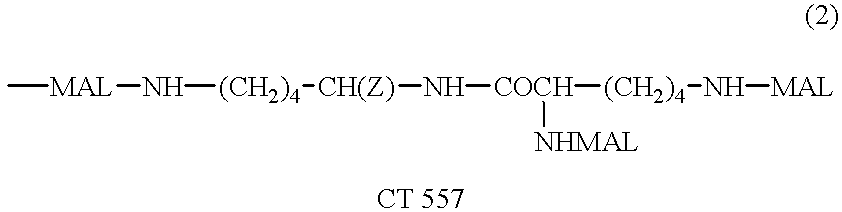
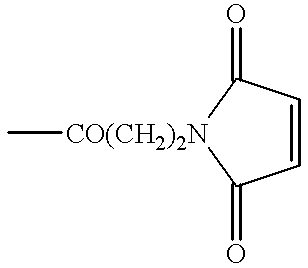
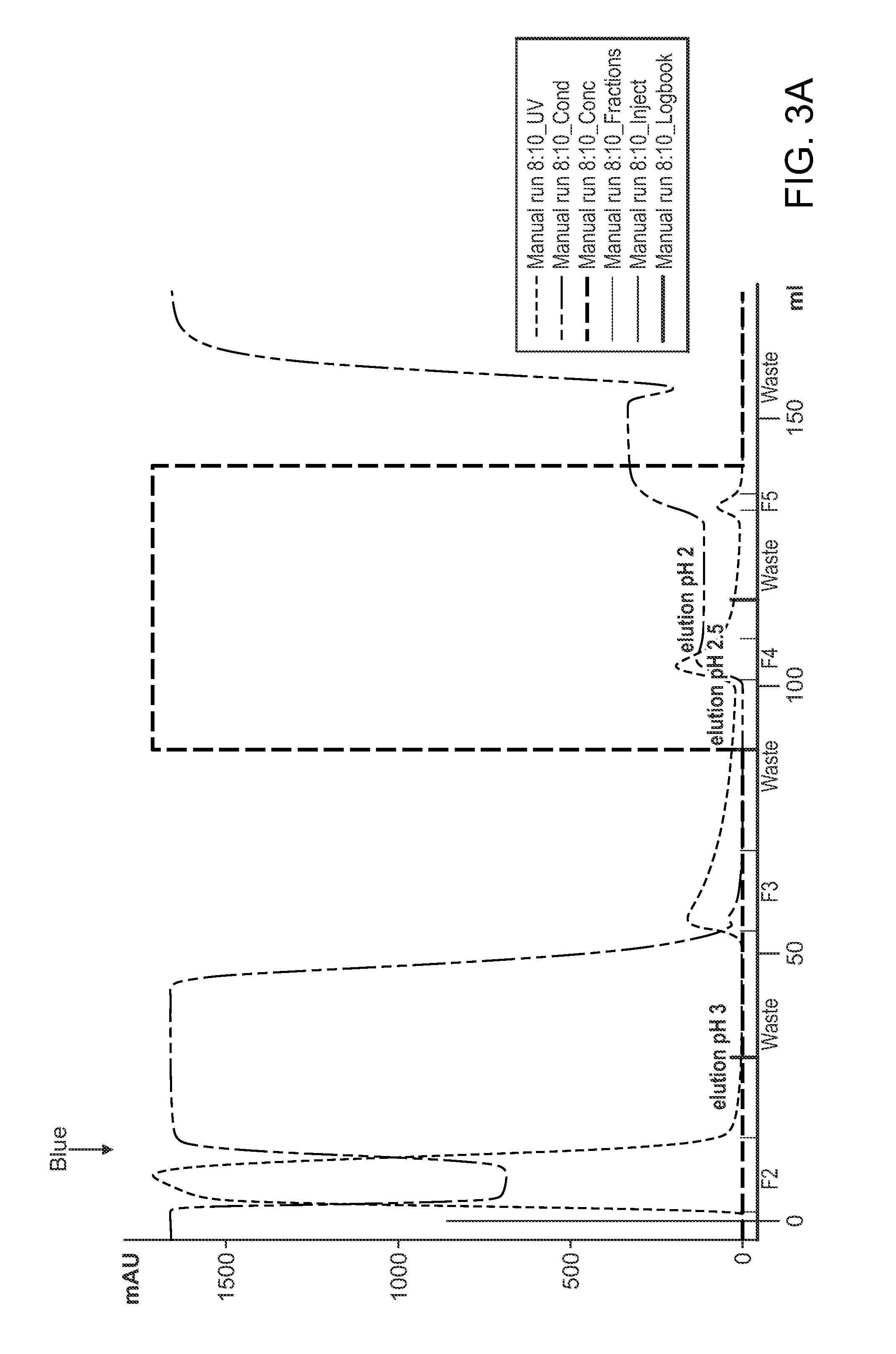
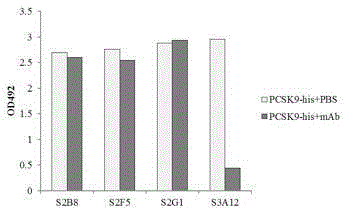
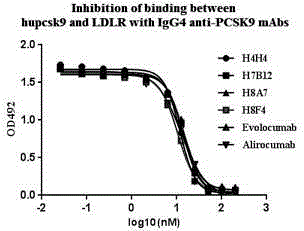
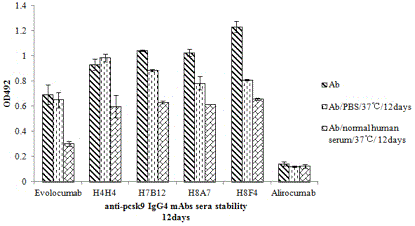
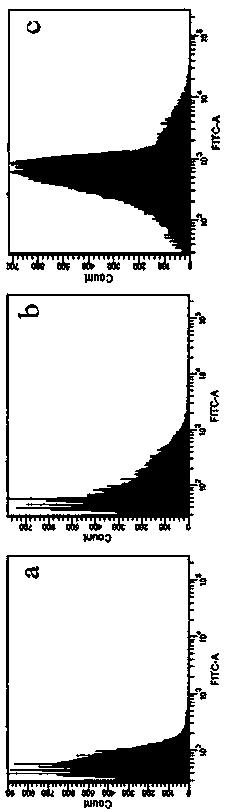
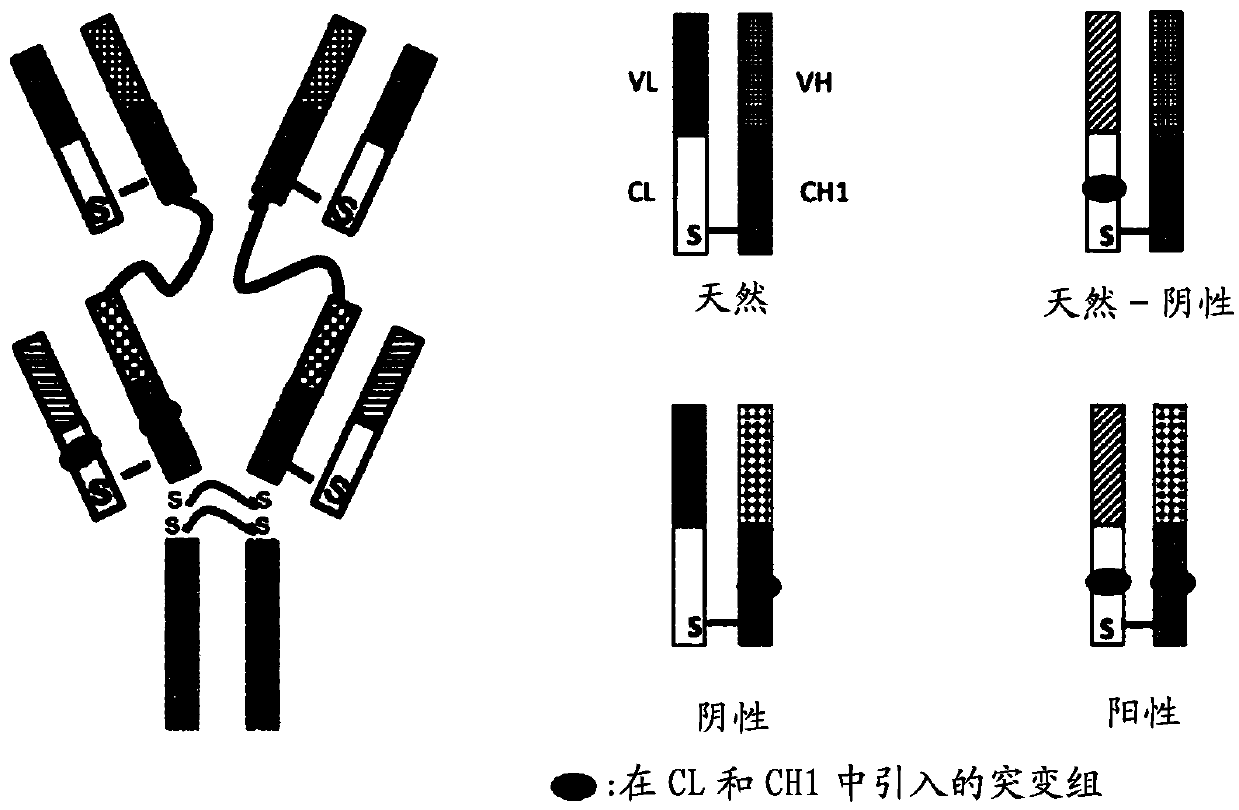
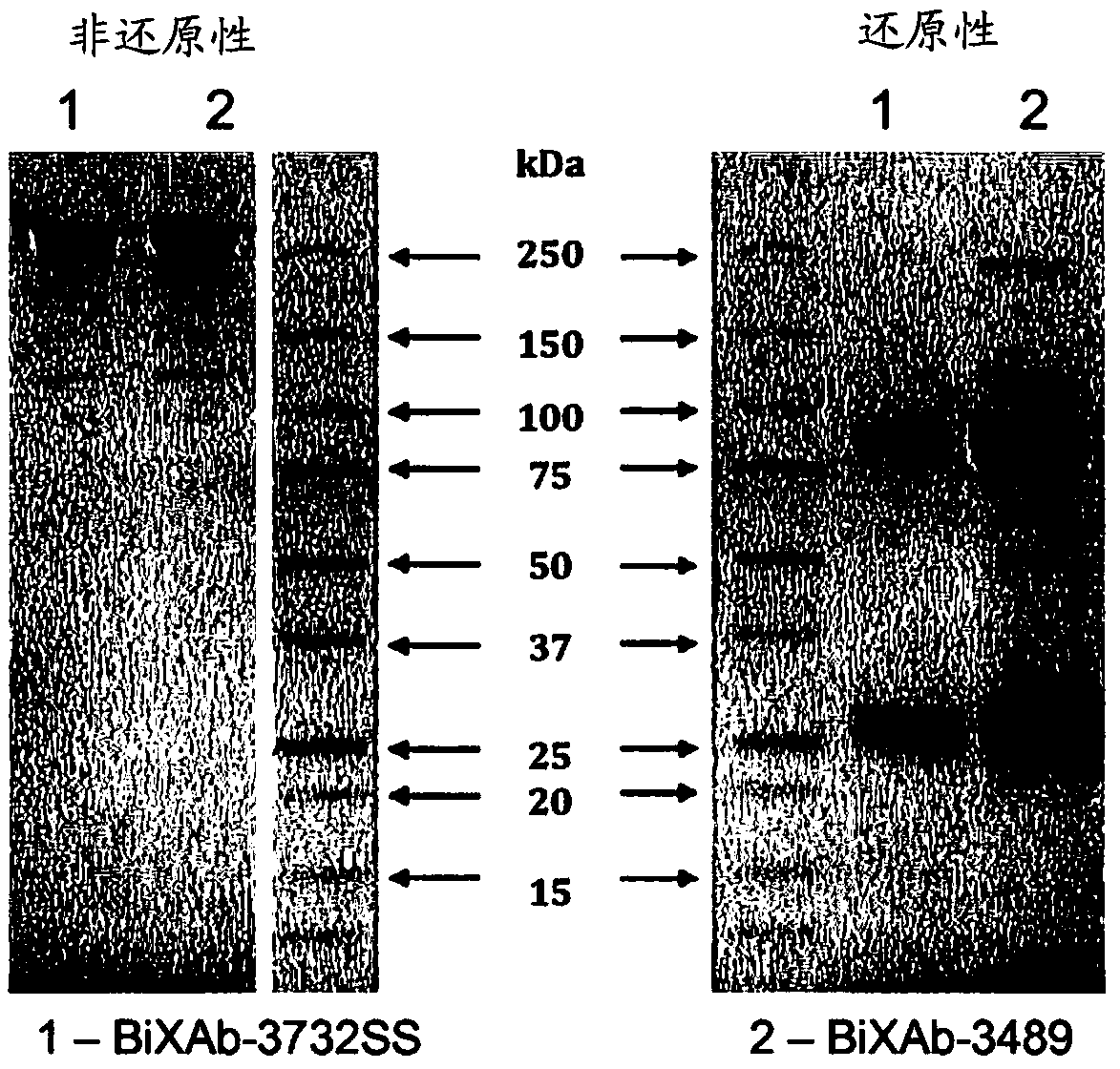
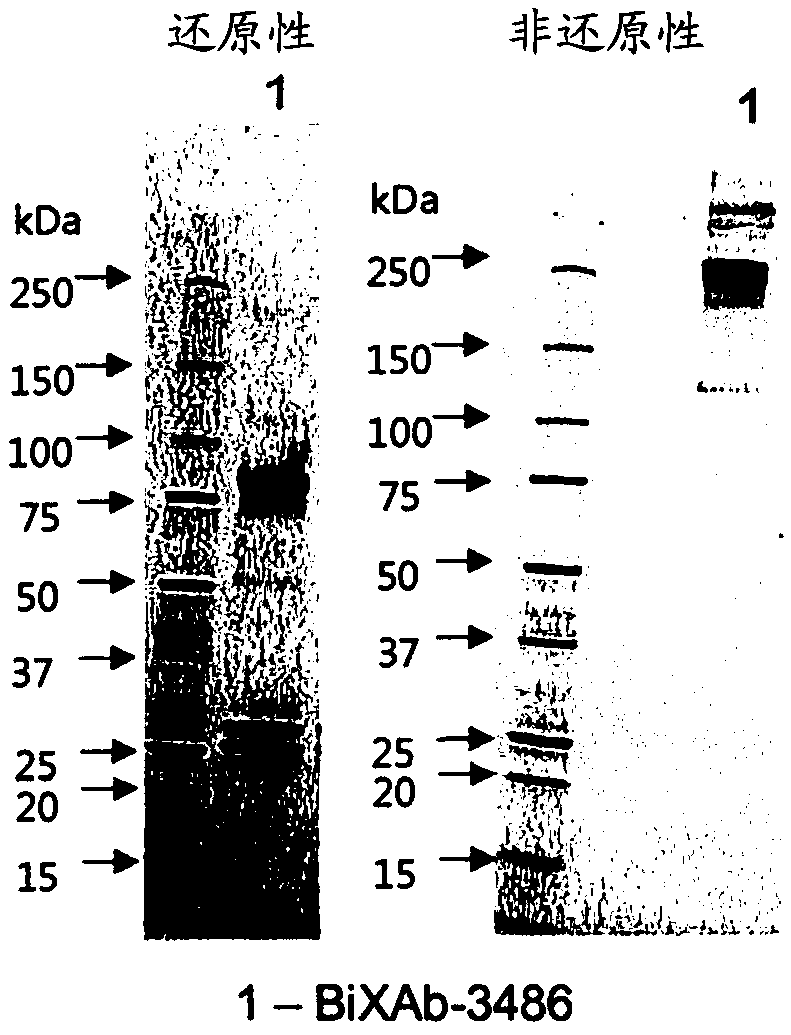
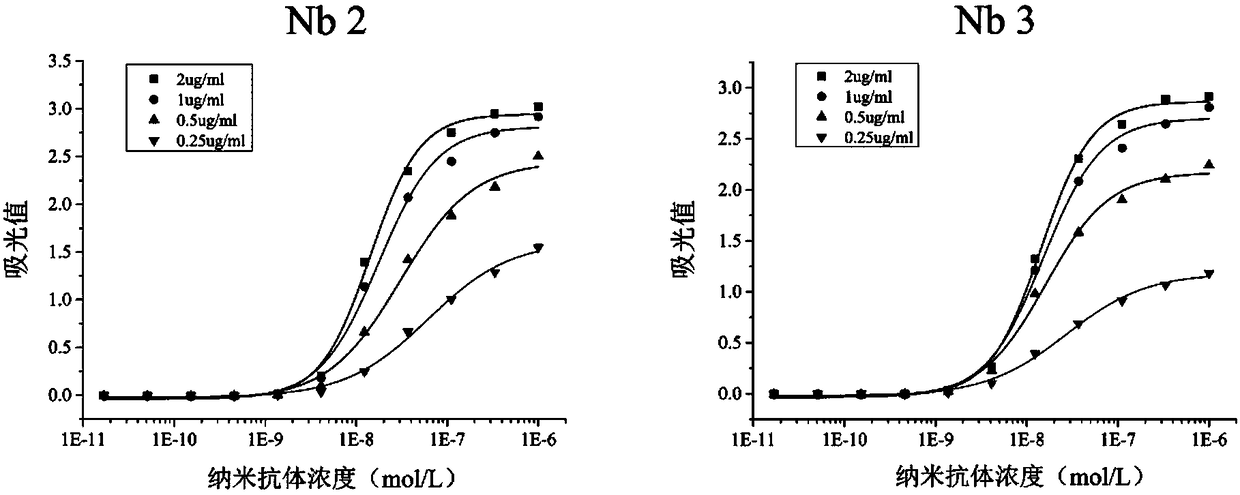
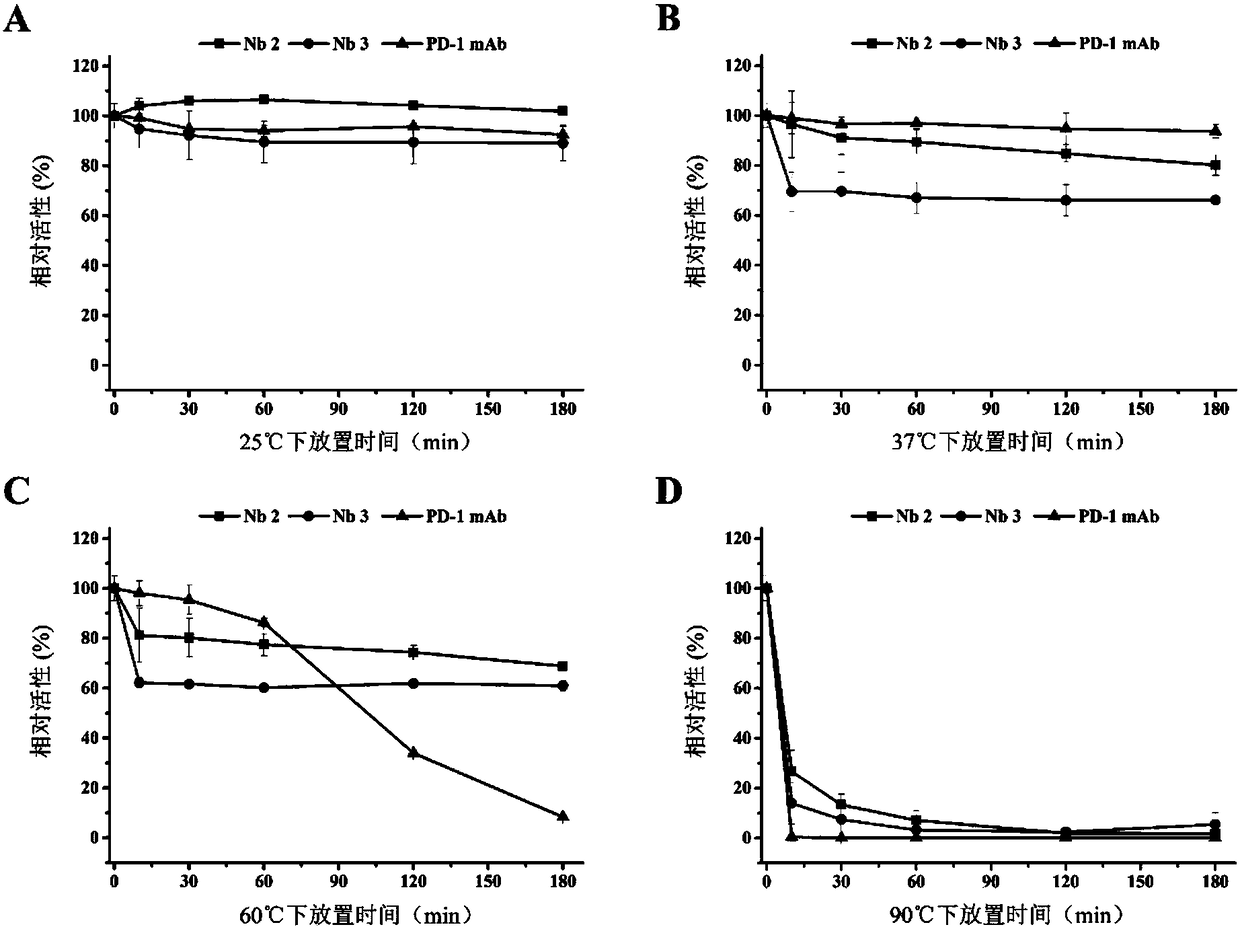
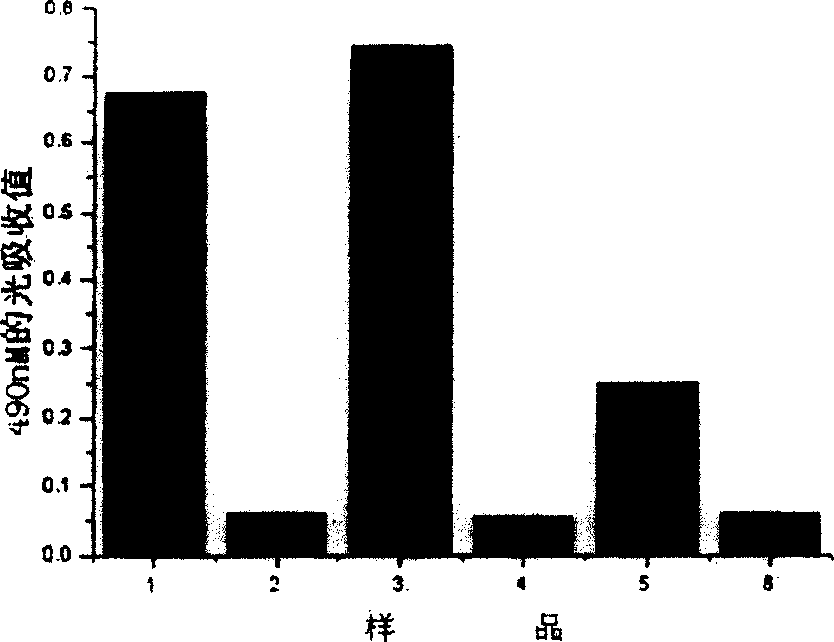
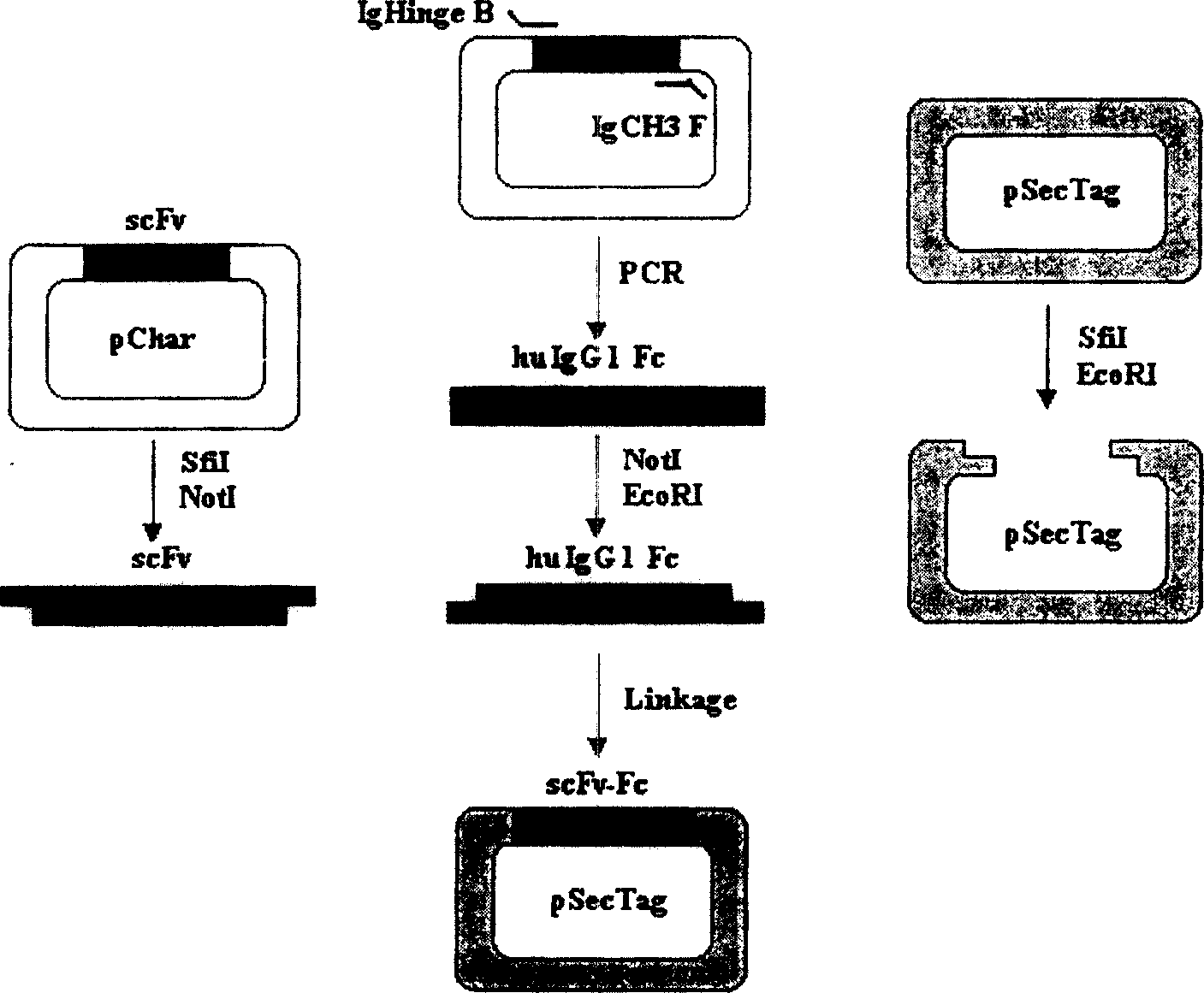
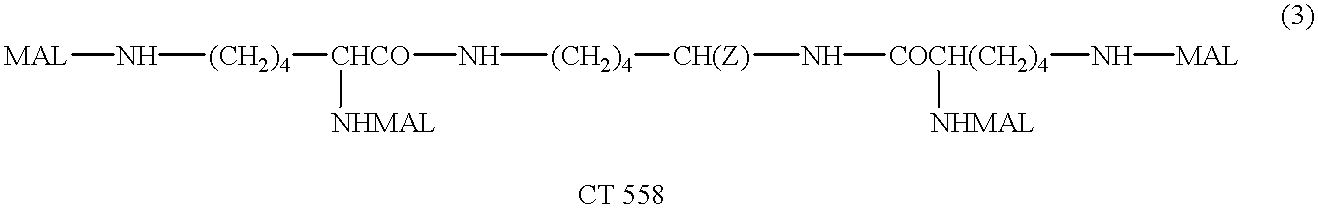Patents
Literature
68 results about "Complete antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Humanized antibodies directed against A33 antigen
InactiveUS6307026B1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkLymphatic Spread
