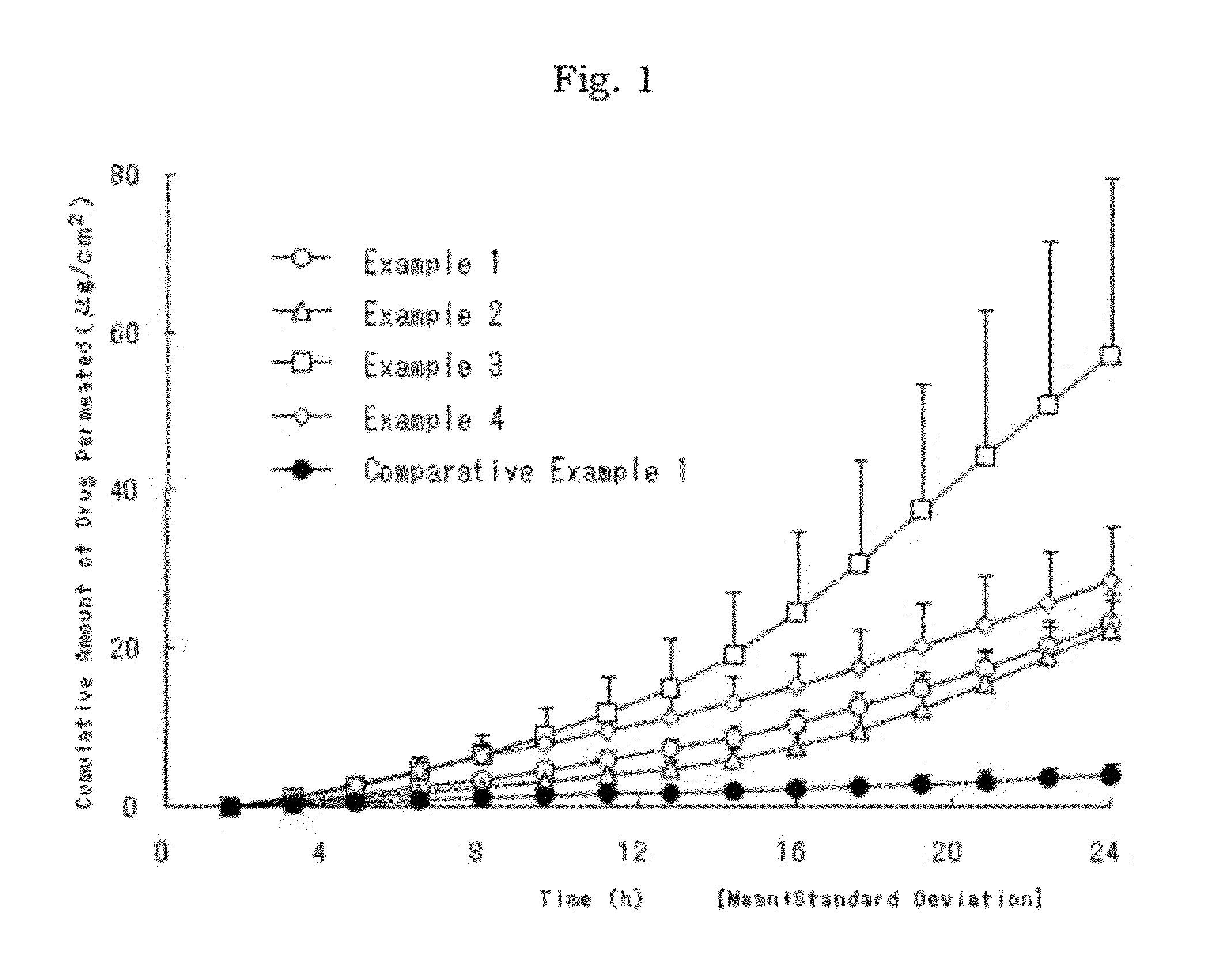
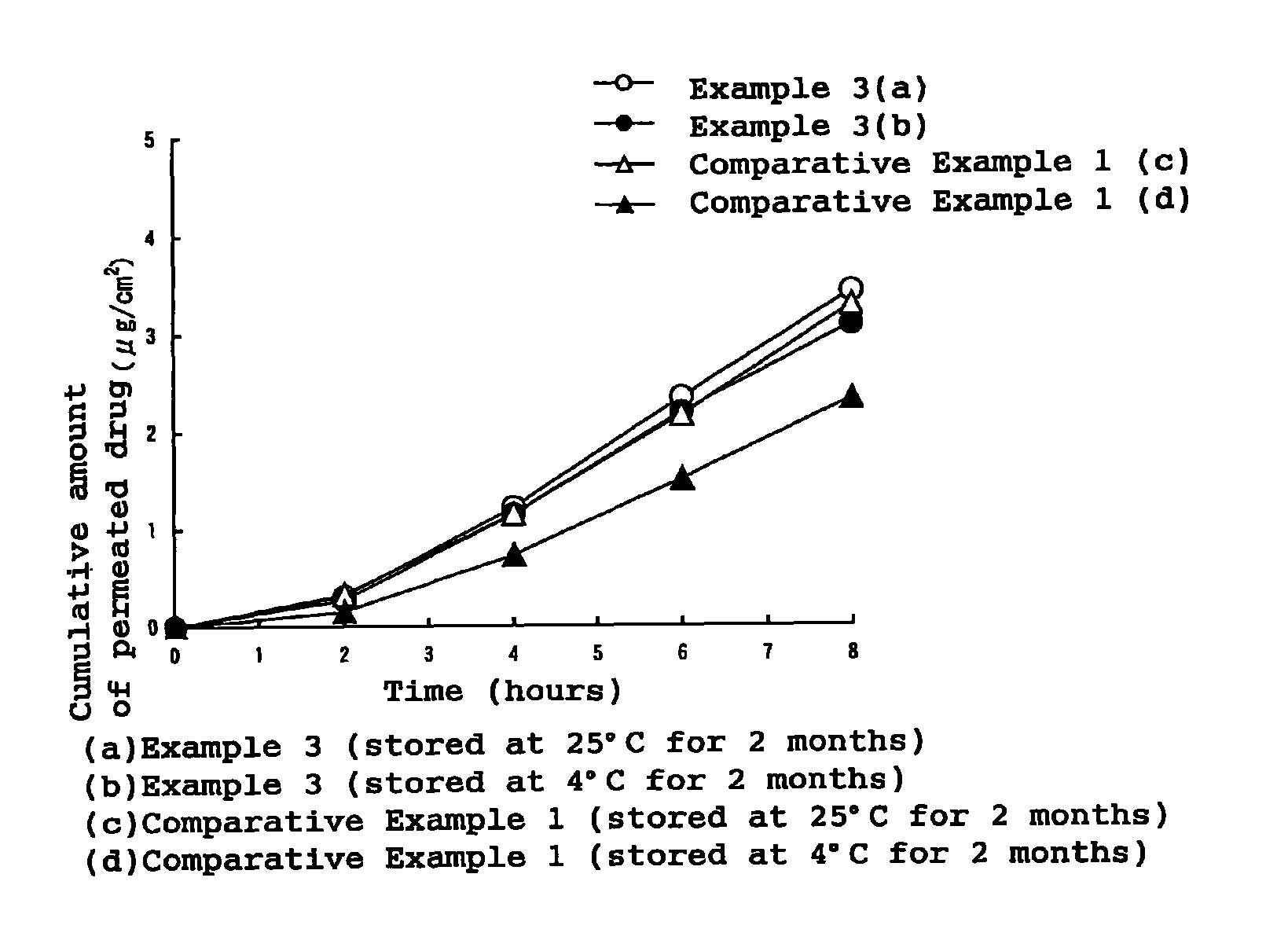
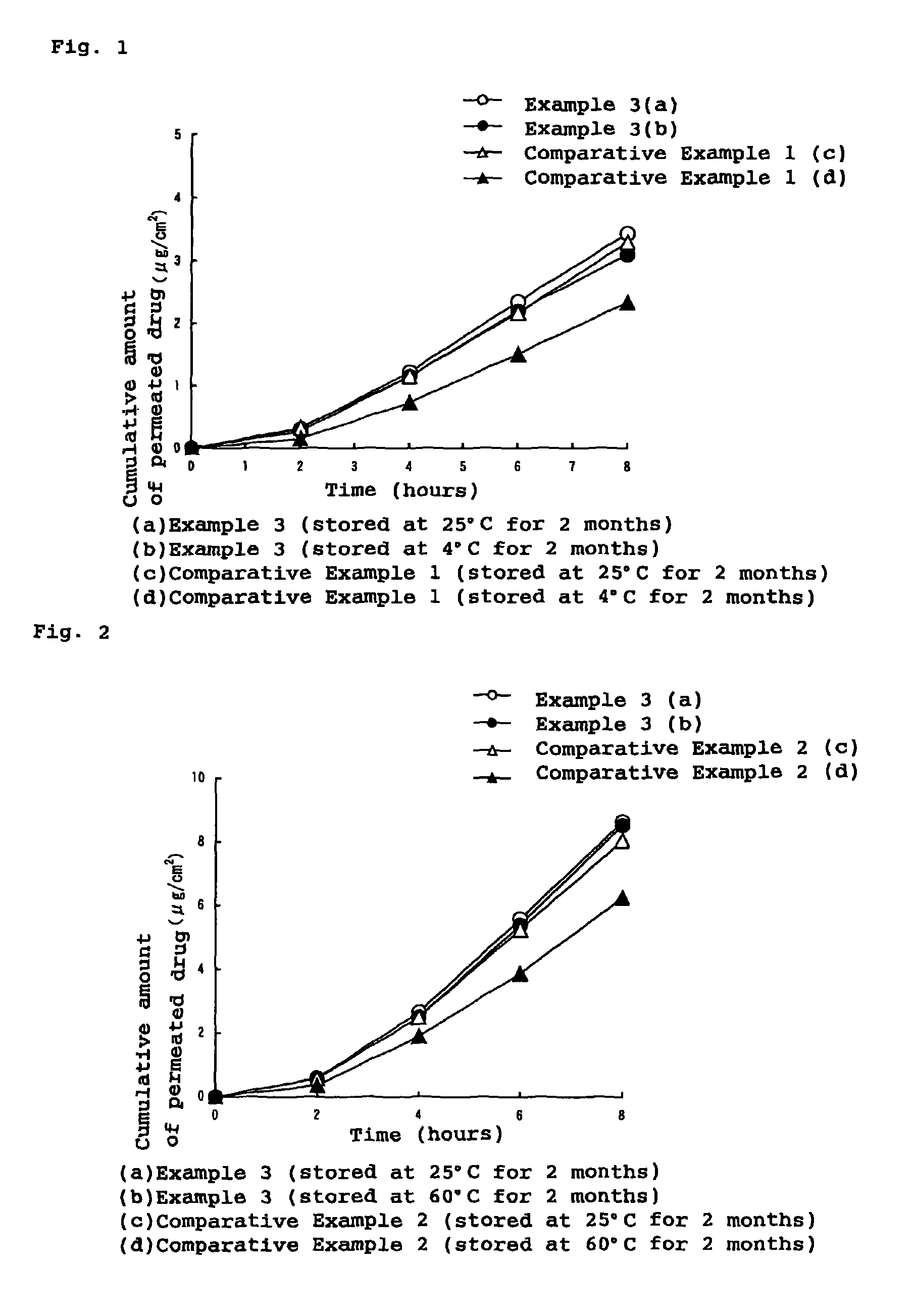
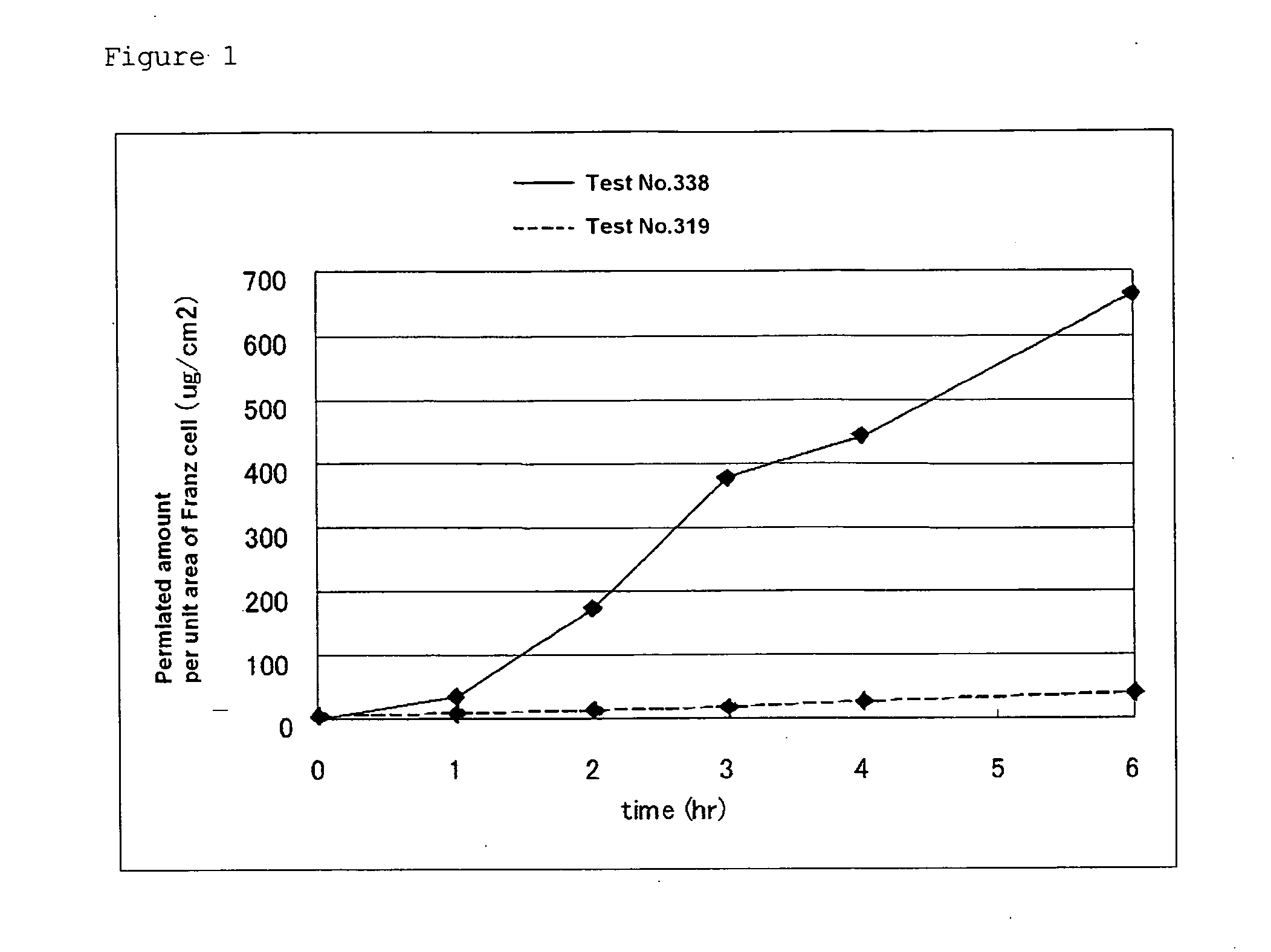
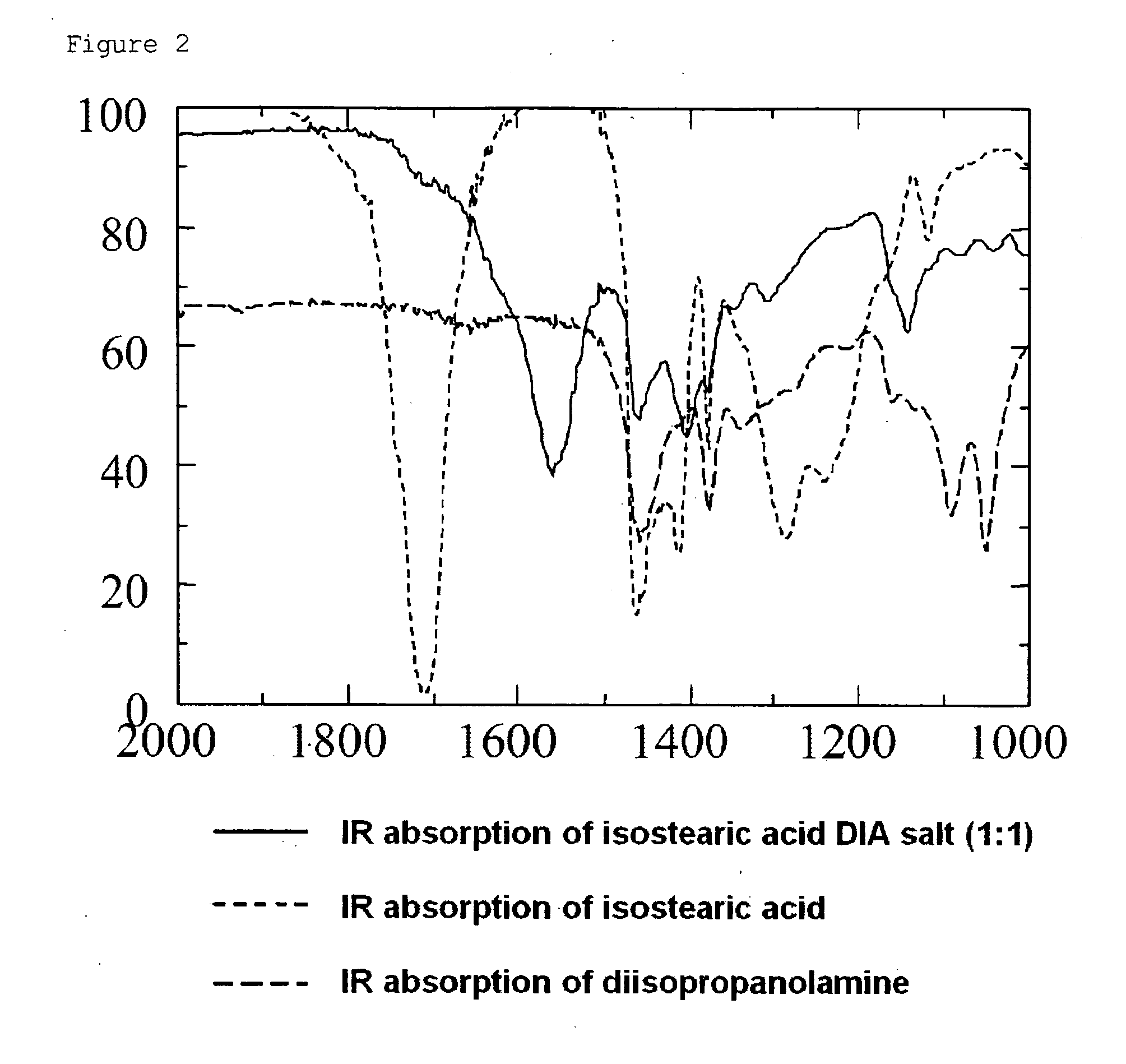
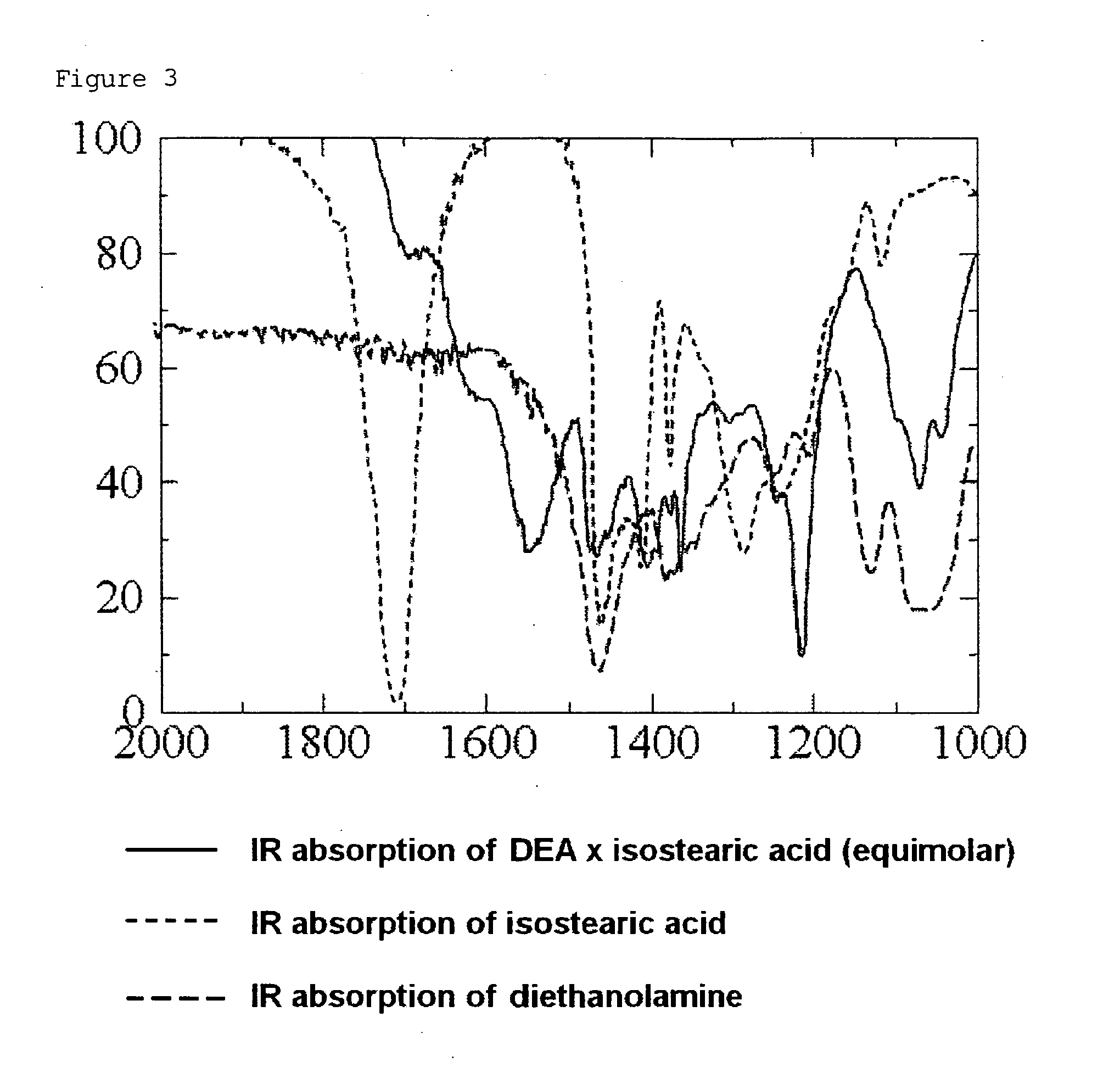
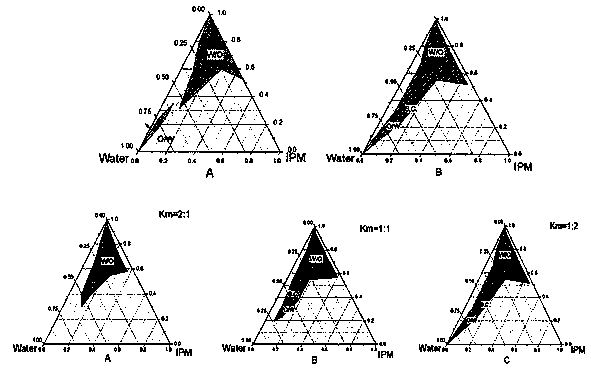
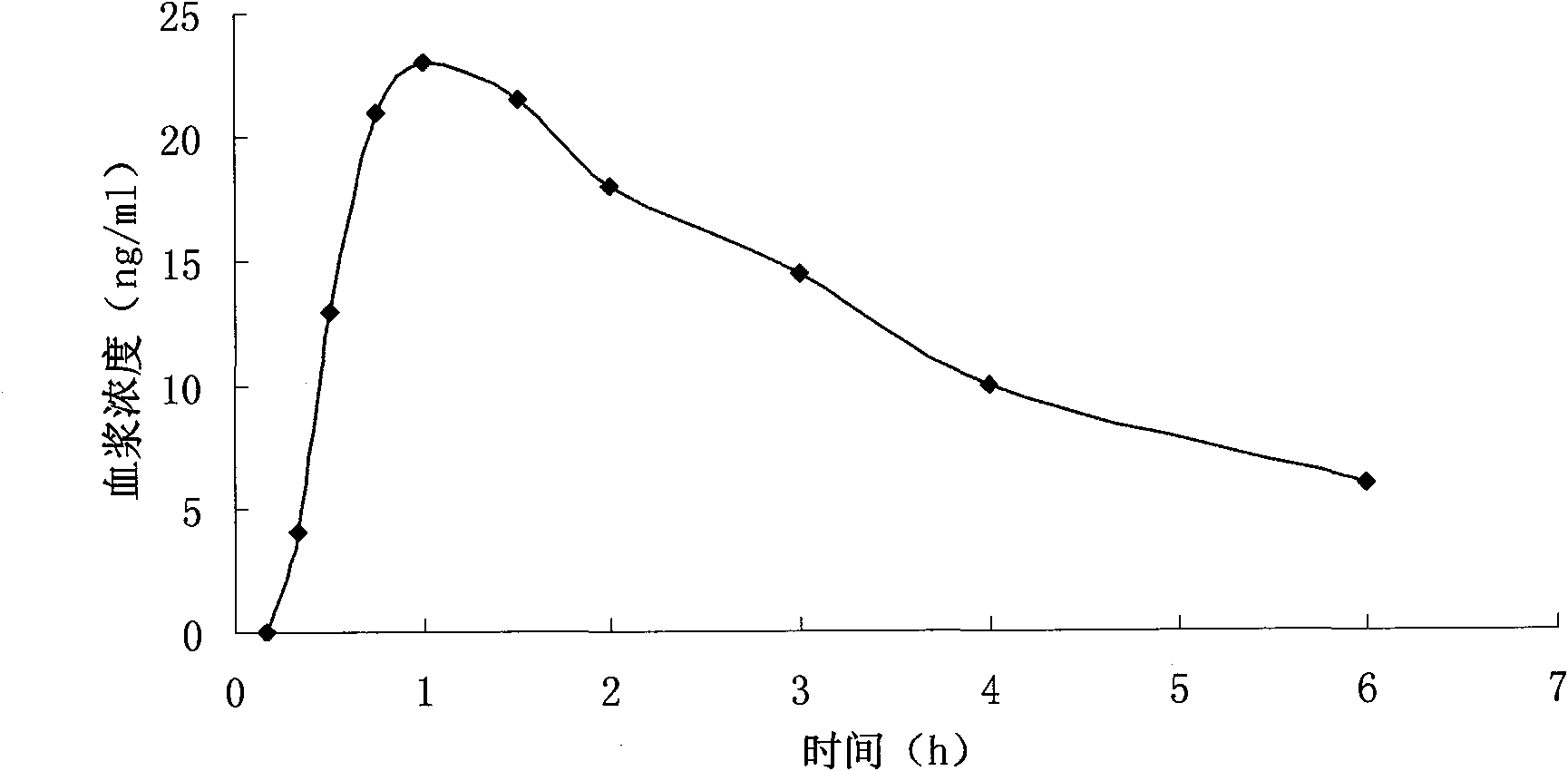
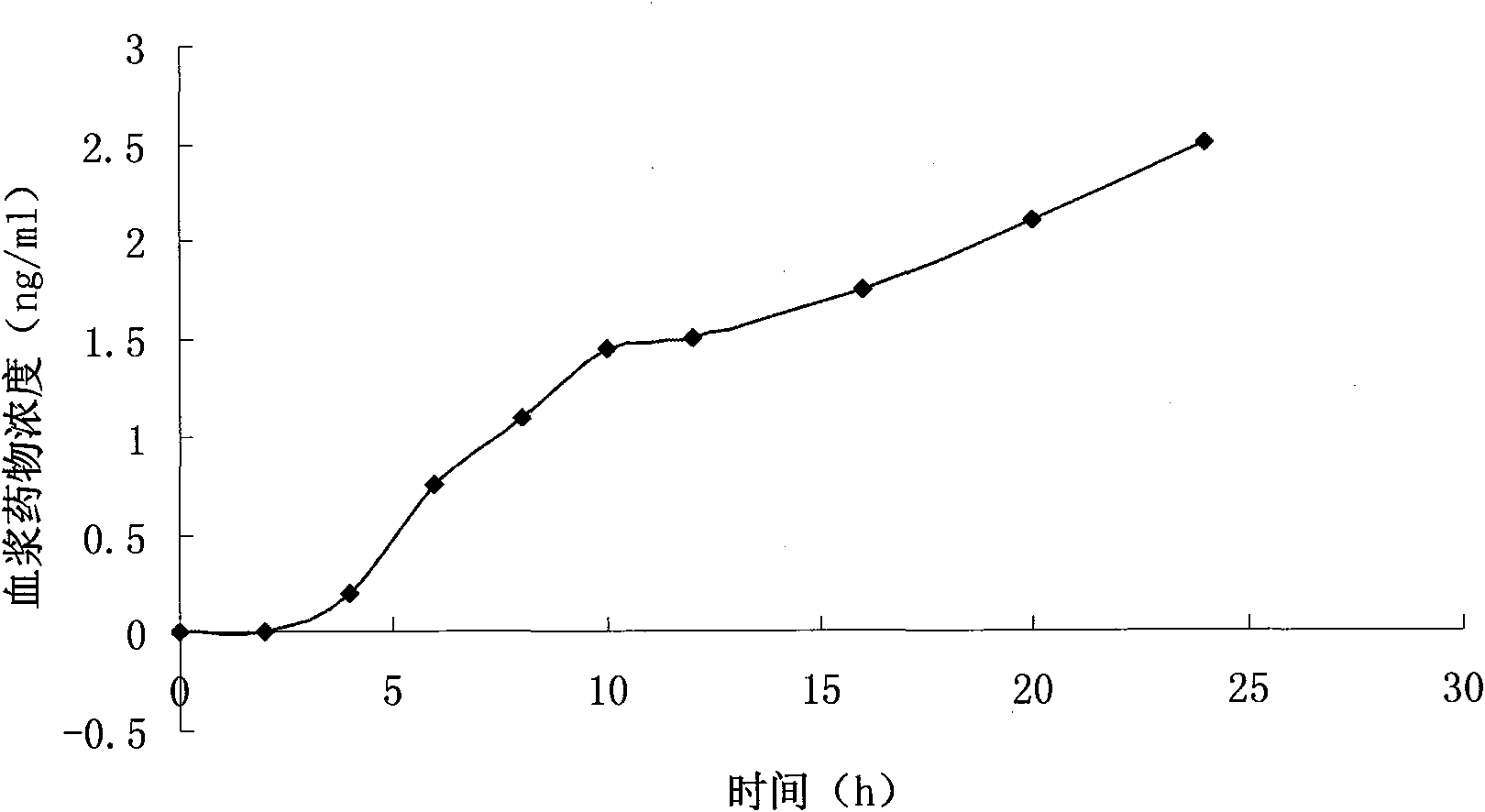
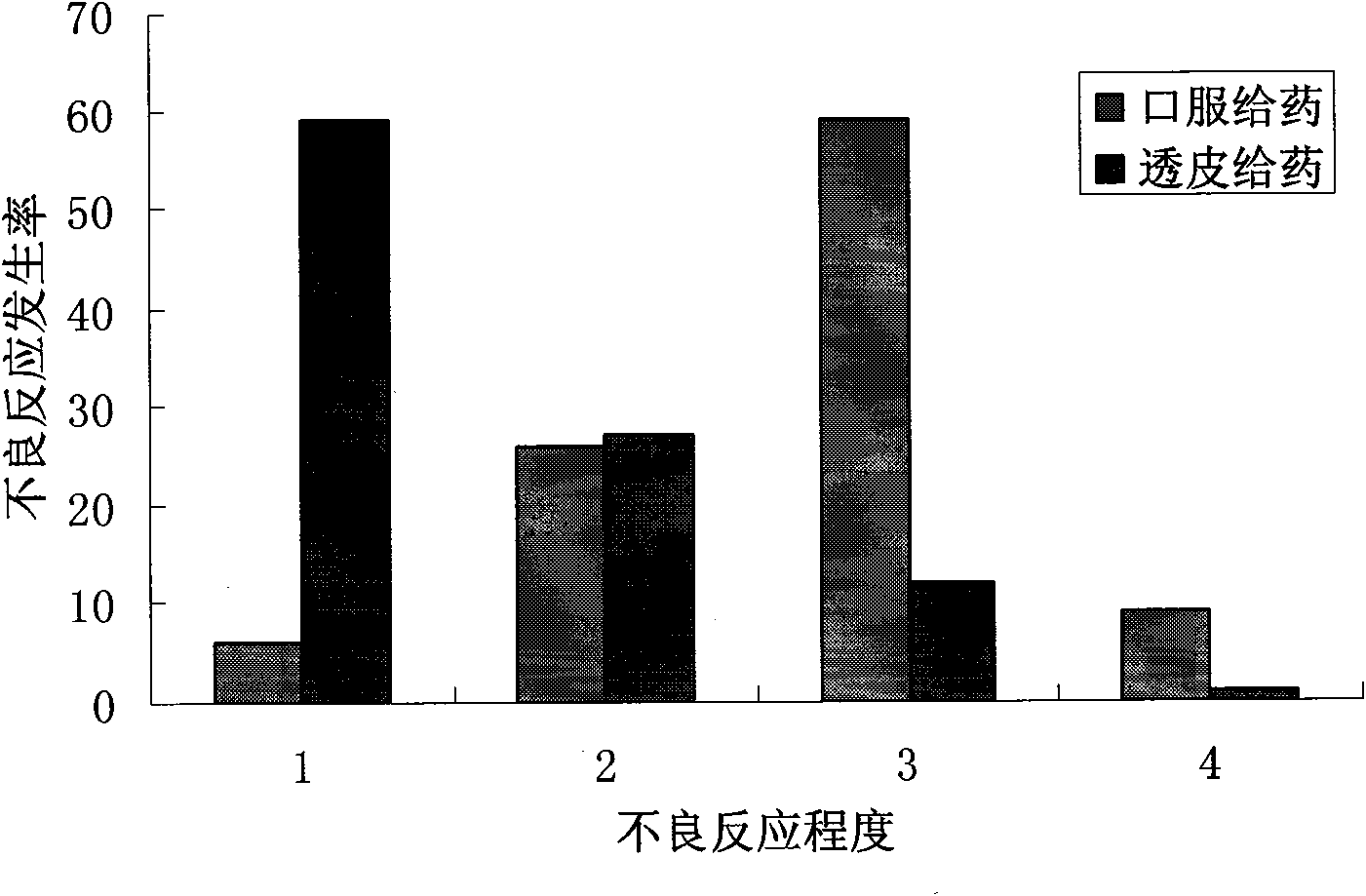
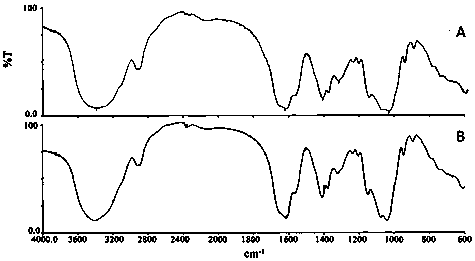
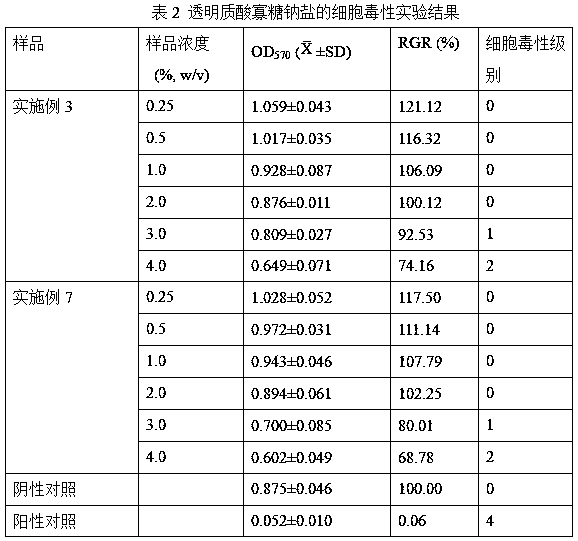
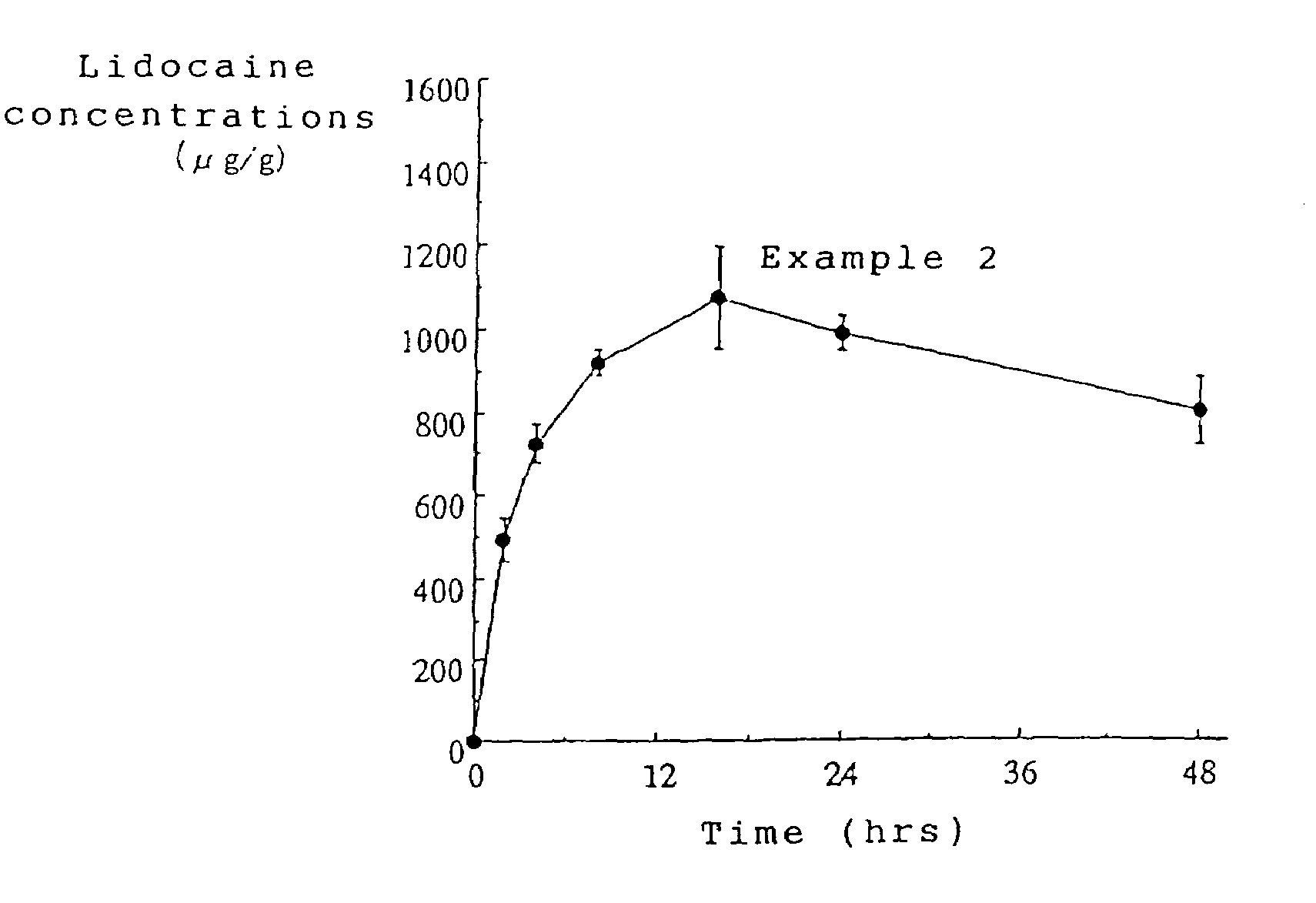
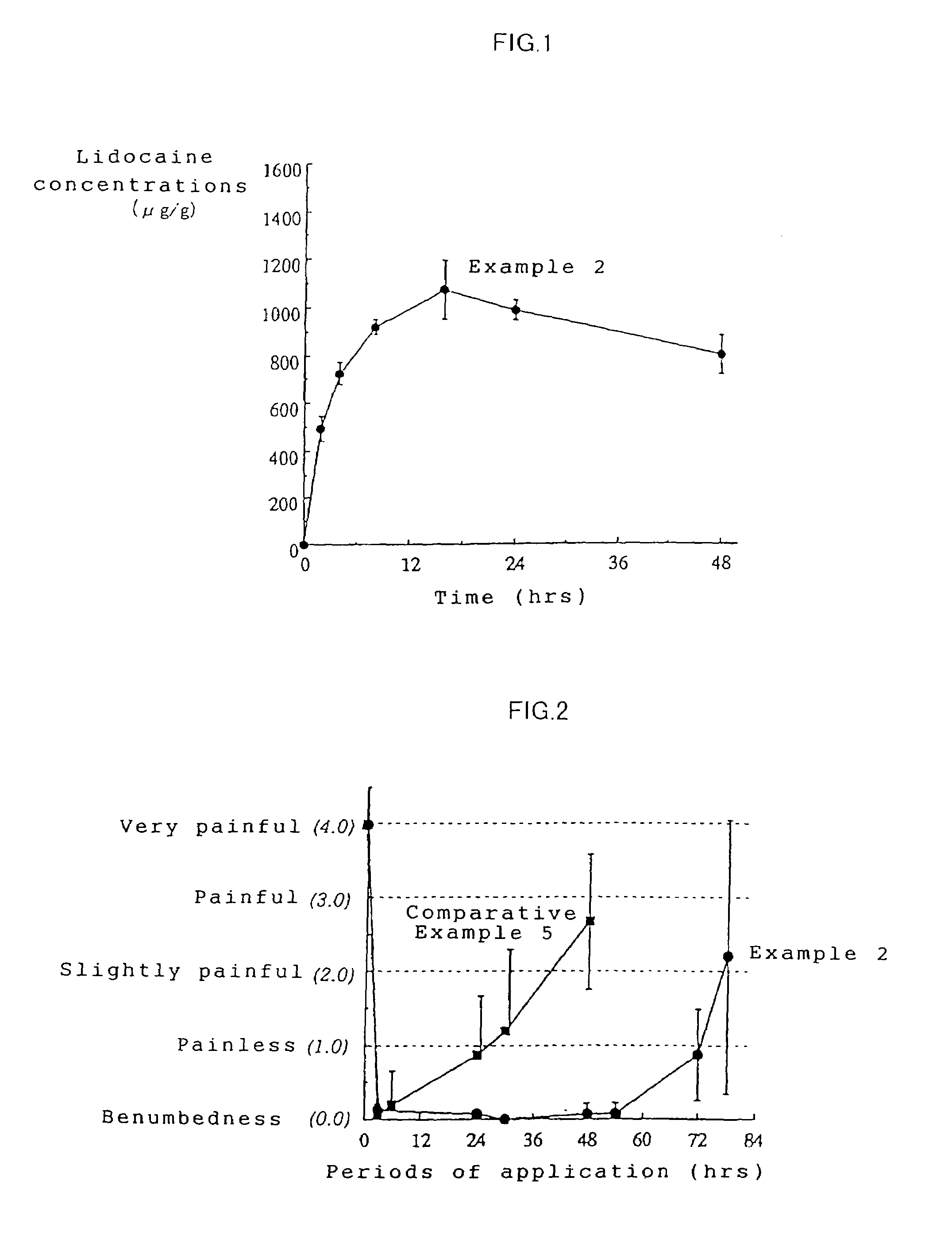
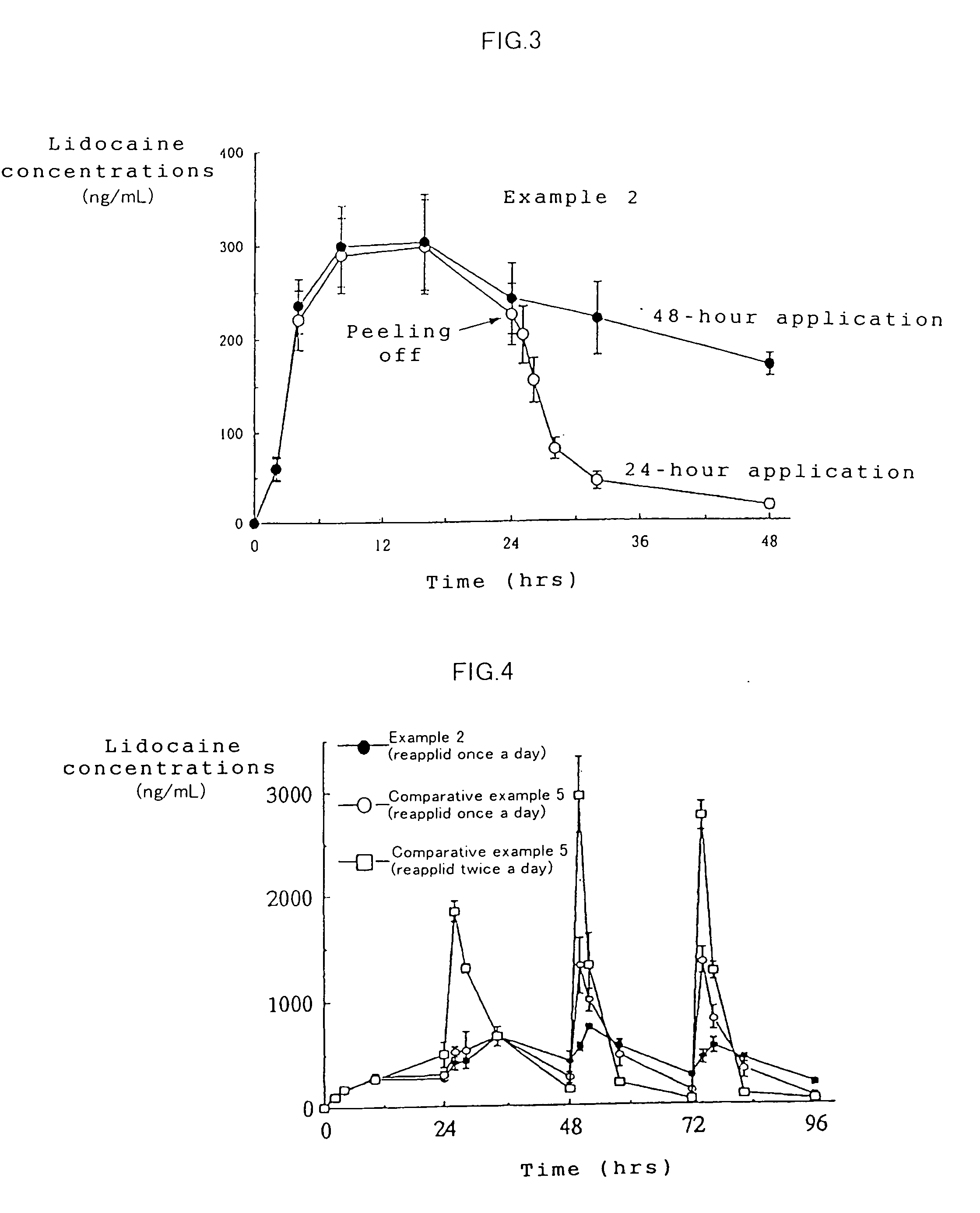
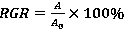Patents
Literature
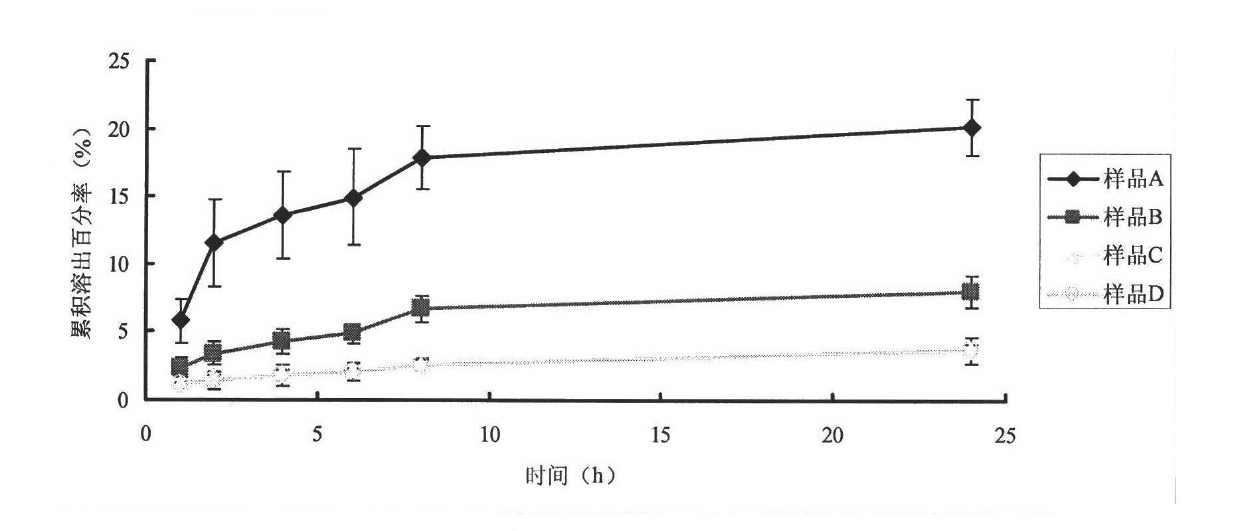
1983 results about "Transdermal absorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transdermal Absorption. What this means is that anything we put on our skin has the possibility of being absorbed. This process is called Transdermal Absorption. And this is the process that makes things like sore muscle rubs and topical medicines work.
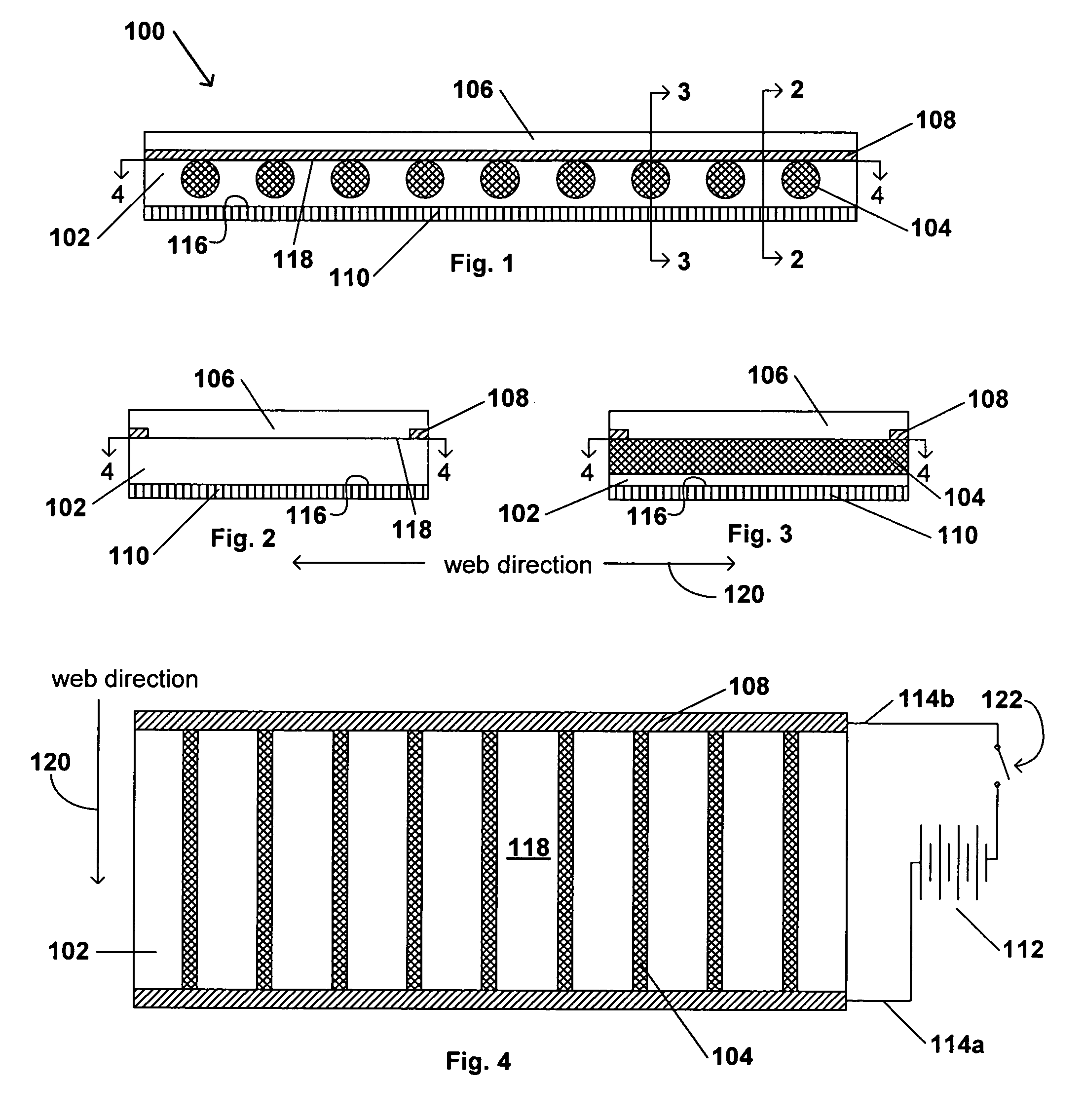
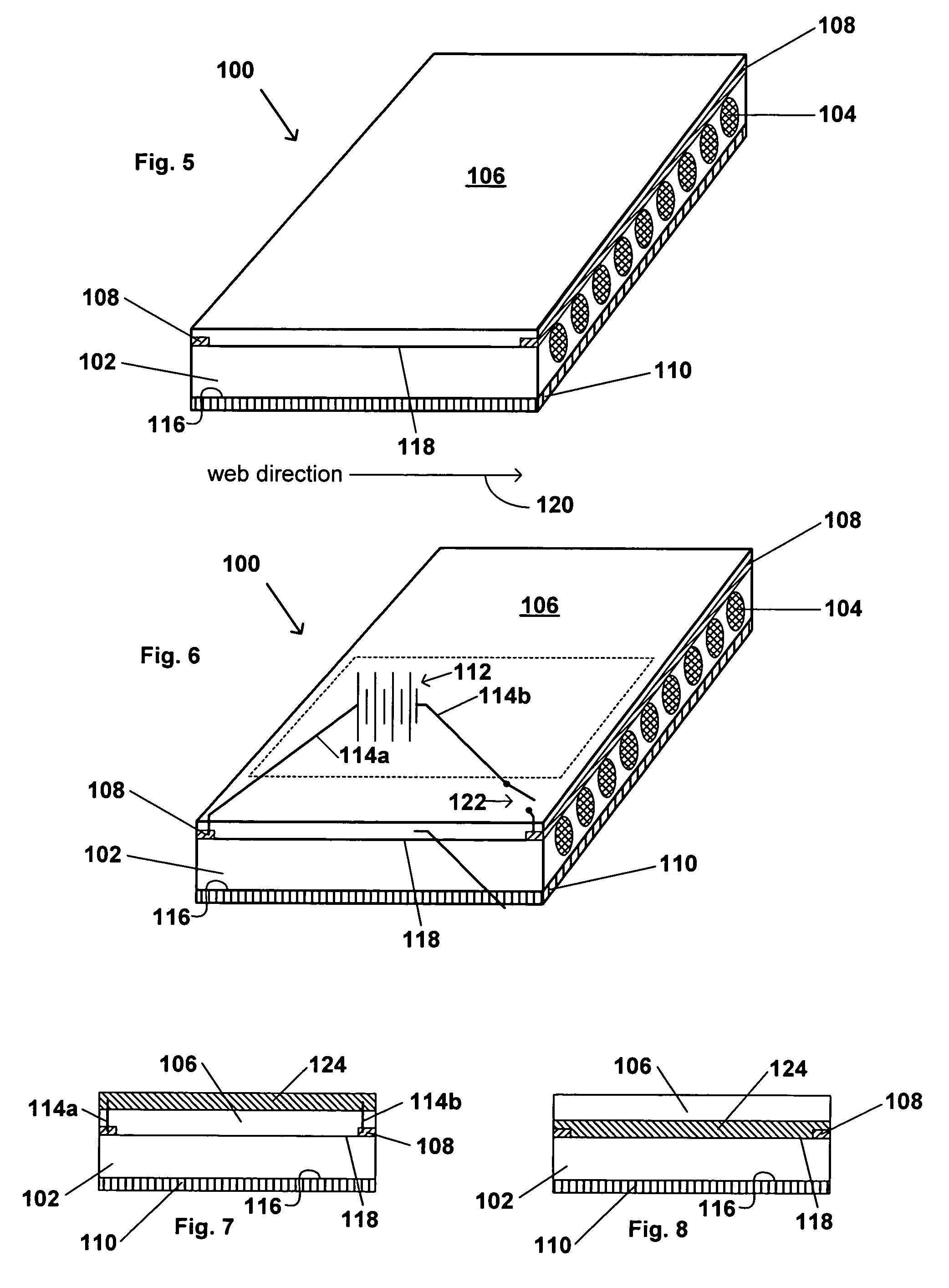
Transdermal contraceptive delivery system and process
InactiveUS7045145B1Cut skinReduce concentrationOrganic active ingredientsAdhesive dressingsObstetricsAdhesive
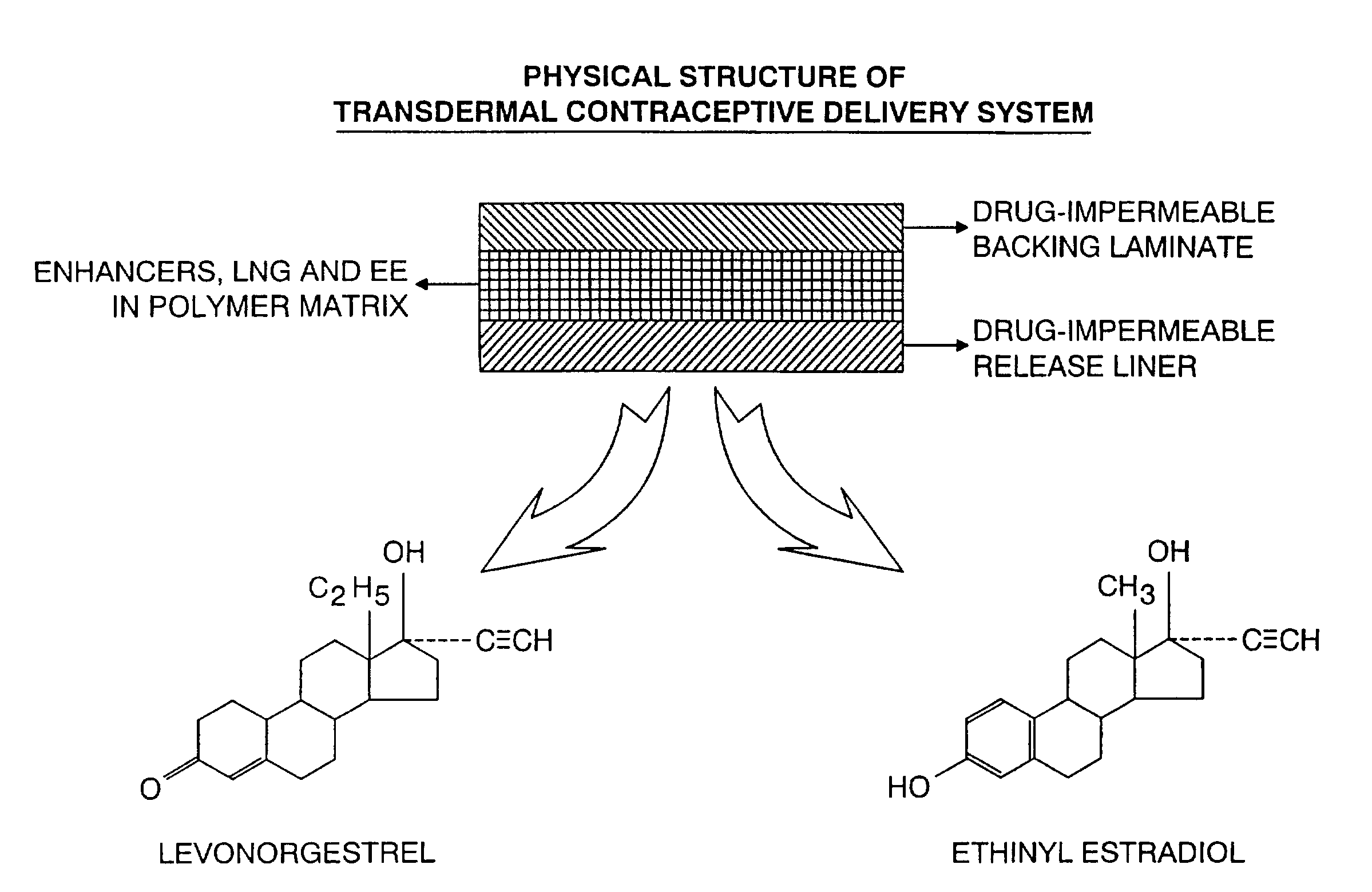
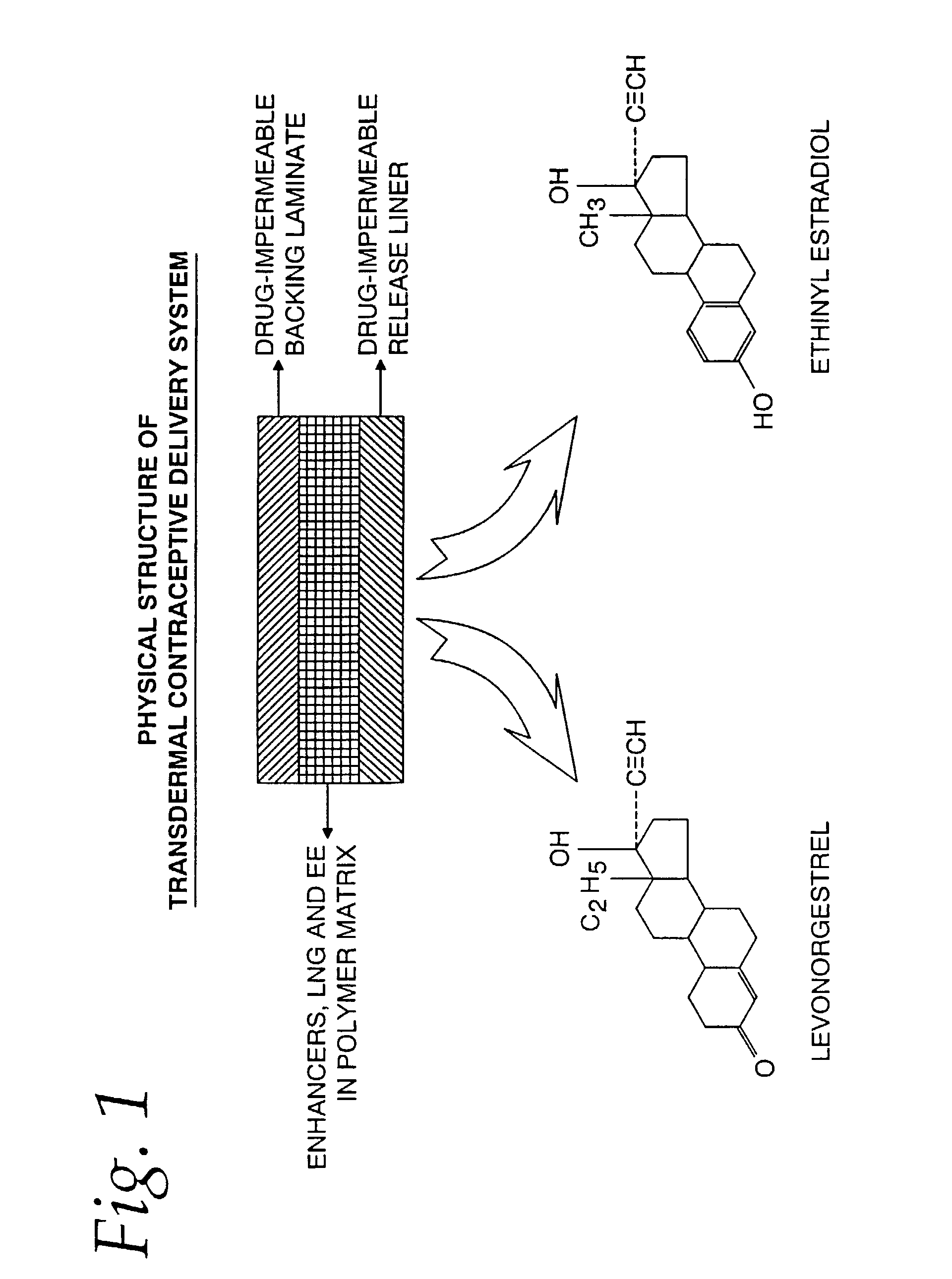
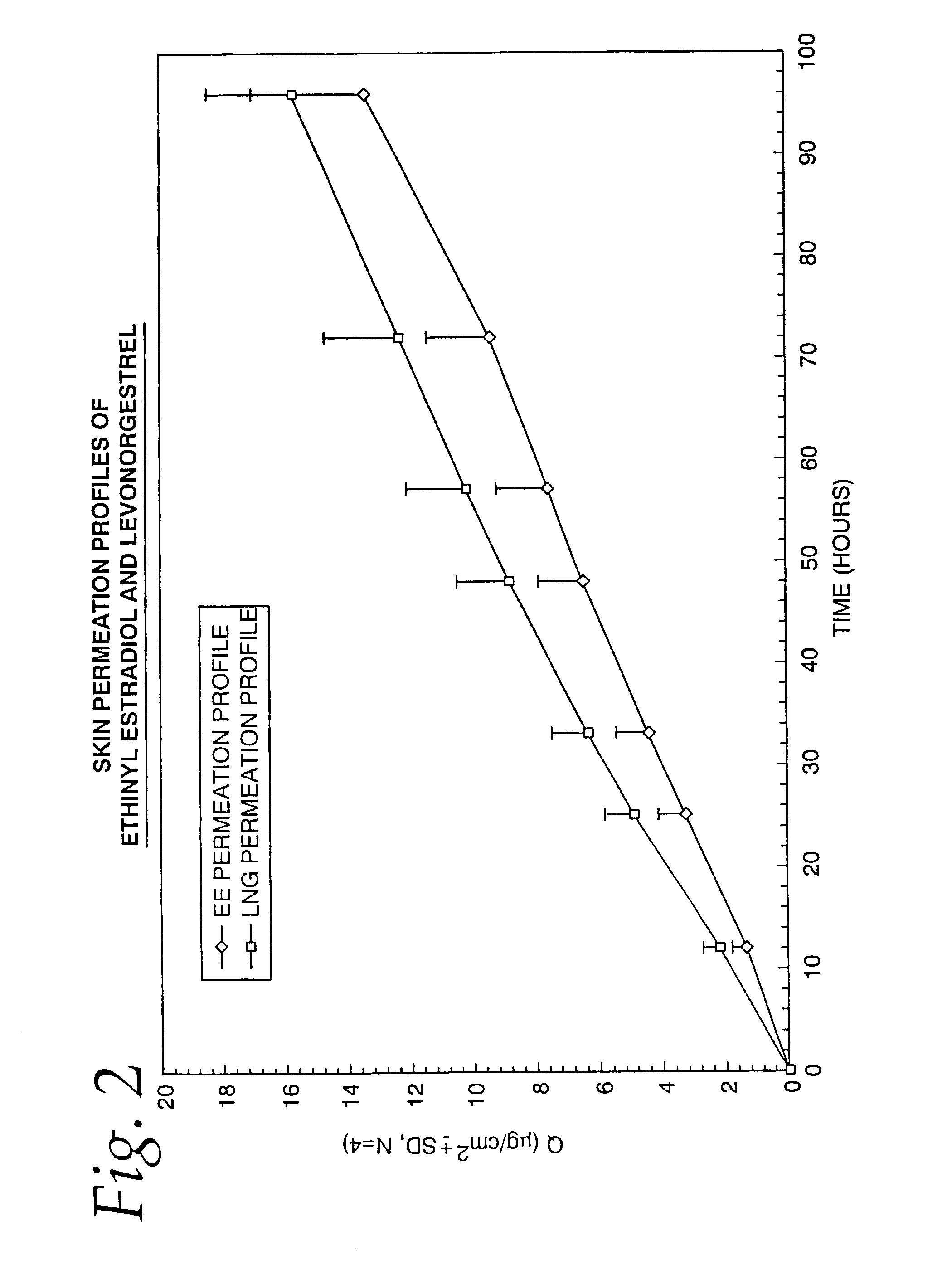
A transdermal contraceptive delivery system (TCDS) for fertility control in women is described. It comprises a backing layer, an adjoining layer of a solid absorption adhesive polymer matrix in which effective daily doses of an estrogen and a progestin are dispersed and released for transdermal absorption. Presently