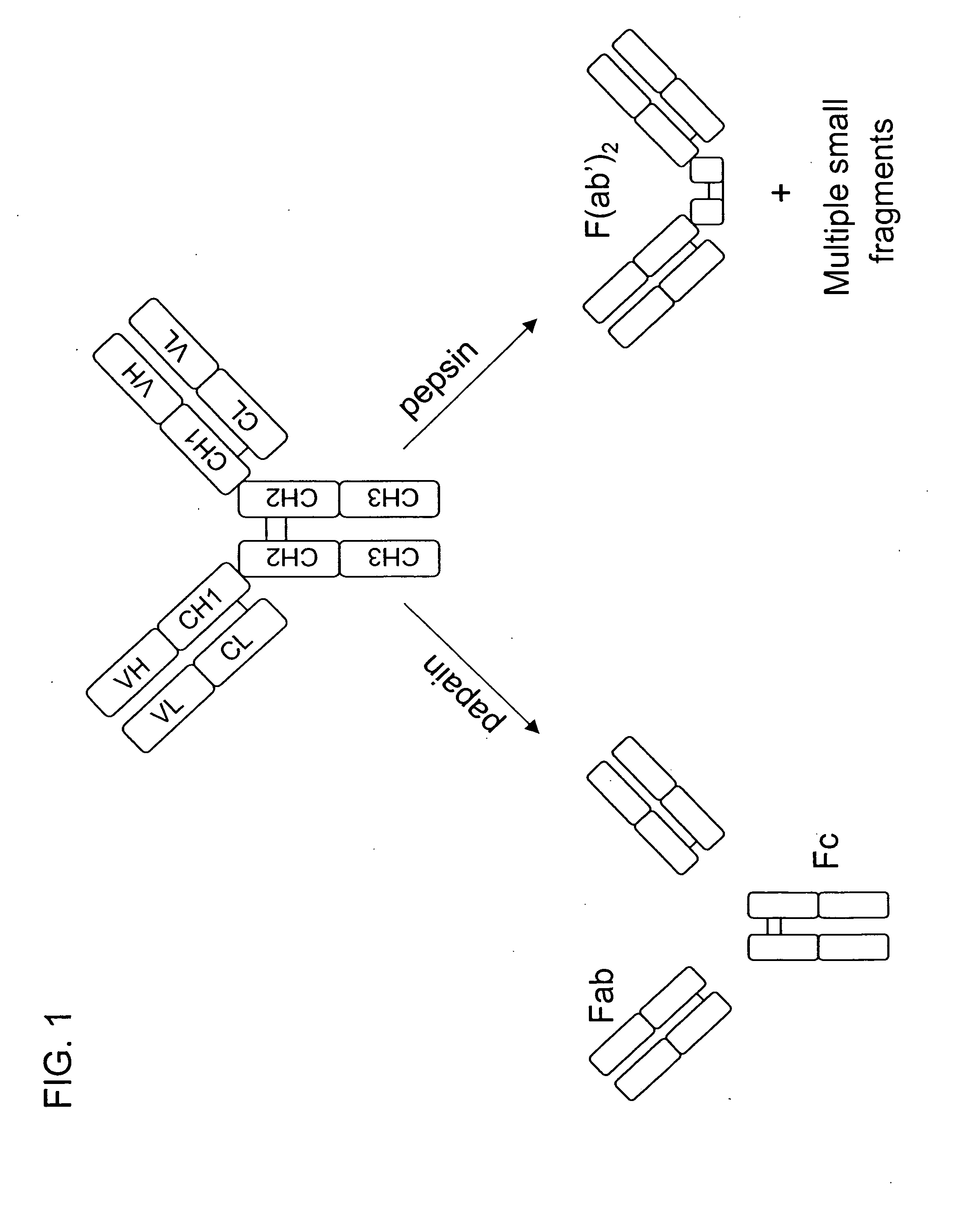
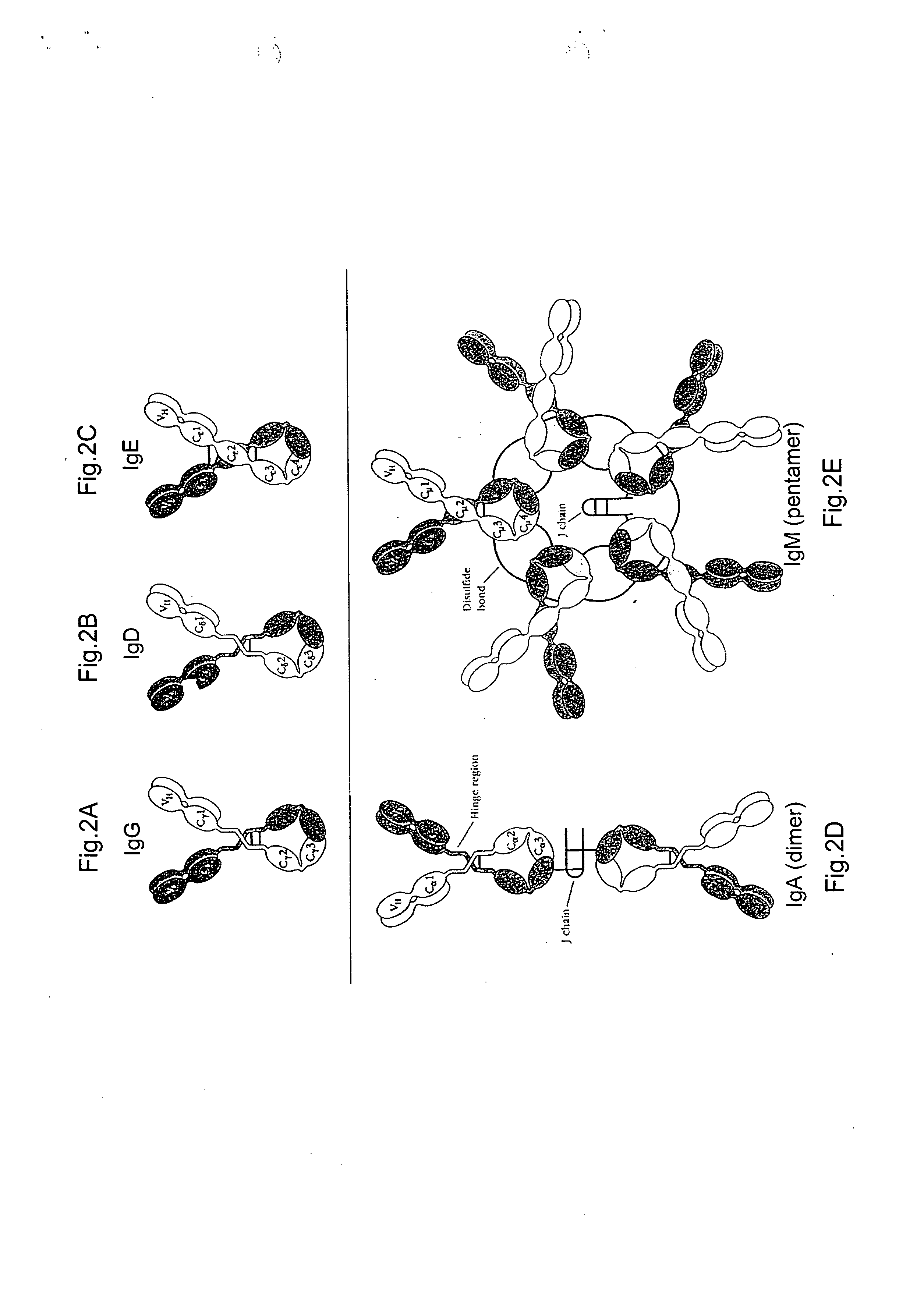
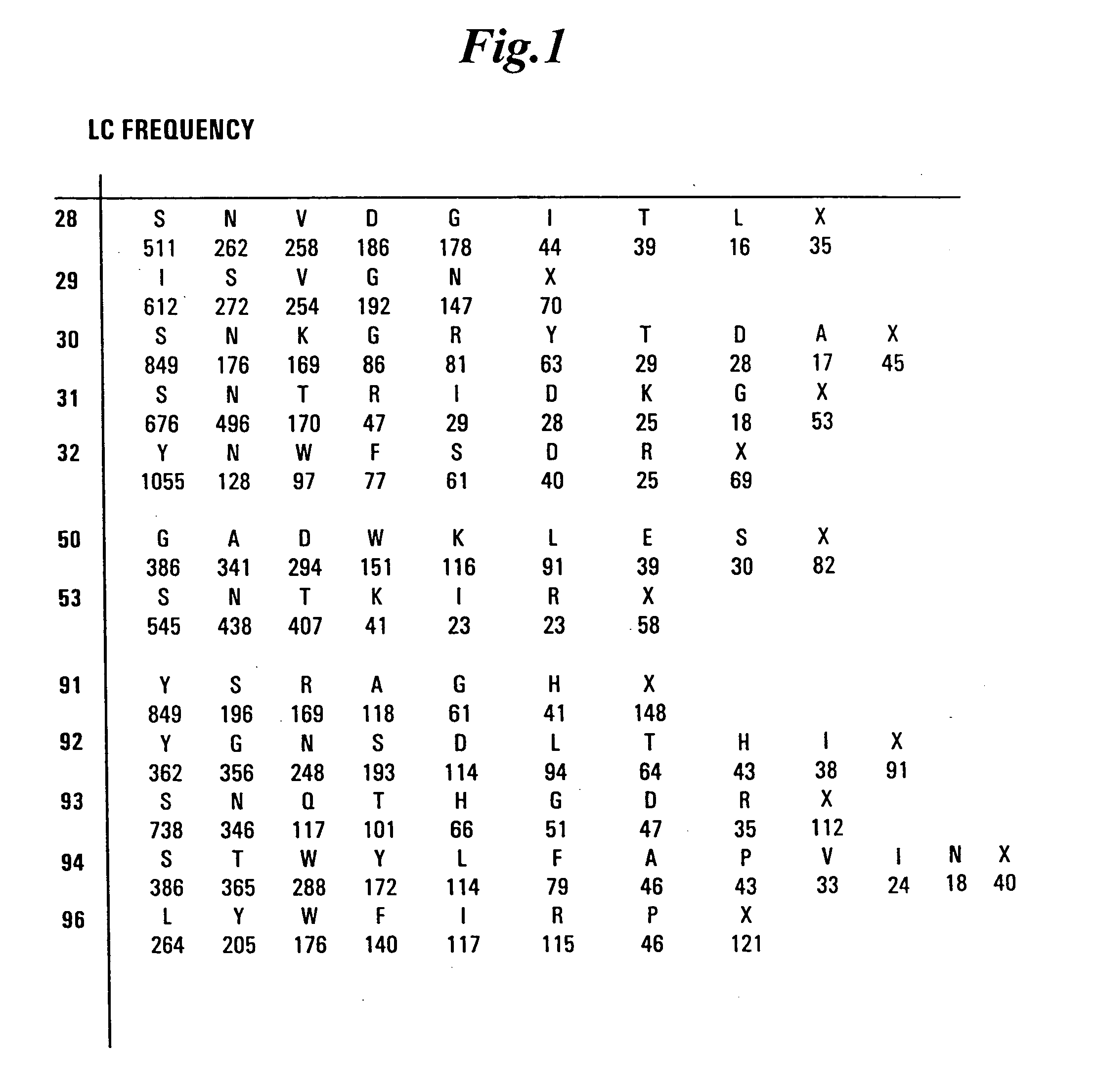
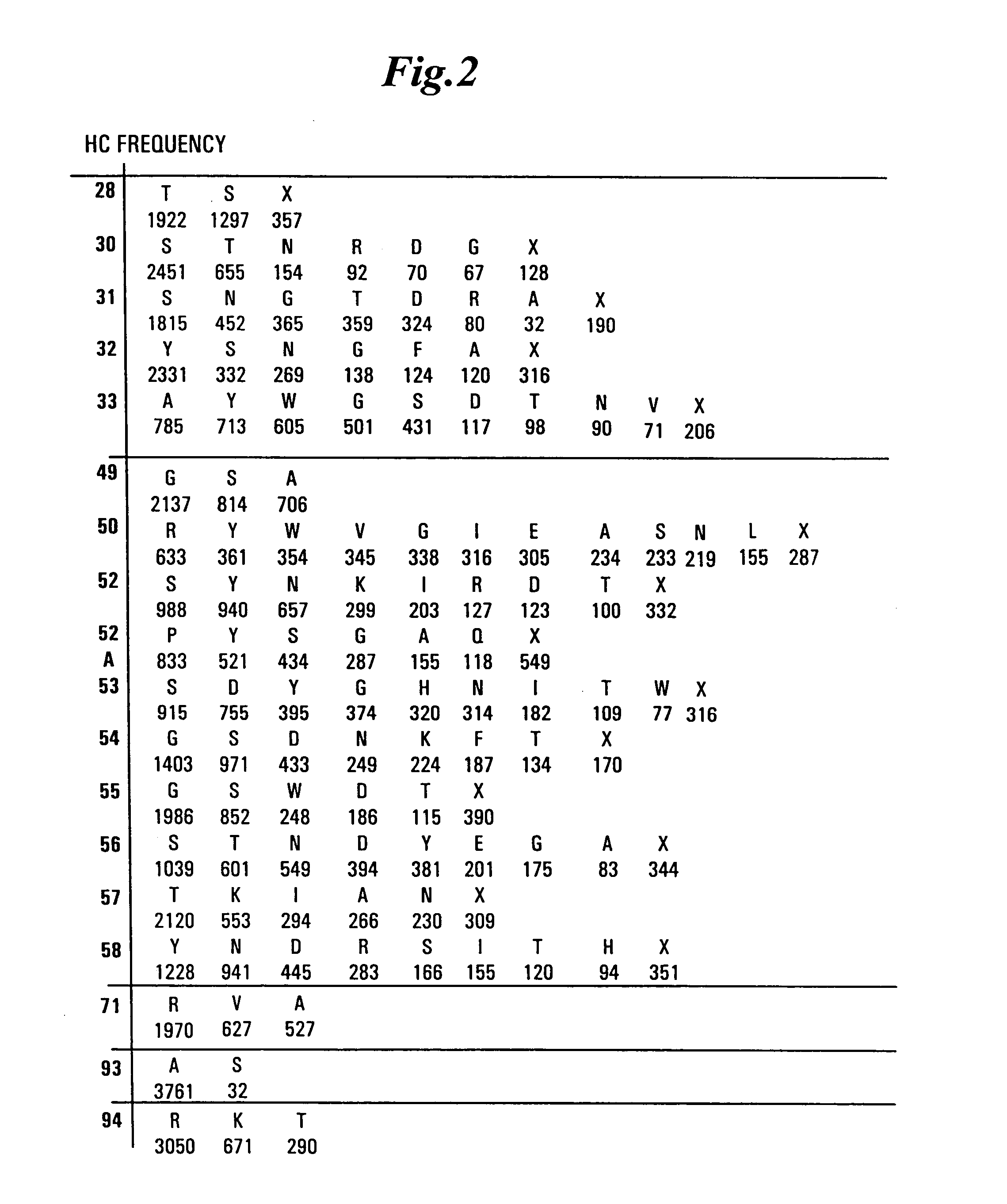
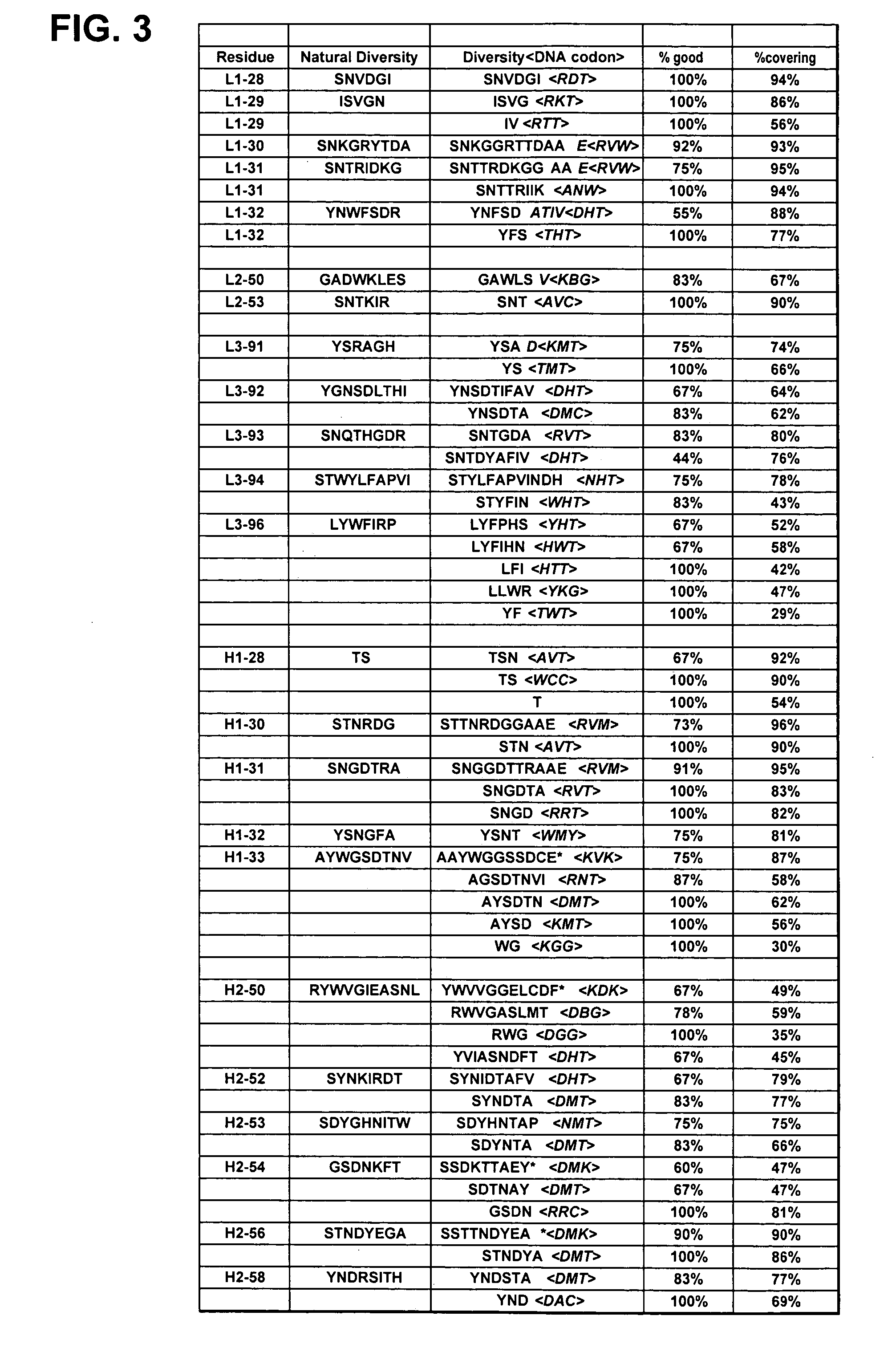
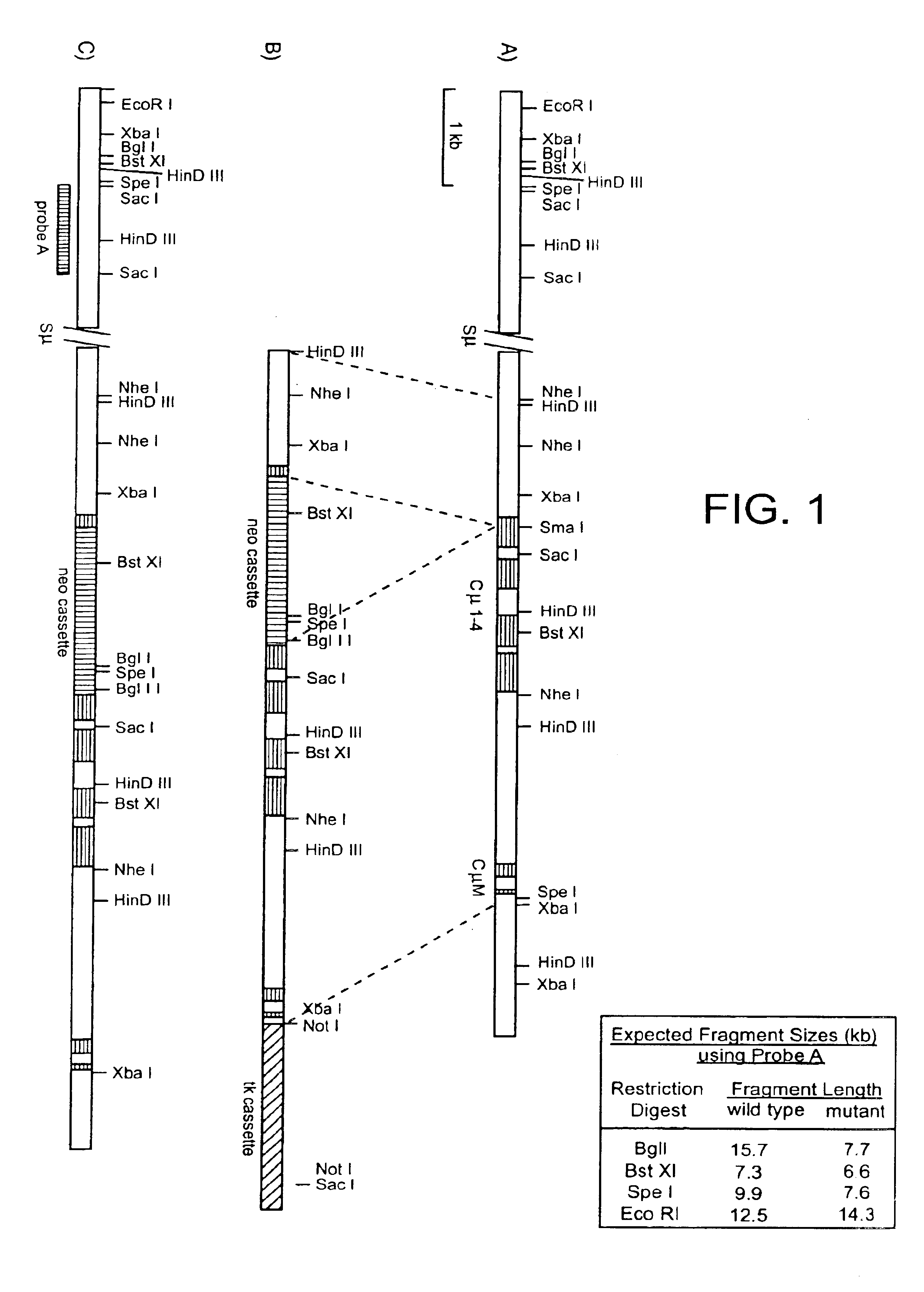
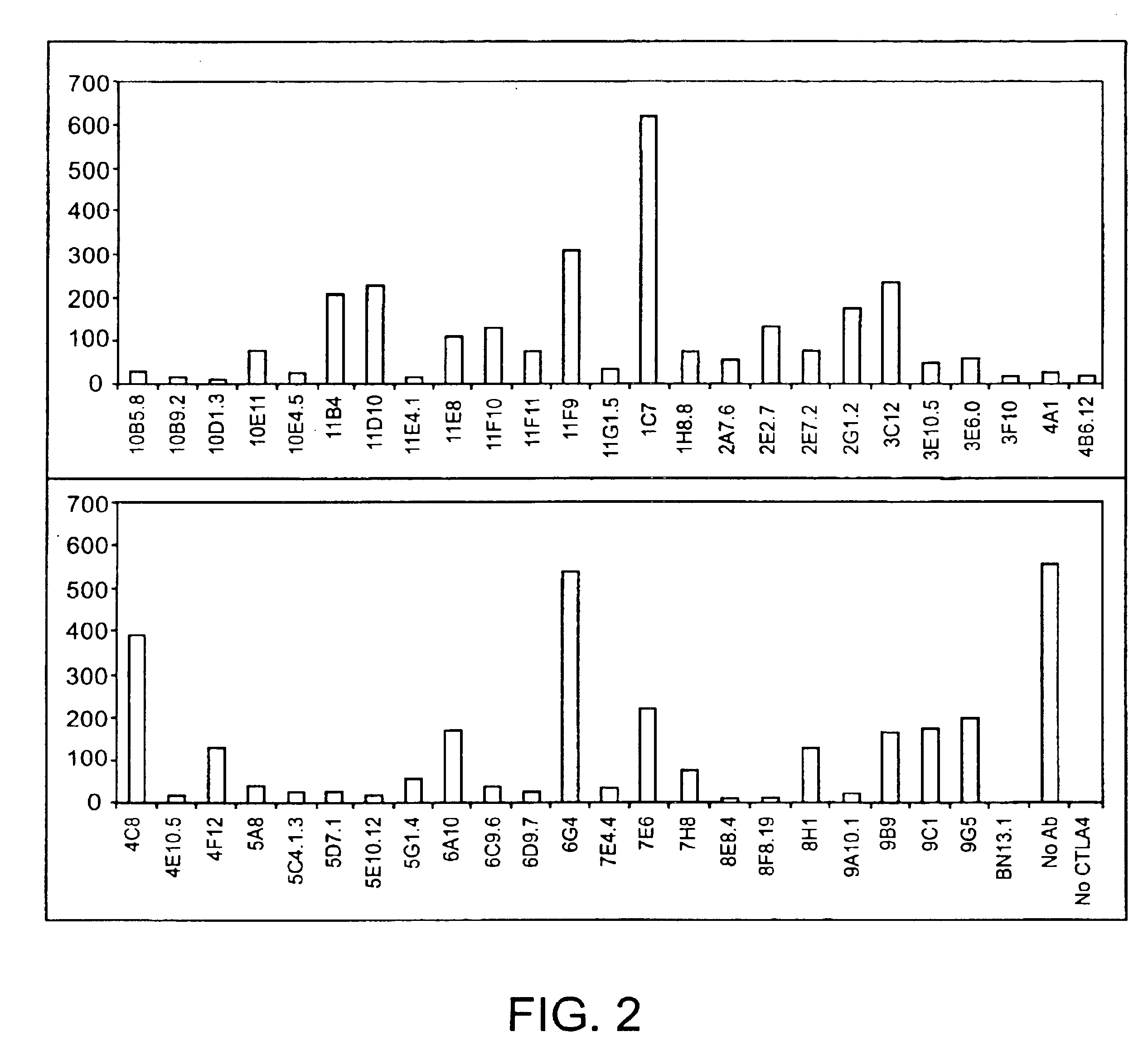
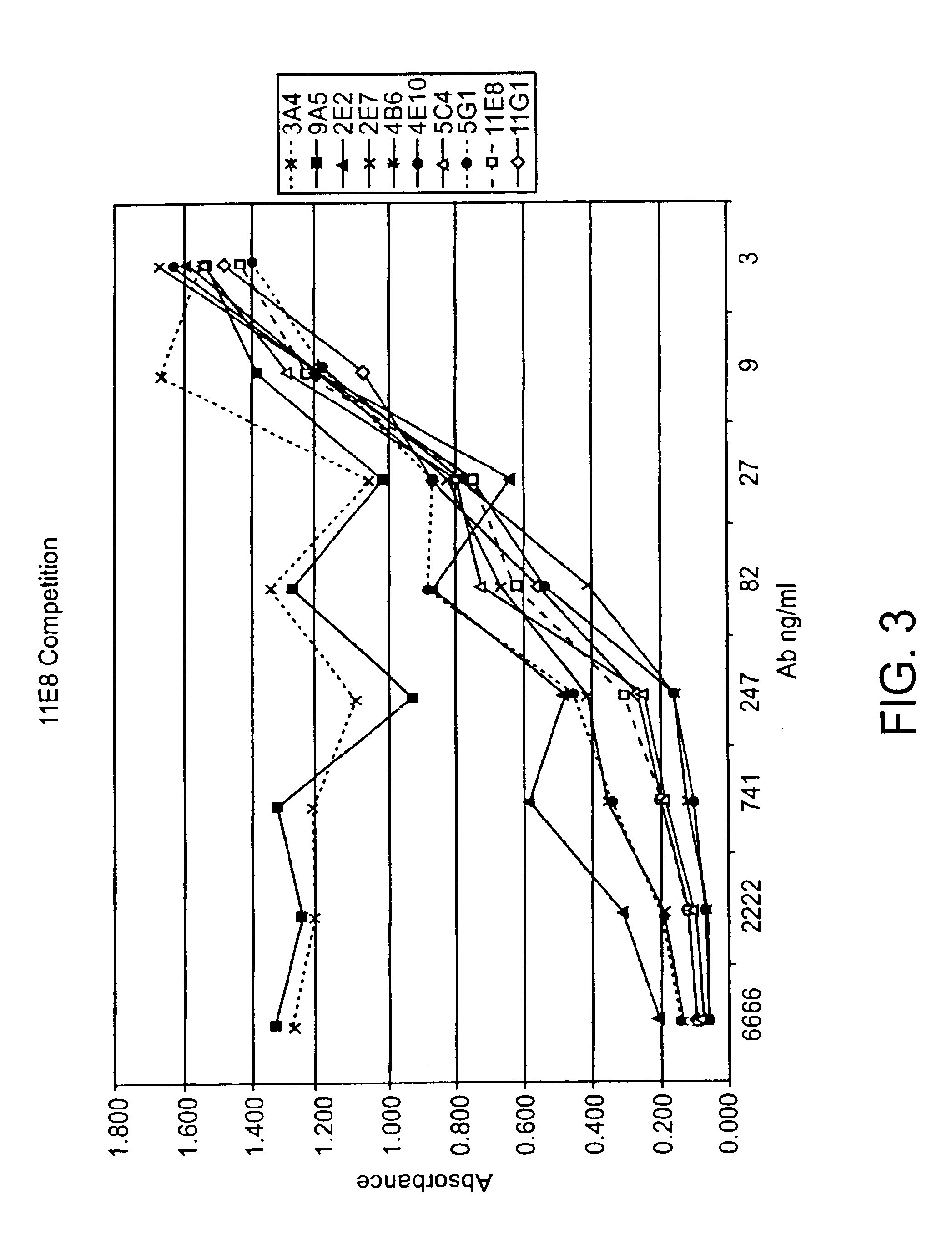
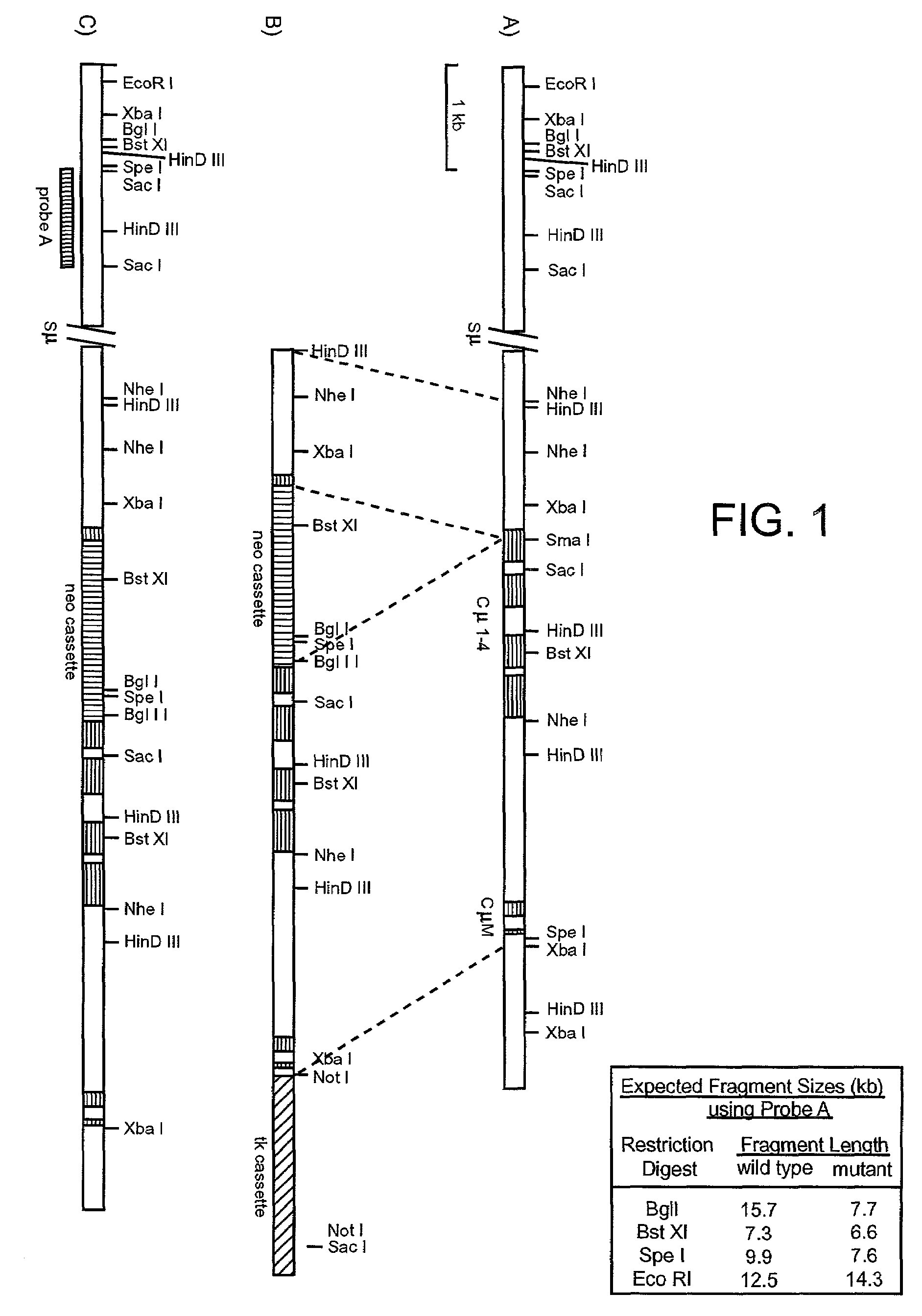
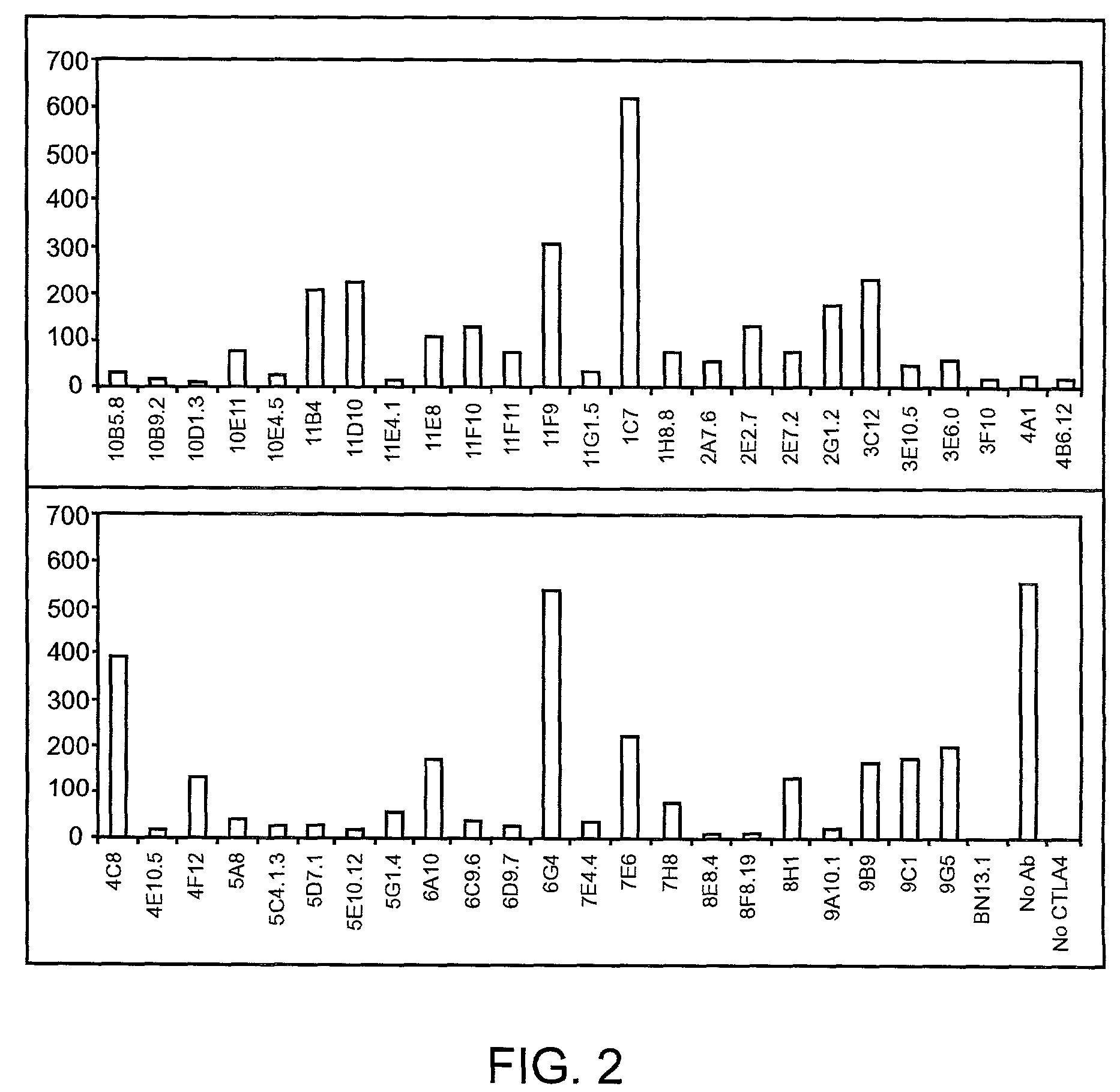
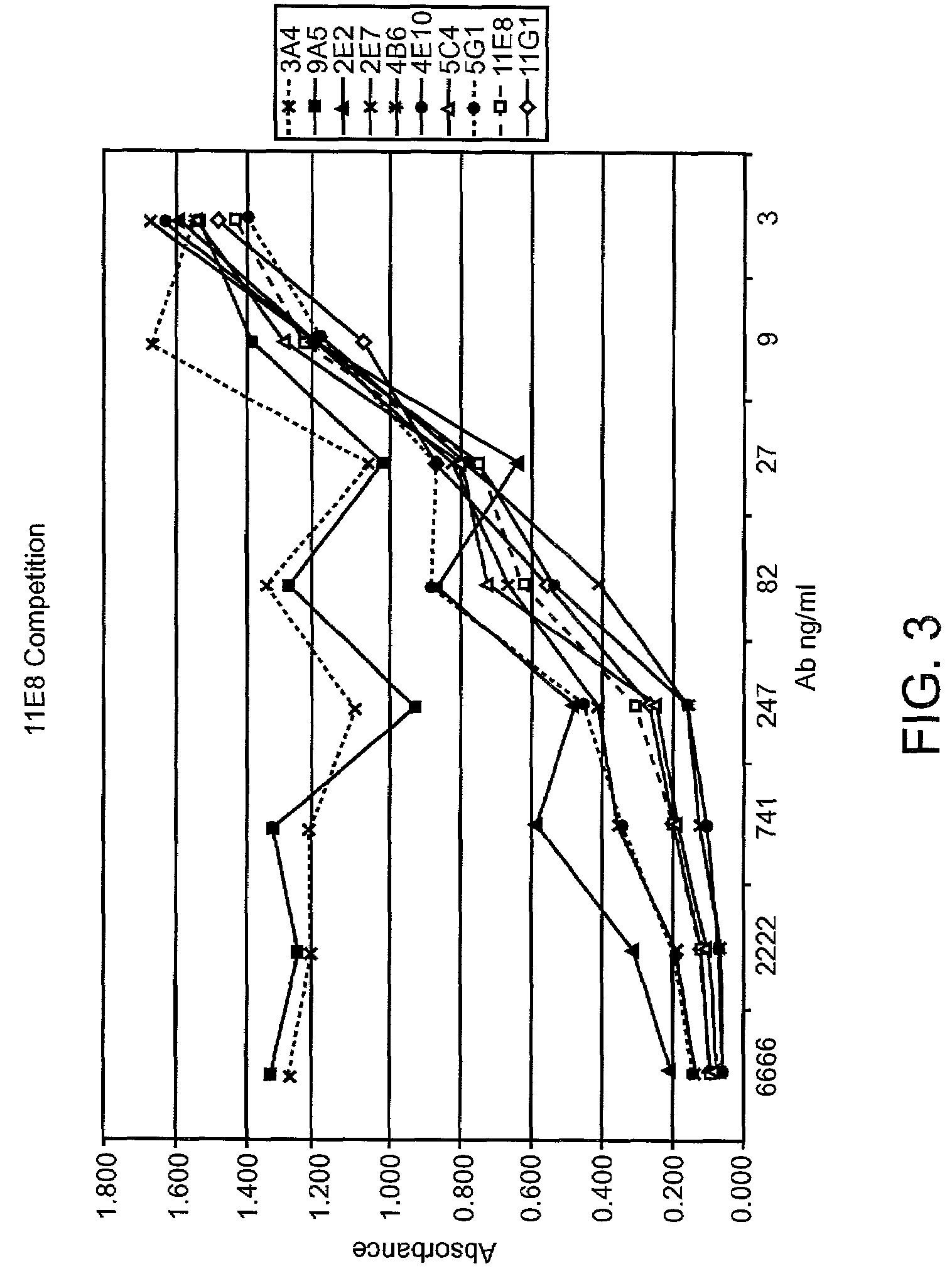
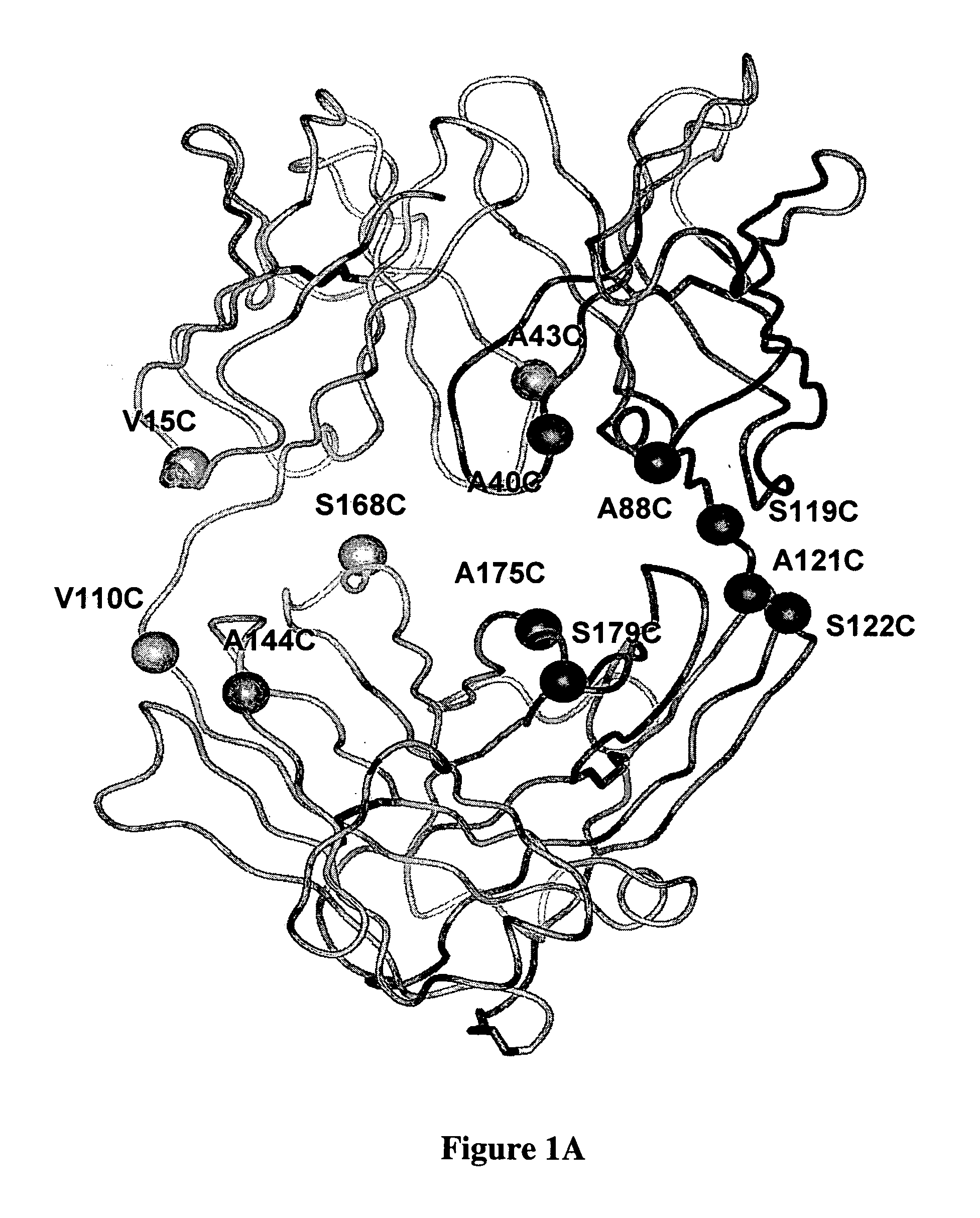
