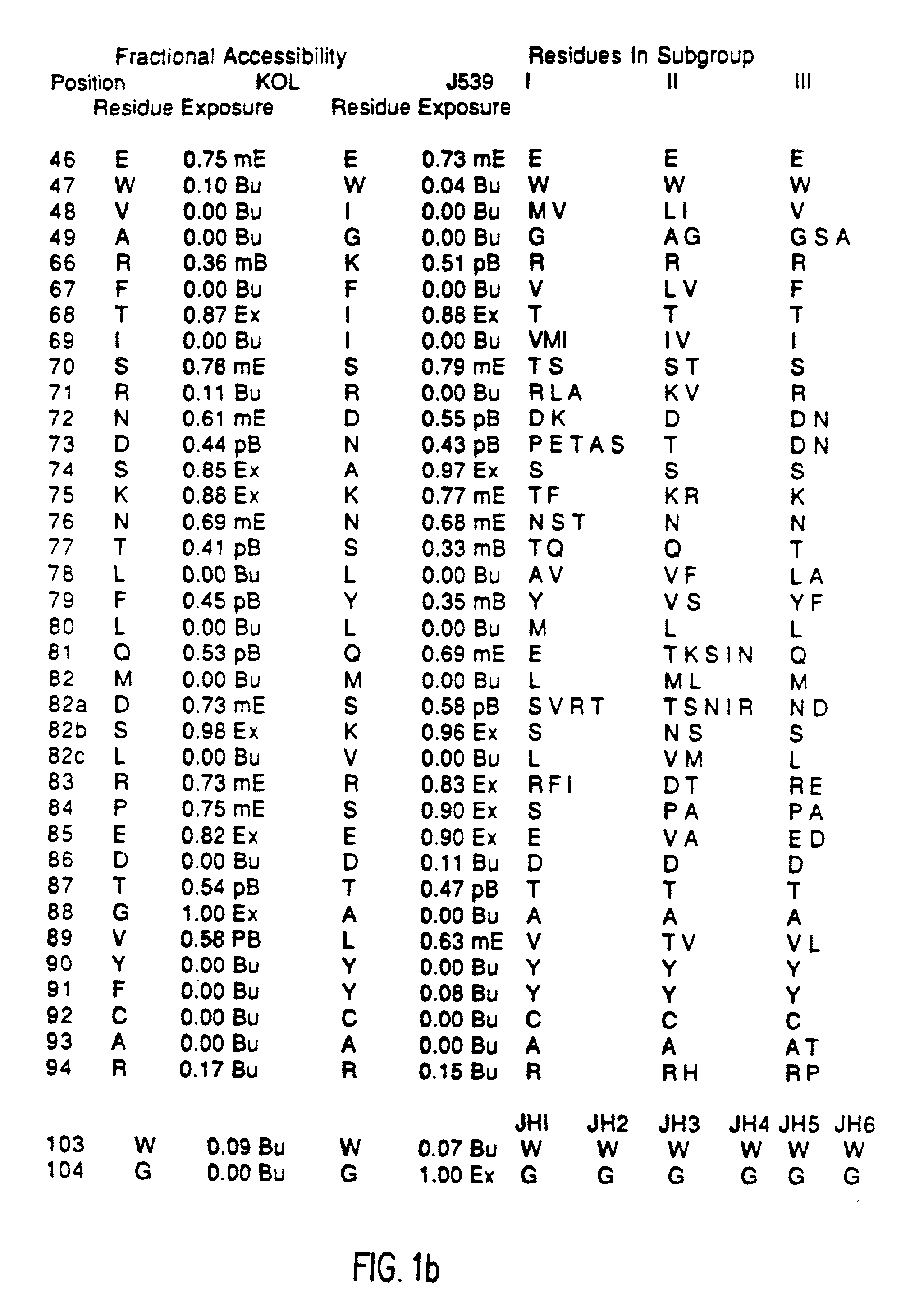
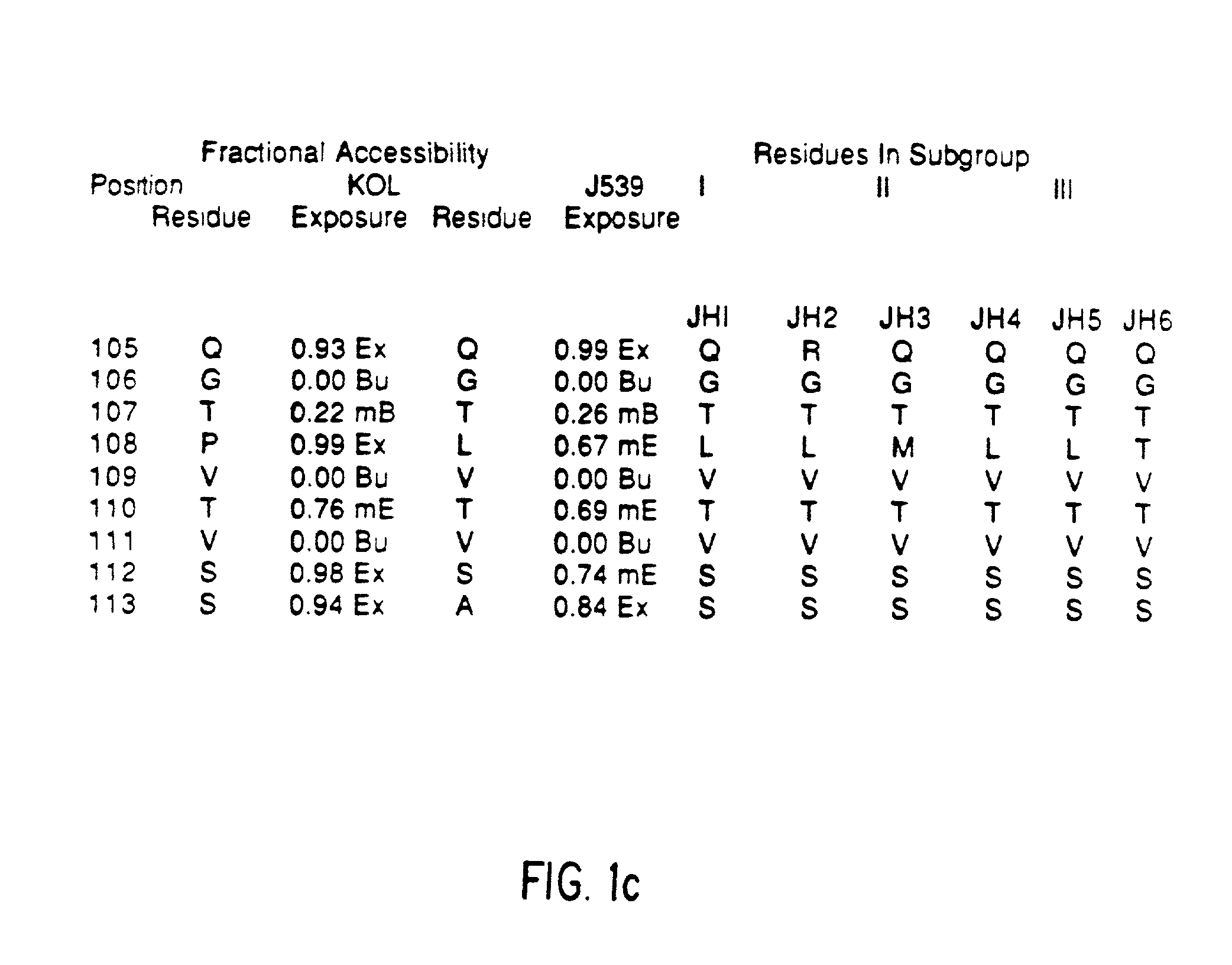
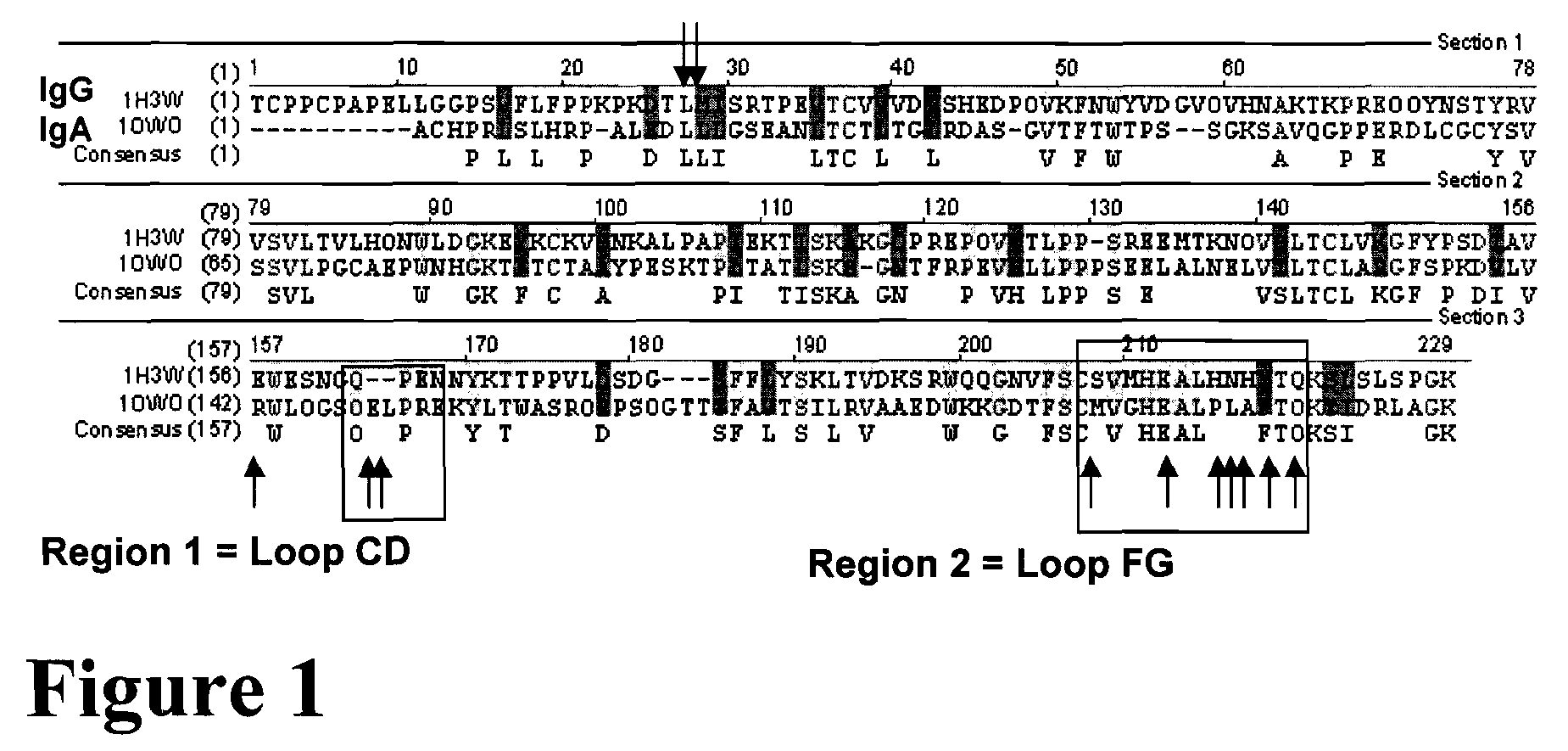
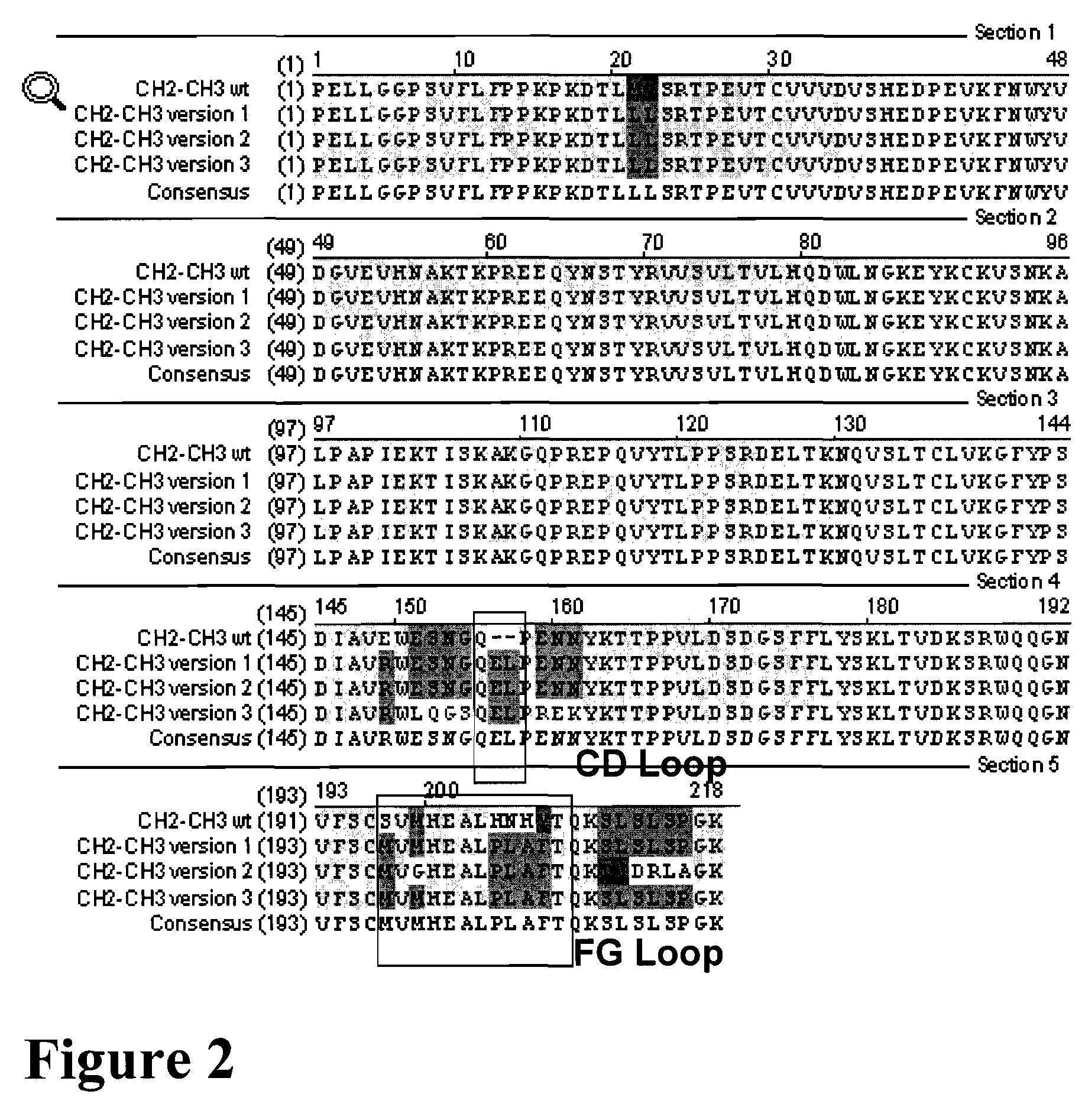
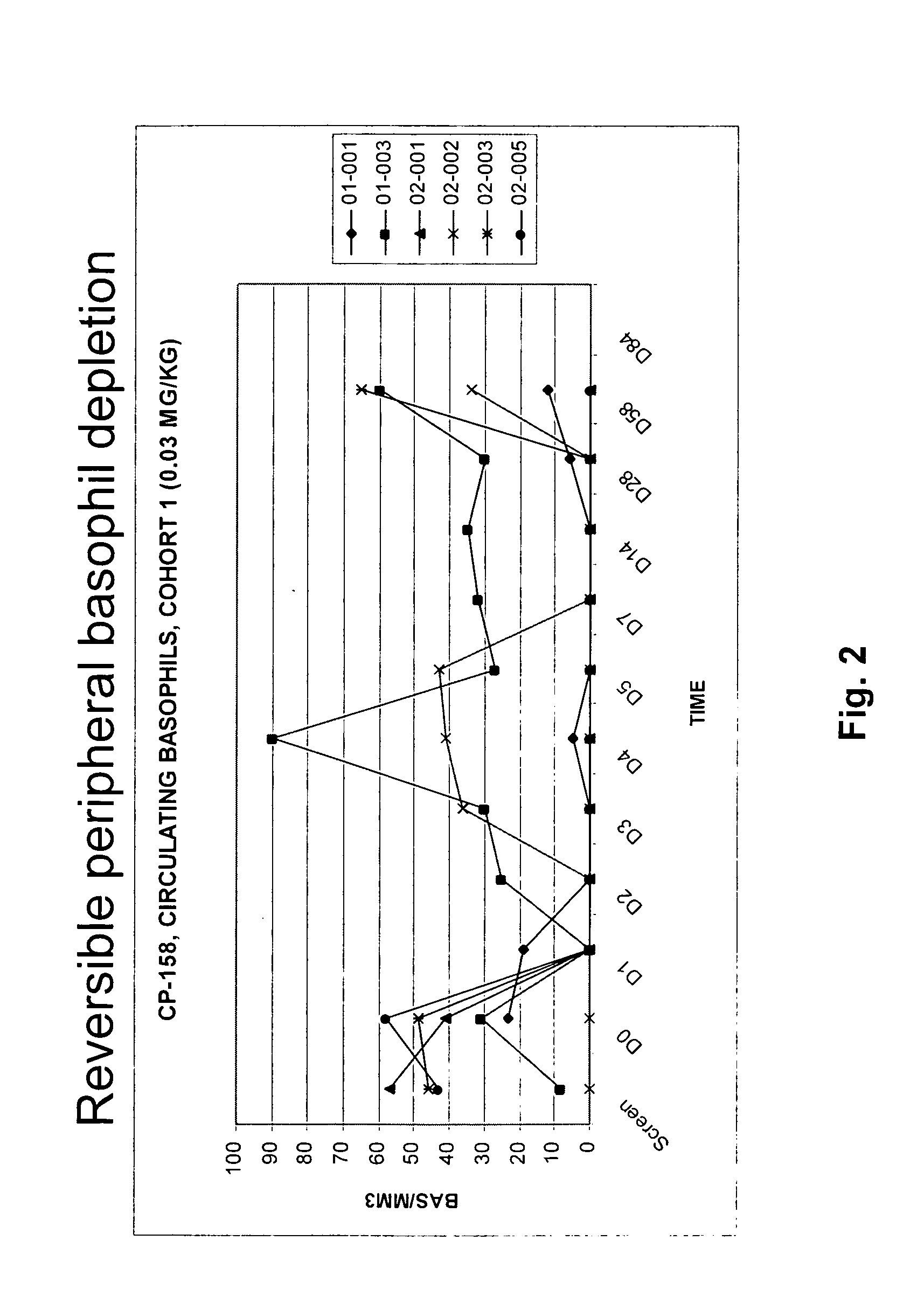
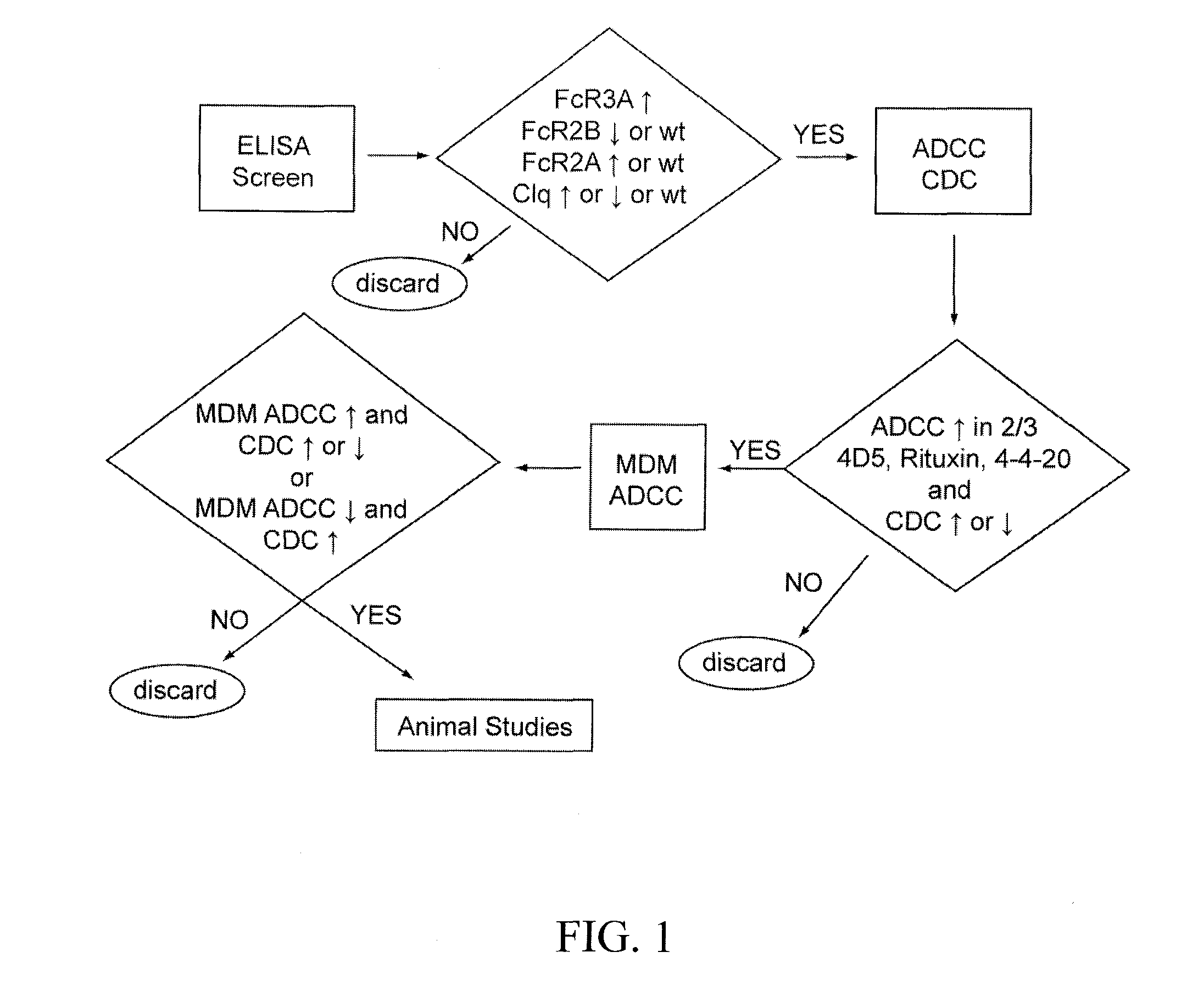
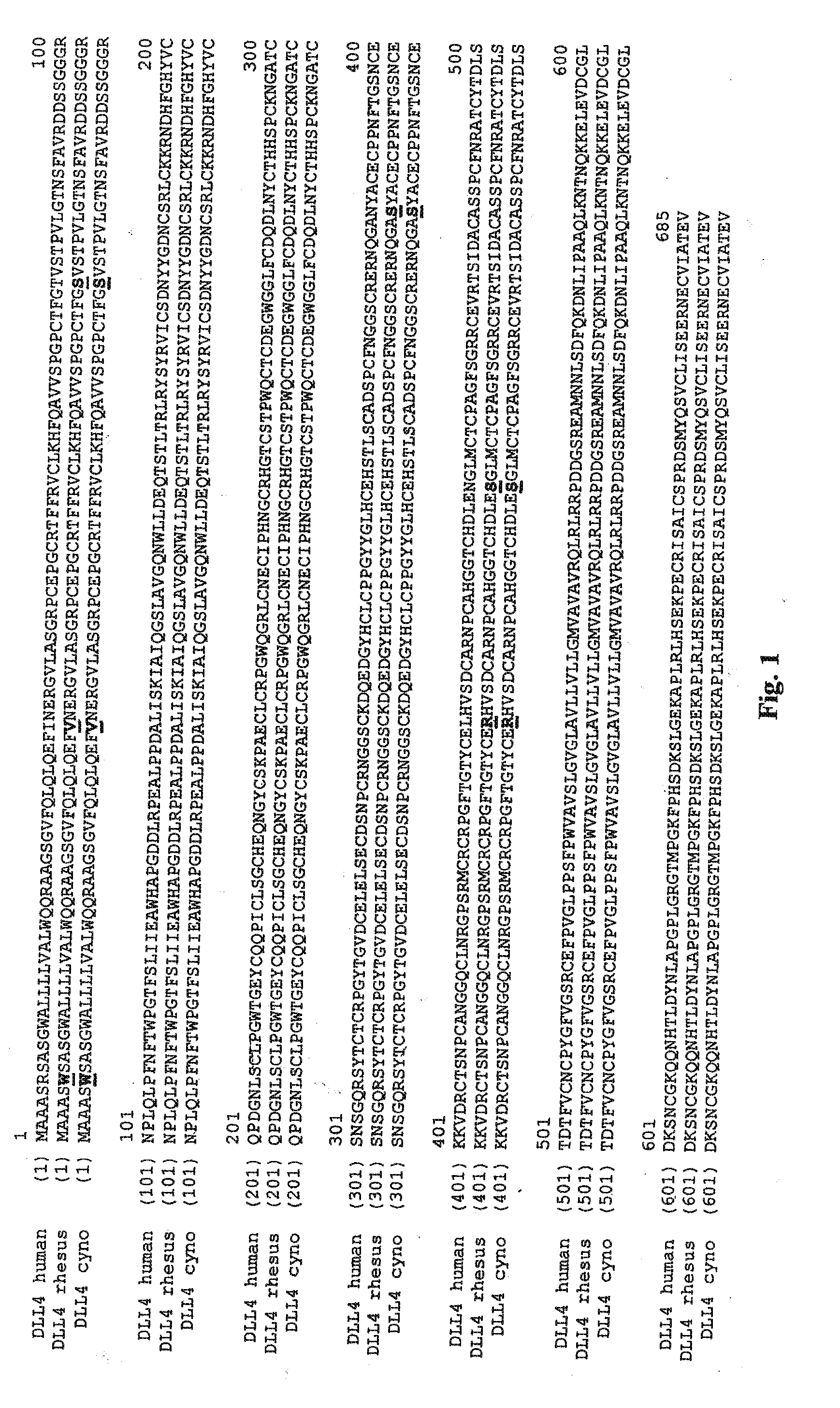
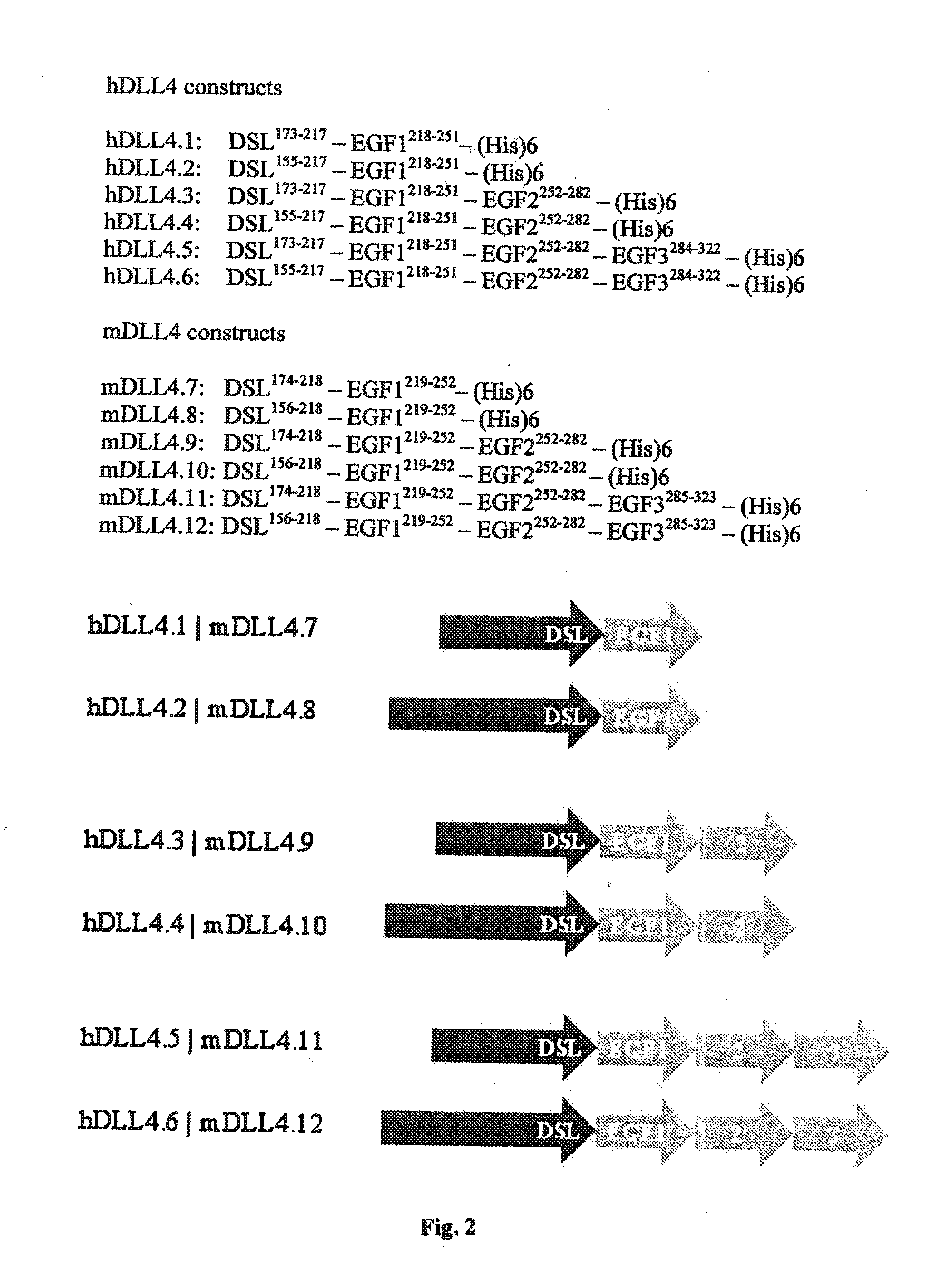
Patents
Literature
784 results about "Intravenous gammaglobulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Initially used as replacement therapy in patients with hypogammaglob-ulinemia, intravenous gamma-globulin (IVIg) preparations are increasingly being used as treatment for various autoimmune disorders.
Synthetic antibody phage libraries
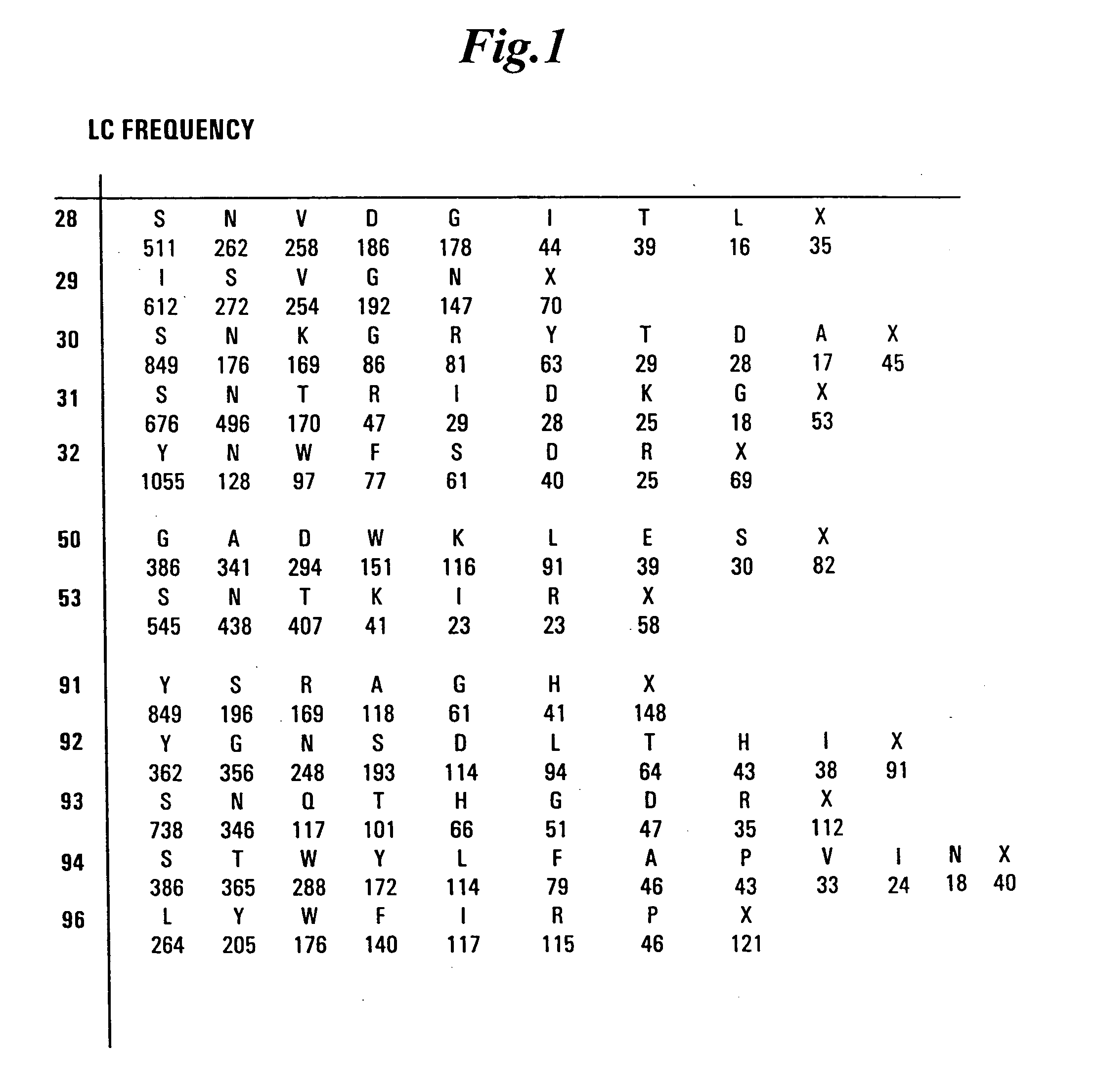
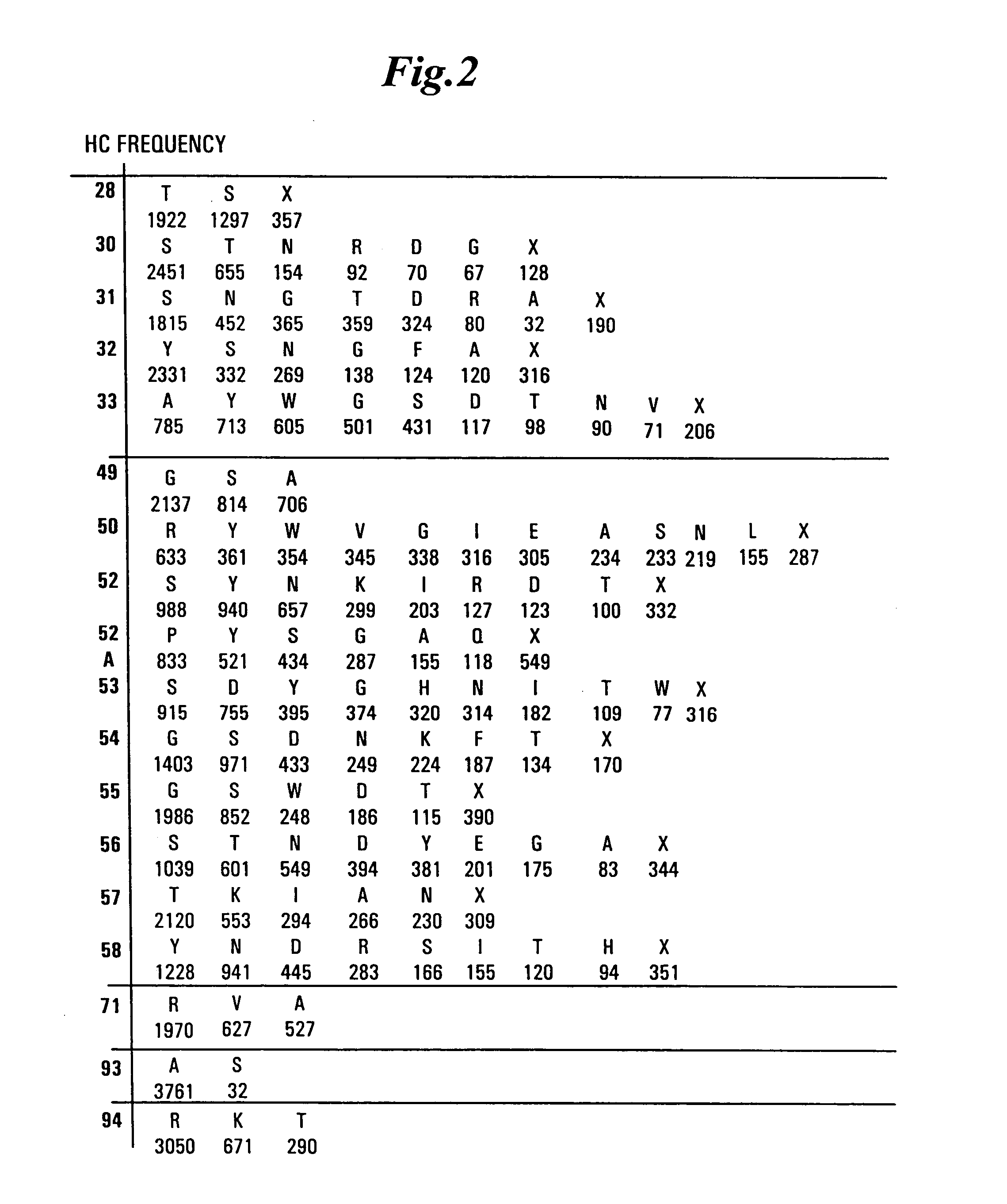
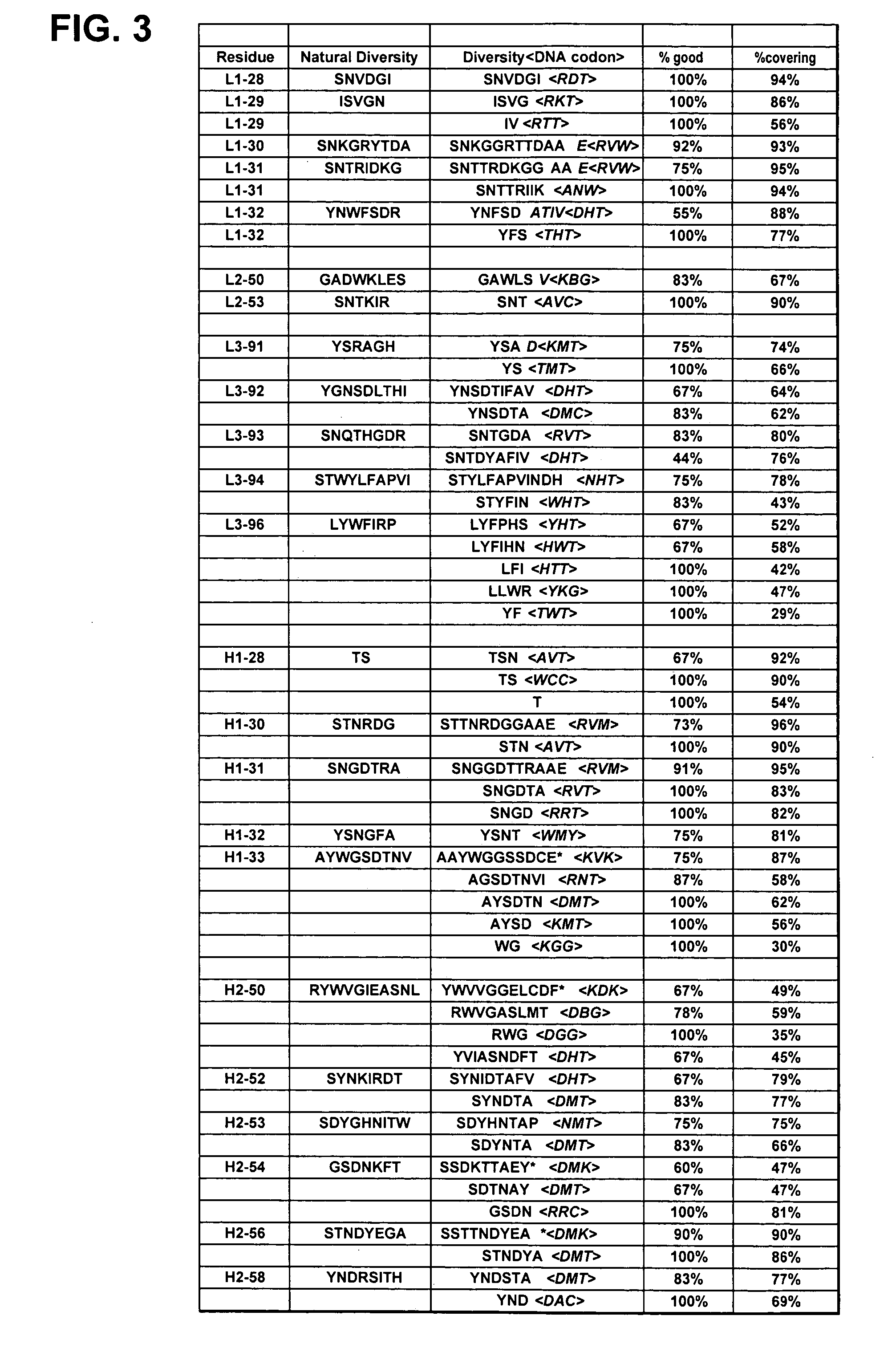
InactiveUS20050079574A1High-quality target binding characteristicGenerate efficientlyAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeterologousIntravenous gammaglobulin
The invention provides immunoglobulin polypeptides comprising variant amino acids in CDRs of antibody variable domains. In one embodiment, the polypeptide is a variable domain of a monobody and has a variant CDRH3 region. These polypeptides provide a source of great sequence diversity that can be used as a source for identifying novel antigen binding polypeptides. The invention also provides these polypeptides as fusion polypeptides to heterologous polypeptides such as at least a portion of phage or viral coat proteins, tags and linkers. Libraries comprising a plurality of these polypeptides are also provided. In addition, methods of and compositions for generating and using these polypeptides and libraries are provided.
