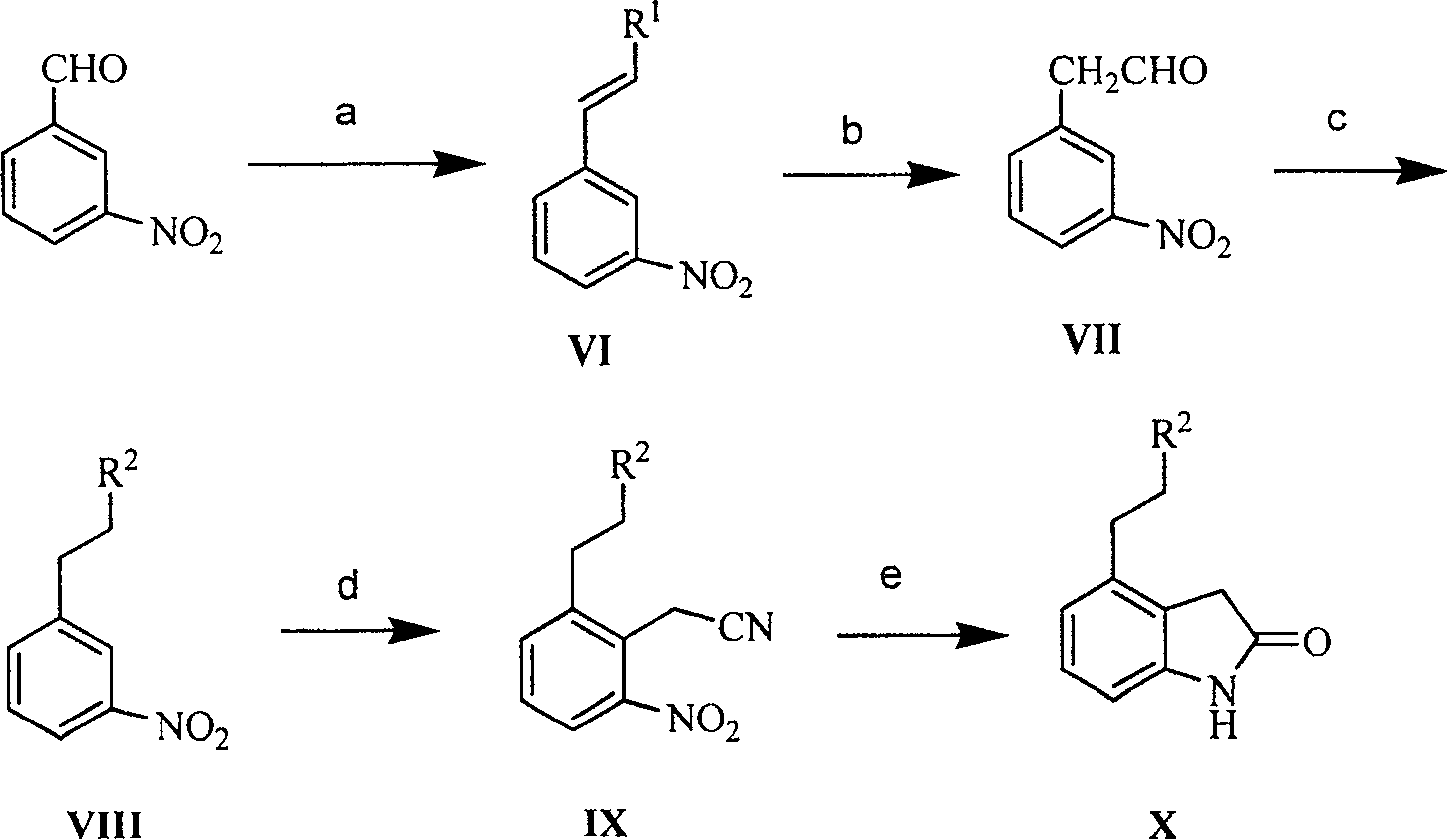
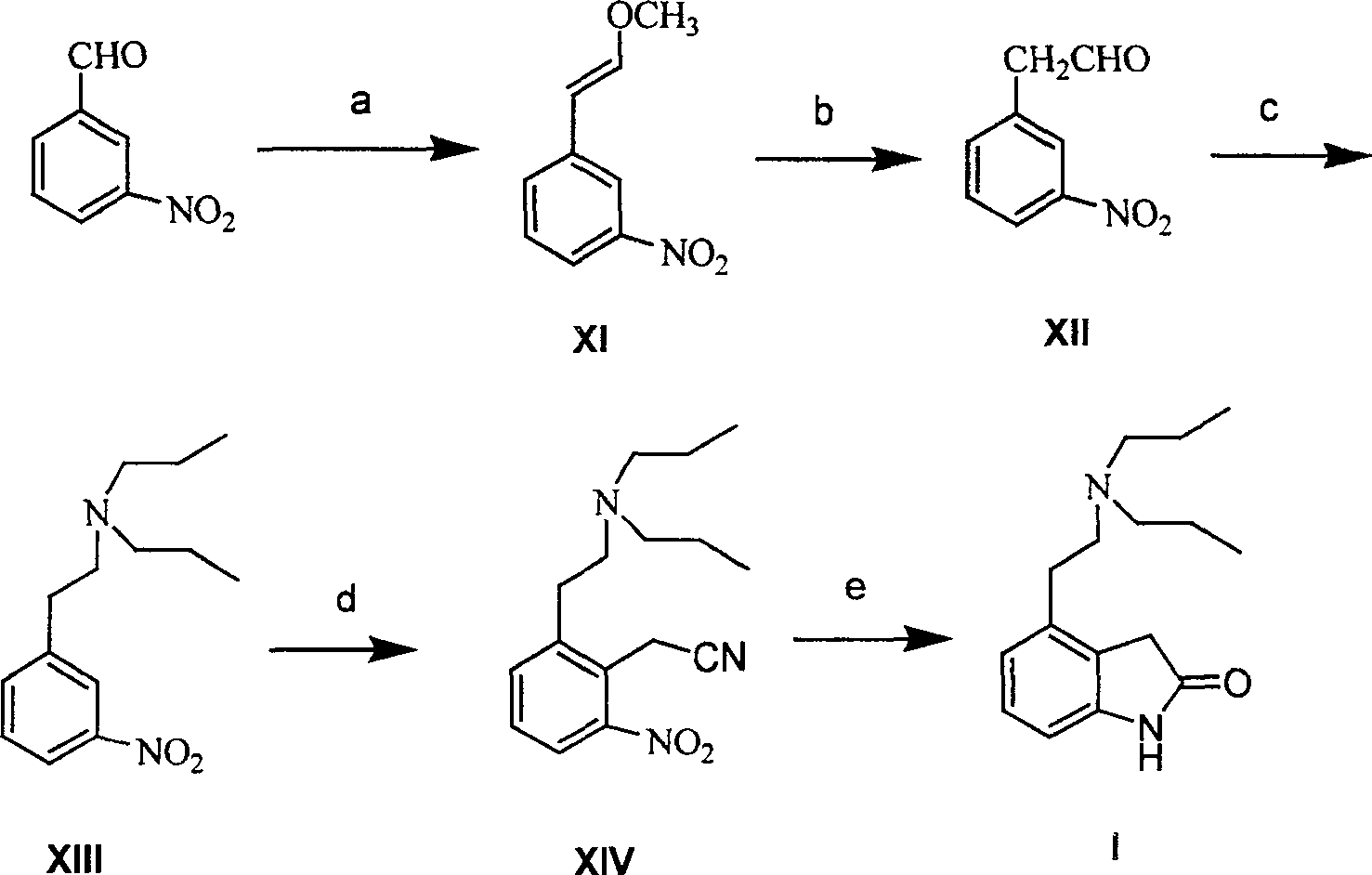
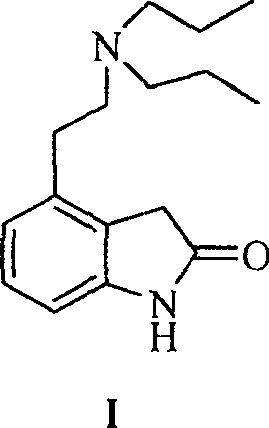
Prepn process of Ropinirole and derivative thereof
A compound and a representative technology, applied in the field of preparation of ropinirole and its derivatives, can solve the problems of eliminating a large number of by-products, high price, etc., and achieve the effects of saving production equipment and costs, short reaction steps, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Preparation of β-methoxy-3-nitrostyrene (XI)
[0082] Methoxymethyltriphenylphosphine chloride (4.75g, 13.86mmol) and anhydrous tetrahydrofuran were mixed and cooled to 0°C, potassium tert-butoxide (1.94g, 17.33mmol) was added with stirring, and the resulting suspension continued to cool to -10°C, add dropwise a toluene solution of 3-nitrobenzaldehyde (1.74g, 11.55mmol) within 30 minutes, after dropping, keep stirring for 1h, add a saturated ammonium chloride solution dropwise at 0°C, and wash the mixture with ethyl acetate Ester extraction (20mL x 2), combined organic layers, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate. The solvent was evaporated to obtain a yellow solid, which was a mixture of cis and trans isomers, with a yield of 88%.
Embodiment 2
[0084] Preparation of 2-(3-nitrophenyl)acetaldehyde (XII)
