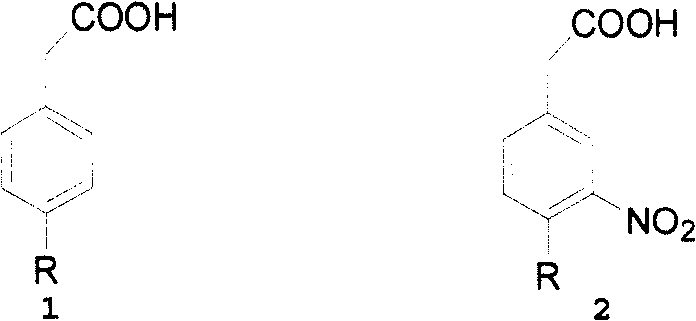Patents
Literature
48 results about "Ropinirole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used alone or with other medications to treat Parkinson's disease.
Pharmaceutical compositions of ropinirole and methods of use thereof
InactiveUS20080004329A1Easy to produceBiocideNervous disorderPharmaceutical drugPharmaceutical medicine
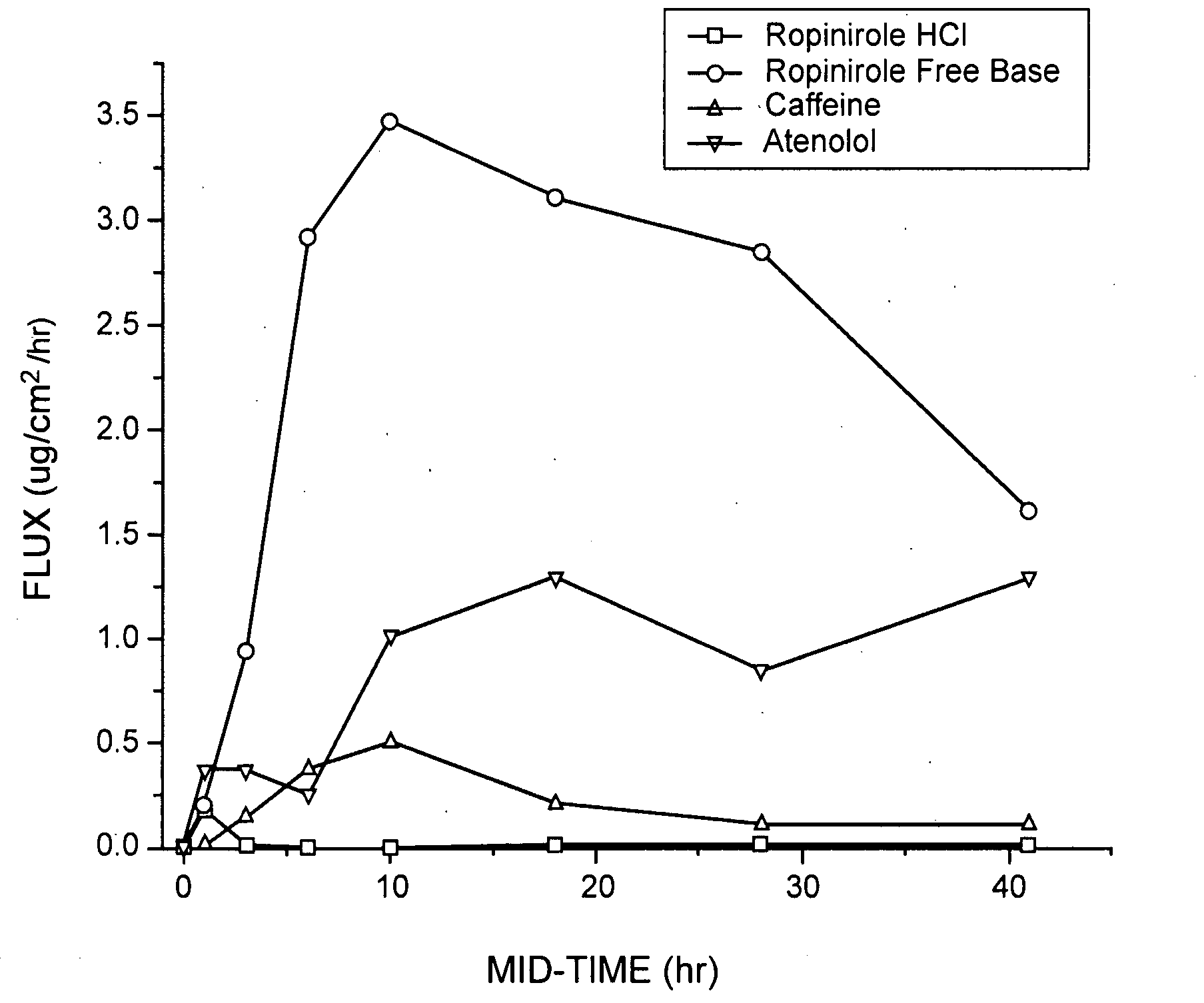
The present invention comprises compositions for pharmaceutical drug delivery of an indolone (e.g., ropinirole), or a pharmaceutically acceptable salt thereof. The composition may, for example, be a gel suitable for transdermal application. The compositions of the present invention typically comprise a hydroalcoholic vehicle, one or more antioxidant, and one or more buffering agent, wherein the pH of the gel is usually between about pH 7 and about pH 9. The compositions may include further components, for example, the hydroalcoholic vehicle may further comprise additional solvent(s), antioxidant(s), cosolvent(s), penetration enhancer(s), buffering agent(s), and / or gelling agent(s). The compositions may be used for the treatment of a variety of neurological disorders.
Owner:JAZZ PHARMA
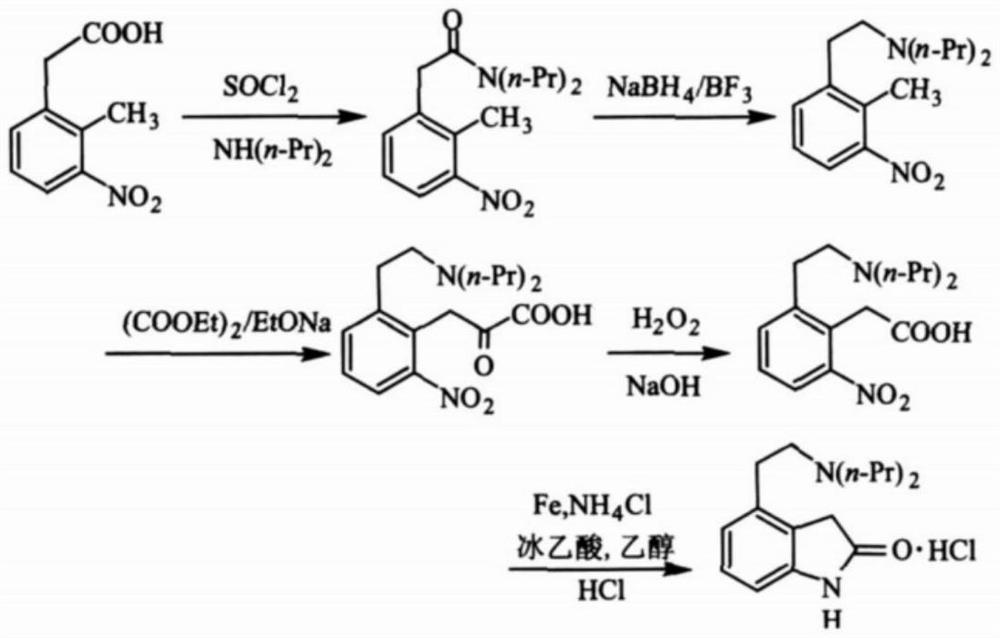
