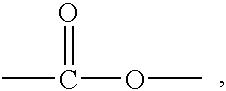
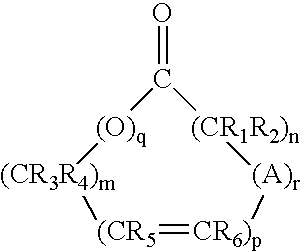
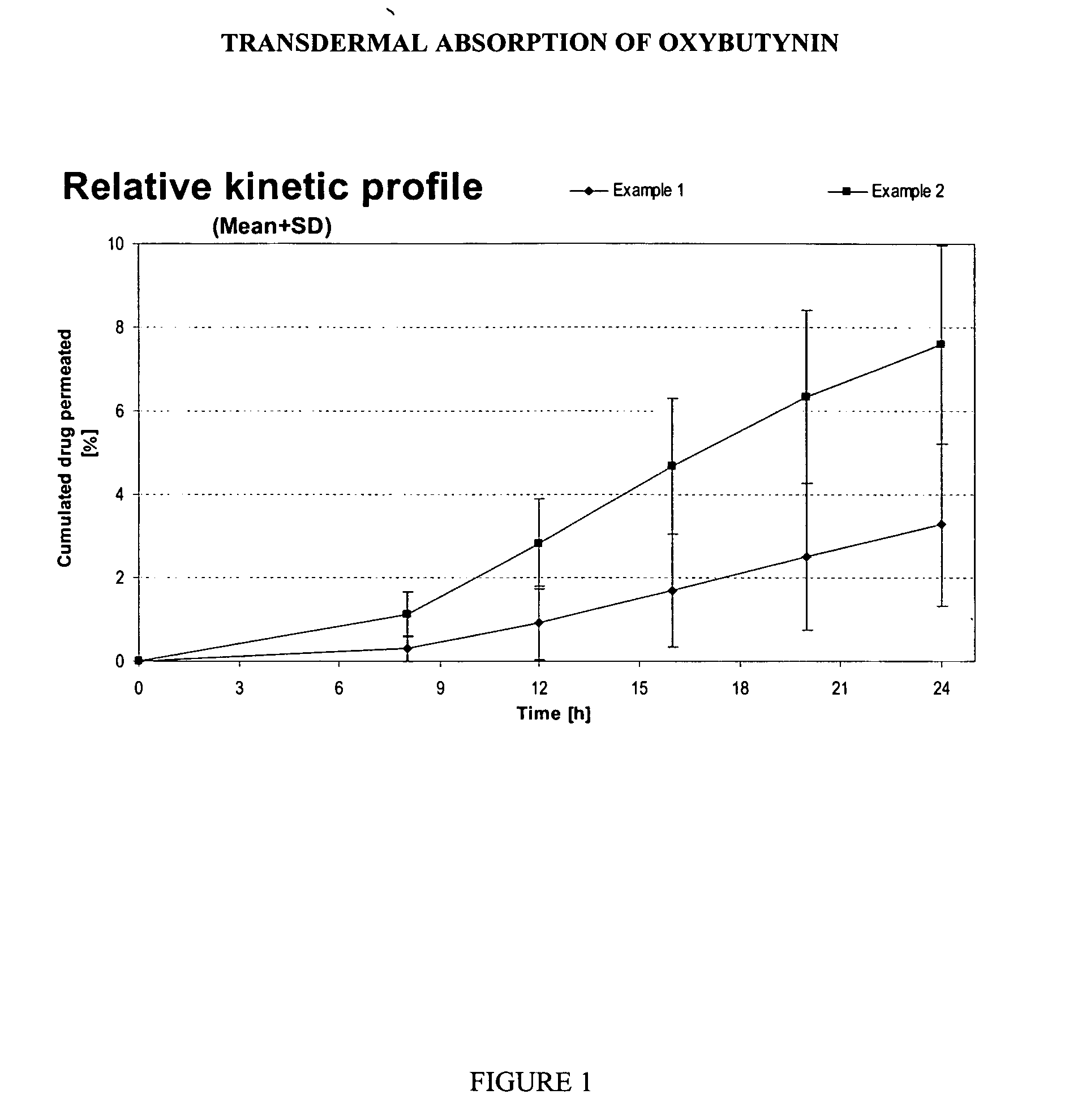
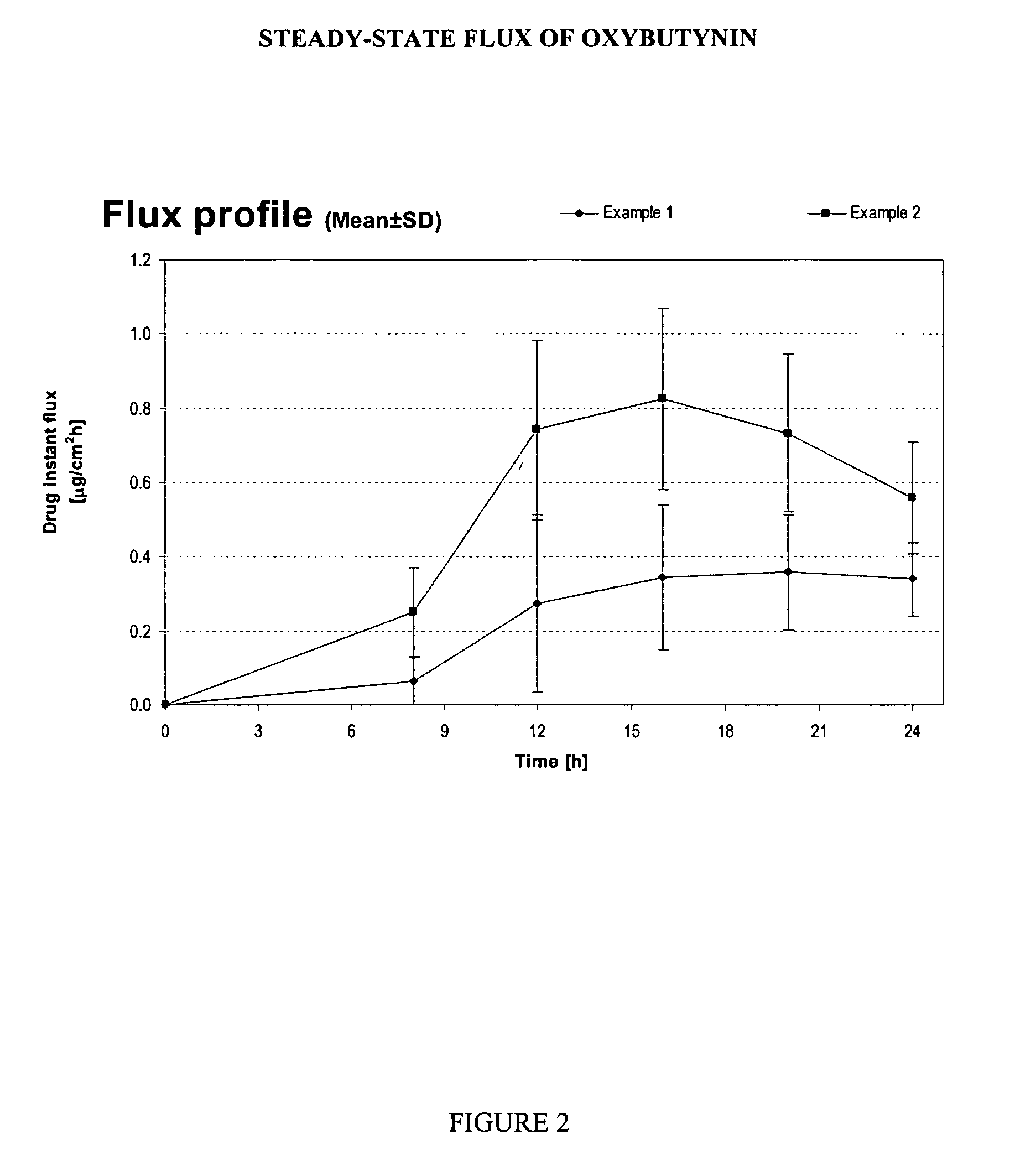
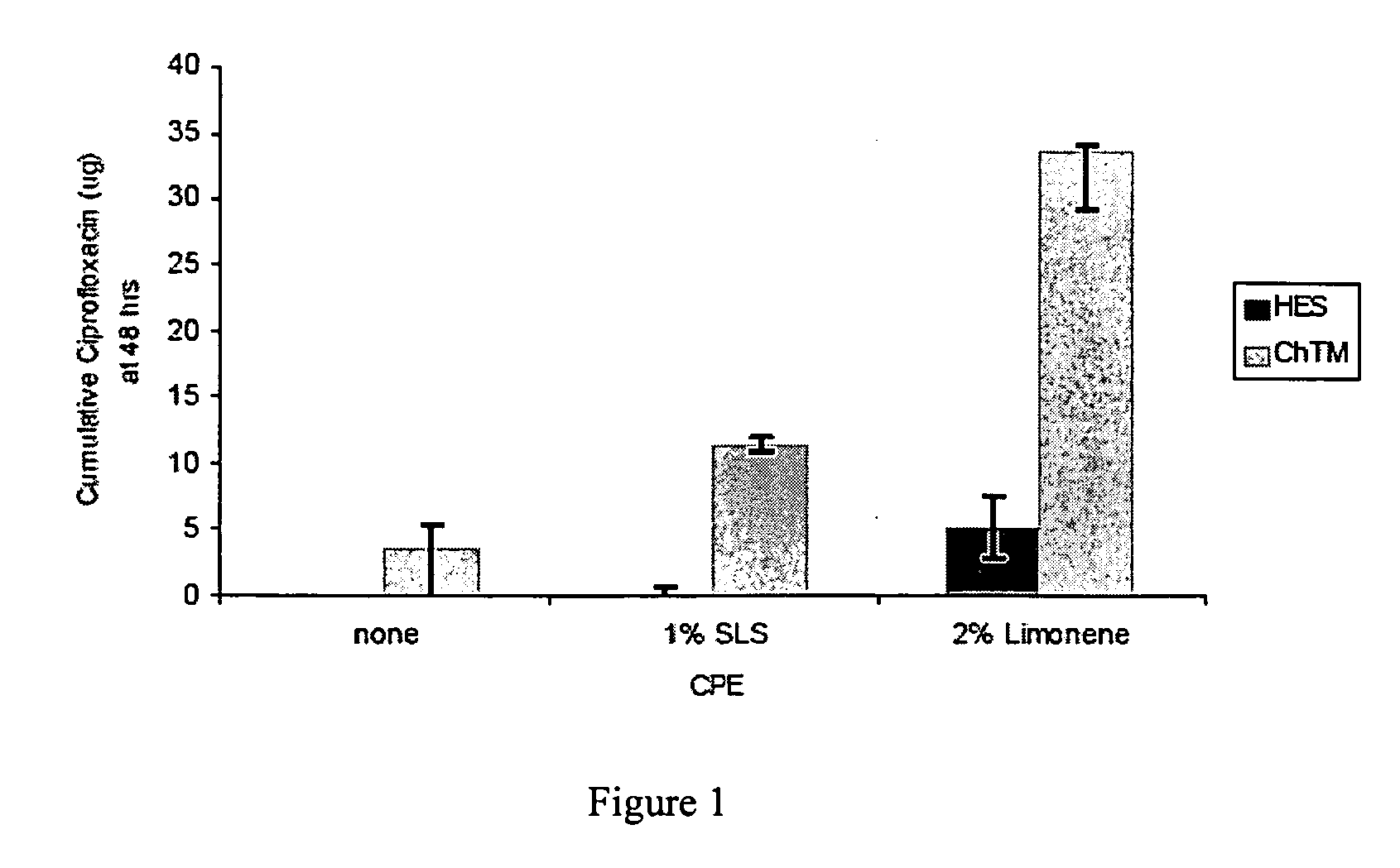
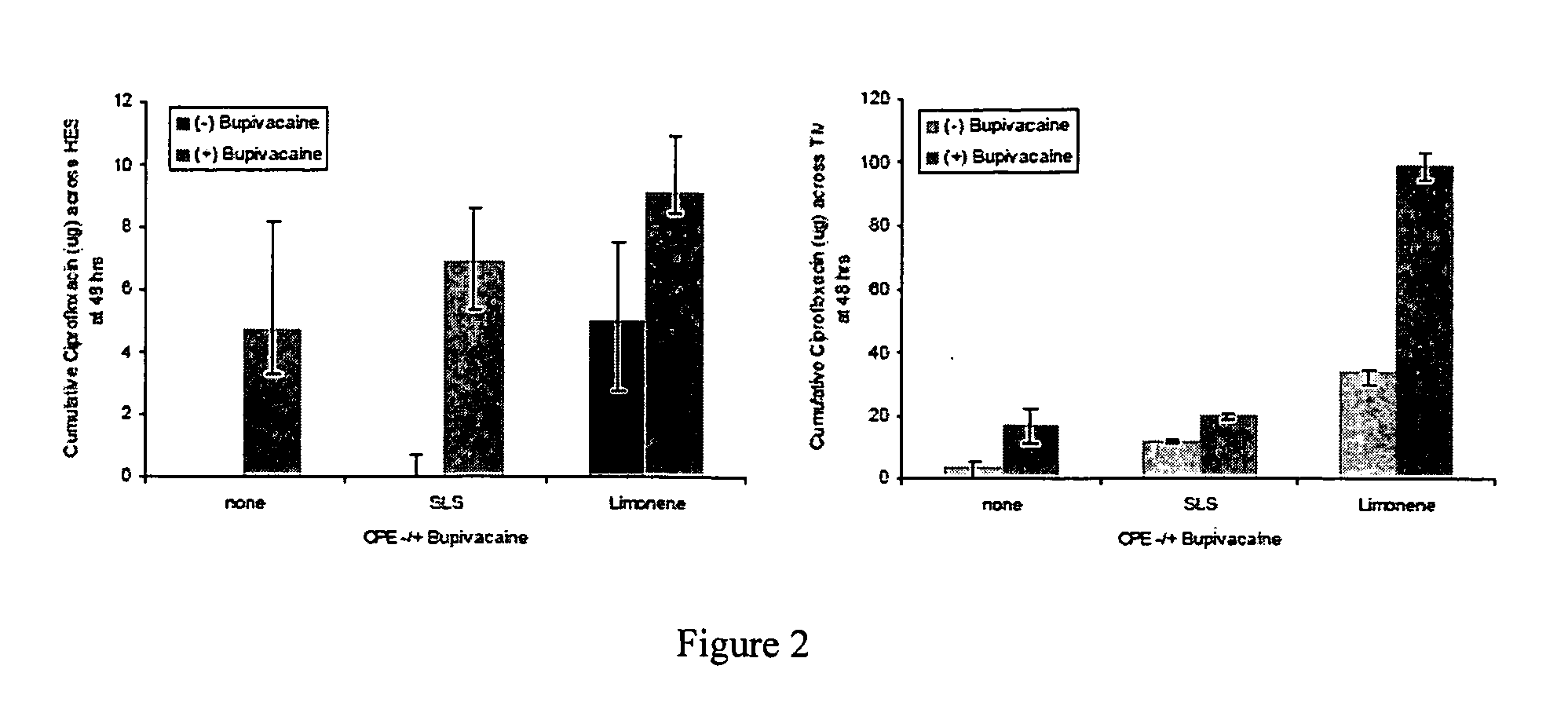
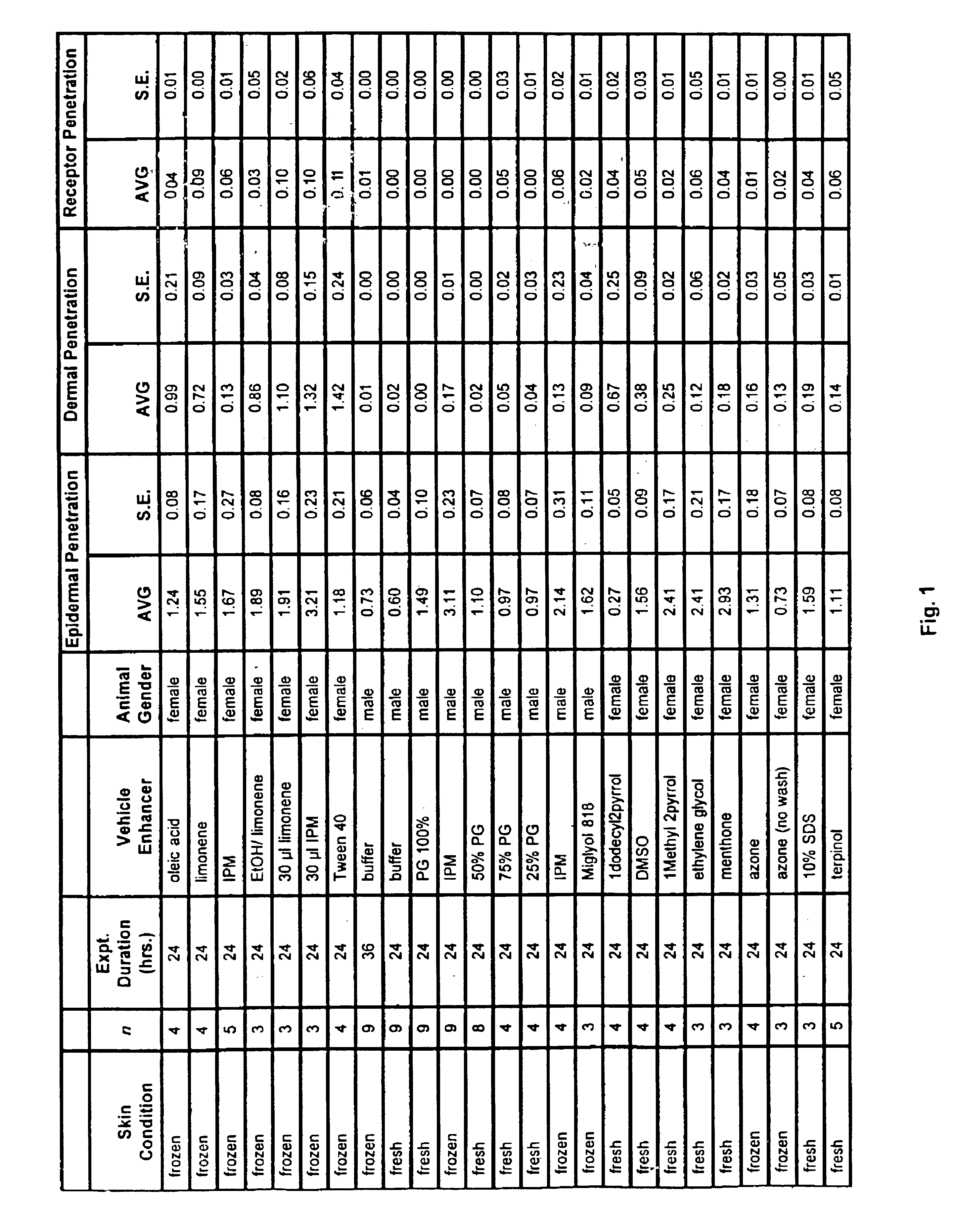
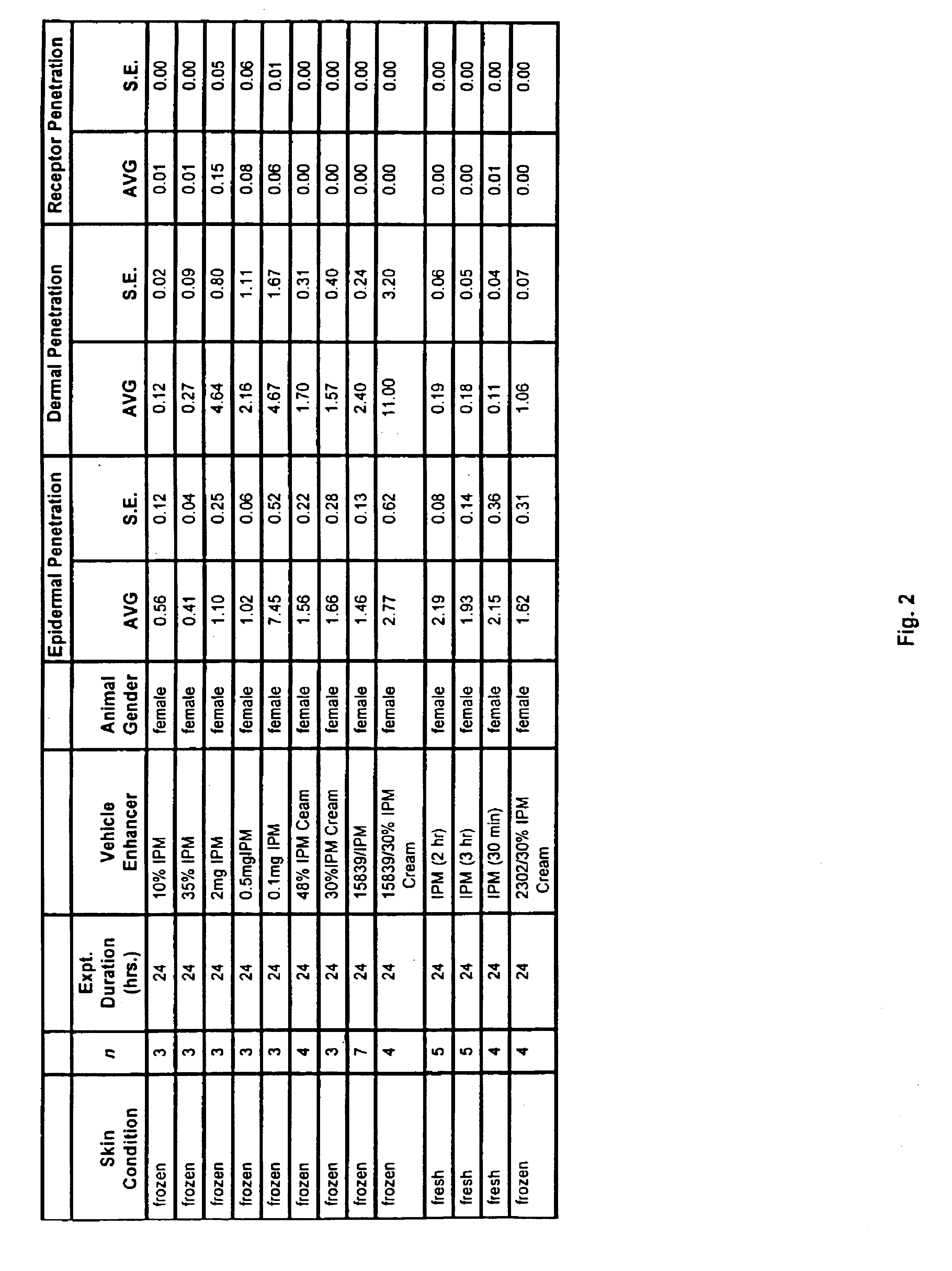
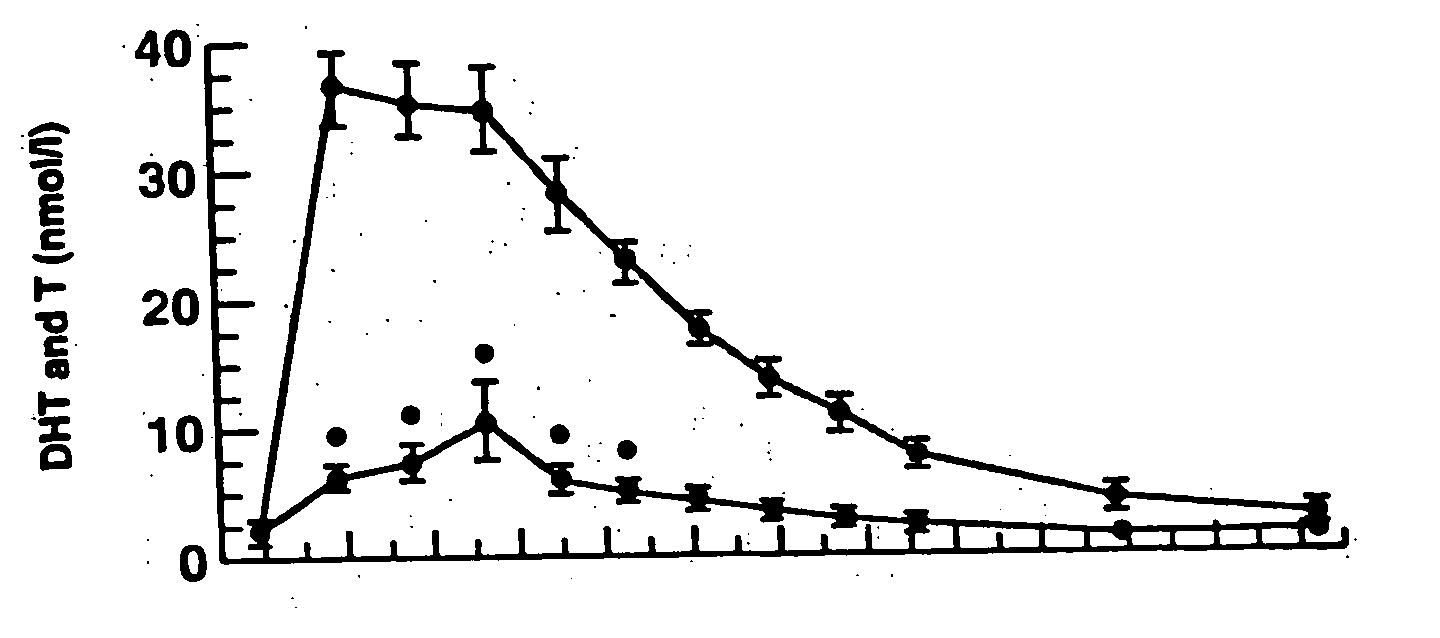
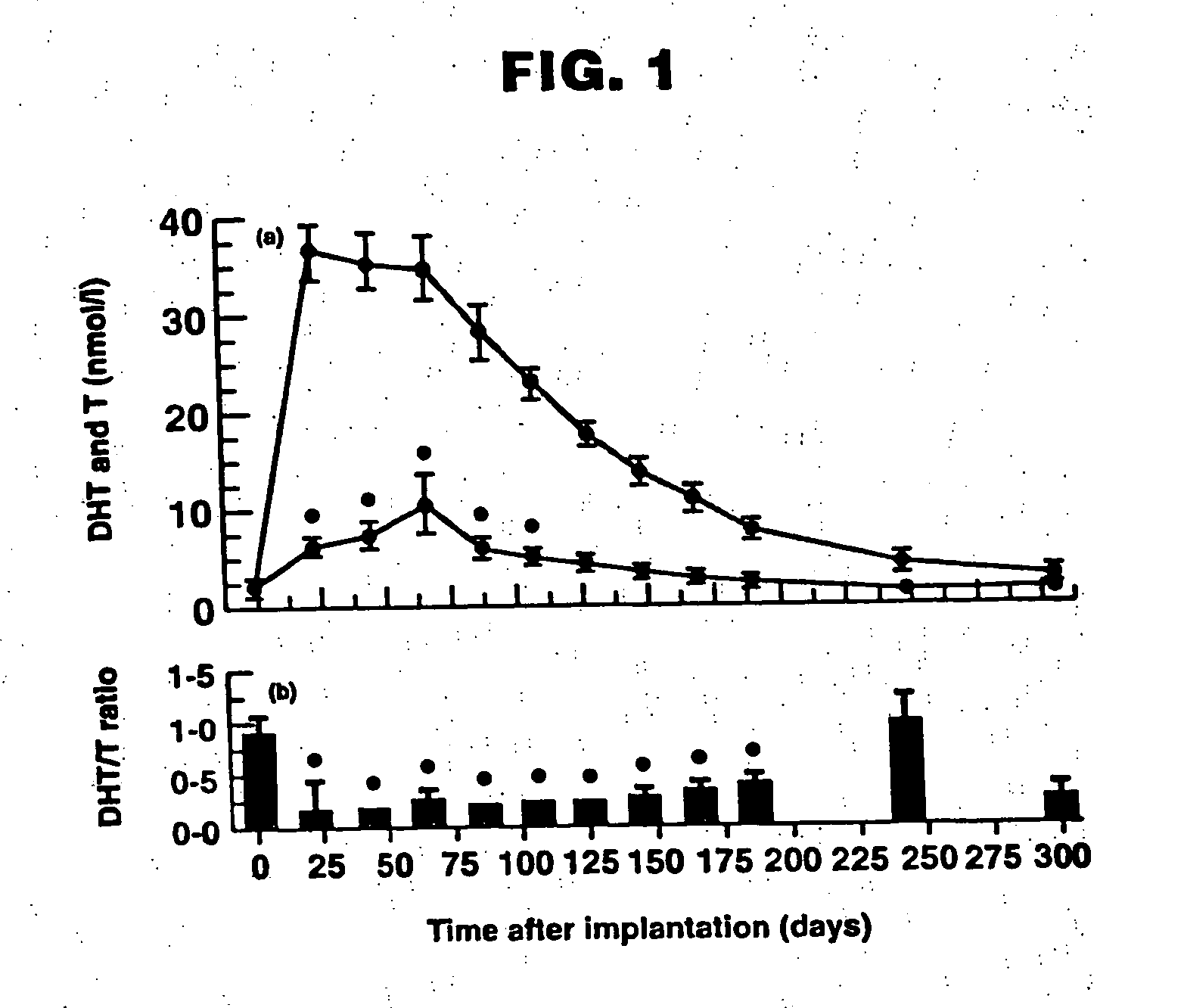
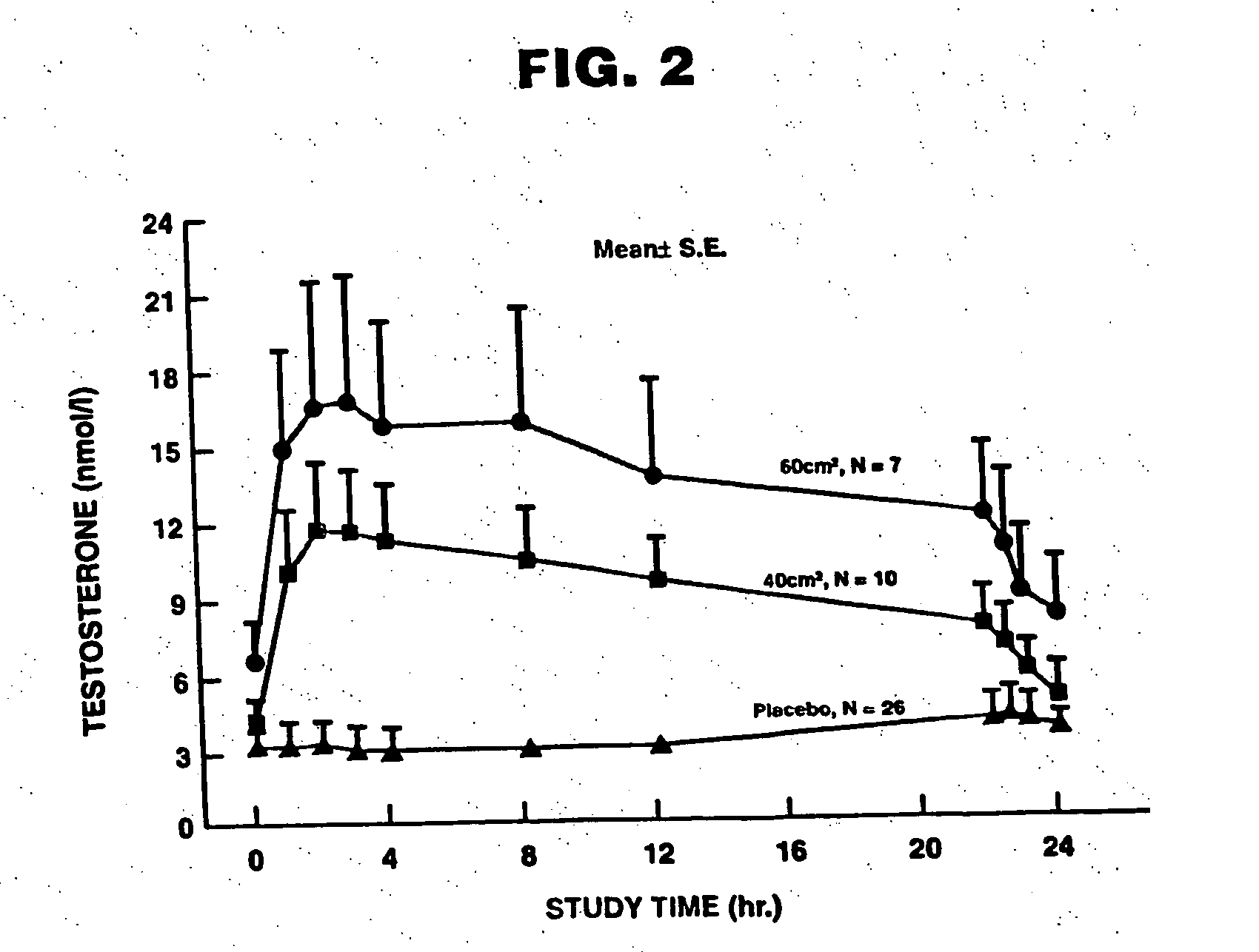
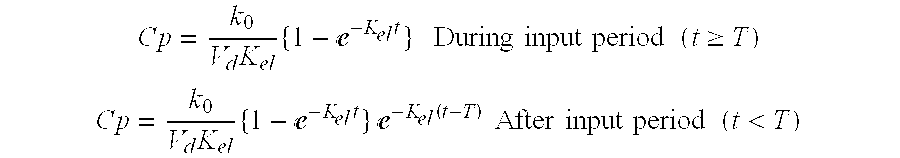Patents
Literature
716 results about "Penetration enhancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