preferred is the use of the synthetic estrogen, ethinyl estradiol, and the synthetic progestin, levonorgestrel. Along with these two steroidal contraceptive agents, a combination of several chemical skin permeation enhancing agents, including capric acid, blended at specific weight ratios, ranging from 2:1:1:0.8 to 6:1:1:0.8, are homogeneously dispersed in the adhesive polymer matrix. The invention also provides a method of fertility control utilizing the transdermal contraceptive delivery system.
Owner:AGILE THERAPEUTICS
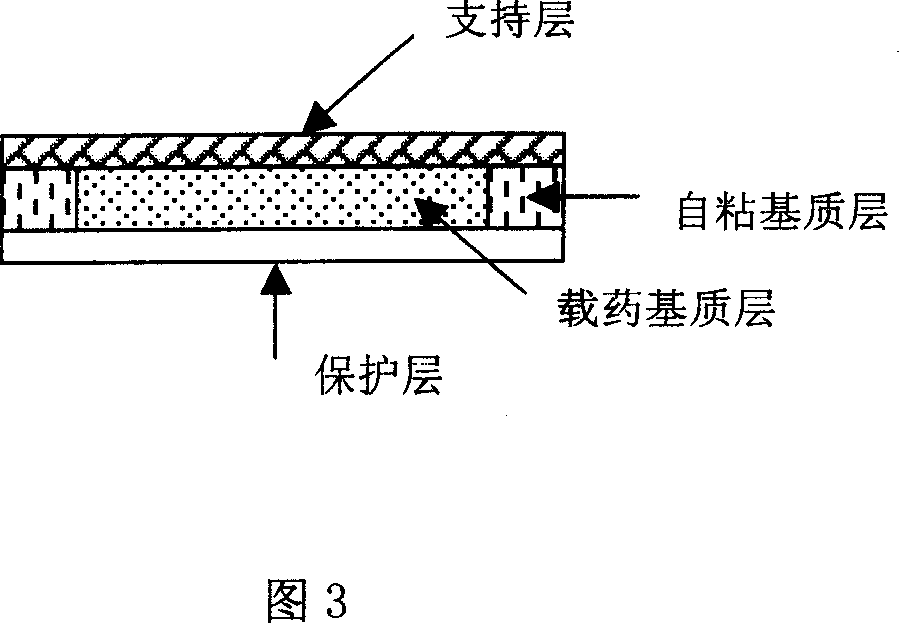
Transdermal Absorption Patch
It is intended to provide a transdermal absorption patch which is highly excellent in transdermal absorption properties and long-lasting drug effect even in the case where a drug-effect component contained in the transdermal absorption patch is a basic drug hardly soluble in a pressure-sensitive adhesive base, has a high stability of the drug contained therein with the passage of time and can achieve improvement in the compliance and simplification of the administration method. These problems can be solved by providing a transdermal absorption patch which contains a basic drug having an octanol / water partition coefficient (logarithm) in the free state of 3 or above, a pressure-sensitive adhesive base and a (meth)acrylic copolymer having carboxyl group.
Owner:HISAMITSU PHARM CO INC
Micro-current Iontophoretic Percutaneous Absorptive Patch
A self-contained, conveniently disposable percutaneous absorptive patch of the present invention utilizes, in a novel ways, the existing percutaneous absorptive principles to delivering topical medications quickly, painlessly, and effectively through the skin barrier. The absorptive