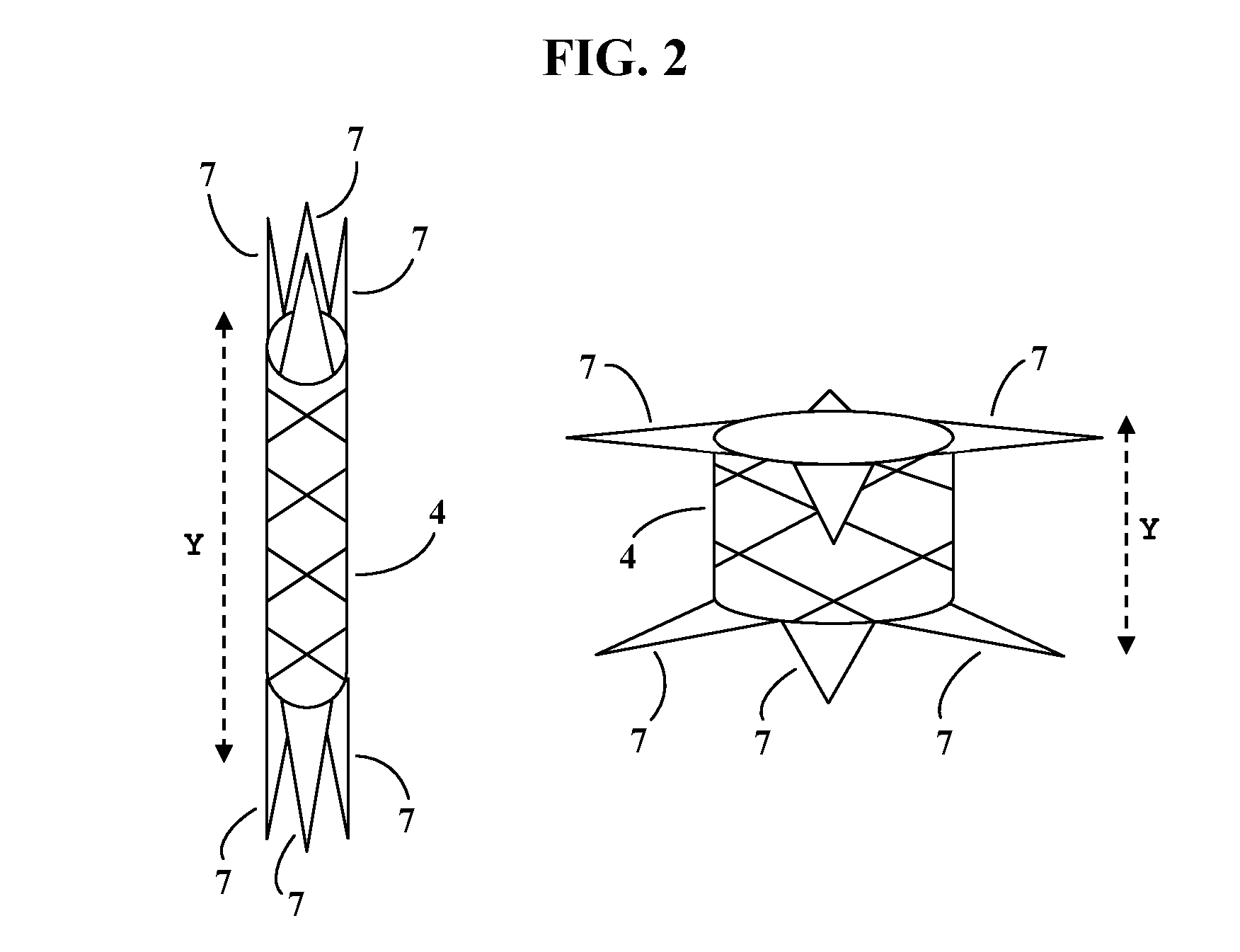
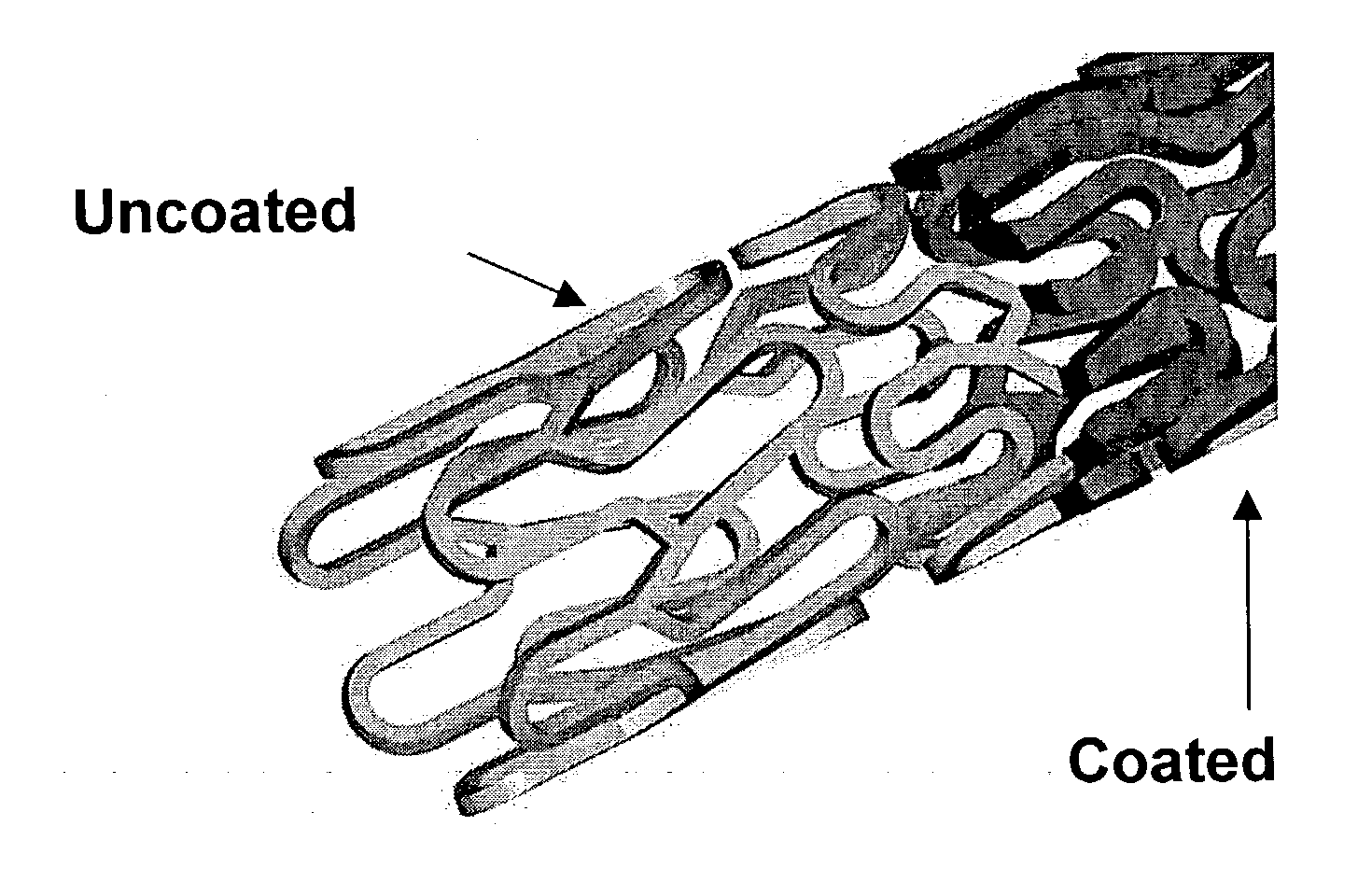
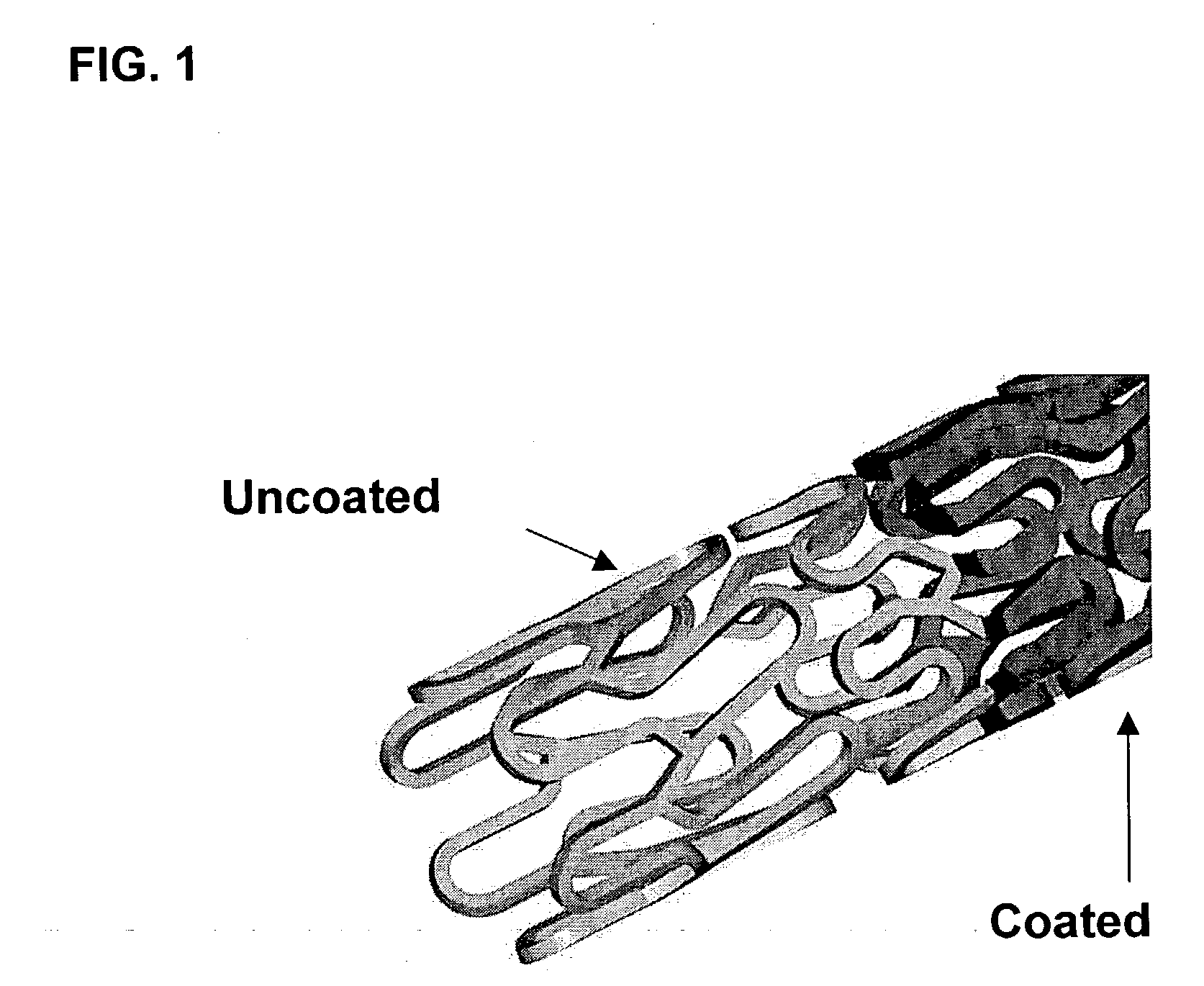
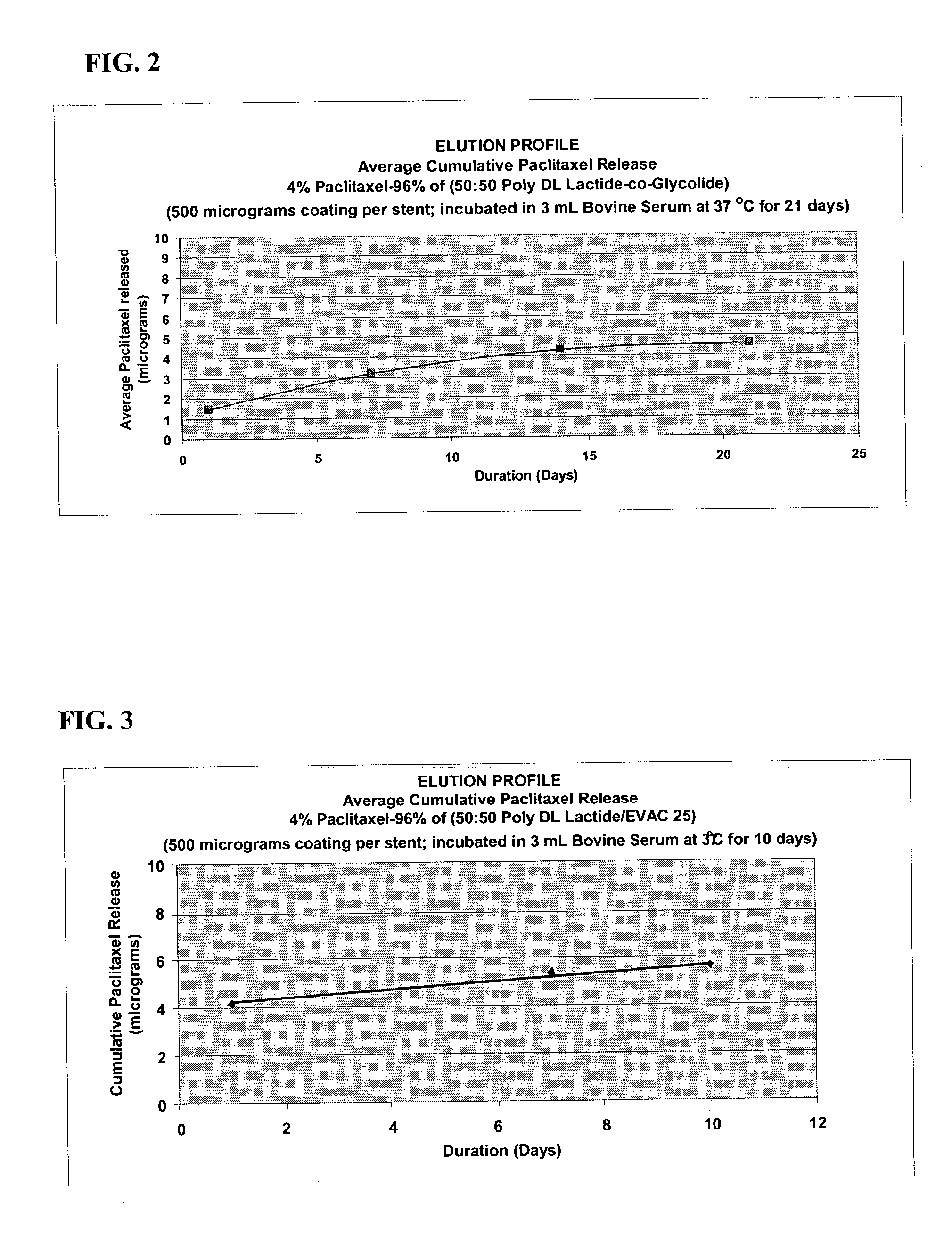
Patents
Literature
604 results about "Implantation Site" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The anatomic site at which a material such as a tissue, graft, device or radioactive material is inserted with some intended degree of permanence. This term may also refer to the site of the uterus at which the early embryo is attached.
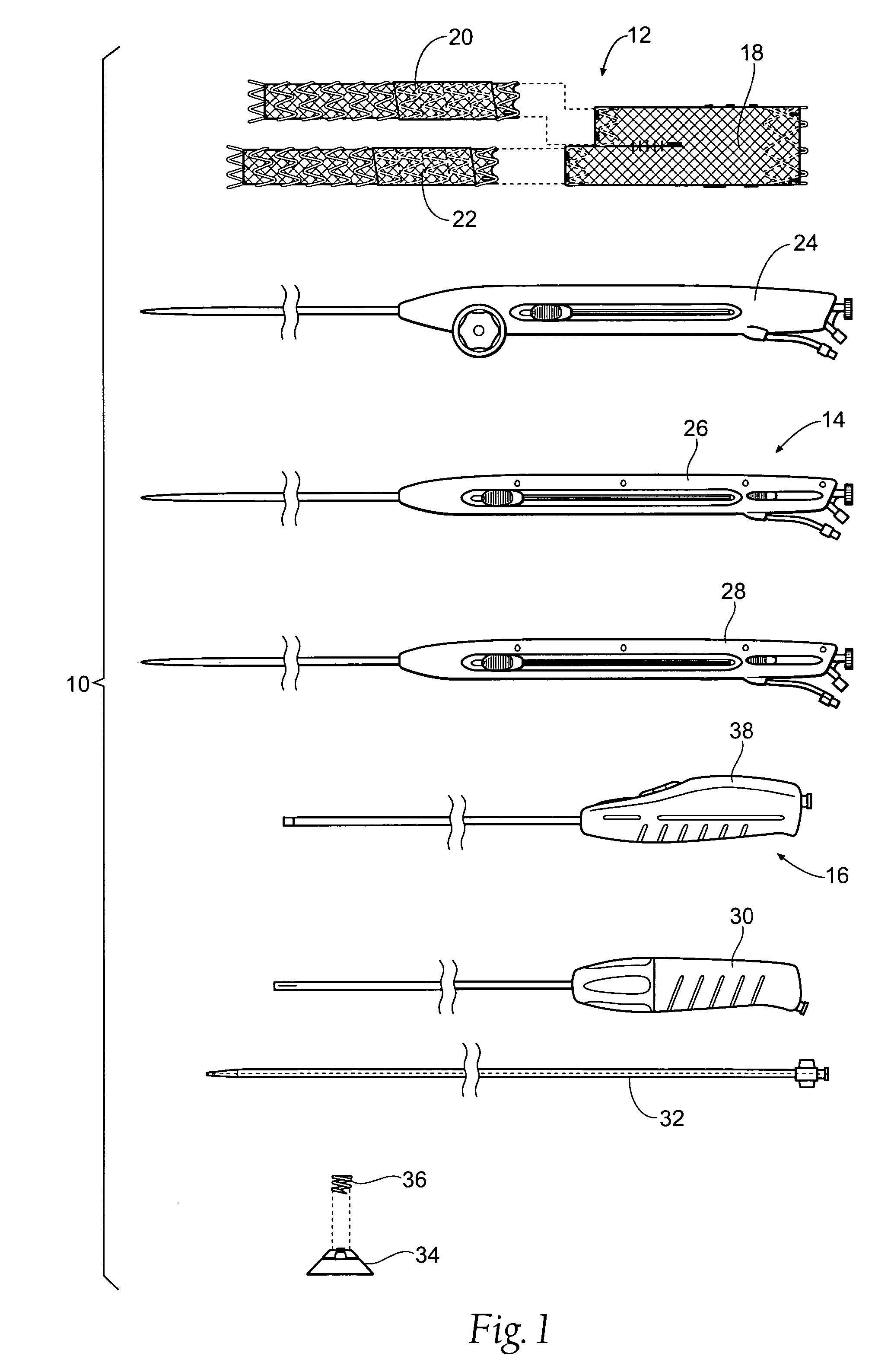
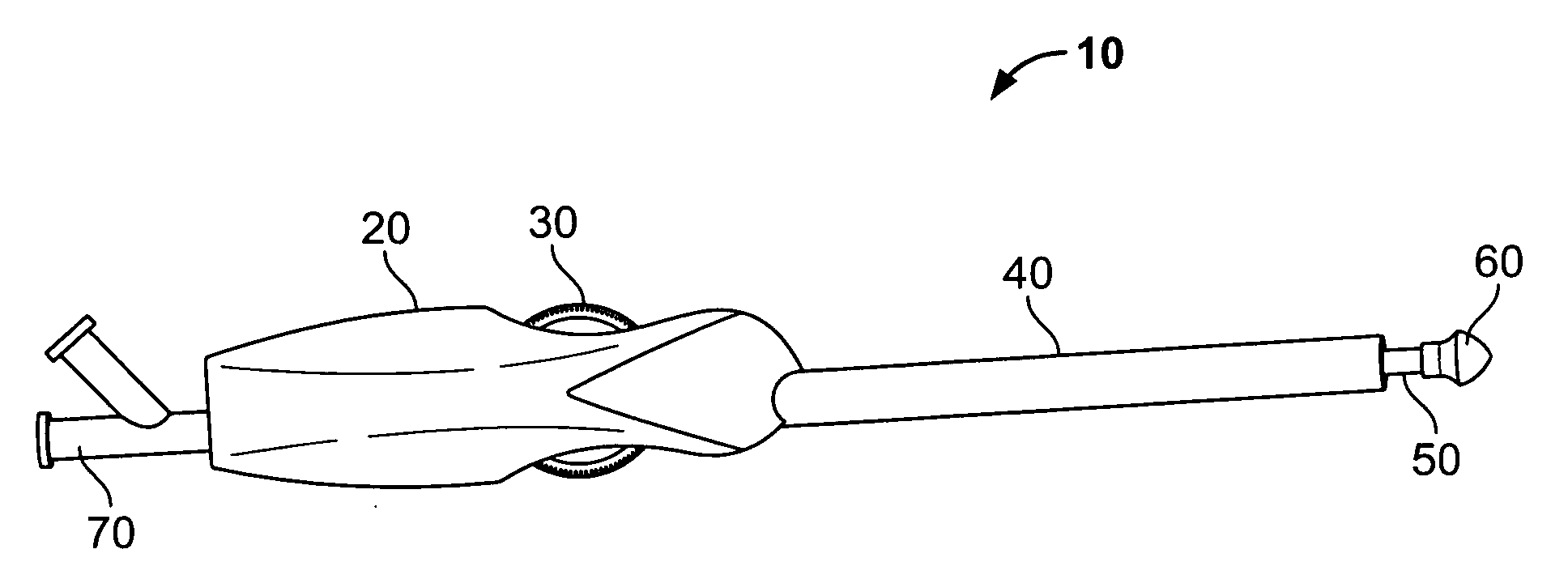
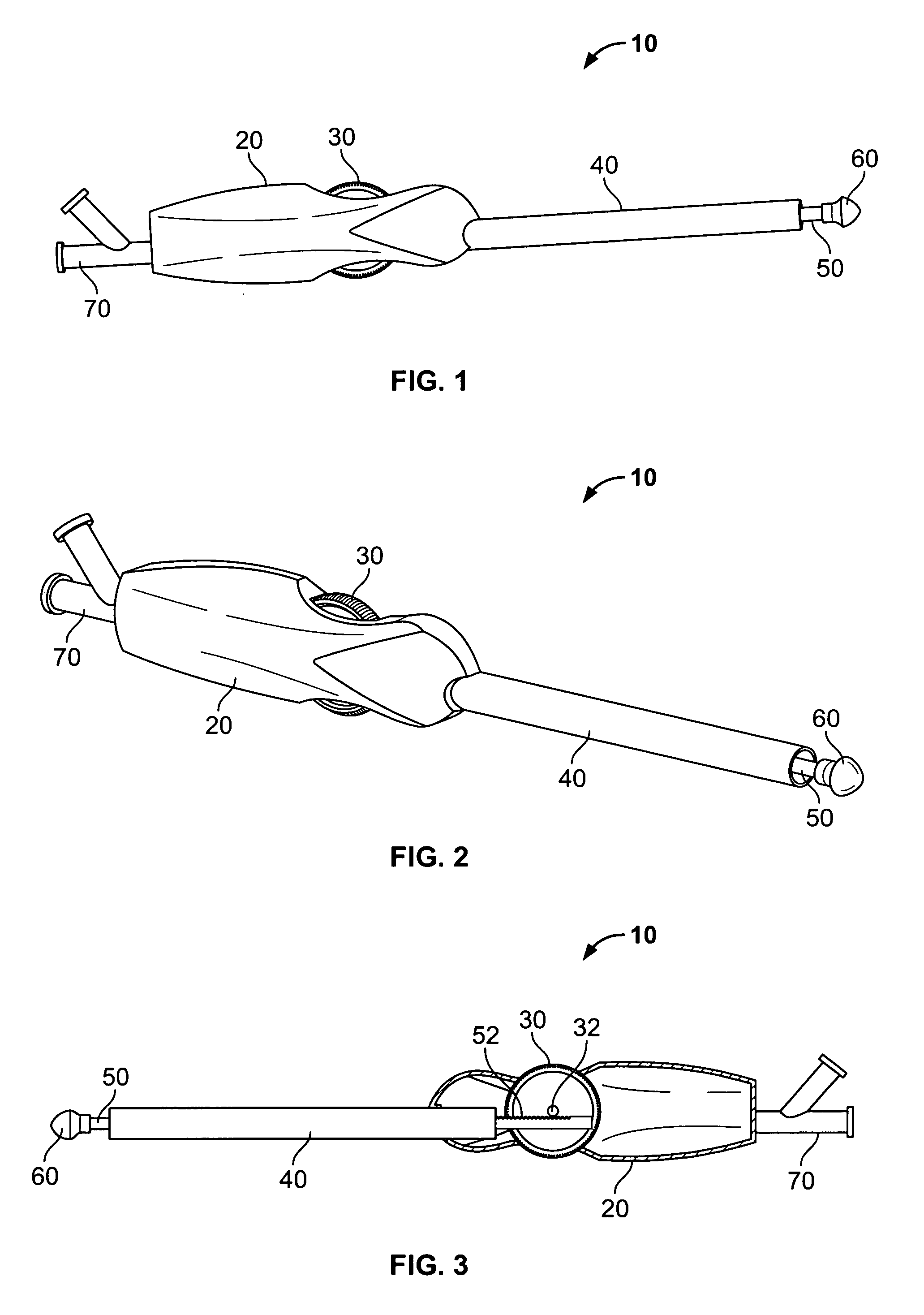
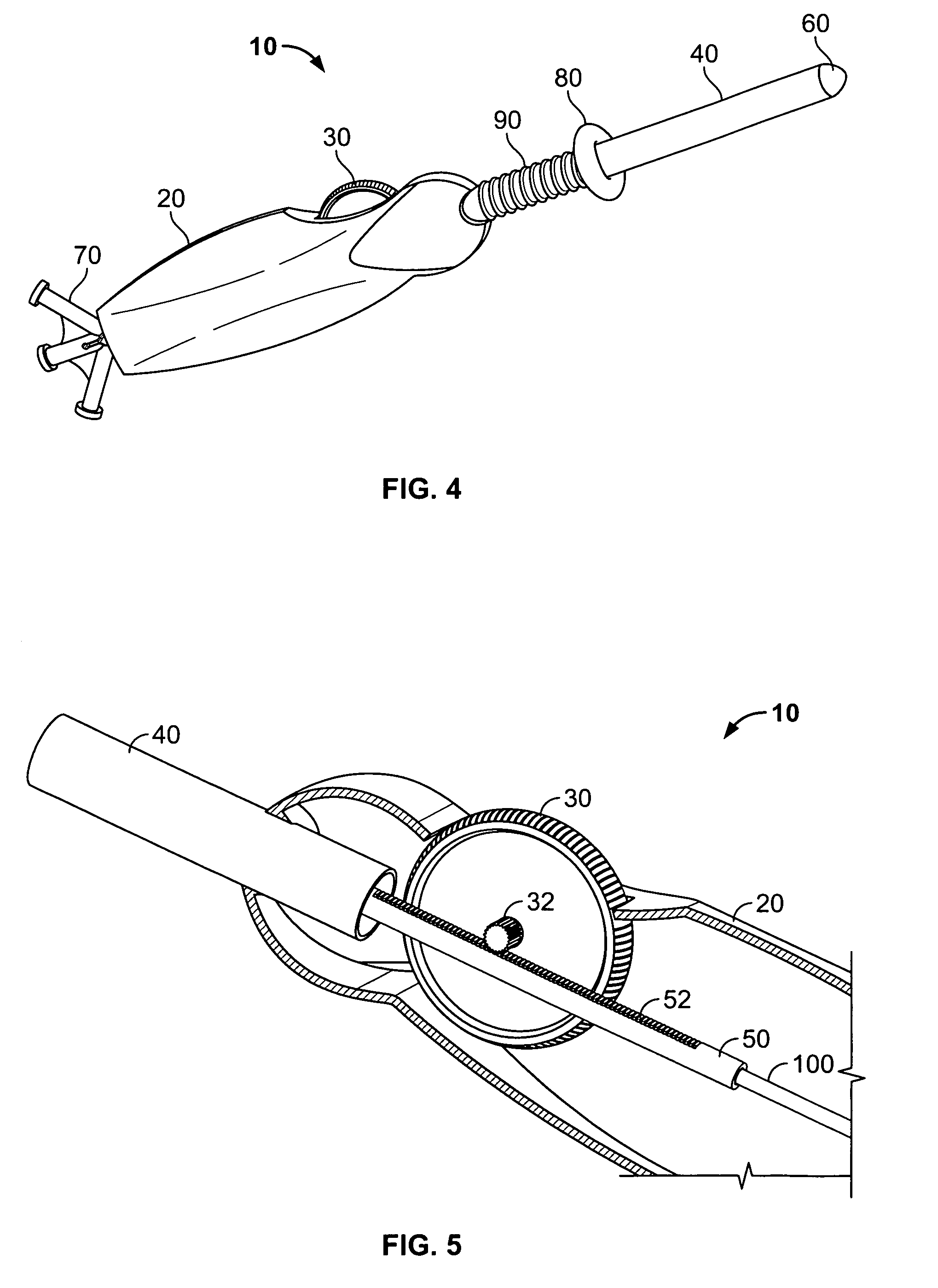
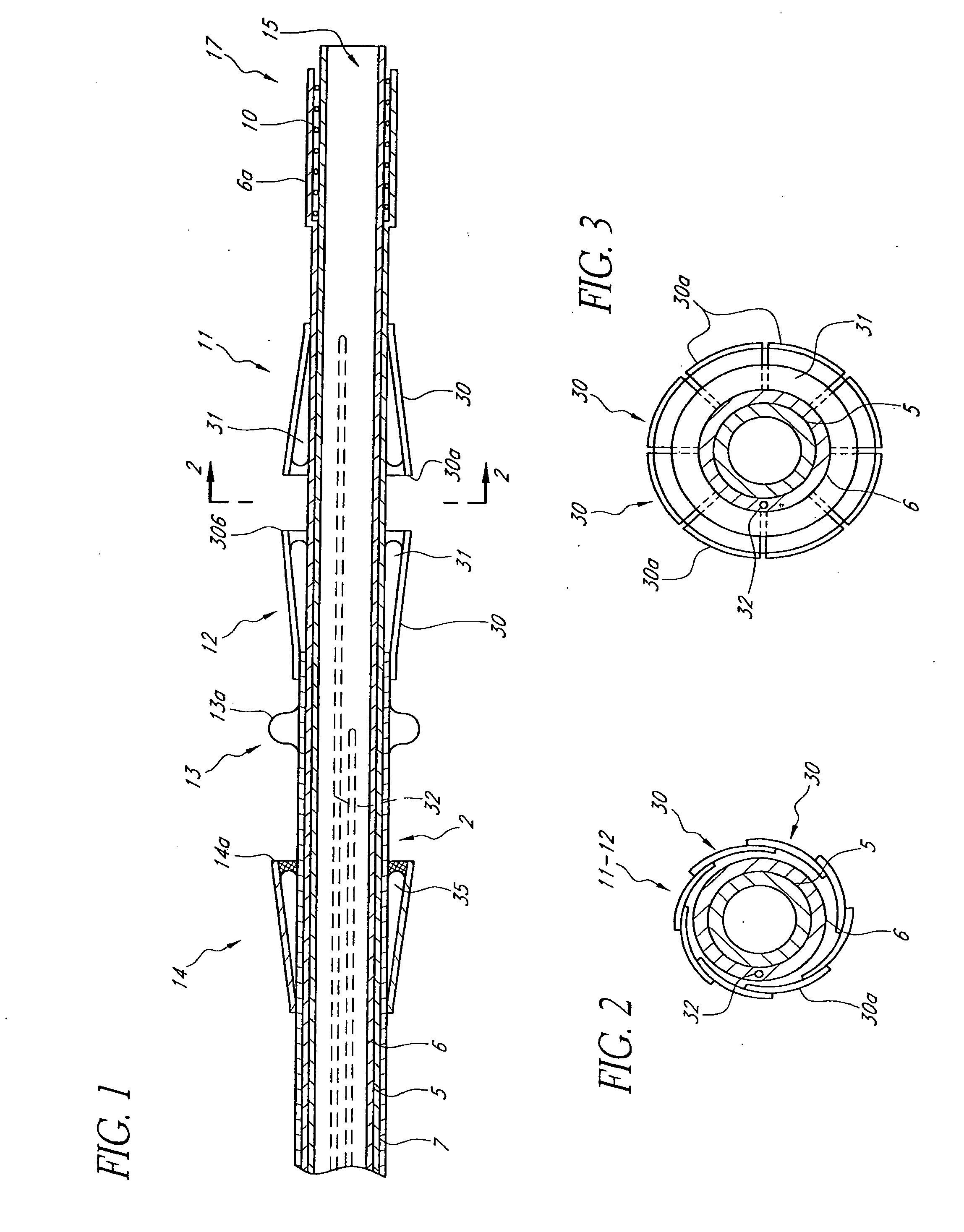
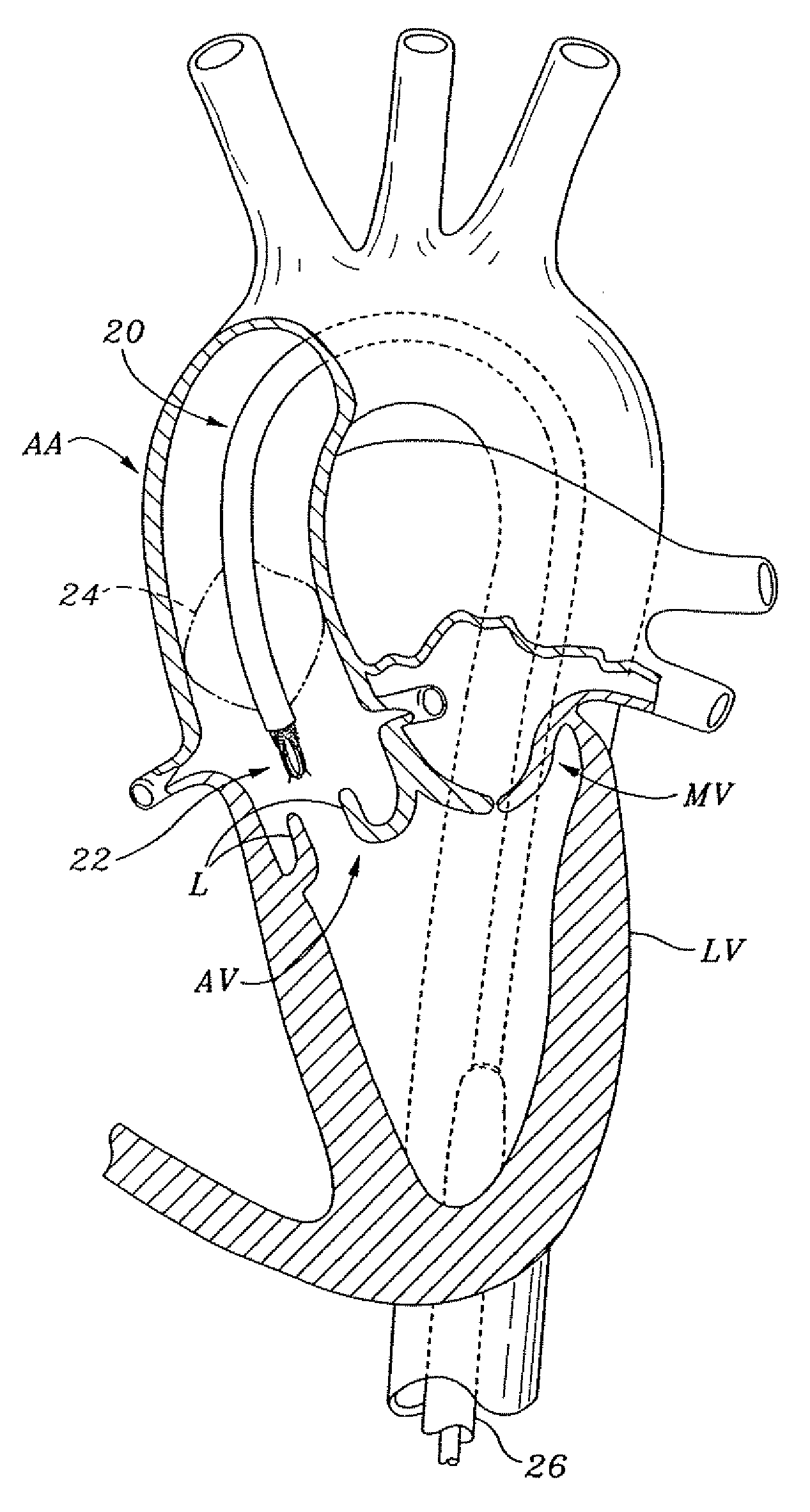
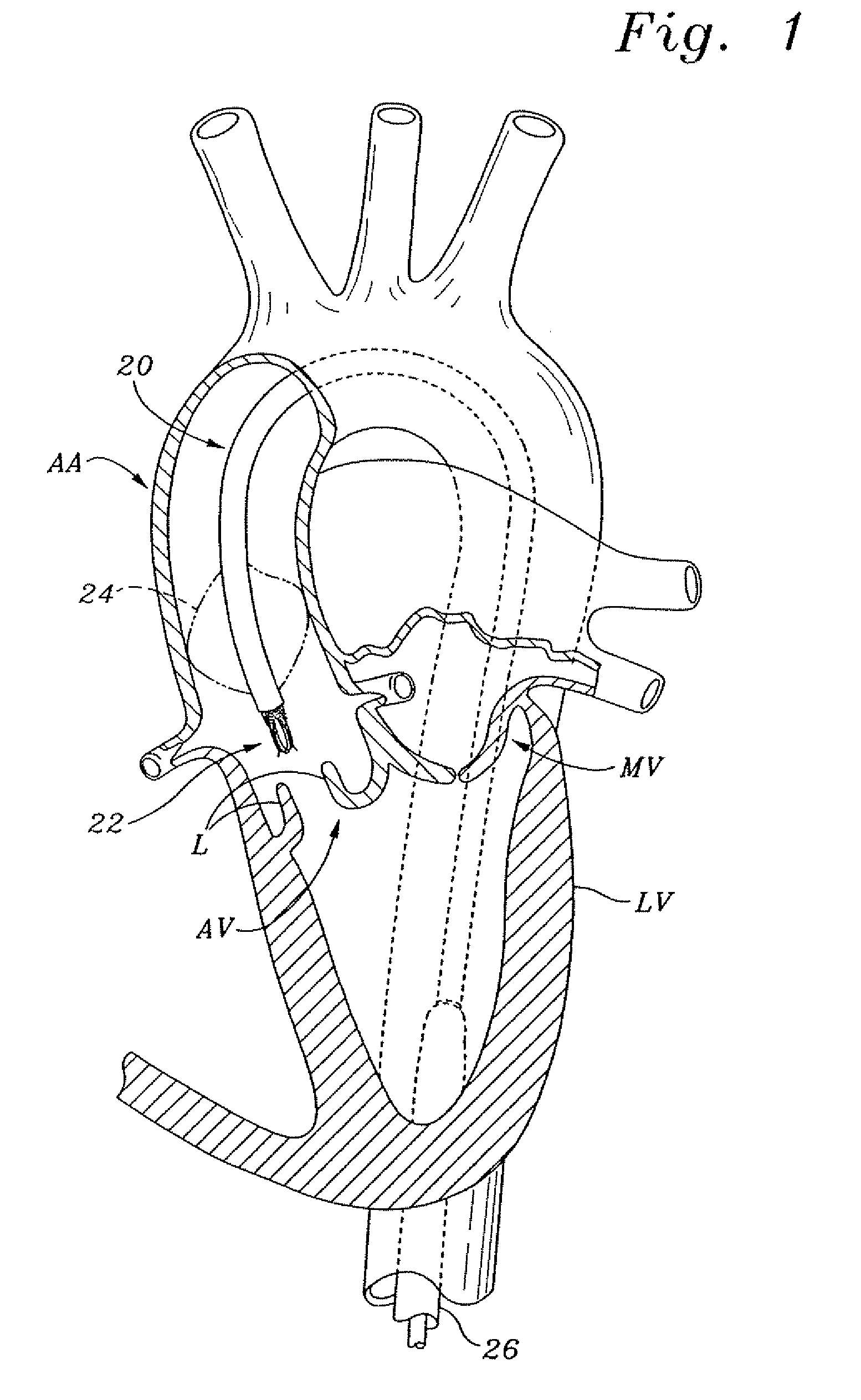
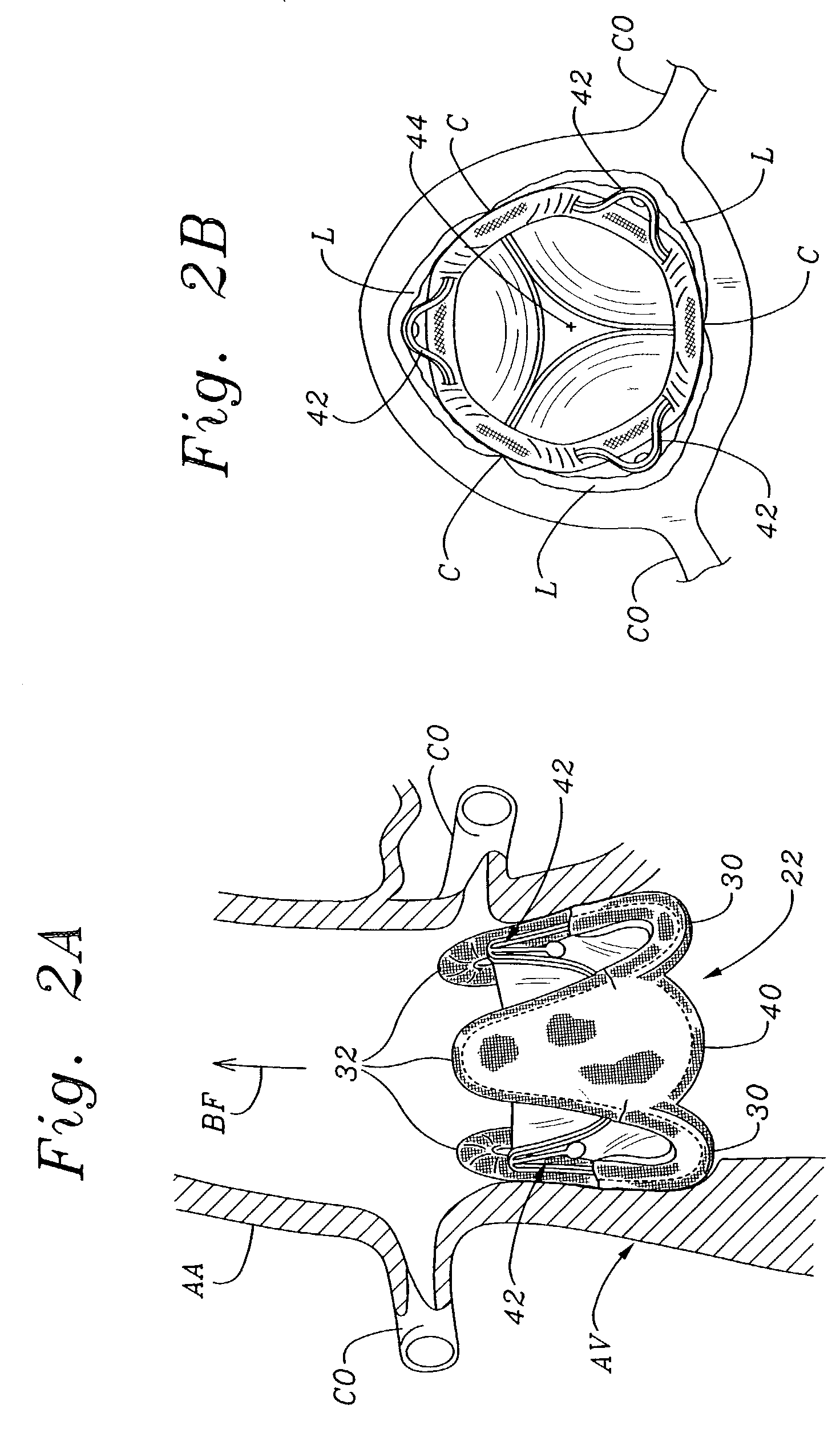
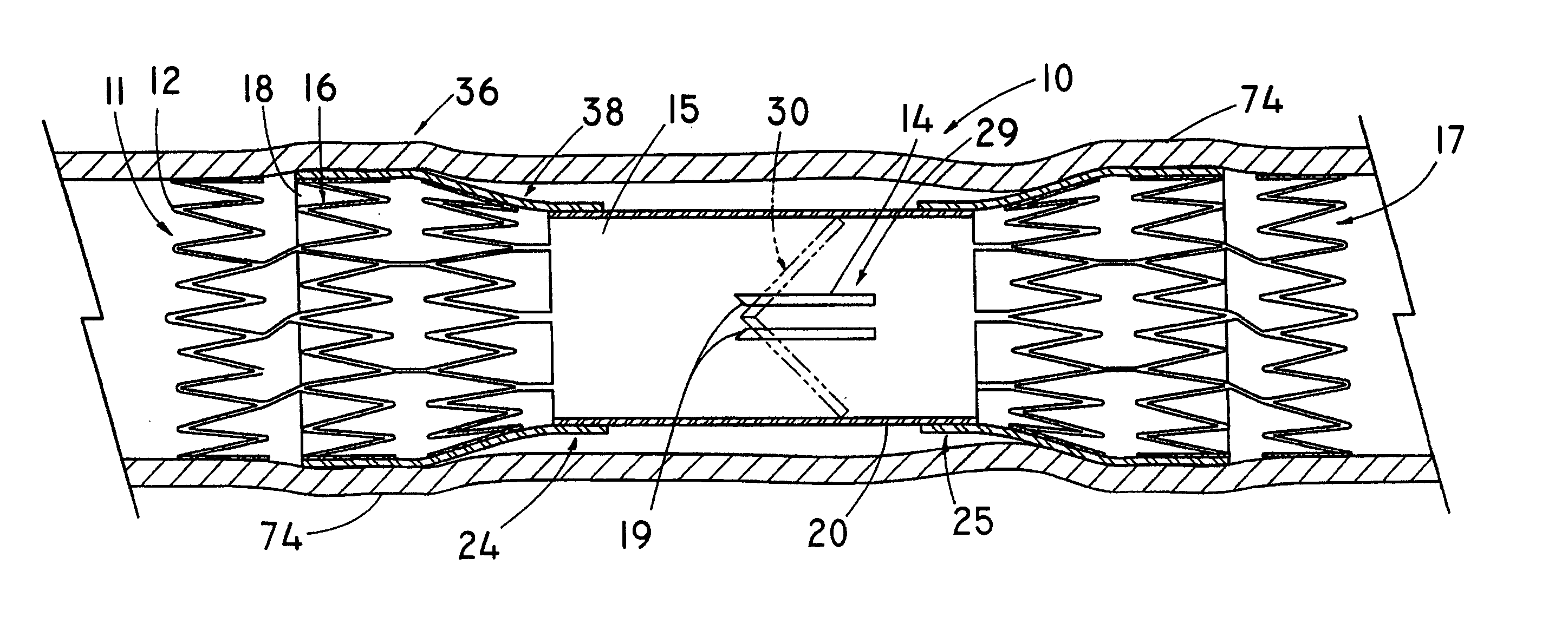
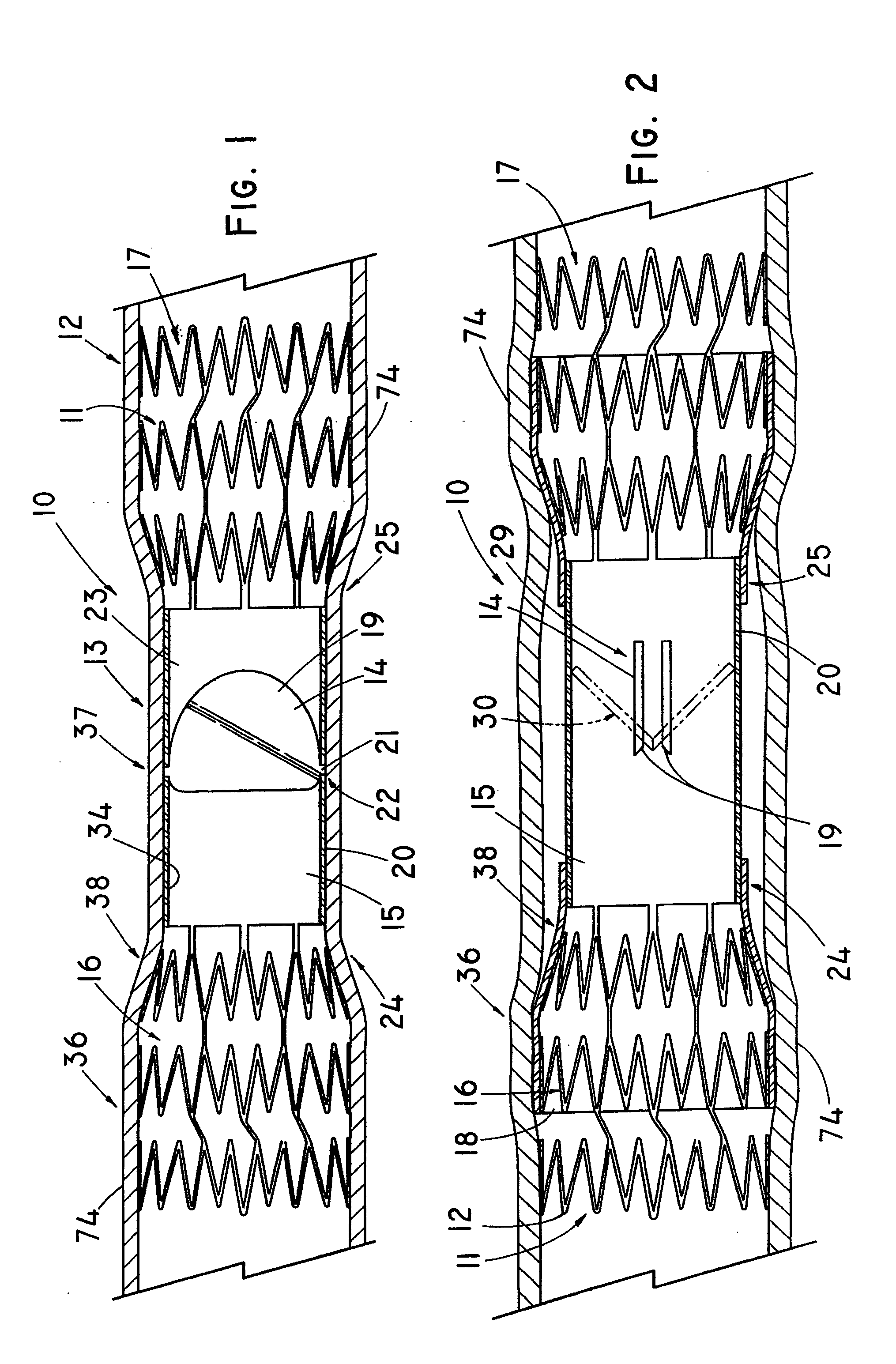
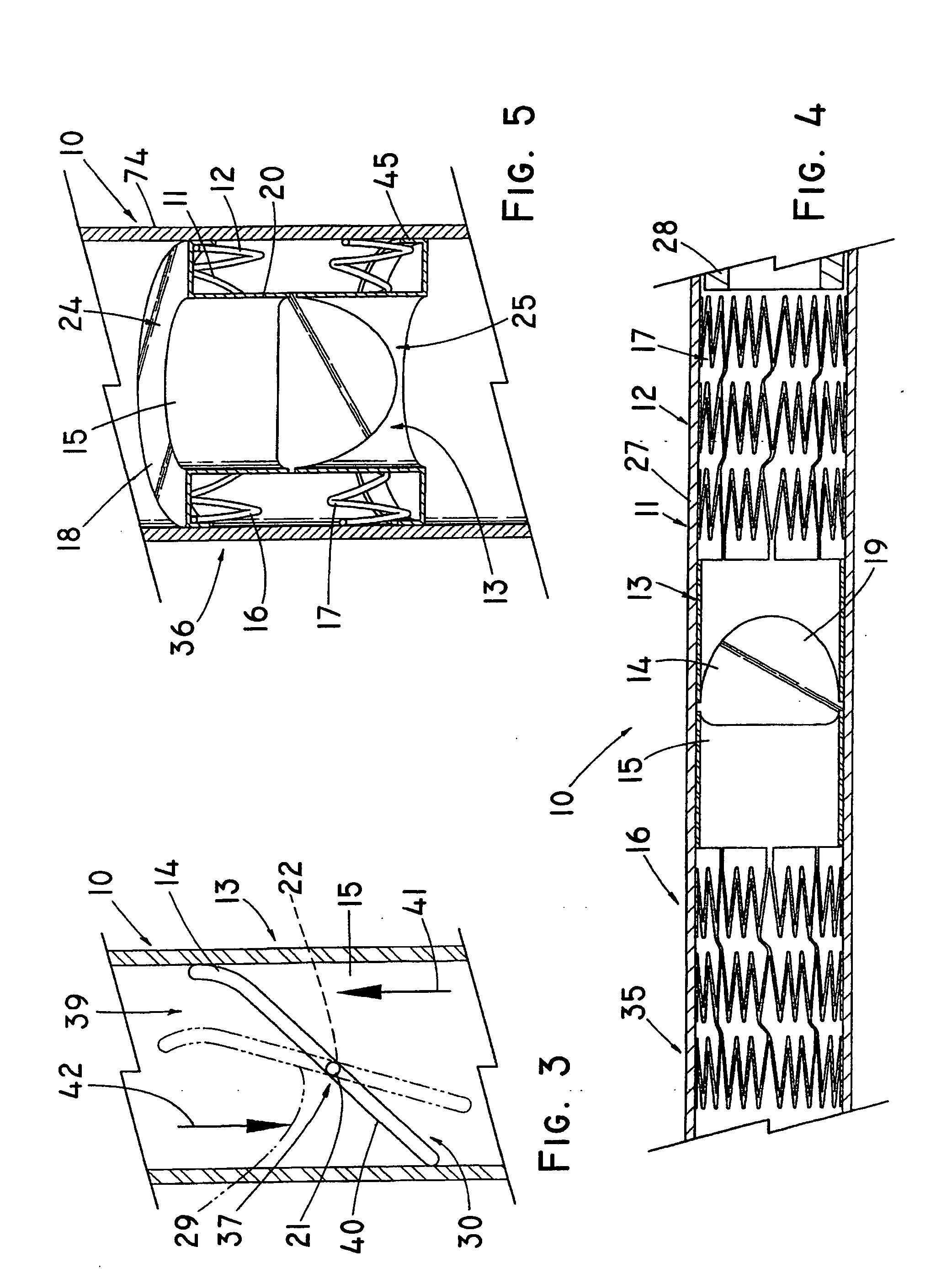
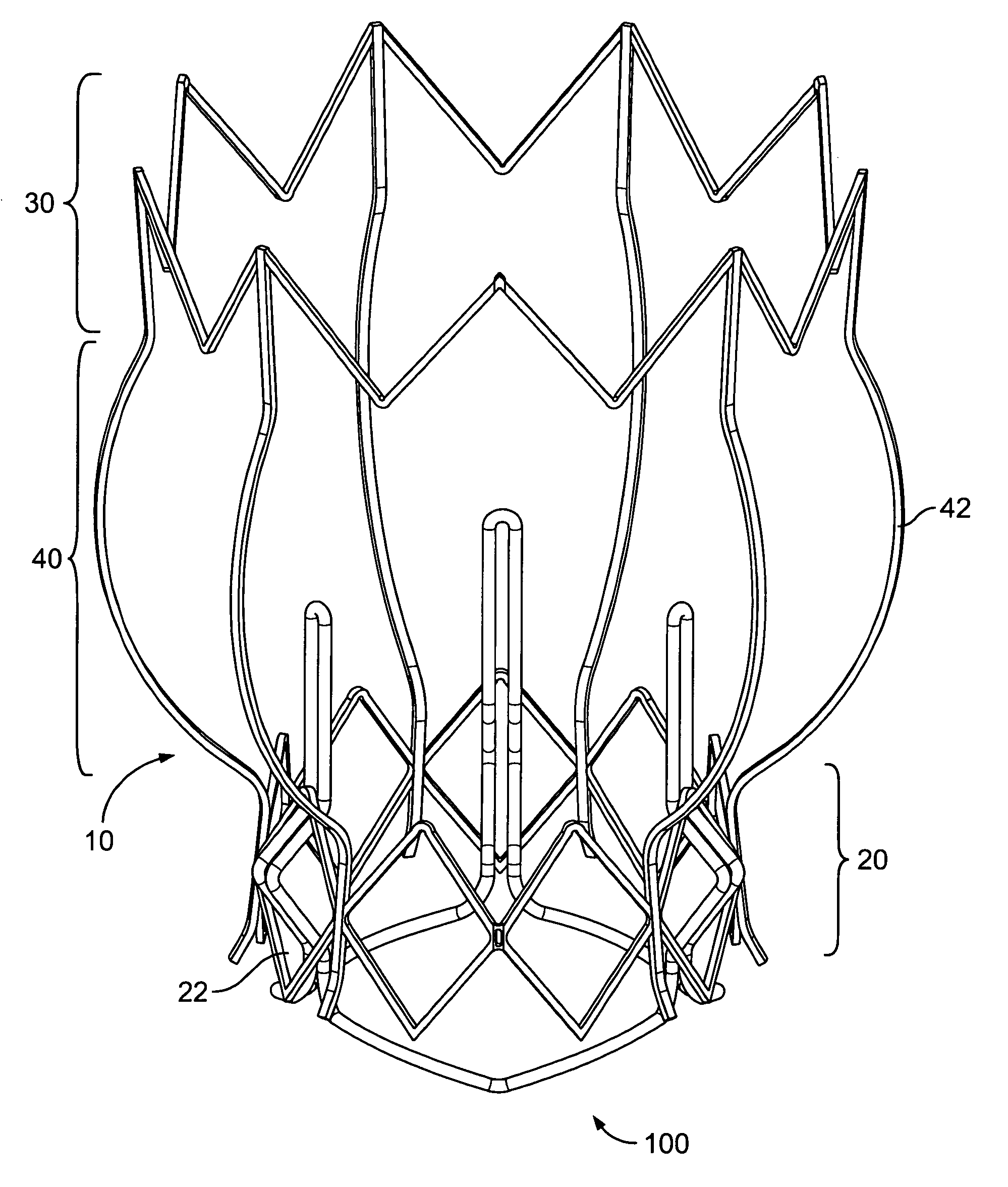
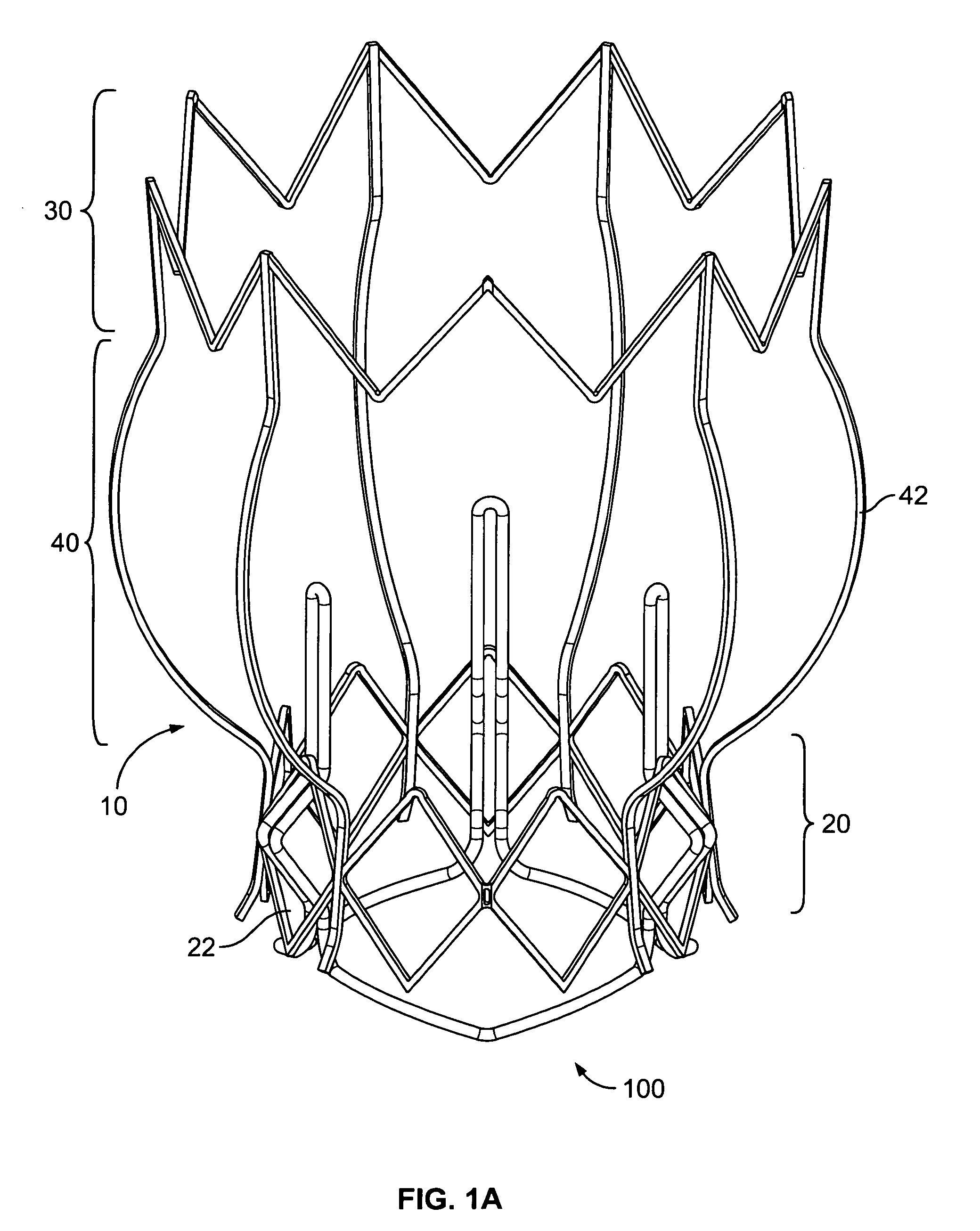
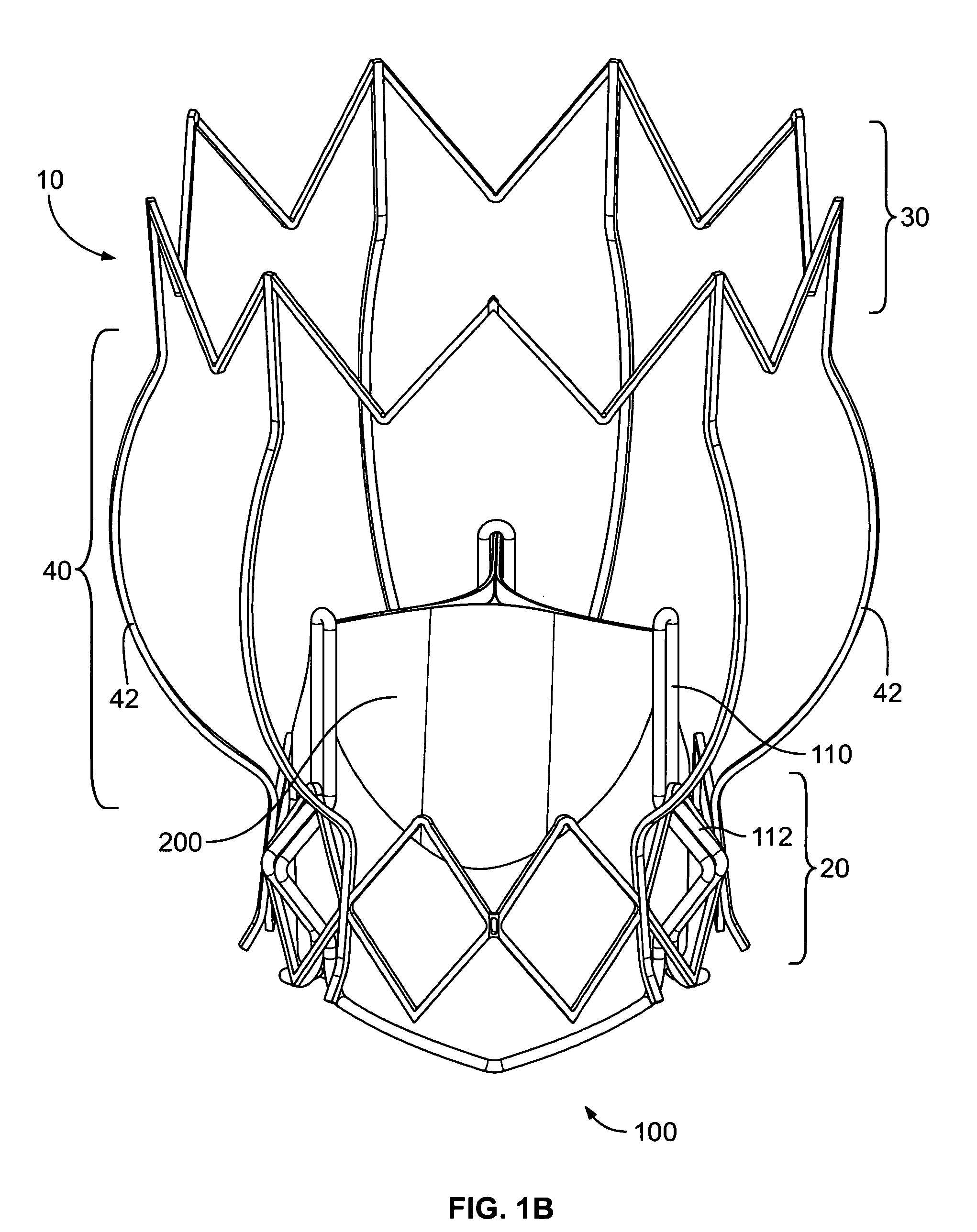
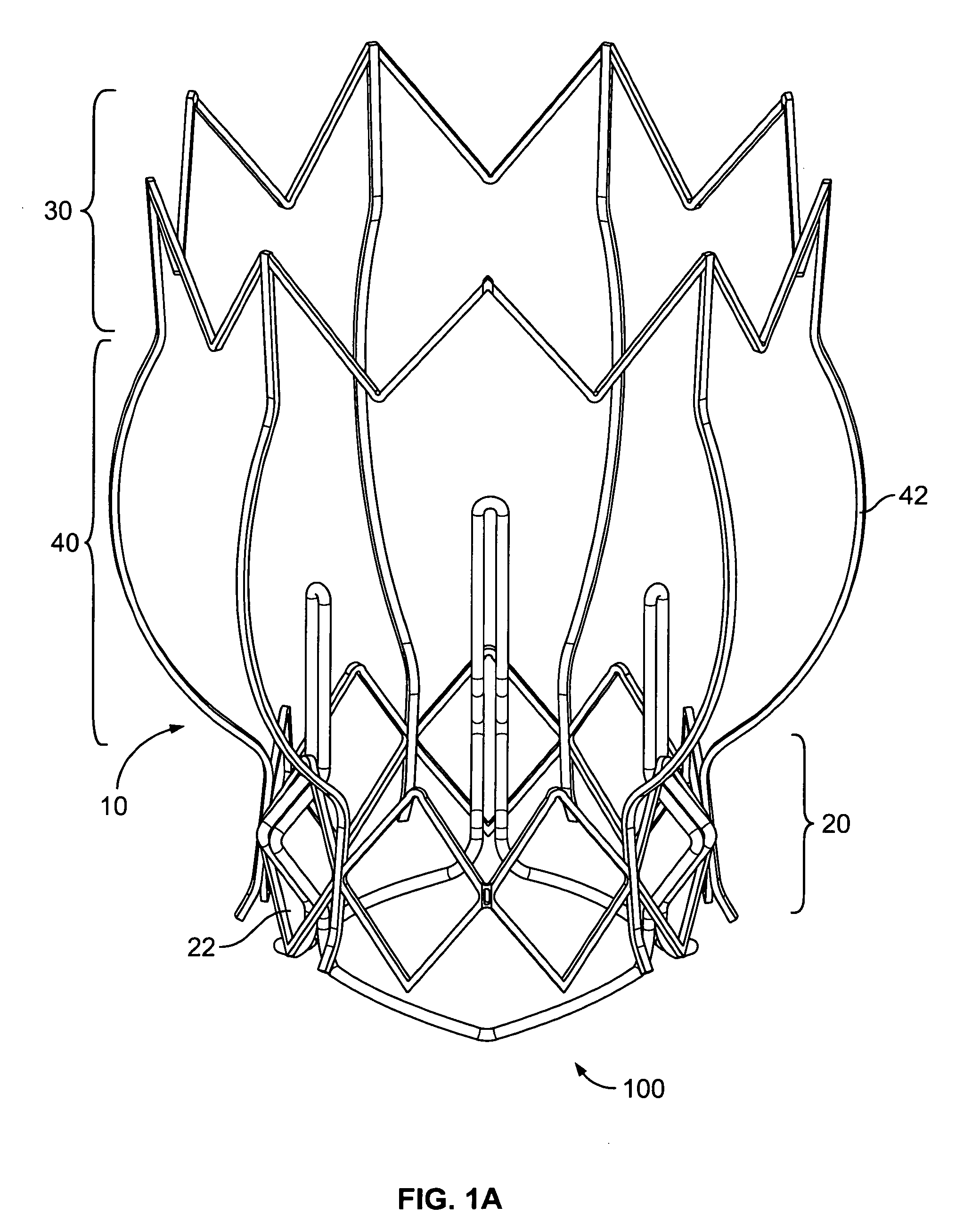
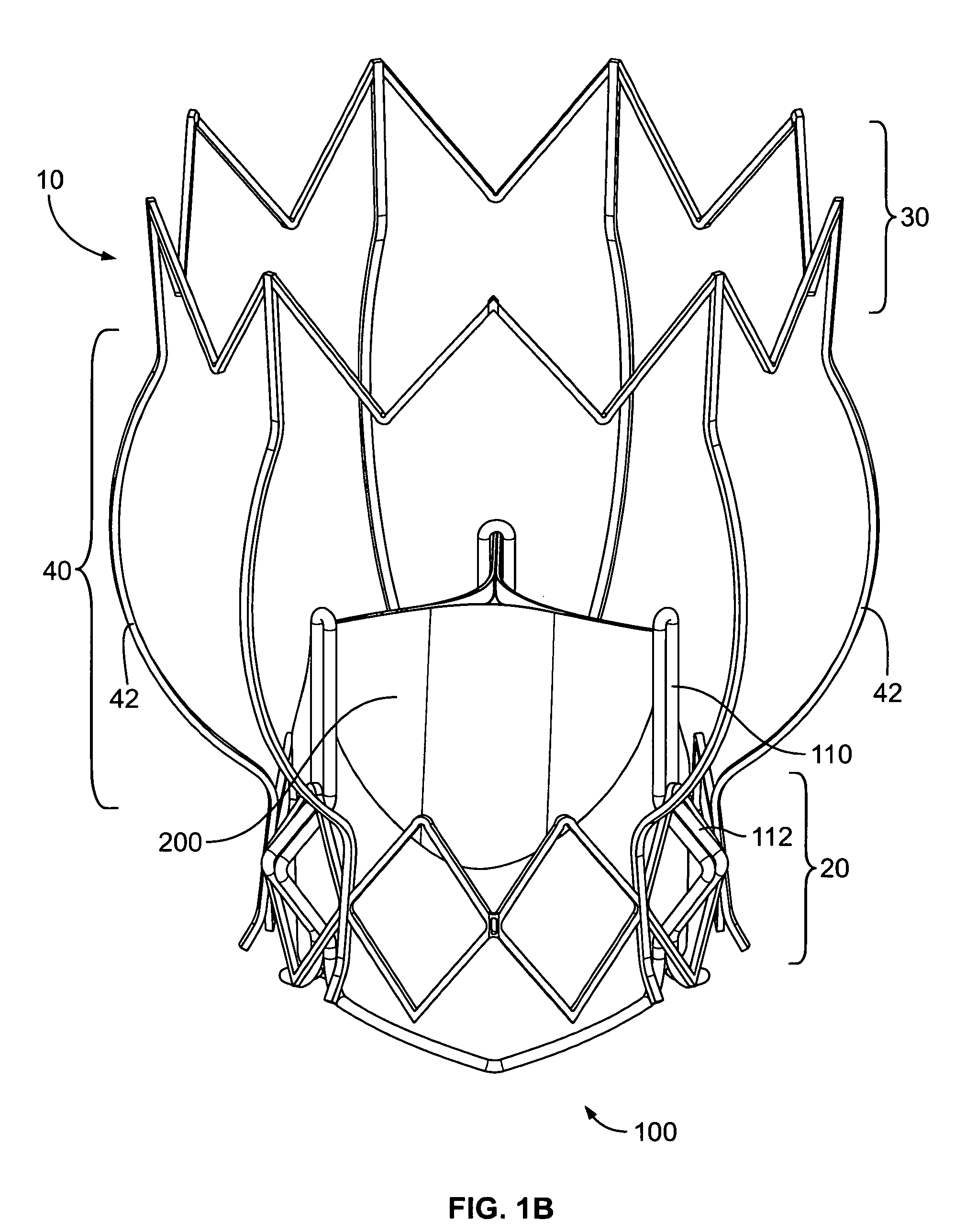
Non-cylindrical prosthetic valve system for transluminal delivery
InactiveUS20070043435A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesCoronary arteriesProsthesis
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable prosthesis frame. If desired, one or more expandable anchors may be used. The prosthesis frame, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the prosthesis frame may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. The prosthesis frame is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. When the outer sheath is retracted, the prosthesis frame expands to an expanded position such that the valve and prosthesis frame expand at the implantation site and the anchor engages the lumen wall. The prosthesis frame has a non-cylindrical configuration with a preset maximum expansion diameter region about the valve opening to maintain the preferred valve geometry. The prosthesis frame may also have other regions having a preset maximum expansion diameter to avoid blockage of adjacent structures such as the coronary ostia.
