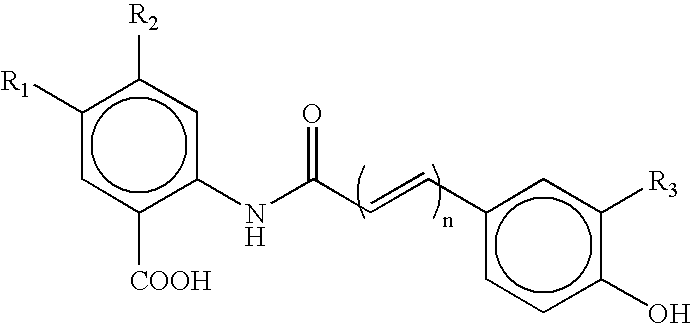
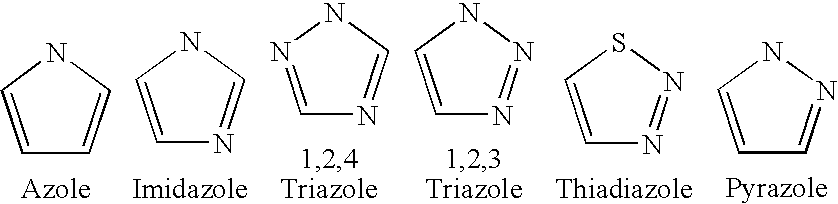
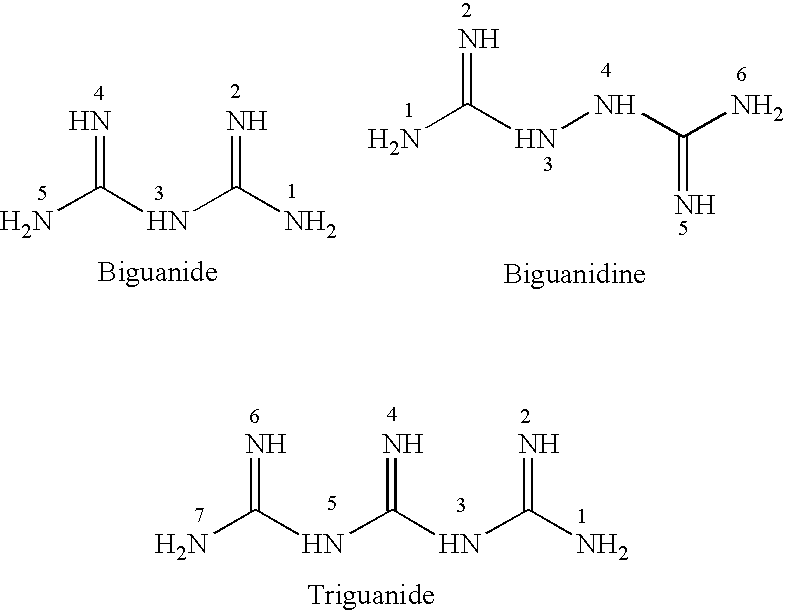
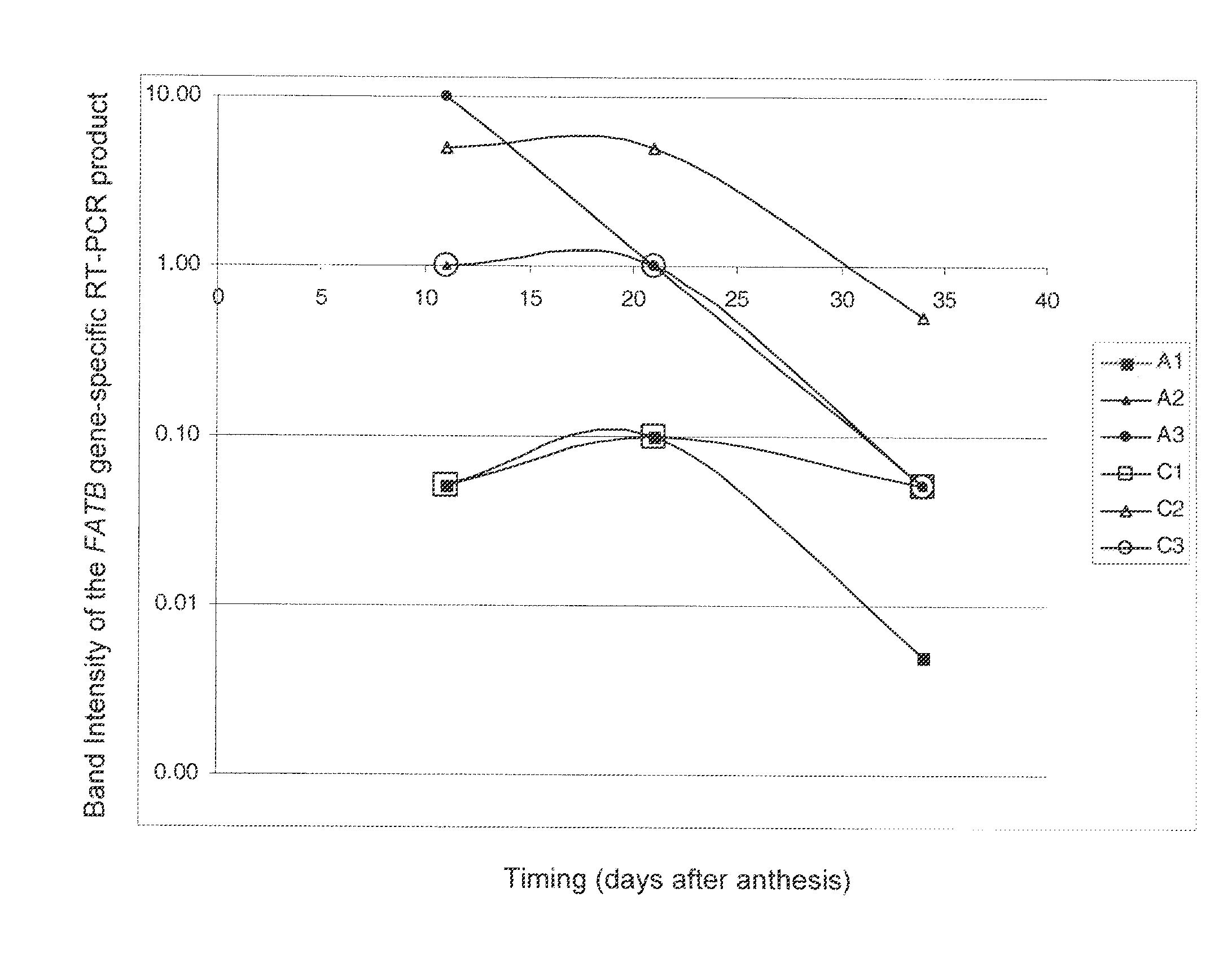Patents
Literature
245346results about "Plant ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Avenanthramide-containing compositions
Methods and compositions for treating or preventing a skin condition, an inflammation, an irritation or an allergy associated with an ectoparasitic infection or infestation on an animal. The methods involve applying to the skin of the animal a pharmaceutical composition that contains a therapeutically effective amount of one or more than one avenanthramide, an optional ecto and / or endo-parasiticidal agent, and a pharmaceutically acceptable diluent or carrier
Owner:CEAPRO
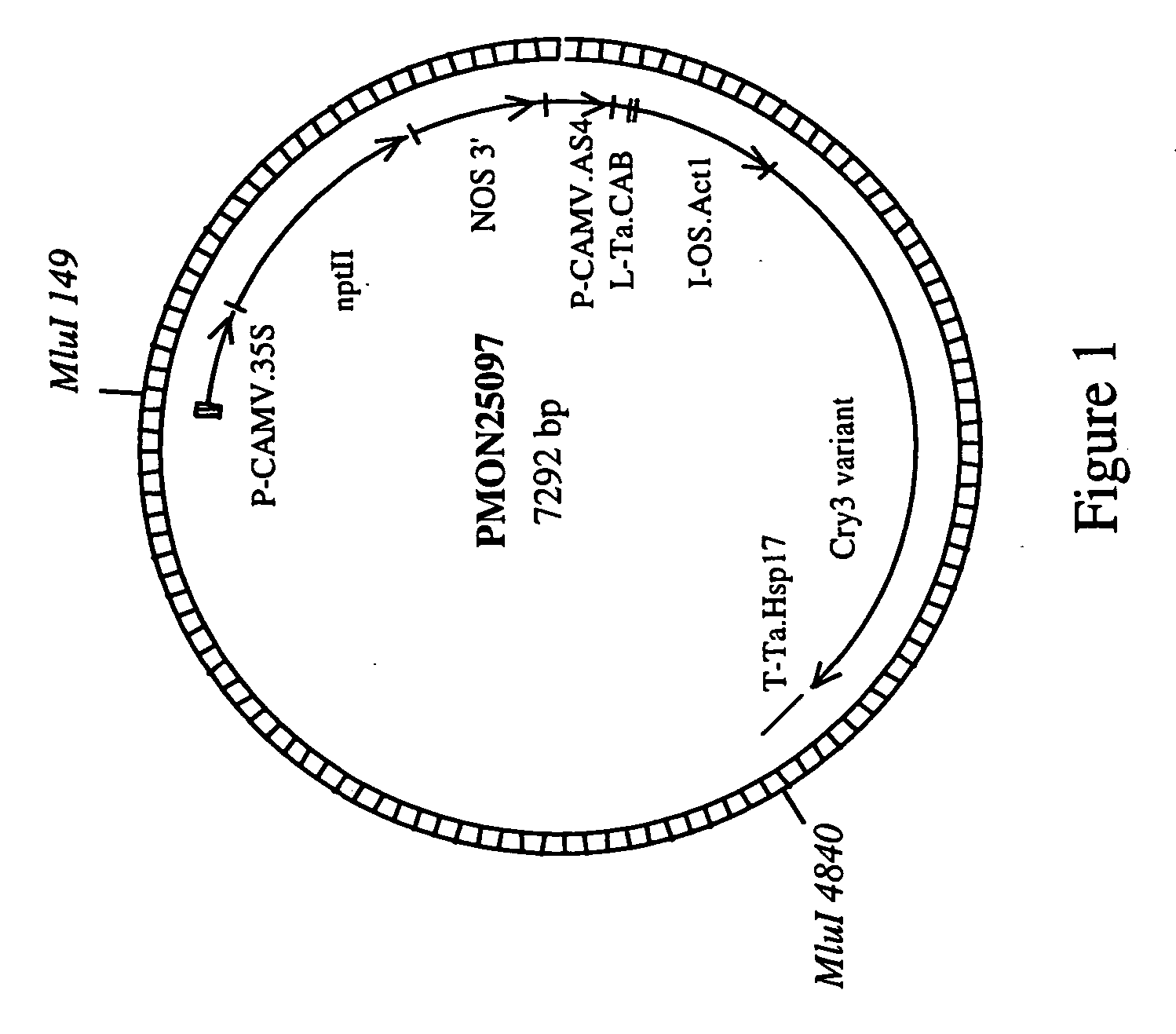
Corn event pv-zmir13 (mon863) plants and compositions and methods for detection thereof
The present invention provides compositions and methods for detecting the presence of the corn event MON863 DNA inserted into the corn genome from the transformation of the recombinant construct containing a Cry3Bb gene and of genomic sequences flanking the insertion site. The present invention also provides the corn event MON863 plants, progeny and seeds thereof that contain the corn event MON863 DNA.
Owner:MONSANTO TECH LLC
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Plants and seeds of corn variety I285291
According to the invention, there is provided seed and plants of the corn variety designated I285291. The invention thus relates to the plants, seeds and tissue cultures of the variety I285291, and to methods for producing a corn plant produced by crossing a corn plant of variety I285291 with itself or with another corn plant, such as a plant of another variety. The invention further relates to corn seeds and plants produced by crossing plants of variety I285291 with plants of another variety, such as another inbred line. The invention further relates to the inbred and hybrid genetic complements of plants of variety I285291.
Owner:MONSANTO TECH LLC
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Skin Firming Anti-Aging Cosmetic Mask Compositions
InactiveUS20040161435A1Promote excess fat reductionPromote cellulite controlBiocideCosmetic preparationsAdditive ingredientPhase mask
I have discovered cosmetic mask compositions suitable for face, neck, chin or body applications. These compositions synergistically combine at least one skin beneficial cosmetic or drug composition with at least one composition to promote excess fat reduction, cellulite control, or muscle toning benefits. The mask composition also contains at least one binder composition that binds with other beneficial ingredients by electrostatic, atomic, or ionic charges to synergistically enhance their topical site-specific benefits. These mask compositions are suitable for a variety of delivery system methods that include peel-off mask, leave-in mask, moisturizing mask, exfoliating mask, prosthetic mask, soaking mask, depilatory mask, foaming mask, rinse-off mask, sloughing mask, rub-off mask, two-phase mask, dual-chamber mask, and self-heating (heat releasing) mask.
Owner:GUPTA SHYAM K
Transdermal delivery system
The present invention relates to the discovery of a transdermal delivery system that can deliver high molecular weight pharmaceuticals and cosmetic agents to skin cells. A novel transdermal delivery system with therapeutic and cosmetic application and methods of use of the foregoing is disclosed.
Owner:ORIX +1
Plant extracts for the preparation of pharmaceutical compositions for the treatment of hepatitis
The use of extracts from the plant Hypericum perforatum in the preparation of pharmaceutical compositions for the treatment of hepatitis C, chronic hepatitis C and related viruses, said pharmaceutical compositions comprising at least one extract of the plant Hypericum perforatum and optionally in addition, one or more pharmaceutically acceptable inactive components, selected from, carriers, coatings, diluents and adjuvants.
