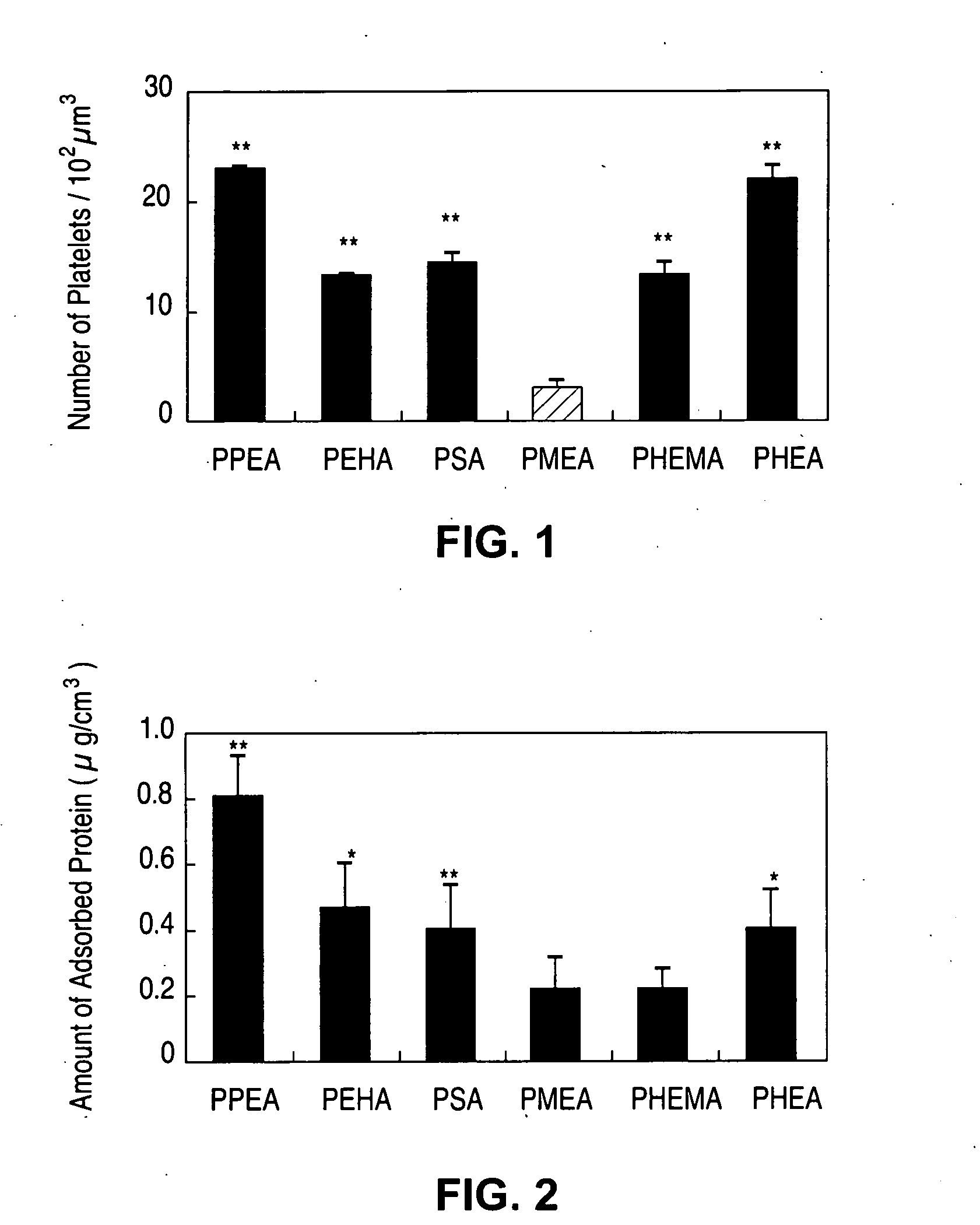
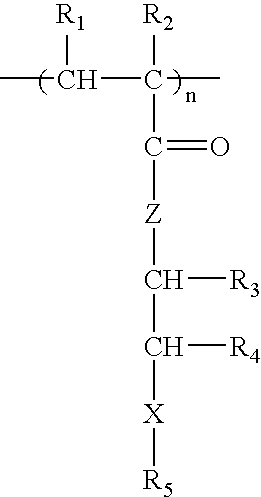
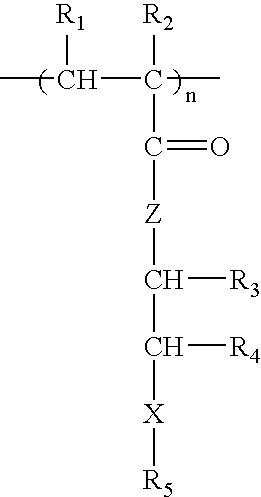
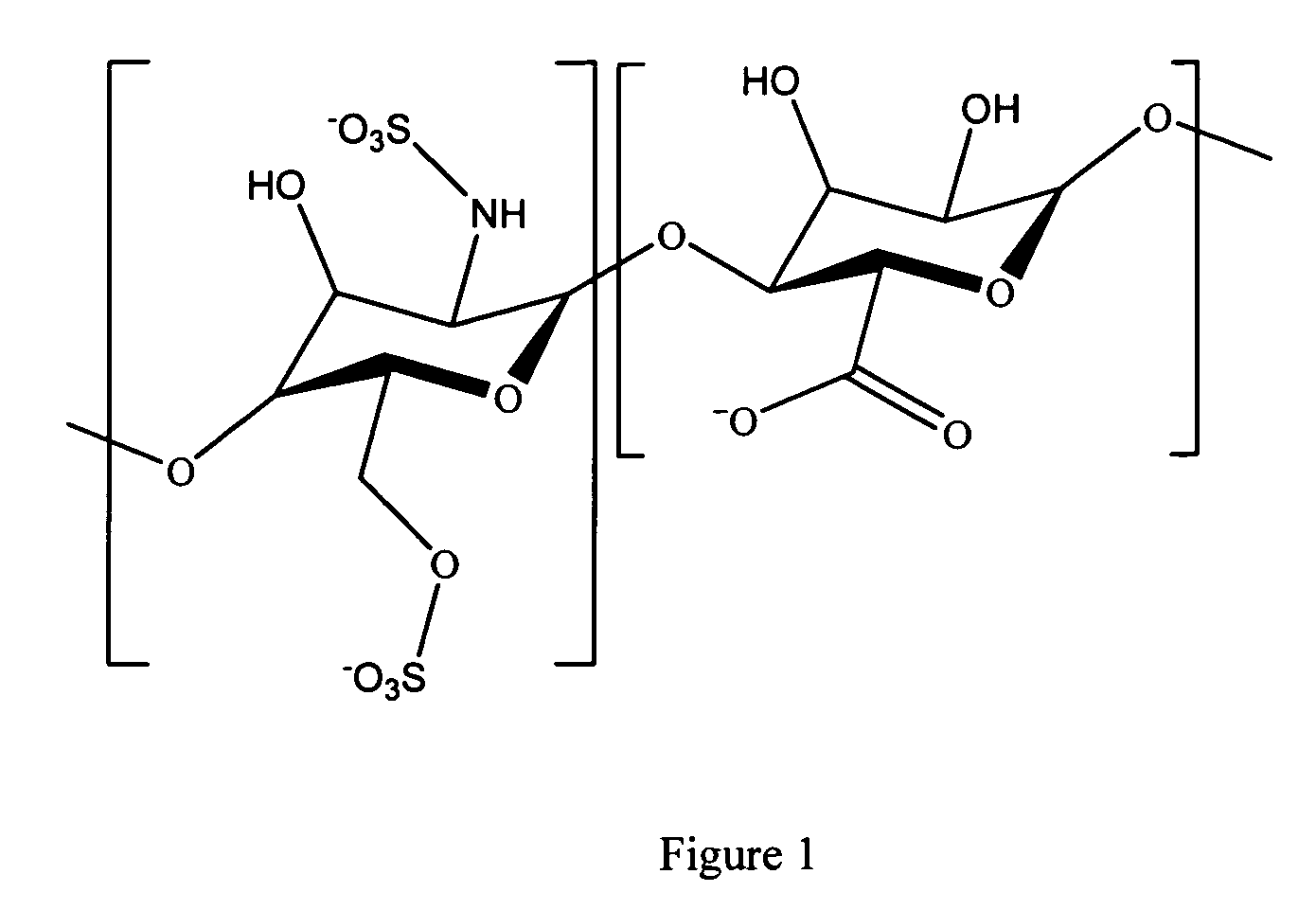
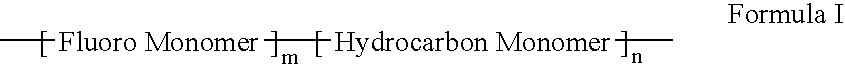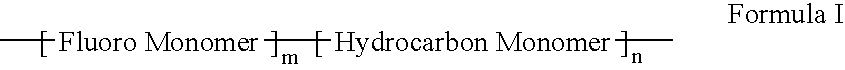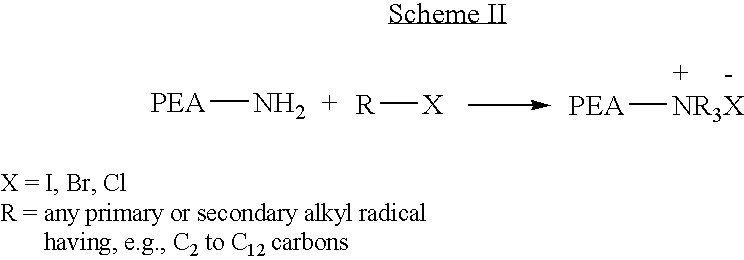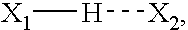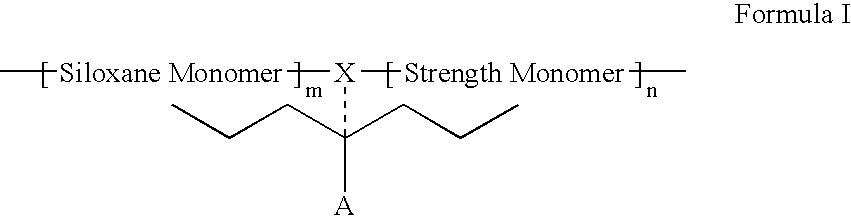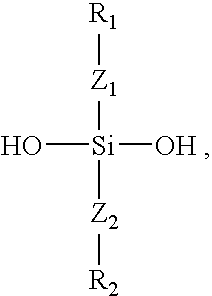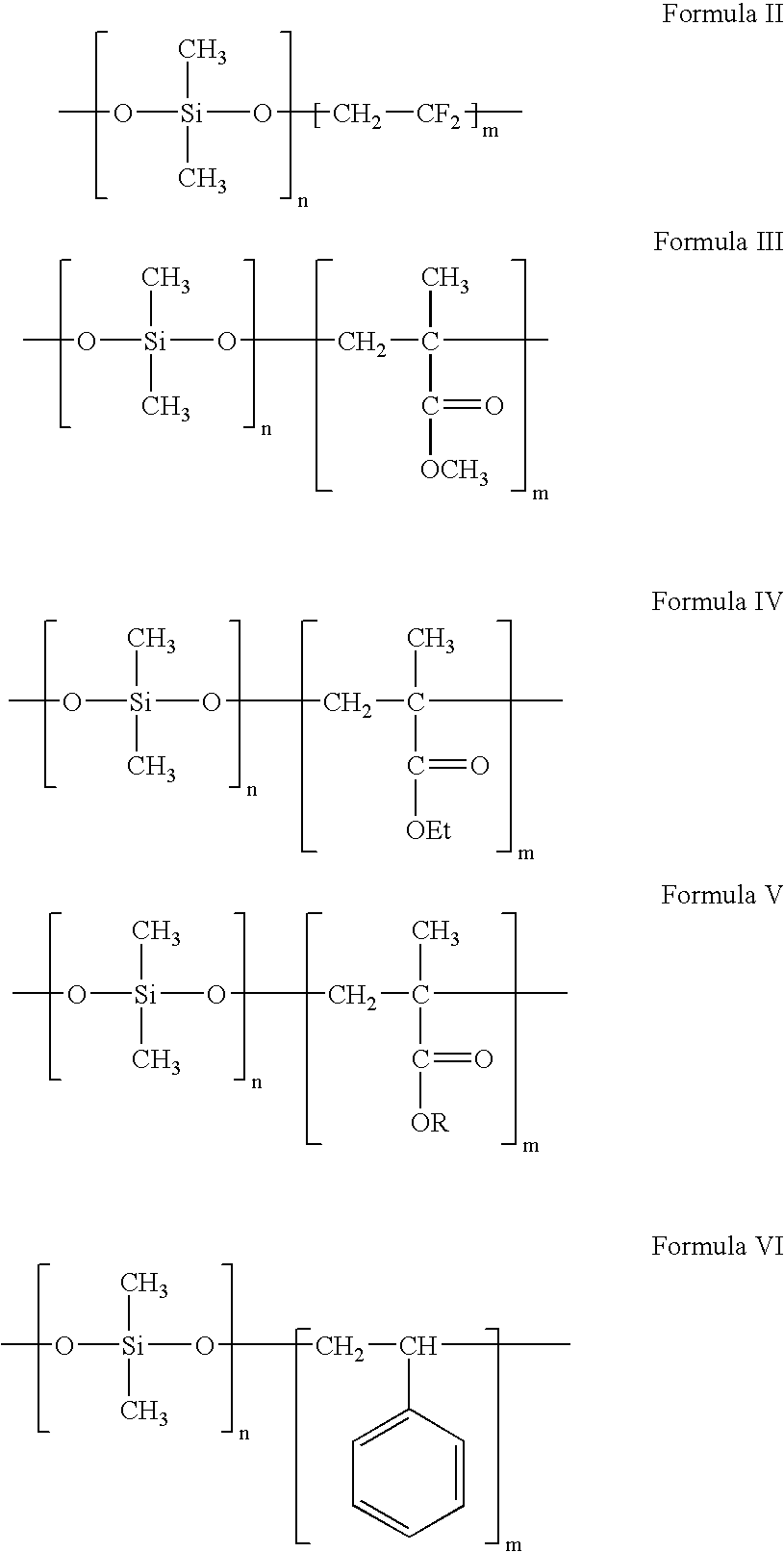Patents
Literature
82 results about "Synthetic graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug eluting implantable medical device
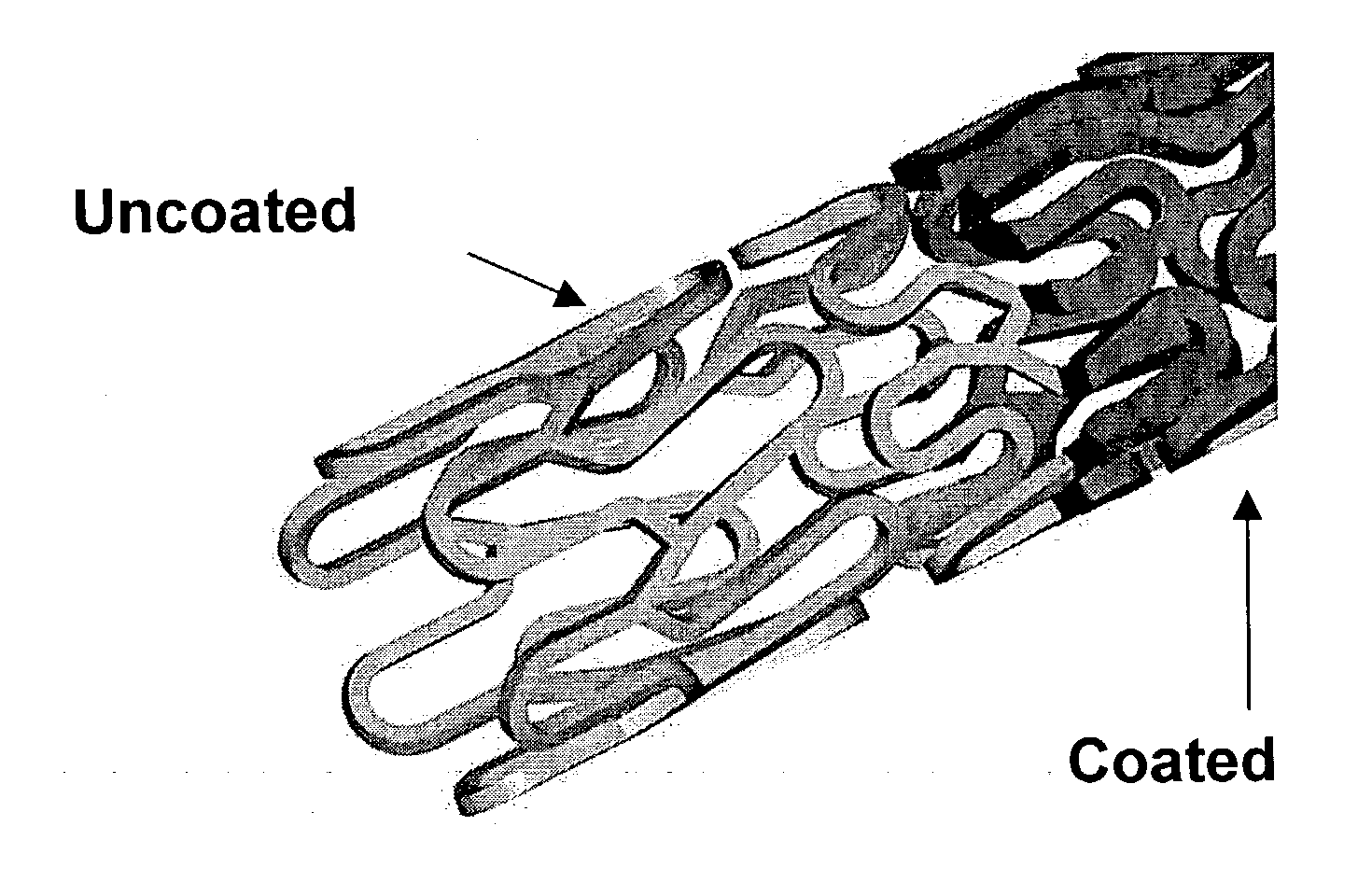
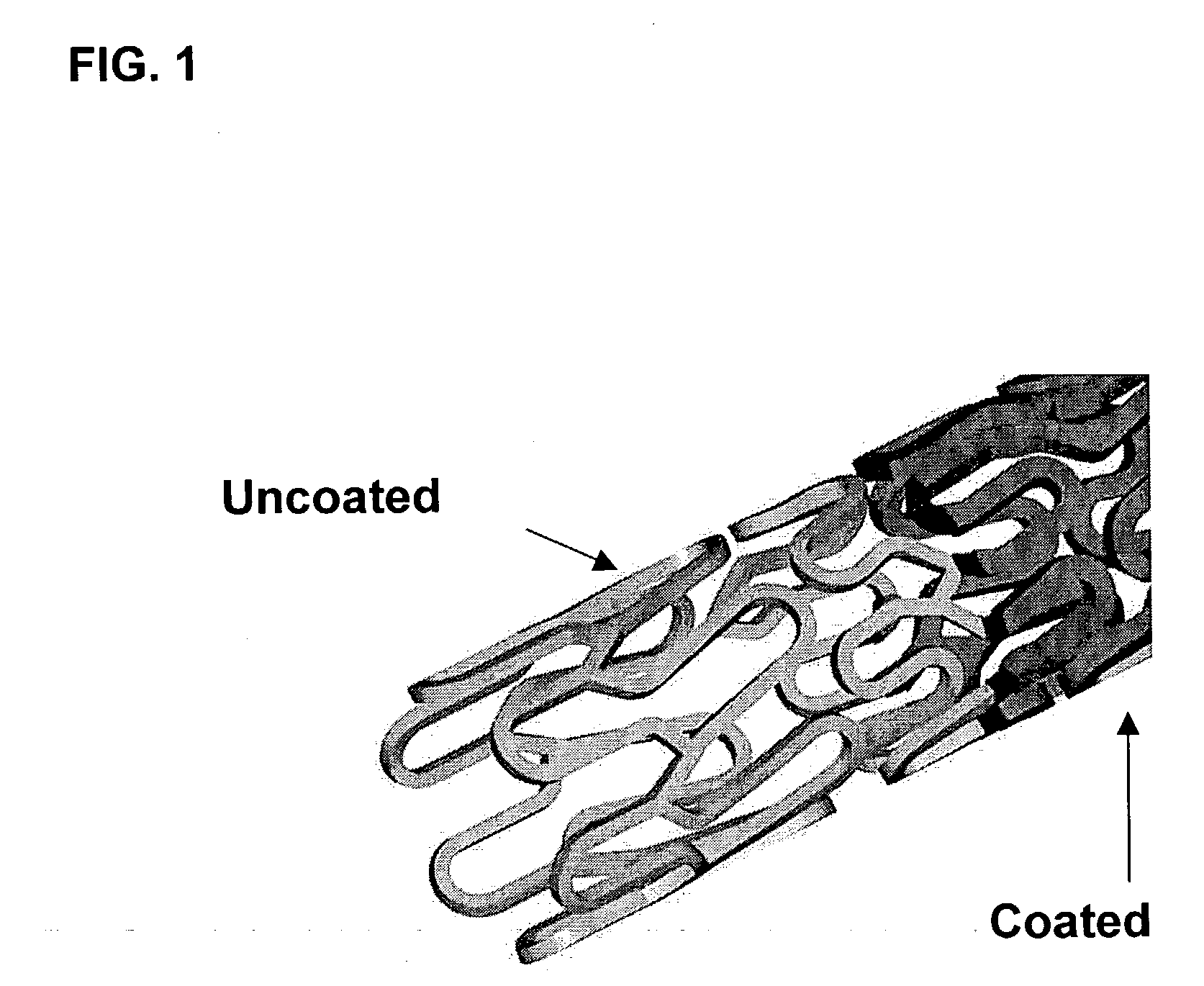
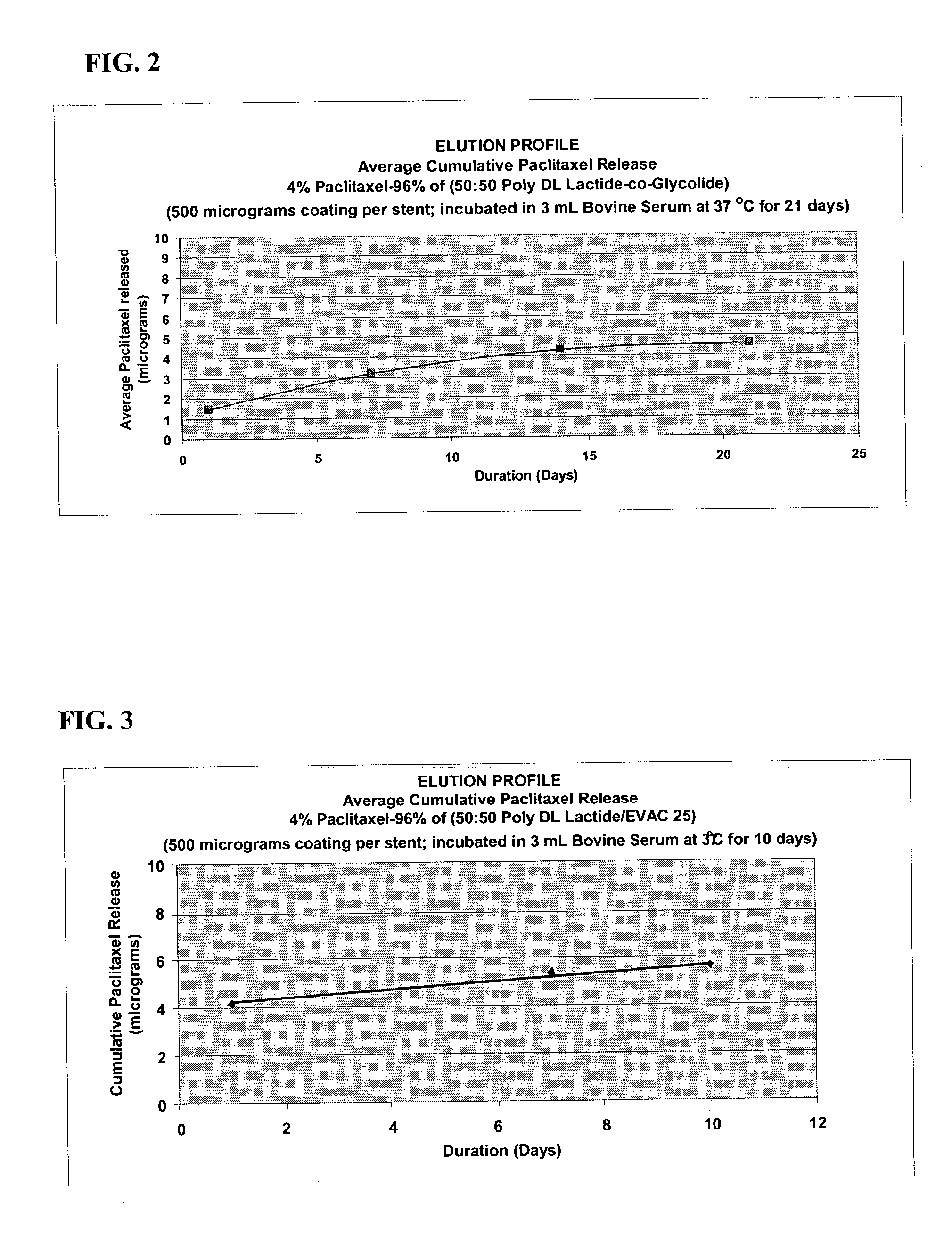
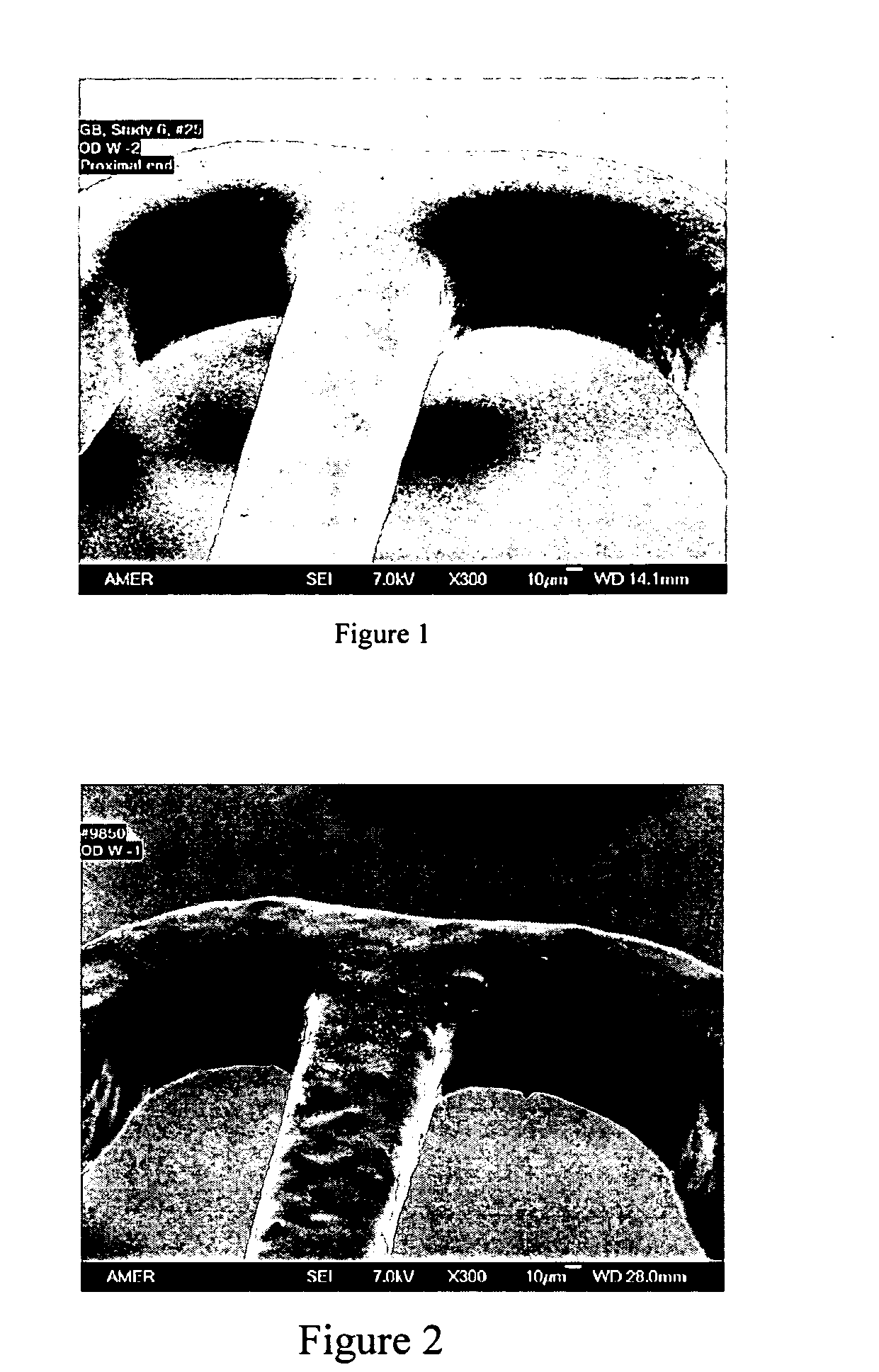
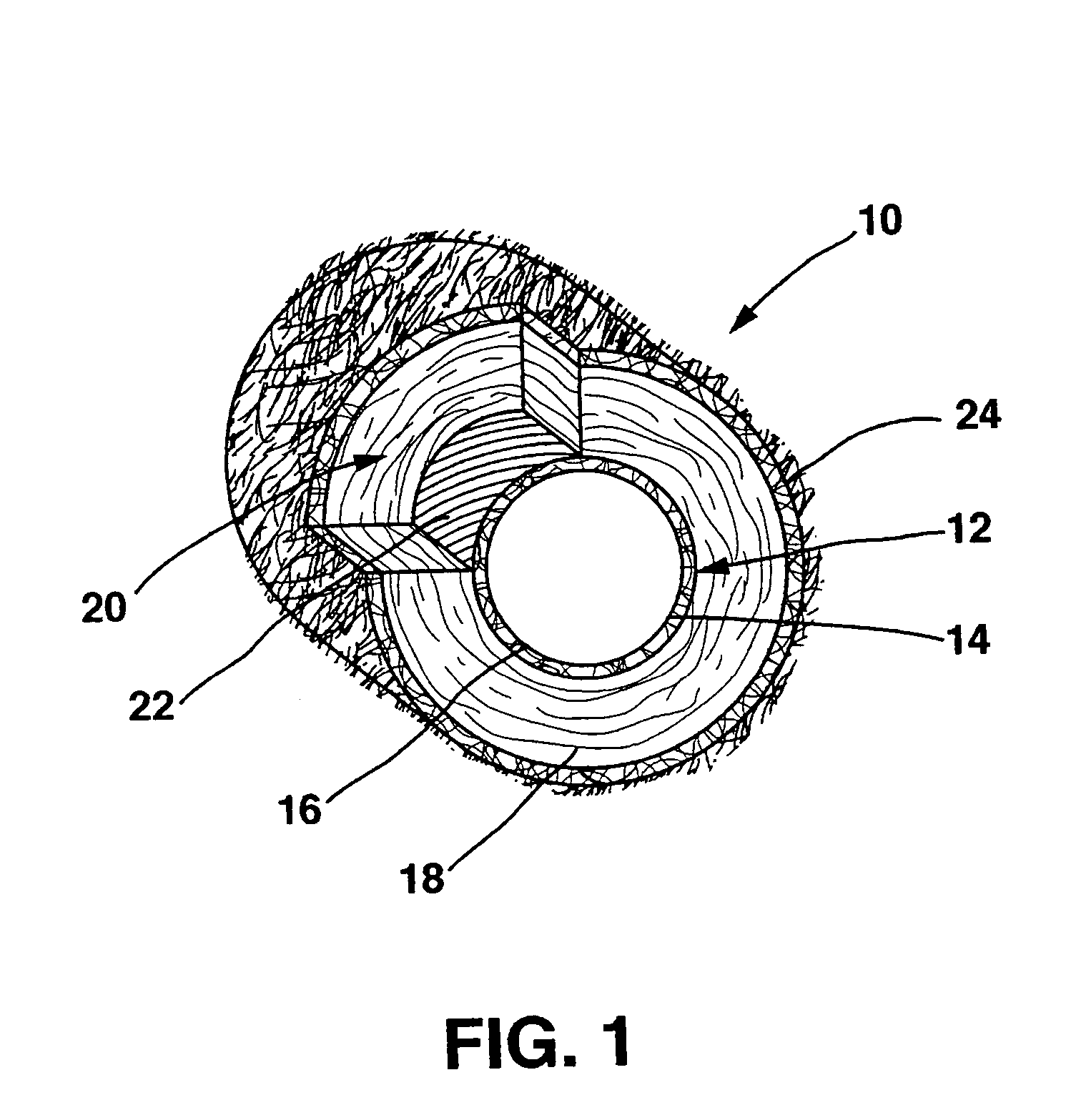
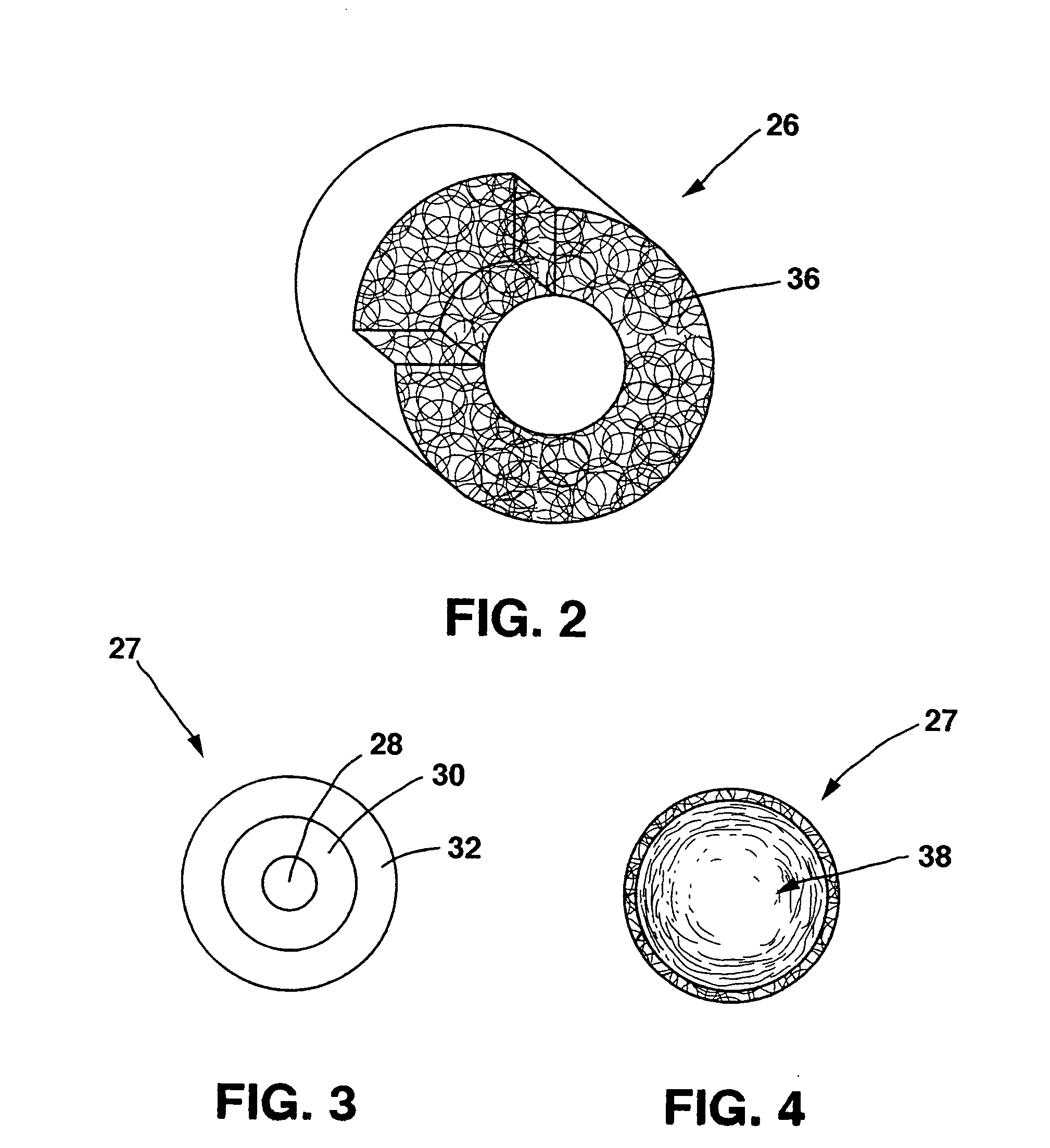
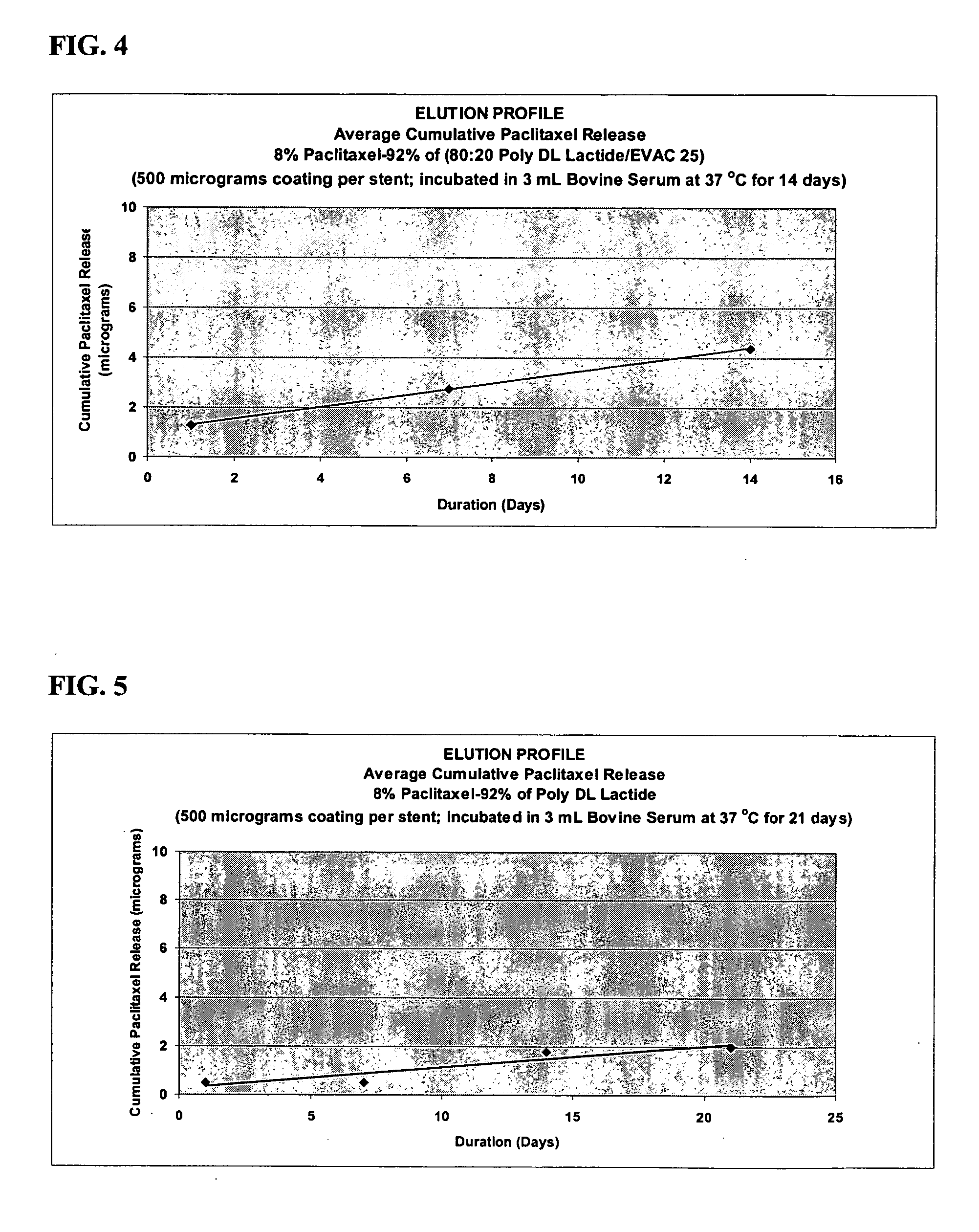
A drug eluting medical device is provided for implanting into vessels or luminal structures within the body of a patient. The coated medical device, such as a