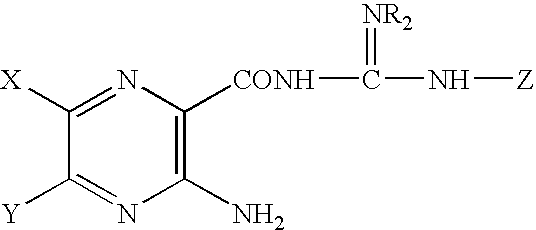
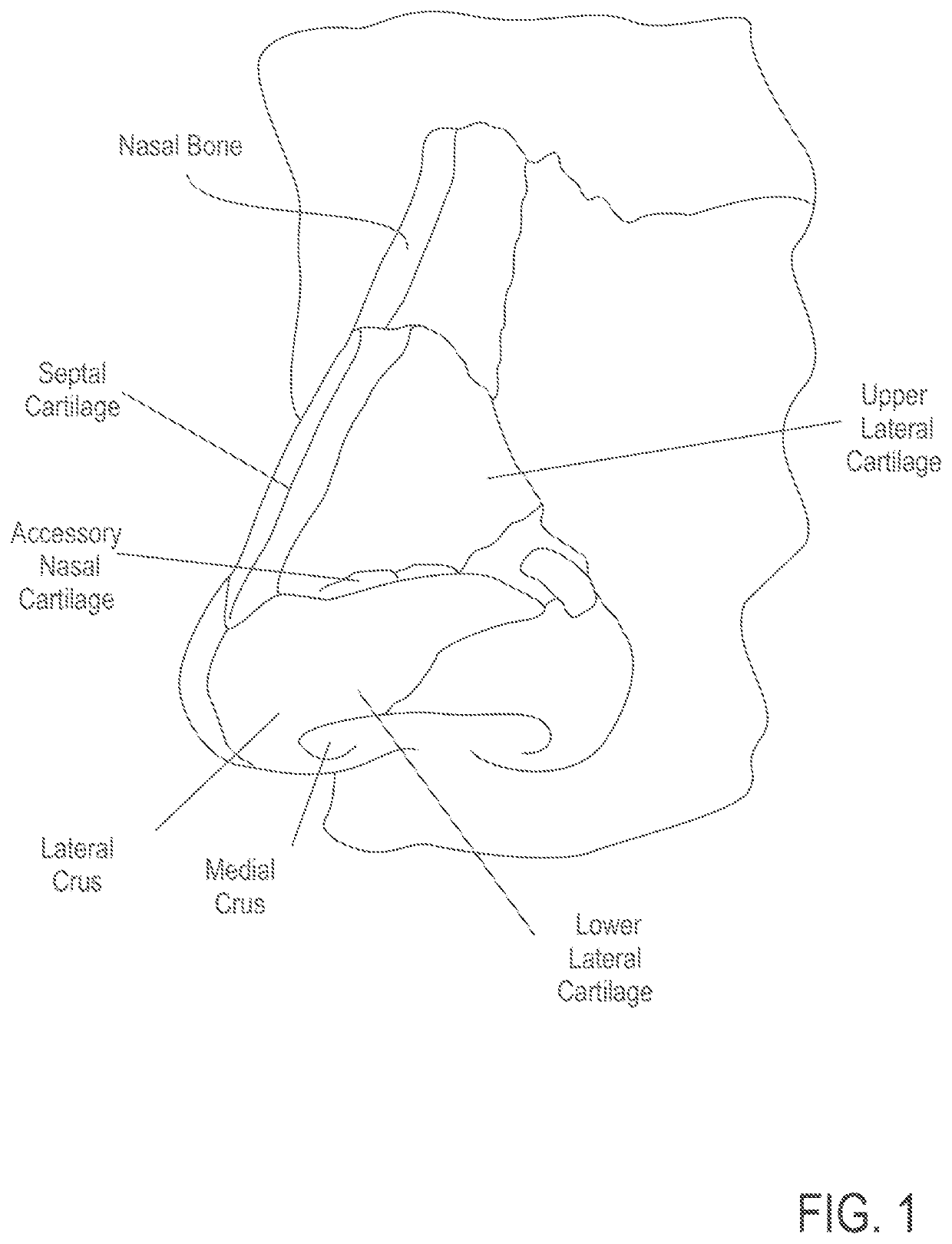
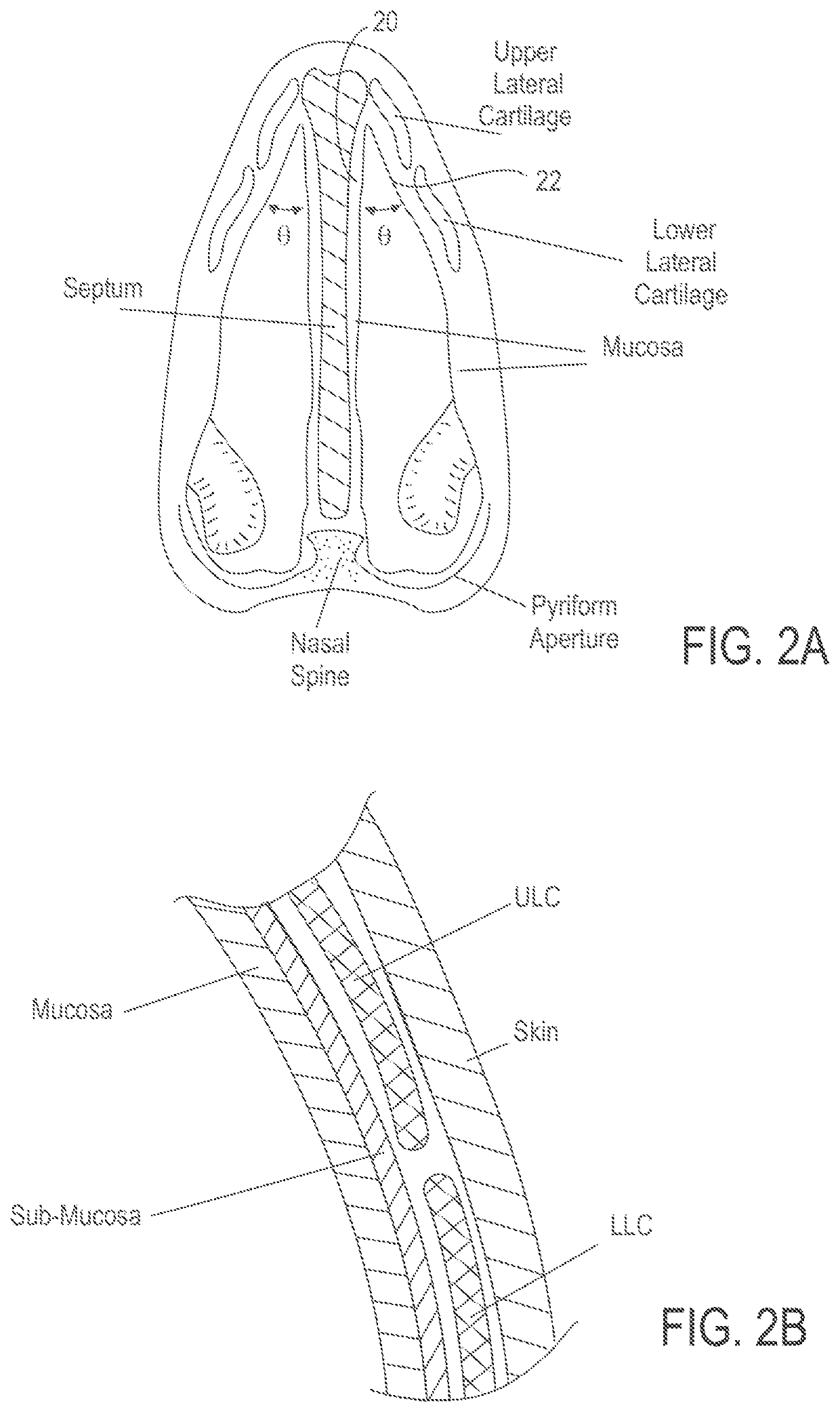
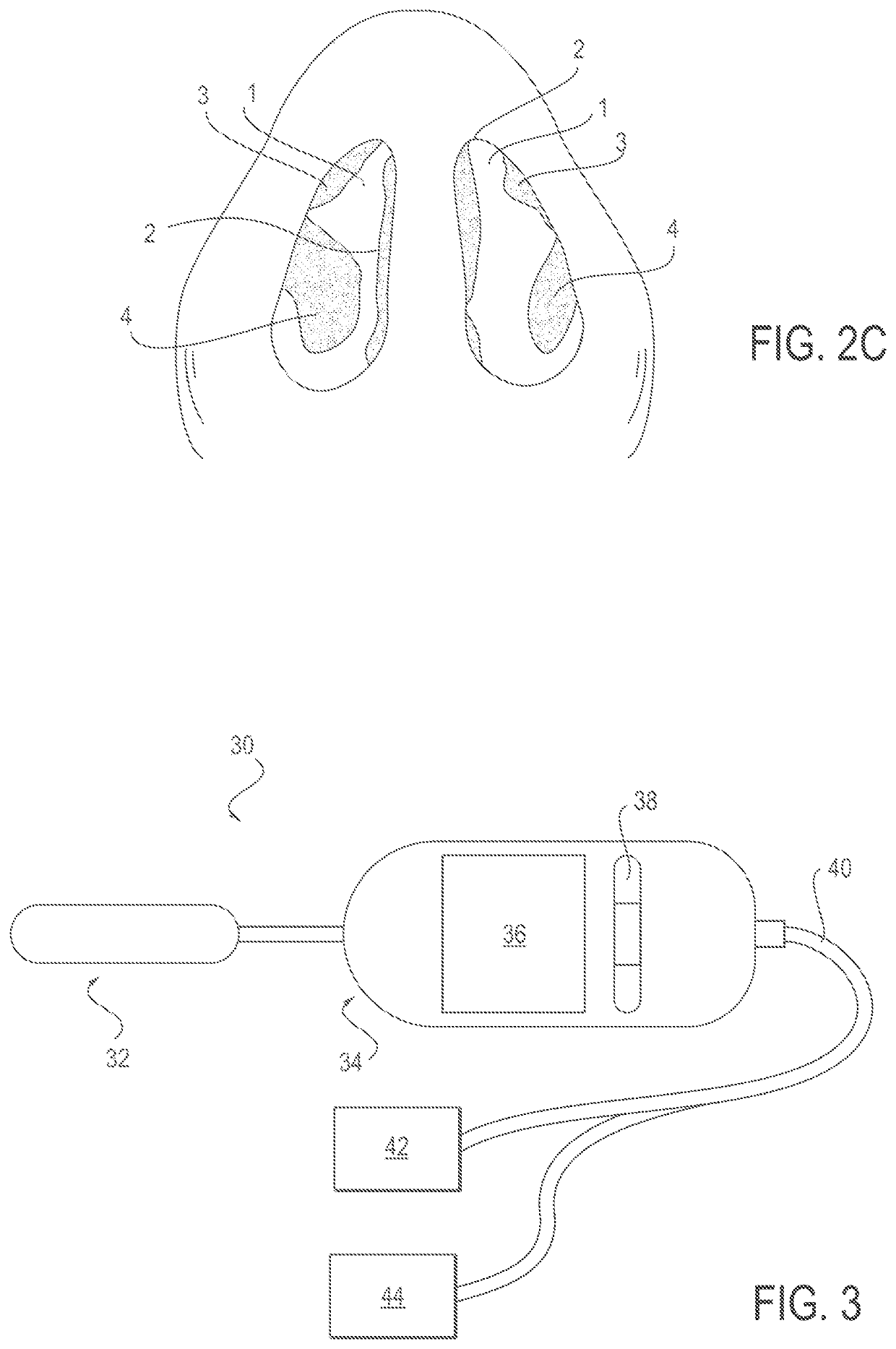
Patents
Literature
66 results about "Nasal airway" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
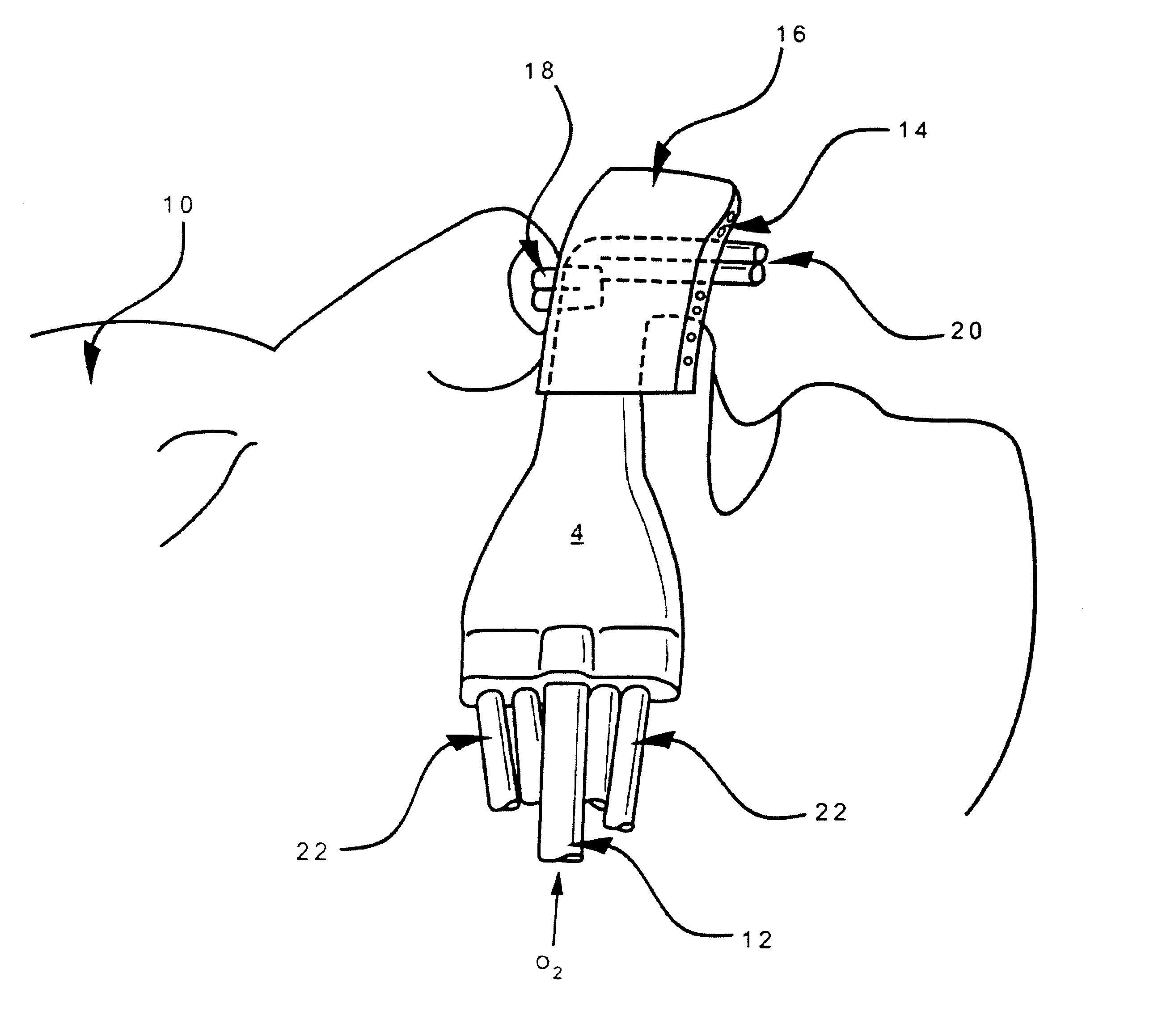
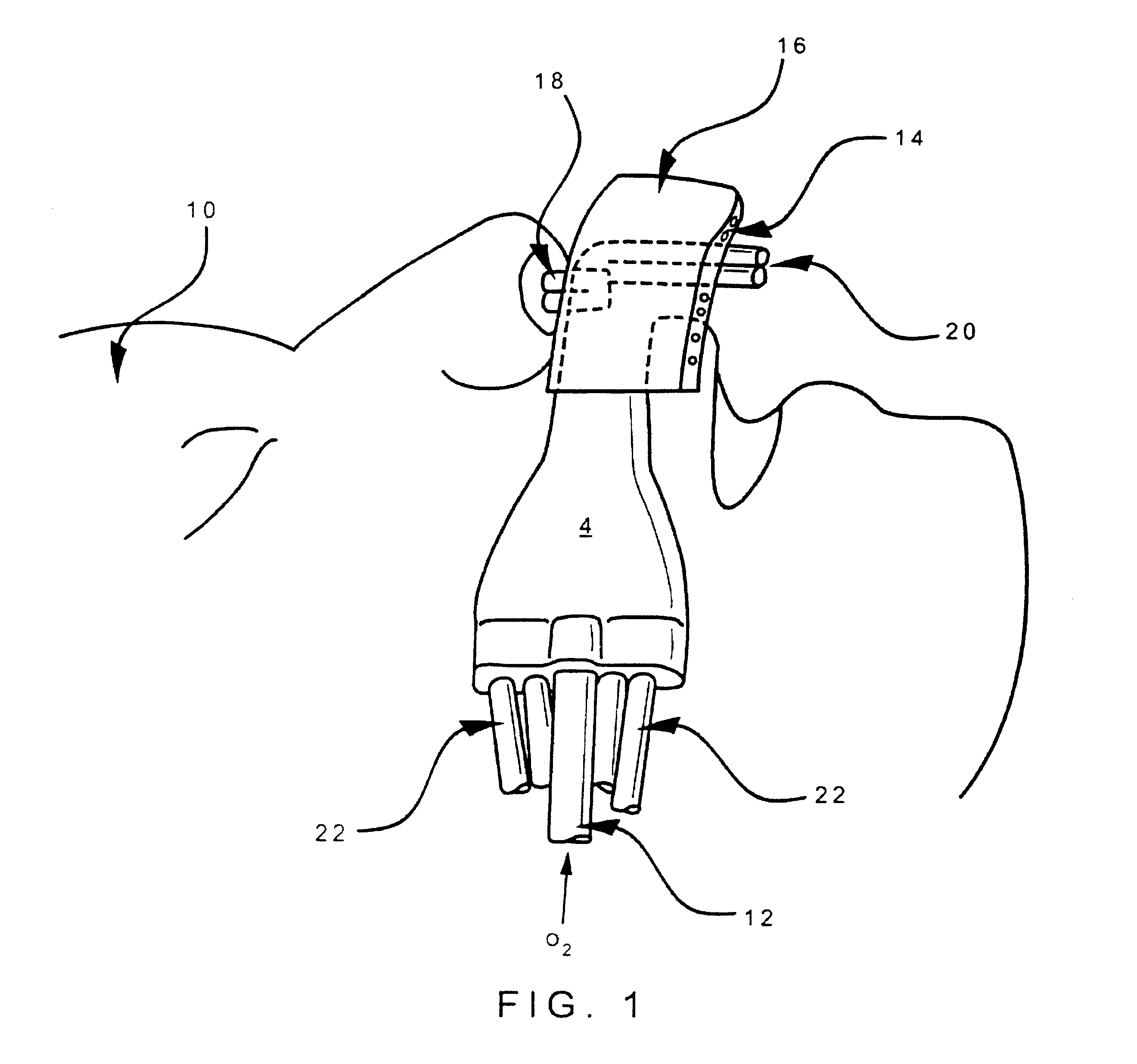
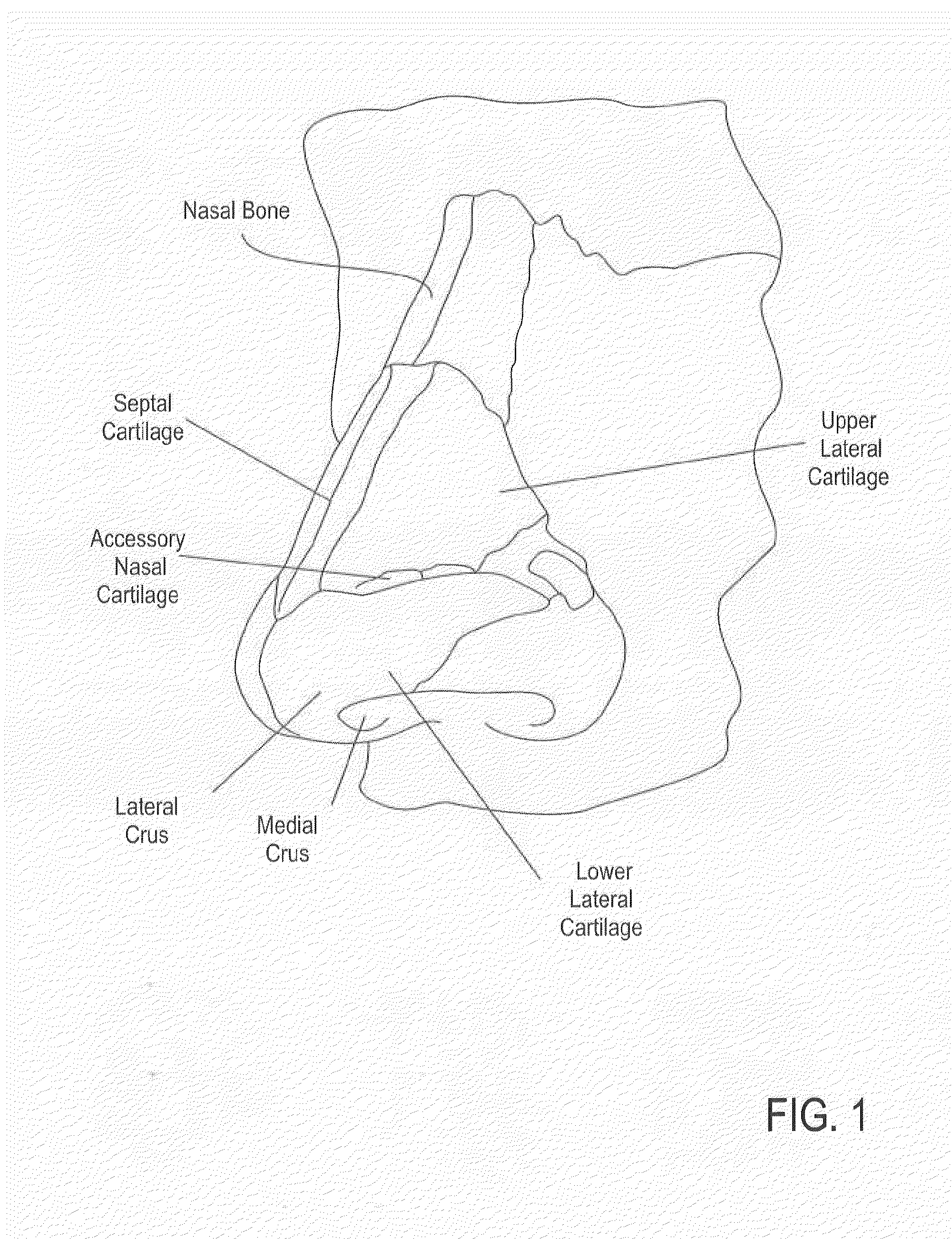
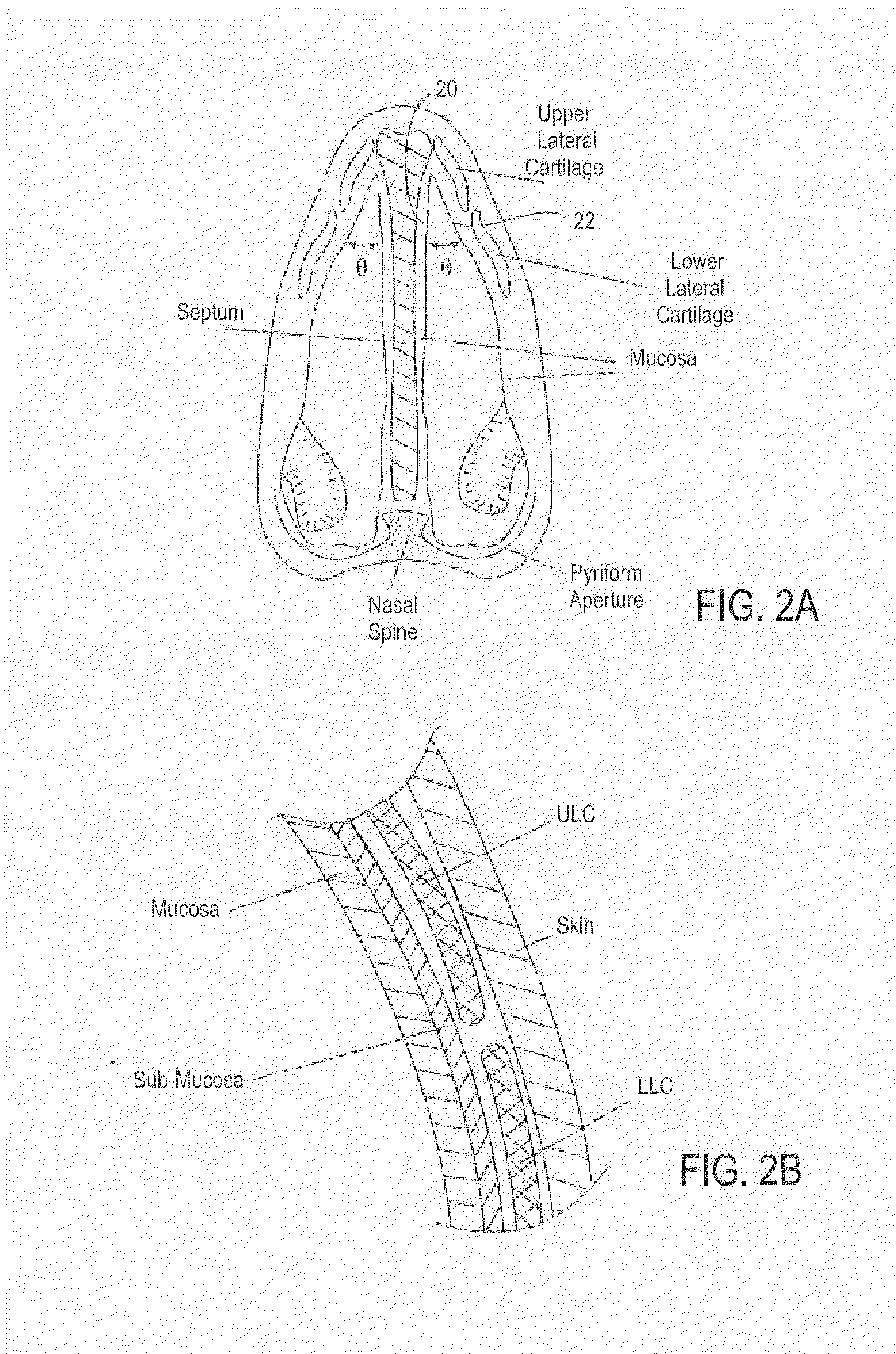
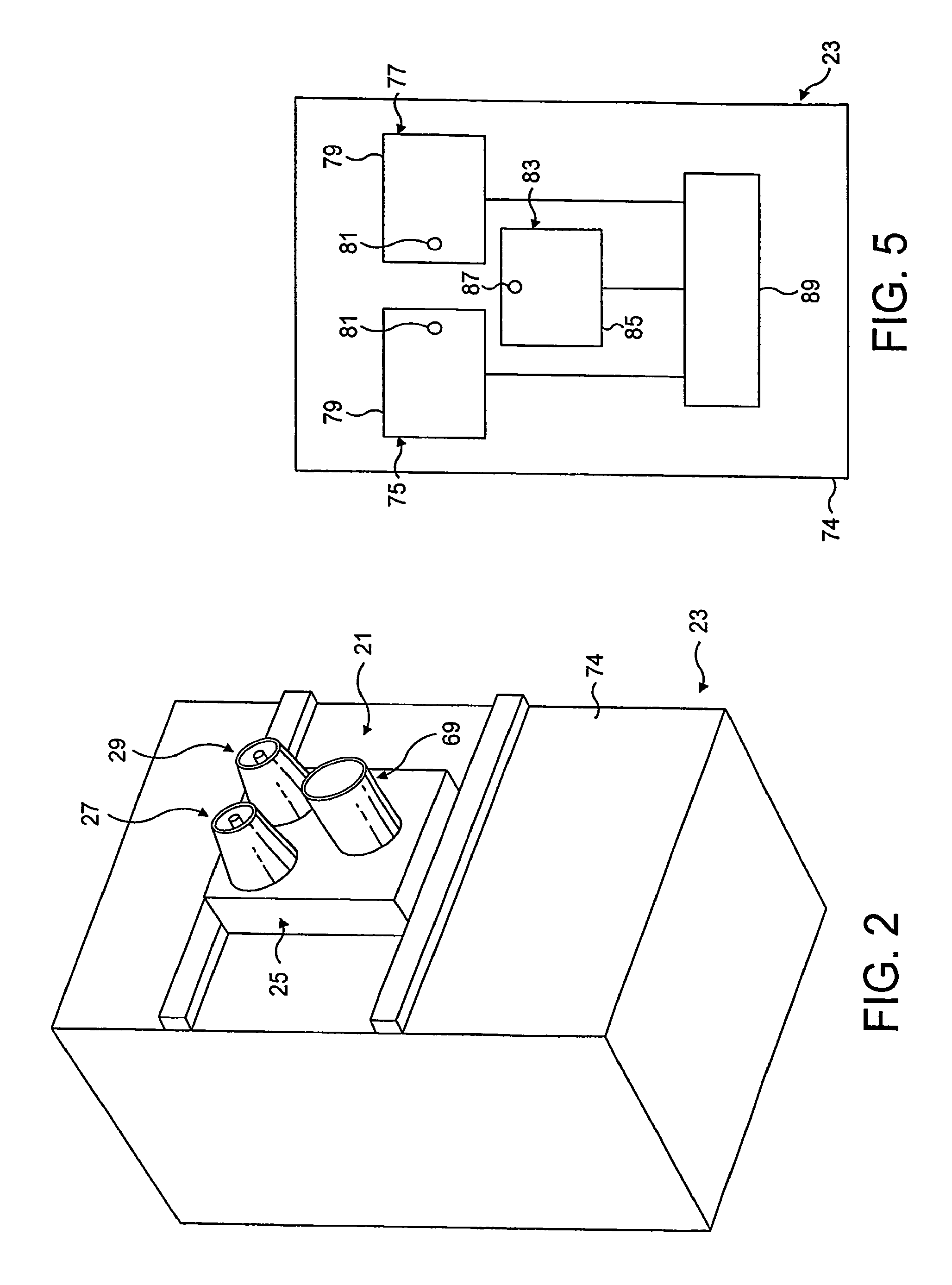
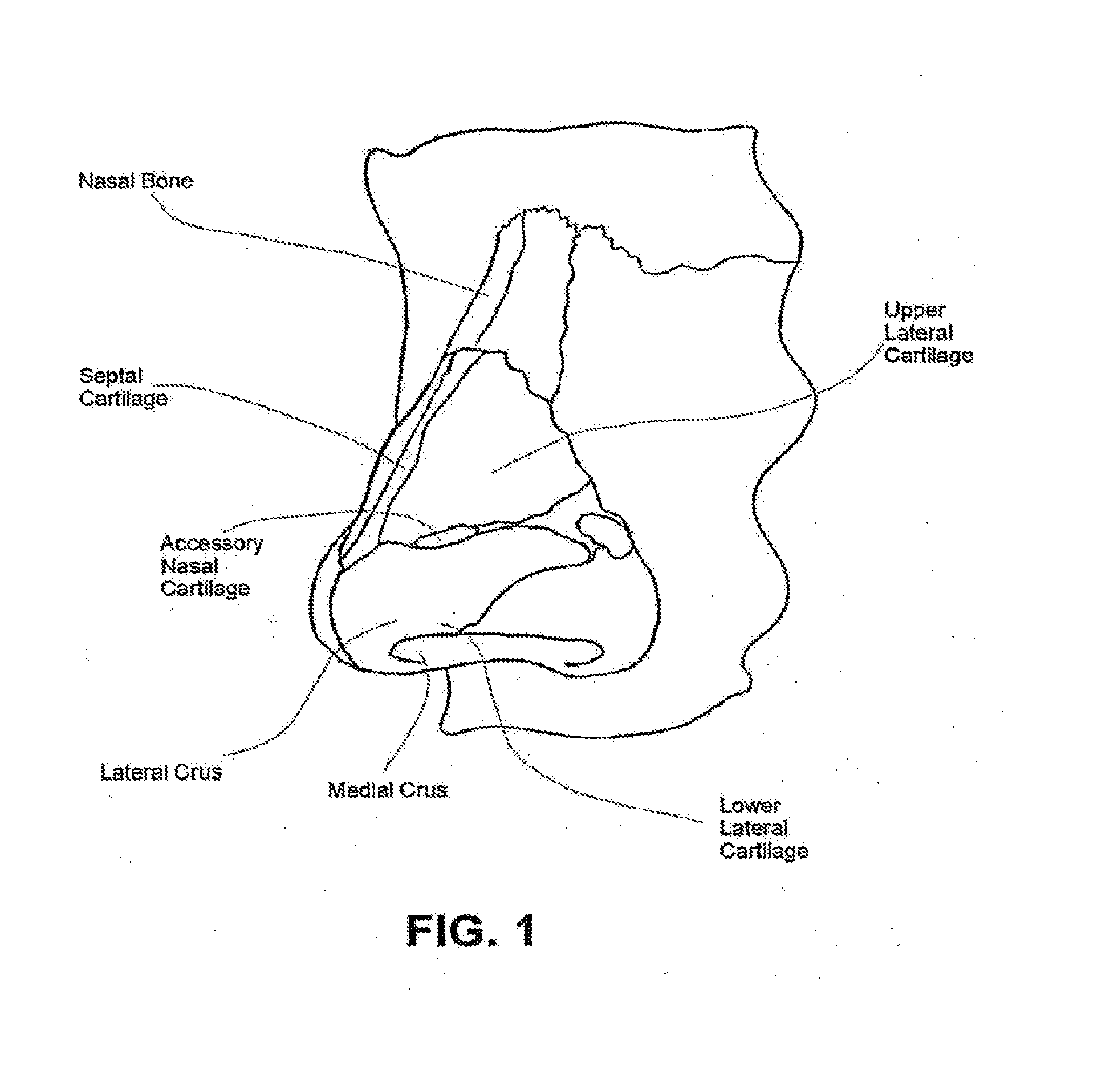
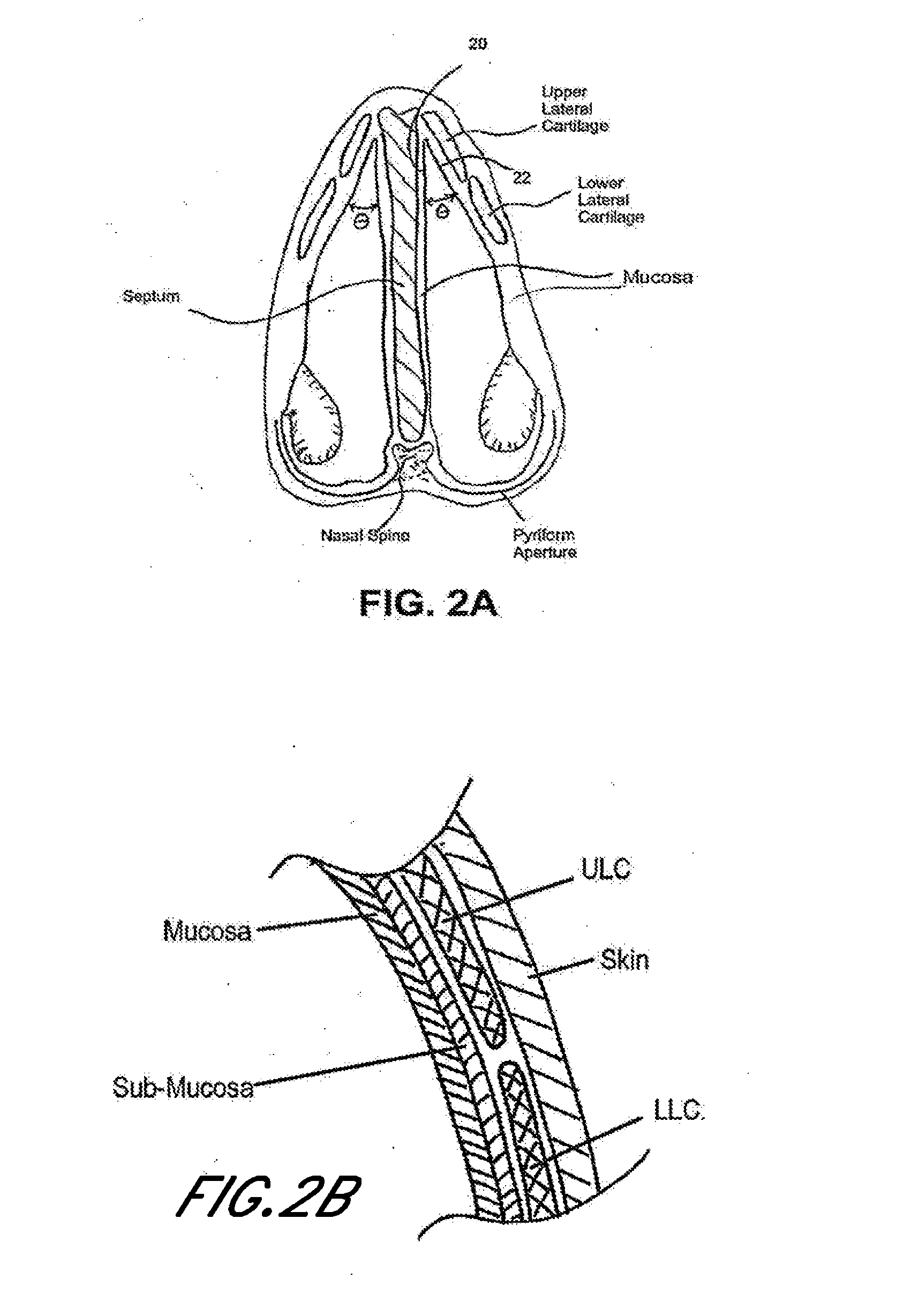
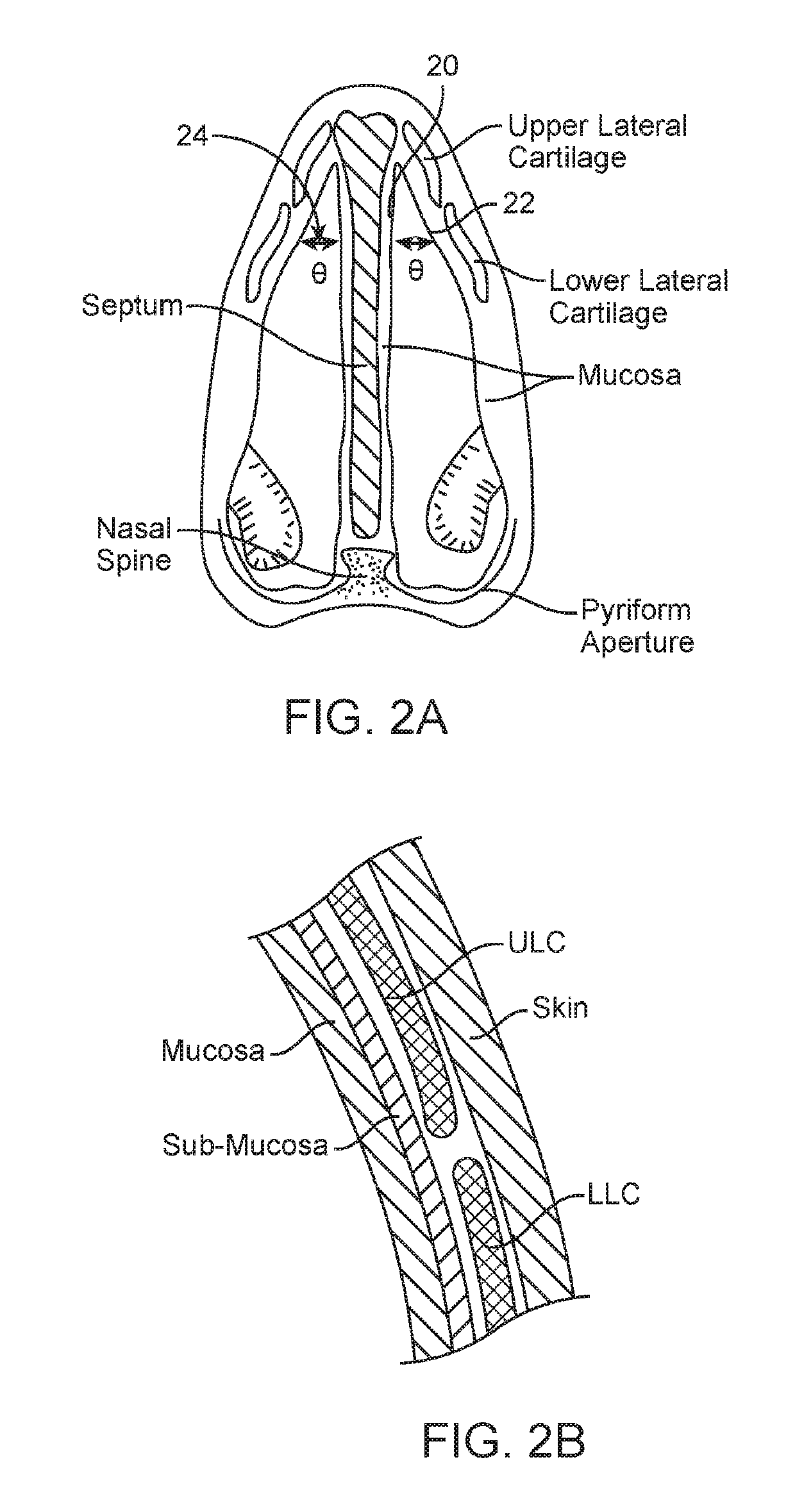
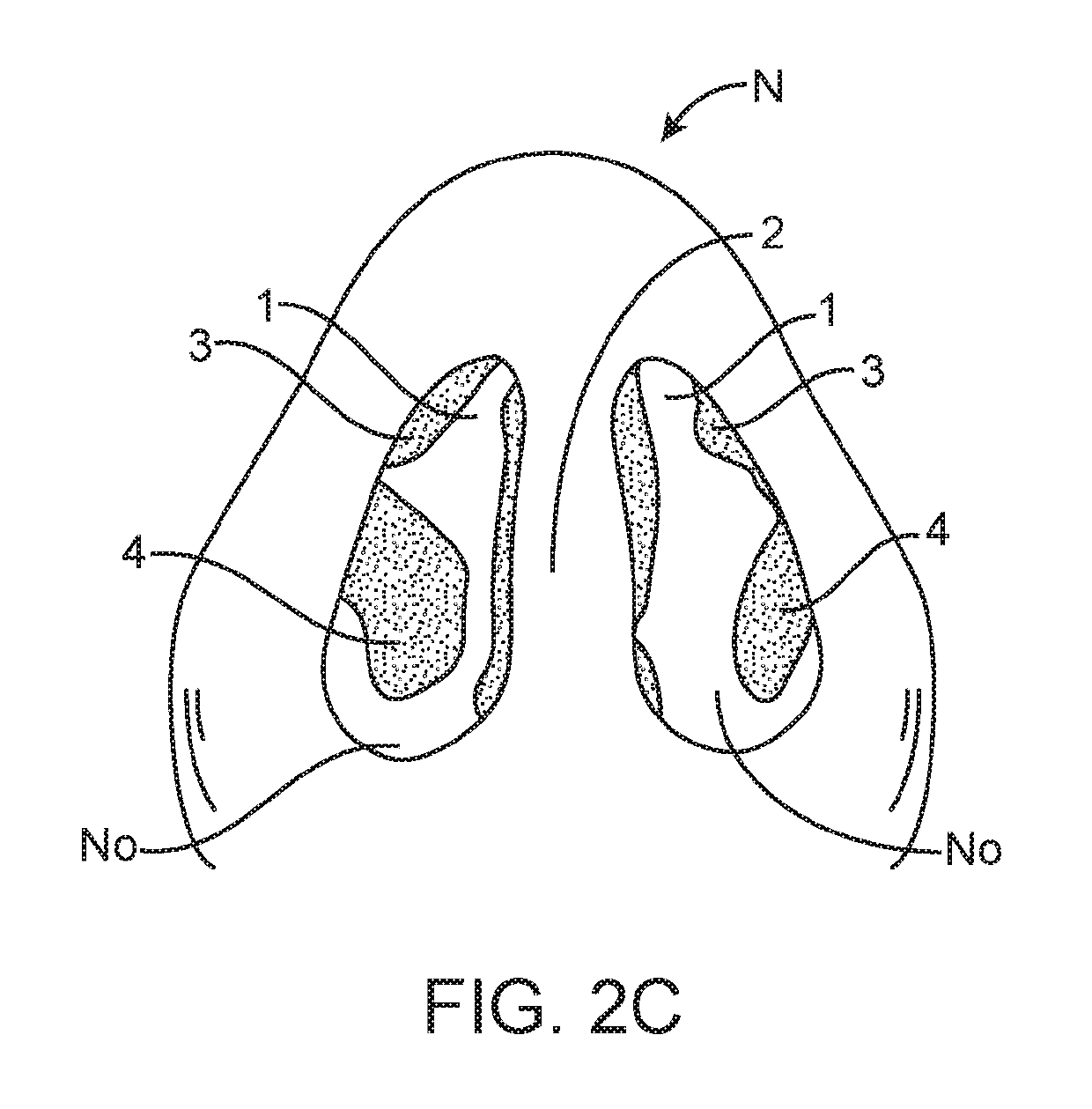
Nasal devices
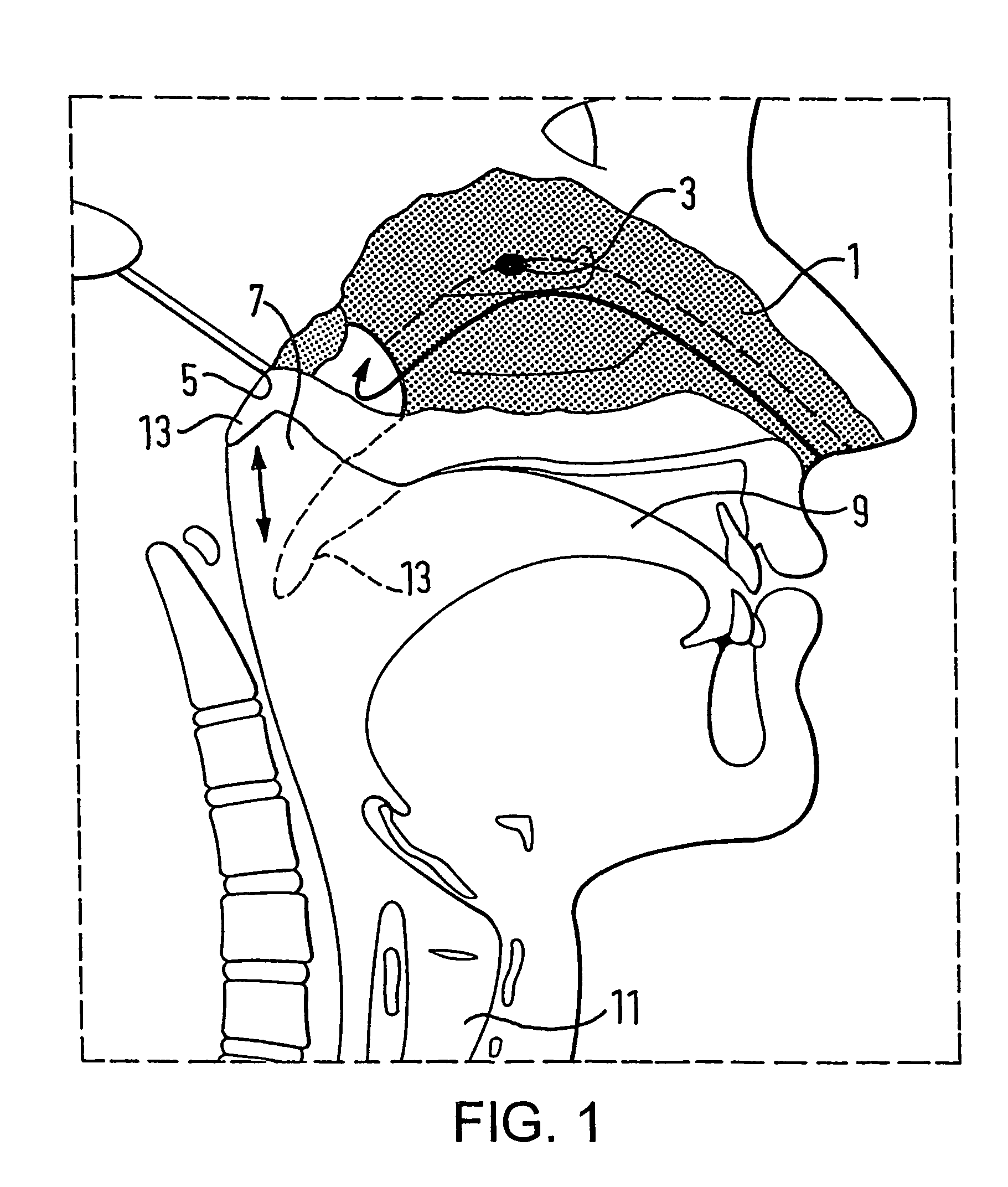
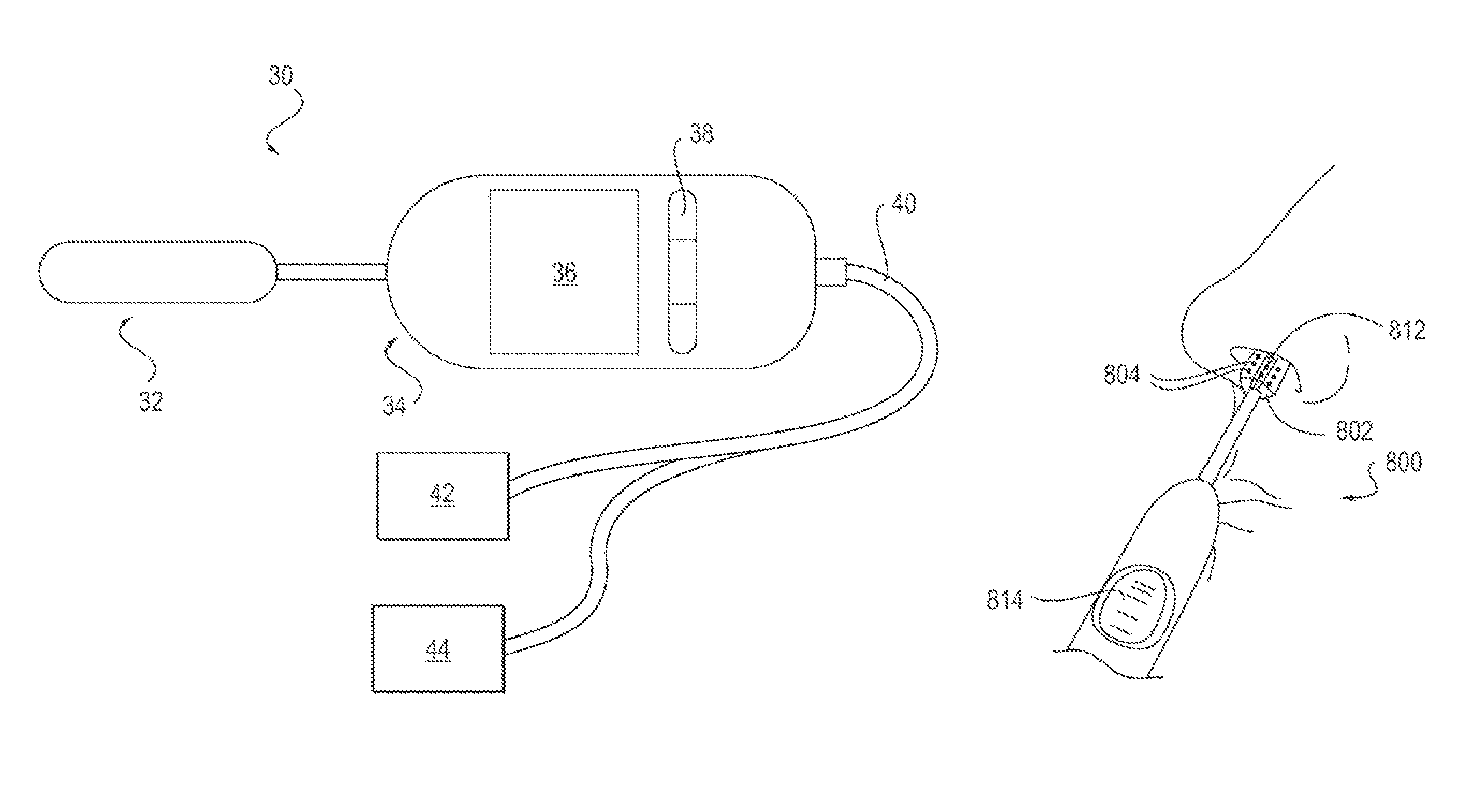
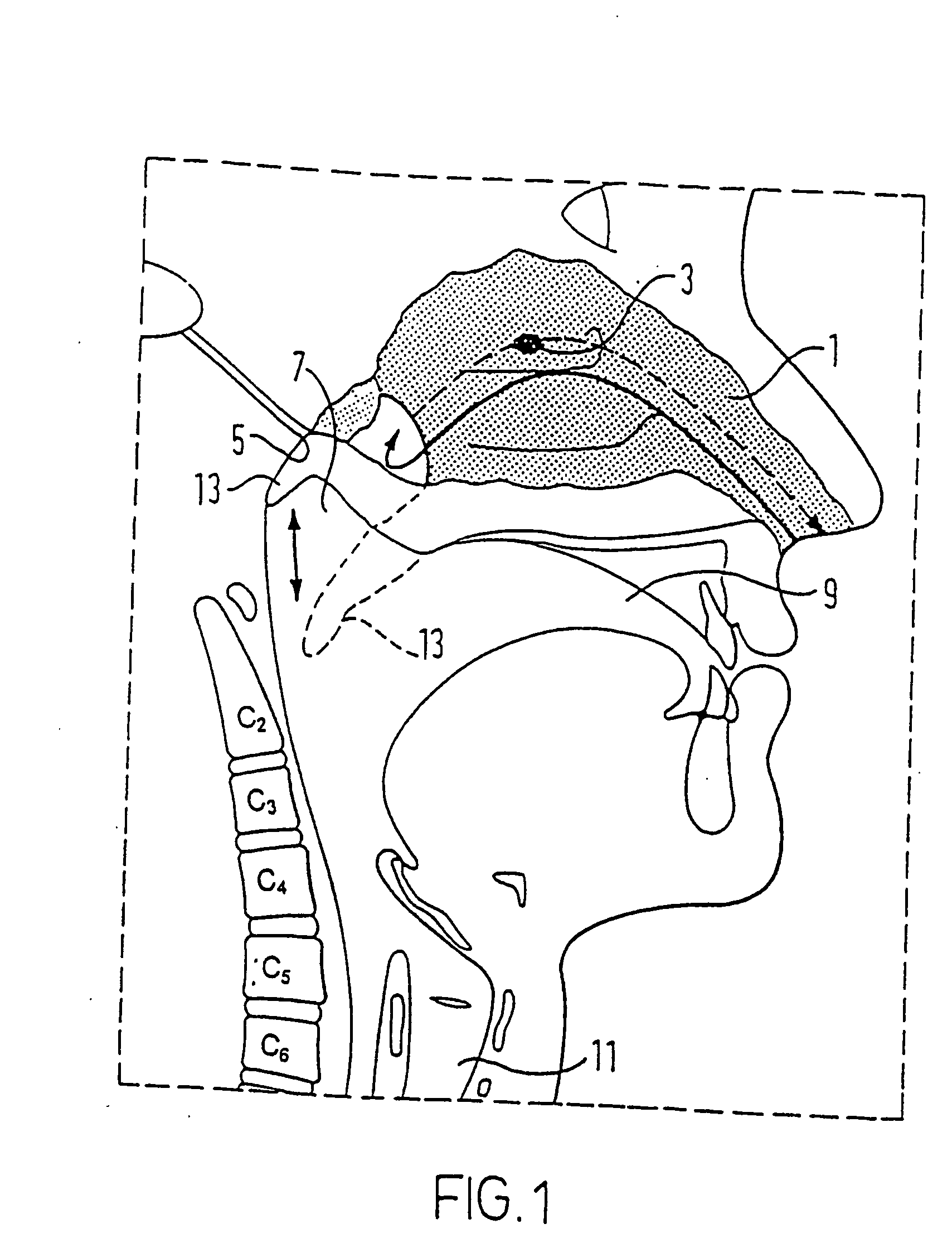
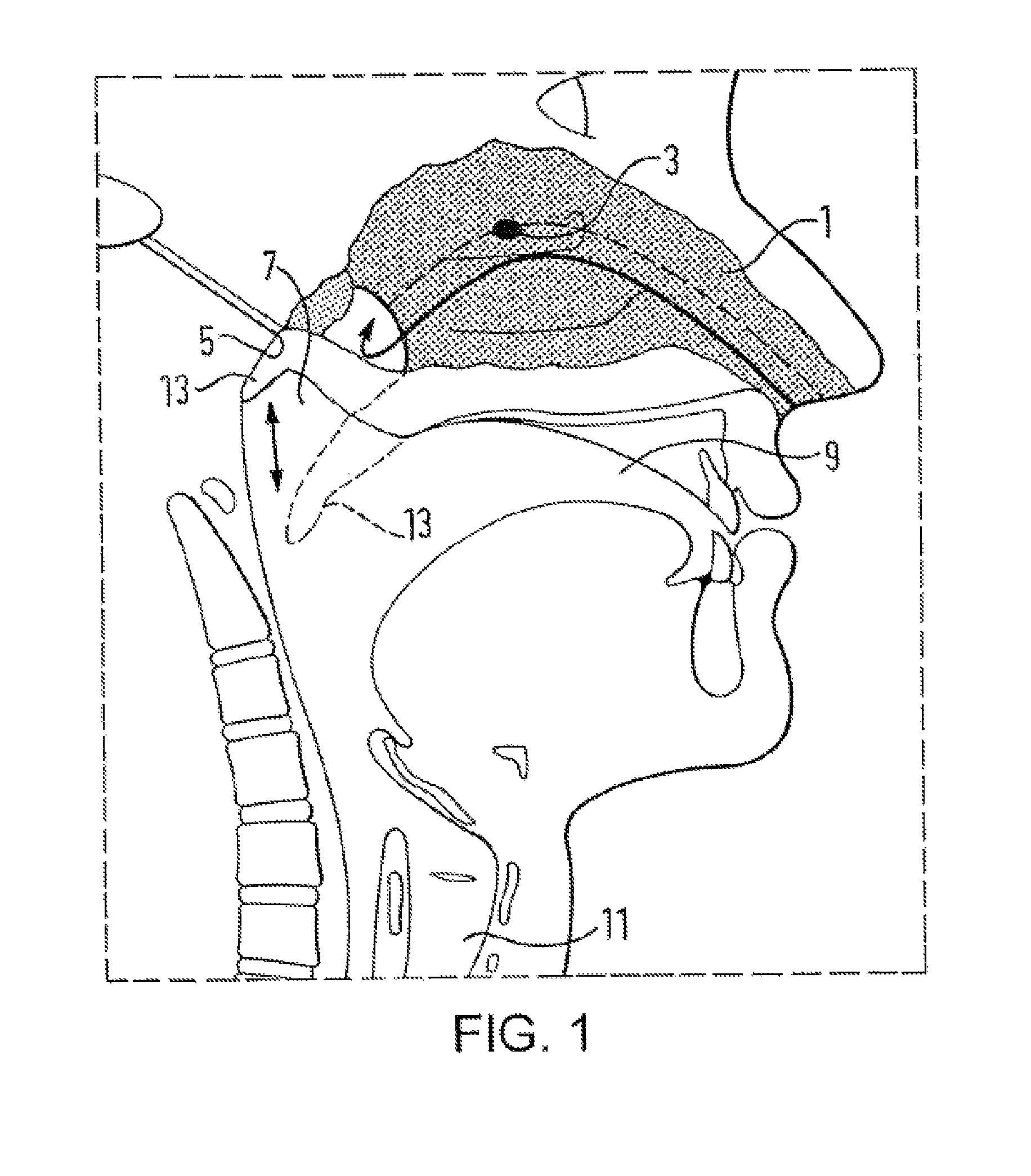
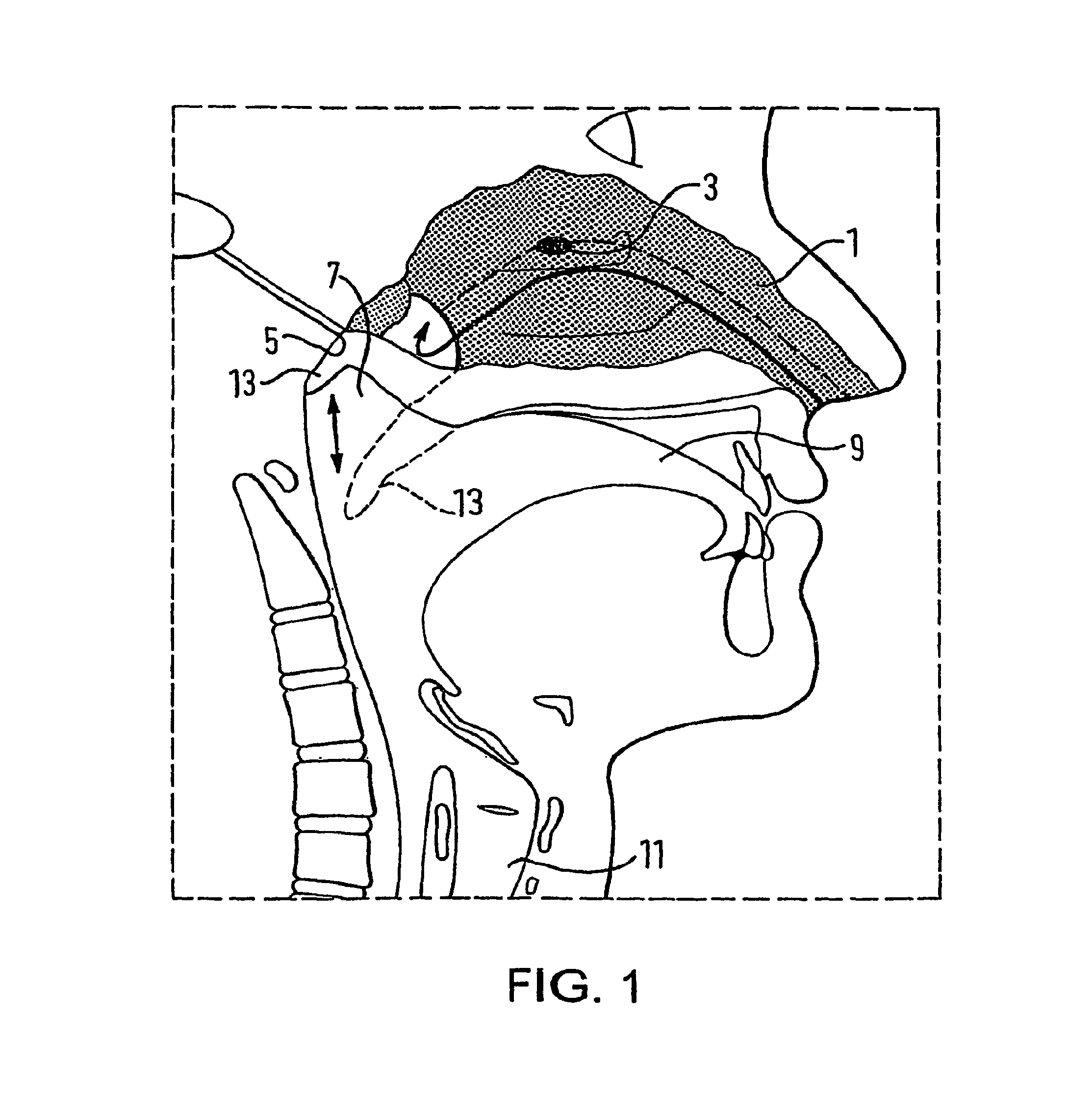
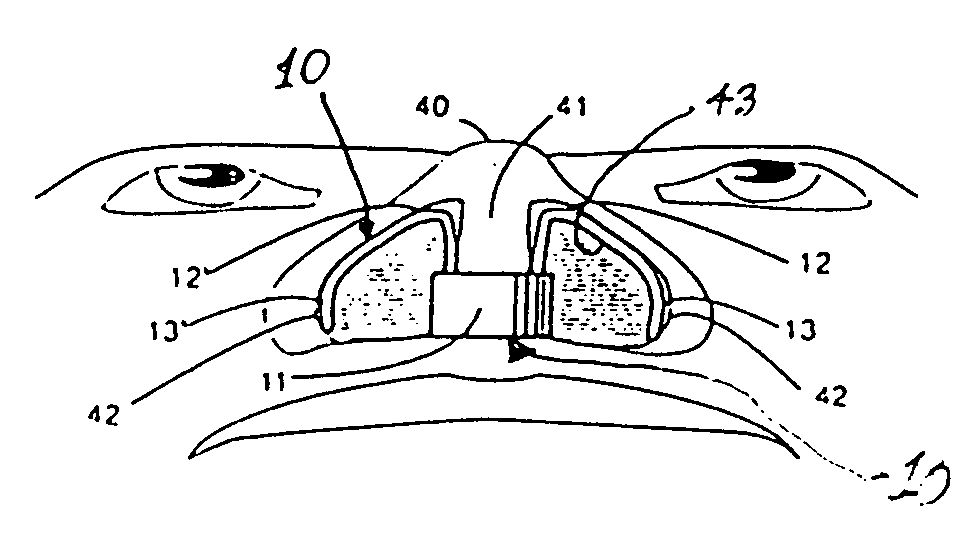
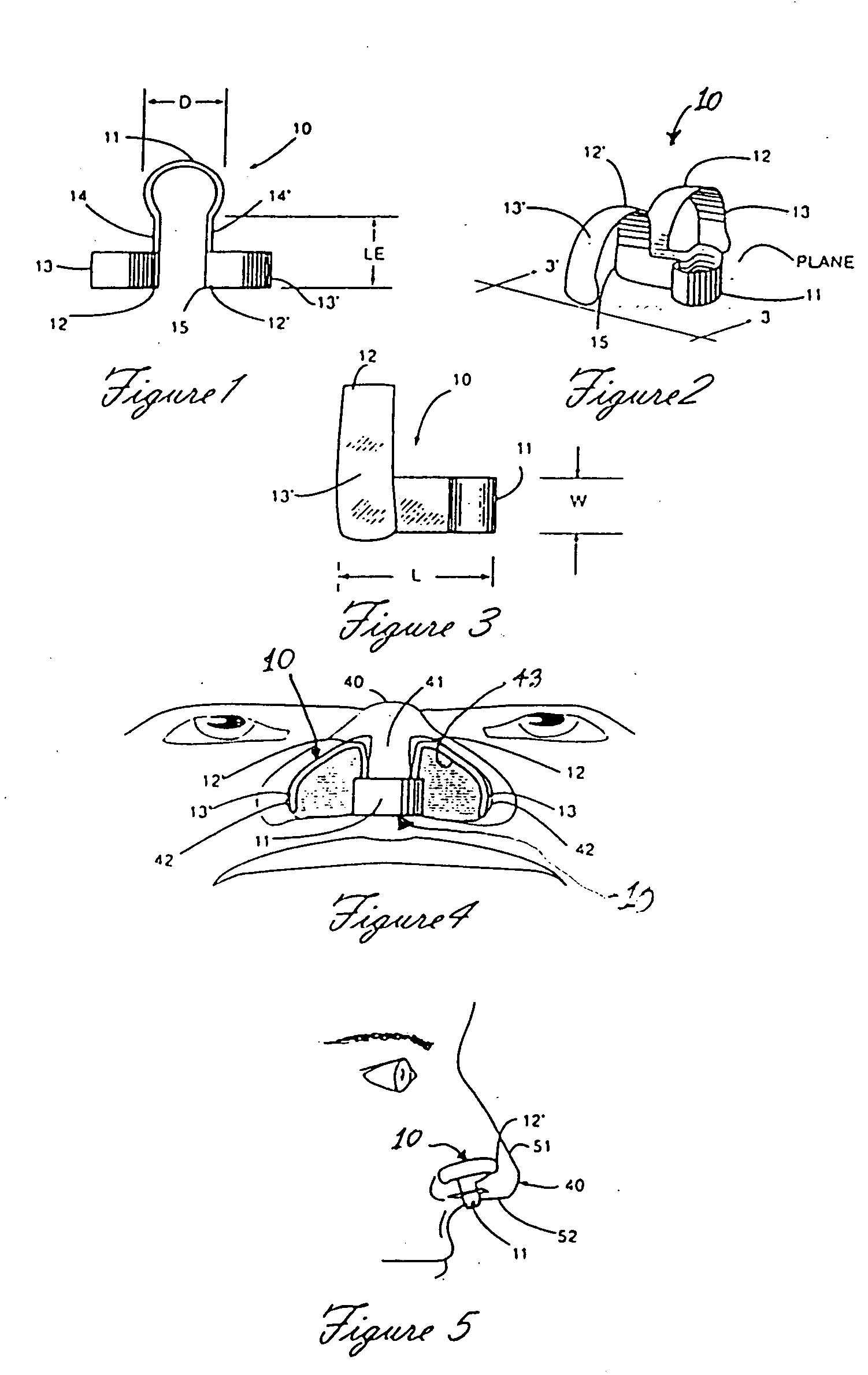
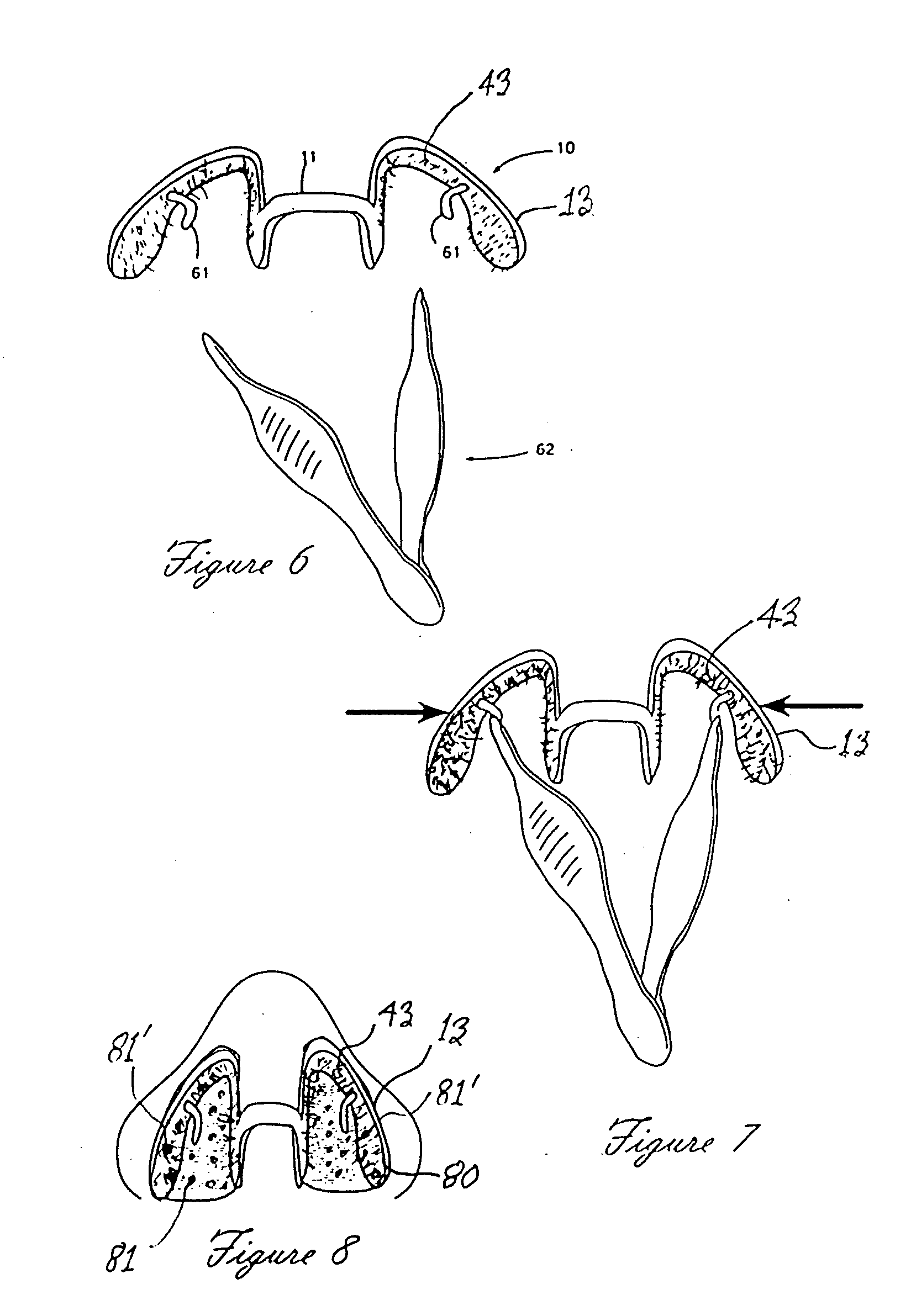
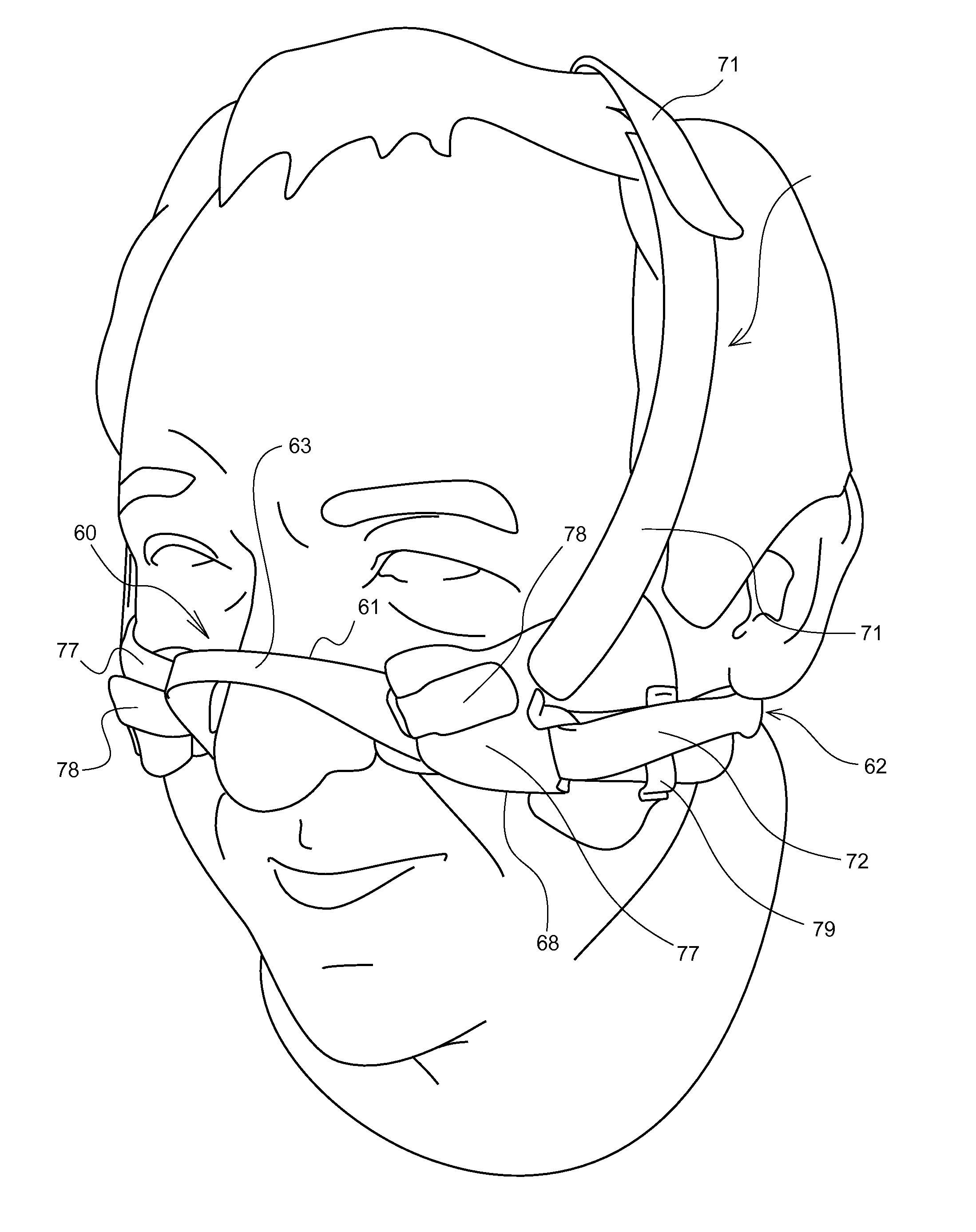
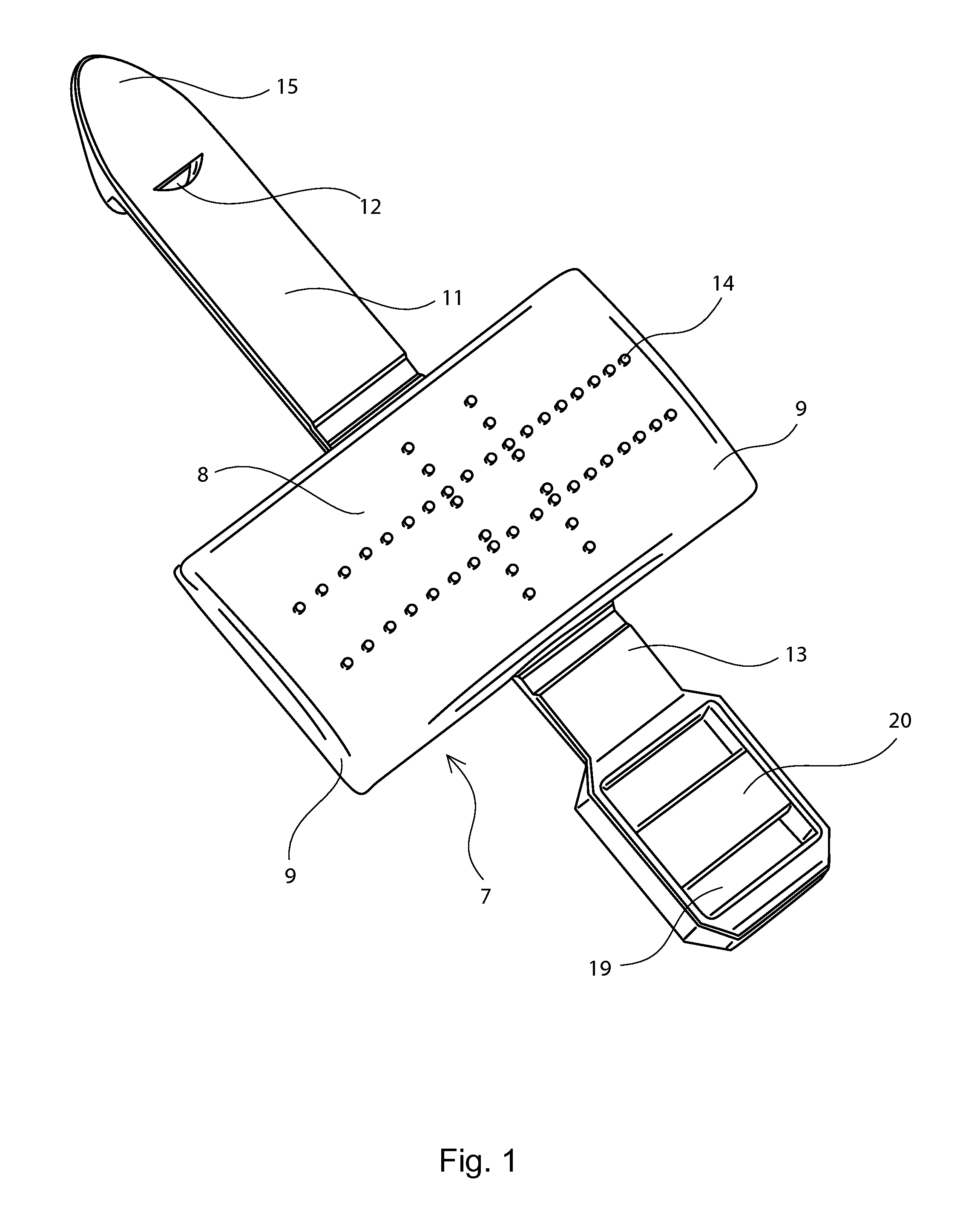
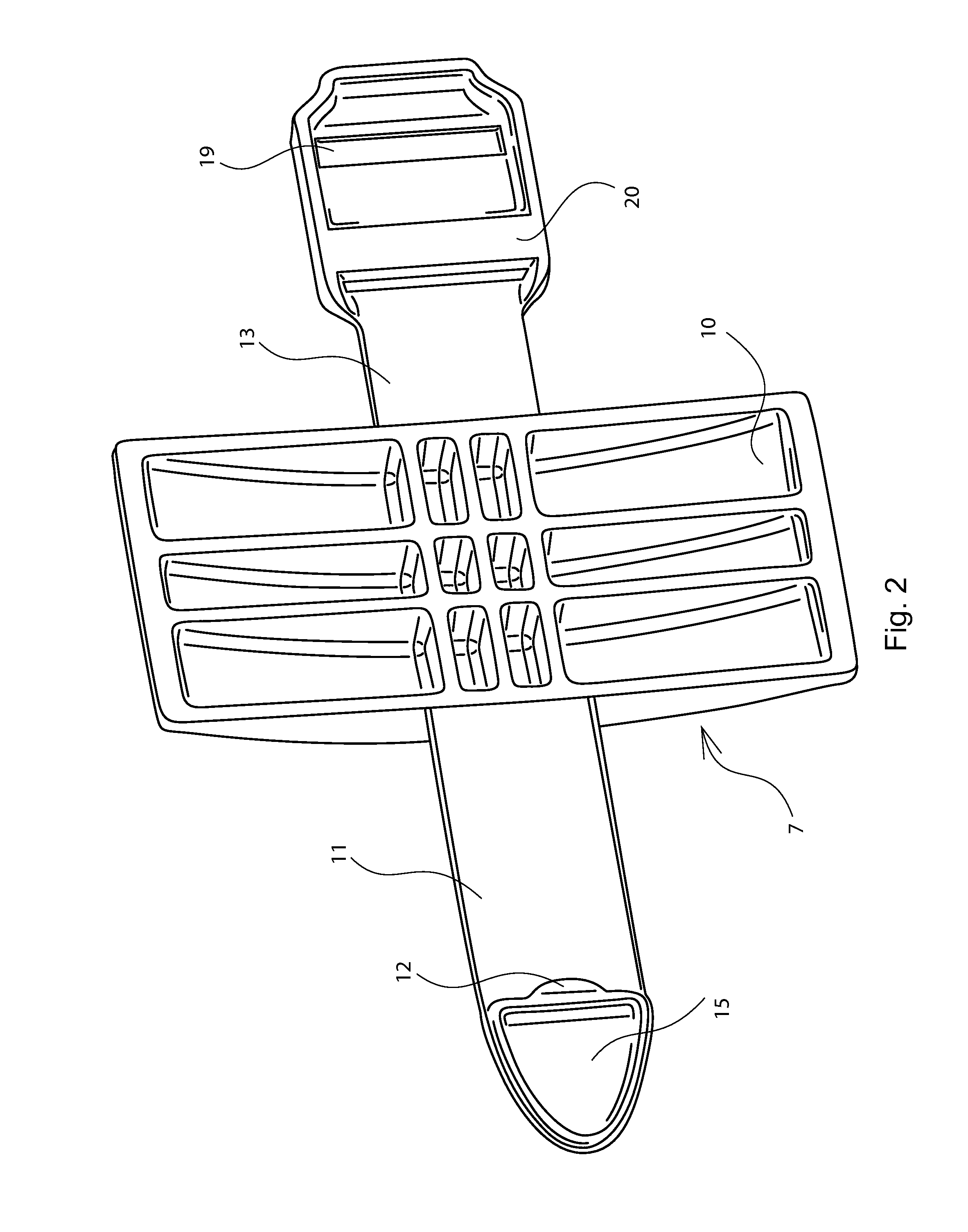
A nasal delivery device and a method of delivering substance to a nasal airway of a subject can be used for mass treatment, especially mass vaccination. The delivery device includes an interface unit, as