Owner:LAVIE DAVID +1
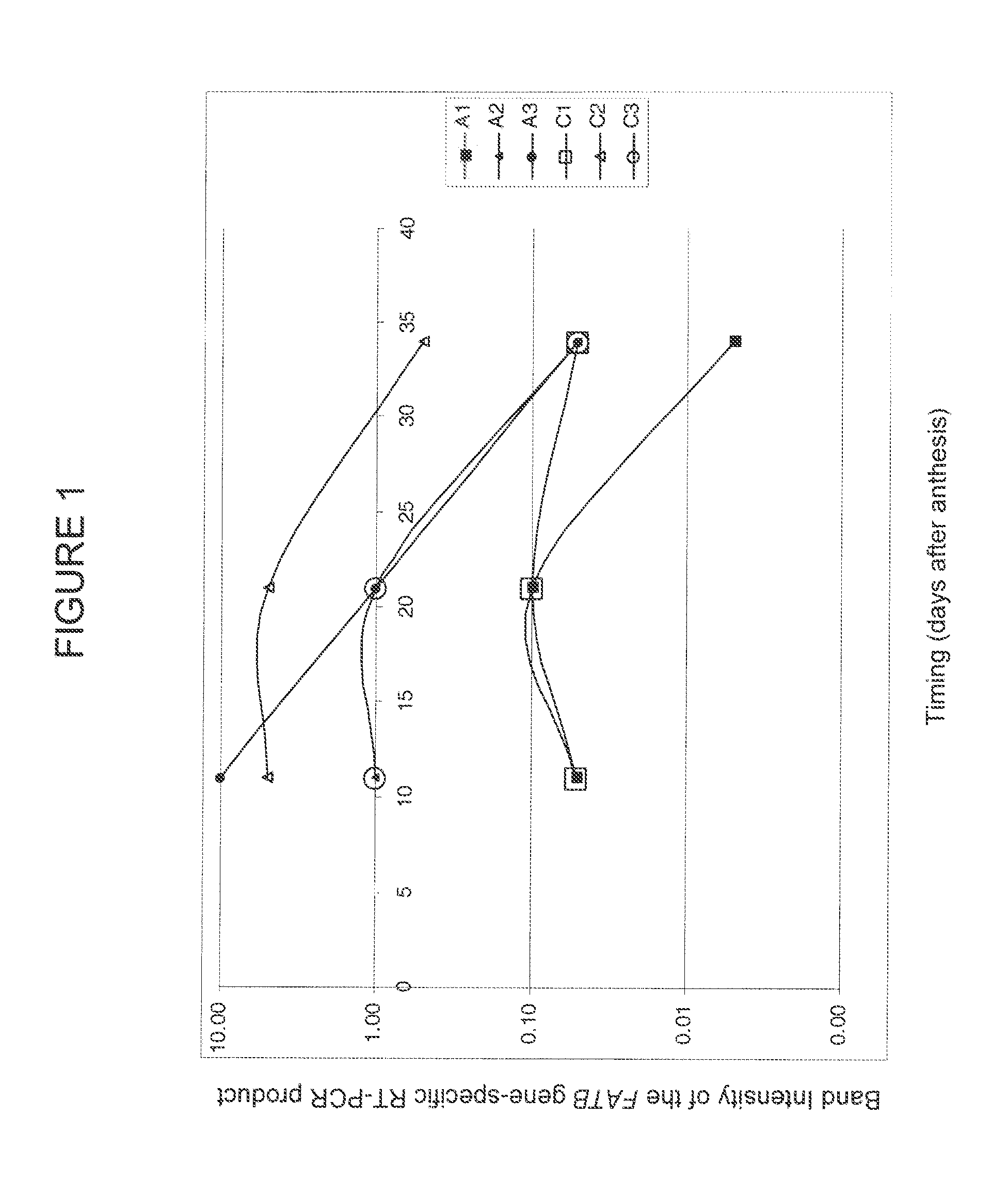
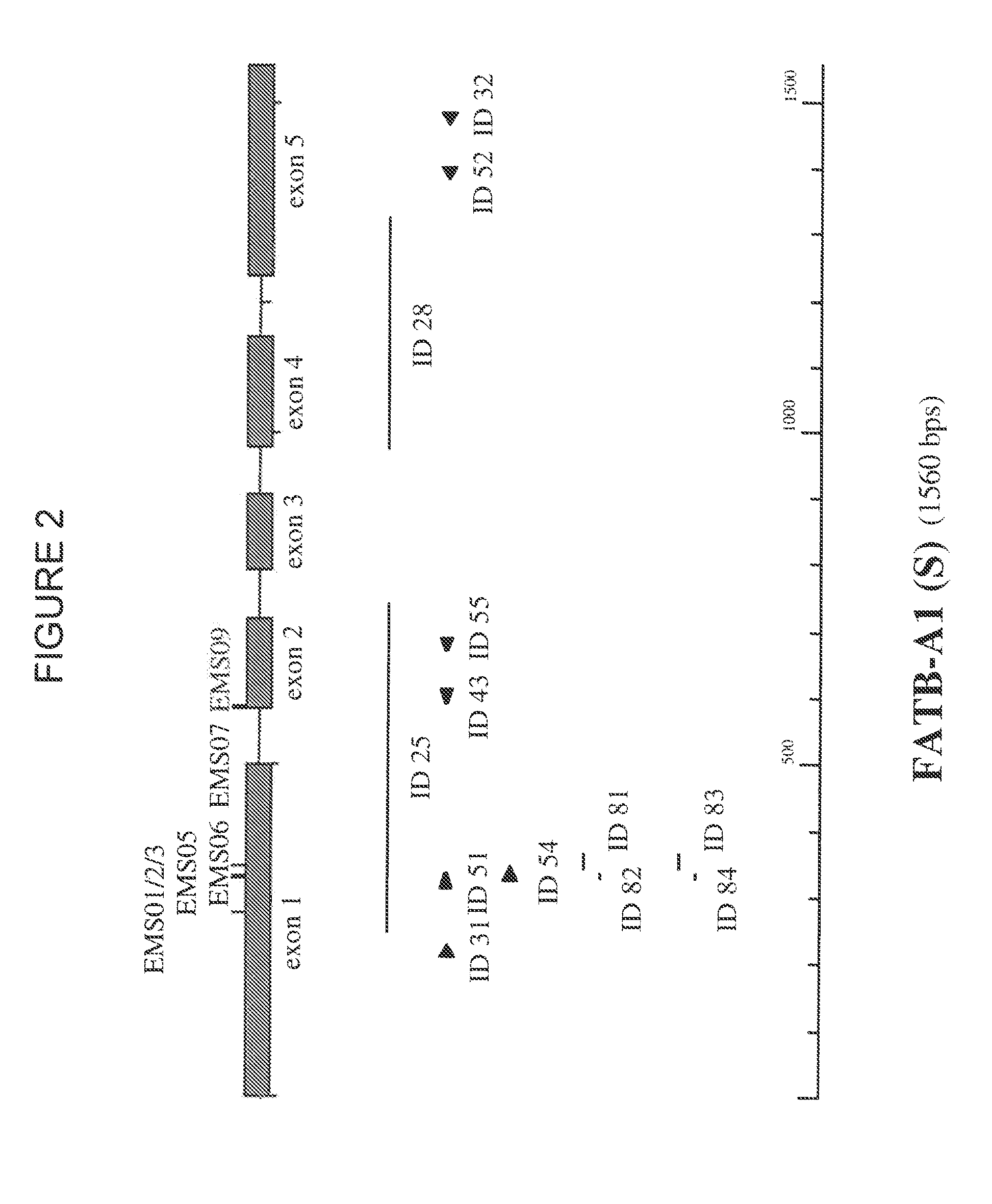
Brassica plant comprising mutant fatty acyl-acp thioesterase alleles
ActiveUS20110145944A1Reduce the amount requiredMinimal level of functional FATB proteinHydrolasesImmunoglobulinsAcyl carrier proteinWild type
The invention relates to crop plants comprising novel seed lipid compositions. Provided are both wild type and mutant nucleic acid molecules encoding Brassica fatty acyl-acyl carrier protein (ACP) thioesterase B proteins (FATB) and the proteins as such. Also provided are Brassica plants, tissue and seeds comprising at least three mutant fatB alleles in their genome, whereby the seed oil fatty acid composition or profile is significantly altered.
Owner:BAYER CROPSCIENCE NV
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Novel epsp synthase genes conferring herbicide resistance
Compositions and methods for conferring herbicide resistance or tolerance to bacteria, plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a polypeptide that confers resistance or tolerance to glyphosate herbicides are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated nucleic acid molecules corresponding to glyphosate resistant nucleic acid sequences are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NOS:2, 4, 6, 8, 10, 12, or 14 or the nucleotide sequence set forth in SEQ ID NOS:1, 3, 5, 7, 9, 11, 13, 28, 29, 30, 31, 32, 33, or 34.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
