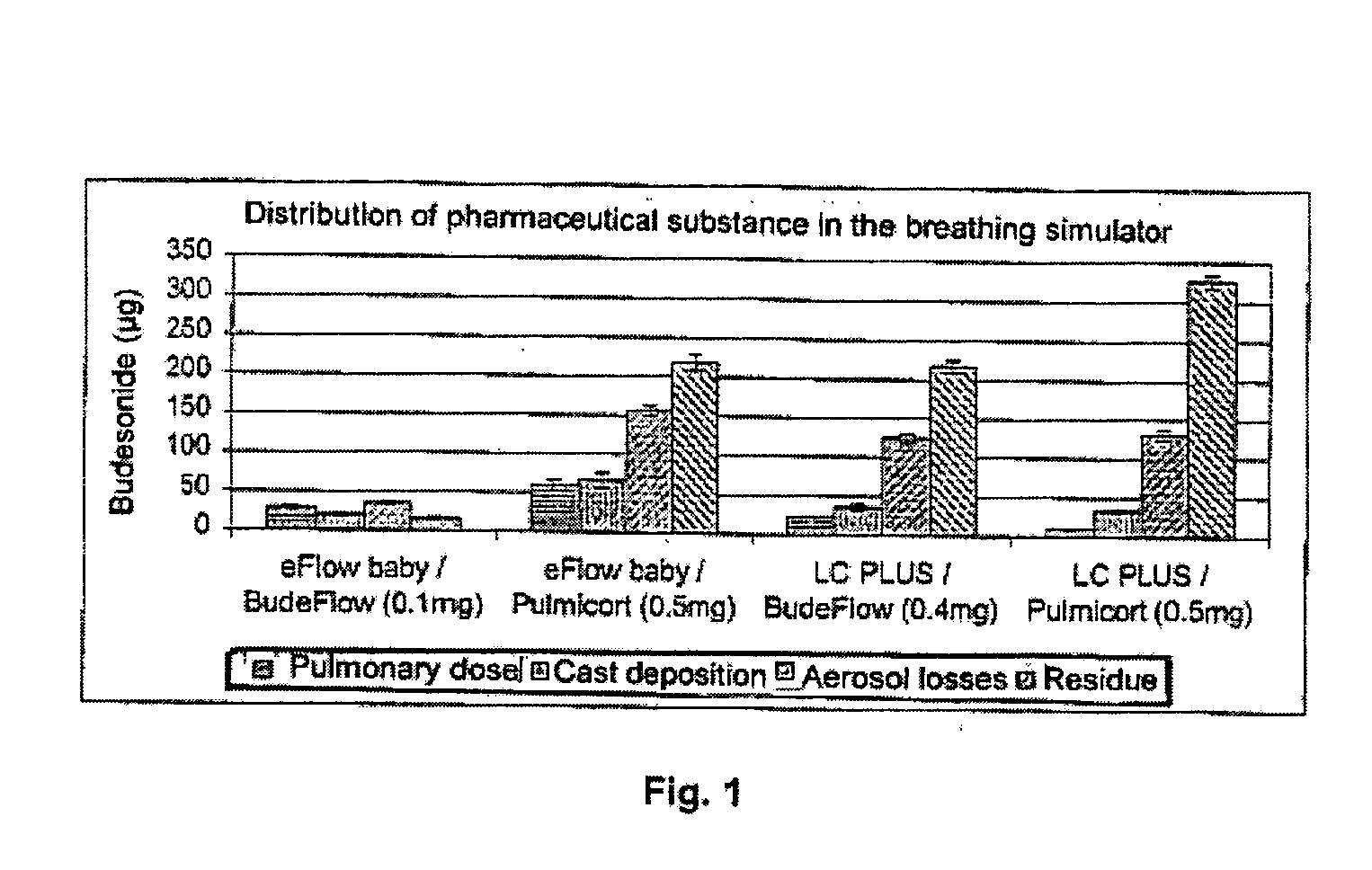
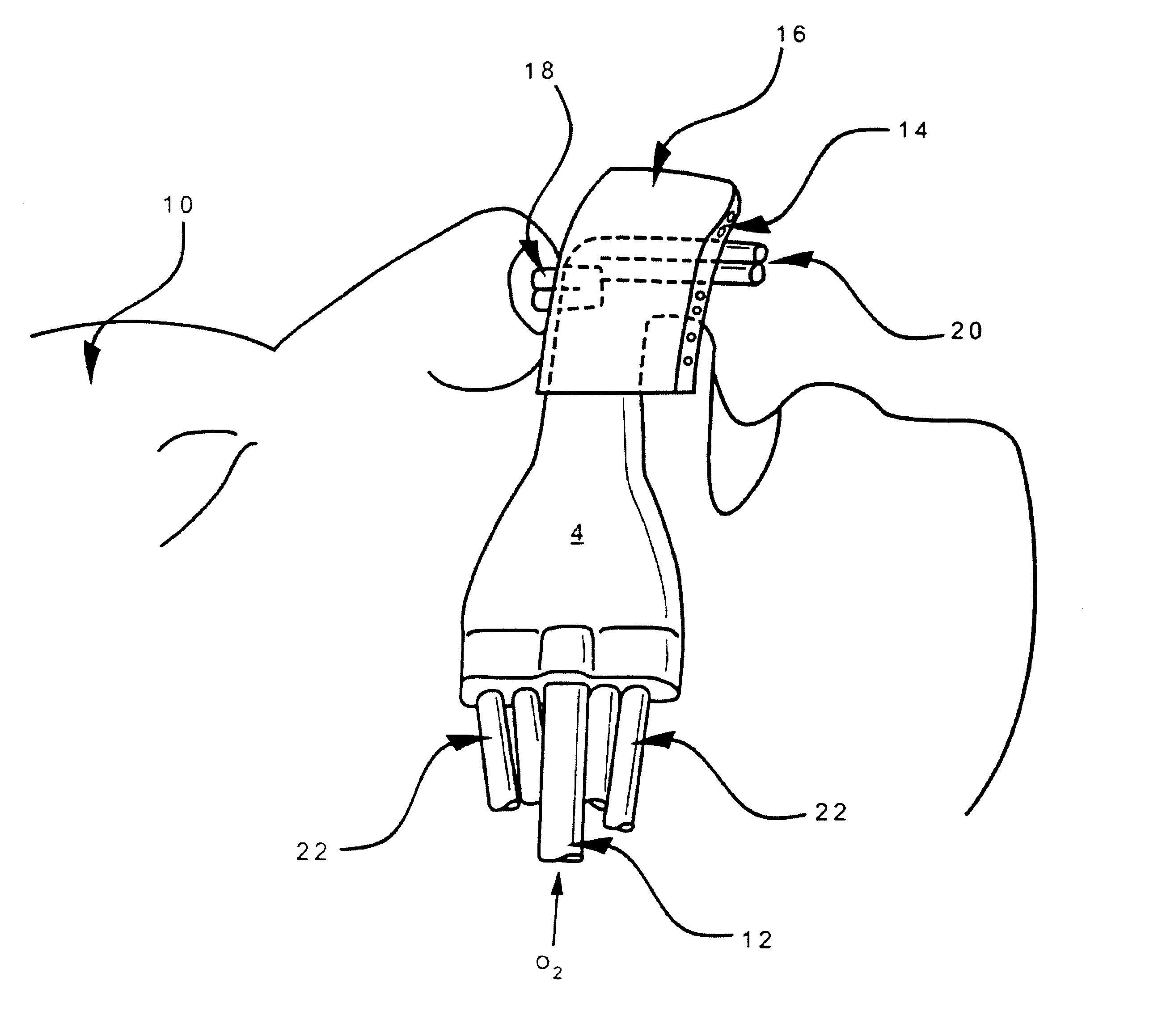
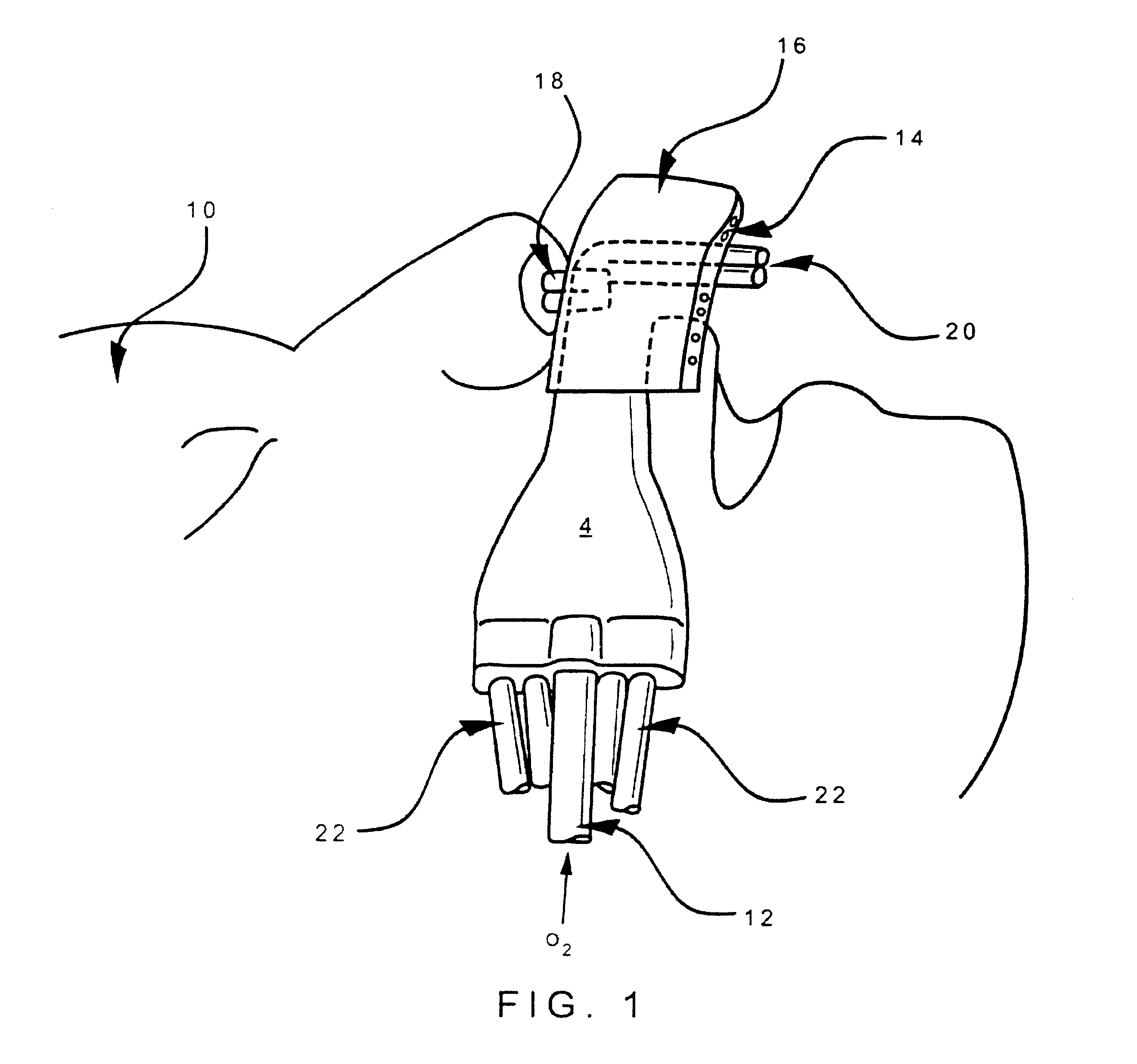
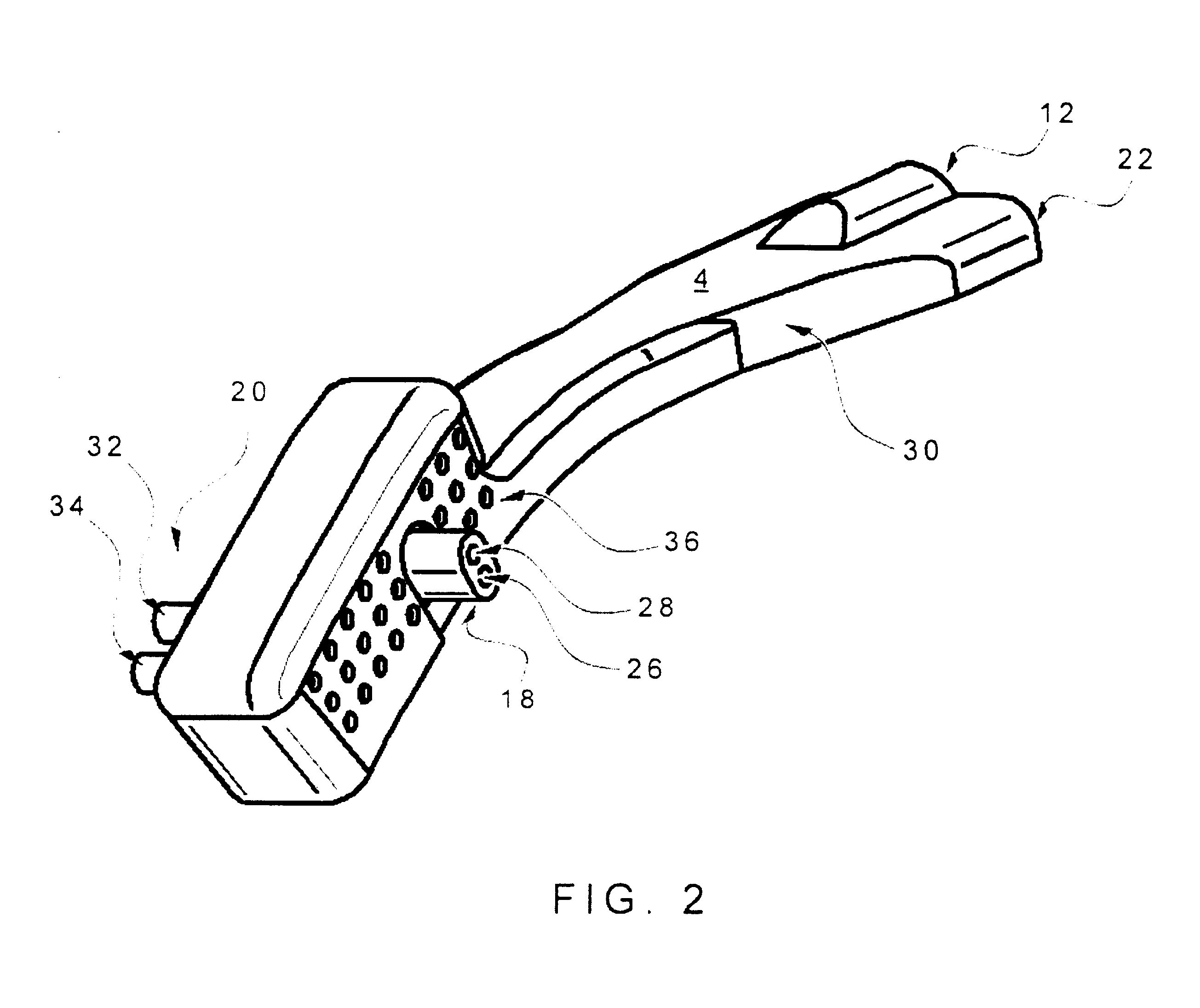
Patents
Literature
3556 results about "Inhalation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
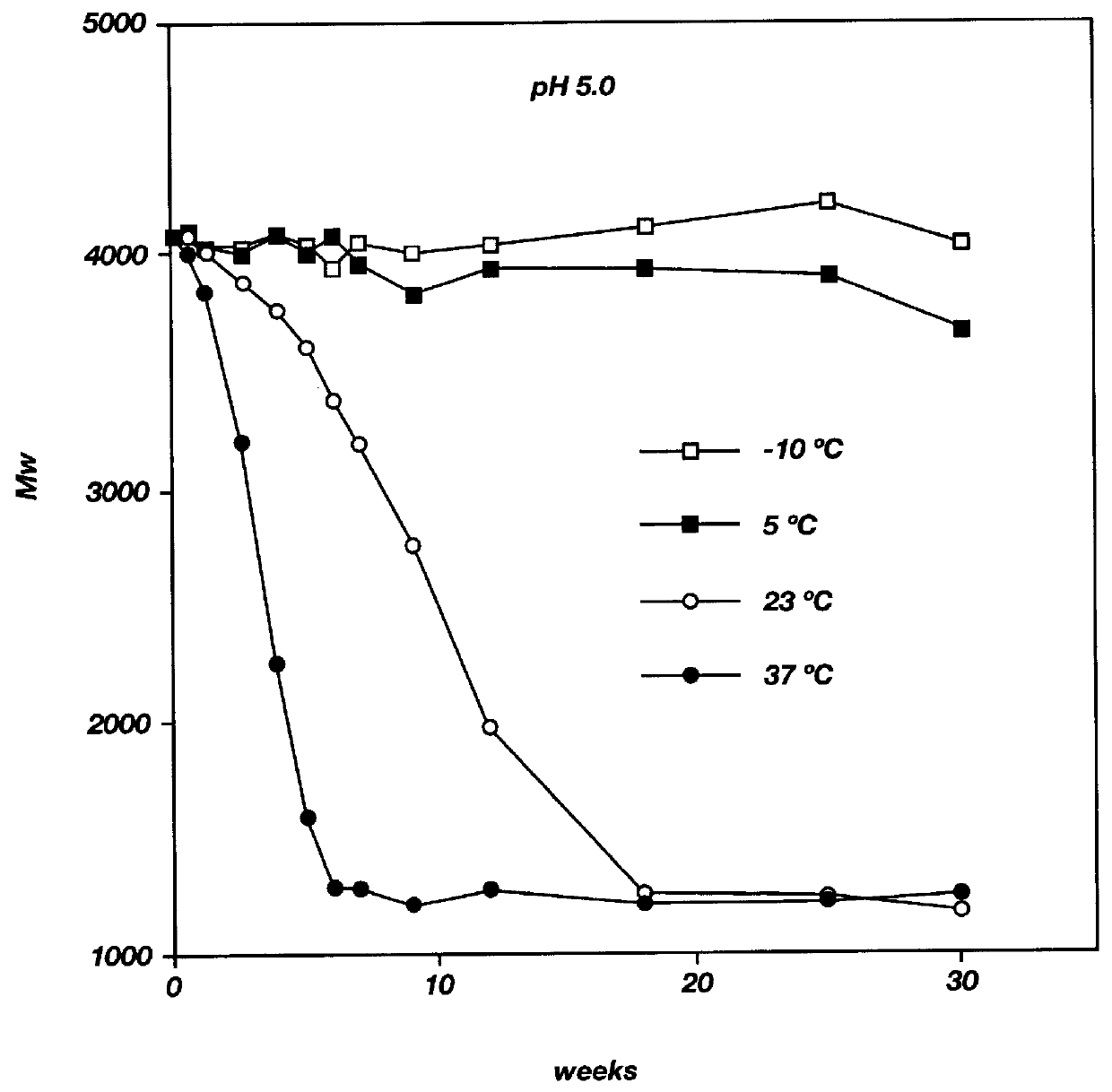
Inhalation (also known as inspiration) happens when air or other gases enter the lungs.
Electronic cigarette
InactiveUS20050016550A1Efficient disseminationGood adhesionTobacco preparationCigar manufactureInhalationEngineering
An electronic cigarette is provided. The electronic cigarette includes a casing having an inhalation hole and a substantially cylindrical configuration, ejection means (first ejection means) provided in the casing and at least one ejection head. Pressure in a cavity filled with a liquid flavor generating medium is changed by driving an actuator to eject the flavor generating medium as droplets from a nozzle in communication with the cavity. Control means provided in the casing controls the driving of the ejection means.
Owner:SEIKO EPSON CORP
Control of supplemental respiratory oxygen
InactiveUS7331343B2Healthy blood oxygen saturationOperating means/releasing devices for valvesRespiratory masksLoop controlInhalation
Methods and systems for supplying supplemental oxygen to patients for use in sub-acute care which maintains healthy blood oxygen content in the patients by controlled dosing of oxygen with a measured response to the patient's actual blood oxygen content are disclosed. The dosing can be provided by simple ON / OFF control over the delivery of oxygen or the amount of oxygen delivered to the patient with each inhalation can be varied in response to the patient's need as determined by a more sophisticated control scheme, such as a PID loop control algorithm, that utilizes the difference between the patient's actual blood oxygen content and a target blood oxygen content and / or trends in the blood oxygen content. The systems and methods are particularly directed at patients receiving supplemental oxygen in a sub-acute care environment.
Owner:MINNESOTA INNOVATIVE TECH & INSTR MITI
Electronic vaporizing device and methods for use
InactiveUS20130298905A1Increase heatImproved vaporizing capabilityTobacco devicesInhalatorsCelluloseBrick
Devices and methods for vaporizing active ingredients of a selected substance for inhalation using a portable vaporization device are provided herein. In certain aspects, the device includes a portable power source, a heating portion, an inhalation sensor, a temperature sensor, a distal light source, and a grinding portion. In response to an inhalation by a user, the power source energizes a heating element of the heating portion so as to heat air flow to a desired vaporization temperature within a few seconds of detecting inhalation, using convection and radiative heating. The device may include a receptacle for receiving a cartridge containing a pre-prepared substance, such as a liquid, gel, powder, or solid brick, and a grinding portion to allow a user to grind intact portions of cellulose-based material into smaller pieces to facilitate vaporization by manually rotating portions of the device relative to each other.
Owner:UPTOKE
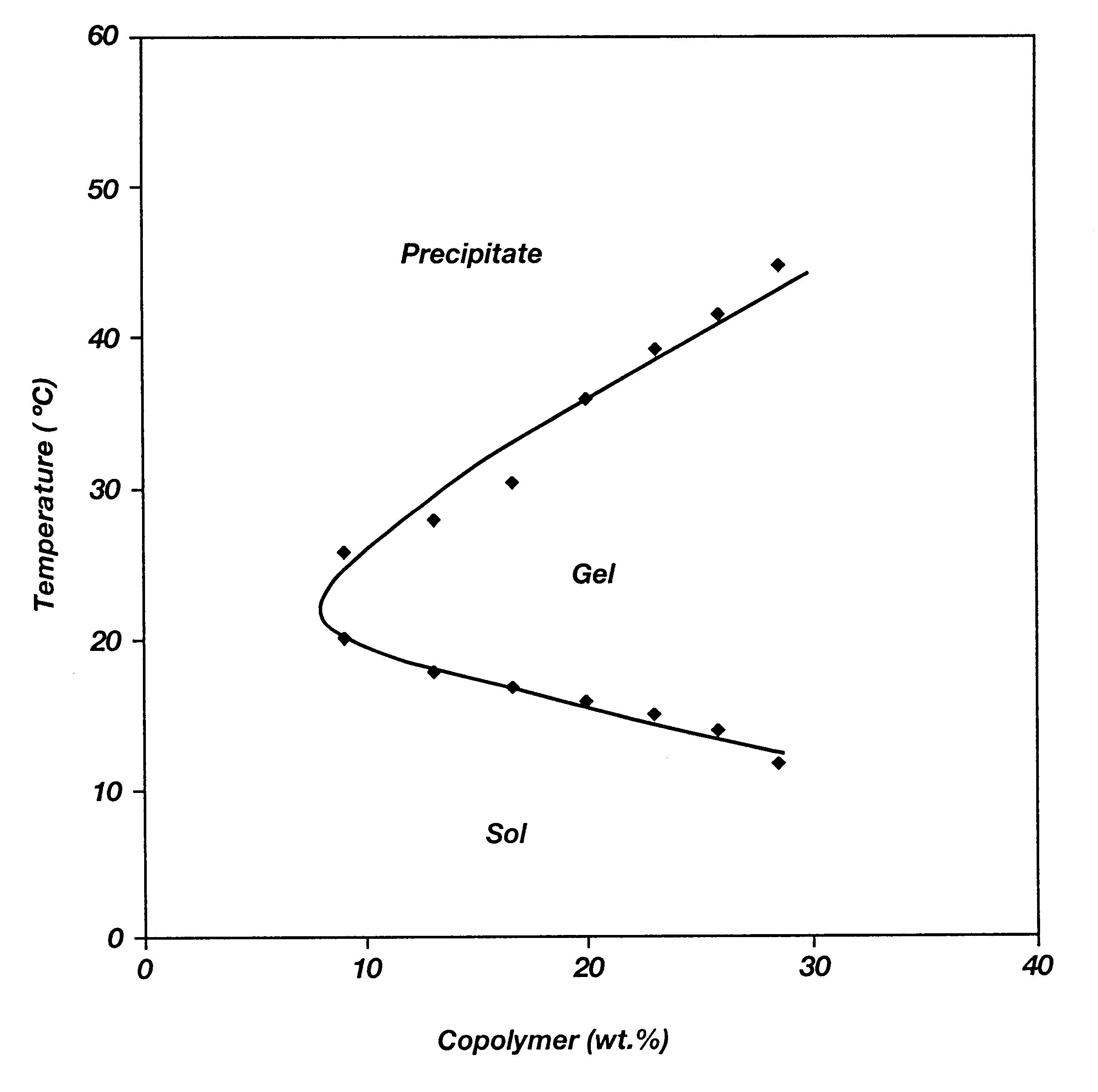
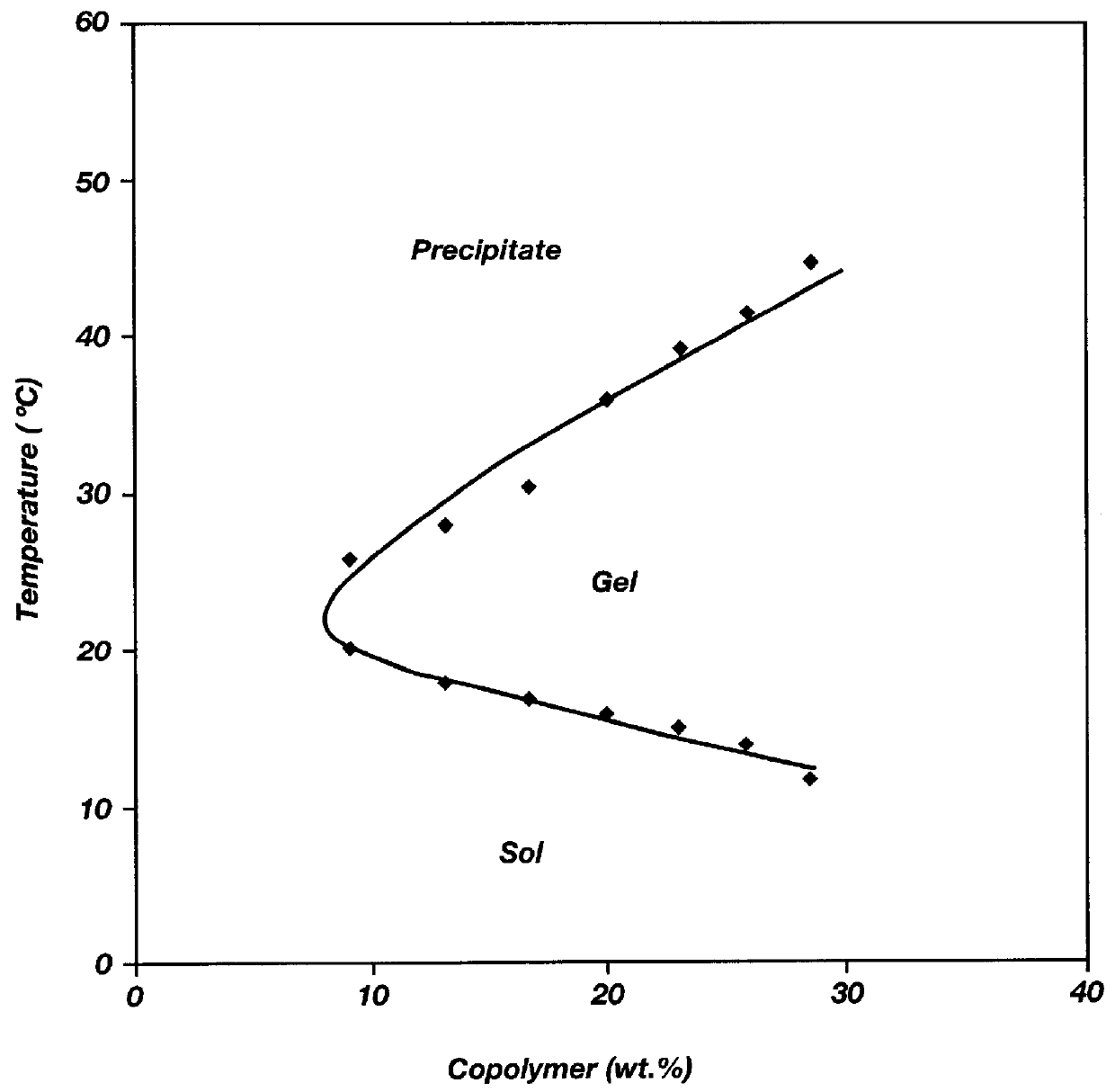
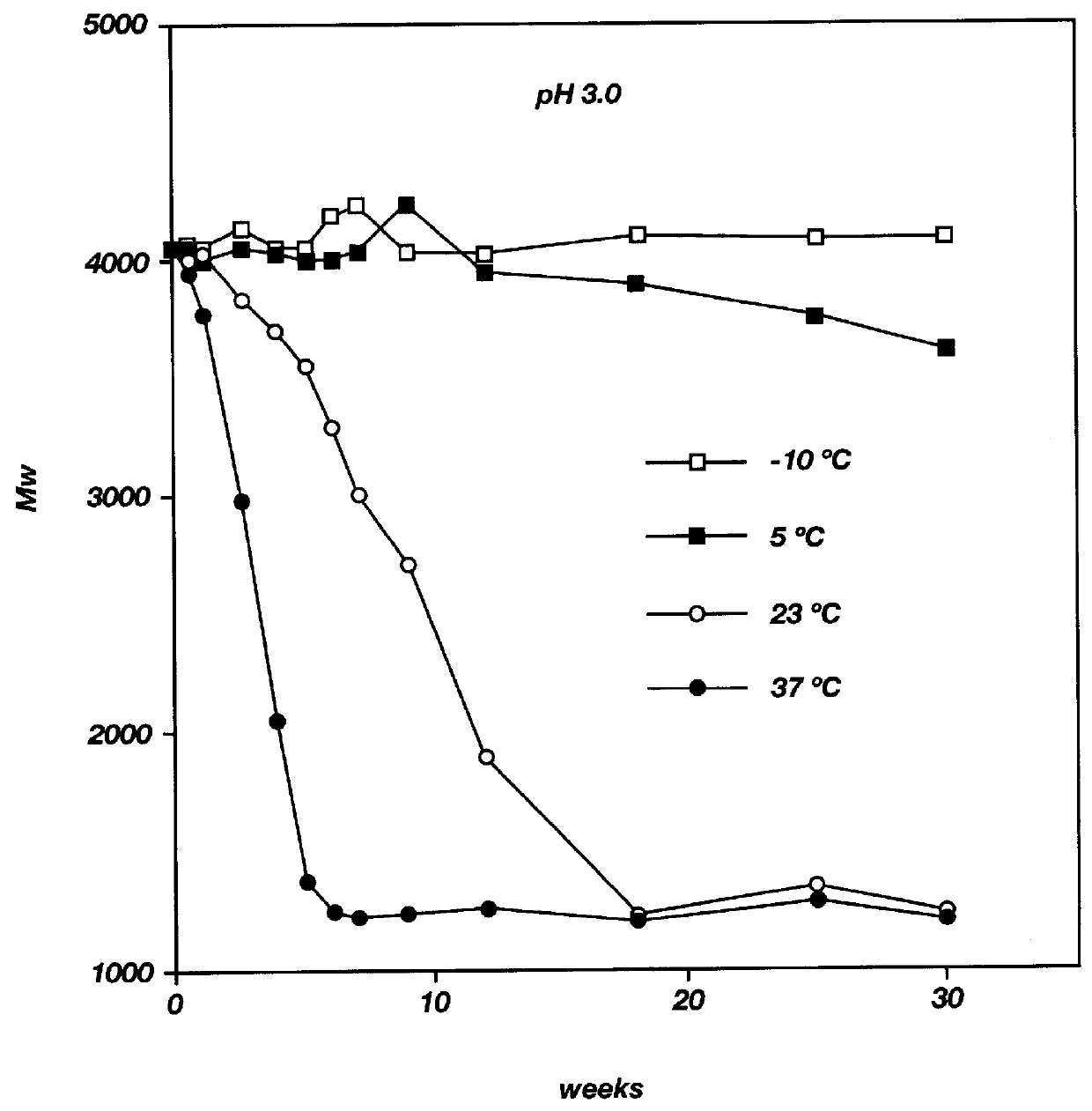
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Smoking articles and use thereof for yielding inhalation materials
The present invention describes articles, such as smoking articles, that can provide an inhalable substance in a form suitable for inhalation by a consumer. The article comprises a cartridge with an inhalable substance medium therein, control housing that includes an electrical energy source and an electrical power source, and a heating member that may be located in either the cartridge or the control housing. The control housing further may include puff-actuated current actuation components and current regulation components.
Owner:RAI STRATEGIC HLDG INC
Low temperature electronic vaporization device and methods
ActiveUS20130042865A1Maintain efficiencyReduce the temperatureInput/output for user-computer interactionTobacco treatmentInhalationEnvironmental health
Low temperature electronic vaporization devices and method are described herein for emulating smoking wherein the devices generate an aerosol for inhalation by a subject by heating a viscous material that can have a tactile response in the mouth or respiratory tract.
