Method for rapidly extracting plasma of COVID-19 patient in rehabilitation period by automatic separation system
A COVID-19, separation system technology, applied in the preparation methods of peptides, chemical instruments and methods, from serum immunoglobulins, etc., can solve the problems of donors' intolerance of blood collection activities and unfavorable donors' plasma collection work.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1, rapid separation of plasma from convalescent patients with COVID-19
[0054] 1. Peripheral blood (more than 250mL) donated by a COVID-19 patient (in this paper, the patient is marked as patient X) collected by venipuncture during the recovery period is placed in a blood collection bag (the collection bag is filled with anticoagulant citrate Natrate), put the blood collection bag on a horizontal shaker and mix well for 15 minutes; Recovered persons after confirmation of antibody titer / serum neutralizing antibody titer]
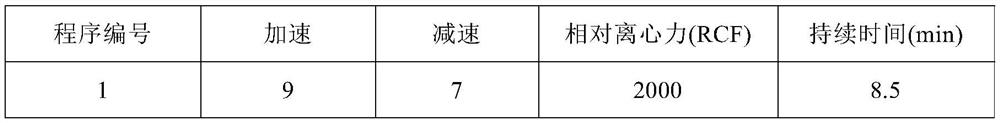
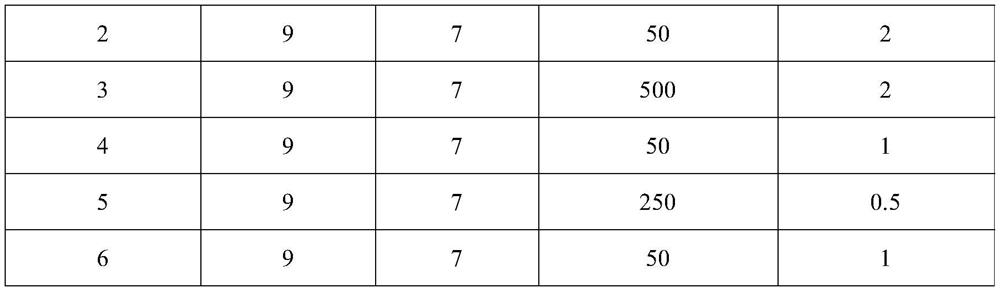
[0055] 2. Using ThermoGenesis Corp.'s closed multicomponent automated cell separation system, namely Multicomponent Automated Cell Separation System (MACSS), insert the plastic needle of the disposable separation cup into the sterile interface on the blood collection bag, and insert the blood collection bag Hang it up so that 60ml of blood naturally flows into the central compartment in the separation cup; 0.6ml of separation aid is pre-add...
Embodiment 2
[0066] Embodiment 2, the separation process of preparing component I+II+III and component II and component I+III from raw plasma,
[0067] 1. Preparation of buffer solution:
[0068] ①Ph4.75 0.15mol / L phosphate buffer: Na required per 1kg 2 HPO 4 12H 2 O is 53g, glacial acetic acid 27mL adjusts pH to 4.75, and the buffer solution that obtains is called Buffer A for short;
[0069] ②Ph5.25 0.5mol / L phosphate buffer: Na required per 1kg 2 HPO 4 12H 2 O is 160.6g, 24mL of glacial acetic acid adjusts pH to 5.25, and the damping fluid that obtains is called for short Buffer B;
[0070] ③ Component I+III balance liquid for pressure filtration: 0.13kg of 95% ethanol, Na 2 HPO 4 12H 2 O is 1.9g, 0.39mL of glacial acetic acid;
[0071] ④1mol / L sodium chloride solution: 58.5g of NaCl is required for every 1kg, add water to 1kg;
[0072] ⑤1mol / L sodium bicarbonate solution: 84.0g of NaCl is required for every 1kg, add water to 1kg;
[0073] ⑥1mol / L HCL solution: make 10.0L ...
Embodiment 3
[0085] Example 3, Precipitation and Dissolution of Component II and Purification of Immunoglobulin G by Column Chromatography
[0086] 1. Preparation of buffer solution:
[0087] ① Chromatographic equilibrium solution: add Na per 1kg 2 HPO 4 12H 2 O 3g, glacial acetic acid 0.27mL, add low-temperature water for injection to dissolve and adjust pH to 6.6-7.0, conductivity 1.3-1.5ms / cm, temperature 0-0.5°C;
[0088] ②Ph4.0 phosphate buffer: add Na per 1kg 2 HPO 4 12H 2 O 17.9g, NaCl 29.2g, glacial acetic acid about 1.5mL, dissolved in low-temperature water for injection;
[0089] ③pH4.0 acetic acid eluent: add NaAc·3H per 1kg 2 O 20.7g, NaCl 58.5g, glacial acetic acid 20.5mL, add water for injection to adjust pH=4.0;
[0090] 2. Dissolve and dilute the component II precipitate obtained in Example 2 with 10 times water for injection, the dissolution temperature is 0-4°C, and the dissolution time is ≥2h. After dissolution, adjust the pH to 6.6-7.0, conductivity 1.4ms, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com