A33 antigen binding proteins are described for use in the diagnosis or treatment of colorectal tumors and metastases arising therefrom. The binding protein may be a humanized A33 antibody, including complete antibody molecules, fragments thereof, and particularly, multivalent monospecific proteins comprising two, three, four or more antibodies or fragments thereof, bound to each other by a cross-linking agent. For diagnosis or therapy, the humanized A33 antibody may be linked to a reporter or effector molecule.
Owner:CELLTECH LTD
Human antibody and expression thereof
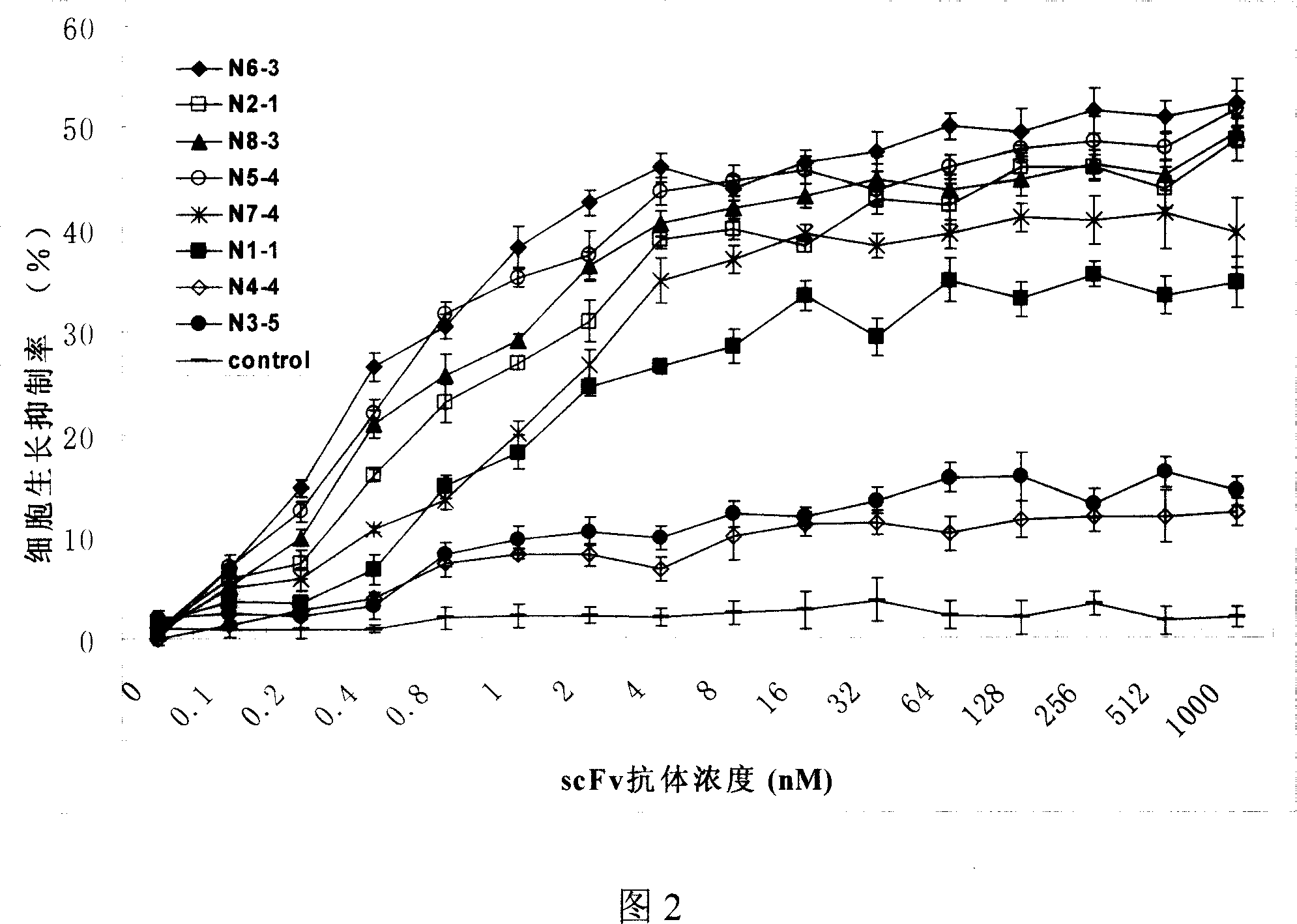
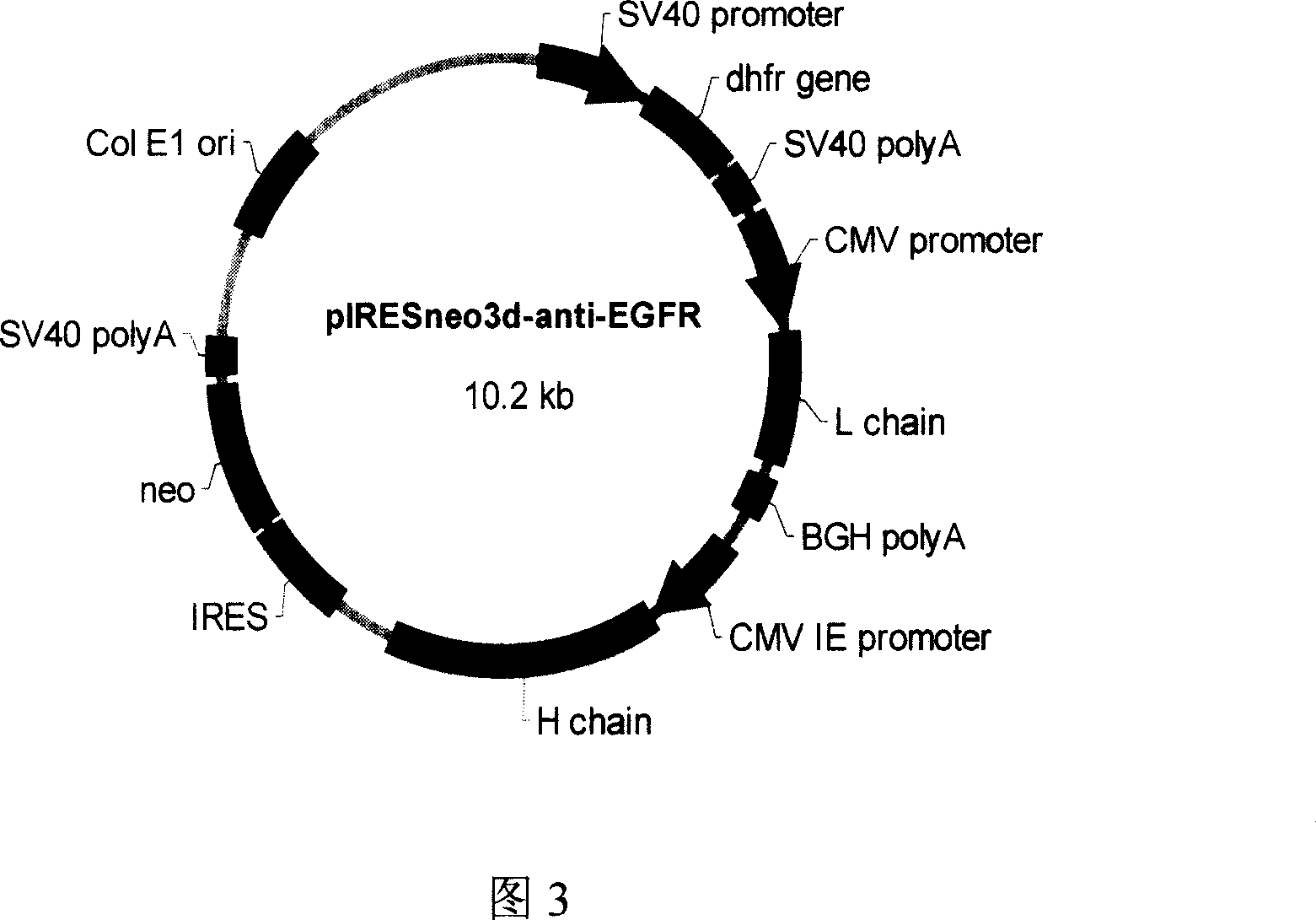
The invention discloses a full-human anti-EGFR scFv antibody segment and entire antibody, which contains heavy chain of antibody and nucleic acid and amino acid of variable area of light chain as well as high-effective expressing method of entire antibody in the CHO cell.
