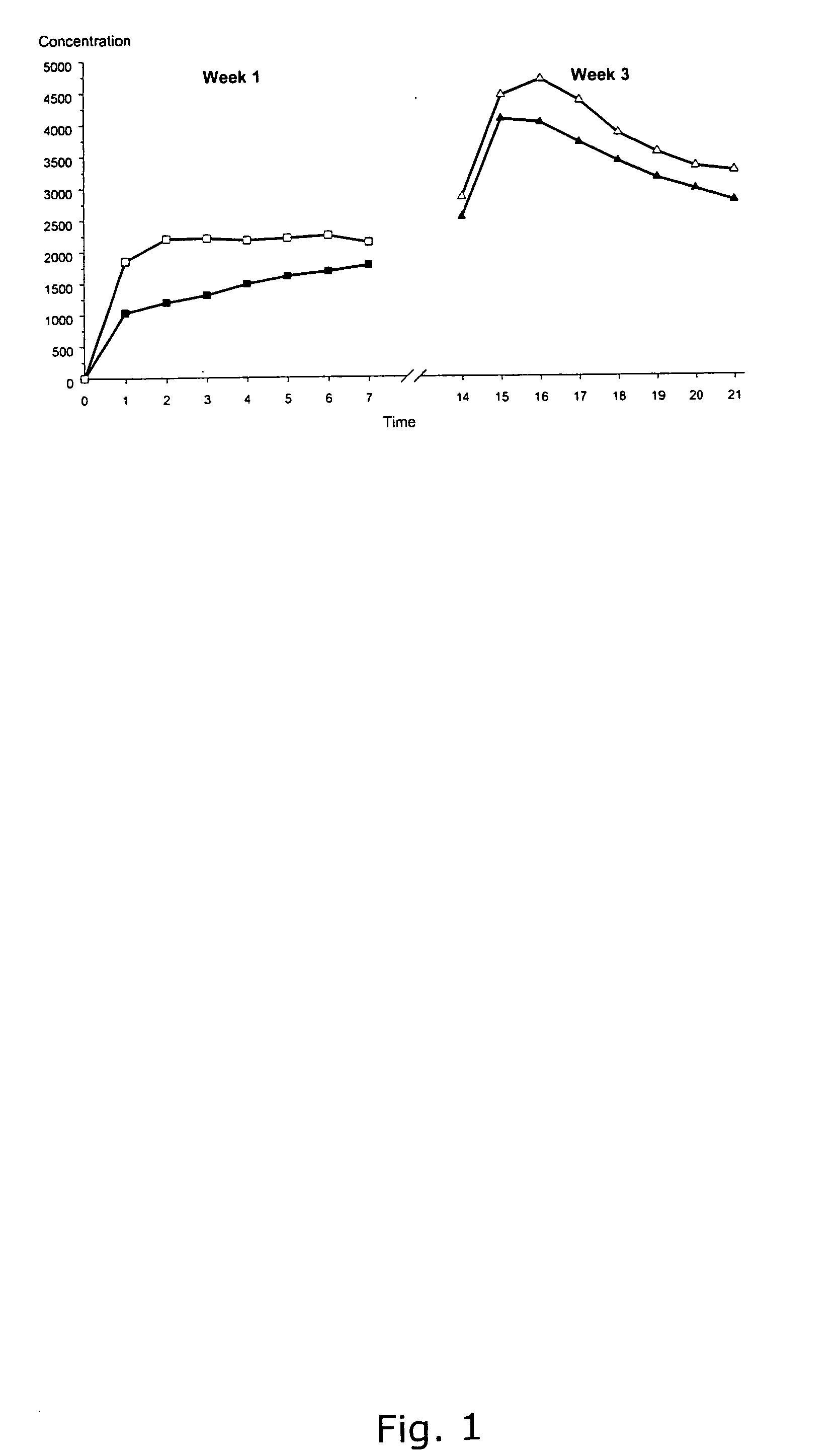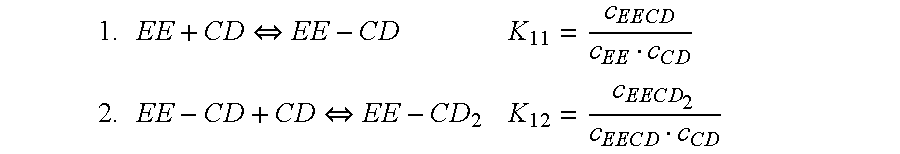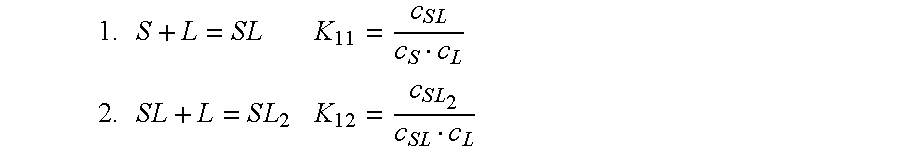Patents
Literature
97 results about "Ethinylestradiol