Patents
Literature
3987 results about "Growth cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell growth (or interphase) is shorthand for the idea of "growth in cell populations" by means of cell reproduction. It is the stage which cells are preparing for the next division, biochemical activities and reactions are taking place, however no obvious changes can be seen at this stage.
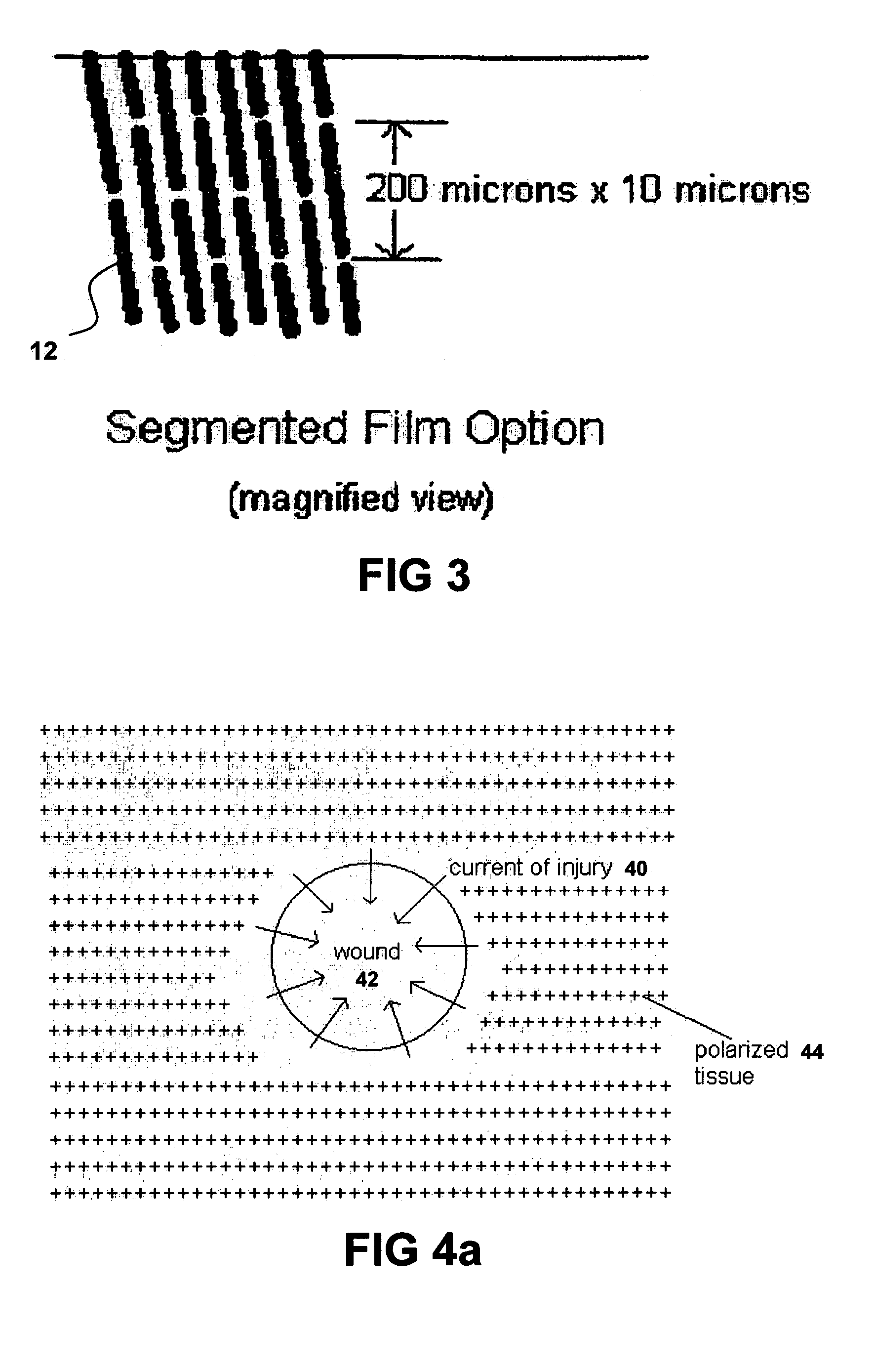
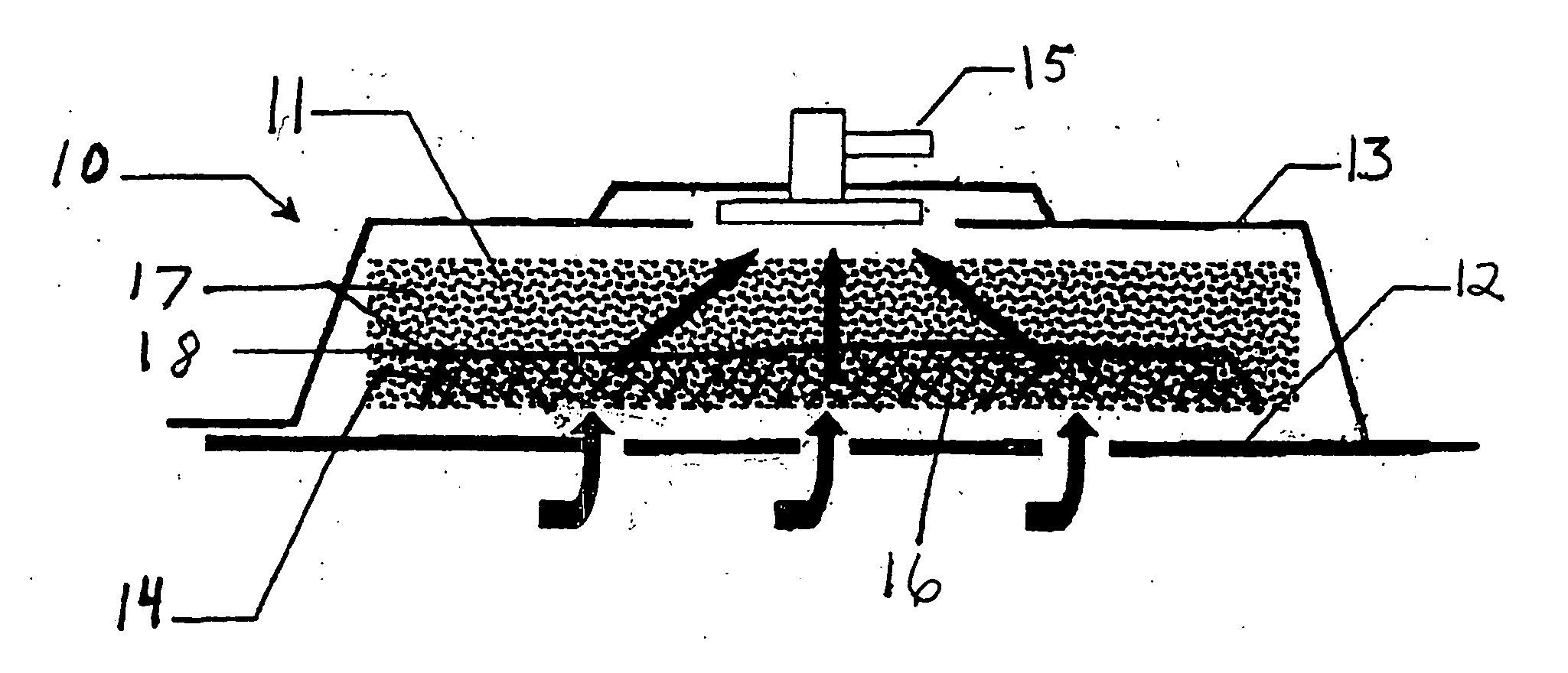
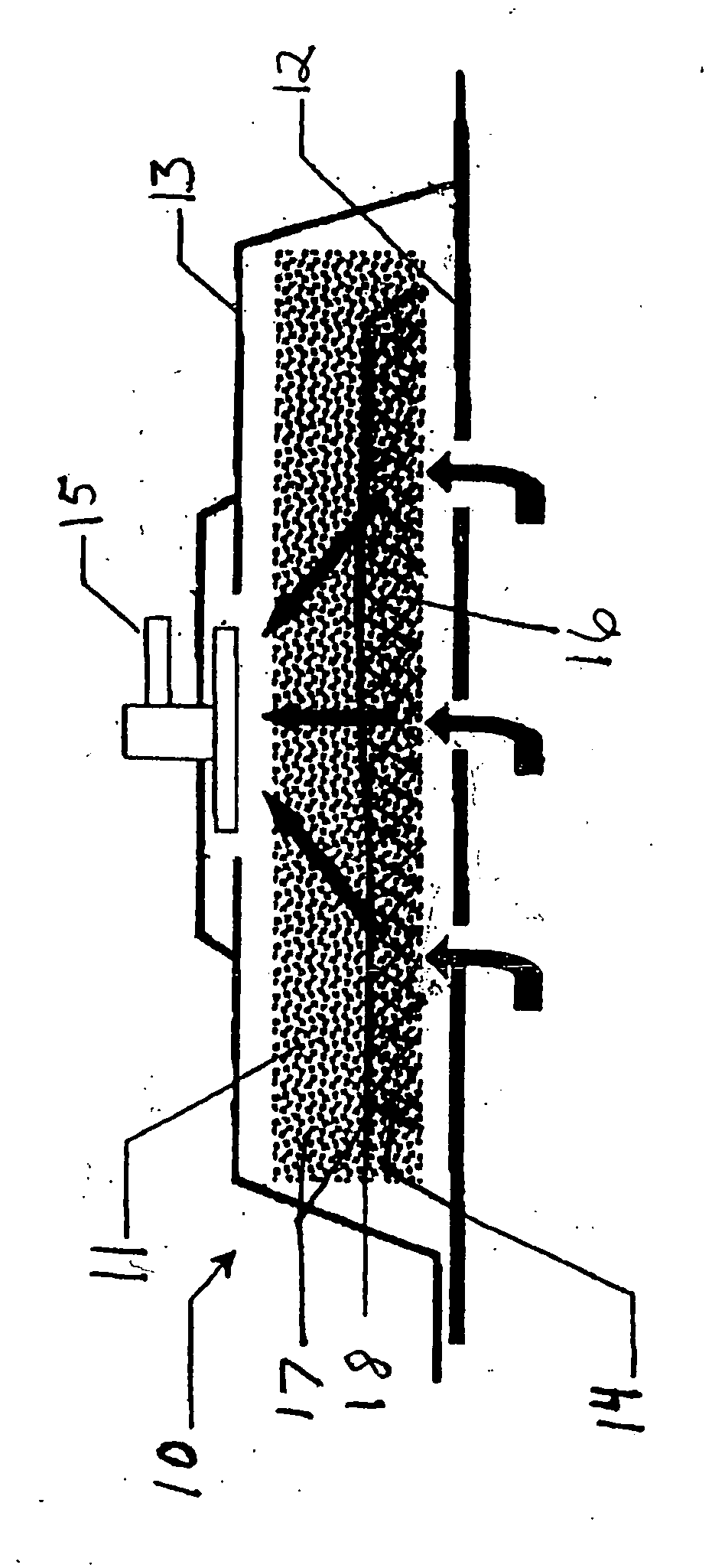
Biocompatible wound dressing
InactiveUS7070584B2Promote cell growthPrevent vacuum leakageWound drainsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
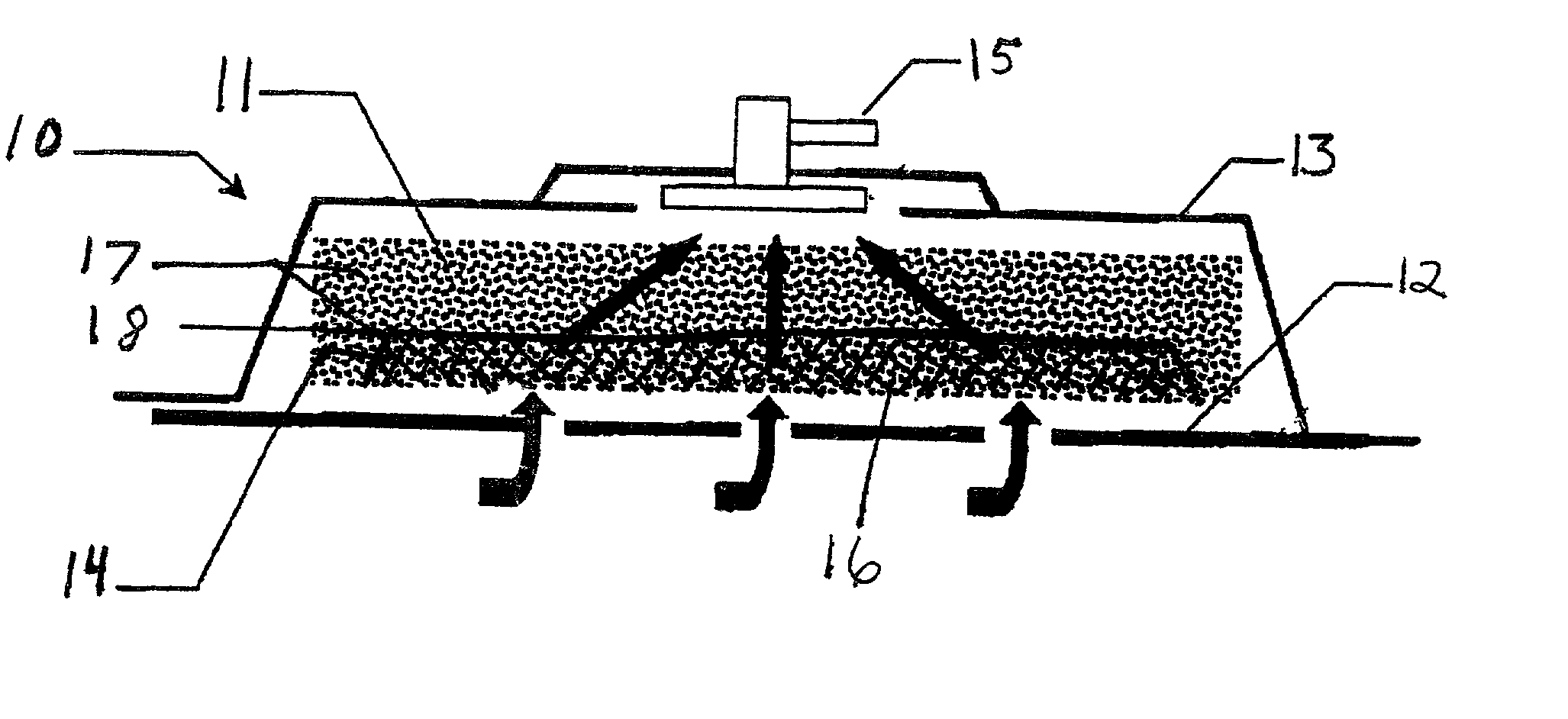
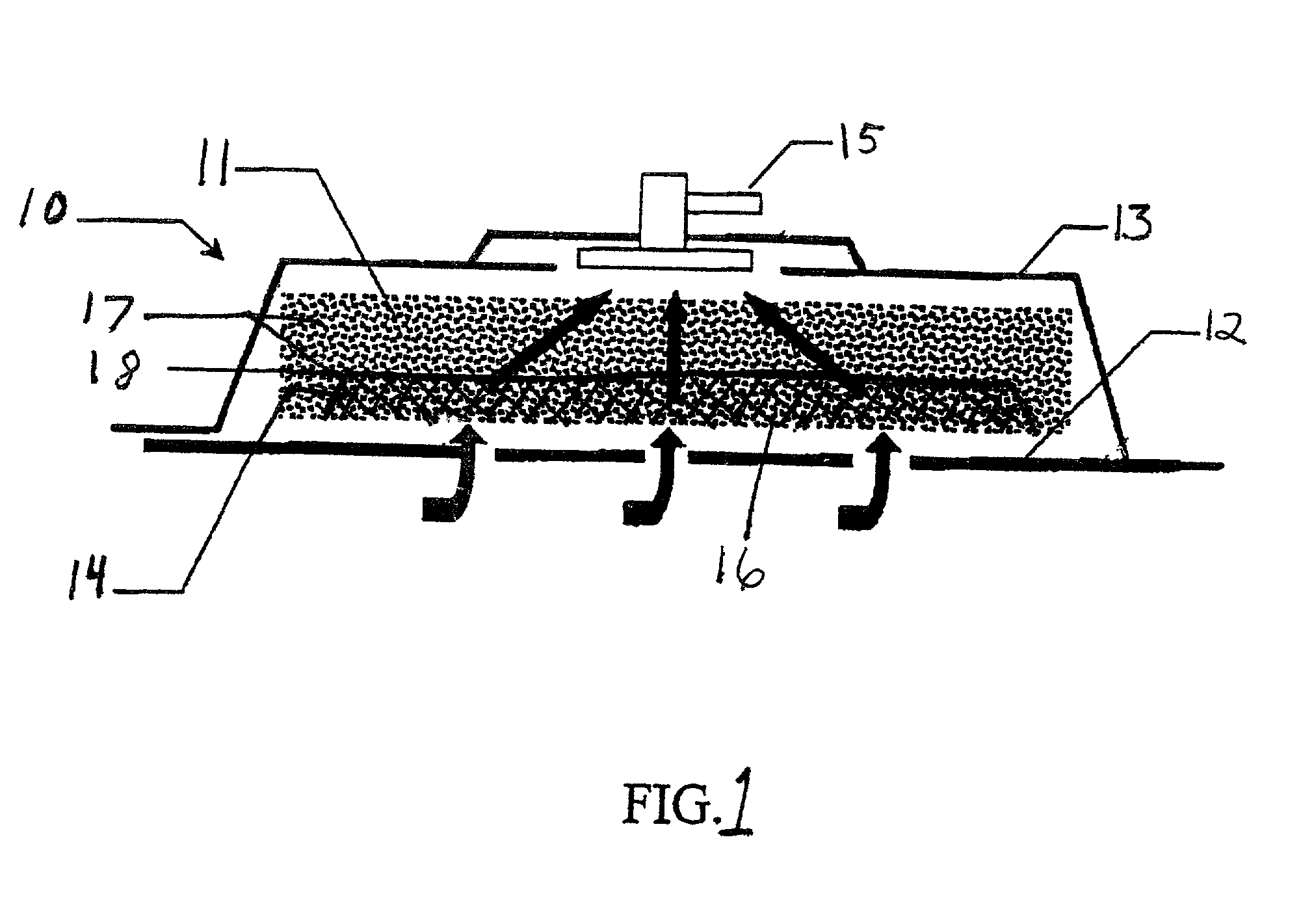
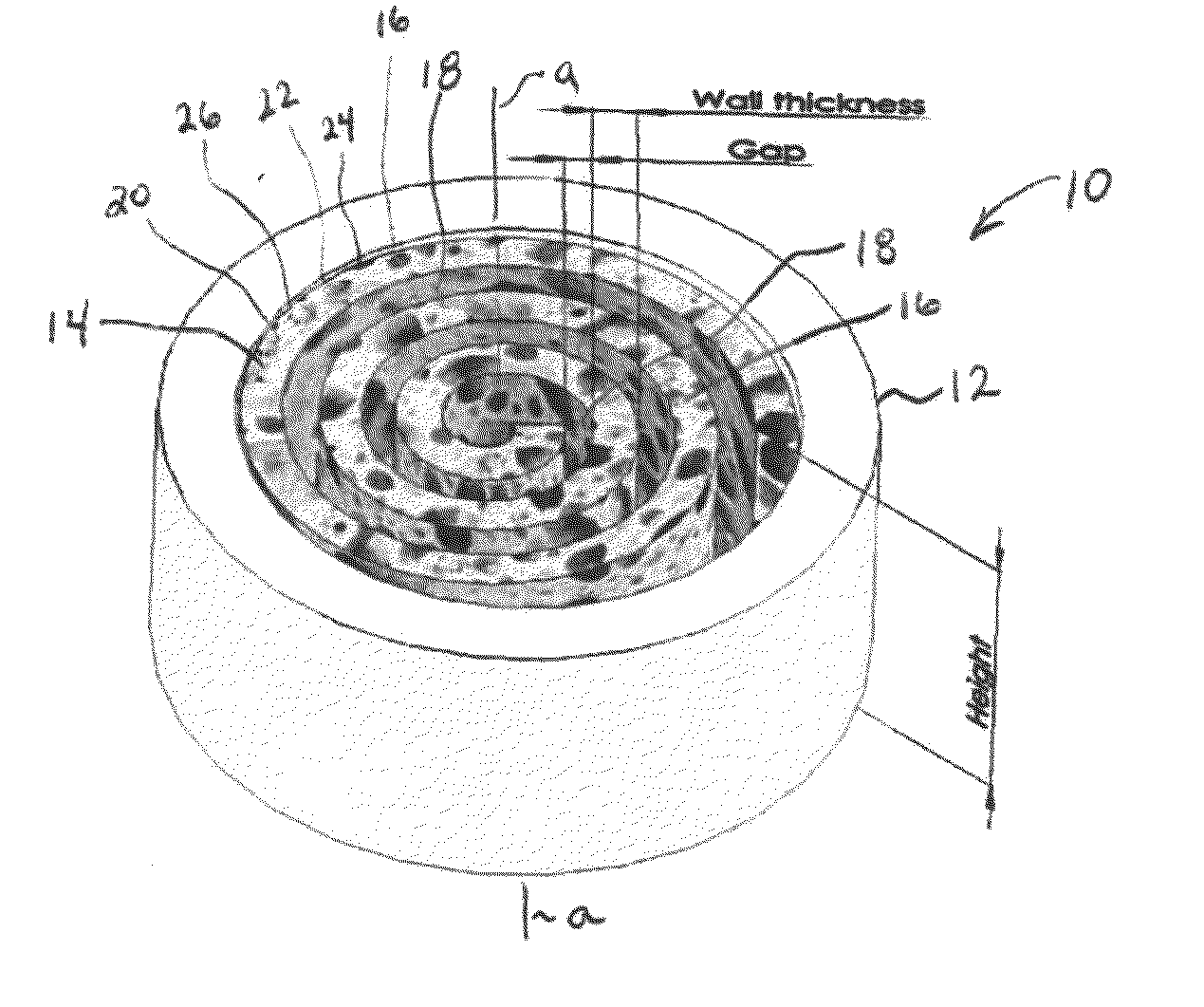
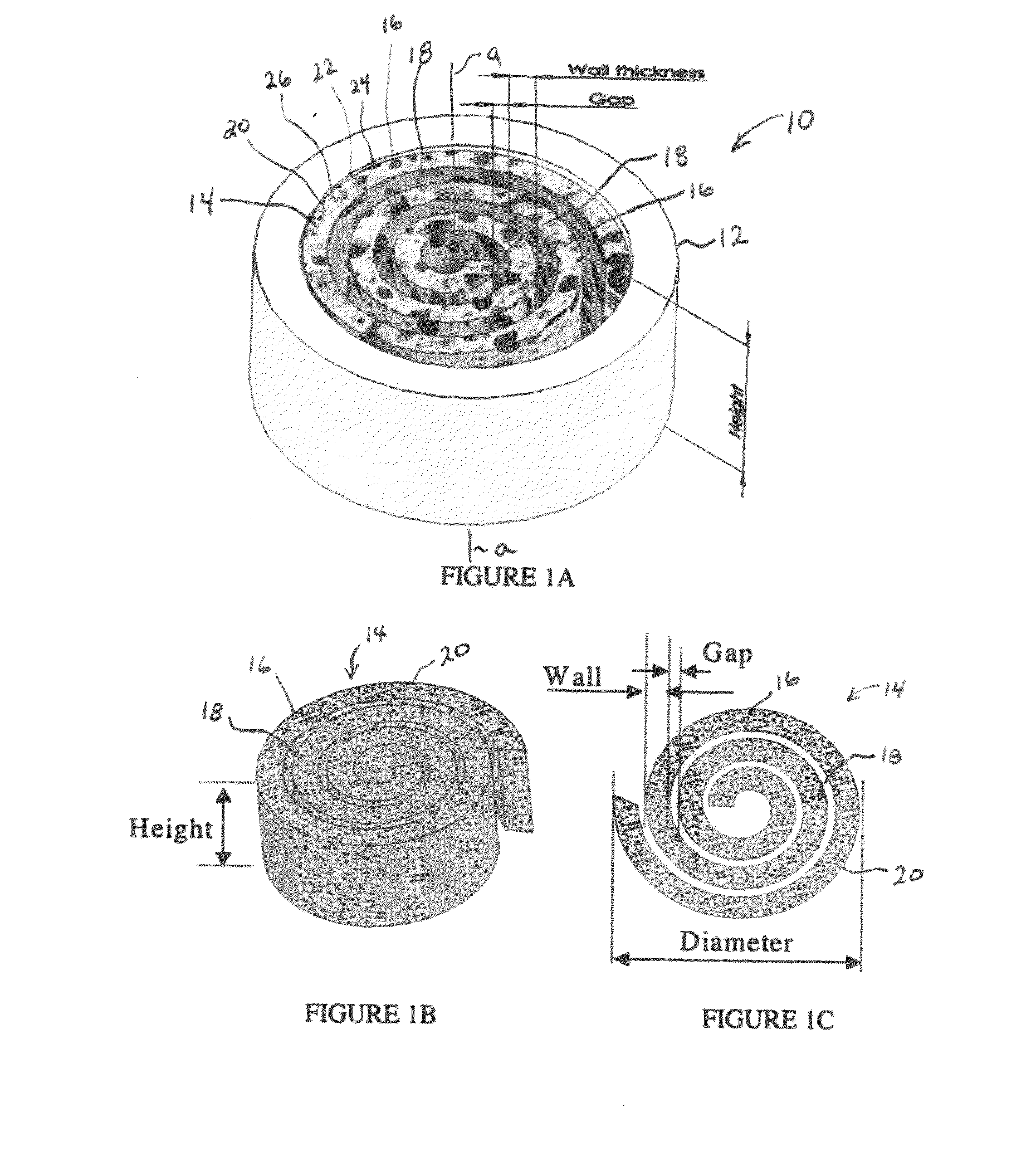
Synergetic functionalized spiral-in-tubular bone scaffolds
InactiveUS20100310623A1Increase the number ofIncrease alkaline phosphatase activityPeptide/protein ingredientsBone implantPorous sheetCell seeding
An integrated scaffold for bone tissue engineering has a tubular outer shell and a spiral scaffold made of a porous sheet. The spiral scaffold is formed such that the porous sheet defines a series of spiral coils with gaps of controlled width between the coils to provide an open geometry for enhanced cell growth. The spiral scaffold resides within the bore of the shell and is integrated with the shell to fix the geometry of the spiral scaffold. Nanofibers may be