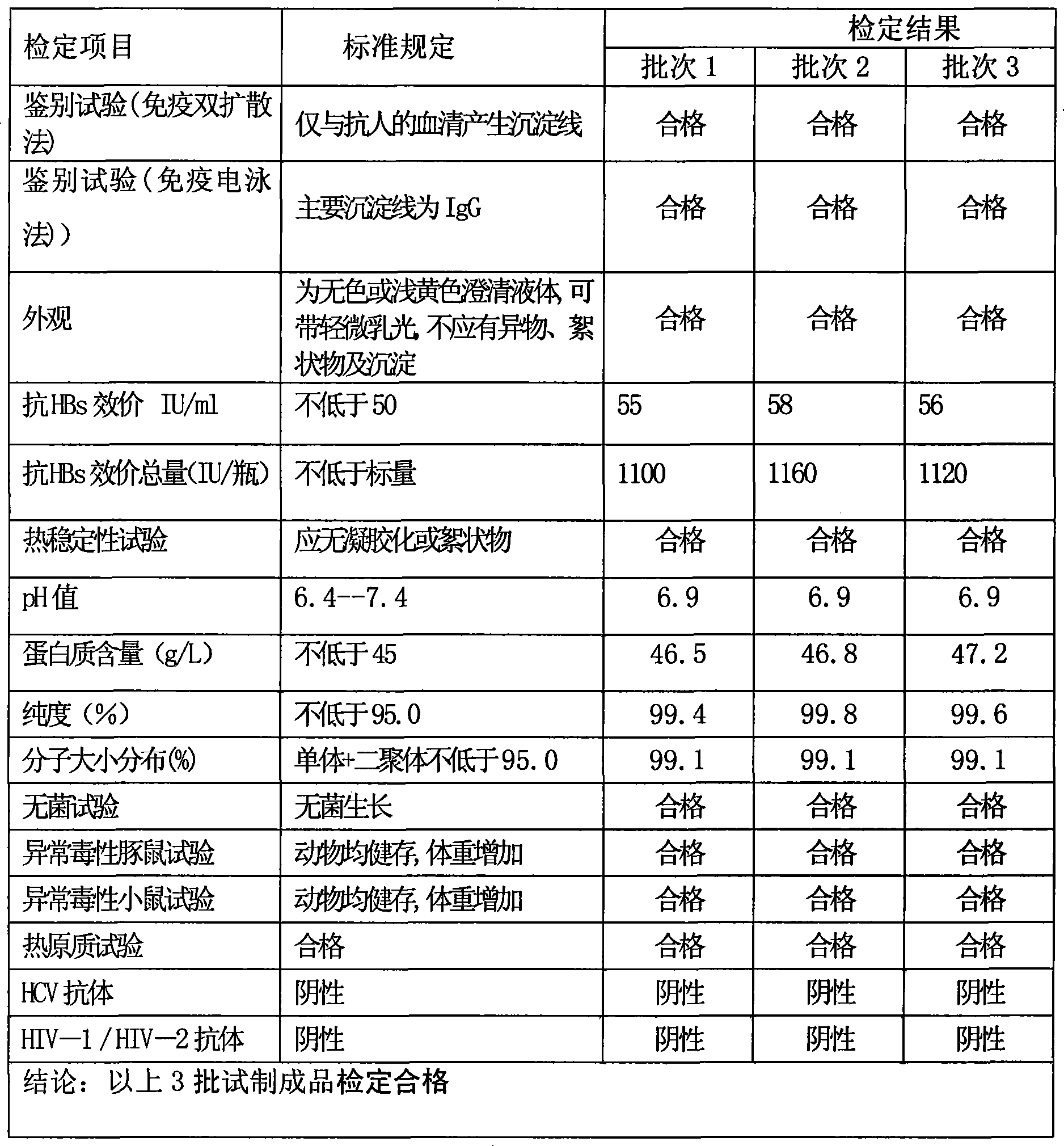
Intravenous injection employ persons hepatitis B immune globulin and method of preparing the same
ActiveCN101249265AReduce infection rateImprove survival ratePowder deliverySerum immunoglobulinsInfection rateFiltration
The invention discloses hepatitis B immunoglobulin used in intravenous injection and a preparation method thereof. The hepatitis B immunoglobulin used in intravenous injection comprises a high-titer hepatitis B virus antibody, and the content of the immunoglobulin is not less than 95.0 percent of the total protein content; the titer of the hepatitis B antibody is more than 50IU / ml, and the sum of an IgG monomer and a dimmer is not less than 95 percent; the hepatitis B immunoglobulin is prepared through the multi-step separation, virus inactivation, ultra-filtration and dealcoholization and the filling of the raw plasma. The hepatitis B immunoglobulin used in intravenous injection is an effective drug used for preventing the patients with liver transplantation from being re-infected by the hepatitis B virus, and can obviously reduce the hepatitis B virus infection rate of the patients with liver transplantation and improve the survival rate of the patients; By overcoming the present technical bottleneck and adopting the unique process parameter control, the preparation process can produce immunoglobulin with anti-hepatitis B specificity with a conventional device of cold ethanol precipitation and pressure filtration and separation, and is suitable for scale industrial production. The products have good anti-hepatitis B activity, and are safe and reliable.
Owner:广东双林生物制药有限公司
Functional dendritic polymer gene vector system of targeted malignant cerebroma
InactiveCN101337076AGrowth inhibitionEfficient transfectionGenetic material ingredientsPharmaceutical non-active ingredientsCyclic peptidePolyamide
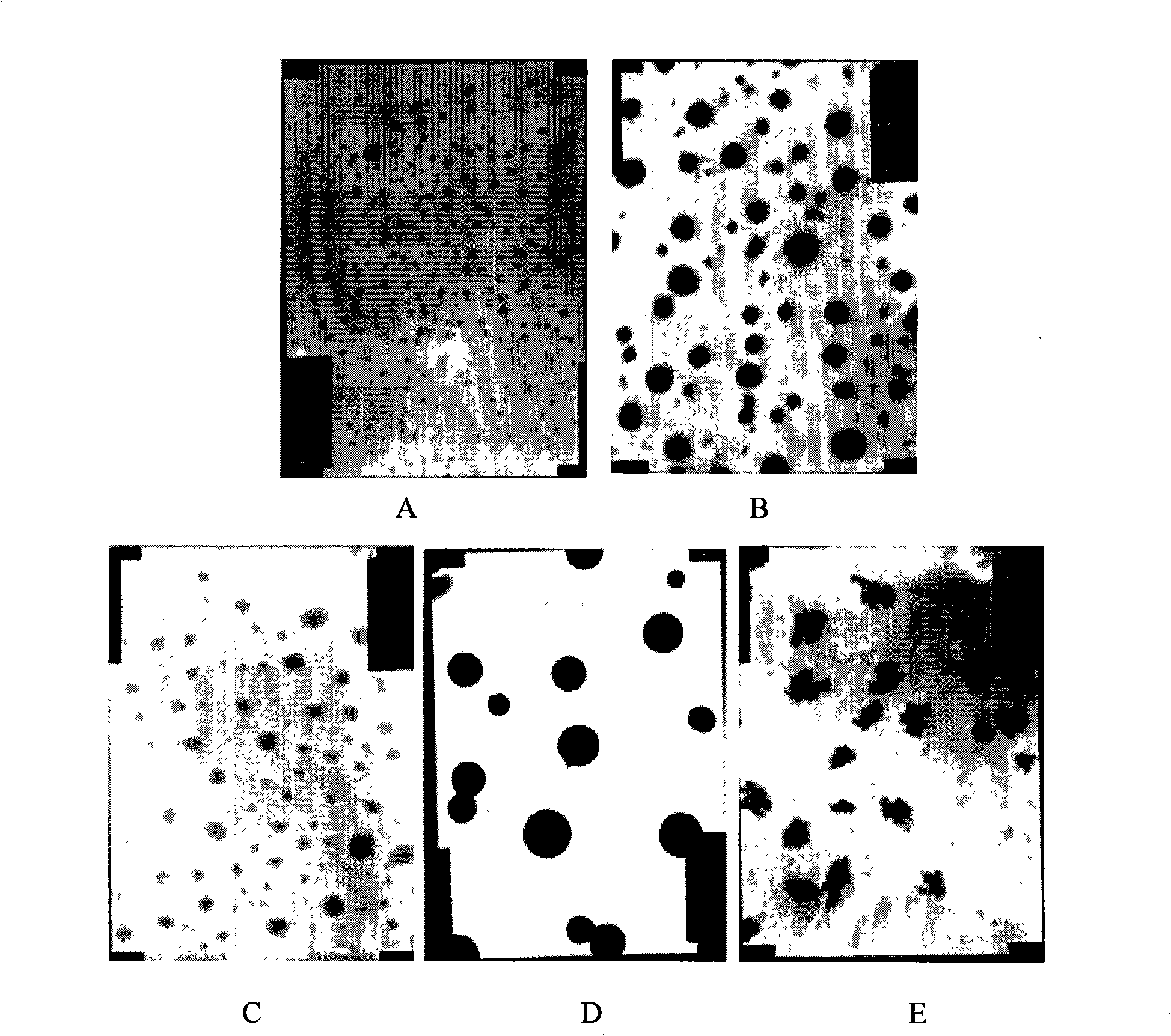
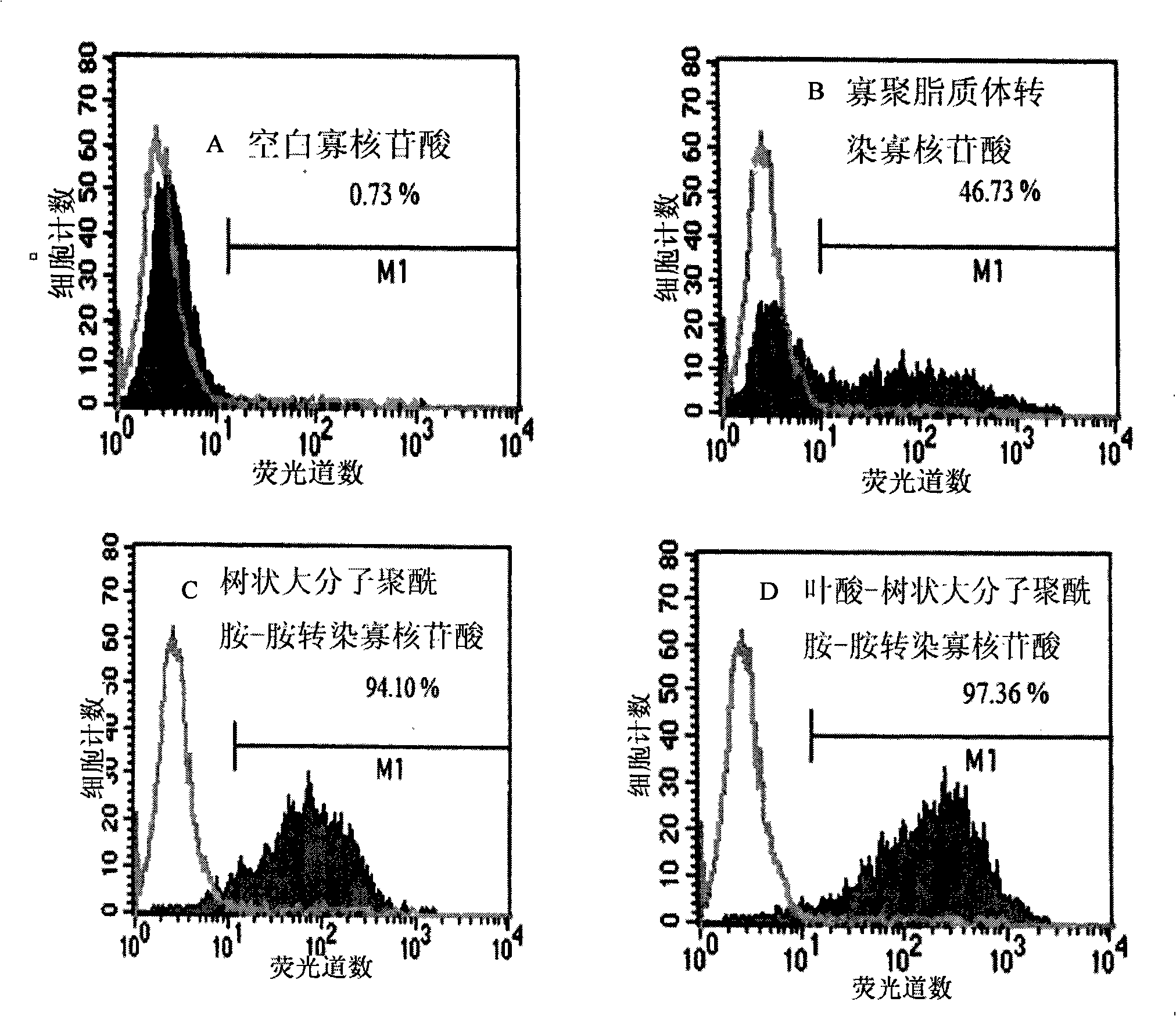
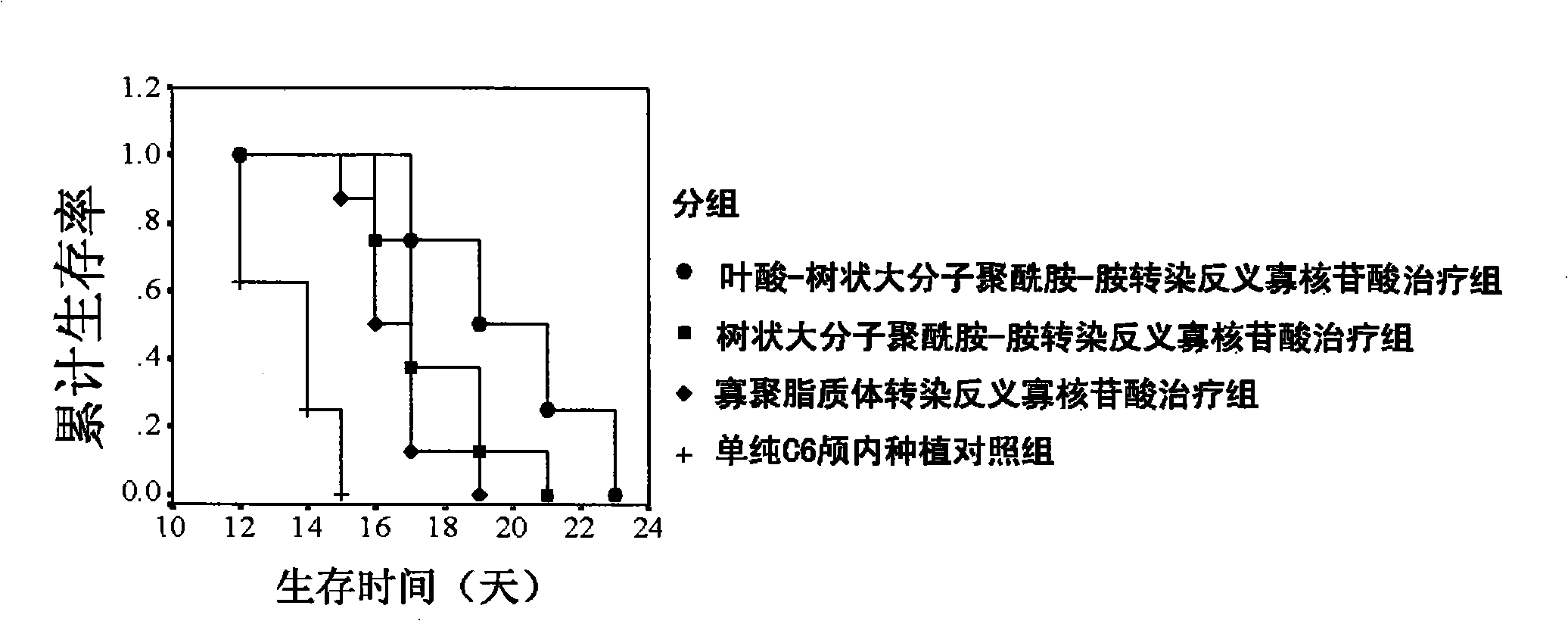
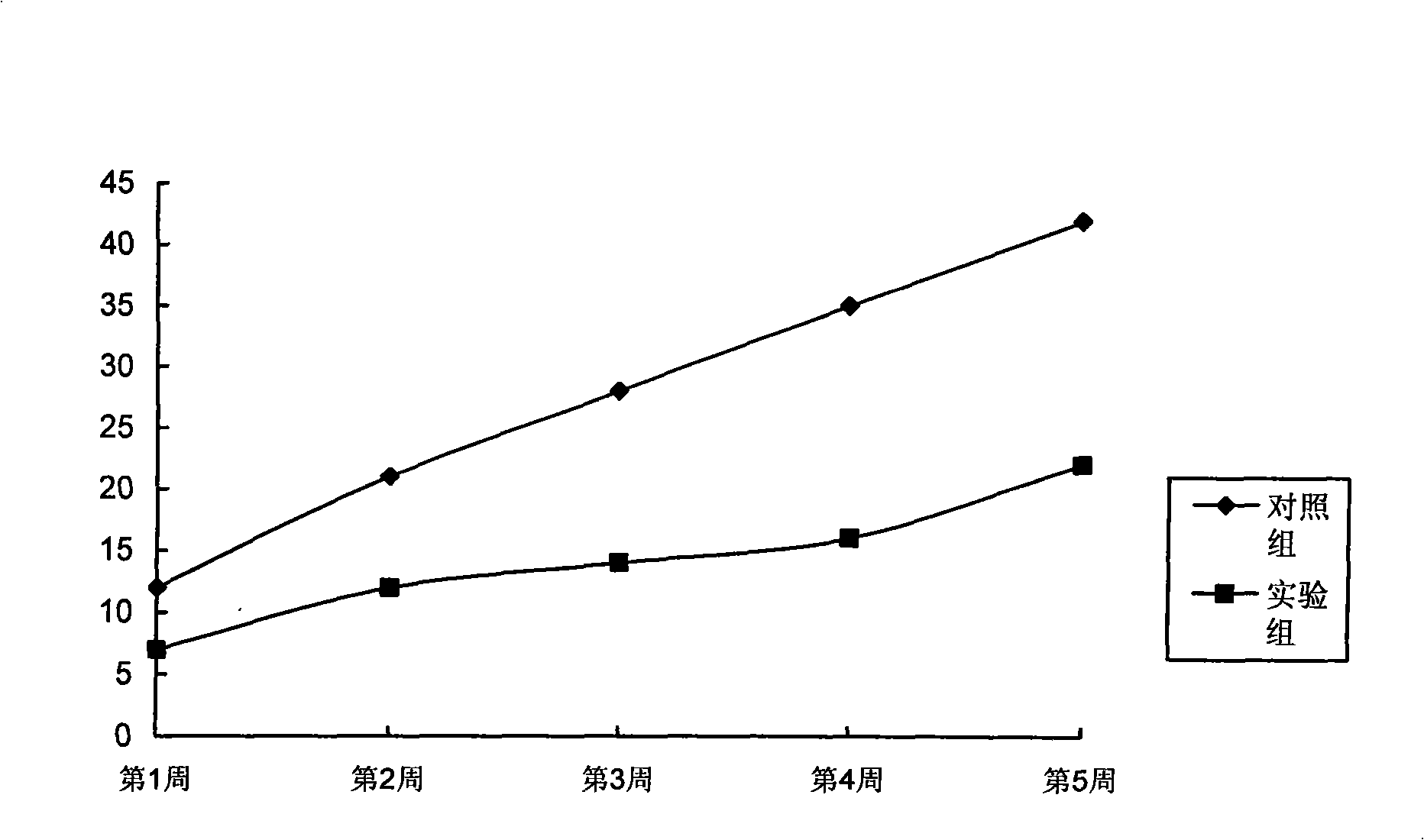
The invention relates to a functionalized arborescent macro radical carrier system for targeting malignant cerebroma. The functionalized arborescent macro radical carrier system is obtained by taking one or more of the following combinations as the targeting functional factor: transferrin and transferrin monoclonal antibody, or polyclonal antibody, folic acid (FA) and epidermal growth factor receptor monoclonal antibody, or polyclonal antibody and RGD cyclic peptide, chemically connecting the combinations to arborescent macro radical polyamide-amine (PAMAM) carrier system, and then compounding the combinations with treatment factor oligonucleotide, siRNA molecule or plasmid DNA. Through intra-tumor stereotactic injection, carotid injection or tail vein, the distribution of genes in the malignant cerebroma can be improved, high expression can be realized, and the effective cerebroma inhibition can be realized. Compared with the commercialized gene carrier Oligofectamin and unmodified PAMAM, the FA-PAMAM can remarkably improve the targeting delivery ability of the in vitro and in vivo genes.