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
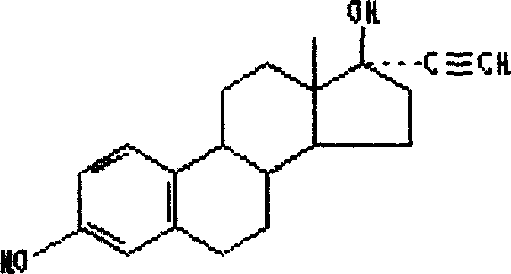
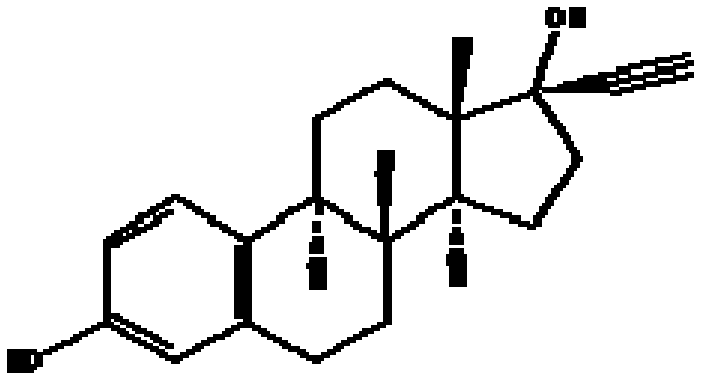
Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. It is also