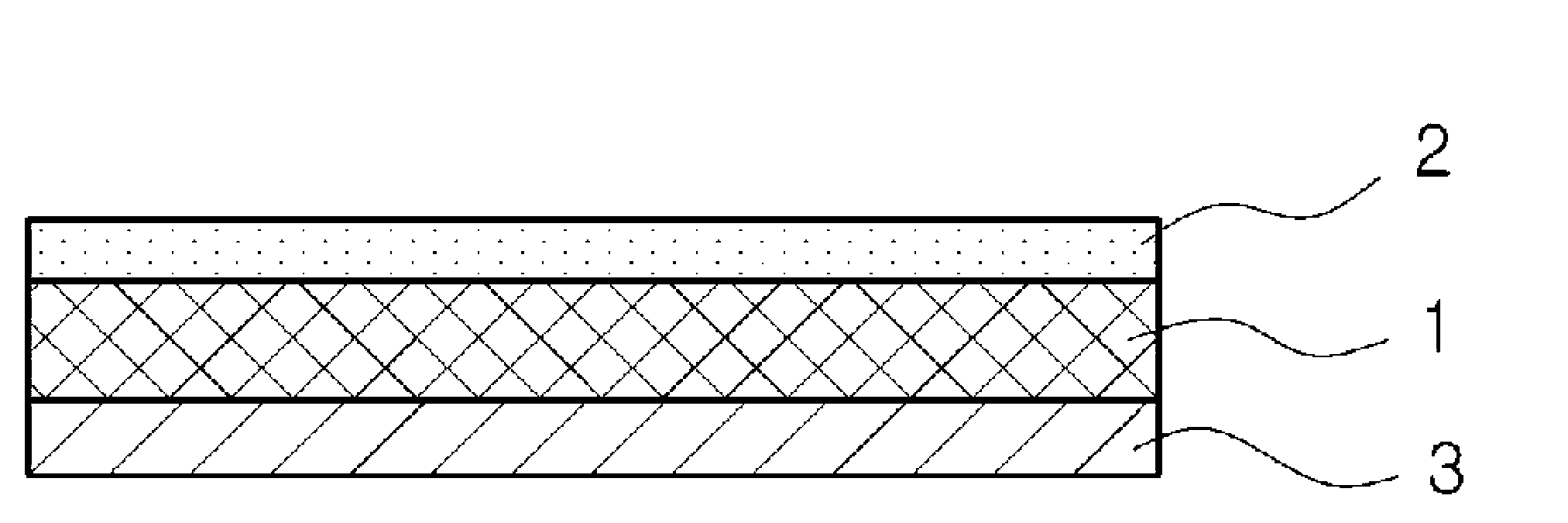
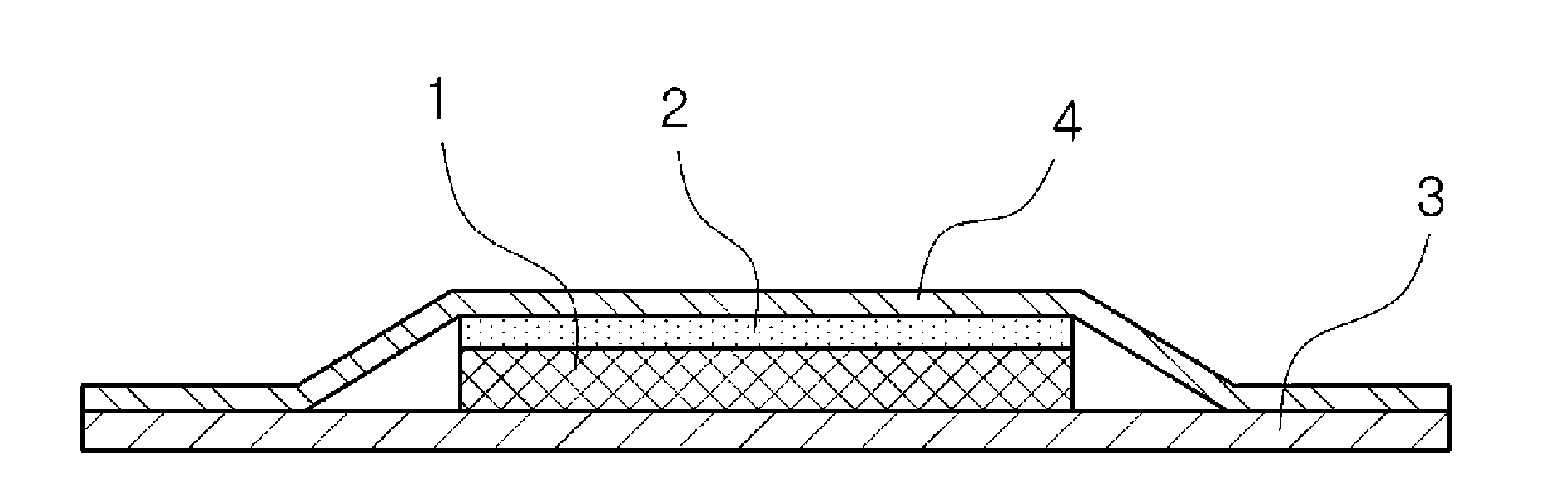
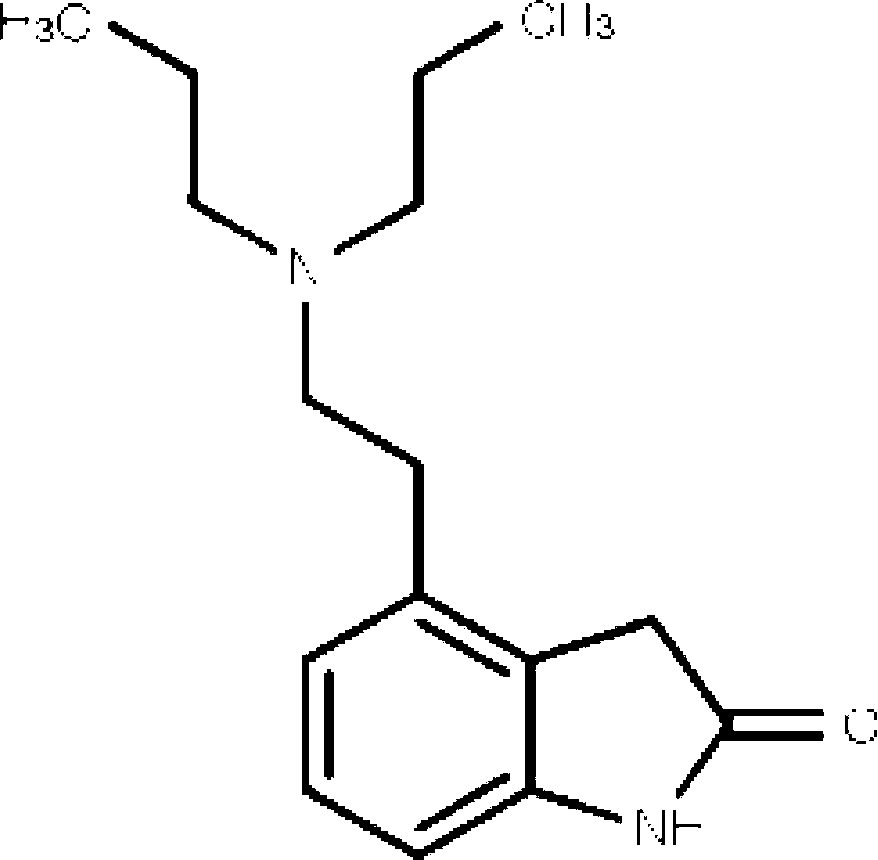
Transdermal absorption preparation
An absorption enhancer and preparation technology, applied in nervous system diseases, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of high polarity and difficult percutaneous absorption, and achieve the effect of good skin permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
[0067] The same ingredients as in Examples 1 to 7 in Table 1 were put into 175 g of toluene and completely dissolved, and the dissolved solution was coated on a polyester film (thickness: 75 μm) coated with silicone resin, dried to a thickness of 110 μm, and laminated on poly Ester film (thickness: 12μm) completes the mount.
Embodiment 8~14
[0069] The same ingredients as in Examples 8 to 14 in Table 1 and Table 2 were put into 175 g of toluene and completely dissolved, and then the dissolved solution was coated on a polyester film (thickness: 75 μm) coated with silicone resin and dried to a thickness of 73 μm. The layer is finished in a polyester film (thickness: 12μm).
[0070] Table 1
[0071]
[0072]
[0073] Table 2
[0074]
[0075]
[0076]
[0077] Franz diffusion cell (effective area: 0.64cm 2 , the volume of the aqueous phase: ) under the effect of the sink (sink condition) under the skin penetration test. As the aqueous phase, phosphate buffered saline (Phosphatebuffered saline, PBS) with a pH value of 7.4 was used.
[0078] First, fill the Franz diffusion cell with water-soluble phase and maintain it at 32±0.5°C, then cut the sample into a circle (with an area of 0.64cm 2 ) was attached to the central part of the prepared skin (human cadaver skin epidermis), the skin with the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com