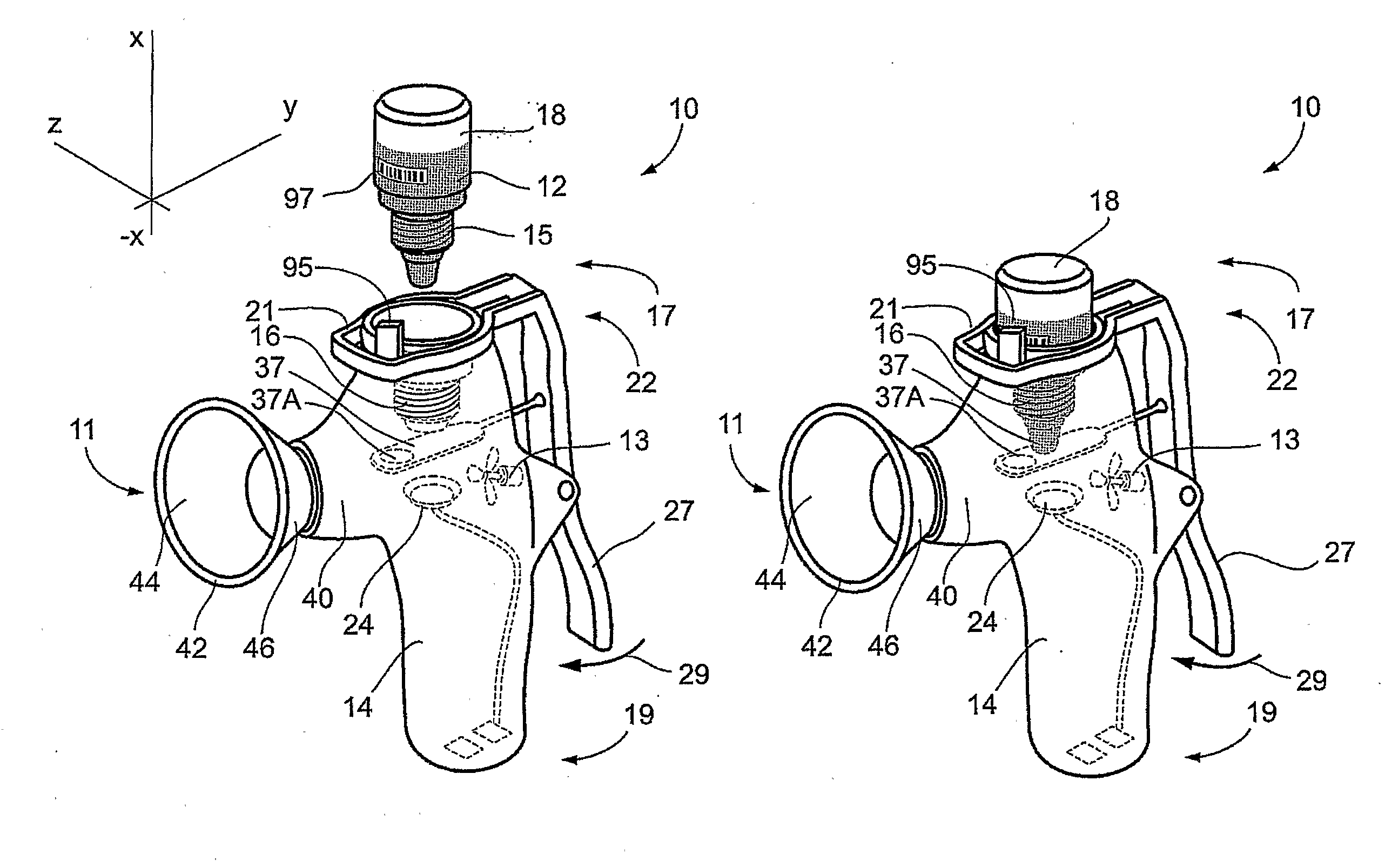
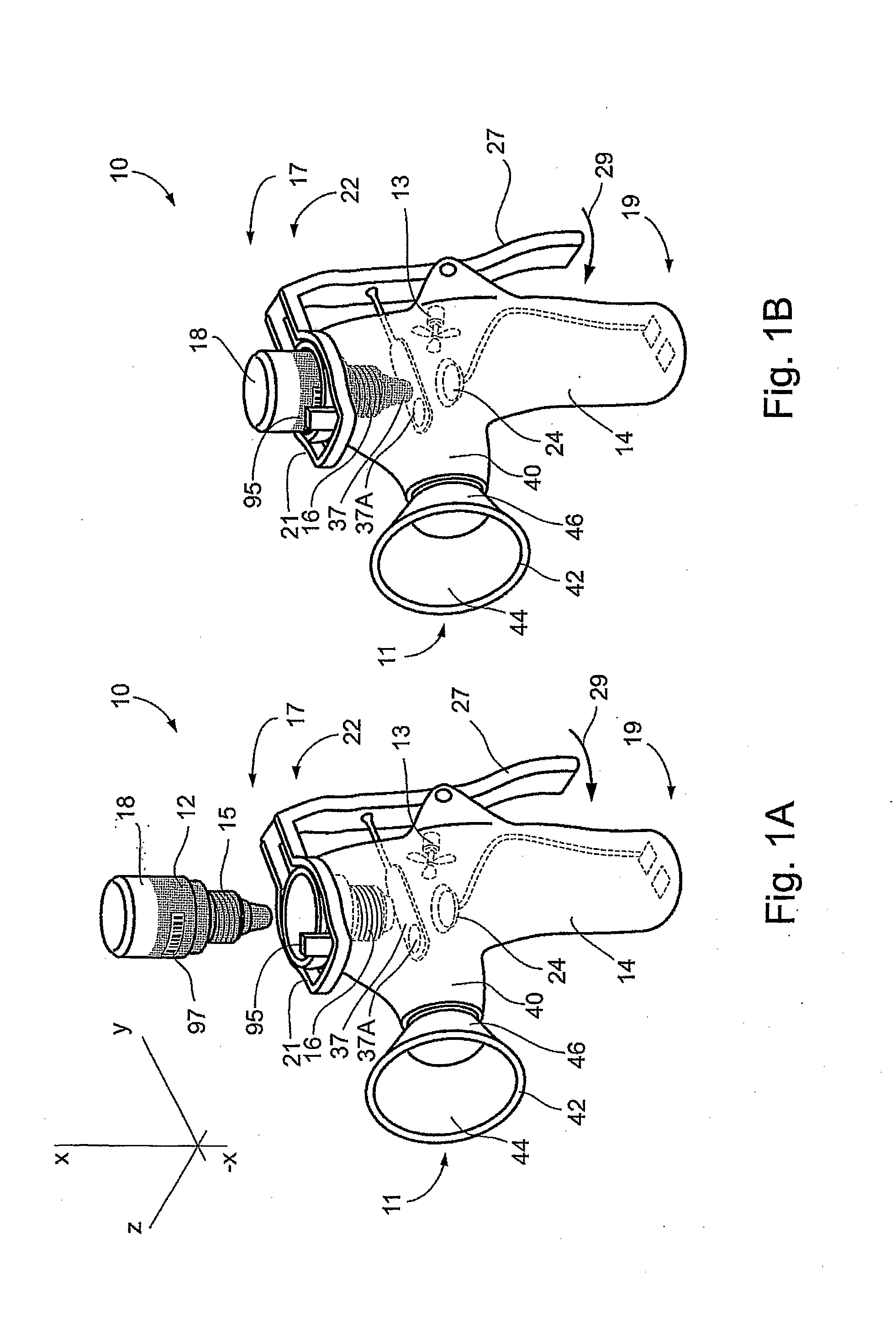
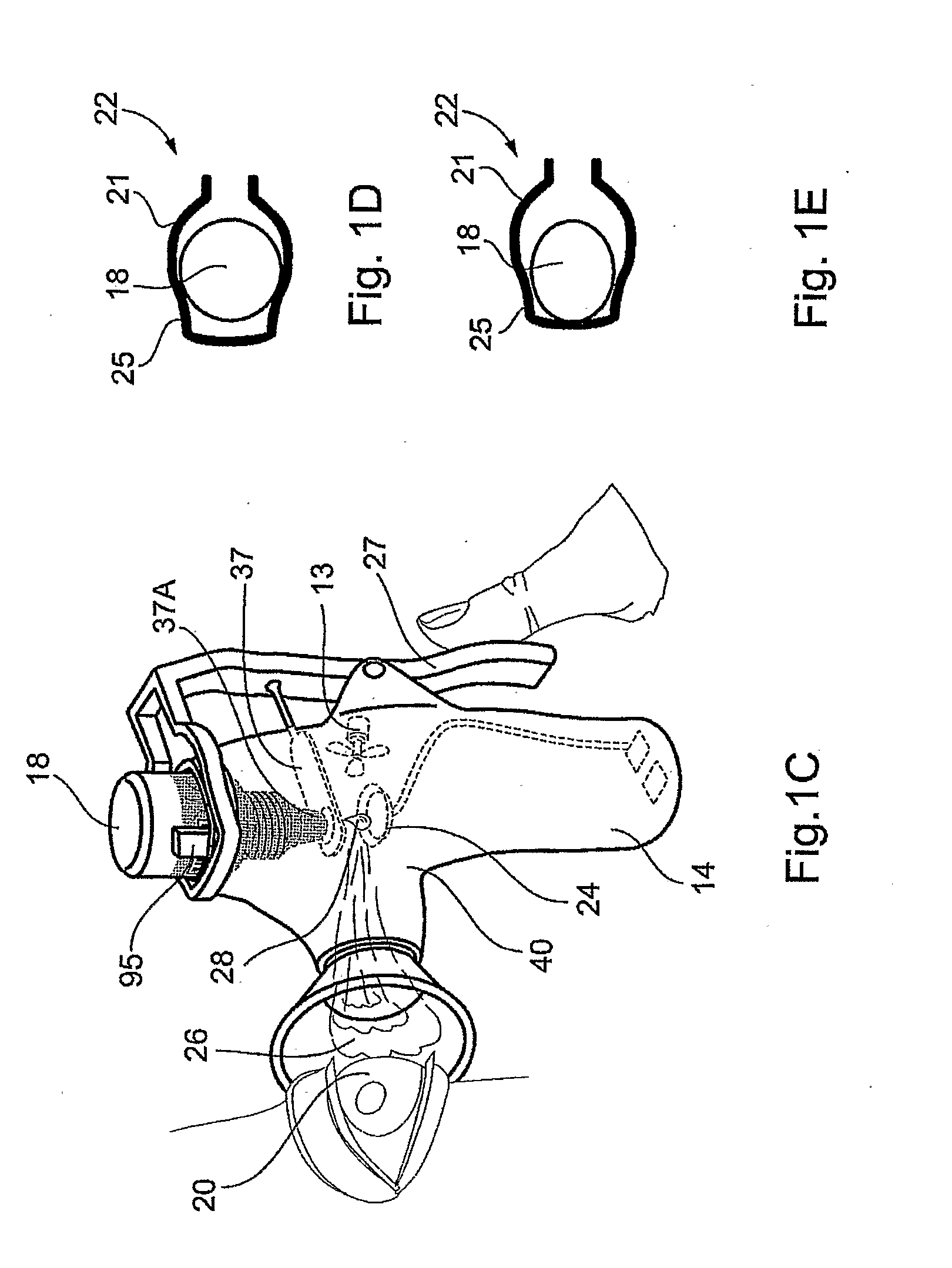
Method and Device for Ophthalmic Administration of Active Pharmaceutical Ingredients
InactiveUS20080233053A1Efficient transferImprove bioavailabilityOrganic active ingredientsPeptide/protein ingredientsAdditive ingredientBioavailability
Disclosed is the use of a mist of a pharmaceutical composition for ophthalmic delivery of a protein or peptide active pharmaceutical ingredient, a related method of treatment and a device useful in implementing the use and method. Disclosed is also the use of a mist for ophthalmic delivery of a pharmaceutical composition including a highly irritating penetration enhancer and an ophthalmically acceptable carrier, a related method of treatment and a device useful in implementing the use and method. Disclosed is also a device for ophthalmic administration configured to direct a mist of a pharmaceutical composition to the eye only when the eye is open. Disclosed is also a self-sterilizing device for ophthalmic administration. Disclosed is also a device and a method for increasing the bioavailability of an ophthalmically administered API in a pharmaceutical composition.
