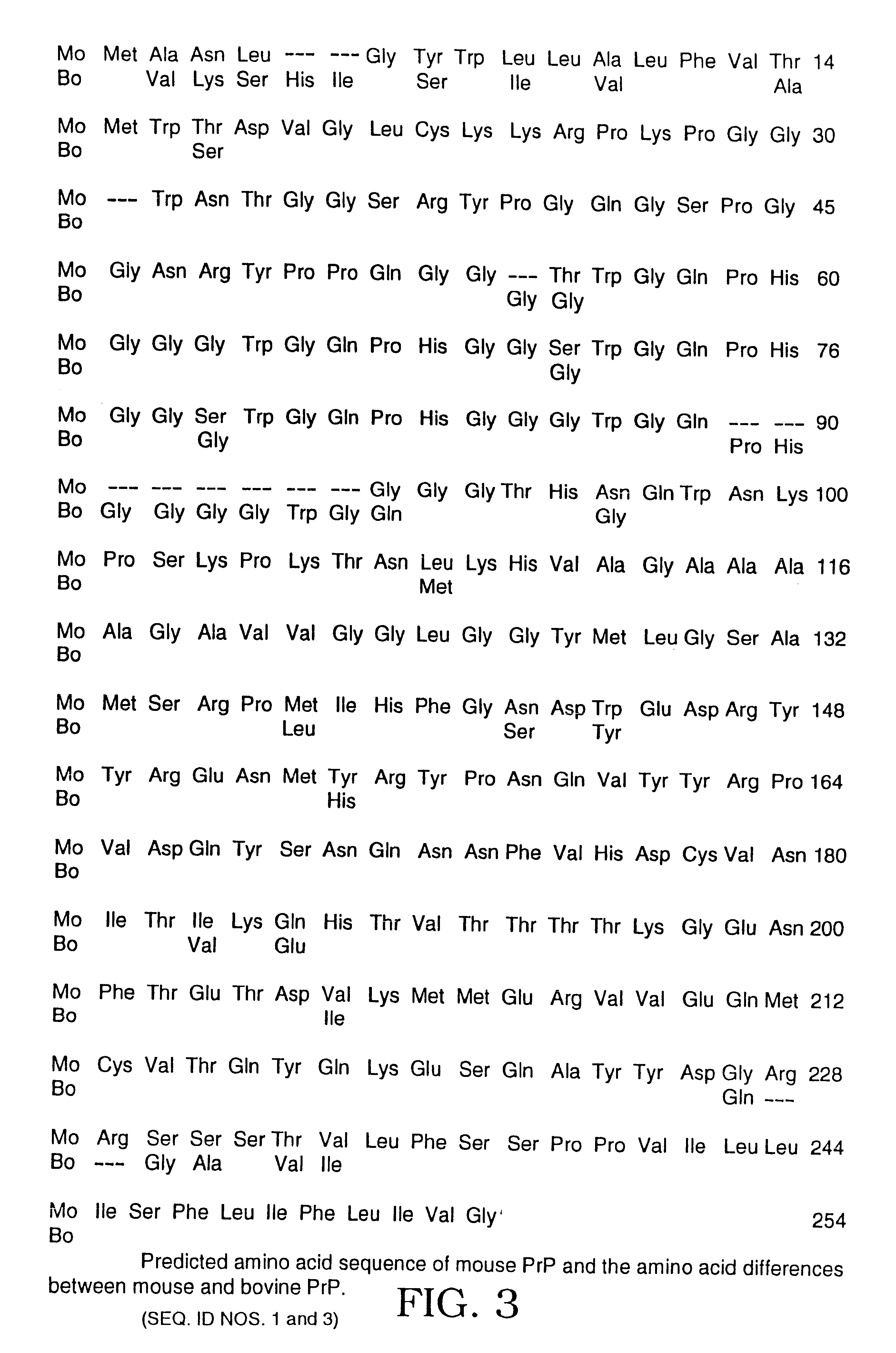Patents
Literature
416 results about "Antibody production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
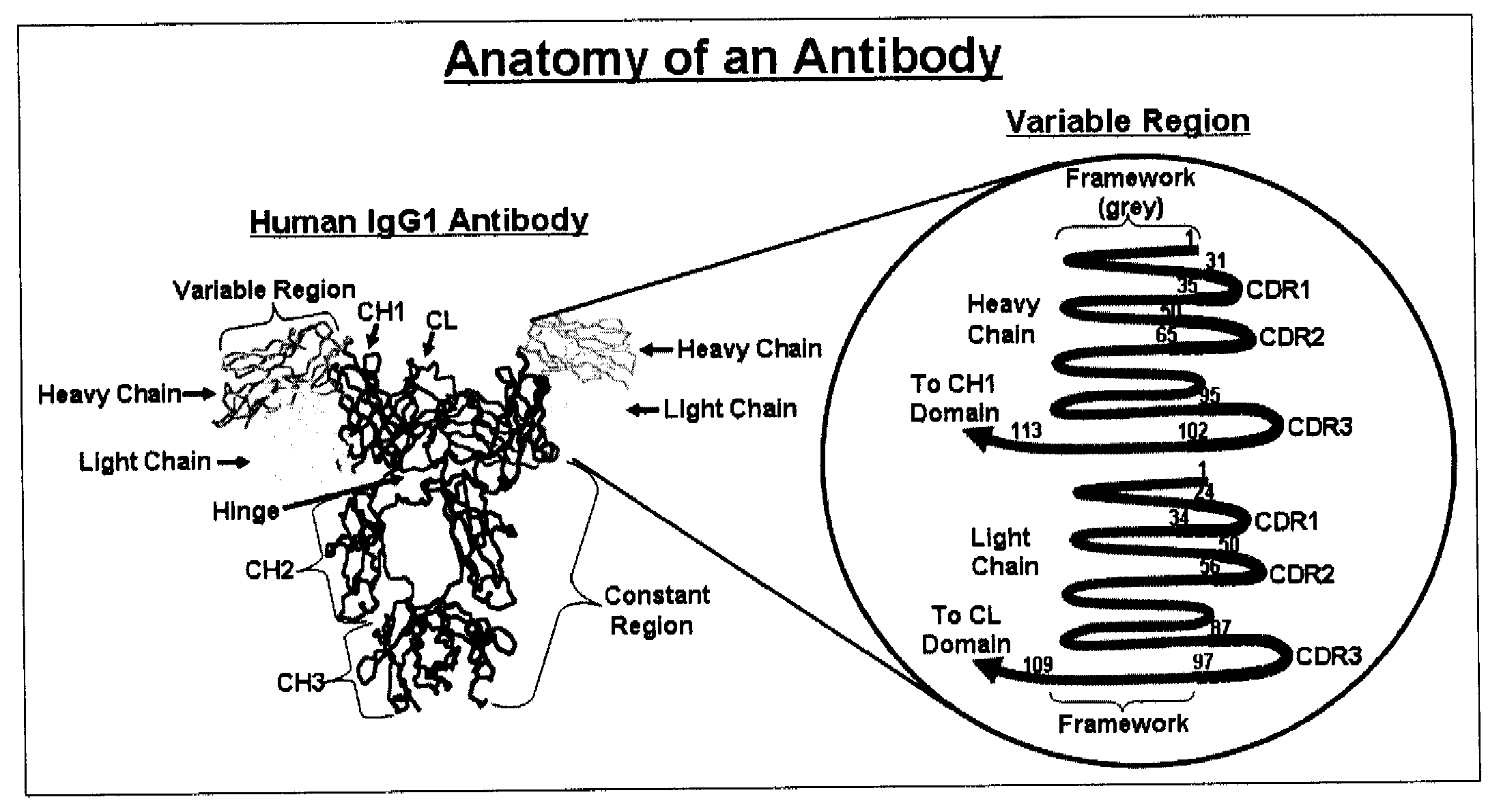
Antibodies and methods for generating genetically altered antibodies with enhanced effector function
InactiveUS20050054048A1Increase variabilityBetter pharmacokinetic profileAnimal cellsSugar derivativesGenetic diversityMonoclonal antibody
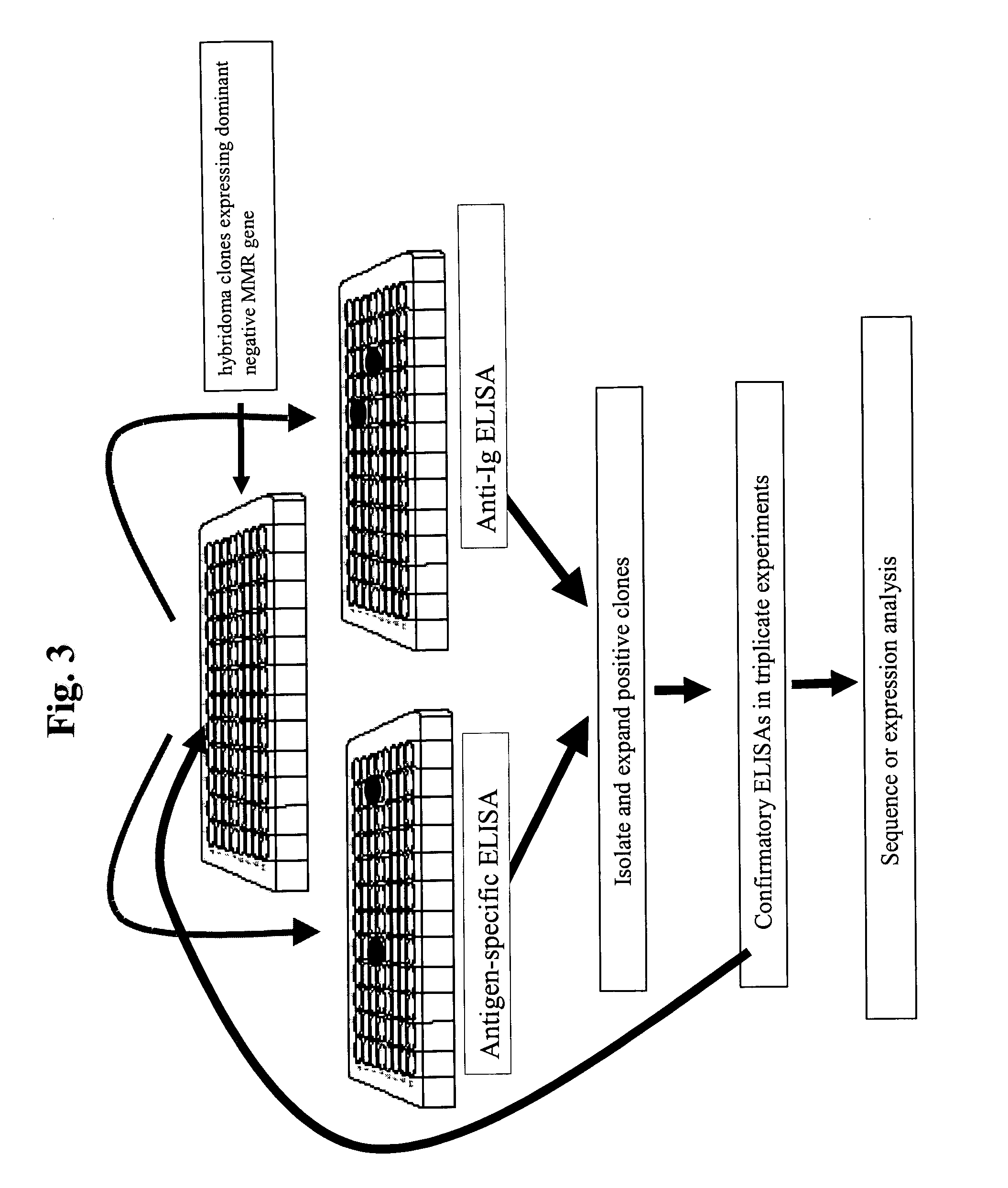
Dominant negative alleles of human mismatch repair genes can be used to generate hypermutable cells and organisms. By introducing these genes into cells and transgenic animals, new cell lines and animal varieties with novel and useful properties can be prepared more efficiently than by relying on the natural rate of mutation. These methods are useful for generating genetic diversity within immunoglobulin genes directed against an antigen of interest to produce altered antibodies with enhanced biochemical activity. Moreover, these methods are useful for generating antibody-producing cells with increased level of antibody production. The invention also provides methods for increasing the effector function of monoclonal antibodies and monoclonal antibodies with increased effector function.
Owner:EISAI INC
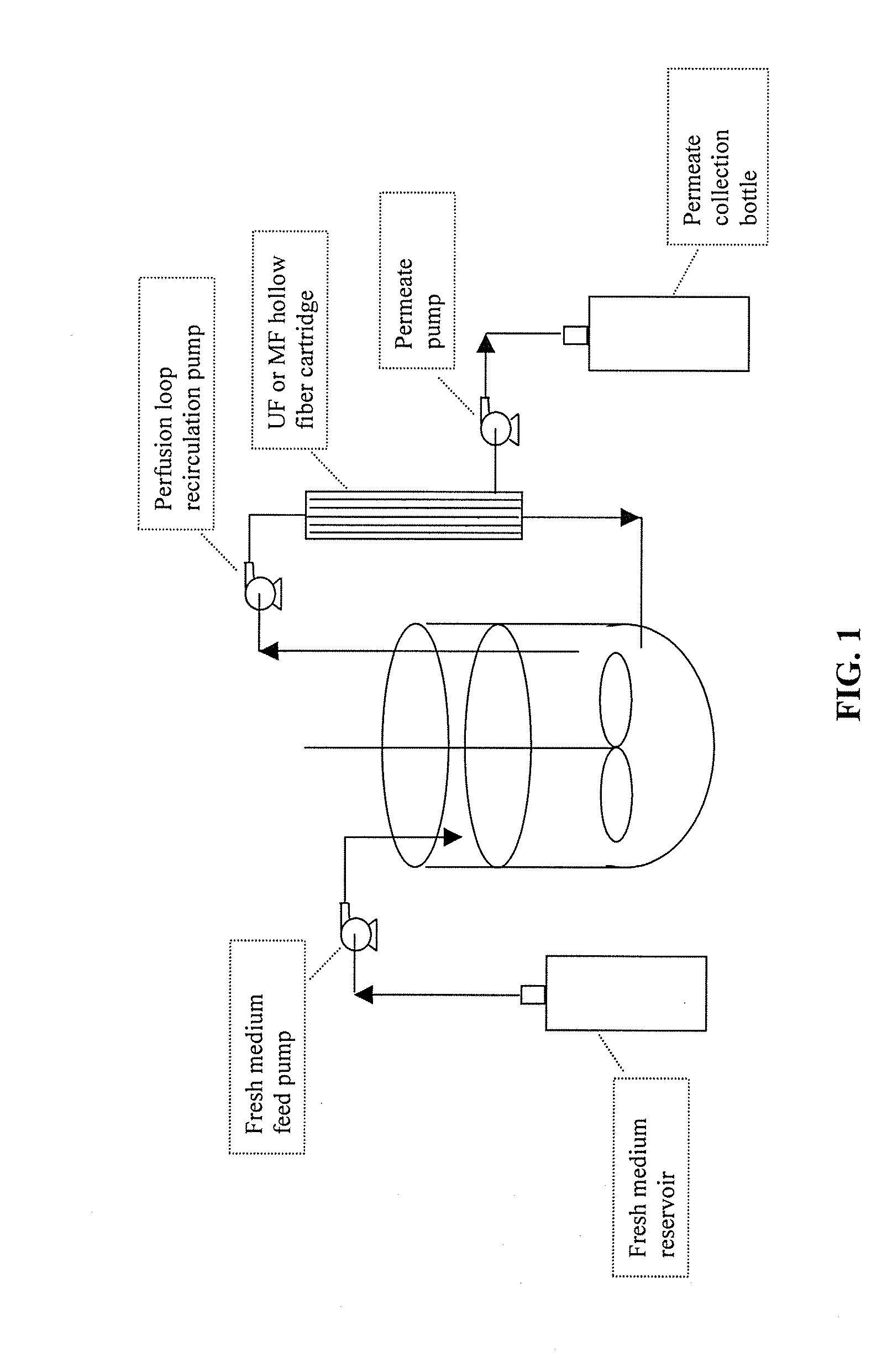
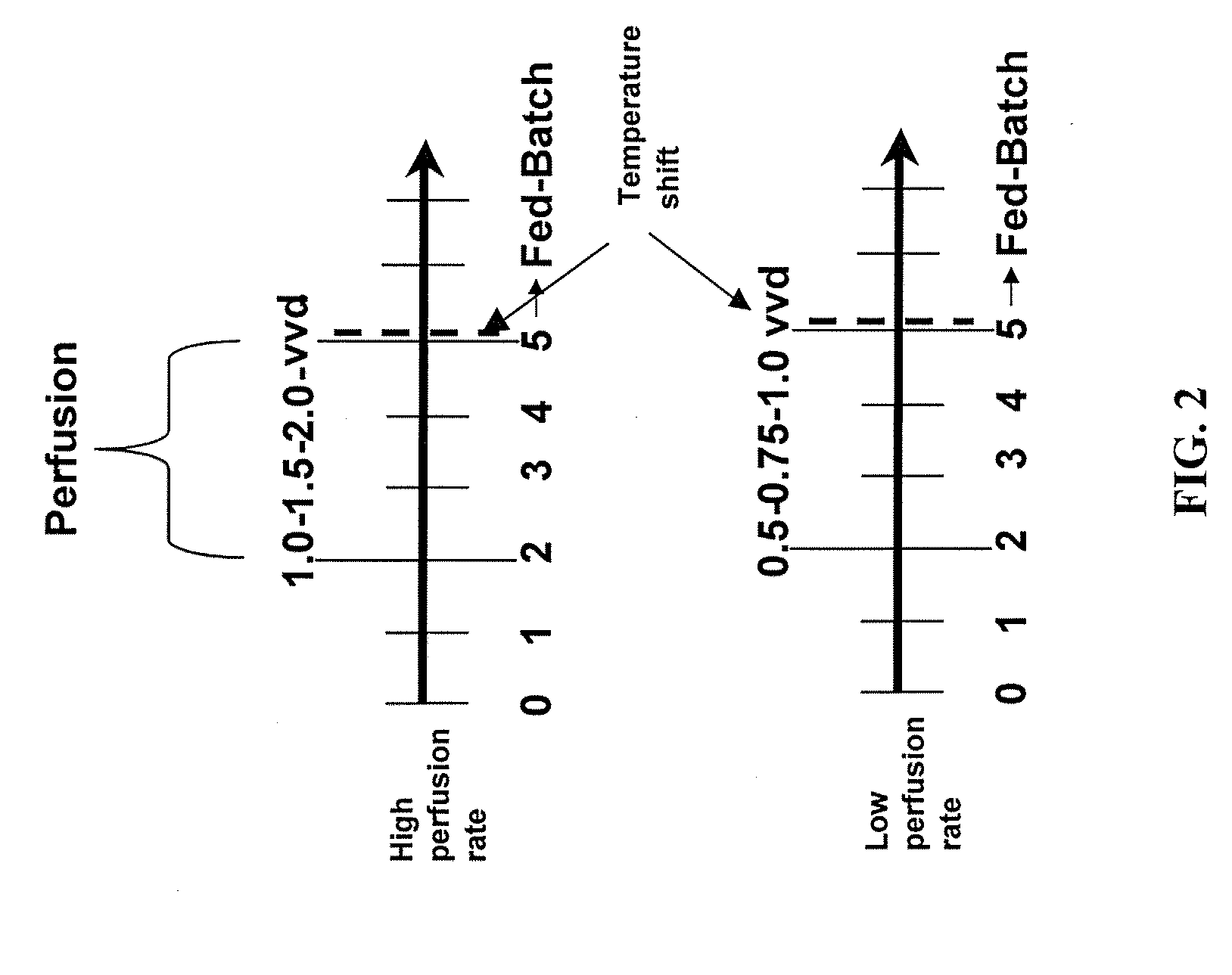
Use of perfusion to enhance production of fed-batch cell culture in bioreactors
InactiveUS20090042253A1Bioreactor/fermenter combinationsBiological substance pretreatmentsFiltrationFeed pump
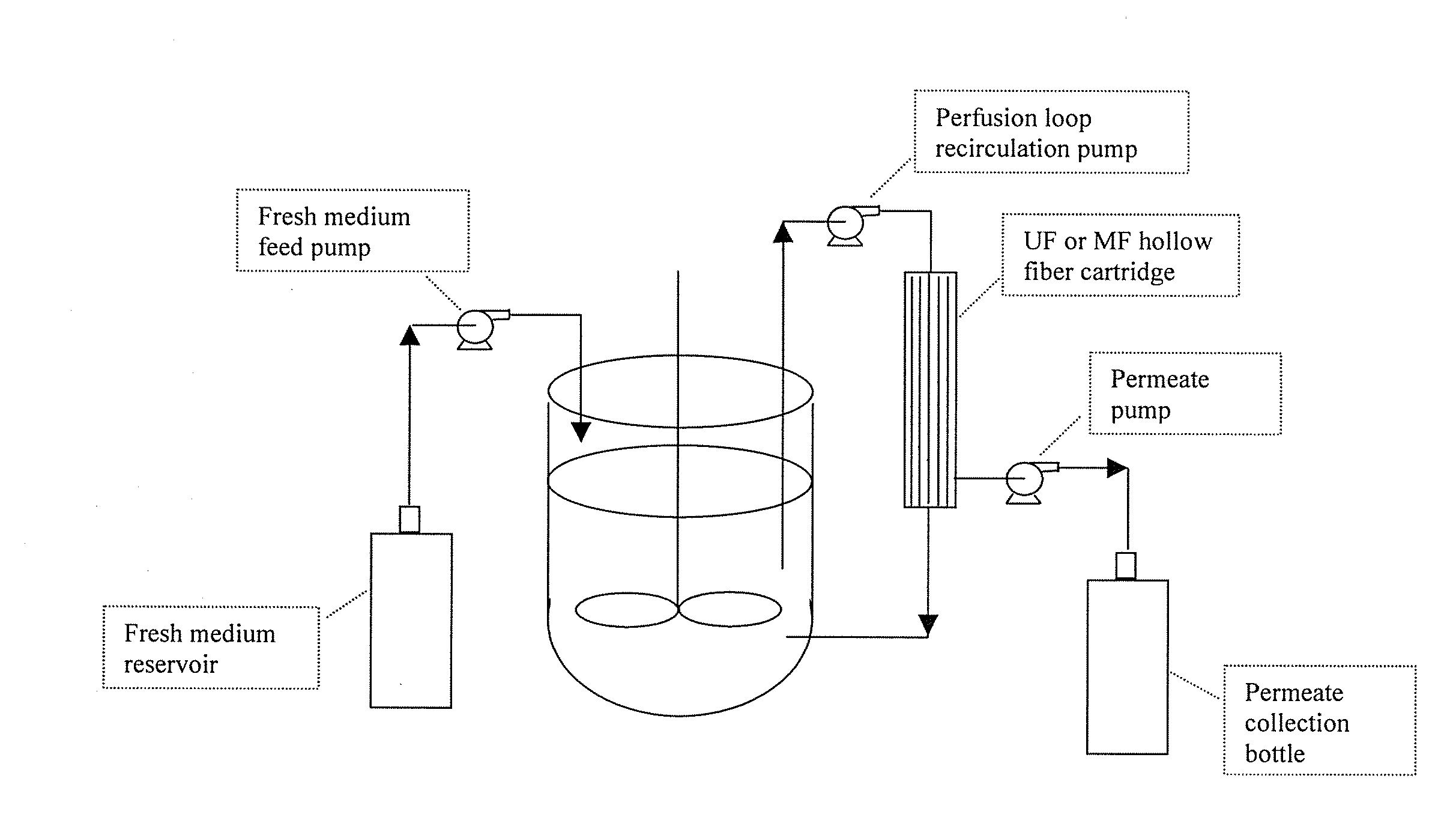
The invention relates to methods of improving protein production, e.g., large-scale commercial protein production, e.g., antibody production, utilizing a modified fed-batch cell culture method comprising a cell growth phase and a polypeptide production phase. The modified fed-batch cell culture method combines both cell culture perfusion and fed-batch methods to achieve higher titers of polypeptide products. Because the modified fed-batch cell culture method of the invention produces higher polypeptide product titers than fed-batch culture alone, it will substantially improve commercial-scale protein production. The invention also relates to a perfusion bioreactor apparatus comprising a fresh medium reservoir connected to a bioreactor by a feed pump, a recirculation loop connected to the bioreactor, wherein the recirculation loop comprises a filtration device, e.g., ultrafiltration or microfiltration, and a permeate pump connecting the filtration device to a permeate collection container.
