Patents
Literature
860 results about "Blister pack" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Blister pack is a term for several types of pre-formed plastic packaging used for small consumer goods, foods, and for pharmaceuticals. The primary component of a blister pack is a cavity or pocket made from a formable web, usually a thermoformed plastic. This usually has a backing of paperboard or a lidding seal of aluminum foil or plastic. A blister that folds onto itself is often called a clamshell.
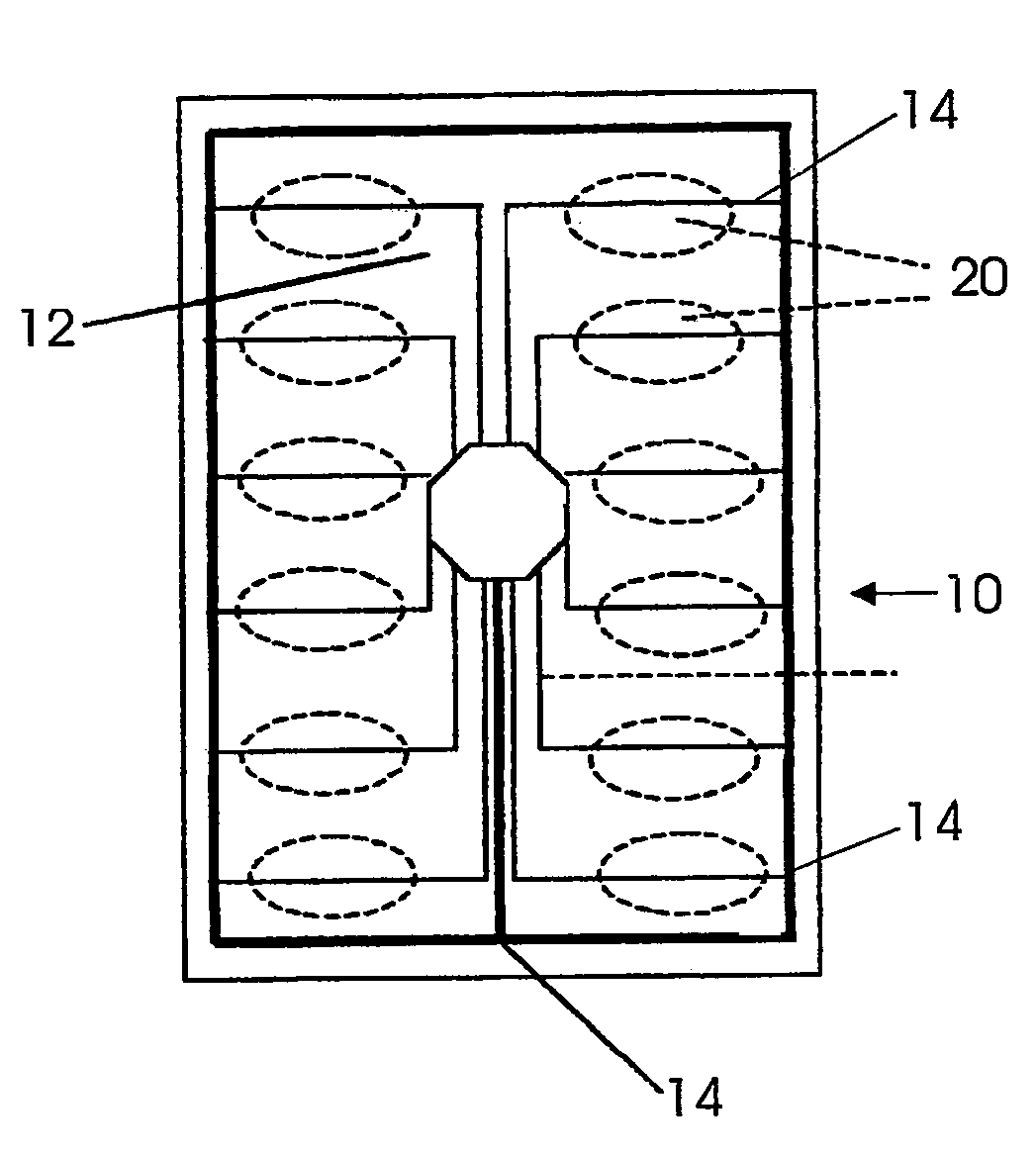
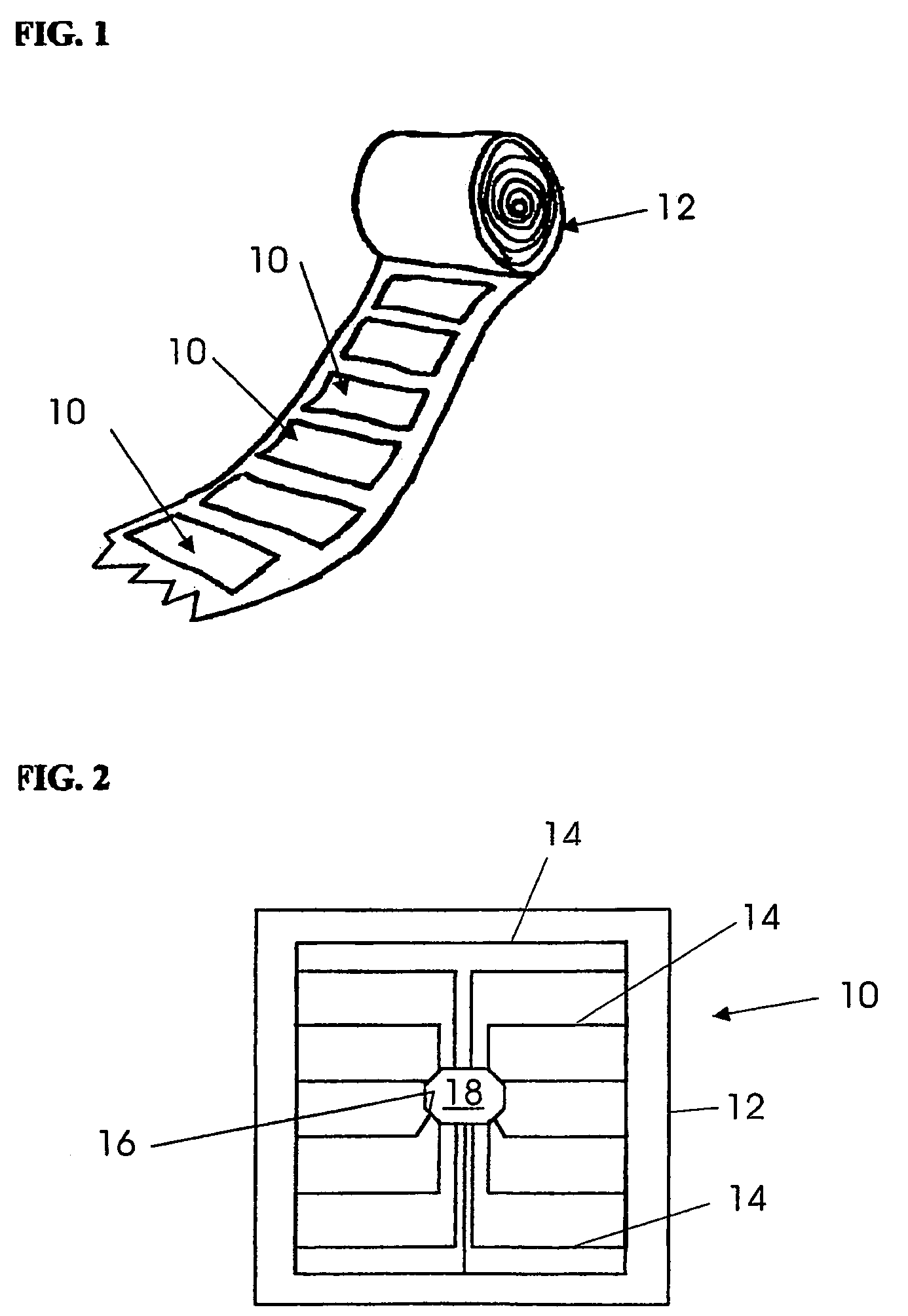
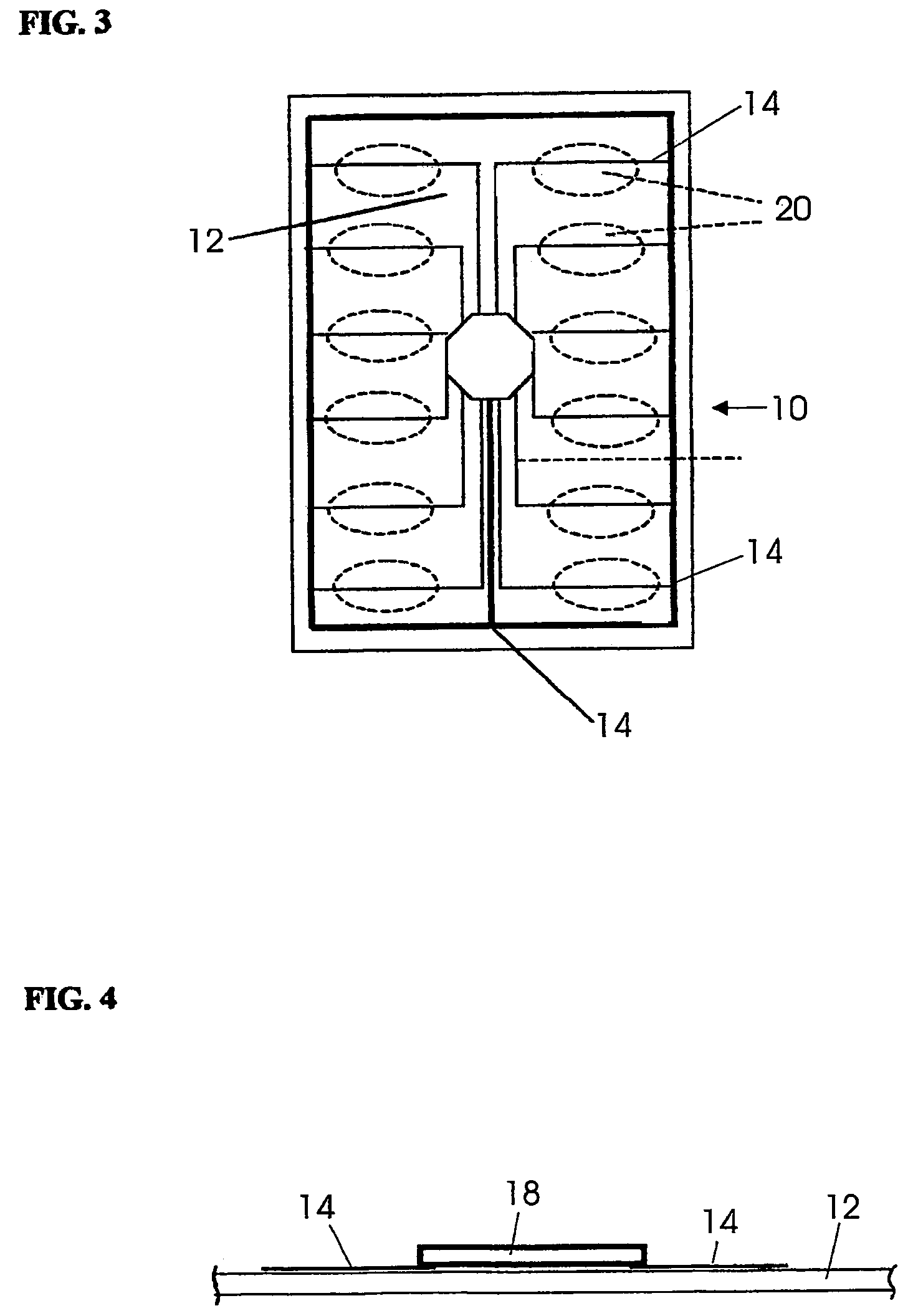
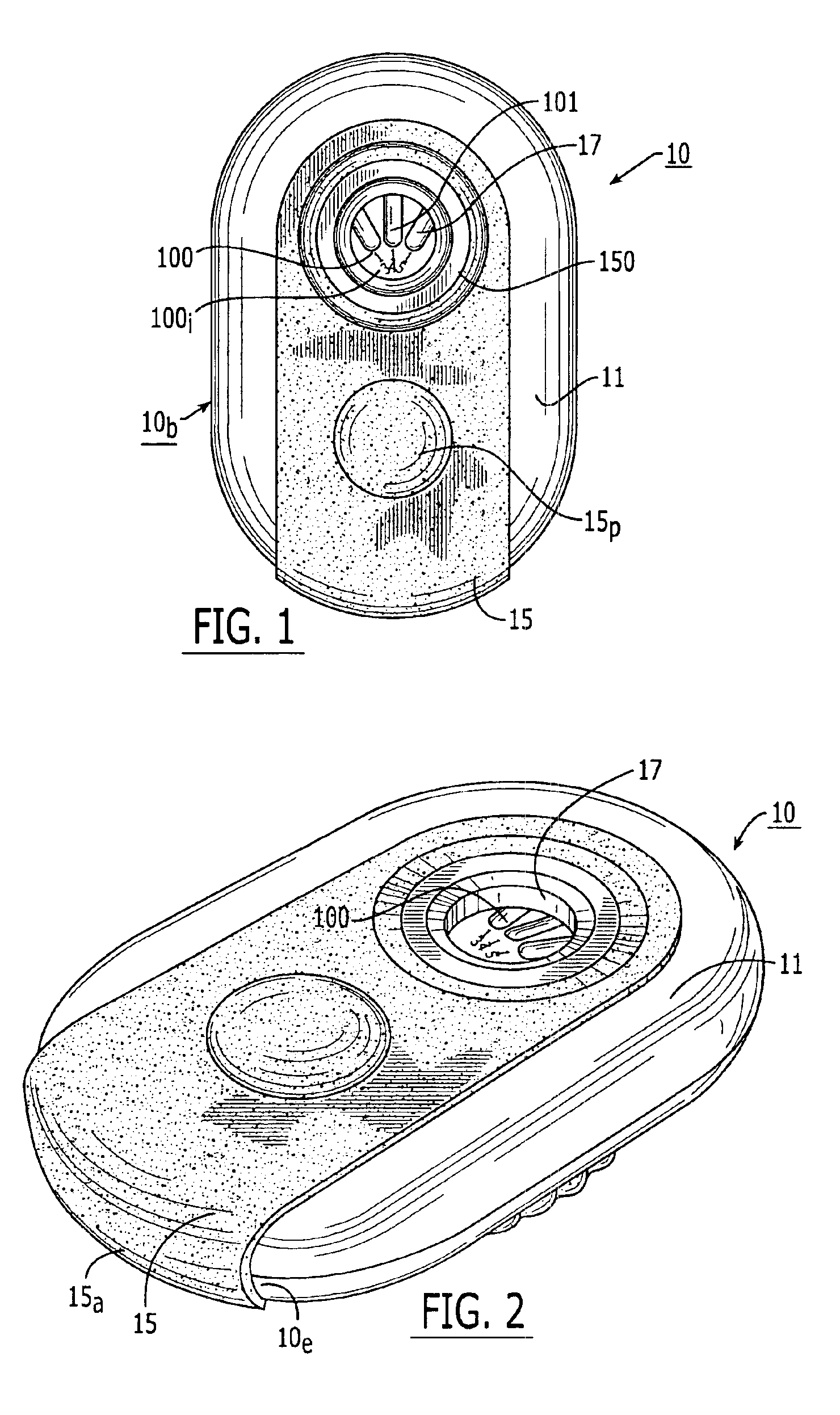
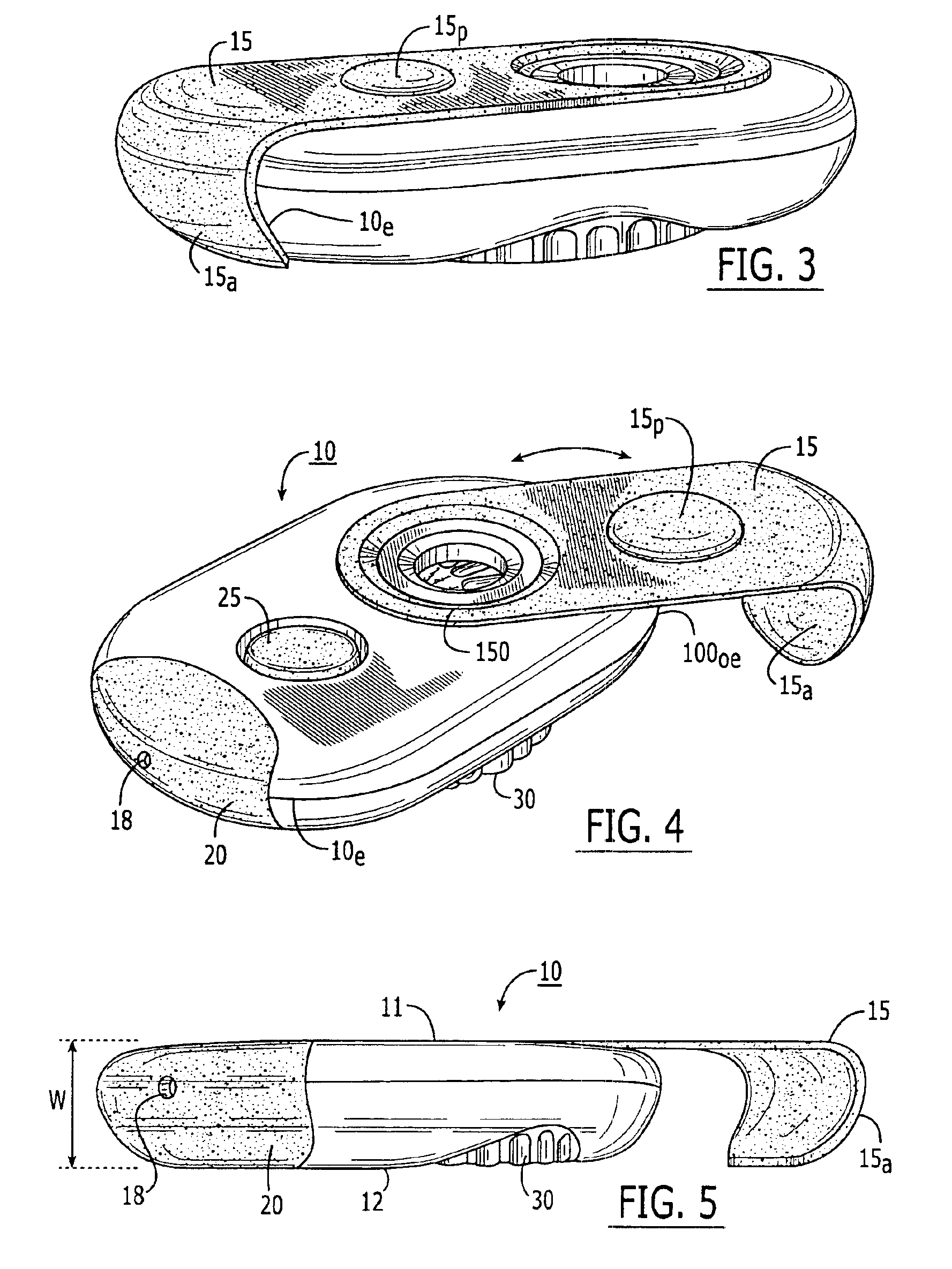
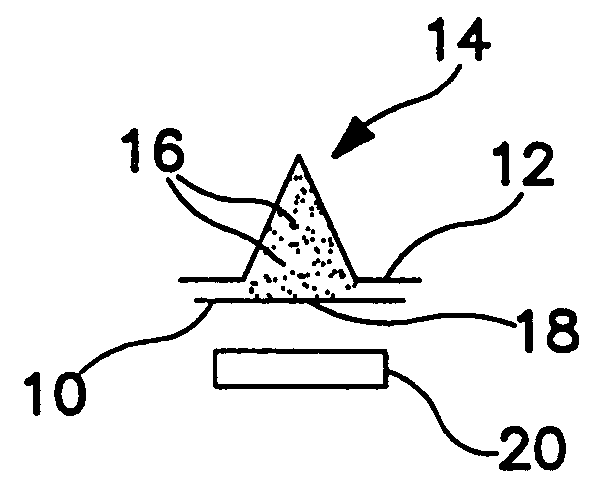
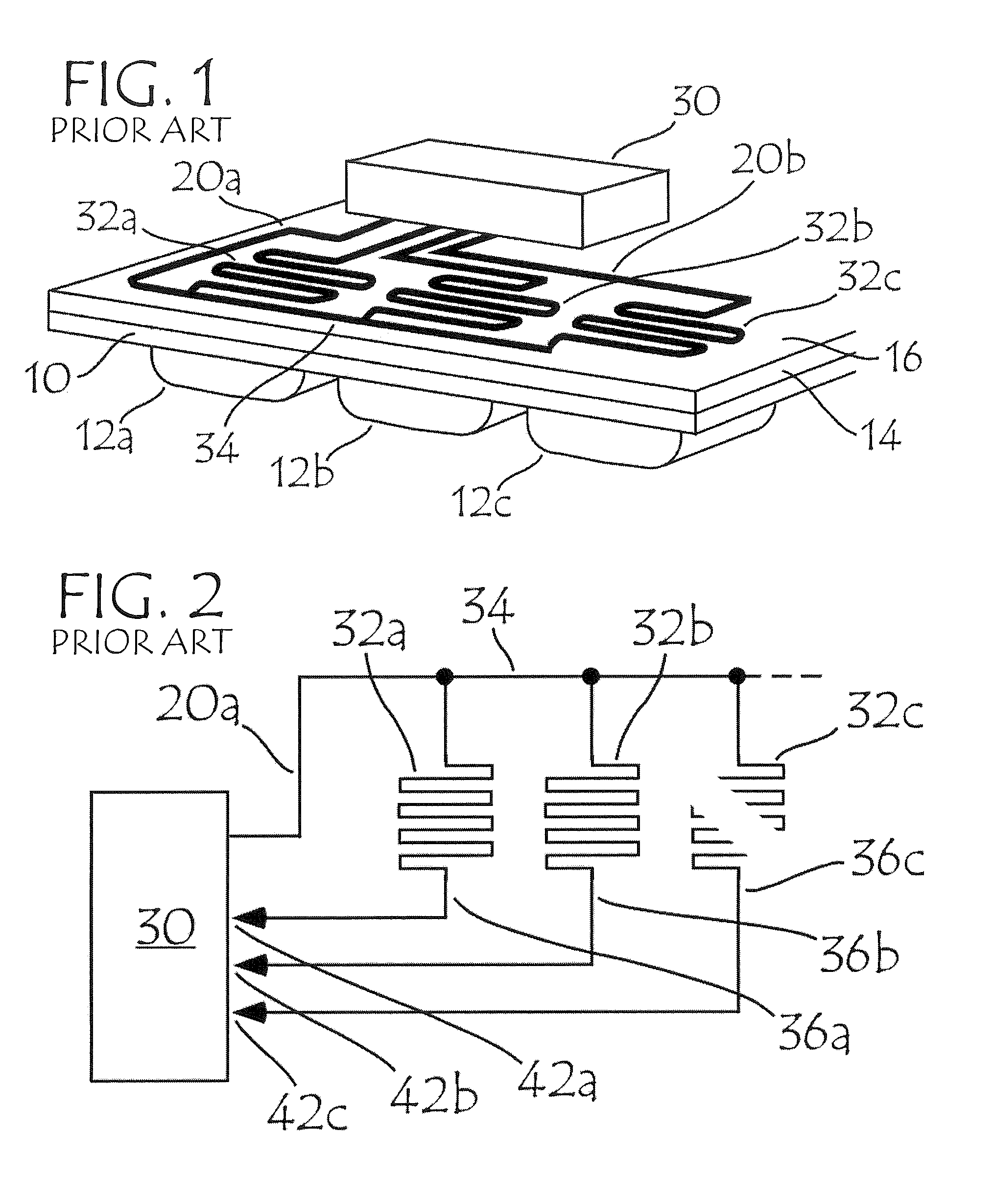
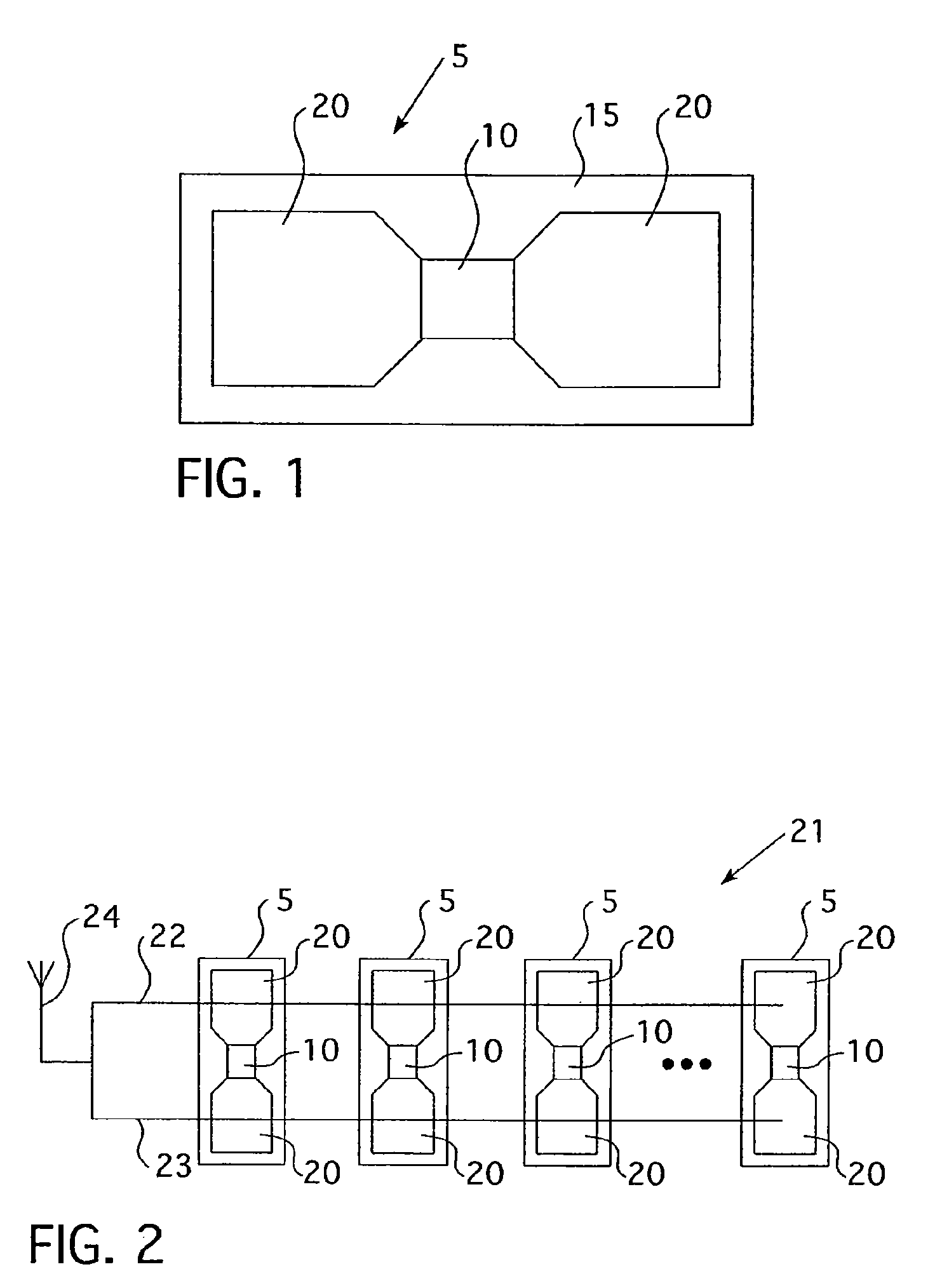
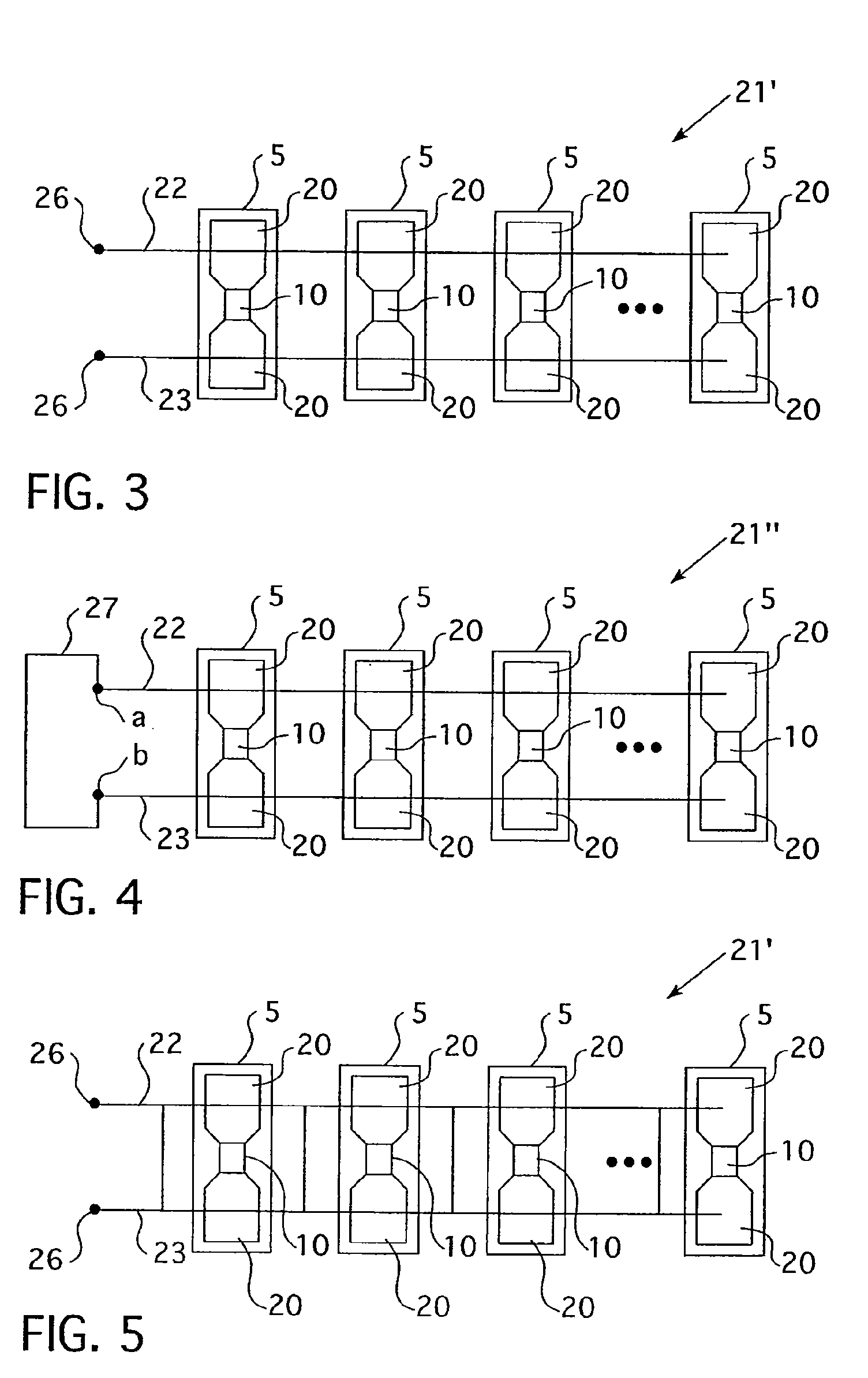
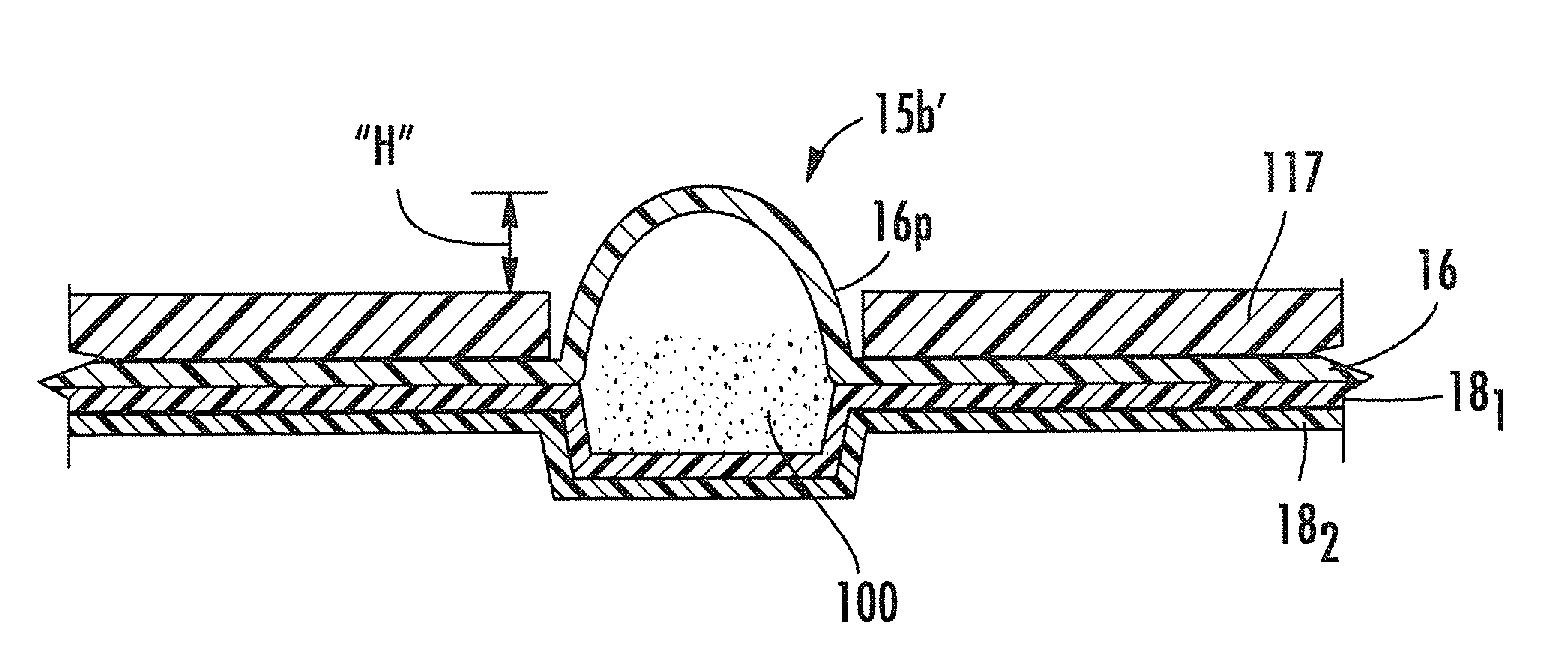
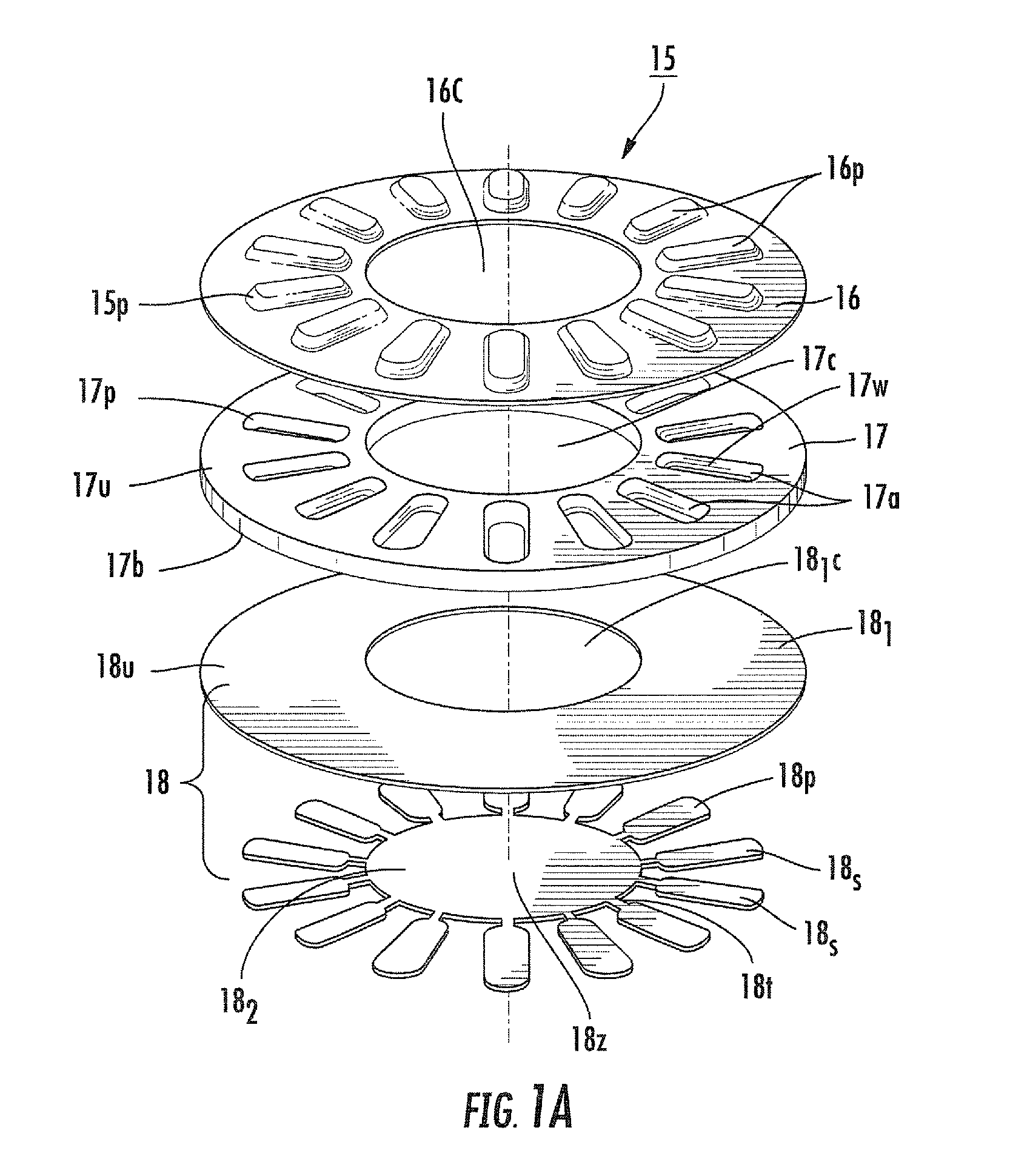
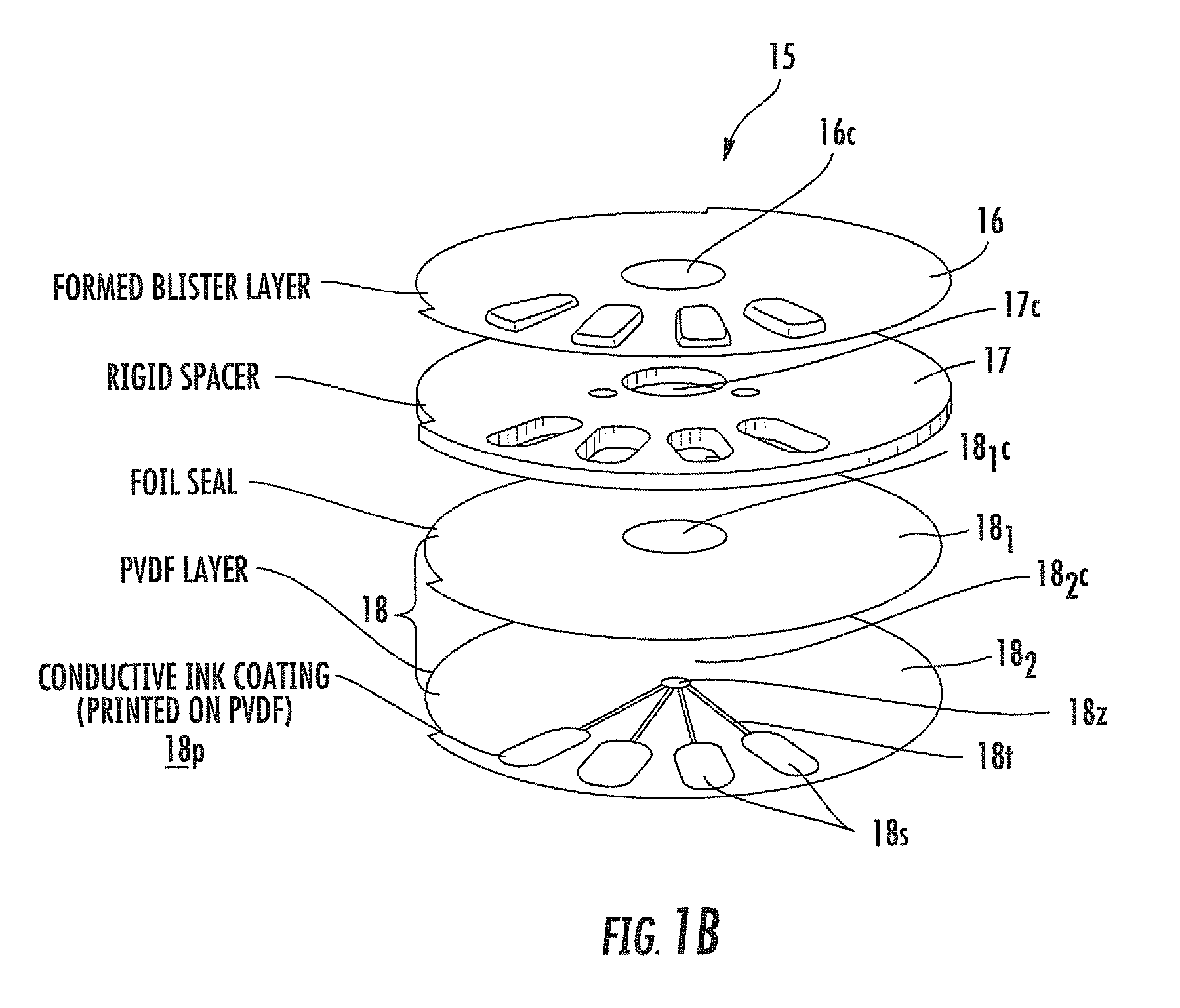
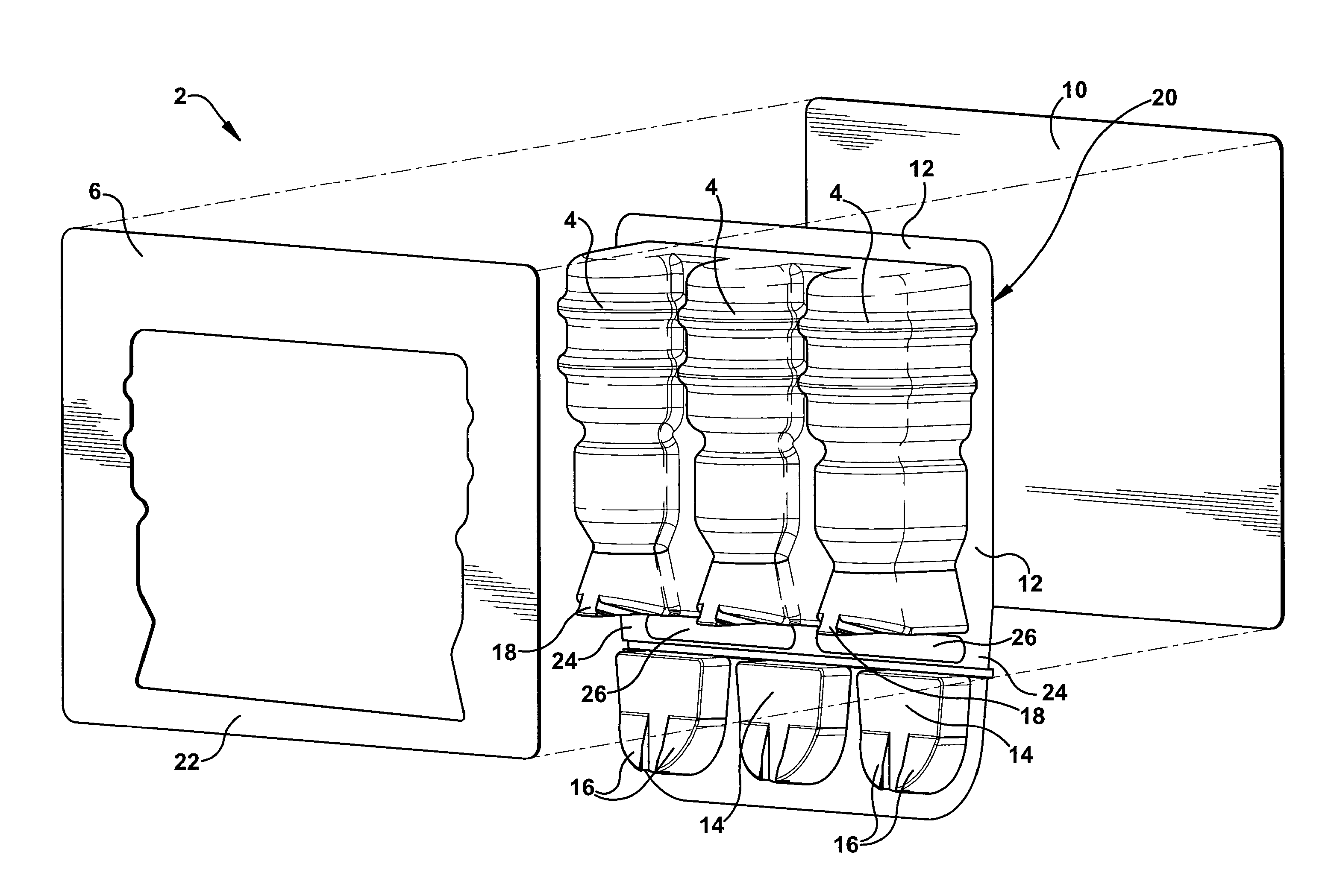
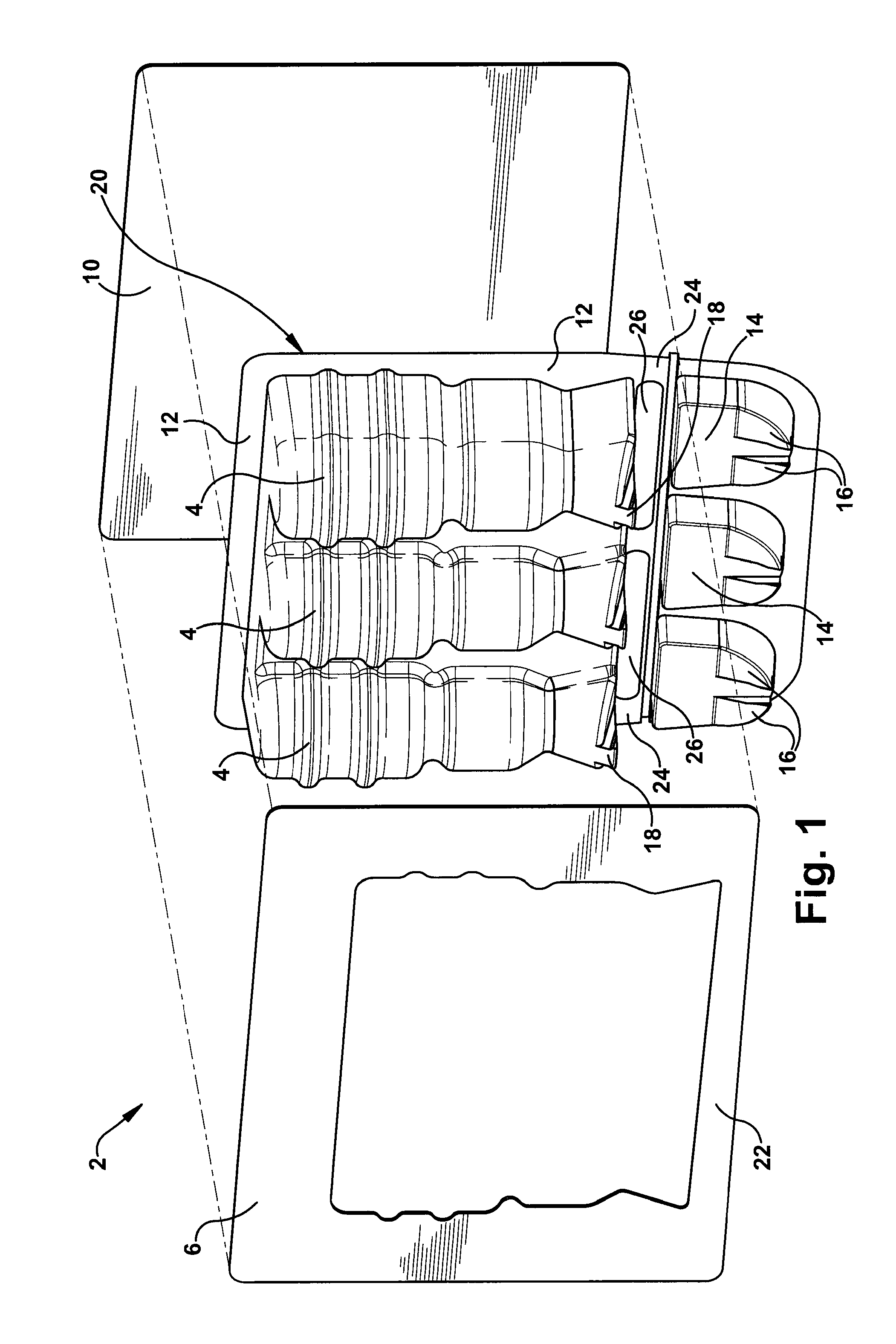
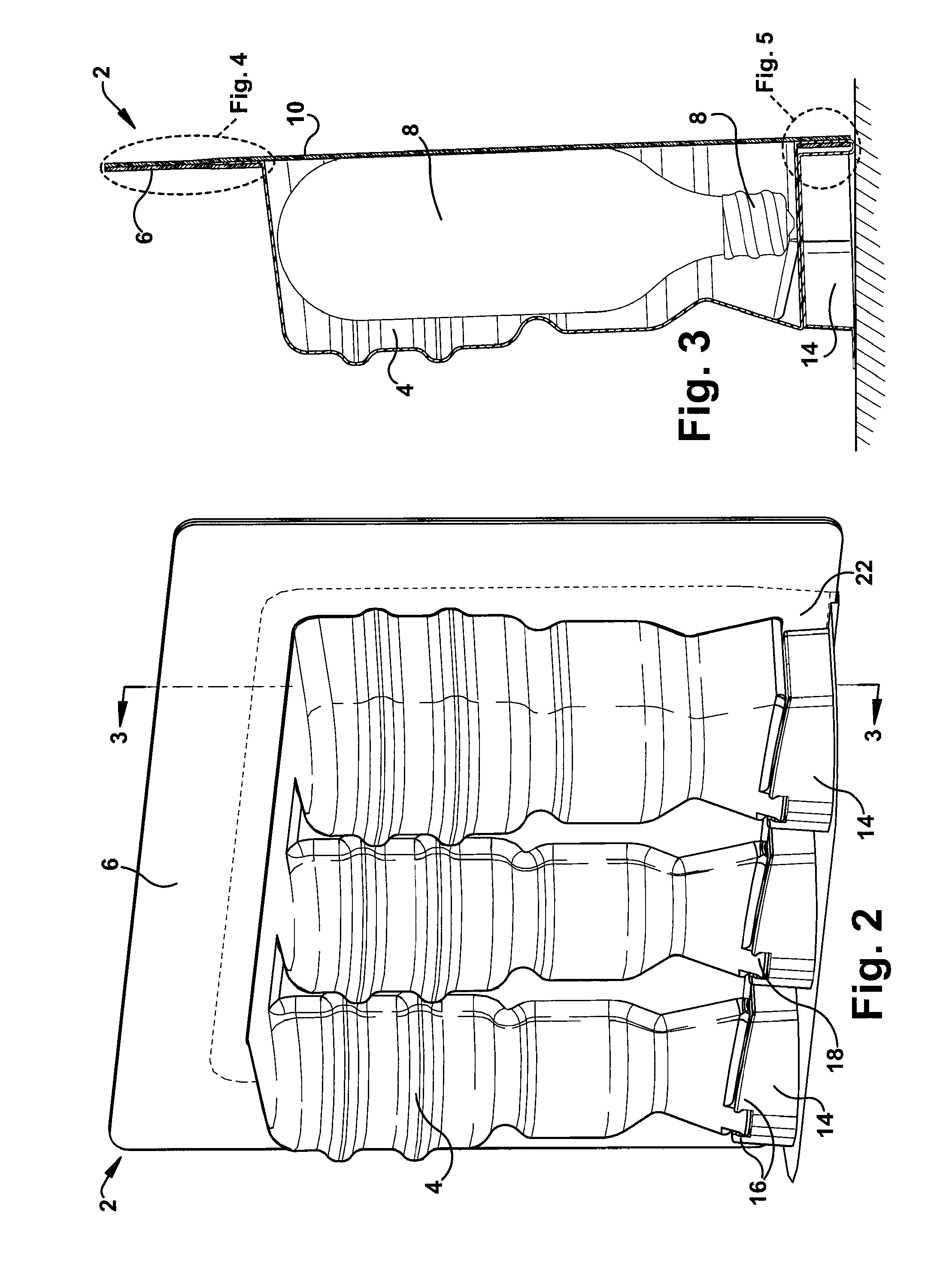
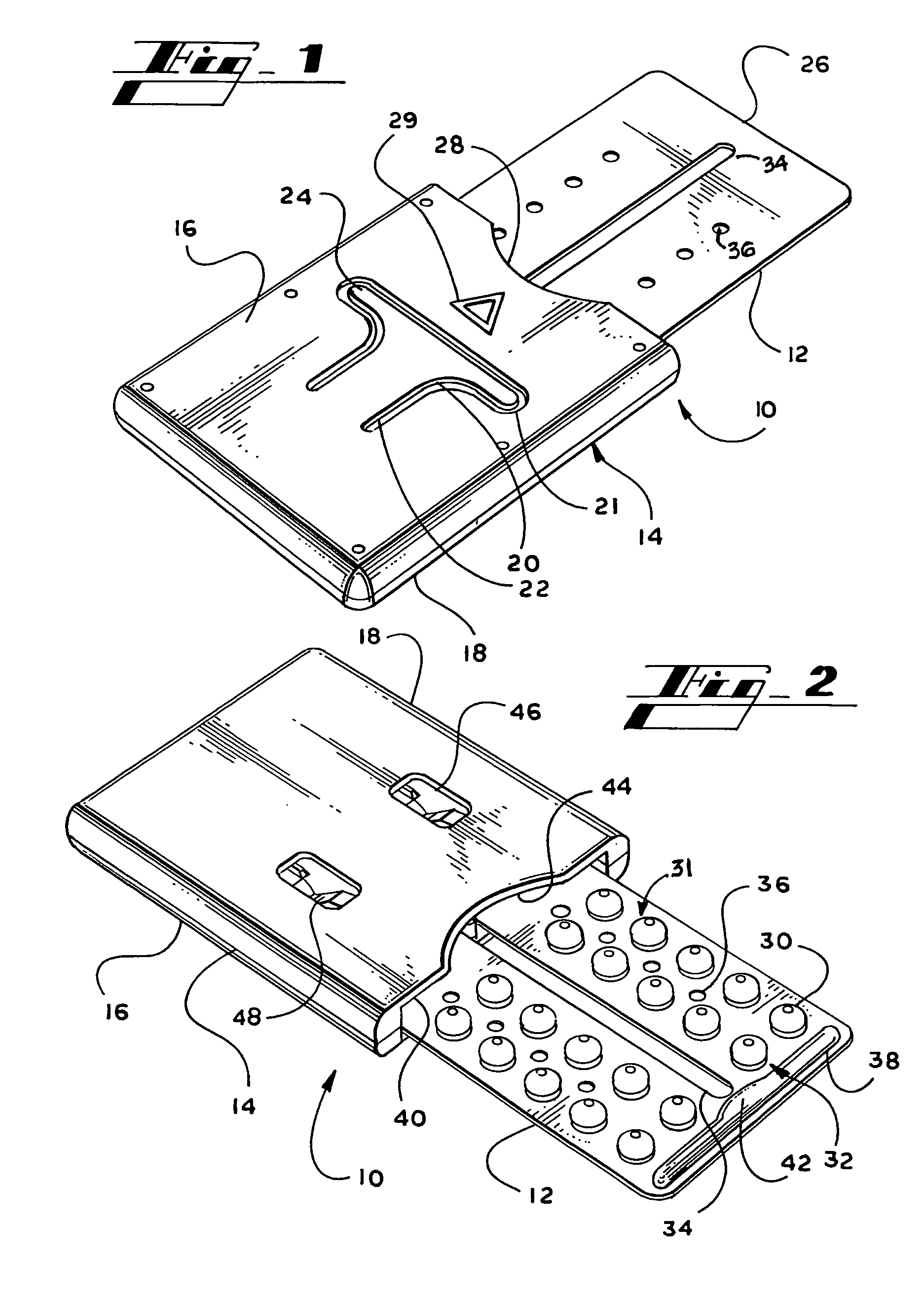
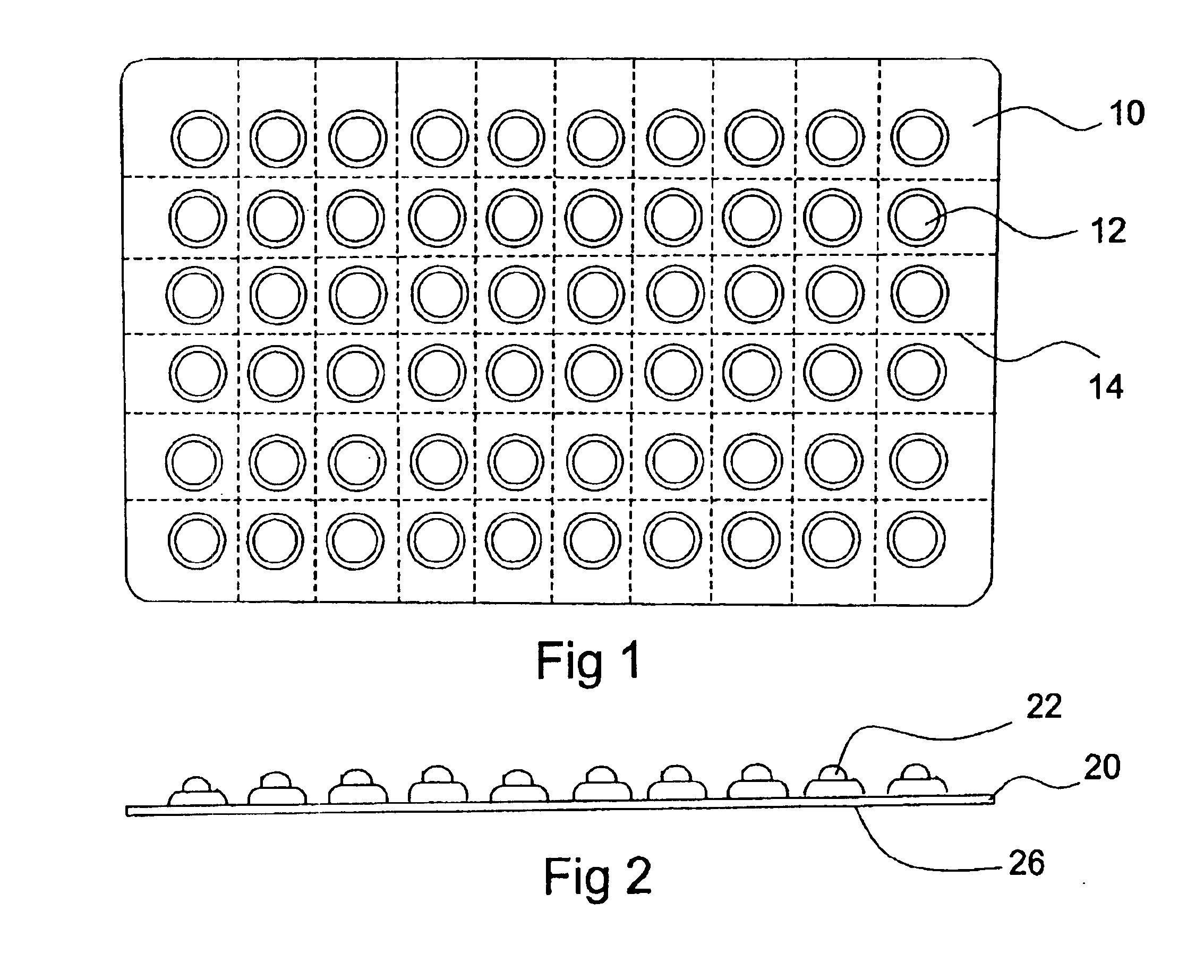
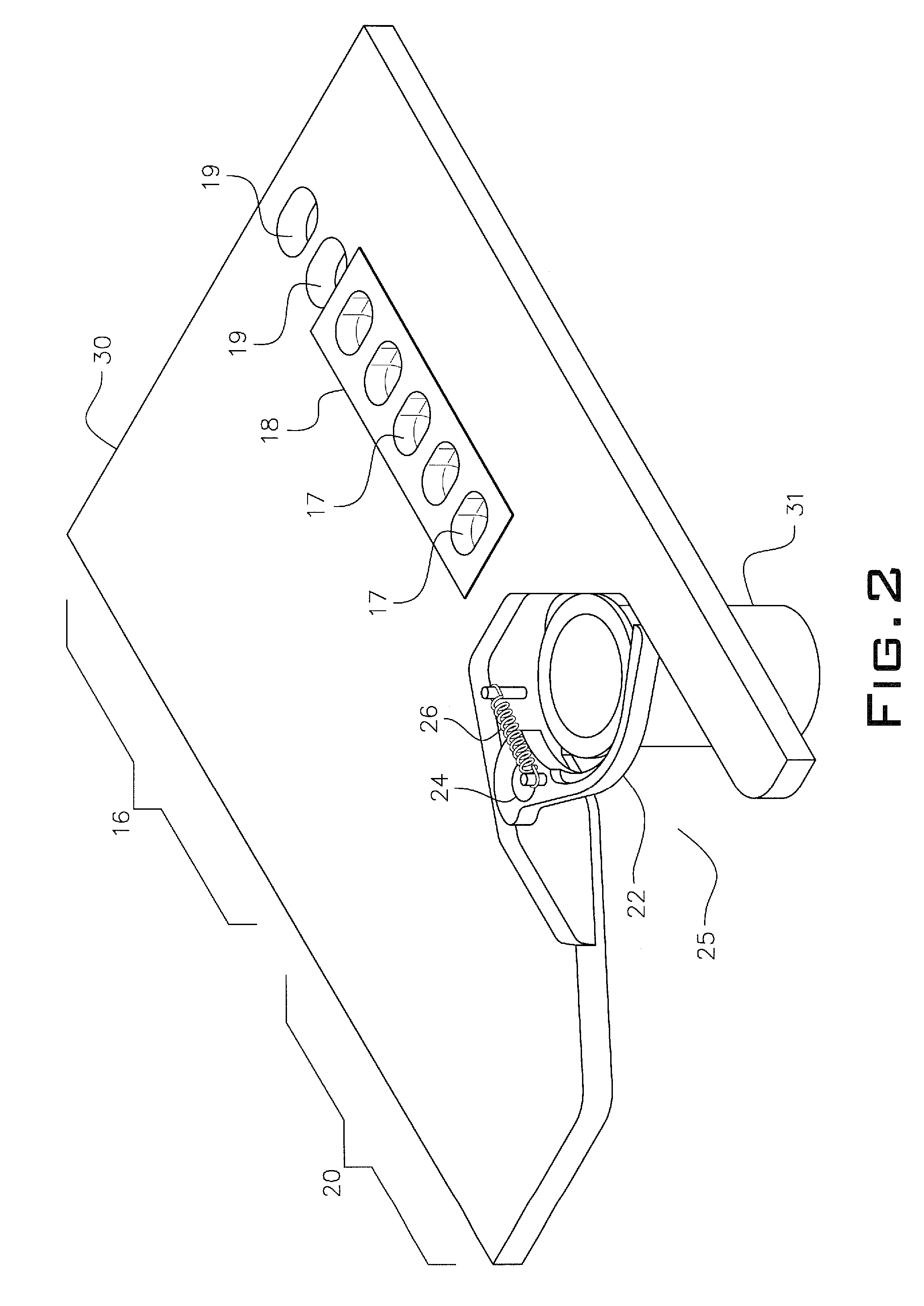
Blister package with electronic content monitoring system
InactiveUS7113101B2Universal applicabilityEfficient operabilityContainer decorationsLevel indicationsMonitoring systemBlister pack
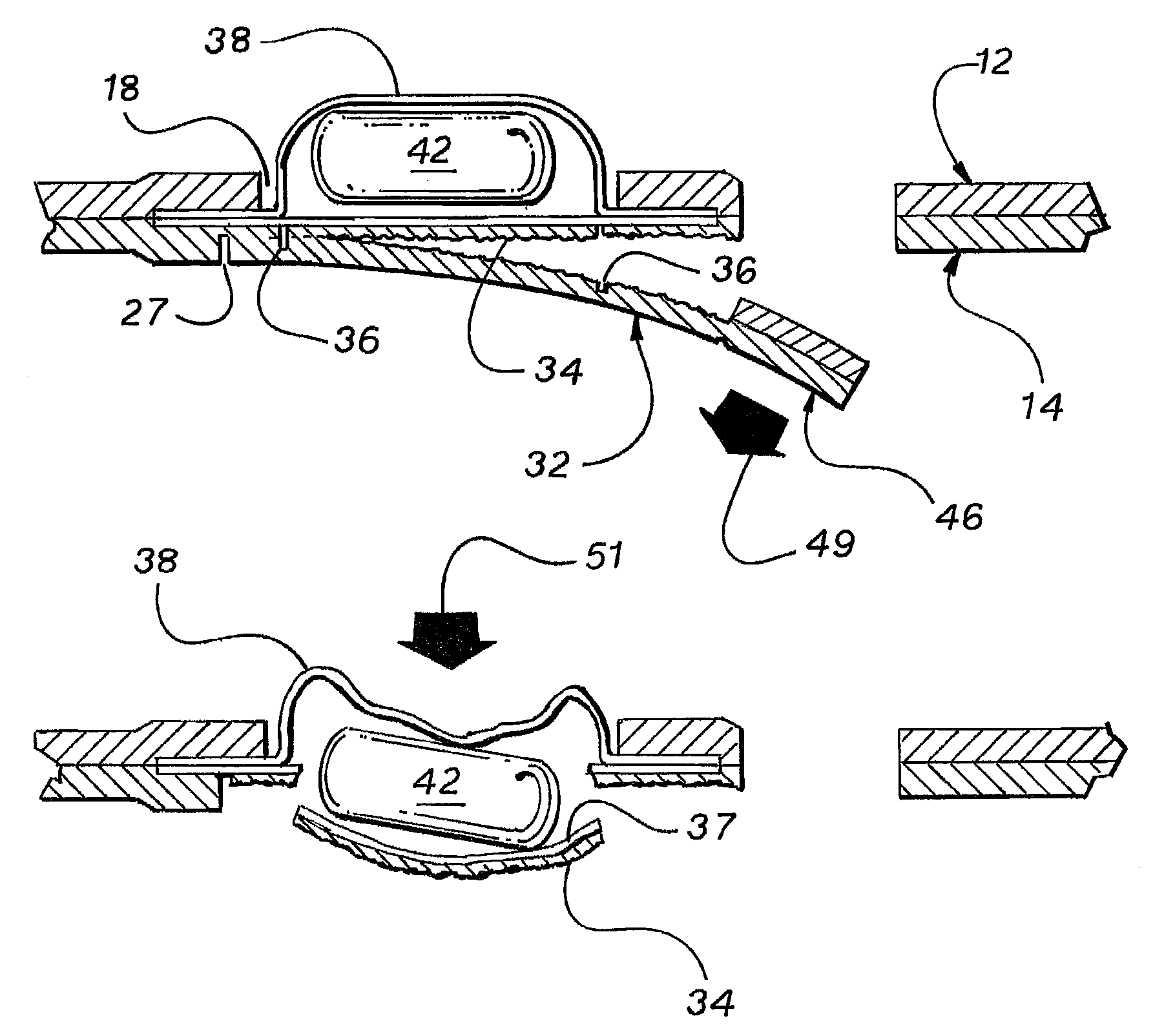
A replicate can be secured to a blister package intended to contain articles, such as pills, and is used to record the removal of individual articles from the blisters. To remove an article from a blister one will usually press against the blister to push the article through a frangible closure seal, breaking the seal in the process. The replicate includes a backing sheet which carries a plurality of traces alignable with corresponding blisters so that when the article is removed from the blister it will not only break the seal but it will also break the corresponding trace. All of the traces are connected to an integrated circuit which may also be formed or provided on the backing sheet, as is a power source for the integrated circuit. The breaking of the trace is an event that is recorded in the integrated circuit for later accessability. The replicate may be secured to the blister package after the package has been produced by conventional form-fill-seal equipment. The individual traces can be formed into a grid of closely spaced traces so that alignment of the traces with the individual blisters is less critical. The replicates may be formed by printing or other conventional methods on a roll of lidstock. After forming the individual replicates are severed from the roll of lidstock for securement to a blister package.
