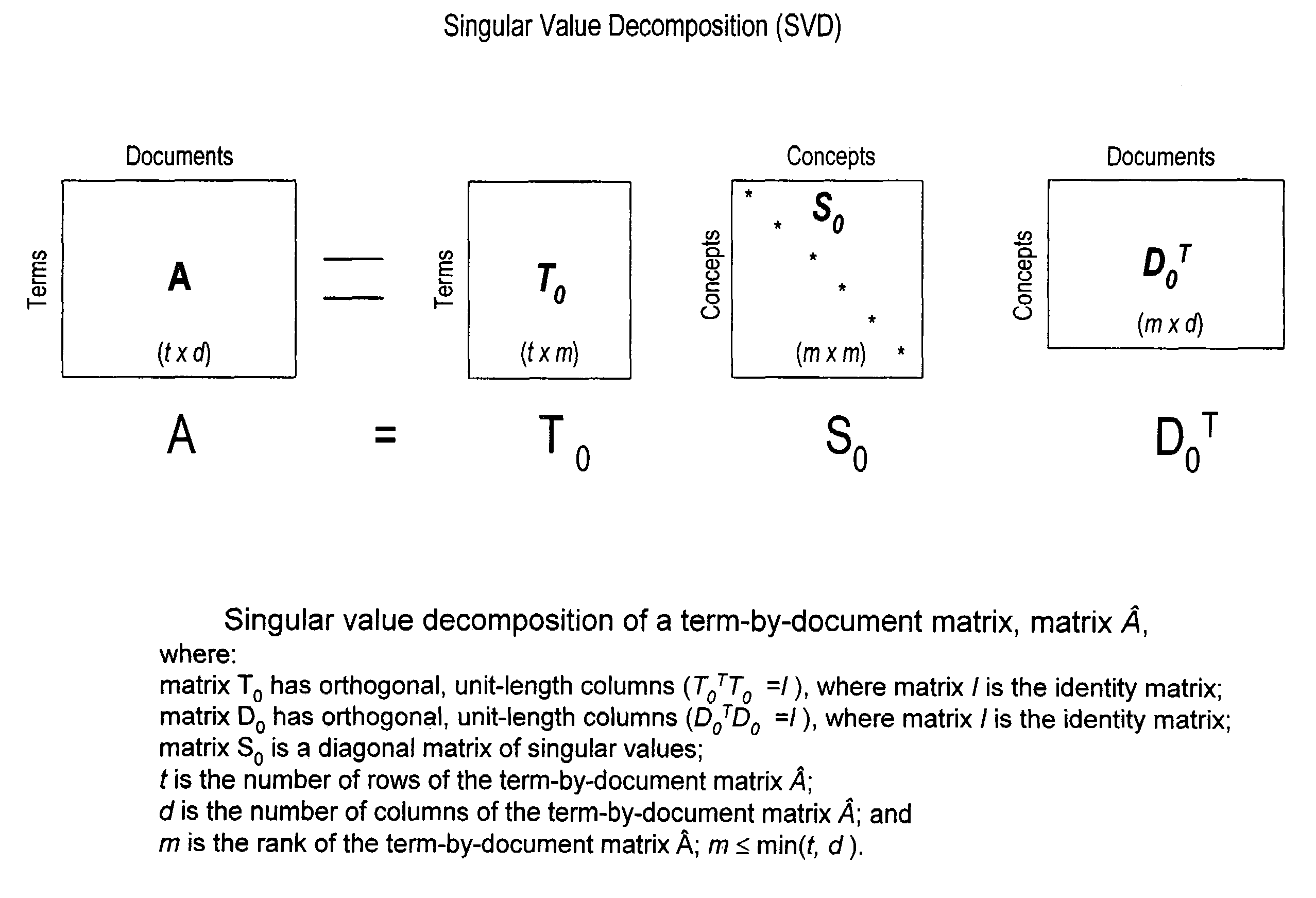Patents
Literature
2152 results about "Matrix form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Matrix, a set of numbers arranged in rows and columns so as to form a rectangular array. The numbers are called the elements, or entries, of the matrix. Matrices have wide applications in engineering, physics, economics, and statistics as well as in various branches of mathematics.
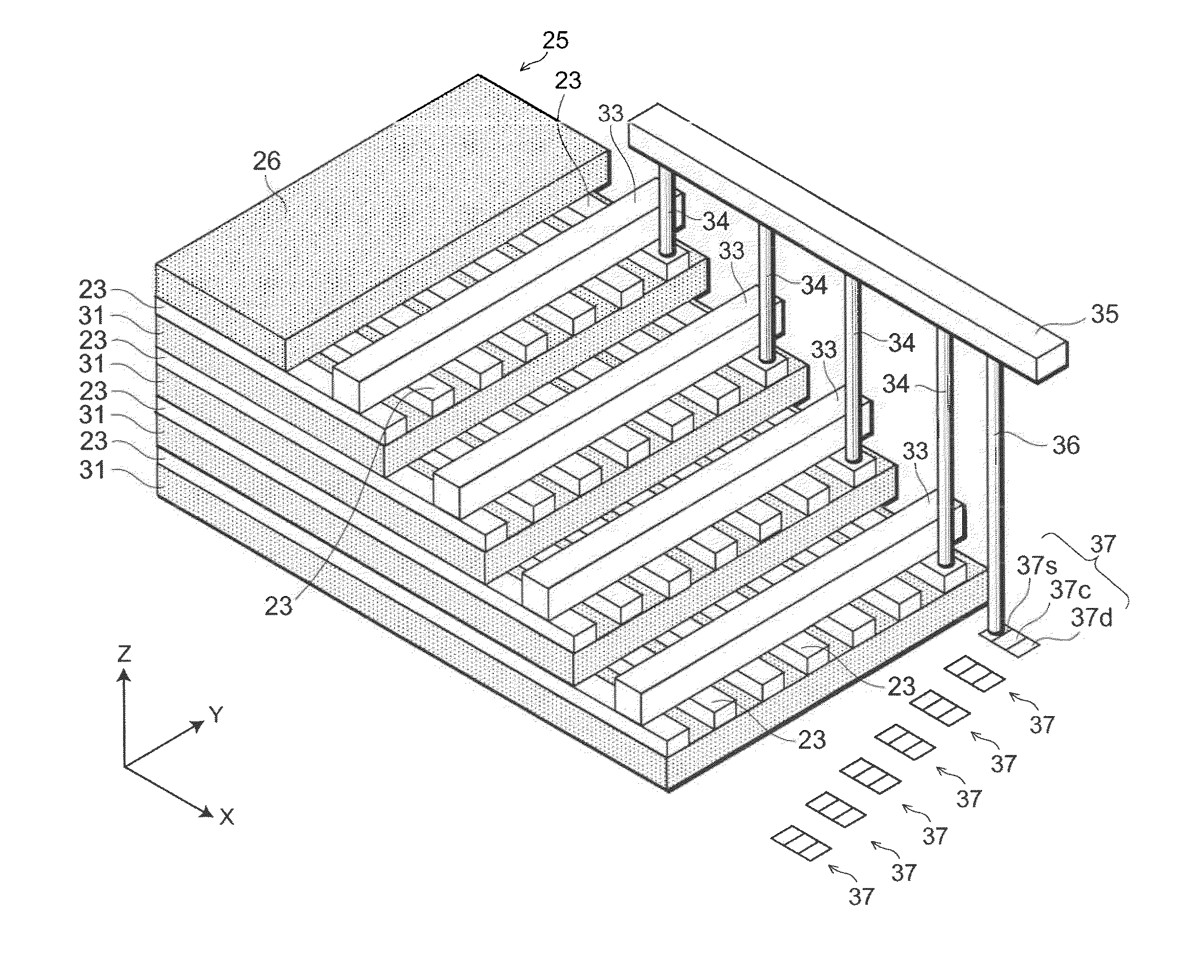
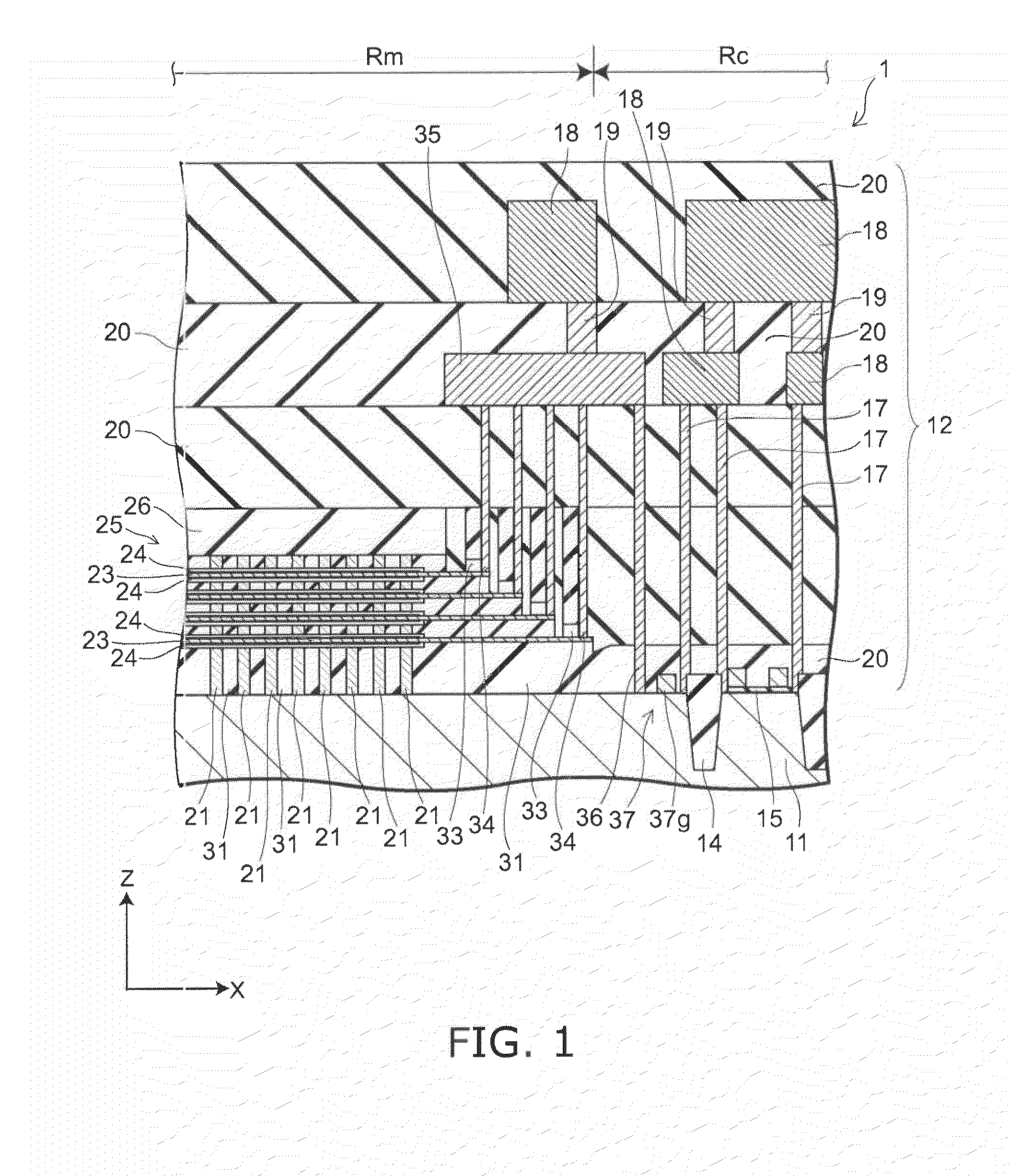
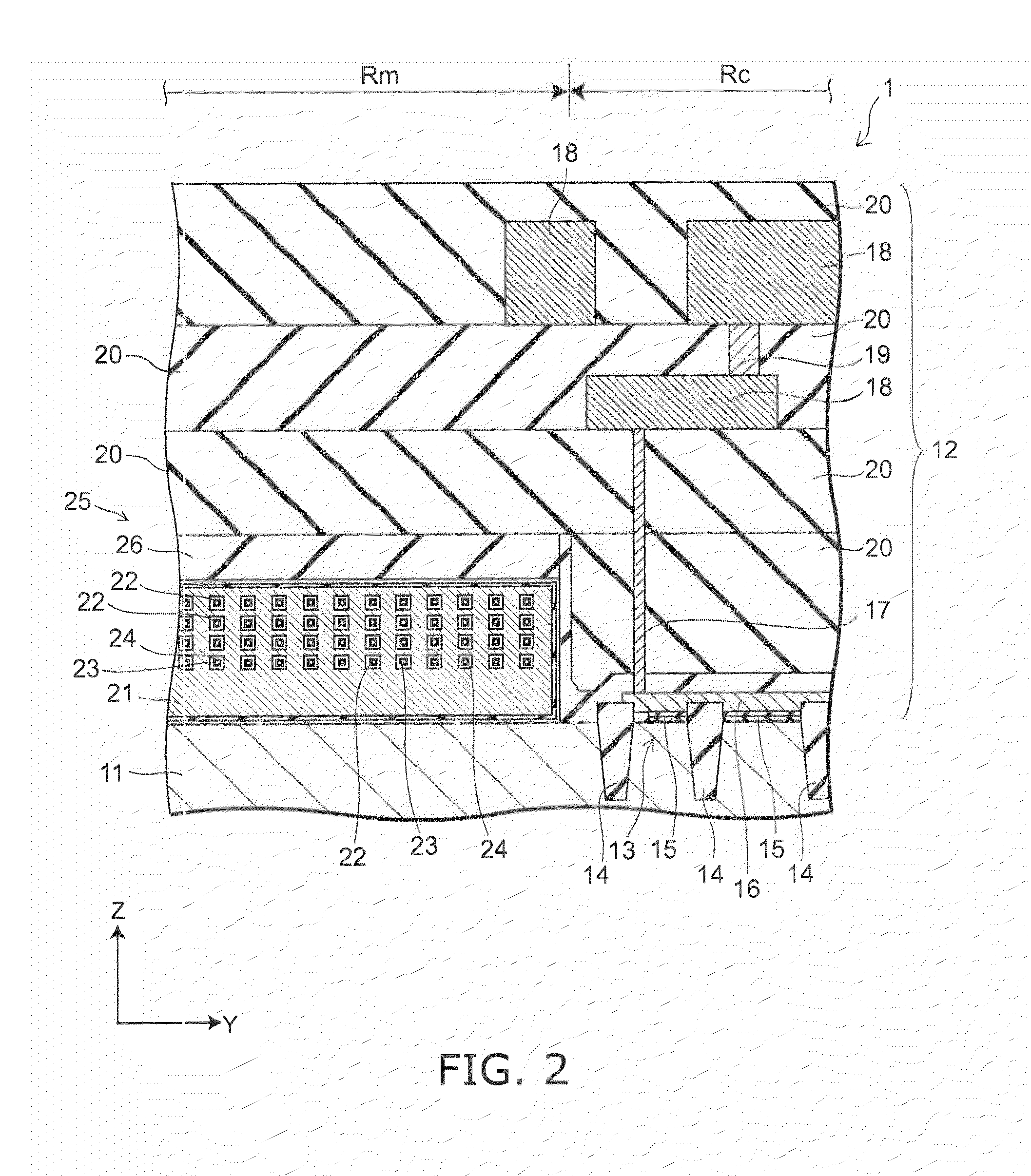
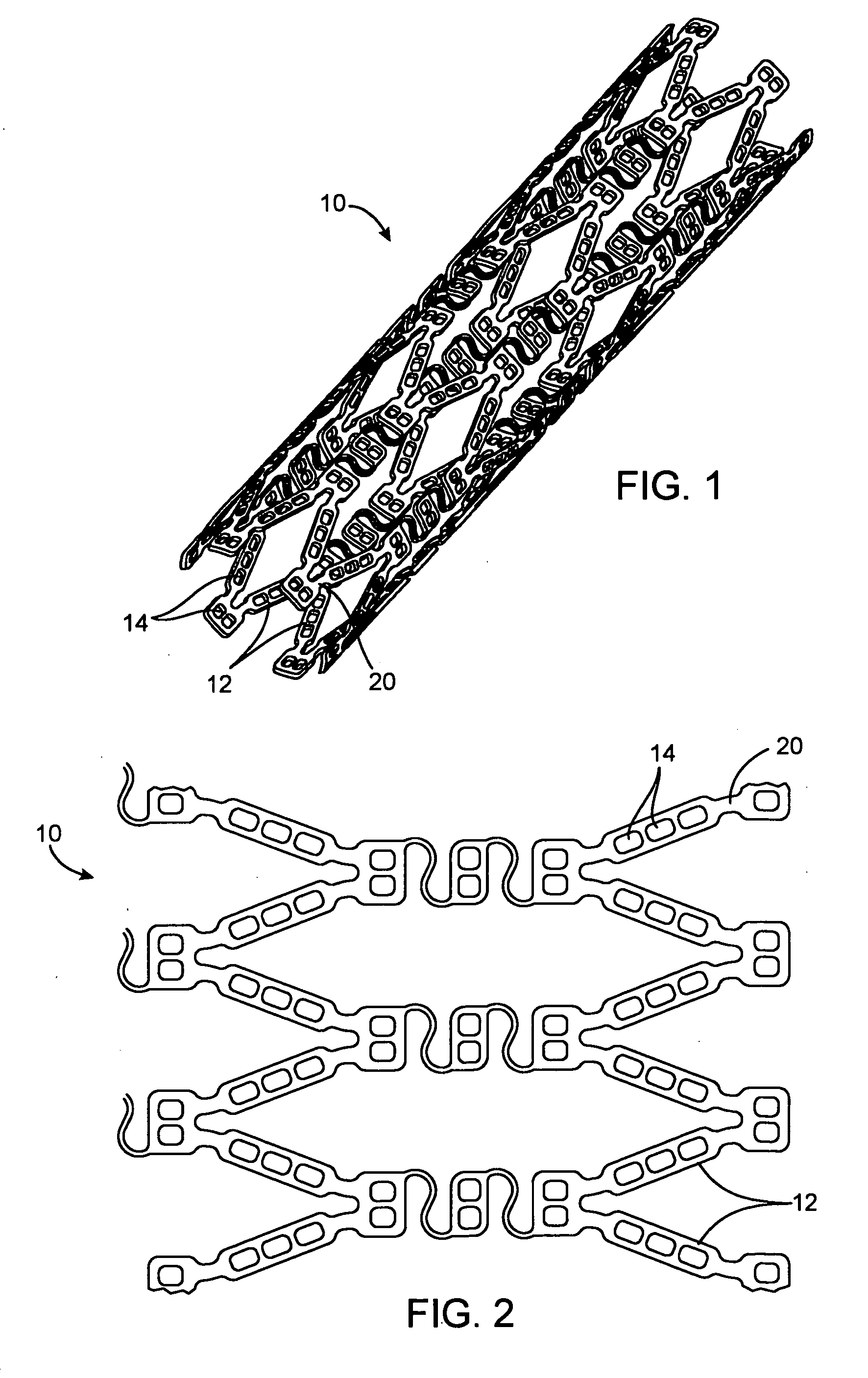
Semiconductor memory and method for manufacturing same
InactiveUS20110284946A1Increase bit densitySolid-state devicesSemiconductor/solid-state device manufacturingSemiconductorSilicon
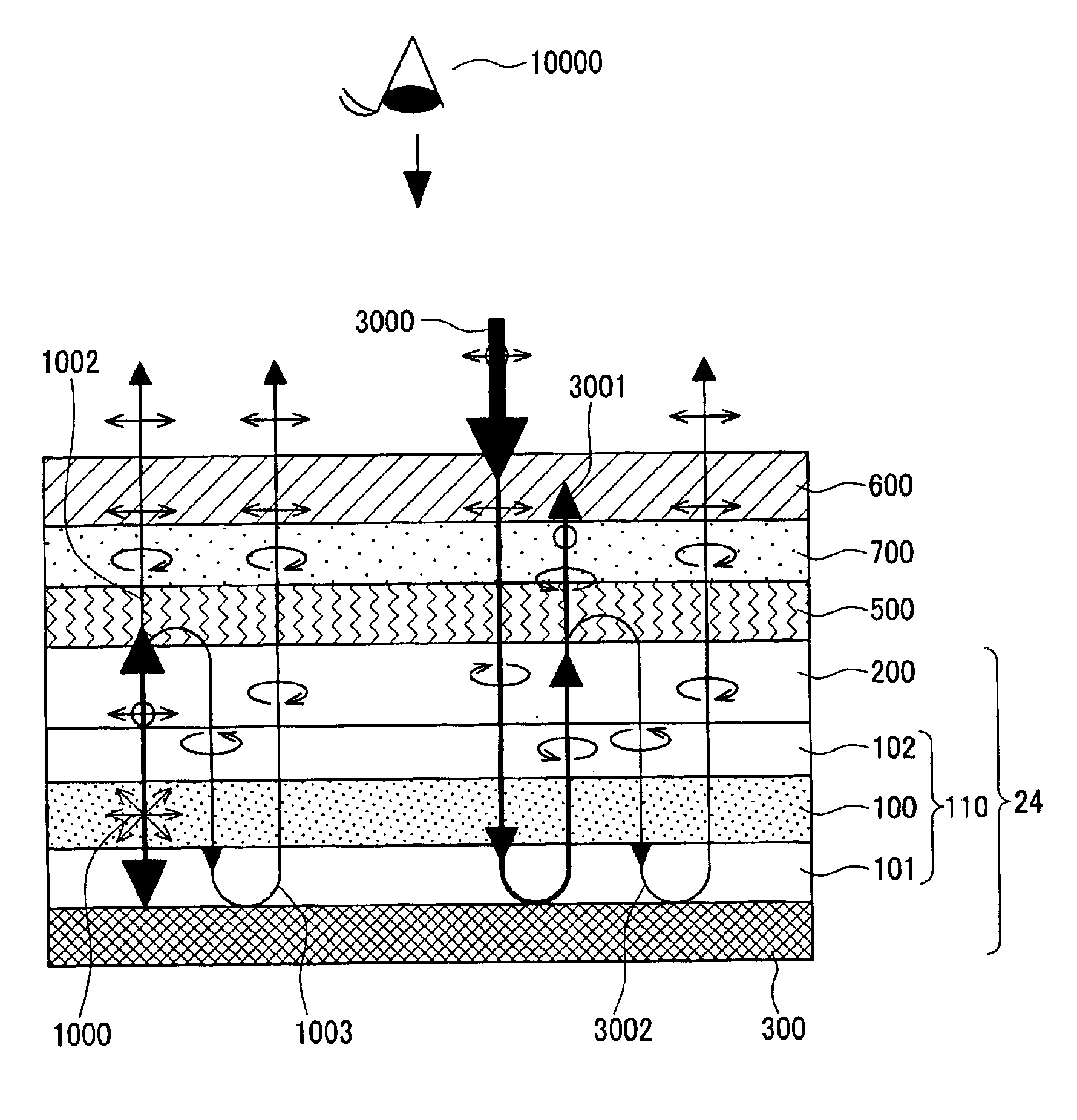
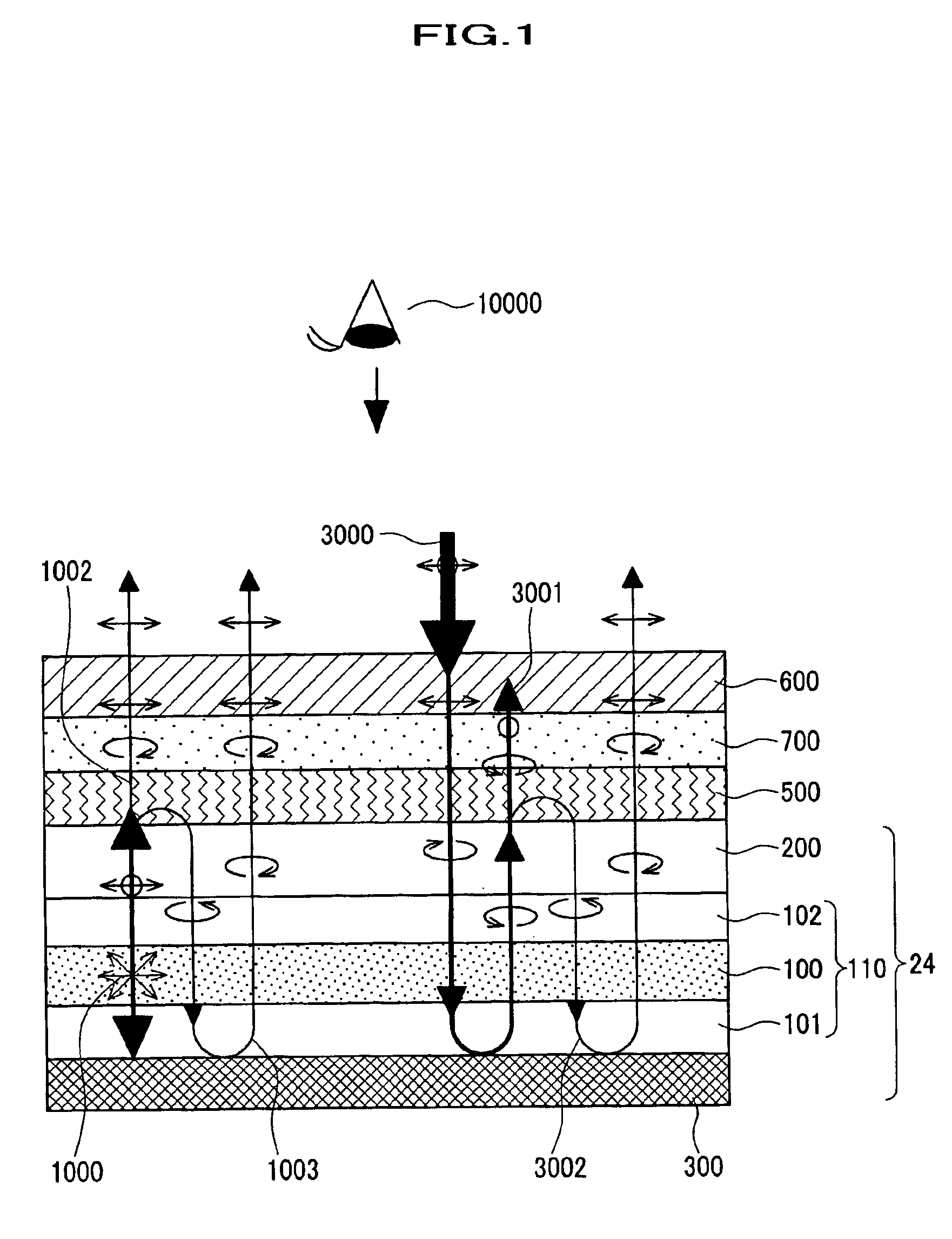
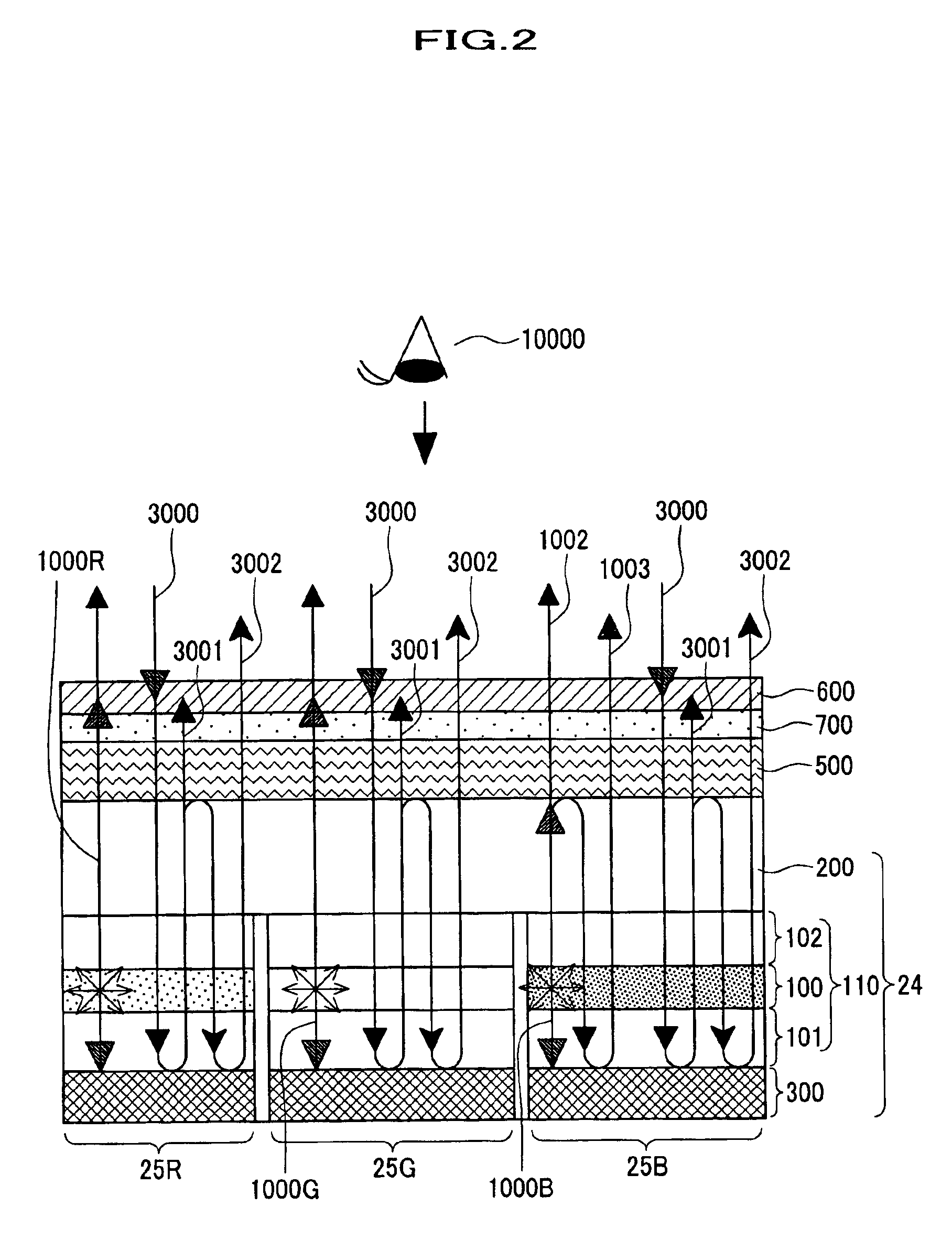
A semiconductor memory capable of increasing bit density by three-dimensional arrangement of cells and a method for manufacturing the same are provided.In a semiconductor memory 1, gate electrode films 21 are provided on a silicon substrate 11. The gate electrode films 21 are arranged in one direction parallel to the upper surface of the silicon substrate 11 (X direction). Each gate electrode film 21 has a lattice plate-like shape, having through holes 22 in a matrix form as viewed in the X direction. Silicon beams 23 are provided passing through the through holes 22 of the gate electrode films 21 and extending in the X direction. Further, an ONO film 24 including a charge storage layer is provided between the gate electrode film 21 and the silicon beam 23.