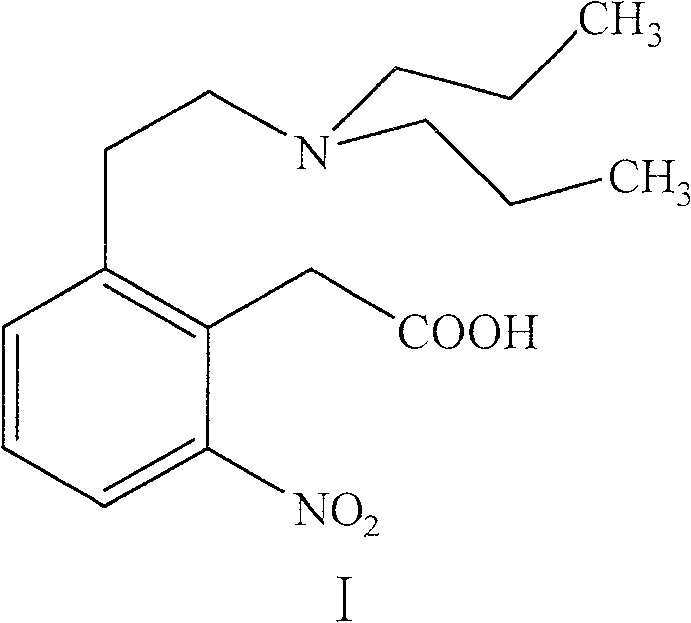
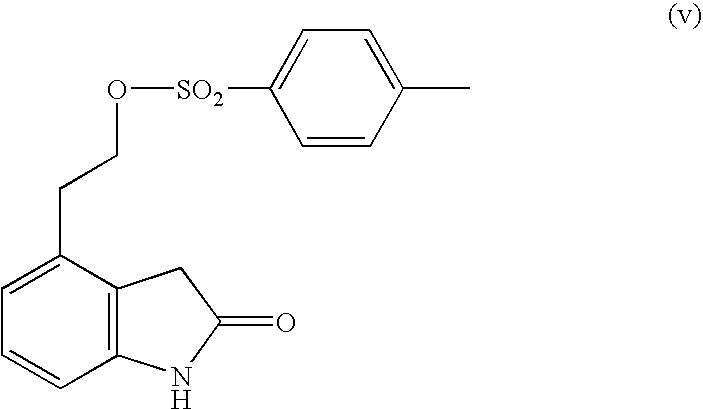
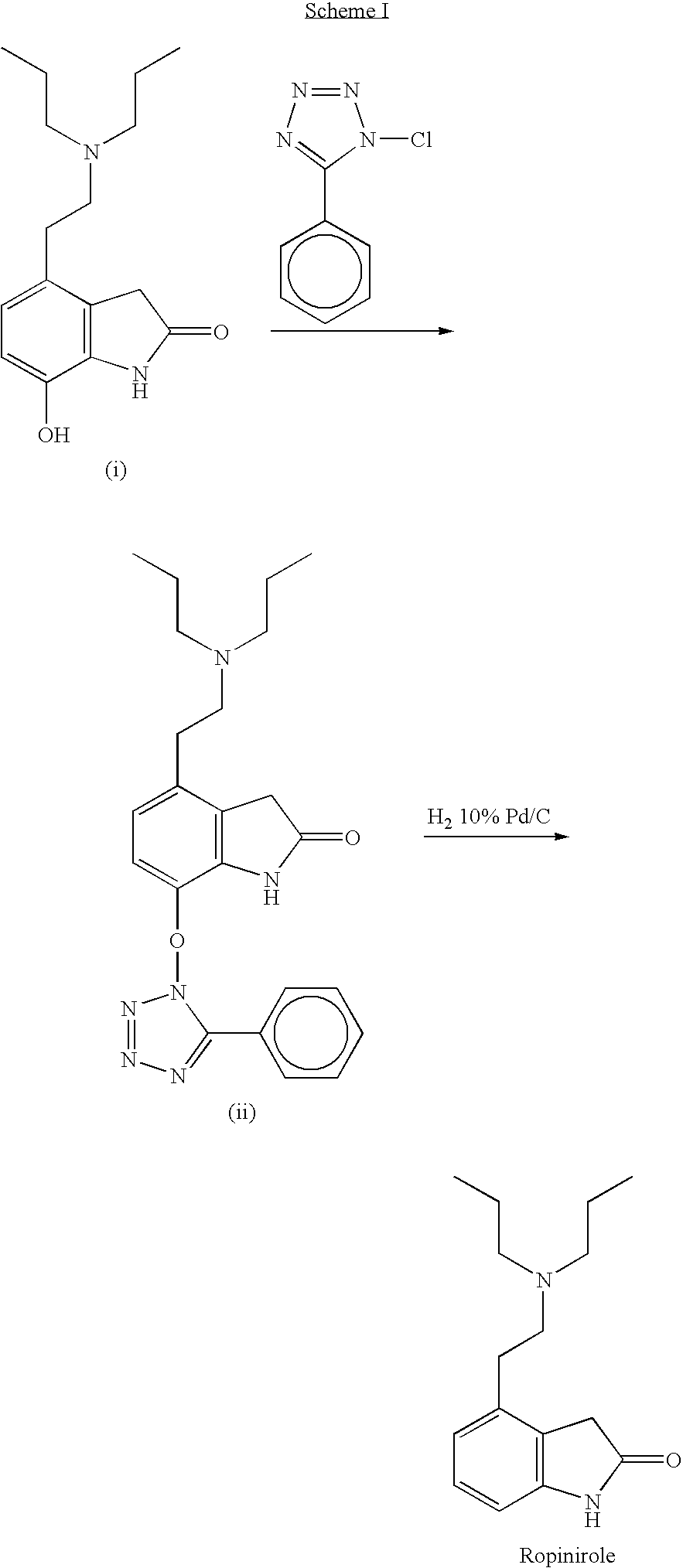
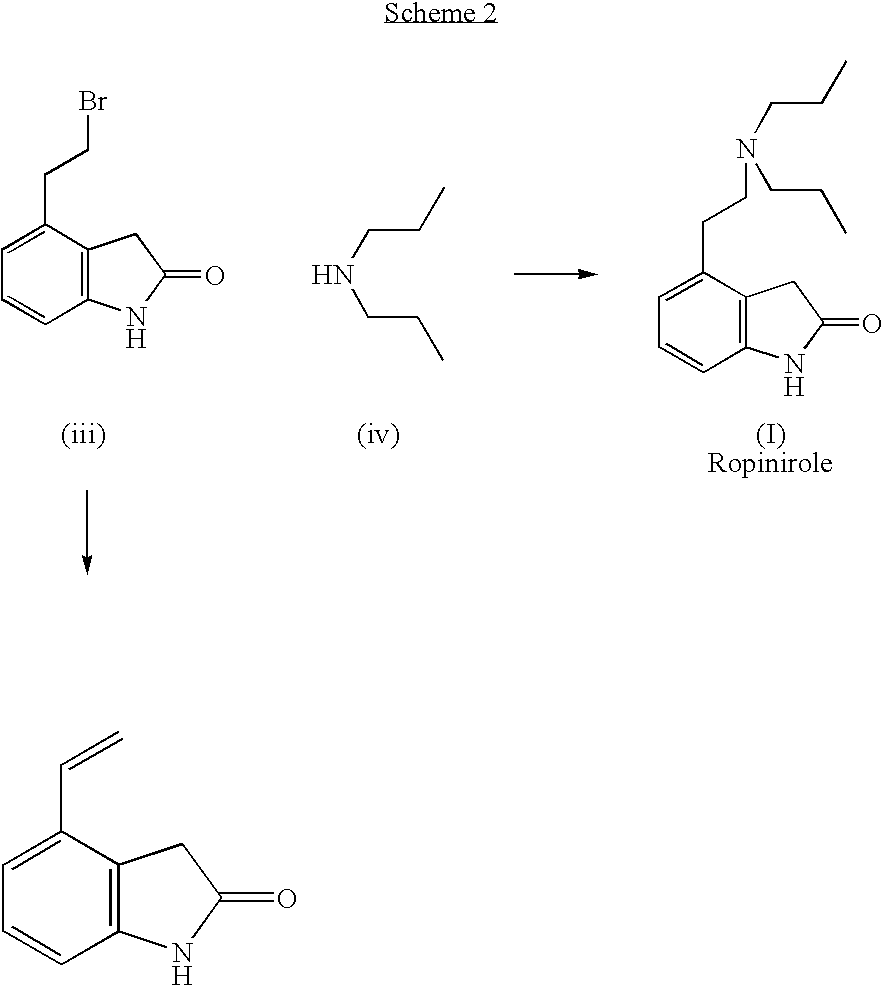
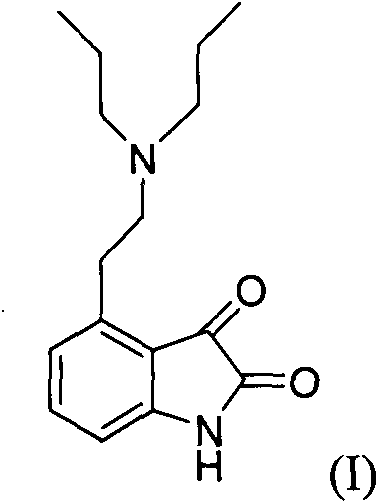
Process for the preparation of 4-(2-dipropylaminoethyl)-1,3-dihydro-2H-indol-2-one hydrochloride
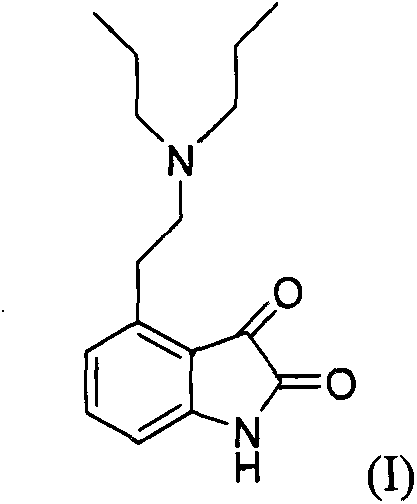
The present invention discloses a novel process and novel intermediates for the Preparation of 4-[2-(di-n-propyl amino) ethyl]-1,3-dihydro-2H-indol-2-one, commonly known as Ropinirole (I) and pharmaceutical composition comprising the same. Further the present invention also discloses a method of treatment for cardiovascular disorders and Parkinson's disease.
Owner:USV LTD
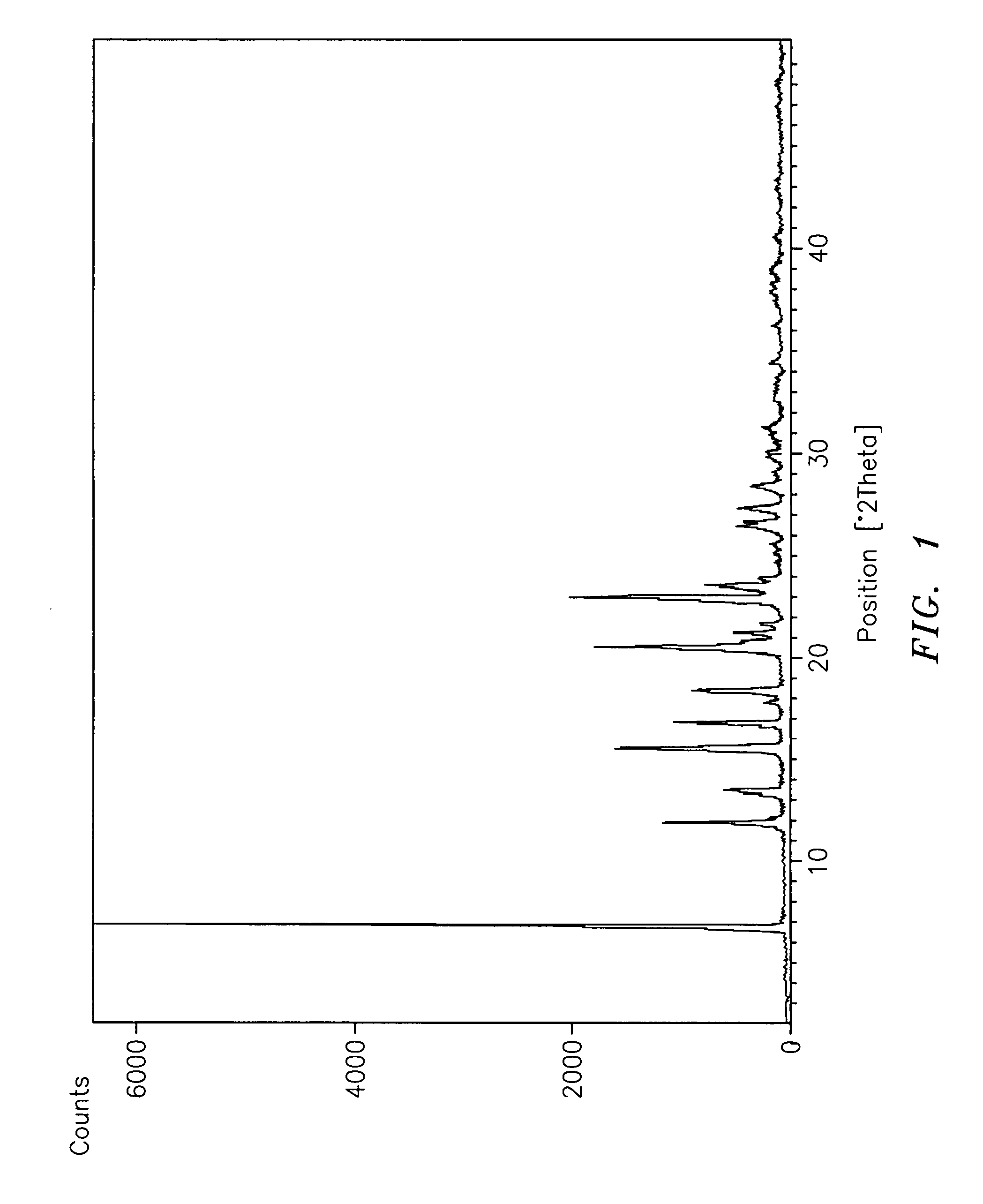
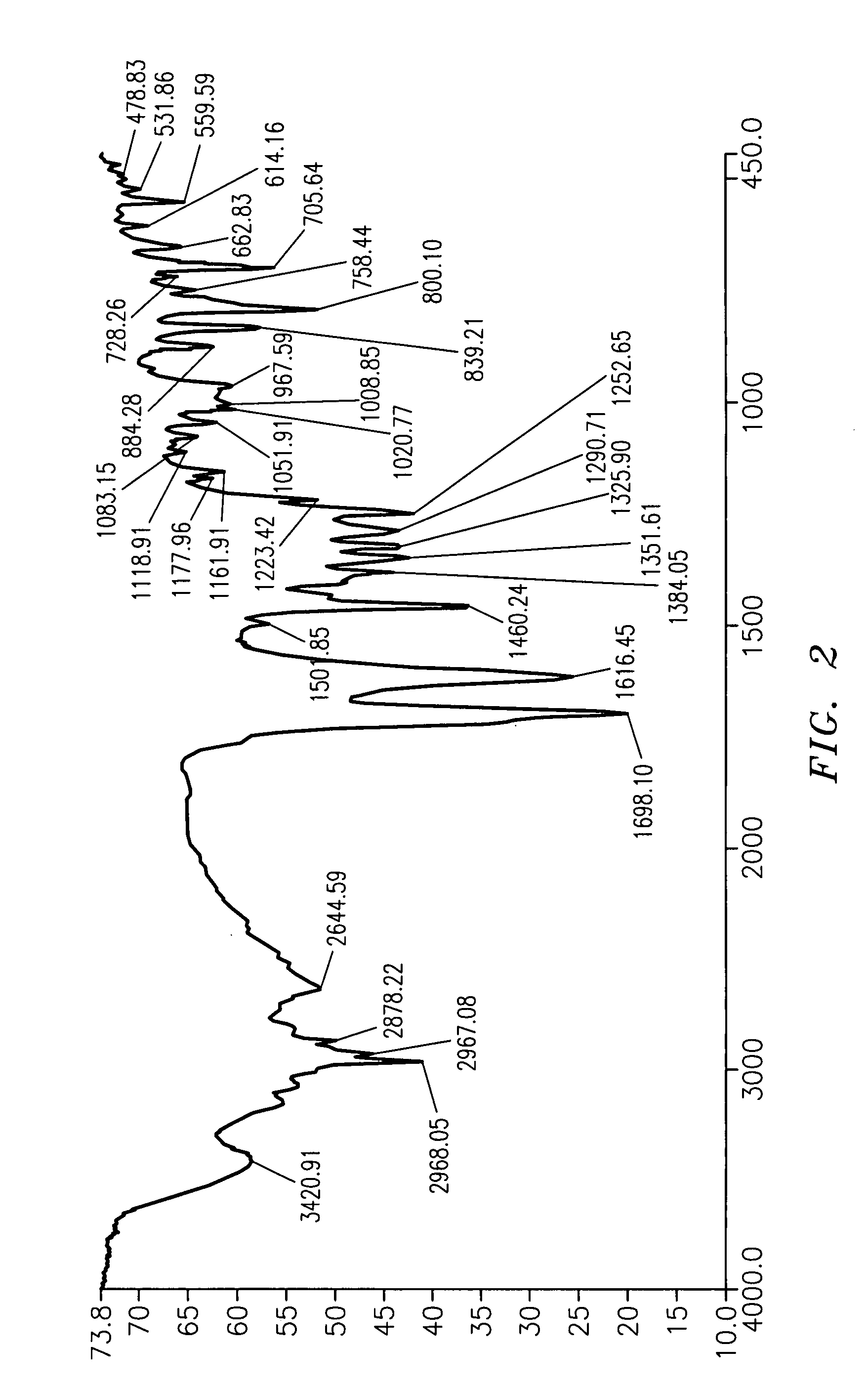
Subtantially pure ropinirole hydrochloride, polymorphic form of ropinirole and process for their preparation
InactiveUS20070254941A1Low costProcess easy and friendlyBiocideOrganic chemistryRopinirole HydrochlorideImpurity
Ropinirole hydrochloride substantially free of impurities and a process for its preparation is provided. Also provided is ropinirole base substantially in polymorph Form A and a process for its preparation. Pharmaceutical compositions containing the same are also provided.