Owner:INTELLIGENT DEVICES SEZC
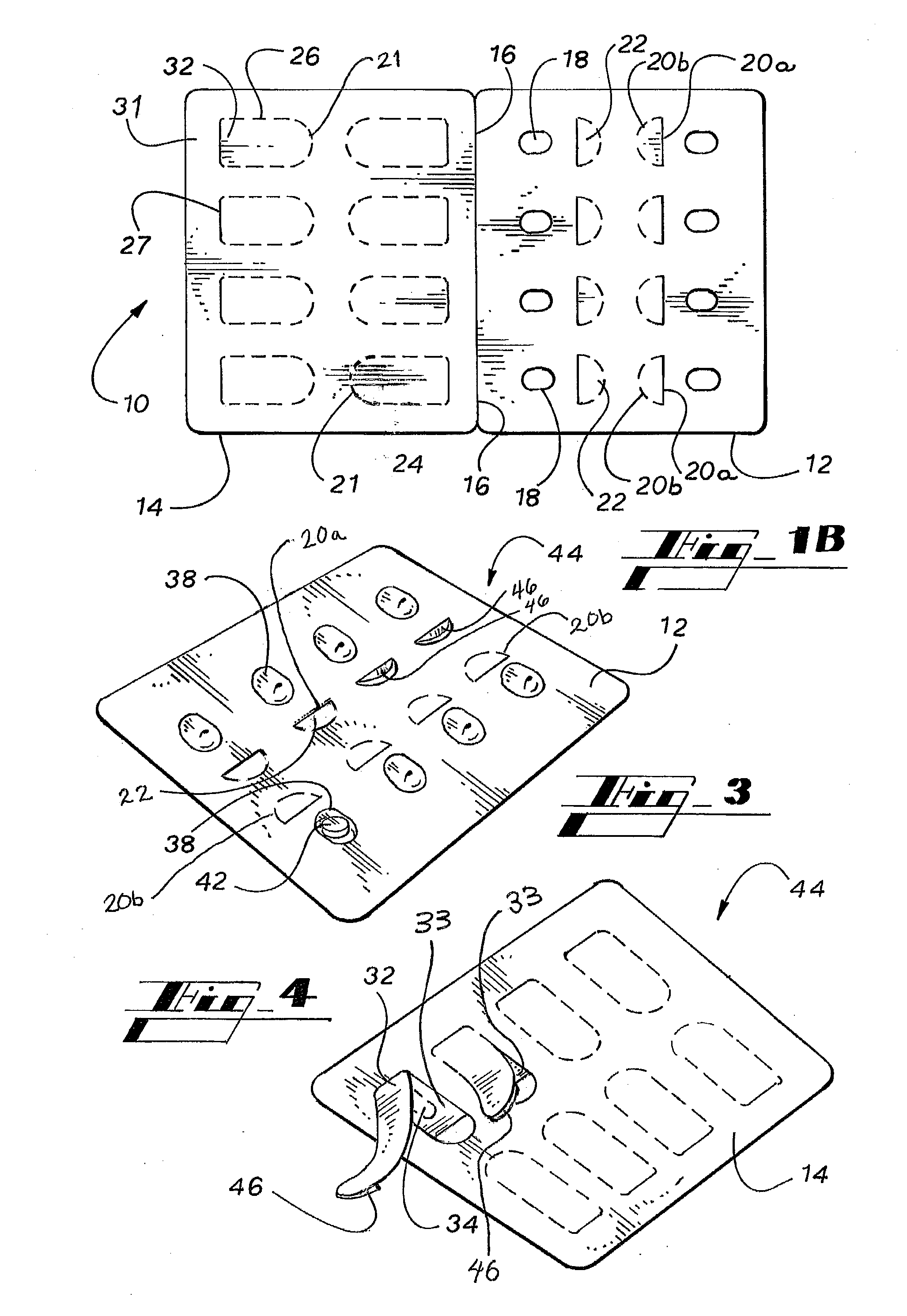
Dry powder inhalers, related blister devices, and associated methods of dispensing dry powder substances and fabricating blister packages
InactiveUS6889690B2Easy to optimizeLimit amount of resistanceSmall article dispensingLiquid surface applicatorsPowder InhalerInhalation
The present invention includes dry powder inhalers and associated multi-dose dry powder packages for holding inhalant formulated dry powder substances and associated fabrication and dispensing methods. The multi-dose package can include a platform body comprising at least one thin piezoelectric polymer material layer defining at least a portion of a plurality of spatially separated discrete elongate dry powder channels having an associated length, width and height; and a metallic material attached to selected portions of the piezoelectric polymer material including each of the regions corresponding to the elongate dry powder channels to, in operation, define active energy releasing vibratory channels. In operation, the elongate channels can be selectively individually activated to vibrate upon exposure to an electrical input.The dry powder inhaler includes an elongate body having opposing first and second outer primary surfaces with a cavity therebetween and having opposing top and bottom end portions and a multi-dose sealed blister package holding a plurality of discrete meted doses of a dry powder inhalable product located in the cavity of the elongate body. The inhaler also includes an inhalation port formed in the bottom end portion of the elongate body, the inhalation port configured to be in fluid communication with at least one of the discrete meted doses during use and a cover member that is pivotably attached to the elongate body so that it remains attached to the body during normal operational periods of use and moves to a first closed position to overlie the inhalation port at the bottom end portion of the body during periods of non-use and moves to a second open position away from the inhalation port during periods of use to allow a user to access the inhalation port.