Owner:KK TOSHIBA
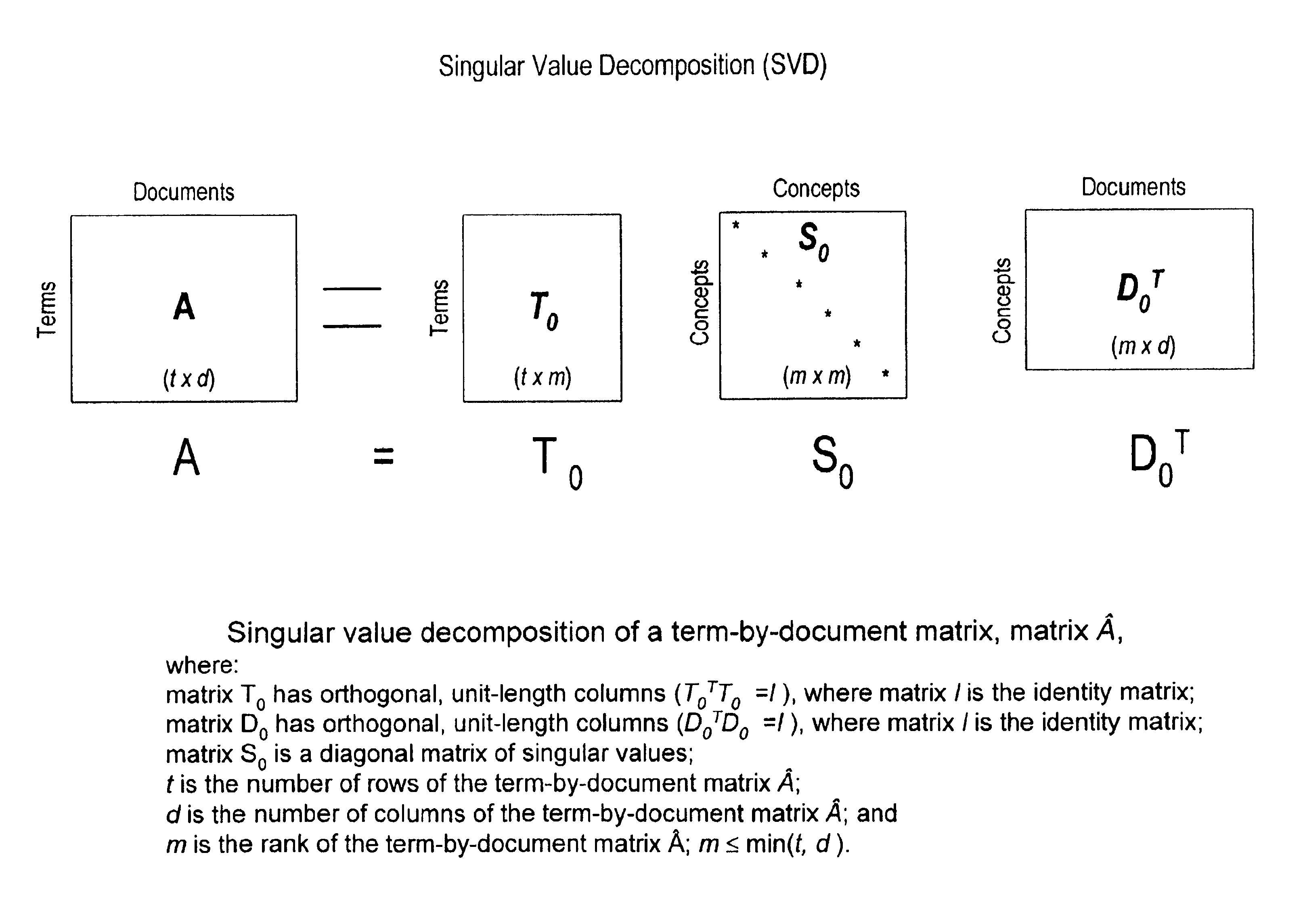
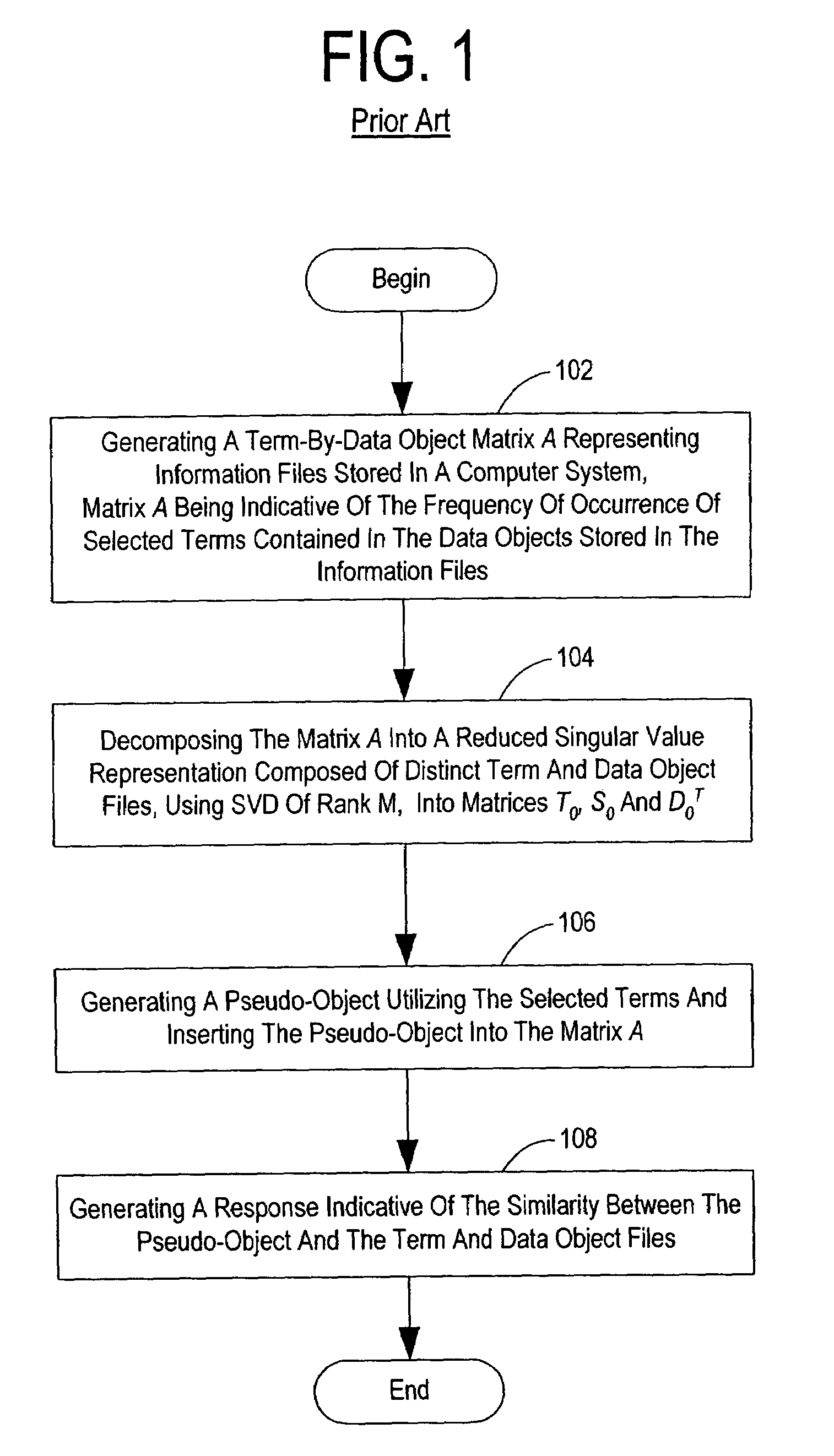
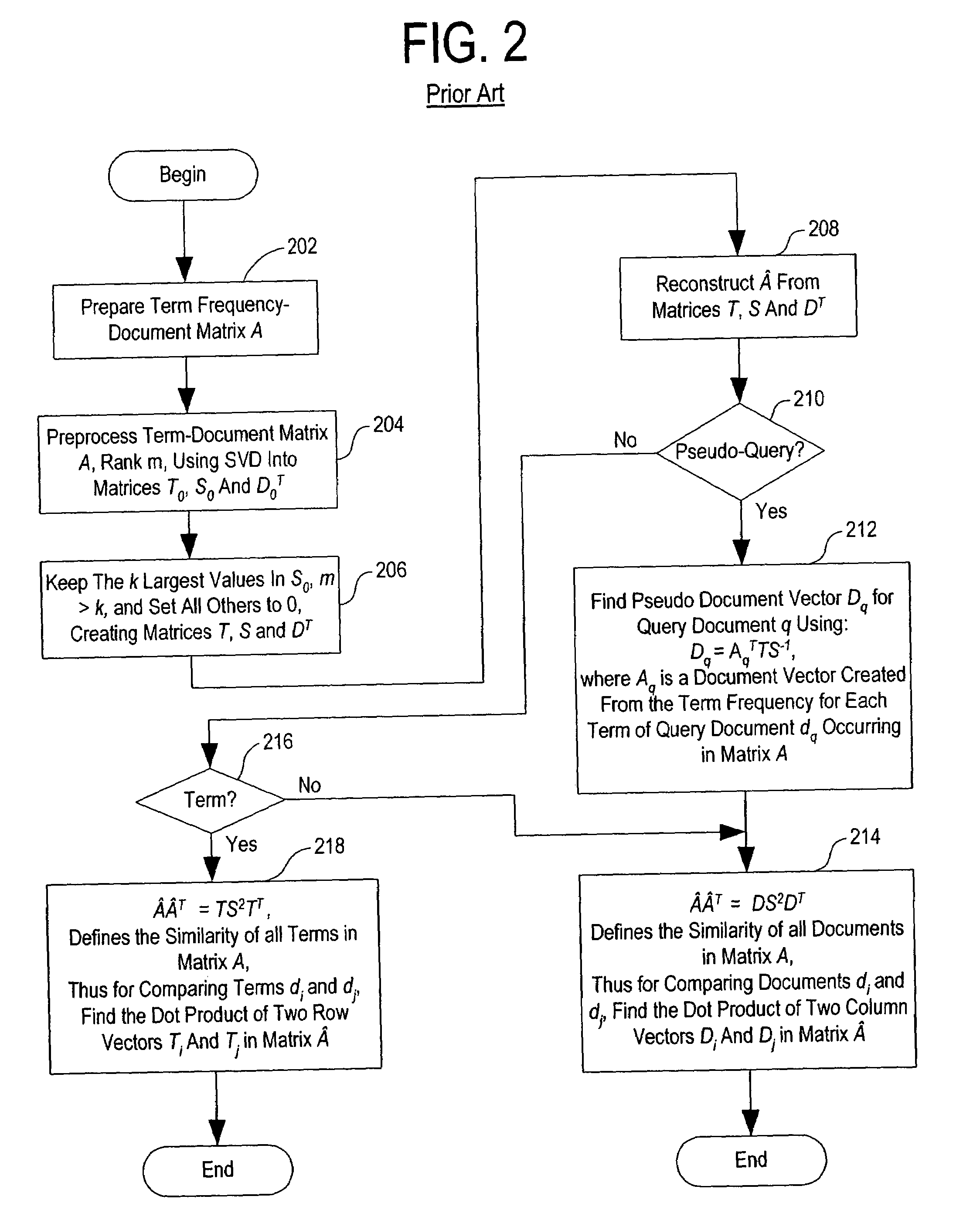
Method and system for optimally searching a document database using a representative semantic space
InactiveUS6847966B1Reduced dimensionData processing applicationsDigital data information retrievalSingular value decompositionSubject matter
A term-by-document matrix is compiled from a corpus of documents representative of a particular subject matter that represents the frequency of occurrence of each term per document. A weighted term dictionary is created using a global weighting algorithm and then applied to the term-by-document matrix forming a weighted term-by-document matrix. A term vector matrix and a singular value concept matrix are computed by singular value decomposition of the weighted term-document index. The k largest singular concept values are kept and all others are set to zero thereby reducing to the concept dimensions in the term vector matrix and a singular value concept matrix. The reduced term vector matrix, reduced singular value concept matrix and weighted term-document dictionary can be used to project pseudo-document vectors representing documents not appearing in the original document corpus in a representative semantic space. The similarities of those documents can be ascertained from the position of their respective pseudo-document vectors in the representative semantic space.
