Patents
Literature
500 results about "Pharynx" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The pharynx (plural: pharynges) is the part of the throat behind the mouth and nasal cavity, and above the esophagus and larynx – the tubes going down to the stomach and the lungs. It is found in vertebrates and invertebrates, though its structure varies across species.
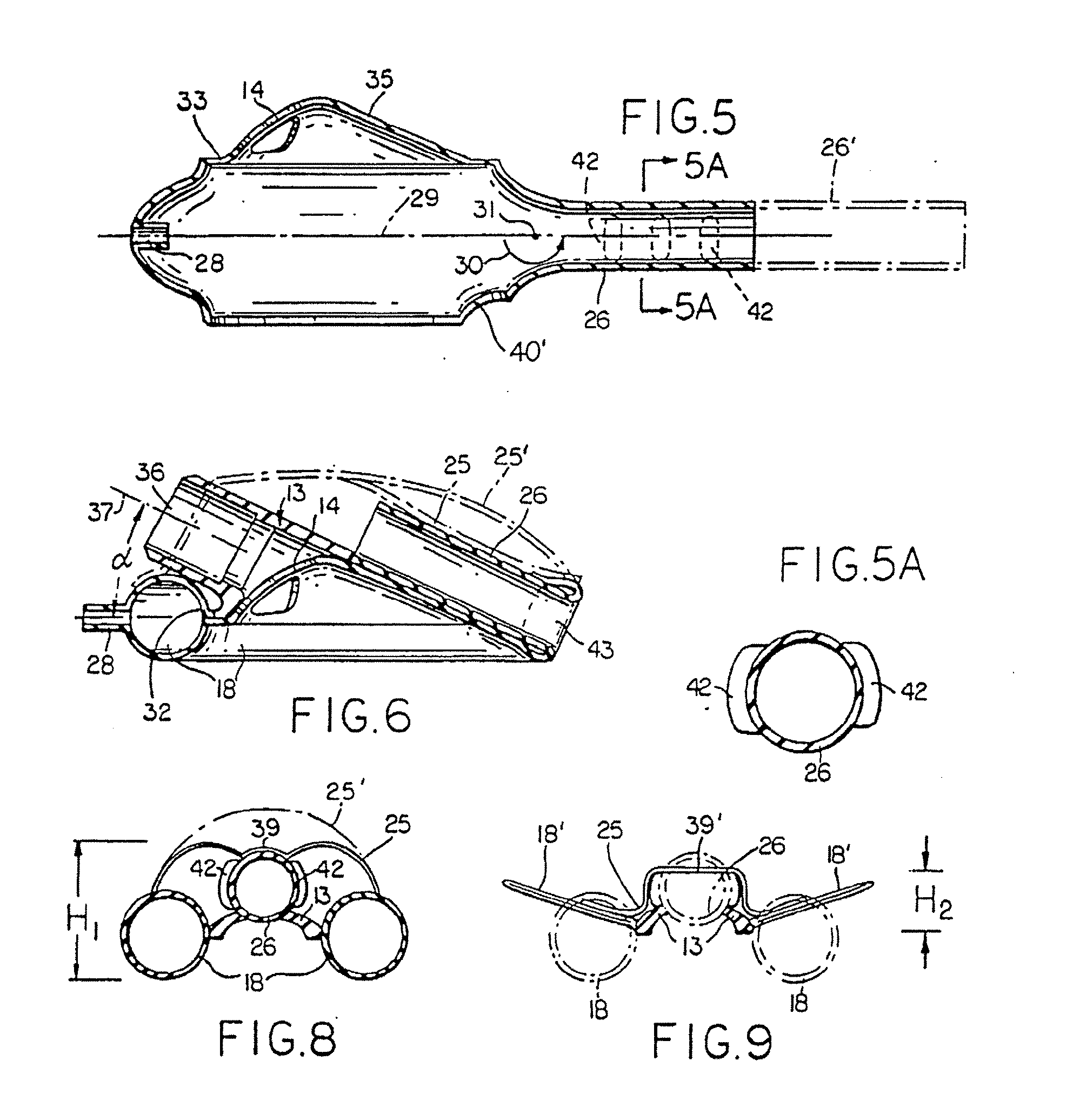
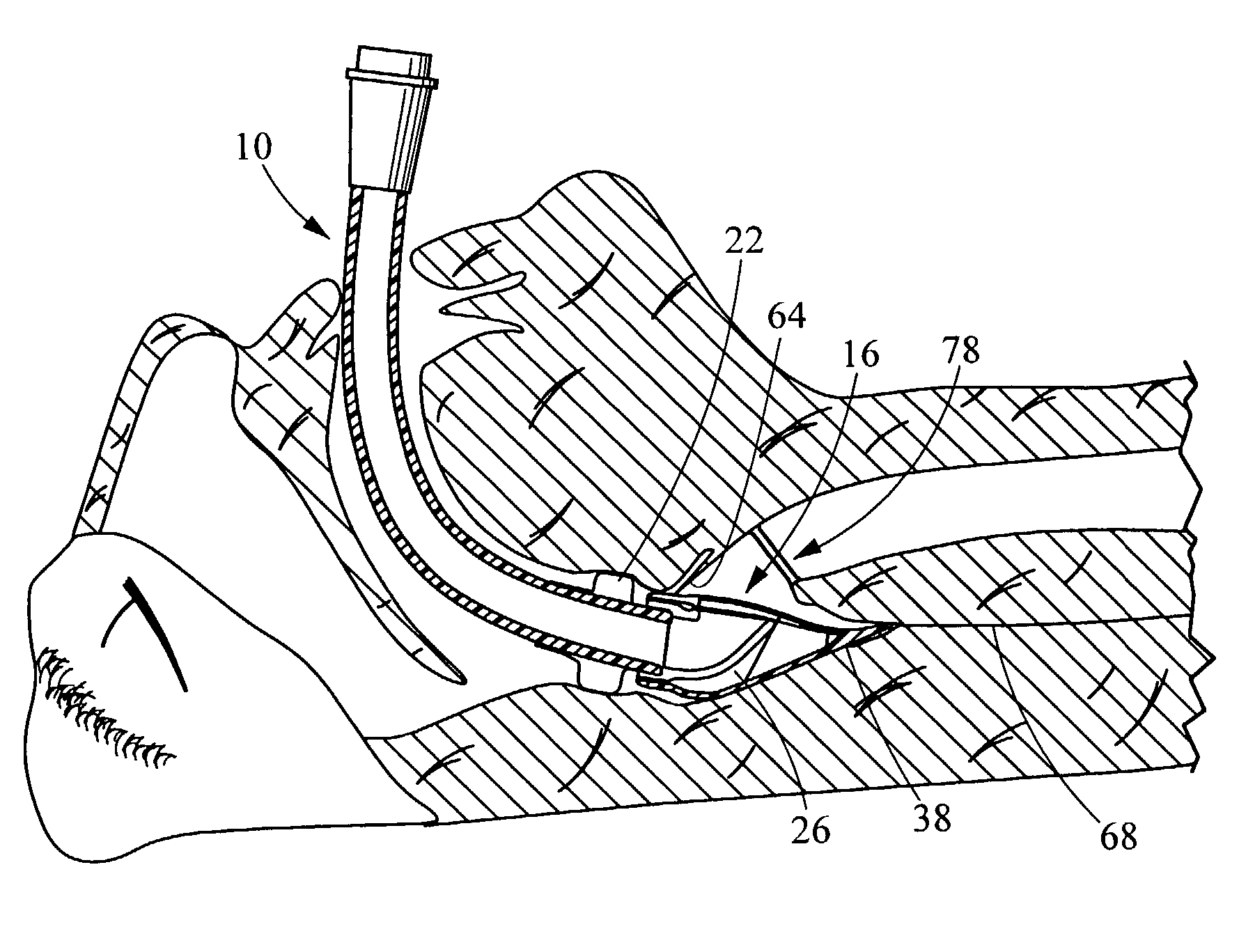
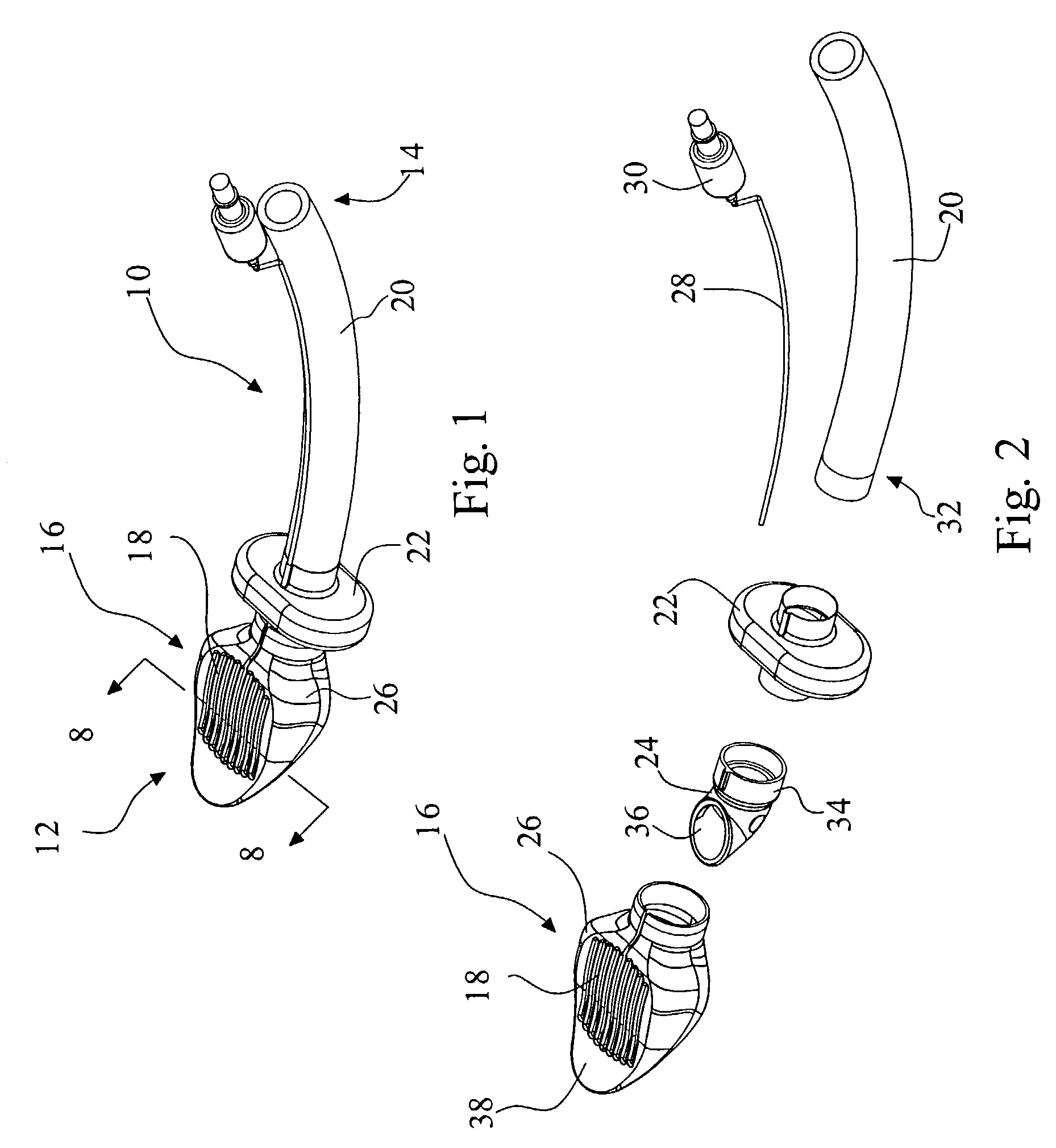
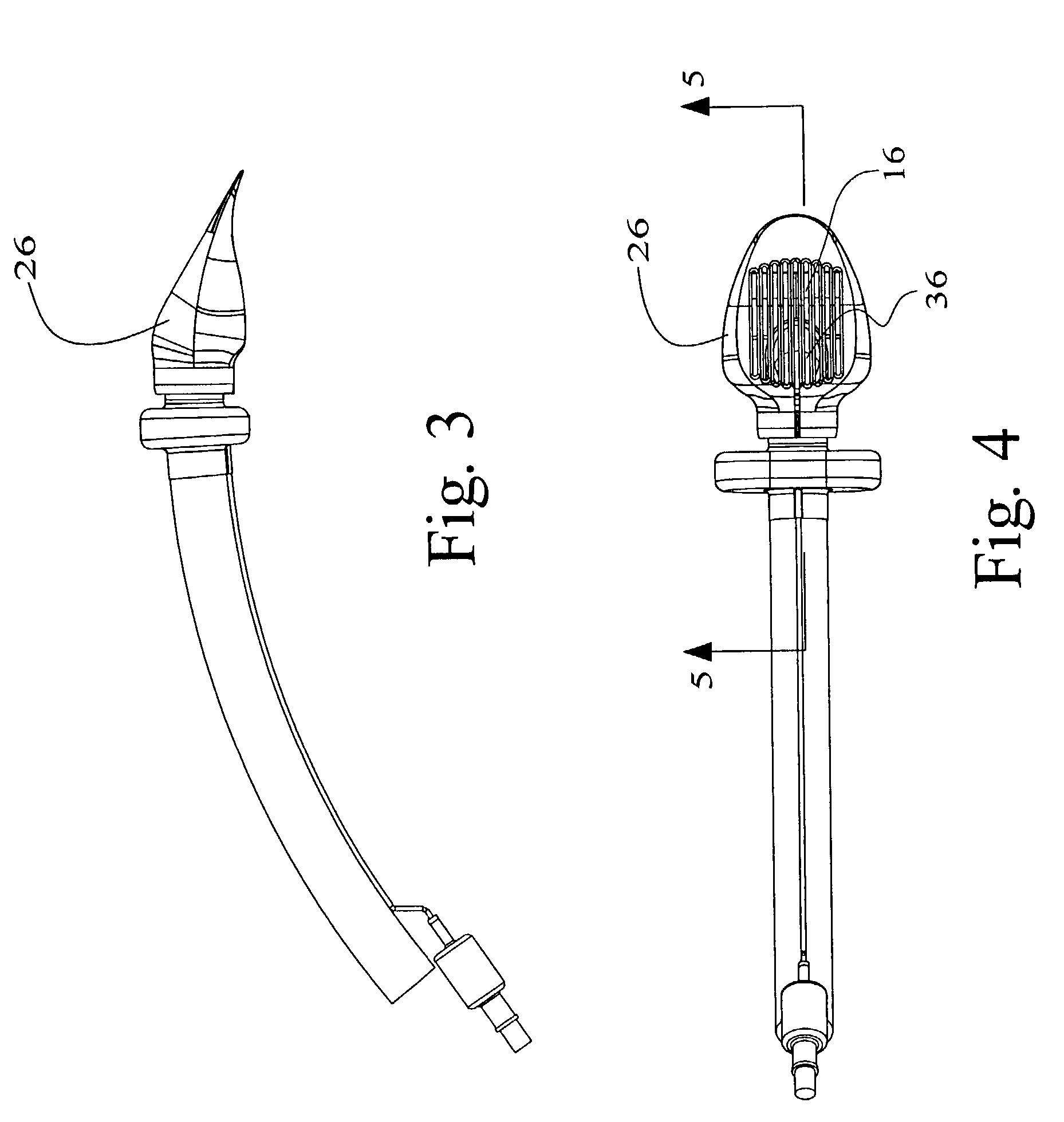
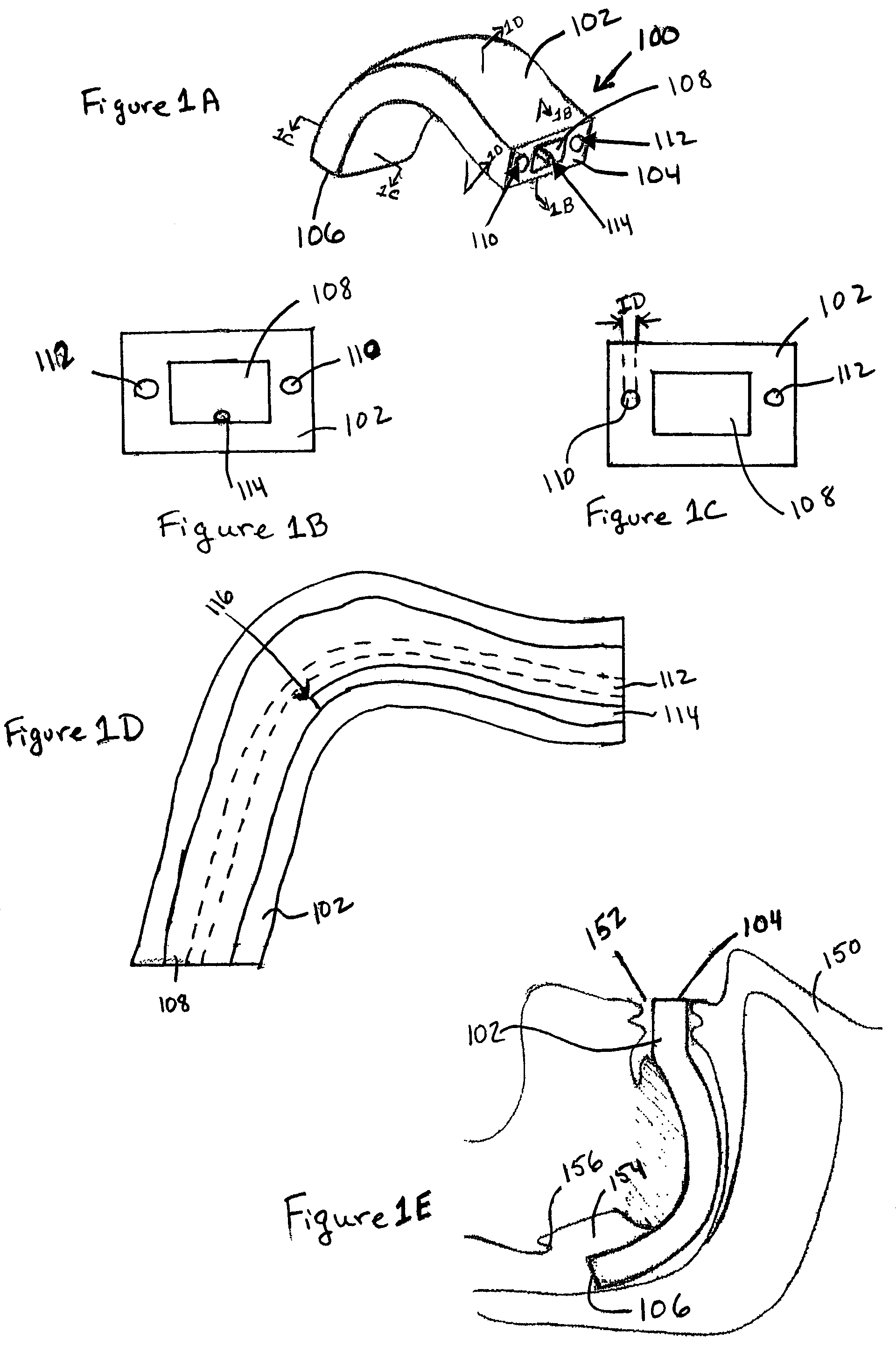
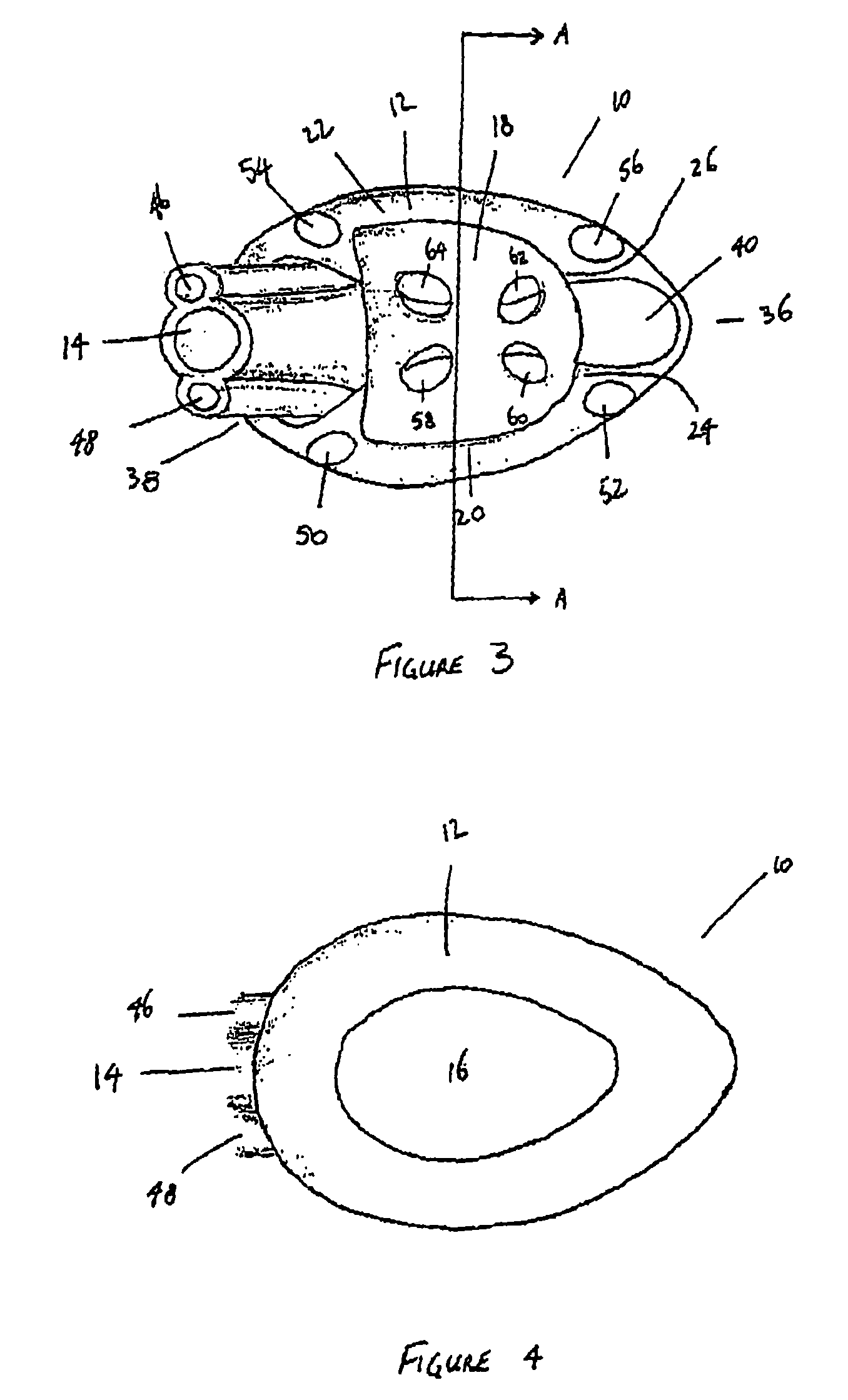
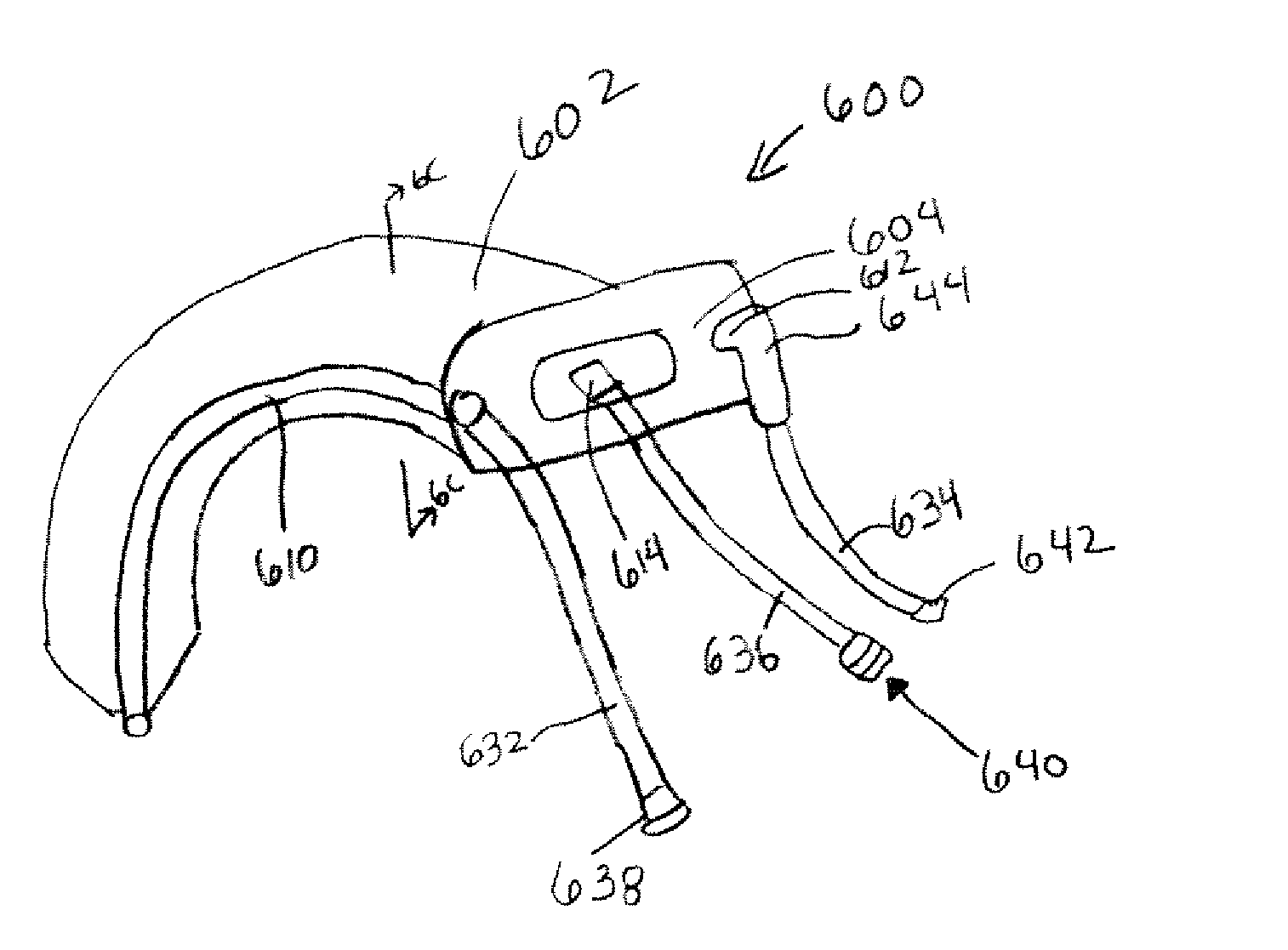
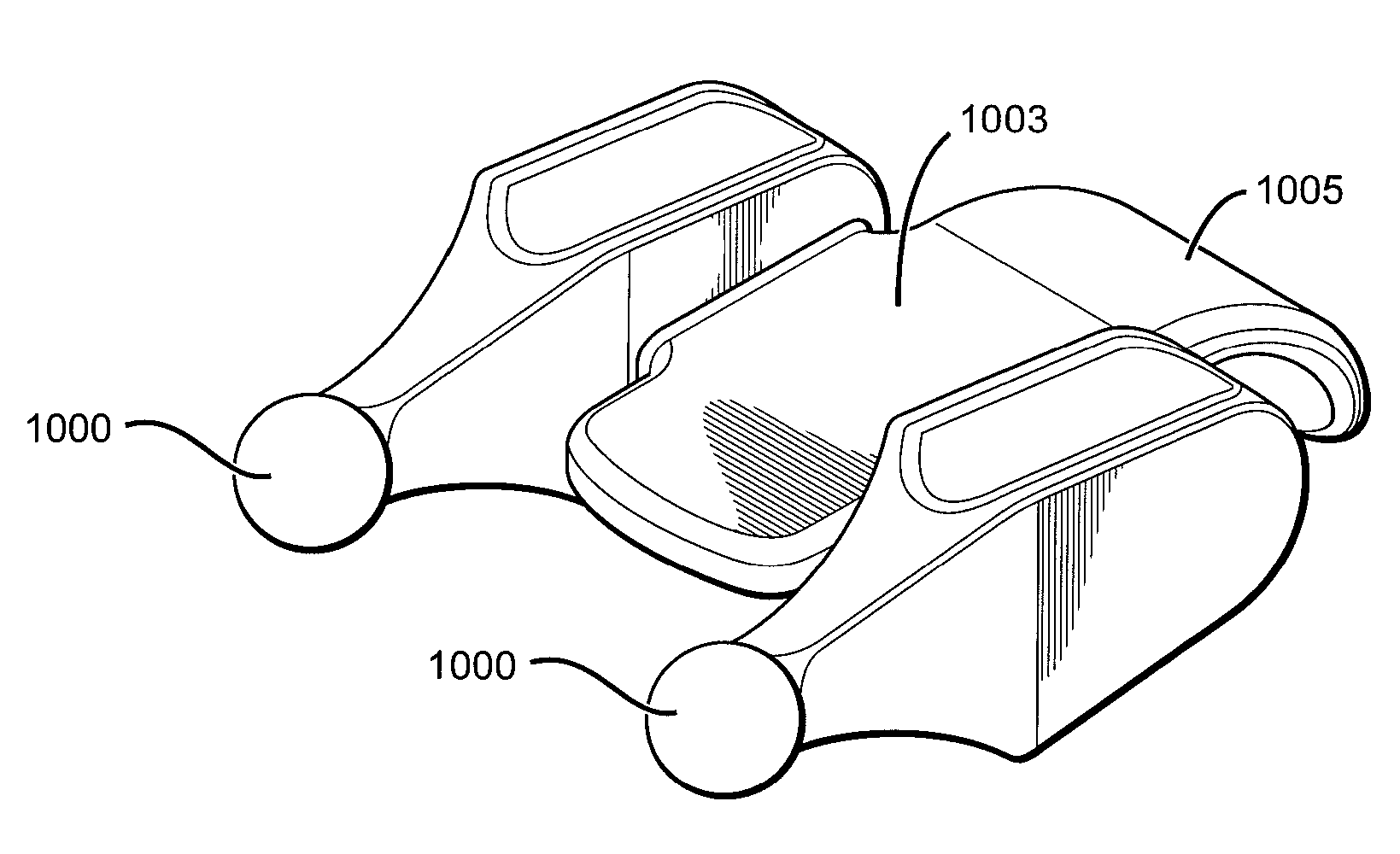
Electrosurgical system and method
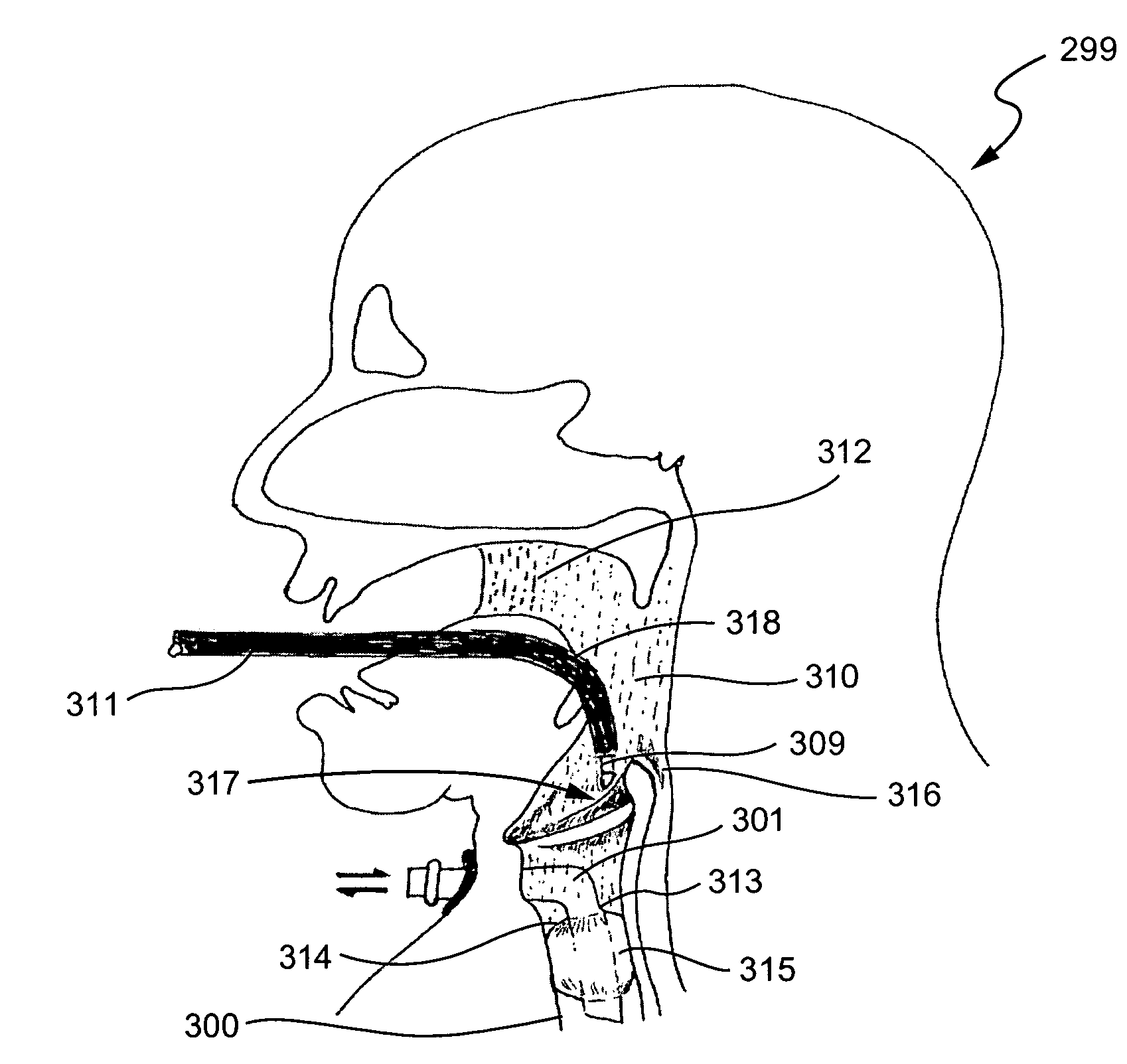
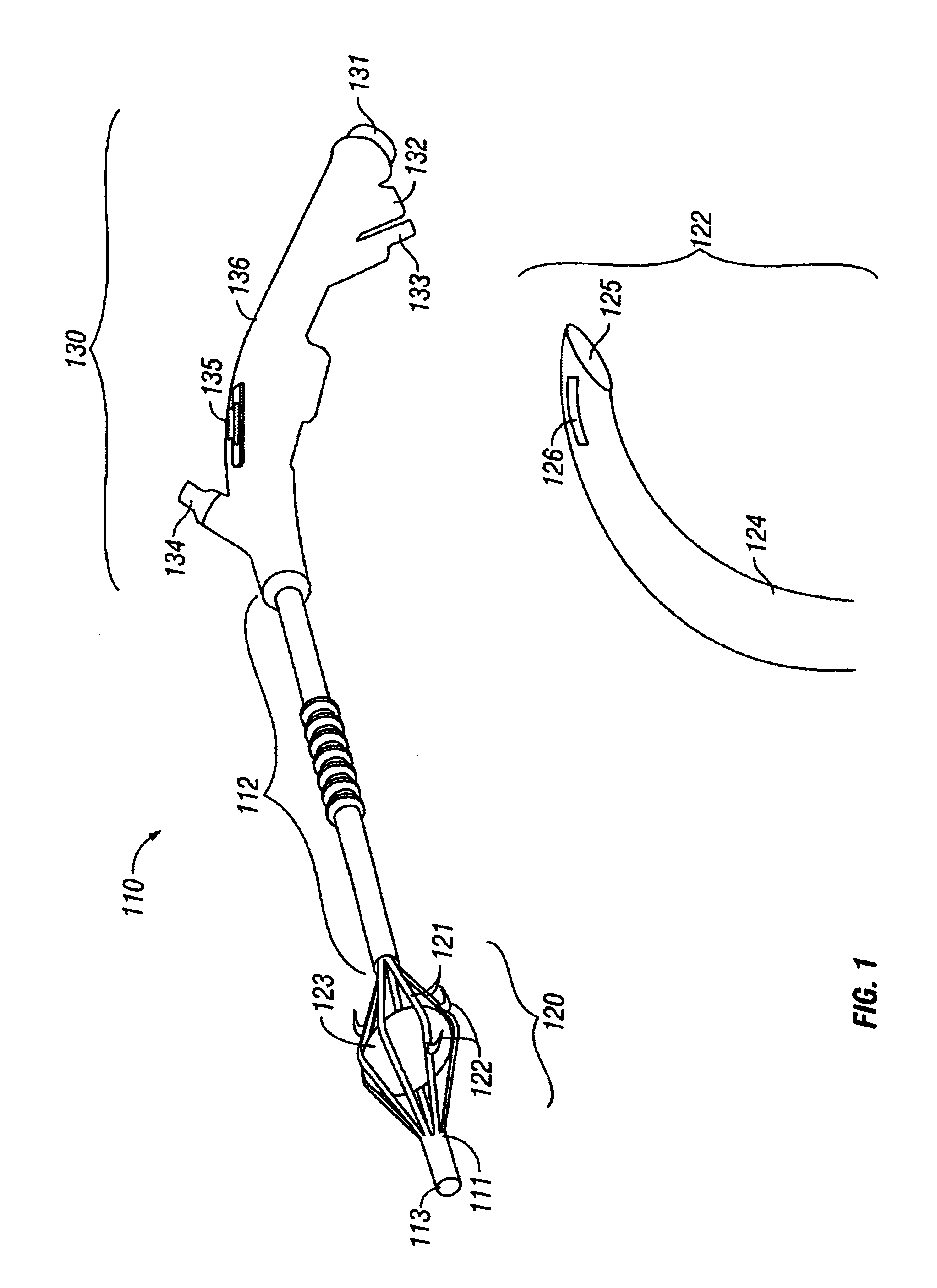
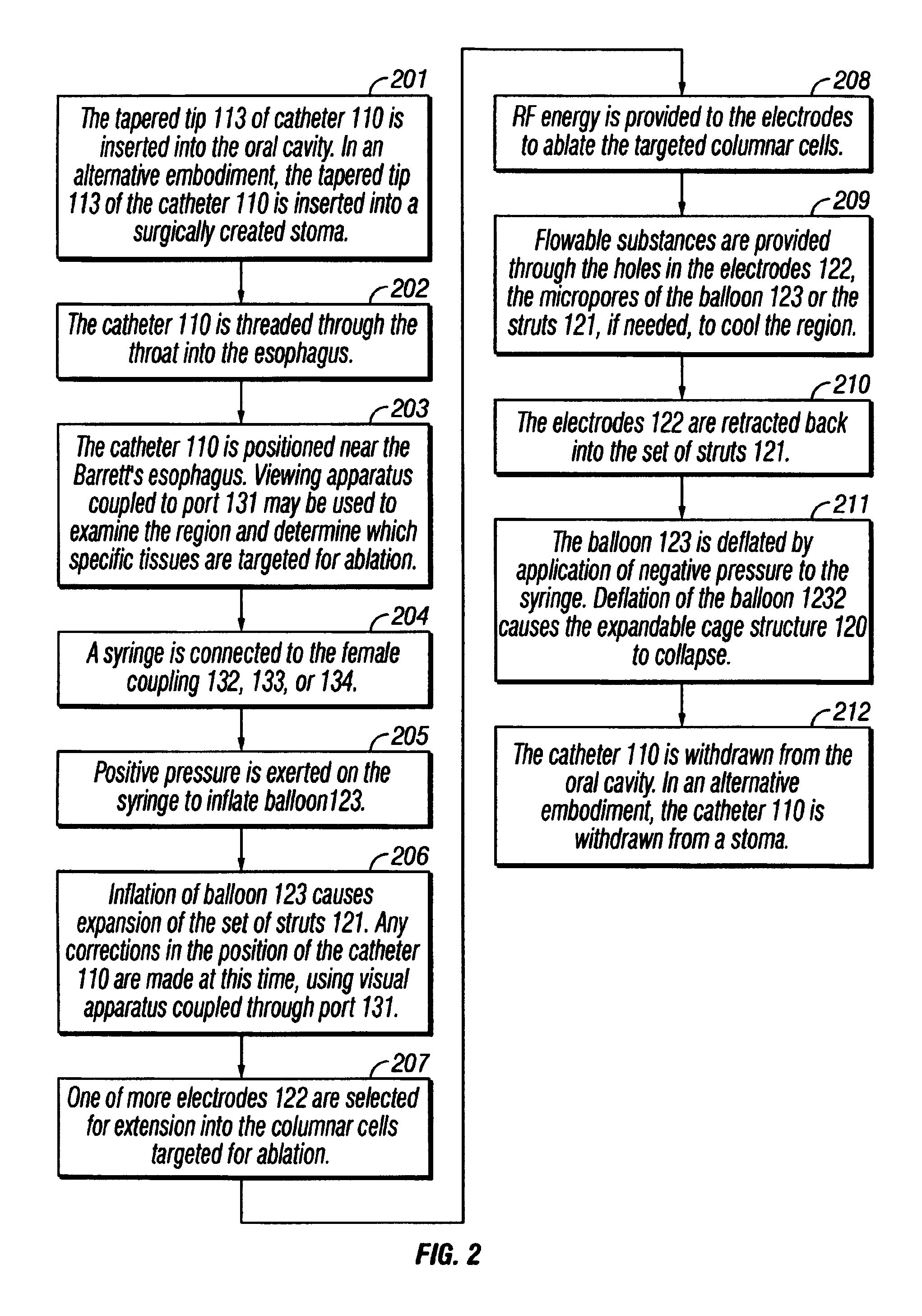
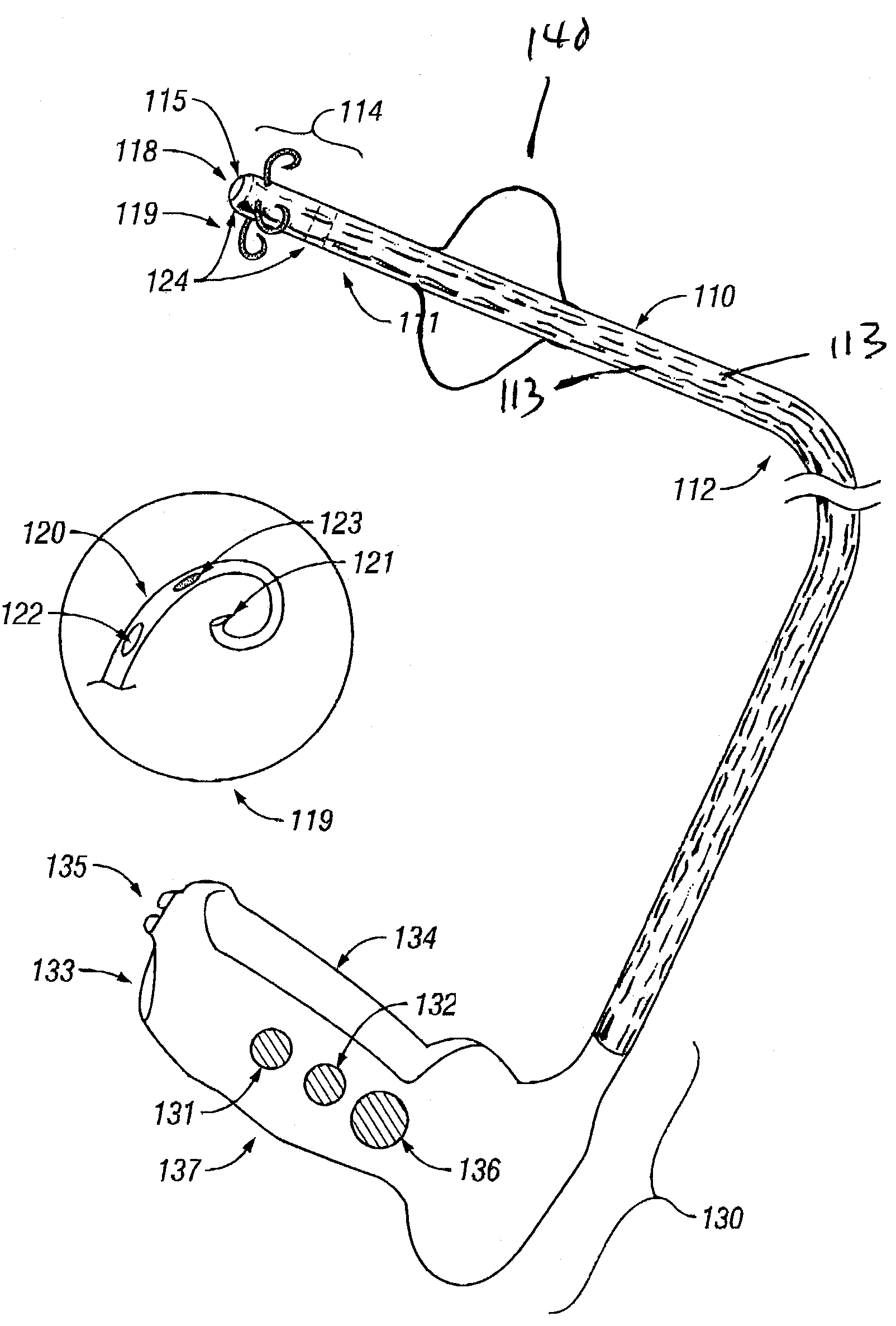
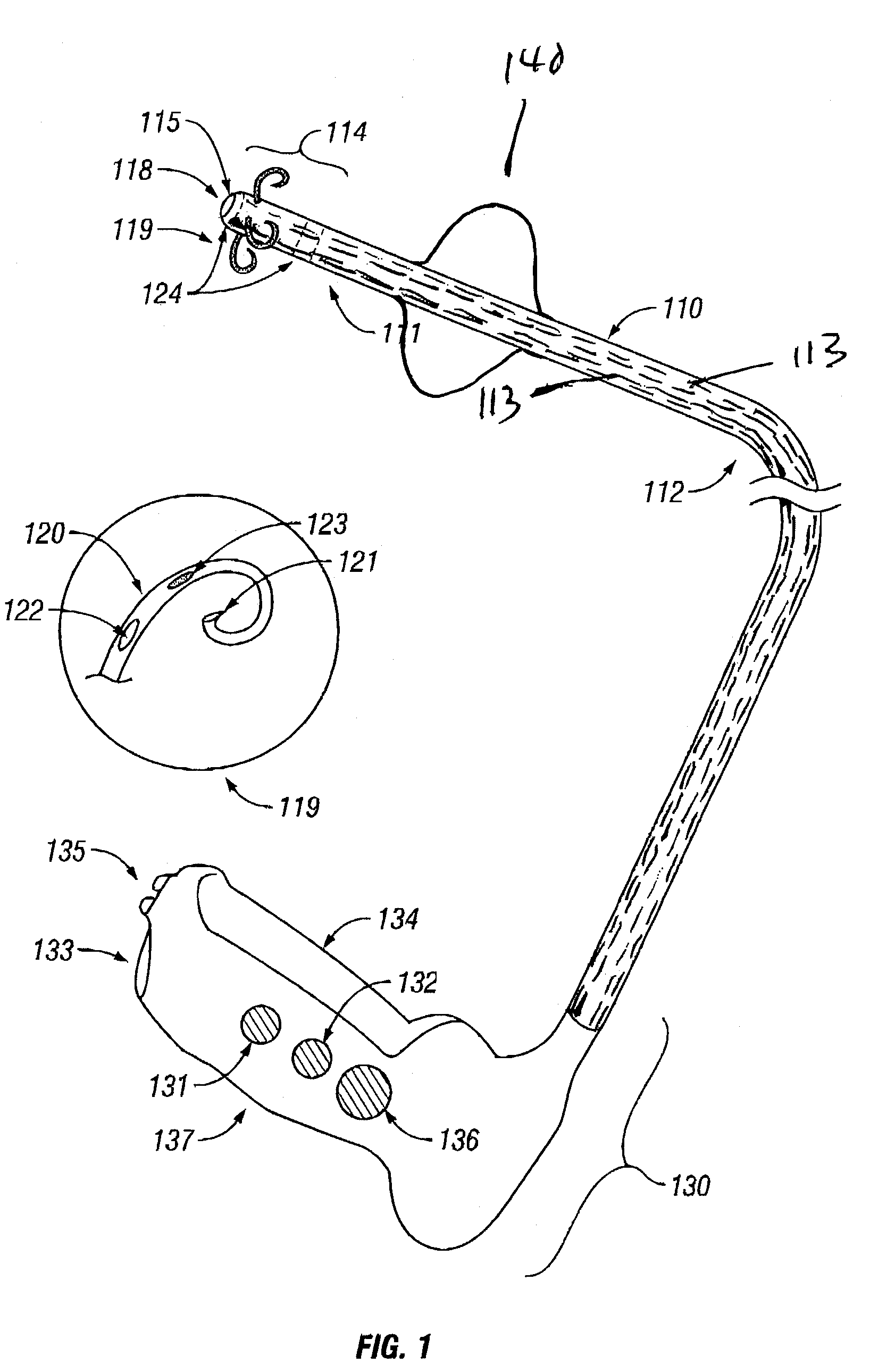
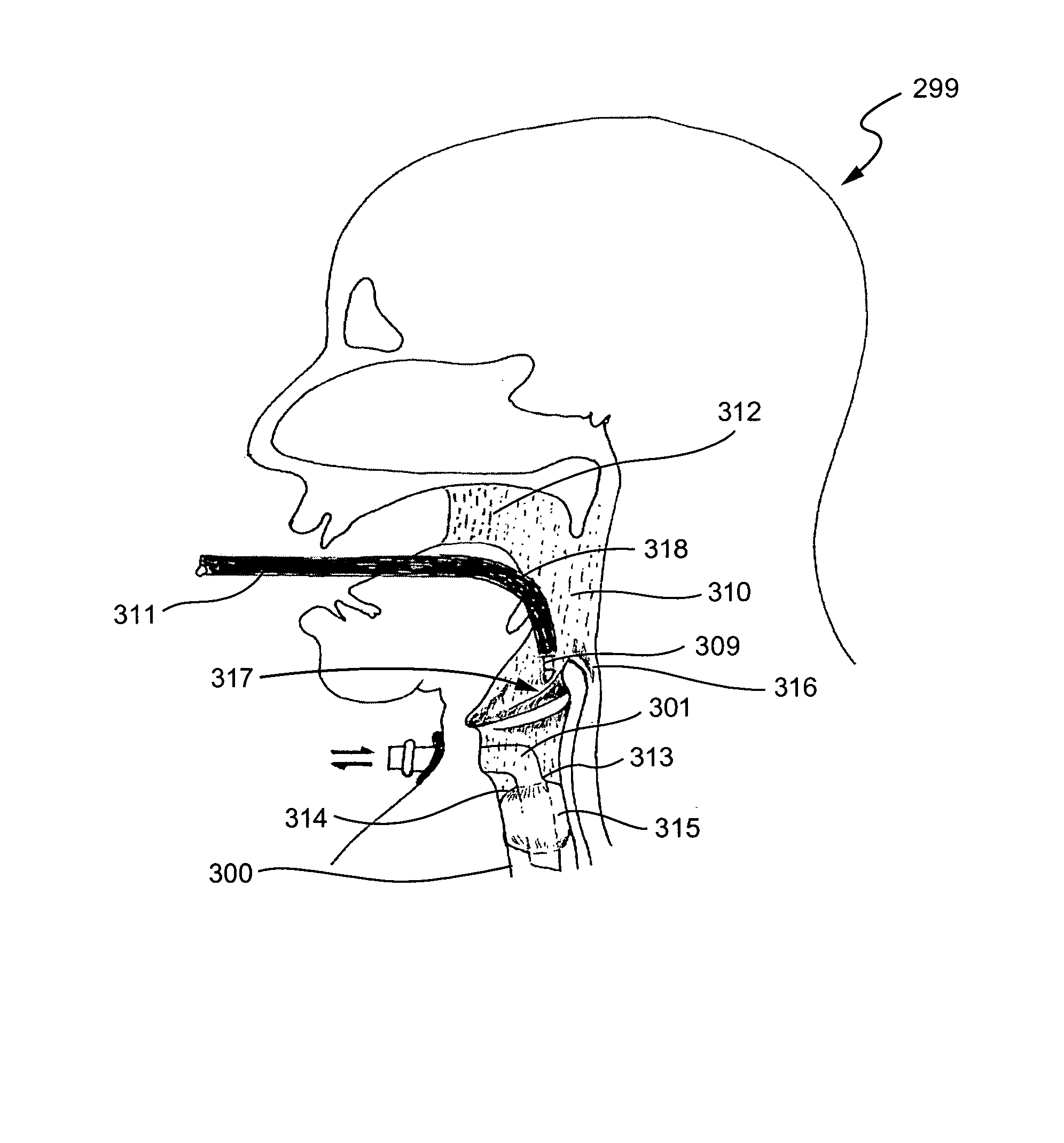
InactiveUS7001380B2Lower impedanceMinimizing char formationCannulasDiagnosticsBenign conditionEnlarged tonsils
A method is disclosed for treating benign conditions, such as enlarged tonsils and / or adenoids located in a patient's throat or nasopharynx, or soft tissue lesions located in a patient's oropharynx or larynx. According to the method, a space containing the patient's nasopharynx, oropharynx or pharynx and larynx is isolated from the patient's trachea and lungs using an inflatable cuff tracheostomy tube or nasotracheal tube inserted in the patient's trachea. The cuff is inflated to occlude the trachea. The patient is placed in a supine position, whereupon at least a portion of the space containing the nasopharynx and / or oropharynx and larynx is filled with saline. An endoscope is then inserted into the space to view the operative site in which the tonsils or tissue lesion are to be treated. An electrosurgical instrument having an active tissue treatment electrode and a return electrode connected to an