Owner:GLENMARK GENERRICS LTD
Novel therapeutic method
InactiveUS20030060499A1Preserving dopaminergic functionReduce the populationBiocideAnimal repellantsMedicinePharmacology
A therapeutic method for preserving the dopaminergic function of patients suffering from Parkinson's disease, which method comprises administering an effective amount of ropinirole or a pharmaceutically acceptable salt or solvate thereof to a human or animal patient in need thereof. Typically, said patient has had Parkinson's disease for a period of less than three years since diagnosis. Preferably the invention comprises administering to said patient an effective amount of ropinirole or a pharmaceutically acceptable salt or solvate thereof, optionally in combination with one or more other dopamine agonists, in the absence of levodopa or any other dopamine precursor, and thereafter treating the patient with levodopa.
Owner:TULLOCH IAN FREDERIC
Process for the preparation of 4-(2-dipropylaminoethyl)-1,3-dihydro-2H-indol-2-one hydrochloride
The present invention discloses a novel process and novel intermediates for the Preparation of 4-[2-(di-n-propyl amino) ethyl]-1,3-dihydro-2H-indol-2-one, commonly known as Ropinirole (I) and pharmaceutical composition comprising the same. Further the present invention also discloses a method of treatment for cardiovascular disorders and Parkinson's disease.
Owner:USV LTD
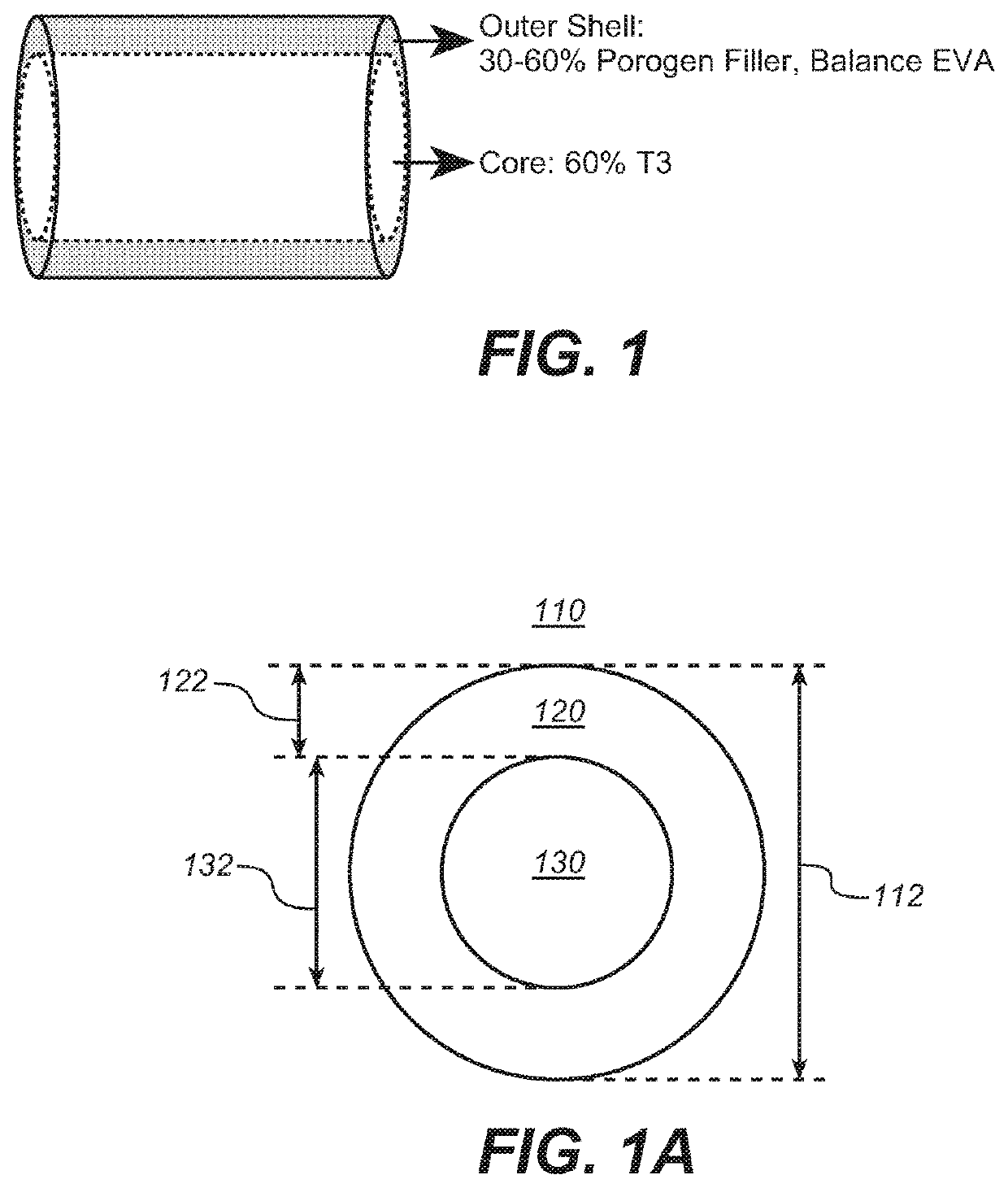
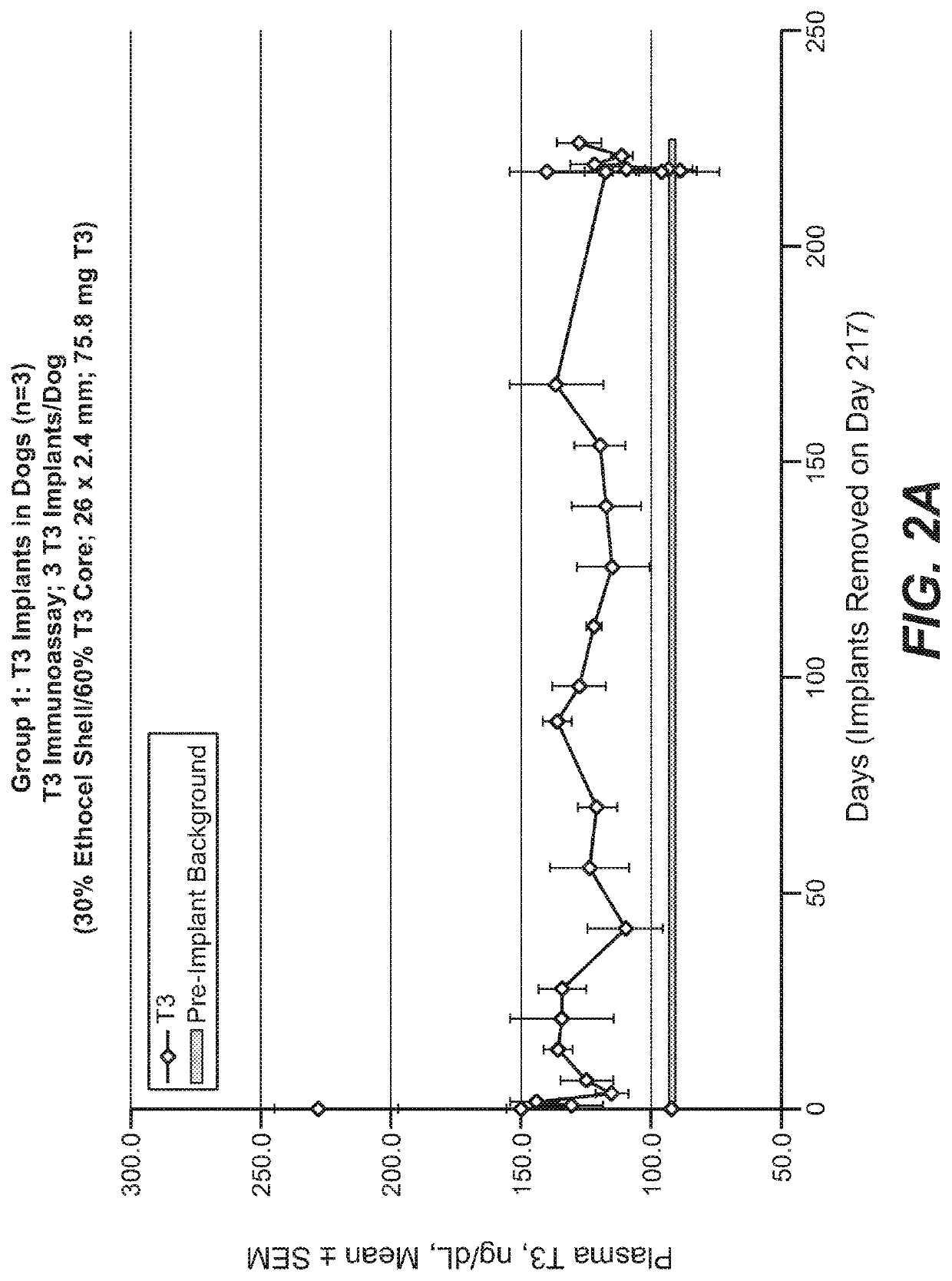
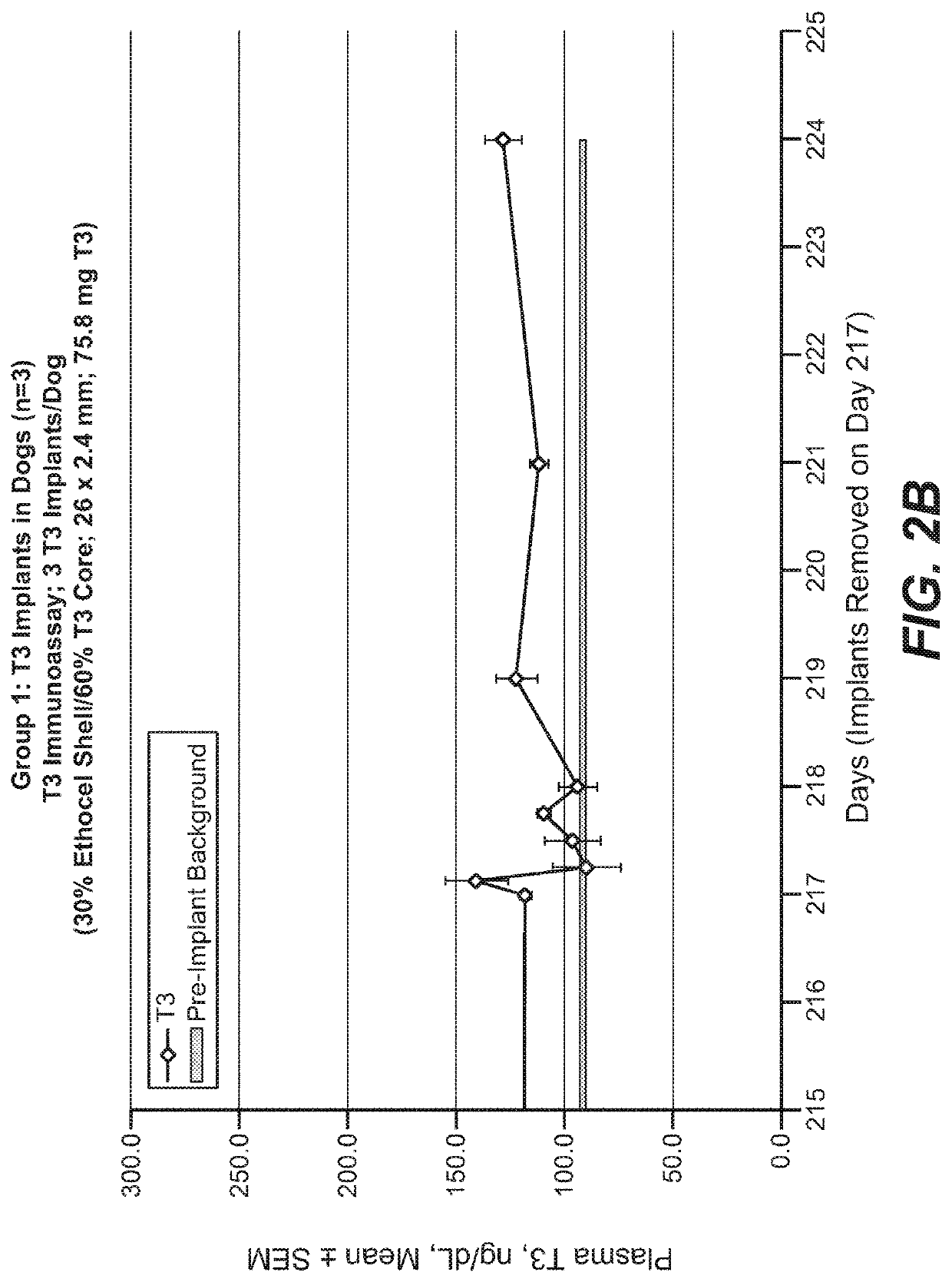
Implantable devices for drug delivery with reduced burst release
InactiveUS20210007973A1Good control and tuning of release rateReduce releasePowder deliveryNervous disorderBiochemistryPharmaceutical Substances