a replaceable unit, having at least one nosepiece unit for fitting to a respective nostril of a subject, a nozzle from which substance is in use delivered, and at least one delivery unit having a substance supply unit for delivering substance to the nozzle of the at least one nosepiece unit. The delivery device also includes an actuation unit for actuating the at least one delivery unit of the interface unit.
Owner:OPTINOSE INC
Apparatus and method for mask free delivery of an inspired gas mixture and gas sampling
InactiveUS20020017300A1Operating means/releasing devices for valvesRespiratory masksOxygen deliveryInspired gas
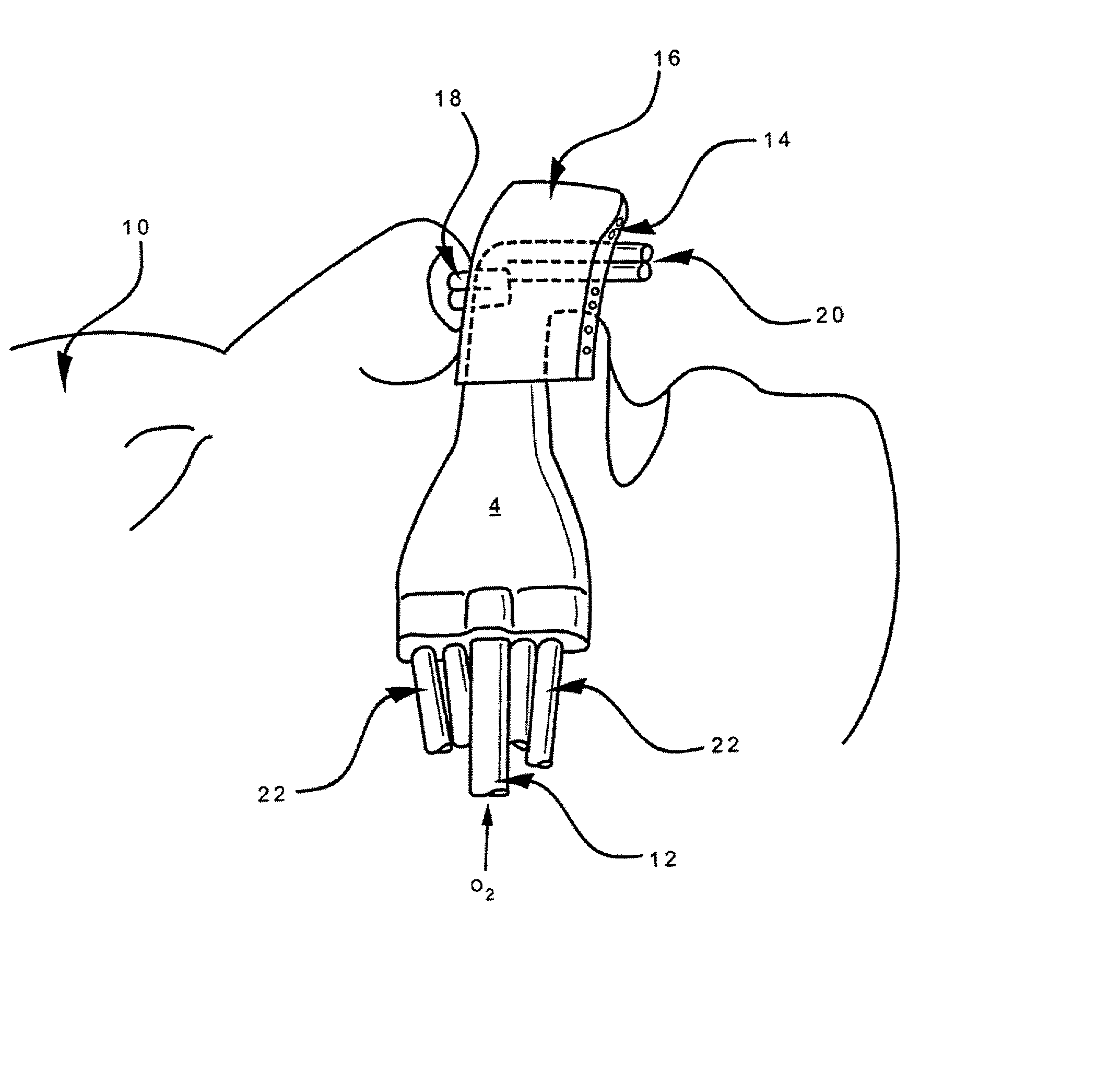
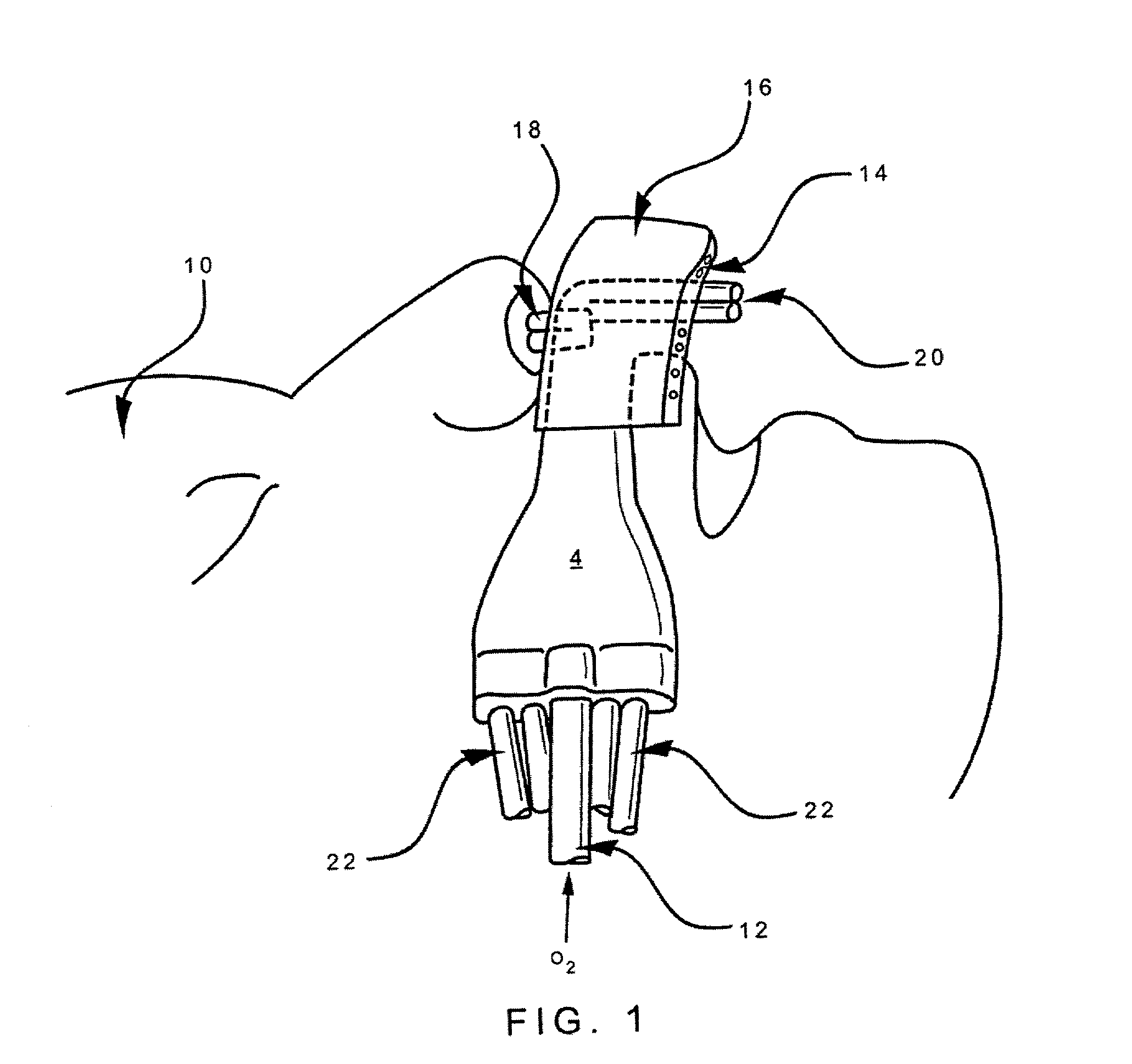
Disclosed is an apparatus and method for the delivery of inspired gas, e.g., supplemental O2, to a person combined with gas sampling, including for the purpose of monitoring of the ventilation of the person. In the invention, the delivery of inspired gas and gas sampling are accomplished without the use of a sealed face mask. The apparatus of one embodiment of the present invention comprises an oxygen delivery device, nasal airway pressure sampling devices, optionally an oral airway pressure sampling device and at least one pressure analyzer connected to the sampling devices which