Owner:PHARMALIGHT
Foamable compositions and kits comprising one or more of a channel agent, a cholinergic agent, a nitric oxide donor, and related agents and their uses
InactiveUS20080317679A1Efficient deliveryMinimizing systemic penetrationOrganic active ingredientsBiocideActive agentNitric oxide
The present invention relates to a foamable therapeutic composition comprising: (a) a therapeutically effective concentration of at least one active agent selected from the group consisting of a channel agent, a cholinergic agent, and a nitric oxide donor; and (b) a foamable carrier comprising:i. about 50% to about 98% of a solvent selected from the group consisting of water; a hydrophilic solvent; a hydrophobic solvent; a potent solvent; a polar solvent, a silicone, an emollient, and mixtures thereof;ii. 0% to about 48% of a secondary solvent selected from the group consisting of water; a hydrophilic solvent; a hydrophobic solvent; a potent solvent; a polar solvent, a silicone, an emollient, a co-solvent, a penetration enhancer and mixtures thereof;iii. a surface-active agent;iv. about 0% to about 5% by weight of at least one polymeric agent; andv. a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition;wherein the composition is housed in a container and is substantially flowable, andwhich upon release expands to form a breakable foam; andwherein the foamable carrier is selected to generate a foam of good to excellent quality.The invention further provides a method of treating, alleviating or preventing a disorder of mammalian subject, comprising administering such a composition to an afflicted target site.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Pharmaceutical composition and method for transdermal drug delivery
InactiveUS20050042268A1Improves transdermal penetrationSmall amountOrganic active ingredientsPharmaceutical delivery mechanismHormones regulationTransdermal medication
A pharmaceutical composition for transdermal administration of a hormone (e.g., testosterone), which includes urea and / or a derivative thereof as a penetration enhancer, and methods utilizing same for treating medical conditions in which elevating a hormone serum level is beneficial are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Pharmaceutical composition and method for transdermal drug delivery
InactiveUS20050020552A1Increase concentrationImproves transdermal penetrationOrganic active ingredientsAerosol deliveryIsostearic acidHormones regulation
A pharmaceutical composition for transdermal administration of a hormone (e.g., testosterone), which includes isostearic acid as a penetration enhancer, and methods utilizing same for treating medical conditions in which elevating a hormone serum level is beneficial are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Topical Composition for Treating Pain
ActiveUS20080311167A1Ameliorate and eliminate painFree from painBiocideHydrocarbon active ingredientsSequelaPreventing pain
Topical compositions having as the active ingredient a lipid, fatty acid ester, natural wax, sterol, or combinations thereof referred to herein as “lipophilic vehicle” or “LV” and methods of use, have been developed for the amelioration or prevention of pain or the sequelae of pain. The composition may be in the form of an ointment, cream, gel, lotion, spray, foam, paste, patch, suspension or dispersion. In the preferred embodiment, the formulation is a gel. The LV may contain a penetration enhancer, most preferably one with membrane disruptive properties. The formulation may be applied to or impregnated into a gauze, wrap, bandage, cotton-tipped stick, adhesive bandage strip, or other support wrap or medical bandage or wound cover. For example, the compositions may be are incorporated onto or into disposables such as hemorrhoid wipes, sponge, mouth guards, dental trays; needles or catheters; adult diapers; gloves, socks or wrist bands, for ease of application. The composition is applied topically to a site at or adjacent to a painful region. The composition is reapplied as necessary. Pain relief is typically obtained within minutes and lasts for periods of variable duration ranging from minutes to several hours and even, in some cases, days. The composition is variably effective to treat visceral, somatic and neuropathic pain both acute and chronic as well as muscle pain and stiffness and joint pain and stiffness.
Owner:EPICENTRX
Antifungal nail lacquer and method using same
InactiveUS6224887B1Effective preventionEffective treatmentBiocideCosmetic preparationsAntifungalLacquer
A nail lacquer effective for the treatment or prevention of fungal infections, such as, onychomycosis, includes fungicidally effective amount of ciclopirox, econazole, or other antifungal agent in a clear, stable, film-forming lacquer vehicle which includes a water-insoluble film-forming polymer; 2-n-nonyl-1,3-dioxolane or similar penetration enhancer; and volatile solvent. A plasticizer for the film-forming polymer which is also compatible with the other components may be included although the preferred penetration enhancers may also function as plasticizer. The composition, when applied to the nails provides a hard, clear, water-resistant film containing the antifungal agent. The film is resistant to multiple washings and is effective in the treatment of onychomycosis.
Owner:MACROCHEM CORP
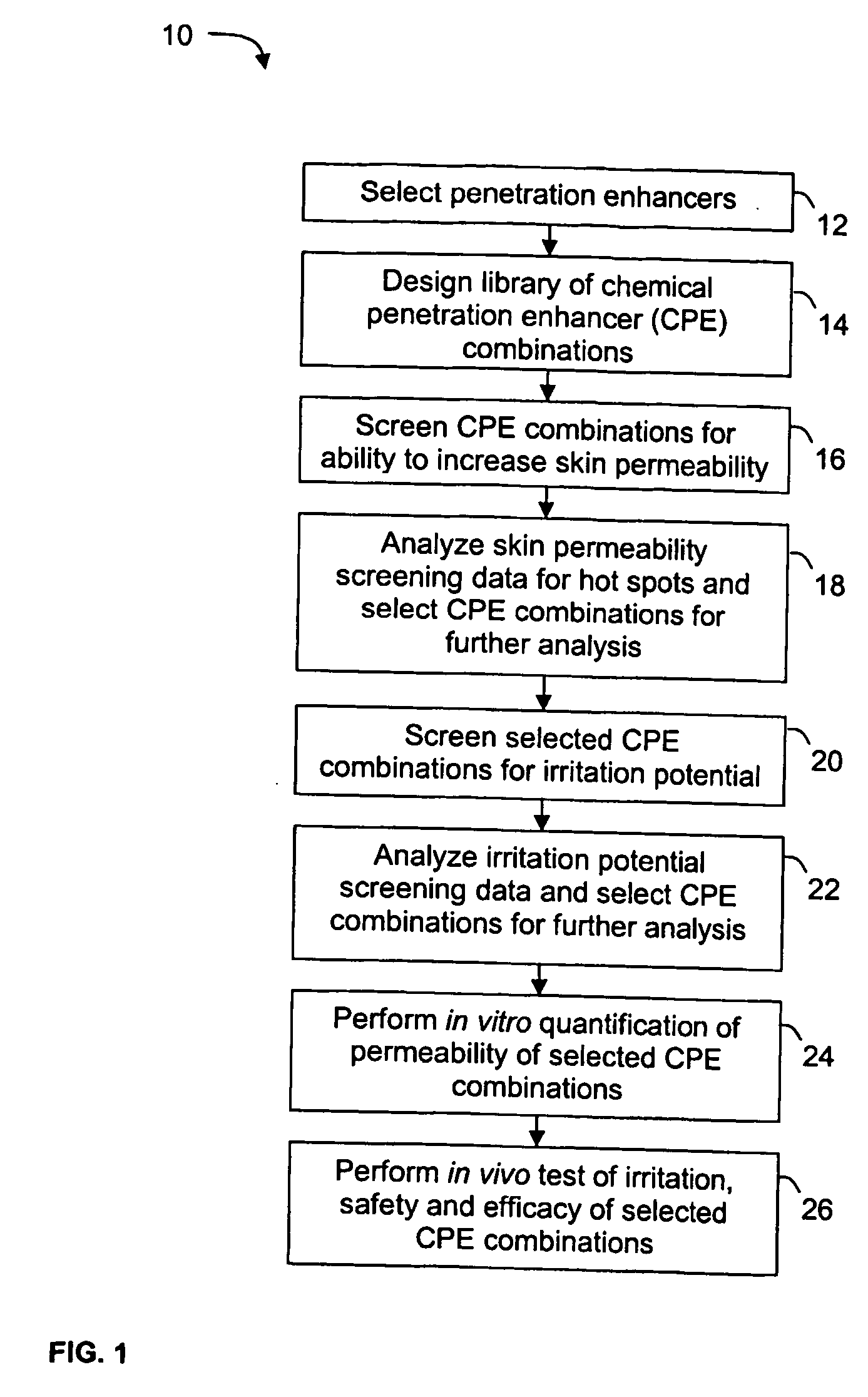
Penetration Enhancer Combinations for Transdermal Delivery
InactiveUS20070269379A1Easy to transportLess irritatingOrganic active ingredientsBiocideHigh-Throughput Screening MethodsIrritation
A high throughput screening and isolation system identifies rare enhancer mixtures from a candidate pool of penetration enhancer combinations. The combinations are screened for high penetration but low irritation potential using a unique data mining method to find new potent and safe chemical penetration enhancer combinations. The members of a library of chemical penetration enhancer combinations are screened with a high throughput device to identify “hot spots”, particular combinations that show higher chemical penetration enhancement compared to neighboring compositions. The irritation potentials of the hot spot combinations are measured to identify combinations that also show low irritation potential. A active component, such as a drug, is then combined with the combination in a formulation which is tested for the ability of the drug to penetrate into or through skin. It is then assessed whether the formulation can deliver the quantity of drug required, and animal tests are conducted to confirm in vivo the ability of the chemical penetration enhancer combinations to facilitate transport of sufficient active molecules across the skin to achieve therapeutic levels of the active molecule in the animal's blood. The invention provides specific unique and rare mixtures of chemical penetration enhancers that enhance skin permeability to hydrophilic macromolecules by more than 50-fold without inducing skin irritation, such as combinations of sodium laurel ether sulfate and 1-phenyl piperazine, and combinations of N-lauryl sarcosine and Span 20 / sorbitan monolaurate.
Owner:RGT UNIV OF CALIFORNIA
Pharmaceutical composition and method for transdermal drug delivery
InactiveUS20050025833A1Increase concentrationImproves transdermal penetrationPowder deliveryOrganic active ingredientsAmmonium compoundsCompound (substance)
A pharmaceutical composition for transdermal administration of a hormone (e.g., testosterone), which includes a quaternary ammonium compound as a penetration enhancer, and methods utilizing same for treating medical conditions in which elevating a hormone serum level is beneficial are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
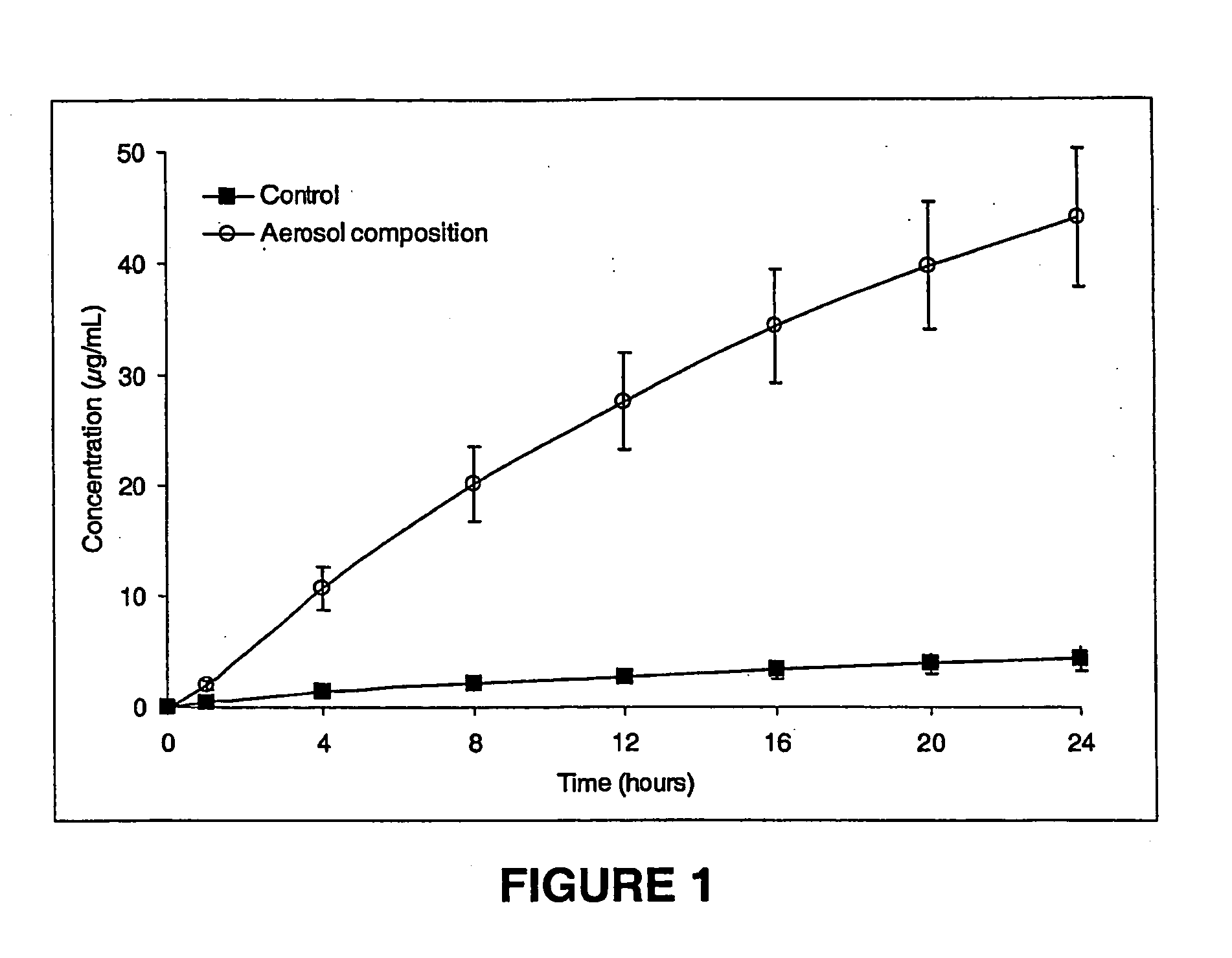
Transdermal aerosol compositions
InactiveUS20050186141A1Overcome disadvantagesCosmetic preparationsToilet preparationsMedicineActive agent
The present invention provides a pharmaceutical composition for transdermal delivery comprising: one or more physiologically active agents; one or more dermal penetration enhancers; a pharmaceutically acceptable carrier comprising a volatile solvent; and a hydrofluorocarbon propellent; wherein the carrier and penetration enhancers combine to provide a single-phase solution of the one or more physiologically active agents.