Owner:KLDISCOVERY ONTRACK LLC
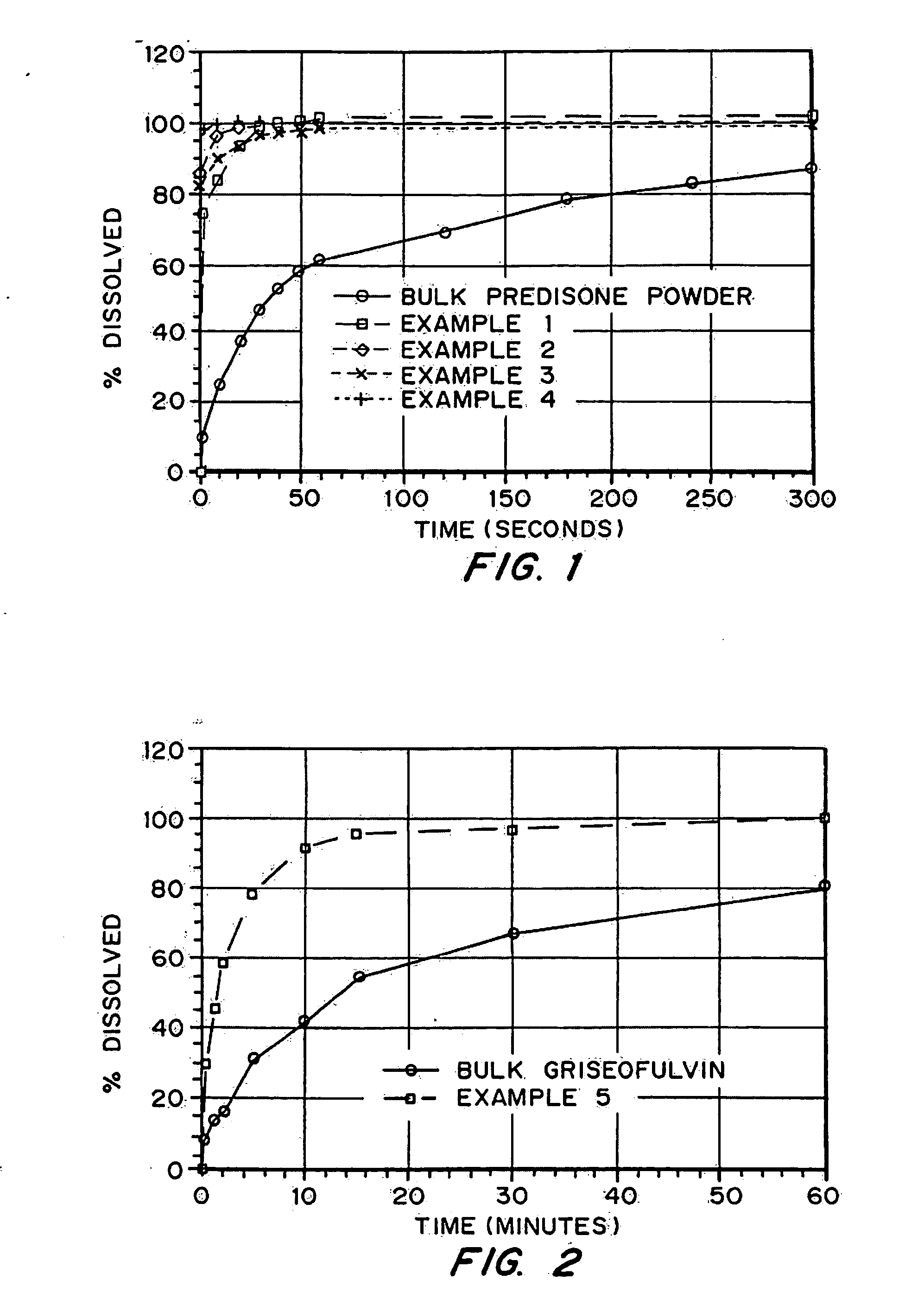
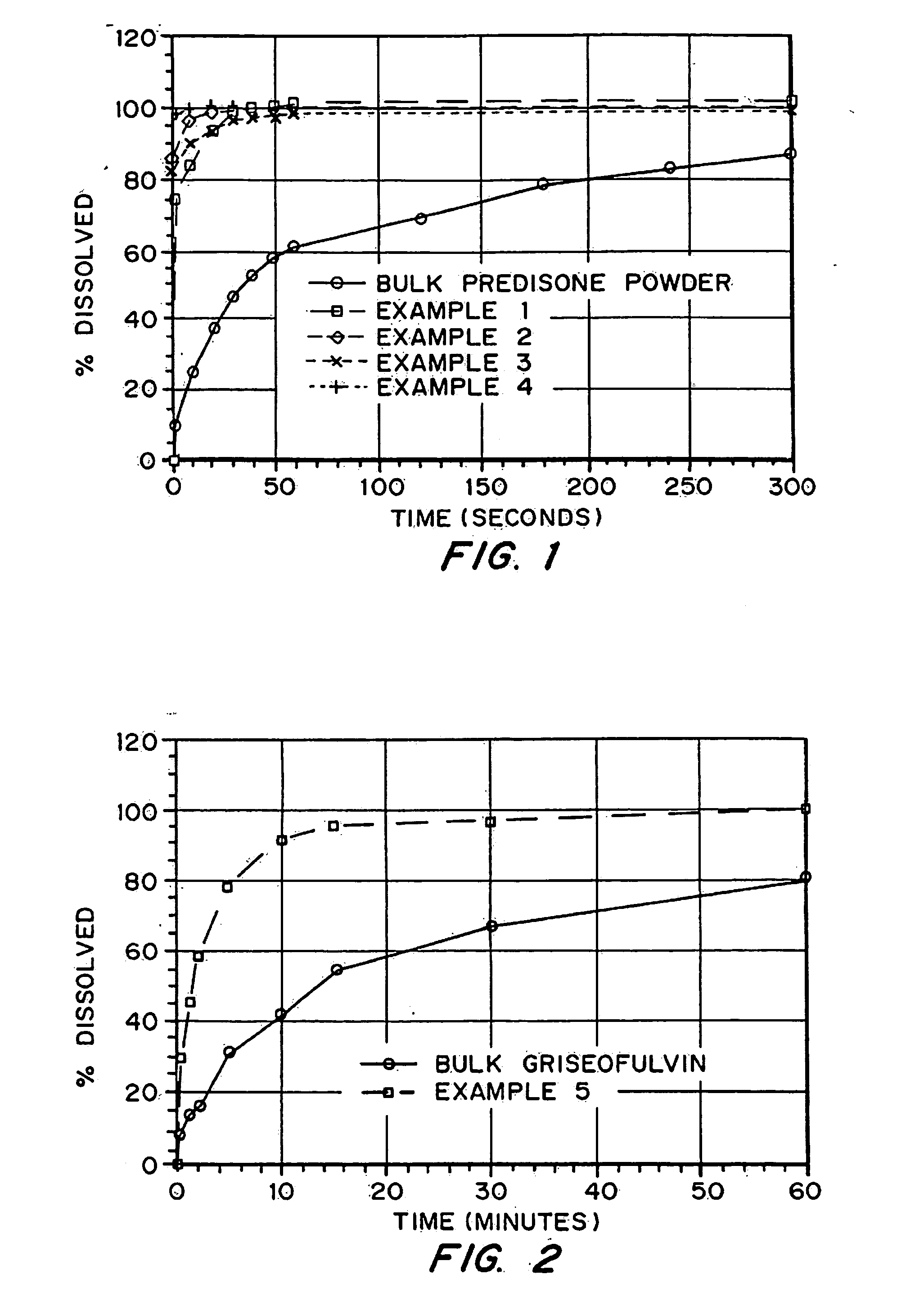
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
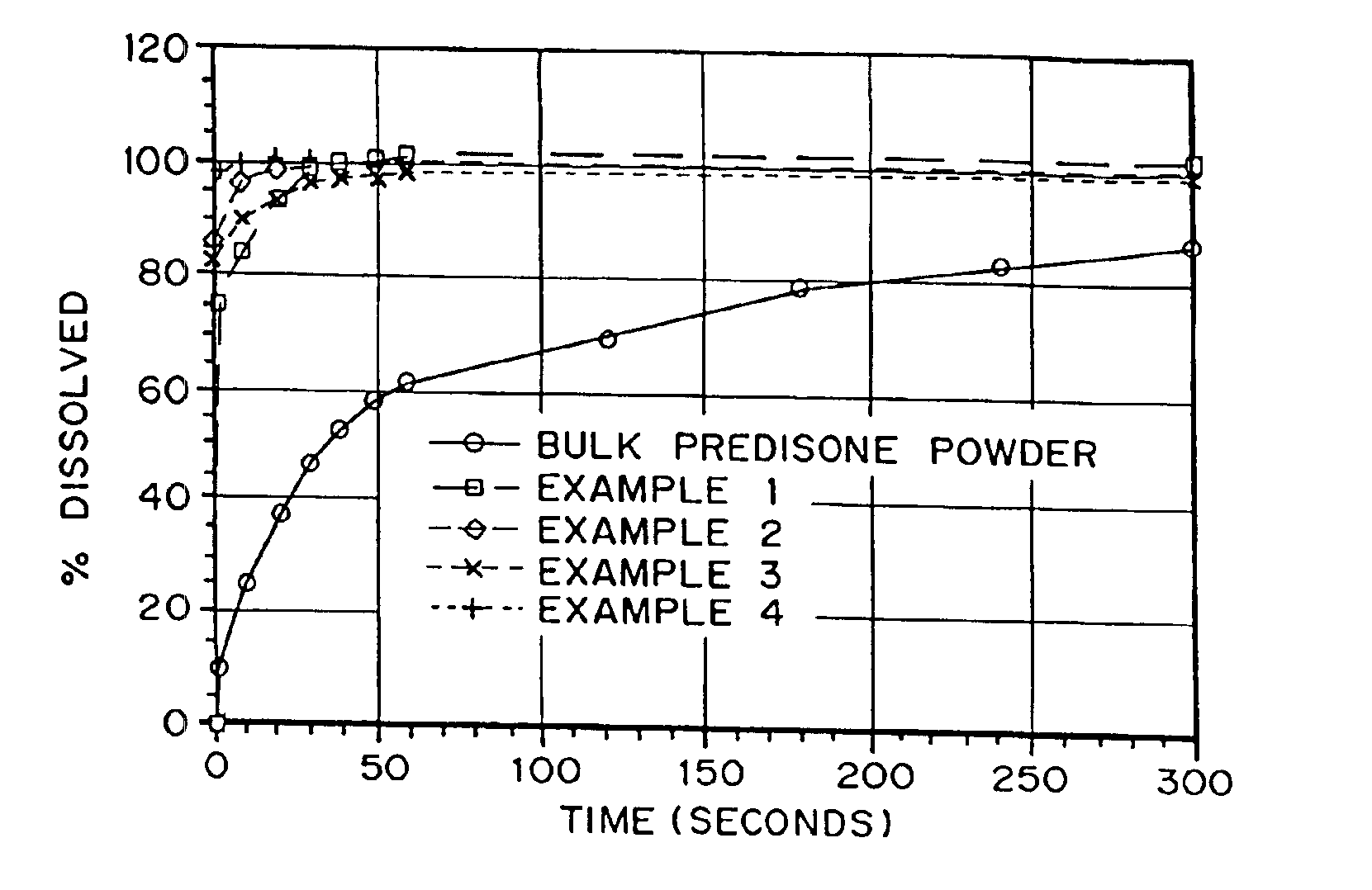
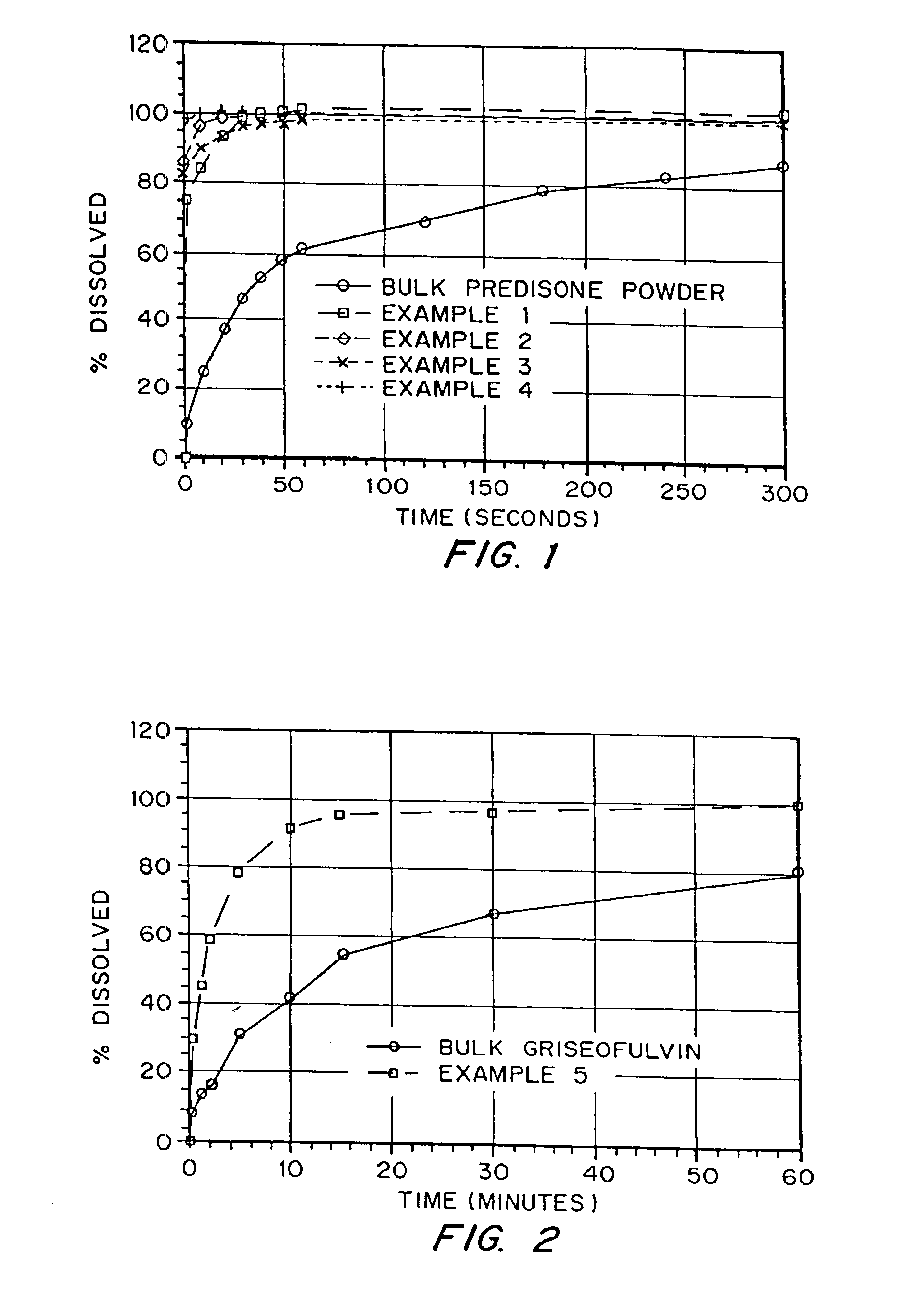
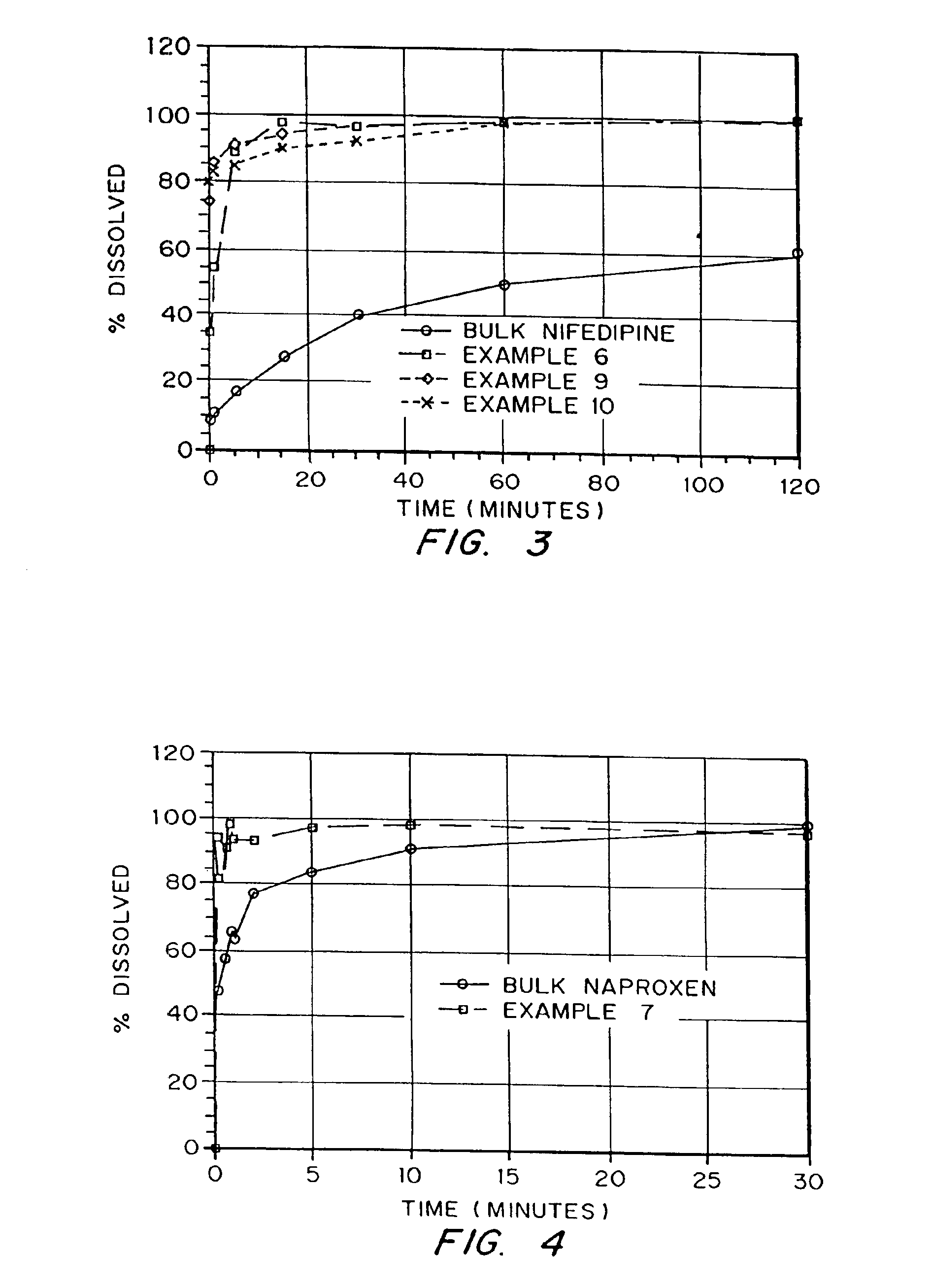
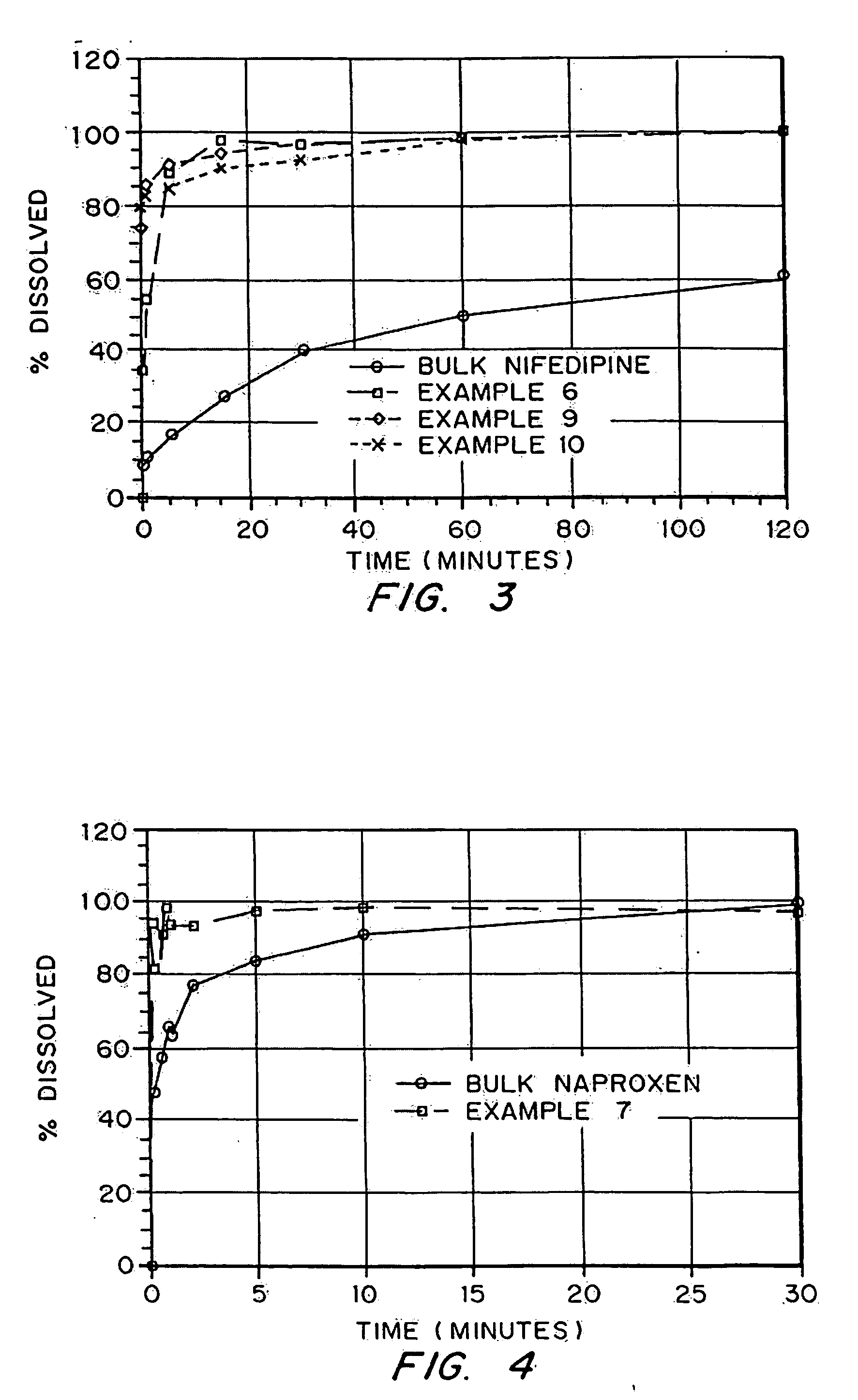
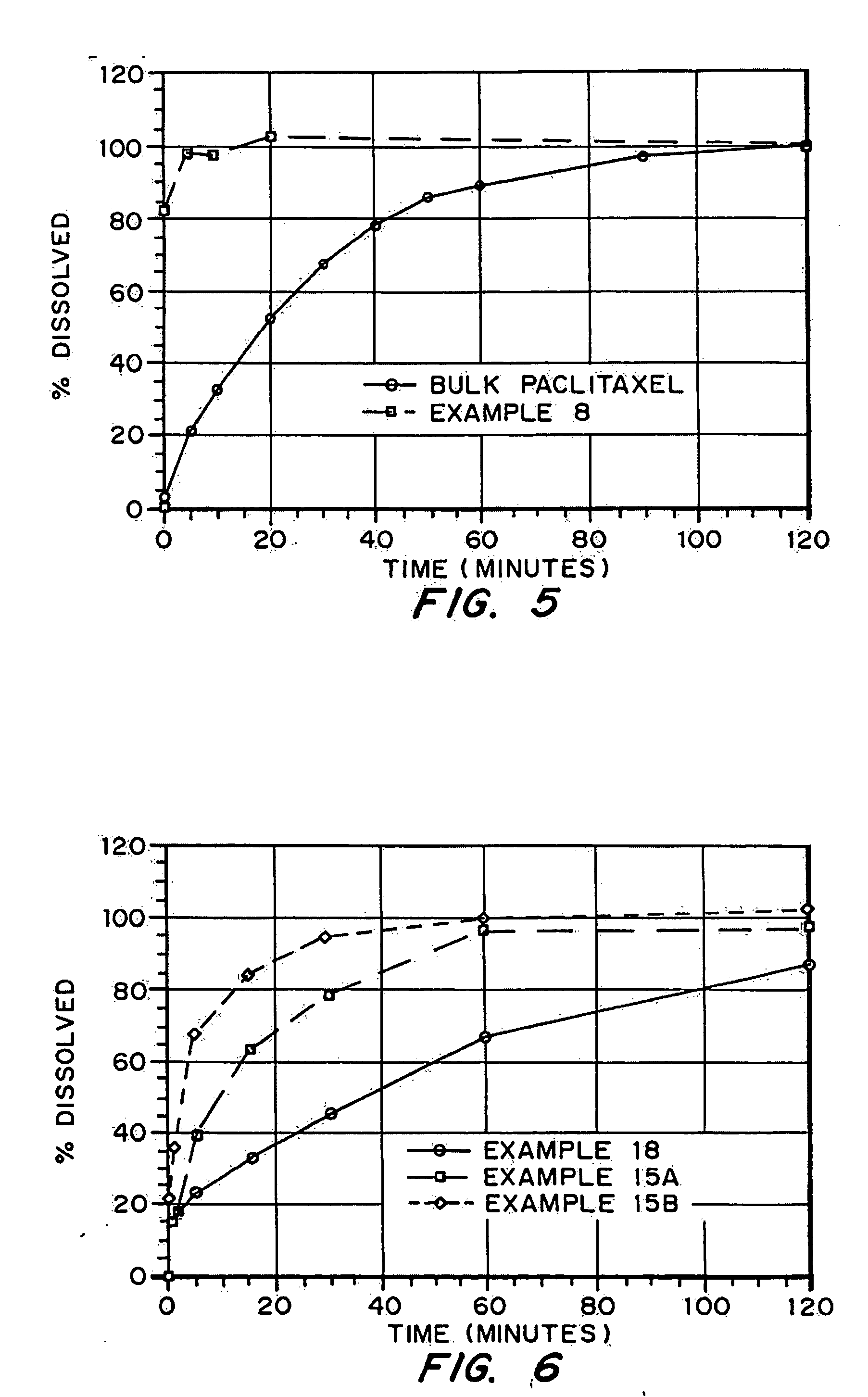
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Binding agent for solid block functional material
InactiveUS6258765B1Fit tightlyEasy to cleanInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsBULK ACTIVE INGREDIENTActive ingredient
A solid functional material comprises a functional agent such as a cleaning composition, a sanitizing agent, where a rinse agent, etc. in a solid block format. The solid block is formed by a binding agent that forms the active ingredients into a solid block. The binding agent comprises a phosphonate or amino acetate sequestrant, a carbonate salt and water in an E-Form hydrate. These materials at a specific mole ratio form a novel binding agent that can form functional materials into a solid matrix form.