Owner:JLI NAT SETTLEMENT TRUST
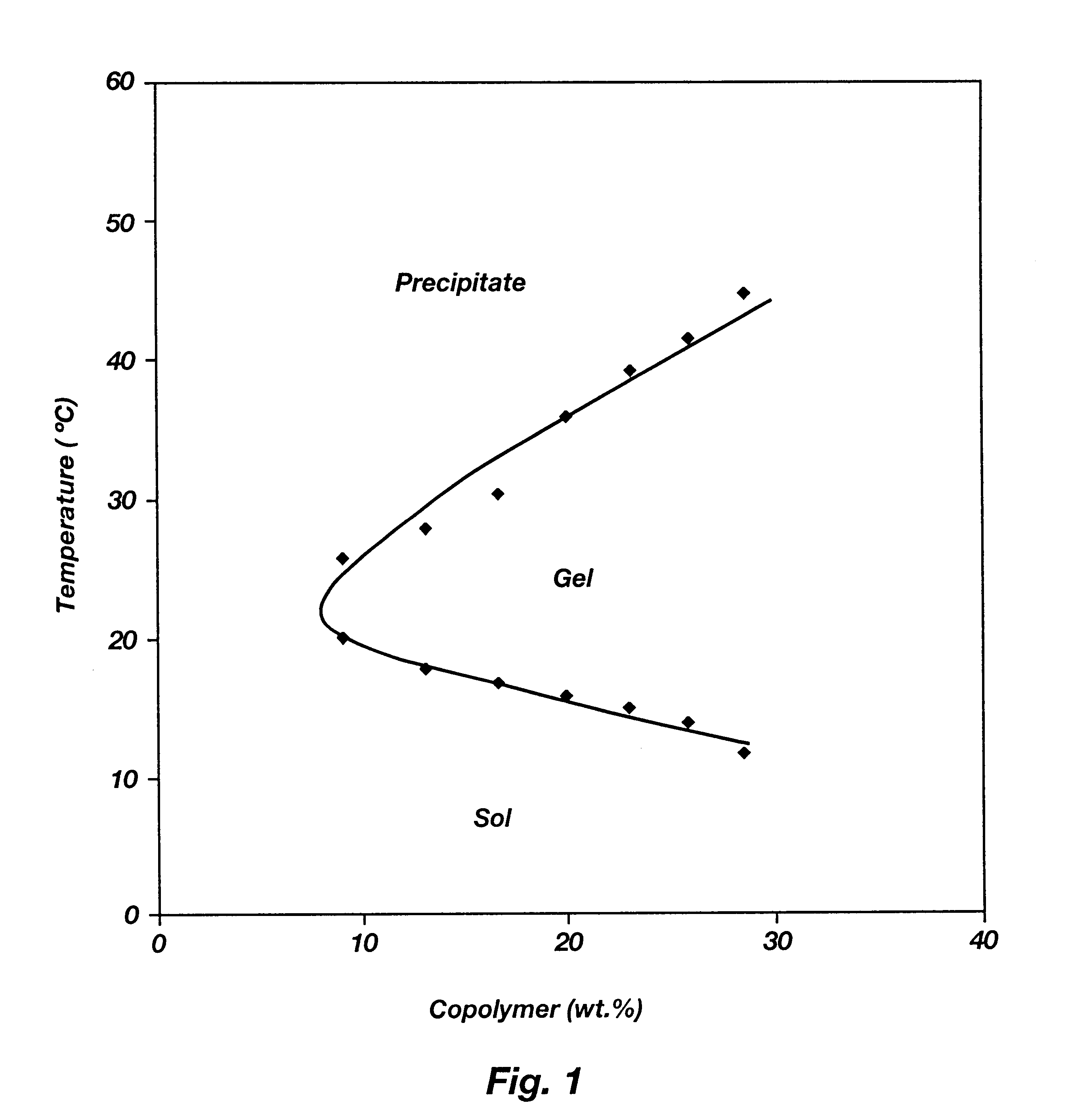
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
E-Cigarette With Vitamin Infusion
InactiveUS20100200008A1Prevent negative consequenceImprove health benefitsTobacco preparationTobacco devicesInhalationEnvironmental health
Owner:TAIEB ELI
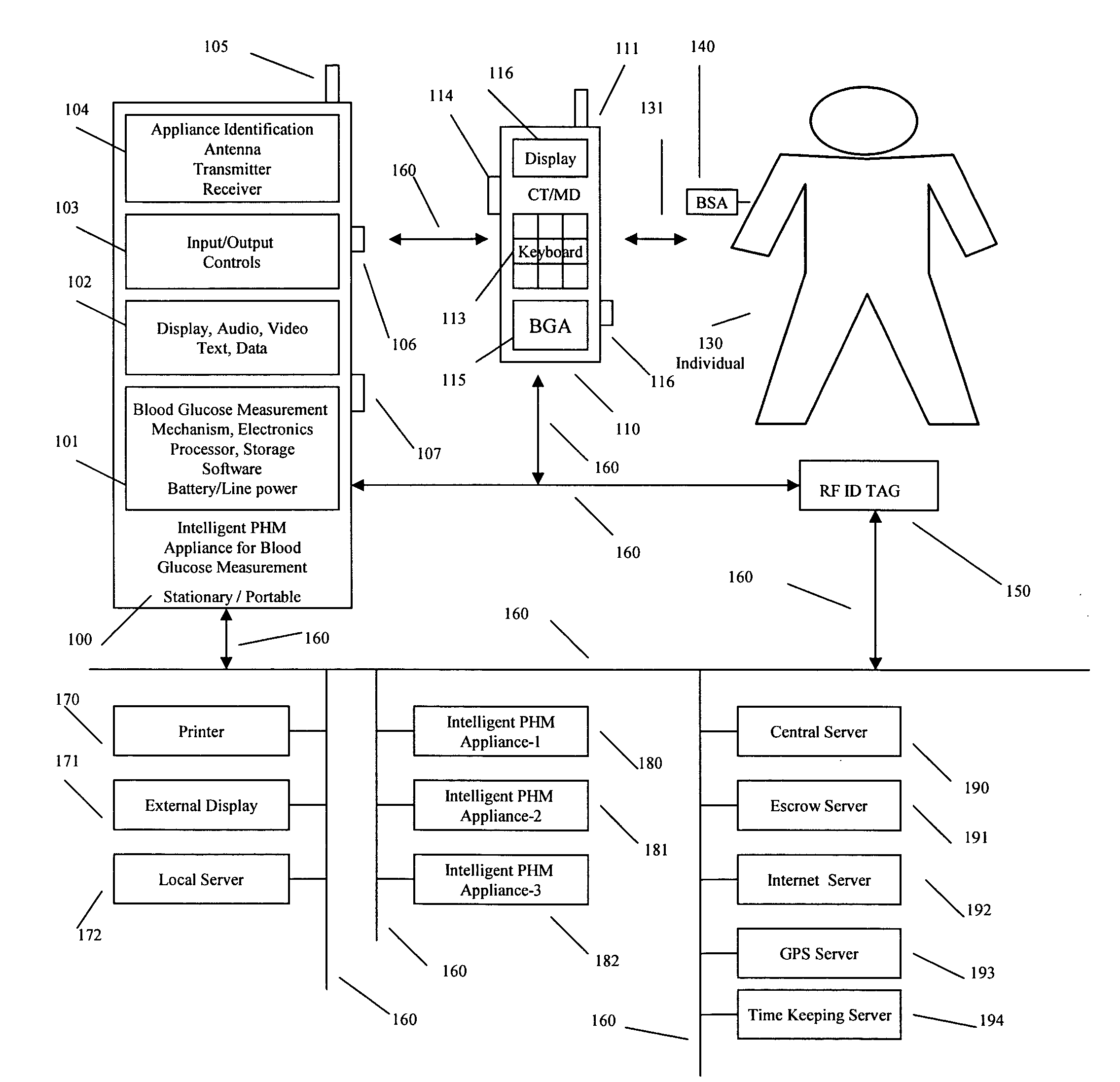
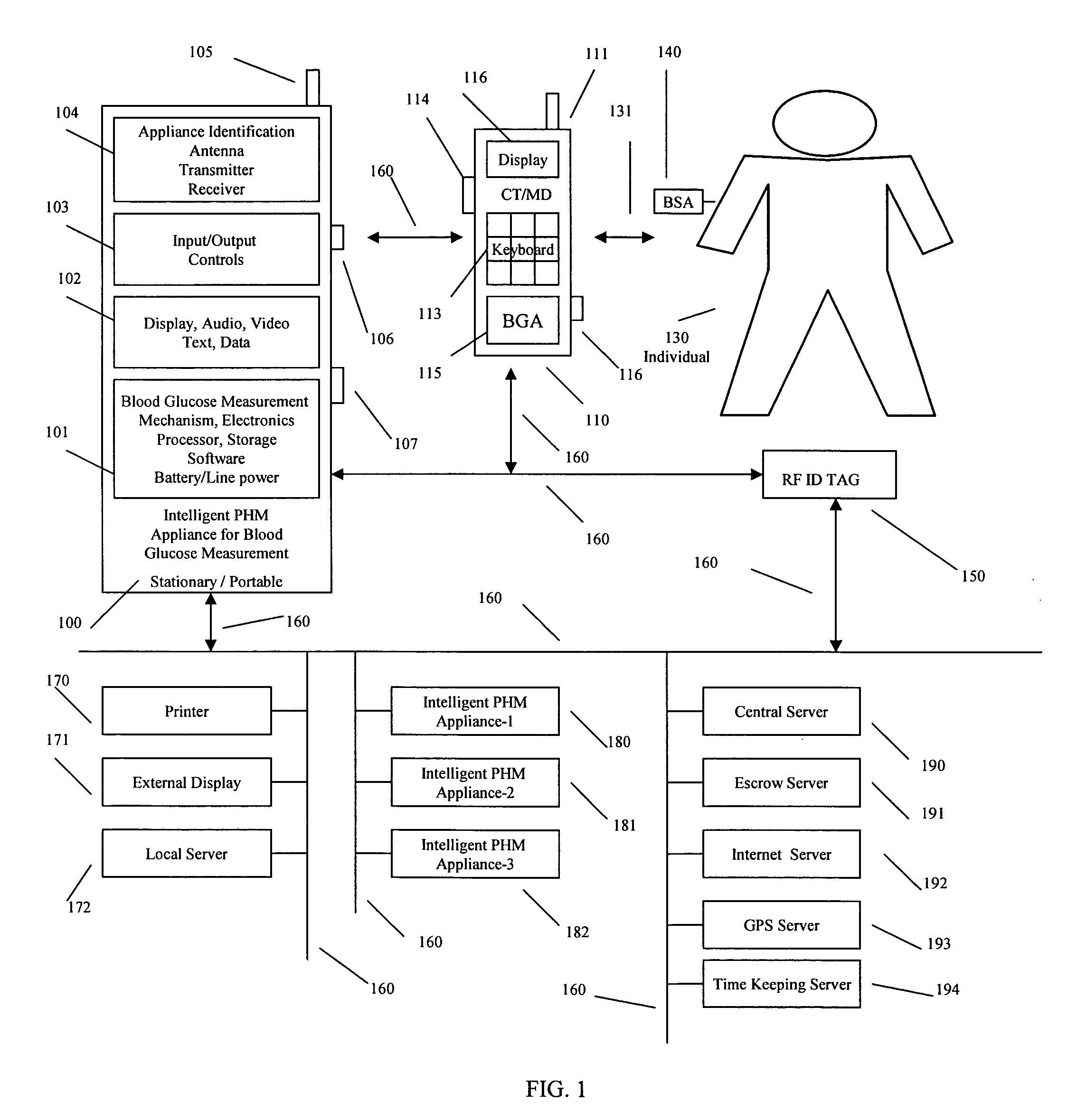
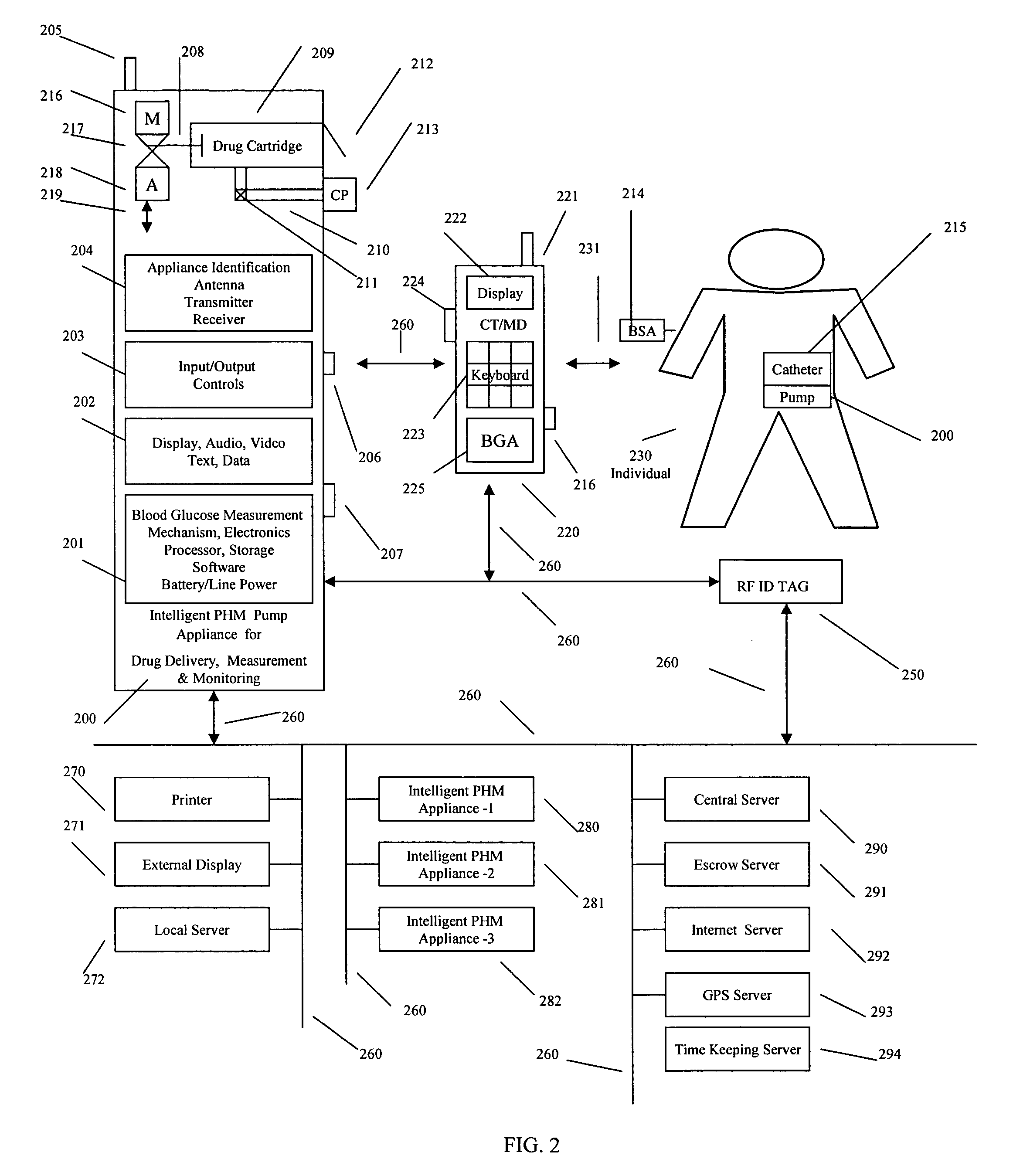
Intelligent personal health management appliances for the measurement and monitoring of health factors and controlled delivery of drugs
InactiveUS20080139907A1Precise positioningPointing accuratelyPhysical therapies and activitiesElectrotherapyDiseaseDiabetes mellitus
A system, method and apparatus for real time measurement and monitoring of various personal health parameters including controlled delivery of drugs / medications by intelligent pump appliances, intelligent inhalation appliances and intelligent skin patch appliances used in a standalone manner or in a wired or wireless networked configuration, in conjunction with various peripheral devices, other intelligent appliances, servers, RF ID Tags and stationary / mobile devices. The intelligent appliances relate to the measurement, monitoring and delivery of insulin / other drugs for