Owner:SINO CELL TECH INC
Methods of Purifying Antibodies
ActiveUS20130317200A1Rapid and efficient separationFavorable manufacturing characteristicHybrid immunoglobulinsFermentationHeavy chainBinding site
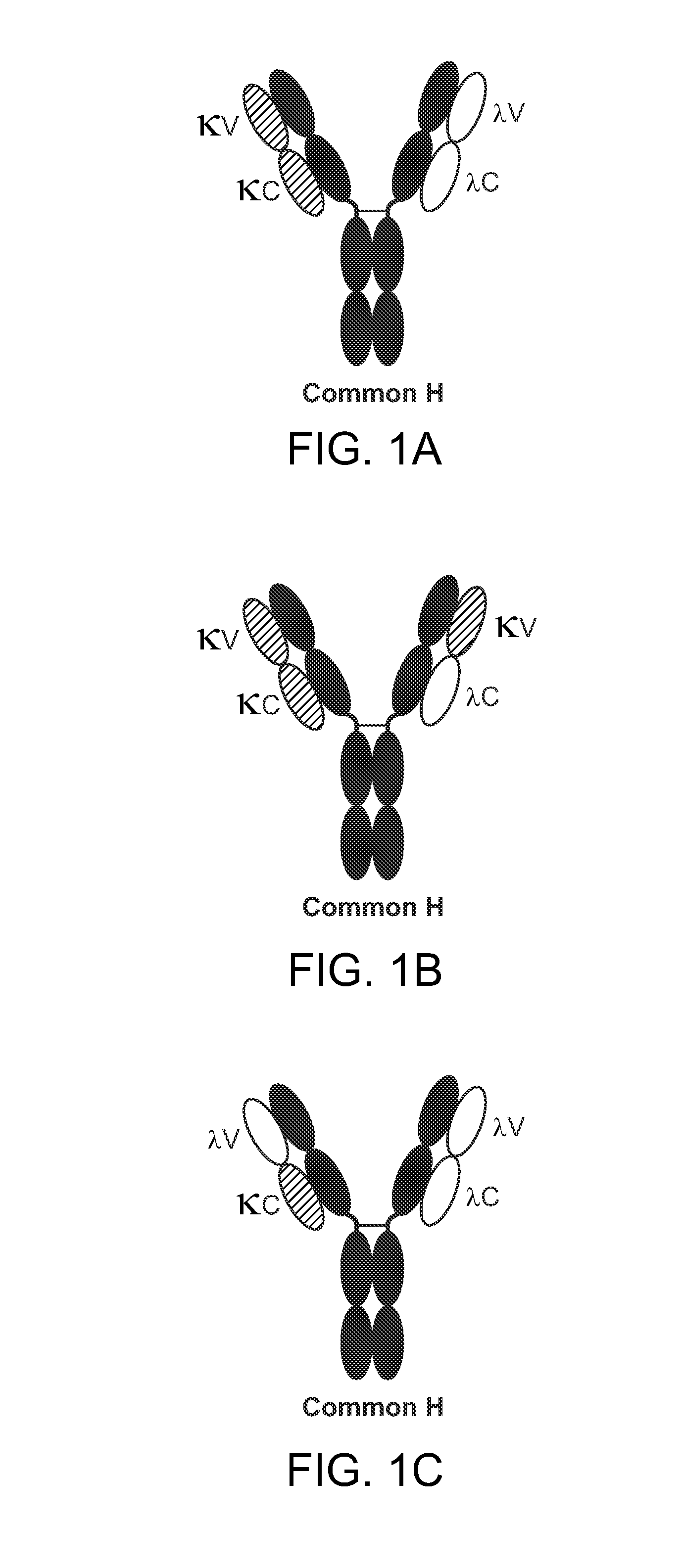
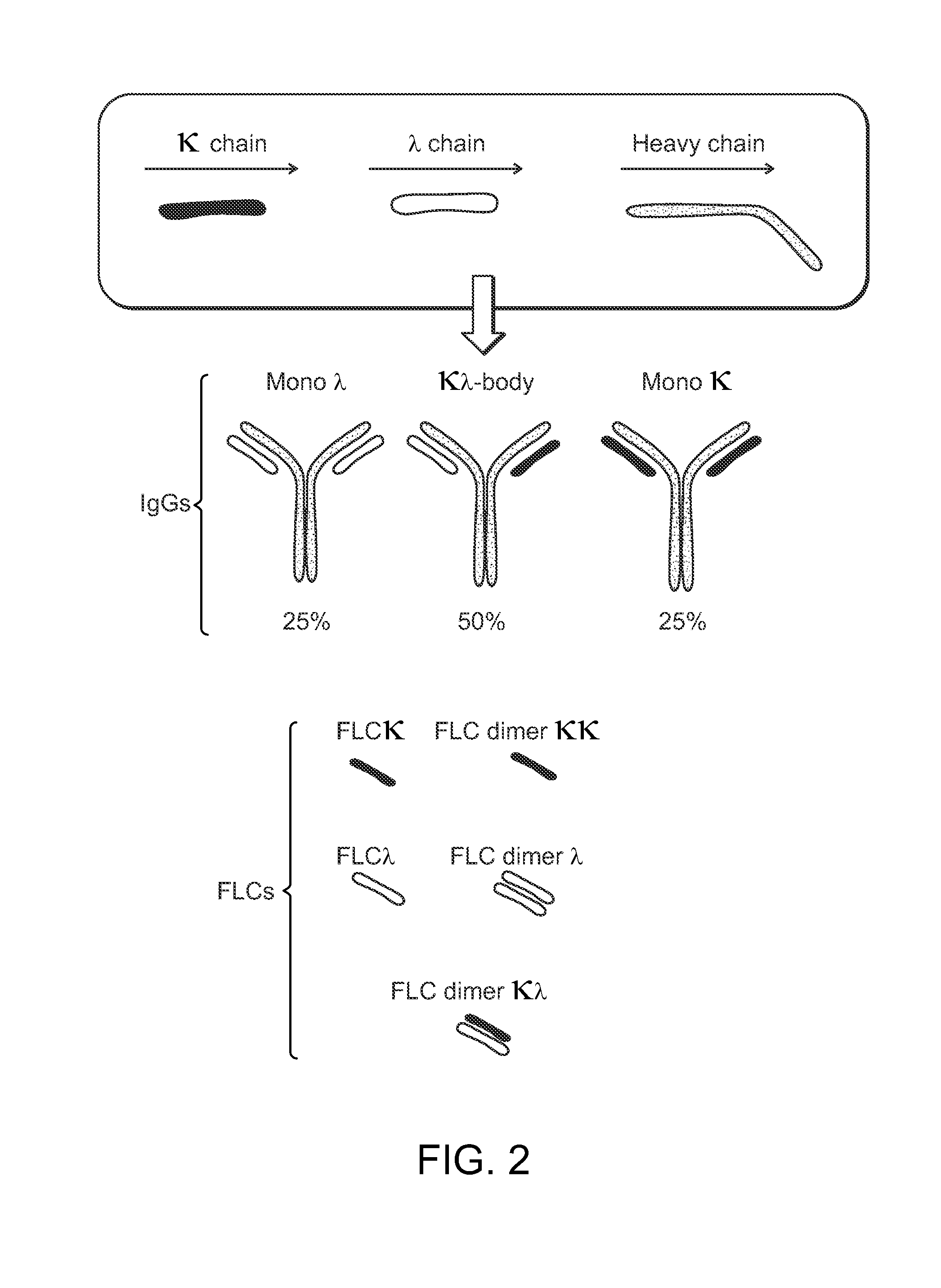
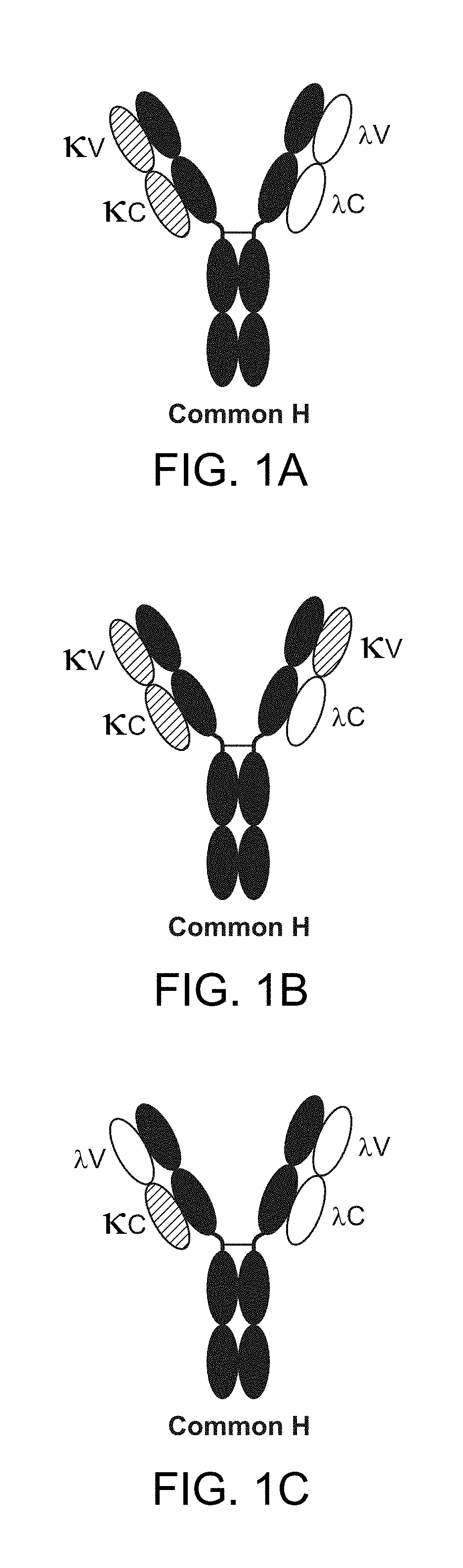
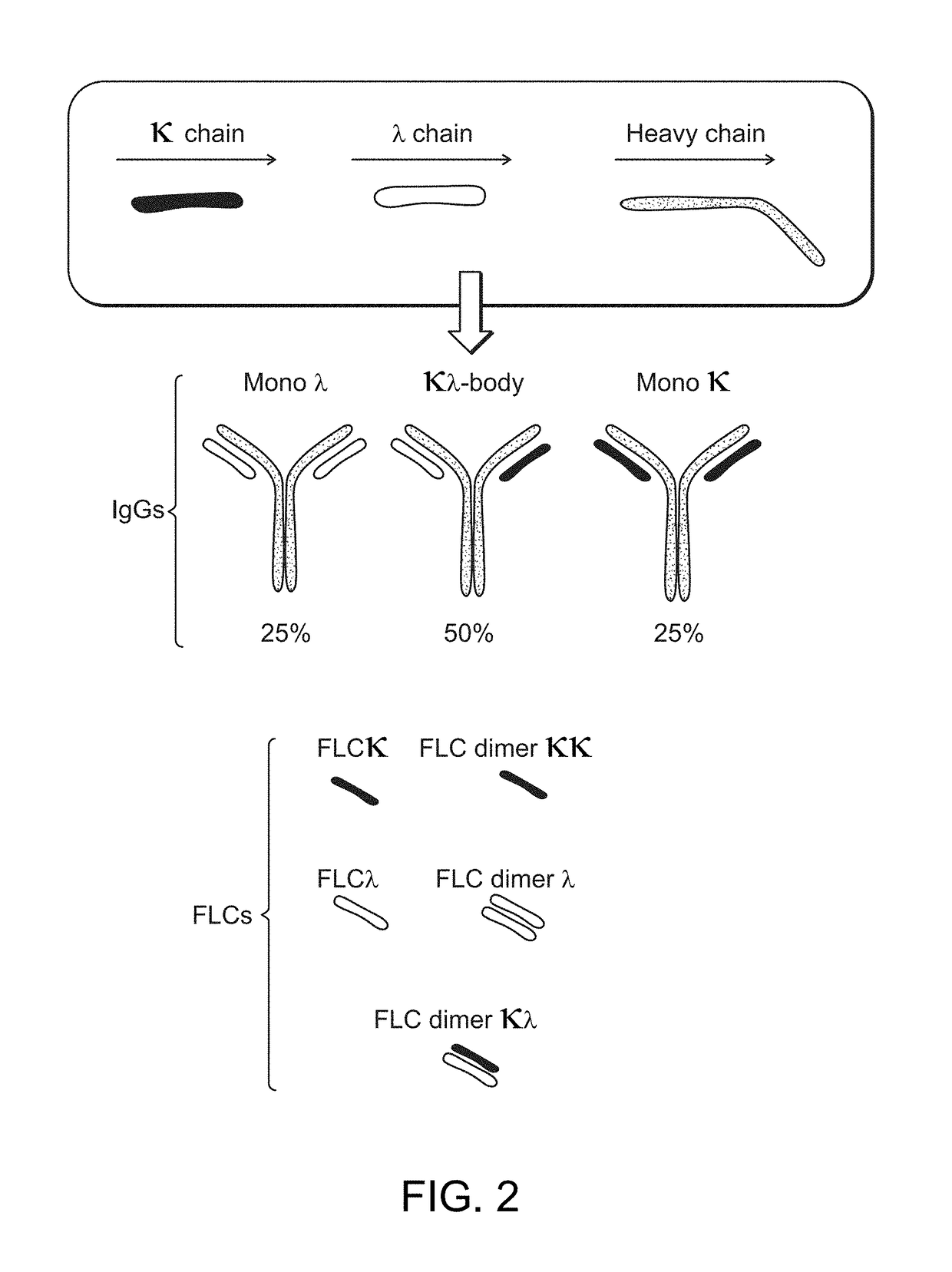
The invention provides methods of purifying antibodies using various antibody-specific purification media to rapidly and efficiently separate mixtures of antibodies, antibody fragments and / or antibody components to isolate a desired antibody product from the mixture. The invention relates to the purification of bispecific monoclonal antibodies carrying a different specificity for each binding site of the immunoglobulin molecule, e.g., antibodies composed of a single heavy chain and two different light chains, one containing a Kappa constant domain and the other a Lambda constant domain, including antibodies of different specificities that share a common heavy chain. The invention also provides the methods of efficiently purifying intact antibodies by separating the intact antibody from non-intact antibodies including free light chains.
Owner:NOVIMMUNE
Anti-human PCSK9 monoclonal antibody
The invention discloses a novel anti-human PCSK9 monoclonal antibody or its fragment prepared by using a bacteriophage antibody library technology for screening and using a gene engineering method. The novel anti-human PCSK9 monoclonal antibody has high affinity to human PCSK9. The antibody can be a full-length antibody, a basically complete antibody, a Fab fragment, a F(ab')2 fragment or a single-chain Fv fragment. The monoclonal antibody or its fragment is used for treating disease mediated by human PCSK9, and the disease comprises, but not limited to, primary hypercholesterolemia, combined hyperlipidemia and familial hypercholesterolemia.
