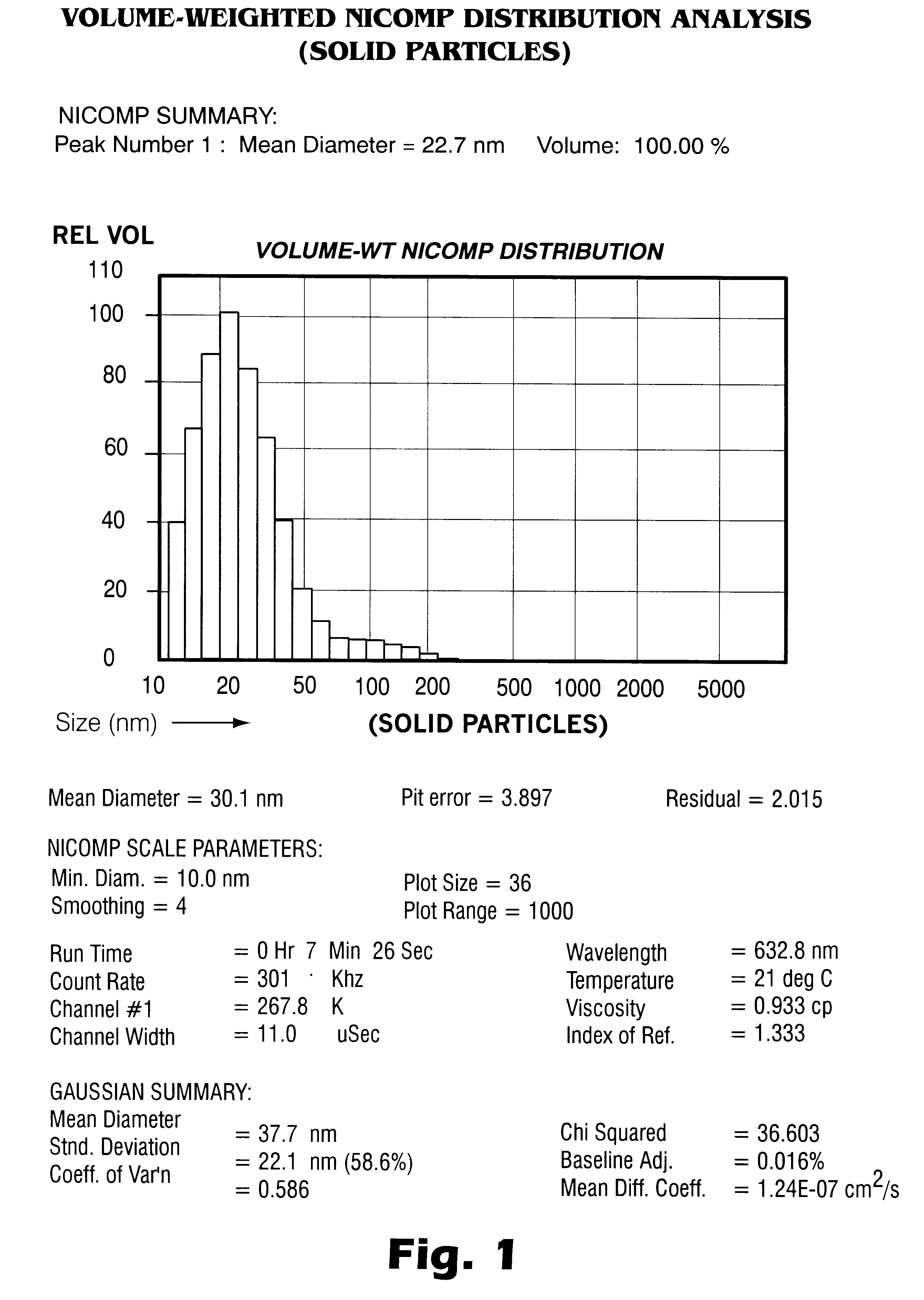
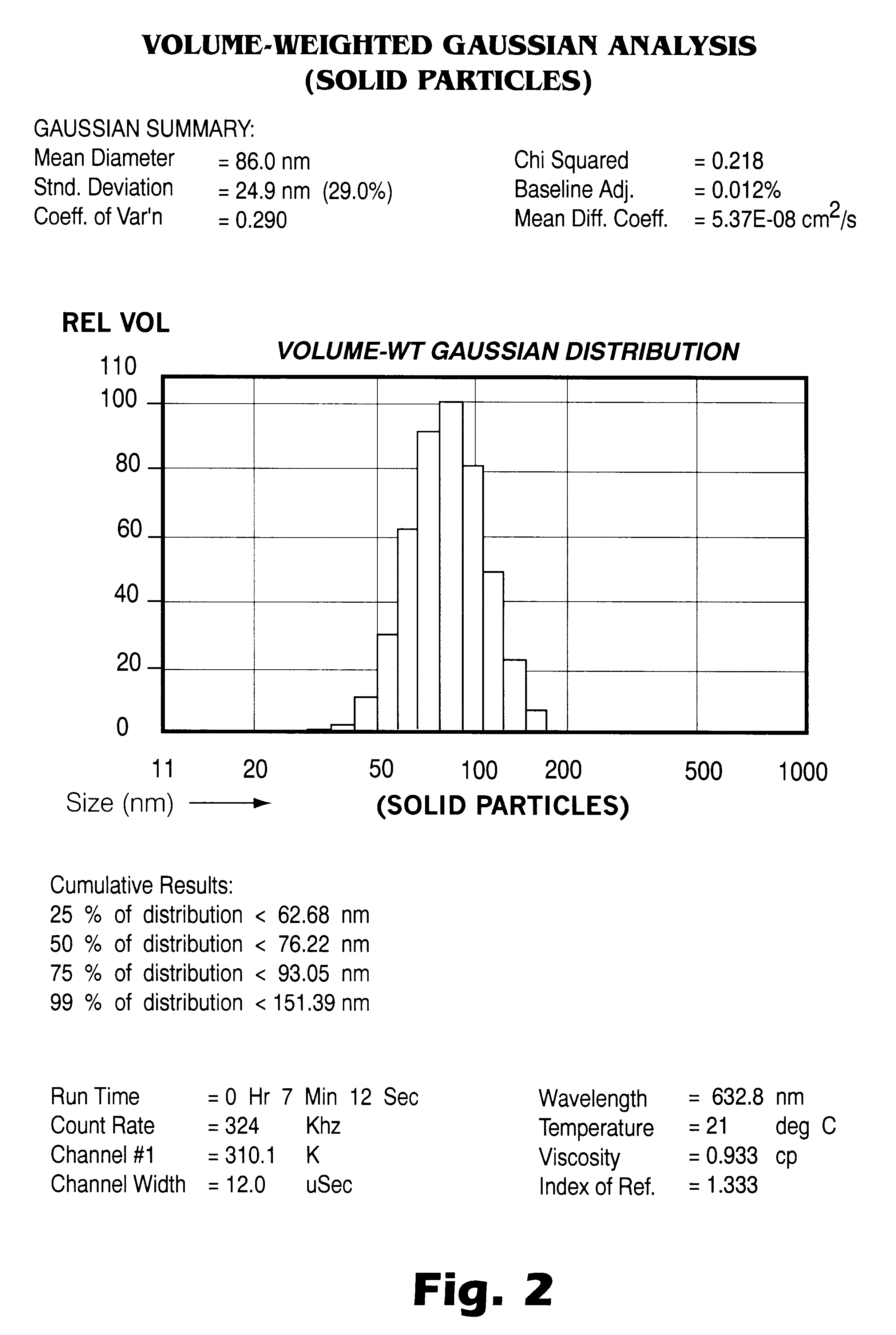
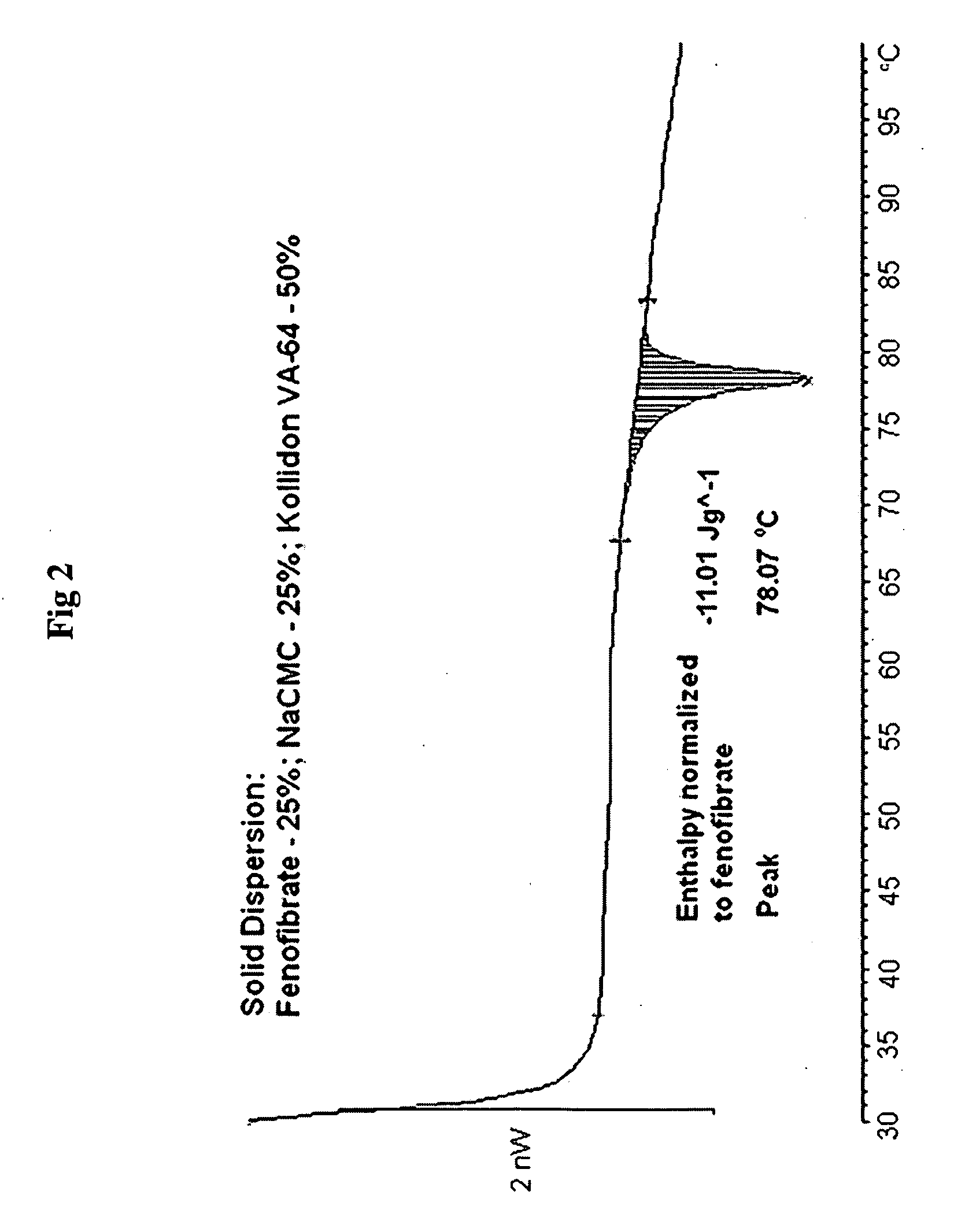
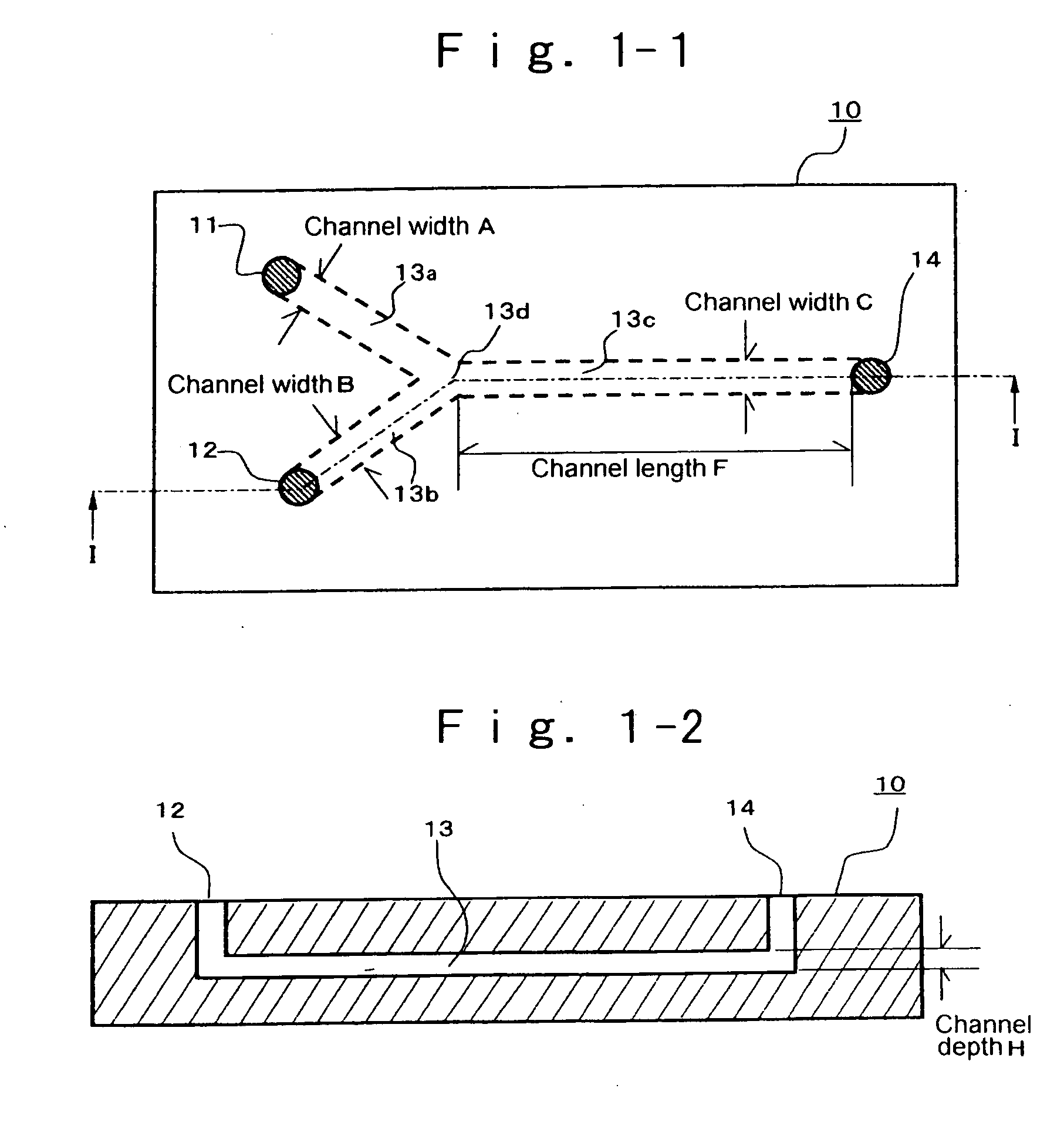
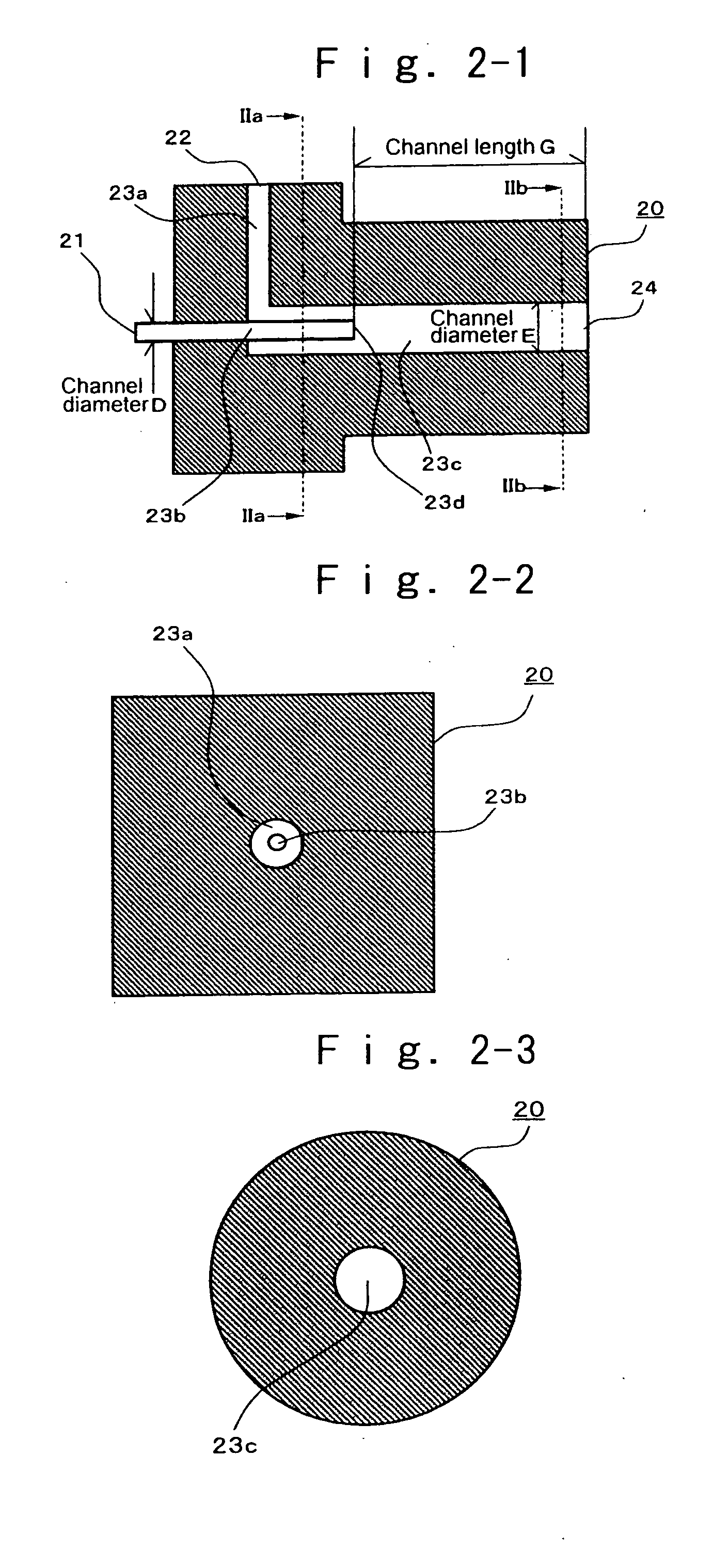
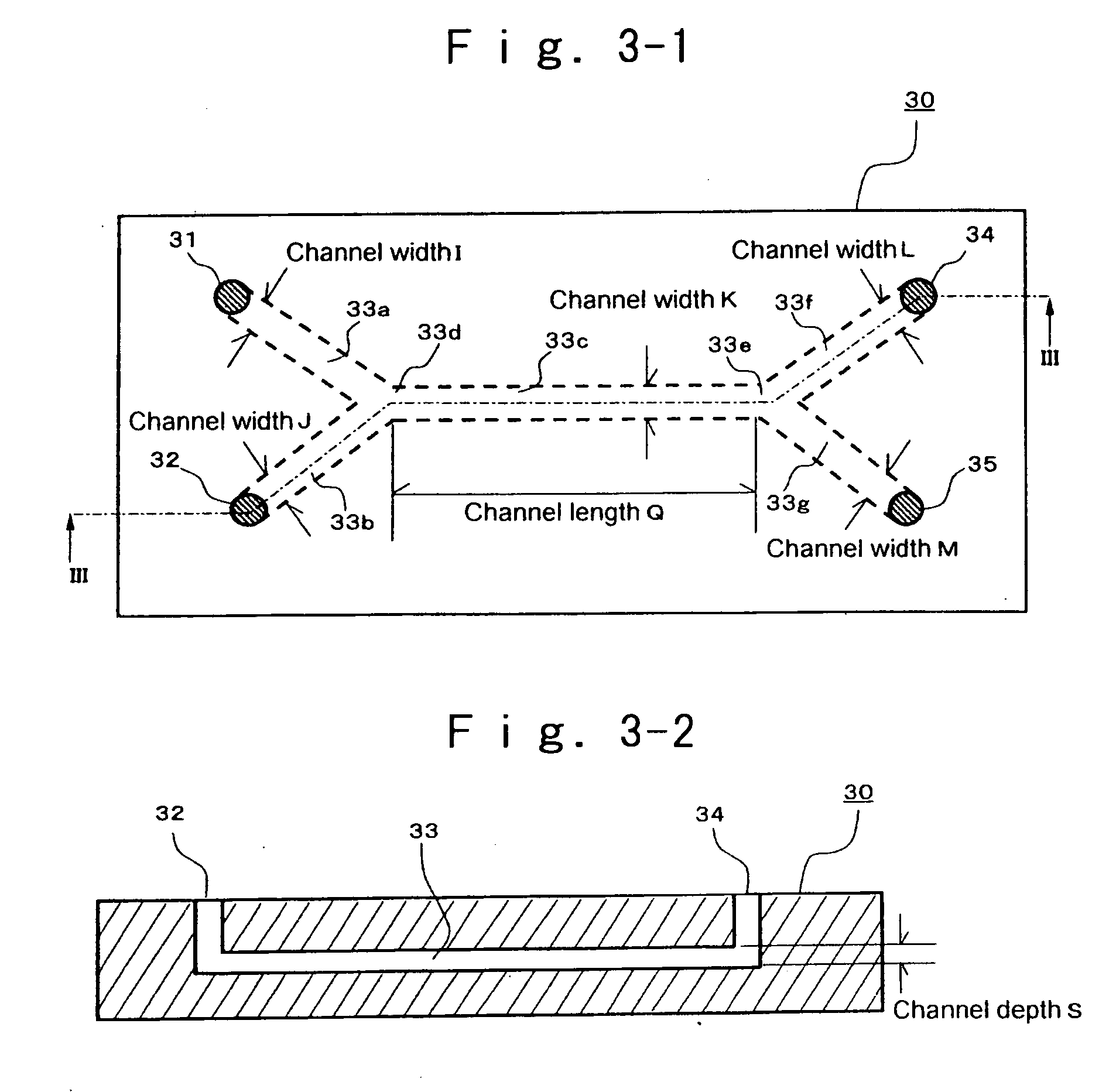
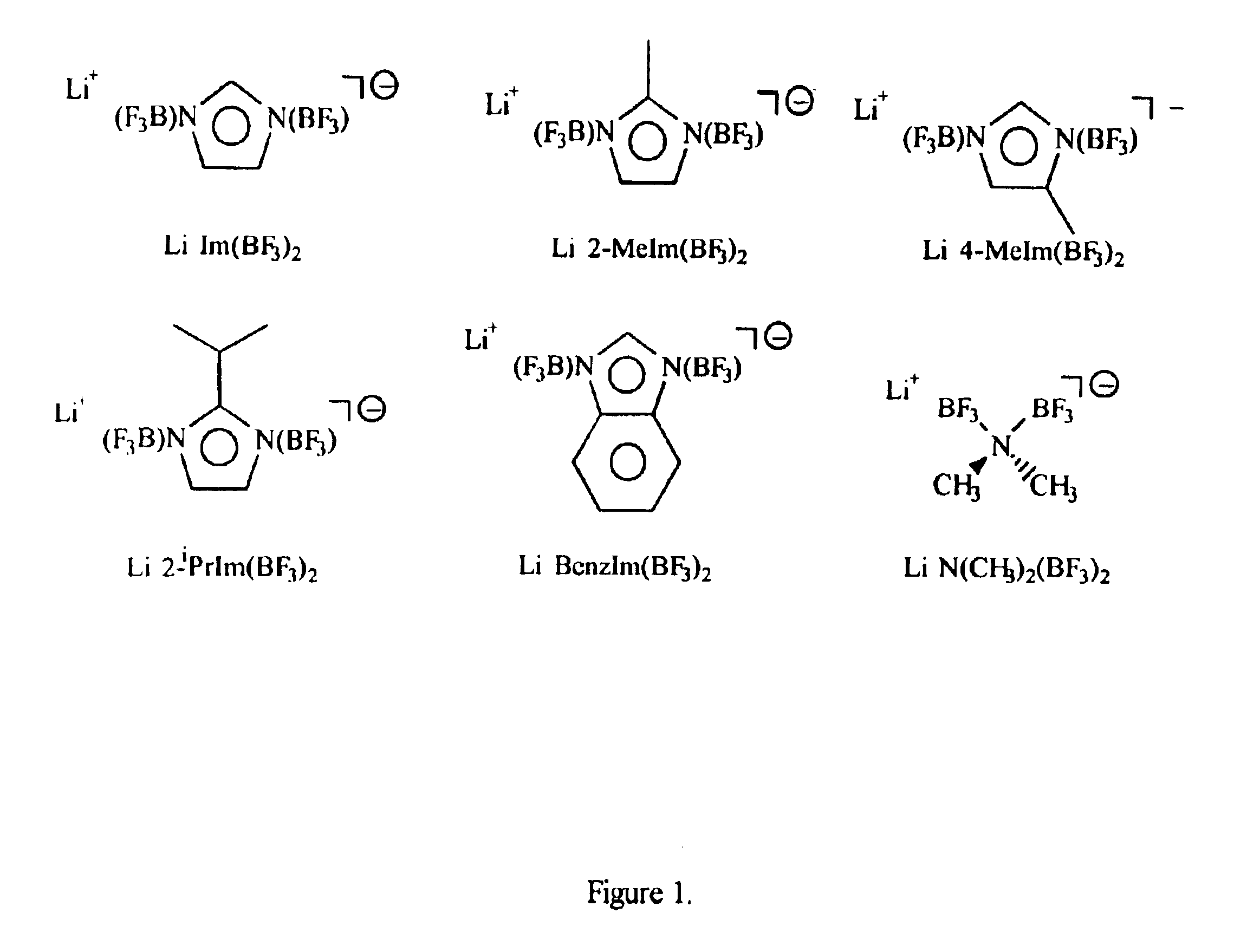
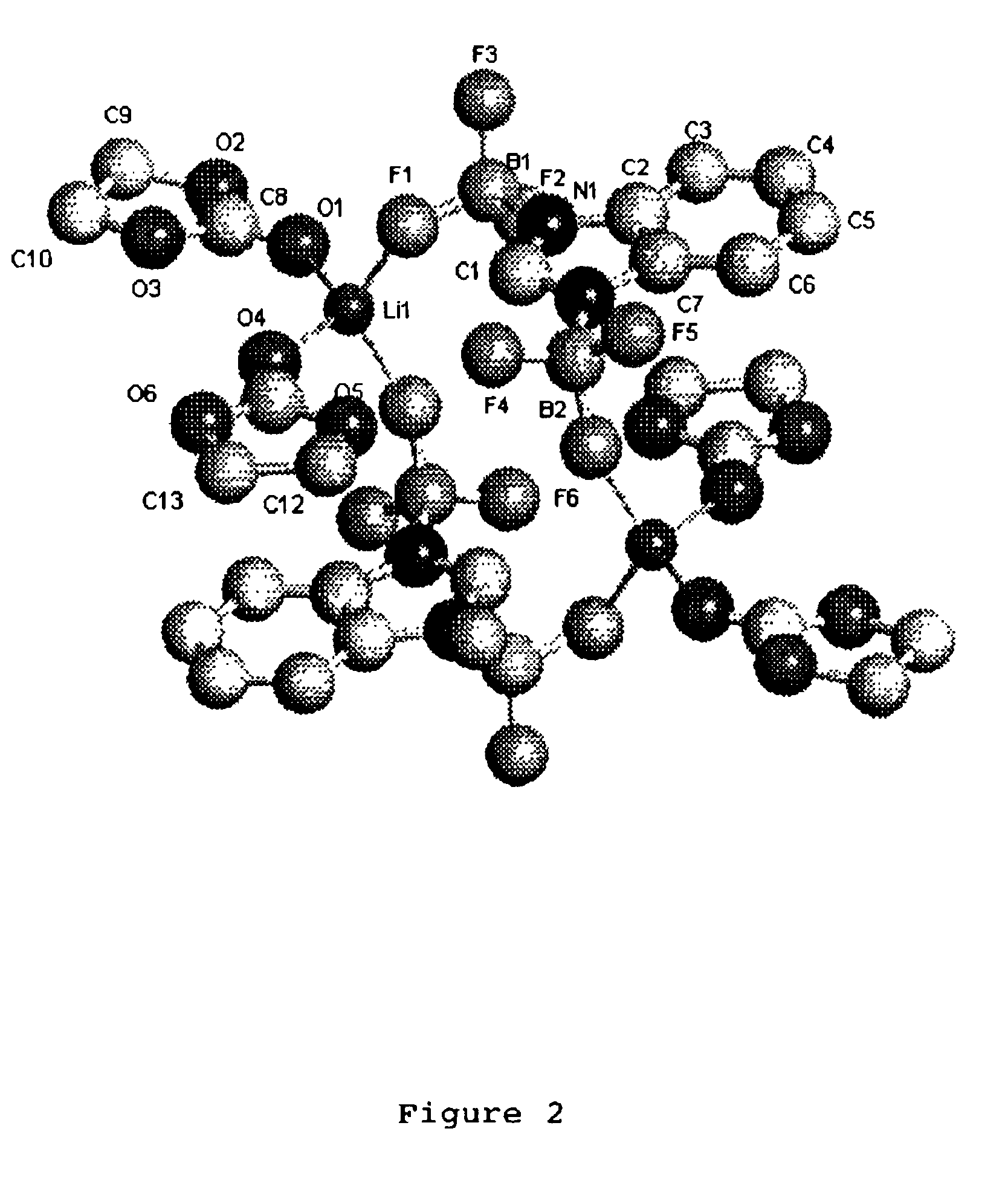
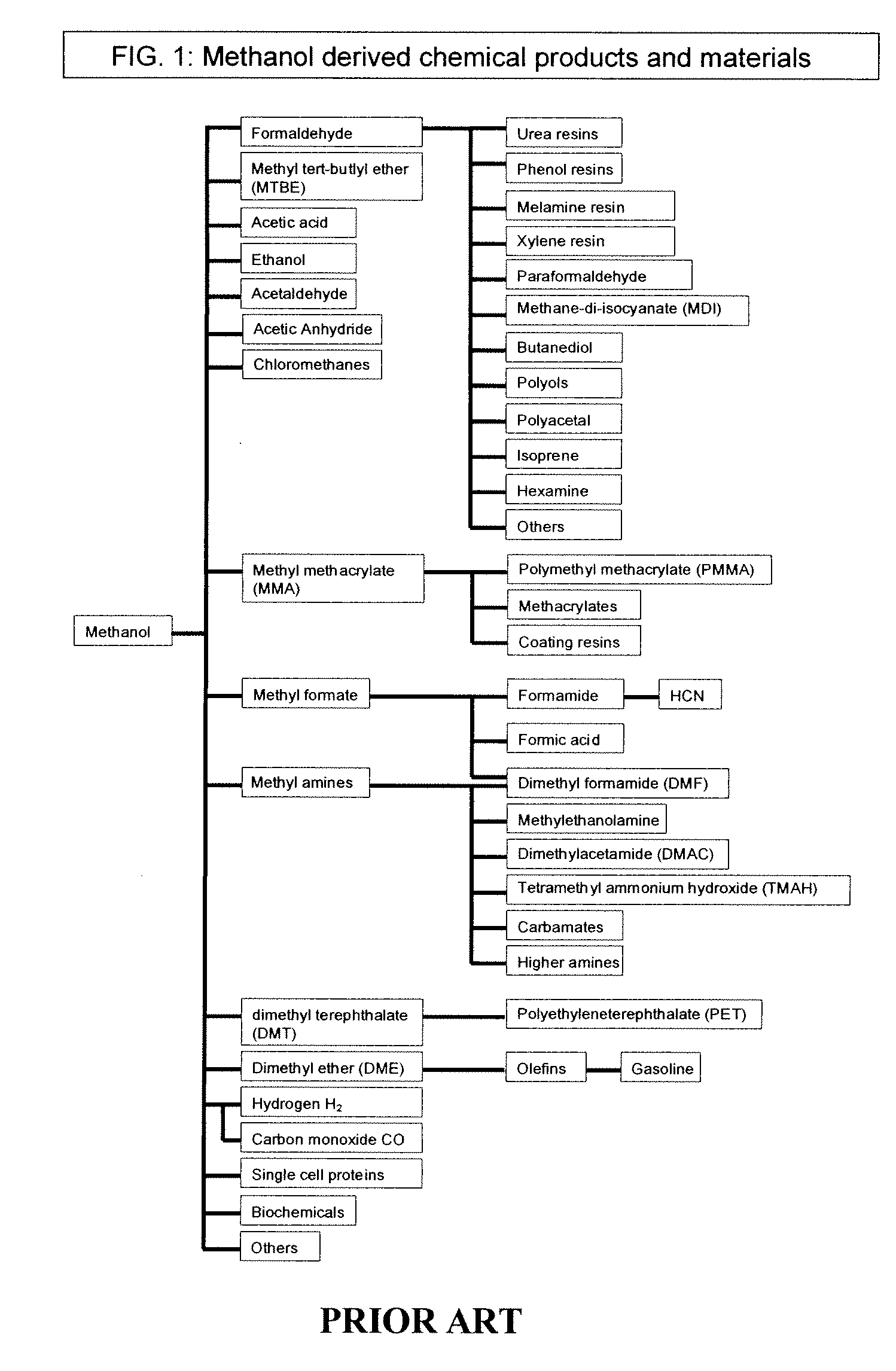
Patents
Literature
4970 results about "Aqueous medium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Permanent aqueous mounting medium is a unique aqueous-based mounting medium designed for the permanent preservation of tissue sections and cell smears which have been immunohistochemically stained with chromogens which cannot be dehydrated with organic solvents.