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
ADMINISTRATION OF GLUTATHIONE (REDUCED) VIA INTRAVENOUS OR ENCAPSULATED IN LIPOSOME FOR THE AMELIORATION OF TNF-alpha EFFECTS AND FLU-LIKE VIRAL SYMPTOMS AND TREATMENT AND PREVENTION OF VIRUS
InactiveUS20070077258A1Improve edemaReduce oxygenationSsRNA viruses negative-senseBiocideWhole bodyTumor necrosis factor alpha
The invention is a method of treatment of the symptoms related to inflammation that accompanies the release of Tumor Necrosis Factor-alpha in diseases such as viral infection such as those affecting the respiratory tract by providing systemic glutathione (reduced) by oral administration of glutathione (reduced) in a liposome encapsulation or by the intravenous administration of reduced glutathione. The administration of a therapeutically effective amount of oral liposomal glutathione (reduced) results in improvement of symptoms of disease induced by the release of TNF-α in infectious disease states such as respiratory and other viruses. The product is novel in that it is stable across the temperature ranges encountered in shipping and does not need to be refrigerated for storage. Compounds enhancing the effect of the liposomal glutathione as well as intravenous glutathione are contemplated such as Selenium.
Owner:GUILFORD F TIMOTHY
Application of T-2 toxin in the preparation of drugs for treating bone cancers and myeloproliferative disorder
ActiveCN101513399AGrowth inhibitionEfficient killingSkeletal disorderAntineoplastic agentsMyeloproliferative DisordersDrug administration
The invention discloses a new use of T-2 toxin, that is, application of T-2 toxin in the preparation of drugs for treating bone cancers and myeloproliferative disorder, wherein the curative dose thereof is 0.1-20 mg / Kg (weight), preferably 0.5-8 mg / Kg (weight), during drug administration, the method comprises oral administration, intravenous injection, hypodermic injection and intramuscular injection. The invention establishes animal or cell models of multiple myeloma, osteosarcoma and myelodysplastic syndrome, and inspects the inhibition of the T-2 toxin on the tumors or tumor cells by injection or cell co-culture. The experiment shows that the T-2 toxin is capable of inhibiting the growth of tumor cells and has good killing effect on the bone cancers and myeloproliferative disorder.