Owner:GENENTECH INC
Readily Isolated Bispecific Antibodies with Native Immunoglobulin Format
ActiveUS20100331527A1Improve abilitiesReduces and eliminates bindingHybrid immunoglobulinsSerum immunoglobulinsImmunoglobulin heavy chainHeavy chain
A bispecific antibody format providing ease of isolation is provided, comprising immunoglobulin heavy chain variable domains that are differentially modified in the CH3 domain, wherein the differential modifications are non-immunogenic or substantially non-immunogenic with respect to the CH3 modifications, and at least one of the modifications results in a differential affinity for the bispecific antibody for an affinity reagent such as Protein A, and the bispecific antibody is isolable from a disrupted cell, from medium, or from a mixture of antibodies based on its affinity for Protein A.
Owner:REGENERON PHARM INC
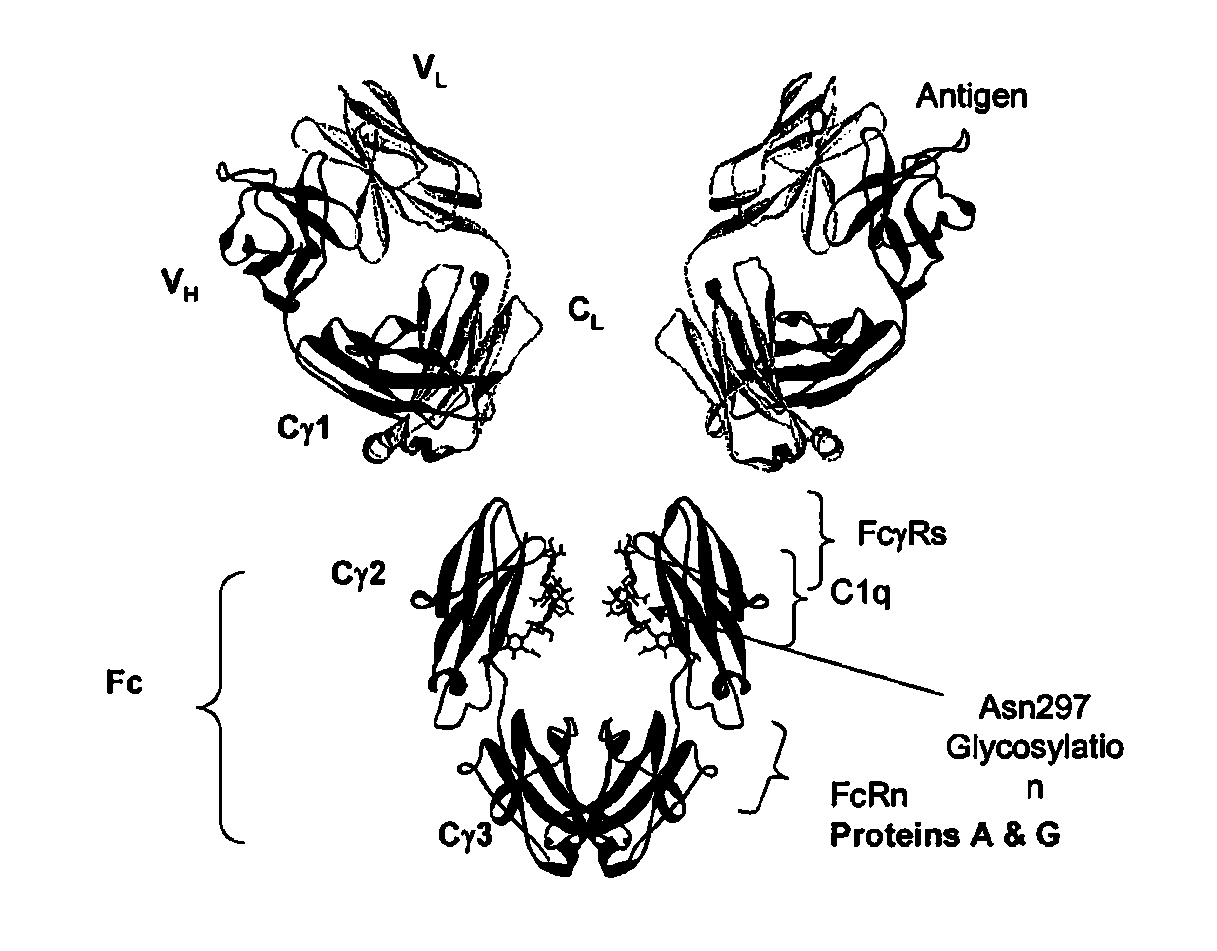
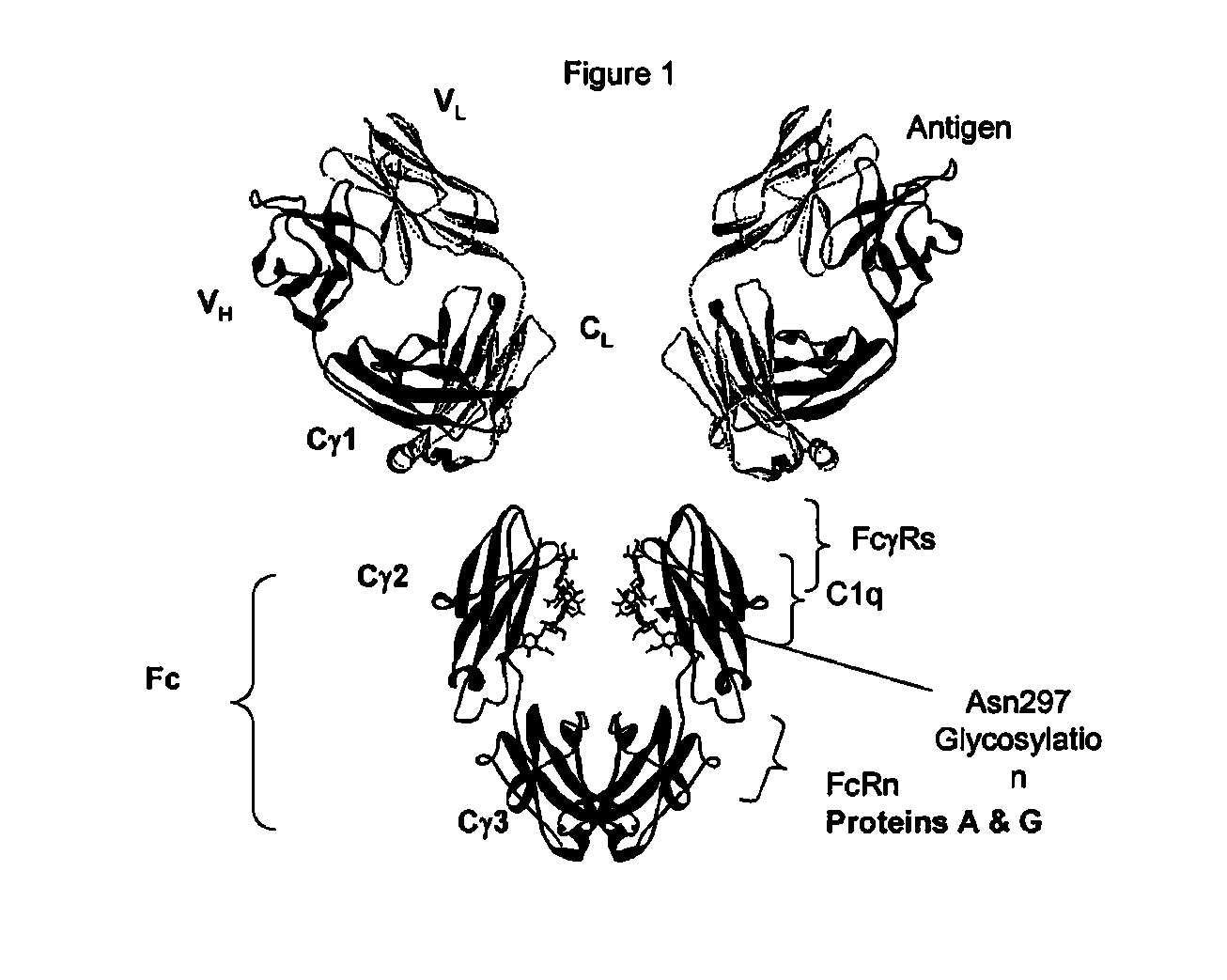
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
Molecules with extended half-lives, compositions and uses thereof
InactiveUS20030190311A1High affinityExtended half-lifeCompounds screening/testingFungiIntravenous gammaglobulinIn vivo
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Increasing antibody affinity by altering glycosylation of immunoglobulin variable region
InactiveUS6933368B2Enhanced antigen binding propertyAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody AffinitiesImmunoglobulin Variable Region
The present invention provides methods for producing mutationally-altered immunoglobulins and compositions containing such mutationally-altered immunoglobulins, wherein the mutationally-altered immunoglobulins have at least one mutation that alters the pattern of glycosylation in a variable region and thereby modifies the affinity of the immunoglobulin for a preselected antigen. The methods and compositions of the invention provide immunoglobulins that possess increased affinity for antigen. Such glycosylation-altered immunoglobulins are suitable for diagnostic and therapeutic applications.
Owner:FACET BIOTECH CORP
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
Binding domain-immunoglobulin fusion proteins
InactiveUS20050175614A1Reduced ability to dimerizeHybrid immunoglobulinsAntipyreticCrystallographyAntigen
The invention relates to novel binding domain-immunoglobulin fusion proteins that feature a binding domain for a cognate structure such as an antigen, a counterreceptor or the like, a hinge region polypeptide having either zero or one cysteine residue, and immunoglobulin CH2 and CH3 domains, and that are capable of ADCC and / or CDC while occurring predominantly as monomeric polypeptides. The fusion proteins can be recombinantly produced at high expression levels. Also provided are related compositions and methods, including immunotherapeutic applications.