Owner:WYETH LLC
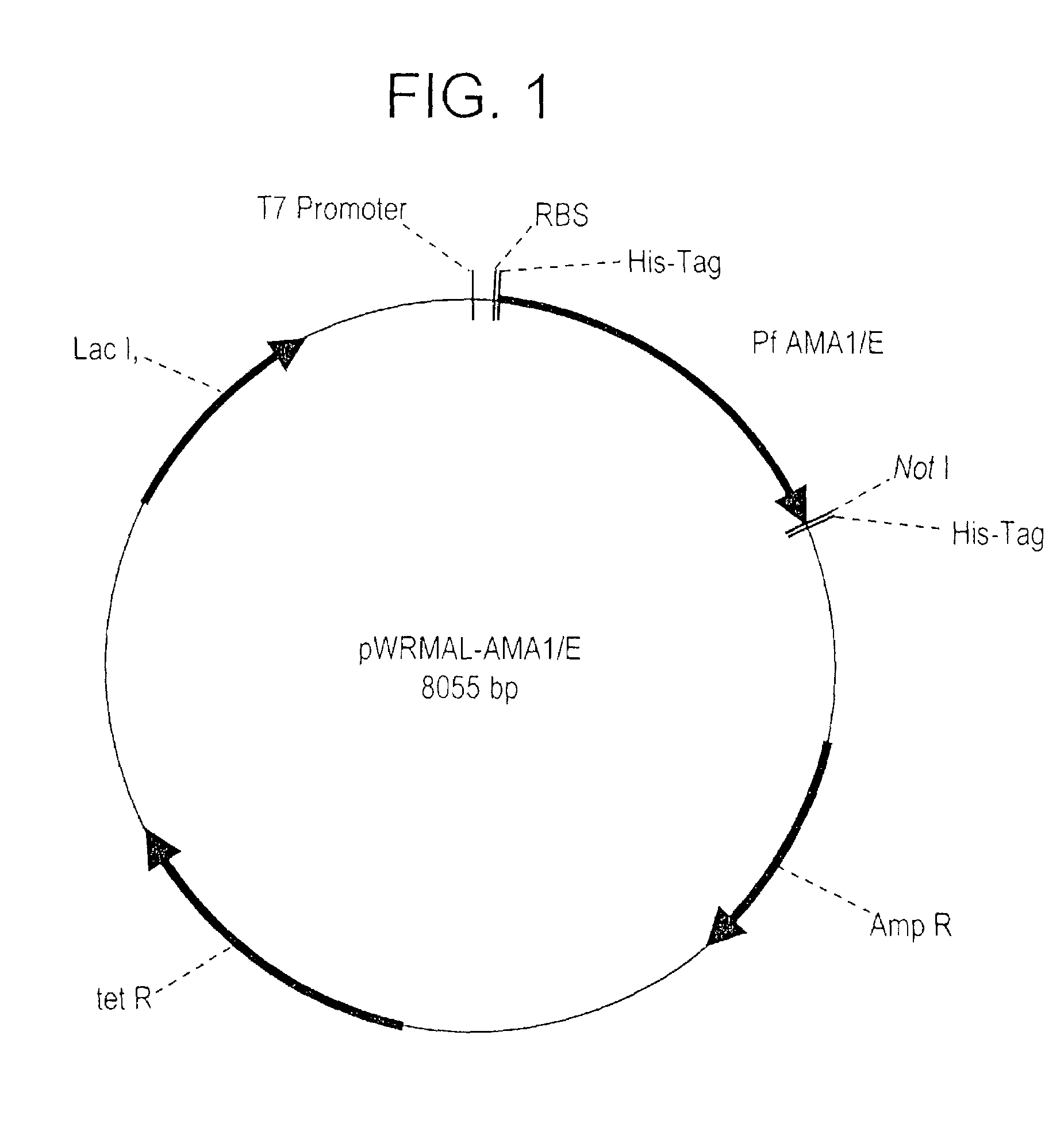
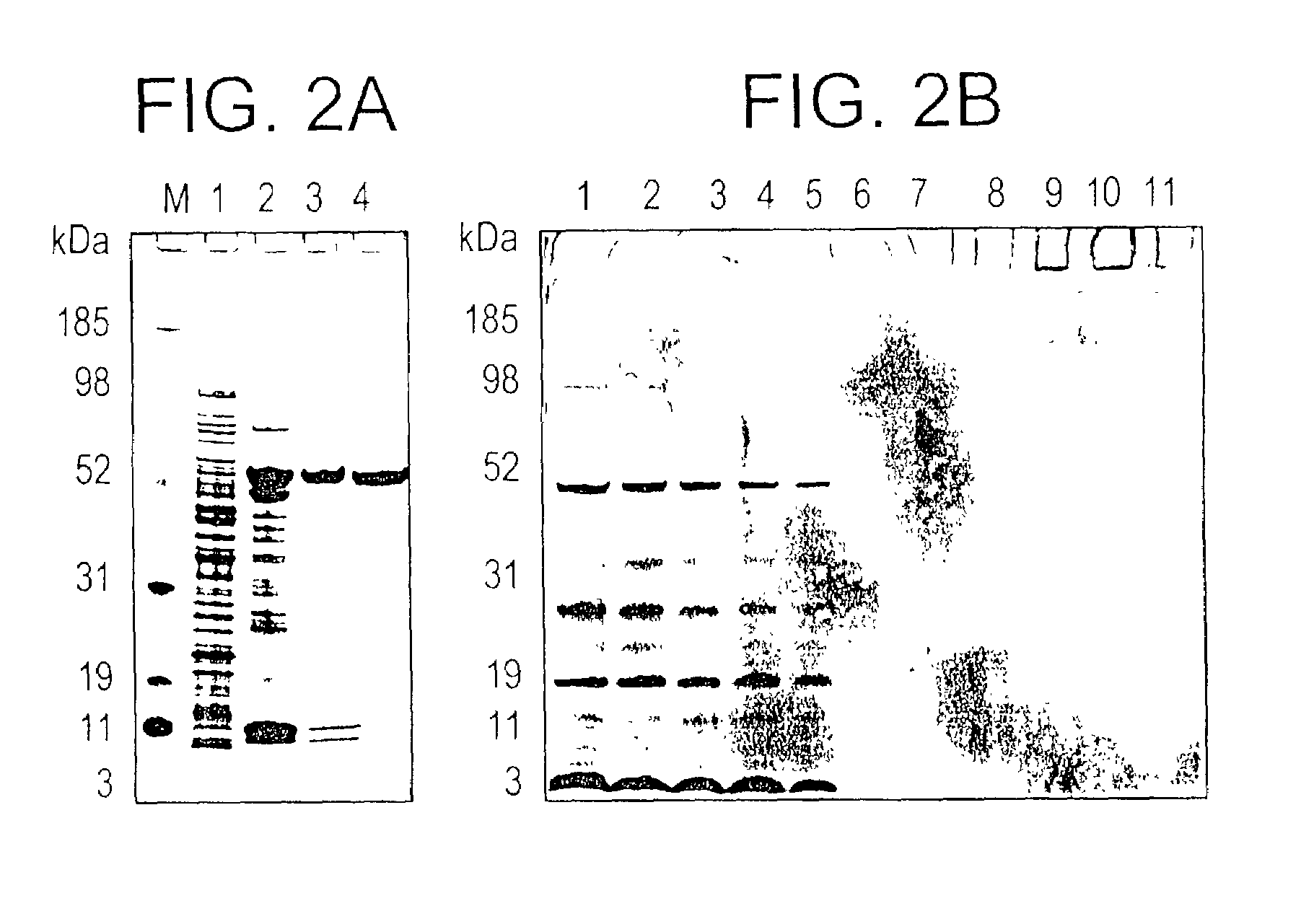
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7029685B2Eliminate the problemImprove responseProtozoaFermentationADAMTS ProteinsMalarial parasites
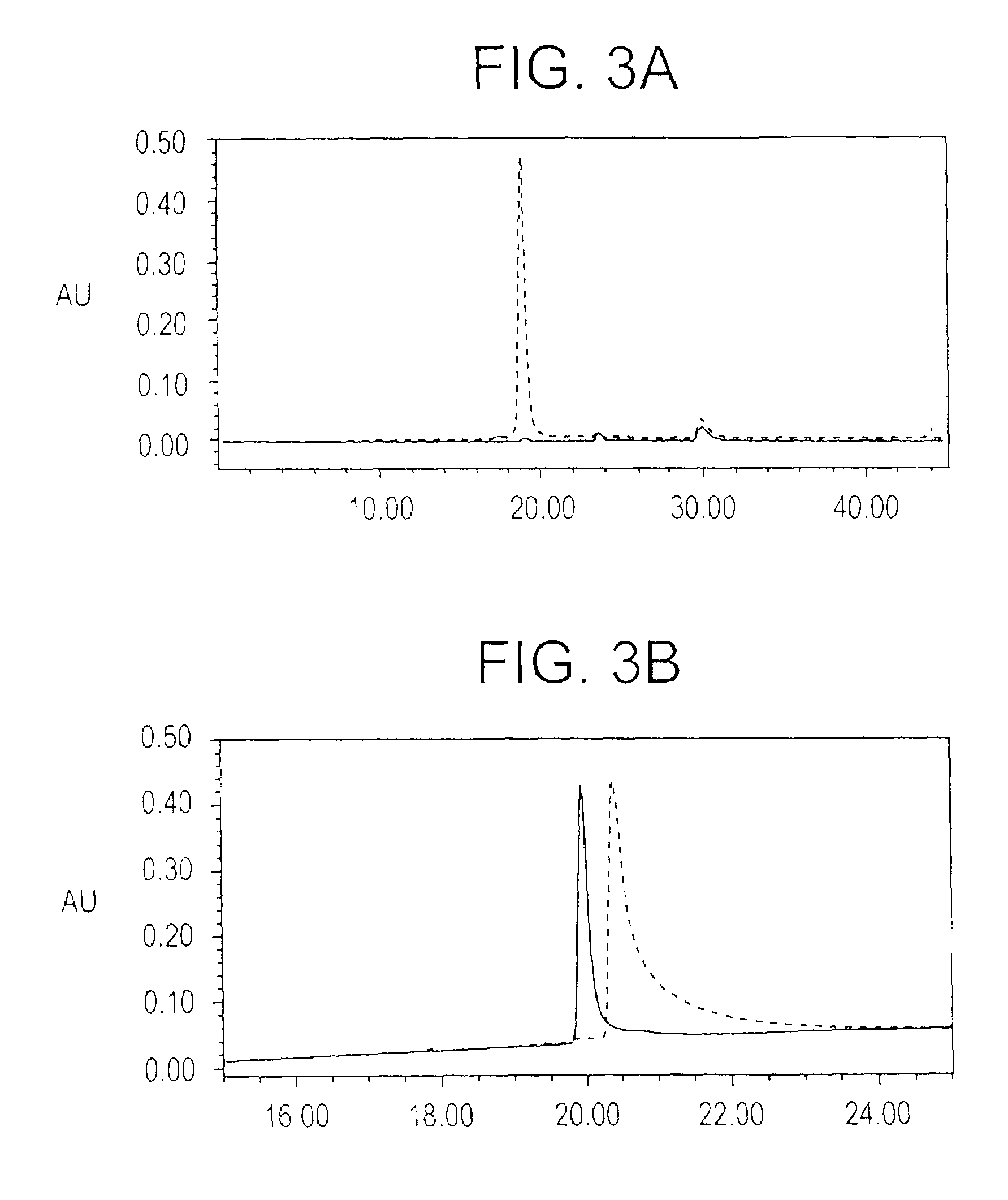
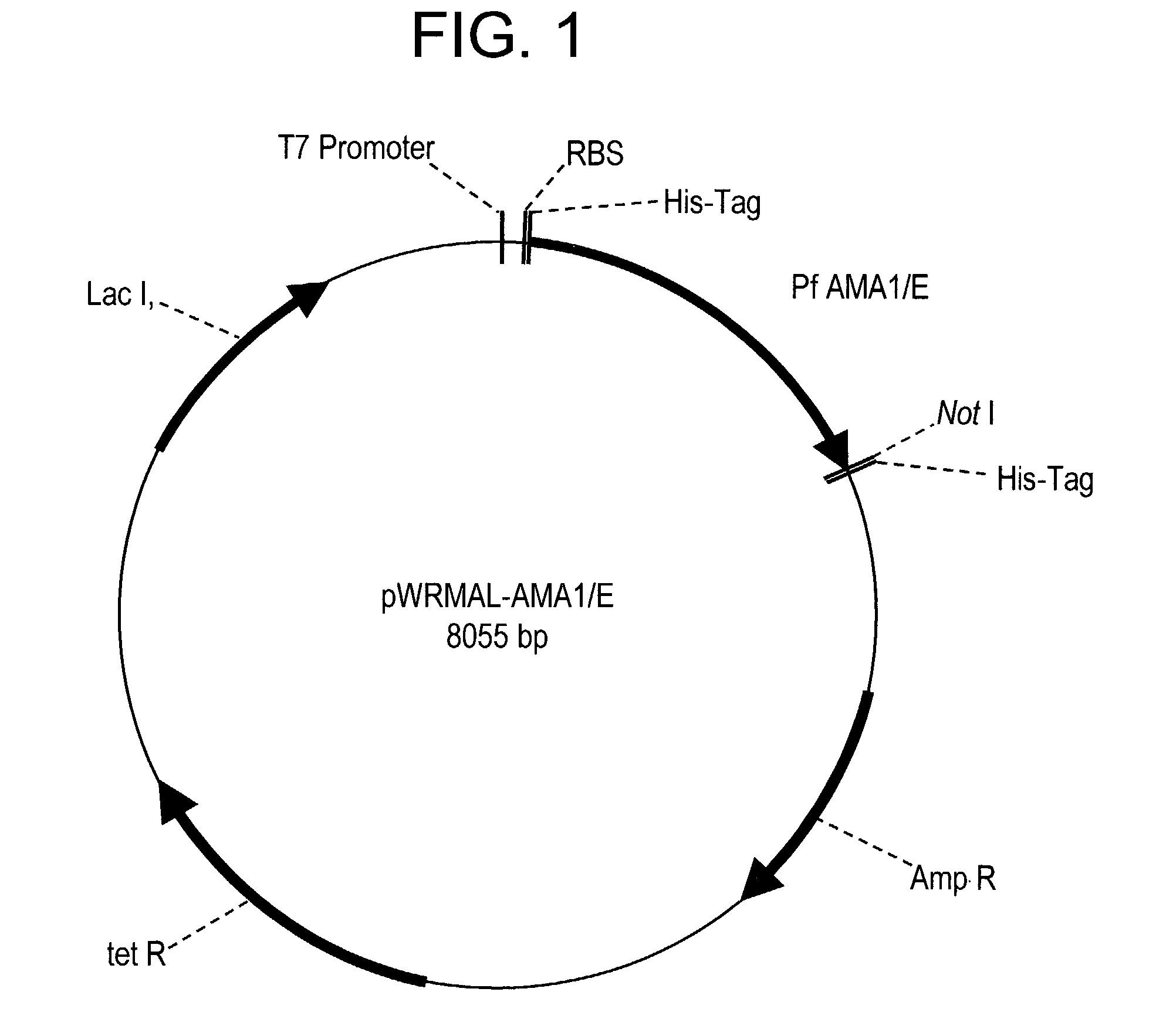
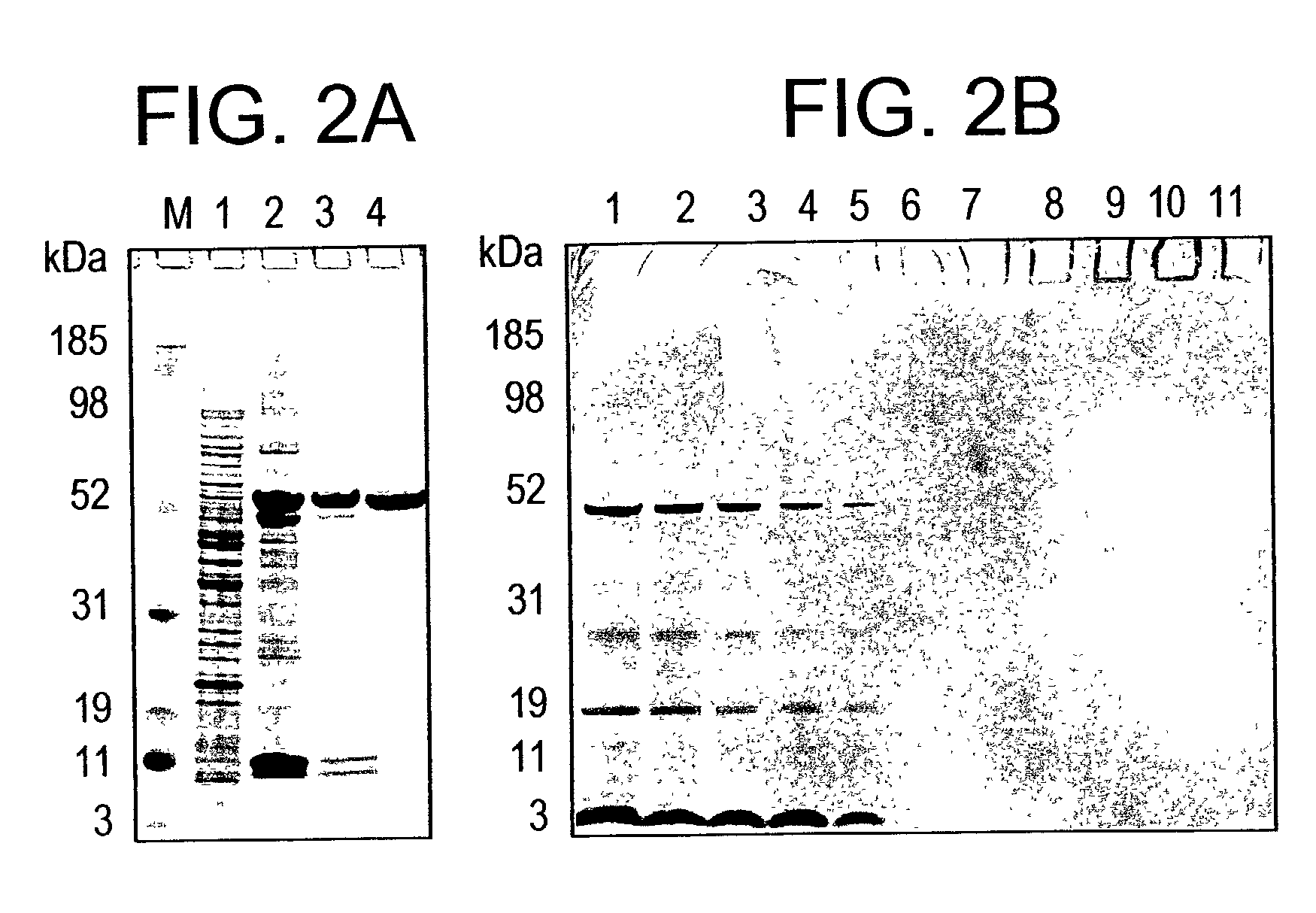
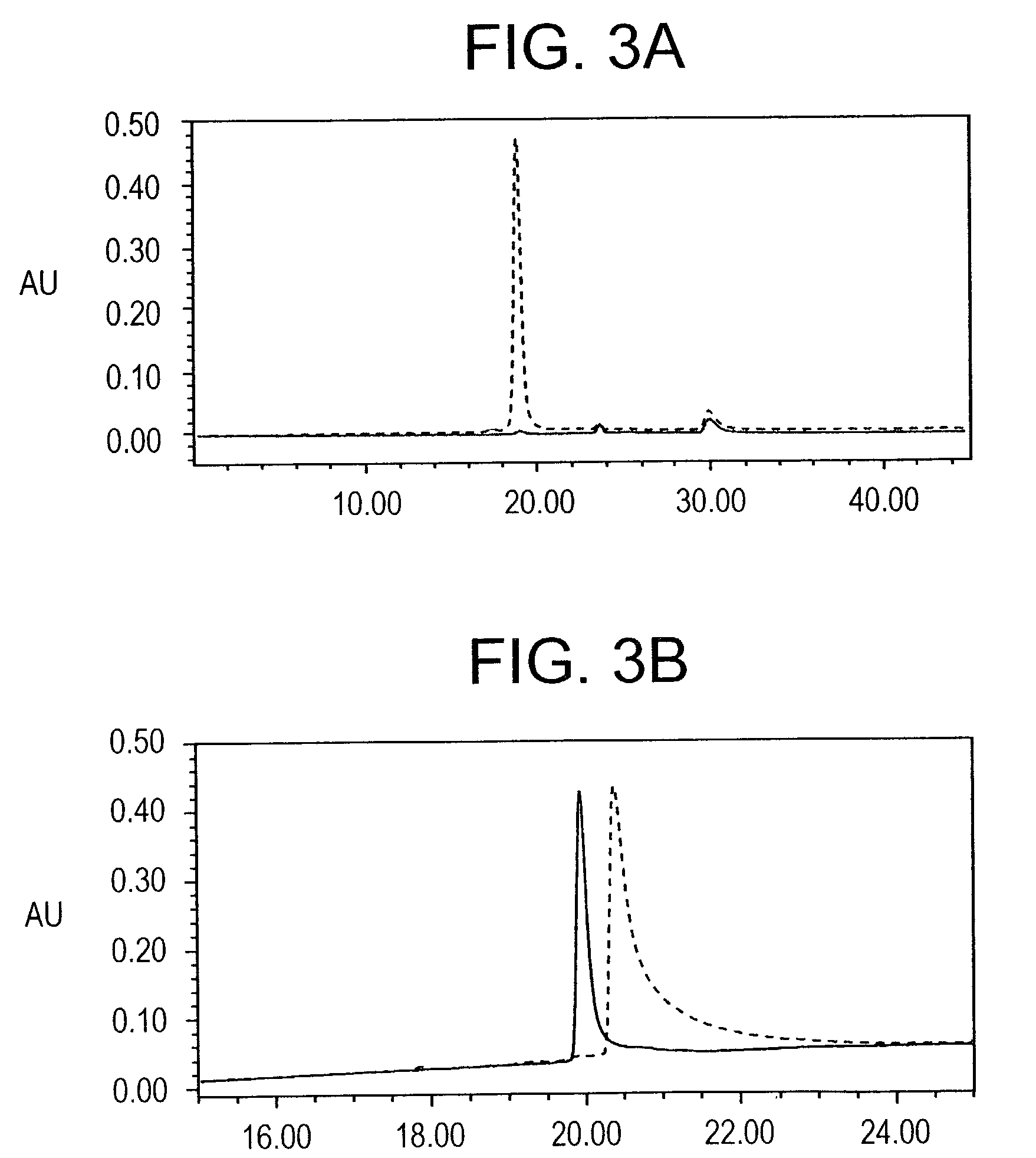
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a protein for use alone, or as part of, a vaccine to prevent malaria.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7060276B2Eliminate the problemImprove responseSugar derivativesViral antigen ingredientsADAMTS ProteinsPlasmodium falciparum