principles include the epidermal microcuts / micro-needles, permeation enhancers, and micro-current iontophoresis. Most importantly, the transdermal absorptive patch of the present invention introduces a novel incorporation of self-producing micro-electrical currents and micro-electrodes into the existing conventional iontophoretic principle.
Owner:VOLT KEVIN
Multiple-layered liposome and preparation method thereof
InactiveUS20070082042A1Good skin permeabilityImprove stabilityDermatological disorderLiposomal deliverySterolIntercellular space
Disclosed are multilayered liposomes for transdermal absorption and a method of preparing the liposomes. The multilayered liposomes are prepared using a mixture of oil-phase components comprising squalane, sterols, ceramides, neutral lipids or oils, fatty acids and lecithins, is 200 to 5000 nm in particle size, and is capable of entrapping a physiologically active substance. The multilayered liposomes entrap a larger amount of a physiologically active substance and are structurally stable when encapsulating the physiologically active substance, compared to unilamellar liposomes. Also, they are prepared by a simple and cost-effective process not using a high-pressure homogenizer but using a general homo mixer. Further, since the multilayered liposomes are prepared in a larger size than the intercellular spaces in the stratum corneum, they overcome the tension of surrounding cells when passing through the intercellular spaces and are thus able to penetrate into the dermal layer, compared to nano-sized unilamellar liposomes. Thus, the multilayered liposomes are useful for enhancing the transdermal absorption of physiologically active substances.
Owner:BIOSPECTRUM