Owner:BEIJING WISDOMAB BIOTECHNOLOGY CO LTD +2
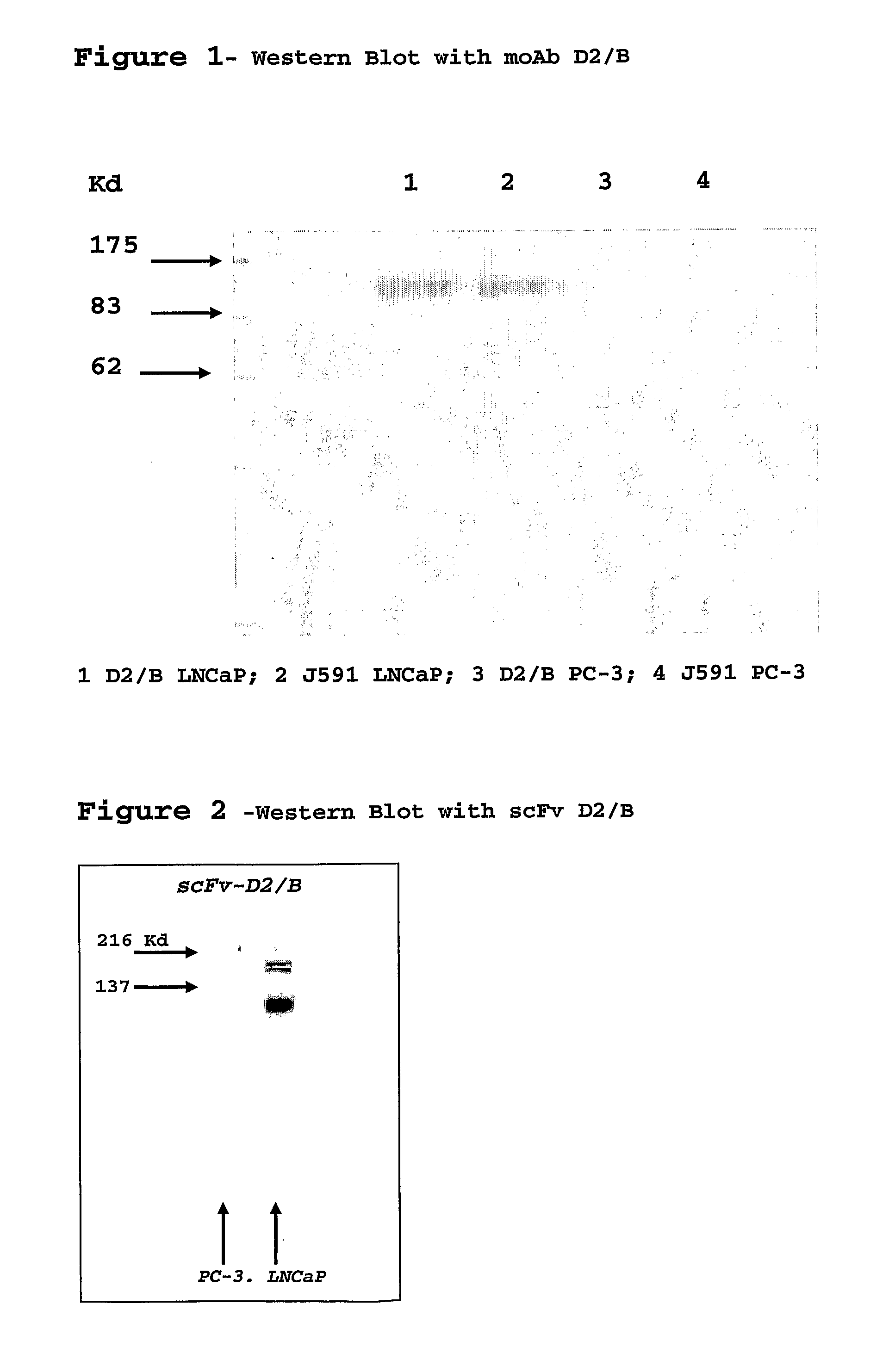
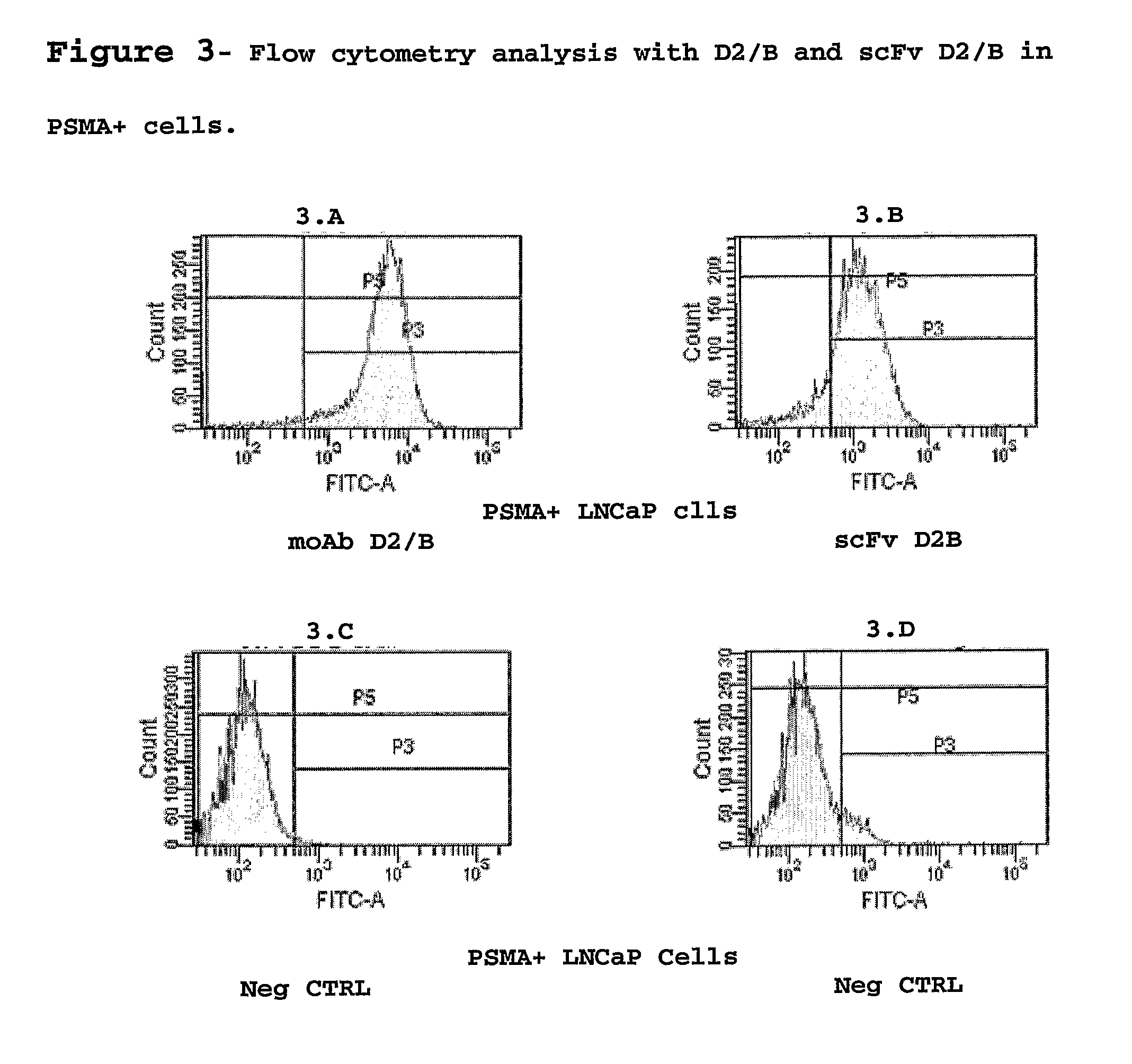
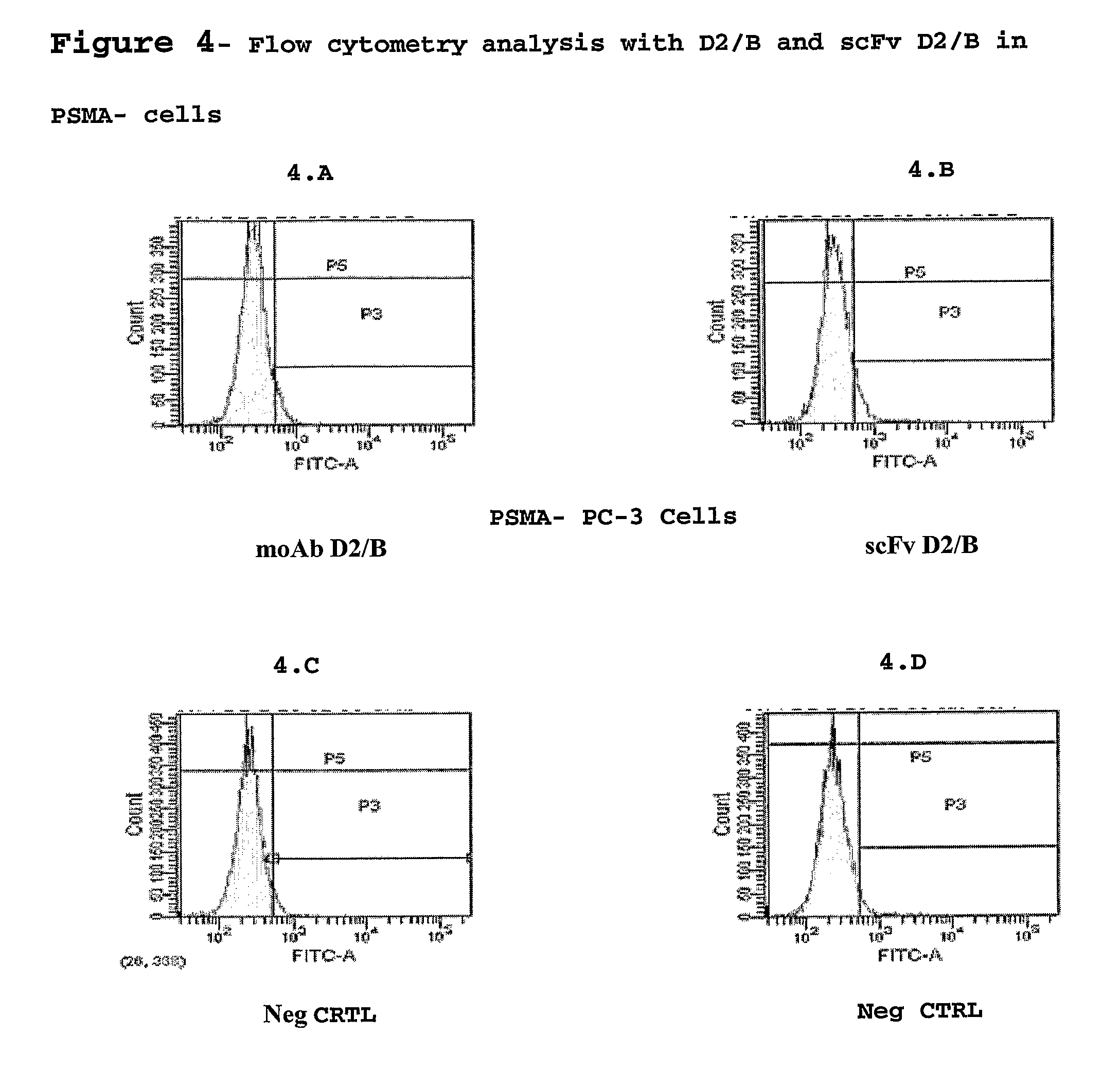
Isolated monoclonal antibody or fragment thereof binding prostate specific membrane antigen, conjugates and uses thereof
ActiveUS8703918B2Ultrasonic/sonic/infrasonic diagnosticsAntibody mimetics/scaffoldsProstate specific membraneAntibody fragments
An isolated monoclonal antibody or fragment thereof binding prostate specific membrane antigen (PSMA) preferably in its native form on the surface of tumour cells. A conjugate of the antibody with an active ingredient and modified forms of the antigen-binding antibody fragment are also provided. The complete antibody and the antigen-recognising fragment thereof are used alone or conjugated for the treatment and the diagnosis of tumours or tissues associated to the tumour overexpressing the PSMA antigen, preferably prostatic neoplastic diseases.
Owner:MARCO COLOMBATTI +2
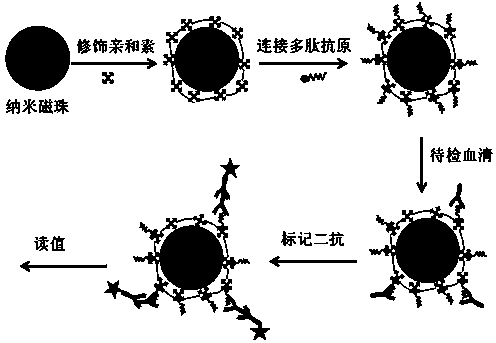
Method for detecting Miltenberger blood group antibody
InactiveCN104360087AAchieving Simultaneous DetectionEnsure safetyBiological testingFluorescenceMagnetic bead
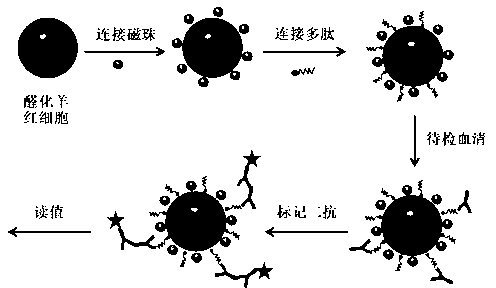
The invention discloses a method for detecting a Miltenberger blood group antibody. The method comprises the steps of coupling nano magnetic beads with avidin to form avidin magnetic beads; coupling the avidin magnetic beads with a multipeptide antigen with a biotin-modified tail end, and closing non-coupled sites to obtain immune magnetic beads; uniformly mixing the immune magnetic beads with a detection sample, then performing incubation and washing, adding fluorescence labeled second antibody or enzyme-labeled second antibody, performing incubation and washing, and measuring a result. In the mode, the method for detecting the Miltenberger blood group antibody based on nano magnetic bead immunoassay is low in operation difficulty and is simple and convenient to operate, can be used for simultaneously detecting complete antibodies and incomplete antibodies; the detection process is short in time, and the result is easy to judge; automation is easily realized, and the detection result is accurate; in combination with a detector, semi-quantitative or quantitative detection can be realized; clinical safe, effective and scientific blood transfusion can be guaranteed.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Bispecific antibodies targeting EGFR and HER2
ActiveCN109563166AHybrid immunoglobulinsPeptide/protein ingredientsAntiendomysial antibodiesCetuximab
The present disclosure relates to bispecific antibodies targeting EGFR and HER2, and methods for the production of these antibodies. The bispecific antibodies consist of one complete antibody on whichtwo VH-VL chains are attached via a linker to each NH terminal region of both VH chains of the antibody. The bispecific antibodies constructed use the amino acid sequences of the heavy chain (VH) andthe light chain (VL) variable regions of two monoclonal antibodies targeting EGFR and HER2, namely cetuximab and trastuzumab, respectively
Owner:BIOMUNEX PHARMA +3
Anti to human alkaline fibroblast growth factor human s c F v antibody and application thereof
ActiveCN102558351AHigh affinityImprove featuresDigestive systemImmunoglobulins against growth factorsSingle-Chain AntibodiesFibrosis
The invention discloses a human single-chain antibody fragment (scFv) antibody of anti-recombination basic fibroblast growth factor and an application thereof. An amino acid sequence of heavy chain variable area of the human scFv antibody is shown in SEQ ID NO. 1, and an amino acid sequence of light chain variable area of the human scFv antibody is shown in SEQ ID NO. 2. A gene sequence encoding the heavy chain variable area is shown in SEQ ID NO. 3. A gene sequence encoding the light chain variable area is shown in SEQ ID NO. 4. The human scFv antibody has the characteristics of high affinity and specificity, and can be directly developed to be used as an antibody drug for human because the human scFv antibody is fully human antibody. The gene encoding the human scFv antibody can be constructed and expressed to obtain various forms of micromolecular genetic engineering antibodies, such as, fragment antigen-binding (Fab) antibody, F(ab)2, single chain antibody, Nanobody, antibody fusion protein, immunoglobulin G (IgG) complete antibody, etc., which can be used for preparing antibody drugs for diagnosing and treating tumor and / or inhibiting viscera fibrosis.
Owner:JINAN UNIVERSITY
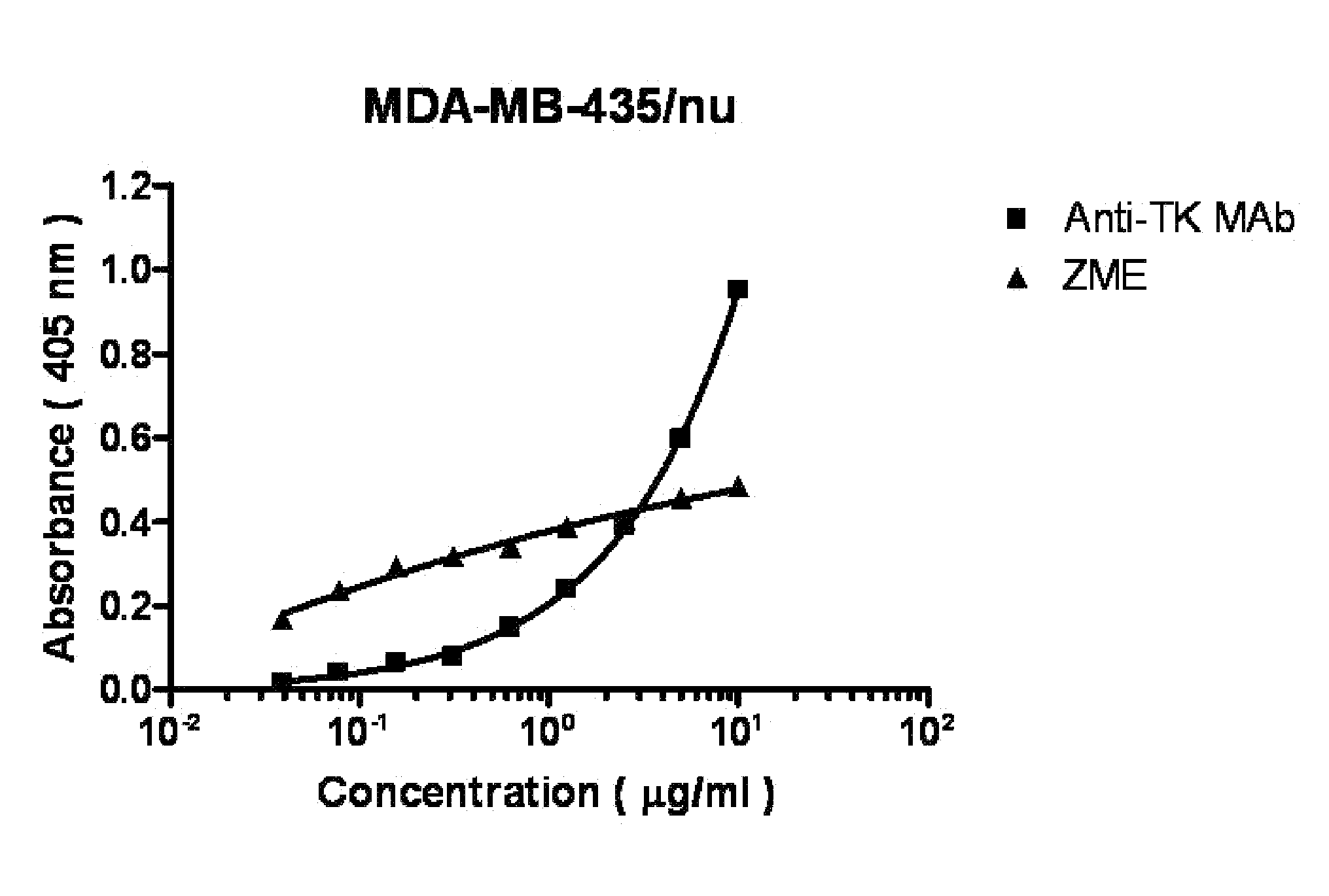
Monoclonal antibodies to human thymidine kinase to treat cancer
InactiveUS20100143290A1Good effectReduce cell proliferationNervous disorderPeptide/protein ingredientsMammalApoptosis
A method of treatment of cancer, viral infections, and the like administers anti-TK1 antibody, constituted as the complete antibody or a fragment thereof. The antibody binds to the surface of cells expressing TK1 thereon. The antibody, with or without another agent bound thereto, may effect complement mediated lysis, antibody-dependent cell-mediated cell cytotoxicity, apoptosis, an immune response by the mammal, a reduction in cellular replication, a combination thereof, or the like for such cells. The antibody may be coupled to an immune response stimulator, a cytotoxin, an enzyme, a combination, or the like to effect the treatment desired.