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a vaccine.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
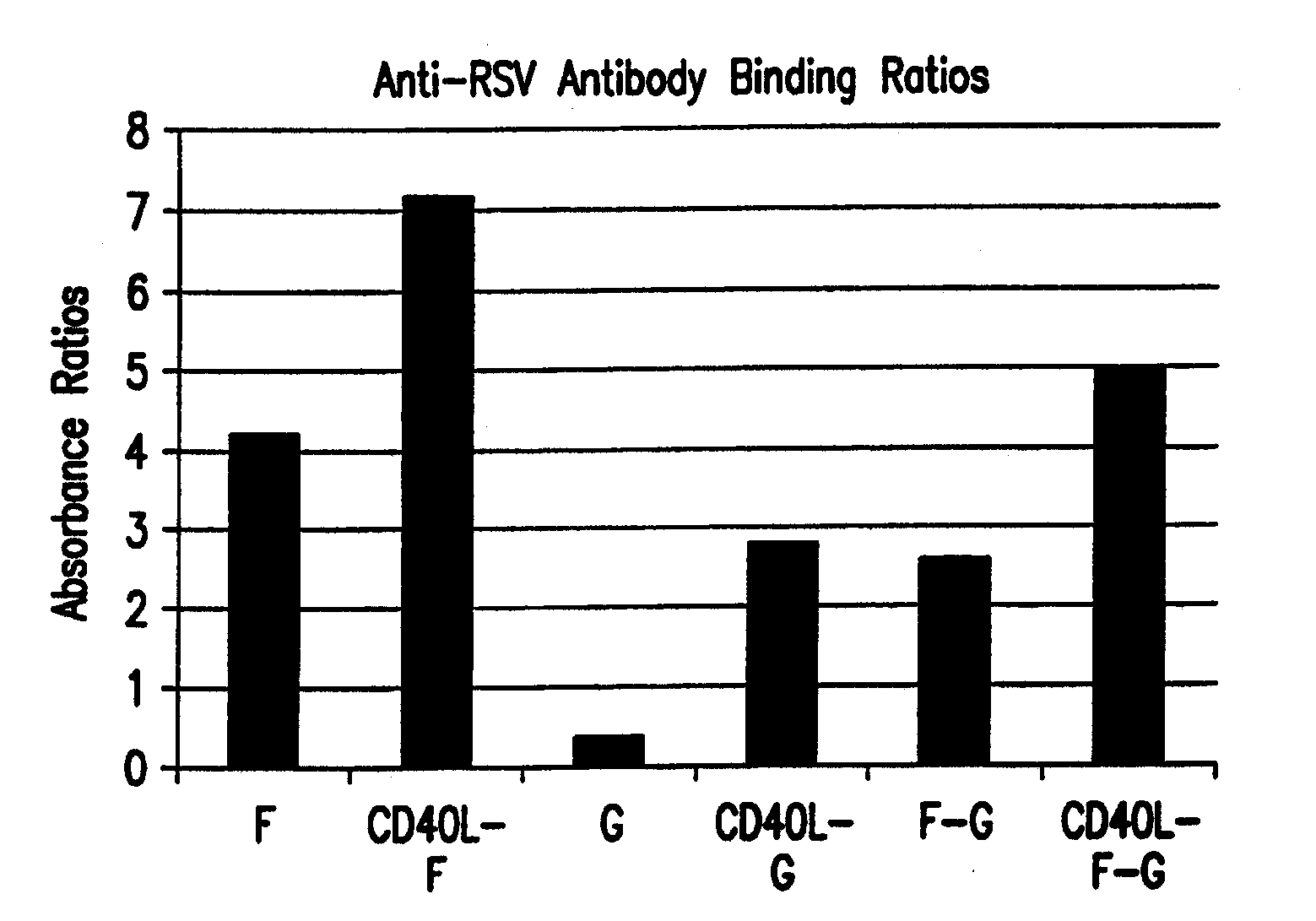
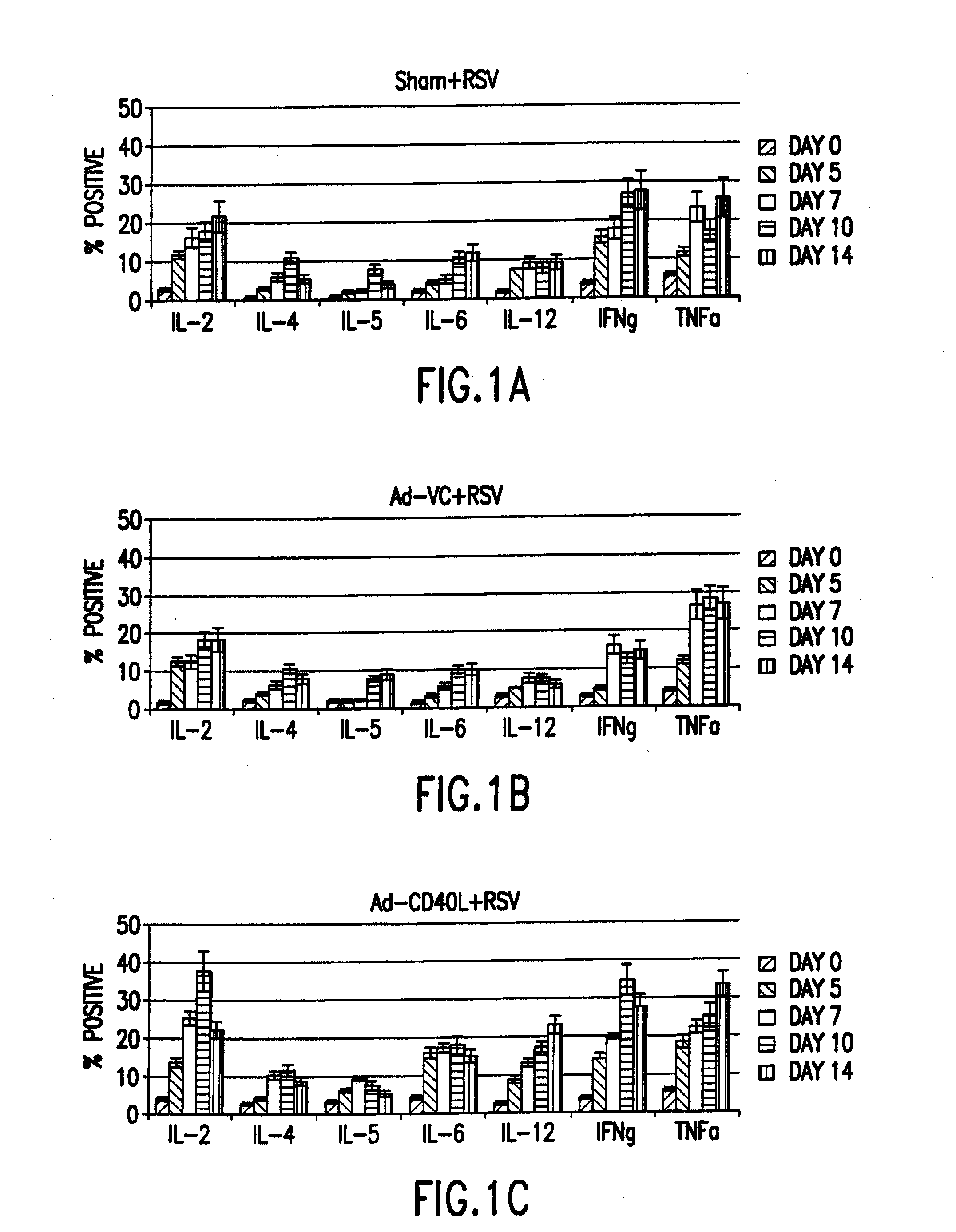
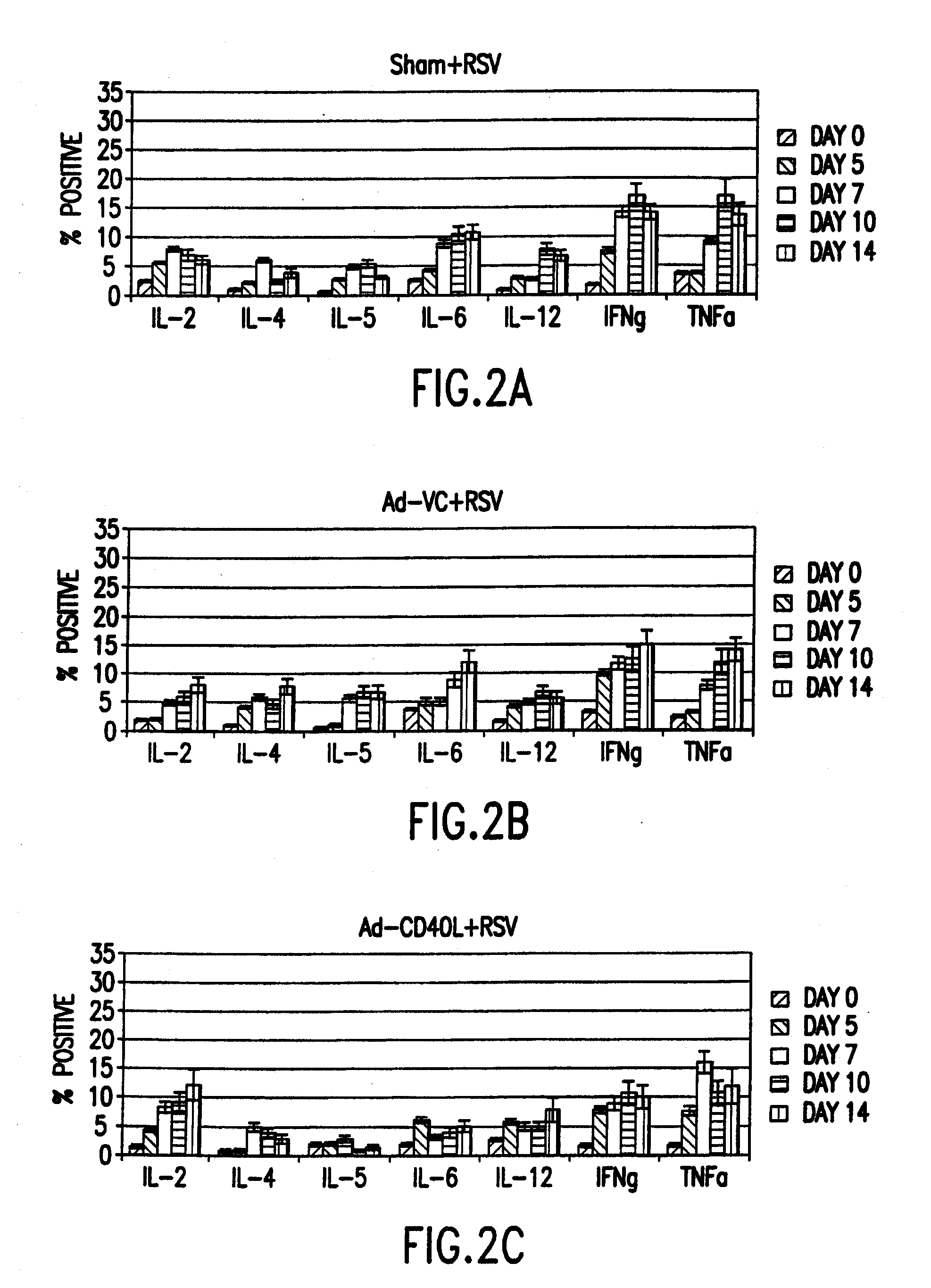
Cd40 ligand adjuvant for respiratory syncytial virus
The present invention provides methods and adjuvants for enhancing an immune response to RSV in a host, wherein the methods and adjuvants comprise a source of a CD40 binding protein. Preferably, the CD40 binding protein is CD40L and the source is a vector comprising a promoter operatively linked to a CD40L coding region. The enhanced immune response produced by the adjuvants and methods of the current invention includes both increased expression of Th1 cytokines and increased production of antibody.