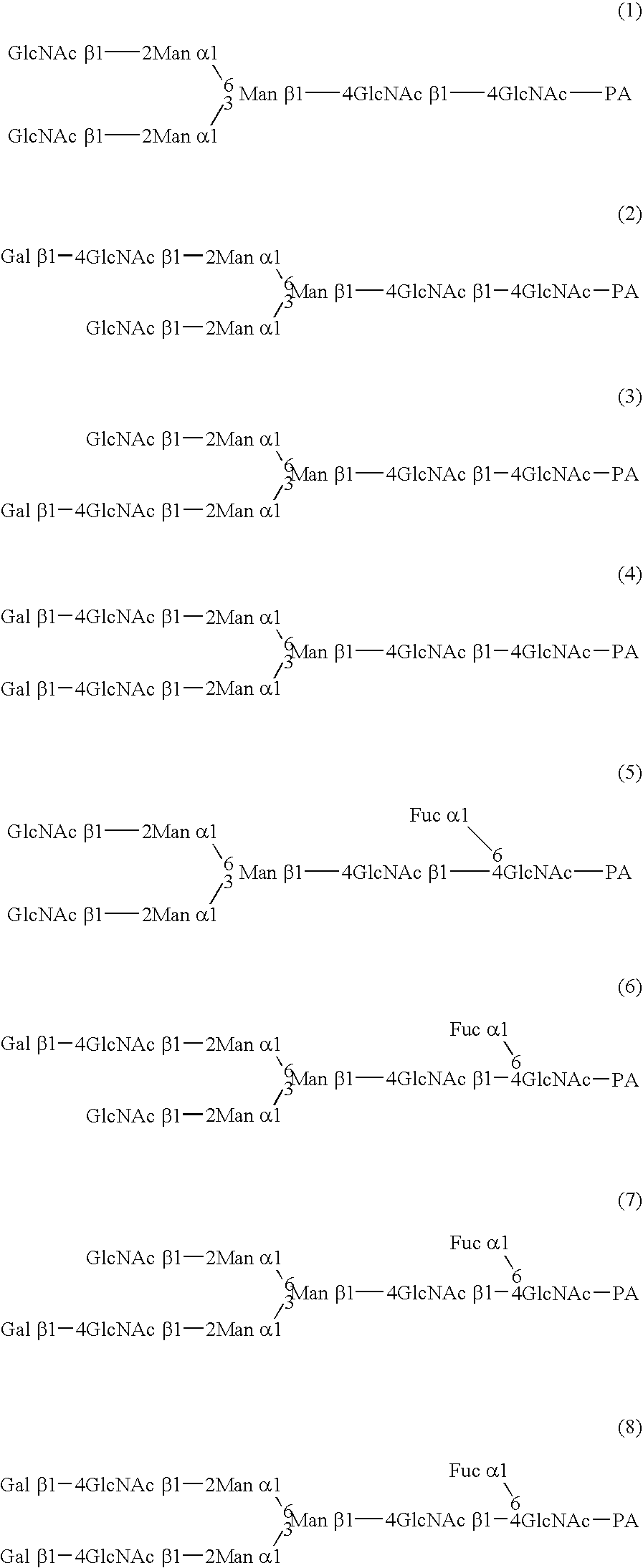Patents
Literature
17032results about "Immunoglobulins against cell receptors/antigens/surface-determinants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human antibodies derived from immunized xenomice
Fully human antibodies against a specific antigen can be prepared by administering the antigen to a transgenic animal which has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled. Various subsequent manipulations can be performed to obtain either antibodies per se or analogs thereof.
