Patents
Literature
879 results about "Drugs solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
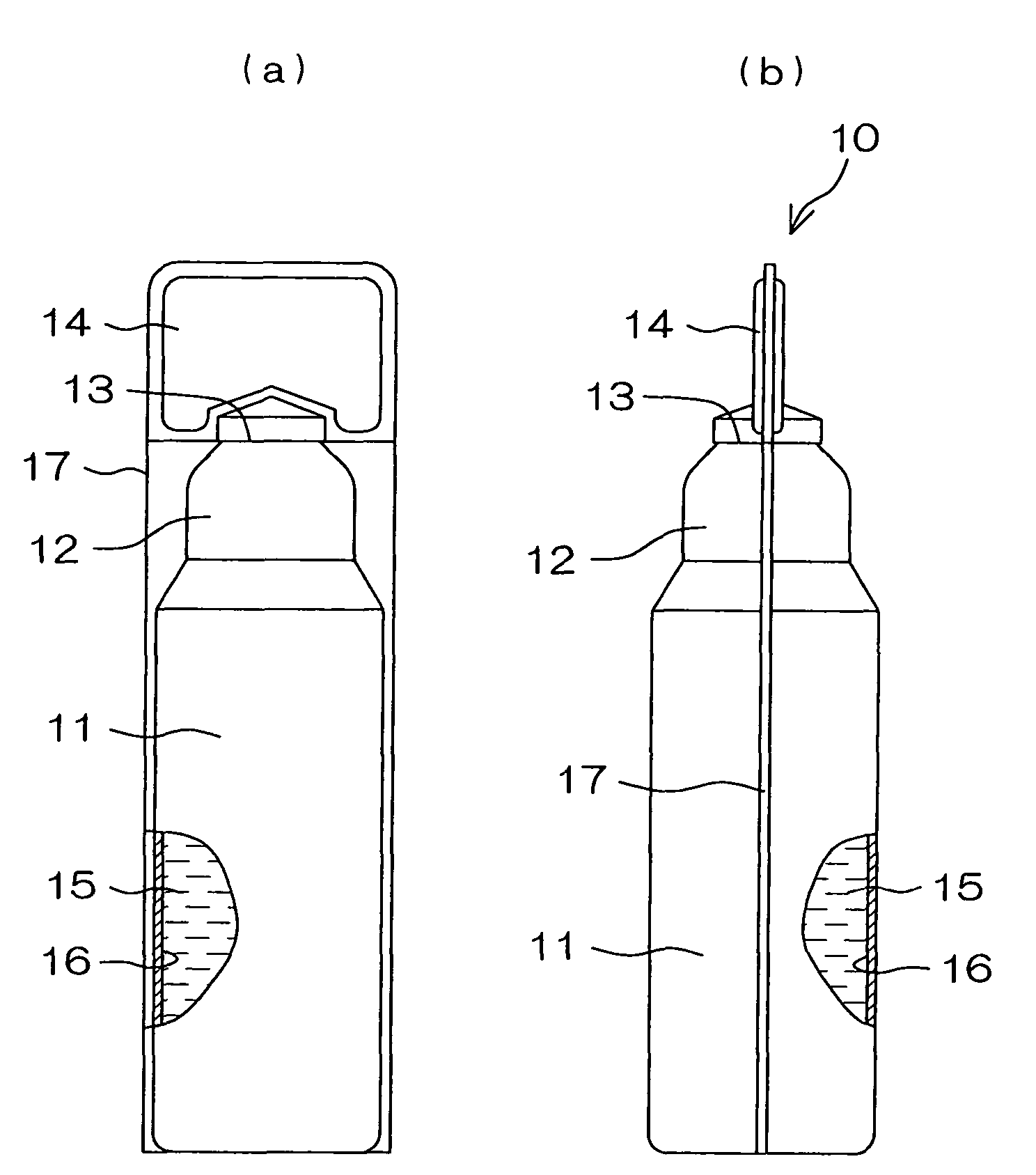
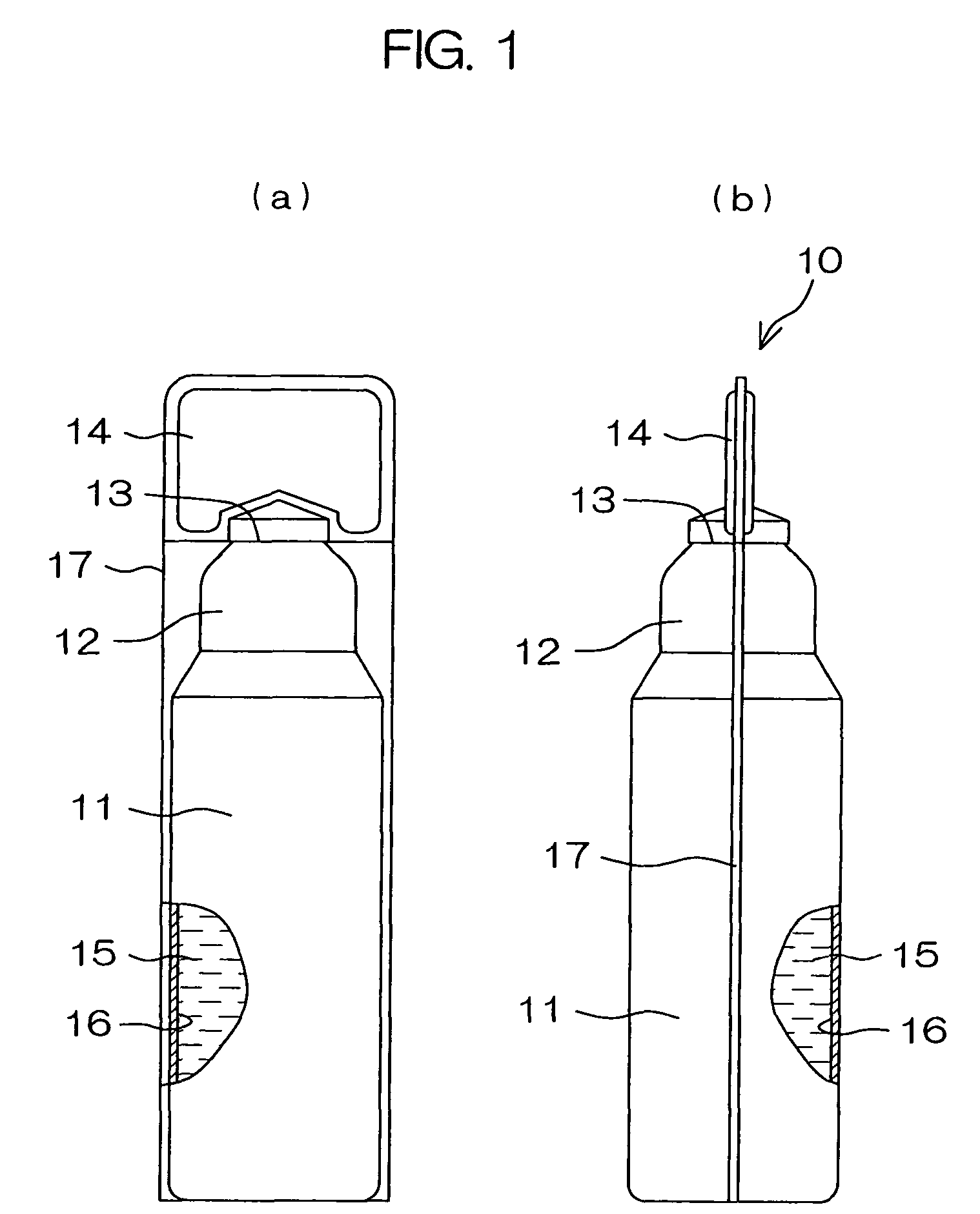
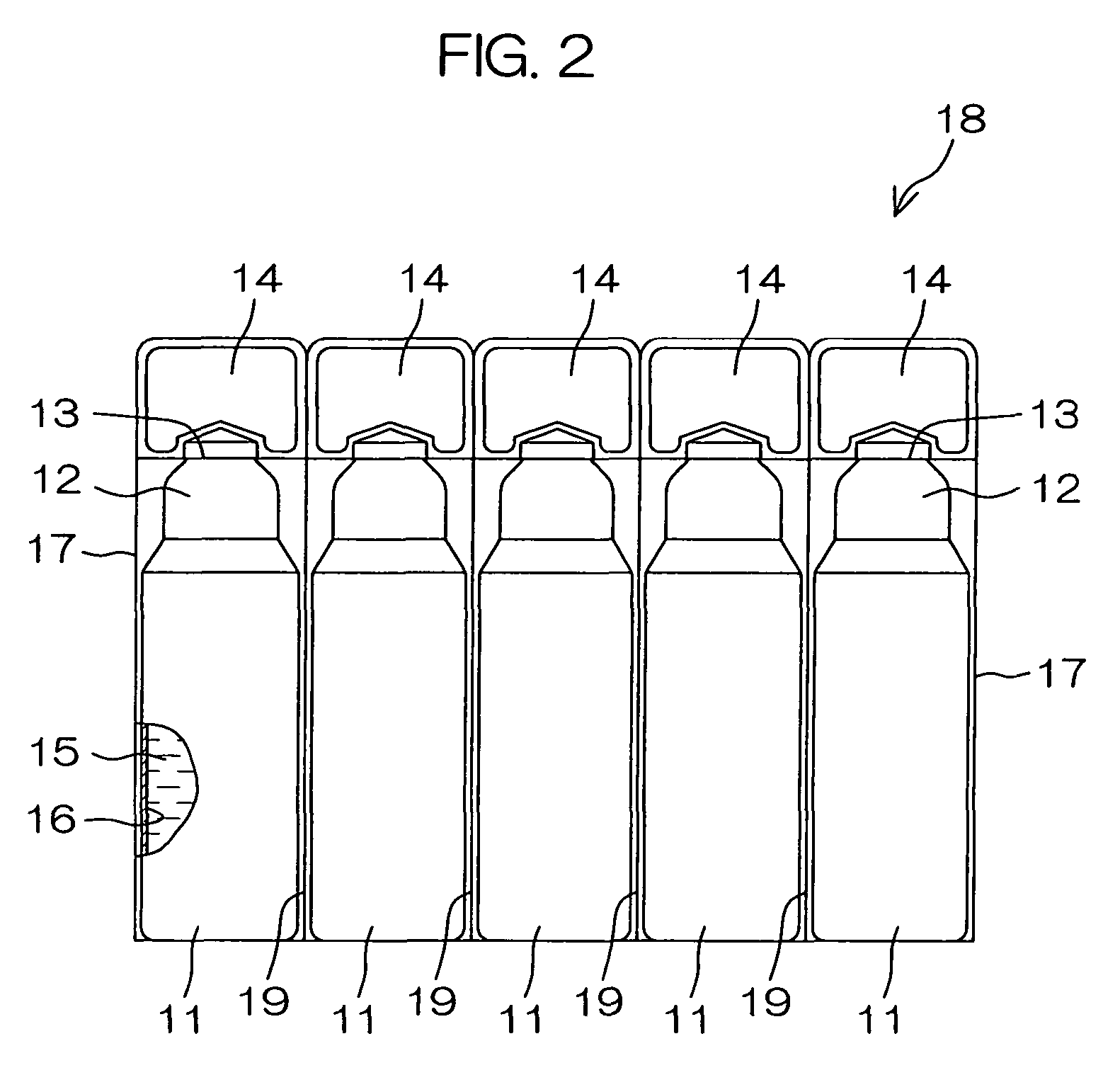
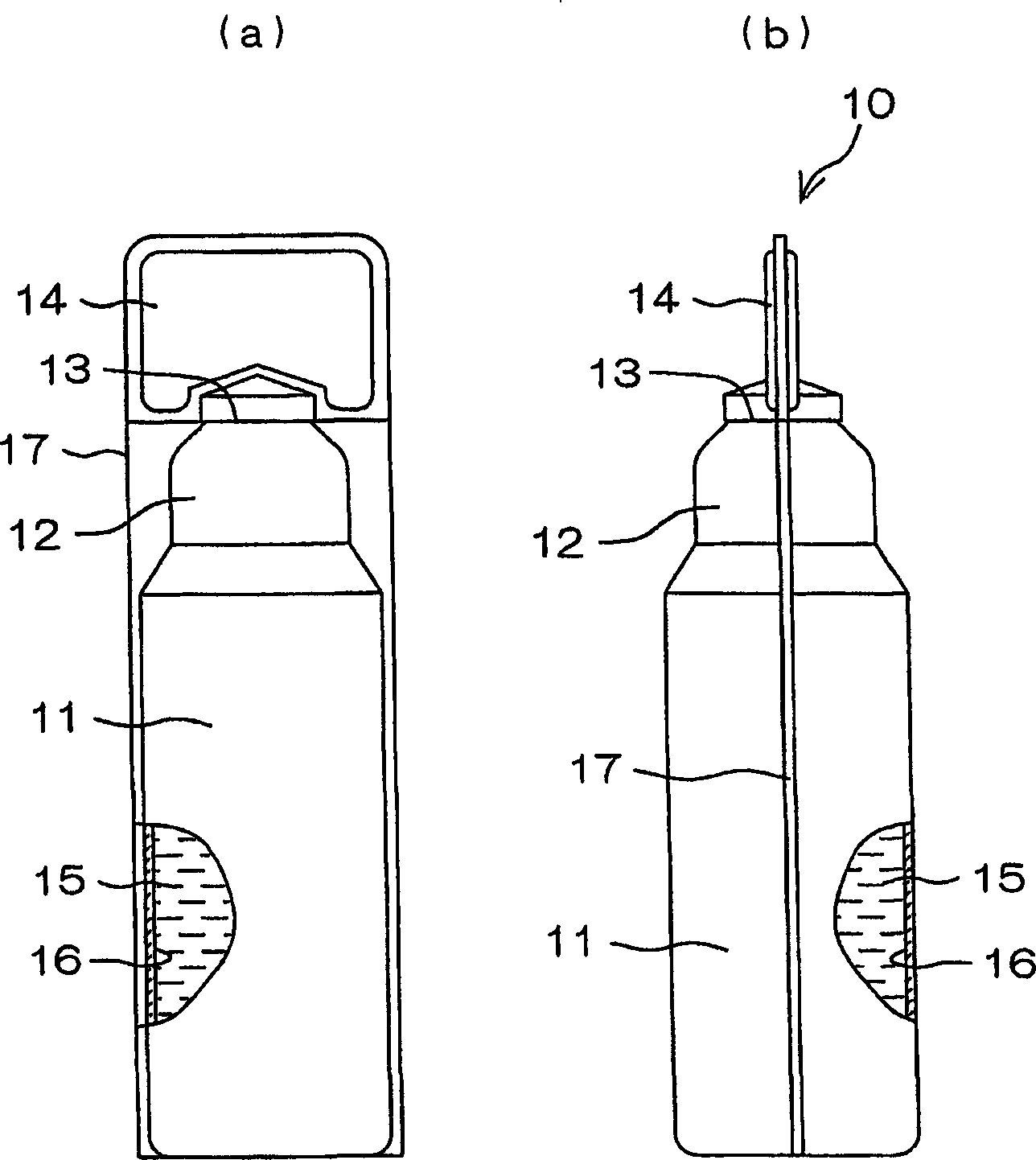
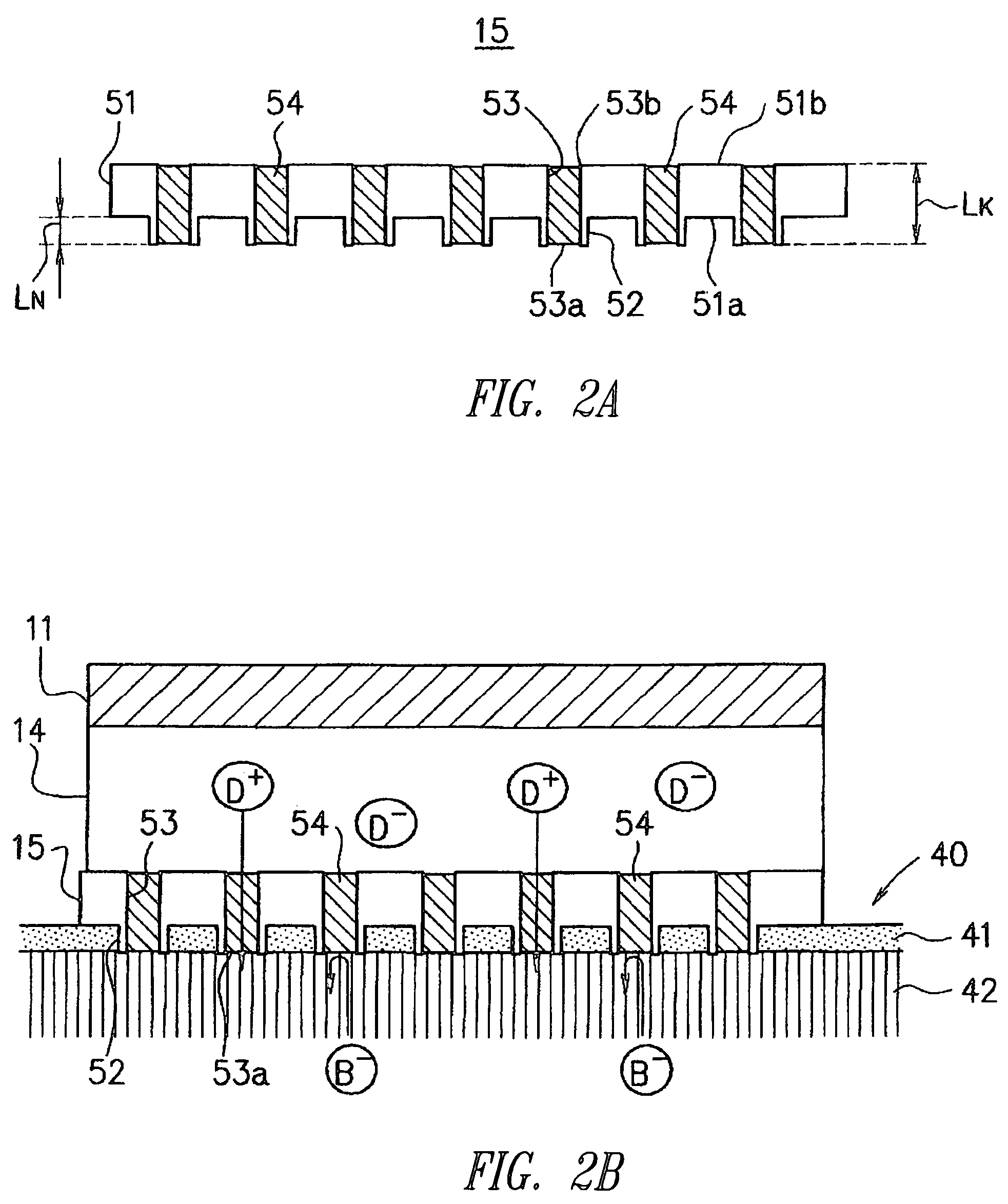
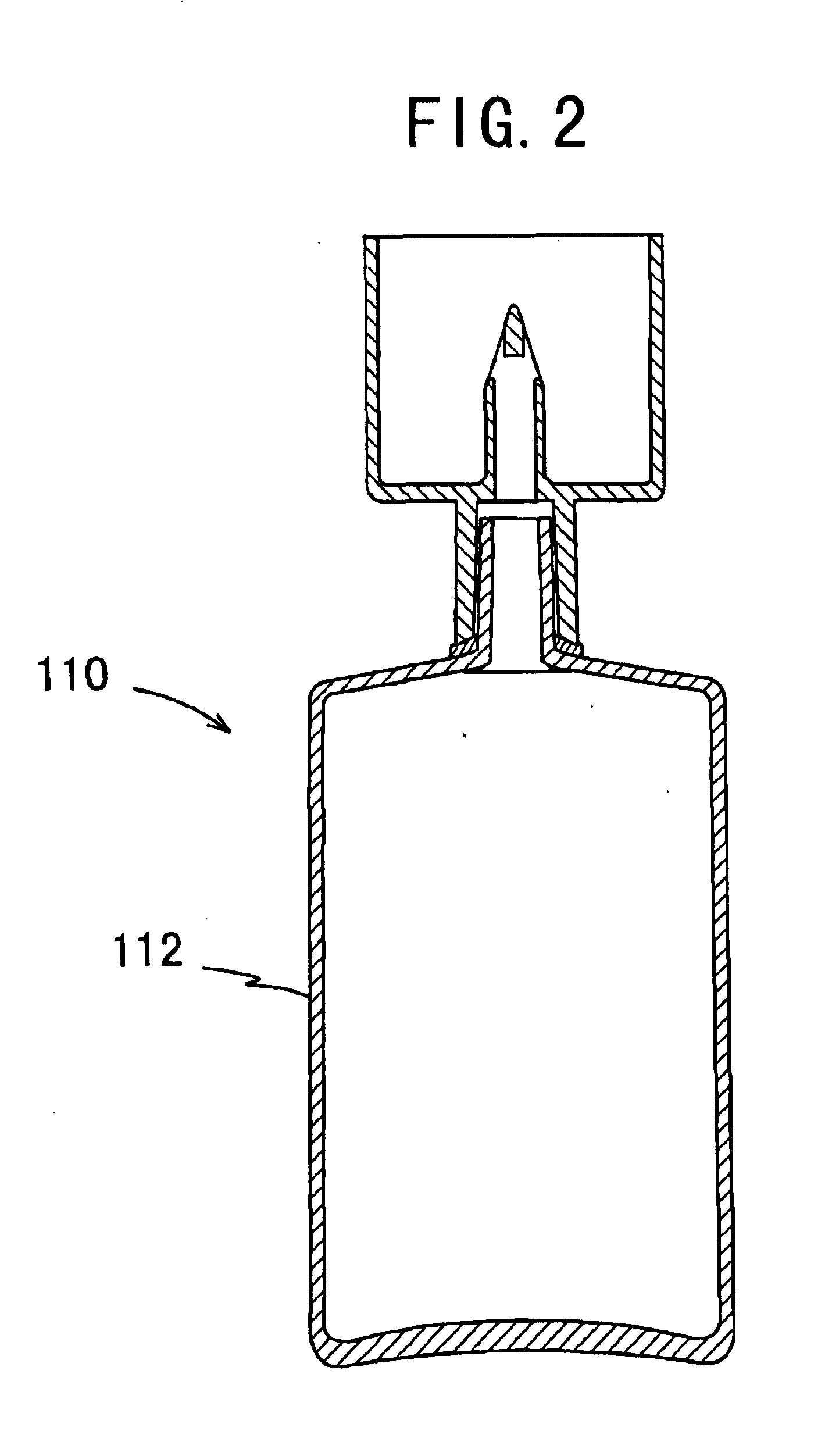
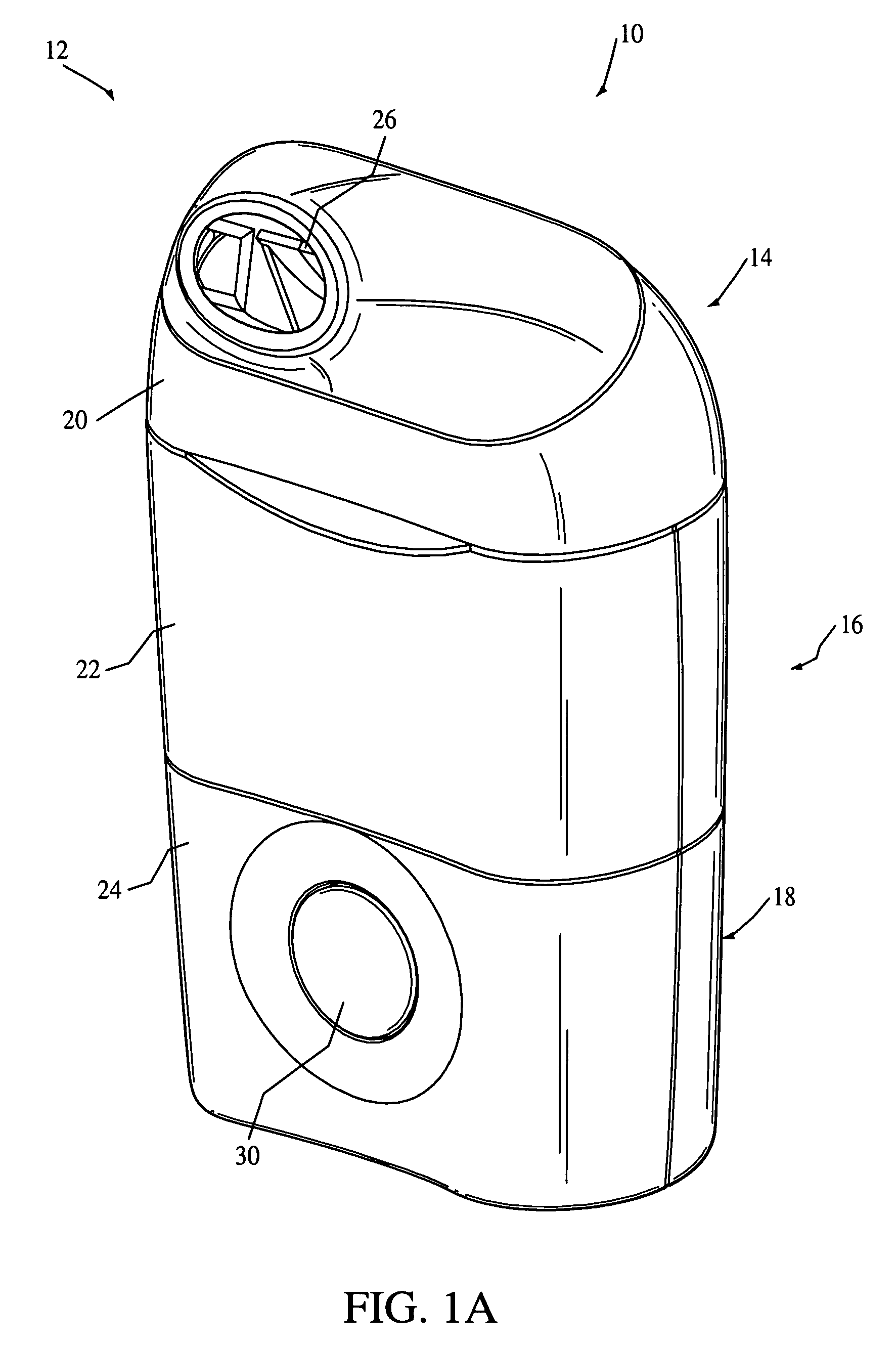
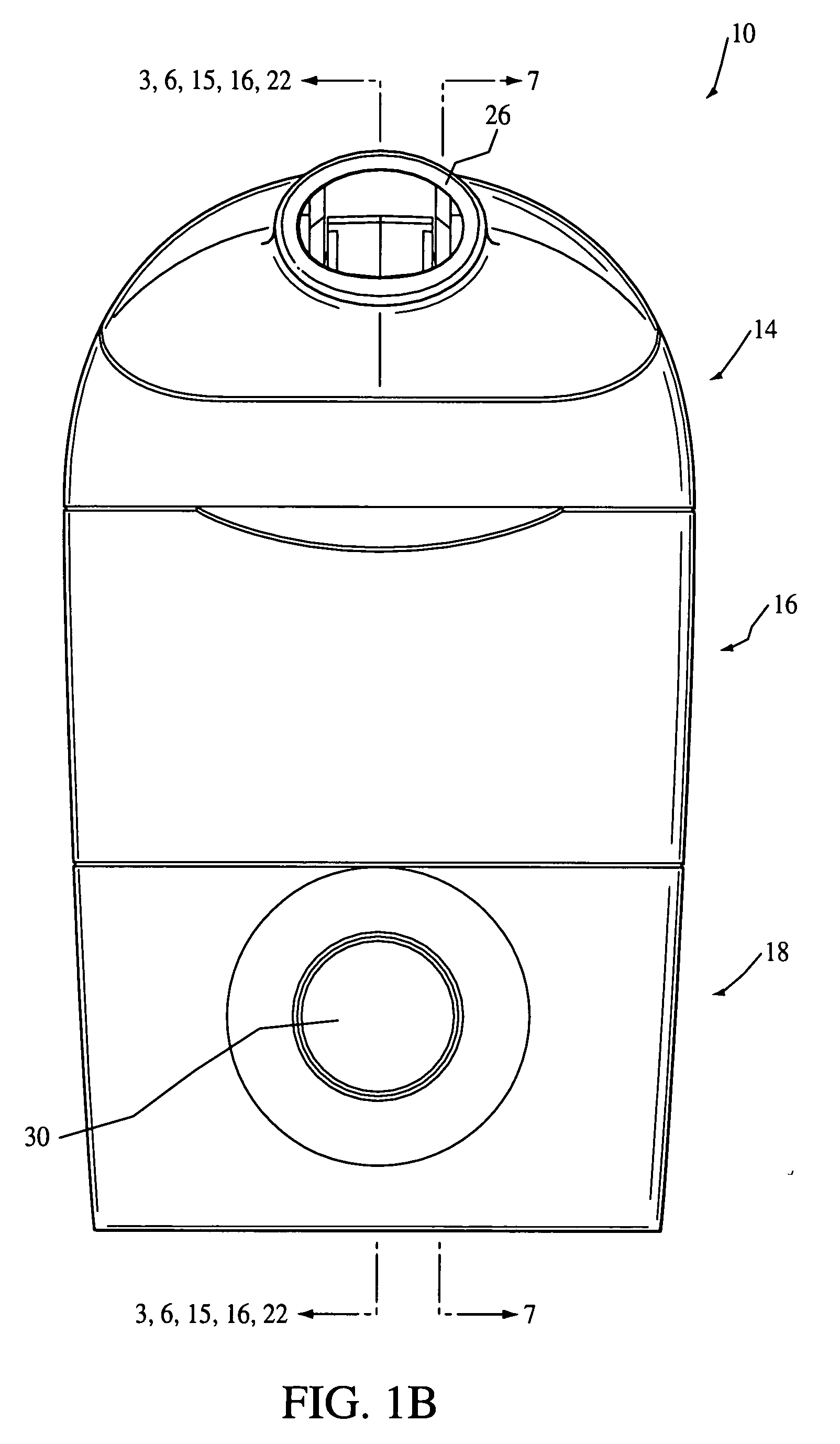
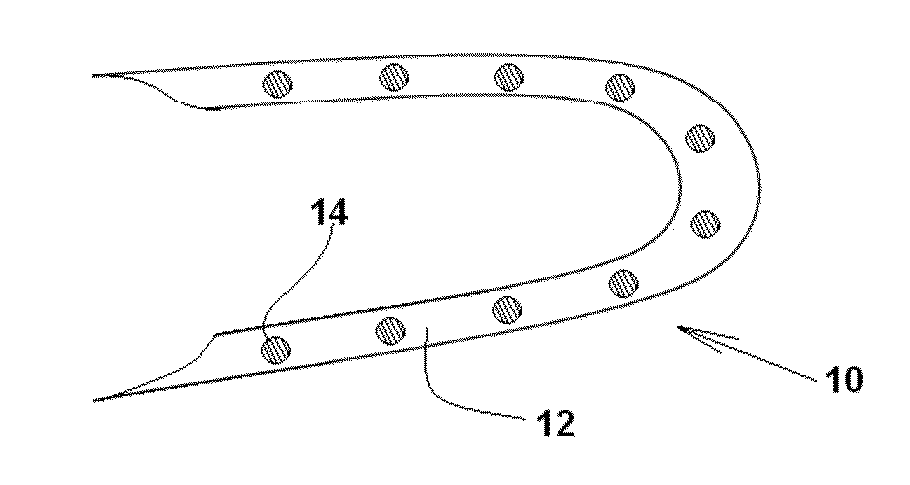
Drug solution filling plastic ampoule and process for producing the same
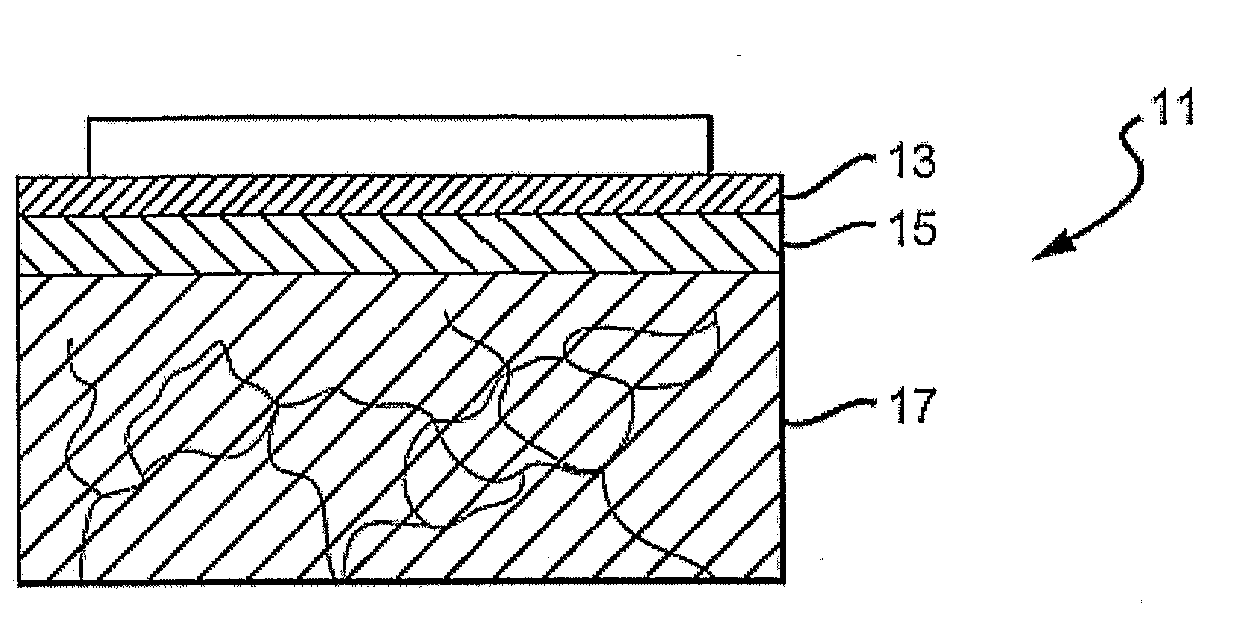
A drug solution filling plastic ampoule having gas, steam and light ray barrier properties, a drug permeation preventing capability and an absorption / adsorption preventing capability, and a production method for the plastic ampoule. The drug solution filling plastic ampoule includes a container body, a fusion-bonded portion which seals a mouth of the container body, and a wrench-off holder tab connected to the fusion-bonded portion. The ampoule is formed from a parison including two or more layers, at least one of which is a functional layer having at least one characteristic property selected from the group consisting of a gas permeation preventing capability, a steam permeation preventing capability, a light ray permeation preventing capability, a drug permeation preventing capability and a drug absorption / adsorption preventing capability.