Owner:SHENZHEN ICARBONX INTELLIGENT PEPTIDE PHARM TECH CO LTD
Use of cytokine levels in intravenous immunoglobulin treatment of alzheimer's disease
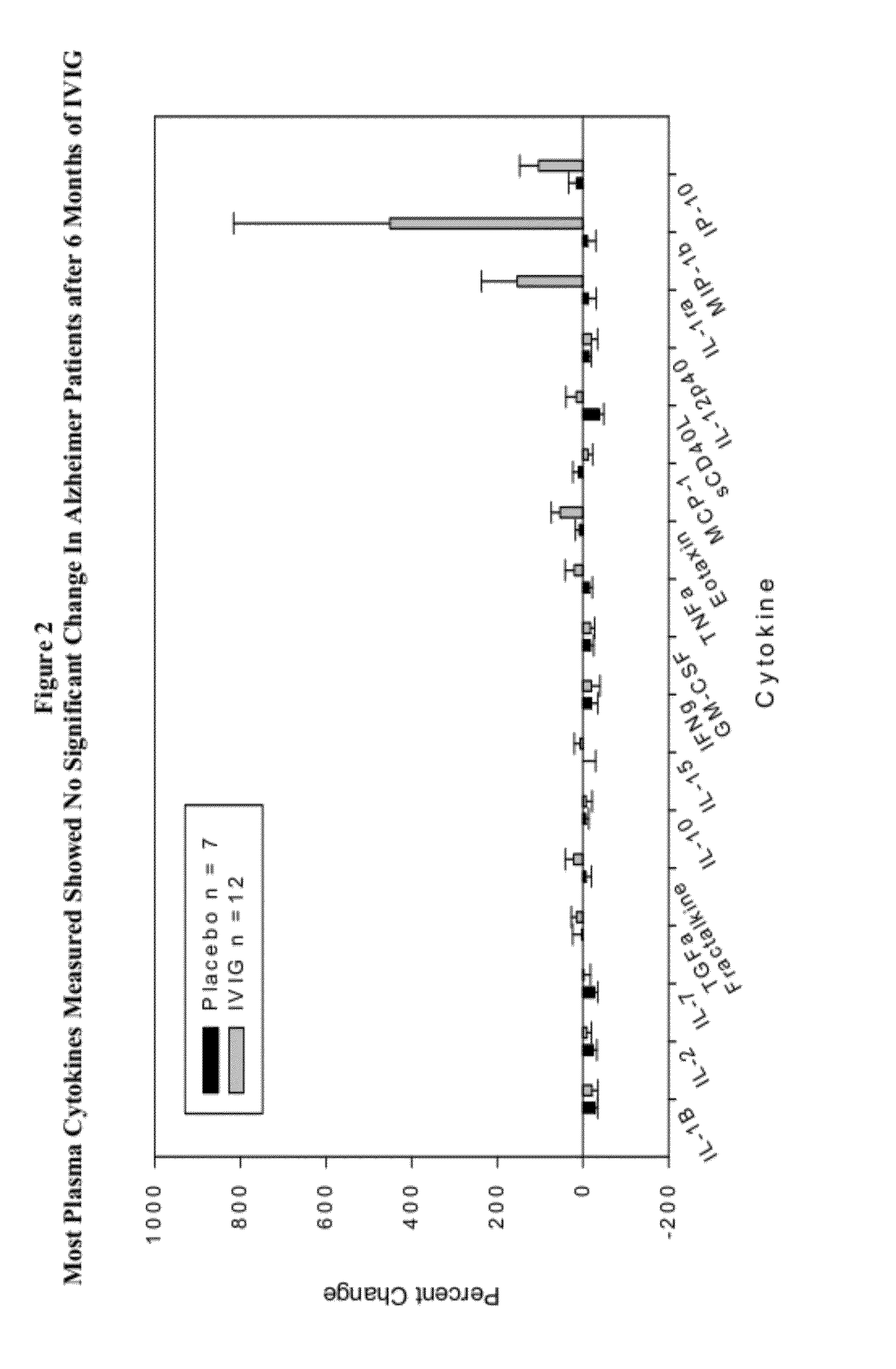
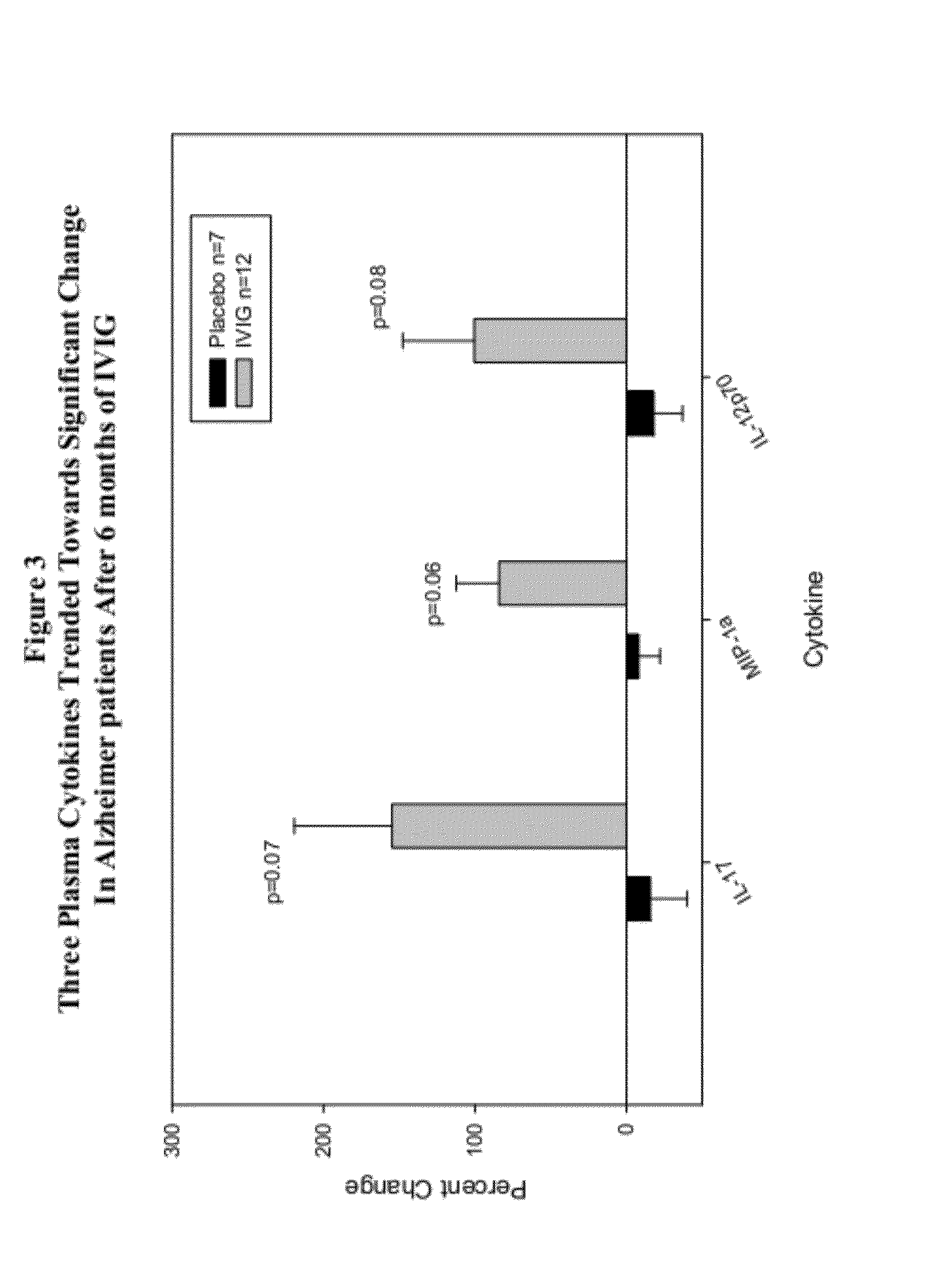
InactiveUS20120251524A1Guaranteed monitoring effectGood curative effectNervous disorderElectrolysis componentsCytokineDisease progression
The present invention relates to the use of the level of certain cytokines in a patient's blood as an objective measure for the purpose of assessing disease progression in patients suffering from Alzheimer's disease and for the purpose of determining therapeutic effectiveness of a treatment regimen. Methods for treating Alzheimer's disease and monitoring therapeutic effectiveness are provided.
Owner:BAXALTA GMBH
Method for detecting activated blood coagulation factor XI in human intravenous immunoglobulin
ActiveCN103185710AAvoid preactivationIndirectly reflect changes in production contentFluorescence/phosphorescenceInitiation factorFactor ii
The invention discloses a method for detecting an activated blood coagulation factor XI in human intravenous immunoglobulin. The method comprises the steps as follows: (1) mixing an IVIG (intravenous immunoglobulin) sample with specially-preprocessed platelet-poor plasma (PPP); (2) adding a thrombin specific fluorogenic substrate with a phospholipid agent into a reaction system; (3) adding an initiation factor for starting a TGT (thrombin generation test), wherein the initiation factor comprises a calcium ion source and the phospholipid agent; (4) obtaining a thrombin generation curve providing parameters by the optimized reaction system; and (5) negatively correlating the peak time of thrombin (TTP) of the detected sample with the FXIa (activated blood coagulation factor XI) level in the sample. The FXIa level in the detected sample can be indirectly obtained by comparing the TTP of the detected sample with that of an FXIa standard sample. The method is suitable for detecting residual micro FXIa in human intravenous immunoglobulin (IVIG) products and is used for extracorporal evaluation of thrombosis risk of related products.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Method for efficiently preparing Enterobacter sakazakii polyclonal antibody
InactiveCN105693855AEfficient preparationNovel methodSerum immunoglobulinsImmunoglobulins against bacteriaImmune profilingNew Zealand white rabbit
The invention belongs to the technical field of immunoassay, and concretely relates to a method for efficiently preparing an Enterobacter sakazakii polyclonal antibody. The method comprises the following steps: immunizing New Zealand white rabbits with Enterobacter sakazakii granular antigens through adopting a subcutaneous multi-point injection and ear vein venous injection combination technology, and further purifying antiserum by a Protein A affinity chromatography column to rapidly obtain the high-tilter Enterobacter sakazakii polyclonal antibody. Compared with traditional immunization methods, the method disclosed in the invention has the advantages of shortening of the immunization time by 7-14d, and great increase of the antibody tilter. A West-blotting and IFAT combination technology is used to detect the specificity of the antibody, and a result shows that the prepared antibody has very high specificity to Enterobacter sakazakii. The method provided by the invention is simple and efficient, provides technical support for preparation of the Enterobacter sakazakii polyclonal antibody, and is helpful for developing the immunodetection technology of the Enterobacter sakazakii.
Owner:OCEAN UNIV OF CHINA
Systemic administration of nac for vaccination prophylaxis
InactiveUS20100233193A1Organic active ingredientsSnake antigen ingredientsWhole bodyAntiviral therapy
Owner:GUILFORD F TIMOTHY +1
Systemic administration of nac as an adjunct in the treatment of terrorism exposures such as radiation and for prophylaxis
The invention is for the combination and related methods of N-acetyl-cysteine oral, inhaled, or intravenous, or glutathione inhaled or intravenous, generally in combination with antibiotic and / or antiviral therapy to ameliorate the toxic effects of infection with materials used in Bioterror incidents such as Bacillus anthracis and smallpox virus, and alternatively, upon exposure to radiation, during testing, and vaccination, as treatment prior to treatment with antibiotic or antiviral therapy to ameliorate the toxic effects of infection and exposure with these organisms.
Owner:GUILFORD F TIMOTHY +1
Method for preparing immunoglobulin G by rapidly extracting plasma of COVID-19 patient in rehabilitation period
ActiveCN112010968AHigh antibody titerFast access to plasmaSerum immunoglobulinsImmunoglobulins against virusesIntravenous gammaglobulinErythroid cell
The invention relates to a method for preparing immunoglobulin G by rapidly extracting plasma of a COVID-19 patient in a rehabilitation period, and more particularly, to a method for preparing intravenous injection COVIDI-19 immunoglobulin G by rapidly separating component plasma from whole blood by using a closed multicellular component automatic separation system (MACSS), for example, to prepareintravenous injection superimmunoglobulin. The intravenous immunoglobulin can be used for treating patients infected by novel coronavirus. The method comprises the following steps: separating human peripheral blood into three component layers, namely an erythrocyte layer, a cell concentration layer and a plasma layer, by using a closed multi-cell component automatic separation system, and then carrying out virus inactivation on the obtained plasma to obtain plasma capable of being used for preparing intravenous immunoglobulin G. The invention also relates to a method for preparing an intravenous immunoglobulin G preparation by using the plasma component obtained by the above method. According to the method, the plasma obtaining speed is high, and the antibody titer of the prepared plasmaand intravenous immunoglobulin G is high.
Owner:深圳博雅感知药业有限公司
Rabbit bordetella bronchiseptica subunit vaccine
ActiveCN106620685APrevention of Bordetella septicemiaAntibacterial agentsBacterial antigen ingredientsAdjuvantBordetella bronchiseptica
The invention provides a rabbit bordetella bronchiseptica subunit vaccine, particularly relates to rabbit bordetella bronchiseptica (QDBb01 strain with the preservation number of CCTCC M 2016607) identified through isolated culture, Bb gene specific primer PCR amplification and sequencing. Rabbit bordetella bronchiseptica OMP (osteoblast milk protein) is extracted, antigenic protein with the protein molecular weight larger than 40 kDa is purified, then the protein concentration is measured with a Bradford protein content measuring method and diluted to be 150 ug / ml, the subunit vaccine ( about 30 ug / ml for finished products) is prepared by emulsifying the protein and a aluminum hydroxide adjuvant in a volume ratio being 1:4, the vaccine is injected subcutaneously into a rabbit with the immunity weight being about 1.5 kg in the dosage of 1 ml for each rabbit, after 14 days, ear intravenous infusion challenge is performed with the rabbit bordetella bronchiseptica QDBb01 strain (LD50 being 4.8*105 CFU) according to 100 LD50, the rabbit is observed for 7 days after challenge, and the challenge protection rate of the rabbit is 10 / 10 through statistics.