deposited on the porous sheet to enhance cell penetration into the spiral scaffold. The spiral scaffold may have alternating layers of polymer and ceramic on the porous sheet that have been built up using a layer-by-layer method. The spiral scaffold may be seeded with cells by growing a cell sheet and placing the cell sheet on the porous sheet before it is rolled.
Owner:UNIV OF CONNECTICUT
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
Antagonist antibody for the treatment of cancer
ActiveUS20100047257A1High degreeStrong cytotoxicitySugar derivativesBiological material analysisSynovial sarcomaAntibody fragments
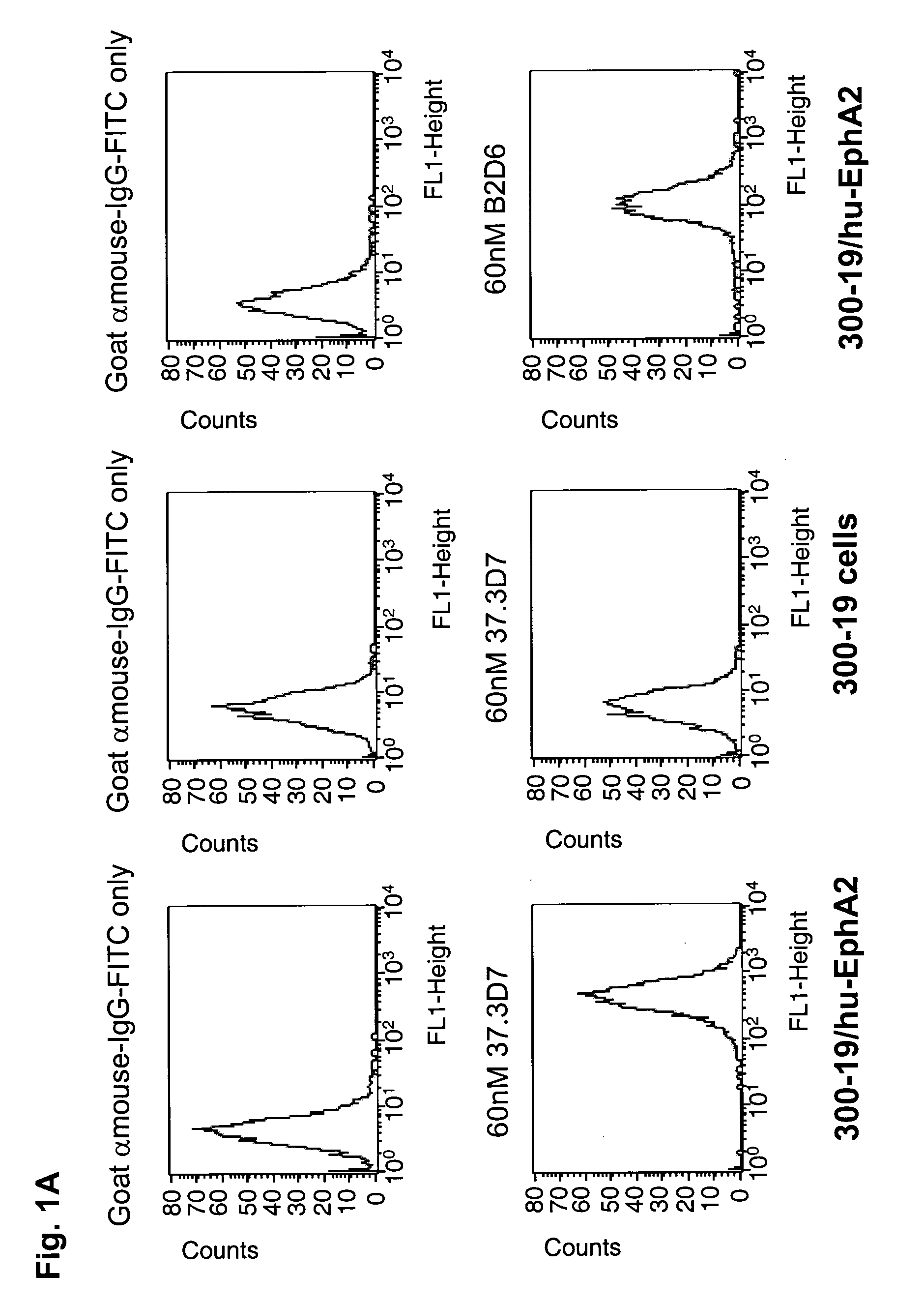
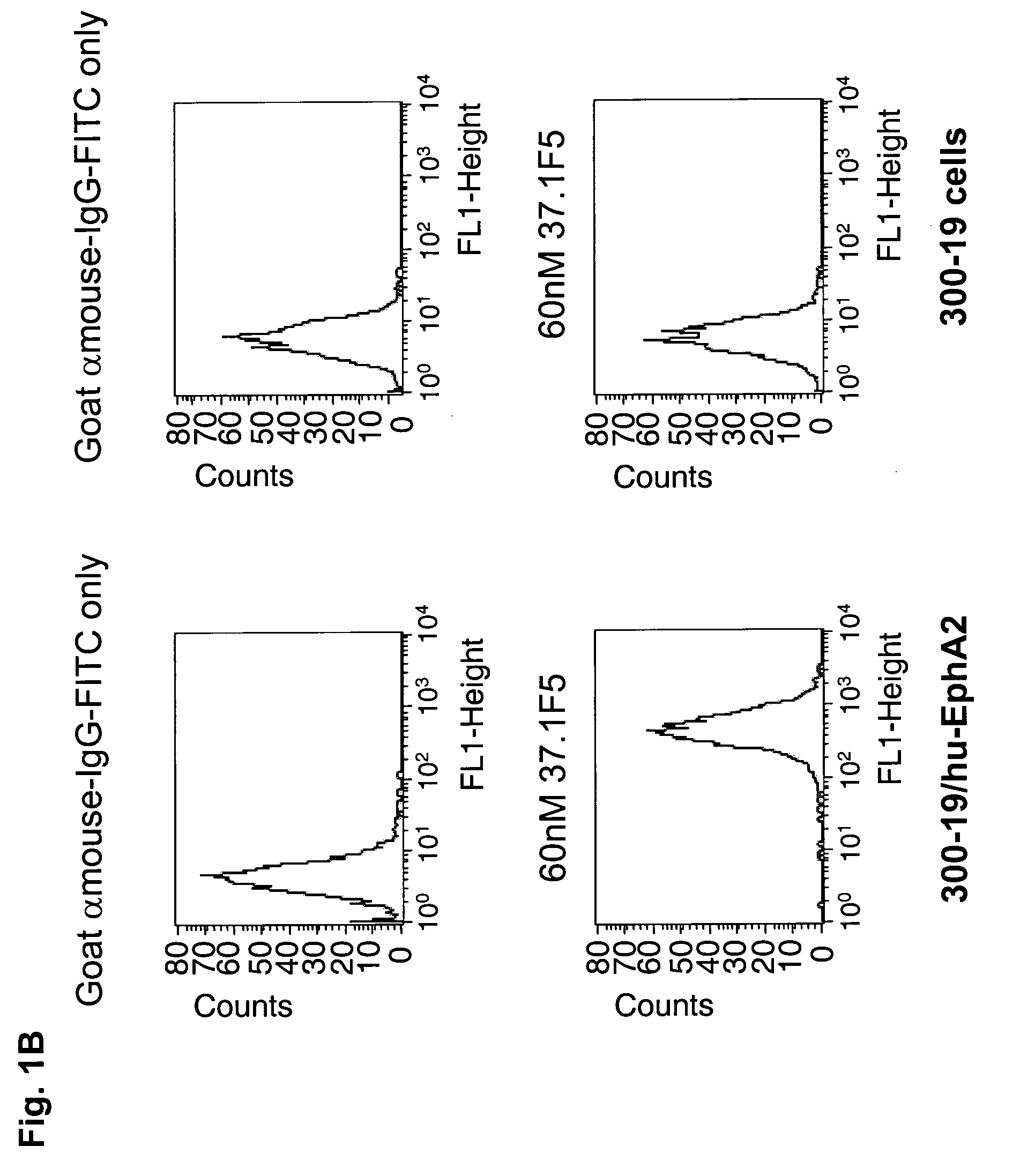
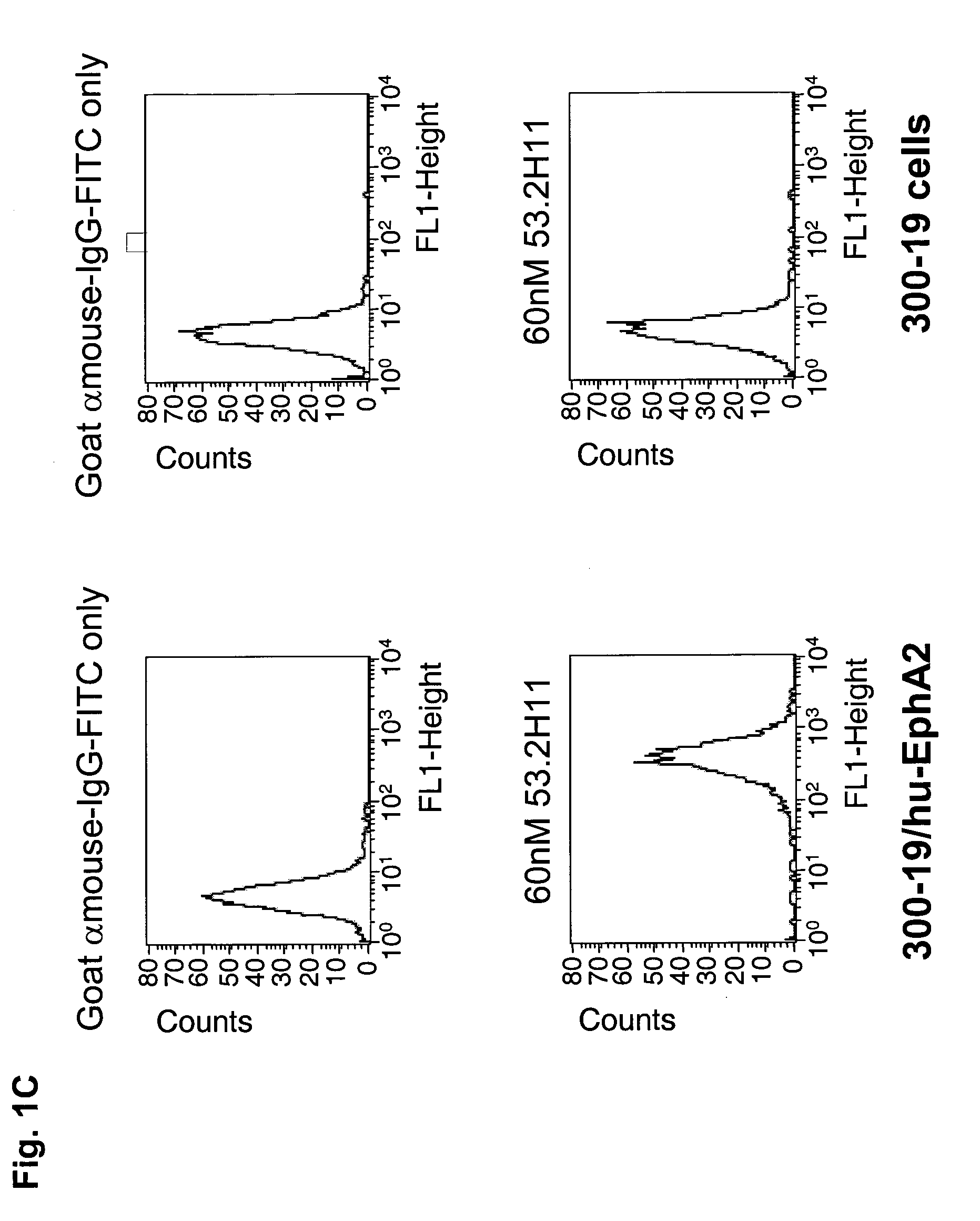
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to, and inhibit A class of Eph receptors, antagonize the effects of growth factors on the growth and survival of tumor cells, and which have minimal agonistic activity or are preferentially devoid of agonist activity. Said antibodies and fragments thereof may be used in the treatment of tumors that express elevated levels of A class of Eph receptors, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma and pancreatic cancer, and said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of A class of Eph receptors. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate are disclosed are all embodiments of the invention. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the A class of Eph receptors.