Owner:ACRUX DDS
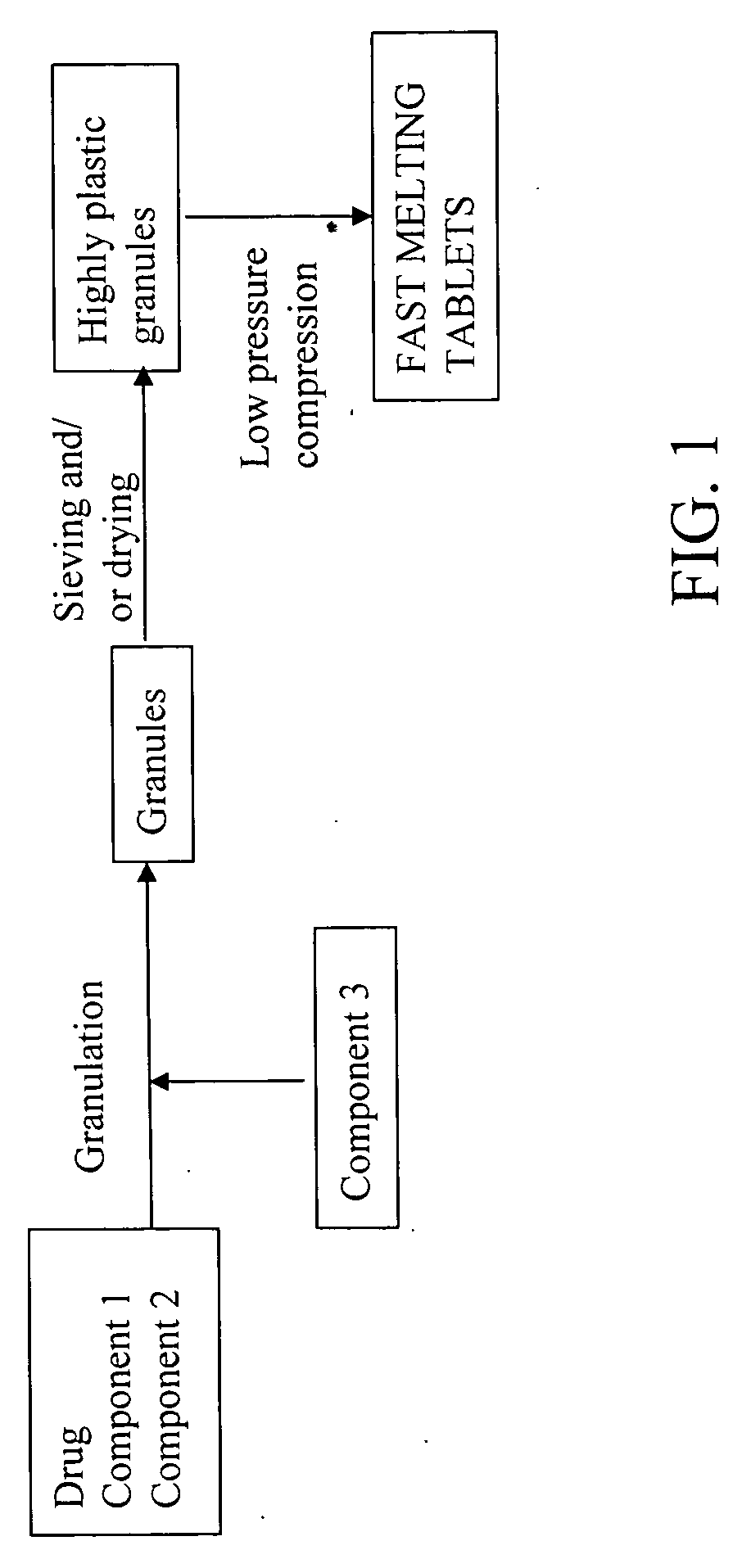
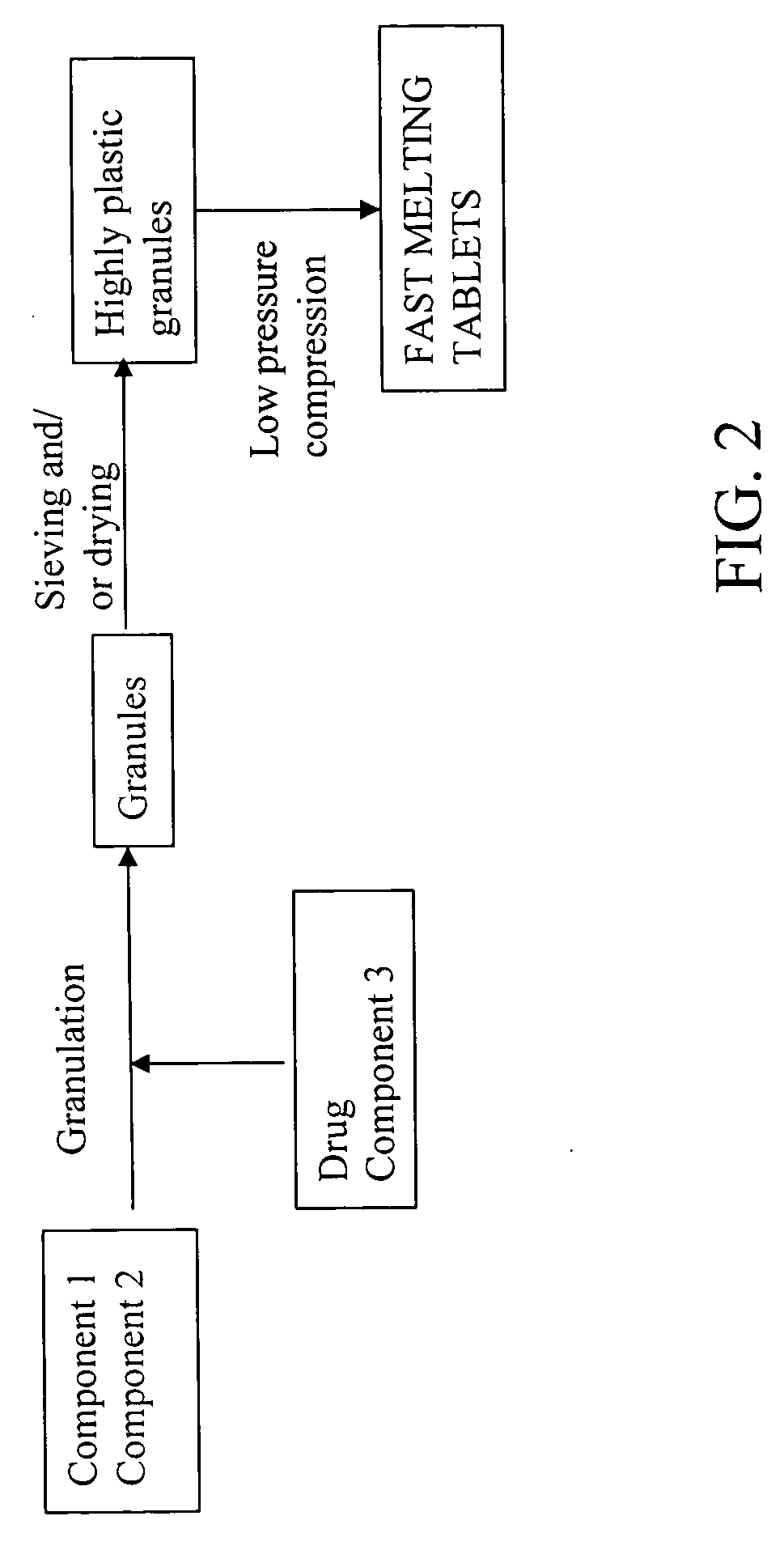
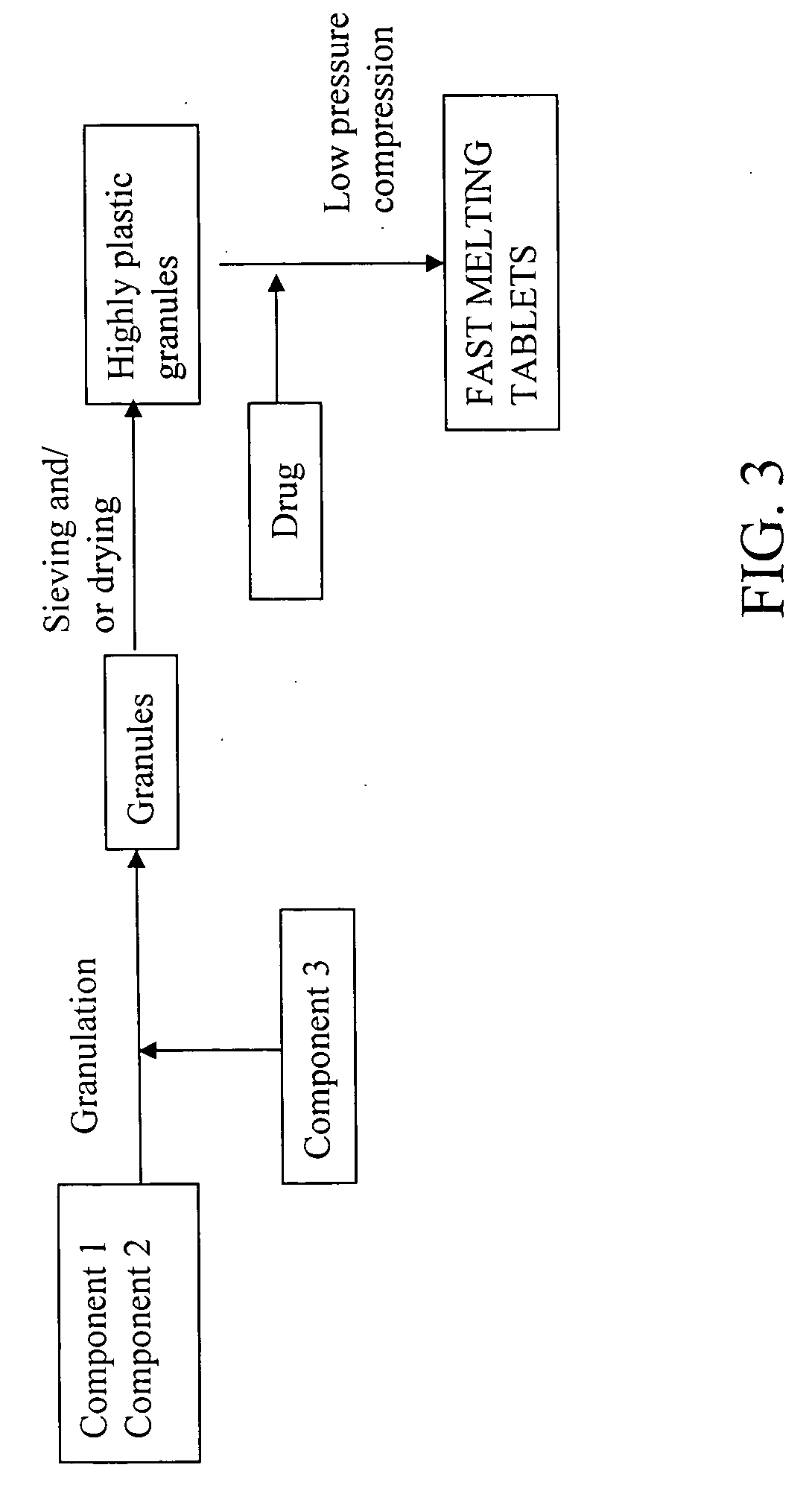
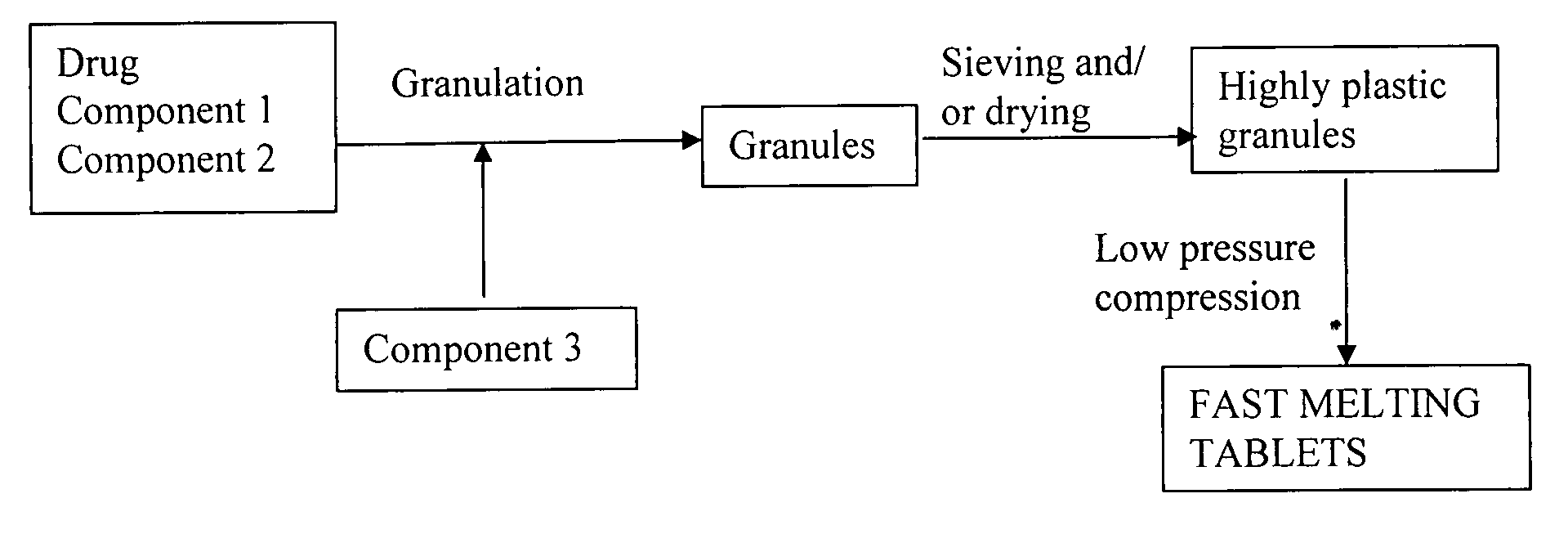
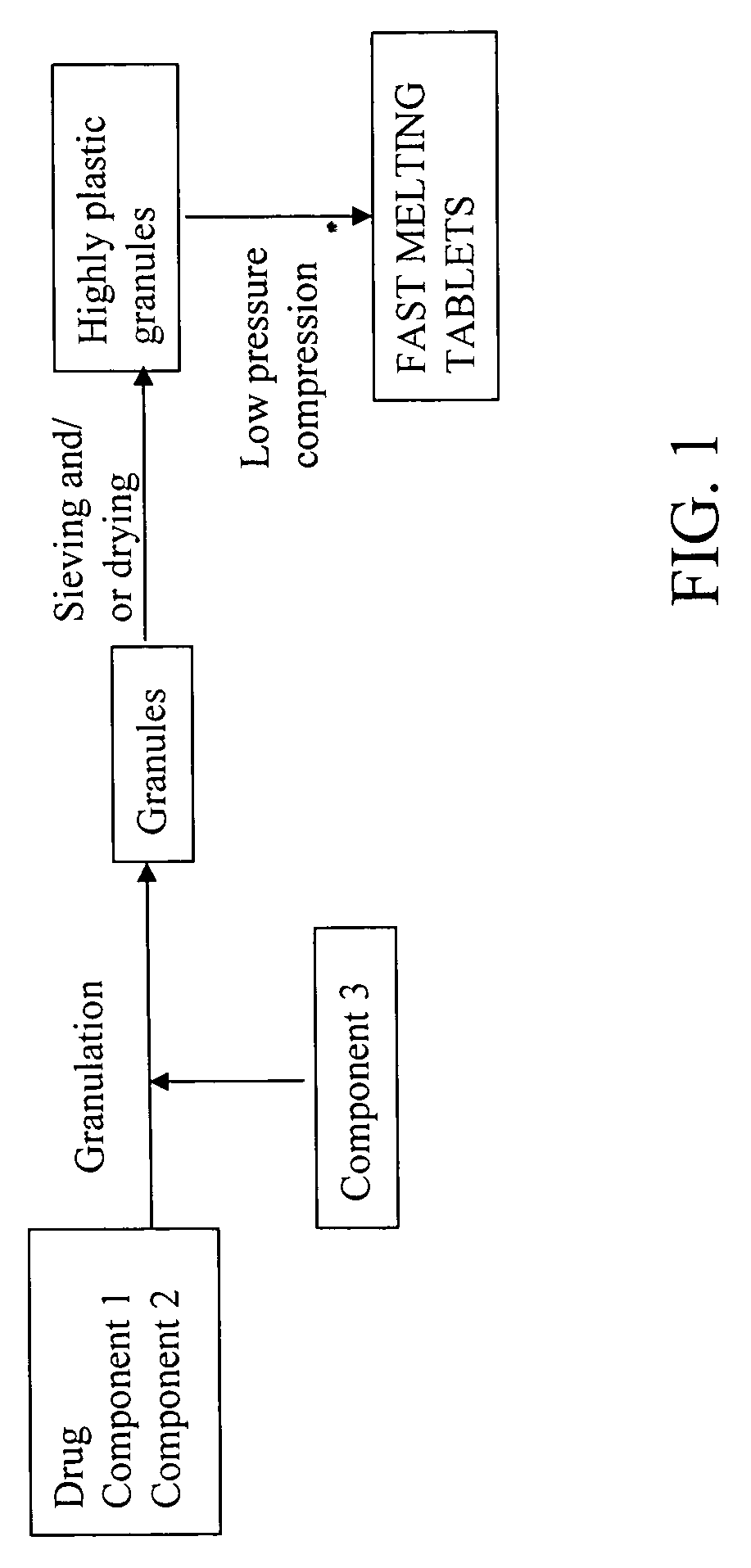
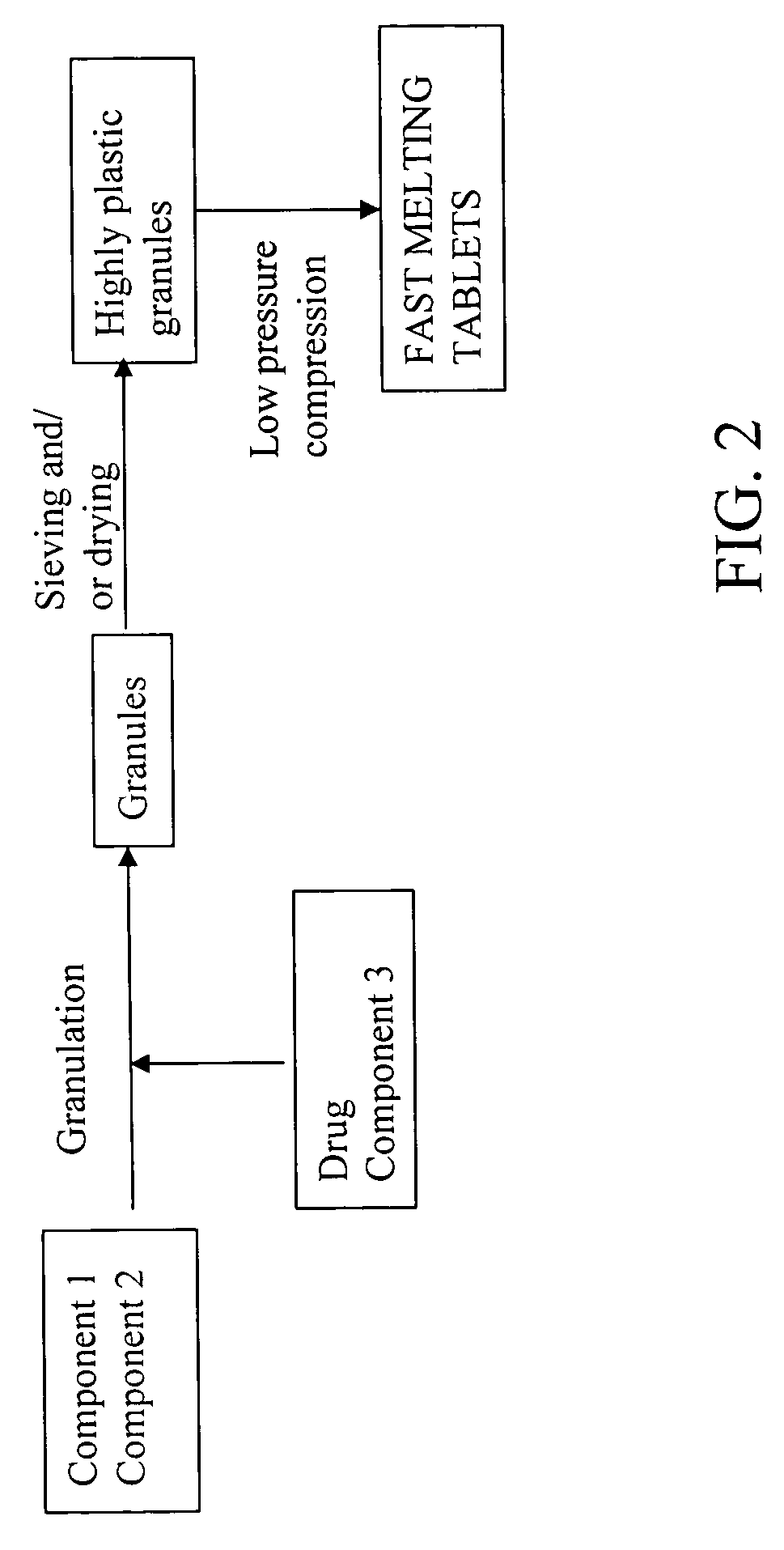
Highly plastic granules for making fast melting tablets
InactiveUS20050013857A1Increases tablet strengthIncrease tablet strengthPowder deliveryPill deliveryPlastic materialsHardness
A fast-melting pharmaceutical tablet comprises a porous, plastic substance, a water penetration enhancer and a binder. One or more drugs can be incorporated into the formulation at different stages of the process so as to afford a pharmaceutically active tablet. Methods of making the pharmaceutical tablet entail combining the porous, plastic material, the water penetration enhancing agent, and the binder so as to form highly plastic granules, which are compressed into tablets. The resulting tablets dissolve rapidly in the mouth and have good hardness with low brittleness. The tablets are particularly valuable to those who have difficulty swallowing conventional pills.