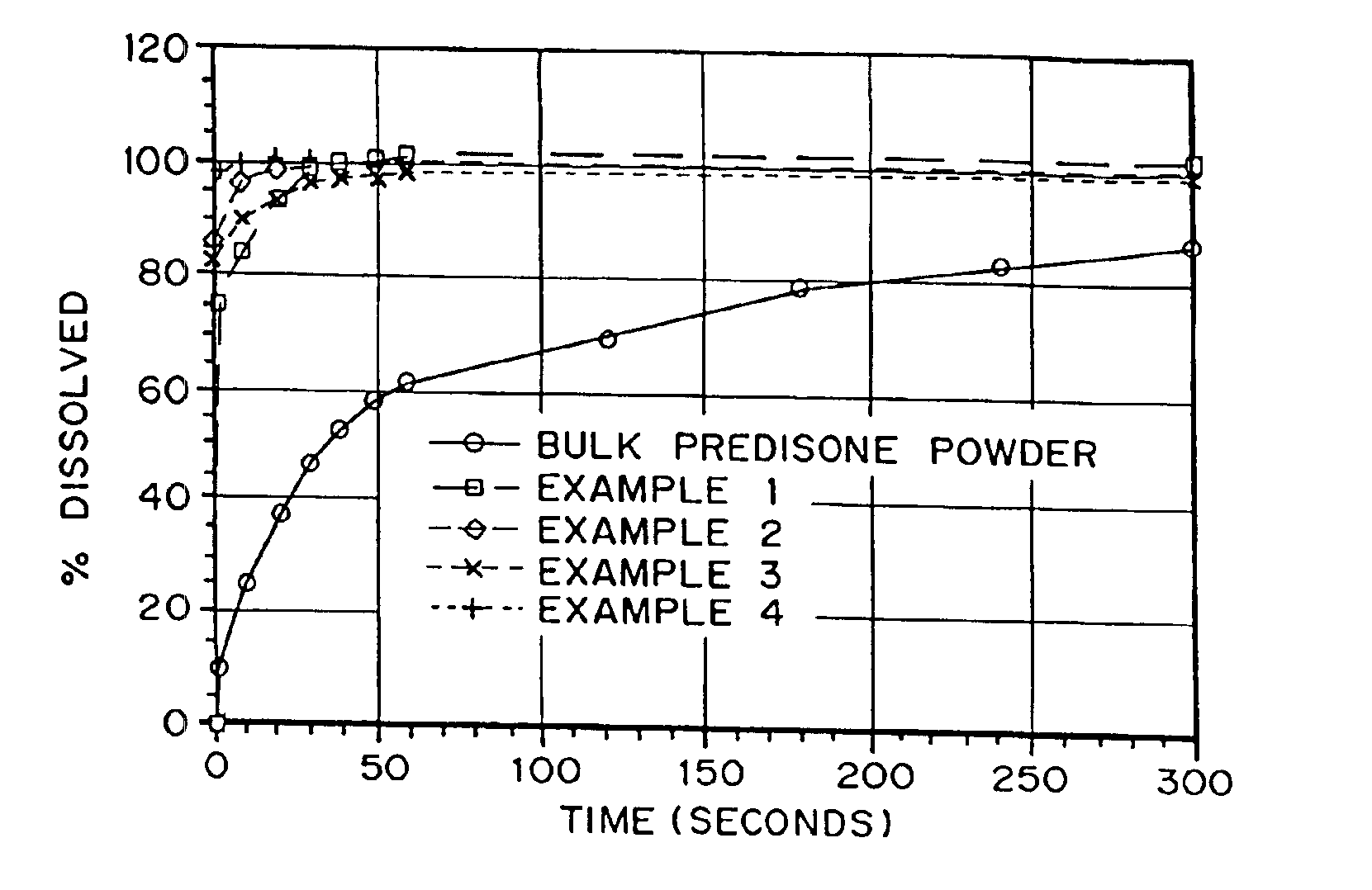
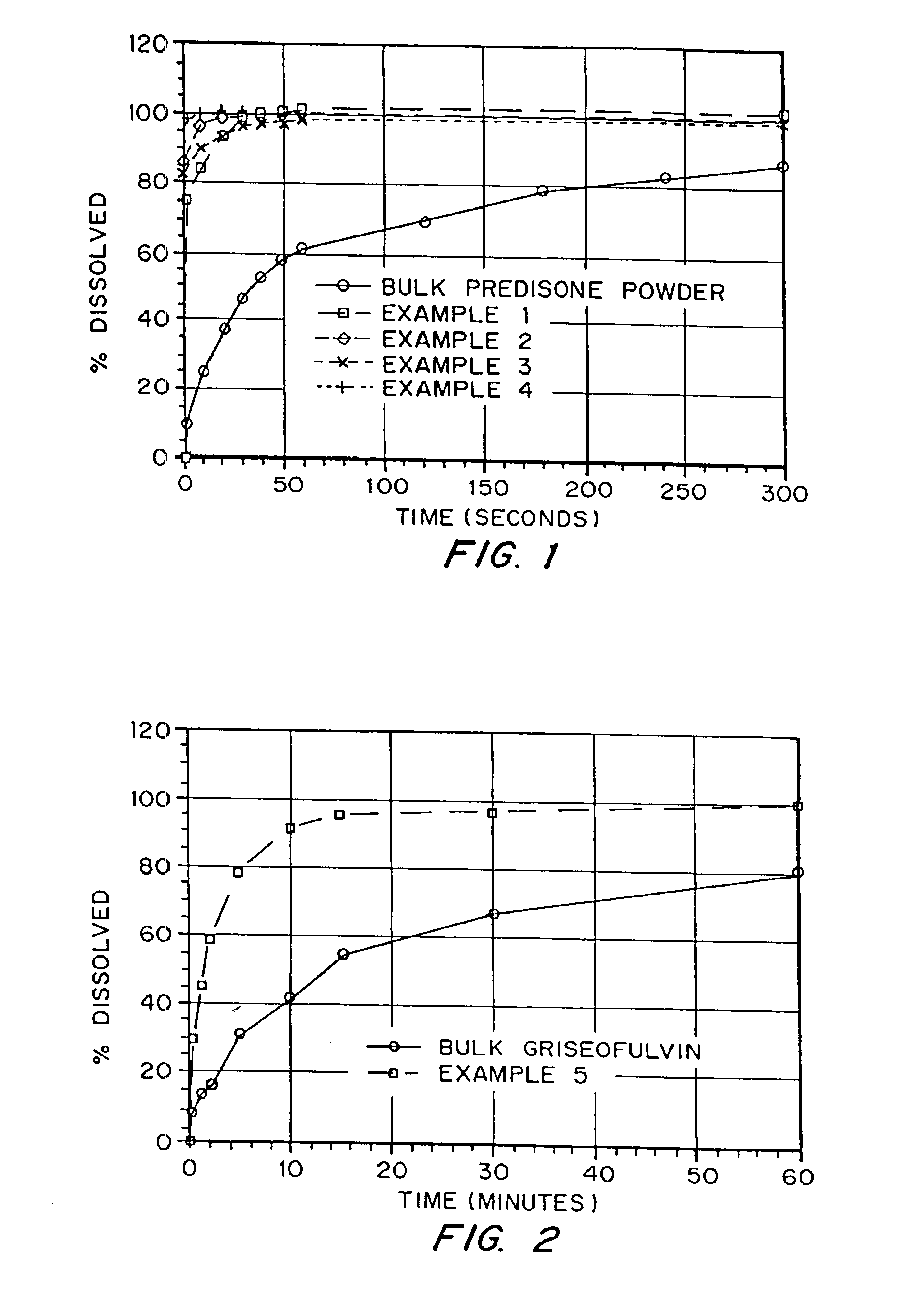
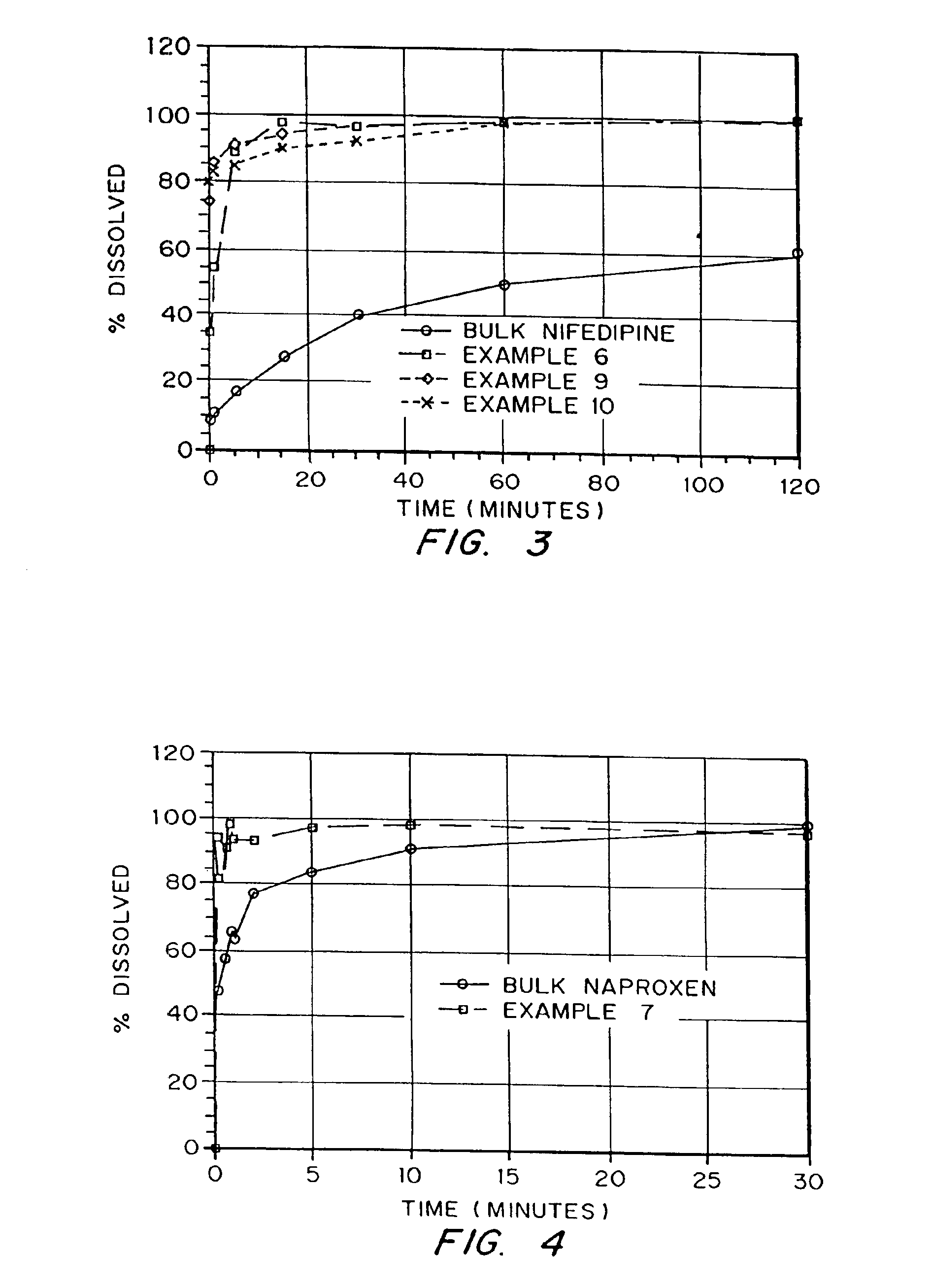
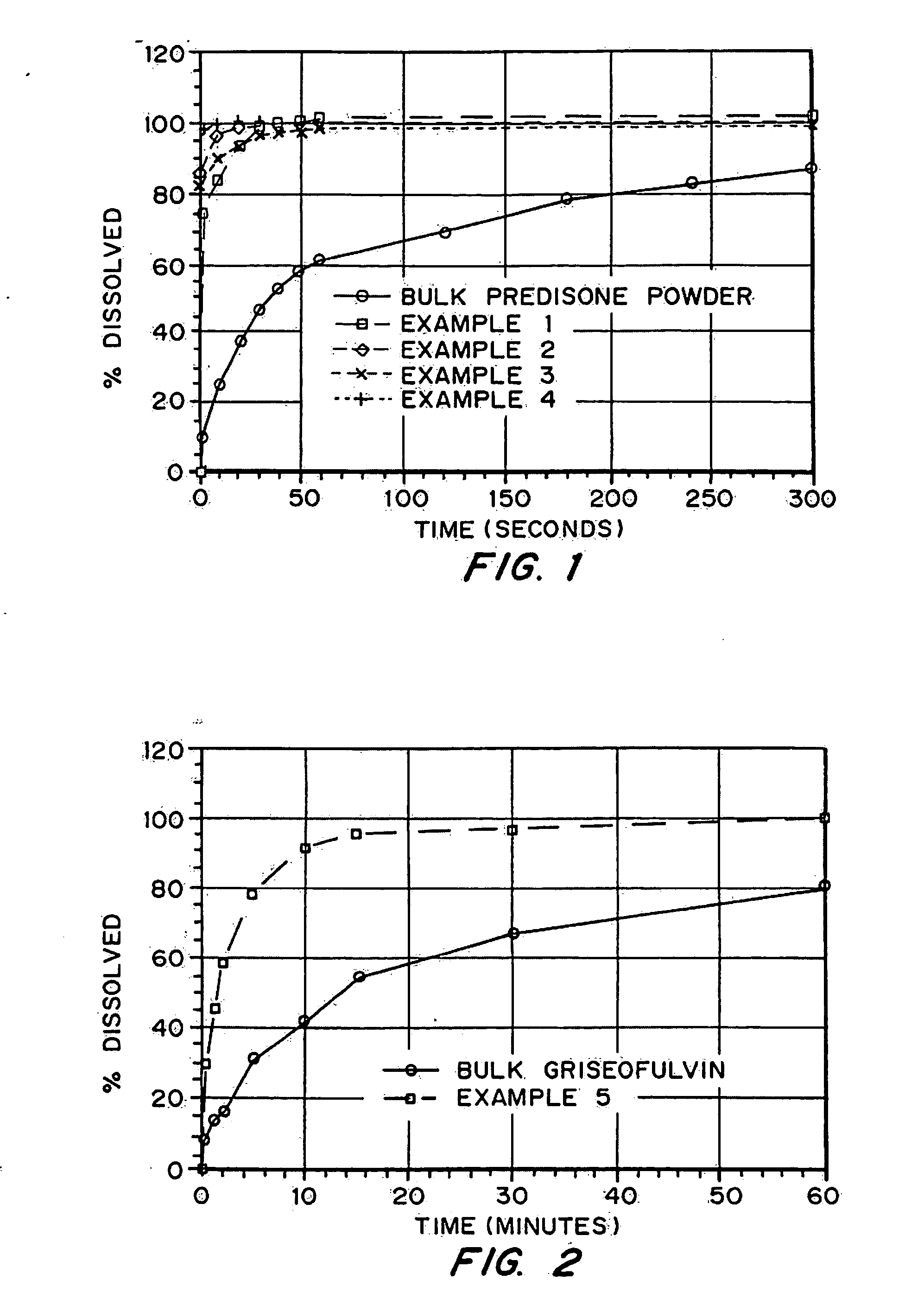
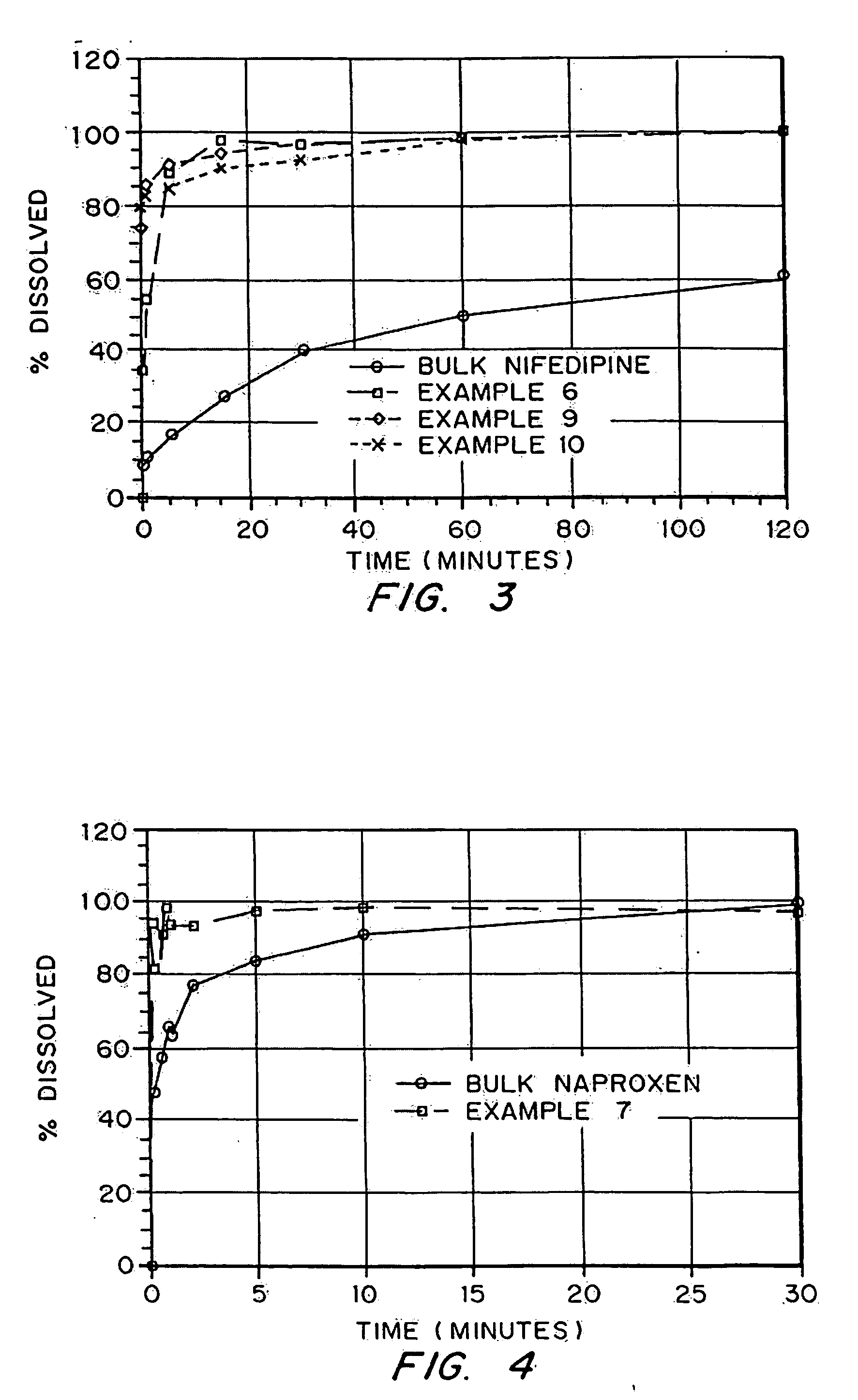
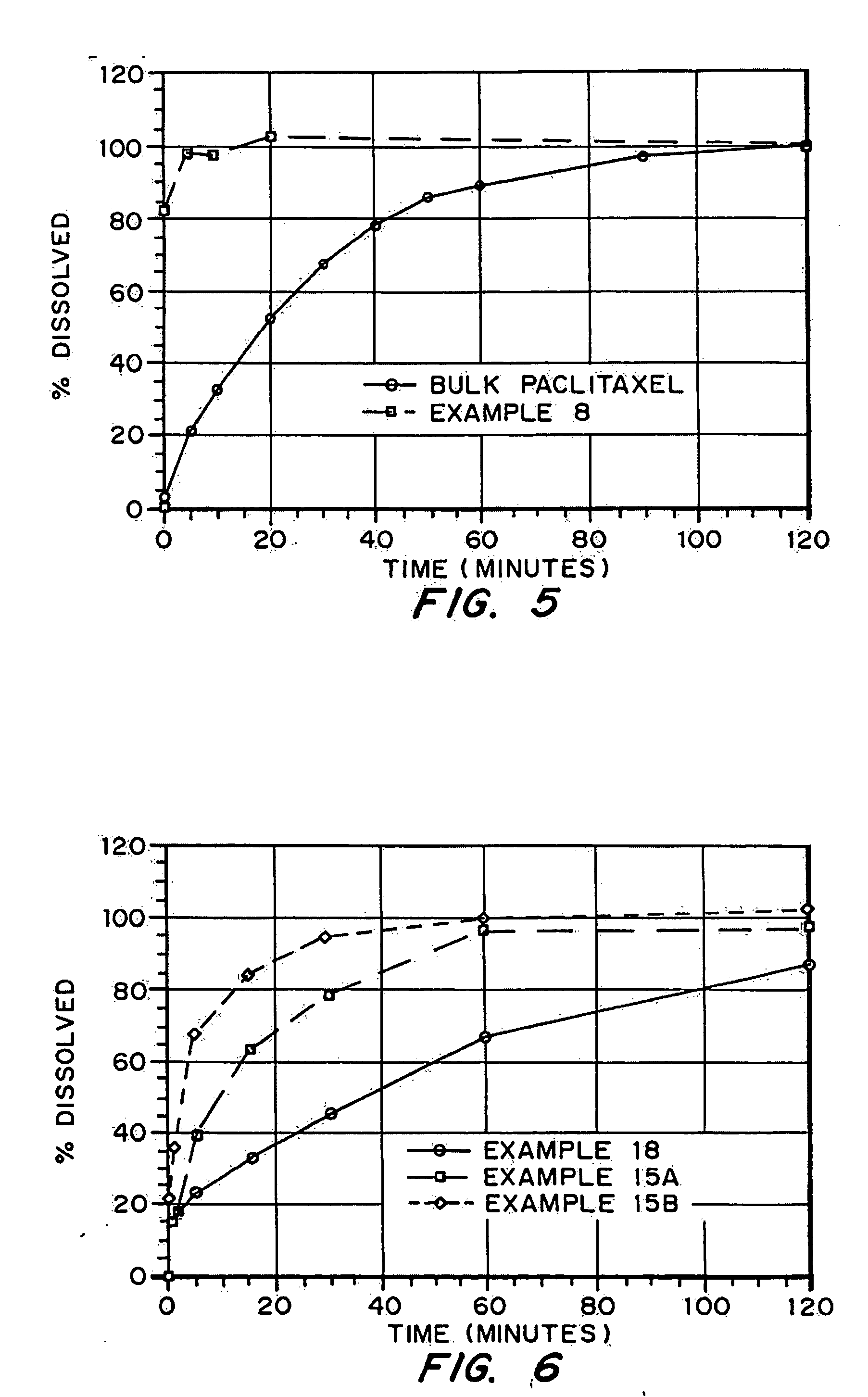
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
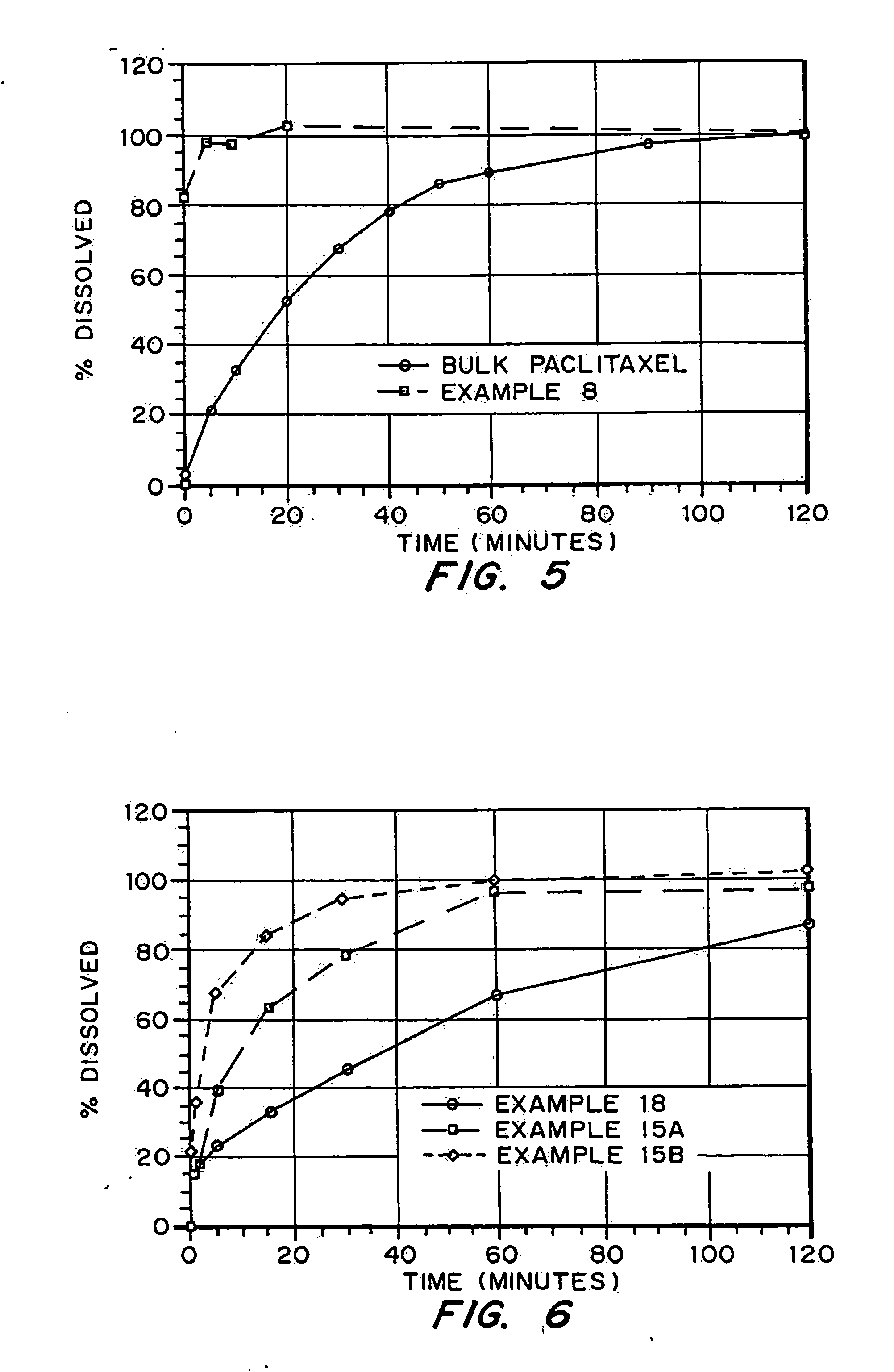
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Diaper providing temperature insensitive liquid handling
The present invention relates to absorbent articles, such as diapers and sanitary napkins, and acquisition members useful for such articles. More specifically, the invention relates to an acquisition member comprising a multitude of fibers and a latex binder, the liquid acquisition member having a void volume of at least 7 cubic centimeter per gram in the temperature range from 20° C. to 40° C., the binder comprising a dispersion of a polymer substance in an essentially aqueous medium, the polymer substance being capable of forming a film, said film having a tan δ value, as defined herein, at 20° C. and at 40° C., wherein the tan δ value at 40° C. is not greater than said tan δ value at 20° C.