Anti-inflammatory analgesic adhesive patch for external use
ActiveUS20120283671A1Promote absorptionLess irritatingAntipyreticAnalgesicsAdditive ingredientTackifier
An external patch containing diclofenac hydroxyethylpyrrolidine prepared by laminating an adhesive layer on a backing, wherein said adhesive layer is characterized by comprising 5-50% by weight of styrene•isoprene•styrene block copolymer, 20-50% by weight of a tackifier resin, 5-70% by weight of a softening agent, and 0.5-20% by weight of one or more solubilizers selected from N-methyl-2-pyrrolidone, propylene glycol and dimethyl sulfoxide as essential ingredients, and 0.5-20% by weight of diclofenac hydroxyethylpyrrolidine as an active ingredient. The patch has excellent transdermal absorption, less skin-irritation and excellent stability of the drug.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
Transdermal absorption promoter, and external skin formulation thereof
InactiveUS20130084257A1Small smellImprove securityCosmetic preparationsBiocideIrritationMethyl group
The present invention provides a substance which promotes the transdermal absorption of a pharmacologically active component while little irritating the skin. The present invention relates to a transdermal absorption promoter which comprises, as the active component, at least one member selected from among isopulegol, 2-(menthoxy)ethanol and 2-methyl-3-(menthoxy)propane-1,2-diol; and an external skin formulation which comprises a pharmacologically active component such as a psychotropic component, an anti-inflammatory component, an analgesic component, an antipyretic component, a whitening component or a hair growth-promoting component, together with the aforesaid transdermal absorption promoter.