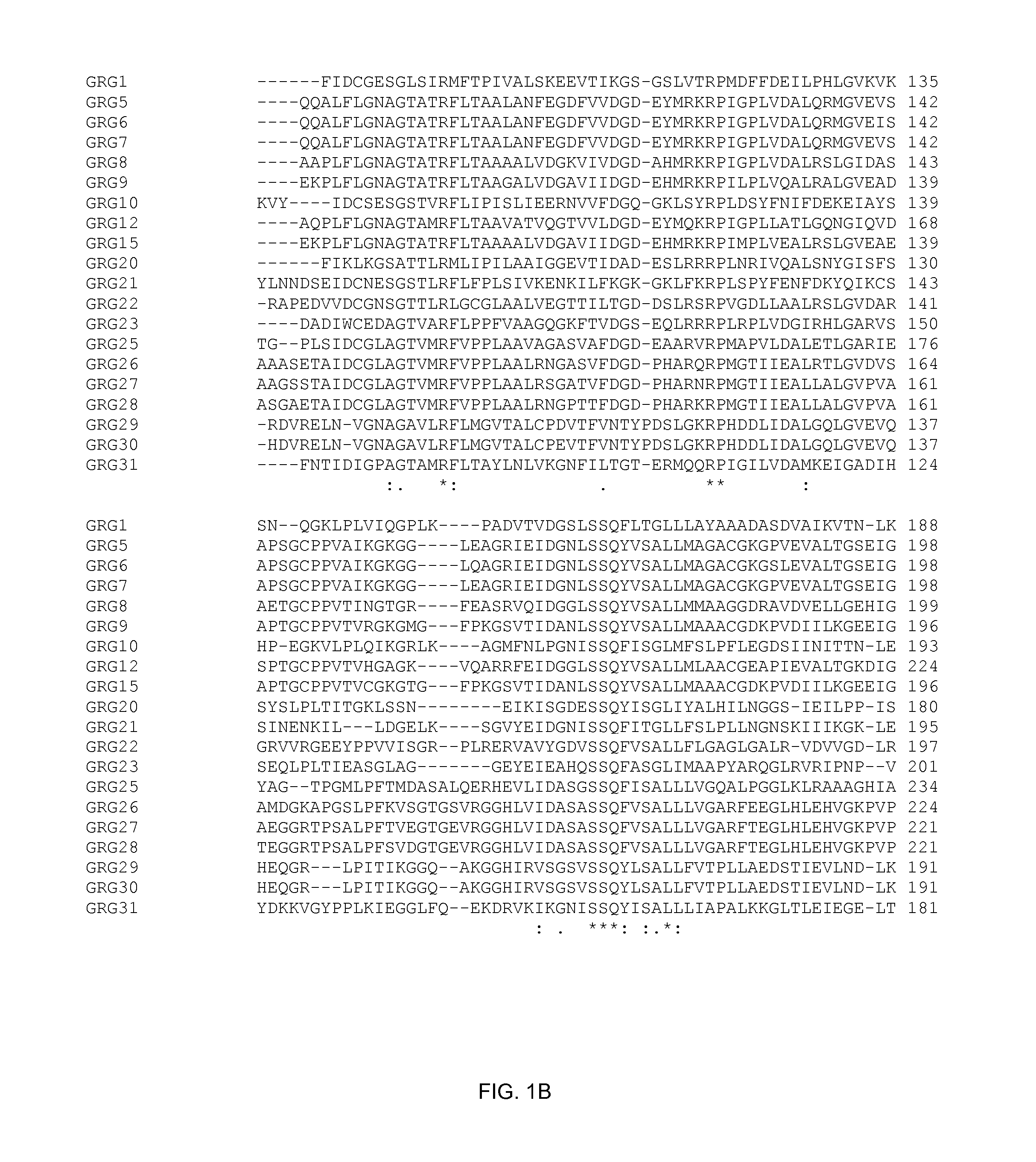
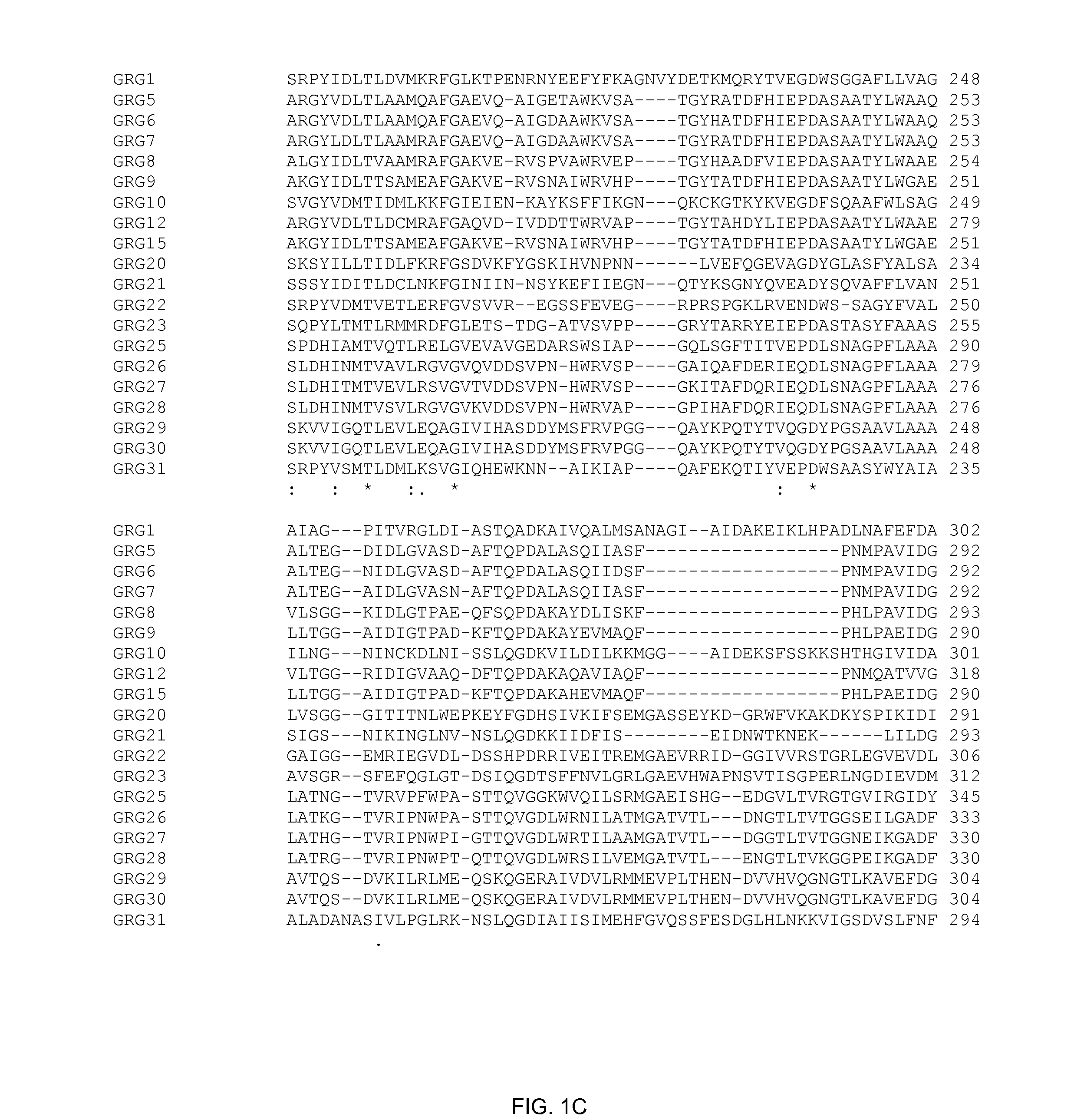
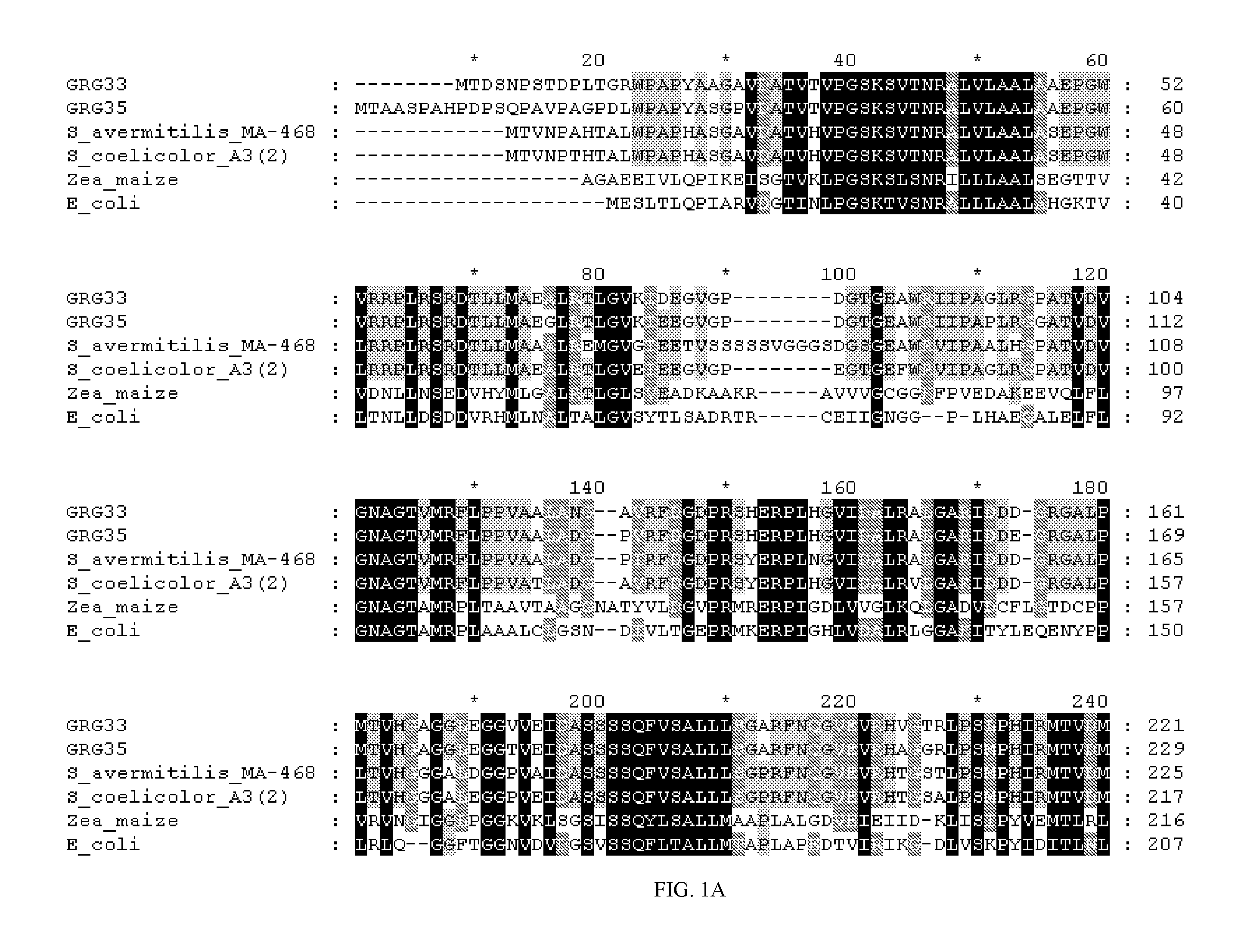
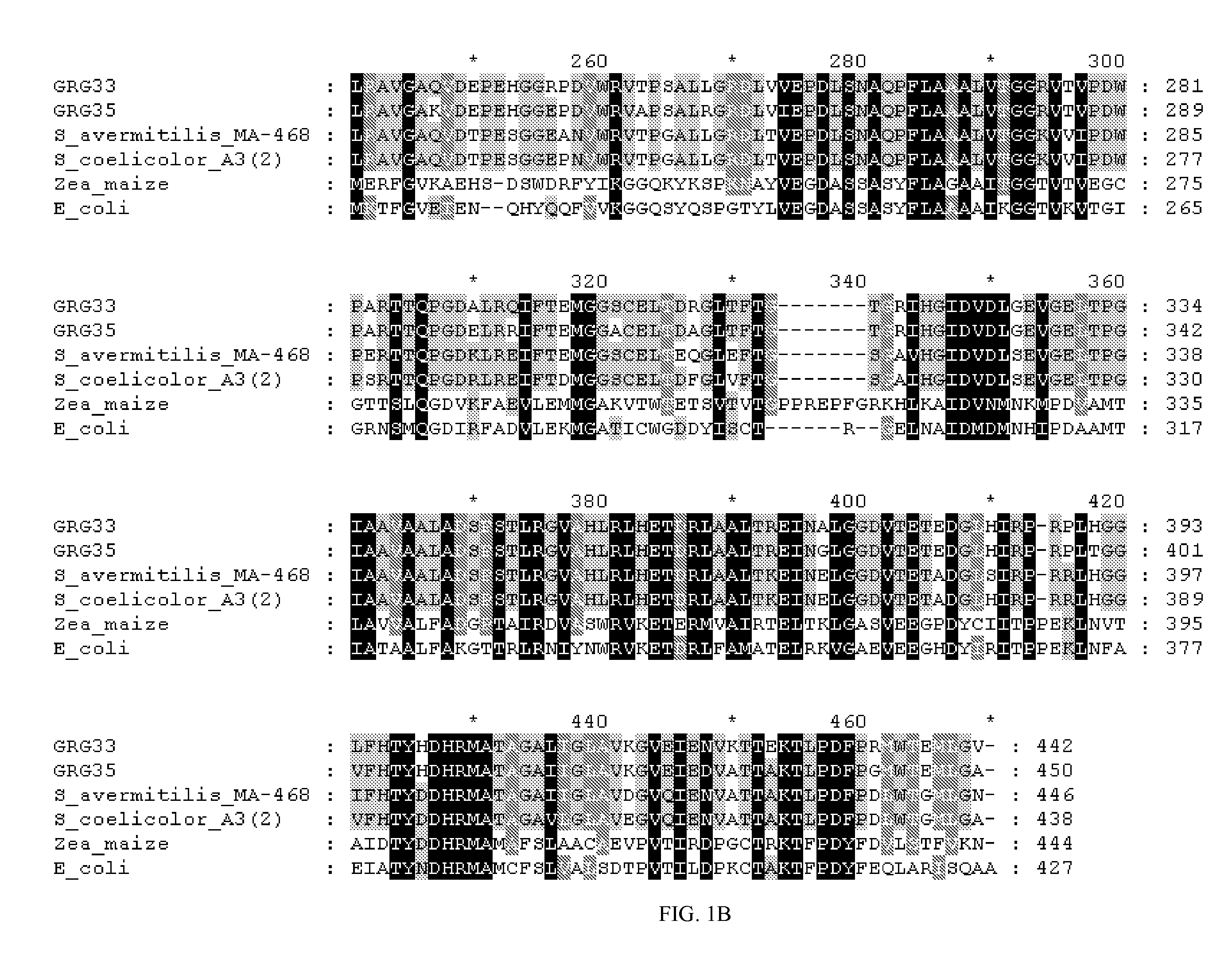
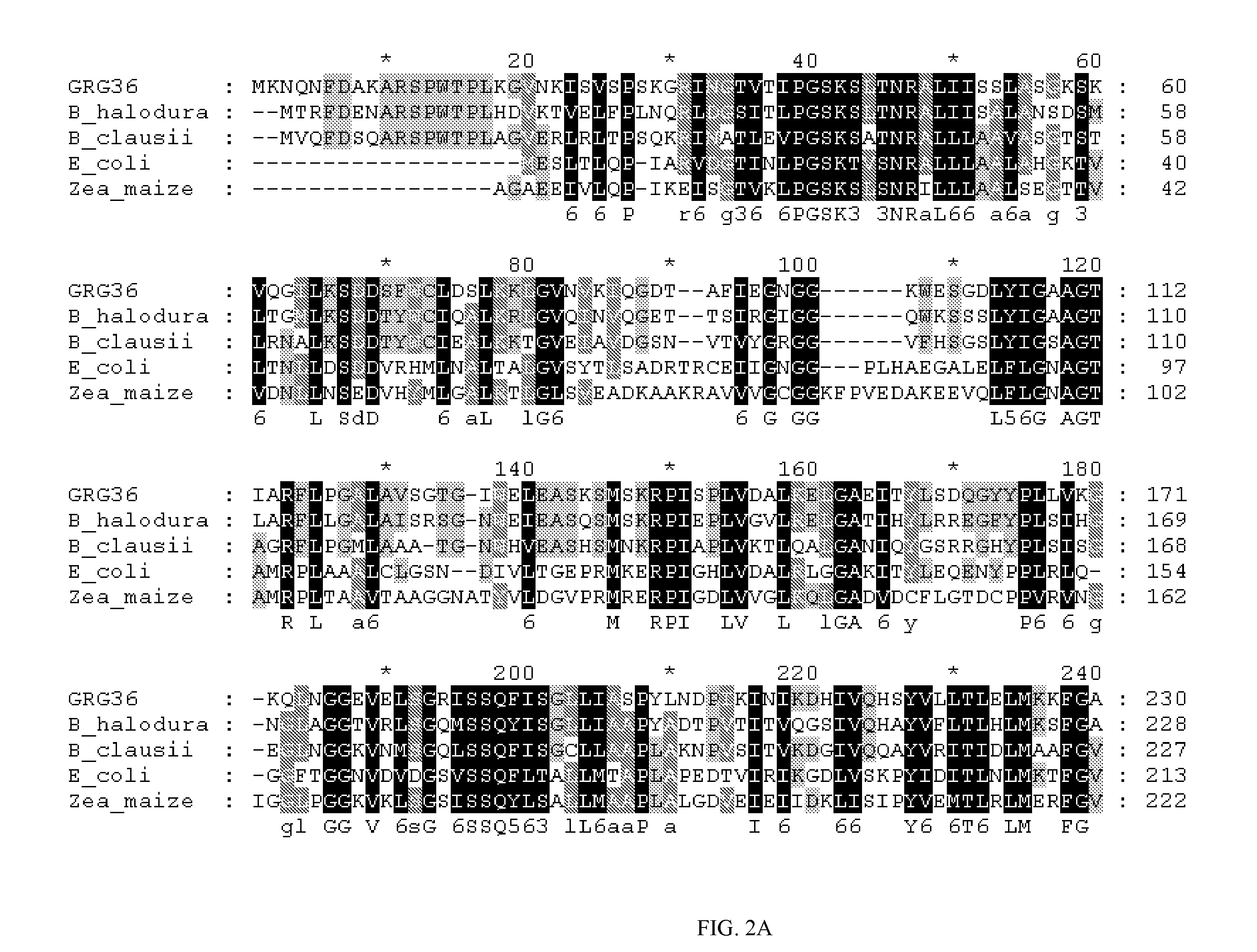
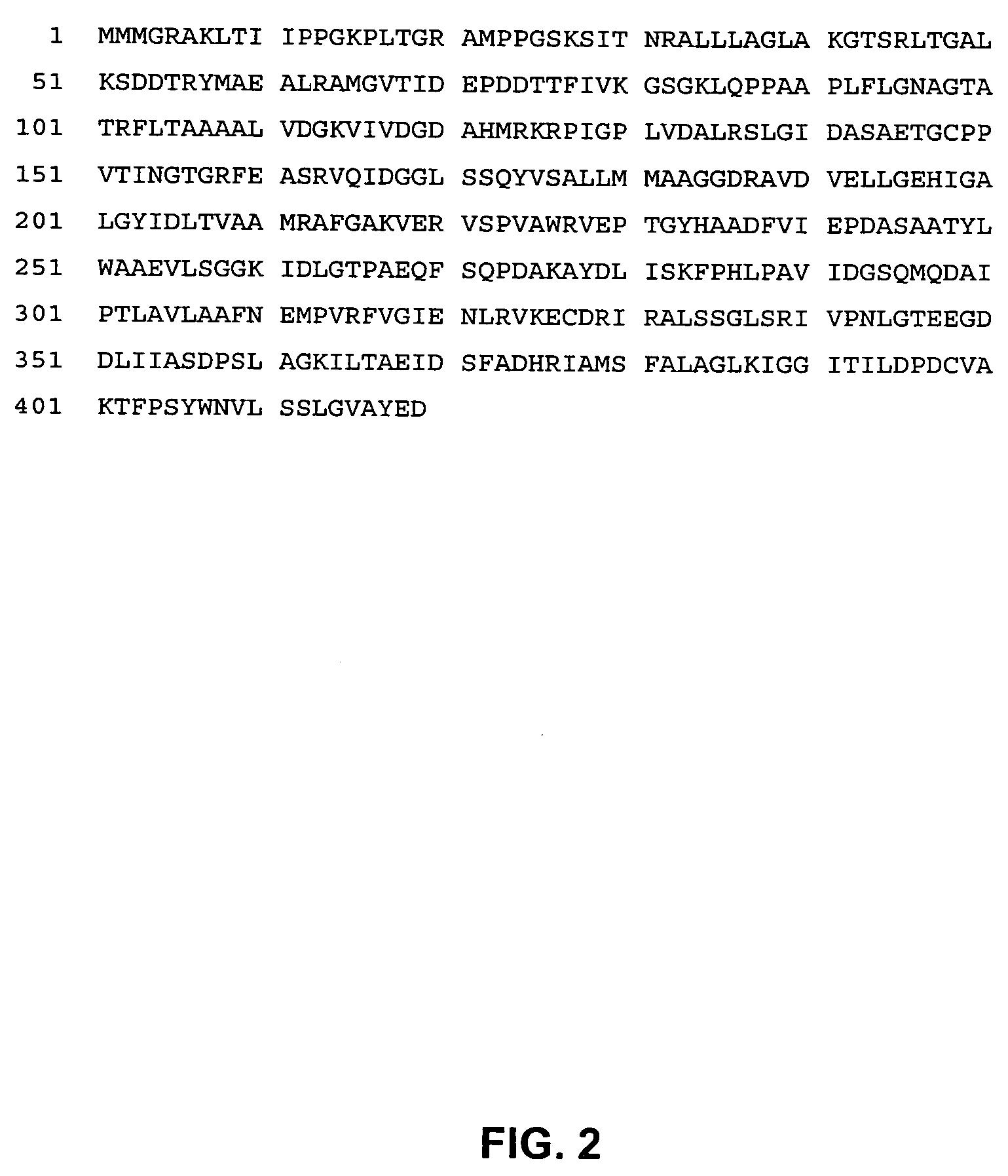
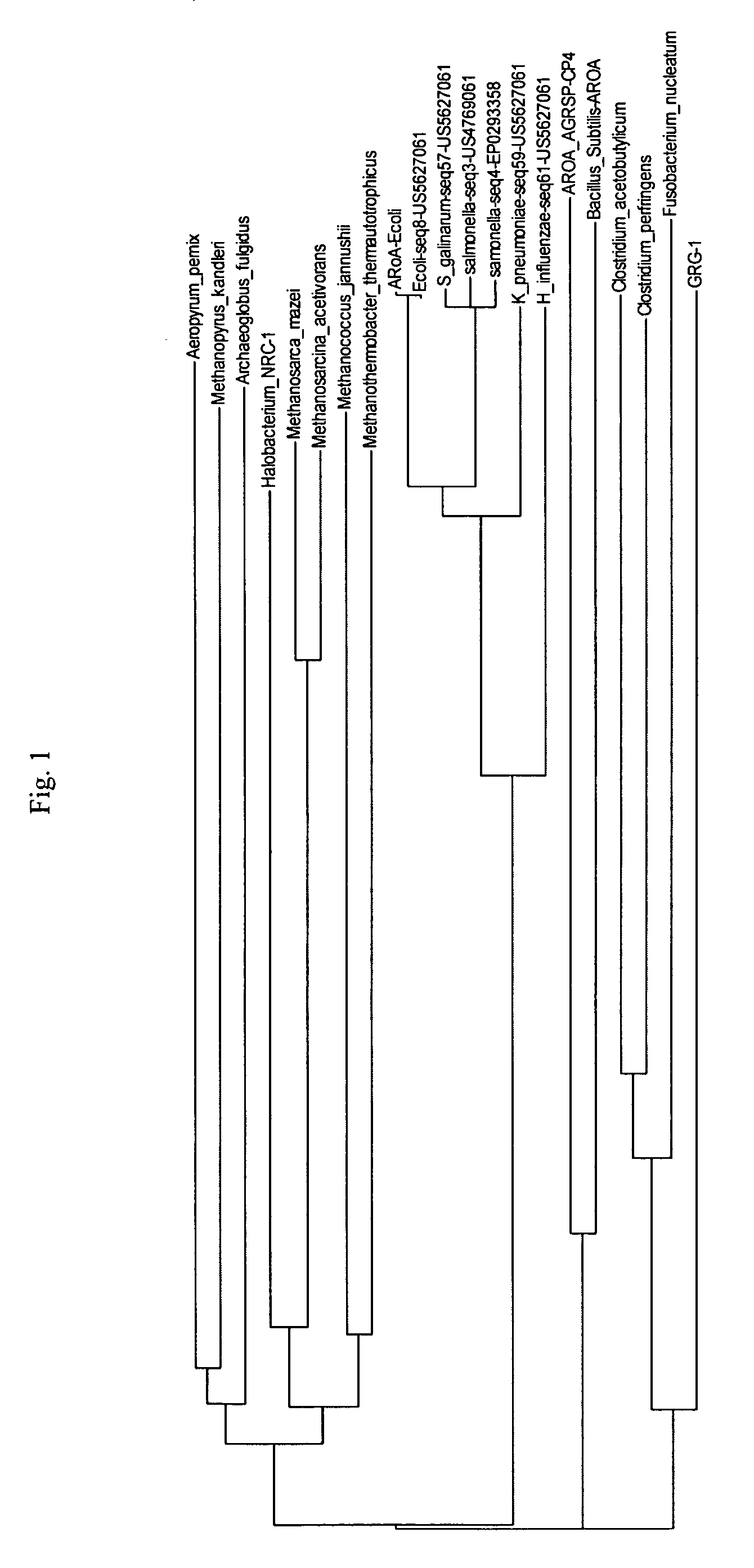
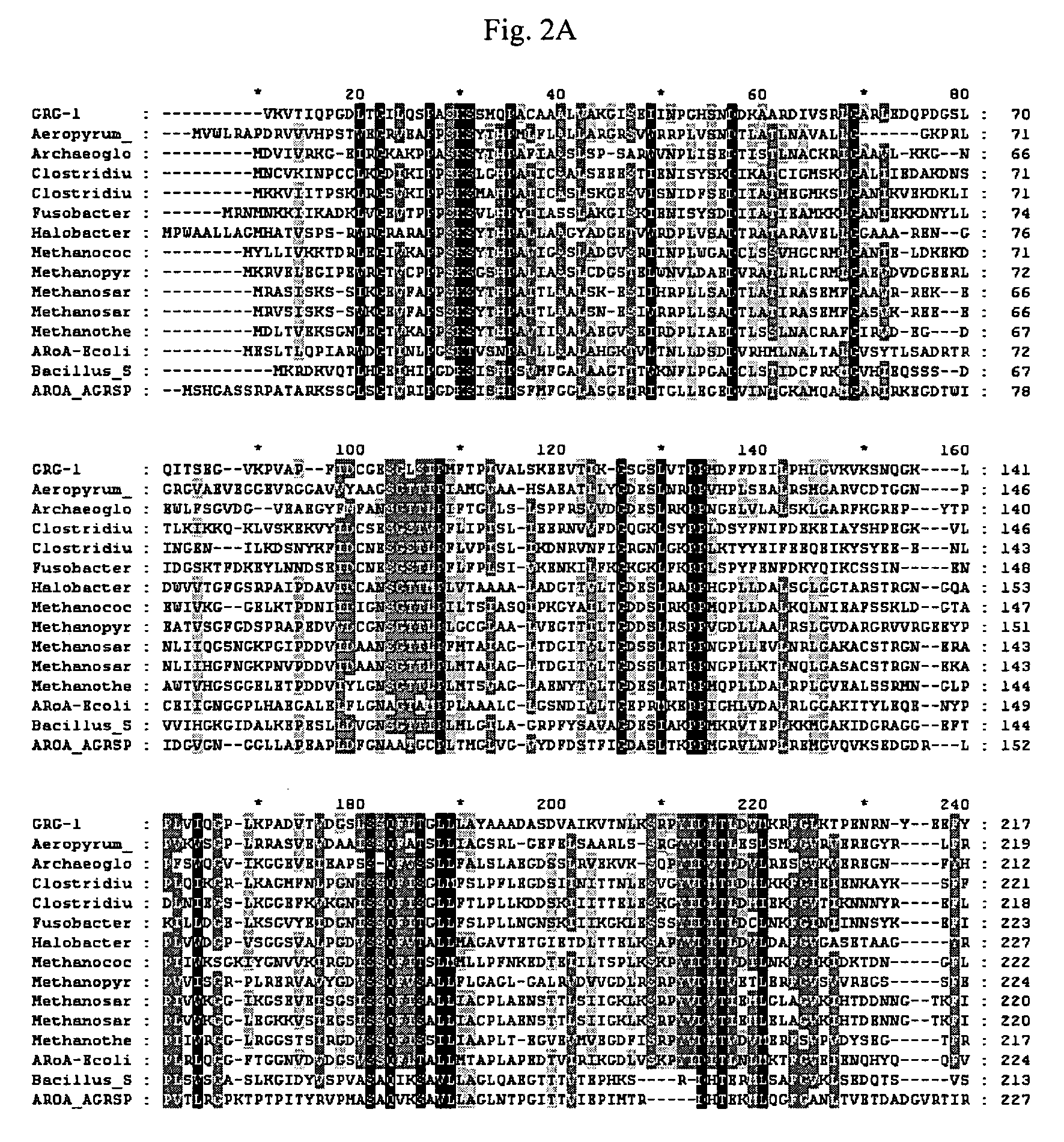
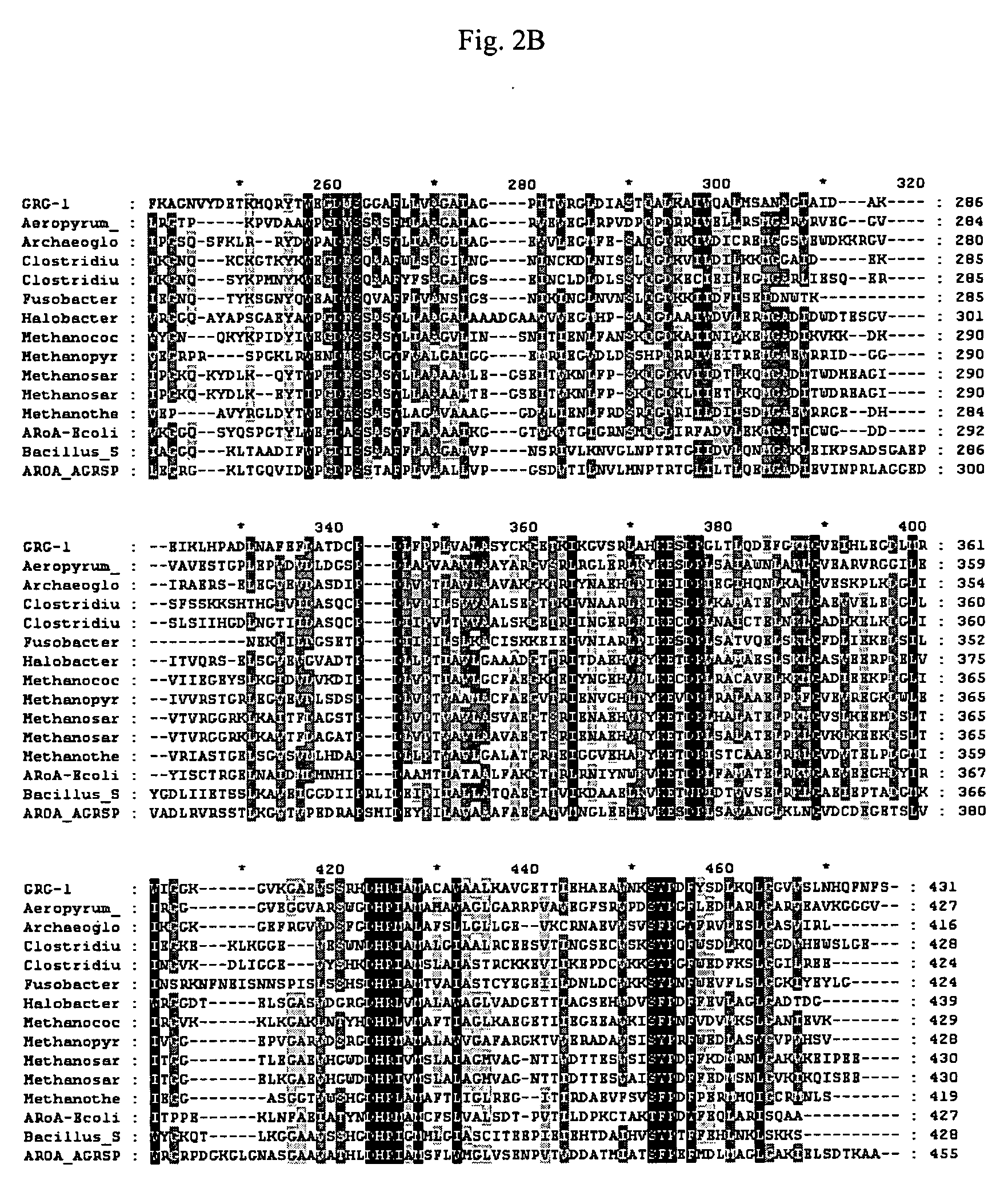
Grg33, grg35, grg36, grg37, grg38, grg39 and grg50: novel epsp synthase genes conferring herbicide resistance
Compositions and methods for conferring herbicide resistance to bacteria, plants, plant cells, tissues and seeds are provided. Compositions include nucleic acid molecules encoding herbicide resistance or tolerance polypeptides, vectors comprising those nucleic acid molecules, and host cells comprising the vectors. The nucleotide sequences of the invention can be used in DNA constructs or expression cassettes for transformation and expression in organisms, including microorganisms and plants. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, the present invention provides for isolated nucleic acid molecules comprising the nucleotide sequence set forth in SEQ ID NO:1, 3, 4, 6, 7, 9, 10, 12, 