Owner:OTSUKA PHARM FAB INC
Drug solution filling plastic ampoule and production method therefor
The invention provides a plastic ampoule filled with medicinal liquid, which has the functions of blocking gas, water vapor and light and preventing drug penetration, absorption and adsorption, and a preparation method thereof. The plastic ampoule 10 for filling medicinal solution of the present invention comprises a container main body 11, a fused portion 13 closing its opening 12 and a holding portion 14 connected thereto for twisting. The ampoule 10 is formed using a parison having two or more layers, at least one of which is a material selected from the group consisting of gas transmission, water vapor transmission, light transmission, drug transmission and A functional layer that prevents at least one of drug absorption and adsorption properties. That is, the parison is extruded from a multi-layer blow mold, clamped by the lower parting die to form the main body part 11 of the container, and after filling the liquid medicine 15 therein, the opening part 12 is clamped by the upper parting die to form a fusion joint. Part 13 and holding part 14, thereby making the product of the present invention.
Owner:OTSUKA PHARM FAB INC
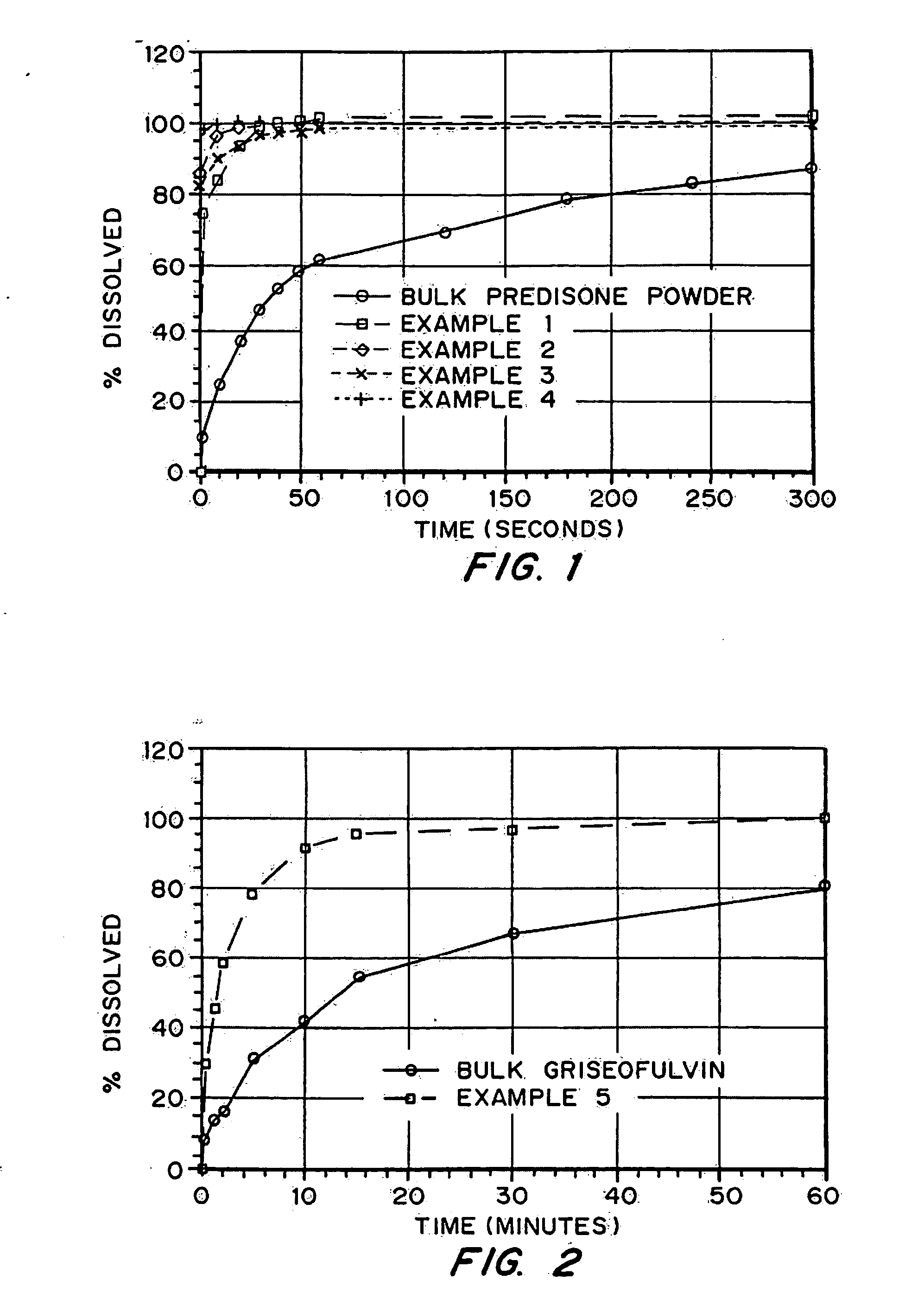
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
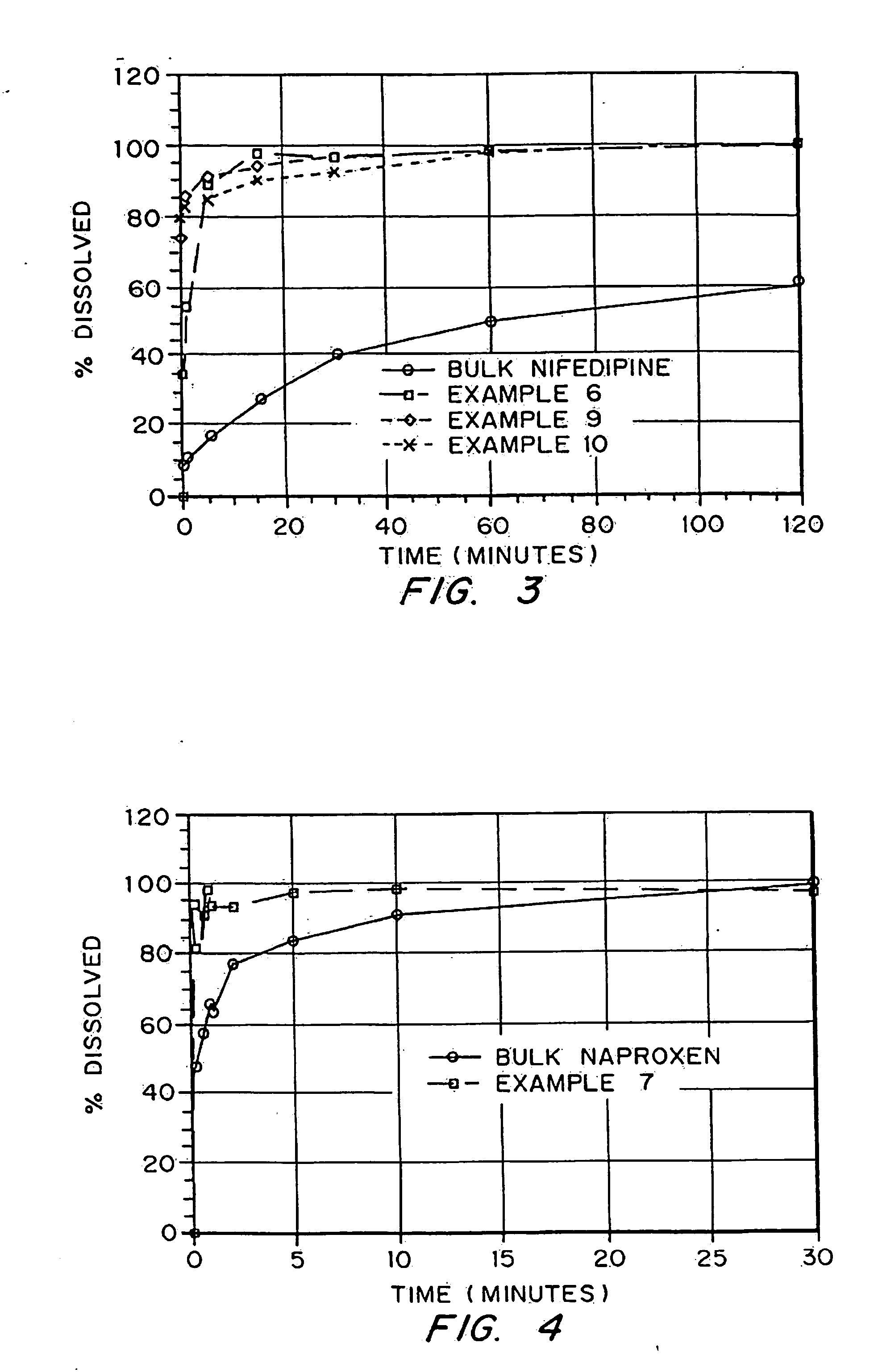
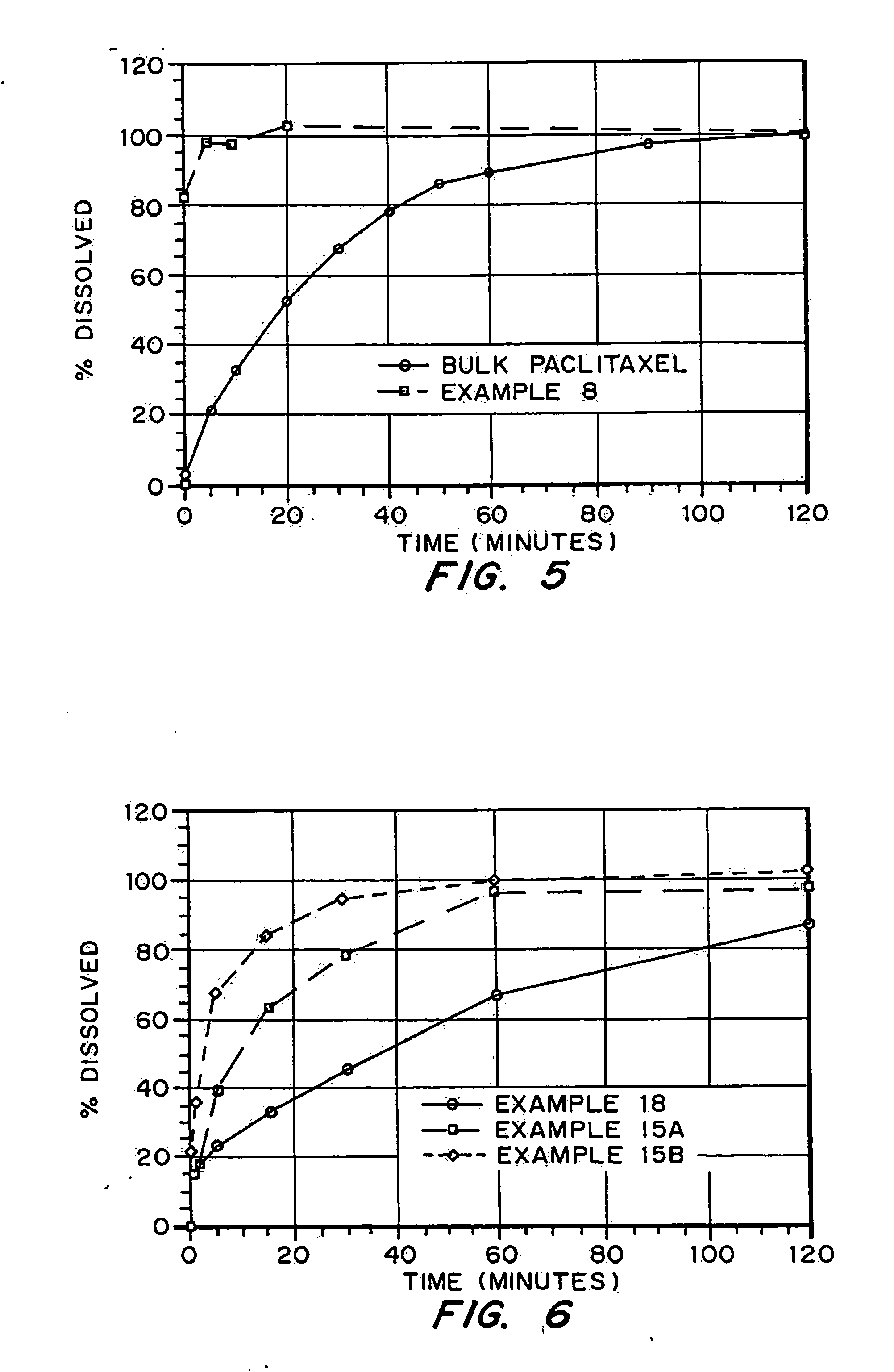
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Solid solution perforator for drug delivery and other applications
InactiveUS6945952B2Fast biodegradationBarrier property can be diminished and controlledSurgeryMicroneedlesDrug reservoirDrugs solution
A solid drug solution perforator (SSP) system and an associated drug reservoir are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, for nutrient delivery and for drug targeting. For drug delivery, the SSP system includes an active drug ingredient and a matrix of perforator material that biodegrades or dissolves quickly upon contact with a patient's body. The SSP system provides a skin barrier perforator and a controller for prompt initiation and cut-off drug delivery. In a preferred method of transdermal drug delivery, an SSP system containing a selected drug penetrates into an epidermis or dermis, and the drug is promptly released from the (dissolving) SSP system perforator. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator. Formulation and fabrication procedures for the SSP and associated reservoir are also provided. An SSP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