electrosurgical generator is then inserted into the space, either along side the endoscope or through the endoscope's working channel. The generator is then operated to apply a radio frequency voltage between the active and return electrodes of the electrosurgical instrument, whereby a conduction path is formed between the active and return electrodes, at least partially through the saline, whereupon the active electrode is manipulated to debulk or otherwise treat the soft tissue lesion or enlarged tonsils and / or adenoids.
Owner:GYRUS MEDICAL LTD
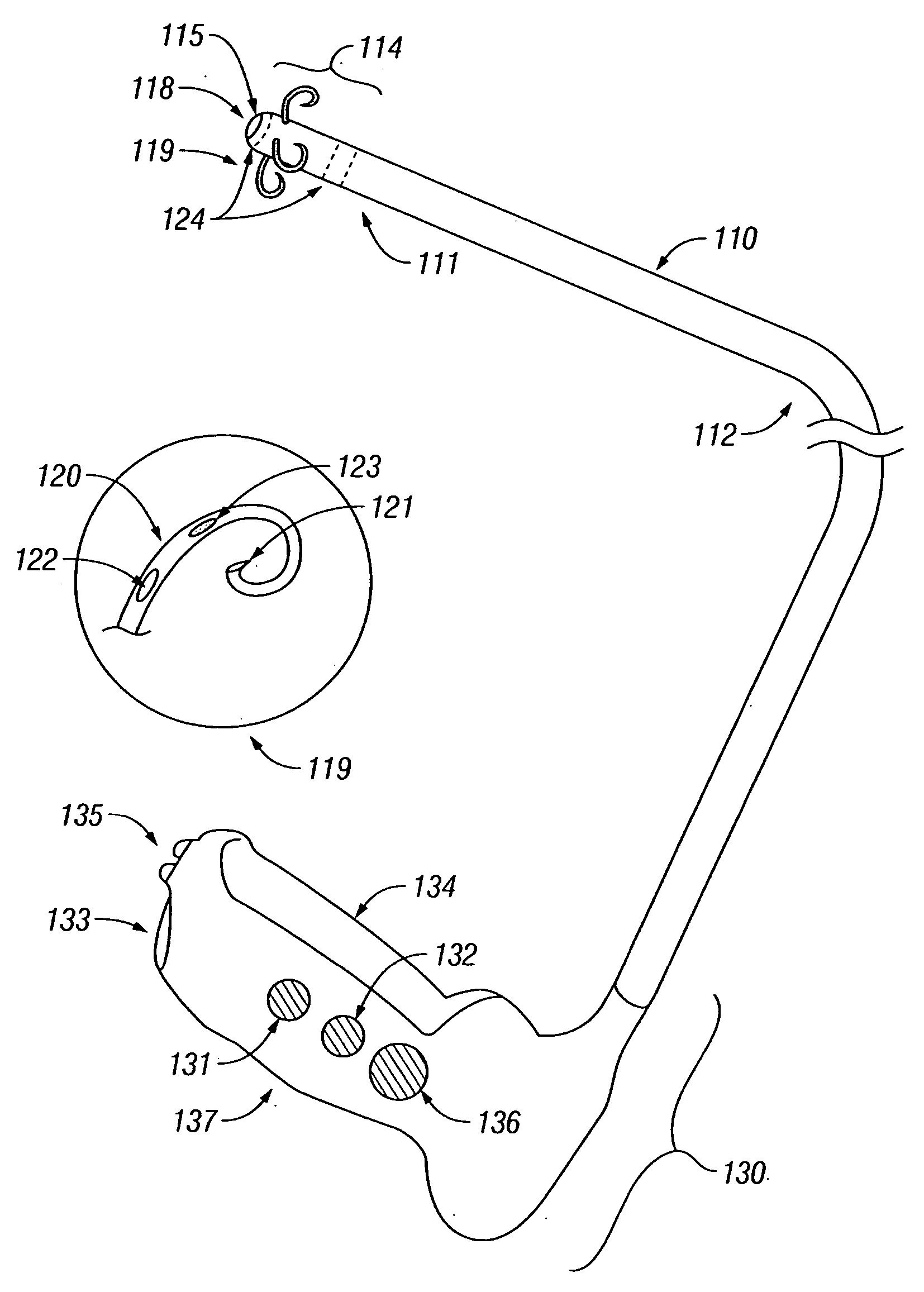
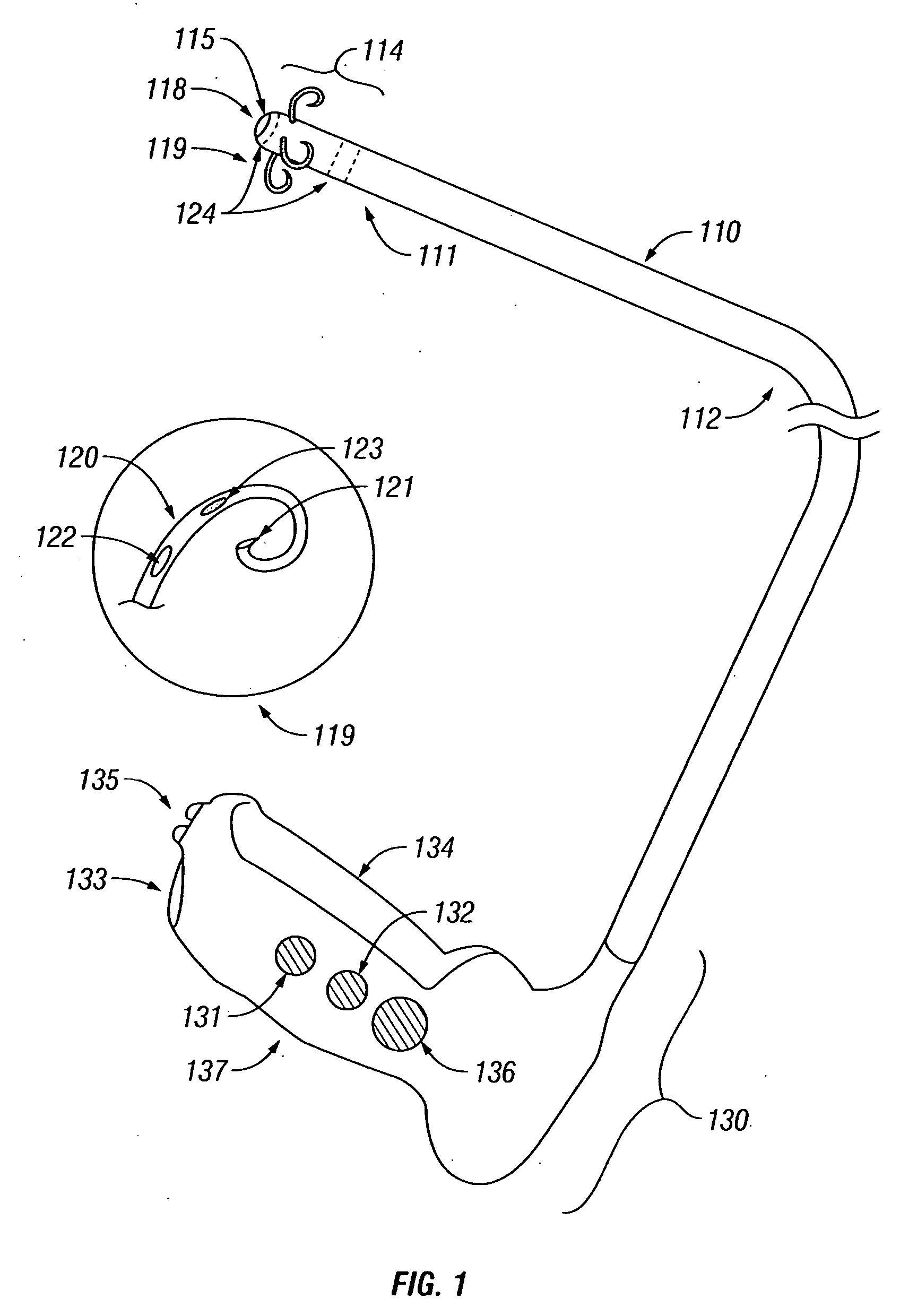
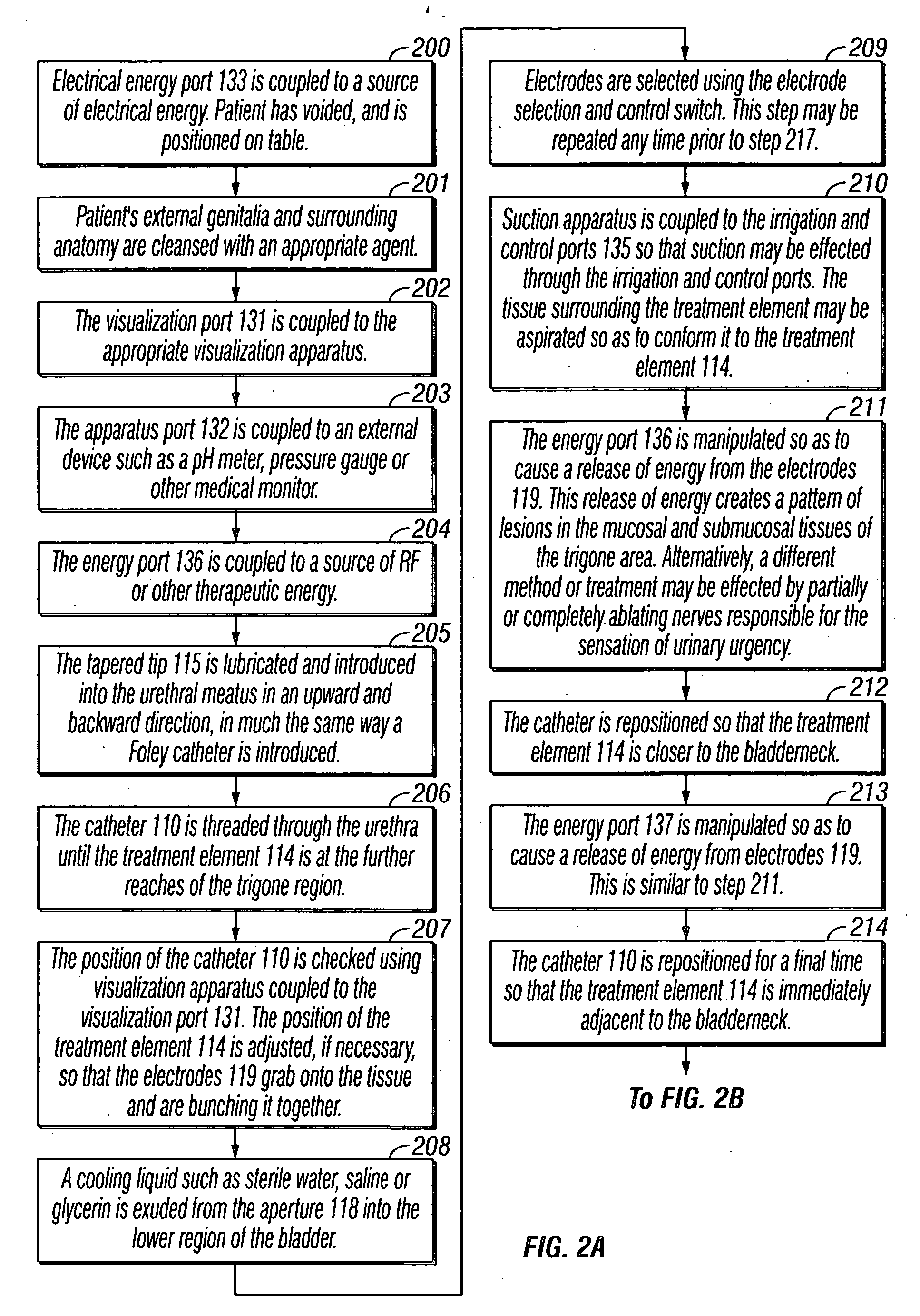
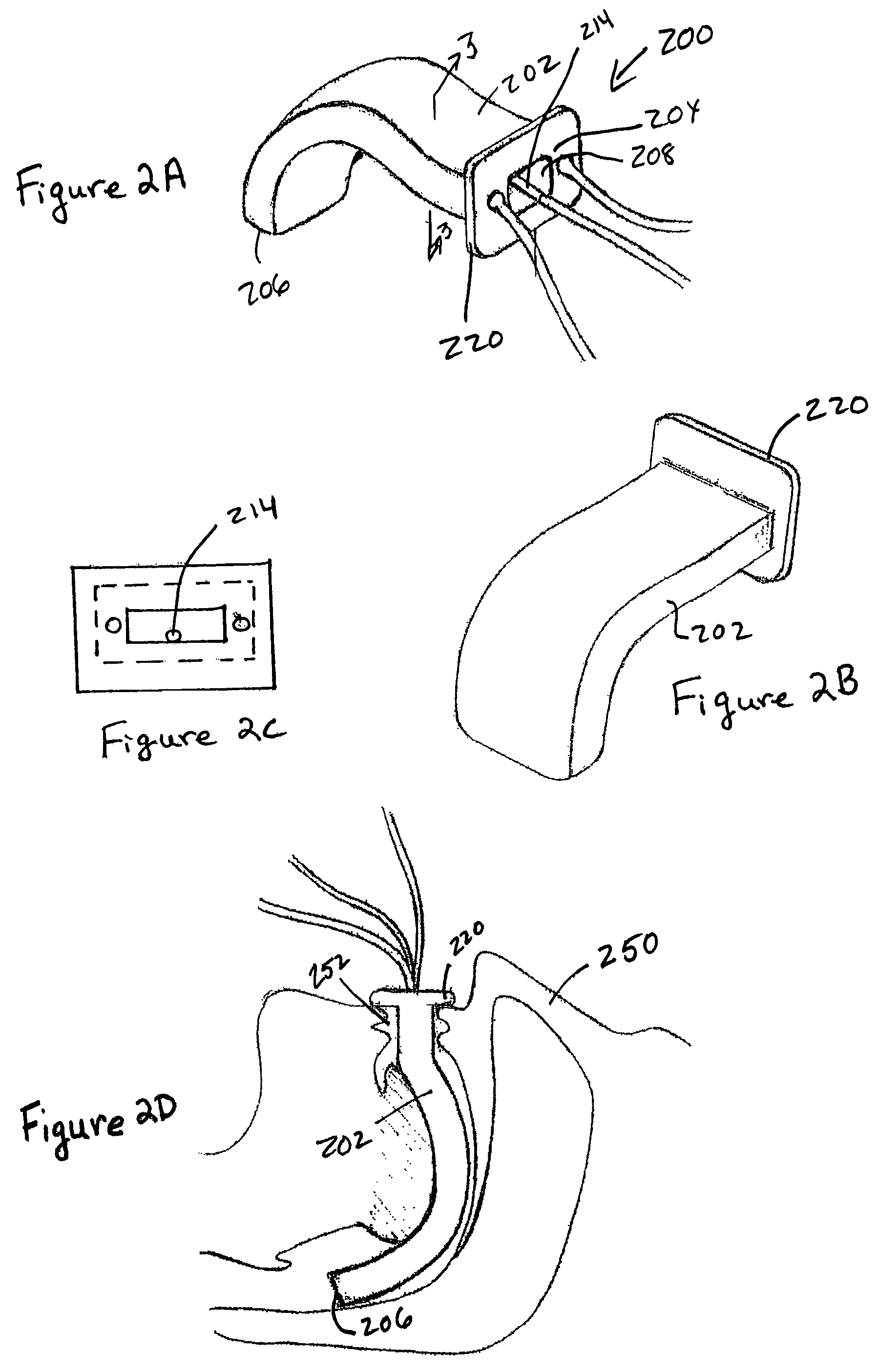
Treatment of tissue in sphincters, sinuses and orifices
Owner:NOVASYS MEDICAL
Treatment of urinary incontinence and other disorders by application of energy and drugs
The invention provides a method and system for treating disorders in parts of the body. A particular treatment can include on or more of, or some combination of: ablation, nerve modulation, three-dimensional tissue shaping, drug delivery, mapping stimulating, shrinking and reducing strain on structures by altering the geometry thereof and providing bulk to particularly defined regions. The particular body structures or tissues can include one or more of or some combination of region, including: the bladder, esophagus, vagina, penis, larynx, pharynx, aortic arch, abdominal aorta, thoracic, aorta, large intestine, sinus, auditory canal, uterus, vas deferens, trachea, and all associated sphincters. Types of energy that can be applied include radiofrequency, laser, microwave, infrared waves, ultrasound, or some combination thereof. Types of substances that can be applied include pharmaceutical agents such as analgesics, antibiotics, and anti-inflammatory drugs, bulking agents such as biologically non-reactive particles, cooling fluids, or dessicants such as liquid nitrogen for use in cryo-based treatments.