13, 15, 17, 18, 20, 21, or 23, a nucleotide sequence encoding the amino acid sequence shown in SEQ ID NO:2, 5, 8, 11, 14, 16, 19, or 22, the herbicide resistance nucleotide sequence deposited in a bacterial host as Accession Nos. NRRL B-30932, B-30933, B-30934, B-30945, B-30946, B-30947, or B-30948, as well as variants and fragments thereof.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
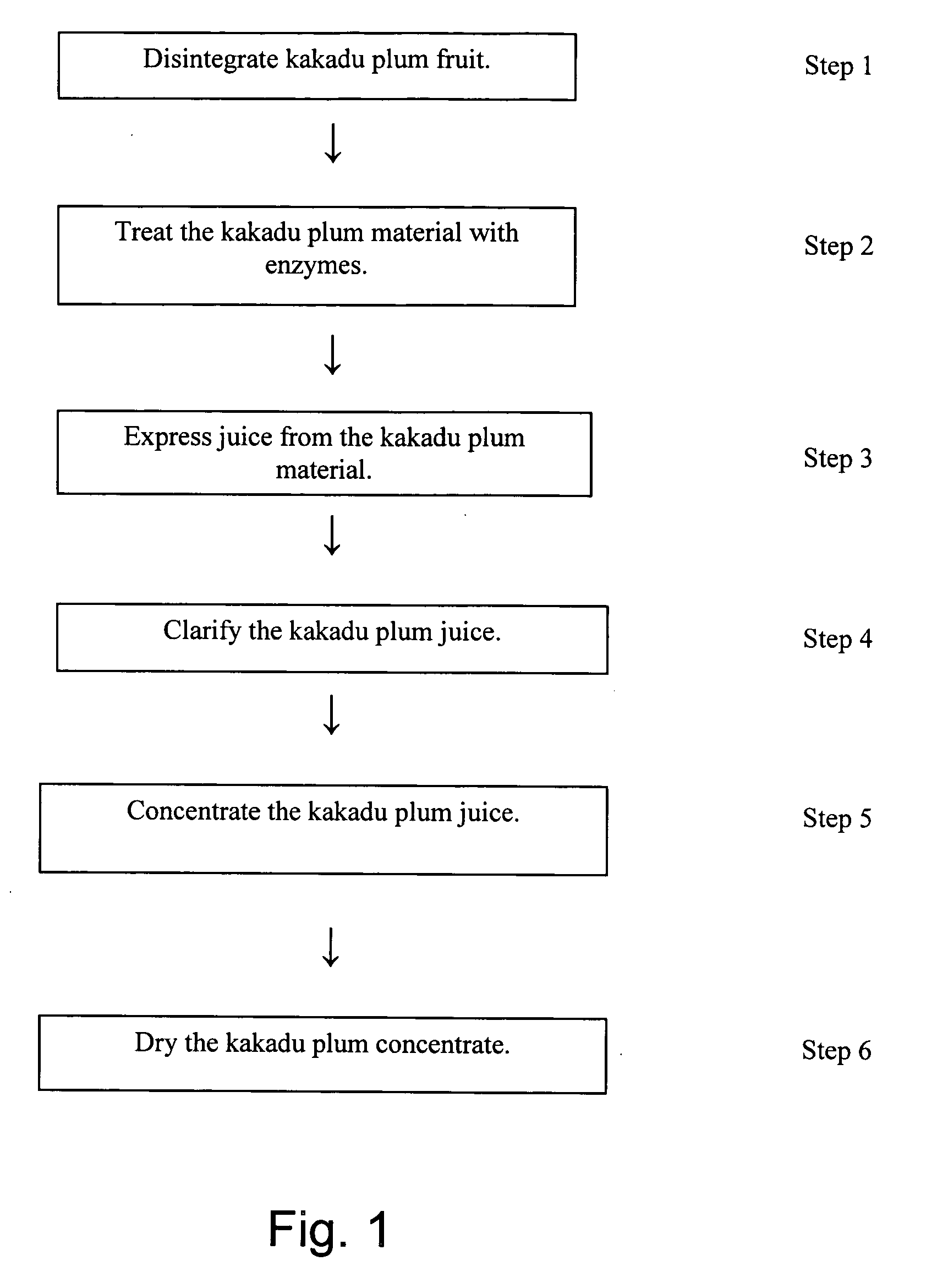
Method of preparing kakadu plum powder
ActiveUS20050163880A1High viscosityImpede efficient processingBiocideUnknown materialsAbsorption capacityUltrafiltration
A process for producing a kakadu plum powder having an increased amount of naturally occurring ascorbic acid and high ORAC value. The process of preparing the extract includes the following: disintegrating kakadu plum fruit; treating the disintegrated kakadu plum material with enzymes to at least partially digest the material; juicing the kakadu plum material and drying the juice to produce a powder. In a preferred embodiment, the kakadu plum juice is further clarified with ultrafiltration and concentrated by performing reverse osmosis on the kakadu plum juice. The resultant kakadu plum powder has a natural ascorbic acid content of at least about 15% and a naturally occurring Oxygen Reduction Absorption Capacity value of at least 1500.