Owner:LALLATIN NATHANIEL C
Novel Miltenberger blood group antibody detecting method
ActiveCN103344772ASolve the problem of shortage of sourcesSolve Simultaneous DetectionBiological testingFluorescenceMagnetization
The invention discloses a non-blood red cell dependent type Miltenberger blood group antibody detecting method. The detecting method comprises the following steps of: coupling sheep red blood cell subjected to hydroformylation and magnetization with polypeptide antigen containing a connecting chain to form an artificial screening cell; and uniformly mixing the artificial screening cell with a detecting sample for cultivating and washing, and measuring the result after adding fluorescence-labeled secondary antibody for cultivating and washing. By virtue of the way, the non-blood red cell dependent type Miltenberger blood group antibody detecting method provided by the invention is free of centrifuging for the washing due to the application of magnetic force, simple and convenient to operate, capable of realizing simultaneously detecting complete antibody and incomplete antibody, short in detecting process time consumption, easy to automate, accurate in detected result and capable of realizing semi-quantitative or quantitative detection by combining with a detecting apparatus, and the result is easy to judge. Besides, the detecting method can guarantee safe, efficient and scientific blood transfusion in clinic.
Owner:SUZHOU GUOKE MEDICAL TECH DEV CO LTD
Anti-cancer surface antigen of p185 monoantigen embedded antibody and its preparation
InactiveCN1613873APrevention is cheapMedicinal safe and effectiveImmunoglobulins against animals/humansFermentationSingle-Chain AntibodiesWilms' tumor
This invention relates to a mono-chain antibody-humanscFv-Fc embedded antibody and its preparation, specialized by hybrid tumor cell strain prepared by surface embedded immune method to transfect mammary animal cell CHO with GS low expression in form of mono-chain embedded antibody gene with pEE14 as recombination antibody carrier to screen CHO cell strains of recombination antibody. The embedded antibody molecule consists of two similar recombined mono-chains, of which each has an antigen combining region connected with heavy and light chains to form antibody to identify antigen. It is smaller a third than a complete antibody due to constant regions CHI structure zones of light and heavy chains removed, so that it is easier to penetrate into tumor and is not easy to be degraded like protein peptide too soon.
Owner:HEFEI HANKEMAB BIOTECH CO LTD
CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) nanoantibody and preparation method and application thereof
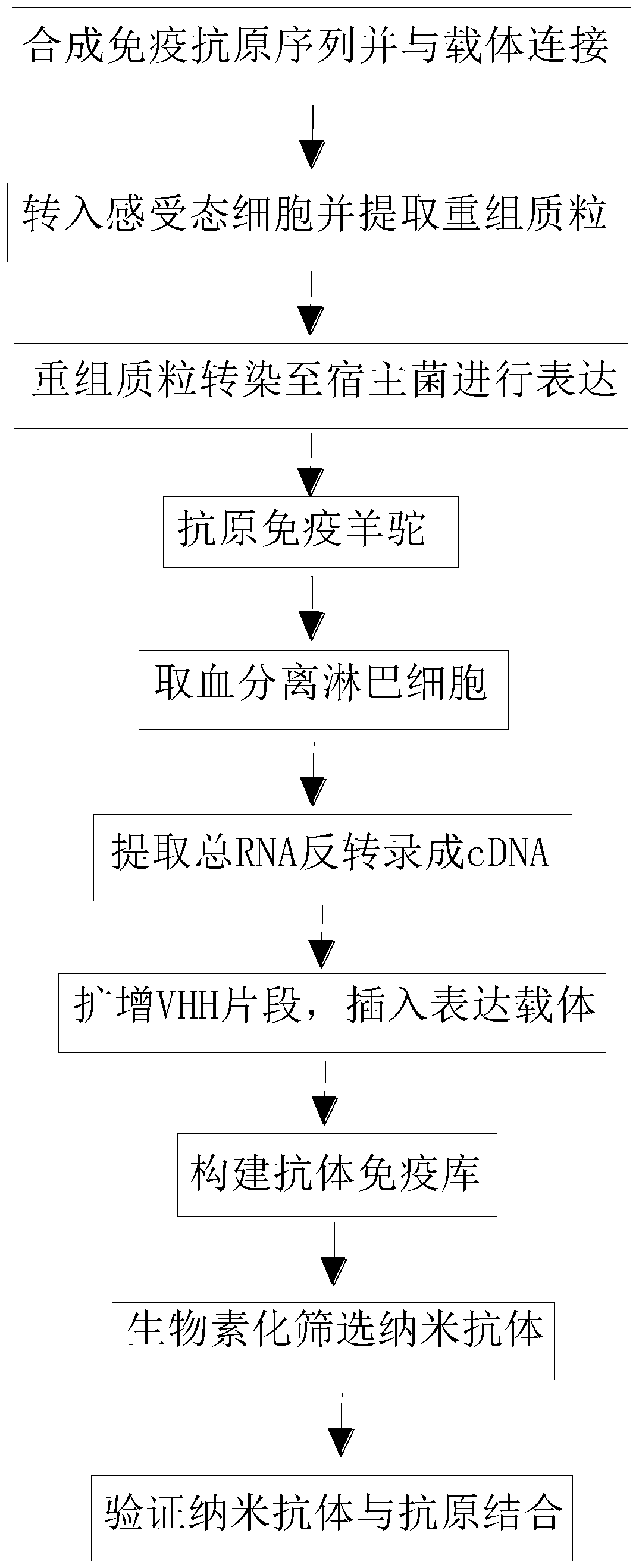
InactiveCN110256563AStable structureDifficult to identifyMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCompetent cellTotal rna
The invention belongs to the technical field of biomedicine and particularly relates to a CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) nanoantibody and a preparation method and application thereof. The ability to bind with antigens can be improved or collaboratively enhanced through the CTLA-4 antibody. The CTLA-4 antibody has no complete antibody structure and lacks Fc end and Y-shaped structure; the antigens bound to the CTLA-4 antibody are not easily recognizable and can escape easily from being caught by the immune system. The technical means includes mainly: carrying out antigen-vector connecting; transferring to competent cells, and extracting recombinant plasmids; transfecting to host bacteria for expressing; immunizing camels with an antigen protein; collecting peripheral blood, extracting total RNA of a lymphocyte sample, inversely transcribing the total RNA into cDNA, performing amplifying to obtain a VHH library fragment, inserting the VHH library fragment into an expression vector, and transferring to the competent cells to construct an antibody immunization library; screening nanoantibodies in the antibody immunization library; verifying binding of the screened nanoantibodies with a CTLA-4 antigen.
Owner:SHIHEZI UNIVERSITY
PD-1 nanoantibody as well as cloning and expression method and application thereof
ActiveCN108299561ASmall molecular weightStrong specificityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPD-L1T cell
The invention discloses a PD-1 nanoantibody as well as a cloning and expression method and an application thereof. The PD-1 nanoantibody has small molecular weight, high specificity and high affinityand can inhibit the interaction between PD-L1 and PD-1 molecules, specifically recognize cell surface natural conformation PD-1 and block combination of PD-L1 and PD-1 to keep activity of T cells; thePD-1 nanoantibody has the combining capacity the same as a PD-1 monoclonal antibody (IgG complete antibody); the PD-1 nanoantibody can reverse and activate the PD-L1 inhibited state of T cells. Meanwhile, compared with the PD-1 monoclonal antibody, the nano antibody has higher relative activity and better thermal stability and has the potential of being developed into an immune checkpoint inhibitor after the same high-temperature treatment. Besides, the cloning and expression method of the nanoantibody has higher yield, the obtained protein purity can reach 93% or above, and the preparation method is simple and low in cost and has good application prospects in preparation of anti-tumor drugs.
Owner:JINAN UNIVERSITY
Methods of purifying antibodies
ActiveUS10047144B2Favorable manufacturing characteristicHybrid immunoglobulinsImmunoglobulins against cytokines/lymphokines/interferonsHeavy chainBispecific monoclonal antibody
The invention provides methods of purifying antibodies using various antibody-specific purification media to rapidly and efficiently separate mixtures of antibodies, antibody fragments and / or antibody components to isolate a desired antibody product from the mixture. The invention relates to the purification of bispecific monoclonal antibodies carrying a different specificity for each binding site of the immunoglobulin molecule, e.g., antibodies composed of a single heavy chain and two different light chains, one containing a Kappa constant domain and the other a Lambda constant domain, including antibodies of different specificities that share a common heavy chain. The invention also provides the methods of efficiently purifying intact antibodies by separating the intact antibody from non-intact antibodies including free light chains.
Owner:NOVIMMUNE
Monoclonal antibodies to human thymidine kinase to treat cancer
ActiveUS20100143244A1Good effectReduce cell proliferationUltrasonic/sonic/infrasonic diagnosticsSurgeryApoptosisComplete antibody
A method of treatment of cancer, viral infections, and the like administers anti-TK1 antibody, constituted as the complete antibody or a fragment thereof. The antibody binds to the surface of cells expressing TK1 thereon. The antibody, with or without another agent bound thereto, may effect complement mediated lysis, antibody-dependent cell-mediated cell cytotoxicity, apoptosis, an immune response by the mammal, a reduction in cellular replication, a combination thereof, or the like for such cells. The antibody may be coupled to an immune response stimulator, a cytotoxin, an enzyme, a combination, or the like to effect the treatment desired.
Owner:SAVOY PHARMA
Anti-phosphatidylinositol proteoglycan 3 complete humanized antibody
InactiveCN105037540AImmunoglobulins against animals/humansAntibody ingredientsPhage antibodiesAntigen
The invention discloses a complete humanized antibody which is selected from humanized high-capacity phage antibody library and is high-affinity-combined with phosphatidylinositol proteoglycan 3. The invention includes a selection method of the antibody, an antibody coding sequence and corresponding amino acid residue sequence, and especially includes three CDR-zone sequences respectively at a heavy chain and a light chain, combination characters of an antigen and the construction method of the complete antibody. The antibody is a complete humanized antibody, is used for treatment in human body, is low in immunogenicity, is less in toxic and side effects, and has a potential value of treating liver cancer and melanin tumor.
Owner:BEIJING BIYANG BIOTECH
Recombinant Kluyveromyces sp. expressing antibody or antibody analogue, and construction method and use thereof
InactiveCN101413002AExtended half-lifePromote phagocytosisFungiMicroorganism based processesYeastHeavy chain
The invention discloses a recombinant yeast Kluyveromyces for expressing antibodies or antibody analogues and application thereof. The invention provides a method for constructing the recombinant yeast Kluyveromyces for expressign antibodies or antibody analogues. The method comprises the following steps: encoding genes of the antibodies or the antibody analogues are guided into the yeast Kluyveromyces to obtain recombinant bacteria; the antibody analogues are fusion proteins formed by Fc fragments of the antibodies and proteins or protein subunits; the encoding genes of the antibodies are heavy chain and light chain encoding genes of the antibodies. The recombinant yeast Kluyveromyces for expressing antibodies or antibody analogues can be cultured to produce complete antibodies or antibody analogues. The invention uses the yeast Kluyveromyces to prepare the antibodies or antibody analogues to preliminarily overcome the difficulties of difficult antibody production, high purity and large quantity requirements for products and provide good application prospects.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Neutralizing monoclonal antibody resisting to tetanus toxin and application thereof
ActiveCN105542004AStrong specificityProlonged deathAntibacterial agentsImmunoglobulins against bacteriaLethal doseComplete antibody
The invention discloses a neutralizing monoclonal antibody resisting to tetanus toxin. Hybridoma cells are obtained from tetanus toxin heavy chain C fragment immune mice, light chain and heavy chain variable region genes of the antibody are taken, human-mouse chimeric complete antibody expression vectors are built, CHO cell transfection is carried out, purification is carried out, and then the antibody is obtained. The antibody has the activity of specific binding to tetanus toxin, the monoclonal antibody can partially protect mice against attack of tetanus toxin, and four antibodies can completely protect mice against a twofold lethal dose of tetanus toxin in combination.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Anti-cancer surface antigen of p185 mono-antigen mosaic antibody and its preparation
InactiveCN1238381CMedicinal safe and effectiveReduce workloadImmunoglobulins against animals/humansFermentationSingle-Chain AntibodiesWilms' tumor
This invention relates to a mono-chain antibody-humanscFv-Fc embedded antibody and its preparation, specialized by hybrid tumor cell strain prepared by surface embedded immune method to transfect mammary animal cell CHO with GS low expression in form of mono-chain embedded antibody gene with pEE14 as recombination antibody carrier to screen CHO cell strains of recombination antibody. The embedded antibody molecule consists of two similar recombined mono-chains, of which each has an antigen combining region connected with heavy and light chains to form antibody to identify antigen. It is smaller a third than a complete antibody due to constant regions CHI structure zones of light and heavy chains removed, so that it is easier to penetrate into tumor and is not easy to be degraded like protein peptide too soon.