Owner:VERATHON
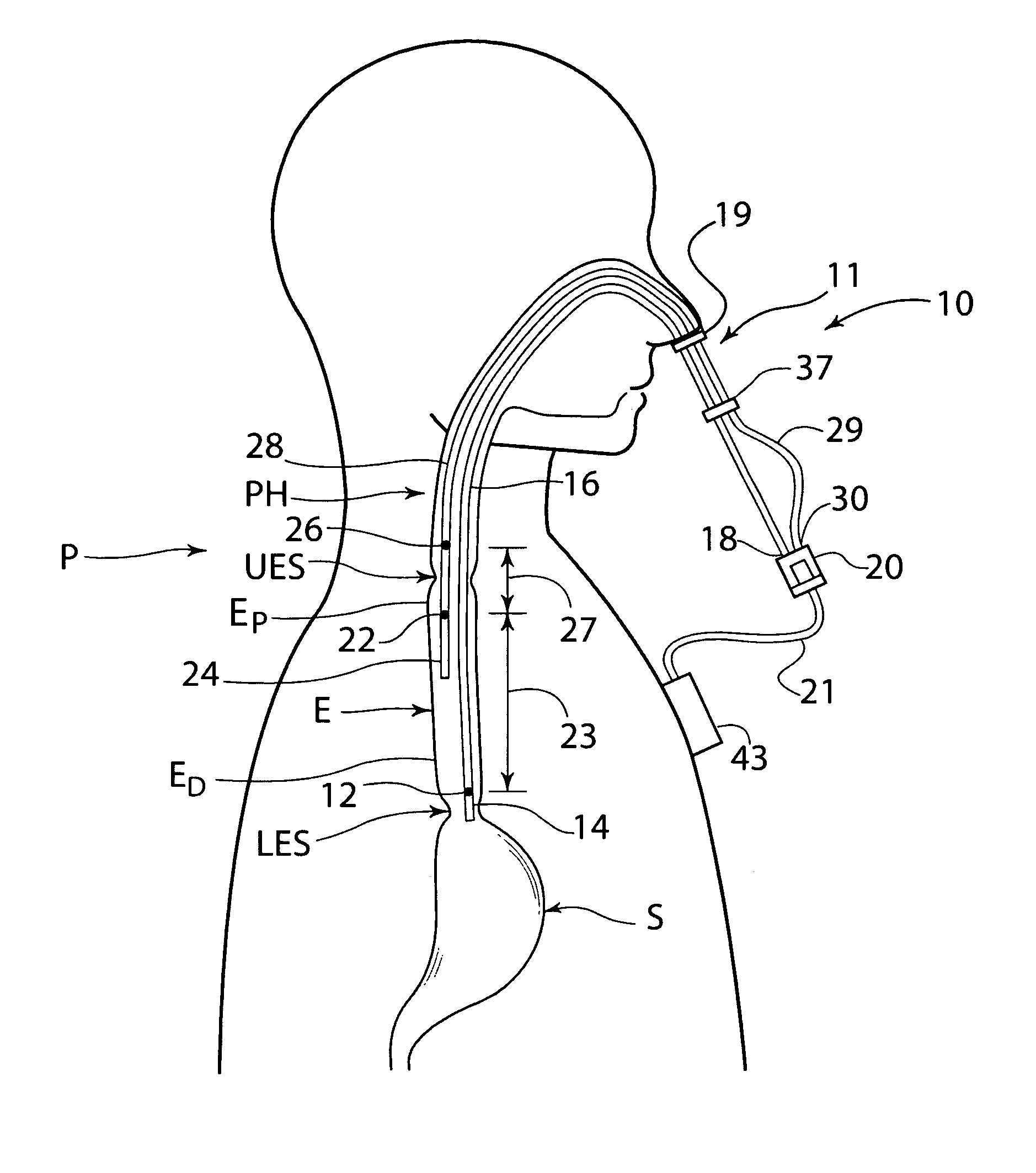
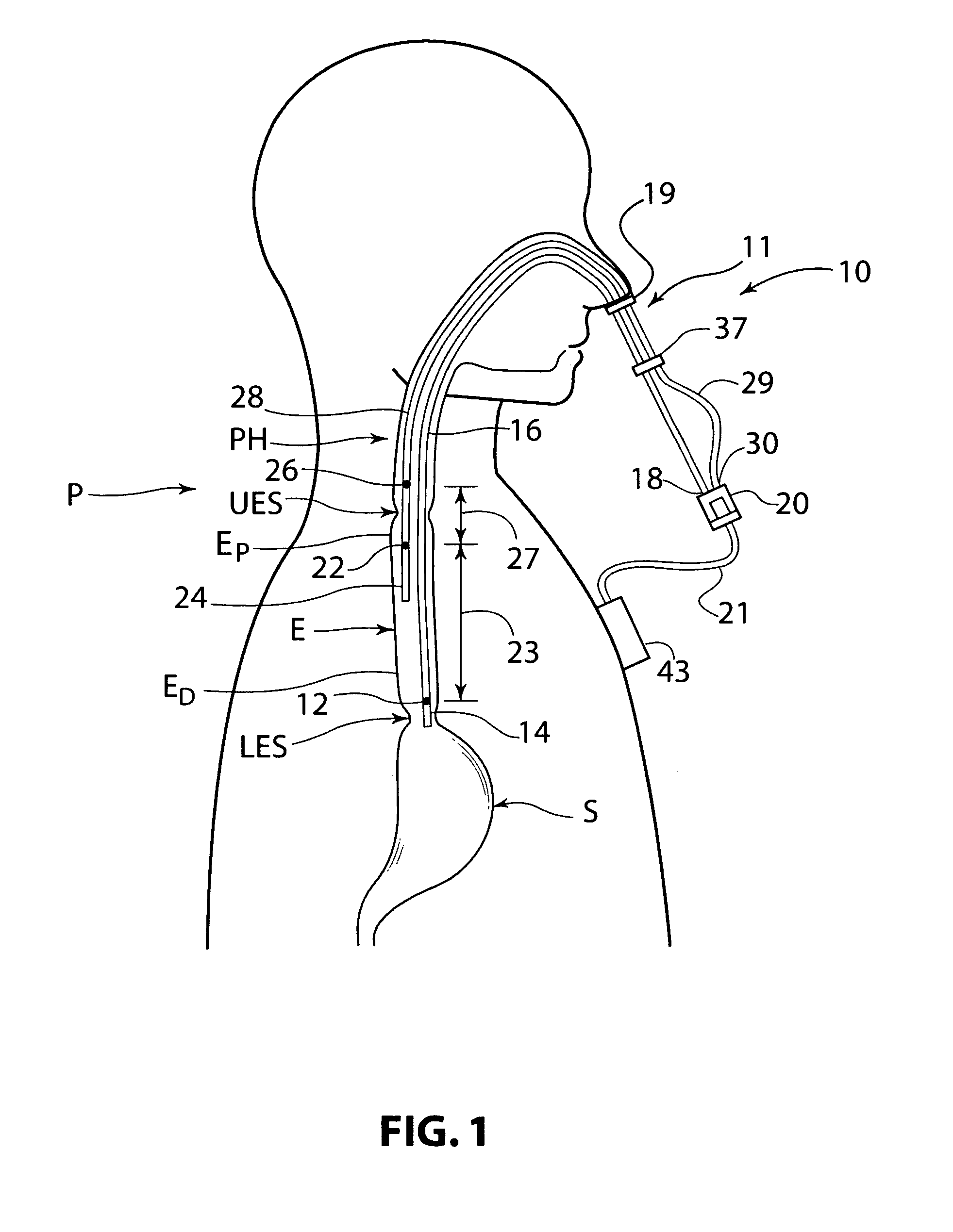
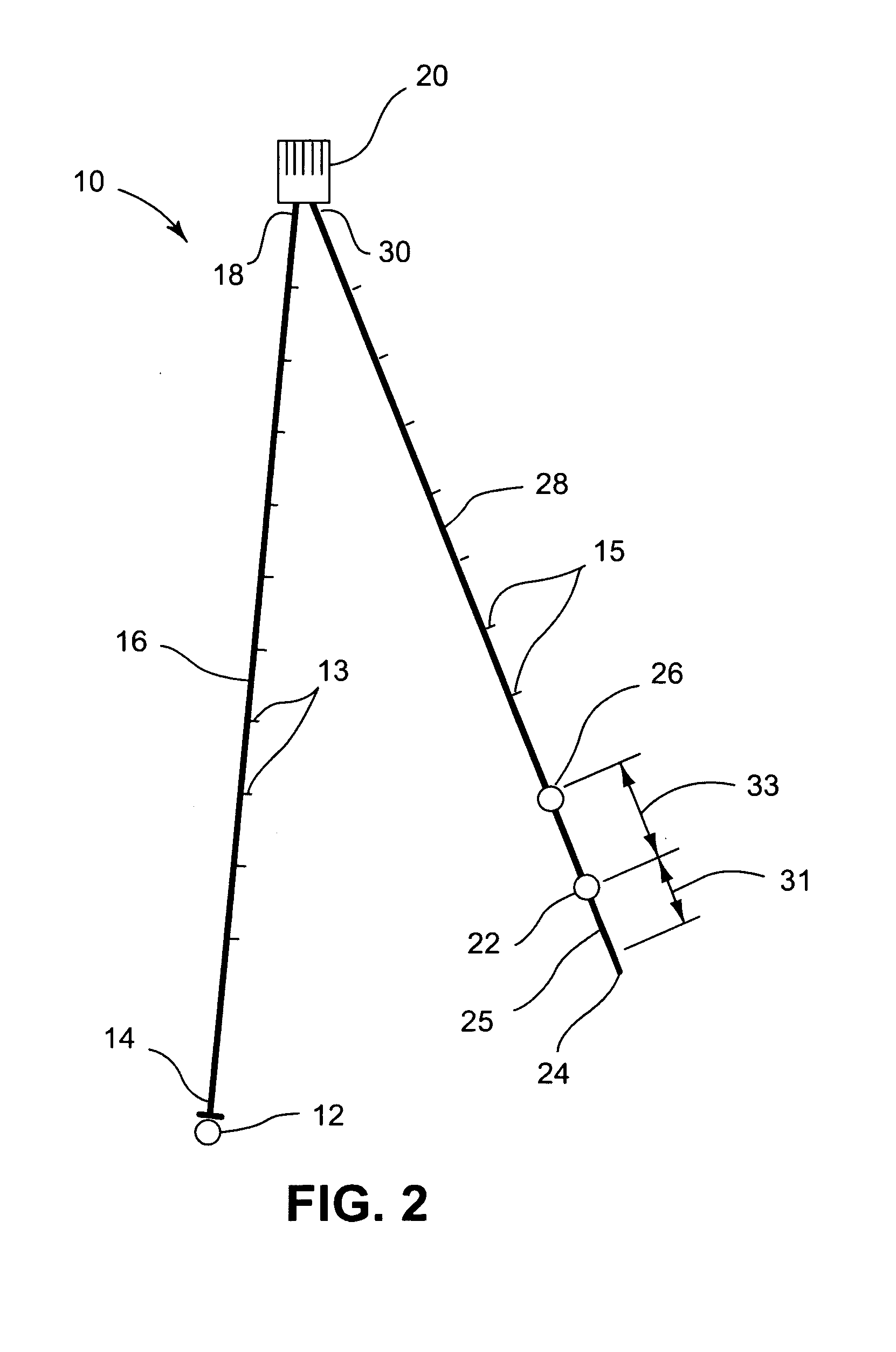
Pharyngoesophageal monitoring systems
Pharyngoesophageal monitoring systems are provided that monitor the environment of the pharynx and esophagus, and in some embodiments, detect and monitor refluxate, and may further record other physical episode data. The system may be provided, in some embodiments, as a bifurcated, adjustable, multiple internal reference probe, and methods thereof, to detect acid reflux and to monitor pH levels of acid reflux episodes simultaneously at multiple locations within the pharyngoesophageal passage. Some embodiments provide a recorder and one or a plurality of sensor arrangements, the recorder being responsive to the sensors and capable of correlation of signals generated by the sensor arrangements.
Owner:DIVERSATEK HEALTHCARE INC
Treatment of urinary incontinence and other disorders by application of energy and drugs
The invention provides a method and system for treating disorders in parts of the body. A particular treatment can include on or more of, or some combination of: ablation, nerve modulation, three-dimensional tissue shaping, drug delivery, mapping stimulating, shrinking and reducing strain on structures by altering the geometry thereof and providing bulk to particularly defined regions. The particular body structures or tissues can include one or more of or some combination of region, including: the bladder, esophagus, vagina, penis, larynx, pharynx, aortic arch, abdominal aorta, thoracic, aorta, large intestine, sinus, auditory canal, uterus, vas deferens, trachea, and all associated sphincters. Types of energy that can be applied include radiofrequency, laser, microwave, infrared waves, ultrasound, or some combination thereof. Types of substances that can be applied include pharmaceutical agents such as analgesics, antibiotics, and anti-inflammatory drugs, bulking agents such as biologically non-reactive particles, cooling fluids, or dessicants such as liquid nitrogen for use in cryo-based treatments.
Owner:VERATHON
Nasopharyngeal airway with reflectance pulse oximeter sensor
InactiveUS6266547B1Improve quality of careImproved pulse oximetry readingCatheterSensorsNasopharyngeal airwayMedicine
A combined nasopharyngeal airway and pulse oximeter sensor capable of monitoring the posterior pharynx, posterior soft palate or nasal mucosa is disclosed. The nasopharyngeal airway includes a thickened wall section over approximately one-third of its circumference. Pulse oximeter sensor elements may be embedded in the airway. The pulse oximeter sensor elements may include a light source, which preferably emits light at wavelengths around 660 nm (red) and around 940 nm (near infrared), and a light detector. The pulse oximeter sensor elements may be connected to a pulse oximeter monitor (spectrophotometer) or other external device for analysis.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Laryngeal mask airway device
InactiveUS7305985B2Avoid disadvantagesDeep shapeTracheal tubesRespiratory apparatusLaryngeal airwayDistal portion
Owner:TELEFLEX LIFE SCI PTE LTD
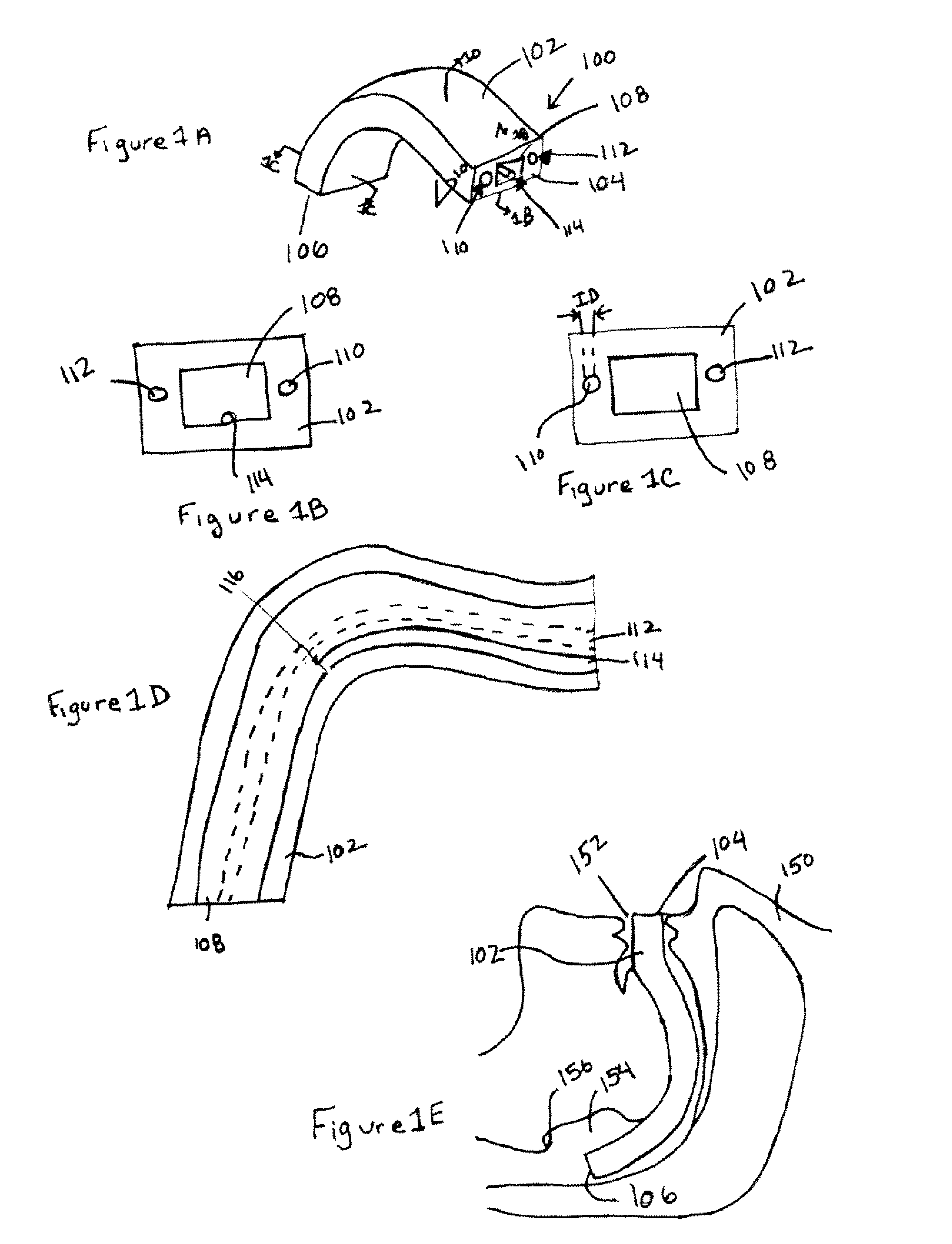
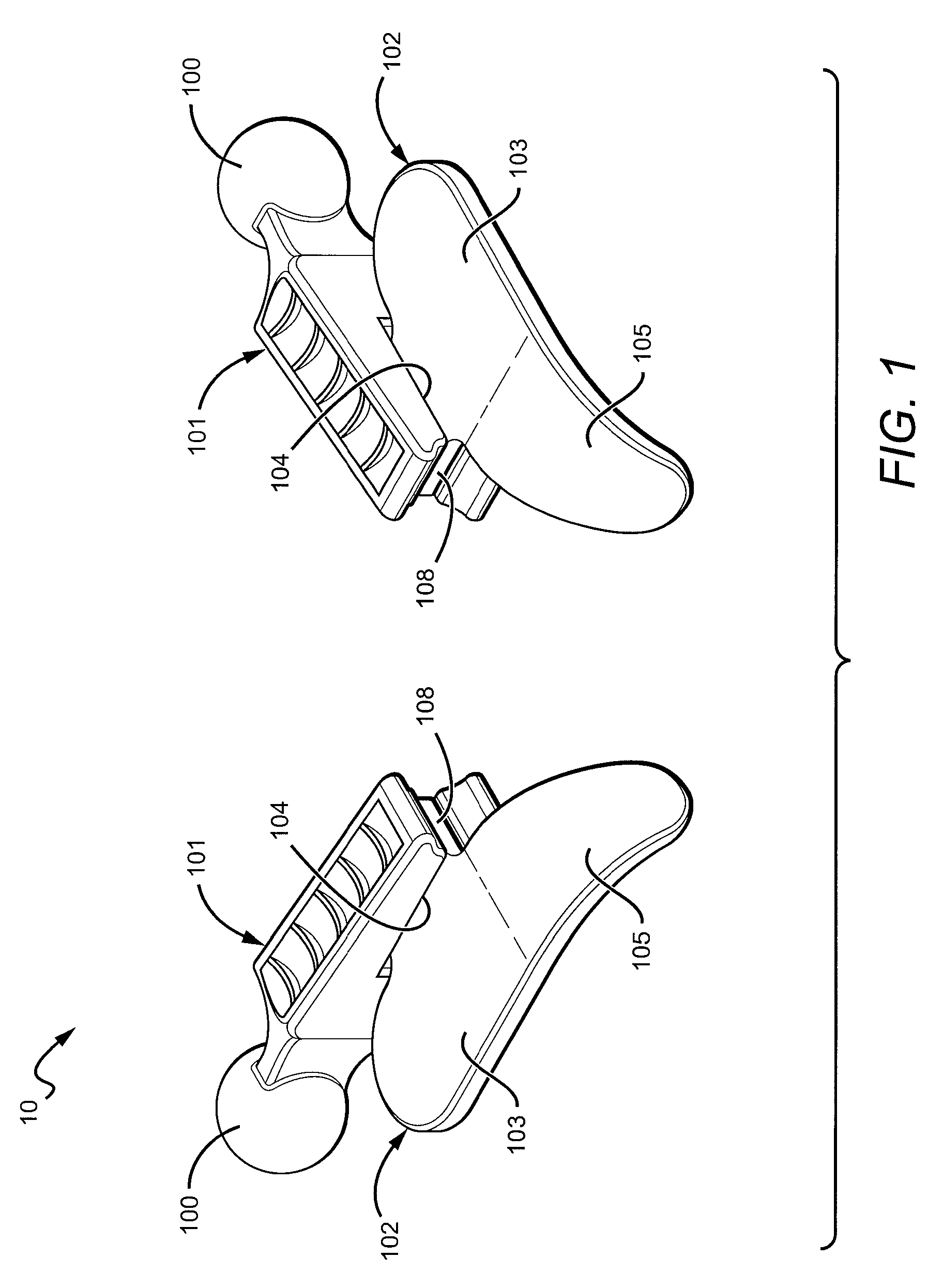
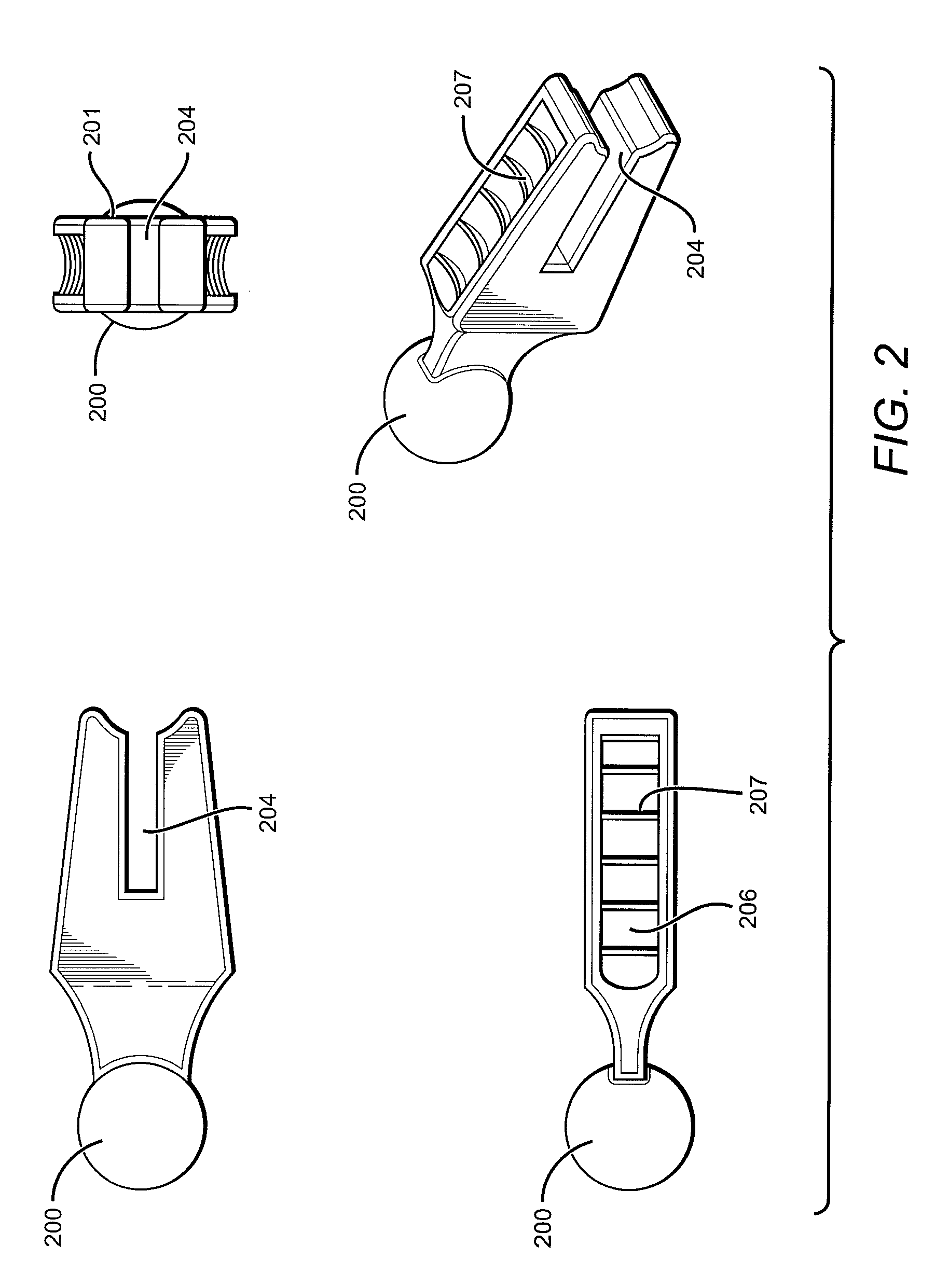
Electrosurgical system and method
InactiveUS20050090819A1Lower impedanceChar formation undesirableCannulasDiagnosticsBenign conditionNose
A method is disclosed for treating benign conditions, such as enlarged tonsils and / or adenoids located in a patient's throat or nasopharynx, or soft tissue lesions located in a patient's oropharynx or larynx. According to the method, a space containing the patient's nasopharynx, oropharynx or pharynx and larynx is isolated from the patient's trachea and lungs using an inflatable cuff tracheostomy tube or nasotracheal tube inserted in the patient's trachea. The cuff is inflated to occlude the trachea. The patient is placed in a supine position, whereupon at least a portion of the space containing the nasopharynx and / or oropharynx and larynx is filled with saline. An endoscope is then inserted into the space to view the operative site in which the tonsils or tissue lesion are to be treated. An electrosurgical instrument having an active tissue treatment electrode and a return electrode connected to an electrosurgical generator is then inserted into the space, either along side the endoscope or through the endoscope's working channel. The generator is then operated to apply a radio frequency voltage between the active and return electrodes of the electrosurgical instrument, whereby a conduction path is formed between the active and return electrodes, at least partially through the saline, whereupon the active electrode is manipulated to debulk or otherwise treat the soft tissue lesion or enlarged tonsils and / or adenoids.