the treatment of diabetes and other diseases. The method also additionally includes the application of intelligent appliances for pain management including visualization of organs and body locations exhibiting pain.
Owner:IP HLDG LLC
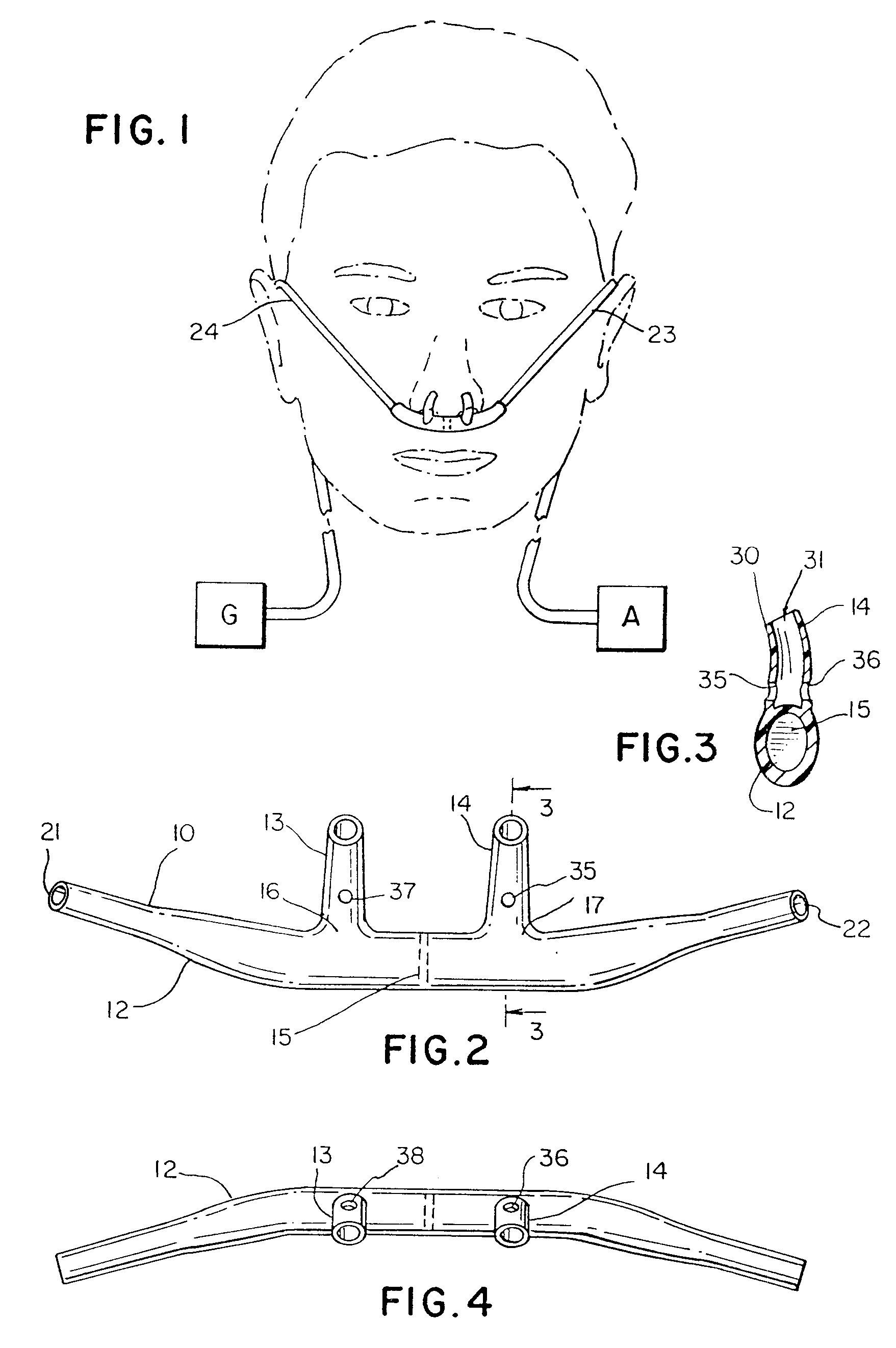
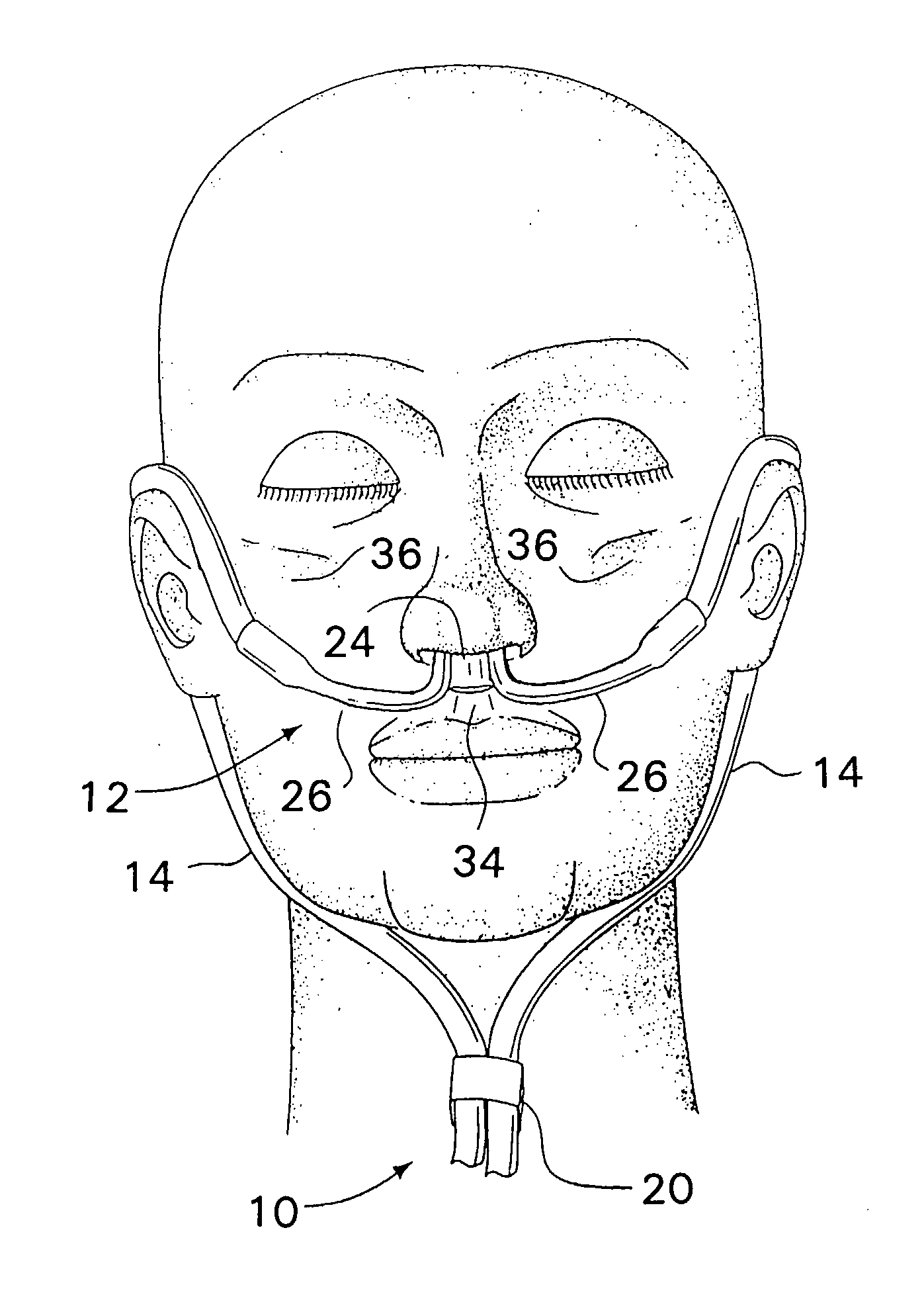
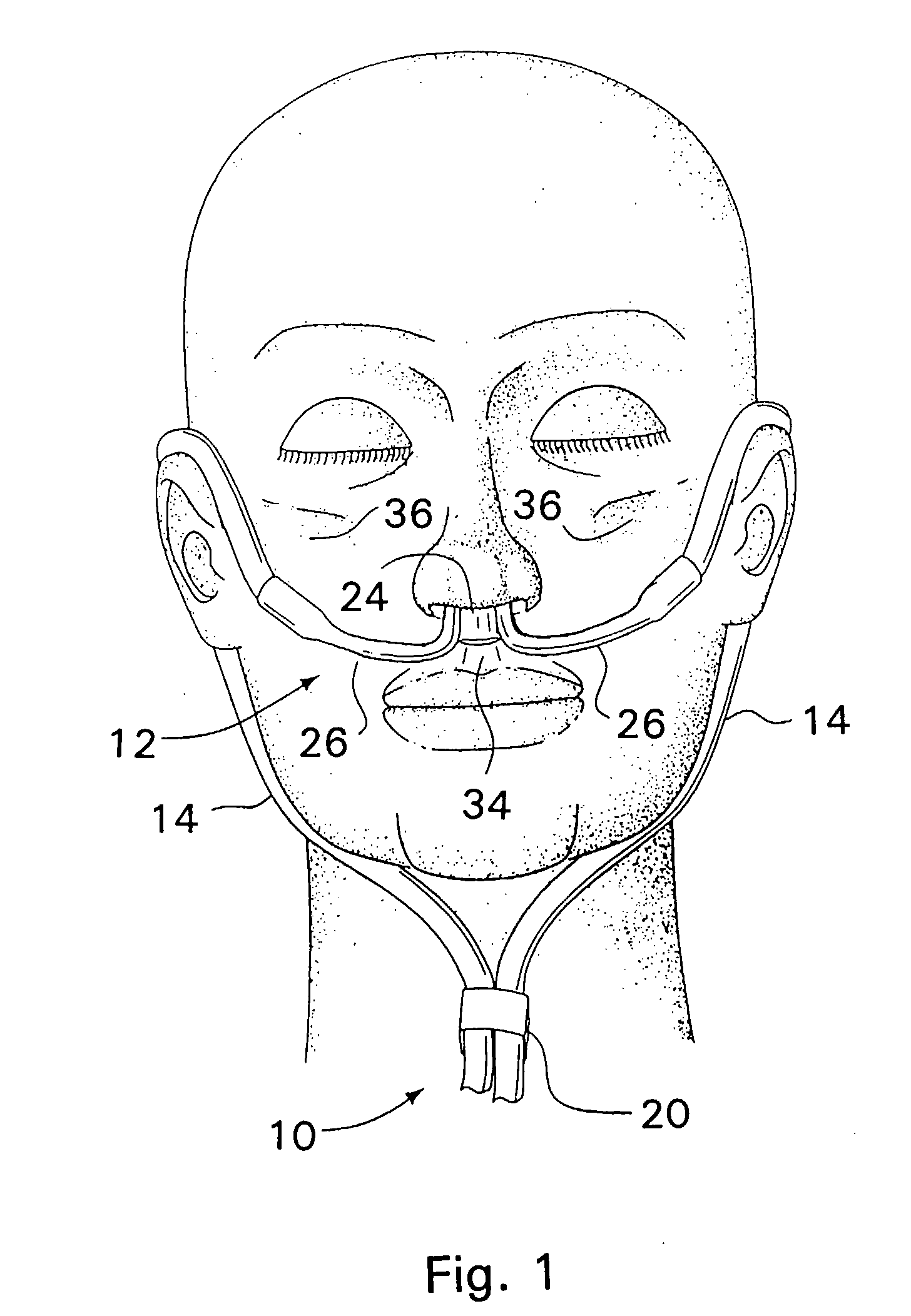
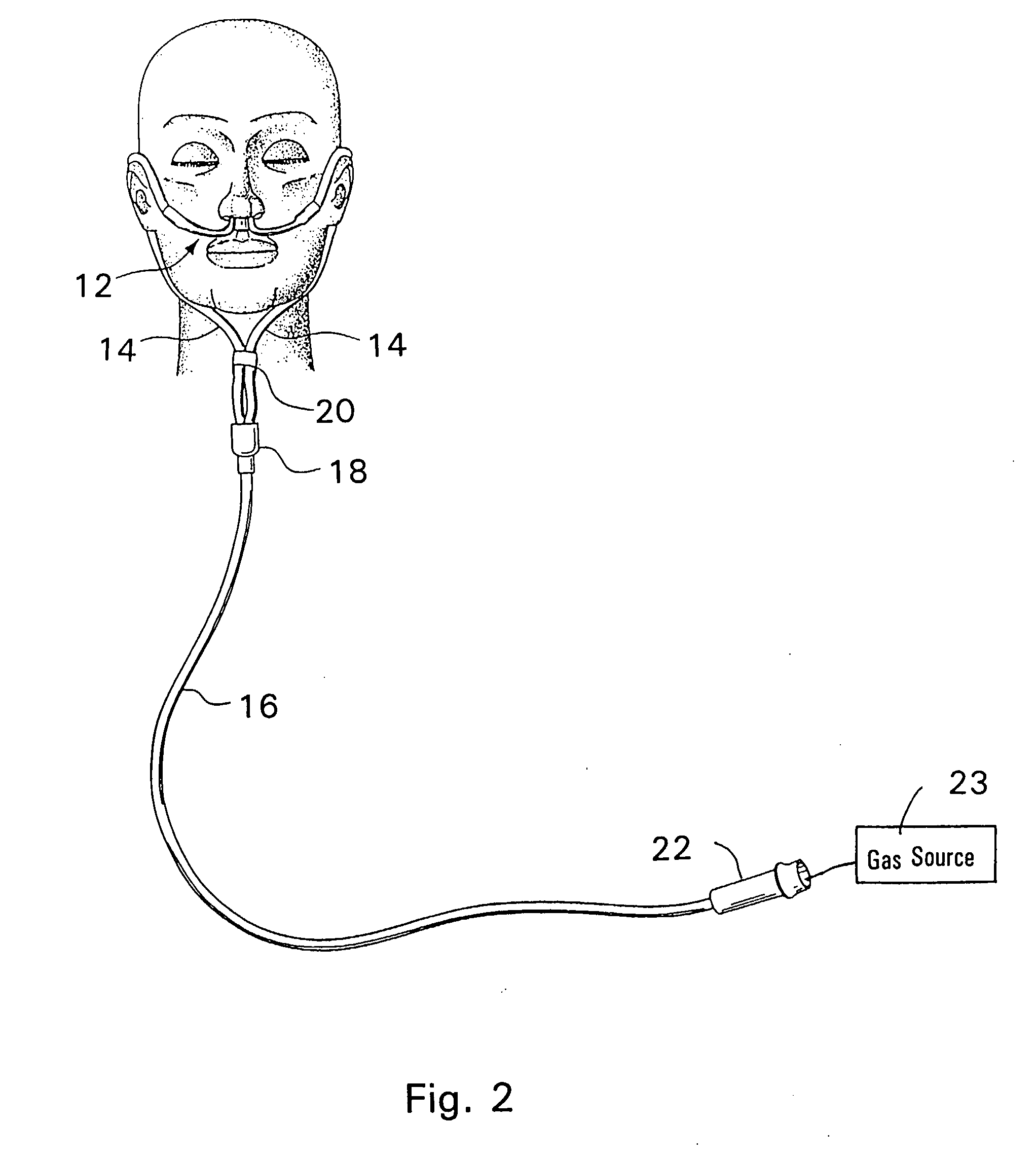
Nasal cannula
InactiveUS6439234B1Reduces and eliminates incidenceAccurate monitoringOperating means/releasing devices for valvesRespiratory masksGas analysisNose
Owner:SALTER LABS
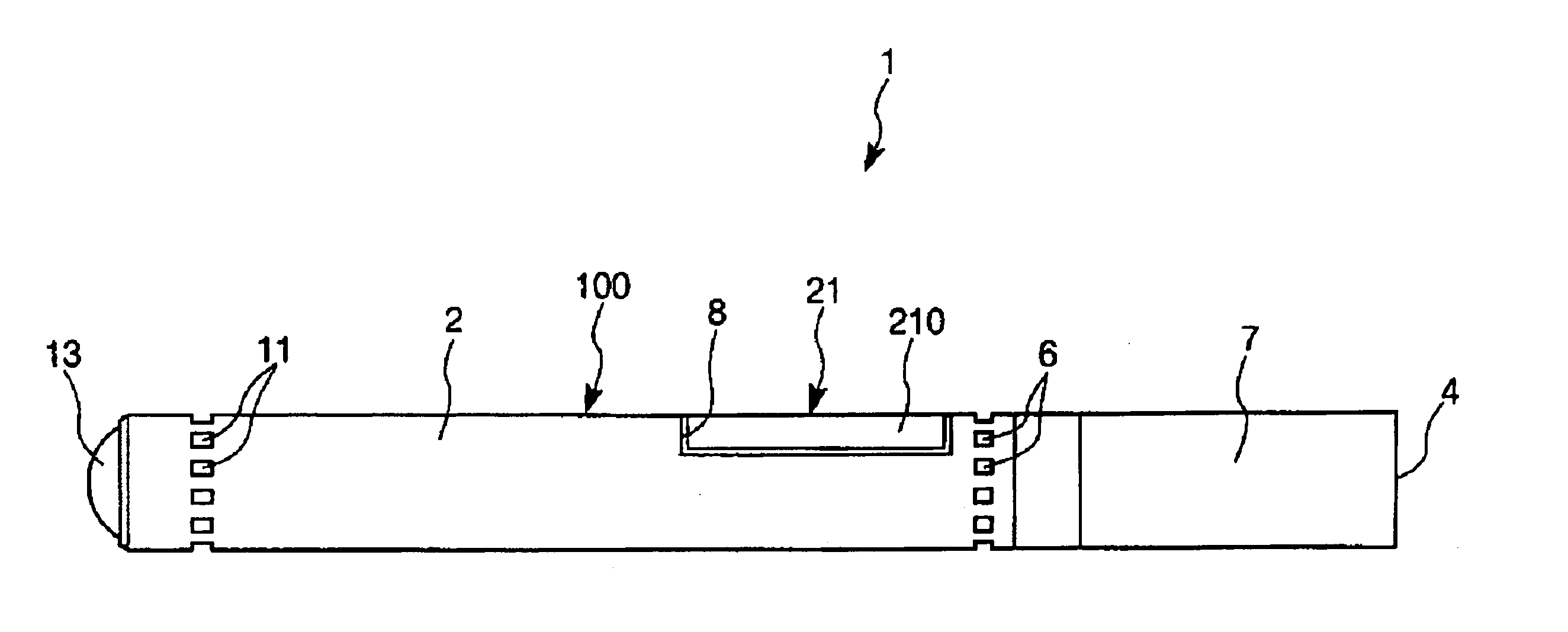
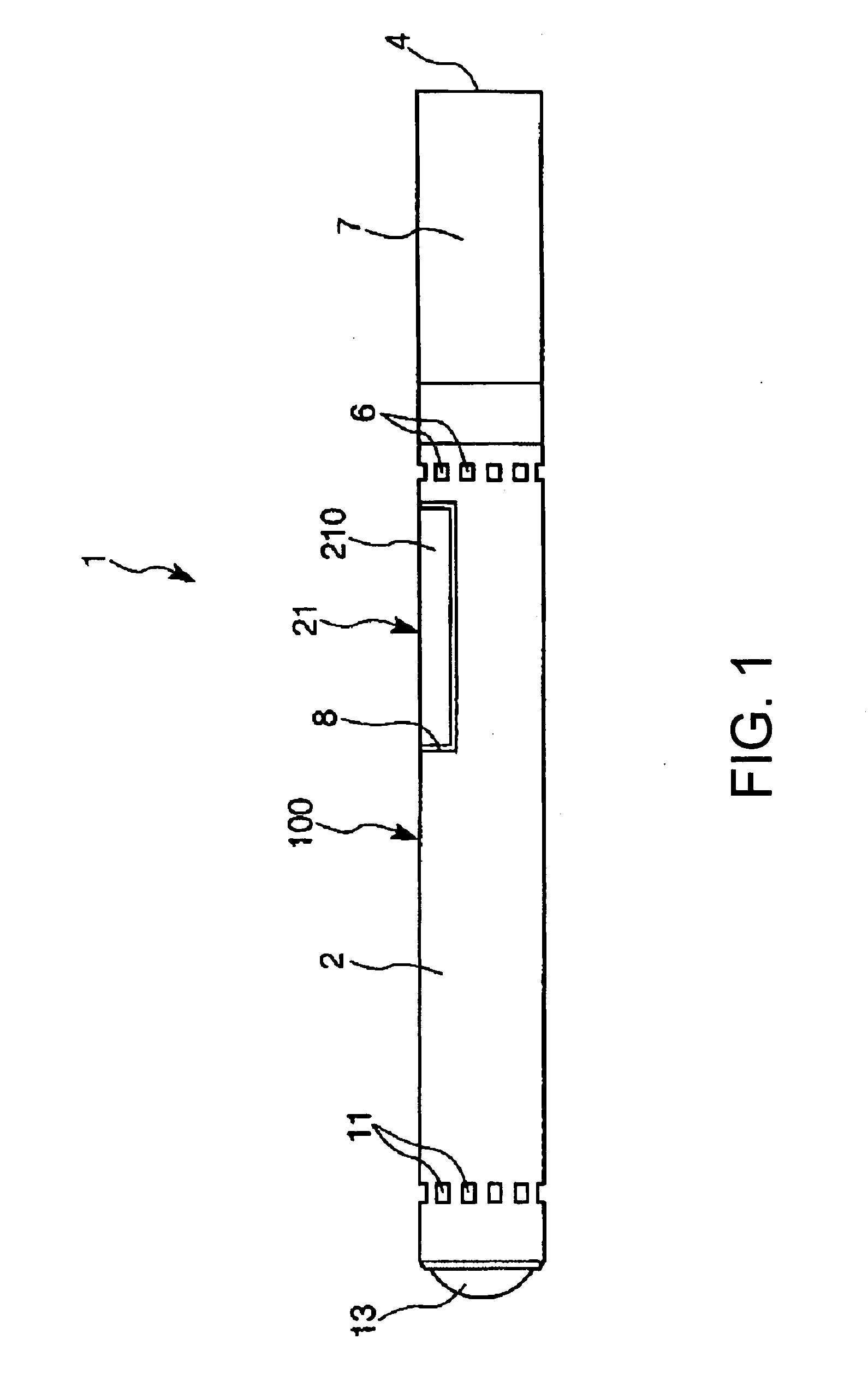
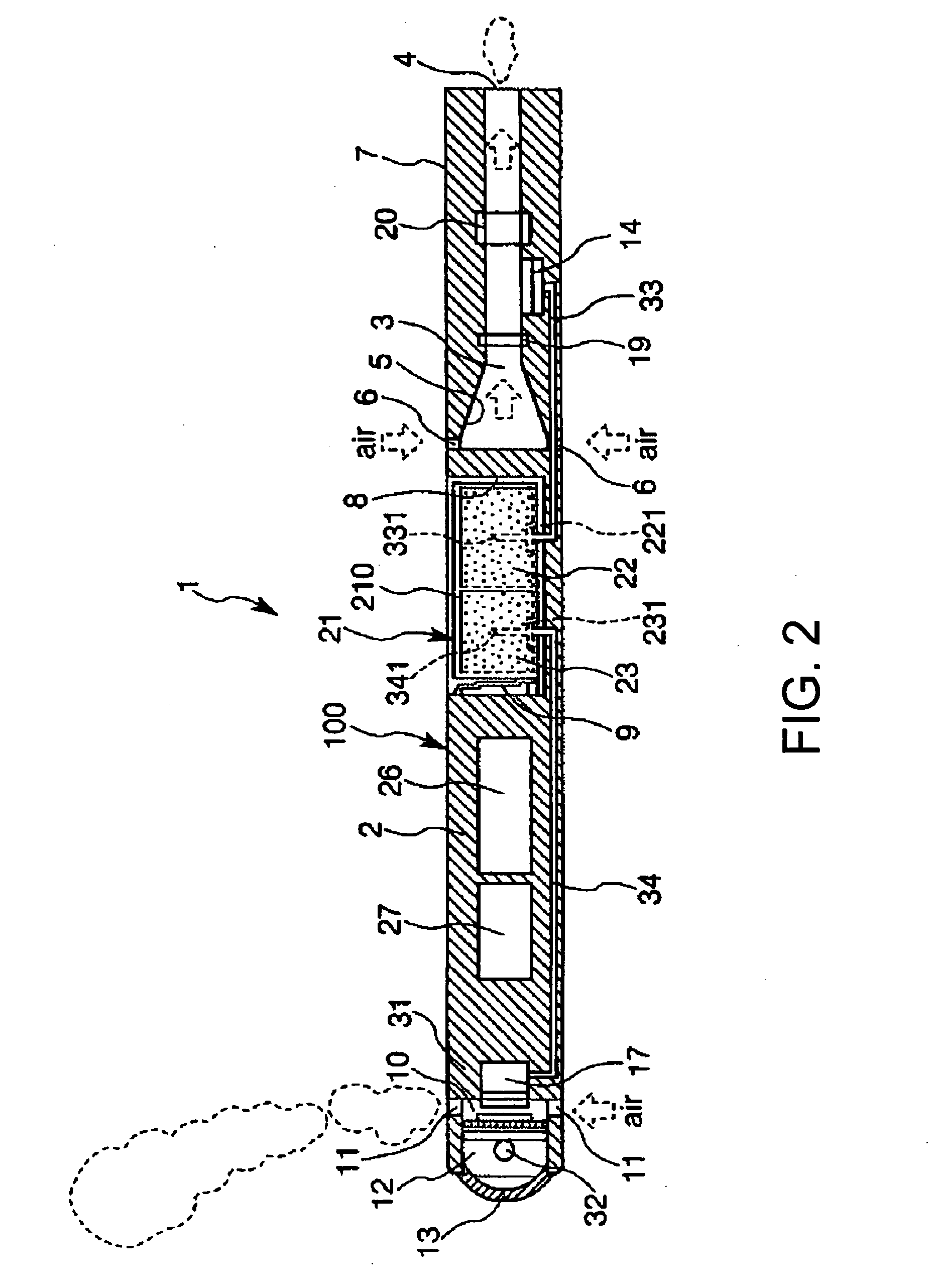
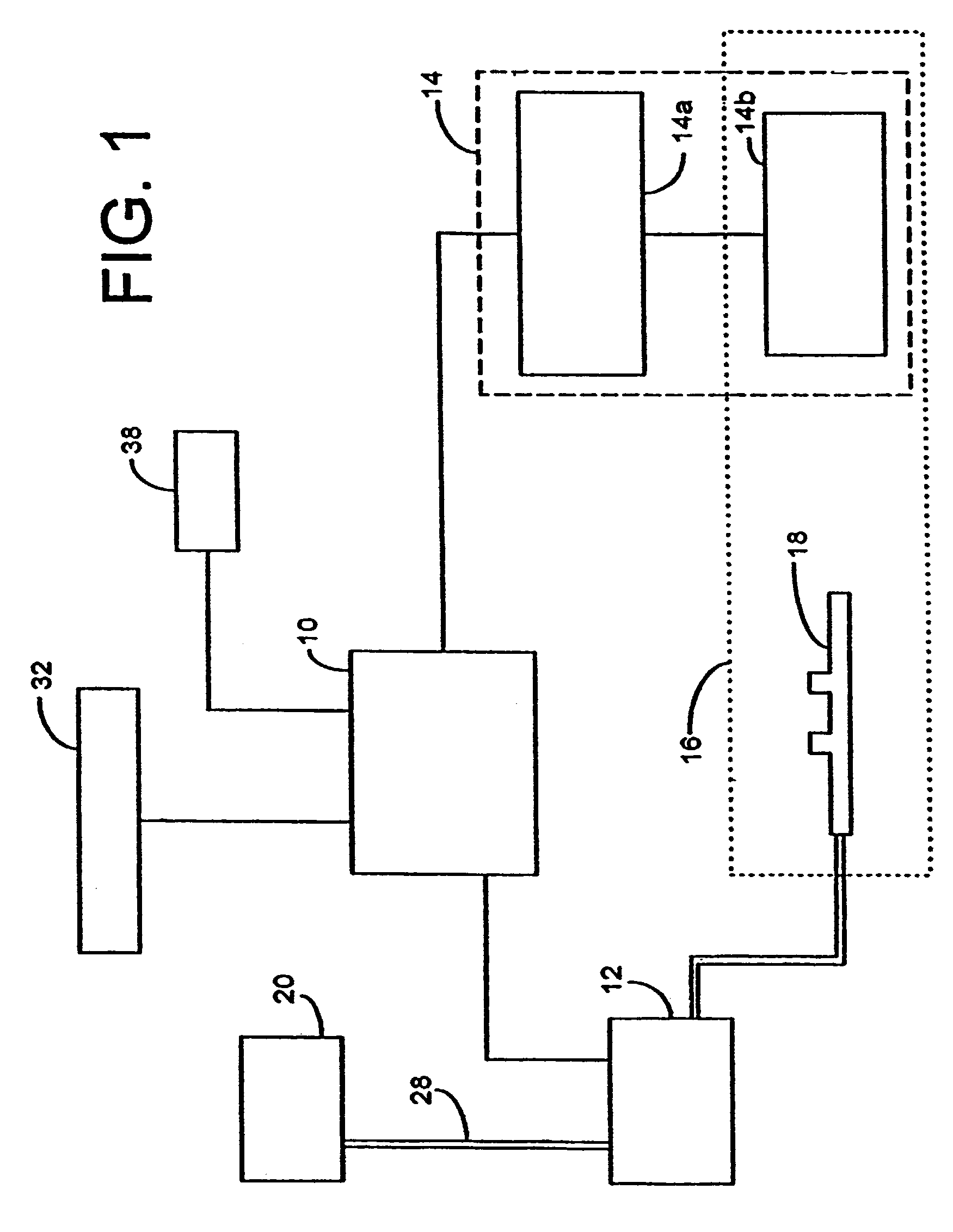
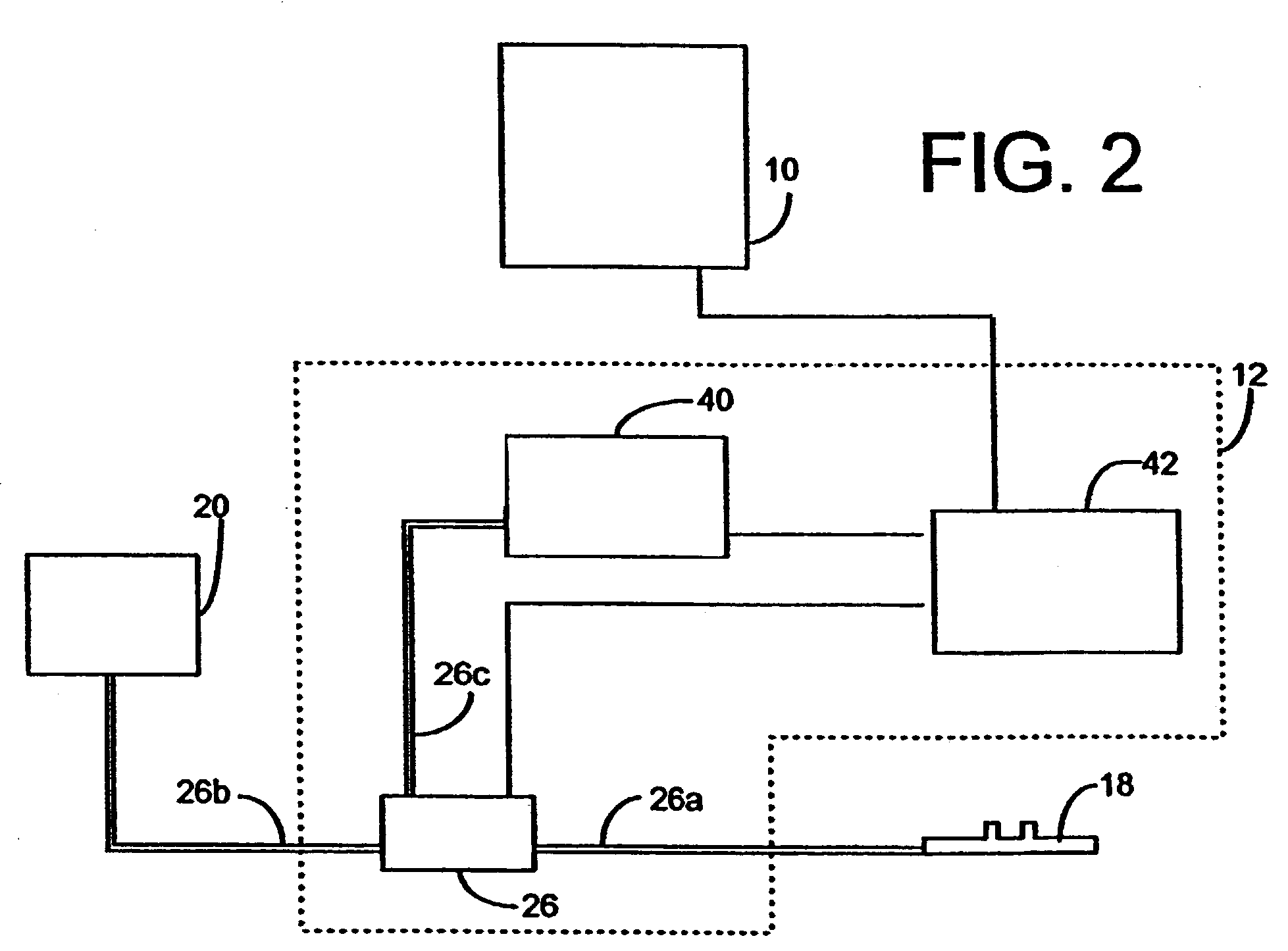
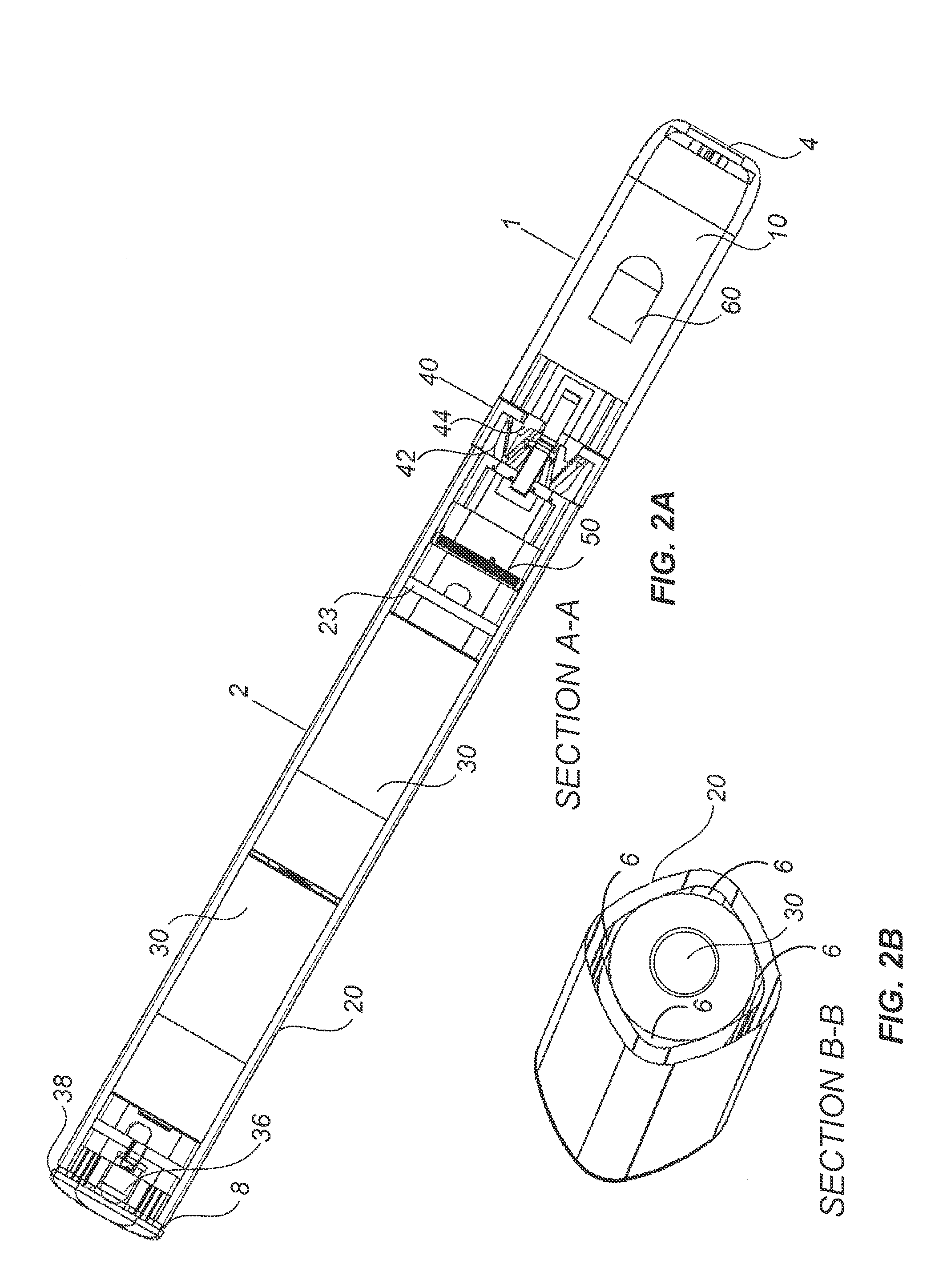
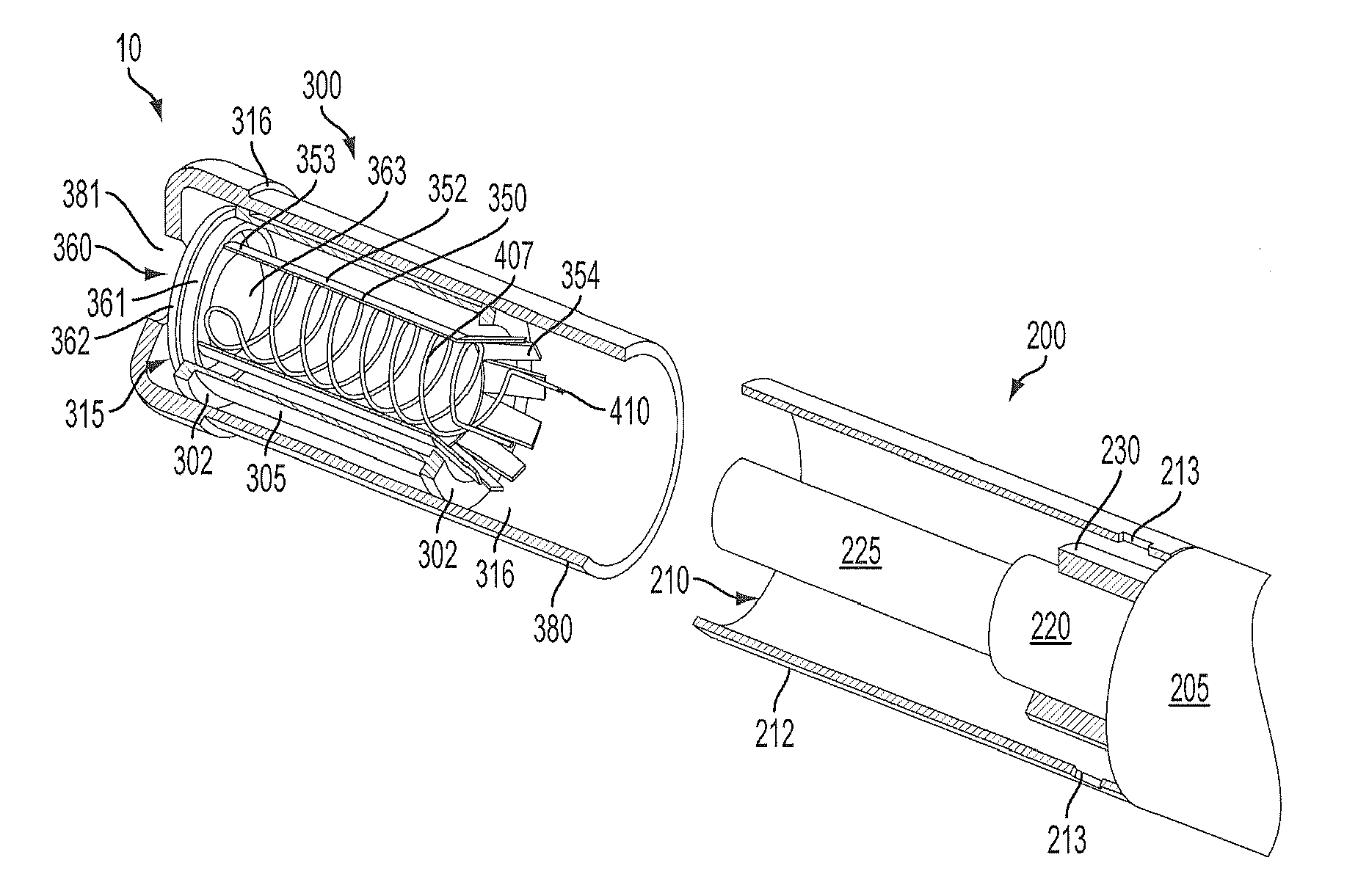
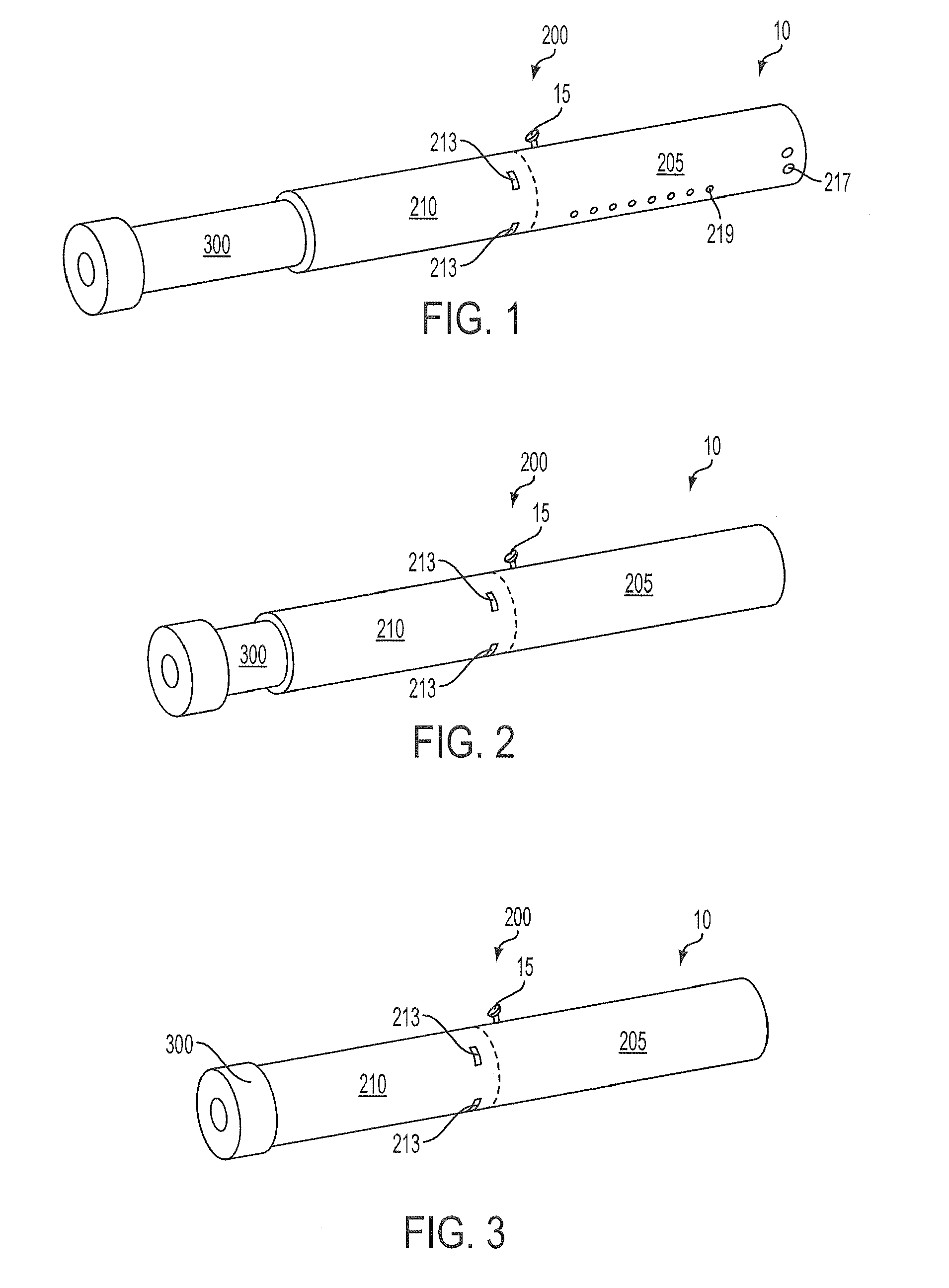
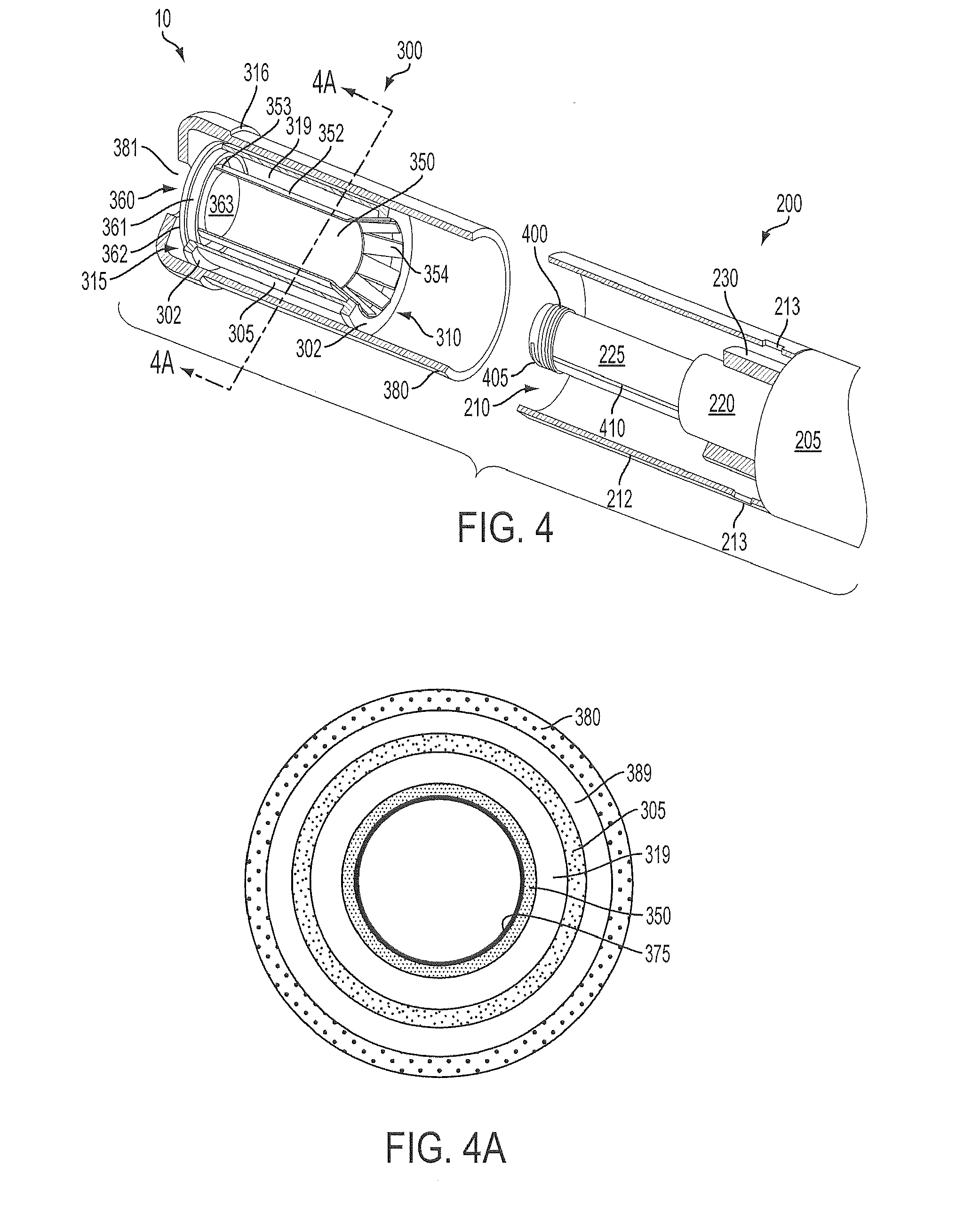
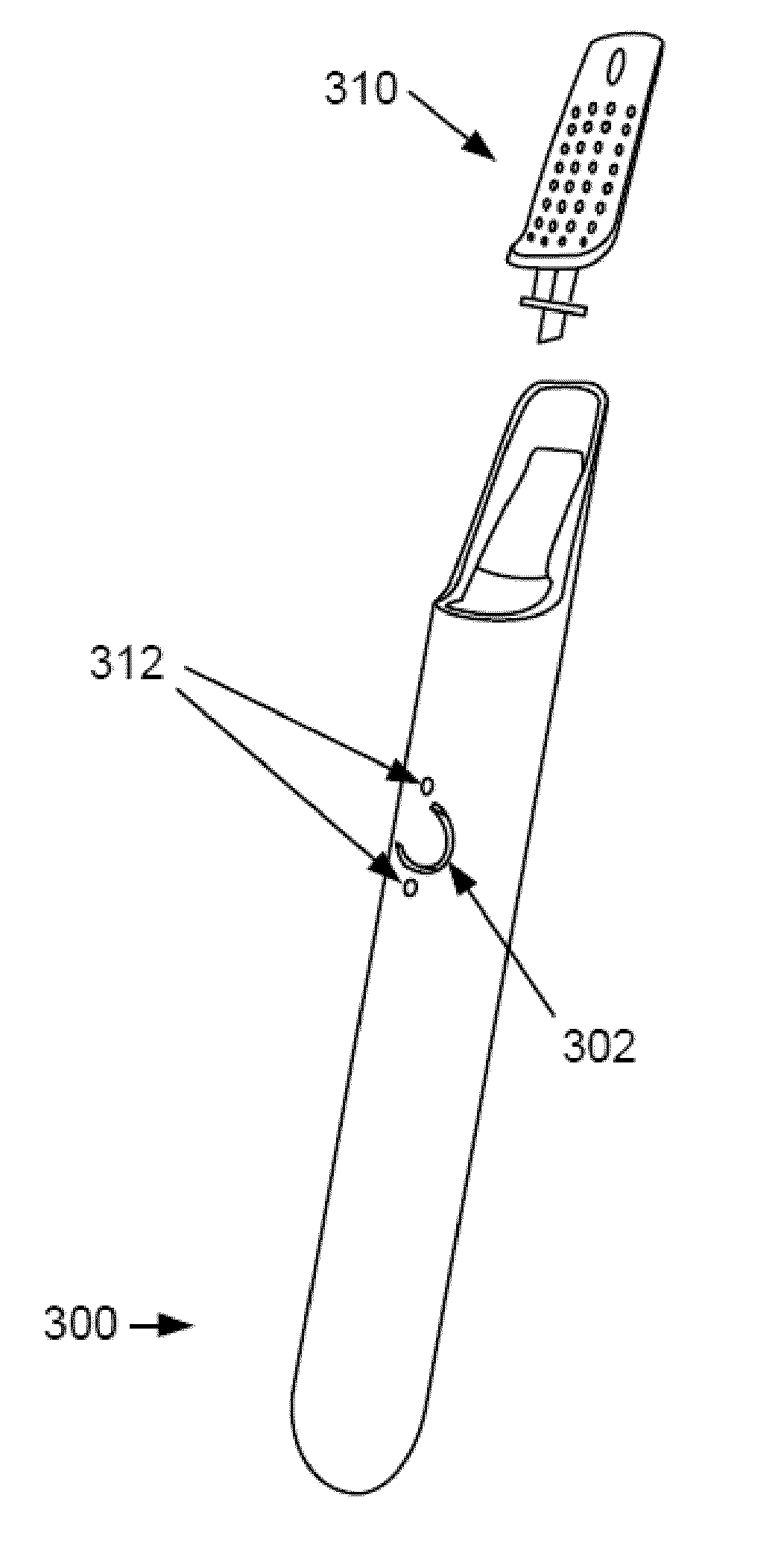
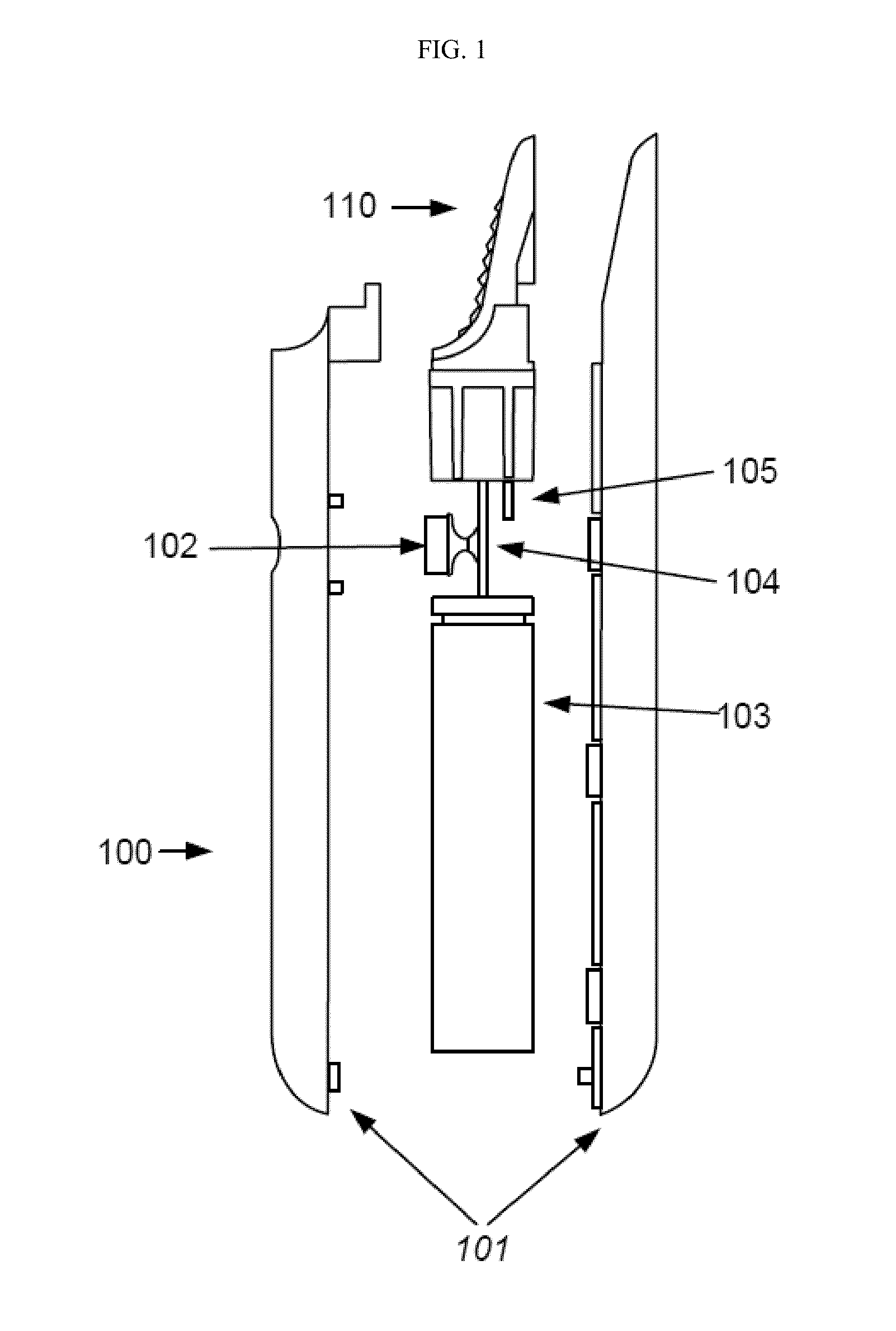
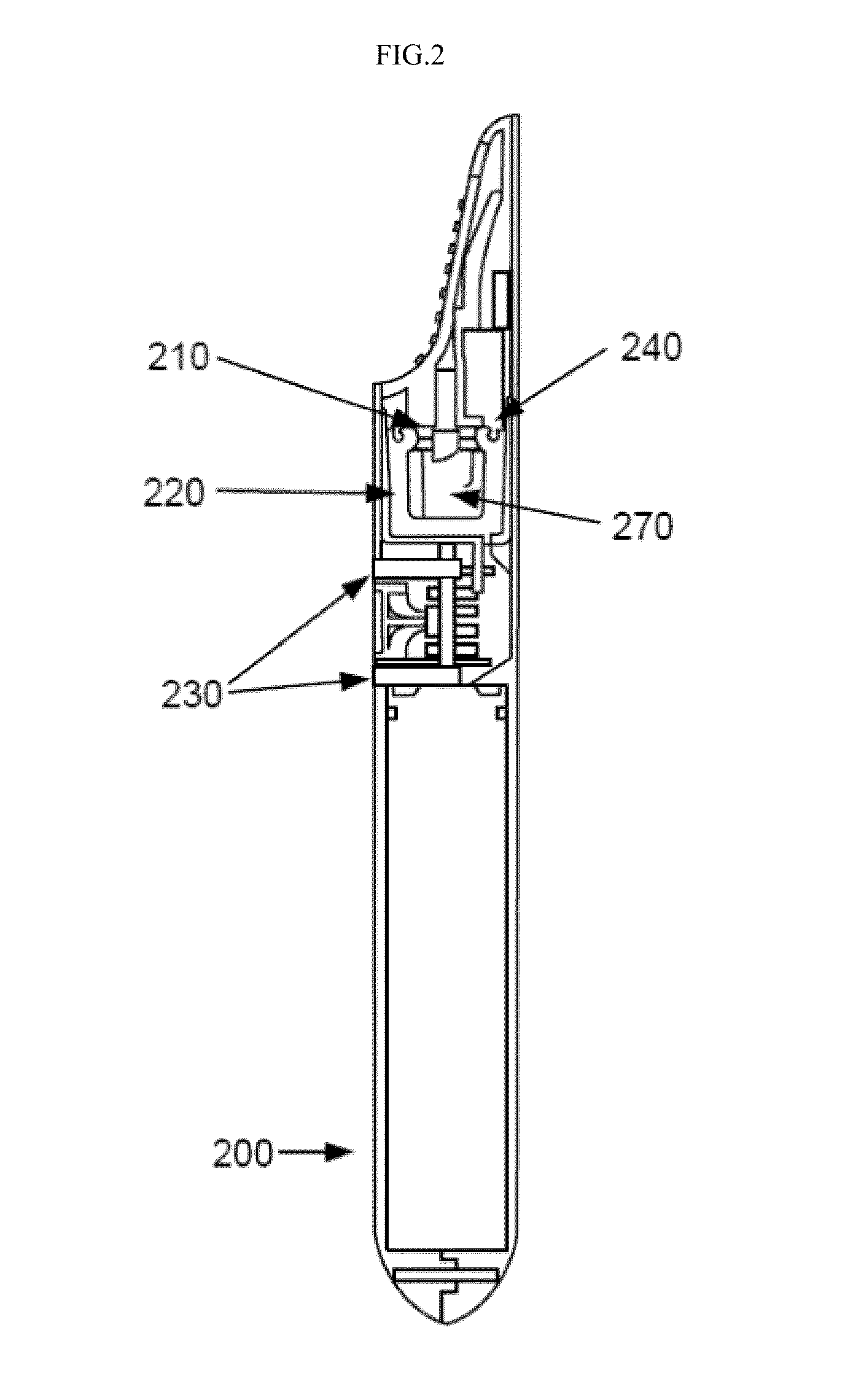
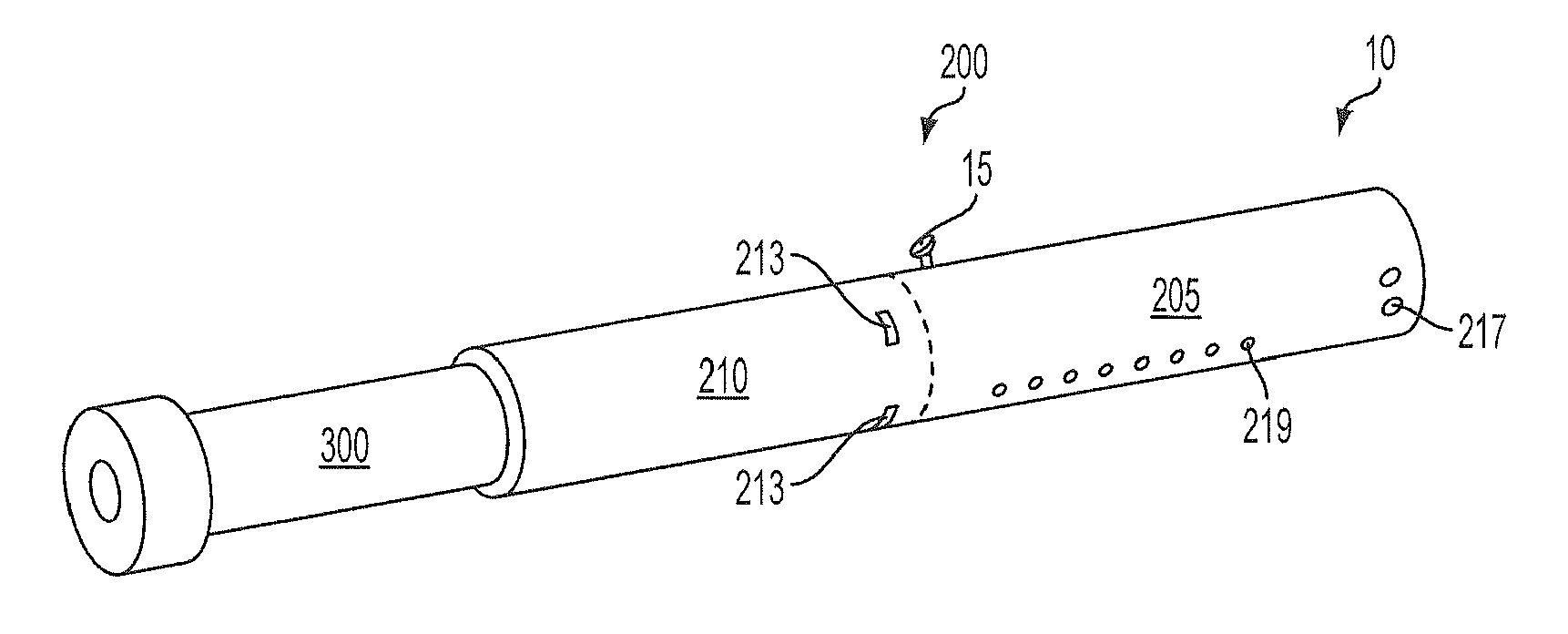
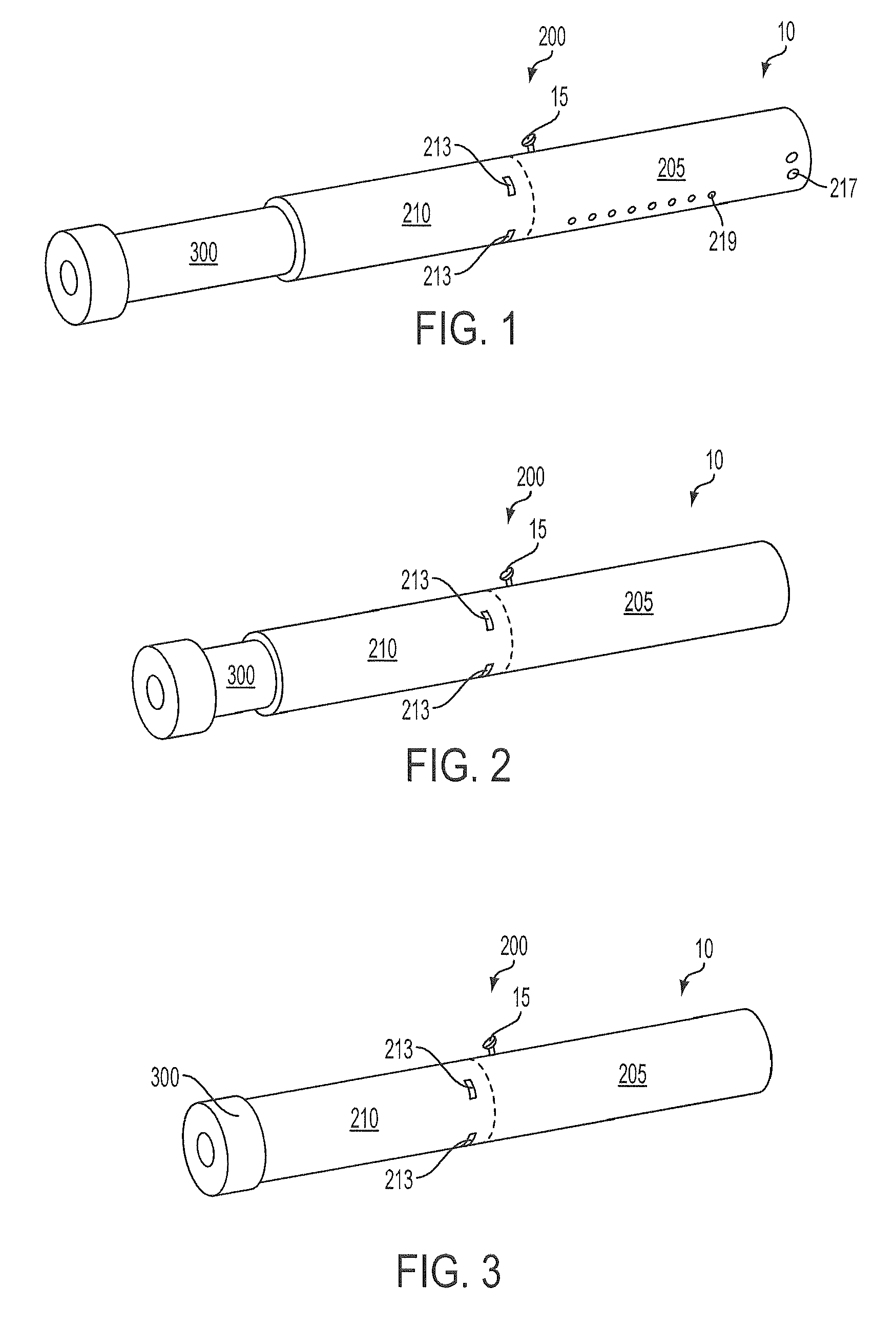
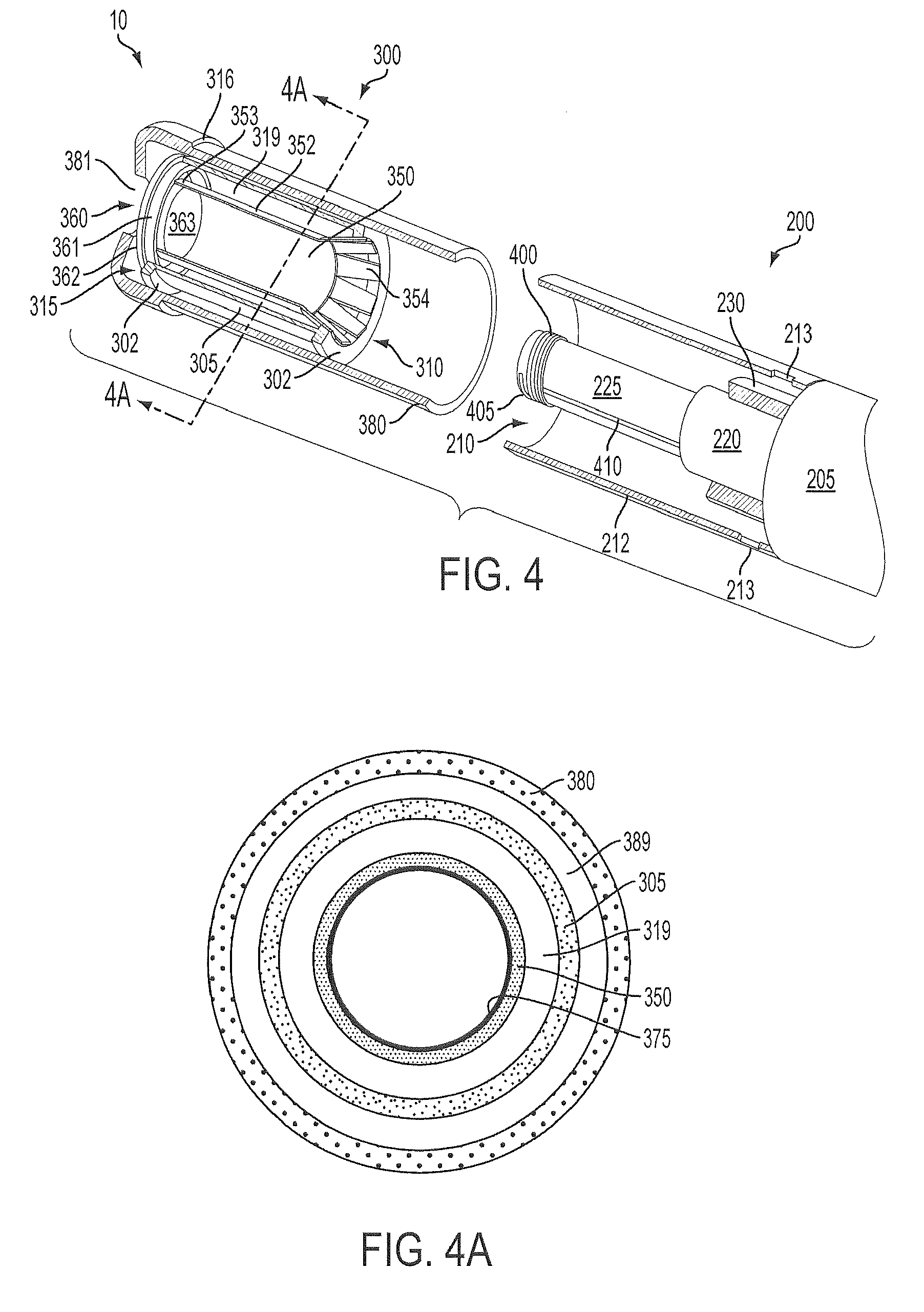
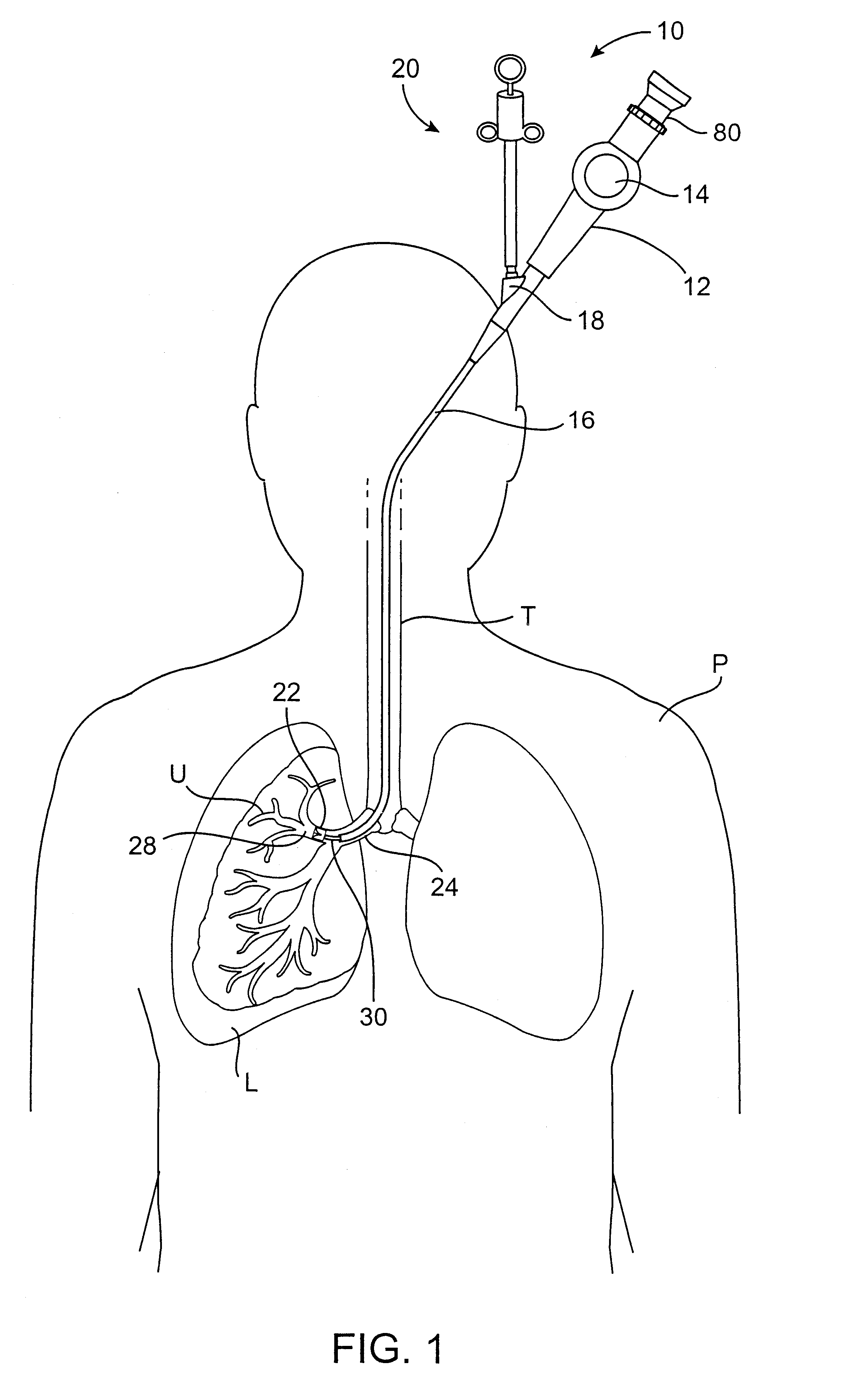
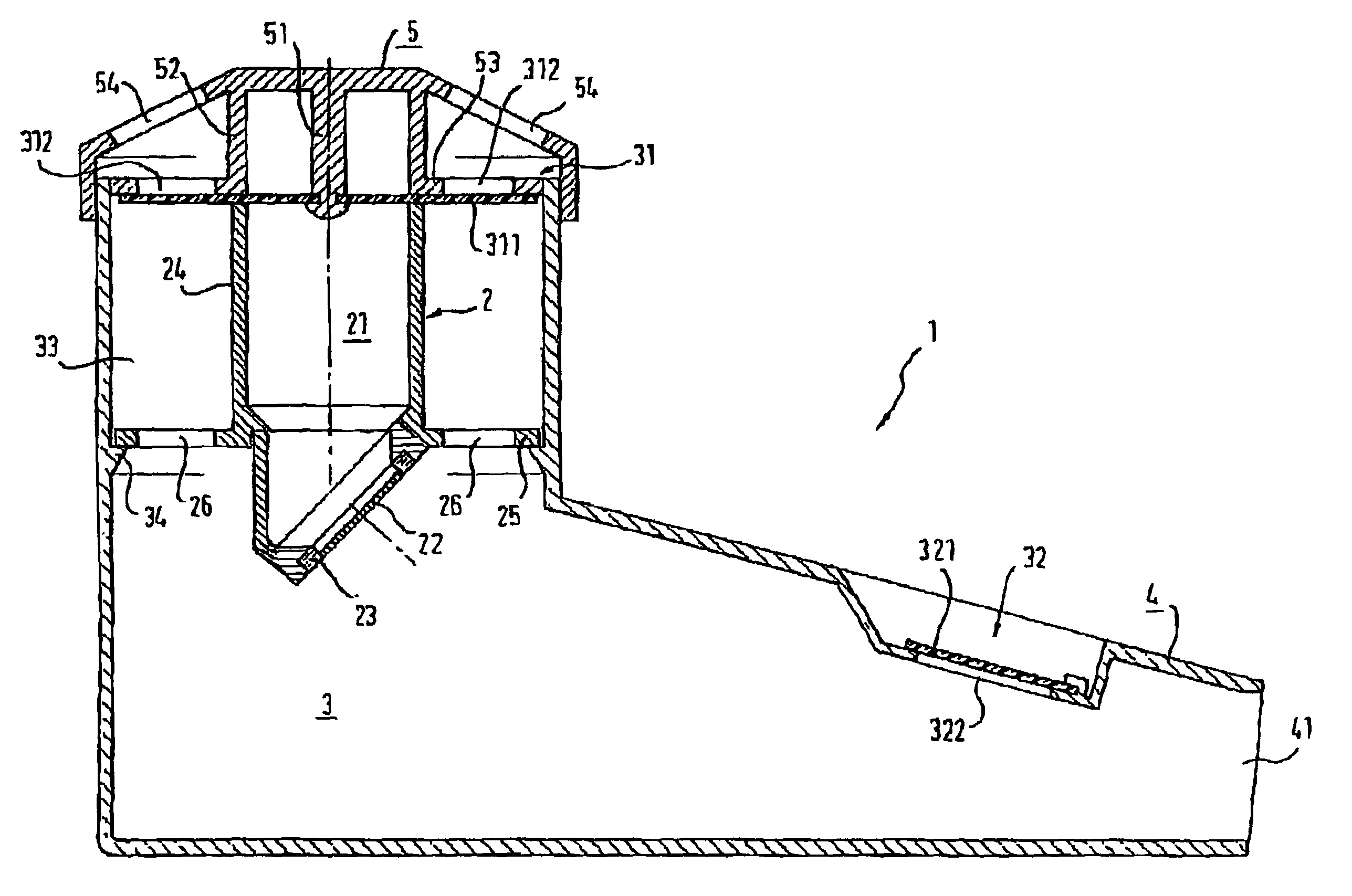