Owner:HEFEI HANKEMAB BIOTECH CO LTD
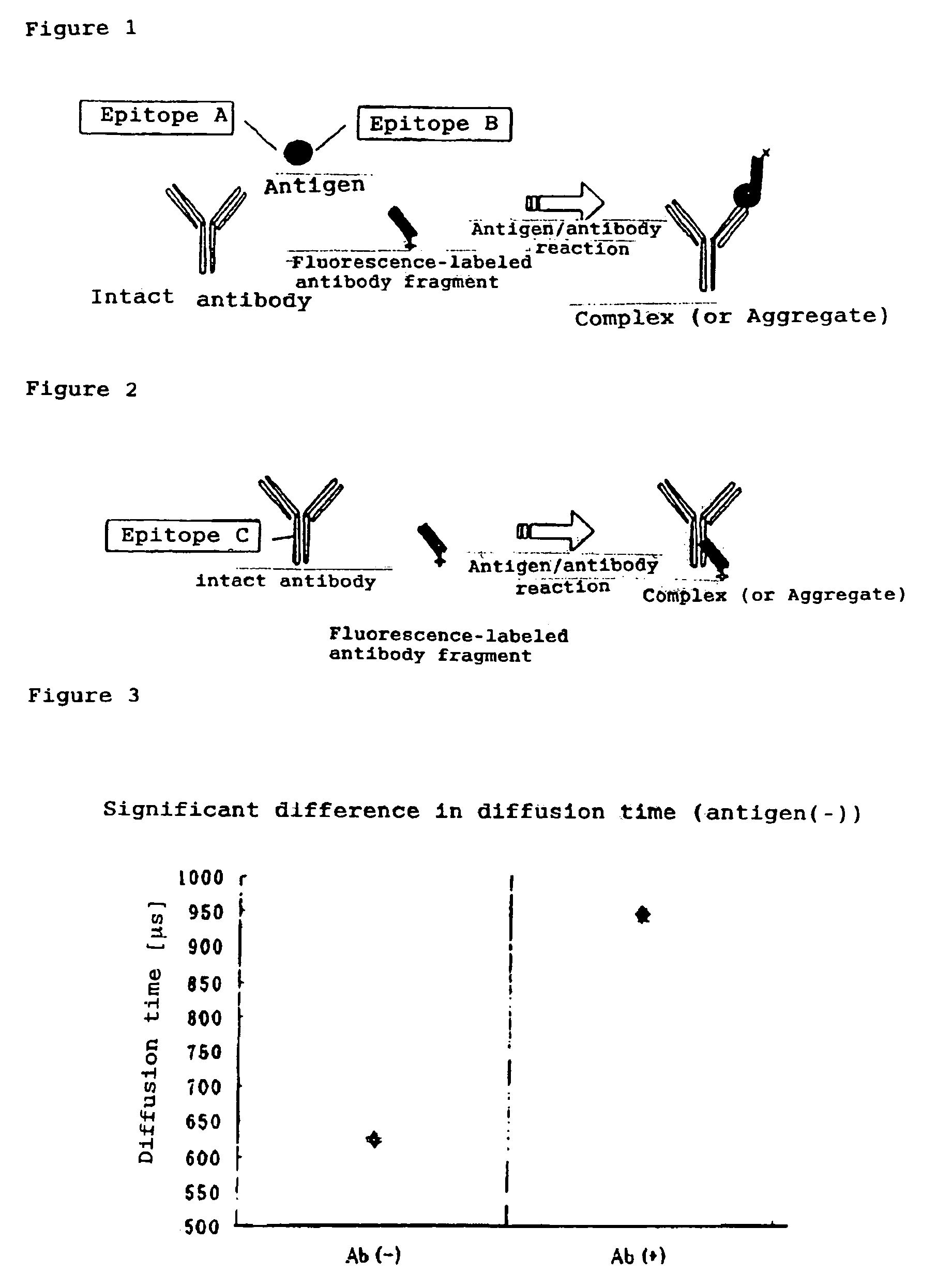
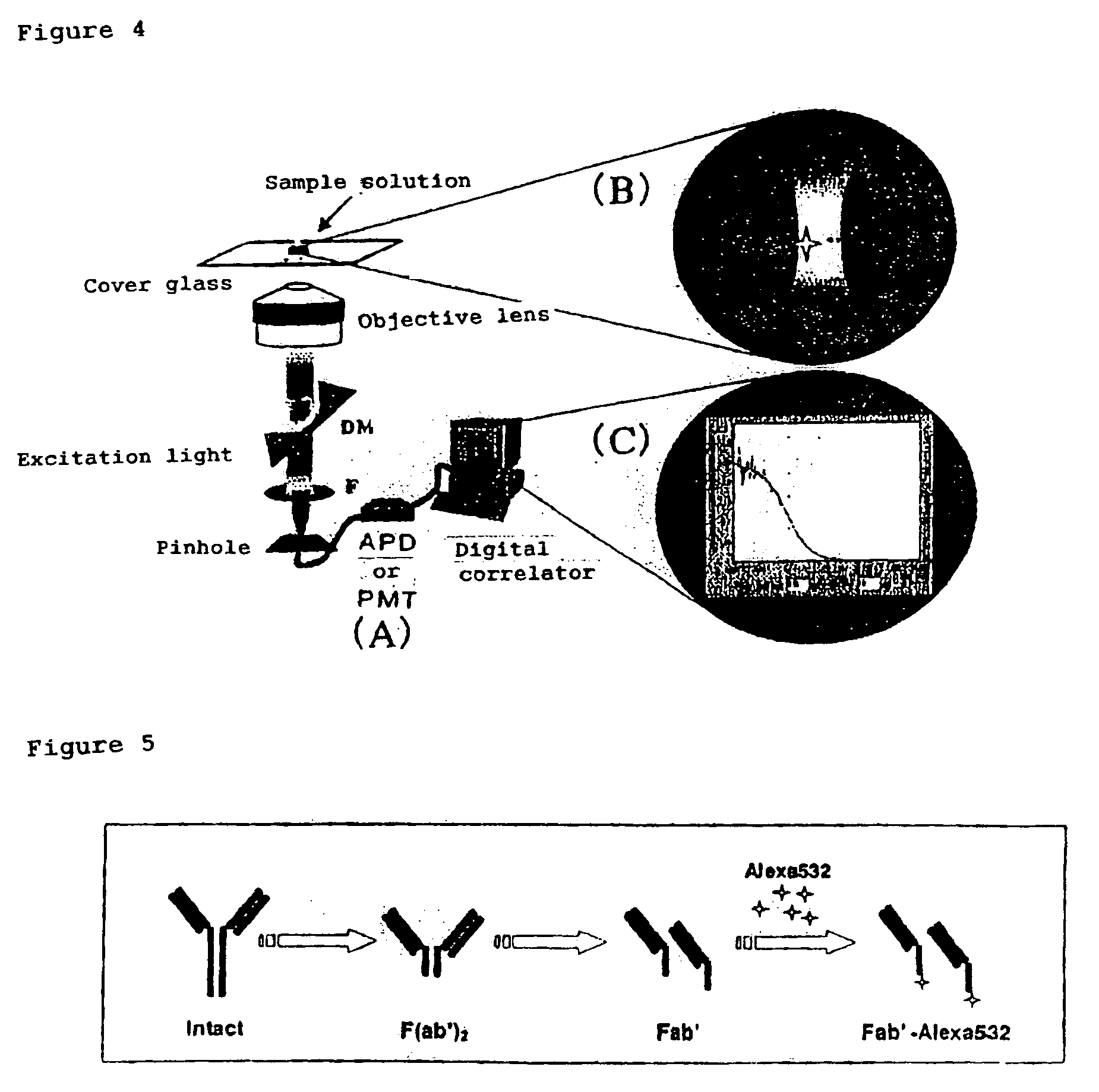
Method of quickly detecting and/or assaying antigen by fluorescence correlation spectrometry
InactiveUS7476545B2Quick and convenient methodAvoid narrow scopeMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceAntibody fragmentsAntigen binding
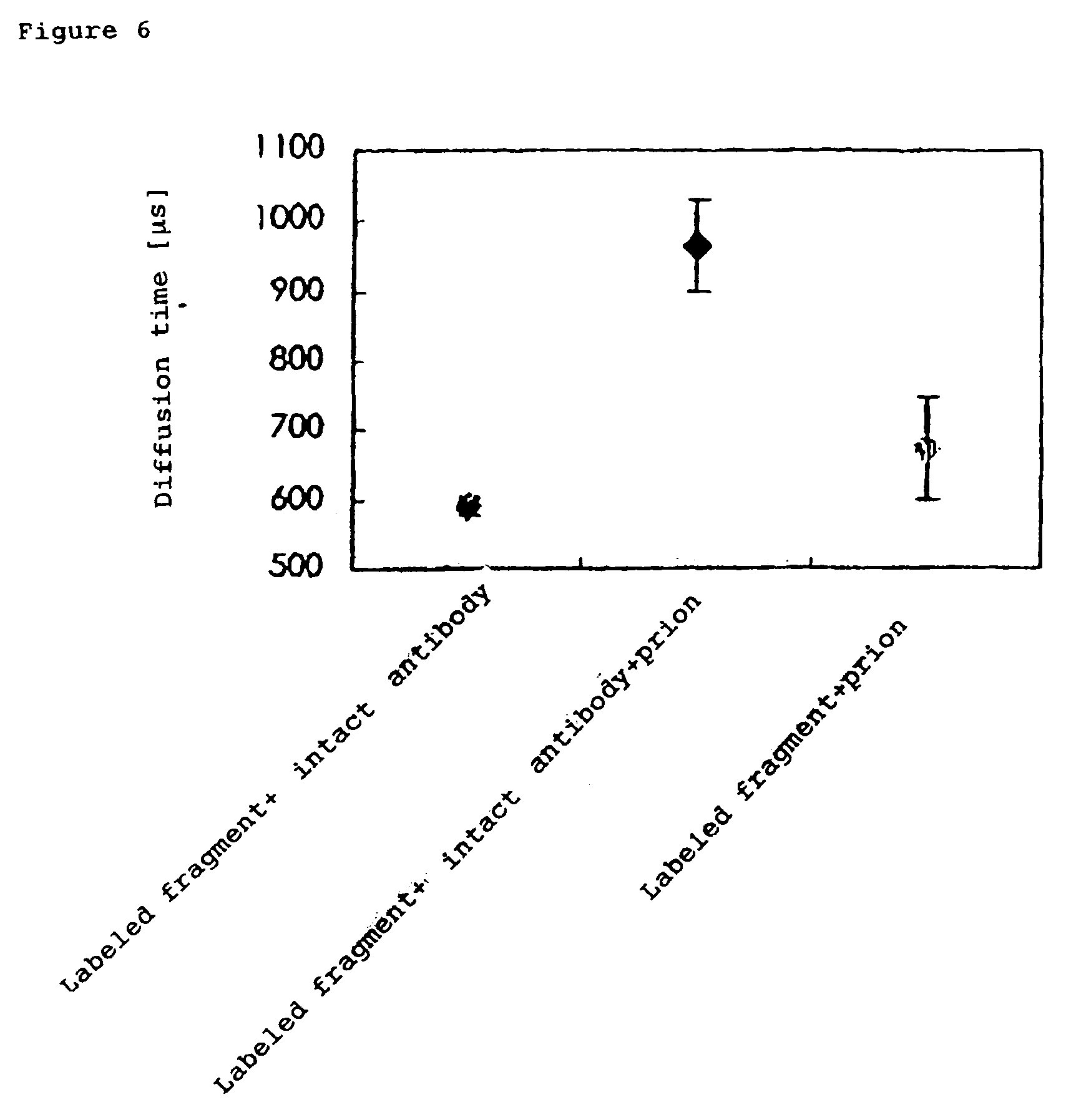
The present invention provides a method of quickly and accurately detecting and / or assaying an antigen using fluorescence correlation spectroscopy (FCS), which involves a fluorescence-labeled antibody fragment and a non-fluorescence-labeled intact antibody that form a complex with the antigen. There is a significant difference in diffusion rate between the fluorescence-labeled antibody fragment not bonded to the antigen and the complex formed by the the fluorescence-labeled antibody fragment, the antigen, and the non-fluorescence-labeled intact antibody, and this diffusion rate can be determined using FCS. The antigen can be an antigenic protein, such as an abnormal prion or a harmful protein contained in a food material. According to this method, antigens over a wide scope can be assayed regardless of the shape or molecular weight.
Owner:JAPAN SCI & TECH CORP
Pharmaceutical composition of F(ab')2 antibody fragments
InactiveUS7485303B2Serum immunoglobulinsImmunoglobulins against cytokines/lymphokines/interferonsSerum igeMammal
The present invention is directed to a pharmaceutical composition comprising F(ab′)2 antibody fragments that are preferably free from albumin and of whole antibodies and also substantially free of pyrogens, and an effective amount of a pharmaceutically acceptable carrier. It is also directed to a method for the production of a pharmaceutical composition comprising F(ab′)2 antibody fragments using serum or blood plasma of a mammal that has been previously immunized as a source of antibodies. The serum or blood plasma is digested with an enzyme pepsin, followed by separation and purification until the pharmaceutical composition of F(ab′)2 fragments is free of albumin and complete antibodies, and substantially free of pyrogens.
Owner:INST BIOCLON DE C V
Preparing method and medical application of optimized plant source recombination humanized bevacizumab
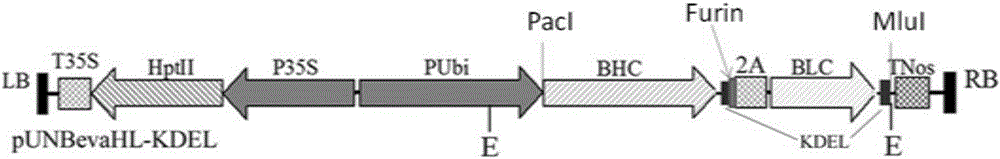
InactiveCN106222194AIncrease productionReduce plant-specific glycosylationVaccinesBiological material analysisPlant SourcesLung cancer
The invention belongs to the technical field of biological medicine, and particularly relates to a preparing method and medical application of optimized plant source recombination humanized bevacizumab. Heavy-chain and light-chain fusion protein of the recombinant antibody bevacizumab is expressed in plants, heavy chain and light chain are expressed in an proportion appropriate to 1:1 by adding 2A sequence to fusion protein, the proportion promotes assembly of a complete antibody obviously, and results show that the yield of antibodies expressed with the system is high. Meanwhile, due to the fact that a stable tetramer structure is formed through equal-proportion assembly of the heavy chain and light chain of the recombinant antibody, the number of unassembled polypeptides easy to degrade is reduced, and then pure and consistent monoclonal antibodies are obtained. The bevacizumab developed with a new method is applied to medicine to be used for treating breast cancer, lung cancer, spongioblastoma, kidney cancer, cervix uterus cancer, ovarian cancer, colon cancer and rectal cancer.
Owner:深圳麦客思鱼生物科技发展有限公司
Selection of human monoclonal antibodies by mammalian cell display
InactiveUS20100292089A1Improve screening efficiencyLibrary screeningImmunoglobulins against virusesFab FragmentsSingle-Chain Antibodies
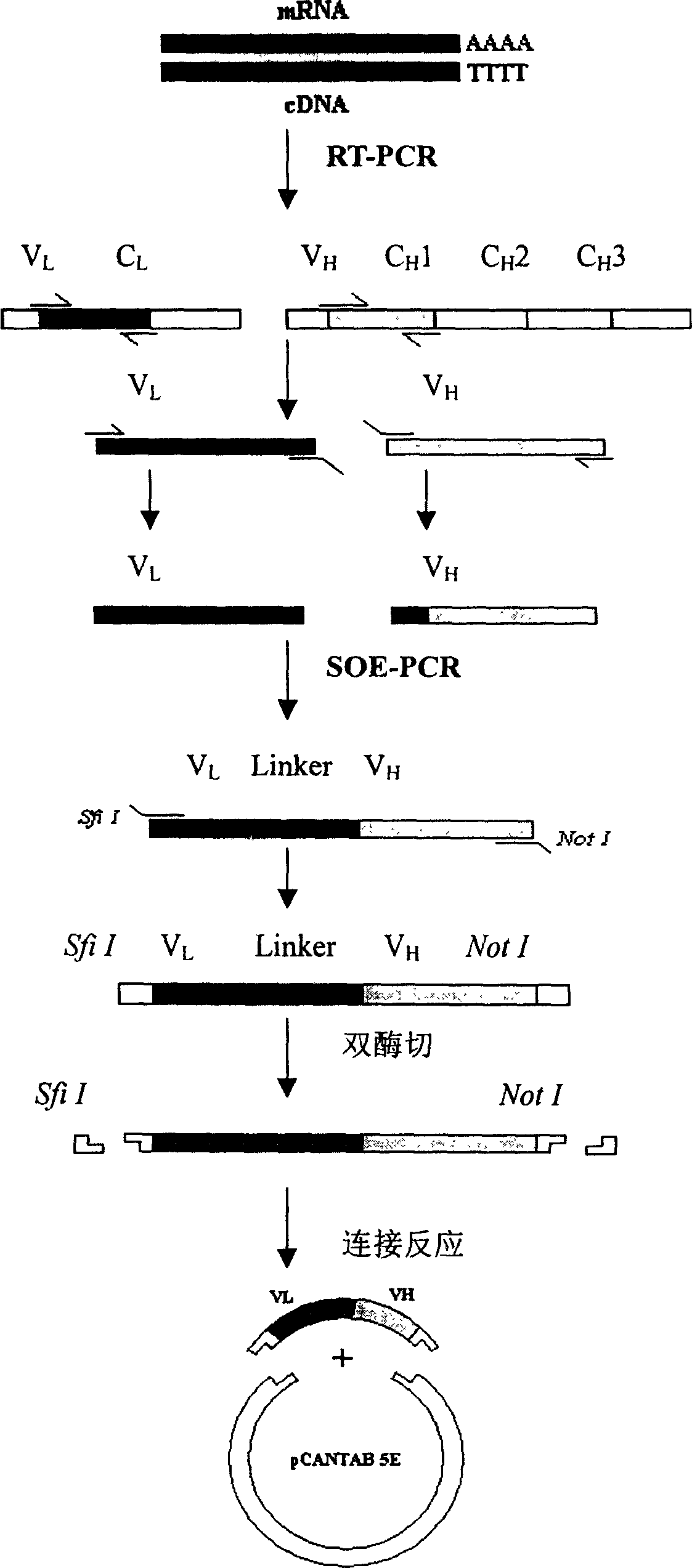
The application provides a method of isolating a eukaryotic cell expressing an antibody of desired specificity, preferably a monoclonal single chain antibody (scFv). The application further provides methods which allow to clone the variable regions of said antibody from that isolated eukaryotic cell and to recombinantly produce antibodies comprising said variable regions as fusion protein with a purification tag, eg. as Fc-fusion, as a Fab fragment, or as whole antibodies, such as IgG, IgE, IgD, IgA and IgM. Said methods also allows to recombinantly produce antibodies with desired specificity in a fully species specific form, preferably as fully human antibodies.
Owner:ELATOS GMBH
Method for detecting residual quantity of Pichia pastoris host protein in recombinant human lysozyme
ActiveCN109799335ALow cross-reactivityNo cross reactionMaterial analysisPichia pastorisTotal protein
The invention discloses a method for detecting residual quantity of Pichia pastoris host protein in a recombinant human lysozyme raw material. The method includes the steps of preparing recombinant human lysozyme; preparing Pichia pastoris host protein; detecting total protein content of the Pichia pastoris host protein; preparing antiserum of the Pichia pastoris host protein; eliminating a crossreaction between the recombinant human lysozyme and immune rabbit serum of the Pichia pastoris host protein and immune sheep serum of the Pichia pastoris host protein; preforming two-dimensional electrophoresis on the Pichia pastoris host protein; detecting antibody coverage rate of the Pichia pastoris host protein; and preforming enzyme-linked immunosorbent assay. By adopting the detection method, the Pichia pastoris host protein in the recombinant human lysozyme is continuously detected for 3 batches, and the content of the Pichia pastoris host protein is all less than 0.05%, and meets the standard of less than 0.1% specified in the Pharmacopoeia of the People's Republic of China. The detection method has the advantages of strong specificity, complete antibody coverage rate, no cross reaction and the like, and can be used for detecting the content of the Pichia pastoris host protein.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Anti-HER2 dual-targeting antibodies, and preparation method and application thereof
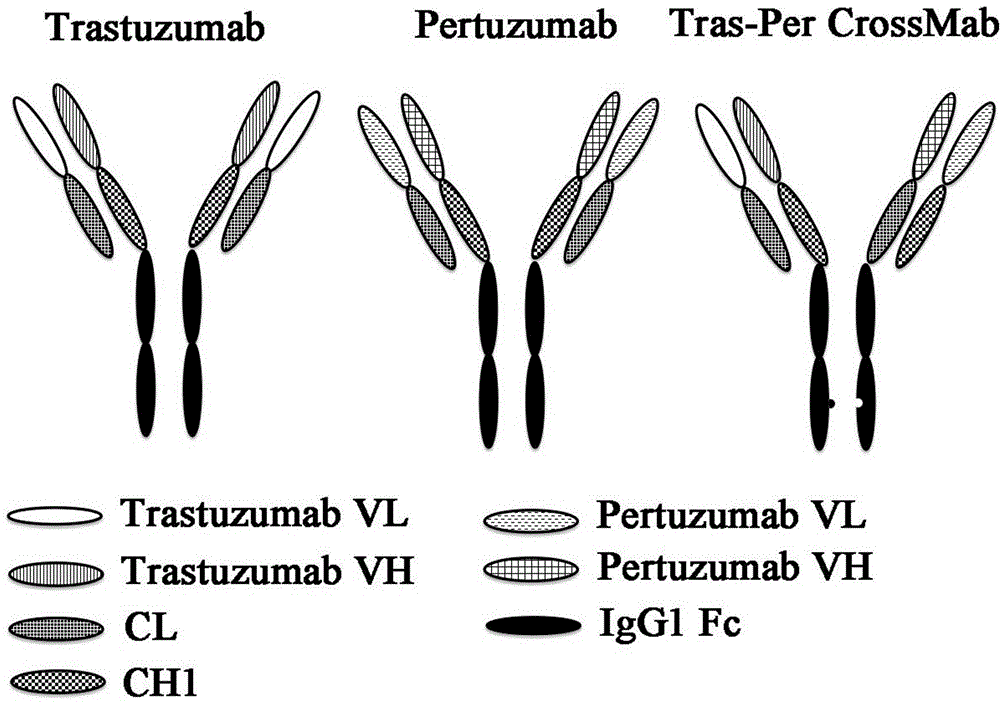
InactiveCN104610453AInhibit synthesisHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAmino acid
The invention discloses anti-HER2 dual-targeting antibodies, a preparation method thereof and application of the anti-HER2 dual-targeting antibodies in the preparation of antibody tumor drugs. More specifically, the invention discloses the dual-targeting antibodies Tras-Per CrossMab and Tras-Permut CrossMab with structures and functions similar to the structures and functions of complete antibodies, and amino acid sequences and the preparation method thereof, and application of the dual-targeting antibodies in the preparation of antitumor drugs. According to the invention, the functions similar to the functions of the complete antibodies are retained; HER2 target protein is dually targeted; and antitumor activity of the anti-HER2 dual-targeting antibodies is better than the antitumor activity of single-targeting anti-HER2 antibodies-Trastuzumab and Pertuzumab.