Owner:THE PROCTER & GAMBLE COMPANY
Ocular delivery of polymeric delivery formulations
InactiveUS20060210604A1Large doseEliminate lossSenses disorderPharmaceutical delivery mechanismControlled releaseMetabolite
The present invention provides a flowable composition suitable for use as a controlled release implant. The flowable composition can be administered into the ocular region of a mammal. The composition includes: (a) a biodegradable, biocompatible thermoplastic polymer that is at least substantially insoluble in aqueous medium, water or body fluid; (b) a biological agent, a metabolite thereof, a biological agently acceptable salt thereof, or a prodrug thereof; and (c) a biocompatible organic liquid, at standard temperature and pressure, in which the thermoplastic polymer is soluble. The present invention also provides methods of medical treatment that include administering the flowable composition into the ocular region of a mammal.
Owner:QLT USA INC
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Water soluble metal and semiconductor nanoparticle complexes
The invention provides a water soluble complex comprising an inner core of a metal or semi-conductor nanoparticle. The nanoparticle is coated with a hydrophobic ligand, which is encapsulated in a micelle. In an aqueous medium, the micelle comprises a hydrophilic shell and a hydrophobic core, the hydrophilic shell comprising a plurality of hydrophilic moieties, the hydrophobic core comprising a plurality of hydrophobic moieties, each hydrophobic moiety comprising at least one chain, each chain comprising a minimum of 8 atoms; wherein the total number of atoms in all chains for each moiety comprises at least 24 atoms. The micelle has a minimum average diameter of approximately 5 nm and a maximum average diameter of approximately 45 nm.
Owner:THE ROCKEFELLER UNIV
Processes to generate submicron particles of water-insoluble compounds
InactiveUS6177103B1Rapid of surface stabilizedRapid attainmentOrganic active ingredientsPowder deliveryWater insolubleChemical compound
Submicron particles of water-insoluble compounds, particularly drugs, are prepared by simultaneously stabilizing microparticulate suspensions of same with surface modifier molecules by rapid expansion into an aqueous medium from a compressed solution of the compound and surface modifiers in a liquefied gas and optionally homogenizing the aqueous suspension thus formed with a high pressure homogenizer.
Owner:JAGOTEC AG +1
Core-shell fluoropolymer dispersions
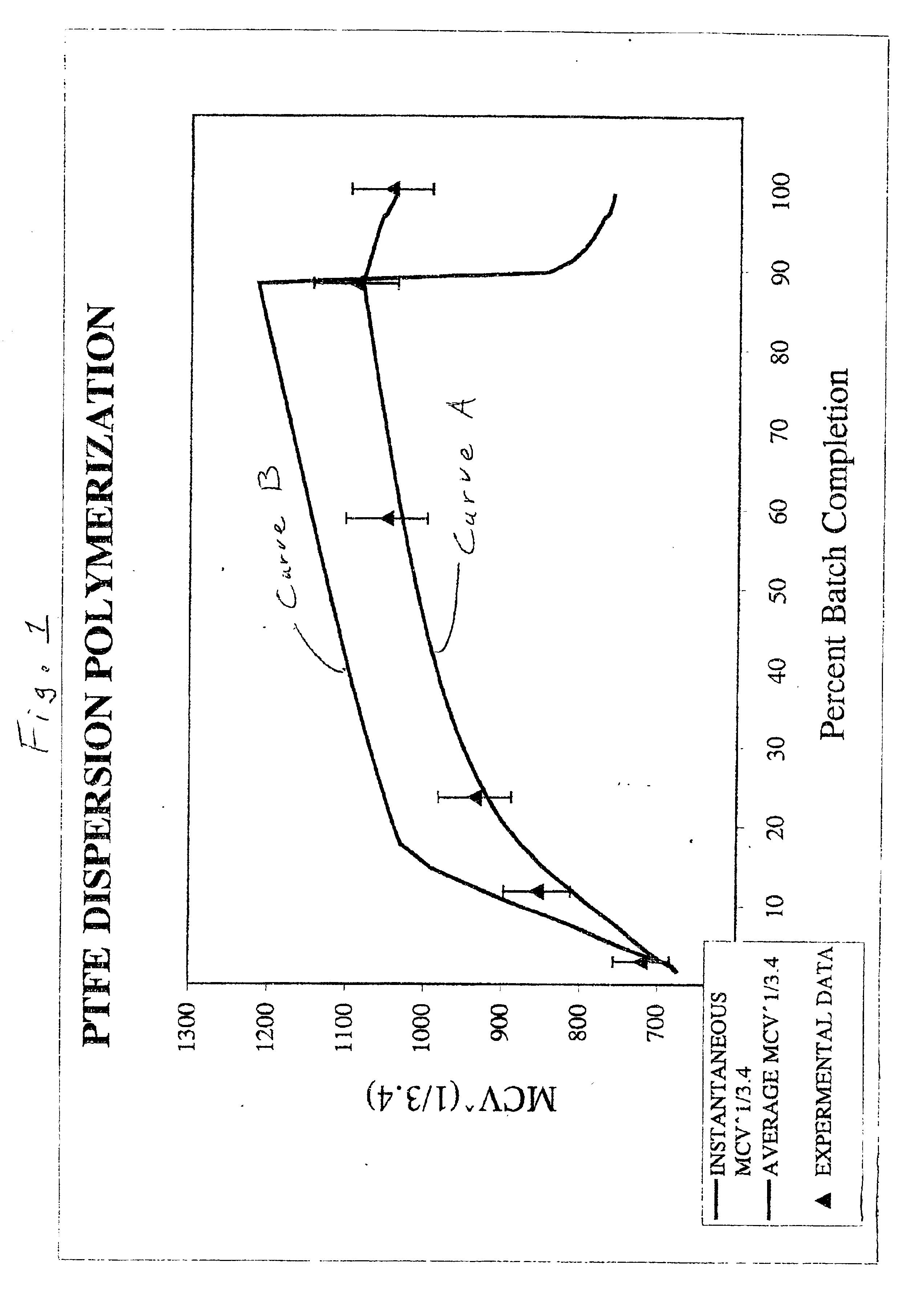
InactiveUS6841594B2High shear stabilityExtend elastic lifeSynthetic resin layered productsCellulosic plastic layered productsTetrafluoroethylenePolymer science
A dispersion of non-melt-processible fluoropolymer particles having an SSG of less than about 2.225 in aqueous medium. The fluoropolymer particles comprise a core of high molecular weight polytetrafluoroethylene having an average melt creep viscosity greater than about 1.5×1010 Pa·s and a shell of lower molecular weight polytetrafluoroethylene or modified polytetrafluoroethylene. The shell has an average melt creep viscosity greater than about 9×109 Pa·s and comprises about 5 to about 30% by weight of the particles. The fluoropolymer in the dispersion of the invention is fibrillating.
Owner:THE CHEMOURS CO FC LLC
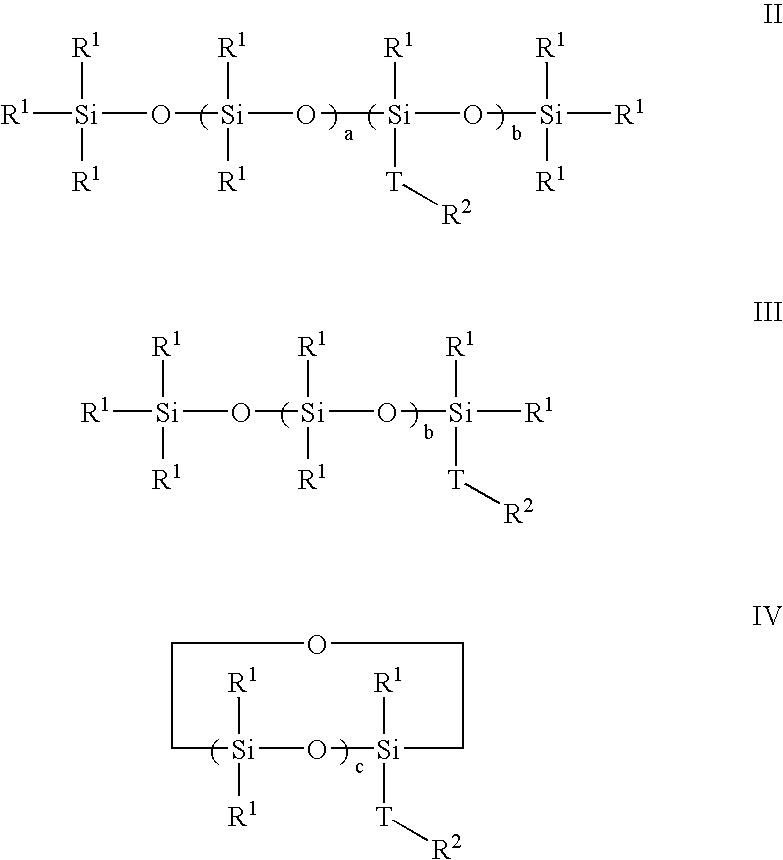