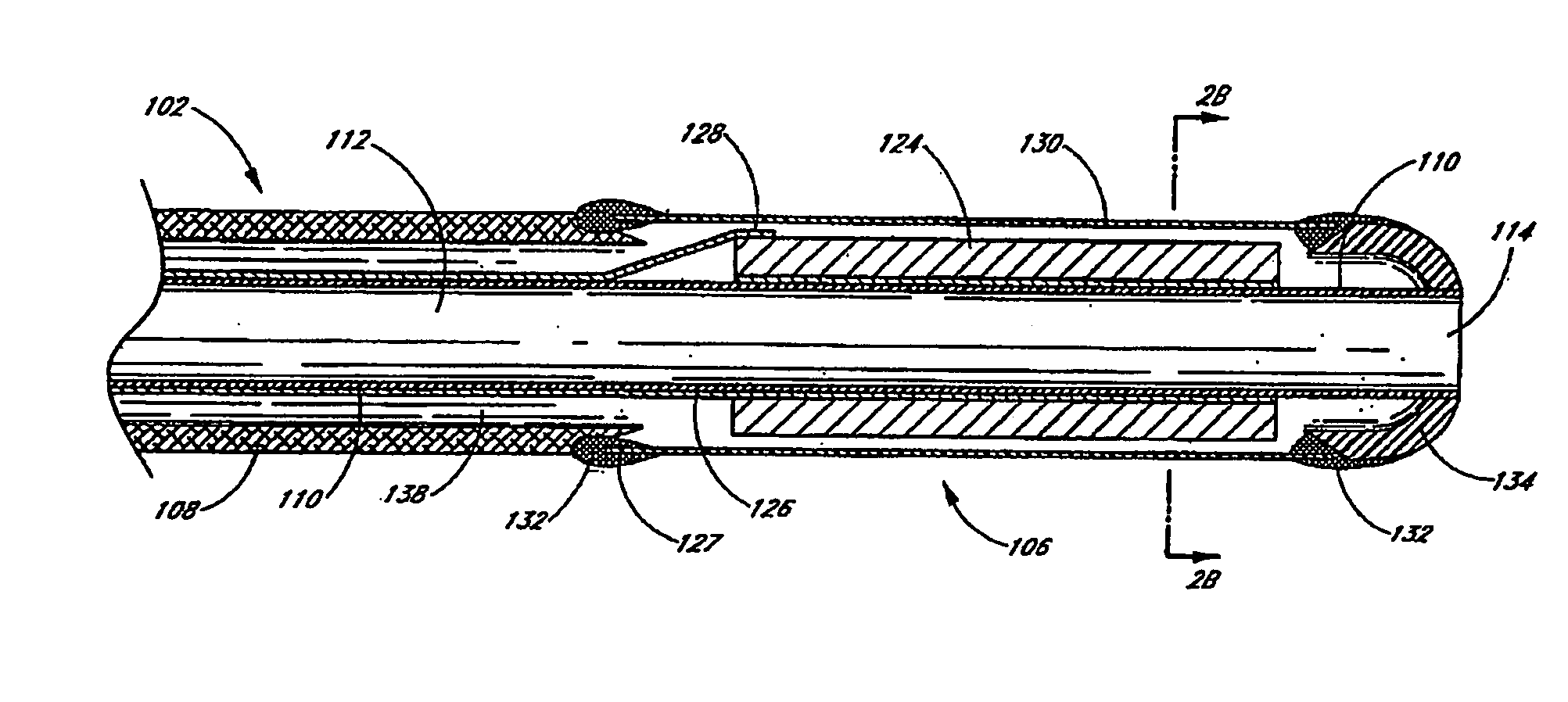
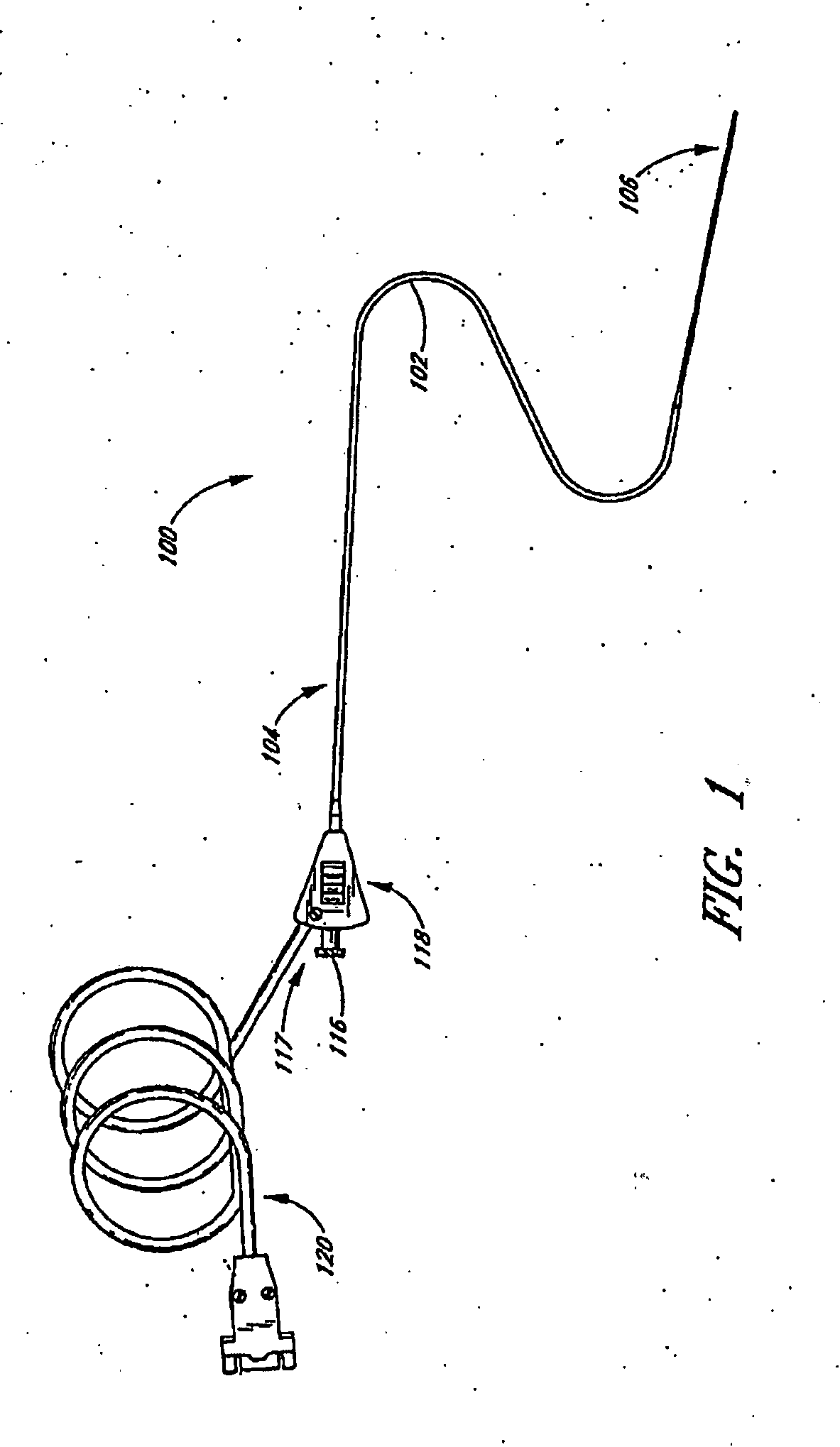
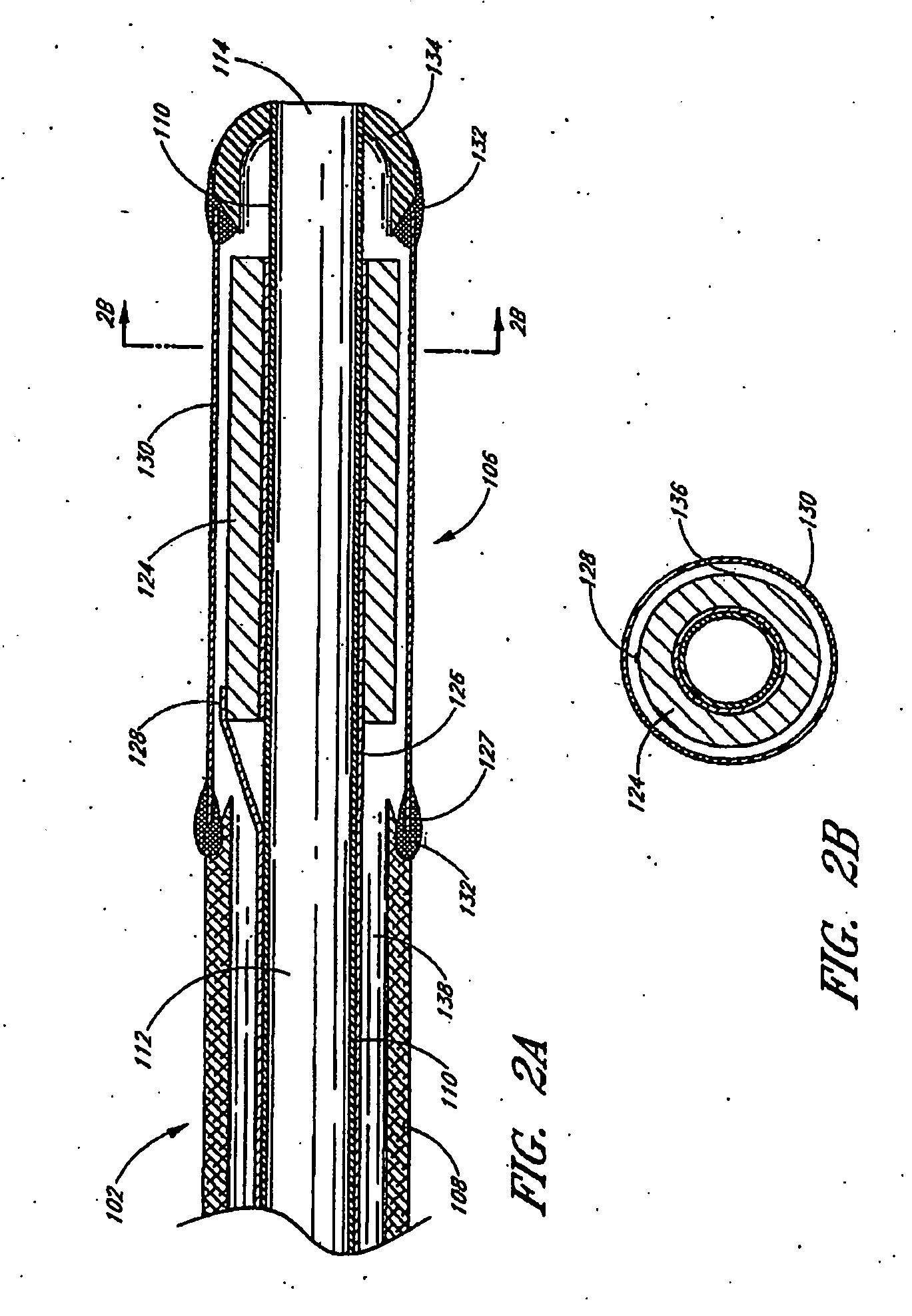
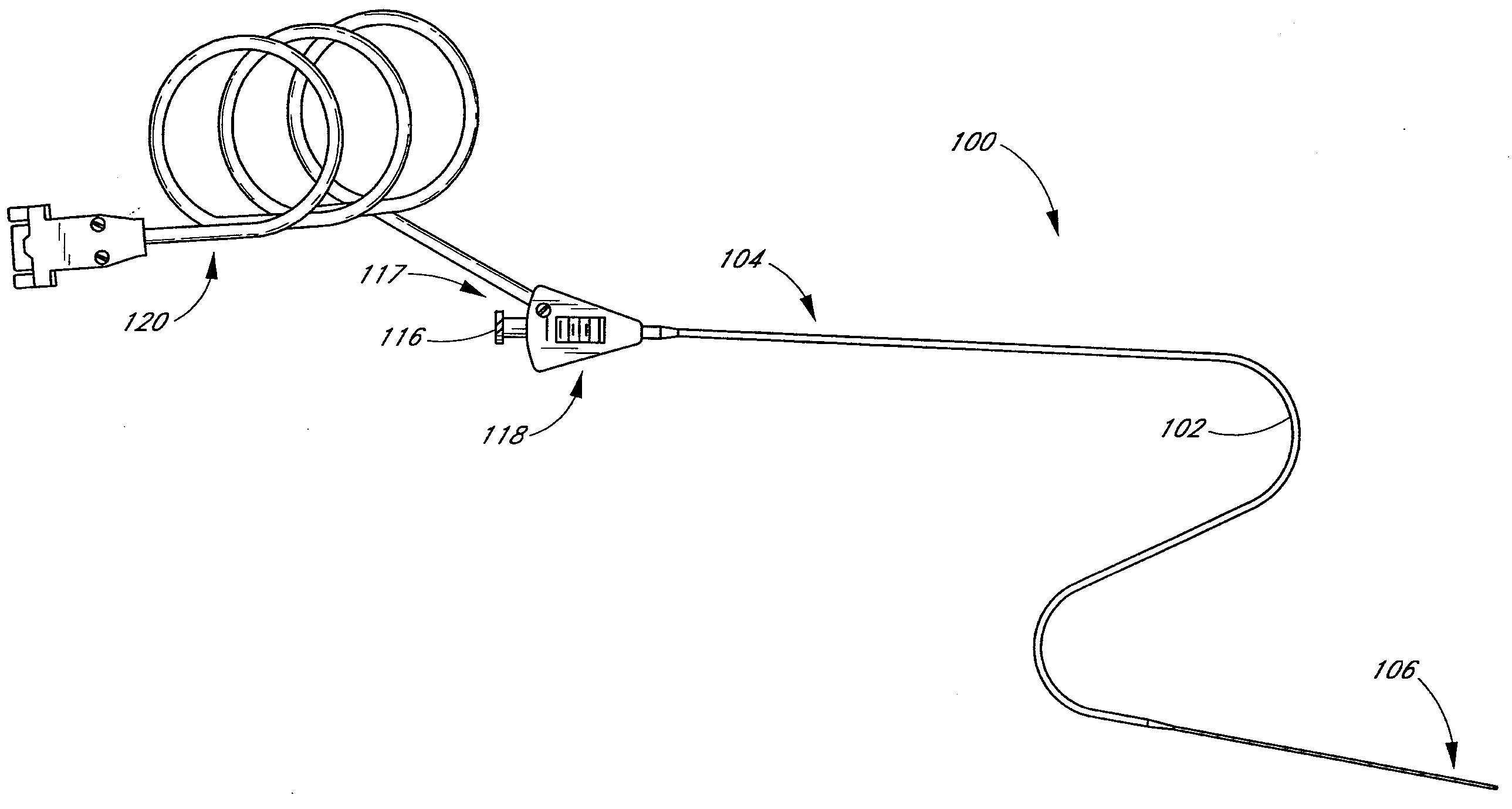
Small vessel ultrasound catheter
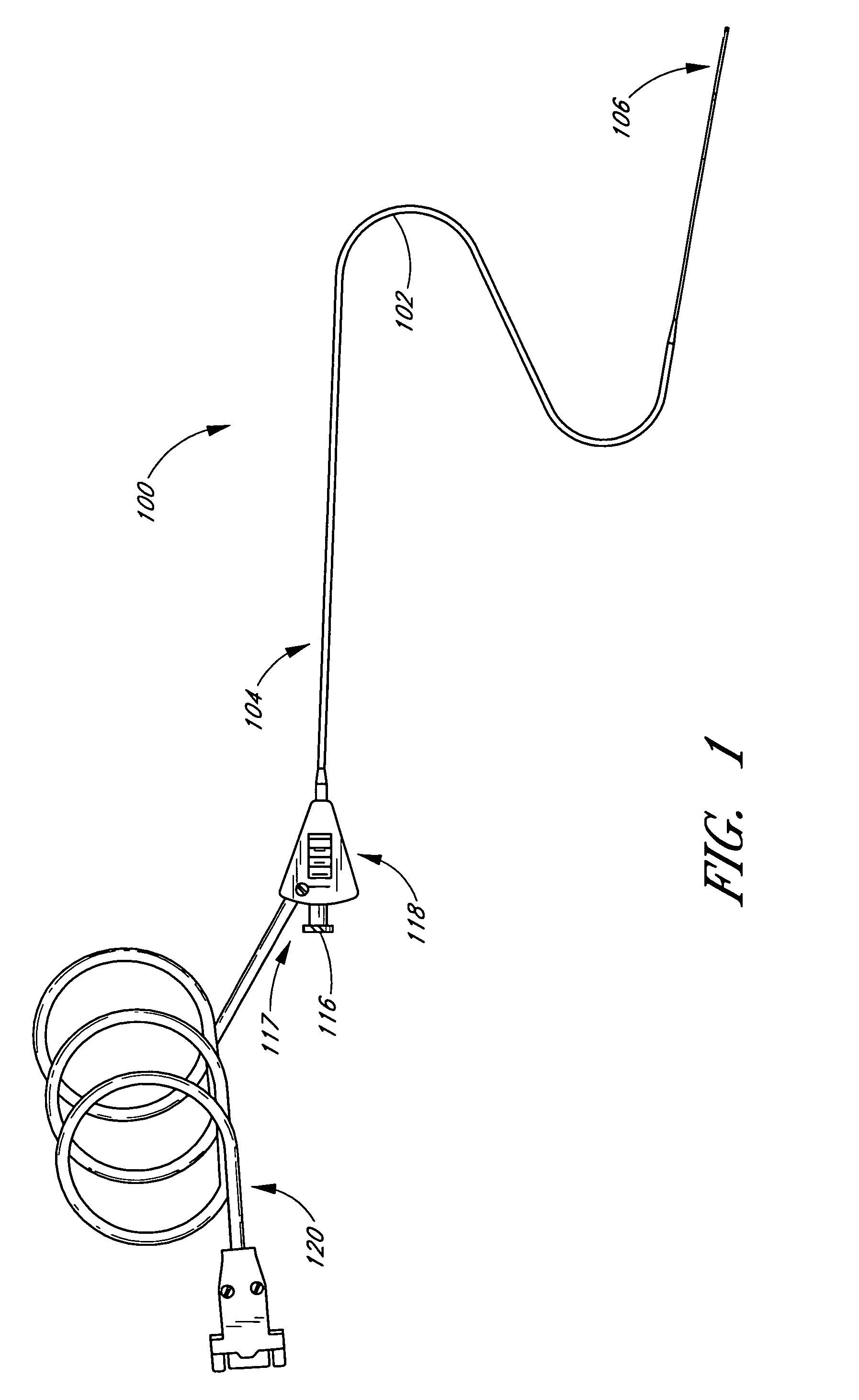
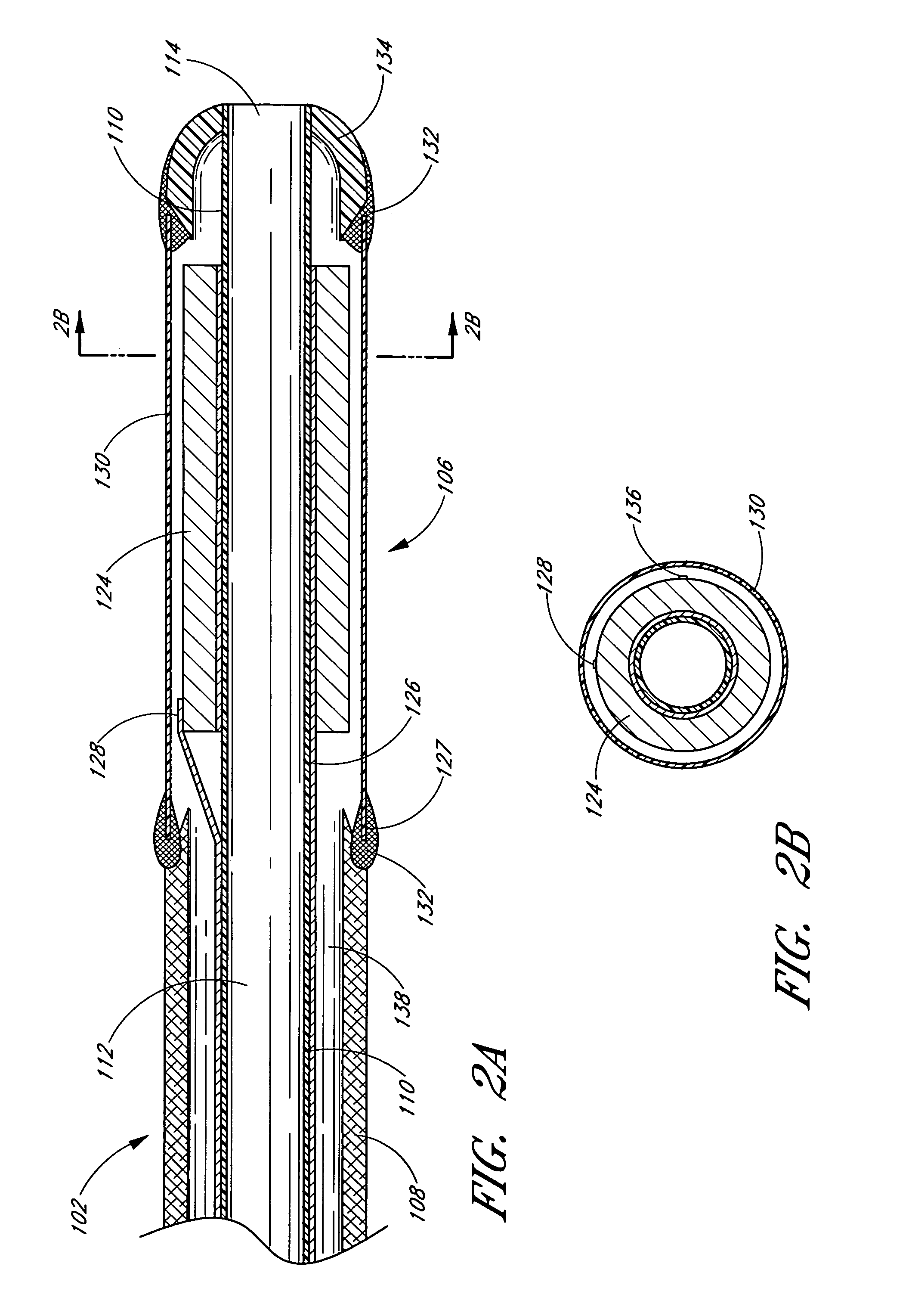
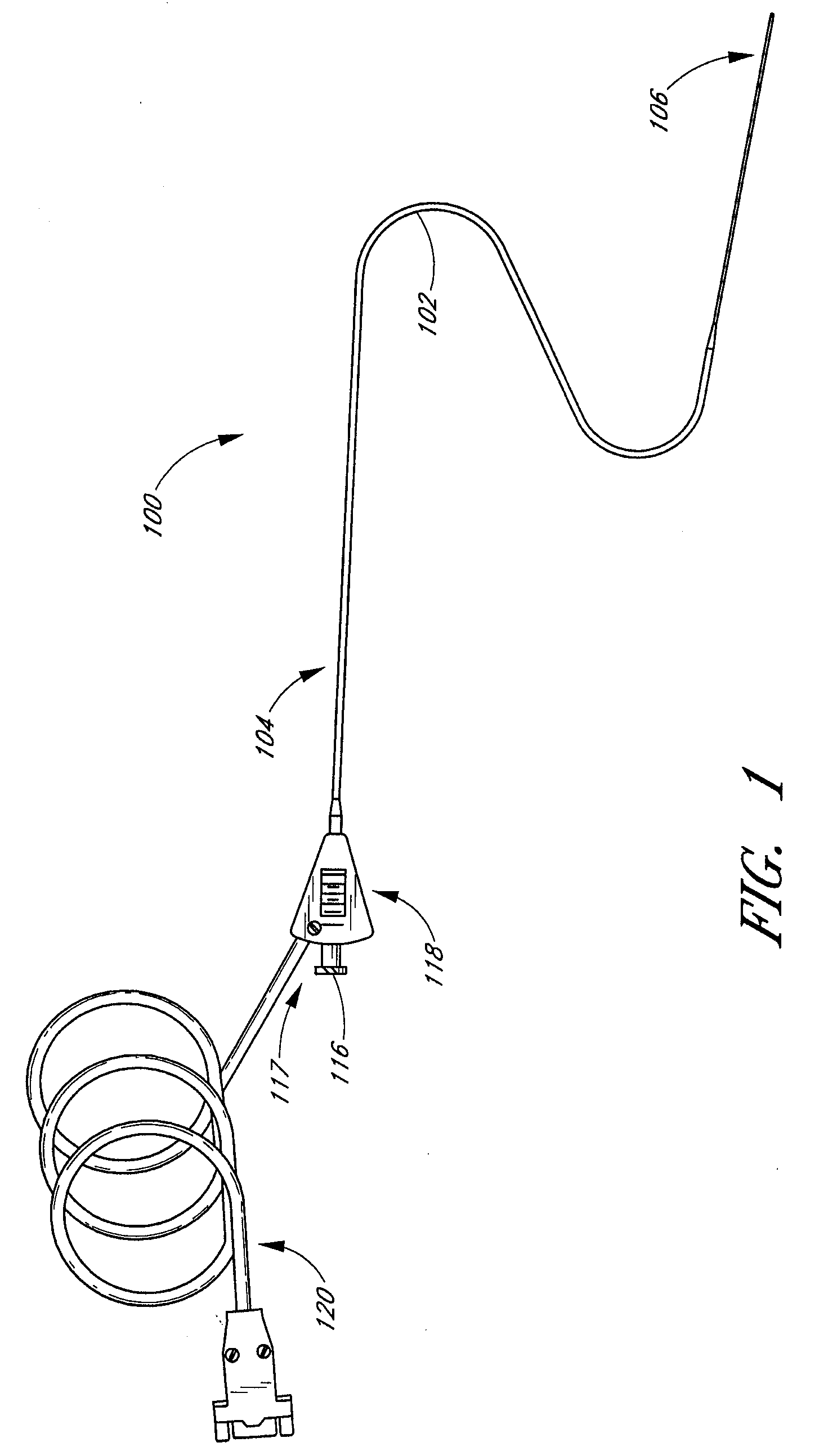
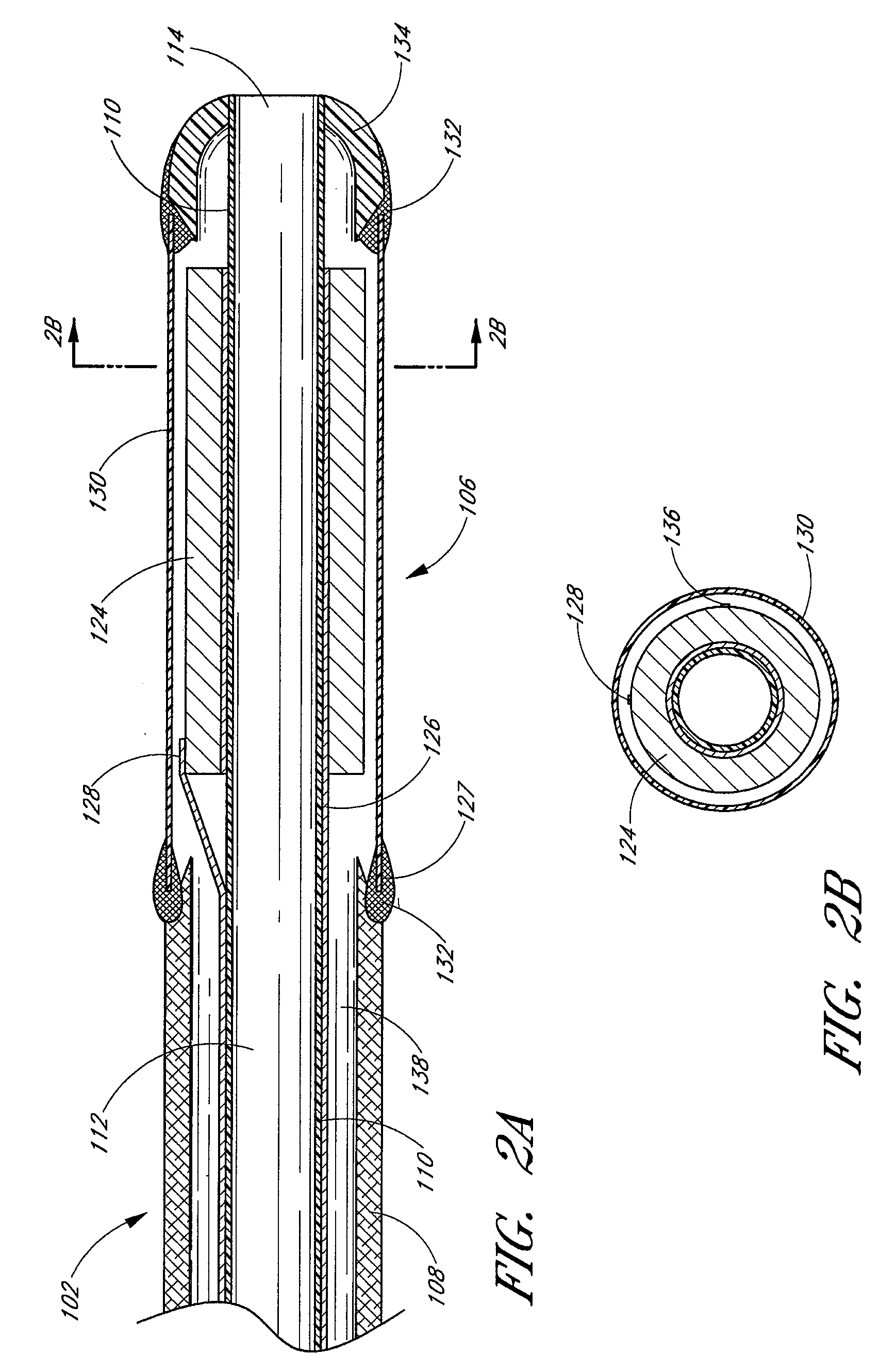
InactiveUS20050215942A1Increase stiffnessSufficient flexibilityUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDrugs solutionVascular ultrasound
An ultrasound catheter adapted for accessing small vessels in the distal anatomy is disclosed. The ultrasound catheter comprises an elongate tubular body formed with a delivery lumen. The flexibility and dimensions of the tubular body allow access to the distal anatomy by advancement over the guidewire. An ultrasound radiating member is provided along the distal end portion of the tubular body for emitting ultrasound energy at a treatment site. A drug solution may also be delivered through the delivery lumen and out an exit port to the treatment site.
Owner:EKOS CORP
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Administration instrument for medical use
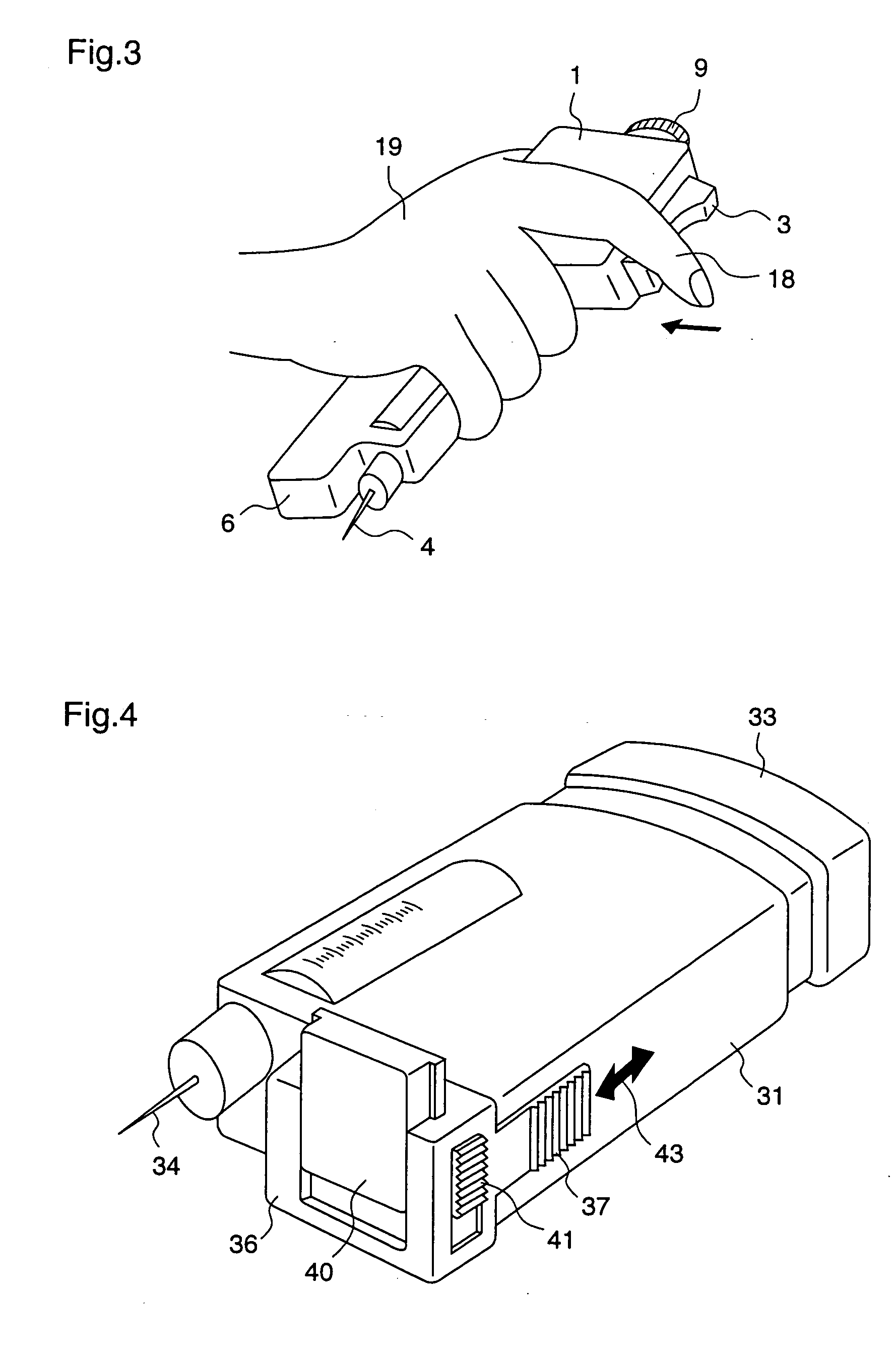
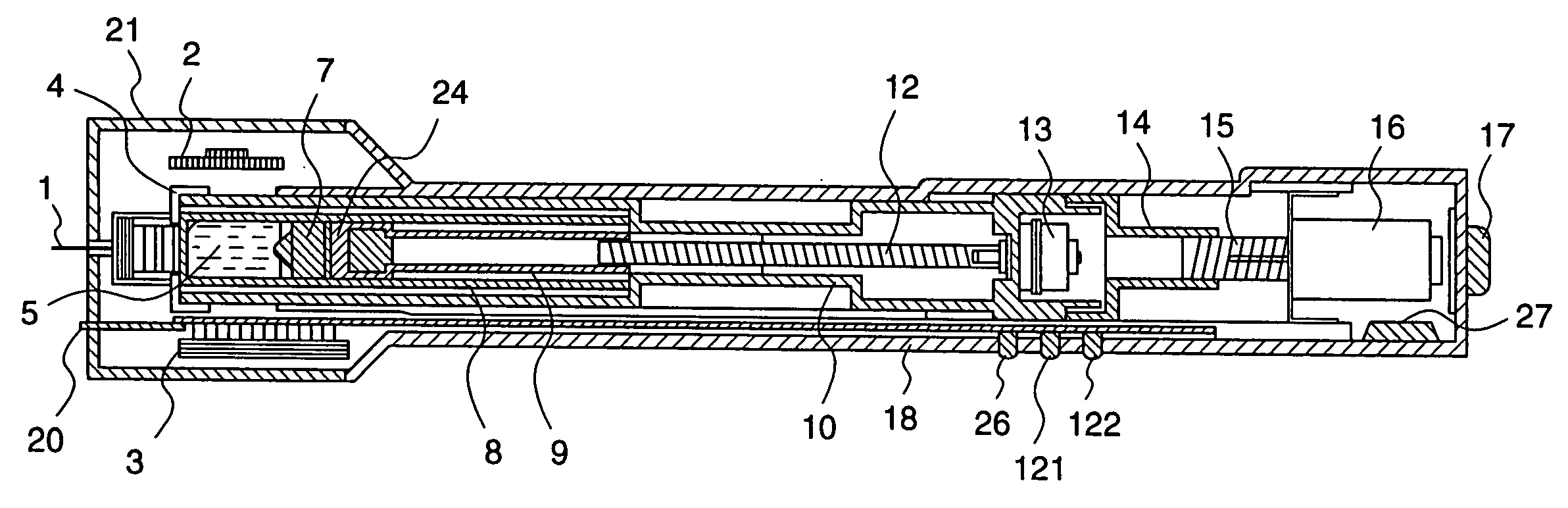
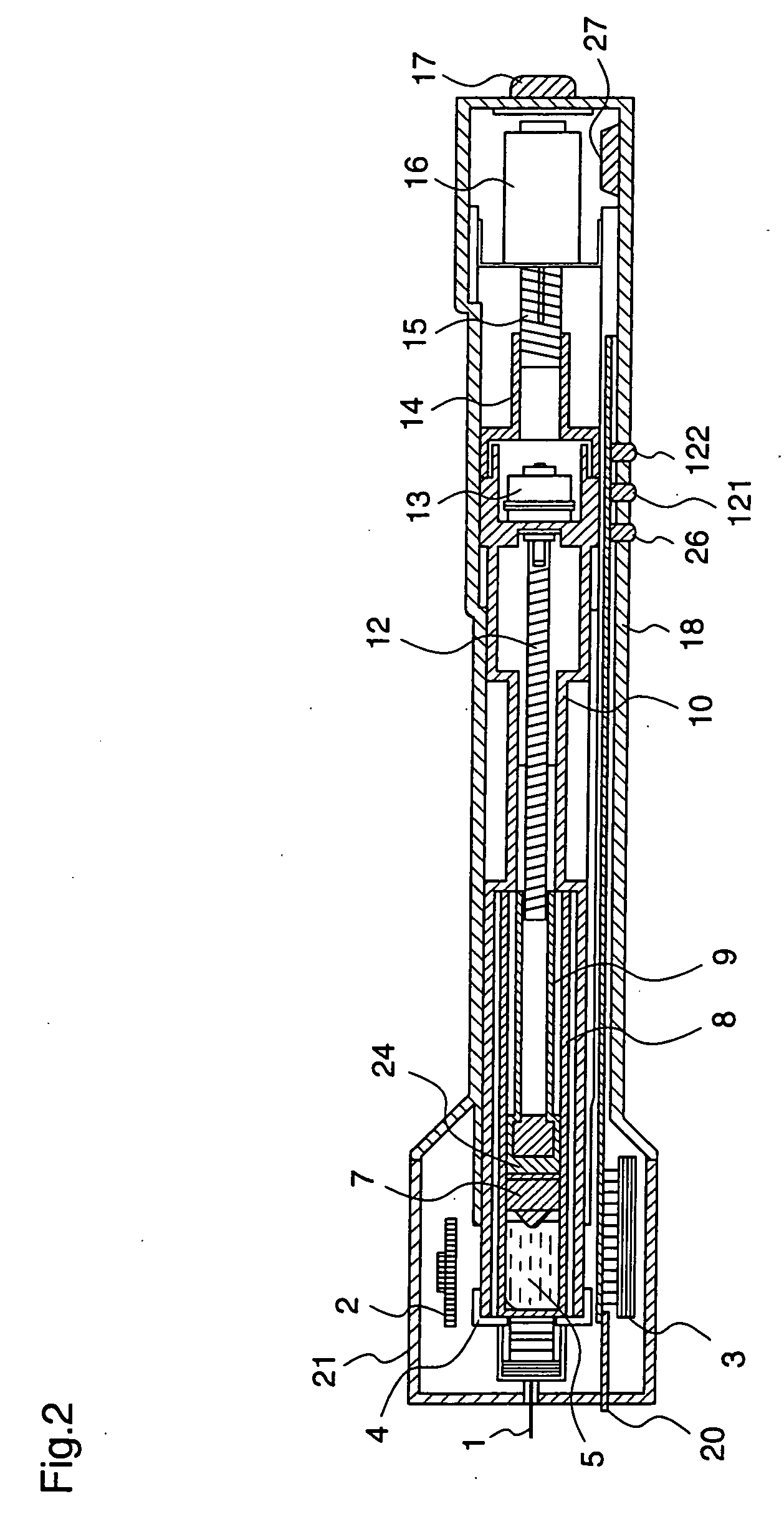
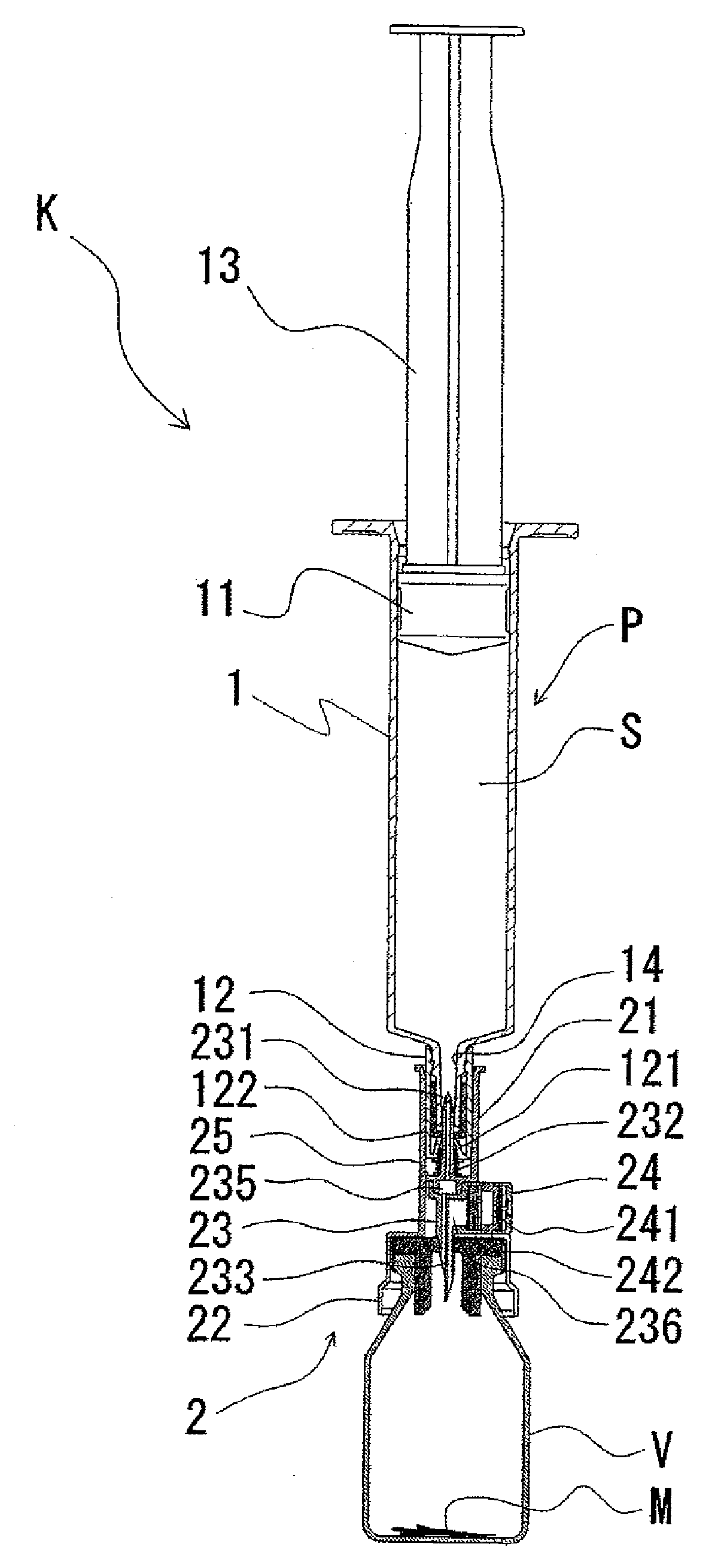
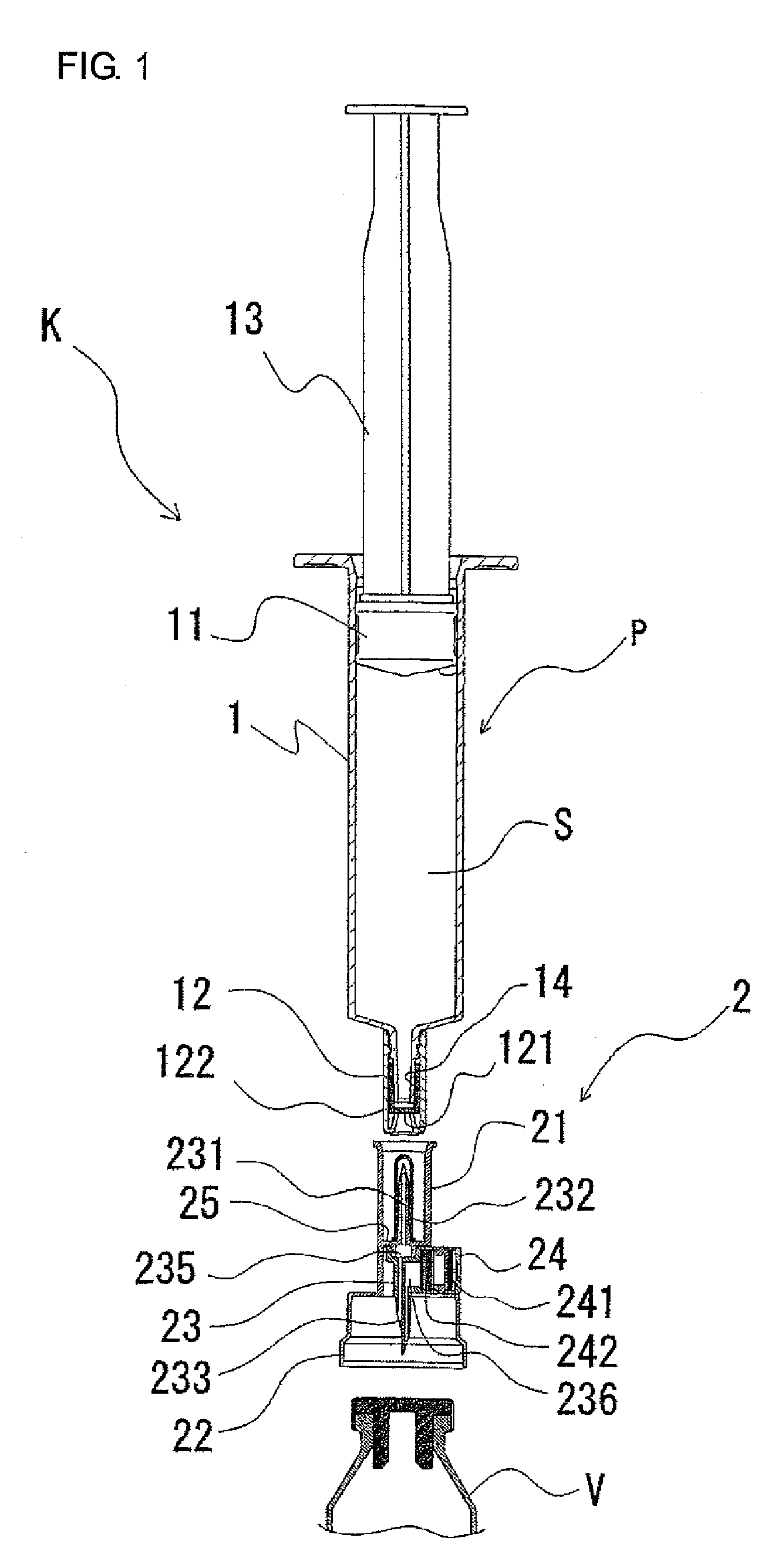
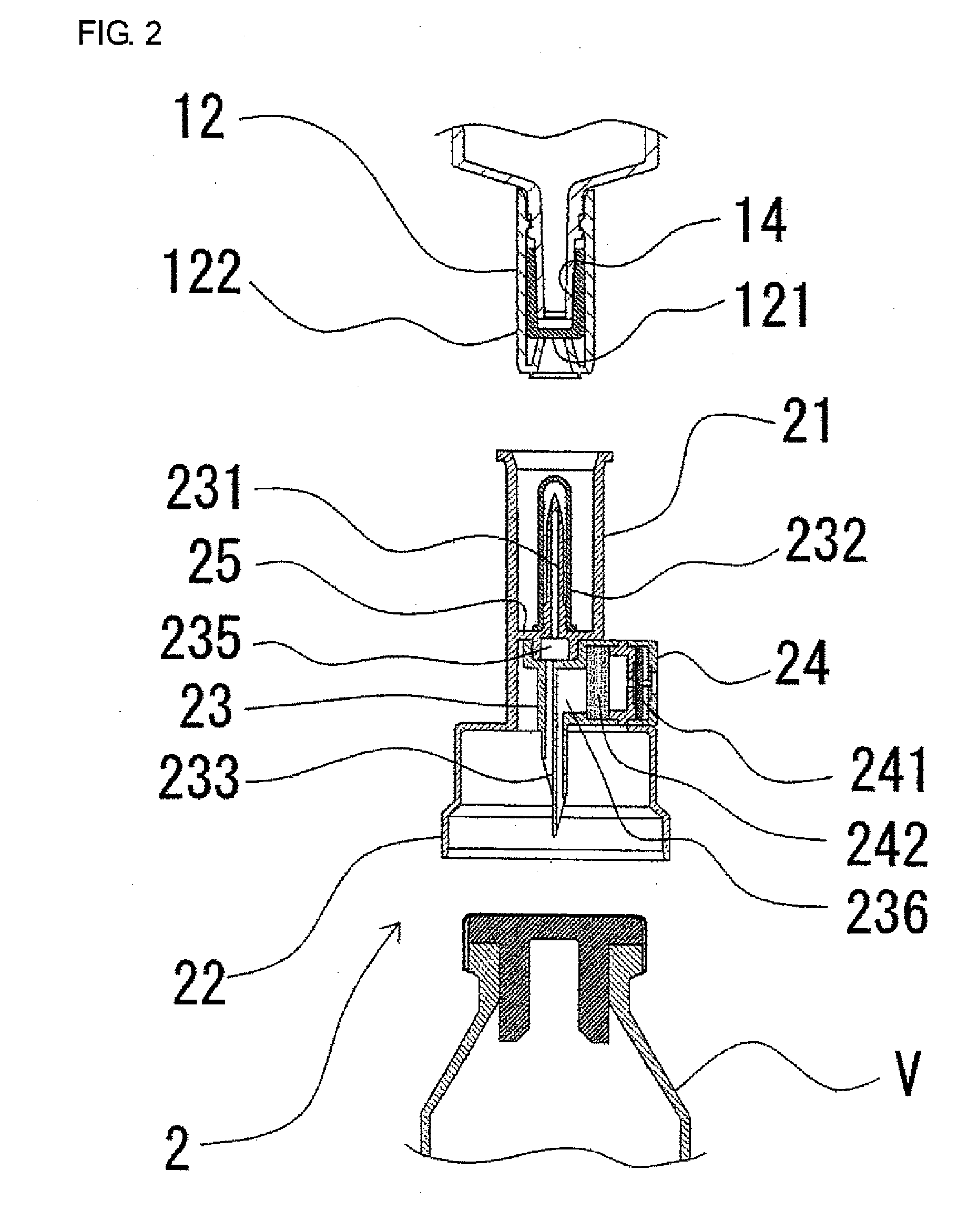
ActiveUS20050090781A1Not increasing physical and mental painAmpoule syringesMedical devicesPain durationDrugs solution
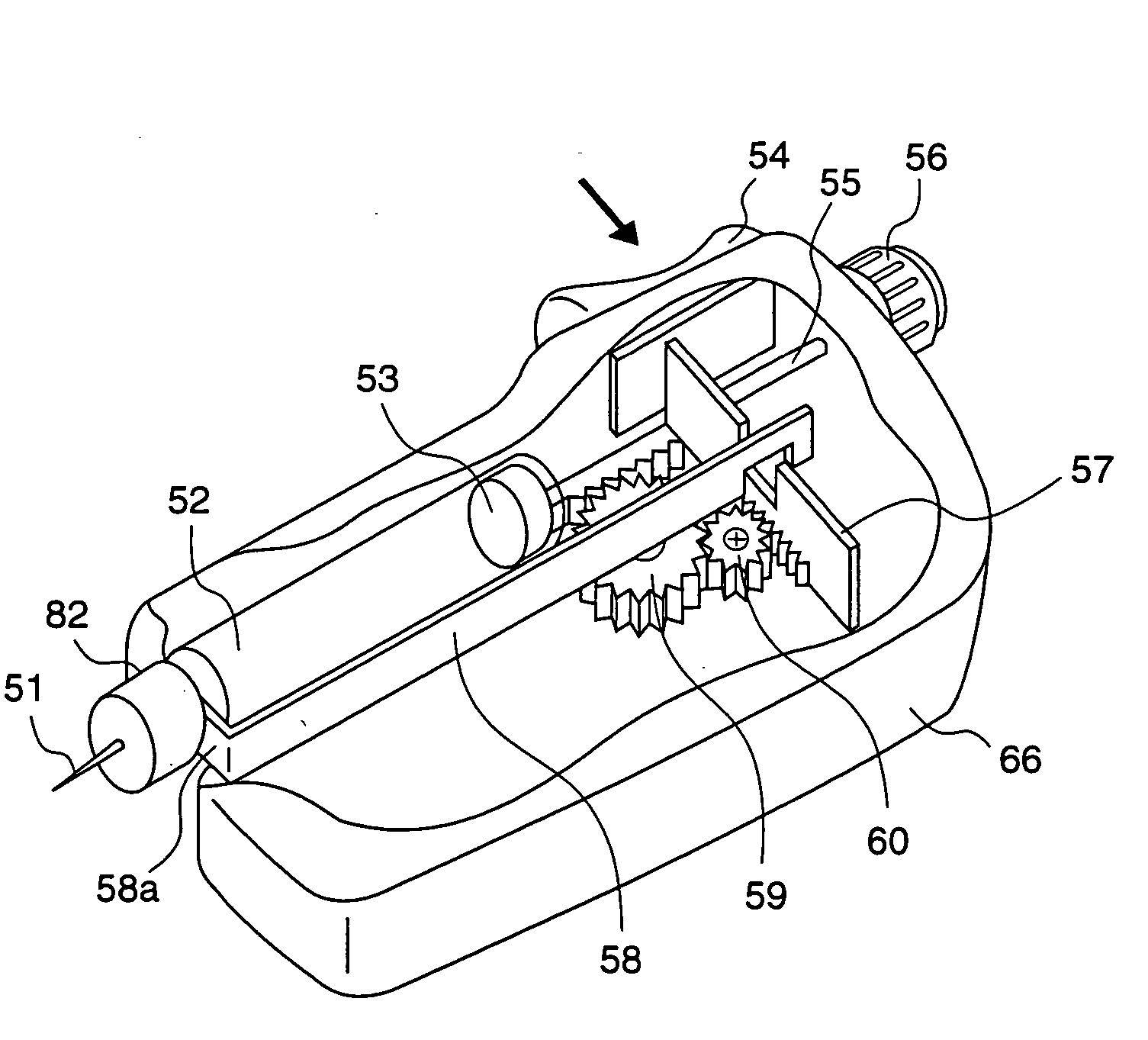
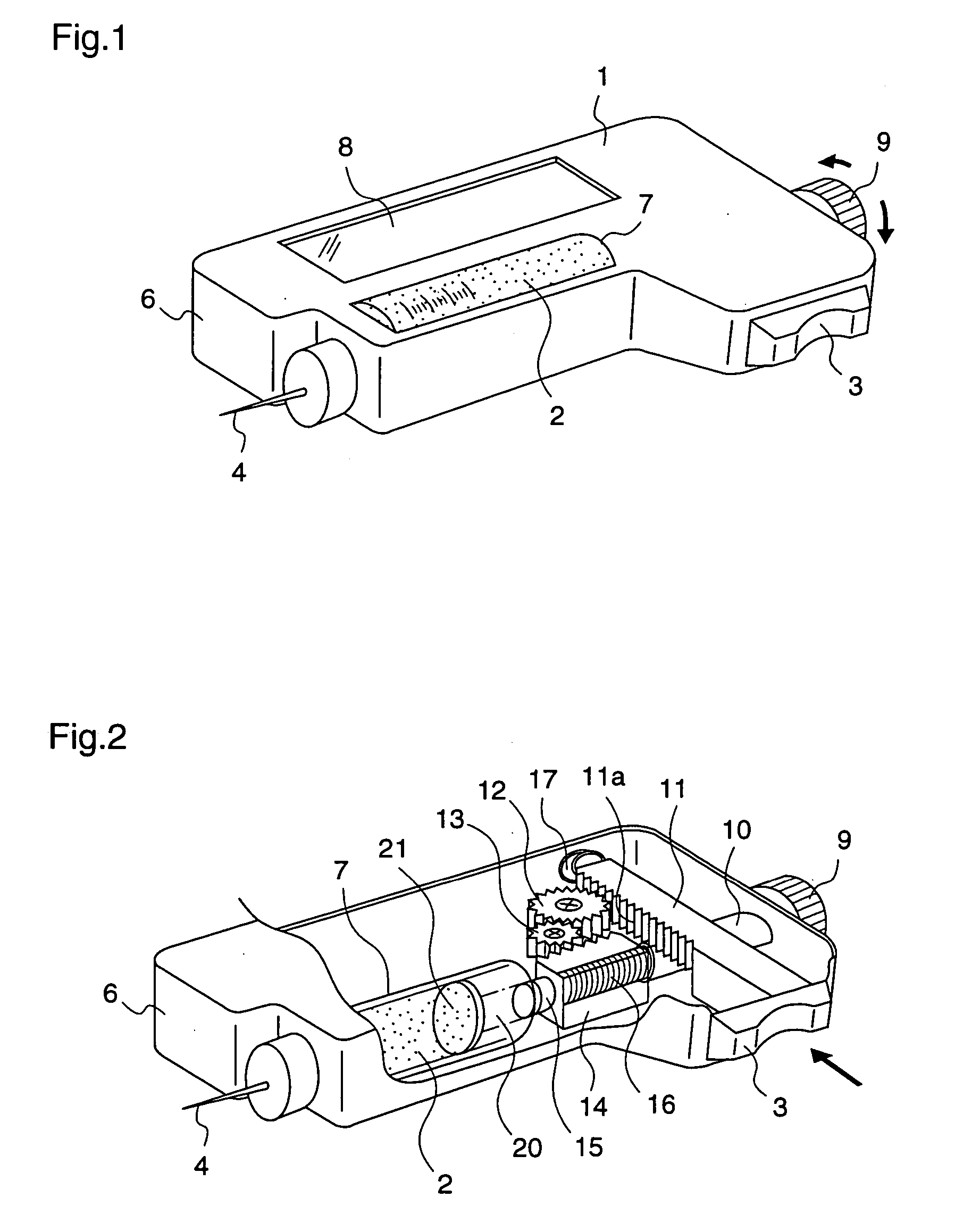
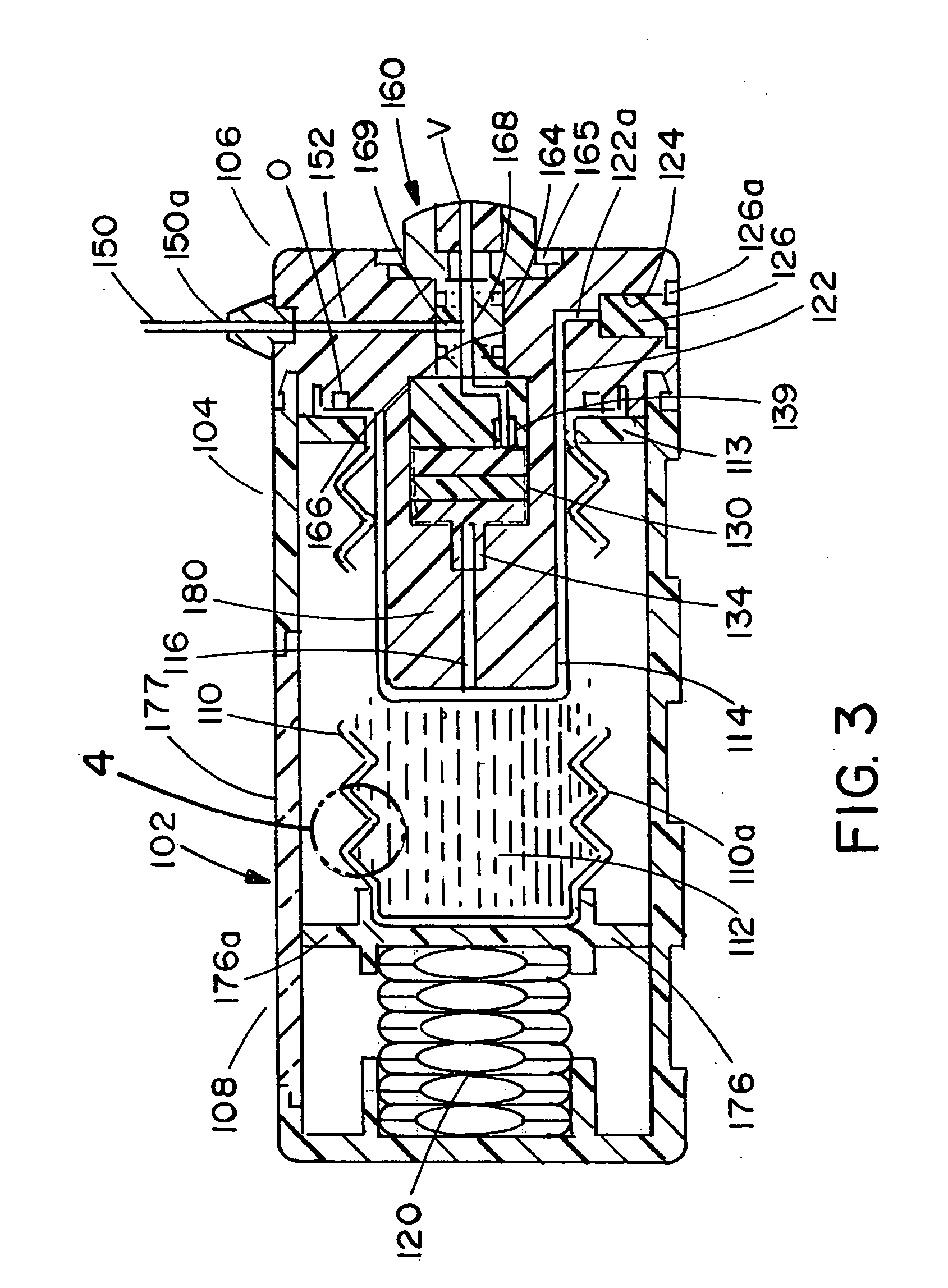
The present invention relates to an administration instrument for medical use that can perform injection of a drug solution with stability and with great reliability. For example, at the administration, it is possible to prevent a force that presses the injection button from acting in a direction of inserting the needle that is inserted into the skin deeper, and enables the administration under a stable state where the needle does not wobble, thereby alleviating physical and mental pain of the administration patient. With a structure in which an injection button (3) is pressed at an angle that is not parallel to the needle with respect to a direction in which the needle (4) is inserted into the skin, it is possible to prevent the force of pressing the injection button from being transmitted in a direction of inserting the needle into the skin deeper than the initial insertion of the needle, thereby achieving an administration under a stable state.