Owner:ECOLAB USA INC
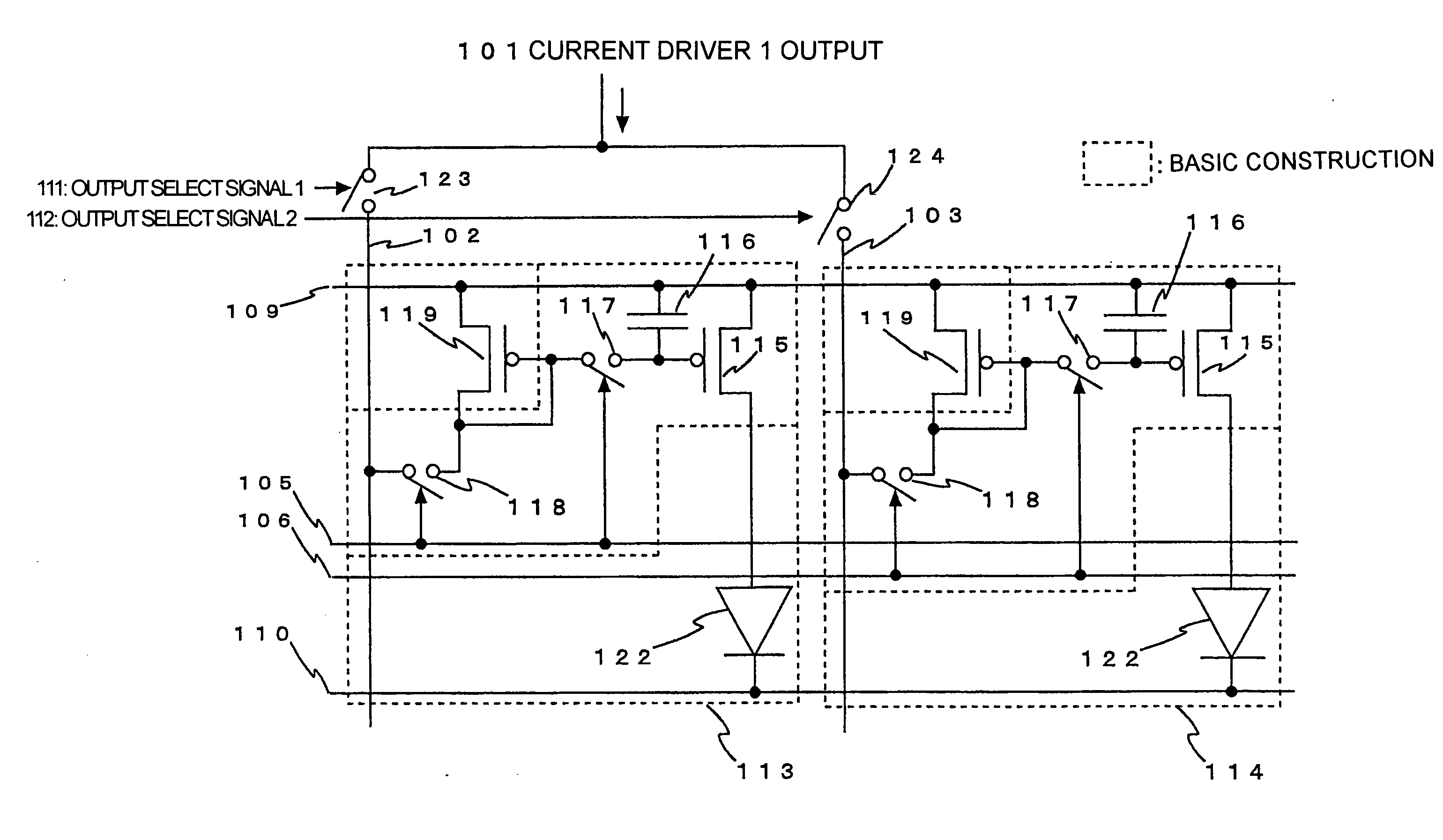
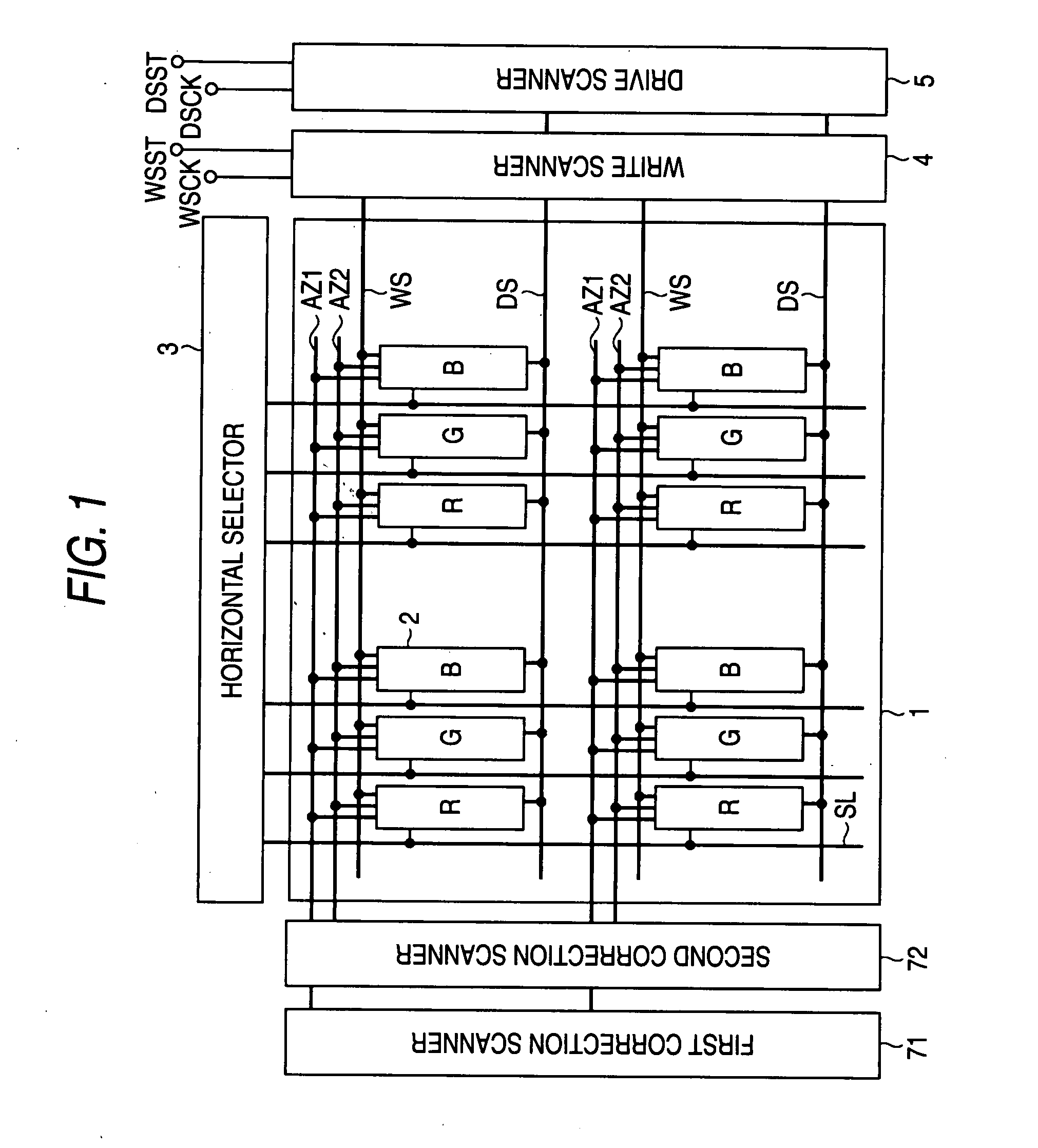
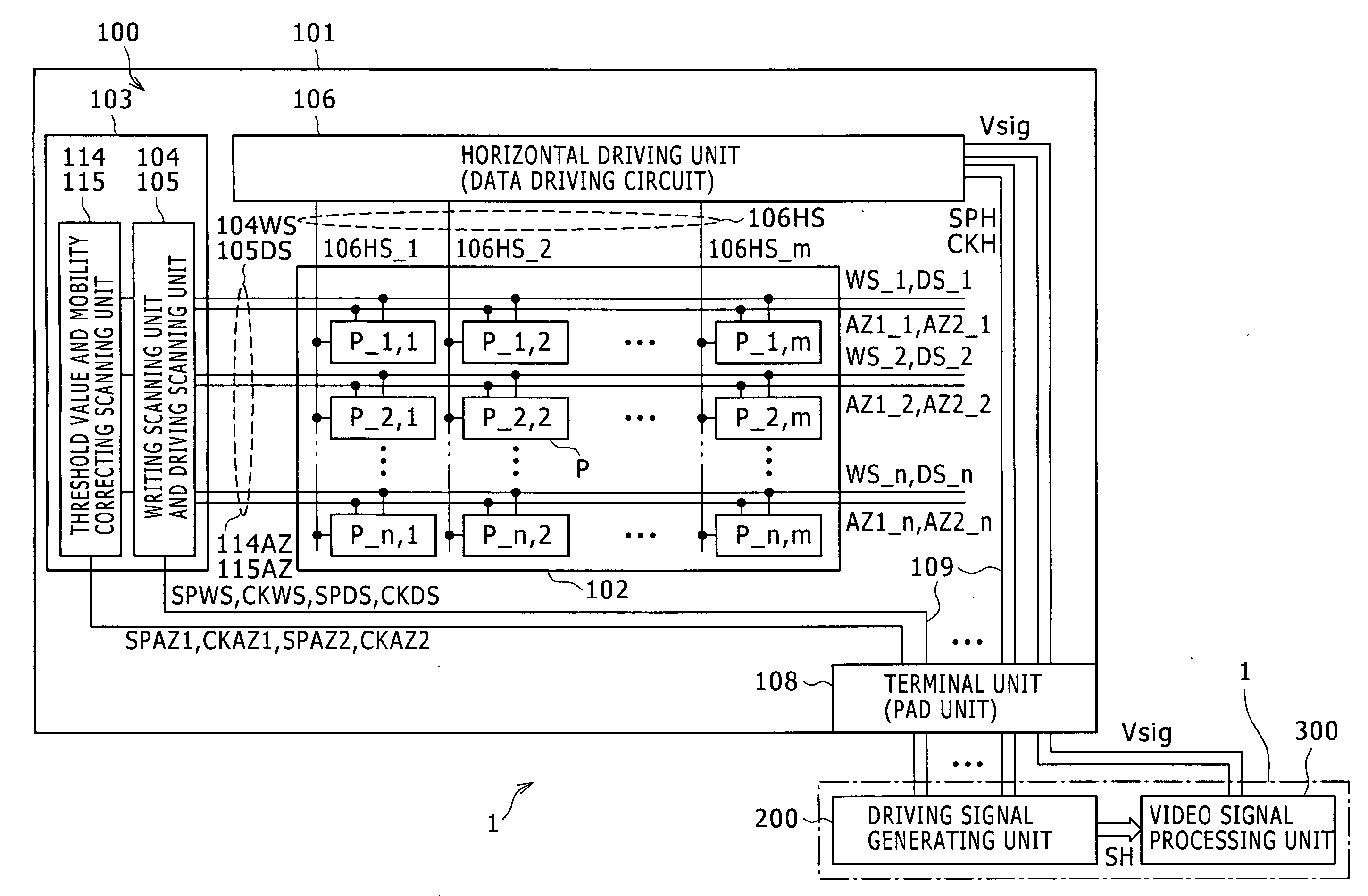
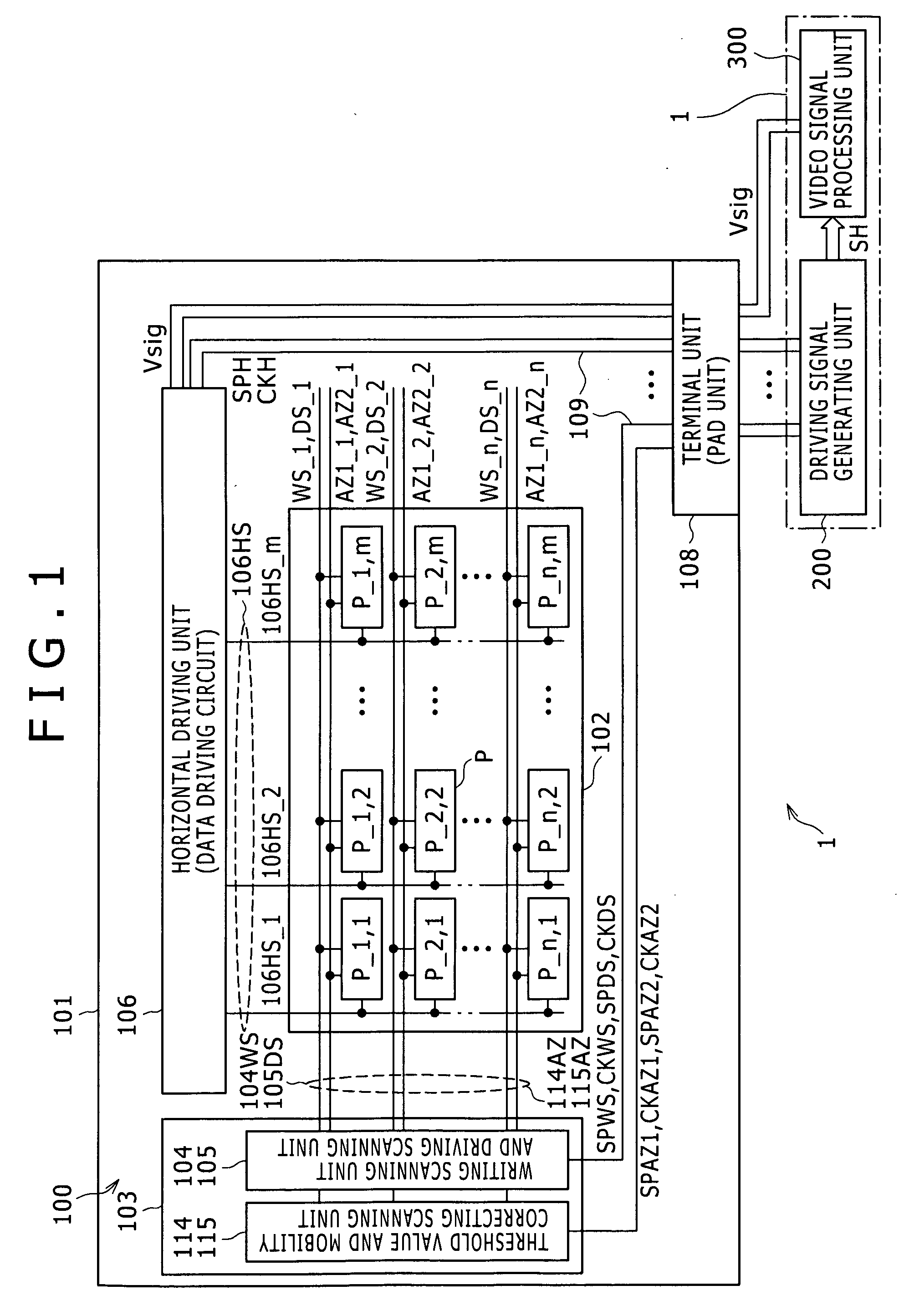
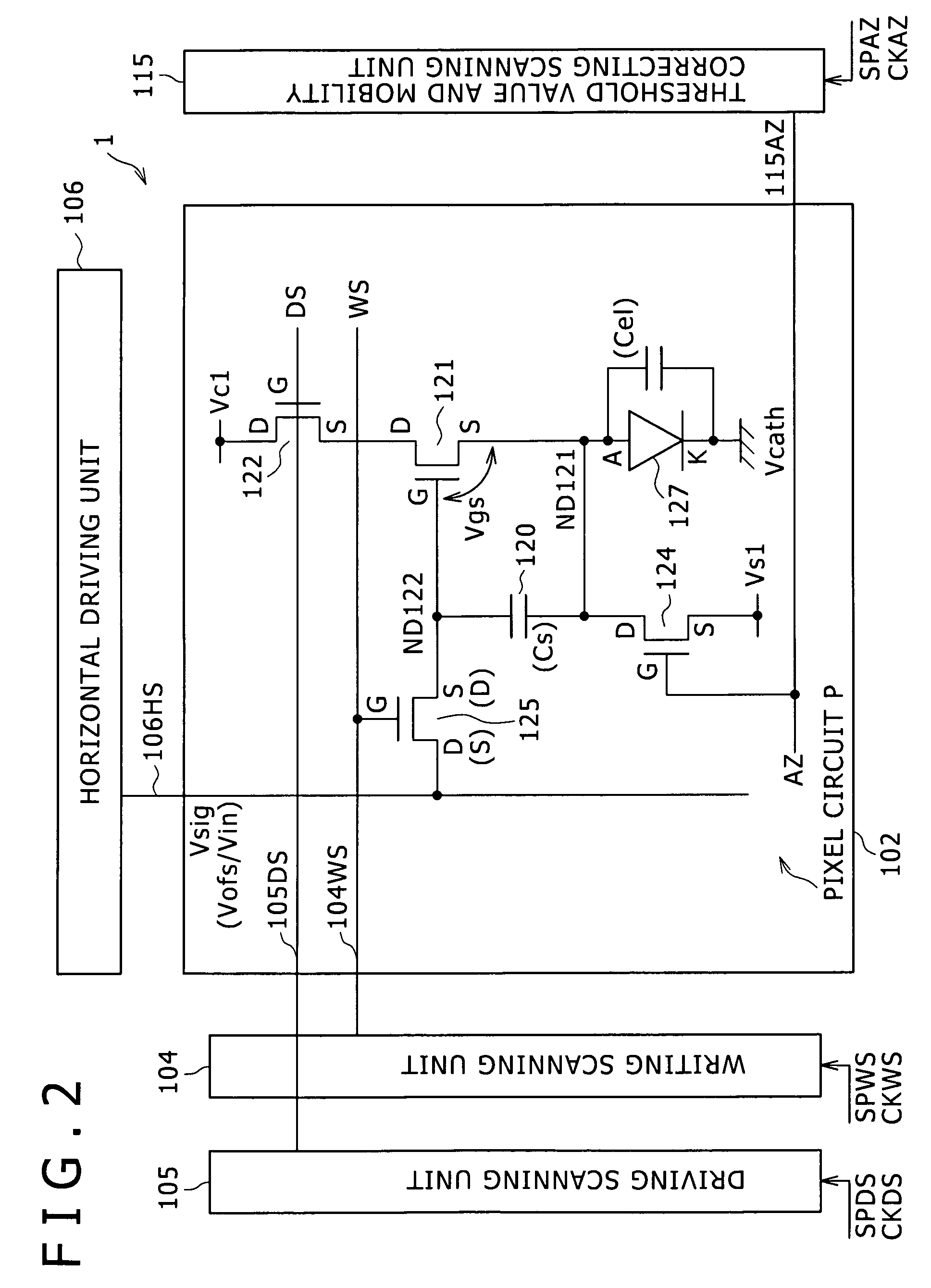
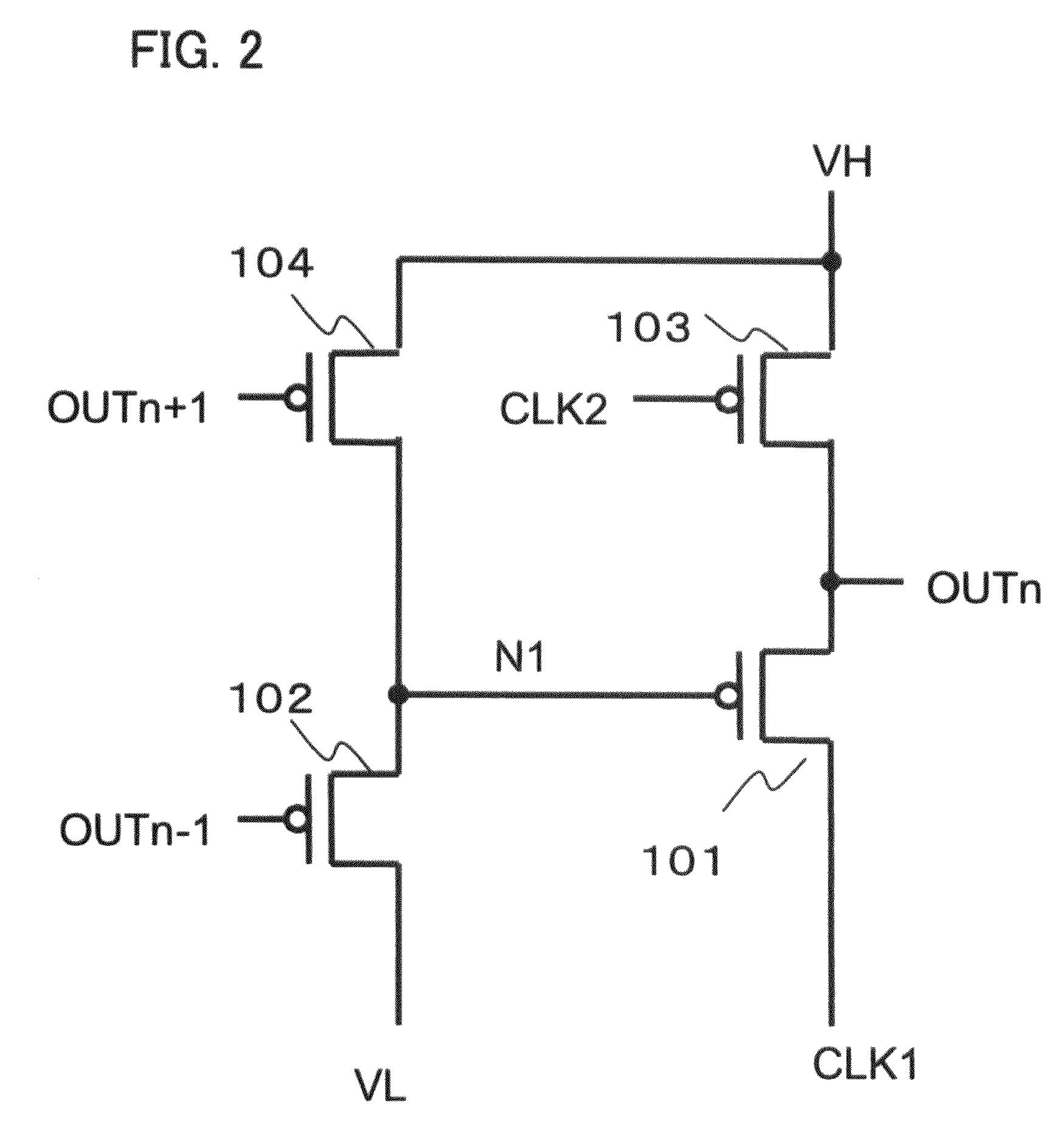
Semiconductor device provided with matrix type current load driving circuits, and driving method thereof
A semiconductor device to which active drive current programming is applied, comprising current load cells each having a current load and a current load driving circuit, which are arranged in a matrix, capable of reducing the circuit scale of a current driver with little change made in the structure of the current load driving circuit, and a driving method of the same. A current load cell (113, 114) includes a current load driving circuit which is provided with a transistor (115) connected in series with a current load (122) between first and second power supplies (109, 110); a capacitance (116) connected between the control terminal of the transistor (115) and the first power supply (109); and switches (117, 118) connected between the control terminal of the transistor (115) and a corresponding data line. The output (101) of a current driver is connected to a plurality of data lines via a selector (123, 124), and the plural data lines connected to one output of the current driver via the selector and at least one of the switches of each of the current load cells corresponding to the respective data lines are drive-controlled in a time division manner during one horizontal period.
Owner:HANNSTAR DISPLAY CORPORATION
Display device and electronic equipment
ActiveUS20080042948A1Amount of signal becomes smallReduce semaphoreElectrical apparatusStatic indicating devicesScan lineControl signal
A display device is disclosed. The display device includes: a pixel array part; and a drive part that drives the pixel array part. The pixel array part includes row-wise first scan lines and second scan lines, column-wise signal lines, pixels arranged in a matrix form on parts where the lines intersect, and power supply lines and ground lines that supply power to the respective pixels. The drive part includes a first scanner that sequentially supplies first control signals to the respective first scan lines and line-sequentially scans the pixels in units of rows, a second scanner that sequentially supplies second control signals to the respective second scan lines according to the line-sequential scan, and a signal selector that supplies video signals to the column-wise signal lines according to the line-sequential scan.
Owner:JOLED INC
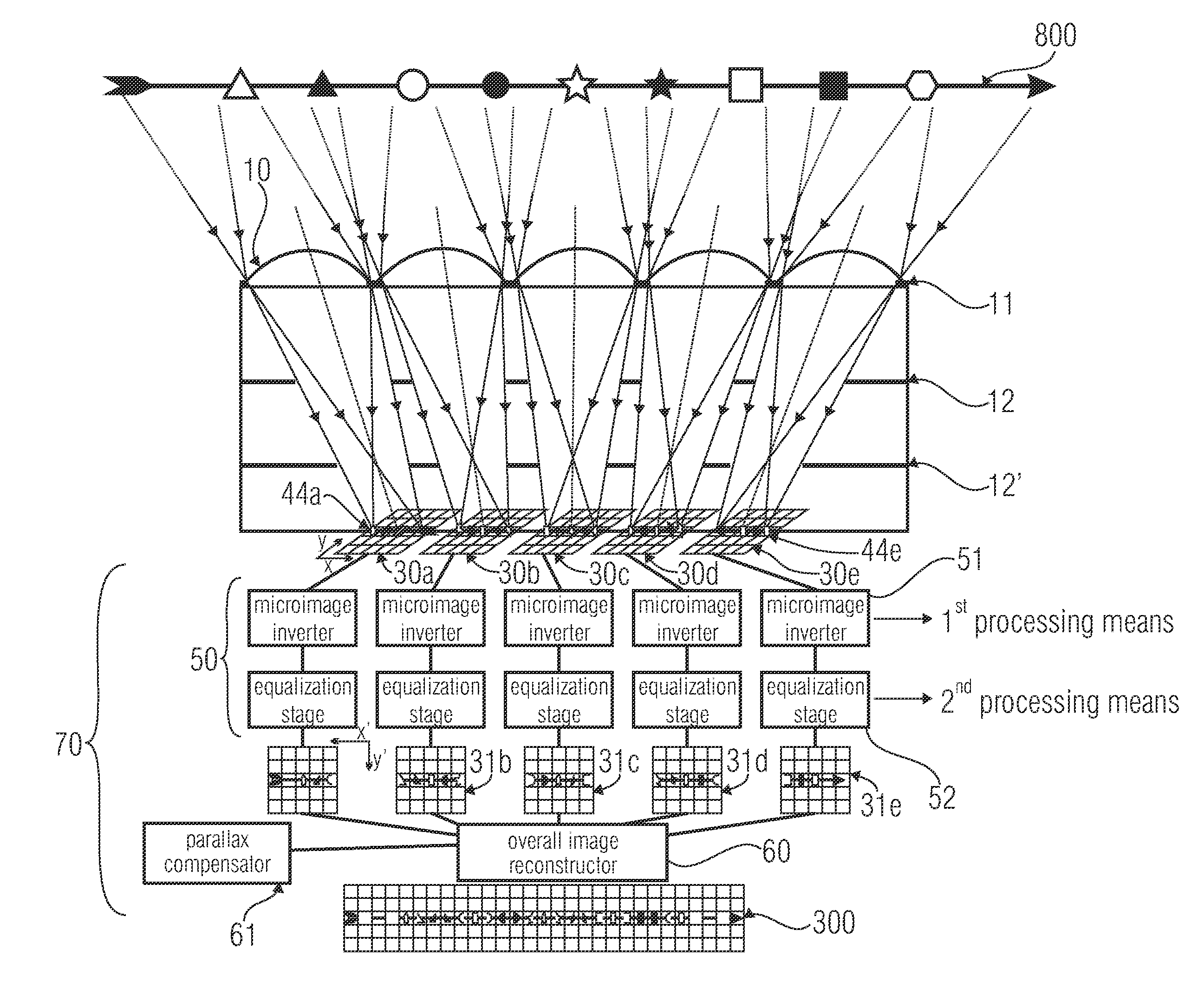
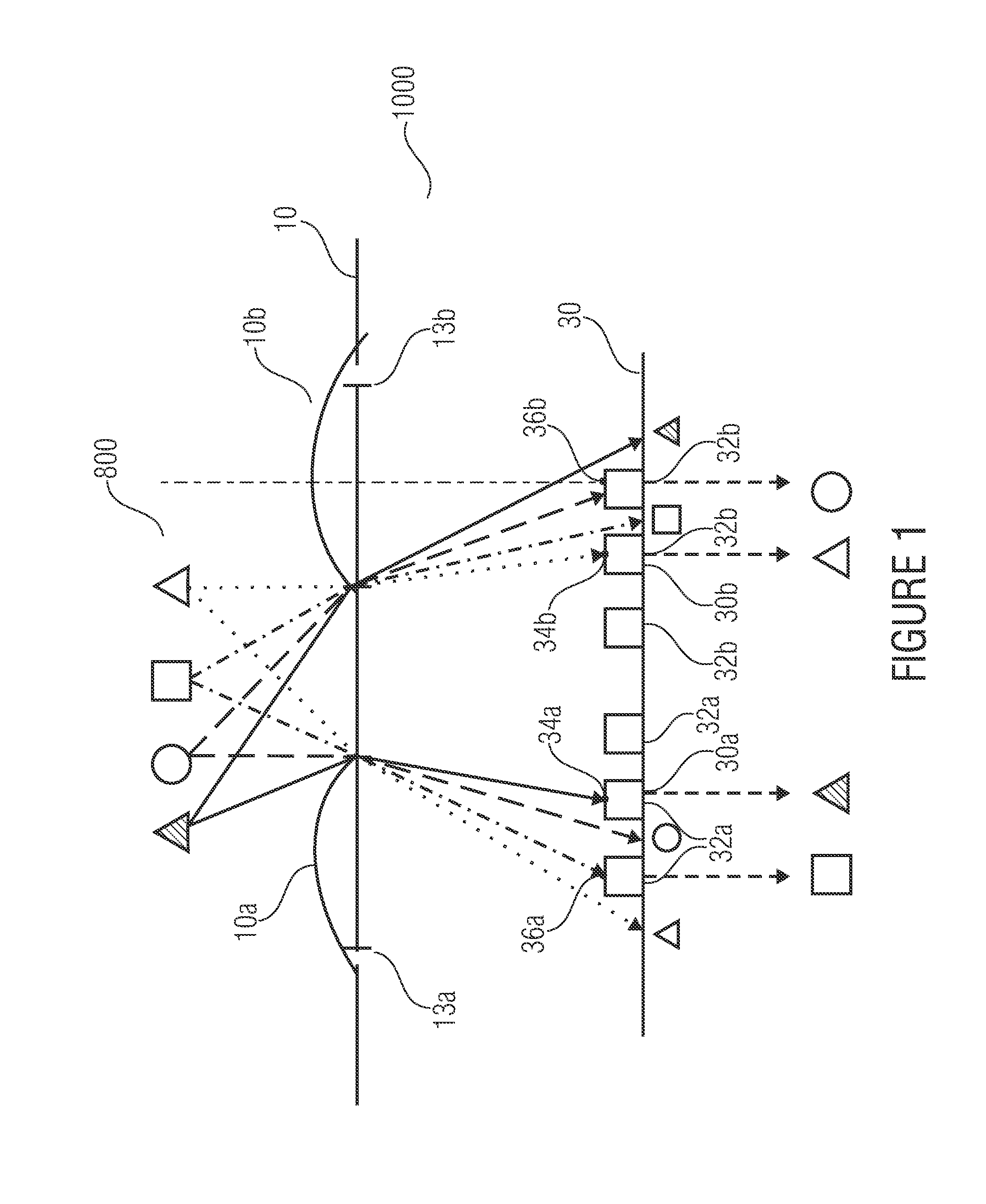
Device, image processing device and method for optical imaging
ActiveUS20110228142A1Short focal lengthShorten build lengthTelevision system detailsSolid-state devicesImaging processingGrating
An optical device for imaging is disclosed having at least one micro lens field with at least two micro lenses and one image sensor with at least two image detector matrices. The at least two image detector matrices each include a plurality of image detectors and there is an allocation between the image detector matrices and the micro lenses, so that each micro lens together with an image detector matrix forms an optical channel. The center points of the image detector matrices are shifted laterally by different distances, with respect to centroids, projected onto the image detector matrices, of the micro lens apertures of the associated optical channels, so that the optical channels have different partially overlapping detection areas and so that an overlapping area of the detection areas of two channels is imaged onto the image detector matrices offset with respect to an image detector raster of the image detector matrices. Further, an image processing device and a method for optical imaging are described.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Display device and folding portable terminal
InactiveUS20050024339A1Small sizeFunctionalInput/output for user-computer interactionDevices with multiple display unitsDisplay devicePolarizer
It is an object to provide a highly functioned and highly value-added display device and mobile terminal. It is another object to provide a display device and mobile terminal characterized by including a plurality of pixels arranged in a matrix form and a first display screen over one surface of a substrate having a translucency, a second display screen over the opposite surface of the one surface of the substrate, each of the plurality of pixels having a light emitting element for emitting light toward the first display screen and the second display screen, and a first polarizer over one surface of the substrate and a second polarizer over the opposite surface of the one surface of the substrate.