Polymerization of halogen-containing monomers using siloxane surfactant
Halogenated polymers are prepared by a process comprising polymerizing at least one halogen-containing monomer in an aqueous medium containing monomer, radical initiator, and siloxane surfactant. The medium may optionally contain one or more of an antifoulant, a buffering agent and a chain-transfer agent.
Owner:ARKEMA INC
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Compositions comprising lipophilic active compounds and method for their preparation
ActiveUS20090098200A1Quick releaseImprove bioavailabilityPowder deliveryBiocideHydrophilic polymersCompound (substance)
Compositions are provided comprising a lipophilic active compound, e.g., a human or veterinary drug or a nutraceutical, interwoven with a polymeric matrix formed by two or more polymers, wherein one of the polymers is an amphiphilic polymer and the other polymer is either an amphiphilic polymer with a different hydrophobic-hydrophilic balance or a hydrophilic polymer, and the active lipophilic compound has modified physicochemical properties. The composition forms colloidal nanodispersion upon contact with aqueous media.
Owner:SOLUBEST
Organic pigment fine-particle, and method of producing the same
A method of producing a fine particle of an organic pigment, containing the steps of: flowing a solution of an organic pigment dissolved in an alkaline or acidic aqueous medium, through a channel which provides a laminar flow; and changing a pH of the solution in the course of the laminar flow.
Owner:FUJIFILM CORP
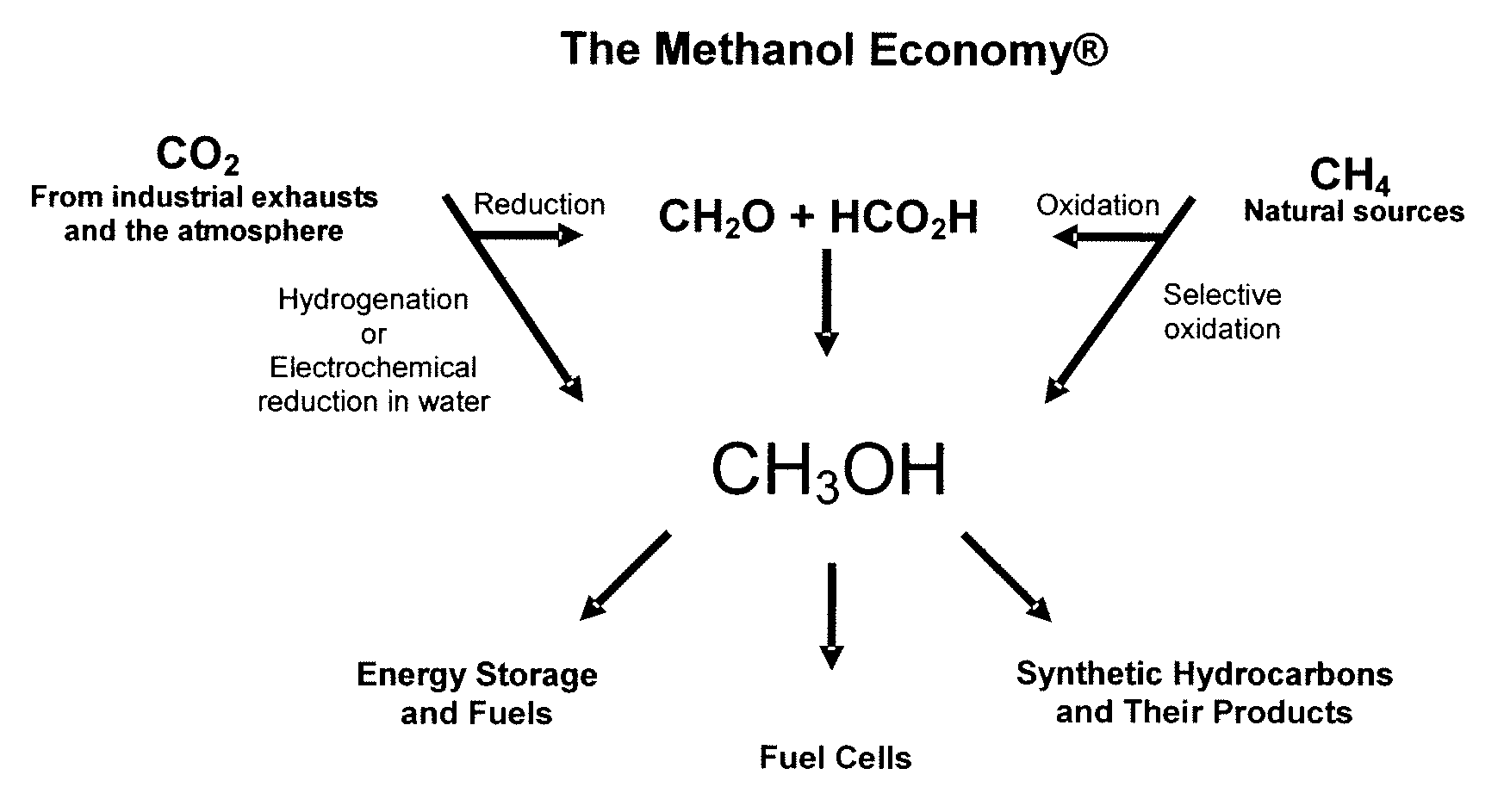
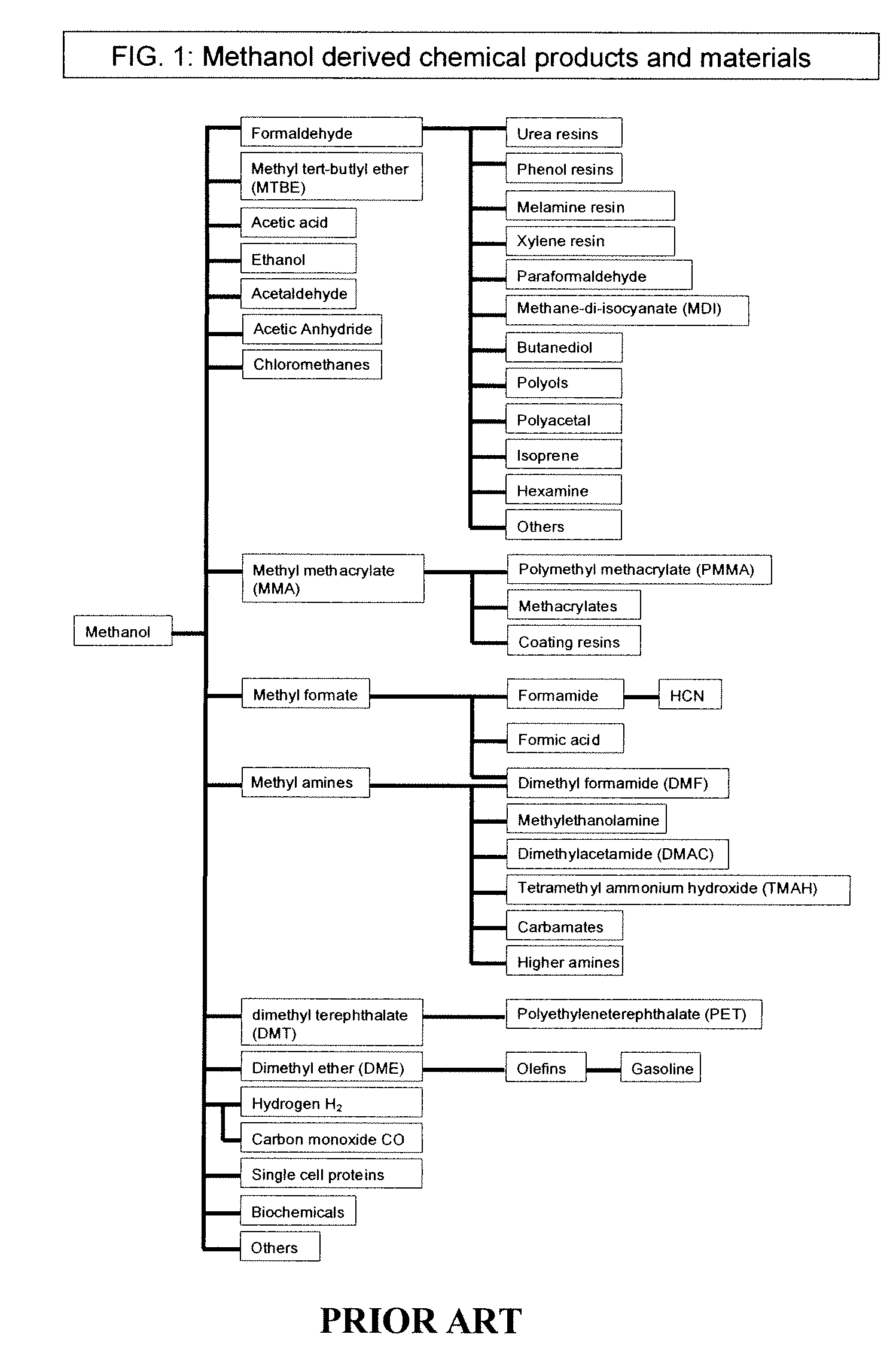
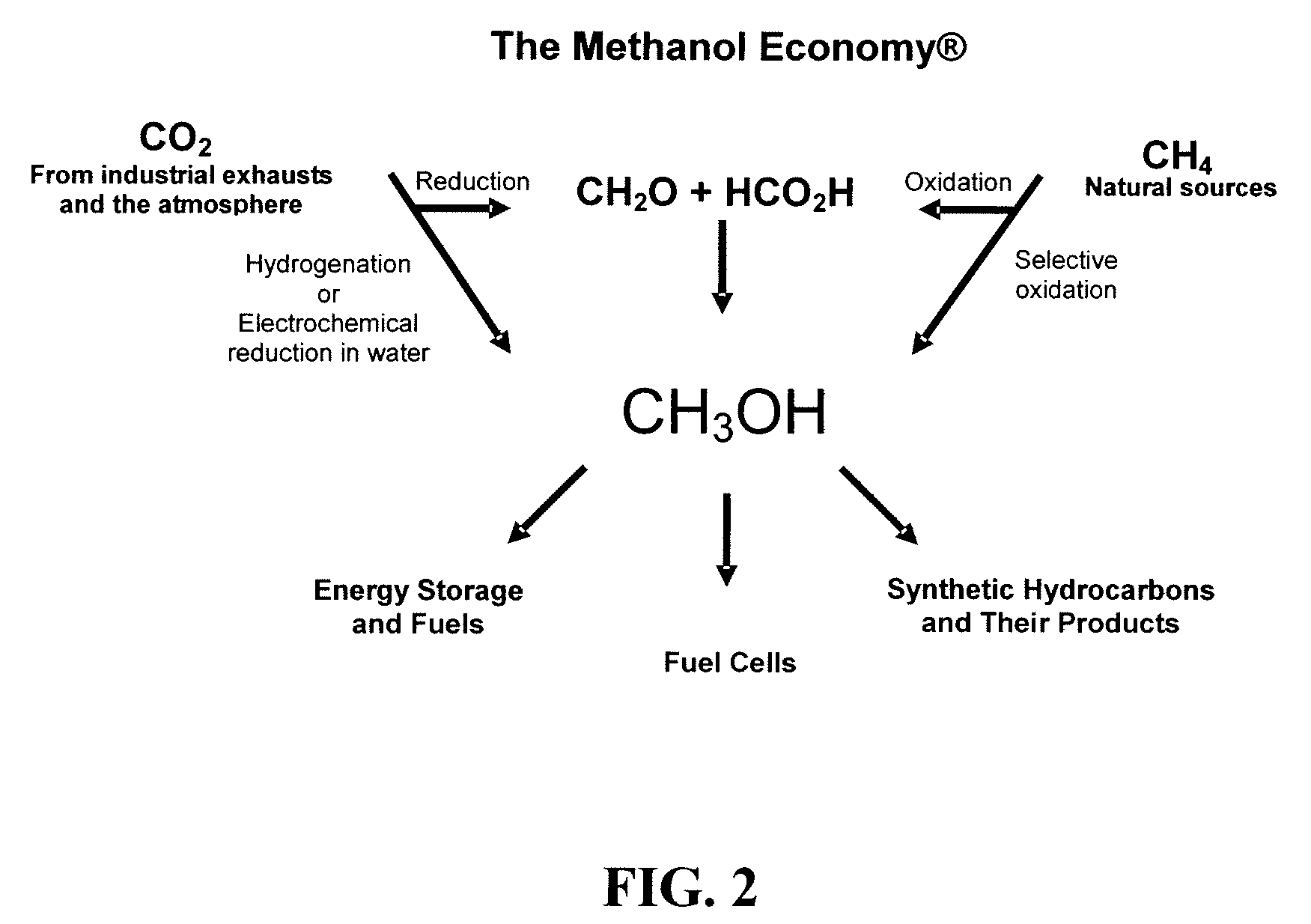
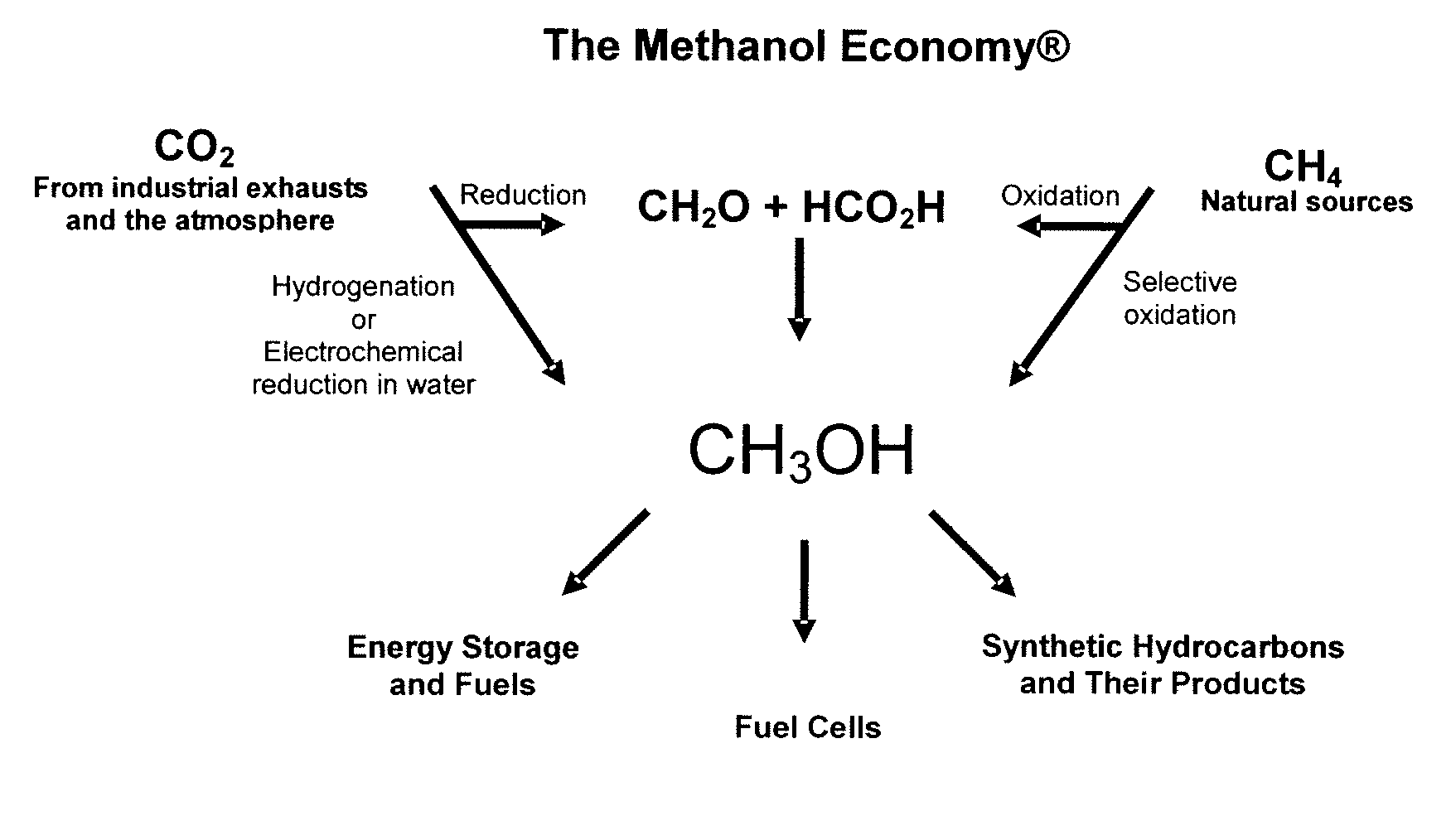
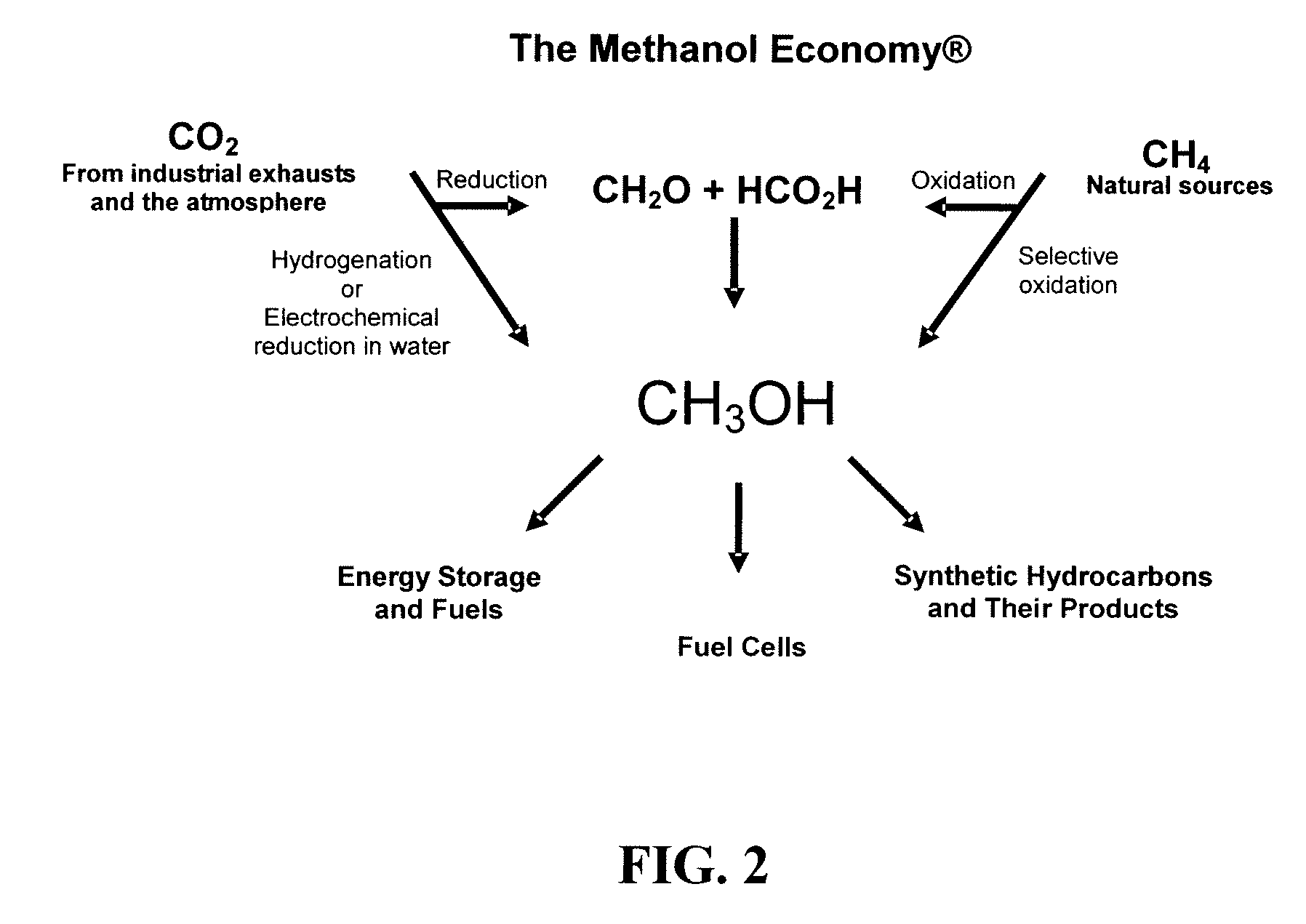
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one cell compartment and a metal cathode electrode in another cell compartment that also contains an aqueous solution comprising methanol and an electrolyte of one or more alkyl ammonium halides, alkali carbonates or combinations thereof to produce therein a reaction mixture containing carbon monoxide and hydrogen which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode.
Owner:UNIV OF SOUTHERN CALIFORNIA
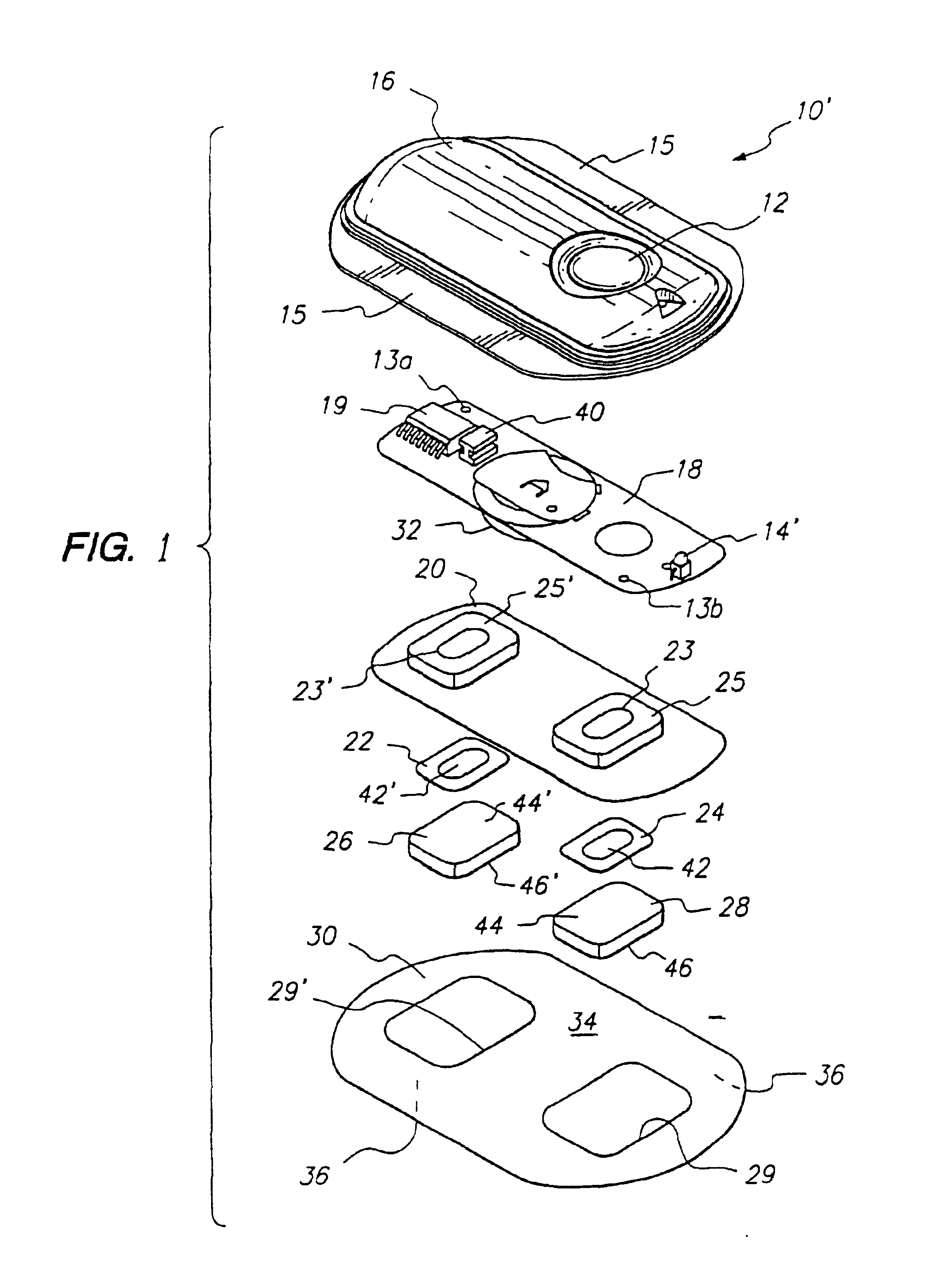
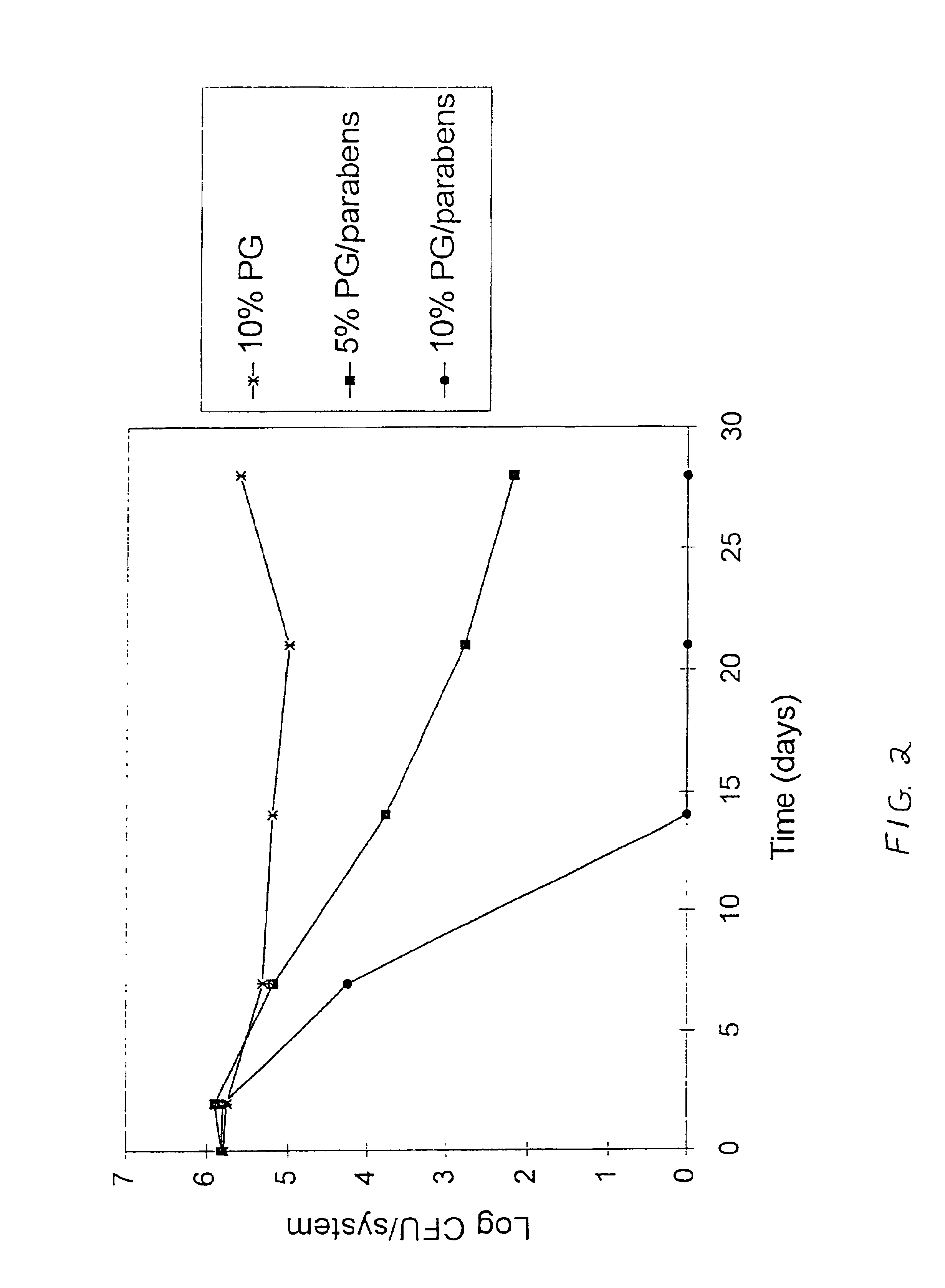
Transdermal electrotransport delivery device including an antimicrobial compatible reservoir composition
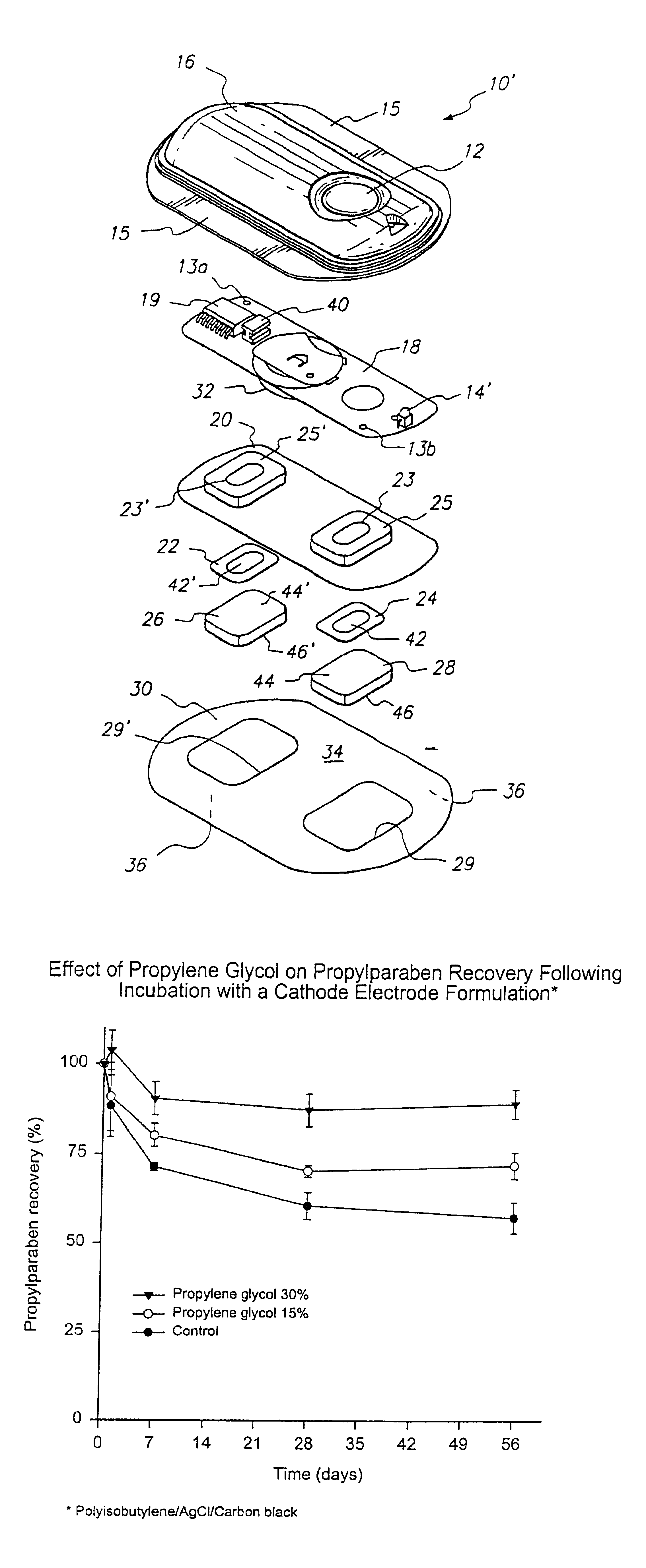
InactiveUS7054682B2Reducing its effectivenessInhibit microbial growthNervous disorderElectrotherapyMedicineAqueous medium
A transdermal electrotransport drug delivery device having an anode, a cathode and a source of electrical power electrically connected to the anode and the cathode. At least one of the anode and the cathode includes an electrode and a reservoir comprised of a housing composed of a polymeric material and an aqueous medium in contact with the housing. The aqueous medium includes (i) a drug or an electrolyte salt or a mixture thereof, (ii) propylene glycol, and (iii) an antimicrobial agent in an amount sufficient to inhibit microbial growth in the aqueous medium. The propylene glycol prevents the antimicrobial agent from being adsorbed by other materials used in the construction of the delivery device. A process for preparing a transdermal electrotransport drug delivery device is also provided.
