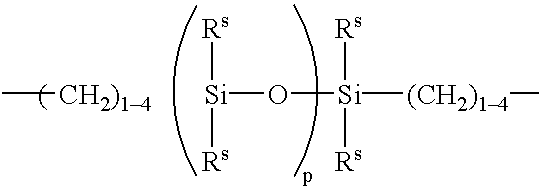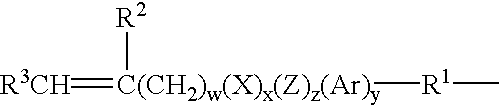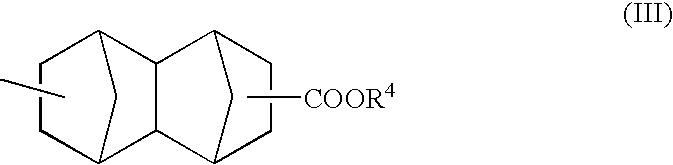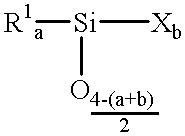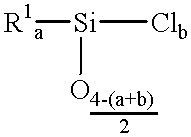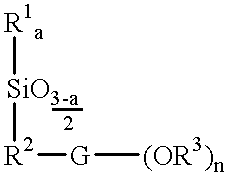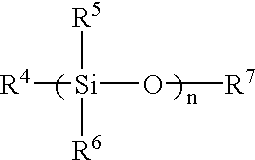Patents
Literature
8752 results about "Methacrylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
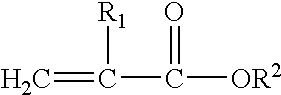
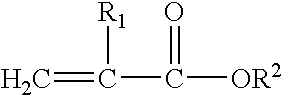
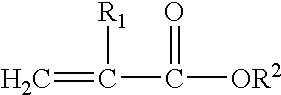
Methacrylate refers to derivatives of methacrylic acid. These derivatives include the parent acid (CH₂C(CH₃)CO₂H), salts (e.g., CH₂C(CH₃)CO⁻₂Na⁺), esters (e.g. CH₂C(CH₃)CO₂CH₃, or methyl methacrylate) and the polymers of these species.
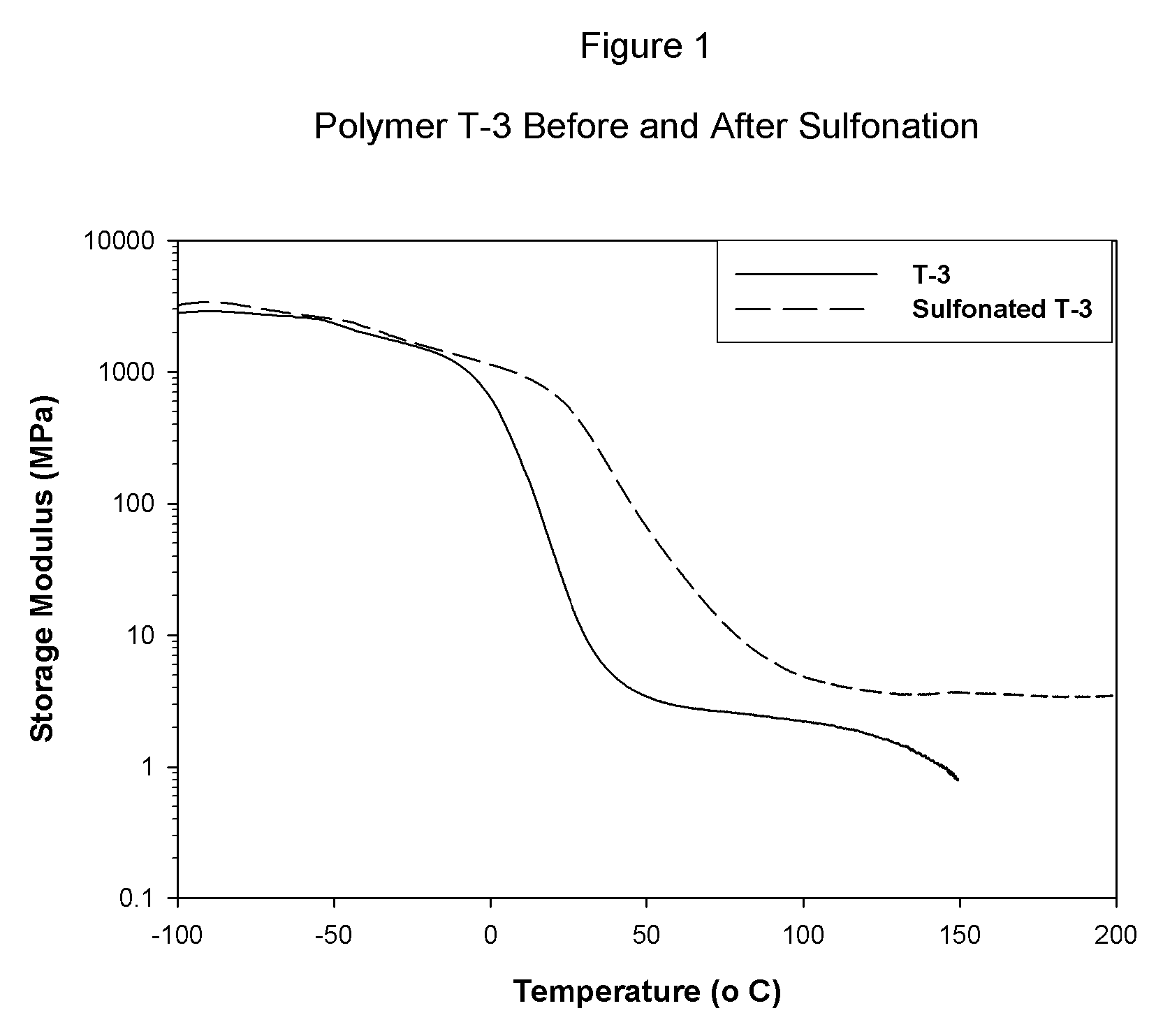
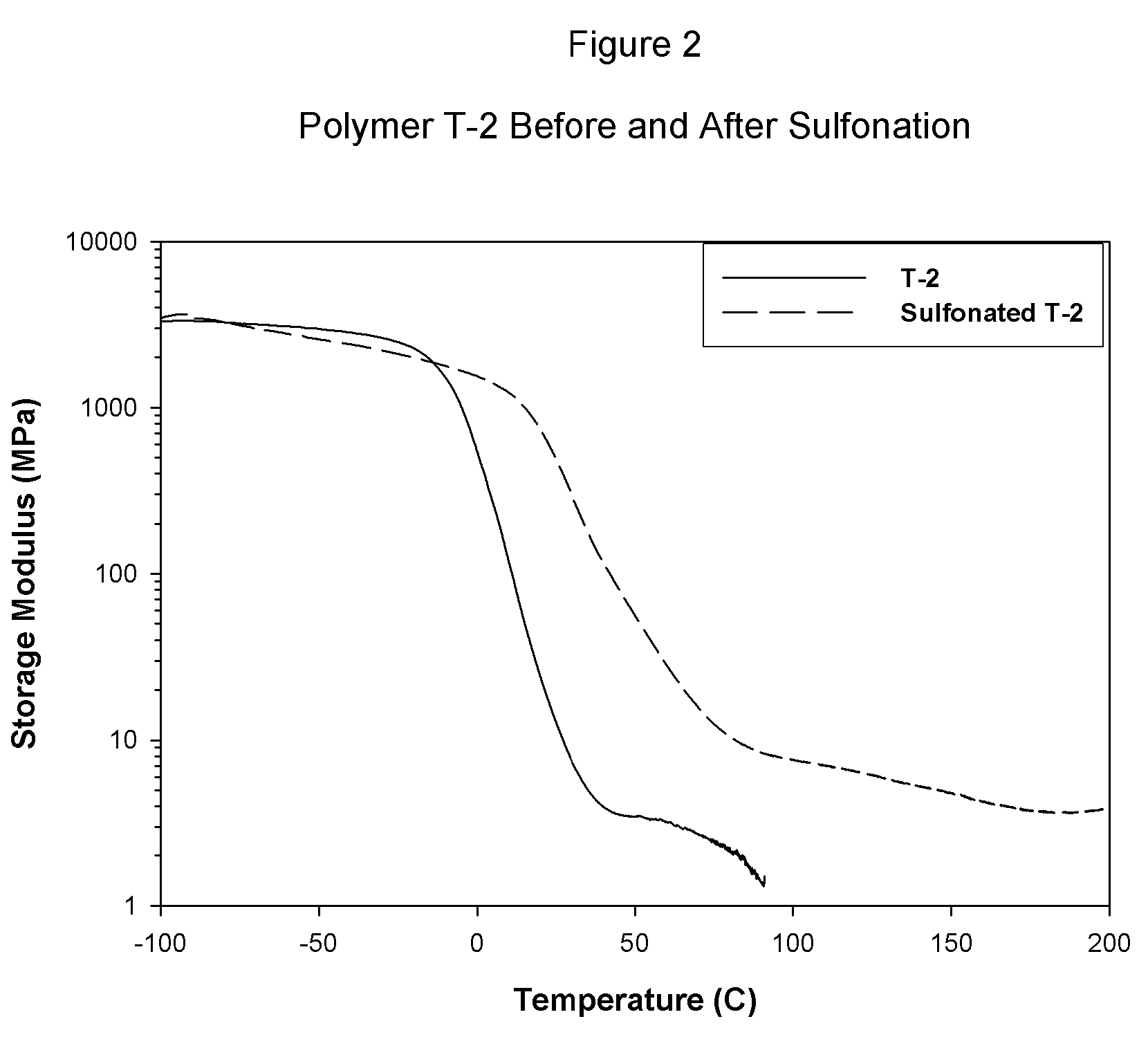
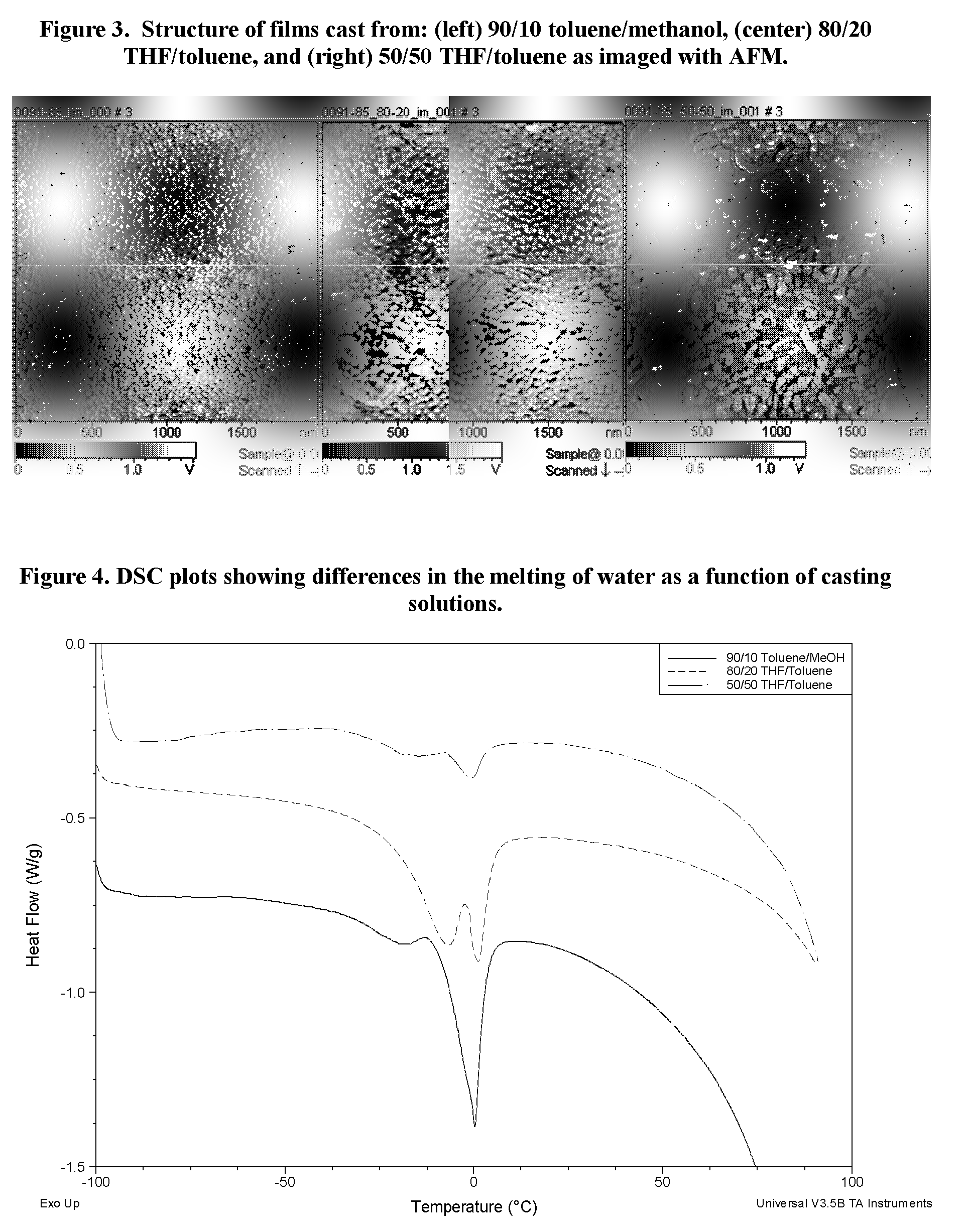
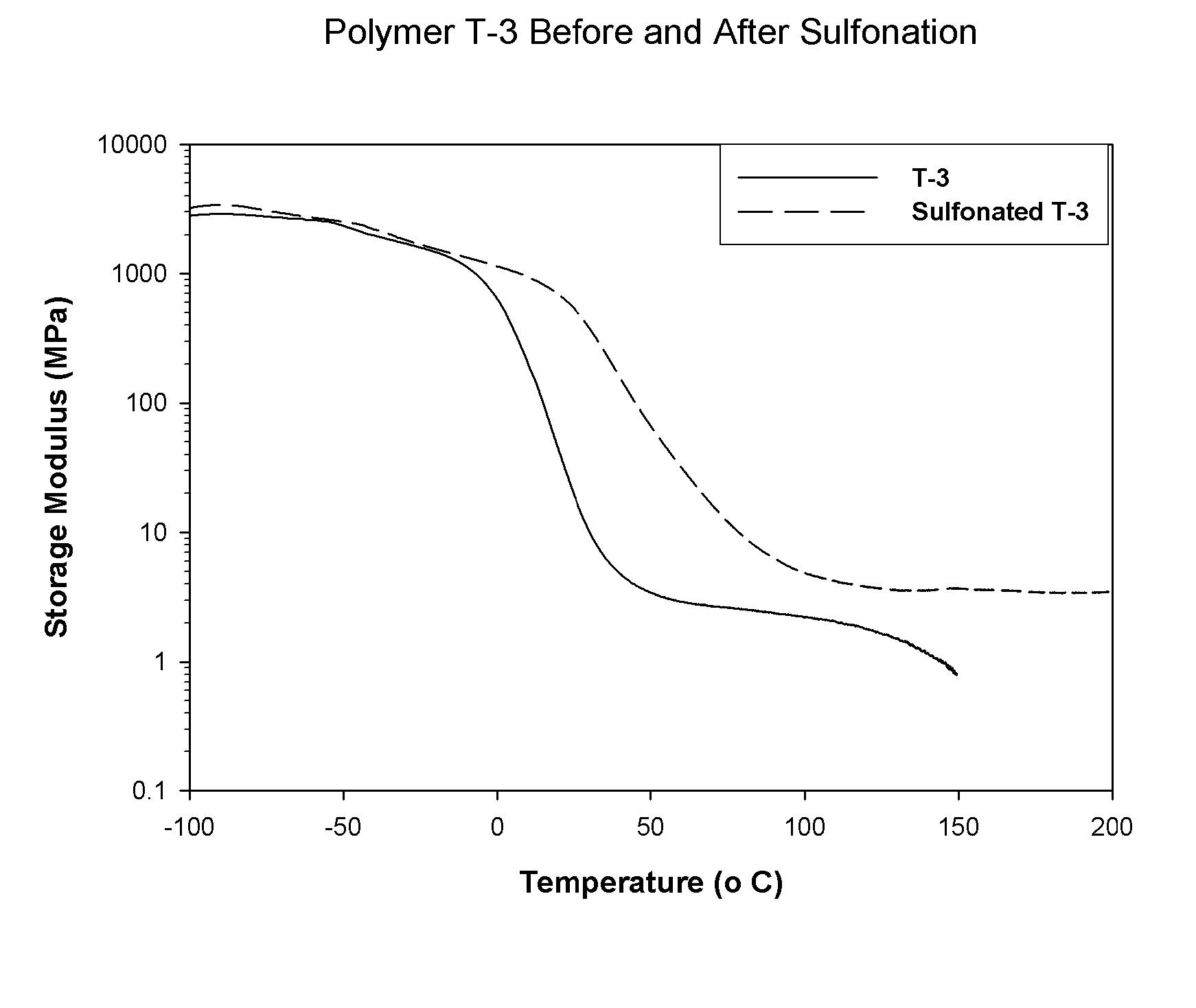
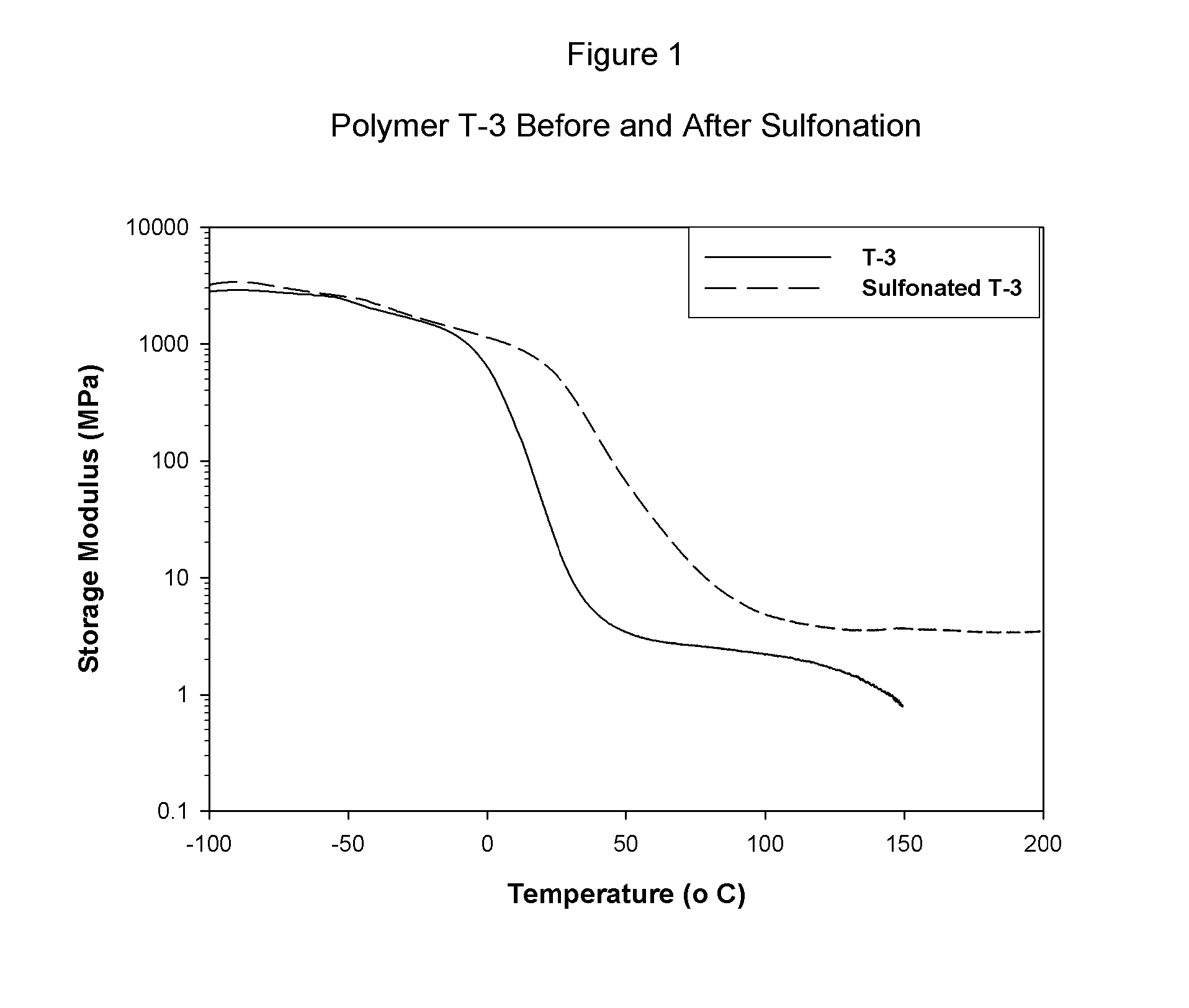
Sulfonated block copolymers, method for making same, and various uses for such block copolymers
ActiveUS7737224B2Reduced responseHigh propertySemi-permeable membranesNegative electrodesMethacrylatePolymer science
The present invention is a, solid block copolymer comprising at least two polymer end blocks A and at least one polymer interior block B wherein each A block is a polymer block resistant to sulfonation and each B block is a polymer block susceptible to sulfonation, and wherein said A and B blocks do not contain any significant levels of olefinic unsaturation. Preferably, each A block comprising one or more segments selected from polymerized (i) para-substituted styrene monomers, (ii) ethylene, (iii) alpha olefins of 3 to 18 carbon atoms; (iv) hydrogenated 1,3-cyclodiene monomers, (v) hydrogenated monomers of conjugated dienes having a vinyl content less than 35 mol percent prior to hydrogenation, (vi) acrylic esters, (vii) methacrylic esters, and (viii) mixtures thereof; and each B block comprising