Owner:ORIEL THERAPEUTICS INC
Carded blister pack
InactiveUS20040026293A1Easy to foldSmall article dispensingContainer decorationsBlister packAssembly machine
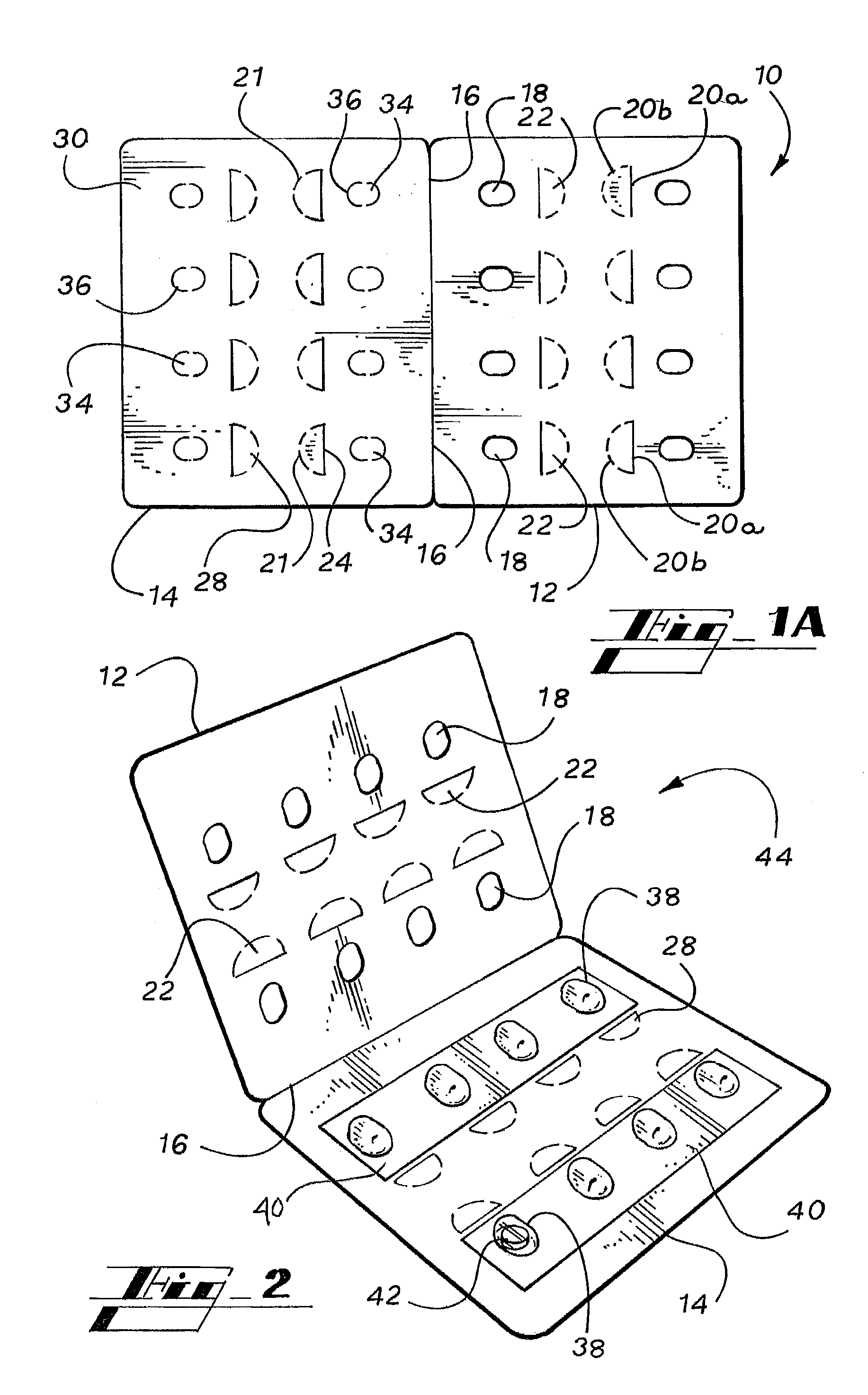
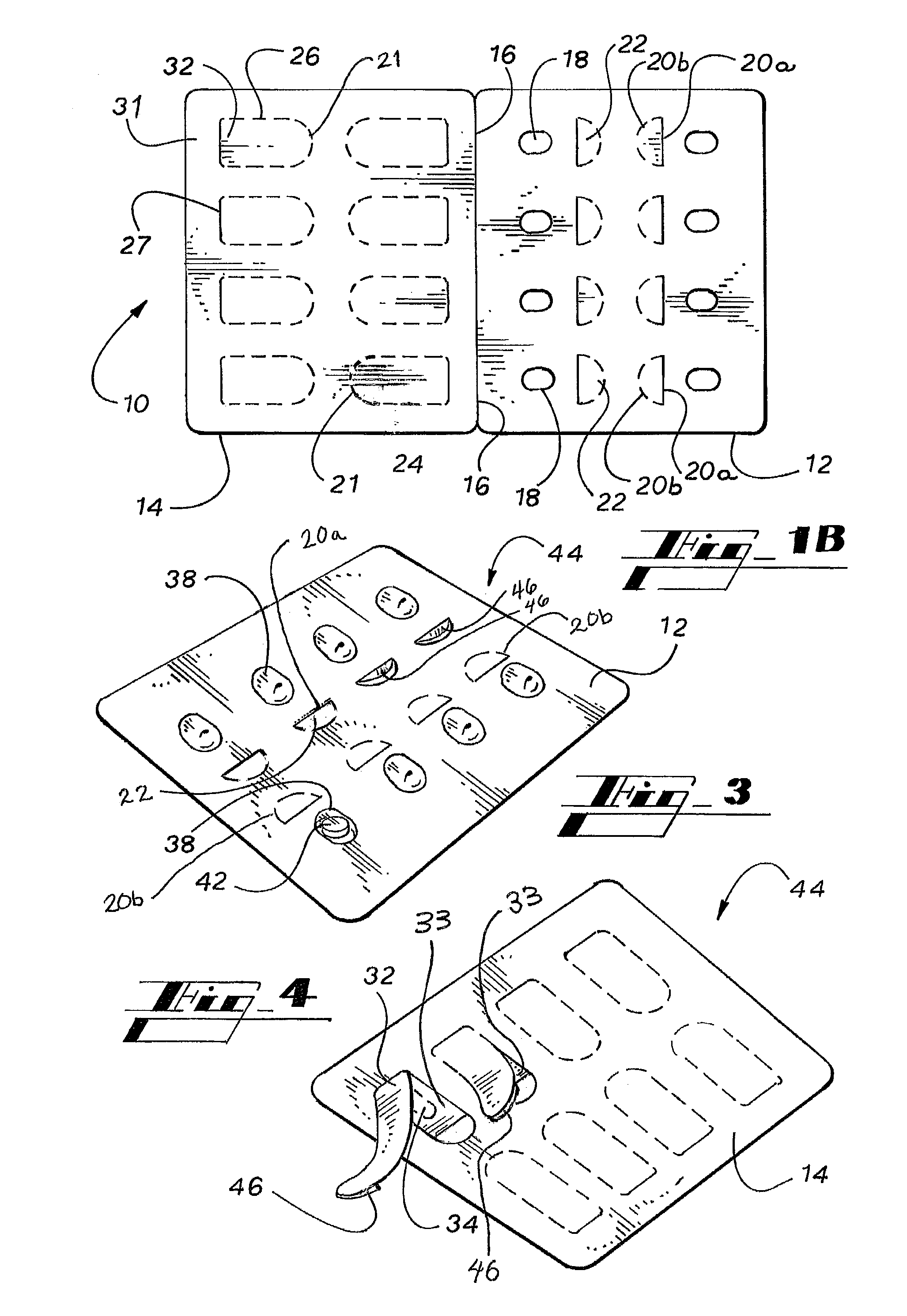
A blister pack (20) folded wallet has a mounting card (12), with a [hinged] spine segment (15), and an adhesive edge strip (16). Overlaid by a pre-formed blister pack (11), for mutual (edge) entrainment; a dedicated assembly machine entrains mounting cards and blister packs stored in respective magazines. Child resistance is available through cover latching and / or paper reinforced foil laminate (161), with through apertures (166) and perforations (163, 165) for selective localised paper patch (169) removal, over individual blister pockets (168).
Owner:HUGHES DAVID
Integrated System for Collecting, Processing and Transplanting Cell Subsets, Including Adult Stem Cells, for Regenerative Medicine
InactiveUS20080171951A1Accurate collectionMinimize risk of contaminationBioreactor/fermenter combinationsNervous disorderFluid transportTissue repair
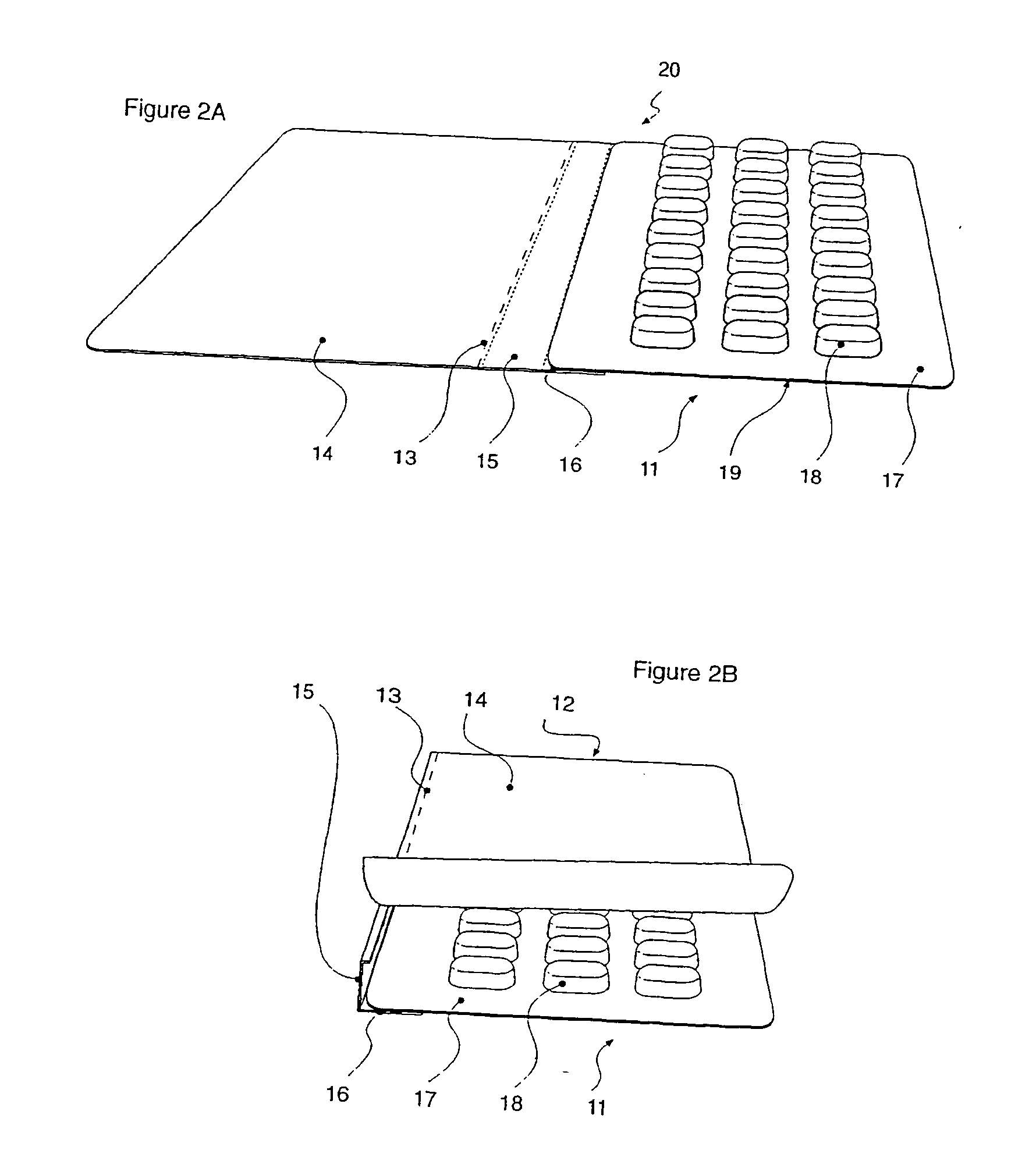
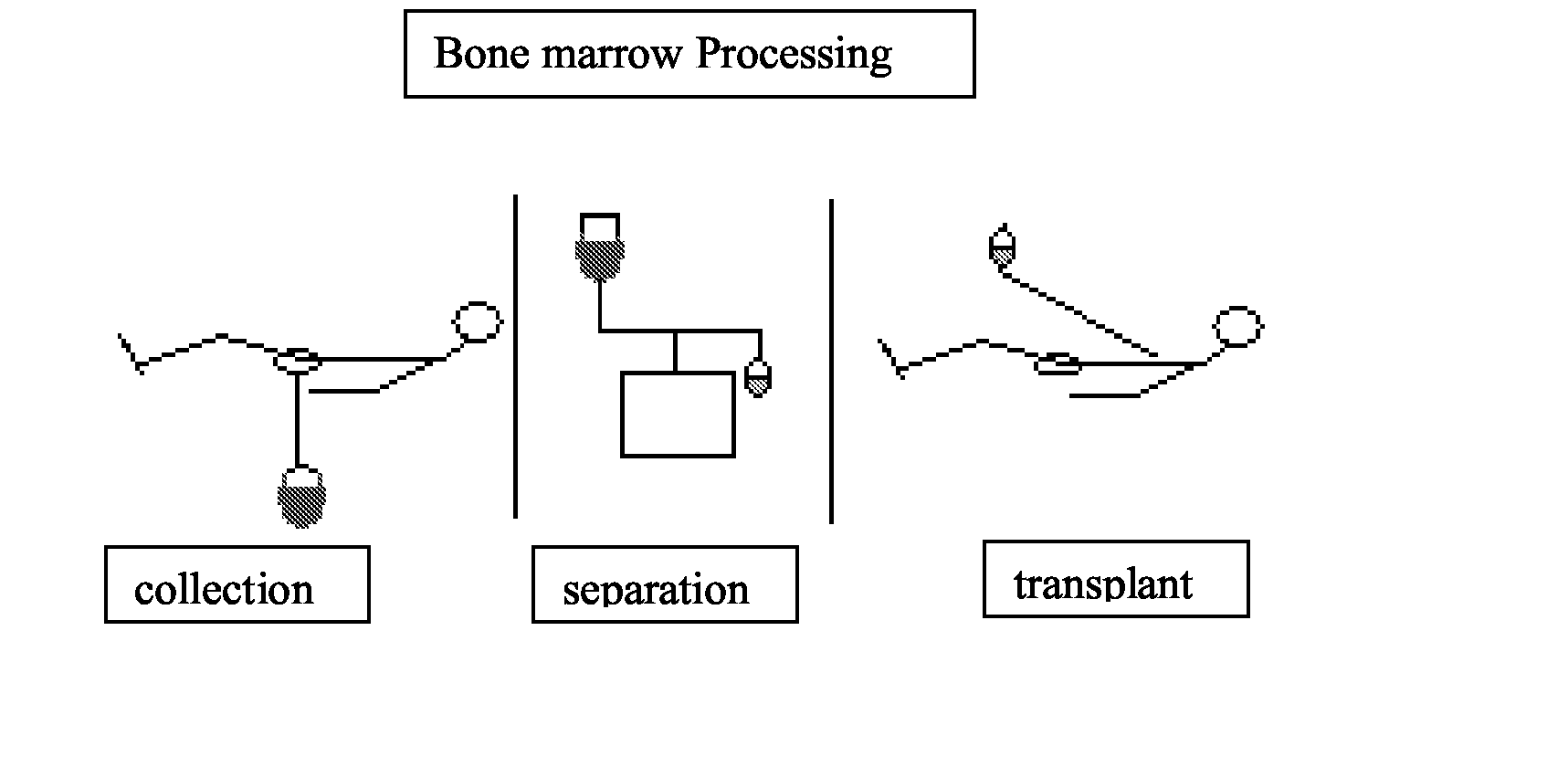
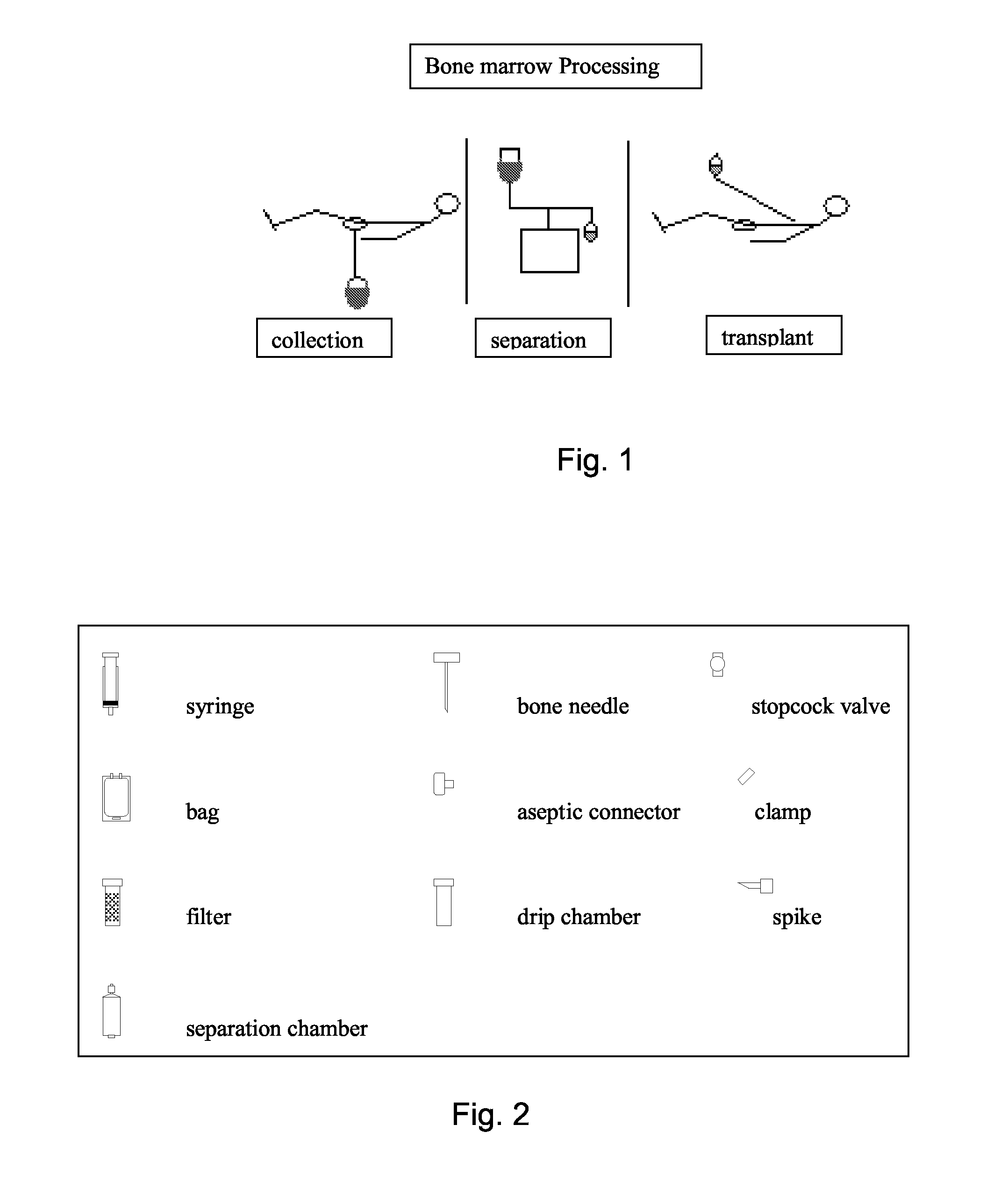
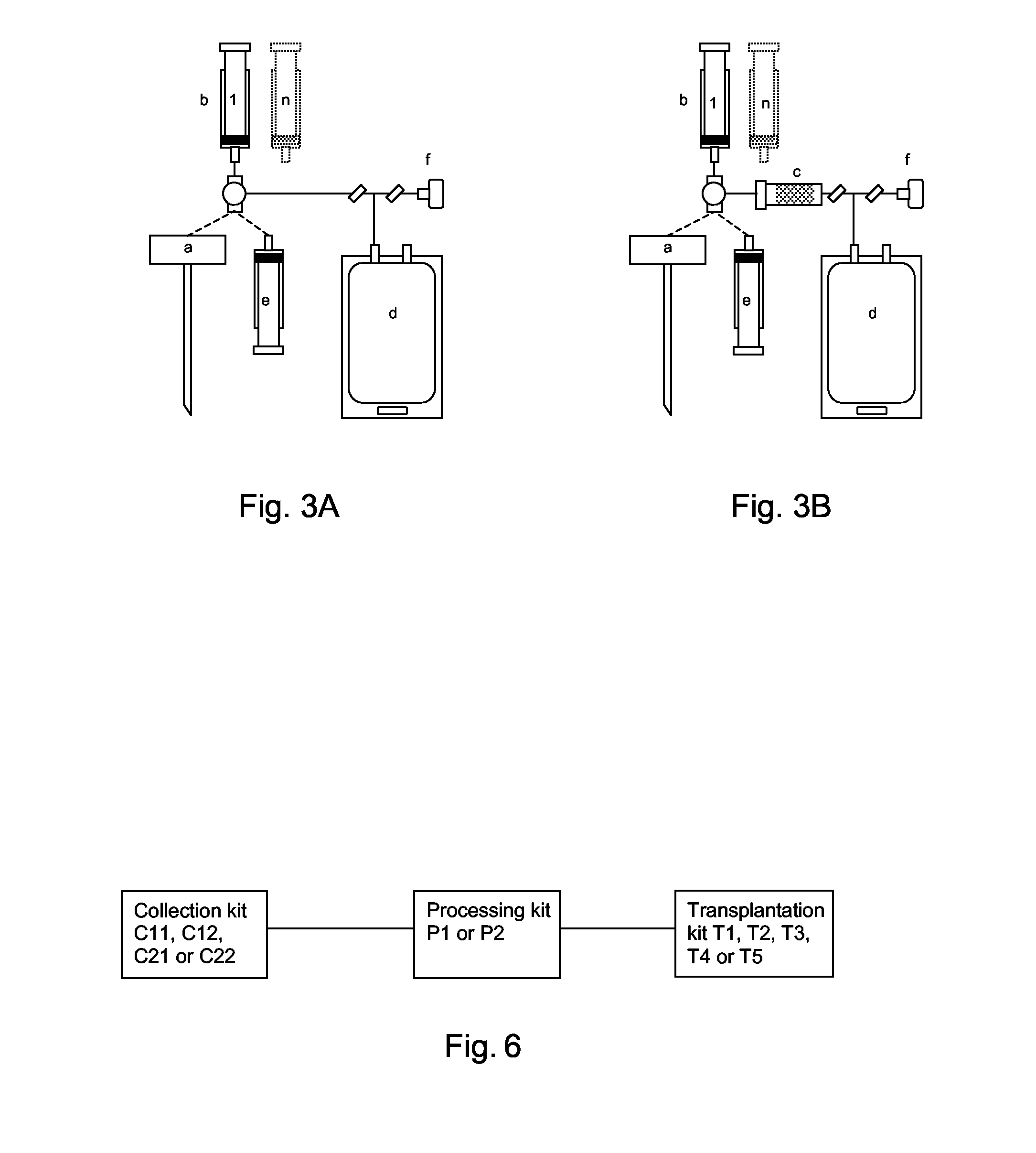
A system for the extraction, collection, processing and transplantation of cell subsets, including adult stem cells and platelets, in particular for tissue repair in regenerative medicine, comprises a set of disposable fluid-transport elements that are pre-connected or that include aseptic connectors for making interconnections between them in an aseptic manner or are adapted to be aseptically connected. The set usually includes three kits of disposable sterile elements, a collection kit, a processing kit, and a transplantation kit packaged in a blister pack on a support such as a tray, having one compartment for receiving each inter-connectable kit of the set. The set includes an extracting device, for example including a needle for bone puncture or vein puncture, for extracting bone marrow or other sources of cell subsets from a patient.
Owner:BIOSAFE SA
Packaging and delivery of pharmaceuticals and drugs
Owner:MICRODOSE THERAPEUTX INC
In situ heat induced antigen recovery and staining apparatus and method
InactiveUS6855292B2Bioreactor/fermenter combinationsBiological substance pretreatmentsAntigenMicroscope slide
An automated in situ heat induced antigen recovery and staining method and apparatus for treating a plurality of microscope slides. The process of heat induced antigen recovery and the process of staining the biological sample on the microscope slide are conducted in the same apparatus, wherein the microscope slides do not need to by physically removed from one apparatus to another. Each treatment step occurs within the same reaction compartment. The reaction conditions of each reaction compartment for treating a slide can preferably be controlled independently, including the individualized application of reagents to each slide and the individualized treatment of each slide. The reagents are preferably held in a reagent dispensing strip similar to a “blister pack”.
Owner:ANGROS LEE
Assays Based on Liquid Flow over Arrays
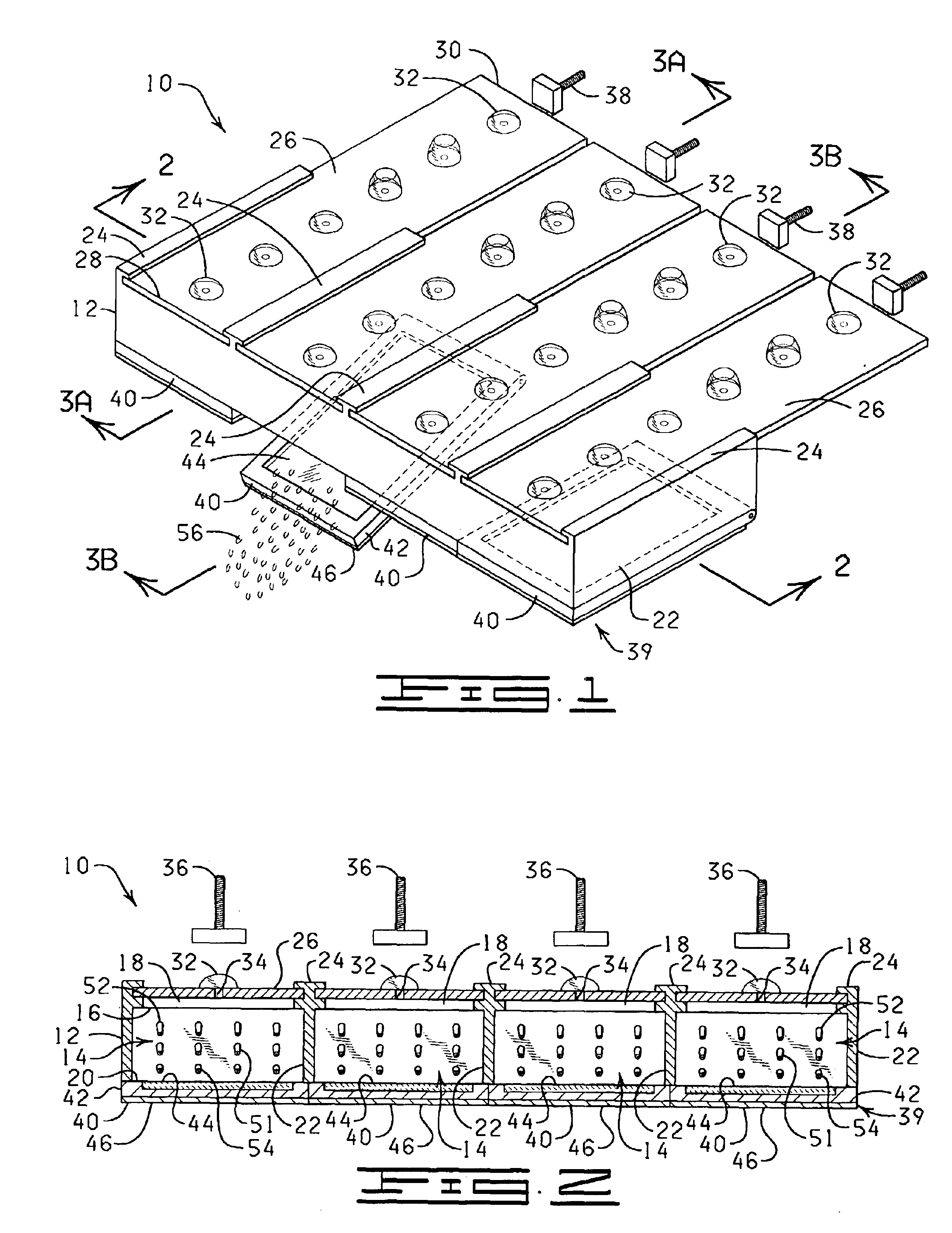
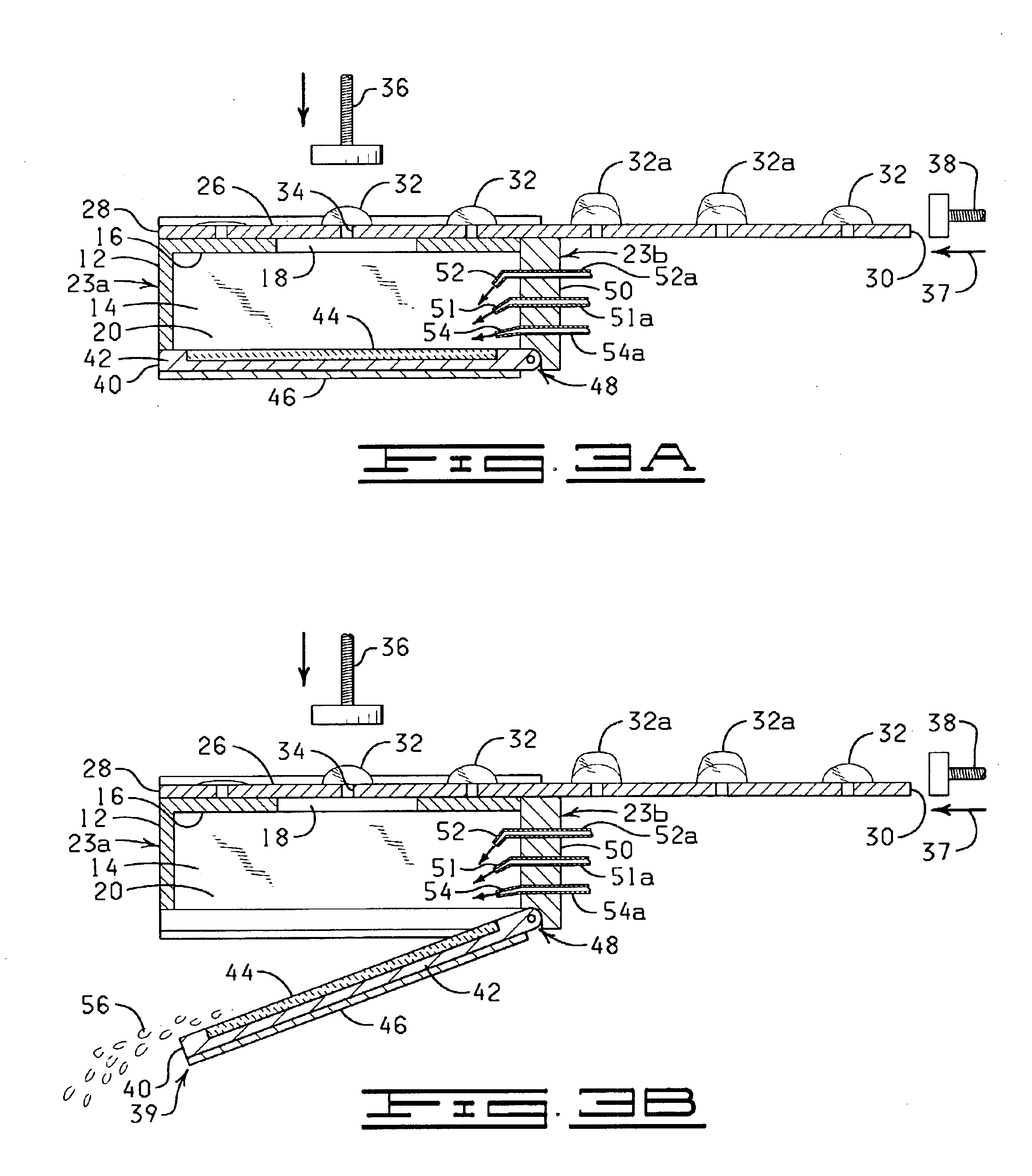
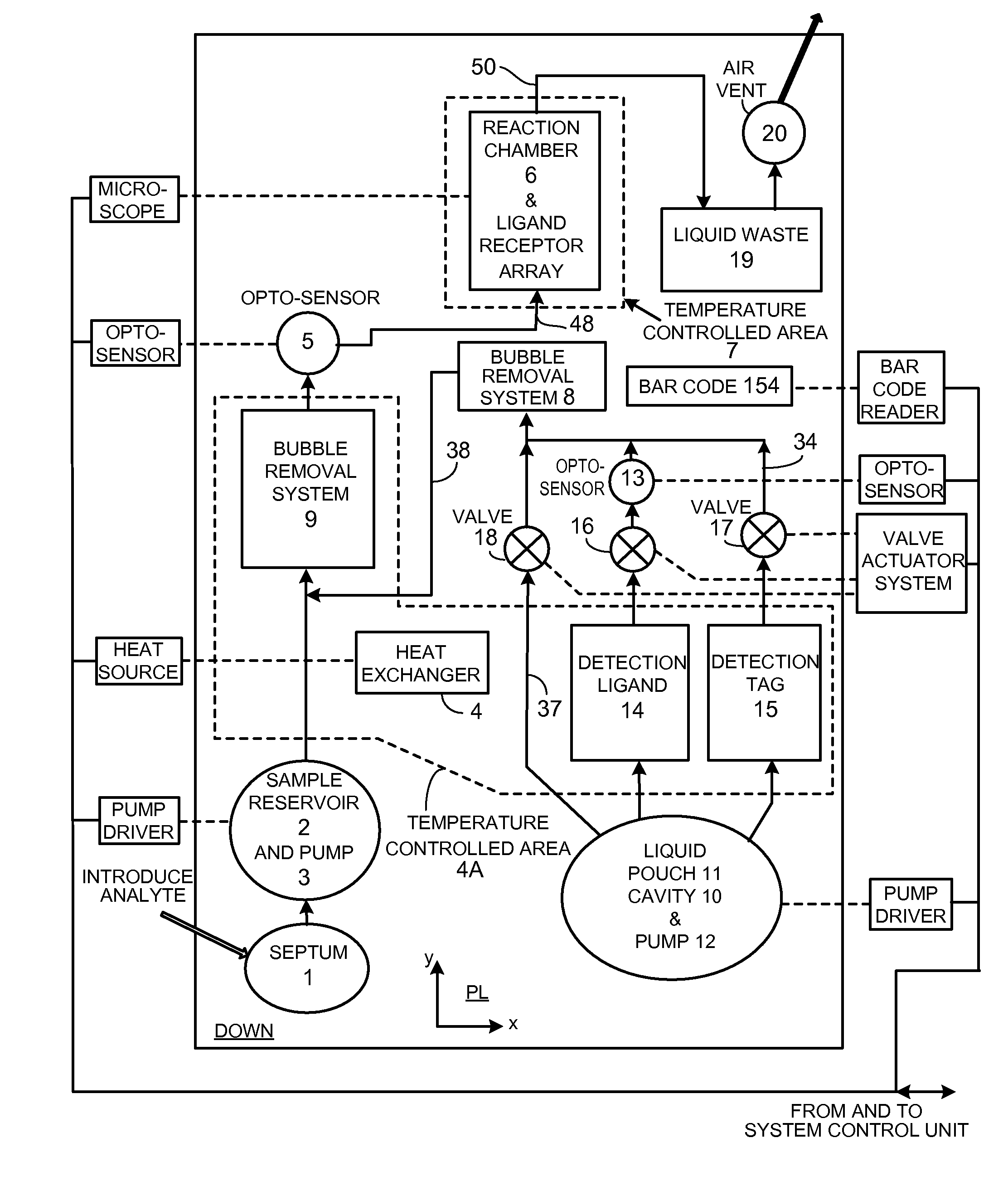
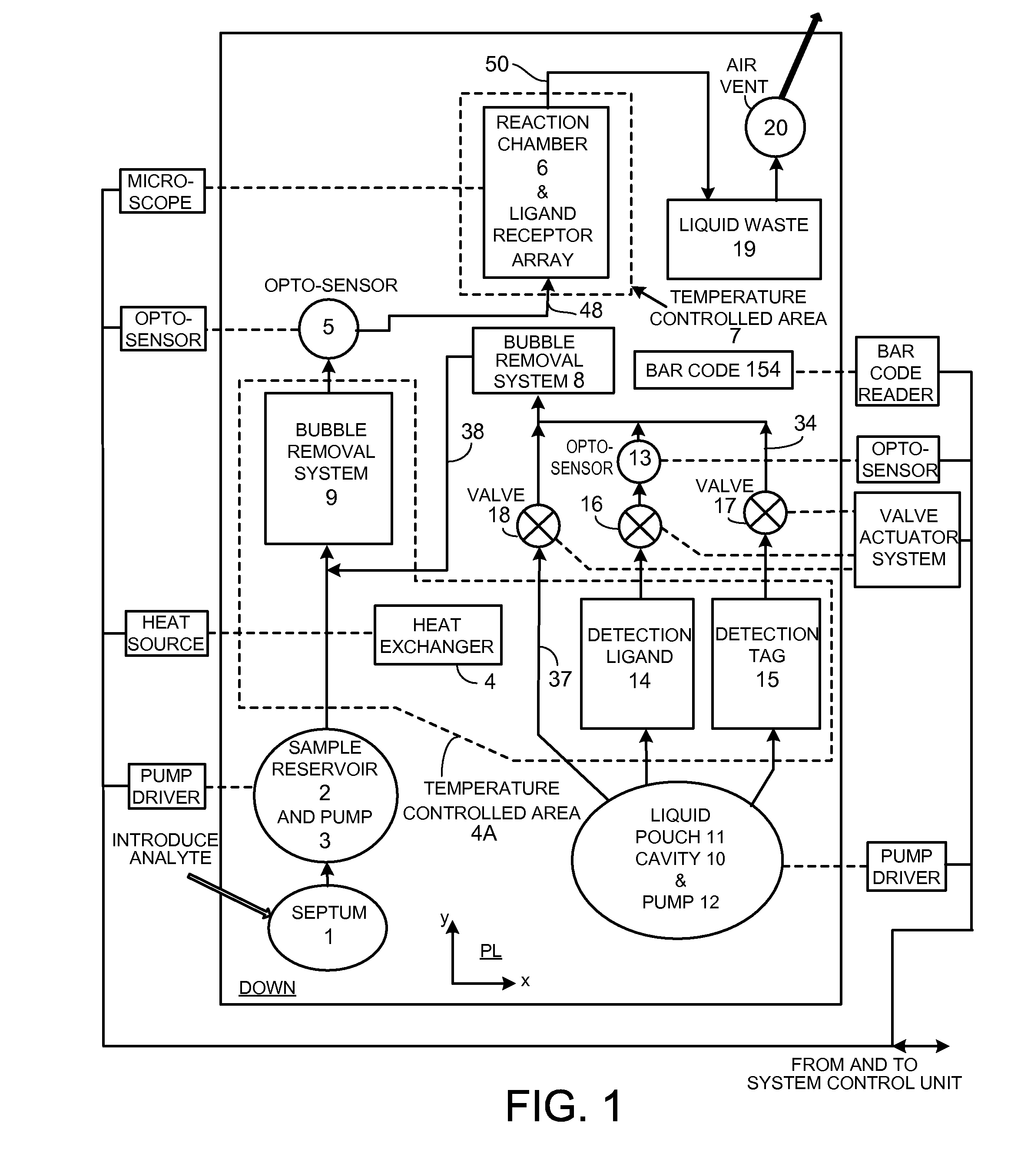
InactiveUS20110207621A1Highly consistent quantitative multiplex assaysLow costLibrary screeningFlexible member pumpsFritBlister pack
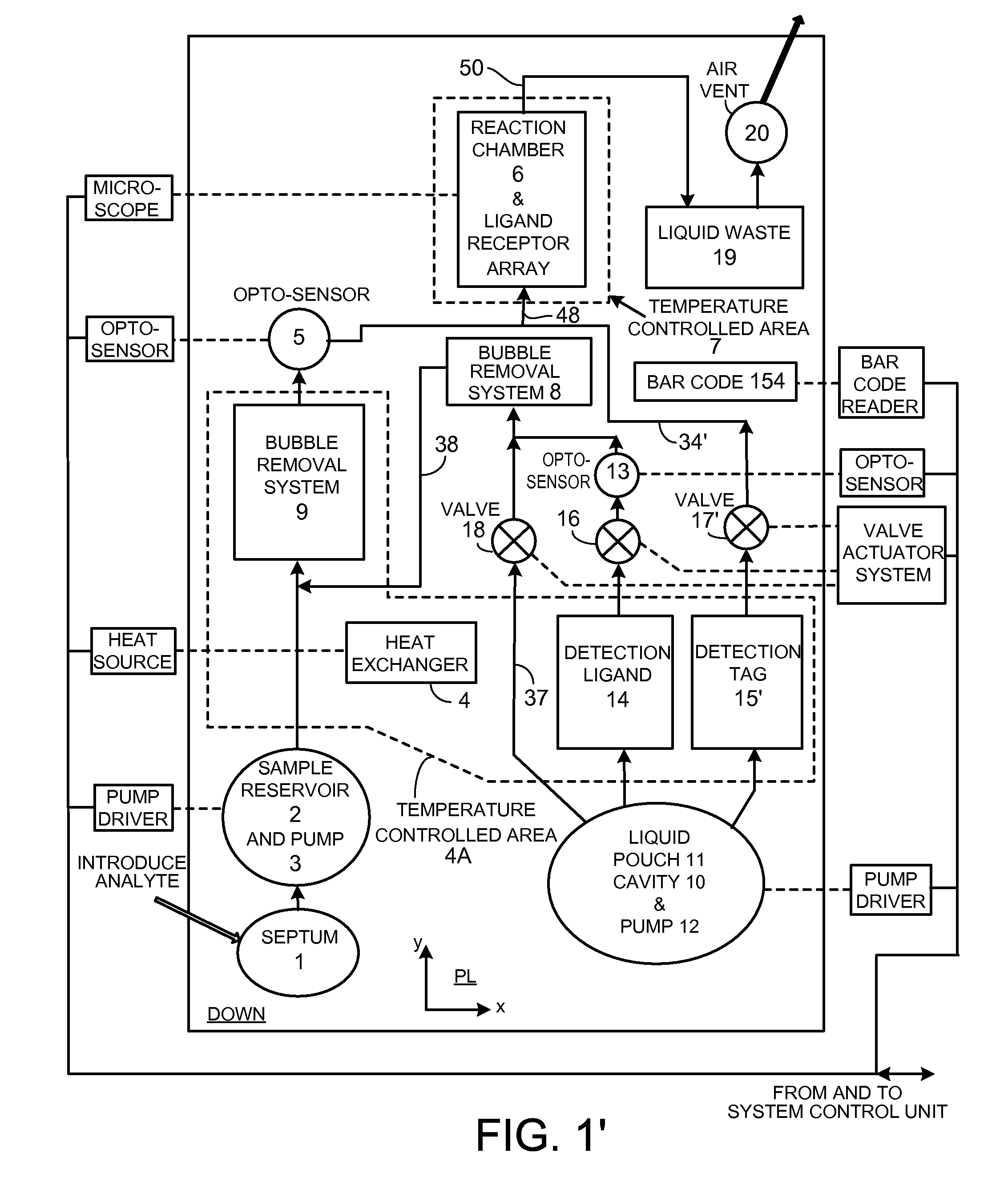
Flow-through assay reaction chamber (6) of cassette has back and forth liquid mixing in narrow gap (G) over array of capture agent (S), with net flow advance to waste confinement (19), produced by reversible pumps (3 or 12), operable with rolling diaphragm action with at least limited elastic recovery that advance sample or buffer liquids through conditioning paths (4A, 8, 8′, 9, 14, 15, 15′) before reaching the reaction chamber (6). A single pump produces accurate flow control, liquid conditioning, e.g., liquefying dry reagent from internal surfaces of flow-dividing material (14a, 15A, 15A′, e.g. open cell foam or frit), heating (4A), and air bubble removal (8, 8′, 9), as well as replenishment of reagent while accomplishing mixing within the flow-through reaction chamber (6). Lower viscosity buffer liquid is arranged to propel higher viscosity reagent, the flow-dividing storage material preserving reagent concentration. A blister pack (11) acts as a reversible pump (12) in producing accurate forward and backward flows with the net flow advance. Cascaded bubble traps (8, 9) on the cassette render the system tolerant of minor pumping error during cassette priming.