Owner:张帆
Serum-free culture medium for protecting disulfide bond integrity of antibody in animal cell culture process
ActiveCN108690825AClear compositionReduce pollution sourcesAnimal cellsCulture processSerum igeComplete antibody
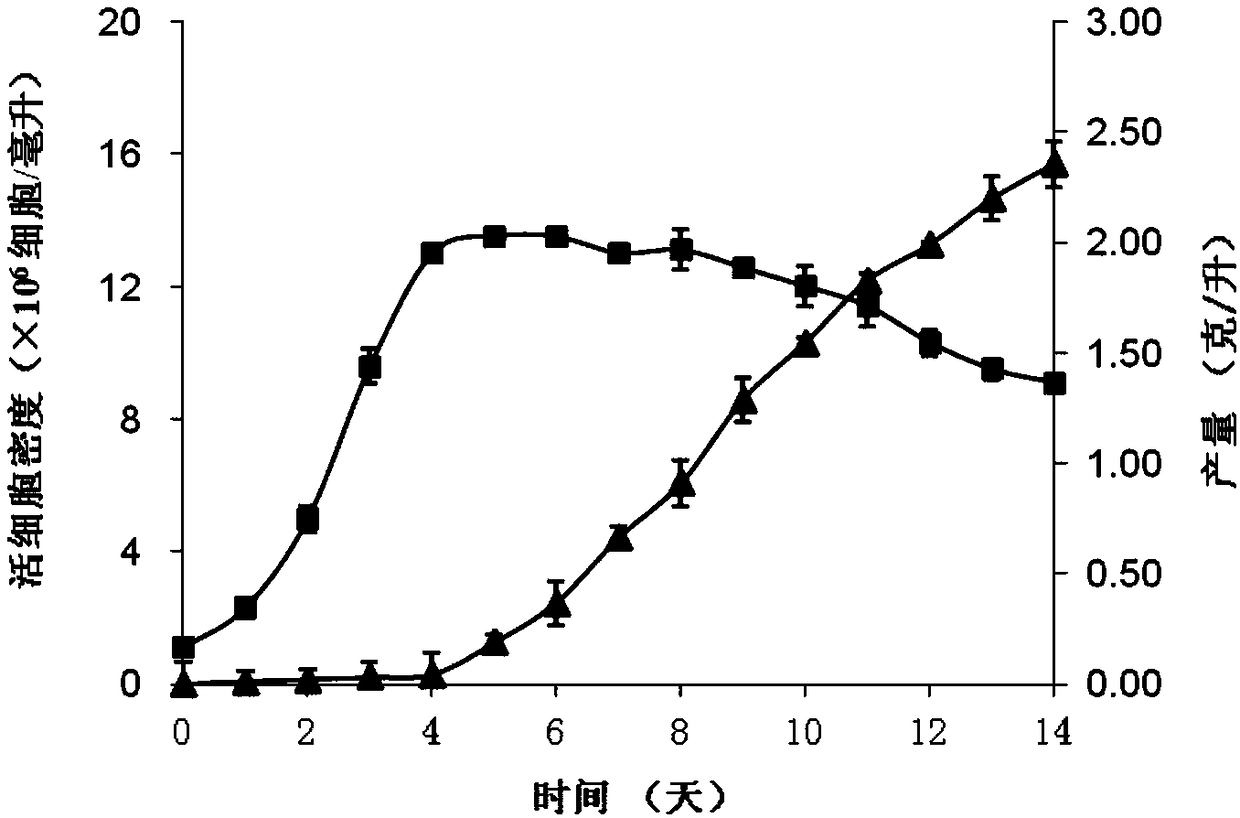
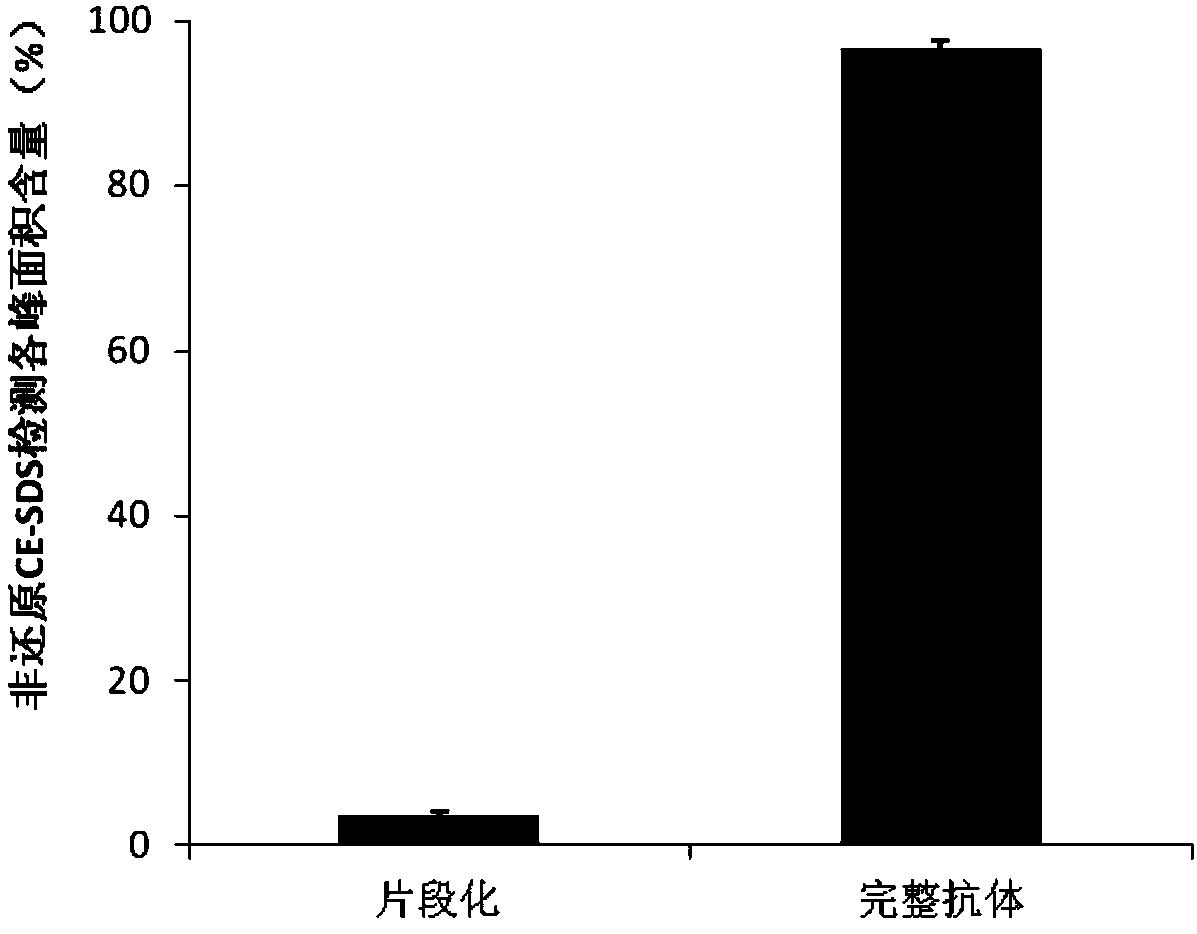
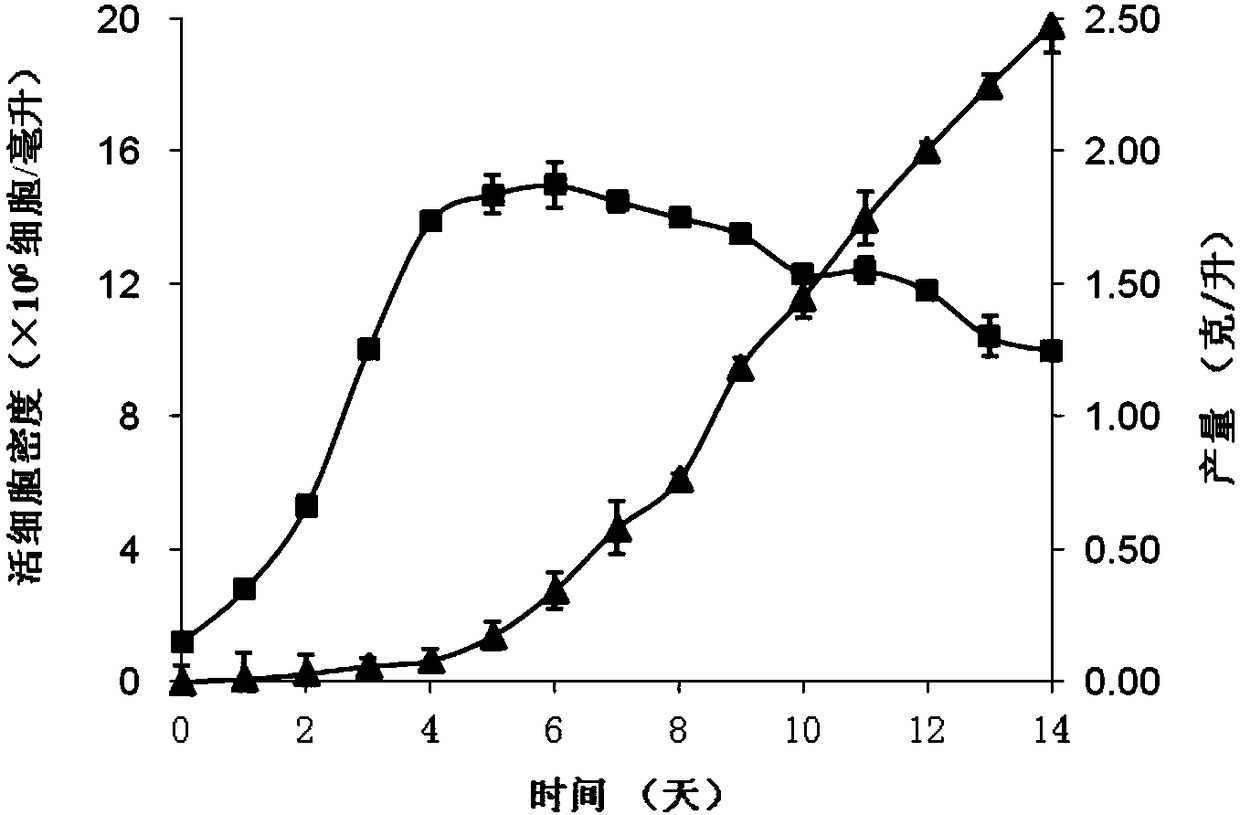
The present invention relates to a serum-free culture medium for protecting the disulfide bond integrity of an antibody in an animal cell culture process, wherein specifically the culture medium comprises a reducing substance, a neutral substance and an oxidizing substance. According to the present invention, the culture medium can support the rapid growth of cells and the high expression of antibodies in animal cell culture processes; and by designing the type and the concentration of reducing substance in the culture medium, the disulfide bond integrity of antibodies can be well protected, and the content of the finally obtained complete antibody is higher than 95%, such that the effectiveness and the safety of antibody production based on animal cell culture processes can be ensured.
Owner:上海倍谙基生物科技有限公司
PD-1 (programmed death 1) nanoantibody and preparation method and application thereof
ActiveCN110256562AStable structureDifficult to identifyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCompetent cellTotal rna
The invention belongs to the technical field of biomedicine and particularly relates to a PD-1 (programmed death 1) nanoantibody and a preparation method and application thereof. The ability to bind with antigens can be improved or collaboratively enhanced through the PD-1 nanoantibody. The PD-1 nanoantibody has no complete antibody structure and lacks Fc end and Y-shaped structure; the antigens bound to the PD-1 nanoantibody are not easily recognizable and can escape easily from being caught by the immune system. The technical means includes mainly: performing antigen-vector connecting; transferring to competent cells, and extracting recombinant plasmids; transfecting to host bacteria for expressing; immunizing camels with antigen proteins; collecting peripheral blood, extracting total RNA of a lymphocyte sample, reversely transcribing the total RNA into cDNA, performing amplifying to obtain a VHH library fragment, inserting the VHH library fragment into an expression vector, and transferring to the competent cells to construct an antibody immunization library; screening nanoantibodies from the antibody immunization library; verifying the binding of the screened nanoantibodies with a PD-1 antigen.