segments of one or more polymerized vinyl aromatic monomers selected from (i) unsubstituted styrene monomers, (ii) ortho-substituted styrene monomers, (iii) meta-substituted styrene monomers, (iv) alpha-methylstyrene, (v) 1,1-diphenylethylene, (vi) 1,2-diphenylethylene and (vii) mixtures thereof. Also claimed are processes for making such block copolymers, and the various end uses and applications for such block copolymers.
Owner:KRATON POLYMERS US LLC
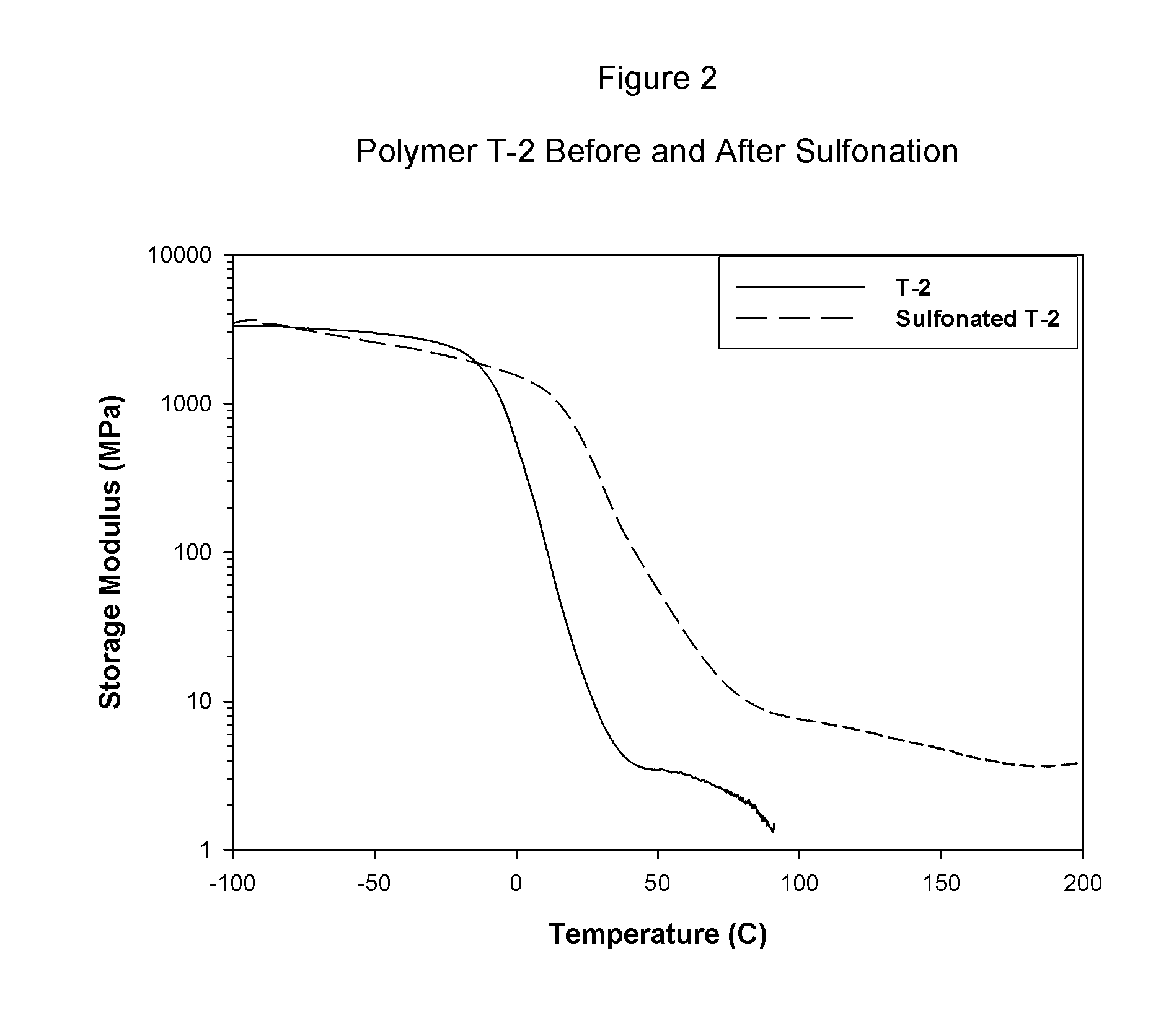
Sulfonated block copolymers, method for making same, and various uses for such block copolymers
ActiveUS20070021569A1High water transport propertyImprove wet strengthSemi-permeable membranesNegative electrodesMethacrylatePolymer science
The present invention is a, solid block copolymer comprising at least two polymer end blocks A and at least one polymer interior block B wherein each A block is a polymer block resistant to sulfonation and each B block is a polymer block susceptible to sulfonation, and wherein said A and B blocks do not contain any significant levels of olefinic unsaturation. Preferably, each A block comprising one or more segments selected from polymerized (i) para-substituted styrene monomers, (ii) ethylene, (iii) alpha olefins of 3 to 18 carbon atoms; (iv) hydrogenated 1,3-cyclodiene monomers, (v) hydrogenated monomers of conjugated dienes having a vinyl content less than 35 mol percent prior to hydrogenation, (vi) acrylic esters, (vii) methacrylic esters, and (viii) mixtures thereof; and each B block comprising segments of one or more polymerized vinyl aromatic monomers selected from (i) unsubstituted styrene monomers, (ii) ortho-substituted styrene monomers, (iii) meta-substituted styrene monomers, (iv) alpha-methylstyrene, (v) 1,1-diphenylethylene, (vi) 1,2-diphenylethylene and (vii) mixtures thereof. Also claimed are processes for making such block copolymers, and the various end uses and applications for such block copolymers.