stent, vascular, or synthetic graft comprises a coating consisting of a controlled-release matrix of a bioabsorbable, biocompatible, bioerodible, biodegradable, nontoxic material, such as a Poly(DL-Lactide-co-Glycolide) polymer, and at least one pharmaceutical substance, or bioactive agent incorporated within the matrix or layered within layers of matrix. In particular, the drug eluting medical device when implanted into a patient, delivers the drugs or bioactive agents within the matrix to adjacent tissues in a controlled and desired rate depending on the drug and site of implantation.
Owner:ORBUSNEICH MEDICAL PTE LTD
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
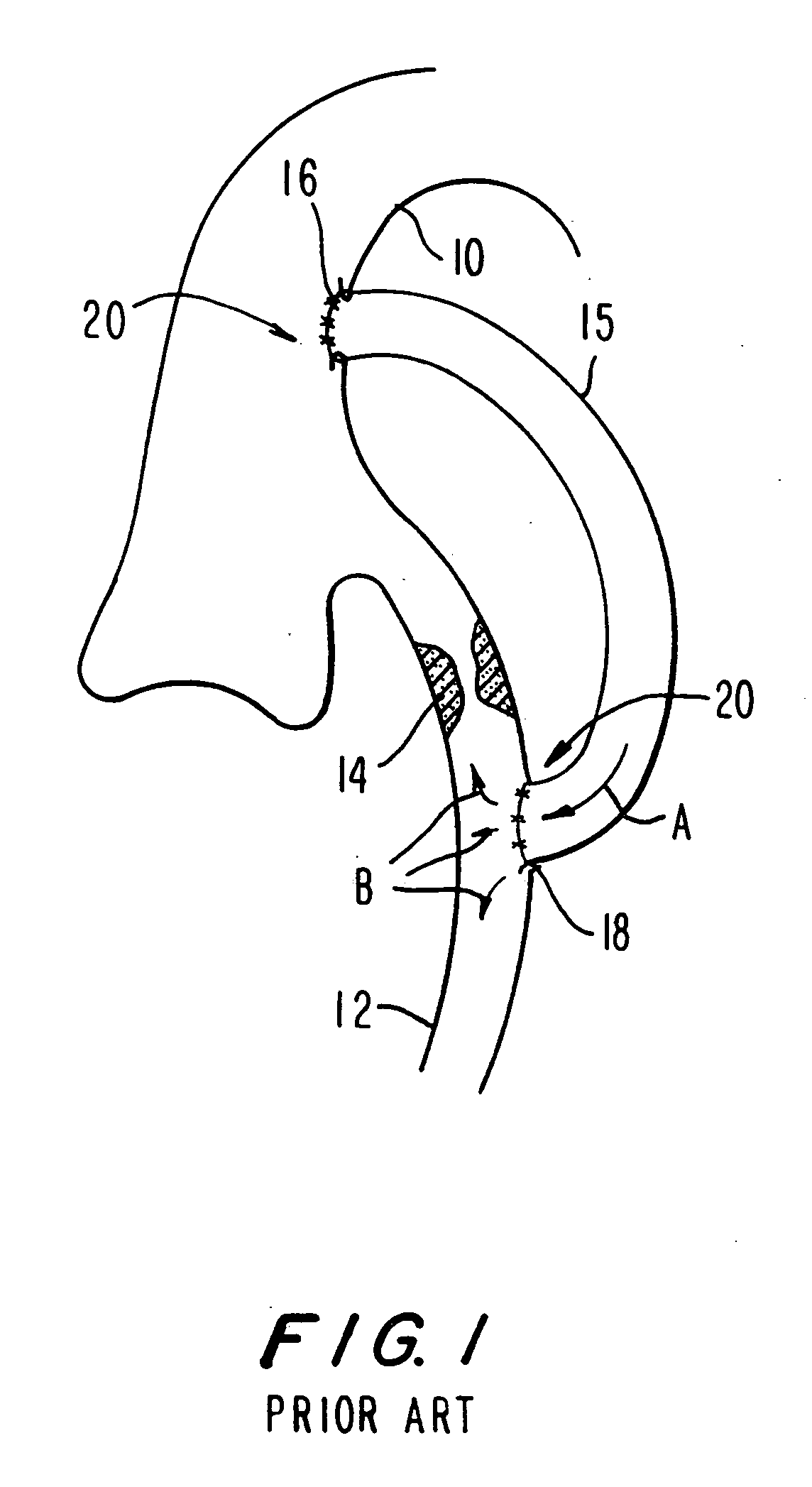
Method of securing tissue
Disclosed is an anastomosis catheter, for achieving a tissue to tissue or synthetic graft to tissue attachment. The catheter includes a plurality of deployable tissue anchors, which may be laterally deployed into surrounding tissue. The anchors may be used to achieve end to end or end to side anastomoses. Methods are also disclosed.