Owner:TRUBION PHARM INC
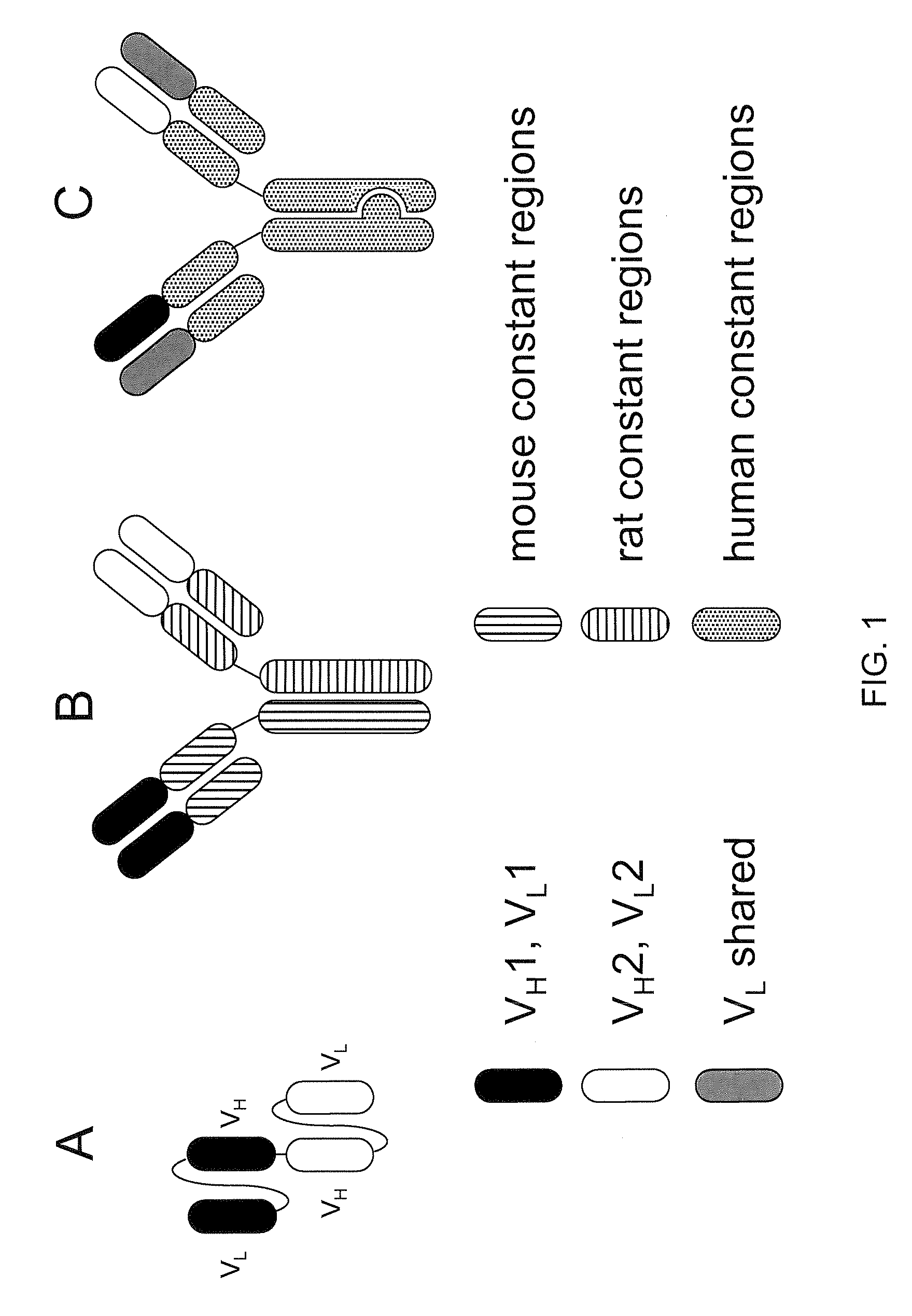
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
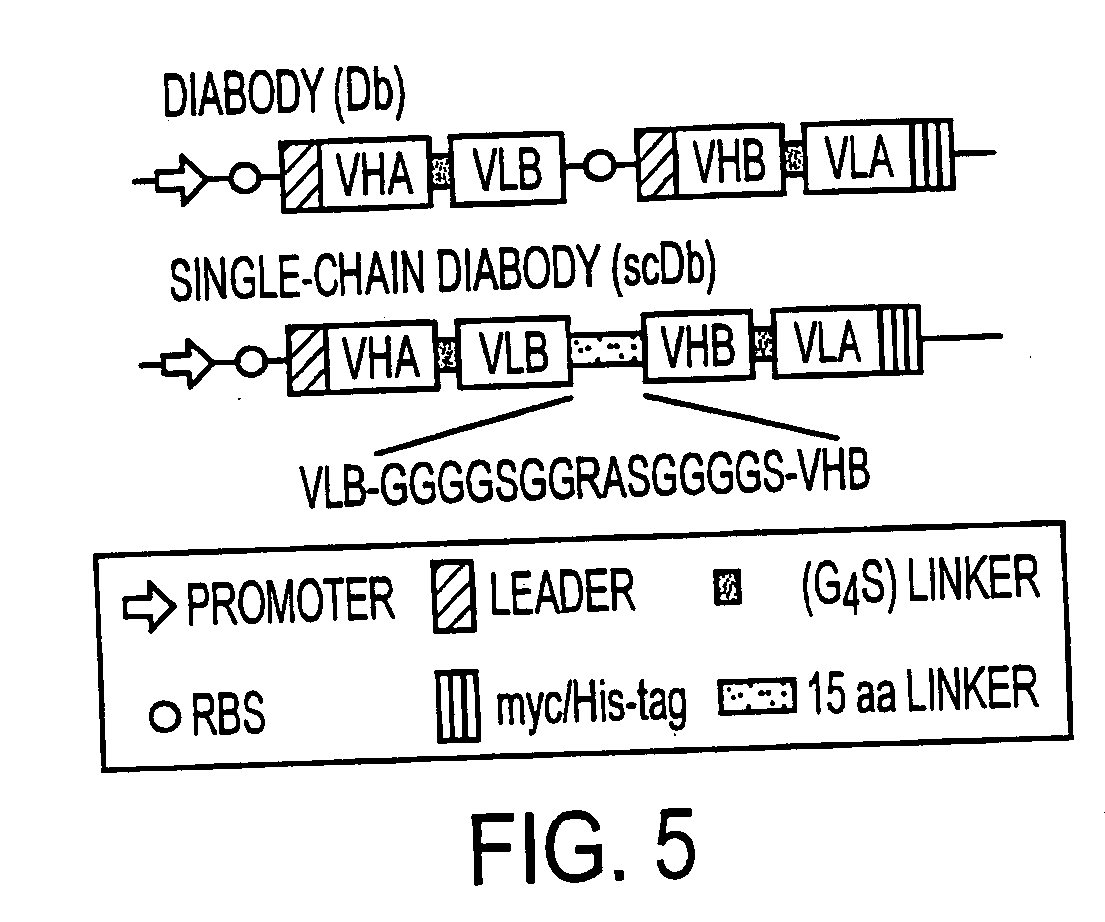
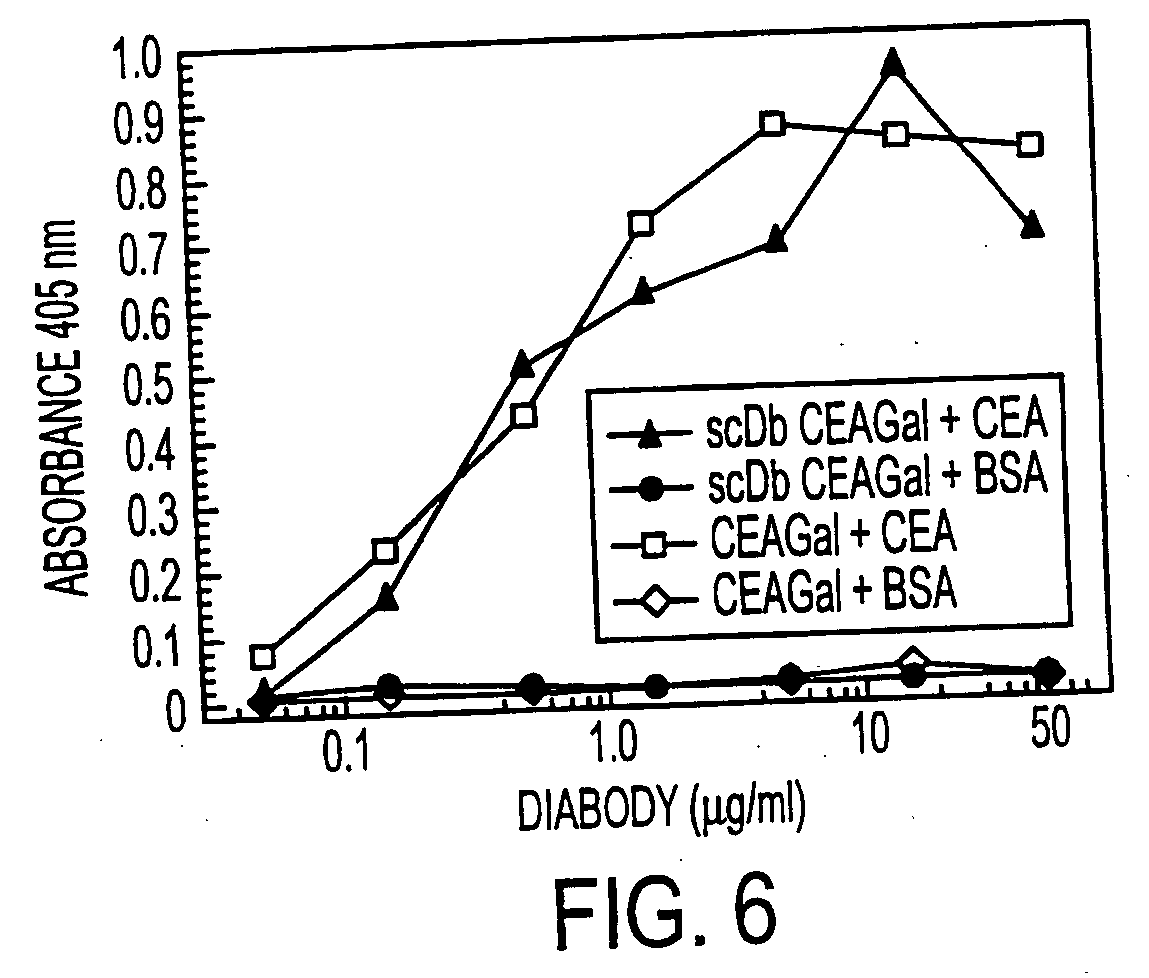
Single-chain multiple antigen-binding molecule, its preparation and use
InactiveUS20050004352A1Reduce dissociationLess complexOrganic active ingredientsFungiAntigen bindingVariable domain
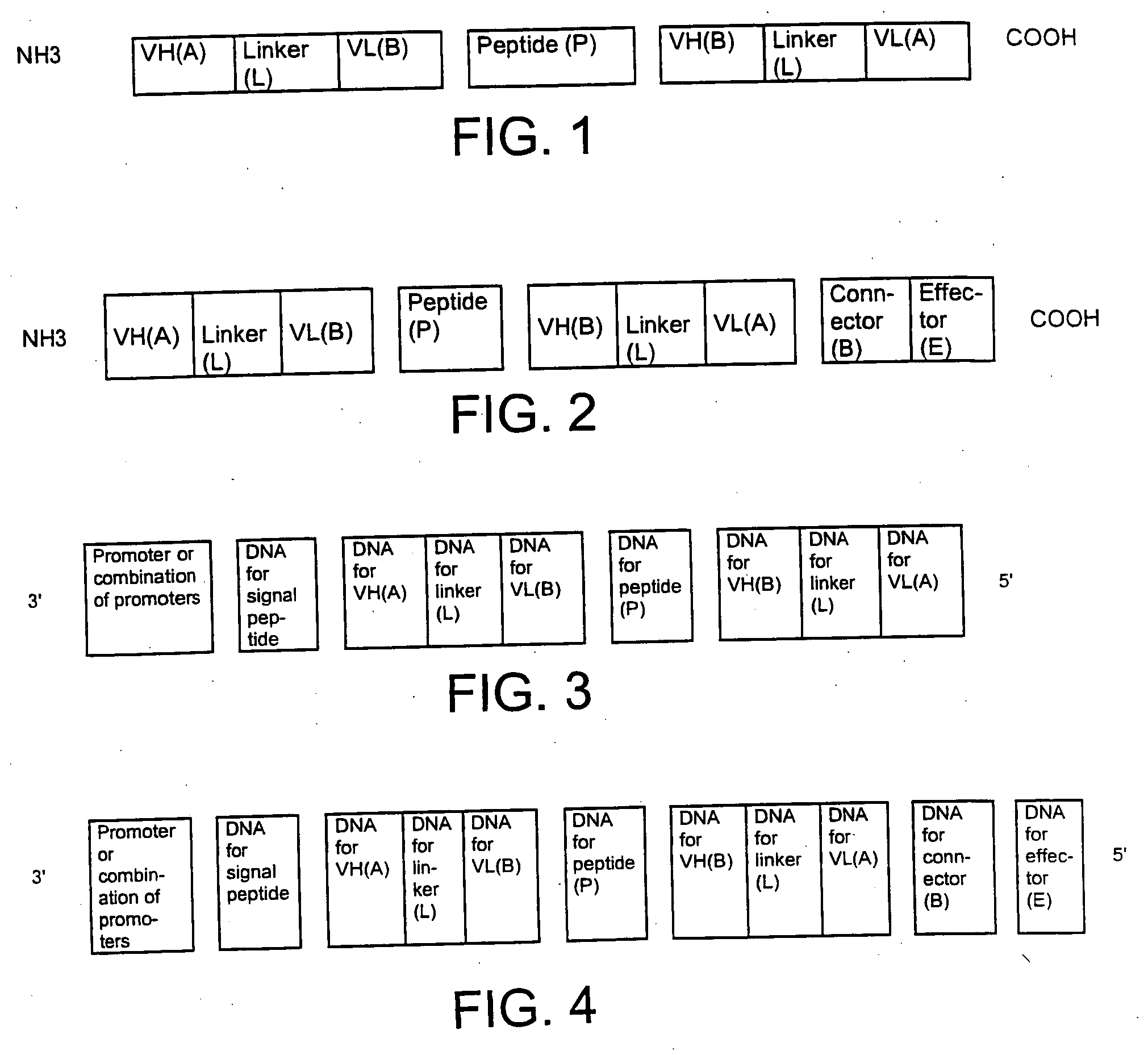
The present invention relates to a single-chain, multiple antigen-binding molecule with diverse variable domains of a heavy and of a light chain of an immunoglobulin, which are connected in the form of a VH-VL construct, which are in turn connected together via a peptide, and to the preparation and use thereof as pharmaceutical or diagnostic aid.
Owner:AFFITECH RESEARCH AS
Fc VARIANTS WITH ALTERED BINDING TO FcRn
ActiveUS20090041770A1Extended half-lifeImmunoglobulins against growth factorsAntibody ingredientsTherapeutic intentCancer research
The present application relates to optimized IgG immunoglobulin variants, engineering methods for their generation, and their application, particularly for therapeutic purposes.
Owner:XENCOR
Production of humanized antibodies in transgenic animals