Owner:YEBIO BIOENG OF QINGDAO
Liposome preparation for anticoagulant thrombolytic difunctional fusion protein and preparation method thereof
InactiveCN102144974ASmall particle sizeGuaranteed Particle SizePeptide/protein ingredientsPharmaceutical non-active ingredientsCholesterolPhospholipid
The invention relates to the field of biopharmaceutics, in particular to a liposome preparation for anticoagulant thrombolytic difunctional fusion protein, namely anticoagulant thrombolytic difunctional fusion protein of 12 peptides of hirudin and reteplase (HV12p-rPA) and a preparation method thereof. The anticoagulant thrombolytic difunctional fusion protein (HV12p-rPA) constructed in the laboratory has the dual effects of anticoagulation and thrombolysis by structural identification, expression, chromatography renaturation, purification and extracorporal and intracorporal pharmacodynamic experiments. In the preparation method, the liposome preparation is prepared from the anticoagulant thrombolytic difunctional fusion protein serving as a raw material, and a liposome is prepared by an optimized membrane dispersion-probe ultrasonic method; and the optimized membrane dispersion-probe ultrasonic method is characterized in that a prescription which is most suitable for improving the envelop rate of the HV12p-rPA liposome is selected by taking a ratio of phospholipid to cholesterol, the concentration of protein medicaments, the volume of buffer solution and water-bath ultrasonic time as influence factors, and the grain diameter of the HV12p-rPA liposome is reduced and homogenized further by probe ultrasonic, so that the HV12p-rPA liposome of which the grain diameter is between 140 and 145 nanometers and which is used for intravenous injection is prepared. By the preparation method, the envelop rate of the prepared HV12p-rPA liposome is over 90 percent, and extracorporal thrombolytic activity and extracorporal anticoagulant activity are 23,810 international unit (IU) .mg<1> and 414 antithrombin unit (ATU) .mg<1> respectively.
Owner:SICHUAN UNIV
Non-viral vector, and preparation method and application thereof
InactiveCN102030754AGood compatibilityOvercome securityOrganic chemistryGenetic material ingredientsDrugWilms' tumor
The invention discloses a non-viral vector, and a preparation method and application thereof. The non-viral vector is nanoparticles prepared by blending polyethyleneimine and a compound shown in a formula V. A vector of a medicament provided by the invention is the non-viral vector provided by the invention. The medicament comprises the non-viral vector provided by the invention and a therapeuticDNA wrapped in the non-viral vector. The target tumor of the medicament is highly expressed by a folate receptor FRalpha. The tumor which can be targeted by the medicament is a folate receptor FRalpha negatively-expressed tumor and heart. After being intravenously injected into a tumor-bearing mouse inoculated with the tumor of folate receptor positive cells, the non-viral vector provided by the invention can carry gene specificity to the tumor cells and continuously and effectively express target genes in the tumor cells.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Rifabutin for the treatment of acinetobacter baumannii
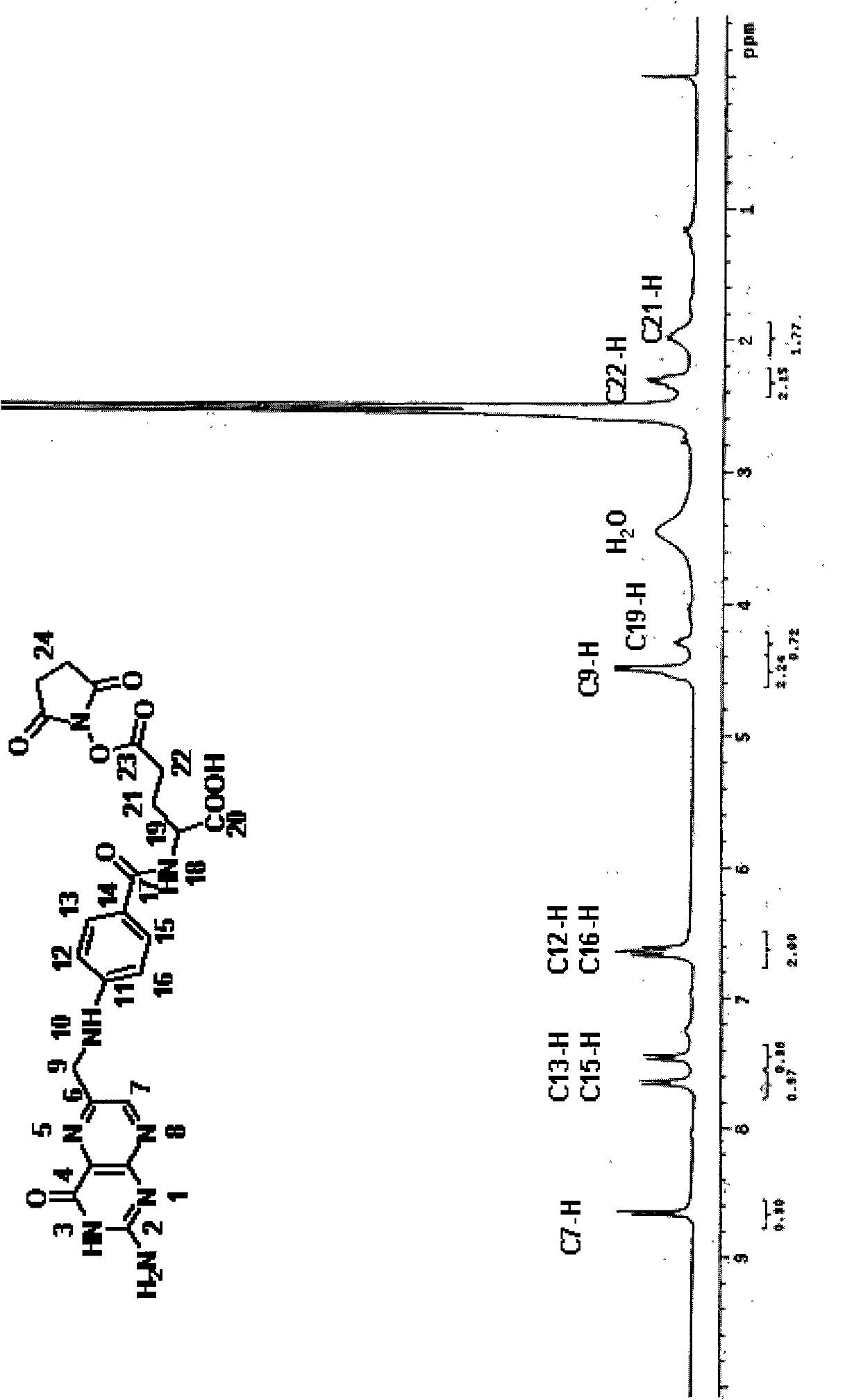
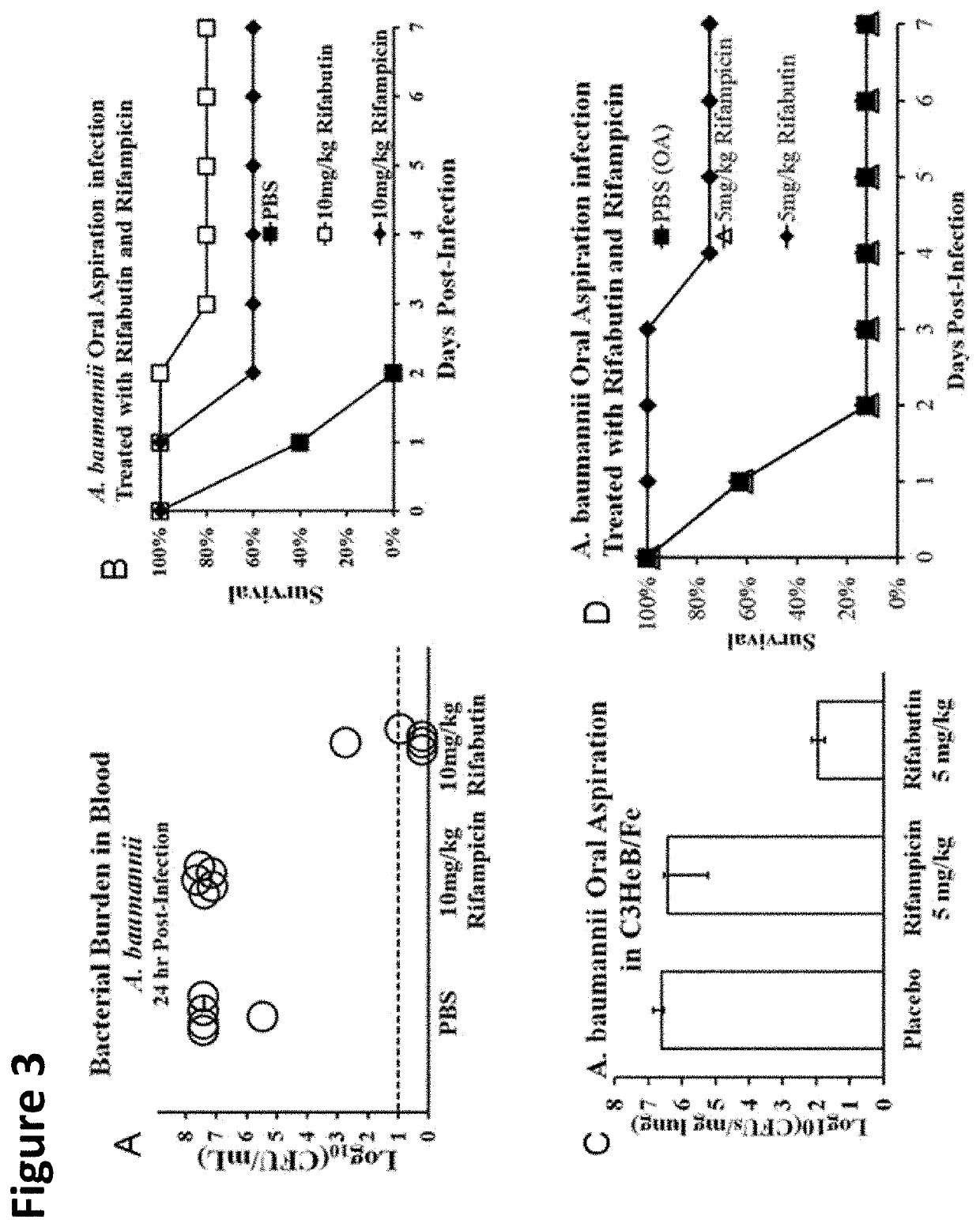
ActiveUS20200222373A1Reduction in CFUsModest survival benefitAntibacterial agentsOrganic active ingredientsRifabutinEfficacy
Owner:UNIV OF SOUTHERN CALIFORNIA
Acid-modified arabinogalactan protein composition
InactiveUS20030211077A1Antibacterial agentsOrganic active ingredientsDrug withdrawal symptomsArabinogalactan protein
An acid-modified arabinogalactan protein composition, having an arabinose-galactose ratio of less than 3.5:1 or less than 80% of the arabinose:galactose ratio of the arabinogalactan protein component of the composition prior to acid modification, prepared from Astragalus membranaceus, especially from the roots of Atragalus membranaceus, is capable of reconstitution into an aqueous intravenously injectable formulation; and is useful for stimulating hematopoiesis, inducing the proliferation or maturation of megakaryocytes, stimulating the production of IL-1beta, Il-6, TNF-alpha, IFN-gamma, GM-CSF, or G-CSF, stimulating the production or action of neutrophils, treating neutropenia, anemia, or thrombocytopenia, accelerating recovery from exposure (e.g. accidental or non-therapeutic exposure, as well as therapeutic exposure) to cytotoxic agents or radiation, treating cachexia, emesis, or drug withdrawal symptoms, or modifying biological responses or protecting hepatic cells in hepatitis B, in a mammal when intravenously administered to the mammal.