Owner:AKINA INC
Transdermal drug delivery systems containing quaternary ammonium salts and methods of using the same
InactiveUS20030091620A1Improve permeabilityReduce skin irritationAntibacterial agentsNervous disorderIrritationReducer
A transdermal drug delivery system is disclosed, which includes a polymer, a drug and an amount of a quaternary ammonium salt that is sufficient to act as a penetration enhancer. The quaternary ammonium salt may also be present in an amount sufficient to act as an irritation reducer. Further, the transdermal drug delivery system may also contain a co-enhancer, which provides a synergistic skin permeation enhancing effect when combined with the quaternary ammonium salt. A method for enhancing the transdermal delivery of a drug is also disclosed.
Owner:FIKSTAD DAVID +3
Transdermal and topical administration of drugs using basic permeation enhancers
InactiveUS20050074487A1Improve throughputEffective amountCosmetic preparationsBiocideActive agentIrritation
Methods are provided for enhancing the permeability of skin or mucosal tissue to topical or transdermal application of pharmacologically or cosmeceutically active agents. The methods entail the use of a base in order to increase the flux of the active agent through a body surface while minimizing the likelihood of skin damage, irritation or sensitization. The permeation enhancer can be an inorganic or organic base. Compositions and transdermal systems are also described.
Owner:DERMATRENDS INC
Regeneration fluid for SCR denitration catalyst
InactiveCN101574671AHigh activityEasy to cleanDispersed particle separationCatalyst regeneration/reactivationAdditive ingredientAmmonium paratungstate
The invention discloses a regeneration fluid for an SCR denitration catalyst, which comprises the following components: 0.001 to 1 weight percent of penetration enhancer JFC, 0.001 to 1 weight percent of surfactant OP-10, 0 to 1 weight percent of peregal, 0.6 to 4 weight percent of ammonium metavanadate, 5.5 to 12.5 weight percent of ammonium paratungstate, 0 to 6.5 weight percent of ammonium paramolybdate, and the balance of deionized water and acid. The regeneration fluid has the advantages that the regeneration fluid can also supplement active ingredients during the washing, and the activity recovery of the catalyst after the regeneration is up to between 90 and 105 percent; a nonionic surfactant is added into the regeneration fluid, so that the regeneration fluid improves the cleaning capability on the catalyst and cannot cause the damage to a catalyst carrier and other effective ingredients; and the regenerated catalyst fully can continue to be normally used, and the service life can reach more than 95 percent of that of a new catalyst.
Owner:COUNTRY JIANGSU CATALYST REGENERATION TECH
Antifungal nail lacquer and method using same
A nail lacquer for the treatment or prevention of fungal infections, such as, onychomycosis, includes fungicidally effective amount of ciclopirox, econazole, or other antifungal agent in a compatible film-forming lacquer vehicle which includes a water-insoluble film-forming polymer; pentadecalactone, or similar cyclic lactone compound or derivative thereof, and volatile solvent. The pentadecalactone functions as a plasticizer for the film-forming polymer and as a penetration enhancer for the antifungal agent. The composition, when applied to the nails provides a hard, clear, water-resistant film containing the antifungal agent. The compositions are used for the treatment of onychomycosis.
Owner:CPEX PHARMACEUTICALS INC
Use of allantoin as a pro-collagen synthesis agent in cosmetic compositions
InactiveUS20080108681A1Improving appearance and texture and firmnessIncrease synthesisCosmetic preparationsOrganic active ingredientsFine lineWrinkle skin
Compositions comprising allantoin and an acceptable carrier and methods of using such compositions to increase pro-collagen synthesis in skin are disclosed. Compositions of the present invention may be used to decrease the signs of skin aging such as wrinkles and fine lines. Compositions of the present invention may be topically administered, orally administered or parenterally, such as administration by injection. When topically administered, additive ingredients such as penetration enhancers, fragrances, and moisturizers and cosmetic adjuvants may be included in compositions of the present invention.
Owner:ACCESS BUSINESS GRP INT LLC
Permeation enhancer comprising genus Curcuma or germacrone for transdermal and topical administration of active agents
InactiveUS20050244522A1Increase permeationImprove permeabilityBiocideOrganic active ingredientsTetraglycolGermacrone
A formulation, method and system for the topical, transdermal or transmucosal administration of a therapeutically effective active agent. Particularly, the invention provides a formulation, system and method for enhancing the permeation or penetration of active agents across the dermal or mucosal surfaces of a mammalian subject. The formulation includes a plant extract of the genus Curcuma of the family Zingiberaceae, a germacrone, or a natural or synthetic constituent thereof, which has been found to increase penetration of the active agent across the dermal or mucosal surface. If desired, a secondary permeation enhancer of a polyalcohol, a monoalkyl ether of diethylene glycol, a tetraglycol, or a mixture thereof can be used for certain active agents for optimal permeation enhancement.
Owner:ANTARES PHARMA IPL
Sustained release delivery of one or more agents
InactiveUS20100209477A1Improve permeabilityReduce the amount requiredBiocideOrganic active ingredientsCross-linkExcipient
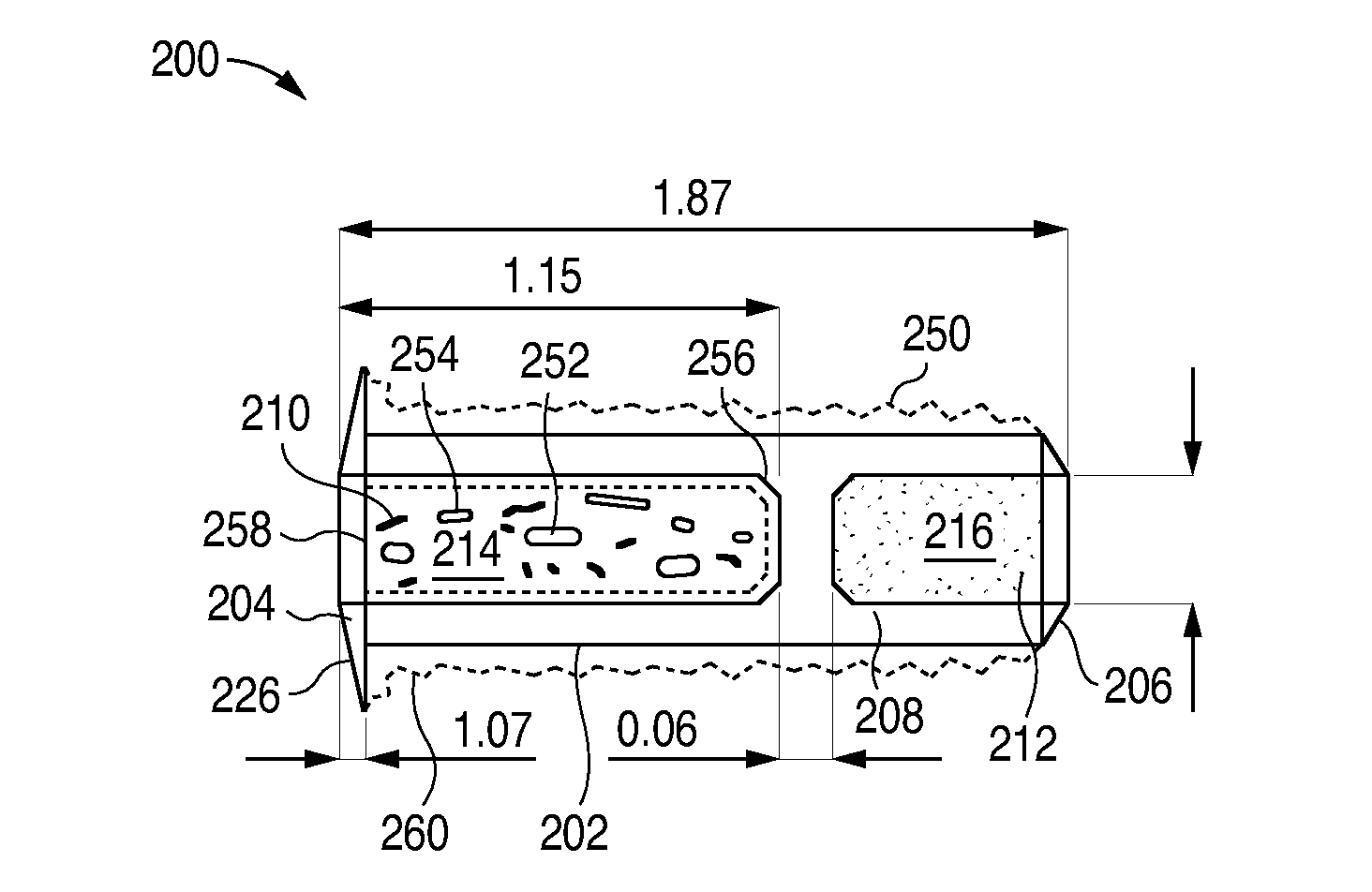
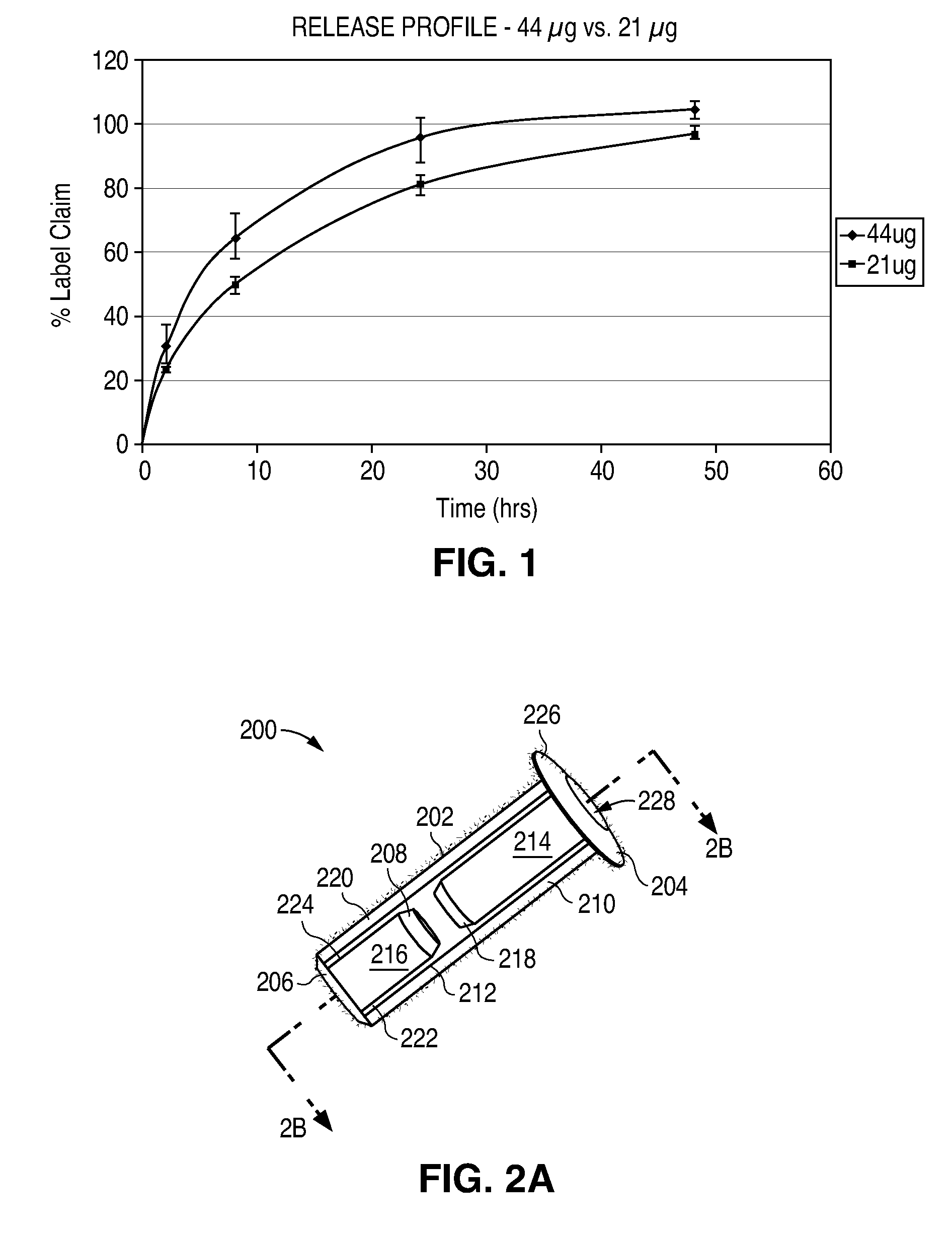
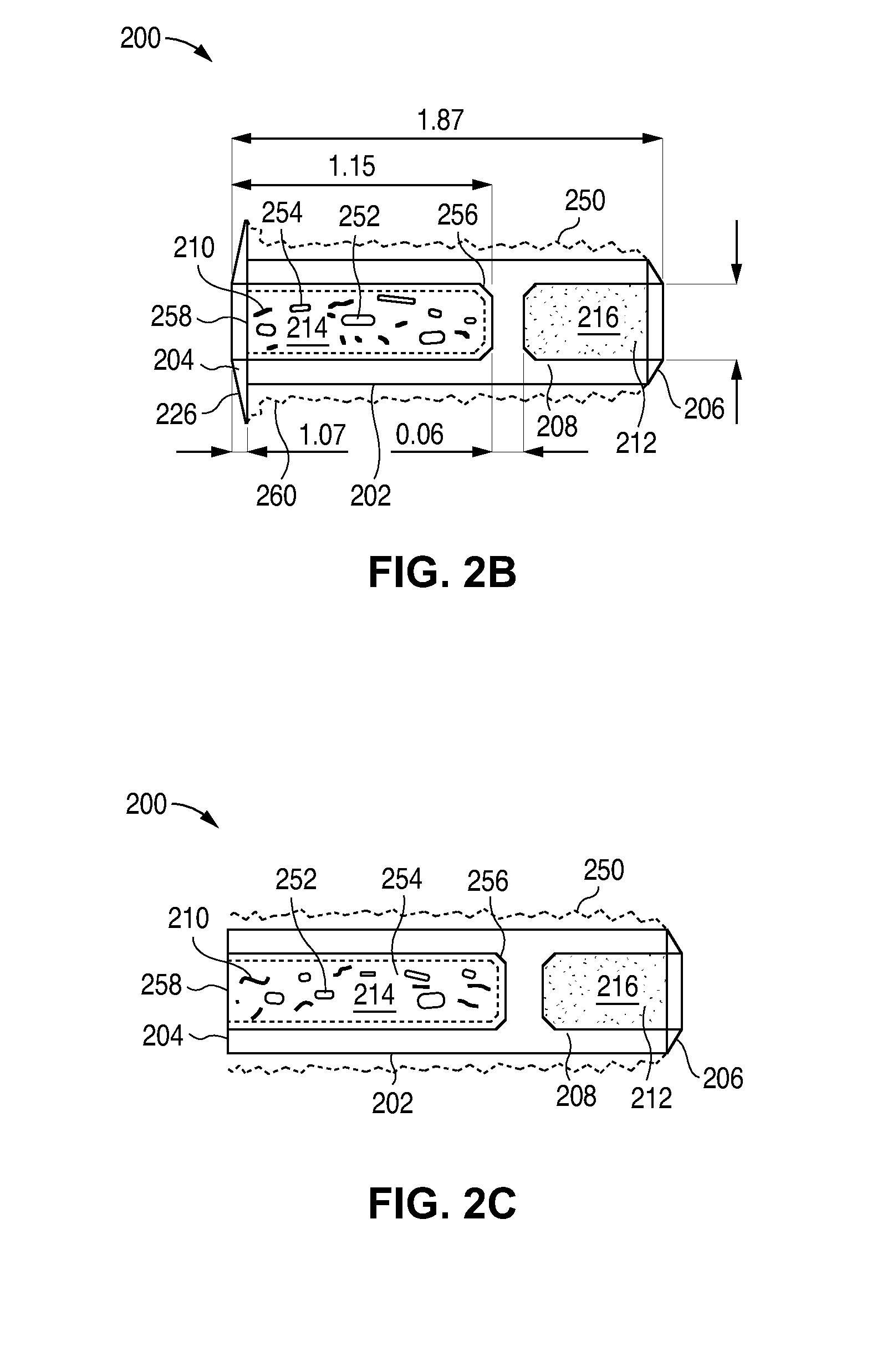
The lacrimal implant delivery systems and methods described herein provide for controlled release of a therapeutic agent for the treatment of disease, including the treatment of glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agents. Treatment of disease, including glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agent in conjunction with penetration enhancer, such as benzalkonium chloride, and / or artificial tears is also provided. Also provided are implants containing a drug core emplacable in a punctum adjacent to an eye of a patient for controlled release of a therapeutic agent such as latanoprost for the treatment of glaucoma, the drug core containing a polymer such as cross-linked silicone, a therapeutic agent, and an excipient, wherein the excipient can increase the rate of release of the agent from the drug core, or can increase the drug loading in the core without loss of desirable homogeneity of the agent within the core, or can improve retention of the agent in the eye or in tear fluid, or can increase corneal penetration of the agent into the eye.