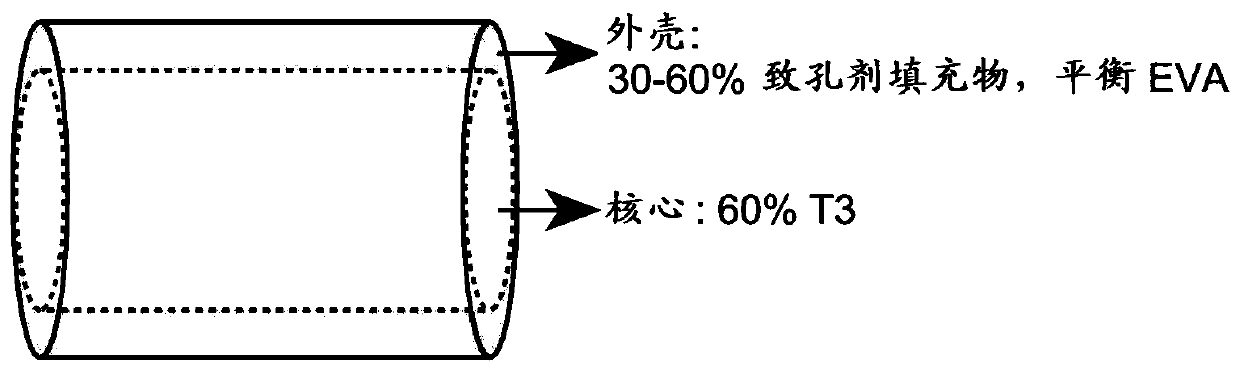
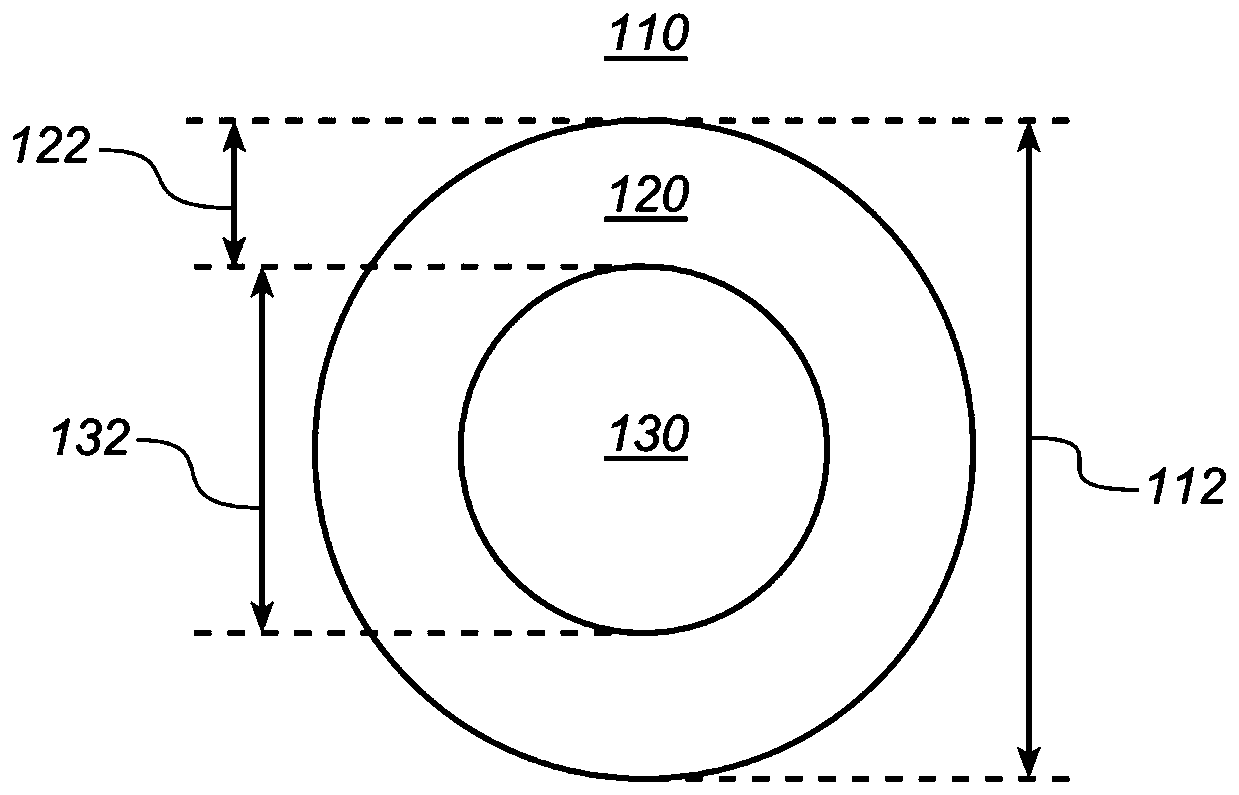
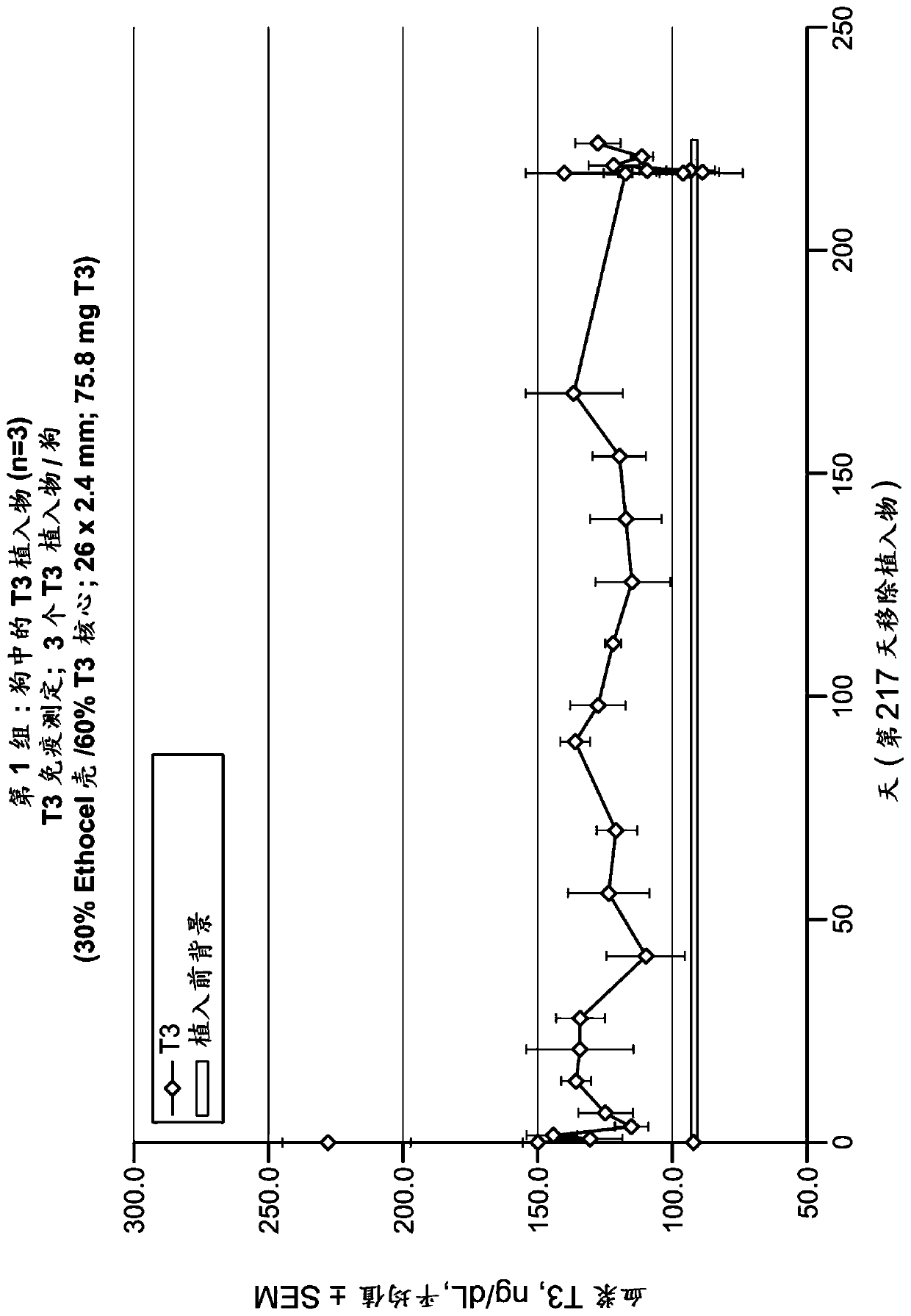
The invention provides implantable drug delivery devices comprising a core comprising a polymer (or polymer blend) and one or more drugs or pharmaceutical substances, and an outer shell comprising a polymer (or polymer blend) and one or more porogen materials. The invention reduces burst release of drug. Pharmaceuticals such as triiodothyronine (T3) or ropinirole can be delivered by the devices.
Owner:FEDSON INC
Transdermal absorption preparation
InactiveUS20130165875A1Improve skinImprove permeabilityOrganic active ingredientsNervous disorderSide effectSkin penetration
The present, invention relates to a transdermal absorption preparation. The transdermal absorption preparation of the present invention comprises a drug-containing adhesive layer, a drug-protective layer and a release layer, wherein the drug-containing adhesive layer contains ropinirole or a salt thereof, rubber, an adhesion-imparting resin, an antioxidant, and a transdermal absorption promoter. Also, the present invention provides a transdermal absorption preparation comprising a drug-containing adhesive layer, a drug-protective layer, and a release layer, wherein the drug-containing adhesive layer contains ropinirole or a salt thereof, acrylic rubber, an anti-crystallisation agent, and a transdermal absorption promoter. When the transdermal absorption preparation of the present invention is used for the treatment of Parkinson's disease or restless leg syndrome, no side effect due to an increase in the initial drug concentration in blood occurs and the skin penetration effect of the drug is excellent.