Owner:AVANTRA BIOSCI CORP
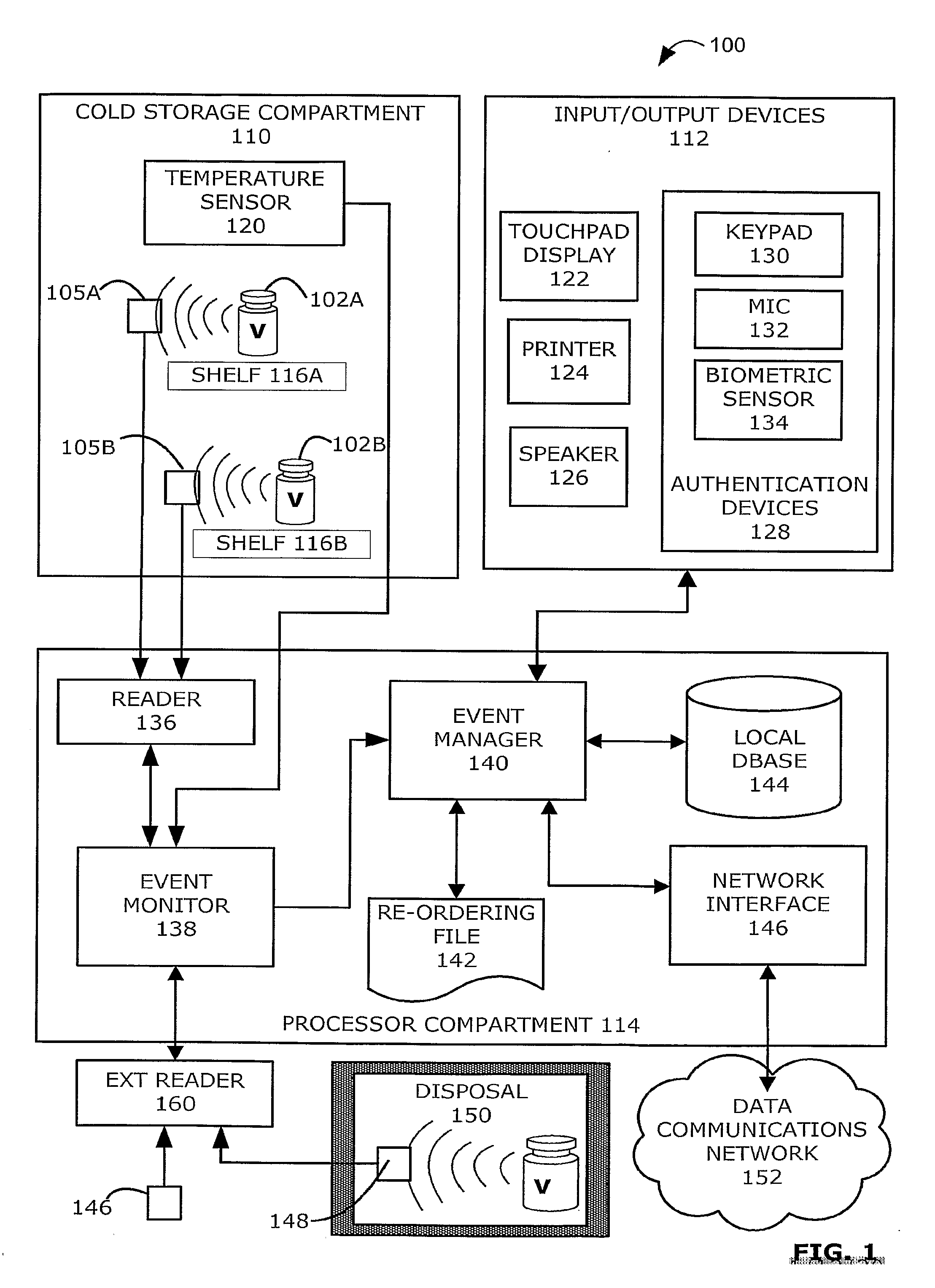
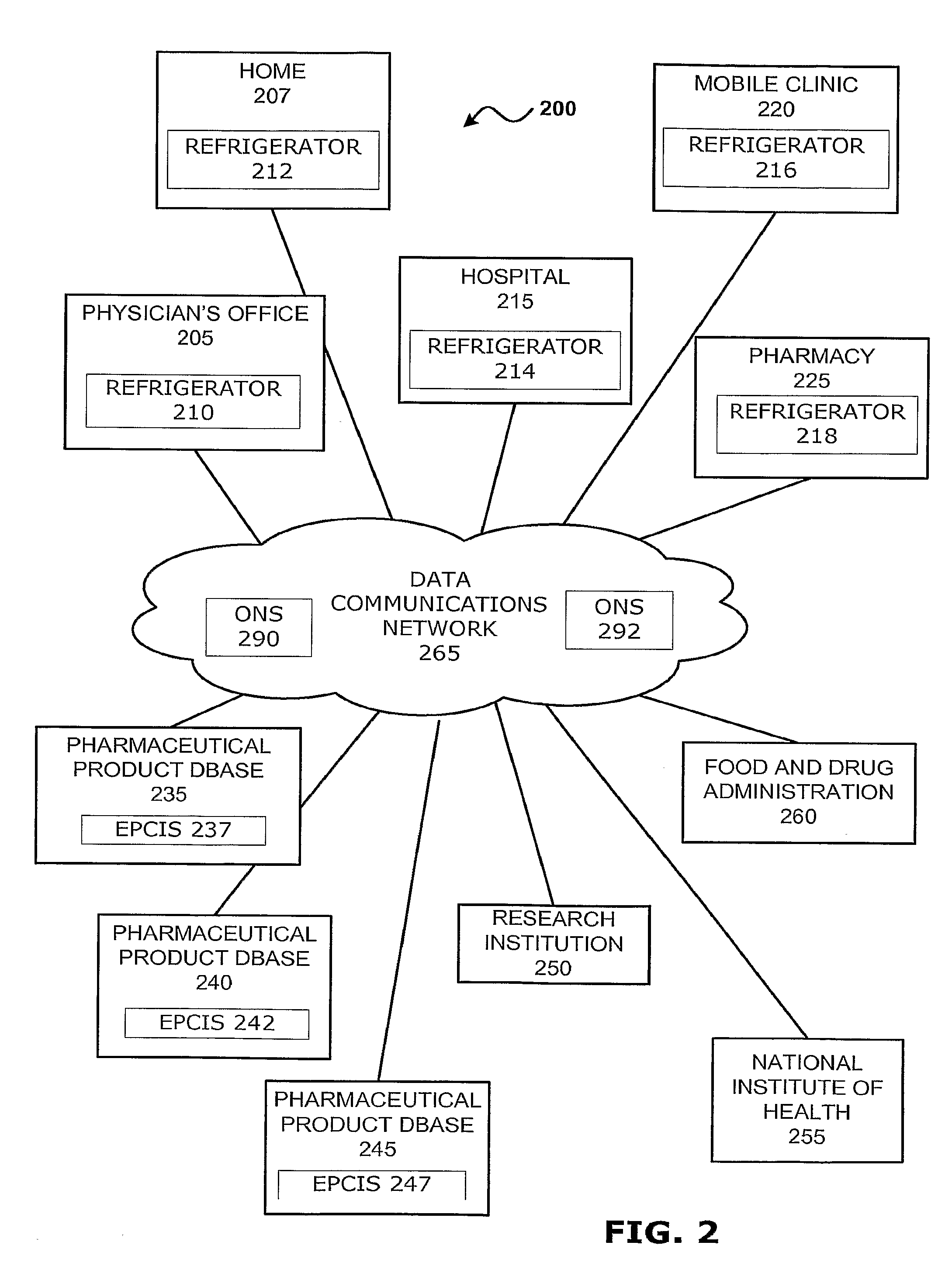
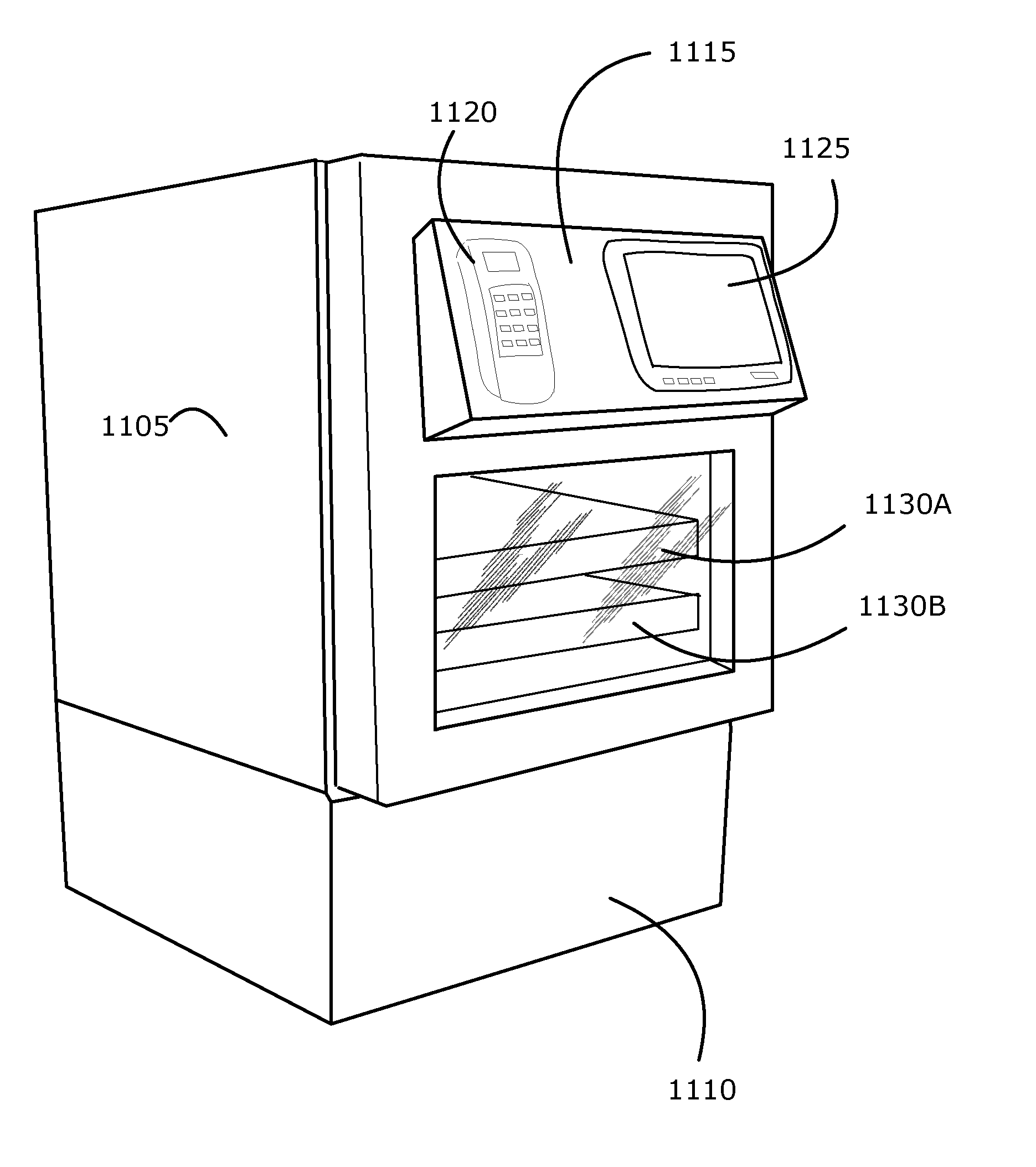
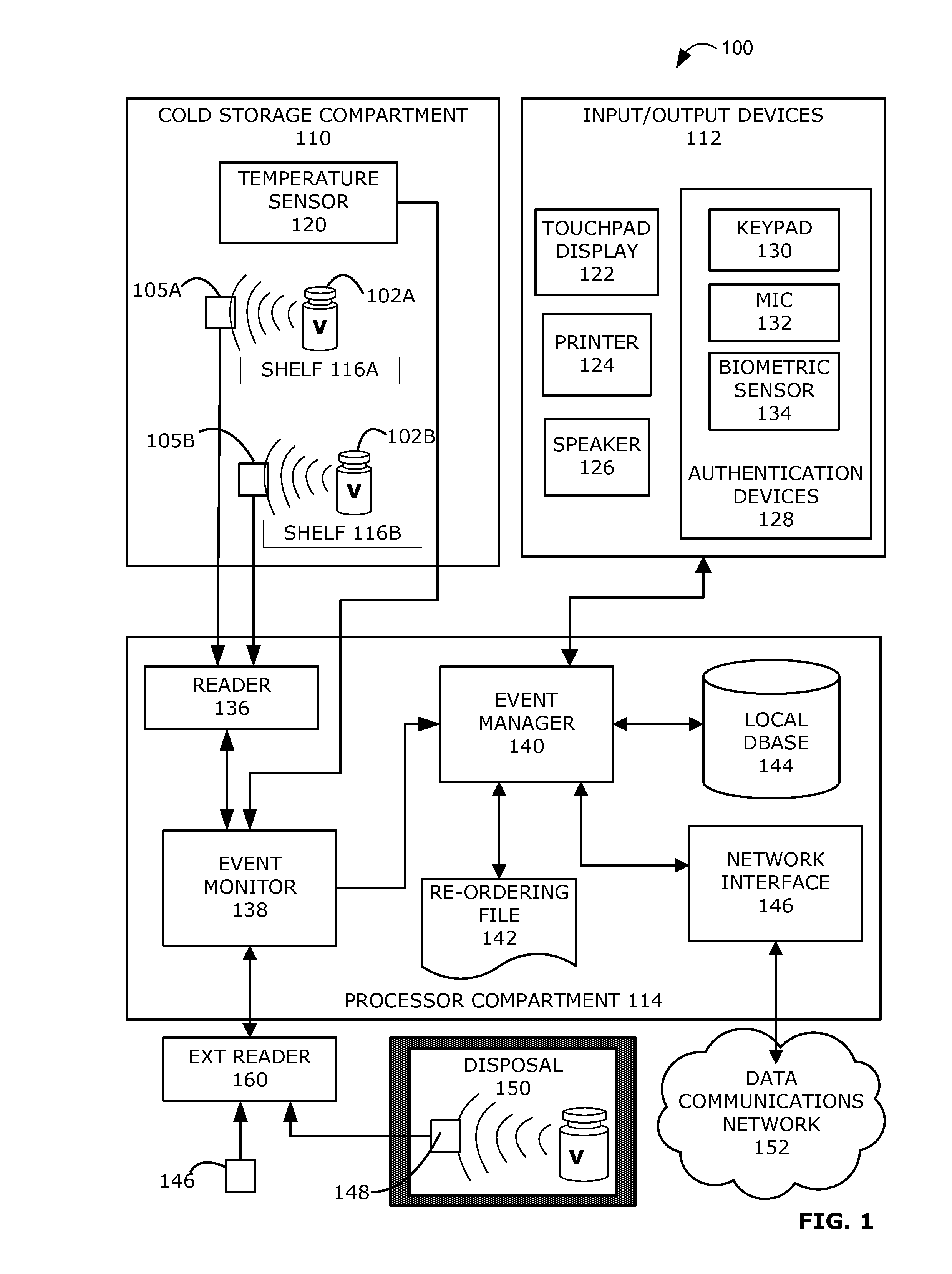
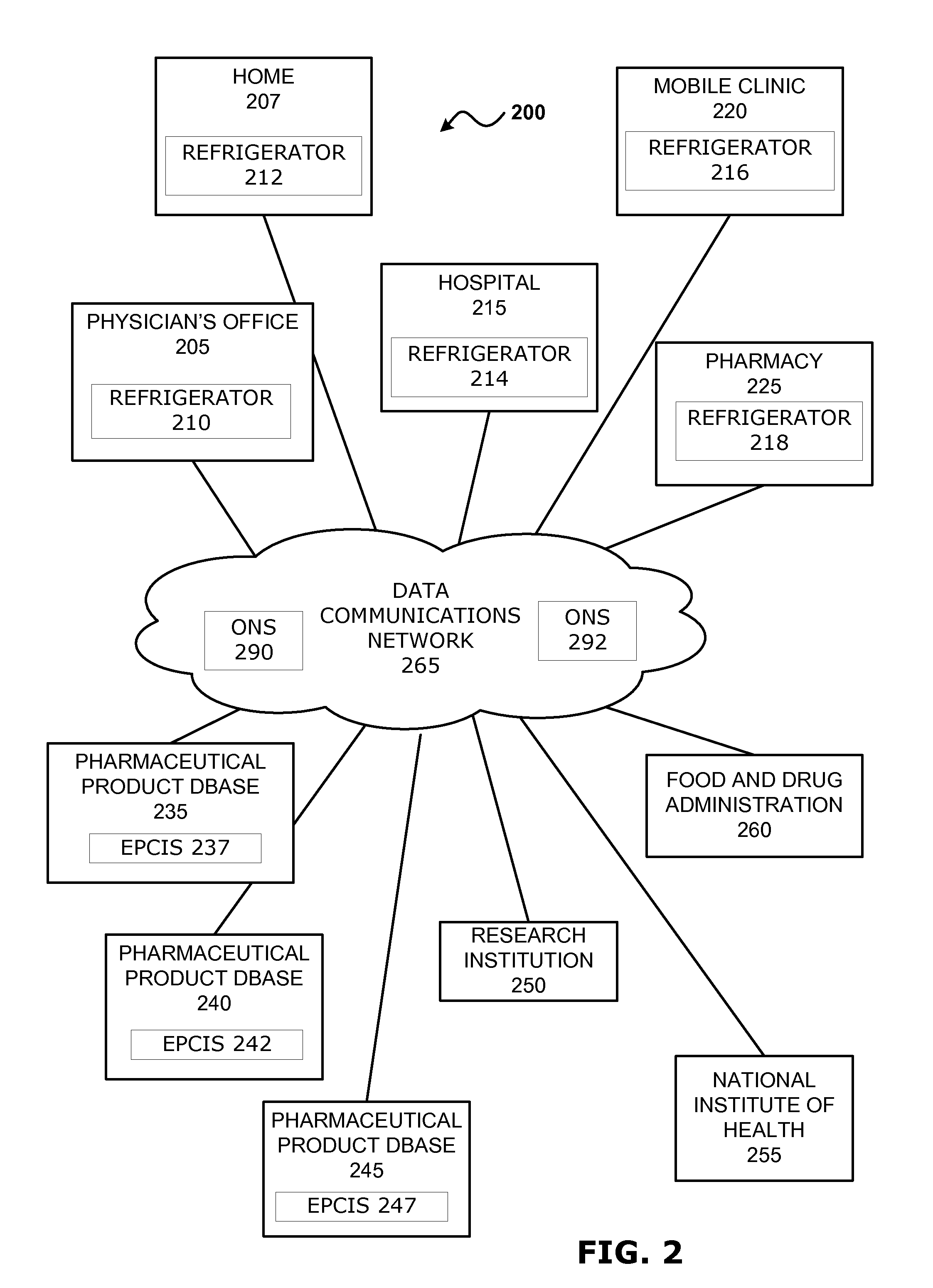
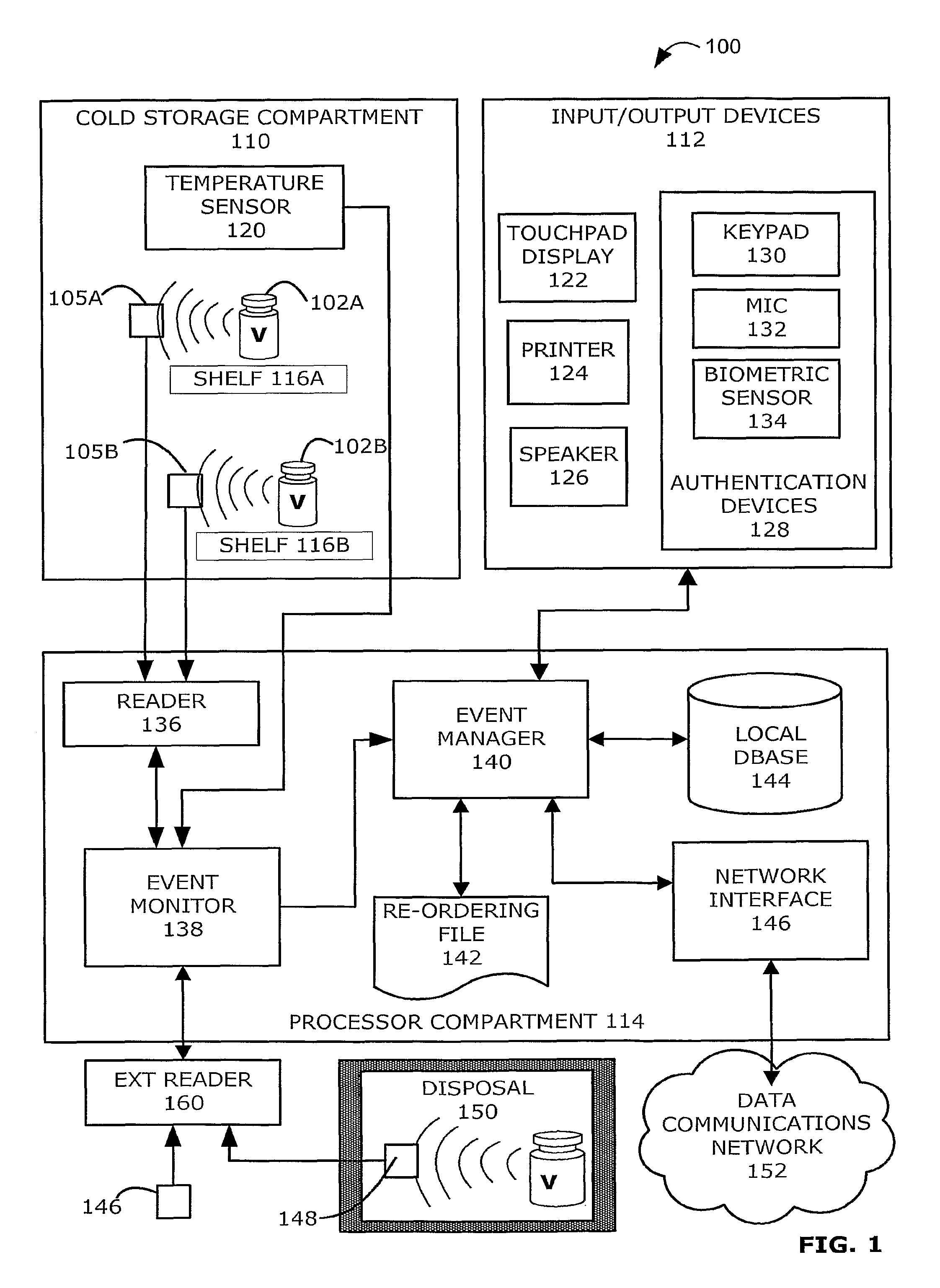
Intelligent Refrigerator for Storing Pharmaceutical Product Containers
Intelligent refrigerator system for storing pharmaceutical product containers, such as vials, ampules, syringes, bottles, medication tubes, blister packs and cartons, at the point of dispensing. Embodiments of the invention use product identification technology, such as radio-frequency identification (RFID) tags and readers, to uniquely identify containers as they are added to or removed from the cold storage compartment of the refrigerator, and automatically retrieve from a local or remote database a variety of details associated with the containers and their contents, such as manufacturing data, expiration dates, time out of refrigeration, inventory levels, safety information, usage statistics, known contraindications and warnings, etc. If the details indicate that there is a problem with a particular pharmaceutical (e.g., that it is counterfeit, expired, suspect, spoiled, recalled or almost depleted), then a message or warning is automatically delivered to a human operator via an attached output device, such as a display screen, speaker or printer. Embodiments of the invention may also be configured to monitor and report temperature faults, power failures and other anomalies associated with the refrigerator or cold storage compartment.