Owner:AMGEN FREMONT INC
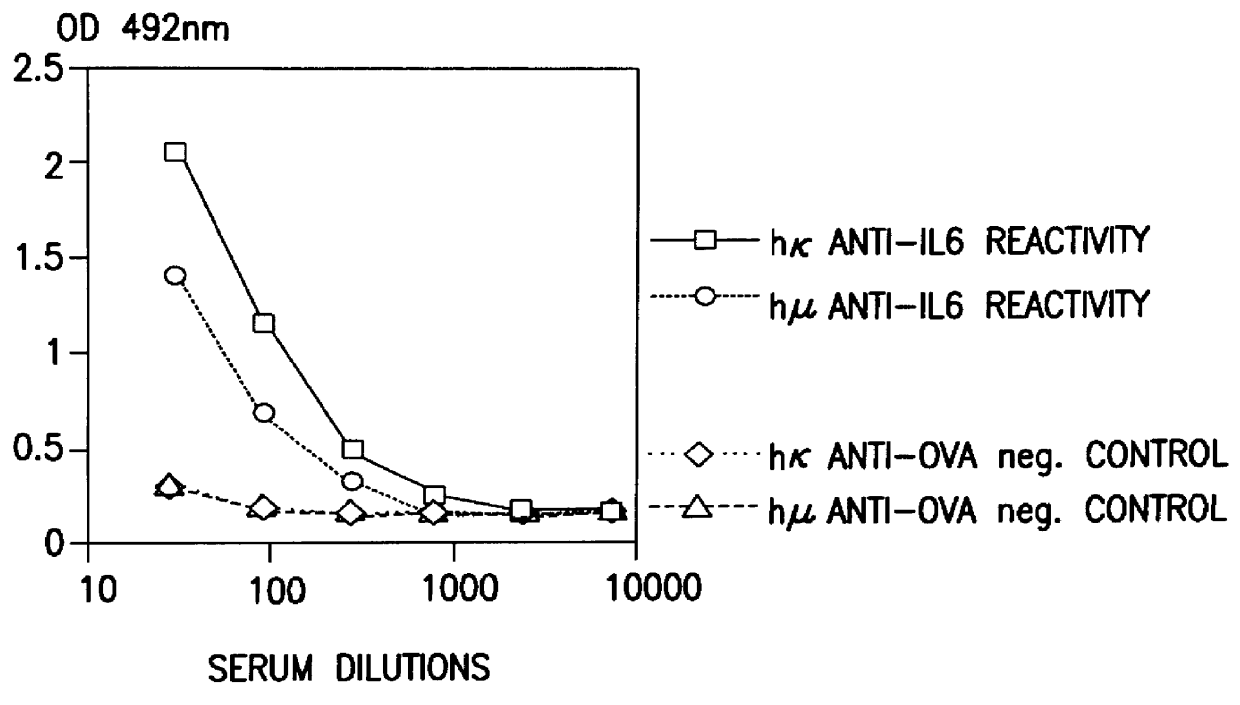
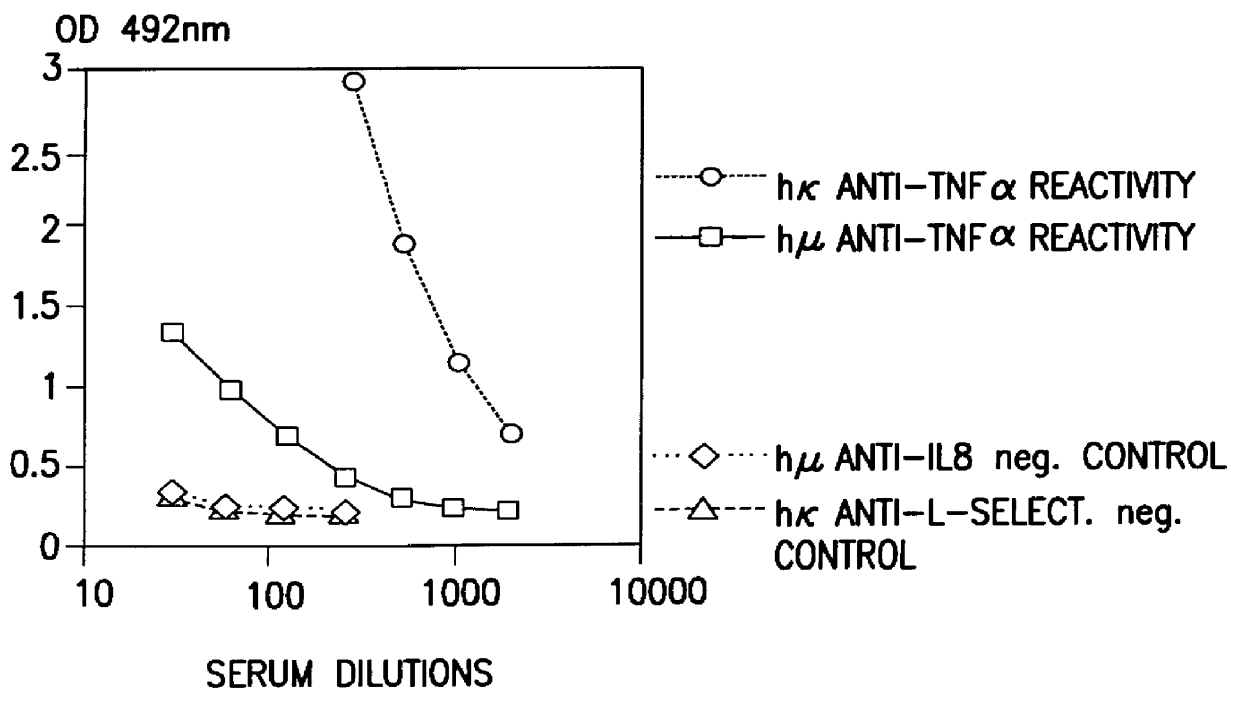
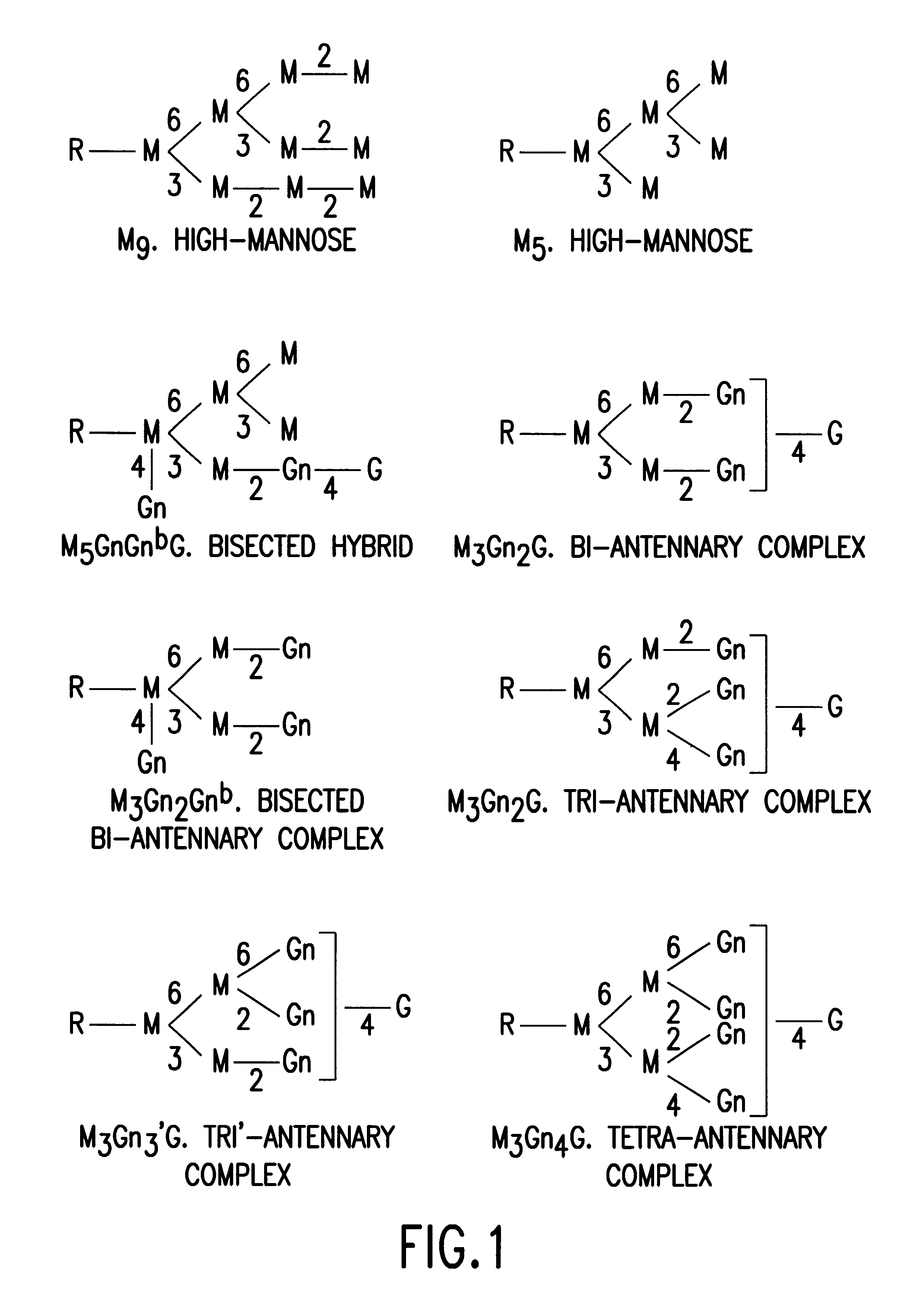
Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity
InactiveUS6602684B1Increase healing valueEnhanced Fc-mediated cellular cytotoxicityNanotechFungiAntibody fragmentsADAMTS Proteins
The present invention relates to the field glycosylation engineering of proteins. More particular, the present invention is directed to the glycosylation engineering of proteins to provide proteins with improved therapeutic properties, e.g., antibodies, antibody fragments, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin, with enhanced Fc-mediated cellular cytotoxicity.
Owner:ROCHE GLYCART AG
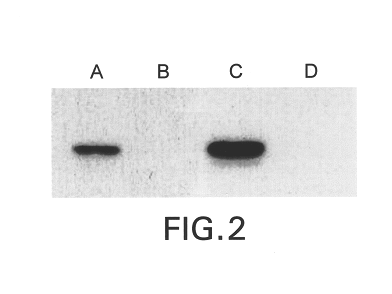
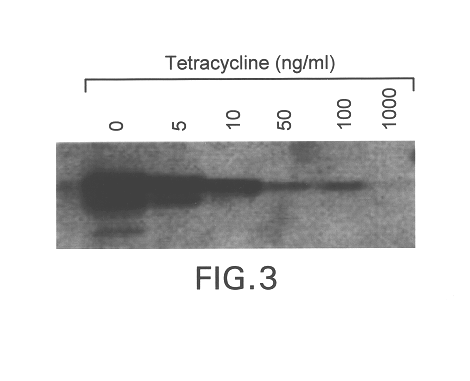
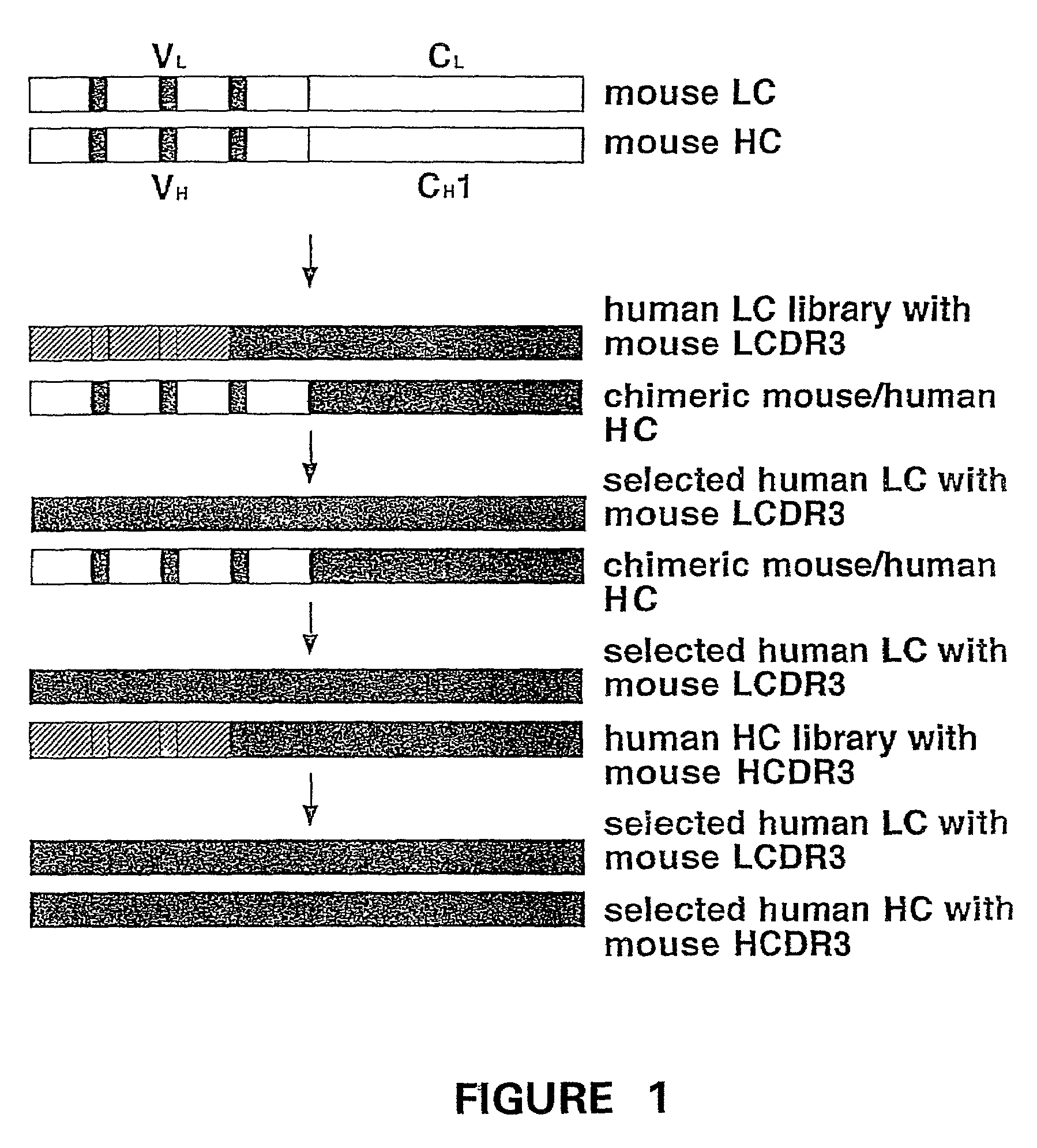
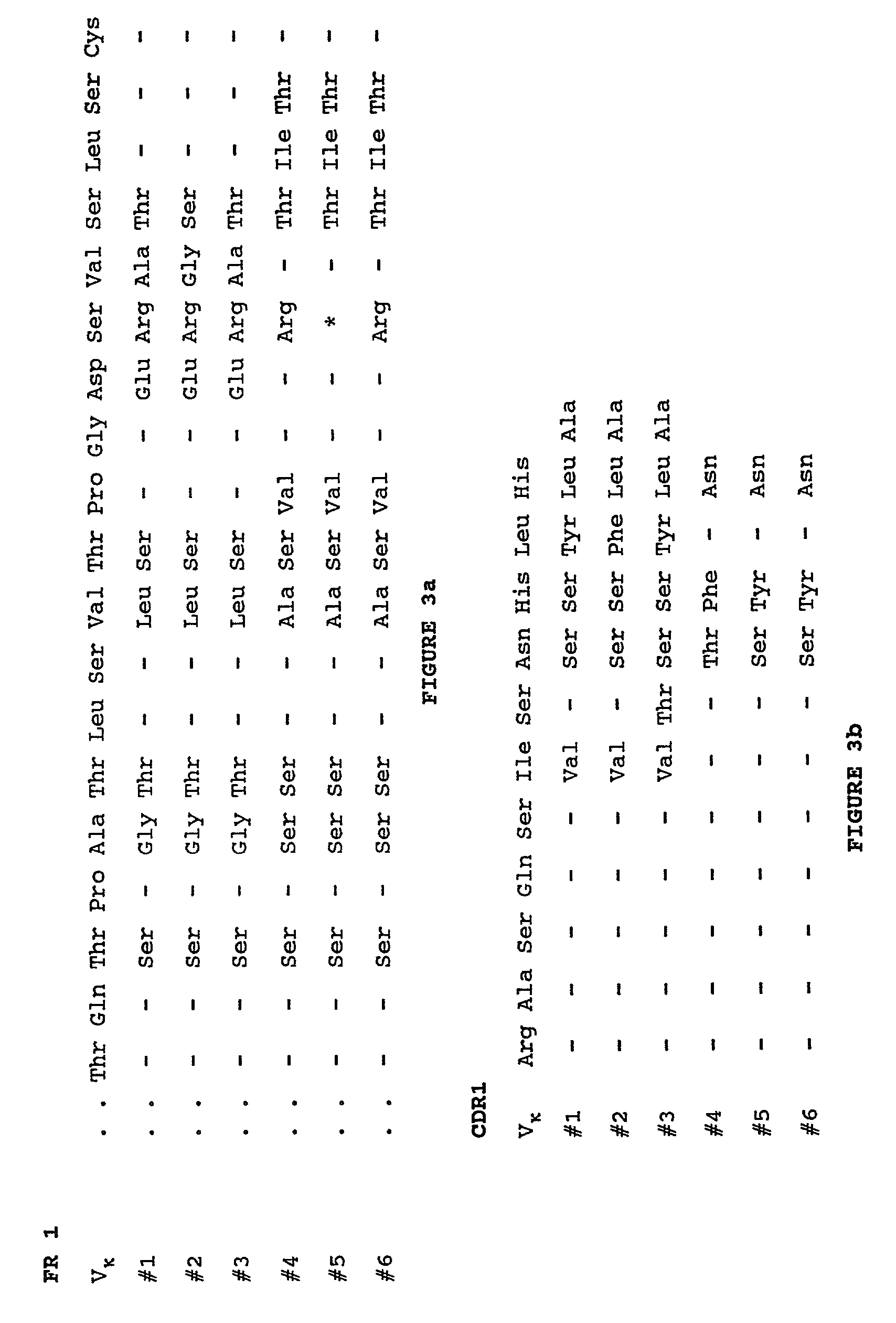
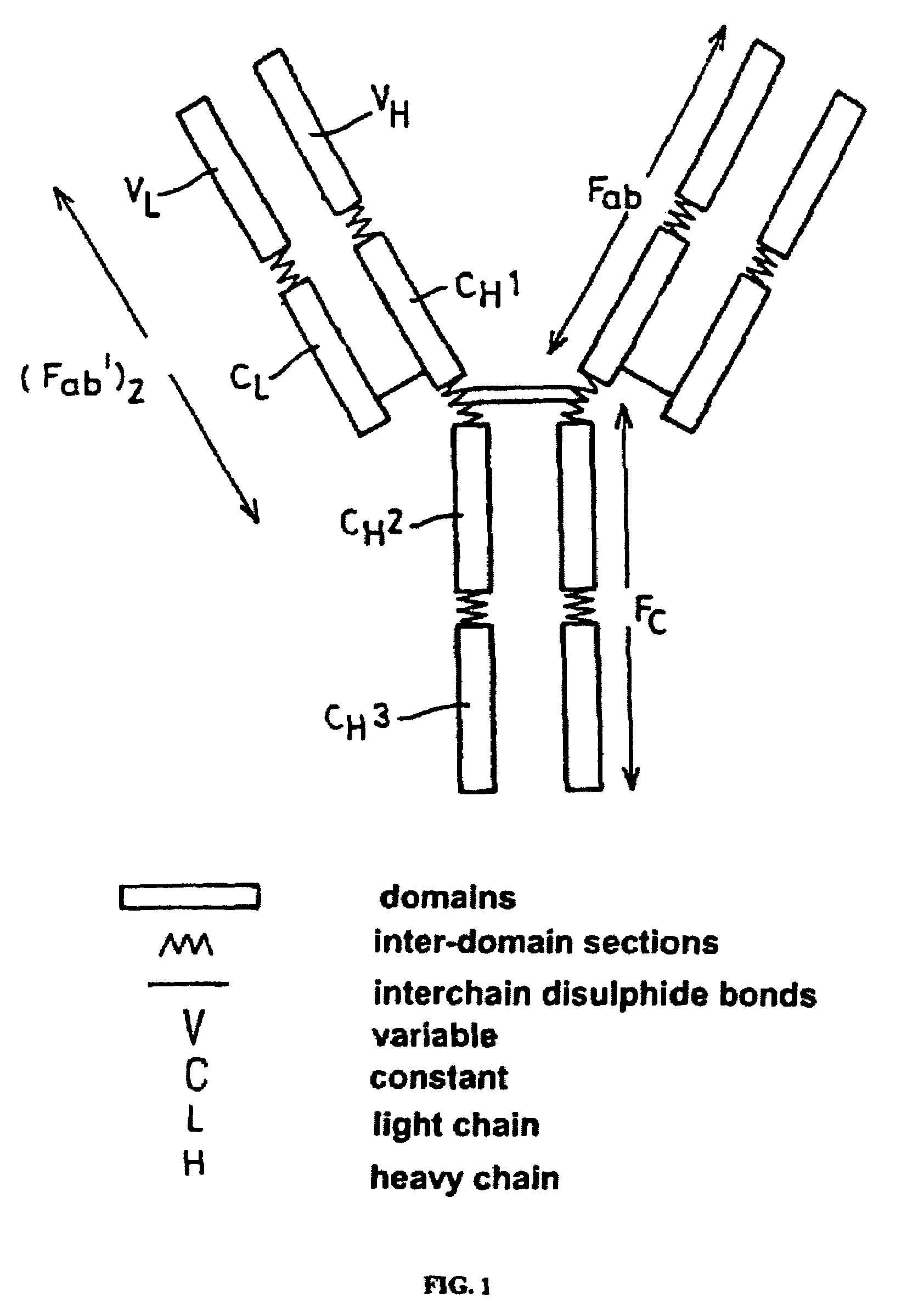
Humanization of murine antibody
InactiveUS7087409B2Hybrid immunoglobulinsSugar derivativesComplementarity determining regionHeavy chain
A humanized murine antibody is provided. The amino acid sequences of a light chain complementarity determining region from a mouse antibody are grafted onto a human light chain, and a heavy chain complementarity determining region from a mouse antibody are grafted onto a human antibody heavy chain to produce libraries from which a humanized murine antibody having the desired specificity is selected.