Owner:ICURE PHARML CORP
Osmotic pumping sustained preparation of ropinirole hydrochloride and its preparing method
InactiveCN1712002AReduce stimulationThe effect of small individual differencesOrganic active ingredientsRopinirole HydrochlorideDrug release
The invention discloses a ropinirole hydrochloride osmotic pump type controlled release preparation and a preparation method thereof. The osmotic pump controlled release preparation consists of three parts: tablet core, semipermeable membrane and drug release hole. The tablet core is composed of ropinirole, osmotic pressure enhancer and other excipients, the semipermeable membrane contains film-forming materials and plasticizers, and the hole-making methods include mechanical drilling and laser drilling. Through the joint application of osmotic pressure enhancers and the adjustment of semi-permeable membrane parameters, the drug release rate can be controlled. After oral administration, the ropinirole osmotic pump-type controlled-release tablet can release the drug continuously for 24 hours or longer, which is more conducive to the clinical treatment of Parkinson's patients and is convenient for patients to take the drug.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
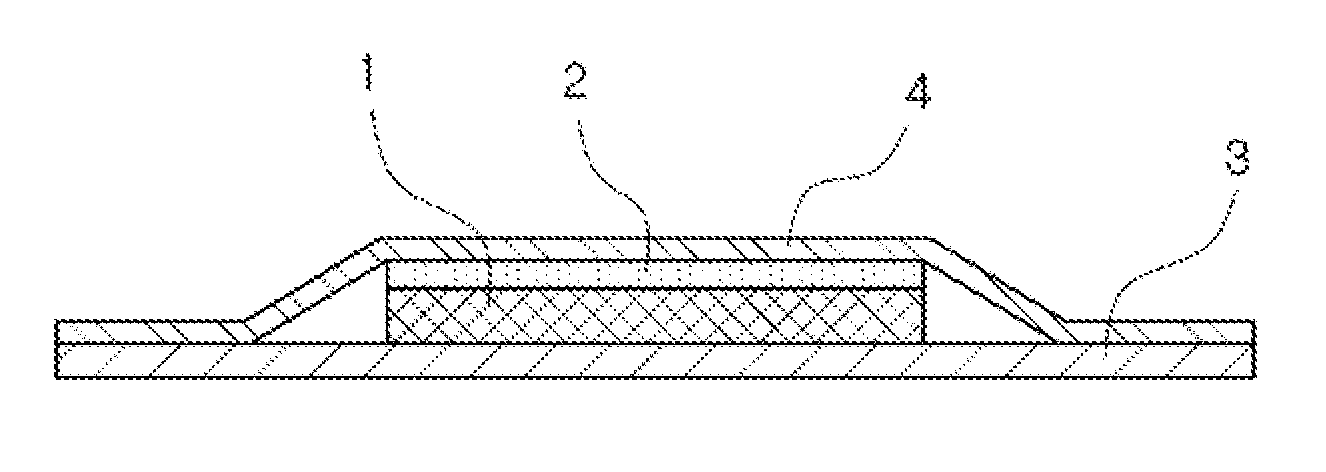
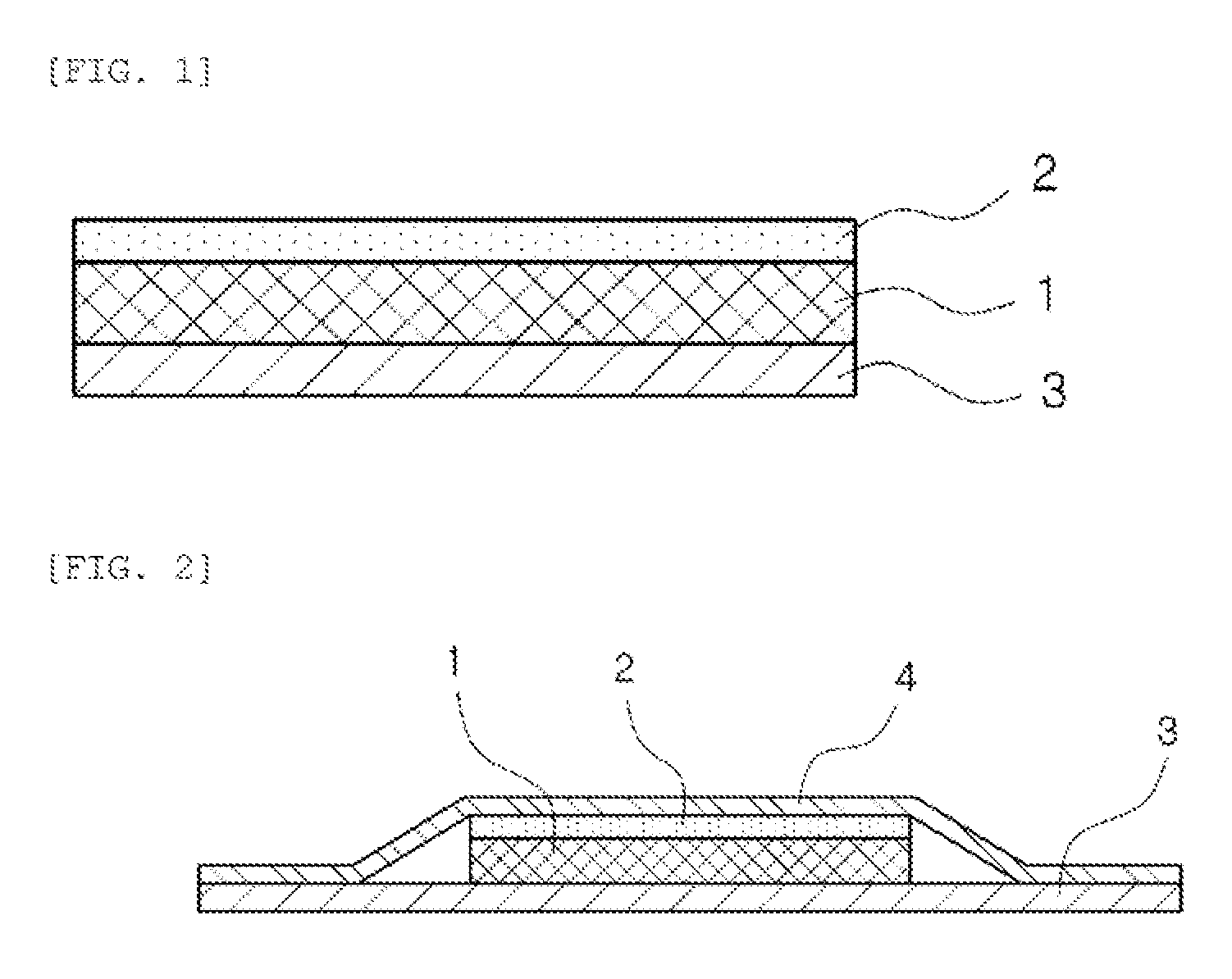
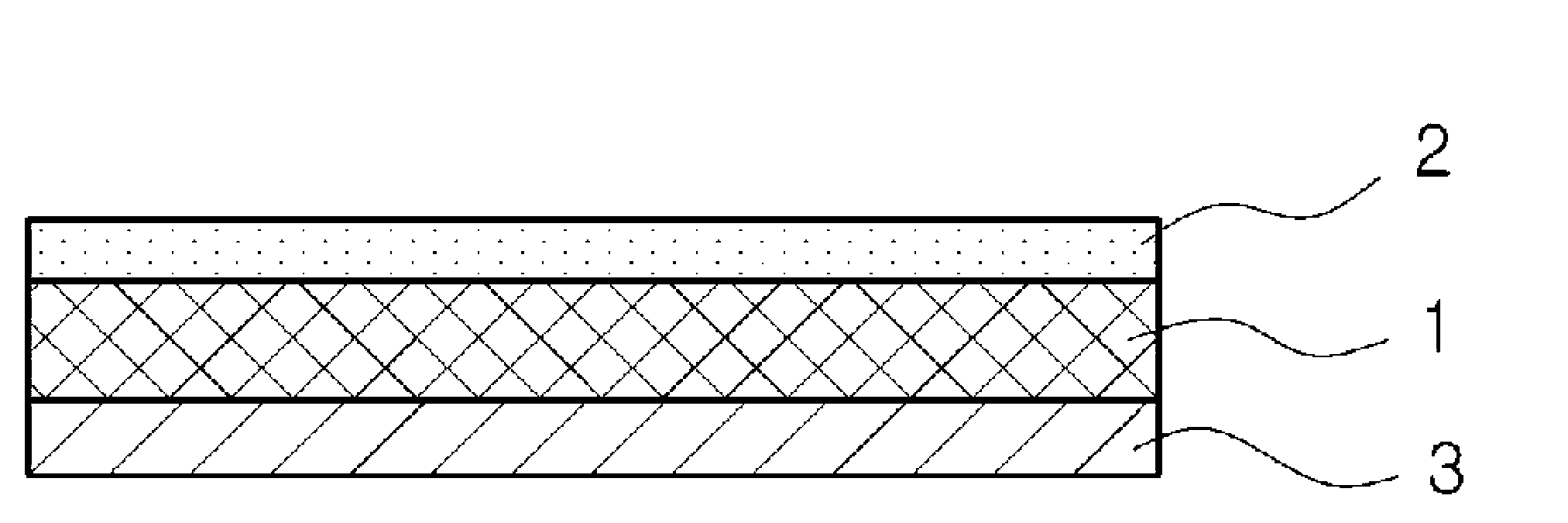
Method for producing patch, patch and package
A method for producing a patch comprising a support layer and an adhesive agent layer arranged on at least one surface of the support layer, the method comprising:step A) obtaining an adhesive agent layer composition comprising free ropinirole in an amount which results in a content of 5 to 13.2% by mass in an obtained adhesive agent layer;step B) heating the adhesive agent layer composition at a temperature in a range from 50 to 76° C. for 5 minutes to 24 hours; andstep C) cooling the heated adhesive agent layer composition to normal temperature at an average rate of temperature drop of 1 to 20° C. / hour, thereby obtaining the adhesive agent layer comprising the free ropinirole at a supersaturated concentration in a dissolved form.
Owner:HISAMITSU PHARM CO INC
Novel therapeutic method
InactiveUS20010056115A1Preserving functionPreserving dopaminergic functionBiocideCarbohydrate active ingredientsPharmacologyRopinirole
A therapeutic method for preserving the dopaminergic function of patients suffering from Parkinson's disease, which method comprises administering an effective amount of ropinirole or a pharmaceutically acceptable salt or solvate thereof to a human or animal patient in need thereof. Typically, said patient has had Parkinson's disease for a period of less than three years since diagnosis. Preferably the invention comprises administering to said patient an effective amount of ropinirole or a pharmaceutically acceptable salt or solvate thereof, optionally in combination with one or more other dopamine agonists, in the absence of levodopa or any other dopamine precursor, and thereafter treating the patient with levodopa.
Owner:TULLOCH IAN FREDERIC
Transdermal absorption preparation
ActiveCN102946873AImprove permeabilityFully viscousOrganic active ingredientsNervous disorderSide effectSkin penetration
The present invention relates to a transdermal absorption preparation. The transdermal absorption preparation of the present invention comprises a drug-containing adhesive layer, a drug-protective layer and a release layer, wherein the drug-containing layer contains ropinirole or a salt thereof, rubber, an adhesion-imparting resin, an antioxidant, and a transdermal absorption promoter. Also, the present invention provides a transdermal absorption preparation comprising a drug-containing adhesive layer, a drug-protective layer and a release layer, wherein the drug-containing layer contains ropinirole or a salt thereof, acrylic rubber, an anti-crystallization agent, and a transdermal absorption promoter.; When the transdermal absorption preparation of the present invention is used for the treatment of Parkinson's disease or restless leg syndrome, no side effect due to an increase in the initial concentration in blood occurs and the skin penetration effect of the drug is excellent.