Owner:BOSTON SCI SCIMED INC
Progenitor endothelial cell capturing with a drug eluting implantable medical device
InactiveUS20050271701A1Increased formationSmooth migrationStentsHeart valvesProgenitorDrug-Coated Stents
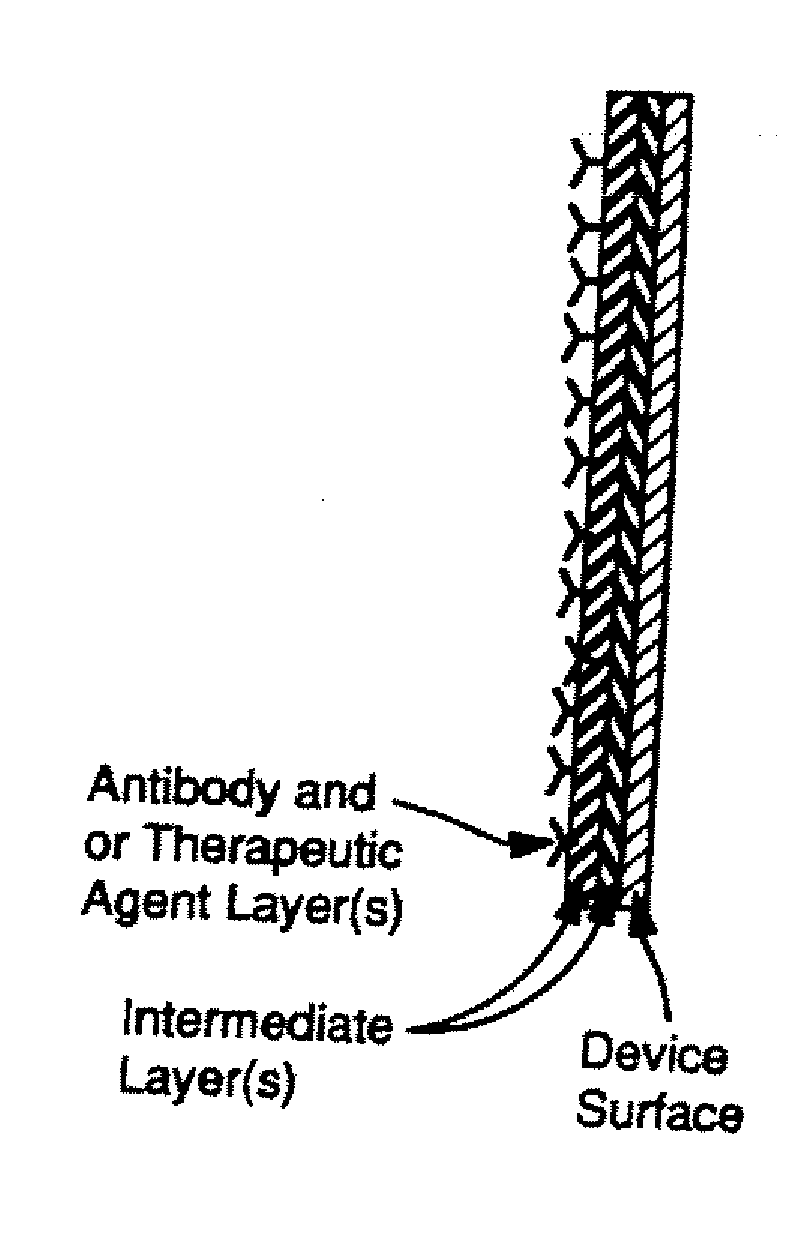
A medical device for implantation into vessels or luminal structures within the body is provided. The medical device, such as a stent and a synthetic graft, is coated with a pharmaceutical composition consisting of a controlled-release matrix and one or more pharmaceutical substances for direct delivery of drugs to surrounding tissues. The coating on the medical device further comprises a ligand such as an antibody or a small molecule for capturing progenitor endothelial cells in the blood contacting surface of the device for restoring an endothelium at the site of injury. In particular, the drug-coated stents are for use, for example, in balloon angioplasty procedures for preventing or inhibiting restenosis.