Owner:ALZA CORP
Materials and process for enhancing selective separations
Use of a Maillard reaction product as an adjuvant in a variety of applications including solid-liquid separations, corrosion inhibition, emulsification, dust suppression, slow release fertilization, viscosity modification and others and especially as a depressant or collector in separation processes, including the selective separation of solids and / or ionic species from aqueous media, such as in the process of froth flotation.
Owner:GEORGIA PACIFIC CHEM LLC
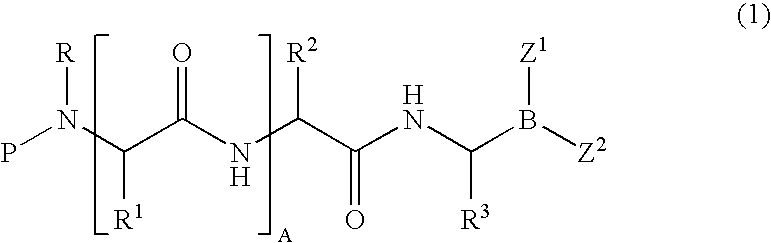
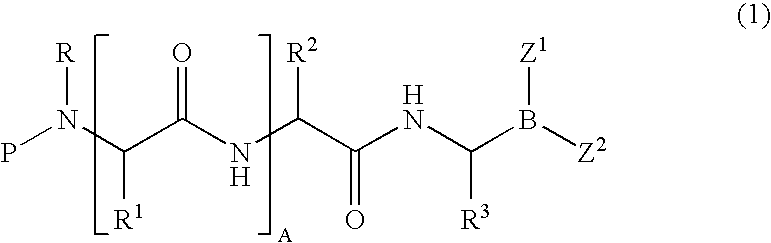
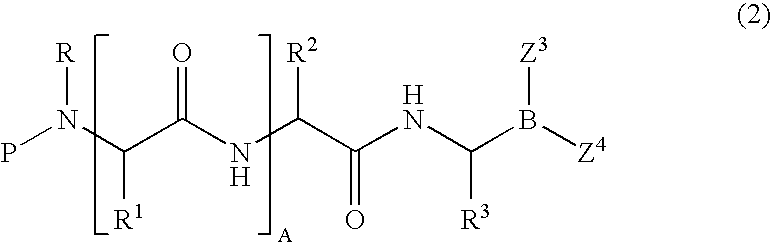
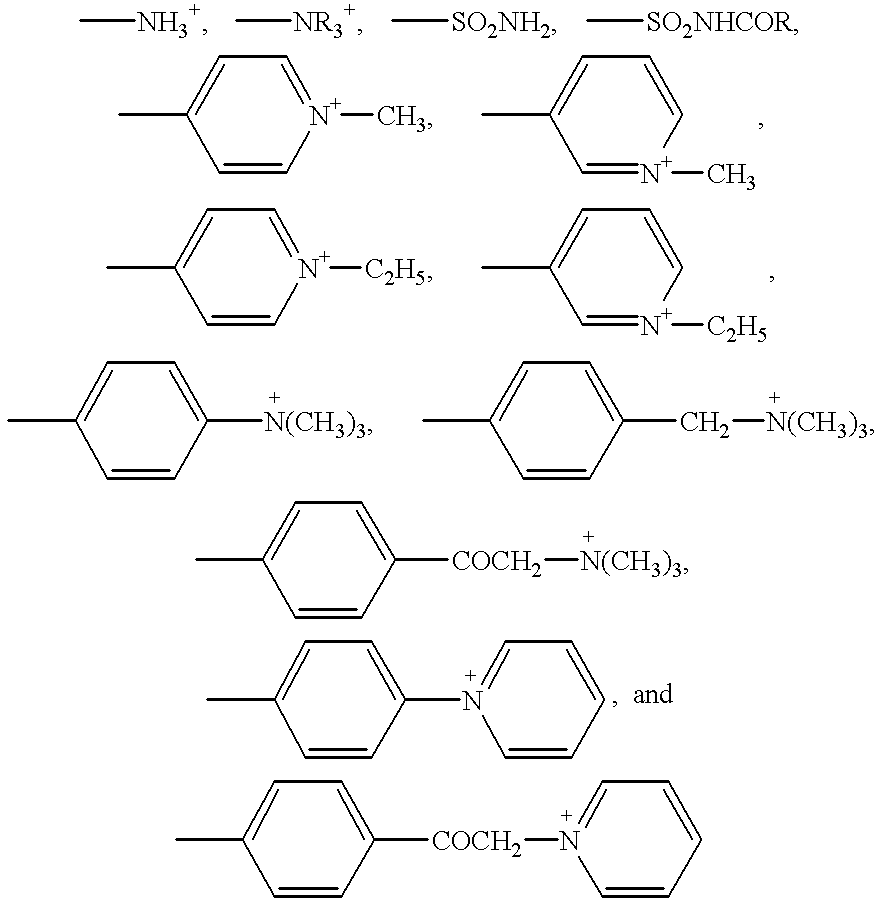
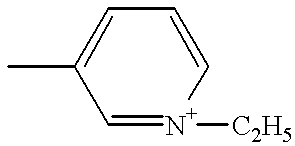
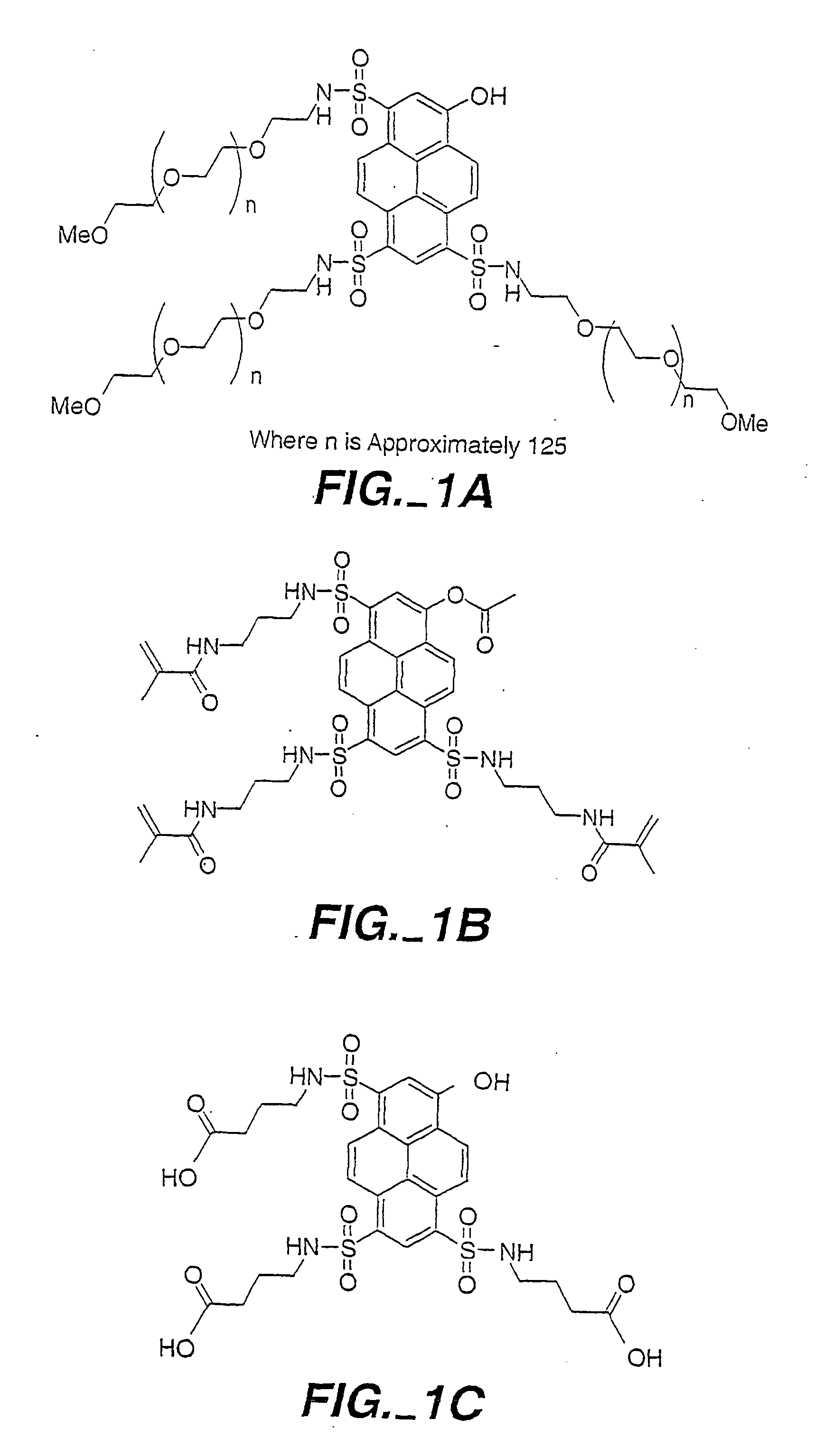
Formulation of boronic acid compounds
The present invention provides stable compounds prepared from boronic acid and lyophilized compounds thereof of the formula (1): in which Z1 and Z2 are moieties derived from sugar. The invention also provides methods for preparing such compounds. Lyophilizing a mixture comprising a boronic acid compound and a moiety derived from sugar produces a stable composition that readily releases the boronic acid compound upon reconstitution in aqueous media.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Ink, ink-jet recording process, recording unit, ink cartridge and ink-jet recording apparatus
InactiveUS6375317B1Stable formationQuality improvementMeasurement apparatus componentsInksPolymer scienceAqueous medium
An ink comprising particles of self-dispersing carbon black having at least one hydrophilic group at the surface thereof, and calcium in an aqueous medium. The ink can form images excellent in fastness properties such as water fastness and light fastness and character quality, and can be stably ejected from a recording head irrespective of printing environment.
Owner:CANON KK
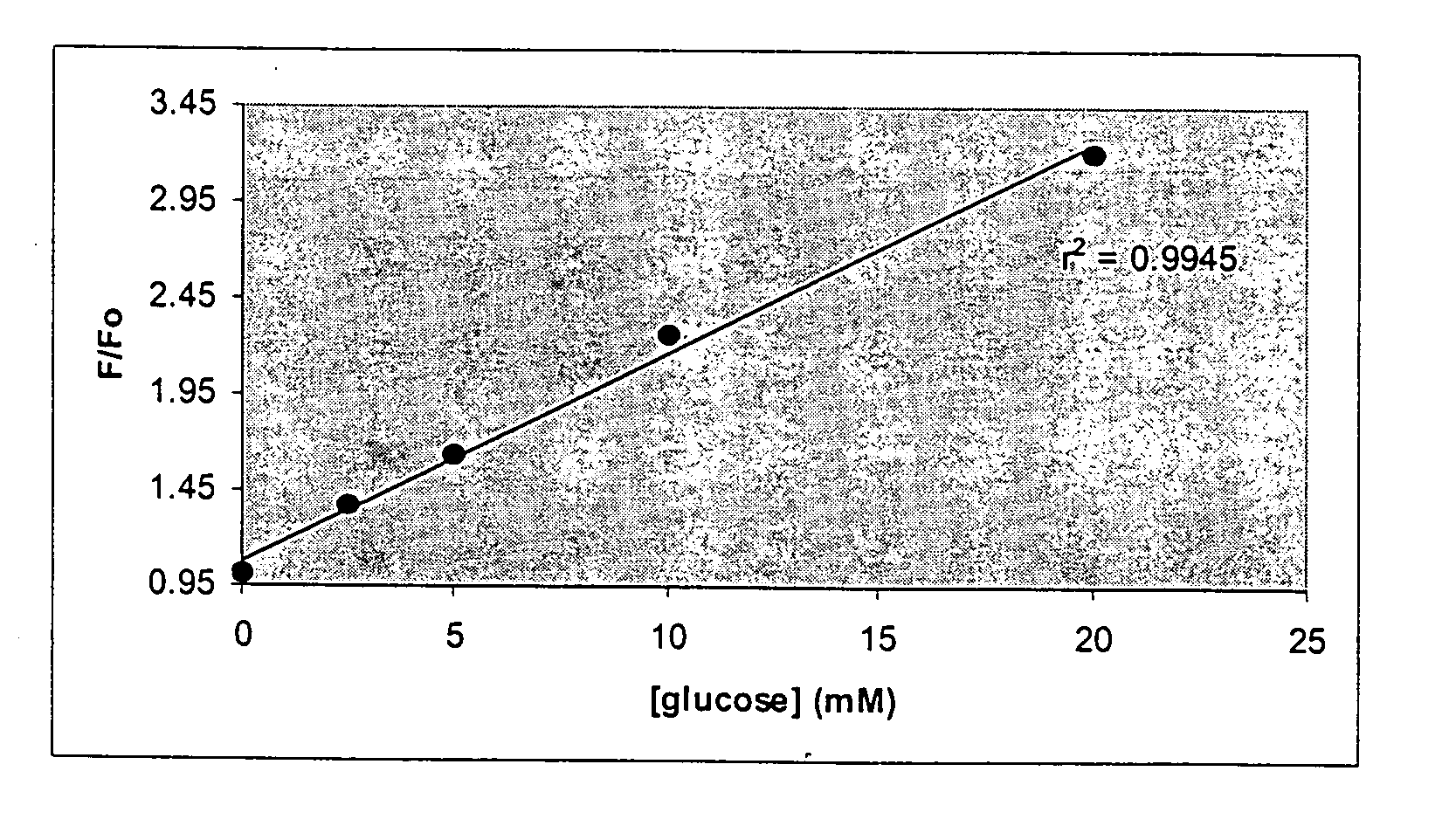
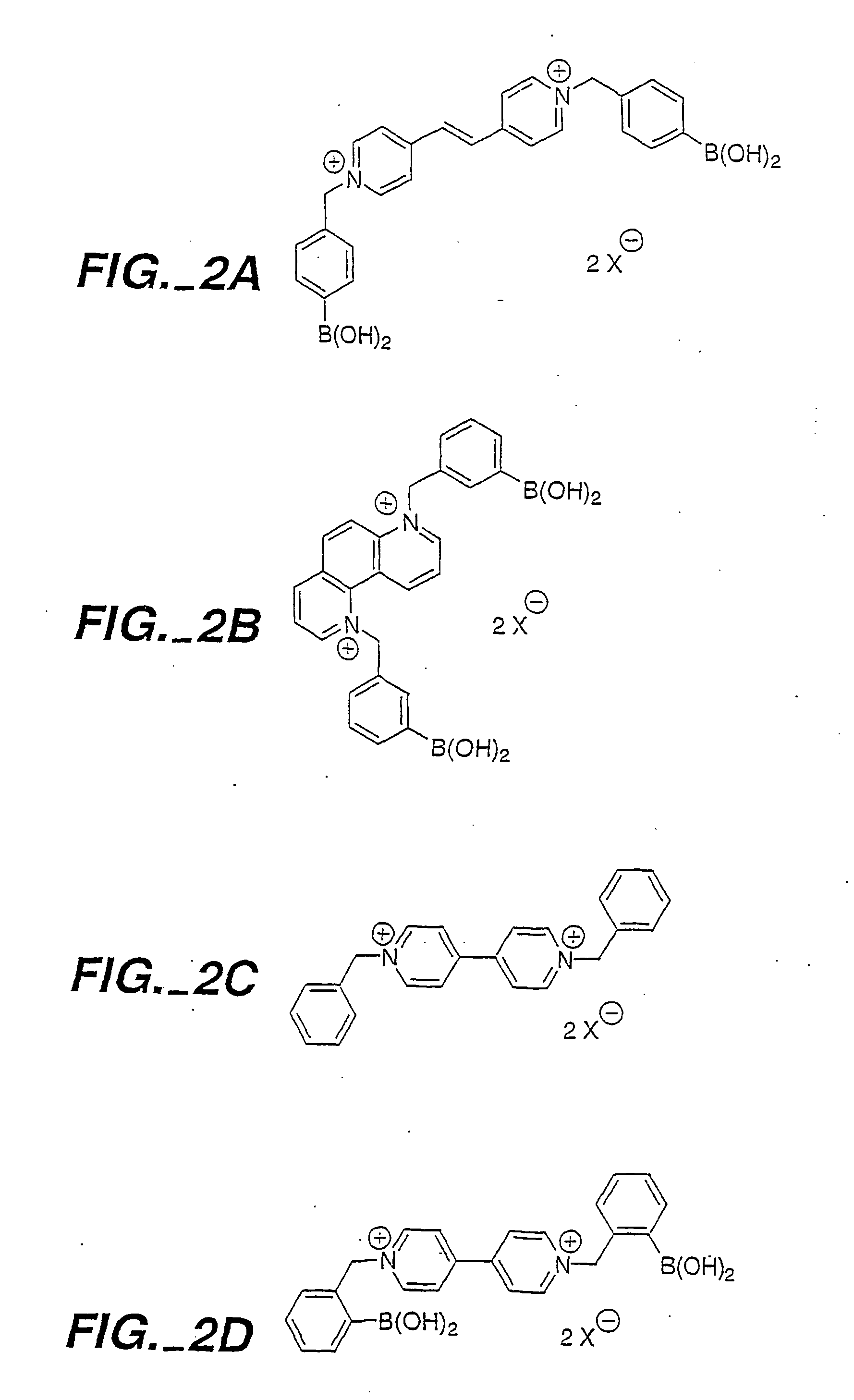
Optical determination of glucose utilizing boronic acid adducts
InactiveUS20060083688A1Efficient excitationHigh selectivityUltrasonic/sonic/infrasonic diagnosticsMaterial nanotechnologyIn vivoAdduct
The present invention concerns an improved optical method and optical sensing device for determining the levels of polyhydroxyl-substituted organic molecules in vitro and / or in vivo in aqueous media. The range of detection is between about 400 and 800 nm. In particular, a sensory devise is implemented in a mammal to determine sugar levels. Specifically, a dye is combined with a conjugated nitrogen-containing heterocyclic aromatic boronic acid-substituted bis-onium compound in the presence of a sugar, such as fructose or glucose. The viologens are preferred as the aromatic conjugated nitrogen-containing boronic acid substituted compounds. The method is useful to determine sugar levels in a human being.
