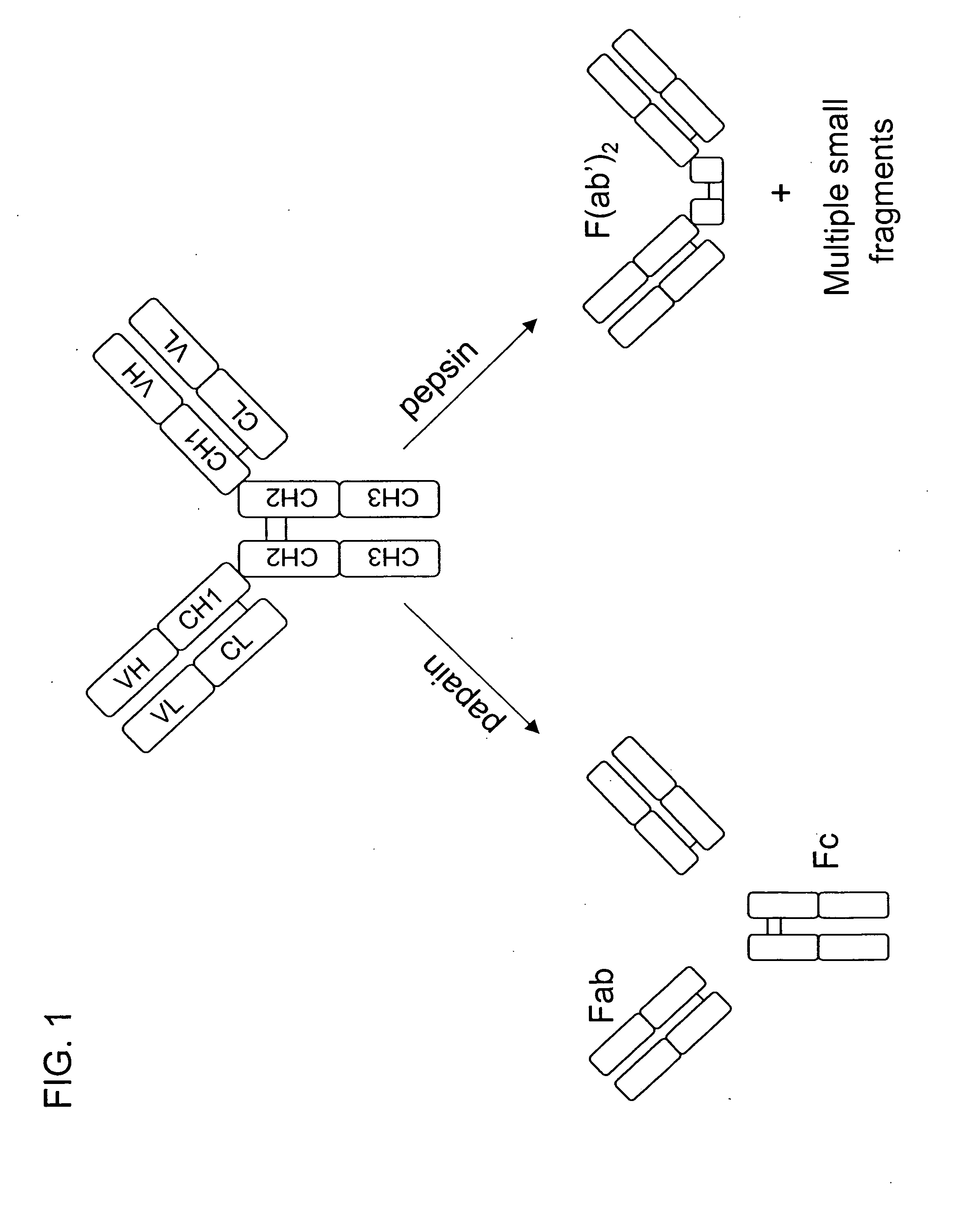
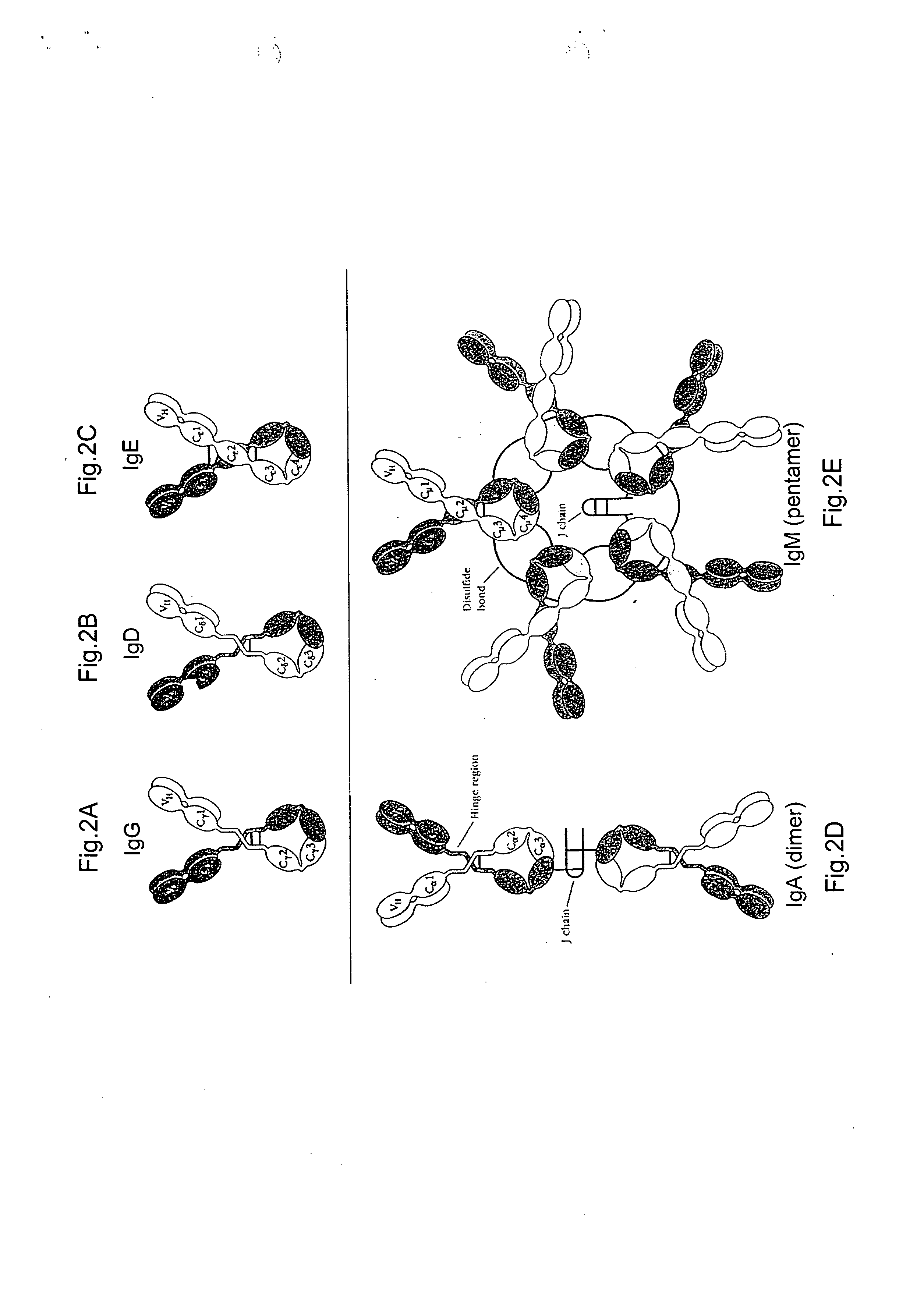
Multivalent antibodies and uses therefor
a multi-purpose, antibody technology, applied in the field of multi-purpose antibodies, can solve the problems of severe combined immune deficient (scid) mice lacking the ability to produce their own immunoglobulins, and the limitation of the clinical use of rodent monoclonal antibodies is an anti-globulin response during therapy, so as to achieve the effect of rapid production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Multivalent Antibodies
[0393] The construct used to generate a tetravalent anti-HER2 antibody, called an “Octopus antibody” (OctHER2), is illustrated in FIG. 5 herein. The backbone of this Octopus antibody is the recombinant, humanized monoclonal antibody 4D5 variant 8 (rhuMAb 4D5-8) (U.S. Pat. No. 5,821,337, Carter et al., expressly incorporated herein by reference). The heavy chain of rhuMAb 4D5-8 was subcloned into the pRK5 vector (EP 307,247, published Mar. 15, 1989). The VH-CH1 region of the heavy chain was removed by mutagenesis, and three unique restriction sites (BamHI; NheI; BspEI) were inserted. These sites were incorporated into PCR primers designed to amplify the VH-CH1 region from different antibodies. The resulting fragments were subcloned into the vector to create the Octopus heavy chain. Co-expression of the Octopus heavy chain with the appropriate light chain in a pRK5 vector in mammalian cell transfections results in the completed Octopus antibody (...
example 2
Evaluation of Anti-HER2 Octopus Antibodies
[0395] OctHER2 was expressed in transiently transfected 293 cells (Graham et al. J. Gen. Virol. 36:59-72 (1977)) and purified over a Protein A sepharose column. The complete antibody is approximately 245 kDa, as compared to the 150 kDa molecular weight of the parent antibody. The Octopus heavy chain is 75 kDa (without carbohydrate), and the light chain is 30 kDa.