Owner:MERCK SHARP & DOHME LLC
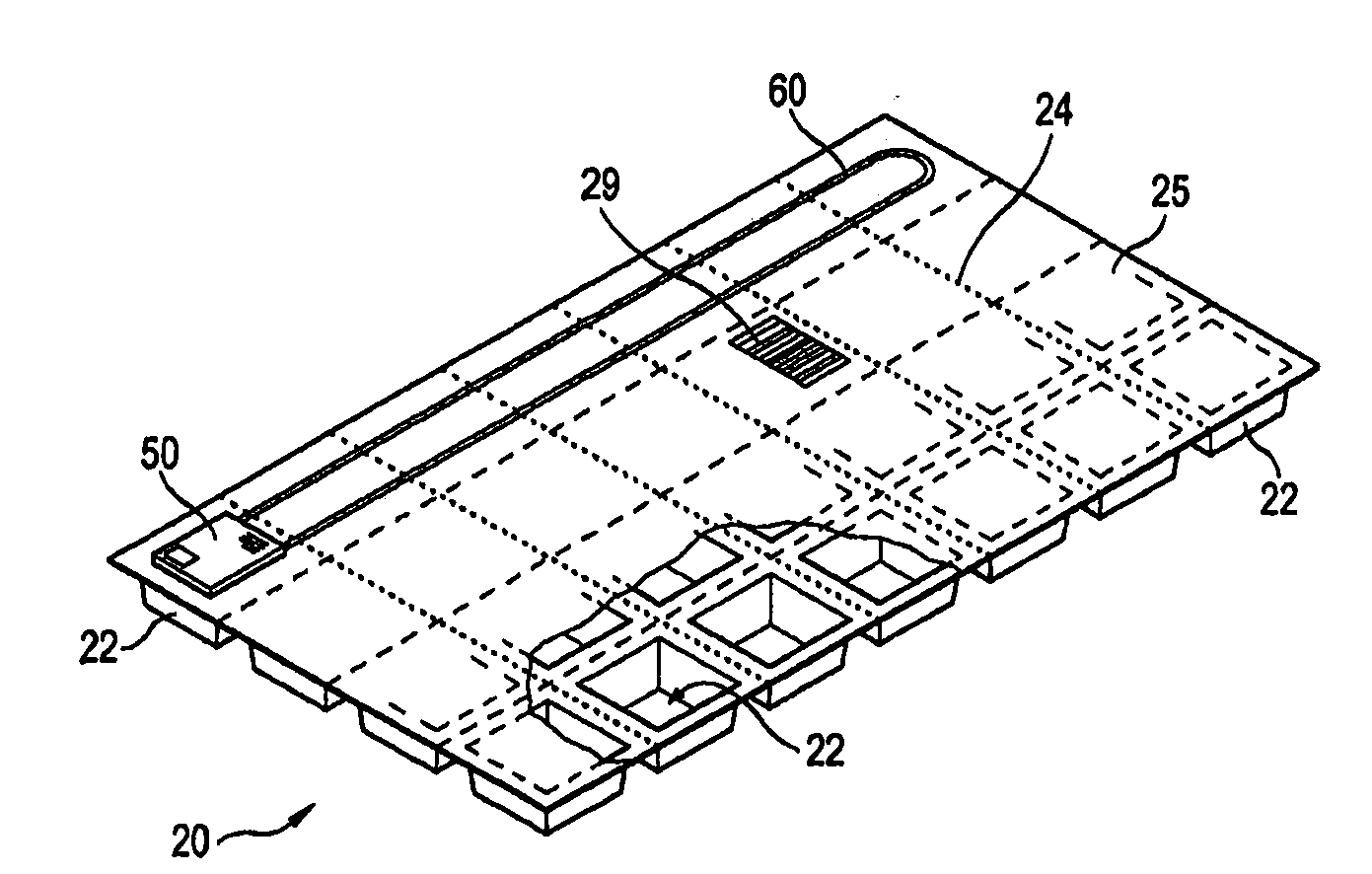
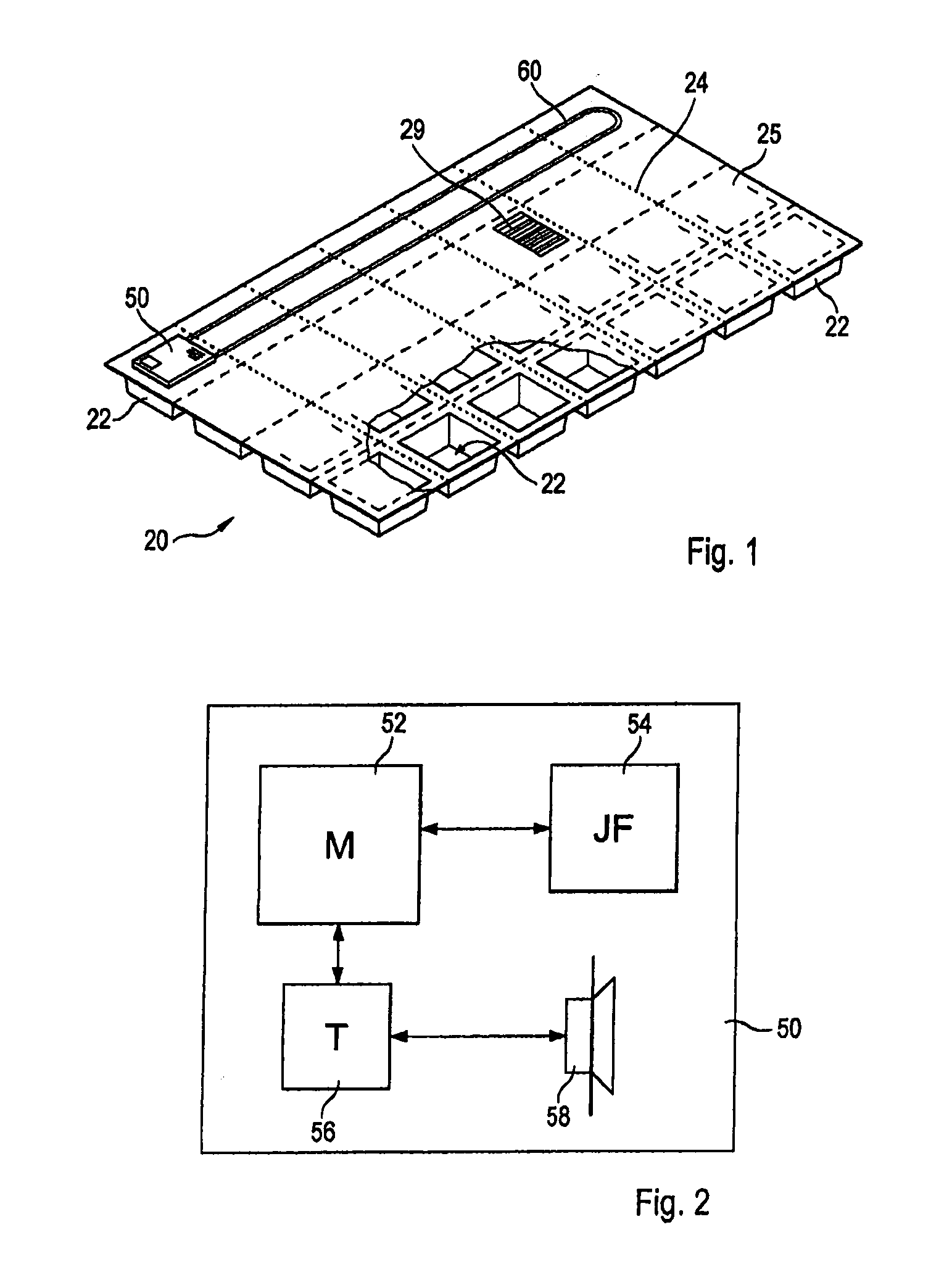
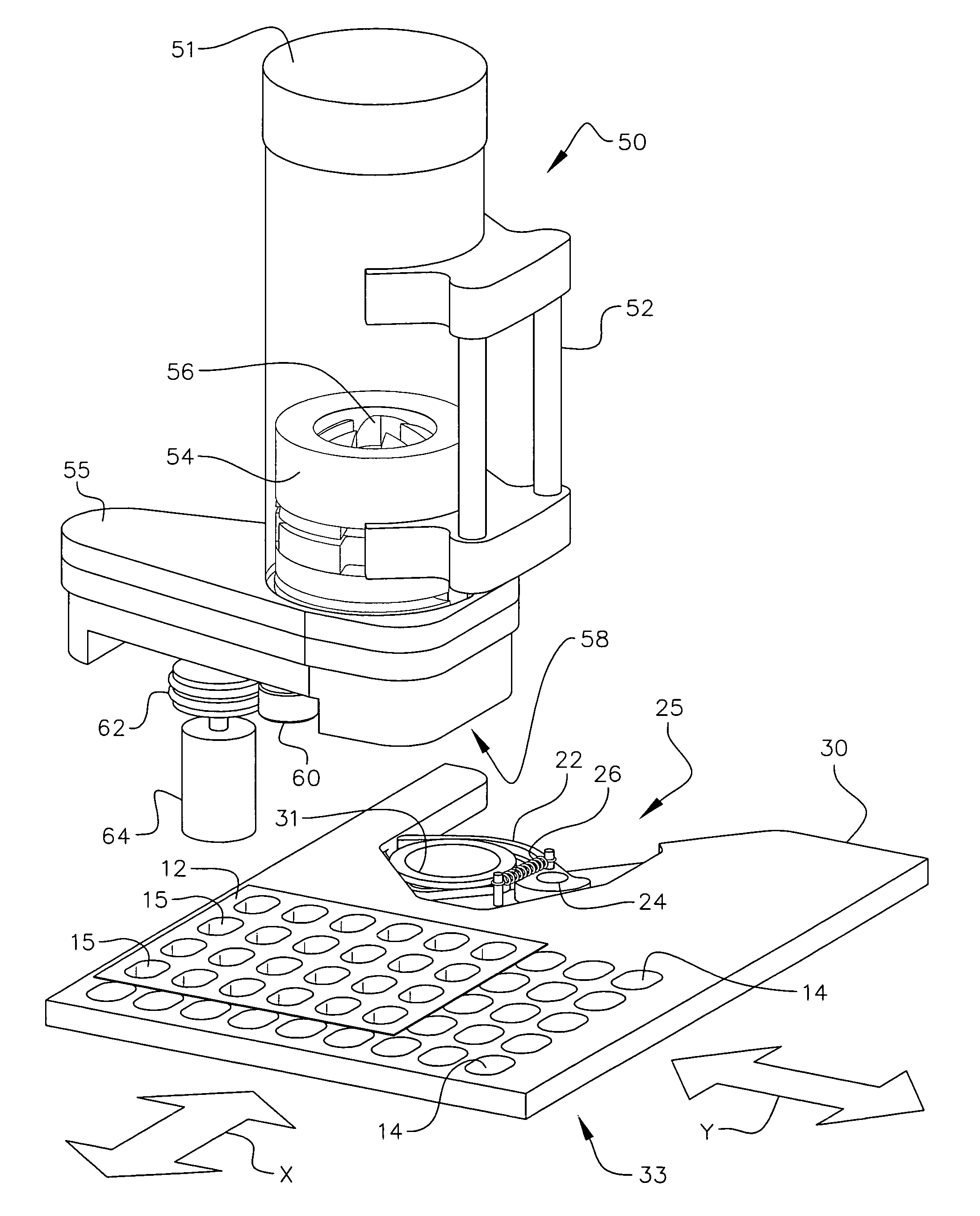
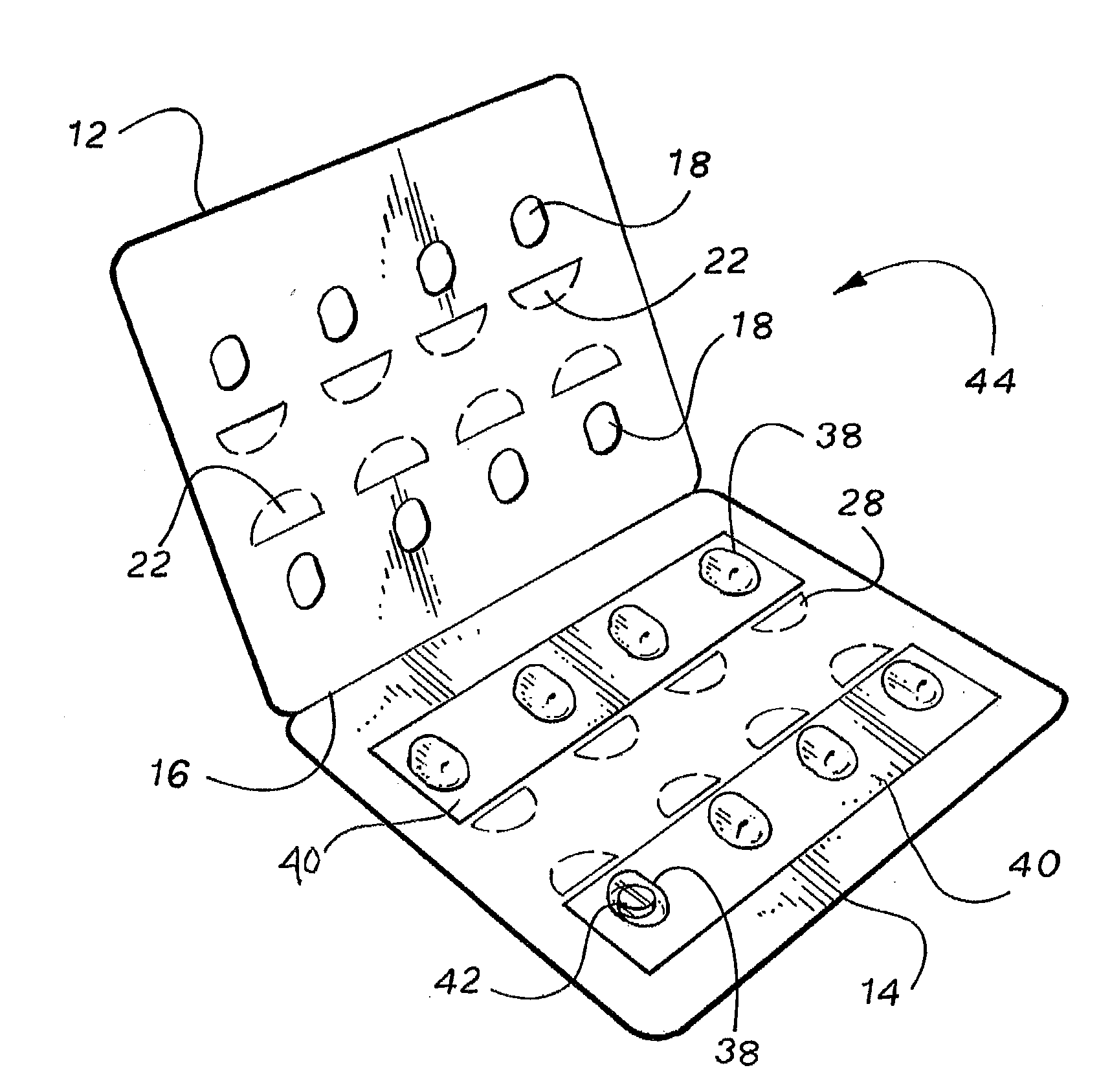
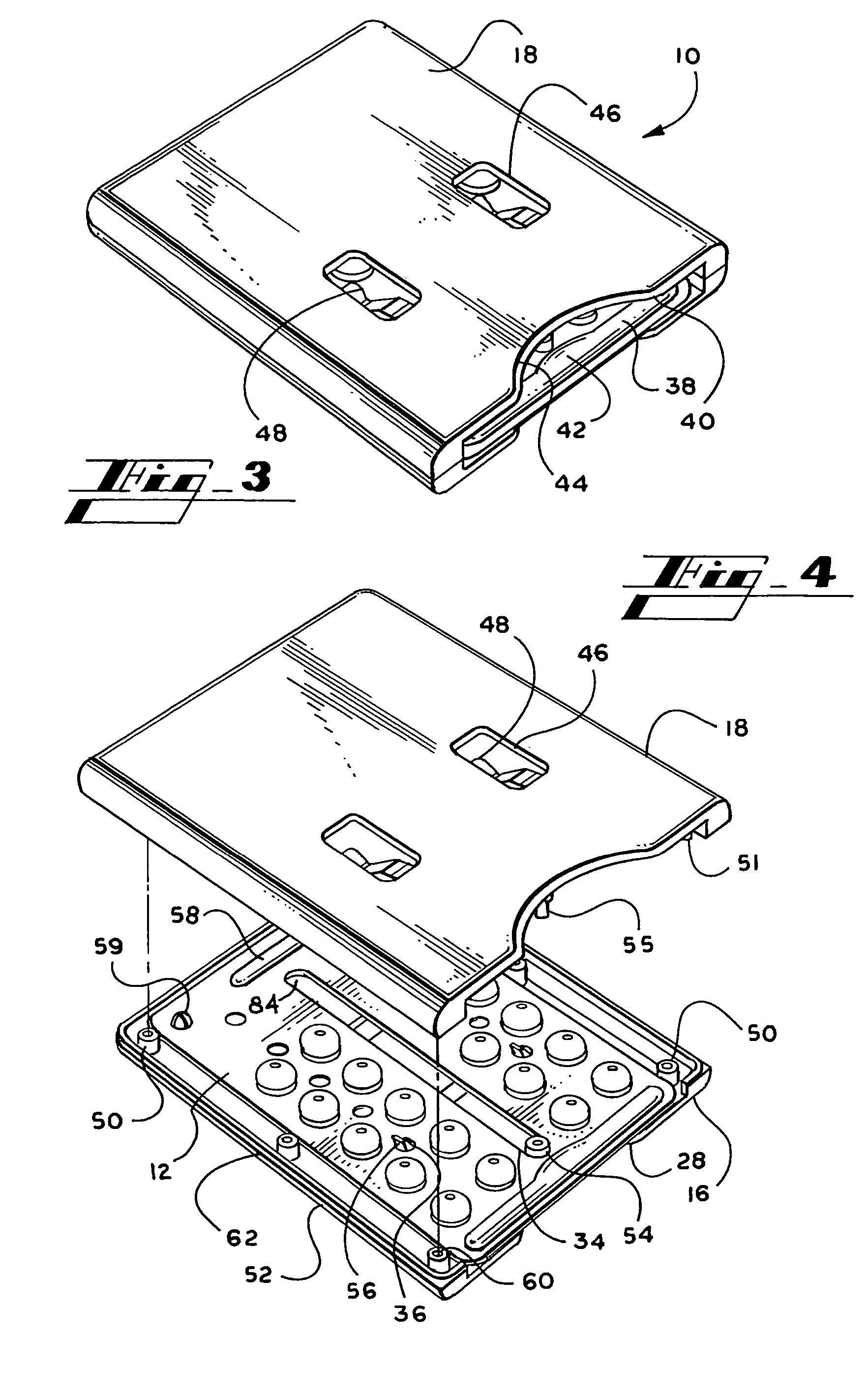
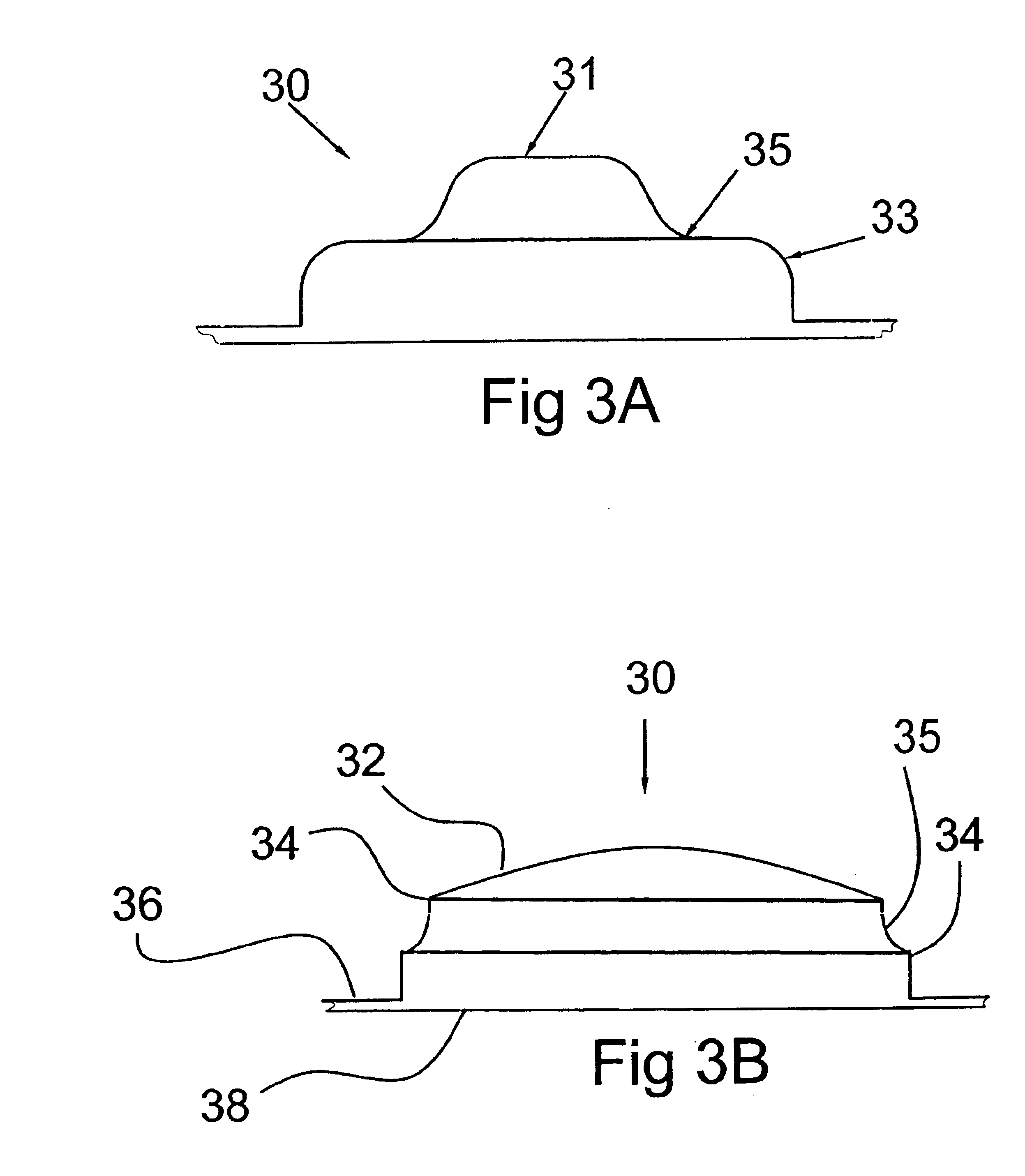
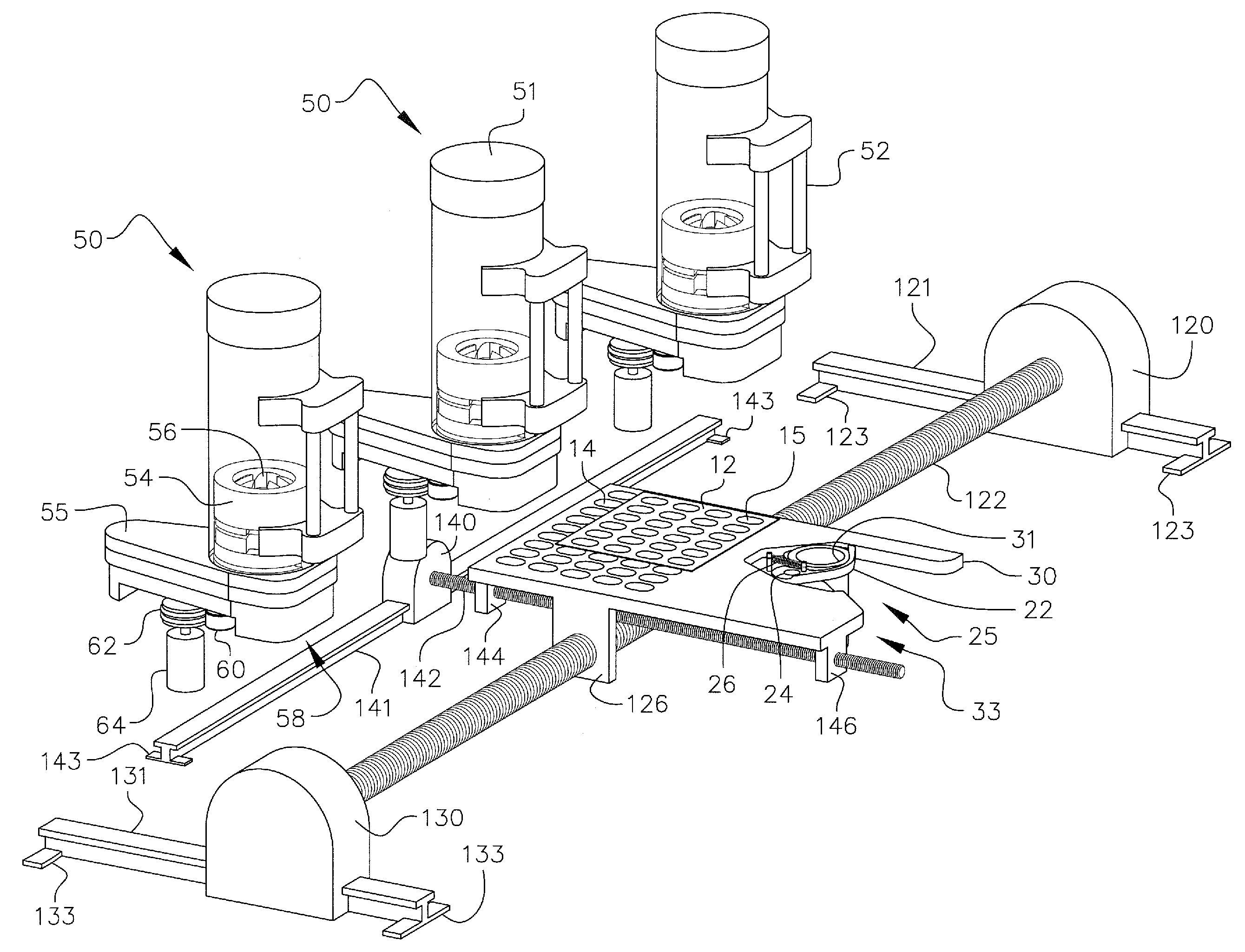
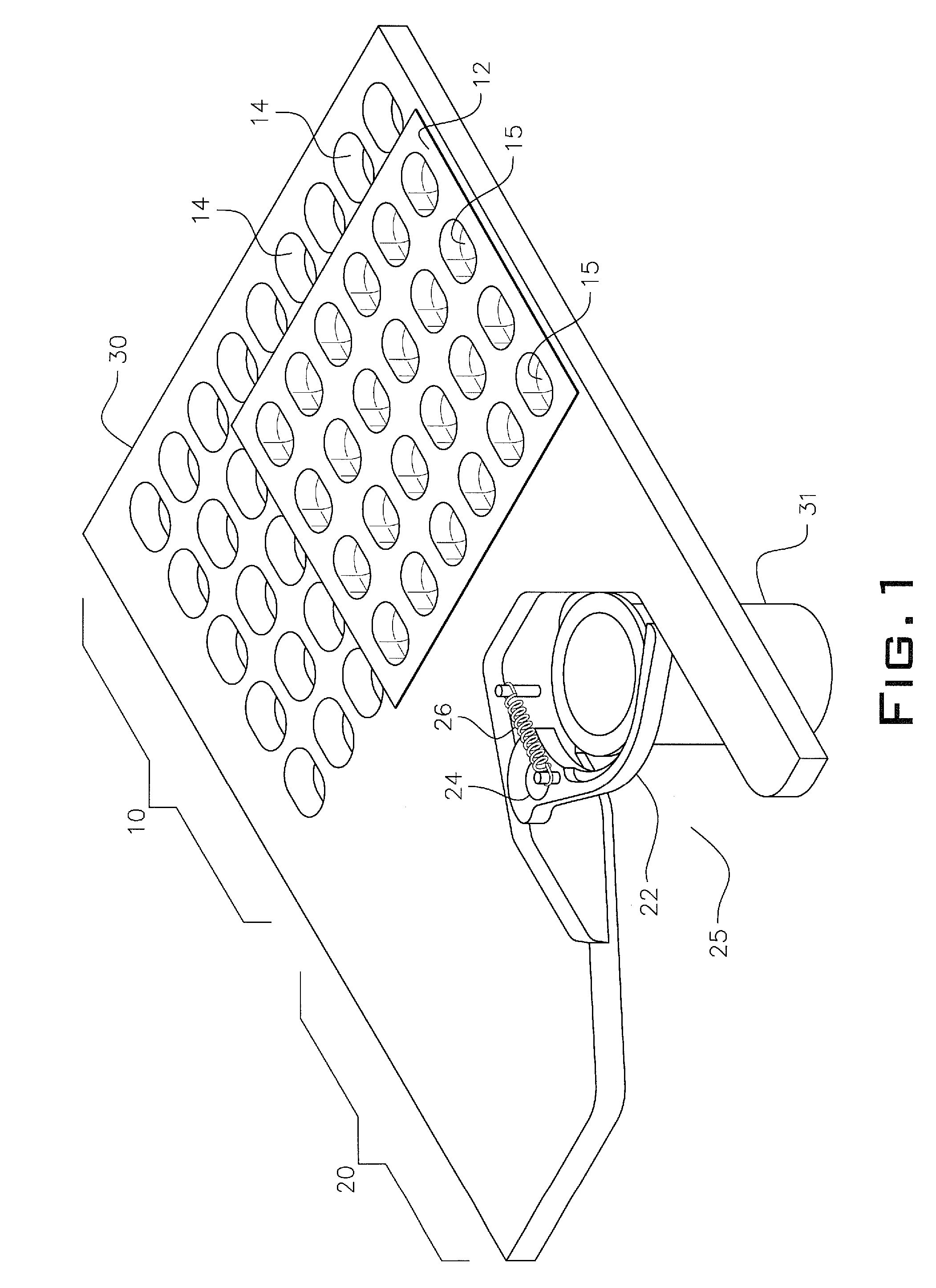
Personalized blister pack
InactiveUS20090277815A1Method securityImprove securitySmall article dispensingContainer/bottle contructionPersonalizationEngineering
A personalized blister pack (20) for the automated packaging of an individually defined product composition, particularly drugs, of a defined person, particularly a patient, for a defined time period comprises a plurality of receiving compartments (22), each closed by a film (25), for the products of the product composition, wherein the receiving compartments (22) are each associated with a defined usage time within the defined time period and disposed in matrix form, comprising a number of lines for usage days and columns for usage times during the day, and a memory device (50) for storing the usage data of the products of the product composition of the defined person for the defined time period, comprising a device (58) for generating a usage reminder signal as a reminder of a defined product usage time based on the stored usage data. The blister pack (20) enables a fast, safe and inexpensive supply of the needs of the person for a defined time period, such as one week, and by means of the memory device, which may comprise a reminder function, supports the planned use of the products.
Owner:KOHL EDWIN
Machine to automate dispensing of pills
A device having a plurality of cassettes, each filed with a supply of pills and positionable over a target location. The device has a platen beneath the target location with receptacles configured to hold both vials and blister packs. The platen or the cassette is movable so that any blister of the blister pack or the vial can be positioned under the target location to receive a quantity of pills from a cassette.
Owner:QEM INC
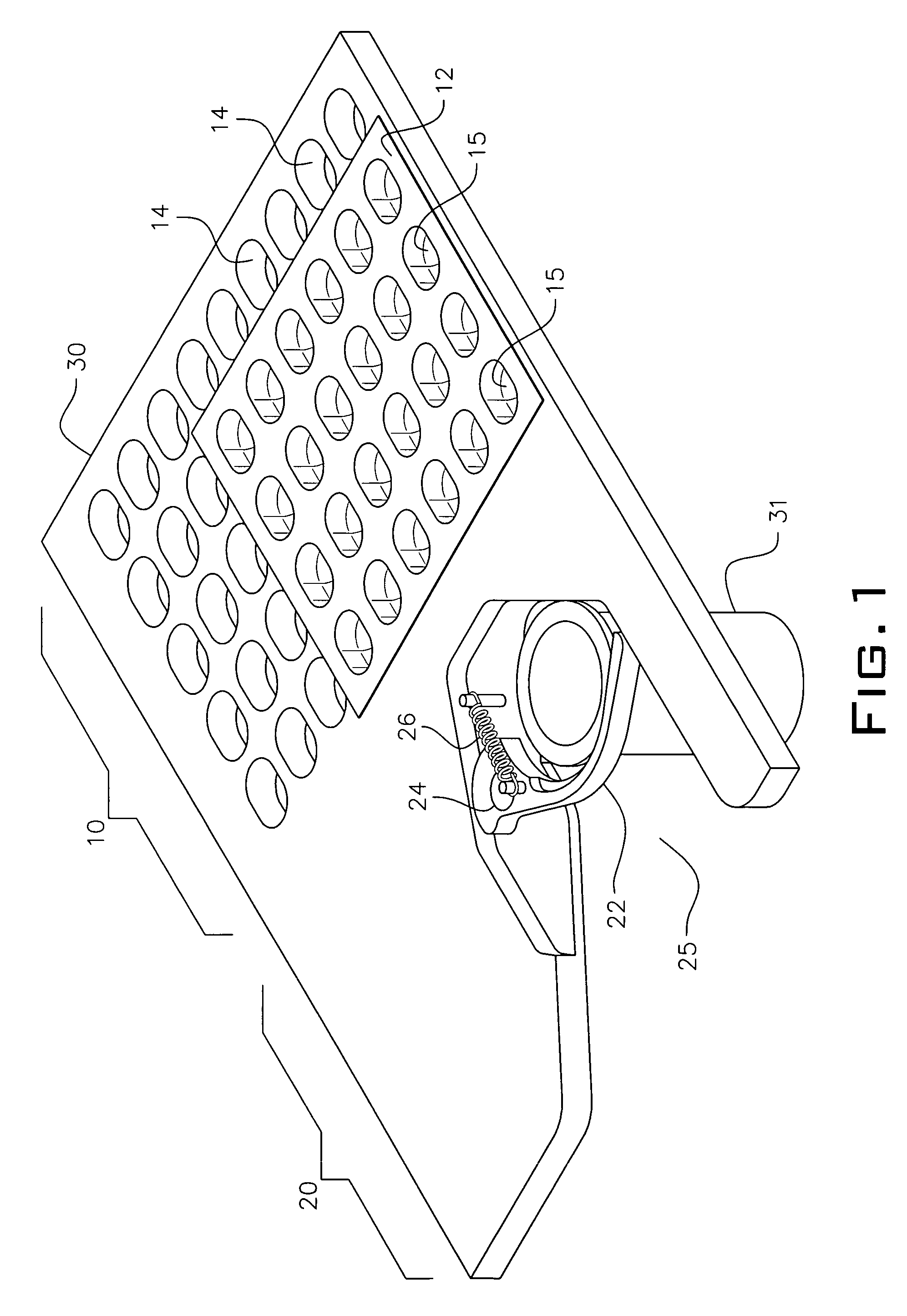
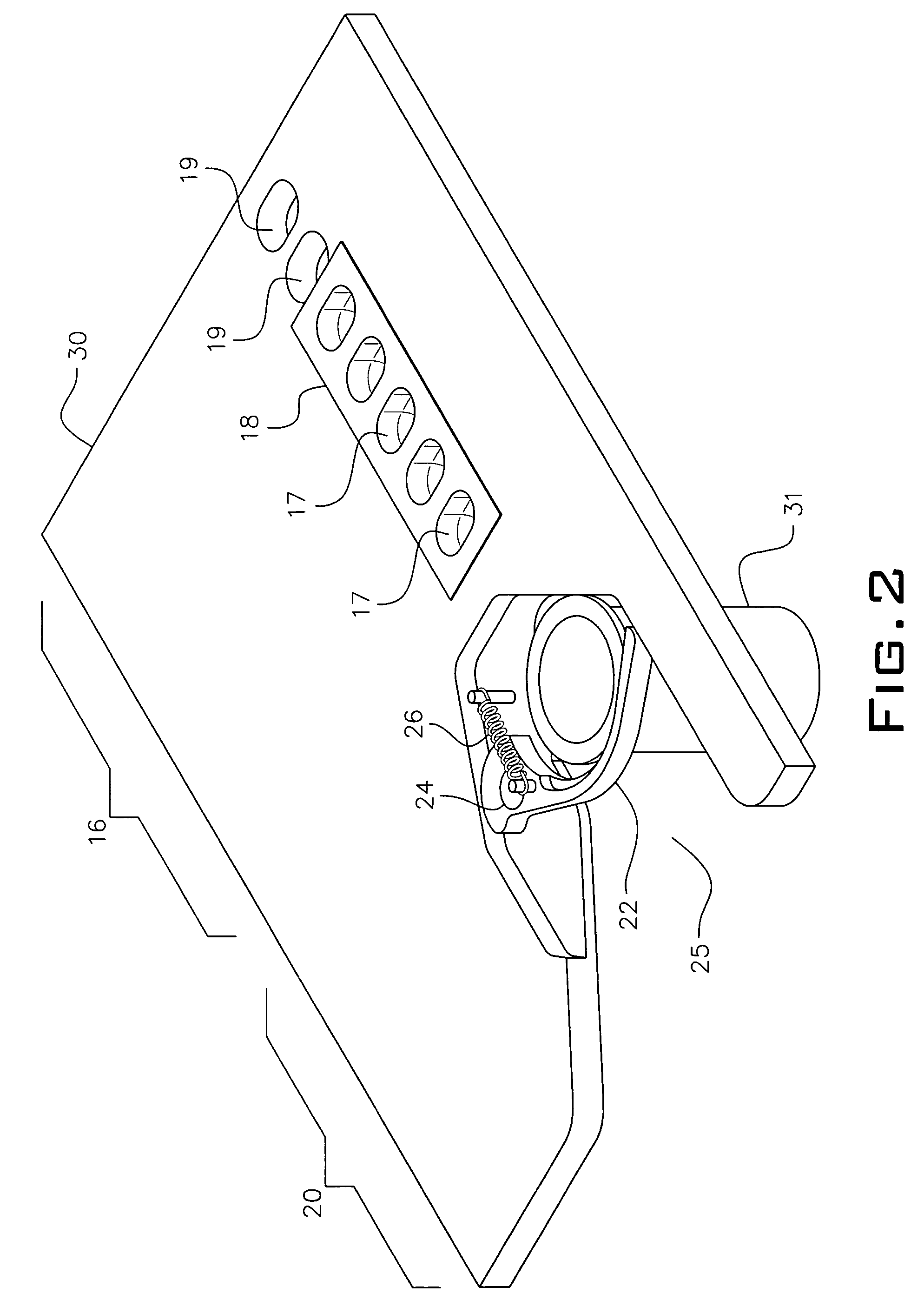
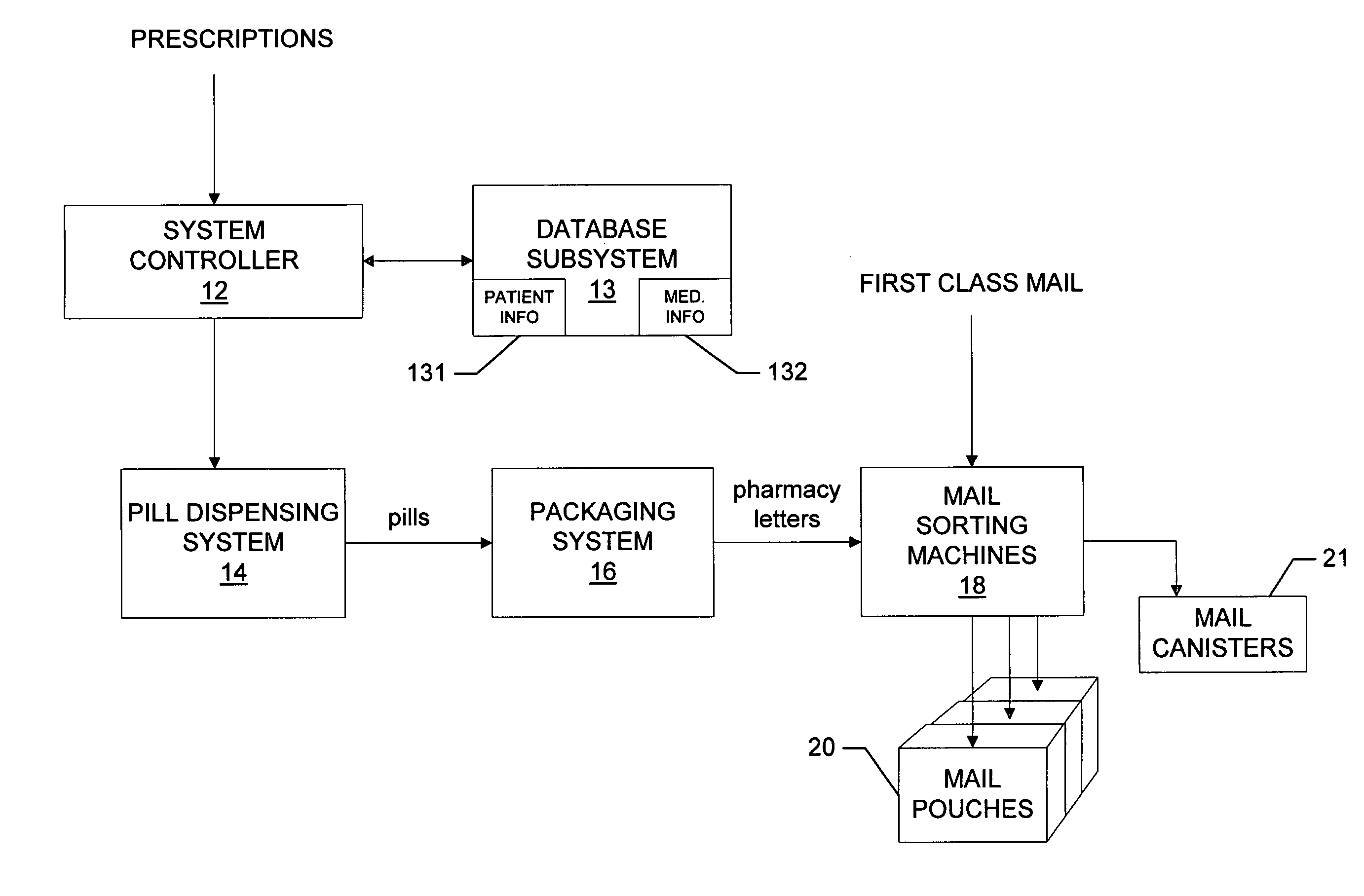
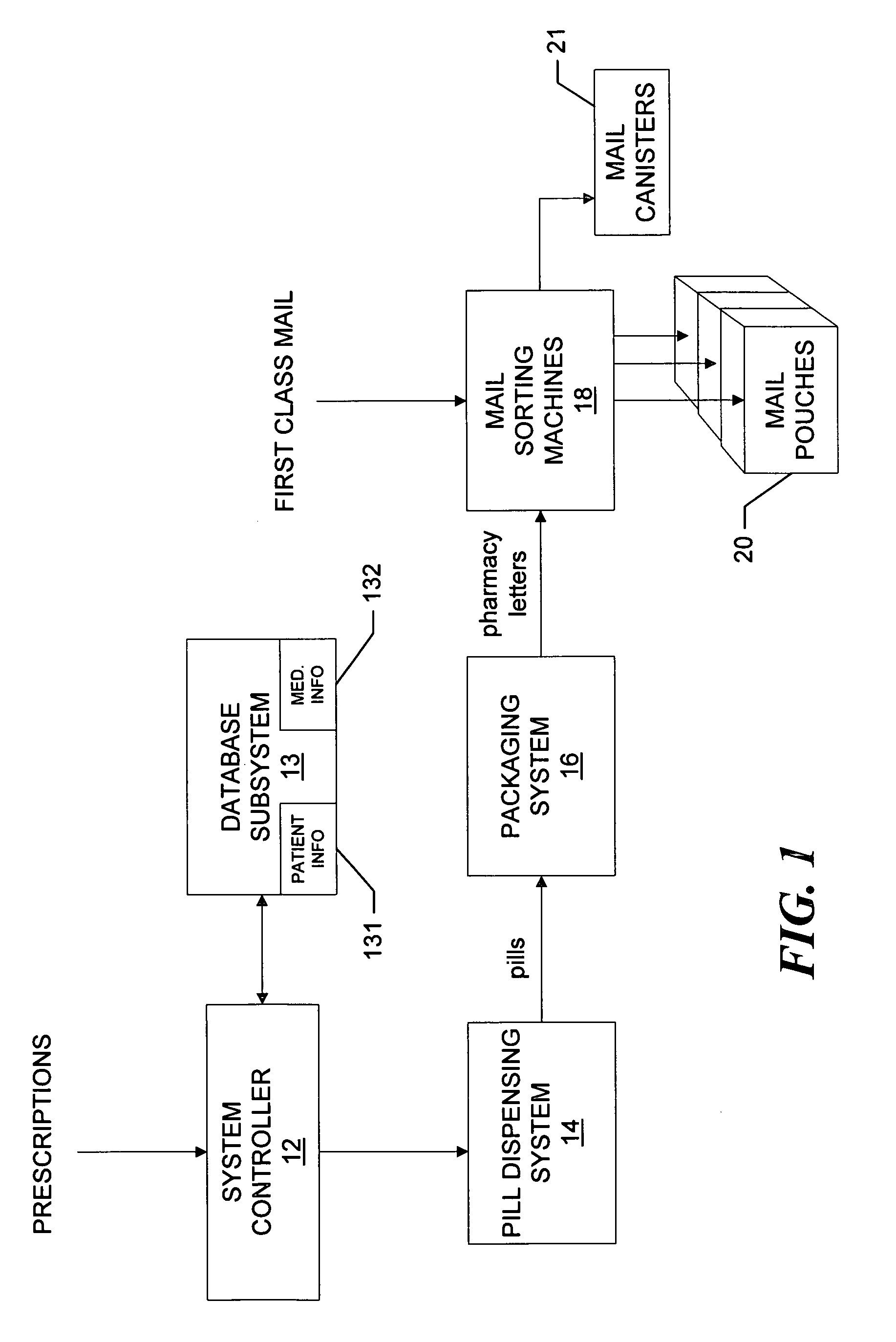
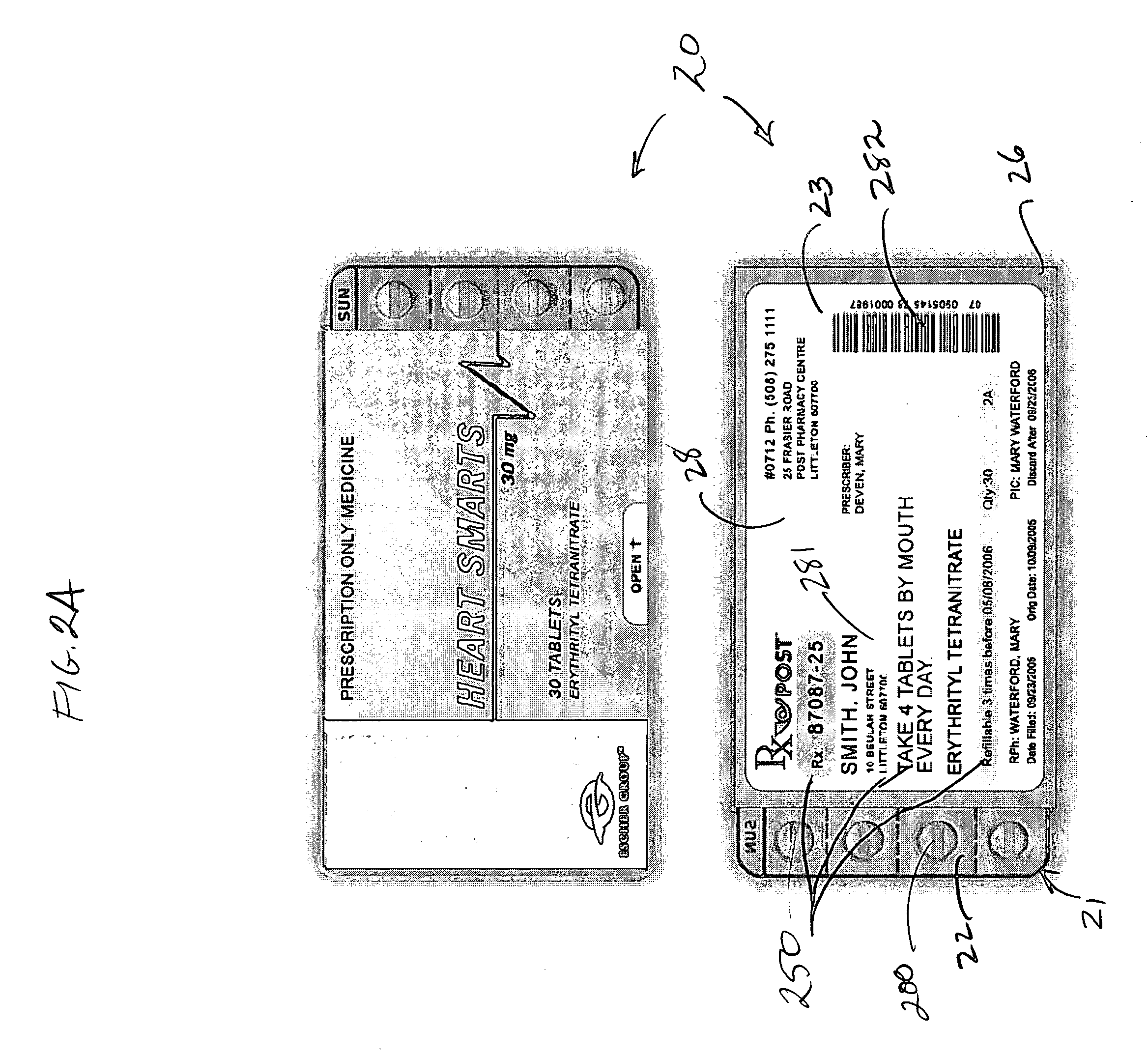
System and method for dispensing, sorting and delivering prescription and non-prescription medications through the post office
ActiveUS20060122729A1Controlling coin-freed apparatusDigital data processing detailsDispensaryNon prescription
Owner:ESCHER GROUP
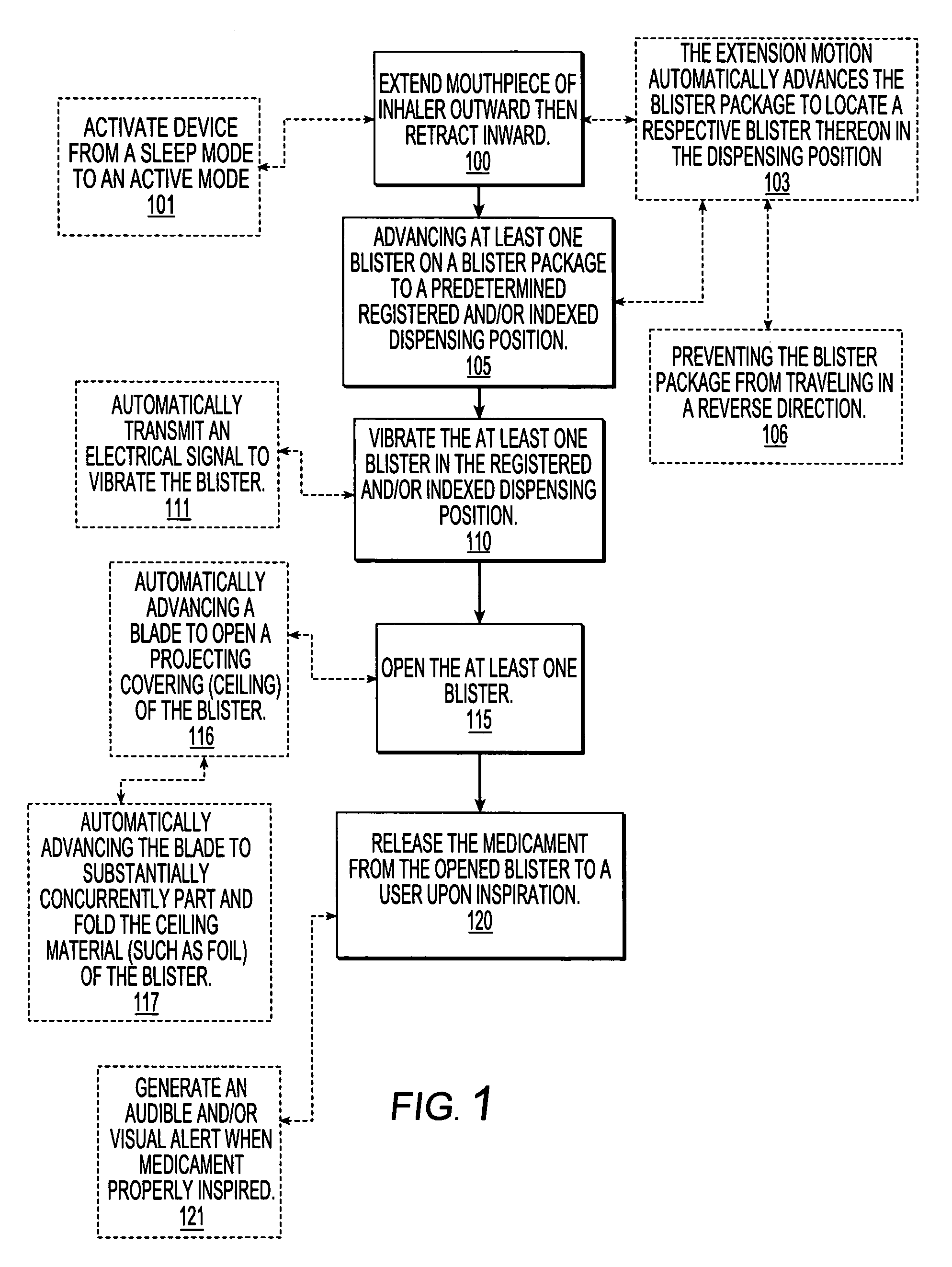
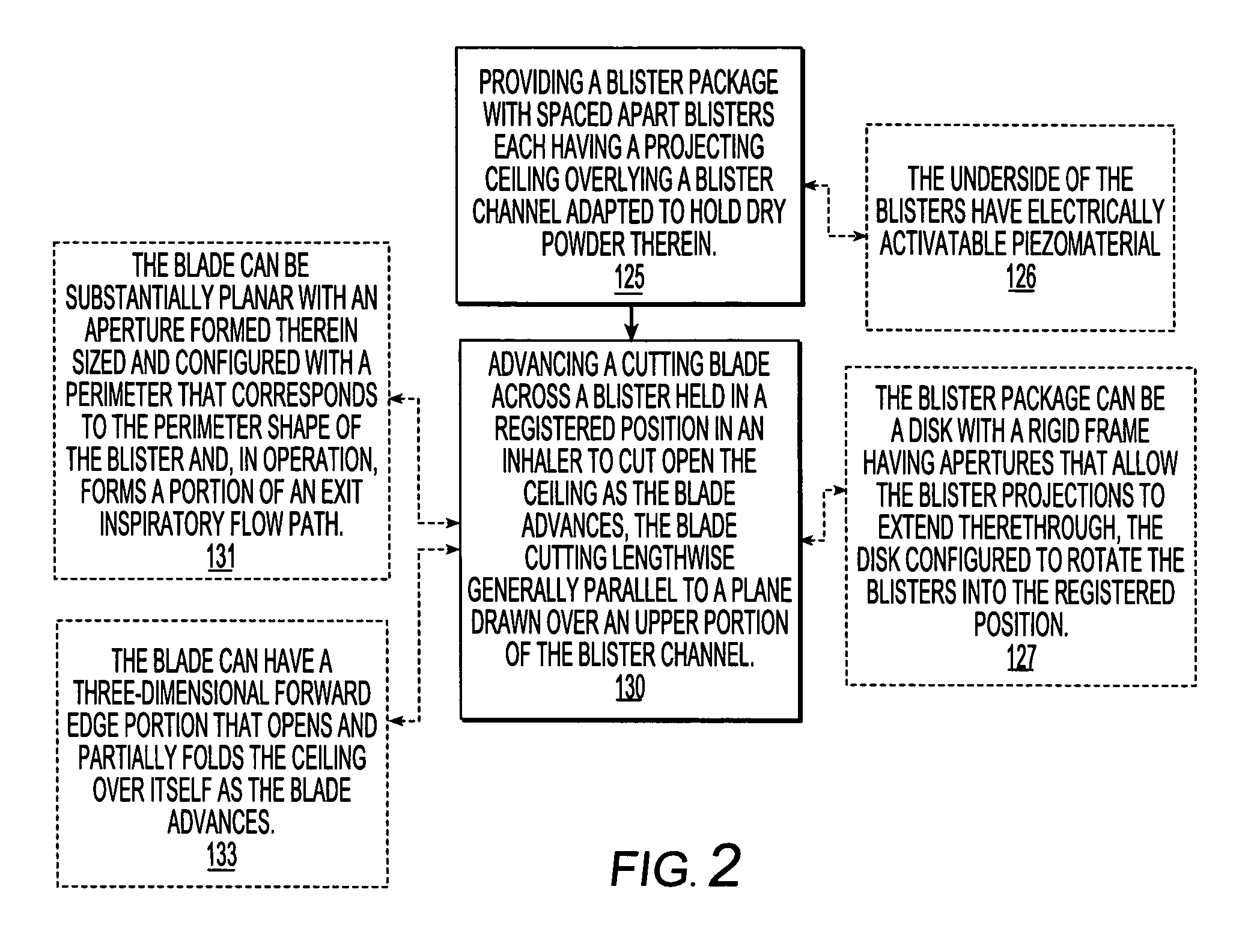
Dry powder inhalers, related blister package indexing and opening mechanisms, and associated methods of dispensing dry powder substances