[0085] Compound XI (17.90 g, 0.10 mol) was dissolved in THF, 2 mol / L HCl 60 ml was added with stirring, and the resulting mixture was heated at 80°C for reaction. THF was distilled off under reduced pressure, the residue was dissolved in ethyl acetate and water, the aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with 5% sodium bicarbonate and saturated sodium chloride solution, and dried over anhydrous sodium sulfate. The solvent was evaporated to obtain the product with a yield of 95%.
Embodiment 3
[0087] Preparation of 3-(di-n-propylamino)ethylnitrobenzene (XIII)
[0088] Compound XII (23.39g, 0.14mol) was dissolved in a mixed solvent of THF and methanol, di-n-propylamine (24.70mL, 0.18mol) and sodium cyanoborohydride (12.47g, 0.20mol) were added accordingly, and the reaction mixture was heated at room temperature Stir the reaction. Add 50ml of 1mol / L HCl to terminate the reaction. The resulting mixture was evaporated under reduced pressure, the residue was extracted with ethyl acetate, the extract was washed with saturated sodium chloride solution, and then dried with anhydrous sodium sulfate. After recovering the solvent, the residue was purified by column chromatography to obtain the target compound , yield 80%.
[0089] 1 H-NMR (CDCl 3 , 200MHz): δ 8.10(s1H), 8.05(d, 1H), 7.55(d, 1H), 7.49(t, 1H), 2.85(dd, 2H), 2.70(dd, 2H), 2.49(t, 4H ), 1.45(m, 4H), 0.90(t, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com