Owner:MEDTRONIC COREVALVE
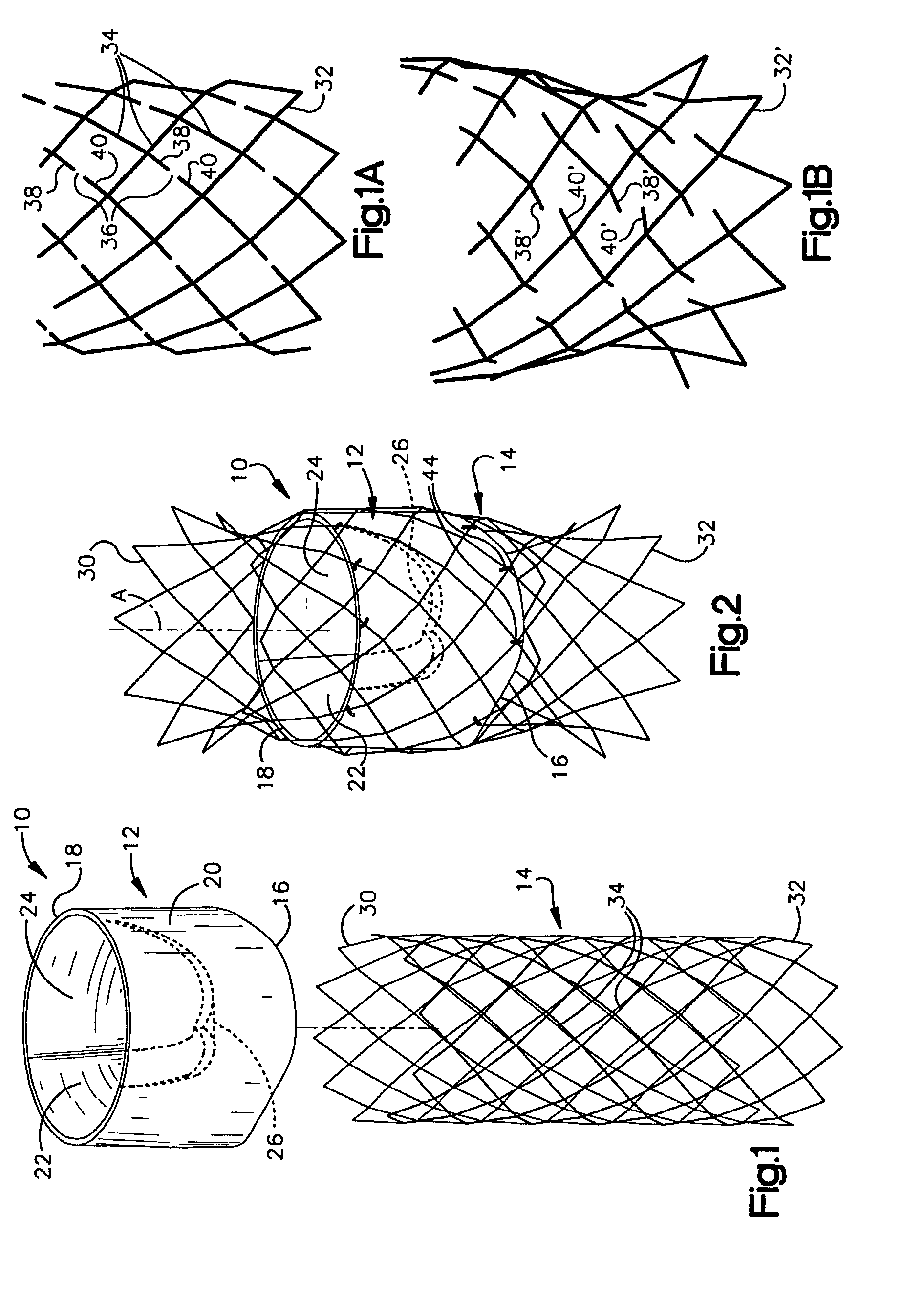
Endovascular aneurysm devices, systems, and methods
Devices, systems, and methods for implanting prostheses in the body lumens rely on tacking or anchoring the prostheses with separately introduced fasteners. After initial placement, a fastener applier system is introduced within the expanded prostheses to deploy a plurality of fasteners to at least one prosthesis end. The fasteners are usually helical fasteners which are releasably restrained on the fastener driver, and are delivered by rotation of the fastener driver. The fasteners may be applied singly, typically in circumferentially spaced-apart patterns about the interior of at least one end of the prosthesis. A lumen extension or lumens may be coupled to the prosthesis to extend the reach of the prosthesis within the implantation site. Fasteners may also be applied to the lumen extensions.
Owner:MEDTRONIC VASCULAR INC
Heart valve prosthesis and sutureless implantation of a heart valve prosthesis
InactiveUS20030040792A1Reduced cross-sectional dimensionReduce exerciseStentsBalloon catheterDirect visionProsthesis
Owner:EDWARDS LIFESCI CARDIAQ
Kit enabling a prosthetic valve to be placed in a body enabling a prosthetic valve to be put into place in a duct in the body
InactiveUS20050043790A1Destruction damageRisk of destructionHeart valvesBlood vesselsProsthetic valveInsertion stent
The present invention is an assembly comprising a prosthetic valve to be implanted; a radially expandable stent comprising at least one zone intended to be expanded to allow the stent, in the expanded state, to bear against the wall of the body duct to be fitted with the valve, this bearing making it possible to immobilize this stent with respect to this wall; and means for mounting the valve with respect to the stent, making