Owner:GYRUS MEDICAL LTD
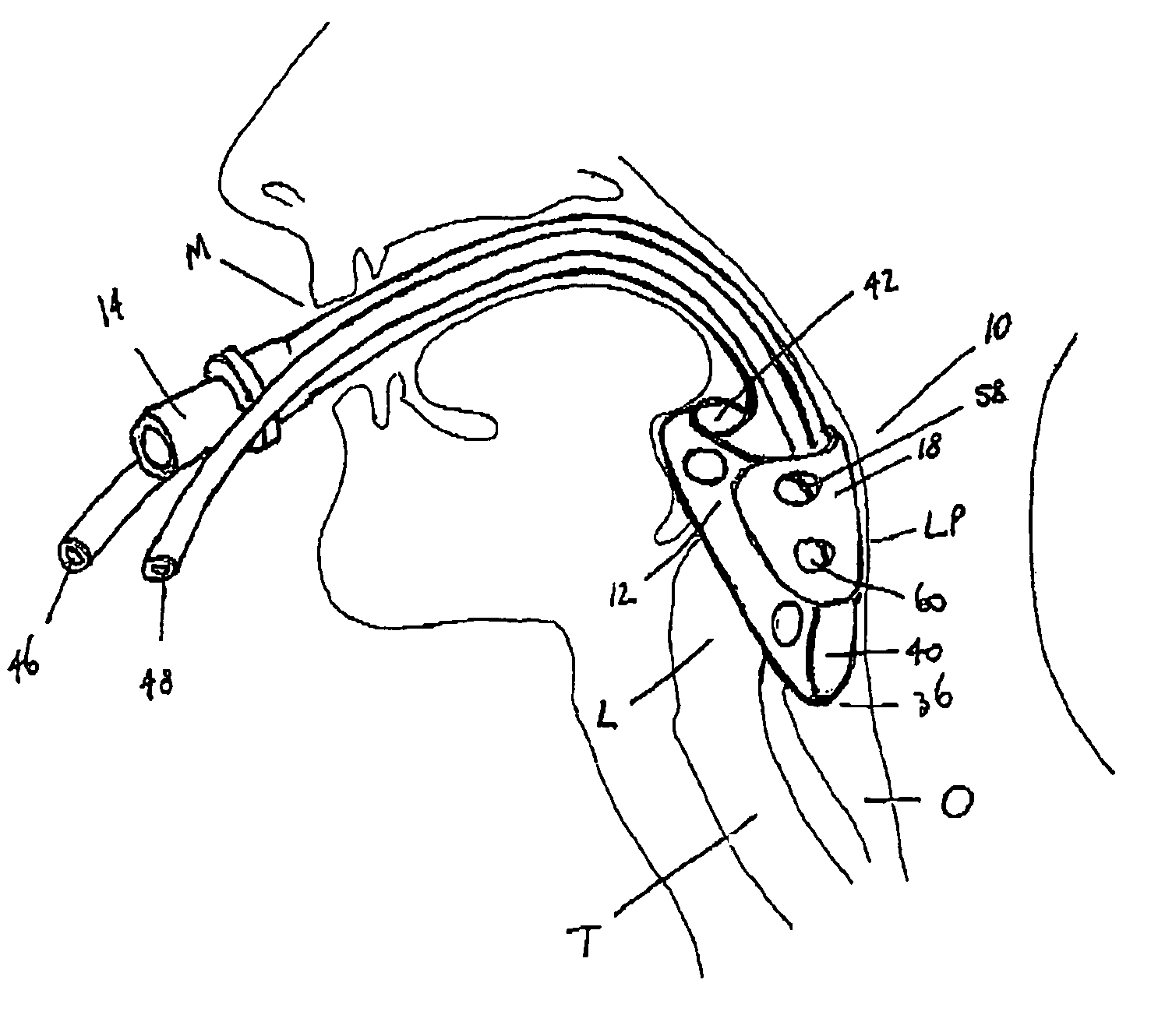
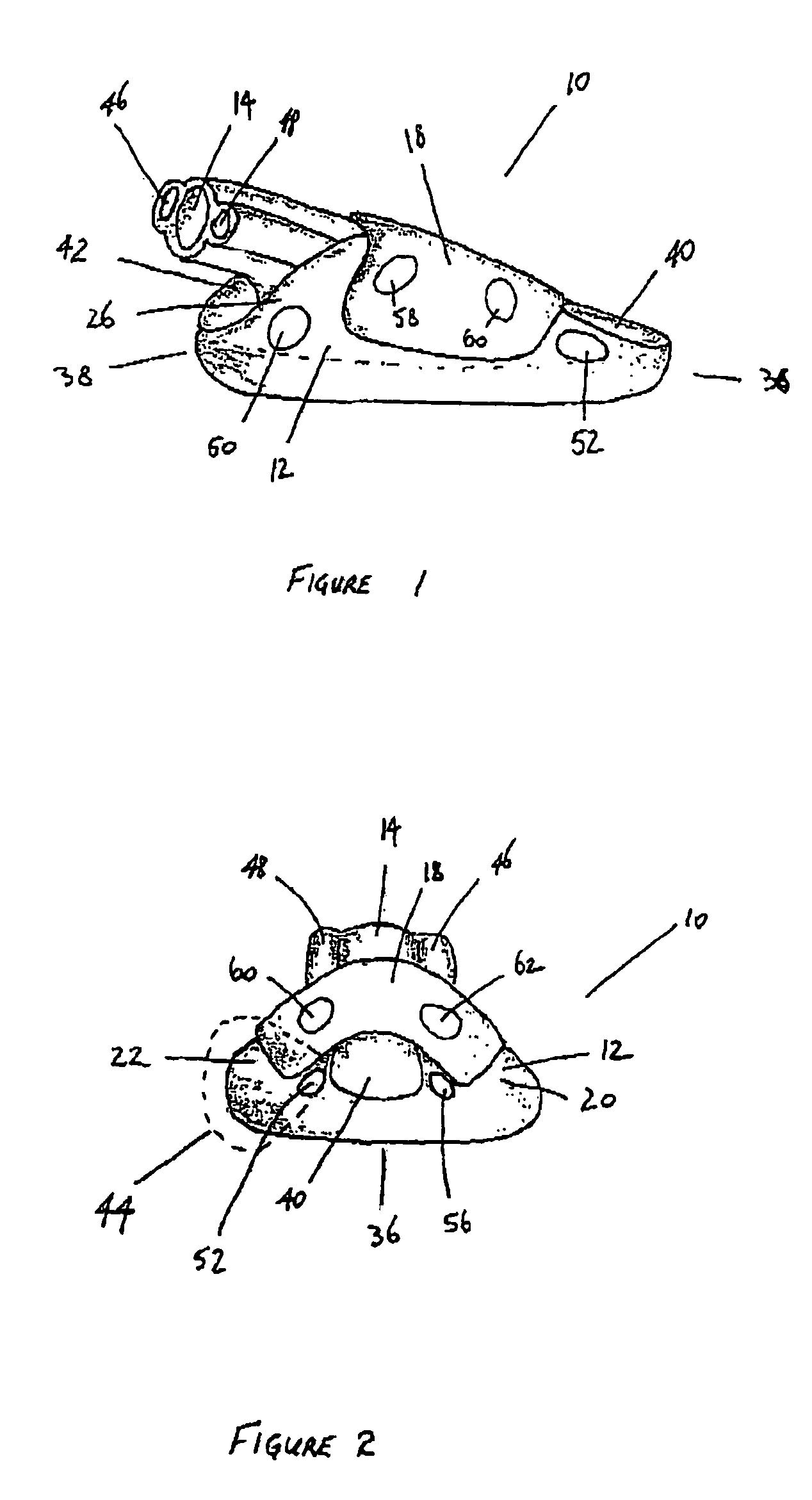
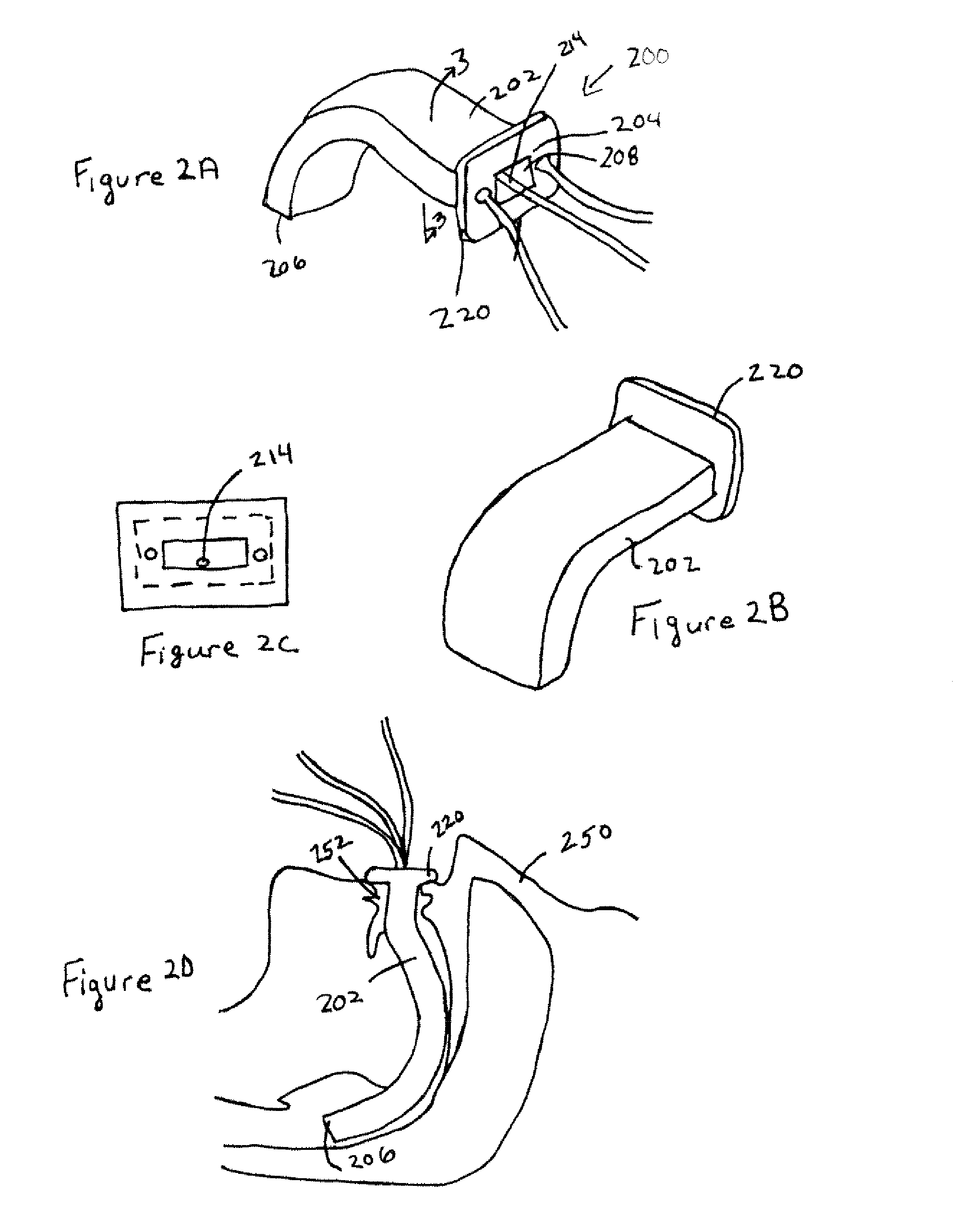
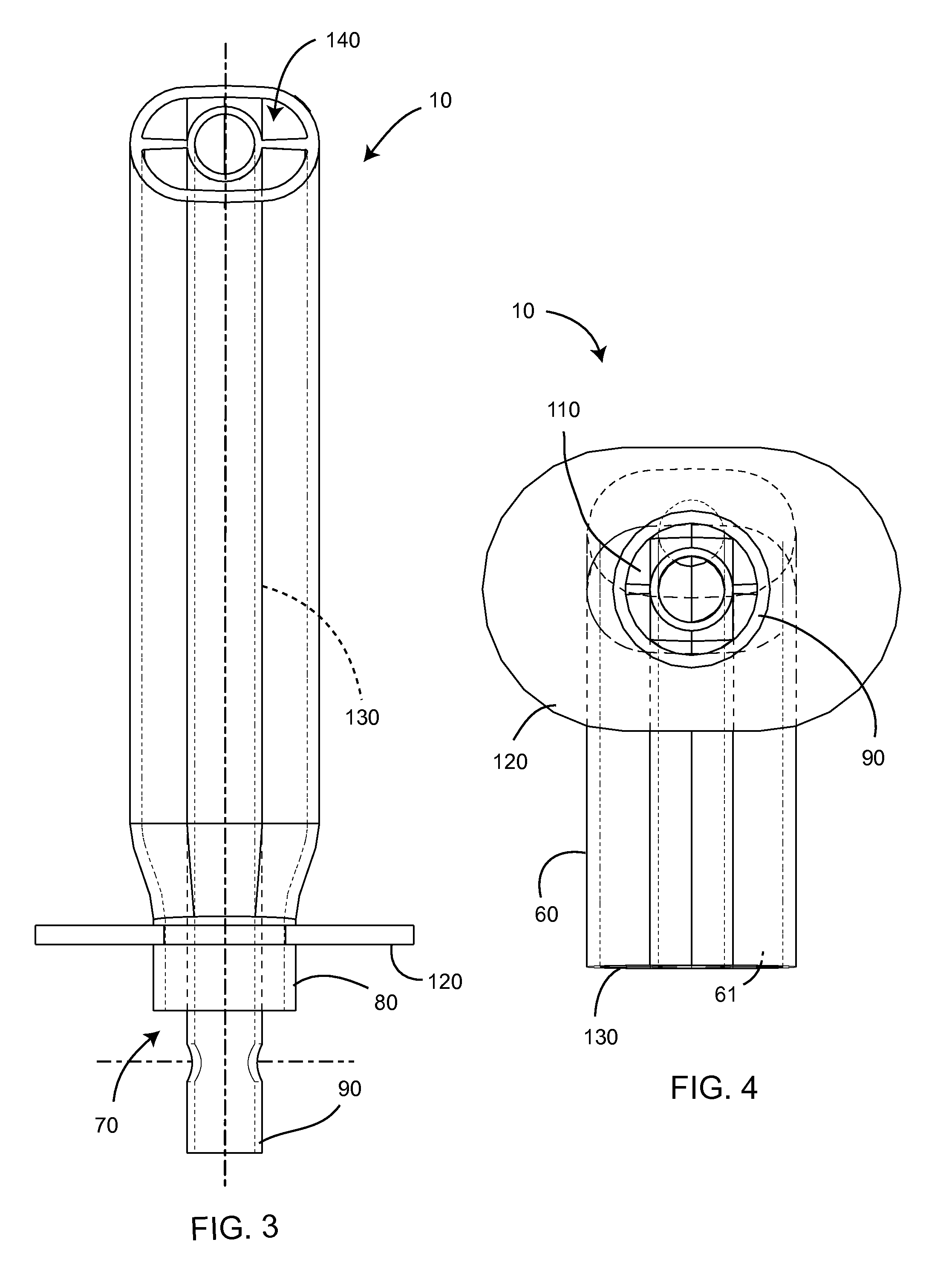
Laryngeal mask
ActiveUS20060180156A1Easy to insertPromote formationTracheal tubesMedical devicesPharynxLaryngeal cartilage
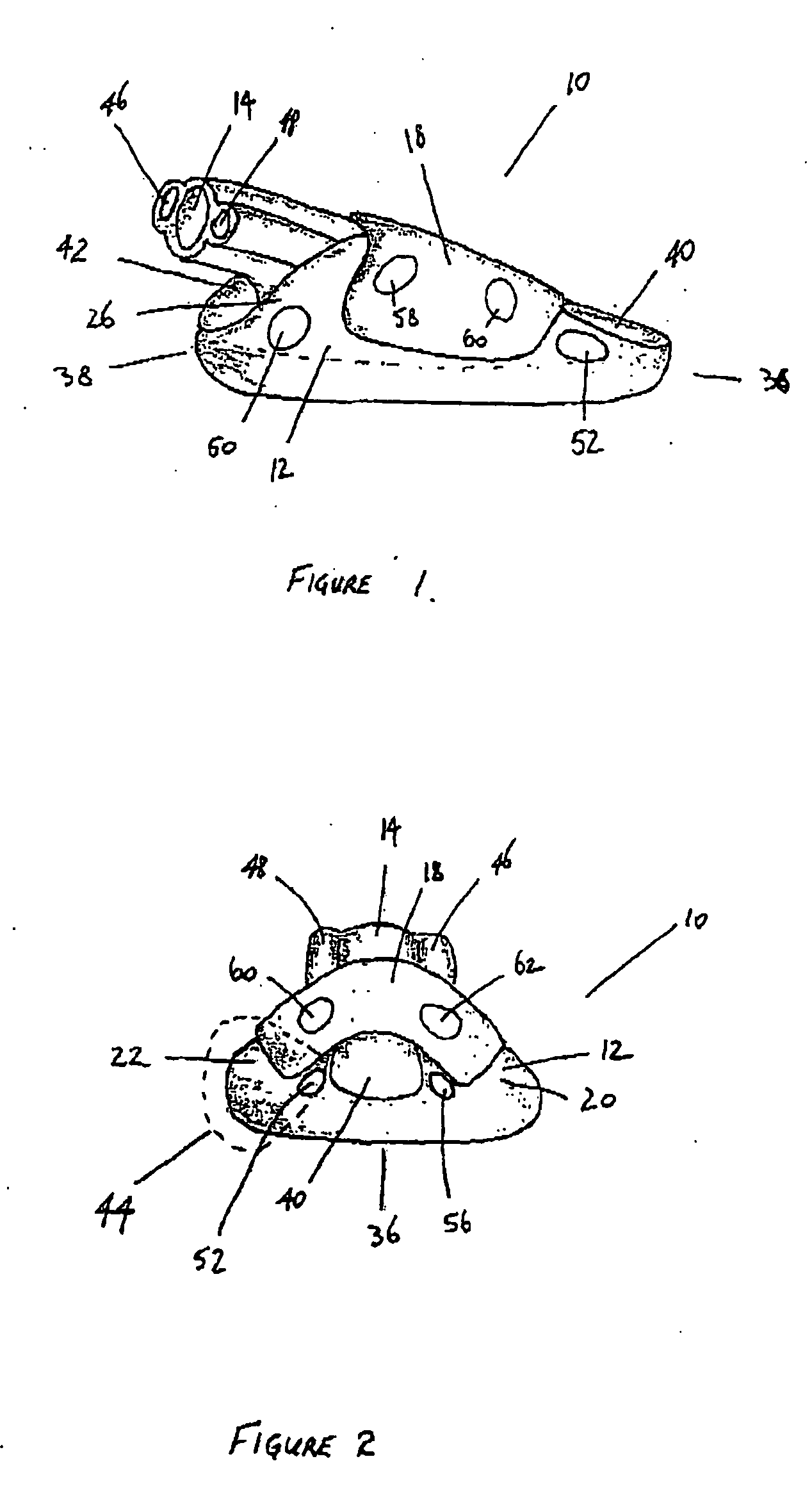
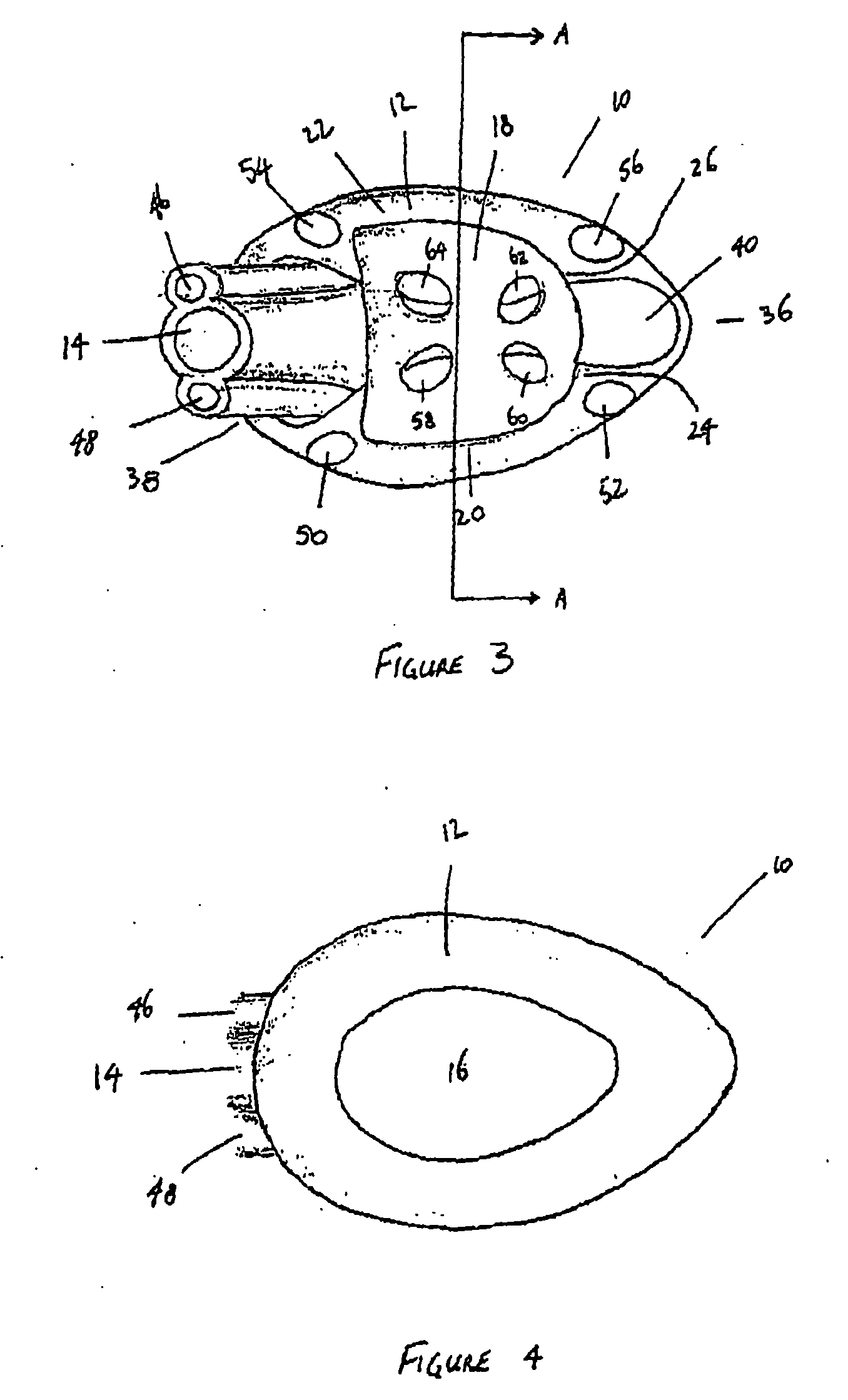
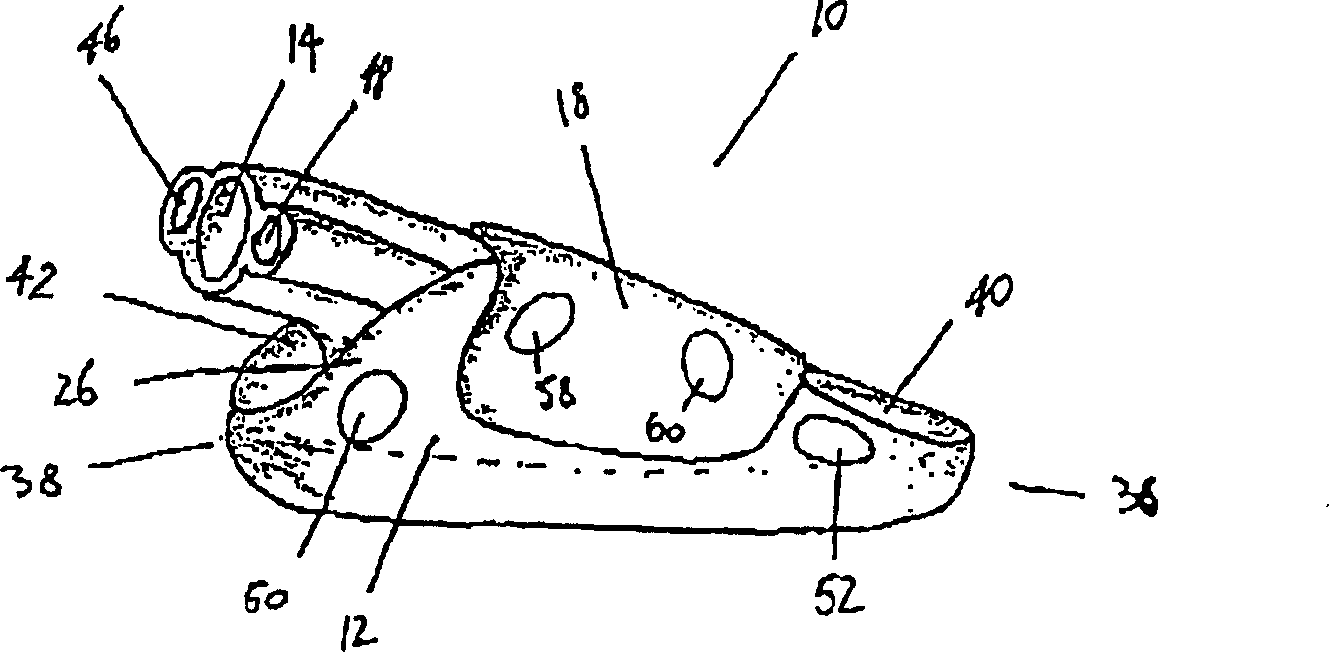
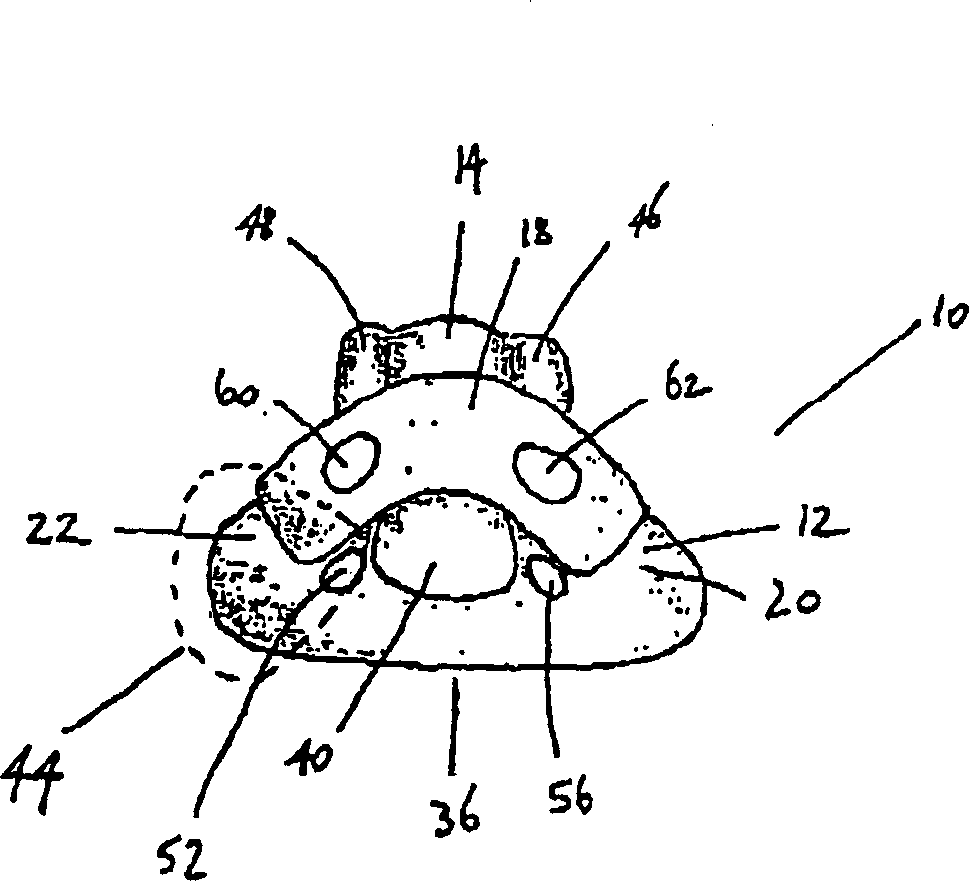
A device for maintaining an airway in a patient. This device includes a mask having a resilient conformable peripheral portion that is shaped such that the mask forms a seal with the larynx when the mask is positioned in the laryngo pharynx to thereby prevent ingress of extraneous fluids into the larynx. The peripheral portion of the mask define at least one cavity for providing fluid communication to the esophagus when the mask is inserted into the laryngo pharynx. An airway tube is connected to or formed with the mask for passing gas to the larynx when the mask is properly inserted into the laryngo pharynx. The airway tube preferably is curved as it leaves the mask.
Owner:BASKA MEENAKSHI
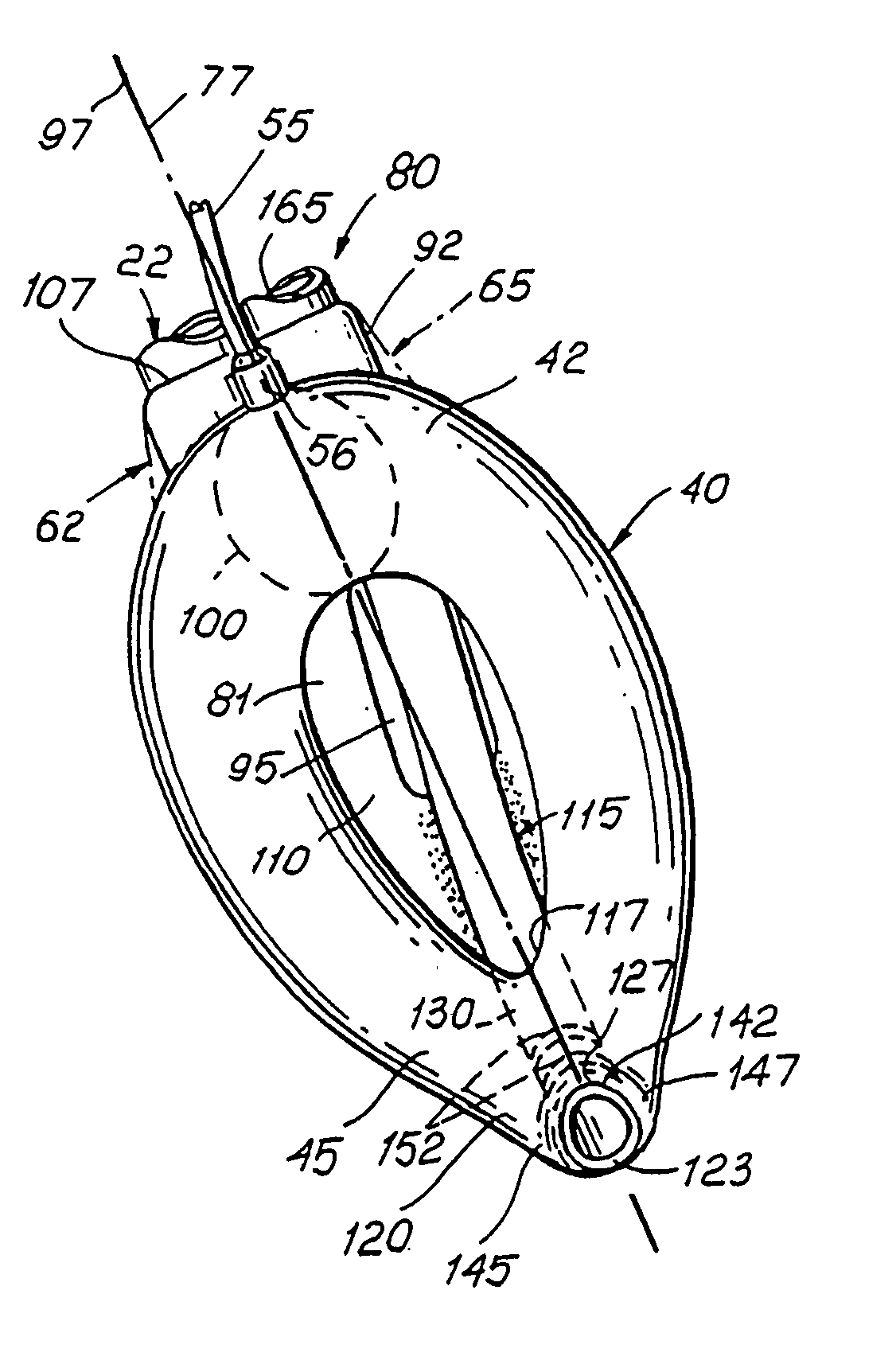
Gastro-Laryngeal Mask
A gastro-laryngeal mask features softly compliant construction of the distal half of the mask, wherein the mask is of generally elliptical configuration, with an inflatable peripheral cuff to seal and support the mask around the laryngeal inlet. A back cushion is inflatable to engage the back wall of the pharynx and thus to forwardly load the peripheral-cuff seal to the laryngeal inlet. An evacuation tube for external removal of a possible gastric discharge completes an evacuation or discharge passage contained within the mask and opening through the distal end of the peripheral cuff. Special provision is made for assuring integrity of the discharge passage within the flexible distal half of the mask, i.e., assuring against collapse of the distal-end half of the softly compliant evacuation tube in the distal region of the mask, such that inflation of the mask does not compromise viability of the evacuation tube by compressing softly compliant material of the evacuation tube during periods of mask inflation. The special provision also favors such collapse of the mask when deflated as to provide a leading flexible edge for piloting a safe and correct advancing insertional advance of the deflated mask in the patient's throat, in avoidance of epiglottis interference and to the point of locating engagement in the upper sphincter of the oesophagus.
Owner:THE LARYNGEAL MASK +1
Perilaryngeal oral airway with flexible tip guide
InactiveUS7040312B2Facilitating positive pressure ventilationShorten the lengthTracheal tubesRespiratory apparatusOral airway insertionDistal portion
An oral airway consists of an elongate tubular member having a leading distal end and a proximal end, the leading distal end leading the elongate tubular member as it is inserted into the mouth and pharynx of a patient. An opening at the leading distal end of the elongate tubular member is inclined so that a posterior portion of the distal portion extends beyond an anterior portion of the distal end. A flexible tip portion extends distally beyond the opening to aid in insertion of the oral airway into the mouth and pharynx of a patient. The tip is configured to flex toward the opening as it abuts tissue within the mouth and pharynx of a patient.