Owner:SHIHEZI UNIVERSITY
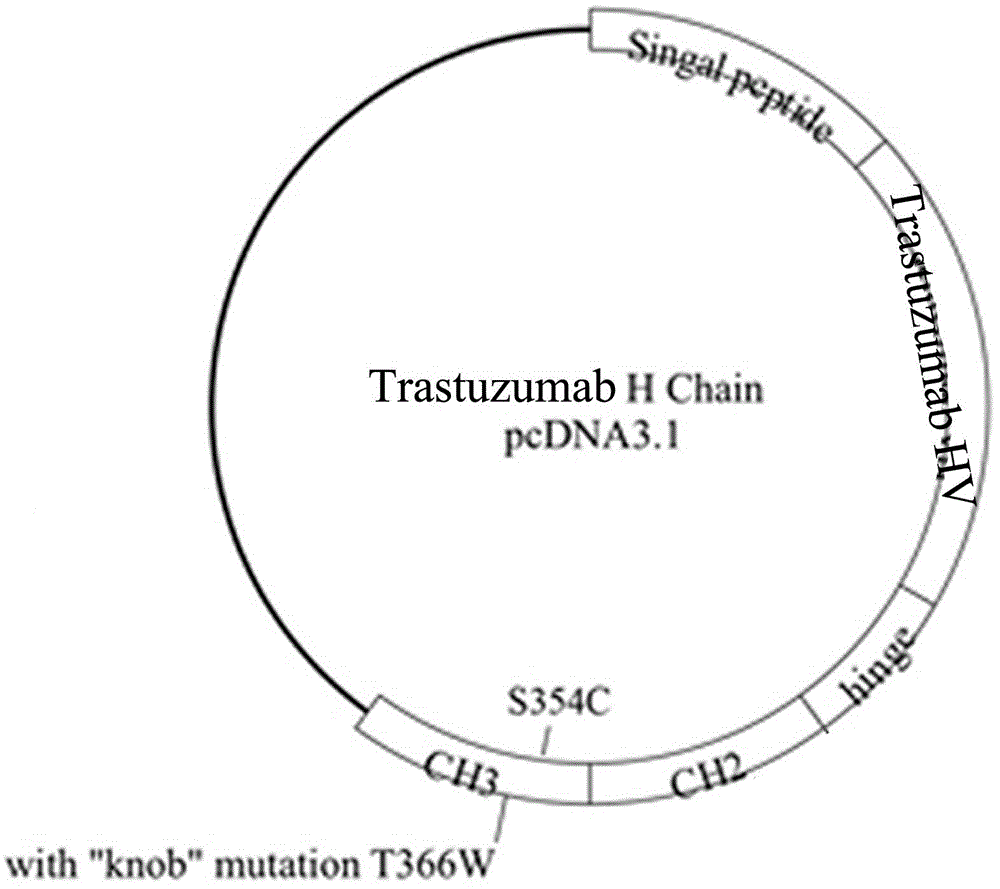
Humanized antibody expression vector and construction method thereof
ActiveCN103898141AImprove shear efficiencyImprove balanceVector-based foreign material introductionAntibody expressionHumanized antibody
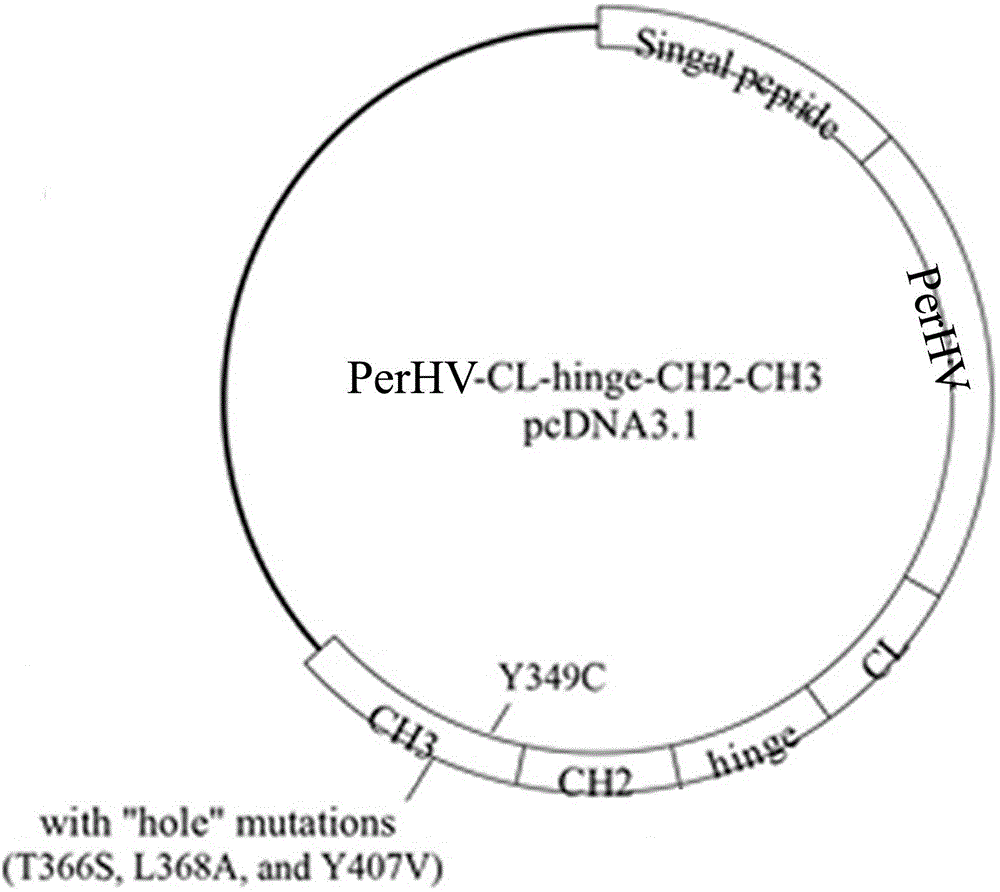
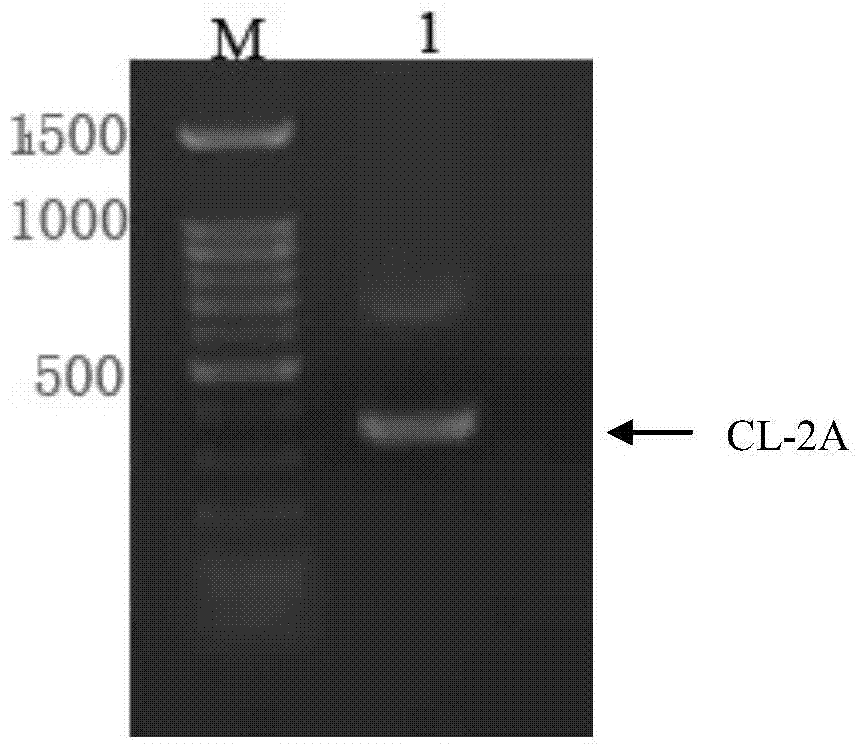
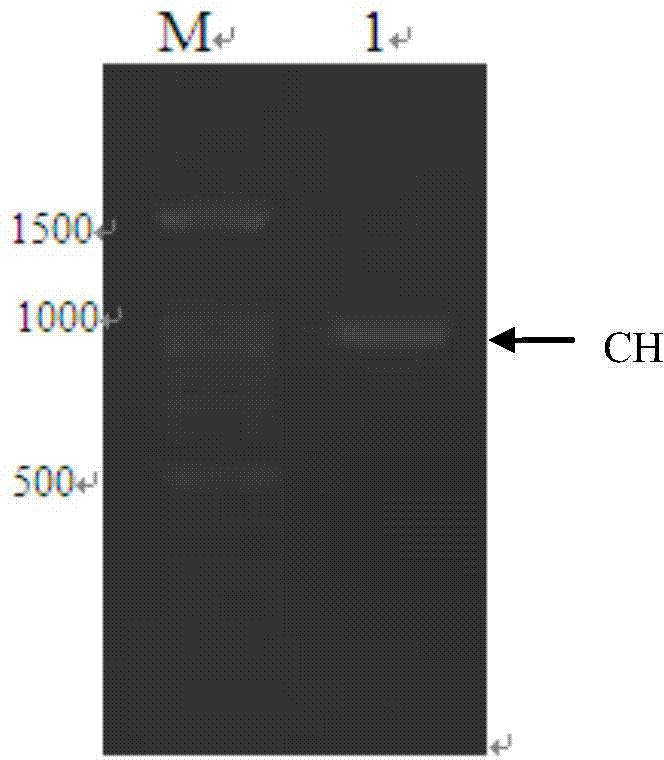
The invention discloses a humanized antibody expression vector and a construction method thereof. The construction method comprises the steps of: respectively connecting a 2A sequence-containing light chain constant region gene sequence CL-2A and a heavy chain constant region gene sequence CH with a pMD18-T vector to obtain a pMD18-T-CL-2A plasmid and a pMD18-T-CH plasmid; connecting the pMD18-T-CL-2A plasmid and a eukaryotic expression vector after being subjected to double digestion to obtain a CL-2A-containing eukaryotic expression vector; and then connecting the CL-2A-containing eukaryotic expression vector with a pMD18-T-CH plasmid to obtain a CL-2A-CH-containing eukaryotic expression vector. The construction method of the expression vector disclosed by the invention can be used for rapidly constructing a full-length complete antibody sequence only by inserting a variable region sequence of a mouse or a humanized antibody into a corresponding locus and rapidly constructing a chimeric antibody, the humanized antibody and the like, and is simple and practical.
Owner:SHENZHEN ZHONGKE AMSHENN MEDICINE CO LTD
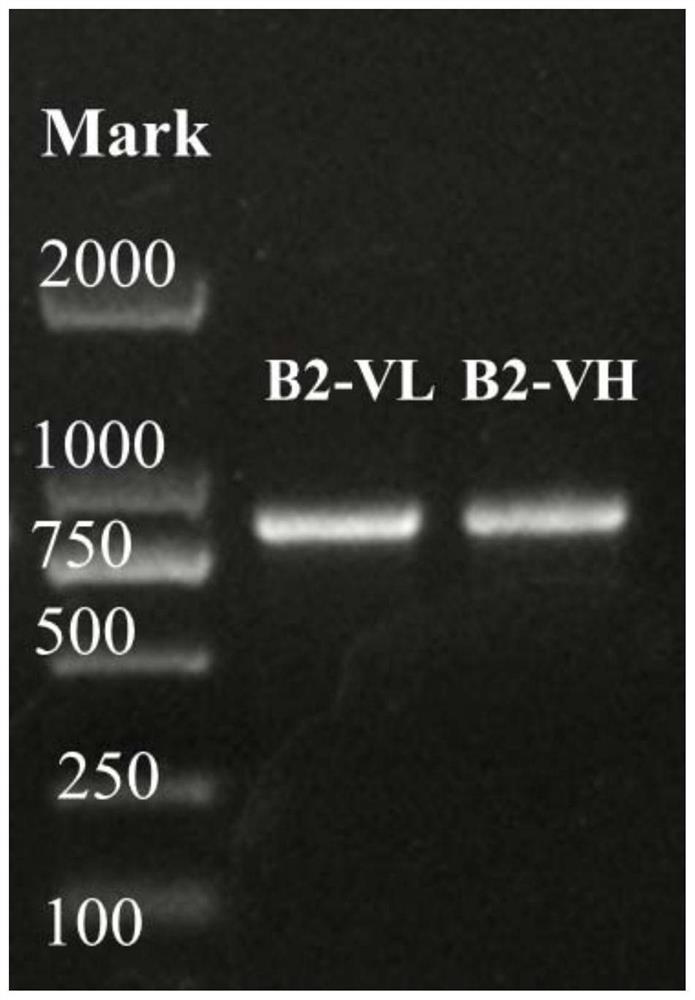
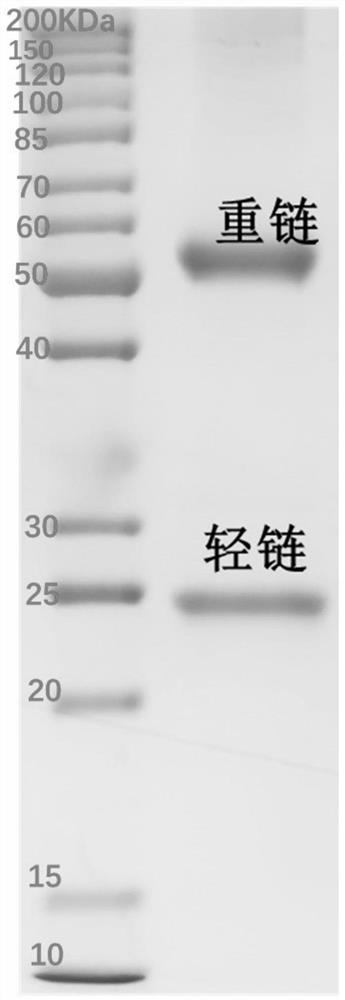
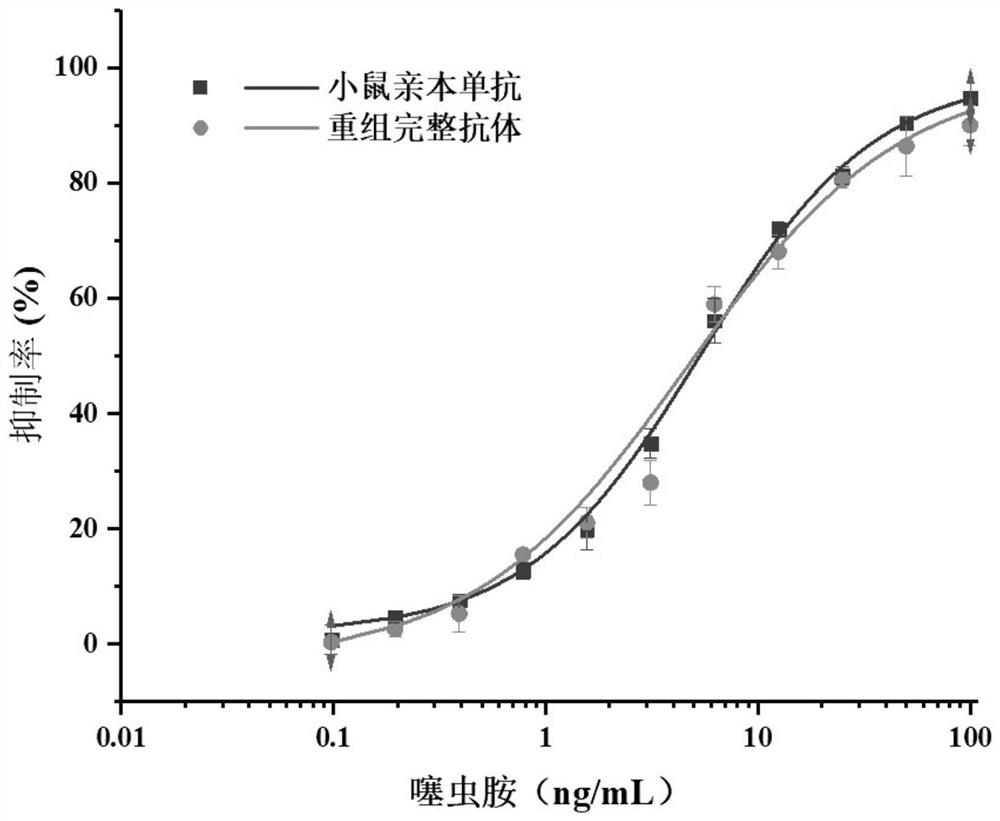
Variable region sequence of broad-spectrum antibody resisting clothianidin and dinotefuran and preparation of recombinant complete antibody of variable region sequence
ActiveCN112062852AHigh purityAvoid mutationImmunoglobulinsFermentationImmune profilingConstant region gene
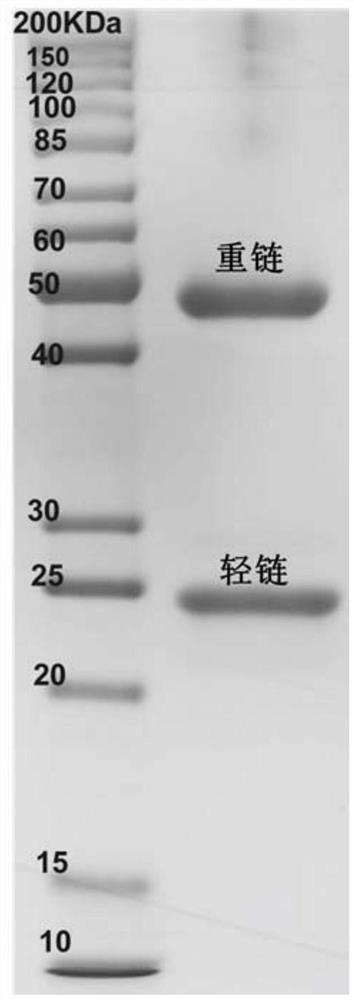
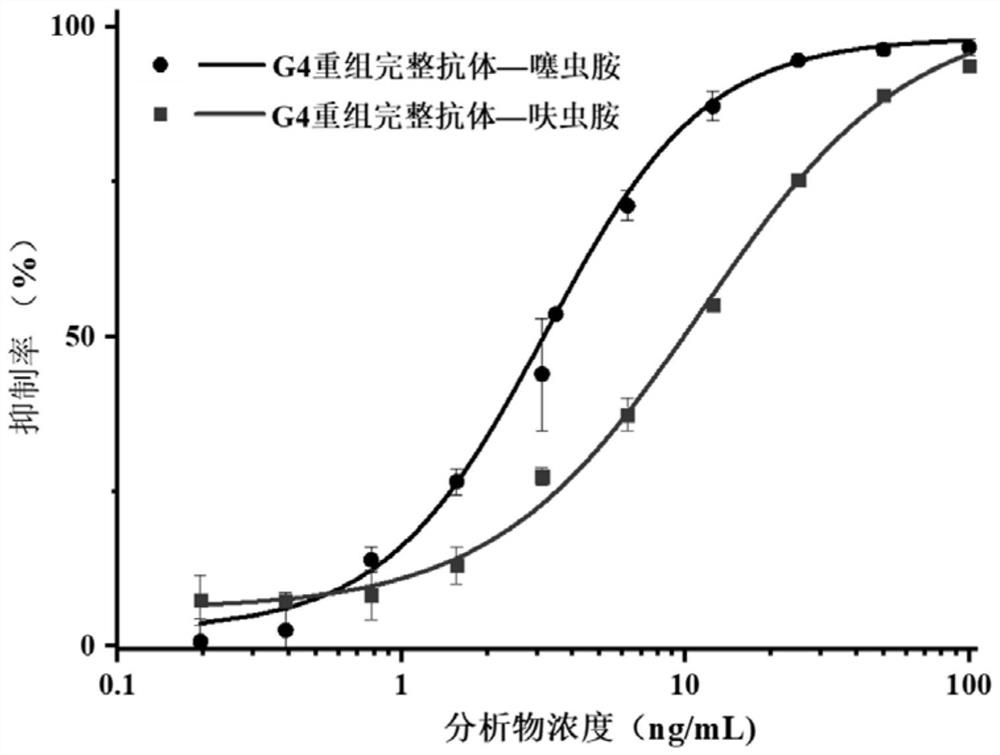
The invention discloses a variable region sequence of a broad-spectrum antibody resisting clothianidin and dinotefuran. The amino acid sequence of a heavy chain variable region coding gene is shown asSEQ ID NO: 2. The invention also discloses a broad-spectrum recombinant complete antibody resisting clothianidin and dinotefuran. The broad-spectrum recombinant complete antibody resisting clothianidin and dinotefuran comprises a heavy chain constant region, a heavy chain variable region, a light chain constant region and a light chain variable region, and the amino acid sequence of the heavy chain variable region coding gene is shown as SEQ ID NO: 2. The sequence genes obtained by the invention are respectively connected to an expression vector containing a heavy chain constant region gene and a light chain constant region gene, and a recombinant complete antibody is obtained by adopting mammalian cell expression of a double-plasmid system, so that the recognition activity of a murine parent antibody is ensured, the broad-spectrum antibody resisting clothianidin and dinotefuran can be stably produced on a large scale, and a reliable core reagent is provided for various immunoassay methods for synchronously detecting the clothianidin and the dinotefuran.
Owner:ZHEJIANG UNIV
Variable region sequence of specific anti-clothianidin antibody, and preparation and application of recombinant complete antibody thereof
ActiveCN112194725AStable production in large quantitiesGuaranteed recognition activityHybrid immunoglobulinsBiological material analysisImmune profilingAntiendomysial antibodies
The invention discloses a variable region sequence of a specific anti-clothianidin antibody. The amino acid sequence of a heavy chain variable region coding gene is shown as SEQ ID NO:2. The inventionalso discloses an anti-clothianidin recombinant complete antibody, which comprises a heavy chain constant region, a heavy chain variable region, a light chain constant region and a light chain variable region; and the amino acid sequence of the heavy chain variable region coding gene is shown as SEQ ID NO:2. The invention also discloses an antibody expression plasmid. The invention further discloses an immune test strip for rapidly detecting clothianidin residues. The sequence gene obtained by the invention is respectively connected to an expression vector containing a heavy chain constant region gene and a light chain constant region gene; the recombinant complete antibody is obtained by adopting mammalian cell expression of a double-plasmid system, so that the recognition activity of amouse parent antibody is ensured; the anti-clothianidin antibody can be stably produced in batches; and a reliable core reagent is provided for various immunoassay methods for clothianidin residue detection.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com