InactiveUS7129084B2Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Isolation of proteins
InactiveUS20050176122A1Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
Owner:UPFRONT CHROMATOGRAPHY
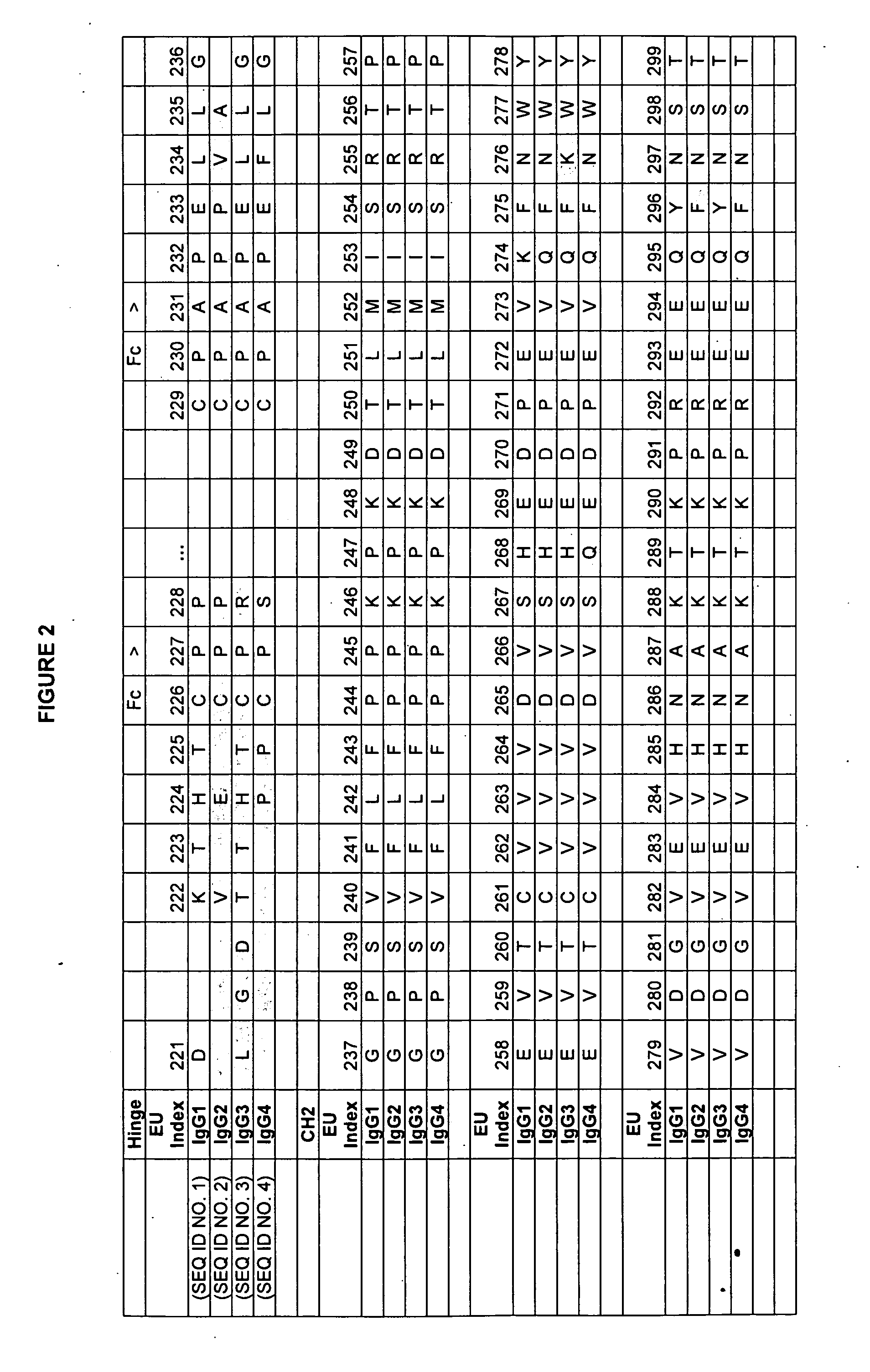
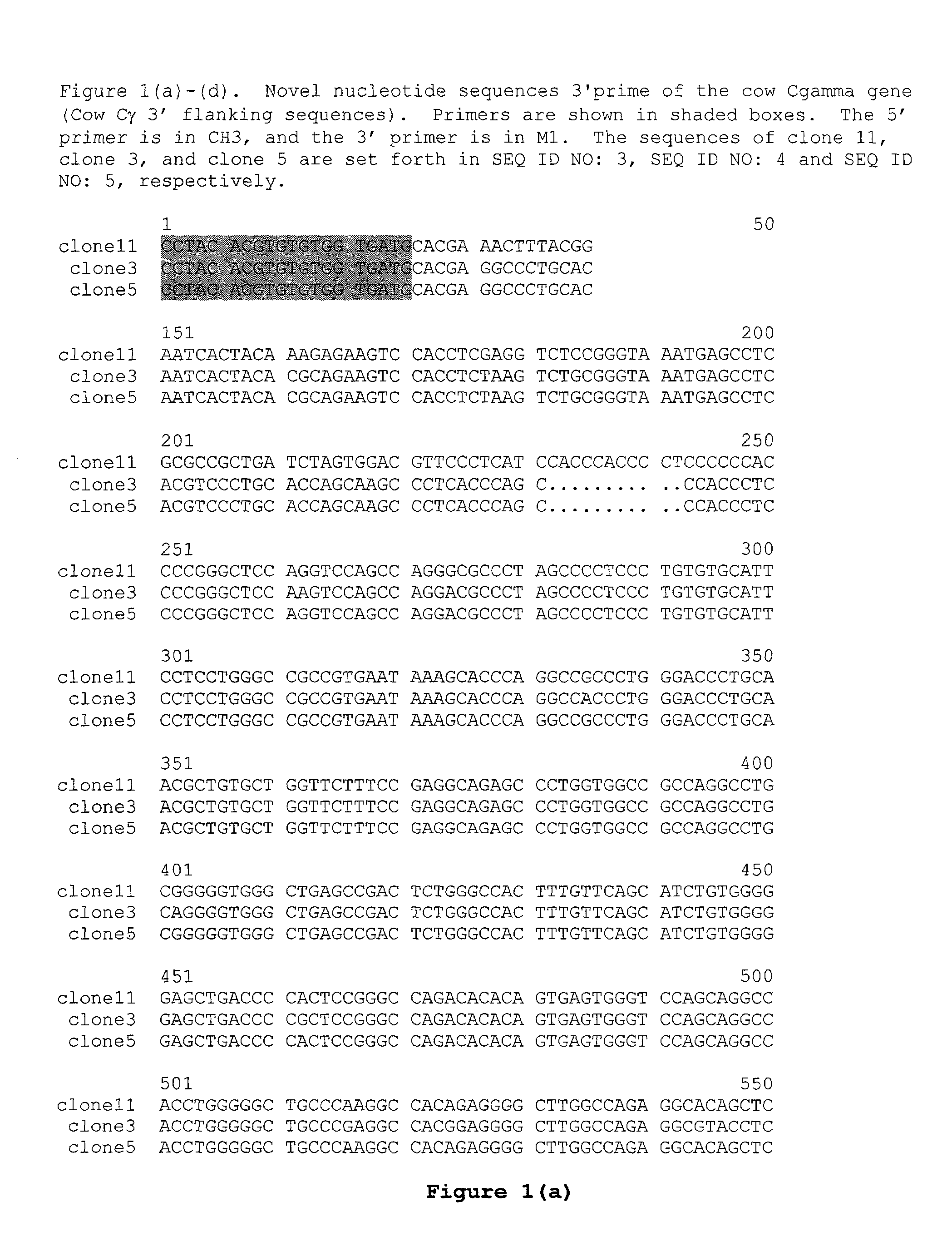
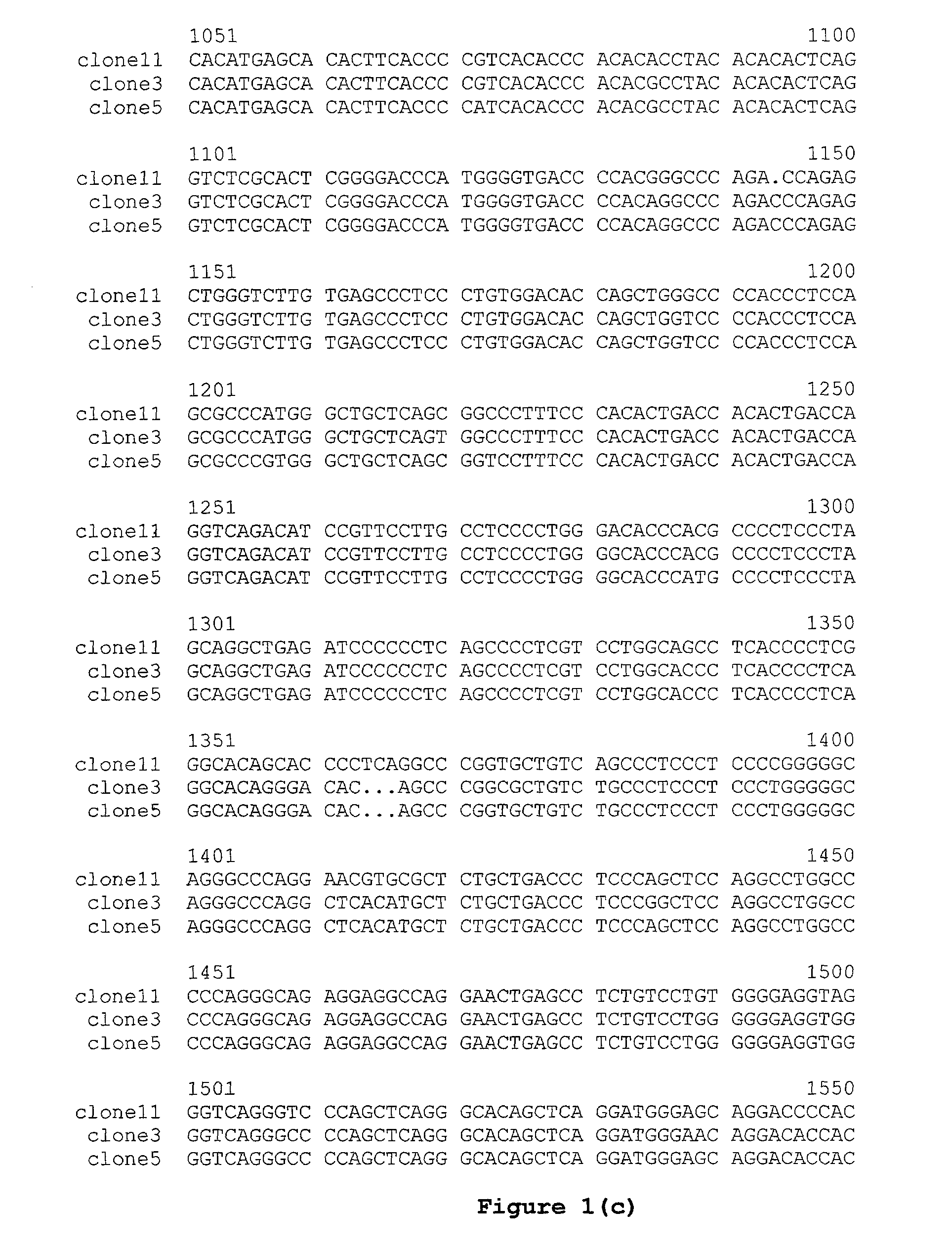
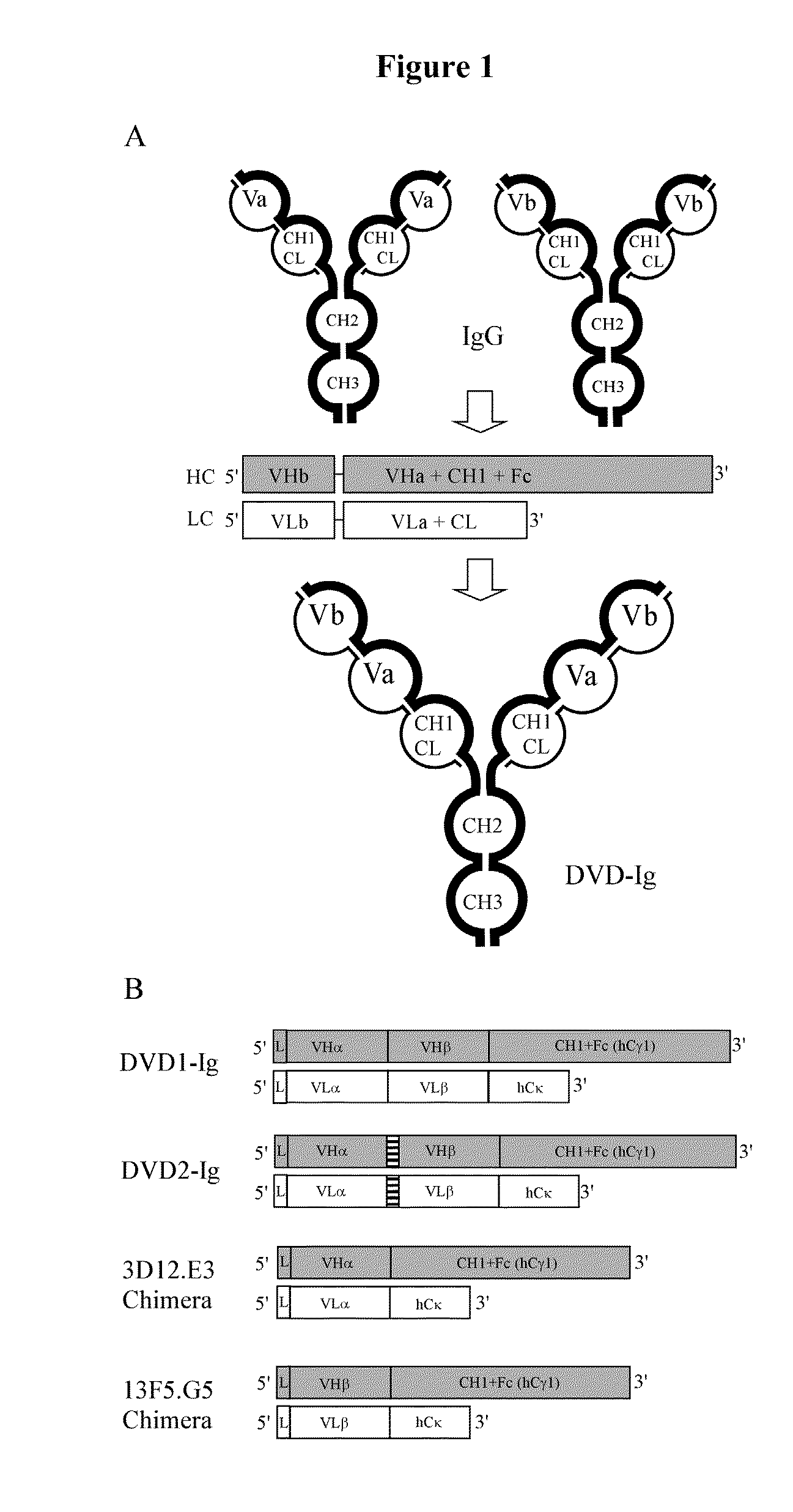
Dual Variable Domain Immunoglobulins and Uses Thereof
The present invention relates to engineered multivalent and multispecific binding proteins, methods of making, and specifically to their uses in the prevention, diagnosis, and / or treatment of disease.
Owner:ABBVIE INC
Immunoglobulin fusion proteins
ActiveUS7867491B2Extended half-lifeImprove expression levelPeptide/protein ingredientsAntipyreticFc domainBioactive molecules
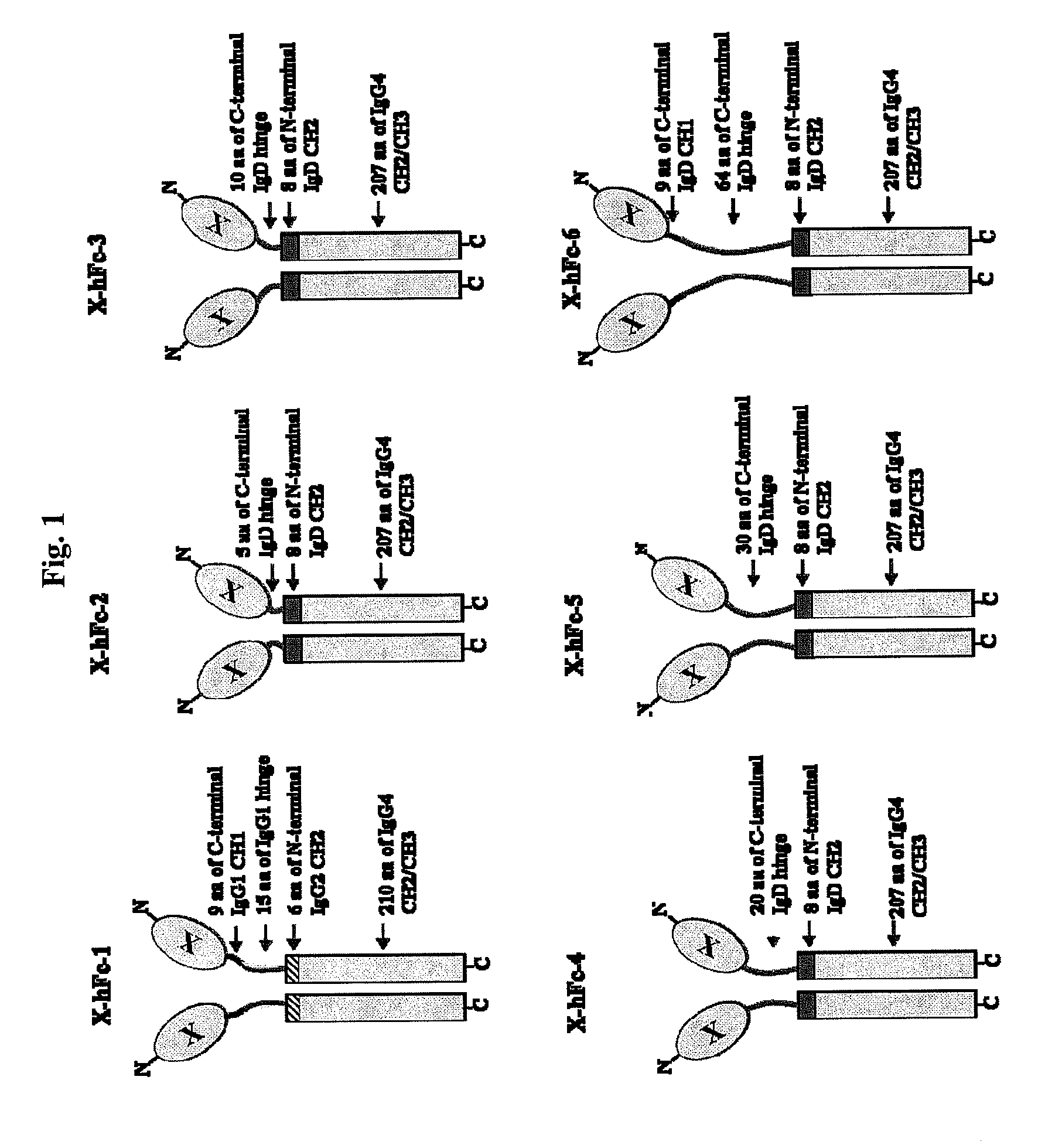
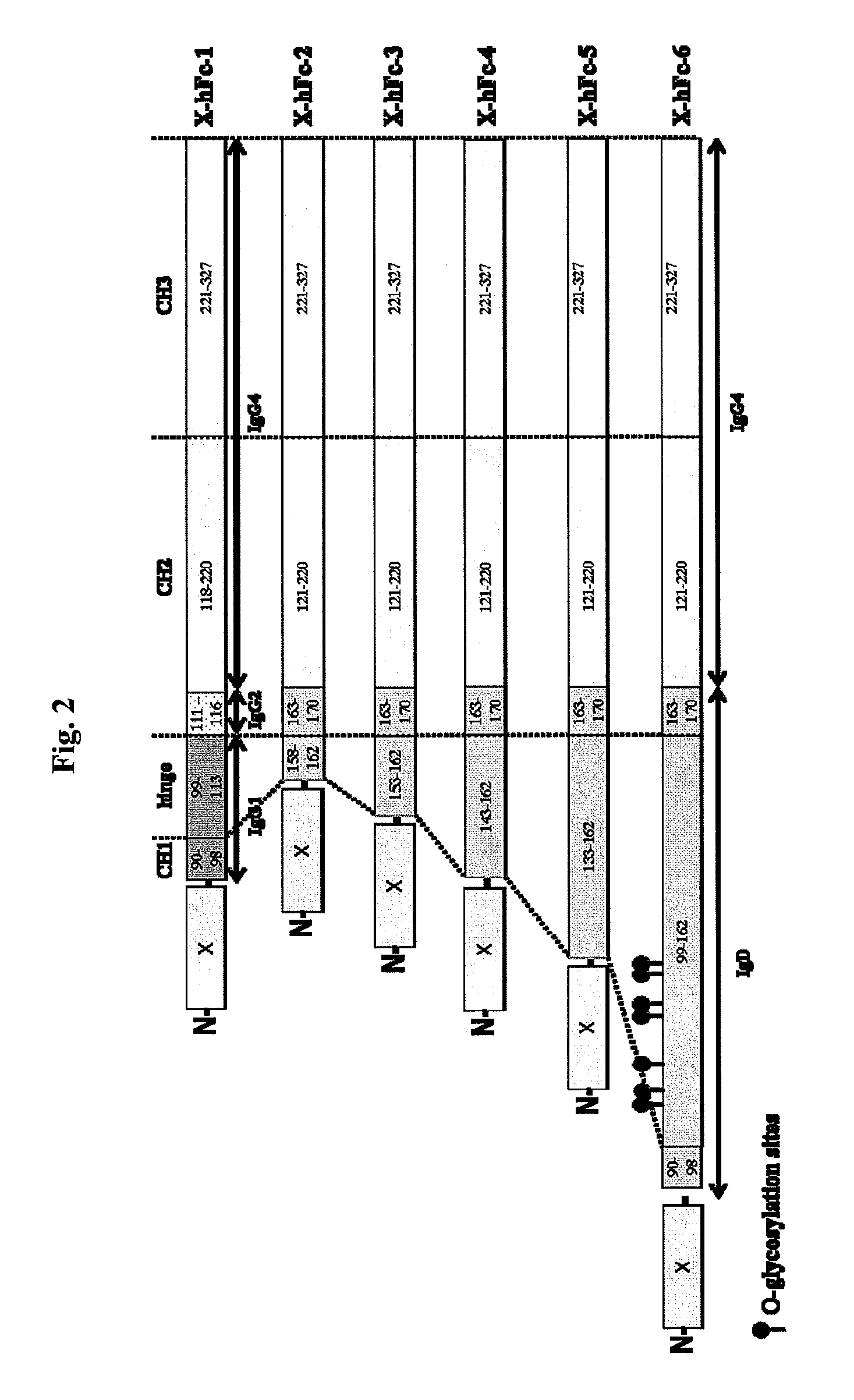
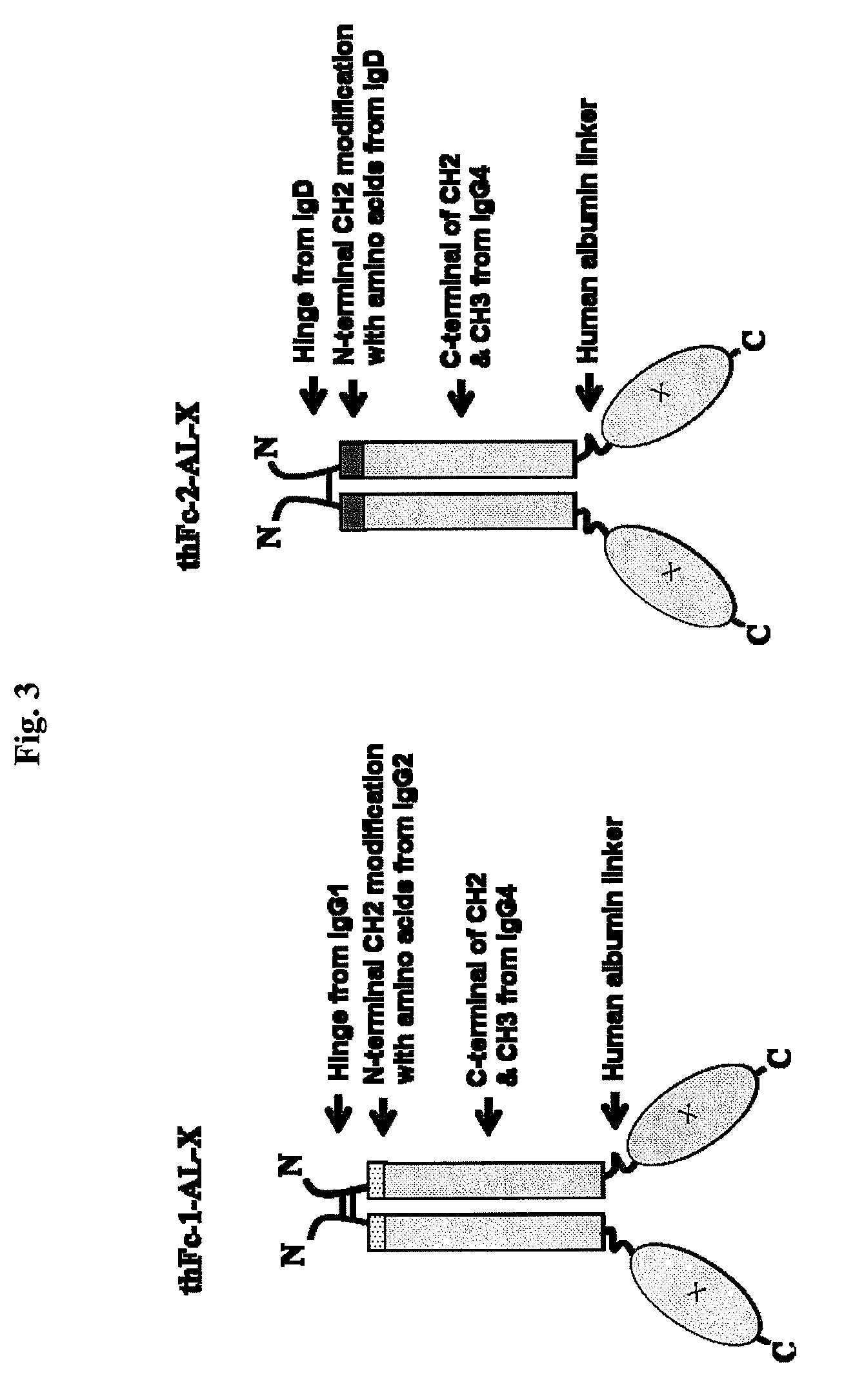
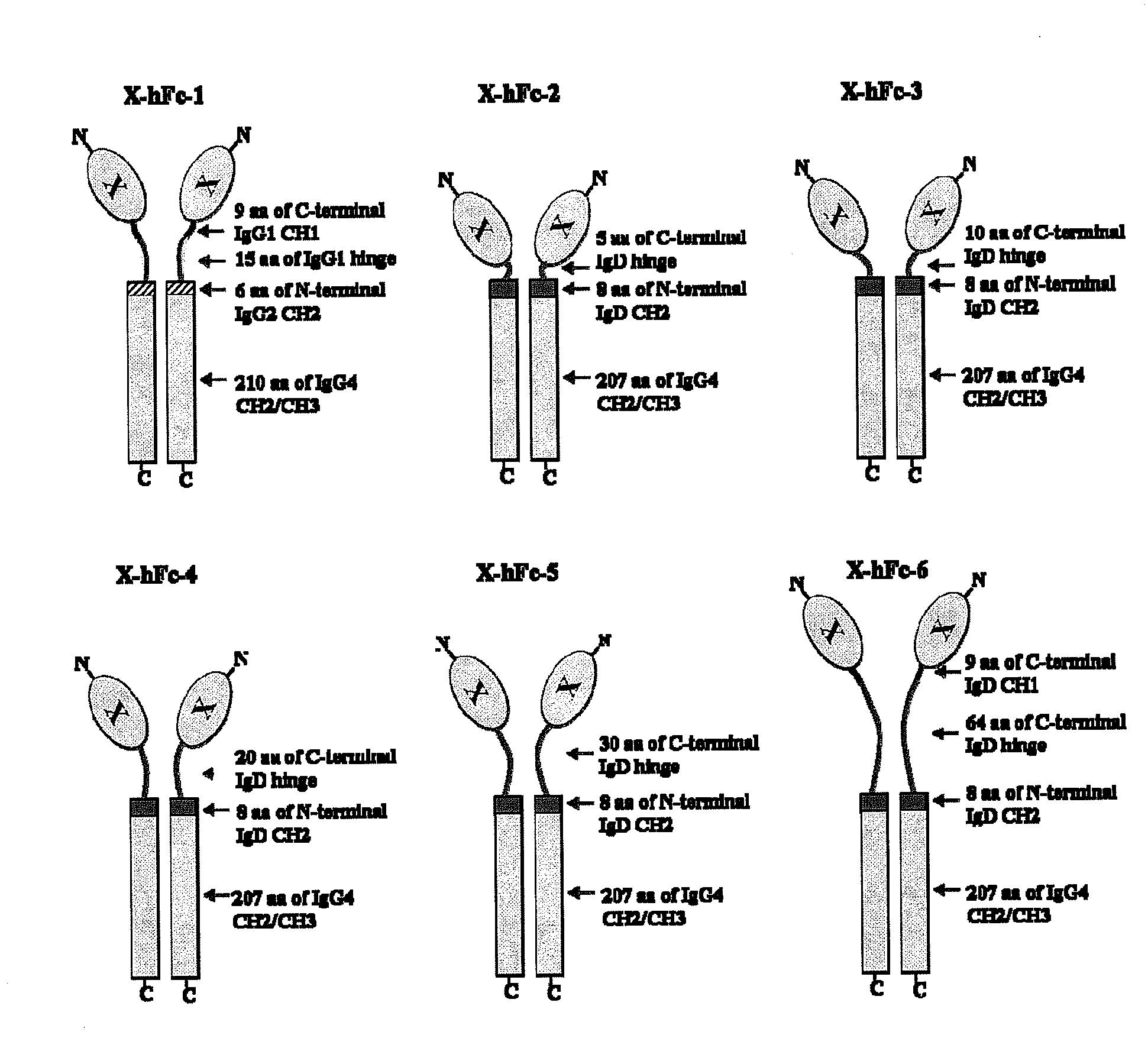
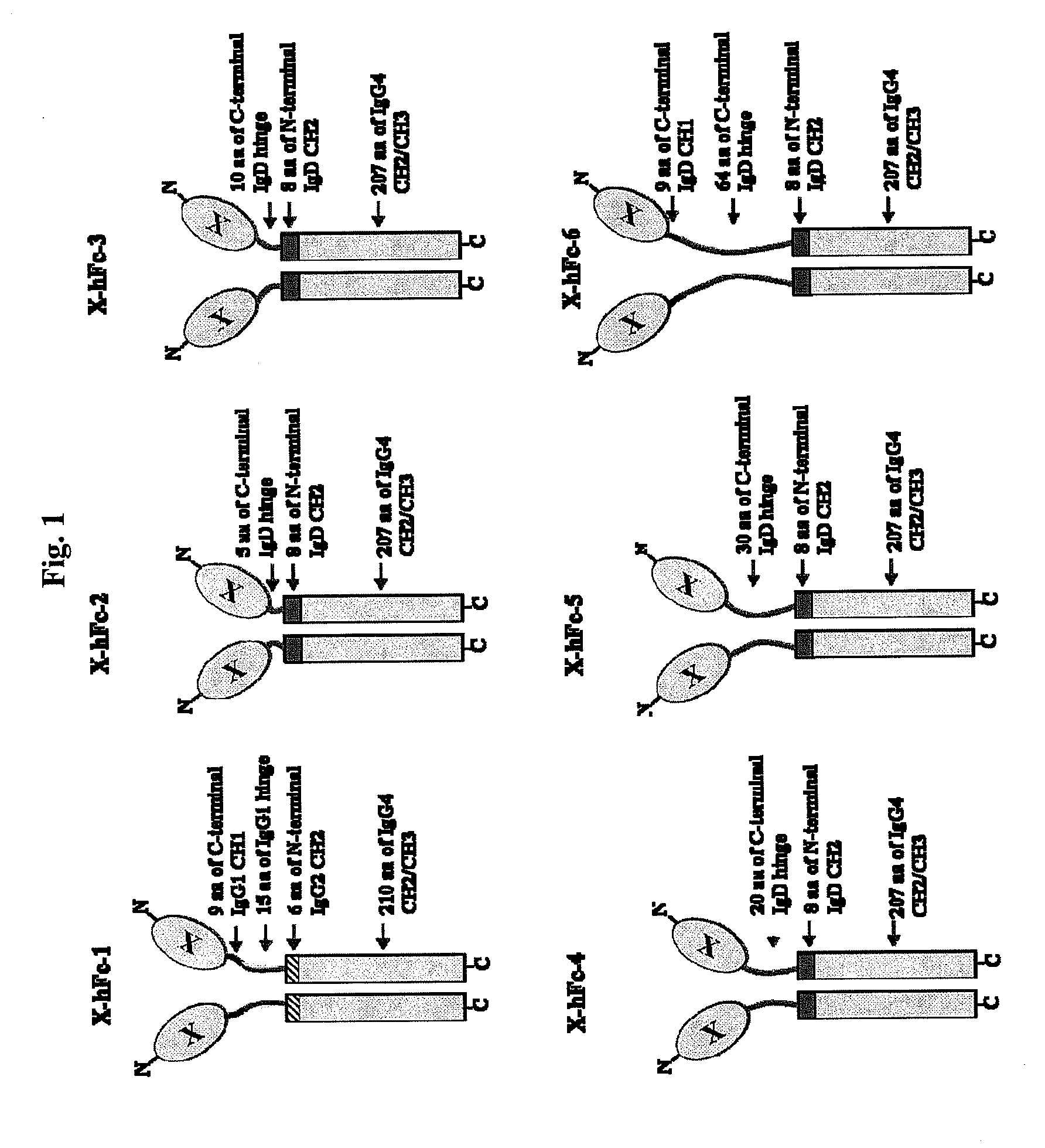
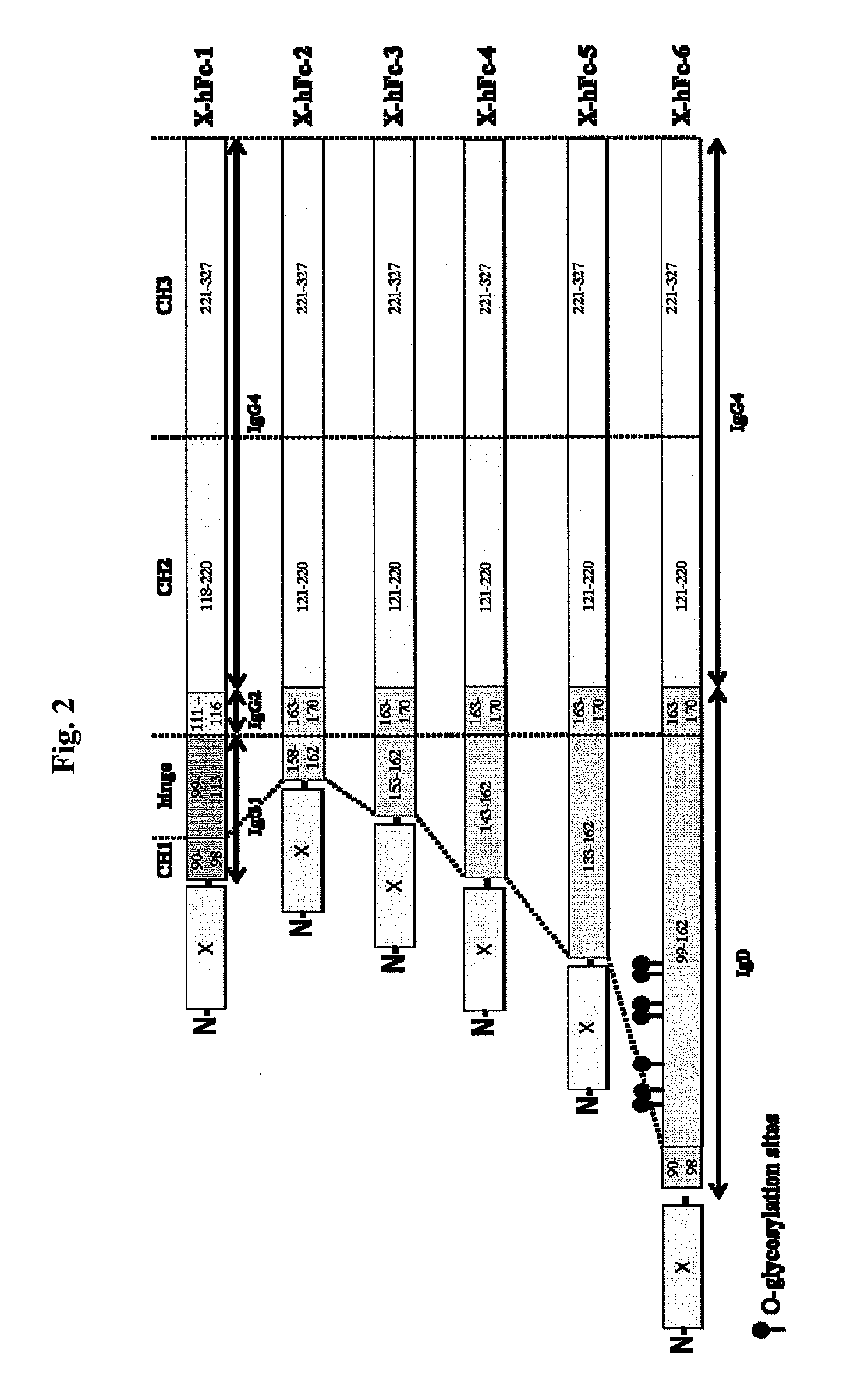
Disclosed are fusion proteins comprising a biologically active molecule and an immunoglobulin (Ig) Fc domain which is linked to the biologically active molecule. The Fc domain is a hybrid human Fc domain of (i) IgG1, IgG2 or IgG4 or (ii) IgG4 and IgD. The hybrid Fc is useful as a carrier of biologically active molecules.
Owner:POSTECH ACADEMY IND FOUND OF POHANG UNIV OF SCI & TECH POSTECH +1
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS8217147B2High affinityLow affinityAntibody mimetics/scaffoldsImmunoglobulinsDiseaseTherapeutic antibody
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcγRIIIA and / or FcγRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcγR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MACROGENICS INC
Immunoglobulin variants and uses thereof
InactiveUS20060024300A1Enhanced effector functionImprove stabilitySenses disorderNervous disorderDiseaseAutoimmune disease
Owner:GENENTECH INC
Pharmaceutically acceptable FN3 polypeptides for human treatments
InactiveUS20060246059A1Optimal folding and stability and solubilityImproved biophysical propertyBacteriaPeptide/protein ingredientsWAS PROTEINBiology
Disclosed herein are proteins that include an immunoglobulin fold and that can be used as scaffolds. Also disclosed herein are nucleic acids encoding such proteins and the use of such proteins in diagnostic methods and in methods for evolving novel compound-binding species and their ligands.