Owner:ORBUSNEICH MEDICAL PTE LTD
Medical device with coating that promotes endothelial cell adherence
InactiveUS7037332B2Prevent restenosisMaterial nanotechnologyPeptide/protein ingredientsCell Surface AntigensPercent Diameter Stenosis
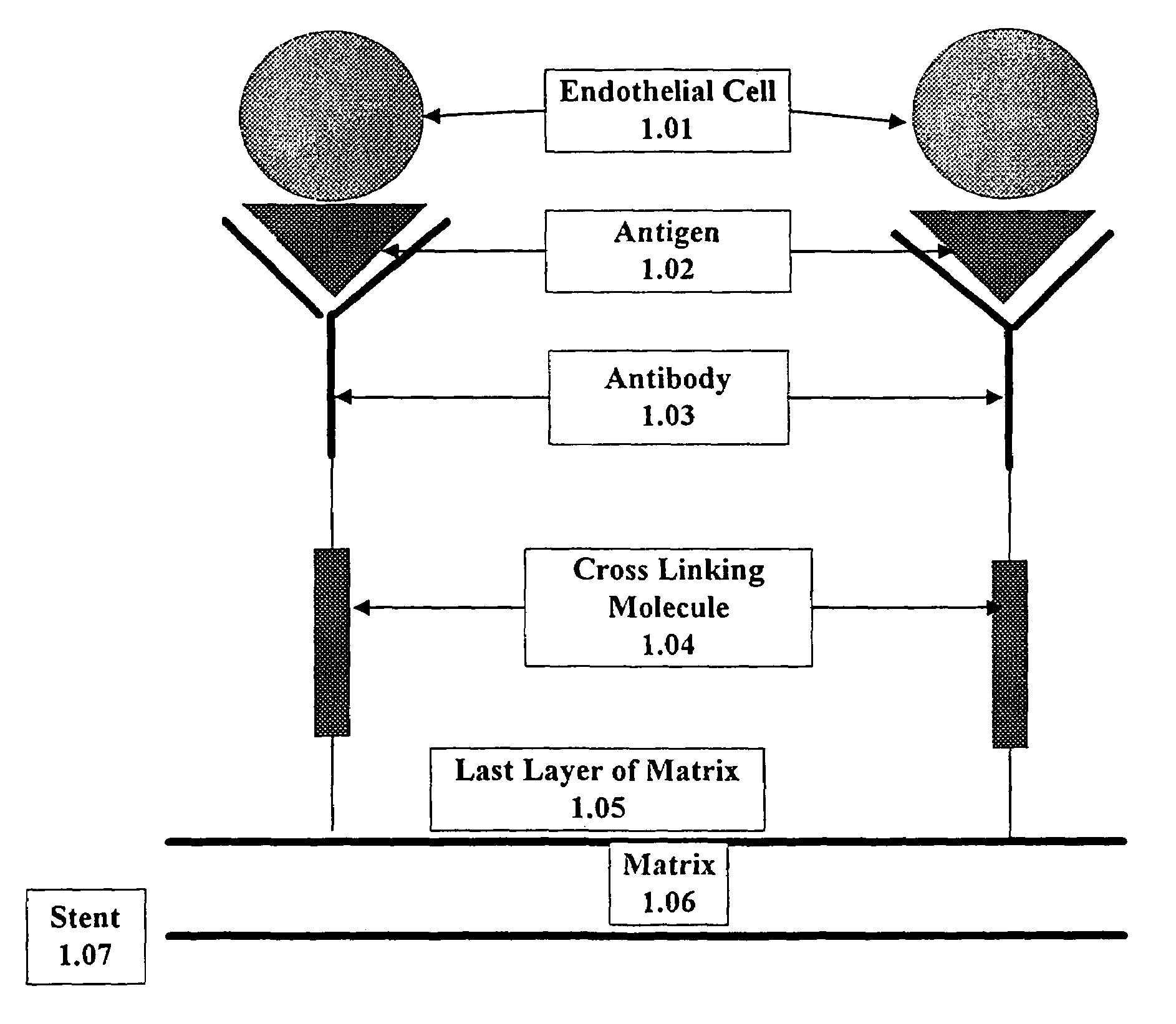
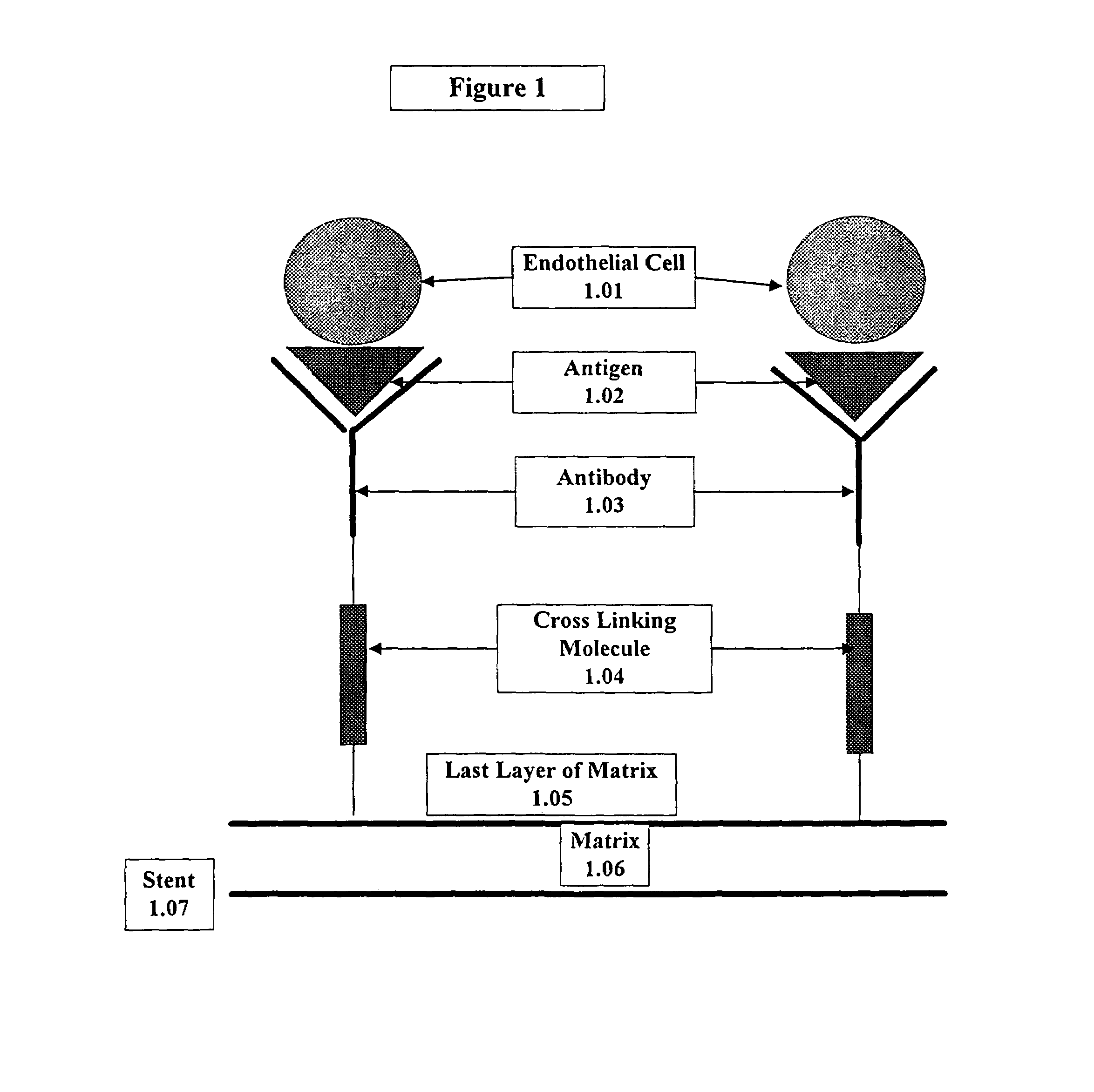
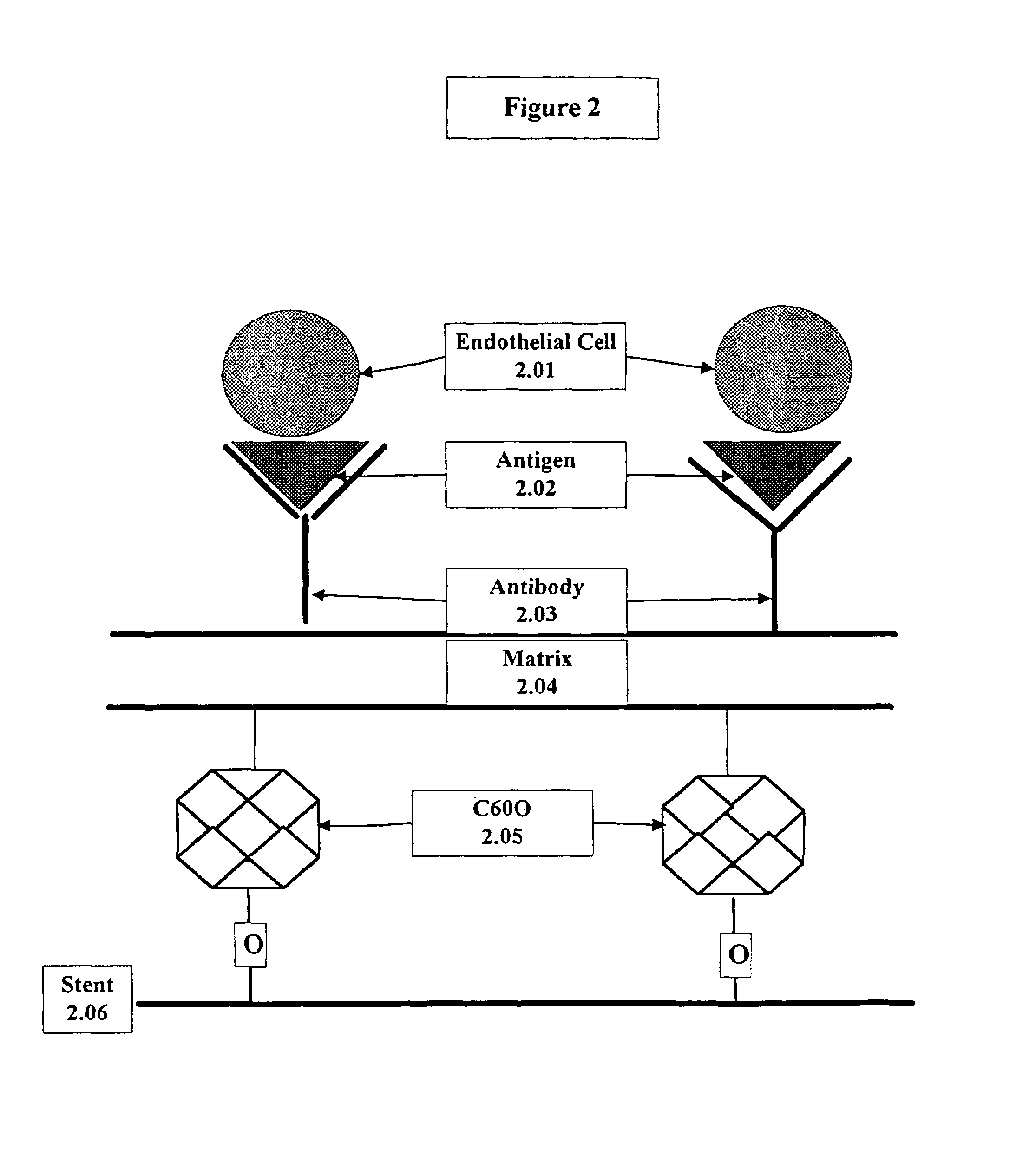
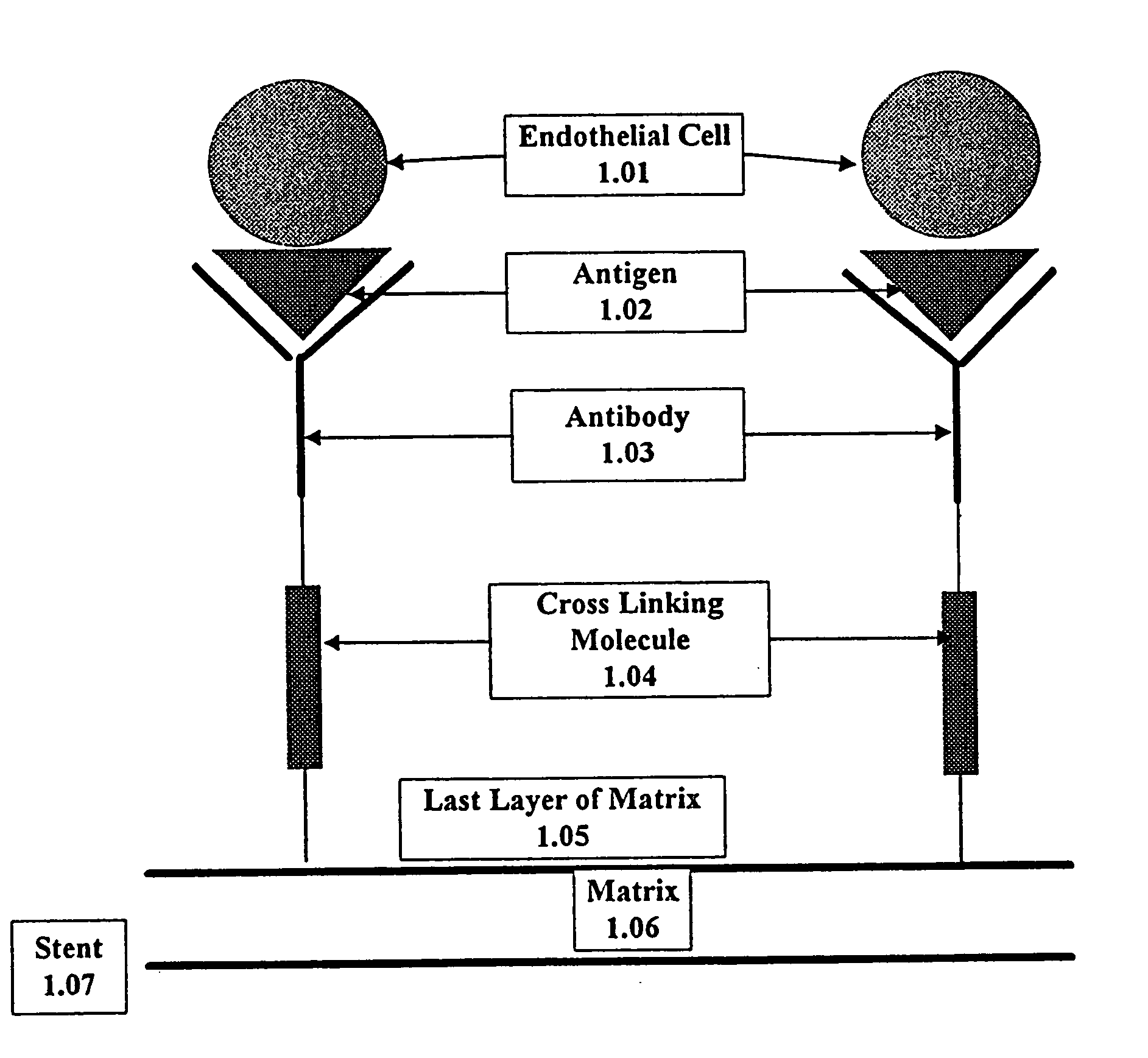
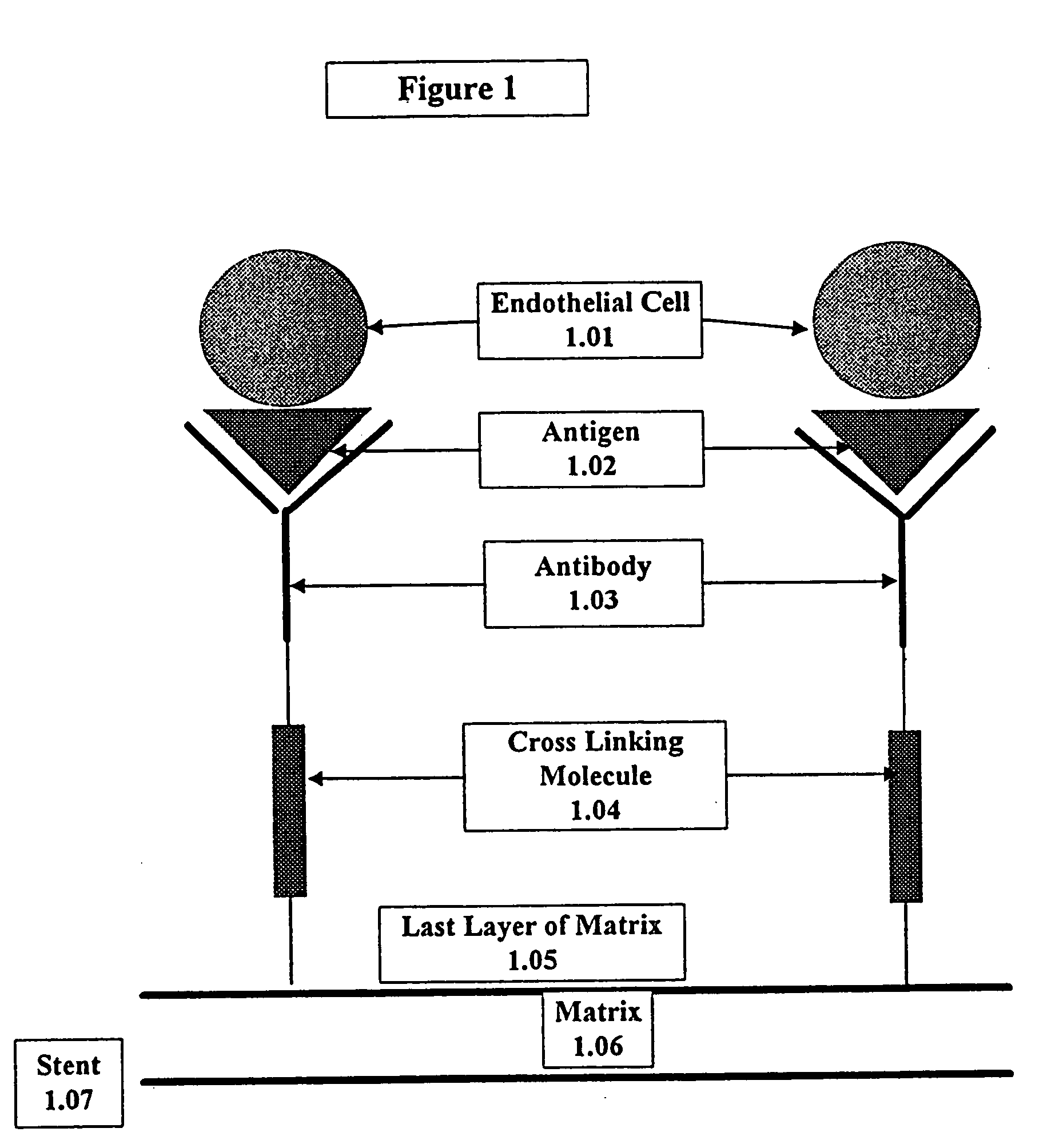
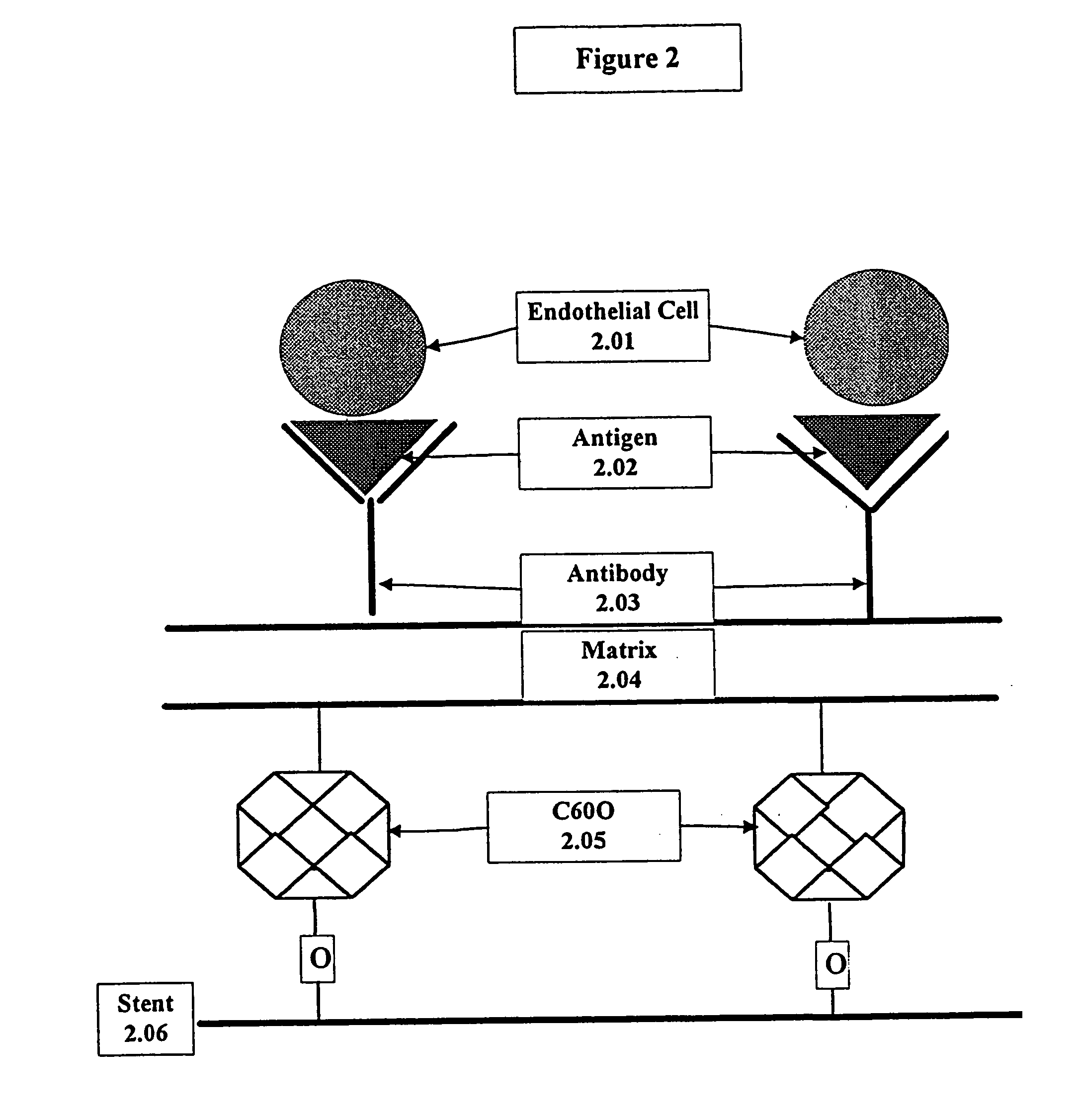
A medical device coated with one or more antibodies and one or more layers of a matrix is disclosed. The antibodies or fragments thereof react with an endothelial cell surface antigen. Also disclosed are compositions and methods for producing the medical device. The matrix coating the medical device may be composed of a synthetic material, such as a fullerene, or a naturally occurring material. The fullerenes range from about C60 to about C100. The medical device may be a stent or a synthetic graft. The antibodies promote the adherence of cells captured in vivo on the medical device. The antibodies may be mixed with the matrix or covalently tethered through a linker molecule to the matrix. Following adherence to the medical device, the cells differentiate and proliferate on the medical device. The antibodies may be different types of monoclonal antibodies. By facilitating adherence of cells to the surface of the medical device, the disclosed methods and compositions will decrease the incidence of restenosis as well as other thromboembolic complications resulting from implantation of medical devices.
Owner:ORBUSNEICH MEDICAL PTE LTD
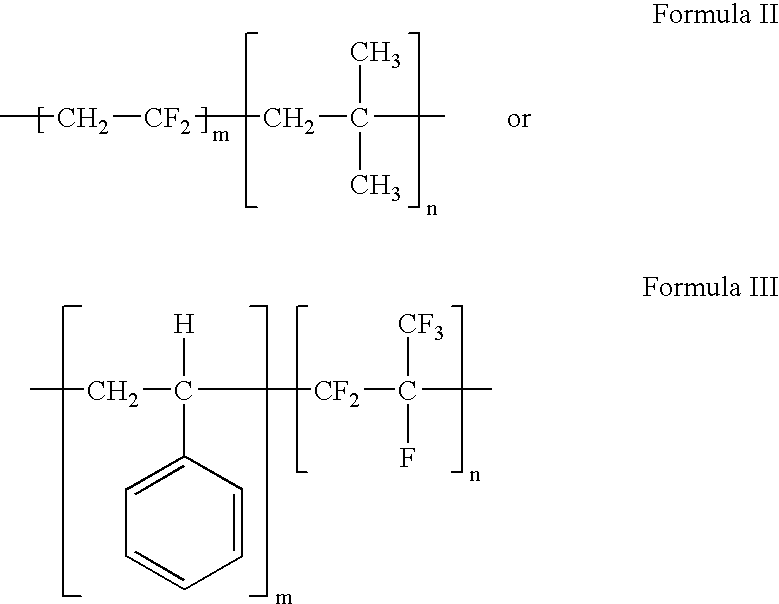
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS20060134165A1Provide mechanical strengthGive flexibilityStentsSurgeryAbnormal tissue growthPolymer science
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
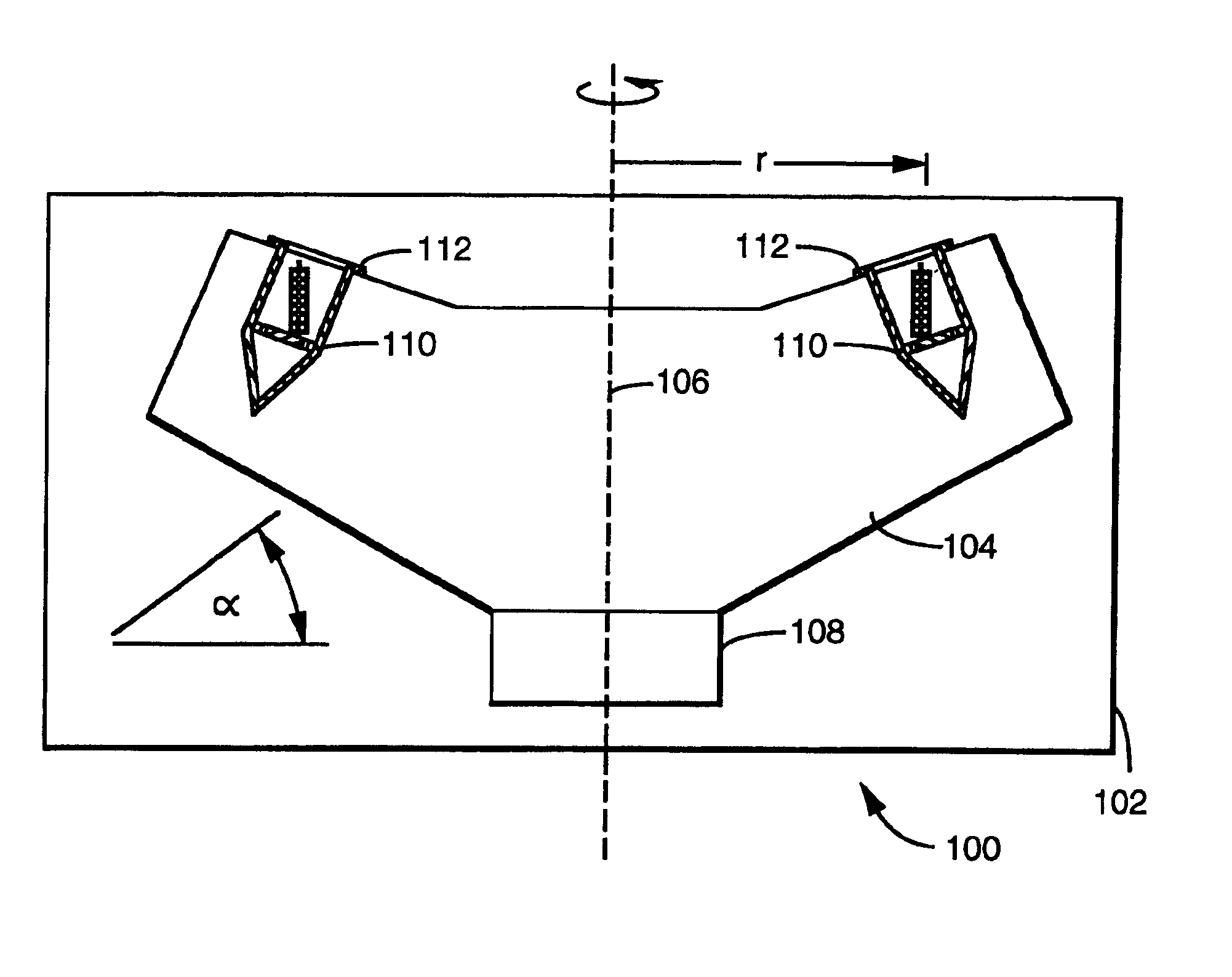
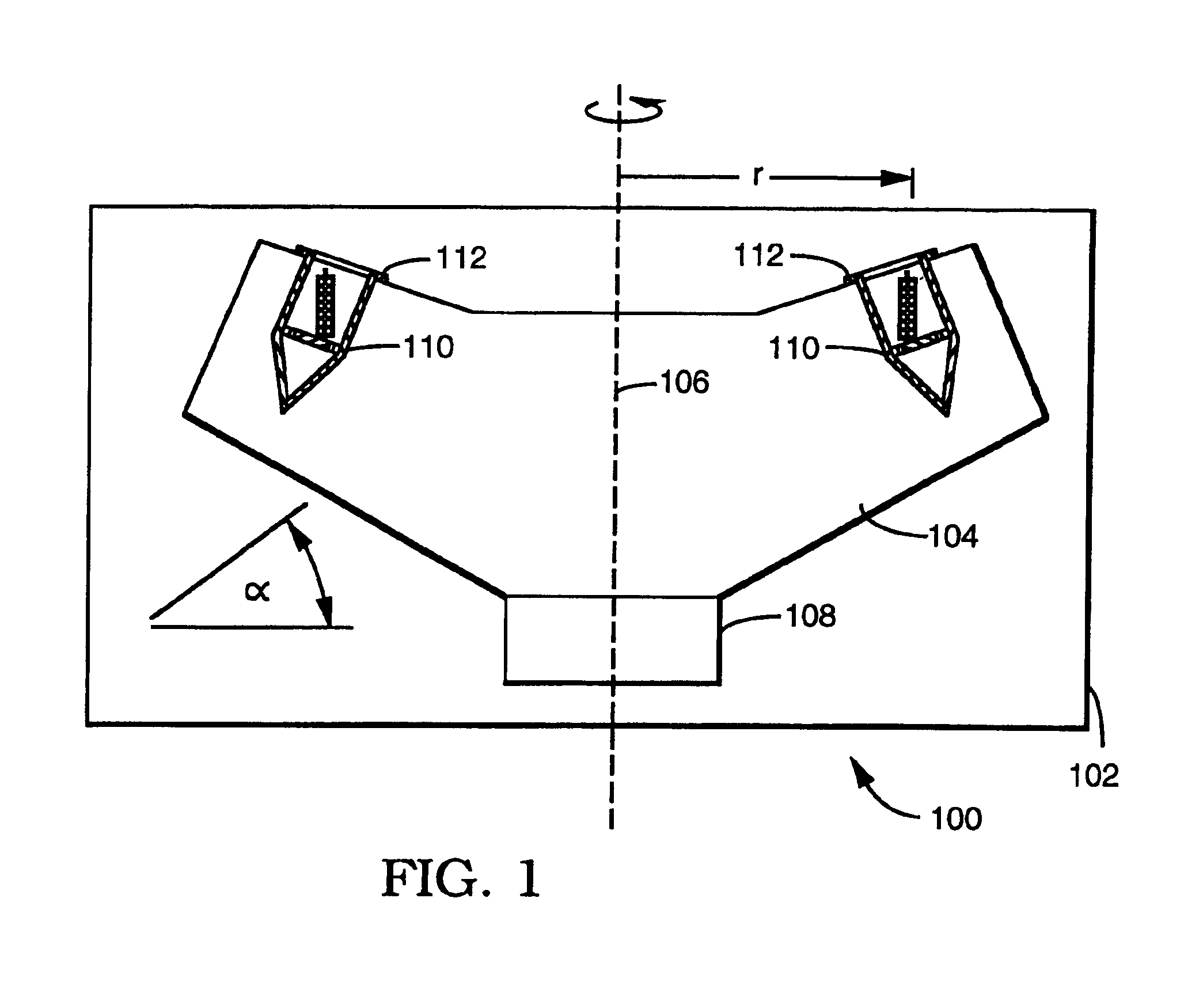
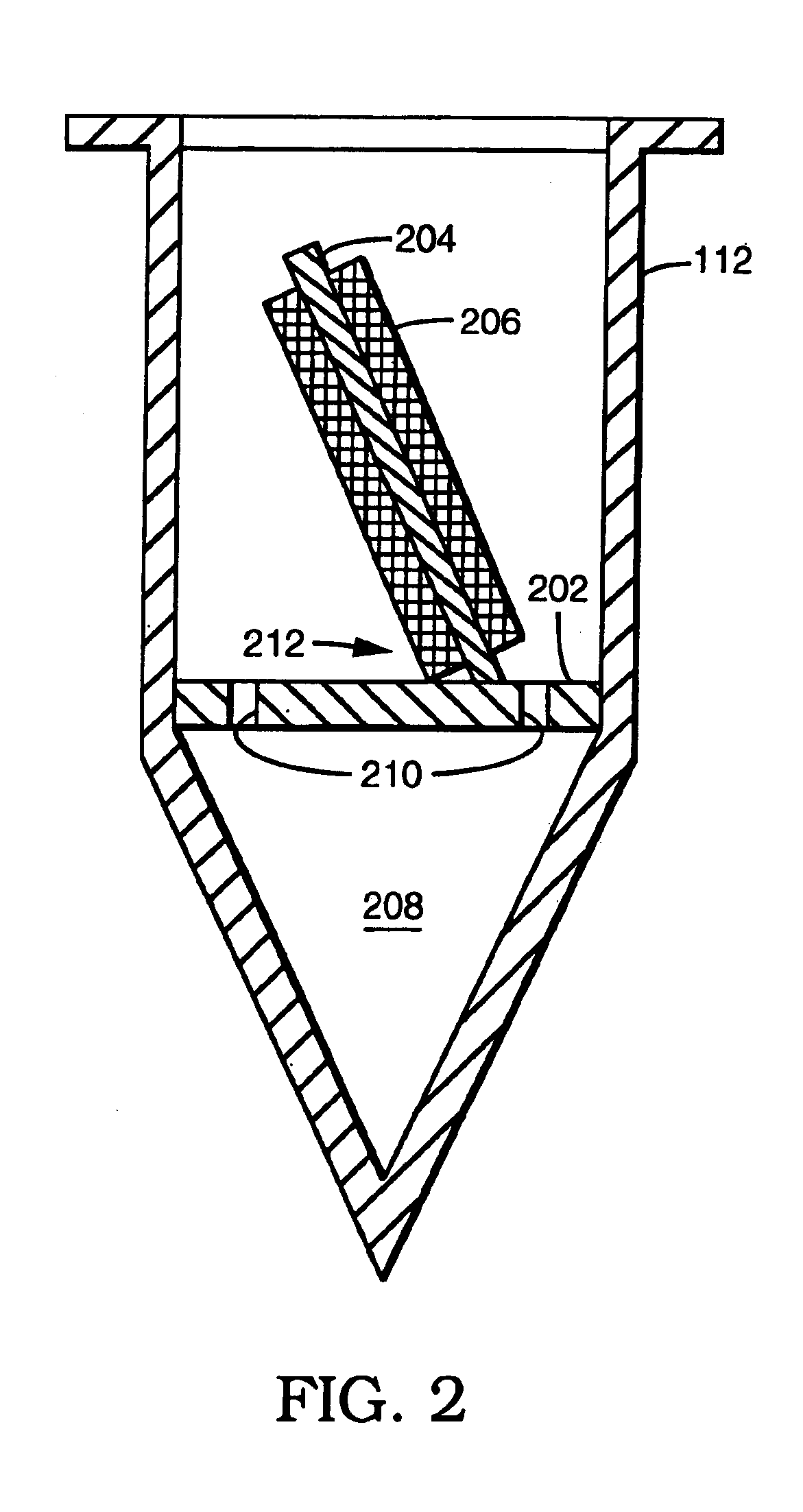
System for the process of coating implantable medical devices