it possible to connect the valve to the stent in such a way that the placement of the stent allows the valve to be mounted in the body duct, and expansion means such as a balloon catheter being provided to trigger expansion of the stent at the implantation site. According to the invention, the valve and the stent are designed in such a way that, at the moment when the stent is expanded, the valve is situated outside the zone or zones of the stent that are subjected to said expansion means. The invention thus consists in separating the valve and said zone or zones to be expanded, so that the expansion of the stent can be effected with an expansion force suitable for perfect anchoring of this stent in the wall of the body duct to be fitted with the valve, and without any risk of destruction or damage of the valve.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Rolled minimally-invasive heart valves and methods of manufacture
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. The valves are rolled into a first, contracted configuration for minimally invasive delivery using a catheter, and then unrolled or unfurled at the implantation site. One- and two-piece stents may be used in conjunction with a plurality of flexible leaflet-forming membranes. The stents may include an annulus section, a sinus section with the membranes attached over sinus apertures, and an outflow section. Lockout tabs and making slots secure the stents in their expanded shapes. Alignment structure ensures concentric unfurling of the stent. Anchoring elements at the stent edges or in the stent body secure the valve within the annulus. A method of manufacture includes shape setting the sheet-like stent to ensure an outward bias during deployment. The stent may also include dear tracks for engagement with a gear mechanism for deployment. The stent is desirably made of a superelastic material such as Nitinol and may have areas removed or thinned to reduce the bending stresses when rolled into its small spiral for catheter delivery.