Owner:PHC HLDG CORP
Small vessel ultrasound catheter
ActiveUS7384407B2Promote progressReduced bending resistanceUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDrugs solutionVascular ultrasound
An ultrasound catheter adapted for accessing small vessels in the distal anatomy is disclosed. The ultrasound catheter comprises an elongate tubular body formed with a delivery lumen. The flexibility and dimensions of the tubular body allow access to the distal anatomy by advancement over the guidewire. An ultrasound radiating member is provided along the distal end portion of the tubular body for emitting ultrasound energy at a treatment site. A drug solution may also be delivered through the delivery lumen and out an exit port to the treatment site.
Owner:EKOS CORP
Medical automatic medicator
ActiveUS20050209569A1Control depthReduce painAmpoule syringesAutomatic syringesDrugs solutionNeedle insertion
After pressing a part of the exterior of a body of an administration instrument against a body region of a patient to which a drug solution is to be administered, an injection needle that is housed in the instrument body is automatically protruded from the body to insert the needle into the body region, and further, the injection needle being inserted into the body region is automatically housed in the instrument body to remove the needle from the body region. Thereby, the pain of the patient during needle insertion and needle removal is reduced, and administration at a constant speed is possible during injection of the drug solution. Furthermore, even when two kinds of drug solutions or a dissolving and mixing type drug solution are / is used, mixing can be easily and reliably carried out.
Owner:PHC HLDG CORP
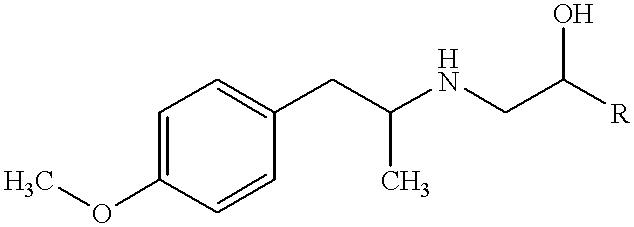
Aripiprazole oral solution
The present invention provides for a pharmaceutical solution suitable for oral administration comprising aripiprazole, a pharmaceutically suitable solvent system, one or more taste-enhancing / masking agents and one or more agents selected from the group consisting of lactic acid, acetic acid, tartaric acid and citric acid, wherein said solution has a pH from 2.5 to 4.5.