Owner:UNITED STATES OF AMERICA
Methods for improving antibody production
InactiveUS20080138898A1Improve propertiesEnhance antibody productionSugar derivativesTissue cultureHeavy chainAntibody production
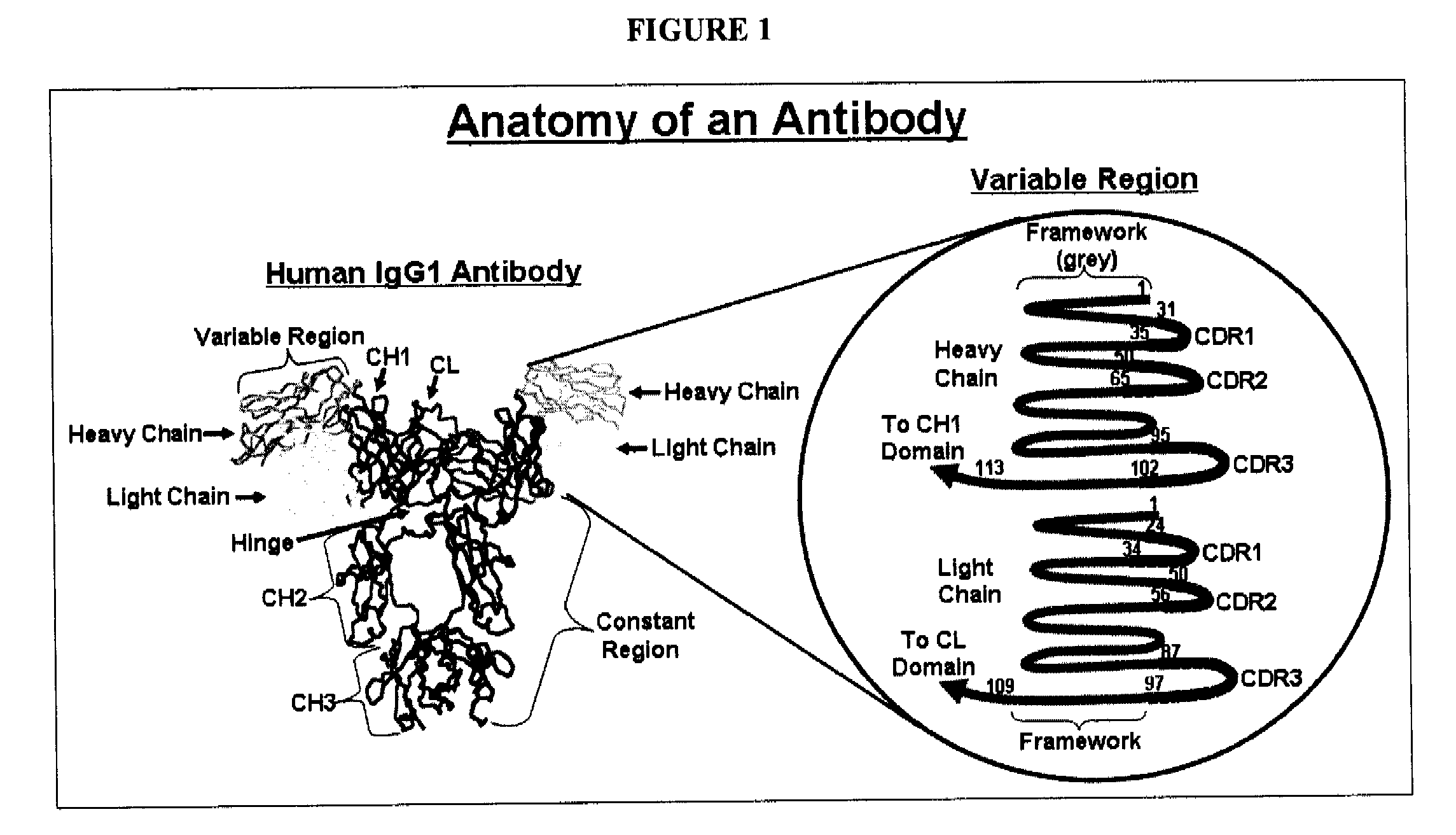
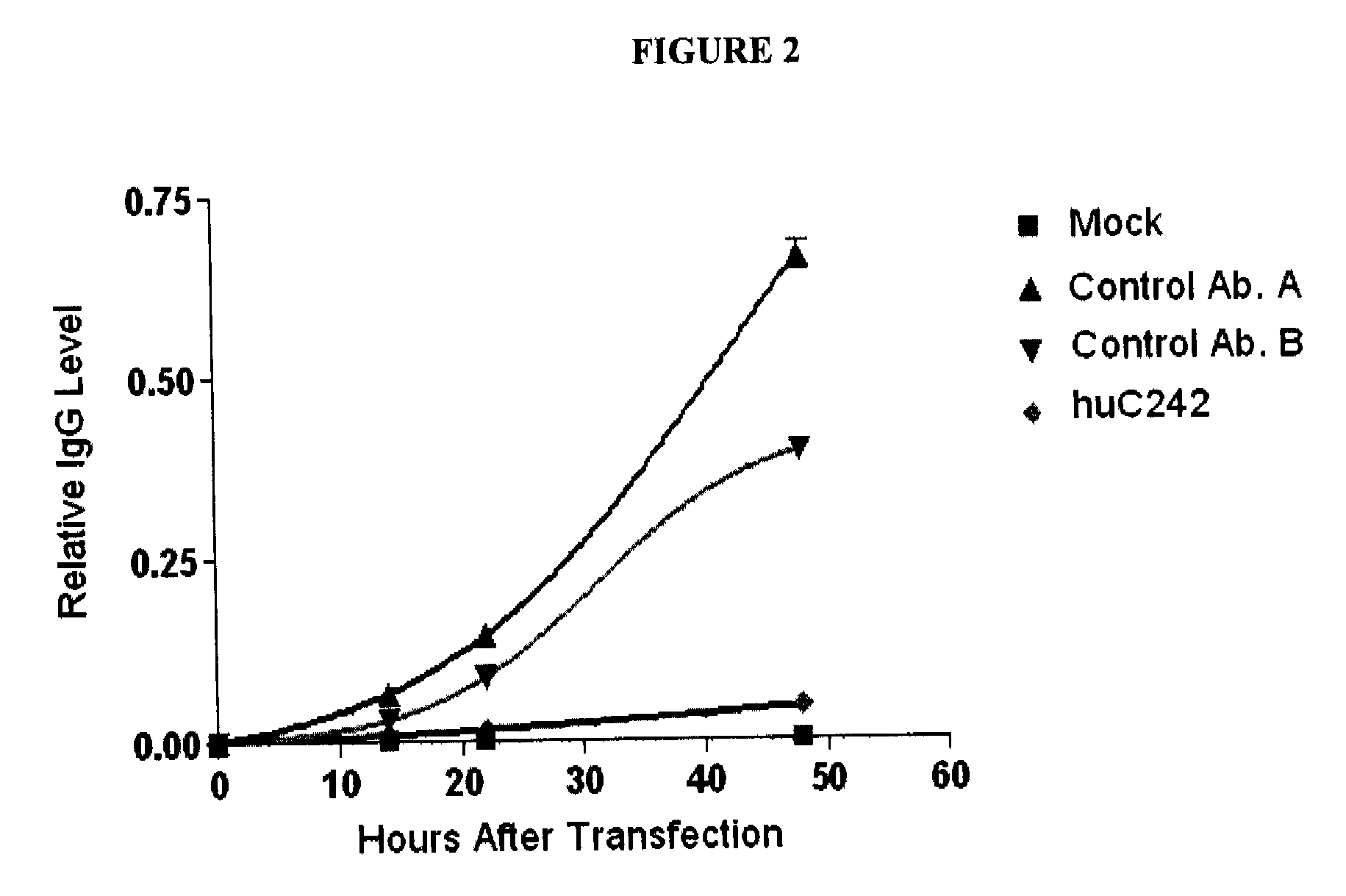
The present invention encompasses manufacturing of antibody variants, such as variant of huC242, or fragments thereof, wherein the variants are manufactured by substituting one or more amino acid residues in a parent antibody. Such substitution(s) is preferably done in a variable region framework sequence of the parent antibody comprising a heavy and a light chain. As a consequence of such substitution(s), variant antibodies or fragments thereof show enhanced antibody synthesis when introduced in a host cell as compared to the parent antibody.
Owner:IMMUNOGEN INC
Immunonanotherapeutics that Provide IgG Humoral Response Without T-Cell Antigen
ActiveUS20100183727A1Improve responseSsRNA viruses negative-senseAntibacterial agentsNanocarriersImmune therapy
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides synthetic nanocarriers capable of eliciting an immune system response in the form of antibody production, wherein the nanocarriers lack any T cell antigens. In some embodiments, the invention provides nanocarriers that comprise an immunofeature surface, which provides high avidity binding of the nanocarriers to antigen presenting cells. The invention provides pharmaceutical compositions comprising inventive nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive nanocarriers and pharmaceutical compositions thereof.
Owner:MASSACHUSETTS INST OF TECH +2
Stem cell mediated treg activation/expansion for therapeutic immune modulation
ActiveUS20080159998A1High activityInduce upregulationBiocideGenetic material ingredientsAntigenT cell
Disclosed are cells, methods of modulating cells, and therapeutic uses of the cells for the immune modulation of mammals in need thereof. Immune modulation including alteration of cytokine profile, cytotoxic activity, antibody production and inflammatory states is achieved through the administration of various cell types that have been unmanipulated or manipulated in order to endow specific biological activity. Cellular subsets and administration of the subsets in combination with various agents are also provided. One embodiment teaches the previously unknown finding that adipose tissue derived mononuclear cells contain T cells with immune regulatory properties that alone or synergistically with various stem cells induce immune modulation upon administration. Another embodiment is the finding that stimulation of stem cell activation results in stem cell secondary activation of immune modulatory cells, one type which is T regulatory cells (Tregs). One specific embodiment involves extraction of a heterogenous stem cell pool, which contains T regulatory cells, treatment in culture of the population with agents known to stimulate stem cell activation, then subsequent extraction and administration of the purified Tregs. Other embodiments include expansion of Tregs in the presence of antigen in order to generate anti-specific Tregs.
Owner:XON CELLS
Novel method for down-regulation of amyloid
Disclosed are novel methods for combatting diseases characterized by deposition of amyloid. The methods generally rely on immunization against amyloid precursor protien (APP) or beta amyloid (Abeta). Immunization is preferably effected by administration of analogues of autologous APP or Abeta, said analogues being capable of inducing antibody production against the autologous amyloidogenic polypeptides. Especially preferred as an immunogen is autologous Abeta which has been modified by introduction of one single or a few foreign, immunodominant and promiscuous T-cell epitopes. Also disclosed are nucleic acid vaccination against APP or Abeta and vaccination using live vaccines as well as methods and means useful for the vaccination. Such methods and means include methods for the preparation of analogues and pharmaceutical formulations, as well as nucleic acid fragments, vectors, transformed cells, polypeptides and pharmaceutical formulations.
Owner:H LUNDBECK AS
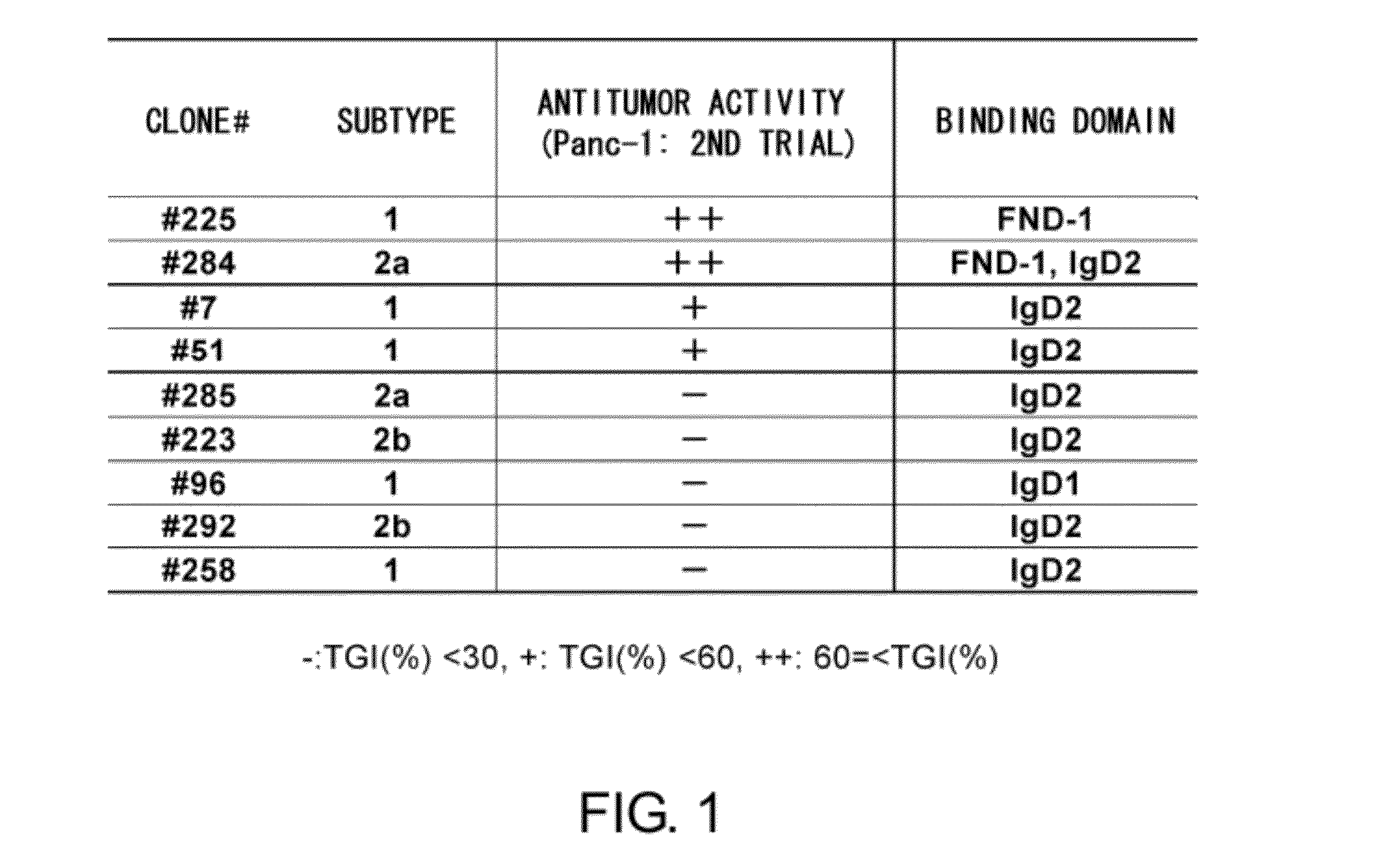
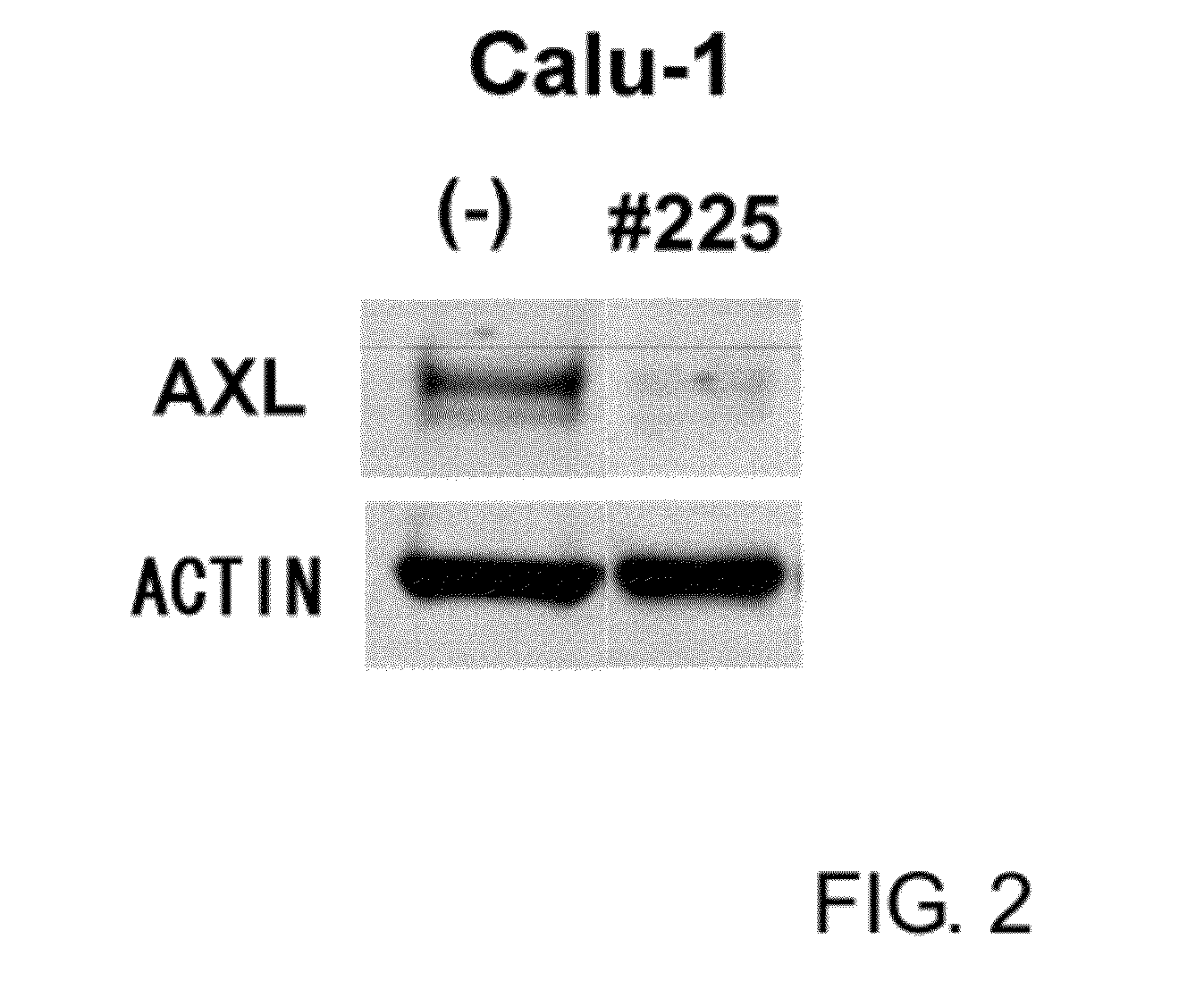
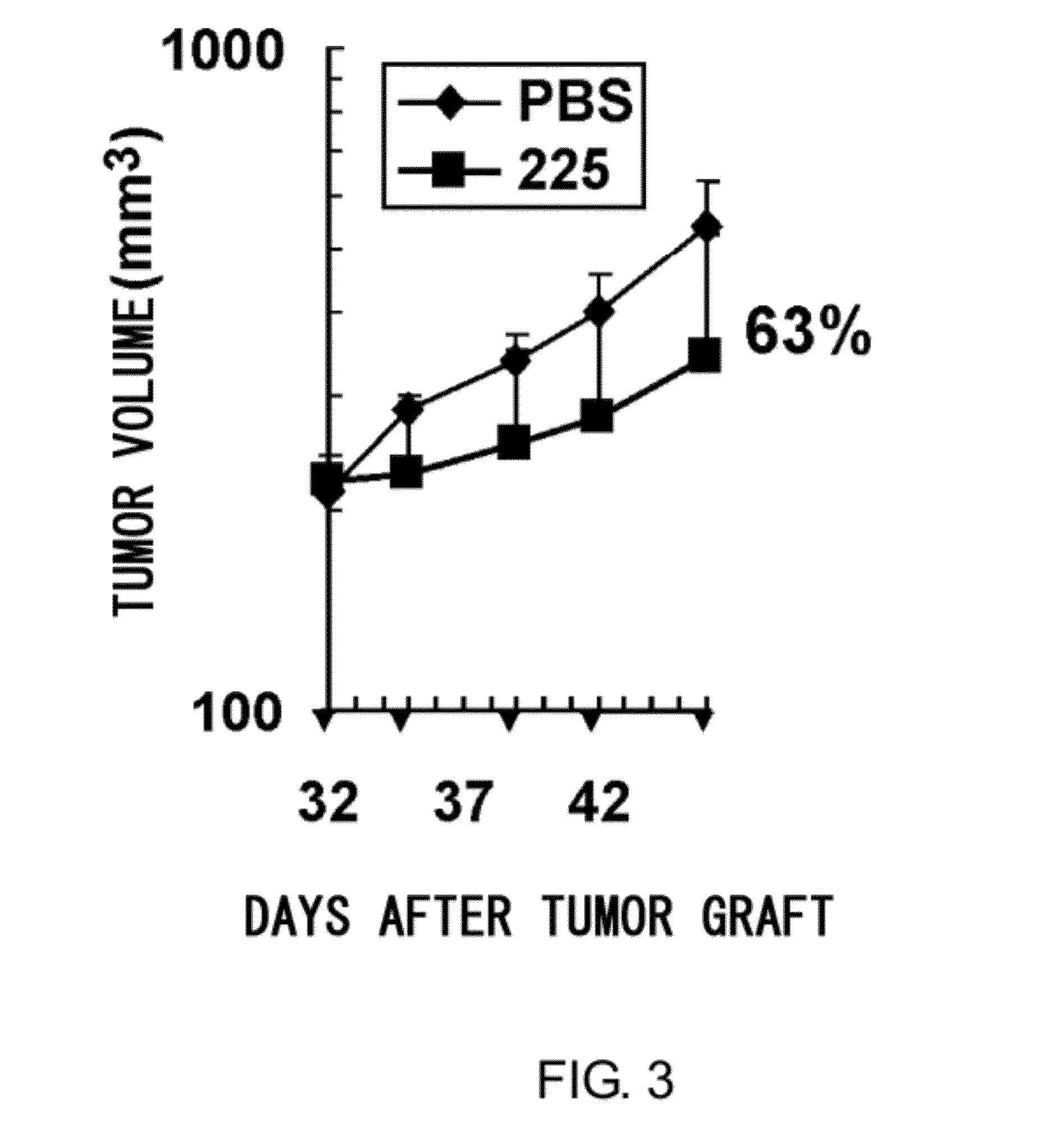
Anti-axl antibody
ActiveUS20120121587A1Low immunogenicityHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiagnostic agentHumanized antibody
An objective of the present invention is to decrease the immunogenicity of mouse-derived anti-AXL antibodies in humans by humanizing them. The present invention provides antibodies that can bind to a specific region in Anexelekto (AXL) and humanized antibodies that are produced based on such antibodies. The anti-AXL antibodies of the present invention have high antitumor activity, and are useful as agents for decreasing the AXL expression level, antitumor agents, and diagnostic agents for cancer.