Owner:KRATON POLYMERS US LLC
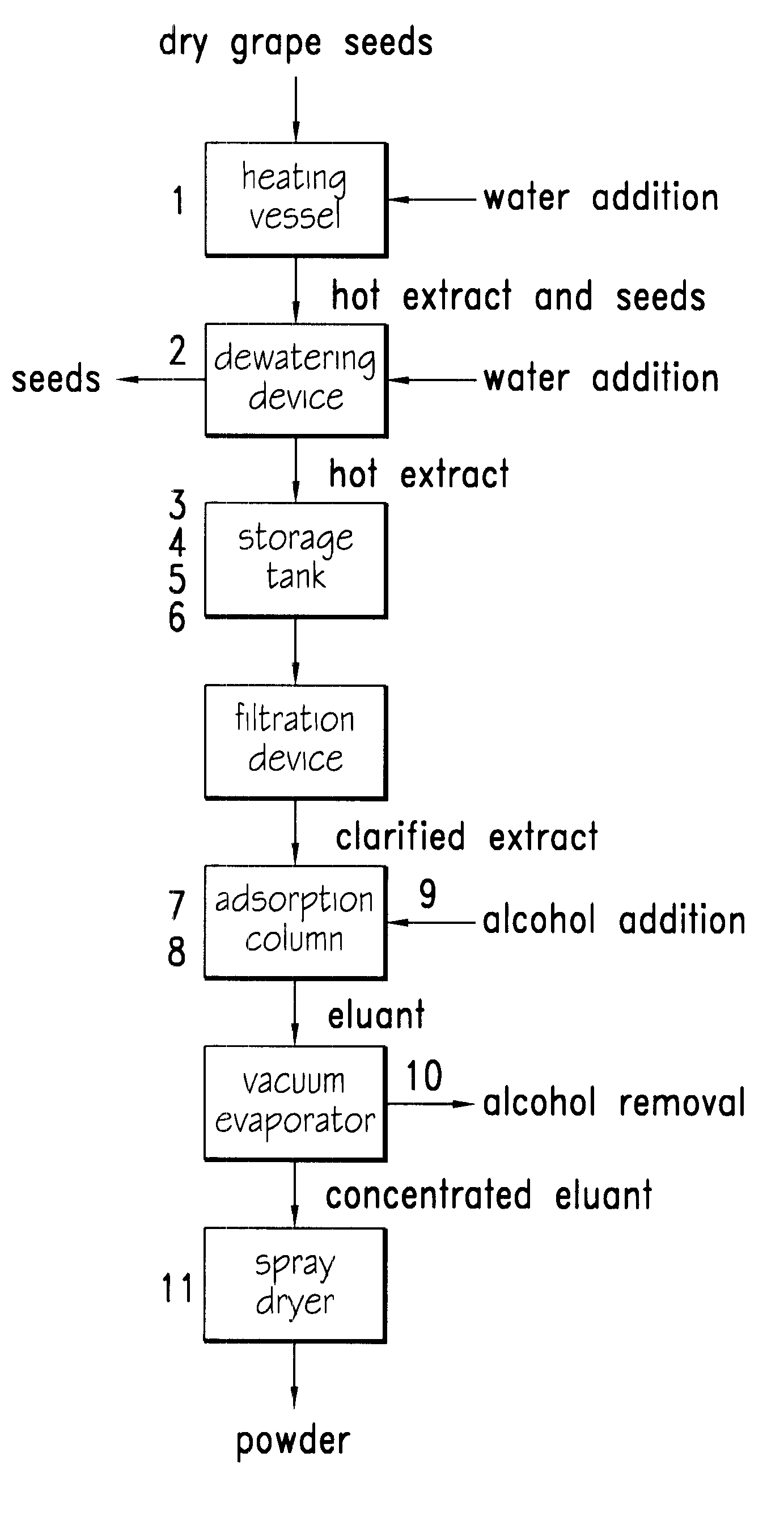
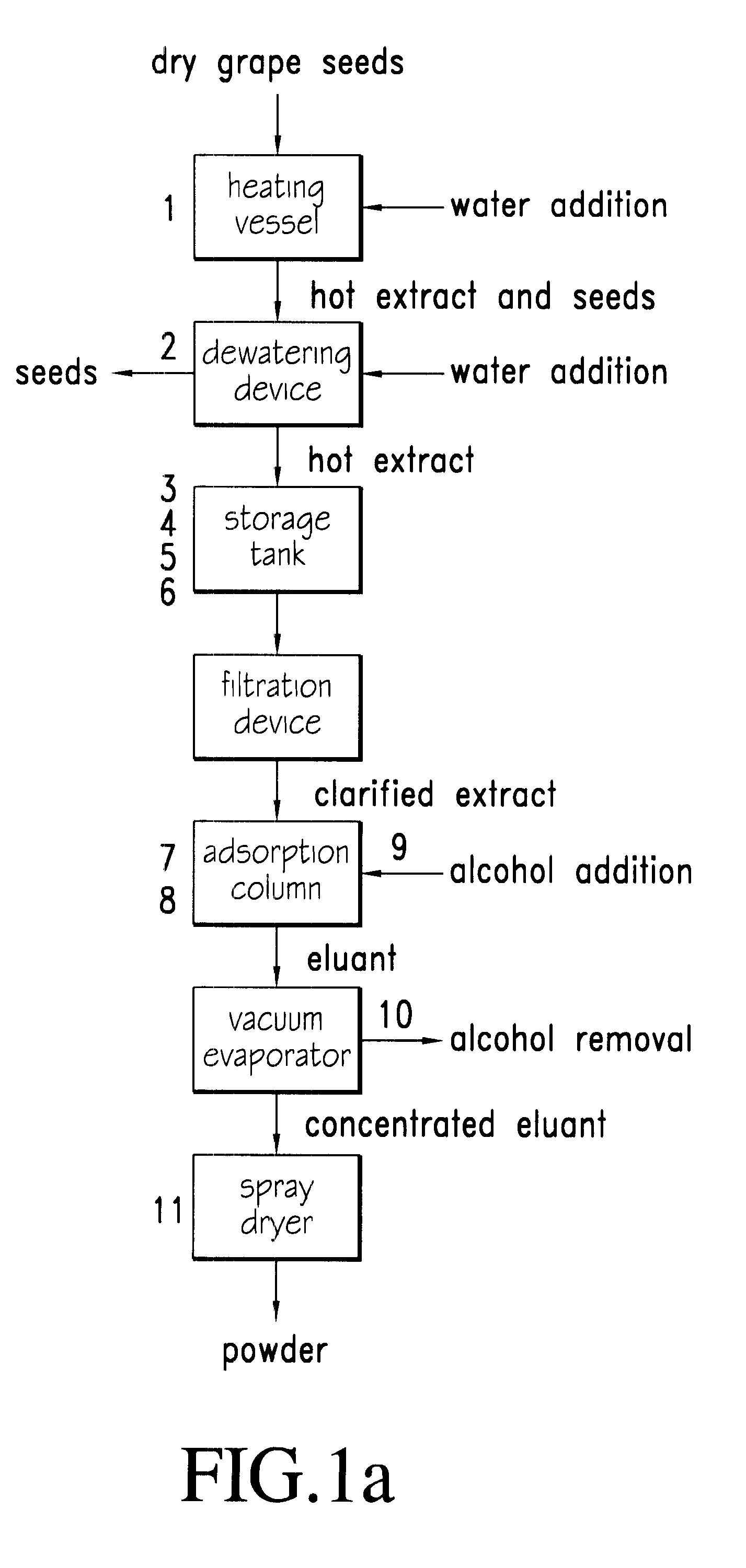
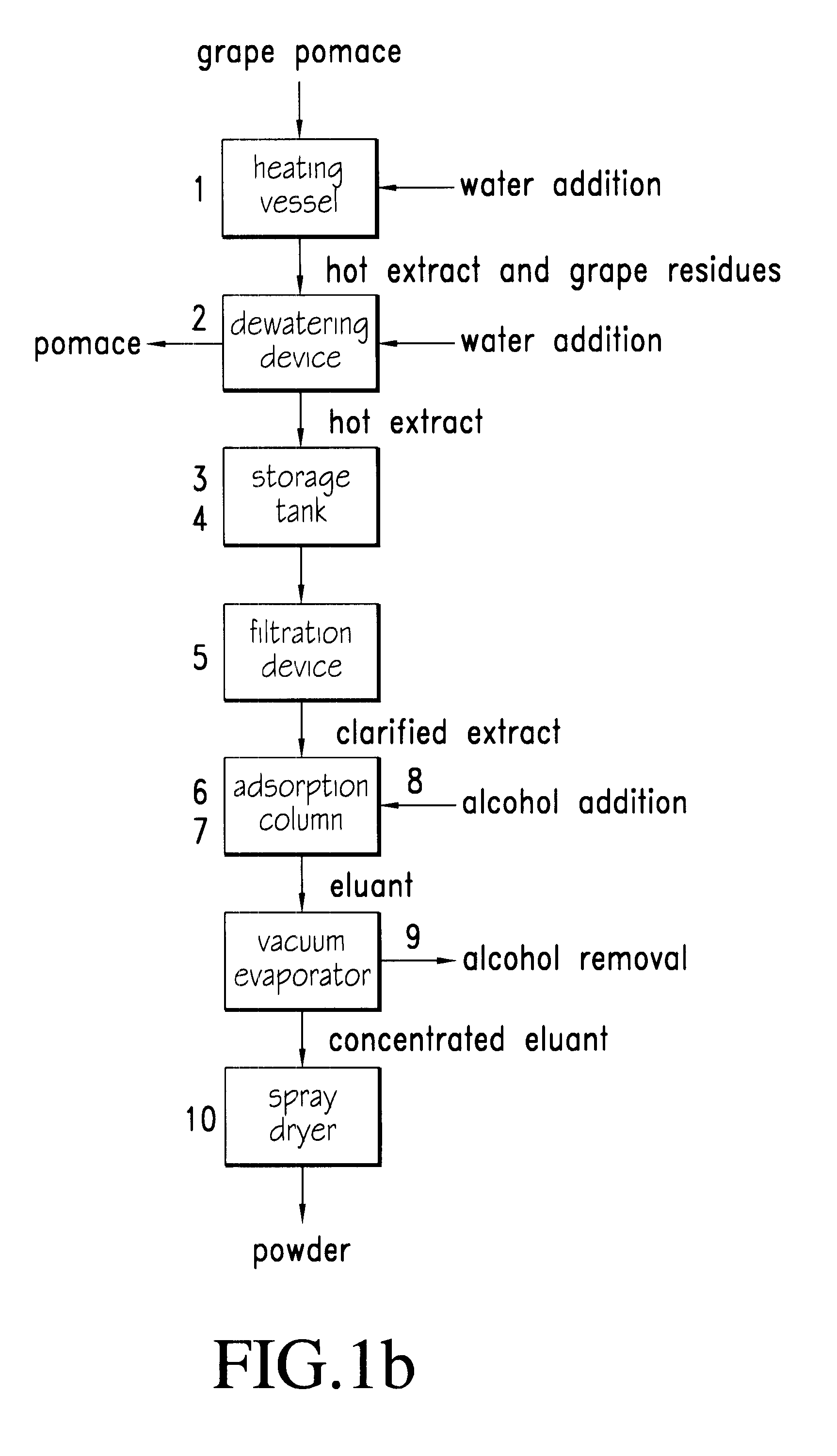
Process for extraction, purification and enrichment of polyphenolic substances from whole grapes, grape seeds and grape pomace
InactiveUS6544581B1Maximize concentration and purificationMaximizing extractionSolid waste disposalFatty substance preservation using additivesMethacrylateFiltration
The present invention provides a novel process for extraction, purification and concentration of polyphenol substances from whole grapes, grape seeds and grape pomace without the need for membrane filtration. Aspects of several embodiments of the novel processes include hot water extraction, a dual pH treatment of the hot water extracts, and the uses of a copolymer of trimethylolpropane trimethacrylate as an adsorbent resin to maximize the concentration and purification of the beneficial polyphenolic substances.
Owner:CANANDAIGUA WINE +1
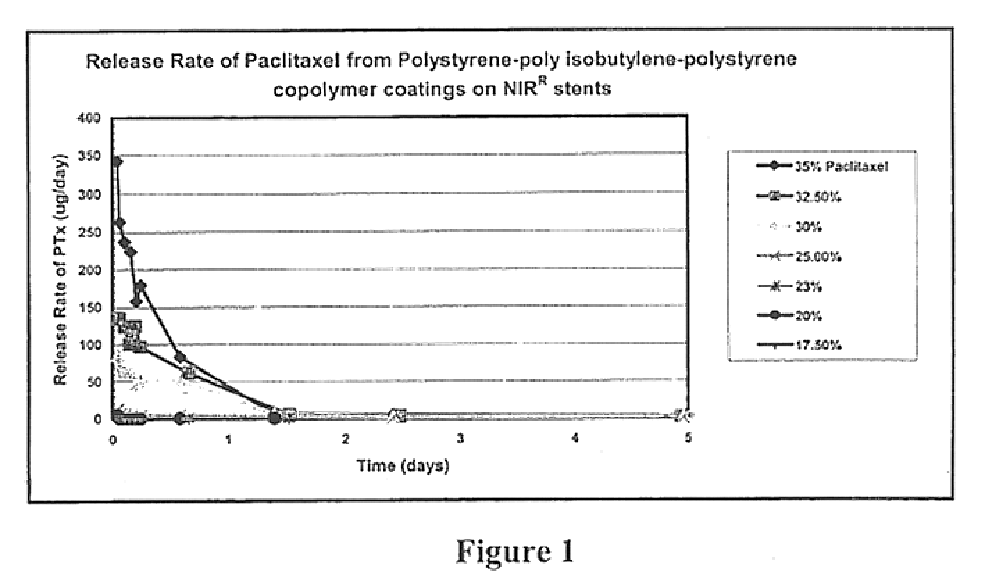
Drug delivery compositions and medical devices containing block copolymer
InactiveUS6855770B2Good mechanical integrityGood biocompatibilityOrganic active ingredientsGenetic material ingredientsThermoplasticMethacrylate
A composition for delivery of a therapeutic agent is provided. The composition comprises: (a) a biocompatible block copolymer comprising one or more elastomeric blocks and one or more thermoplastic blocks and (b) a therapeutic agent, wherein the block copolymer is loaded with the therapeutic agent. The block copolymer is preferably of the formula X—(AB)n, where A is an elastomeric block, B is a thermoplastic block, n is a positive whole number and X is a seed molecule. The elastomeric blocks are preferably polyolefin blocks, and the thermoplastic blocks are preferably selected from vinyl aromatic blocks and methacrylate blocks. According to another aspect of the invention, a medical device is provided, at least a portion of which is insertable or implantable into the body of a patient. The medical device comprises (a) the above biocompatible block copolymer and (b) a therapeutic agent, wherein the block copolymer is loaded with the therapeutic agent. According to another aspect of the present invention, a method of treatment is provided in which the above device is implanted or inserted into a patient, resulting in the release of therapeutic agent in the patient over an extended period. According to yet another aspect of the invention, a coated medical device is provided which comprises: (a) an intravascular or intervascular medical device and (b) a coating over at least a portion of the intravascular or intervascular a medical device, wherein the coating comprises the above biocompatible block copolymer.