Owner:PHARMAGENESIS
Compositions comprising an Anti-lag-3 antibody or an Anti-lag-3 antibody and an Anti-pd-1 or Anti-pd-l1 antibody
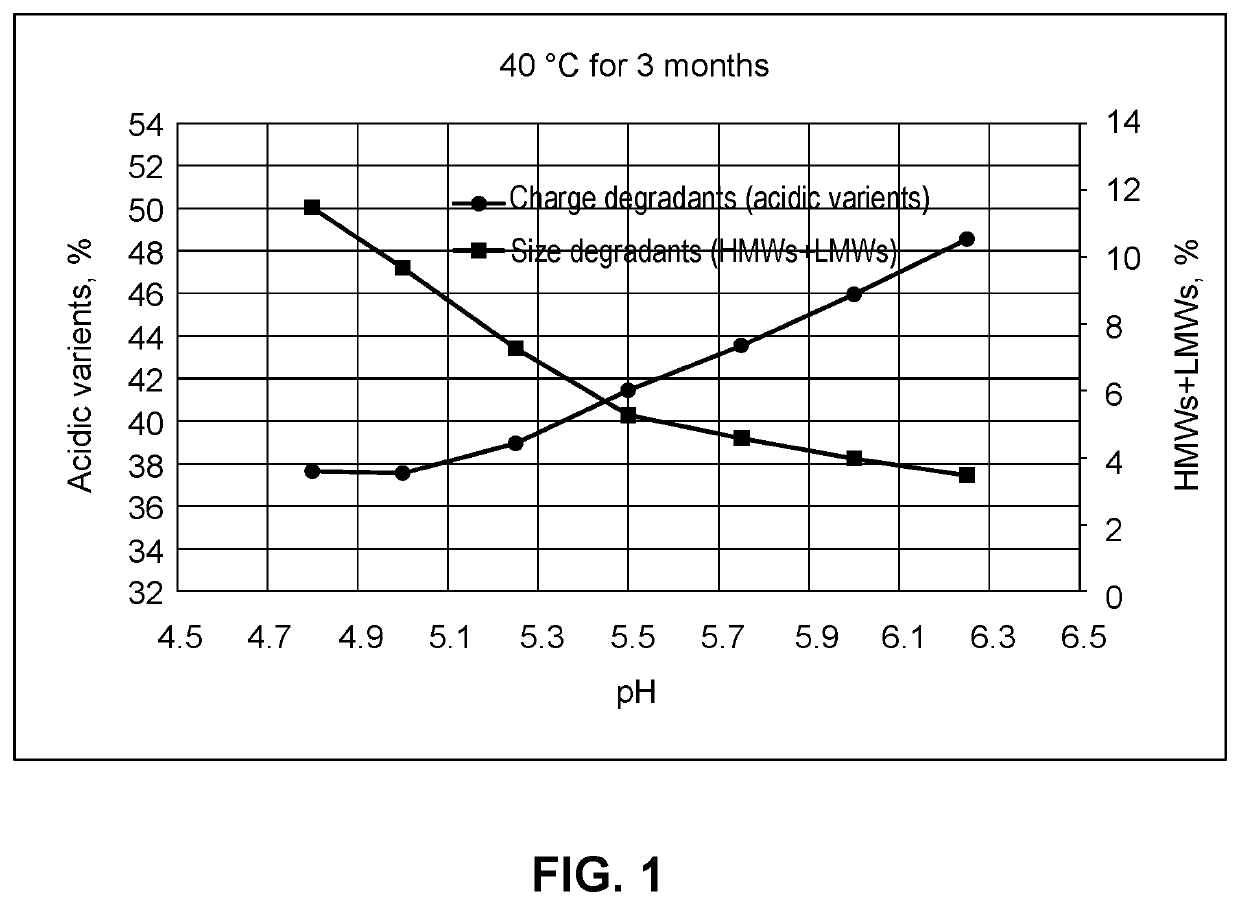
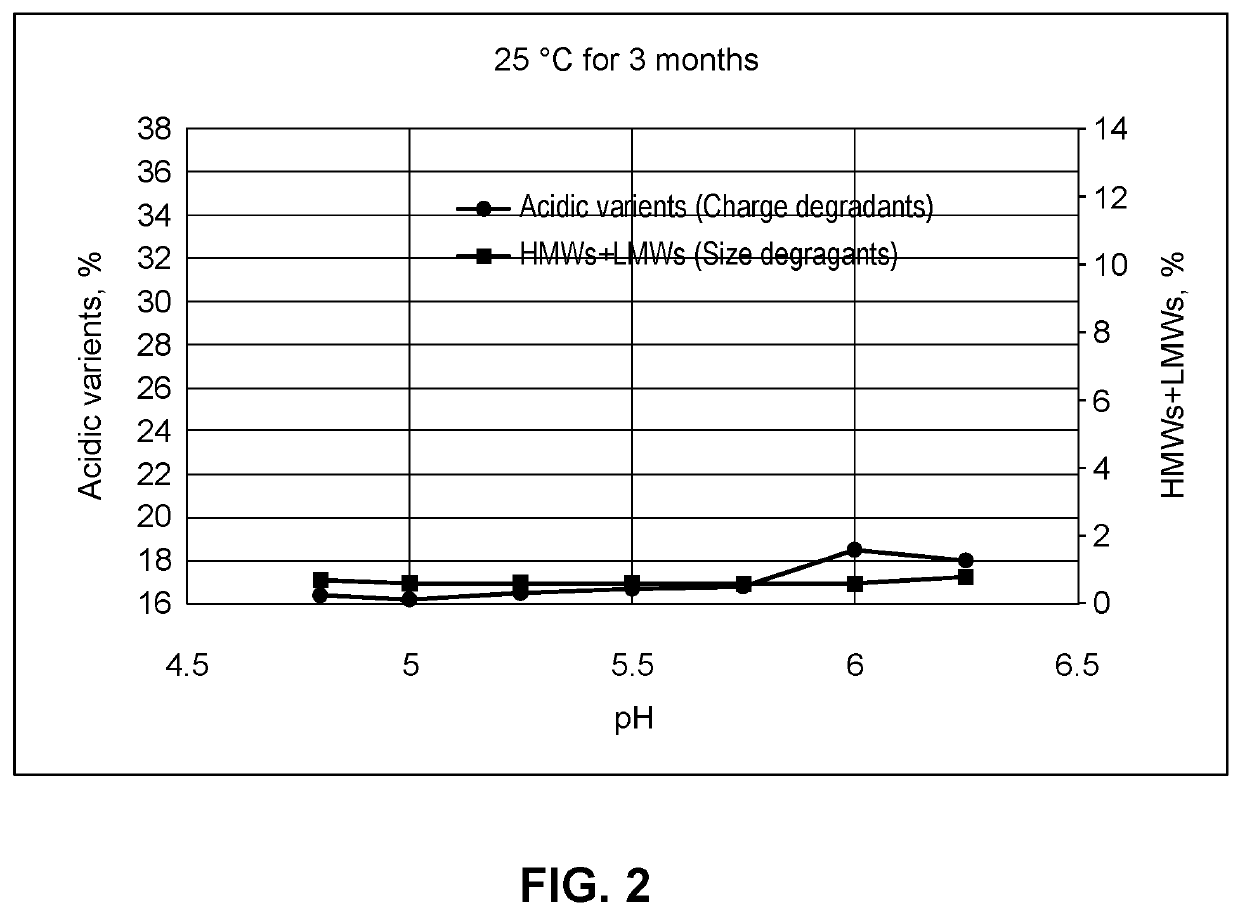
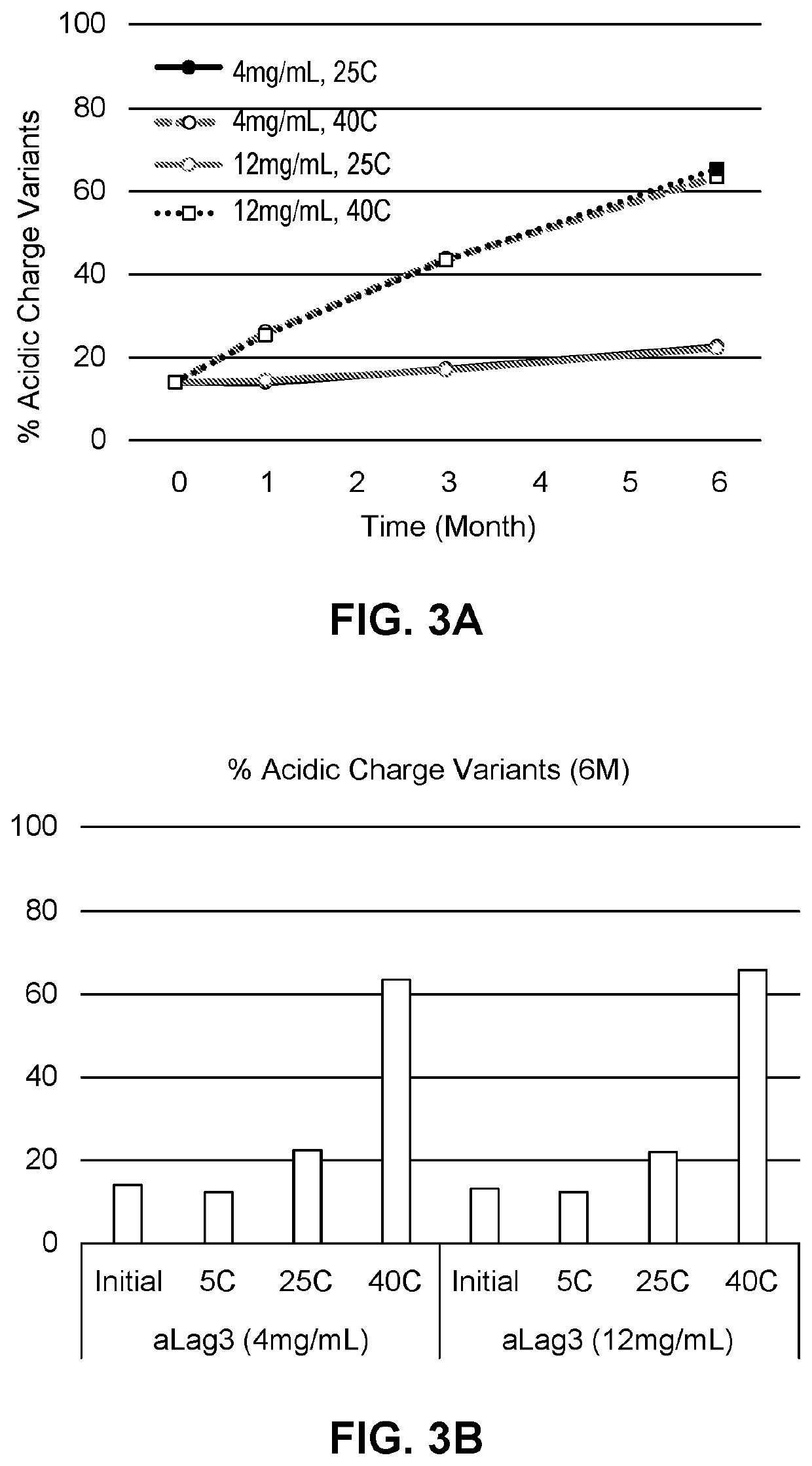
PendingUS20210283251A1Shorten the counting processReduce concentrationInorganic non-active ingredientsPharmaceutical containersAntiendomysial antibodiesIntravenous IG
This provides pharmaceutical compositions that comprise (i) an anti-LAG-3 antibody or antigen binding fragment thereof or (ii) an anti-LAG-3 antibody or antigen binding fragment thereof and an anti-PD-1 antibody, anti-PD-L1 antibody, or antigen binding fragment thereof. Also provided are pharmaceutical compositions that comprise a buffering agent, stabilizing or bulking agent, and a surfactant. The disclosure also provides a vial, syringe, intravenous bag, or kit that comprises the compositions, and methods for using the compositions.