Owner:EDWARDS LIFESCIENCES CORP
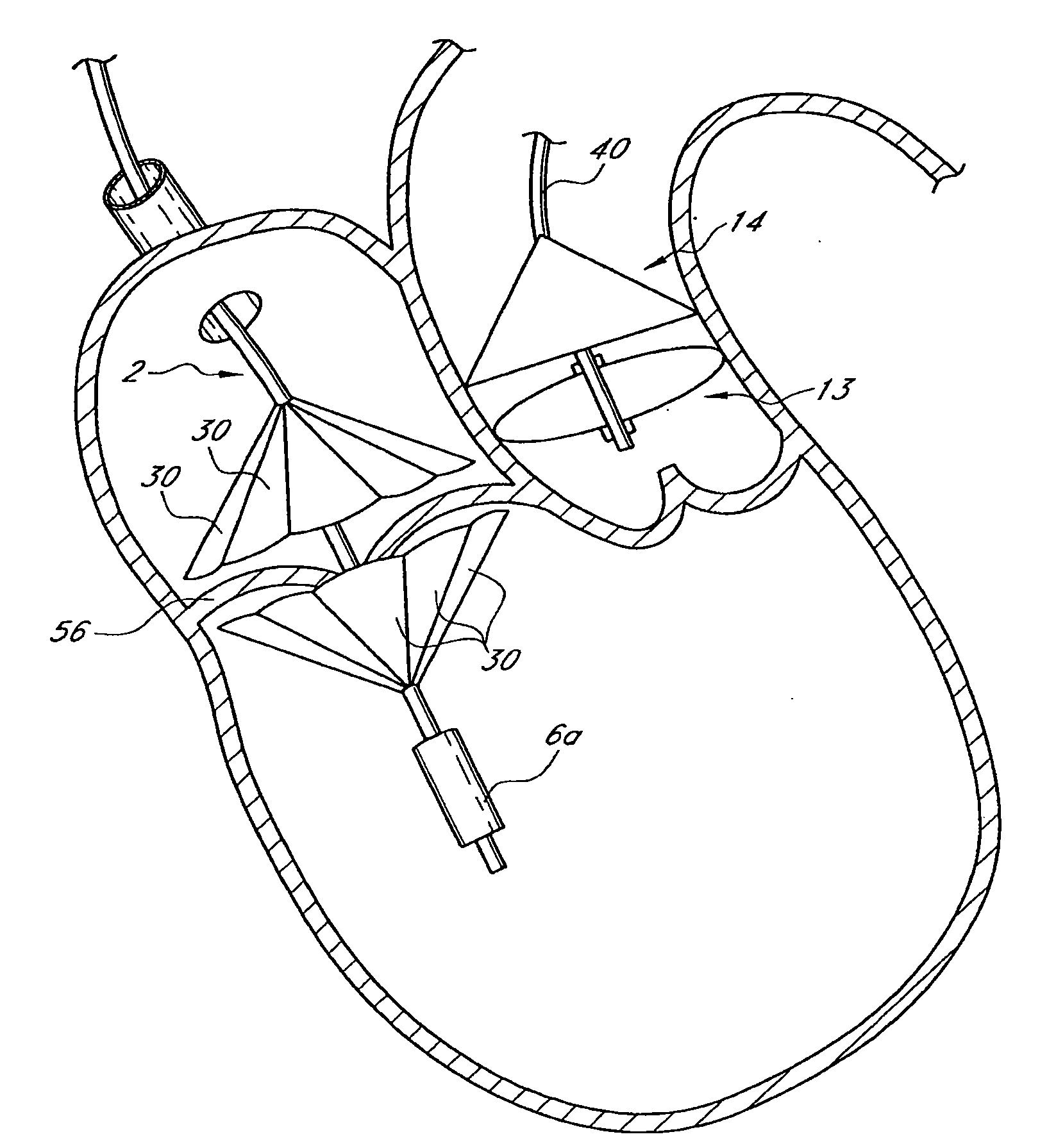
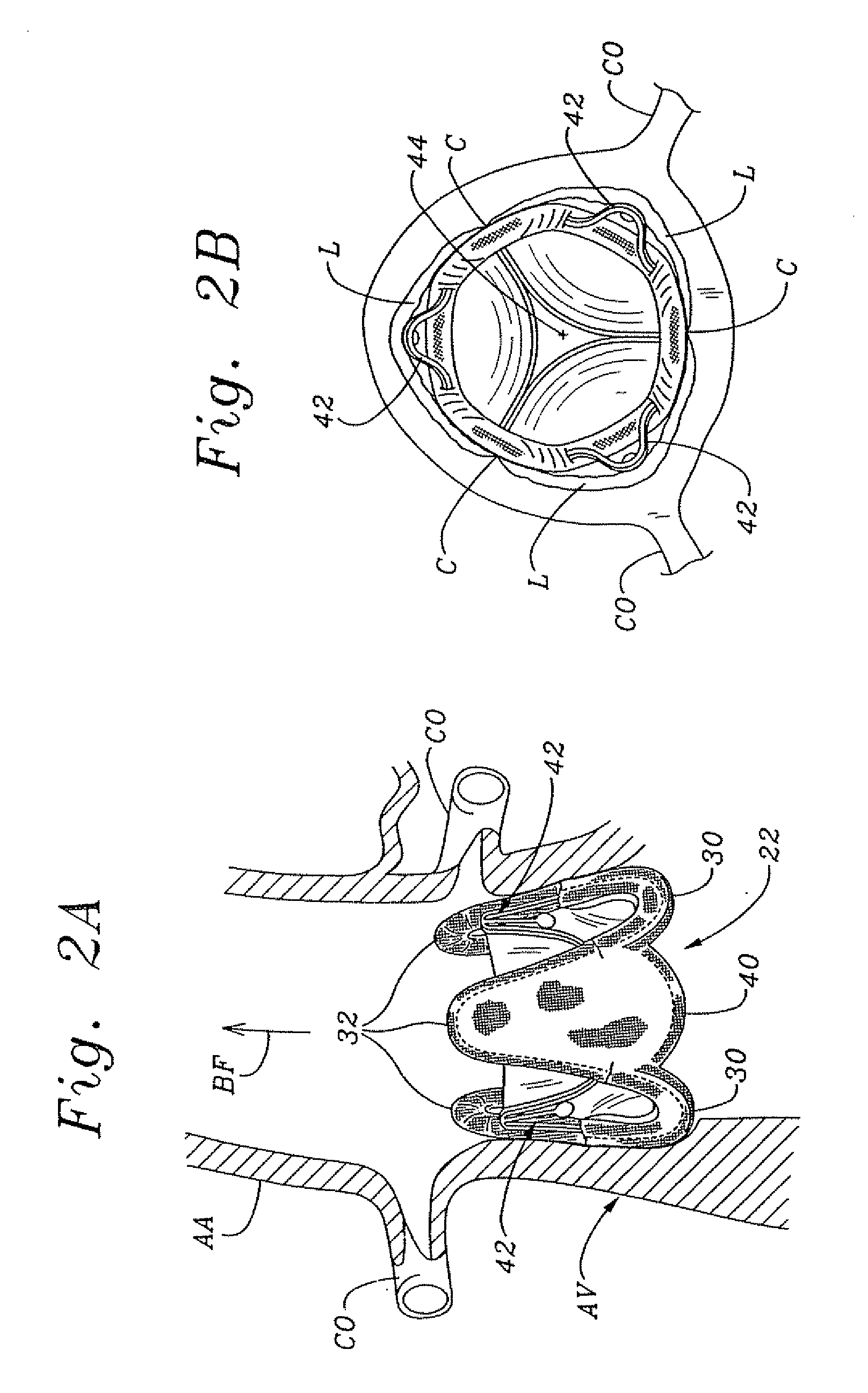
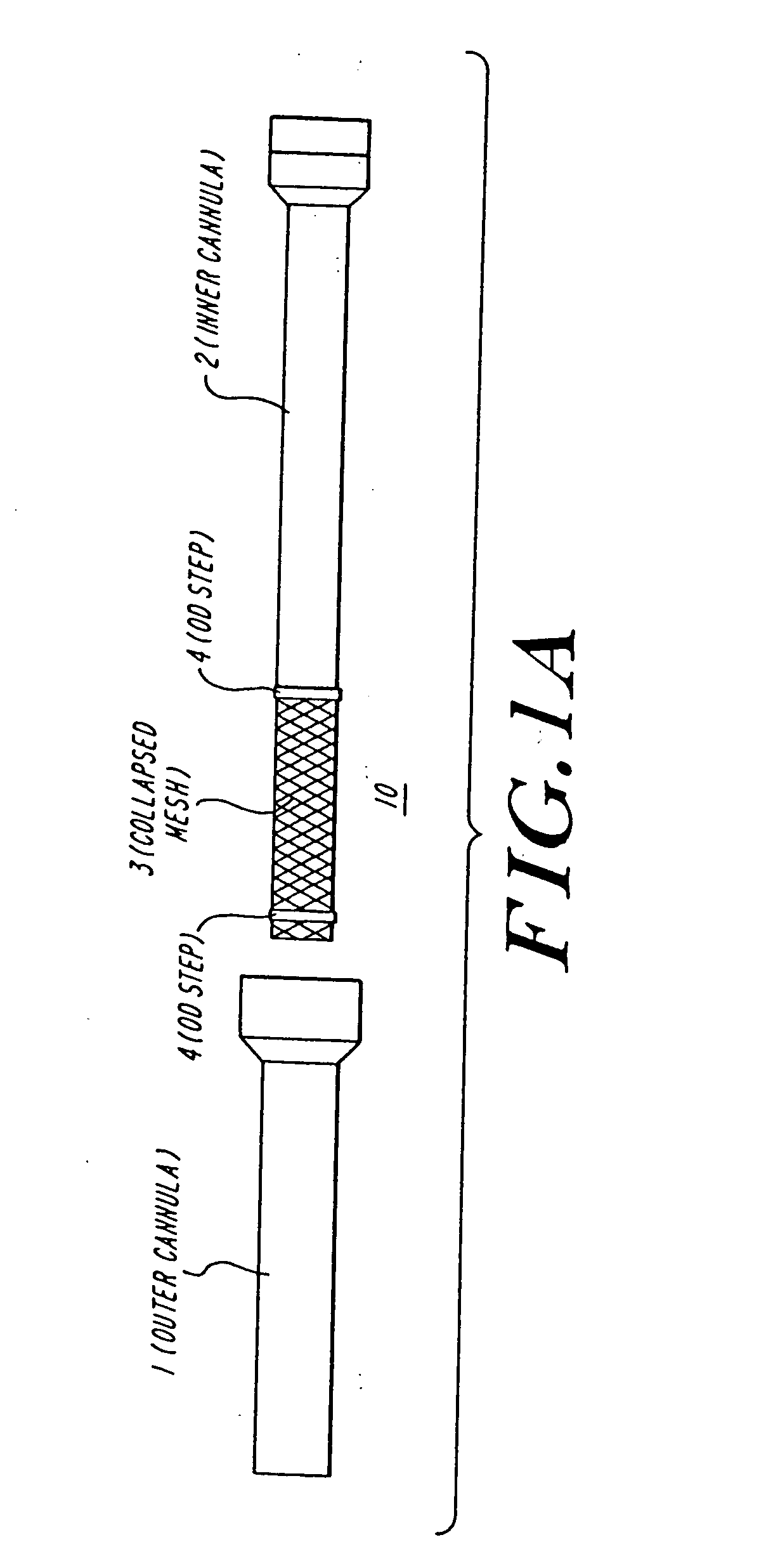
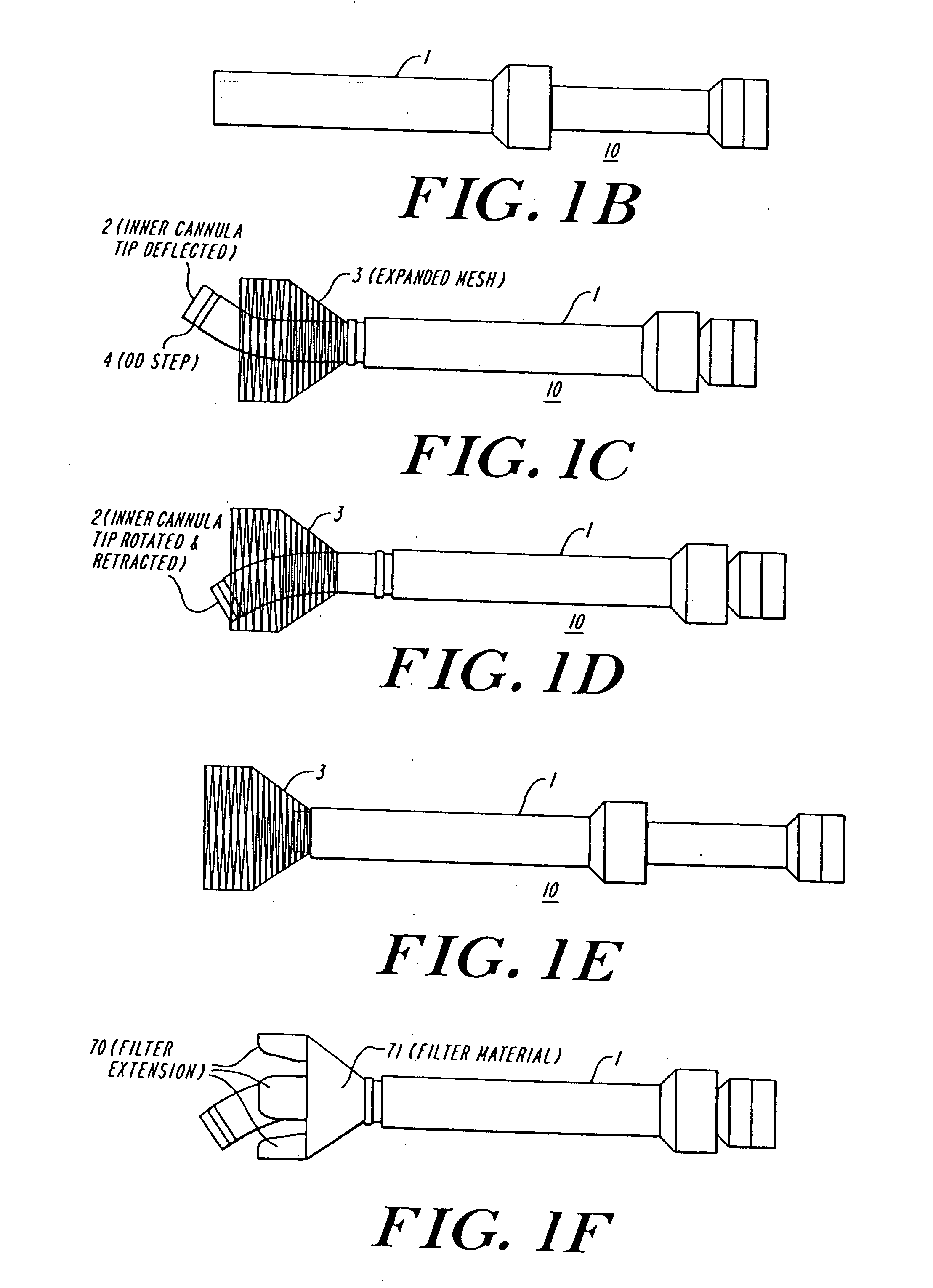
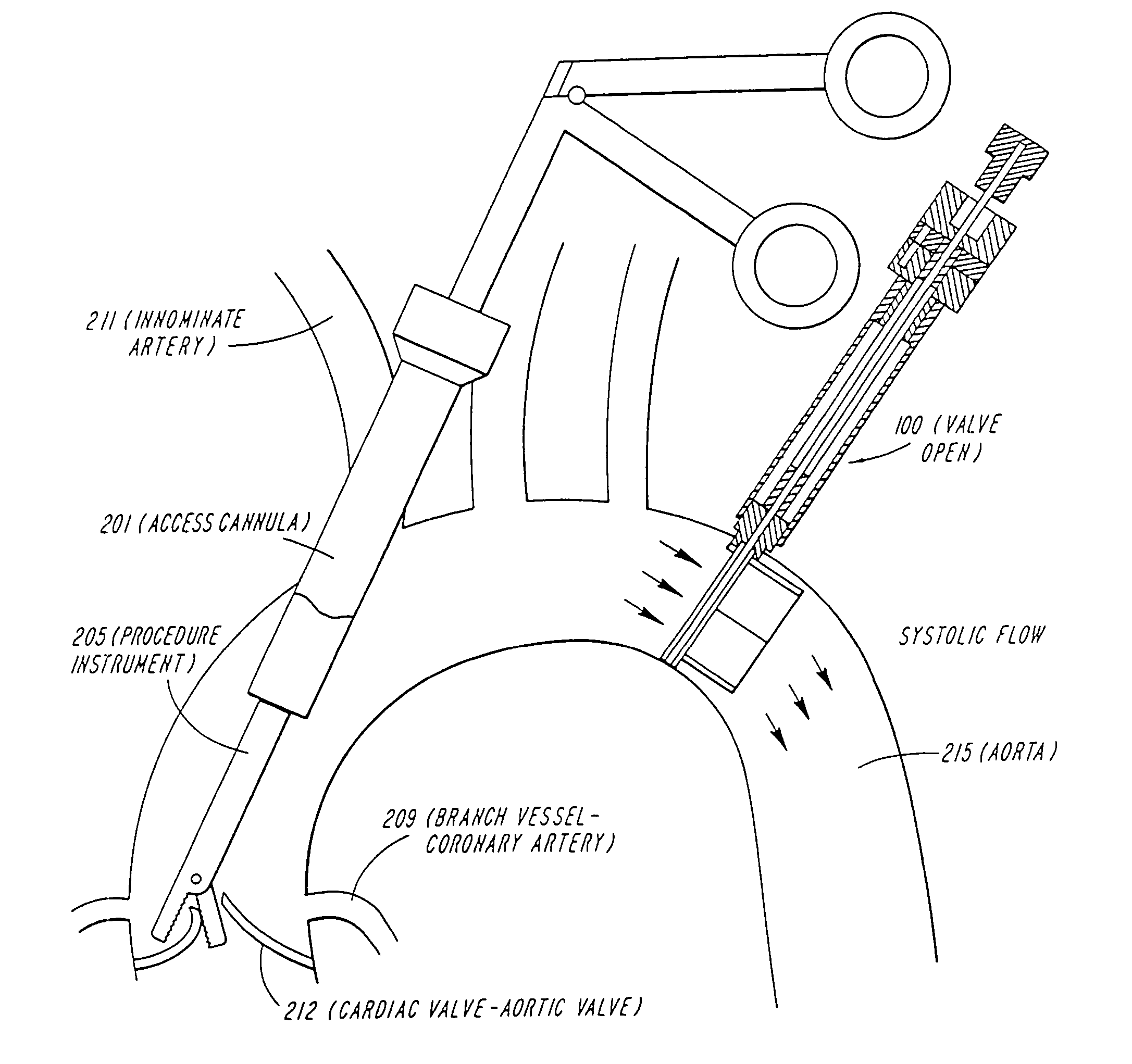
Cardiac valve procedure methods and devices
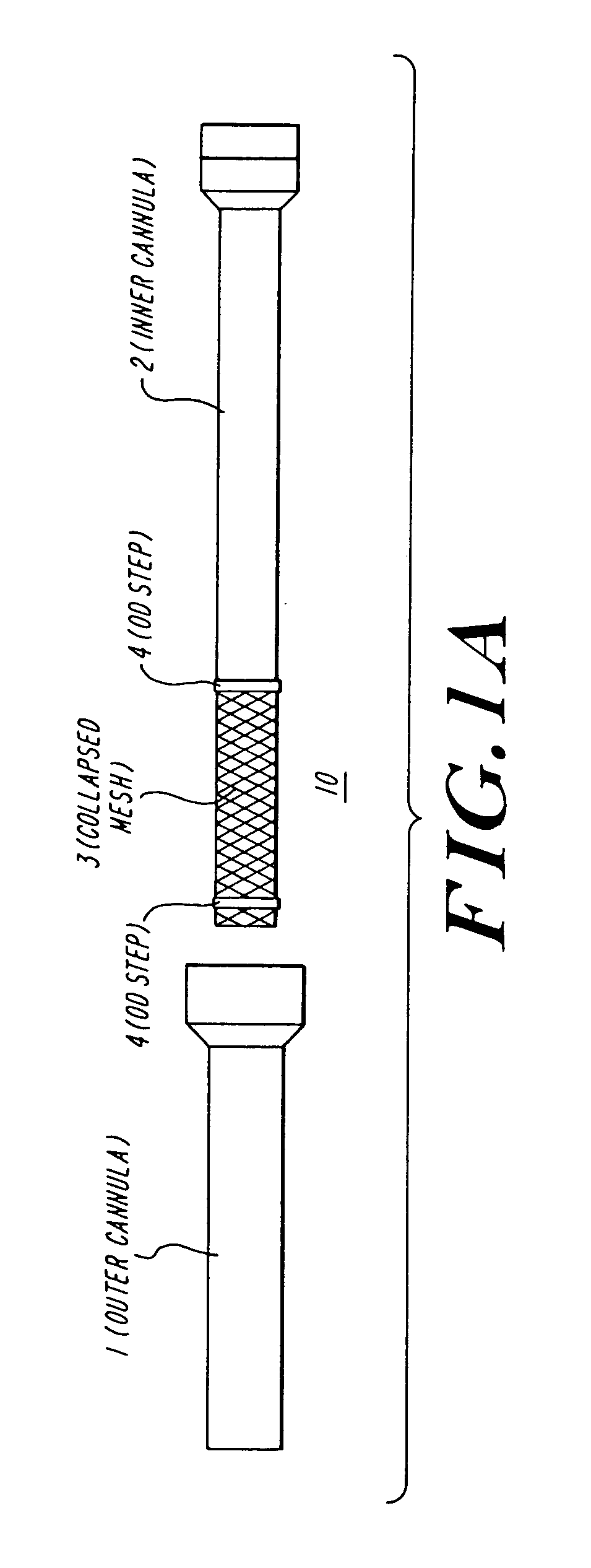
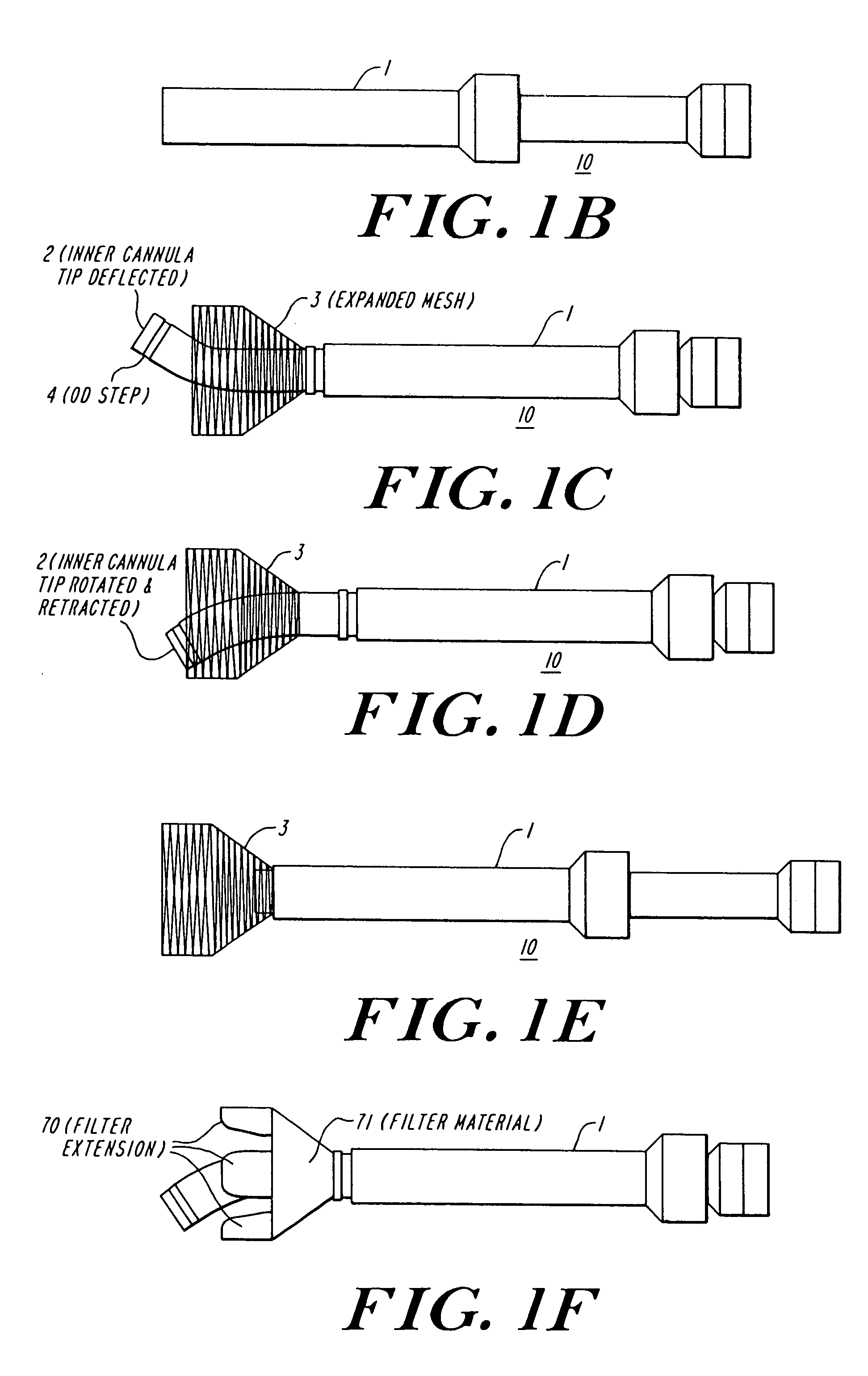
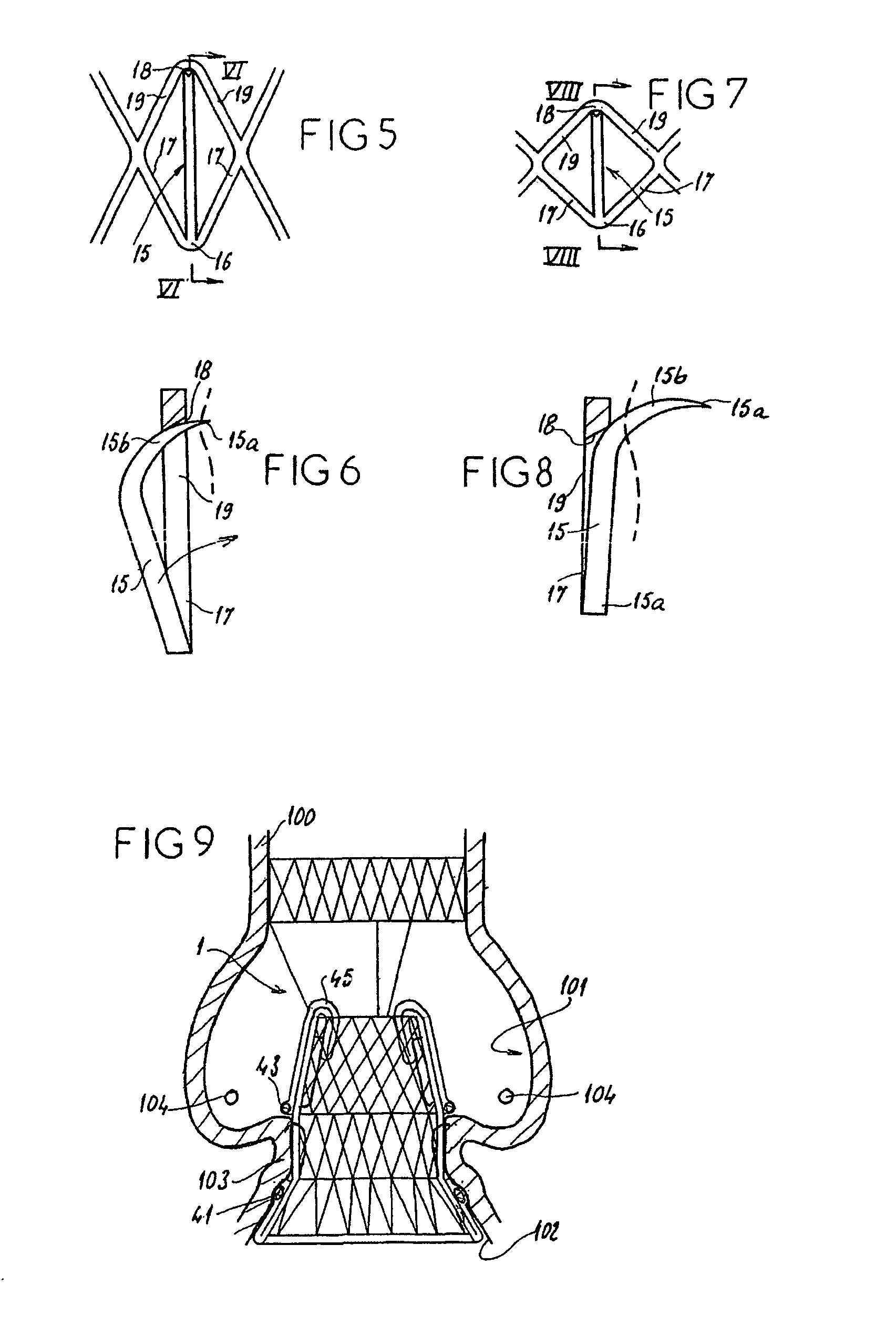
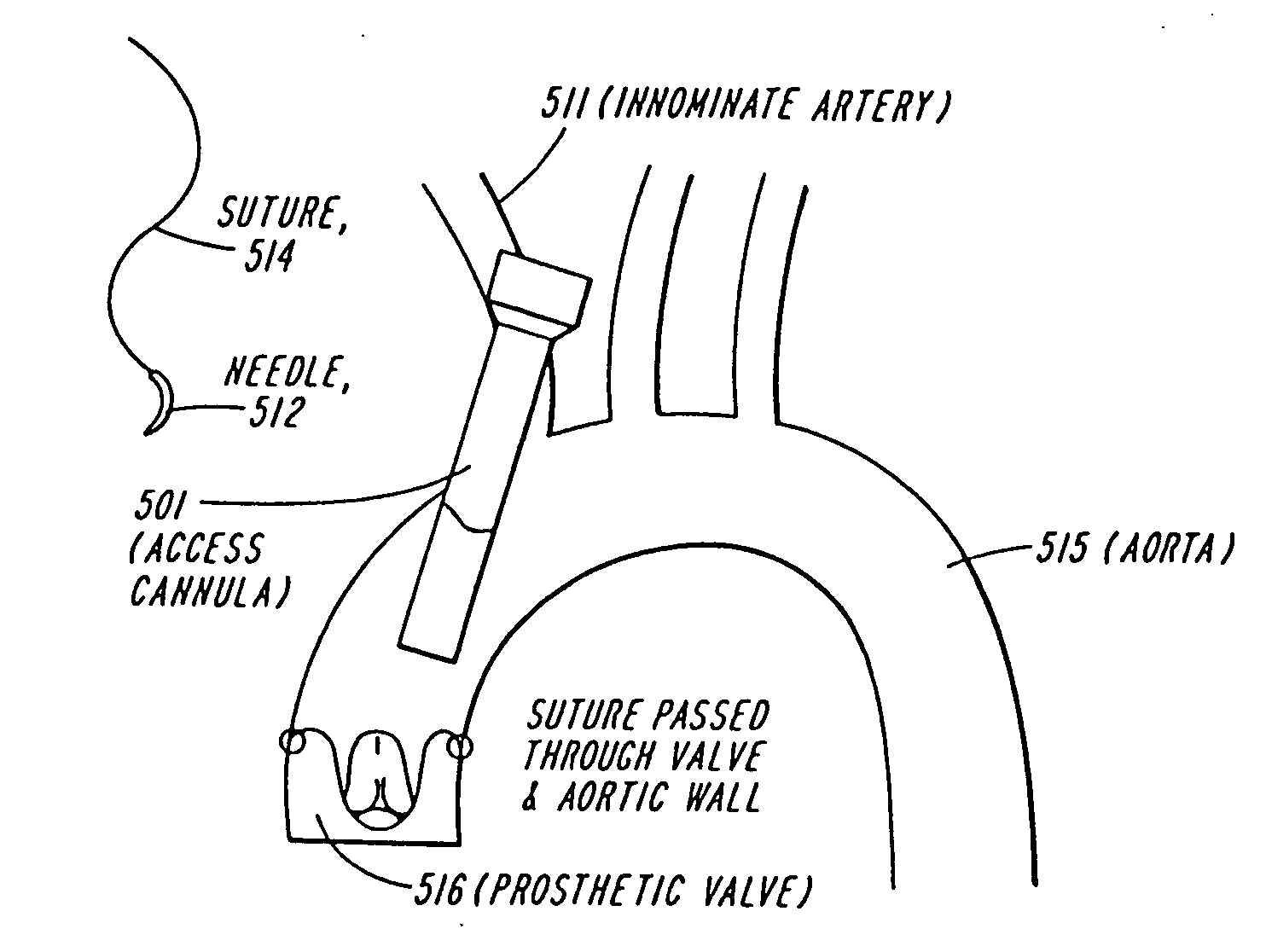
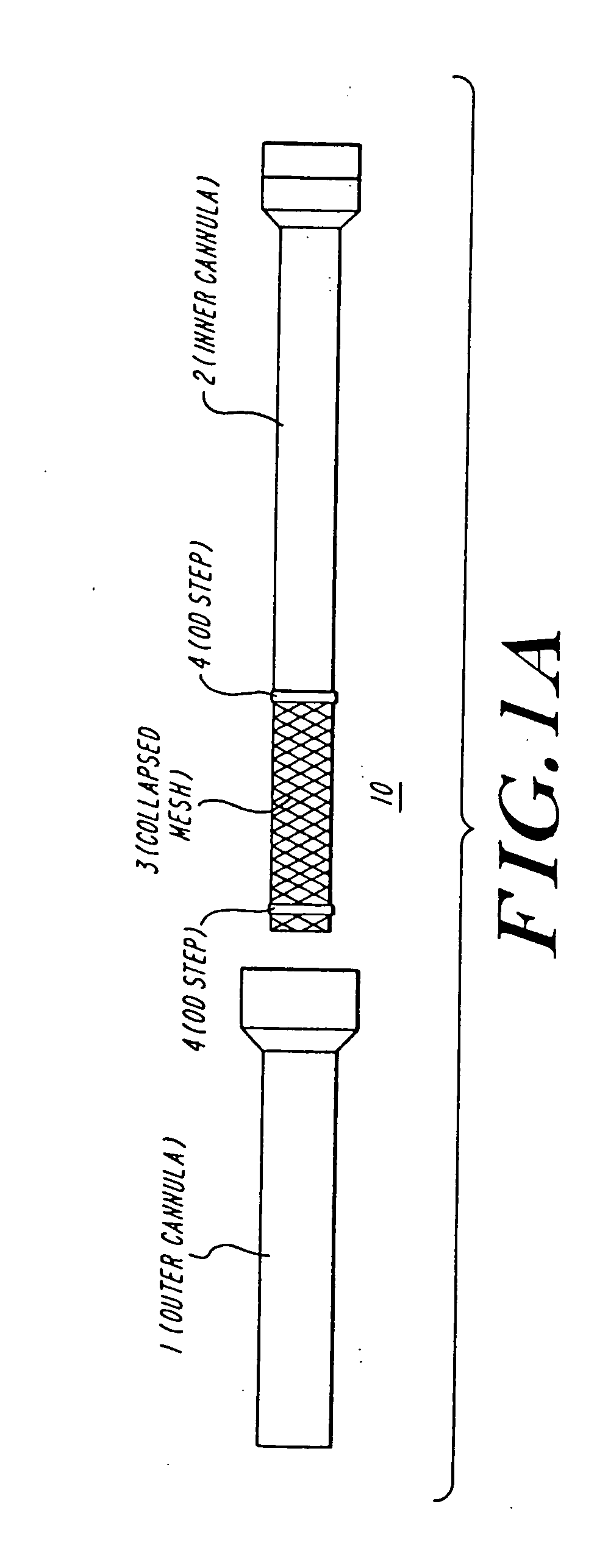
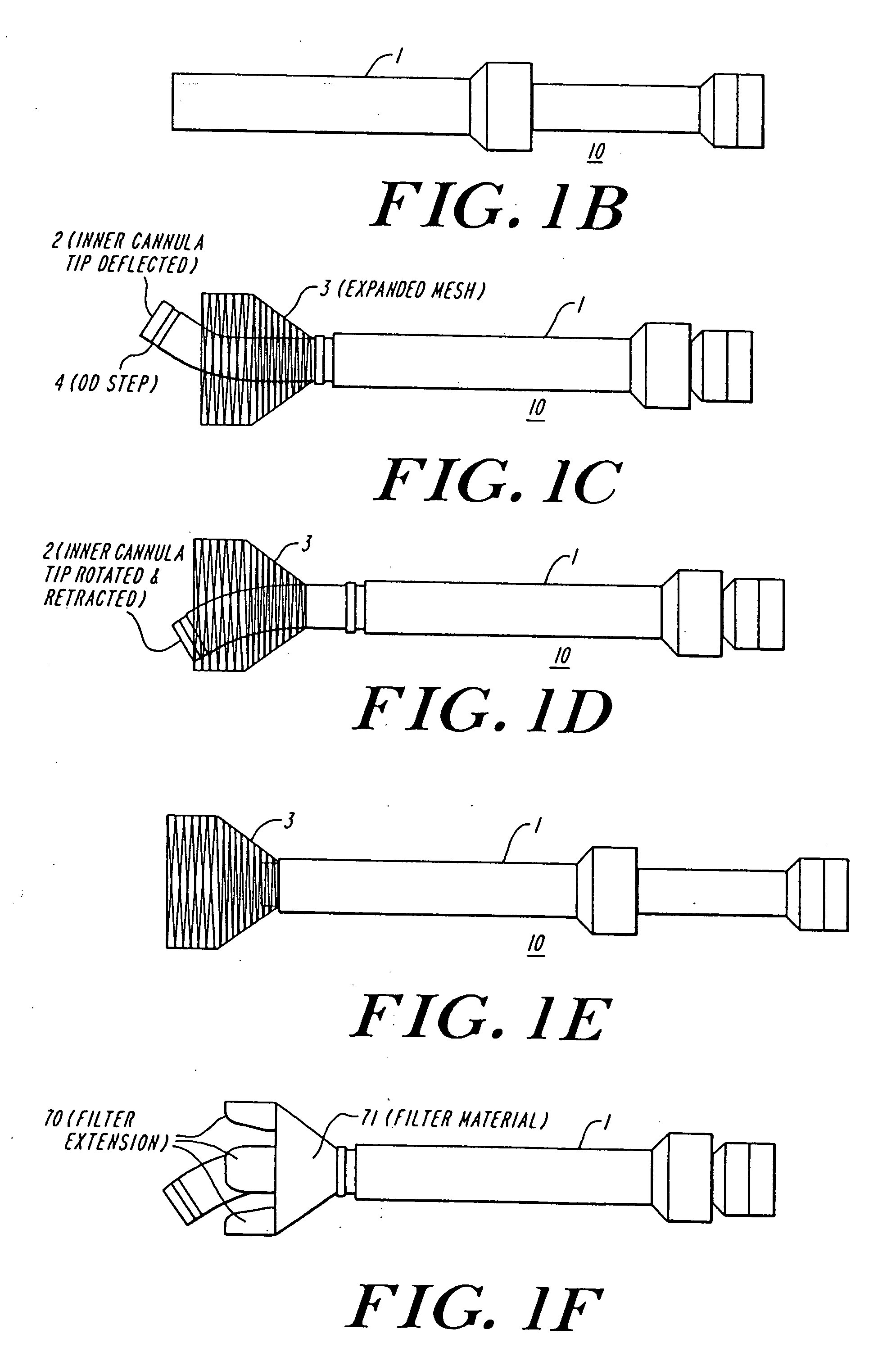
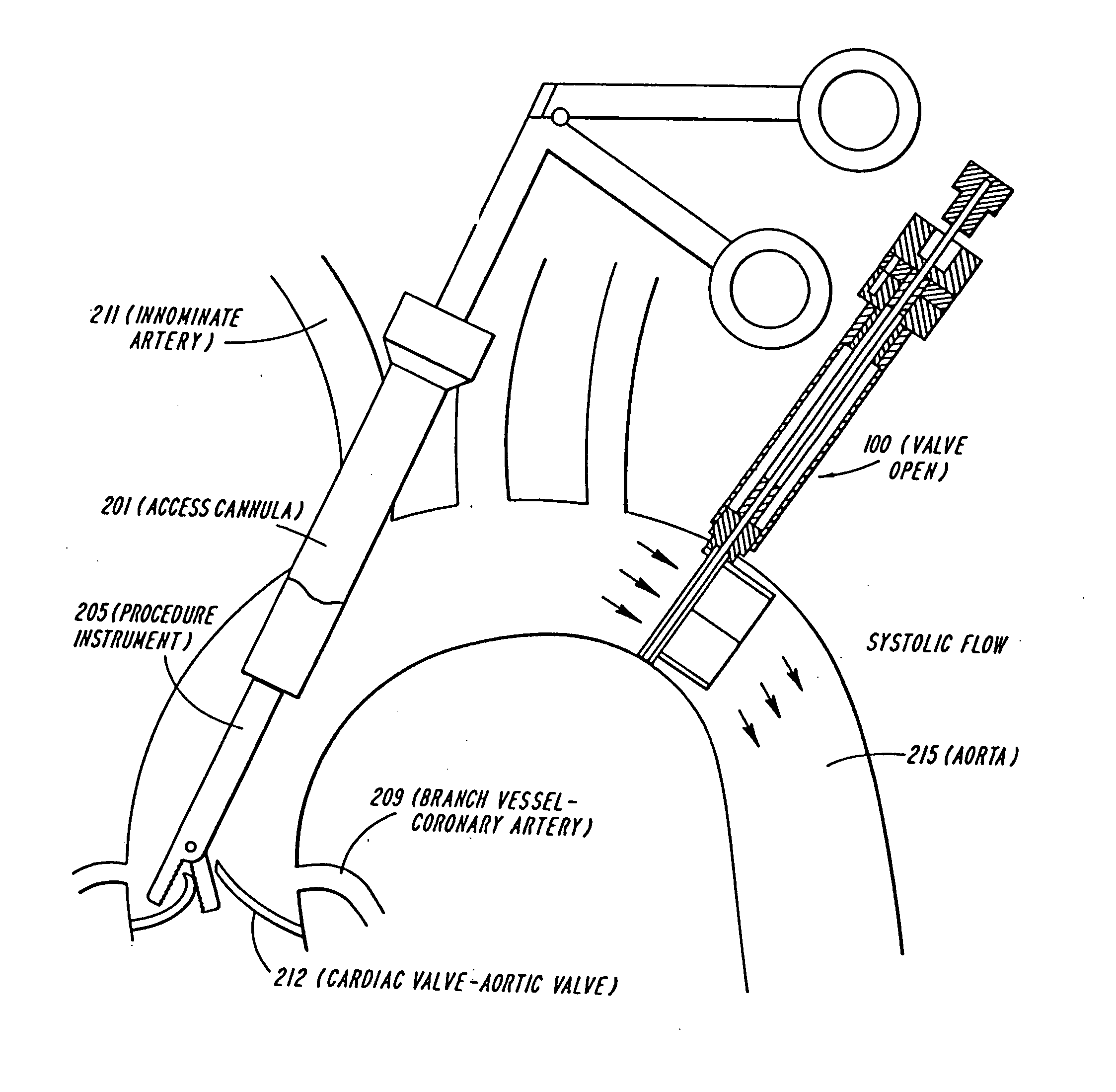
The present invention discloses devices and methods for performing intravascular procedures with out cardiac bypass. The devices include various embodiments of temporary filter devices, temporary valves, and prosthetic valves.The temporary filter devices have one or more cannulae which provide access for surgical tools for effecting repair of the cardiac valves. A cannula may have filters of various configurations encircling the distal region of the cannula, which prevent embolitic material from entering the coronary arteries and aorta.The temporary valve devices may also have one or more cannulae which guide the insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel. In one embodiment, the temporary valve is a disc of flexible, porous, material that acts to filter blood passing therethrough. A set of valve leaflets extend peripherally from the disc. These leaflets can alternately collapse to prevent blood flow through the valve and extend to permit flow.The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. In one embodiment, the prosthetic valves have at least one substantially rigid strut, at least two expandable fixation rings located about the circumference of the base of the apex of the valve, and one or more commissures and leaflets. The prosthetic valves are introduced into the vascular system a compressed state, advanced to the site of implantation, expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
Kit enabling a prosthetic valve to be placed in a body enabling a prosthetic valve to be put into place in a duct in the body
The present invention is an assembly comprising a prosthetic valve to be implanted; a radially expandable stent comprising at least one zone intended to be expanded to allow the stent, in the expanded state, to bear against the wall of the body duct to be fitted with the valve, this bearing making it possible to immobilize this stent with respect to this wall; and means for mounting the valve with respect to the stent, making it possible to connect the valve to the stent in such a way that the placement of the stent allows the valve to be mounted in the body duct, and expansion means such as a balloon catheter being provided to trigger expansion of the stent at the implantation site. According to the invention, the valve and the stent are designed in such a way that, at the moment when the stent is expanded, the valve is situated outside the zone or zones of the stent that are subjected to said expansion means. The invention thus consists in separating the valve and said zone or zones to be expanded, so that the expansion of the stent can be effected with an expansion force suitable for perfect anchoring of this stent in the wall of the body duct to be fitted with the valve, and without any risk of destruction or damage of the valve.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Cardiac valve procedure methods and devices