Owner:SCI MED LIFE SYST
Hydrogel from polysiloxane-containing urethane prepolymer, tris (trimethylsiloxy) silylpropyl methacrylate, and a hydrophilic comonomer
A polyurethane based prepolymer is provided and useful in biomedical devices which provides high oxygen permeability and superior physical properties. A hydrogel is produced from a comonomer mixture containing a polysiloxane-containing urethane prepolymer, tris(trimethylsiloxy)-silylpropyl methacrylate and a hydrophilic comonomer. The hydrogel is especially useful for biomedical materials such as contact lenses and implants.
Owner:BAUSCH & LOMB INC
Materials for making hydrophobic intraocular lens
Owner:BENZ RES & DEV
Monomer substituted photoacid generator of fluoroalkylsulfon and a polymer thereof
InactiveUS7534844B2Reduce solubilityImprove solubilityOrganic chemistryPhotomechanical apparatusSolubilityMethacrylate
The present invention relates to a novel compound with a fluoroalkylsulfonium photoacid generating group and novel copolymers having superior solubility in organic solvents, which is prepared from radical polymerization of the novel compound with methacrylate monomers.
Owner:IUCF HYU (IND UNIV COOP FOUND HANYANG UNIV)
Liquid inks comprising a stable organosol
InactiveUS20020128349A1Good dispersionAvoid resistanceDuplicating/marking methodsInksMethacrylateDispersion stability
A liquid ink comprises (a) a carrier liquid having a Kauri-Butanol number less than 30; (b) a graft copolymer comprising a (co)polymeric steric stabilizer covalently bonded to a thermoplastic (co)polymeric core that is insoluble in the carrier liquid; and (c) a colorant, wherein the steric stabilizer comprises units derived from 3,3,5-trimethylcyclohexyl methacrylate, and the thermoplastic (co)polymeric core comprising units derived from at least a polymerizable monomer selected from the group consisting of (meth)acrylates having aliphatic amino radicals, nitrogen-containing heterocyclic vinyl monomers, N-vinyl substituted ring-like amide monomers, aromatic substituted ethylene monomers containing amino radicals, and nitrogen-containing vinylether monomers. The organosol provides improved liquid electrophotographic and electrographic ink compositions with improved dispersion stability, chargeability, and blocking resistance so that improved print quality or ink transfer performance are obtained.
Owner:S PRINTING SOLUTION CO LTD
Positive resist composition and method of forming resist pattern from the same
InactiveUS20040110085A1Small line edge roughnessHigh resolutionRadiation applicationsSemiconductor/solid-state device manufacturingSolubilityMethacrylate
There is provided a positive type resin composition comprising (A) a resin component comprising within the principal chain a structural unit derived from a (meth)acrylate ester and incorporating an acid dissociable, dissolution inhibiting group containing a polycyclic group on an ester side chain section, for which the solubility in alkali increases under the action of acid, (B) an acid generator component which generates acid on exposure, and (C) an organic solvent, wherein the component (A) comprises both a structural unit derived from a methacrylate ester and a structural unit derived from an acrylate ester. According to such a resist composition, a resist pattern can be formed which displays little surface roughness and line edge roughness on etching, and also offers excellent resolution and a wide depth of focus range.
Owner:TOKYO OHKA KOGYO CO LTD
High flow engineering thermoplastic compositions and products made therefrom
InactiveUS20040108623A1Improve moldability and flowabilityGood balance of impact and heat resistanceOrganic dyesThermoplasticAcrylate
High flow engineering thermoplastic compositions made from a thermoplastic host polymer and a low molecular weight flow modifier polymer, and products made therefrom. The flow modifier polymer is made by polymerizing at least one vinyl aromatic monomer and at least one (meth)acrylate monomer. The high flow engineering thermoplastics provide improved flowability and processability without sacrificing impact strength or heat resistance
Owner:JOHNSON POLYMER INC
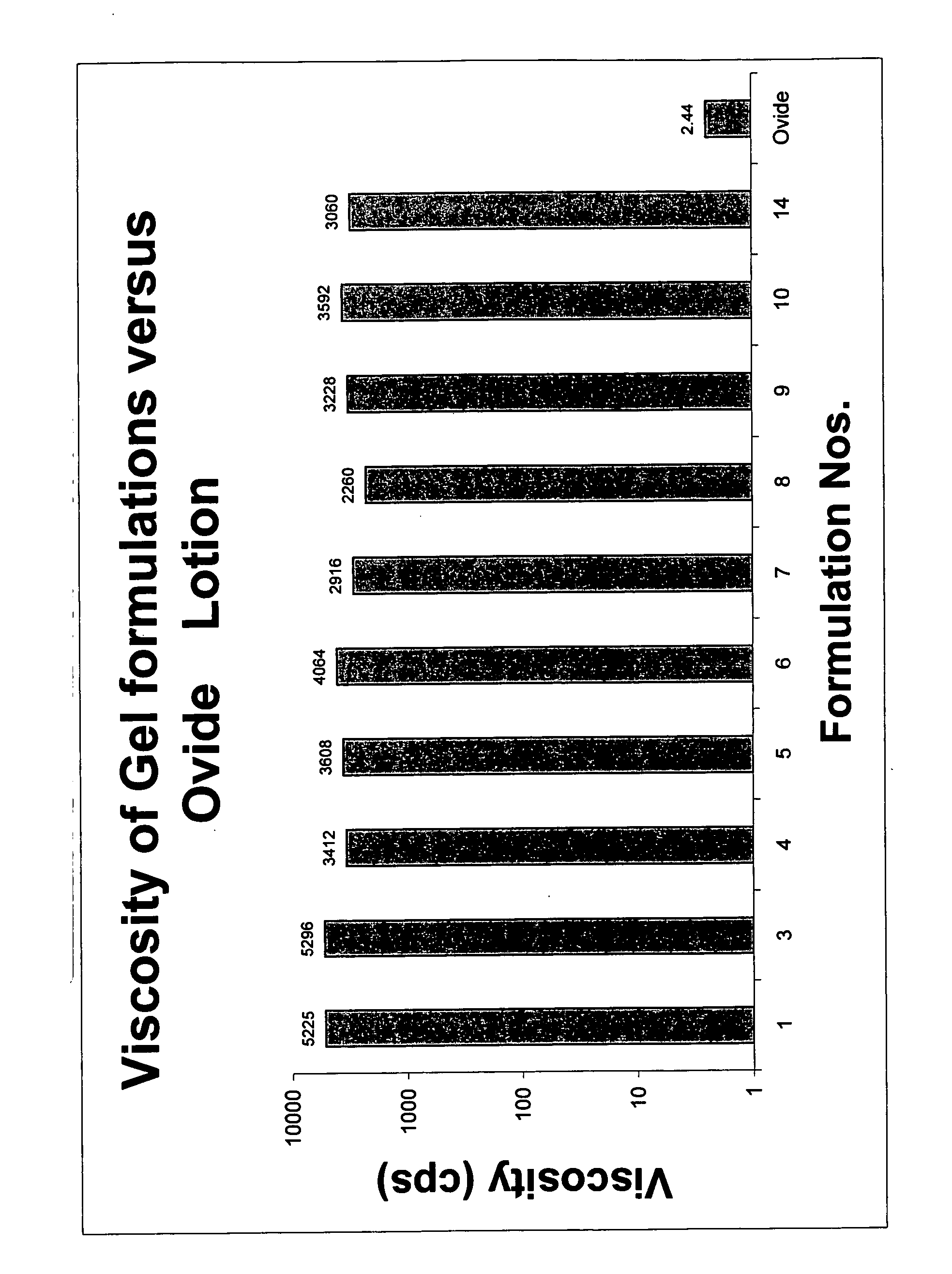
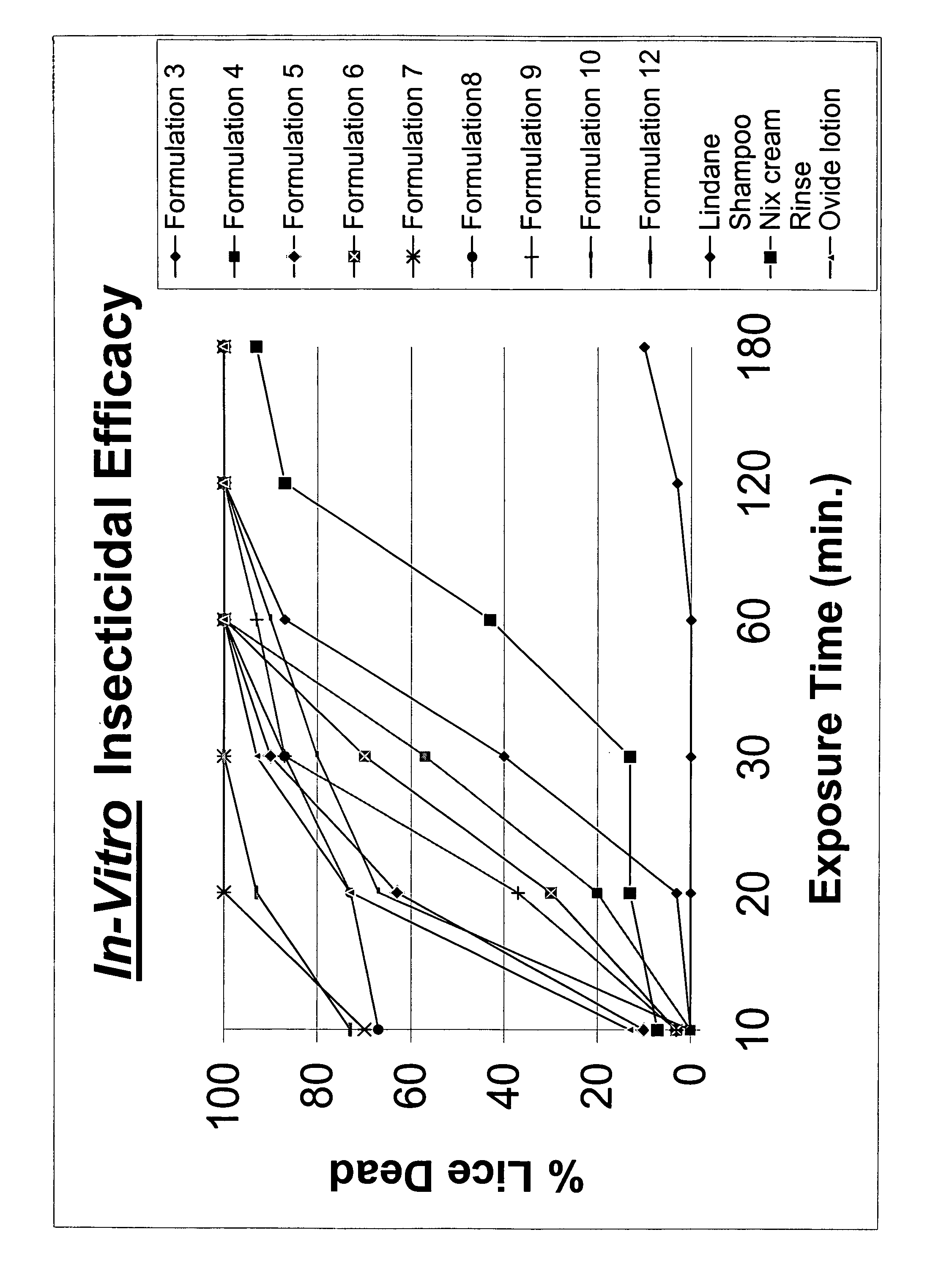
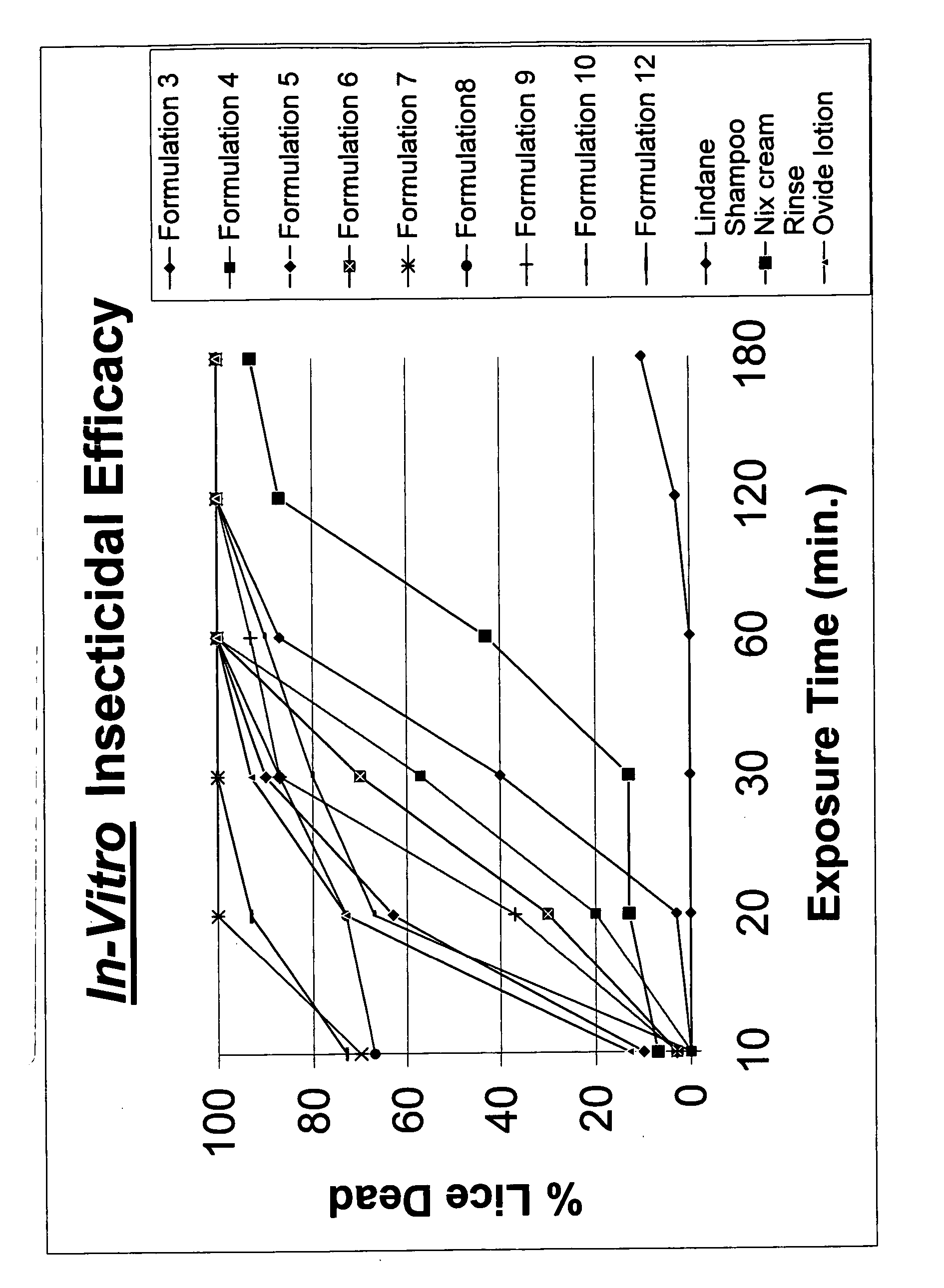
Topical gel formulation comprising insecticide and its preparation thereof
The present invention provides a topical gel pharmaceutical formulation of insecticide suitable for treating an ectoparasite in a mammal, comprising: a) about 0.1-10% by weight of an insecticide; b) at least about 75% by weight of an organic solvent selected from the group consisting of a lower alkyl alcohol, a ketone, a glycol and a mixture thereof, wherein the organic solvent contains at least about 40% by weight of the lower alkyl alcohol; and c) at least one polymer selected from the group consisting of a cellulosic polymer, acrylates, methacrylates, and polyvinyl pyrrolidone. The present invention further provides a process of preparing as well as a method of treating ectoparasites in a mammal using the same.