Owner:BRISTOL MYERS SQUIBB CO
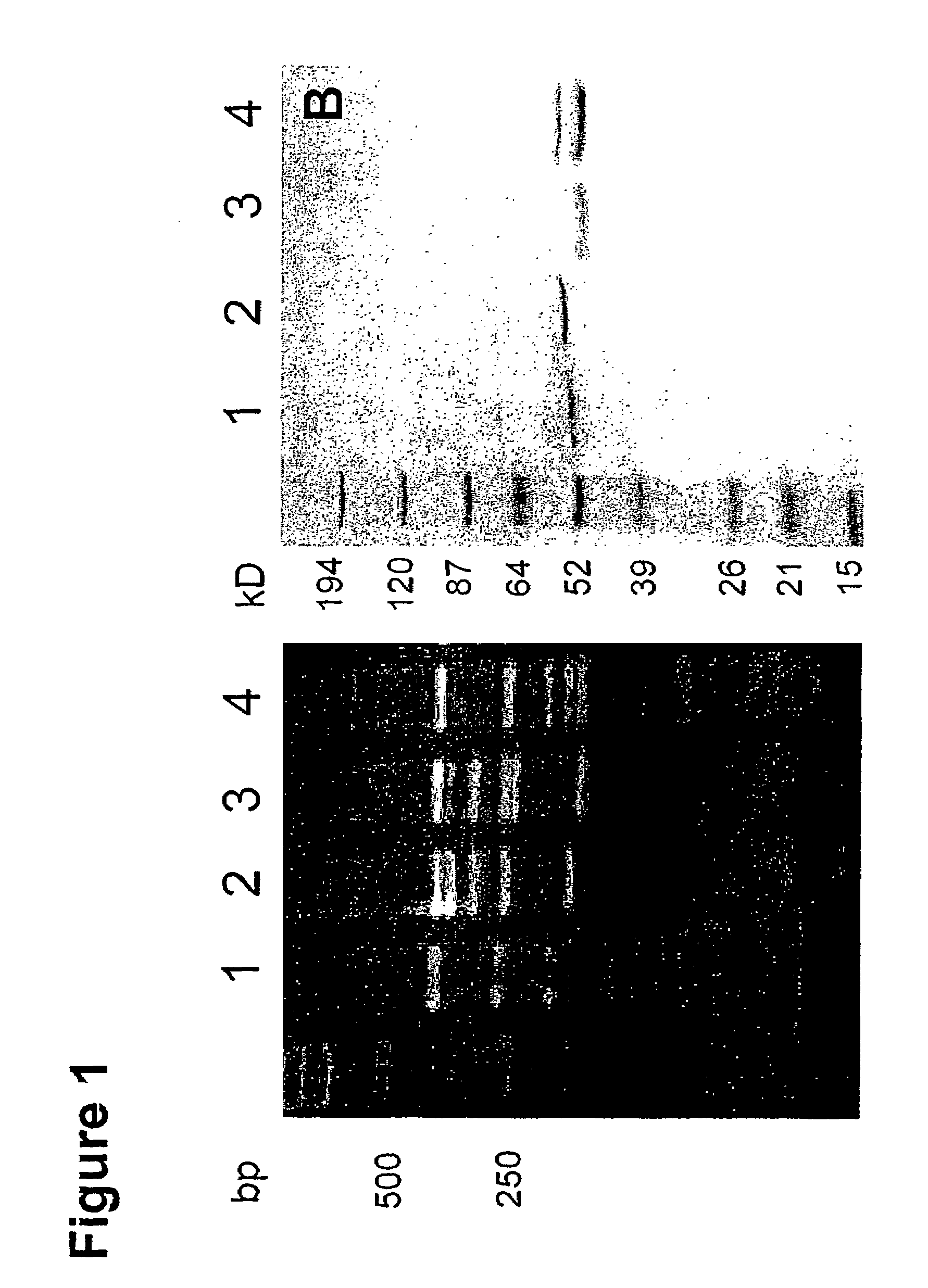
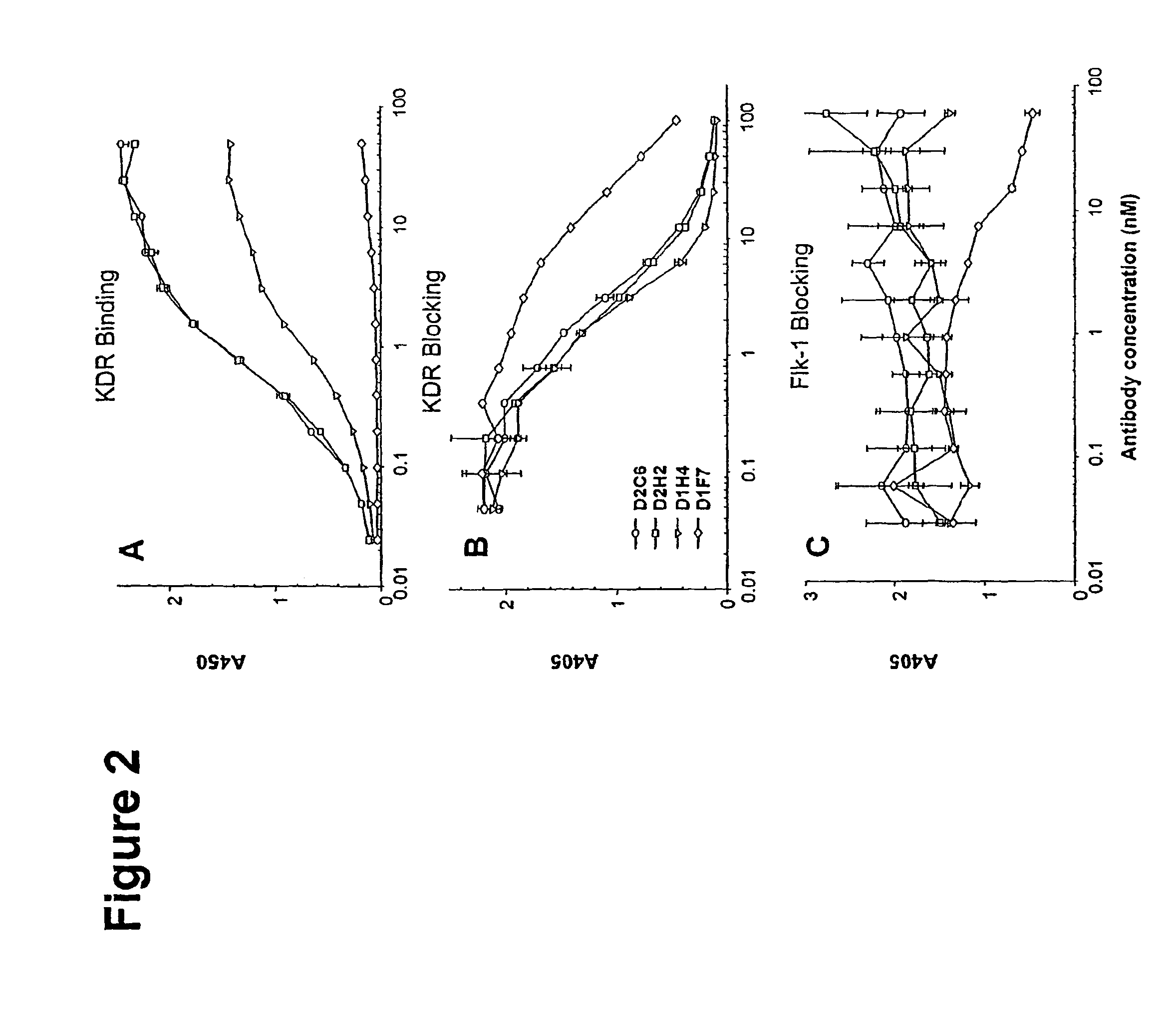
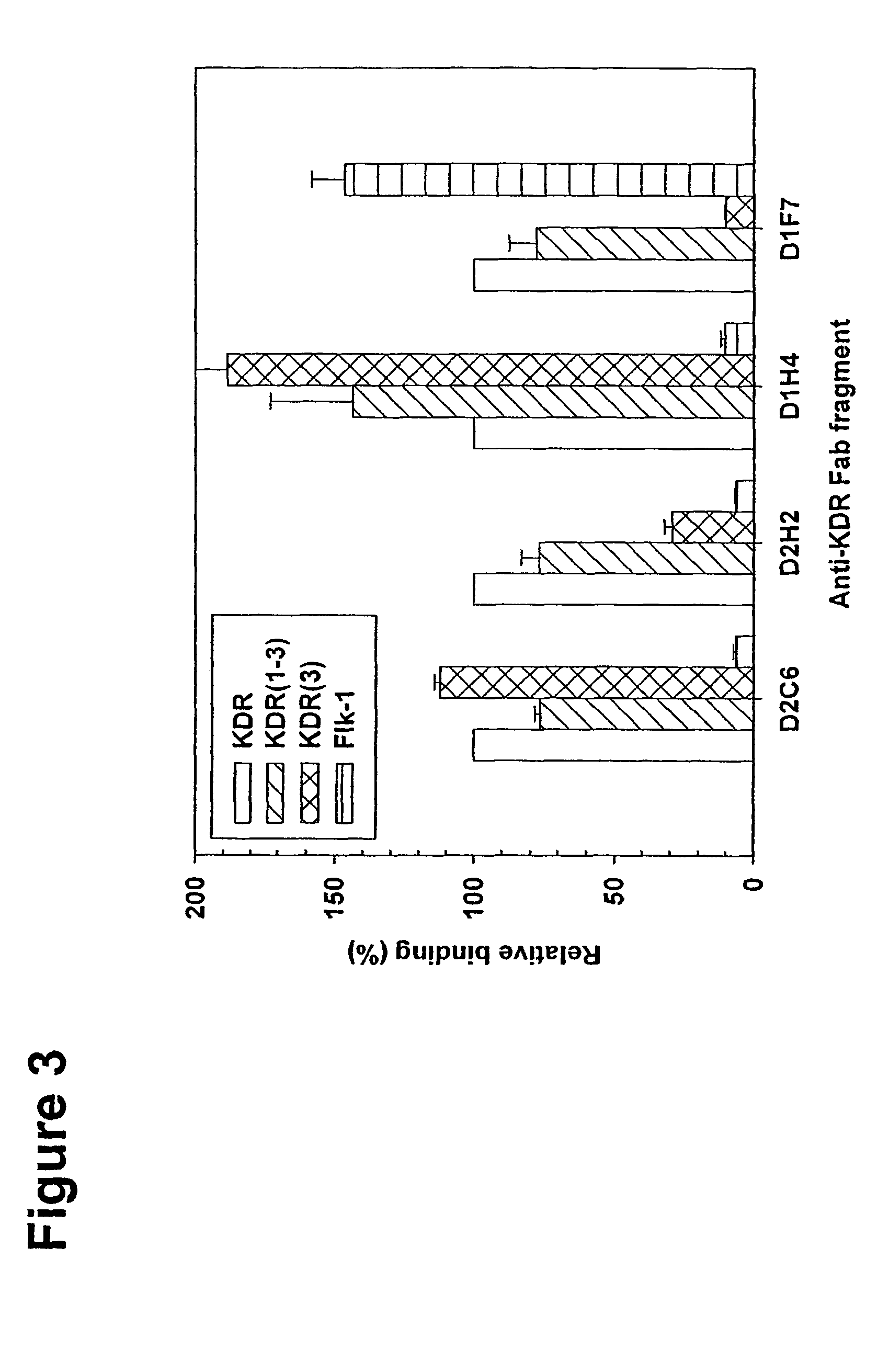
Human antibodies specific to KDR and uses thereof
The invention provides an antibodies that bind to KDR with an affinity comparable to or higher than human VEGF, and that neutralizes activation of KDR. Antibodies include whole immunoglobulins, monovalent Fabs and single chain antibodies, multivalent single chain antibodies, diabodies, triabodies, and single domain antibodies. The invention further provides nucleic acids and host cells that encode and express these antibodies. The invention further provides a method of neutralizing the activation of KDR, a method of inhibiting angiogenesis in a mammal and a method of inhibiting tumor growth in a mammal.
Owner:IMCLONE SYSTEMS
Method for preparing human immunoglobulin concentrates for therapeutic use
InactiveUS7186410B2Simple processHighly compatibleAntibacterial agentsSerum immunoglobulinsAnion-exchange chromatographyBlood plasma
The invention concerns a method for preparing human immunoglobulin concentrates for therapeutic use, from plasma or a plasma fraction. The method comprises pre-purification and a single anion-exchange chromatography carried out at alkaline pH, thereby enabling the immunoglobulins to be retained on the chromatographic support and fractionated. The method enables to obtain IgG, IgA and IgM concentrates.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
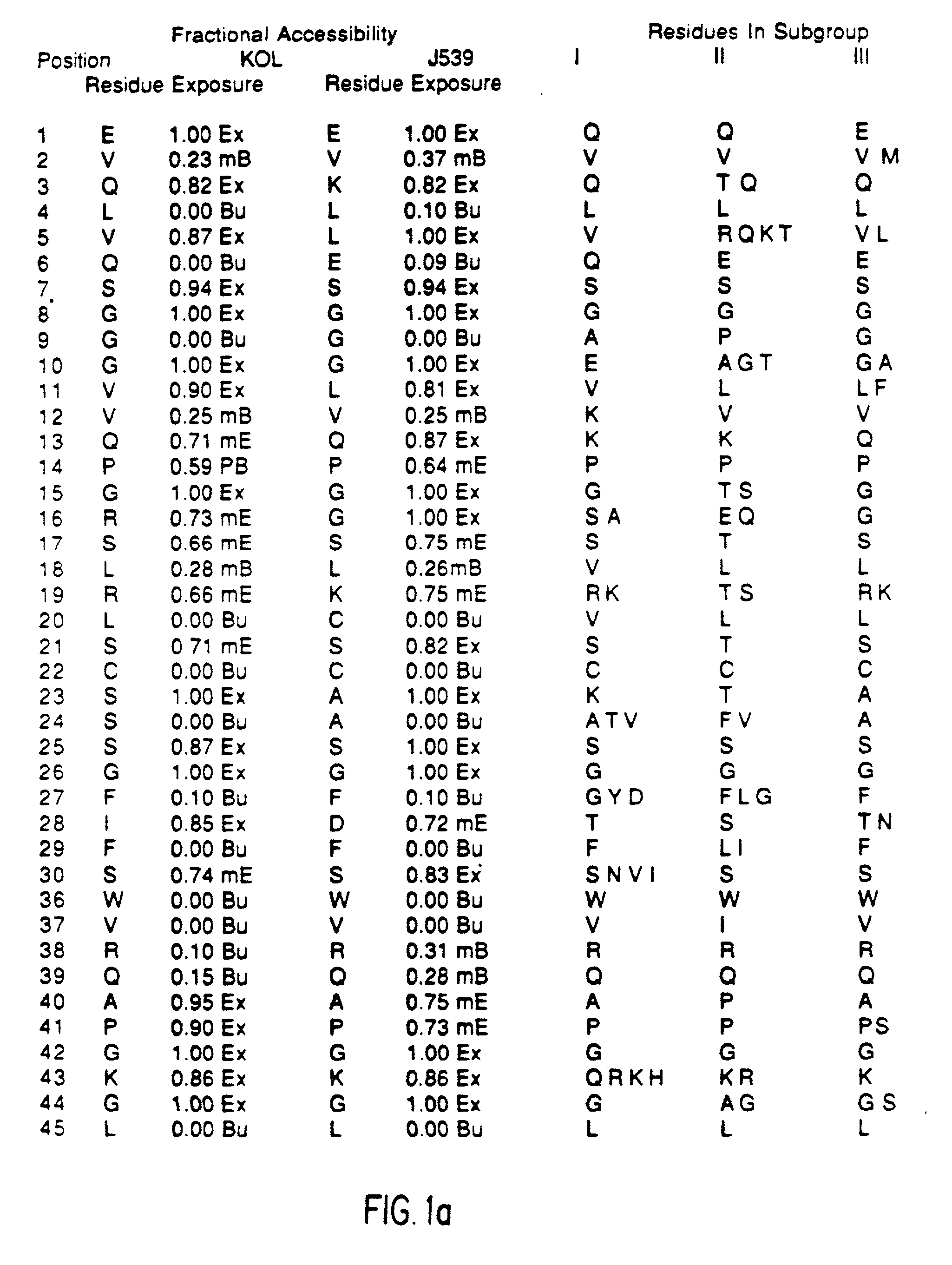
Method for reducing the immunogenicity of antibody variable domains
InactiveUS20020034765A1Altered immunogenicityLow immunogenicityHybrid immunoglobulinsBiological testingBound propertyVariable domain
A unique method is disclosed for identifying and replacing immunoglobulin surface amino acid residues which converts the antigenicity of a first mammalian species to that of a second mammalian species. The method will simultaneously change immunogenicity and strictly preserve ligind binding properties. The judicious replacement of exterior amino acid residues has no effect on the ligind binding properties but greatly alters immunogenicity.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE +1
Binding proteins comprising immunoglobulin hinge and fc regions having altered fc effector functions
Provided herein are binding proteins comprising one or more immunoglobulin Fc region hinge, CH2, and / or CH3 domain wherein one or more hinge and / or constant region CH2 and / or CH3 domain is modified to alter the binding protein's binding affinity and / or specificity for a cognate receptor (e.g., an Fc receptor) and / or to impart one or more new binding specificity(ies) to the hinge and / or constant region that the corresponding unmodified immunoglobulin does not possess (e.g., affinity for distinct class of cognate receptor distinct from the class of cognate receptor to which the unmodified binding protein specifically binds). Binding proteins according to the present invention include, for example, modified antibodies, antibody fragments, recombinant binding proteins, and molecularly engineered binding domain-immunoglobulin fusion proteins, including small modular immunopharmaceutical products (SMIP™ products).
Owner:TRUBION PHARM INC
Immunoglobulin formulation and method of preparation thereof
InactiveUS20050053598A1Fixed volumeNervous disorderAntipyreticPharmaceutical formulationVariable weight
A stable aqueous pharmaceutical formulation comprising a therapeutically effective amount of an antibody, polysorbate 80, a buffer which inhibits polysorbate oxidation is described along with methods of making the preparation. Also described are formulations with high antibody concentrations which maintain fixed volumes and which may be used on patients of variable weight.
Owner:BIOGEN MA INC
Humanized immunoglobulin loci
Owner:THERAPEUTIC HUMAN POLYCLONALS
Stabilized glycoproteins
ActiveUS20040191265A1Increased in half lifeGood storage stabilitySnake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainGlycoprotein i
The present invention provides stabilized immunoglobulin molecules that have increased storage stability and / or in vivo half-lives due to the mutation of one or more amino acids that would otherwise render the immunoglobulin molecules susceptible to degradation. In a preferred embodiment, the stabilized immunoglobulins of the invention have mutations at the heavy chain constant domain hinge region. Such stabilized immunoglobulin molecules, i.e., immunoglobulin molecules with increased storage stability have one or more of the following advantages they are more readily transported and / storable for longer periods and / or less stringent conditions than non-stabilized counterparts; that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such stabilized molecules.
Owner:MEDIMMUNE LLC
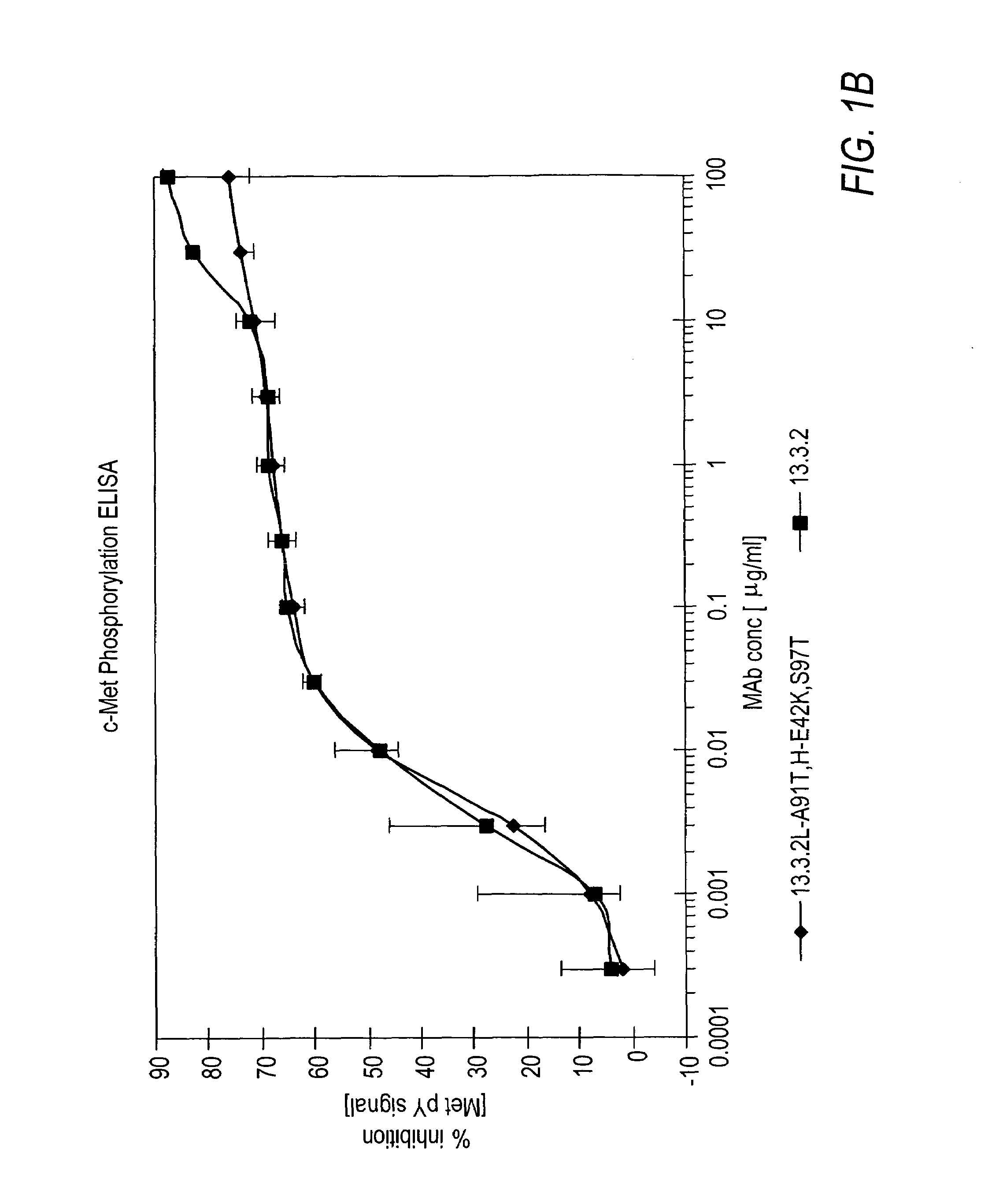
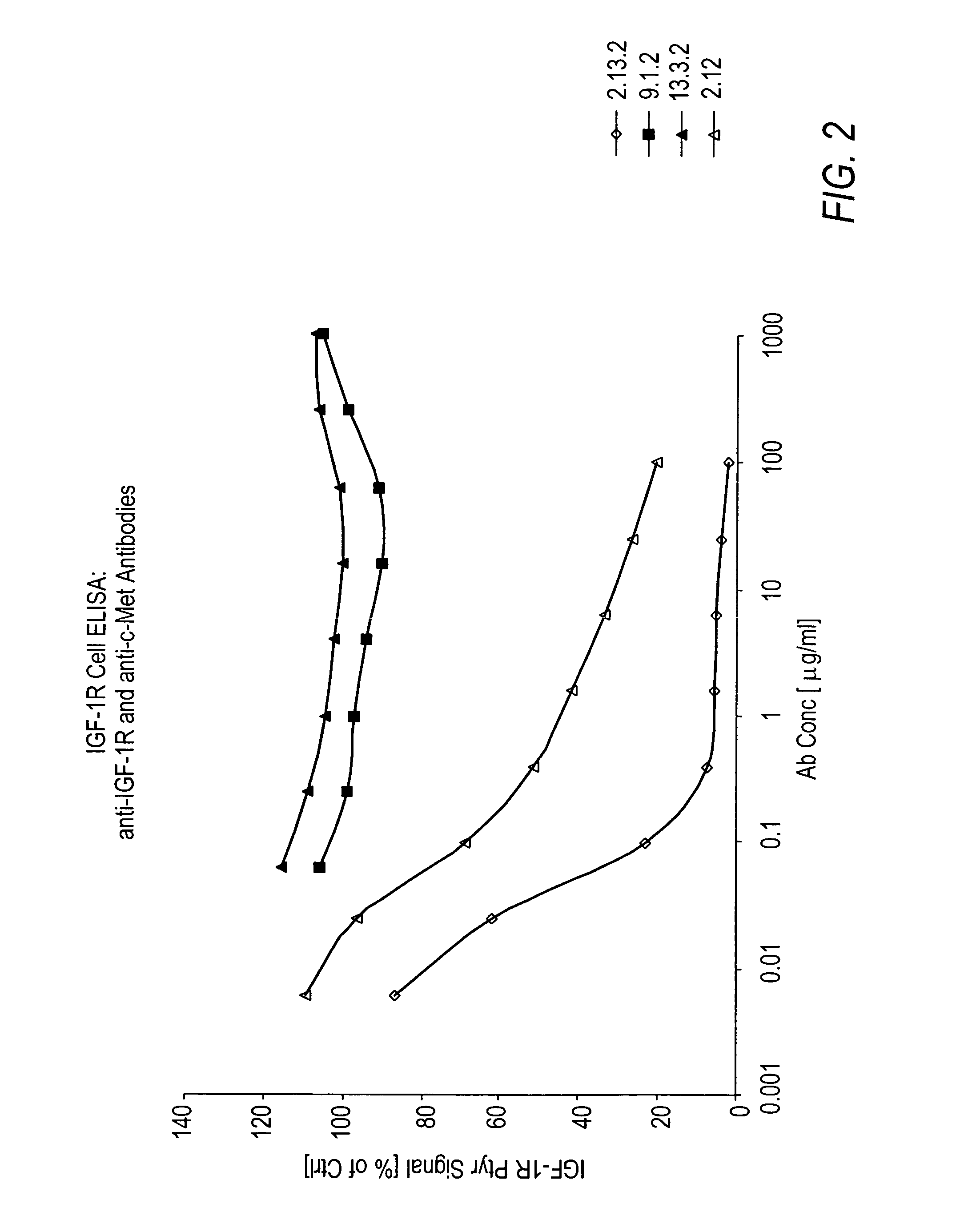
Antibodies to c-Met
ActiveUS7498420B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSingle-Chain AntibodiesHeavy chain
The present invention relates to antibodies including human antibodies and antigen-binding portions thereof that specifically bind to c-Met, preferably human c-Met, and that function to inhibit c-Met. The invention also relates to human anti-c-Met antibodies and antigen-binding portions thereof. The invention also relates to antibodies that are chimeric, bispecific, derivatized, single chain antibodies or portions of fusion proteins. The invention also relates to isolated heavy and light chain immunoglobulins derived from human anti-c-Met antibodies and nucleic acid molecules encoding such immunoglobulins. The present invention also relates to methods of making human anti-c-Met antibodies, compositions comprising these antibodies and methods of using the antibodies and compositions for diagnosis and treatment. The invention also provides gene therapy methods using nucleic acid molecules encoding the heavy and / or light immunoglobulin molecules that comprise the human anti-c-Met antibodies. The invention also relates to transgenic animals or plants comprising nucleic acid molecules of the present invention.