Owner:MATI THERAPEUTICS
Foamable Compositions and Kits Comprising One or More of a Channel Agent, a Cholinergic Agent, A nitric Oxide Donor and Related Agents and Their Uses
InactiveUS20150025060A1Efficient deliveryMinimizing penetrationBiocideAerosol deliveryDiseaseActive agent
Owner:FOAMIX PHARMACEUTICALS LIMITED
Tympanic membrane permeating ear drops and uses thereof
ActiveUS20110166060A1Maximizes exposure and concentrationImprove throughputBiocideSenses disorderMedicineAntibiotic Y
The present invention provides compositions and methods for noninvasive delivery of therapeutic agents across an intact tympanic membrane. For example, the compositions include a penetration enhancer which increases the flux of a therapeutic agent (e.g., antibiotic) across the tympanic membrane. Such compositions are particularly useful in the treatment of otitis media. Additionally, the composition may include a sustained release agents that, in some embodiments form sustained release reservoirs, in situ, once administered to a patient.
Owner:MASSACHUSETTS INST OF TECH +1
Compositions and methods for topical delivery of oligonucleotides
InactiveUS6841539B1Simpler and easy and effective deliverySimple and efficient and convenient deliveryOrganic active ingredientsTissue cultureCuticleLiposome
Owner:IONIS PHARMA INC
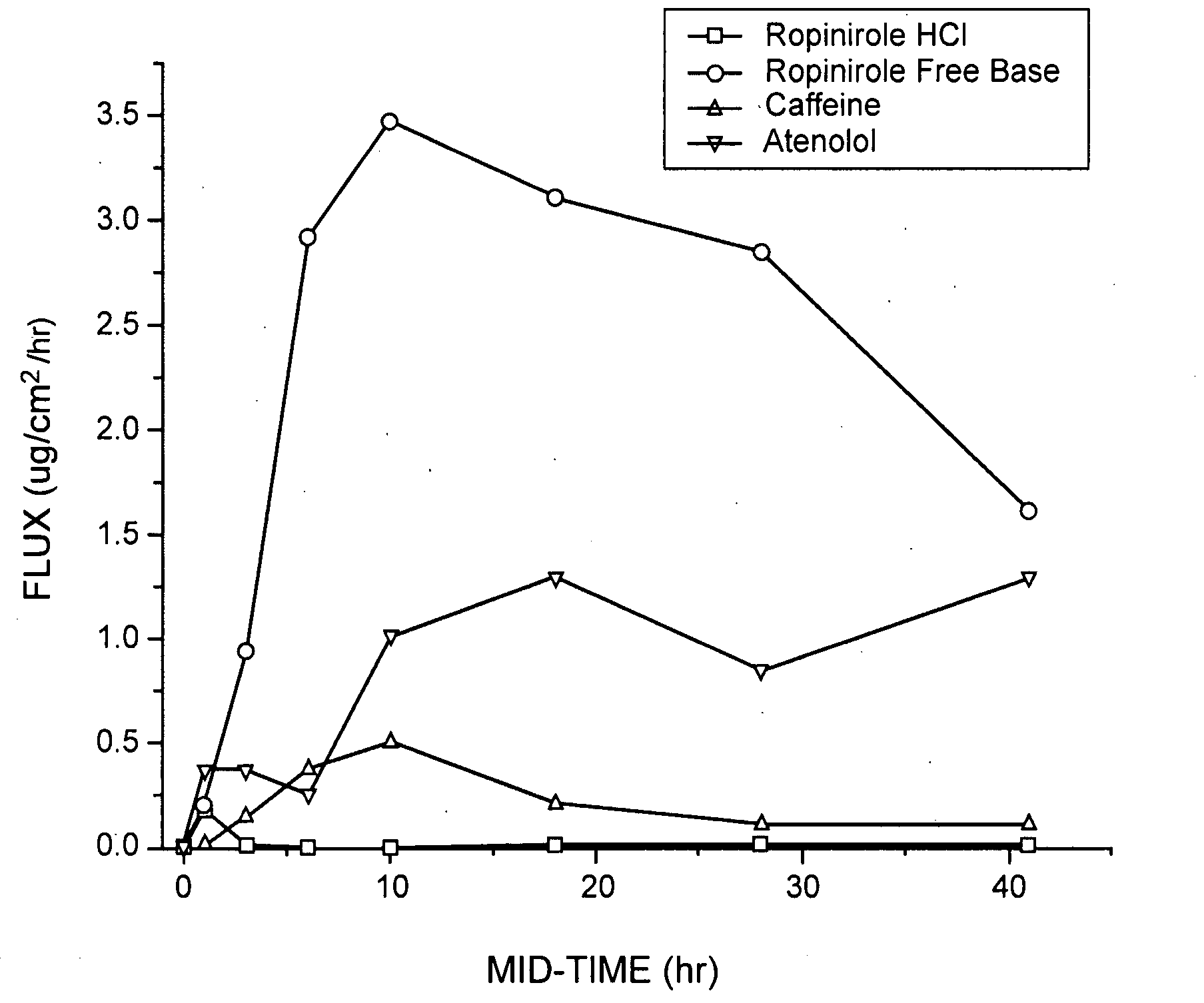
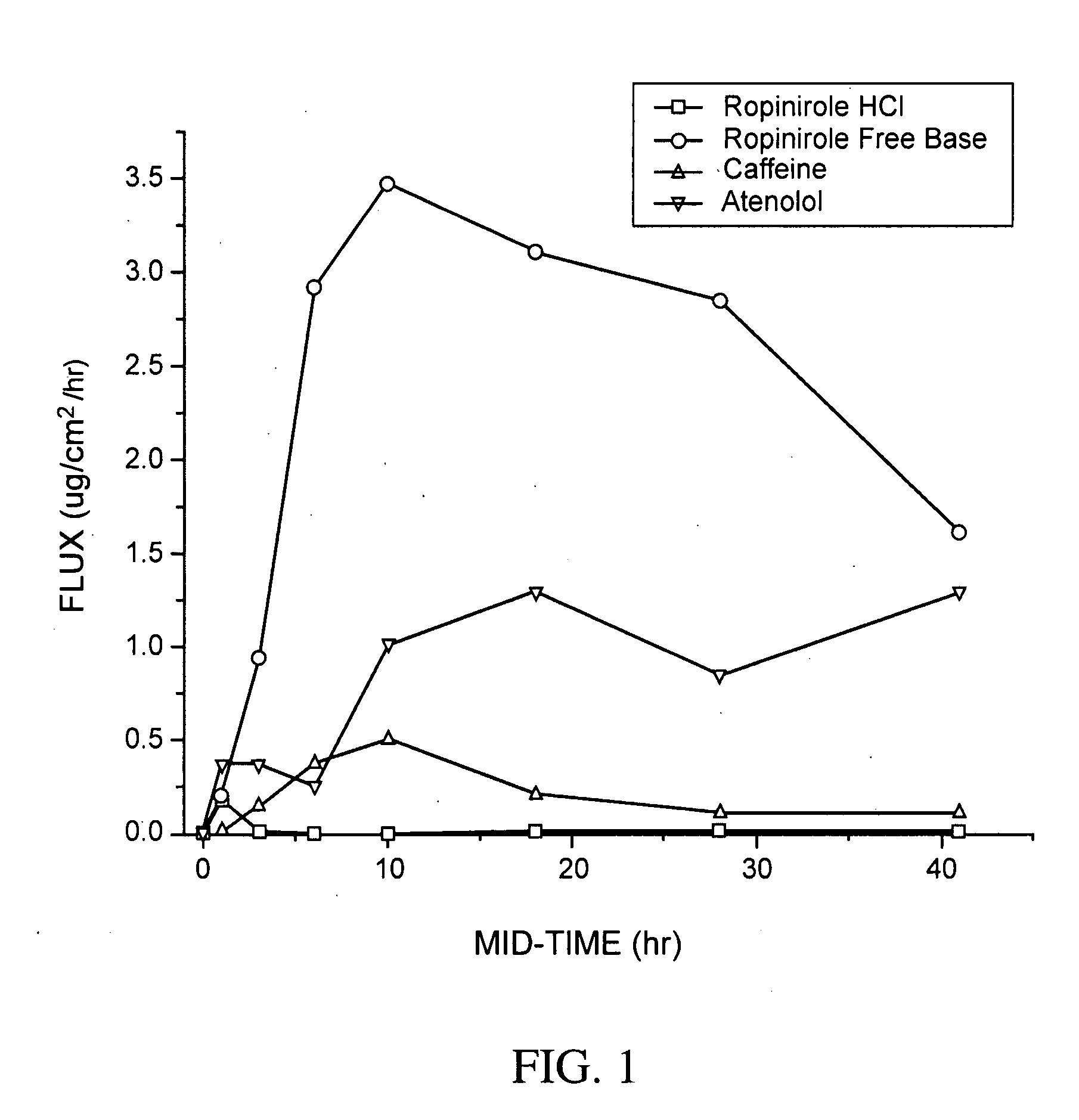
Pharmaceutical compositions of ropinirole and methods of use thereof
InactiveUS20080004329A1Easy to produceBiocideNervous disorderPharmaceutical drugPharmaceutical medicine
The present invention comprises compositions for pharmaceutical drug delivery of an indolone (e.g., ropinirole), or a pharmaceutically acceptable salt thereof. The composition may, for example, be a gel suitable for transdermal application. The compositions of the present invention typically comprise a hydroalcoholic vehicle, one or more antioxidant, and one or more buffering agent, wherein the pH of the gel is usually between about pH 7 and about pH 9. The compositions may include further components, for example, the hydroalcoholic vehicle may further comprise additional solvent(s), antioxidant(s), cosolvent(s), penetration enhancer(s), buffering agent(s), and / or gelling agent(s). The compositions may be used for the treatment of a variety of neurological disorders.
Owner:JAZZ PHARMA
Systems and methods for delivery of biologically active agents
InactiveUS20100196445A1Efficient deliveryPeptide/protein ingredientsMicroneedlesCell-Extracellular MatrixActive agent
The invention provides methods and devices for the delivery of therapeutic or biologically active agents to tissue, for example by facilitating the transport of said agents through the skin of a human or animal. Therapeutic agents include various botulinum neurotoxin (BoNT) and other biologically active agents, which can be delivered across the skin and into the dermis using multiple strategies including microneedle drug delivery, transport moieties, or penetration enhancers such as extracellular matrix-digestive enzymes.
Owner:THE SCRIPPS RES INST +1
Highly plastic granules for making fast melting tablets
A fast-melting pharmaceutical tablet comprises a porous, plastic substance, a water penetration enhancer and a binder. One or more drugs can be incorporated into the formulation at different stages of the process so as to afford a pharmaceutically active tablet. Methods of making the pharmaceutical tablet entail combining the porous, plastic material, the water penetration enhancing agent, and the binder so as to form highly plastic granules, which are compressed into tablets. The resulting tablets dissolve rapidly in the mouth and have good hardness with low brittleness. The tablets are particularly valuable to those who have difficulty swallowing conventional pills.
Owner:AKINA INC
Transdermal delivery rate control using amorphous pharmaceutical compositions
InactiveUS20050175680A1High propensity toward skin irritationDecrease in percutaneous absorption efficiencyOrganic active ingredientsNervous disorderMedicineActive agent
A pharmaceutical composition for transdermal delivery comprising one or more physiologically active agents; one or more dermal penetration enhancers; and a volatile pharmaceutically acceptable carrier comprising a volatile solvent; and wherein the physiologically active agent and dermal penetration enhancer form an amorphous deposit upon evaporation of the volatile carrier, said amorphous deposit forming a reservoir within the stratum corneum; and (A) wherein the composition has a release rate profile of physiologically active agent so as to provide a ratio of the maximum concentration (Cmax) to the average concentration (Cavg) for the physiologically active agent over the dosage interval within the range of 1 to 10.