Owner:GOYAL SANDHYA +4
Disc augmentation using materials that expand in situ
A method of augmenting a nucleus pulposus within an annulus fibrosis. A material having a relatively thin, elongated first state is inserted through the annulus, after which it expands or otherwise assumes a shape that is more rounded when implanted. In the preferred embodiment, for introduction the material is relatively rigid or hard and relatively thin, resembling a needle or a nail. The size, shape, and consistency of the material allow the device to be pushed through the fibers of the annulus fibrosis, preferably without an incision, and into the nucleus pulposus and / or disc space. The resultant shape assists the nucleus pulposis in acting as a “shock absorber,” and the expansion of the material also makes extrusion unlikely. Various materials qualify for this purpose according to the invention. Materials that change shape with temperature include memory-effect alloys such as Nitinol and substances such as stearle methacrylate. Materials that change in shape in the presence of moisture include hydrogels and other substances that imbibe water. Materials that expand due to chemical reaction include various foams, and the like, some of which may be applied in two-part form.
Owner:ANOVA
Pigment ink jet ink composition
An ink jet ink composition comprising an aqueous media and a pigment dispersion comprising a pigment and a polymeric dispersant wherein said polymeric dispersant is a copolymer comprising at least a hydrophobic methacrylate or acrylate monomer containing an aliphatic chain having greater than or equal to 12 carbons; and a hydrophilic methacrylic or acrylic acid monomer; wherein said copolymer comprises at least 10% by weight of the methacrylate or acrylate monomer and at least 5% by weight of the methacrylic or acrylic acid monomer; and wherein the copolymer comprises, in total, 20 to 95 weight % of hydrophobic monomer.
Owner:EASTMAN KODAK CO
Porous films, process for producing the same, and laminate films and recording sheets made with the use of the porous films
InactiveUS6177181B1High in transparency and ink absorptionImprove waterproof performanceMembranesSemi-permeable membranesBoiling pointOptical transmittance
Porous membranes having a micro phase separation structure and showing a light transmittance at the wavelength of 400 nm of not less than 30% are obtained by the dry phase conversion method comprising drying a coating layer of a dope containing a polymer, a good solvent for the polymer and a poor solvent for the polymer which solvent has a higher boiling point than the good solvent. The polymer includes cellulose derivatives, vinyl-series polymers such as acrylonitrile-series polymers and (meth)acrylic acid ester-series polymers, polysulfone-series polymers, and the like. The porous polymer membranes have a porosity of 10 to 60%, a mean pore size of about 0.002 to 0.35 mum and a maximum pore size of not greater than 0.4 mum. These porous membranes shows not only excellent transparency but also high productivity.
Owner:DAICEL CHEM IND LTD
Biomimetic hydrogel materials
Novel biomimetic hydrogel materials and methods for their preparation. Hydrogels containing acrylamide-functionalized carbohydrate, sulfoxide, sulfide or sulfone copolymerized with a hydrophilic or hydrophobic copolymerizing material selected from the group consisting of an acrylamide, methacrylamide, acrylate, methacrylate, vinyl and a derivative thereof present in concentration from about 1 to about 99 wt %. and methods for their preparation. The method of use of the new hydrogels for fabrication of soft contact lenses and biomedical implants.
Owner:RGT UNIV OF CALIFORNIA
Organic-inorganic hybrids surface adhesion promoter
An organic-inorganic hybrid surface adhesion promoter having the general formula, A-B, wherein A is hydrolyzed and polycondensed from a trioxysilane R-Si(OR')3 or its mixture with one or two more silanes, where R' is methyl, ethyl or propyl, and where R is an organic group of methacrylate, epoxy, amine, isolyante, hydroxide or non-halogens or halogens containing alkyl, alkenyl, aryl, alkylary or arylalky, and wherein B is hydrolyzed and polycondensed from an alkoxy silane, chloride silane, or alkoxy or chloride metal compound, whereby B reacts with a substrate to form a uniting group which is selected from the group consisting of Si-O-Si, M-O-M, M-O-S and Si-O-M, M being a metal atom.
Owner:ZENASTRA PHOTONICS INC