Owner:ICURE PHARML CORP
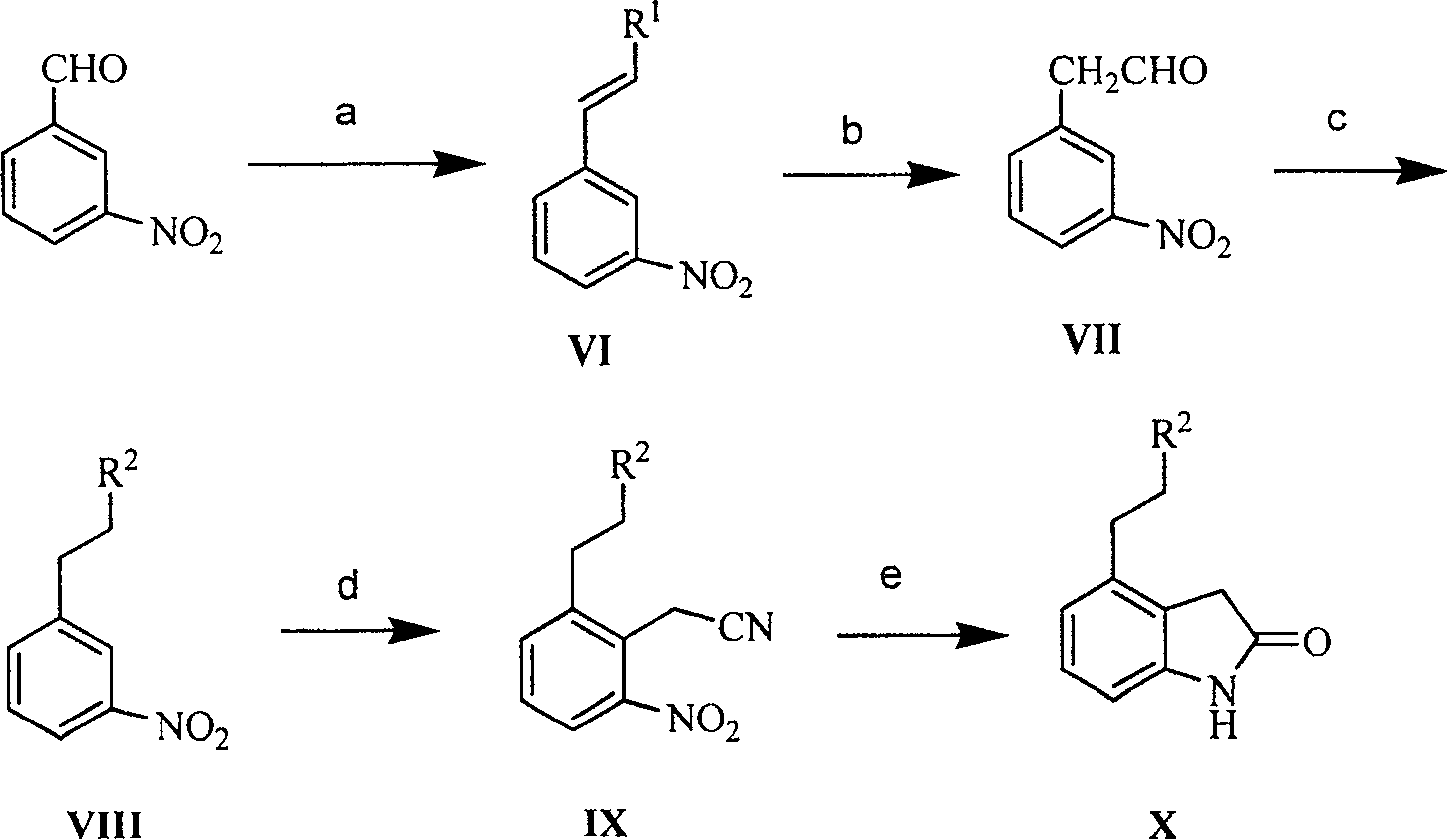
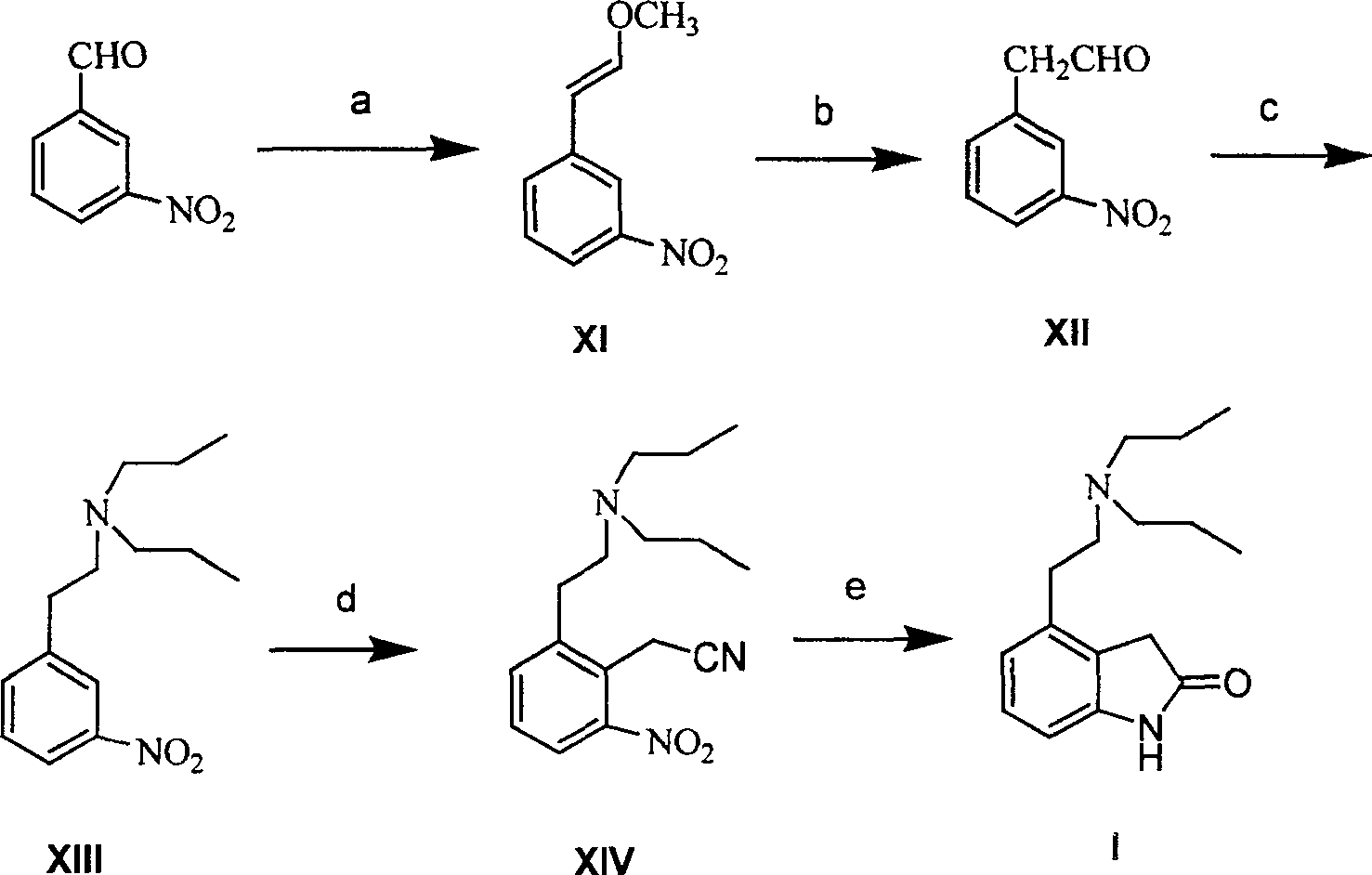
Method for preparing ropinirole
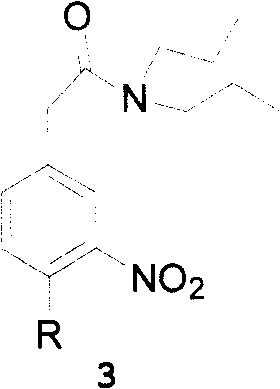
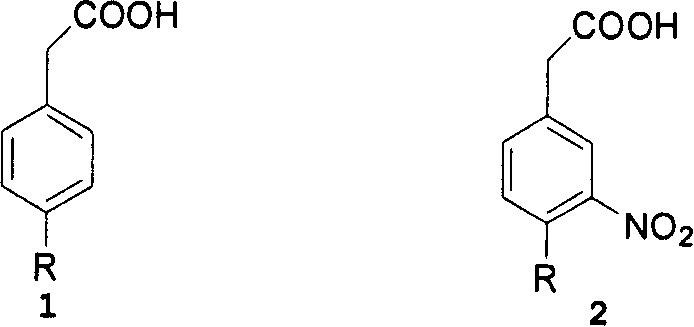
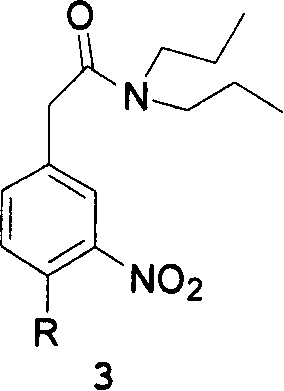
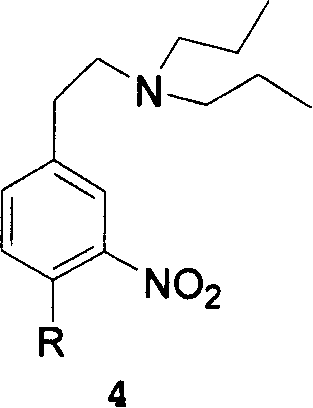
This invention adopts commercially available phenylacetic acid or 4-substituted phenylacetic acid as the starting material, and comprises nitrifying, reacting into acyl chloride and amide, reducing amide, reducing nitryl, condensing, cyclizating, reducing carbanyl and dehalogenating to obtain ropinirole.
Owner:2Y CHEM
Implantable devices for drug delivery with reduced burst release
The invention provides implantable drug delivery devices comprising a core comprising a polymer (or polymer blend) and one or more drugs or pharmaceutical substances, and an outer shell comprising a polymer (or polymer blend) and one or more porogen materials. The invention reduces burst release of drug. Pharmaceuticals such as triiodothyronine (T3) or ropinirole can be delivered by the devices.
Owner:TITAN PHARMA
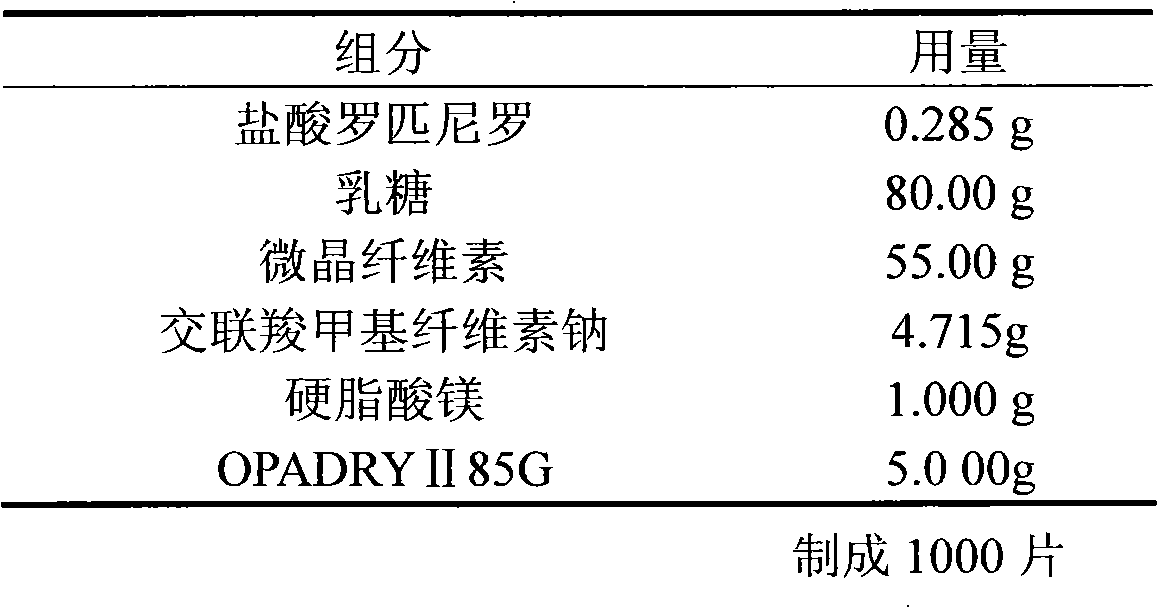
Preparation method of composition containing ropinirole
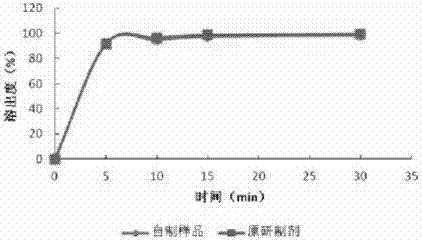
The invention discloses a preparation method of a solid pharmaceutical composition containing ropinirole or derivatives of ropinirole. The preparation method is characterized by comprising the following steps: firstly, dissolving the ropinirole or derivatives of ropinirole into a proper solvent, then adding the solution to a pharmaceutically acceptable carrier; evenly mixing, drying and then mixing with other materials, and finally preparing into an oral solid preparation. By adopting the method, ropinirole can be rapidly dissolved out, the problems of complicated preparation process and poor content uniformity in the preparation process of the ropinirole preparation can be effectively solved; meanwhile, the stability of ropinirole is not affected.
Owner:XIAN LIBANG PHARMA
Method for purifying ropinirole hydrochloride
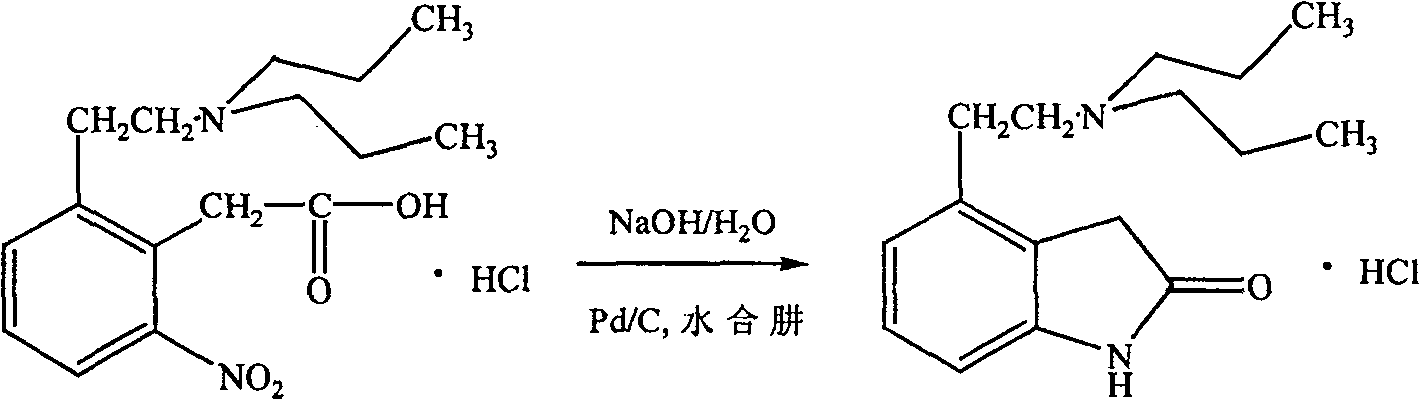
The invention relates to a method for purifying ropinirole hydrochloride (4-2-di-n-propylaminoethyl-1,3-dihydro-2H-indole-2-ketohydrochloride). The method comprises the following steps of: adding ropinirole hydrochloride containing an oxidized impurity structural formula (I) into water, adding an organic solvent, stirring and dissolving at room temperature, adding alkali, stirring, standing, demixing, and removing an aqueous layer; drying the obtained organic layer by using anhydrous magnesium sulfate, adding active carbon, stirring, filtering and concentrating; and adding a certain quantity of organic solvent into the obtained oily matter after concentrating to be dry, slowly adding a certain quantity of concentrated hydrochloric acid, stirring, cooling the solution, performing throwing filtration, and drying, thus obtaining the ropinirole hydrochloride. By adopting the method, oxidized impurities (I) can be effectively removed from the ropinirole hydrochloride, and the ropinirole hydrochloride can be obtained with high yield and purity, so that the oxidized impurities are controlled and the purity of the ropinirole hydrochloride reaches the medicinal standard.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method and compound for treatment of menopausal symptoms
ActiveUS9468631B1Reduces effect menopausal symptomRelieve symptomsOrganic active ingredientsOrganic chemistryHot flashes/flushesDopamine
The subject invention describes a method of use of Ropinirole™ to alleviate and control menopausal symptoms in women, and in particular, hot flashes. The invention describes the use of Ropinirole as a dopamine agonist with affinity for the dopamine D2, D3, or D4 receptors. Ropinirole may also be used to treat menopausal symptoms in conjunction with Tizanidine™ to further reduce the effects menopausal symptoms by providing a sedative and muscle relaxant effect which aids in sleep. The combination of Ropinirole and Tizanidine provides a useful new compound for treatment of menopausal symptoms that are most disruptive to the functioning in activities of daily living.