Owner:ENGINEERED MEDICAL SYST
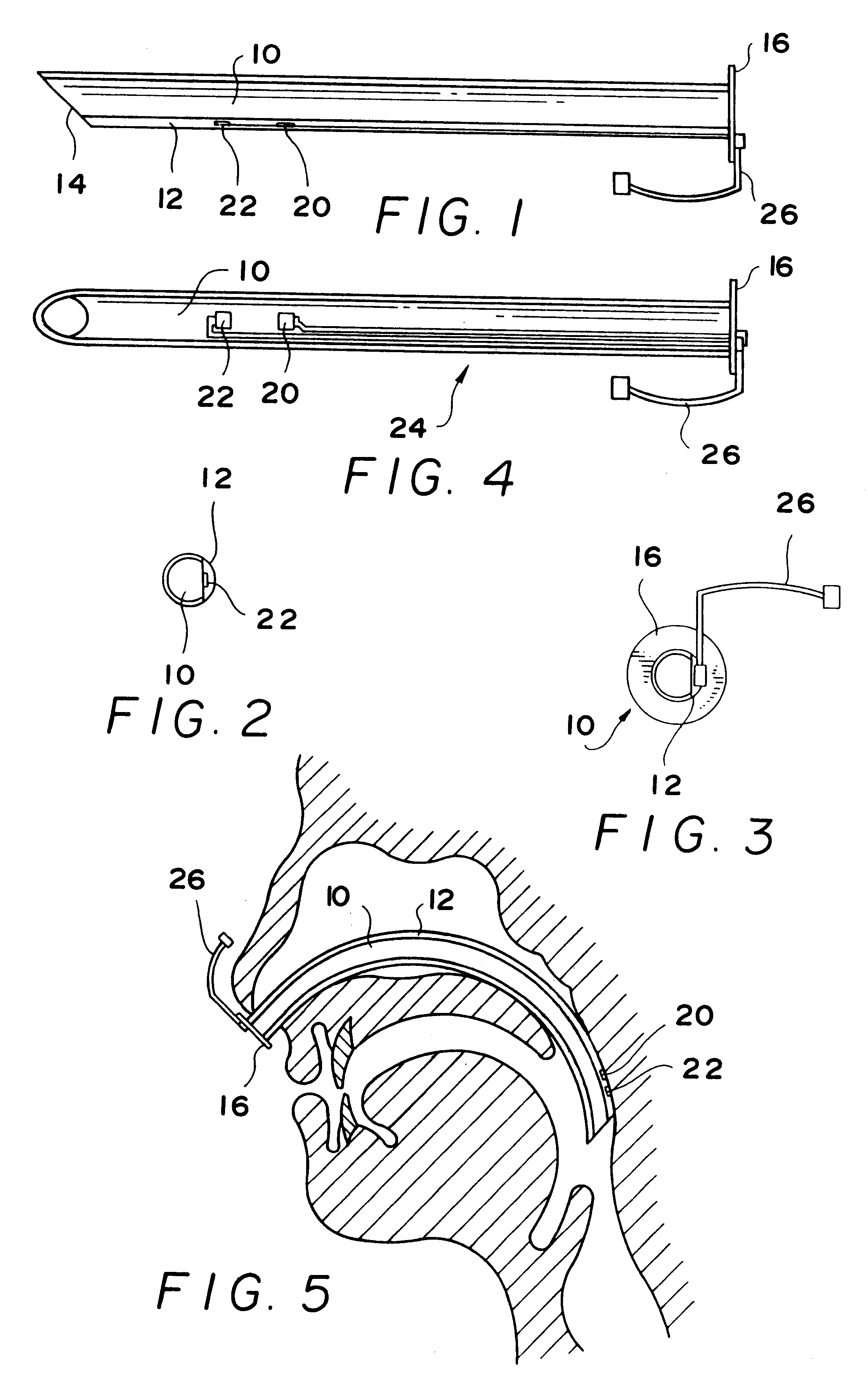
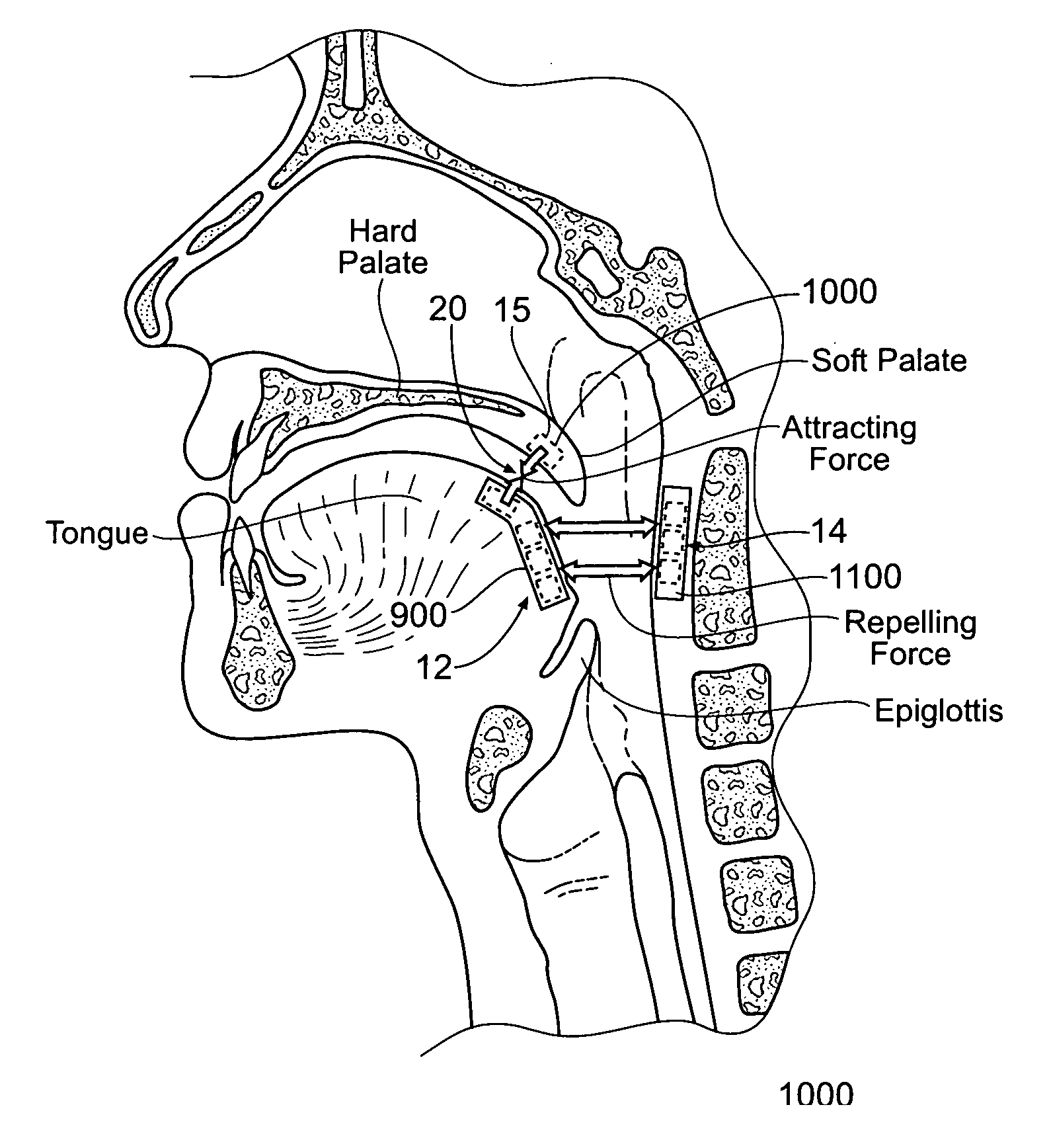
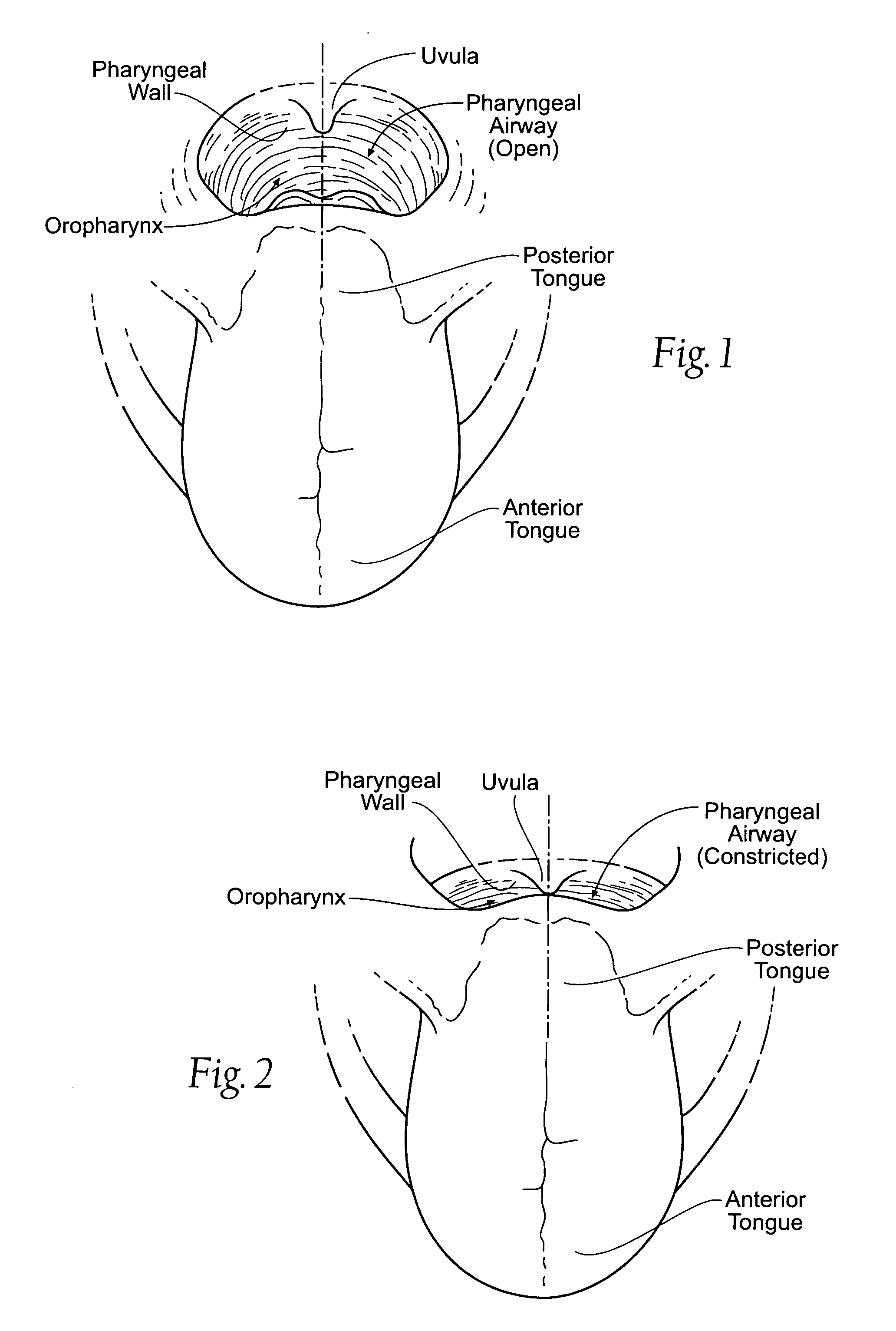
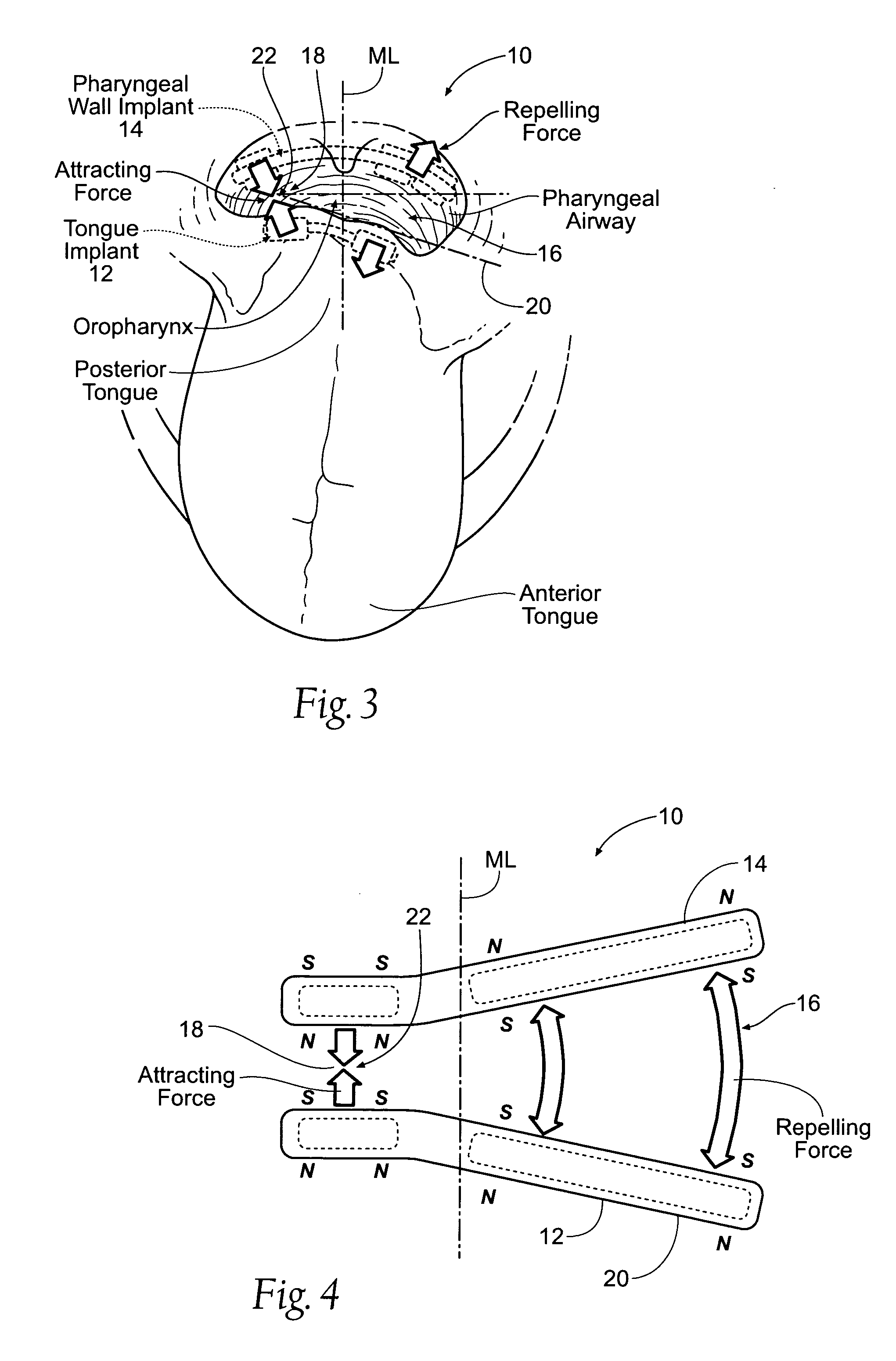
Self-anchoring magnetic force implant devices, systems, and methods
InactiveUS20070209665A1“hinge” structureSnoring preventionNon-surgical orthopedic devicesMagnetic tension forcePharynx
A magnetic force system uses a magnetic implant sized and configured to be inserted in the pharynx and another magnetic implant sized and configured to be inserted in the tongue, palate, or pharynx. The system establishes different regions of magnetic interaction between the two implants across the airway, attracting and repelling, such that attractive interaction in one region of the implants combines with repelling interaction in another region of the implants, to provide a “hinge” structure. Alternatively, a magnetic force system that uses three magnetic implants sized and configured to be inserted in the tongue, pharynx, and palate, respectively. The tongue implant is attracted to the palatal implant, and repels the pharyngeal implant, forming a modified “hinge” structure. Forces of magnetic attracting bring tissue together to form a magnetic hinge joint, providing an anchor to stabilize the regions where repelling forces work to separate tissue to keep the airway open.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Oropharyngeal airway
An oropharyngeal device for insertion into the mouth of a patient. The device includes a body having a distal end and a proximal end with a flange formed at the proximal end. The distal end is inserted into the mouth until the flange is disposed outside and adjacent to the patient's mouth. The flange keeps the proximal device from entering the mouth. The body is sized such that the distal end of the body is disposed within the pharynx above the epiglottis. The device includes a channel that forms an airway between the ends. The device also includes at least three separate conduits integrated into the body for administering oxygen, suctioning, and for assessing ventilation thorough end-tidal carbon dioxide monitoring. The conduits for oxygenation and suctioning extend through the body between its proximal and distal ends. The conduit for end-tidal carbon dioxide monitoring terminates within the channel.
Owner:THOMAS JEFFERSON UNIV
Laryngeal mask
InactiveUS7997274B2Promote formationEasy maintenanceTracheal tubesMedical devicesLarynxLaryngeal Masks
Owner:BASKA MEENAKSHI
Sleep apnea prevention and relief device
A sleep apnea prevention and relief device is provided that is useful for a patient diagnosed with obstructive sleep apnea. For example, a form-fitting neck device is provided with a suction generation or creation means to create continuous or lasting negative pressure upon or around the neck in order to urge substantially outward expansion of the soft tissues that are compressing the pharynx and creating the obstruction.
Owner:SILWA JOHN W +1
Laryngeal mask airway device
InactiveUS20060124132A1Avoid disadvantagesImprove stabilityTracheal tubesRespiratory apparatusLaryngeal airwayDistal portion
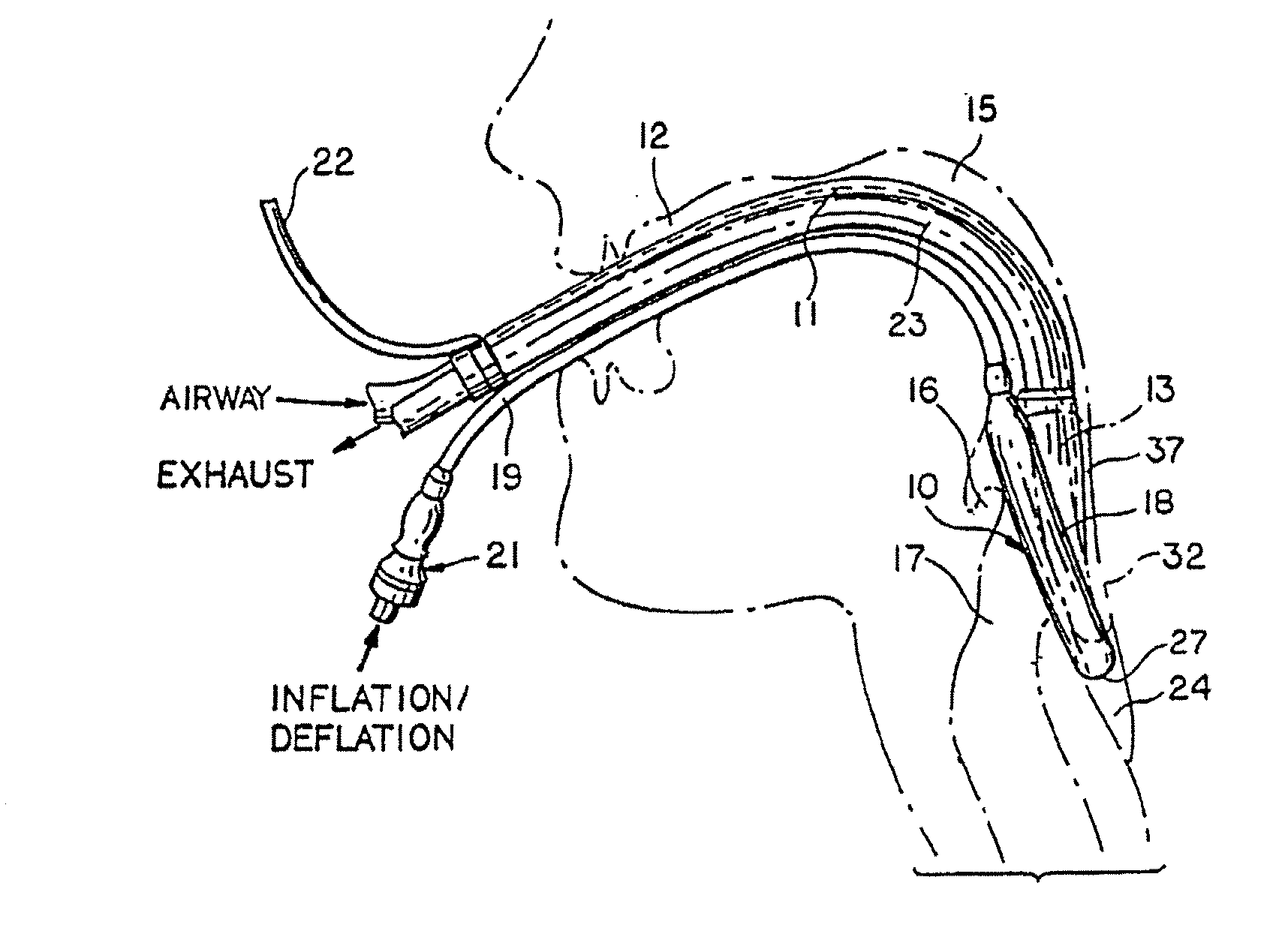
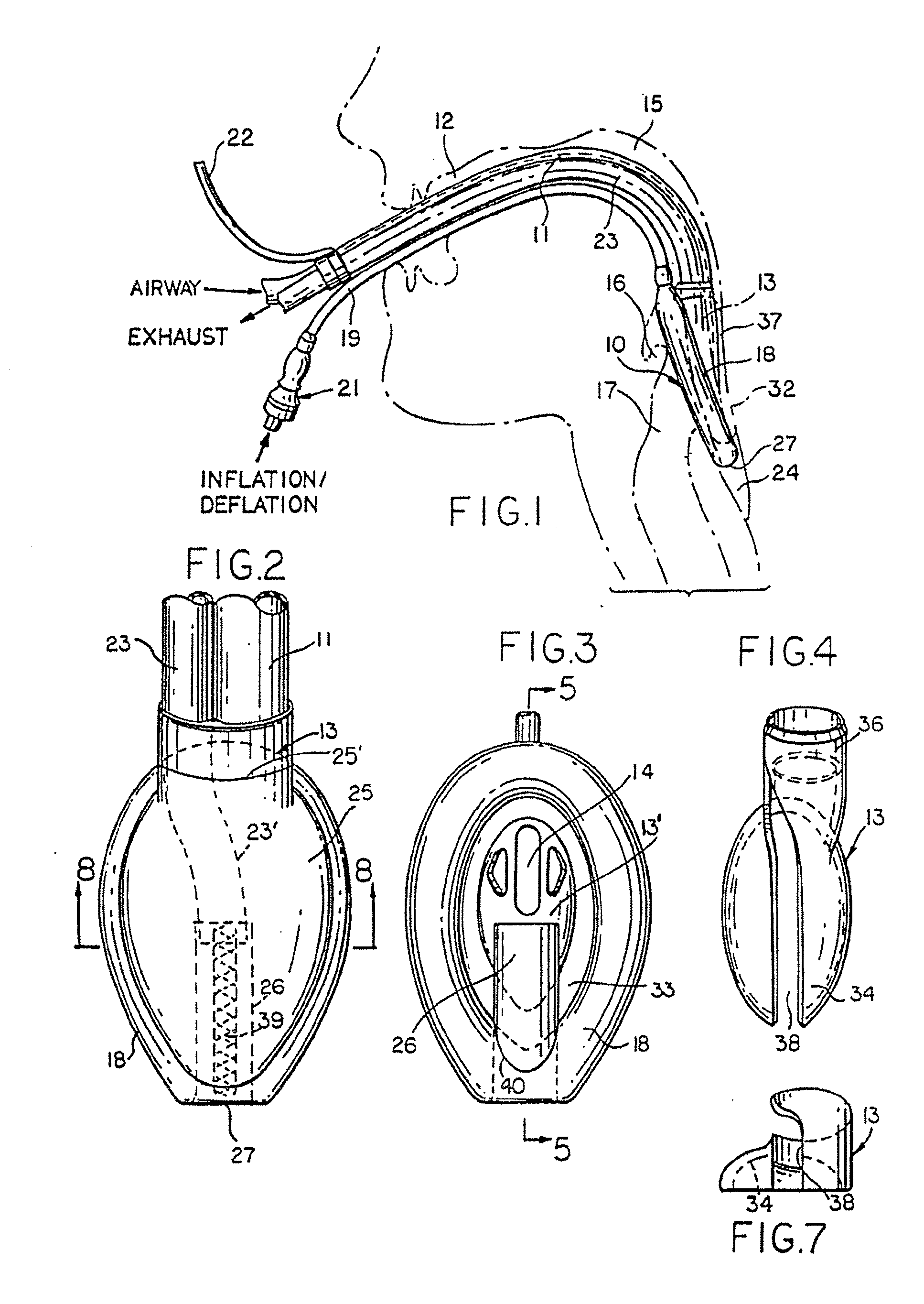
A laryngeal-mask airway device including provision for drainage of the oesophagus comprises an inflatable main-cuff and a backplate having a laryngeal-side and a pharyngeal-side. The backplate also has an external tube-joint adjacent to the proximal region of the main-cuff. The backplate is hermetically bonded to the periphery of the main-cuff establishing separation between a laryngeal-chamber region and a pharyngeal region. An distally open evacuation tube includes a distal portion which longitudinally traverses the interior of the distal region of the main-cuff in sealed relation therewith for operative engagement and communication with the inlet of the oesophagus. The evacuation tube traverses the laryngeal-chamber region generally adjacent to the laryngeal-side of the backplate and passages through a proximally located tube-joint to the pharyngeal region. An airway tube also extends into the tube-joint for communication with an airway port to provide a flowpath between the airway tube and laryngeal-chamber region.