Owner:AMGEN FREMONT INC +1
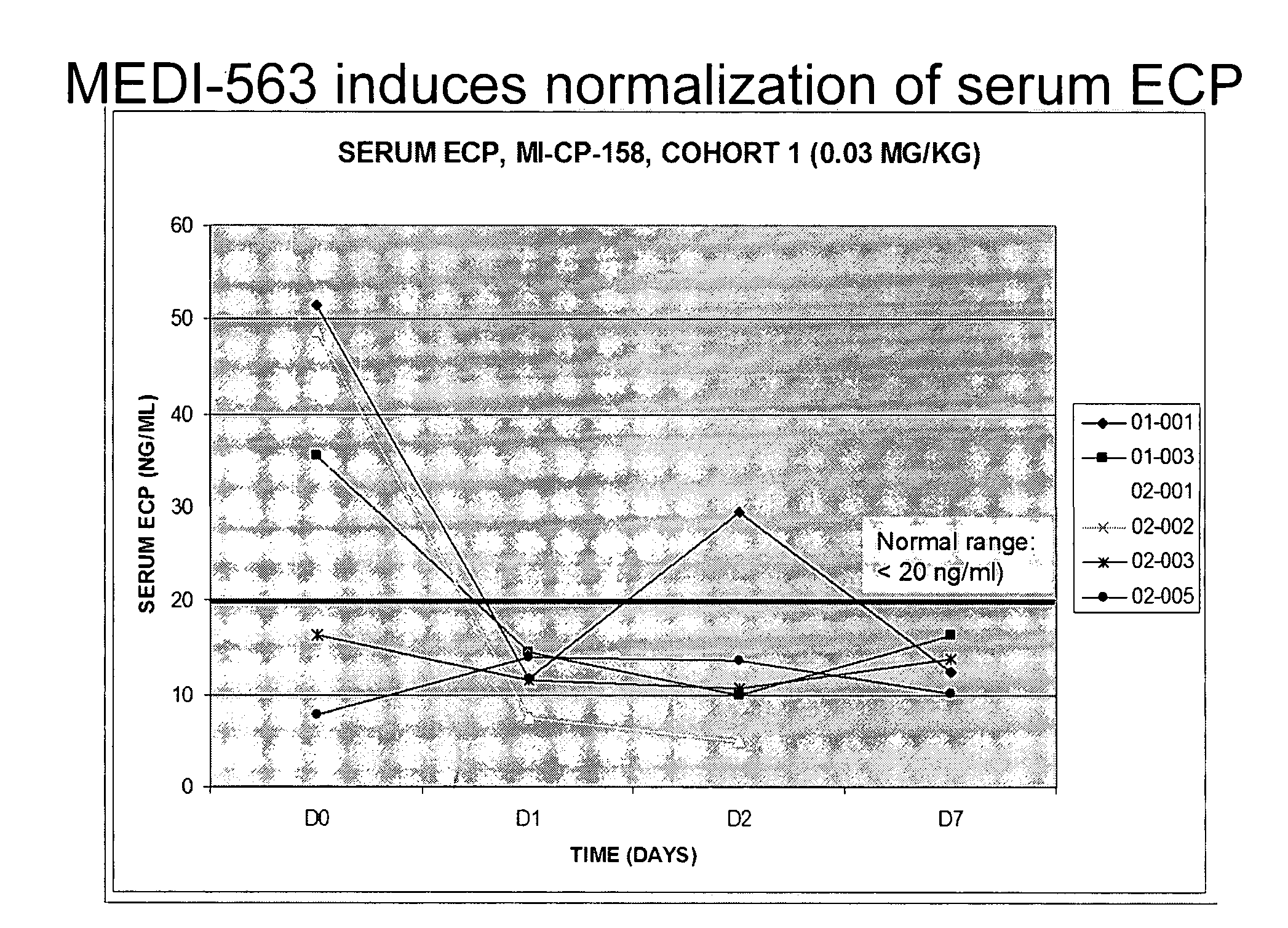
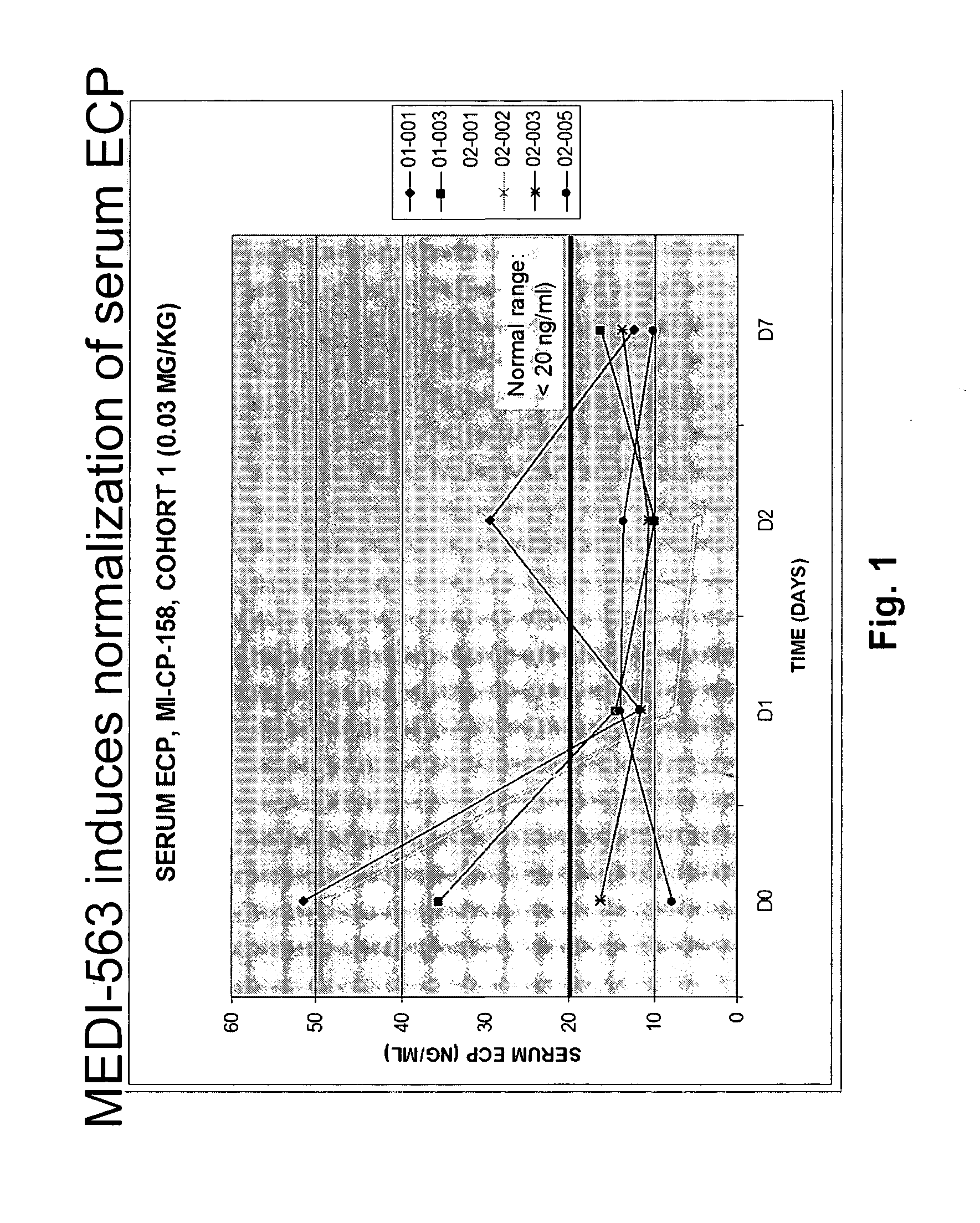
Methods of reducing eosinophil levels
The present invention relates to a method of reducing the numbers of eosinophils in a human subject comprising administration to a subject an IL-5R binding molecule that comprises (a) a region that specifically binds to the IL-5R and (b) an immunoglobulin Fc region. In a specific embodiment, a method of the invention reduces the number of eosinophils in blood, bone marrow, gastrointestinal tract (e g, esophagus, stomach, small intestine and colon), or lung.
Owner:BIOWA +1
Fc Region-Containing Polypeptides That Exhibit Improved Effector Function Due To Alterations Of The Extent Of Fucosylation, And Methods For Their Use
ActiveUS20120219551A1Enhanced antibody effector functionGood curative effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsEffector cellEffector functions
The present invention relates to Fc region-containing polypeptides that exhibit improved effector function due to alterations of the extent of fucosylation, and to methods of using such polypeptides for treating or preventing cancer and other diseases. The Fc region-containing polypeptides of the present invention are preferably immunoglobulins (e.g., antibodies), in which the Fc region comprises at least one amino acid substitution relative to the corresponding amino acid sequence of a wild type Fc region, and which is sufficient to attenuate post-translational fucosylation and mediate improved binding to an activating Fc receptor and reduced binding to an inhibitory Fc receptor. The methods of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection where either an enhanced efficacy of effector cell function mediated by FcγR is desired (e.g., cancer, infectious disease) or an inhibited effector cell response mediated by FcγR is desired (e.g., inflammation, autoimmune disease).
Owner:MACROGENICS INC
Bispecific binding molecules for Anti-angiogenesis therapy
InactiveUS20110172398A1Reduce manufacturing costImproving desired propertySenses disorderSugar derivativesAngiogenesis EffectAntiangiogenic therapy
Bispecific binding molecules, in particular immunoglobulin single variable domains such as VHHs and domain antibodies, comprising a VEGF-binding component and a Dll4-binding component in one molecule. Pharmaceutical compositions containing same and their use in the treatment of diseases that are associated with VEGF- and Dll4-mediated effects on angiogenesis. Nucleic acids encoding the bispecific binding molecules, host cells and methods for preparing same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Immunoglobulin Fusion Proteins
ActiveUS20080300188A1Extended half-lifeImprove expression levelOrganic active ingredientsPeptide/protein ingredientsFc domainBioactive molecules
Disclosed are fusion proteins comprising a biologically active molecule and an immunoglobulin (Ig) Fc domain which is linked to the biologically active molecule. The Fc domain is a hybrid human Fc domain of (i) IgG1, IgG2 or IgG4 or (ii) IgG4 and IgD. The hybrid Fc is useful as a carrier of biologically active molecules.
Owner:POSTECH ACADEMY IND FOUND OF POHANG UNIV OF SCI & TECH POSTECH +1
Human tumor necrosis factor-immunoglobulin(TNFR1-IgG1) chimera composition
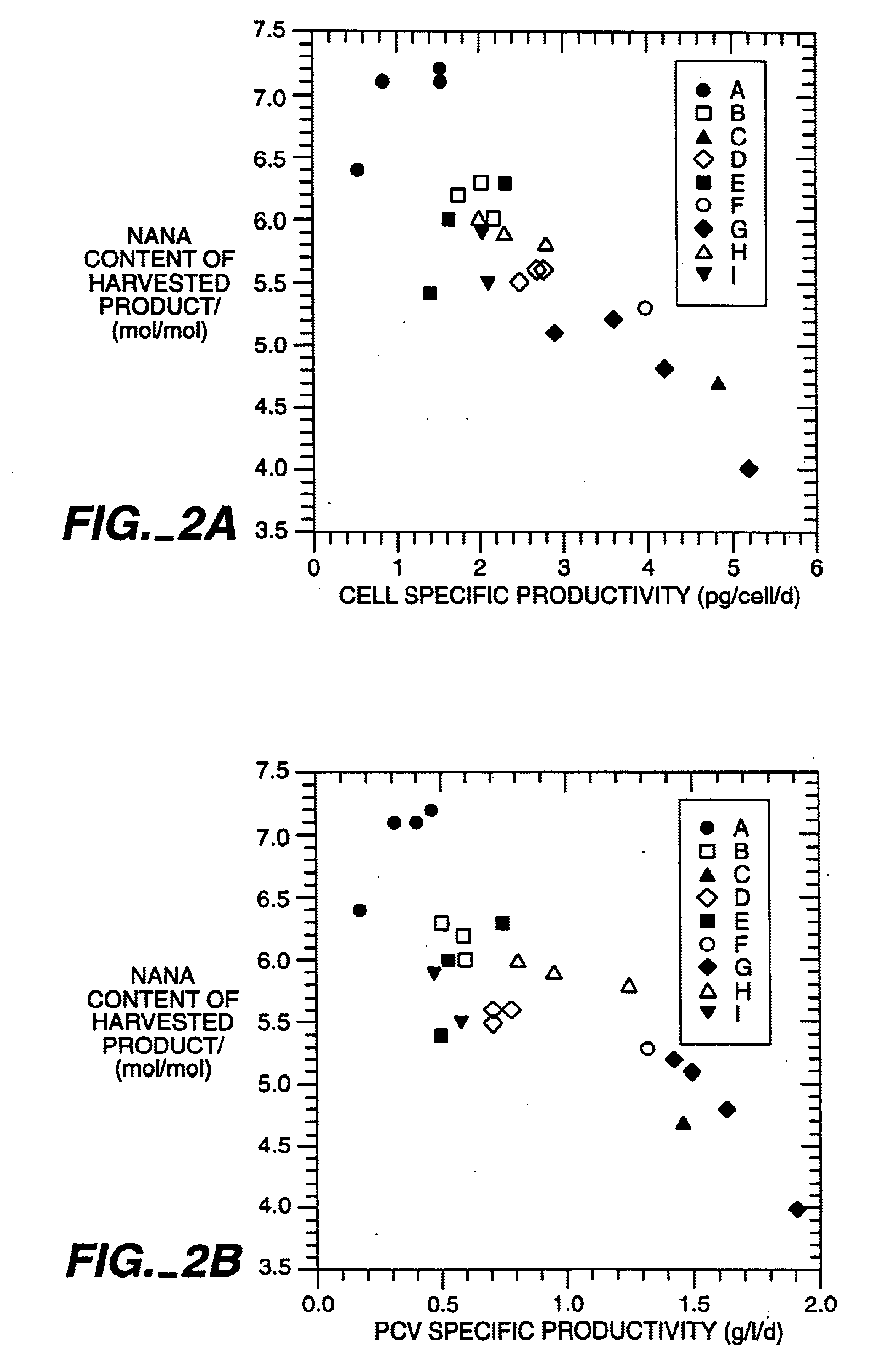
The present invention relates to novel process for the preparation of glycoproteins by mammalian cell culture wherein the sialic acid content of the glycoprotein produced is controlled over a broad range of values by manipulating the cell culture environment. The invention provides for processes in which the sialic acid content of the glycoprotein is modified by changes in cell culture parameters which affect cell specific productivity. Preferred embodiments of the invention include cell culture processes in the osmolality of the cell culture is controlled as well as the concentration of a transcription enhancer during the production phase of the cell culture. The invention further provides for novel preparations of soluble type 1 tumor necrosis factor immunoglobulin G1 and their uses in the treatment of inflammatory or immune related disorders.
Owner:GENENTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com