Owner:THE SCRIPPS RES INST
Cells of which genome is modified
InactiveUS20040110704A1Raise the ratioDecreased and deleted activityAntibacterial agentsAntipyreticGlycosideN-Acetylglucosamine
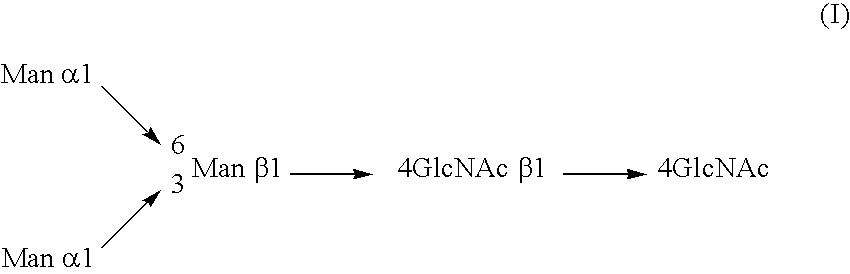
A cell in which genome is modified so as to have a more decreased or deleted activity of an enzyme relating to modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain than its parent cell, and a process for producing an antibody composition using the cell.
Owner:KYOWA HAKKO KOGYO CO LTD
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050014934A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
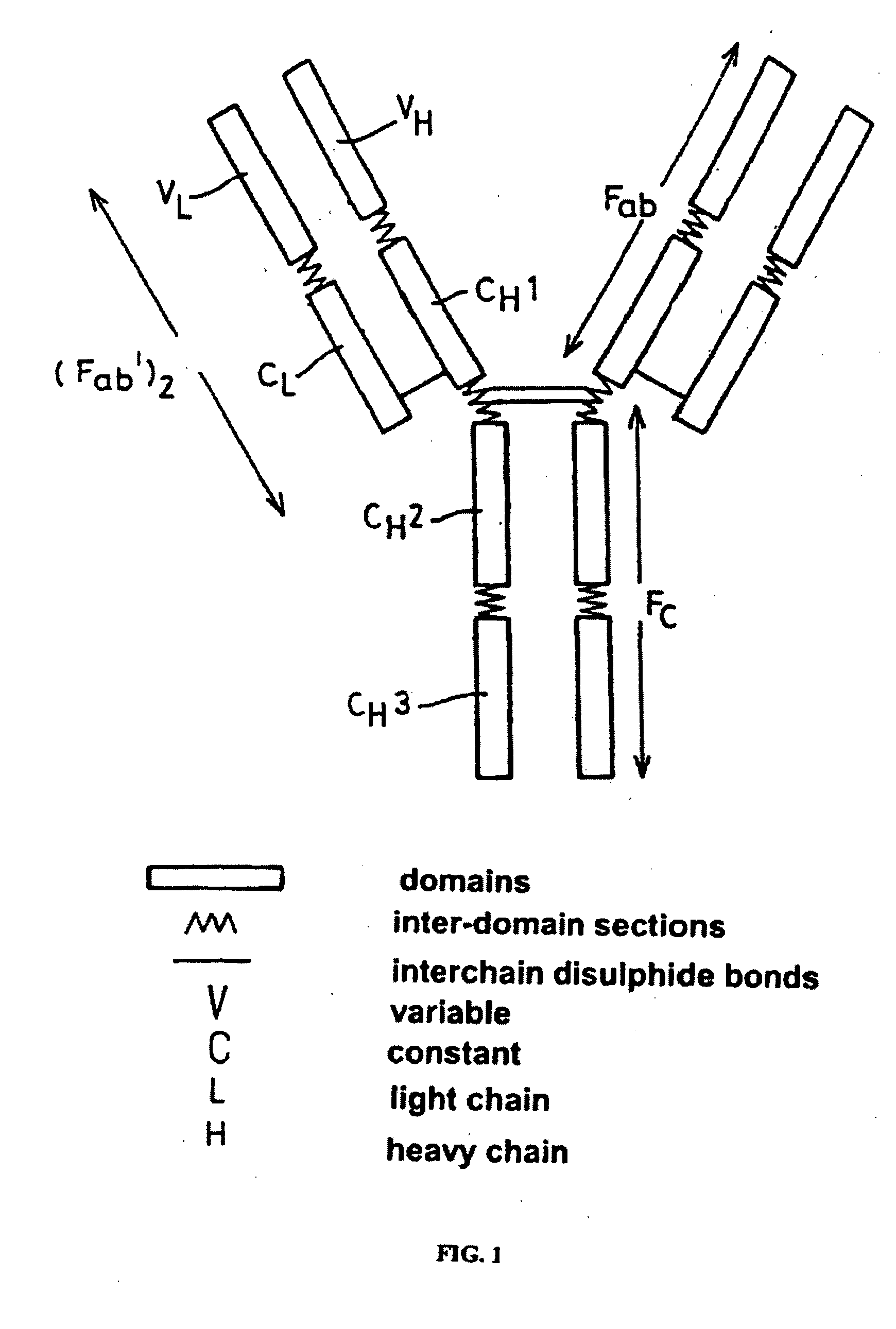
Polypeptide variants with altered effector function
The present invention concerns polypeptides comprising a variant Fc region. More particularly, the present invention concerns Fc region-containing polypeptides that have altered effector function as a consequence of one or more amino acid modifications in the Fc region thereof.
Owner:GENENTECH INC
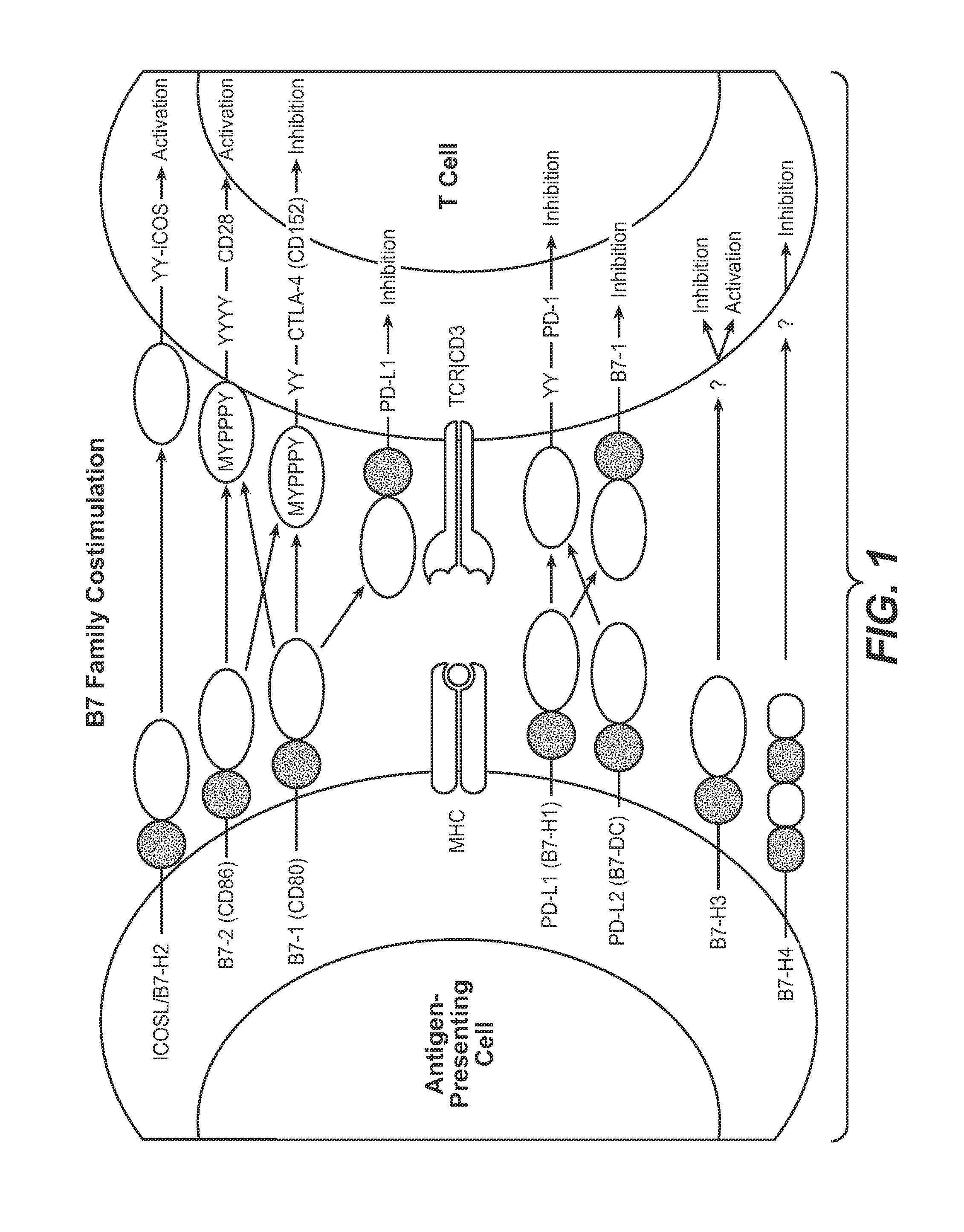
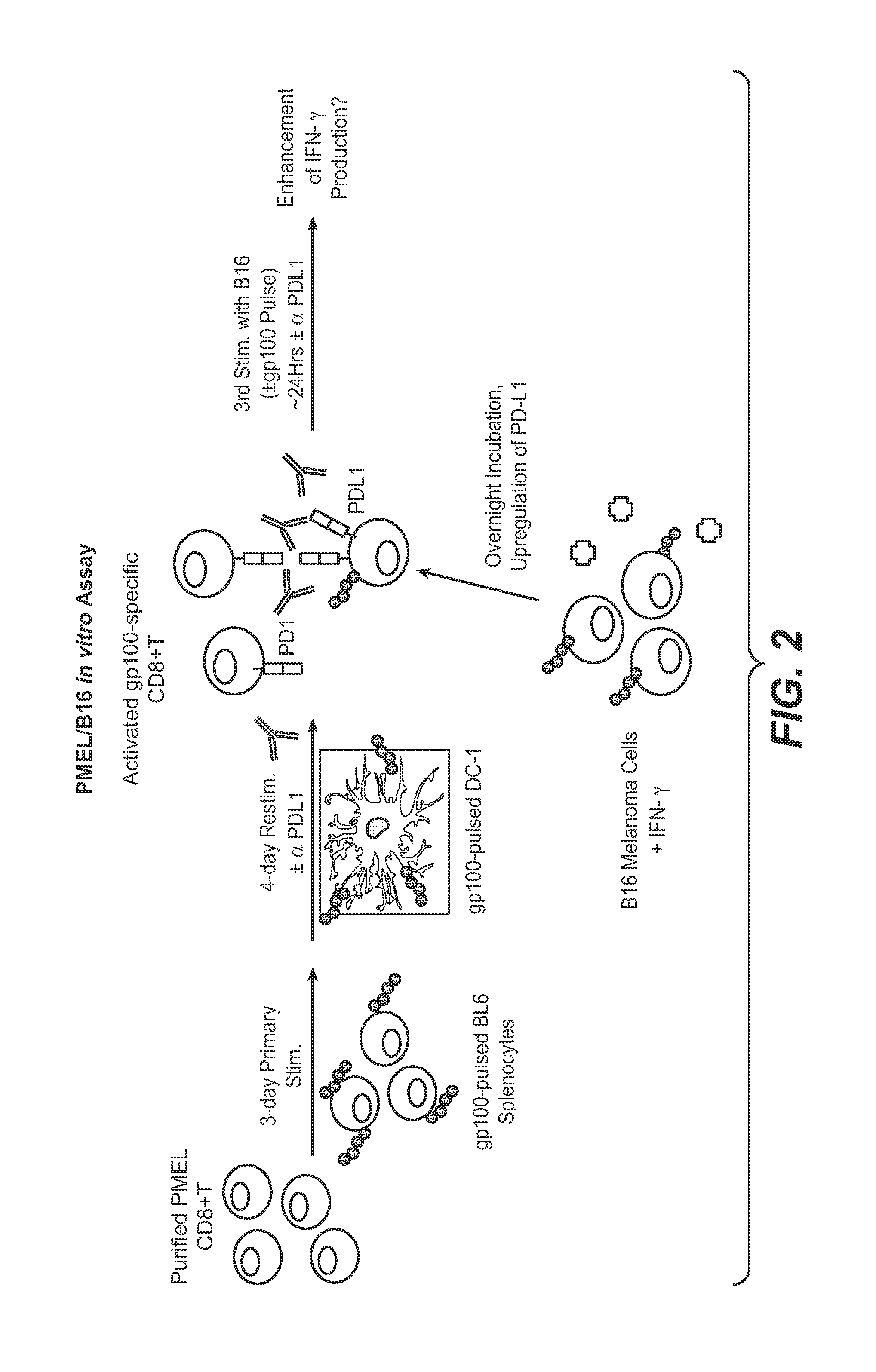
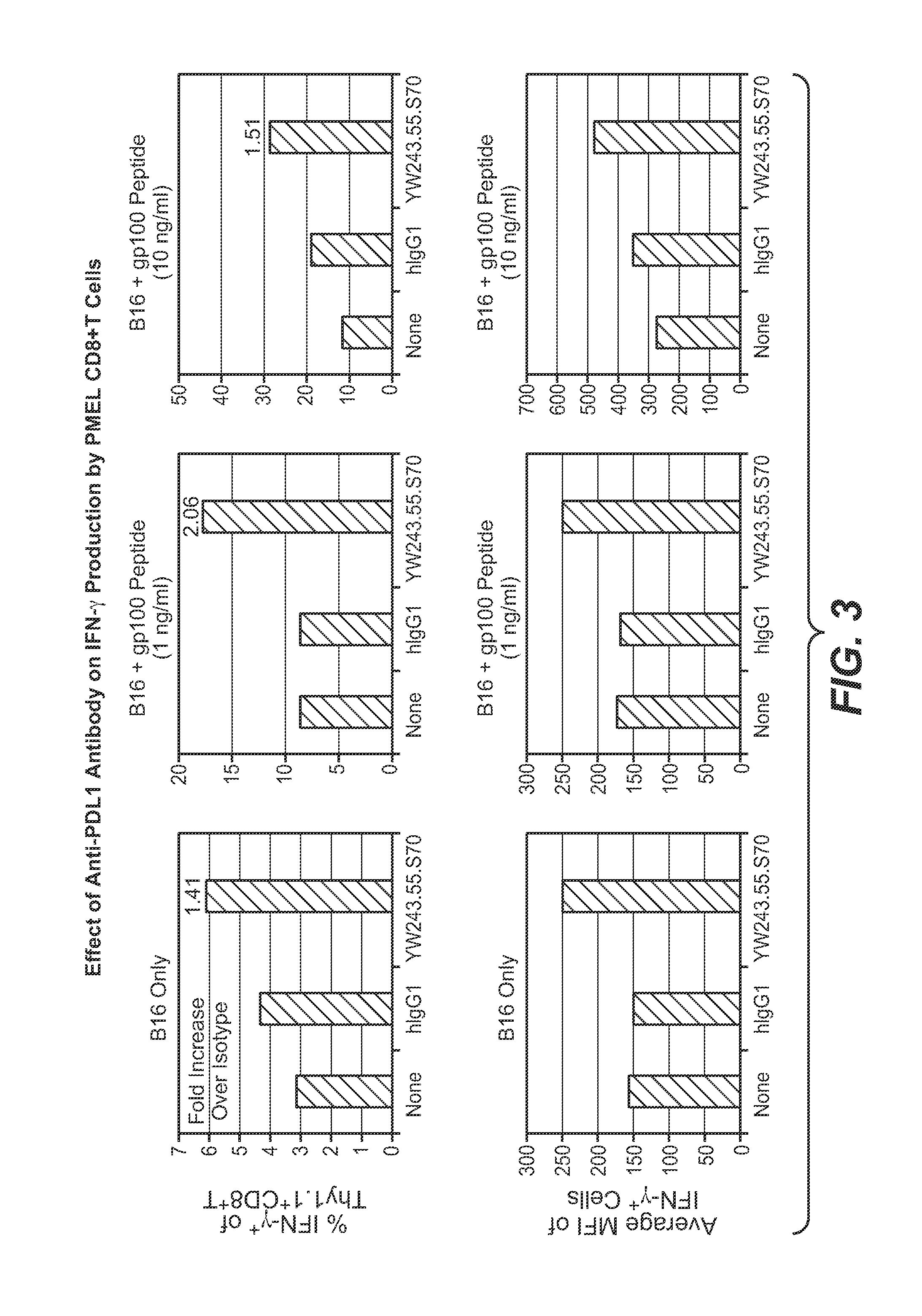
Anti-PD-L1 antibodies, compositions and articles of manufacture
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
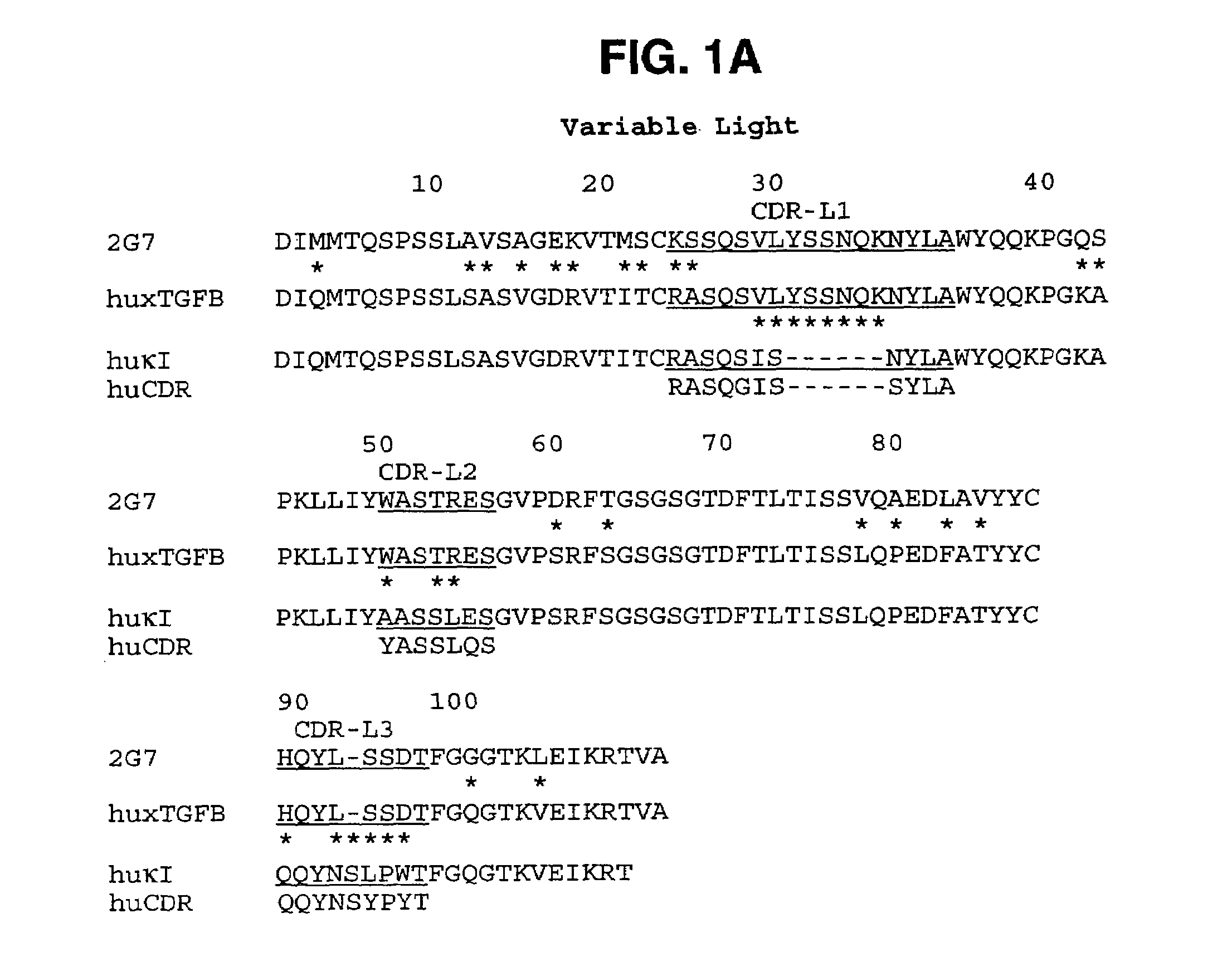
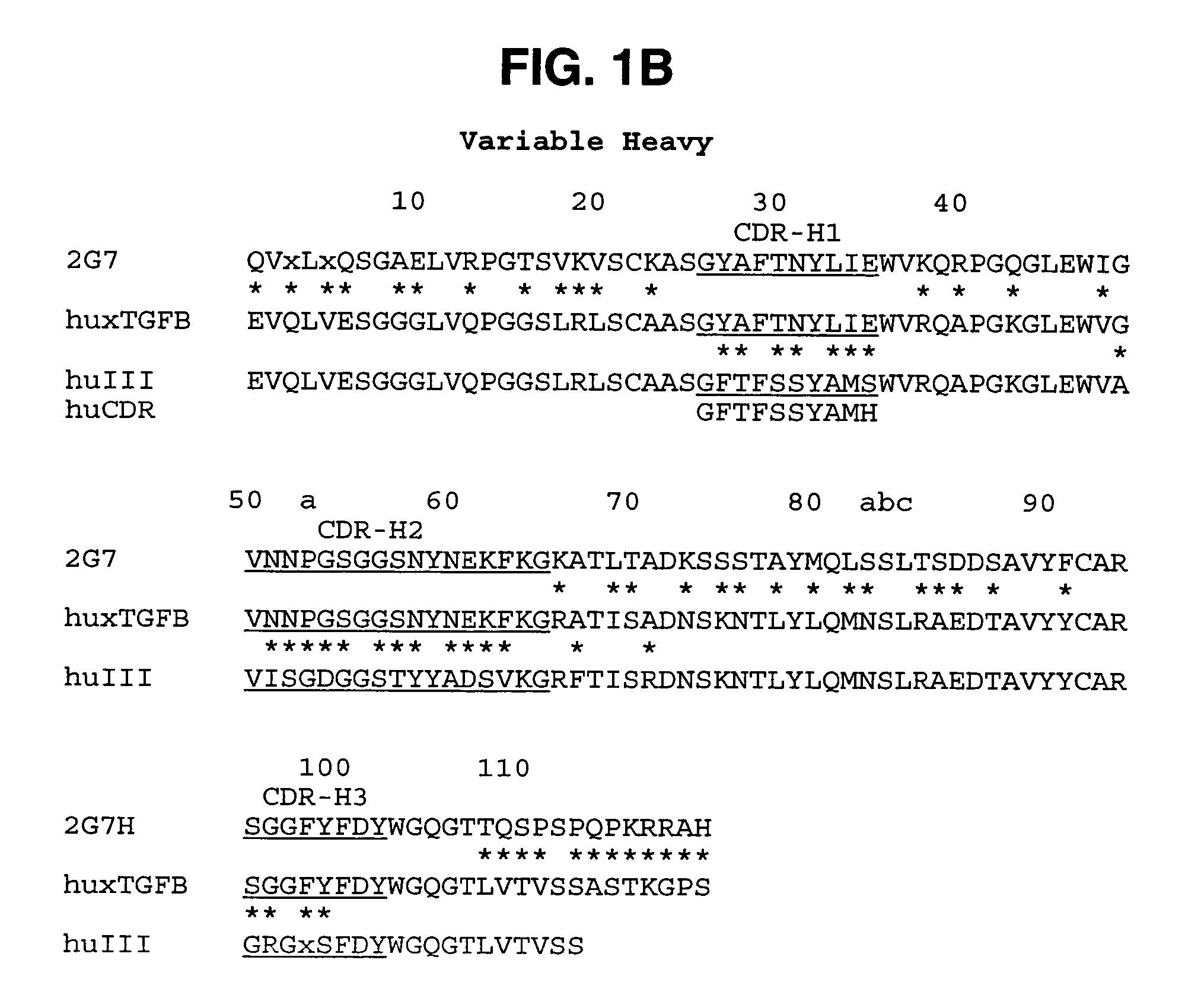
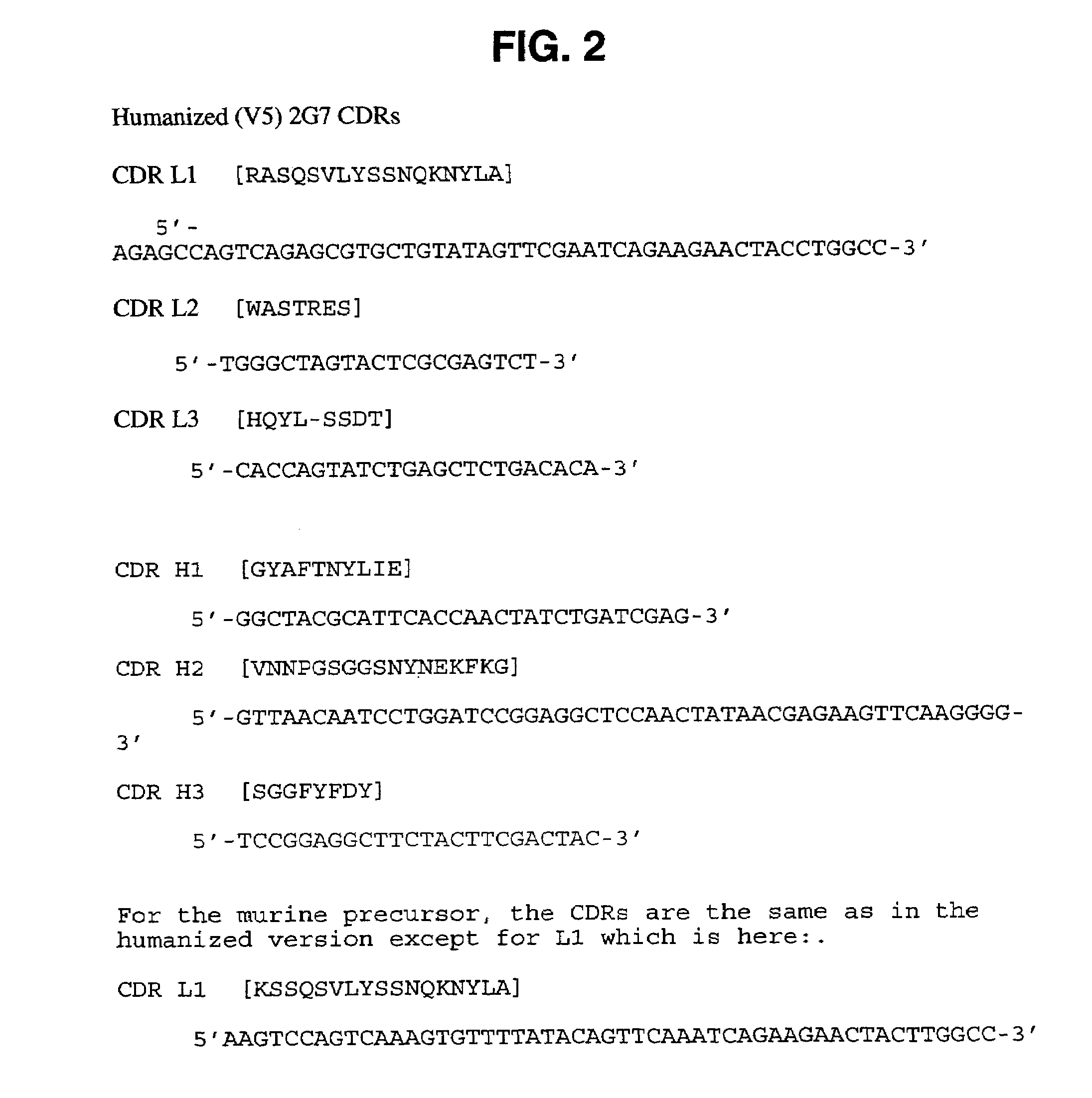
Humanized anti-TGF-beta antibodies
Humanized anti-TGF-beta antibodies are provided, as well as methods for their preparation and use, including methods for treating TGF-beta disorders, for example, cancer. Also provided are articles of manufacture designed for various uses that contain the humanized antibodies.