occasionally used as a component of menopausal hormone therapy for the treatment of menopausal symptoms in combination with progestins. In the past, EE was widely used alone for various indications such as the treatment of gynecological disorders and prostate cancer. It is usually taken by mouth.
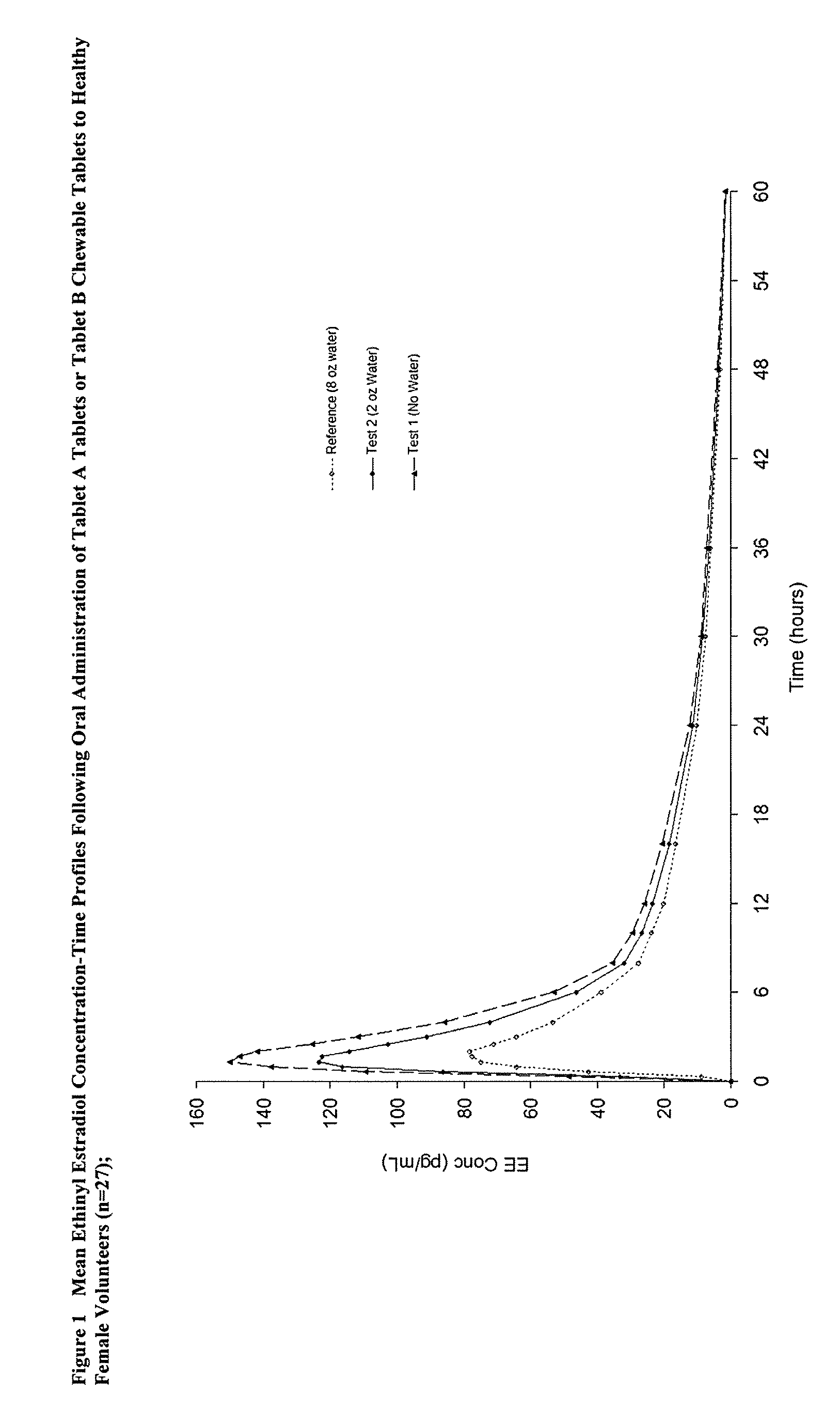
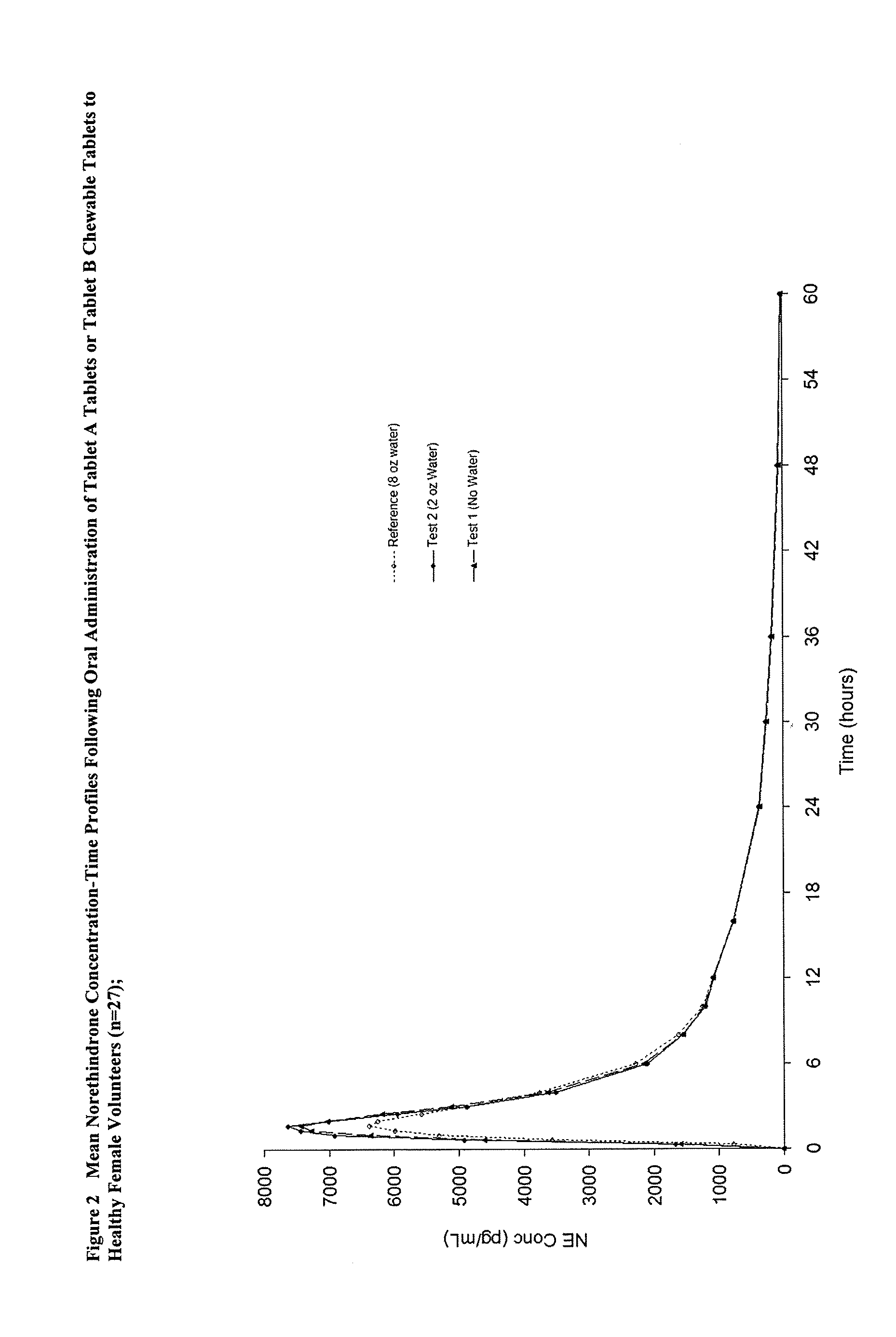