Owner:OTSUKA PHARM CO LTD
Porous drug matrices and methods of manufacture thereof
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
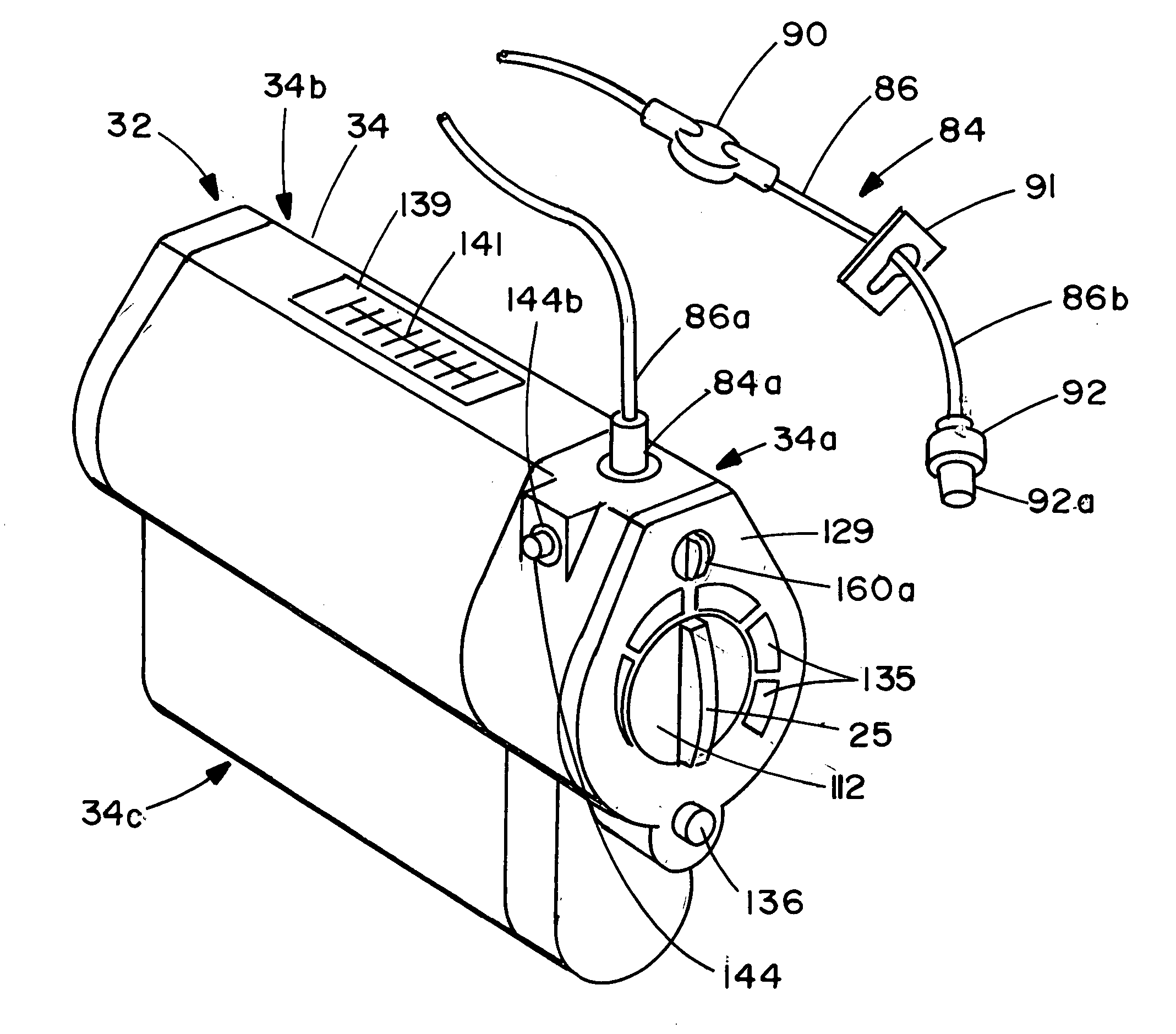
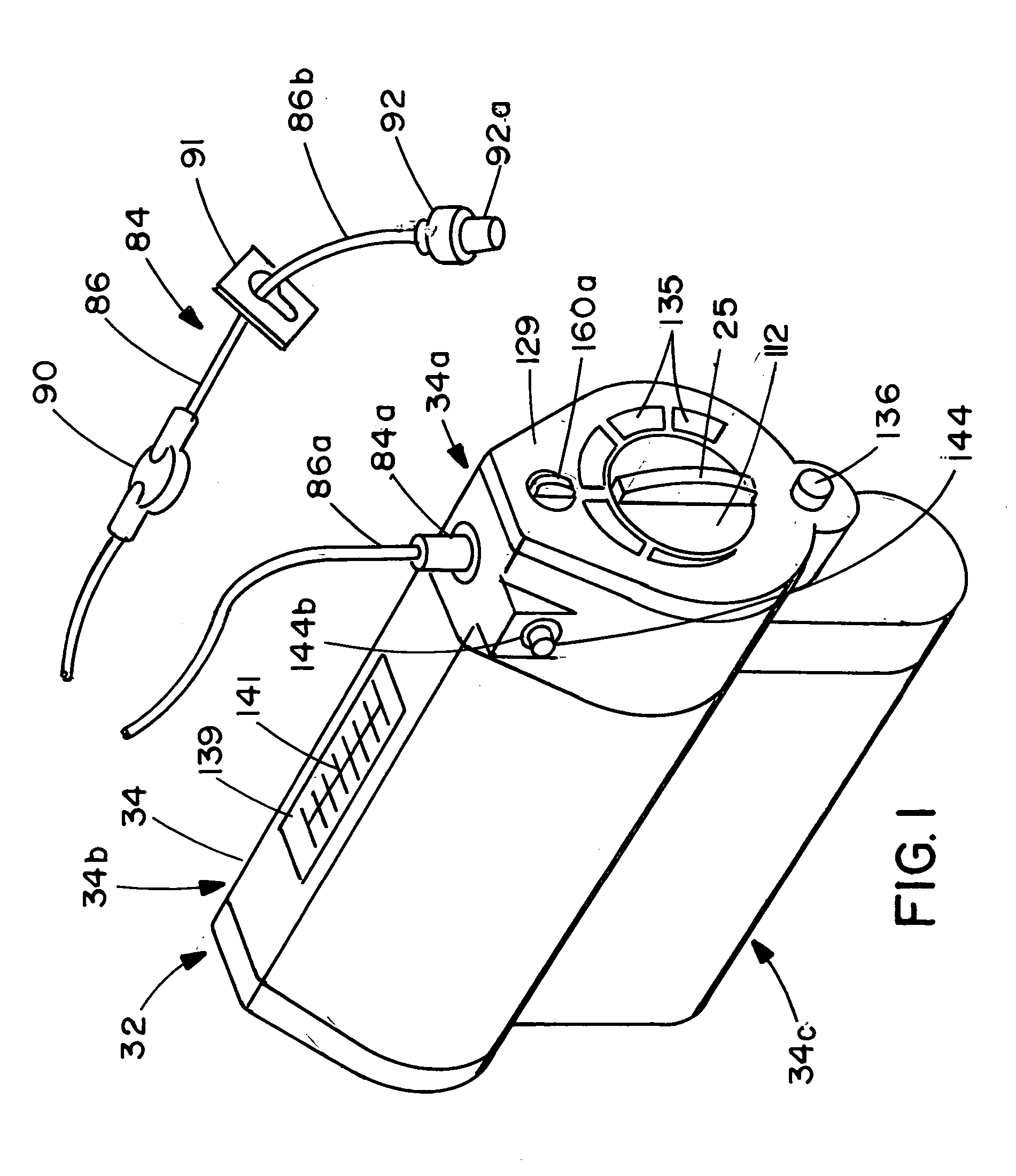
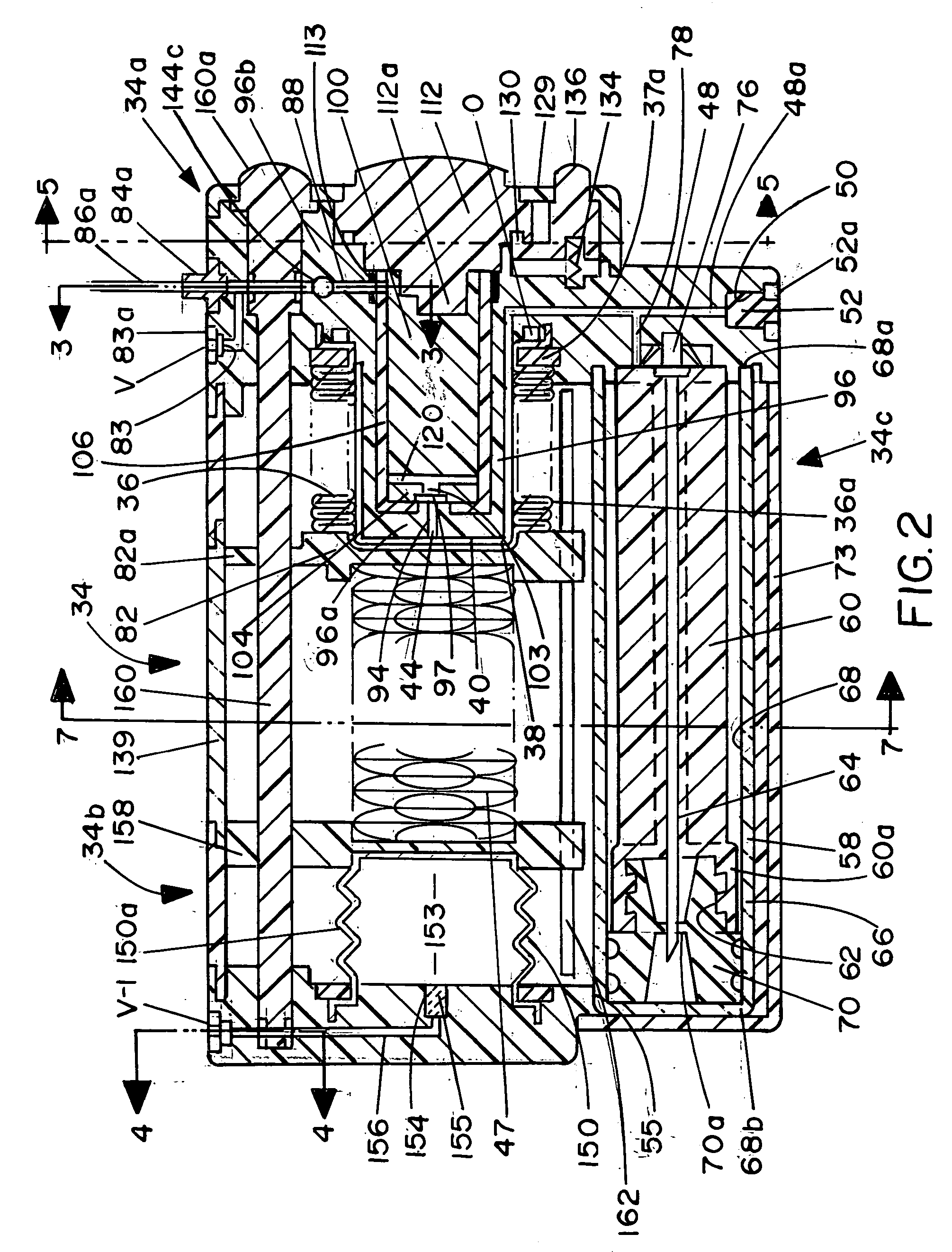
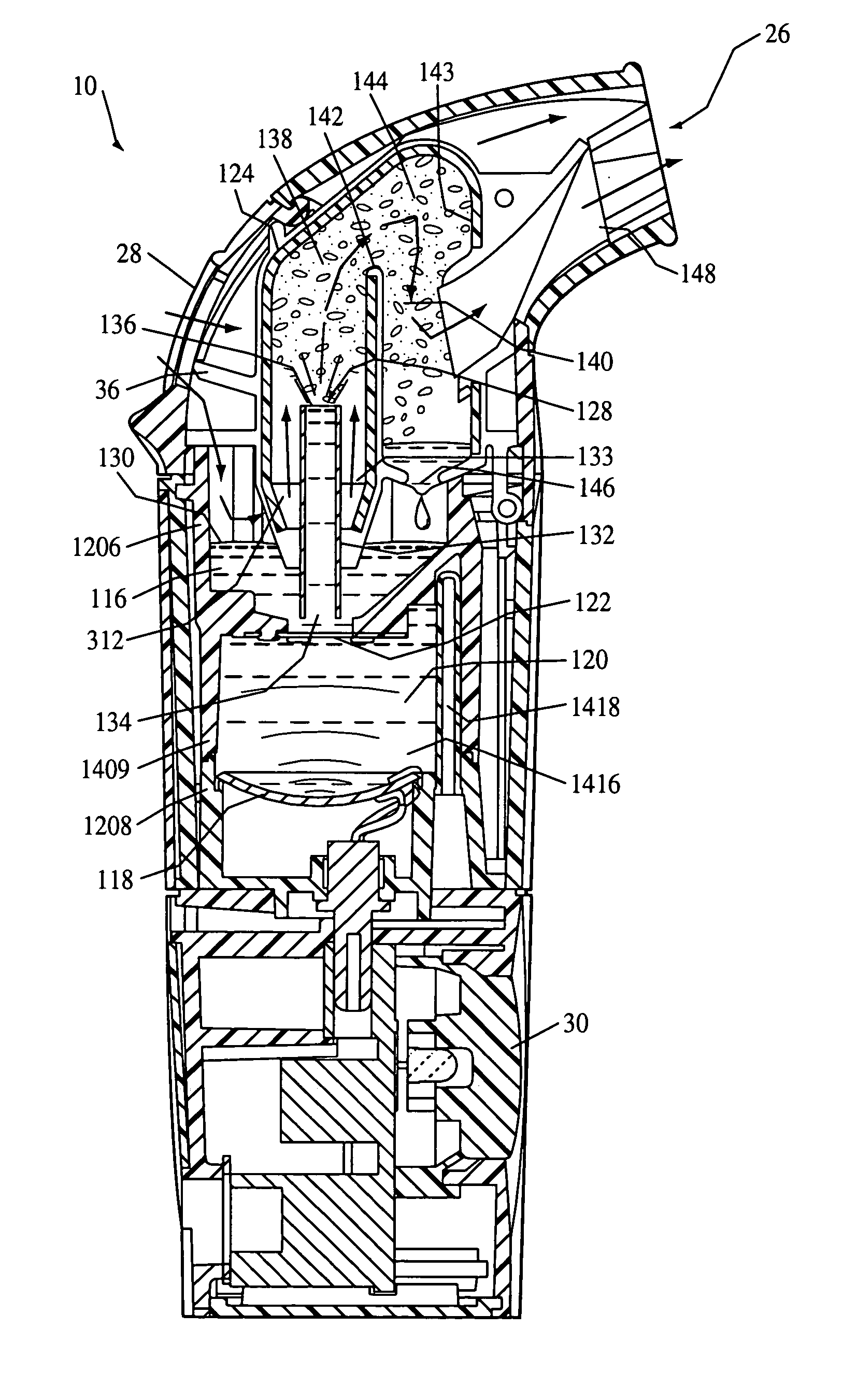
Infusion apparatus
ActiveUS20050277882A1Easy to useEasy constructionAmpoule syringesAutomatic syringesDrugs solutionStored energy
A compact fluid dispenser for use in controllably dispensing fluid medicaments, such as, antibiotics, oncolytics, hormones, steroids, blood clotting agents, analgesics, and like medicinal agents from prefilled containers at a uniform rate. The dispenser uniquely includes a stored energy source that is provided in the form of a substantially constant-force, compressible-expandable wave spring that provides the force necessary to continuously and uniformly expel fluid from the device reservoir. The device further includes a fluid flow control assembly that precisely controls the flow of medicament solution to the patient.
Owner:MARSHALL S KRIESEL REVOCABLE TRUST
Infusion rate adjusting device for drug solution injector
Owner:NIPRO CORP
Drug solution preparing kit
InactiveUS20090326506A1Easy to handleReduce pressureDiagnosticsSurgeryDrugs solutionAerosol dispersion
To provide a drug solution preparing kit which can be handled with ease in a substantially aseptic manner, and has no risk that a leakage of a drug solution such as a splash and a dispersion of an aerosol to an ambient environment occurs upon preparation of the drug solution There is provided a drug solution preparing kit including a pre-filled syringe and a transfusing tool, wherein the pre-filled syringe includes a sealing member which seals the tip end and can not be removed from the tip end, and that the transfusing tool includes a one-way valve which can discharge only gas from the system in an irreversible manner, and a filter which is provided so as to adjoin to the second communication channel with respect to the one-way valve.
Owner:NIPRO CORP
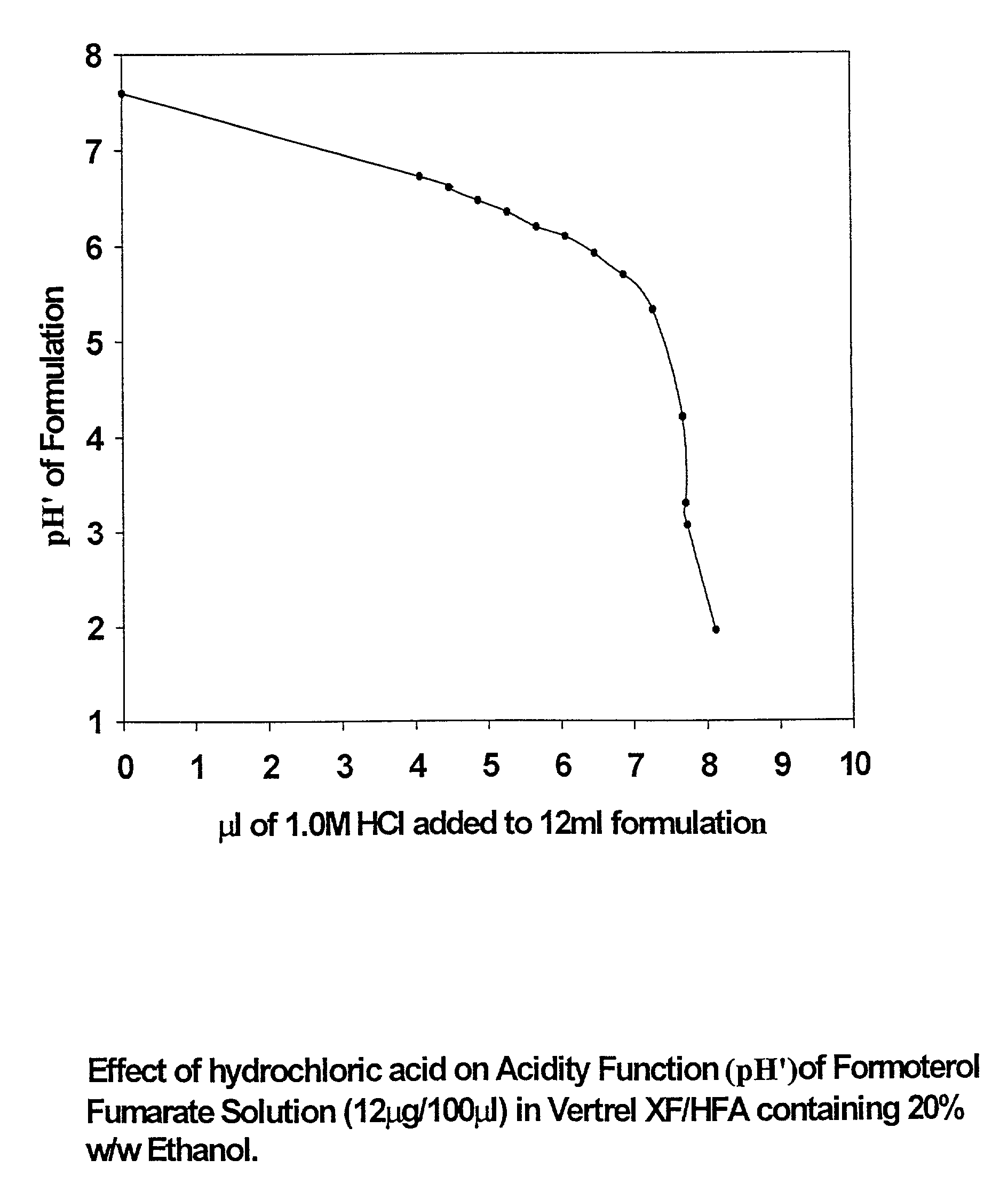
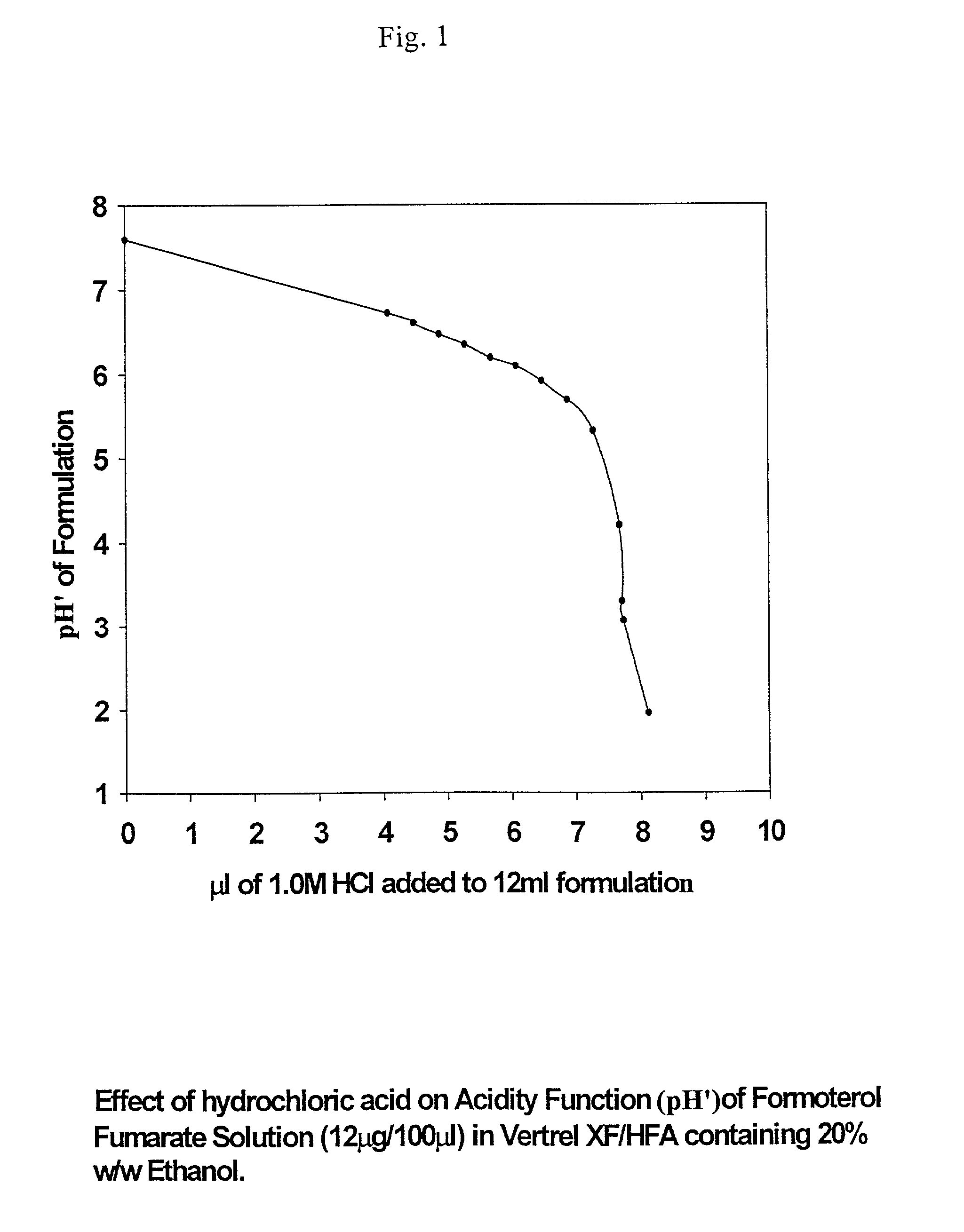
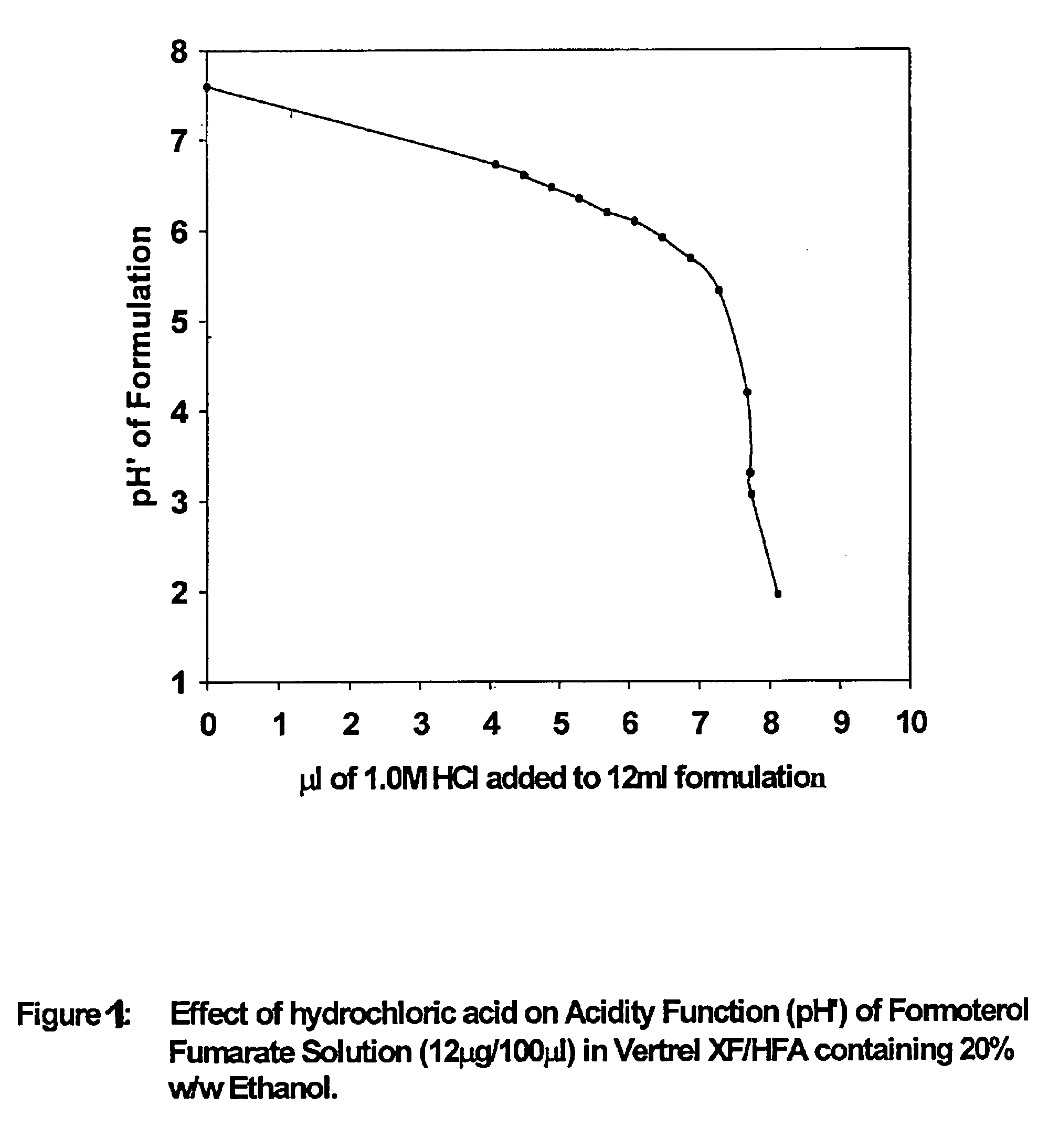
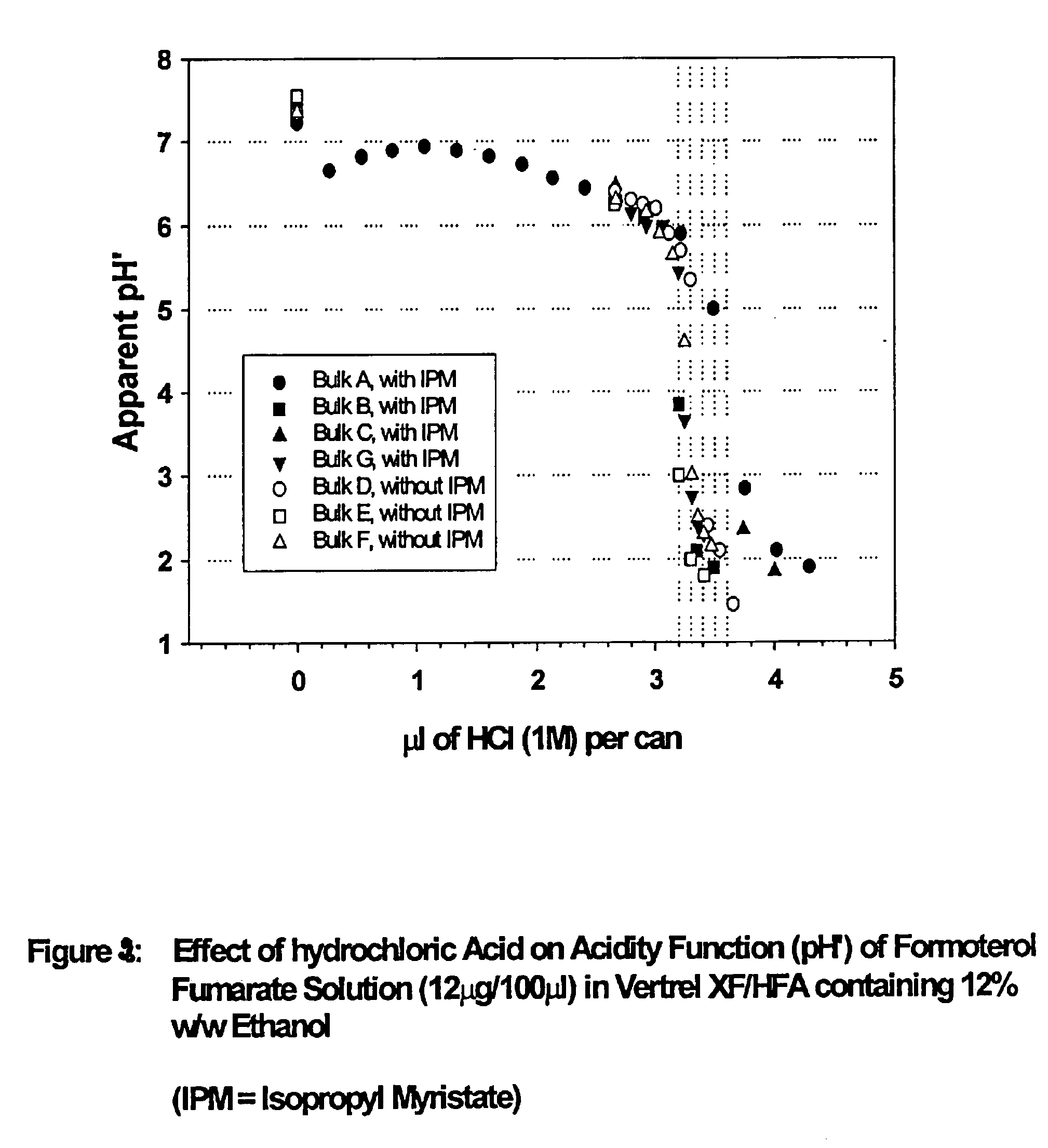
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
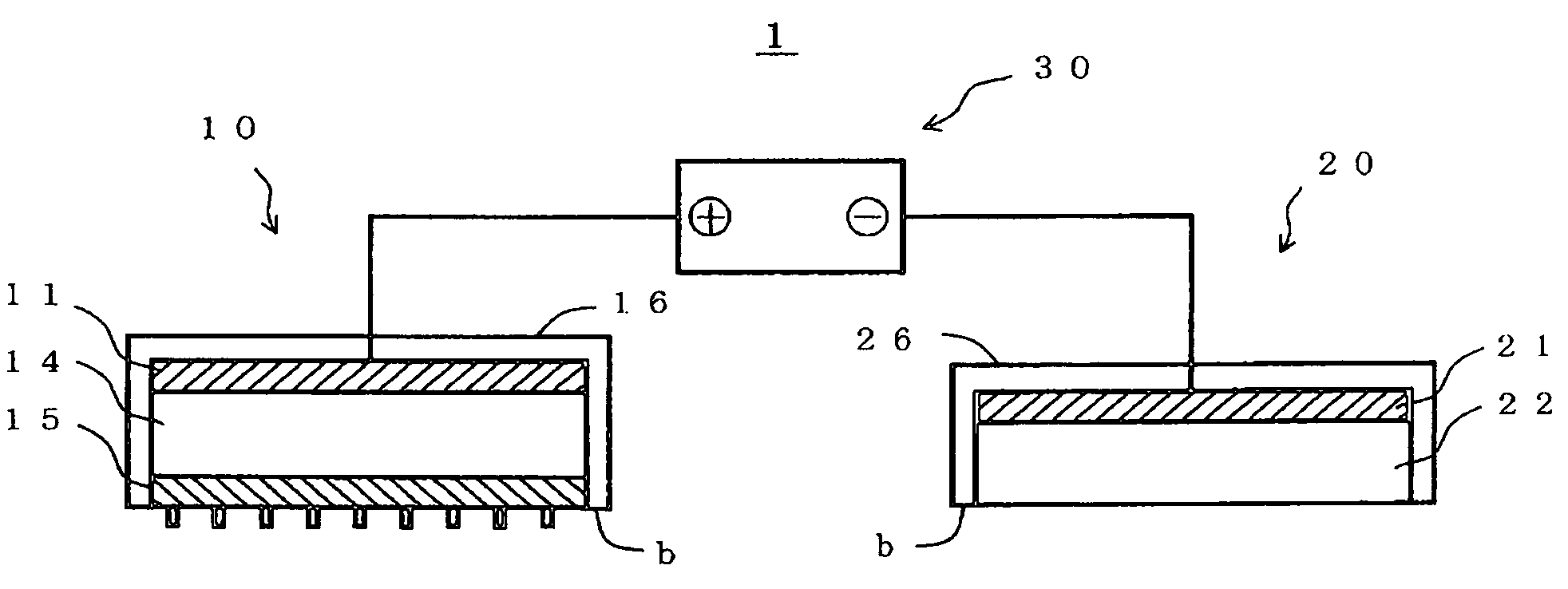
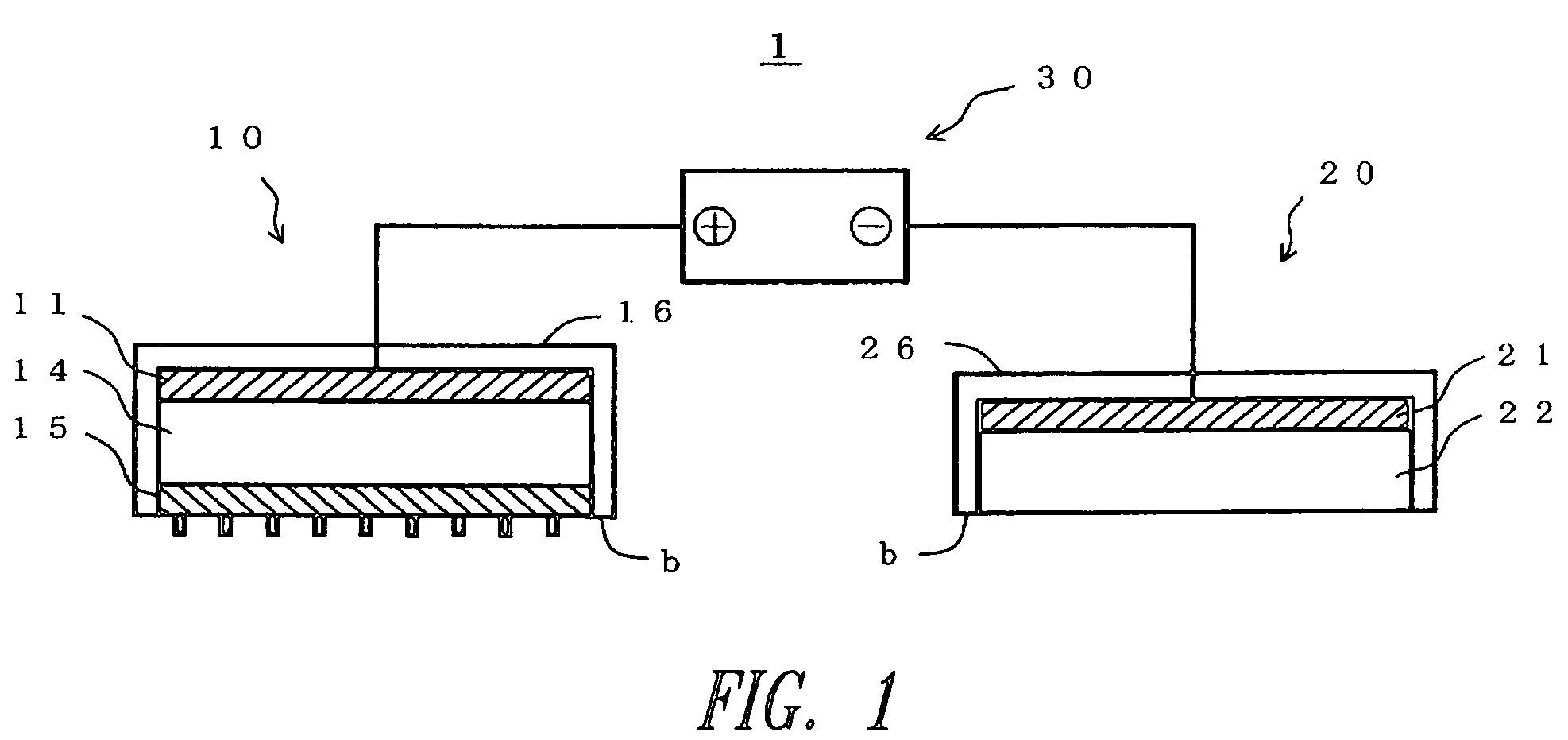
Iontophoresis device
InactiveUS7437189B2Improve efficiencyLower average currentElectrotherapyInfusion needlesDrugs solutionPermeation
An iontophoresis device includes: a first electrode; a biological interface contact member including a substrate having a front surface and a rear surface, and a plurality of needles that protrude from the front surface of the substrate and can be inserted into a biological interface, the biological interface contact member allowing selective permeation of ions of a first polarity; and a drug holding part applied with an electrical potential or voltage through the first electrode and holding a drug solution containing drug ions charged in the first polarity, the drug holding part being interposed between the first electrode and the biological interface contact member.