Owner:RGT UNIV OF CALIFORNIA
Method or producing an organic pigment dispersion liquid and organic pigment fine particles obtained by the method
A method of producing an organic pigment dispersion liquid, which has the steps of: providing an alkaline or acidic solution with an organic pigment dissolved therein and an aqueous medium, wherein a polymerizable compound is contained in at least one of the organic pigment solution and the aqueous medium; mixing the organic pigment solution and the aqueous medium; and thereby forming the pigment as fine particles; then polymerizing the polymerizable compound to form a polymer immobile from the pigment fine particles.
Owner:FUJIFILM CORP
Porous drug matrices and methods of manufacture thereof
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Substrate-enhanced microbial fuel cells
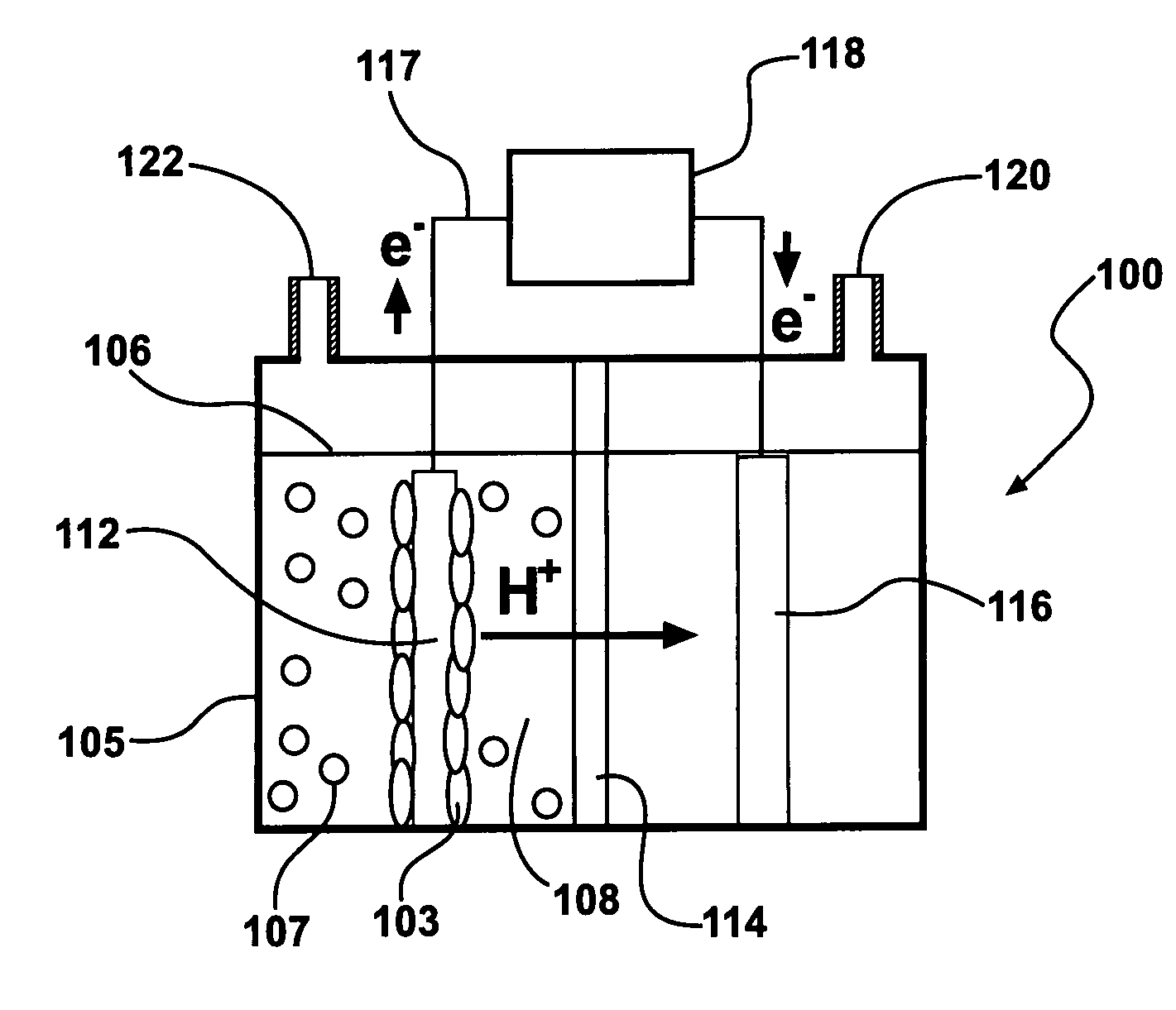
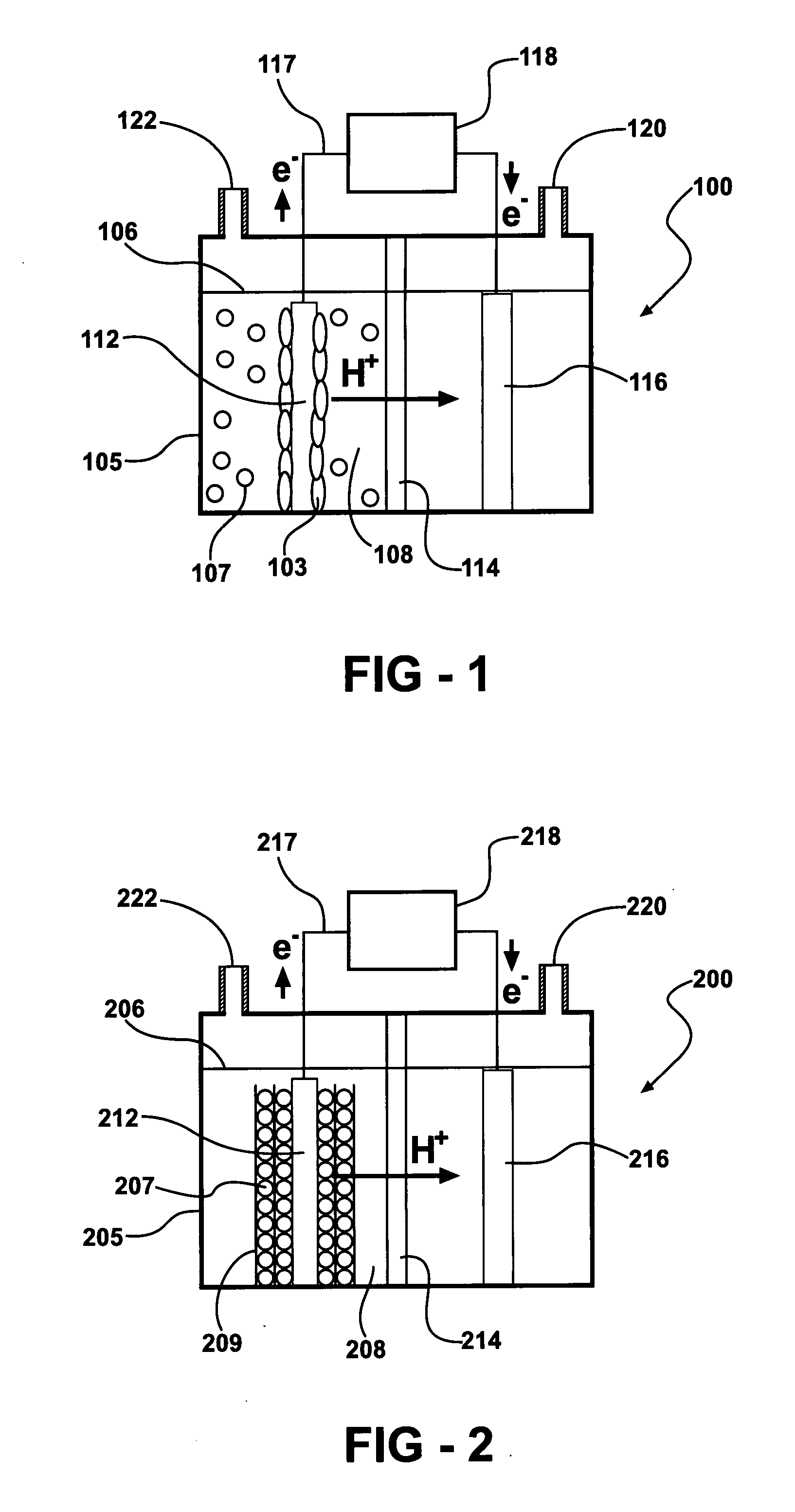
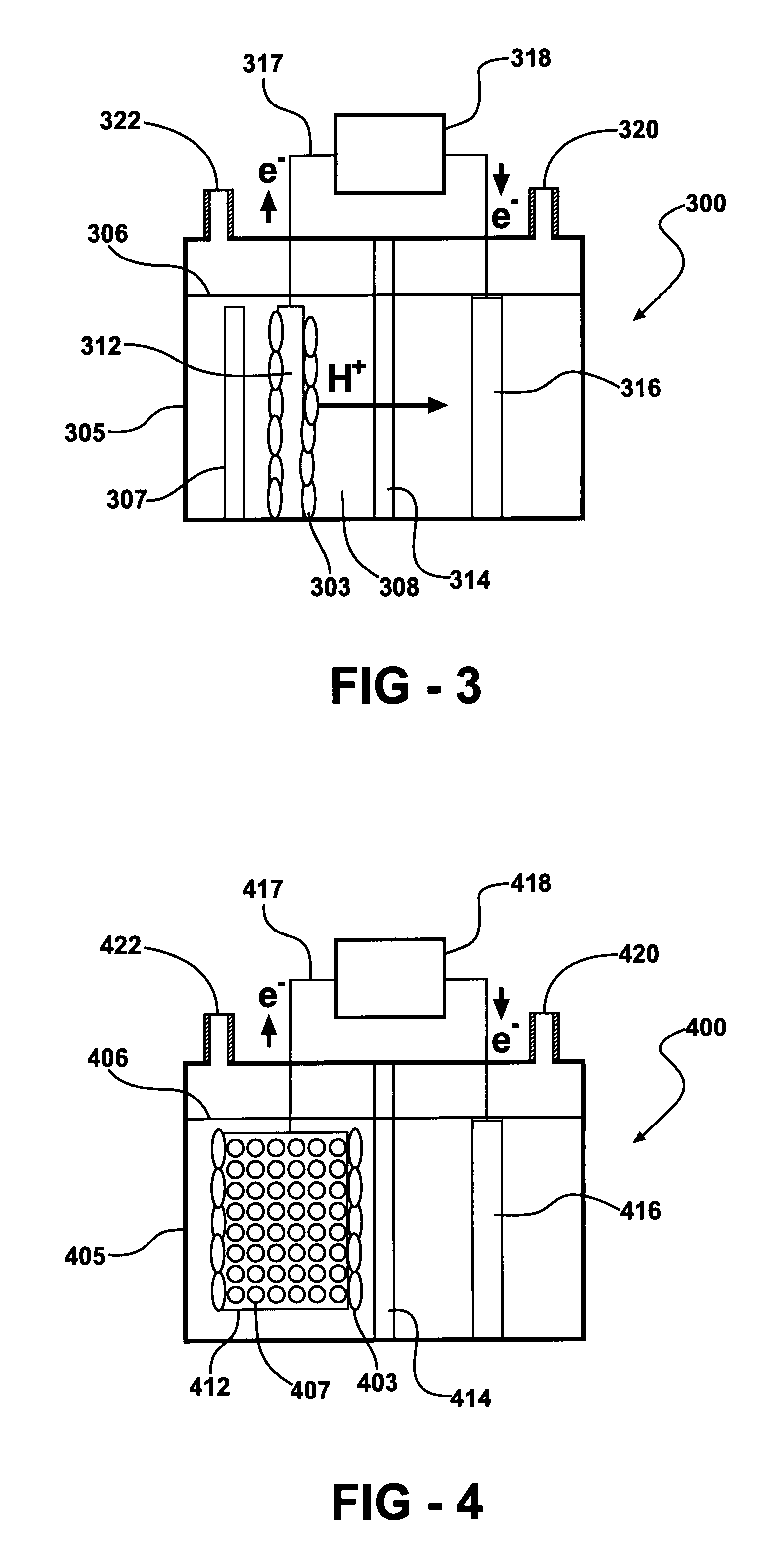
A microbial fuel cell configuration of the invention includes a substrate particularly formulated for a microbial fuel cell configured to produce electricity and / or a modified microbial fuel cell configured to produce hydrogen. A substrate formulation according to one embodiment includes a solid biodegradable organic material in a package porous to bacteria. A microbial fuel cell provided according to embodiments of the present invention includes an anode, a cathode, an electrically conductive connector connecting the anode and the cathode, a housing for an aqueous medium, the aqueous medium in contact with the anode, and a solid form of a biodegradable organic substrate disposed in the aqueous medium, the solid form of a biodegradable organic substrate formulated to support electron generation and transfer to the anode by anodophilic bacteria over a selected minimum period of time.
Owner:PENN STATE RES FOUND
Novel nutritional food products for improved digestion and intestinal absorption
InactiveUS20100239559A1Sustained stabilityAvoiding unstable breakdown of the enzyme and large overdosingHydrolasesPeptide/protein ingredientsDigestionNovel food
The present invention is directed to novel food products, e.g., nutritional food products and infant formula, which contain one ore more enzymes selected from lipase, protease, and amylase that have been formulated / stabilized to have sustained stability in an aqueous medium. Such formulations are intended to provide a greater degree of compliance based on their ability to be incorporated into aqueous media while avoiding unstable breakdown of the enzyme and large overdosing due to expected breakdown when exposed to an aqueous environment, including saliva. Further described in the invention are additives packaged with instructions for combination with an aqueous medium, and instructions for the administration of the resulting mixture to a subject. In certain embodiments, enzyme insufficient patients, e.g., infants and elderly persons, would find particular benefit from the food products described herein.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Formulations for cell-schedule dependent anticancer agents
InactiveUS20060121085A1Strong specificityEliminate side effectsPowder deliveryBiocideMetaboliteAnticarcinogen
The present invention provides a flowable composition suitable for use as a controlled release implant. The composition includes: (a) a biodegradable, biocompatible thermoplastic polymer that is at least substantially insoluble in aqueous medium, water or body fluid; (b) a cell-cycle dependent biological agent, a schedule-dependent biological agent, a metabolite thereof, a pharmaceutically acceptable salt thereof, or a prodrug thereof; and (c) a biocompatible organic liquid, at standard temperature and pressure, in which the thermoplastic polymer is soluble. The present invention also provides a method of treating cancer in a mammal. The present invention also provides a method of blocking, impeding, or otherwise interfering with cell cycle progression at the G1-phase, G1 / S interphase, S-phase, G2 / M interface or M-phase of the cell cycle in a mammal. The methods includes administering to a mammal an effective amount of a flowable composition of the present invention.
Owner:QLT USA INC
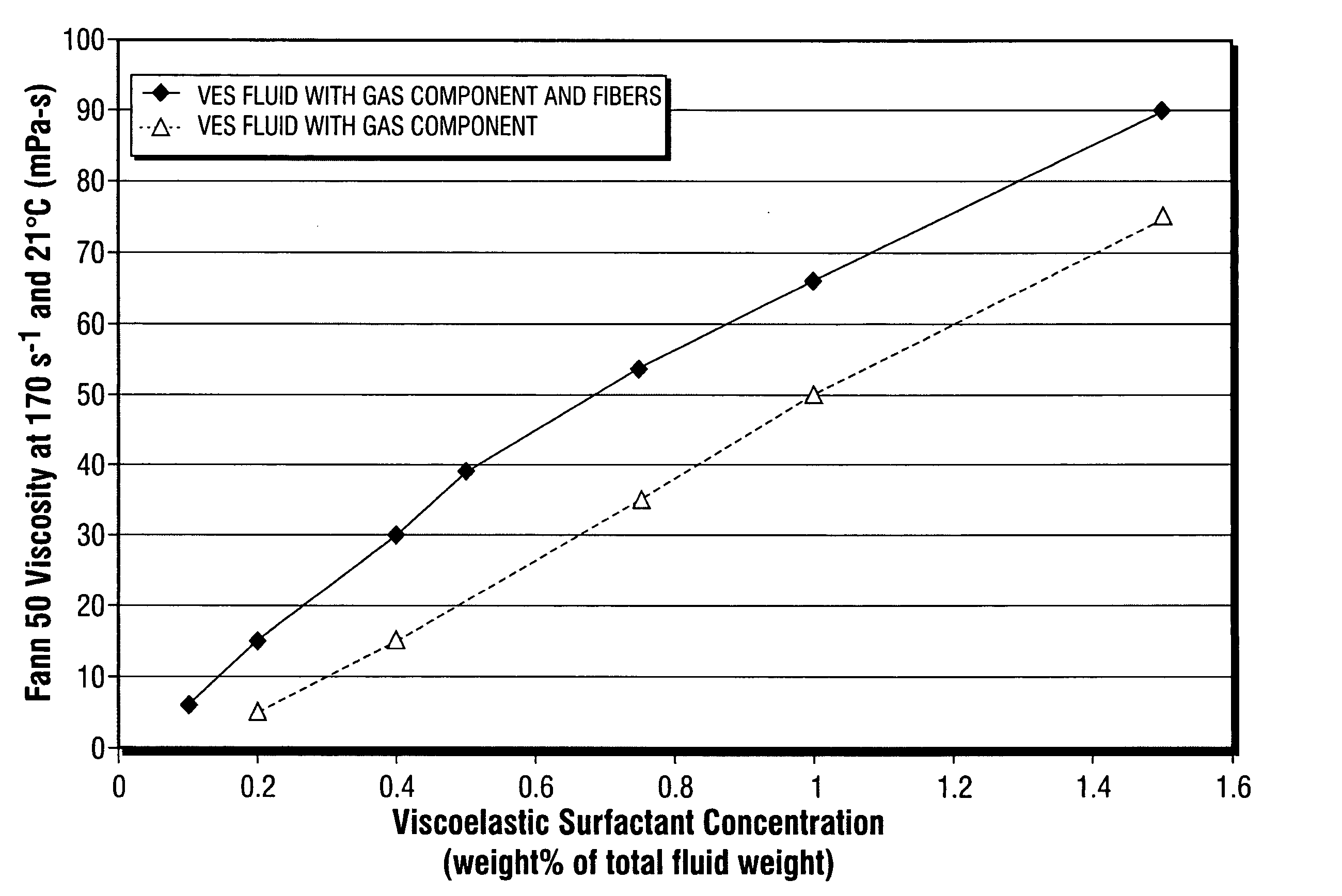
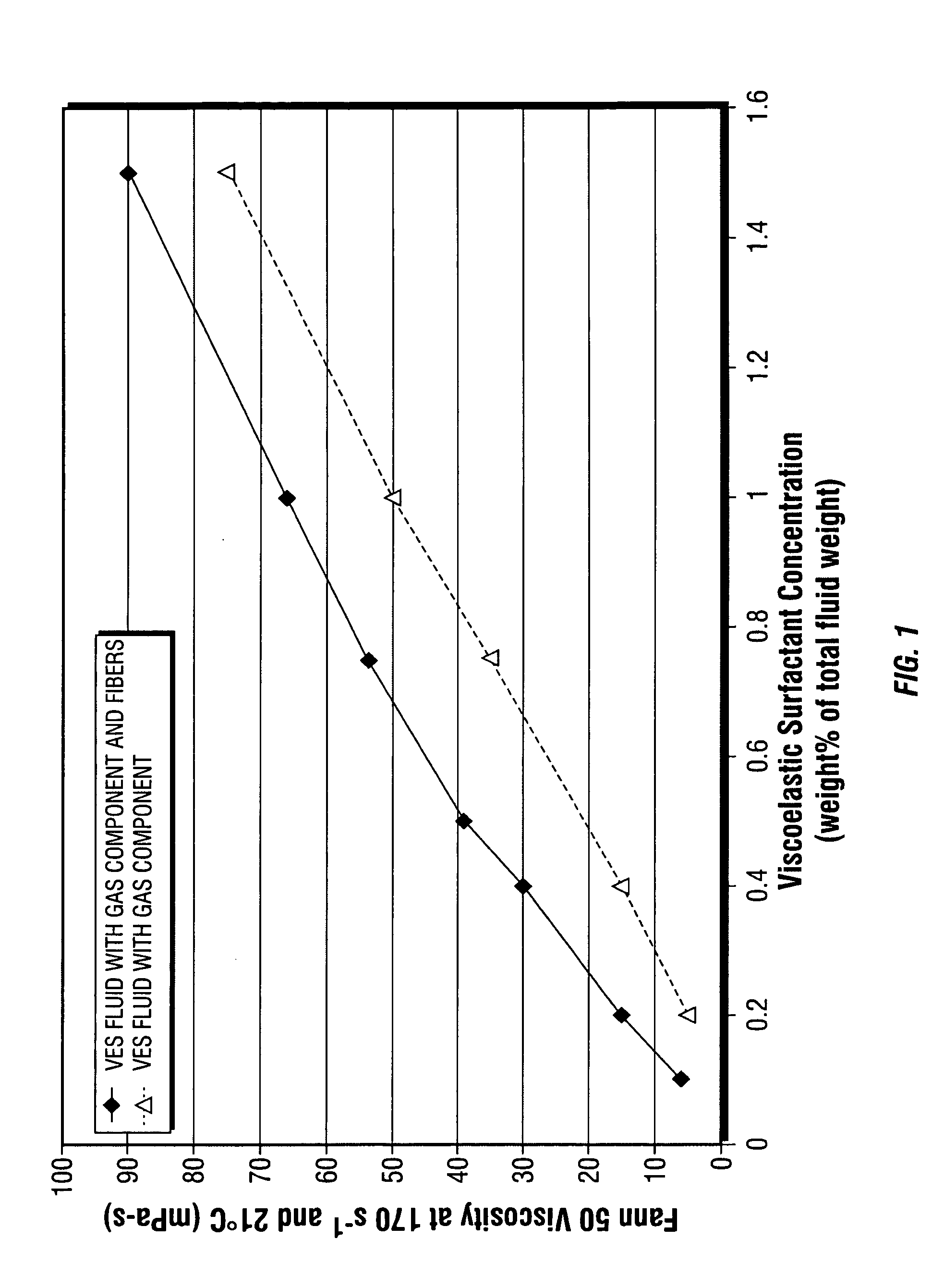
Fiber laden energized fluids and methods of use thereof
ActiveUS20060054324A1Good proppant suspensionGood transport propertyFluid removalFlushingFiberThermodynamics
The present invention relates to aqueous oilfield treatment fluids containing a gas component and fibers, wherein the fluids may further include a viscosifying agent and / or proppant. The fluids have good proppant suspension and transport properties as well as excellent gas phase stability. Use of fluids comprising an aqueous medium, a gas component, viscosifying agent, and fibers for hydraulically fracturing a subterranean formation, cleanup operations, and gravel packing a wellbore, are also disclosed.