The present invention discloses devices and methods for performing intravascular procedures with out: cardiac bypass. The devices include various embodiments of temporary filter devices, temporary valves, and prosthetic valves. The temporary filter devices have one or more cannulae which provide access for surgical tools for effecting repair of the cardiac valves. A cannula may have filters of various configurations encircling the distal region of the cannula, which prevent embolitic material from entering the coronary arteries and aorta. The temporary valve devices may also have one or more cannulae which guide the insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel. In one embodiment, the temporary valve is a disc of flexible, porous, material that acts to filter blood passing therethrough. A set of valve leaflets extend peripherally from the disc. These leaflets can alternately collapse to prevent blood flow through the valve and extend to permit flow. The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. In one embodiment, the prosthetic valves have at least one substantially rigid strut, at least two expandable fixation rings located about the circumference of the base of the apex of the valve, and one or more commissures and leaflets. The prosthetic valves are introduced into the vascular system a compressed state, advanced to the site of implantation, expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
Apparatus and method for implanting collapsible/expandable prosthetic heart valves
Apparatus for delivering a prosthetic heart valve into a patient by means that are less invasive than conventional open-chest, open-heart surgery. The prosthetic valve may be collapsed while in a delivery device. When the valve reaches the desired implant site in the patient, the valve can be released from the delivery device, which allows the valve to re-expand to the configuration in which it can function as a heart valve. For example, the delivery device may be constructed to facilitate delivery of the prosthetic valve into the patient via the apex of the patient's heart.
Owner:ST JUDE MEDICAL
Two-piece percutaneous prosthetic heart valves and methods for making and using them
A percutaneous heart valve assembly includes a gasket member or other first prosthesis and a valve member or other second prosthesis. The first and second prostheses are contractible from an enlarged or relaxed condition into a contracted or delivery condition. The prostheses may be loaded into the same or separate catheters or other delivery devices for delivery through a patient's vasculature to an implantation site, e.g., a biological annulus within a heart. The first prosthesis may be deployed adjacent or within the biological annulus and secured at least partially into the biological annulus. The second prosthesis may then be deployed adjacent the biological annulus, expanded, and docked to the first prosthesis. In one embodiment, the first and / or second prostheses may be advanced over one or more sutures or other filaments secured to tissue surrounding or adjacent the biological annulus.
Owner:MEDTRONIC INC
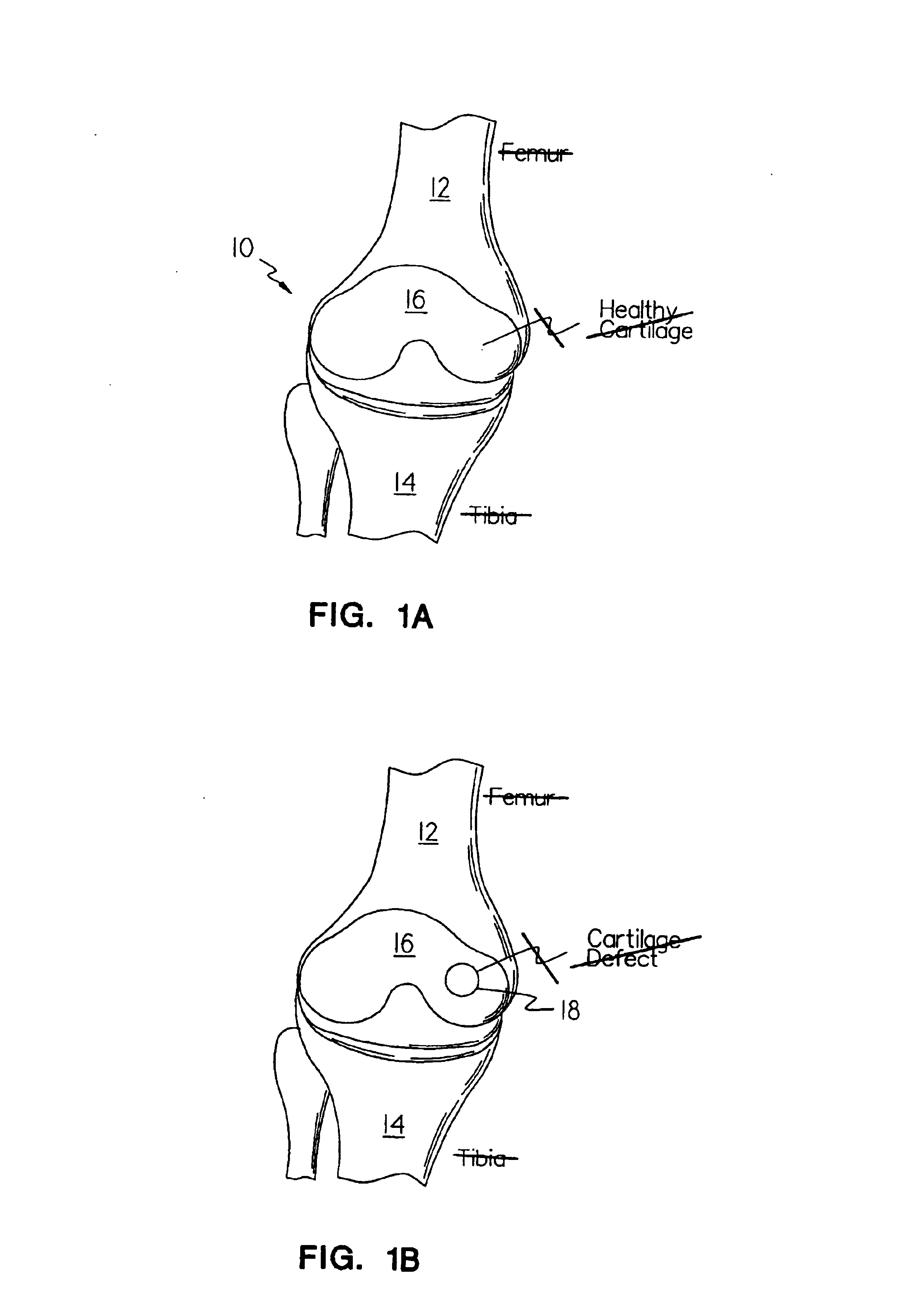
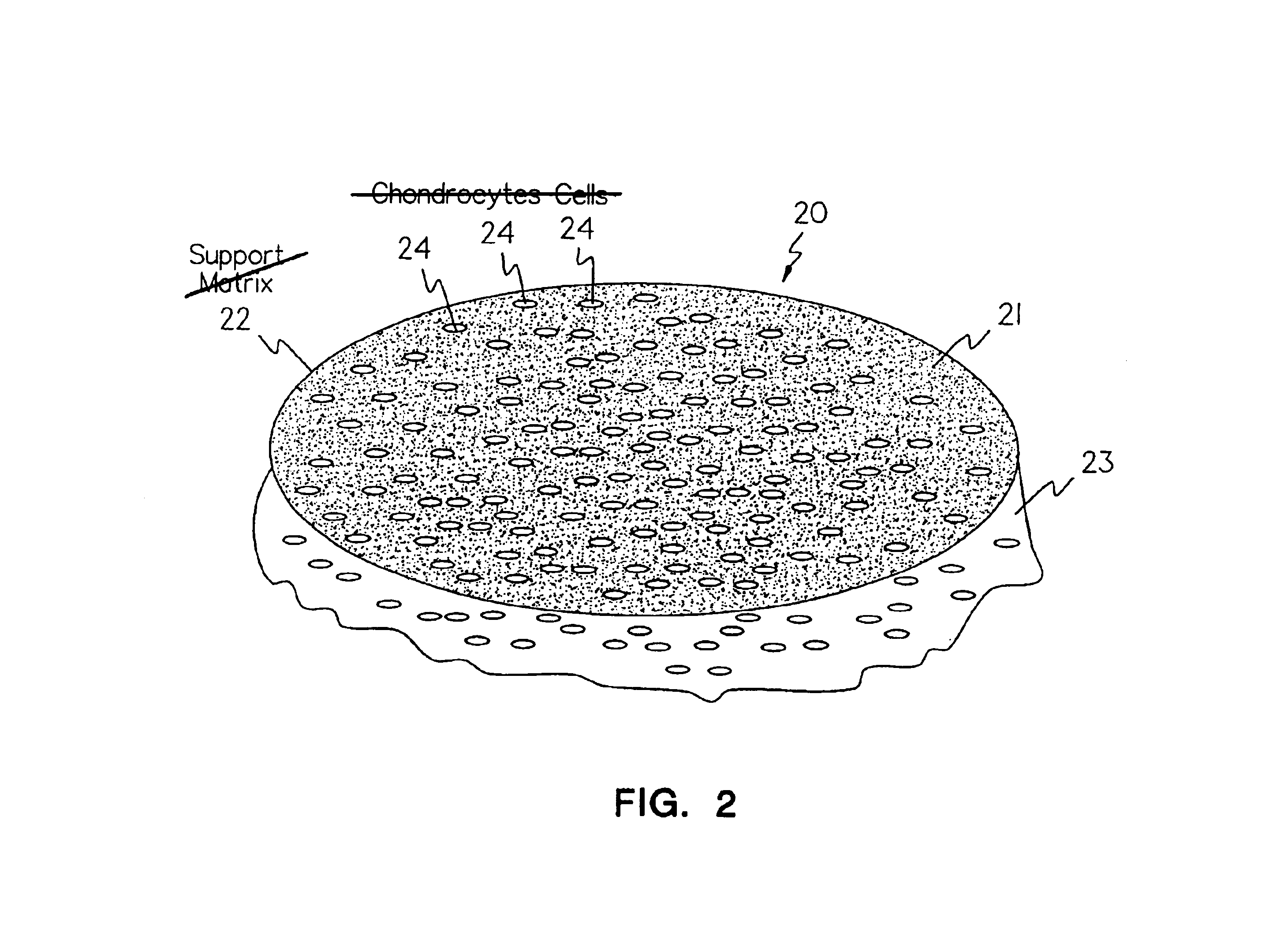
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
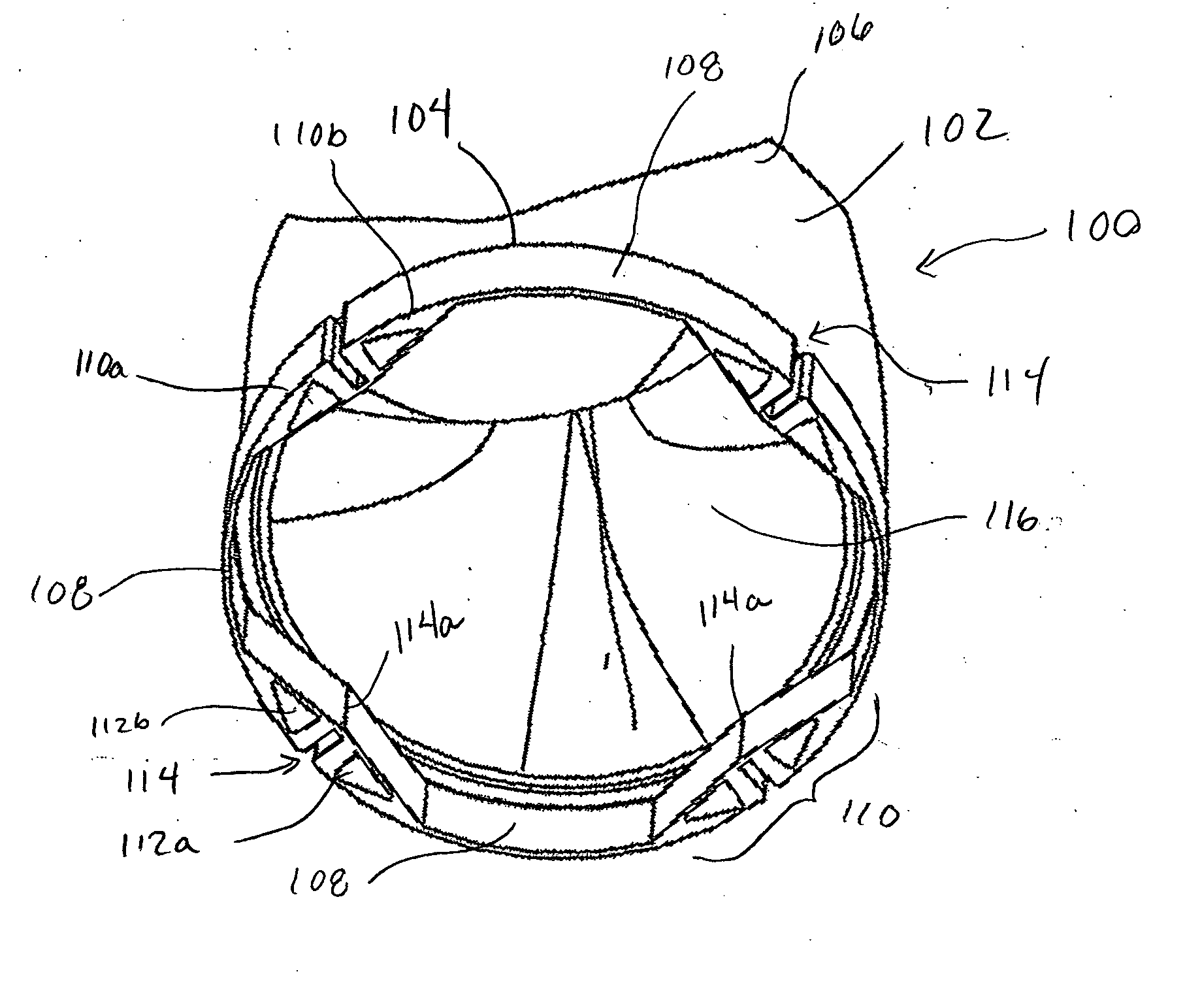
Prosthetic heart valves
A prosthetic heart valve (10) (e.g., a prosthetic aortic valve) is designed to be somewhat circumferentially collapsible and then re-expandable. The collapsed condition may be used for less invasive delivery of the valve into a patient. When the valve reaches the implant site in the patient, it re-expands to normal operating size, and also to engage surrounding tissue of the patient. The valve includes a stent portion (200) and a ring portion (100) that is substantially concentric with the stent portion but downstream from the stent portion in the direction of blood flow through the implanted valve. When the valve is implanted, the stent portion engages the patient's tissue at or near the native valve annulus, while the ring portion engages tissue downstream from the native valve site (e.g., the aorta).