Owner:ACCESS BUSINESS GRP INT LLC +1
Genes conferring herbicide resistance
Compositions and methods for conferring herbicide resistance to bacteria, plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a polypeptide that confers resistance or tolerance to glyphosate herbicides are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated nucleic acid molecules corresponding to glyphosate resistant nucleic acid sequences are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:3 or the nucleotide sequence set forth in SEQ ID NO:1 or 2.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
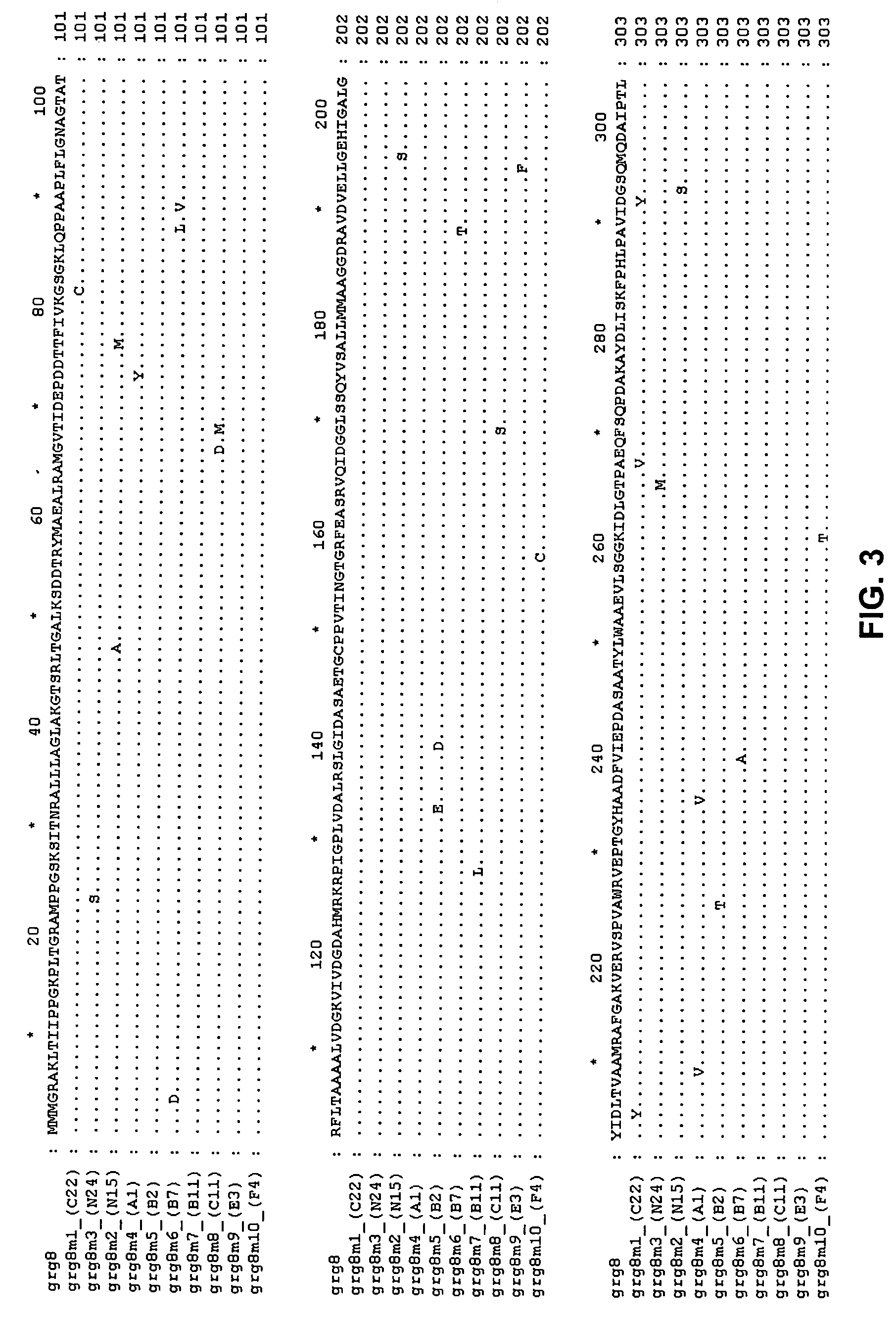
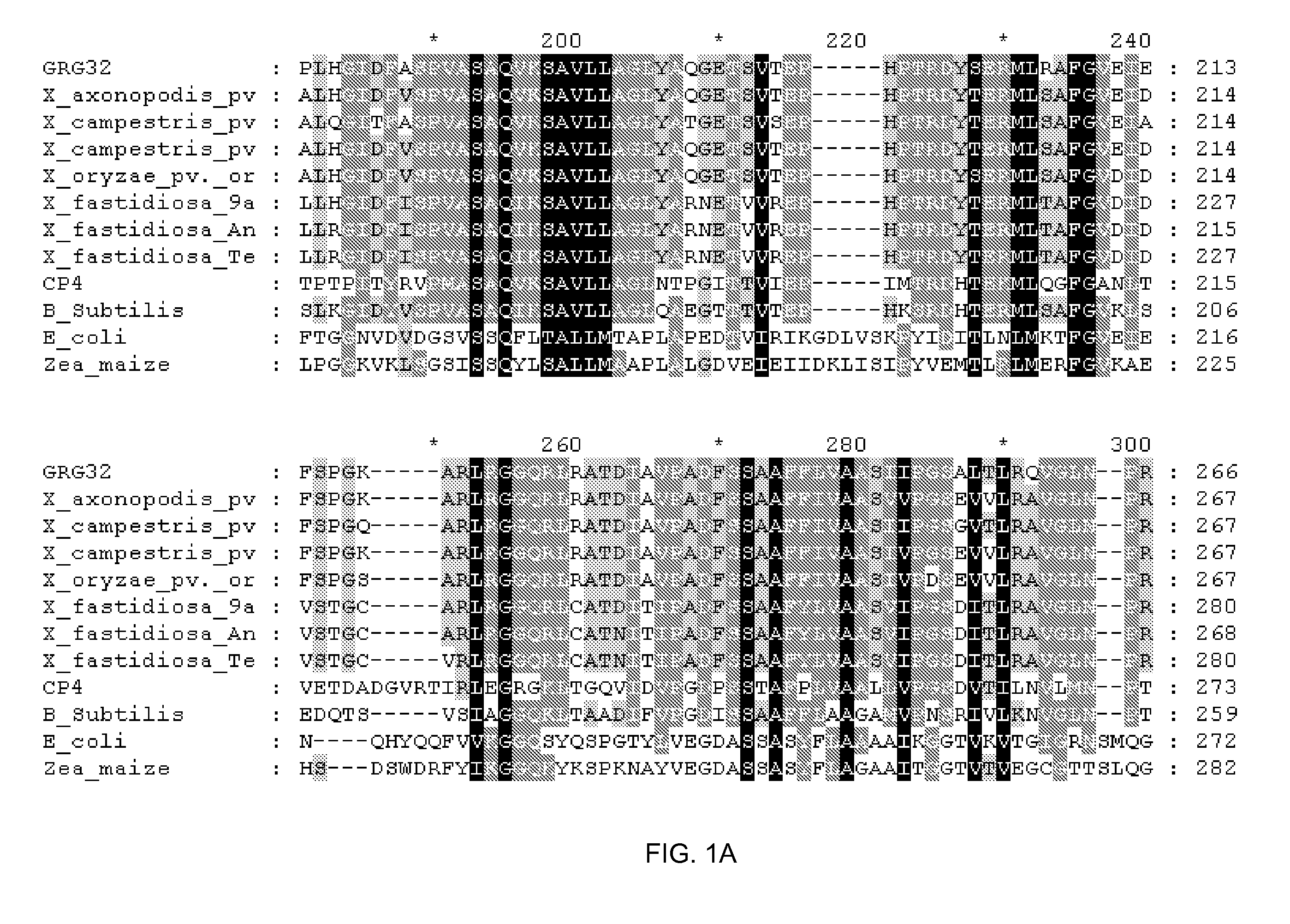
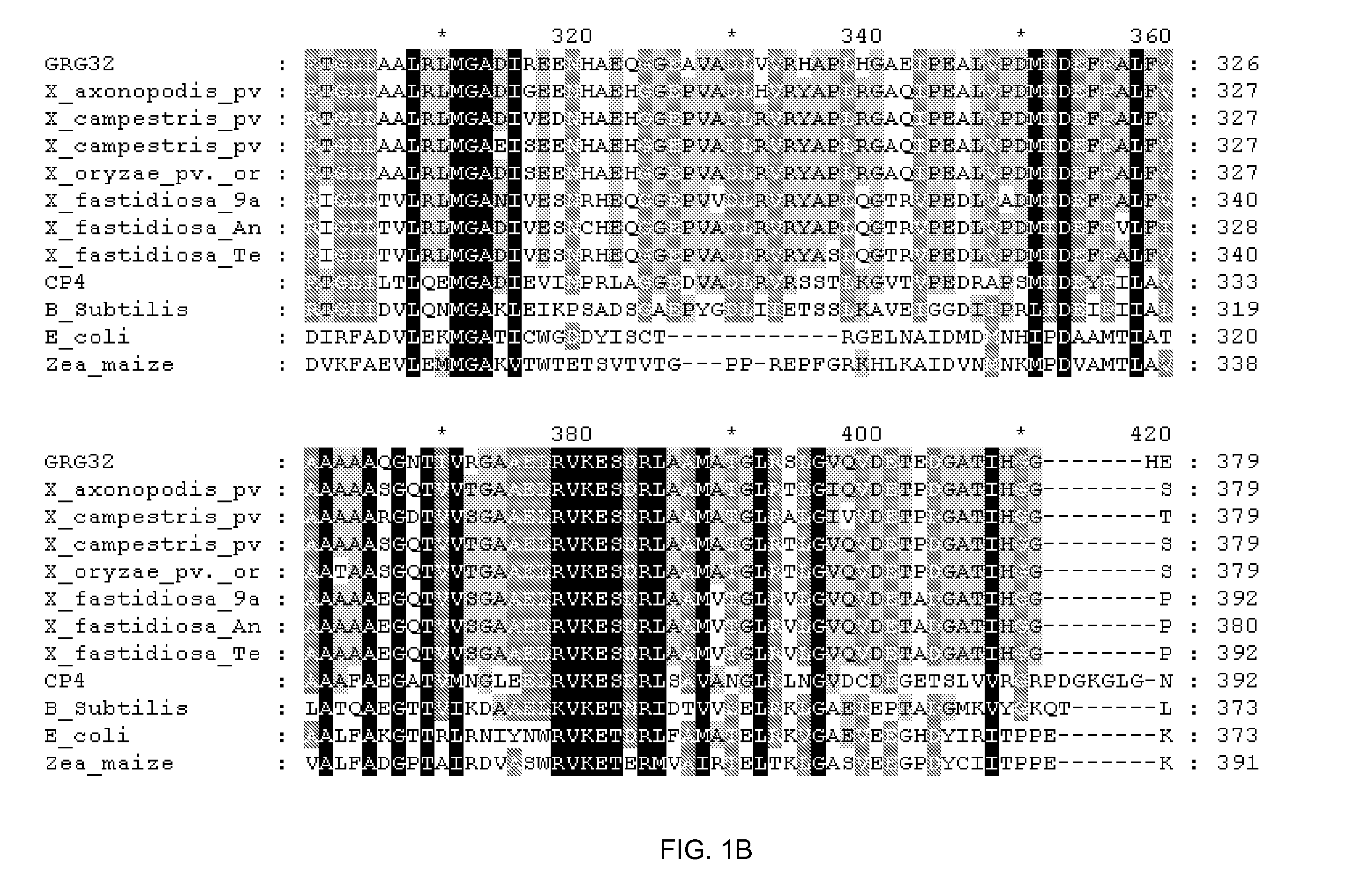
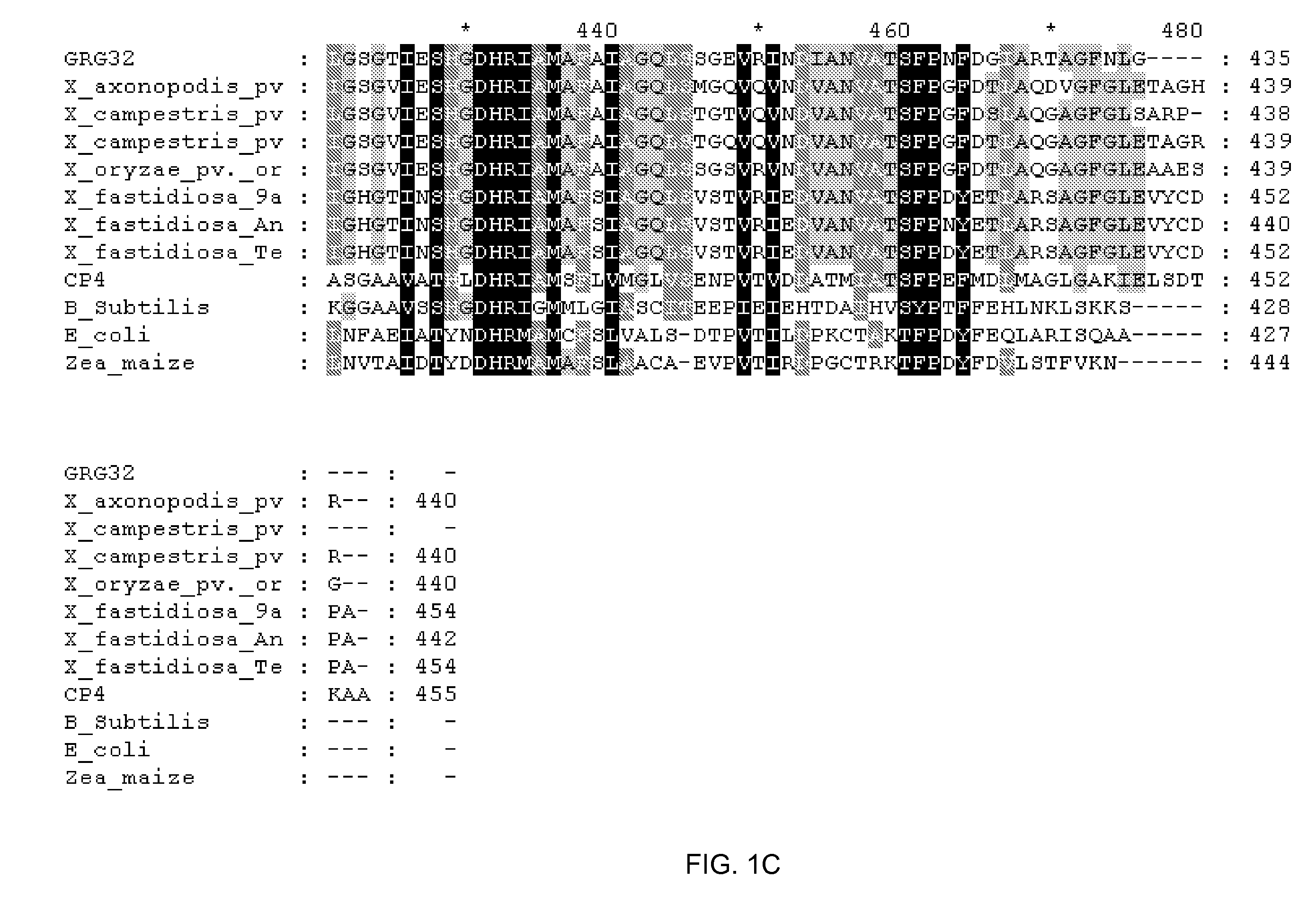
Grg32: a novel epsp synthase gene conferring herbicide resistance
Compositions and methods for conferring herbicide resistance to bacteria, plants, plant cells, tissues and seeds are provided. Compositions include nucleic acid molecules encoding herbicide resistance or tolerance polypeptides, vectors comprising those nucleic acid molecules, and host cells comprising the vectors. The nucleotide sequences of the invention can be used in DNA constructs or expression cassettes for transformation and in organisms, including microorganisms and plants. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, the present invention provides for isolated nucleic acid molecules comprising the nucleotide sequence set forth in SEQ ID NO:1 or 14, a nucleotide sequence encoding the amino acid sequence shown in SEQ ID NO:2, the herbicide resistance nucleotide sequence deposited in a bacterial host as Accession Nos. NRRL B-30931, as well as variants and fragments thereof.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
Topical formulations and methods of use
InactiveUS20060216251A1Treating and protectingLow elastic modulusBiocideCosmetic preparationsWrinkle skinDihydrolipoic acid
A topical composition comprising a lipoic acid, a carnitine, and a carnosine in a suitable vehicle for topical application and a method for treating skin is provided. The present compositions are useful in improving the appearance of aged skin characterized by wrinkles and loss of elasticity. Preferred components include R-lipoic acid or R-dihydrolipoic acid, acetyl-1-carnitine, and 1-carnosine.
Owner:TRACIE MARTYN INT
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Process for preparing materials for extraction
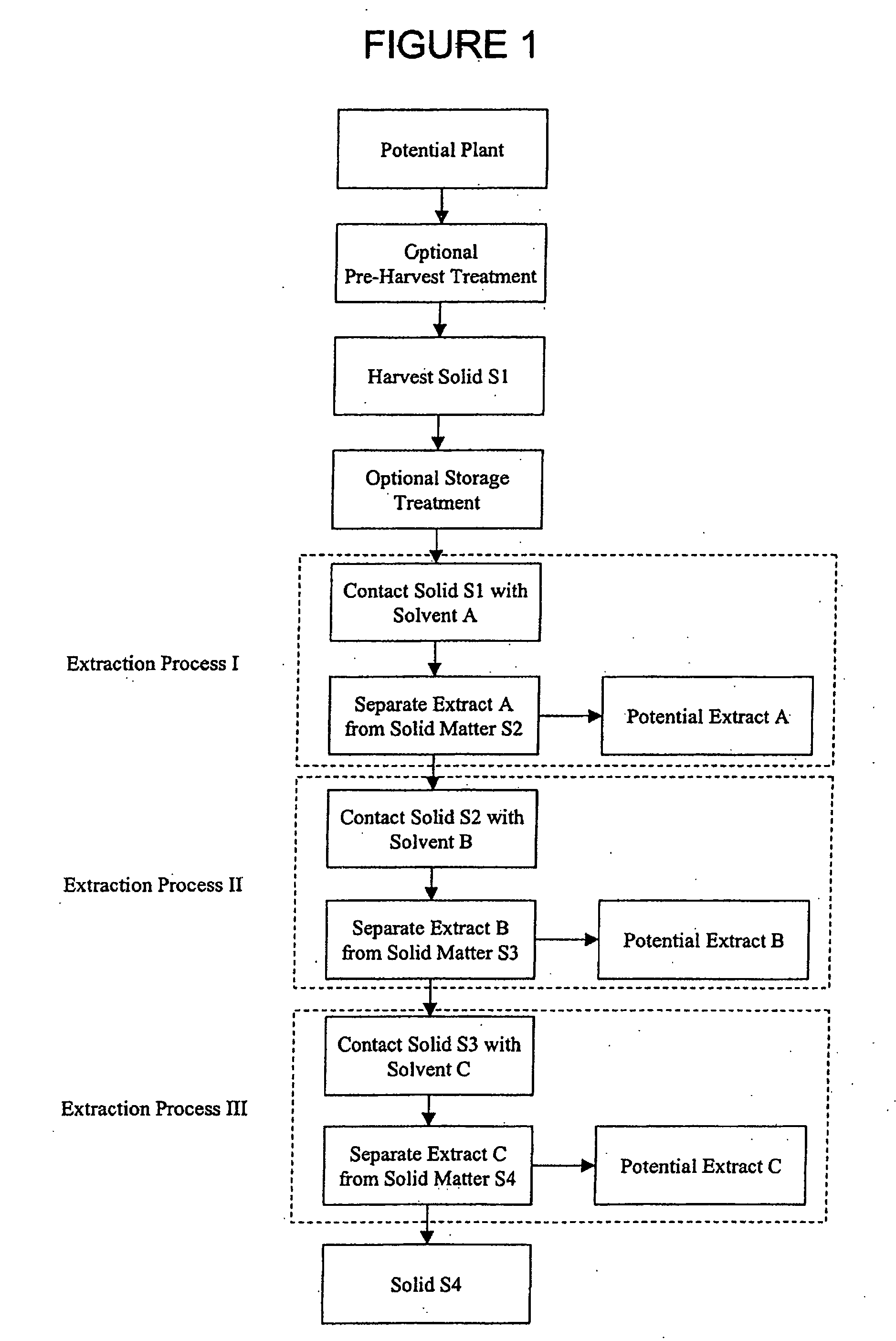