determine the phase of the person's respiration cycle and the person's primary airway. The oxygen delivery device is connected to a controller such that it delivers a higher flow of oxygen to the person during the inhalation phase of the person's respiratory cycle. The invention thus increases end tidal oxygen concentrations. The invention further comprises carbon dioxide sampling tubes that continuously sample gas from two nasal sites and the mouth. The nasal sampling tubes are connected to a switching valve that is in turn connected to a capnometer which determines carbon dioxide concentration during exhalation. The oral gas sampling site is connected to a second capnometer.
Owner:SCOTT LAB
Mask free delivery of oxygen and ventilatory monitoring
InactiveUS6938619B1Increase oxygen concentrationFast sensingOperating means/releasing devices for valvesRespiratory masksOxygen deliveryAirway devices
Disclosed is an apparatus and method for the delivery of supplemental oxygen gas to a person combined with the monitoring of the ventilation of the person with both being accomplished without the use of a sealed face mask. Preferred embodiments of the present invention combine an oxygen delivery device, a nasal airway pressure sampling device, an oral airway pressure sampling device, and a pressure analyzer connected to the sampling devices to determine the phase of the person's respiration cycle and the person's primary airway. The oxygen delivery device is connected to a controller such that it delivers a higher flow of oxygen to the person during the inhalation phase of the person's respiratory cycle. The invention thus increases end tidal oxygen concentrations with improved efficiency comparative to known open airway devices. Embodiments of the invention can include carbon dioxide sampling tubes that continuously sample air from the nose and mouth to determine carbon dioxide concentration during exhalation.
Owner:SCOTT LAB
Methods and devices to treat nasal airways
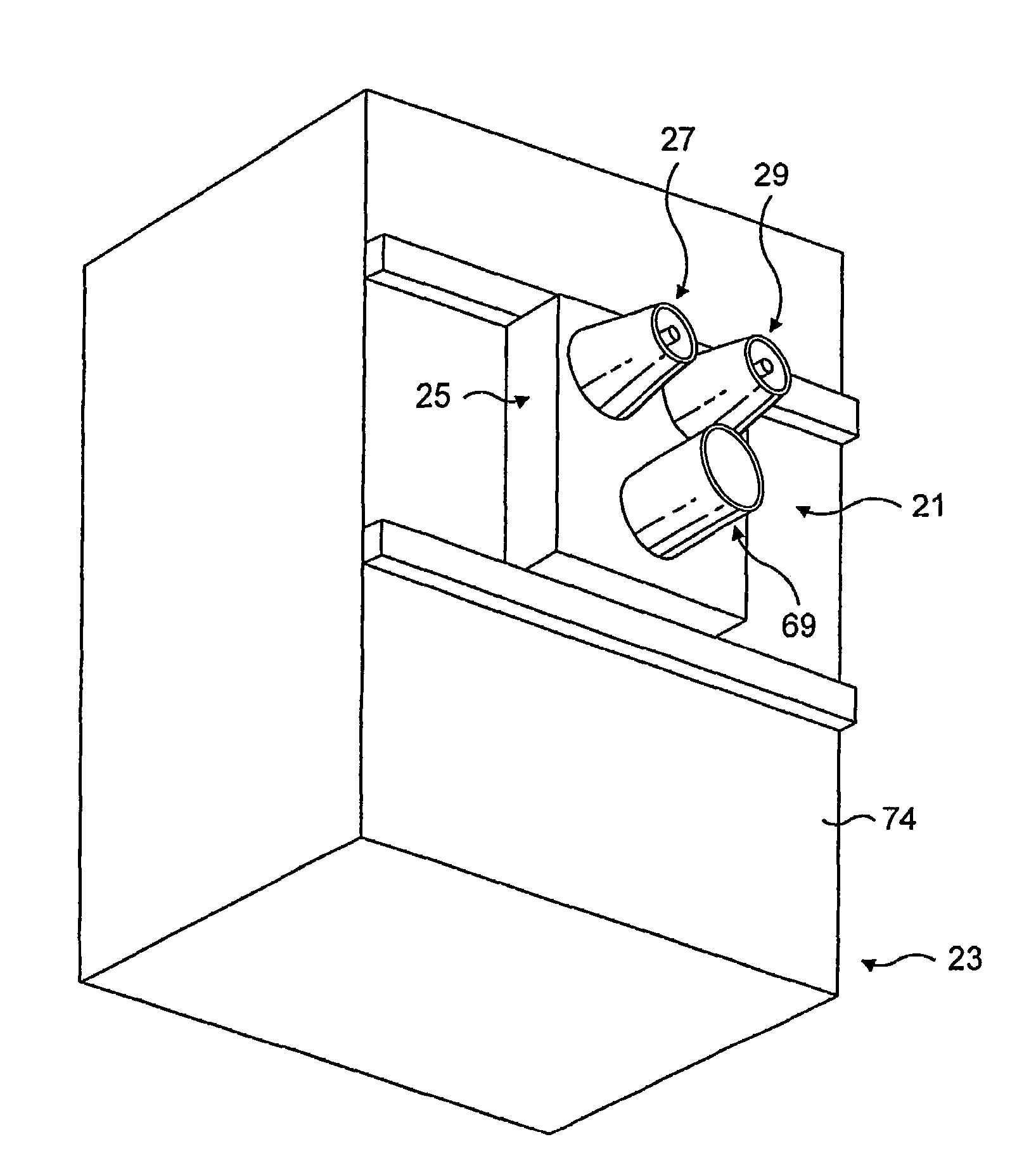
ActiveUS8986301B2Increase ratingsWithout weakeningUltrasound therapyElectrotherapyNostrilSurgical incision
Owner:AERIN MEDICAL INC
Methods and devices to treat nasal airways
ActiveUS20140088463A1Decrease airflow resistance perceived airflowIncrease ratingsElectrotherapySurgical needlesSmall airwaysNostril
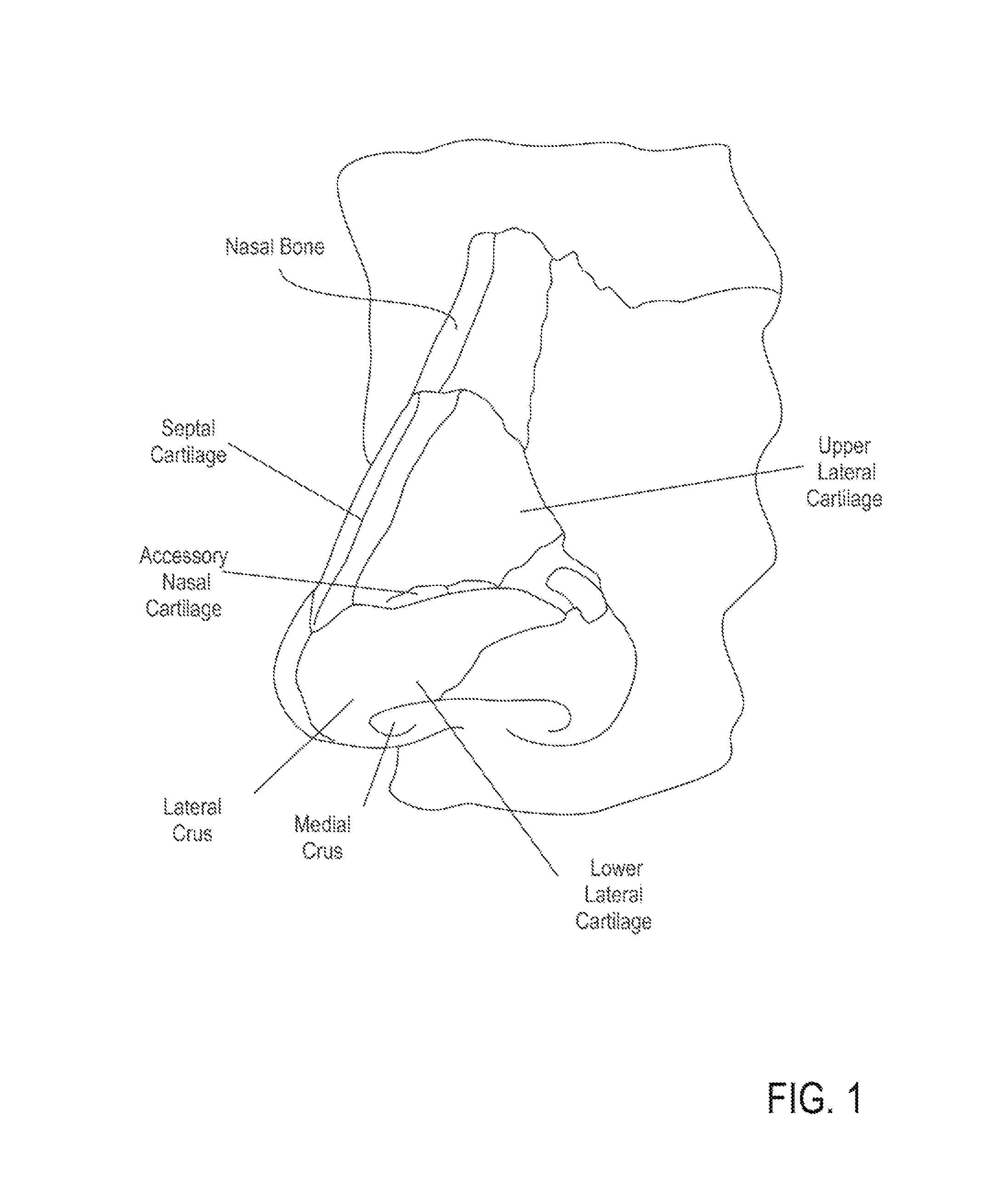
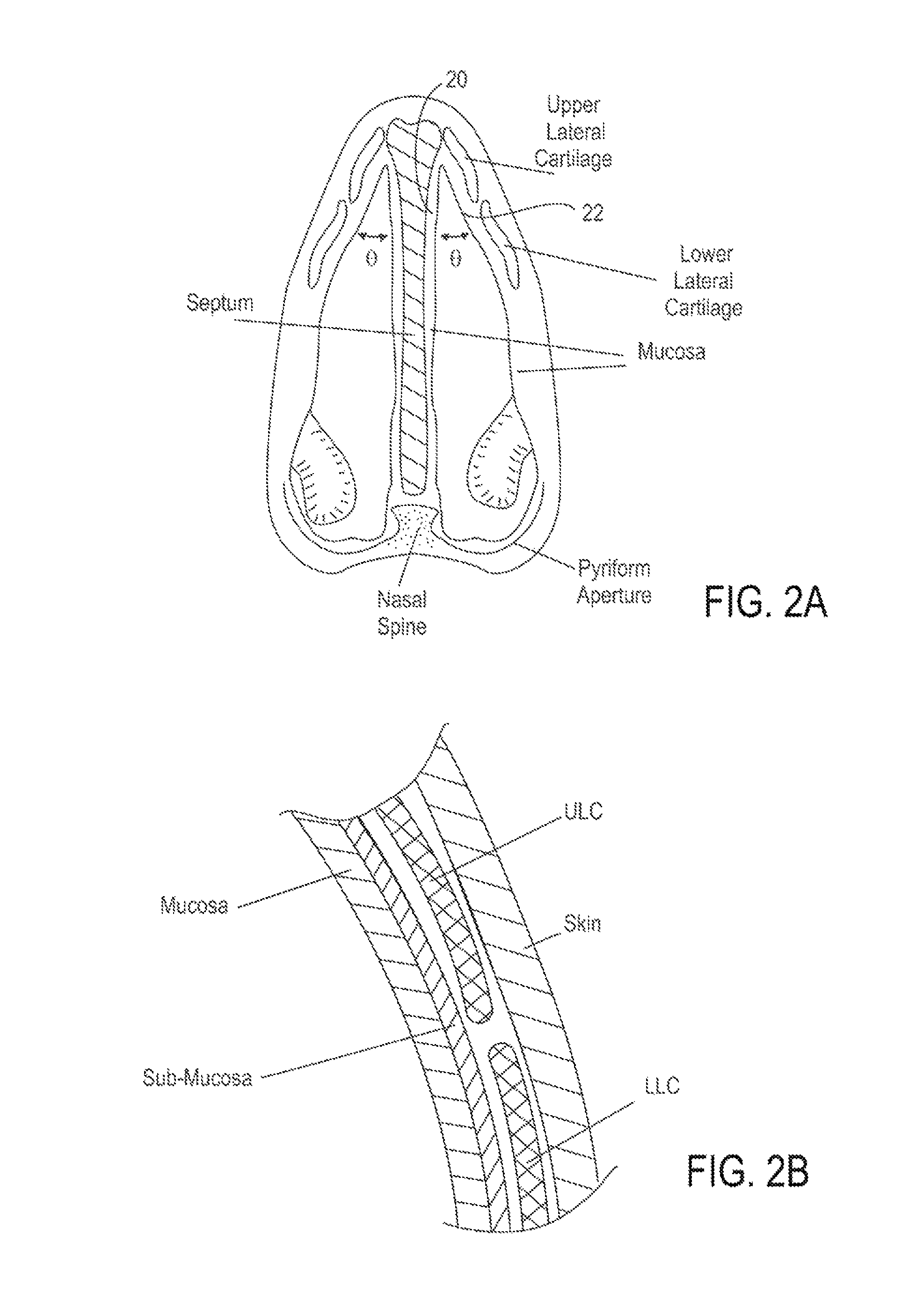
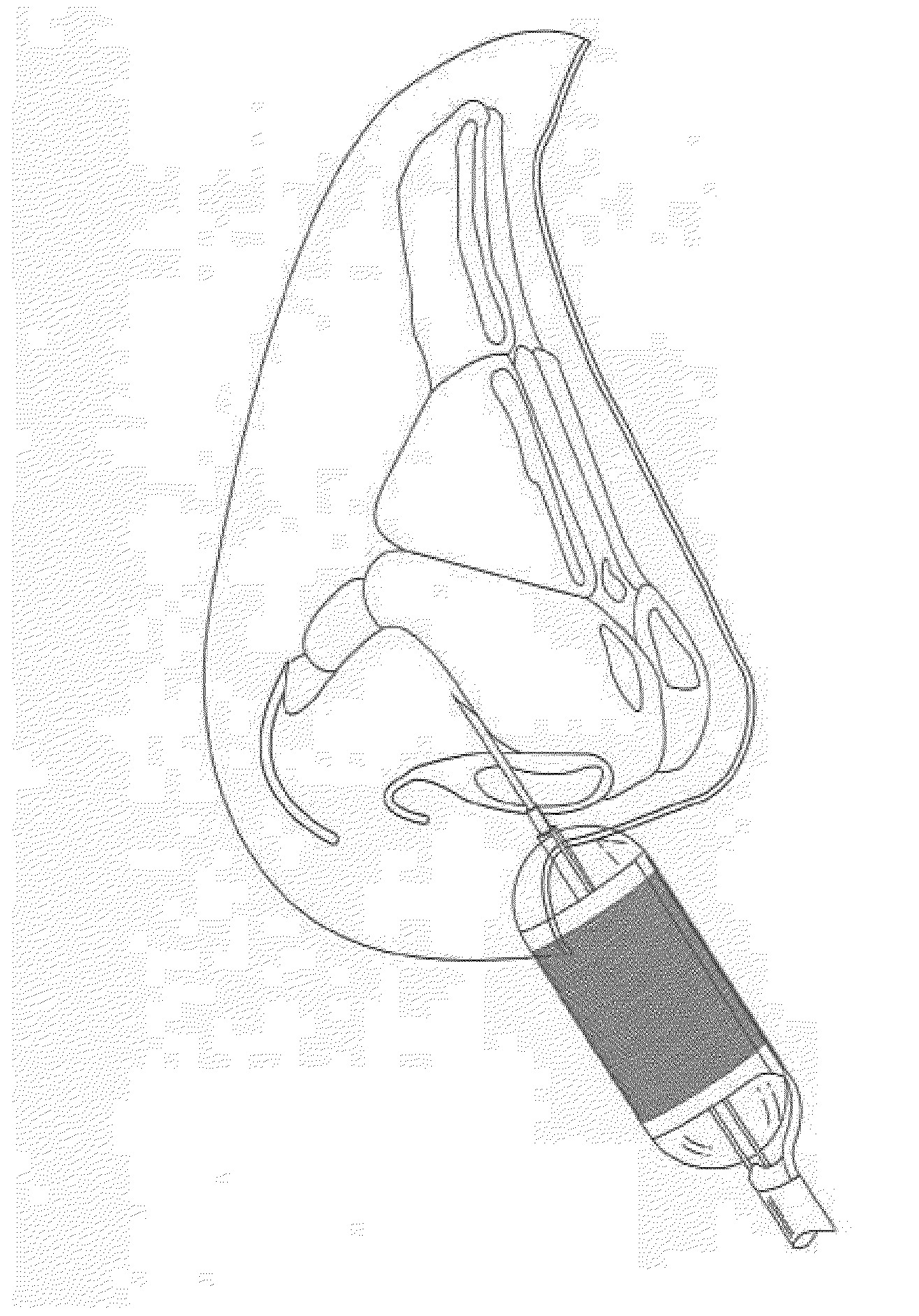
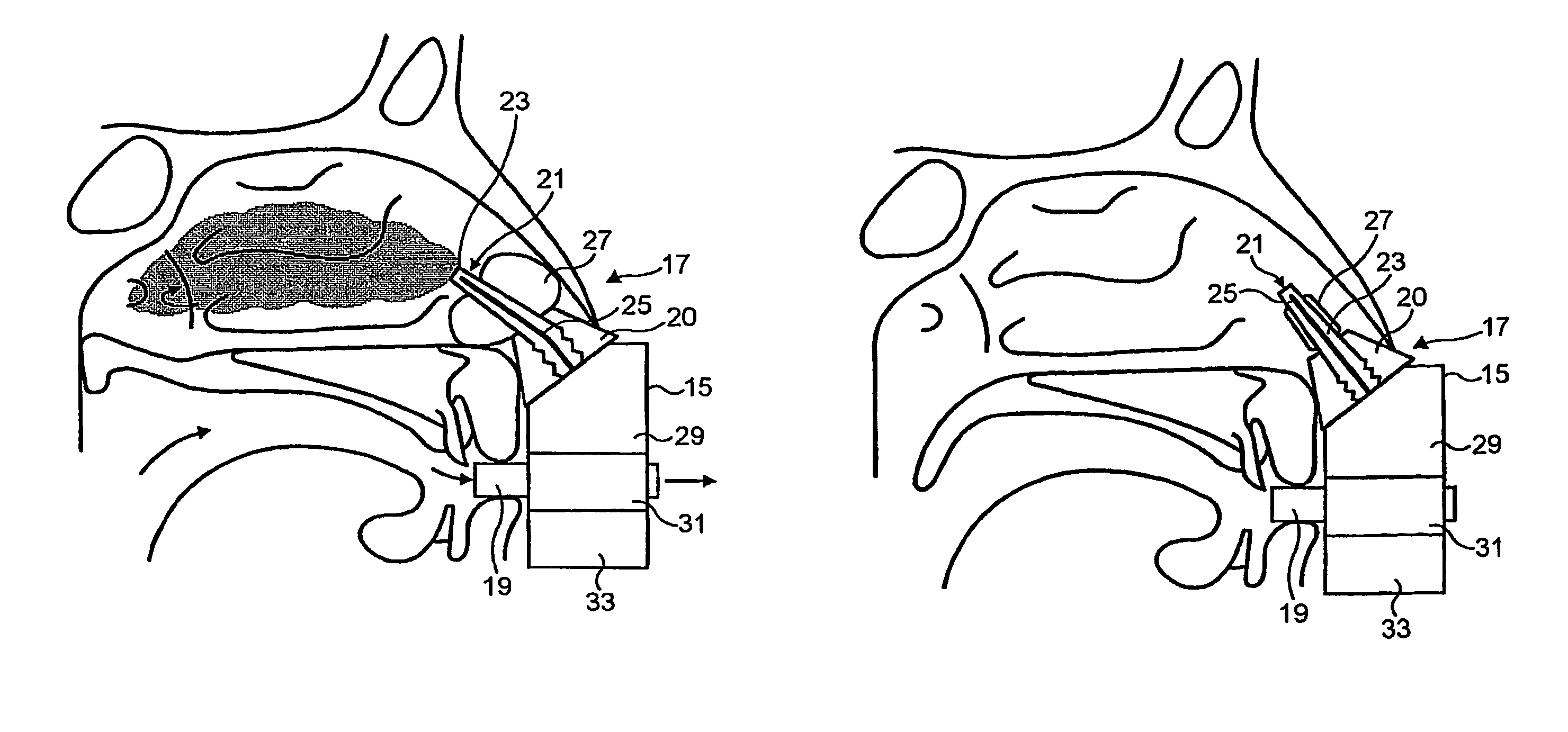
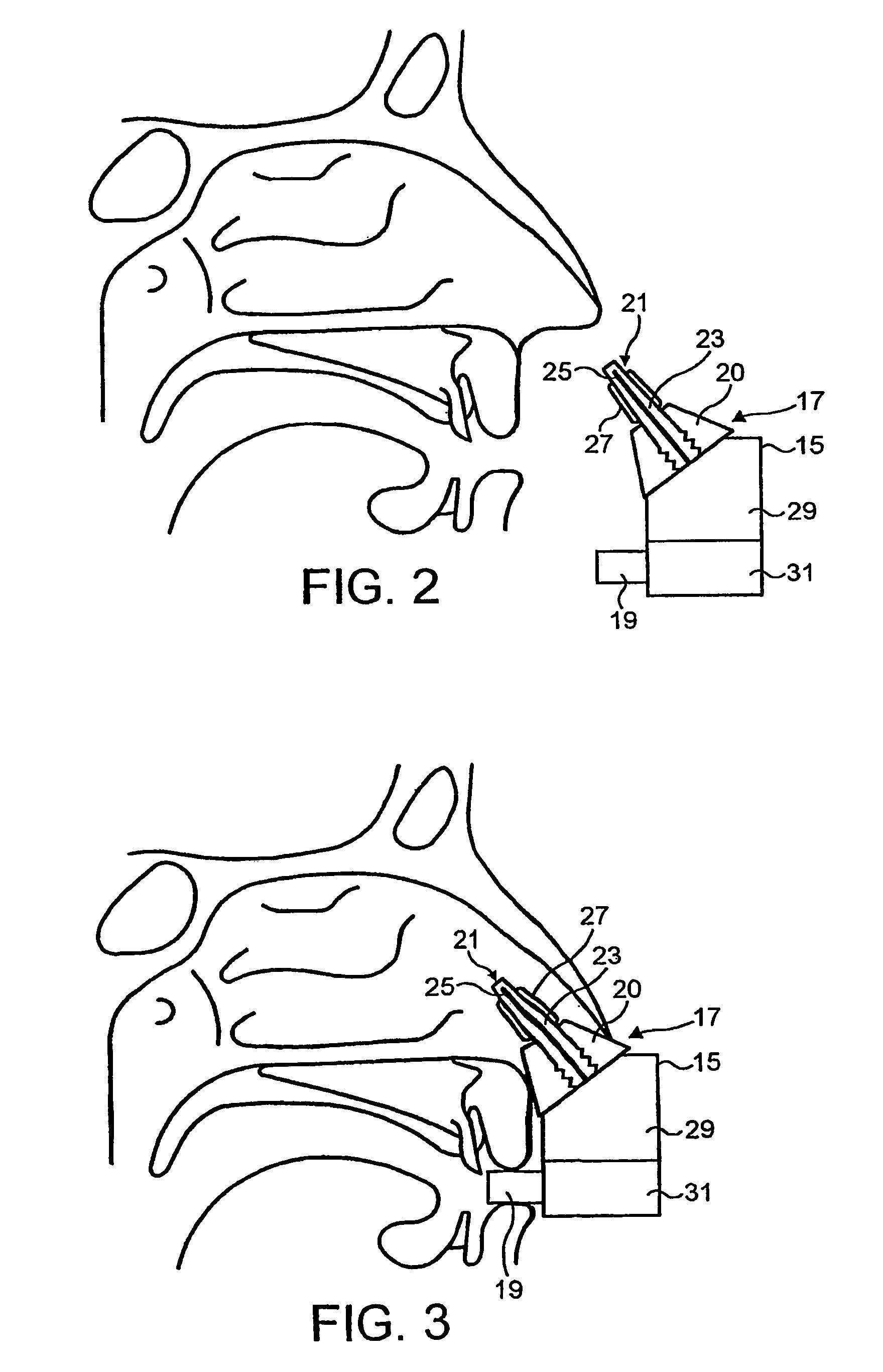
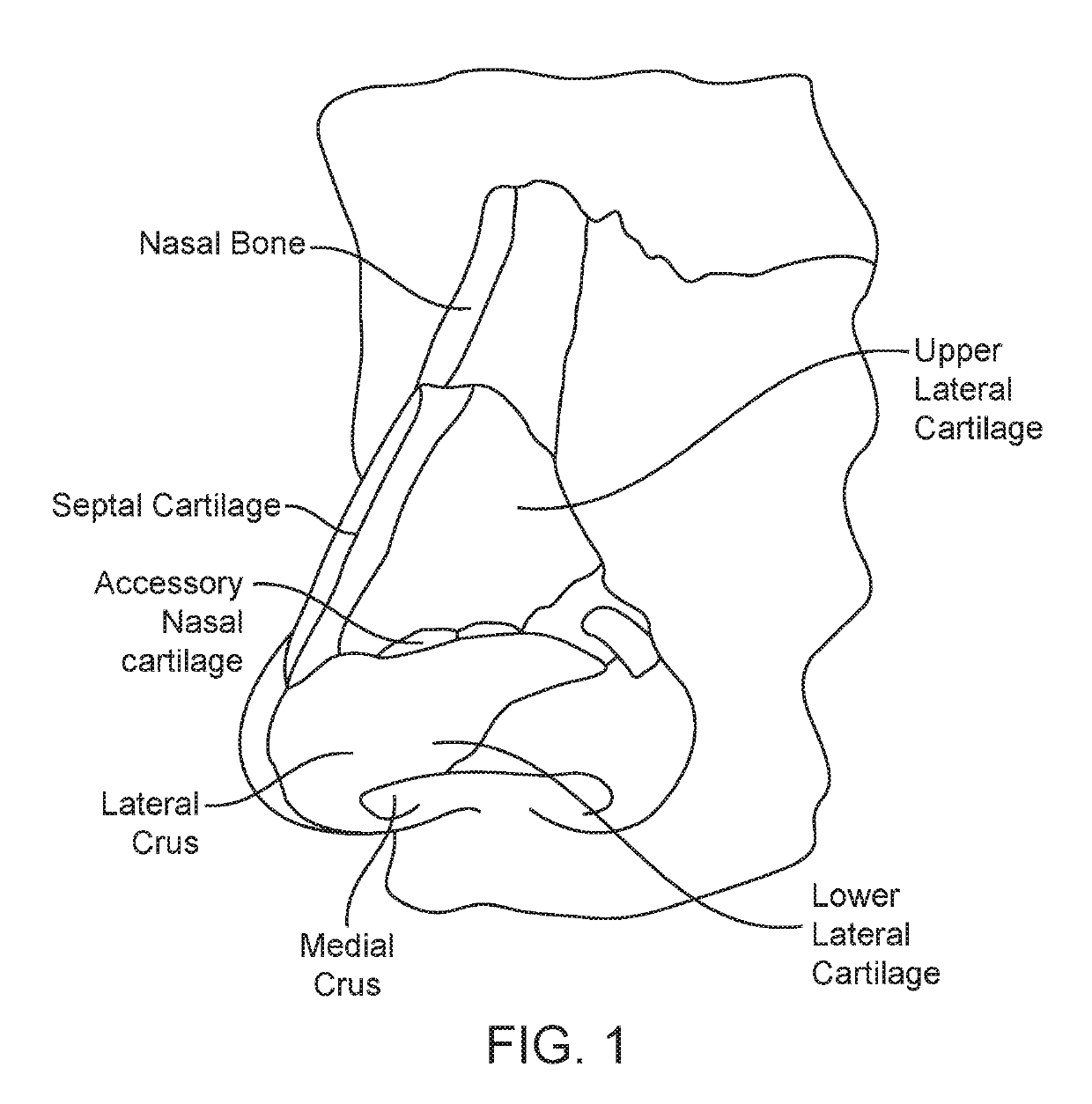