Owner:ST JUDE MEDICAL
Rolled minimally invasive heart valves
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. The valves are rolled into a first, contracted configuration for minimally invasive delivery and then unrolled or unfurled at the implantation site. One- and two-piece stents may be used in conjunction with a plurality of flexible leaflet-forming membranes. The one-piece stents may include an annulus anchoring section, a sinus section with the membranes attached over sinus apertures, and a rigid outflow section. The two-piece stent may include a primary stent to provide a tubular base at the annulus, and a secondary stent having the membranes that couples within the primary stent. Lockout tabs to secure the stents in their expanded shapes are provided. Also, alignment structure may be provided to ensure concentric unfurling. Anchoring barbs at the stent edges or in the stent body secure the valve within the annulus. Methods of implantation are also provided.
Owner:EDWARDS LIFESCIENCES CORP
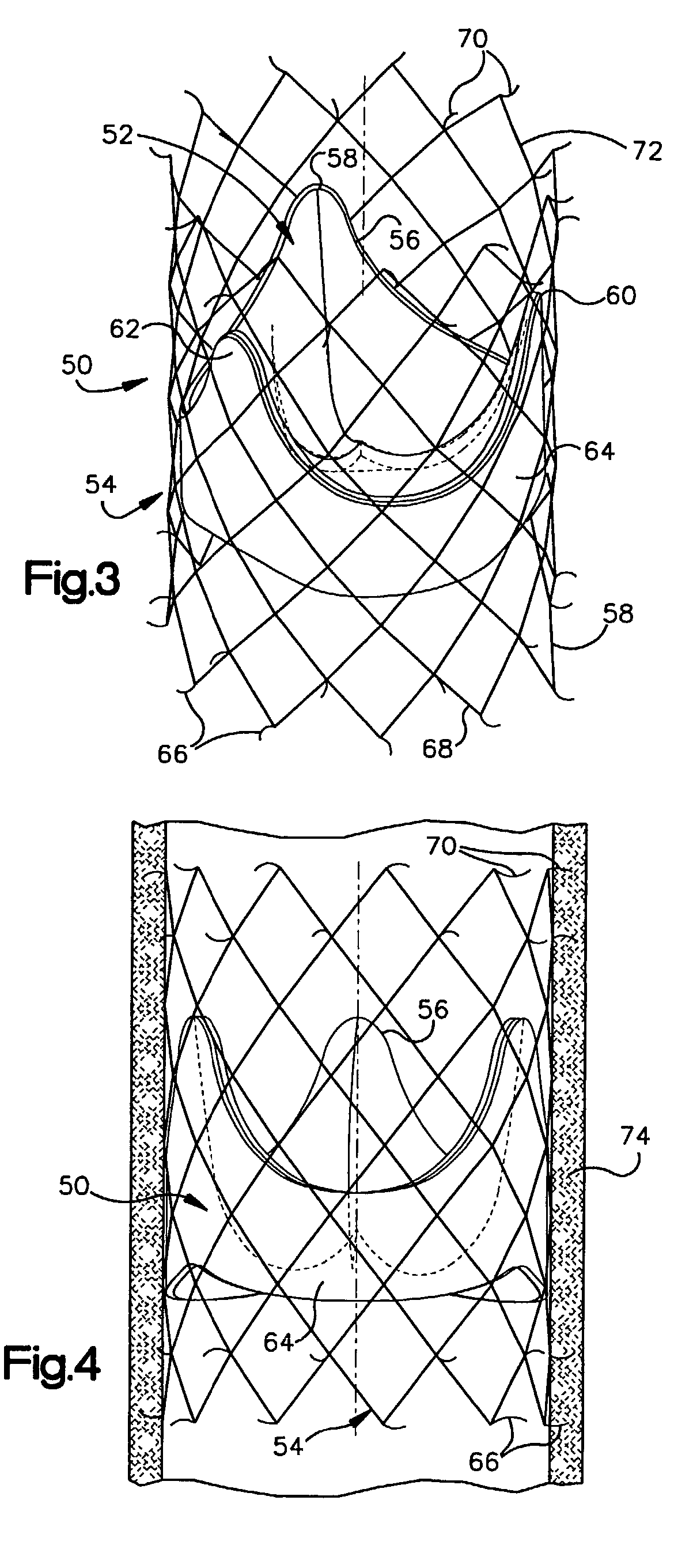
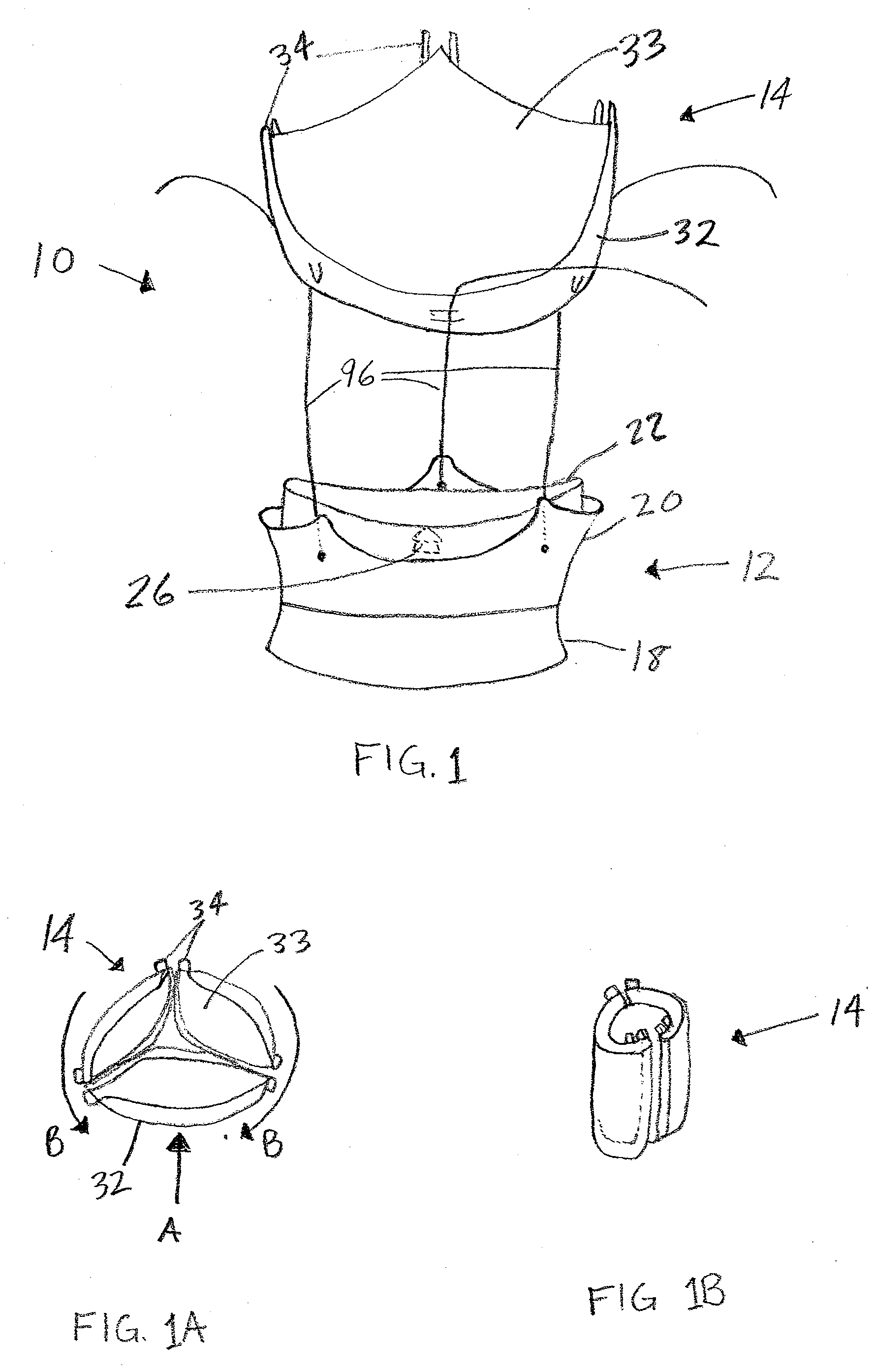
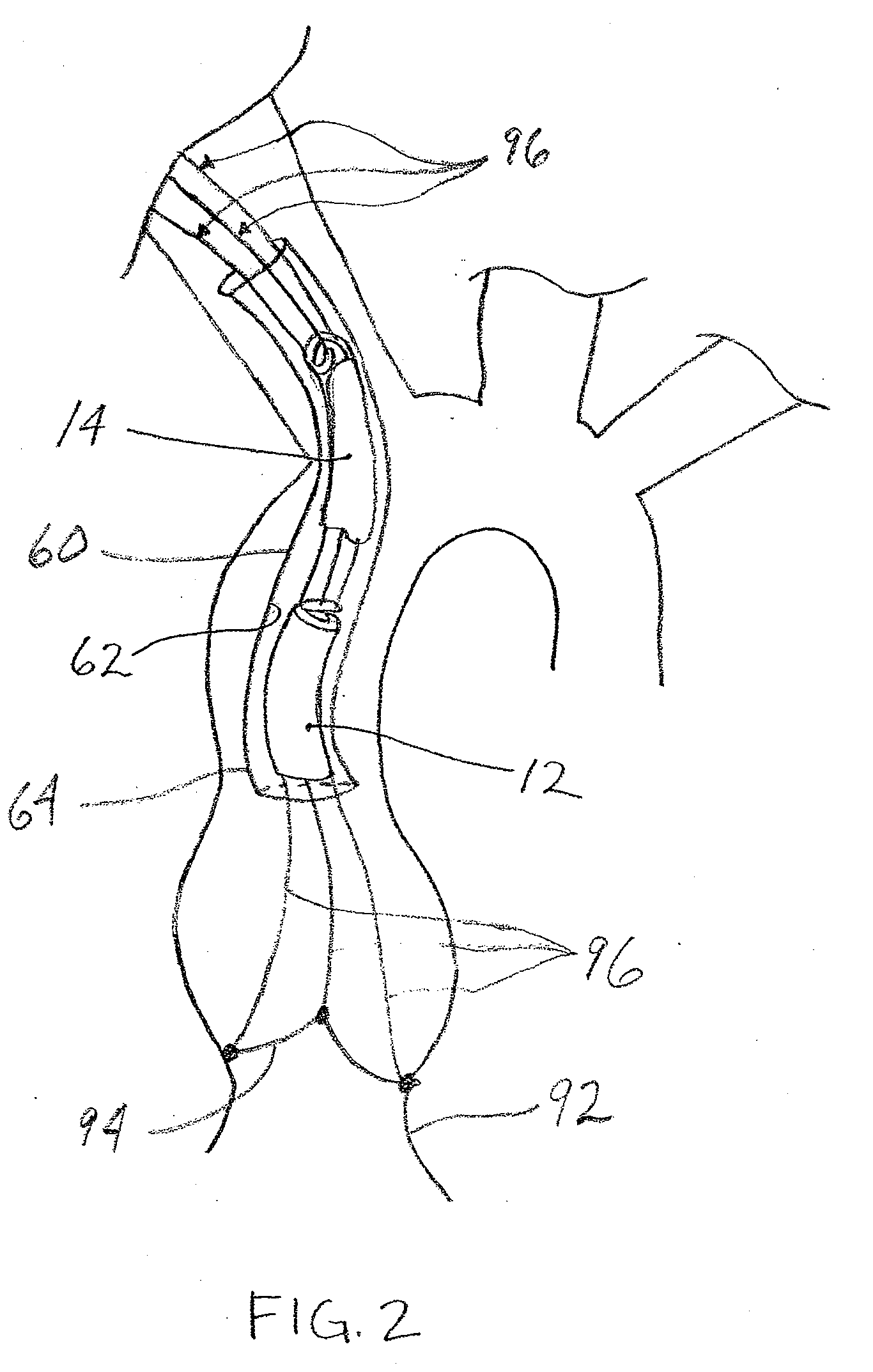
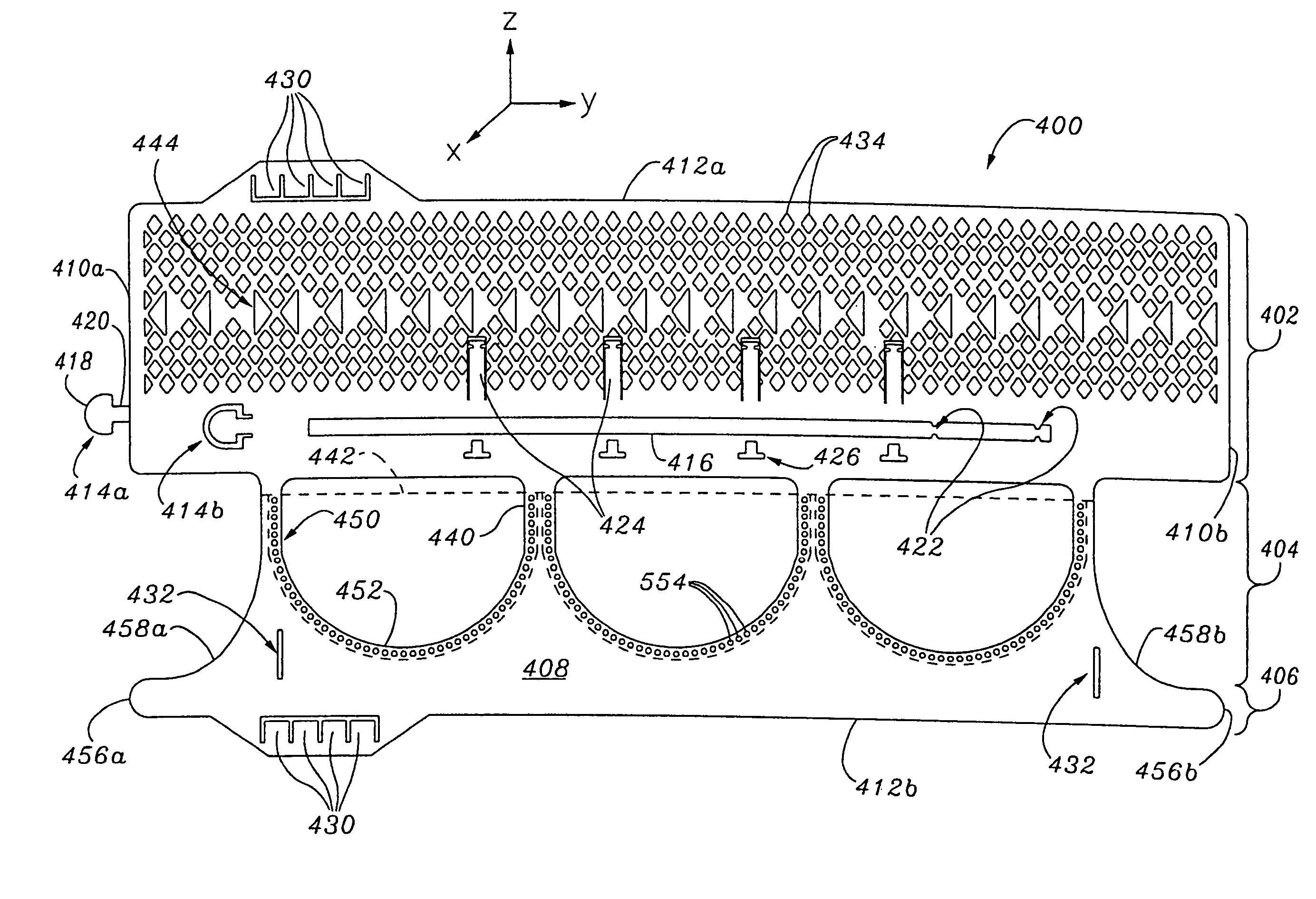
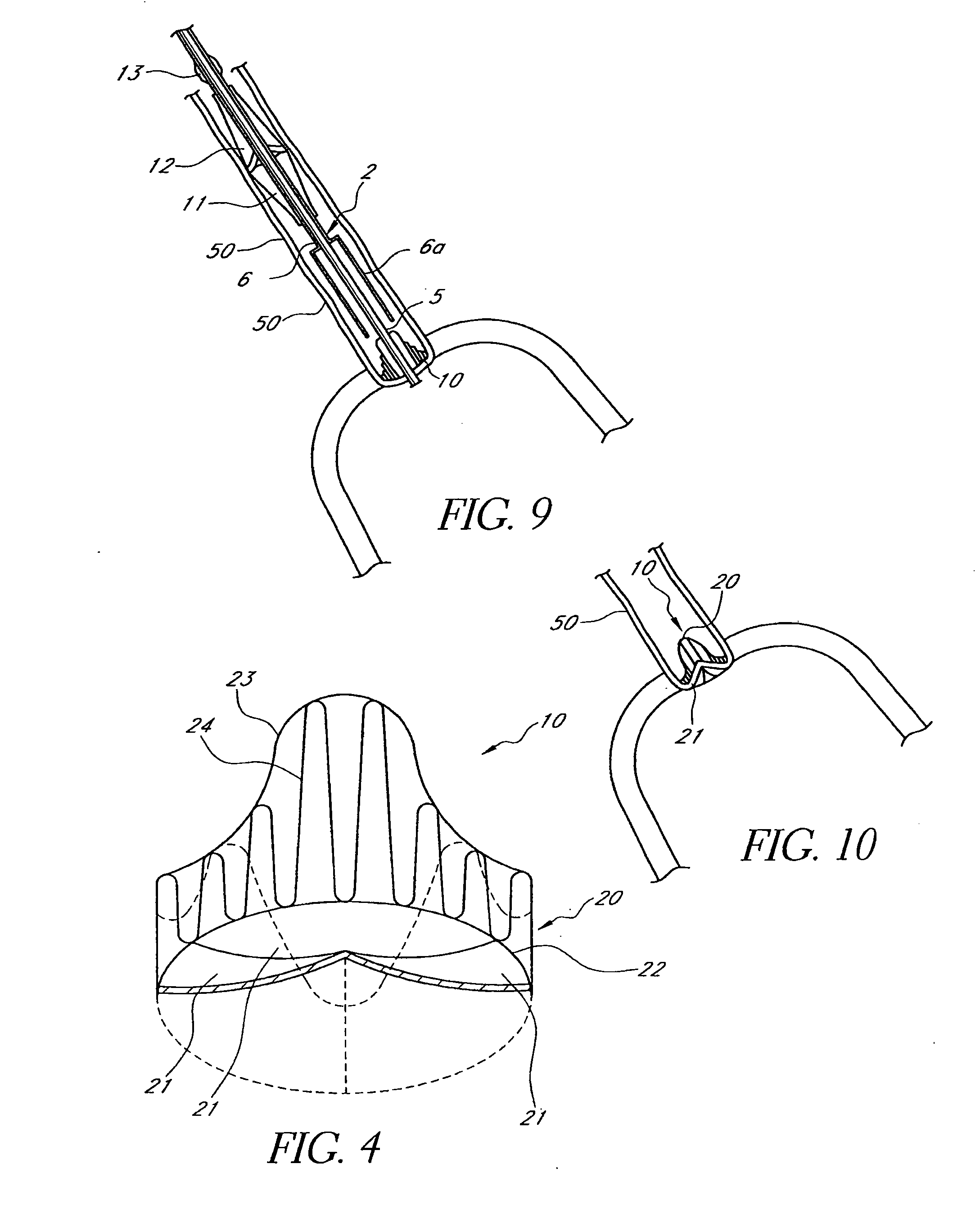
Prosthetic Valve for Transluminal Delivery
InactiveUS20100004740A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesVenous accessImplantation Site
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchors may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the valve support may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. A radial restraint, comprising a wire, thread or cuff, may be used to ensure expansion does not exceed the preset diameter. The valve support may optionally comprise a drug elution component. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. A blood pump may be inserted into the catheter to ensure continued blood flow across the implantation site during implantation procedure. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand at the implantation site and the anchor engages the lumen wall. Insertion of the catheter may optionally be performed over a transseptally delivered guidewire that has been externalized through the arterial vasculature. Such a guidewire provide dual venous and arterial access to the implantation site and allows additional manipulation of the implantation site after arterial implantation of the prosthetic valve. Additional expansion stents may be delivered by venous access to the valve.
Owner:MEDTRONIC COREVALVE
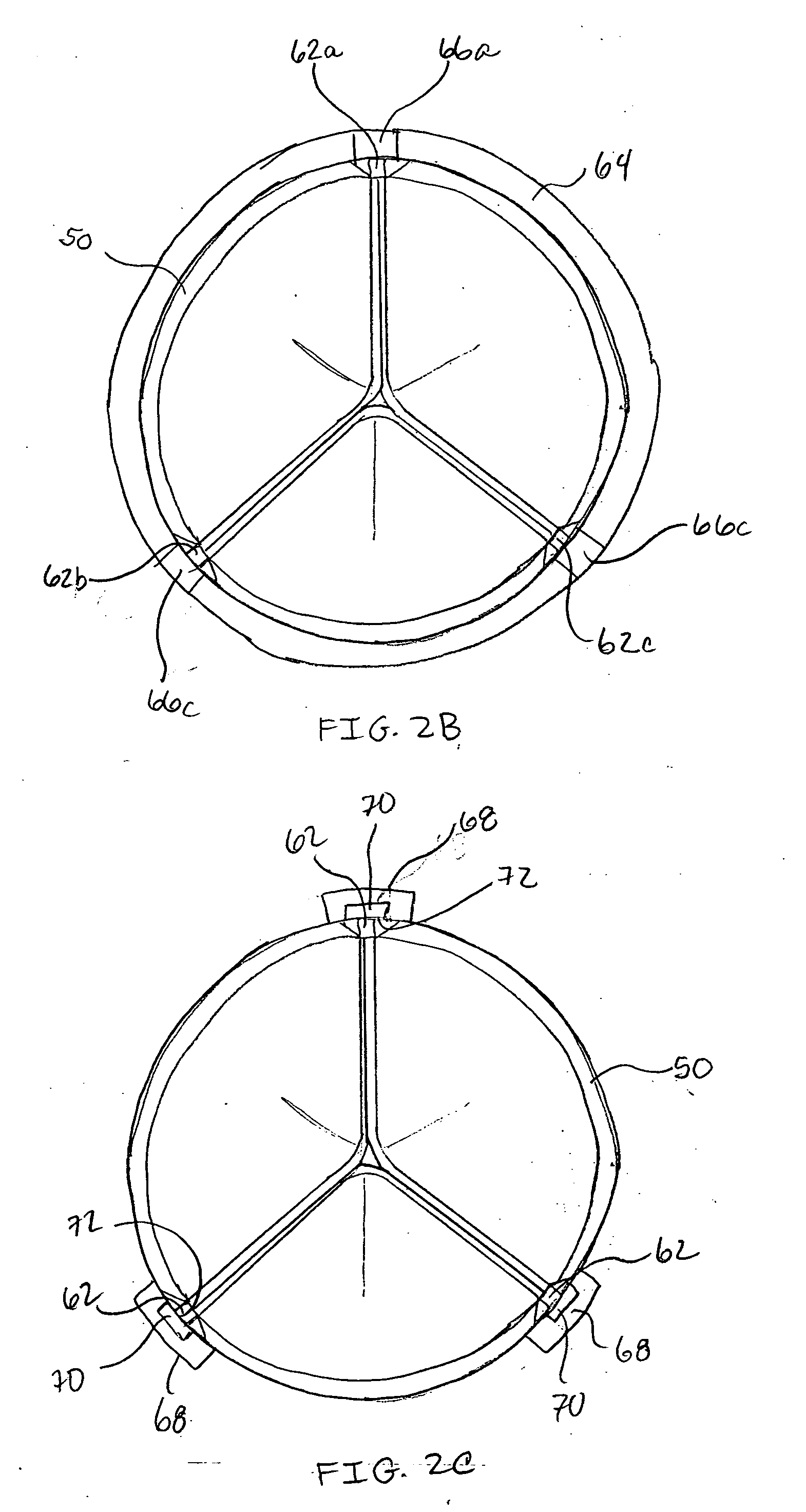
Prosthetic cardiac valves and systems and methods for implanting thereof
Implantable prosthetic valve systems and methods for implanting them are provided. Magnets are employed within one or more components of the valve systems to facilitate anchoring of the prosthetic valve at a target implant site, delivery of the prosthetic valve to the target implant site or both.
Owner:MEDTRONIC INC
Minimally-invasive heart valve with cusp positioners
A prosthetic heart valve having an internal support frame with a continuous, undulating leaflet frame defined therein. The leaflet frame has three cusp regions positioned at an inflow end intermediate three commissure regions positioned at an outflow end thereof. The leaflet frame may be cloth covered and flexible leaflets attached thereto form occluding surfaces of the valve. The support frame further includes three cusp positioners rigidly fixed with respect to the leaflet frame and located at the outflow end of the support frame intermediate each pair of adjacent commissure regions. The valve is desirably compressible so as to be delivered in a minimally invasive manner through a catheter to the site of implantation. Upon expulsion from catheter, the valve expands into contact with the surrounding native valve annulus and is anchored in place without the use of sutures. In the aortic valve position, the cusp positioners angle outward into contact with the sinus cavities, and compress the native leaflets if they are not excised, or the aortic wall if they are. The support frame may be formed from a flat sheet of Nitinol that is bent into a three-dimensional configuration and heat set. A holder having spring-like arms connected to inflow projections of the valve may be used to deliver, reposition and re-collapse the valve, if necessary.