Methods of coating an implantable device and a system for performing such methods are disclosed. An embodiment of the method includes applying a coating substance to the surface of an implantable device, and rotating the implantable device in a centrifuge. The method can uniformly coat the implantable device with the coating substance and to remove unwanted accumulations of coating substance entrained between struts or crevices in the implantable device body. This system is applicable to methods for coating intraluminal stents, synthetic grafts, and stent coverings with therapeutic compositions comprising therapeutic agents mixed with a polymeric matrix and a solvent.
Owner:ABBOTT CARDIOVASCULAR
Progenitor Endothelial Cell Capturing with a Drug Eluting Implantable Medical Device
InactiveUS20070123977A1Stimulating positive blood vessel remodelingEnhance and accelerate formationStentsSurgeryProgenitorDrug-Coated Stents
A medical device for implantation into vessels or luminal structures within the body is provided, which stimulates positive blood vessel remodeling. The medical device, such as a stent and a synthetic graft, is provided with a coating with a pharmaceutical composition containing a controlled-release matrix and one or more pharmaceutical substances for direct delivery of drugs to surrounding tissues. The coating on the medical device further comprises one or more barrier layers, and a ligand such as a peptide, an antibody or a small molecule for capturing progenitor endothelial cells in the blood contacting surface of the device for restoring an endothelium at the site of injury. In particular, the drug-coated stents are for use, for example, in balloon angioplasty procedures for preventing or inhibiting restenosis.
Owner:ORBUSNEICH MEDICAL PTE LTD
Progenitor Endothelial Cell Capturing with a Drug Eluting Implantable Medical Device
InactiveUS20070129789A1Stimulating positive blood vessel remodelingEnhance and accelerate formationOrganic active ingredientsSurgeryProgenitorDrug-Coated Stents
A medical device for implantation into vessels or luminal structures within the body is provided, which stimulates positive blood vessel remodeling. The medical device, such as a stent and a synthetic graft, is coated with a pharmaceutical composition consisting of a controlled-release matrix and one or more pharmaceutical substances for direct delivery of drugs to surrounding tissues. The coating on the medical device further comprises a ligand such as a peptide, an antibody or a small molecule for capturing progenitor endothelial cells in the blood contacting surface of the device for restoring an endothelium at the site of injury. In particular, the drug-coated stents are for use, for example, in balloon angioplasty procedures for preventing or inhibiting restenosis.