Wet-stick adhesives
A wet-skin adhesive includes a pressure-sensitive adhesive component that includes at least one monoethylenically unsaturated (meth)acrylic acid ester comprising an alkyl group having at least 4 carbons on average and at least one monoethylenically unsaturated reinforcing monomer; and a film-forming component that includes at least one monoethylenically unsaturated (meth)acrylic acid ester comprising an alkyl group having less than 4 carbons on average and at least one hydrophilic acidic monomer, wherein the wet-stick pressure-sensitive adhesive has an initial wet skin adhesion of at least about 0.8 N / dm. Advantageously, the wet-stick adhesive has an initial wet skin adhesion that is at least about 65% of an initial dry skin adhesion.
Owner:3M INNOVATIVE PROPERTIES CO
Thermoset materials with improved impact resistance
The present invention relates to a thermoset material with improved impact resistance comprising: 99 to 20% of a thermoset resin, 1 to 80% of an impact modifier comprising at least one copolymer chosen from copolymers comprising S-B-M, B-M and M-B-M blocks, in which: each block is connected to the other by means of a covalent bond or of an intermediate molecule connected to one of the blocks via a covalent bond and to the other block via another covalent bond, M is a PMMA homopolymer or a copolymer comprising at least 50% by weight of m thyl methacrylate, B is incompatible with the thermoset resin and with the M block and its glass transition temperature Tg is less than the operating temperature of the thermoset material, S is incompatible with the thermoset resin, the B block and the M block and its Tg or its melting temperature M.t. is greater than the Tg of B. S is advantageously polystyrene and B polybutadiene. The thermoset resin advantageously originates from the reaction of a thermosetting epoxy resin and of a hardener.
Owner:ATOFINA
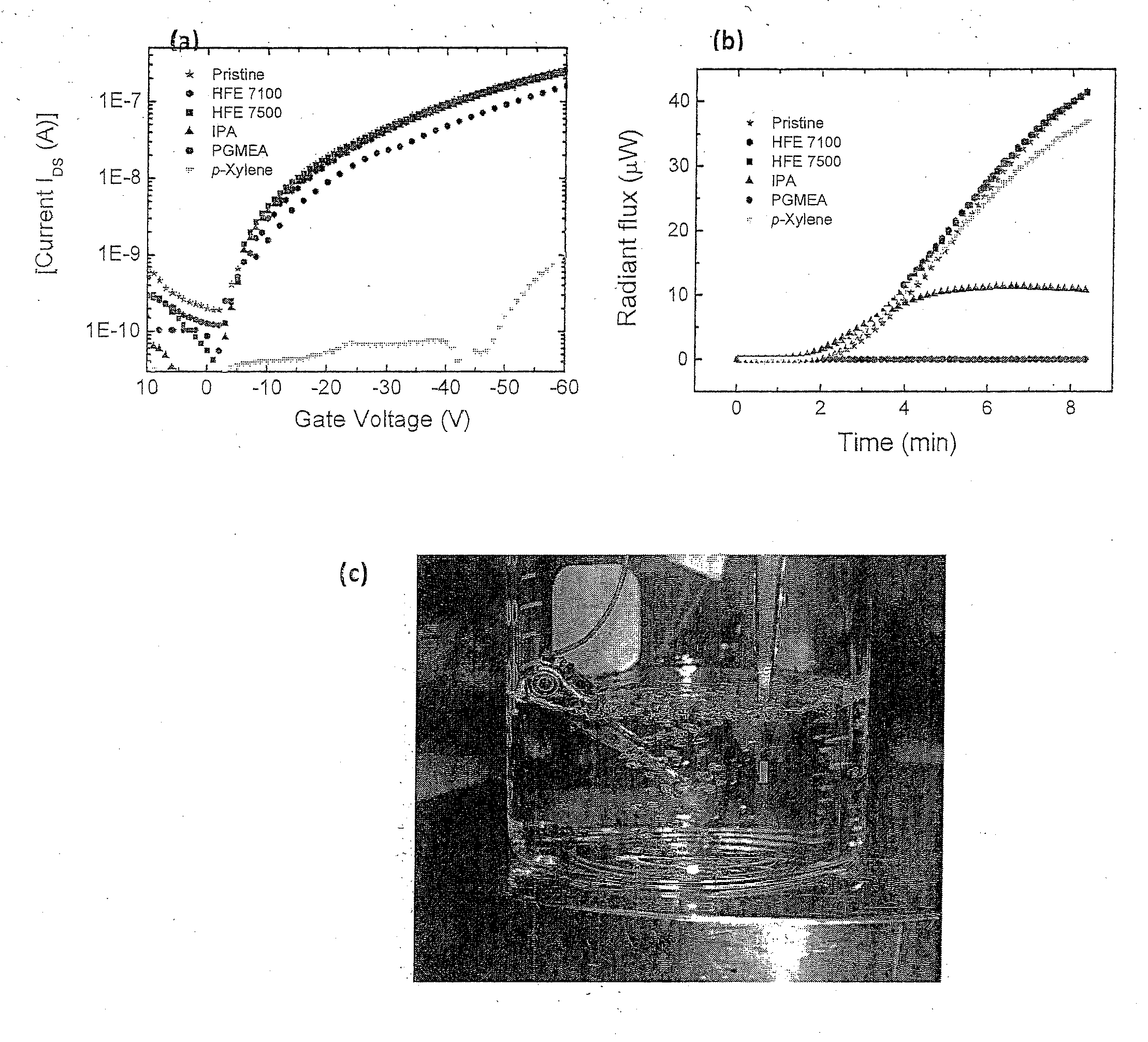
Orthogonal Procesing of Organic Materials Used in Electronic and Electrical Devices
ActiveUS20110159252A1Avoid damageImprove throughputOrganic chemistryPhotosensitive materialsOrganic structureMethacrylate
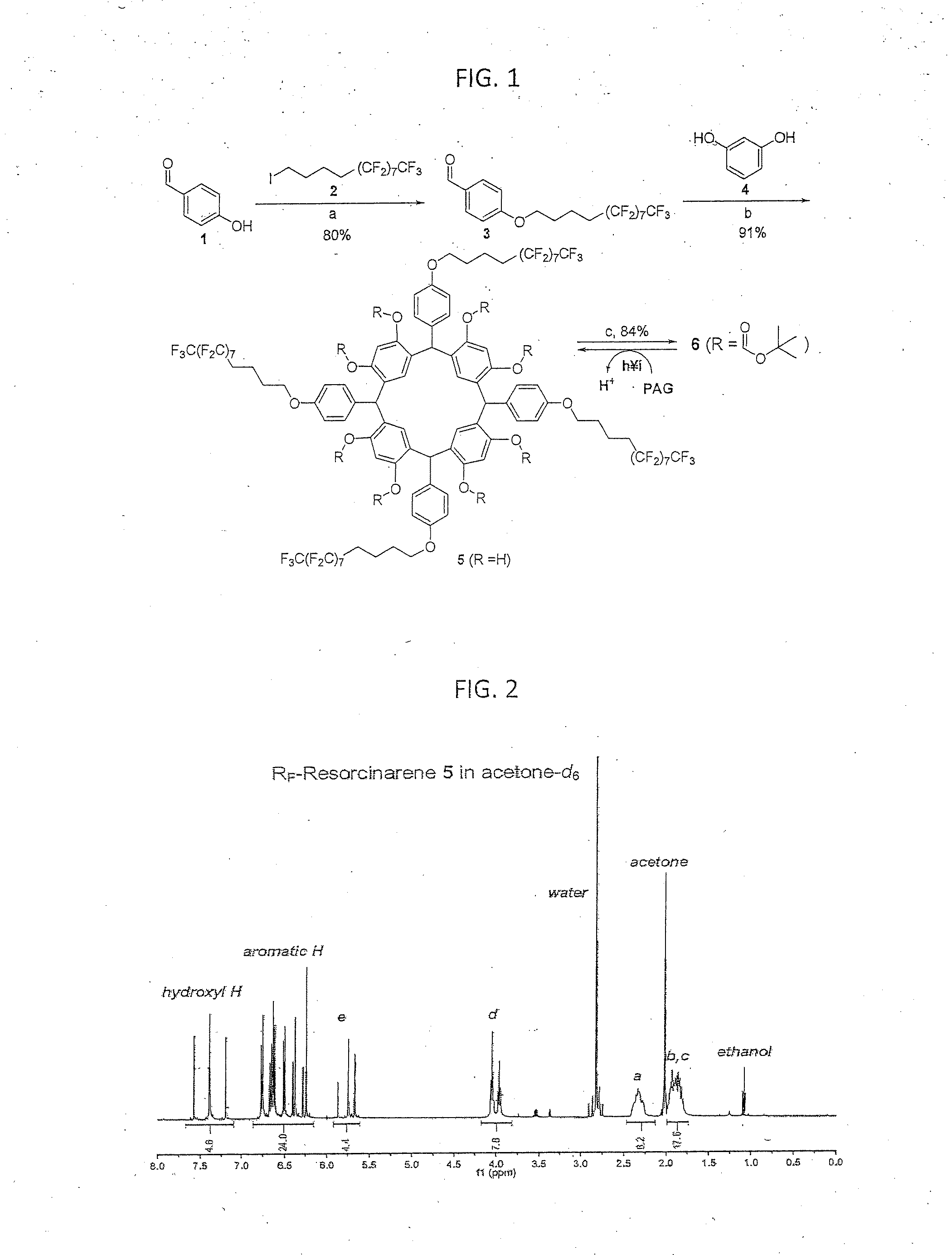
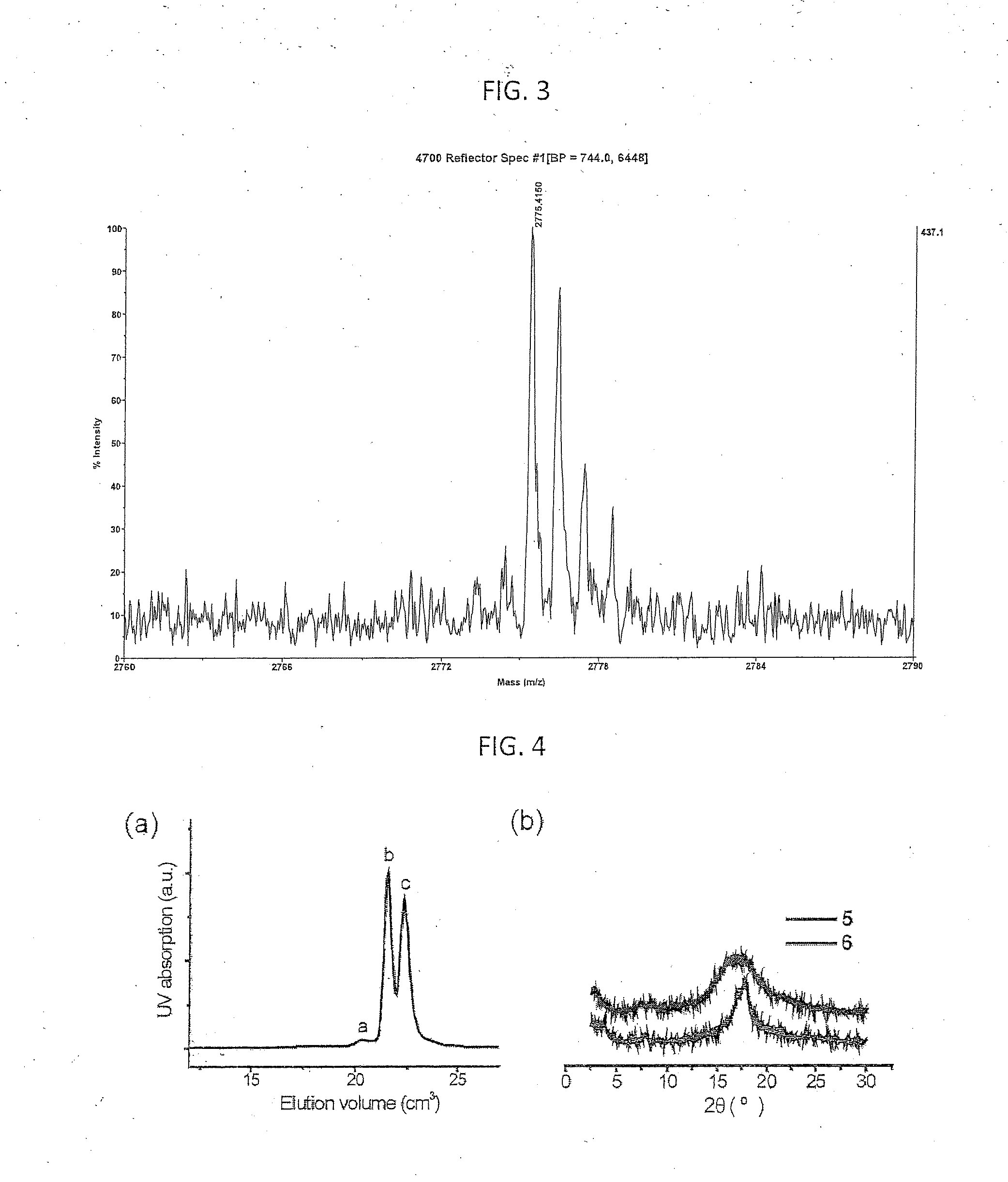
An orthogonal process for photolithographic patterning organic structures is disclosed. The disclosed process utilizes fluorinated solvents or supercritical CO2 as the solvent so that the performance of the organic conductors and semiconductors would not be adversely affected by other aggressive solvent. One disclosed method may also utilize a fluorinated photoresist together with the HFE solvent, but other fluorinated solvents can be used. In one embodiment, the fluorinated photoresist is a resorcinarene, but various fluorinated polymer photoresists and fluorinated molecular glass photoresists can be used as well. For example, a copolymer perfluorodecyl methacrylate (FDMA) and 2-nitrobenzyl methacrylate (NBMA) is a suitable orthogonal fluorinated photoresist for use with fluorinated solvents and supercritical carbon dioxide in a photolithography process. The combination of the fluorinated photoresist and the fluorinated solvent provides a robust, orthogonal process that is yet to be achieved by methods or devices known in the art.
Owner:CORNELL UNIVERSITY
Resist composition
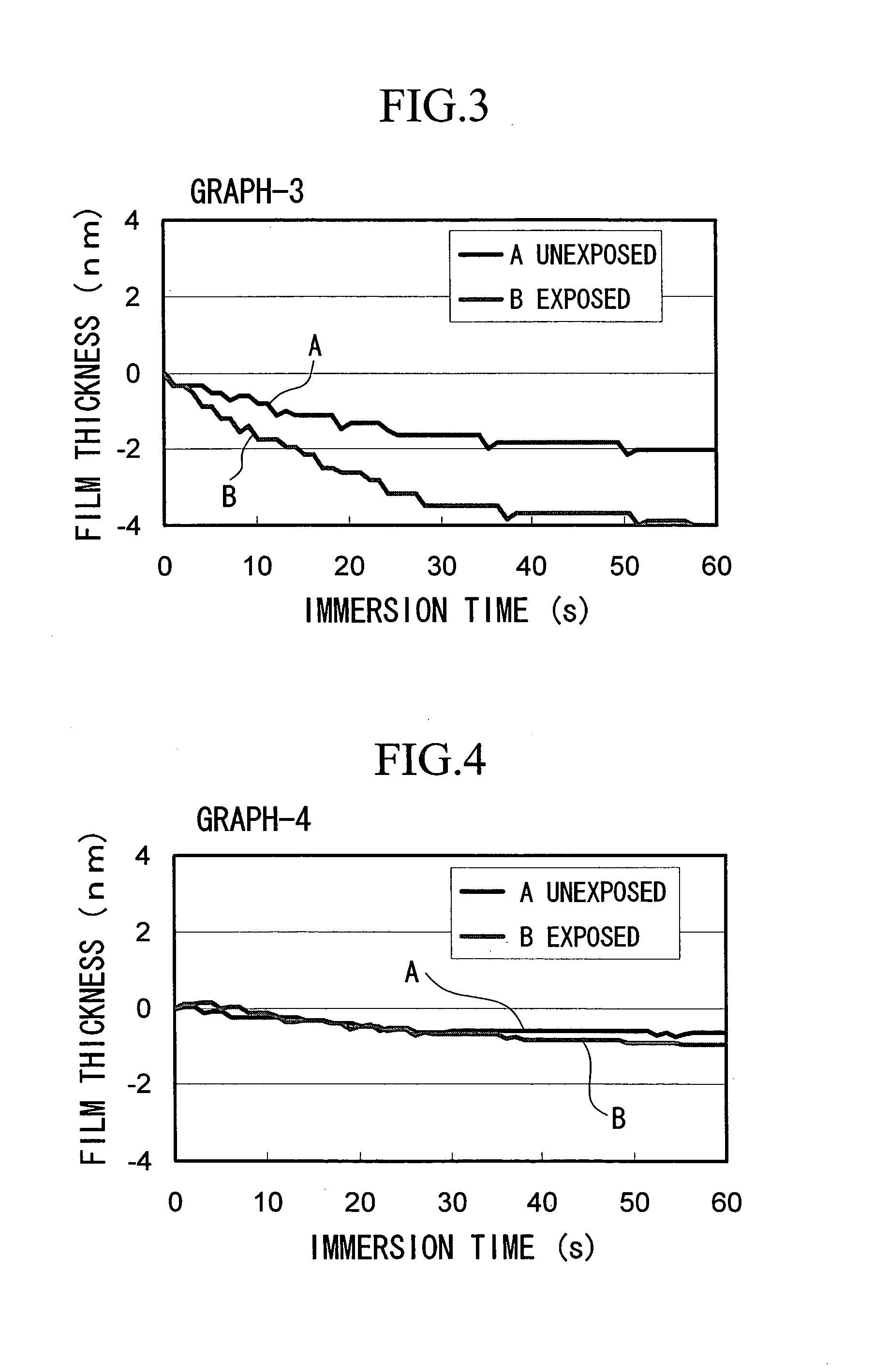
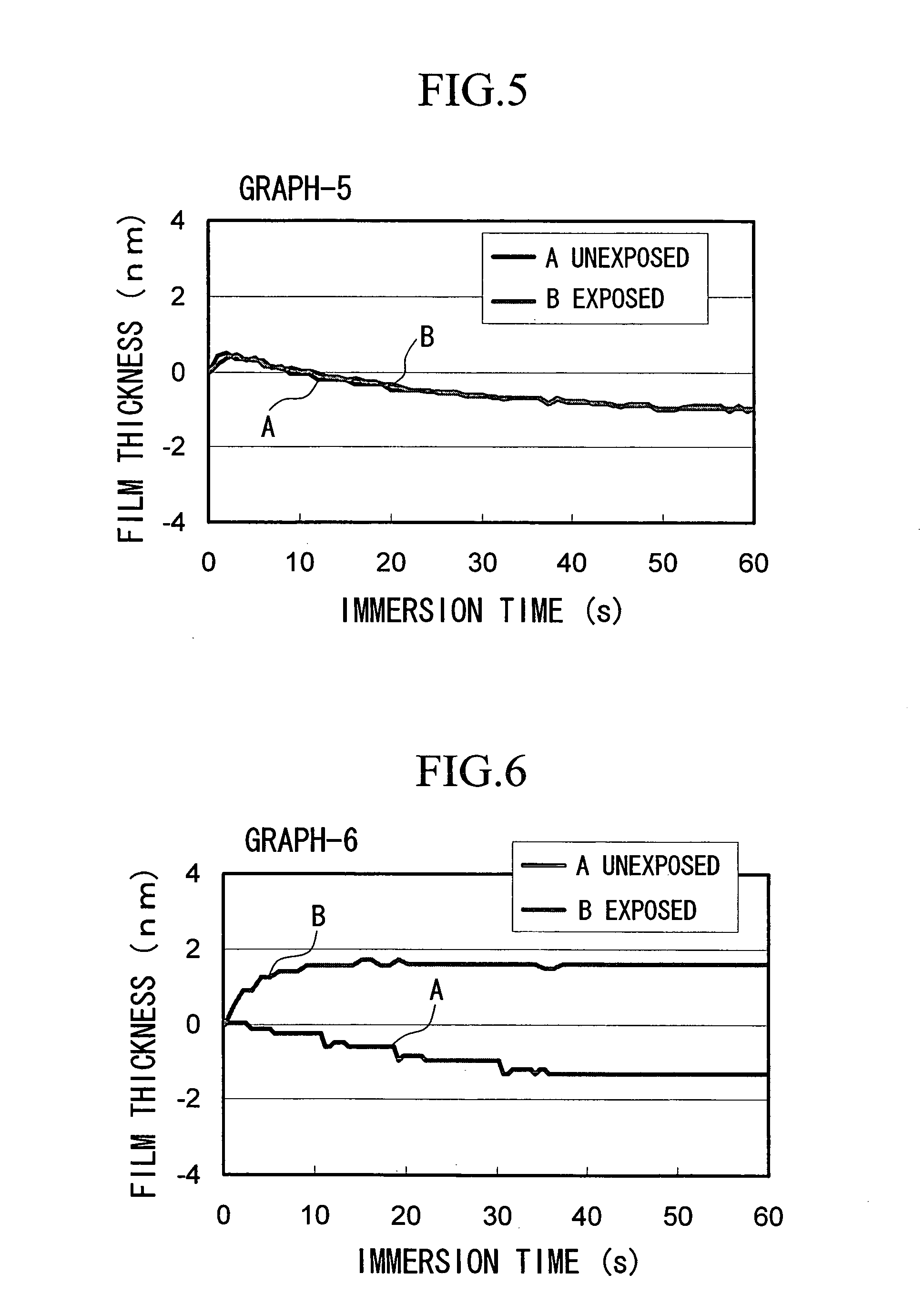
ActiveUS20050014090A1Minimal deterioration in sensitivitySmall swellingRadiation applicationsSemiconductor/solid-state device manufacturingMethacrylateSolubility
A resist composition which is stable relative to solvents used in immersion lithography processes and displays excellent sensitivity and resist pattern profile, and a method of forming a resist pattern that uses such a resist composition are provided. The resist composition is in accordance with predetermined parameters, or is a positive resist composition comprising a resin component (A) which contains an acid dissociable, dissolution inhibiting group and displays increased alkali solubility under the action of acid, an acid generator component (B), and an organic solvent (C), wherein the component (A) contains a structural unit (a1) derived from a (meth)acrylate ester containing an acid dissociable, dissolution inhibiting group, but contains no structural units (a0), including structural units (a0-1) containing an anhydride of a dicarboxylic acid and structural units (a0-2) containing a phenolic hydroxyl group.