InactiveUS20060122410A1Improve extraction efficiencyQuality improvementFungiUnicellular algaeArachidonic acid supplementationFermentation
The present invention relates to a process for preparing a biomass, such as from a microbial fermentation, for an extraction process to separate desired chemicals, nutritional products, bioactive components, proteins, carbohydrates, and lipids, from the biomass. Particularly preferred substances to extract include docosahexaenoic acid, docosapentaenoic acid, and arachidonic acid. The present invention also includes extracting the prepared biomass. Biomasses to be treated in accordance with the methods of the invention include plant, animal, and microbial biomass, particularly a microorganism such as Crypthecodinium cohnii and a fungus such as Mortierella alpina.
Owner:MARTEK BIOSCIENCES CORP
Herbal and pharmaceutical drugs enhanced with probiotics
The curative action of drugs, including herbal remedies, allopathic remedies, and periodontal remedies, is enhanced and accelerated by administering such drugs in combination or association with probiotics, especially those of genus Lactococcus, Lactobacillus, Pediococcus, Streptococcus, Propionibacterium, Brevibacterium, Penicillium, and Saccharomyces.
Owner:REDDY MALIREDDY S
Plant extracts and dermatological uses thereof
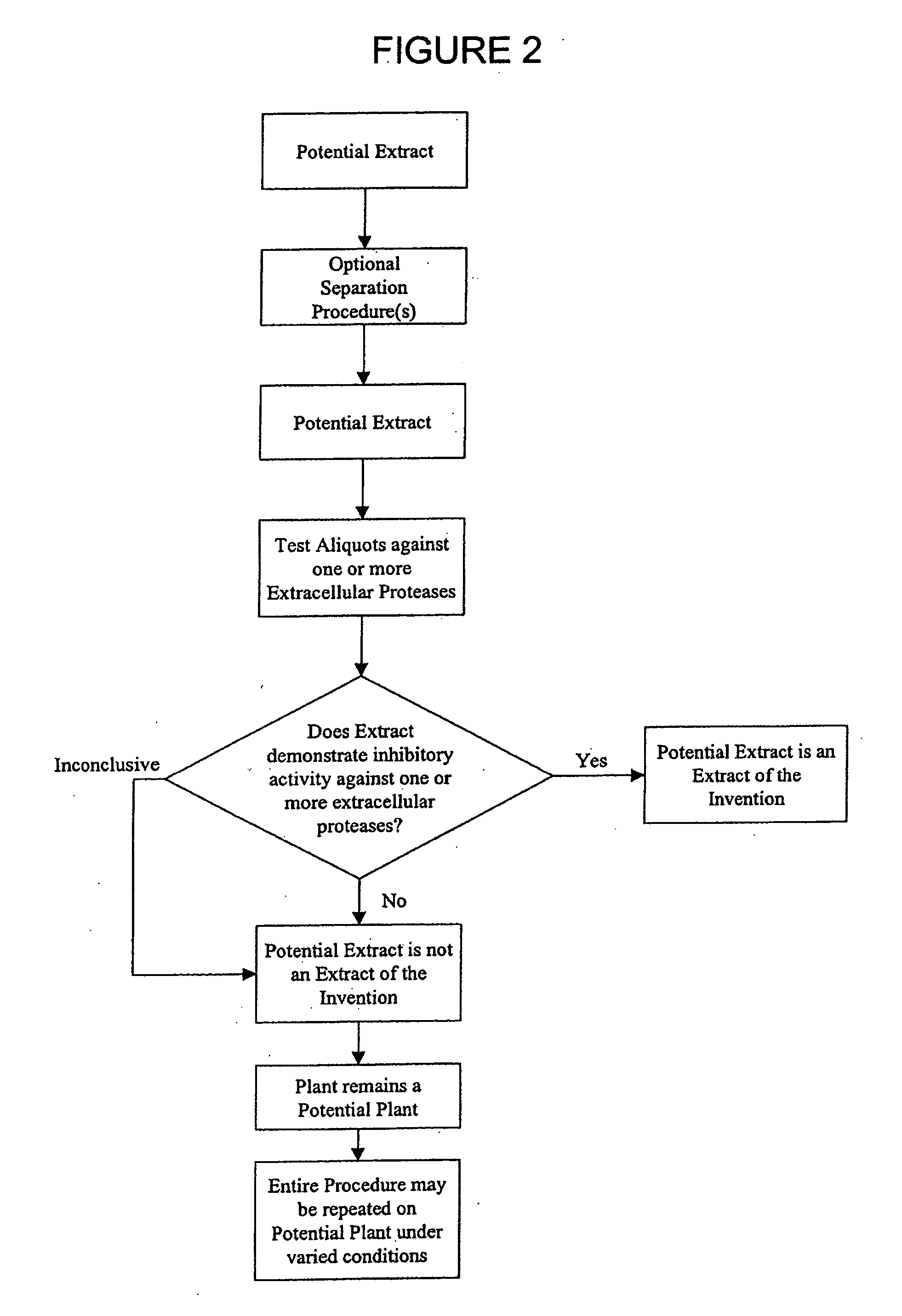
InactiveUS20070122492A1Good for healthGood lookingBiocideCompound screeningMatrix metalloproteasesSkin lines
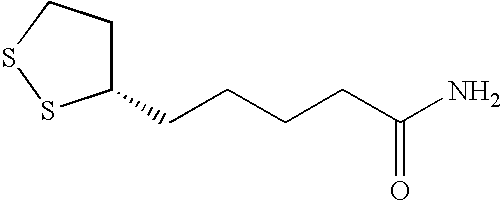
The present invention provides for plant extracts and dermatological formulations comprising one or more plant extracts that are capable of inhibiting one or more extracellular proteases selected from the group of: matrix metalloprotease-1 (MMP-1), matrix metalloprotease-2 (MMP-2), matrix metalloprotease-3 (MMP-3), matrix metalloprotease-9 (MMP-9) and human leukocyte elastase (HLE). The present invention further provides for a rapid method for screening plant extracts to identify those having the above activity that are suitable for incorporation into the dermatological formulations of the invention. The invention also provides for the use of the plant extracts as dermatological agents suitable for the treatment or prevention of various dermatological conditions, including wrinkling or sagging of the skin, irradiation induced skin and / or hair damage, deepening of skin lines, elastotic changes in the skin, as well as for the routine care of the skin, hair and / or nails.