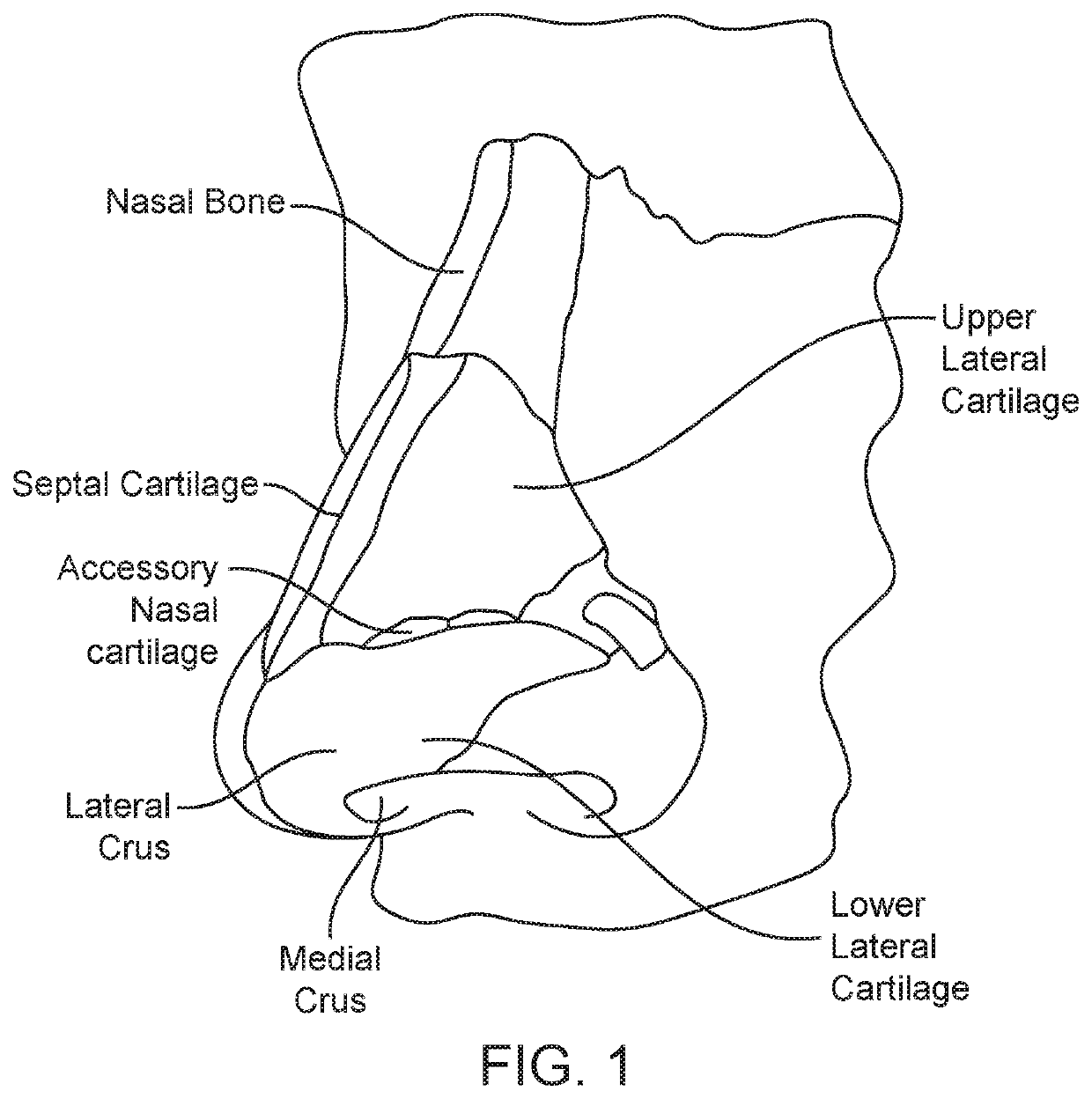
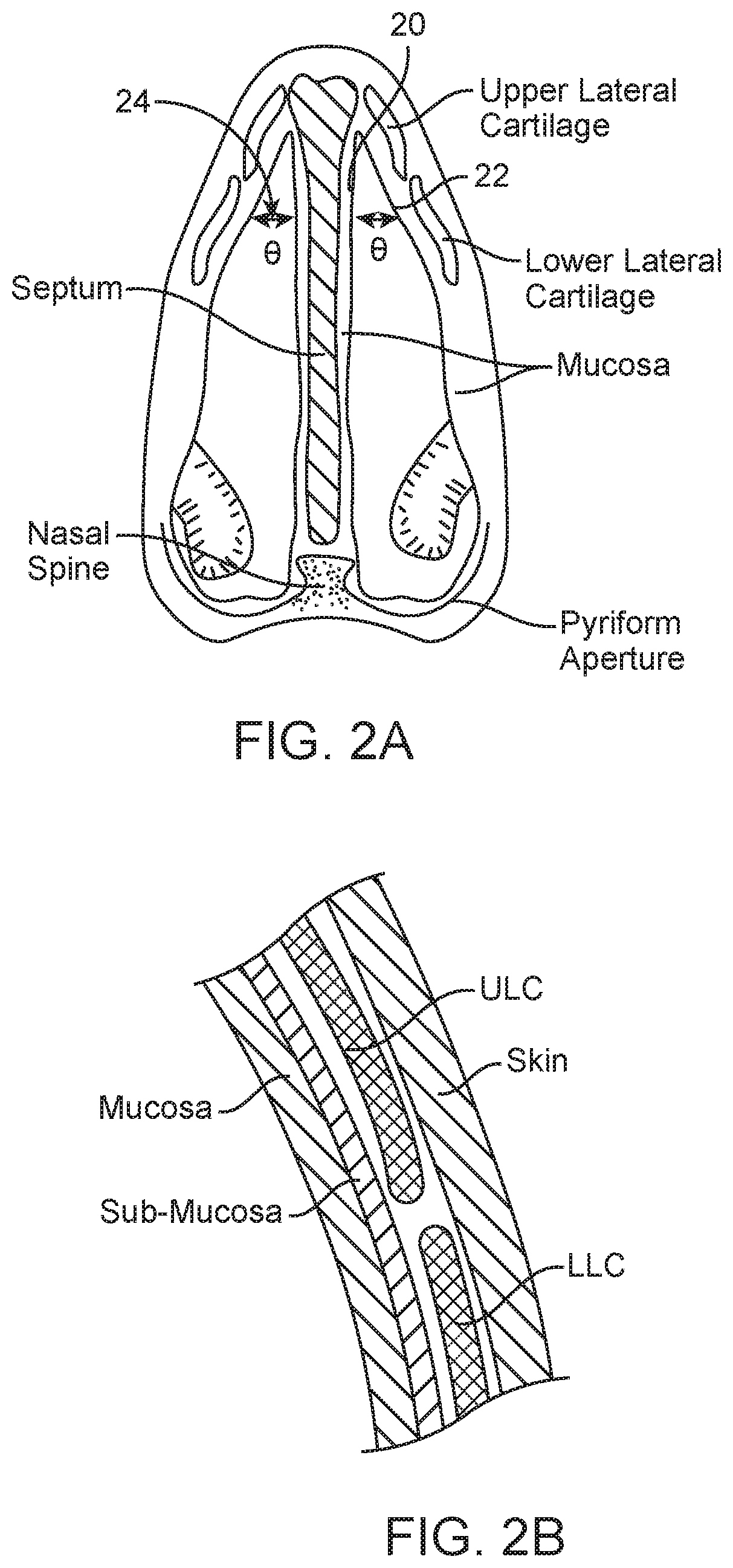
A method is described for modifying at least one property of a tissue of or near a nasal valve of a nose, without using a surgical incision or an implant, to decrease airflow resistance or perceived airflow resistance in a nasal airway. The method may involve contacting a treatment element of a treatment device with the at least one tissue inside the nasal airway, with sufficient force to at least temporarily deform the at least one tissue, applying energy to, or removing energy from, the at least one tissue, using the treatment element, and removing the treatment element from the nostril.
Owner:AERIN MEDICAL
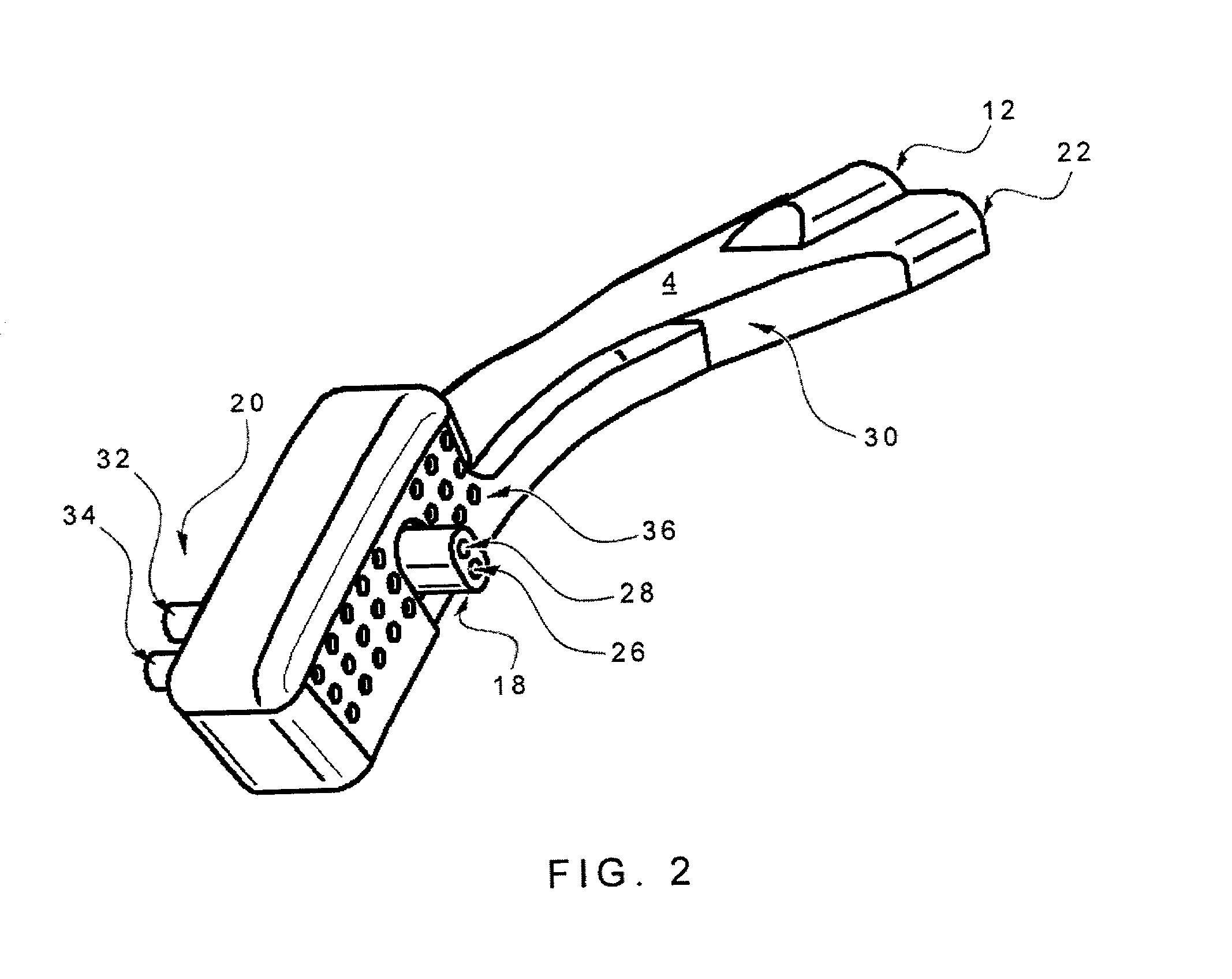
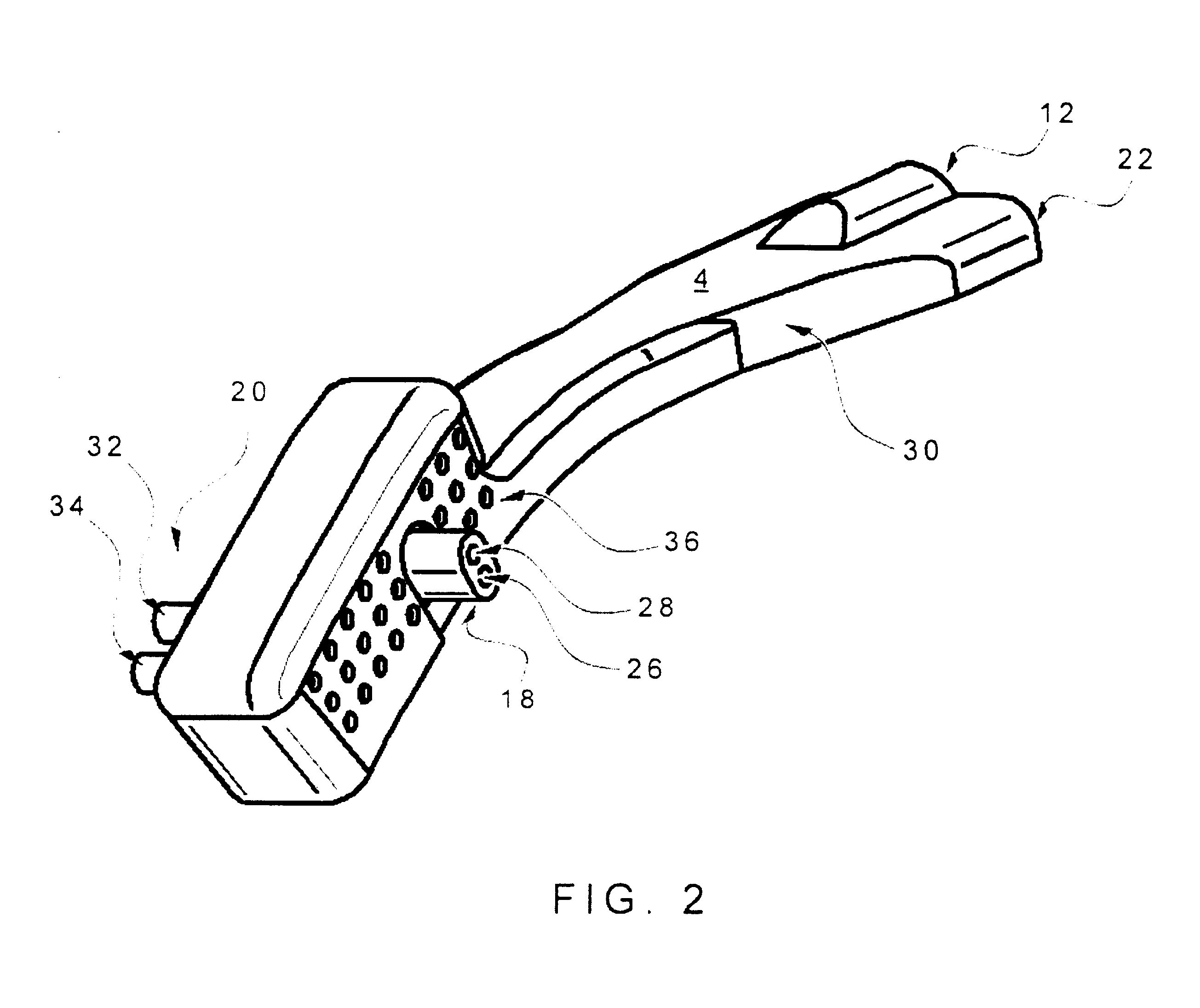
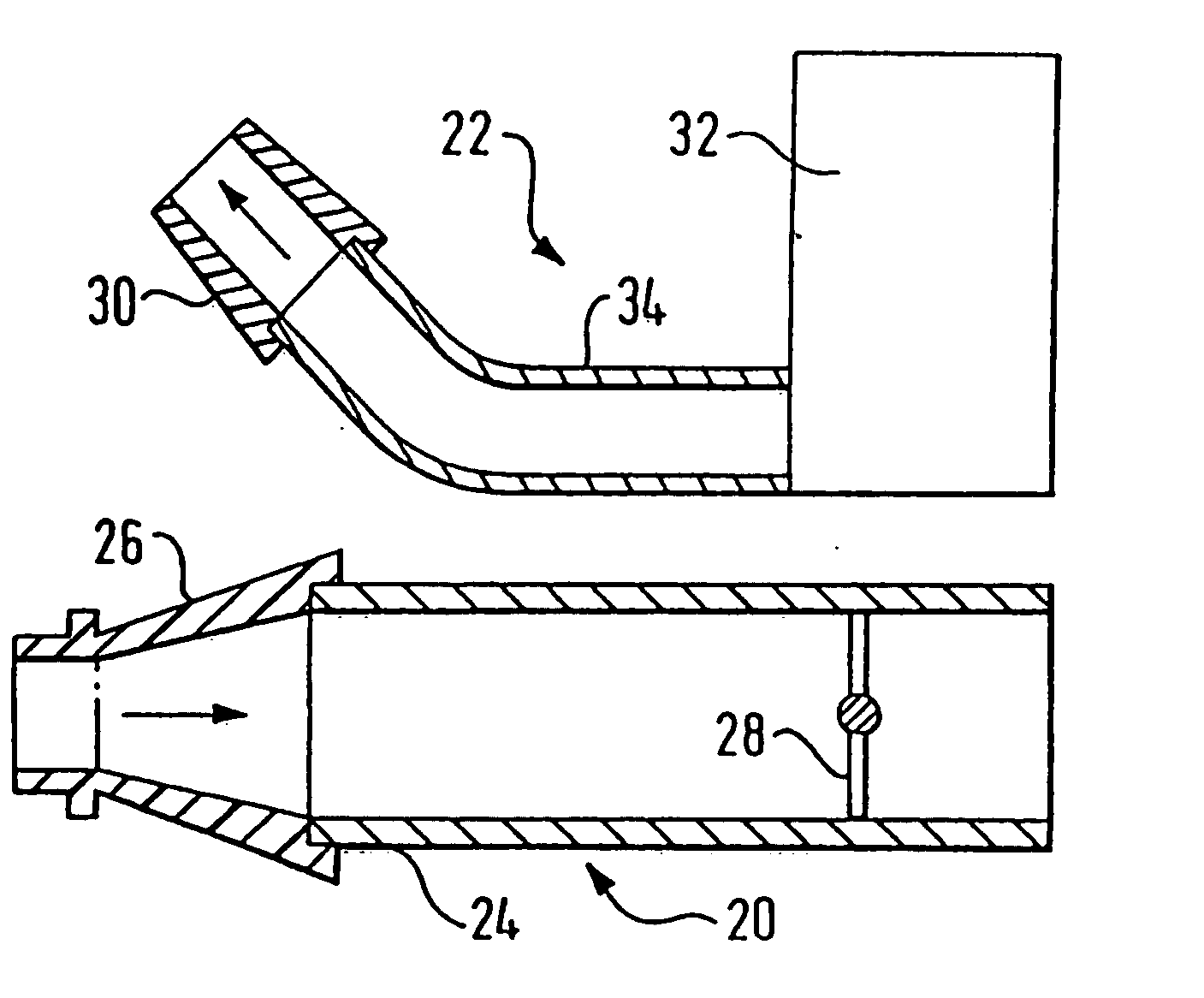
Nasal delivery device
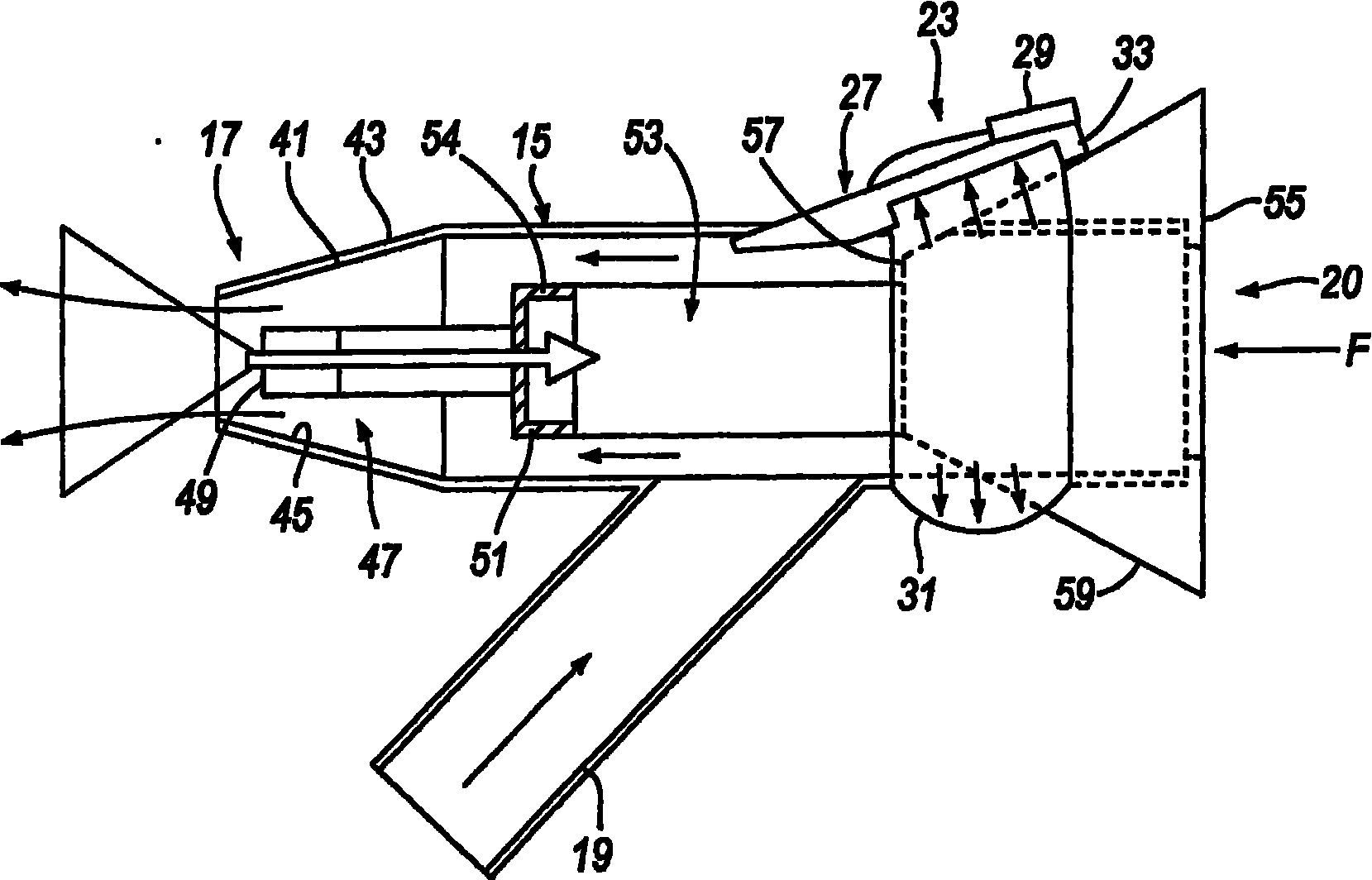
ActiveUS20060219240A1Prevent escapeImprove complianceRespiratorsCannulasNasal cavityPosterior region
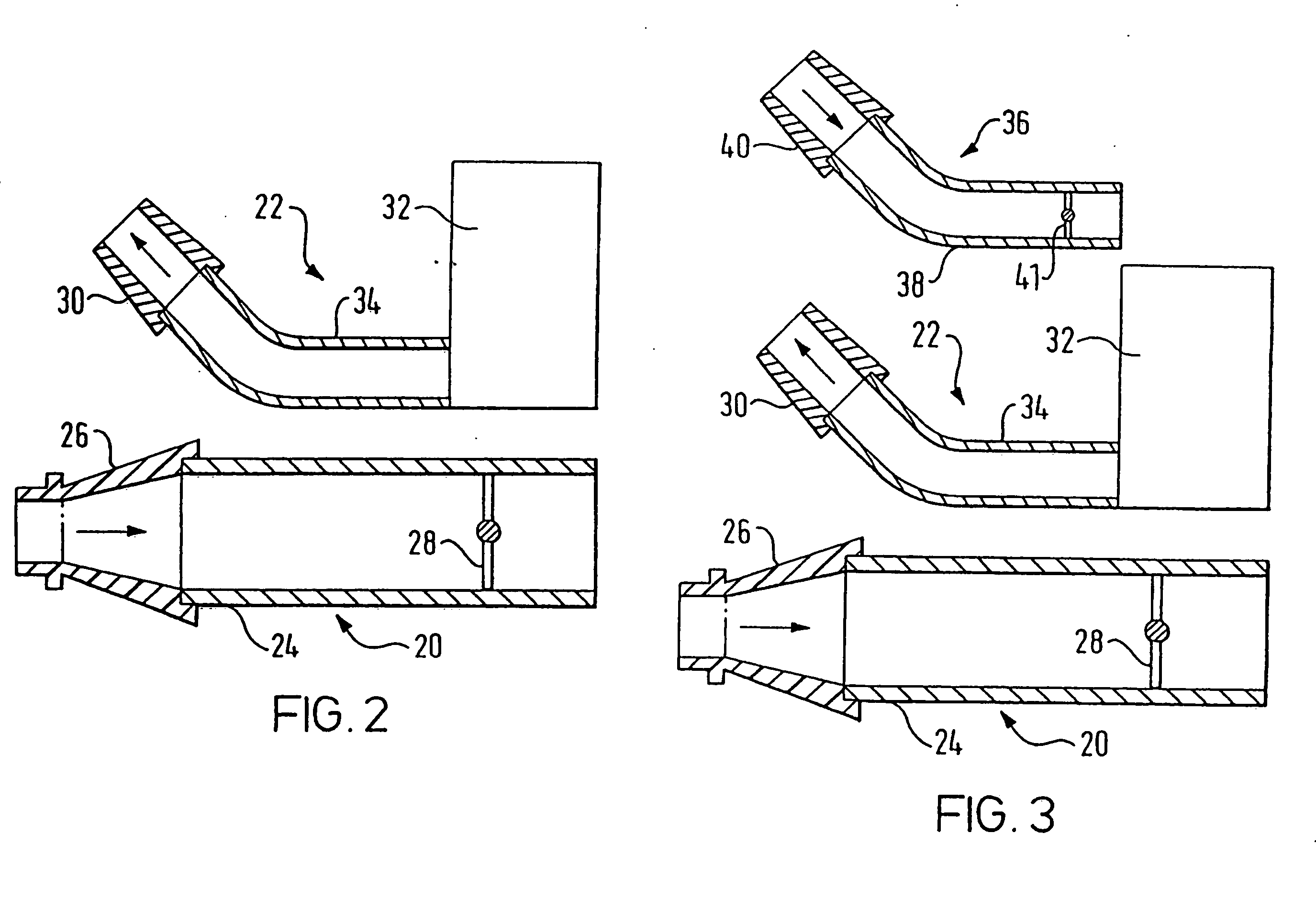
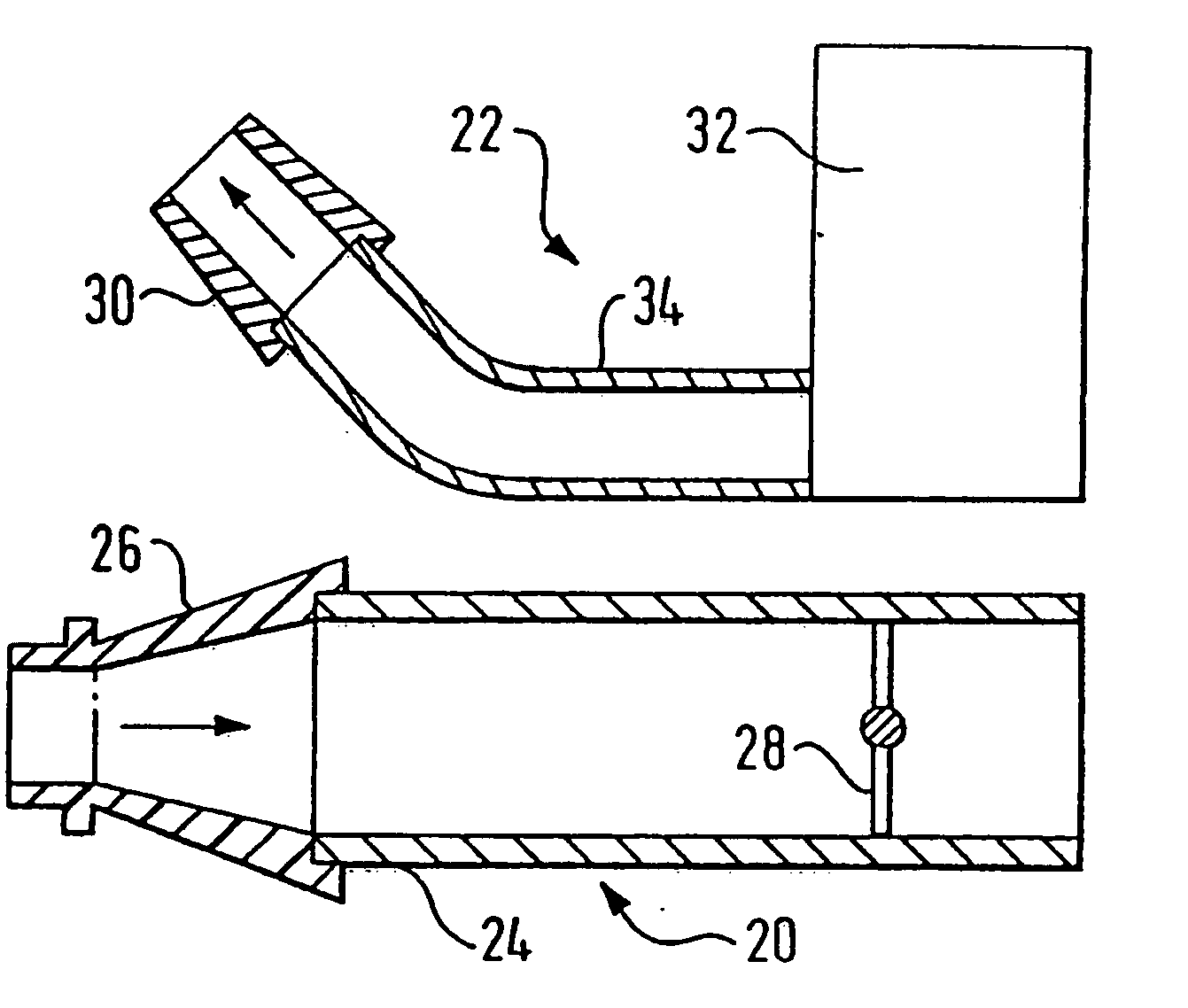
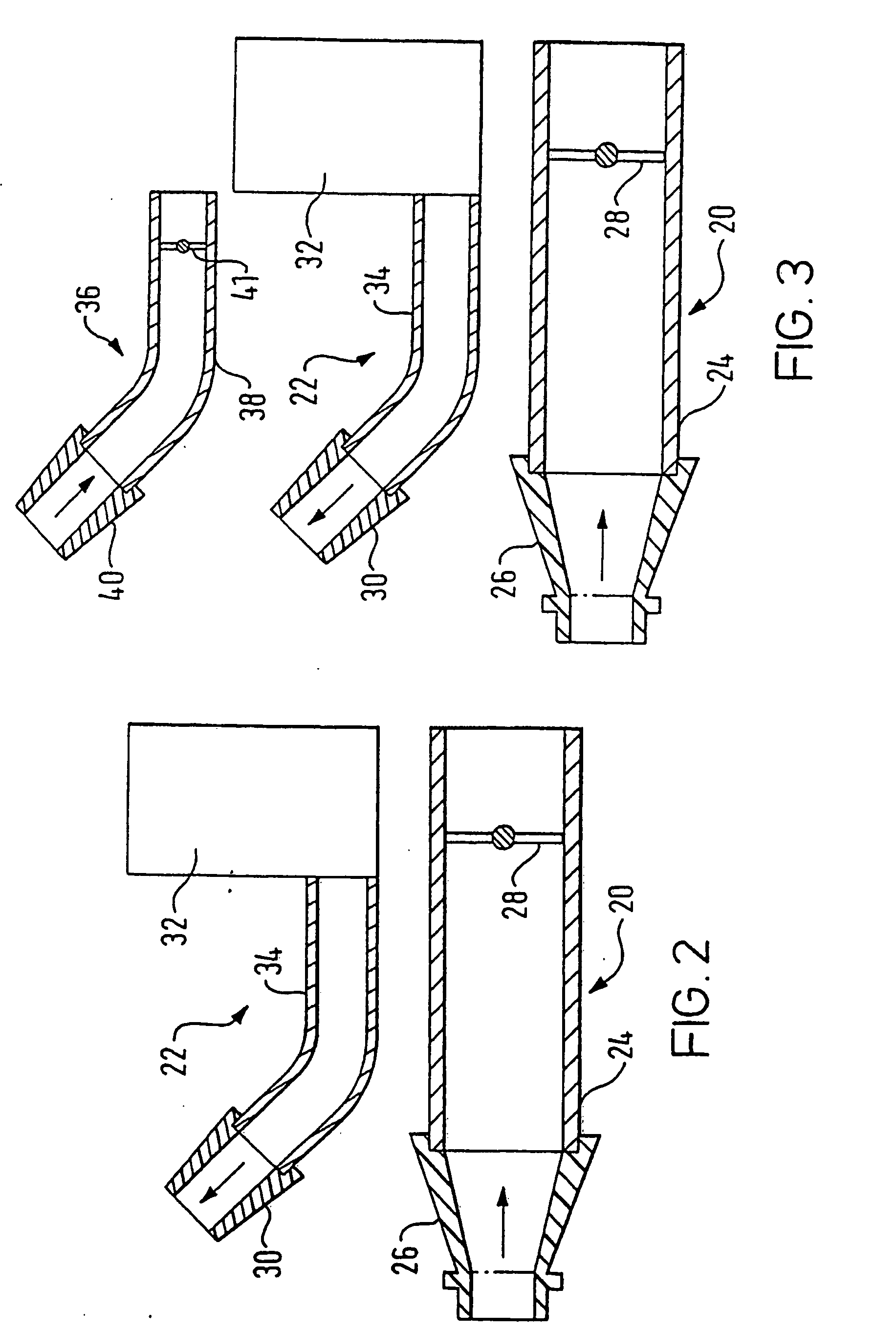
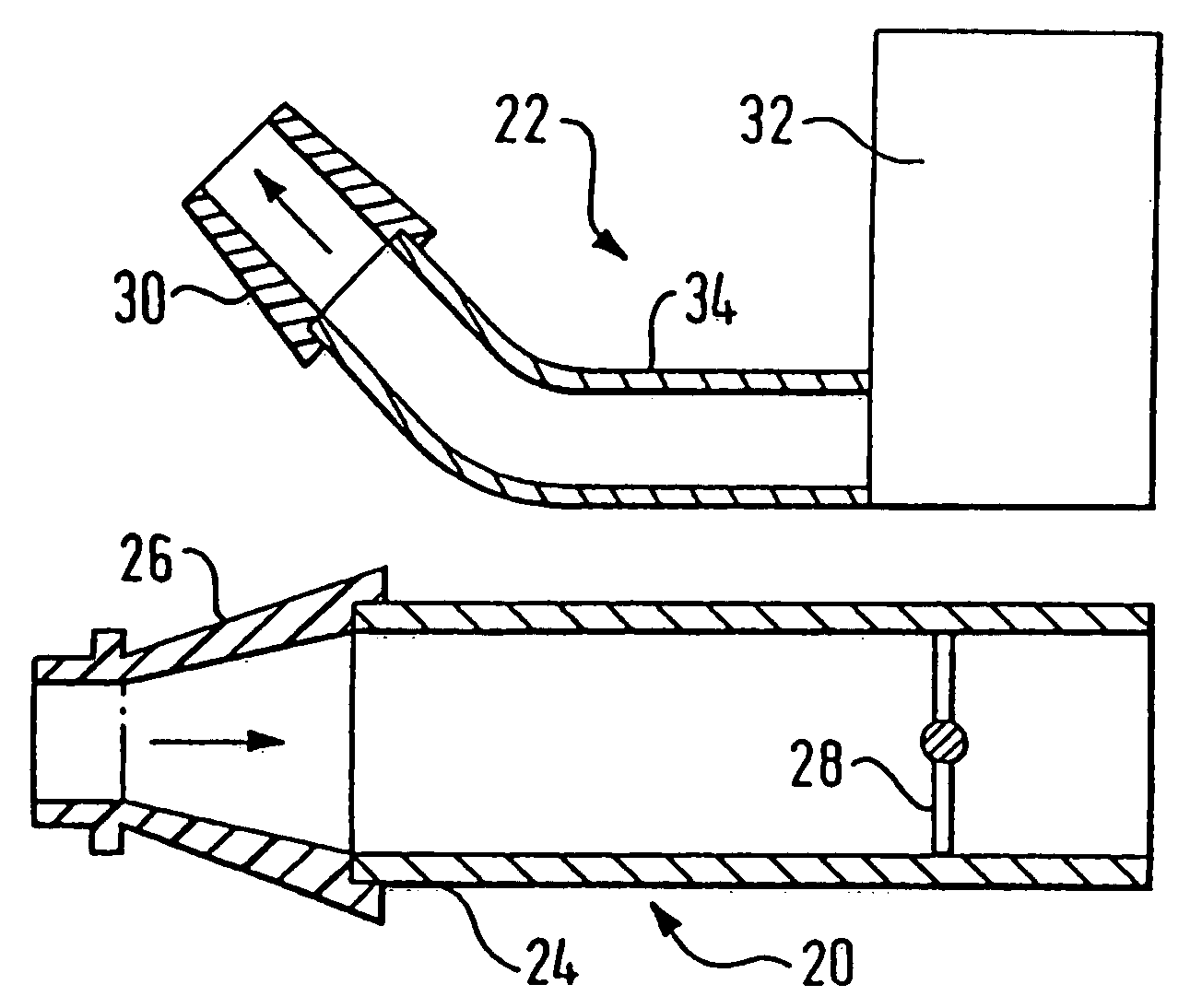
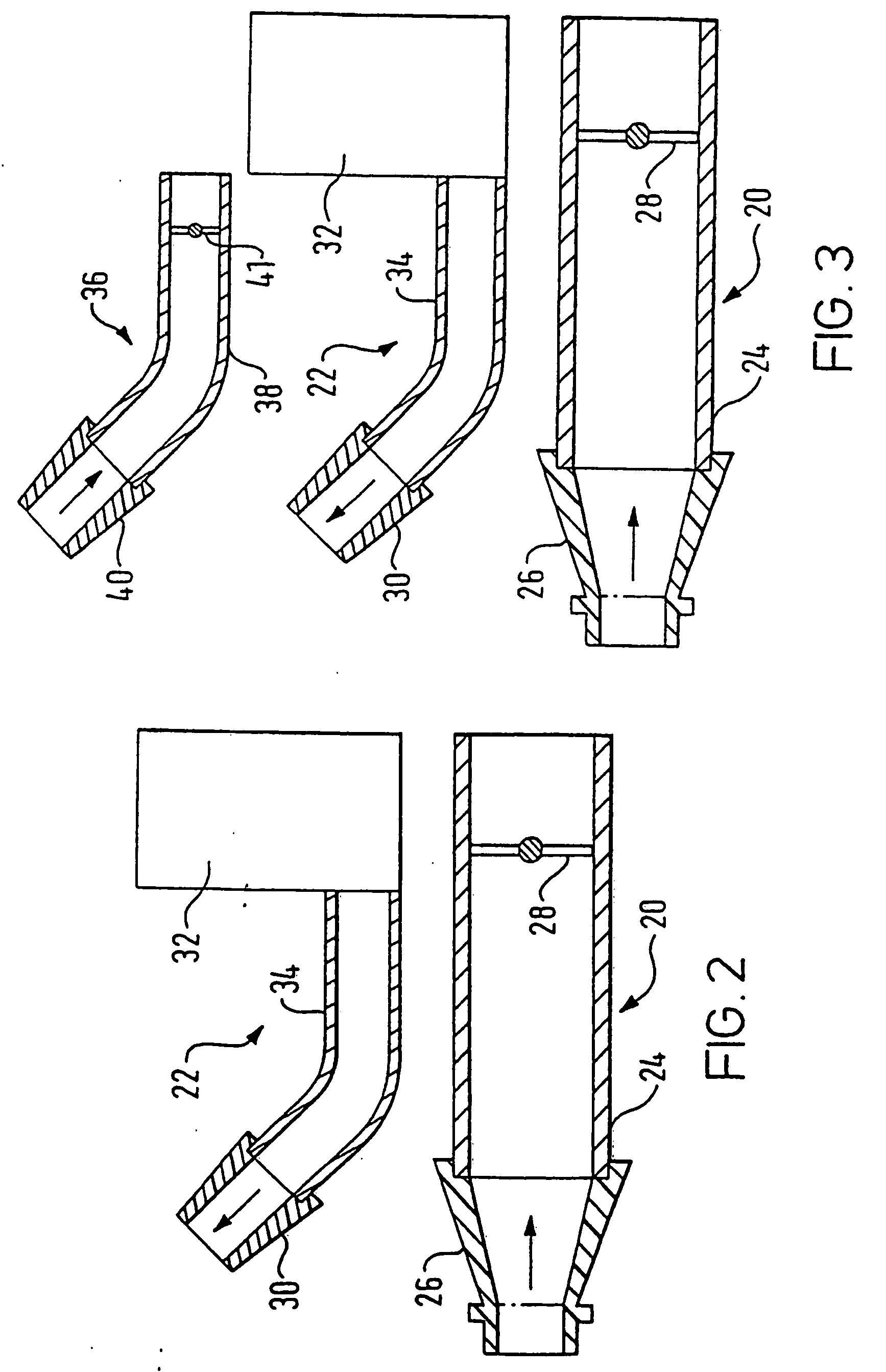
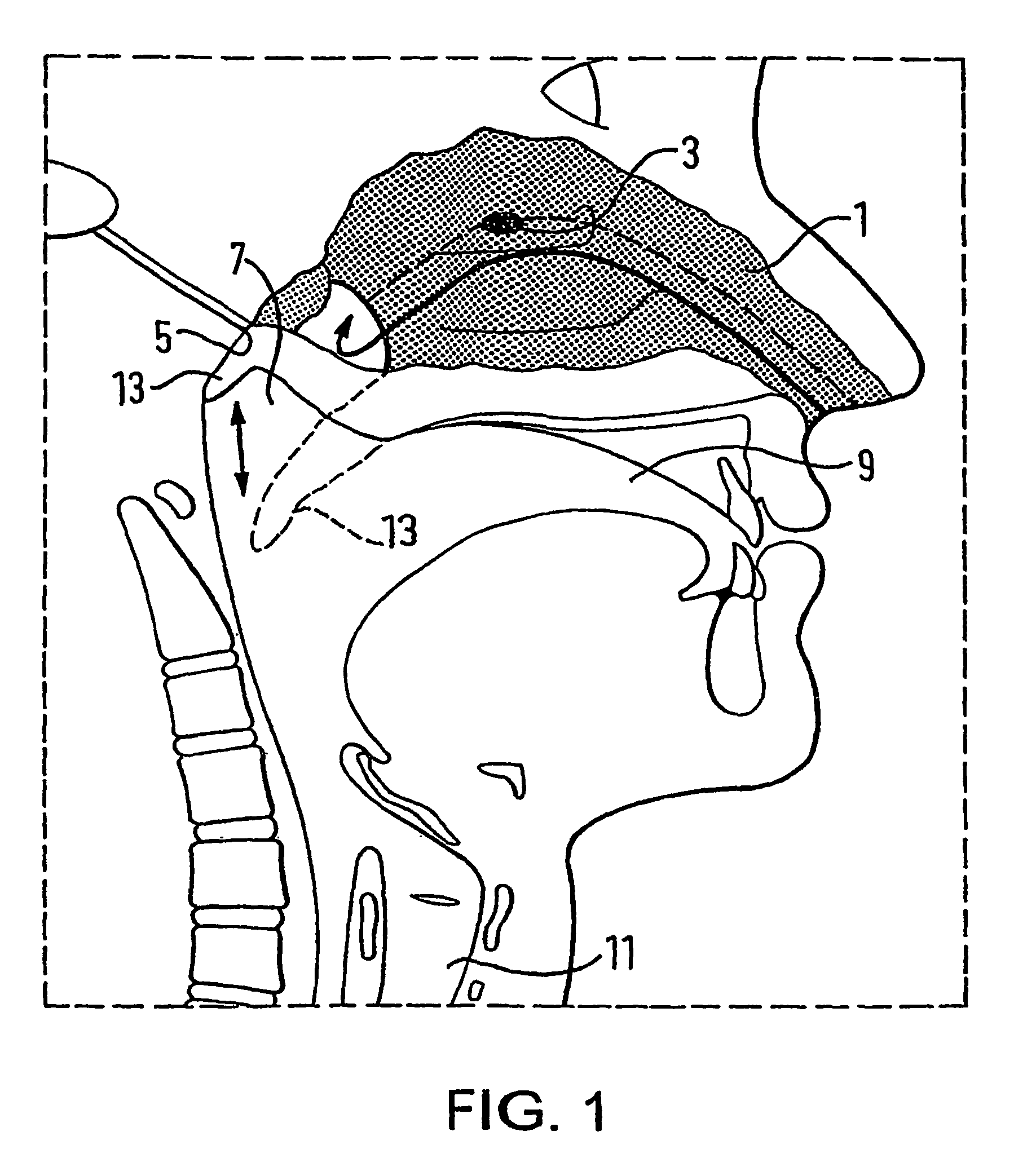
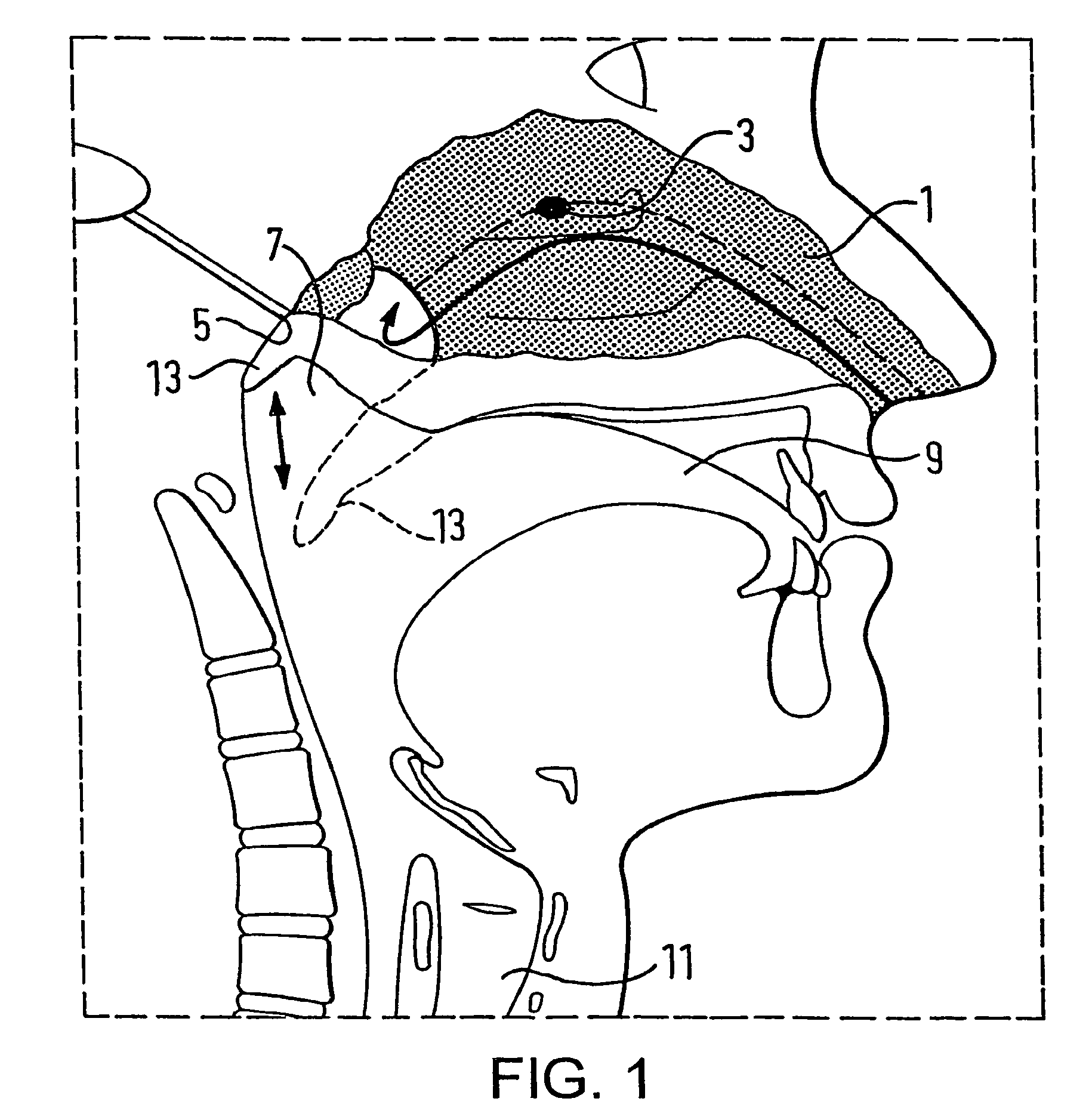
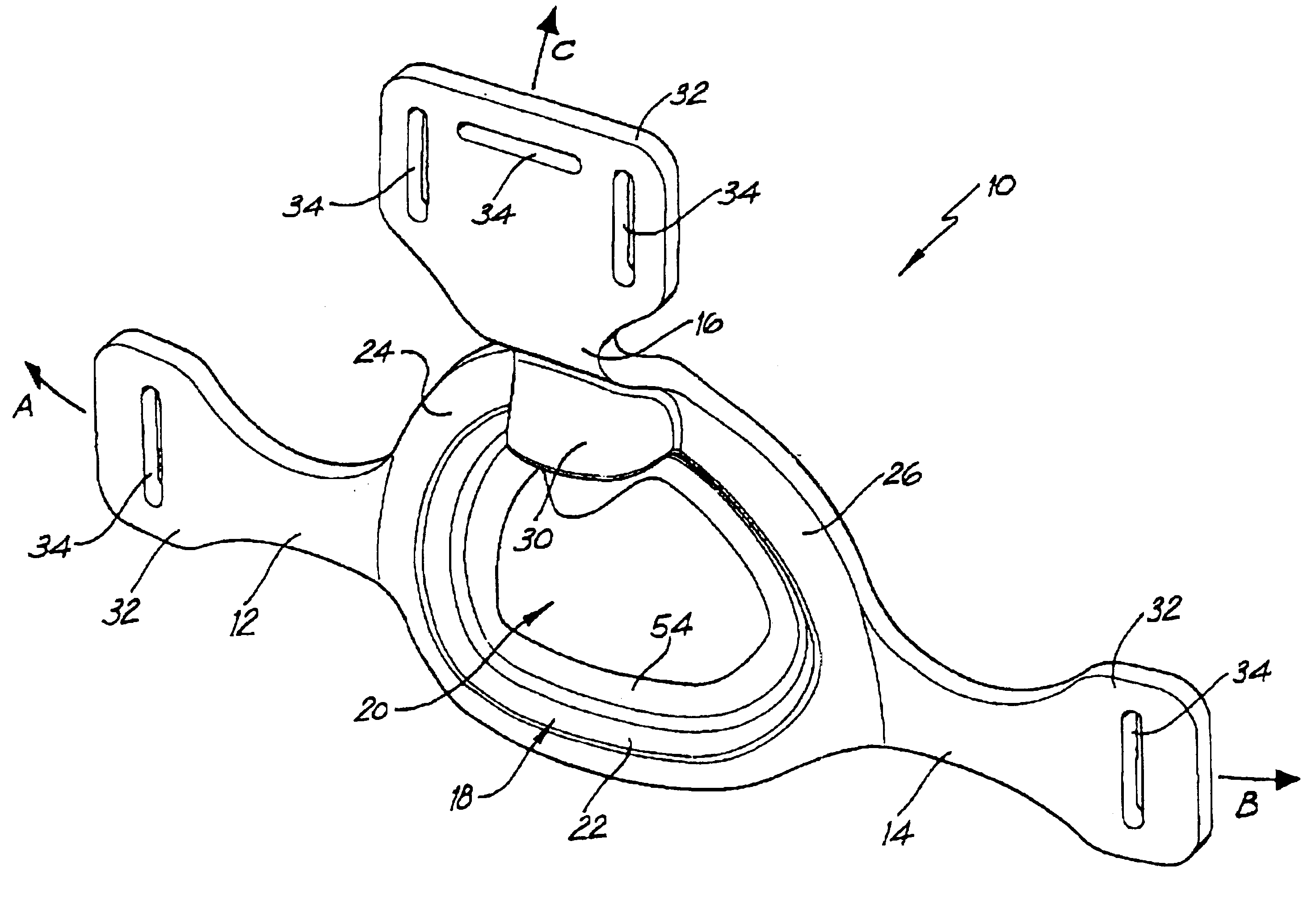