Owner:ORBUSNEICH MEDICAL PTE LTD
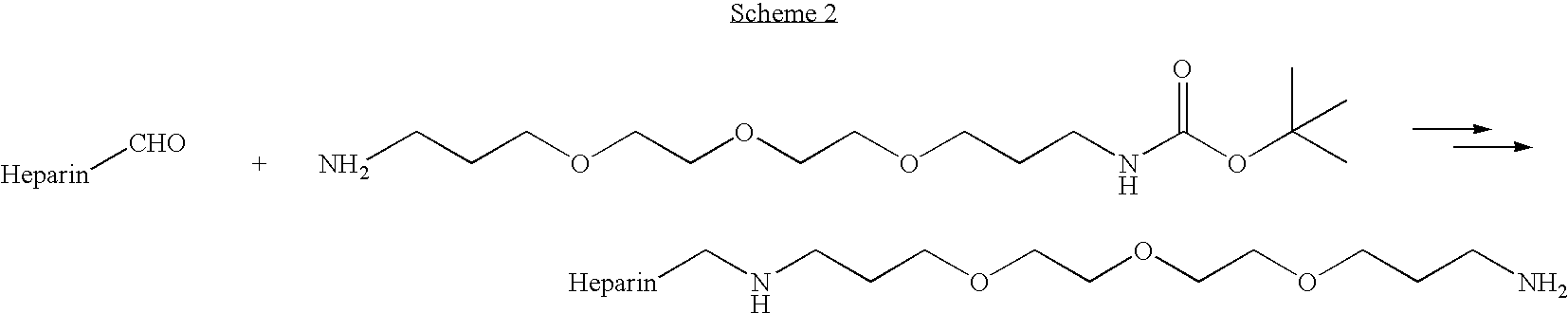
Antifouling heparin coatings
A medical device comprising a coating thereon comprising a biocompatible polymer and heparin is provided herein. Heparin is coupled with the biocompatible polymer via a spacer having a grouping that renders a binding site of the heparin molecule accessible by a binding protein. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Medical device with coating that promotes endothelial cell adherence
InactiveUS20050043787A1Prevent restenosisPreventing other thromboembolic complicationMaterial nanotechnologyPeptide/protein ingredientsAntigenPolyethylene glycol
This invention provides compositions and methods for producing a medical device coated with a matrix and an antibody which reacts with an endothelial cell antigen. The matrix coating the medical device may be composed of synthetic material, such as polyurethane, poly-L-lactic acid, cellulose ester or polyethylene glycol. In another embodiment, the matrix is composed of naturally occurring materials, such as collagen, fibrin, elastin, amorphous carbon. In a third embodiment, the matrix may be composed of fullerenes. The fullerenes range from about C60 to about C100. The medical device may be a stent or a synthetic graft. The antibodies promote adherence of endothelial cells on the medical device. The antibodies may be mixed with the matrix or covalently tethered through a linker molecule to the matrix. Following adherence to the medical device, the endothelial cells differentiate and proliferate on the medical device. The antibodies may be different types of monoclonal antibodies. Methods of preparing such composition and methods of treating a mammal with atherosclerosis or other types of vessel obstruction are disclosed. By facilitating adherence of endothelial cells to the surface of the medical device, the methods and compositions of this invention will decrease the incidence of restenosis as well as other thromboembolic complications resulting from implantation of medical devices.