Owner:BRISTOL MYERS SQUIBB CO
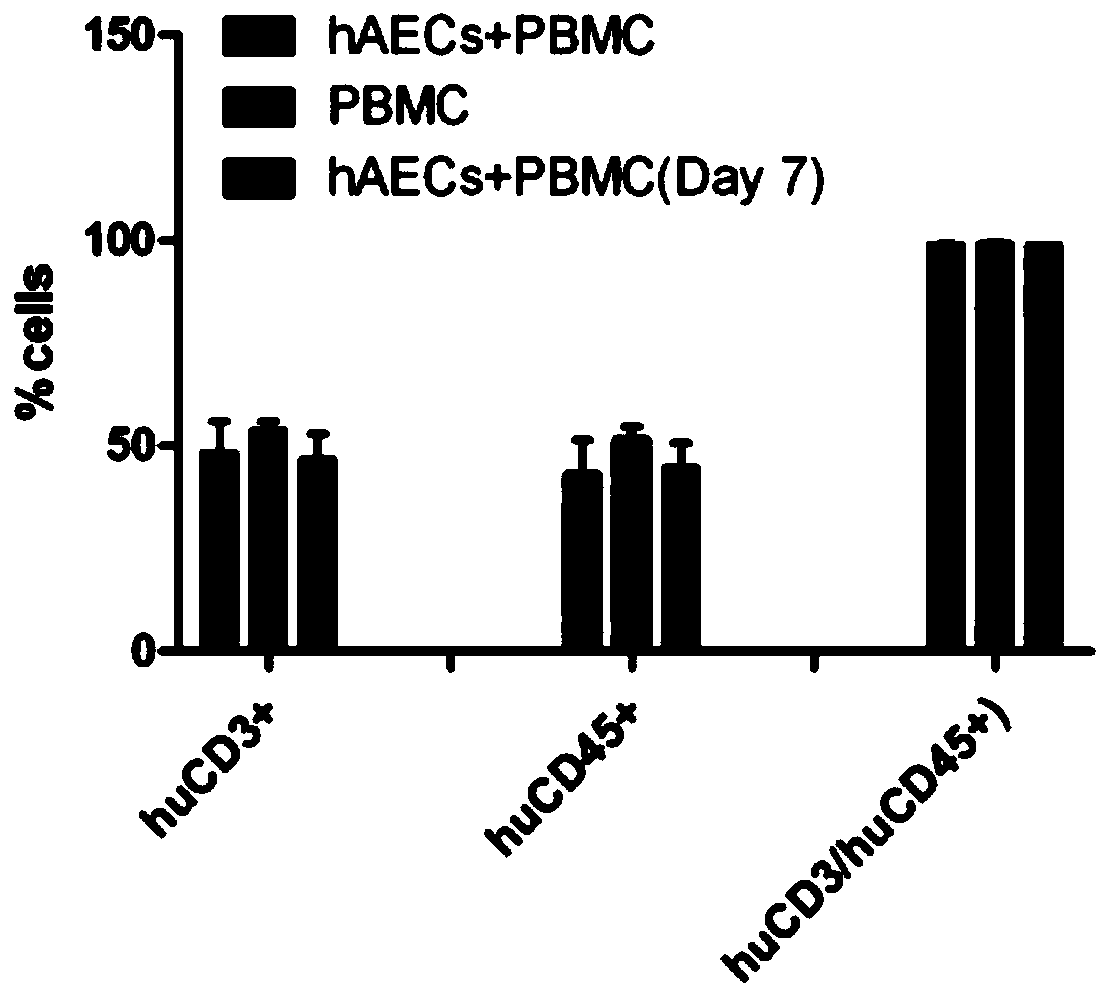
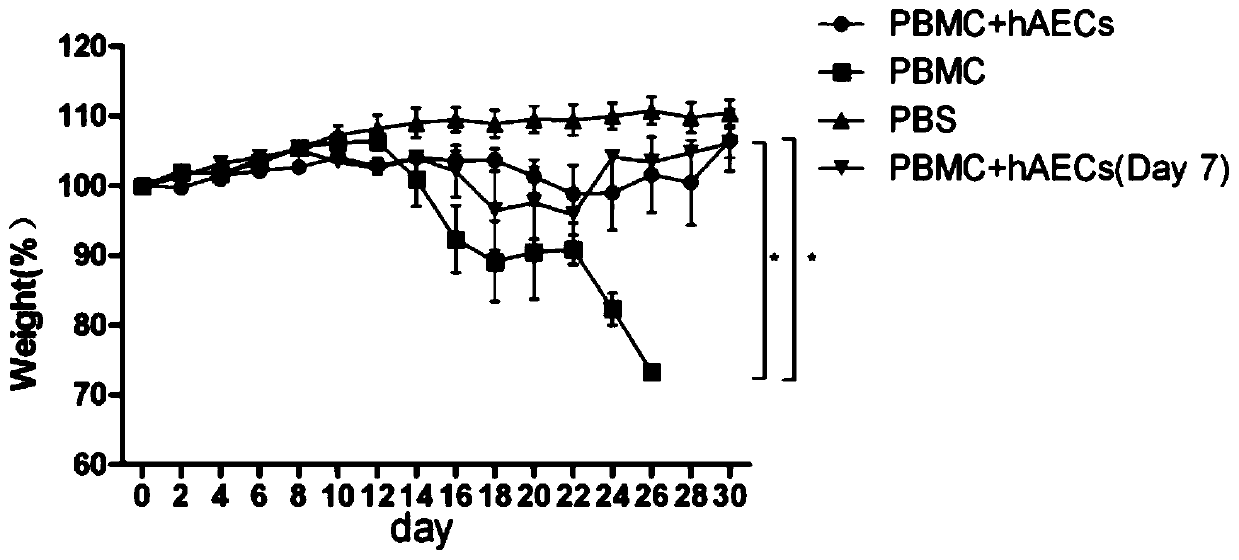
Use of human amniotic epithelial cells in treatment of graft-versus-host disease
ActiveCN110090227AReduce infiltrationReduce lesionsCell dissociation methodsEpidermal cells/skin cellsIntramedullary injectionIntravenous IG
The invention relates to a use of human amniotic epithelial cells (hAECs) in treatment of graft-versus-host disease (aGVHD). The invention discloses a method for treating and / or improving the graft-versus-host disease by using effective dose of the amniotic epithelial cells or a cell preparation containing the amniotic epithelial cells alone or in combination with other drugs. The amniotic epithelial cells can be given to patients by intravenous injection or intramedullary injection, the dosage range given at each time is about 10<3>-10<9> cells, the infiltration of inflammation cells caused by aGVHD to target organs can be effectively alleviated, the lesion of target organs is also significantly alleviated, the hAECs are found out to have an apoptosis-promoting effect on multiple leukemiacell lines and be used for treating the graft-versus-host disease.
Owner:ZHEJIANG UNIV +1
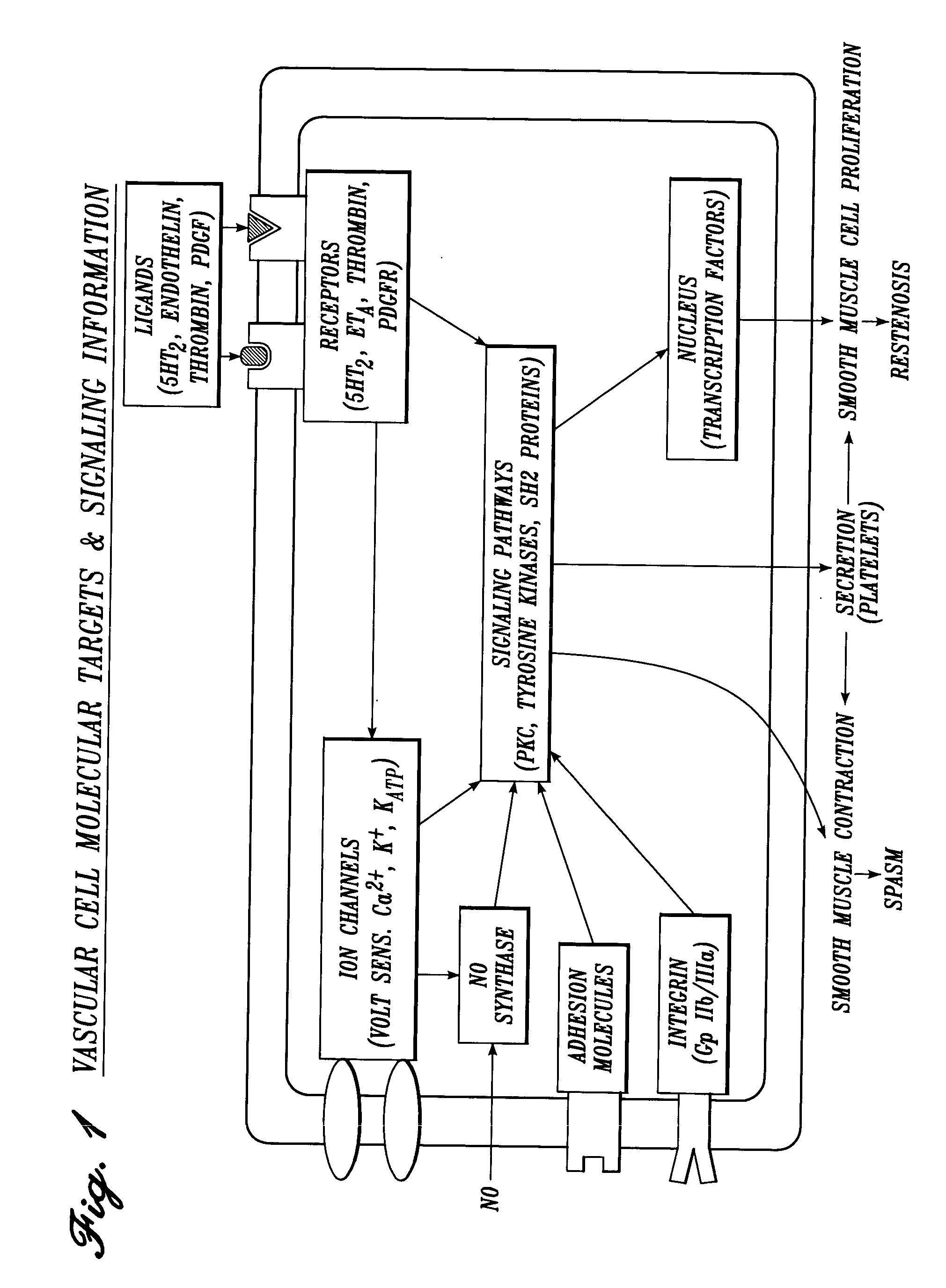
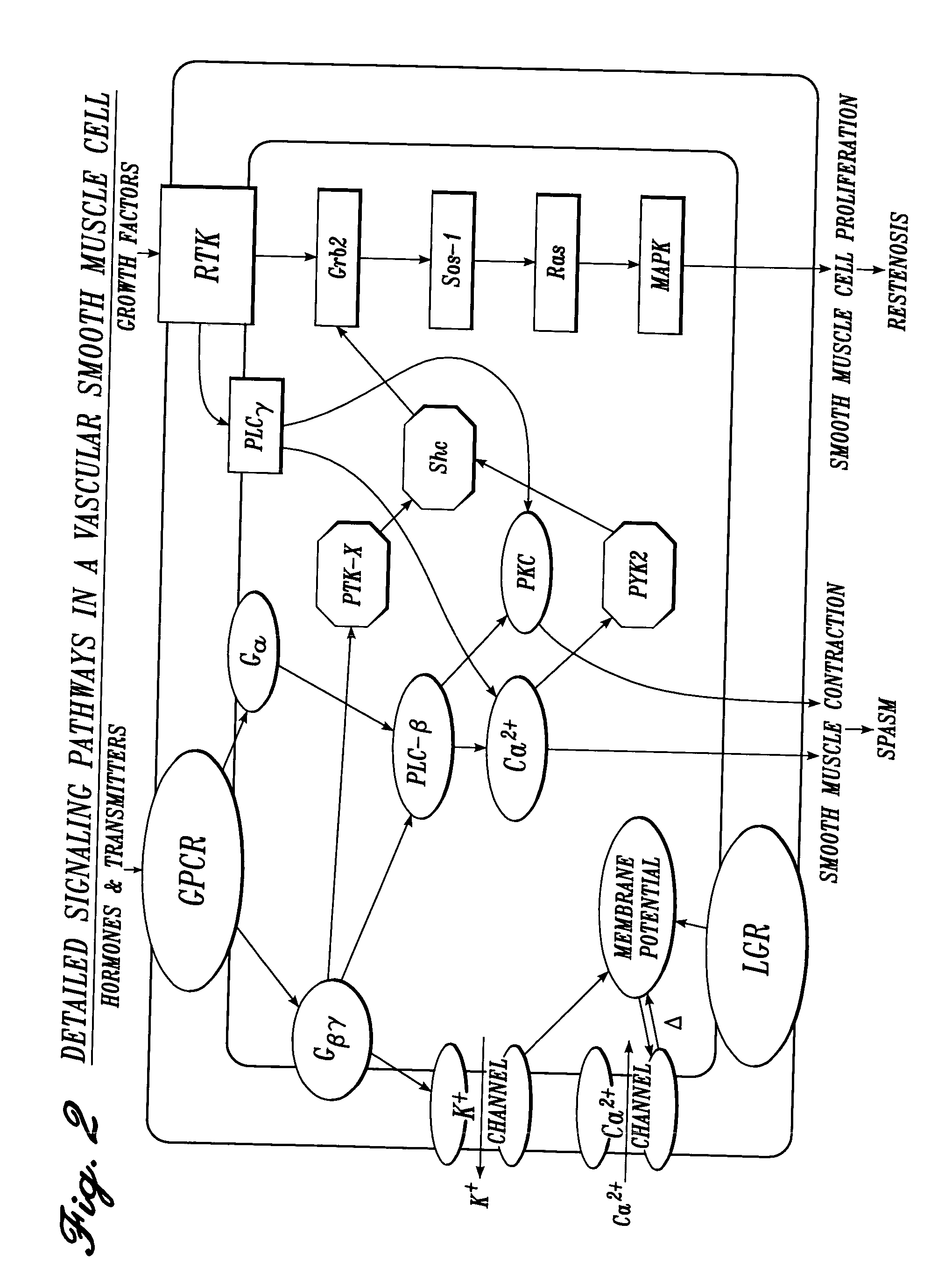
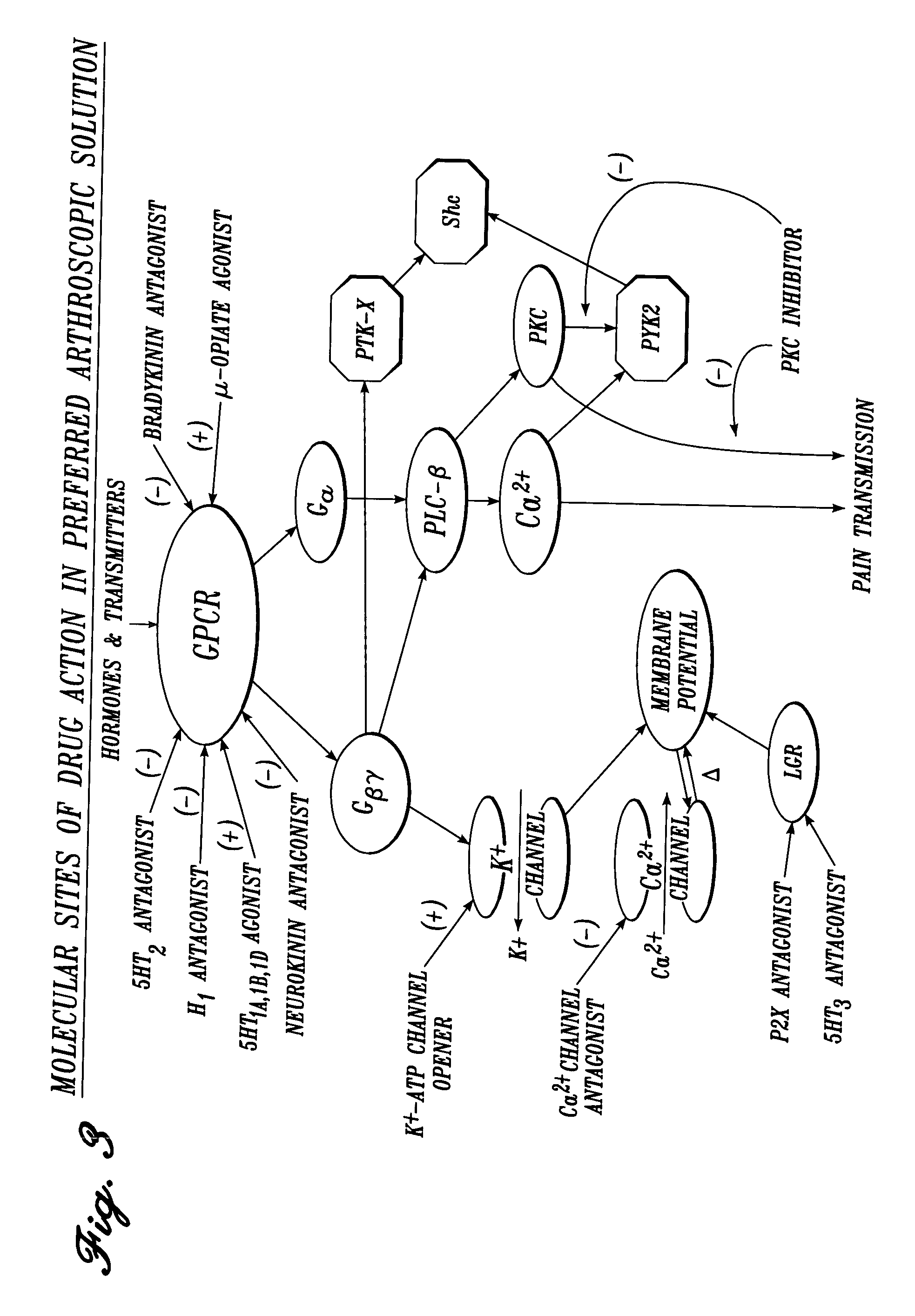
Vascular irrigation solution and method for inhibition of pain, inflammation, spasm and restenosis
InactiveUS20040127884A1Limit their usefulnessLow costTripeptide ingredientsMedical devicesProtein-Tyrosine KinasesPercent Diameter Stenosis
A method and solution for perioperatively inhibiting a variety of pain, inflammation, spasm and restenosis processes resulting from cardiovascular or general vascular therapeutic and diagnostic procedures. The solution preferably includes multiple pain and inflammation inhibitory agents and spasm inhibitory agents at dilute concentration in a physiologic carrier, such as saline or lactated Ringer's solution. Specific preferred embodiments of the solution of the present invention for use in cardiovascular and general vascular procedures also include anti-restenosis agents. The solution is introduced luminally to continuously irrigate an arterial site during an operative / interventional or diagnostic procedure for preemptive inhibition of pain and inflammation, vascular and non-vascular smooth muscle spasm, and restenosis while avoiding undesirable side effects associated with oral, intramuscular, subcutaneous or intravenous application of larger doses of the agents. One preferred solution to inhibit pain, inflammation, vasospasm and restenosis includes a serotonin2 antagonist, a cyclooxygenase inhibitor, an endothelin antagonist, an ATP-sensitive K<+> channel antagonist, a Ca<2+> channel antagonist, a nitric oxide donor, an anti-thrombin agent, a glycoprotein IIb / IIIa receptor blocker, a PKC inhibitor and a protein tyrosine kinase inhibitor.
Owner:OMEROS CORP
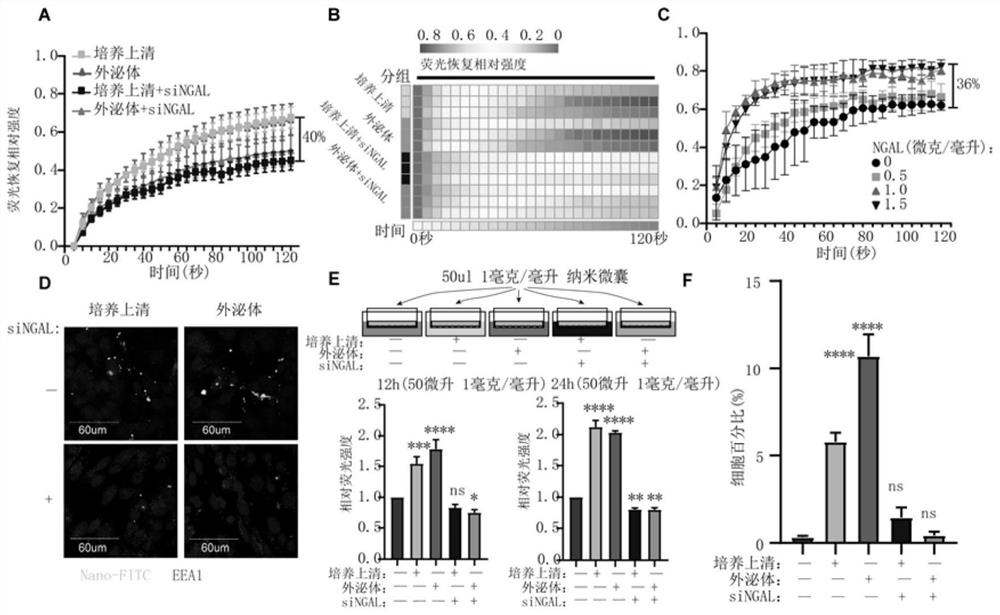