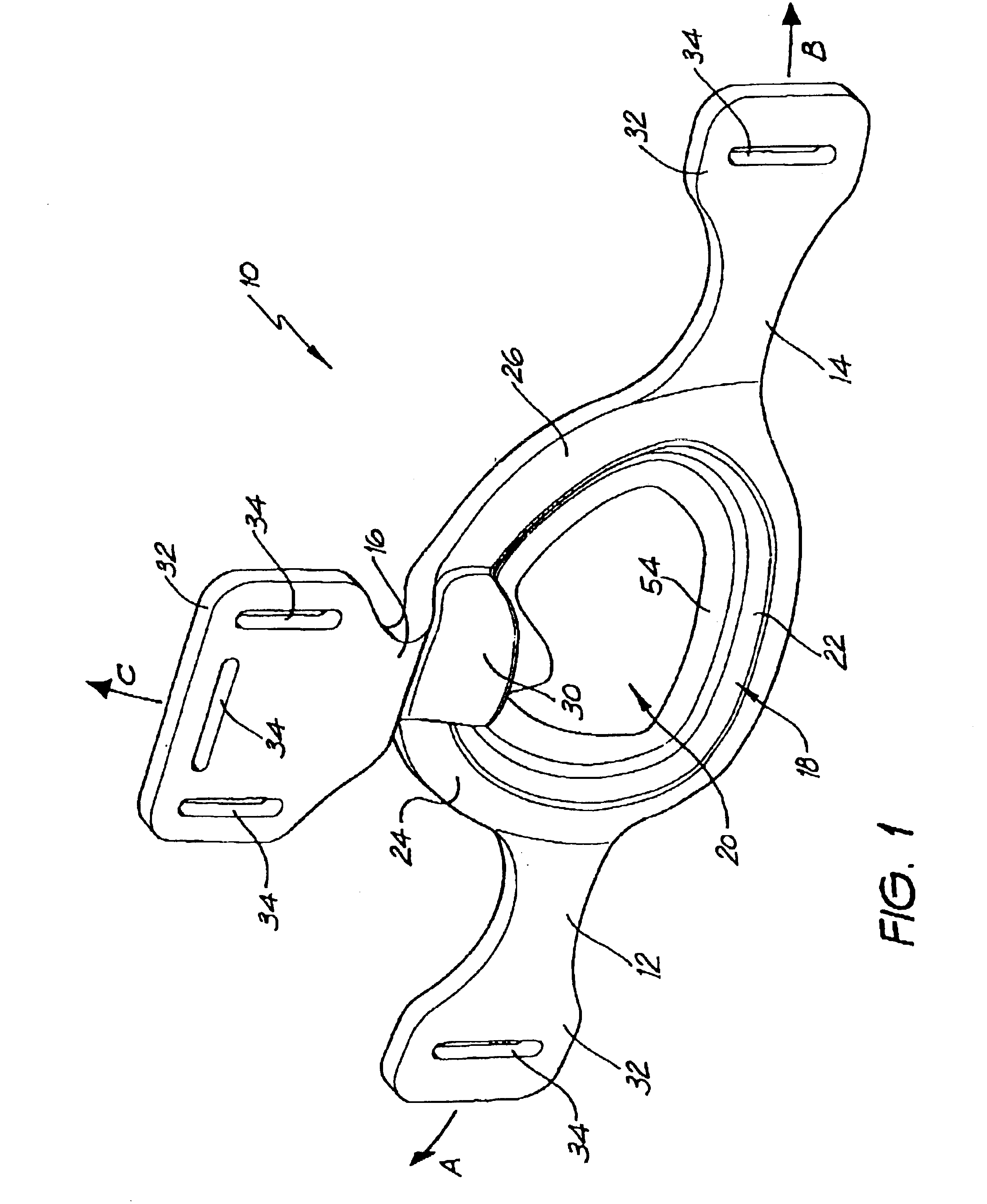
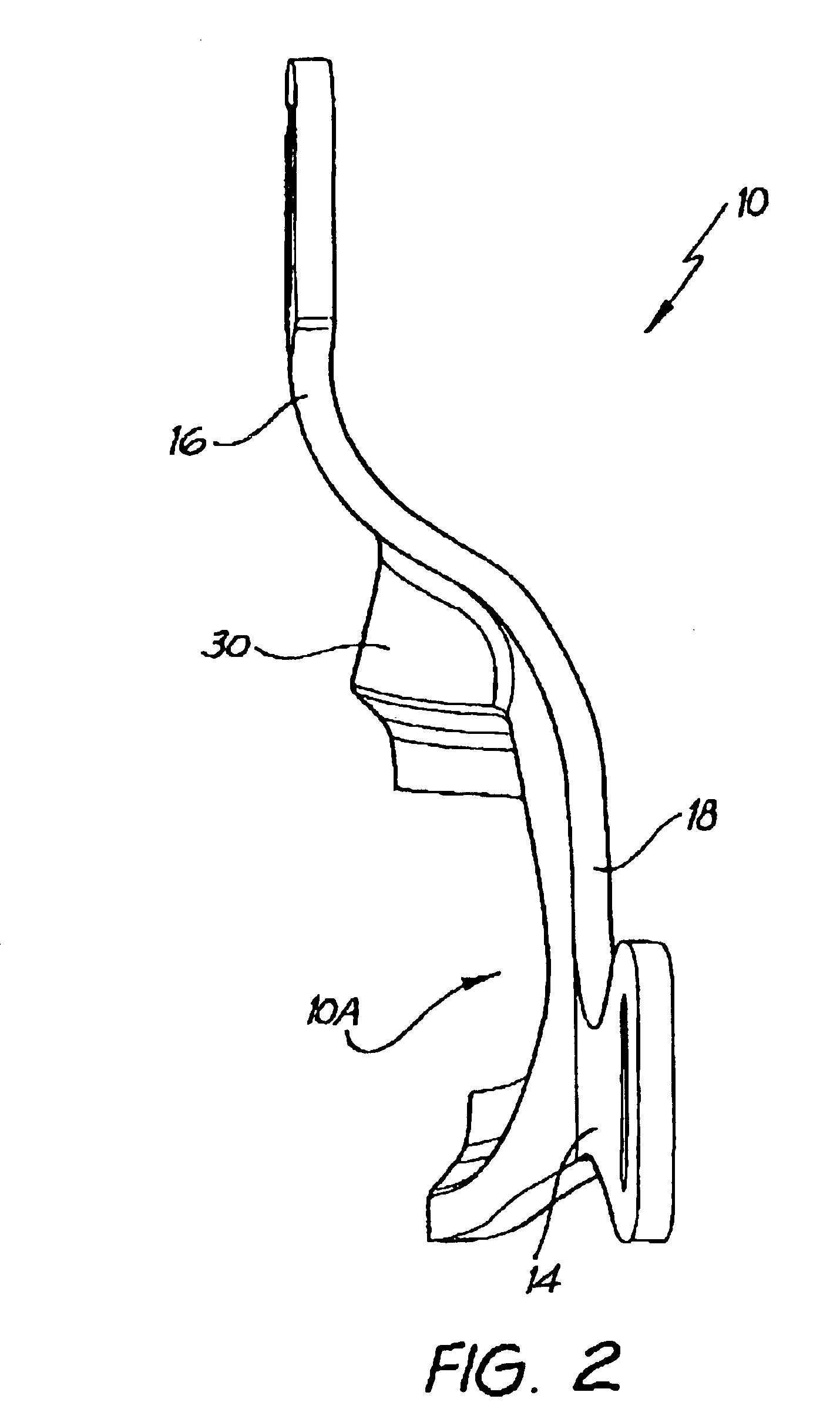
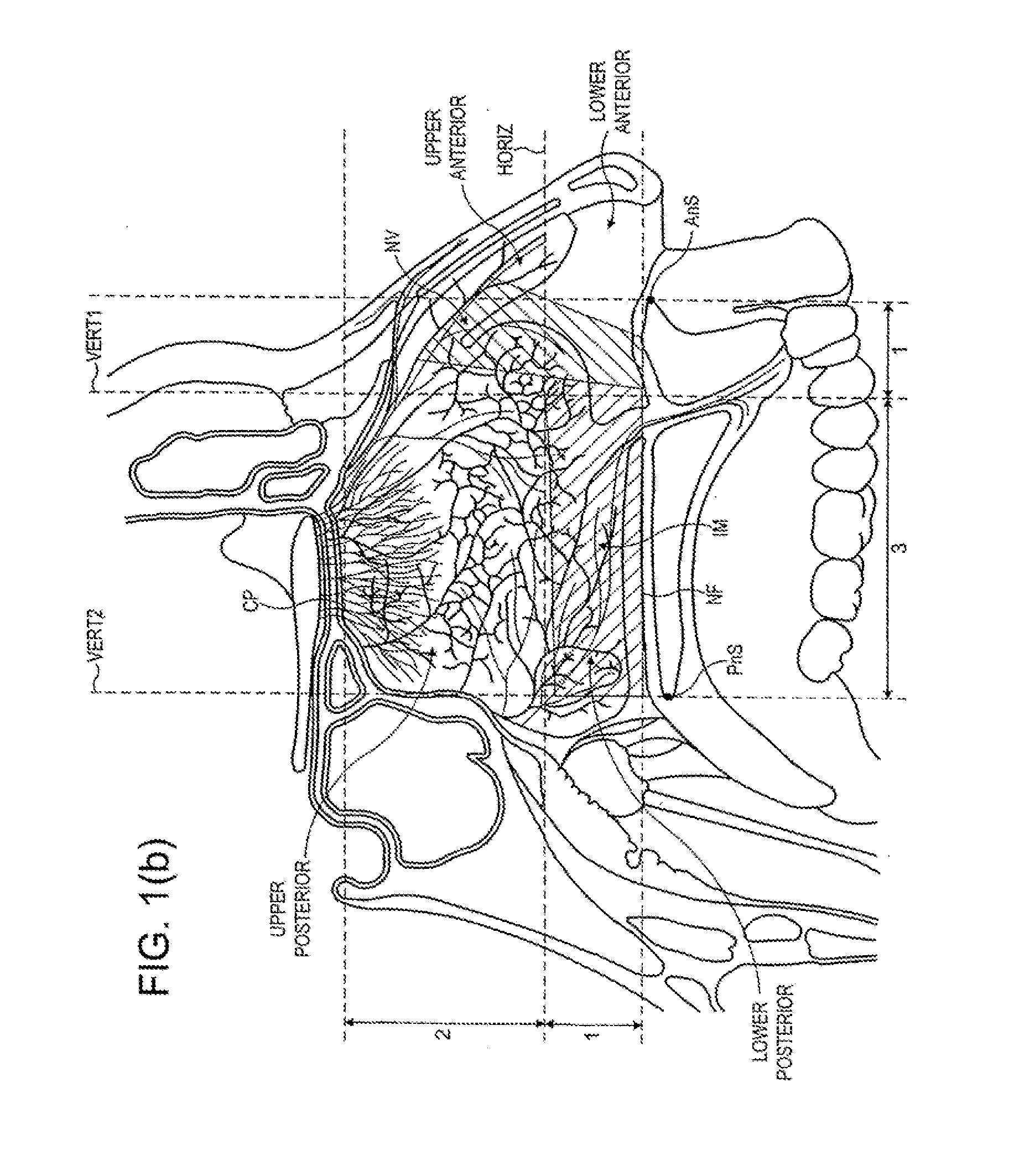
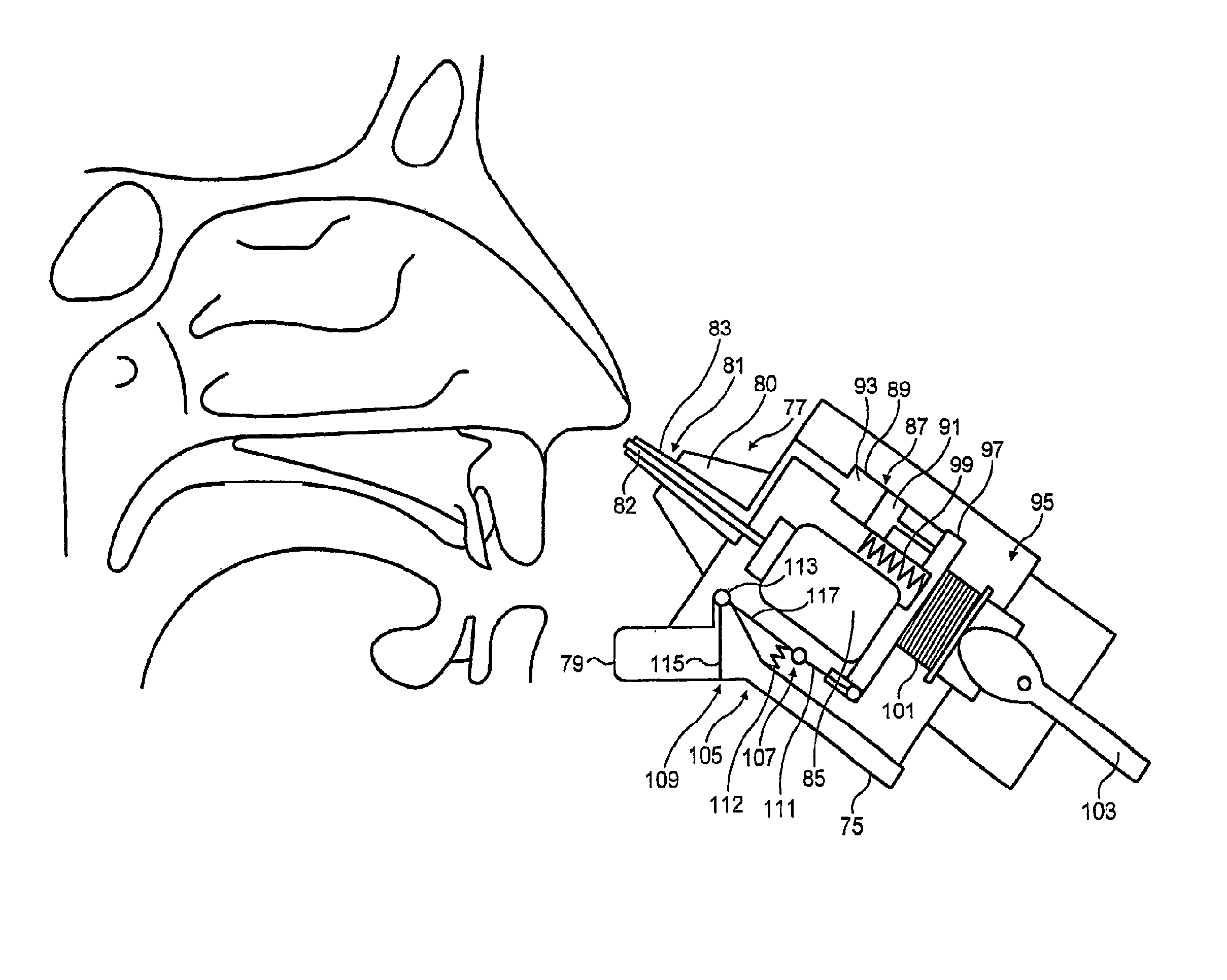
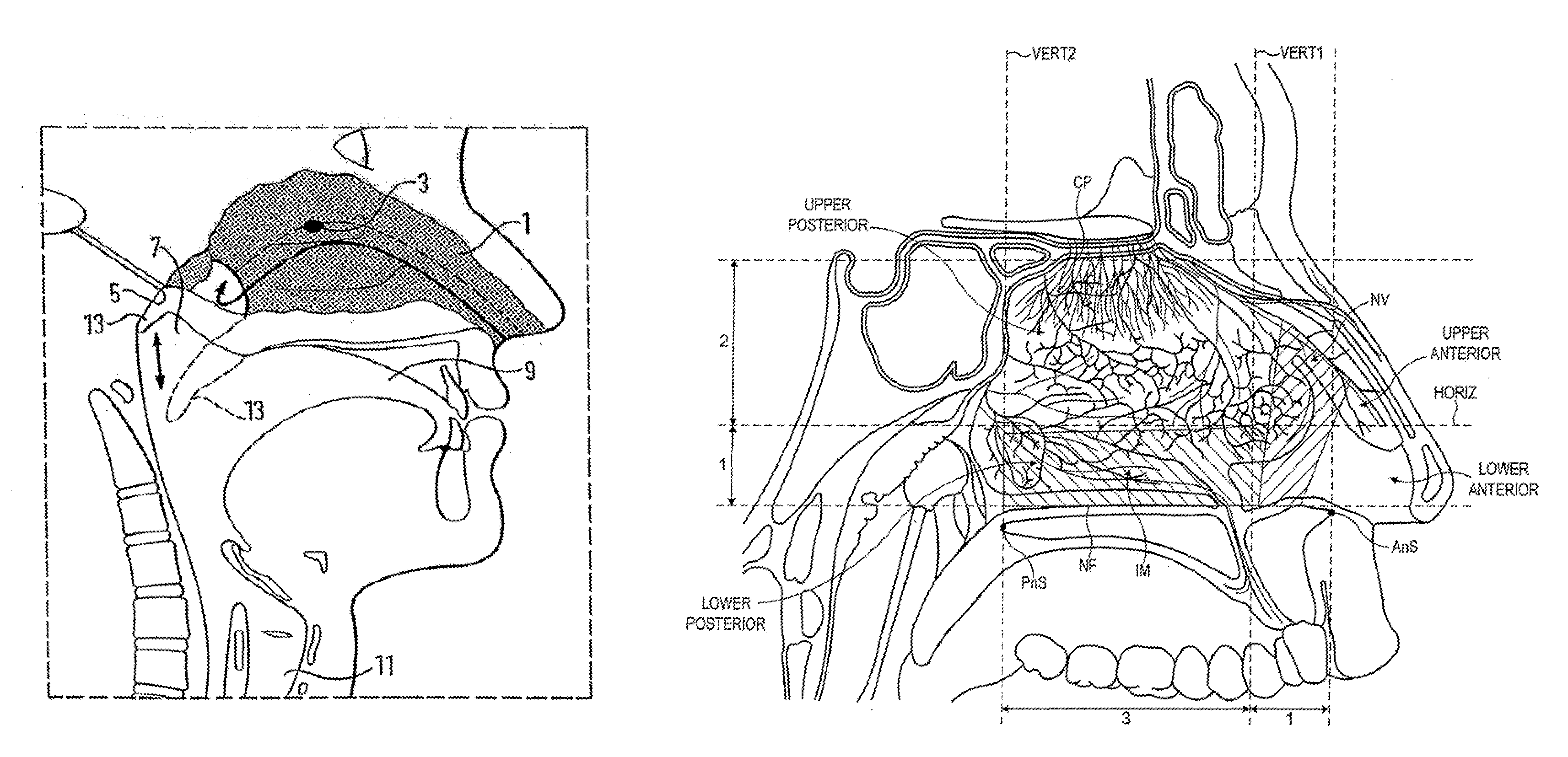
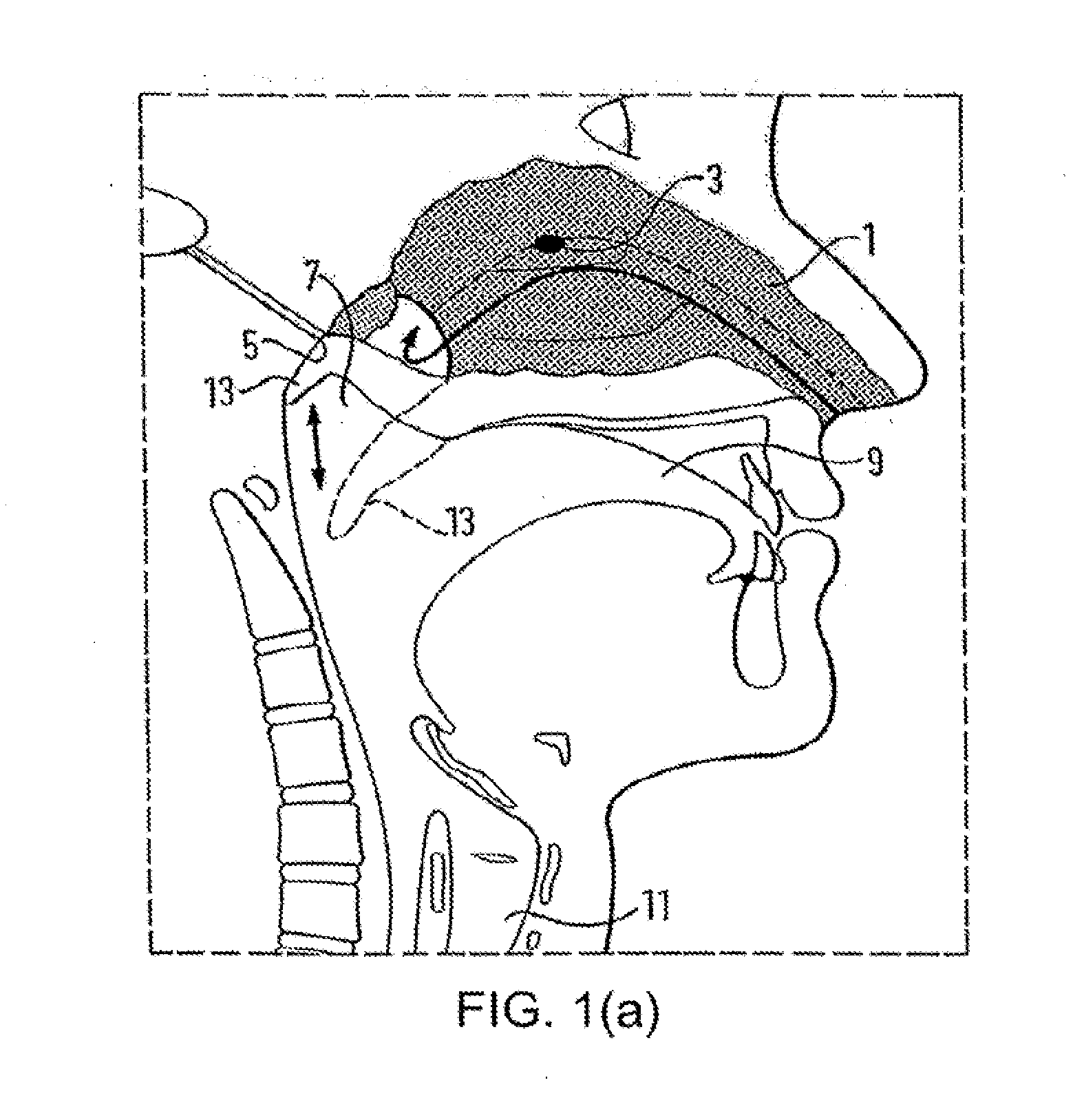
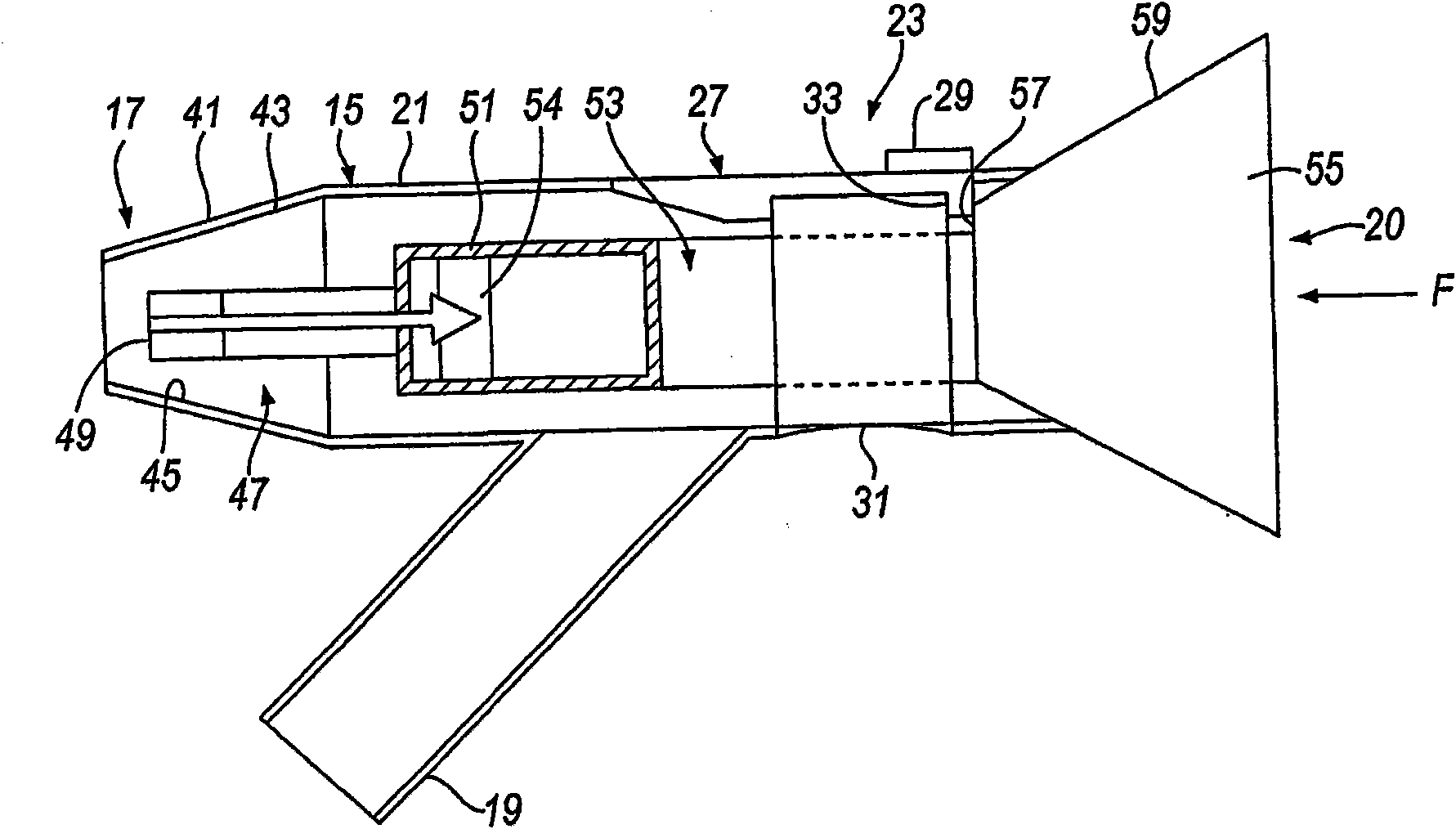
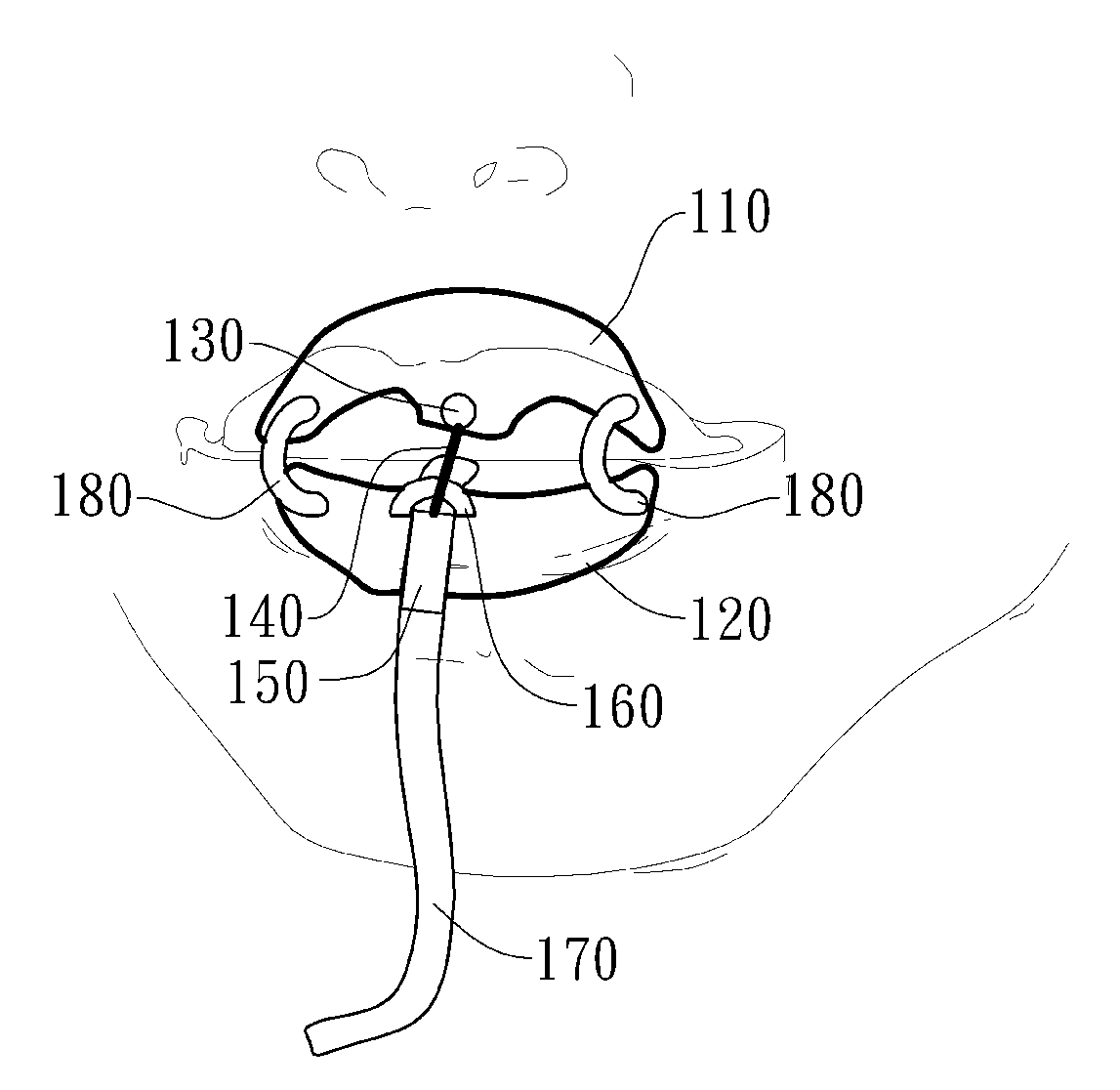
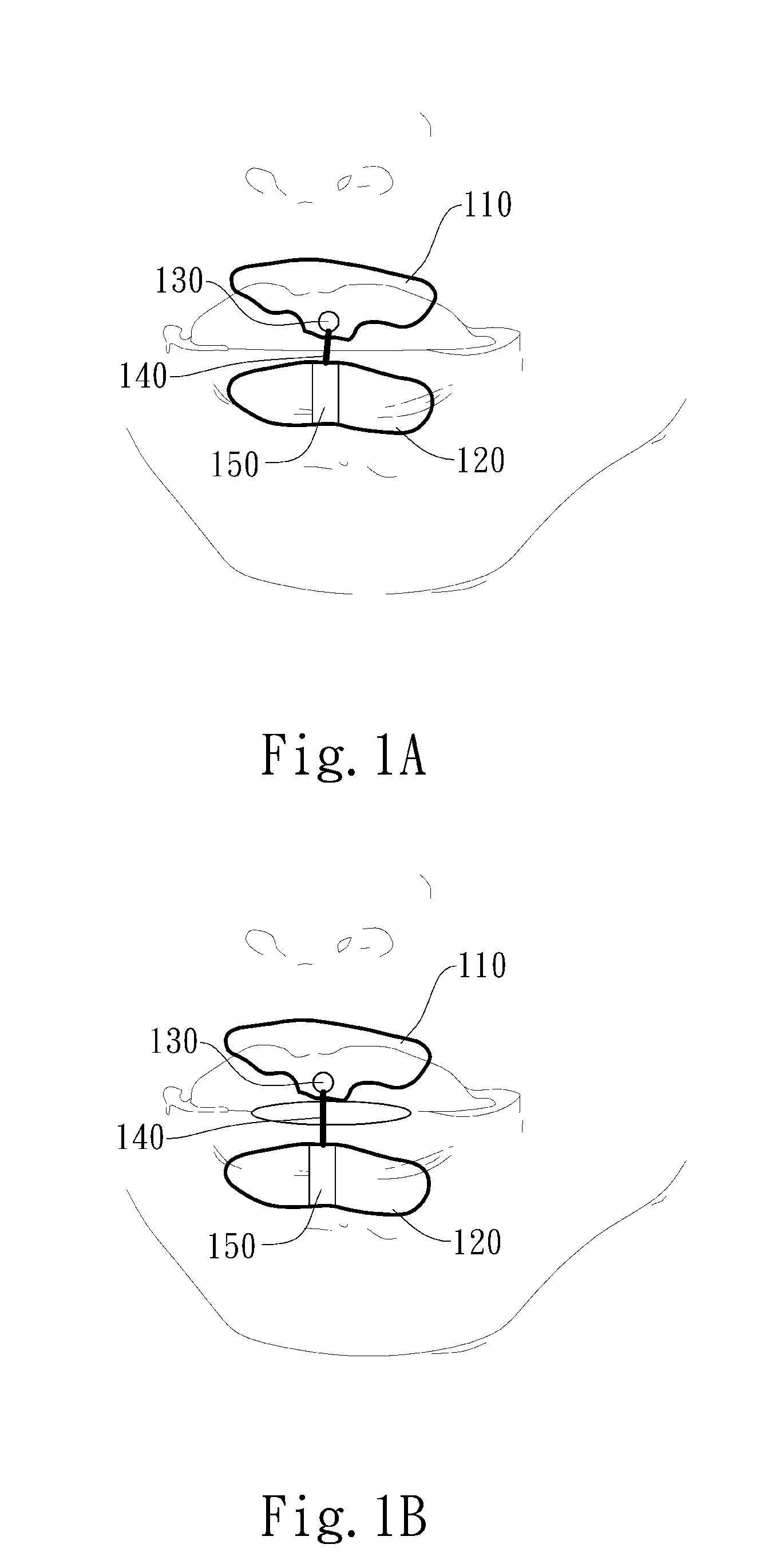
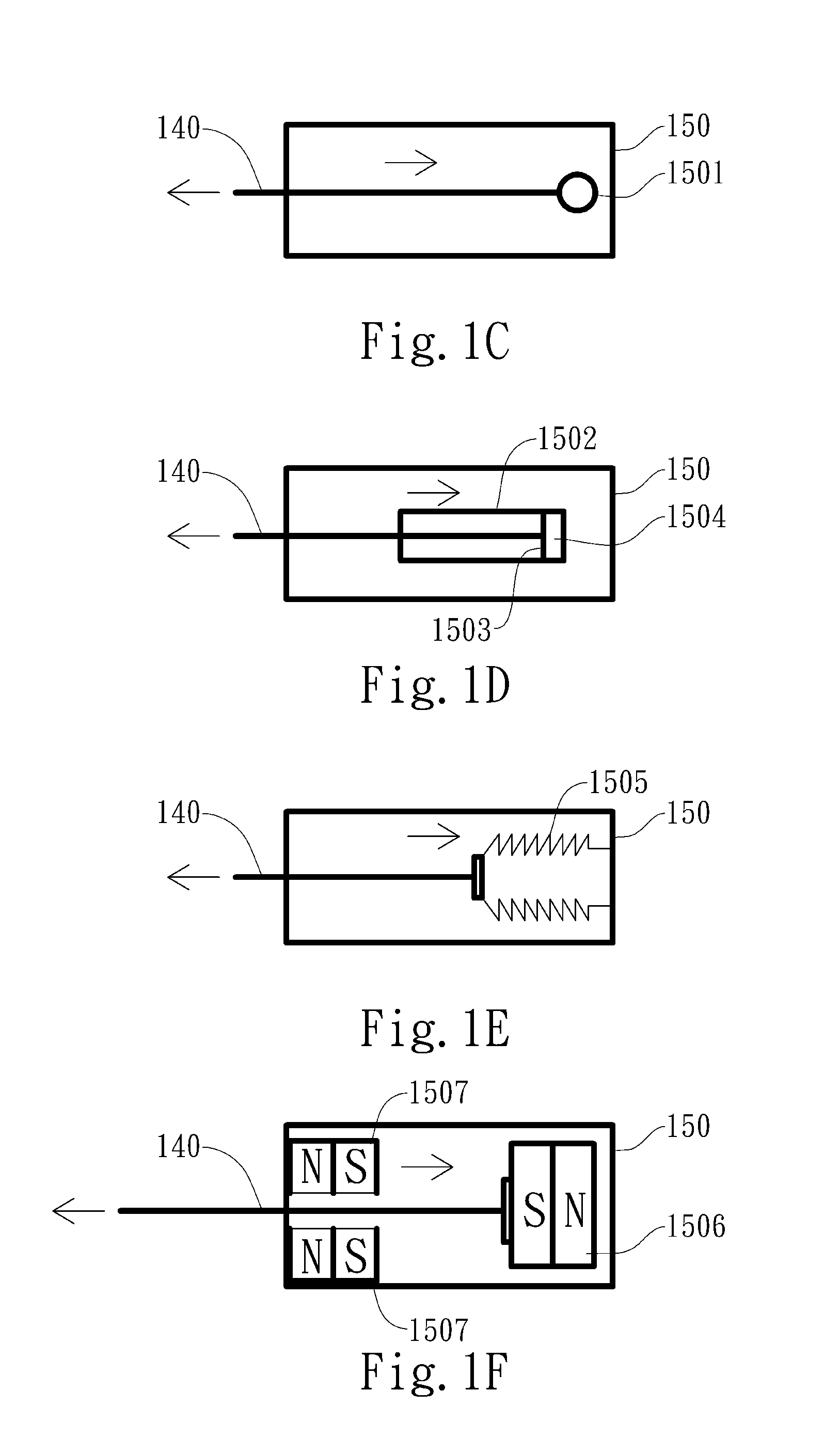
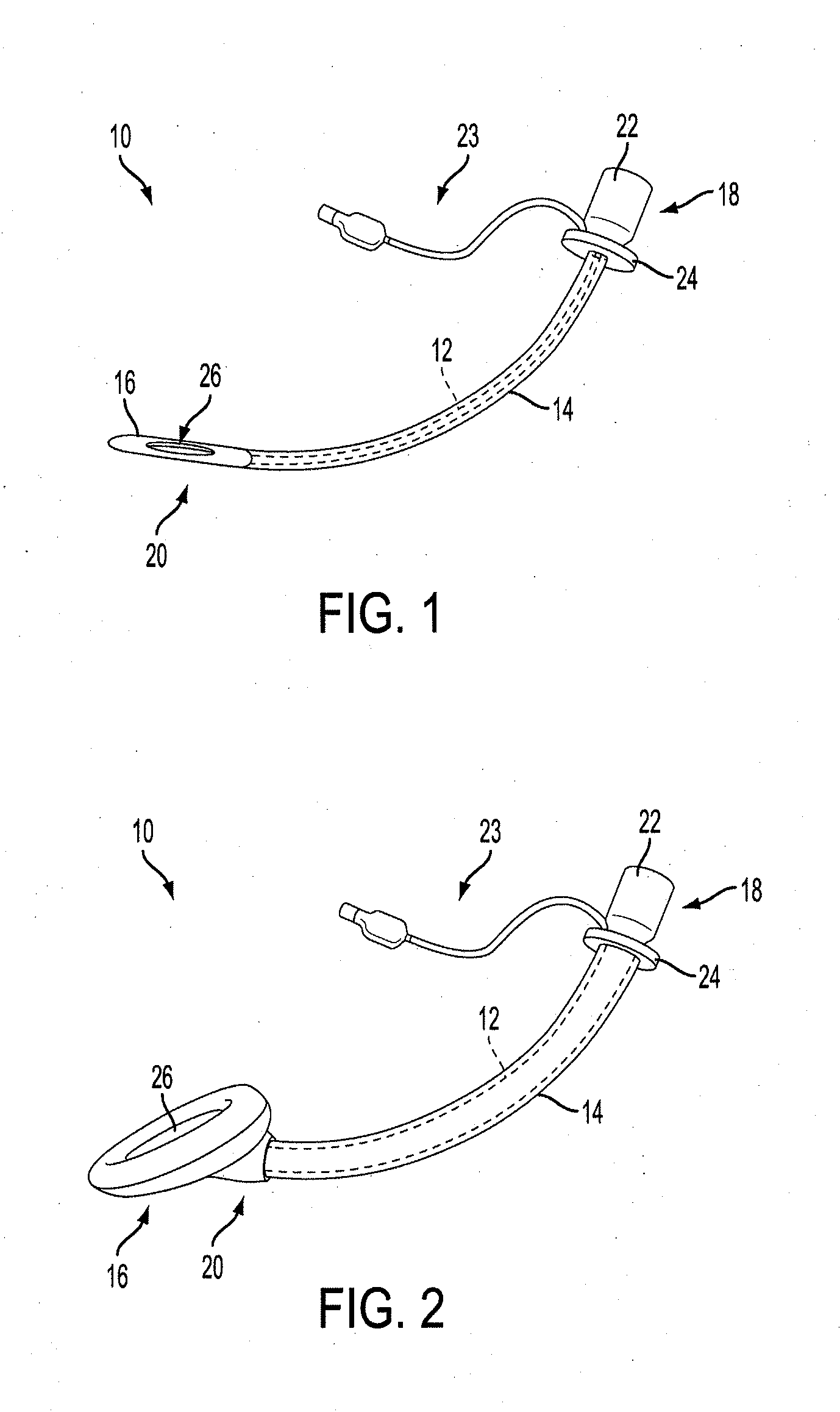
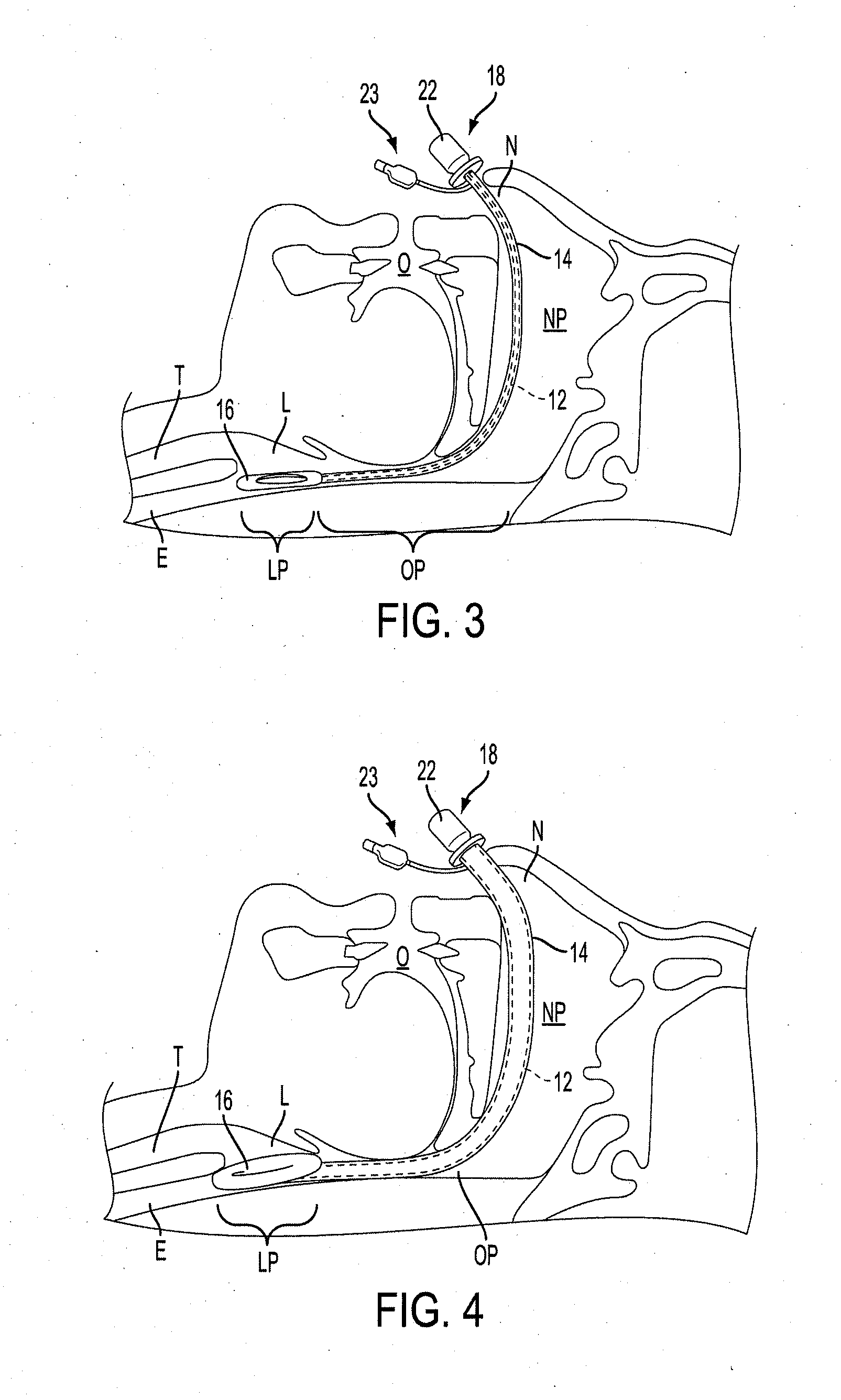
A delivery device (20, 22) for and a method of delivering a substance to the nasal airway (1) of a subject, in particular the posterior region of the nasal airway, the delivery device comprising: a closure unit for causing the closure of the oropharyngeal velum of the subject; and a delivery unit for delivering a gas flow entraining a substance to one of the nostrils of the subject at such a driving pressure as to flow around the posterior margin of the nasal septum and out of the other nostril of the subject, wherein the delivery unit comprises a nosepiece (30, 40, 58, 82, 102, 132) which includes an outlet through which the gas flow is in use delivered to the one nostril and a sealing member for sealing the one nostril to the outlet such as in use to prevent the escape of the gas flow through the one nostril.