Owner:BIOPHARMACOPAE DESIGN INT
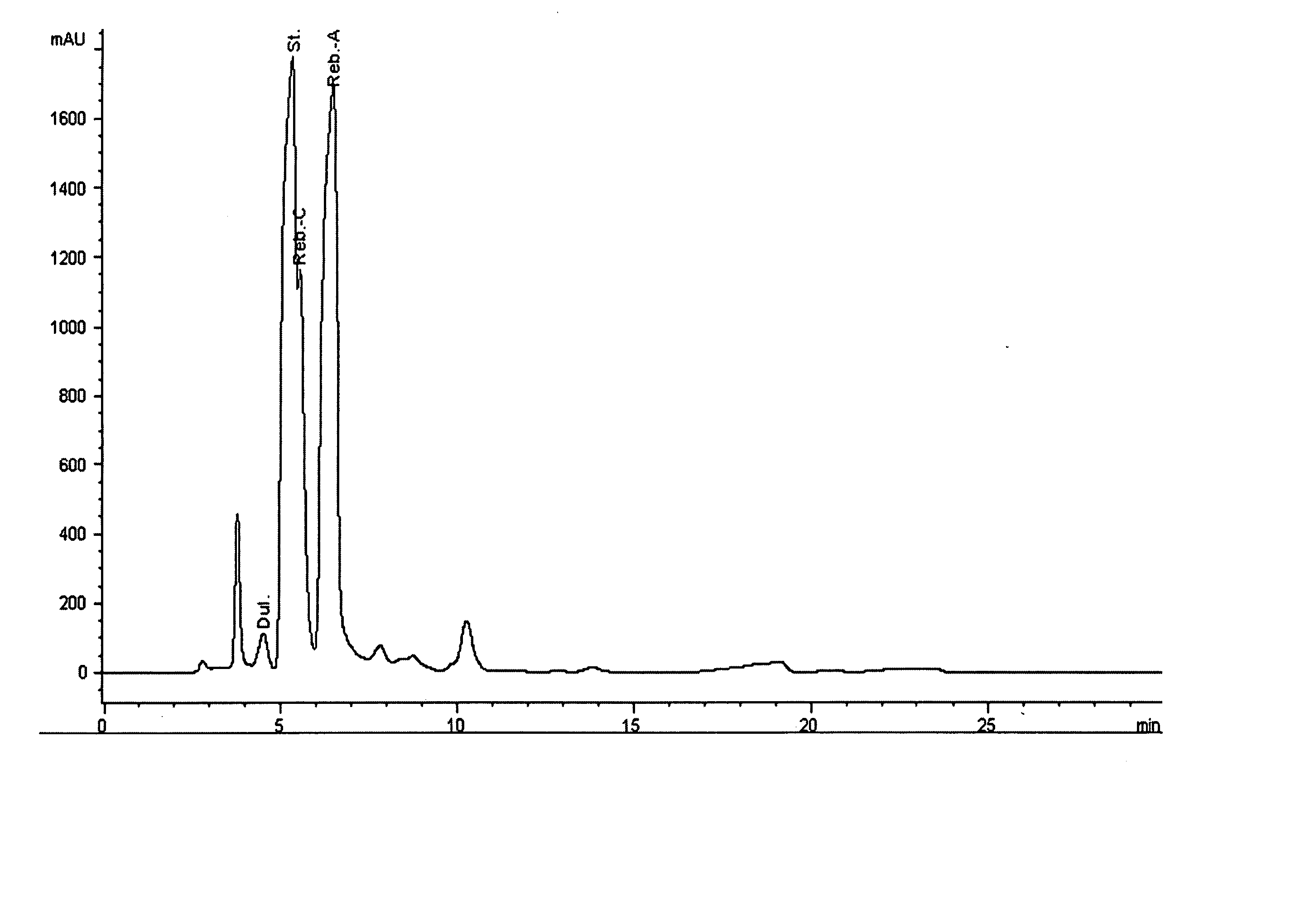
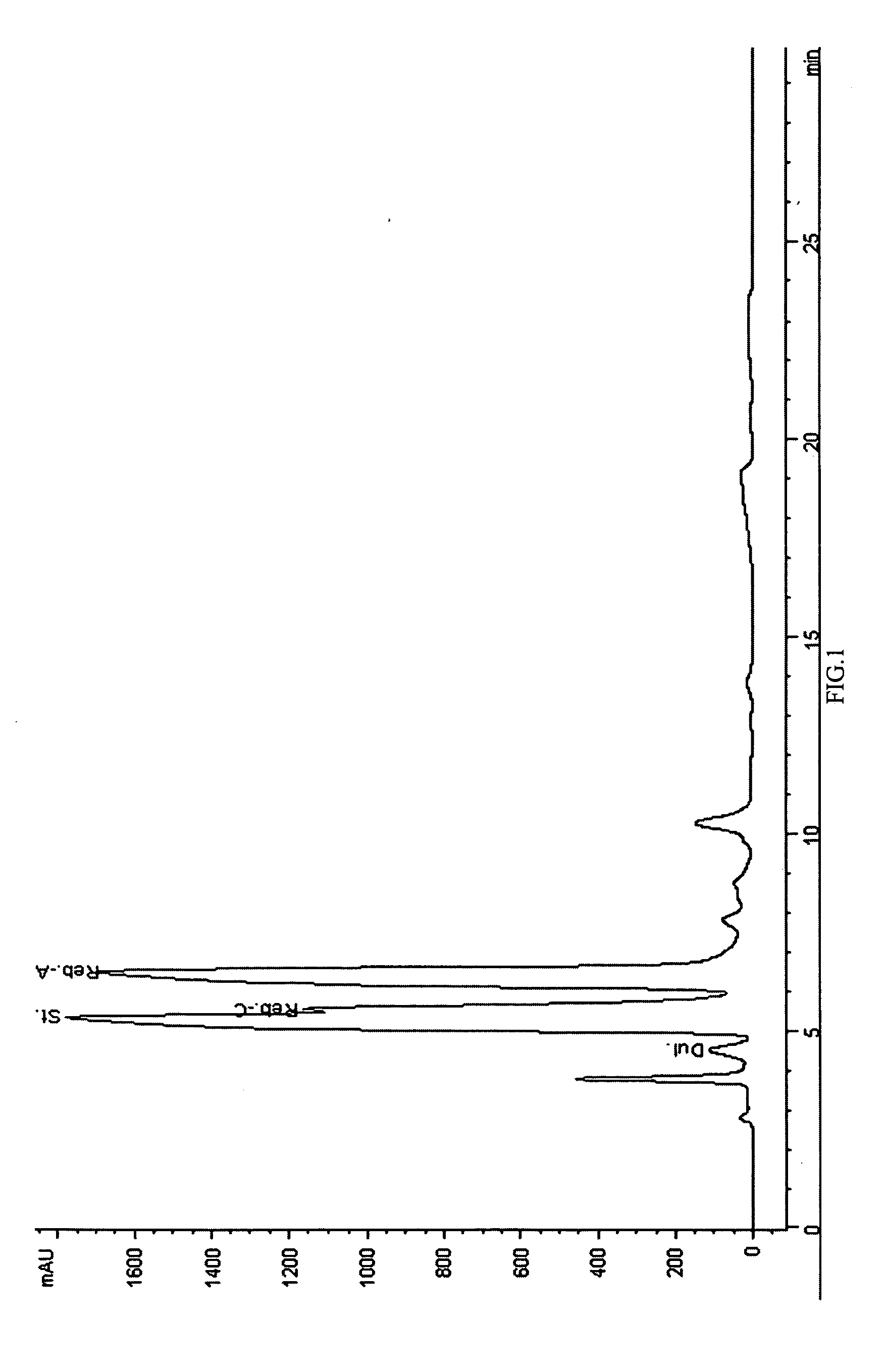
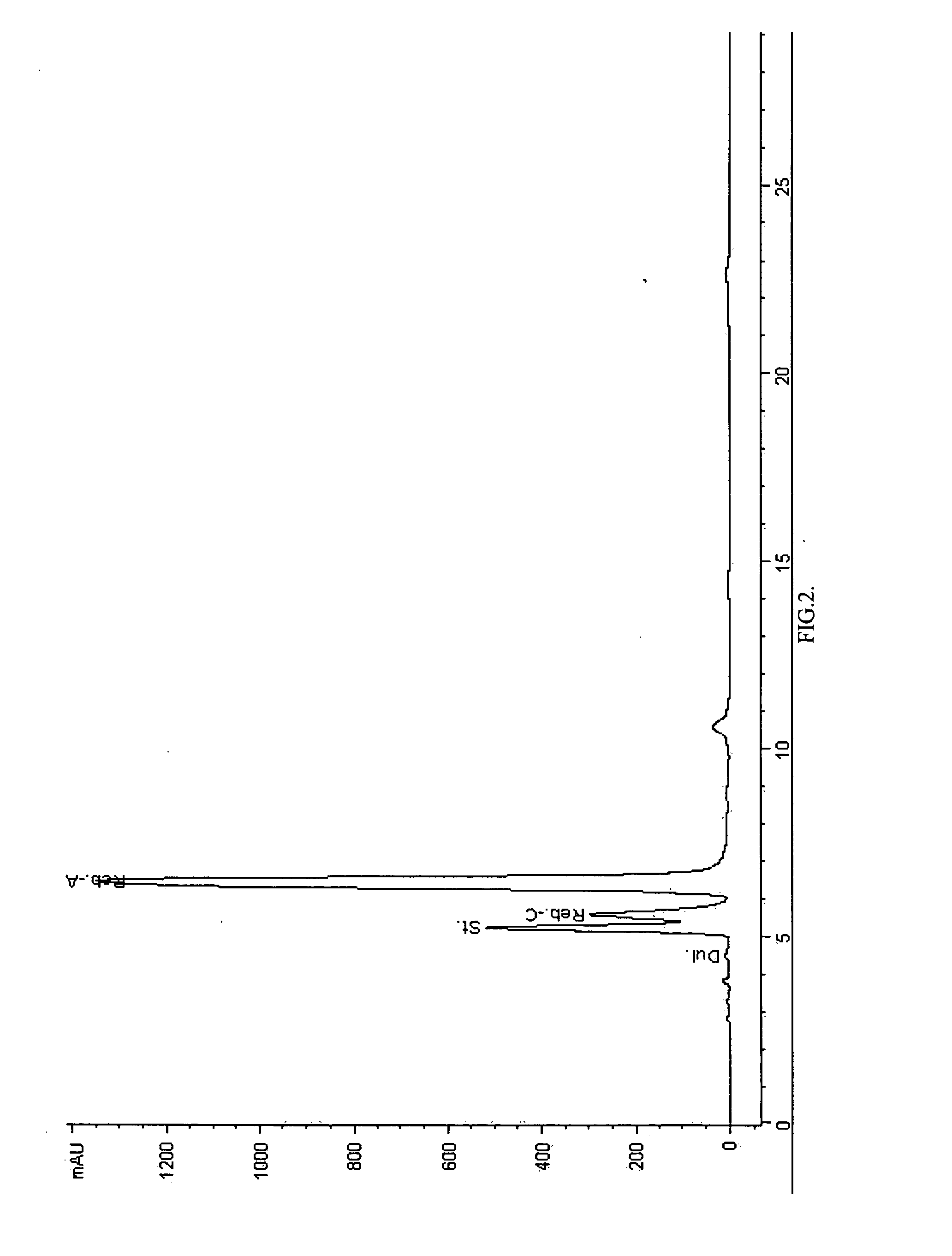
Extraction, separation and modification of sweet glycosides from the stevia rebaudiana plant
ActiveUS20060134292A1Increasing of degree of transglycosylationDelayed reaction timeBiocideAnimal repellantsPectinaseSodium Bentonite
The invention disclosed relates to a method for the extraction of sweet glycosides from the Stevia rebaudiana Bertoni plant and recovery of individual rebaudioside A and stevioside. The extraction is developed in the presence of pectinase, and the extract is purified using cyclodextrin and bentonite. High purity rebaudioside A is obtained by crystallization and recrystallization from ethanol. High purity stevioside is prepared from the filtrate by purification with cyclodextrin, bentonit, and ion exchange resins. The enzymatic modification of the rebaudioside A, stevioside and the purified extract is carried out using the transferring enzymes derived from Thermoactinomyces vilgaris and Bacillus halophilus.
Owner:PURECIRCLE SDN BHD
Anti-infection augmentation foamable compositions and kit and uses thereof
Anti-infective foamable composition and kits include a foamable carrier; a therapeutically safe and effective concentration of an anti-infective agent; an augmenting agent selected from the group consisting of a keratolytic agent and a skin penetration enhancer; and a propellant. The composition is housed in a container and upon release is expandable to form a breakable foam. The foamable carrier is selected to generate a foam of good or excellent quality in the presence of the augmenting agent and anti-infective agent. Methods for treating, alleviating or preventing a disorder of the skin, a body cavity or mucosal surface, wherein the disorder involves a fungal, bacterial or viral infection as one of its etiological factors, is described.