Owner:TAKASAGO INTERNATIONAL CORPORATION
External patch containing estrogen and/or progestogen
InactiveUS8486442B2Stable drug releaseLess irritatingBiocideOrganic active ingredientsIrritationProlonged release
An external patch capable of stable prolonged release and transdermal absorption of active ingredient hormones (estrogens and / or progestogens) contained in a pressure sensitive adhesive layer, which external patch ensures low irritation on the skin. In particular, an external patch comprising a support and, superimposed thereon, a pressure sensitive adhesive layer, characterized in that the pressure sensitive adhesive layer comprises, as indispensable components, 5 to 50 wt. % of styrene / isoprene / styrene block copolymer, 20 to 70 wt. % of tackifier resin, 10 to 60 wt. % of softener and 1 to 20 wt. % of polyvinylpyrrolidone and contains, as an active ingredient, estrogen and / or progestogen.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
Skin preparation for external use characterized by containing sugar derivative of alpha, alpha-trehalose
ActiveUS20070003502A1Prevent and improve rough skinPrevent and improve and suntanAntibacterial agentsCosmetic preparationsScattering effectSugar derivatives
The present invention has an object to provide an external dermatological formulation having satisfactory blood flow-promoting effect, antiinflammatory effect, antibacterial effect, moisturizing effect, whitening effect, UV-absorbing effect, UV-scattering effect, antioxidant effect, hair growing effect, hair nourishing effect, emusifying effect, astringent effect, wrinkle-reducing effect, cell-activating effect and / or transdermal absorption-promoting effect with a satisfactory safety and skin feeling; The object is solved by providing an external dermatological formulation comprising a saccharide derivative of α,α-trehalose and one or more members selected from substances having any one of blood flow-promoting effect, antiinflammatory effect, antibacterial effect, moisturizing effect, whitening effect, UV-absorbing effect, UV-scattering effect, antioxidant effect, hair growing efect, hair nourishing effect, emulsifying effect, astringent effect, wrinkle-reducing effect, cell-activating effect and transdermal absorption-promoting effect.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Transdermal patch containing rasagiline for treatment or prophylaxis of nervous system disease and its preparation process
InactiveUS20090136549A1Improve skin penetrationTreatment or prophylaxis of nervous system diseasesBiocideOrganic active ingredientsTransdermal patchPharmacy
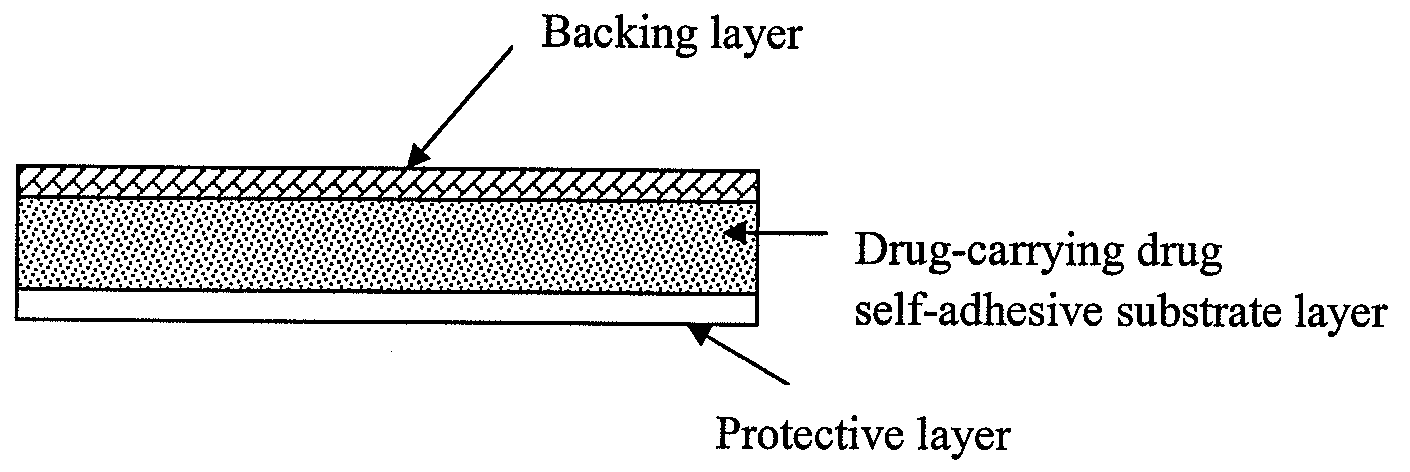
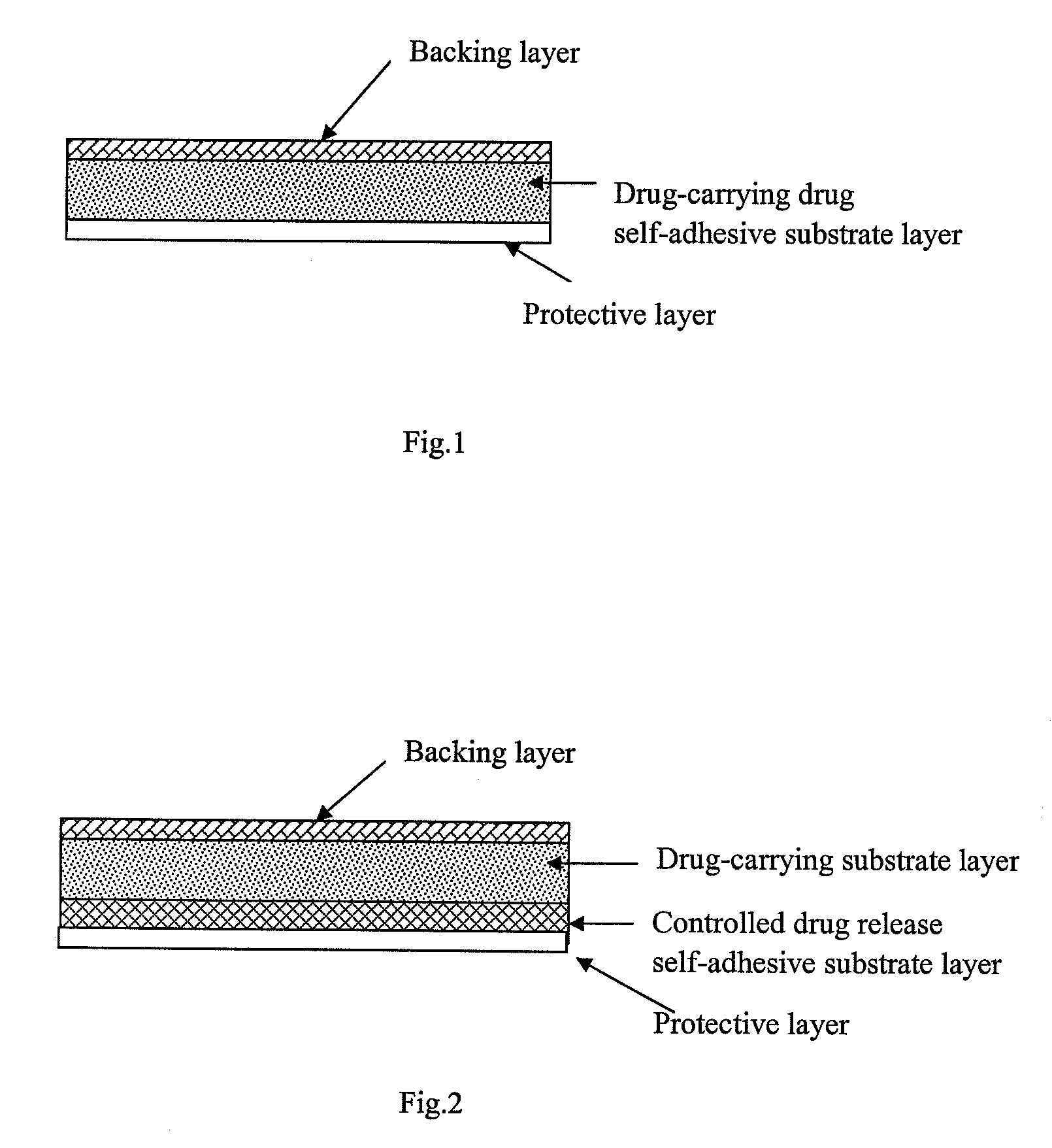
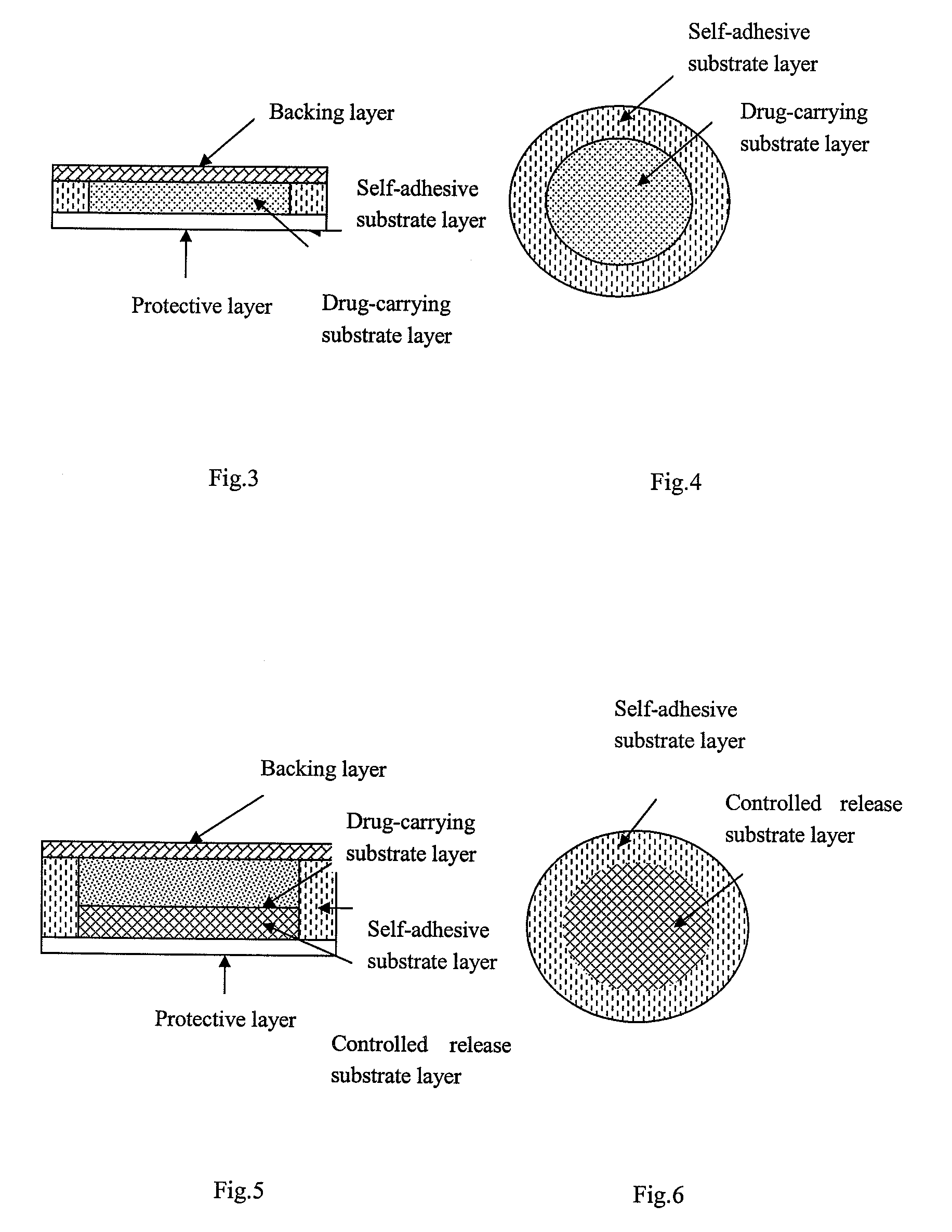
The present invention relates to a rasagiline transdermal patch for treatment or prophylaxis of nervous system diseases, in which the patch comprises an inert backing layer chemically inert to substrate ingredients, a substrate layer comprising rasagiline or a pharmaceutically acceptable salt thereof, and a protective layer to be peeled off before use. The substrate layer is an adhesive system comprising an organic polymer material as basis and an inorganic or organic material as filler, and a plurality of micro-reservoirs containing rasagiline. The substrate further comprises one or more substances for enhancing the transdermal absorption of rasagiline, in which the above organic polymer material in the substrate is used for the reservoir of rasagiline and as adhesive.
Owner:CHONGQING PHARMA RES INST +1
Sterile polymerized covering dressing for wound surface
Owner:JIANGSU HUAYI CELL & TISSUE ENG CO LTD
Skin preparation for external use characterized by containing sugar derivative of a,a-trehalose
InactiveCN1761450AAnti-inflammatoryHas whitening effectAntibacterial agentsOrganic active ingredientsAntiinflammatory EffectSugar derivatives
The present invention has an object to provide an external dermatological formulation having satisfactory blood flow-promoting effect, antiinflammatory effect, antibacterial effect, moisturizing effect, whitening effect, UV-absorbing effect, UV-scattering effect, antioxidant effect, hair growing effect, hair nourishing effect, emusifying effect, astringent effect, wrinkle-reducing effect, cell-activating effect and / or transdermal absorption-promoting effect with a satisfactory safety and skin feeling; The object is solved by providing an external dermatological formulation comprising a saccharide derivative of alpha , alpha -trehalose and one or more members selected from substances having any one of blood flow-promoting effect , antiinflammatory effect, antibacterial effect, moisturizing effect, whitening effect, UV-absorbing effect, UV-scattering effect, antioxidant effect, hair growing efect, hair nourishing effect, emulsifying effect, astringent effect, wrinkle-reducing effect, cell-activating effect and transdermal absorption-promoting effect.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
External preparation composition comprising fatty acid-based ionic liquid as active ingredient
ActiveUS20100256174A1Promote transdermal absorptionPoor transdermal absorbabilityBiocideNervous disorderIonic compositionBULK ACTIVE INGREDIENT
Disclosed is an external preparation composition having good transdermal absorbability. An external preparation composition having excellent transdermal absorbability can be produced by dissolving a medicinal substance or a salt thereof in a fatty acid-based ionic liquid to form a composite ionic composition of the medicinal substance. The external preparation composition can be used as a liquid preparation, an ointment, a cream, a plaster or the like, and enables to provide a preparation having excellent transdermal absorbability.