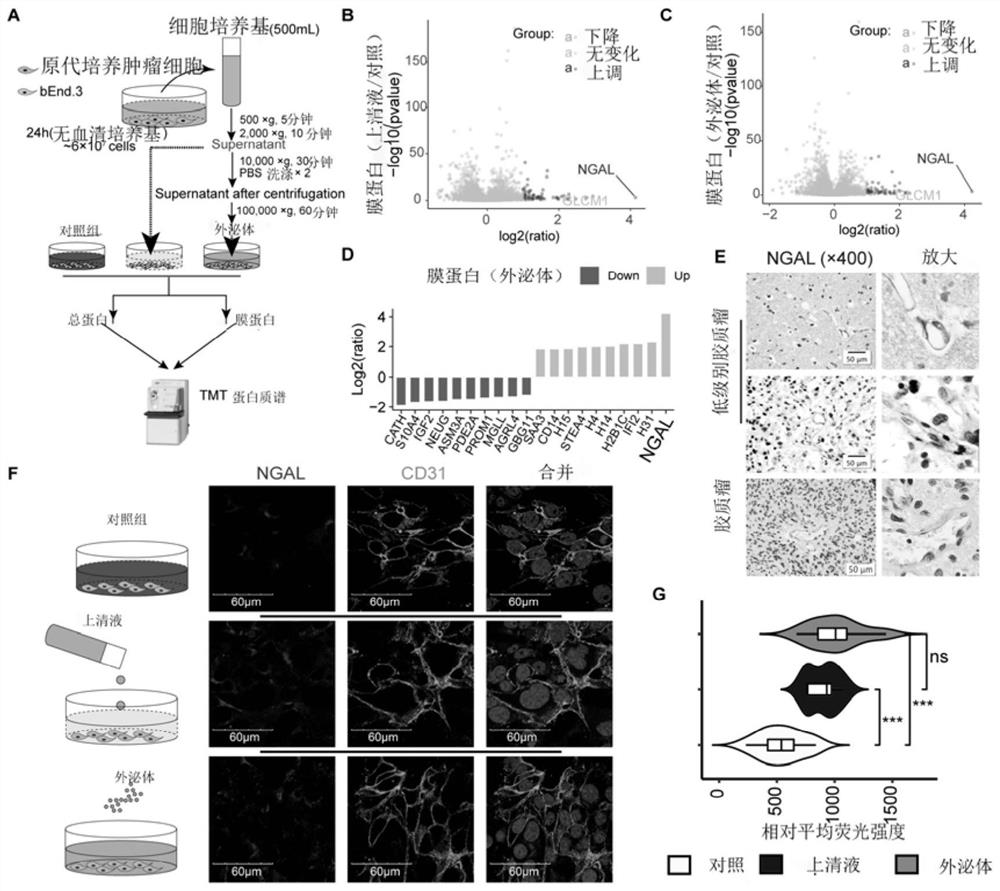
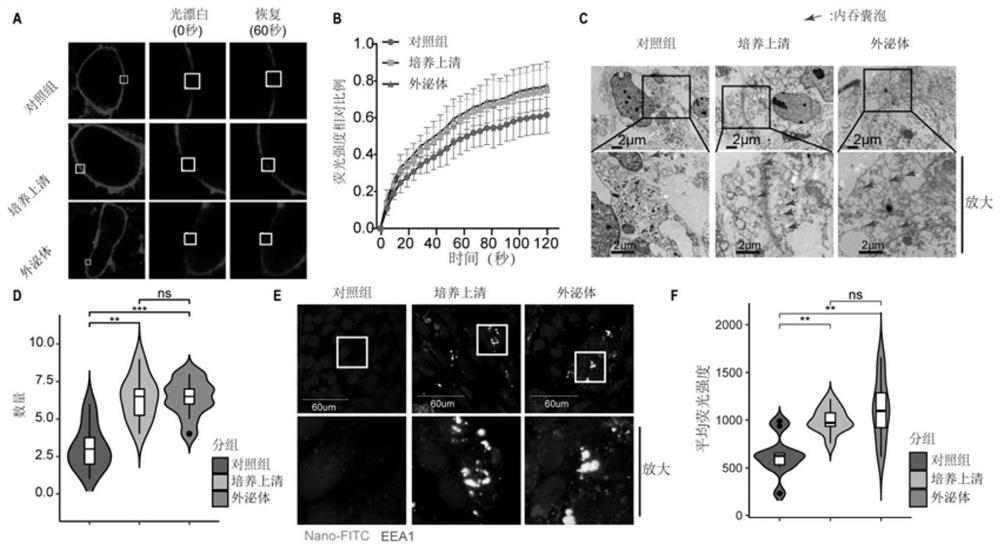
Use of neutrophil gelatinase-associated lipocalin to promote crossing of therapeutic antibodies across blood-brain barrier
PendingCN112494647AImprove permeabilityIncrease the total amount across the BBBAntibody ingredientsImmunoglobulinsA lipoproteinIntravenous gammaglobulin
The invention discloses a use of neutrophil gelatinase-associated lipocalin in promoting a therapeutic antibody to cross a blood-brain barrier. The therapeutic antibody is a nanocapsule containing immune globulin. The neutrophil gelatinase-associated lipocalin enters blood through intravenous injection to play a role in increasing the permeability of a blood-brain barrier. According to the presentinvention, lipoprotein related to neutrophil gelatinase is intravenously injected into blood circulation of a glioma animal model, the permeability of a blood-brain barrier can be improved, and therefore the total amount of therapeutic antibodies crossing the blood-brain barrier is increased, and particularly, when the therapeutic antibodies select nanocapsules containing immune globulin, the effect that the medicine passes through the blood-brain barrier can be further improved, a better treatment effect is achieved, and extremely high clinical transformation significance is achieved.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Method for rapidly extracting plasma of COVID-19 patient in rehabilitation period by automatic separation system
ActiveCN112225799AHigh speedHigh titerSerum immunoglobulinsInorganic non-active ingredientsIntravenous gammaglobulinEngineering
The invention relates to a method for rapidly extracting plasma of a COVED-19 patient in a rehabilitation period by an automatic separation system. The method comprises the following steps of: separating peripheral blood taken from a human body into three component layers, namely an erythrocyte layer, a cell concentration layer and a plasma layer, by using a closed multi-cell component automatic separation system, performing virus inactivation on the obtained plasma, and optionally cryopreserving the plasma to obtain the plasma which can be used for preparing intravenous immunoglobulin G. Theinvention also comprises a method for preparing an intravenous immunoglobulin G preparation. The method comprises the following steps: obtaining a plasma component by the method, precipitating the plasma by a low-temperature ethanol method to obtain components I + II + III, separating a component II from a component I + III, respectively preparing an immunoglobulin G semi-finished product 1 and animmunoglobulin G semi-finished product 2, performing sterilization and virus inactivation to obtain an immunoglobulin G stock solution. and further preparing the intravenous immunoglobulin G preparation for intravenous injection. The method provided by the invention achieves excellent technical effects as described in the specification.