Dry powder inhalers with a multi-dose dry powder package for dispensing pharmaceutical grade formulations of inhalable dry powder, include: (a) a blister package comprising a plurality of spaced apart sealed blisters thereon, each blister having a projecting ceiling and a floor defining a blister channel therebetween, the blister channel comprising a dry powder therein; (b) a movable blade cartridge holding a blade at a forward portion thereof; and (c) an extendable mouthpiece attached to the movable blade cartridge. In operation, a user pulls the mouthpiece outward and then pushes the mouthpiece inward to cause the blister package to advance to position a blister in a selected dispensing position in the inhaler and to cause the blade cartridge to move the blade across a blister ceiling held in the dispensing position in the inhaler to thereby open the blister held in the dispensing position.
Owner:ORIEL THERAPEUTICS INC
Intelligent refrigerator for storing pharmaceutical product containers
Intelligent refrigerator system for storing pharmaceutical product containers, such as vials, ampules, syringes, bottles, medication tubes, blister packs and cartons, at the point of dispensing. Embodiments of the invention use product identification technology, such as radio-frequency identification (RFID) tags and readers, to uniquely identify containers as they are added to or removed from the cold storage compartment of the refrigerator, and automatically retrieve from a local or remote database a variety of details associated with the containers and their contents, such as manufacturing data, expiration dates, time out of refrigeration, inventory levels, safety information, usage statistics, known contraindications and warnings, etc. If the details indicate that there is a problem with a particular pharmaceutical (e.g., that it is counterfeit, expired, suspect, spoiled, recalled or almost depleted), then a message or warning is automatically delivered to a human operator via an attached output device, such as a display screen, speaker or printer. Embodiments of the invention may also be configured to monitor and report temperature faults, power failures and other anomalies associated with the refrigerator or cold storage compartment.
Owner:MERCK SHARP & DOHME LLC
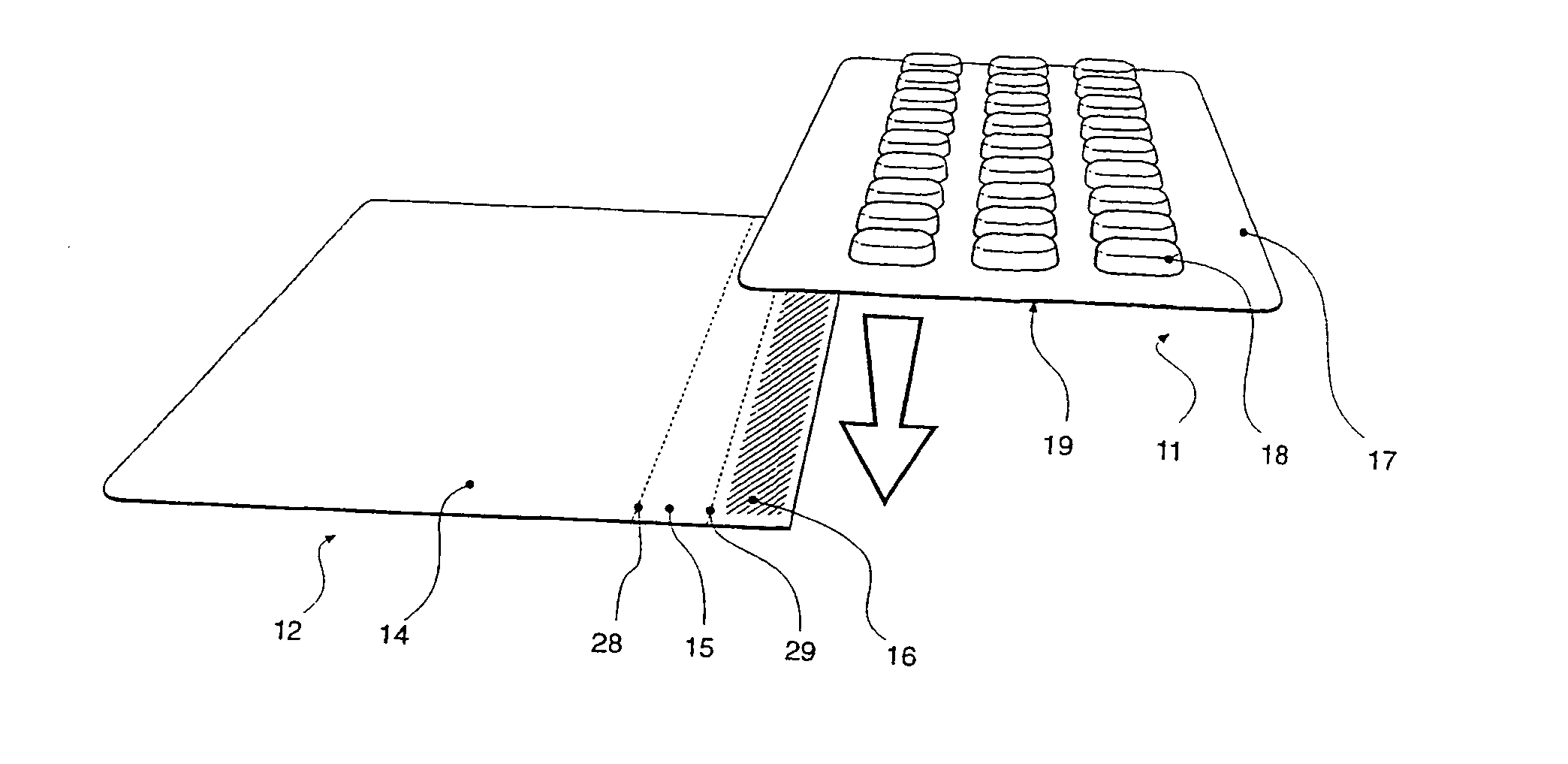
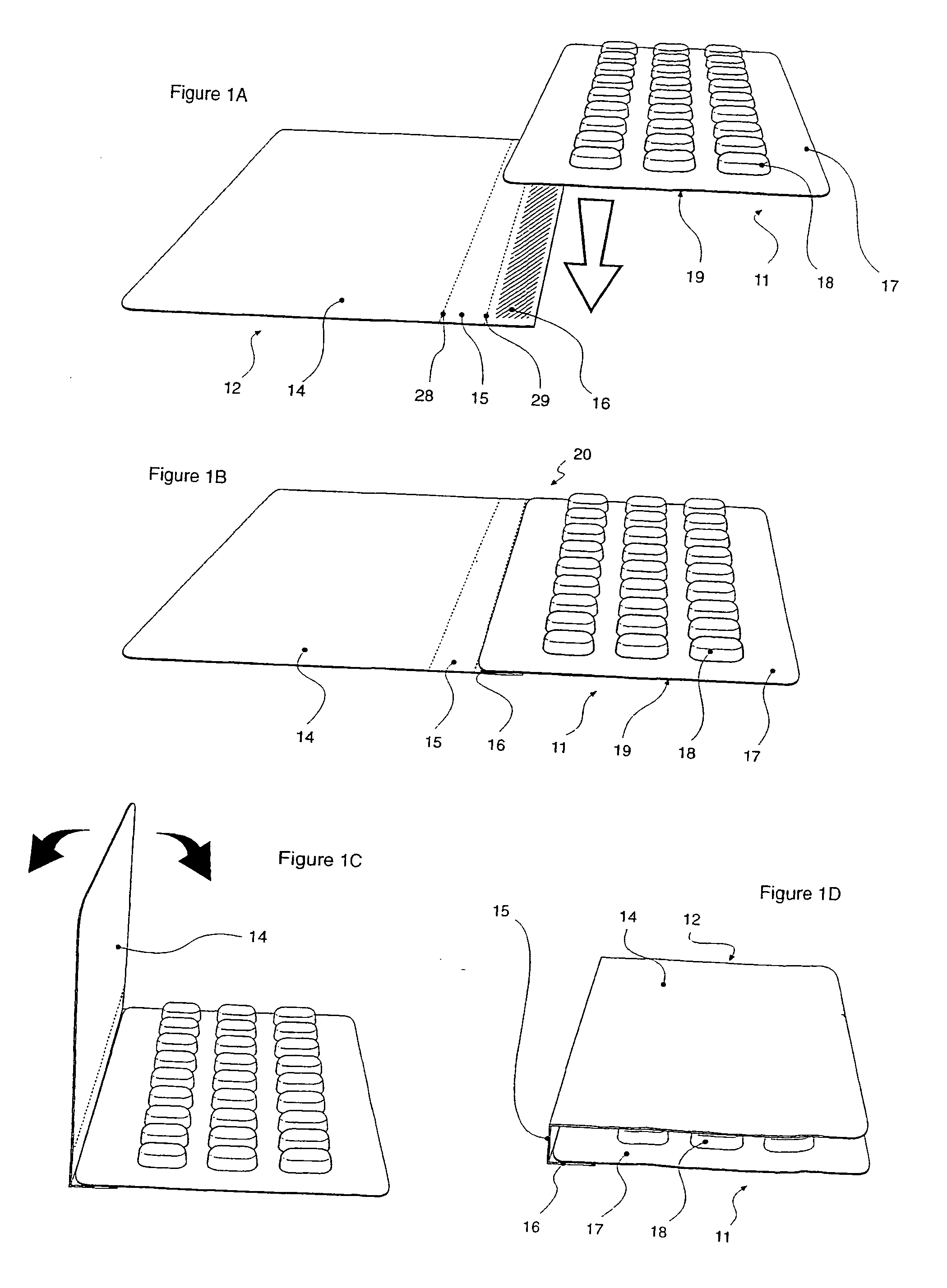
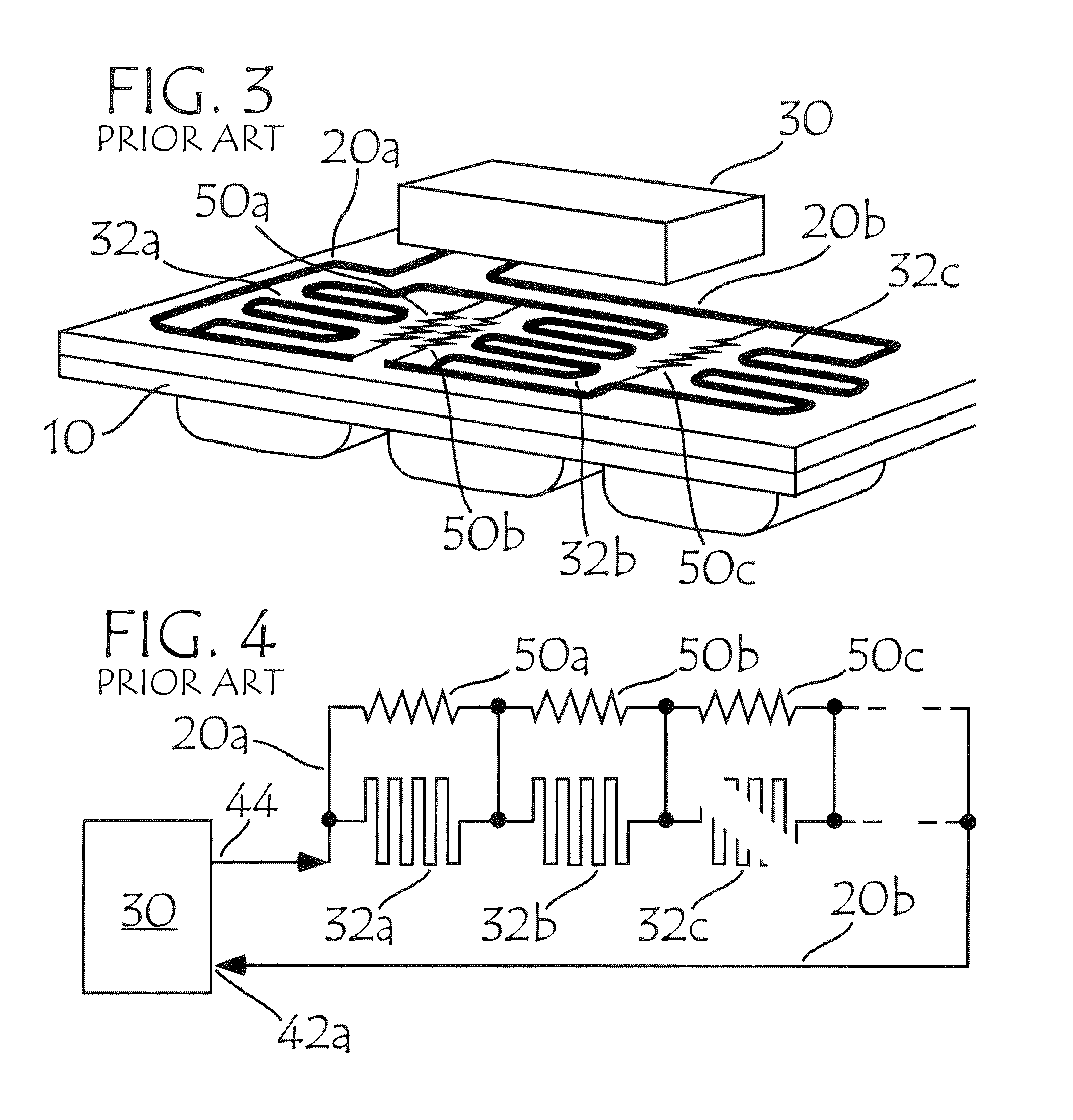
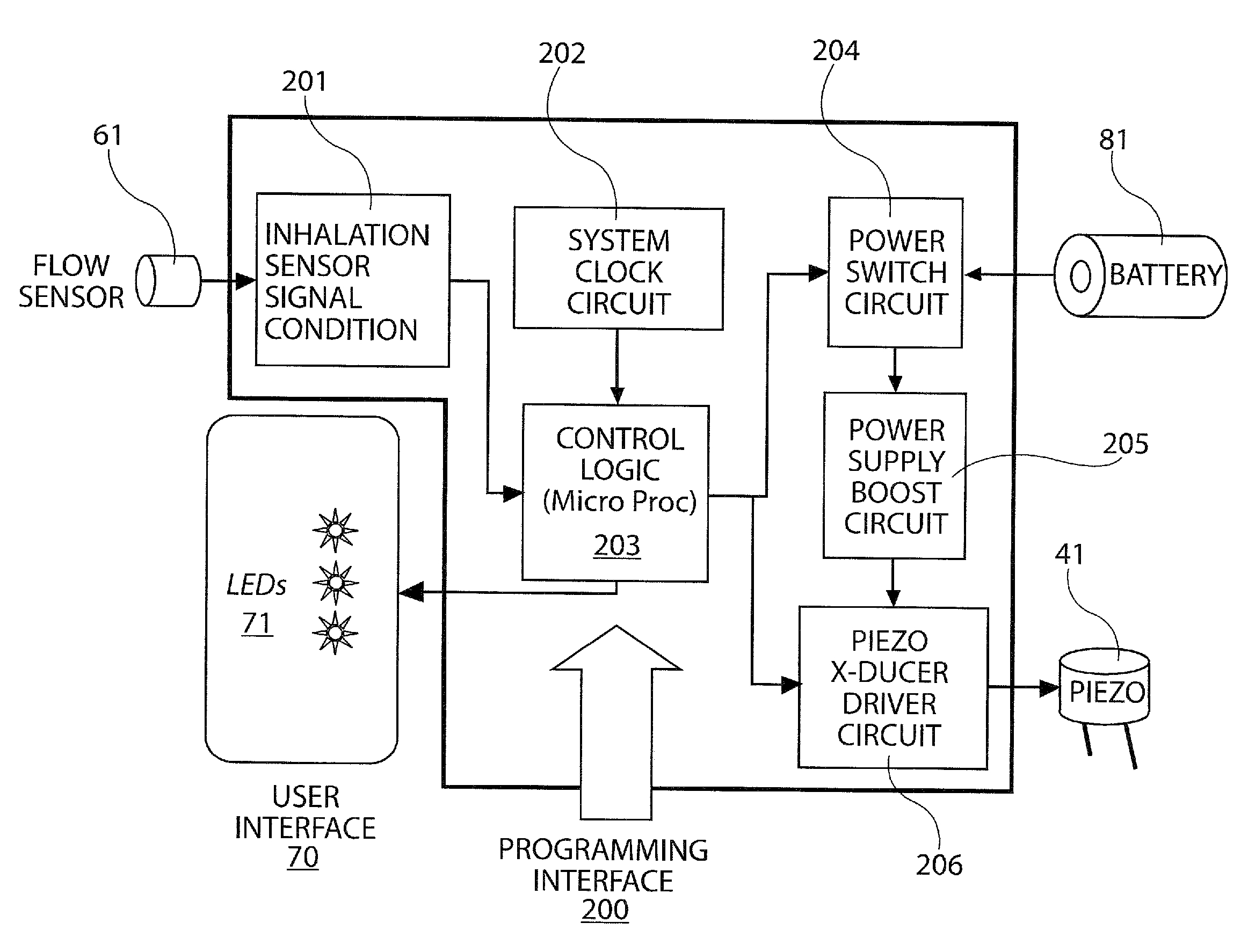
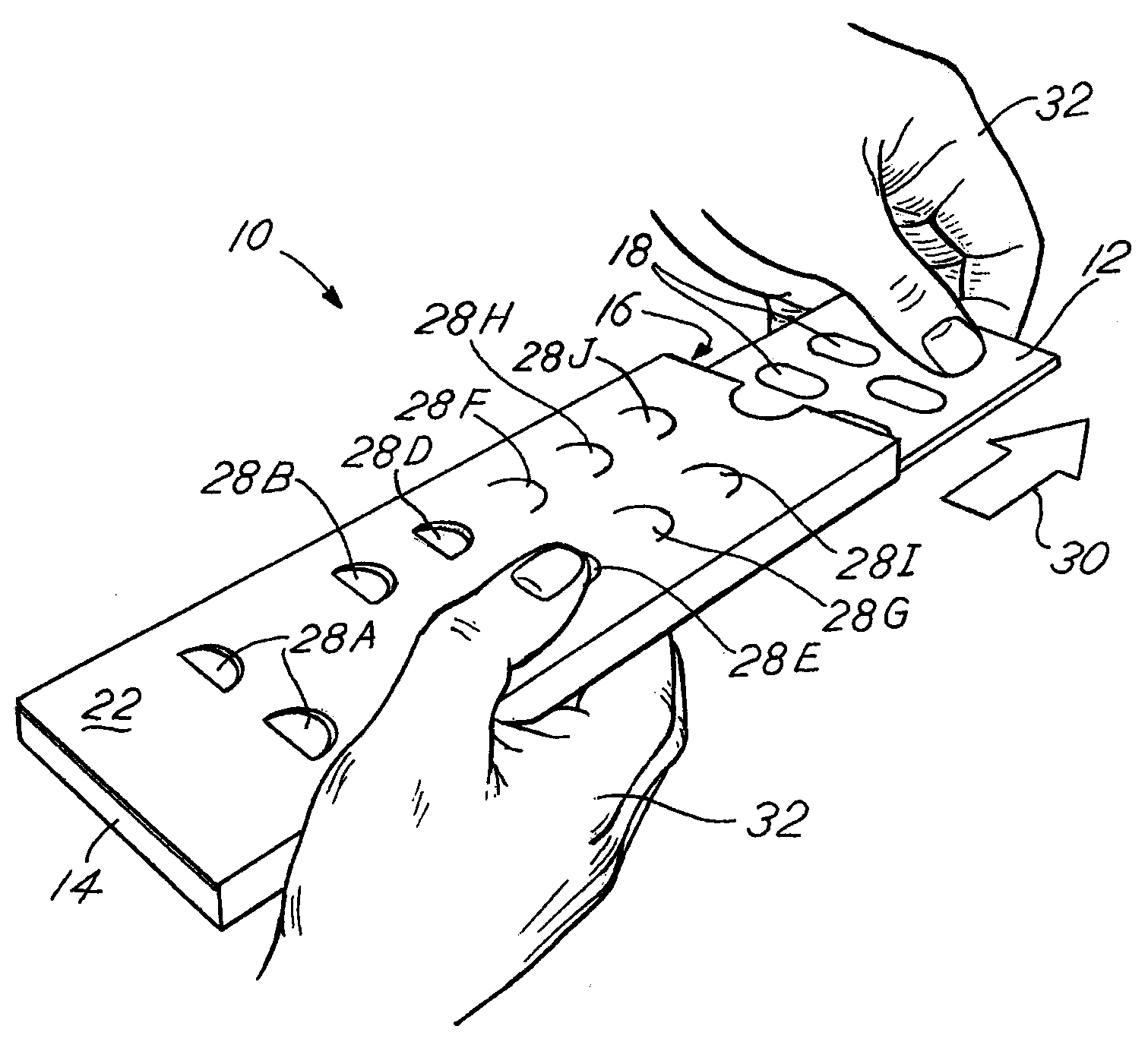
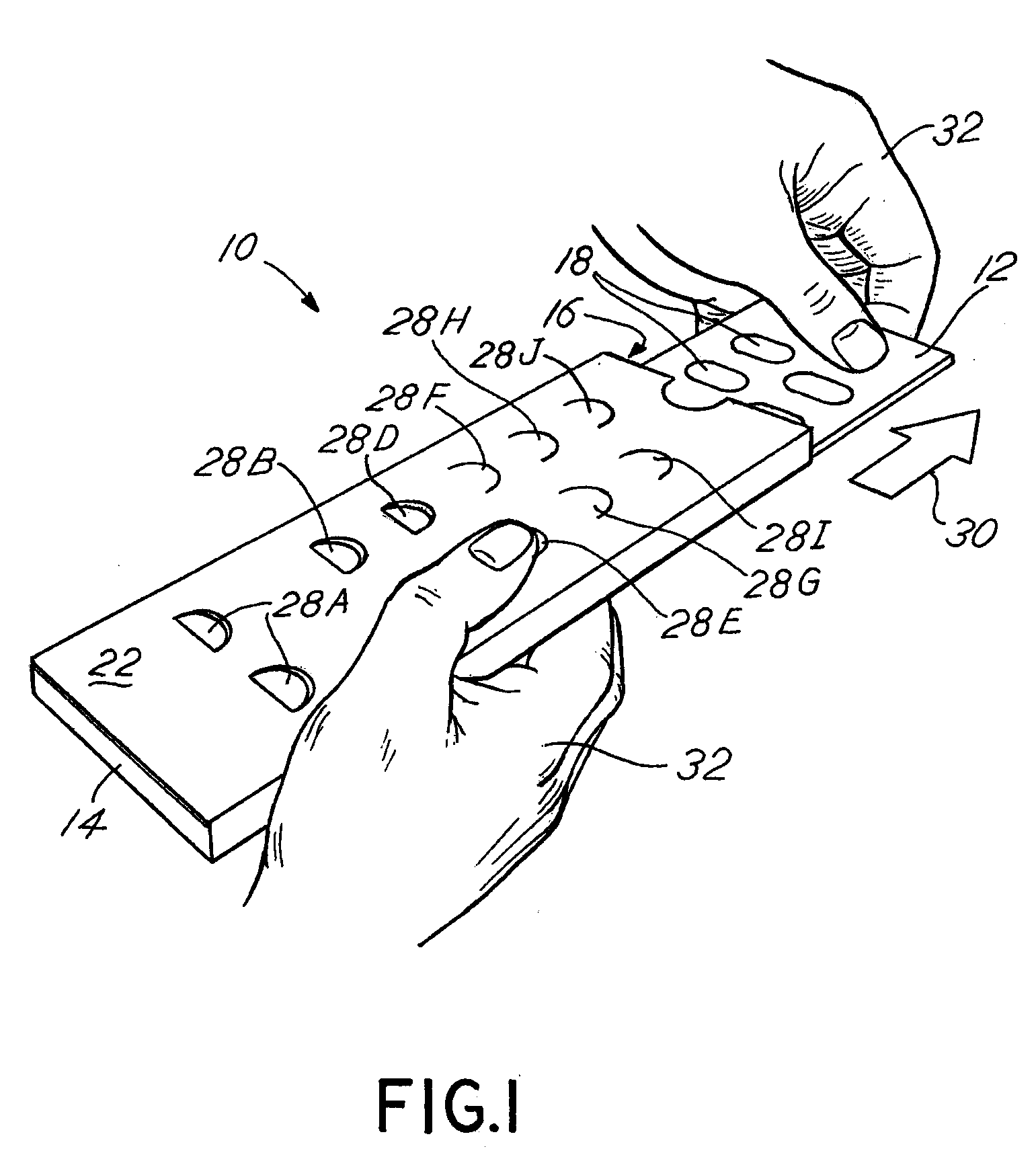
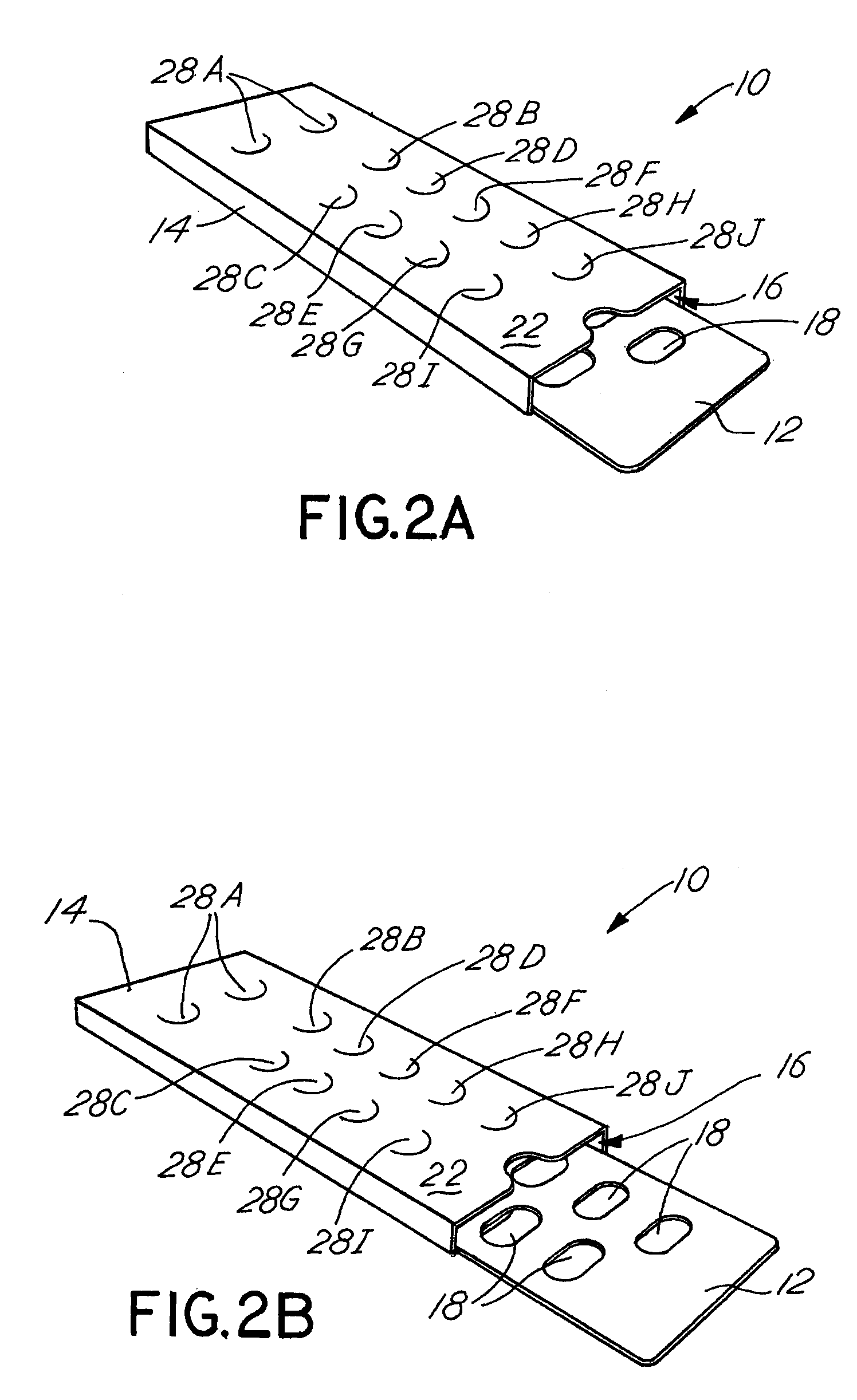
Blister pack content usage monitoring
ActiveUS8960440B1Save battery powerExtended service lifeSmall article dispensingPharmaceutical containersElectrical resistance and conductanceVoltage ratio
A system is provided for monitoring the removal of blister pack contents. An array of spatially-extended, electrically parallel breakable traces made from electrically resistive material is formed behind a corresponding array of blisters of a blister card. Then this array is connected in series with a reference resistor to form a voltage divider. All resistive traces are formed from the same materials in a single operation. Blister breakage is determined using changes in the ratio of the resistances of the array and the divider. A predictive algorithm is used to adjust the threshold resistance ratio change that signals blister breakage and voltage ratios are used to adjust for battery output changes over time. Breakage events and their time of occurrence are recorded in nonvolatile memory for later retrieval. Additional resistors can be used for activating the system and detecting tampering.