Owner:SEMICON ENERGY LAB CO LTD
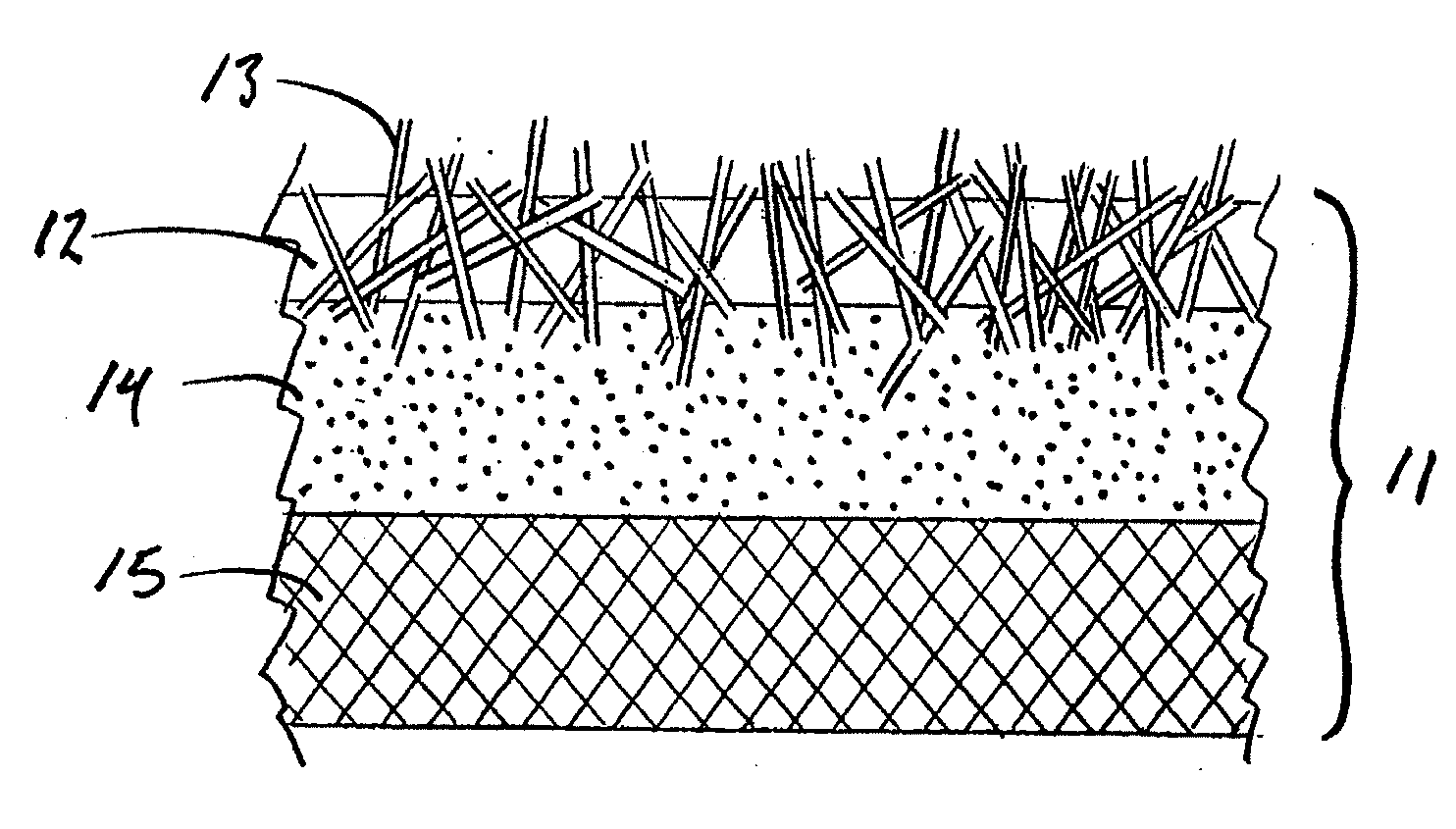
Reinforced, laminated, impregnated, and composite-like materials as crosslinked polyvinyl alcohol hydrogel structures
InactiveUS6855743B1High mechanical strengthIncrease modulusFibre treatmentSynthetic resin layered productsPorosityCross-link
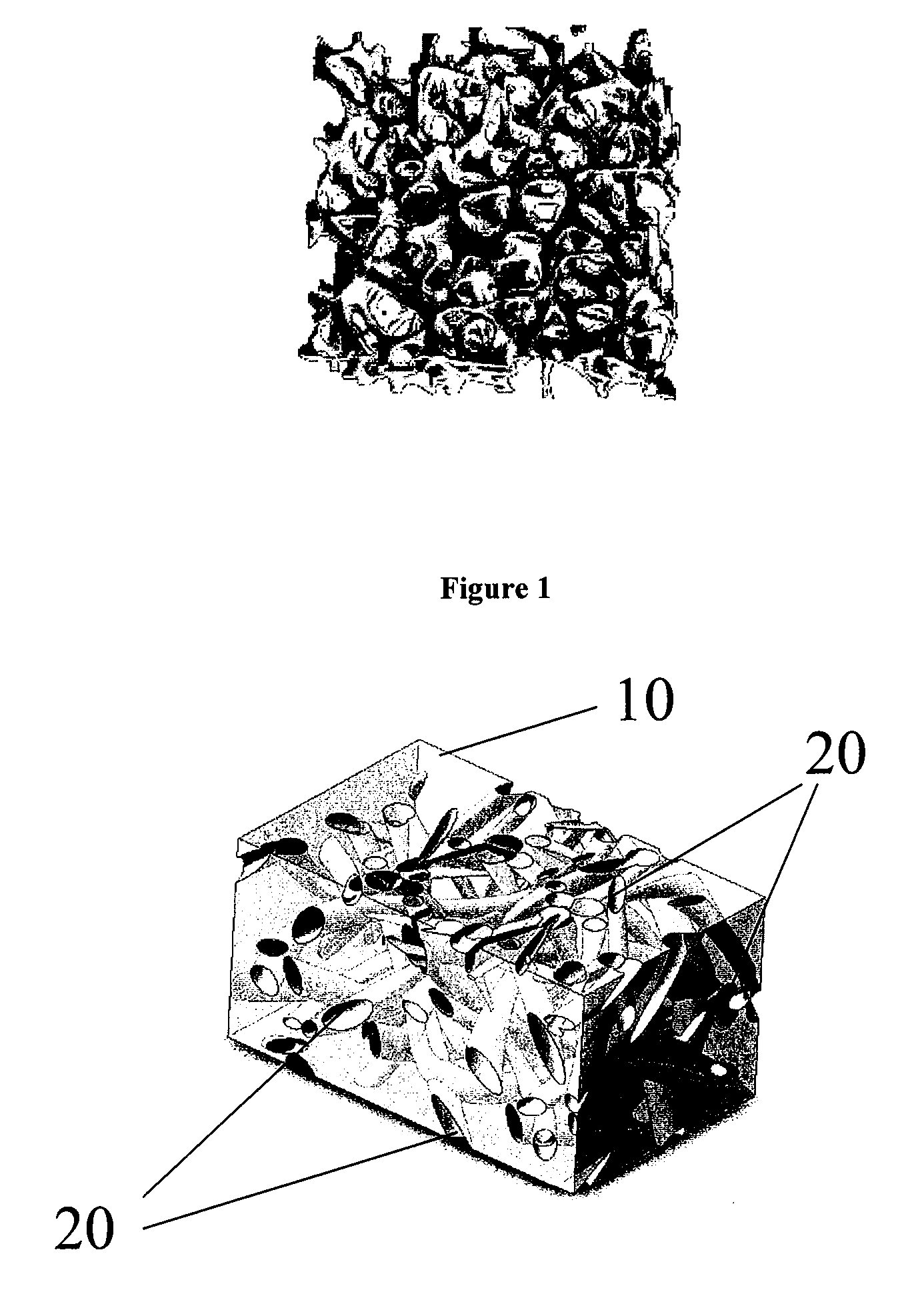
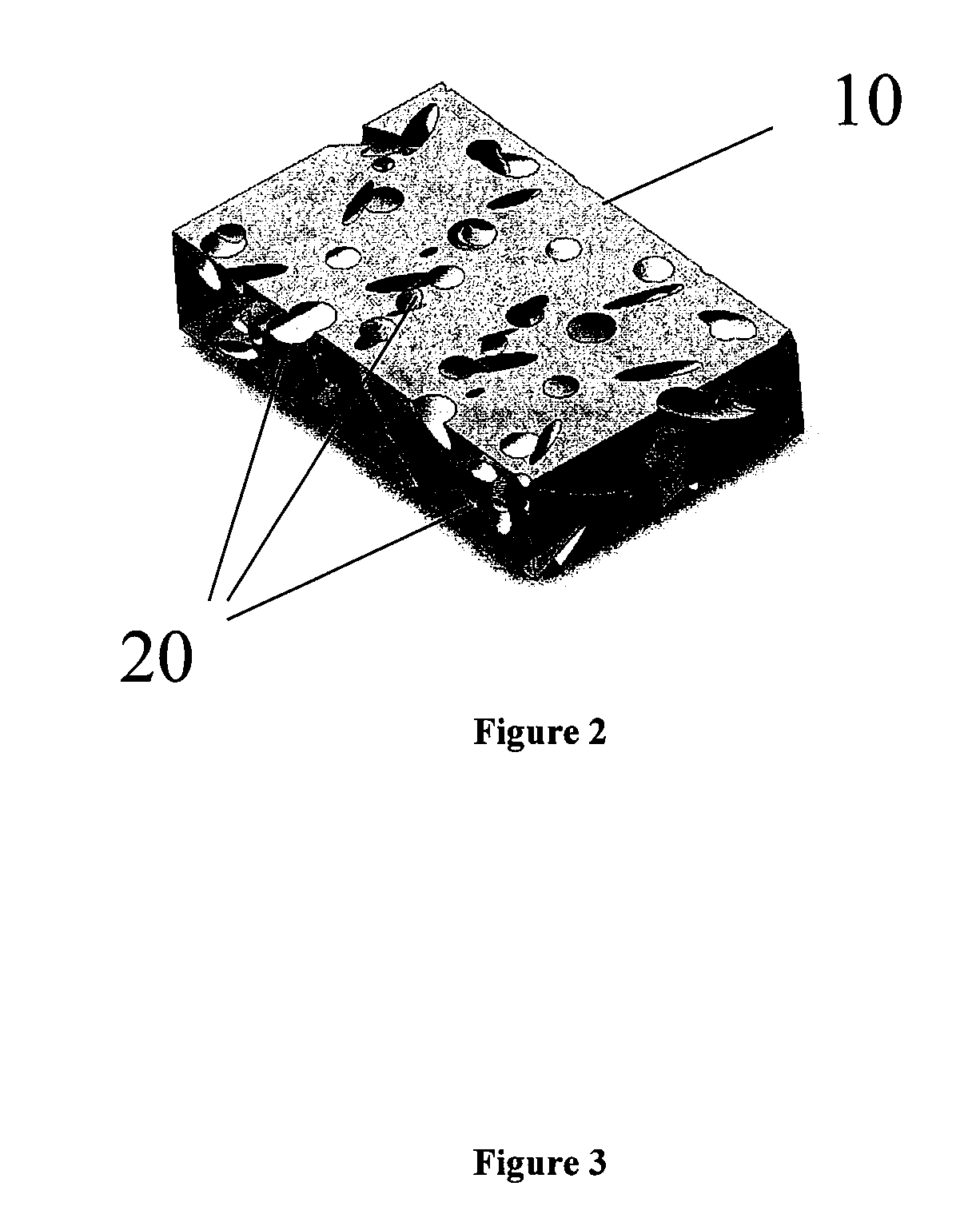
Reinforced, laminated, impregnated, and materials with composite properties as cross linked polyvinyl alcohol hydrogel structures in bulk or cellular matrix forms that can take essentially any physical shape, or can have essentially any size, degree of porosity and surface texture. They have a wide range of physical properties, unusual and unique combinations of physical properties and unique responses to stress fields, which allows for their use in many end use applications.
Owner:HYDROMEDICA
Miniaturized cell array methods and apparatus for cell-based screening
InactiveUS7160687B1Amount of timeImprove throughputOptical radiation measurementBioreactor/fermenter combinationsToxin detectionA domain
The present invention describes methods and cassettes for cell-based toxin detection and organ localization. The cassettes includes an array containing cells and a matrix of openings or depressions, wherein each region of the substrate enclosed by the opening or depression in the matrix forms a domain individually addressable by microfluidic channels in the device.
Owner:CELLOMICS +1
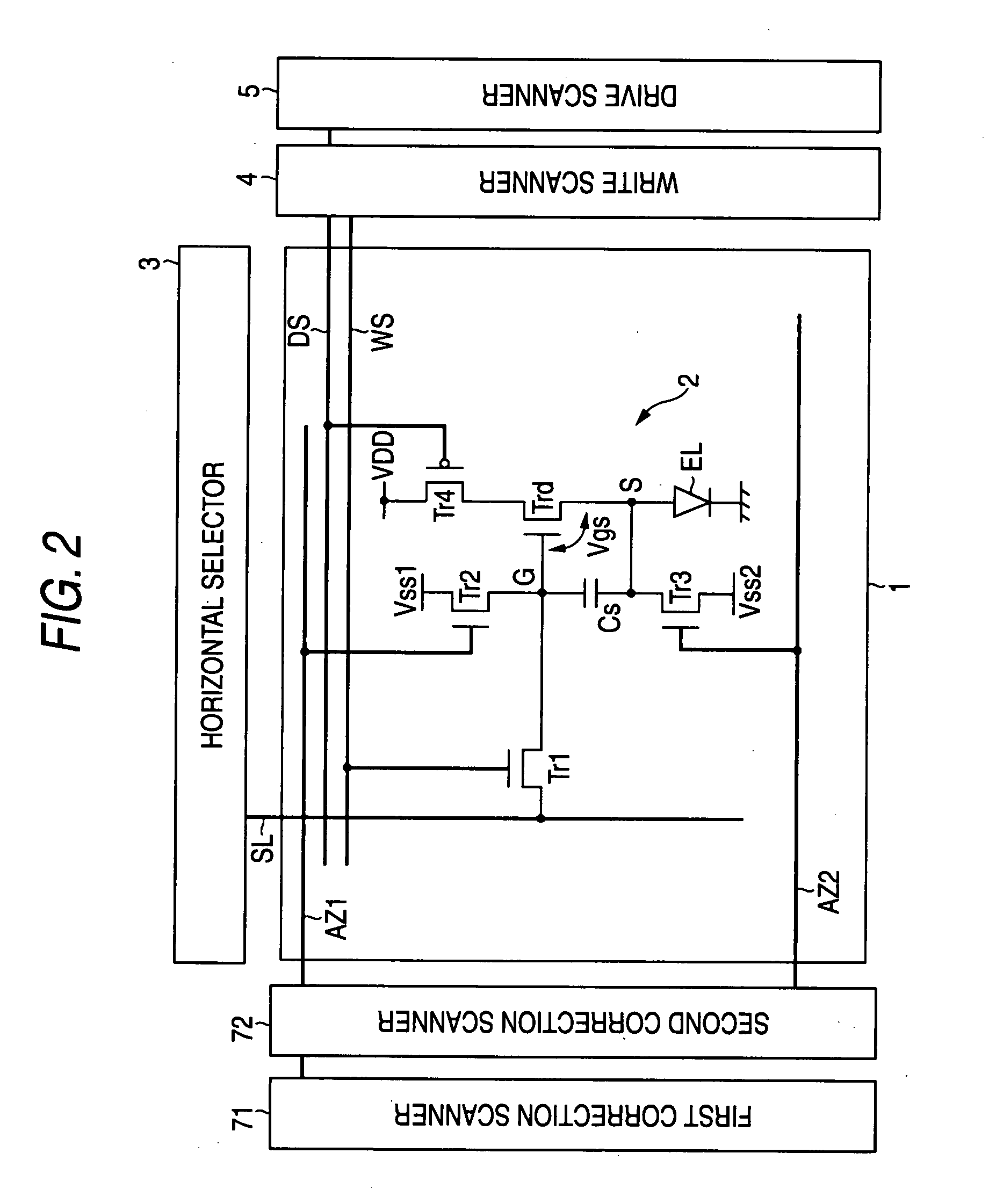
Display device and driving method thereof
InactiveUS20080198103A1Slow effectSuppress brightness changesElectrical apparatusElectroluminescent light sourcesDisplay deviceEngineering
Disclosed herein is a display device including: a pixel array unit having pixel circuits arranged in a form of a matrix; and a control unit having a writing scanning unit for outputting, to the sampling transistor, a writing scanning pulse. The control unit effects control to supply a control input terminal of the drive transistor with a fixed potential for a threshold value correcting operation for retaining a voltage corresponding to a threshold voltage of the drive transistor in the storage capacitor. When setting a voltage across the storage capacitor to the threshold voltage of the drive transistor by repeating the threshold value correcting operation a plurality of times on a time division basis, the control unit effects control to perform each the threshold value correcting operation and the sampling transistor to a conducting state.
Owner:SONY CORP
Direct-view MEMS display devices and methods for generating images thereon
InactiveUS20070205969A1Improve image qualityReduce power consumptionCathode-ray tube indicatorsOptical elementsData setDisplay device
A direct-view display includes an array of MEMS light modulators and a control matrix formed on a transparent substrate, where each light modulator can be driven into at least two states, and a controller for controlling the states of each light modulator in the array. The control matrix transmits data and actuation voltages to the array. The controller includes an input, a processor, a memory, and an output. The input receives image data encoding an image frame for display. The processor derives a plurality of sub-frame data sets from the image data, where each sub-frame data set indicates desired states of light modulators in multiple rows and multiple columns of the array. The memory stores the plurality of sub-frame data sets. The output outputs the plurality of sub-frame data sets according to an output sequence to drive light modulators into the states indicated in the sub-frame data sets.
Owner:SNAPTRACK
Touch and proximity sensitive display panel, display device and touch and proximity sensing method using the same
InactiveUS20110025635A1Reduce thicknessLow costEnergy efficient ICTElectrical apparatusCapacitanceTouch Senses
A touch and proximity sensitive display panel, a display device, and a touch and proximity sensing method using the same are disclosed. The display panel includes a plurality of pixels arranged in a matrix form, a pixel substrate having a pixel electrode arranged in an image output direction, a common substrate having a common electrode arranged at a position facing the pixels, and a panel controller that identifies touch and proximity positions of a touch object by sensing electrostatic capacitances of the pixel electrodes through the data lines in a touch-sensing mode. The display panel can sense the touch and proximity of the touch object without an additional touch screen.