Owner:EDWARDS LIFESCIENCES CORP
Targeted and high density drug loaded polymeric materials
ActiveUS20060002852A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
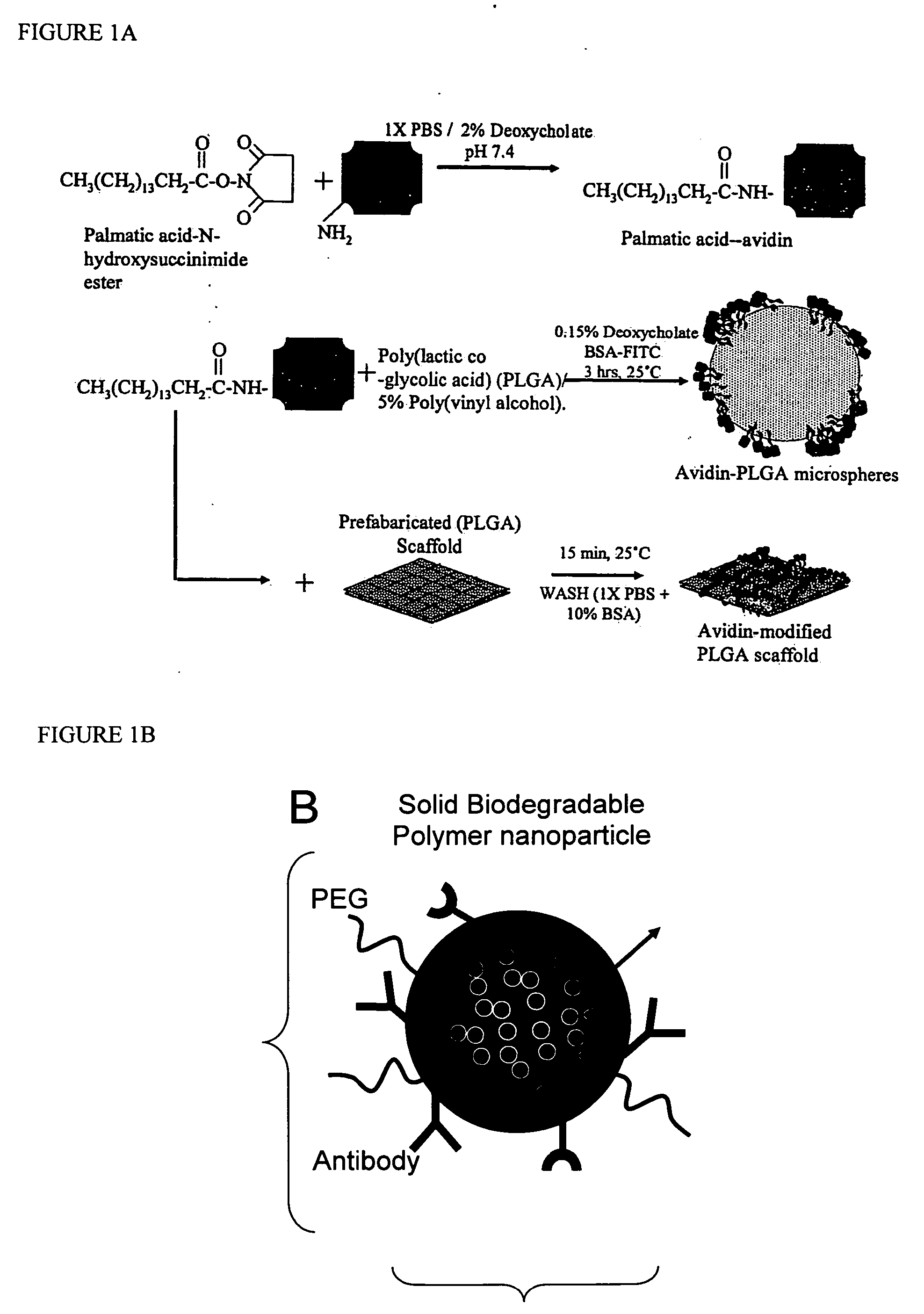
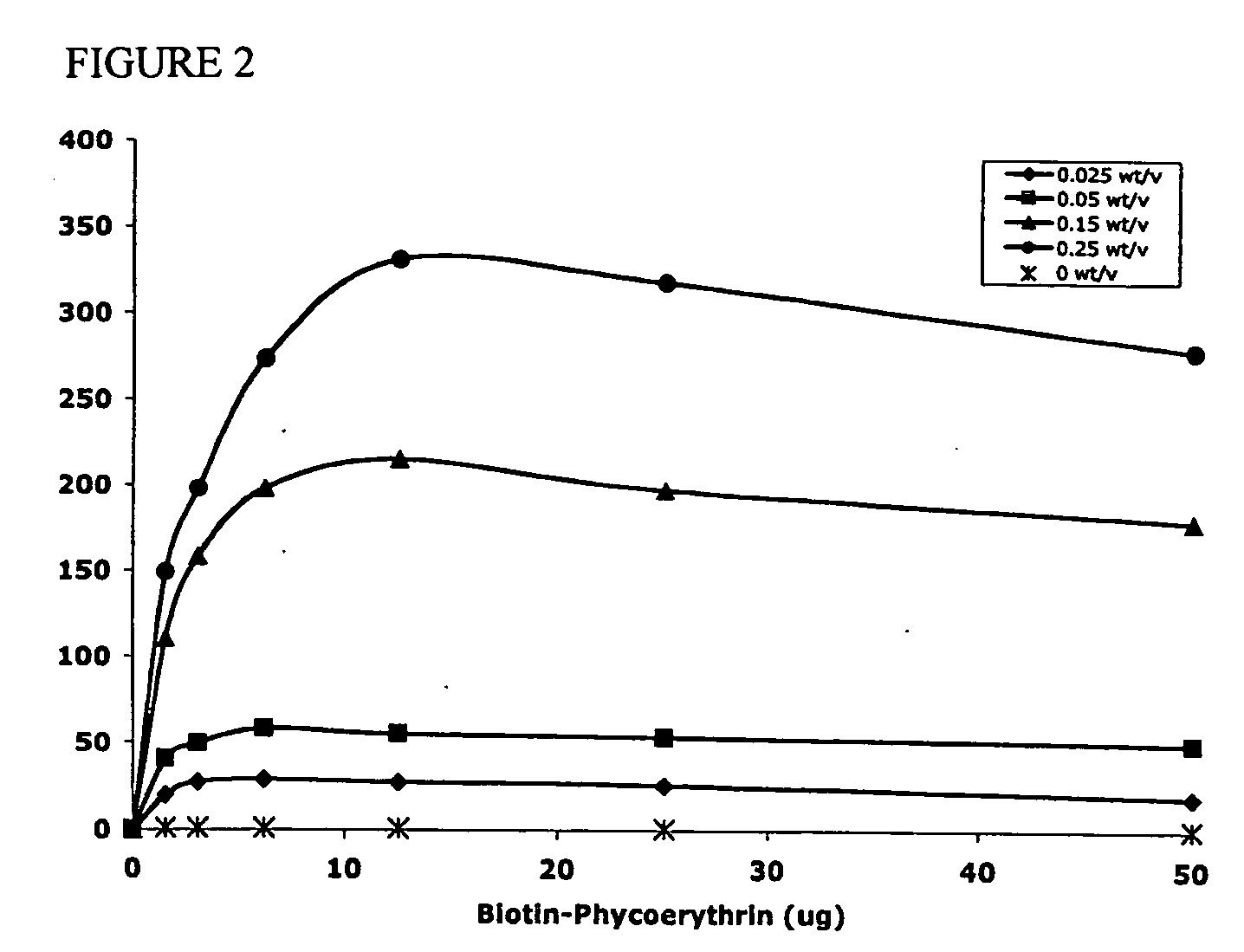
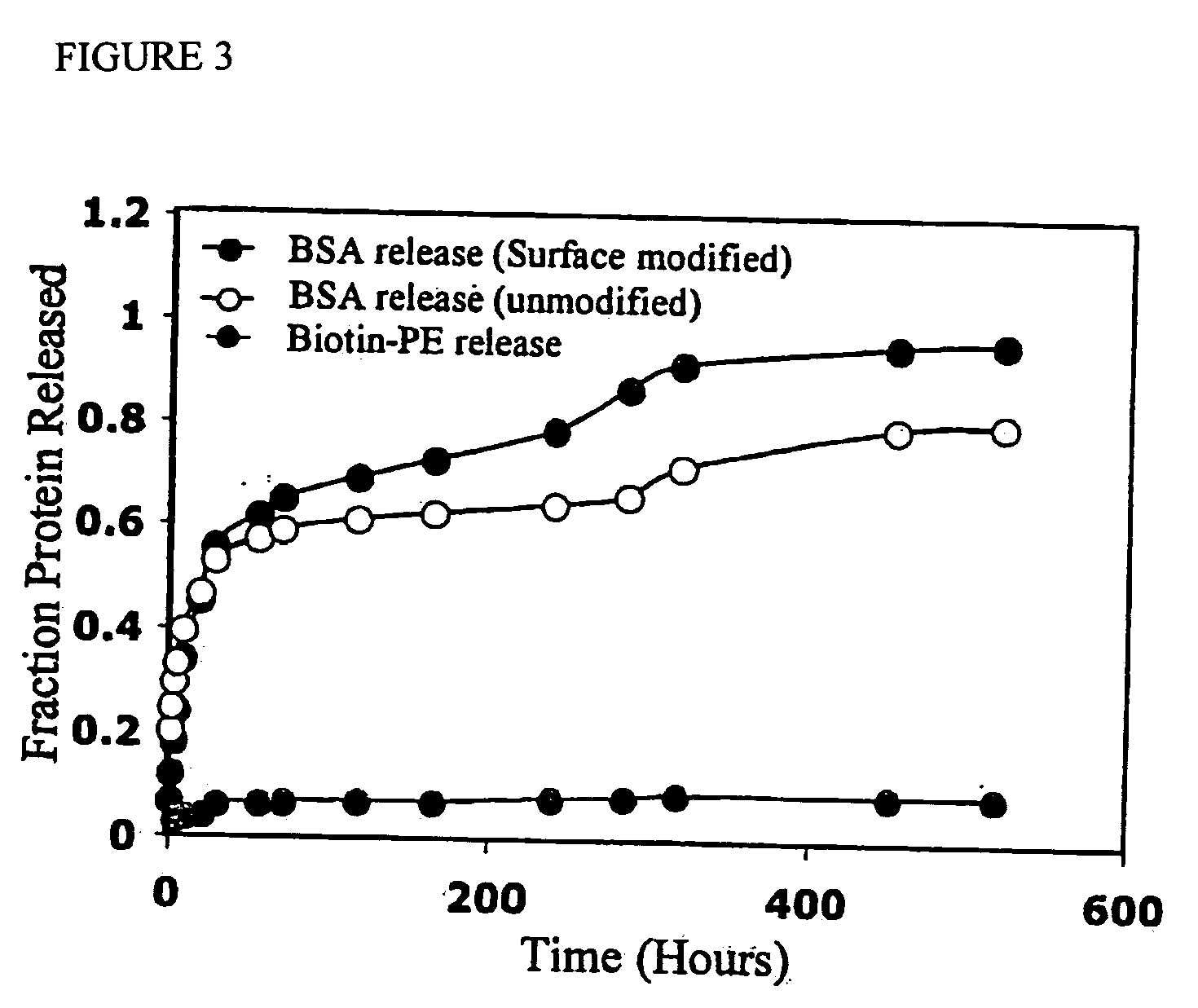
Polymeric delivery devices have been developed which combine high loading / high density of molecules to be delivered with the option of targeting. As used herein, “high density” refers to microparticles having a high density of ligands or coupling agents, which is in the range of 1000-10,000,000, more preferably between 10,000 and 1,000,000 ligands per square micron of microparticle surface area. A general method for incorporating molecules into the surface of biocompatible polymers using materials with an HLB of less than 10, more preferably less than 5, such as fatty acids, has been developed. Because of its ease, generality and flexibility, this method has widespread utility in modifying the surface of polymeric materials for applications in drug delivery and tissue engineering, as well other other fields. Targeted polymeric microparticles have also been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Cardiac valve procedure methods and devices
The present invention discloses devices and methods for performing intravascular procedures with out cardiac bypass. The devices include various embodiments of temporary filter devices, temporary valves, and prosthetic valves. The temporary filter devices have one or more cannulae which provide access for surgical tools for effecting repair of the cardiac valves. A cannula may have filters of various configurations encircling the distal region of the cannula, which prevent embolitic material from entering the coronary arteries and aorta. The temporary valve devices may also have one or more cannulae which guide the insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel. In one embodiment, the temporary valve is a disc of flexible, porous, material that acts to filter blood passing therethrough. A set of valve leaflets extend peripherally from the disc. These leaflets can alternately collapse to prevent blood flow through the valve and extend to permit flow. The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. In one embodiment, the prosthetic valves have at least one substantially rigid strut, at least two expandable fixation rings located about the circumference of the base of the apex of the valve, and one or more commissures and leaflets. The prosthetic valves are introduced into the vascular system a compressed state, advanced to the site of implantation, expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
Minimally-invasive heart valve with cusp positioners
A prosthetic heart valve having an internal support frame with a continuous, undulating leaflet frame defined therein. The leaflet frame has three cusp regions positioned at an inflow end intermediate three commissure regions positioned at an outflow end thereof. The leaflet frame may be cloth covered and flexible leaflets attached thereto form occluding surfaces of the valve. The support frame further includes three cusp positioners rigidly fixed with respect to the leaflet frame and located at the outflow end of the support frame intermediate each pair of adjacent commissure regions. The valve is desirably compressible so as to be delivered in a minimally invasive manner through a catheter to the site of implantation. Upon expulsion from catheter, the valve expands into contact with the surrounding native valve annulus and is anchored in place without the use of sutures. In the aortic valve position, the cusp positioners angle outward into contact with the sinus cavities, and compress the native leaflets if they are not excised, or the aortic wall if they are. The support frame may be formed from a flat sheet of Nitinol that is bent into a three-dimensional configuration and heat set. A holder having spring-like arms connected to inflow projections of the valve may be used to deliver, reposition and re-collapse the valve, if necessary.
Owner:EDWARDS LIFESCIENCES CORP
Methods and apparatuses for deploying minimally-invasive heart valves
A system for delivering and deploying a self-expandable heart valve includes a deployment mechanism that engages the valve and regulates the rate of expansion of both the proximal and distal ends thereof. The deployment mechanism may include a plurality of distal fingers and a plurality of proximal fingers that engage the end portions of the heart valve. Controlled radial movement of the fingers regulates the expansion of the heart valve such that the proximal and distal ends radially expand at the same rate. A stabilization balloon may be used to axially and radially locate the deployment mechanism relative to the site of implantation. Methods of operation of the delivery and deployment mechanism include regulating the rate of self-expansion of the valve and forcing the valve outward into its fully expanded configuration utilizing the same or different means.
Owner:EDWARDS LIFESCIENCES CORP
Percutaneously placed prosthesis with thromboresistant valve portion
InactiveUS20050182483A1Prevent and limit refluxAvoid poolingBall valvesVenous valvesVenous ValvesBlood flow
A venous valve prosthesis having a substantially non-expandable, valve portion comprising a valve-closing mechanism, such as a pair of opposing leaflets; and an anchoring portion, such as one or more self-expanding frames or stents that are expandable to anchor the prosthesis at the implantation site. In one embodiment, the rigid valve portion includes a deposition of material such as pyrolitic carbon to reduce the thrombogenecity of the blood-contacting surfaces. The anchoring portions preferably include a covering, such as a tubular construct of synthetic or collagen-derived material (such as a bioremodelable ECM material), which attaches about the support structure such that blood flow is directed through the valve mechanism as it transitions from the larger diameter anchoring portion to the intermediate, smaller-diameter portion of the prosthesis. In another embodiment, the valve support housing and valve-closing elements are delivered in a collapsed, folded, and / or dissembled state sized for delivery, then manipulated in situ to the second expanded configured following deployment.
Owner:COOK INC
Cardiac valve procedure methods and devices
ActiveUS20050015112A1Improve performanceReduce riskHeart valvesSurgeryCoronary arteriesProsthetic valve
Devices and methods for performing intravascular procedures without cardiac bypass include embodiments of temporary filter devices, temporary valves, and prosthetic valves. The temporary filter devices have a cannula which provides access for surgical tools for effecting repair of cardiac valves. The cannula may have filters which prevent embolitic material from entering the coronary arteries and aorta. The valve devices may also have a cannula for insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel and operate to prevent blood flow and to permit flow through the valve. The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. The prosthetic valves are introduced into the vascular system in a compressed state, advanced to the site of implantation, and expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
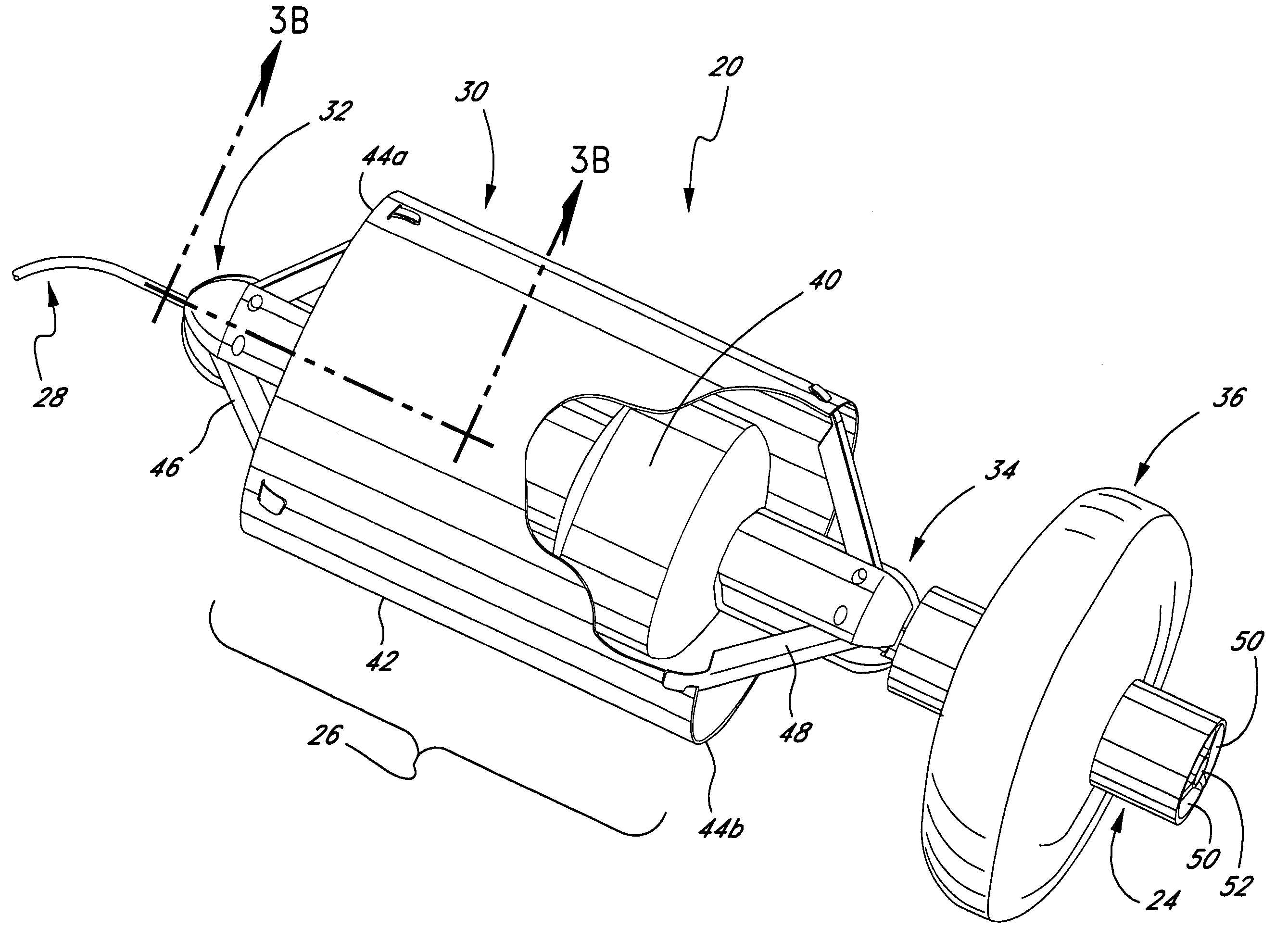
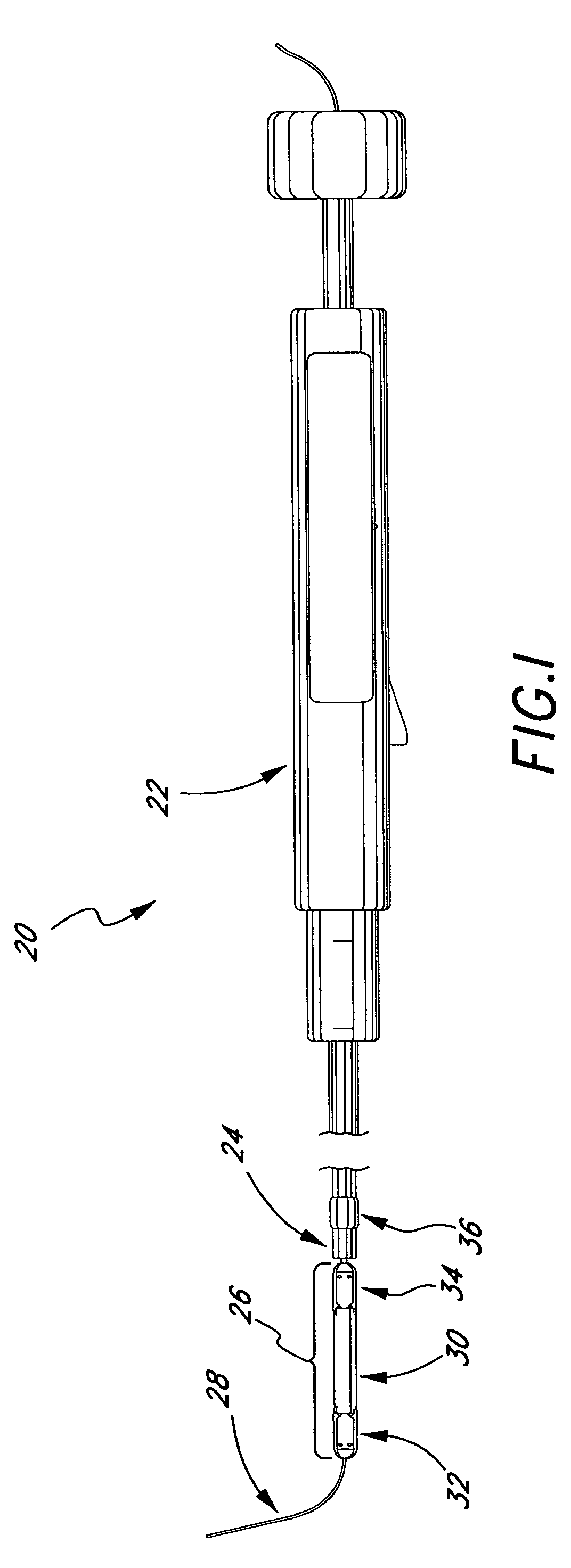
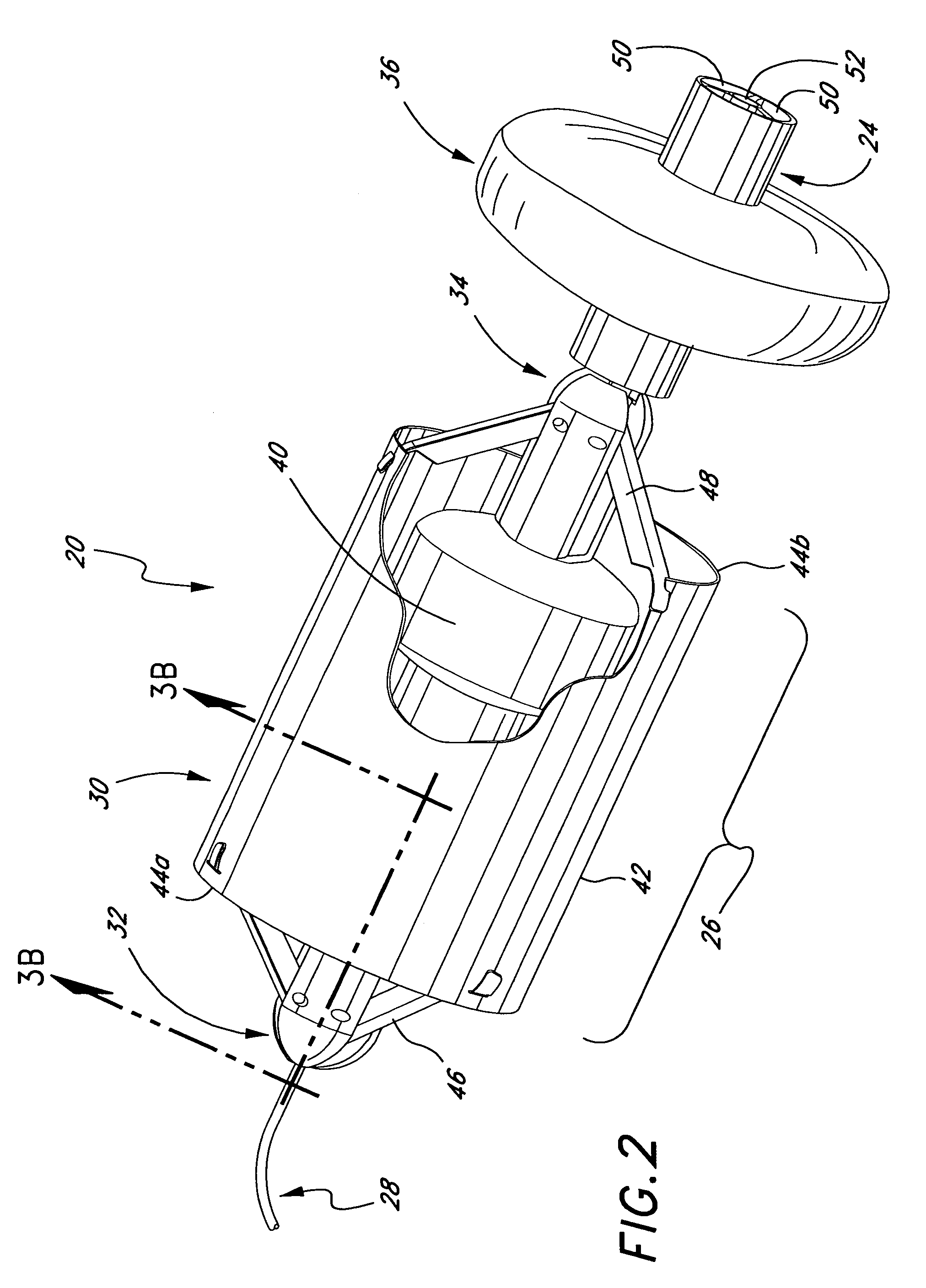
Medical implant deployment tool
ActiveUS20070112355A1Safely delivering proper level of forceSuture equipmentsInternal osteosythesisPath lengthImplantation Site
A medical implant deployment tool and deployment method are disclosed. One aspect of the invention provides an implant system including an implant adapted for endovascular delivery and deployment; and a deployment tool adapted to deploy the implant, with the deployment tool having an actuation controller; a plurality of actuation elements adapted to apply forces to one or more implant deployment mechanisms and each adapted to extend along an actuation element path within a patient's vasculature; and an actuation element compensation mechanism adapted to compensate for differences in length between the actuation element paths. Another aspect of the invention provides a method of deploying an implant including the steps of endovascularly delivering an implant and implant deployment mechanisms to an implant site and applying an actuation force to the implant deployment mechanisms through actuation elements extending through the patient's vasculature while compensating for differences in length between actuation element path lengths to deploy the implant.