Owner:TELEFLEX LIFE SCI PTE LTD
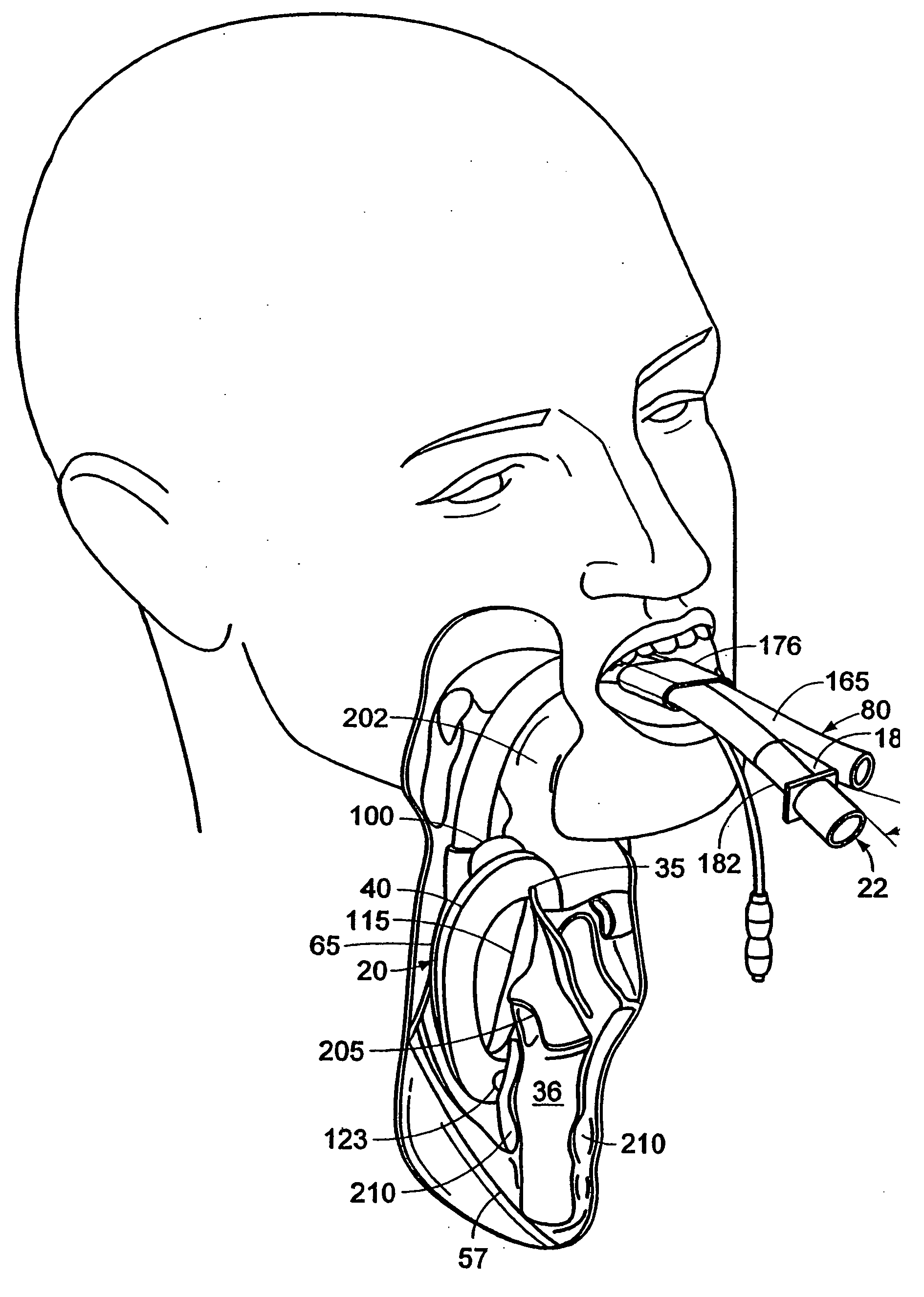
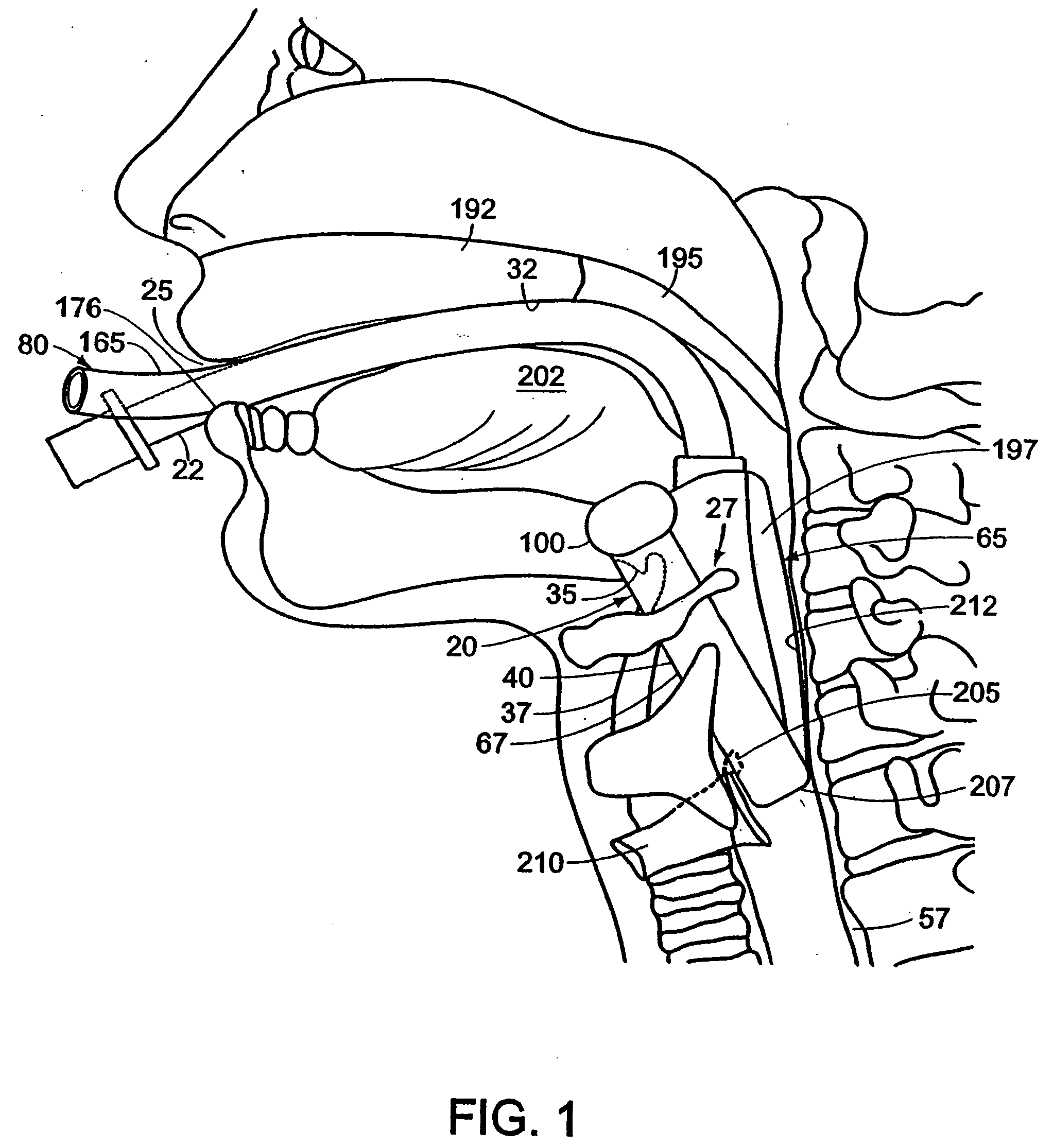
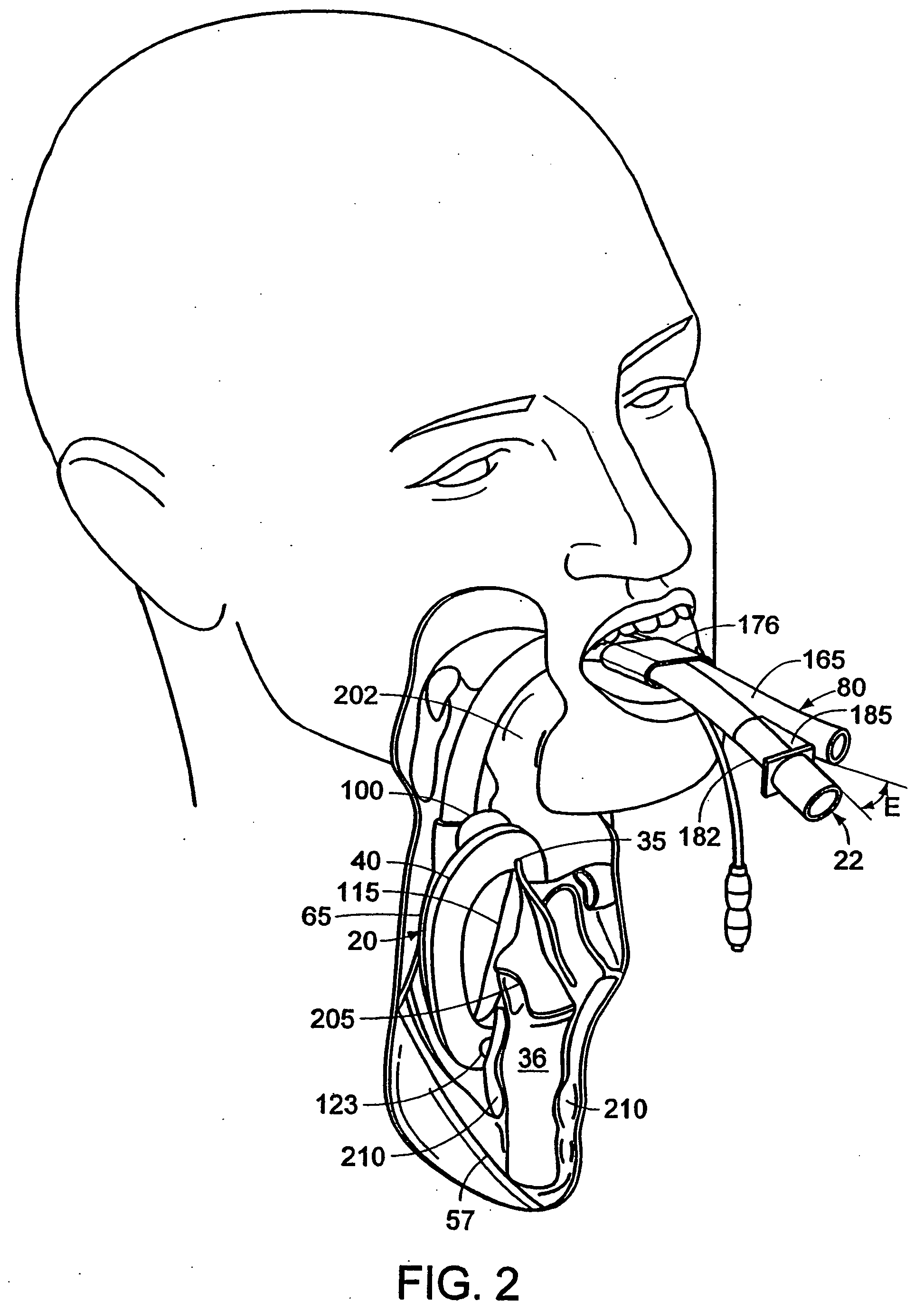
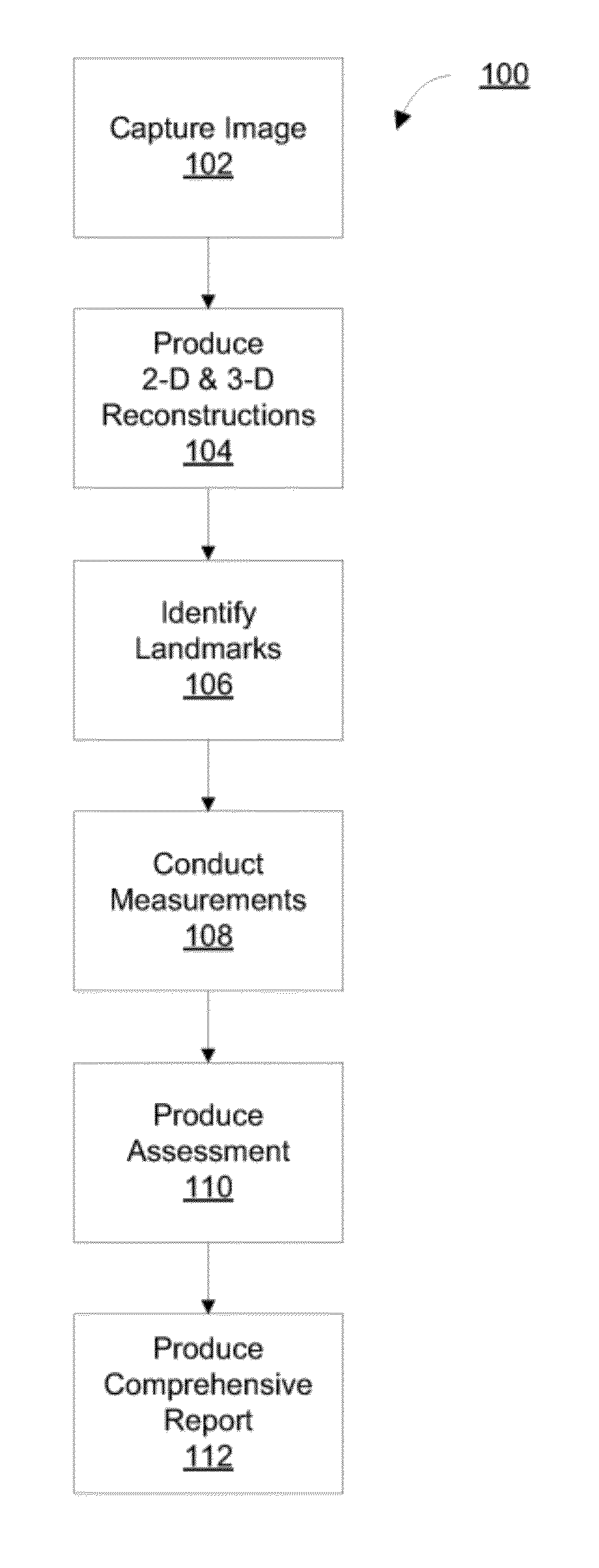
Diagnosing Airway Obstructions
InactiveUS20120022365A1Well representedReliable diagnosisUltrasonic/sonic/infrasonic diagnosticsComputerised tomographsPartial obstructionEpiglottis
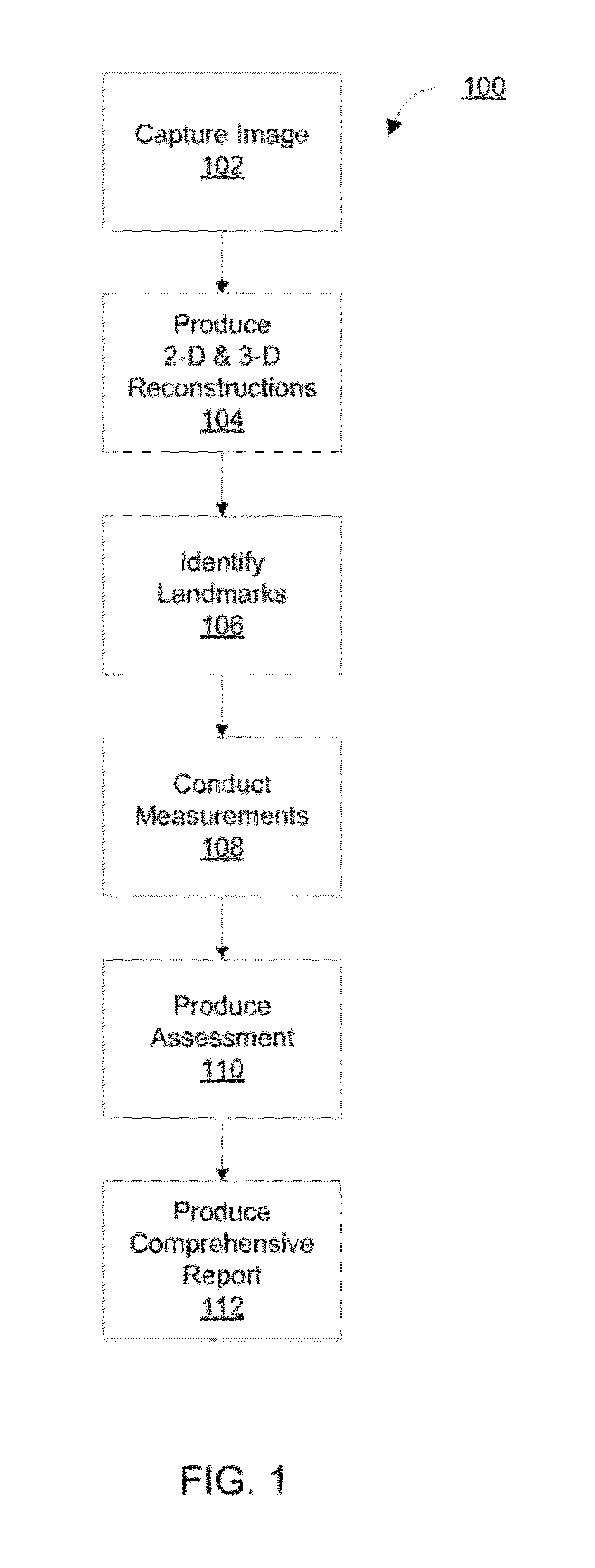
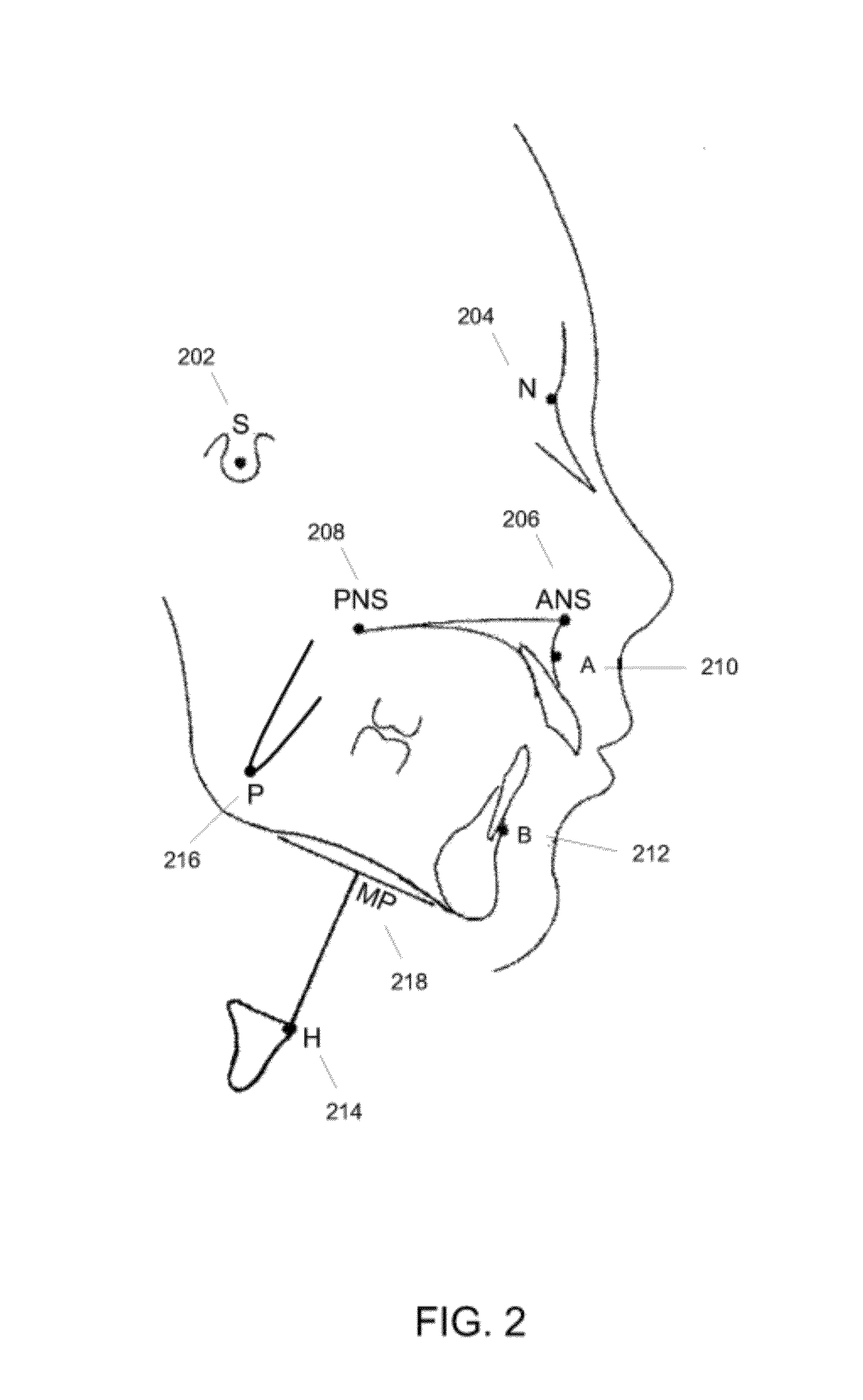
Methods, devices, and computer program products facilitate characterization of a patient's upper airway including nose, palate, oral cavity, epiglottis, pharynx and larynx. Identification of full or partial obstructions in the upper airway enables the production of a full assessment the patient's airway. A report is produced that details the various contributing factors to the patient's airway obstructions. The comprehensive assessments can be utilized to effectively carry out various surgical and non-surgical procedures for treating sleep apnea.
Owner:MANSFIELD ENTERPRISES
Laryngeal mask
A device (10) for maintaining an airway in a patient comprises a mask (12), the mask having a resilient conformable peripheral portion (20, 22) shaped such that the mask (12) forms a seal with the larynx when the mask is positioned in the laryngo pharynx to thereby prevent ingress of extraneous fluids into the larynx, the peripheral portion (20, 22) of the mask defining at least one cavity (32, 34) for providing fluid communication to the oesophagus when the mask is inserted into the laryngo pharynx, and an airway tube (14) connected to or formed with the mask for passing gas to the larynx when the mask is properly inserted into the laryngo pharynx. The airway tube (14) preferably is curved as it leaves the mask.
Owner:卡纳格.巴斯卡 +1
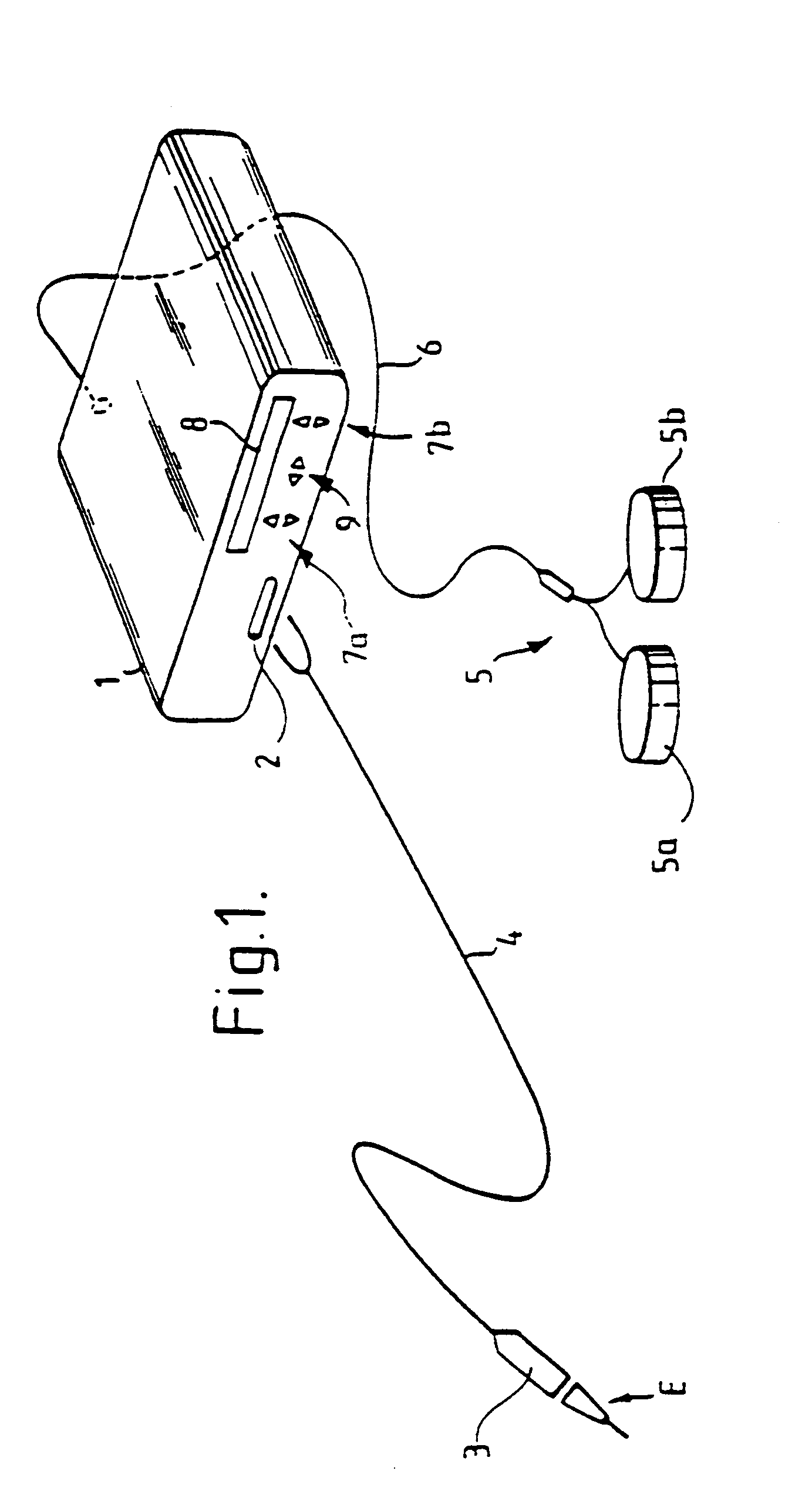
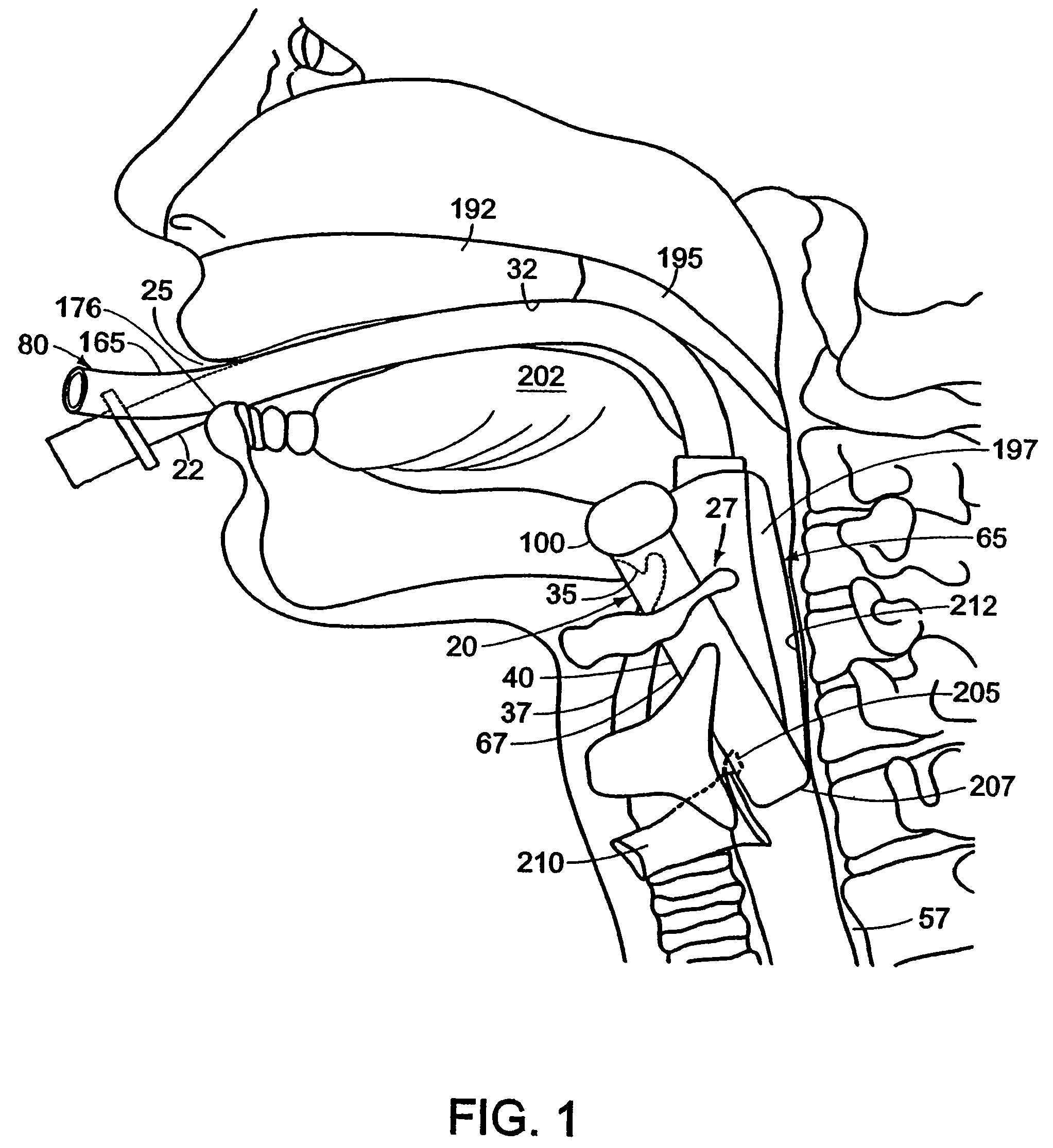
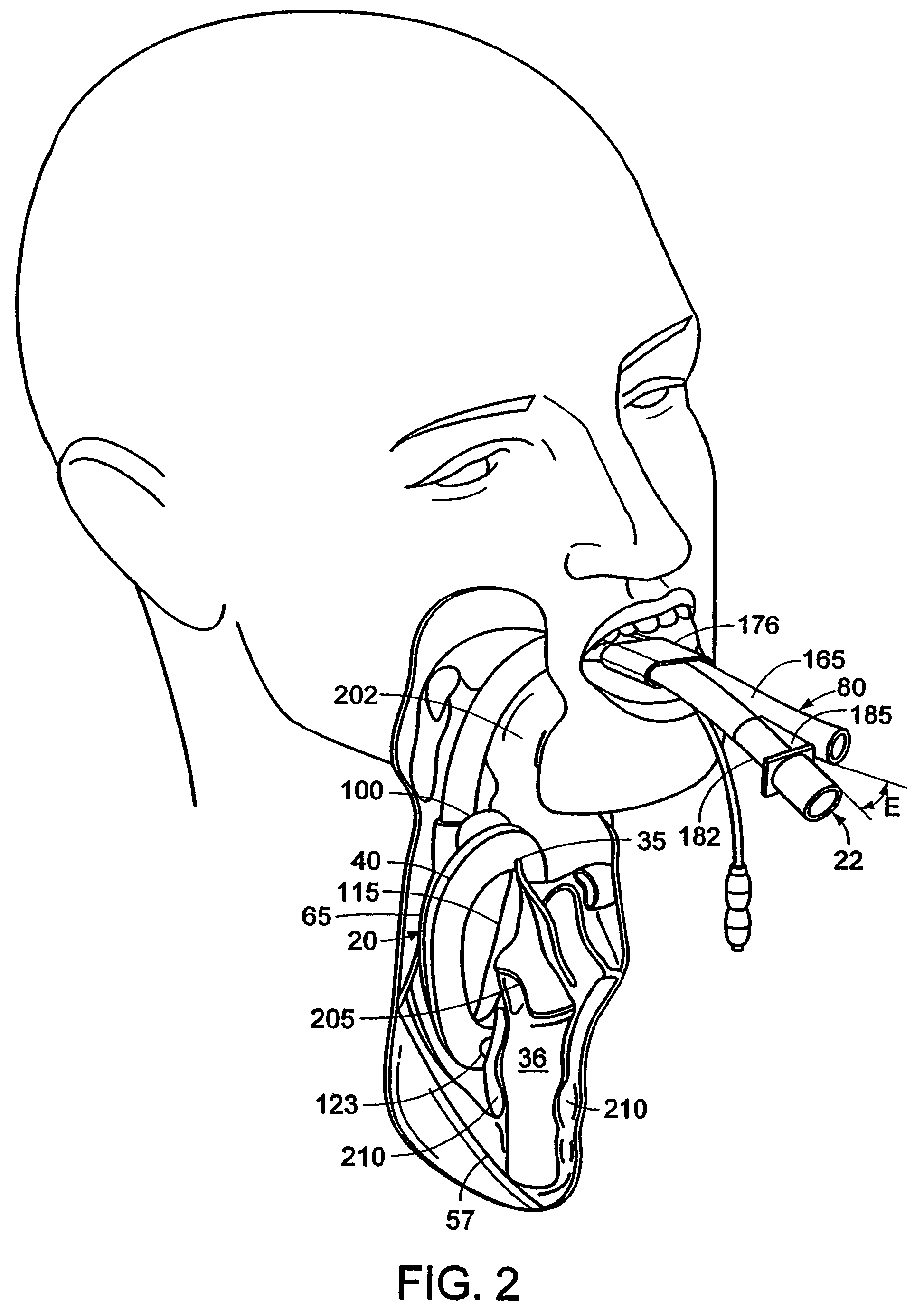
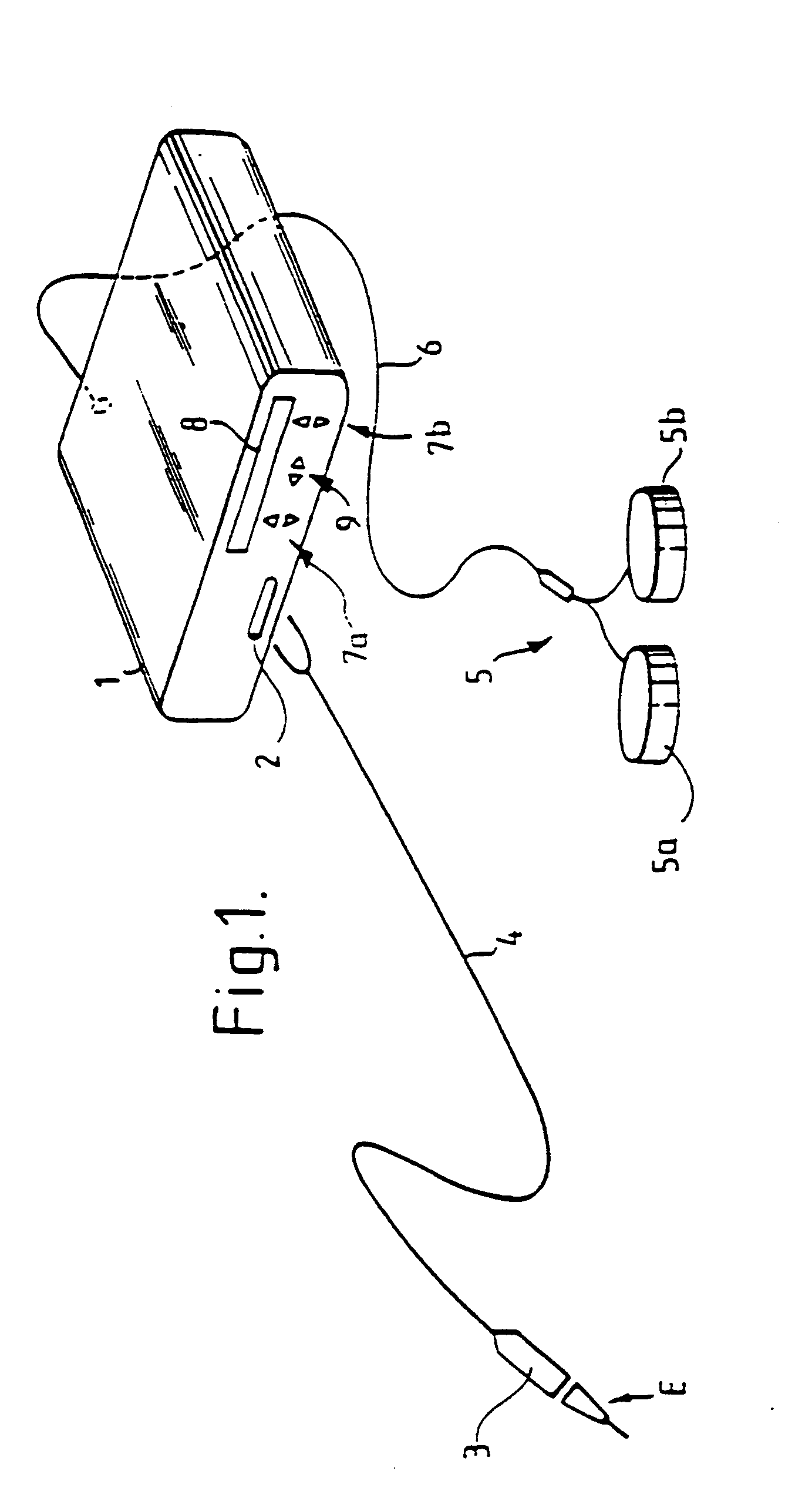
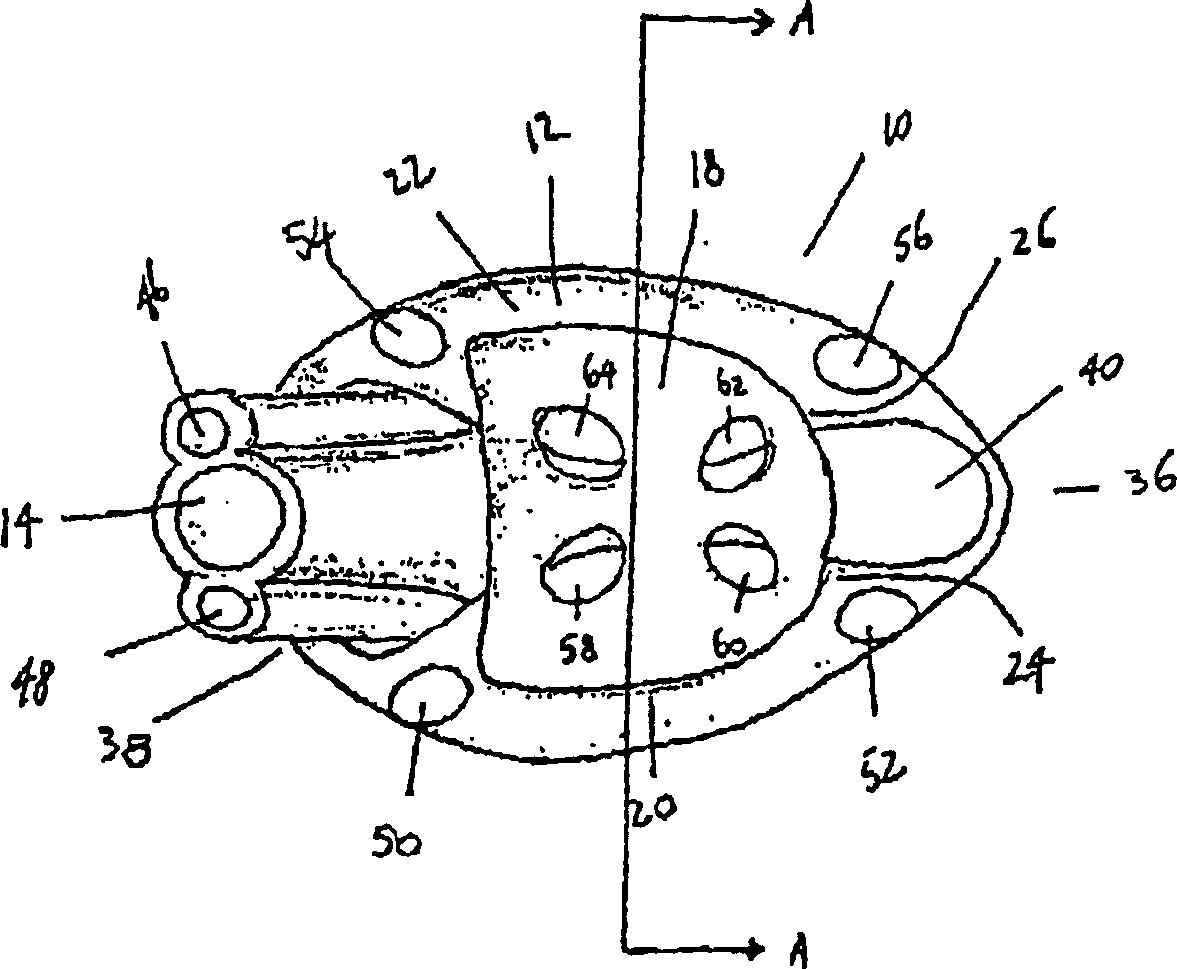
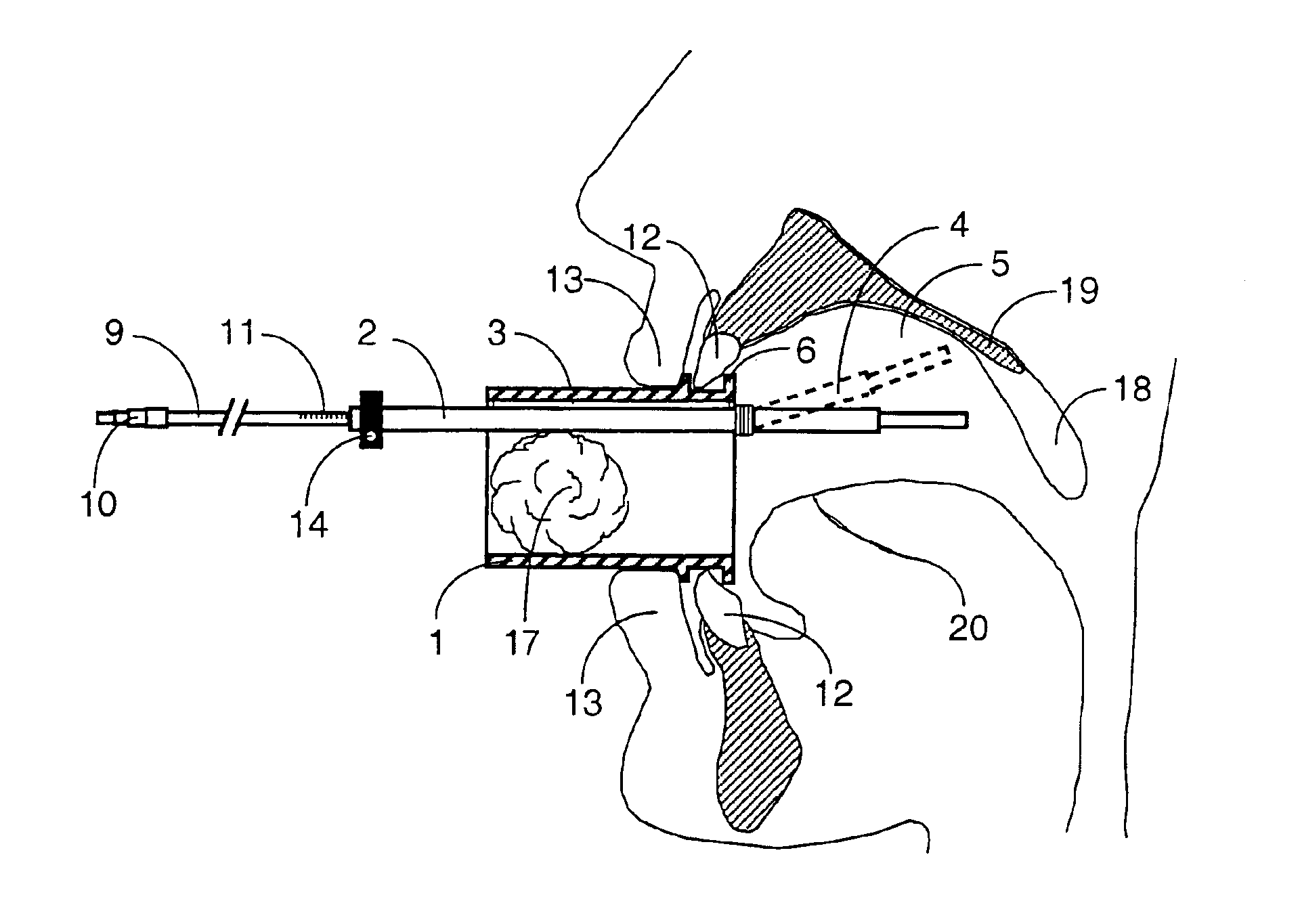
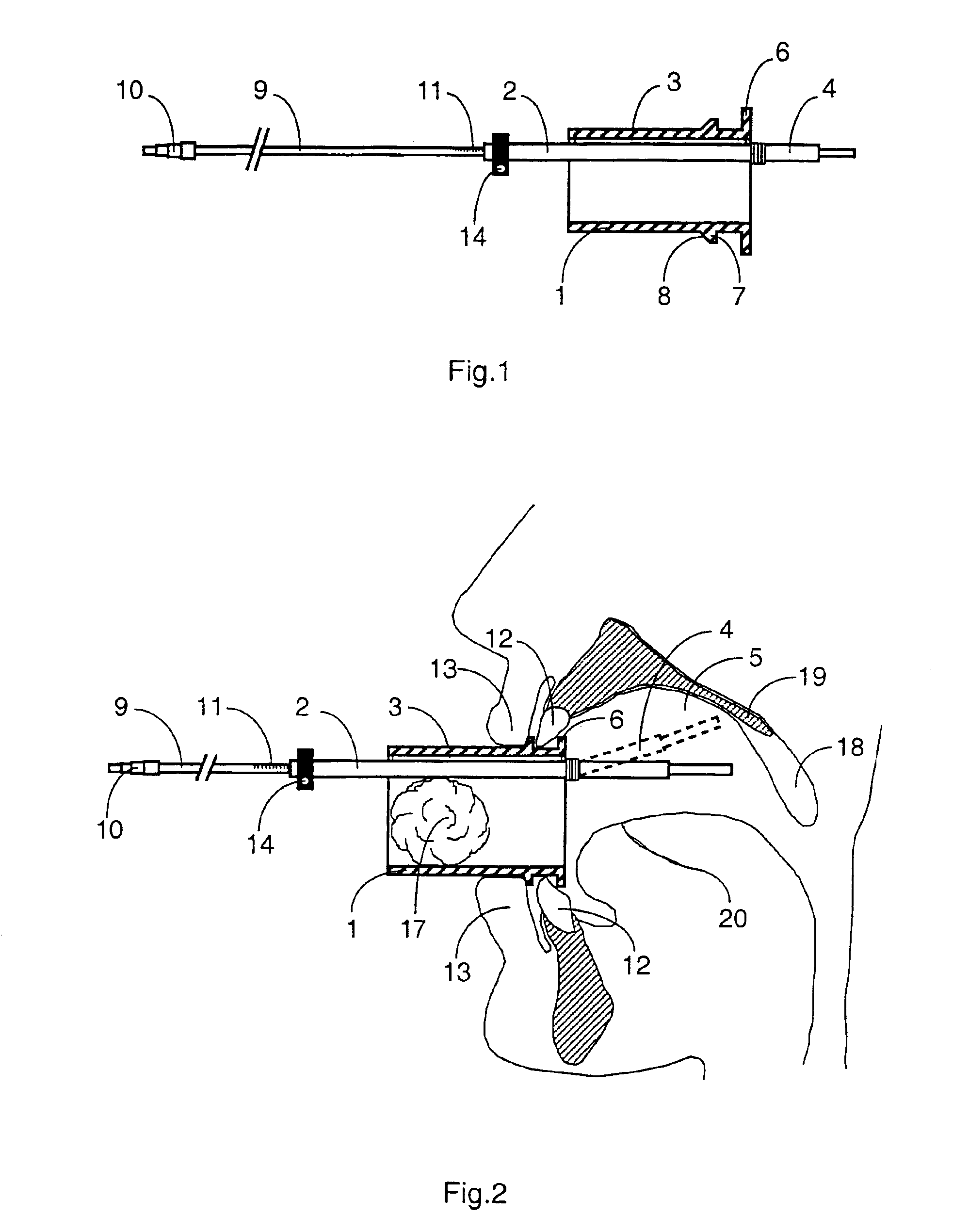
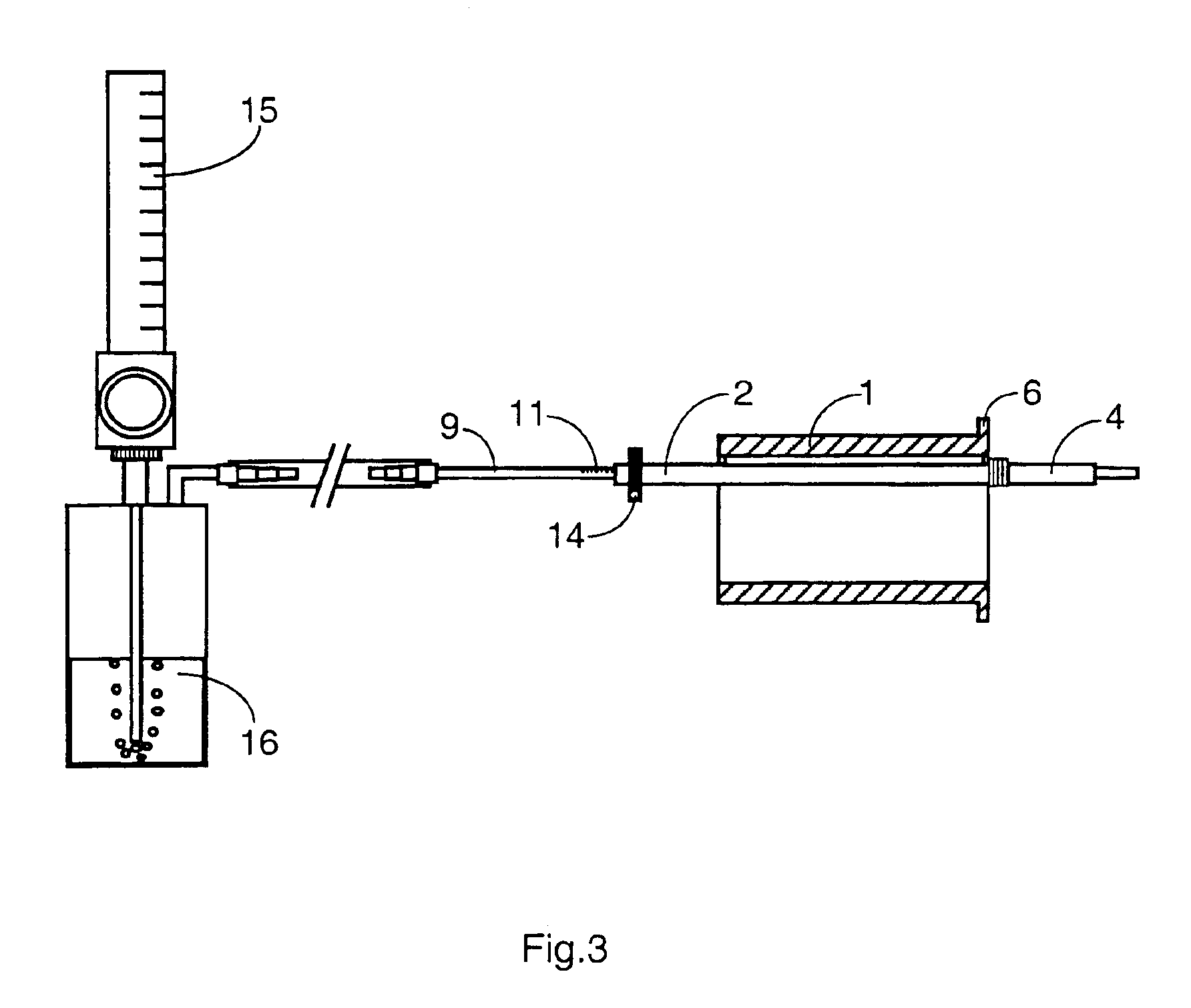
Mouthpiece intended for a device used to assess the sensitivity of the pharynx and a device comprising same
InactiveUS6916287B2Limiting undesirable effectSimple, inexpensive means of evaluating the sensitivityBronchoscopesLaryngoscopesVisual observationPharynx
To assess the sensitivity of a subject's pharynx, an open mouthpiece (1) fitted with a thin guide tube (2), having an articulated end (4), is inserted into the subject's mouth. A pipe (9) is inserted into the guide tube, under visual observation through the mouthpiece, until it touches the subject's palate. The position on a measurement scale (11) provided on the pipe facing a second end of the guide tube is noted, and the pipe is withdrawn over a preset distance, for example 1 cm, and fixed in this position onto the guide tube. A variable gas flow is injected into the pipe and reaches the pharyngeal mucous situated facing the pipe. The sensitivity of the subject's pharynx, measured by the threshold of sense perception, is determined by the lowest flow value perceived by the subject.
Owner:UNIV JOSEPH FOURIER
Oropharyngeal Airway
An oropharyngeal device for insertion into the mouth of a patient. The device includes a body having a distal end and a proximal end with a flange formed at the proximal end. The distal end of the body is inserted into the mouth of the patient until the flange at the proximal end is disposed outside and adjacent to the patient's mouth. The flange keeps the proximal device from entering the mouth. The body is sized such that the distal end of the body is disposed within the pharynx above the epiglottis. The device includes a channel through the body that forms an airway between its proximal and distal ends. The device also includes at least three separate conduits integrated into the body for administering oxygen, suctioning, and for assessing ventilation through end-tidal carbon dioxide monitoring. The conduits for oxygenation and suctioning extend through the body between its proximal and distal ends. The conduit for end-tidal carbon dioxide monitoring extends along and is attached to a side wall of the channel and terminates within the channel.
Owner:GANESH ARJUNAN +2
Tablet forming candy with refreshing, pharynx-clearing and throat-smoothing action
The invention relates to a compound extraction of refreshing and throat beneficiary effect, comprising essentially 1 to 5 portion of tea extraction, 0.5 to 5 portion of guanala extraction, 0.2 to 5 portion of balloonflower root extraction, 0.2 to 5 portion of Sterculiae Scaphigera extractuibm extraction, 10 to 60 portion of xylitol, 0.2 portion of grosvenor momordica extraction, o.1 to 1 portion of ginger oil, and 0.1 to 1 portion of menthol. The invention still relates to a sugar-free refreshing and throat beneficiary tabletting candy, comprising the compound extraction as the principal agent and medically acceptable accessories.