Owner:SANOFI SA
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of skin diseases or disorders
Methods of treating, preventing, correcting and / or managing skin diseases or disorders characterized by overgrowths of the epidermis, keratoses, scleroderma, cutaneous vasculitis, acne or wrinkles are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent. Specific second active ingredients are capable of affecting or inhibiting cell growth or proliferation, removing or improving acne scars, or reducing or correcting wrinkle lines. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Autogenic living scaffolds and living tissue matrices: methods and uses thereof
ActiveUS20050226856A1Preventing host rejectionThicker and strongBiocideSkin implantsTransdifferentiationOrganism
A 3-dimensional structure comprising suitable cells (or entities) and the ECM (or matrix) that has been completely produced and arranged by these cells (or entities) that promotes the differentiation, dedifferentiation and / or transdifferentiation of cells and / or formation of tissue in vitro and in vivo, while at the same time promoting cell growth, proliferation, migration, acquisition of in vivo-like morphology, or combinations thereof, and that 1. provides structural and / or nutritional support to cells, tissue, organs, or combinations thereof, termed an “Autogenic Living Scaffold” (ALS); or 2. is capable of being transformed into a more complex tissue (or matrix) or a completely different type of tissue (or matrix), termed a “Living Tissue Matrix” (LTM). Autogenic means it is self-produced. The living cells that produce the LTM or ALS, or are added to Autogenic Living Scaffolds, may be genetically engineered or otherwise modified. The matrix component of the ALS or LTM provides a structural framework for cells that guide their direction of growth, enables them to be correctly spaced, prevents overcrowding, enables cells to communicate between each other, transmit subtle biological signals, receive signals from their environment, form bonds and contacts that are required for proper functioning of all cells within a unit such as a tissue, or combinations thereof. The ALS or LTM may thus provide proper or supporting mechanical and chemical environments, signals, or stimuli to other cells, to the cells that produce the ALS, to surrounding tissue at an implantation site, to a wound, for in vitro and ex vivo generation and regeneration of cells, tissue and organs, or combinations thereof. They may also provide other cells with nutrients, growth factors, and / or other necessary or useful components. They may also take in or serve as buffers for certain substances in the environment, and have also some potential at adapting to new environments.
Owner:GENESIS TECH LTD
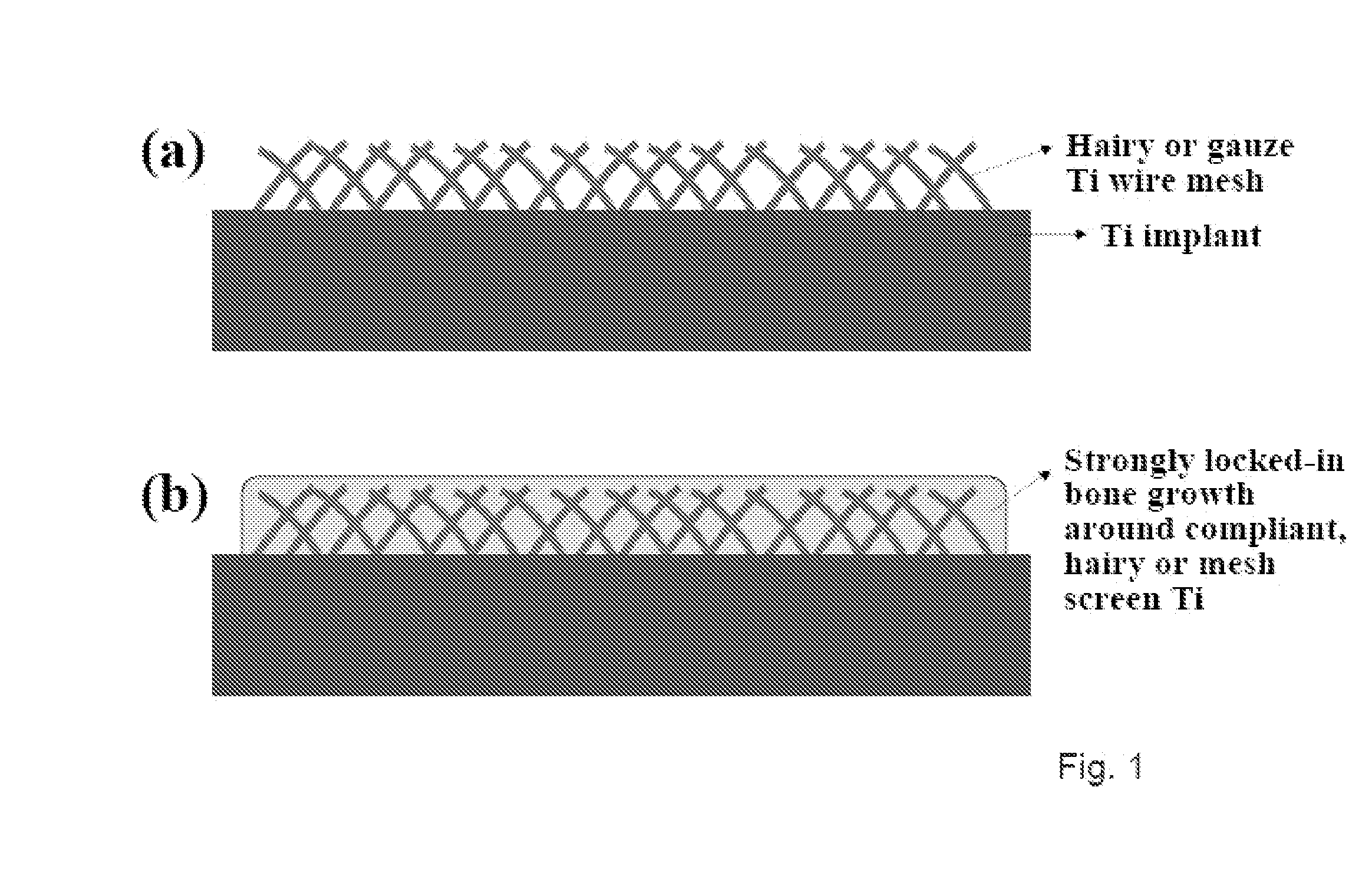
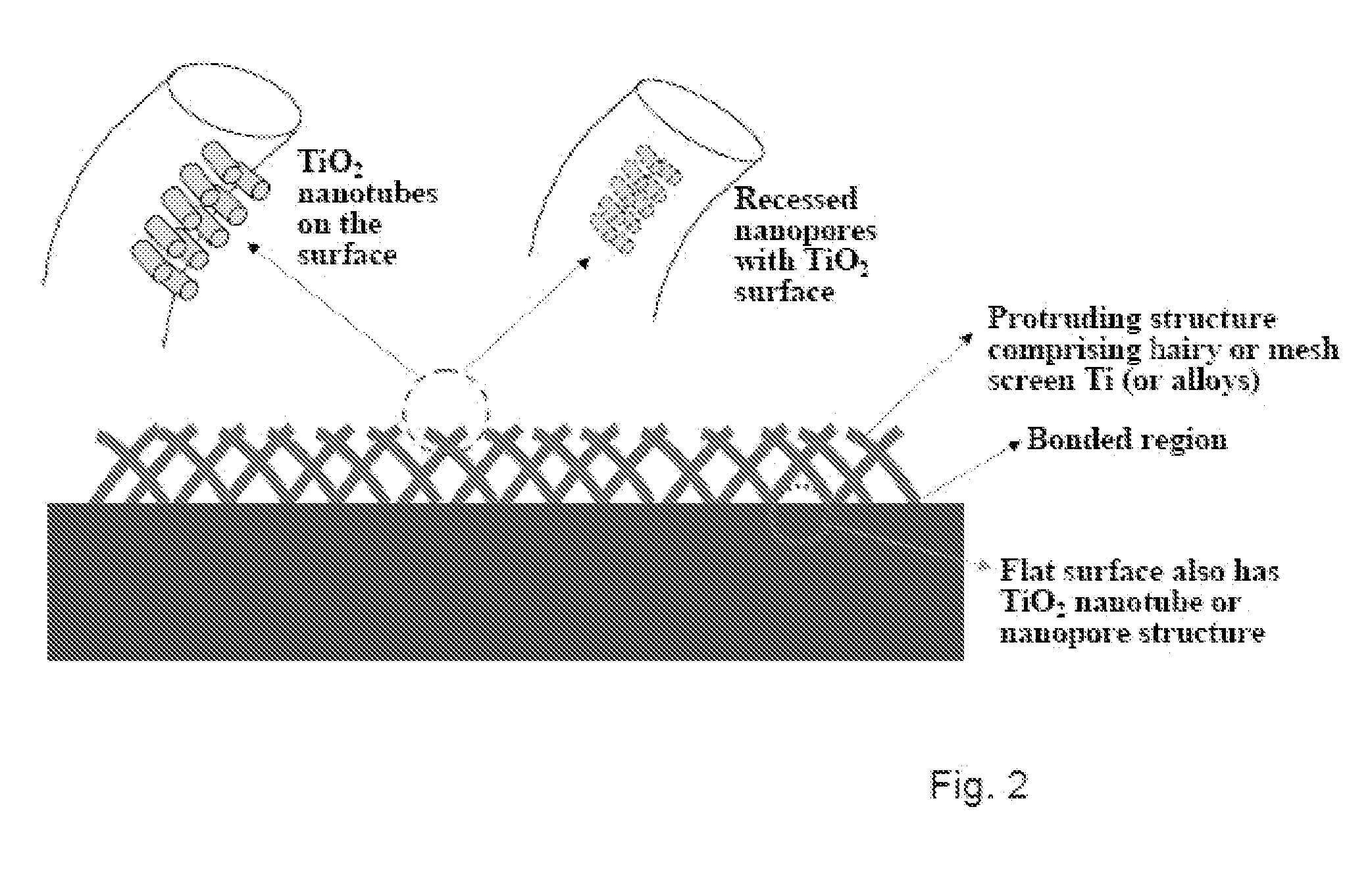
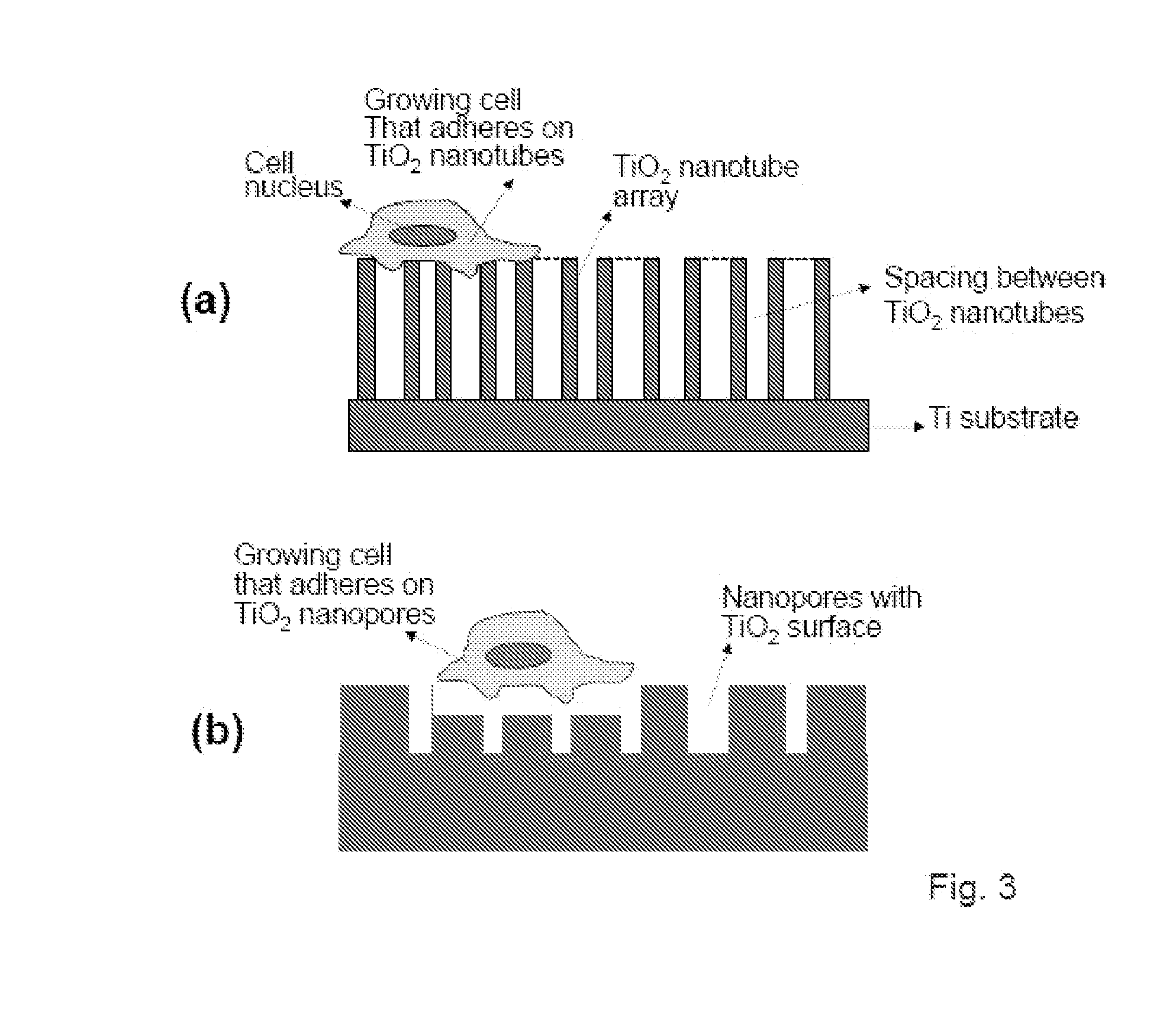
Compositions comprising nanostructures for cell, tissue and artificial organ growth, and methods for making and using same
ActiveUS20090220561A1Improve bone formationIncreased durabilityBioreactor/fermenter combinationsElectrolysis componentsIn vivoNanostructure
The invention provides articles of manufacture comprising biocompatible nanostructures comprising nanotubes and nanopores for, e.g., organ, tissue and / or cell growth, e.g., for bone, kidney or liver growth, and uses thereof, e.g., for in vitro testing, in vivo implants, including their use in making and using artificial organs, and related therapeutics. The invention provides lock-in nanostructures comprising a plurality of nanopores or nanotubes, wherein the nanopore or nanotube entrance has a smaller diameter or size than the rest (the interior) of the nanopore or nanotube. The invention also provides dual structured biomaterial comprising micro- or macro-pores and nanopores. The invention provides biomaterials having a surface comprising a plurality of enlarged diameter nanopores and / or nanotubes.
Owner:RGT UNIV OF CALIFORNIA
Pluripotential embryonic stem cells and methods of making same
The present invention provides a non-mouse, including human, pluripotential embryonic stem cell which can:(a) be maintained on feeder layers for at least 20 passages; and(b) give rise to embryoid bodies and multiple differentiated cell phenotypes in monolayer culture.The invention further provides a method of making a pluripotential embryonic stem cell comprising culturing germ cells and germ cell progenitors in a composition comprising a growth enhancing amount of basic fibroblast growth factor, leukemia inhibitory factor, membrane associated steel factor, and soluble steel factor to primordial germ cells under cell growth conditions, thereby making a pluripotential embryonic stem cell.Also provided are compositions useful to produce the pluripotent embryonic stem cells and methods of screening associated with the method of making the embryonic stem cell.