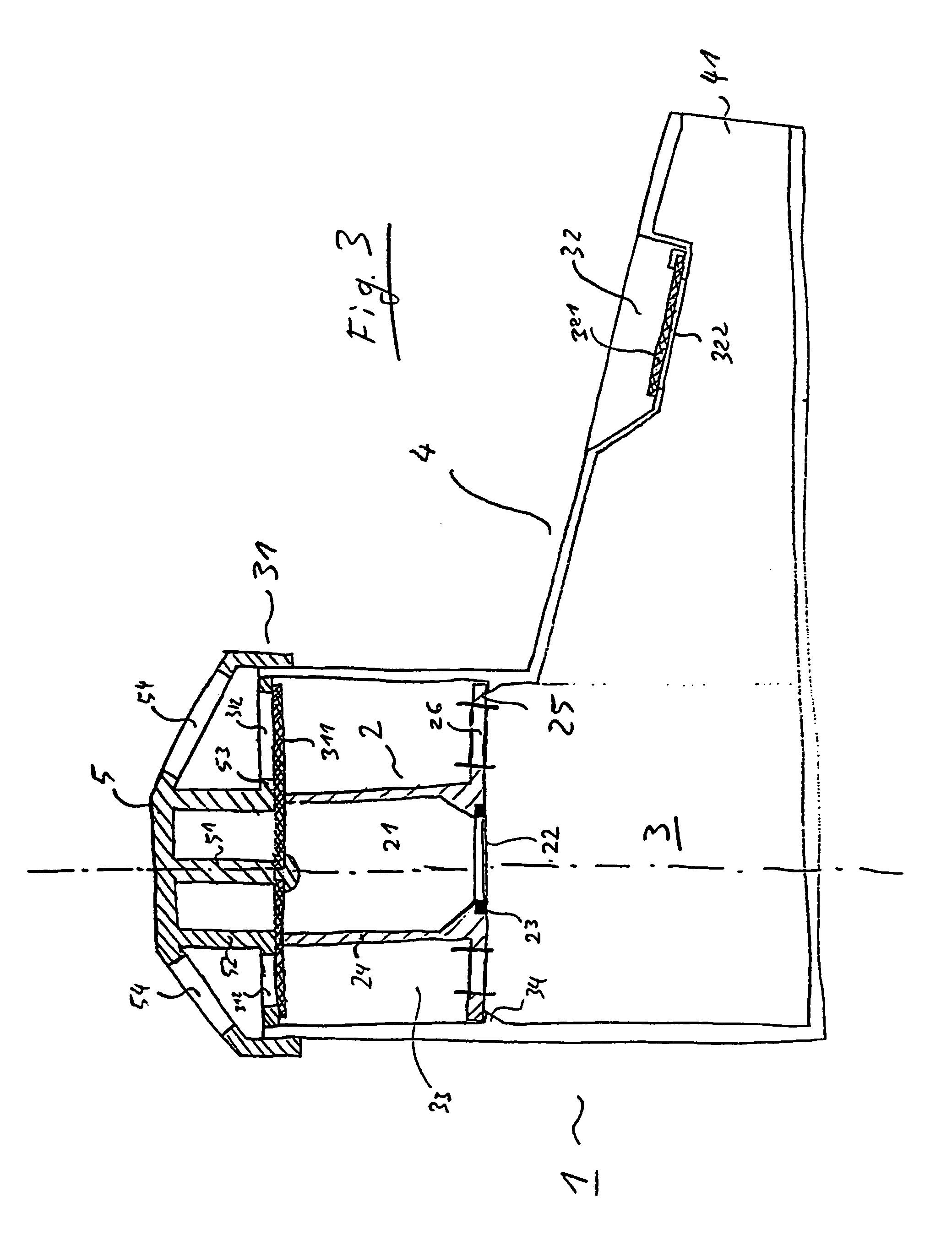
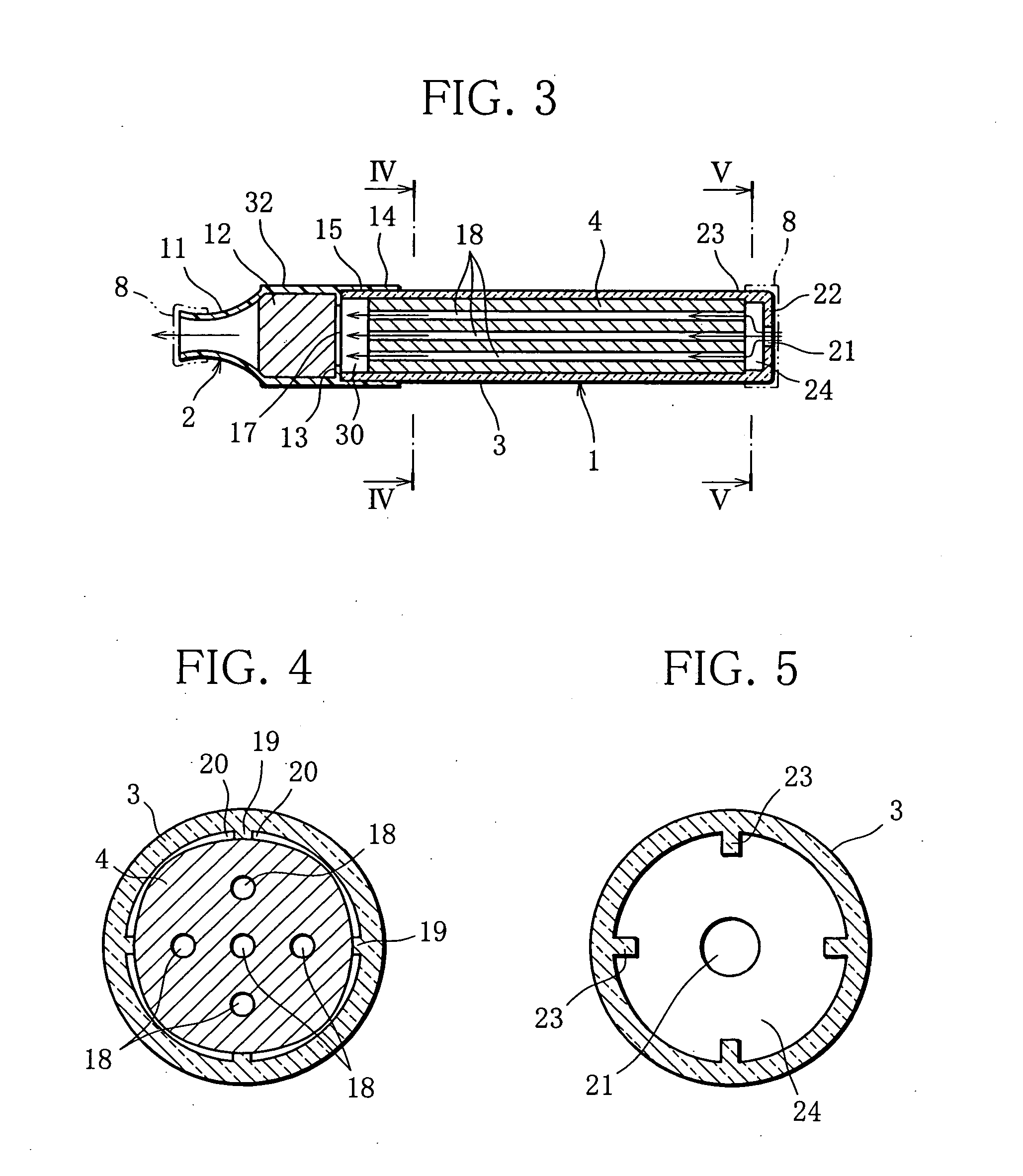
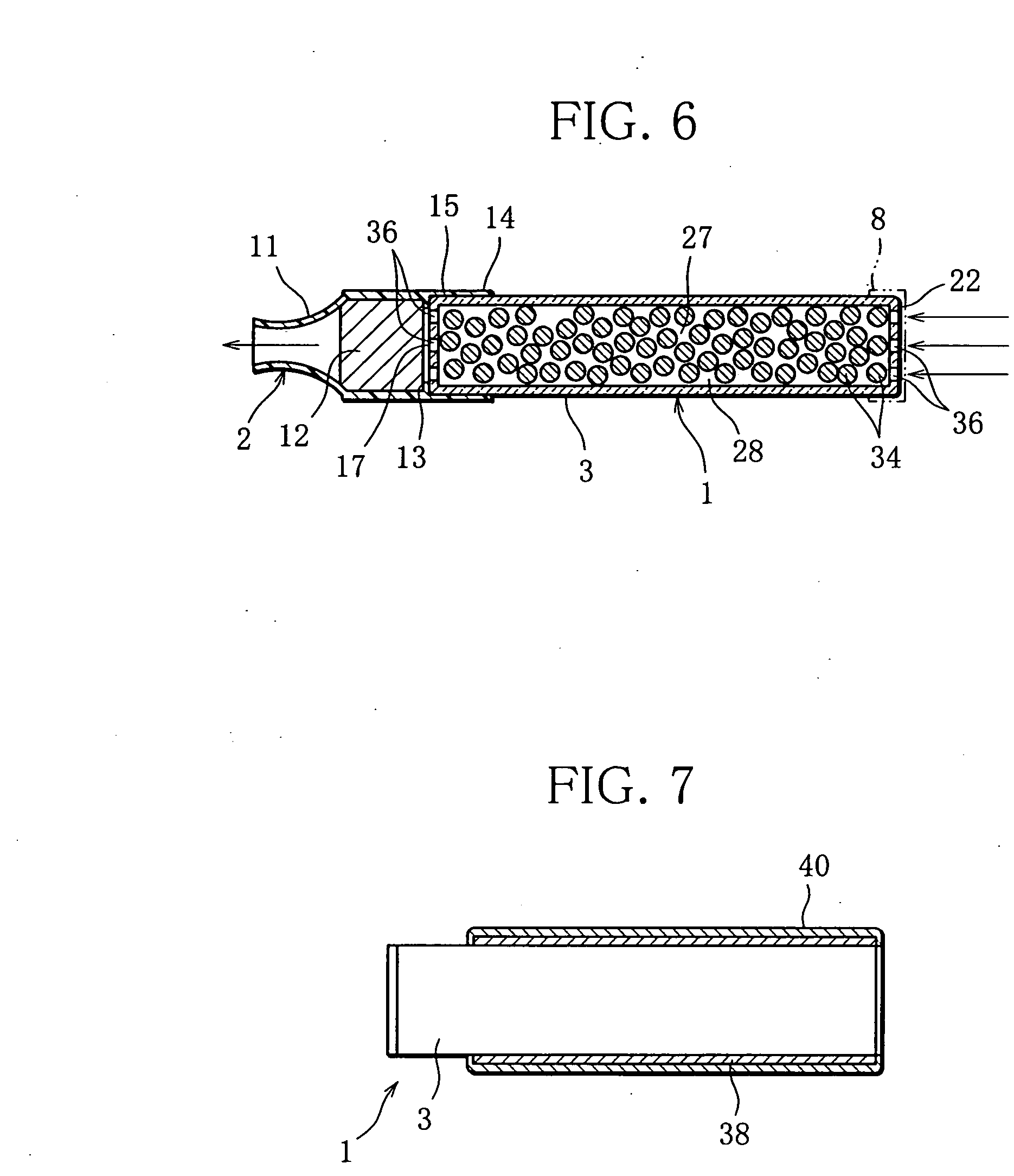
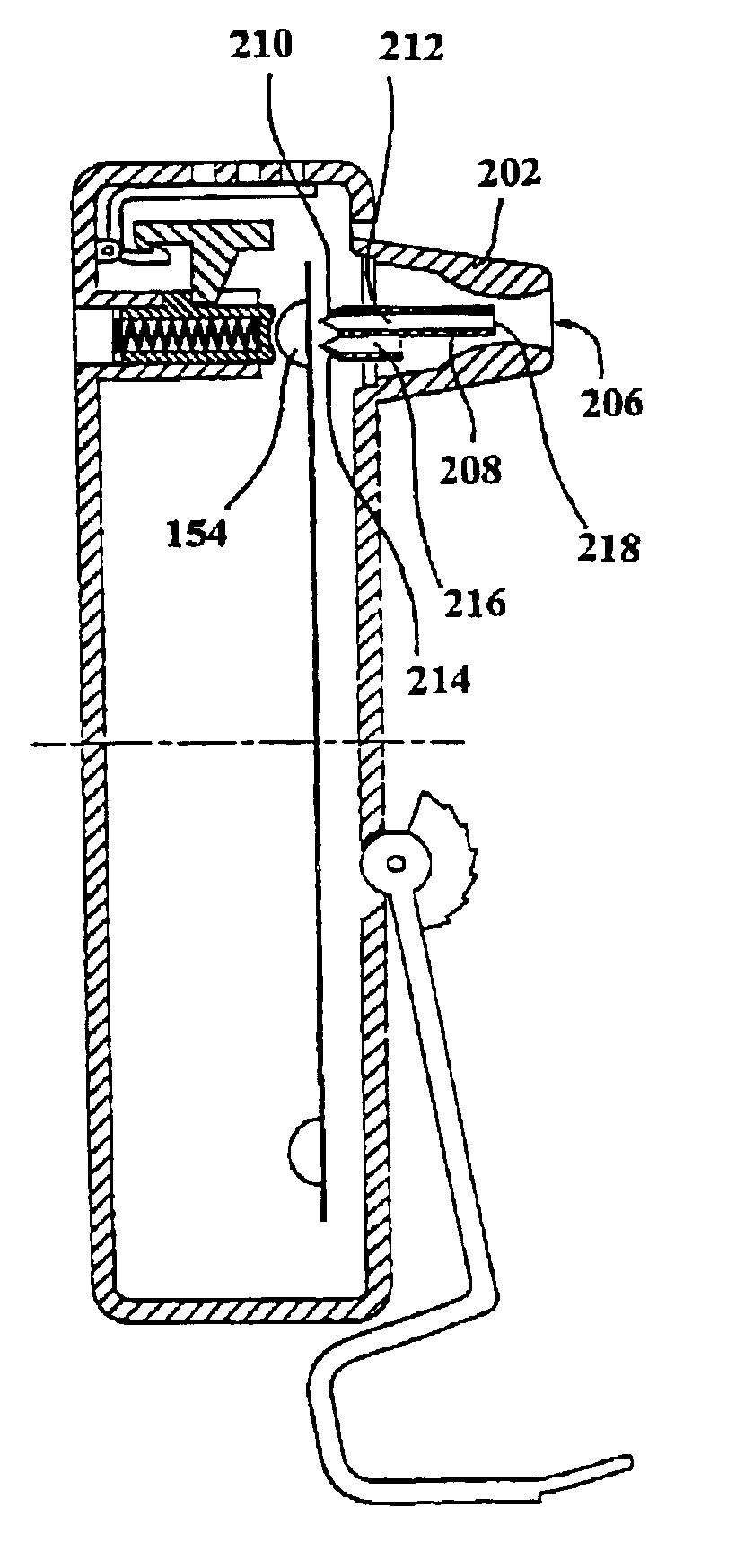
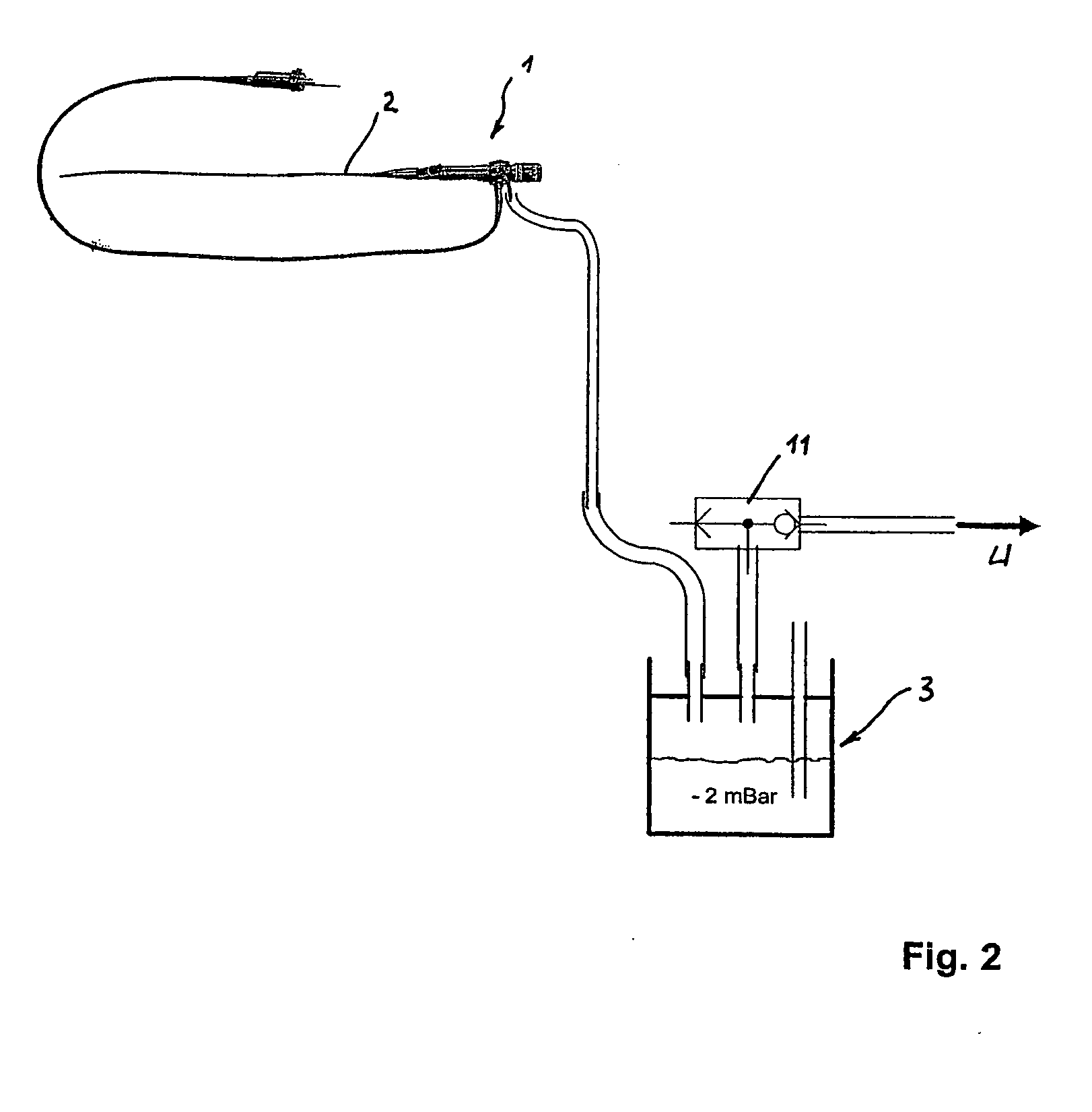
Inhalation device and heating unit therefor
The invention relates to a heating unit for an inhalation device for the inhalation administration of an inhalation mixture of air and at least one additive material, having a fuel storage (252) which is filled or can be filled with a thermally combustible solid or liquid fuel (258), and with a combustion chamber (256) for the combustion of the fuel (258), which is essentially sealed from the surroundings by a combustion chamber wall (222). The invention further relates to an inhalation device (210) with such a heating unit.According to the invention, the combustion chamber (256) is designed for forming a flame, and the combustion chamber wall (222) has at least some micro openings (224). The micro openings are designed in such a way that the sum of the outer side lengths of all micro openings (224) is at least 140 mm, and the sum of the outer side lengths of the micro openings (224) per surface in the area of the combustion chamber wall (222) averages at least 80 mm / cm2.Application as cigarette substitute or as aid for nicotine withdrawal.