Owner:CHUGAI PHARMA CO LTD
Control of antibody responses to synthetic nanocarriers
Disclosed are synthetic nanocarrier compositions that comprise B cell antigen for desired antibody production and an off-target response attenuating polymeric coating as well as related methods.
Owner:SELECTA BIOSCI
Genetically altered antibody-producing cell lines with improved antibody characteristics
InactiveUS20050048621A1Increase variabilityBetter pharmacokinetic profileAnimal cellsSugar derivativesBiological bodyGenetic diversity
Dominant negative alleles of human mismatch repair genes can be used to generate hypermutable cells and organisms. Cells may be selected for expression of activation-induced cytidine deaminase (AID), stimulated to produce AID, or manipulated to express AID for further enhancement of hypermutability. These methods are useful for generating genetic diversity within immunoglobulin genes directed against an antigen of interest to produce altered antibodies with enhanced biochemical activity. Moreover, these methods are useful for generating antibody-producing cells with increased level of antibody production.
Owner:EISAI INC
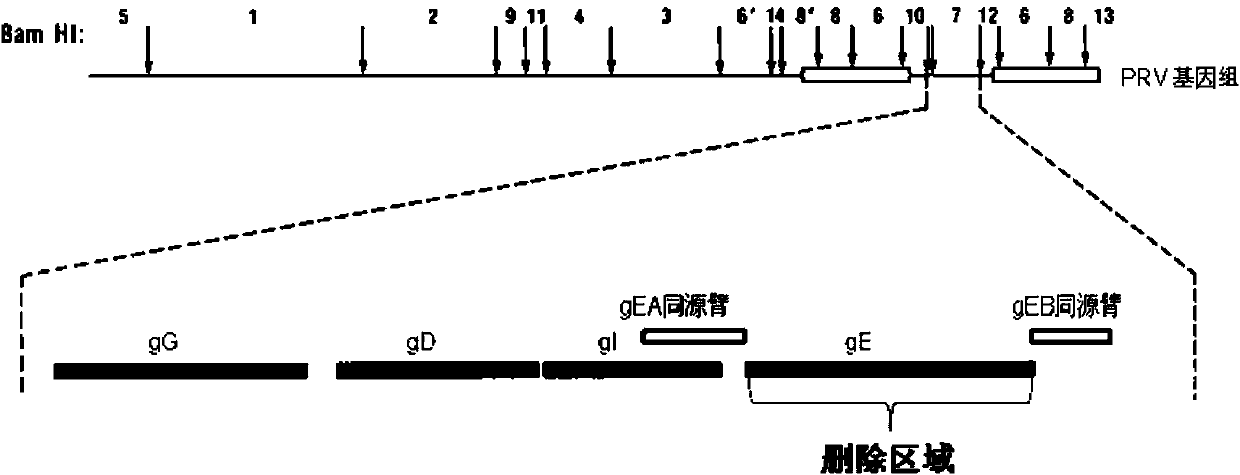
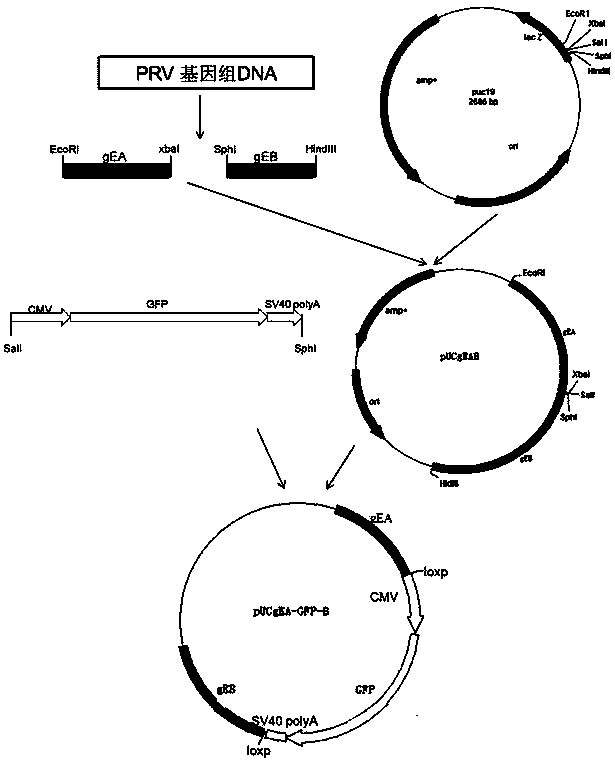
Porcine pseudorabies virus gene deletion strain, vaccine composition, and preparation method and application of vaccine composition
ActiveCN103923884ASymptoms relieved or improvedMicroorganism based processesAntiviralsVirus antigenTGE VACCINE
The invention provides a porcine pseudorabies virus gene deletion strain, a vaccine composition, and a preparation method and an application of the vaccine composition. The vaccine composition comprises an immunizing dose of an attenuated livetotivirus antigen and an inactivated totivirus antigen of the porcine pseudorabies virus gene deletion strain or its culture. The vaccine composition can effectively induce the antibody production, can effectively protect pigs, and can be used as a marking vaccine to effectively differentiate wild strains and vaccine strains.
Owner:PU LIKE BIO ENG
Antibody production
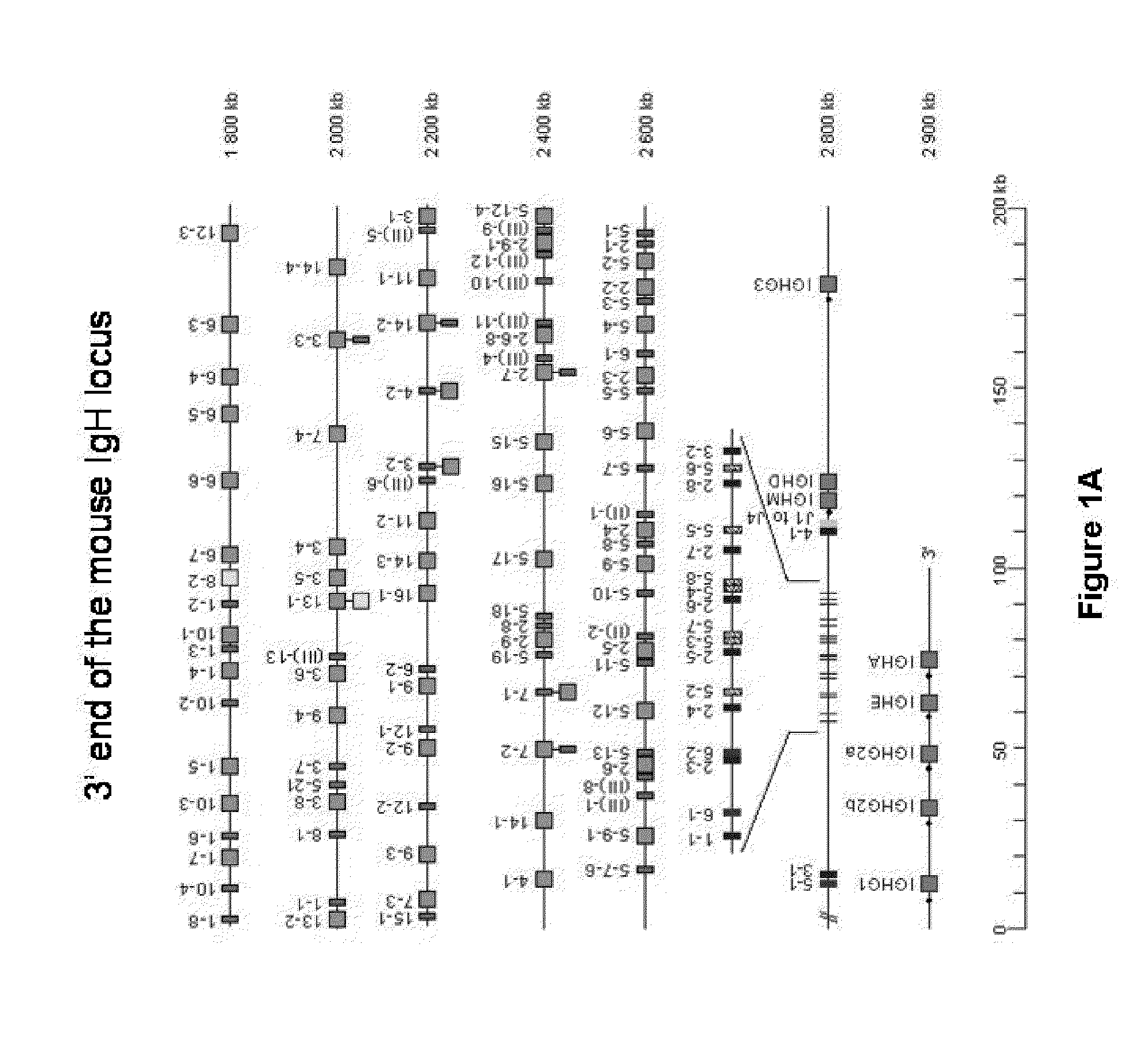
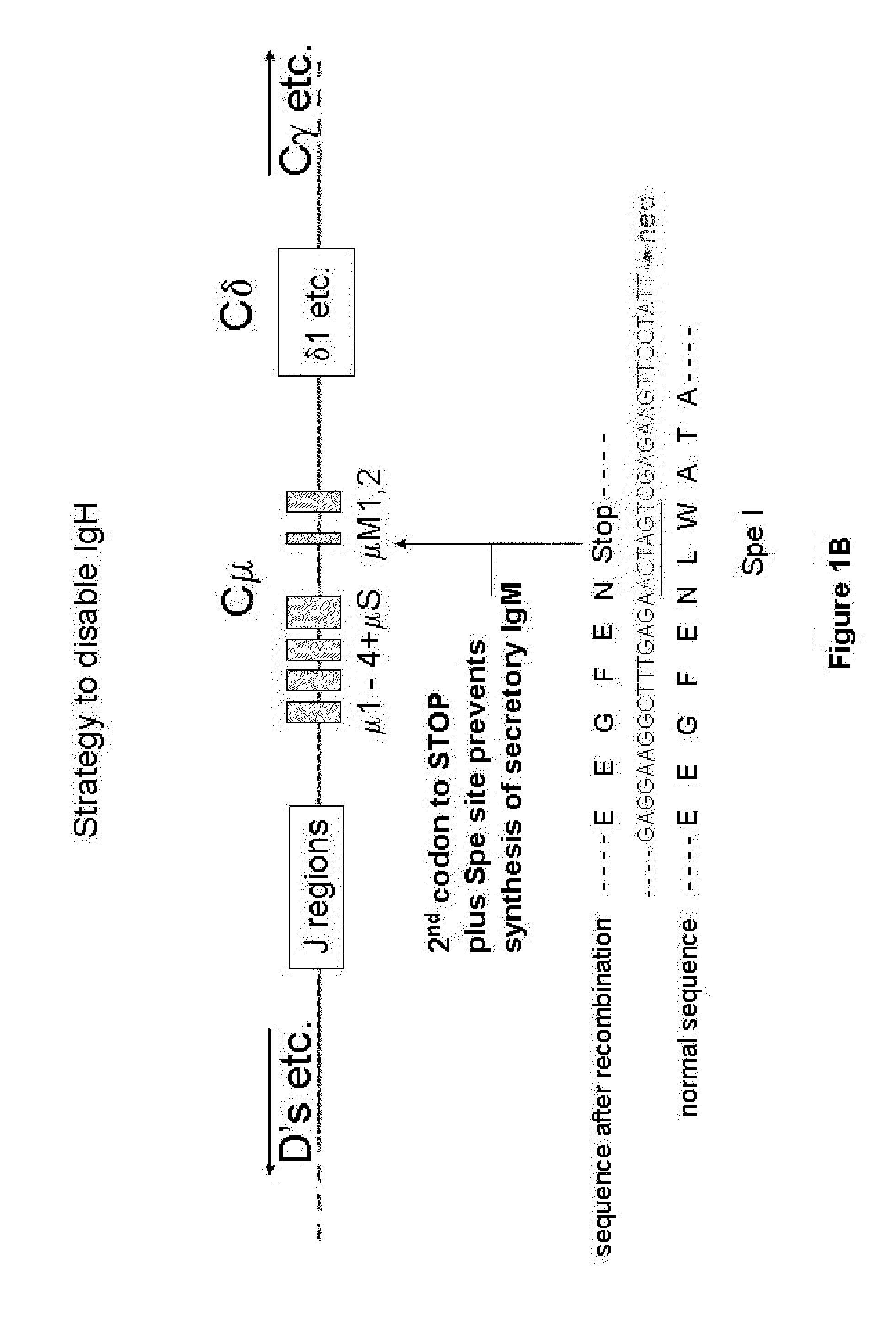
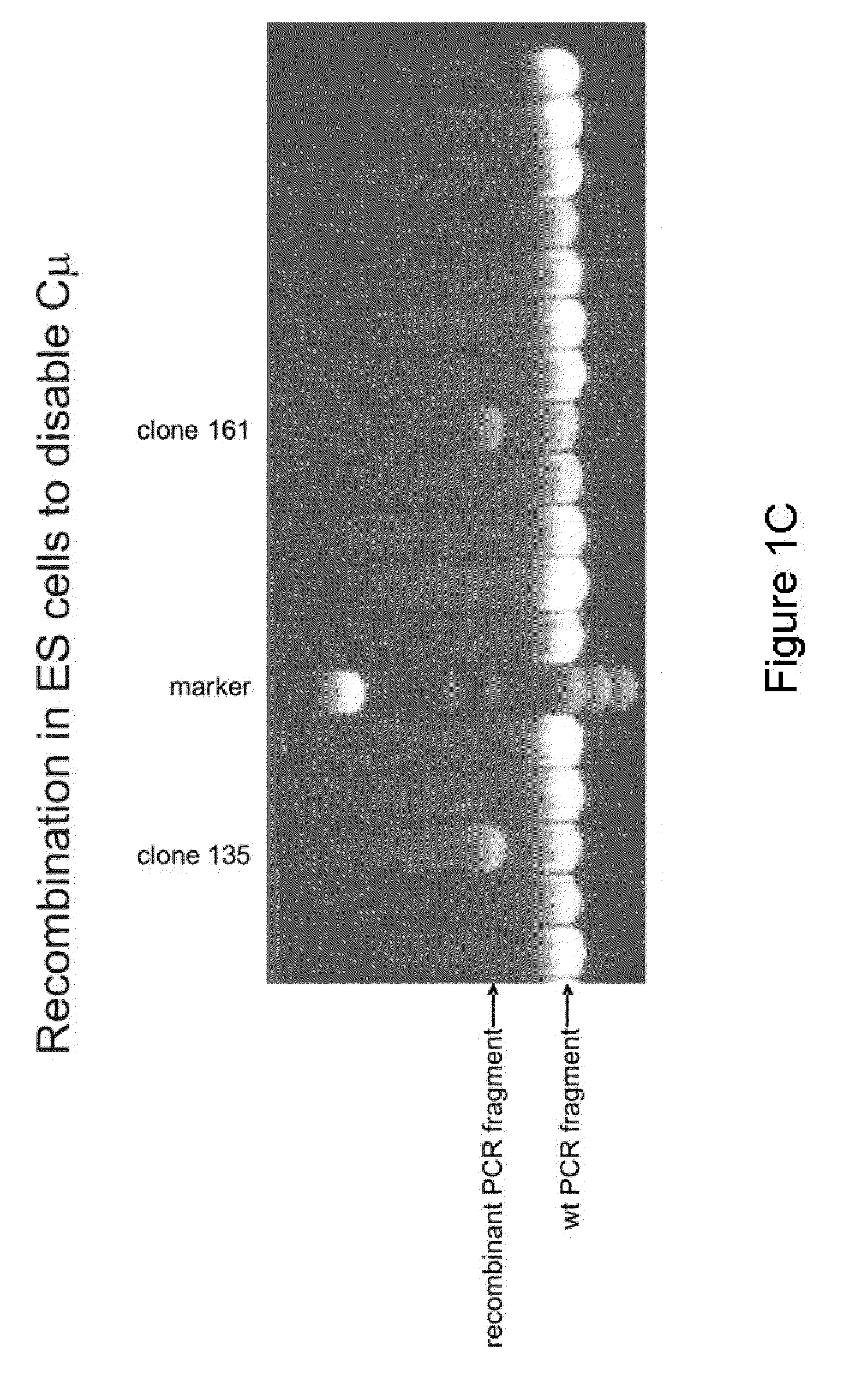
InactiveUS20110314563A1Antibody repertoire and diversityIncrease probabilityImmunoglobulinsImmunological disordersImmunoglobulin heavy chainAntigen challenge