Owner:VANDERBILT UNIV
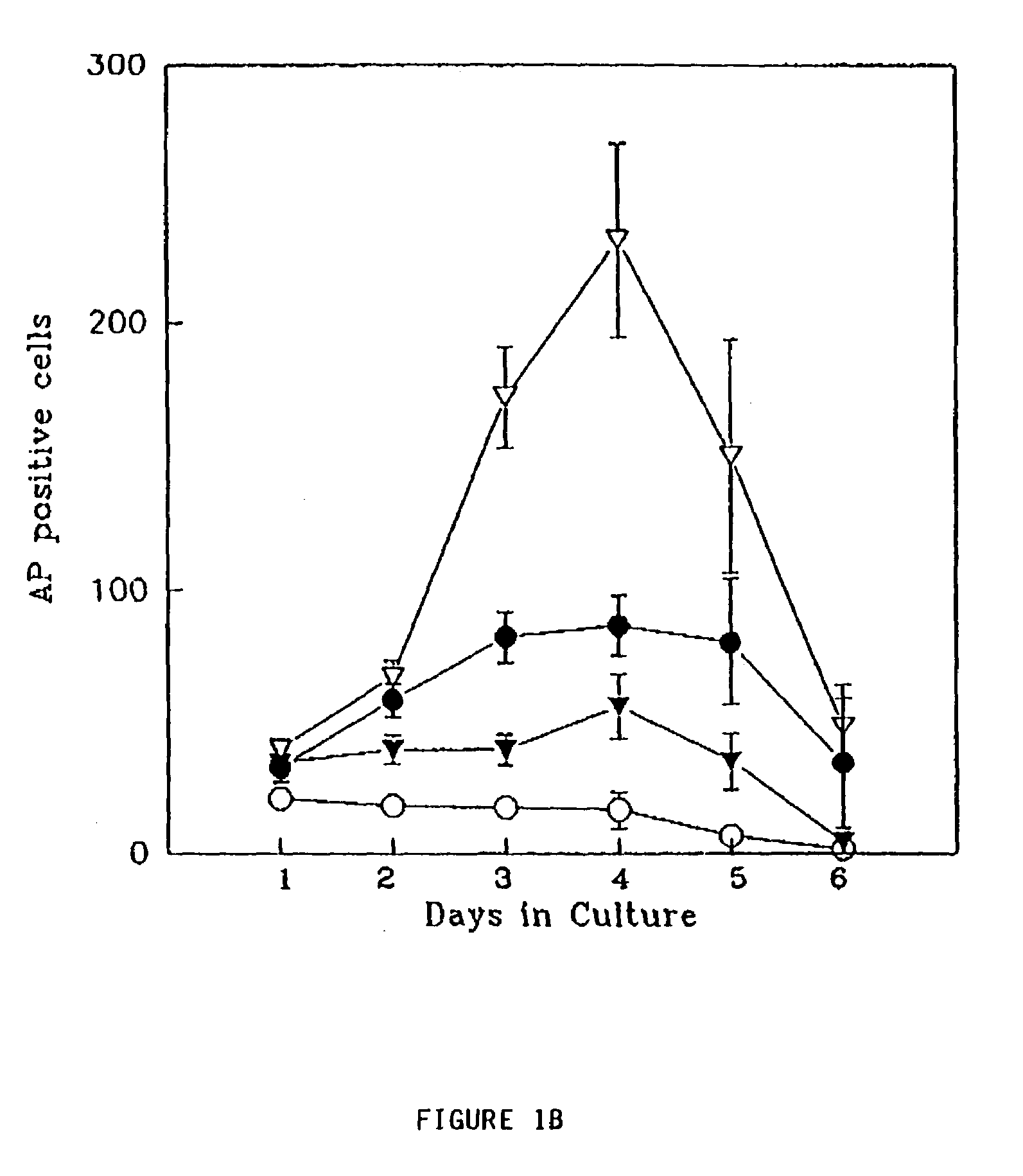
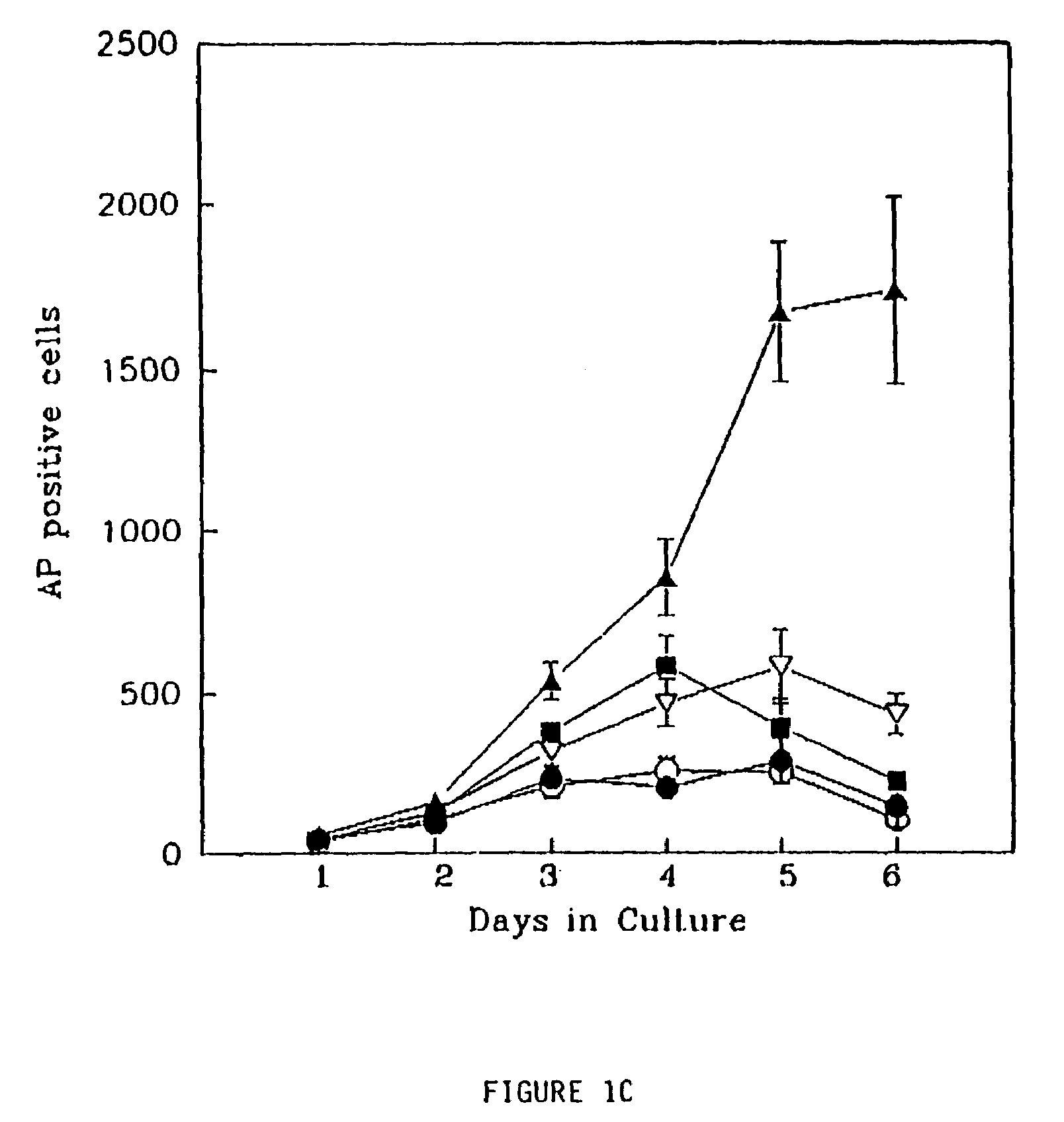
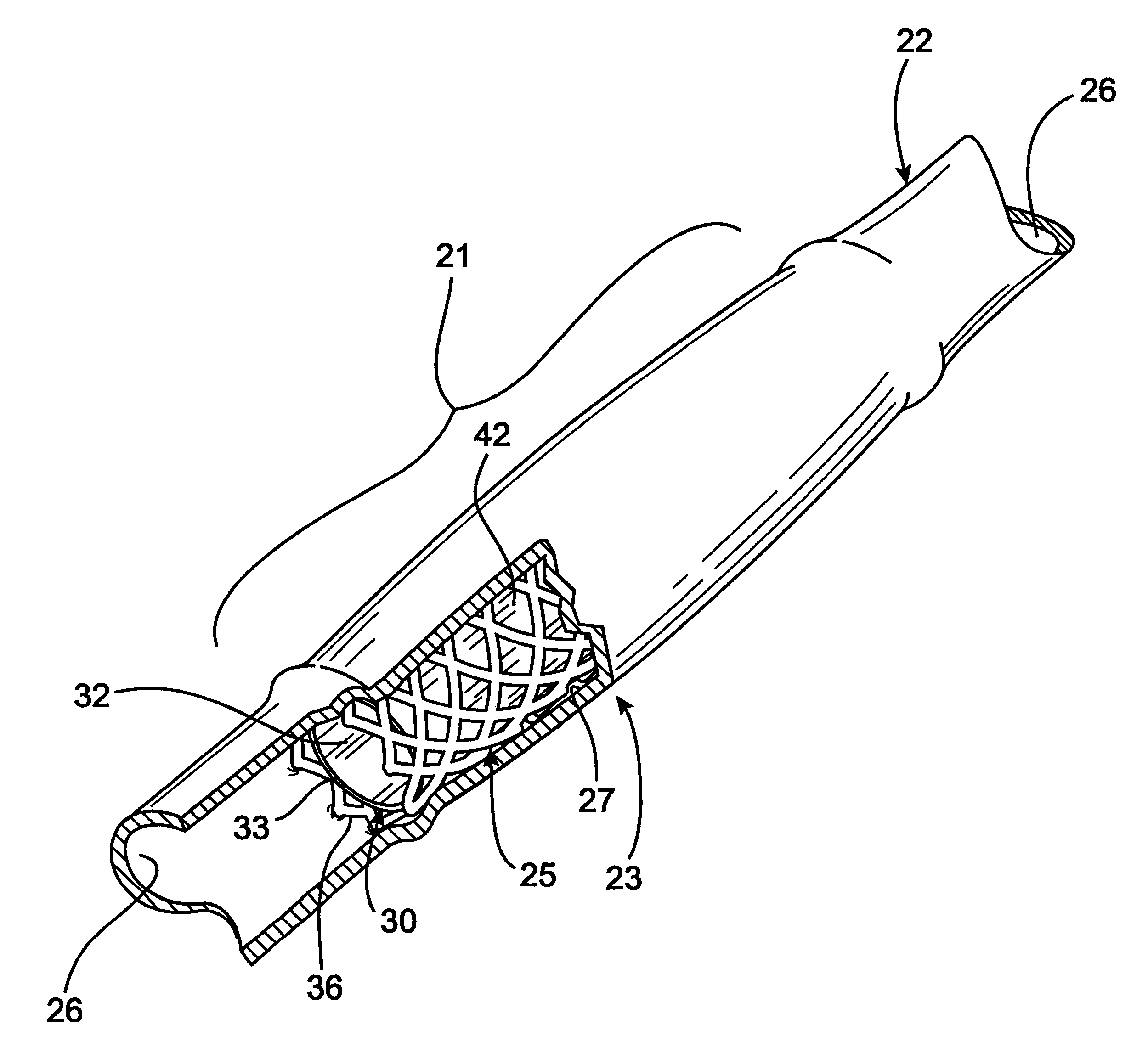
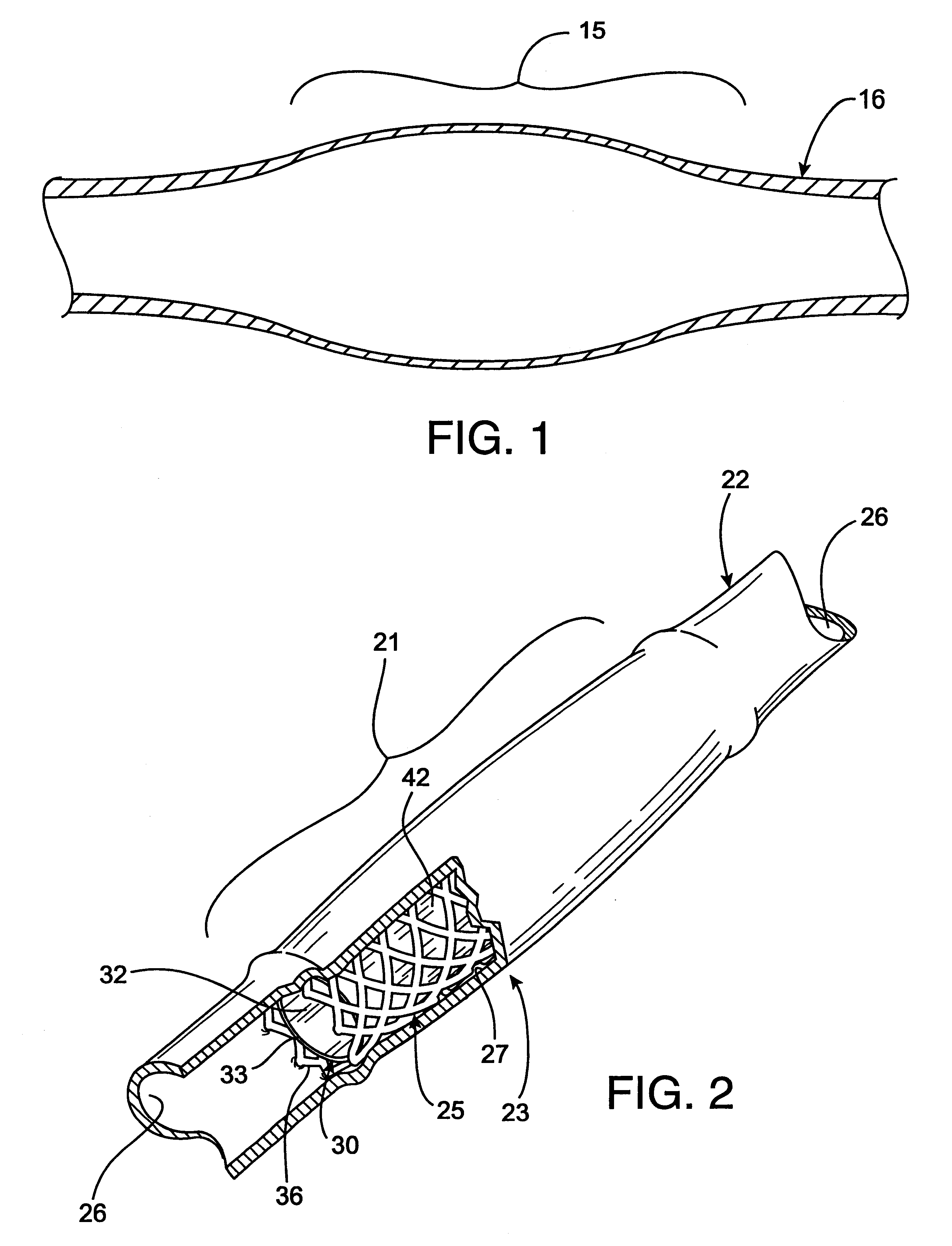
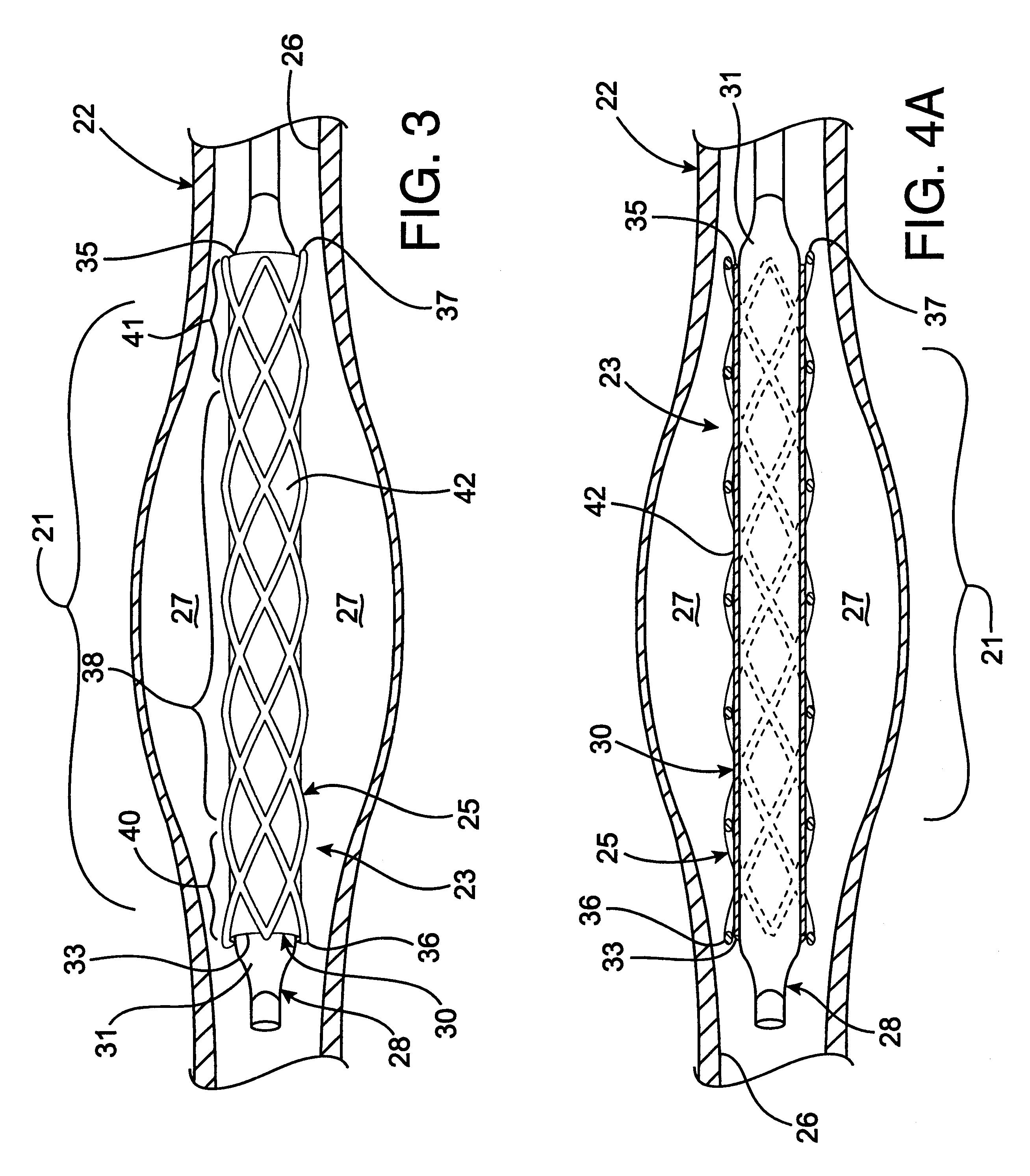
Radioactive intraluminal endovascular prosthesis and method for the treatment of aneurysms
A method for increasing the rate of thrombus formation and / or proliferative cell growth of a selected region (21) of cellular tissue (22) including the step of endovascularly irradiating the selected region (21) with radiation, having a dose range of endovascular radiation of about 1 Gy to about 600 Gy at a low dose rate of about 1 cGy / hr to about 320 cGy / hr, to increase thrombus formation and / or cell proliferation of the affected selected region (21). Preferably, the delivery means includes a deformable endovascular prosthesis (25) adapted for secured positioning adjacent to the selected region (21) of cellular tissue (22), and a radioactive source. This source cooperates with the deformable endovascular device (25) in a manner endovascularly irradiating the selected region with radiation, having the above-indicated dose range and low dose rate of endovascular radiation to increase thrombus formation and / or cell proliferation of the affected selected region (21).