Owner:ATLAB INC
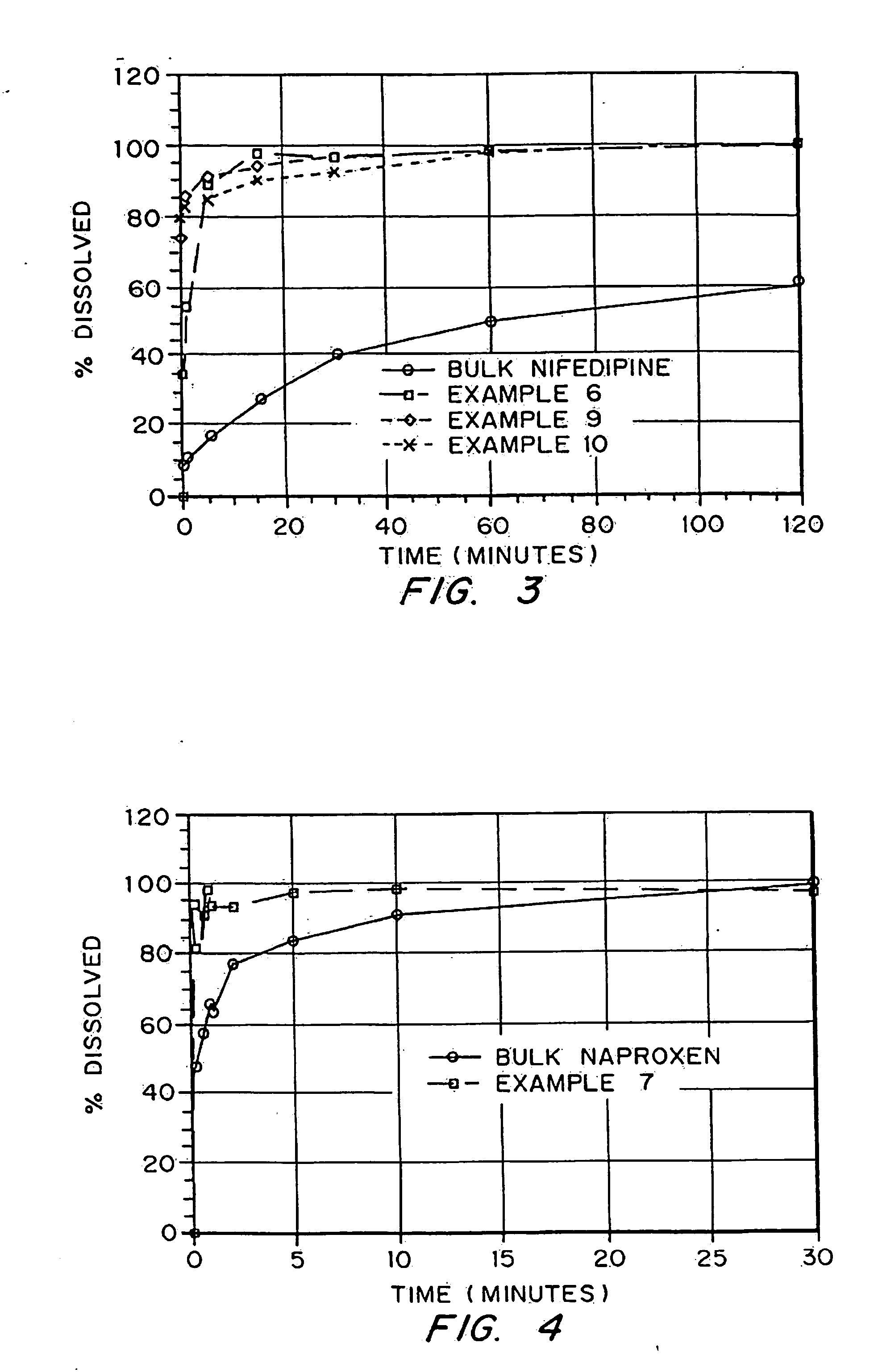
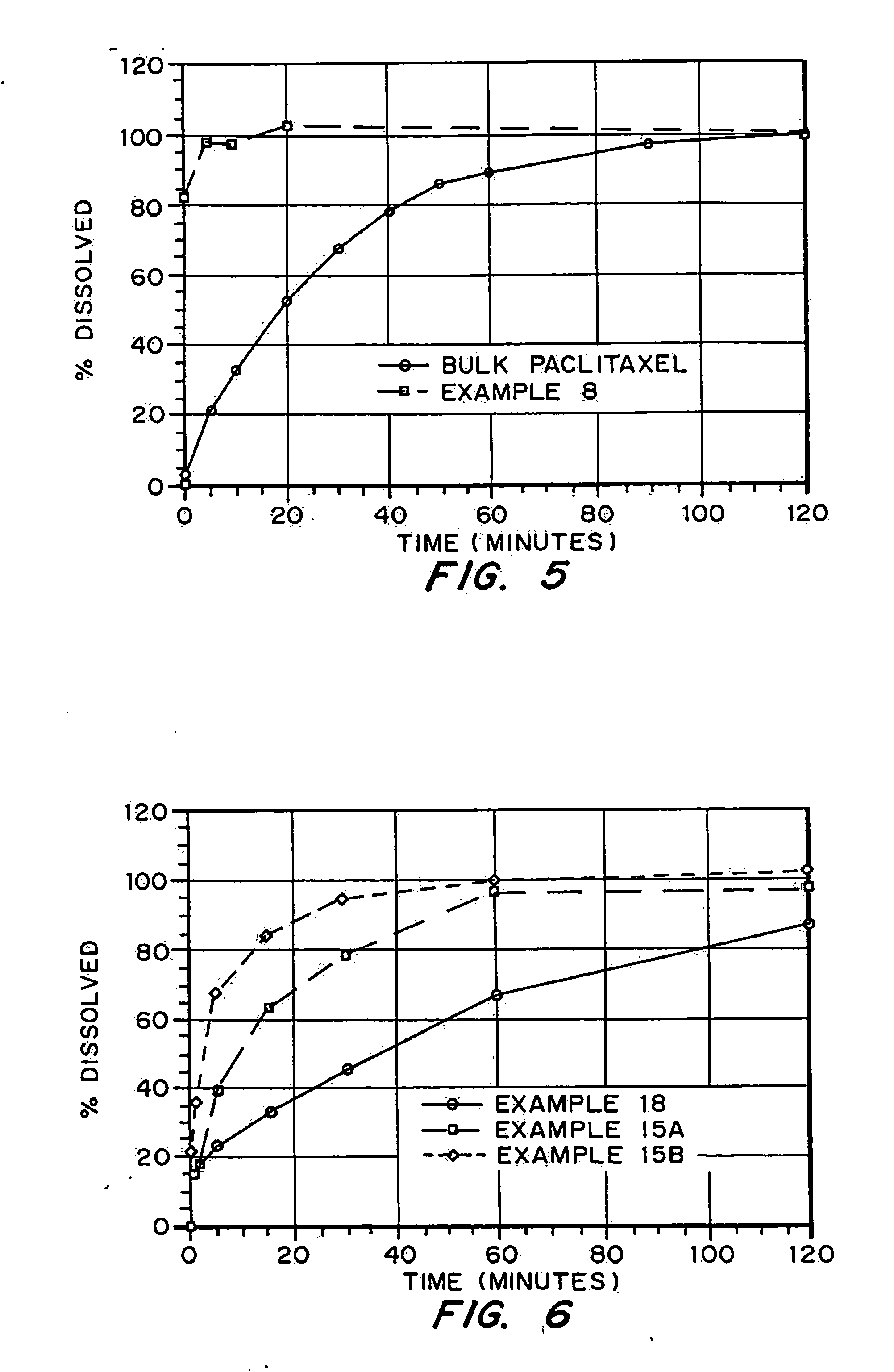
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
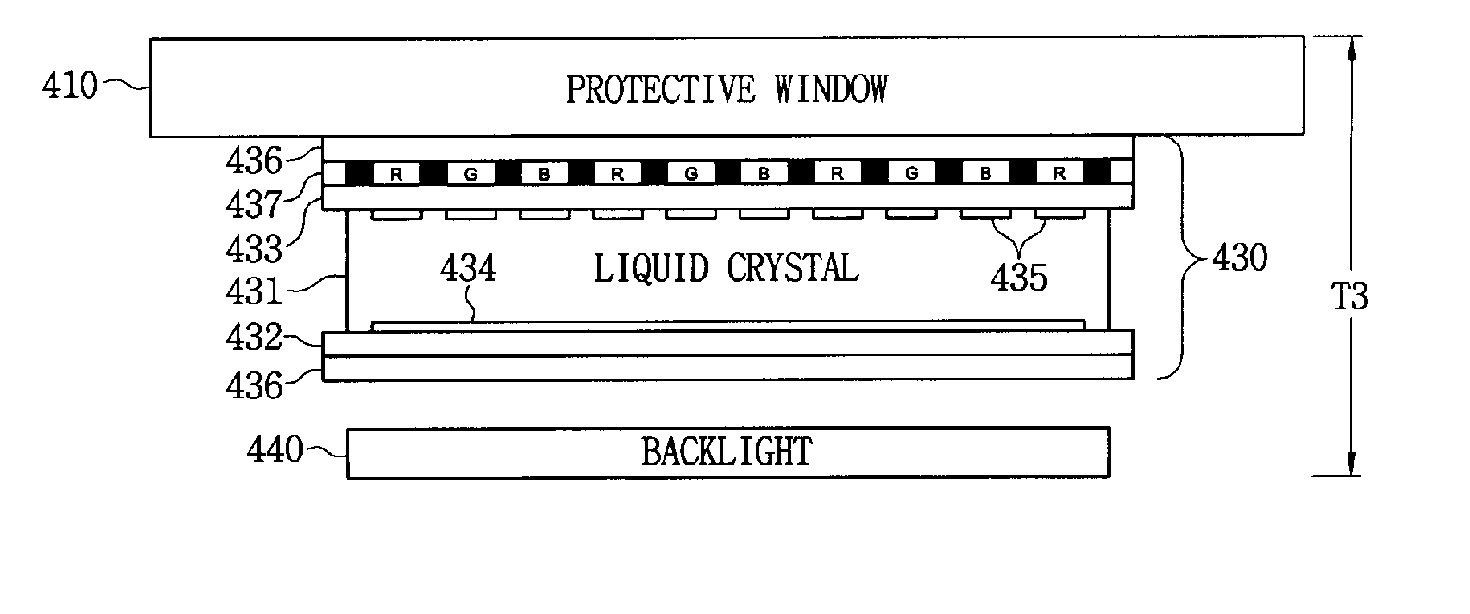
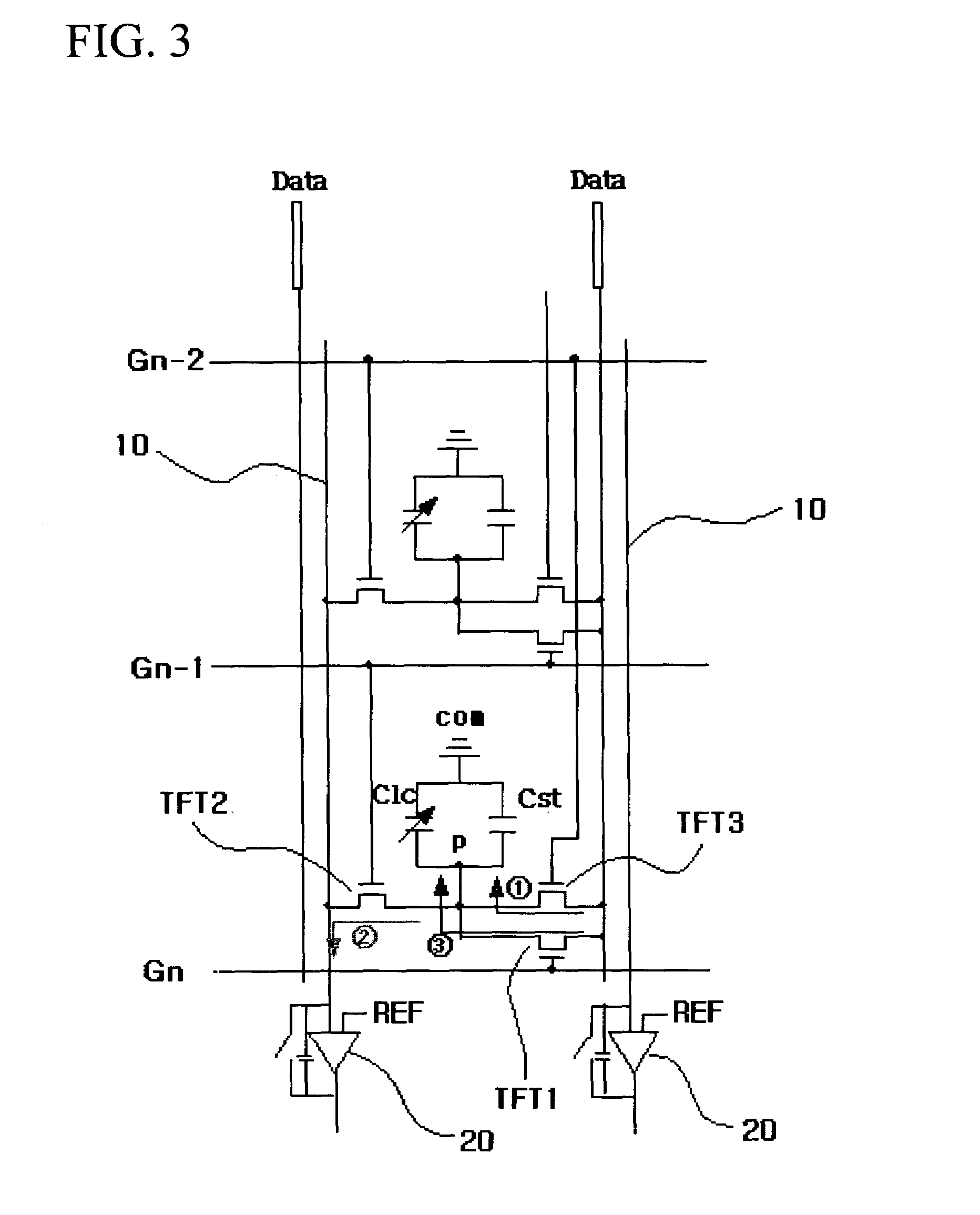
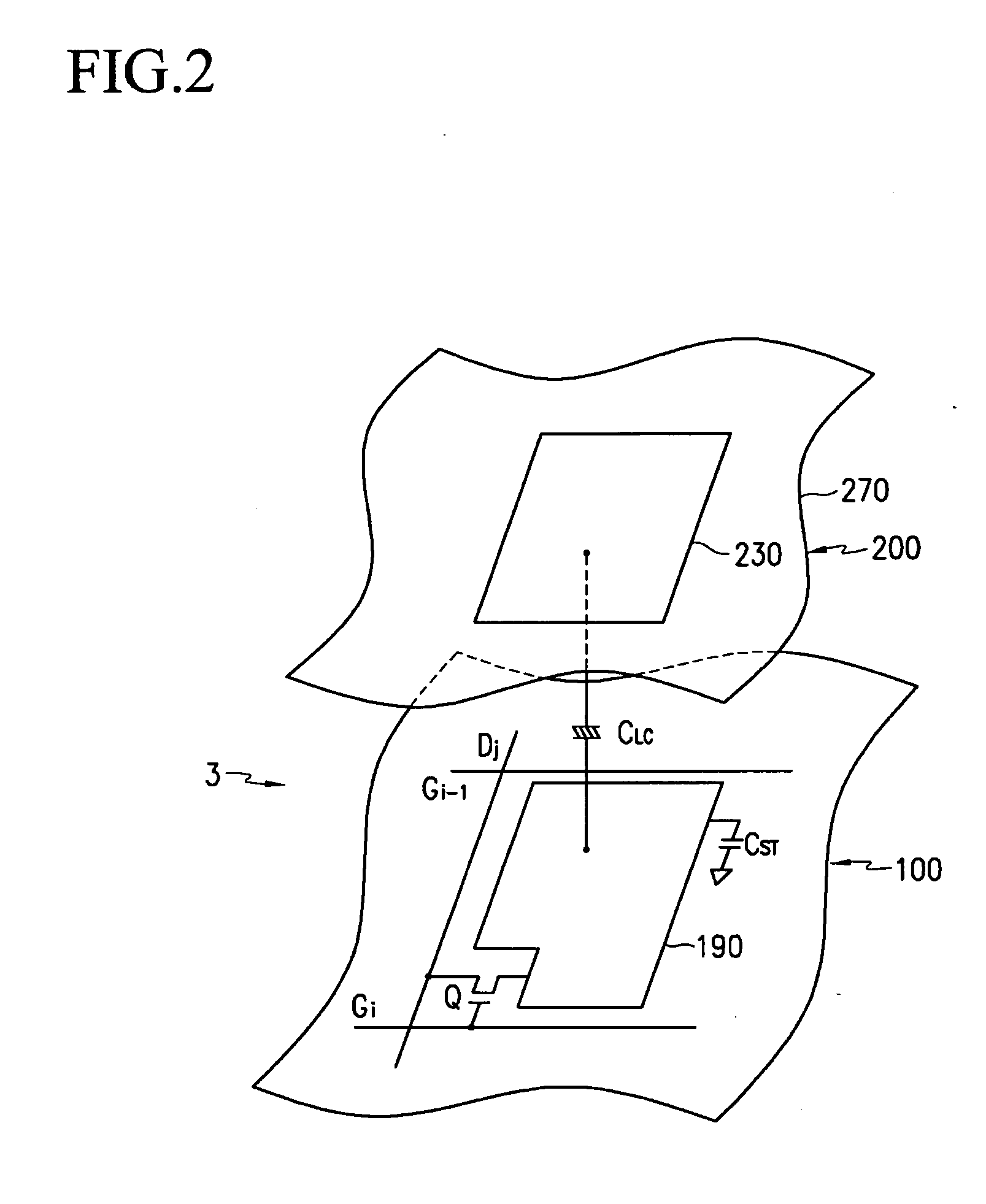
Liquid crystal display device having touch screen function and method of fabricating the same
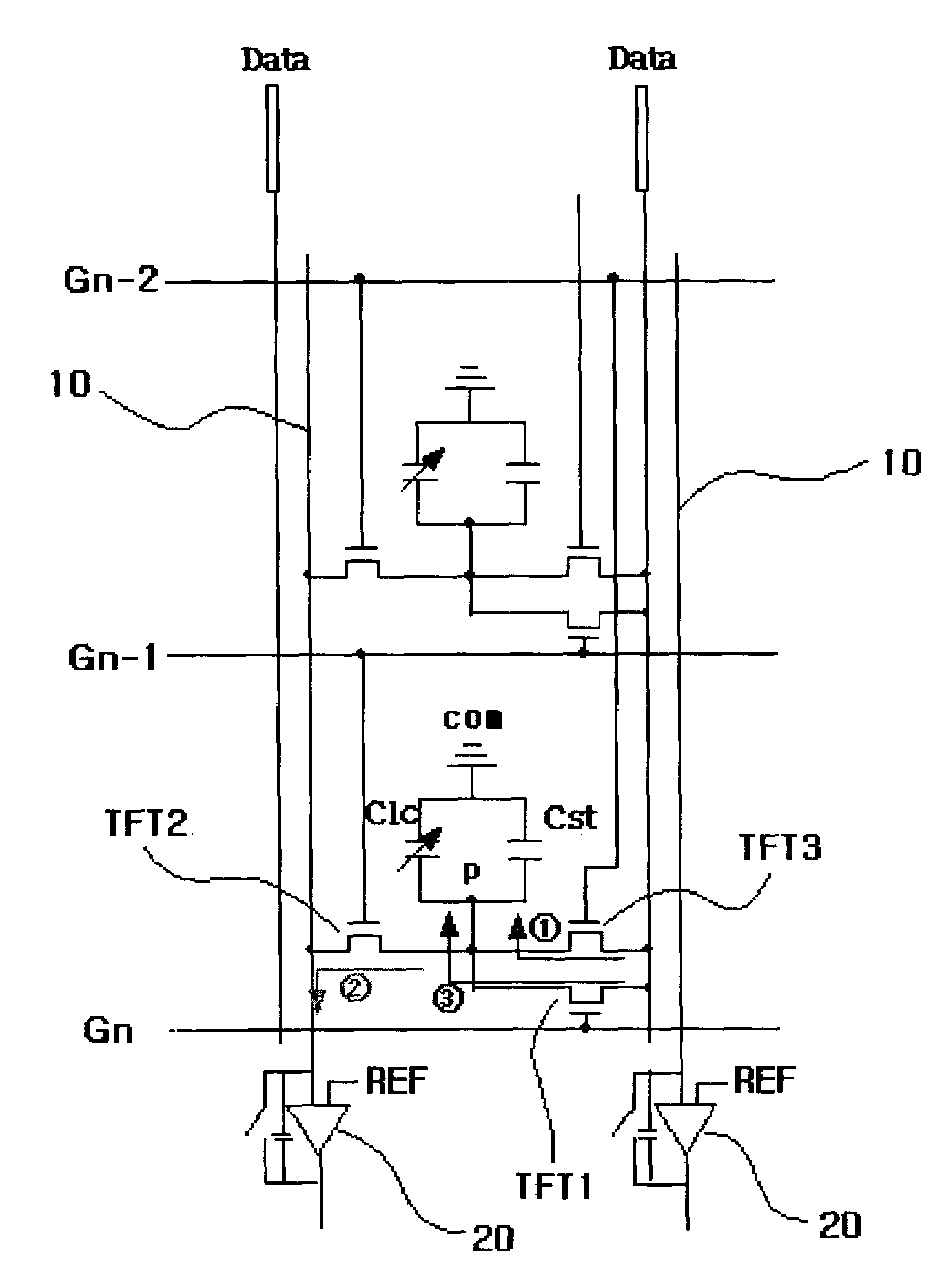
The present invention relates to an LCD device having a touch screen function. The LCD device of the present invention includes a plurality of gate lines, a plurality of data lines intersecting the gate lines, and a plurality of signal lines that are insulated from and juxtaposed with the data lines. First to third switching elements are formed in each of a plurality of pixel regions in the form of a matrix, which are surrounded by the gate lines and the data lines. Here, a gate electrode of the first switching element is connected to a gate line Gn, a source electrode thereof is connected to the data line, and a drain electrode thereof is connected to a pixel electrode. Further, liquid crystal capacitance and storage capacitance are formed between the pixel electrode and a common electrode. At this time, the liquid crystal capacitance is changed due to variation in the liquid crystal cell gap. The second and third switching elements are designed to read variation in the liquid crystal capacitance. A source electrode of the second switching element is connected to the pixel electrode, a drain electrode thereof is connected to the signal line, and a gate electrode thereof is connected to a previous gate line Gn-1. Furthermore, a source electrode of the third switching element is connected to the data line, a drain electrode thereof is connected to the pixel electrode, and a gate electrode thereof is connected to a second previous gate line Gn-2. Each signal line is connected to each signal amplifier.
Owner:SAMSUNG DISPLAY CO LTD
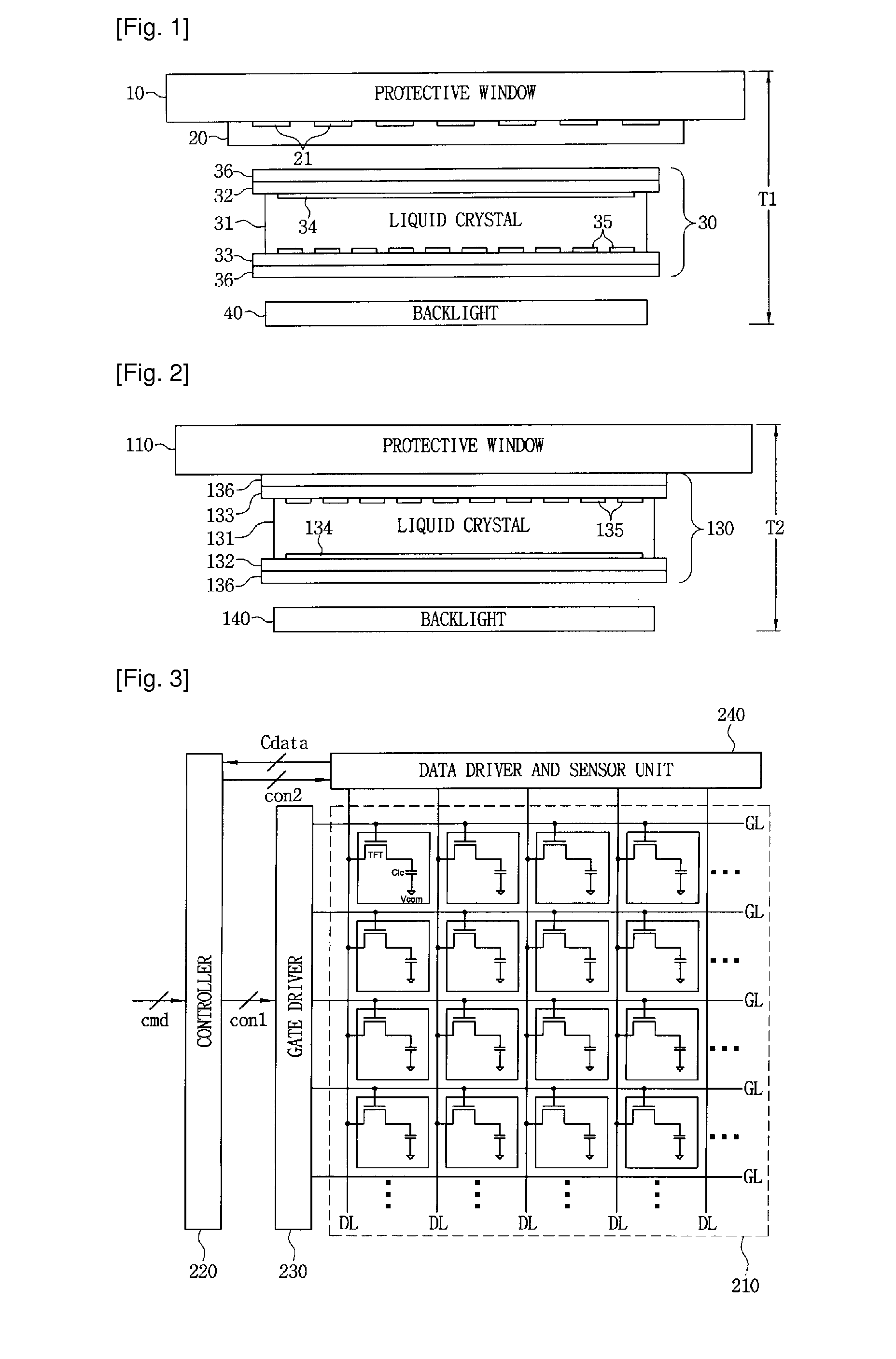
Display device
ActiveUS20090046077A1Stable touch panel operationSuppress couplingNon-linear opticsInput/output processes for data processingCapacitanceCapacitive coupling
A display device includes: a first substrate and a second substrate disposed to face each other; a display medium layer interposed between the first substrate and the second substrate; a plurality of pixel electrodes arranged in a matrix between the first substrate and the display medium layer; a first transparent electrode, interposed between the second substrate and the display medium layer, for detecting a touched location; and a second transparent electrode, interposed between the first transparent electrode and the display medium layer, for receiving a display signal. The display device detects the touched location using a capacitive coupling method and displays an image. A shield electrode for suppressing capacitive coupling is formed between the first transparent electrode and the second transparent electrode.
Owner:SHARP KK
Capacitance type touch panel
ActiveUS20090096760A1Non-linear opticsInput/output processes for data processingCapacitanceLiquid-crystal display
The present invention relates to a liquid crystal display (LCD). The LCD includes a plurality of display units formed with a first substrate, a color matrix formed on the first substrate, and a common electrode formed on the color matrix, a second substrate spaced from the first substrate, a pixel electrode matrix formed on the second substrate, a liquid crystal material disposed between the common electrode and the pixel electrode matrix. The LCD includes a touch sensing member integrated onto the color matrix of the first substrate.
Owner:AU OPTRONICS CORP
Biodegradable therapeutic implant for bone or cartilage repair
The exemplary embodiments of the present invention relate to an at least partially biodegradable implant suitable for implantation into a subject for repairing a bone or cartilage defect, comprising a matrix forming an open-celled structure having a plurality of interconnected spaces, whereas the channels of the matrix are substantially completely filled with metallic material particles, and wherein at least one of the metallic material or the matrix material is at least partially degradable in-vivo. Furthermore, the present invention relates to a method for repairing a bone or cartilage defect in a living organism, comprising implanting an implant according to the exemplary embodiments of the present invention into the defective bone or cartilage, or replacing the defective bone or cartilage at least partially.