Owner:KNOBLER ROBERT L
Oral solid medicine composition containing ropinirole
ActiveCN101574341AFix stability issuesImprove stabilityOrganic active ingredientsNervous disorderDiseaseTraditional medicine
The invention discloses an oral solid medicine composition containing ropinirole. The oral solid medicine composition comprises a medicine containing part and hollow white granules, wherein the medicine containing part contains ropinirole and pharmaceutically acceptable carriers, and the hollow white granules contain no ropinirole and are formed by mixing pharmaceutically acceptable carriers. Theoral solid medicine composition can be prepared with simple process, has better uniformity of preparation content and stability and can be used for treating parkinson disease (PD).
Owner:AVENTIS PHARMA HAINAN
Method for producing patch, patch and package
ActiveUS9320728B2StorabilityImprove the level ofOrganic active ingredientsNervous disorderRopiniroleChemistry
A method for producing a patch comprising a support layer and an adhesive agent layer arranged on at least one surface of the support layer, the method comprising:step A) obtaining an adhesive agent layer composition comprising free ropinirole in an amount which results in a content of 5 to 13.2% by mass in an obtained adhesive agent layer;step B) heating the adhesive agent layer composition at a temperature in a range from 50 to 76° C. for 5 minutes to 24 hours; andstep C) cooling the heated adhesive agent layer composition to normal temperature at an average rate of temperature drop of 1 to 20° C. / hour, thereby obtaining the adhesive agent layer comprising the free ropinirole at a supersaturated concentration in a dissolved form.
Owner:HISAMITSU PHARM CO INC
Method for producing patch, patch and package
ActiveCN104138366AImprove permeabilityFor long-term storageOrganic active ingredientsNervous disorderPolymer scienceRopinirole
A method for producing a patch comprising a support layer and an adhesive agent layer arranged on at least one surface of the support layer, the method comprising: step A) obtaining an adhesive agent layer composition comprising free ropinirole in an amount which results in a content of 5 to 13.2% by mass in an obtained adhesive agent layer; step B) heating the adhesive agent layer composition at a temperature in a range from 50 to 76°C for 5 minutes to 24 hours; and step C) cooling the heated adhesive agent layer composition to normal temperature at an average rate of temperature drop of 1 to 20°C / hour, thereby obtaining the adhesive agent layer comprising the free ropinirole at a supersaturated concentration in a dissolved form.
Owner:HISAMITSU PHARM CO INC
Method for preparing ropinirole hydrochloride
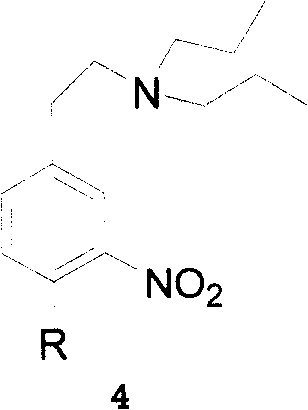
InactiveCN108440376AHigh yieldEasy to makeOrganic chemistryRopinirole HydrochlorideEthane Dichloride
The invention belongs to the technical field of medicinal chemistry and organic chemistry, and particularly relates to a method for preparing ropinirole hydrochloride. A novel compound 4 is synthesized by the aid of the method and is used as a raw material for preparing the ropinirole hydrochloride. The particular method includes dissolving the compound 4 in one or a plurality of types of ethyl alcohol / methanol / ethyl acetate to obtain liquid, adding Pd / C into the ethyl alcohol / methanol / ethyl acetate, and carrying out reaction to obtain compounds 5; dissolving the compounds 5, p-toluenesulfonylchloride and pyridine in one or a plurality of types of dichloromethane / trichloromethane / 1, 2-dichloroethane / pyridine, and carrying out reaction to obtain compounds 6; dissolving the compounds 6, NaIand dipropyl amine in one or a plurality of types of DMF (dimethyl formamide) / DMSO (dimethylsulfoxide) / Toluene, and carrying out reaction to obtain ropinirole; dissolving the ropinirole in 1, 4 dioxane with hydrochloric acid, and carrying out reduced-pressure compression to obtain the ropinirole hydrochloride. A proportion of the compounds 5 to the p-toluenesulfonyl chloride to the pyridine is equal to 1:1.2:1.2. A proportion of the compounds 6 to the NaI to the dipropyl amine is equal to 1:1:1.2. The novel method for preparing the ropinirole hydrochloride has the advantages that the method includes simple and convenient steps and can be practically put into production, and raw materials for the ropinirole hydrochloride are simple and are easily available.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
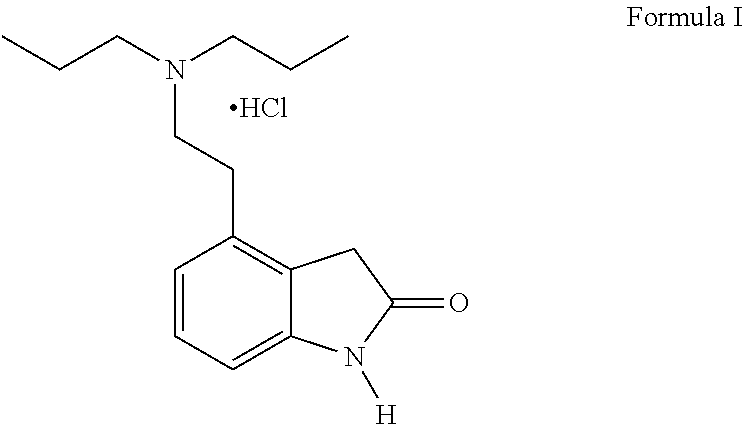
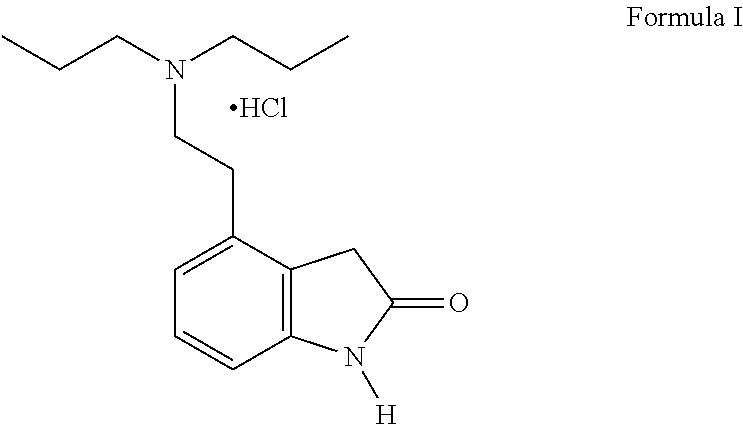
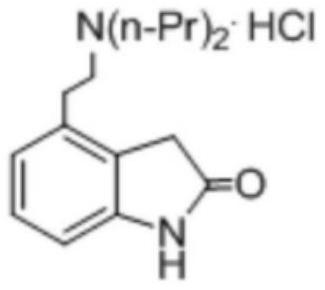
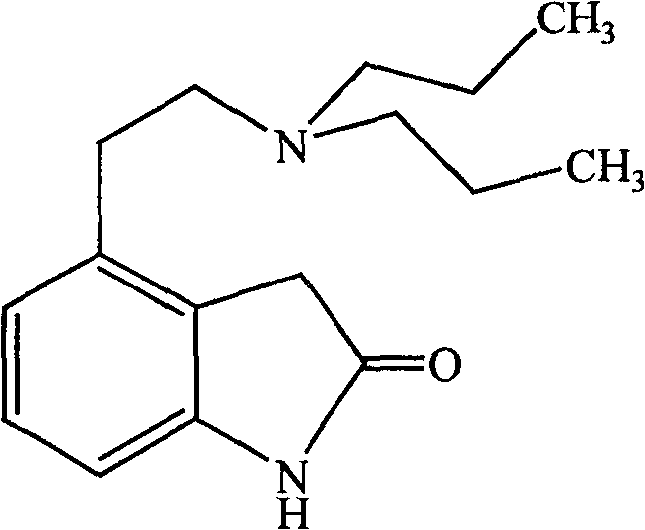
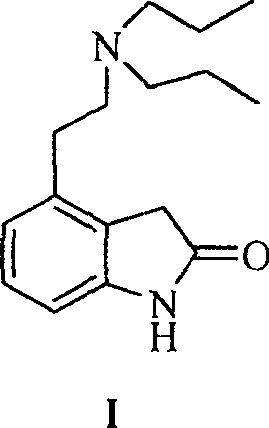
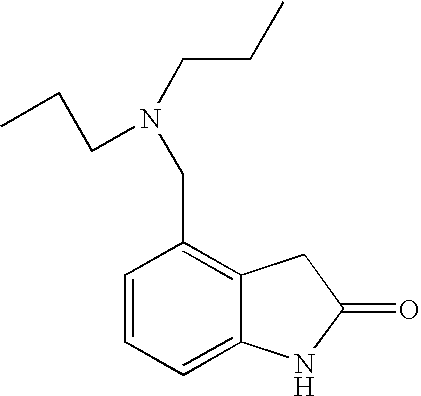
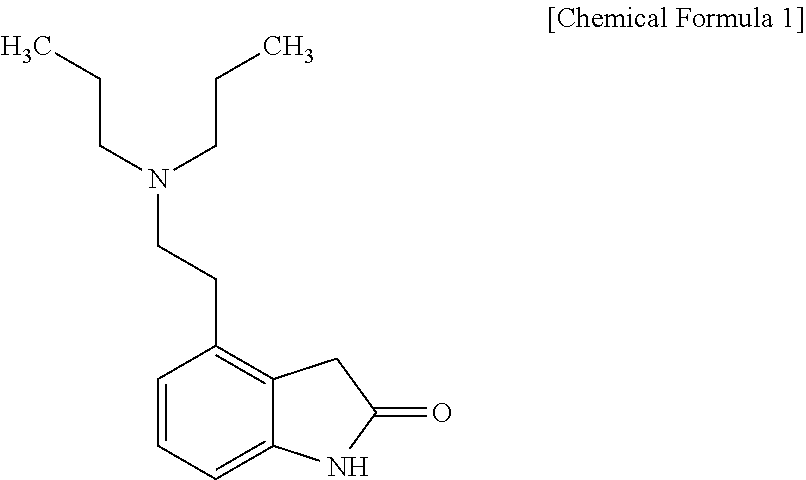
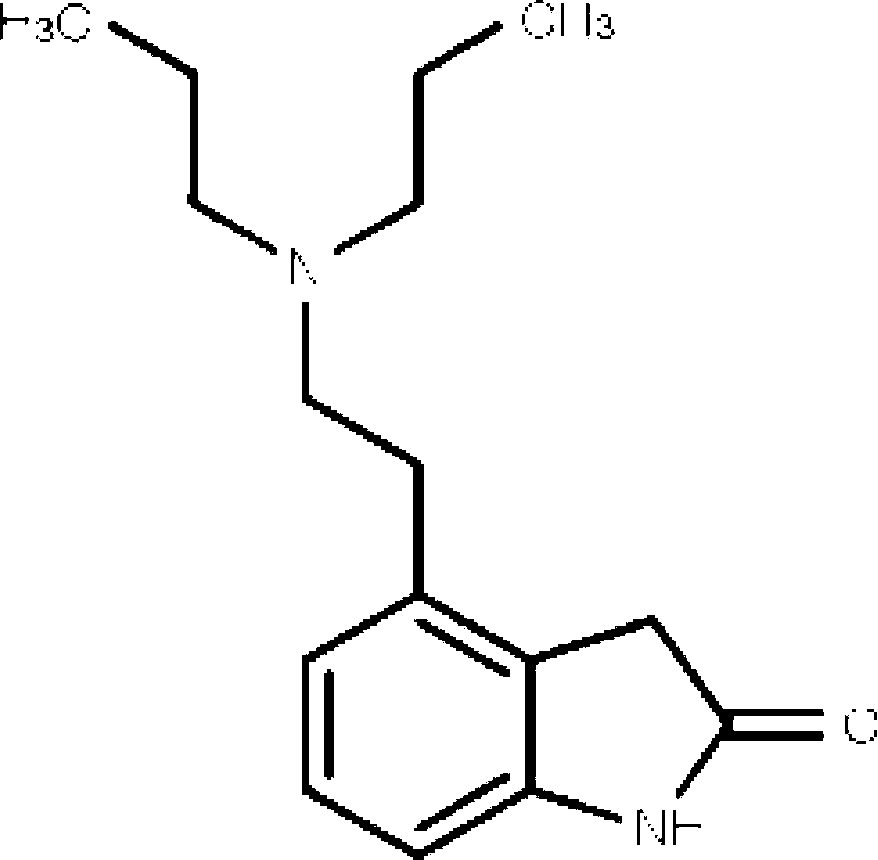
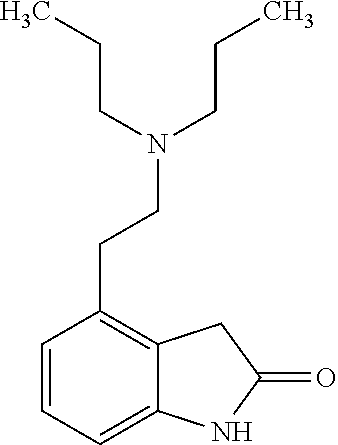
4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one (Ropinirole) a New Inhibitor of Jack bean Urease Enzyme: An Example of Drug Repurposing
This invention provides that Ropinirole (4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one) possess potent urease inhibitory potential (in vitro) with 97.3% inhibition and IC50=11.7+0.46 μM, when compared to the standard inhibitors i.e. acetohydroxamic acid (IC50=41.5+1.50 μM), and thiourea (IC50=21.0+0.50 μM).