Owner:MEDRX CO LTD
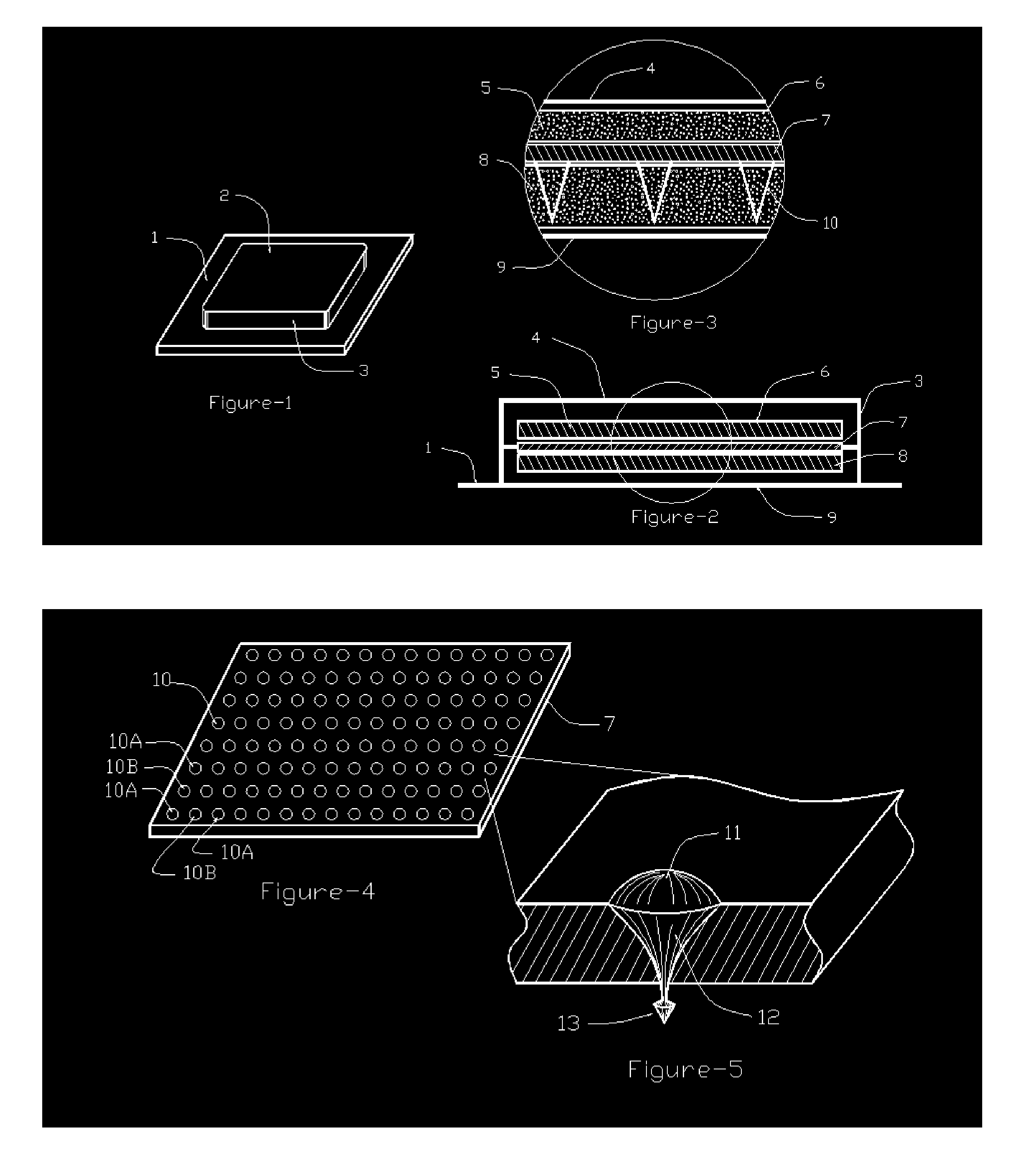
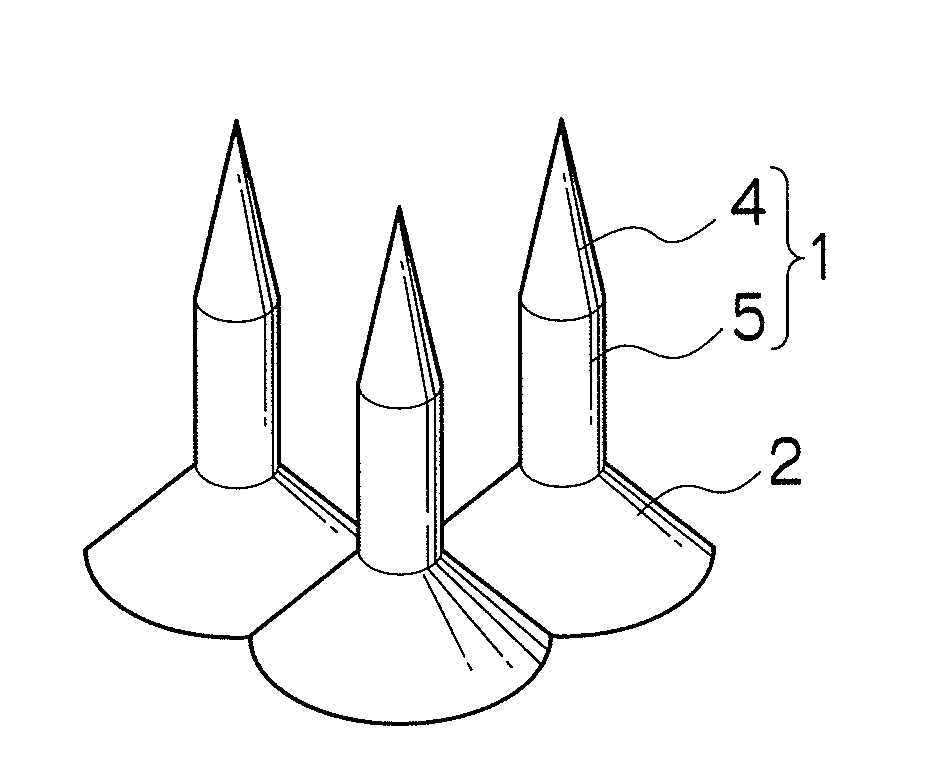
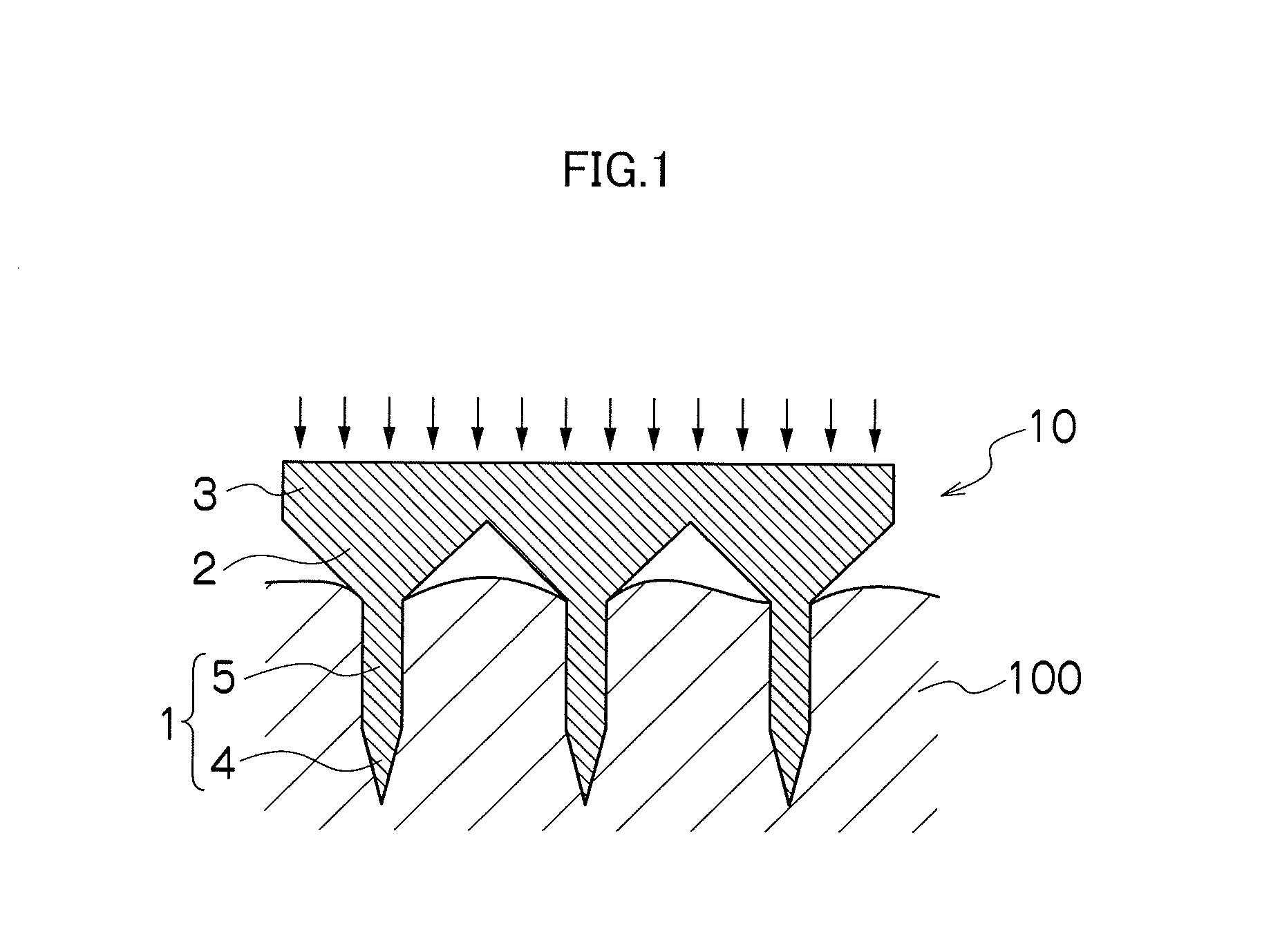
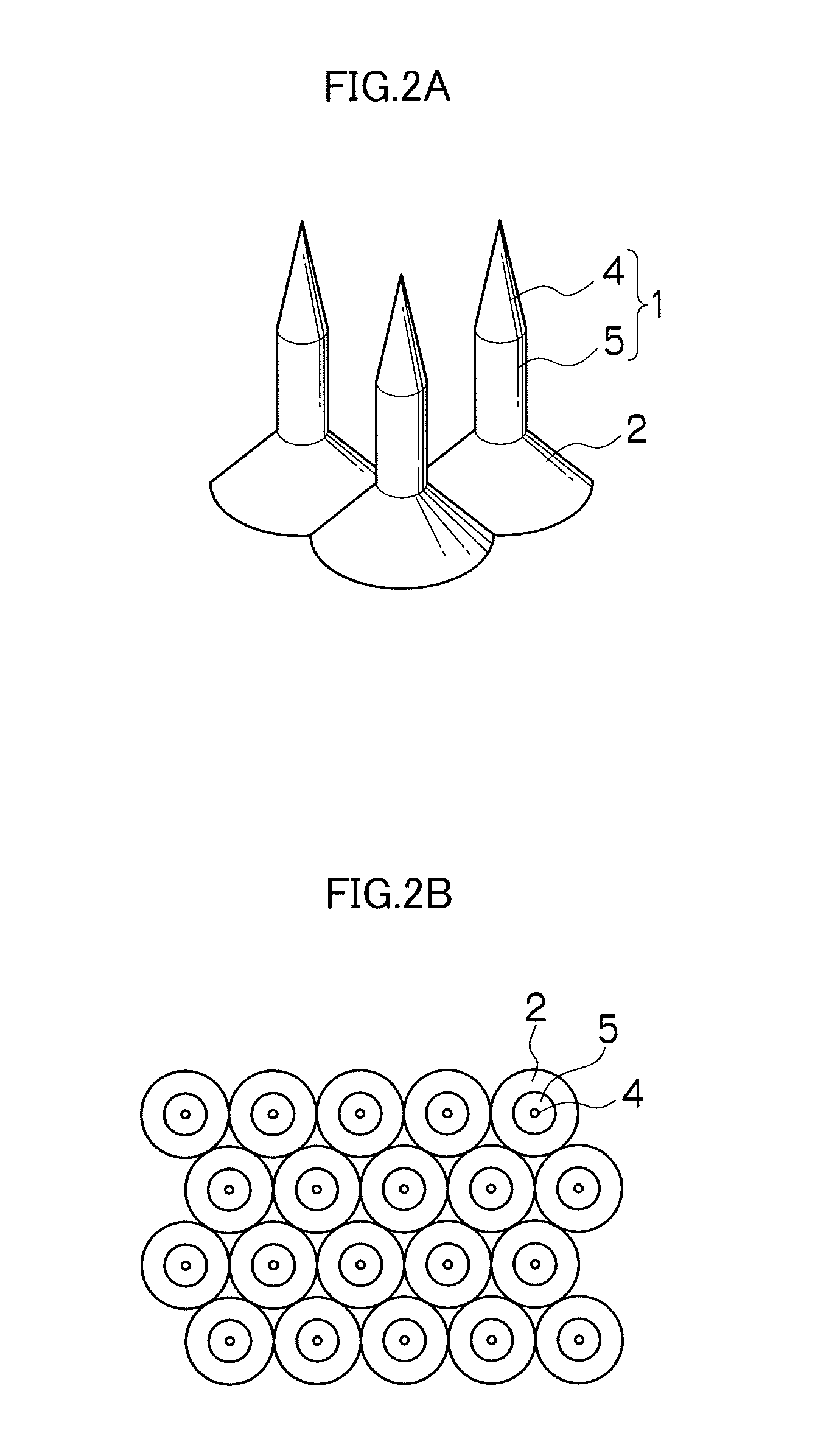
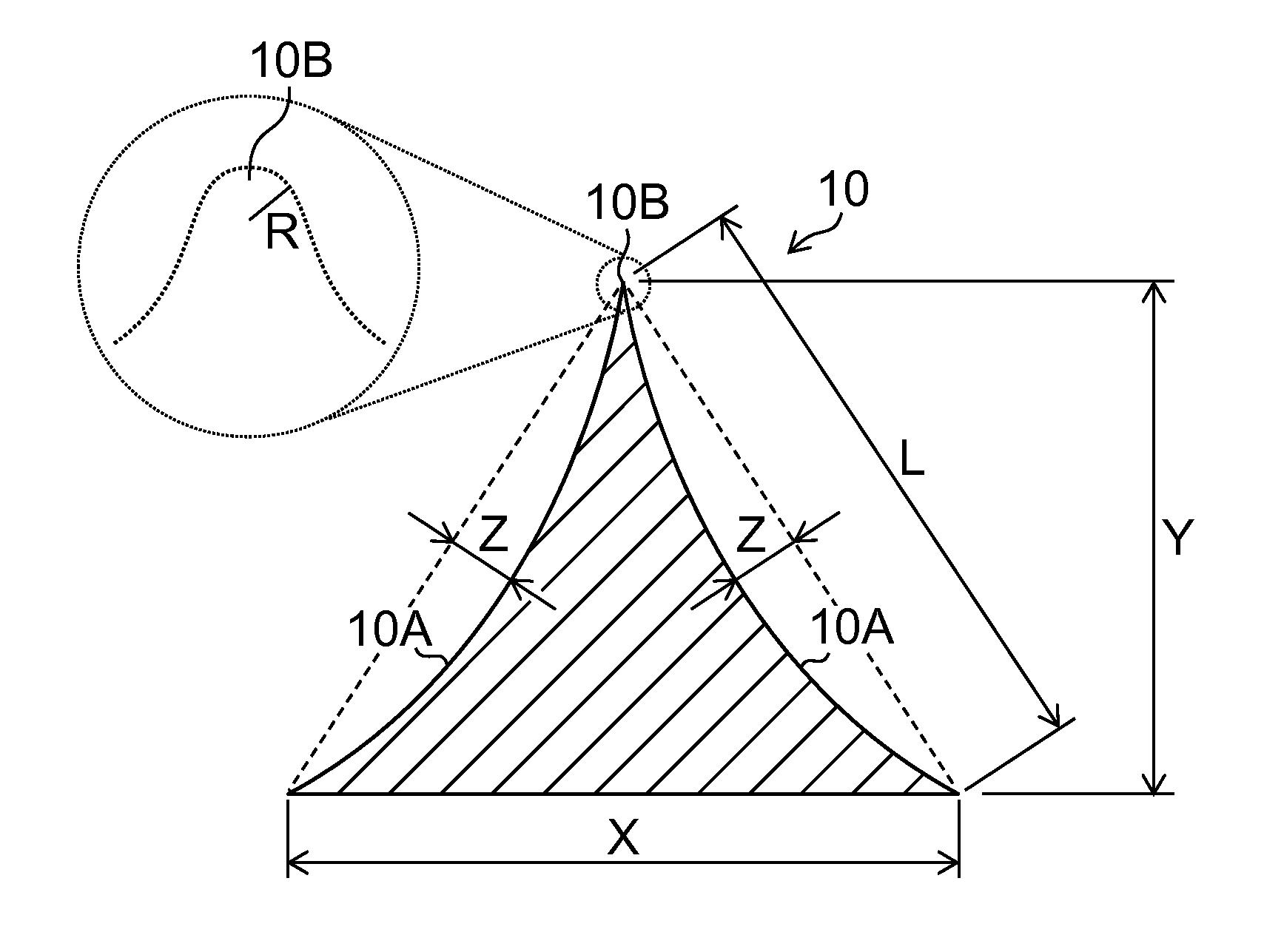
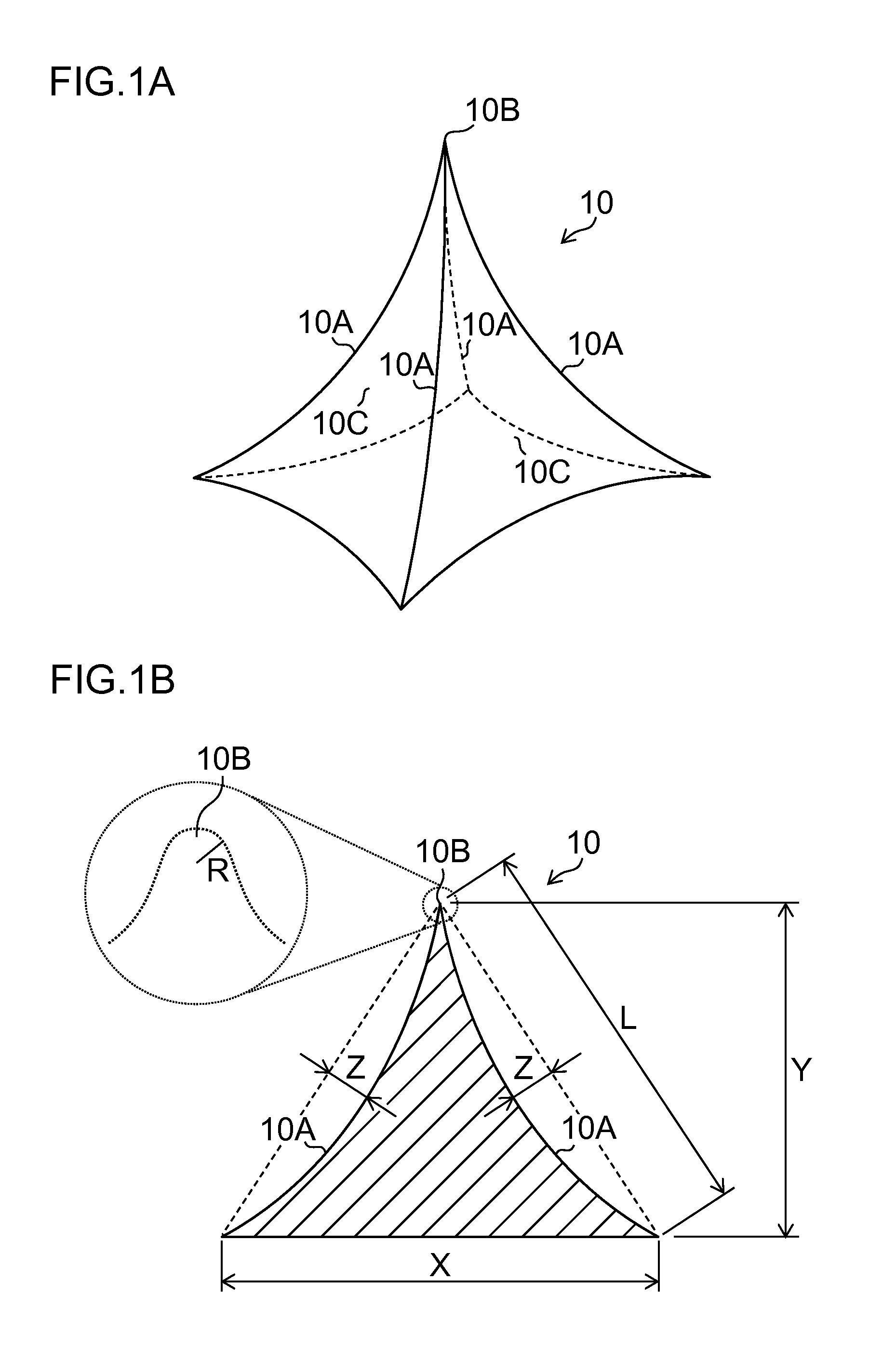
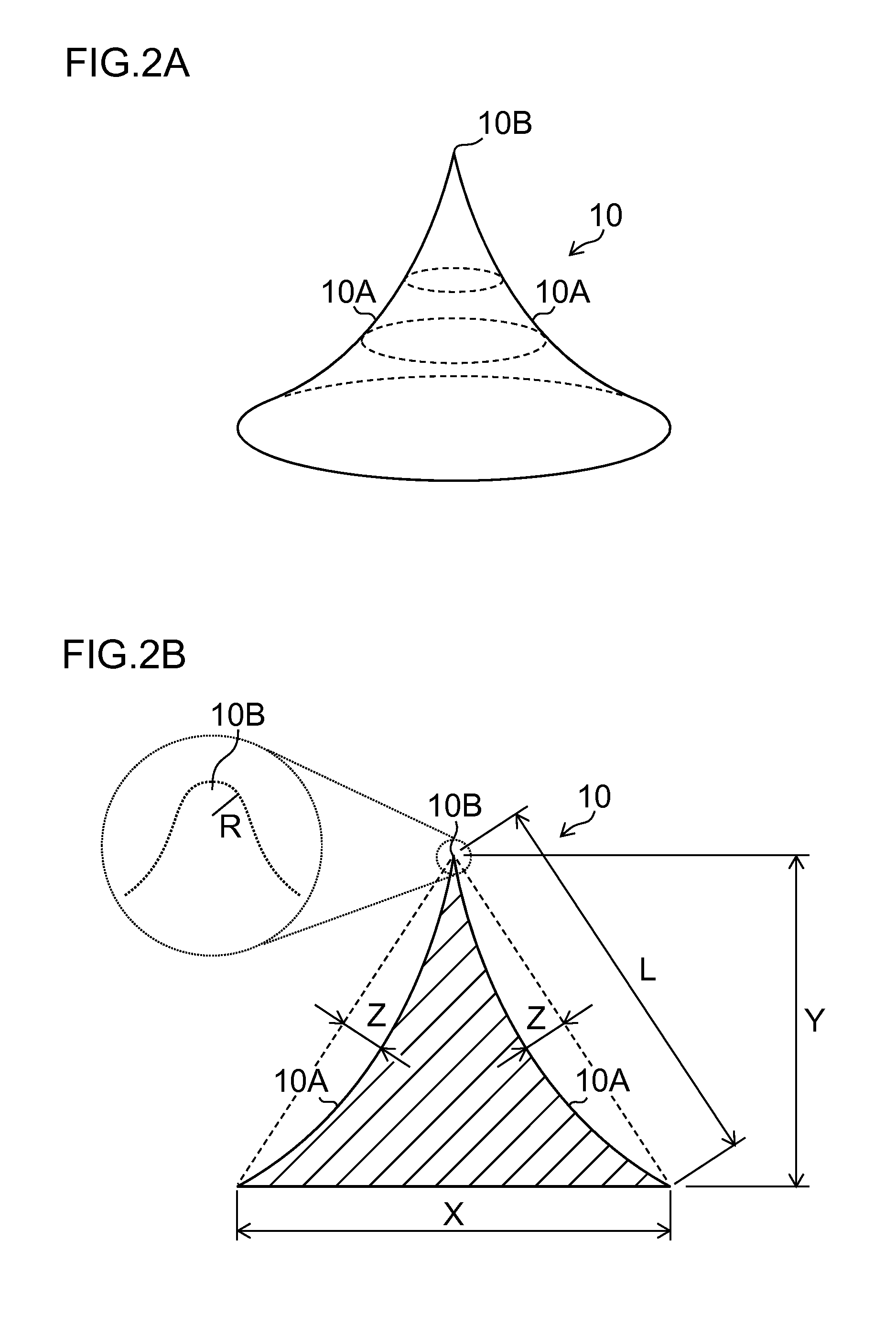
Needle array transdermal absorption sheet and method for manufacturing needle array transdermal absorption sheet
A needle array transdermal absorption sheet to be attached onto a skin for supplying a drug into the skin, includes: a plurality of needle portions each having a tapered shape, each of the needle portions including a needle having a conical or pyramidal shape and a body part which has a columnar shape and whose end surface is connected to a base of the needle; a sheet portion having a flat-plate shape; and a plurality of frustum portions each having a frustum shape, the frustum portions which are arranged on a surface of the sheet portion in a manner that perimeters of larger bases of adjacent frustum portions are in contact with each other on the surface of the sheet portion, and smaller bases of which are respectively connected to the body parts of the needle portions.