Antigen Binding
[0396] Binding of OctHER2 to antigen, HER2 extracellular domain (HER2 ECD), was analyzed using a HER2 ELISA assay (Sias et al. J. Immunol. Methods 132:73-80 (1990)). Ninety-six well plates were coated with the HER2 extracellular domain (ECD) (WO90 / 14357), and incubated with different dilutions of anti-HER2 antibodies. After washing to remove unbound antibody, a secondary peroxidase-conjugated antibody was then added to detect the anti-HER2 antibody bound to the ECD. The appropriate substrate was then added, and the wells were visualized and then quantitated on a pla...
example 3
Evaluation of Anti-DR5 Octopus Antibodies
[0406] DR5 a member of the TNF receptor superfamily that binds the trimeric Apo2L / TRAIL (Apo2L). After Apo2L receptors bind Apo2L and are clustered, death domains in the cytosolic region of the receptors induce caspases to trigger cellular apoptosis. Two versions of anti-DR5 Octopus constructs were made: one from 16E2, an anti-DR5 cloned from a single-chain human Fv phage library (see WO98 / 51793, expressly incorporated herein by reference); the second anti-DR5 Octopus antibody was made from Mab 3H3.14.5 (the “3H3” antibody; ATCC HB-12534, WO99 / 64461), a murine anti-DR5 MAb that induces apoptosis when it is crosslinked. Since anti-Death receptor monoclonal antibodies may require crosslinking to trigger apoptosis, they are candidates for the Octopus antibody construct. The anti-DR5 Octopus antibodies were prepared by replacing the variable domains of the OctHER2 construct described above with the VL and VH domains from 16E2 or 3H3.
[0407] The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com