Owner:OPTINOSE INC
Nasal delivery device
InactiveUS20060225732A1Prevent escapeImprove complianceRespiratorsCannulasNasal cavityPosterior region
A delivery device (20, 22) for and a method of delivering a substance to the nasal airway (1) of a subject, in particular the posterior region of the nasal airway, the delivery device comprising: a closure unit for causing the closure of the oropharyngeal velum of the subject; and a delivery unit for delivering a gas flow entraining a substance to one of the nostrils of the subject at such a driving pressure as to flow around the posterior margin of the nasal septum and out of the other nostril of the subject, wherein the delivery unit comprises a nosepiece (30, 40, 58, 82, 102, 132) which includes an outlet through which the gas flow is in use delivered to the one nostril and a sealing member for sealing the one nostril to the outlet such as in use to prevent the escape of the gas flow through the one nostril.
Owner:OPTINOSE INC
Nasal delivery device
InactiveUS20060219241A1Prevent escapeImprove complianceRespiratorsCannulasNasal cavityPosterior region
A delivery device (20, 22) for and a method of delivering a substance to the nasal airway (1) of a subject, in particular the posterior region of the nasal airway, the delivery device comprising: a closure unit for causing the closure of the oropharyngeal velum of the subject; and a delivery unit for delivering a gas flow entraining a substance to one of the nostrils of the subject at such a driving pressure as to flow around the posterior margin of the nasal septum and out of the other nostril of the subject, wherein the delivery unit comprises a nosepiece (30, 40, 58, 82, 102, 132) which includes an outlet through which the gas flow is in use delivered to the one nostril and a sealing member for sealing the one nostril to the outlet such as in use to prevent the escape of the gas flow through the one nostril.
Owner:OPTINOSE INC
Nasal devices
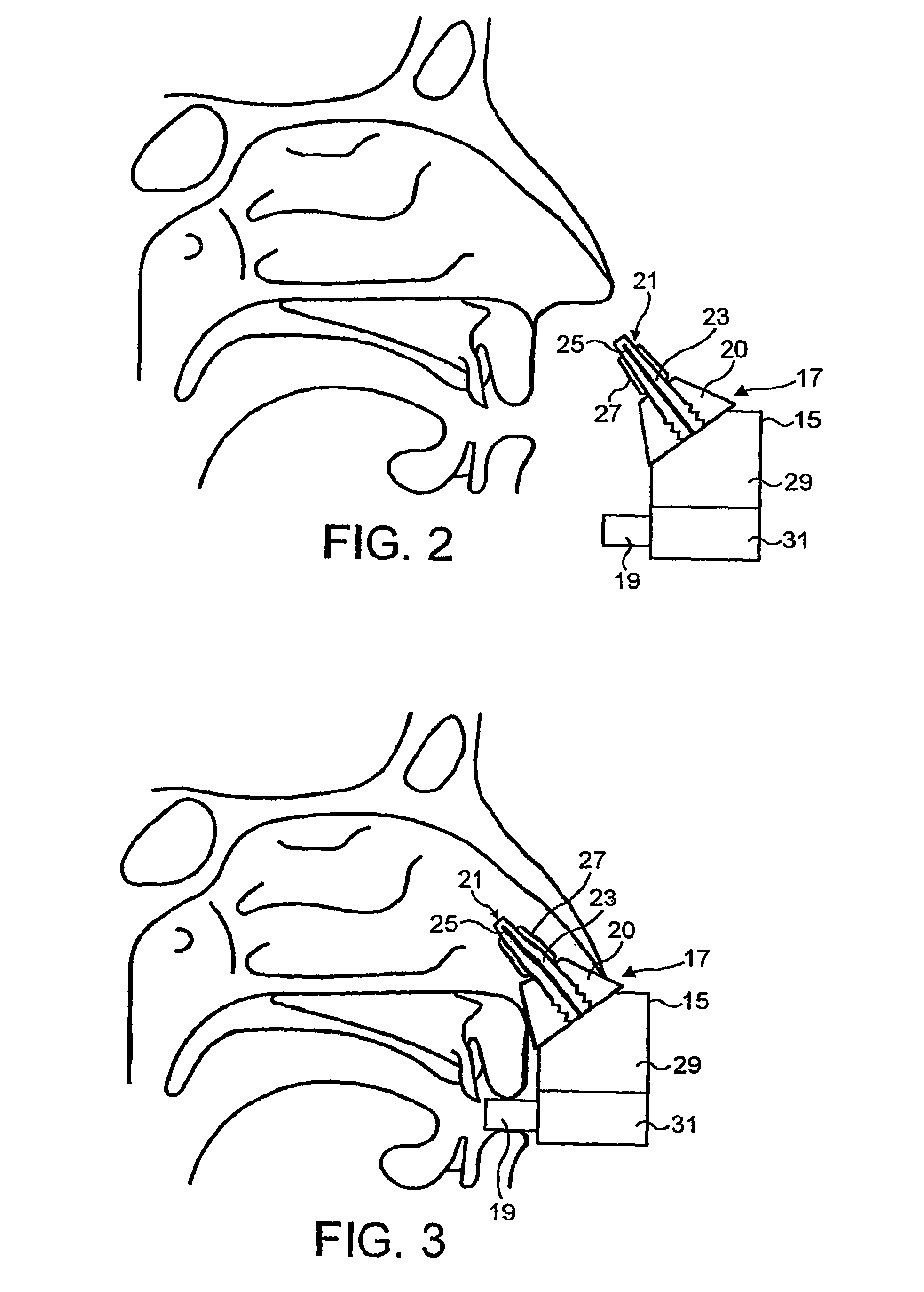
ActiveUS7975690B2Substance may accumulateReduce deliveryPowder deliverySenses disorderNasal cavityWhole body
The present invention relates to a nasal delivery device for and a method of delivering a substance, in particular one of a liquid, as a suspension or solution, or a powder containing a medicament, especially systemic or topical pharmaceuticals, or a vaccine to the nasal airway of a subject.
Owner:OPTINOSE INC
Nasal devices
A nasal delivery device and a method of delivering substance to a nasal airway of a subject, can be used for mass treatment, especially mass vaccination. The delivery device can include an interface unit, as a replaceable unit, having at least one nosepiece unit for fitting to a respective nostril of a subject, a nozzle from which substance is in use delivered, and at least one delivery unit having a substance supply unit for delivering substance to the nozzle of the at least one nosepiece unit. The delivery device can also include an actuation unit for actuating the at least one delivery unit of the interface unit.
Owner:OPTINOSE INC
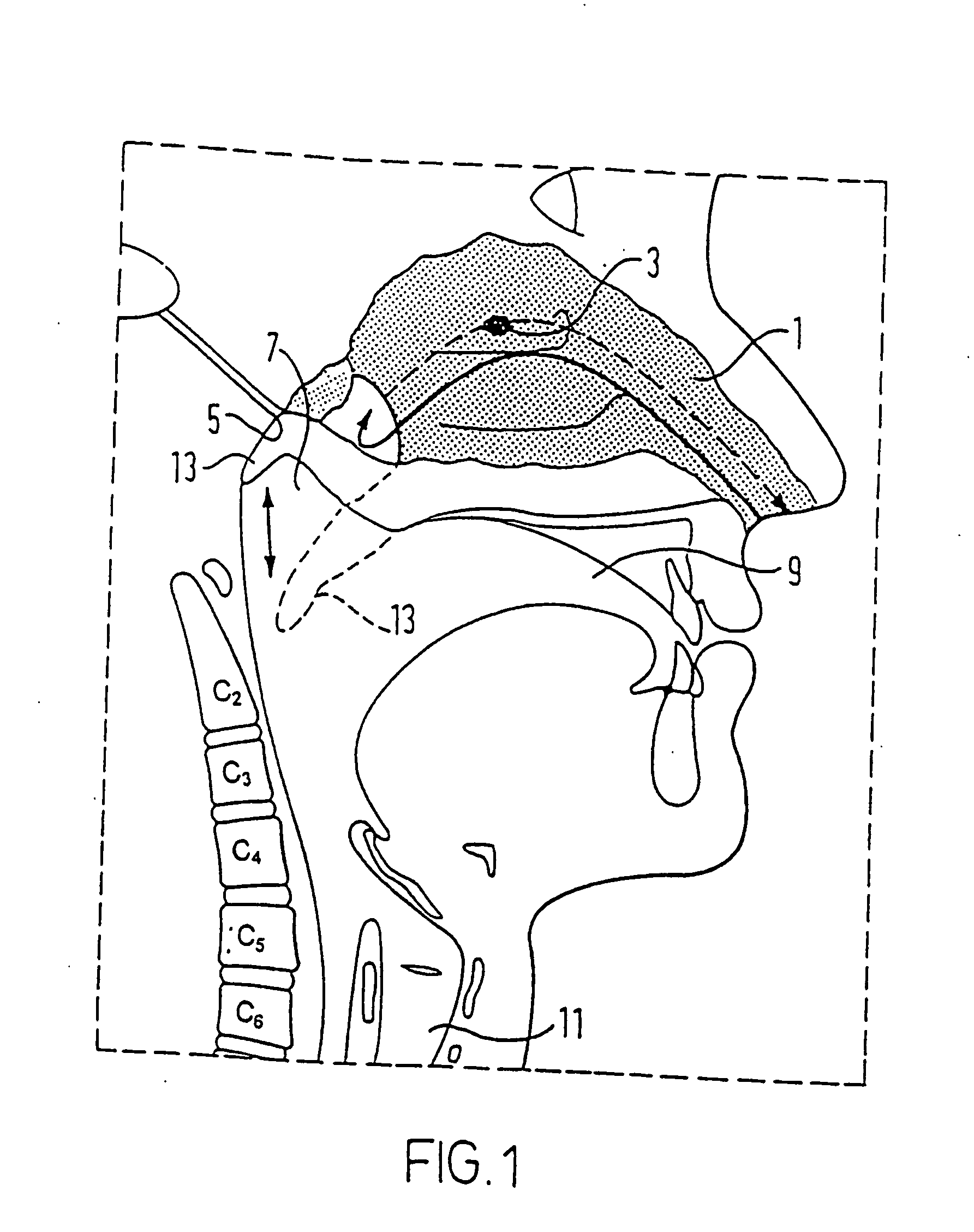
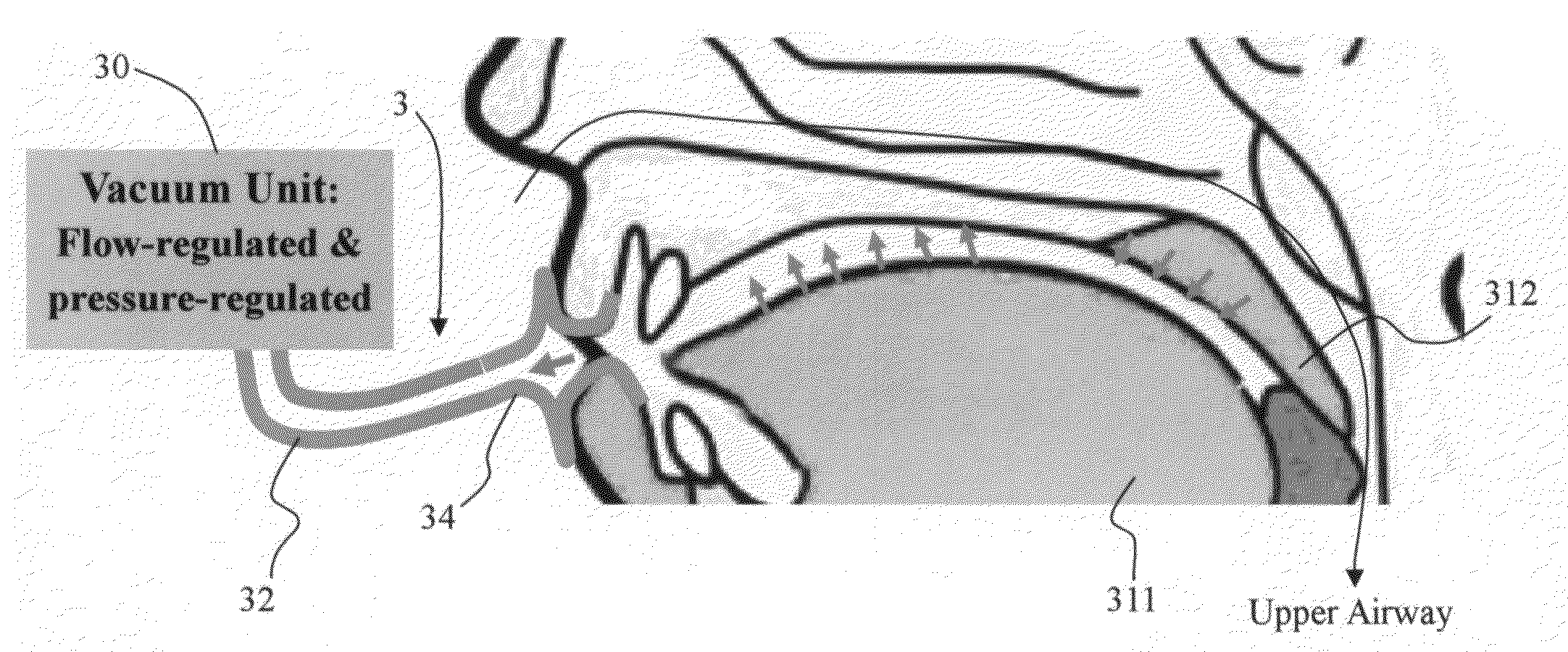
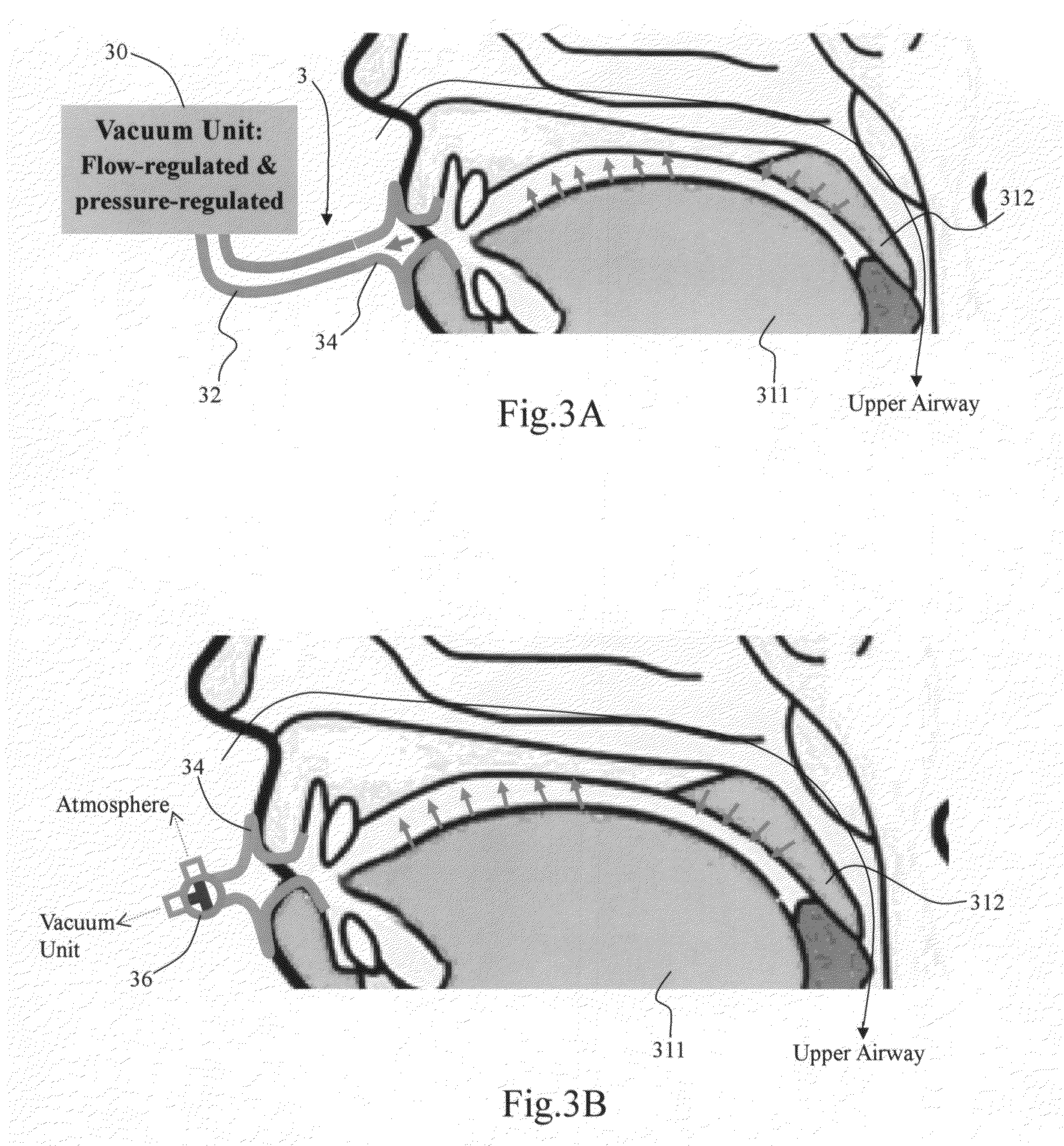
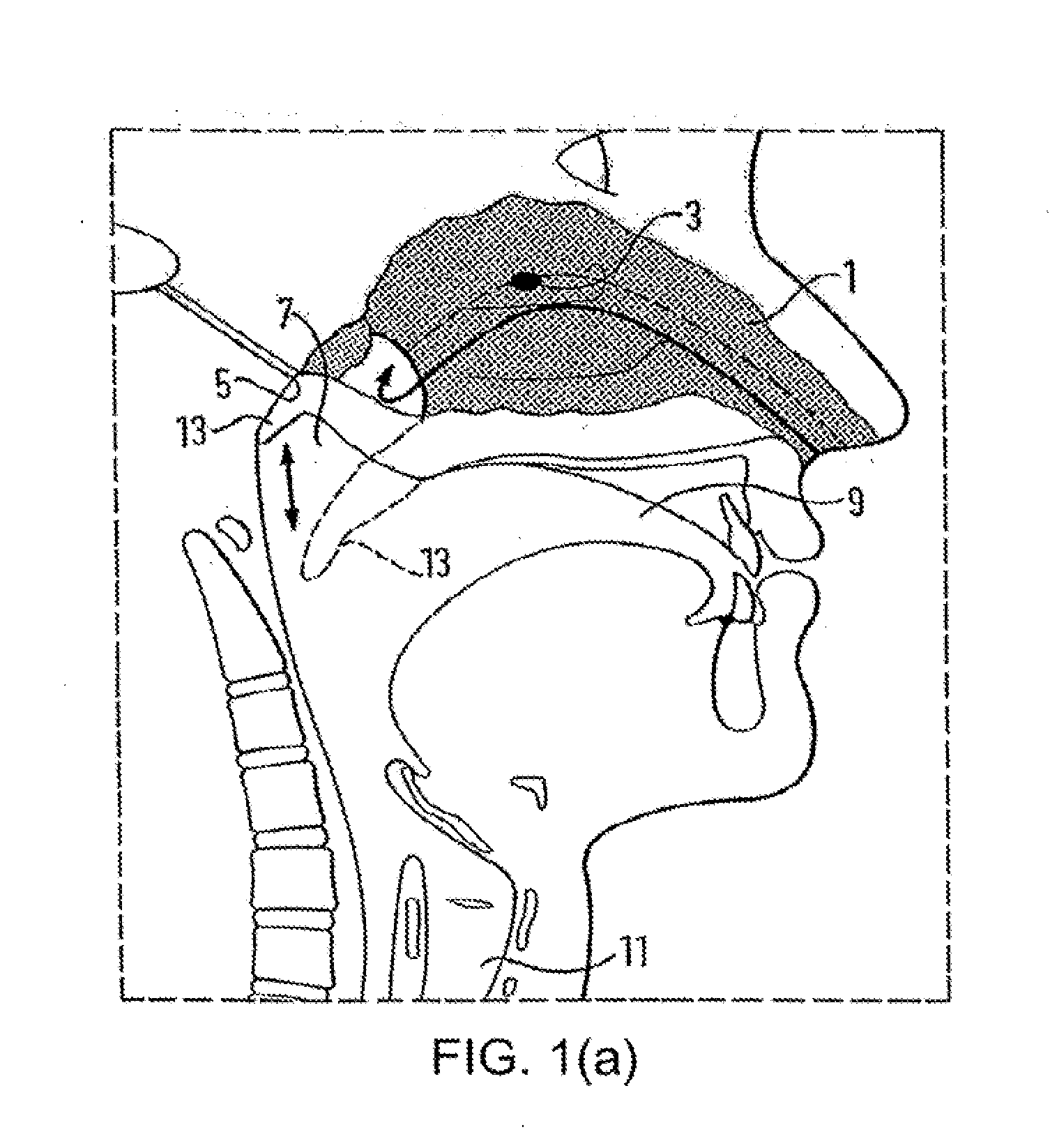
Method and apparatus for treating obstructive sleep apnea by using negative oral pressure to a patient
ActiveUS20070277818A1Improve efficacyImprove complianceRespiratorsBreathing masksNasal cavitySoft palate
The present invention provides a method and apparatus for treating obstructive sleep apnea by using negative oral pressure to a patient. The present apparatus includes a vacuum unit for controlling and maintaining negative pressure of an oral cavity of the patient, a tube with one end thereof connecting to the vacuum unit to suck out air in the oral cavity to generate the negative pressure therein, and a mouthpiece connecting to the other end of the tube and fitting into and sealing the patient's mouth to prevent the oral cavity from air leakage. By using negative pressure in the oral cavity, the patient's soft palate is pulled toward the oral cavity and the patient's tongue is pulled toward an upper palate so as to maintain the patient's nasal air passageway open.
Owner:SOMNICS INC
Nasal mask with integral mouldable straps
InactiveUS6886564B2Simple and cheap to manufactureRespiratory masksBreathing masksThree dimensional shapeNasal mask
A mask for supplying gas under pressure to the nasal airway of a human includes a series of flexible stretchable straps formed from an elastomeric material for locating the mask against the human face. The straps are integrally formed or joined to define a perimeter enclosing an aperture adapted to fit around the nasal area of a human. The straps are configured to approximate to the three dimensional shape of the region around the nasal area of the human face as they arm stretched, to form a tight fitting seal. The mask may be used with or without a manifold.
Owner:AUSTRALIAN CENT FOR ADVANCED MEDICAL TECH
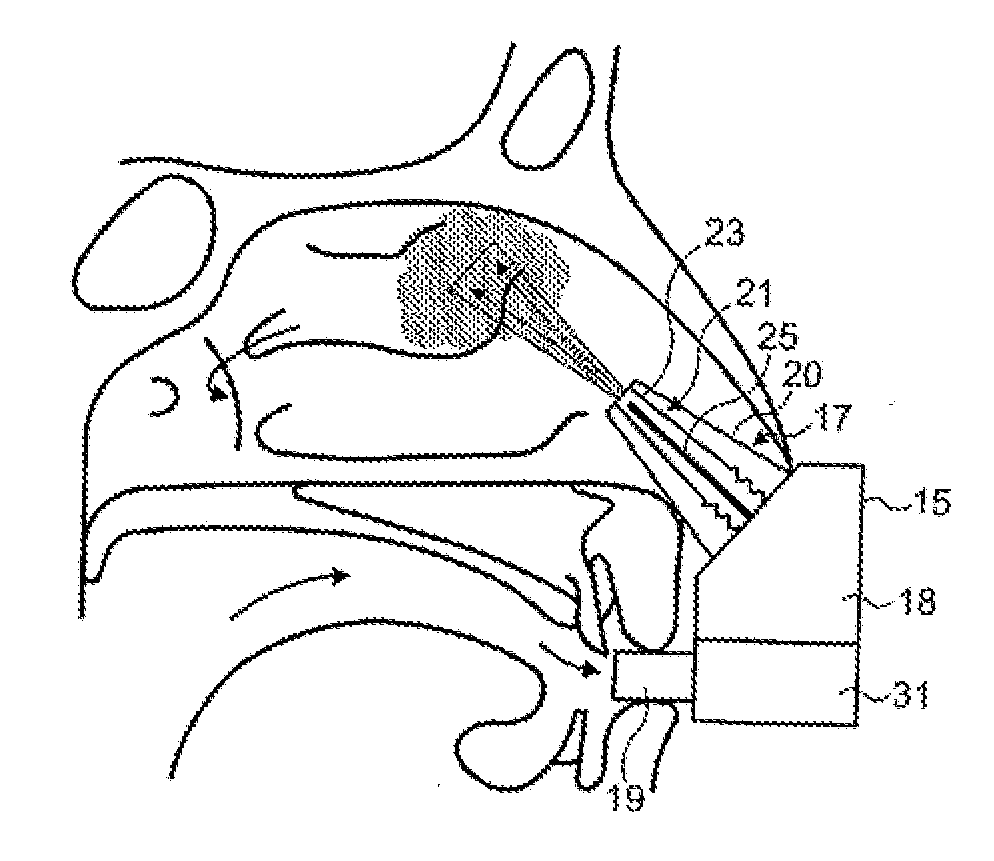
Methods and devices to treat nasal airways
ActiveUS20140316396A1Easy to breatheReduce airflow resistanceElectrotherapySurgical needlesNasal valveSurgery
Methods and devices for treating nasal airways are provided. Such devices and methods may improve airflow through an internal and / or external nasal valve, and comprise the use of mechanical re-shaping, energy application and other treatments to modify the shape, structure, and / or air flow characteristics of an internal nasal valve, an external nasal valve or other nasal airways.