Owner:SILLER FRIEDRICH
Inhaler for multiple dosed administration of a pharmacological dry powder
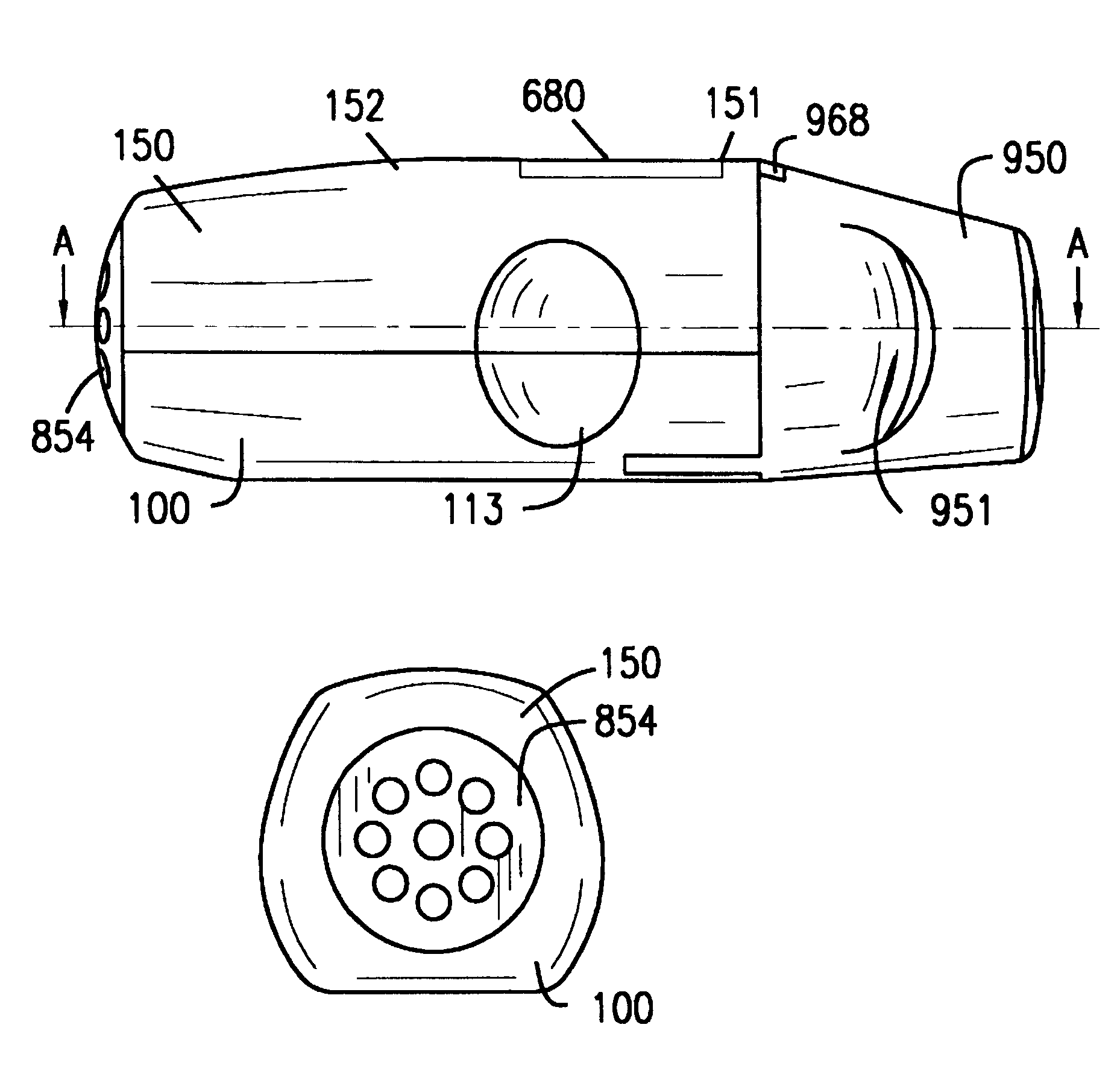
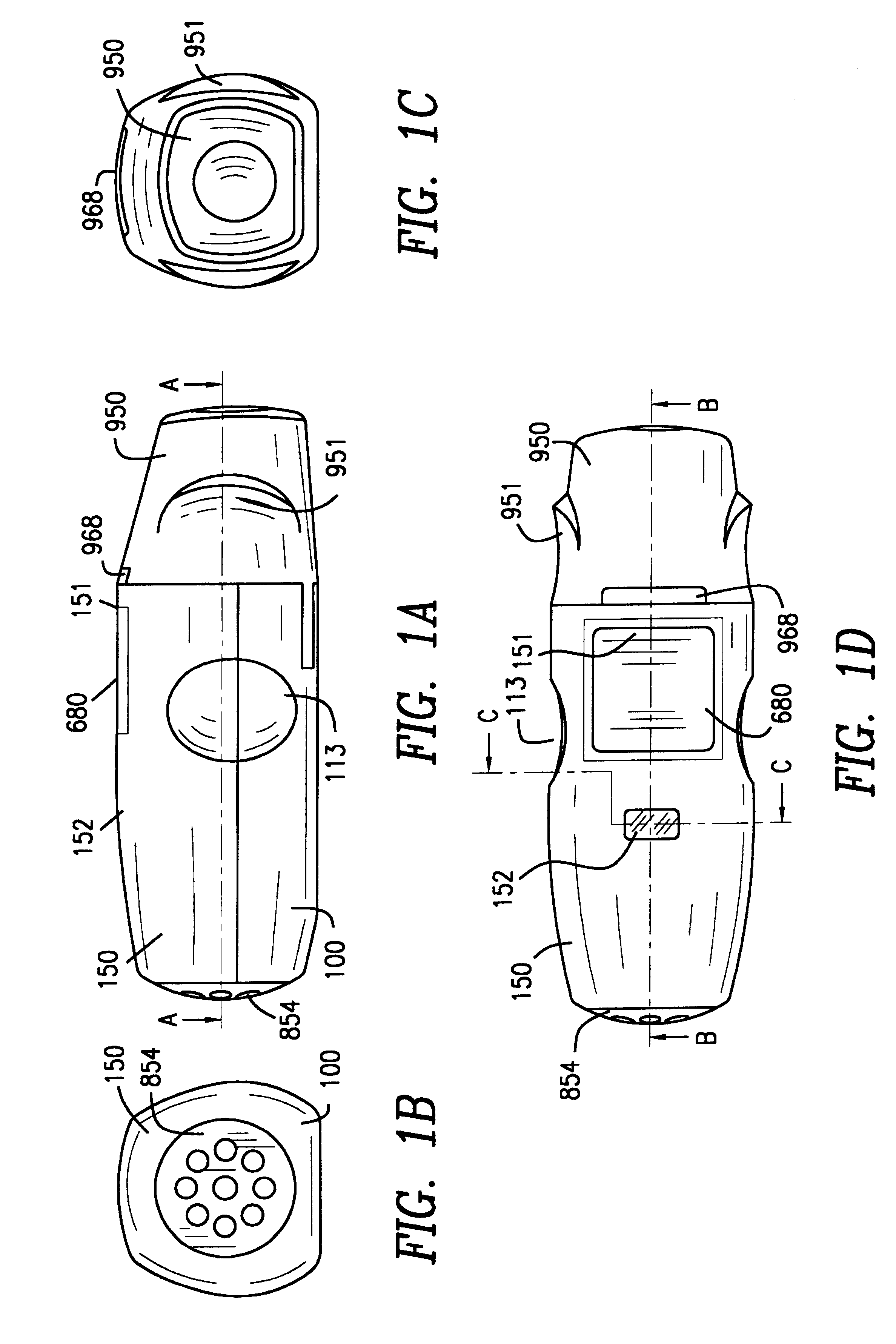
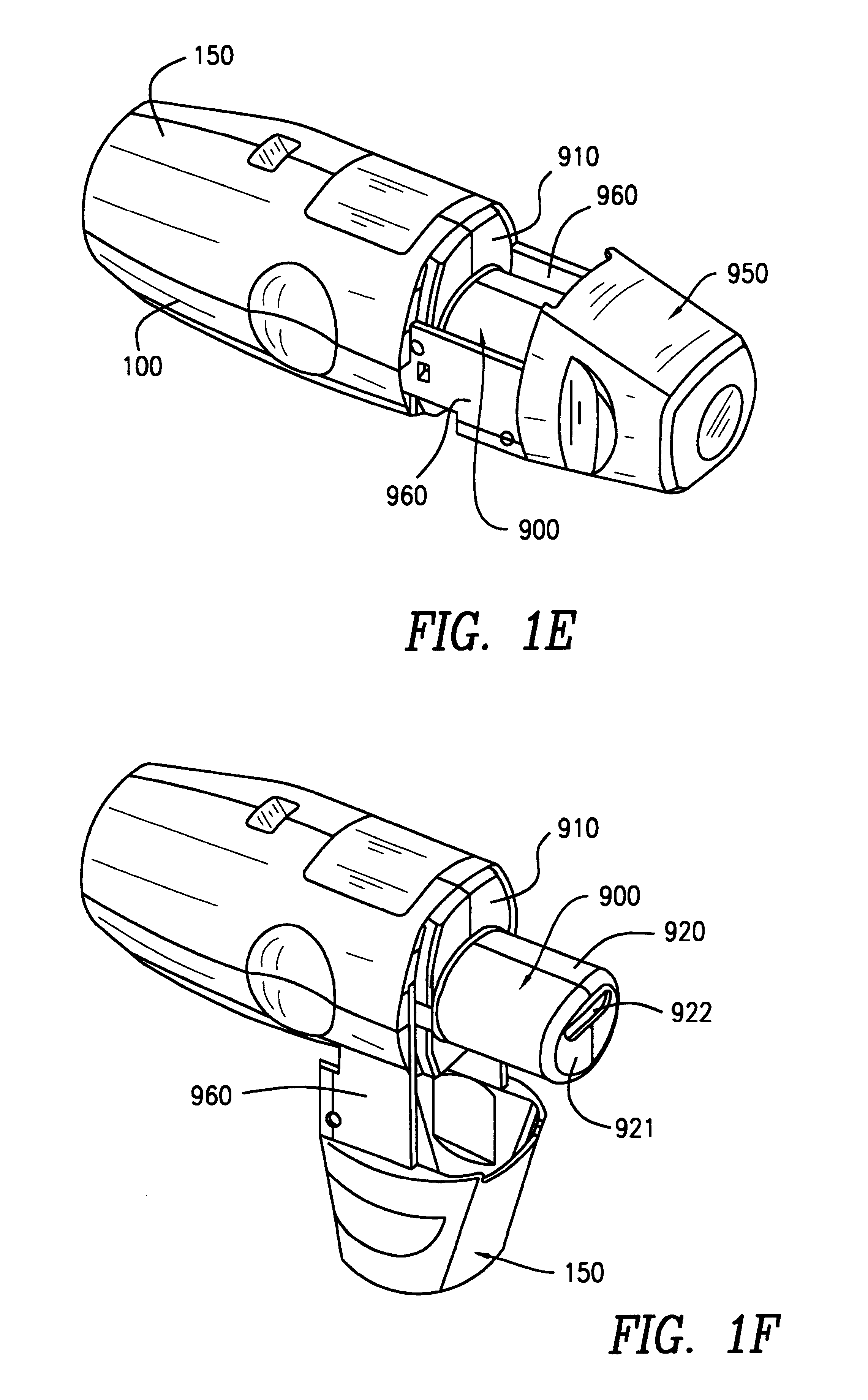
InactiveUS6182655B1Avoid possibilityReducing powder flow rateRespiratorsLiquid surface applicatorsMouth pieceEngineering
An inhaler for multiple dosed administration of a pharmacological dry powder consists externally of a housing (100,150) and of a protective cap (950) which can be removed from a special mouthpiece (900) fitted on the housing. Arranged on the inside there are a slide rail (200), a dosing slide (300), a shutter (400), a carriage (500), a funnel arrangement (600), a counter device (700), a valve shield (800) and a valve guide (850). Removal of the protective cap (950) initiates the dosing, with a dose received in the dosing cavity (302) being transported to the mouth-piece (900) by means of the dosing slide (300). Only upon application of a defined minimum intensity of inhalation is the shutter (400) moved by the suctioned valve shield (800), as a result of which the dose is released for inhalation. Completed with an electronic module and a controllable nozzle, all inhalation-relevant data can be recorded and the flow conditions regulated.
Owner:JAGOTEC AG
Smoking articles and use thereof for yielding inhalation materials
The present invention describes articles, such as smoking articles, that can provide an inhalable substance in a form suitable for inhalation by a consumer. The article comprises a cartridge with an inhalable substance medium therein, control housing that includes an electrical energy source and an electrical power source, and a heating member that may be located in either the cartridge or the control housing. The control housing further may include puff-actuated current actuation components and current regulation components.
Owner:RAI STRATEGIC HLDG INC
Methods and devices for use in performing pulmonary procedures
Systems, methods and devices for performing pulmonary procedures, and in particular treating lung disease. A flow control element includes a valve that prevents airflow in the inhalation direction but permits airflow in the exhalation direction. The flow control element is guided to and positioned at the site by a bronchoscope that is introduced into the patient's trachea and used to view the lungs during delivery of the flow control element. The valve may include one, two or more valve elements, and it may be collapsible for easier delivery. A source of vacuum or suction may be used to increase the amount of fluid withdrawn from the lung tissue. A device for measuring hollow structures, such as bronchioles, and a device for removing a previously-placed flow control element are disclosed as well.
Owner:FOUNDRY LLC THE
Methods and compositions for pulmonary administration of a TNFa inhibitor
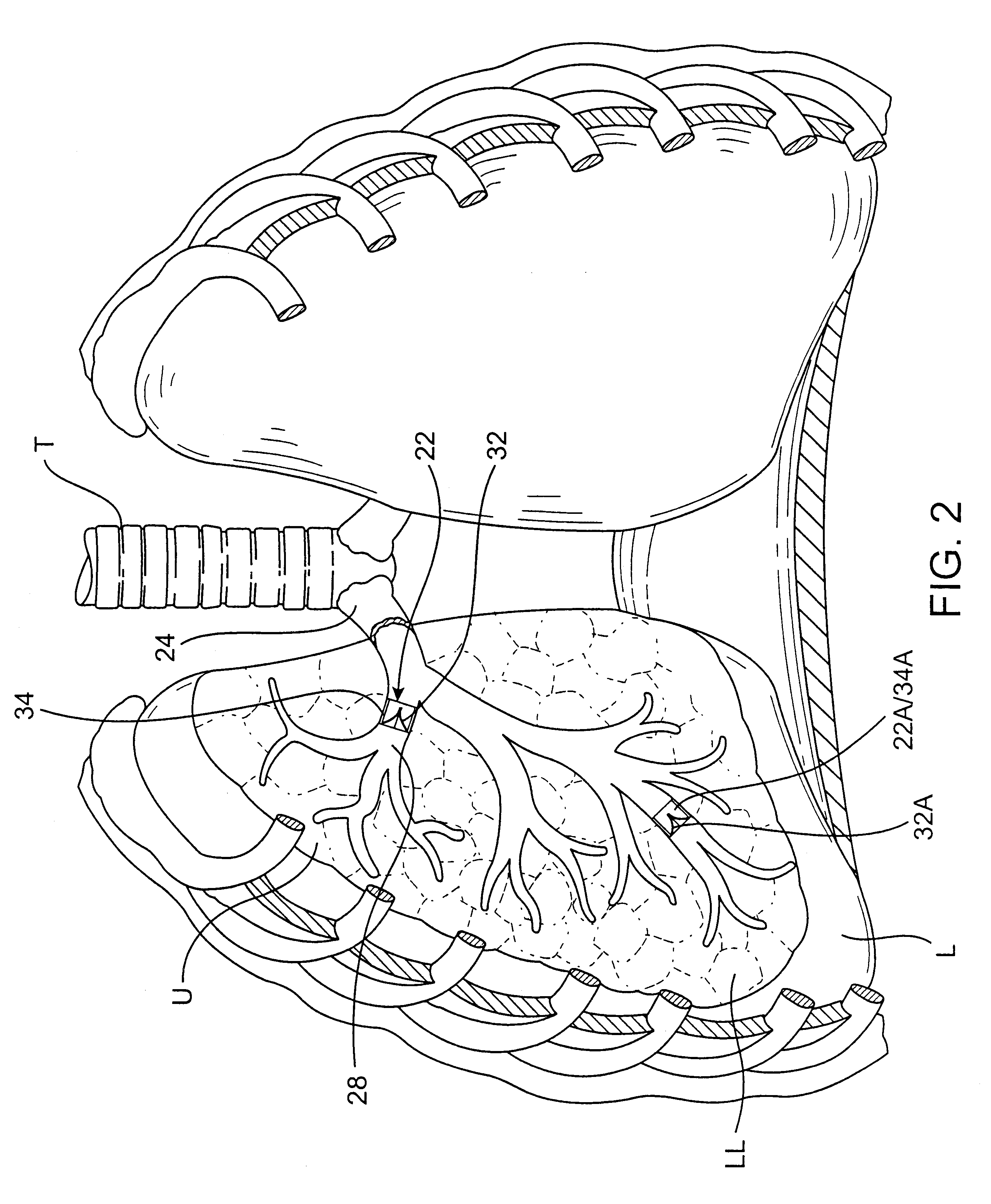
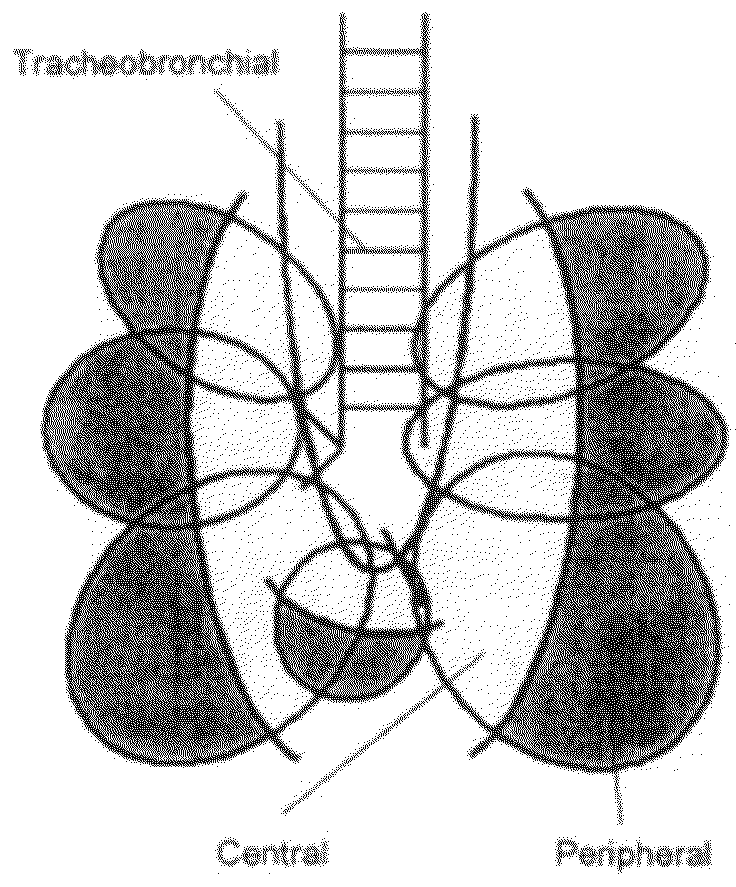
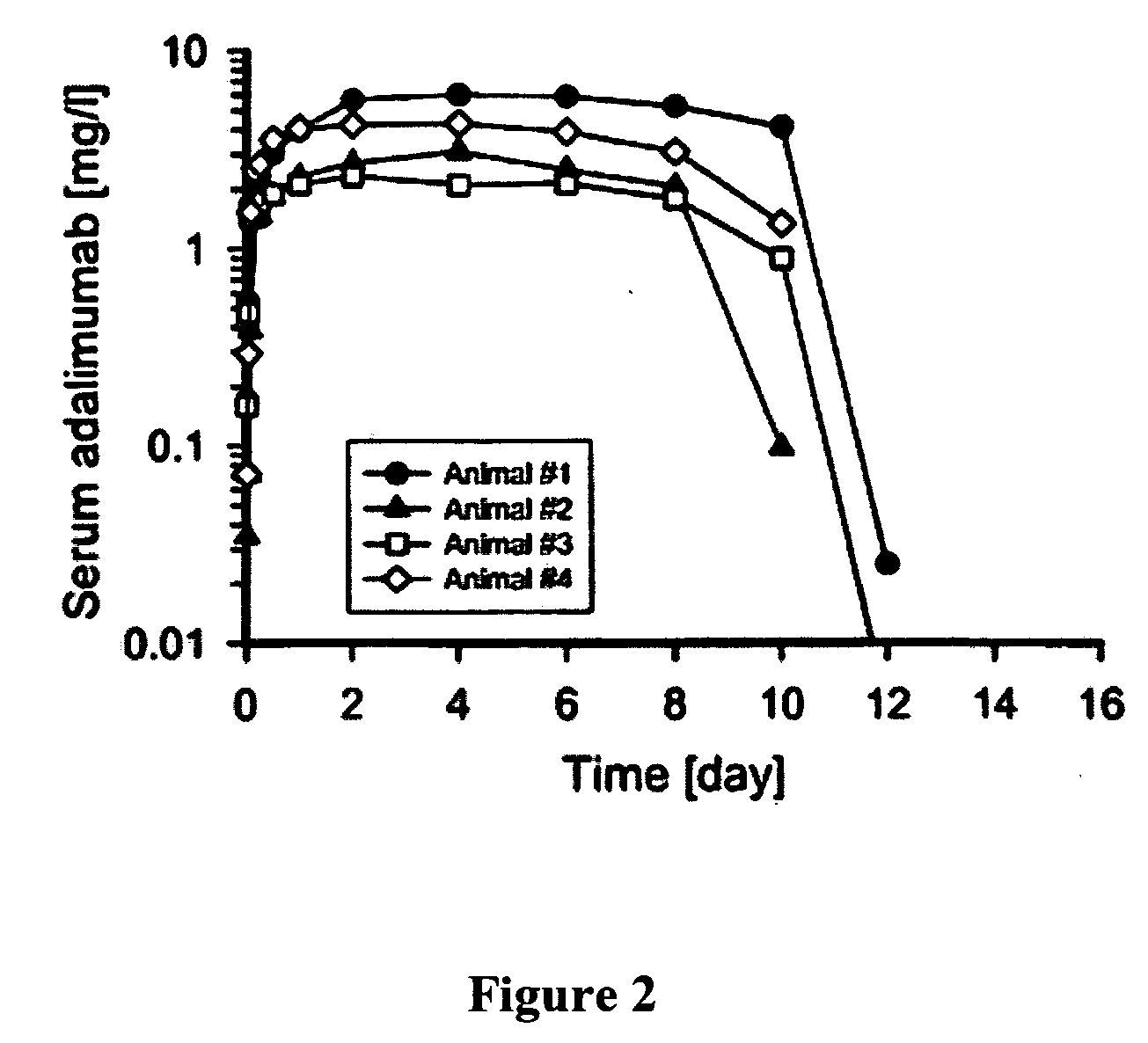
The invention describes methods of pulmonary delivery of a TNFα inhibitor to a subject having a disorder in which TNFα is detrimental, such that the disorder is treated. Also included is a method of achieving systemic circulation of a TNFα inhibitor in a subject comprising administering the TNFα inhibitor to the central lung region or the peripheral lung region of the subject via inhalation, such that systemic circulation of the TNFα inhibitor is achieved.
Owner:ABBVIE BIOTECHNOLOGY LTD
Inhalation nebulizer
InactiveUS6962151B1Prevent escapePrevent inflowMovable spraying apparatusSpray nozzlesNebulizerInhalation
An inhalation nebulizer (1) includes an aerosol generator (2) that has a diaphragm (22) vibrated by a vibration generator (23). The inhalation nebulizer (1) includes a liquid storage container (21) that is in fluid contact with the diaphragm (22). A liquid contained in the storage container (21) is atomized into a mixing chamber (3) through openings in the diaphragm and can subsequently be inhaled by a patient.