Owner:BOSTON SCI SCIMED INC
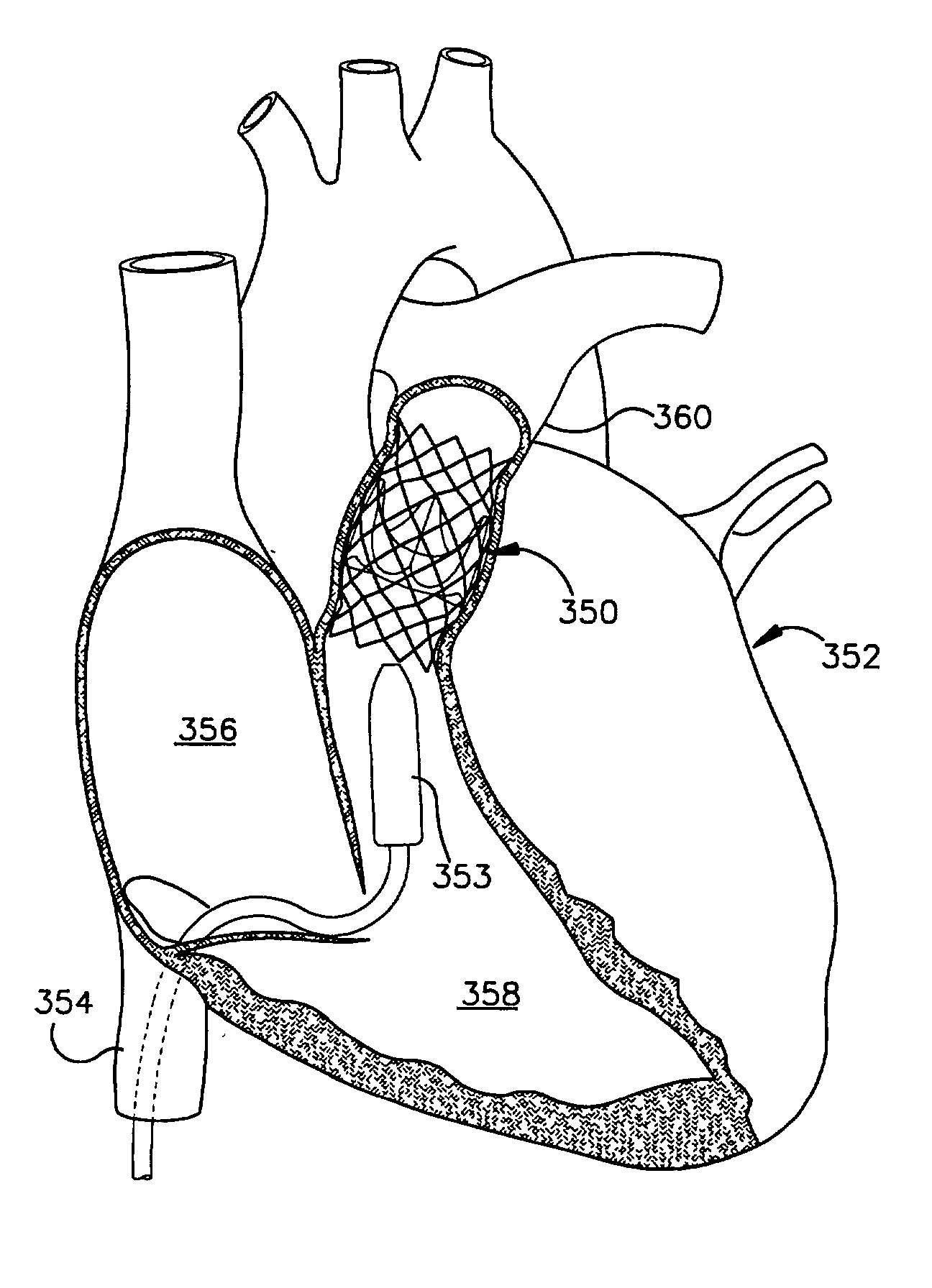
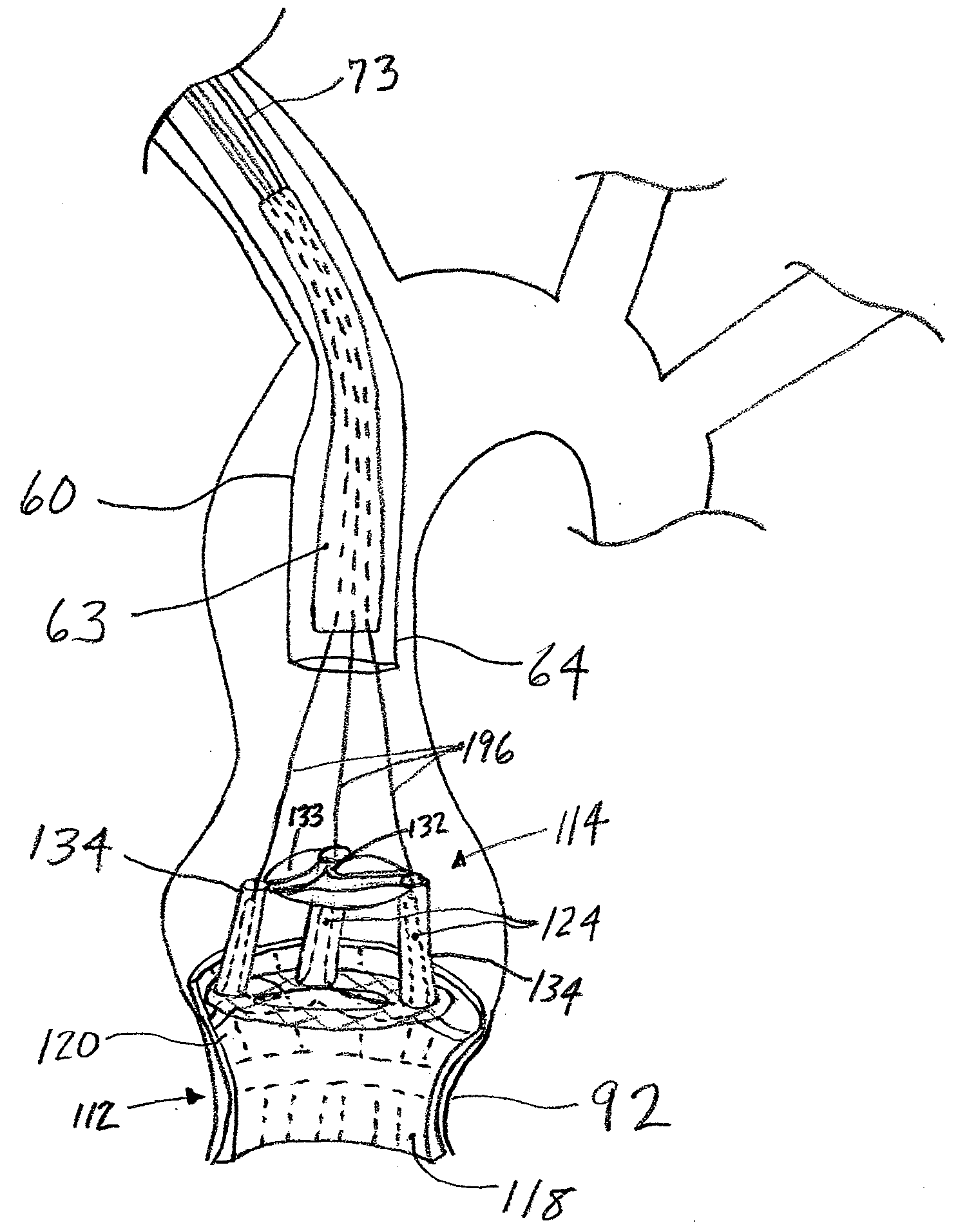
Leadless Cardiac Pacemaker with Secondary Fixation Capability
The invention relates to leadless cardiac pacemakers (LBS), and elements and methods by which they affix to the heart. The invention relates particularly to a secondary fixation of leadless pacemakers which also include a primary fixation. Secondary fixation elements for LBS's may either actively engage an attachment site, or more passively engage structures within a heart chamber. Active secondary fixation elements include a tether extending from the LBS to an anchor at another site. Such sites may be either intracardial or extracardial, as on a vein through which the LBS was conveyed to the heart, the internal or external surface thereof. Passive secondary fixation elements entangle within intraventricular structure such as trabeculae carneae, thereby contributing to fixation of the LBS at the implant site.
Owner:NANOSTIM
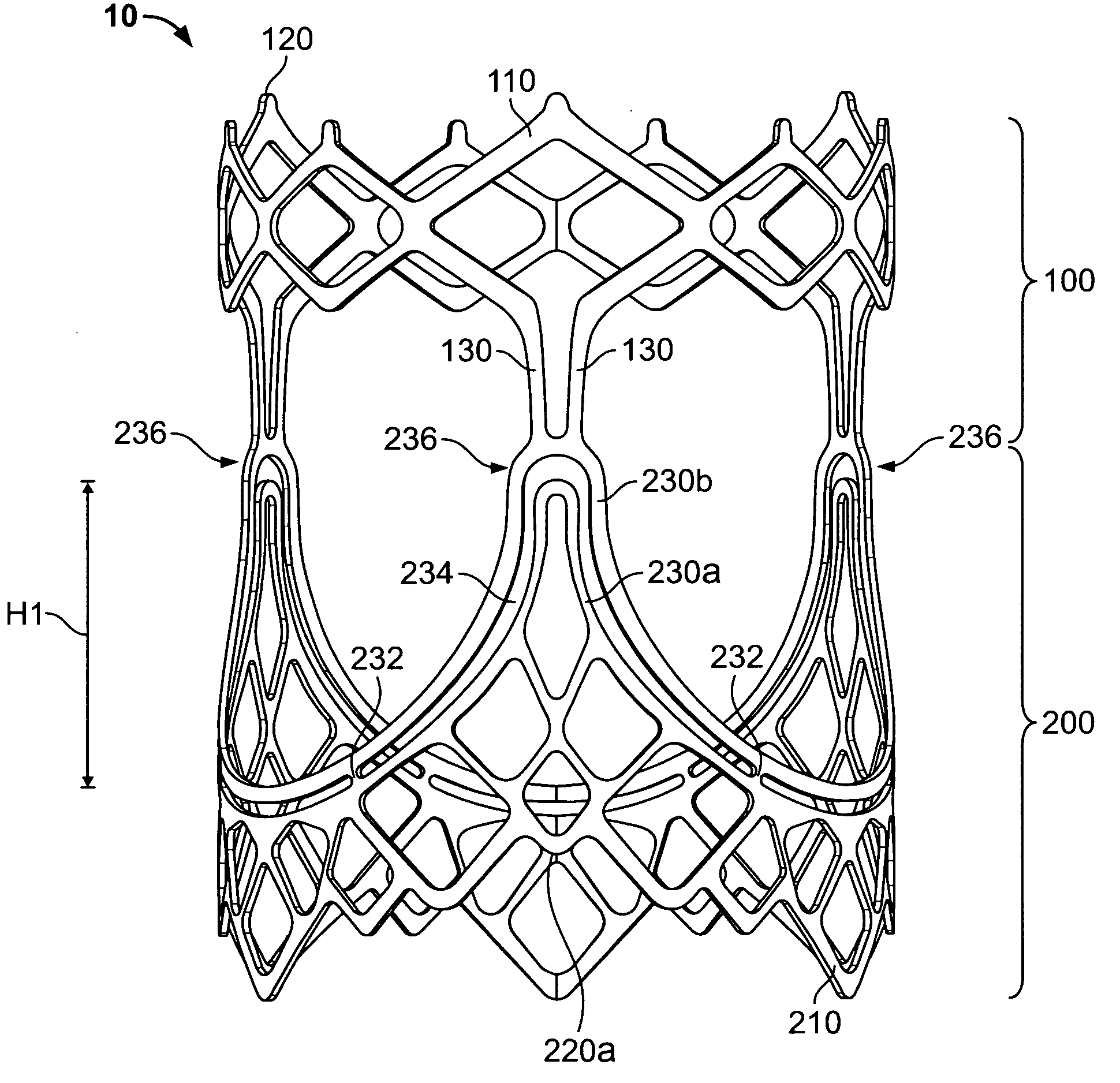
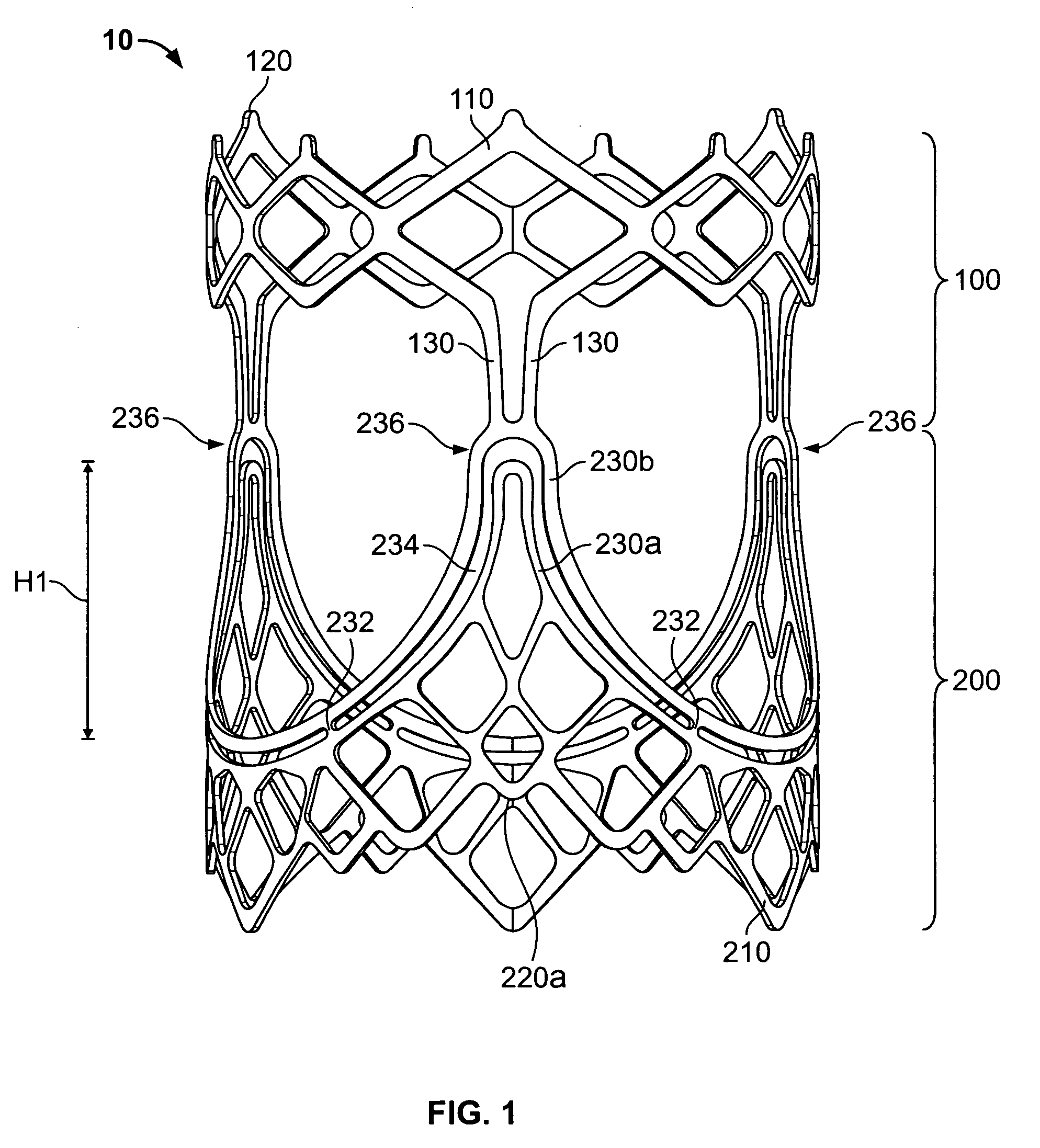
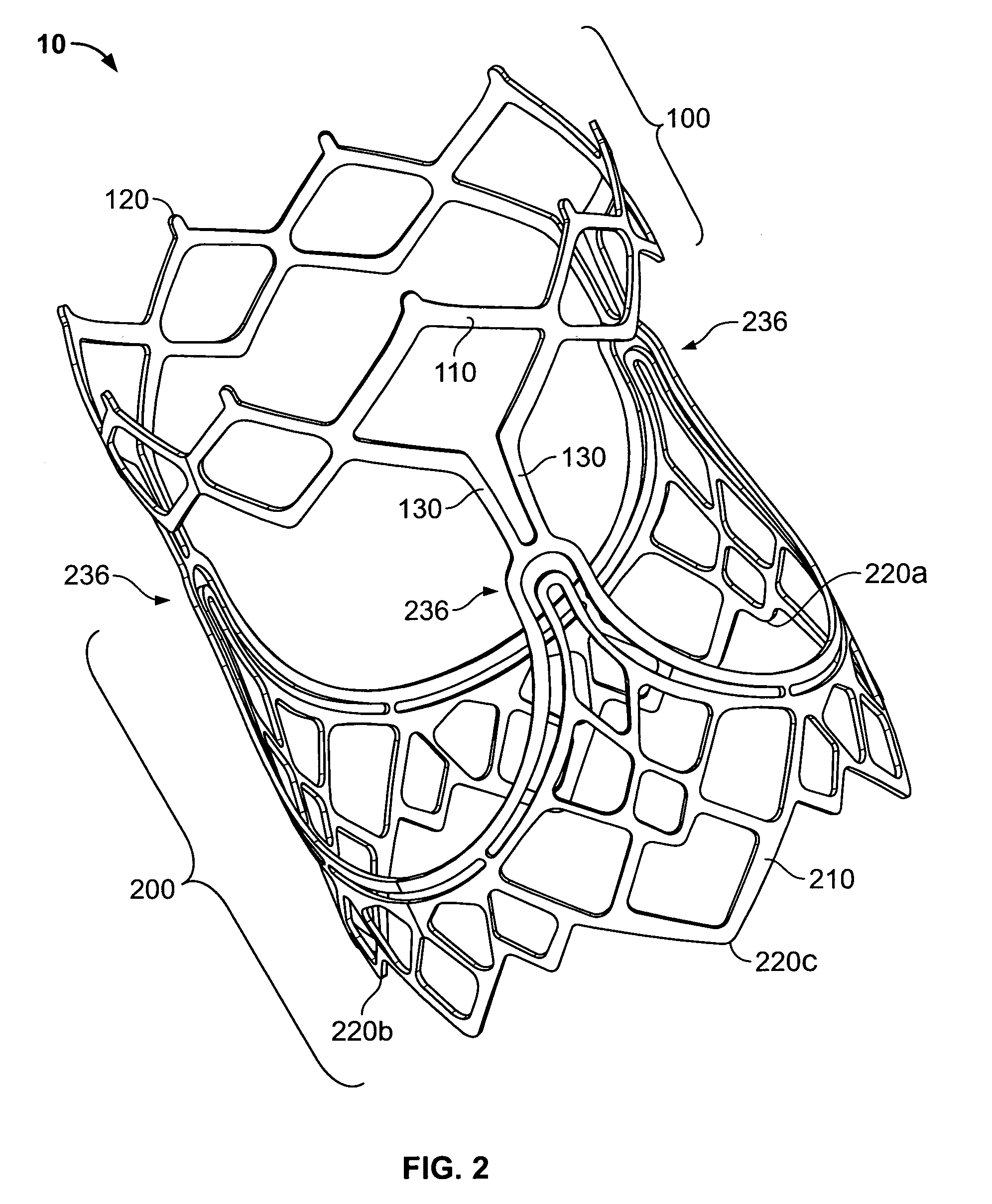
Two-stage collapsible/expandable prosthetic heart valves and anchoring systems
ActiveUS8454686B2Small diameterLow profileBalloon catheterHeart valvesProsthetic heartImplantation Site
Prosthetic heart valve apparatus is adapted for delivery into a patient in a circumferentially collapsed condition, followed by circumferential re-expansion at the implant site in the patient. The apparatus includes an annular anchoring structure that can be implanted in the patient first. The apparatus further includes an annular valve support structure, which supports a flexible leaflet structure of the valve. The support and leaflet structures are initially separate from the anchoring structure, but they can be implanted in the patient by interengagement of the support structure with the already-implanted anchoring structure.
Owner:ST JUDE MEDICAL LLC
Deployment system for an expandable device
The present invention is directed to a deployment system for an endoluminal device. The deployment system includes a confining sheath placed around a compacted endoluminal device. A deployment line is provided in the system that is an integral extension of the sheath. As the deployment line is actuated, the sheath retracts from around the compacted endoluminal device. As the sheath retracts from around the endoluminal device, material from the sheath may be converted into deployment line. Once the sheath is retracted from around the compacted endoluminal device, the endoluminal device expands in configuration and repairs vascular or cardiac structures of an implant recipient. Any remaining sheath material is removed from the implantation site along with the deployment line. The deployment system also includes an endo-prosthesis mounting member placed between the endoluminal device and an underlying catheter. The endo-prosthesis mounting member serves to cushion and retain the endoluminal device when constrained by the sheath and may assist in expansion of the endoluminal device when unconstrained by the sheath. The present invention is also directed to a deployment system having a deployment assembly that simultaneously expands an endo-prosthesis mounting member while removing a sheath from an expandable medical device.
Owner:WL GORE & ASSOC INC
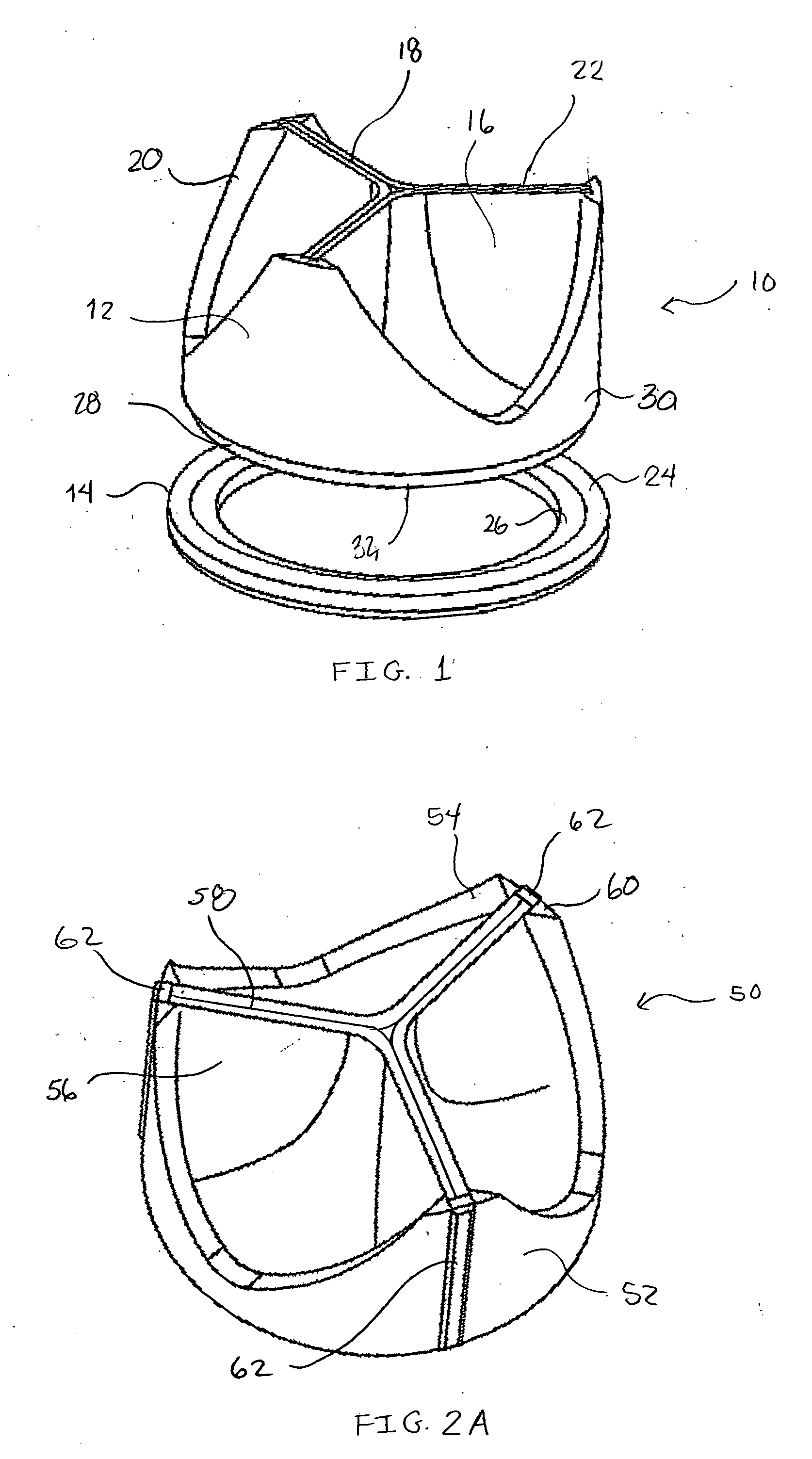
Valve prosthesis
The present disclosure relates to valve replacement devices that are foldable for catheter-based deployment to the site of implantation, as well as systems for the delivery of valve prostheses, including prostheses having the special characteristics of the disclosed valve replacement devices. The devices include highly effective adhering mechanisms for secure and enduring precision implantation. The adhering mechanisms may employ a unique sealing mechanism that includes a cuff that expands slowly whereby the device is not secured in place until the completion of the implantation procedure. The implanted device, optionally together with the cuff, prevents perivalvular leaks and incorporate an appropriate leaflet system for reliable functioning in situ.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Drug eluting implantable medical device
A drug eluting medical device is provided for implanting into vessels or luminal structures within the body of a patient. The coated medical device, such as a stent, vascular, or synthetic graft comprises a coating consisting of a controlled-release matrix of a bioabsorbable, biocompatible, bioerodible, biodegradable, nontoxic material, such as a Poly(DL-Lactide-co-Glycolide) polymer, and at least one pharmaceutical substance, or bioactive agent incorporated within the matrix or layered within layers of matrix. In particular, the drug eluting medical device when implanted into a patient, delivers the drugs or bioactive agents within the matrix to adjacent tissues in a controlled and desired rate depending on the drug and site of implantation.
Owner:ORBUSNEICH MEDICAL PTE LTD
Self-aligning stent graft delivery system, kit, and method
ActiveUS20050049667A1Reduce the possibilityIncreases blood-tight vascular connectionStentsBlood vesselsDistal portionStent grafting
A delivery system and kit for endovascularly delivering a prosthesis along a guidewire to an curved implantation site includes a control handle having a handle body with a prosthesis movement control assembly movably disposed thereto. A proximal end of a catheter is connected to the control handle. A prosthesis delivery assembly is movably disposed in the catheter. The delivery assembly has a guidewire lumen slidably receiving therein the guidewire and having a curved distal end orienting the delivery assembly when passing through a curved vessel portion. A method for automatic endovascular alignment of the prosthesis includes loading it in the distal delivery assembly, positioning the guidewire into the curved implantation site, and threading the guidewire lumen over the guidewire with the delivery assembly and into the implantation site to at least approximately align the curved distal portion to a curve of the curved implantation site and, thereby, orient the prosthesis.
Owner:BOLTON MEDICAL INC
Two-stage collapsible/expandable prosthetic heart valves and anchoring systems
ActiveUS20100204785A1Improve securityLow profileBalloon catheterHeart valvesProsthesisProsthetic heart
Prosthetic heart valve apparatus is adapted for delivery into a patient in a circumferentially collapsed condition, followed by circumferential re-expansion at the implant site in the patient. The apparatus includes an annular anchoring structure that can be implanted in the patient first. The apparatus further includes an annular valve support structure, which supports a flexible leaflet structure of the valve. The support and leaflet structures are initially separate from the anchoring structure, but they can be implanted in the patient by interengagement of the support structure with the already-implanted anchoring structure.
Owner:ST JUDE MEDICAL LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com