Owner:湖南新中意食品有限公司
Medicine capable of relieving cough and moistening lung
InactiveCN101455806ANo side effectsShort course of treatmentRespiratory disorderPlant ingredientsSide effectChinese traditional
The invention provides a drug with effects of relieving cough and moistening lung. The drug is prepared from the following Chinese medicine materials: prepared rehmannia root, semen ginkgo, bulbus fritillariae cirrhosae, rhizoma polygonati, stigmata maydis, orange peel, folia eriobotryae, angelica decursiva, fructus aristolochiae, almond, semen lepidii, bamboo juice, honeysuckle, rice, medlar, stemona root, oroxylum indicum, liquorice, fructus schizandrae, rhizoma phragmitis, corn mint, figwort, lily, aster and rhizoma polygonati officinalis. The drug uses traditional Chinese herbal medicines as raw materials and is prepared through a scientific formulation and repeated validation of clinical test and meets the theory of traditional Chinese medicine; and the drug has the advantages of no toxicity, no side effect, short treatment course and high radical treatment efficiency. The drug can be used for treating common cold or cough, dry pharynx and other symptoms caused by acute and chronic bronchitis, and has the efficacies of replenishing qi, nourishing yin, moistening lung, relieving cough and the like; therefore, the drug has good social benefits when widely popularized.
Owner:朱新华
Oral airway
An oral airway for insertion into the mouth and pharynx of a patient is disclosed and adapted to connect to an anesthesia breathing connector, a suction tube, or a nasal cannula, interchangeably as needed, without necessitating the removal of the oral airway from the patient. A tubular member has a connector at a proximal end and includes a first portion that is adapted for fixing with the anesthesia breathing connector. A second portion of the connector is adapted for receiving the suction tube. At least one protuberance between the first and second portions receives and retains a portion of the nasal cannula, or alternately, at least one aperture is included in the second portion for receiving a portion of the nasal cannula. A mouth guard extends outwardly from the connector. Alternately, a suction tube guide within the tubular member may be slidably positionable between a retracted and extended position.
Owner:GUERRA PHILLIP B +1
Medicine plaster for curing laryngopharyngitis and its preparation method
InactiveCN1443555AEffective treatmentWell mixedUnknown materialsRespiratory disorderPeppermintsPlatycodon grandiflorus
The present invention relates to a medicine plaster for external application for curing larynopharyngitis. It is a medicinal small granule made up by using one kind of the Chinese medicinal materialsof licorice, platycodon root, trichosanthes rind, anemarrhena root, scutellaria roiot, peppermint and others or several kinds of them, and said small medicinal granule is adhered on the adhesive plaster so as to obtain the invented medicine plaster. When it is used, the small medicine granule can be mounted and acted on the neck and pharynx zone on the back of hand.
Owner:胡世英
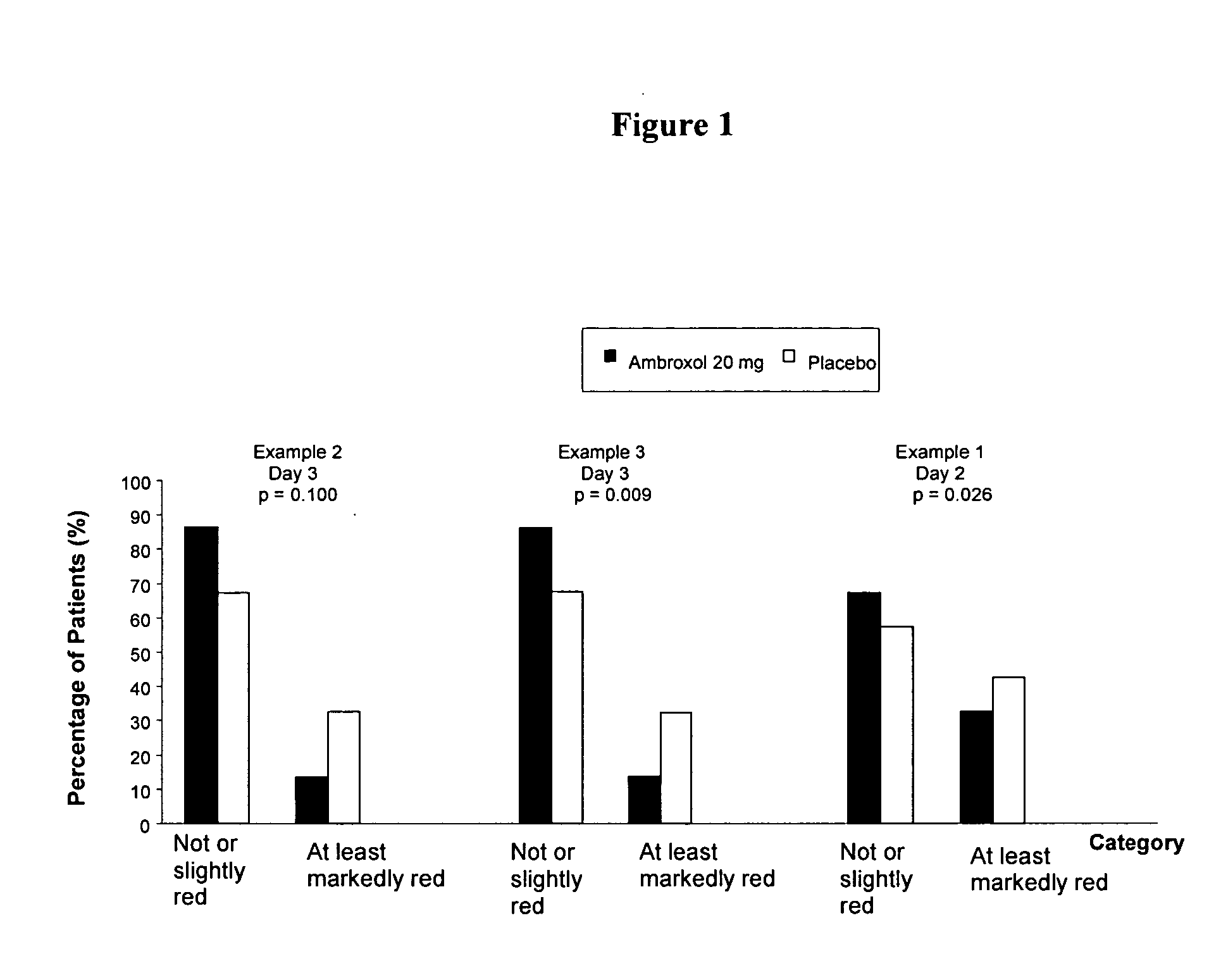
Ambroxol for the treatment of inflammation in the pharynx
The invention relates to the use of ambroxol (trans-4-(2-amino-3,5-dibromobenzylamino)-cyclohexanole) and the pharmacologically acceptable salts thereof for preparing a pharmaceutical composition for the treatment of inflammation in the pharynx.
Owner:BOEHRINGER INGELHEIM PHARM KG
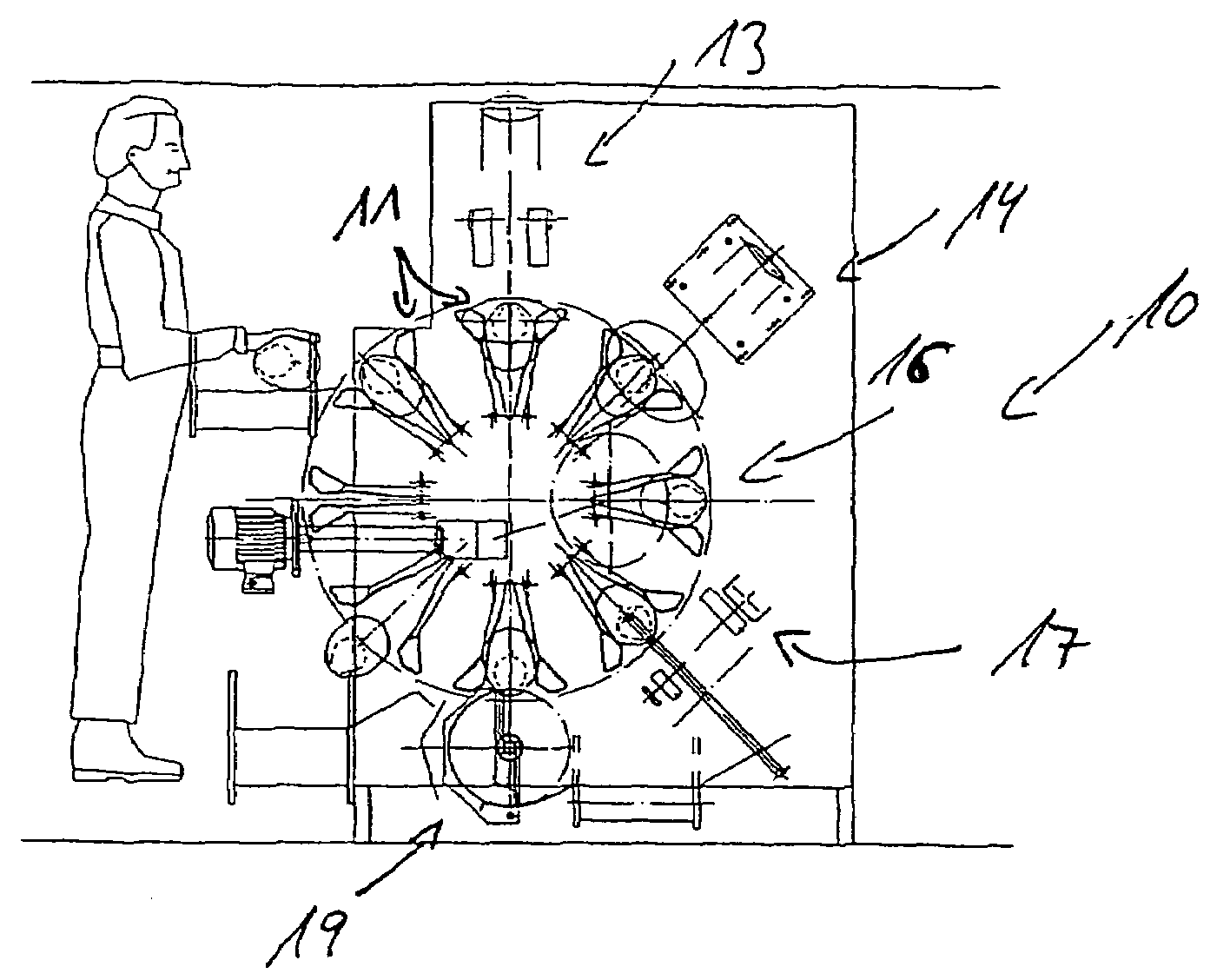
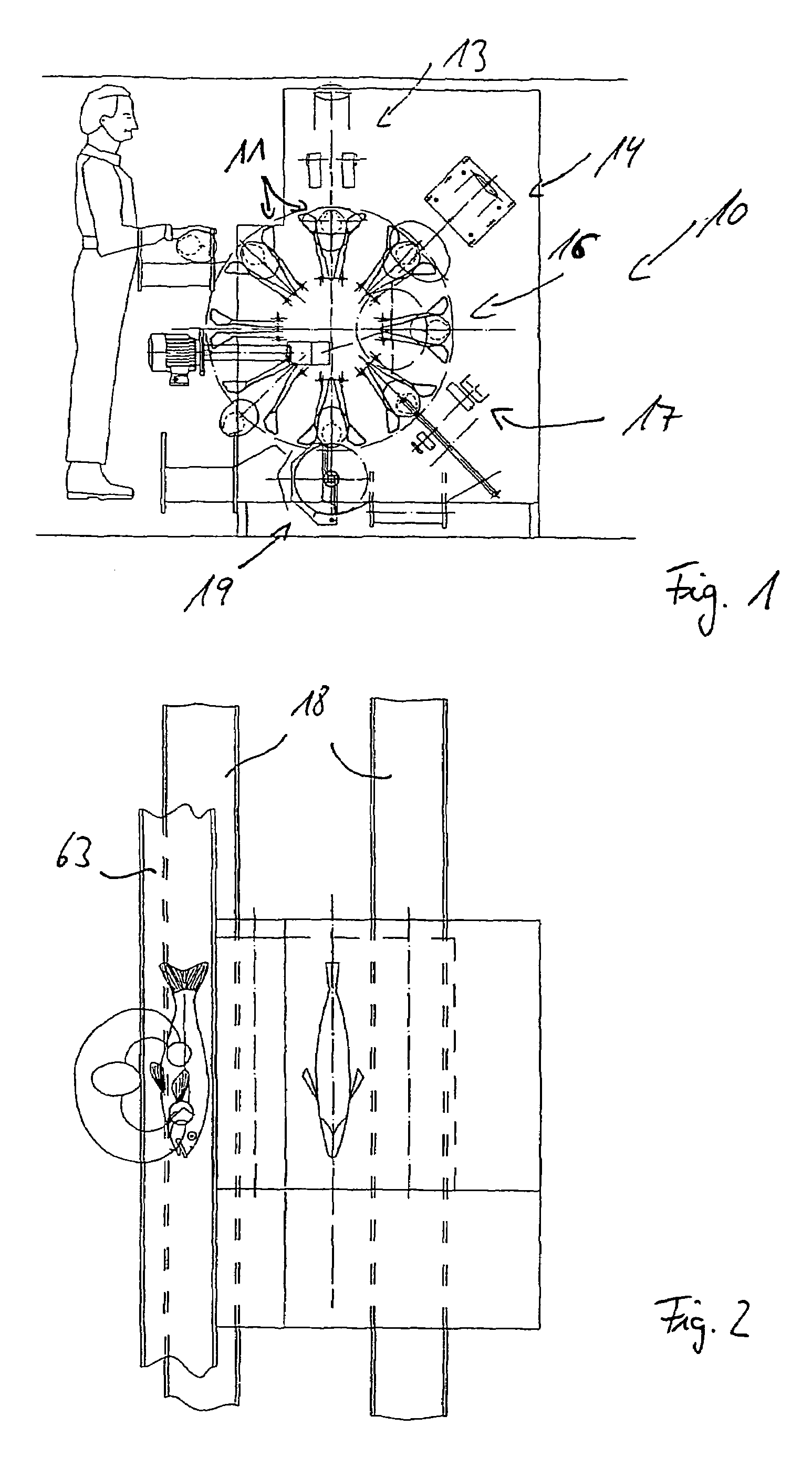
Method and device for slaughtering fish in particular white fish
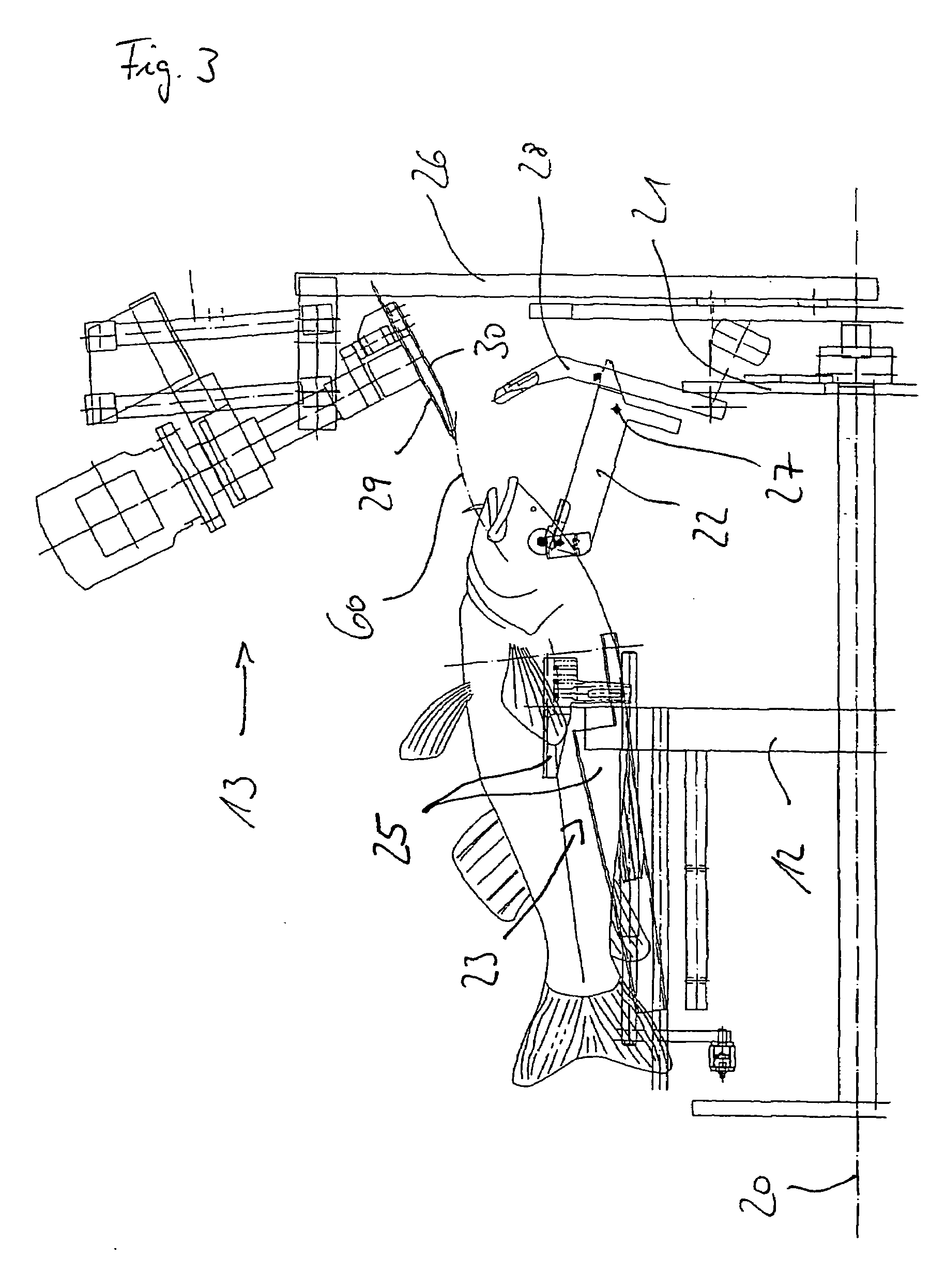
InactiveUS7056202B2Increased length rangeImprove cut qualityAnimal stomach clearanceKosher slaughtering devicesAbdominal cavityPharynx
A device and method for slaughtering fish, in particular white fish, has at least one fish receptacle for positioning and receiving the fish, a throat cutting apparatus for cutting through the throat in preparation for the pharynx cut, a pharynx cutting apparatus for completely cutting through the pharynx, a slaughtering apparatus for opening the abdominal cavity, a gut severing apparatus for releasing the entrails from the abdominal cavity and a peripheral fish receiving drum with which the fish can be moved to the individual processing stations. The fish receiving drum is driven intermittently in rotation about a horizontal shaft. The fish are movable transversely to their longitudinal axis on an essentially vertical circular path. The fish receptacle includes pectoral fin receptacles and a torso clamp for fixing the fish torsos. Also, a head support with a head clamp is provided.