Owner:TOKYO OHKA KOGYO CO LTD
Injection molding method for neutral and acidic-group containing (meth)acrylate copolymers
InactiveUS20020160042A1Reduce contentOrganic dyesPharmaceutical non-active ingredientsMeth-Additive ingredient
The invention relates to a process for producing mouldings by injection moulding, the steps in the process being A) Melting a mixture made from a) a (meth)acrylate copolymer composed of from 40 to 100% by weight of free-radical-polymerized C1-C4-alkyl esters of acrylic or methacrylic acid and from 0 to 60% by weight of (meth)acrylate monomers having an anionic group in the alkyl radical, where the copolymer comprises b) from 0.1 to 3% by weight of a release agent, and, where appropriate, the mixture may comprise c) from 0 to 50% by weight of a drier, d) from 0 to 30% by weight of a plasticizer, e) from 0 to 100% by weight of additives or auxiliaries, f) from 0 to 100% by weight of an active pharmaceutical ingredient, g) from 0 to 20% by weight of another polymer or copolymer, where the amounts given for components b) to g) are based on the (meth)acrylate copolymer a) and the mixture prior to melting has a content of more than 0.5% by weight of low-boiling constituents with vapour pressure of at least 1.9 bar at 120° C., B) Devolatilizing the mixture in the thermoplastic state at temperatures of at least 120° C., thereby lowering to not more than 0.5% by weight the content of the low-boiling constituents with vapour pressure of at least 1.9 bar at 120° C., C) Injecting the molten and devolatilized mixture into the mould cavity of an injection mould, the temperature of the mould cavity being below the glass transition temperature of the (meth)acrylate copolymer by at least 10° C., cooling the molten mixture, and removing the resultant moulding from the mould.
Owner:PFIZER INC +1
Methods of using two-part self-adhering dental compositions
ActiveUS20050014861A1High bonding strengthSimple methodImpression capsTooth crownsArylHigh concentration
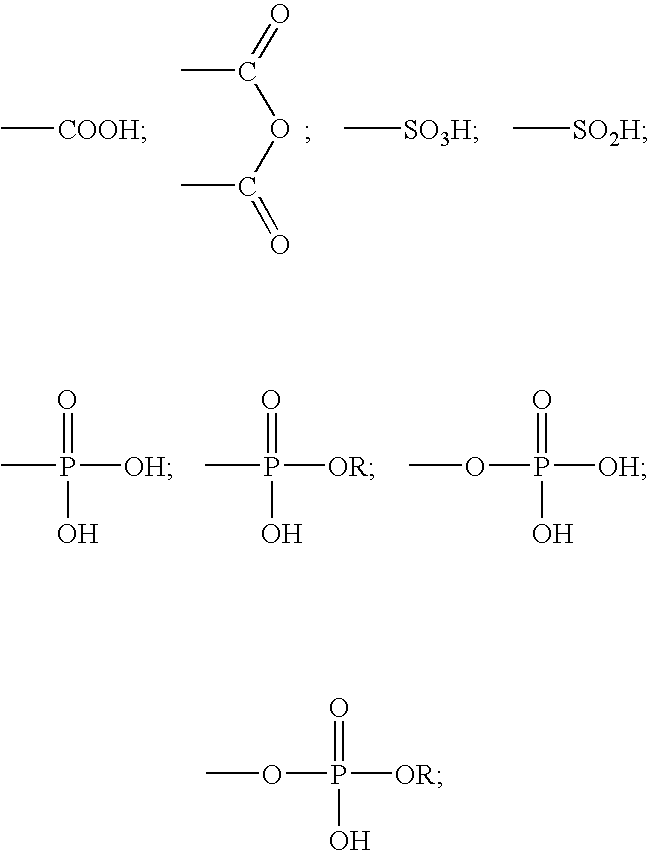
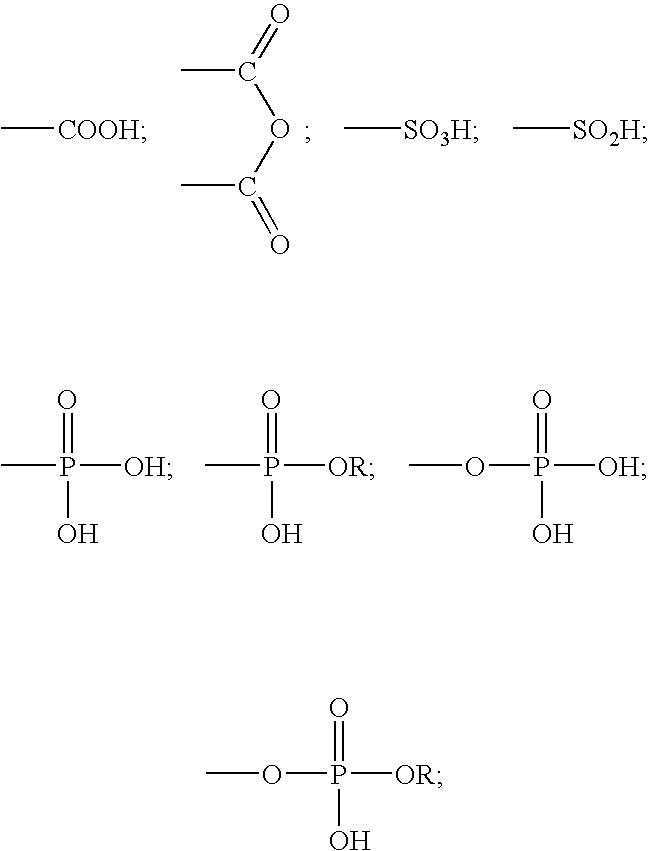
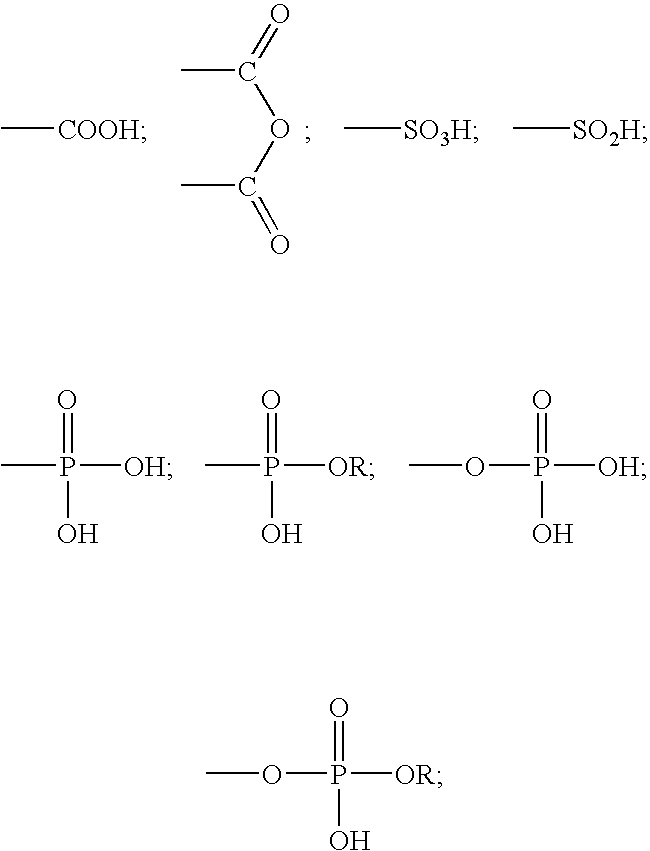
A method for providing a dental composition comprising providing a paste / paste two-part self-adhering dental composition comprising (a) at least one acidic compound containing at least one acidic moiety selected from the group consisting of where R is an alkyl or aryl group; (b) at least one polymerizable monomer without any acidic group where the polymerizable group is selected from the group consisting of an acrylate, a methacrylate and a vinyl group; (c) at least one finely divided filler; (d) a reducing agent; and (e) an oxidizing agent; and providing instructions for mixing the two pastes immediately prior to application where the ratio of a first paste containing (a) or a higher concentration of (a) to a second paste not containing (a) or containing a lower concentration of (a) is greater than 1:1 (by volume). The method also includes mixing the two pastes and applying the mixed composition to a dental substrate.
Owner:THE KERR
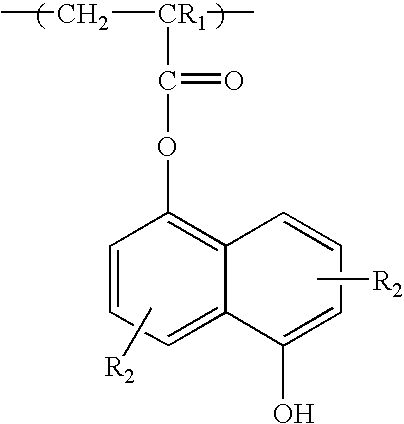
(Meth)acrylate esters of organosiloxane polyols, process for their preparation, and their use as radiation-curable materials
Owner:EVONIK DEGUSSA GMBH
Silyl (METH) acrylate copolymers, processes for preparing the same, antifouling paint compositions containing the silyl (METH) acrylate copolymers, antifouling coating films formed from the antifouling paint compositions, antifouling methods using the antifouling paint compositions, and hulls or underwater structures coated with the antifouling coating films
InactiveUS6458878B1Improve antifouling performanceSuppressed hydrolysis rateAntifouling/underwater paintsPaints with biocidesMeth-Unsaturated monomer
Disclosed is a silyl (meth)acrylate copolymer which comprises 20 to 80% by weight of (a) silyl (meth)acrylate constituent units (I), 0.01 to 40% by weight of (b) acrylic unsaturated monomer constituent units (II) and 5 to 79.9% by weight of Ĉ unsaturated monomer constituent units other than the constituent units (a) and (b). Also disclosed is a process for preparing the silyl (meth)acrylate copolymer, an antifouling paint composition comprising the copolymer, a coating film formed from the paint composition, a hull with the coating film and an antifouling method using the paint composition.
Owner:CHUGOKU MARINE PAINTS
Polymer binder for electrochemical device comprising multiply stacked electrochemical cells
ActiveUS20060275661A1Increase in bulk energy densityHighly-integratedLarge-sized flat cells/batteriesFinal product manufactureMethacrylateMeth-
Disclosed is an electrochemical device, which comprises: (A) a binder comprising polymer particles obtained from the polymerization of: (a) 20-70 parts by weight of a (meth)acrylic acid ester monomer; (b) 20-60 parts by weight of a vinyl monomer; and (c) 0.01-30 parts by weight of an unsaturated carboxylic acid monomer, based on 100 parts by weight of a binder polymer; and (B) electrochemical cells stacked multiply by using the binder, wherein the binder allows electrode active material particles in an electrode to be fixed and interconnected among themselves and between the electrode active material and a collector, and the electrode and a separator that is in contact with the electrode are bonded to each other by way of hot fusion. The binder is also disclosed. The binder has excellent adhesion and thermal bonding characteristics, and thus is useful for an electrochemical device comprising multiply stacked electrochemical cells, and can improve the overall quality of a battery.
Owner:LG CHEM LTD
Aqueous ink composition and method of manufacturing the same
InactiveUS20050004261A1Improve printing qualityReduce blurDuplicating/marking methodsInksMethacrylatePolymer science
An aqueous ink composition comprising: a pigment, having a particle diameter as determined by the light scattering method of no less than 20 nm and no more than 200 nm; and a water dispersible polymer, having a styrene-equivalent number average molecular weight of as determined by gel permeation chromatography no less than 5000 and no more than 200000, having a surface tension of no less than 20 mN / m and no more than 40 mN / m, and wherein the abovementioned pigment is a polymer-coated pigment that is coated with the abovementioned water dispersible polymer, is provided. The water-dispersible polymer may be a copolymer of monomers, mainly comprising acrylic acid and / or methacrylic acid and an acrylate and / or methacrylate. With this ink composition, the printing quality can be improved.