Owner:ORBUSNEICH MEDICAL PTE LTD
Plasticizers for coating compositions
A biocompatible plasticizer useful for forming a coating composition with a biocompatible polymer is provided. The coating composition may also include a biobeneficial polymer and / or a bioactive agent. The coating composition can form a coating on an implantable device. The implantable device can be used to treat or prevent a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
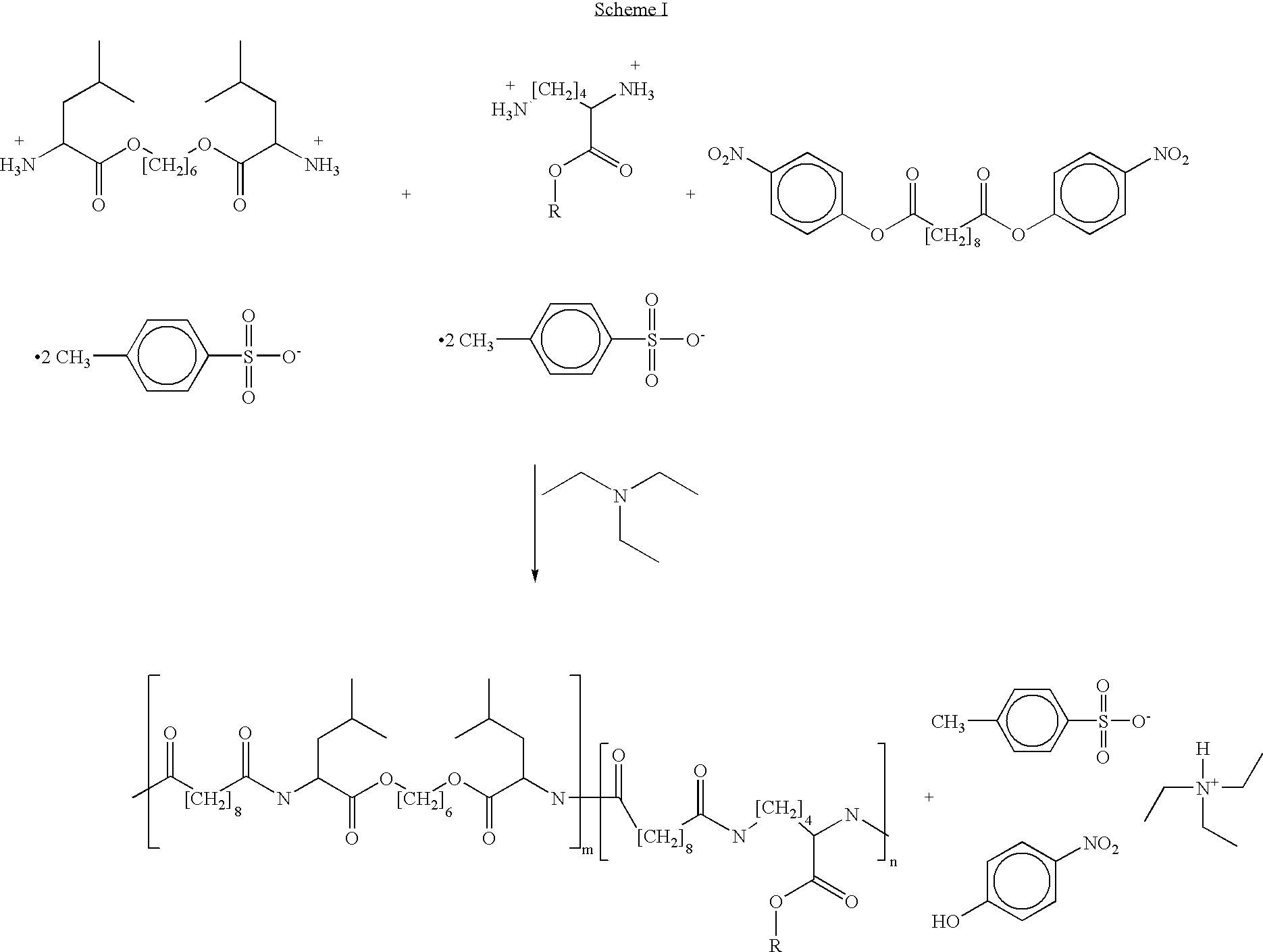
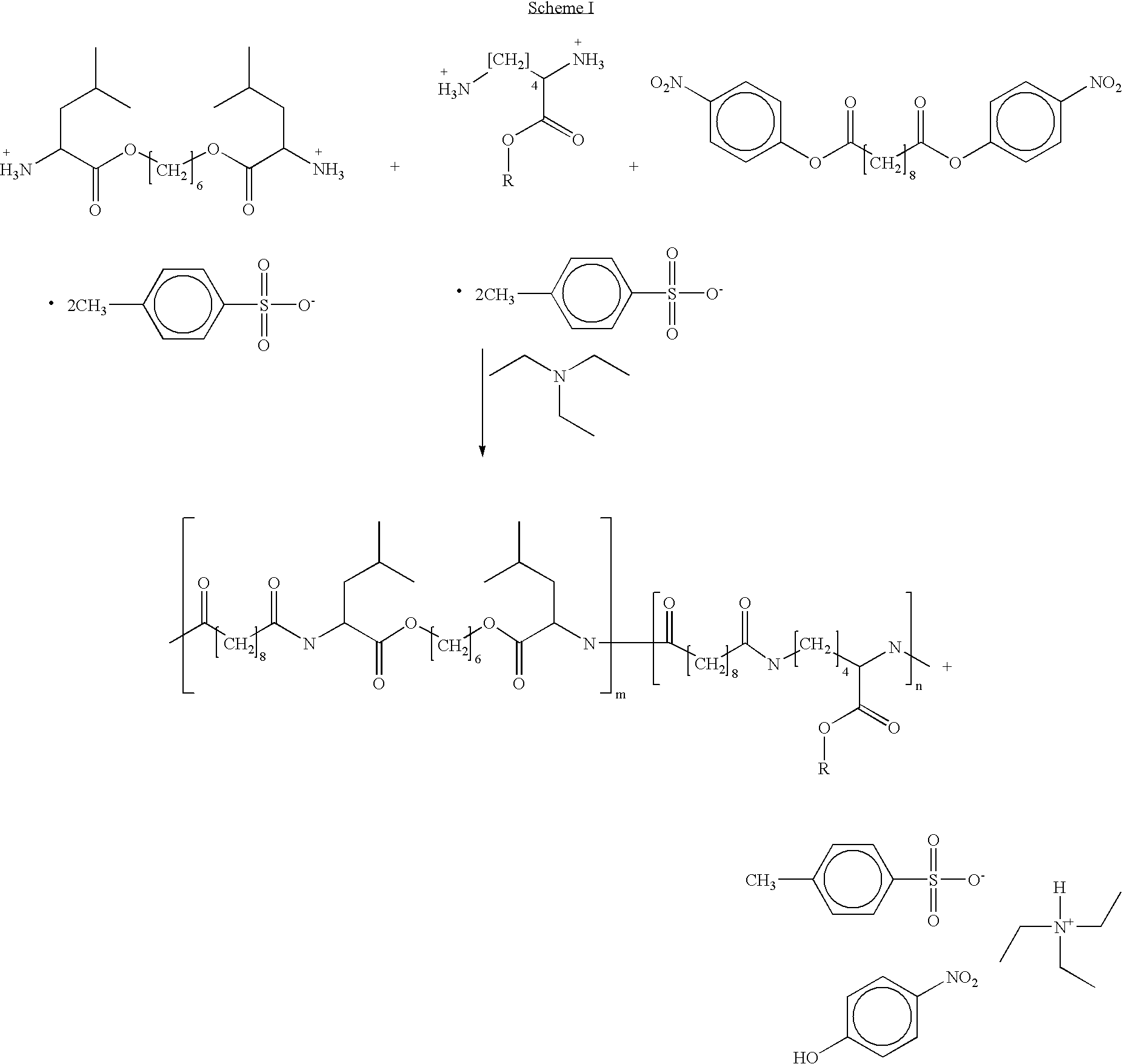
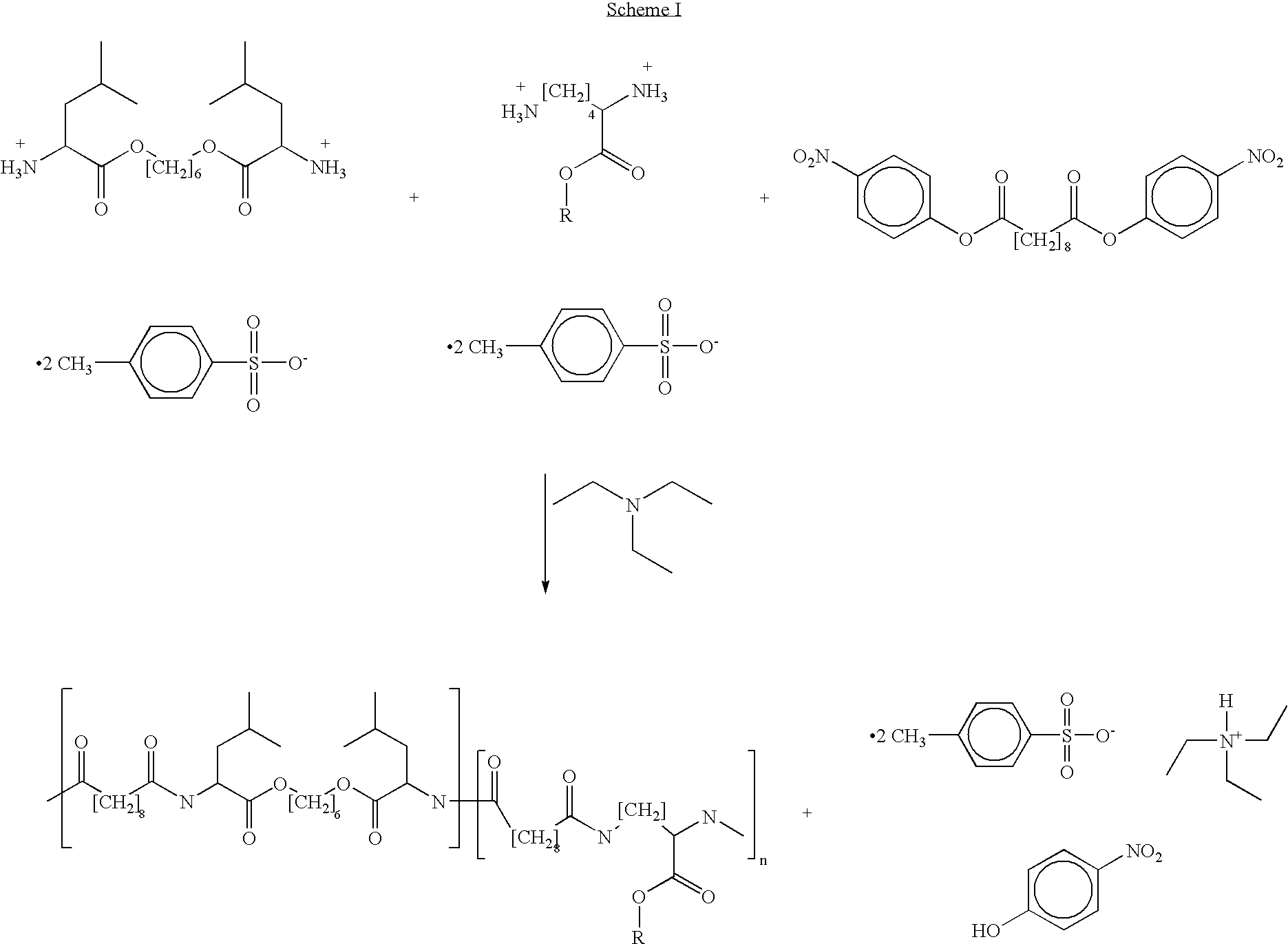
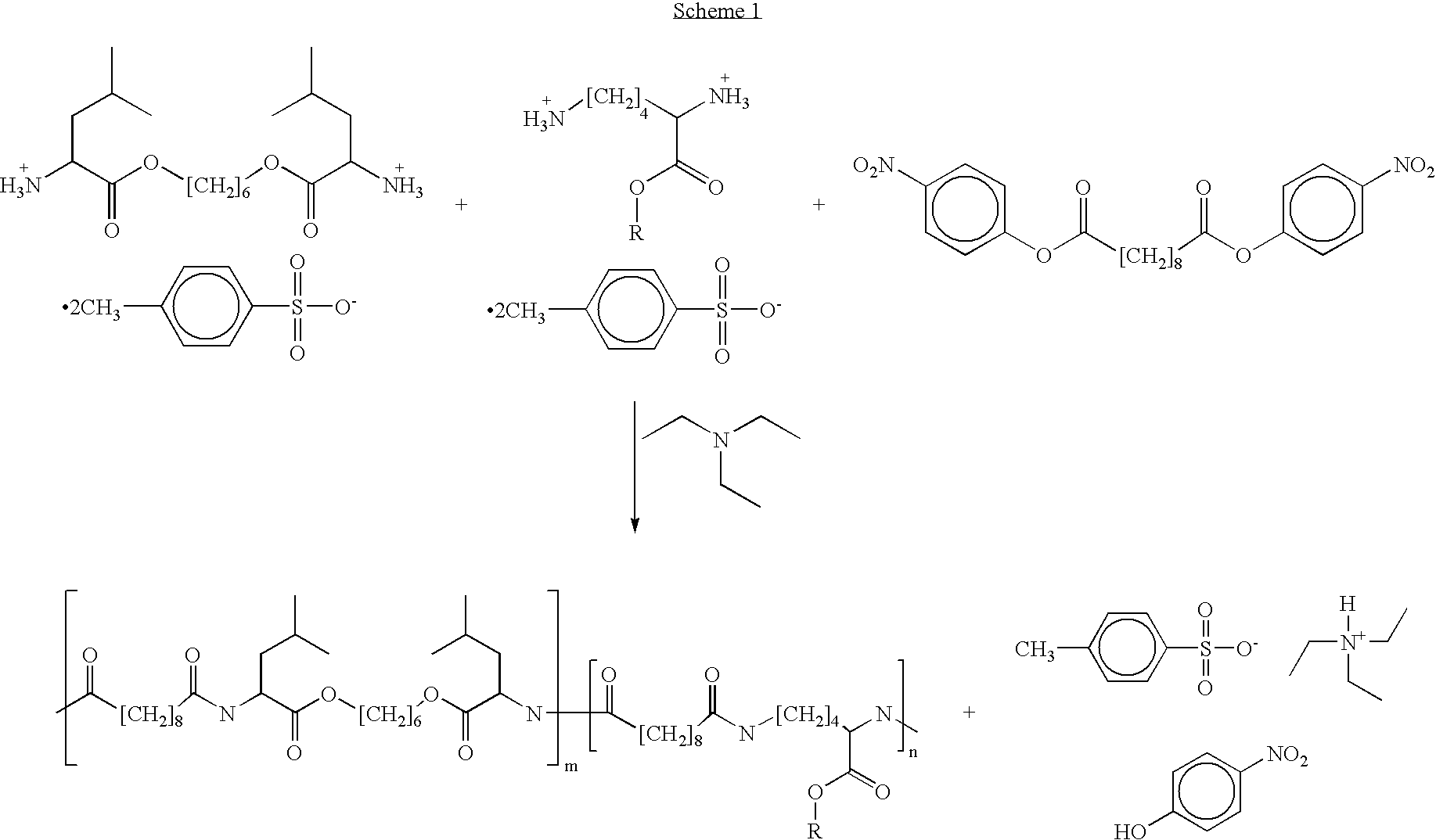
Blends of poly(ester amide) polymers
ActiveUS7166680B2Improved stability and drug release rate and mechanical characteristicReduce degradationSurgeryCatheterDiseasePEA polymer
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Progenitor Endothelial Cell Capturing with a Drug Eluting Implantable Medical Device
InactiveUS20070141107A1Stimulating positive blood vessel remodelingEnhance and accelerate formationStentsSurgeryProgenitorDrug-Coated Stents
A medical device for implantation into vessels or luminal structures within the body is provided, which stimulates positive blood vessel remodeling. The medical device, such as a stent and a synthetic graft, is provided with a coating with a pharmaceutical composition containing a controlled-release matrix and one or more pharmaceutical substances for direct delivery of drugs to surrounding tissues. The coating on the medical device further comprises one or more barrier layers, and a ligand such as a peptide, an antibody or a small molecule for capturing progenitor endothelial cells in the blood contacting surface of the device for restoring an endothelium at the site of injury. In particular, the drug-coated stents are for use, for example, in balloon angioplasty procedures for preventing or inhibiting restenosis.