Owner:ACRUX DDS
Anti-wrinkle composition
ActiveUS20070166267A1Easy to superviseAccurate balanceBiocideOrganic active ingredientsWrinkle skinDimethyl isosorbide
A composition is disclosed for treating the skin comprising an acylated short chain bioactive peptide and Lycium barbarum extract product. Also disclosed is a method for topically administering the composition in an amount therapeutically effective to reduce wrinkles by building the dermal fibroblast matrix. The composition may contain dimethylisosorbide or ethoxydiglycol as solubilizing and penetration enhancers for the hydrophobically modified peptide.
Owner:GRANT INDS
Transdermal delivery of hormones without the need of penetration enhancers
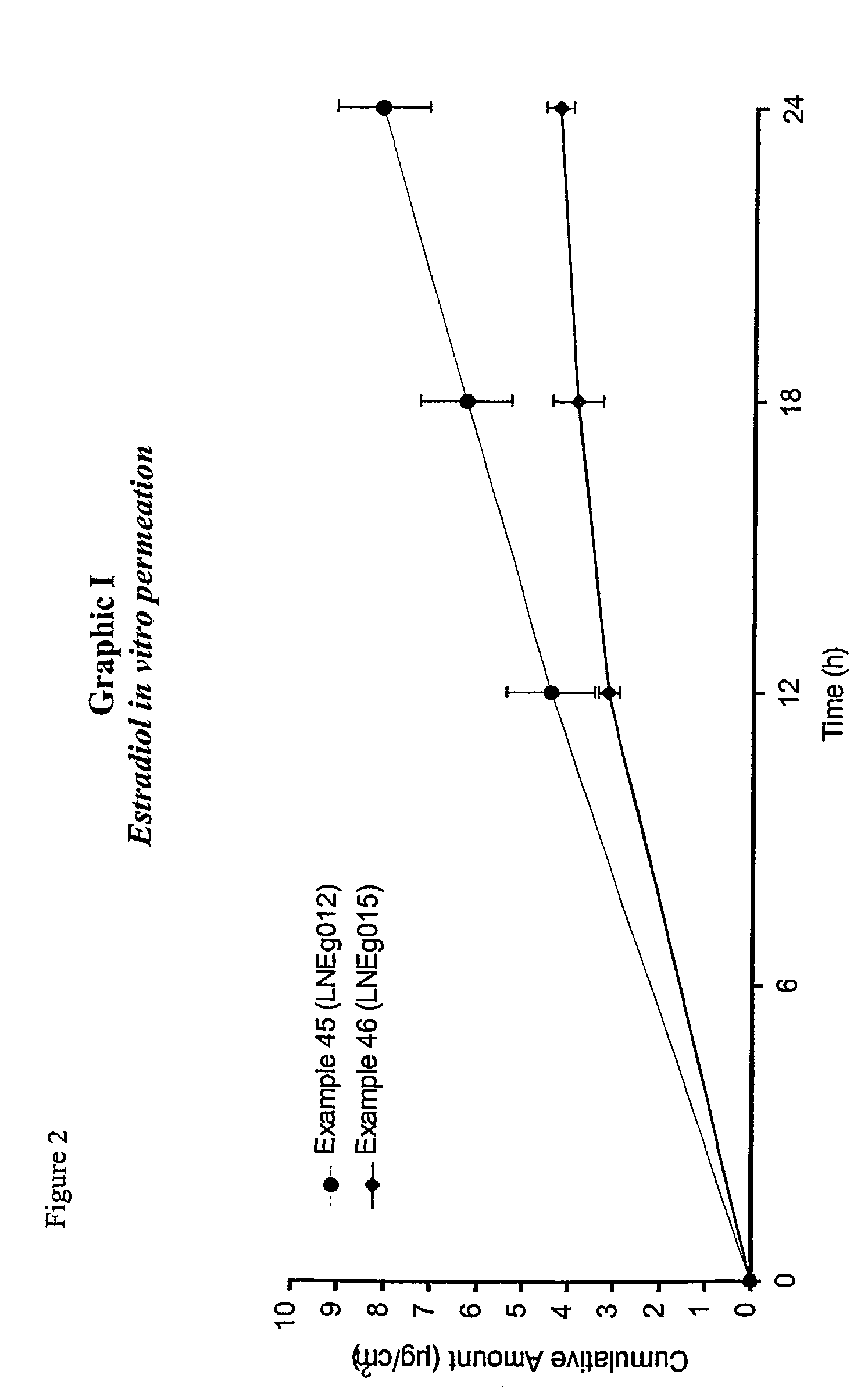
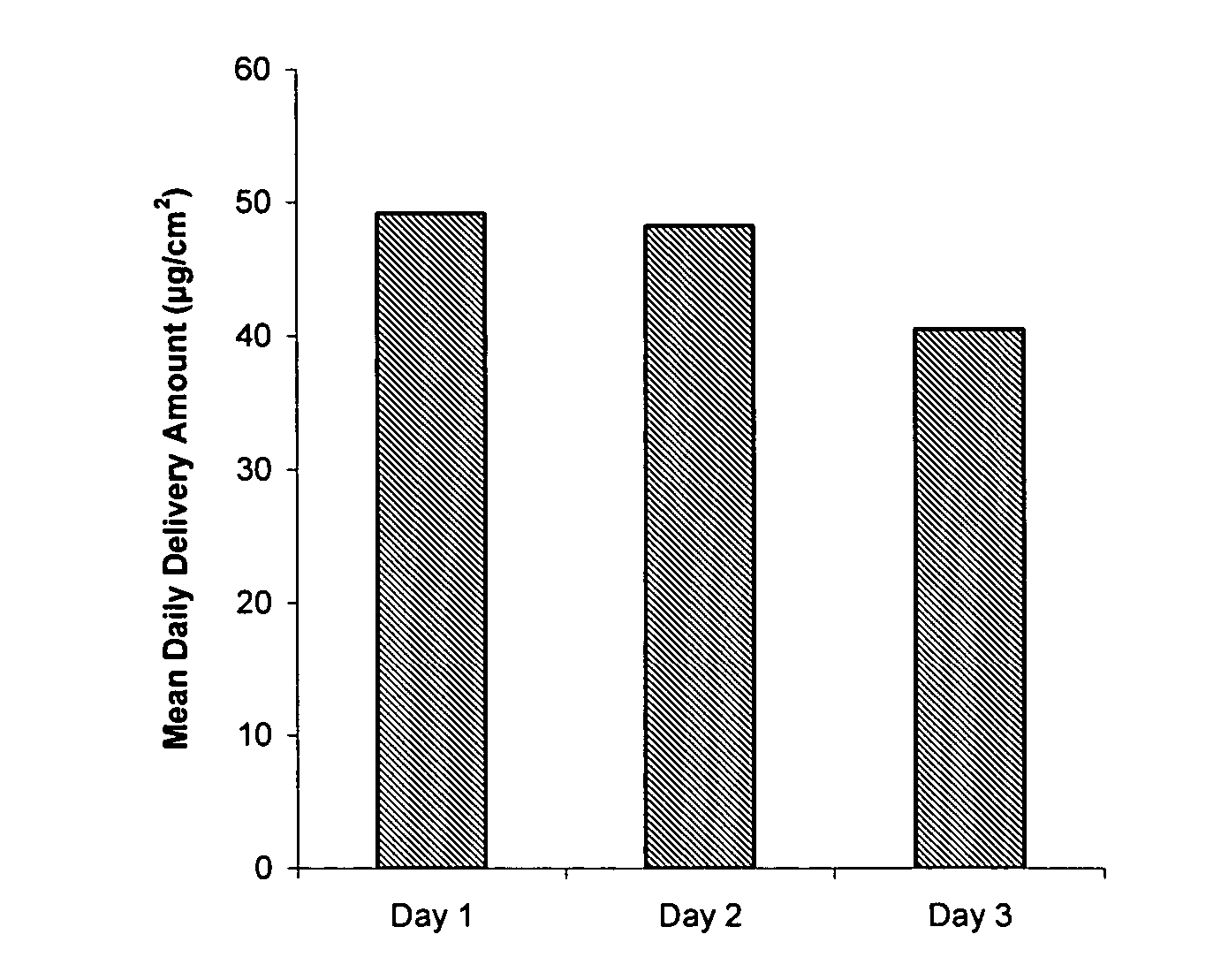
ActiveUS20050142175A1High plasma levelInhibit ovulationBiocideOrganic active ingredientsEthinyl oestradiolHormones regulation
The present invention relates to a patch comprising a drug-containing layer with low content of hormones, such as gestodene, and optionally an estrogen (e.g. ethinyl estradiol). Upon administering the patch to a woman, plasma levels of at least 1.0 ng / ml of Gestodene is achieved at steady state conditions without the need of incorporating penetration enhancers or permeation enhancers in the drug-containing layer. Satisfactorily plasma levels of the hormones is also achieved throughout a period of at least 1 week, making the patch applicable for being used in female contraception with the concept of administering the patch ones weekly.
Owner:LUYE PHARMA SWITZERLAND AG
Prostate hypertrophy treatment composition and method
InactiveUS20050271597A1Improve permeabilityHigh permeation rate prevents undesirable modifications of the hormone within the skinOrganic active ingredientsPowder deliveryPhysiologyProgesterones
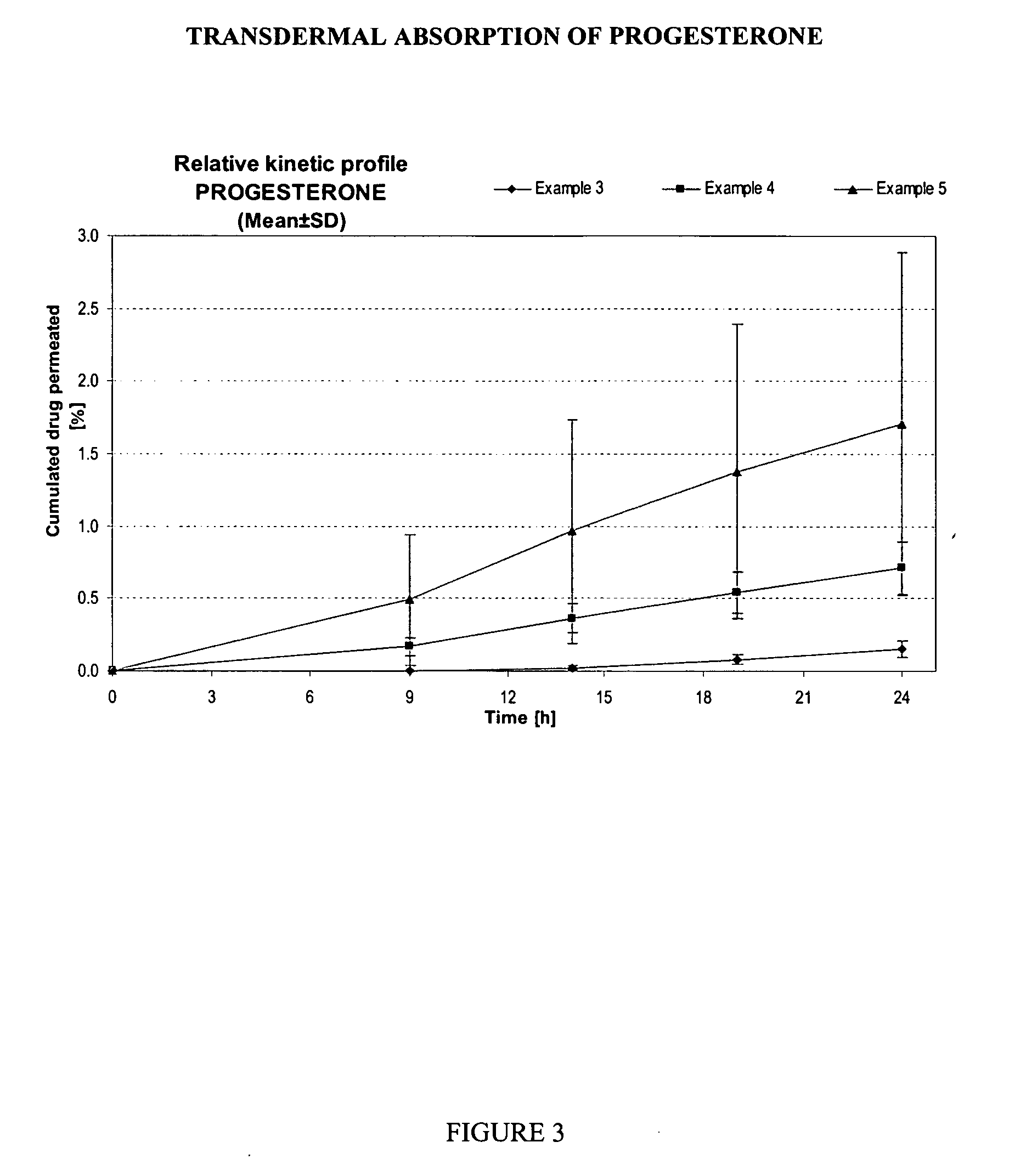
The present invention provides a method and composition for treatment of benign prostate hyperplasia (BPH) in men via a transscrotal delivery system. The composition of the present invention includes the steroid hormone progesterone containing permeation enhancers that greatly facilitate permeation through the skin, thus preventing modification of the constituents therein and providing continuous and sustained delivery of progesterone for several hours that mimics the circadian rhythm of endogenous progesterone. The progesterone composition preferably is capable of delivering an effective dosage amount of about 65-100 mg of progesterone per ml when applied directly onto the surface of scrotum.
Owner:KEITH ALEC D
Composition for transdermal and/or transmucosal administration of active compounds that ensures adequate therapeutic levels
InactiveUS7214381B2Easy to usePromote absorptionOrganic active ingredientsGogglesActive agentTreatment level
The present invention refers to a pharmaceutical composition suitable for the transdermal or transmucosal administration of one or more active agents, in form of a gel or a solution, comprising as a permeation enhancers a combination of: a) saturated fatty alcohol of formula CH3—(CH2)n—CH2OH or saturated fatty acid CH3—(CH2)n—CH2COOH wherein n is an integer number 8÷22, preferably 8÷12, most preferably 10, or unsaturated fatty alcohol or fatty acid of formula: CH3(CnH2(n-1))—OH or CH3(CnH2(n-1))—COOH wherein n is an integer number 8÷22, b) a ternary vehicle or carrier consisting of a C1÷C4 alkanol, a polyalcohol in particular propylenglycol and water, c) optionally also a monoalkylether of diethylenglycol.
Owner:ANTARES PHARMA IPL
Transdermal delivery system for alkaloids of aconitum species
InactiveUS20050042271A1Long lasting potencyMinor side effectsBiocideAerosol deliveryBlood plasmaAnalgesic agents
The present invention provides a composition of transdermally administered alkaloids from aconitum plant for ameliorating pain and inflammation. In one aspect, an aconitum alkaloid is delivered in a sufficient amount to achieve and maintain a blood plasma aconitum alkaloid level of about 0.5 ng / mL to about 400 ng / mL. Aconitum alkaloids may be delivered by themselves, or in combination with other elements, such as additional analgesics, other drugs, or positive health promoting substances. Various formulations for the transdermal delivery of aconitum alkaloids are disclosed, and may include selected penetration enhancers.
Owner:XEL HERBACEUTICALS INC
Pharmaceutical composition and method for treating hypogonadism
InactiveUS20050113353A1High densityIncrease libidoElcosanoid active ingredientsOintment deliveryAnabolic steroidAlcohol
A pharmaceutical composition useful for treating hypogonadism is disclosed. The composition comprises an androgenic or anabolic steroid, a C1-C4 alcohol, a penetration enhancer such as isopropyl myristate, and water. Also disclosed is a method for treating hypogonadism utilizing the composition.
Owner:UNIMED PHARMA LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com