Owner:ISOSTENT
Articles comprising large-surface-area bio-compatible materials and methods for making and using them
ActiveUS20100303722A1Improve cell adhesionAccelerated cell growth characteristicImmobilised enzymesBioreactor/fermenter combinationsCell culture mediaBone growth
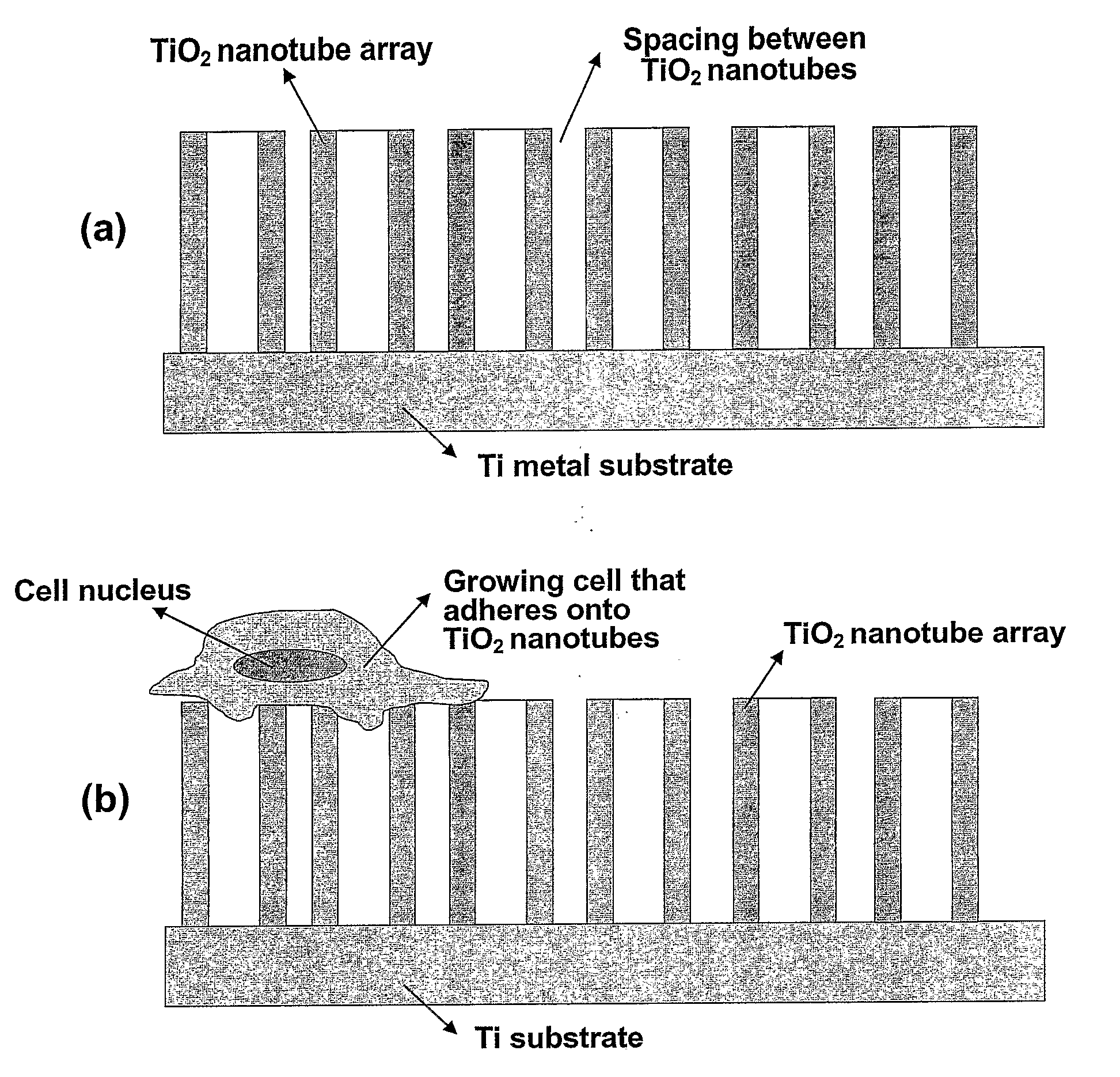
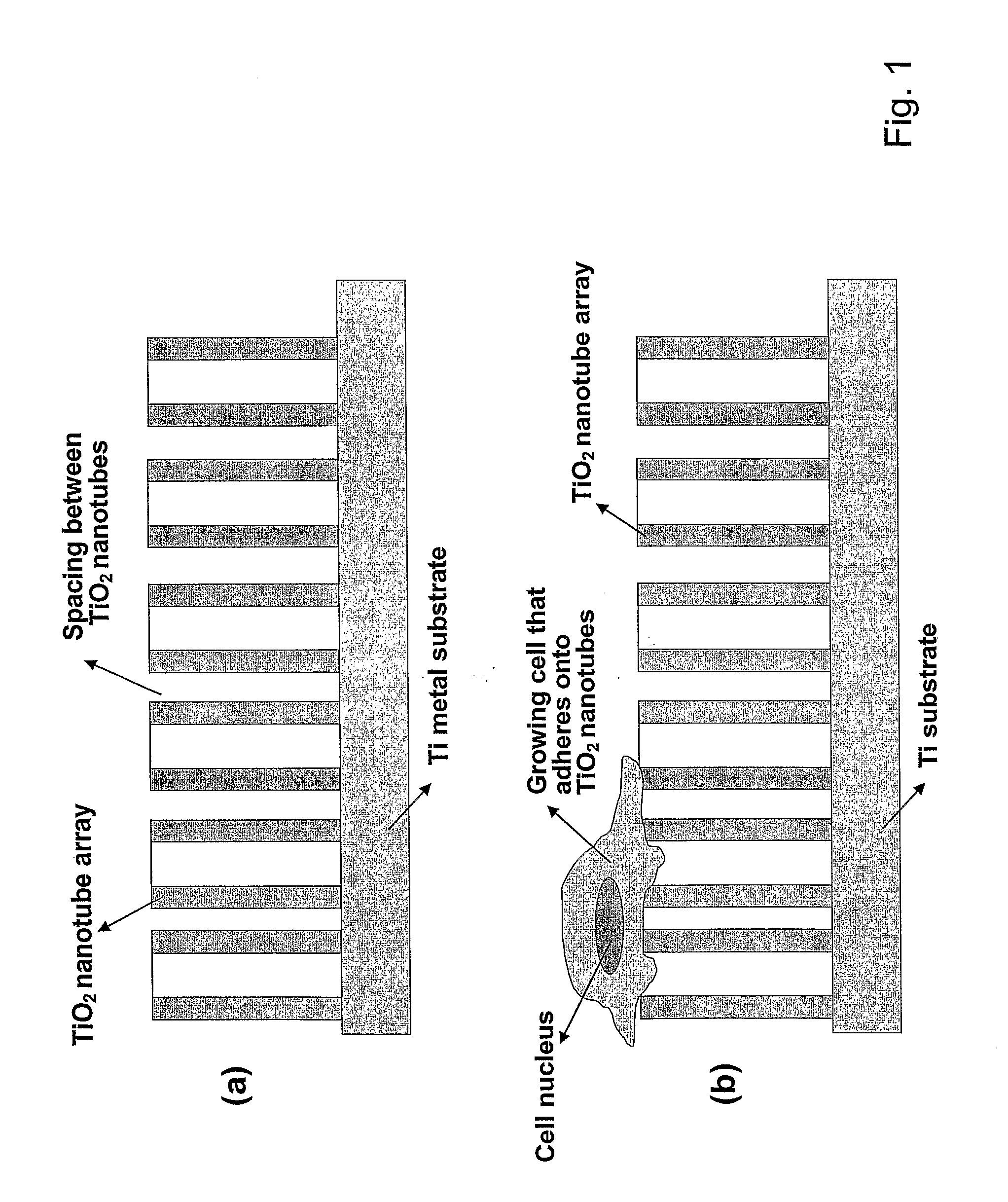
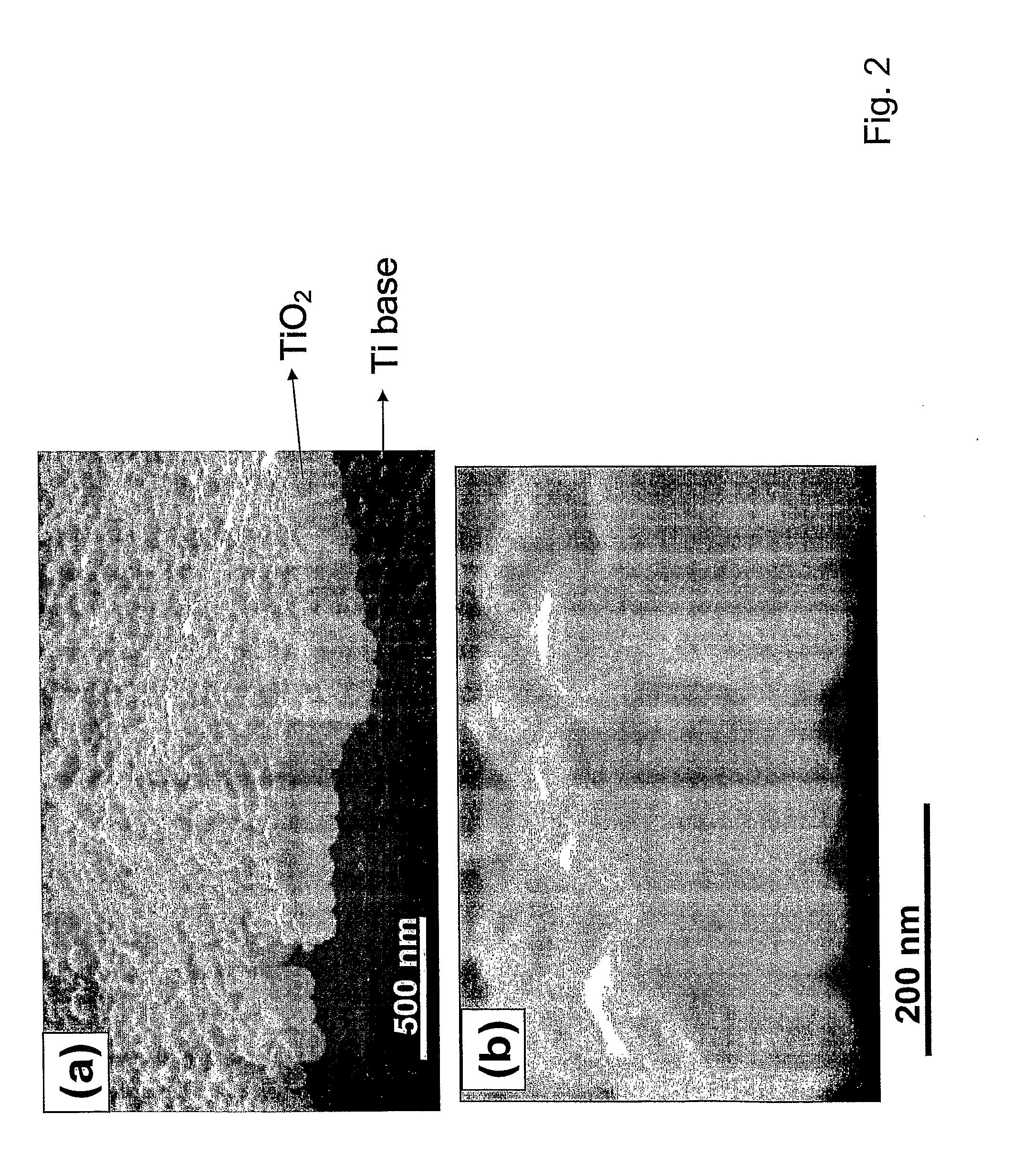
The present invention provides articles of manufacture comprising biocompatible nanostructures comprising significantly increased surface area for, e.g., organ, tissue and / or cell growth, e.g., for bone, tooth, kidney or liver growth, and uses thereof, e.g., for in vitro testing of drugs, chemicals or toxins, or as in vivo implants, including their use in making and using artificial tissues and organs, and related, diagnostic, screening, research and development and therapeutic uses, e.g., as drug delivery devices. The present invention provides biocompatible nanostructures with significantly increased surface area, such as with nanotube and nanopore array on the surface of metallic, ceramic, or polymer materials for enhanced cell and bone growth, for in vitro and in vivo testing, cleansing reaction, implants and therapeutics. The present invention provides optically transparent or translucent cell-culturing substrates. The present invention provides biocompatible and cell-growth-enhancing culture substrates comprising elastically compliant protruding nanostructure substrates coated with Ti, TiO2 or related metal and metal oxide films.
Owner:RGT UNIV OF CALIFORNIA
Meniscal repair scaffold
ActiveUS20050234549A1Rapid and effective tissue regenerationEncourage healingJoint implantsLigamentsMeniscal repairTissue repair
Methods and apparatus for treating meniscal tissue damage are disclosed, including a biocompatible meniscal repair device comprising a biocompatible tissue repair scaffold and a cell growth conduit flap. The tissue repair scaffold is adapted to be placed in contact with a defect in the meniscus and can preferably provide a structure for supporting meniscal tissue and / or encouraging tissue growth. The cell growth conduit flap, which is attached to the tissue repair scaffold, allows communication between the synovium and the tissue repair scaffold.
Owner:DEPUY SYNTHES PROD INC
Stimulation of cell growth at implant surfaces
Owner:BOSTON SCI SCIMED INC
Biocompatible wound dressing
InactiveUS20060189910A1Prevent vacuum leakagePromote cell growthAdhesive dressingsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
Bone replacement materials
ActiveUS20070203584A1Easy adhesionFacilitate cell growthAdditive manufacturing apparatusBone implantBone tissueBone tissue engineering
Particular aspects provide novel devices for bone tissue engineering, comprising a metal or metal-based composite member / material comprising an interior macroporous structure in which porosity may vary from 0-90% (v), the member comprising a surface region having a surface pore size, porosity, and composition designed to encourage cell growth and adhesion thereon, to provide a device suitable for bone tissue engineering in a recipient subject. In certain aspects, the device further comprises a gradient of pore size, porosity, and material composition extending from the surface region throughout the interior of the device, wherein the gradient transition is continuous, discontinuous or seamless and the growth of cells extending from the surface region inward is promoted. Additional aspects provide a device for bone tissue engineering, comprising a metal or metal-based composite member / material comprising an interior porous structure, wherein the pore size, porosity and material composition is selected to provide a device having an optimal density and / or elastic modulus and / or compression strength for a specific recipient. Novel methods for fabricating the devices are also provided.
Owner:WASHINGTON STATE UNIVERSITY
Multilayered cell culture apparatus
ActiveUS20070026516A1Effective trainingProvide consistencyBioreactor/fermenter combinationsBiological substance pretreatmentsCulture cellCell growth
A multilayered cell culture apparatus for the culturing of cells is disclosed. The cell culture apparatus is defined as an integral structure having a plurality of cell culture chambers in combination with tracheal space(s). The body of the apparatus has imparted therein gas permeable membranes in combination with tracheal spaces that will allow the free flow of gases between the cell culture chambers and the external environment. The flask body. also includes an aperture that will allow access to the cell growth chambers by means of a needle or cannula. The size of the apparatus, and location of an optional neck and cap section, allows for its manipulation by standard automated assay equipment, further making the apparatus ideal for high throughput applications.
Owner:CORNING INC
Method for creating a cell growth surface on a polymeric substrate
InactiveUS6617152B2Extraordinary levels of cell attachmentPromote cell adhesionBioreactor/fermenter combinationsBiological substance pretreatmentsCell adhesionAdhesion process
A method, apparatus and product for producing an advantaged cell growth surface. According to the present invention, a stream of plasma is comprised of activated gaseous species generated by a microwave source. This stream is directed at the surface of a polymer substrate in a controlled fashion such that the surface is imparted with attributes for cell adhesion far superior to that of untreated polymer or polymer treated by other known methods.
Owner:CORNING INC
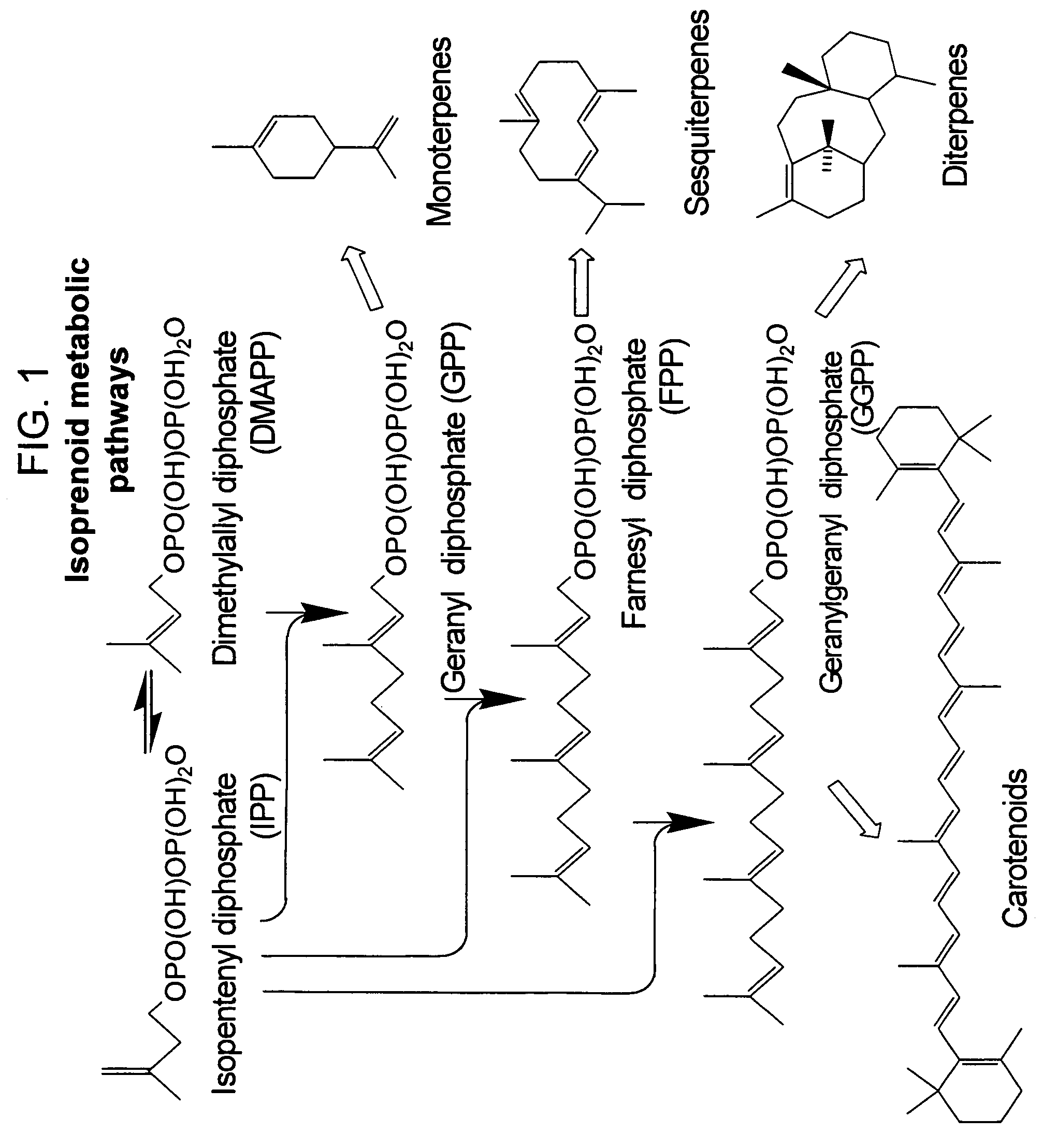
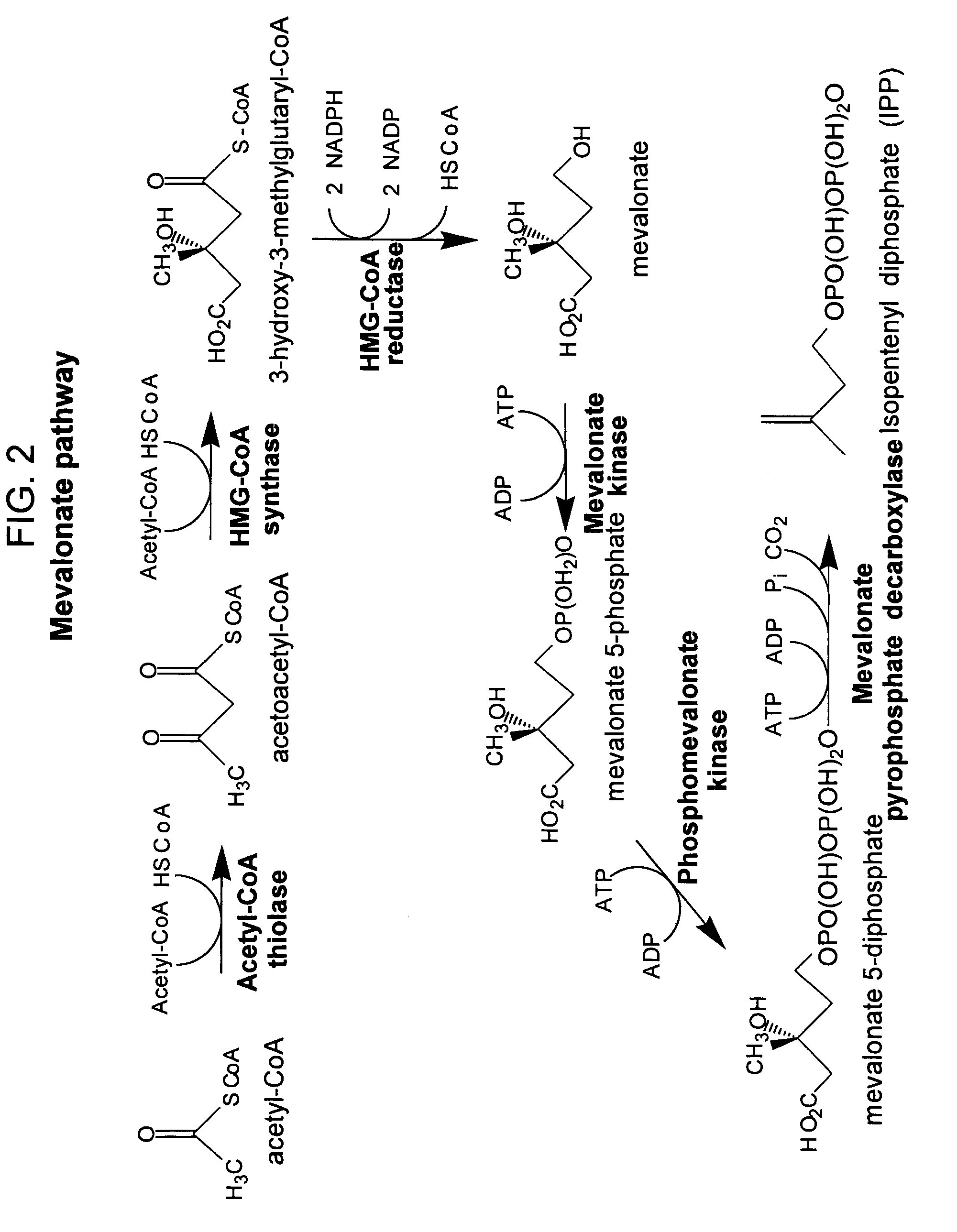
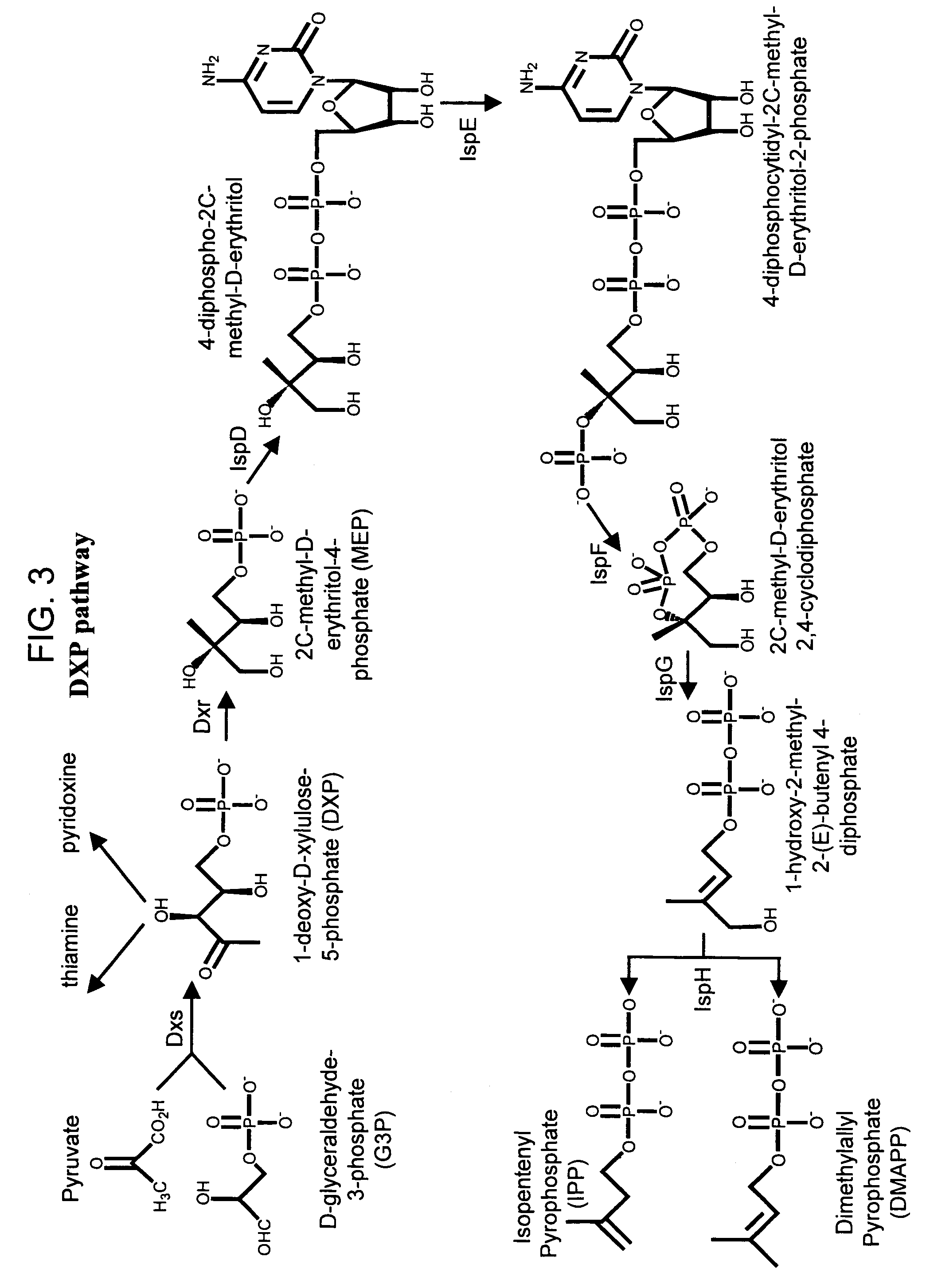
Method for enhancing production of isoprenoid compounds
The present invention provides methods of producing an isoprenoid or an isoprenoid precursor in a genetically modified host cell. The methods generally involve modulating the level of hydroxymethylglutaryl-CoA (HMG-CoA) in the cell, such that the level of HMG-CoA is not toxic to the cell and / or does not substantially inhibit cell growth, but is maintained at a level that provides for high-level production of mevalonate, IPP, and other downstream products of an isoprenoid or isoprenoid pathway, e.g., polyprenyl diphosphates and isoprenoid compounds. The present invention further provides genetically modified host cells that are suitable for use in a subject method. The present invention further provides recombinant nucleic acid constructs for use in generating a subject genetically modified host cell, including recombinant nucleic acid constructs comprising nucleotide sequences encoding one or more mevalonate pathway enzymes, and recombinant vectors (e.g., recombinant expression vectors) comprising same. The present invention further provides methods for identifying nucleic acids that encode HMG-CoA reductase (HMGR) variants that provide for relief of HMG-CoA accumulation-induced toxicity. The present invention further provides methods for identifying agents that reduce intracellular accumulation of HMG-CoA.