Owner:TITI ELLEBEAU INC
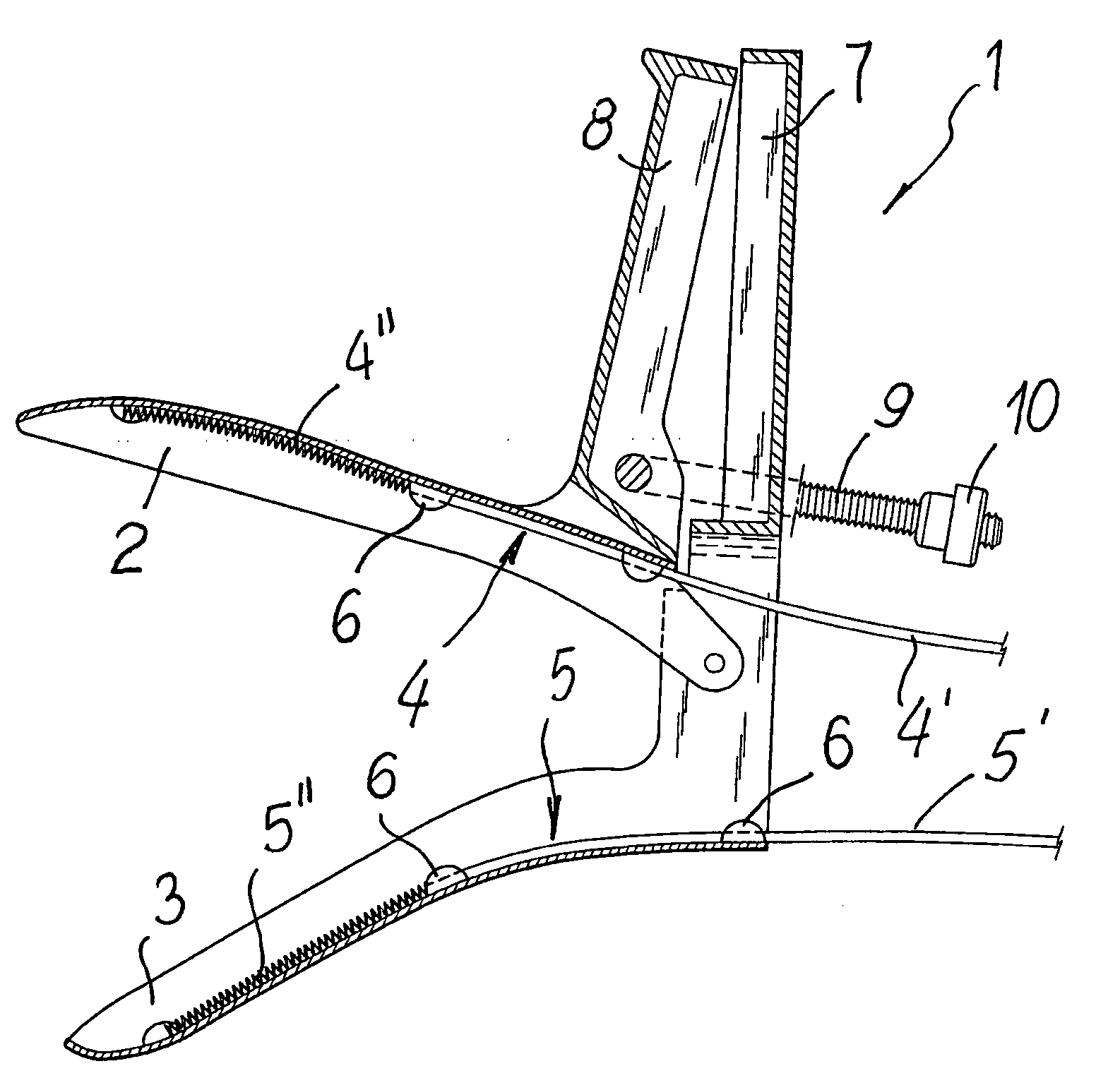
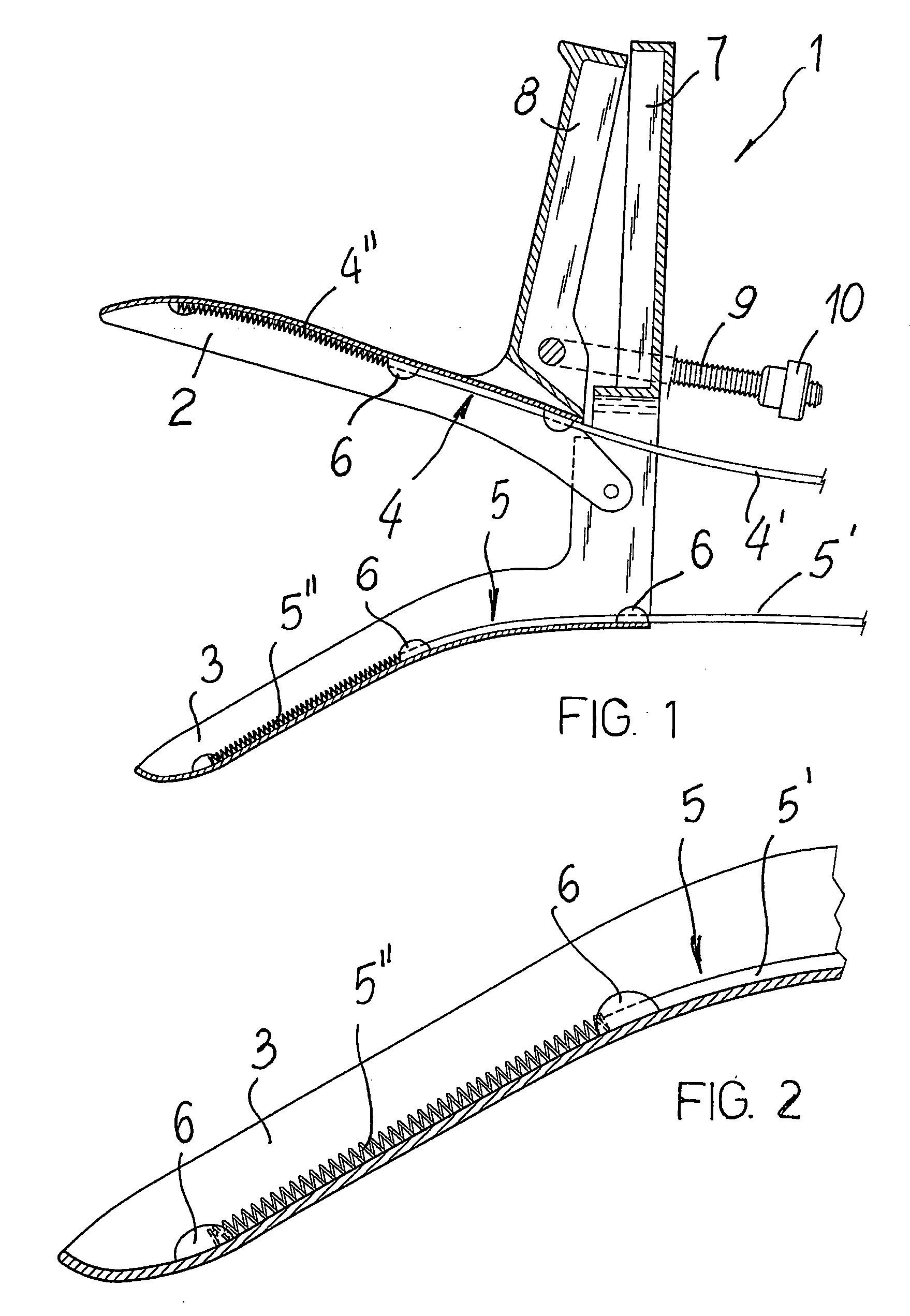
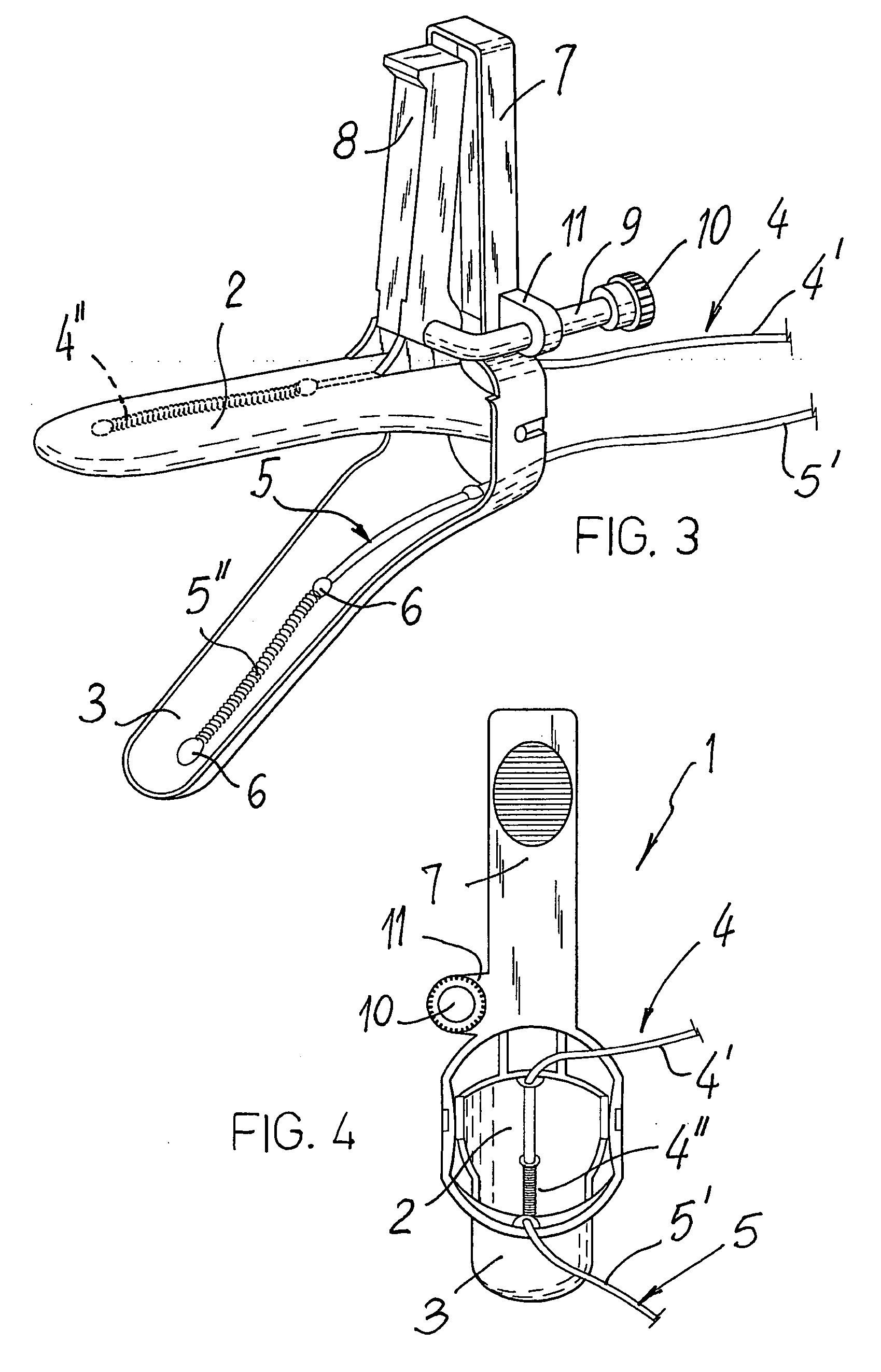
Speculum for the electropharmacological treatment of vaginal diseases
A speculum for the electropharmacological treatment of vaginal diseases has a pair of elongated blades interconnected such that an opening adapted for observation and passage of instruments is defined between the blades. There is at least one electrode connected to one of the blades on an inner face of the blade. The electrode has a proximal insulated portion and a distal uninsulated portion. To treat vaginal diseases, the speculum is placed in a vagina so that the electrode is located proximate to the vaginal disease, and a drug solution is injected into the vagina. An electric current is then applied to the electrode, which causes the migration of the drug solution toward the vaginal disease.
Owner:PHYSION
Pharmaceutical solution formulations for pressurised metered dose inhalers
A method for delivering two or more active drug substances to the lungs by inhalation from a single pressurized metered dose inhaler product, said inhaler containing a HFA / cosolvent based solution formulation wherein all the active drug substances are fully dissolved in the formulation.
Owner:CHIESI FARM SPA
Small vessel ultrasound cathter
InactiveUS20080221506A1Promote progressReduce resistanceUltrasound therapyMulti-lumen catheterDrugs solutionVascular ultrasound
An ultrasound catheter adapted for accessing small vessels in the distal anatomy is disclosed. The ultrasound catheter comprises an elongate tubular body formed with a delivery lumen. The flexibility and dimensions of the tubular body allow access to the distal anatomy by advancement over the guidewire. An ultrasound radiating member is provided along the distal end portion of the tubular body for emitting ultrasound energy at a treatment site. A drug solution may also be delivered through the delivery lumen and out an exit port to the treatment site.
Owner:RODRIGUEZ OSCAR E +7
Drug solution container with a connector for communicating
The drug solution container with a connector for communicating contains a drug solution container having at a tip end thereof an injection needle connecting part, and a hollow connector for communicating attached to a tip end of the drug solution container, and the connector for communicating contains a cylindrical guide part with a bottom capable of being slidably attached to an opening of a vial, and a hollow penetrating member provided at a center of the bottom of the guide part to penetrate the bottom. The penetrating member contains a penetrating needle at a tip end side with respect to the bottom, and a connecting part at a base end side with respect to the bottom, and the connecting part is connected to the injection needle connecting part through a fragile portion. According to the drug solution container with a connector for communicating, an operation for preparation of a drug solution can be easily carried out in a short period of time without causing injury of an operator or coring.
Owner:NIPRO CORP
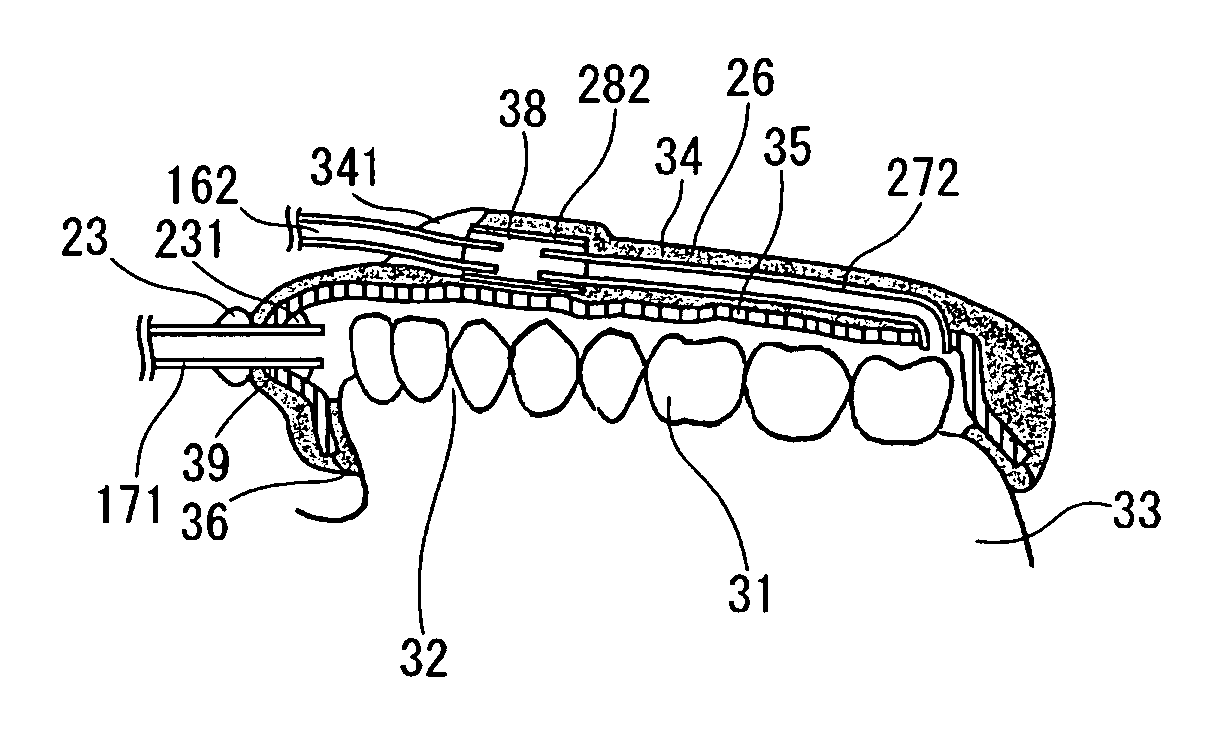
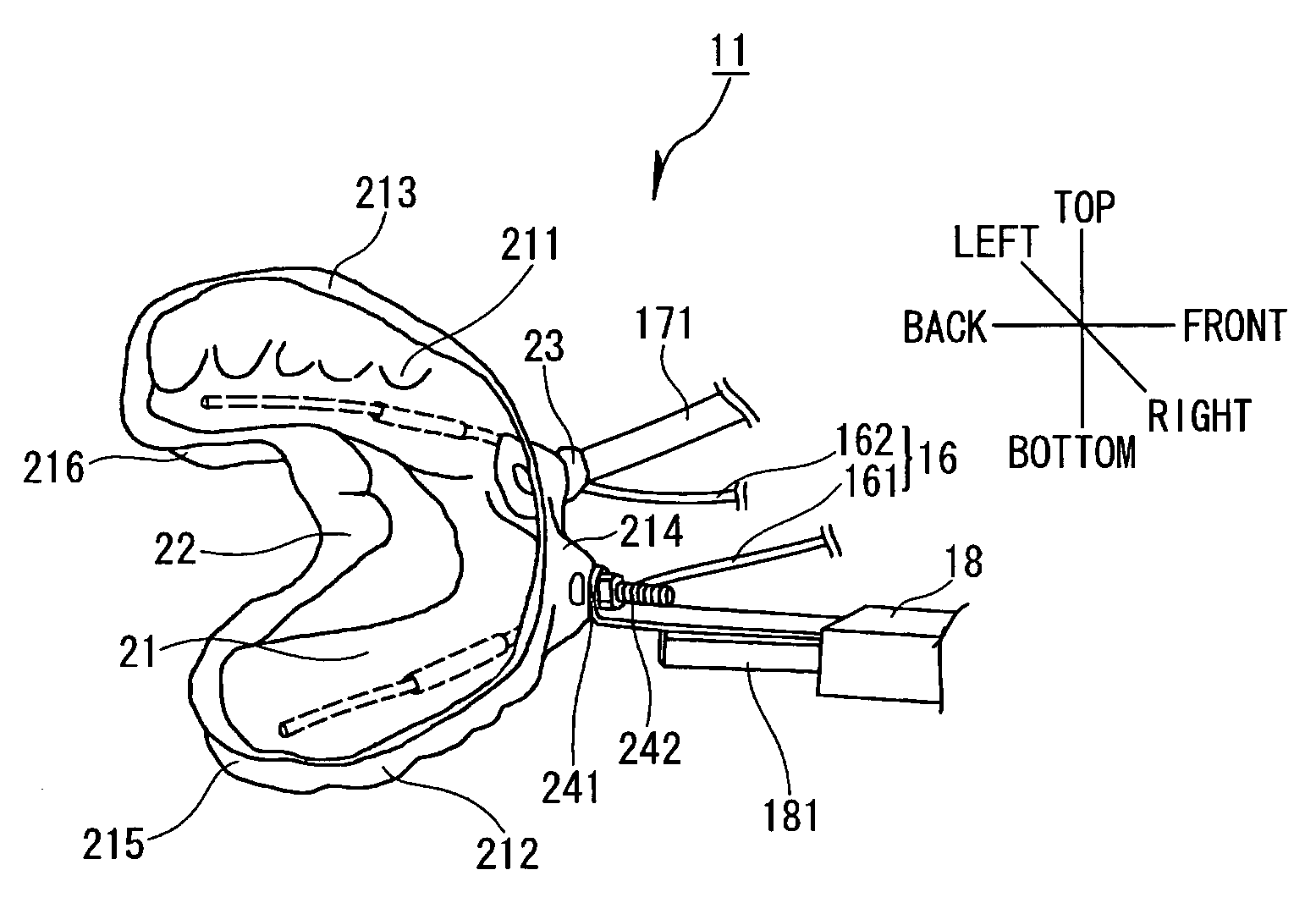
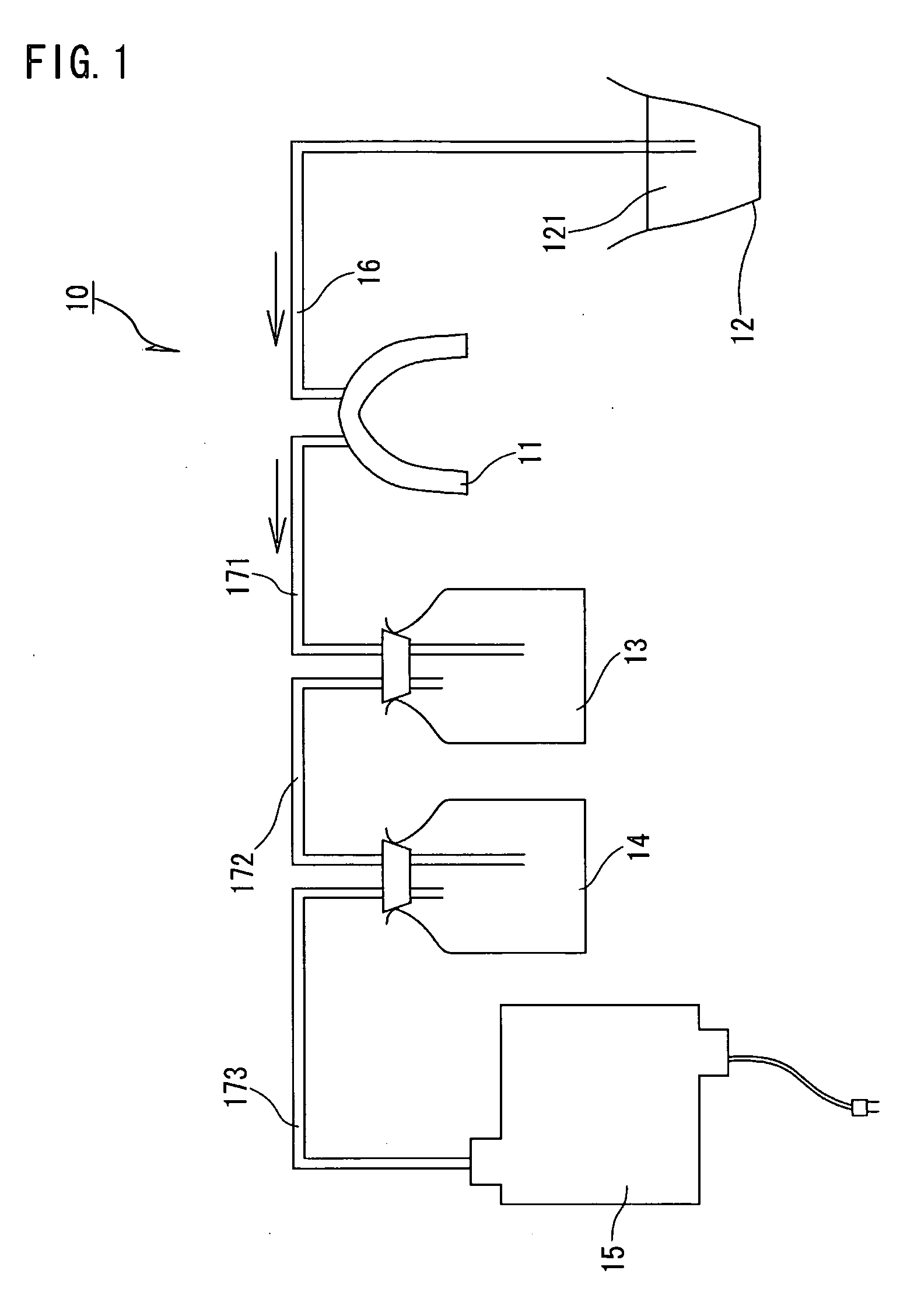
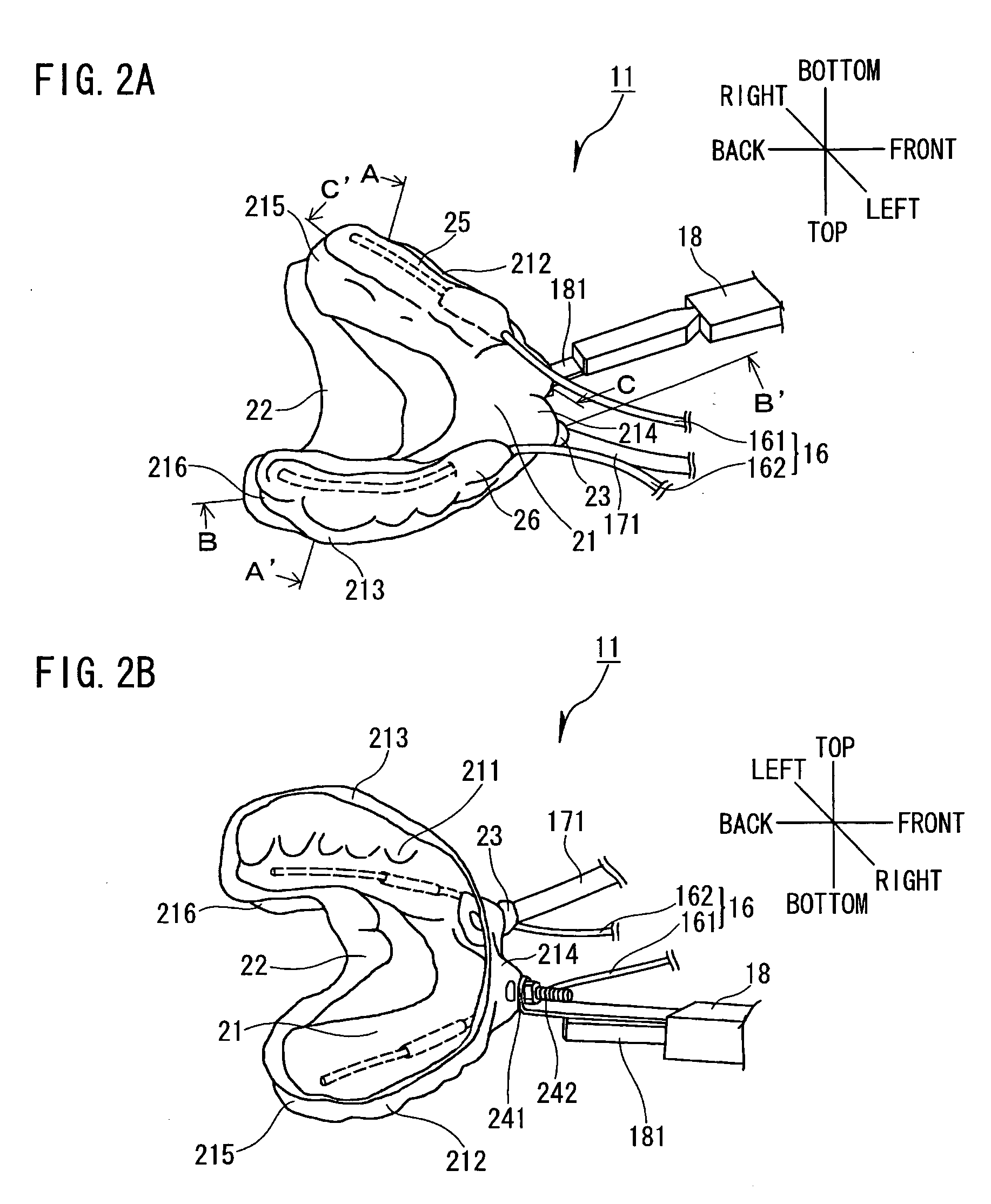
Dental system and method of producing the same
Owner:INOUE YOSHINORI +2
Infusion apparatus with modulated flow control
InactiveUS20050033232A1Easy to useEasy constructionFlexible member pumpsMedical devicesStored energyConstant force
A compact fluid dispenser for use in controllably dispensing fluid medicaments, such as antibiotics, oncolytics, hormones, steroids, blood clotting agents, analgesics, and like medicinal agents from prefilled containers at a uniform rate. The dispenser uniquely includes a stored energy source that is provided in the form of a substantially constant-force, compressible-expandable wave spring that provides the force necessary to continuously and uniformly expel fluid from the device reservoir. The device further includes a fluid flow control assembly that precisely controls the flow of medicament solution to the patient. Additionally, the device includes a novel modulating assembly for controllably modulating the force exerted by the wave spring tending to expel the fluid from the device reservoir.