Owner:FOAMIX PHARMACEUTICALS LIMITED
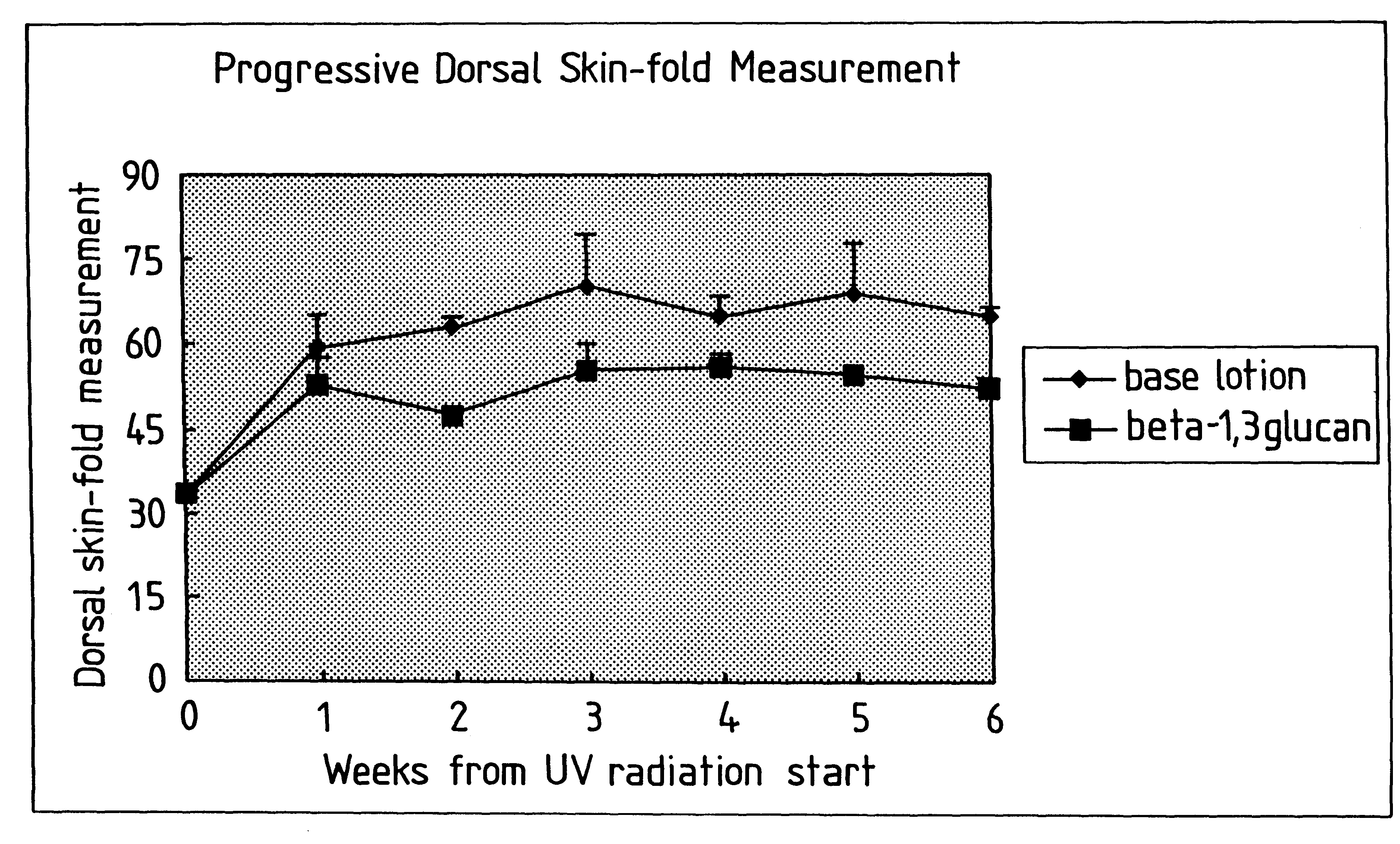
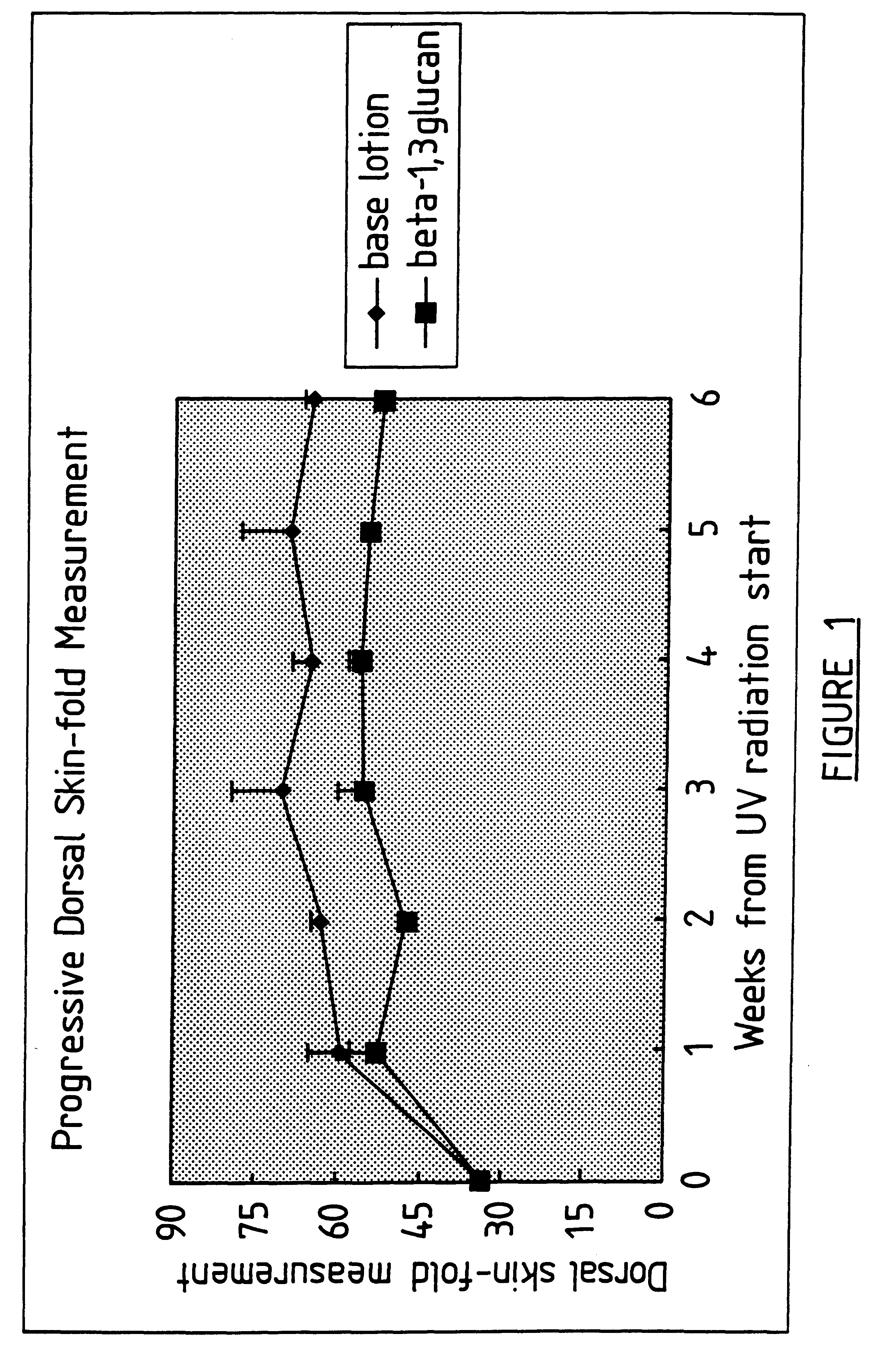
Process for glucan preparation and therapeutic uses of glucan
InactiveUS6242594B1Low costSuitable solubility characteristicAntibacterial agentsOrganic active ingredientsOrganic solventMicroparticle
A process for the production of beta-3-(1,3)(1,6) glucan from a glucan containing cellular source is described, together with compositions and uses / methods of treatment involving glucan. The process of the invention comprises the steps of: (a) extracting glucan containing cells with alkali and heat, in order to remove alkali soluble components; (b) acid extracting the cells of step (a) with an acid and heat to form a suspension; (c) extracting the suspension obtained of step (b) or recovered hydrolyzed cells with an organic solvent which is non-miscible with water and which has a density greater than that of water separating the resultant aqueous phase, solvent containing phase and interface so that substantially only the aqueous phase comprising beta-(1,3)(1,6) glucan particulate material remains; wherein the extraction with said organic solvent provides separation of glucan subgroups comprising branched beta-(1,3)(1,6)-glucan, and essentially unbranched beta-(1,3) glucan which is associated with residual non-glucan contaminents; and (d) drying the glucan material from step (c) to give microparticulate glucan.
Owner:TR THERAPEUTICS
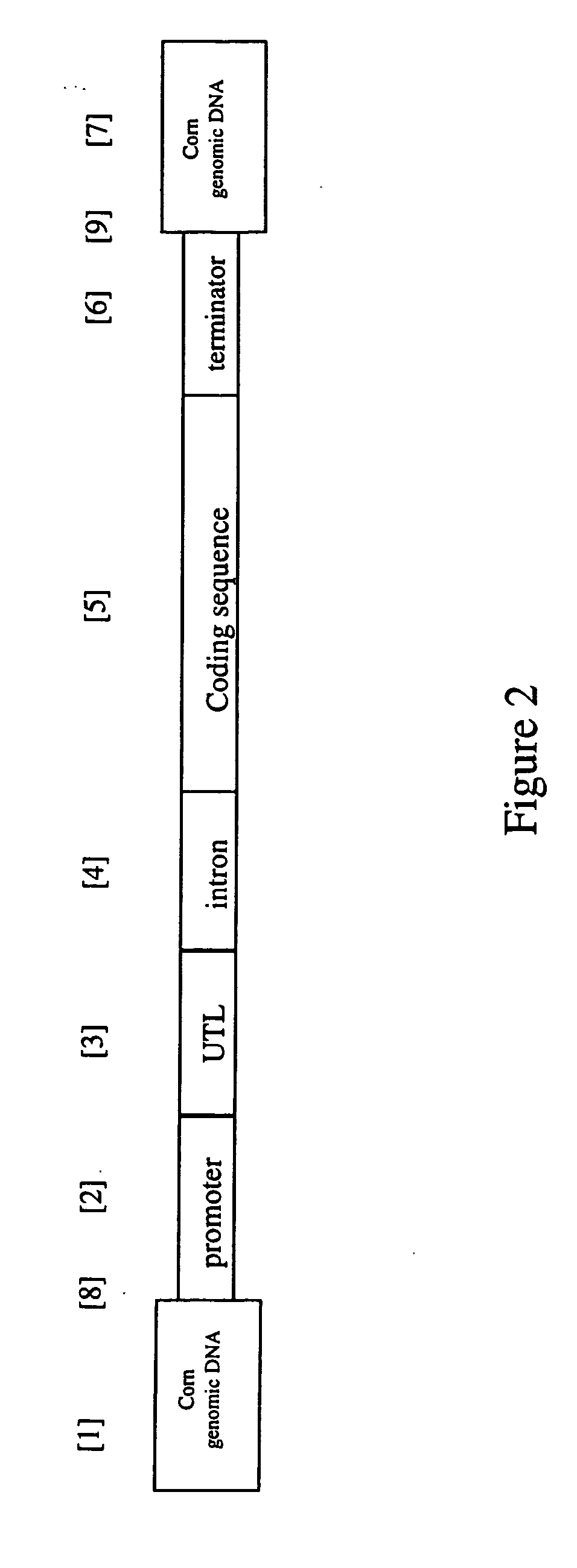
Nucleotide sequences and polypeptides encoded thereby useful for modifying plant characteristics
ActiveUS20070039067A1Increased biomass productionIncrease productionImmunoglobulinsFermentationBiotechnologyNucleotide sequencing
Owner:CERES INC
Power having nutrition of paddy, bean, fruit, vegetables and tea with the functions of equalizing the nutrition, losing weight and reducing blood sugar
ActiveCN101116510ABalanced nutritionHave weight lossPre-extraction tea treatmentMetabolism disorderFiberGlucose polymers
The present invention provides a low-lipid, high-fiber, balanced-nutritional, instant-taking and instant-resolving powder made from all natural components including corn, bean, flower, vegetable, fruit, tea and bi-usage plants for both food and medication. The nutritional powder provided by the present invention has not only the functions to balance the nutrition, but also the effect to decrease the body weight, lower the blood glucose, and effectively prevent and treat the diabetes.
Owner:湖南湘泉药业股份有限公司
Herbal enhanced analgesic formulations
InactiveUS20110028412A1Good analgesic effectIncrease productionBiocideNervous disorderVitamin CWhite willow bark
The analgesic properties of L-tryptophan and 5-HTP can be safely enhanced with the coadministration of salacin. Salacin can be effectively provided in the form of white willow bark along with other ingredients to further enhance the formulation's analgesic effect. As salacin can cause the loss of vitamin C in humans, the formulation advantageously includes a supplemental amount of vitamin C.
Owner:CAPPELLO INC
Method for supplementing the diet
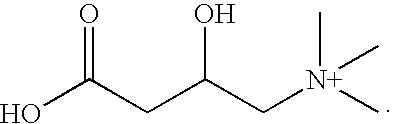
InactiveUS6579544B1Increased susceptibilityPrevent diseaseHeavy metal active ingredientsBiocideDietary supplementAlpha-Lipoic Acid
A dietary supplement blend composition is disclosed, the basic formulation of the composition containing vitamins, minerals, and carotenoids. The composition can also contain bioflavonoids, cartilage protectors such as glucosamine or chondroitin, alpha-lipoic acid, coenzyme Q10, and a source of omega-3 fatty acids such as flax seed oil. The composition is beneficial for improving health and preventing disease, particularly for degenerative conditions. A method for supplementing the diet is also disclosed, wherein the quantity of daily rations of the dietary supplement blend composition is determined based on the person's age, body weight, and quality of diet.
Owner:NUTRIEX
Genes conferring herbicide resistance
Compositions and methods for conferring herbicide resistance to plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a polypeptide that confers resistance or tolerance to glyphosate herbicides are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants. Compositions also comprise transformed plants, plant cells, tissues, and seeds. In particular, isolated nucleic acid molecules encoding glyphosate resistance proteins are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:2 or the nucleotide sequence set forth in SEQ ID NO: 1.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com