Owner:CINVENTION AG
Non-rectangular display apparatus
ActiveUS20080266210A1Reduce distractionsIncrease in sizeStatic indicating devicesDigital storageActive matrixScan line
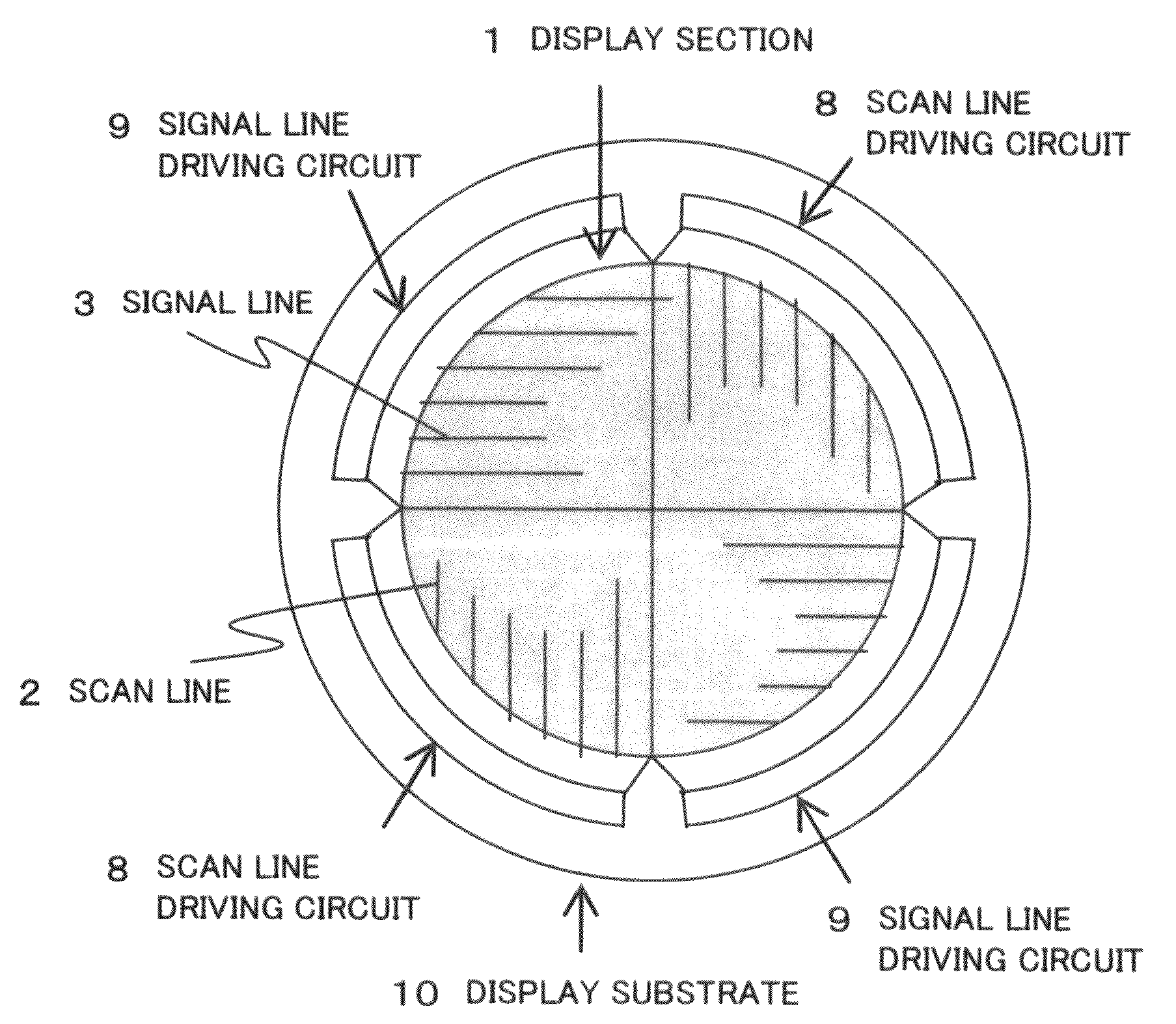
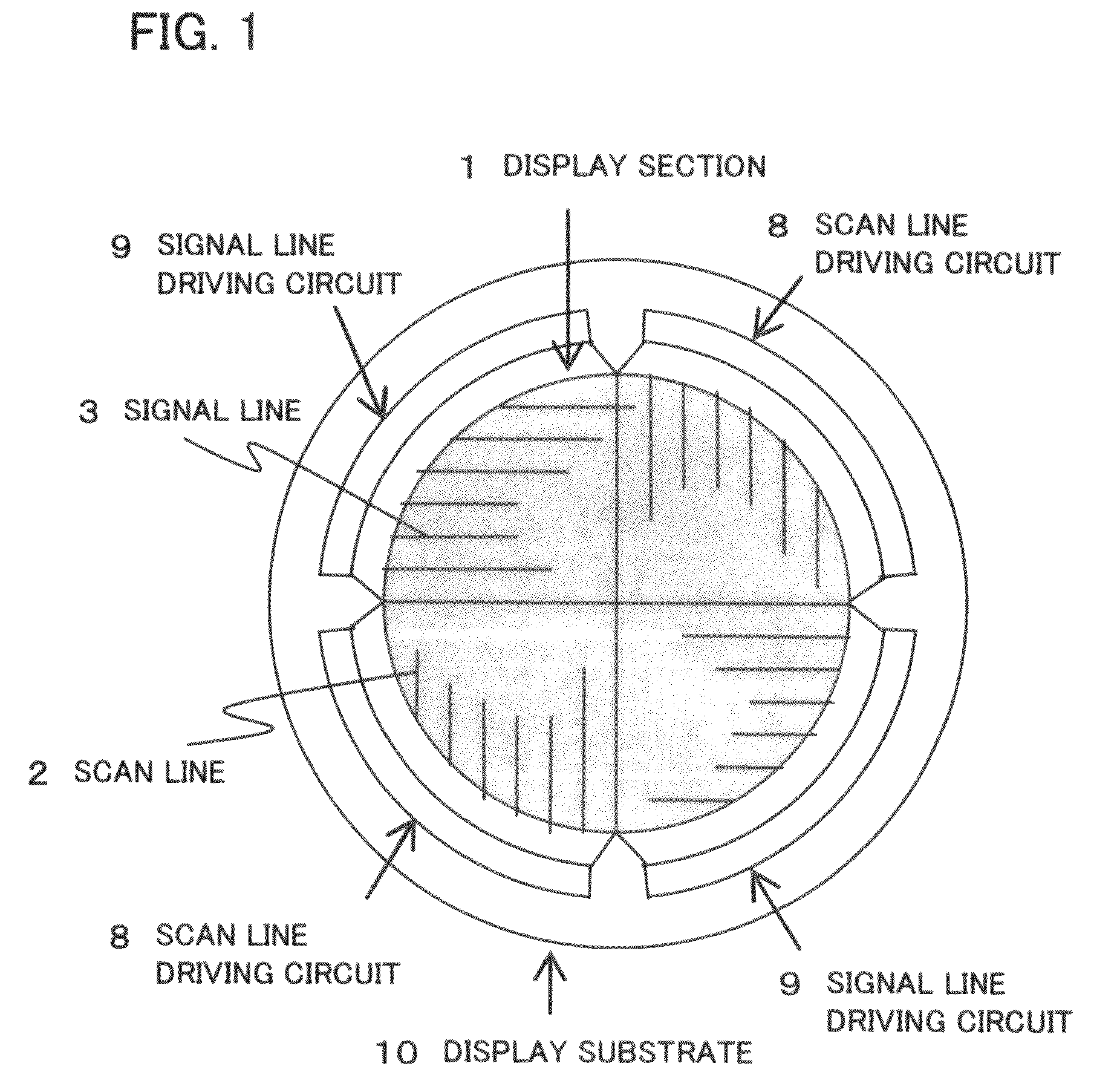
Disclosed is a display apparatus comprising an active matrix display section including a plurality of signal lines and scan lines, arranged in a matrix on a substrate, and a plurality of pixels and active elements arranged at intersections of the signal and scan lines, a scan line driving circuit for driving the scan lines and a signal line driving circuit for driving the signal lines. The display section has a non-rectangular shape. The active elements that make up the scan line driving circuit and / or the signal line driving circuit are formed by the same manufacturing process as that for forming the active elements in the active matrix display section. The scan line driving circuit and / or the signal line driving circuit are each a set of circuit units having the same function. These circuit units are arranged to conform to and extend around the outer circumference of the non-rectangular display section.
Owner:TIANMA MICRO ELECTRONICS CO LTD
Porous drug matrices and methods of manufacture thereof
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Display device
InactiveUS7067985B2Incadescent screens/filtersElectric discharge tubesDisplay deviceLight emitting device
Owner:SAMSUNG DISPLAY CO LTD +1
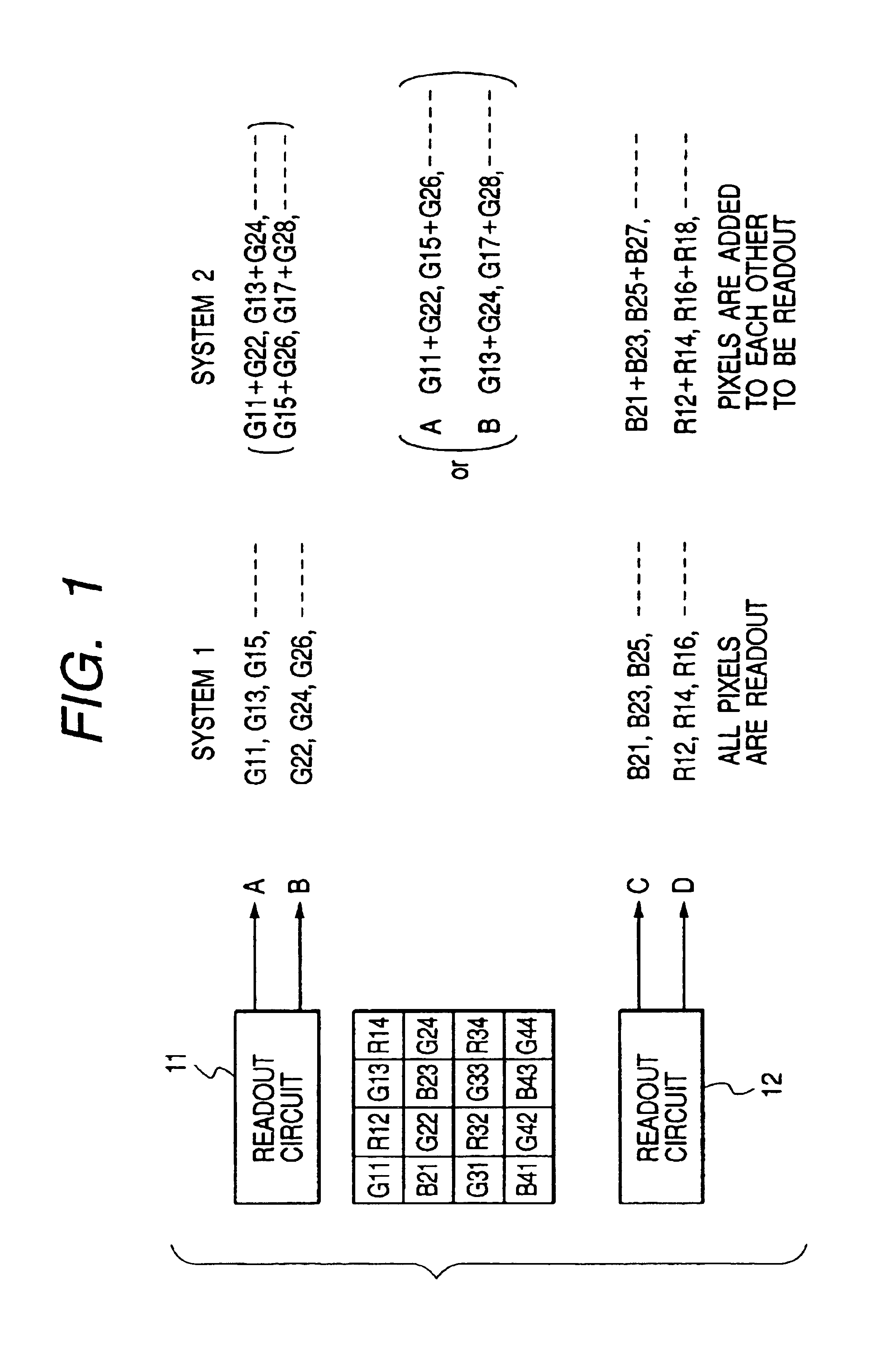
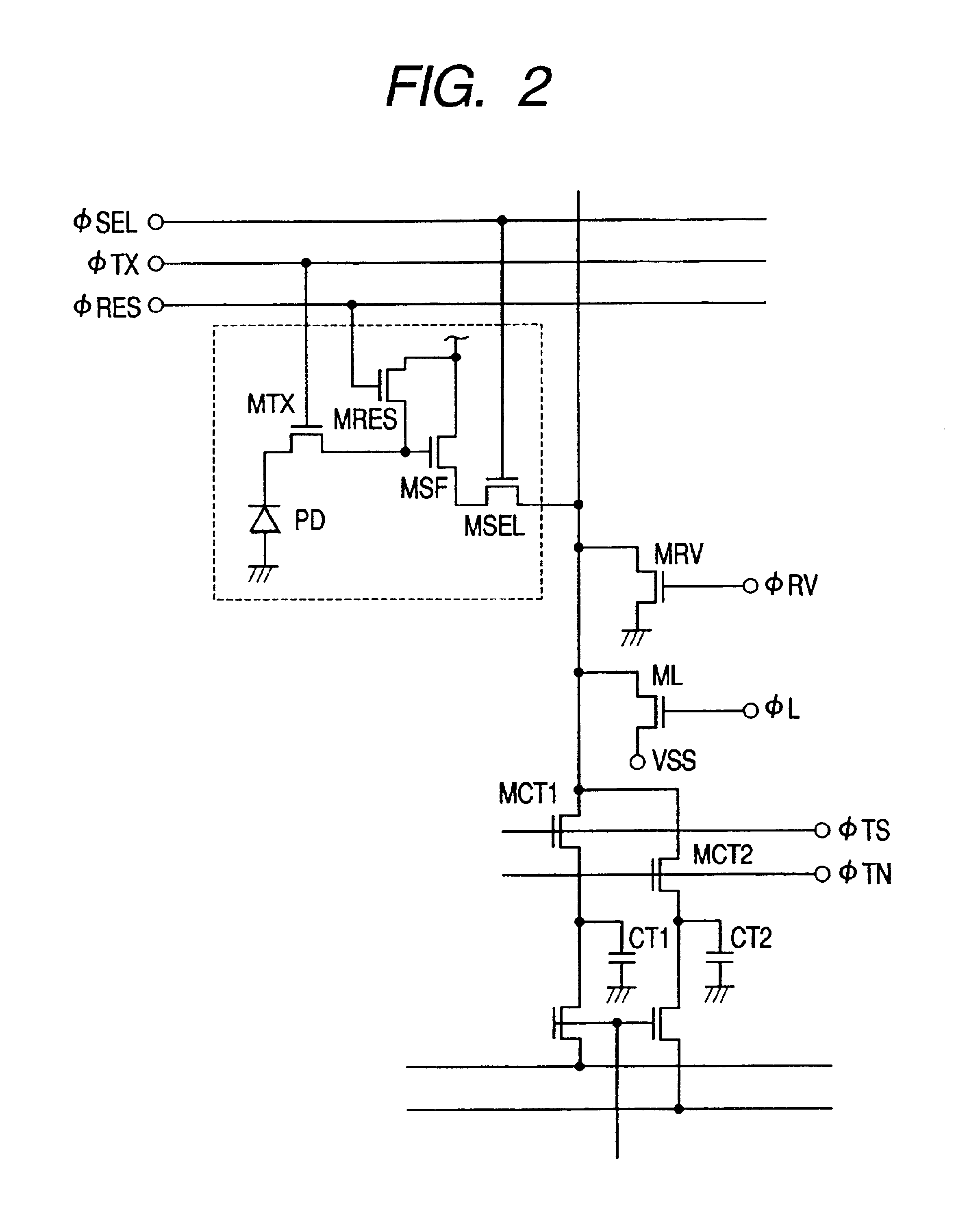
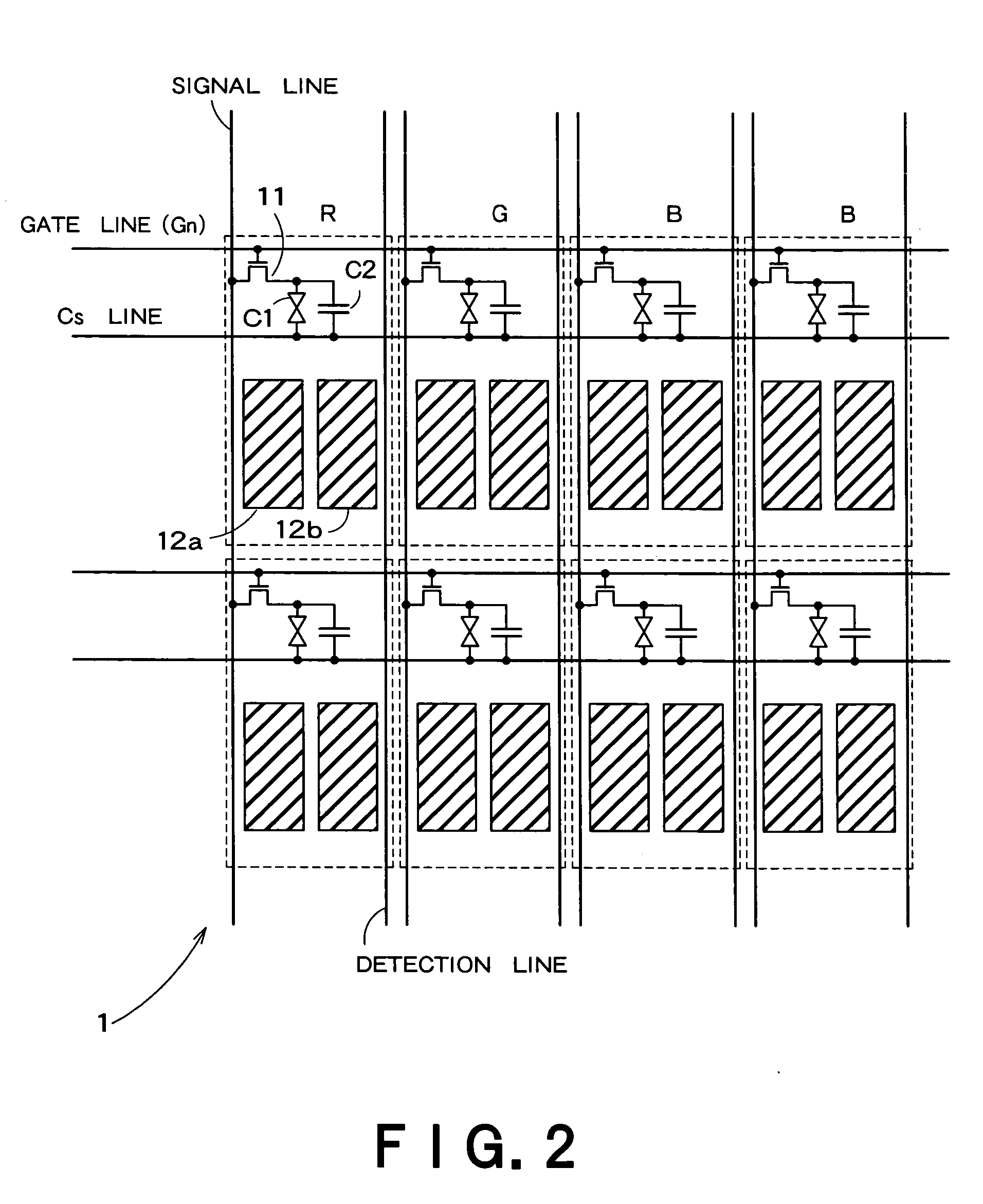
Image pickup apparatus having plural pixels arranged two-dimensionally, and selective addition of different pixel color signals to control spatial color arrangement
InactiveUS6992714B1Preferable image qualityTelevision system detailsTelevision system scanning detailsColor filter arrayComputer science
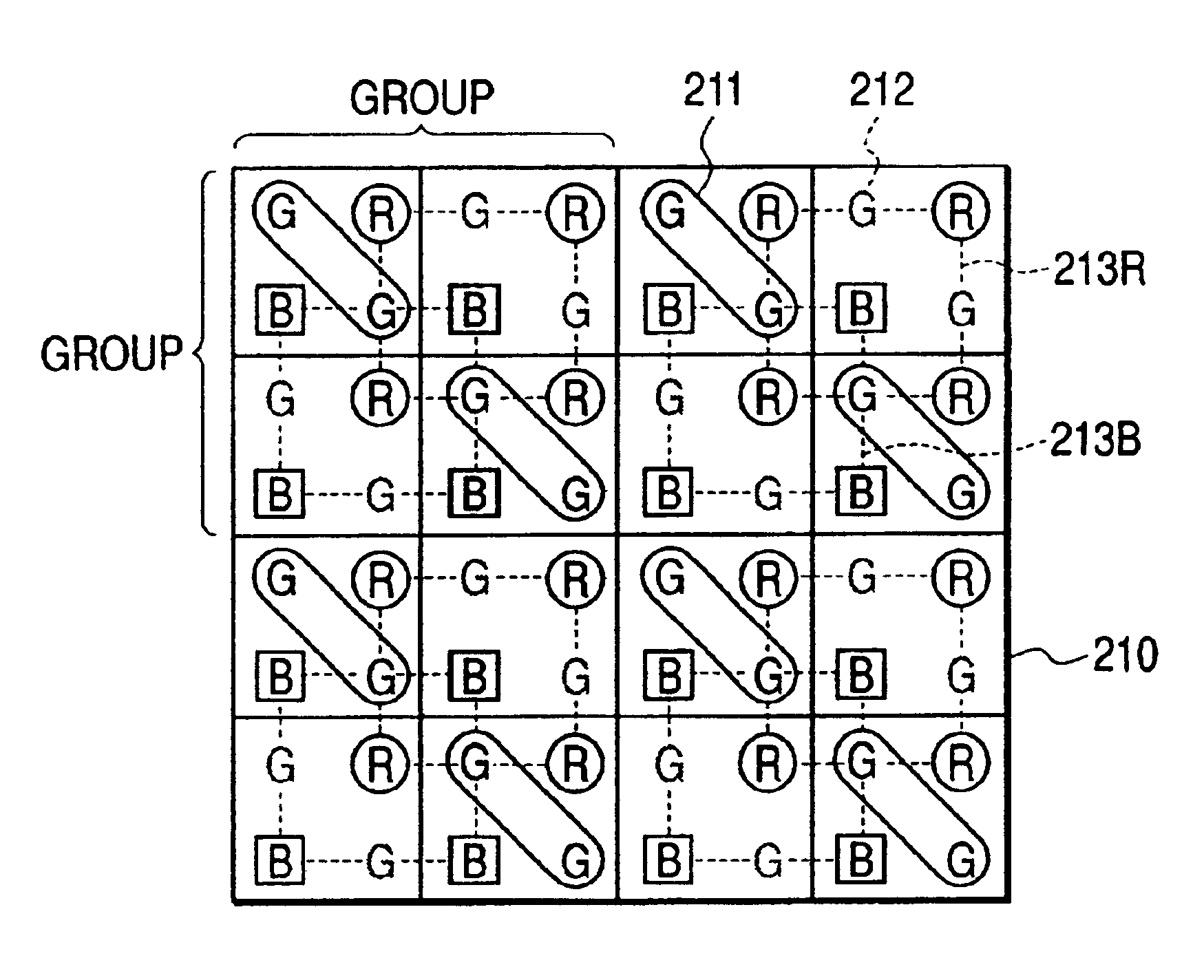
An image pickup apparatus comprises a plurality of pixels arranged in a matrix form, first color filters arranged in an oblique direction for the pixels, second and third color filters arranged so that a same color is arranged in a horizontal direction, a circuit for adding together signals of two or more pixels each having the first color filter, which are adjacent to each other in an oblique direction, and a circuit for adding together the signals of two or more pixels each having the second color filter, which are arranged in a horizontal direction, and for adding together the signals of two or more pixels each having the third color filter, which are arranged in the horizontal direction.
Owner:CANON KK
Method and system for optimally searching a document database using a representative semantic space
InactiveUS7483892B1Data processing applicationsDigital data information retrievalSingular value decompositionSubject matter
A term-by-document matrix is compiled from a corpus of documents representative of a particular subject matter that represents the frequency of occurrence of each term per document. A weighted term dictionary is created using a global weighting algorithm and then applied to the term-by-document matrix forming a weighted term-by-document matrix. A term vector matrix and a singular value concept matrix are computed by singular value decomposition of the weighted term-document index. The k largest singular concept values are kept and all others are set to zero thereby reducing to the concept dimensions in the term vector matrix and a singular value concept matrix. The reduced term vector matrix, reduced singular value concept matrix and weighted term-document dictionary can be used to project pseudo-document vectors representing documents not appearing in the original document corpus in a representative semantic space. The similarities of those documents can be ascertained from the position of their respective pseudo-document vectors in the representative semantic space.
Owner:KLDISCOVERY ONTRACK LLC
Full-color organic electroluminescence display
InactiveUS20060033422A1Easy to manufactureGuaranteed flatnessDischarge tube luminescnet screensElectroluminescent light sourcesDisplay deviceOrganic electroluminescence
A full-color organic electroluminescence (OEL) display panel includes a substrate and a plurality of full-color OEL pixel devices in a matrix form as a display frame. Each of the pixel devices is composed of a plurality of sub-pixel regions, corresponding to R, G or B colors. Each of the specific color sub-pixel regions in the pixel device abuts the same specific color sub-pixel region of the adjacent pixel device thereof to form a double-sized area. With this arrangement of sub-pixel regions, it is easier to manufacture high-resolution full-color OLED panels by a metal-mask alignment process and high-resolution full-color PLED panels by an ink-jet printing process.