Owner:RGT UNIV OF CALIFORNIA
Electromagnetic activation of gene expression and cell growth
InactiveUS20050059153A1Accelerating cell cycleReduce inflammationElectrotherapyMutant preparationAngiotensin receptorA-DNA
The invention is directed to a method for accelerating the cell cycle by delivering to a cell an effective amount of electromagnetic energy. The invention also provides a method for activating a cell cycle regulator by delivering to a cell an effective amount of electromagnetic energy. Also provided by the invention is a method for activating a signal transduction protein; a method for activating a transcription factor; a method for activating a DNA synthesis protein; and a method for activating a Receptor. A method for inhibiting an angiotensin receptor as well as a method for reducing inflammation also are provided by the present invention. The invention also is directed to a method for replacing damaged neuronal tissue as well as a method for stimulating growth of administered cells.
Owner:REGENESIS BIOMEDICAL
Nano/micro-textured surfaces and methods of making same by aluminum-induced crystallization of amorphous silicon
InactiveUS20090176018A1Enhanced cell attachmentPromote cell growthVacuum evaporation coatingSputtering coatingAmorphous siliconMicroelectromechanical systems
The present invention discloses a method of surface texturing at nano / micro-scale by aluminum-induced rapid crystallization of amorphous silicon for controlling the wettability of a surface, enhancing cell attachment to a surface, and promoting cell growth on a surface. The present invention can be used in a variety of applications, such as producing superhydrophobic or superhydrophilic surfaces for medical devices, microelectromechanical systems, and microfluidic channels.
Owner:ZOU MIN +1
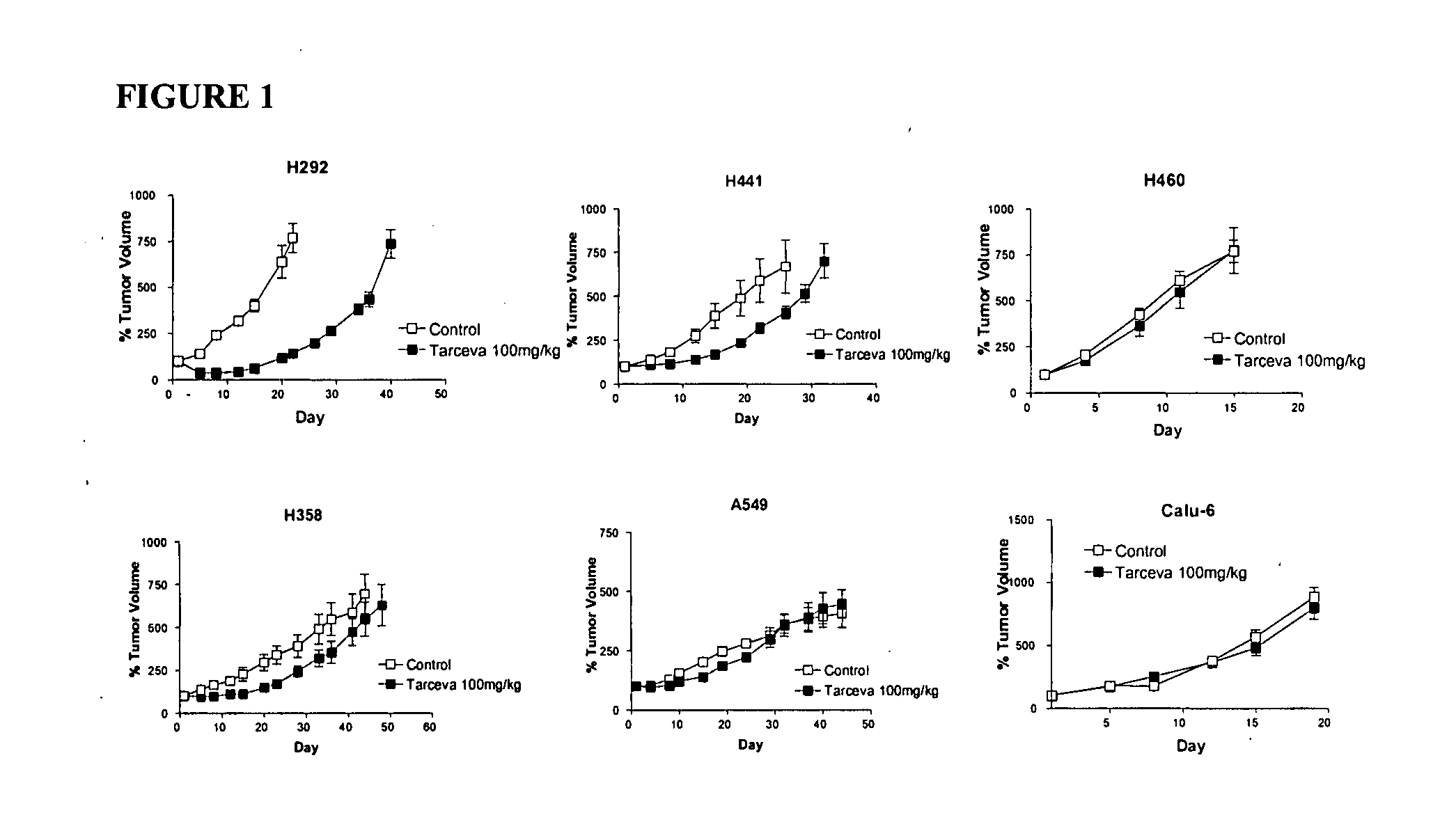
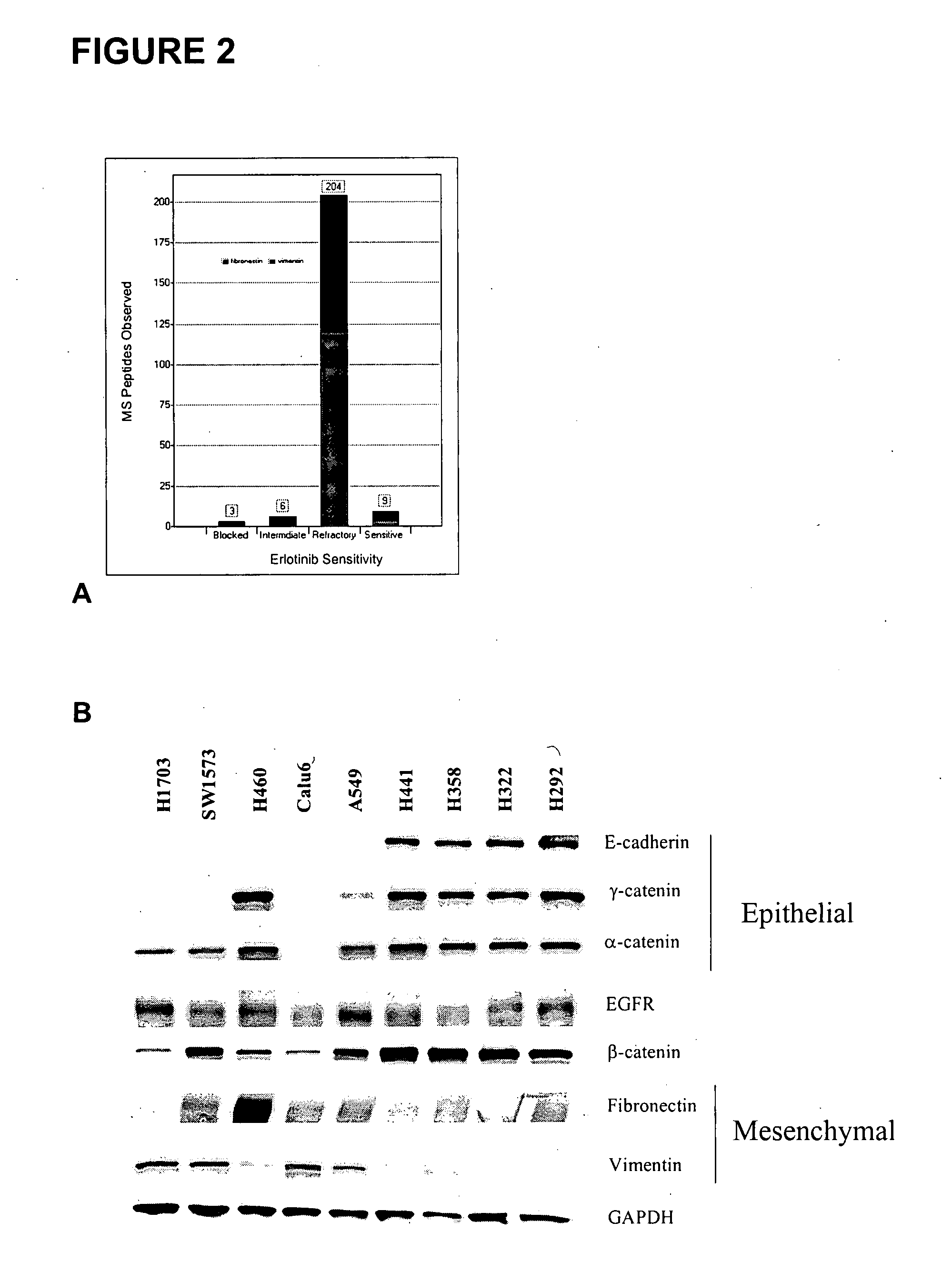
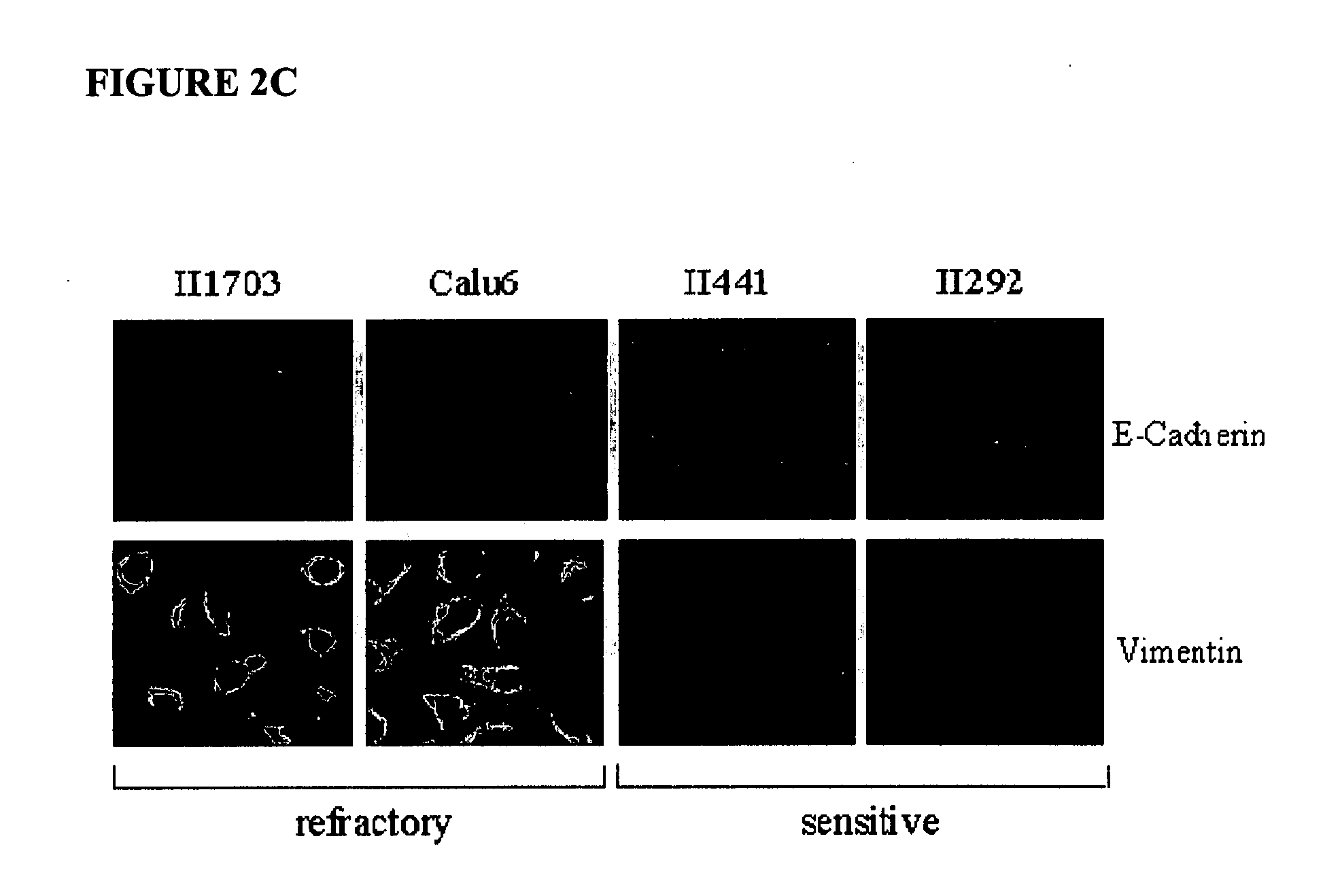
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS20070212738A1Restore sensitivityHigh sensitivityDisease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseKinase
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA LLC
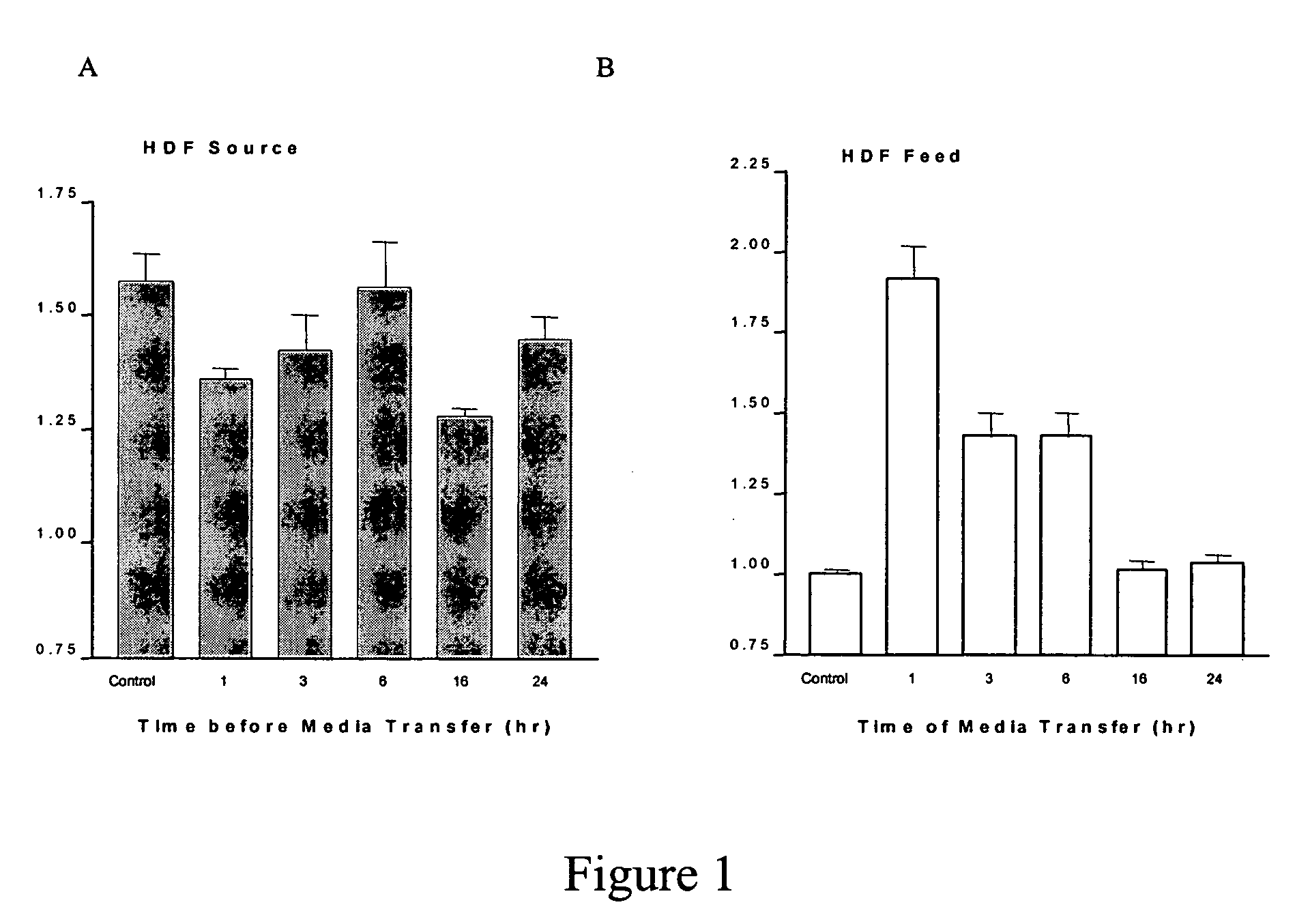
Method and system for the production of cells and cell products and applications thereof