Owner:PARI PHARMA GMBH
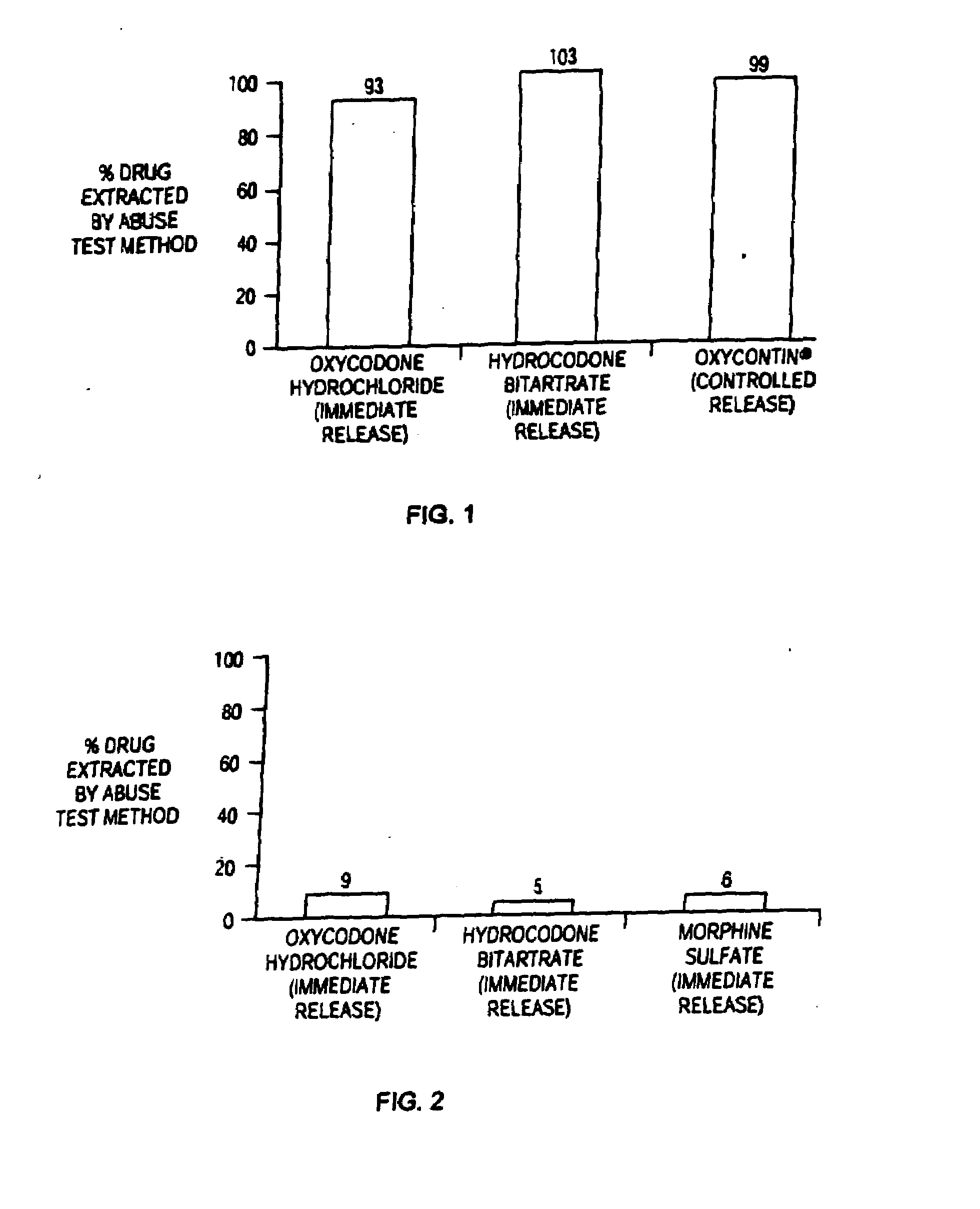
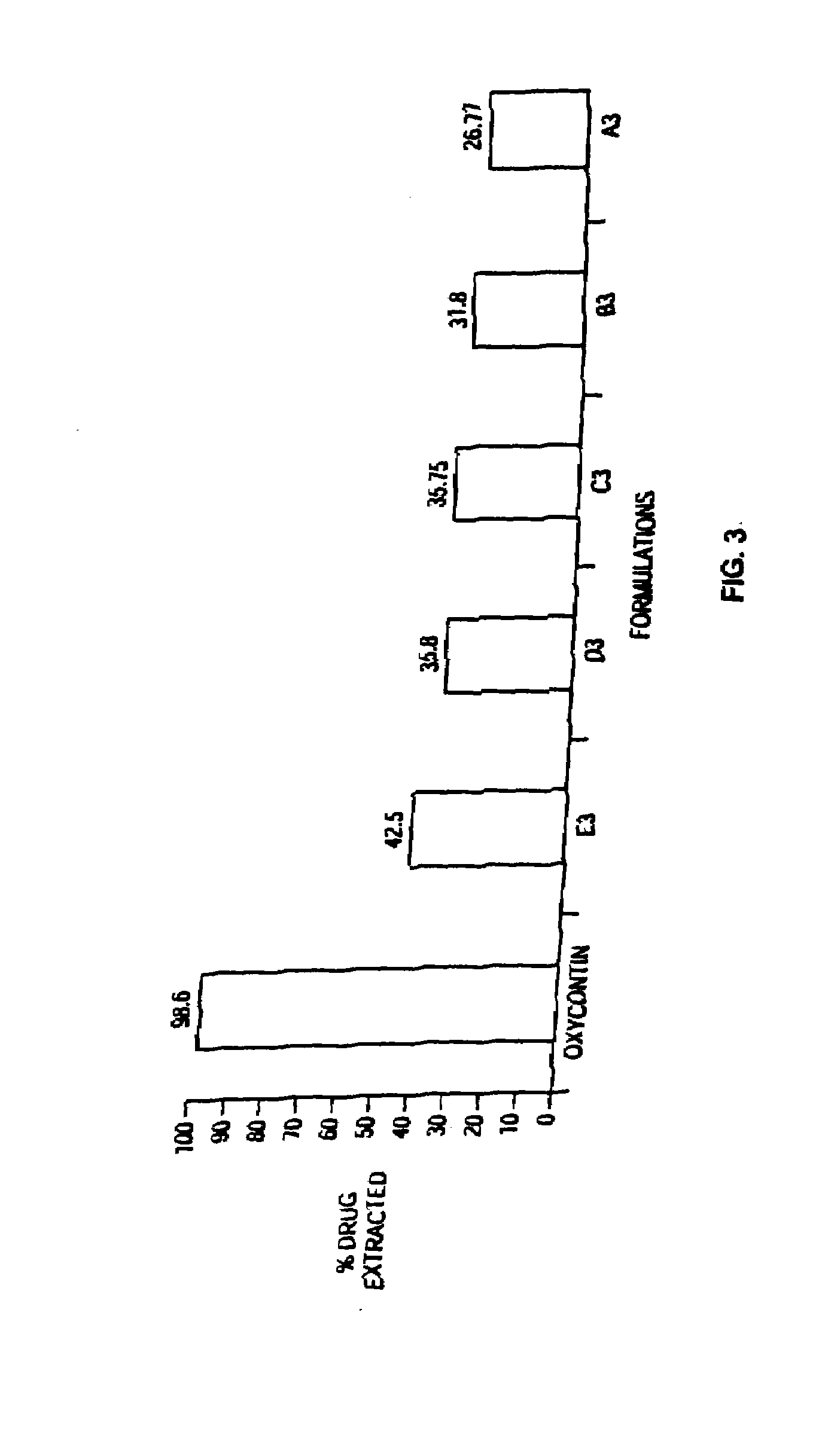
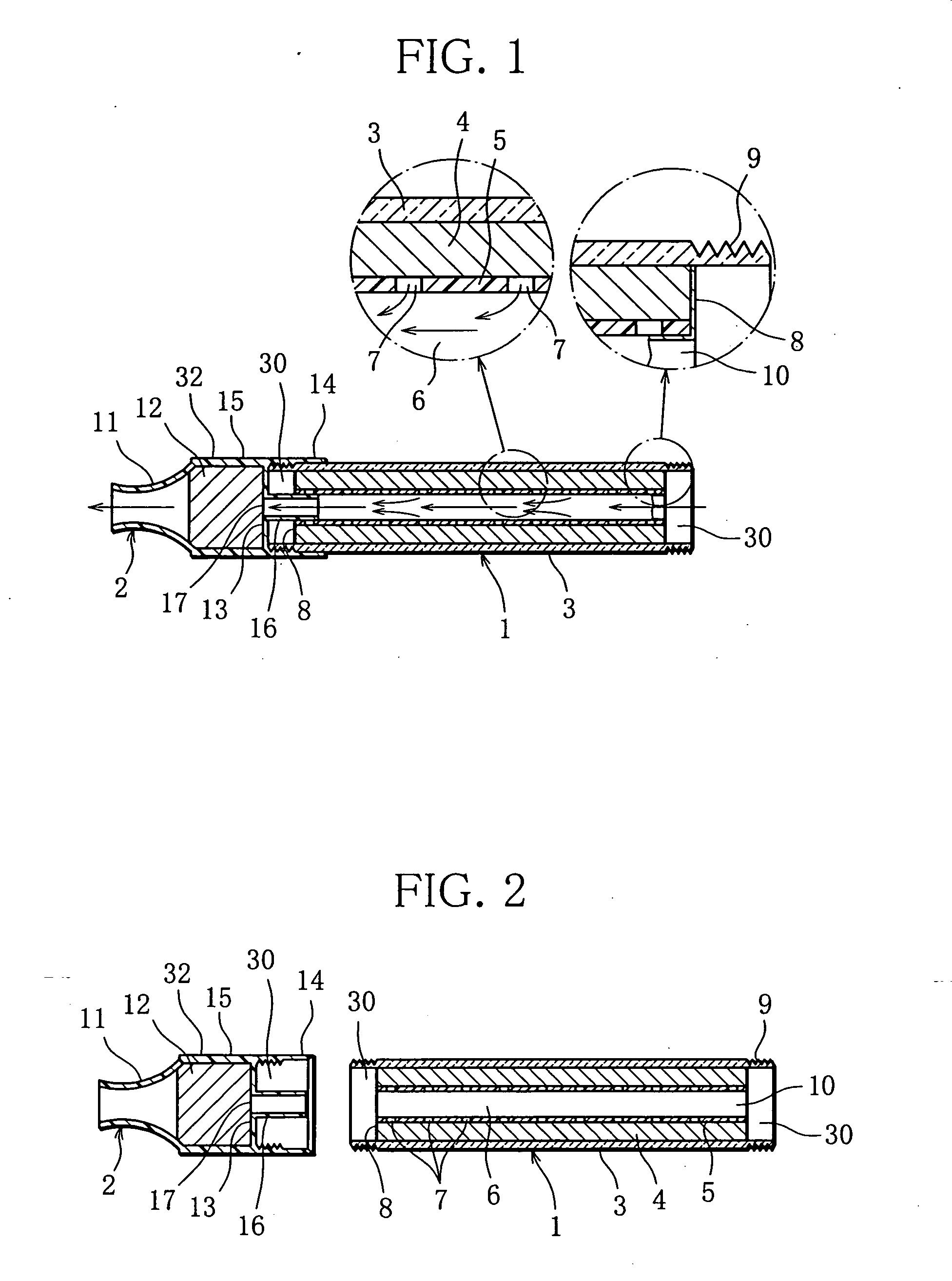
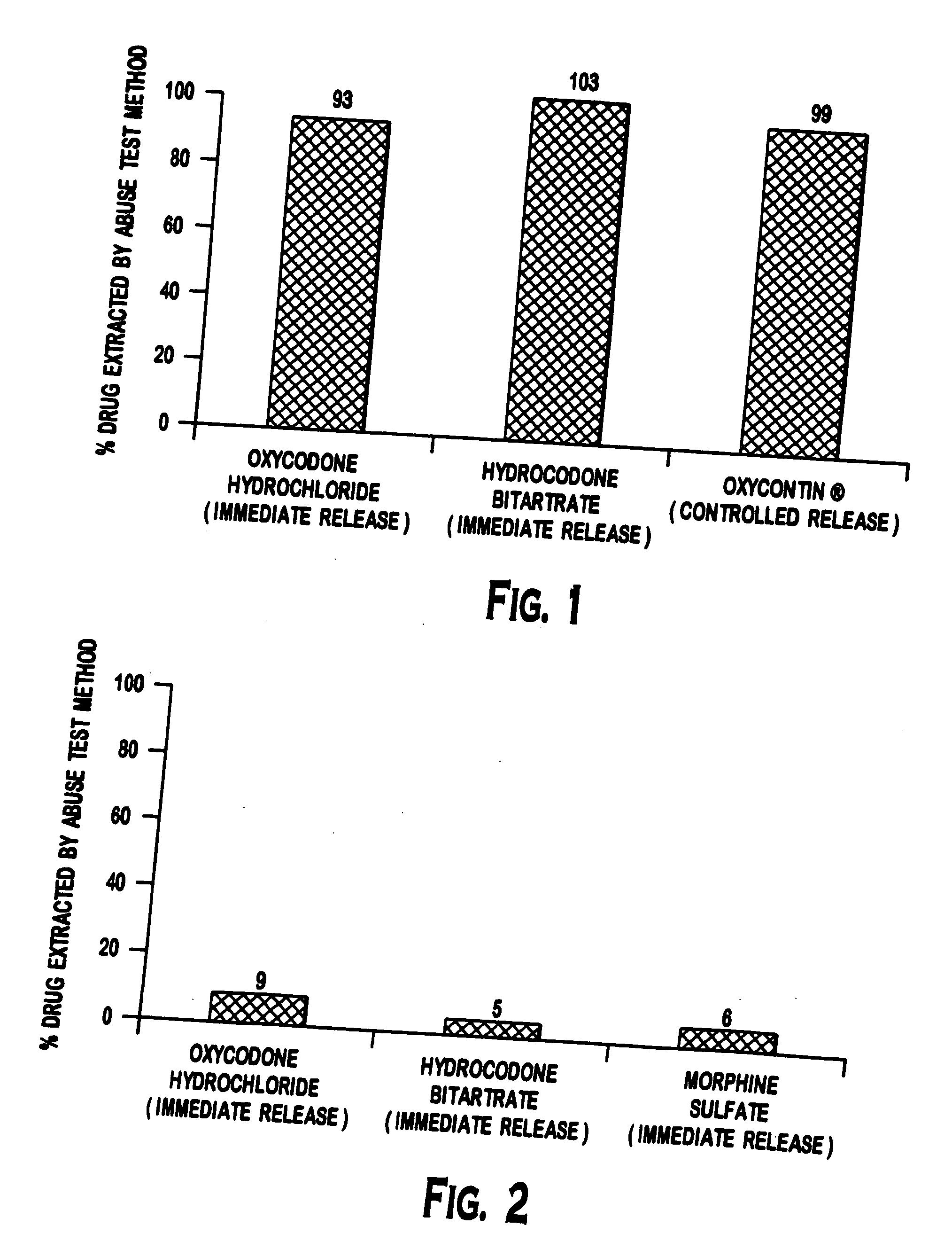
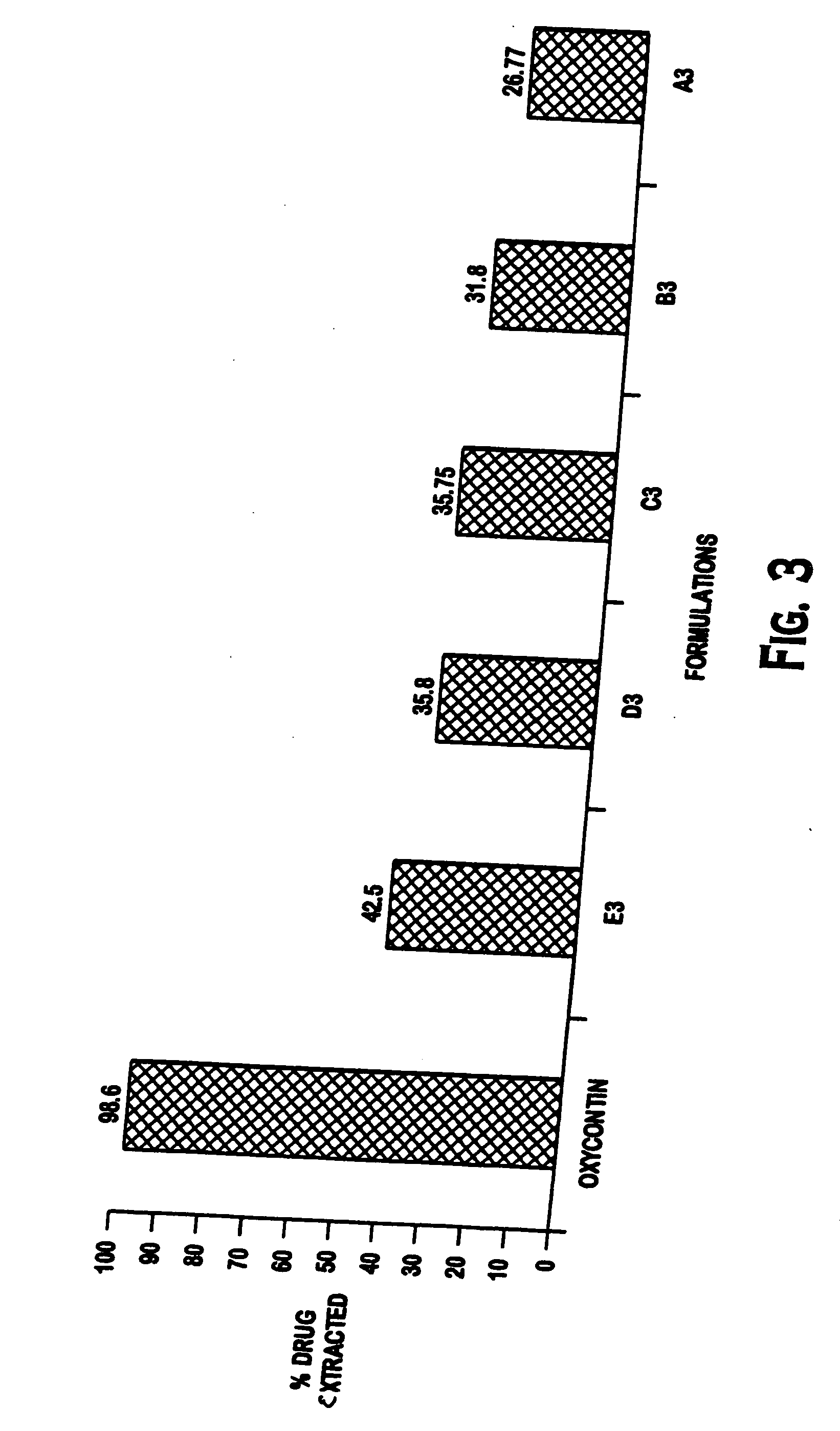
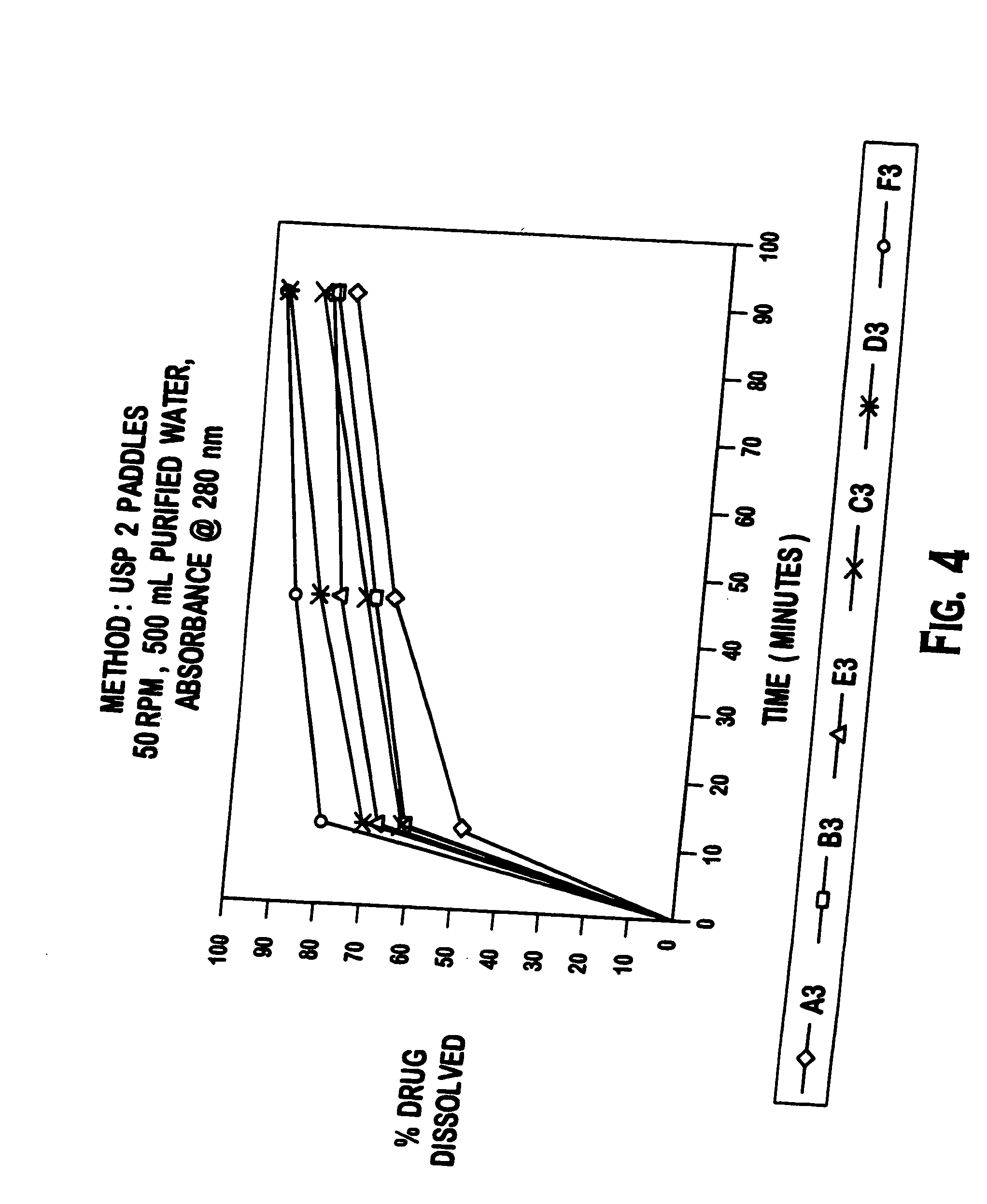
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Nicotine suction pipe and nicotine holder
InactiveUS20060191546A1Prevent natural vaporizationSmall inhalation resistanceTobacco devicesInhalationSilica gel
A nicotine inhalation pipe includes a rodlike nicotine holder (1) and a mouthpiece (2) attached to one end of the holder (1). The holder (1) includes a transparent outer tube (3) having a plurality of openings (36) at both ends thereof, liquid absorbent granules (34) filled in the outer tube (3), and a nicotine inhalation path formed by gaps between the liquid absorbent granules (34) and gaps between the inner peripheral surface of the outer tube (3) and the liquid absorbent granules (34). The liquid absorbent granules (34), which are made of porous silica gel and in which a nicotine solution is absorbed, permit nicotine to be vaporized from the nicotine solution.
Owner:SHUSEI TAKANO
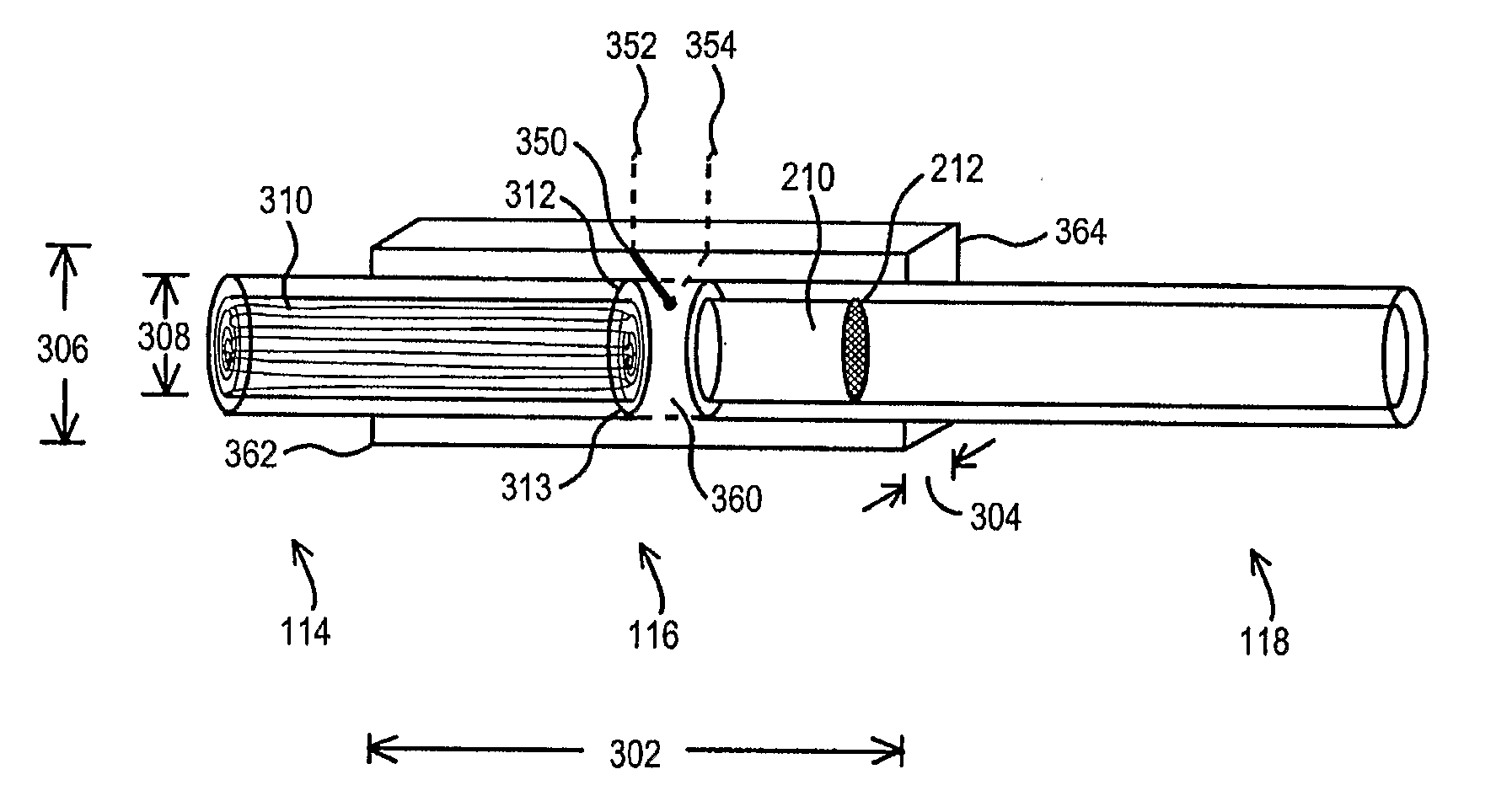
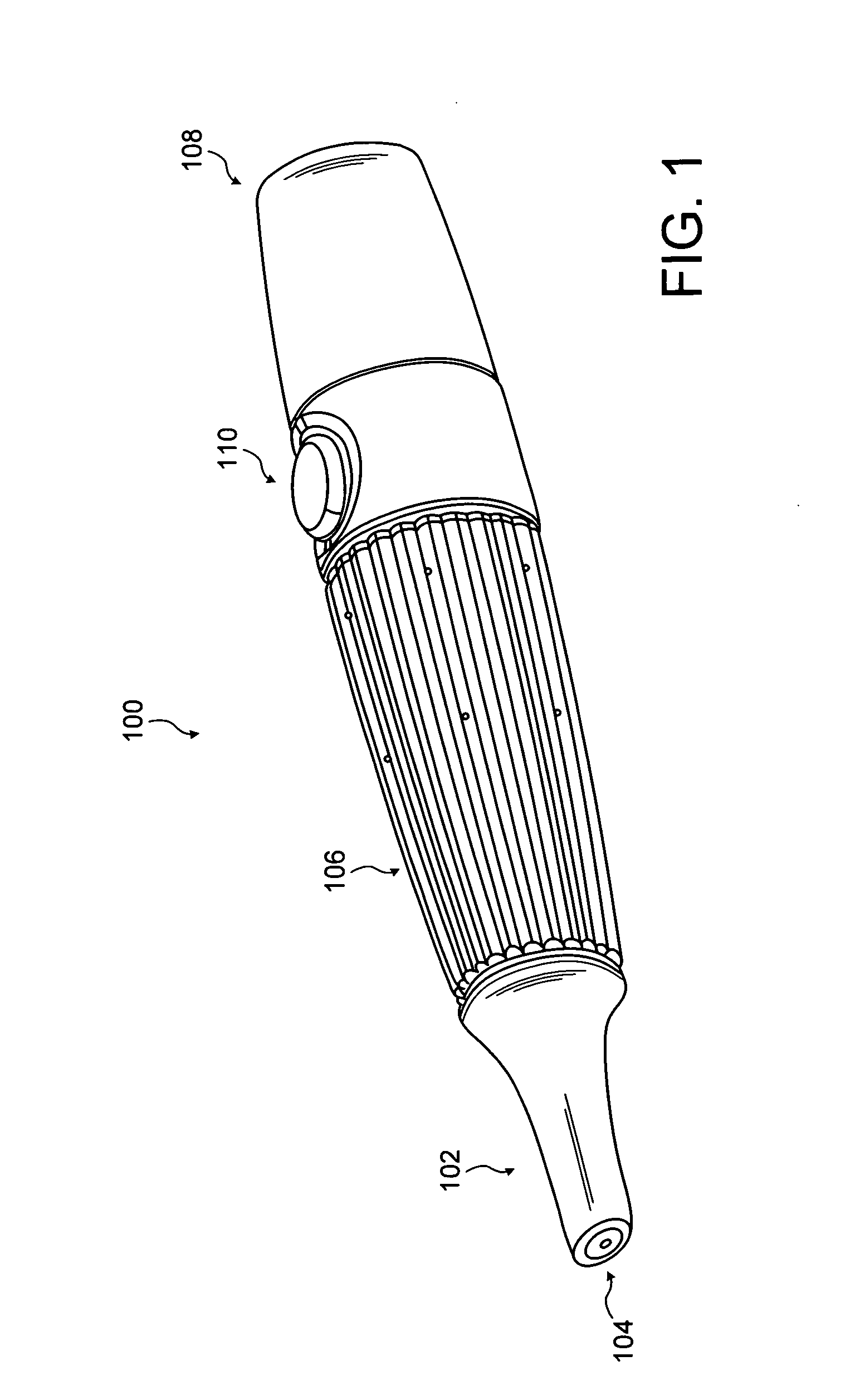
Portable vaporizing device and method for inhalation and/or aromatherapy without combustion
ActiveUS20110120482A1Even heat distributionAvoid problemsRespiratorsMedical devicesAromatherapyCombustion
A hand-held apparatus (100) to vaporize volatile compounds disposed in a solid source material is disclosed. The hand-held apparatus comprises a housing (105), a mouthpiece tube (118) removeably disposed within, and extending outwardly from, said housing, a heating element assembly (114) in communication with said mouthpiece tube, wherein said heating element assembly heats to an operating temperature of about 450° F. in about 60 seconds.
Owner:BRENNEISE JAKE
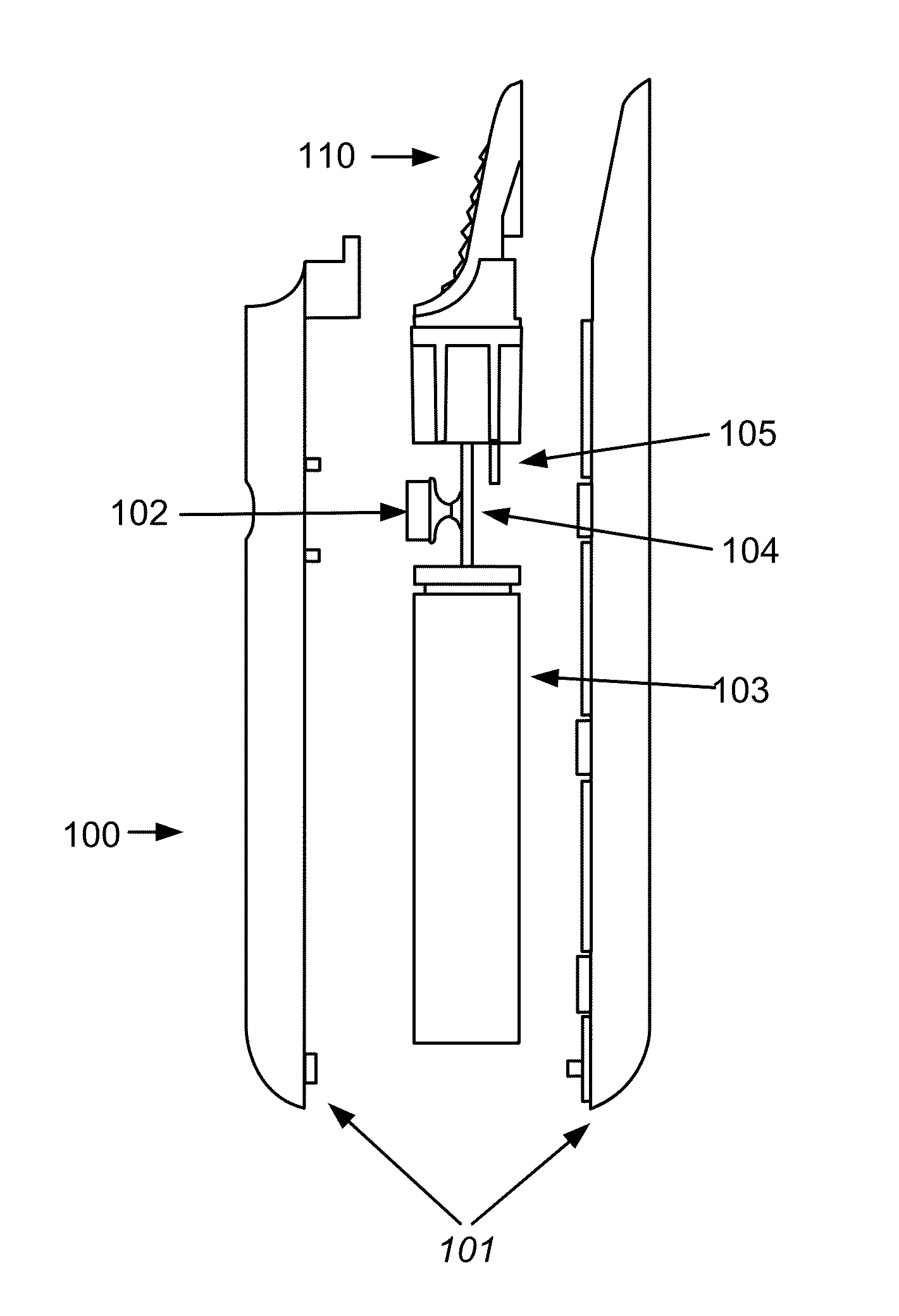
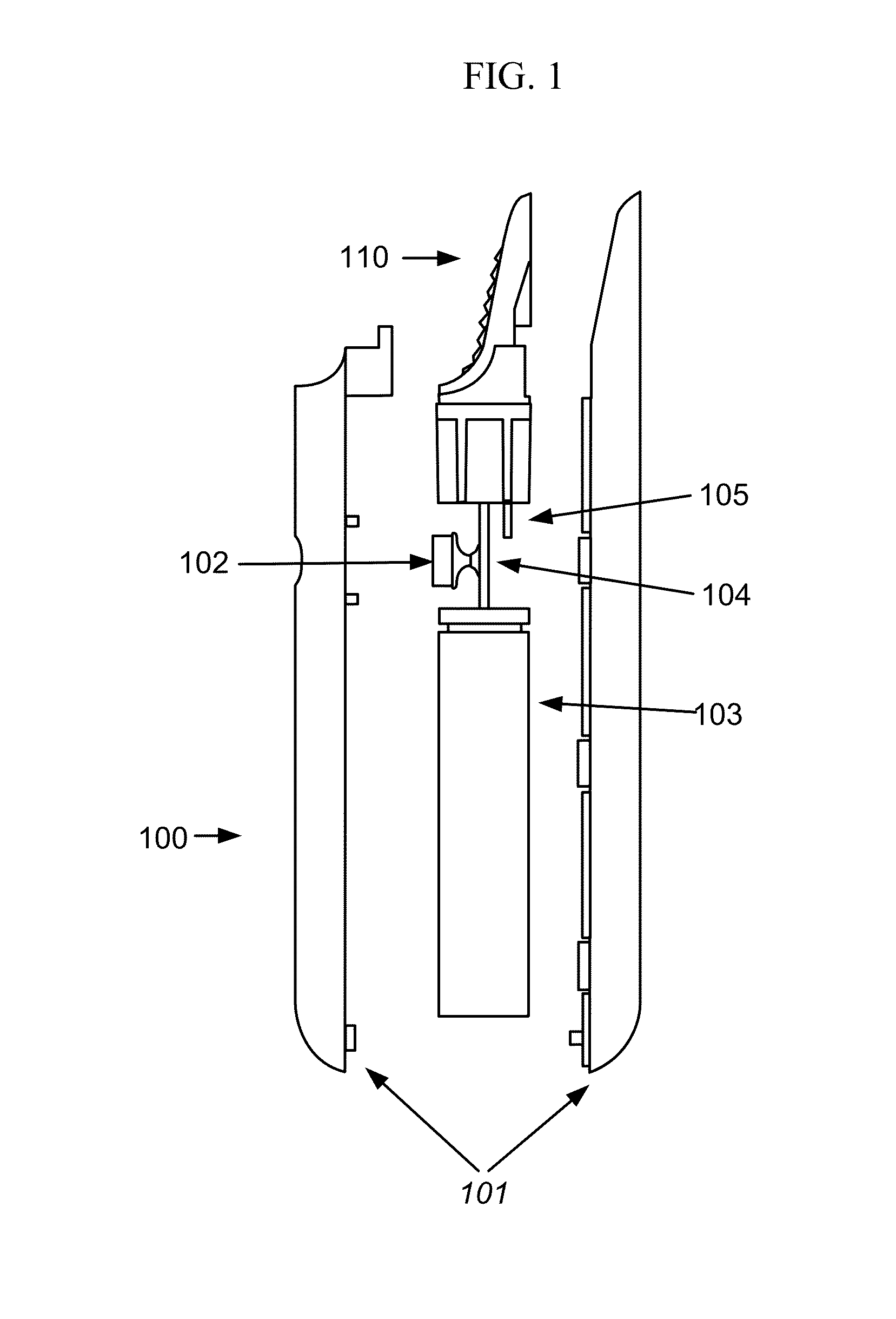
Inhaler
InactiveUS6880555B1Lifting efficiencyGood effectRespiratorsLiquid surface applicatorsInhalationMedicine
An inhaler for medicament in powder form with an opening intended for inhalation. The powder medicament is arranged in the inhaler in a number of enclosures, each enclosure including a specific dose of medicament. A member is provided for enabling access to the dose of medicament. The member is arranged and designed such that it is able to be inserted inside the enclosure and establish at least one outlet passage, between the interior of the enclosure and the inhalation opening, through which outlet passage the medicament is delivered to the patient upon inhalation.