Owner:KHAN JALALUDDIN AZAM JALAL +5
Ropinirole hydrochloride sustained release tablets and preparation method thereof
InactiveCN104473893AEffective treatmentReduce absorption rateOrganic active ingredientsNervous disorderExtended release tabletsPlastic packaging
The invention provides ropinirole hydrochloride sustained release tablets. The ropinirole hydrochloride sustained release tablets are prepared from 2 parts by weight of ropinirole hydrochloride, 50-95 parts by weight of sustained release skeleton material and 3-20 parts by weight of lubricating agent. A preparation method of the ropinirole hydrochloride sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The ropinirole hydrochloride sustained release tablets and the preparation method have the beneficial effects that the ropinirole hydrochloride sustained release tablets can be used for effectively treating Parkinson diseases and adopt the novel sustained release preparations; sustained release refers to reducing the absorption rates of medicines into bodies by reducing the release rates of the medicines from the dosage forms, thus achieving more stable treatment effects; the ropinirole hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Process for the preparation of ropinirole and salts thereof
InactiveUS20120253051A1Sacrificing qualitySacrificing yieldOrganic chemistryMedicinal chemistryRopinirole
The present invention relates to an improved process for the preparation of Ropinirole and pharmaceutical acceptable salts or derivatives thereof, in particular to a process for large scale production of Ropinirole and salts thereof in high yield and high purity and pharmaceutical preparations containing said compounds.
Owner:PHARMATHEN
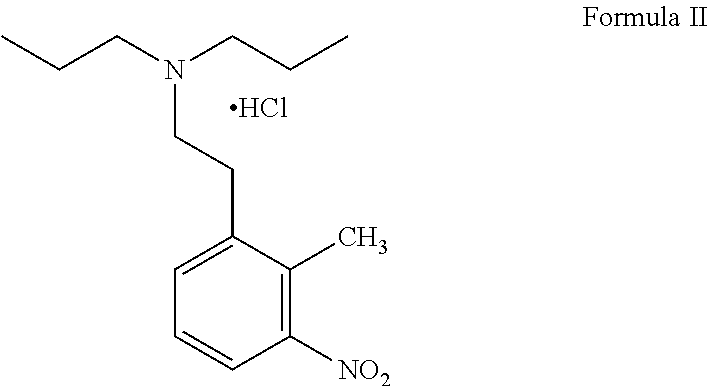
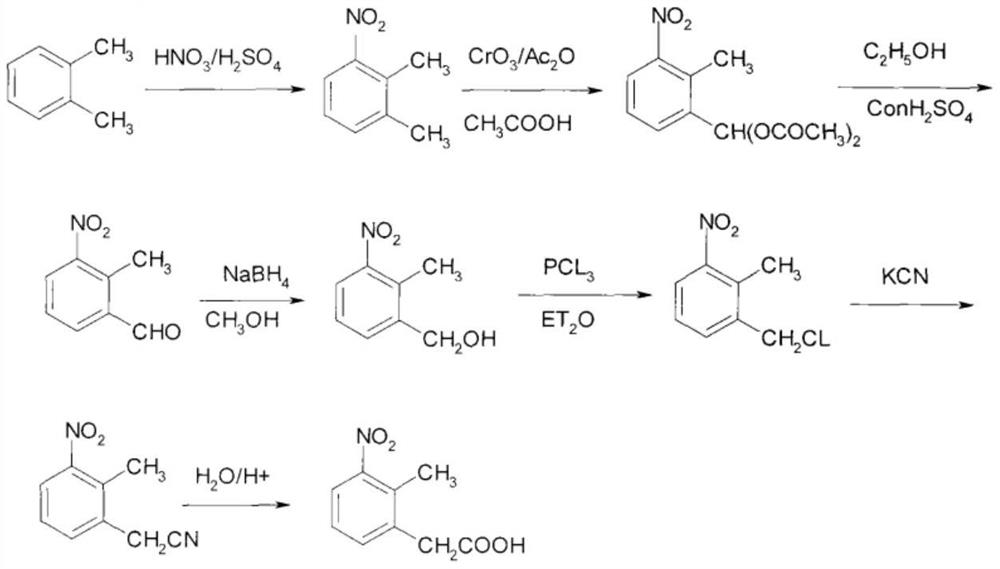
The synthetic method of ropinirole hydrochloride intermediate 2-methyl-3-nitrophenylacetic acid
ActiveCN109810001BReduce pollutionOrganic chemistryOrganic compound preparationPhenylacetic acidOrganic base
The invention discloses a method for synthesizing ropinirole hydrochloride intermediate 2-methyl-3-nitrophenylacetic acid, in which 6-halo-2-nitrotoluene and ethyl haloacetate are combined under the action of a catalyst Reaction to obtain 2-methyl-3-nitrophenylacetic acid, 1) under protective gas, ethyl haloacetate, organic base, catalyst and solvent S1 are mixed, the temperature is raised to 100-120 ° C, and the pressure is raised to 3- At 5 atmospheres, add dropwise a mixture of 6-halogenated-2-nitrotoluene and solvent S2 for 30-60 minutes, increase the temperature to 130-155 °C, increase the pressure to 7-9 atmospheres, and react for 2-4 hours After cooling to room temperature, drip alkaline water, control the temperature not to exceed 90°C, after the dropwise addition, control the temperature to 120-140°C, pressure 2-3 atmospheres, and finish the reaction for 1-2 hours; 2) Filter and remove the precipitate after cooling , the filtrate was adjusted to pH 1 with acid water, added solvent S3 for extraction, layered, the organic layer was washed with water, dried, concentrated and evaporated to remove the solvent to obtain the product. The method has short steps, high yield and less pollution discharge.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
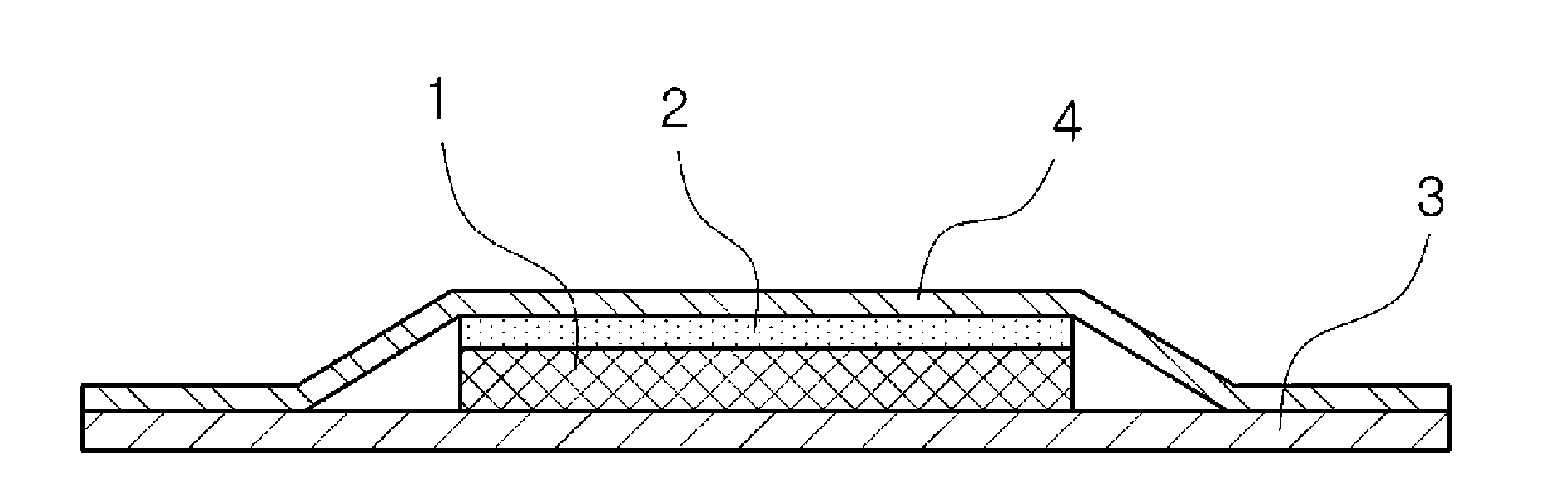
Ropinirole-containing patch and package thereof
ActiveUS9918945B2Improve skinSufficient adhesivenessOrganic active ingredientsNervous disorderRopiniroleChemistry
A ropinirole-containing patch comprises an adhesive agent layer and a support layer, the adhesive agent layer containing ropinirole and / or a pharmaceutically acceptable salt thereof, wherein a content of the ropinirole and / or the pharmaceutically acceptable salt thereof in terms of free ropinirole in the adhesive agent layer satisfies the following two conditions:5 to 13.2% by mass relative to a total mass of the adhesive agent layer, and0.12 to 2.7 mg / cm2 per unit area of the adhesive agent layer.
Owner:HISAMITSU PHARM CO INC
Double-skin milk and ropinirole mixed nutrient solution and preparation method thereof
The invention discloses a double-skin milk and ropinirole mixed nutrient solution and a preparation method thereof and relates to the technical field of food processing. The double-skin milk and ropinirole mixed nutrient solution mainly contains 3,000g of water, 50-60g of cream, 30-40g of chocolate, 50-60g of red beans, 20-30g of fruits, 200-250g of white sugar, 30-40g of pudding, 40-50g of dried rose petals, 5-10g of ginger juice, 5-10g of acanthopanax bark, 5-10g of plantain seed, 10-20g of Chinese waxgourd peel, 10-20g of Chinese yam, 20-25g of safflowers, 20g of black beans, 20g of Sprite and 20g of ropinirole. The method comprises the steps of selecting materials, mincing, melting, cooling, mixing and stirring, cupping, and charging, thereby completing preparation. The double-skin milk and ropinirole mixed nutrient solution is diverse in flavor and unique in taste, so that the requirements of people on good food are met; the preparation method is simple and is low in cost, so that the increase of the economic benefit is facilitated; and the dried rose petals can be used for treating gynecological diseases such as infrequent menstruation, abdominal pain due to blood stasis, irregular menstruation, amenorrhea and dysmenorrhea.
Owner:安徽省鸿运生物医药股份有限公司
Method for preparing ropinirole
This invention adopts commercially available phenylacetic acid or 4-substituted phenylacetic acid as the starting material, and comprises nitrifying, reacting into acyl chloride and amide, reducing amide, reducing nitryl, condensing, cyclizating, reducing carbanyl and dehalogenating to obtain ropinirole.
Owner:2Y CHEM
Process for preparing ropinirole and its salt
InactiveCN100560567CEasy to operateGood response controllabilityOrganic chemistryRopiniroleStereochemistry
Owner:北京德众万全医药科技有限公司
Ropinirole-containing patch and method for improvng skin permeability of ropinirole
PendingUS20220362214A1Good skin permeabilityOrganic active ingredientsNervous disorderSkin permeabilityAdhesive
A ropinirole-containing patch comprising an adhesive agent layer and a backing layer, whereinthe adhesive agent layer containing:at least one selected from the group consisting of ropinirole and a pharmaceutically acceptable salt thereof;a fatty acid metal salt having 7 or more carbon atoms; andan adhesive agent.
Owner:HISAMITSU PHARM CO INC
Prepn process of Ropinirole and derivative thereof
Owner:浙江凯普化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one (Ropinirole) a New Inhibitor of Jack bean Urease Enzyme: An Example of Drug Repurposing 4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one (Ropinirole) a New Inhibitor of Jack bean Urease Enzyme: An Example of Drug Repurposing](https://images-eureka.patsnap.com/patent_img/e064eb23-1691-4eb9-a24d-51608a1f5352/US20170252323A1-20170907-D00001.png)
![4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one (Ropinirole) a New Inhibitor of Jack bean Urease Enzyme: An Example of Drug Repurposing 4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one (Ropinirole) a New Inhibitor of Jack bean Urease Enzyme: An Example of Drug Repurposing](https://images-eureka.patsnap.com/patent_img/e064eb23-1691-4eb9-a24d-51608a1f5352/US20170252323A1-20170907-D00002.png)