Owner:GENENTECH INC
Methods of modifying eukaryotic cells
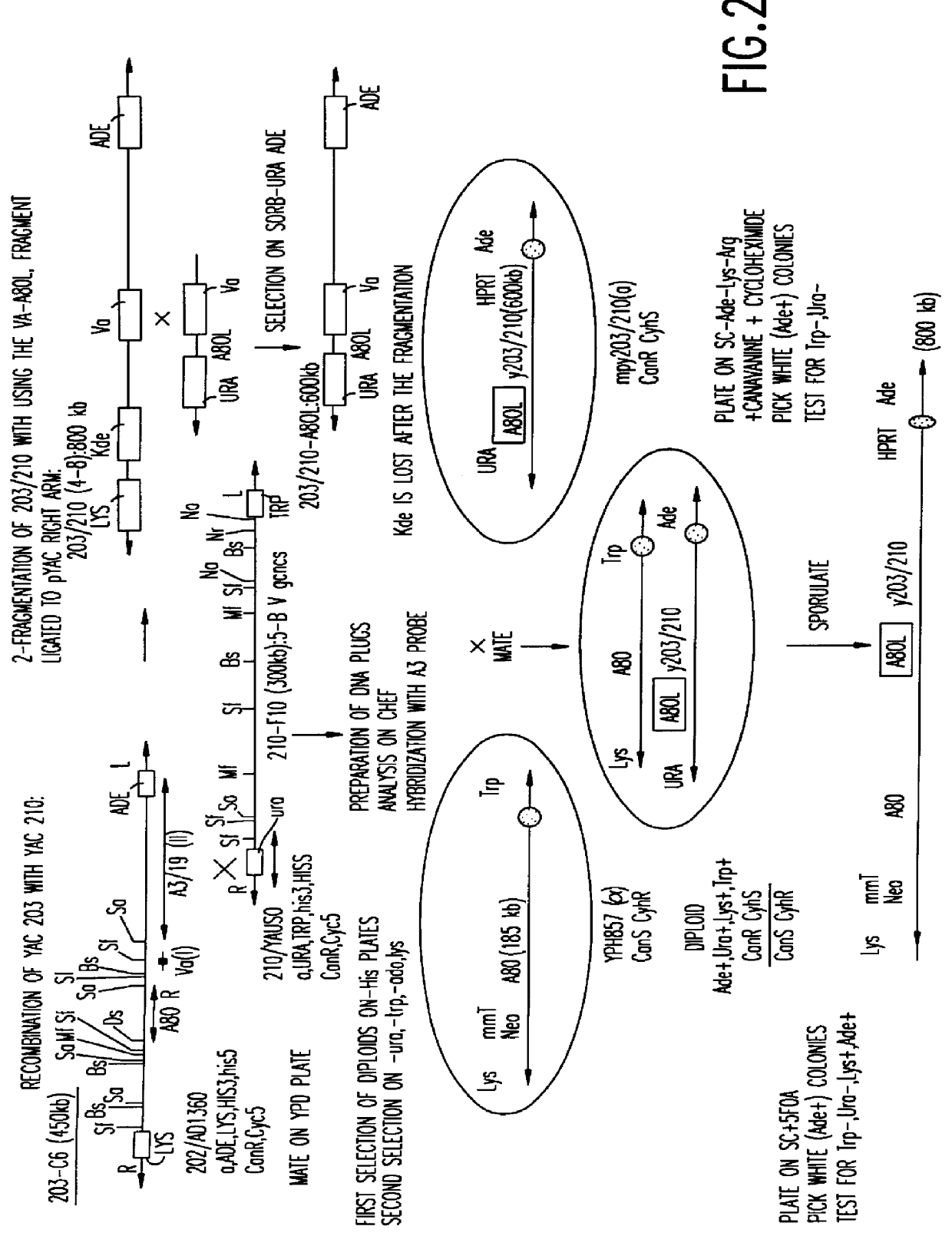
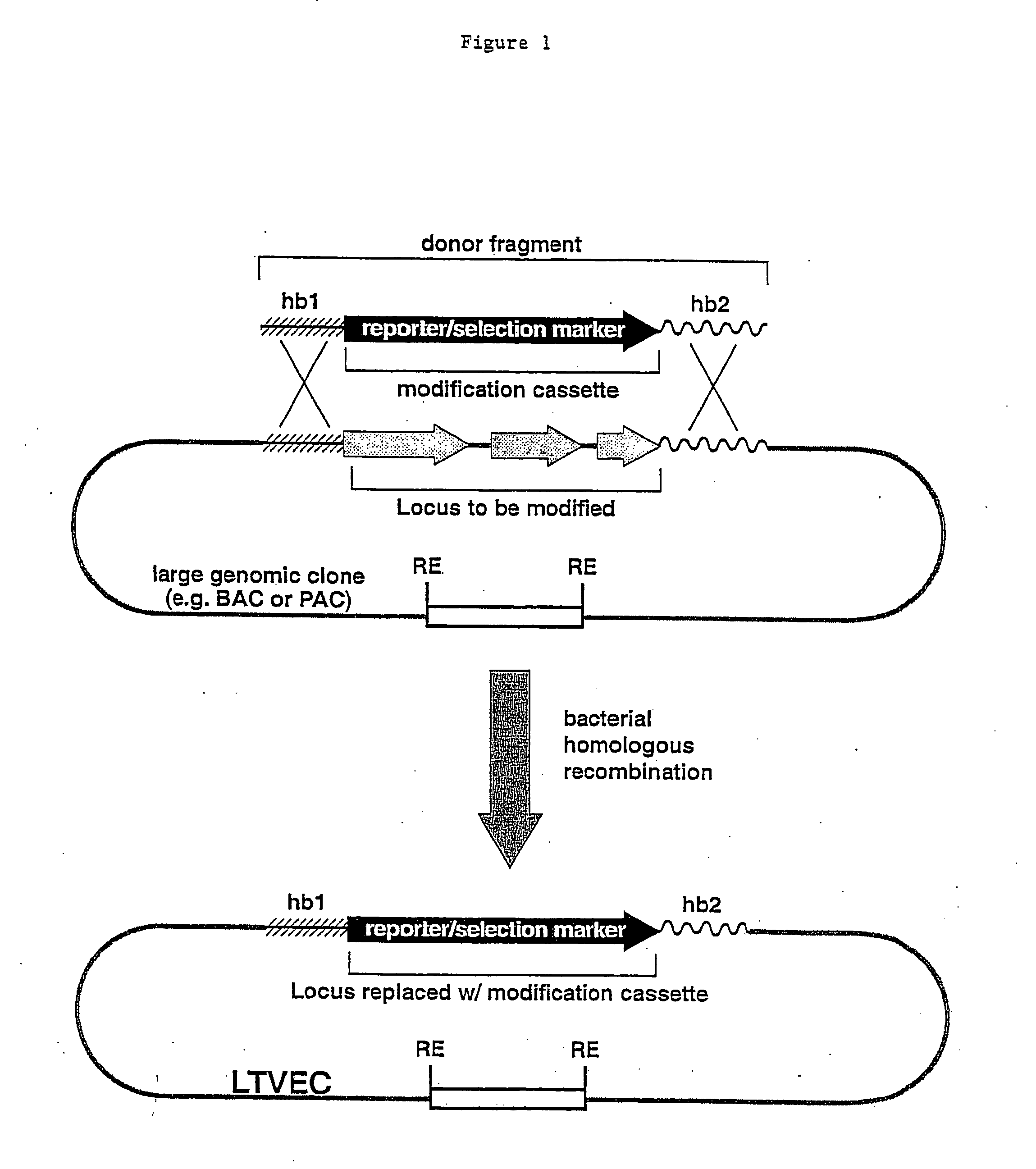
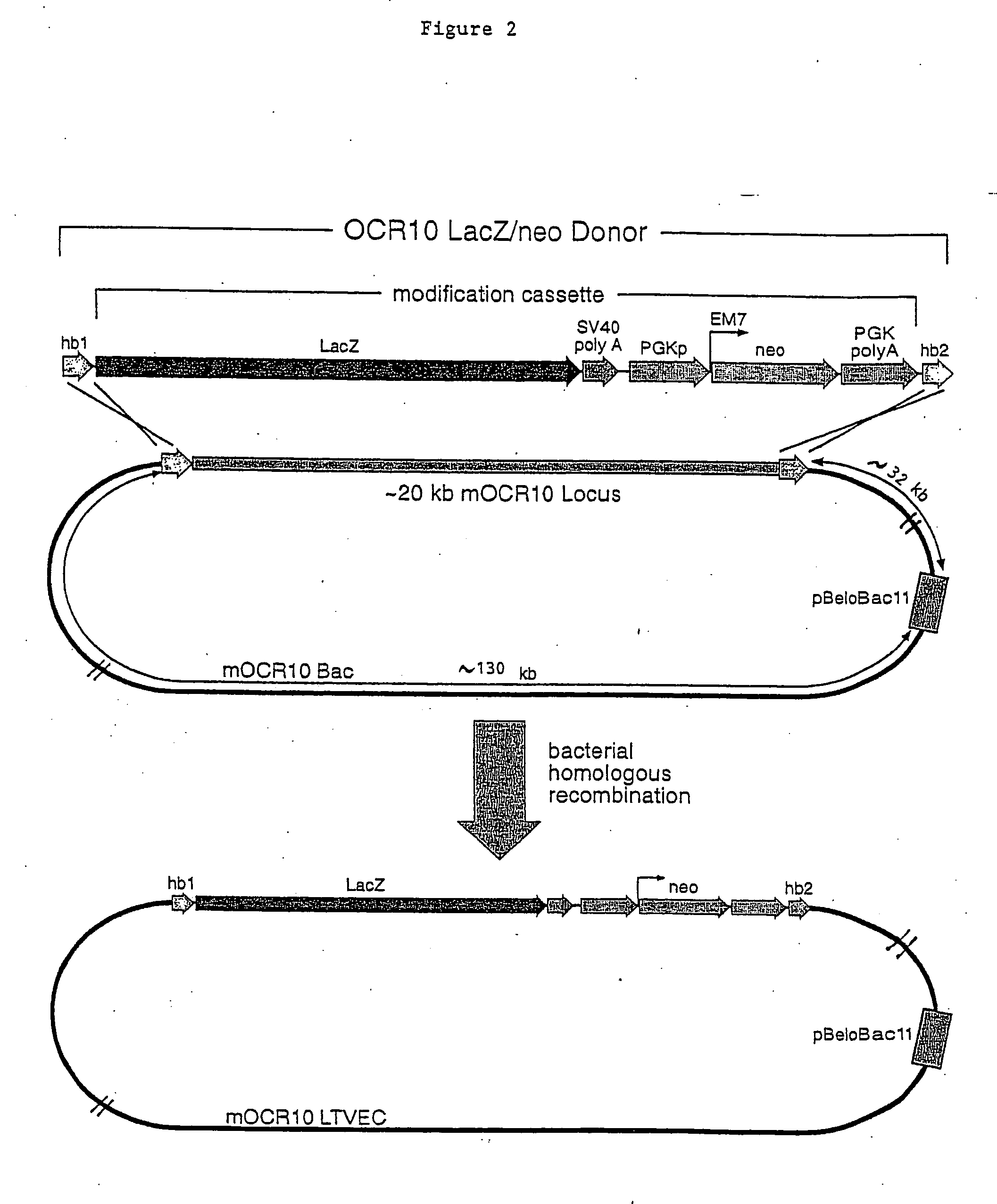
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous gene(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
Multivalent antibodies and uses therefor
The present application describes engineered antibodies, with three or more functional antigen binding sites, and uses, such as therapeutic applications, for such engineered antibodies.
Owner:GENENTECH INC
Synthetic antibody phage libraries
InactiveUS20050079574A1High-quality target binding characteristicGenerate efficientlyAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeterologousIntravenous gammaglobulin
The invention provides immunoglobulin polypeptides comprising variant amino acids in CDRs of antibody variable domains. In one embodiment, the polypeptide is a variable domain of a monobody and has a variant CDRH3 region. These polypeptides provide a source of great sequence diversity that can be used as a source for identifying novel antigen binding polypeptides. The invention also provides these polypeptides as fusion polypeptides to heterologous polypeptides such as at least a portion of phage or viral coat proteins, tags and linkers. Libraries comprising a plurality of these polypeptides are also provided. In addition, methods of and compositions for generating and using these polypeptides and libraries are provided.