ActiveUS20090269841A1Minimization requirementsAvoid possibilityBioreactor/fermenter combinationsBiological substance pretreatmentsCell culture mediaComputer module
Owner:BIOVEST INT
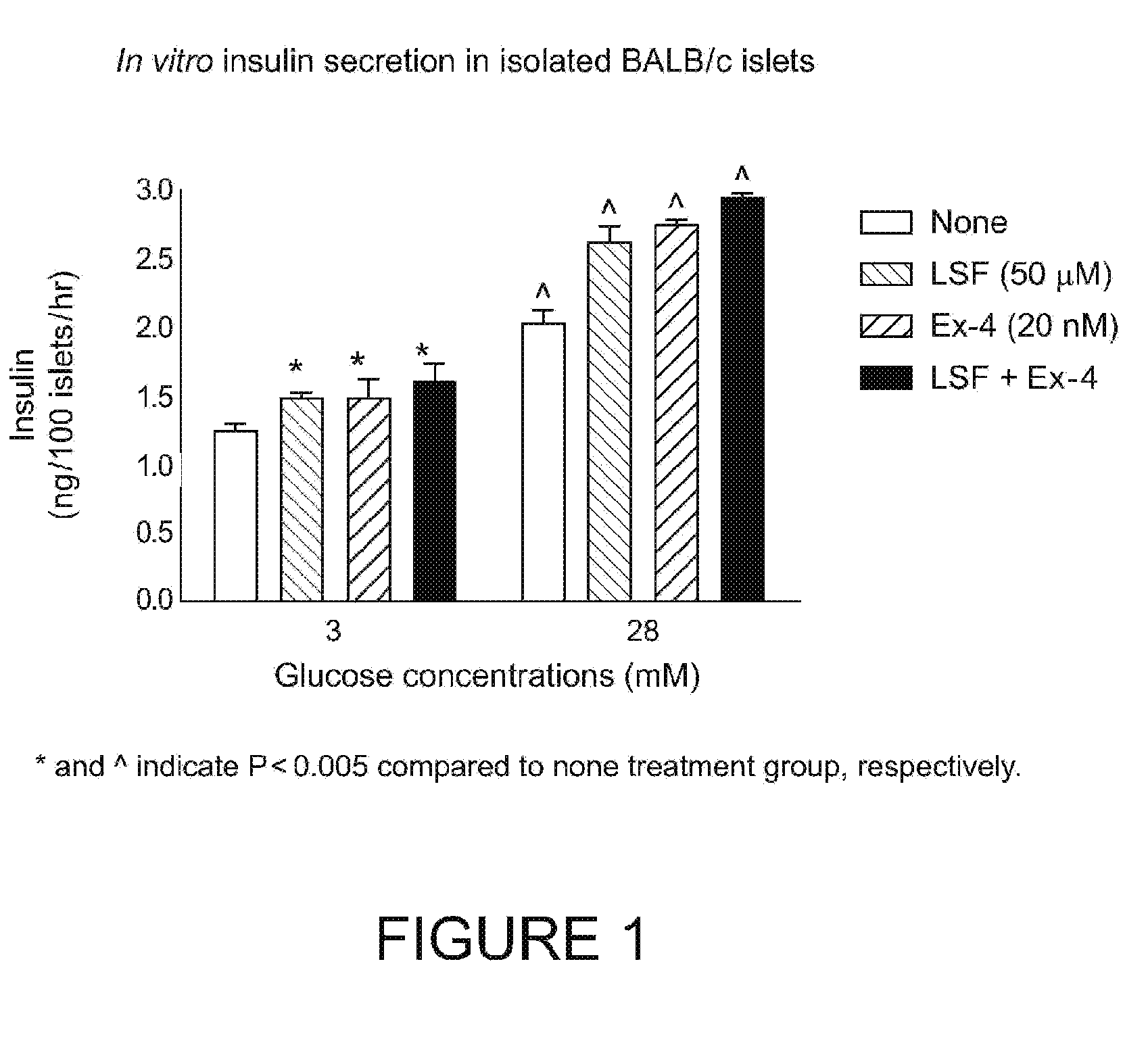
Pharmaceutical compositions and methods for restoring β-cell mass and function
ActiveUS7393827B2Increase the number ofInhibition formationOrganic active ingredientsPeptide/protein ingredientsAdjuvantCell mass
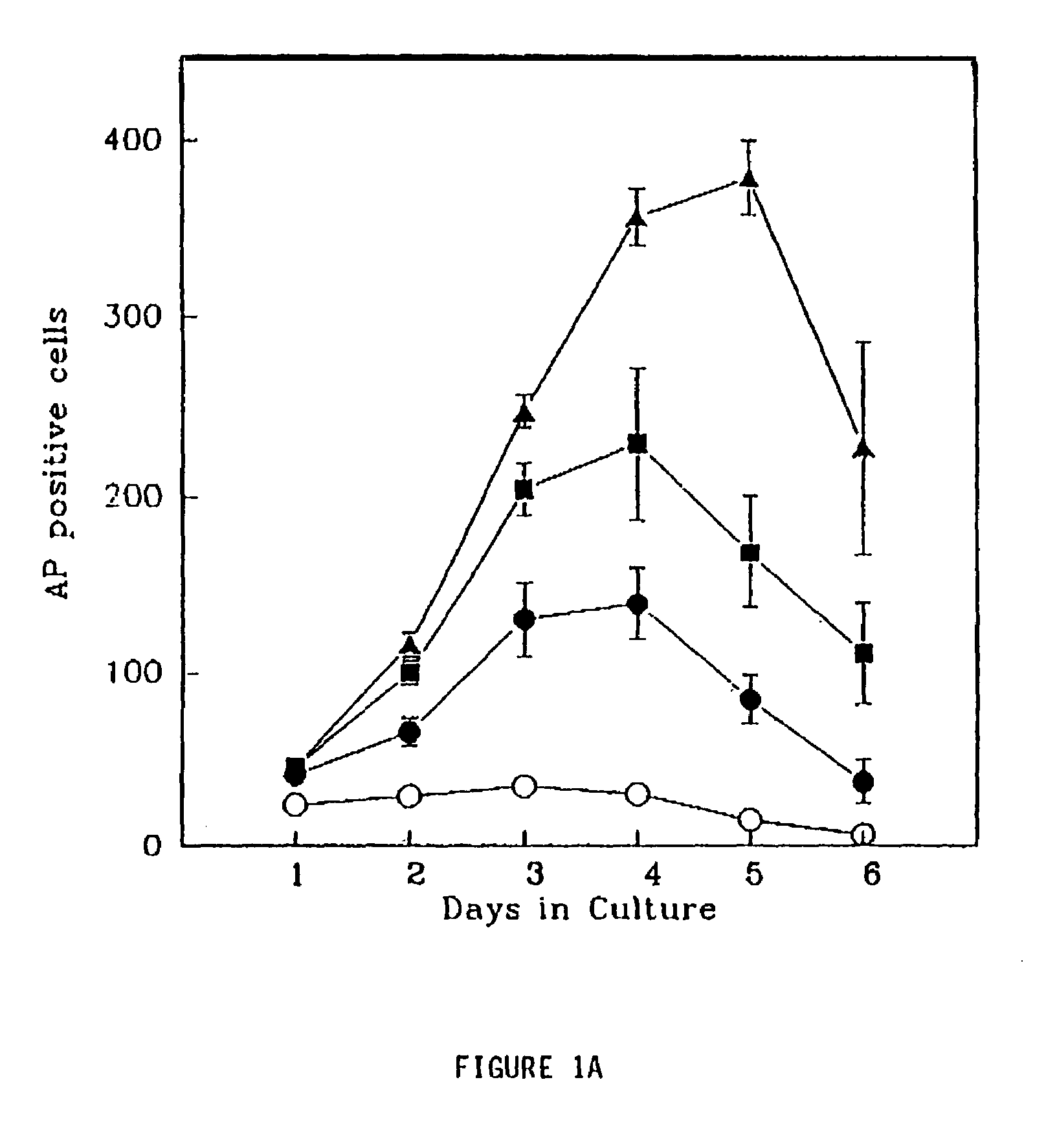
Pharmaceutical compositions and methods for using are provided for restoring β-cell mass and function in a mammal in need thereof. The pharmaceutical compositions have a biological response modifier and a β-cell growth factor in admixture with a pharmaceutically acceptable carrier, adjuvant or vehicle.
Owner:DIAKINE THERAPEUTICS
Muscle regeneration promoter
ActiveUS20100008907A1Promote muscle regenerationIncrease the number ofPeptide/protein ingredientsMuscular disorderCell growthSignal Pathways
Owner:OSAKA UNIV
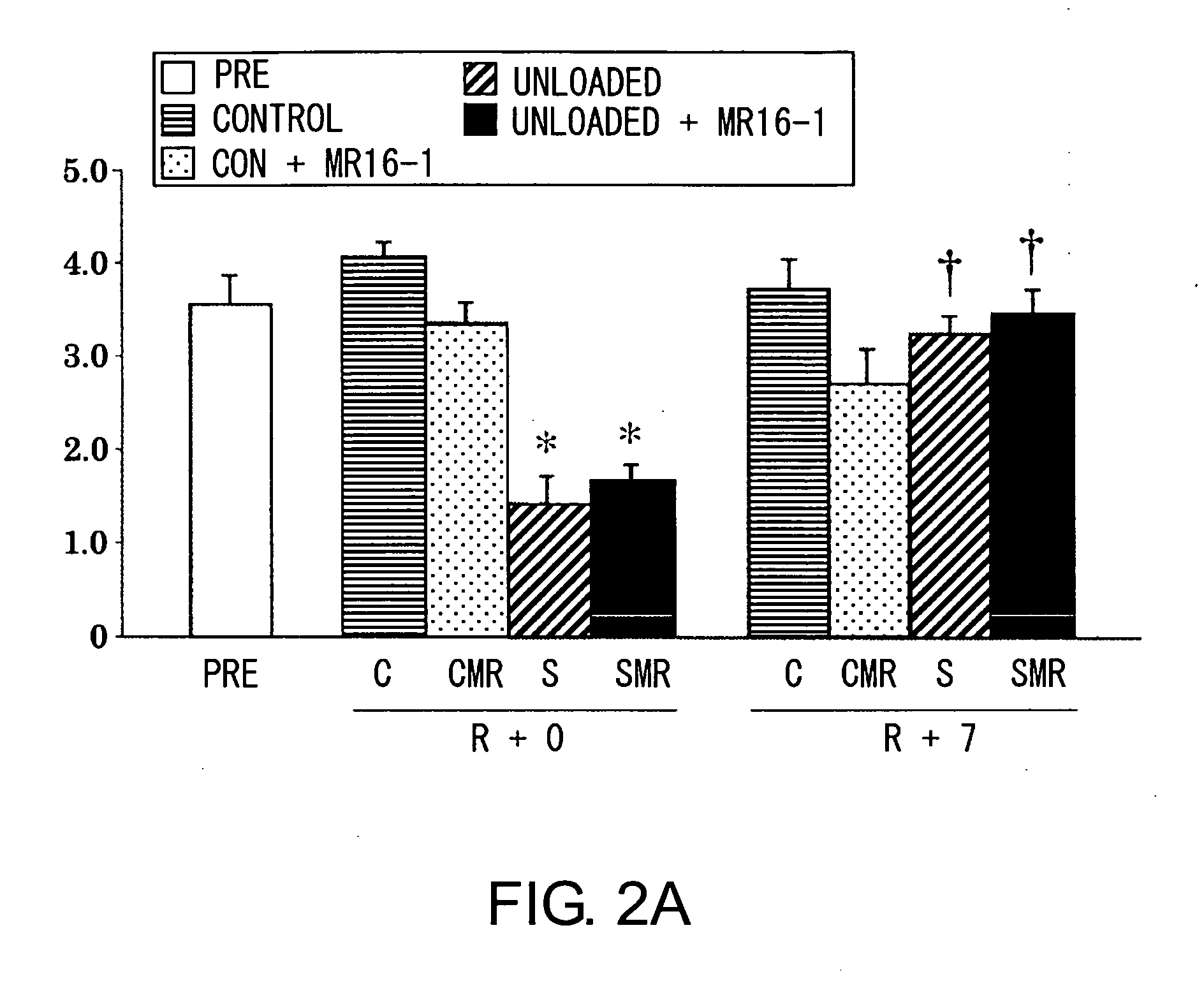
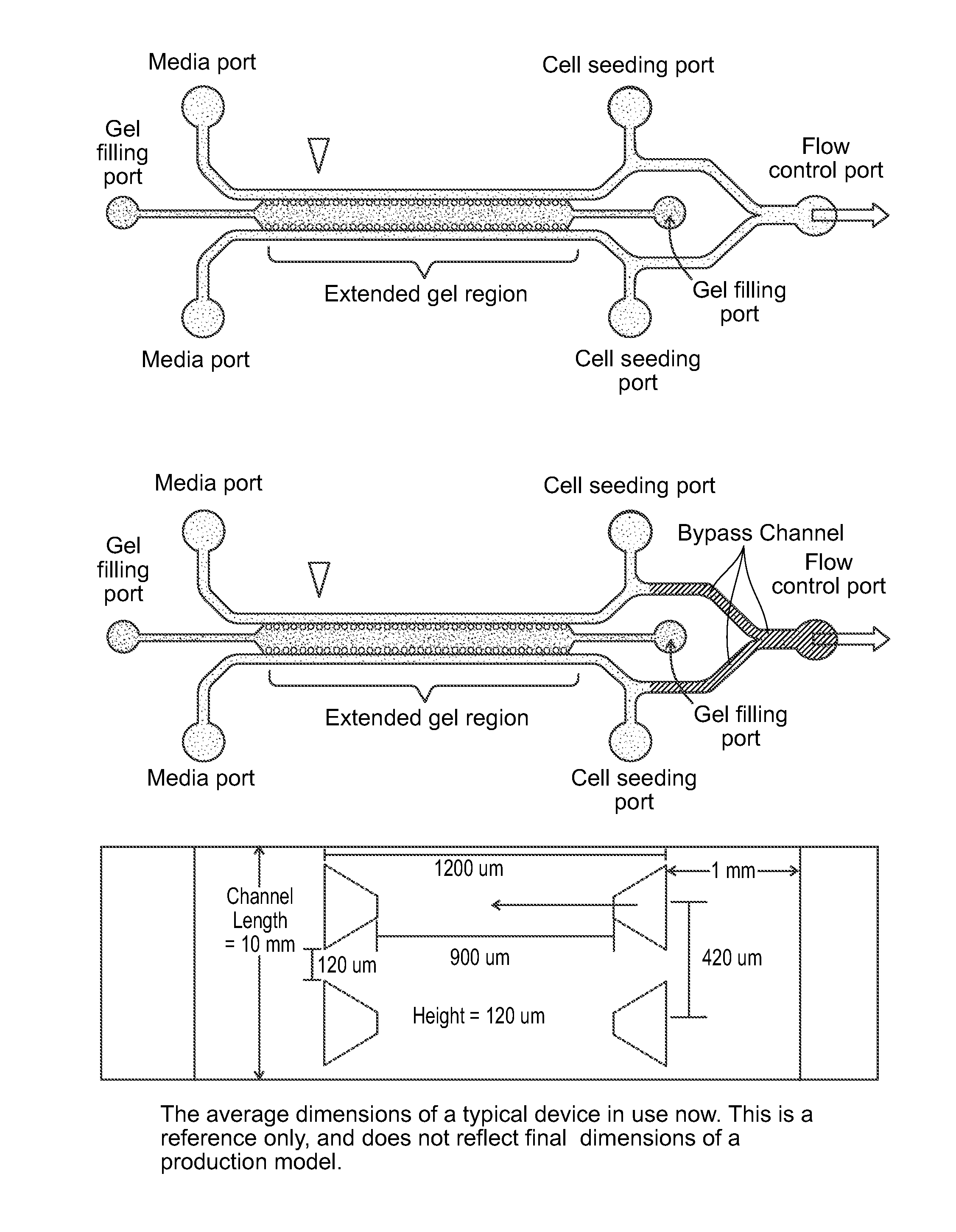
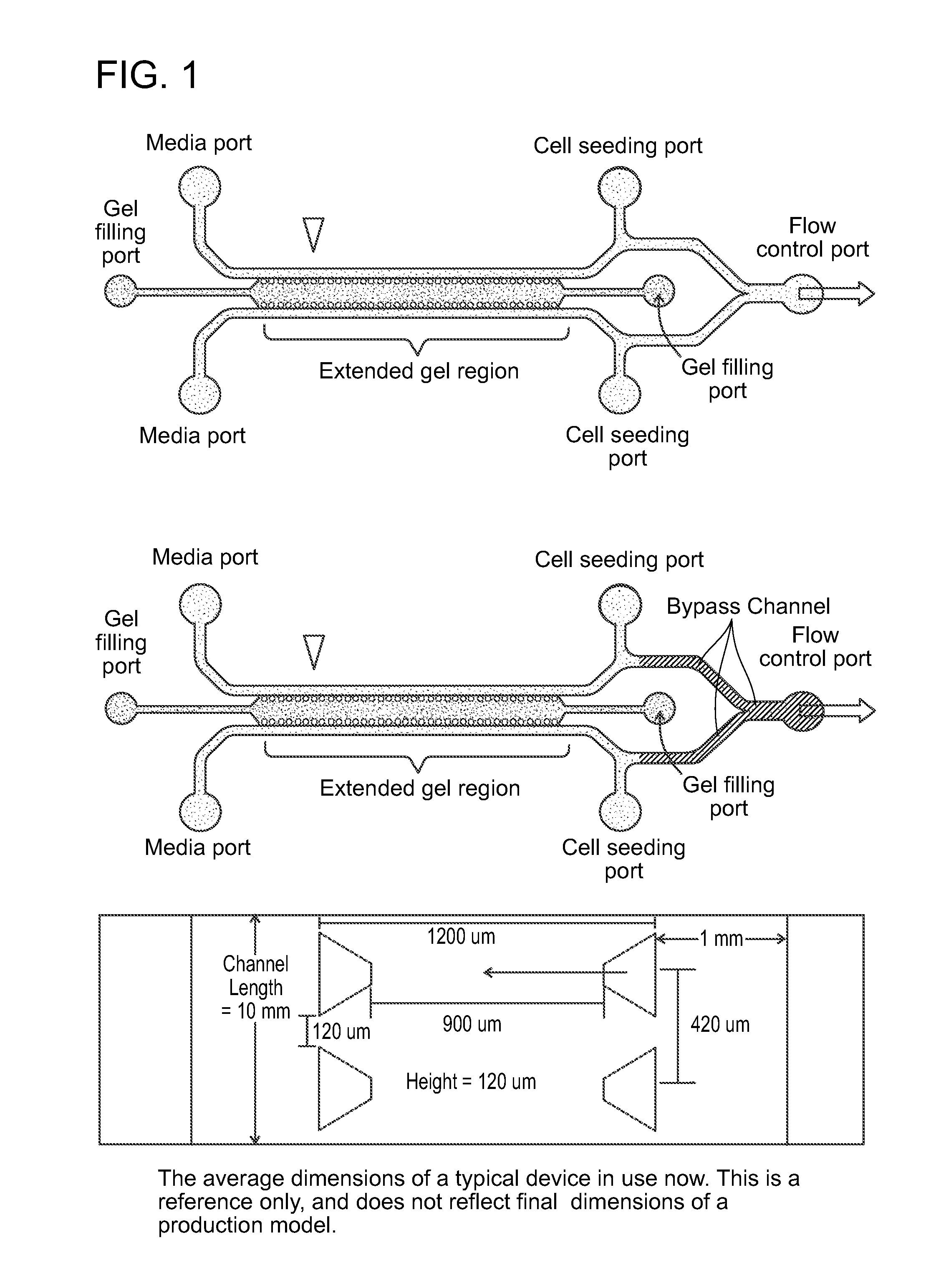
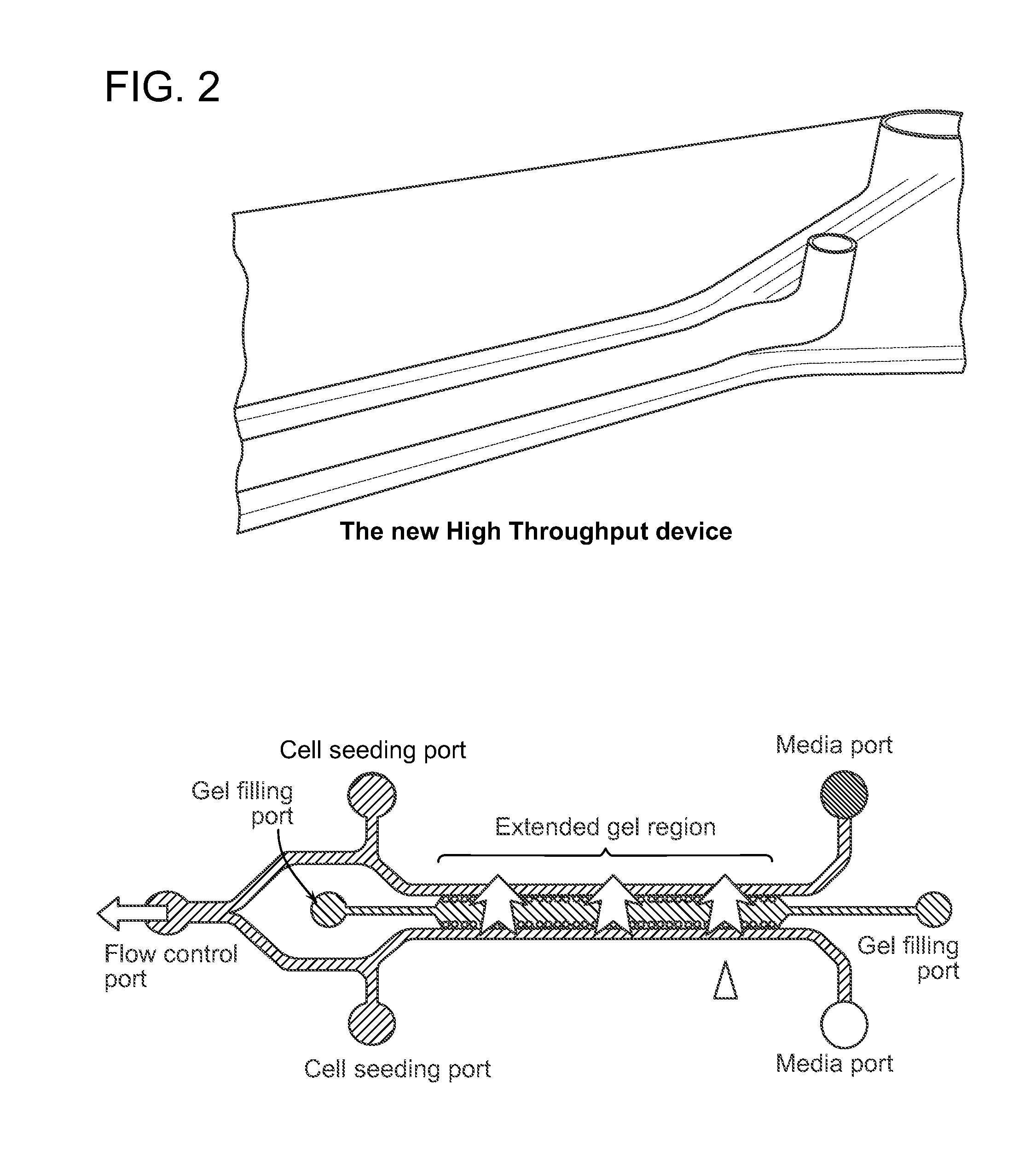
Device For High Throughput Investigations Of Multi-Cellular Interactions
ActiveUS20140057311A1Detailed analysisImprove throughputBioreactor/fermenter combinationsBiological substance pretreatmentsCancer cellBlood vessel
Provided herein are microfluidic devices that can be used as a 3D bioassay, e.g., for drug screening, personalized medicine, tissue engineering, wound healing, and other applications. The device has a series of channels {e.g., small fluid channels) in a small polymer block wherein one or more of the channels can be filled with a biologically relevant gel, such as collagen, which is held in place by posts. As shown herein, when the device is plated with cells such as endothelial cells, new blood vessels grow in the gel, which is thick enough for the cells to grow in three dimensions. Other channels, e.g., fluid channels, allow drugs or biological material to be exposed to the 3D cell growth. Cells, such as endothelial cells, can be cultured and observed as they grow on the surface of a 3D gel scaffold, where e.g., rates of angiogenesis can be measured, as well as intervascularization and extravascularization of cancerous cells.