Owner:深圳博雅感知药业有限公司
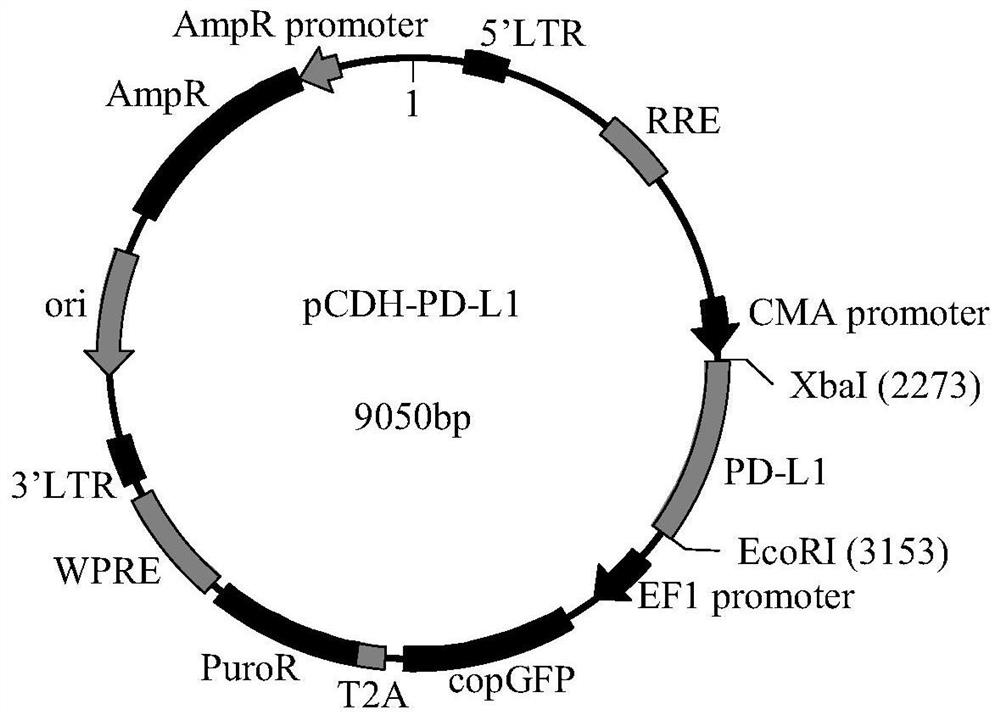
Mesenchymal stem cell strain for overexpression of PD-L1 gene as well as a construction method and application of mesenchymal stem cell strain
PendingCN111961689ARelieve symptomsReduce joint damageAntipyreticAnalgesicsInflammatory factorsBiomedicine
The embodiment of the invention discloses a mesenchymal stem cell strain for overexpression of a PD-L1 gene as well as a construction method and an application of the mesenchymal stem cell strain, andbelongs to the technical field of biomedicine. The method comprises the following steps: 1) constructing a recombinant slow virus carrying a PD-L1 gene; and 2) transfecting mesenchymal stem cells with the recombinant slow virus carrying the PD-L1 gene, and carrying out puromycin screening, so as to obtain a mesenchymal stem cell strain for over-expressing the PD-L1 gene. After the mesenchymal stem cell strain for over-expressing the PD-L1 gene is injected into a rheumatoid arthritis mouse model intravenously, the symptoms of rheumatoid arthritis can be remarkably relieved, the joint injury degree is reduced, the expression of inflammatory factors is regulated, inflammatory response is inhibited, and the mesenchymal stem cell strain has a remarkable curative effect on treatment of rheumatoid arthritis diseases.
Owner:樊克兴
Method for creating autoimmune hepatitis animal model
Owner:CHANGZHI MEDICAL COLLEGE
Mumps virus attacking animal model and establishment method thereof
ActiveCN114794018AIncrease capacityEffective infectionCompounds screening/testingAnimal husbandryIntramuscular injectionIntravenous IG
The invention relates to a mumps virus attacking animal model and an establishment method thereof, and belongs to the technical field of biology. The invention provides a mumps virus counteracting animal model. The mumps virus counteracting animal model is an interferon receptor deletion animal which is intravenously injected with mumps virus. Research finds that compared with common mice, the mumps virus load in blood of the interferon receptor-deleted mice is remarkably improved after the mumps virus is attacked by the interferon receptor-deleted mice, and compared with intramuscular injection and intranasal injection, the mumps virus load in blood of the interferon receptor-deleted mice is remarkably improved after the mumps virus is injected into the interferon receptor-deleted mice through caudal veins, so that the mumps virus load in blood of the interferon receptor-deleted mice is remarkably improved. Intravenous injection of the mumps virus to the interferon receptor-deficient animal can break through species limitation, effective infection of the mumps virus in the animal body is realized, and symptoms similar to human infection (for example, remarkable replication in various visceral organs) are formed, so that the mumps virus challenge animal model can be used as the mumps virus challenge animal model.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD +1
Reagent for predicting treatment reactivity of children with Kawasaki disease and application of reagent
ActiveCN110982890AInnovativeSimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationIntravenous gammaglobulinKawasaki disease
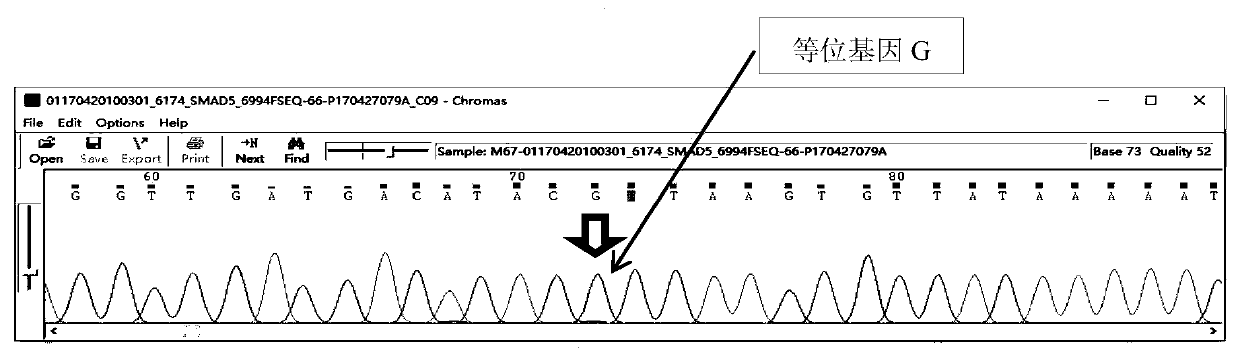
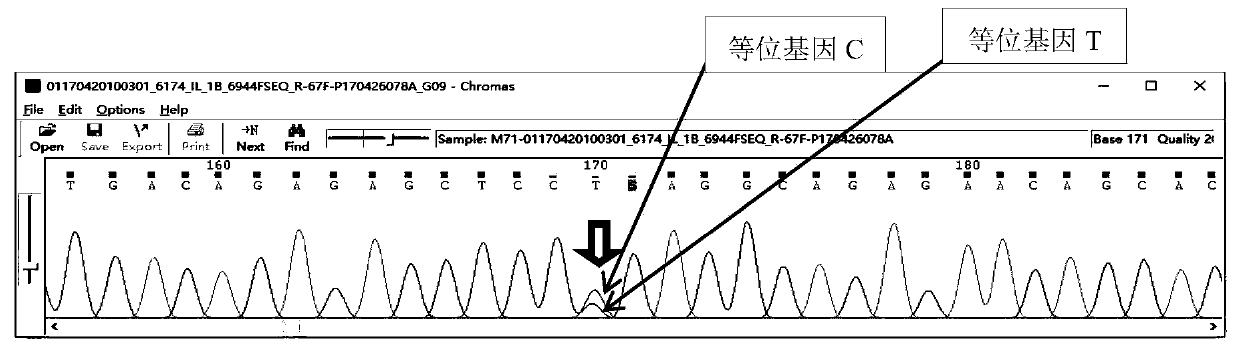
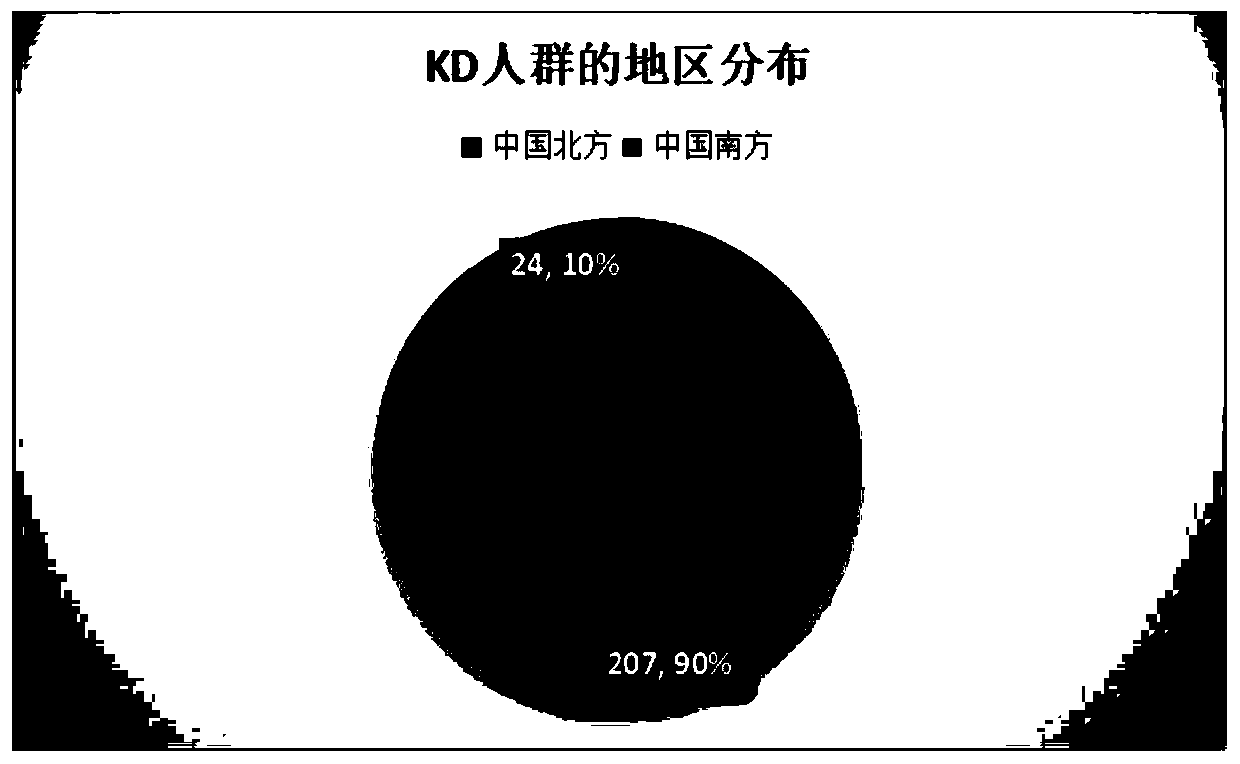
The invention provides a reagent for predicting treatment reactivity of children with Kawasaki disease and an application of the reagent, and mainly provides the reagent for detecting an intravenous human immunoglobulin (IVIG) non-responsive Kawasaki disease susceptibility gene and a kit containing the reagent. The reagent can evaluate the reactivity of the children with the Kawasaki disease to IVIG treatment by detecting 5 SNP loci of 3 genes (rs10056474 or rs746994 of an SMAD5 gene, rs76863441 of a PLA2G7 gene, and / or rs16944 or rs1143627 of an IL-1B gene) of a patient. The kit has the characteristics of simple operation and low costs; and the reagent and kit provided by the invention can provide a basis for screening IVIG non-responsive high-risk children as early as possible and guiding clinical doctors to take reasonable intervention and treatment measures, and is in line with the development trend of precision medicine.
Owner:AFFILIATED CHILDRENS HOSPITAL OF CAPITAL INST OF PEDIATRICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com