Owner:GENENTECH INC
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
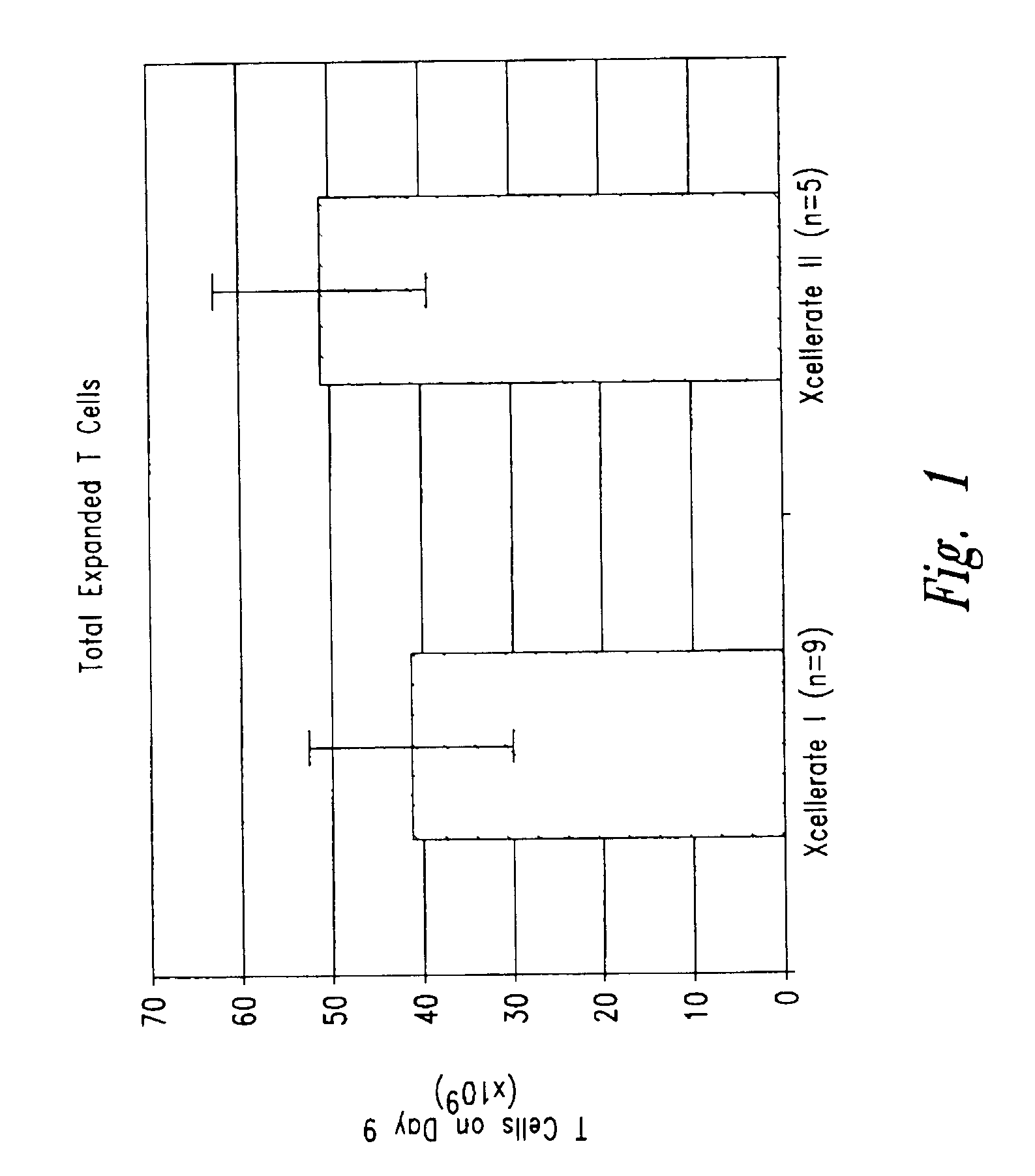
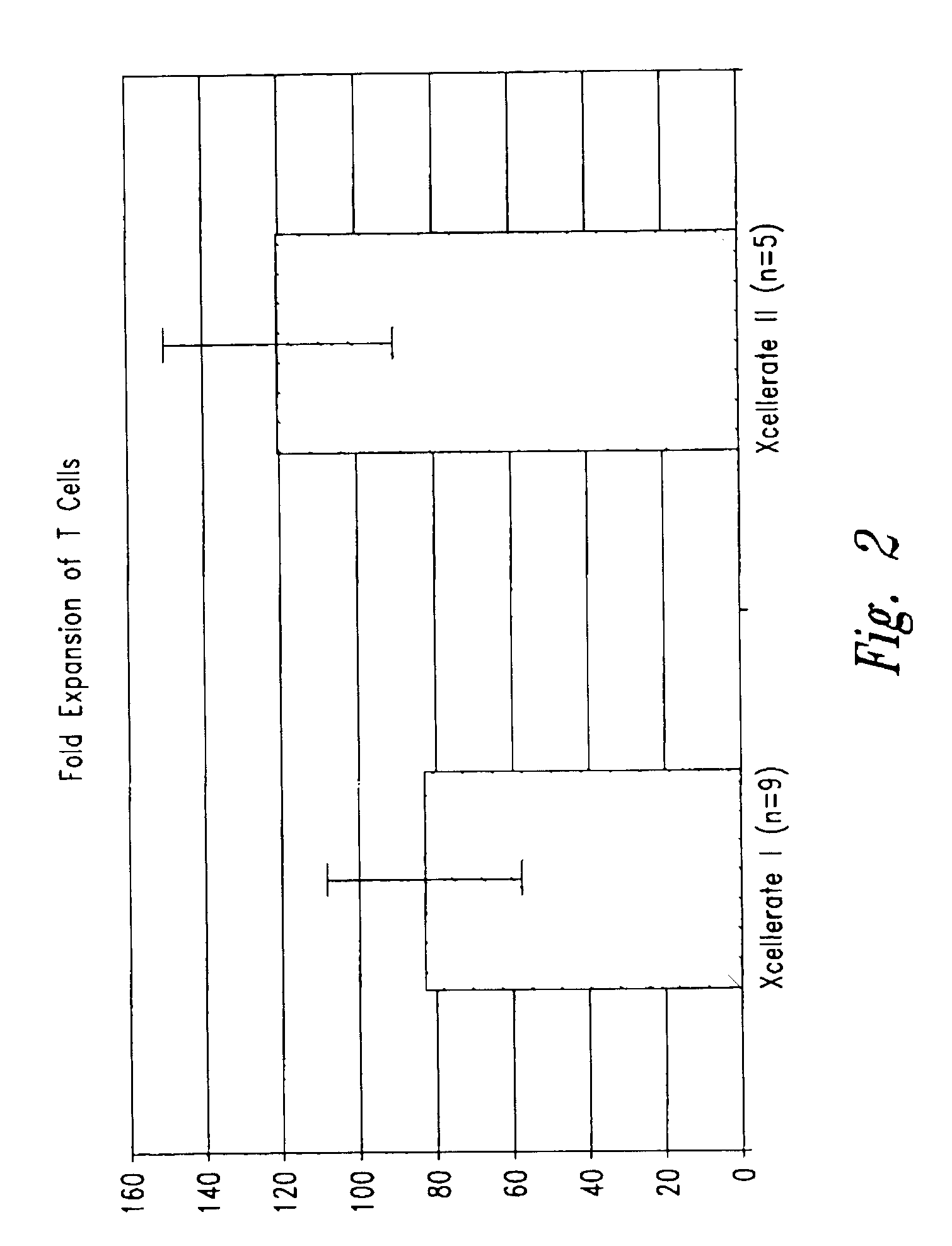
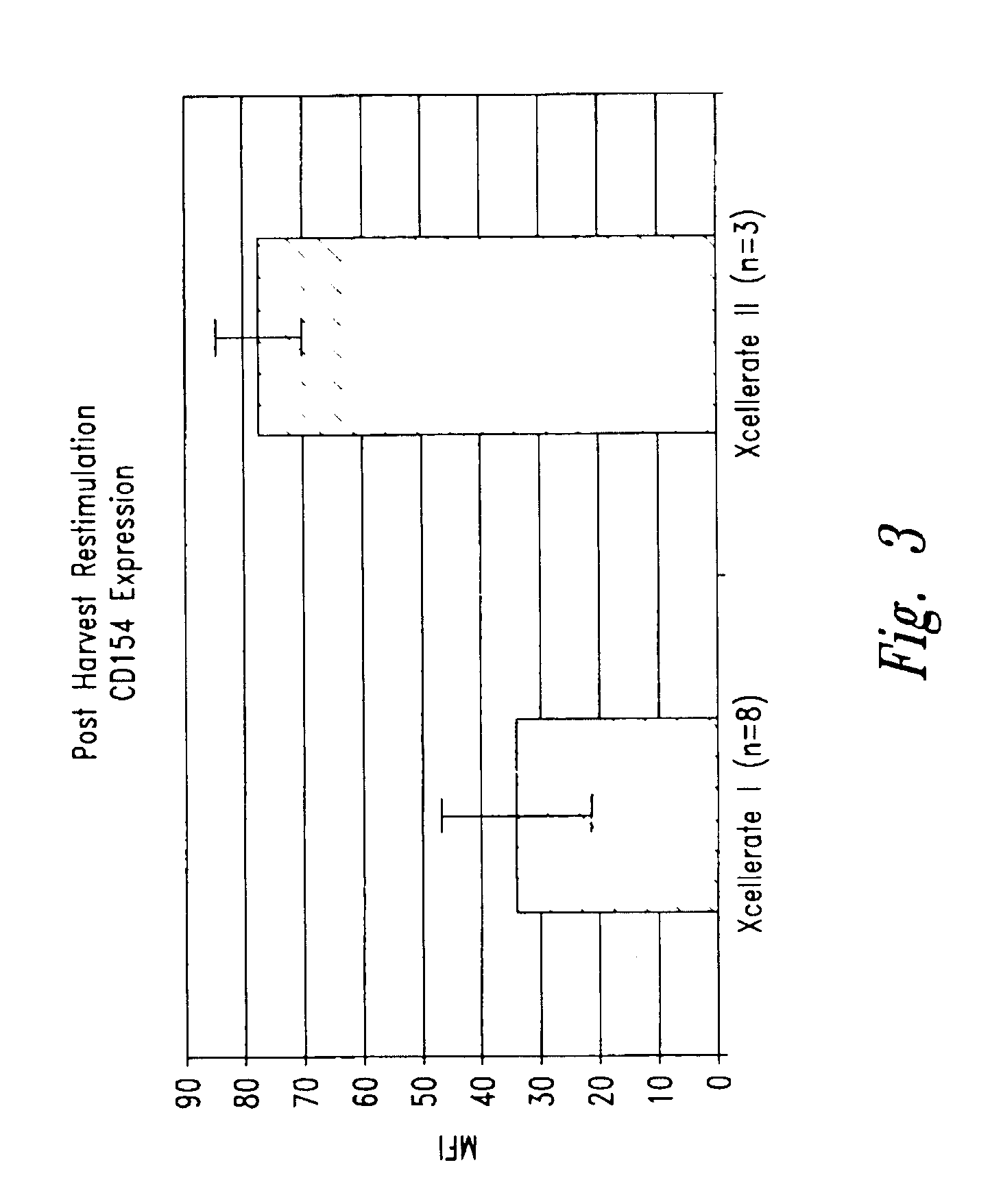
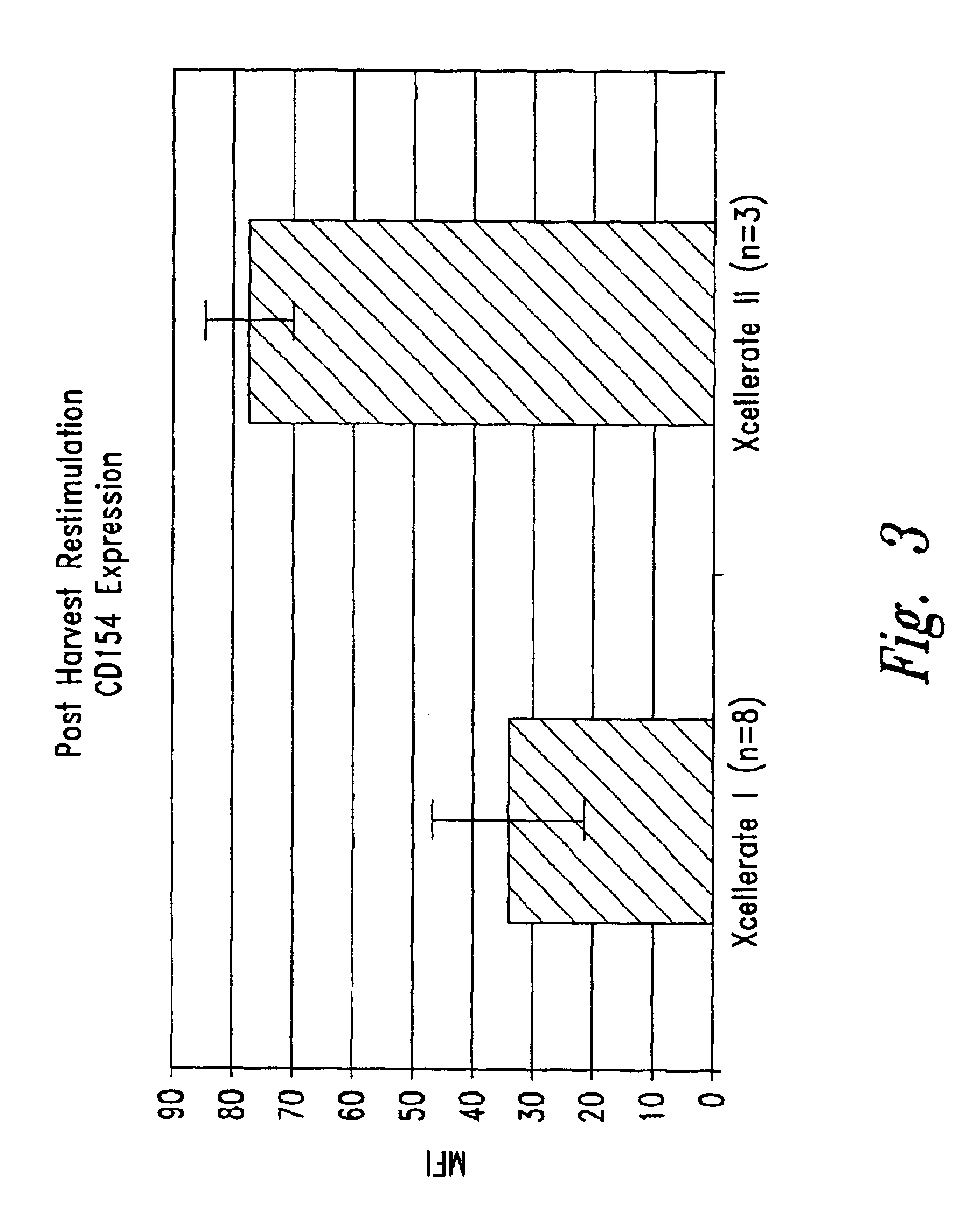
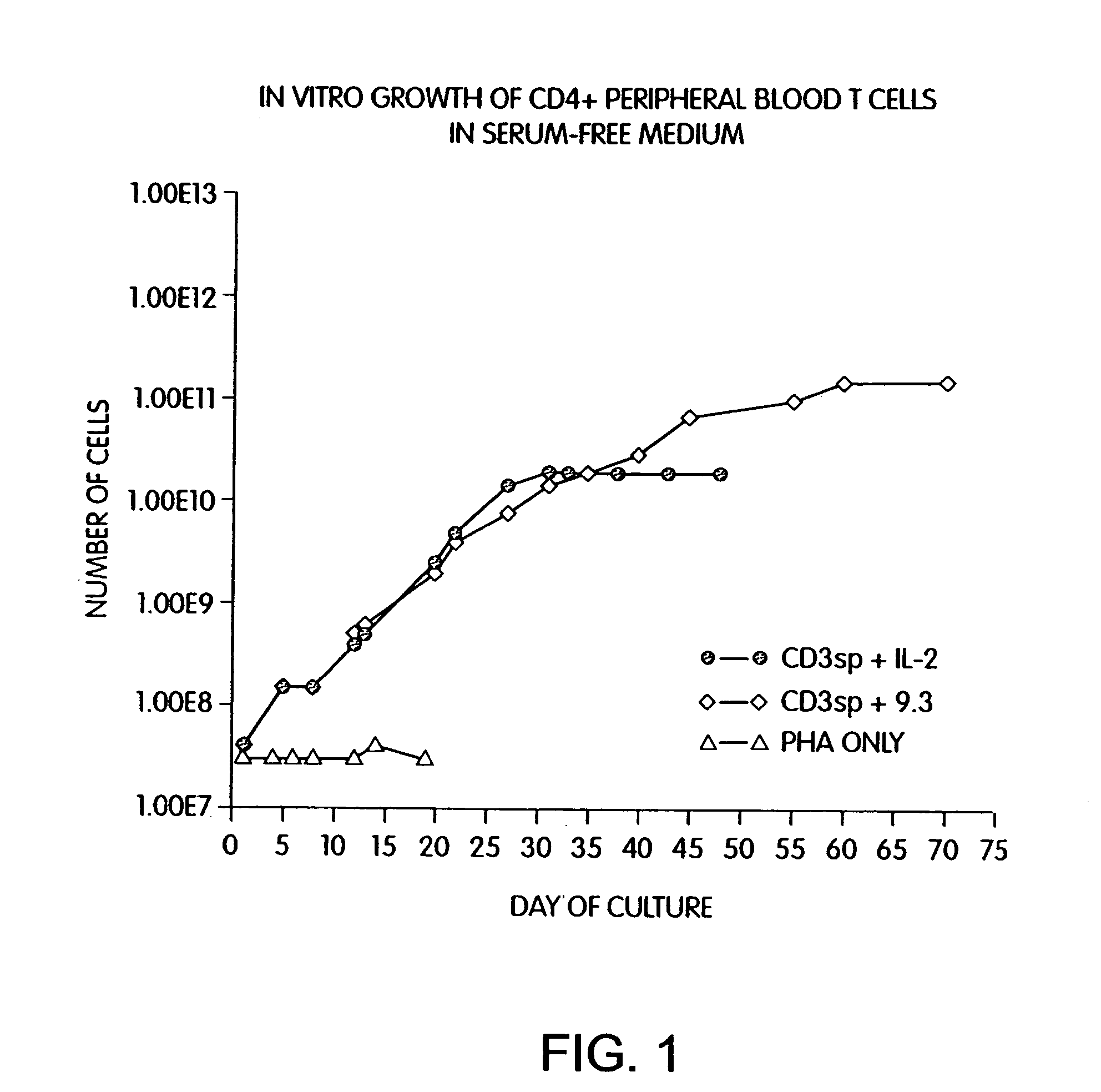
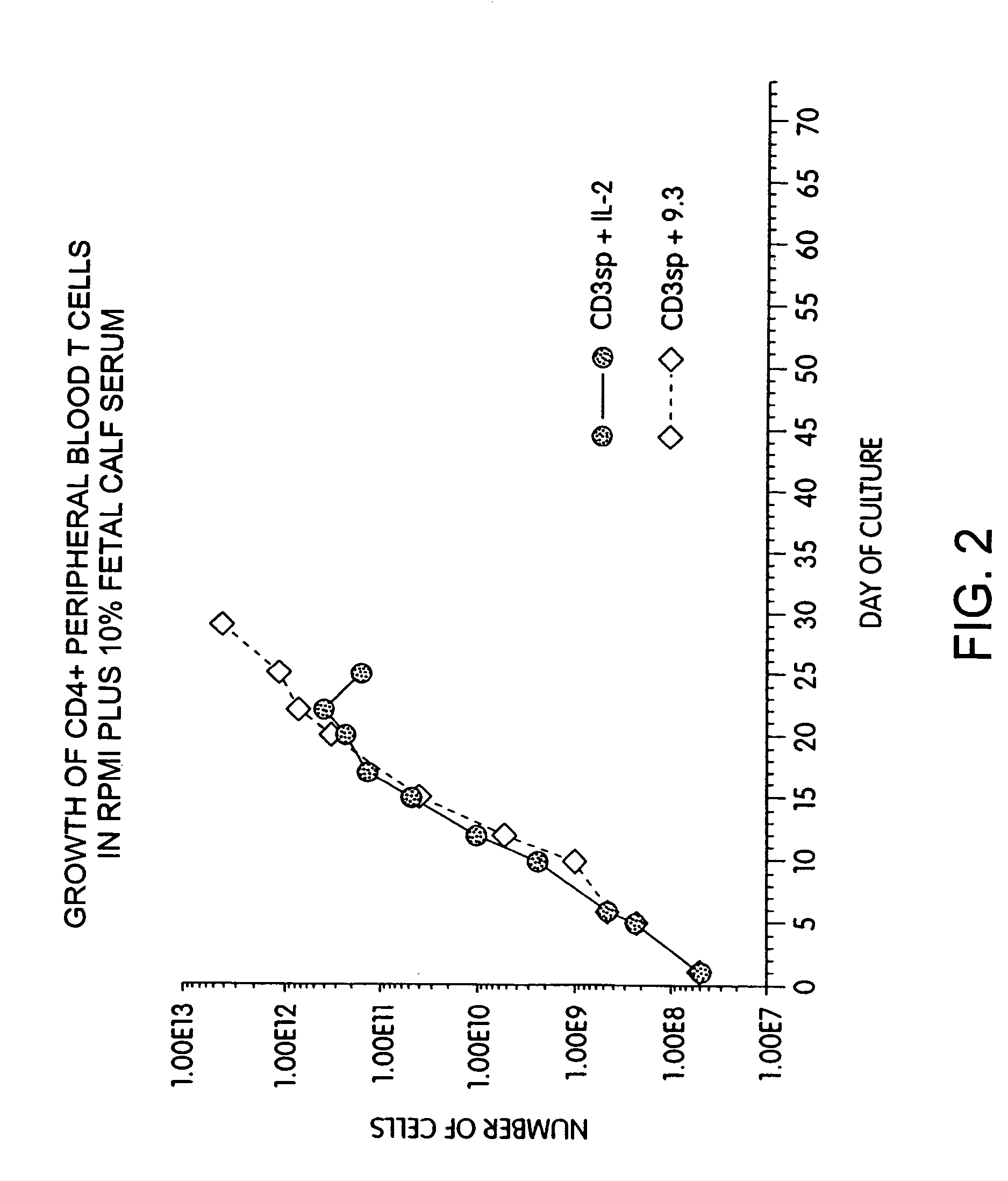
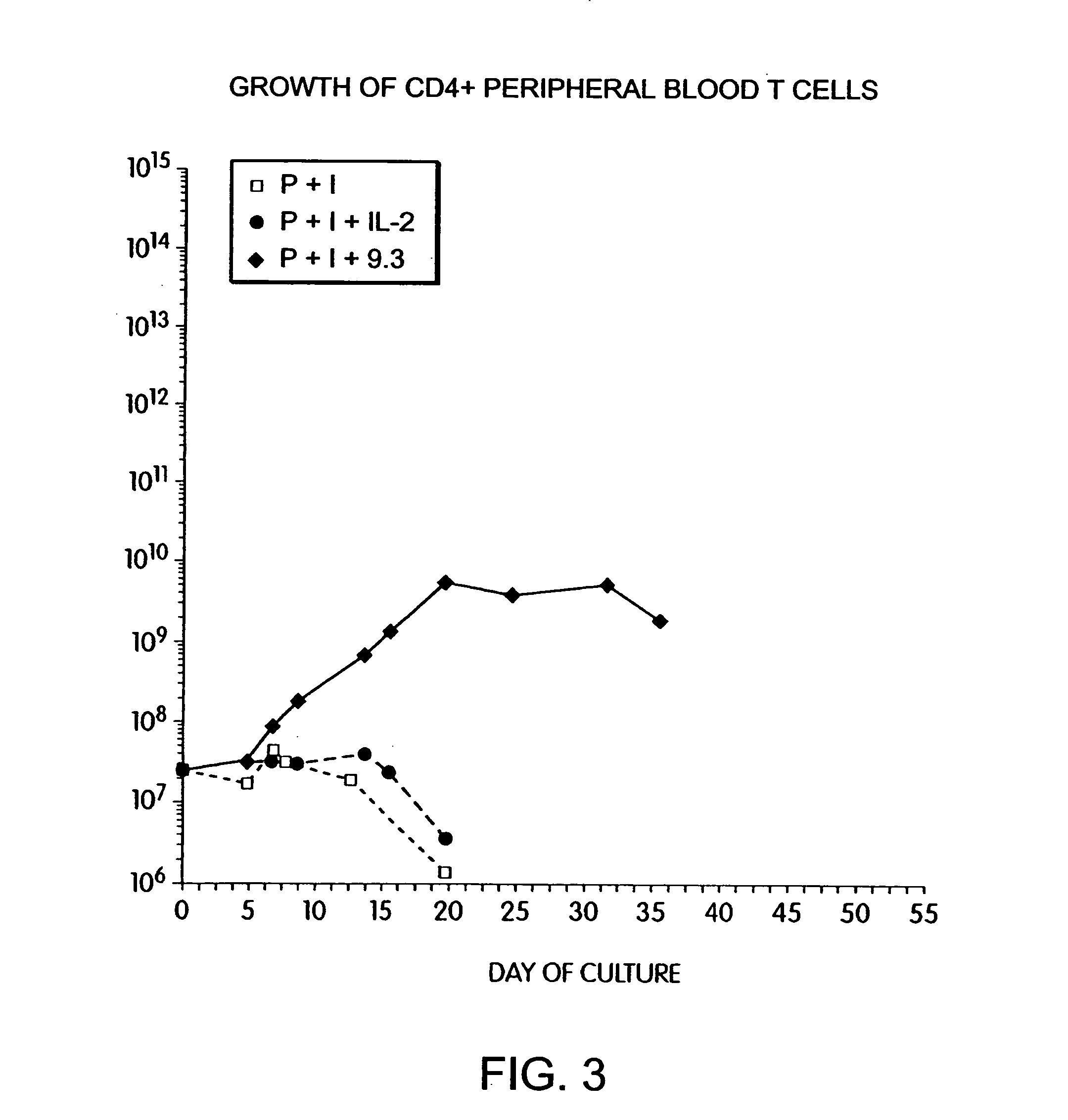
Simultaneous stimulation and concentration of cells
InactiveUS6905874B2Maximizes stimulationBiocideImmunoglobulins against cell receptors/antigens/surface-determinantsT cellCells signal
The present invention relates generally to methods for stimulating cells, and more particularly, to a novel method to concentrate and stimulate cells that maximizes stimulation and / or proliferation of such cells. In the various embodiments, cells are stimulated and concentrated with a surface yielding enhanced proliferation, cell signal transduction, and / or cell surface moiety aggregation. In certain aspects methods for stimulating a population of cells such as T-cells, by simultaneous concentration and cell surface moiety ligation are provided by contacting the population of cells with a surface, that has attached thereto one or more agents that ligate a cell surface moiety and applying a force that predominantly drives cell concentration and cell surface moiety ligation, thereby inducing cell stimulation, cell surface moiety aggregation, and / or receptor signaling enhancement. Also provided are methods for producing phenotypically tailored cells, including T-cells for the use in diagnostics, drug discovery, and the treatment of a variety of indications, including cancer, viral infection, and immune related disorders. Compositions of cells having specific phenotypic properties produced by these processes are further provided.