Owner:FUJIFILM CORP
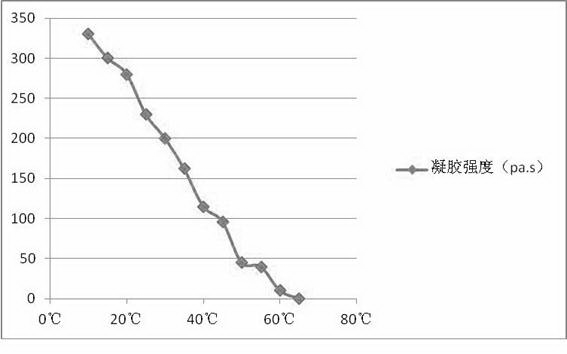
High-temperature stable hydrogel mask
ActiveCN104042449AFast conditioningFast absorptionCosmetic preparationsToilet preparationsPlanting seedSeaweed extract
A high-temperature stable hydrogel mask comprises a carrier net cloth and hydrogel. The hydrogel comprises the following components by weight: 0.1%-1% of a cellulose derivative water-soluble polymer, 0.5%-2% of seaweed extract natural water-soluble polymer, 0.1%-2% of extracellular polysaccharide natural water-soluble polymer generated by microbial fermentation, 0.1%-2% of plant seed or root extracted natural water-soluble polymer, 0.1%-2% of electrolyte required by cross-linking initiation, 0.5%-5% of a skin conditioner, 0.1%-1% of a transdermal absorption promoter, 5%-20% of polyol, 0.1%-1% of a preservative and the balance of pure water. The high-temperature stable hydrogel mask meets the requirements for physicochemical indexes of mask in Light Industry Standard of the People's Republic of China ''QB-T2872-2007 mask''. The hydrogel on the carrier net cloth maintains stable in the temperature range of -15 to 55 DEG C, and improves the stability of the product during transport and storage.
Owner:NOX BELLCOW COSMETICS CO LTD
Skin-contacting heatable dressing
ActiveUS7238196B2Substantial degree of mobility and freedom of movementElectrotherapyAdhesive dressingsFiberPolymer science
A skin-contacting heatable dressing including a pressure-sensitive adhesive layer having a first skin-contacting side and a second side; heat generating conductive carbon fibers contained within the skin-contacting pressure sensitive adhesive layer; and a source of electrical energy electrically connected to the carbon fibers. In one embodiment, the pressure-sensitive adhesive layer may include a transdermally absorbable medicament. In one embodiment, the dressing may include a backing layer. In one embodiment, the source of electrical energy may be a thin film battery, which may supplement or take the place of the backing layer.
Owner:AVERY DENNISON CORP
Rasagiline transparent patch for curing and preventing neurological diseases and the preparing method thereof
The present invention relates to one kind of transdermal rasagiline medicine plaster for preventing and treating neurological diseases and its preparation process. The transdermal rasagiline medicine plaster includes one support layer without chemical reaction with the matrix components, one matrix layer containing rasagiline or its pharmaceutically acceptable salt, and operate protecting layer being torn off before the plaster is used. It features the matrix layer with polymer as the basic material, adhering system with inorganic or organic filler, stored rasagiline and matter(s) to promote the transdermal absorption of rasagiline.
Owner:CHONGQING PHARMA RES INST +1
Method for manufacturing transdermal-absorption sheet
InactiveUS20150238413A1Improve production efficiencyEfficient preparationMouldsMicroneedlesEngineeringPartial filling
Owner:FUJIFILM CORP
Coenzyme q10 containing proliposome and preparation thereof
InactiveUS20060251708A1Improve stabilityMixing of flexible and convenientCosmetic preparationsPeptide/protein ingredientsLipid formationSpray dried
A coenzyme Q10 and ceramide-containing preliposome, and pharmaceutical preparations and cosmetics containing the same. The preliposome can further comprise other lipid components. A granular and lyophilized solid-preparation is produced by lyophilization or spray drying. Then, the coenzyme Q10-containing proliposome is obtained by adding water in the said solid-preparation and shaking. Transdermal absorption of coenzyme is improved together with the effect of coenzyme Q10 in cosmetics and the stability of coenzyme Q10 and liposome, which facilitate the formulation of cosmetics.
Owner:SHANGHAI JAHWA UNITED
Externally used gel preparation containing human umbilical cord mesenchymal stem cell extract as well as preparation method and application of externally used gel preparation
ActiveCN106474155APromote growthShorten healing timeAerosol deliveryOintment deliveryDiseaseMedicine
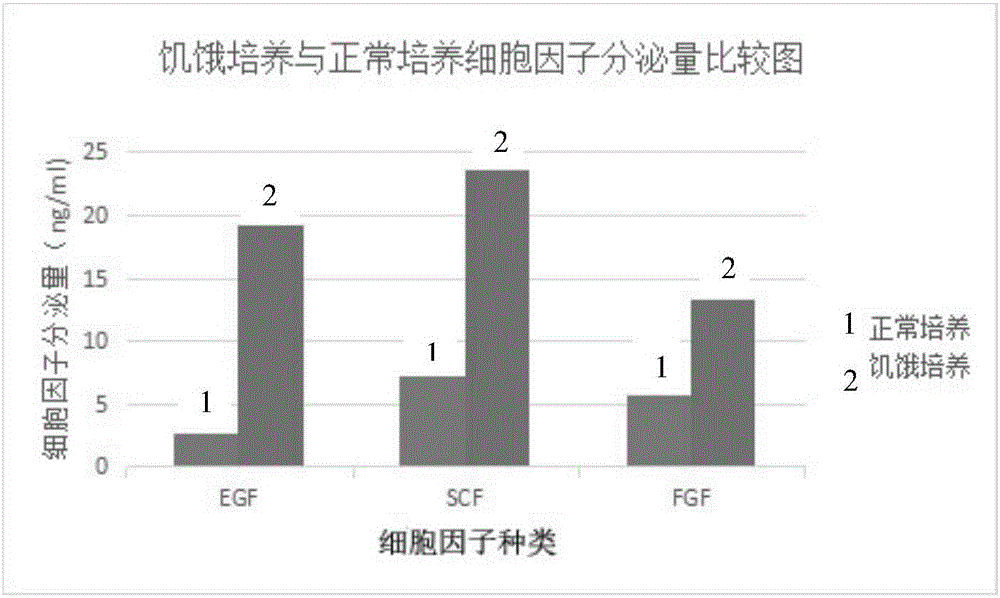
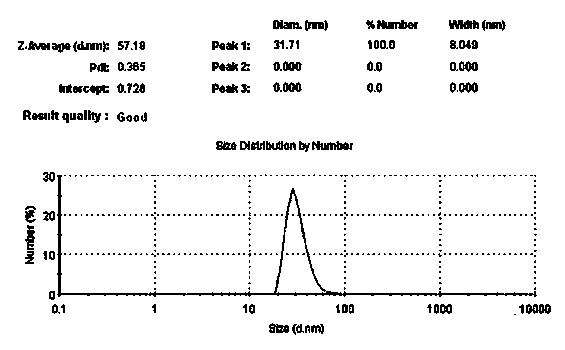
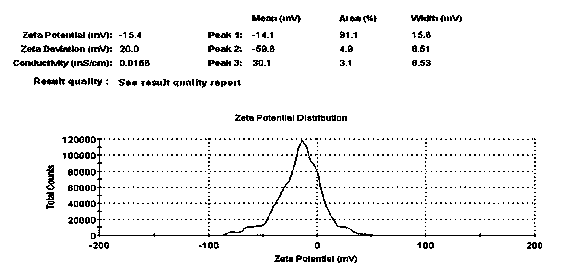
The invention discloses an externally used gel preparation containing human umbilical cord mesenchymal stem cell extract as well as a preparation method and an application of the externally used gel preparation. The externally used gel preparation contains the human umbilical cord mesenchymal stem cell extract and a gel solution, wherein the human umbilical cord mesenchymal stem cell extract is the extract obtained after starvation culture; the gel solution contains sodium alginate, a compound electrolyte injection, a humectant and a penetration enhancer, and a stable and homogeneous gelatinous substance is formed. The externally used gel preparation can be used for preparing drugs for treating skin ulcer, burns, scalds, eczema, trauma and other diseases.
Owner:天津捷渲生物医药科技有限公司 +1
Ginseng-based whitening and anti-aging nanoemulsion essence and preparation method thereof
InactiveCN103462846AEasy to prepareGood repeatabilityCosmetic preparationsToilet preparationsBiotechnologyFruit juice
The invention relates to the technical field of pharmaceutical nanoemulsion drug delivery systems and transdermal absorption, in particular relates to ginseng-containing whitening and anti-aging nanoemulsion essence and a preparation method thereof, and belongs to a new pharmaceutical technology and the field of study on new formulations applied to cosmetics. The nanoemulsion essence is characterized in that a natural or food-grade emulsifier and an oil phase are selected, wherein double distilled water containing fruit juice, vegetable juice or honey is used as an oil phase. The nanoemulsion essence comprises the following components in percent: 10.30 to 80.60% of emulsifier / co-emulsifier, 1.0 to 40.80% of oil phase, 0.10 to 14.0% of ginseng, and the balance of the water phase. The confocal microscopy result shows that the transdermal absorption mechanism of the essence is the mechanism of majorly dispersing among cells of cutaneous appendages at the beginning and then dispersing and penetrating through cytomembrane. The nanoemulsion essence has high absorption, is innocuous, unpoisonous and pollution-free, does not damage the skin, and can completely realize the green, health, whitening, anti-aging and beautifying makeup concept.
Owner:JILIN UNIV
Transdermal absorption preparation of oxybutynin as well as preparation method and medication application thereof
The invention provides a transdermal absorption preparation containing oxybutynin, which comprises gels, ointment and cream and aims at reducing the adverse reaction rate of the oxybutynin and lightening the serious adverse reaction degree, wherein when the content of the oxybutynin in the transdermal absorption preparation is 0.1-30 percent by weight, the content of the oxybutynin in the transdermal absorption preparation is preferentially to be 8-12 percent by weight. The invention also discloses the requirements of the transdermal absorption preparation in the aspects of formula compatibility and proportioning, as well as a preparation method and medication application of the transdermal absorption preparation.
Owner:陕西麦科奥特生物科技有限公司
Method for preparing ultra-low molecular weight hyaluronic acid oligosaccharides and salt thereof through combination of solid-liquid biphasic enzymolysis and ultrafiltration
ActiveCN108220364AReduce dosageIncrease enzyme activityFermentationHigh concentrationUltrafiltration
The invention discloses a method for preparing ultra-low molecular weight hyaluronic acid oligosaccharides and a salt thereof through combination of solid-liquid biphasic enzymolysis and ultrafiltration. The method comprises the following steps: degrading hyaluronic acid and a salt thereof by utilizing hyaluronidase produced by bacilli; preparing high-concentration hyaluronic acid enzymatic hydrolysate by adopting the method of combining a solid-liquid biphasic enzymolysis system and an ultrafiltration system, and then performing inactivating, activated carbon adsorption and impurity removal,filtering and spray-drying to obtain the ultra-low molecular weight hyaluronic acid oligosaccharides and the salt thereof with the molecular weight of less than or equal to 3 kDa. The product preparedby the method is high in stability, ultra-low in molecular weight, good in inflammation resistance and percutaneous absorptivity, high in purity, strong in oxidation resistance, does not have cytotoxicity, has the advantages of repairing skin cell damage and the like, and can be widely applied to the fields of cosmetics, food and medicines. According to the method, the process flow is easy to operate; conditions are mild; various alcohols are not used; the energy consumption is relatively low; the method does not have damage to a product structure, does not have environmental pollution, and is suitable for large-scale industrial production.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD +1
Percutaneous absorption agent containing Ailamode, preparation method and medical uses thereof
InactiveCN101401783AAvoid damageAvoid first pass reactionOrganic active ingredientsAntipyreticPercutaneous absorptionIguratimod
The invention relates to a transdermal absorbent containing Iguratimod, which comprises gellies, ointment, and cream, wherein the mass percentage content of the Iguratimod in the transdermal absorbent is between 0.1 and 10 percent, and preferably the mass percentage content of the Iguratimod in the transdermal absorbent is between 0.4 and 3 percent; and the invention also discloses requirements of the transdermal absorbent on the selections of matrix and a transdermal enhancer. Furthermore, the invention also provides a method for preparing the transdermal absorbent containing the Iguratimod and pharmaceutical application thereof.
Owner:杨喜鸿
Resveratrol flexible liposome and preparation method thereof
InactiveCN101874763AImprove hydrophilicityEfficient penetrationCosmetic preparationsHydroxy compound active ingredientsActive componentMedicine
The invention discloses a resveratrol flexible liposome which is characterized by comprising the following components in mass percent (in terms of the weight of the liposome): 0.1-10% of resveratrol, 0.5-10% of phospholipid and 0.1-30% of flexible agent. In addition, the invention also discloses a preparation method of the resveratrol flexible liposome. The resveratrol flexible liposome of the invention has high flexibility and penetrability and can greatly improve the percutaneous absorption of active components.