Owner:AERIN MEDICAL INC
Nasal administration
InactiveUS20140144443A1Reduce deliveryGood effectRespiratorsOrganic active ingredientsNasal cavityCns effects
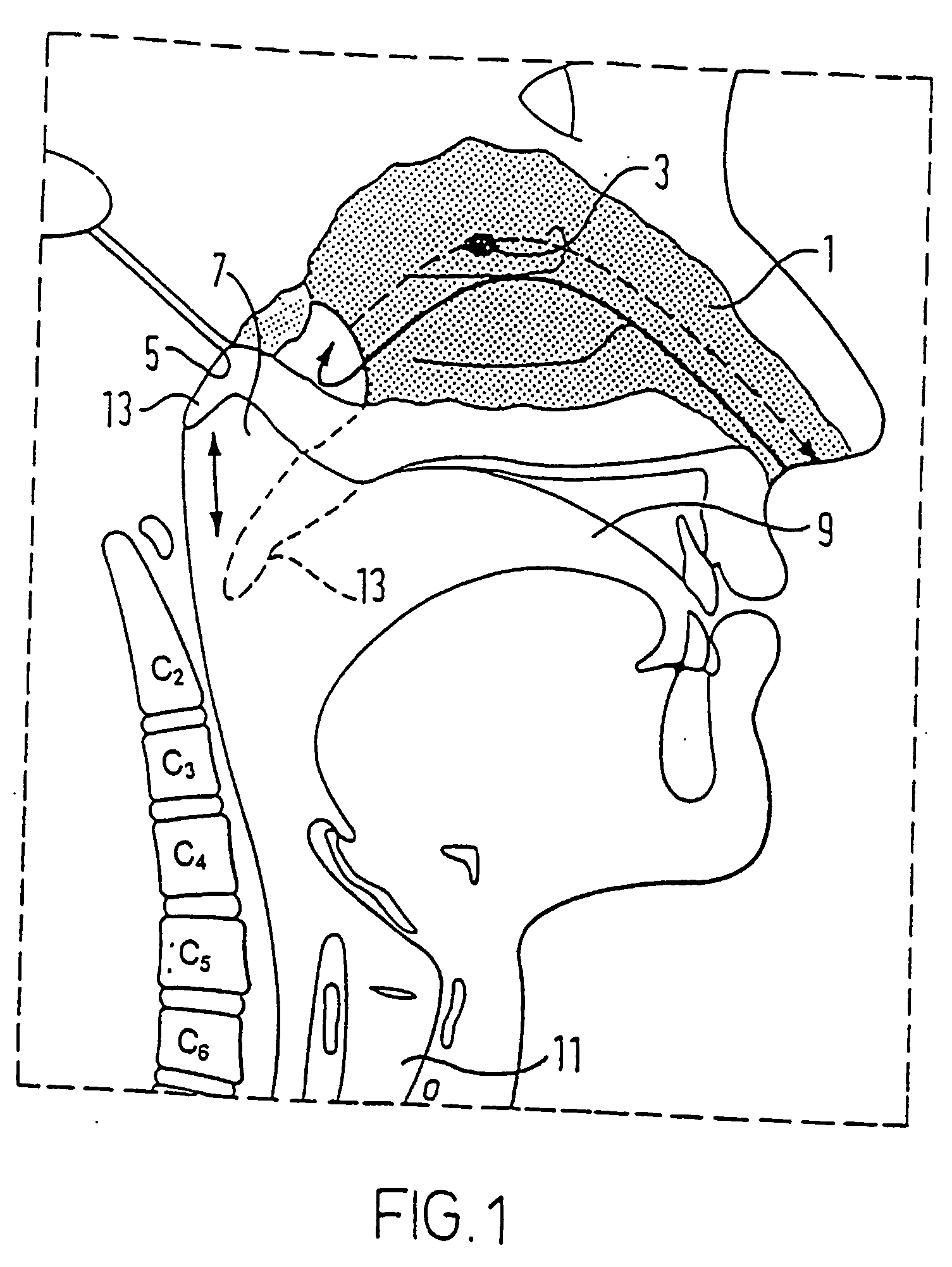
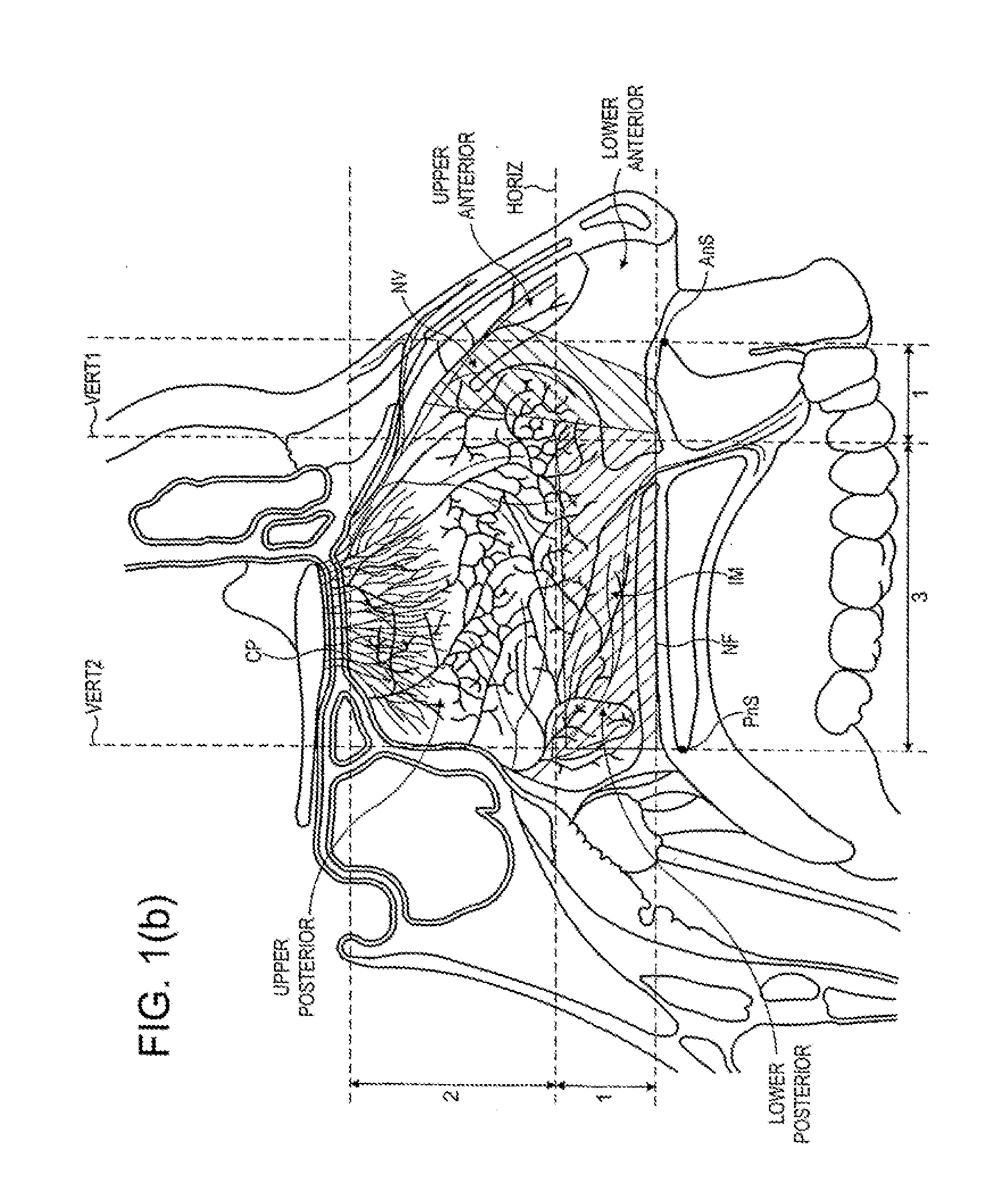
A delivery device for and method of providing for delivery of substance to the central nervous system (CNS) of a subject, the delivery device comprising: a nosepiece unit for insertion into a nasal airway of a subject and comprising an outlet unit which includes a nozzle for delivering substance into the nasal airway of the subject; and a substance supply unit which is operable to deliver a dose of substance to the nozzle; wherein the delivery device is configured such that at least 30% of the dose as initially deposited in the nasal airway is deposited in an upper posterior region of the nasal airway, thereby providing a CNS concentration of the substance, and hence CNS effect, which is significantly greater than that which would be predicted from a counterpart blood plasma concentration of the substance.
Owner:OPTINOSE AS
Nasal devices
A nasal delivery device and a method of delivering substance to a nasal airway of a subject can be used for mass treatment, especially mass vaccination. The delivery device can include an interface unit, as a replaceable unit, having at least one nosepiece unit for fitting to a respective nostril of a subject, a nozzle from which substance is in use delivered, and at least one delivery unit having a substance supply unit for delivering substance to the nozzle of the at least one nosepiece unit. The delivery device can also include an actuation unit for actuating the at least one delivery unit of the interface unit.
Owner:OPTINOSE INC
Nasal devices
A nasal delivery device for and a method of delivering a substance, in particular one of a liquid, as a suspension or solution, or a powder containing a medicament, especially systemic or topical pharmaceuticals, or a vaccine to the nasal airway of a subject.
Owner:OPTINOSE INC
Delivery of gases to the nasal airway
InactiveUS20150090259A1Promote absorptionAvoid possibilityRespiratory masksOxygen respiratorsPharmacologyNasal airway
A delivery device for and a method of delivering gases to the nasal airway, in particular therapeutic gases and gases in combination with active substances, either as powders or liquids, for enhanced uptake of the active substances.
Owner:OPTINOSE AS
Regimens and Compositions for AAV-Mediated Passive Immunization of Airborne Pathogens
InactiveUS20140031418A1Organic active ingredientsBacterial antigen ingredientsRegimenHigh level expression
A prophylactic regimen for passively preventing infection with a pathogen which has a typical route of infection through the nasopharynx region of a subject, e.g., an airborne virus typically transmitted through coughing or sneezing. The method involves specifically targeting a subject's nasopharynx with a viral vector comprising an AAV capsid and carrying a nucleic acid sequence encoding an anti-viral neutralizing antibody construct operably linked to expression control sequences, in order to provide for high levels of expression of the anti-viral neutralizing antibody construct in the nasal airway cells. Optionally, the neutralizing antibody construct is expressed under a promoter which is regulated or induced by a small molecule which is delivered separately from the viral vector. In one embodiment, the method permits transfection of a subject's nasopharynx even where the subject has circulating neutralizing antibodies against the AAV capsid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Nasal administration
InactiveUS20140144442A1Reduce deliveryGreat CNS effectRespiratorsOrganic active ingredientsNasal cavityCns effects
A delivery device for and method of providing for delivery of substance to the central nervous system (CNS) of a subject, the delivery device comprising: a nosepiece unit for insertion into a nasal airway of a subject and comprising an outlet unit which includes a nozzle for delivering substance into the nasal airway of the subject; and a substance supply unit which is operable to deliver a dose of substance to the nozzle; wherein the delivery device is configured such that at least 30% of the dose as initially deposited in the nasal airway is deposited in an upper posterior region of the nasal airway, thereby providing a CNS concentration of the substance, and hence CNS effect, which is significantly greater than that which would be predicted from a counterpart blood plasma concentration of the substance.
Owner:OPTINOSE AS
Nasal Administration
InactiveUS20150367090A1Reduce deliveryGood effectTracheal tubesOrganic active ingredientsNasal cavityPosterior region
A delivery device for and method of providing for delivery of substance to the central nervous system (CNS) of a subject, the delivery device comprising: a nosepiece unit for insertion into a nasal airway of a subject and comprising an outlet unit which includes a nozzle for delivering substance into the nasal airway of the subject; and a substance supply unit which is operable to deliver a dose of substance to the nozzle; wherein the delivery device is configured such that at least 30% of the dose as initially deposited in the nasal airway is deposited in an upper posterior region of the nasal airway, thereby providing a CNS concentration of the substance, and hence CNS effect, which is significantly greater than that which would be predicted from a counterpart blood plasma concentration of the substance.
Owner:OPTINOSE AS
Methods and devices to treat nasal airways
ActiveUS10456185B2Easy to breatheReduce airflow resistanceElectrotherapySurgical needlesNostrilNasal septum
A method for reshaping a nasal airway in a patient involves advancing an inflatable balloon of a reshaping device in an uninflated configuration into a nostril of the patient and between a nasal septum and a lateral wall of the nasal airway. The method then involves inflating the inflatable balloon to an inflated configuration to cause the inflatable balloon to contact nasal mucosa covering the nasal septum and the lateral wall, delivering energy from an energy delivery member attached to or inside of the inflatable balloon, and removing the reshaping device from the nasal airway.
Owner:AERIN MEDICAL
Nasal delivery devices
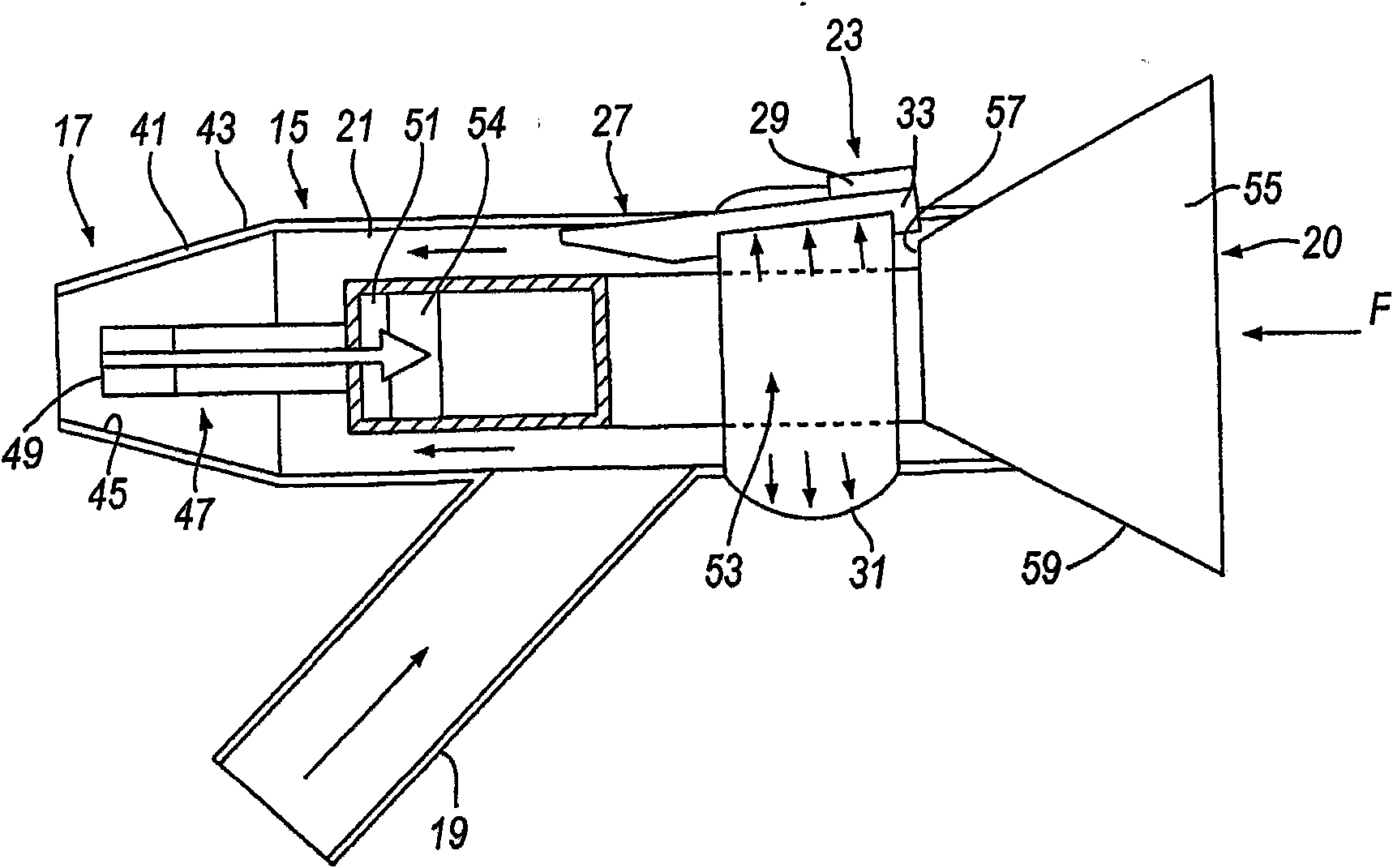
A nasal delivery device for and a method of delivering substance to a nasal airway of a subject, the nasal delivery device comprising : a mouthpiece (519) through which the subject in use exhales to cause closure of the oropharyngeal velum of the subject; a nosepiece (517) for fitting to a nostril of a subject, the nosepiece including a nozzle (549) through which substance is in use delivered to the nasal airway; and a manually-actuatable substance supply unit (520) for delivering substance through the nozzle of the nosepiece.
Owner:OPTINOSE INC
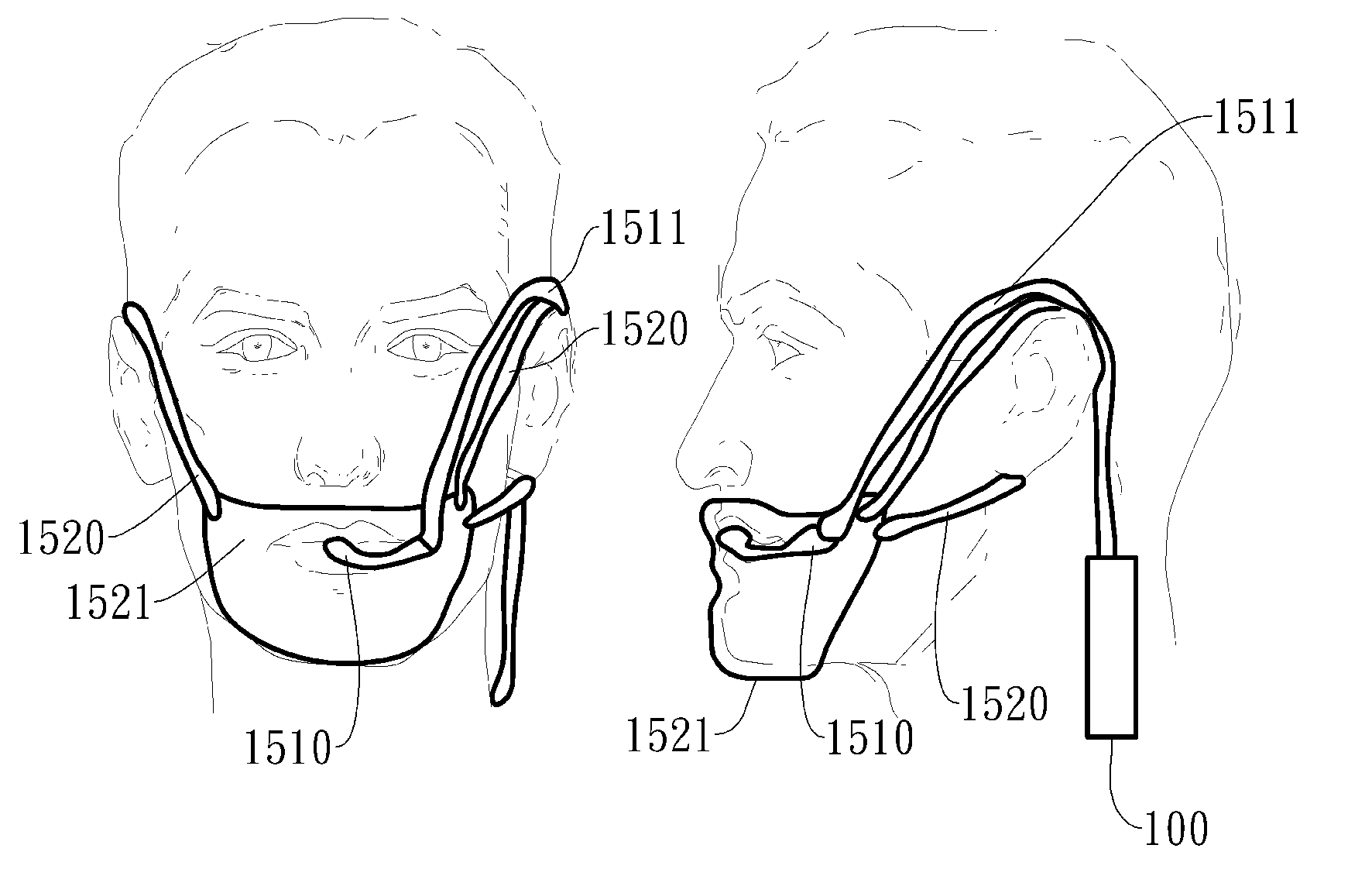
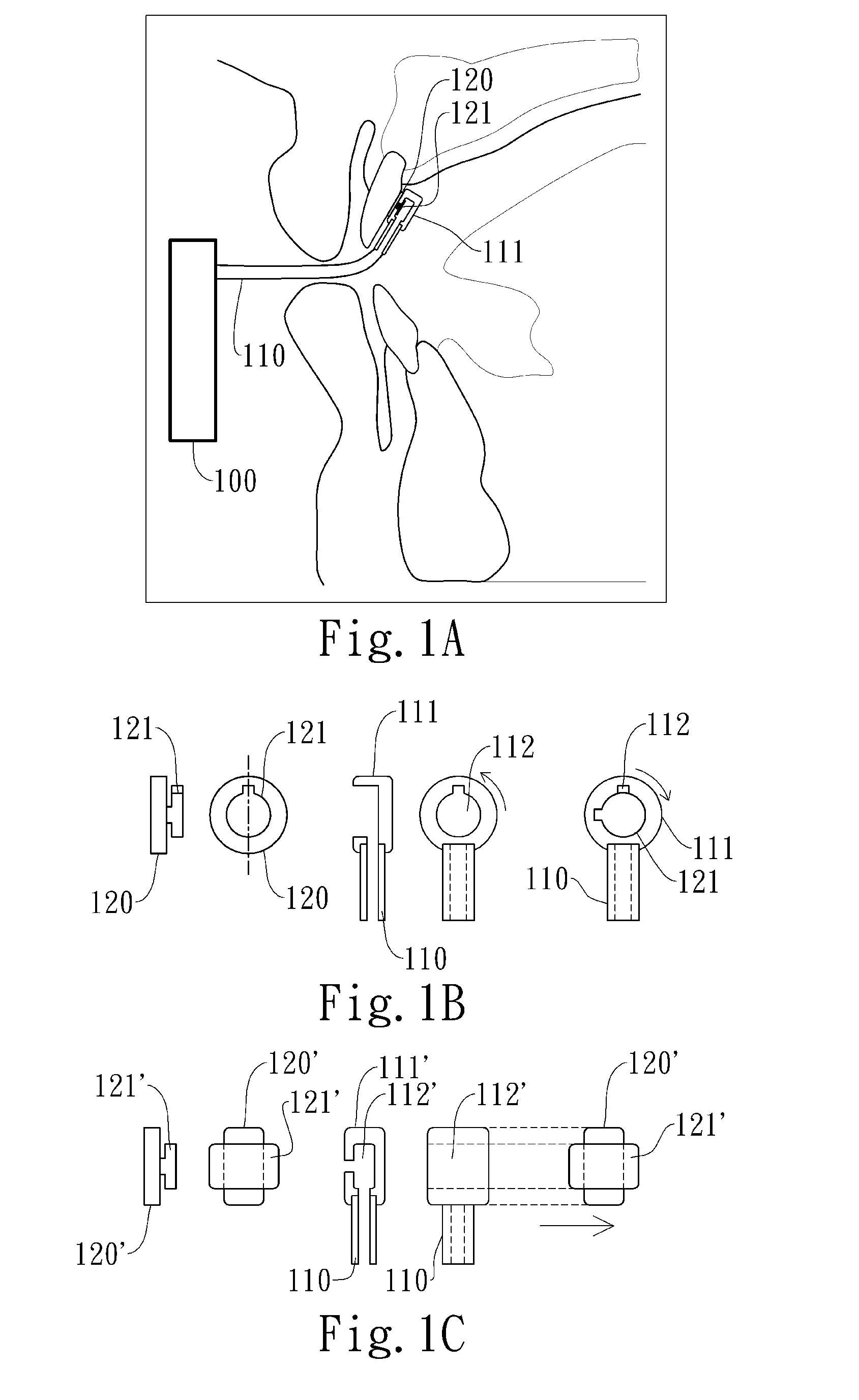
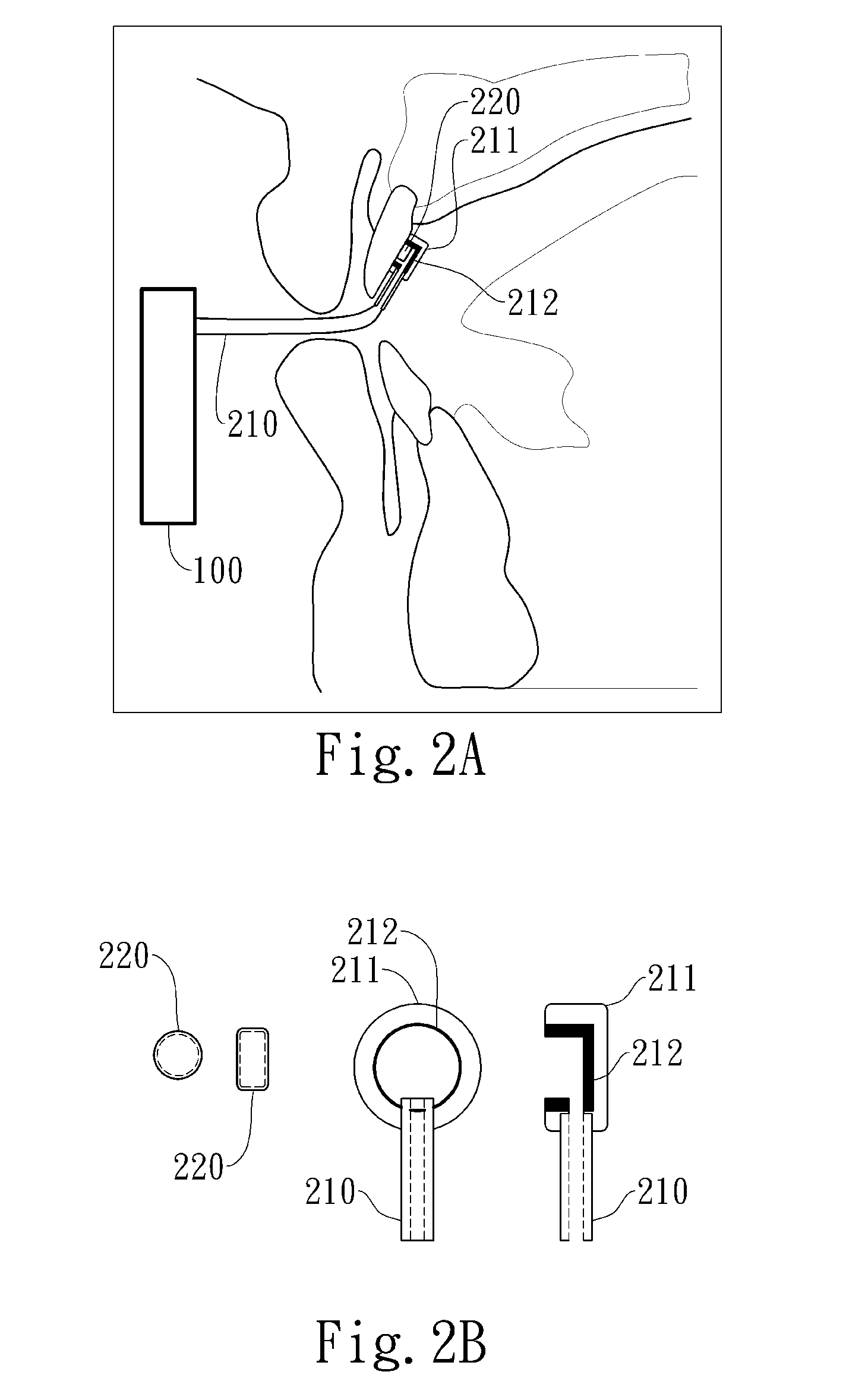
Negative pressure oral apparatus
ActiveUS20110073119A1Inhibit sheddingReduce curingRespiratorsSnoring preventionSleep disordered breathingPalate muscle
This invention provides an oral apparatus and method capable of alleviating or curing snore and obstructive sleep apnea by applying a negative pressure through a mini oral interface to the oral cavity. The mini oral interface creates a secure connection between the interface and mouth or teeth and prevents the interface from falling off. The oral interface also provides fixation between upper and lower lips (or tooth) and prevents opening of patient's mouth during sleeping. The negative pressure pulls the tongue toward upper palate and also pulls the soft palate forward as well. By moving the tongue and the soft palate in a forward direction, the patentcy of the upper airway near the pharynx is maintained to prevent sleep-disordered breathing. The negative pressure will pull the lips inward to close the mouth ad prevent air from entering the oral cavity from atmosphere. The negative pressure will also pull the soft palate into contact with the rear surface of the tongue to create a seal that prevents the air from entering the oral cavity through the nasal airway.
Owner:SOMNICS INC
Nose airway for aromatherapy and detecting airborne pathogens
InactiveUS20050199245A1Controlling and limiting projectionEasy and inexpensive to manufactureFire rescueInvestigating presence of gasesAromatherapyMedicine
A nasal airway for insertion in the nose for the delivery of volatile compositions into air entering the nose or, in a second embodiment, for the detection of targeted airborne pathogenic organisms present in the respirated airstream. The volatile compositions include aromatherapeutic compositions and scents.
Owner:BRENNAN H GEORGE
Devices to dilate nasal airways for various applications involving: activities using goggles with a helmet or goggles alone; swimming with goggles, without or with a swim cap; sleep; sleep with a cpap mask; and for physical activities
InactiveUS20150216710A1Improve nasal patencyMany applicationsGogglesRespiratory masksNasal cavityTotal occlusion
Medical devices are disclosed as providing nasal airway dilation in subjects in need thereof whose nasal airways are partially or completely blocked, such as for sleep; with CPAP masks; for exercise; wearing goggles alone or with helmets; and for swimming. In an aspect, the devices with a group of applications provide nasal dilation to overcome obstruction caused by nasal valve dysfunction. Methods of employing the same to dilate nasal airways are further disclosed.
Owner:NOZEWAIR
Methods of hydrating mucosal surfaces
InactiveUS7056524B2Avoid reabsorptionPromote secretionOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolP2Y2 Receptors
A method of hydrating nasal airway surfaces in a subject in need of such treatment comprises topically applying a sodium channel blocker to a nasal airway surface of the subject in an amount effective to inhibit the reabsorption of water by the surface. The channel blocker may be a pyrazinoylguanidine sodium channel blocker, such as benzamil, phenamil, amiloride, or a pharmaceutically acceptable salts thereof. The method may further comprise the step of topically applying a P2Y2 receptor agonist to a nasal airway surface of the subject in an amount effective to stimulate chloride secretion by the nasal airway surface. In a preferred embodiment, the sodium channel blocker is a covalent conjugate of a pyrazinoylguanidine sodium channel blocker and a non-absorbable carrier moiety (e.g., albumin, polyethylene glycol). Such compounds may also be administered to other mucosal surfaces where it is desired to inhibit the reabsorption of water.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Methods of treating nasal airways
ActiveUS20190336196A1Decrease airflow resistance perceived airflowIncrease ratingsElectrotherapySurgical needlesSurgical incisionNasal valve
A device is described for treating a nasal airway by modifying a property of a nasal tissue of or near a nasal valve of the airway, without using a surgical incision or an implant, to decrease airflow resistance or perceived airflow resistance in the nasal airway. Various embodiments include an elongate shaft, a bipolar radiofrequency delivery member extending from one end of the shaft, and a handle attached to the elongate shaft at an opposite end from the radiofrequency delivery member. The radiofrequency delivery member is sized to be inserted into a nose and configured to at least temporarily deform the nasal tissue and deliver radiofrequency energy. The radiofrequency delivery member includes two rows of protruding electrodes disposed on a tissue contact surface, and the device is configured to deliver radiofrequency energy from one row of electrodes to the other row of electrodes.
Owner:AERIN MEDICAL
Automated negative pressure oral apparatus
InactiveUS20110192404A1Reduce curingPrevent disengagementRespiratorsSnoring preventionSleep disordered breathingPalate muscle
This invention provides an oral apparatus and method capable of alleviating or curing snore and obstructive sleep apnea by applying a negative pressure through a mini oral interface to the oral cavity. The mini oral interface creates a secure connection to mouth and helps a patient's upper and lower lips closed during sleeping. The negative pressure pulls the tongue toward upper palate and also pulls the soft palate forward as well. By moving the tongue and the soft tissue in a forward direction, the patency of the upper airway near the pharynx is maintained to prevent sleep-disordered breathing. The negative pressure will pull the lips inward to close the mouth preventing air from entering the oral cavity from atmosphere. The negative pressure will also pull the soft palate into contact with the rear surface of the tongue to create a seal that prevents the air from entering the oral cavity through the nasal airway.
Owner:SOMNICS INC
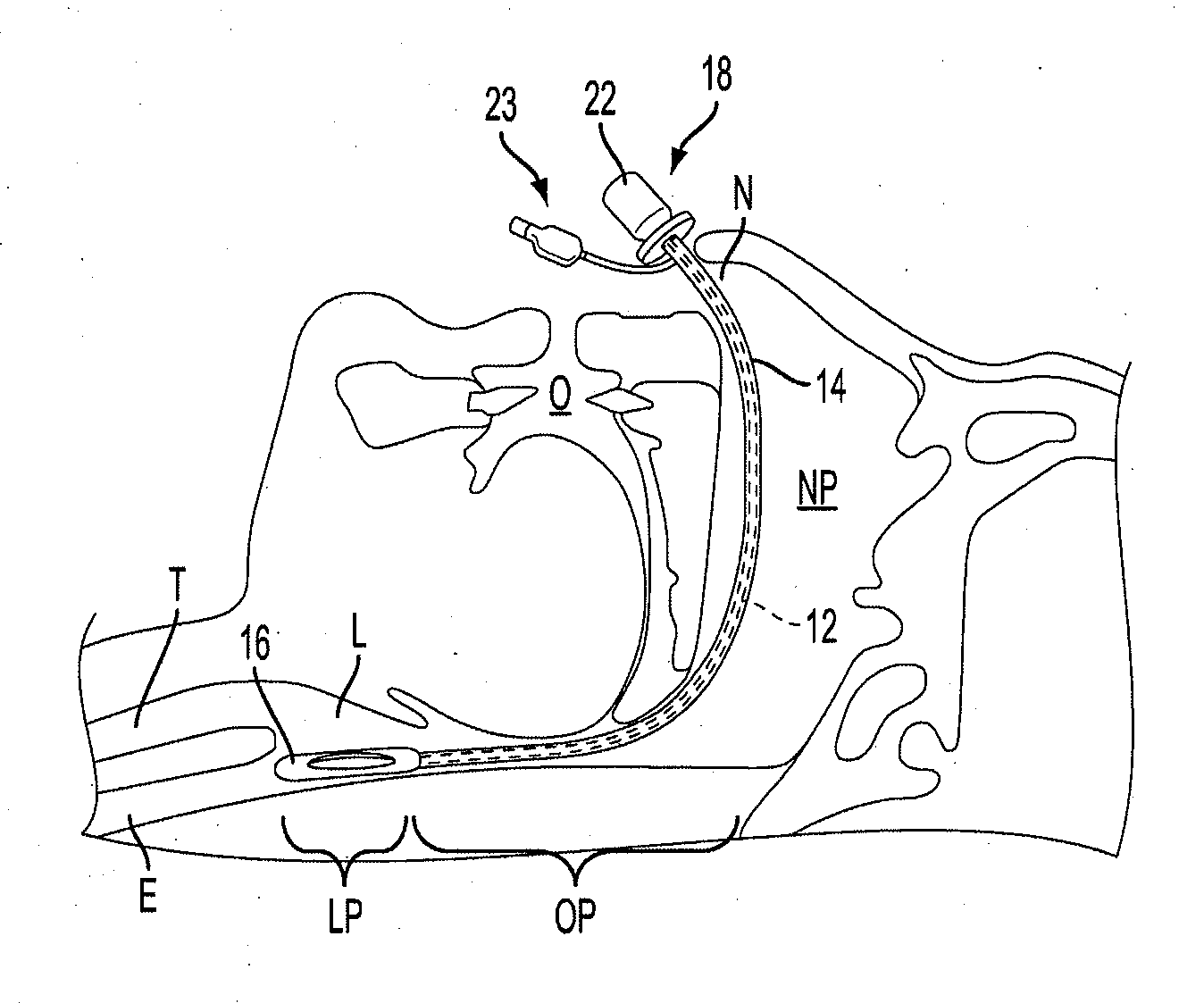
Nasal airway management device
An airway management device including a hollow flexible tube. The tube defines a lumen extending between a proximal end and a distal end. The proximal end is configured to be coupled to a ventilator and / or anesthesia circuit. An opening is provided at or near the distal end to allow passage of air and / or anesthesia therethrough. The tube is configured to be expanded radially after insertion through a patient's nasopharyngeal passageway when the distal end of the tube is positioned proximate the patient's hypopharynx. The device may include an inflatable outer sleeve surrounding and extending along the length of the tube. When the outer sleeve is in a deflated state, the distal end of the tube may be inserted through a patient's nasopharyngeal passageway. When the distal end of the tube is positioned proximate the patient's hypopharynx, the outer sleeve may be inflated and expanded. The device may include an inflatable cuff attached at or near the distal end of the tube and including an opening fluidly coupled with the opening and lumen of the tube to allow passage of air and / or anesthesia therethrough. When in a deflated state, the inflatable cuff may be inserted through the patient's nasopharyngeal passageway. When in an inflated state, the inflatable cuff may expand to form a seal around the patient's supraglottic laryngeal inlet. An intermediate inflatable cuff having a larger diameter than other portions of the outer sleeve when inflated may be positioned along the length of the tube between the proximal and distal ends. The respective inflatable elements of the device may be concurrently or separately inflatable.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods and devices to treat nasal airways
ActiveUS10722282B2Easy to breatheReduce airflow resistanceElectrotherapySurgical needlesNasal foramenBiomedical engineering
Owner:AERIN MEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com