Owner:SEIKO EPSON CORP
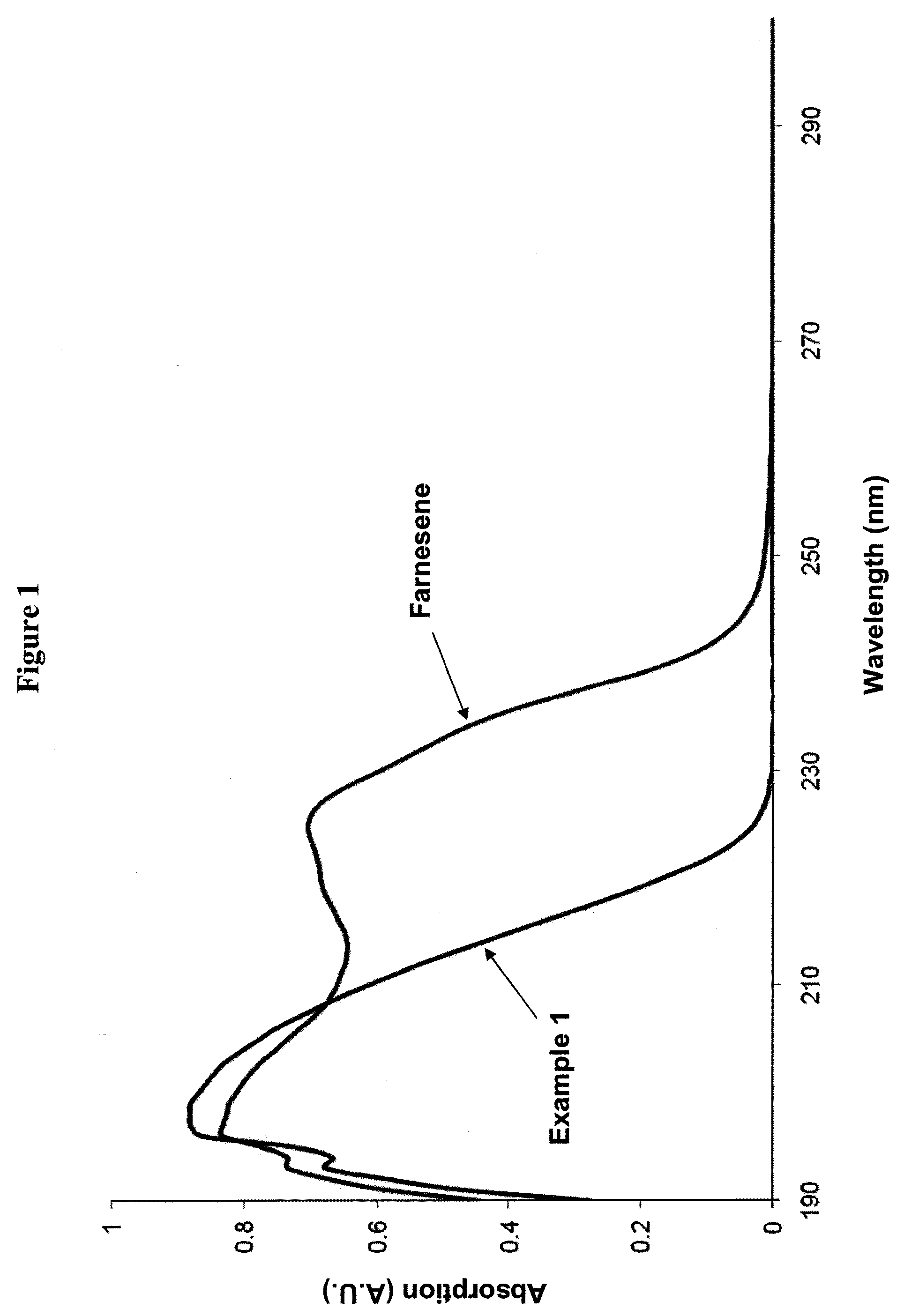
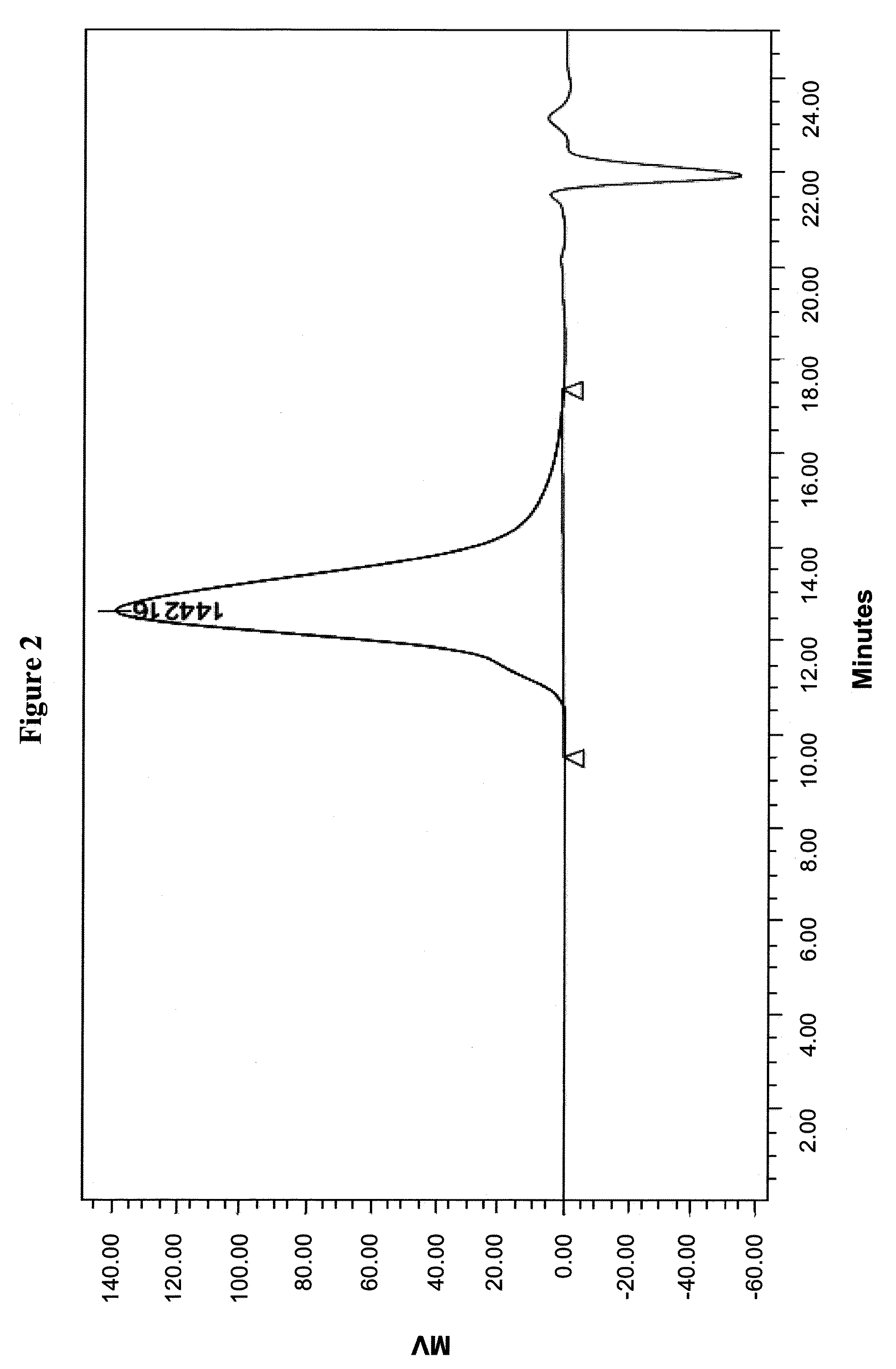
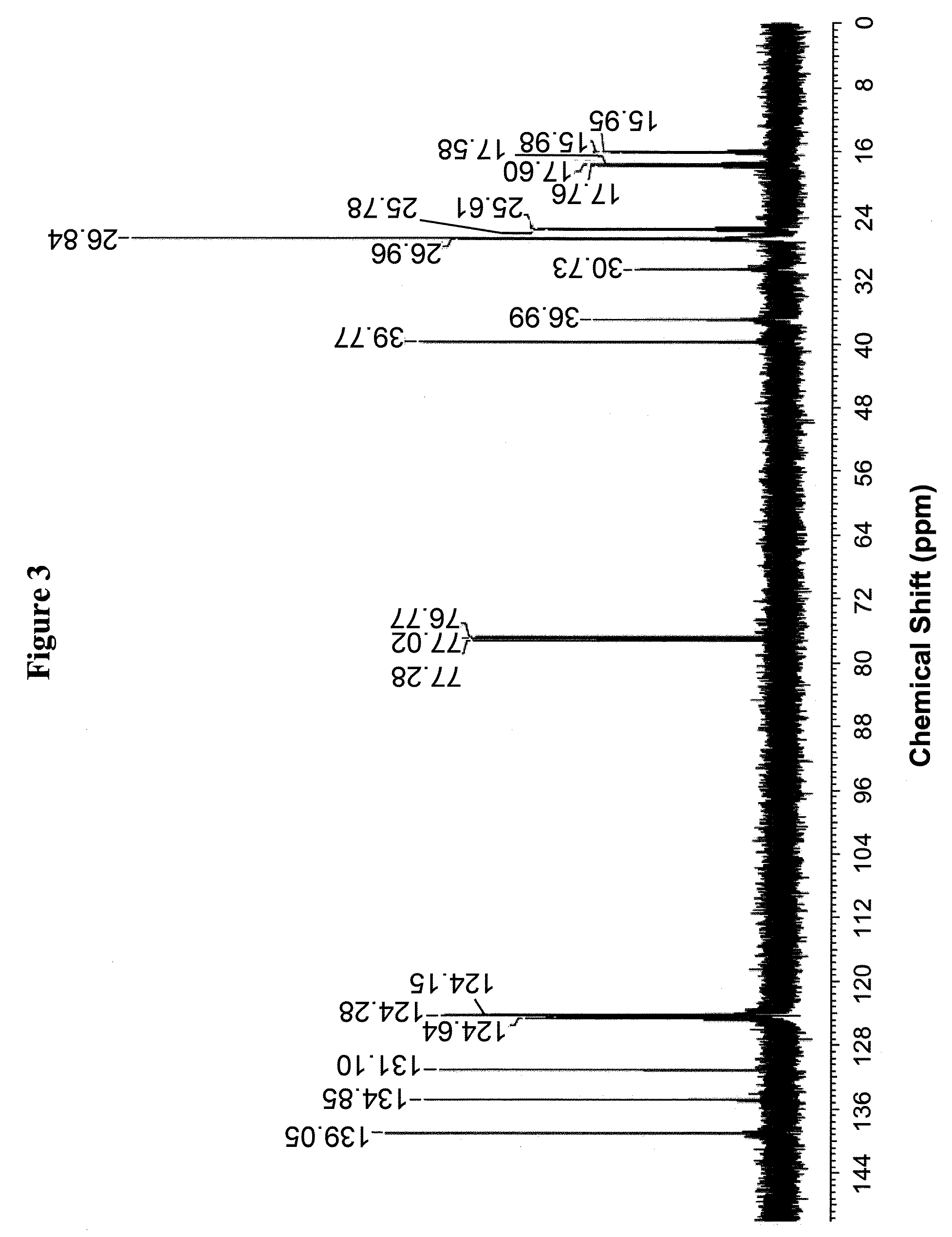
Farnesene interpolymers
Farnesene interpolymer comprises units derived from a farnesene (e.g., α-farnesene or β-farnesene) and units derived from at least one vinyl monomer. The farnesene interpolymer can be prepared by copolymerizing the farnesene and at least one vinyl monomer in the presence of a catalyst. In some embodiments, the farnesene is prepared from a sugar by using a microorganism. In other embodiments, the at least one vinyl monomer is ethylene, an α-olefin, or a substituted or unsubstituted vinyl halide, vinyl ether, acrylonitrile, acrylic ester, methacrylic ester, acrylamide or methacrylamide, or a combination thereof.
Owner:AMYRIS INC
Positive resist containing naphthol functionality
ActiveUS20060105267A1Improve photolithographic effectExcellent etch resistancePhotosensitive material auxillary/base layersPhotosensitive material processingResistMethacrylate
Acid-catalyzed positive resist compositions which are imageable with 193 nm radiation are obtained using a polymer having acrylate / methacrylate monomeric units comprising a naphthol ester group. The resist may optionally contain polymer having acrylate / methacrylate monomeric units with fluorine-containing functional groups. The resists containing the polymer having acrylate / methacrylate monomeric units comprising a naphthol ester group have an improved process window, including improved etch resistance and reduced swelling compared to conventional fluorine-containing 193 nm resist.
Owner:IBM CORP
Polymer coated drug-ion exchange resins and methods
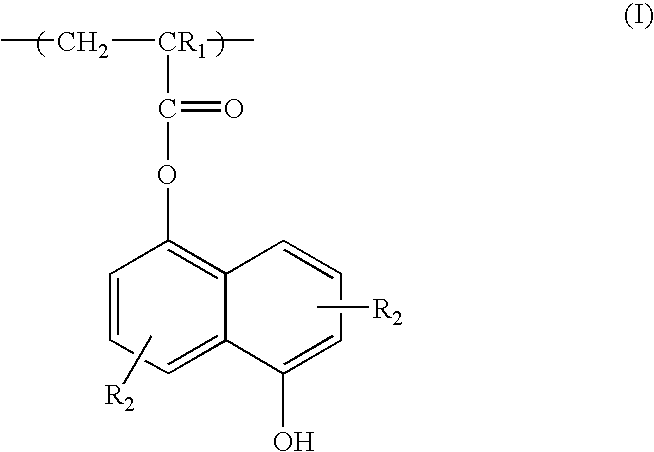
Included are compositions, and methods of making, coated controlled-release drug and ion exchange resin form complexes. Methacrylate coatings, which can be free of plasticizers particularly with Eudragit® NE type polymer, are preferred to enhance the control of drug release from the drug-resin complexes. Liquid formulations including the coated resin forms and a chelating agent to inhibit degradation are also included.
Owner:WOCKHARDT EU OPERATIONS SWISS
Polyvinylidene fluoride weather resistant coating compositions including polymethyl methacrylate
InactiveUS6362271B1Improve adhesionReduce the shrinkage of the polyvinylidene fluorideMixing methodsCoatingsMethacrylatePolymethyl methacrylate
A weather resistant coating composition comprising a miscible polymer blend of highly crystalline polyvinylidene fluoride polymer and polyalkyl methacrylate, such as PVDF and PMMA. The miscible blend comprises from about 50 weight percent to about 90 weight percent polymer comprising polyvinylidene fluoride and from about 10 weight percent to about 50 weight percent polyalkyl methacrylate. The polyalkyl methacrylate having a molecular weight within the range of about 25,000 grams per mole to about 200,000 grams per mole. The crystallinity of the polyvinylidene fluoride polymer is about 20% to about 70%. The weather resistant coating has excellent and unexpected physical characteristics, including solvent resistance and gloss retention.
Owner:SOLVAY SOLEXIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com