Owner:SHANGHAI JAHWA UNITED
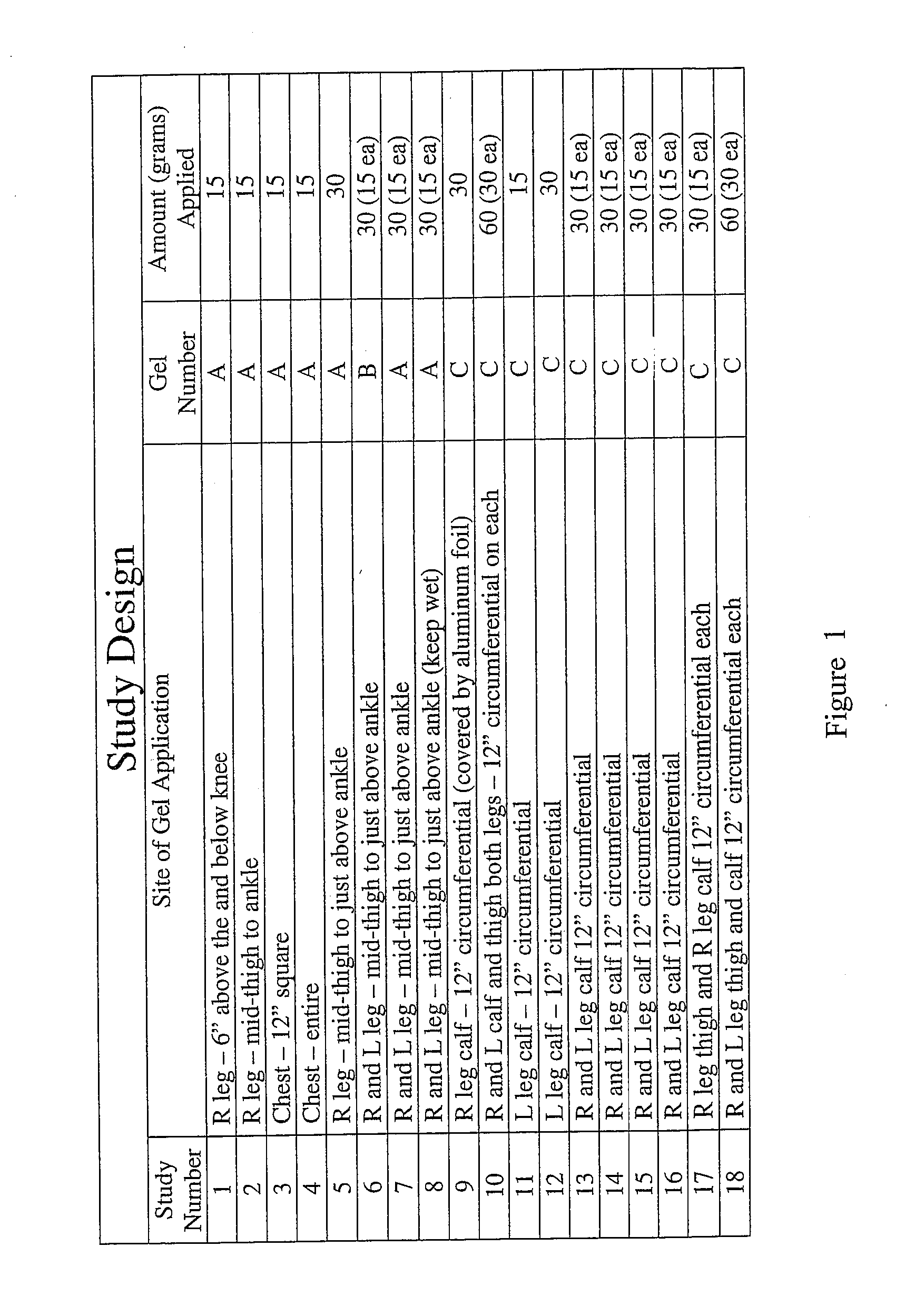
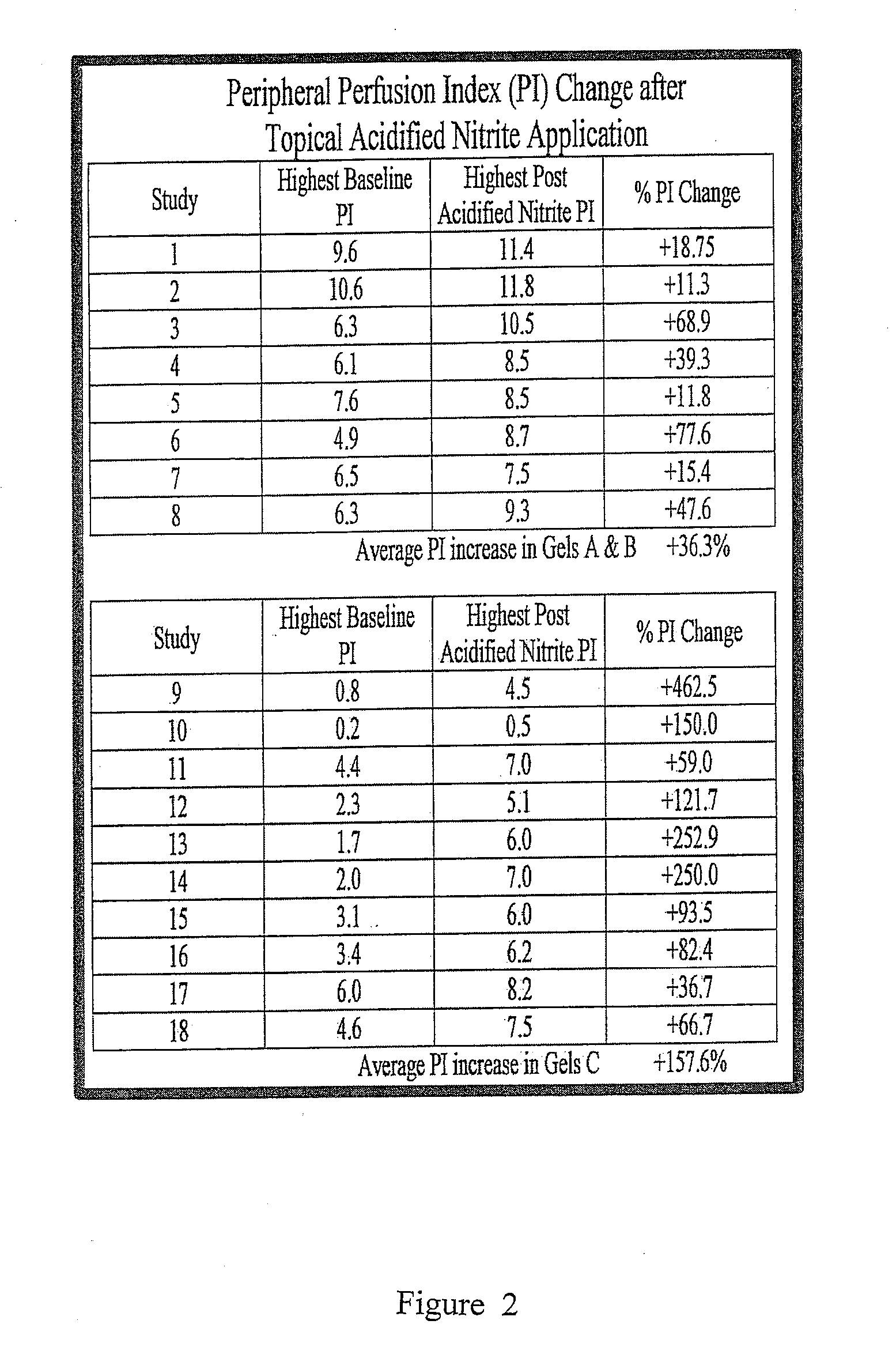
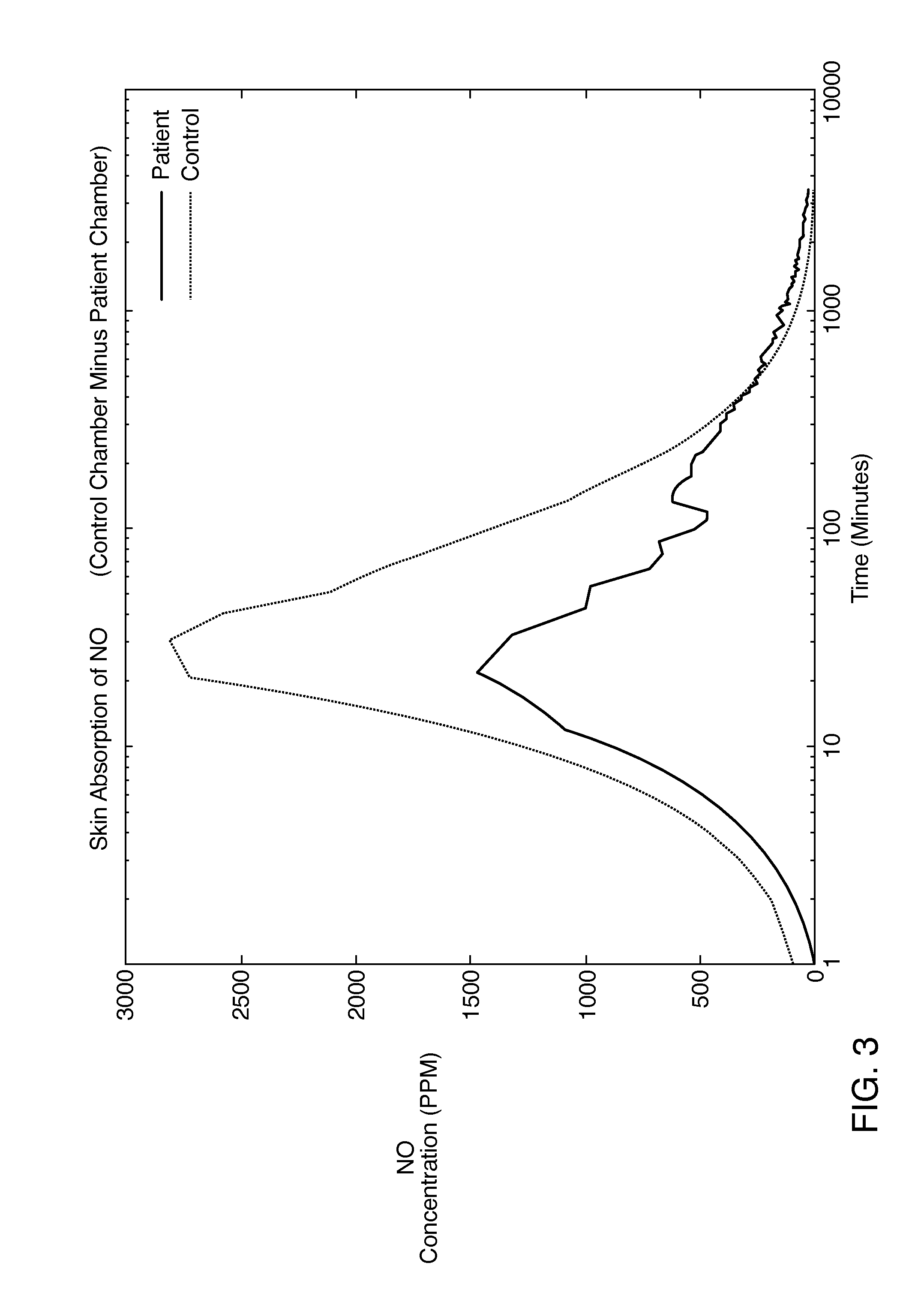
Deep topical systemic nitric oxide therapy apparatus and method
ActiveUS20140335207A1Short timeLong length of timeBiocideInorganic active ingredientsNitric oxide therapyWhole body
A topical mixture that produces nitric oxide and a method for using the topical mixture to increase the vasodilation of a bloodstream via transdermal absorption of the nitric oxide. The nitric oxide can then affect subcutaneous tissues. The systemic vasodilation of a mammal may be increased via a topical application of an appropriate nitric oxide producing substance.
Owner:SYK TECH LLC
Flexible nanoliposomes with charges for cosmetics and preparation method thereof
ActiveCN102397168AProlong the action timeGive full play to the effect of skin care and nourishing skinCosmetic preparationsToilet preparationsCholesterolFreeze-drying
The invention relates to flexible nanoliposomes with charges for cosmetics and a preparation method thereof. The flexible nanoliposomes with the charges for the cosmetics comprise the following components in part by weight: 1-1000 parts of neutral phospholipid, 0.5-500 parts of phospholipid with the charges, 1-1000 parts of cholesterol, 0.5-500 parts of surface active agent, 0.1-20 parts of hyaluronic acid, 0.5-250 parts of cosmetic active ingredients and 100-8000 parts of freeze drying protective agent. The flexible nanoliposomes with the charges for the cosmetics can obviously improve the stabilities of the cosmetic active ingredients, promote the percutaneous absorption and the interaction efficiency of the active ingredients, and also improve the retention and action time of the active ingredients on the skin surface and the skin deep layer; and the flexible nanoliposomes with the charges for the powdery cosmetic active ingredients are also benefit to the use, transportation and storage. The preparation method of the flexible nanoliposomes with the charges can adopt a mechanized method, so the stability of the product technology and quality is good, and the repeatability is high.
Owner:江妍
Beta-elemi alkene bulk medicament and method of preparing its preparations
InactiveCN101402543AHydrocarbon active ingredientsDistillation purification/separationVolatilesSelf emulsifying
The invention provides a method for preparing high-purity beta elemene from natural plants containing the beta elemene such as curcuma zedoary (earthnuts or tubers of the curcuma zedoary), cedronella (fresh leaves of the cedronella), yellowtop (roots, stems, leaves, flowers and seeds of the yellowtop) and so on, which can improve the production efficiency from starting materials to the high-purity beta elemene and reduce production cost. Compared with the prior art, the method is mainly different in that the roots, stems, leaves, flowers and seeds of the natural plants are taken as raw materials; oleum volatile of specific parts of the natural plants is obtained by methods for extracting different oleum volatiles, and is distilled by molecular distillation method to remove compositions with high boiling points and non-volatile compositions; impurity compositions are removed by the ethanol extraction method and silver nitrate complex extraction method; and finally the beta elemene with the content between 95.0 and 99.9 percent is obtained through reduced pressure distillation or rectification. The bulk pharmaceutical chemicals not only can be prepared into oral dosing preparation such as emulsion oral liquid, self-emulsifying / self-microemulsifying capsules, soft capsules and so on, but also can be prepared into non-alimentary dosing preparation such as emulsion injection, liquid drugs injection, transdermal absorbent, lung sprays, suppository and so on. The method has the advantages of novel design, concise operating steps, mild operating conditions and improvement of the production efficiency of the beta elemene.
Owner:沈阳万爱普利德医药科技有限公司
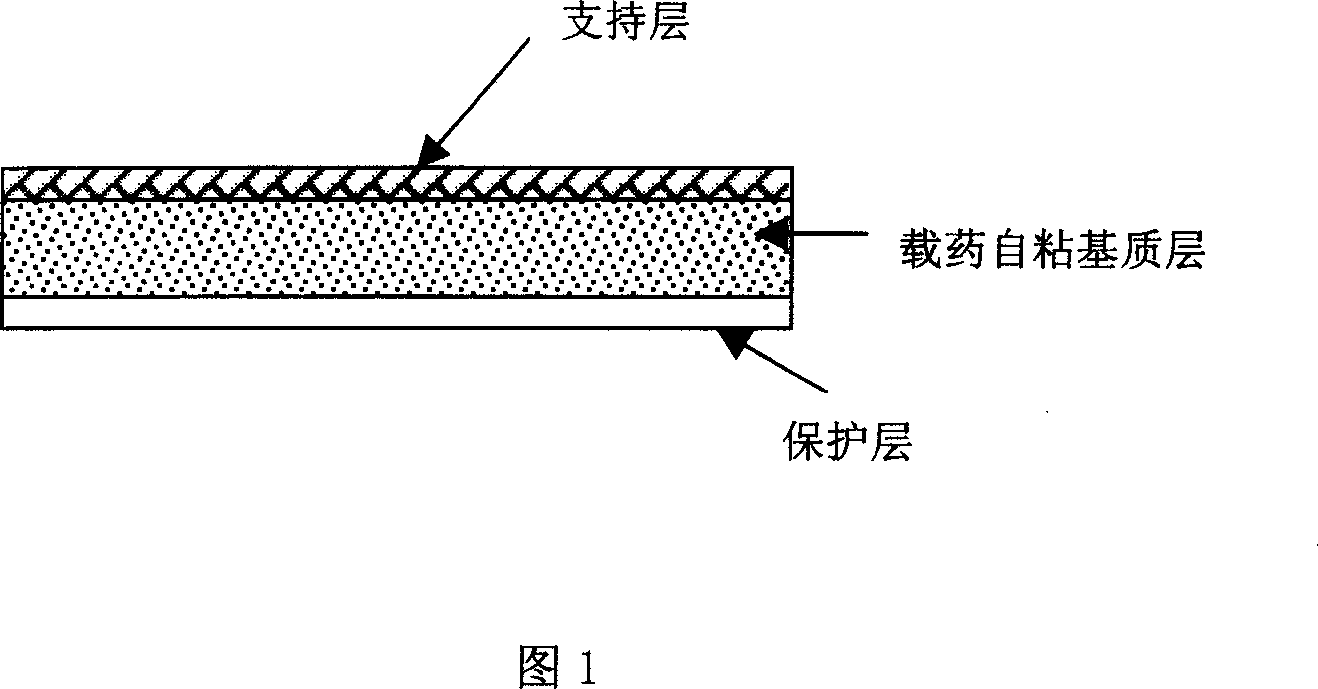
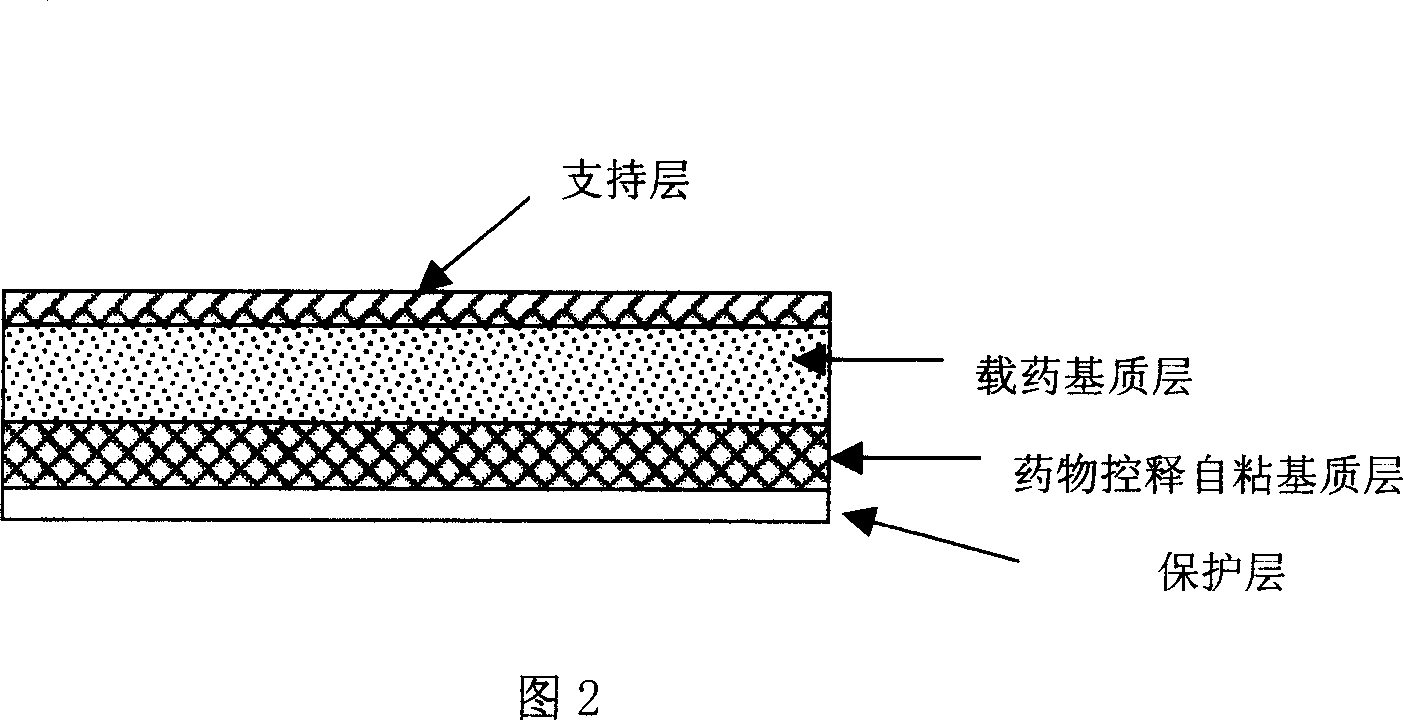
Tape material for transcutaneous absorption
A preparation for transdermal absorption is disclosed which is suited for alleviating lasting pains caused by herpes zoster or postherpetic neuralgia and is practical and more improved in drug efficacy, safety and application characteristics. This tape preparation for transdermal absorption is obtained by causing an adhesive mass prepared by incorporating 1–30 parts by weight of a local anesthetic as an active ingredient in 100 parts by weight of a nonaqueous adhesive mass base comprising 5–50% by weight of a styrene-isoprene-styrene block copolymer, 1–60% by weight of an alicyclic saturated hydrocarbon resin, 5–60% by weight of liquid paraffin and 1–30% by weight of butyl rubber to be supported on a backing.
Owner:YUTOKU PHARMA IND +1
Anti-aging composition containing lycopene and microcapsule and preparation method thereof
InactiveCN105853245AGood water solubilityStrengthen the antioxidant systemCosmetic preparationsToilet preparationsLycopeneOil phase
The invention discloses an anti-aging cosmetic composition containing lycopene. The cosmetic composition is prepared from an oil phase composed of one or more oily components and lycopene, a mixed emulsifier composed of one or more emulsifiers and water, wherein the ratio of the mass of lycopene to the total mass of the oily components is (0.1-5):1,000, an HLB value of the mixed emulsifier is a weighted mean value of HLB values of all the emulsifiers composing the mixed emulsifier, the value taking interval of the HLB value of the mixed emulsifier is 8-18, and the mass ratio of the oil phase to the mixed emulsifier is 1:(0.05-1). According to the anti-aging cosmetic composition, all the ingredients are matched according to a specific proportion, the free radical scavenging capacity of lycopene can be fully achieved, after lycopene is percutaneously absorbed, excessive accumulation of free radicals can be fundamentally inhibited, and an anti-oxidant system of the skin is enhanced, so that aging is delayed.
Owner:FOSHAN QIANRU COSMETICS CO LTD
Total alkaloids extraction of corydalis, its preparation method, medicine composition containing the total alkaloids extraction and application thereof
InactiveCN101054377AEfficient preparation methodIn line with the proportion of natural occurrencePowder deliveryAlkaloids chemistryHarmineFreeze-drying
The present invention discloses a Rhizoma Corydalis Decumbentis total alkaloids extract, its preparation method, pharmaceutical composition containing same. The total alkaloids mainly comprise : macleyine, tetrahydropalmatine, bicucalline, palmatine hydrochloride, 'xiawuning' alkaloid, corydaline harmine, other alkaloid and extract. The preparation method is : using Rhizoma Corydalis Decumbentis as material, adding right amount of polar solvent for leaching, merging the leachate, carrying out large pore adsorption resin chromatography, eluting impurity with diluted acid and alkaline aqueous solution orderly, eluting using polar organic solvent, collecting elution liquor, eliminating impurity further by alumina column adsorption, Collecting after-column liquid, concentrating liquor to obtain the Rhizoma Corydalis Decumbentis total alkaloids extract. The present invention also discloses a pharmaceutical composition containing the total alkaloids extract and uses of the pharmaceutical composition in preparing tablet, capsule, soft capsule, suppository, granula, transdermal absorption agent, drop pills, oral disintegrating agent, slow release agent, freeze-drying powder injection etc.
Owner:SHANGHAI INST OF PHARMA IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com