Owner:THE GENERAL HOSPITAL CORP +3
Methods for promoting nerve regeneration and neuronal growth and elongation
ActiveUS20070239080A1Increase amplitudeUltrasonic/sonic/infrasonic diagnosticsChiropractic devicesNervous systemRisk stroke
A method of enhancing the regeneration of injured nerves has the step of administering an effective exposure of pressure pulses or acoustic shock waves in a pulse or wave pattern to the zone of injury of the nerve during the regeneration process. The inventive method may include enhancing the stimulation of neuronal cell growth or regeneration by administering an effective exposure of pressure pulses or acoustic shock waves in a pulse or wave pattern to stimulate neuronal cell growth or regeneration, wherein the administering of the treatment is applied to a patient who has a pathological condition where neuronal repair can be facilitated including peripheral nerve damage caused by injury or disease such as diabetes, brain damage associated with stroke, and for the treatment of neurological disorders related to neurodegeneration, including Parkinson's disease, Alzheimer's disease and amyotrophic lateral, sclerosis multiple sclerosis and disseminated sclerosis. The treatment is ideally suited for neural regeneration after a degenerative condition due to any neurological infections or any other pathological condition.
Owner:SOFTWAVE TISSUE REGENERATION TECH LLC
Stable Composite Material Comprising Supported Porous Gels
ActiveUS20080264867A1Improve throughputLow resistance to hydraulic flowComponent separationIon-exchanger regenerationPorosityFiltration
This invention relates to a stable composite material comprising a support member that has a plurality of pores extending through the support member, and a macroporous crosslinked gel that is located in, and fills, the pores of the support member, in which crosslinked gel is entrapped a stabilising polymer, which stabilising polymer is neutral, linear or branched, non-crosslinked, and substantially water-insoluble. The presence of the stabilising polymer is such that it allows the composite material to largely retain its porosity and morphology after being dried. The invention also relates to a process for preparing the stable composite material described above, and to its use. The stable composite material is suitable, for example, for separation of substances, for example by filtration or adsorption, including chromatography, for use as a support in synthesis or for use as a support for cell growth.
Owner:MERCK MILLIPORE LTD
Direct expression of peptides into culture media
InactiveUS6103495AReduced viabilityImprove breathabilityBacteriaPeptide/protein ingredientsGrowth phaseBiotechnology
Expression systems are disclosed for the direct expression of peptide products into the culture media where genetically engineered host cells are grown. High yield was achieved with novel vectors, a special selection of hosts, and / or fermentation processes which include careful control of cell growth rate, and use of an inducer during growth phase. Special vectors are provided which include control regions having multiple promoters linked operably with coding regions encoding a signal peptide upstream from a coding region encoding the peptide of interest. Multiple transcription cassettes are also used to increase yield. The production of amidated peptides using the expression systems is also disclosed.
Owner:ENTERIS BIOPHARMA
Methods for Rapid Distinction between Debris and Growing Cells
Owner:CALIFORNIA INST OF TECH
Enzyme-mediated modification of fibrin for tissue engineering
The invention provides fibrin-based, biocompatible materials useful in promoting cell growth, wound healing, and tissue regeneration. These materials are provided as part of several cell and tissue scaffolding structures that provide particular application for use in wound-healing and tissue regenerating. Methods for preparing these compositions and using them are also disclosed as part of the invention. A variety of peptides may be used in conjunction with the practice of the invention, in particular, the peptide IKVAV, and variants thereof. Generally, the compositions may be described as comprising a protein network (e.g., fibrin) and a peptide having an amino acid sequence that comprises a transglutaminase substrate domain (e.g., a factor XIIIa substrate domain) and a bioactive factor (e.g., a peptide or protein, such as a polypeptide growth factor), the peptide being covalently bound to the protein network. Other applications of the technology include their use on implantable devices (e.g., vascular graphs), tissue and cell scaffolding. Other applications include use in surgical adhesive or sealant, as well as in peripheral nerve regeneration and angiogenesis.
Owner:CALIFORNIA INST OF TECH
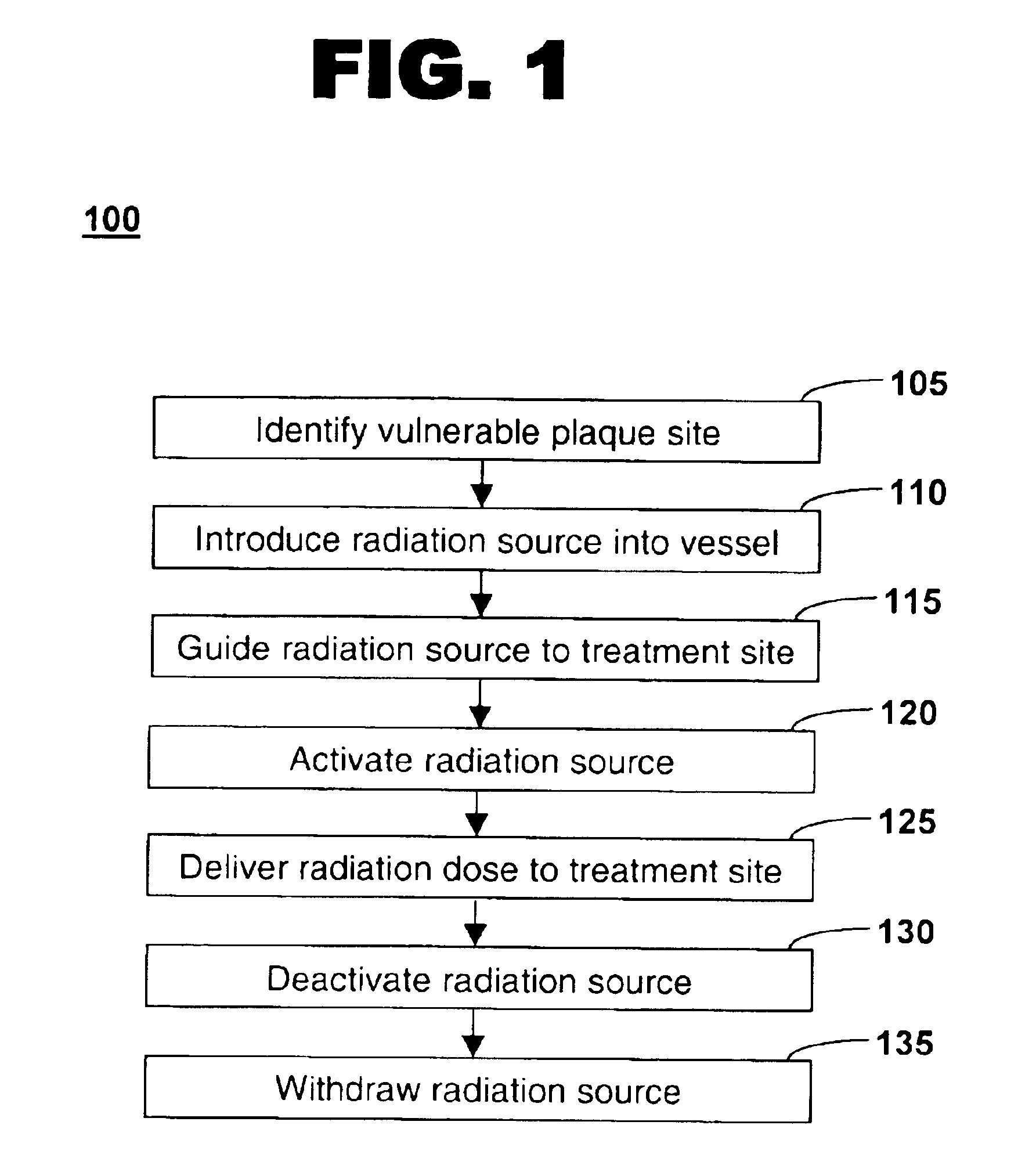
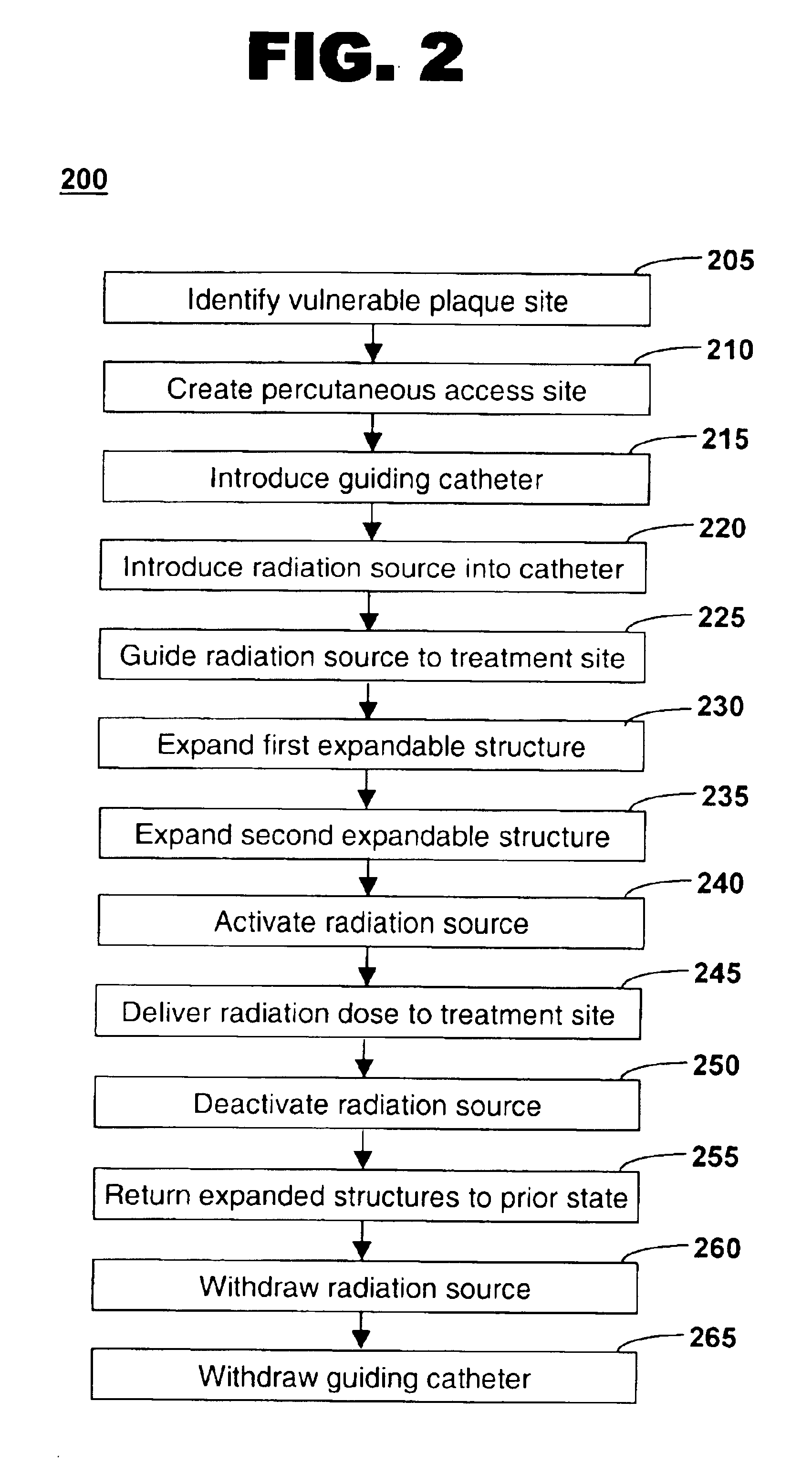
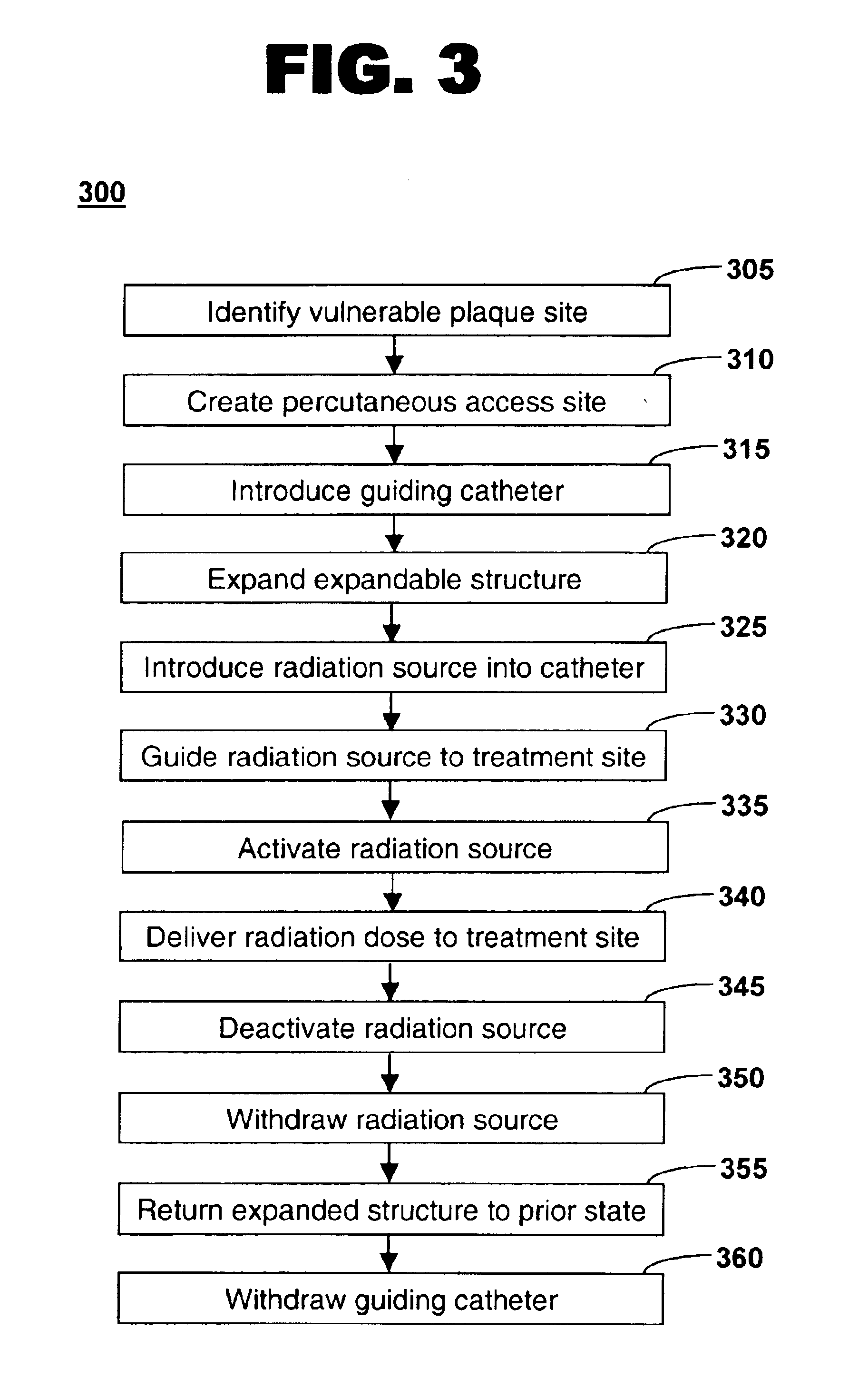
Method of treating vulnerable plaque using a catheter-based radiation system
The invention provides a method of treating vulnerable plaque at a site in a vessel. A vulnerable plaque site is identified for treatment. A radiation source is introduced into a vessel containing a vulnerable plaque site identified for treatment. The radiation source is guided to a position adjacent to the treatment site. A therapeutically effective dose of radiation is delivered to the vulnerable plaque site. As the radiation impinges upon the wall of the lumen, it promotes cell growth. Such growth can serve to strengthen the thin fibrous cap found atop a vulnerable plaque lesion. With the lesion thus stabilized, time is provided for the use of statin drugs or other agents to shrink or remove the lipid pool beneath the cap.
Owner:MEDTRONIC VASCULAR INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com