Owner:BIOQUIDDITY
Nebulizing drug delivery device with barrier
The present invention provides a drug delivery device that uses an aerosol generator to nebulize a drug solution. The drug delivery device includes differently sized guide tubes and separator walls to provide substantially consistent particles that can be varied in size by using different guide tubes. The drug delivery device also includes a barrier that separates a fluid contained in the device from the drug solution at least a portion of the barrier is formed from Polyetheretherketone. The present invention also has a drug detection device that can detect the volume of drug in the device or whether the barrier has been compromised.
Owner:RIC INVESTMENTS LLC
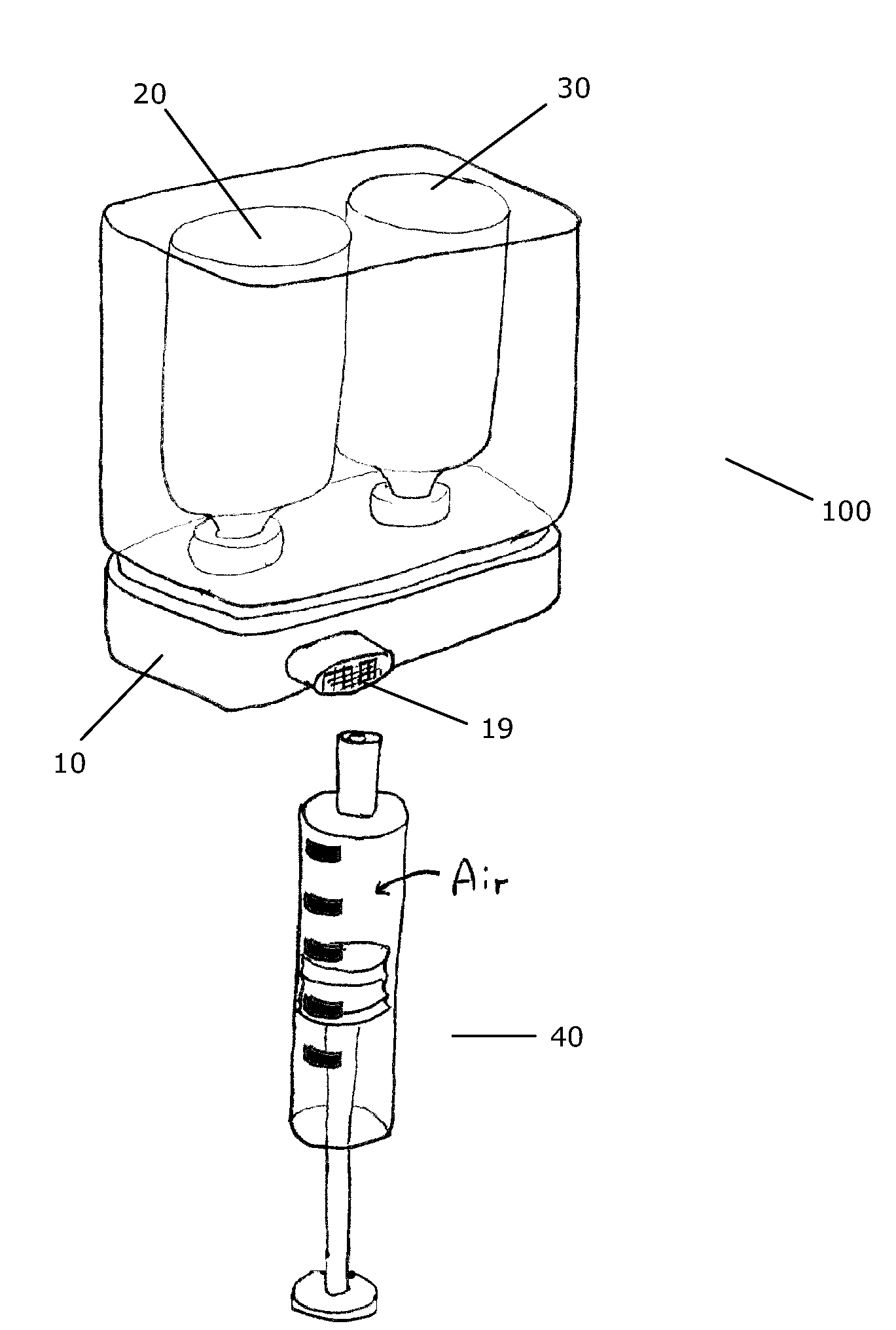
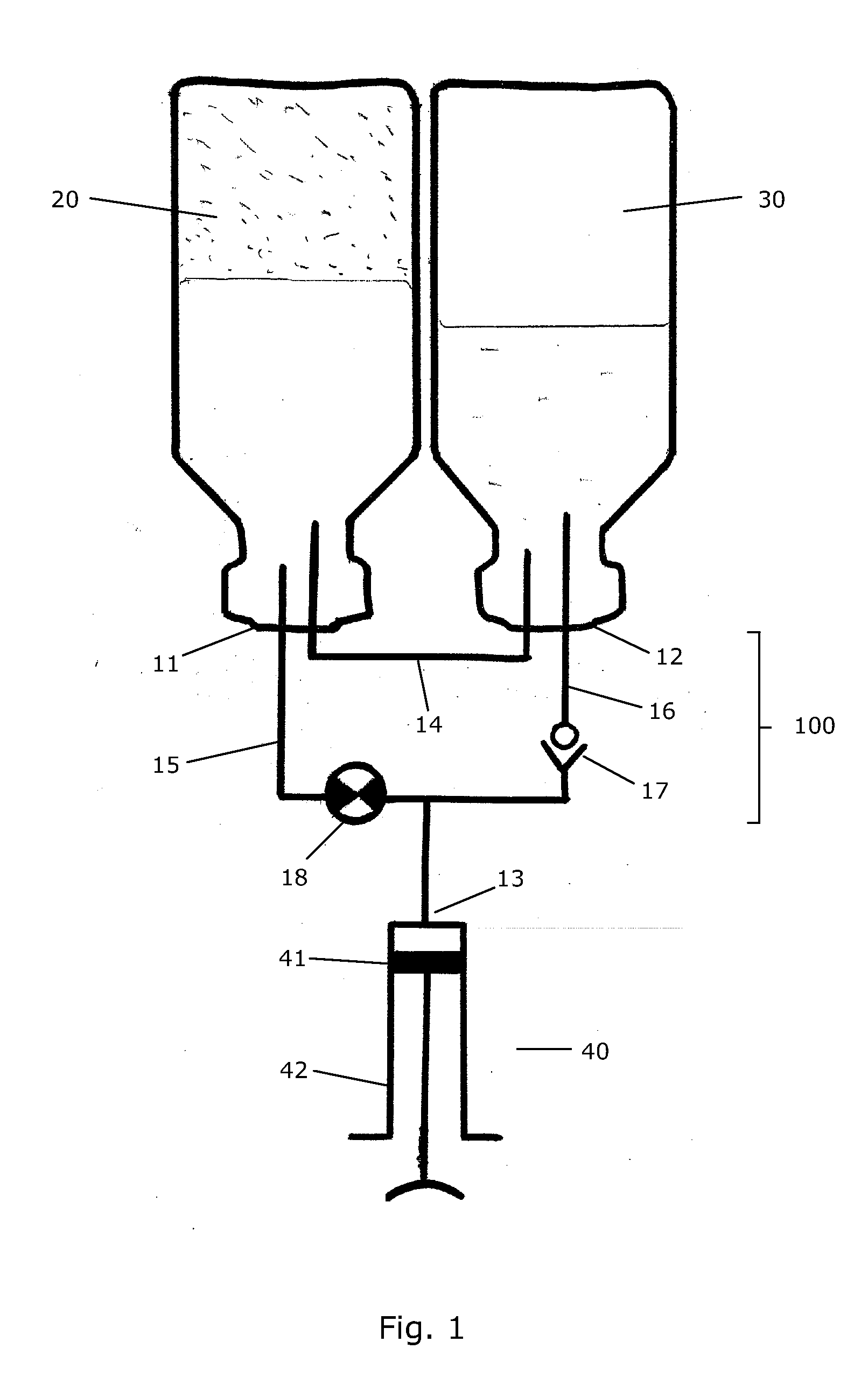
Transfer System for Forming a Drug Solution from a Lyophilized Drug
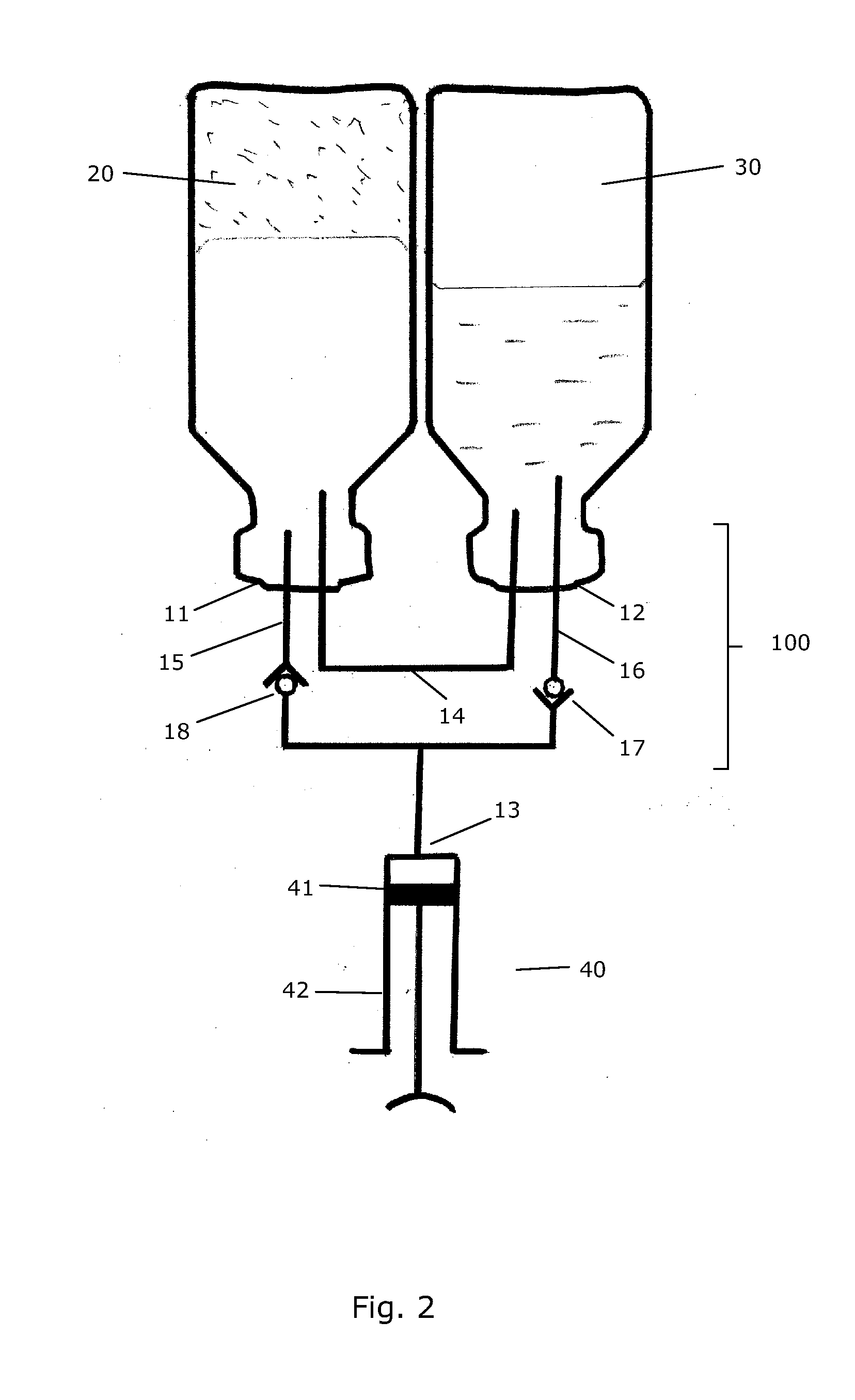
InactiveUS20090099547A1Simple but efficientInfusion syringesPharmaceutical containersDrugs solutionTransfer system
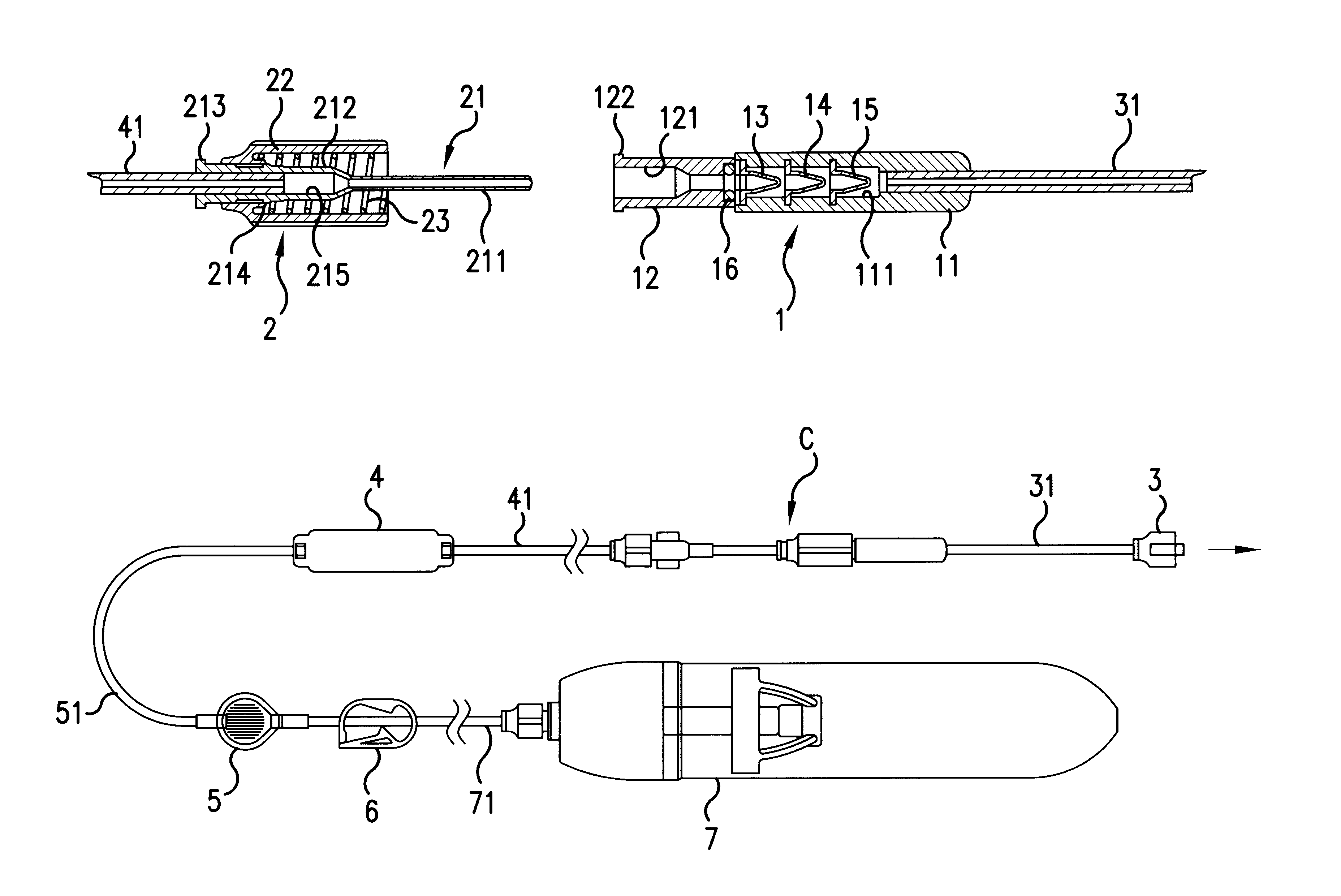
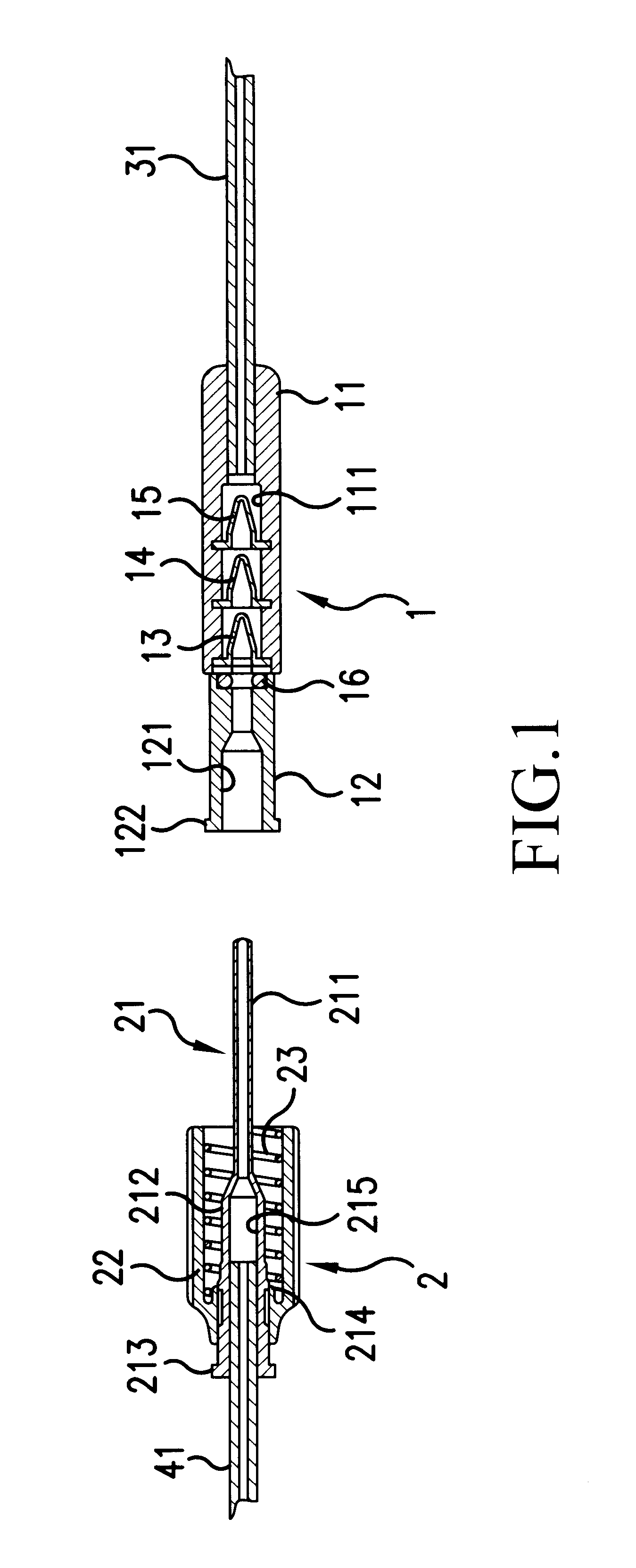
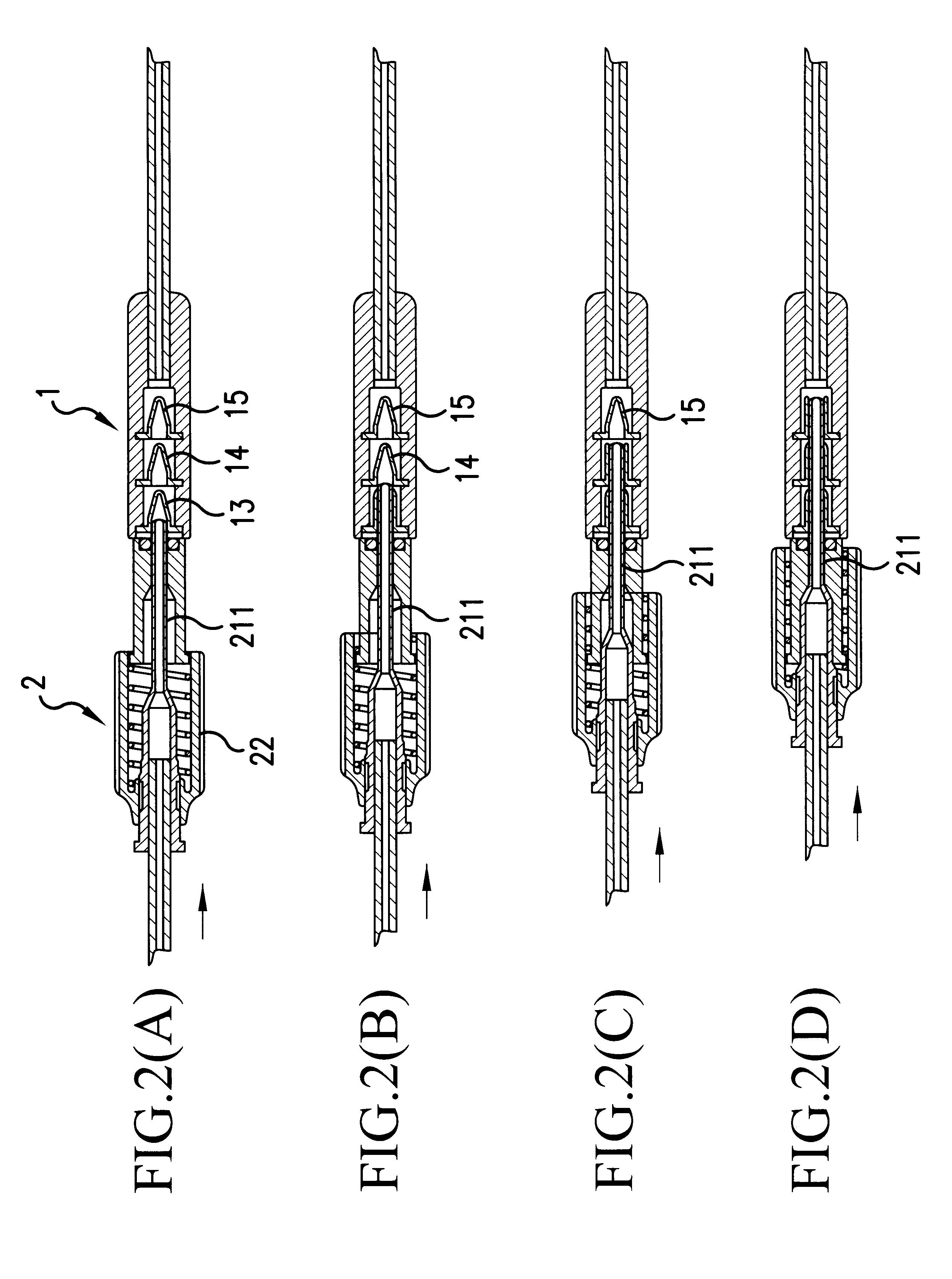
A transfer system (100, 100′, 100″) adapted to allow first contents of a first container (20) and second contents of a second container (30) to mix to form a material. The mixed material is retrieved to a syringe (40). The transfer system (100, 100′, 100″) comprises first (17) and second (18) flow control members for controlling fluid flow between the containers (20, 30) and the syringe (40). The invention further relates to a drug mixing kit comprising a container unit containing first and second containers, and a transfer unit comprising ports for receiving the containers and a syringe and a number of flow channels. The container unit and the transfer unit are adapted to be coupled together to form a drug mixing kit.