Owner:NORDISCHER MASCHINENBAU RUD BAADER GMBH CO KG
Negative pressure oral apparatus
ActiveUS20110073119A1Inhibit sheddingReduce curingRespiratorsSnoring preventionSleep disordered breathingPalate muscle
This invention provides an oral apparatus and method capable of alleviating or curing snore and obstructive sleep apnea by applying a negative pressure through a mini oral interface to the oral cavity. The mini oral interface creates a secure connection between the interface and mouth or teeth and prevents the interface from falling off. The oral interface also provides fixation between upper and lower lips (or tooth) and prevents opening of patient's mouth during sleeping. The negative pressure pulls the tongue toward upper palate and also pulls the soft palate forward as well. By moving the tongue and the soft palate in a forward direction, the patentcy of the upper airway near the pharynx is maintained to prevent sleep-disordered breathing. The negative pressure will pull the lips inward to close the mouth ad prevent air from entering the oral cavity from atmosphere. The negative pressure will also pull the soft palate into contact with the rear surface of the tongue to create a seal that prevents the air from entering the oral cavity through the nasal airway.
Owner:SOMNICS INC
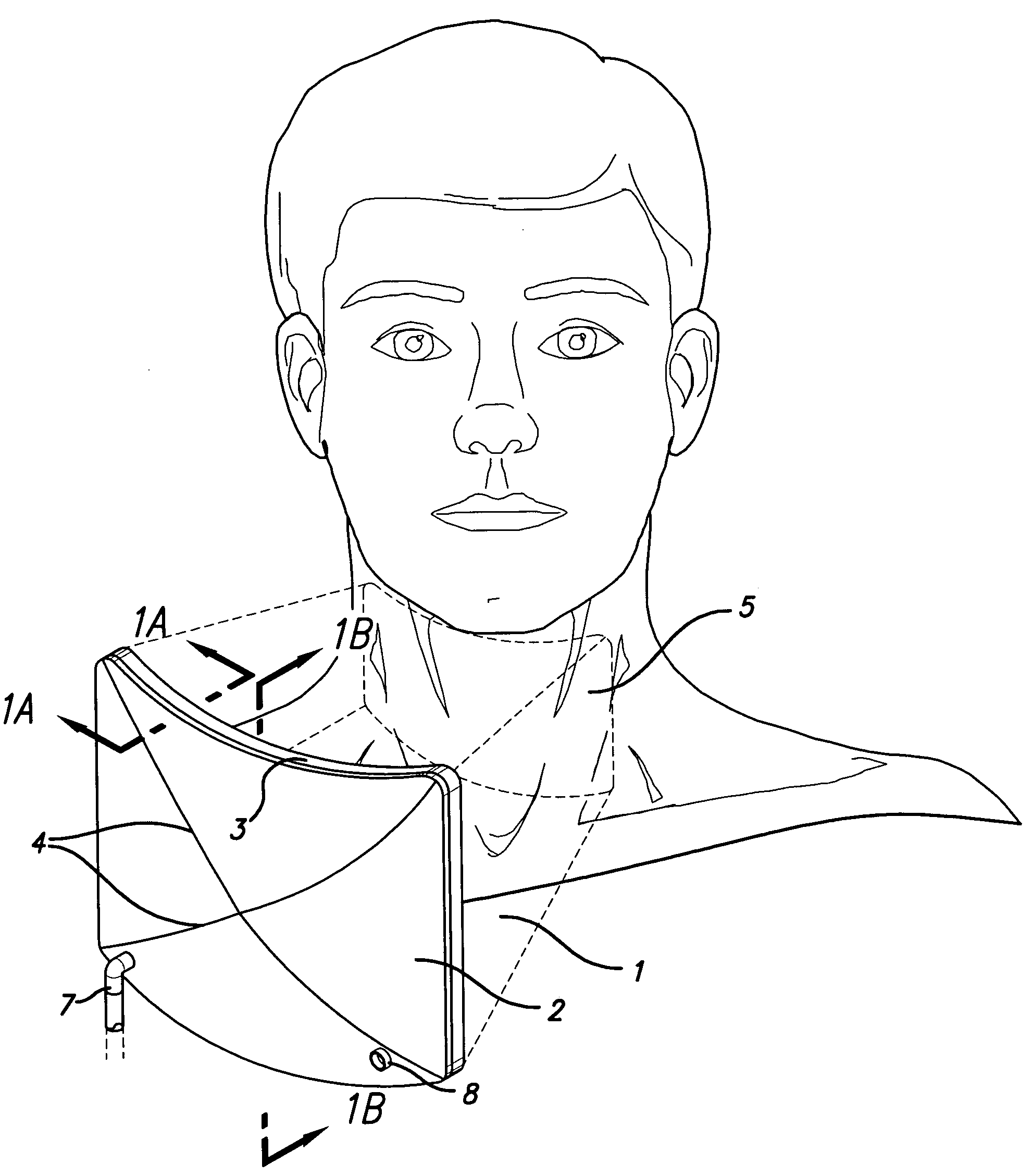
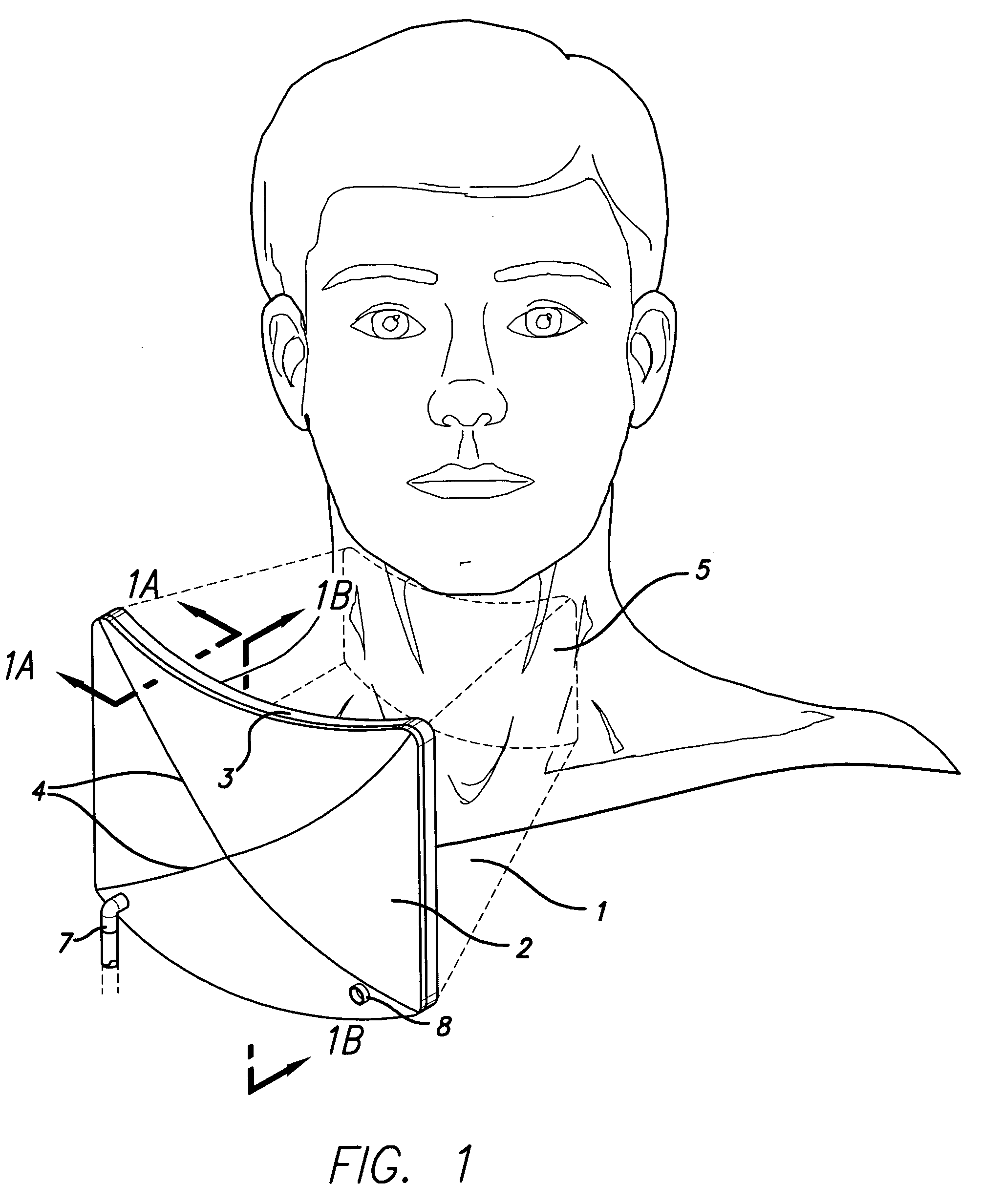
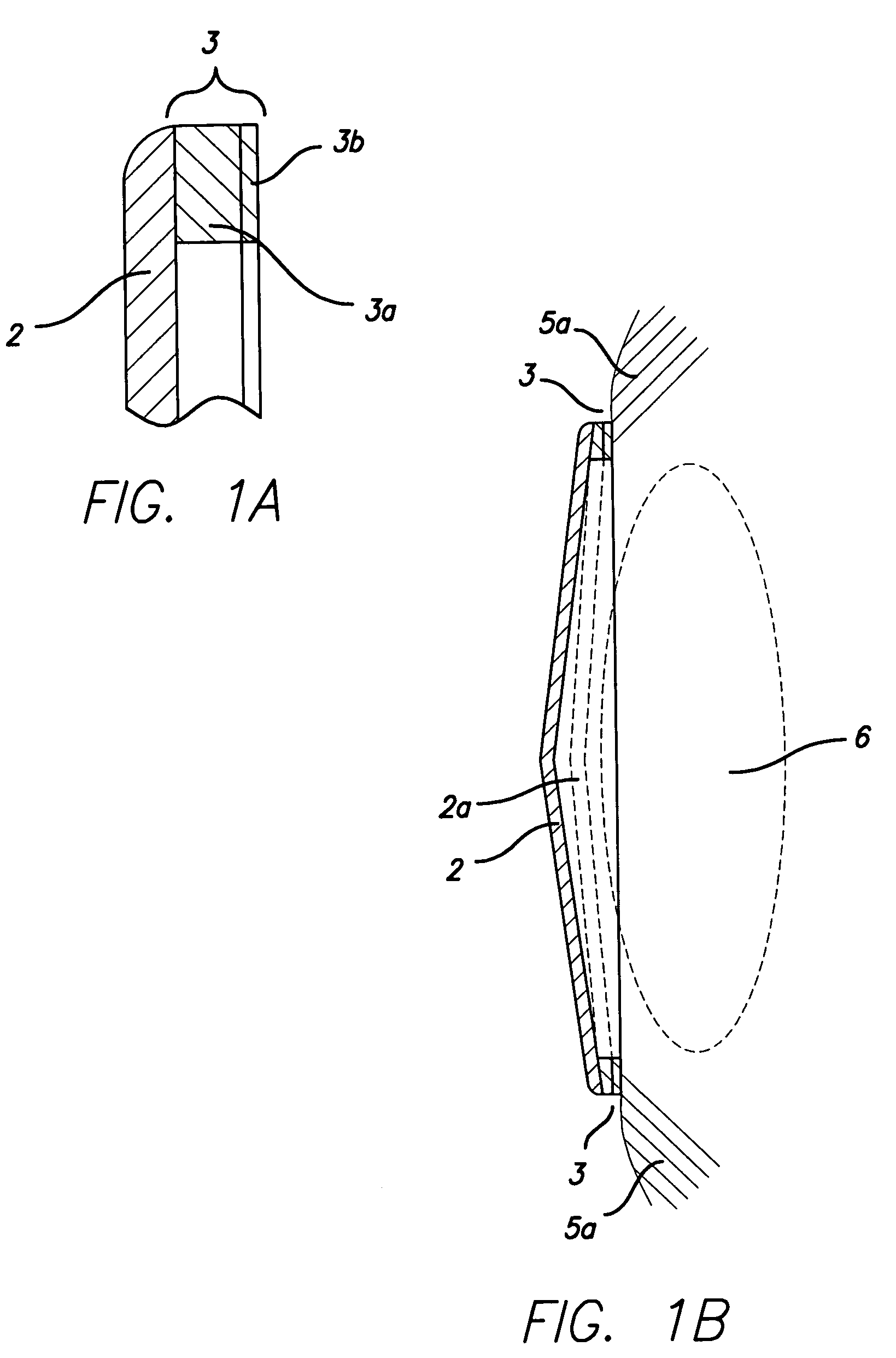
Combination Bite Block, Tongue Depressor/Retractor And Airway
A combination bite block, tongue depressor / retractor and airway for establishing and maintaining an open airway while preventing emergence clenching and the resulting dental and soft tissue damage associated with emergence clenching in procedures where anesthesia and / or sedation are used, or when the patient is not in control of their own airway, regardless of the cause. The inventive subject matter includes a tongue depressor / retractor component in both right and left conformations; and a bite block component. The bite block component is a wedge shaped, compressible component that is inserted between the upper and lower molars on either the right or left side of the mouth. The tongue depressor component is comprised by a flat portion of that is inserted into the side of the bite block, and an optional curved portion that retracts the tongue off of the posterior pharynx when in place.
Owner:CALIFORNIA POLYTECHNIC STATE UNIVERSITY
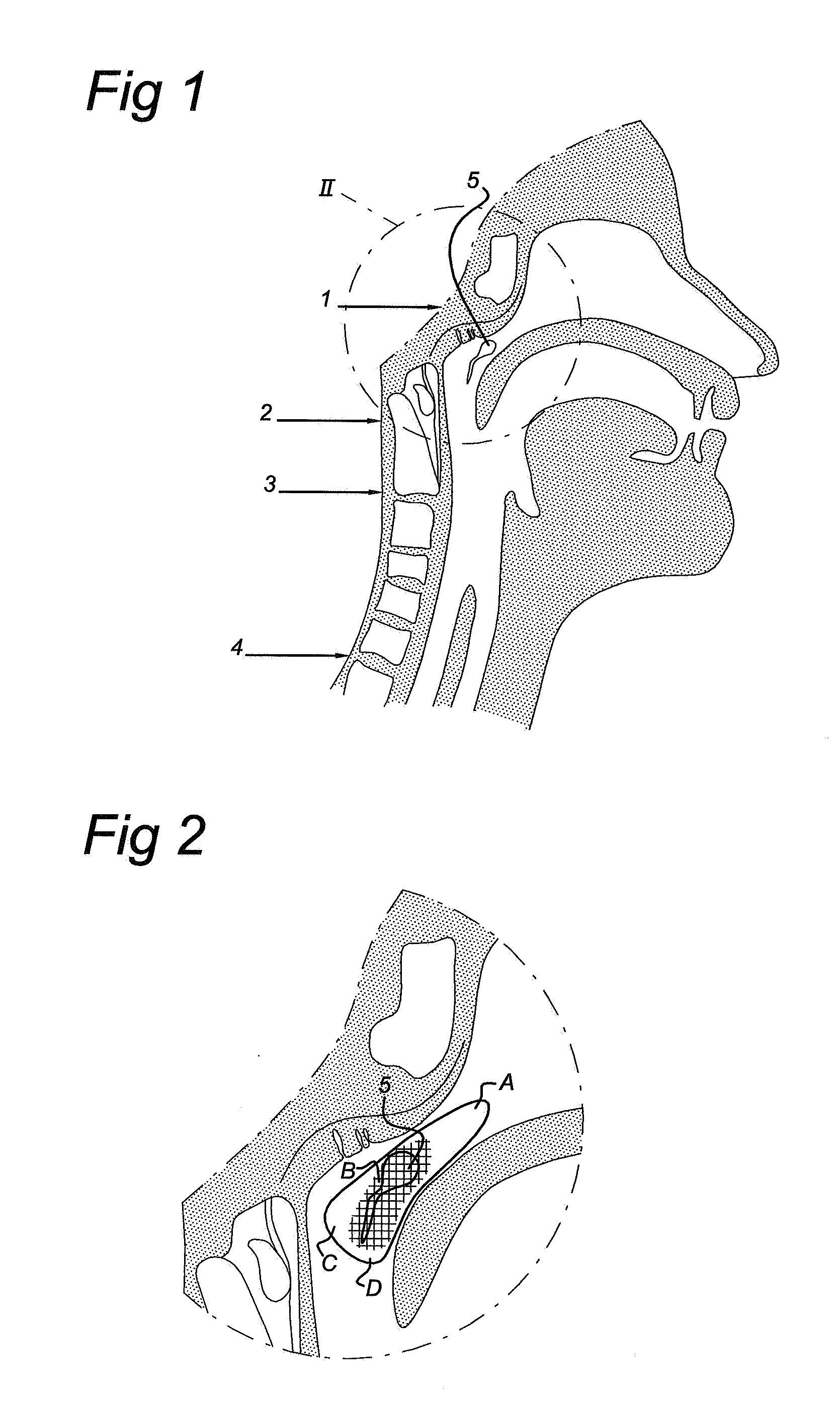
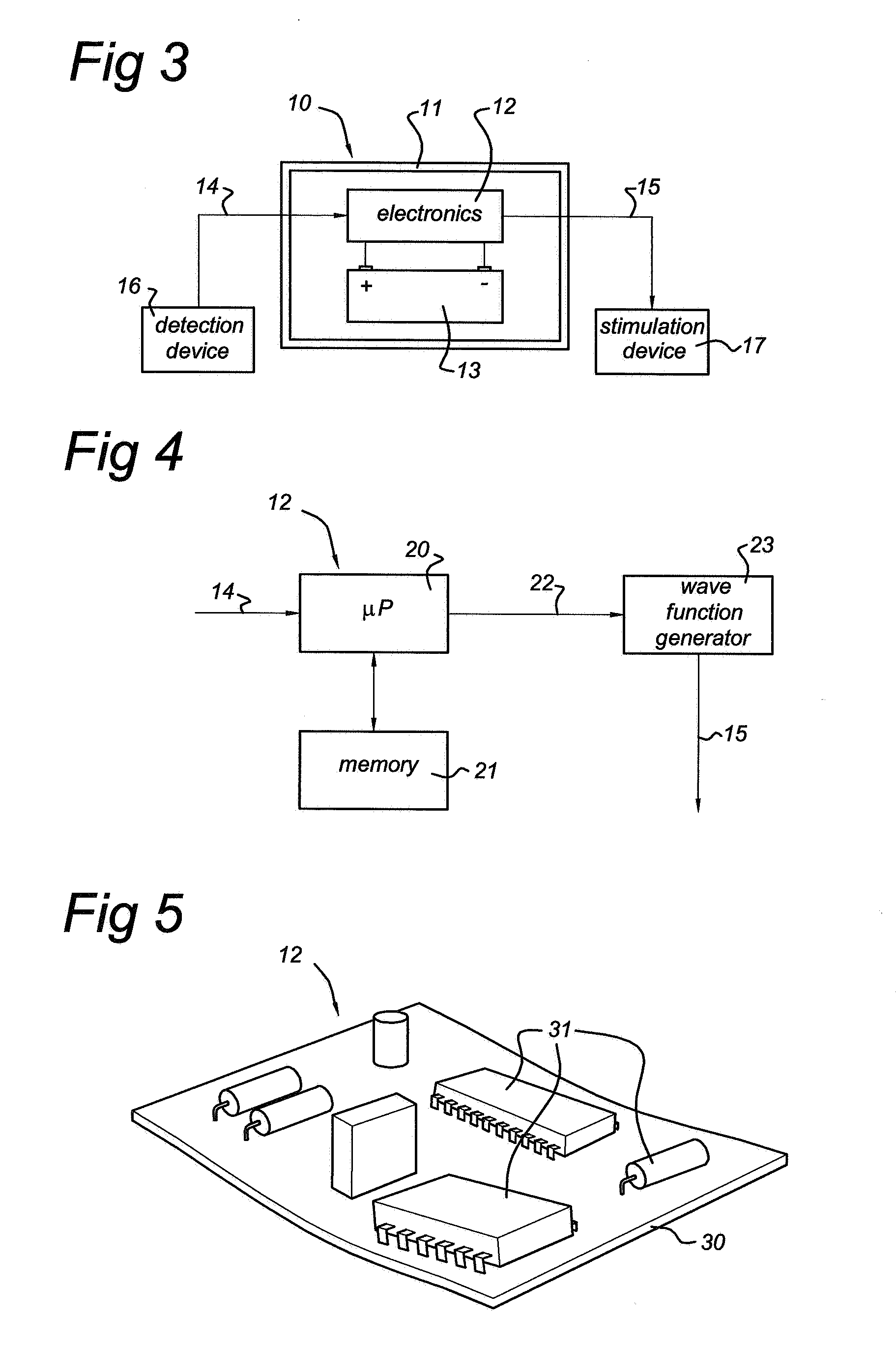
Cardiovertor/defibrillator
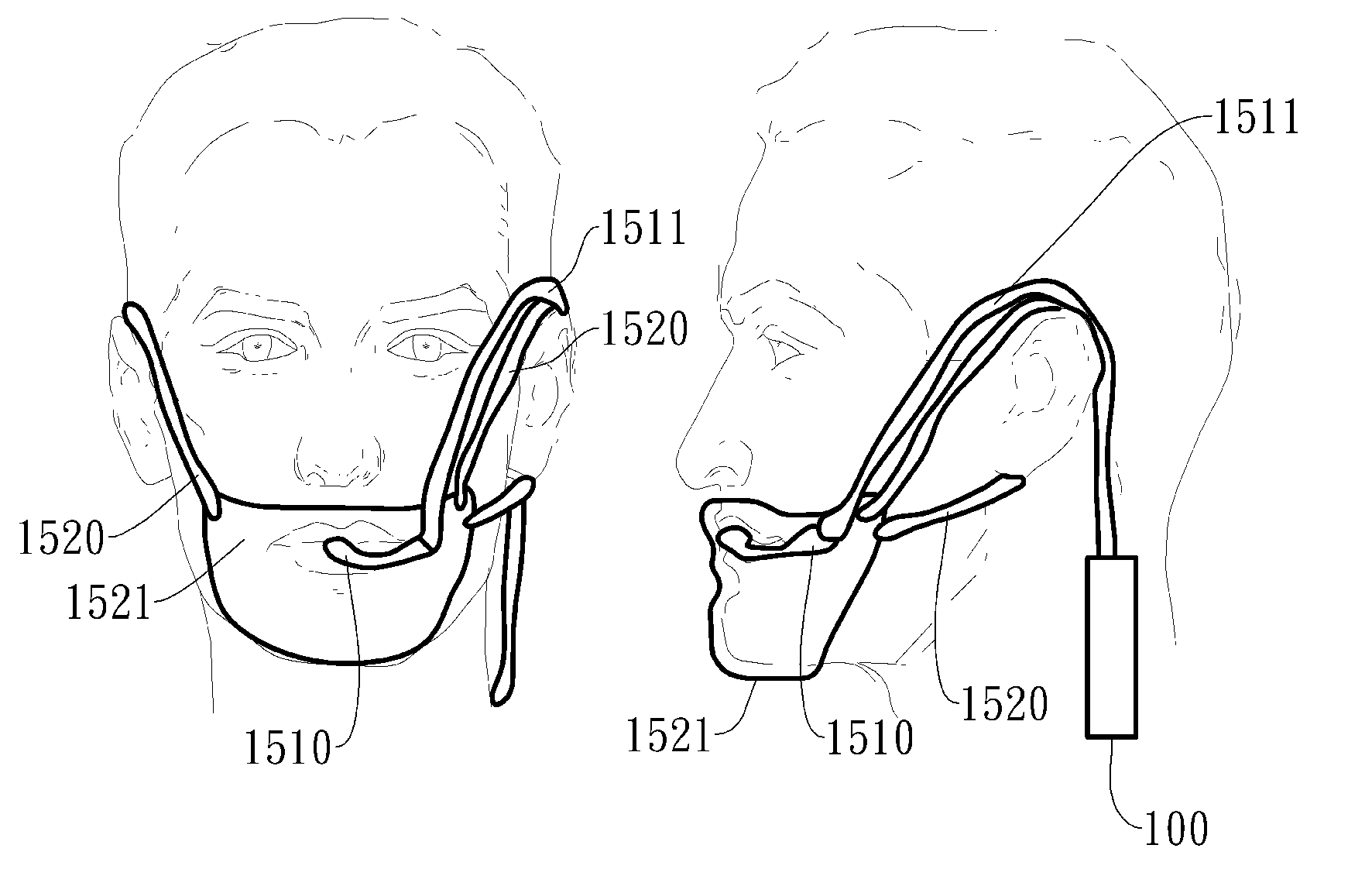
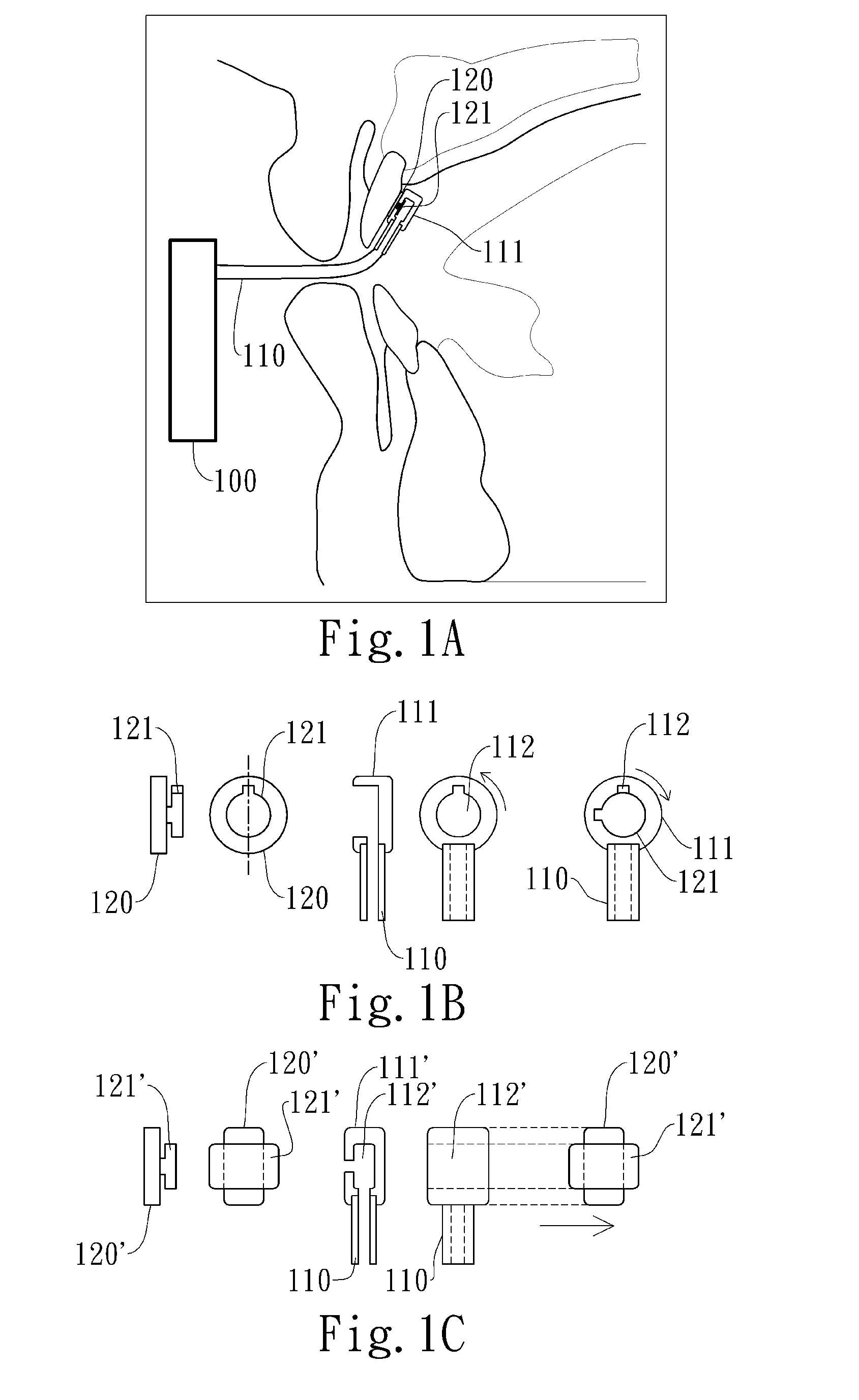
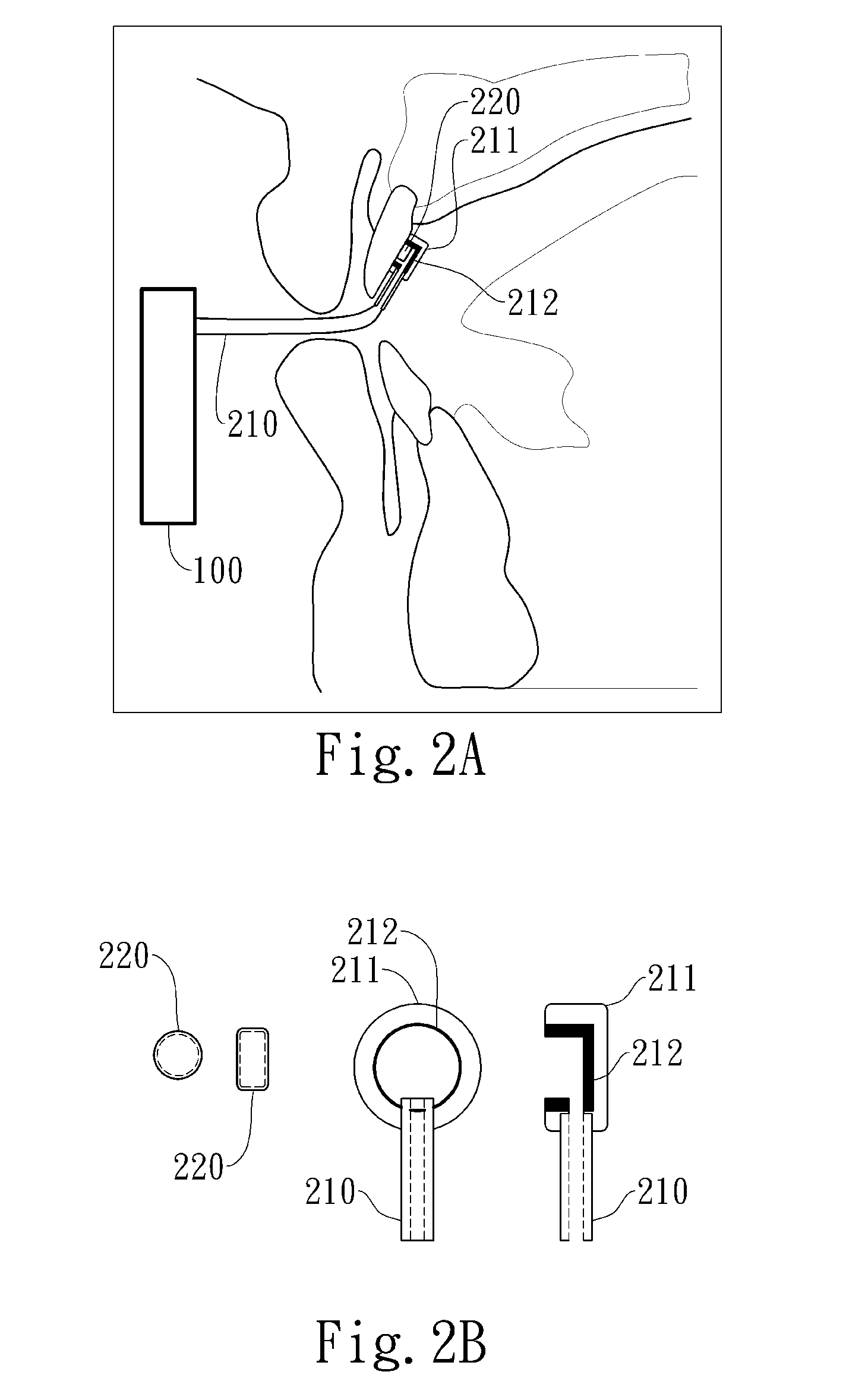
Cardiovertor / defibrillator with electronics (12) that receives a detection signal from a detection device (16) indicating whether a heart of a mammal is fibrillating, processes the detection signal by running a program and generates a control signal for a stimulation device (17) based on the detection signal in order to allow the stimulation device (17) to provoke defibrillation of the heart by a resuscitating stimulationin the area of the pharynx of the mammal. The apparatus has a nose plug.
Owner:LISIAK 1
Automatic throat swab sampling device
ActiveCN111643123ALow costSimple structureBronchoscopesLaryngoscopesPhysical medicine and rehabilitationThroat swab sample
The invention provides an automatic throat swab sampling device which comprises a head positioning unit of a person to be detected, a navigation positioning unit and a sample collection execution unit, the to-be-tested person head positioning unit is used for realizing head positioning, opening the oral cavity of the to-be-tested person and flattening the tongue of the to-be-tested person, and providing an unobstructed space leading to the pharynx for the tail ends of the navigation positioning unit and the sample collection execution unit; the navigation positioning unit enters the oral cavity of the to-be-detected person along with the tail end of the sample collection execution unit and is used for collecting throat images and determining depth information between the depth sensor and the throat of the to-be-detected person; the sample collection execution unit comprises a three-degree-of-freedom guide rail type device and a one-degree-of-freedom tail end execution mechanism; the guide rail type device can achieve pharyngeal wiping action of a person to be tested and control over deep feeding movement and wiping action force control parts in the oral cavity of the person to be tested, and all the units are controlled by a control end in real time. The device provides a basis for full automation of the throat swab collection process.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com