A non-human mammal containing an endogenous lambda light chain gene locus, an endogenous kappa light chain gene locus and an endogenous heavy chain gene locus, each of which can re-arrange so that immunoglobulin heavy and light chain genes are formed and expressed in B-cells following antigen challenge but said loci have been mutated so that the ability to form functional immunoglobulin tetramers comprising re-arranged heavy and light chains produced from said mutated loci has been substantially reduced or eliminated.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
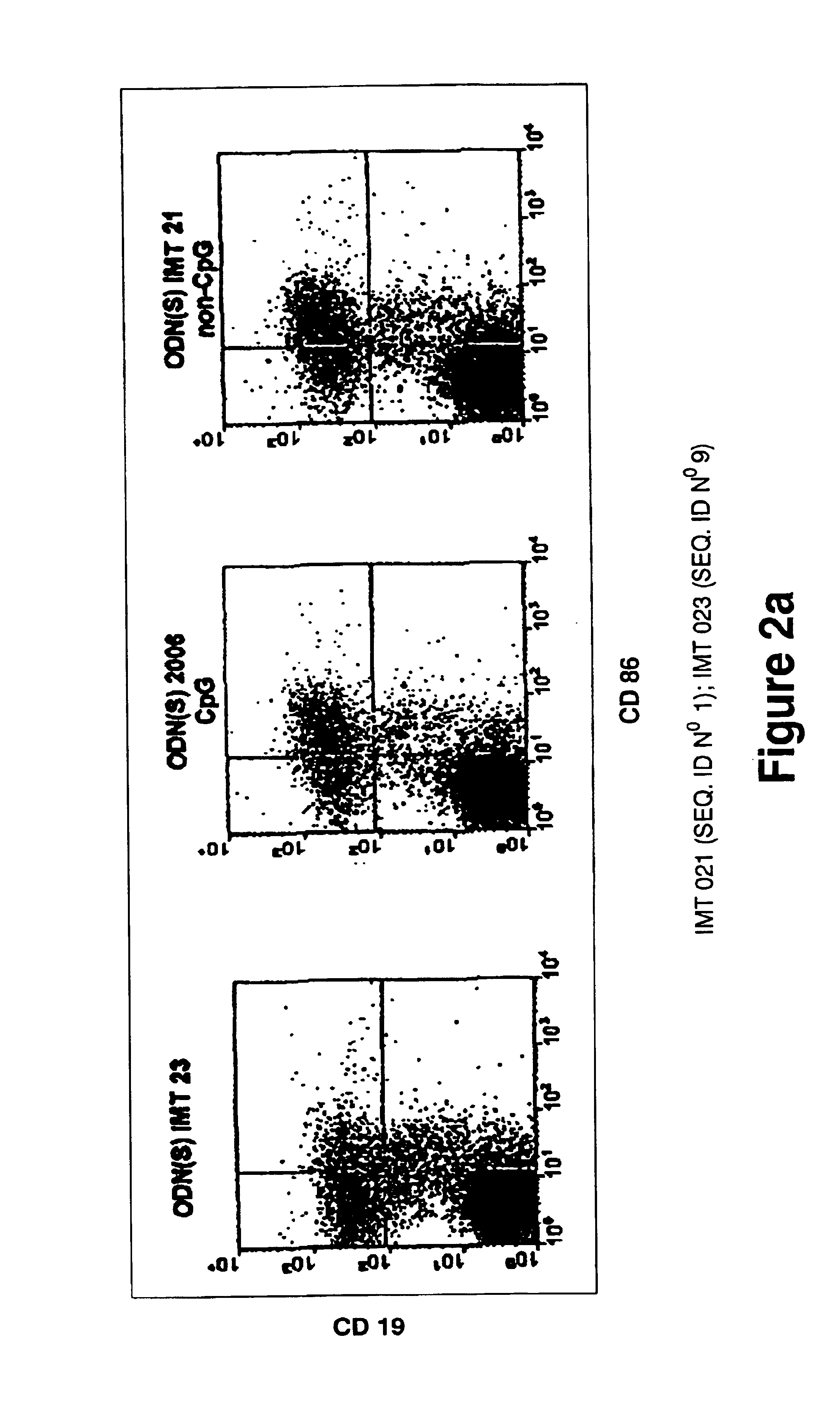
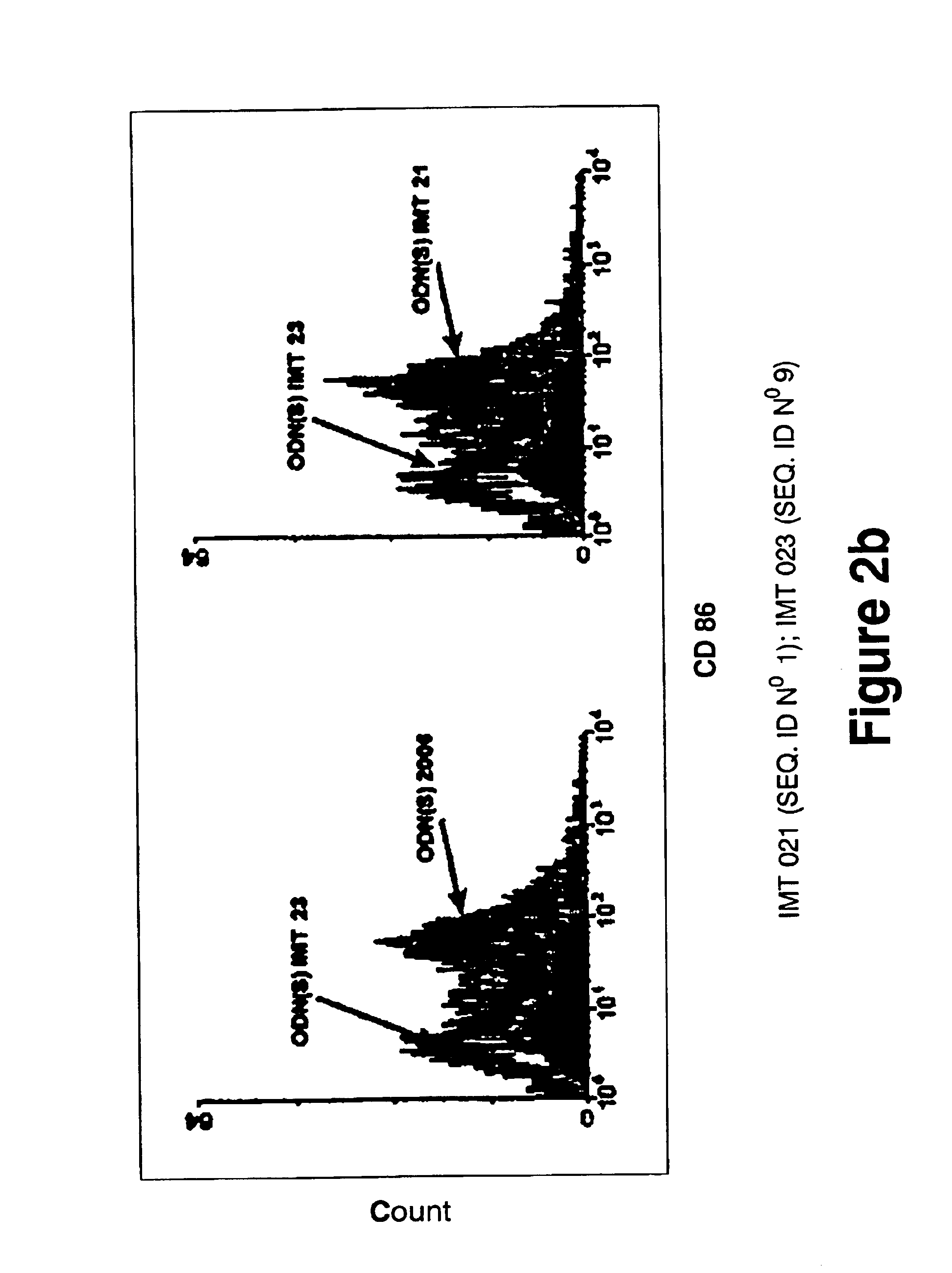
Immunostimulatory oligonucleotides and uses thereof
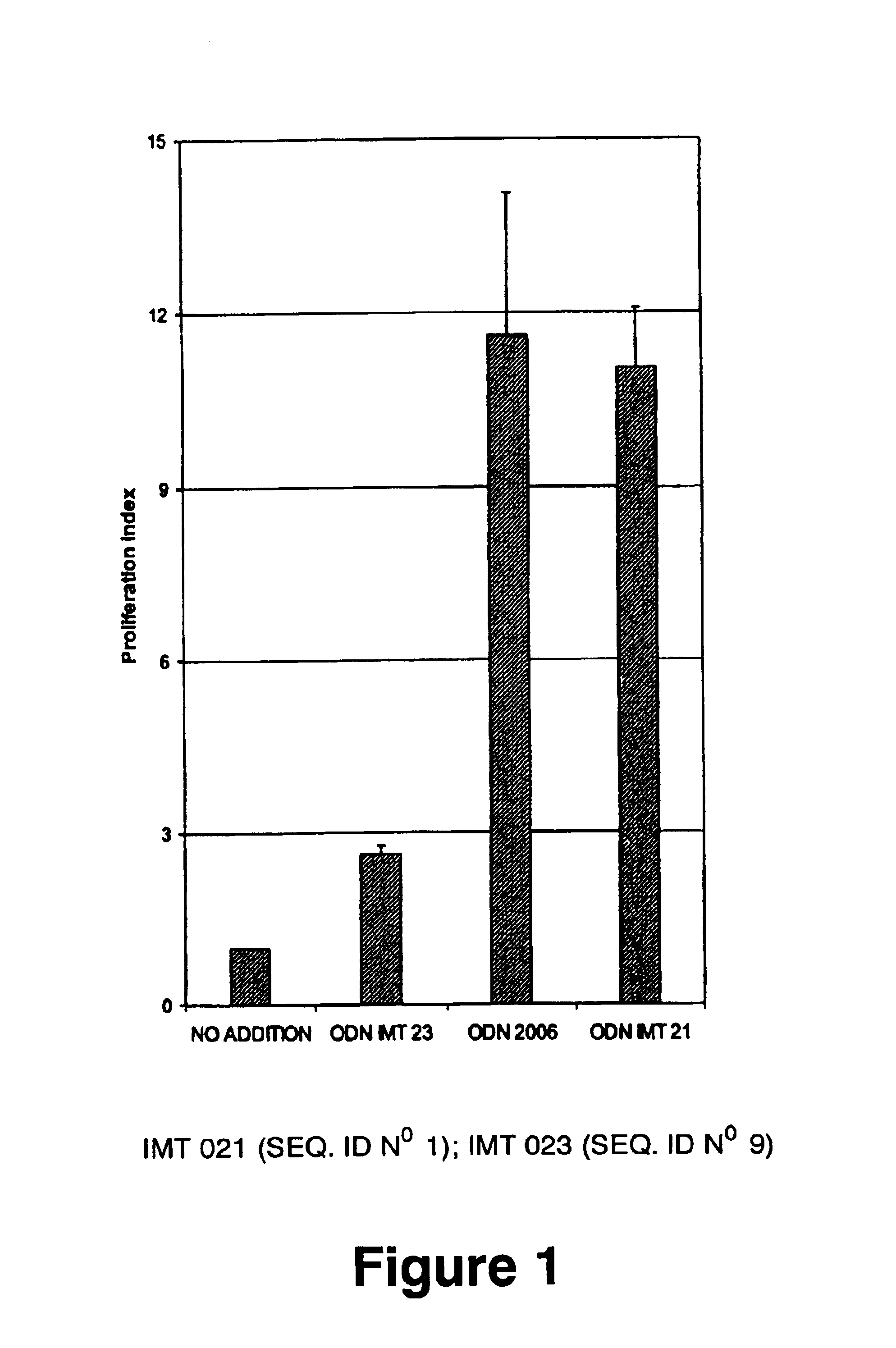
Oligonucleotides containing the non-palindromic sequence motif:X1X2X3X4X5X6X7X8,wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
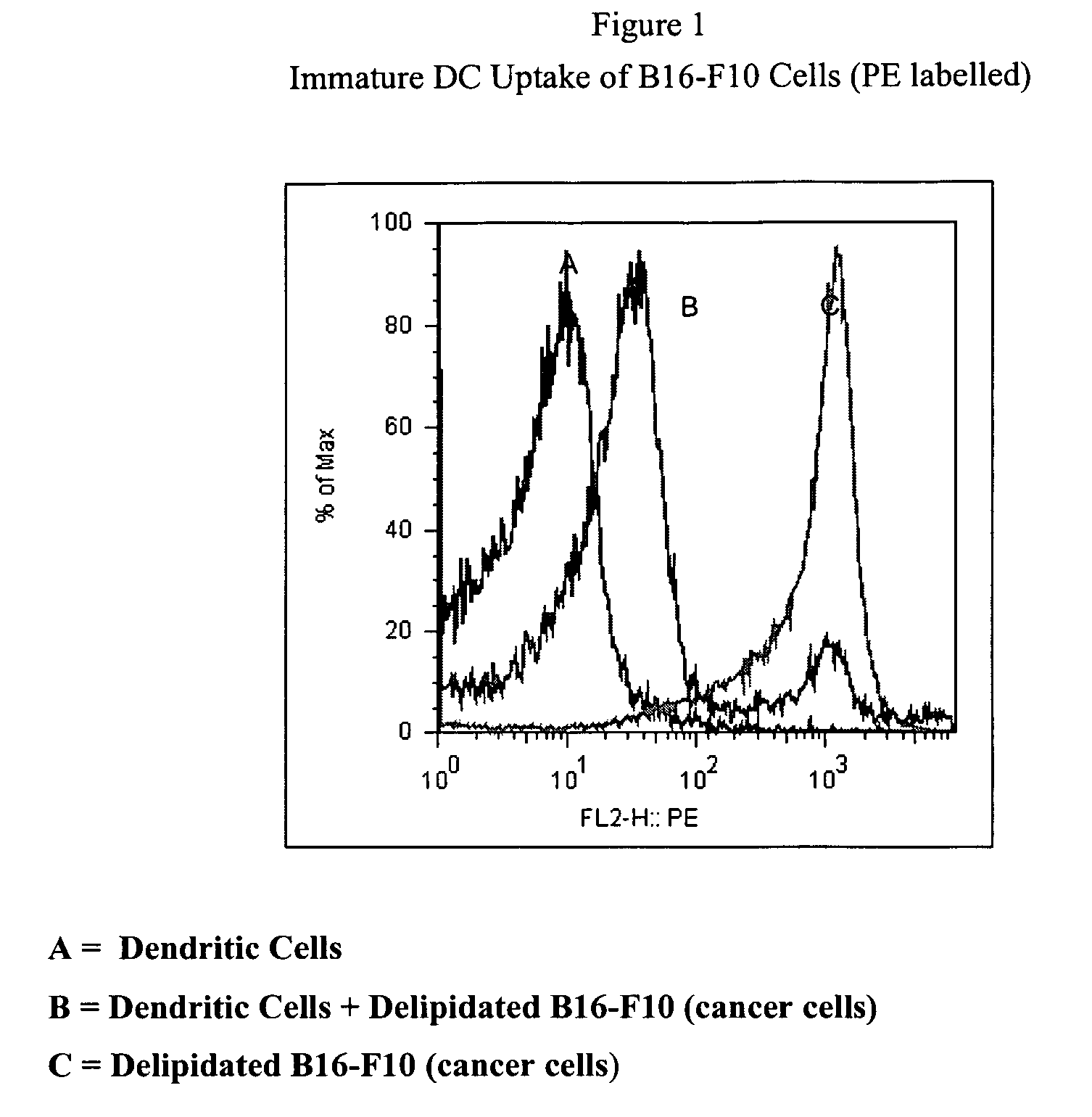
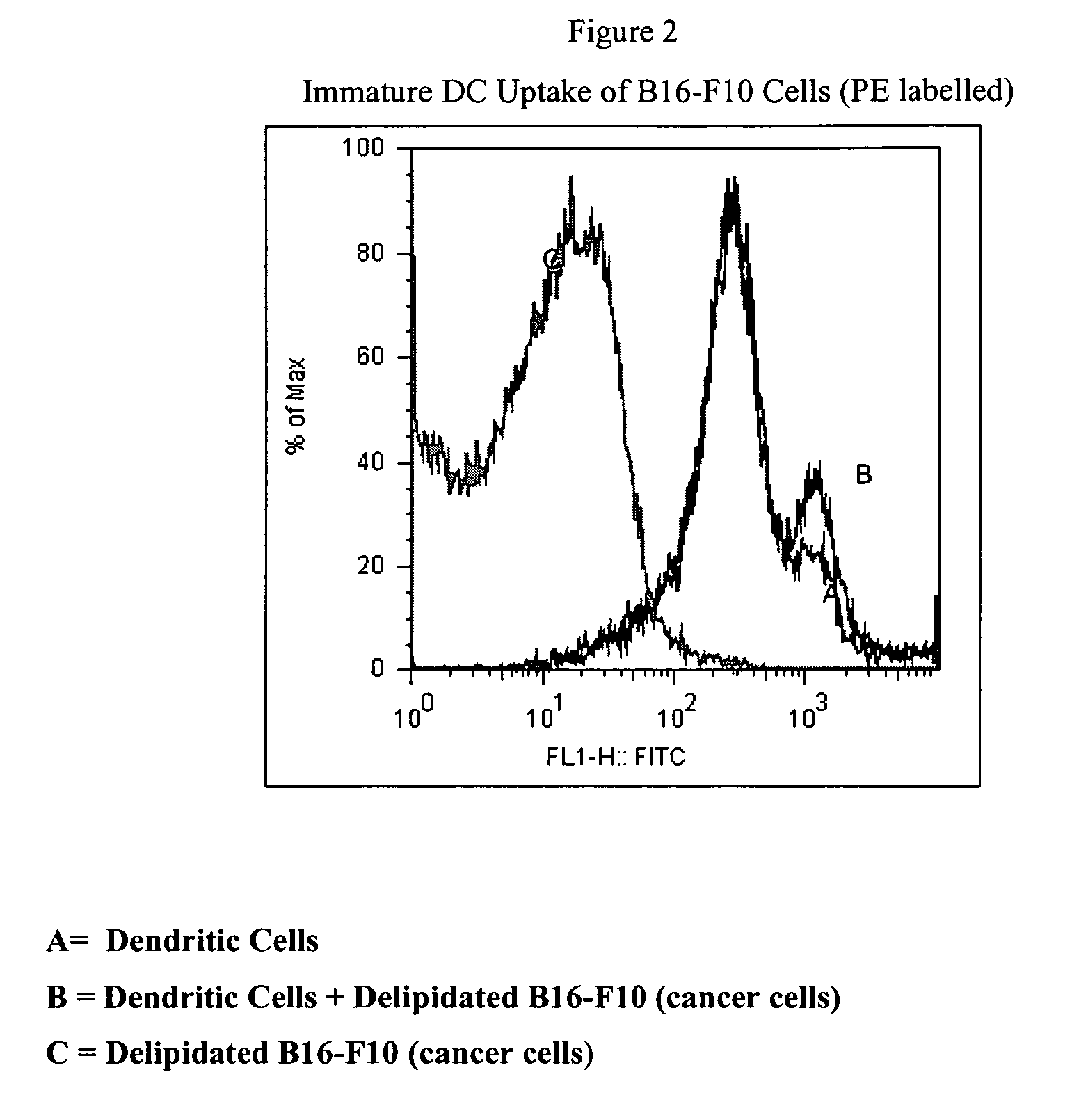
Method of treating cancer cells to create a modified cancer cell that provokes an immunogenic response
InactiveUS7361360B2Preventing occurrence and reoccurrenceSlow onsetSnake antigen ingredientsDead animal preservationLipid formationHeterologous vaccine
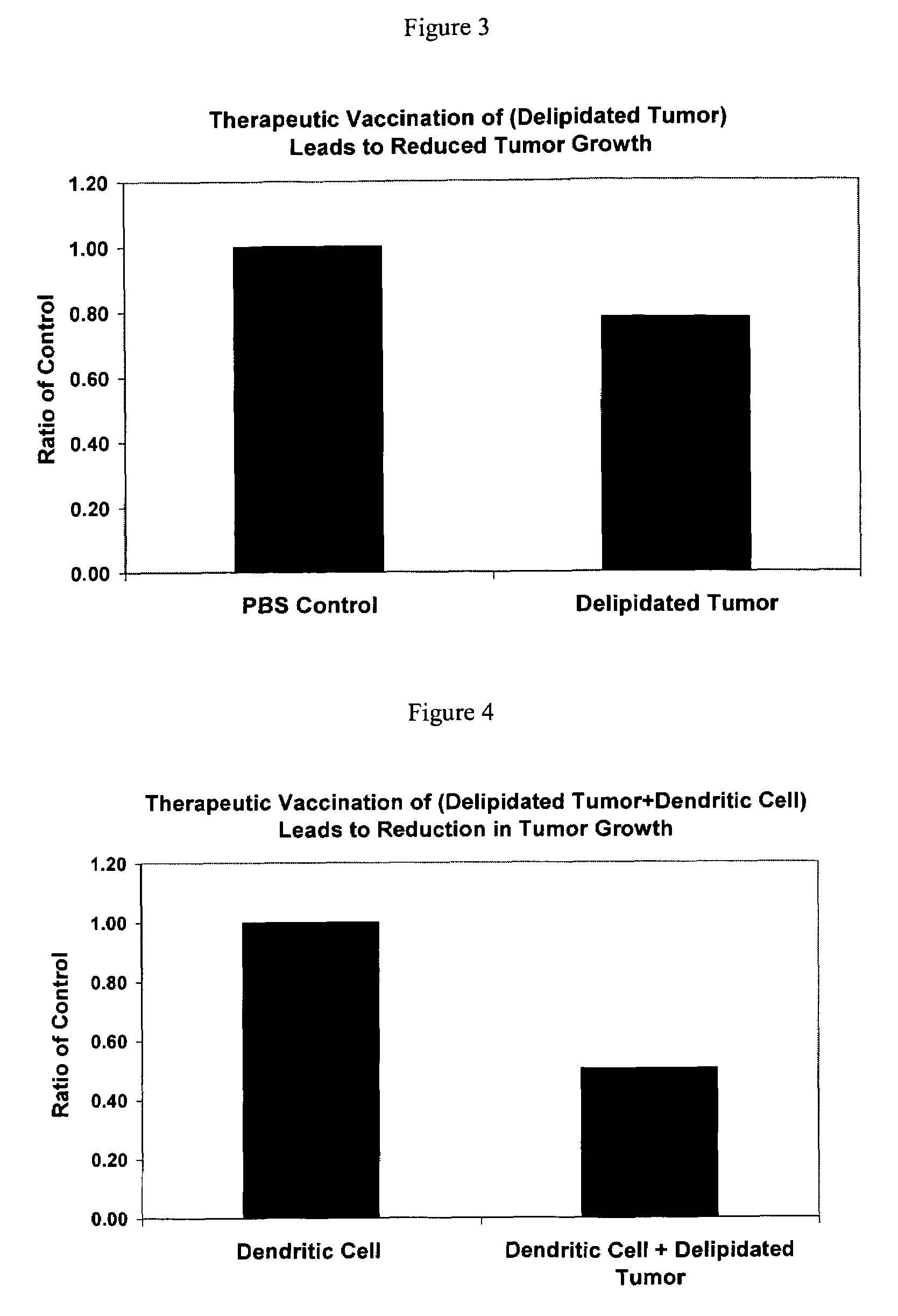
The present invention relates to a delipidation method employing a solvent system useful for extracting lipids from cancer cells, thereby creating a modified cancer cell particle. Upon delipidation of the cancer cells, a portion of the cancer cell antigens remain intact. These exposed antigens, or epitopes, foster and promote antibody production. The resulting modified cancer cell particle, or portions of the cancer cell, initiate a positive immunogenic response when administered to an animal or human and help to treat, prevent or delay the onset of cancer. The present invention provides autologous and heterologous vaccine compositions comprising the modified cancer cell with a pharmaceutically acceptable carrier. The present invention provides method of administering these vaccines to treat, prevent or delay the onset of cancer.
Owner:ELI LILLY & CO +1
Method and apparatus for antibody production and purification
ActiveUS20150111252A1Minimize timeLong productionBioreactor/fermenter combinationsBiological substance pretreatmentsBiochemistryAntibody production