Owner:LIFE TECH CORP
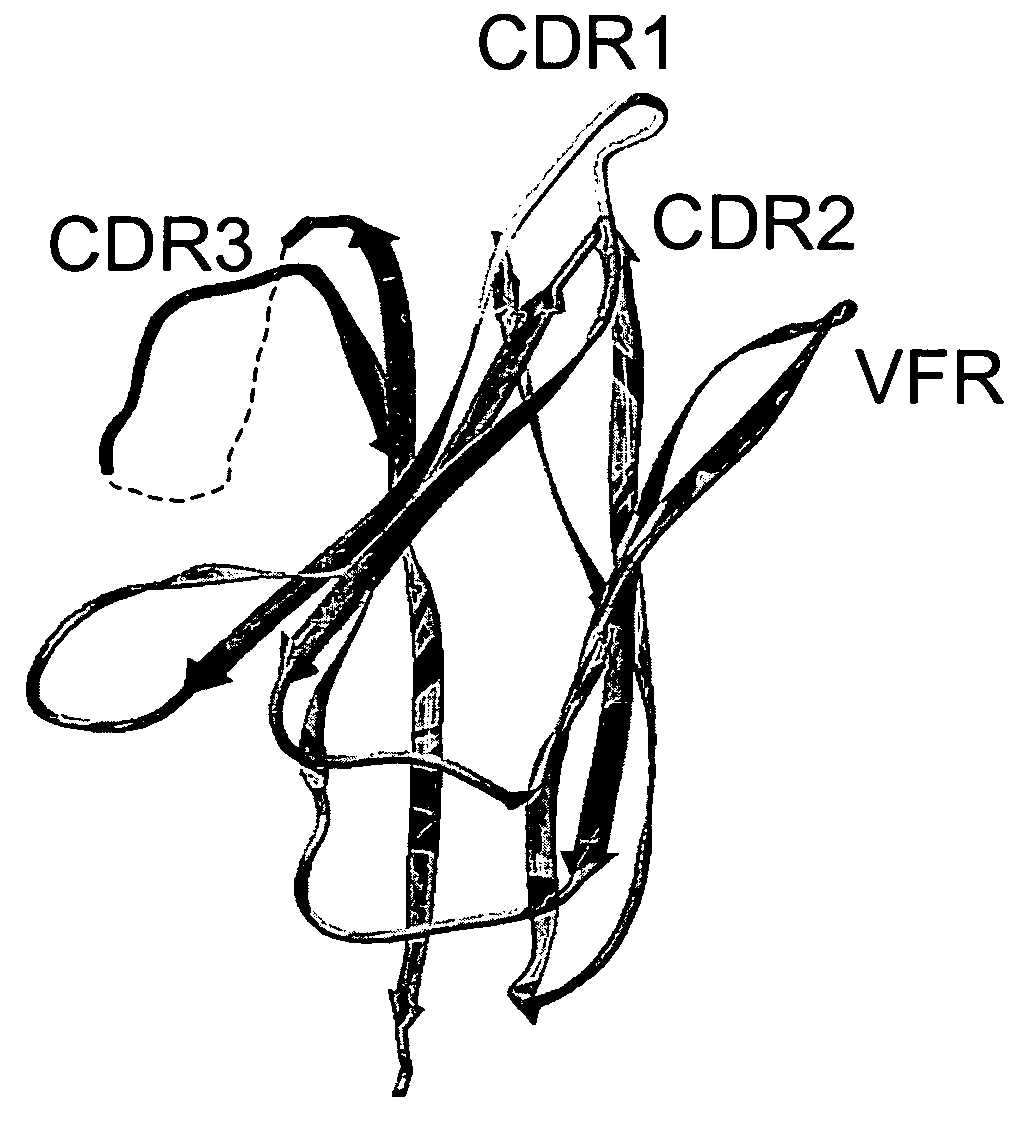
Variable domain library and uses
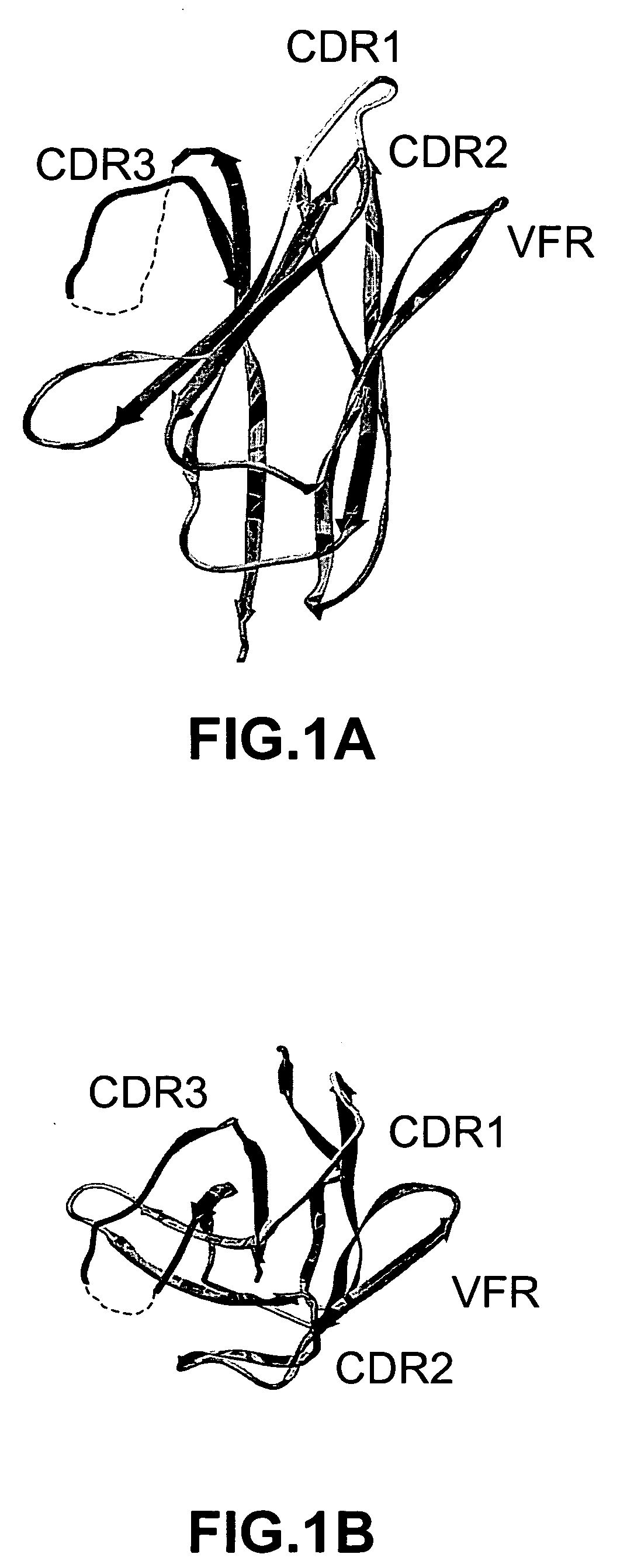
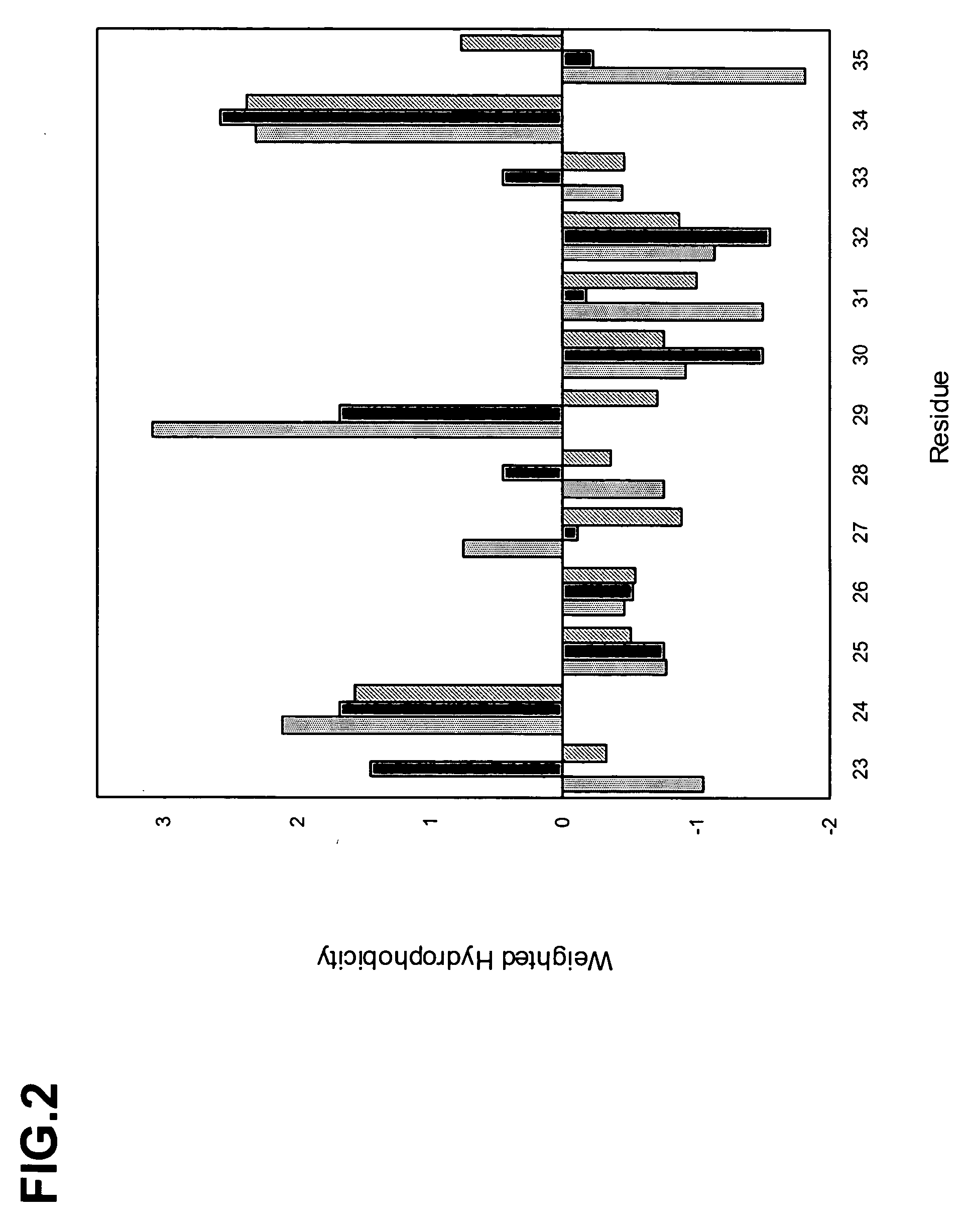
ActiveUS20050266000A1Generate efficientlyQuality improvementImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionAntigen binding
The invention provides polypeptides comprising a variant heavy chain variable framework domain (VFR). In some embodiments, the amino acids defining the VFR form a loop of an antigen binding pocket. In an embodiment, the polypeptide is a variable domain of a monobody and has a variant VFR. The polypeptide may optionally comprise one or more complementary determining regions (CDRs) of antibody variable domains. In an embodiment, the polypeptide is a variable domain of a monobody and has a variant VFR and one or more variant CDRs. Libraries of polypeptides that include a plurality of different antibody variable domains generated by creating diversity in a VFR, and optionally, one or more CDRs are provided and may be used as a source for identifying novel antigen binding polypeptides that can be used therapeutically or as reagents. The invention also provides fusion polypeptides, compositions, and methods for generating and using the polypeptides and libraries.
Owner:GENENTECH INC
Simultaneous stimulation and concentration of cells
InactiveUS6867041B2Maximizes stimulationCulture processArtificial cell constructsDrug discoveryCells signal
The present invention relates generally to methods for stimulating cells, and more particularly, to a novel method to concentrate and / or stimulate cells that maximizes stimulation and / or proliferation of such cells. In the various embodiments, cells are stimulated and concentrated with a surface yielding enhanced proliferation, cell signal transduction, and / or cell surface moiety aggregation. In certain aspects methods for stimulating a population of cells such as T-cells, by simultaneous concentration and cell surface moiety ligation are provided by contacting the population of cells with a surface, that has attached thereto one or more agents that ligate a cell surface moiety and applying a force that predominantly drives cell concentration and cell surface moiety ligation, thereby inducing cell stimulation, cell surface moiety aggregation, and / or receptor signaling enhancement. Also provided are methods for producing phenotypically tailored cells, including T-cells for the use in diagnostics, drug discovery, and the treatment of a variety of indications, including cancer, viral infection, and immune related disorders. Compositions of cells having specific phenotypic properties produced by these processes are further provided.
Owner:LIFE TECH CORP
Methods for selectively stimulating proliferation of T cells
InactiveUS7175843B2Increase the number ofBiocideCell receptors/surface-antigens/surface-determinantsAccessory moleculeExogenous growth
Owner:GENETICS INST LLC +2
Phage antibodies
Peripheral blood leucocytes incubated with a semi-synthetic phage antibody library and fluorochrome-labeled CD3 and CD20 antibodies were used to isolate human single chain Fv antibodies specific for subsets of blood leucocytes by flow cytometry. Isolated phage antibodies showed exclusive binding to the subpopulation used for selection or displayed additional binding to a restricted population of other cells in the mixture. At least two phage antibodies appeared to display hithereto unknown staining patterns of B lineage cells. This approach provides a subtractive procedure to rapidly obtain human antibodies against known and novel surface antigens in their native configuration, expressed on phenotypically defined subpopulations of cells. Importantly, this approach does not depend on immunization procedures or the necessity to repeatedly construct phage antibody libraries.
Owner:JANSSEN VACCINES & PREVENTION BV
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
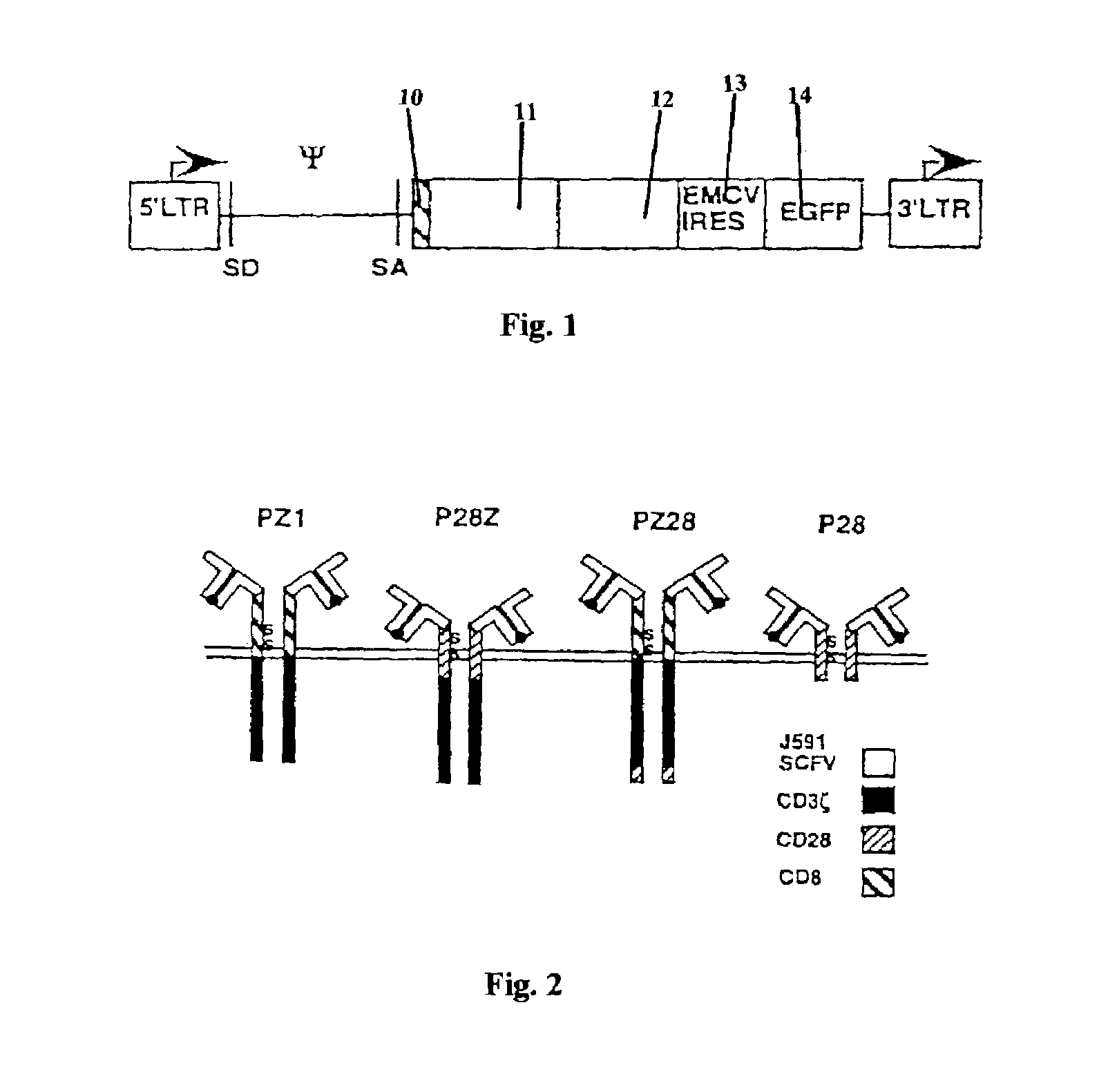
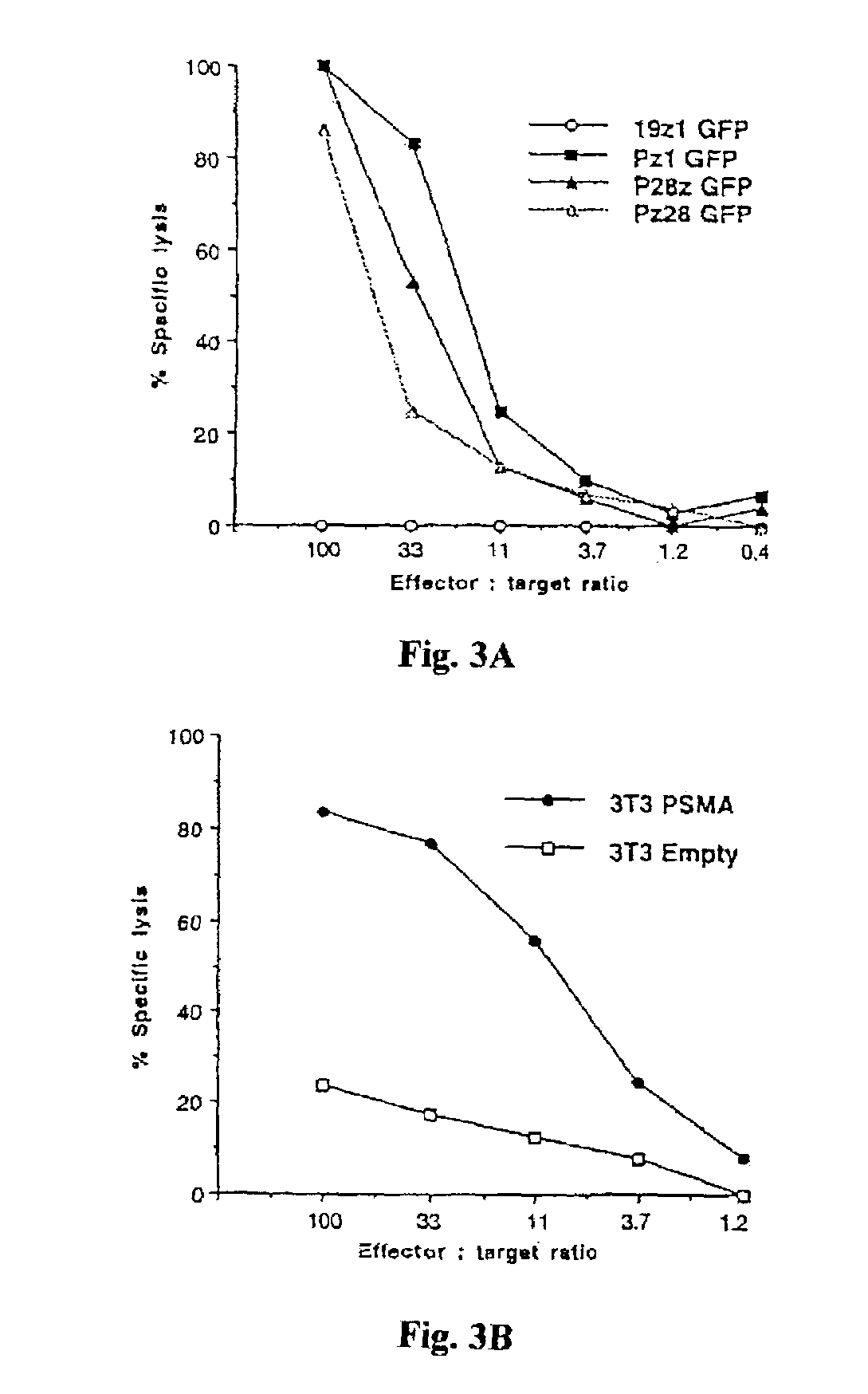
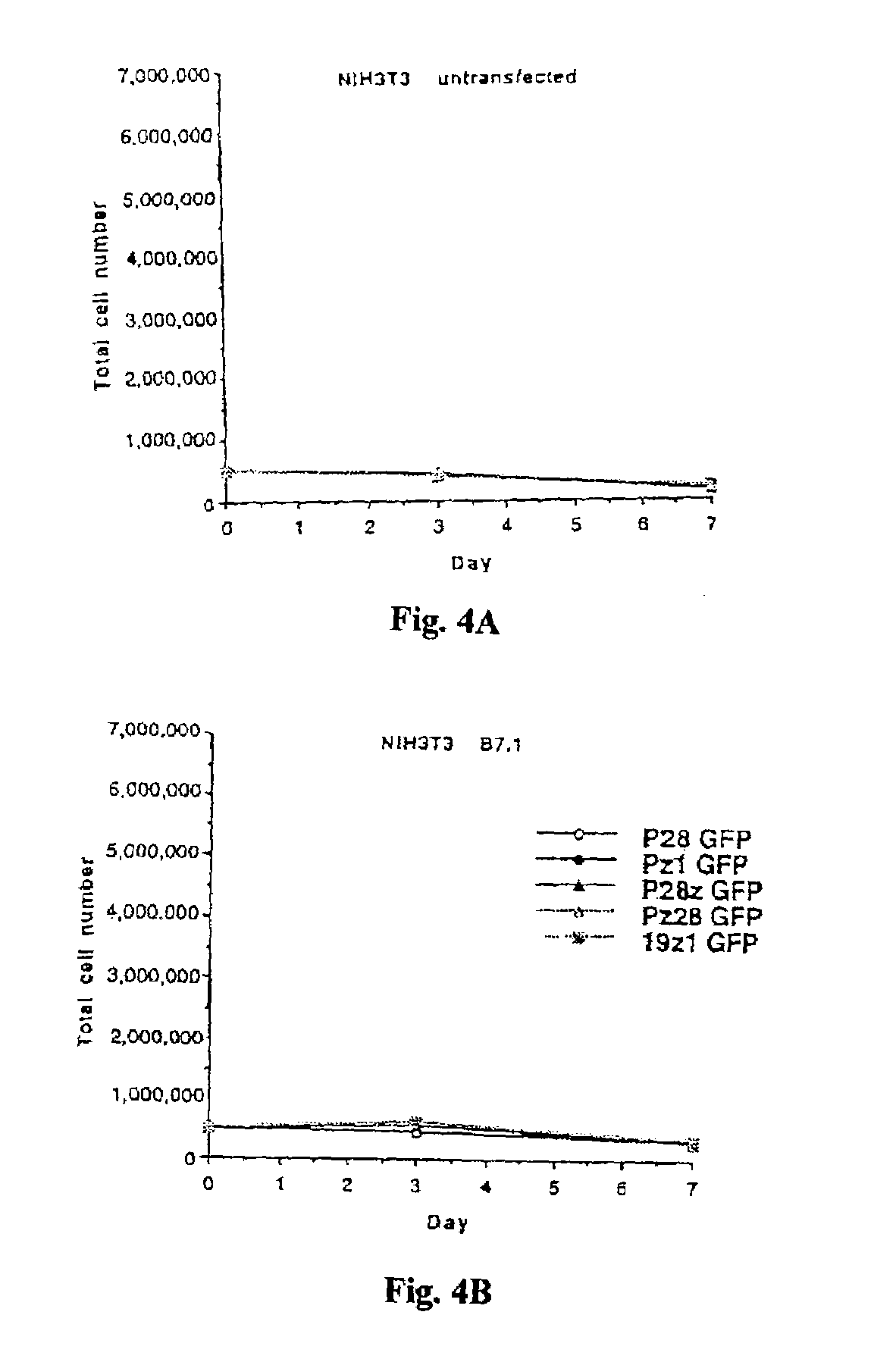
Nucleic acids encoding chimeric T cell receptors
ActiveUS7446190B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCytotoxicityBiological activation
Chimeric T cell receptors (TCR) are provided that combine, in a single chimeric species, the intracellular domain of CD3 ζ-chain, a signaling region from a costimulatory protein such as CD28, and a binding element that specifically interacts with a selected target. When expressed, for example in T-lymphocytes from the individual to be treated for a condition associated with the selected target, a T cell immune response is stimulated in the individual to the target cells. The chimeric TCR's are able to provide both the activation and the co-stimulation signals from a single molecule to more effectively direct T-lymphocyte cytotoxicity against the selected target and T-lymphocyte proliferation.