Owner:SCHLUMBERGER TECH CORP
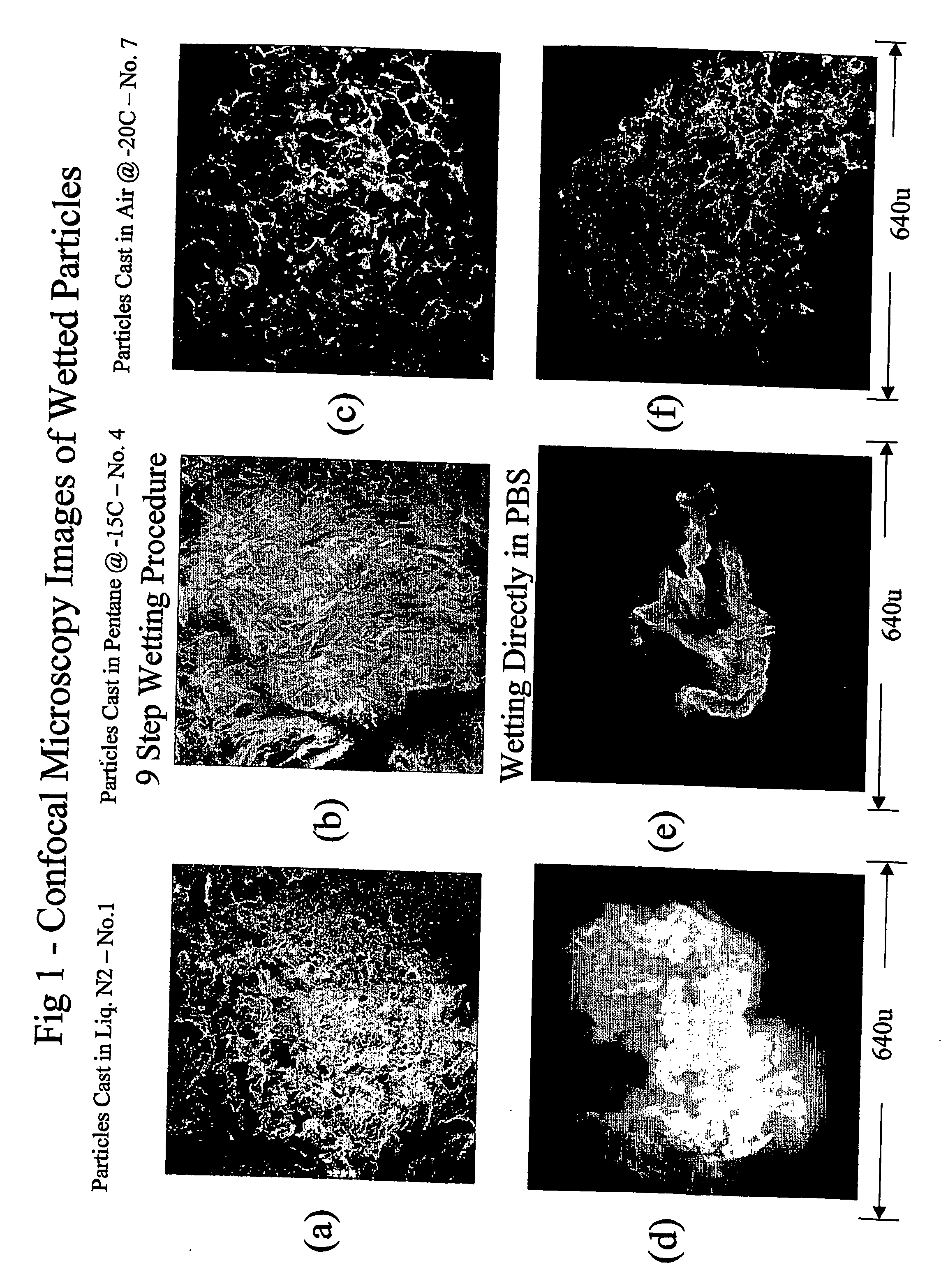
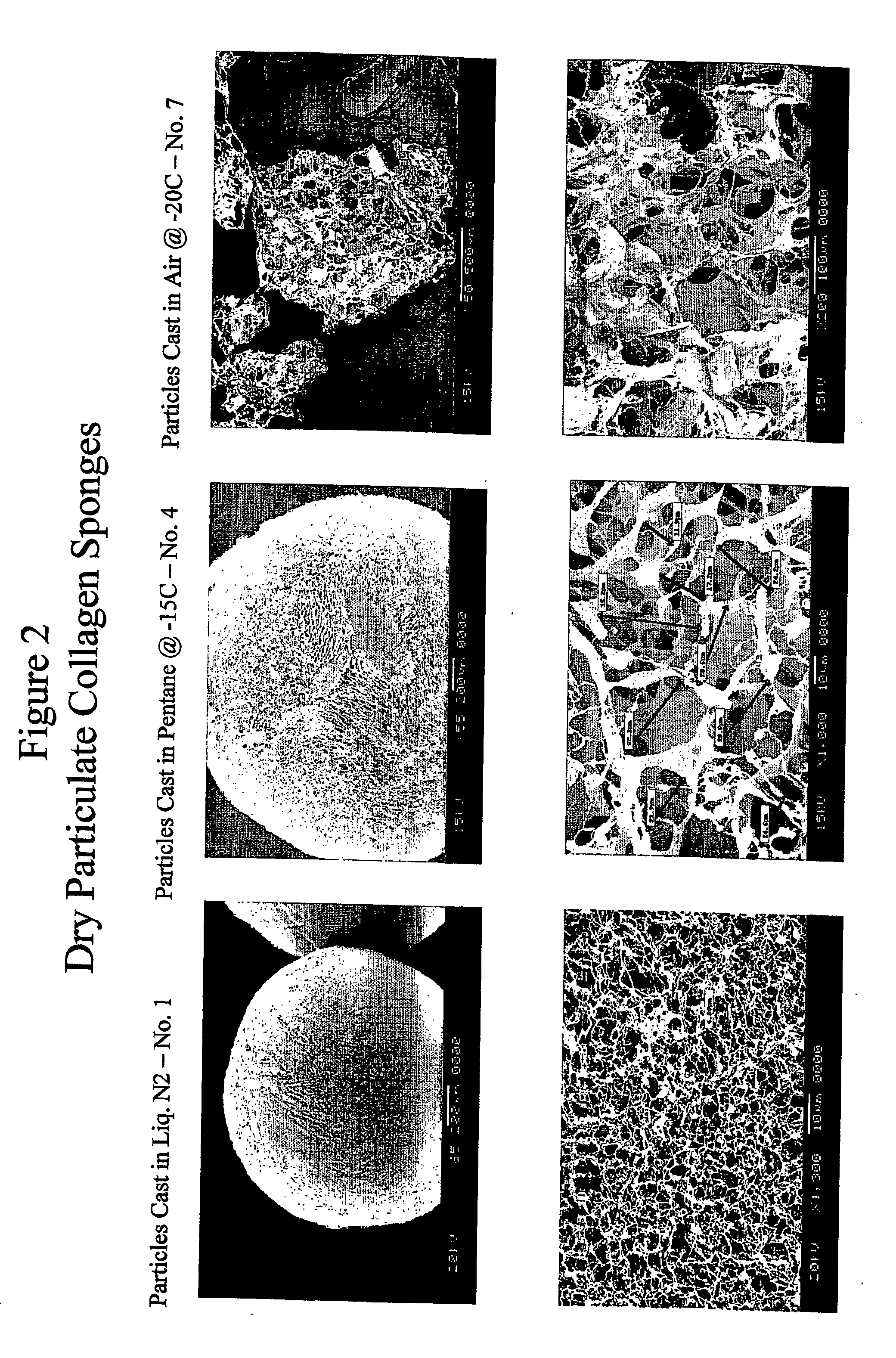
Porous particulate collagen sponges
InactiveUS20060135921A1Low toxicitySatisfactory porosityCosmetic preparationsToilet preparationsParticulatesCross-link
The present invention relates to the development of new porous particulate collagen sponges, combining the desirable features of low toxicity, resorbability, and satisfactory porosity, particularly when wetted in an aqueous medium. Accordingly, the present invention is directed to new porous, particulate, dehydrothermally cross-linked, wetted sponges, as well as a process for making them.
Owner:WIERCINSKI ROBERT A +2
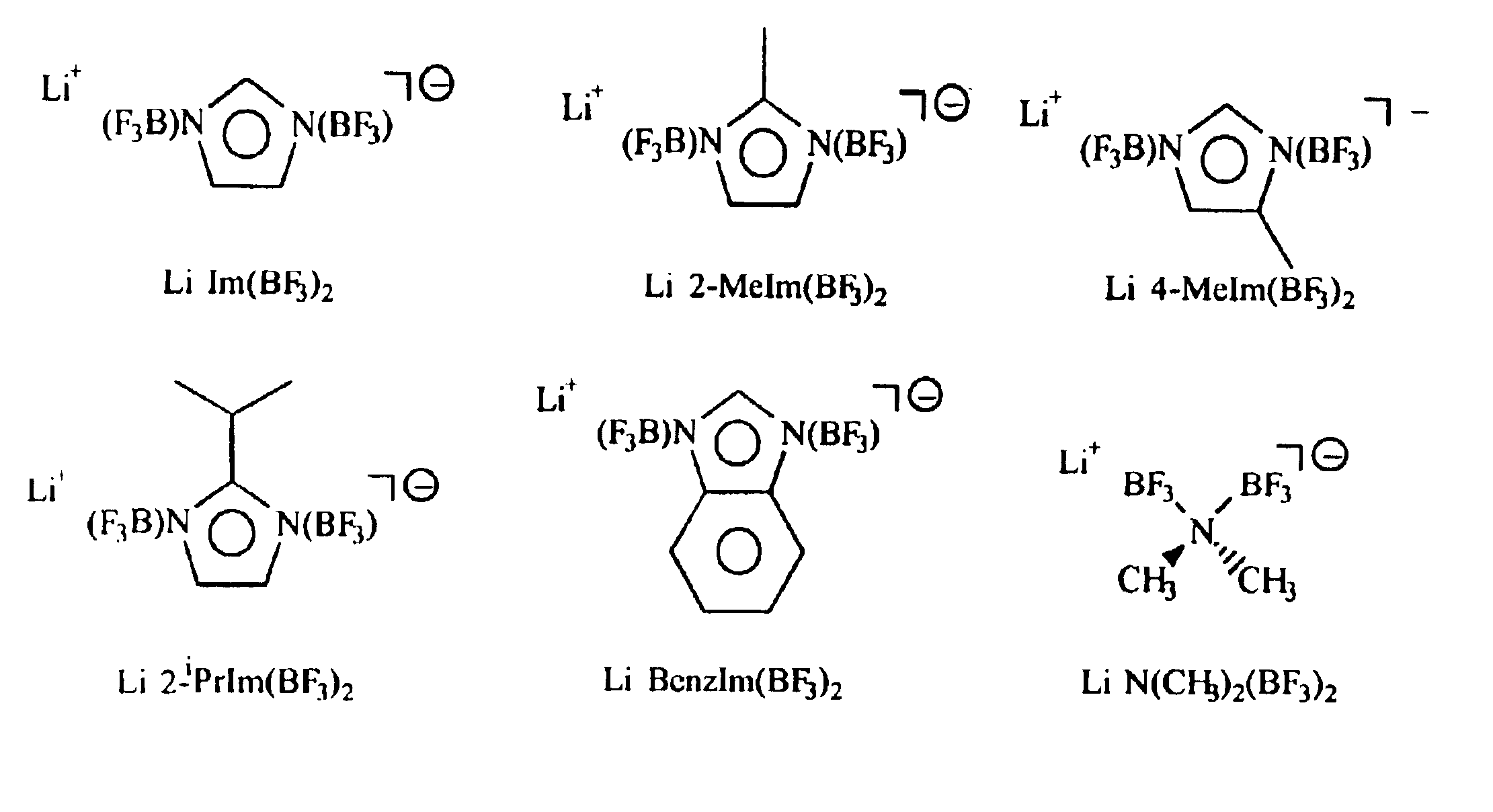
Non-aqueous electrolytes for lithium electrochemical cells
InactiveUS6852446B2Improve conductivityImprove stabilityAlkaline accumulatorsOrganic electrolyte cellsHydrogen atomHydrogen
A non-aqueous electric current producing electrochemical cell is provided comprising an anode and a cathode, an ionically permeable separator interposed between the anode and the cathode, and a non-aqueous electrolyte, the electrolyte comprising an ionically conducting salt in a non-aqueous medium, the ionically conducting salt corresponding to the formula:M+(Z*(J*)j(X*)x)−, wherein:M is a lithium atom,Z* is an anion group containing two or more Lewis basic sites and comprising less than 50 atoms not including hydrogen atoms,J* independently each occurance is a Lewis acid coordinated to at least one Lewis basic site of Z*, and optionally two or more such J* groups may be joined together in a moiety having multiple Lewis acidic functionality,X* independently each occurrence is selected from the group consisting of H, C1-C4 alkyl, alkoxide, halide and mixtures thereof,j is an integer from 2 to 12, andx is an integer from 0 to 4.
Owner:EAGLE PICHER TECH LLC
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one cell compartment and a metal cathode electrode in another cell compartment that also contains an aqueous solution comprising methanol and an electrolyte of one or more alkyl ammonium halides, alkali carbonates or combinations thereof to produce therein a reaction mixture containing carbon monoxide and hydrogen which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode.
Owner:UNIV OF SOUTHERN CALIFORNIA
Photothermographic recording material coatable from an aqueous medium
InactiveUS6143488AImprove imaging effectImprove stabilityRadiation applicationsPhotothermographic systemsSilver iodideWater dispersible
A process for producing a photothermographic recording material having a support and a photo-addressable thermally developable element containing photosensitive silver halide in catalytic association with a substantially light-insensitive silver salt of an organic carboxylic acid, an organic reducing agent for the substantially light-insensitive silver salt of an organic carboxylic acid in thermal working relationship therewith and a binder including a water-soluble binder, a water-dispersible binder or a mixture of a water-soluble binder and a water-dispersible binder, comprising the steps of: (i) producing an aqueous dispersion or aqueous dispersions containing photosensitive silver halide, a substantially light-insensitive silver salt of an organic carboxylic acid, an organic reducing agent for the substantially light-insensitive silver salt of an organic carboxylic acid and a binder including a water-soluble binder, a water-dispersible binder or a mixture of a water-soluble binder and a water-dispersible binder; (ii) coating the aqueous dispersion or aqueous dispersions onto a support thereby forming a photo-addressable thermally developable element on the support, wherein at least 80 mol % of the photosensitive silver halide is silver iodide and the aqueous dispersion further contains or the aqueous dispersions further contain a diazine compound.
Owner:AGFA HEALTHCARE NV
Carbon nanotubes: high solids dispersions and nematic gels thereof
InactiveUS20060099135A1Good dispersionMaterial nanotechnologyNanoinformaticsAqueous mediumSURFACTANT BLEND
Disclosed are high weight fraction carbon nanotube dispersions including an aqueous medium, carbon nanotubes, and at least one surfactant, the surfactant having an aromatic group, an alkyl group having from about 4 to about 30 carbon atoms, and a charged head group. Also disclosed are ultrasonication processes capable of providing stable dispersions of carbon nanotubes having reduced breakage of the carbon nanotubes. The preparation of nematic nanotube gels from the carbon nanotube dispersions are also disclosed. A variety of uses and applications of the carbon nanotube dispersions and nematic nanotube gels are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Pigment ink jet ink composition
An ink jet ink composition comprising an aqueous media and a pigment dispersion comprising a pigment and a polymeric dispersant wherein said polymeric dispersant is a copolymer comprising at least a hydrophobic methacrylate or acrylate monomer containing an aliphatic chain having greater than or equal to 12 carbons; and a hydrophilic methacrylic or acrylic acid monomer; wherein said copolymer comprises at least 10% by weight of the methacrylate or acrylate monomer and at least 5% by weight of the methacrylic or acrylic acid monomer; and wherein the copolymer comprises, in total, 20 to 95 weight % of hydrophobic monomer.
Owner:EASTMAN KODAK CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com