Owner:INTELLIGENT DEVICES SEZC
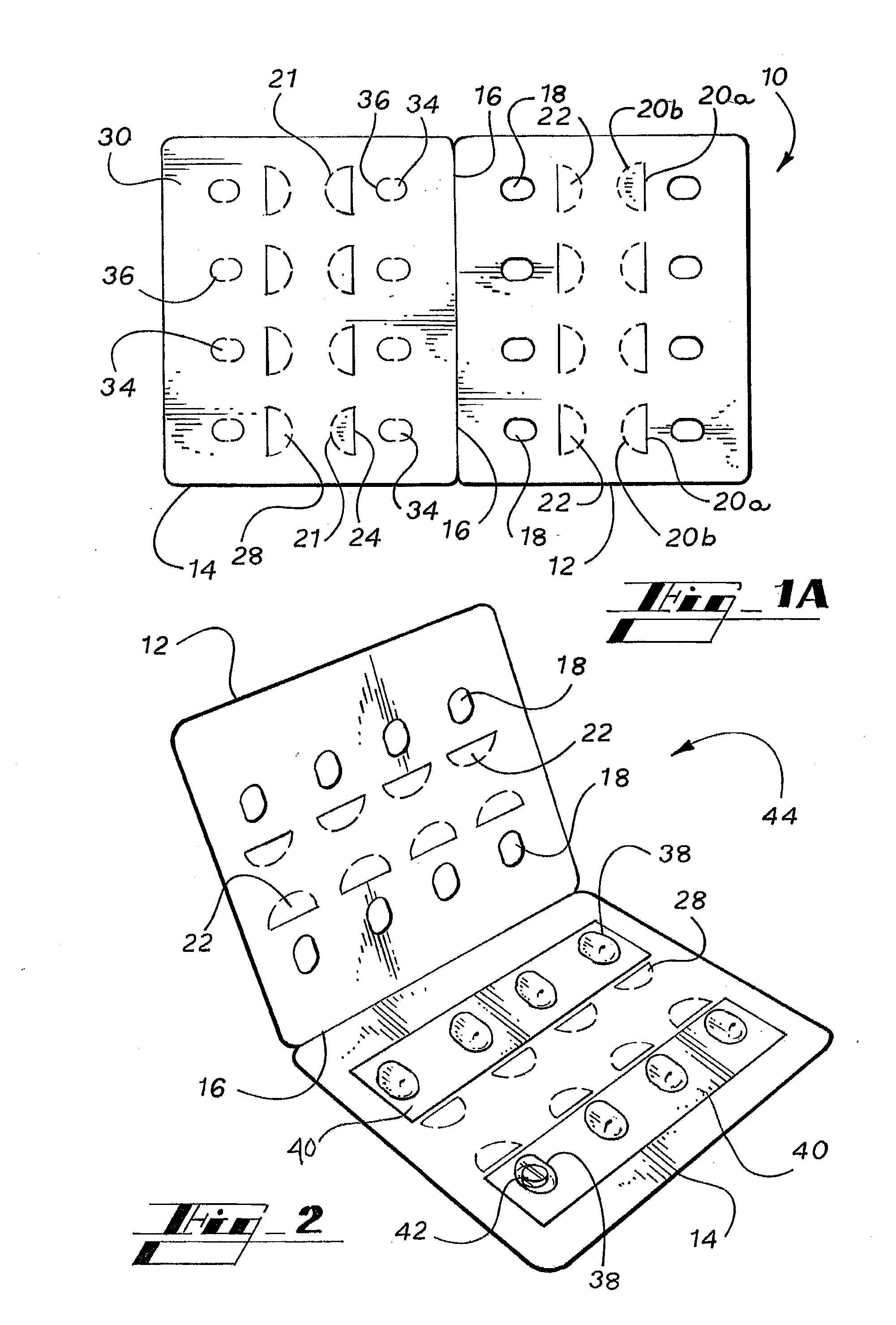
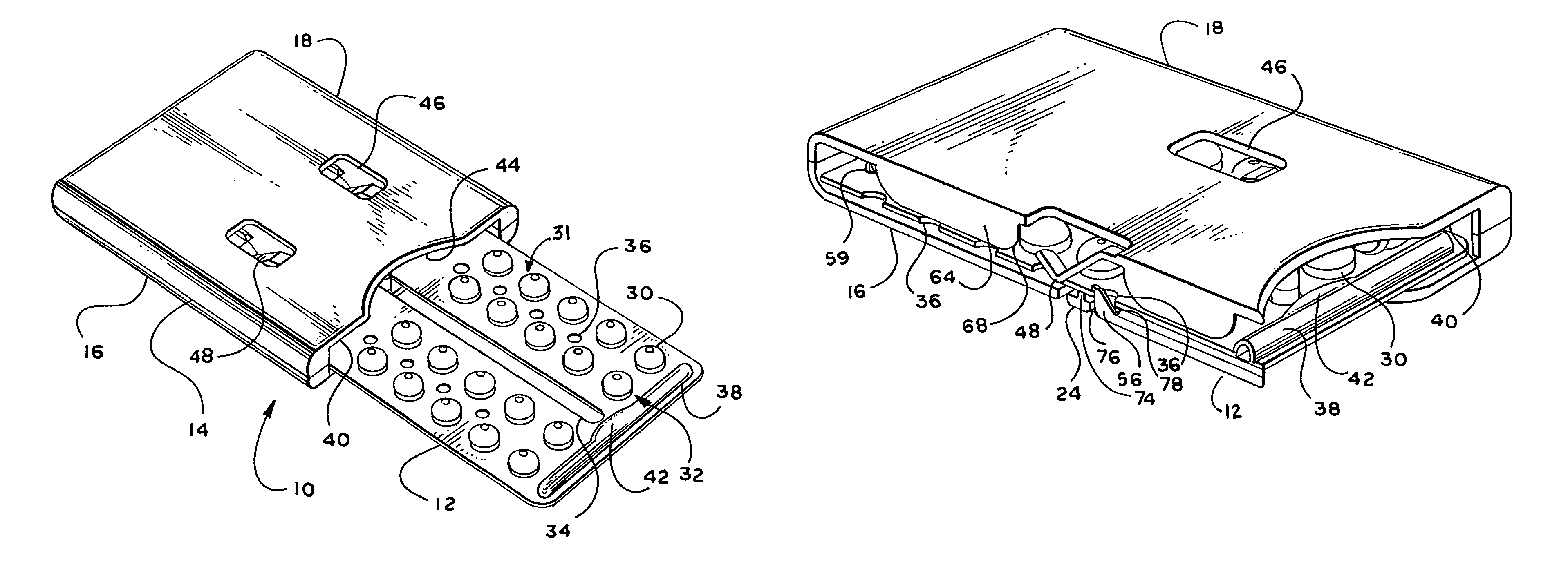
Child-Resistant Blister Package
ActiveUS20060289328A1InexpensiveEasy to makeSmall article dispensingOther accessoriesEngineeringBlister pack
A package includes a blank having a face panel and a back panel. The face panel includes apertures and face tabs. The back panel includes gates that correspond with apertures, and tab strips that overlap the gates and are adjoined to back tabs. A blister pack is sealed between the face panel and the back panel whereby blisters align over gates and protrude through apertures, and tabs and form a composite pull tab. To remove an item from a blister, the pull tab is pressed out of the panels, the tab strip is peeled from the back panel, and pressure is applied to force the item through the backing sheet of the blister pack and the exposed gate.
Owner:WESTROCK MWV LLC
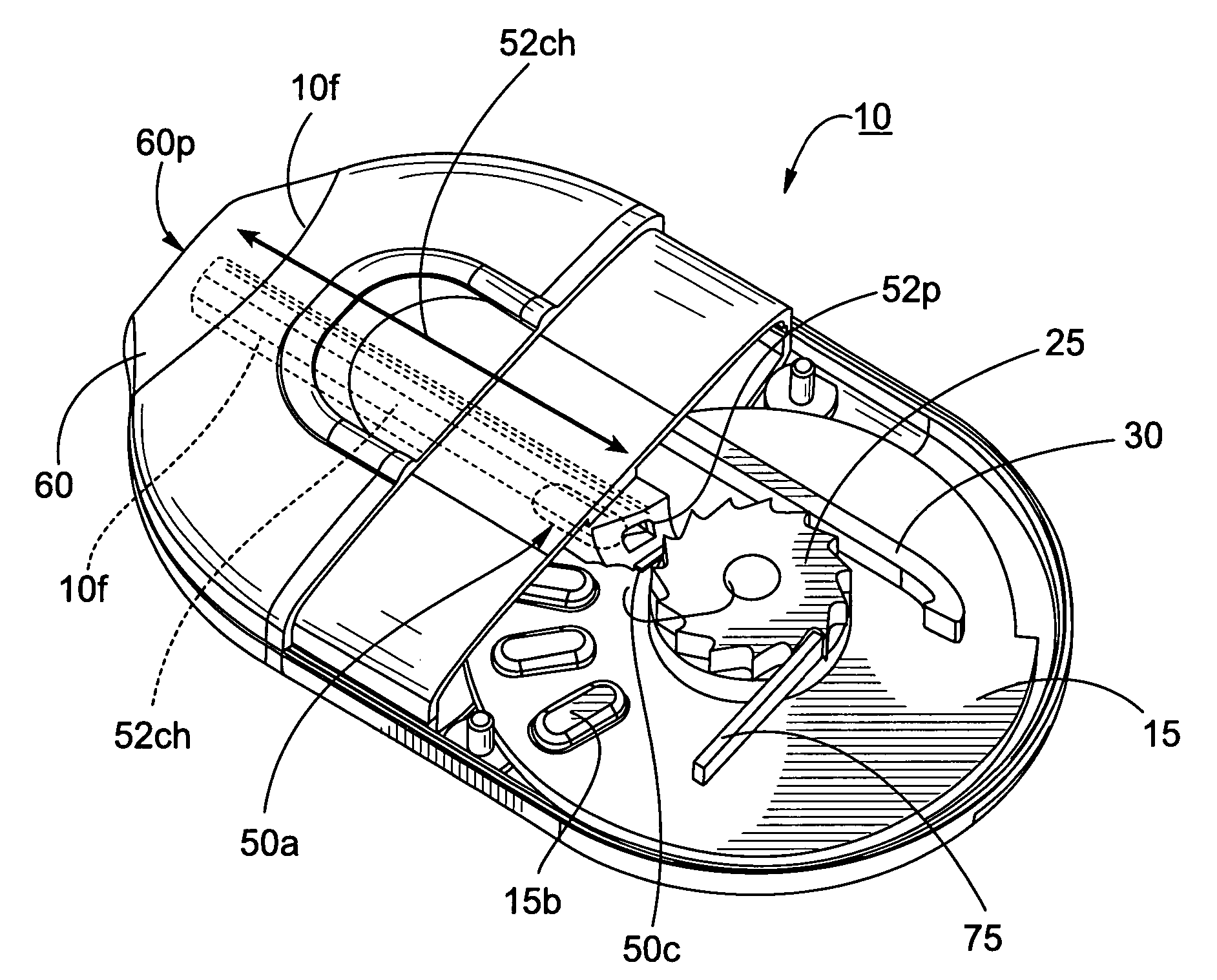
Rotary cassette system for dry powder inhaler
InactiveUS20100294278A1Simpler and more compact assemblyRespiratorsLiquid surface applicatorsEngineeringBlister pack
The present disclosure provides an inhaler having a vibration element for aerosolizing medicament contained in a blister pack, wherein a plurality of individual blister packs are arranged in a rotary cassette that fits within a housing, and wherein the individual blister packs are dragged up into a clamping position between the vibration element and a piercing element. The motion of the blister pack is controlled by a rotary disk within the housing which further coordinates the movement of the piercing and vibrating elements for the piercing and deaggregation, respectively, of the individual blister packs.
Owner:MICRODOSE THERAPEUTX INC
Sublingual drug delivery device
A drug delivery device that aerosolizes a dry powder formulation so that it forms a fine coating in the oral cavity and, more specifically, in the sublingual region of the oral cavity is described herein. In the preferred embodiment, the device contains five main parts: (i) a compressed gas canister, (ii) a dispenser body (also referred to herein as the main housing), (iii) a means for storing one or more doses of a drug formulation, (iv) a means for releasing a dose of the drug formulation such as a gas canister or spring piston and (v) a mouthpiece. Preferred configurations include circular, tubular, and rectangular. The means for storing the drug formulation may be configured to separately store one or more materials. In one embodiment, the means for storing the active agent is in the form of one or more drug discs, where the drug discs contain a plurality of blister packs, each storing one dose of the drug formulation. In another embodiment, the means for storing the active agent is a dosage cartridge containing a single dose of the drug formulation. In yet another embodiment, the drug formulation is stored on a ribbon containing a plurality of blister packs, each storing one dose of the drug formulation.
Owner:BIODEL
Security for blister packs
Various RFID based blister pack embodiments are provided for improving the ability to detect and prevent tampering and counterfeiting of blister packs and / or facilitating the chain of custody tracking of blister packs during manufacture. Some embodiments employ an RFID chip network on the blister pack and some embodiments employ an RFID strap network on the blister pack, wherein the integrity of the network (or lack thereof) is used to indicate the possibility of tampering. Other embodiments determine the possibility of tampering based on the frequency of an RF signal that is received from a chip provided on the blister pack, which will vary depending on whether the associated antenna has been detuned as a result of some type of tampering.
Owner:UNIVERSITY OF PITTSBURGH
Blister packages with frames and associated methods of fabricating dry powder drug containment systems
Owner:ORIEL THERAPEUTICS INC
Intelligent refrigerator for storing pharmaceutical product containers
Intelligent refrigerator system for storing pharmaceutical product containers, such as vials, ampules, syringes, bottles, medication tubes, blister packs and cartons, at the point of dispensing. Embodiments of the invention use product identification technology, such as radio-frequency identification (RFID) tags and readers, to uniquely identify containers as they are added to or removed from the cold storage compartment of the refrigerator, and automatically retrieve from a local or remote database a variety of details associated with the containers and their contents, such as manufacturing data, expiration dates, time out of refrigeration, inventory levels, safety information, usage statistics, known contraindications and warnings, etc. If the details indicate that there is a problem with a particular pharmaceutical (e.g., that it is counterfeit, expired, suspect, spoiled, recalled or almost depleted), then a message or warning is automatically delivered to a human operator via an attached output device, such as a display screen, speaker or printer. Embodiments of the invention may also be configured to monitor and report temperature faults, power failures and other anomalies associated with the refrigerator or cold storage compartment.
Owner:MERCK SHARP & DOHME LLC
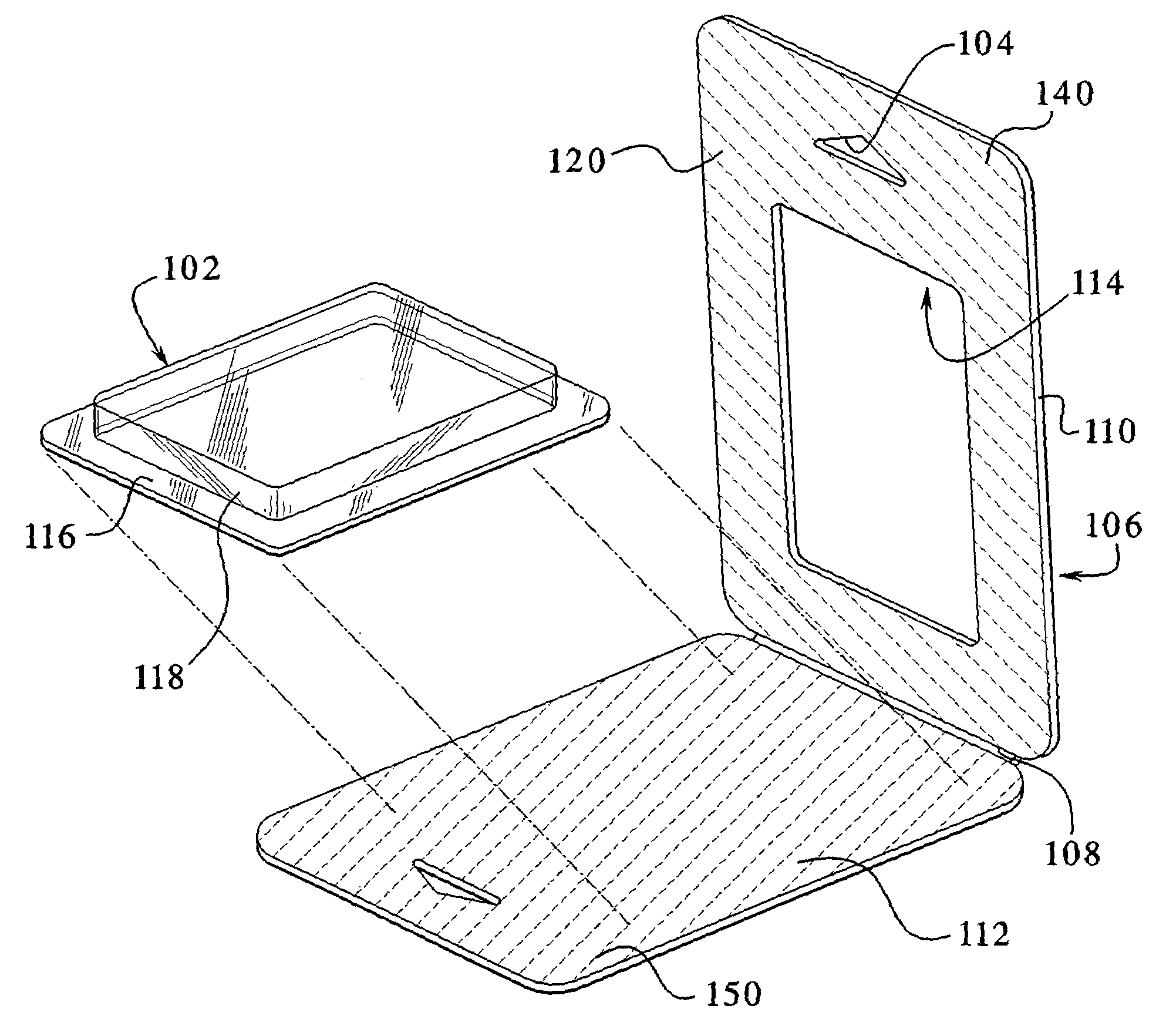
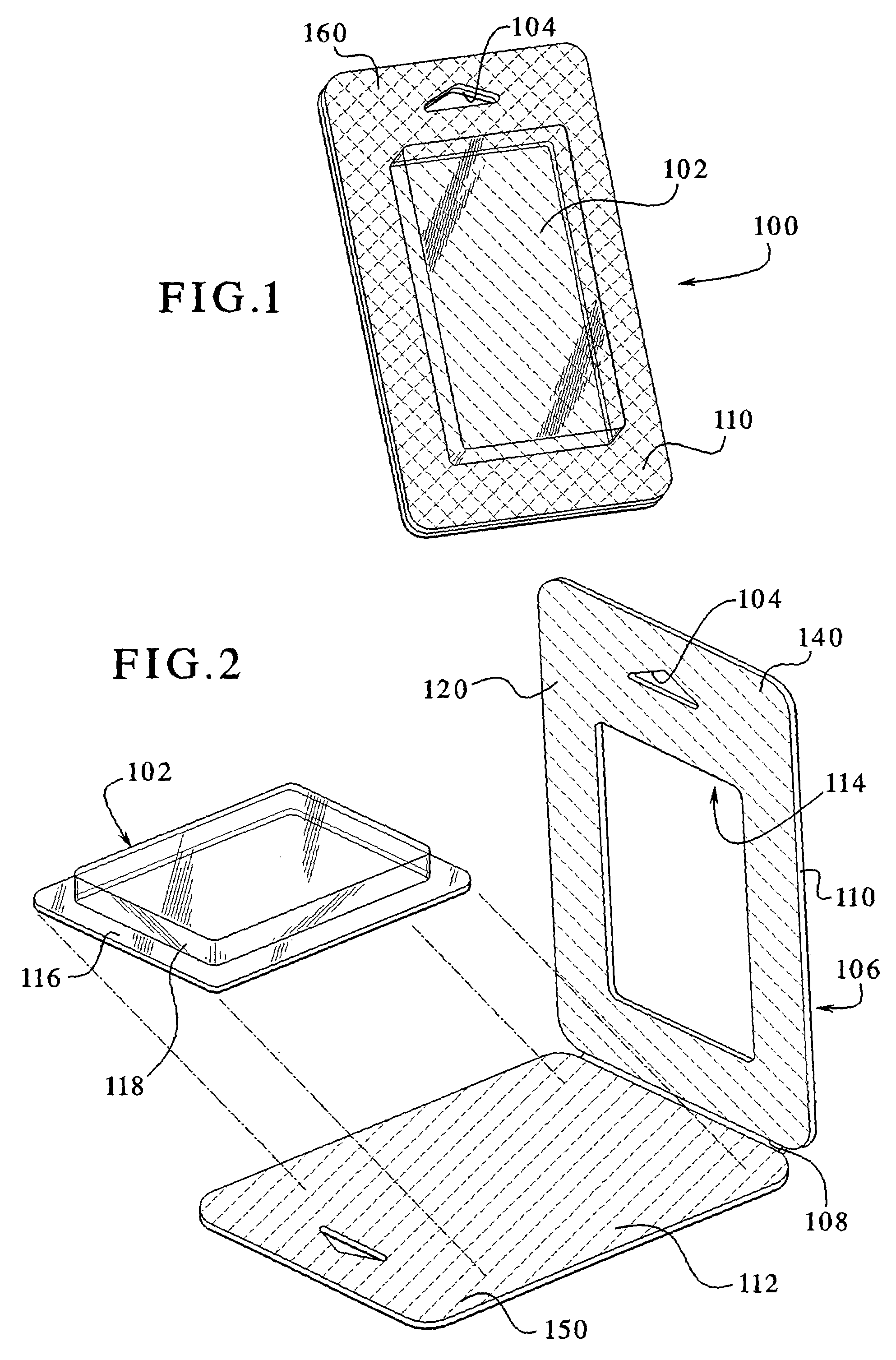
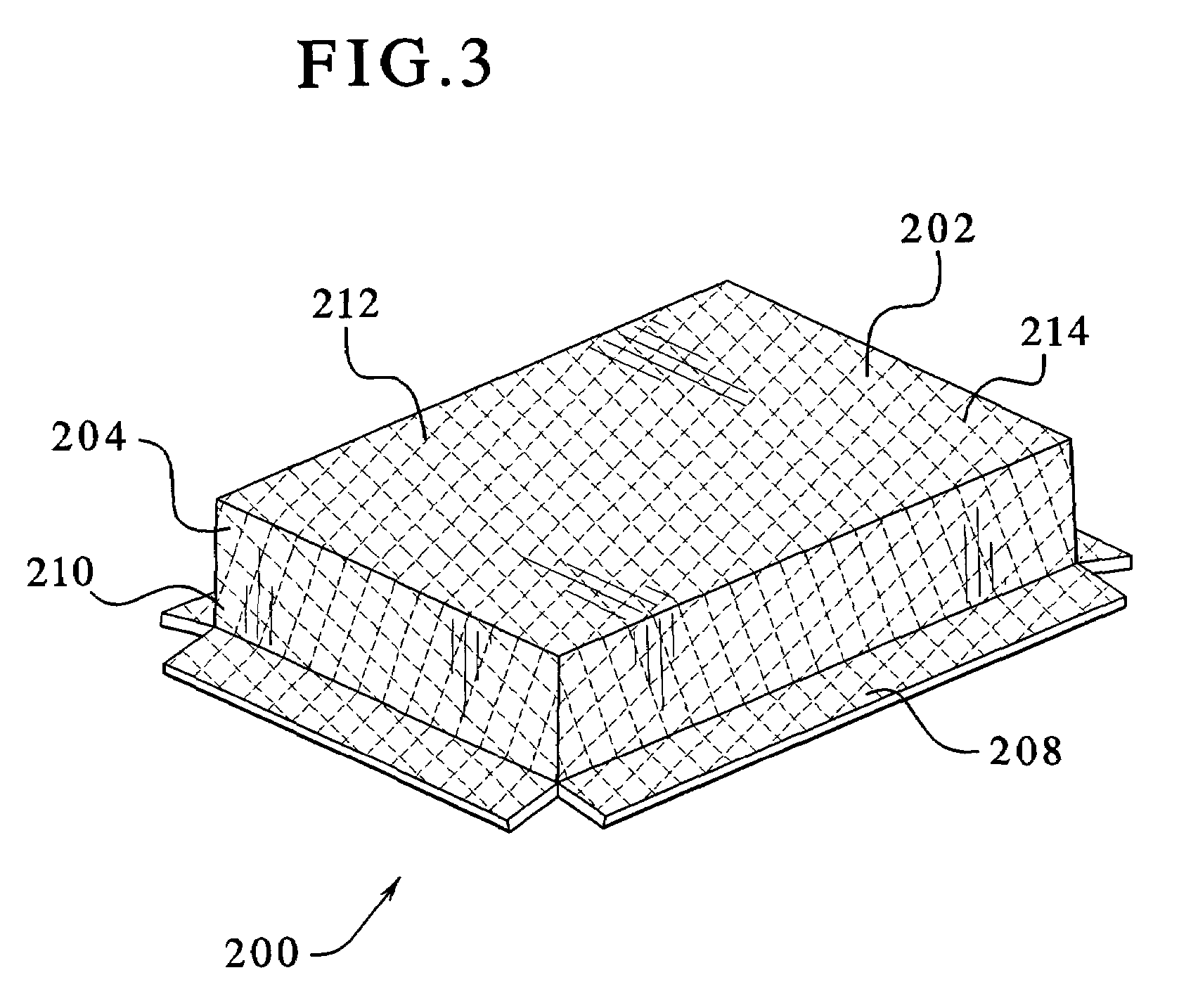
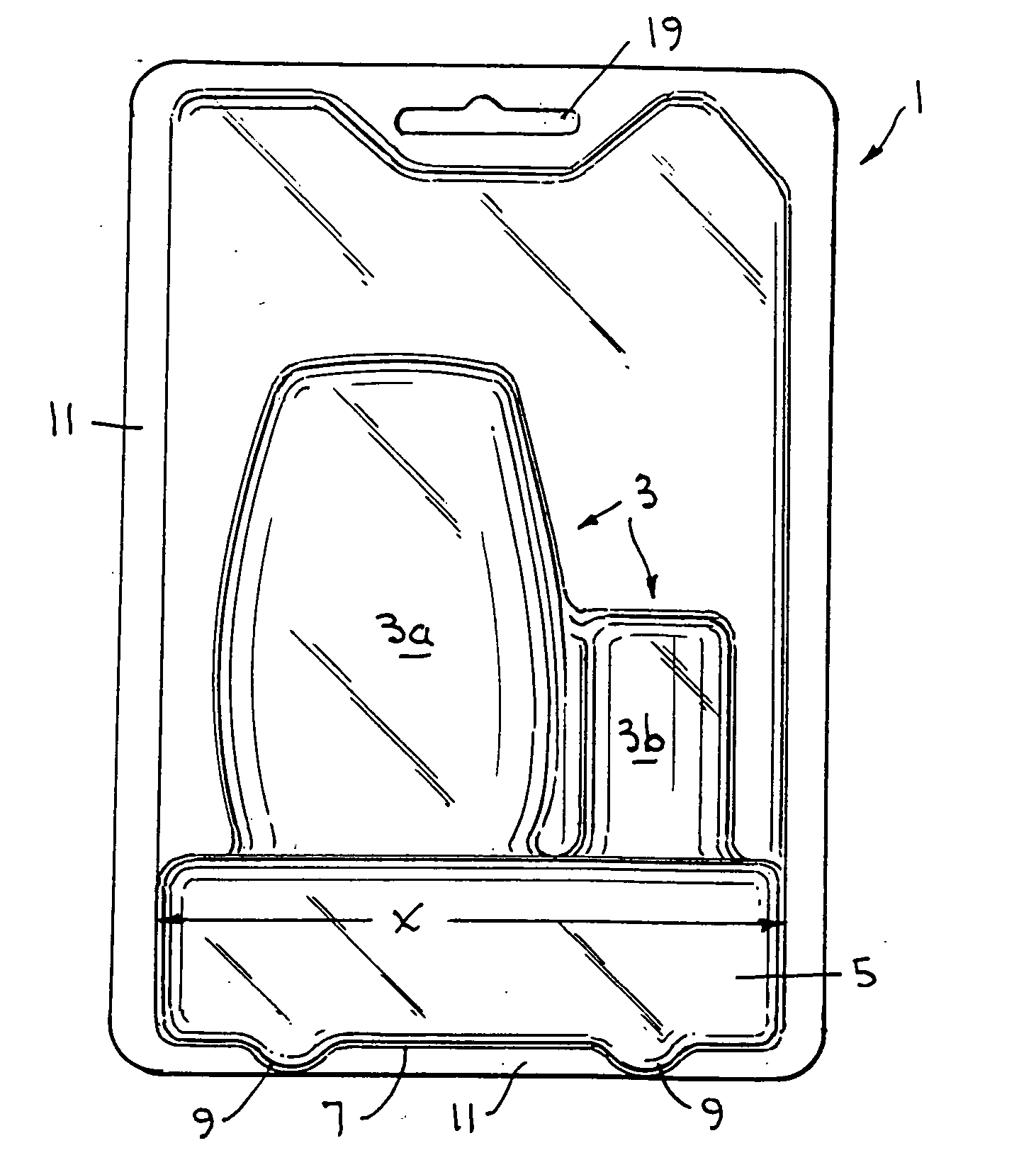
Environmentally separable packaging device with attaching base
The present invention generally relates to a bubble package which allows a product to be displayed independently standing alone or hanging from a bracket / shelf, and also provides the ability to separate the paper and plastic portions of the package into at least two separate environmentally recycled portions. The paper based portion of the package operates as a display card and / or as a stiffener. The sides of the one or more display cards are bonded to one another to enclose the product within the plastic and prevent tampering with the product. The plastic portion, in addition to containing and displaying the product therein, optionally incorporates a base near the bottom of the package. The base protrudes from the bottom of the package and secures back onto the plastic portion via a securing mechanism. This arrangement allows the package to stand independent. Optionally, a corrugated plastic insert can be used to provide additional support to the unit. In a typical arrangement, the plastic portions and paper portions avoid being bonded or directly adhered to one another, allowing the card to be entirely separable and allowing for complete environmental recyclability.
Owner:J & S IMPORTS LLC +2
Unit dose container with locking sleeve
ActiveUS7588149B2Inexpensive and easy to assemblePrevents and at least frustrates unintentional withdrawSmall article dispensingOther accessoriesDetentEngineering
A container includes a slidable tray and a locking sleeve. The tray is a conventional blister package, with blisters formed therein. The tray includes a slot and the sleeve includes a stop that extends through the slot to prevent the tray from sliding out of the sleeve. The sleeve includes detents that engage with holes in the tray to lock the tray in multiple positions. Manipulating a biaser releases the detents from the holes, thereby allowing the tray to slide within the sleeve.
Owner:WESTROCK MWV LLC
Medicament dispenser and associated methods
ActiveUS20070138049A1Reduce usageIncrease contactSmall article dispensingContainer decorationsMedication DispenserBlister pack
A medicament dispenser comprises a housing having opposing first and second walls. A connecting assembly is associated with at least one of the first and second walls to facilitate connection of the first and second walls to one another. A blister pack containing the medicament is pivotally connected to the connecting assembly so as to be rotatable about the connecting assembly and about an axis of rotation substantially orthogonal to the first and second walls to facilitate movement of the blister pack from: i) a closed position in which the blister pack is substantially enclosed within the housing; and ii) an open position in which the blister pack is at least partially rotated from the housing.
Owner:ALLERGAN SALES LLC +1
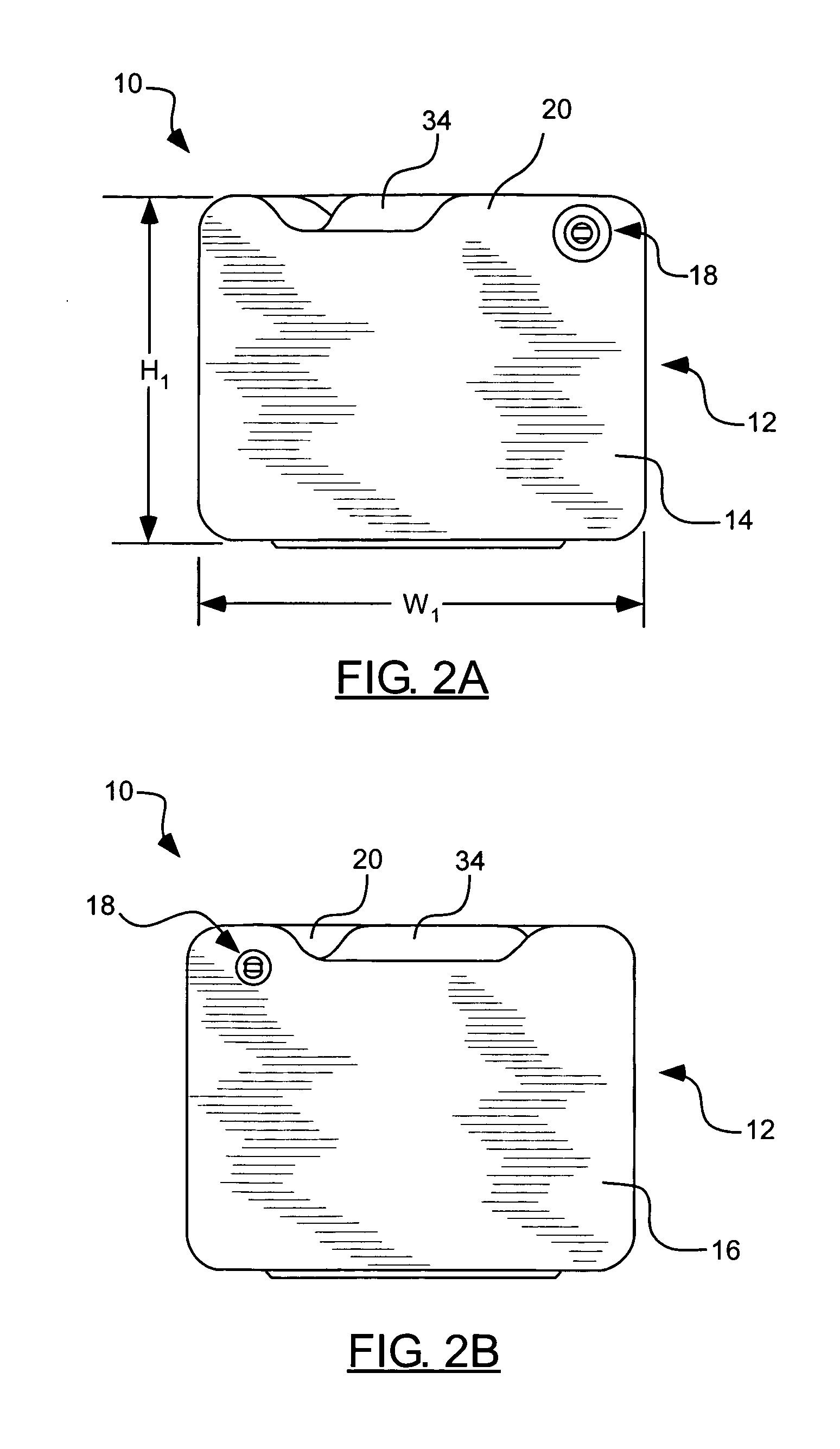
Pilfer-resistant packaging with criss-cross grain pattern
ActiveUS7051876B2Prevent theftEasy and economical to manufactureSmall article dispensingPharmaceutical containersGraphicsTear resistance
A clamshell package for displaying and housing products or other objects that is substantially pilfer-resistant, yet safe to open. The clamshell packaging includes a substantially tear-resistant housing that encloses a display chamber by criss-cross grain material to provide cut or tear resistance in multiple directions. The display chamber of the clamshell package may be either substantially transparent to allow for the product to be displayed or substantially opaque display graphics and / or to prevent the contents from being viewed. The chamber is preferably seamless such that it may not be opened without the use of scissors and, when opened, such opening is readily apparent. Furthermore, when cut open, the material used does not form sharp or jagged edges that may pose a danger to anyone handling the package. In addition to clamshell packages, the tear resistant material may also be used in blister packs and other types of packaging.