Owner:SHL MEDICAL AG
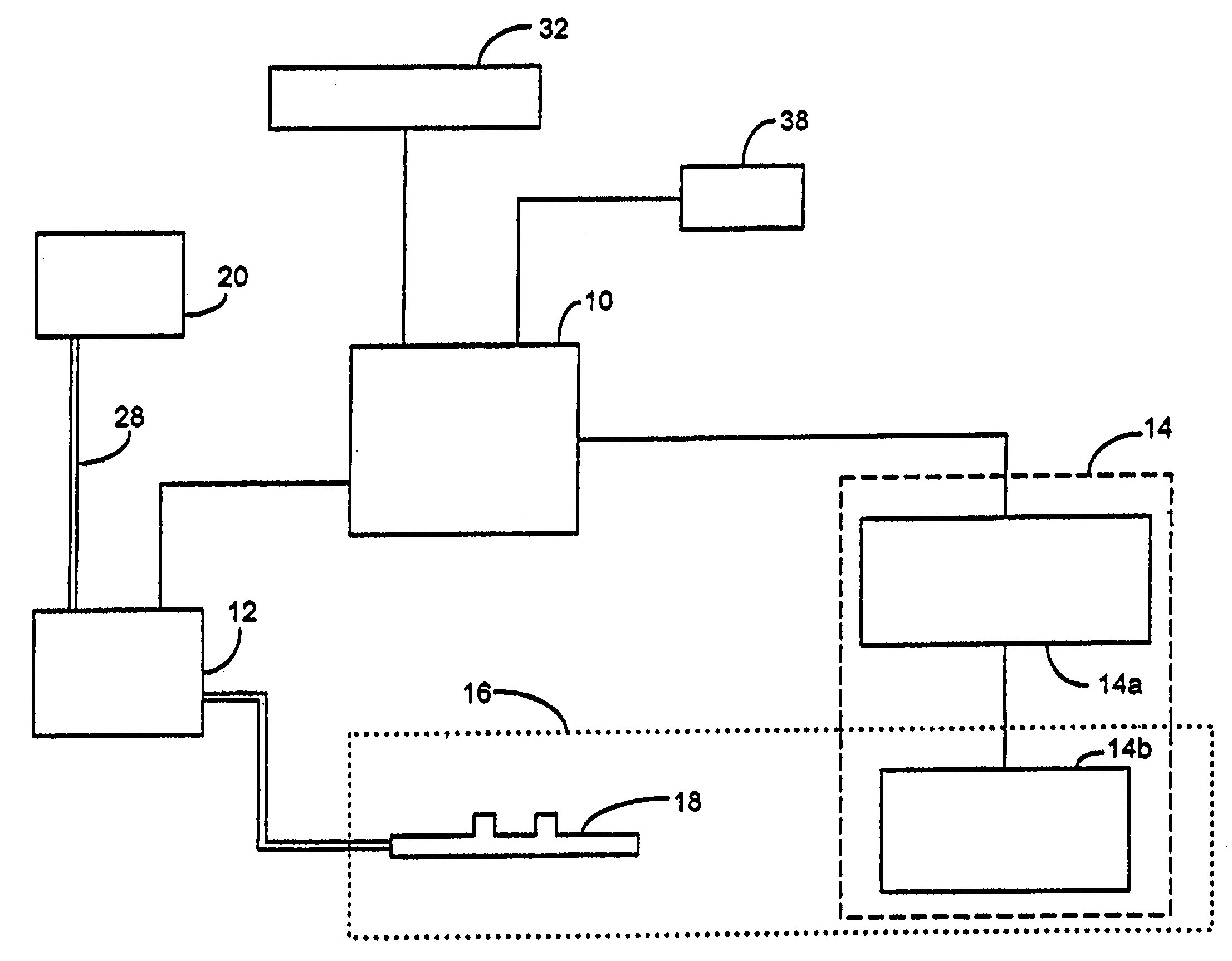
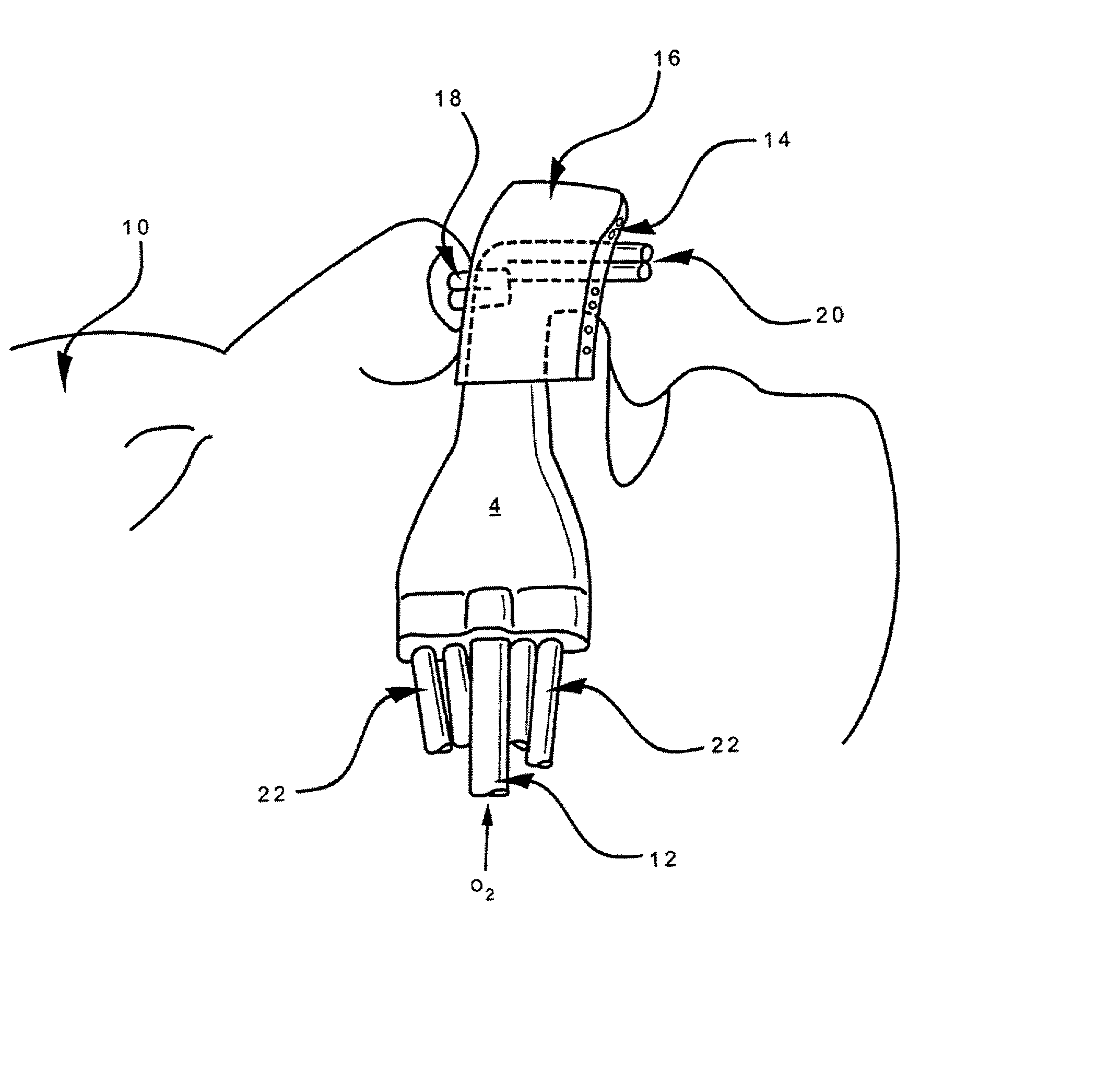
Apparatus and method for mask free delivery of an inspired gas mixture and gas sampling
InactiveUS20020017300A1Operating means/releasing devices for valvesRespiratory masksOxygen deliveryInspired gas
Disclosed is an apparatus and method for the delivery of inspired gas, e.g., supplemental O2, to a person combined with gas sampling, including for the purpose of monitoring of the ventilation of the person. In the invention, the delivery of inspired gas and gas sampling are accomplished without the use of a sealed face mask. The apparatus of one embodiment of the present invention comprises an oxygen delivery device, nasal airway pressure sampling devices, optionally an oral airway pressure sampling device and at least one pressure analyzer connected to the sampling devices which determine the phase of the person's respiration cycle and the person's primary airway. The oxygen delivery device is connected to a controller such that it delivers a higher flow of oxygen to the person during the inhalation phase of the person's respiratory cycle. The invention thus increases end tidal oxygen concentrations. The invention further comprises carbon dioxide sampling tubes that continuously sample gas from two nasal sites and the mouth. The nasal sampling tubes are connected to a switching valve that is in turn connected to a capnometer which determines carbon dioxide concentration during exhalation. The oral gas sampling site is connected to a second capnometer.
Owner:SCOTT LAB
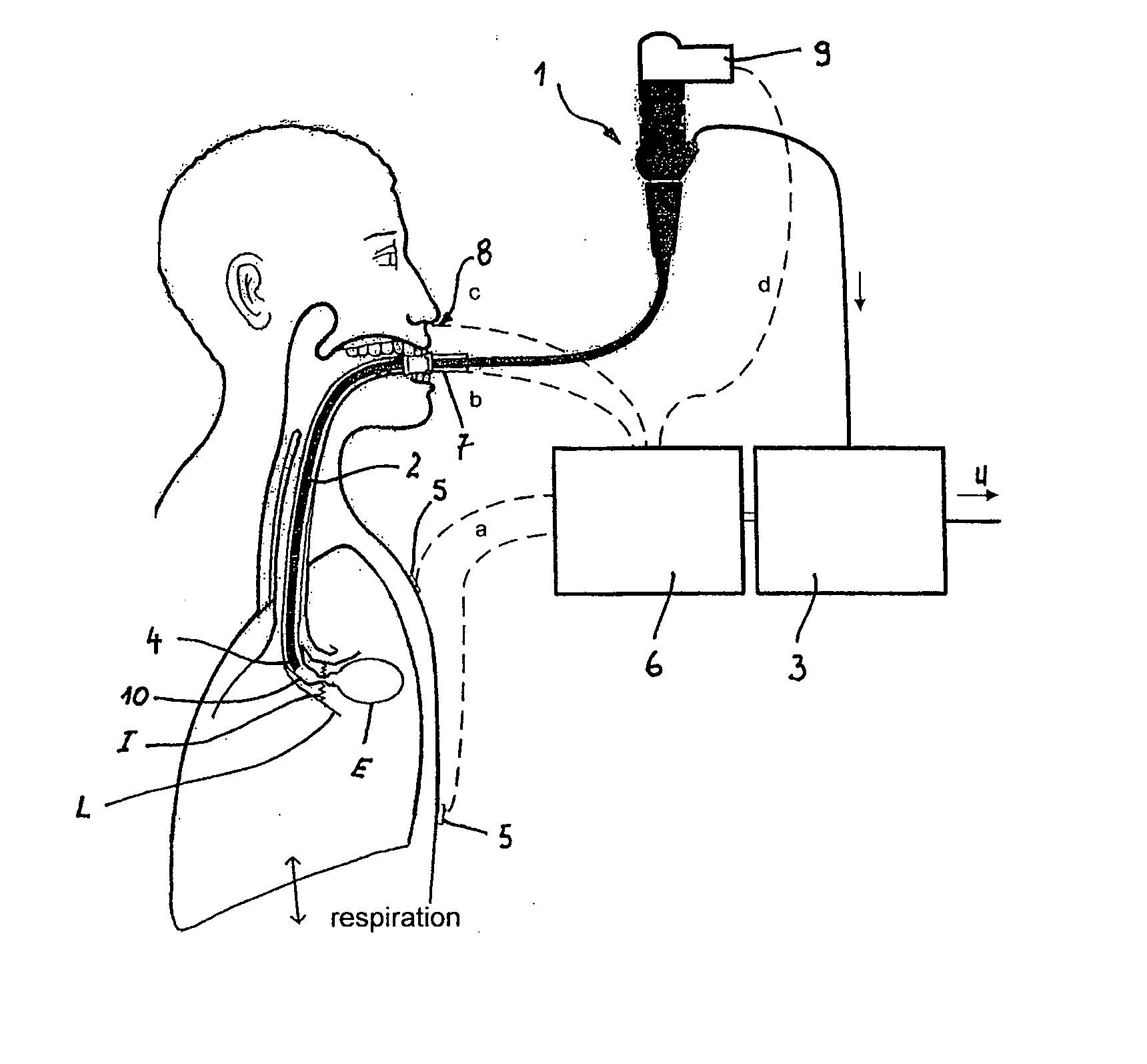
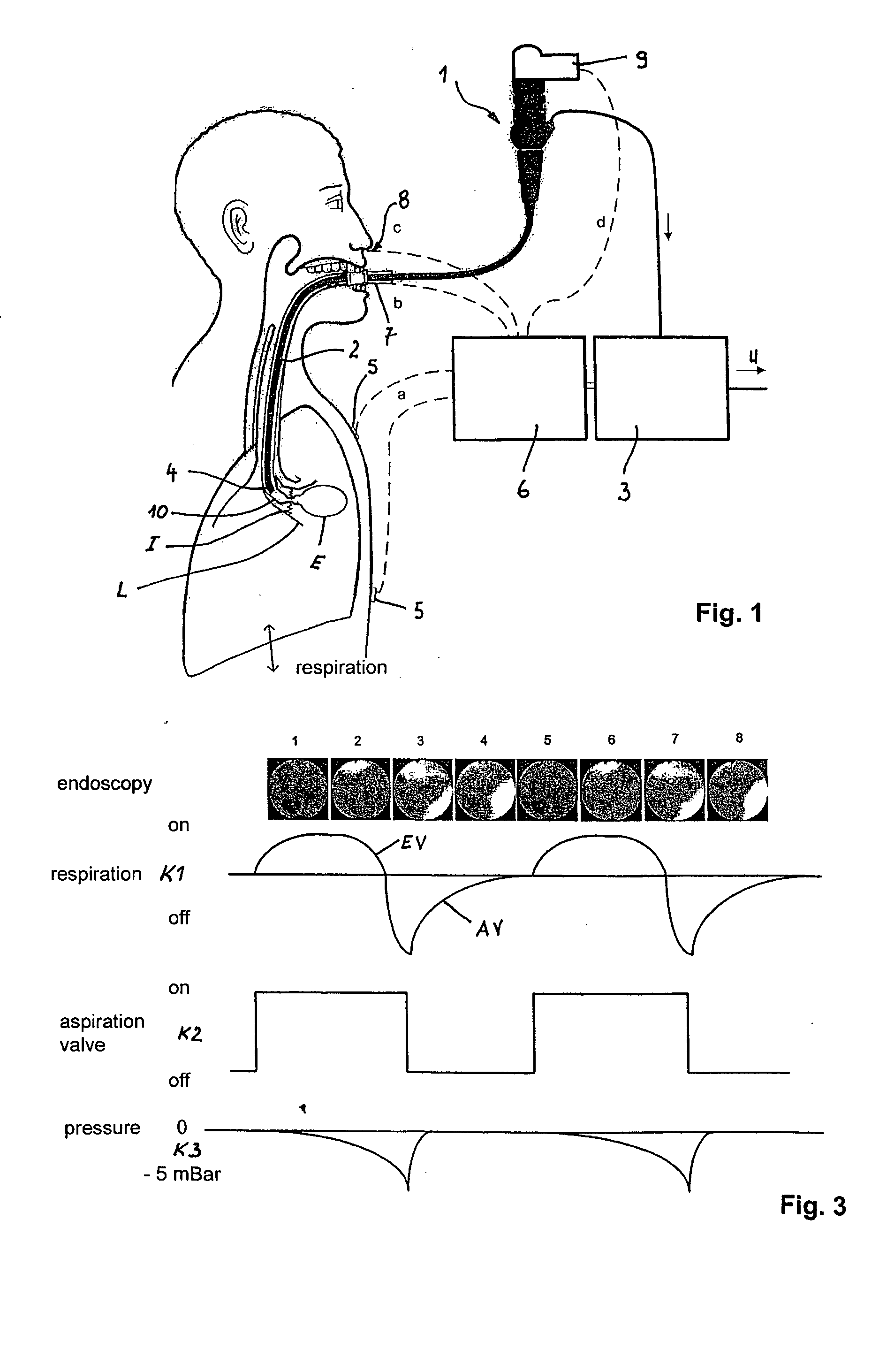
Method and arrangement for reducing the volume of a lung
The invention relates to a method and an arrangement for reducing the volume of a patient's lung. A bronchial catheter (2) is introduced into a hyperexpanded lung area, and air is aspirated from there by means of an aspiration device (3). The associated segmental bronchus is then closed. According to the invention, the patient's spontaneous respiration is recorded by sensors (5), and aspiration of the air is carried out in synchrony with the patient's inhalation action. In order to prevent collapse of the associated segmental bronchus, a pressure generator is provided with which the associated segmental bronchus can be widened, by a compressed gas pulse, in synchrony with the aspiration. The pressure generator can be activated as a function of the aspirated air stream, which is monitored with a measuring device.
Owner:PULMONX
Low temperature electronic vaporization device and methods
InactiveUS20130312742A1Maintain efficiencyReduce the temperatureInput/output for user-computer interactionTobacco treatmentInhalationEnvironmental health
Low temperature electronic vaporization devices and method are described herein for emulating smoking wherein the devices generate an aerosol for inhalation by a subject by heating a viscous material that can have a tactile response in the mouth or respiratory tract.
Owner:JUUL LABS INC
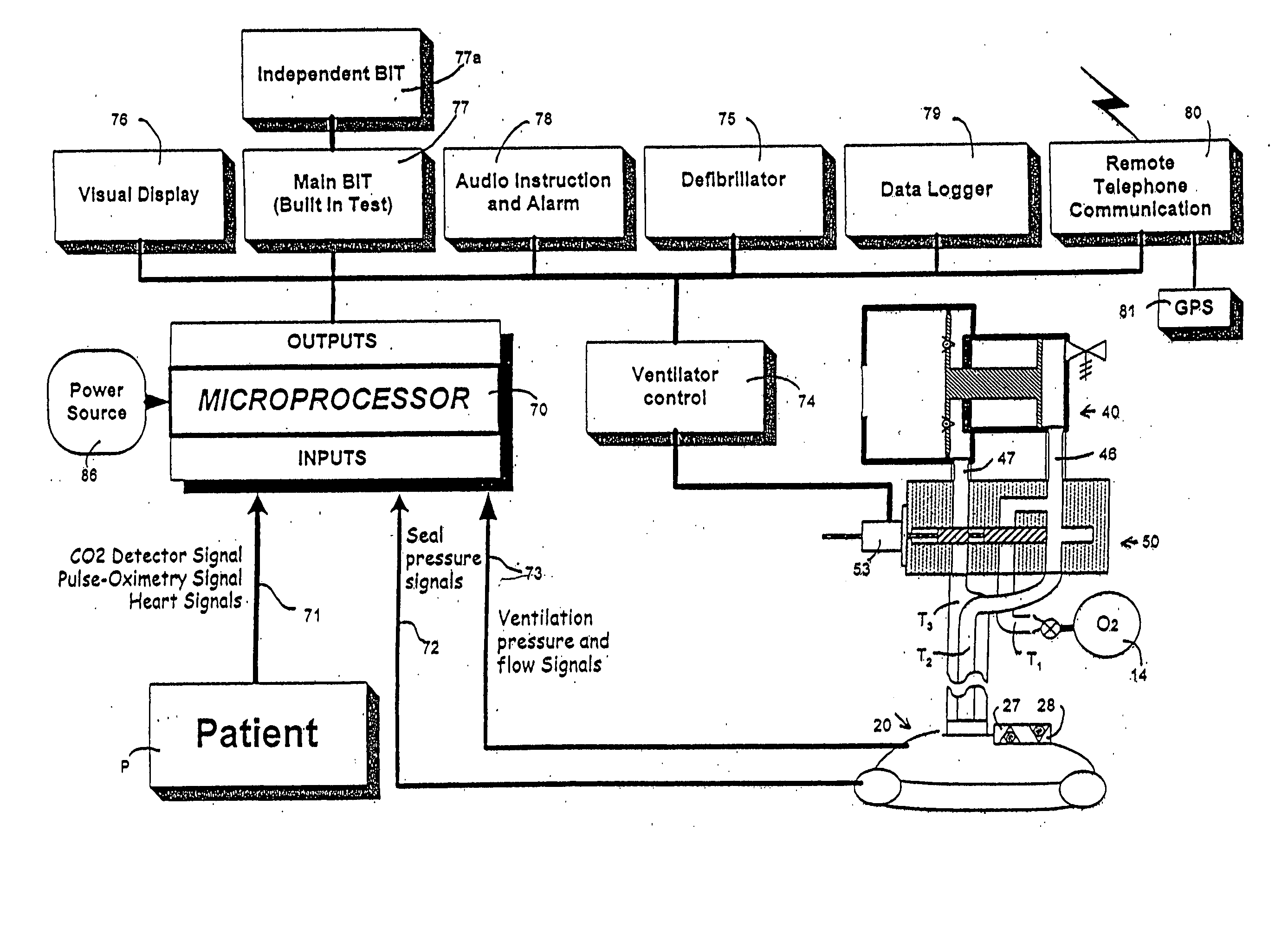
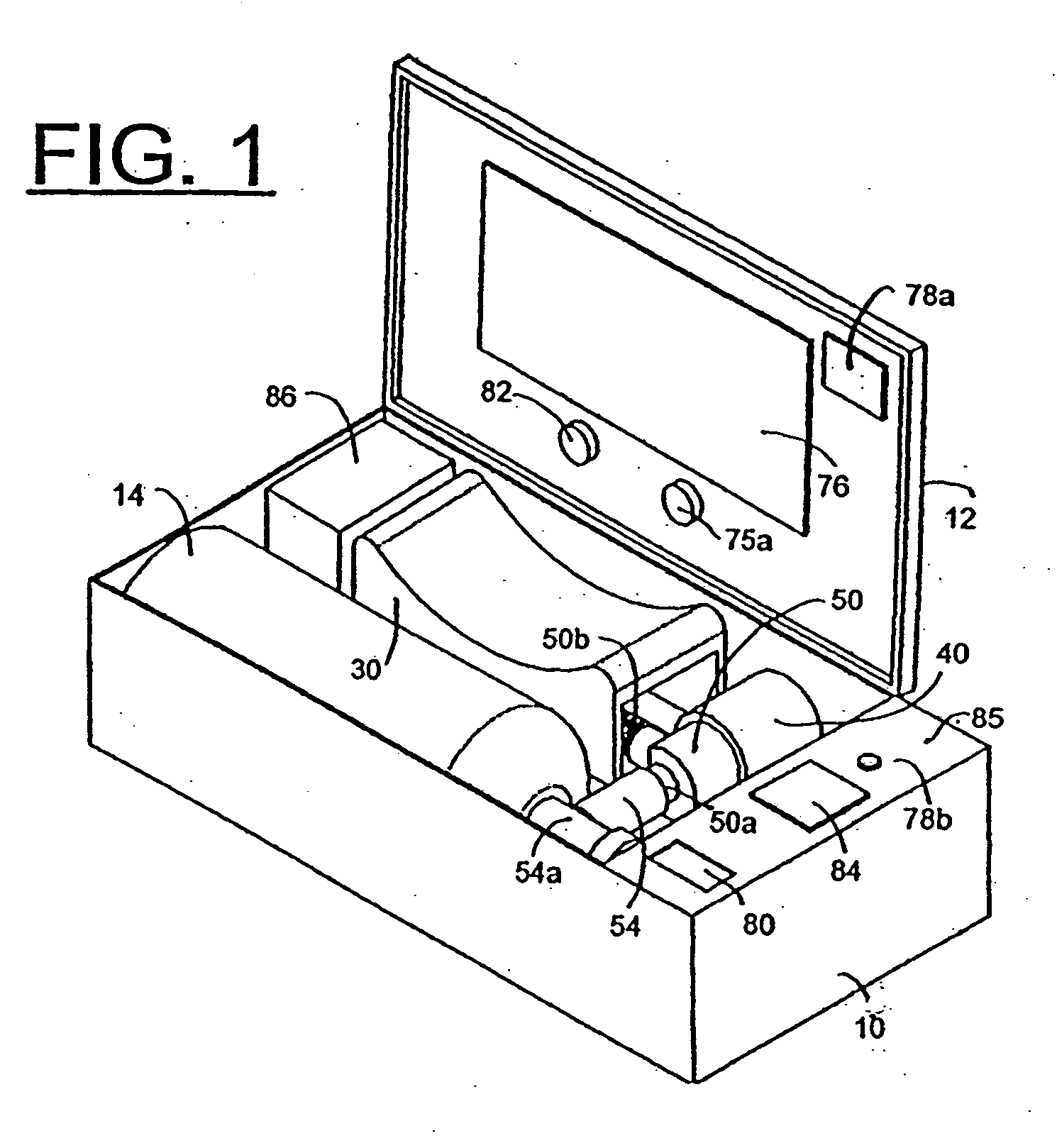
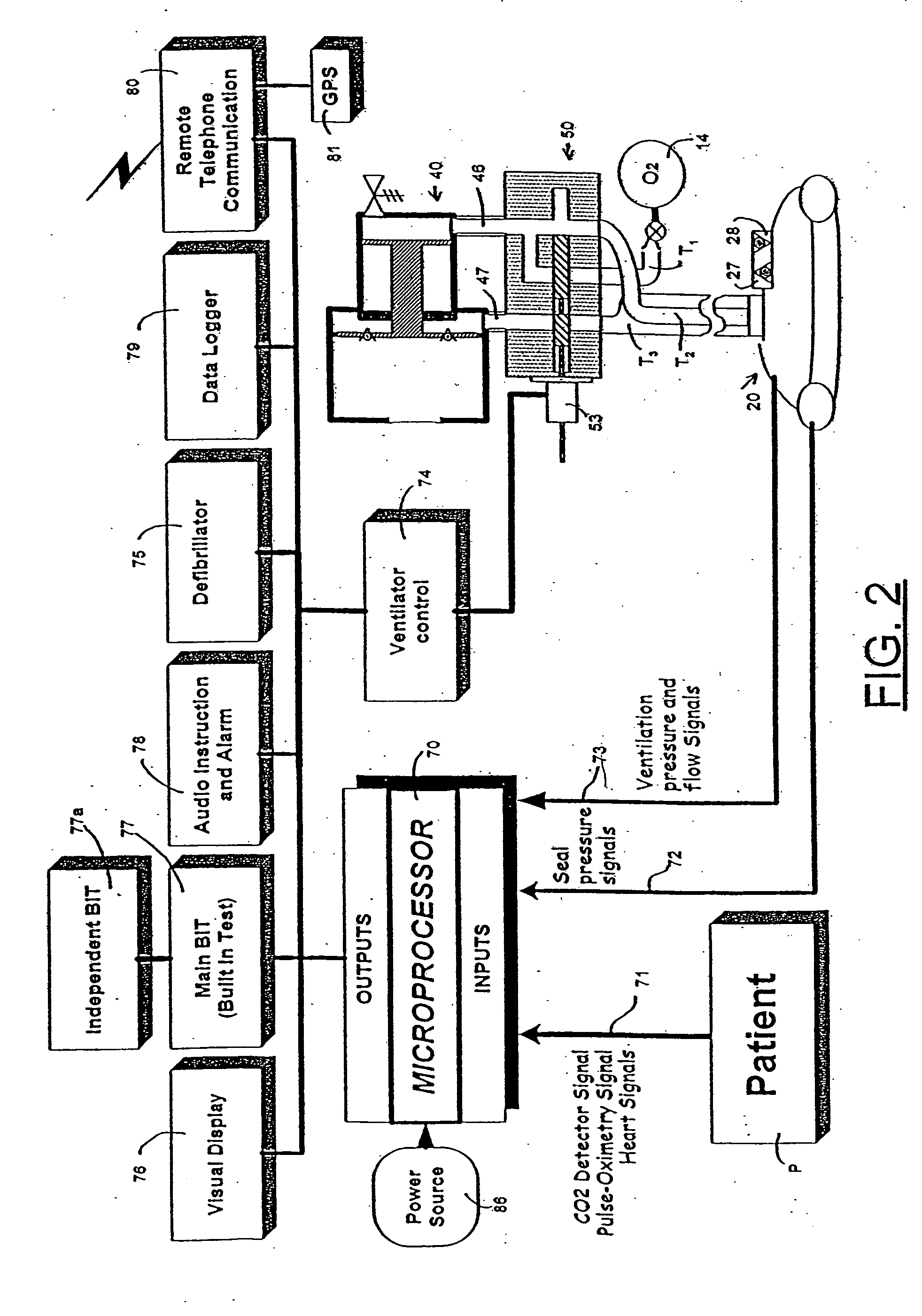
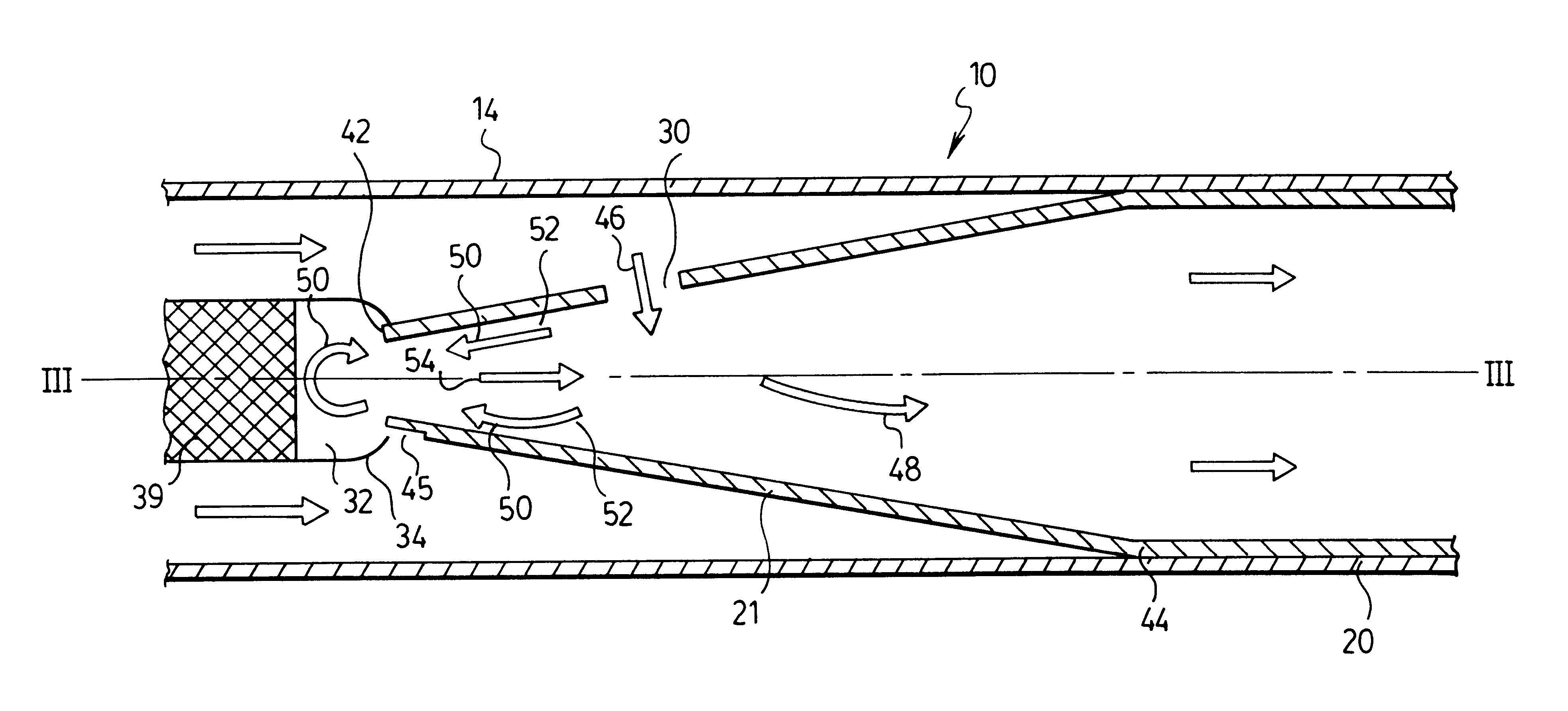
Emergency medical kit, respiratory pump, and face mask particularly useful therein
InactiveUS20050085799A1Efficient driveWide degree of automatic controlRespiratorsElectrocardiographyEmergency medicineNon invasive
An emergency medical kit for use, particularly by a non-professional, to render emergency medical treatment to a patient, includes: a pressurized-oxygen container within a housing; a face mask within the housing for application to the face of a patient requiring cardiopulmonary resuscitation; and a respiratory pump within the housing connected to the pressurized-oxygen container so as to be driven thereby to supply oxygen to the mask for inhalation by the patient, and to discharge the exhalations of the patient via the face mask to the atmosphere. The face mask includes an inflatable seal around its circumference engageable with the face of the patient receiving the mask for sealing the interior of the mask; a pressure sensor sensing the pressure in the inflatable seal; and an indicator for indicating whether the face mask is properly applied to the face of the patient. The kit further includes a neck rest having straps for attaching the face mask thereto in contact with the patient's face when the patient's head is placed on the head rest. According to a most essential aspect of the invention there is provided an emergency, fully automatic kit, based on non-invasive means for performing all stages of the “chain of survival” (including: external defibrillation, ventilation and automatic chest compression) by a single operator.