Owner:ORBUSNEICH MEDICAL PTE LTD
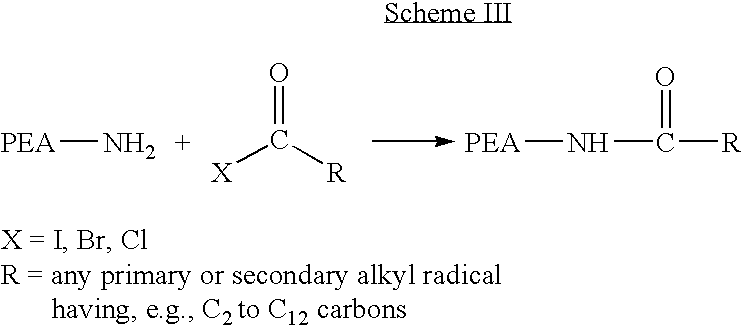
End-capped poly(ester amide) copolymers
Provided herein is an end-capped poly(ester amide) PEA) polymer and the method of making the polymer. The PEA polymer is substantially free of active amino end groups and / or activated carboxyl groups. The PEA polymer can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Blends of poly(ester amide) polymers
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
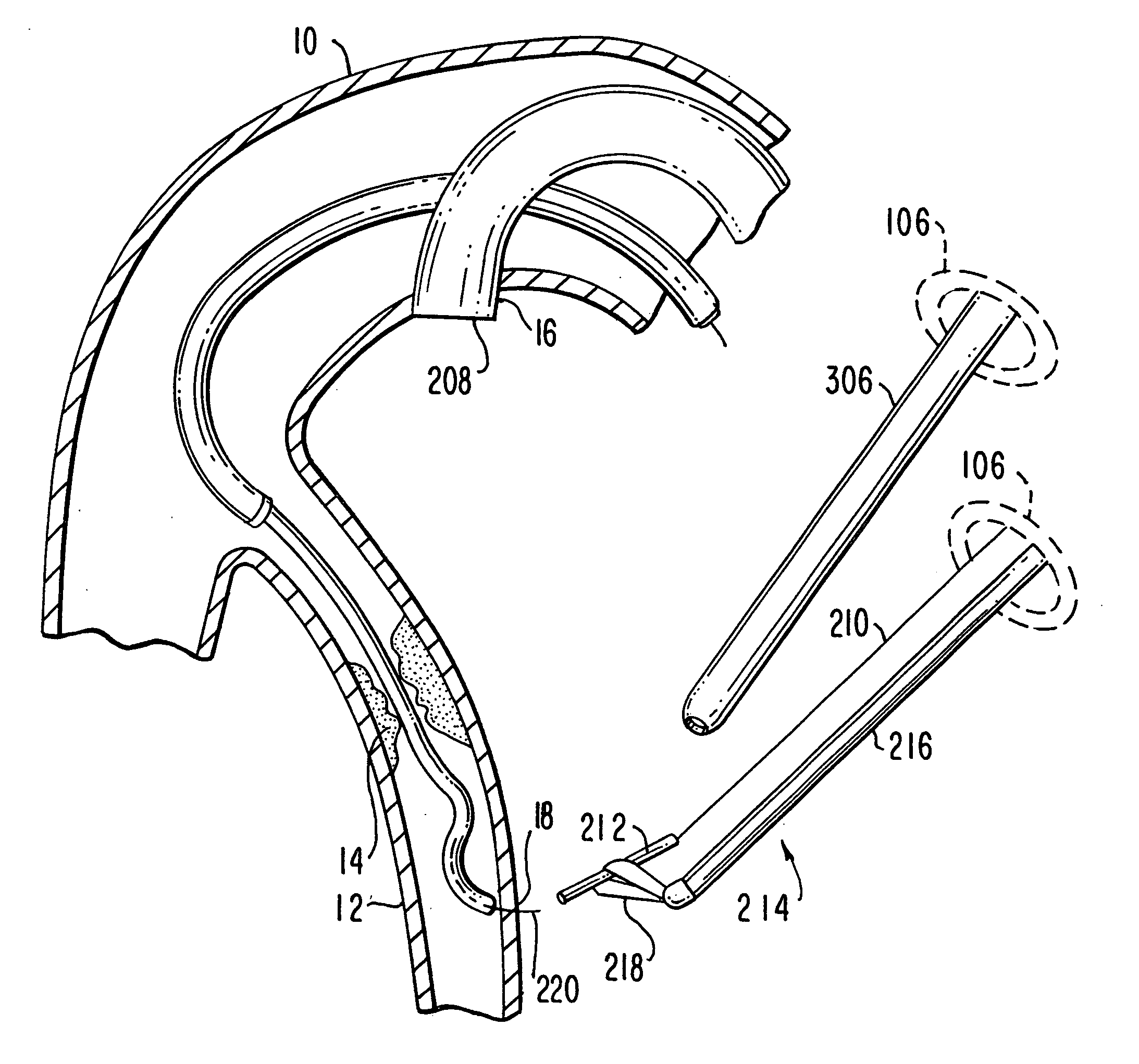
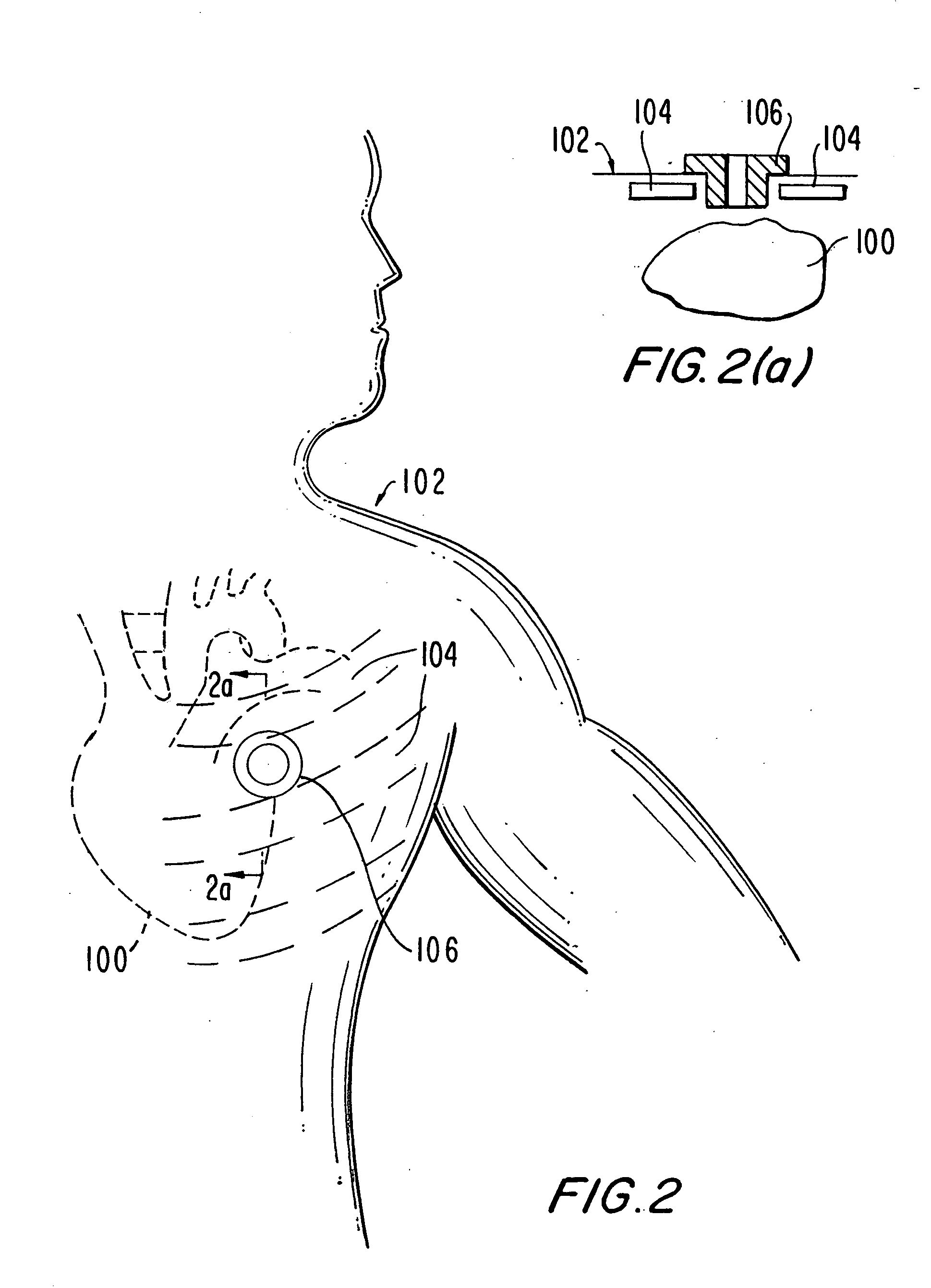
Medical grafting methods and apparatus
Methods and apparatus for delivering and installing a new length of tubing between two sections of a patient's existing body organ tubing and at least partly outside of that existing structure. For example, the new length of tubing may be for the purpose of providing the patient with a coronary bypass. The new tubing may be an artificial graft, a natural graft (harvested elsewhere from the patient), or both. The new tubing is installed at the operative site primarily by providing at least one graft location with instrumentation inserted through the patient's existing tubular body organ structure. Assistance in installing the new tubing may be provided by minimally invasive surgical access openings in the patient's chest. The tubing may be delivered through the patient's existing tubular body structure or, alternatively, through the surgical access openings.
Owner:ST JUDE MEDICAL ATG
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
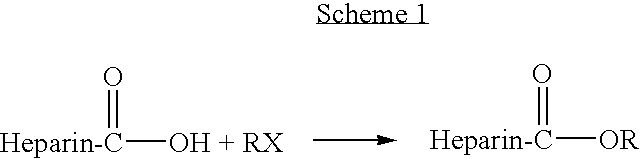
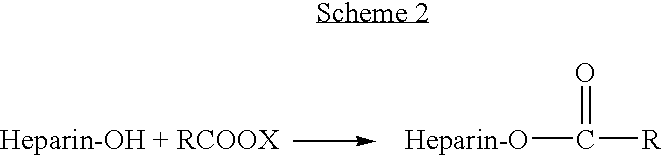
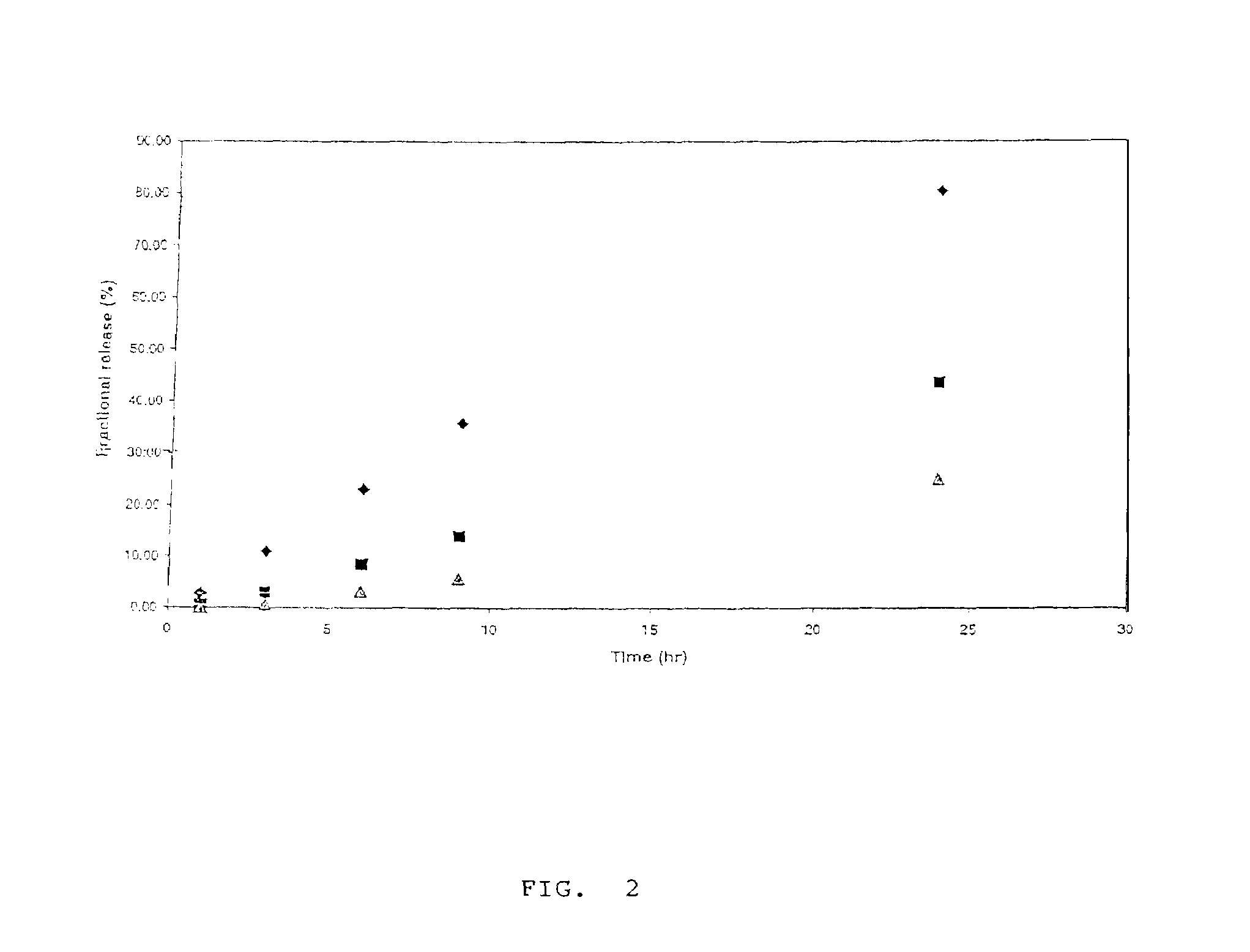
Heparin prodrugs and drug delivery stents formed therefrom
InactiveUS20060014720A1Organic active ingredientsPeptide/protein ingredientsDiseasePercent Diameter Stenosis
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS7390497B2Improved stability and drug release rate and mechanical characteristicOrganic active ingredientsNervous disorderPEA polymerDevice form
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) block copolymers
Owner:ABBOTT CARDIOVASCULAR
Progenitor Endothelial Cell Capturing with a Drug Eluting Implantable Medical Device
InactiveUS20070128723A1Stimulating positive blood vessel remodelingEnhance and accelerate formationStentsGenetically modified cellsAntiendomysial antibodiesEngineering
A medical device for implantation into vessels or luminal structures within the body is provided, which stimulates positive blood vessel remodeling. The medical device, such as a stent and a synthetic graft, is coated with a pharmaceutical composition consisting of a controlled-release matrix and one or more pharmaceutical substances for direct delivery of drugs to surrounding tissues. The coating on the medical device further comprises a ligand such as a peptide, an antibody or a small molecule for capturing progenitor endothelial cells in the blood contacting surface of the device for restoring an endothelium at the site of injury. In particular, the drug-coated stents are for use, for example, in balloon angioplasty procedures for preventing or inhibiting restenosis.
Owner:ORBUSNEICH MEDICAL PTE LTD
Transmural concentric multilayer ingrowth matrix within well-defined porosity
A multilayer ingrowth matrix is constructed within well-defined porosity of a prosthetic material. The matrix consists of either proteinaceous or synthetic layers or gradients, or a combination of proteinaceous and synthetic layers or gradients. Each layer within the matrix is designed to achieve a specific function, such as facilitation of ingrowth of a particular cell type or release of a particular growth factor. The well-defined porosity is in the form of either helically oriented, interconnected transmural ingrowth channels, or a porous wall structure containing uniformly shaped pores (i.e. voids) in a very narrow size range, or a combination of channels and pores. This invention allows for uninterrupted ingrowth of connective tissue into walls of a synthetic graft prosthesis made from the prosthetic material. Furthermore, this invention can produce small diameter prostheses having an internal diameter of 6 mm or less.
Owner:MEDTRONIC INC
Drug eluting implantable medical device
A drug eluting medical device is provided for implanting into vessels or luminal structures within the body of a patient. The coated medical device, such as a stent, vascular, or synthetic graft comprises a coating consisting of a controlled-release matrix of a bioabsorbable, biocompatible, bioerodible, biodegradable, nontoxic material, such as a Poly(DL-Lactide-co-Glycolide) polymer, and at least one pharmaceutical substance, or bioactive agent incorporated within the matrix or layered within layers of matrix. In particular, the drug eluting medical device when implanted into a patient, delivers the drugs or bioactive agents within the matrix to adjacent tissues in a controlled and desired rate depending on the drug and site of implantation.
Owner:ORBUSNEICH MEDICAL PTE LTD
Medical grafting methods and apparatus
InactiveUS20060161195A1Enhance their bio-utilityElasticityStentsBalloon catheterBody organsCoronary arteries
Methods and apparatus for delivering and installing a new length of tubing between two sections of a patient's existing body organ tubing and at least partly outside of that existing structure. For example, the new length of tubing may be for the purpose of providing the patient with a coronary bypass. The new tubing may be an artificial graft, a natural graft (harvested elsewhere from the patient), or both. The new tubing is delivered to and installed at the operative site primarily by working through the patient's existing tubular body organ structure. This avoids the need for any significant surgery on the patient. The artificial grafts may have shapes other than tubular. Certain procedural and apparatus aspects of the invention have uses other than in connection with grafting in general or tubular grafting in particular.
Owner:DONGBU HITEK CO LTD
Progenitor endothelial cell capturing with a drug eluting implantable medical device
InactiveUS8088060B2Stimulating positive blood vessel remodelingEnhance and accelerate formationStentsSurgeryProgenitorDrug-Coated Stents
Owner:ORBUSNEICH MEDICAL PTE LTD
Progenitor endothelial cell capturing with a drug eluting implantable medical device
InactiveUS20120172970A1Stimulating positive blood vessel remodelingAvoid dissectionStentsSurgeryProgenitorDrug-Coated Stents
A medical device for implantation into vessels or luminal structures within the body is provided, which stimulates positive blood vessel remodeling. The medical device, such as a stent and a synthetic graft, is provided with a coating with a pharmaceutical composition containing a controlled-release matrix and one or more pharmaceutical substances for direct delivery of drugs to surrounding tissues. The coating on the medical device further comprises one or more barrier layers, and a ligand such as a peptide, an antibody or a small molecule for capturing progenitor endothelial cells in the blood contacting surface of the device for restoring an endothelium at the site of injury. In particular, the drug-coated stents are for use, for example, in balloon angioplasty procedures for preventing or inhibiting restenosis.
Owner:ORBUSNEICH MEDICAL PTE LTD
Polymers containing siloxane monomers
Owner:ABBOTT CARDIOVASCULAR
Artificial nerve graft constructed based on chip-type acellularized scaffold and preparation method of artificial nerve graft
ActiveCN106730034AAvoid the disadvantages that if the strength is too small, it will easily remain, and if the strength is too high, it will be crushedImproved cell loading capacityTissue regenerationProsthesisAdhesiveSpinal cord
The invention relates to an artificial nerve graft constructed based on a chip-type acellularized scaffold and a preparation method of the artificial nerve graft, belonging to the field of tissue engineering. According to the artificial nerve graft and the preparation method, heterogeneous animal medulla spinalis, skeletal muscle or tendon acellularized tissue slices are adopted as a bracket, genipin is adopted for crosslinking cytokines, autologous ecto mesenchymal stem cells are planted on the bracket, and fibrous protein is taken as an adhesive for constructing the artificial nerve graft, so that the repairing of the nervous tissue is promoted. The artificial nerve graft and the preparation method have the advantages that the preparation cost is low, the period is short, the preparation raw materials are easily available, and the preparation is simple and is easy to operate. The constructed artificial nerve graft has low immunogenicity, contains a large number of autologous stem cells and cell factors, and is beneficial for adjusting the nerve injury microenvironment, and promoting the nerve regeneration and myelinogenesis. The artificial nerve graft can be used for preparing peripheral nerve and medulla spinalis defect, and has the broad application prospect.
Owner:JIANGSU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com