The subject invention pertains to methods and apparatus for the production and purification of cell products, such as immunoglobulins. One aspect of the invention is an integrated cell culture and purification apparatus for the growth and maintenance of cells and the harvest and purification of cell products, such as immunoglobulins. Thus, the apparatus integrates a cell culture function with a purification function. Other aspects of the invention pertain to an automated method for producing immunogenic compositions such as vaccines.
Owner:BIOVEST INT
Methods of treating rheumatoid arthritis using anti-TNF receptor fusion proteins
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:CENTOCOR +1
Immunosuppressant binding antibodies and methods of obtaining and using same
The present invention relates among other things to antibodies that immunospecifically bind to at least one agent of interest (e.g., an immunosuppressive agent), methods for producing such antibodies, and immunoassays that employ said antibodies. Additionally, the present invention also relates to methods for selecting an antibody for use in a diagnostic immunoassay and methods for selecting an antigen for use in a diagnostic immunoassay. The present invention further relates to the improvement of antibody recognition of an active parent drug in the presence of one or more of its major metabolites.
Owner:ABBOTT LAB INC
Antibodies specific for native PrPSc
InactiveUS6372214B1Fast and efficient cost-effective assayFast and efficientImmunoglobulins against animals/humansHybrid cell preparationDiseaseMammal
Owner:THE SCRIPPS RES INST
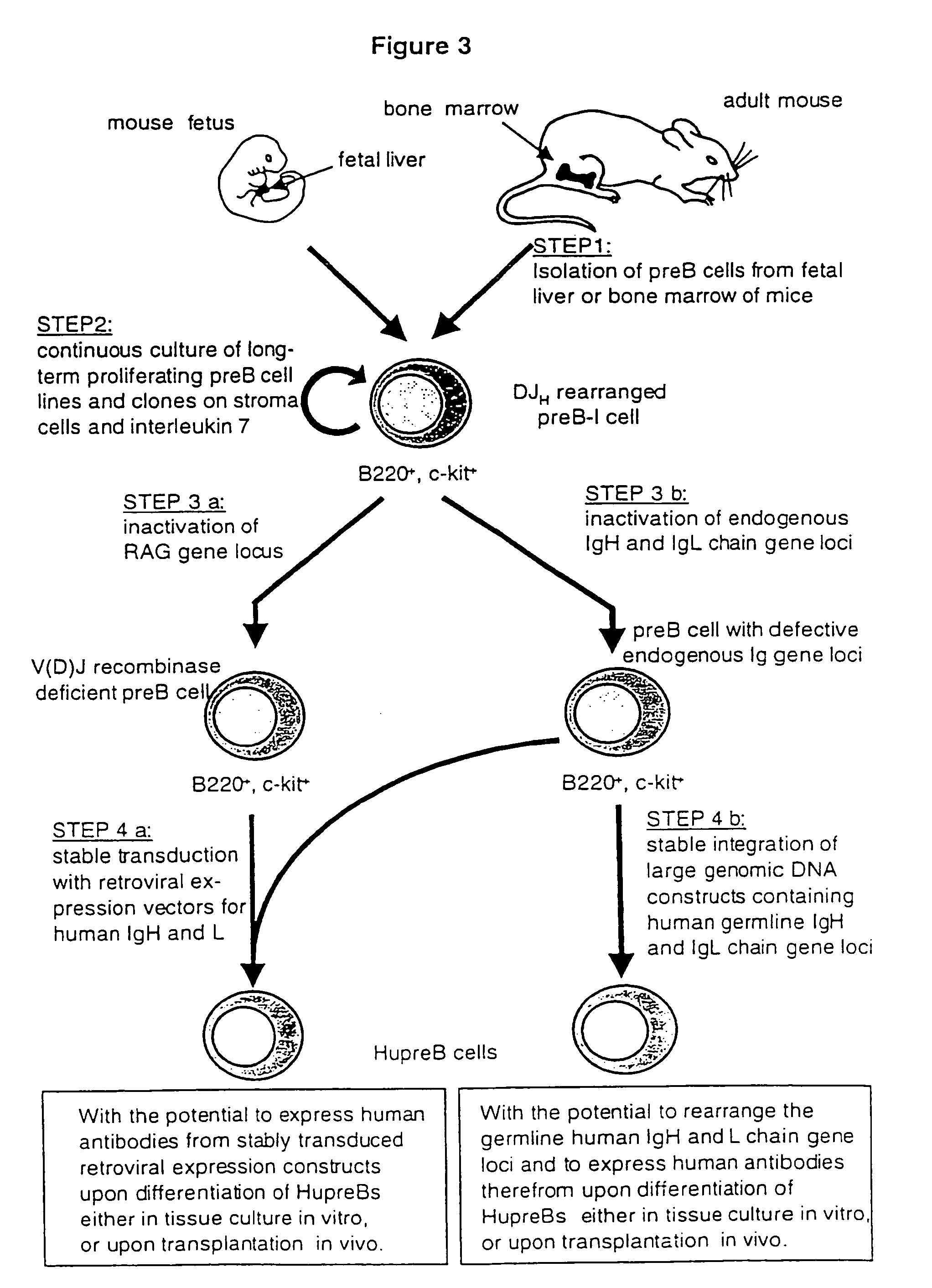
Method for the generation of genetically modified vertebrate precursor lymphocytes and use thereof for the production of heterologous binding proteins
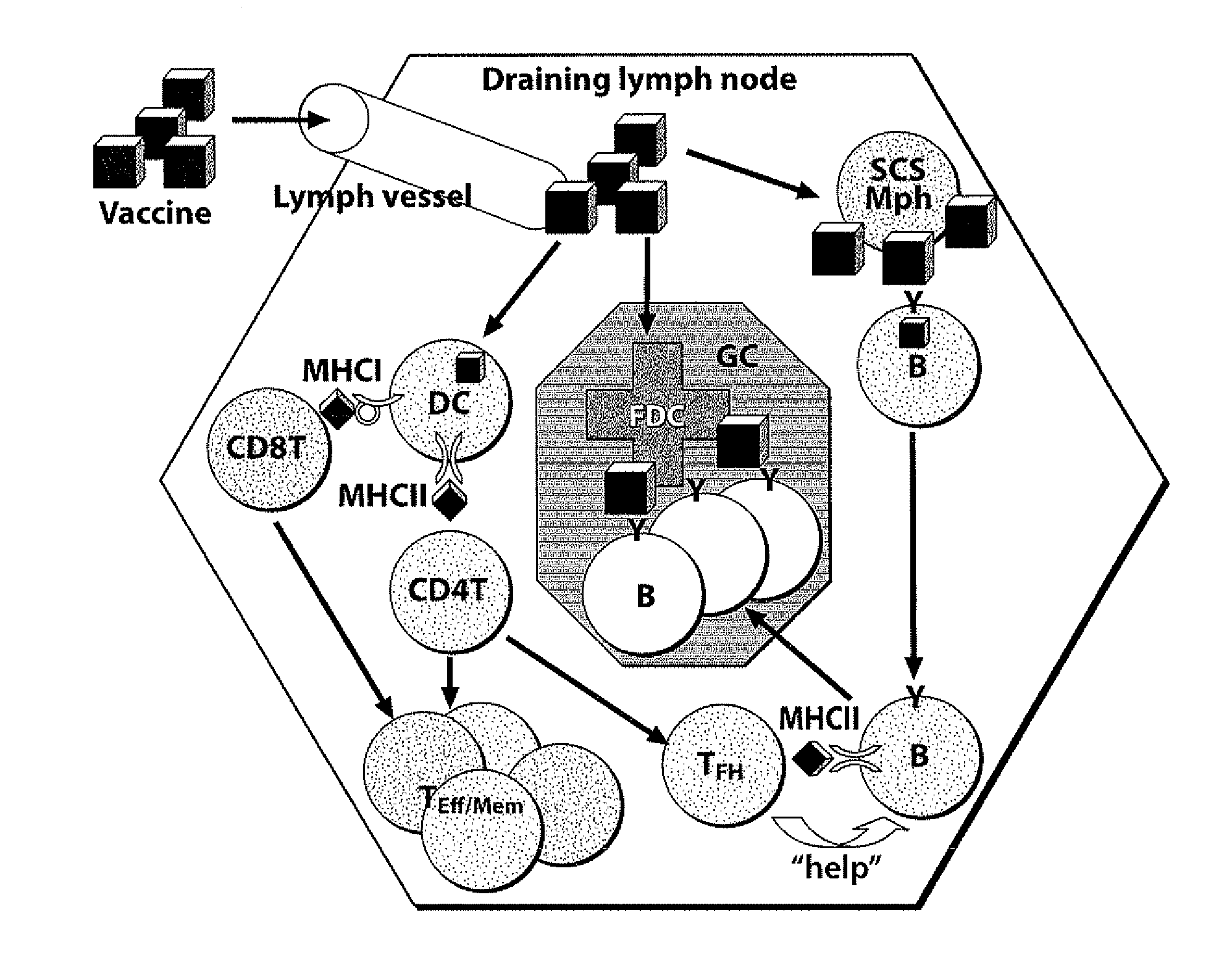
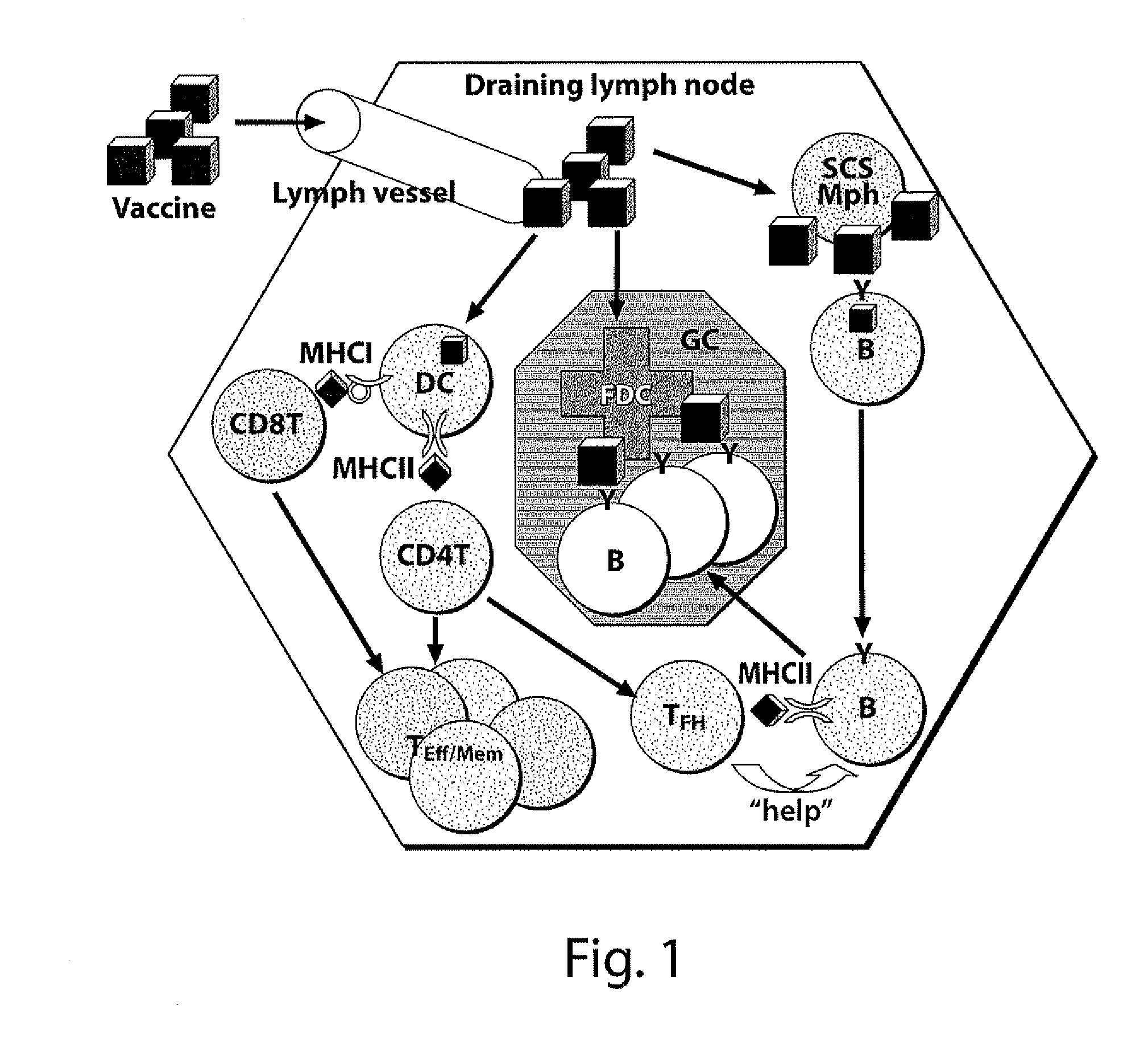
The present invention generally relates to the fields of genetic engineering and antibody production. In particular, it relates to the generation of genetically modified vertebrate precursor lymphocytes that have the potential to differentiate into more mature lymphoid lineage cells, and to the use thereof for the production of any heterologous antibody or binding protein.