Owner:COLBERT PACKAGING CORP
Child-resistant blister package
ActiveUS7401702B2InexpensiveEasy to makeSmall article dispensingOther accessoriesBlister packBlisters
A package includes a blank having a face panel and a back panel. The face panel includes apertures and face tabs. The back panel includes gates that correspond with apertures, and tab strips that overlap the gates and are adjoined to back tabs. A blister pack is sealed between the face panel and the back panel whereby blisters align over gates and protrude through apertures, and tabs and form a composite pull tab. To remove an item from a blister, the pull tab is pressed out of the panels, the tab strip is peeled from the back panel, and pressure is applied to force the item through the backing sheet of the blister pack and the exposed gate.
Owner:WESTROCK MWV LLC
Medication record system and dispenser
InactiveUS6951353B2Reduce the possibilitySimple and reliable processSmall article dispensingContainer/bottle contructionPharmacyMedicine
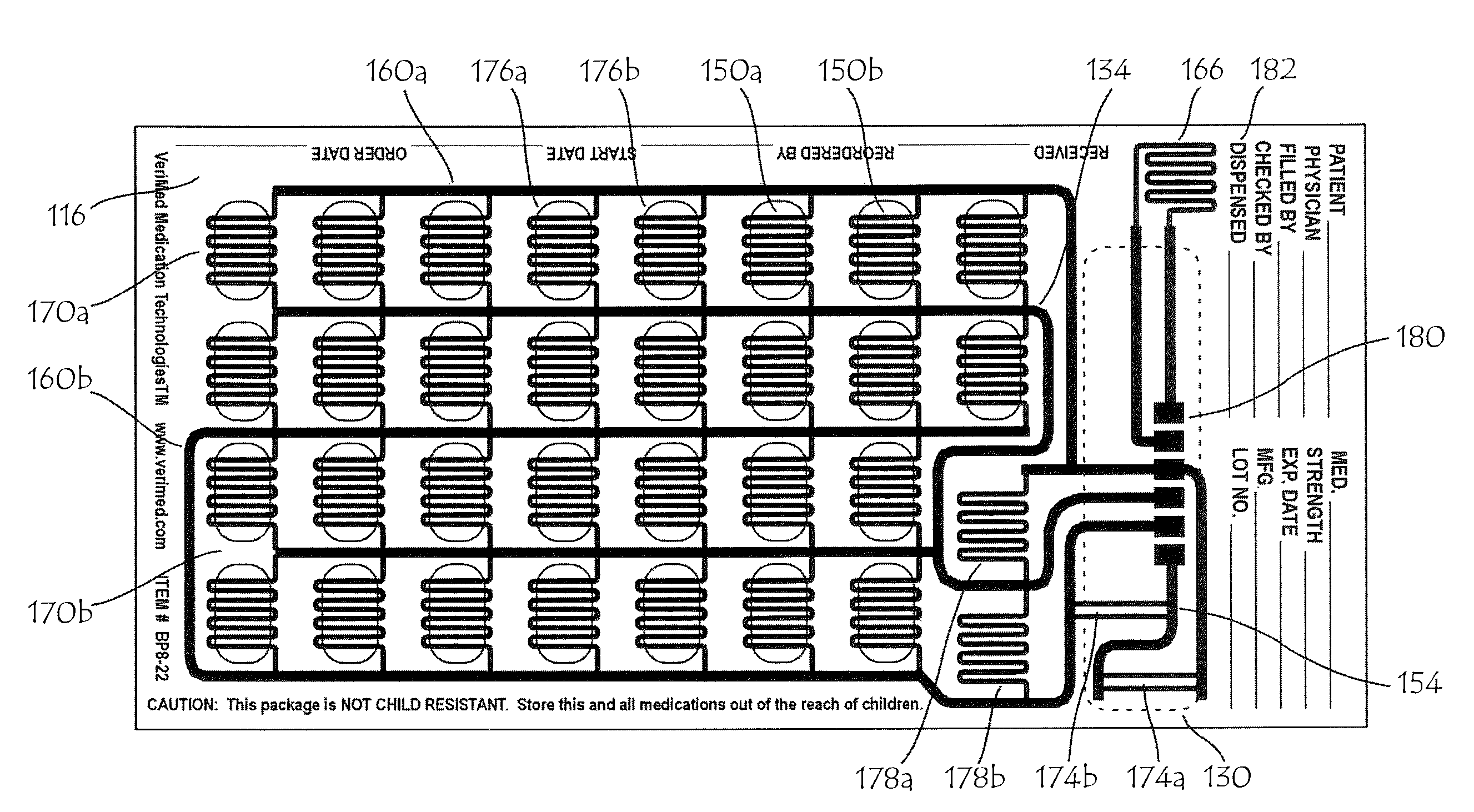
The present invention relates to a medication management system that is simple, reliable and extremely easy to use. It comprises a label having a plurality of raised tabs that are depressed upon taking a medication to provide a tactile and a visual record of medication use. It can be secured to the medication container, and thus is not subject to being misplaced or forgotten. The system can be integrated with a pharmacy's computerized pharmaceutical record and prescription label printing system or it can be a stand-alone paste-on label. Alternatively, it can be used in combination with blister packs to dispense medication while maintaining a record of use. The label can be in the form of an overlay, which is placed over a preprinted container or a container having a prescription label. The use of a pressure sensitive releasable adhesive permits the removal of the overlay label in the event that is it necessary to read information on the underlying label. The underlying label is provided with a plurality of raised tabs that correspond to the number of doses to be taken per day and the number of days for which the medication is to be taken.
Owner:KOZLOWSKI NANCY MS
Method of dispensing pills from a movable platen
A plurality of cassettes, each filed with a supply of pills and positioned over a target location. A platen is provided beneath the target location with receptacles configured to hold both vials and blister packs. The platen or the cassette is movable so that any blister of the blister pack or the vial can be positioned under the target location to receive a quantity of pills from the cassette.
Owner:QEM INC
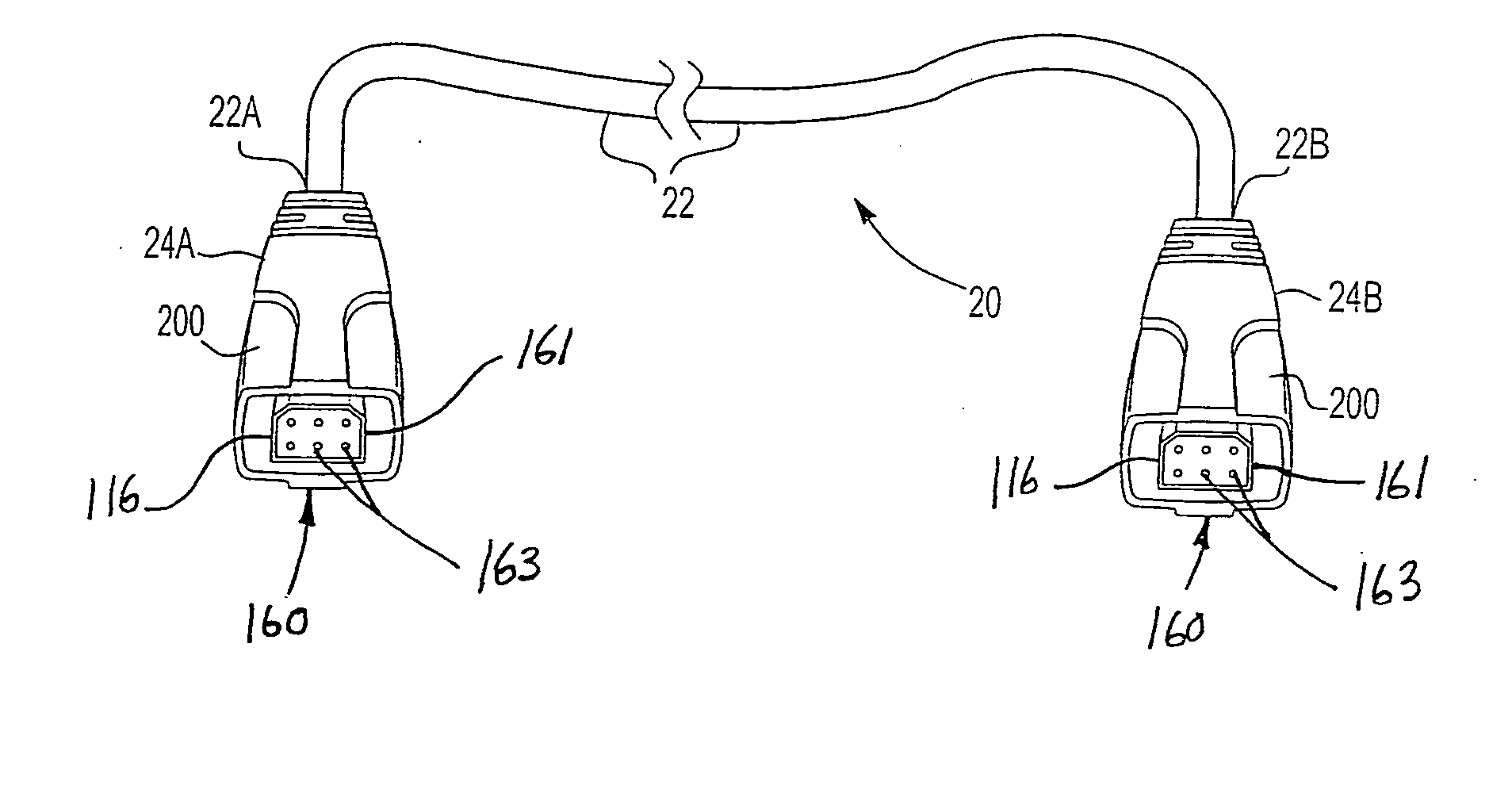
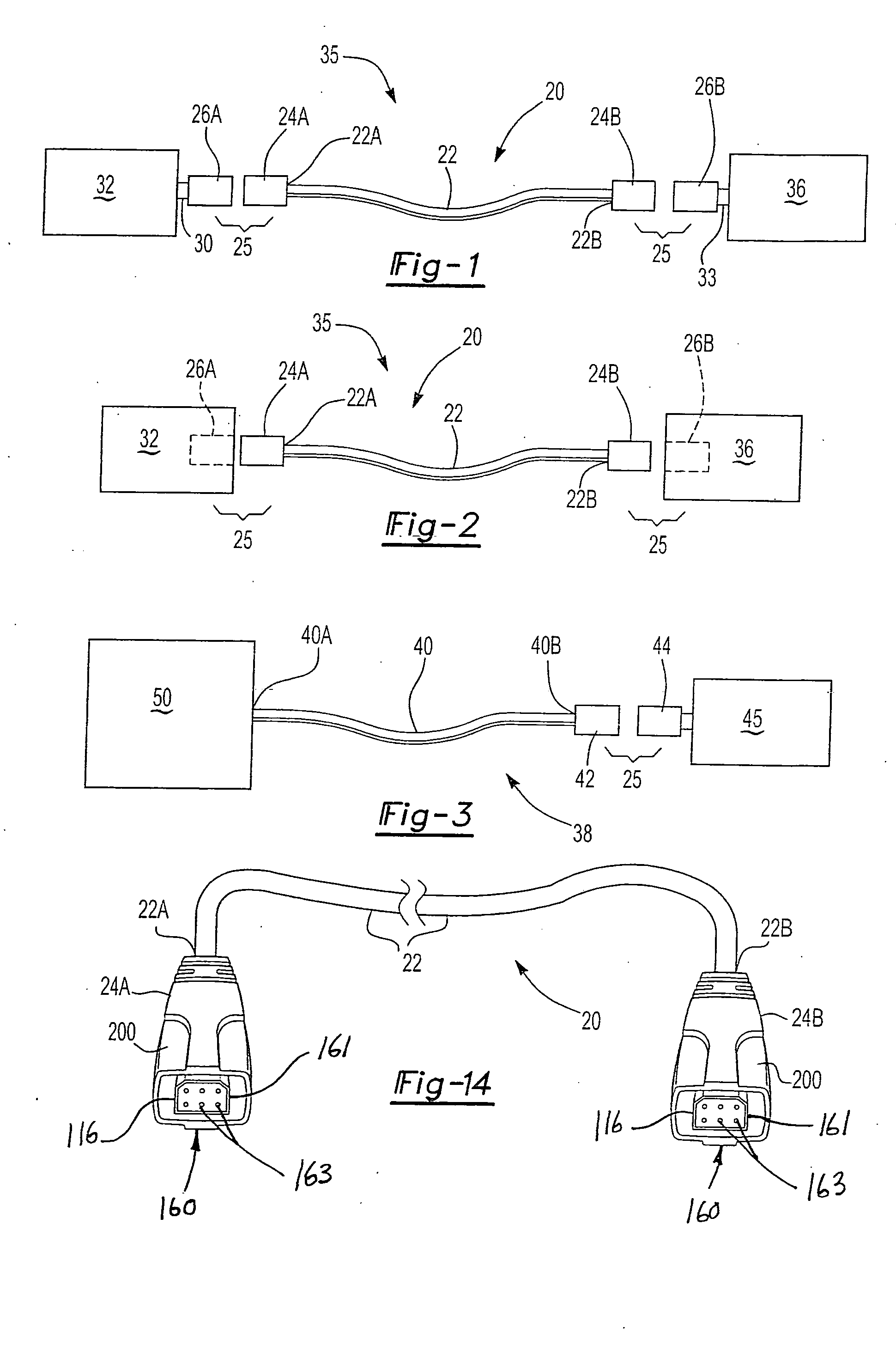
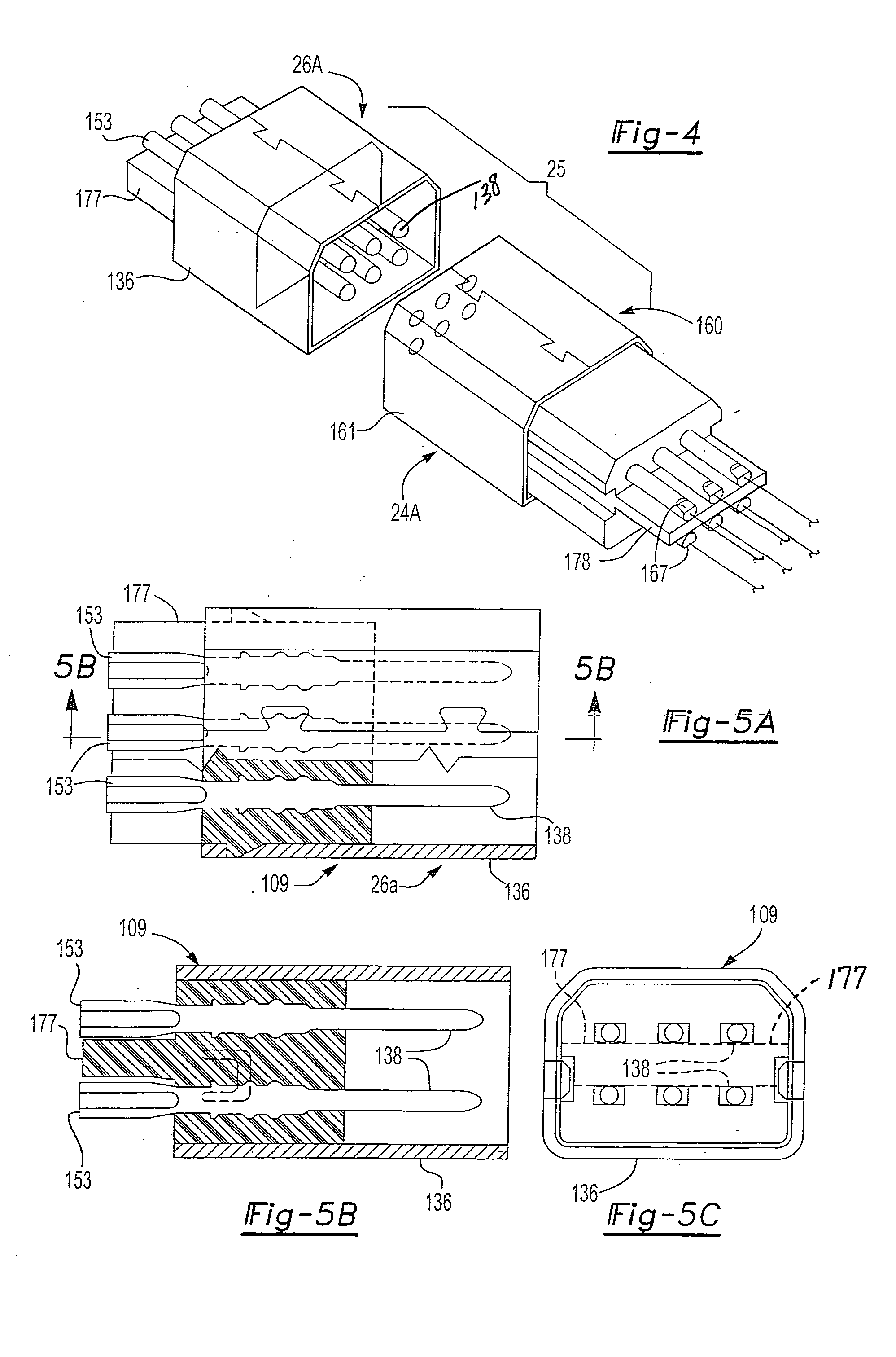
Universal computer cable kit with interchangeable quick connectors
InactiveUS20050070154A1Incorrect coupling preventionElectric connection structural associationsGeneral purpose computerEngineering
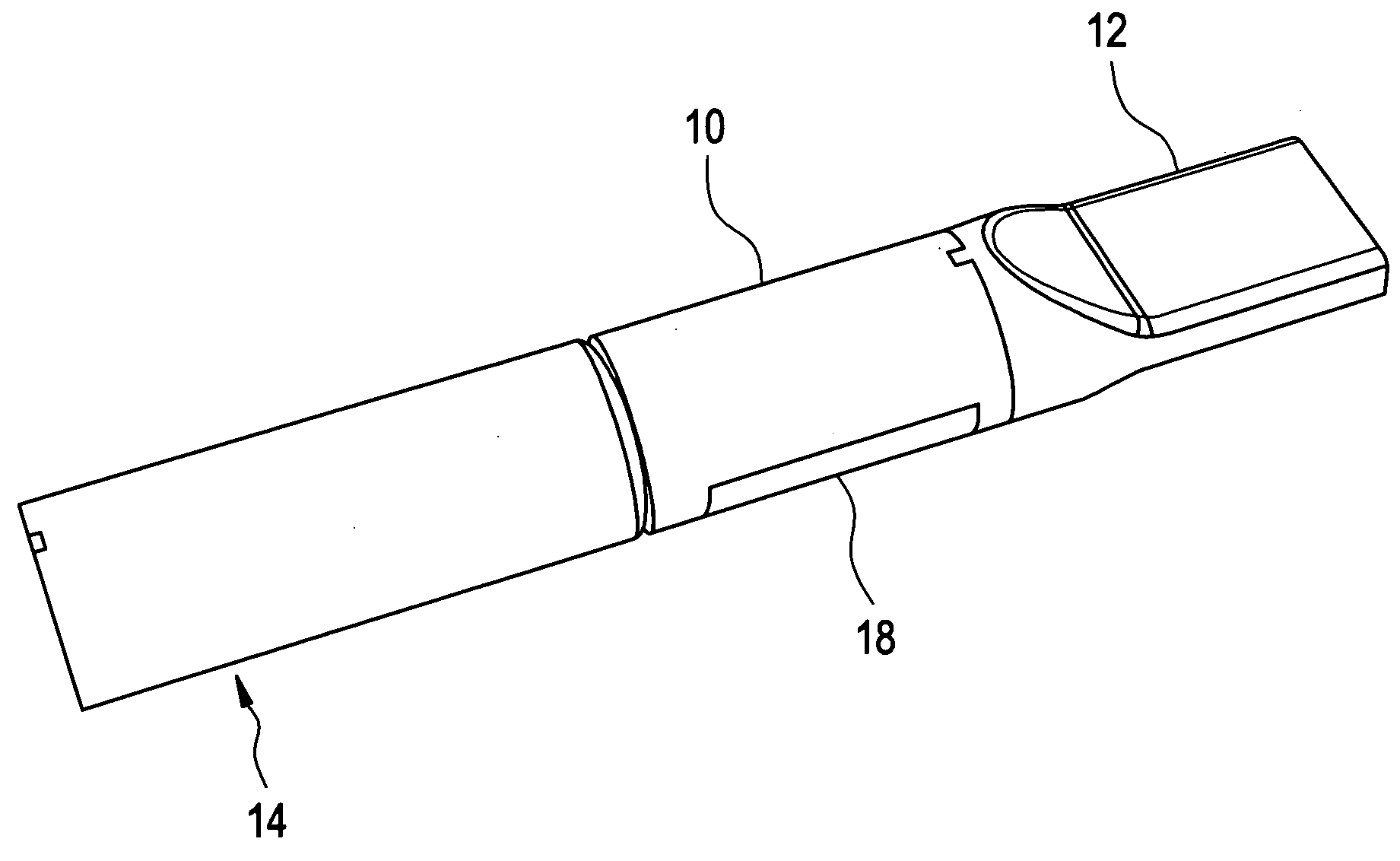
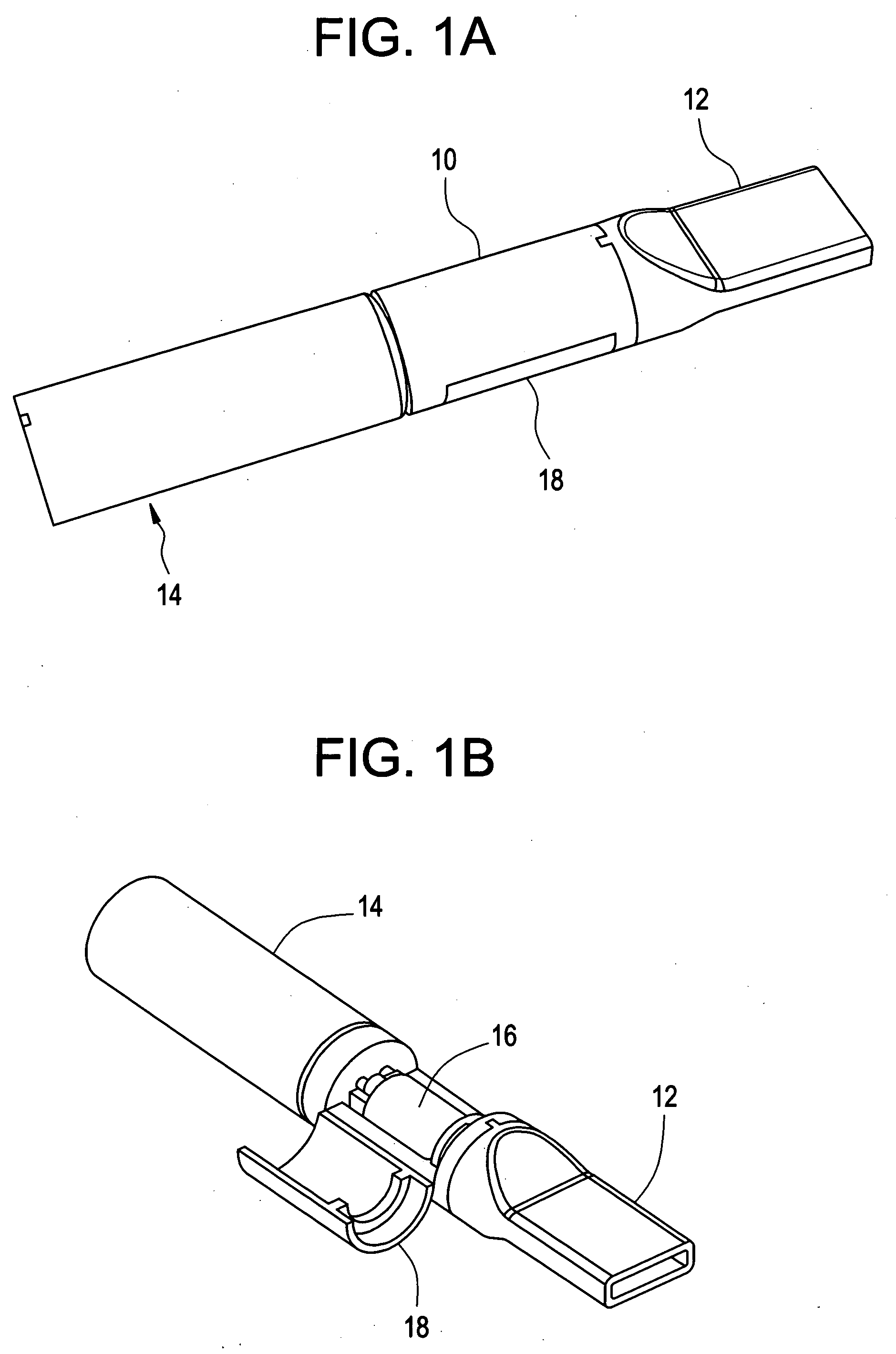
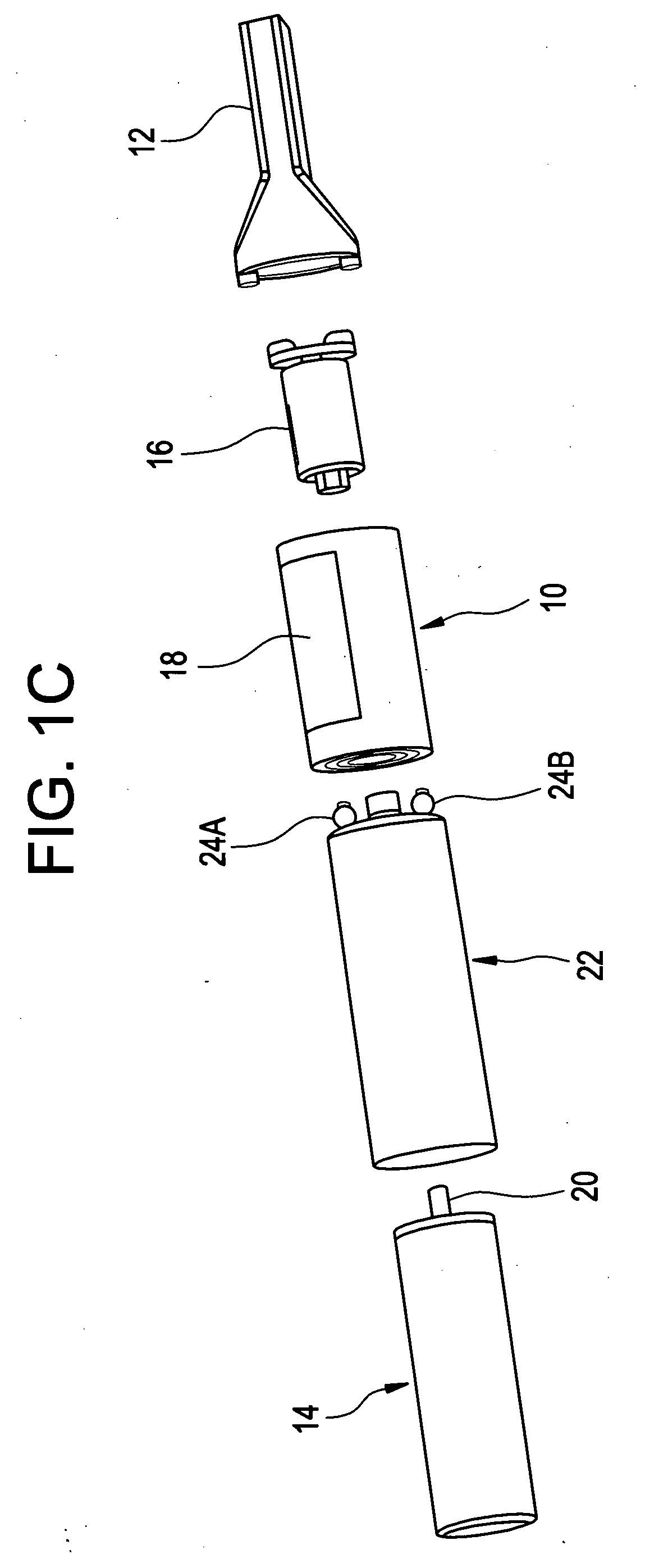
A universal computer cable kit includes a universal cable having quick connector portions on opposing ends thereof, a plurality of interchangeable connectors for attachment to the quick connector portions, a container for storing the interchangeable connectors, and a blister pack for packaging and displaying the kit.
Owner:MILAN HENRY
Display package with stabilizing and indexing means
ActiveUS20050092644A1Provide stabilityImprove package stabilityRacksOther accessoriesEngineeringBlister pack
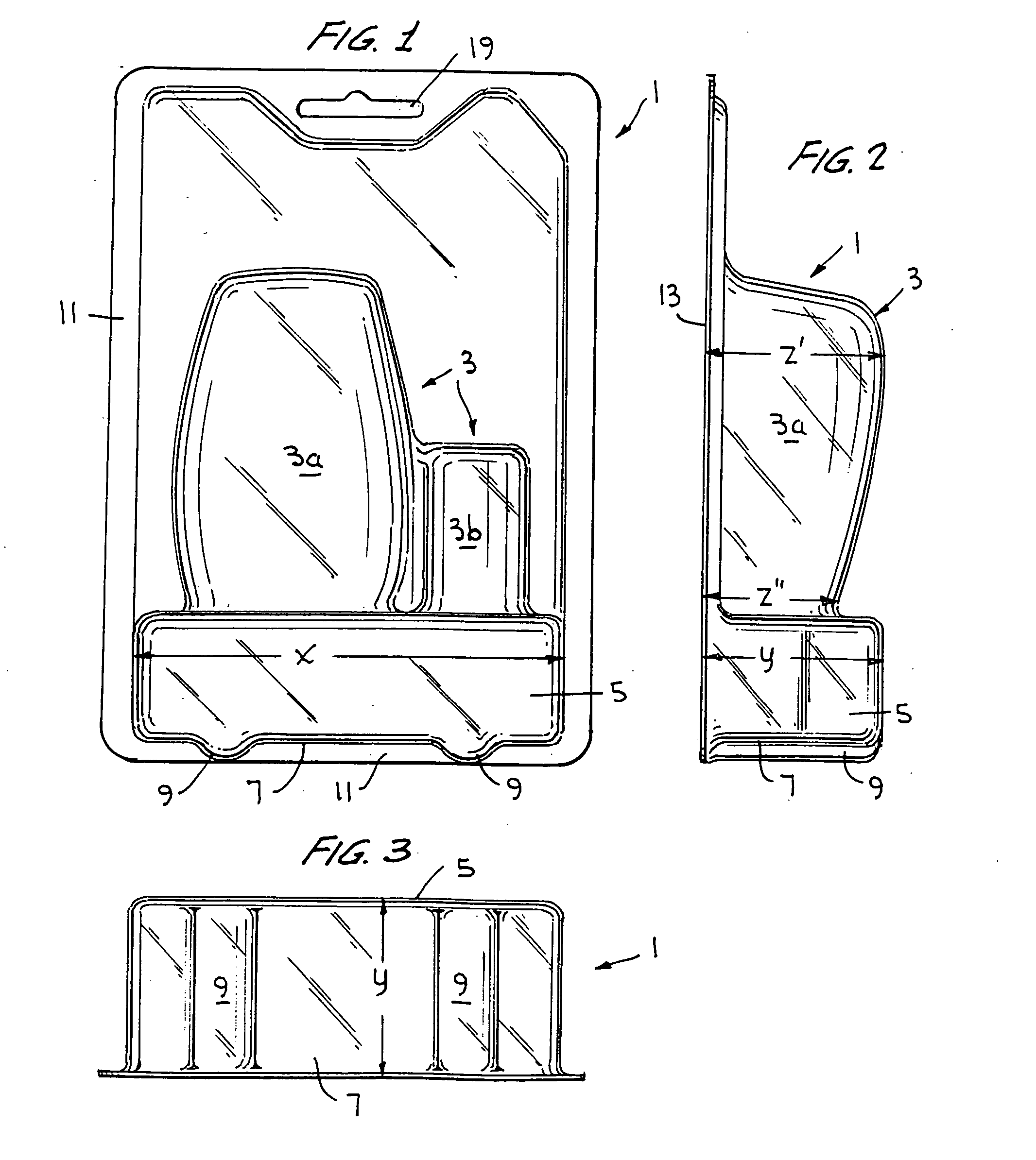
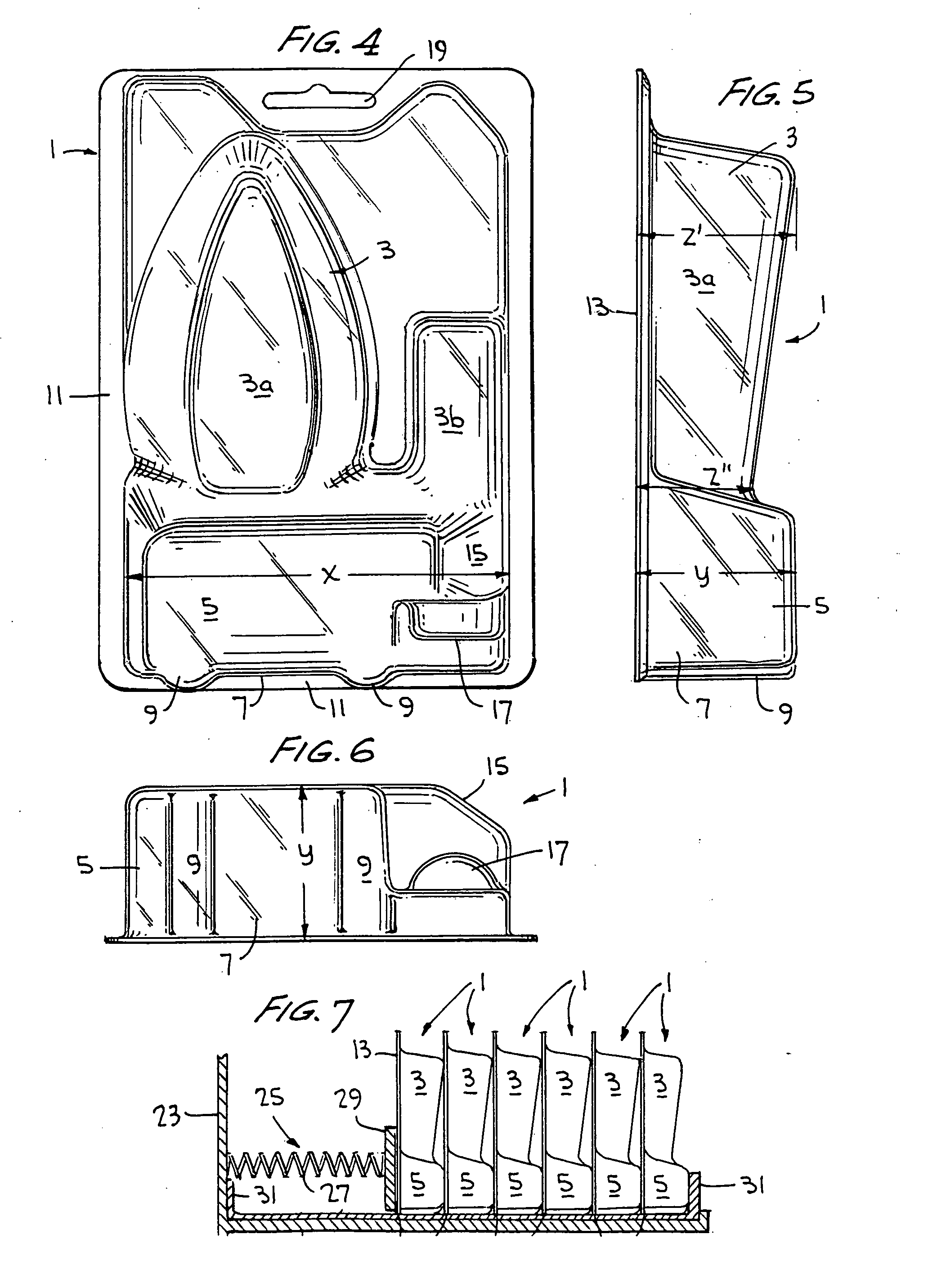
A blister pack and package including such blister pack is described which provides for package stability and self-indexing in relation to adjacently aligned packages. The blister pack is configured to include at least one compartment for enclosing an article, and an outward projecting portion or foot in the bottom portion of the blister pack. The foot is configured to have a width, depth and height sufficient to allow the package to be freestanding and self-indexing. The package is especially suited for use in a merchandise point-of-sale display including a pressure applicator for maintaining displayed packages in a forwardmost position in the display when one or more packages are removed from the display. The foot provides for a predetermined stable spacing during packaging and while in storage and on display, and provides for self-indexing, i.e. maintenance of proper spacing, when a pressure applicator moves the aligned packages forward in a display.
Owner:SC JOHNSON & SON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com