Owner:SLOAN KETTERING INST FOR CANCER RES
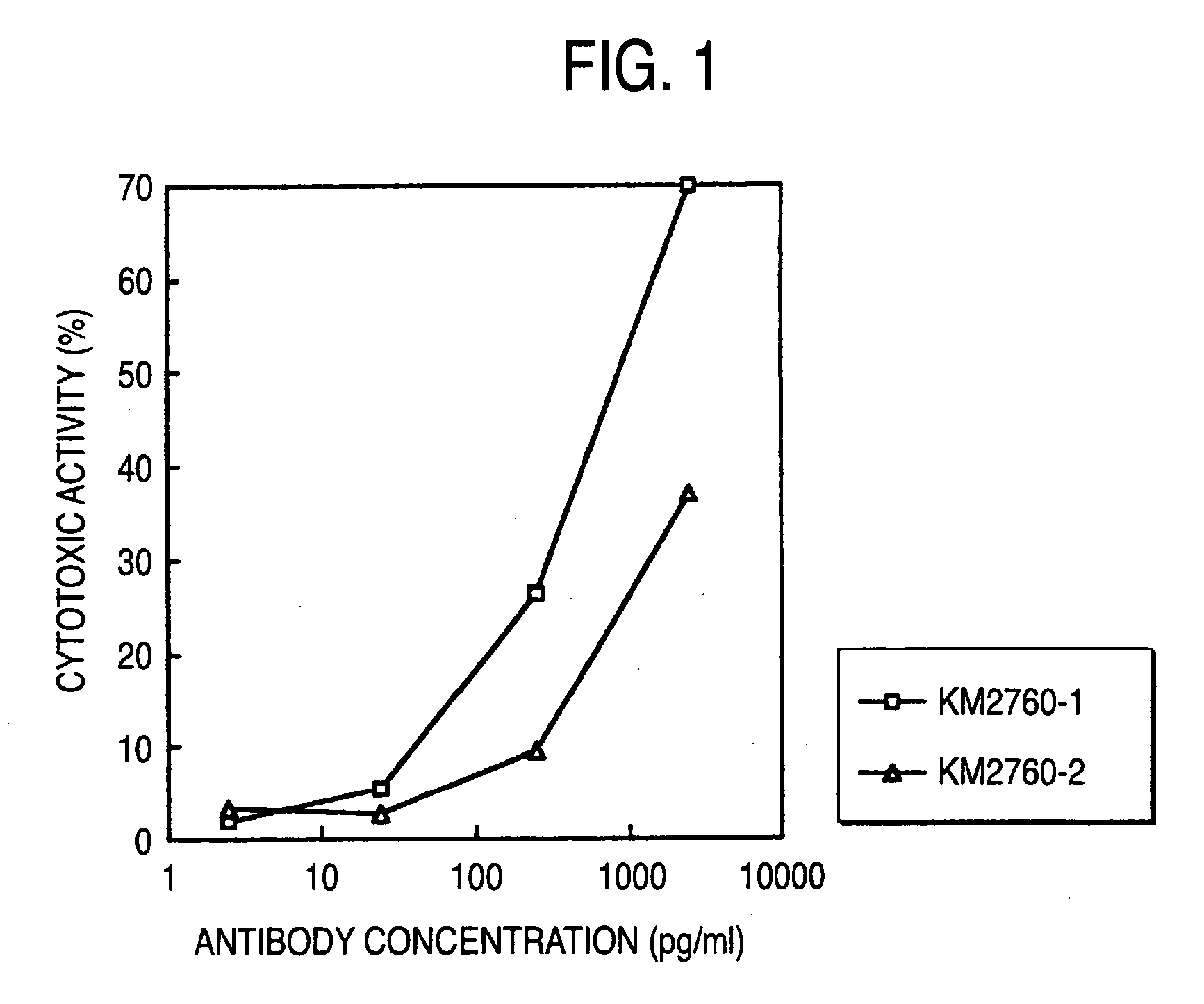
Cells producing antibody compositions with increased antibody dependent cytotoxic activity
The present invention relates to a cell for the production of an antibody molecule such as an antibody useful for various diseases having high antibody-dependent cell-mediated cytotoxic activity, a fragment of the antibody and a fusion protein having the Fc region of the antibody or the like, a method for producing an antibody composition using the cell, the antibody composition and use thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
Anti-PD1 antibodies and their use as therapeutics and diagnostics
ActiveUS8735553B1Inhibition of secretionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathAnti pd1
Owner:BEIGENE SWITZERLAND GMBH
Super humanized antibodies
InactiveUS6881557B2Antibody mimetics/scaffoldsAnalogue computers for chemical processesHuman sequenceHumanized antibody
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
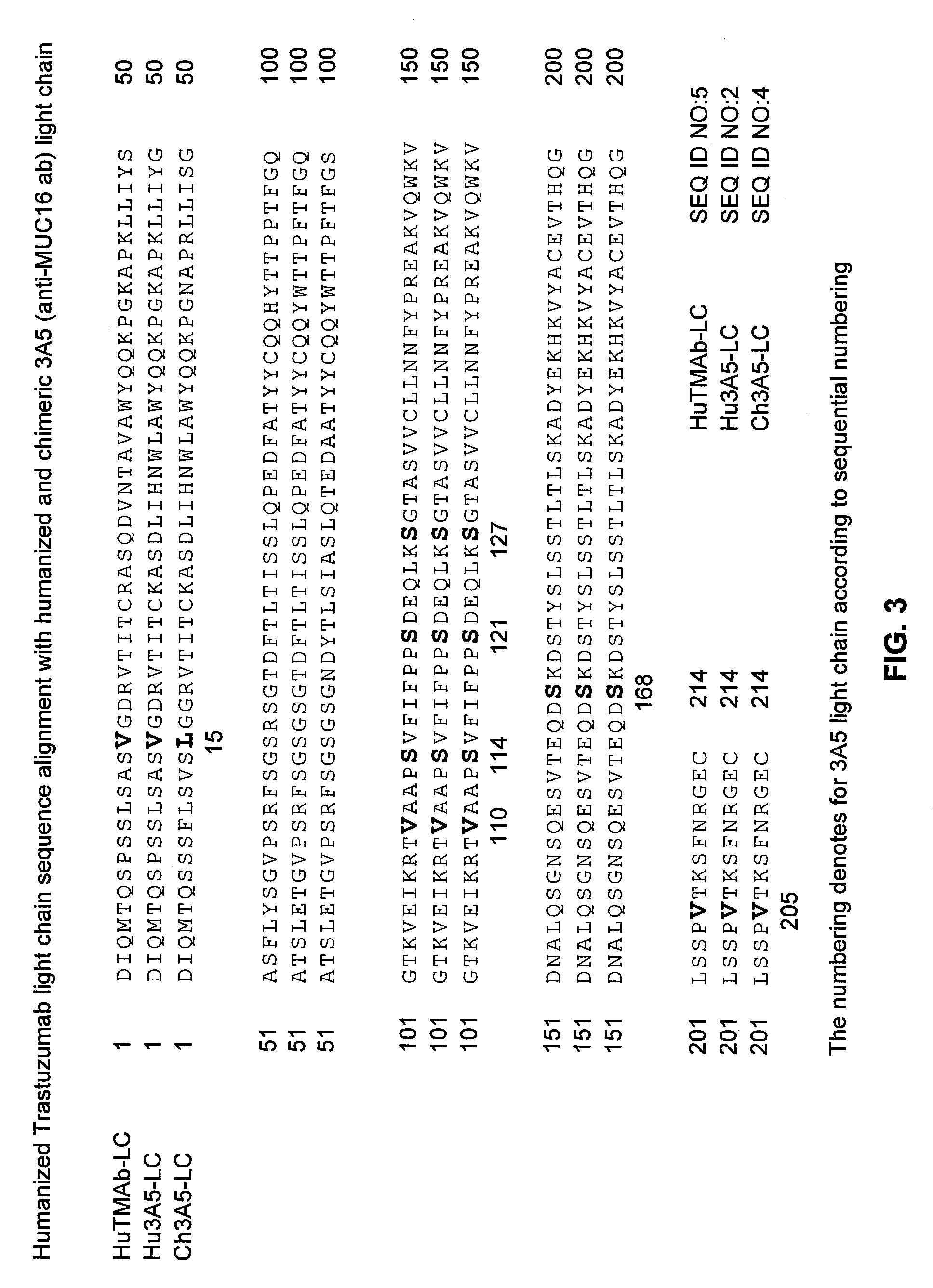
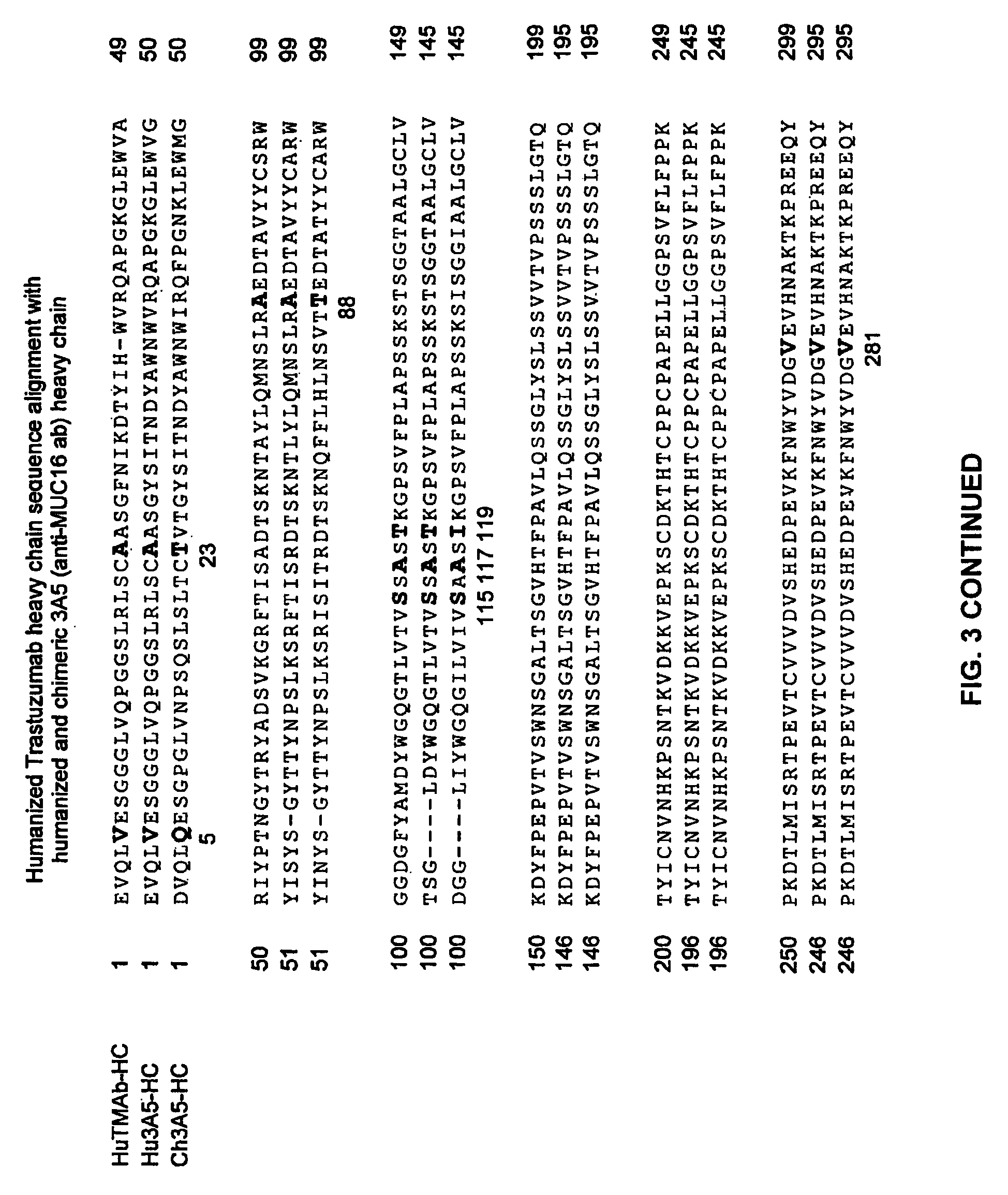
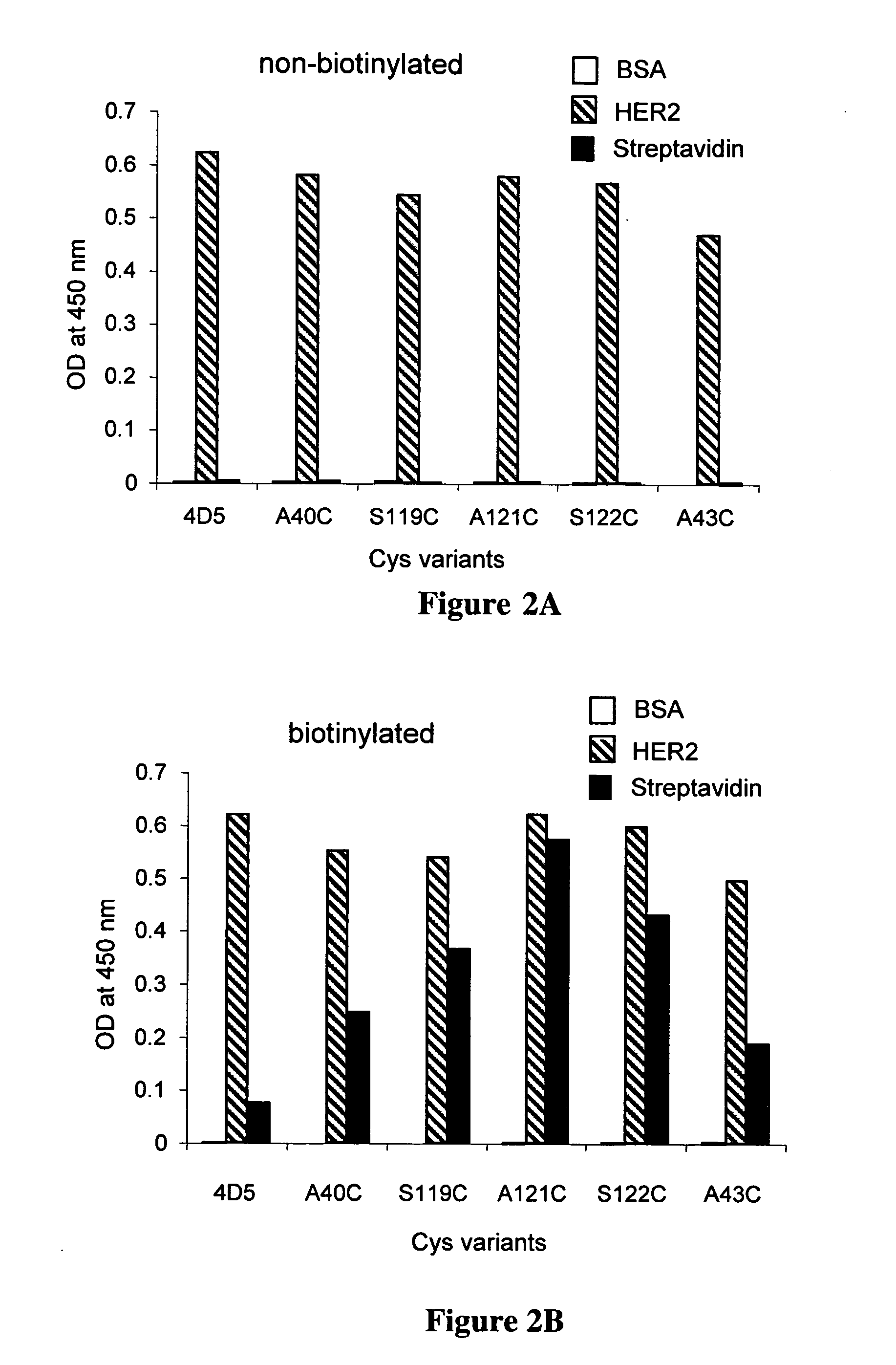
Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates
ActiveUS7723485B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkDrug compound
Cysteine engineered anti-MUC16 antibodies are engineered by replacing one or more amino acids of a parent anti-MUC16 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-MUC16 antibodies are provided. Cysteine engineered anti-MUC16 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-MUC16 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS7217797B2Function increaseExtended half-lifeAnimal cellsSugar derivativesAntiendomysial antibodiesBiochemistry
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Human CTLA-4 antibodies and their uses
InactiveUS7605238B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesVirology
The present invention provides human sequence antibodies against human CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
Cysteine engineered antibodies and conjugates
ActiveUS20070092940A1Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkCysteine thiolate
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab), optionally with an albumin-binding peptide (ABP) sequence, are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered antibody-drug conjugates having Formula I: Ab-(L-D)p I where p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Mammalian cell surface antigens; related reagents
InactiveUS7025962B1Abnormal immune responseFacilitated DiffusionPeptide/protein ingredientsAntibody mimetics/scaffoldsMammalT cell
Owner:MERCK SHARP & DOHME CORP
Compositions for targeting the vasculature of solid tumors
InactiveUS6051230AEffectively compete for bindingReduce TEC-4 or TEC-11 bindingHybrid immunoglobulinsPeptide/protein ingredientsAntigenCell Surface Antigens
The present invention relates generally to methods and compositions for targeting the vasculature of solid tumors using immunological- and growth factor-based reagents. In particular aspects, antibodies carrying diagnostic or therapeutic agents are targeted to the vasculature of solid tumor masses through recognition of tumor vasculature-associated antigens, such as, for example, through endoglin binding, or through the specific induction of endothelial cell surface antigens on vascular endothelial cells in solid tumors.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com