Owner:NOVO NORDISK AS
Apparatus and Methods for Loading a Drug Eluting Medical Device
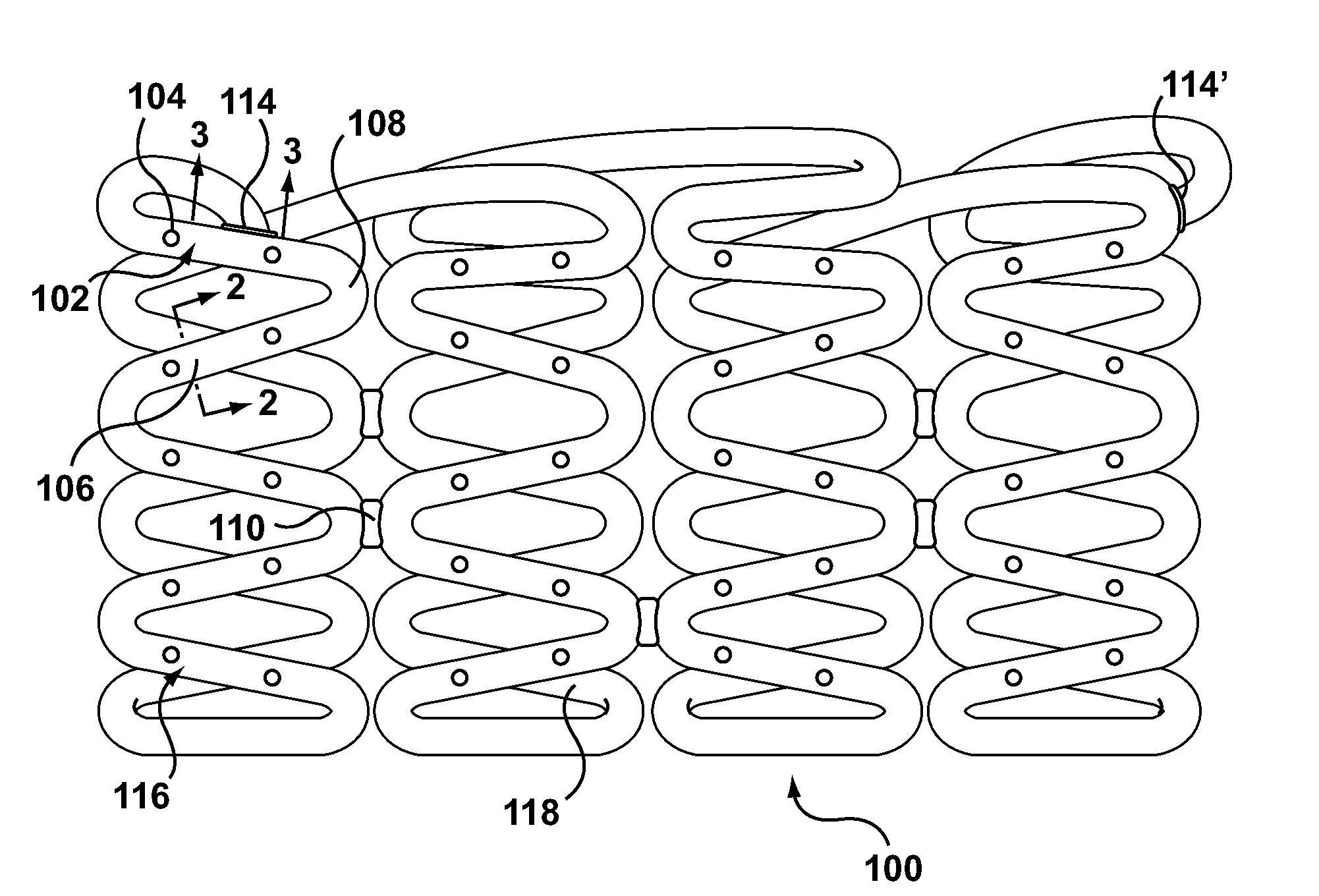
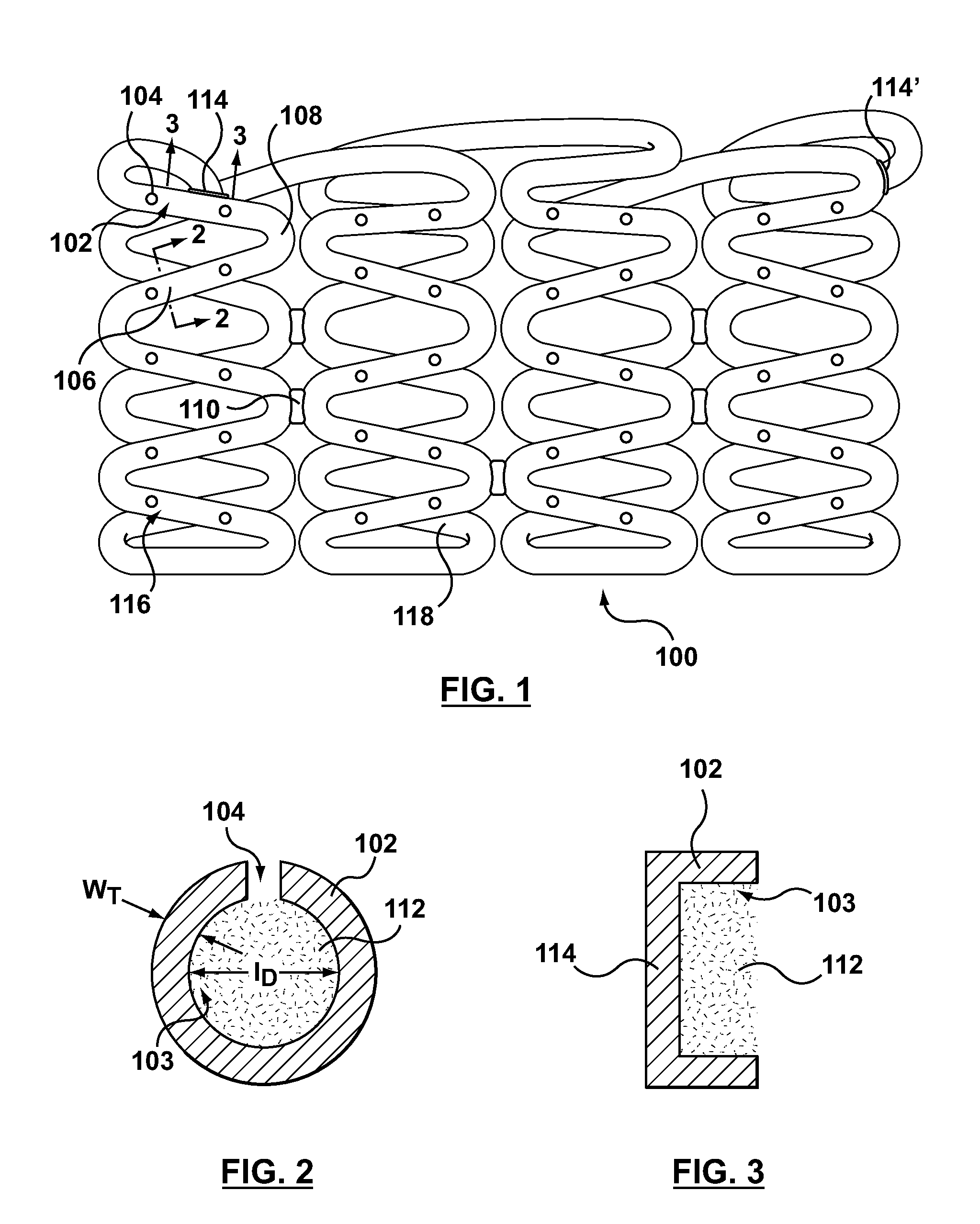
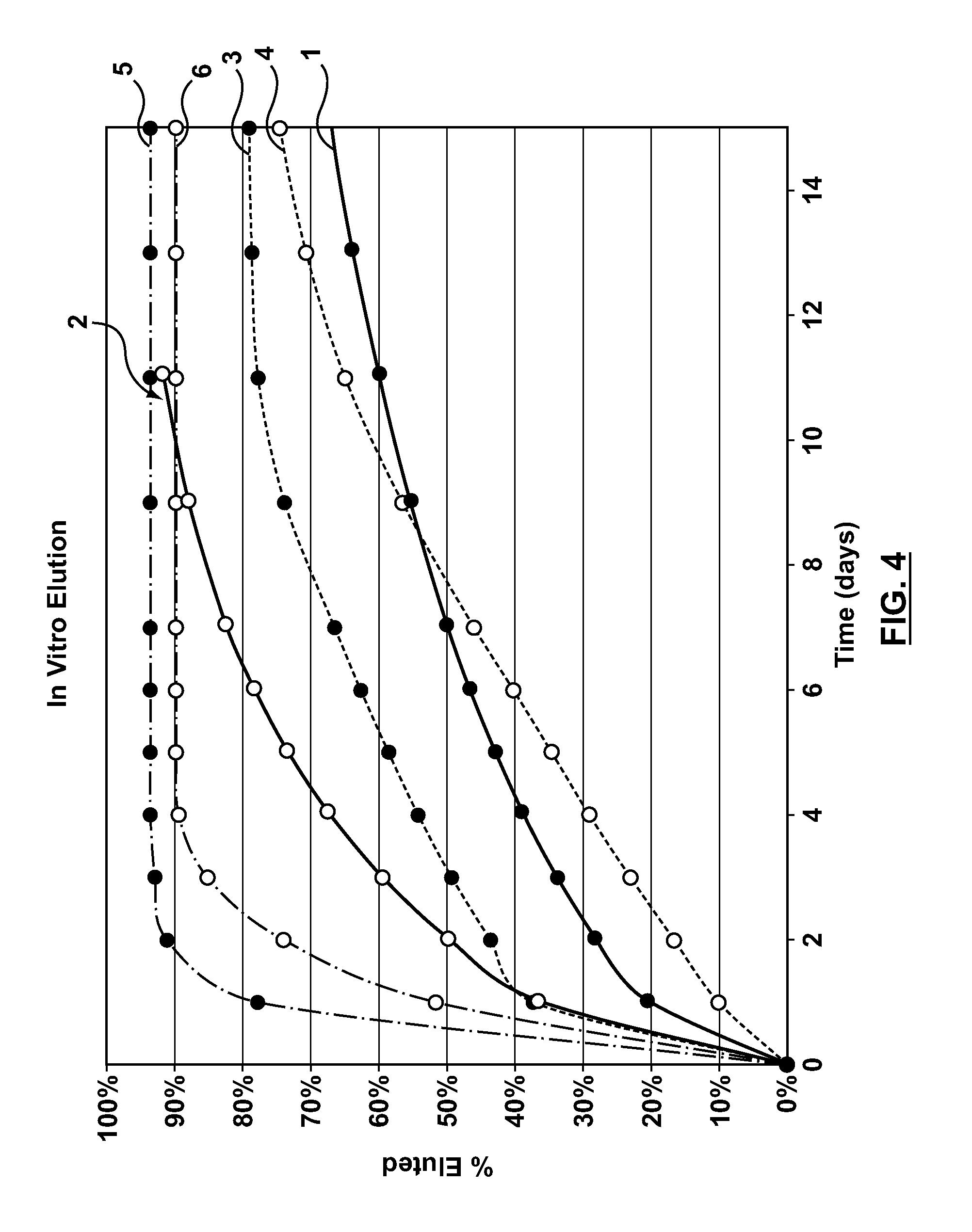
Methods and apparatus are disclosed for loading a therapeutic substance or drug within a lumenal space of a hollow wire having a plurality of side openings along a length thereof that forms a hollow drug-eluting stent with a plurality of side drug delivery openings. Loading a drug within the lumenal space of the hollow stent includes a drug filling step, in which the drug is mixed with a solvent or dispersion medium. The lumenal space may be filled with the drug solution or suspension in a reverse fill process and / or a forward fill process. After the drug filling step, a solvent or dispersion medium extracting step is performed to extract the solvent or dispersion medium from within the lumenal space such that only the drug remains within the hollow stent. A stent cleaning step may be performed to an exterior surface of the hollow stent.
Owner:MEDTRONIC VASCULAR INC
Dental system and method of producing the same
A dental system for cleaning a user's teeth and marginal gingiva with a drug solution includes a mouthpiece made for each user and having substantially the same shape as the teeth and the marginal gingiva of the user. The mouthpiece is designed to produce a gap between the mouthpiece and the teeth together with the marginal gingiva when placed to cover the teeth and the marginal gingiva of the user. The dental system also includes a drug solution supply unit and a drain unit, both connected to the mouthpiece, and a suction unit connected to the drain unit. The mouthpiece adheres to the marginal gingiva by a suction applied from the suction unit. The dental system cleans the teeth and the marginal gingiva with a stream of the drug solution flowing through the gap.
Owner:INOUE YOSHINORI +2
Medical Device with Crystalline Drug Coating
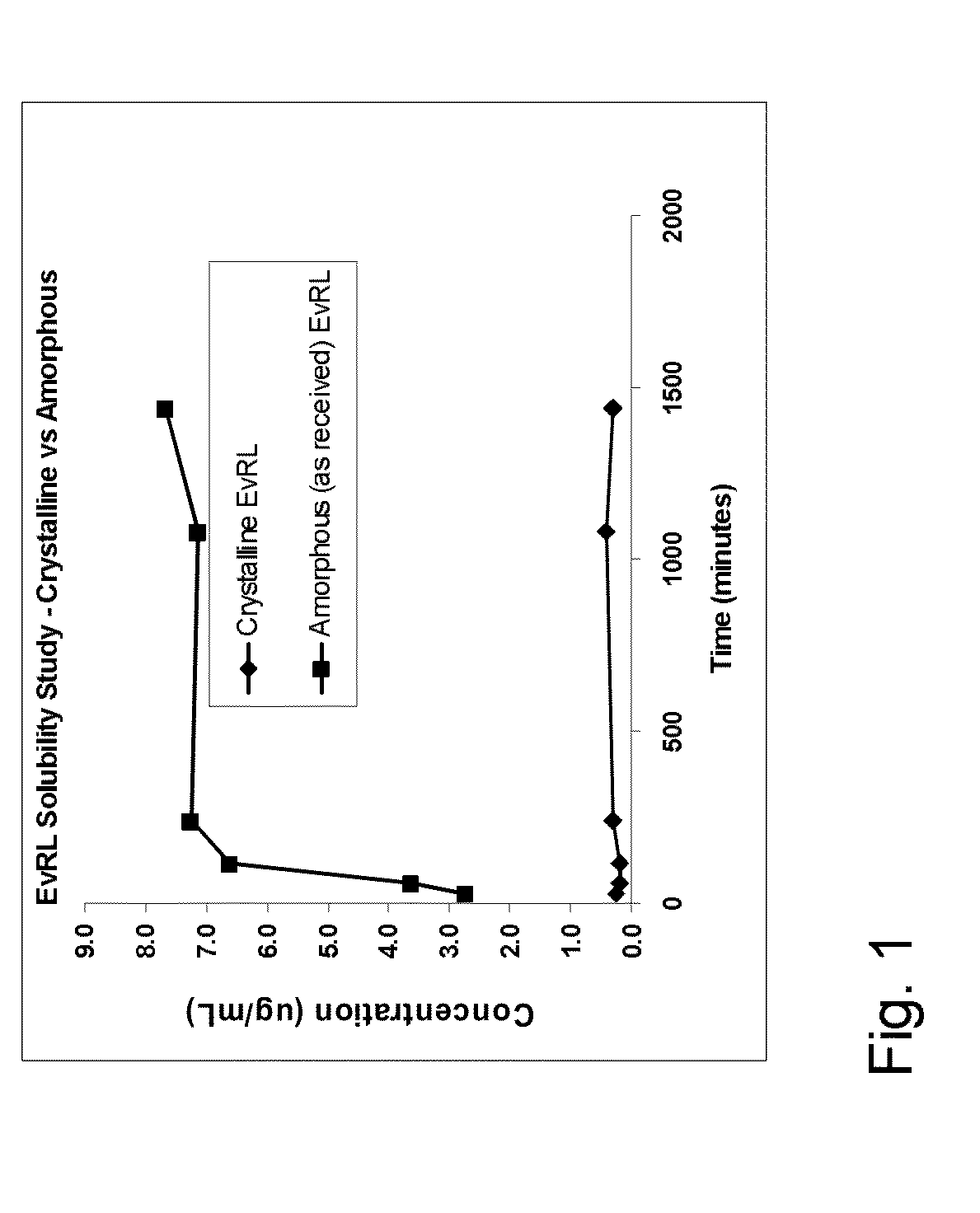
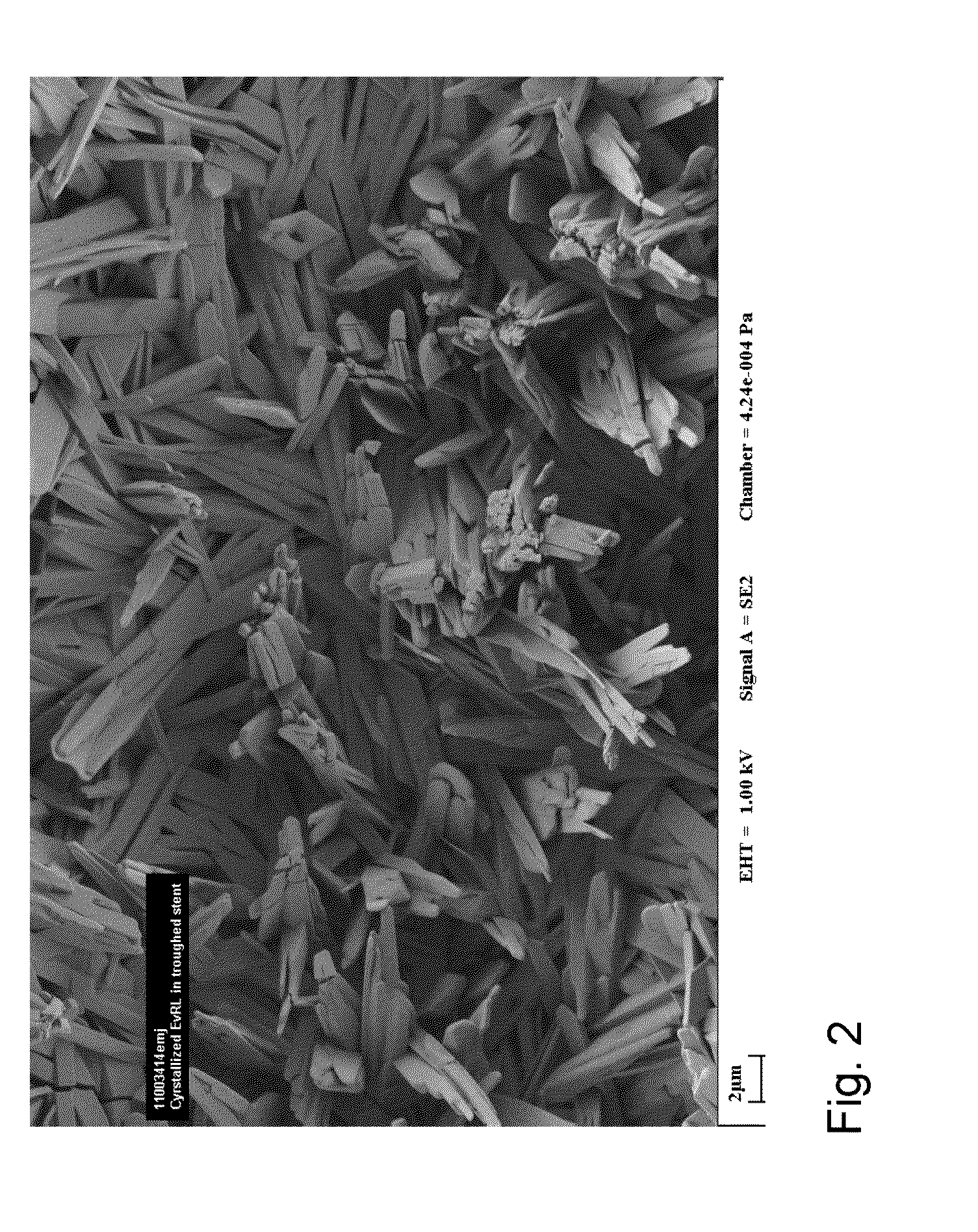
A medical device having a polymer-free outer surface layer comprising a crystalline drug selected from the group consisting of everolimus, tacrolimus, sirolimus, zotarolimus, biolimus, and rapamycin. The device may be produced by a method comprising the steps of providing a medical device; applying a solution of the drug to said portion of the outer surface to form a coating of amorphous drug; and vapor annealing the drug with a solvent vapor to form crystalline drug; wherein a seed layer of a crystalline form of said drug having a maximum particle size of about 10 μm or less is applied to at least said portion of the outer surface of the device before or after applying the drug solution, but before vapor annealing the amorphous coating.
Owner:BOSTON SCI SCIMED INC
Iontophoresis Methods
ActiveUS20090163848A1Reduction in patient discomfortEnhanced and real-time monitoringOrganic active ingredientsElectrotherapyDrugs solutionTympanic Membranes
A method of anesthetizing a tympanic membrane of an ear of a patient using iontophoresis is disclosed. The method involves delivering an anesthetizing drug solution to an ear canal of the patient's ear, wherein the drug solution includes an anesthetic and a buffer, and wherein the drug solution has a pH in the range of about 6.5 to about 7.5; and applying an amount of current to the drug solution, wherein the amount of applied current is increased at a rate of less than about 0.5 milliamp per second until a maximum current is achieved.
Owner:TUSKER MEDICAL
Solid Solution Perforator Containing Drug Particle and/or Drug-Adsorbed Particles
InactiveUS20090035446A1Barrier property can be diminished and controlledFast biodegradationPeptide/protein ingredientsMicroneedlesDrugs solutionDrug reservoir
A solid drug solution perforator containing drug particles and / or drug-adsorbed or loaded particles with an associated drug reservoir (SSPP system) are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, diagnostics, and for nutrient delivery and drug targeting. For drug delivery, the SSPP system includes an active drug ingredient in particulate form or drug adsorbed on the particle surface in a matrix material that dissolves upon contact with a patient's body. In a preferred method of transdermal drug delivery, an SSPP system containing a drug-adsorbed microparticle penetrates into the epidermis or dermis, and the drug is released from the (dissolving) SSPP system perforator and desorbed from the particles. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator Formulation and fabrication procedures for the SSPP and associated reservoir are also provided. An SSPP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
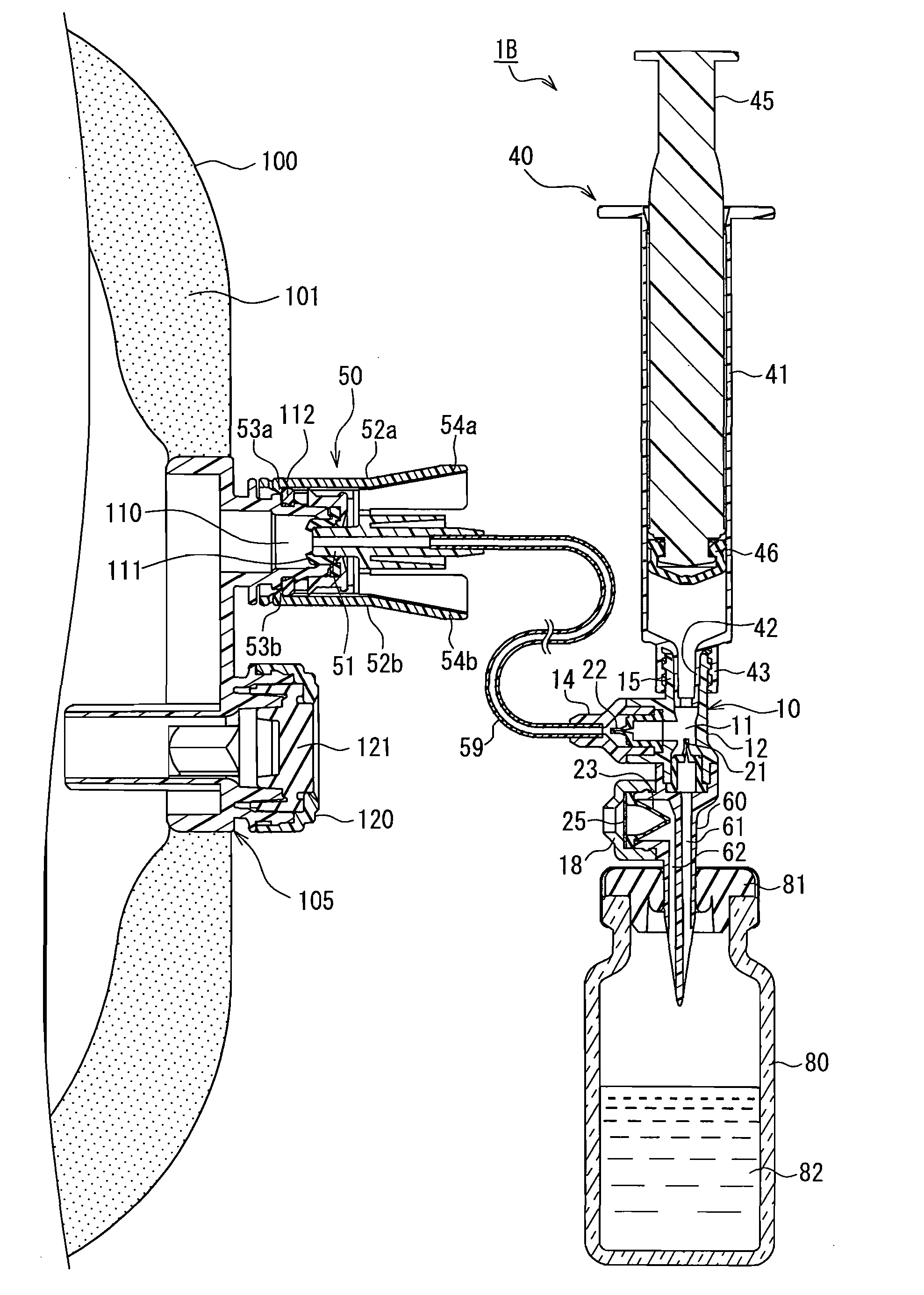
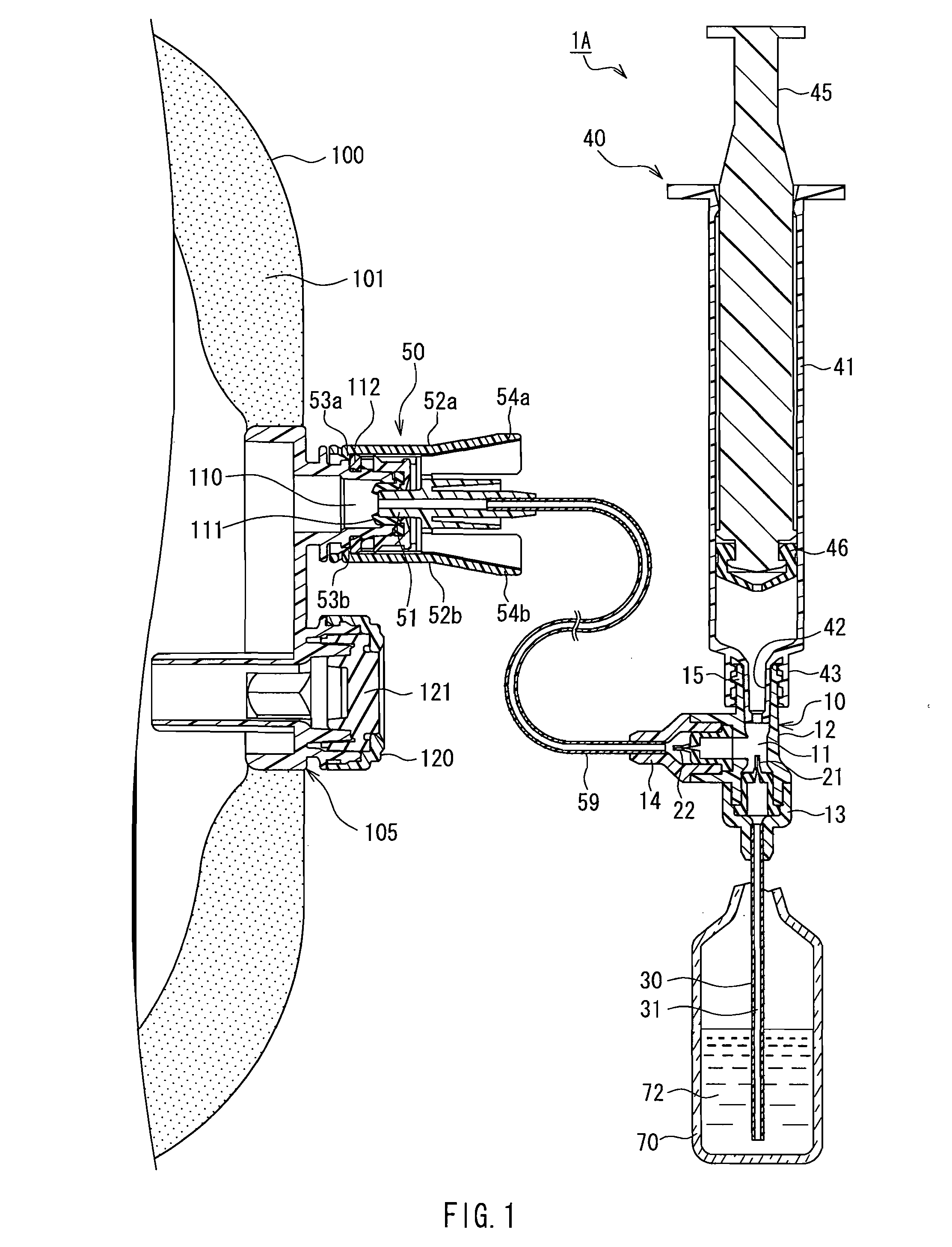
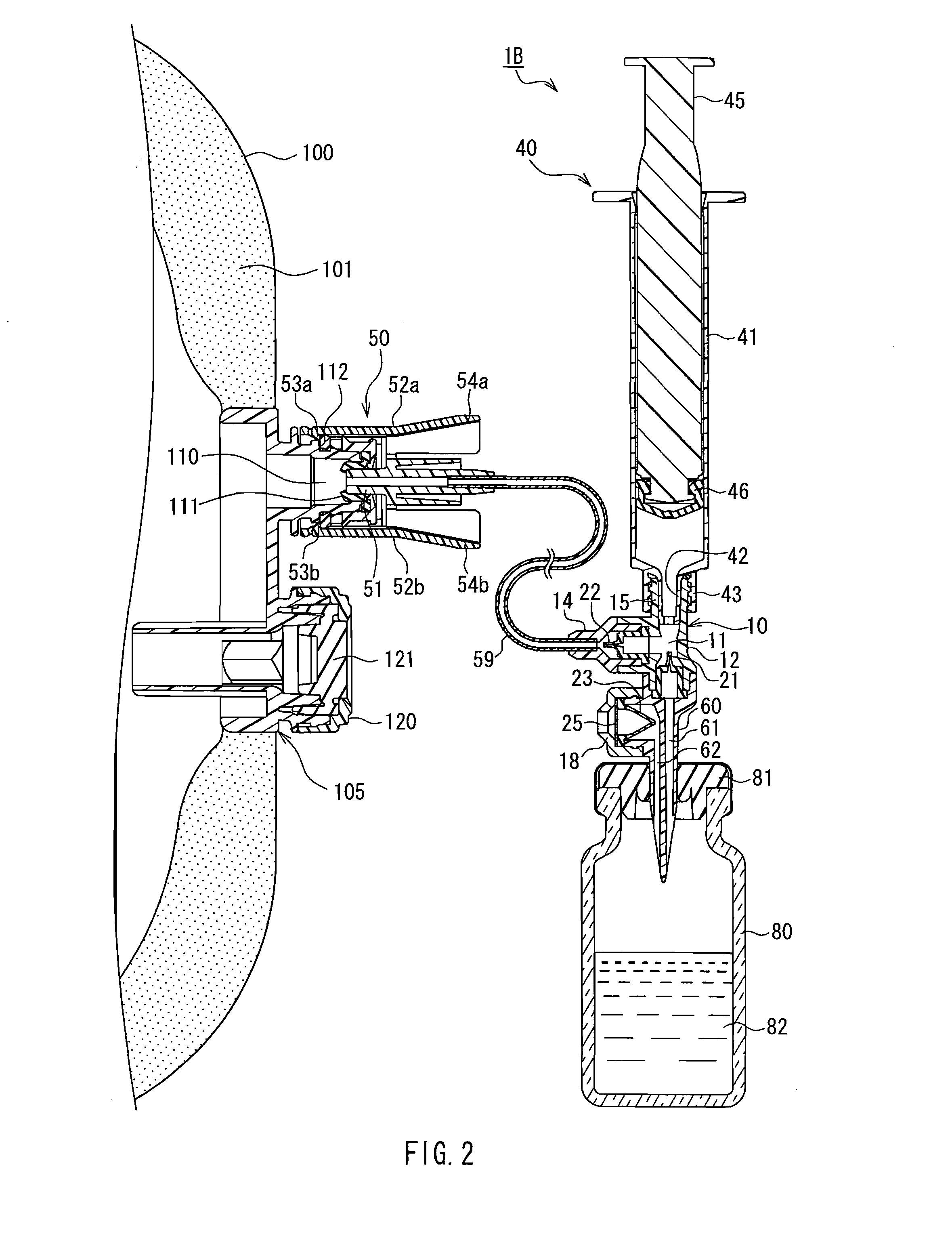
Drug solution delivery device for medical use
A cylindrical body (30) that is inserted into a drug solution container (70), a connection port (15) to which a syringe (40) is connected, and a connector (50) that is connected to a port (110) of an infusion bag (100) communicate with a cavity (11) of a delivery device main body (10). A first check valve (21) that functions to permit the flow of drug solution toward the cavity from the drug solution flow path and restrict the flow of drug solution in the opposite direction is provided on a flow path between a drug solution flow path (31) of the cylindrical body and the cavity. A second check valve (22) that functions to permit the flow of drug solution toward the connector from the cavity and restrict the flow of drug solution in the opposite direction is provided on a flow path between the connector and the cavity. Drawing a plunger (45) of the syringe in and out enables a drug solution (72) contained in the drug solution container to be efficiently transferred to the infusion bag.
Owner:JMS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com