Owner:LURIA ODED +1
Inhaler
InactiveUS6234169B1Reduce negative impactHigh simulationRespiratorsLiquid surface applicatorsParticulatesInhalation
An inhaler for use by an individual to inhale a particulate medicament from a reservoir comprises a chamber having a first end connectable to the reservoir to be in air flow communication therewith, a second end for delivering the medicament to the individual upon inhalation and a conduit defining an air flow path extending between the first end and the second end; and, an orifice in the chamber between the first end and the second end, the orifice utilizing the Coanda Effect when the reservoir is in air flow communication with the chamber and upon inhalation by the individual to draw medicament from the reservoir into the air flow path.
Owner:SANSA BARBADOS
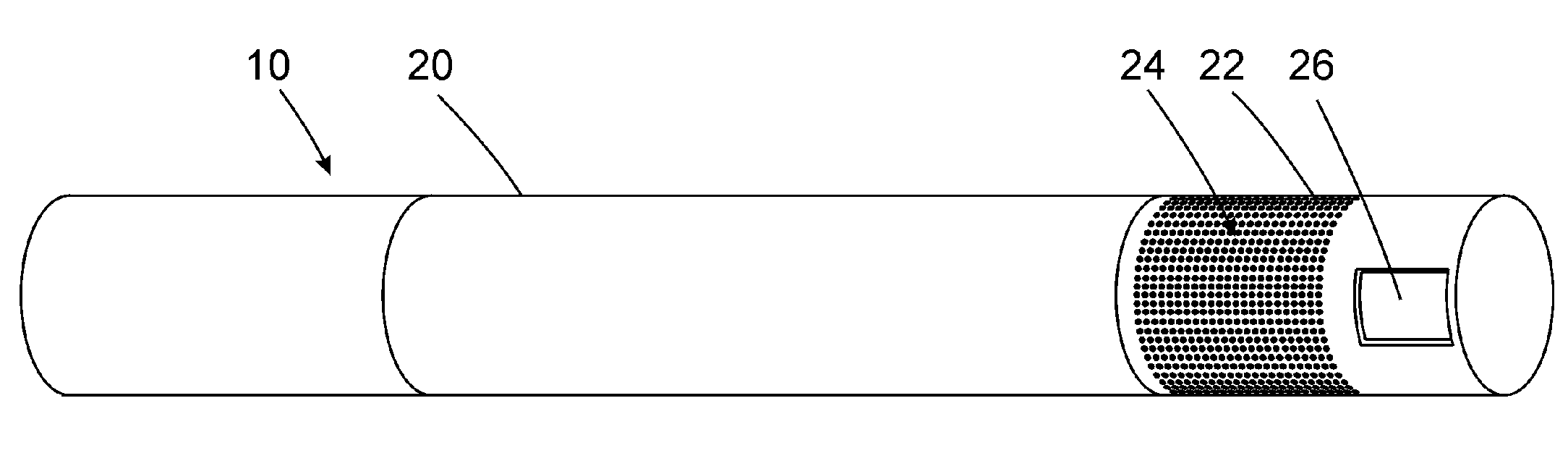
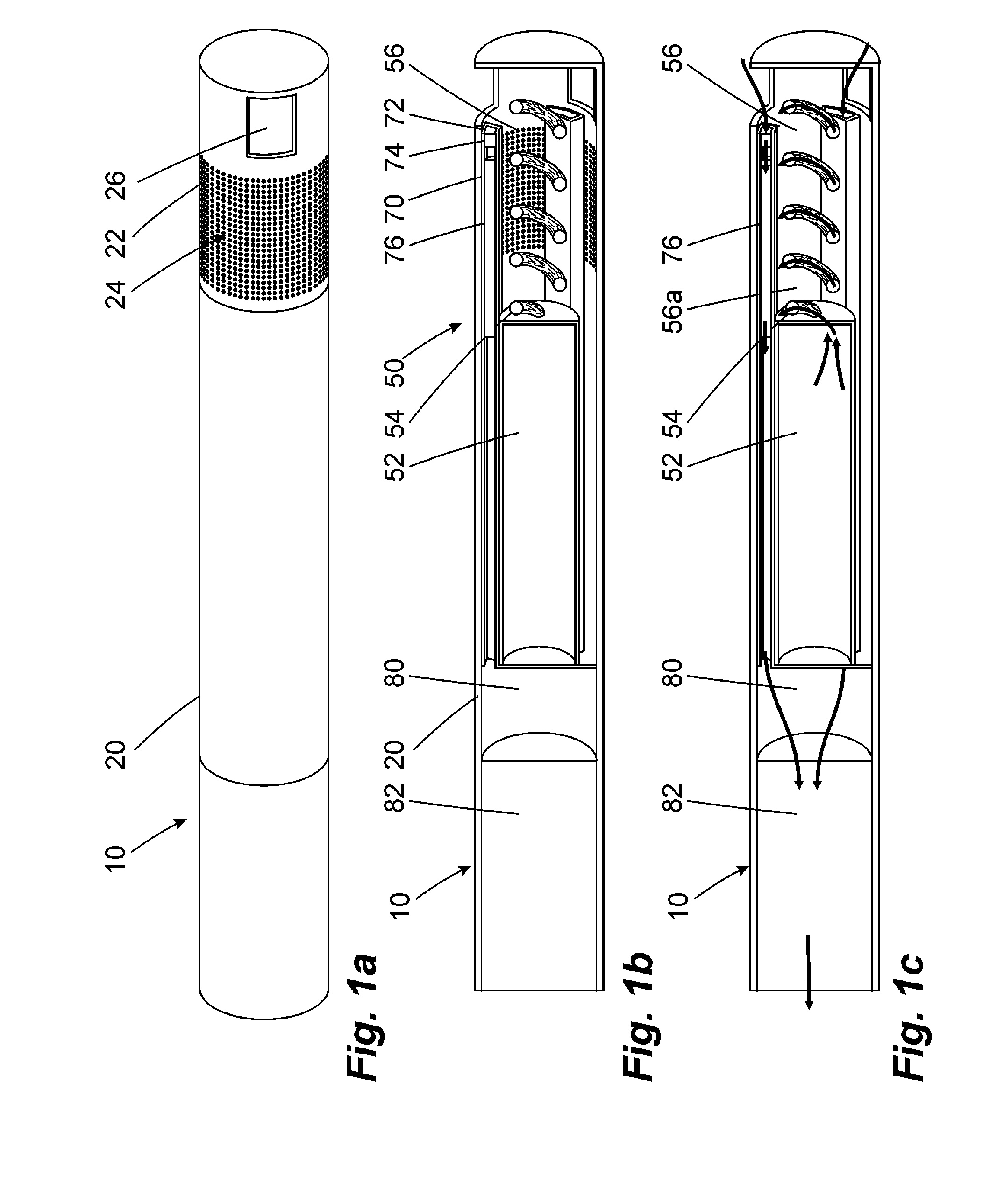
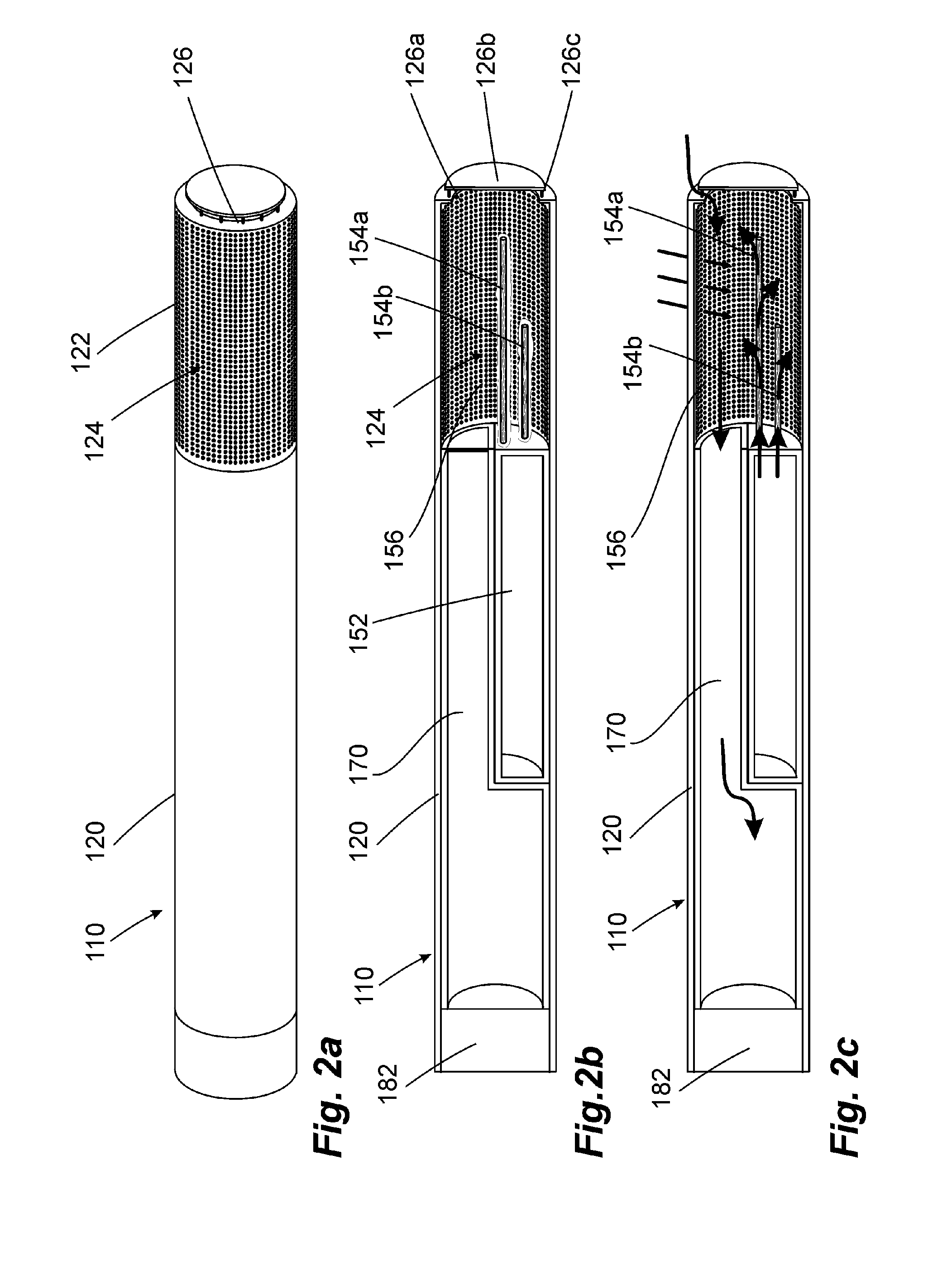
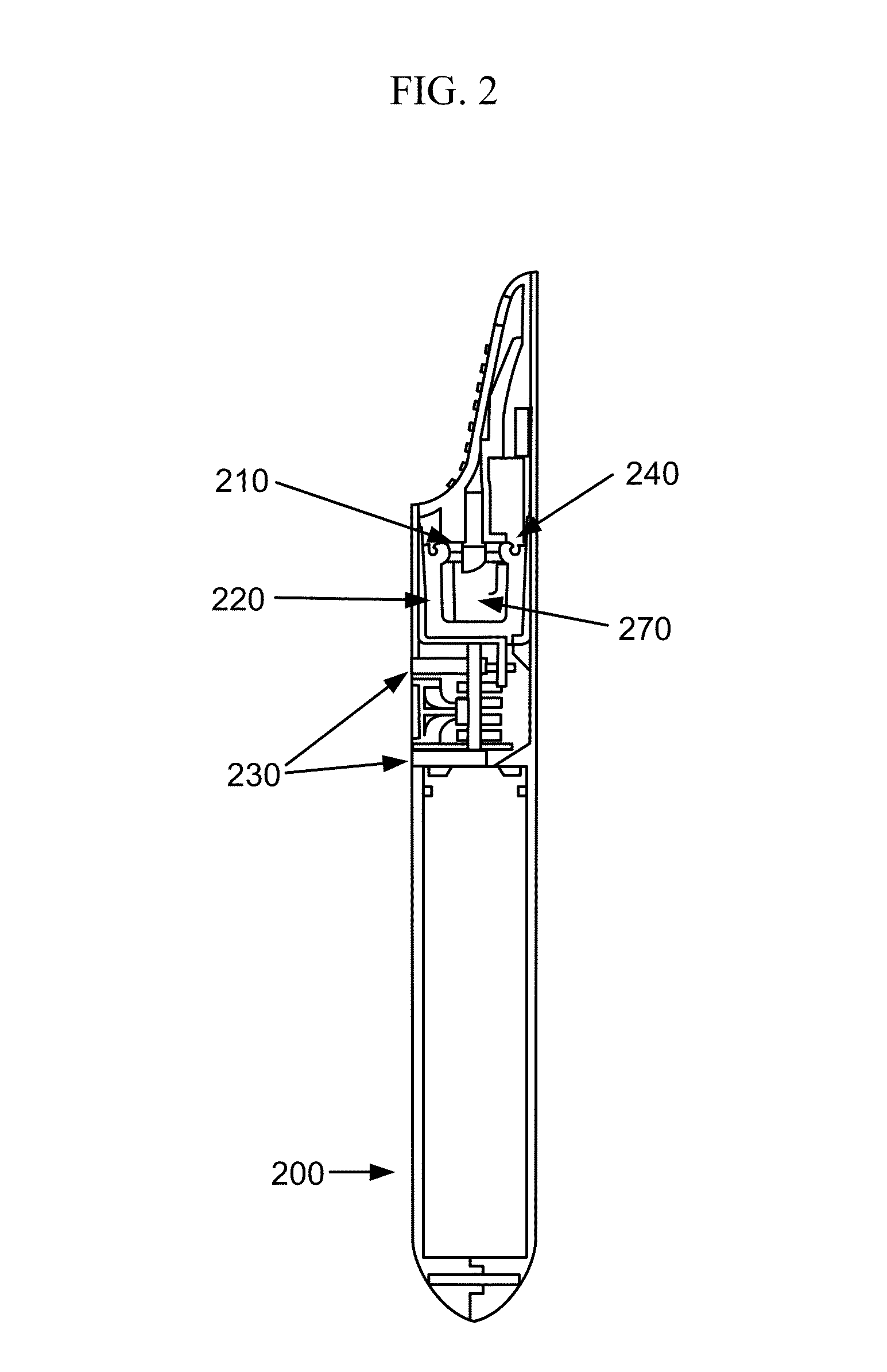
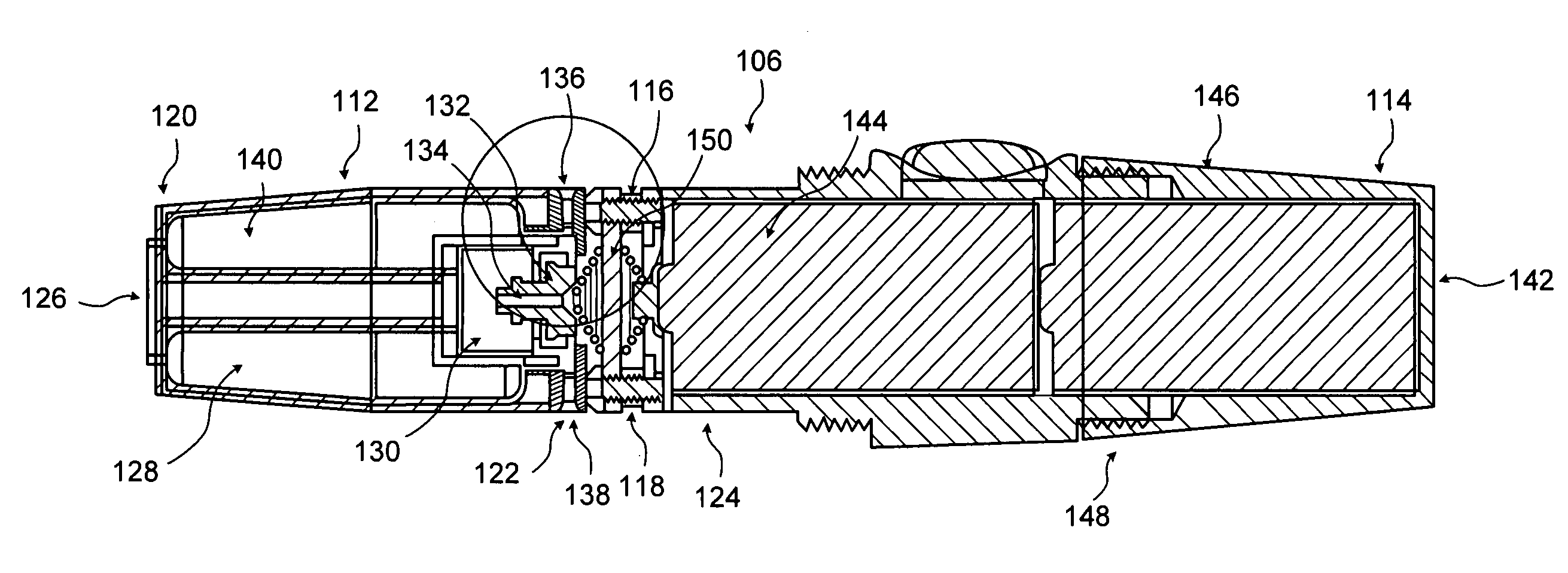
Electronic vapor inhaling device
InactiveUS20120174914A1Realize automatic adjustmentRespiratorsMedical devicesInhalationUnique identifier
An electronic vapor inhaling device has an outer housing that defines an internal cavity having a proximal end and a distal end. A mouthpiece is located at the proximal end. A liquid storage chamber is defined within the outer housing near its proximal end and a battery compartment containing a battery is located near the distal end. An atomizer is positioned between the liquid storage chamber and battery chamber and is in communication with an electronic circuit board. The electronic circuit board is configured to receive input from a unique identifier associated with the electrical resistance of the liquid in the liquid storage chamber and automatically adjust the power supplied to the atomizer. The atomizer rapidly heats the liquid that is injected into an air passageway when the user inhales through the mouthpiece, causing the liquid to vaporize and allow inhalation by the user.
Owner:PIRSHAFIEY NASSER +2
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Mask free delivery of oxygen and ventilatory monitoring
InactiveUS6938619B1Increase oxygen concentrationFast sensingOperating means/releasing devices for valvesRespiratory masksOxygen deliveryAirway devices
Disclosed is an apparatus and method for the delivery of supplemental oxygen gas to a person combined with the monitoring of the ventilation of the person with both being accomplished without the use of a sealed face mask. Preferred embodiments of the present invention combine an oxygen delivery device, a nasal airway pressure sampling device, an oral airway pressure sampling device, and a pressure analyzer connected to the sampling devices to determine the phase of the person's respiration cycle and the person's primary airway. The oxygen delivery device is connected to a controller such that it delivers a higher flow of oxygen to the person during the inhalation phase of the person's respiratory cycle. The invention thus increases end tidal oxygen concentrations with improved efficiency comparative to known open airway devices. Embodiments of the invention can include carbon dioxide sampling tubes that continuously sample air from the nose and mouth to determine carbon dioxide concentration during exhalation.
Owner:SCOTT LAB
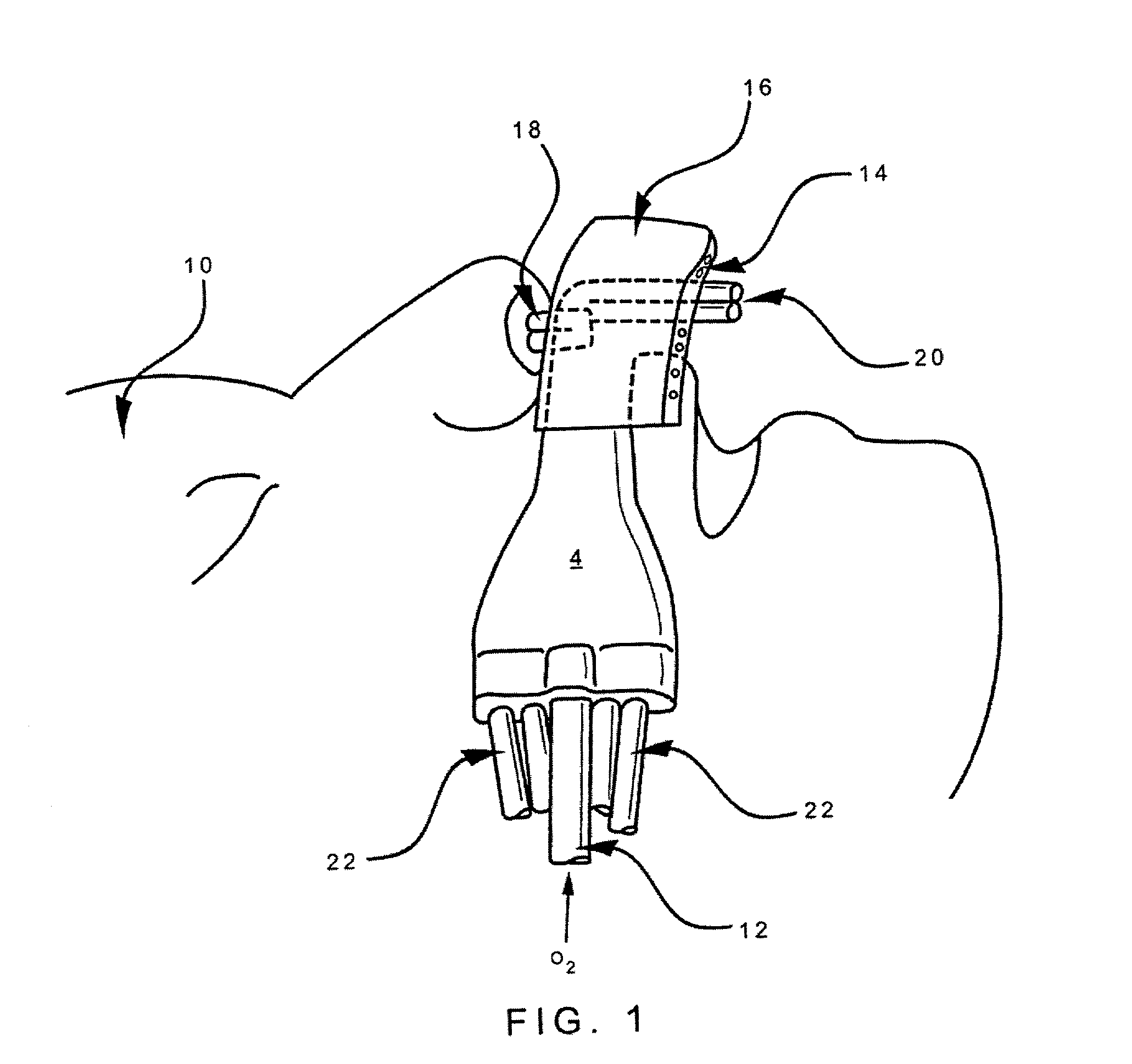
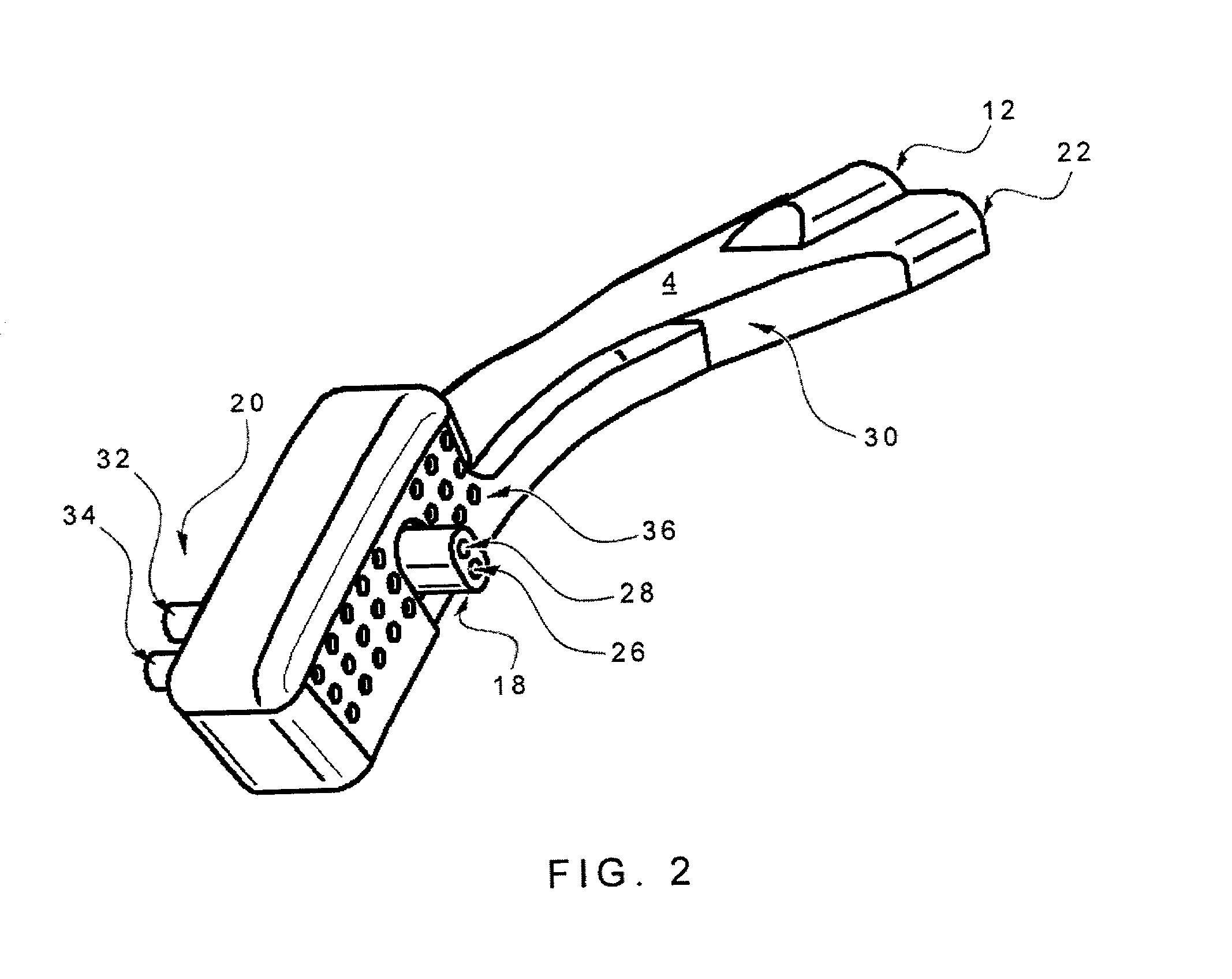
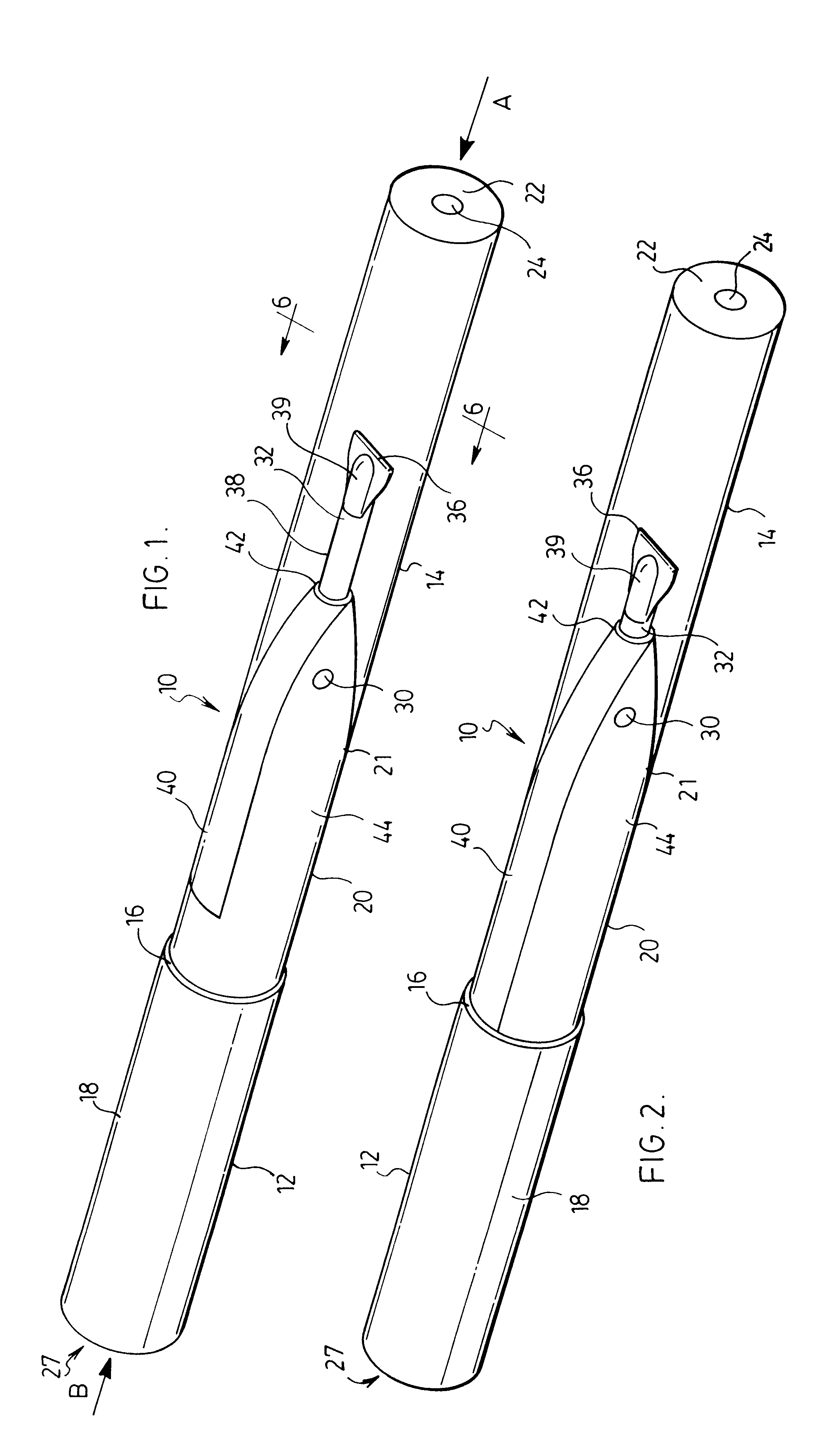
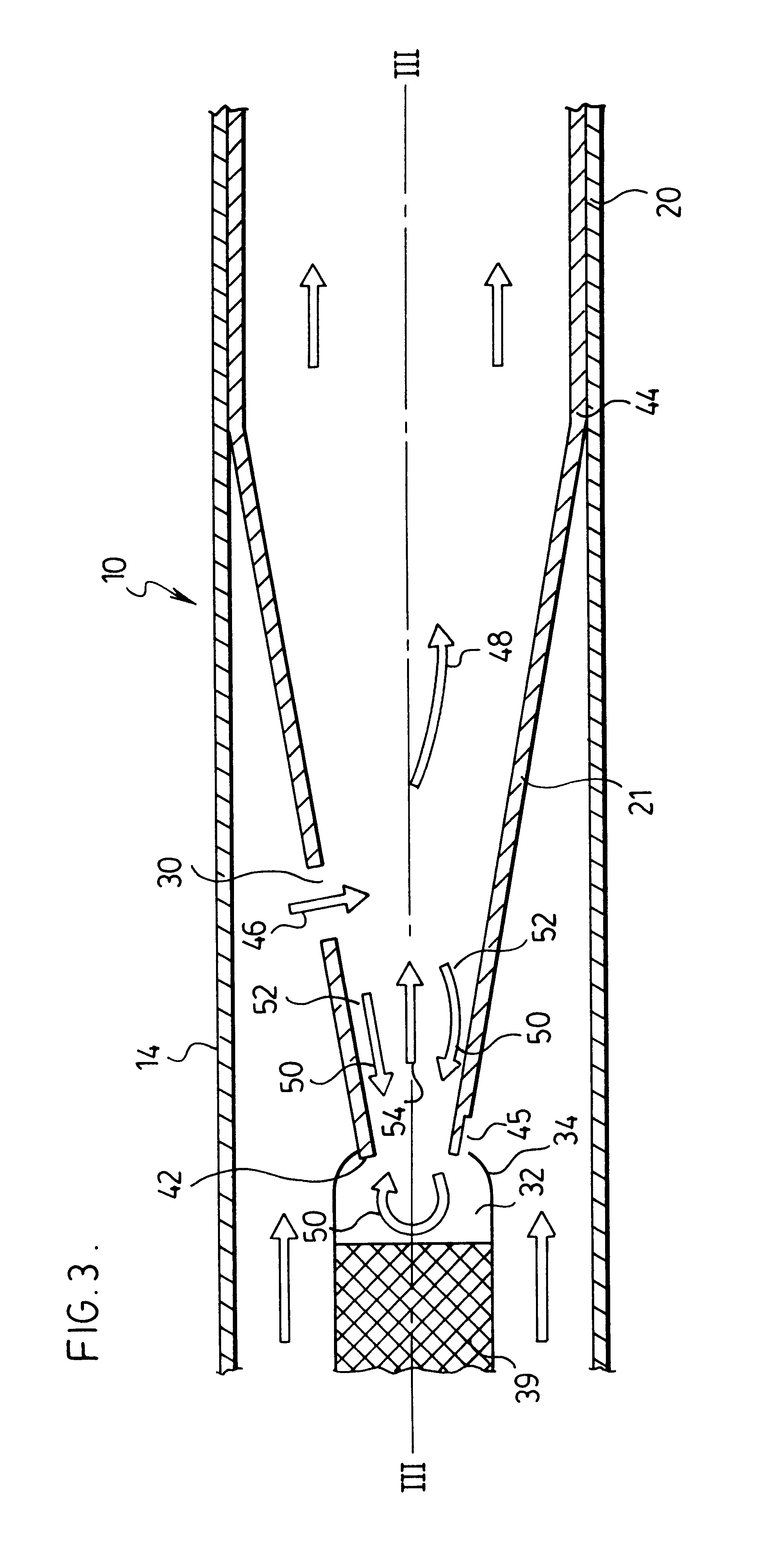
Respiratory Therapy System Including a Nasal Cannula Assembly
InactiveUS20080051674A1Improve fatigueIncrease inhalationOperating means/releasing devices for valvesPhysical therapyNasal cavityInhalation
A nasal cannula, for supplying a respiratory gas to a patient, comprising: a pair of spaced apart supply lines which each have a head at one end thereof with a discharge opening therein. The opposite end of each supply line is connectable to a respiratory gas source. Each head is sized to be snugly received and retained within one of the nasal cavities of the patient while forming a sufficient leakage passage, between a portion of inwardly facing nasal cavity skin of a patient and a portion of an exterior surface of the head, to facilitate exhausting of any excess respiratory gas supplied to the patient through the leakage passage and also facilitate inhalation of any room air required in excess of the respiratory gas to be supplied to the patient. The invention also relates to a respiratory therapy system incorporating the nasal cannula, a method of treating a patient with sleep disorder by using the nasal cannula, a diagnostic tool for measuring nasal cavity pressure of a patient, and a method of using the diagnostic tool for measuring nasal cavity pressure of a patient.
Owner:SALTER LABS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com