Owner:WORLDLED
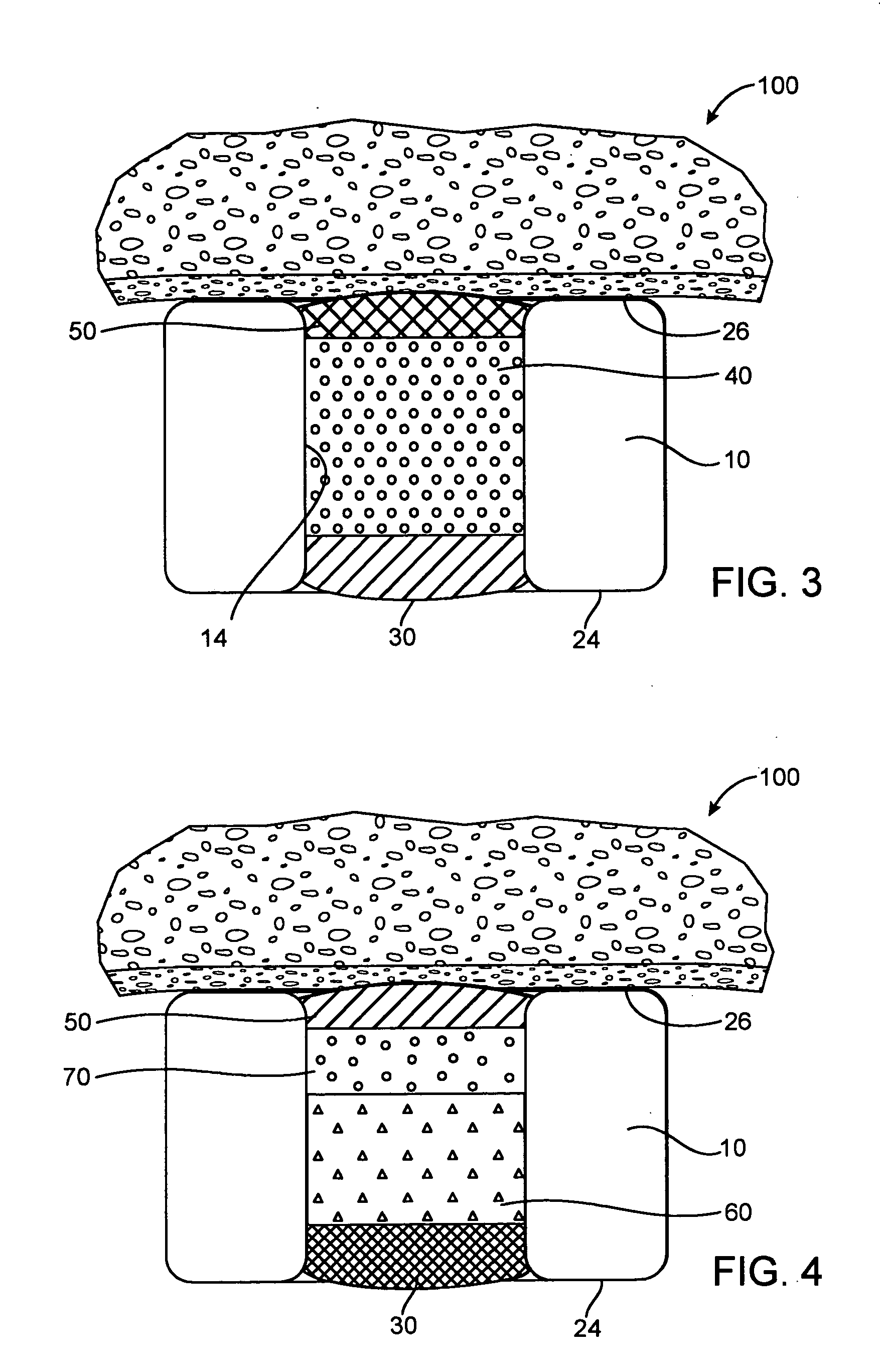
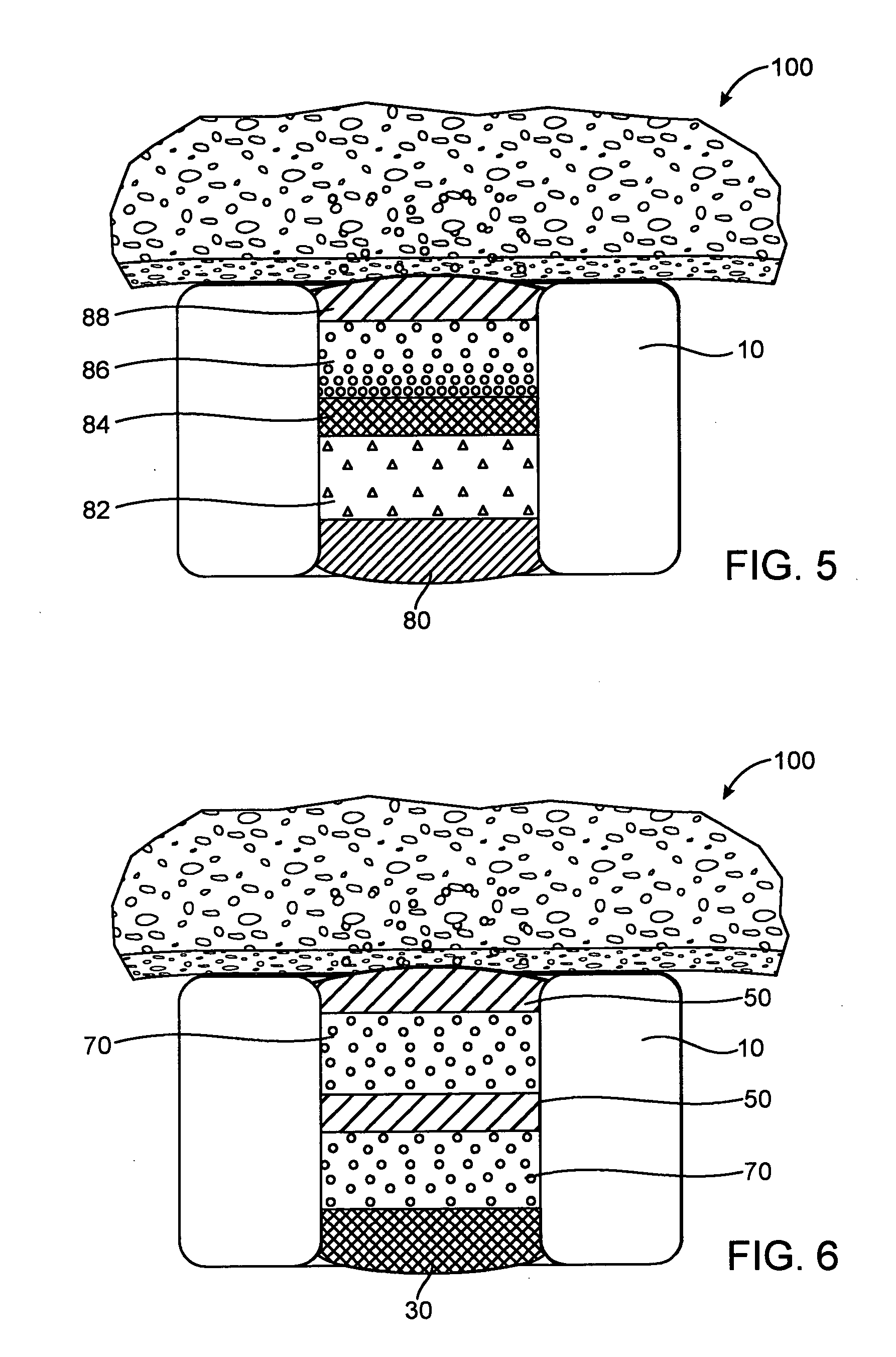
Expandable medical device with beneficial agent matrix formed by a multi solvent system
A multi solvent drug delivery matrix formation method is used to place layers into a reservoir in a stent in a stepwise manner to achieve extended delivery of water soluble, sensitive, or difficult to deliver drugs. The multi solvent matrix formation method allows the formation of a drug reservoir with a layered morphology in which the mixing between layers is limited to allow the different layers to perform different functions in controlling drug delivery. A stent having a drug delivery matrix includes a first beneficial agent layer affixed to the stent by depositing a first solution of a first polymer and a first solvent, and a second beneficial agent layer affixed to the first beneficial agent layer by depositing a second solution of a second polymer and a second solvent. The second solvent is selected so that the first polymer is substantially insoluble in the second solvent to prevent degradation of the first polymer during deposition of the second polymer. A therapeutic agent is provided in the first beneficial agent layer or the second beneficial agent layer to form a drug delivery matrix.
Owner:INNOVATIONAL HLDG LLC
Membranes with Embedded Nanotubes For Selective Permeability
ActiveUS20100025330A1Good membrane permeabilityHigh selectivityCeramic shaping apparatusReverse osmosisFiltrationNanometre
Membranes for filtration by size exclusion are formed from open-ended nanotubes embedded in a polymeric matrix. The matrix forms a layer whose thickness is substantially less than the average length of the nanotubes, allowing the nanotubes to be randomly oriented throughout the matrix while providing channels extending through the layer for the selective passage of molecular species or particles based on size.
Owner:NAGARE MEMBRANES
Thin film transistor array panel and display device
ActiveUS20060164350A1Low production costReduce the number of drivesStatic indicating devicesNon-linear opticsTransistor arrayDisplay device
Disclosed is a thin film transistor array panel. The panel includes a plurality of pixels arranged in the form of a matrix each with a pixel electrode and a switching element connected to the pixel electrode, and a plurality of gate lines connected to the switching elements and extending in the row direction. A pair of the gate lines are connected to pixels in each pixel row. A plurality of data lines are connected to the switching elements, and elongated in the column direction. Each data line is provided between two columns of the pixels. The respective data lines are horizontally bent between the two adjacent gate lines, and vertically extend between the two pixel rows.
Owner:TCL CHINA STAR OPTOELECTRONICS TECH CO LTD
Display device and photoelectric conversion device
ActiveUS20050045881A1Sufficient photoelectric current without further production costTransistorTelevision system scanning detailsDisplay deviceEngineering
A display device has display elements provided inside of pixels, each being formed in vicinity of intersections of signal lines and scanning lines aligned in matrix form; and photoelectric conversion elements, wherein each of the photoelectric conversion elements includes first, second and third semiconductor regions disposed adjacently in sequence in parallel to a surface of a substrate; a first electrode connected to the first semiconductor region; and a second electrode connected to the third semiconductor region, the first semiconductor region being formed by injecting a first conductive impurity in first dose amount; the third semiconductor region being formed by injecting a second conductive impurity in second dose amount; and the second semiconductor region being formed by injecting the first conductive impurity in third dose amount less than the first dose amount.
Owner:JAPAN DISPLAY CENTRAL CO LTD
Personalized blister pack
InactiveUS20090277815A1Method securityImprove securitySmall article dispensingContainer/bottle contructionPersonalizationEngineering
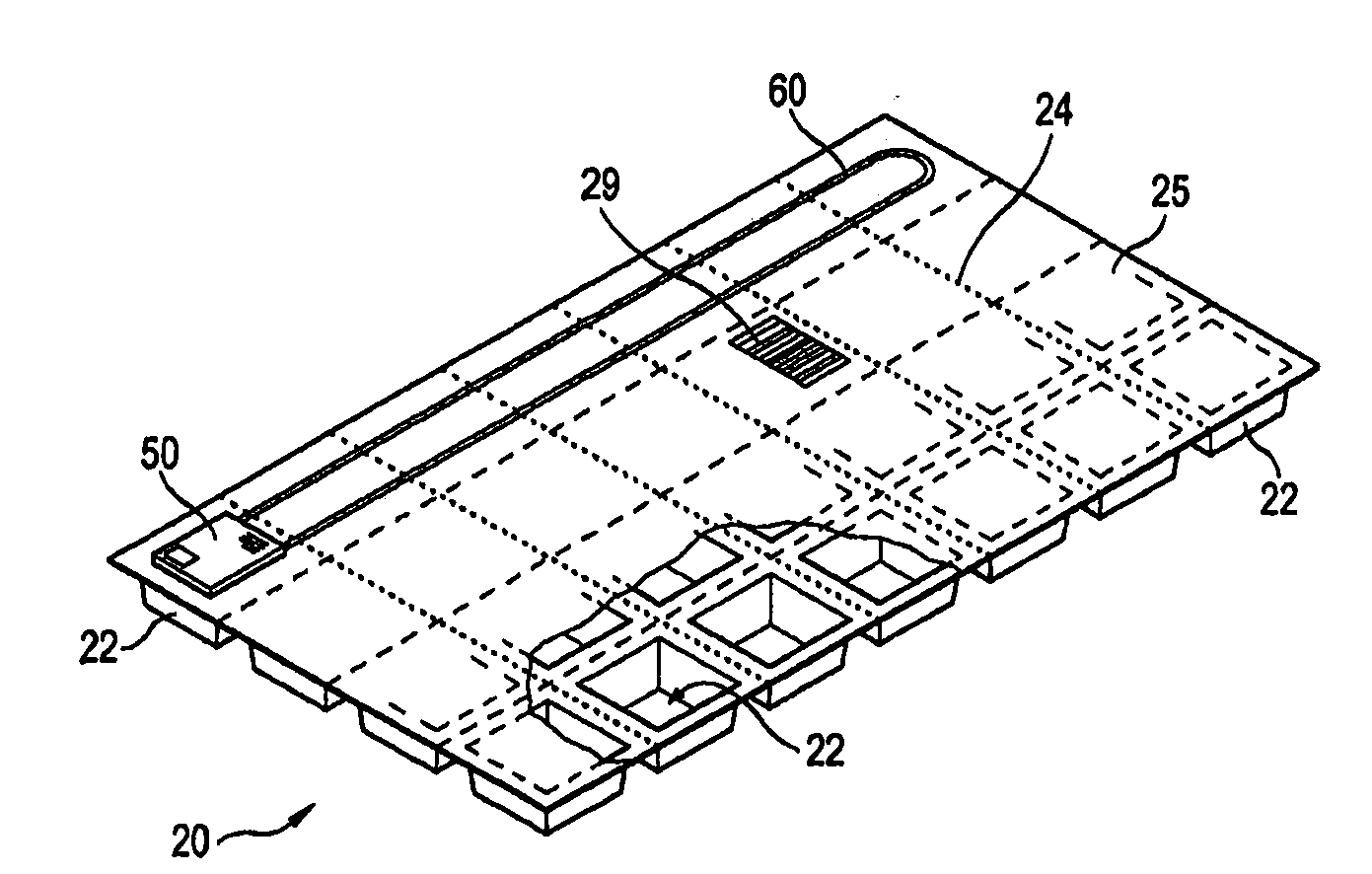
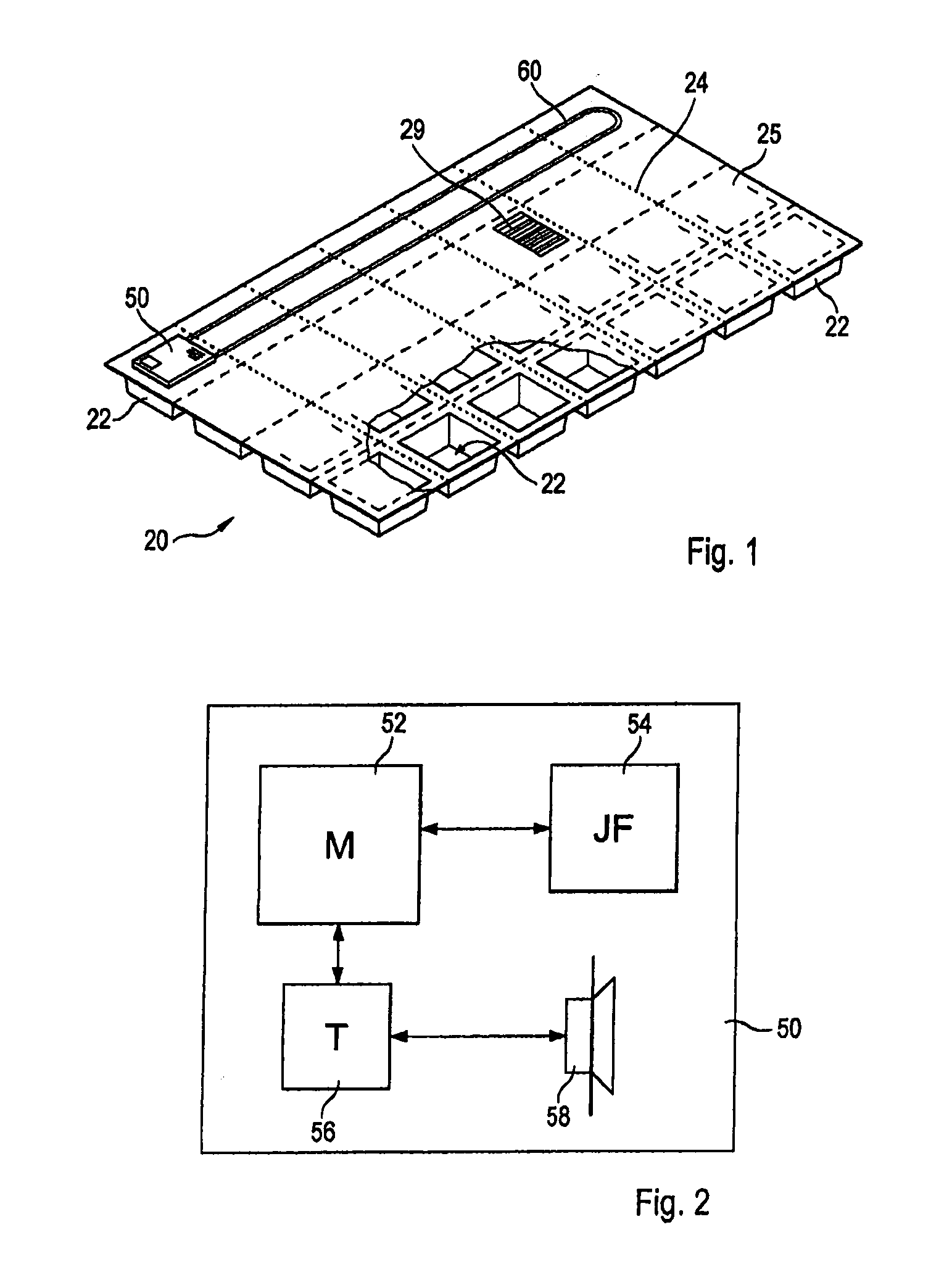
A personalized blister pack (20) for the automated packaging of an individually defined product composition, particularly drugs, of a defined person, particularly a patient, for a defined time period comprises a plurality of receiving compartments (22), each closed by a film (25), for the products of the product composition, wherein the receiving compartments (22) are each associated with a defined usage time within the defined time period and disposed in matrix form, comprising a number of lines for usage days and columns for usage times during the day, and a memory device (50) for storing the usage data of the products of the product composition of the defined person for the defined time period, comprising a device (58) for generating a usage reminder signal as a reminder of a defined product usage time based on the stored usage data. The blister pack (20) enables a fast, safe and inexpensive supply of the needs of the person for a defined time period, such as one week, and by means of the memory device, which may comprise a reminder function, supports the planned use of the products.
Owner:KOHL EDWIN
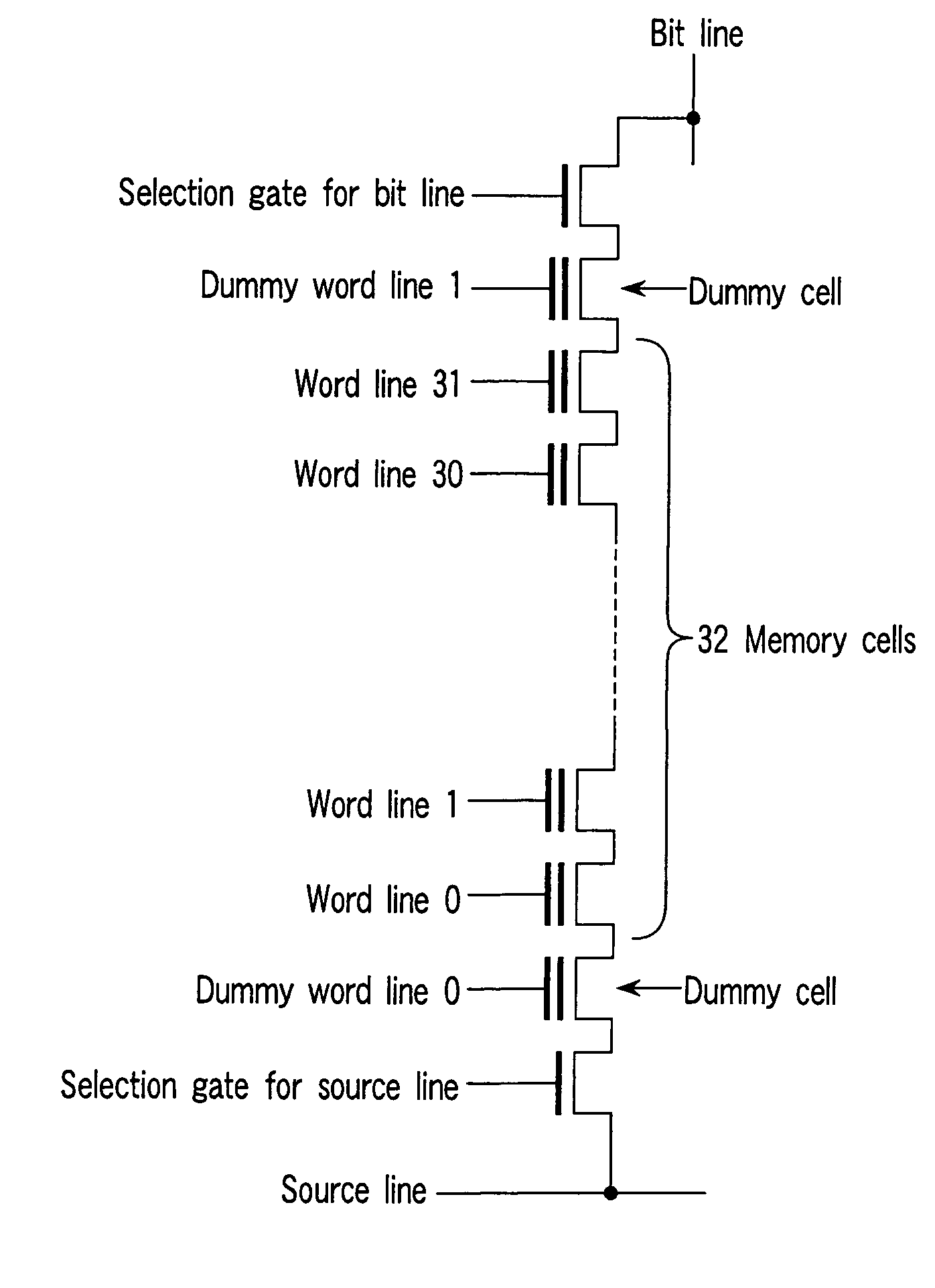
NAND-structured flash memory
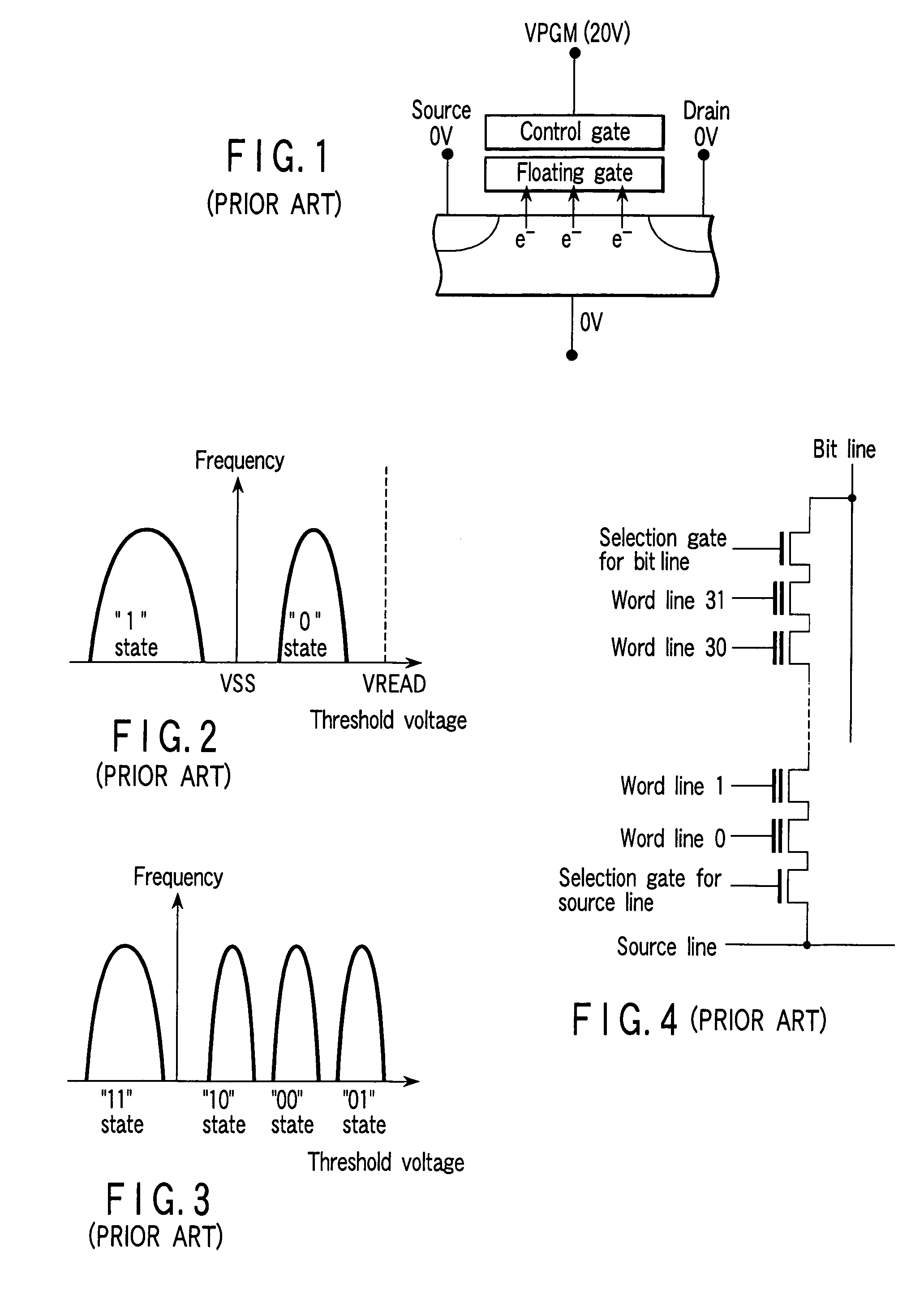
A NAND-structured flash memory comprises a memory cell array wherein plural memory strings are arranged in matrix form, each of the memory cell strings including plural nonvolatile memory cells, the first conducting paths of the memory cells being connected in series, at least one of the memory cells having a function other than an external data storing function, plural first selection transistors having second conducting paths, and one end of the second conducting paths being connected to one end of the series of the first conducting paths, respectively, plural bit lines connected to the other end of the second conducting paths, plural second selection transistors having third conducting paths, and one end of the third conducting paths being connected to one end of the series of the first conducting paths, respectively, and a source line connected to the other end of the third conducting paths.
Owner:KIOXIA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com