Owner:AGENUS INC
Methods of treating psoriasis using anti-TNF receptor fusion proteins
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:CENTOCOR +1
Use of parasitic biological agents for prevention and control of allergic and other IgE-mediated disorders
InactiveUS20070087020A1Reduce and eliminate and ameliorate inappropriate immune responseStimulate immune responseProtozoa antigen ingredientsLeech/worm material medical ingredientsDiseaseInterleukin 10
The present invention describes using, on a repetitive basis, a non-human colonizing helminth compound, in an amount sufficient to establish a transitory parasitic helminth infection and or to simulate in a parasitic helminth infection, thereby having immunosuppressive effect against benign antigens and or stimulating a regulatory immune response characterized by the production of T helper cells 2 (Th2), T regulatory helper cells (TREG) and certain cytokines, including, but not limited to interleukin 10 (IL-10), as a therapy or prophylaxis of allergy and other IgE-mediated disorders, which are marked by an inappropriate IgE immune response including, but not limited to an aberrant and or enhanced IgE antibody production to benign antigens. The invention presents using helminth compound by administering it in a frequency and amount sufficient to eliminate or ameliorate the inappropriate immune response in an asthmatic and or allergic individual.
Owner:MILESTONE RES
Rhodococcus ruber and application of same as immunologic adjuvant in preparing vaccine
ActiveCN109576180AWill not cause accidental infectionReduce pollutionBacteriaMicroorganism based processesSide effectShort terms
The invention discloses rhodococcus ruber and application of same as an immunologic adjuvant in preparing vaccine. The rhodococcus ruber is also called rhodococcus ruber RDC-01, and the preservation number is CGMCC (China General Microbiological Culture Collection Center) NO. 16640. The rhodococcus ruber disclosed by the invention has the function of increasing and regulating the body immunity andis capable of nonspecifically enhancing the activity of TB (Tuberculosis) lymphocyte, macrophagocyte and NK cells and inducing multiple cell factors such as interferon, and the rhodococcus ruber canbe used as the immunologic adjuvant after being inactivated so as to be added in an oil-adjuvant inactive vaccine, so that generation of an animal antibody induced by the vaccine can be obviously promoted; compared with single use of the oil-adjuvant inactive vaccine, a high-titre antibody can be generated, the use is safe, long-term and short-term toxic and side effects are not generated, and anapplication prospect in the field of preparation of vaccines for animals is good.
Owner:北京利昂盛生物技术有限公司
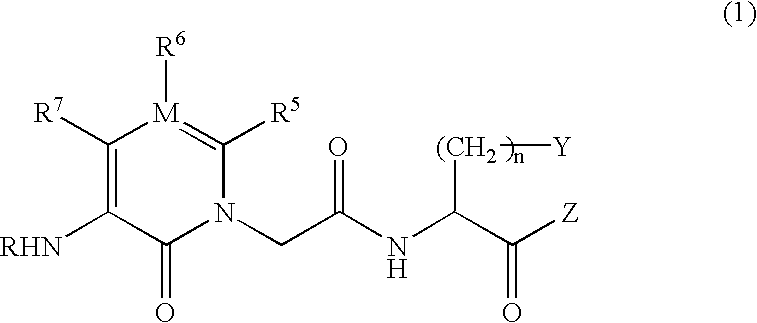
IgE antibody production inhibitors and autoimmune diseases inhibitors
InactiveUS6528514B1High chymase inhibitory actionExcellent IgE antibody production inhibitory actionBiocideNervous disorderDiseaseAryl
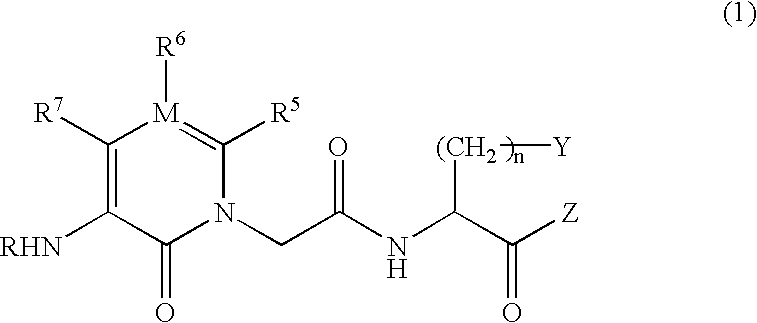
The present invention relates to an IgE antibody production inhibitor and an autoimmune disease suppressant containing a heterocyclic amide compound represented by the following general formula (1) or a pharmaceutically acceptable salt thereof as an active ingredientwherein R represents a hydrogen atom, alkyl, -CHO, -COOH, etc.; R5, R6 and R7 represent each hydrogen, alkyl, aryl, etc.; M represents a carbon atom or a nitrogen atom; Y represents aryl, etc.; and Z represents hydrogen, alkyl, aryl, etc.
Owner:MITSUBISHI TANABE PHARMA CORP
Probiotics as alternative medicines against infectious diseases
An exemplary embodiment providing one or more improvements includes feeding animals with probiotic microbes encapsulated in a mixture of xanthan gum and chitosan, or in gelatin, specifically Pediococcus acidilactici and Saccharomyces boulardii. Such encapsulation protects the viability of the probiotic microbes against unfavorable temperatures. An exemplary embodiment providing one or more improvements includes methods of using viable probiotics in therapy of birds and mammals infected with infectious diseases. Probiotics acted as adjuvants in stimulating antibody reaction and stimulated a cellular immunity response. In particular, probiotics were shown to reduce the number of viable oocytes from fecal samples, stimulate antibody production, and stimulate of proliferation of splenocytes in chickens infected with Elimeria. In addition, probiotics were shown to relieve symptoms of parvovirus infection in dogs.
Owner:IMAGILIN TECH LLC
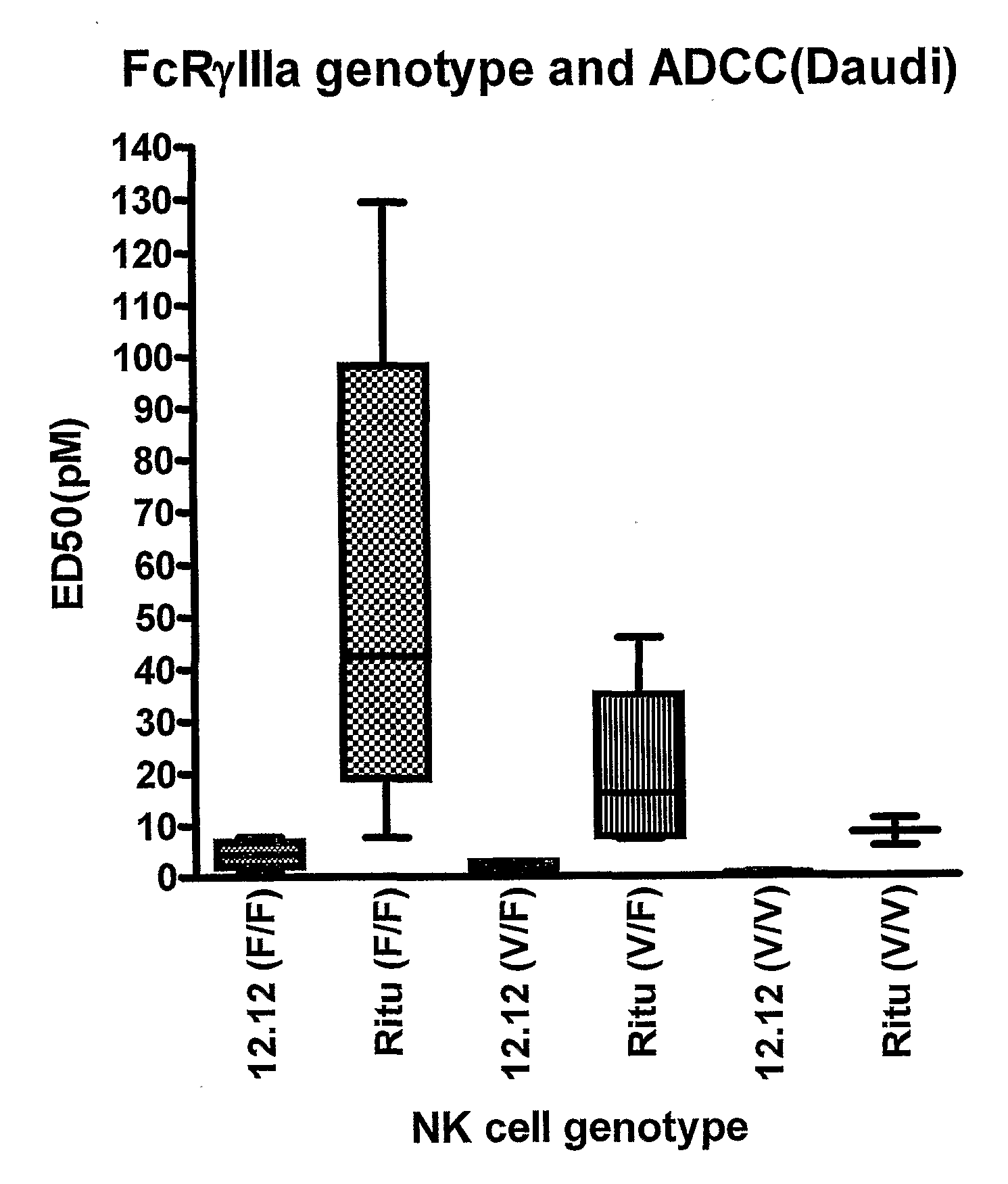
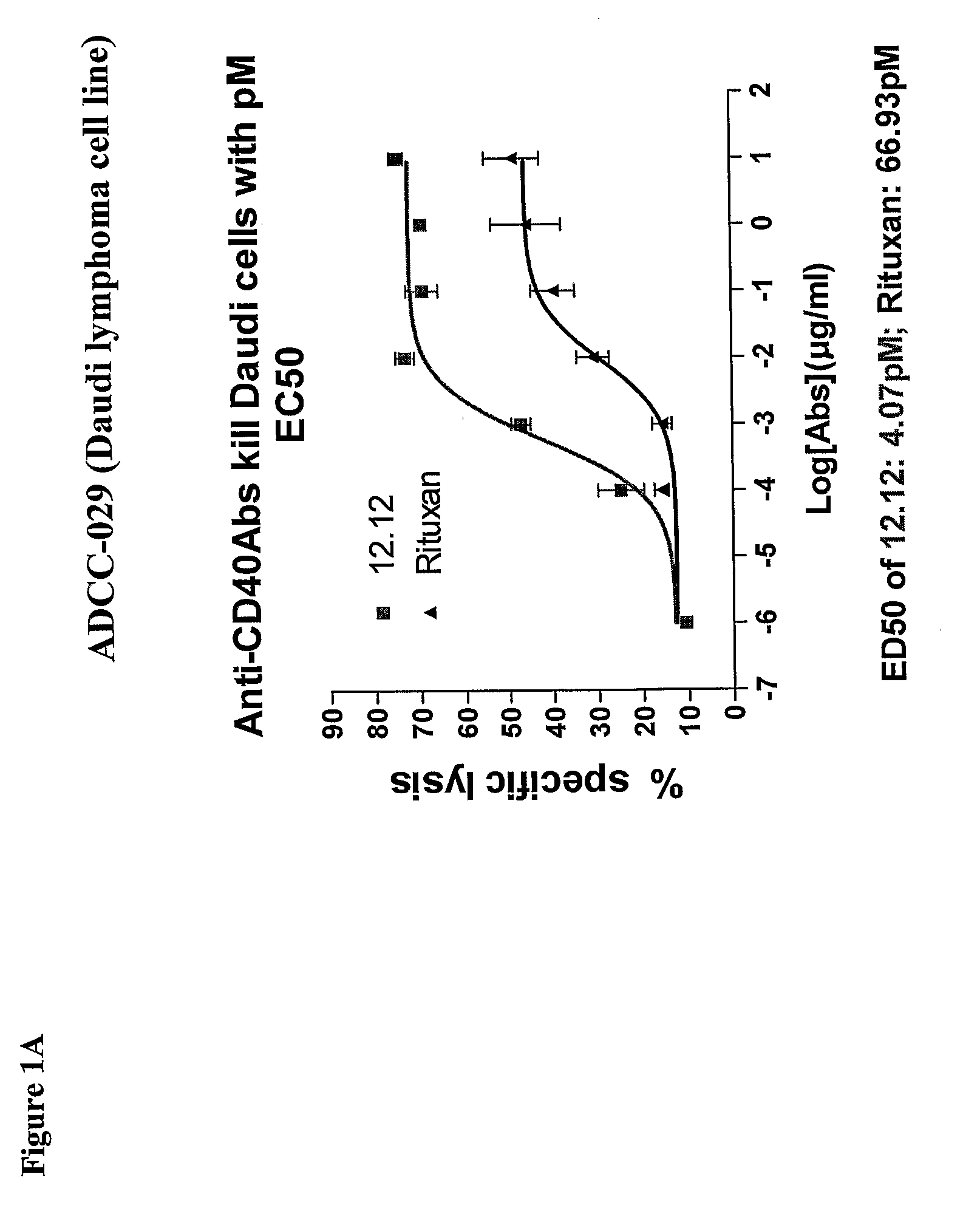
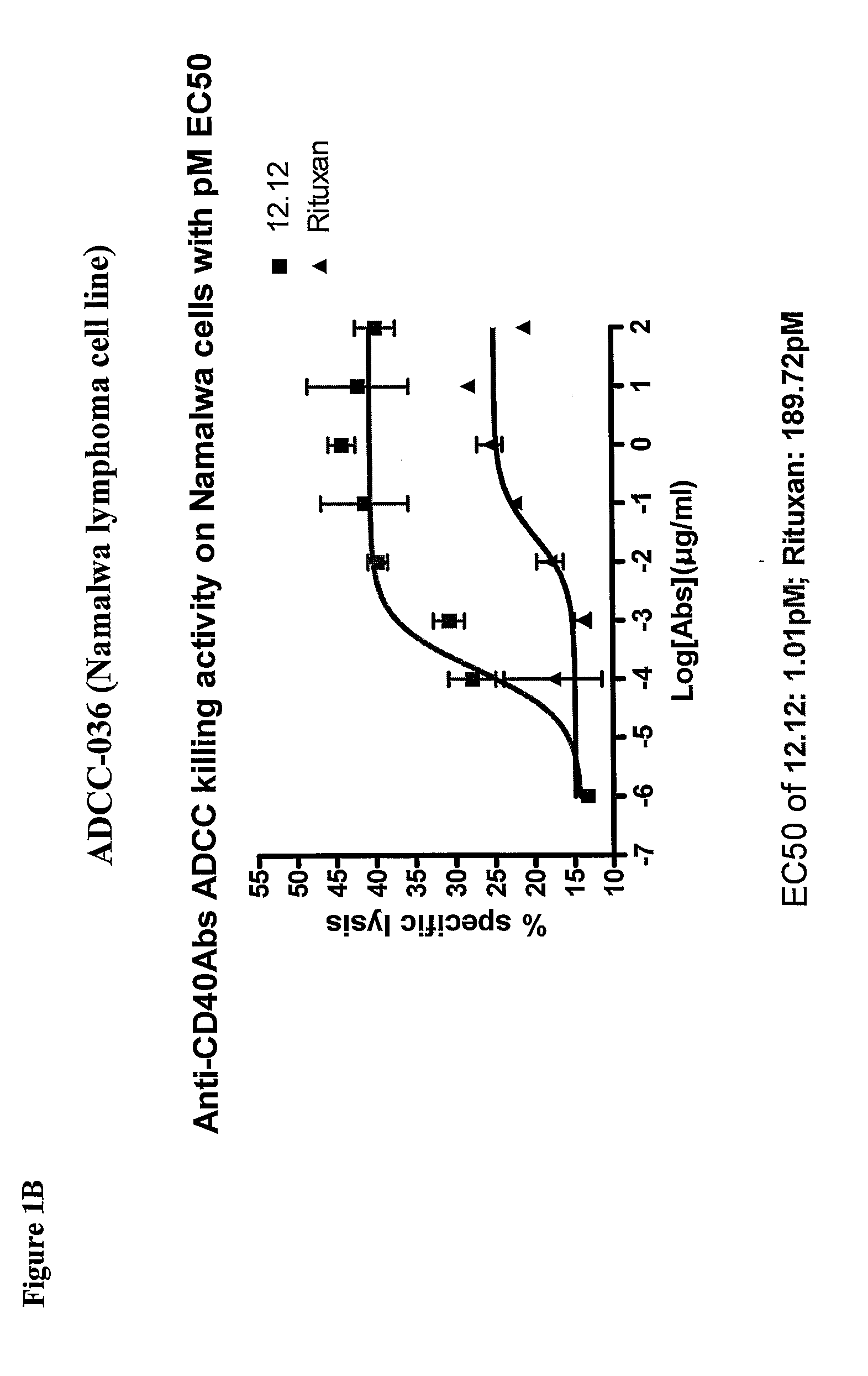
Uses of Anti-cd40 antibodies
Methods for treating a human patient for a cancer or pre-malignant condition that is associated with CD40-expressing cells are provided, where the human patient is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). Also provided are methods of inhibiting antibody production by B cells in a human patient who is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). The methods comprise administering to the human patient a therapeutically or prophylactically effective amount of an anti-CD40 antibody. Methods and kits for identifying a human patient with a cancer or pre-malignant condition that is treatable with an anti-CD40 antibody and which is refractory to treatment with rituximab (Rituxan®), as well as methods and kits for selecting an antibody therapy for treatment of a human patient having a cancer or pre-malignant condition that is refractory to treatment with rituximab (Rituxan®), are also provided. The methods of the present invention find use in treatment of cancers and pre-malignant conditions that are associated with CD40-expressing cells. These methods are particularly advantageous with respect to cancers and pre-malignant conditions that are associated with cells expressing both CD40 and CD20, as the methods enable the treatment of patients having a cancer or pre-malignant condition that is refractory to therapy with other oncotherapeutic agents such as anti-CD20 antibodies.
Owner:XOMA TECH LTD
Method for down-regulating GDF-8 activity using immunogenic GDF-8 analogues
InactiveUS7070784B1Small sizeQuality improvementAntibody mimetics/scaffoldsGenetic material ingredientsMyostatinVaccination
Disclosed are novel methods for increasing muscle mass by means of immunization against Growth Differentiation Factor 8 (GDF-8, myostatin). Immunization is preferably effected by administration of analogues of GDF-8 which are capable of inducing antibody production against homologous GDF-8. Especially preferred as an immunogen is homologous GDF-8 which has been modified by introduction of one single or a few foreign, immunodominant and promiscuous T-cell epitopes while substantially preserving the tertiary structure of the homologous GDF-8. Also disclosed are nucleic acid vaccination against GDF-8 and vaccination using live vaccines as well as methods and means useful for the vaccination. Such methods and means include methods for identification of useful immunogenic GDF-8 analogues, methods for the preparation of analogues and pharmaceutical formulations, as well as nucleic acid fragments, vectors, transformed cells, polypeptides and pharmaceutical formulations.
Owner:PHARMEXA
High titer antibody production
InactiveUS20110229933A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCell culture mediaAntibody production
Owner:MERCK SHARP & DOHME CORP
IMMUNONANOTHERAPEUTICS THAT PROVIDE IgG HUMORAL RESPONSE WITHOUT T-CELL ANTIGEN
ActiveUS20120087890A1Improve responseEffective amountSsRNA viruses negative-senseAntibacterial agentsNanocarriersAntibody production
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides synthetic nanocarriers capable of eliciting an immune system response in the form of antibody production, wherein the nanocarriers lack any T cell antigens. In some embodiments, the invention provides nanocarriers that comprise an immunofeature surface, which provides high avidity binding of the nanocarriers to antigen presenting cells. The invention provides pharmaceutical compositions comprising such nanocarriers. The present invention provides methods of designing, manufacturing maceutical compositions thereof.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com