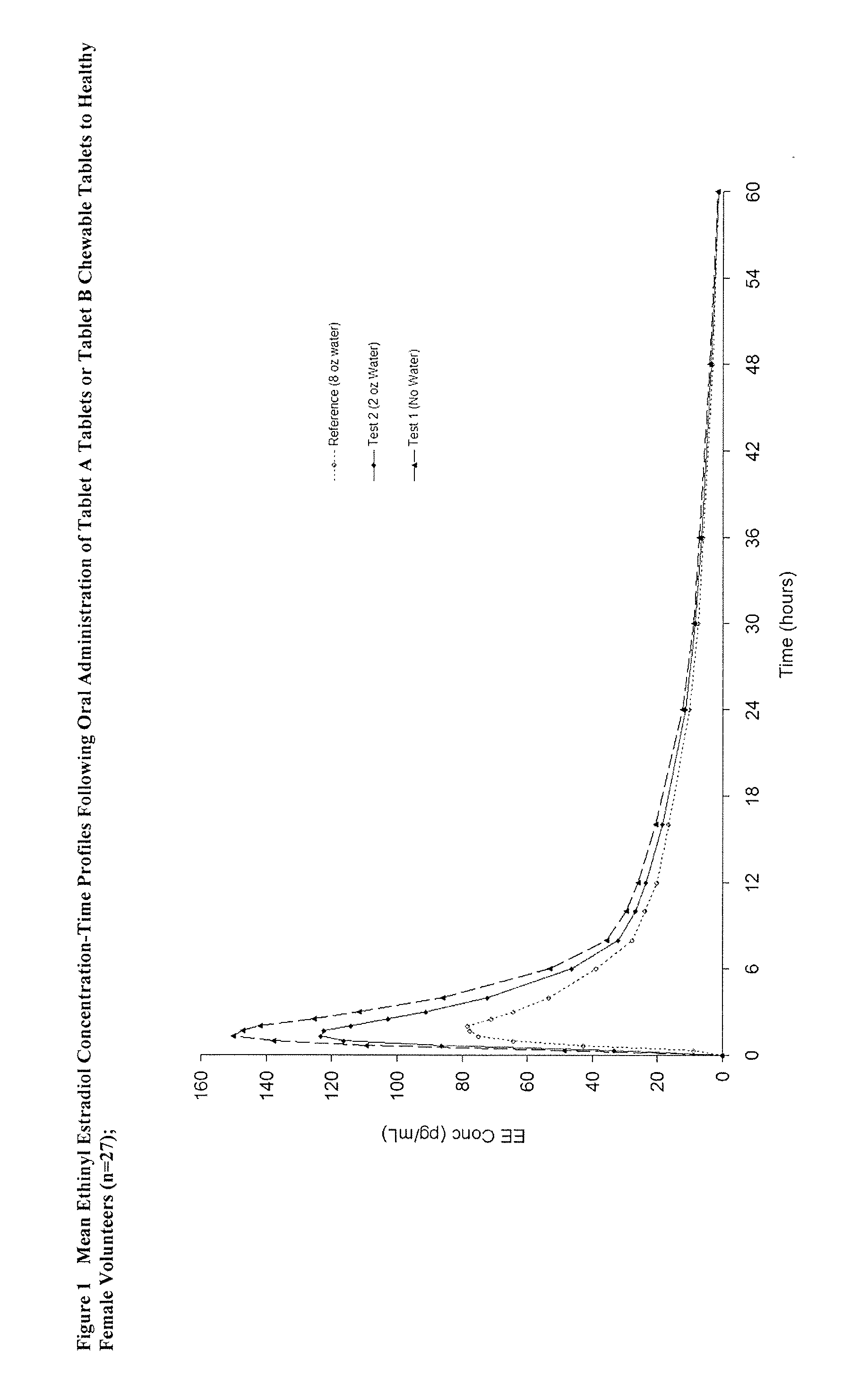
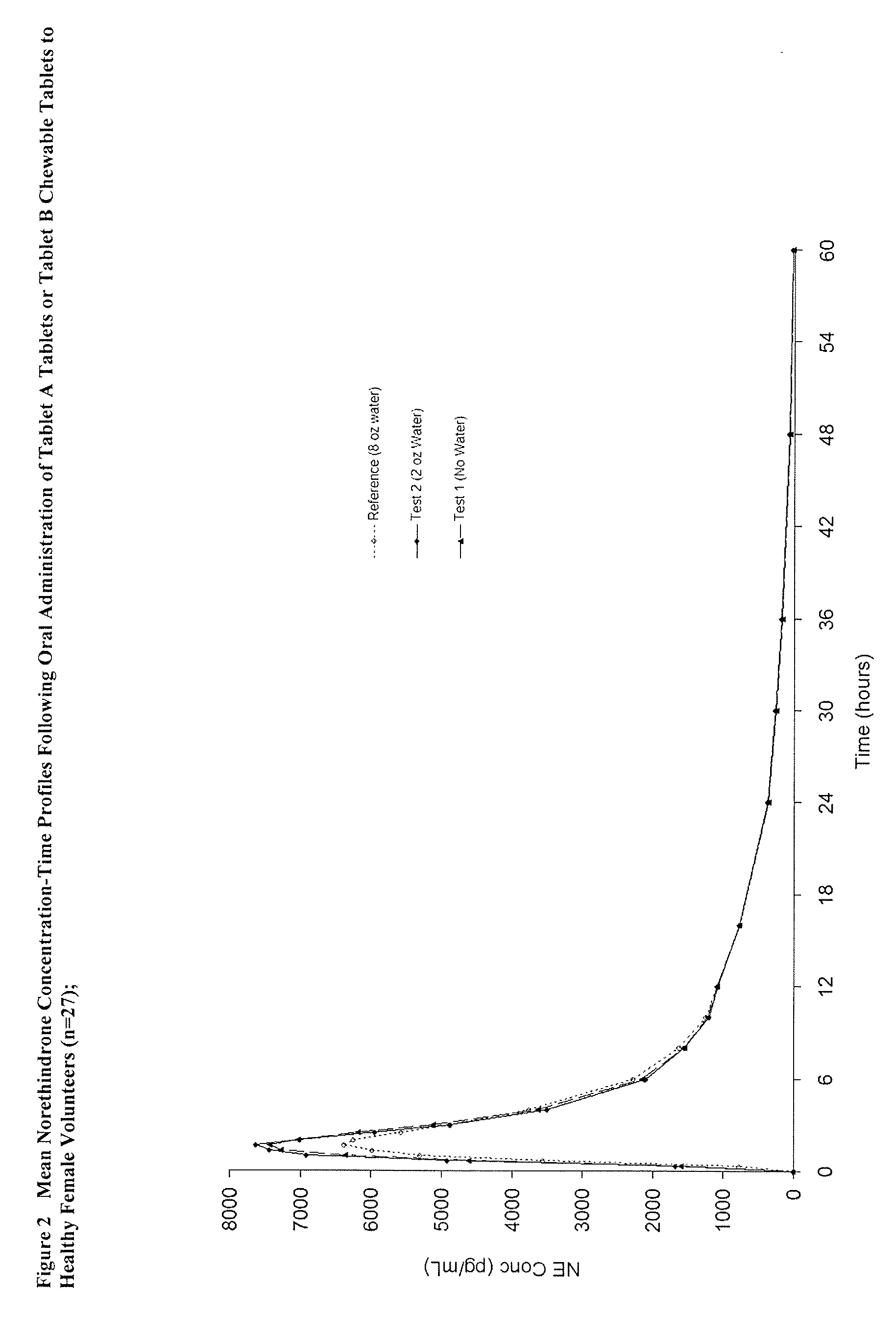
Methods to administer ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20070286819A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPill deliveryHormone replacementBioavailability
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:WARNER CHILCOTT CO LLC
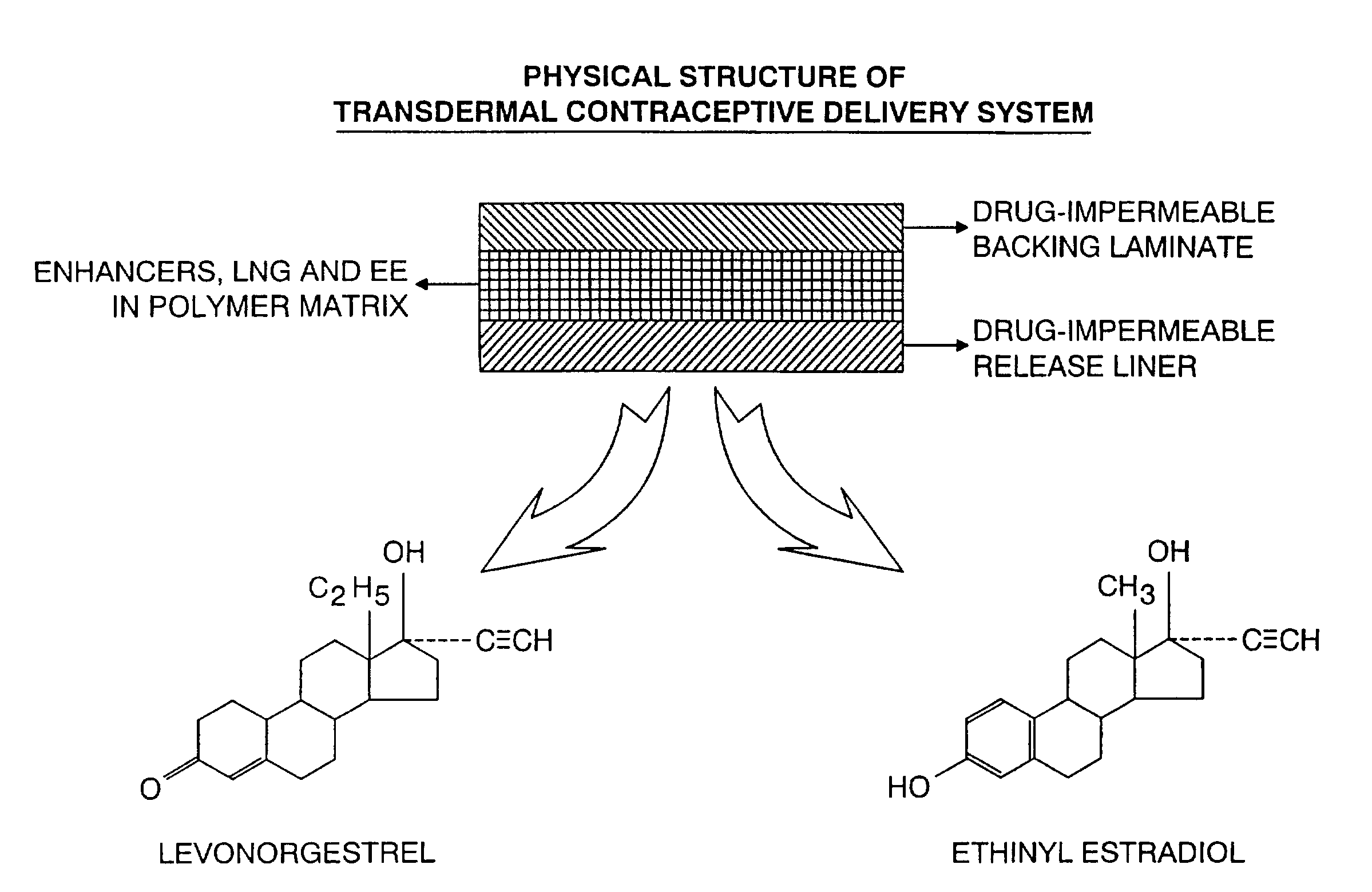
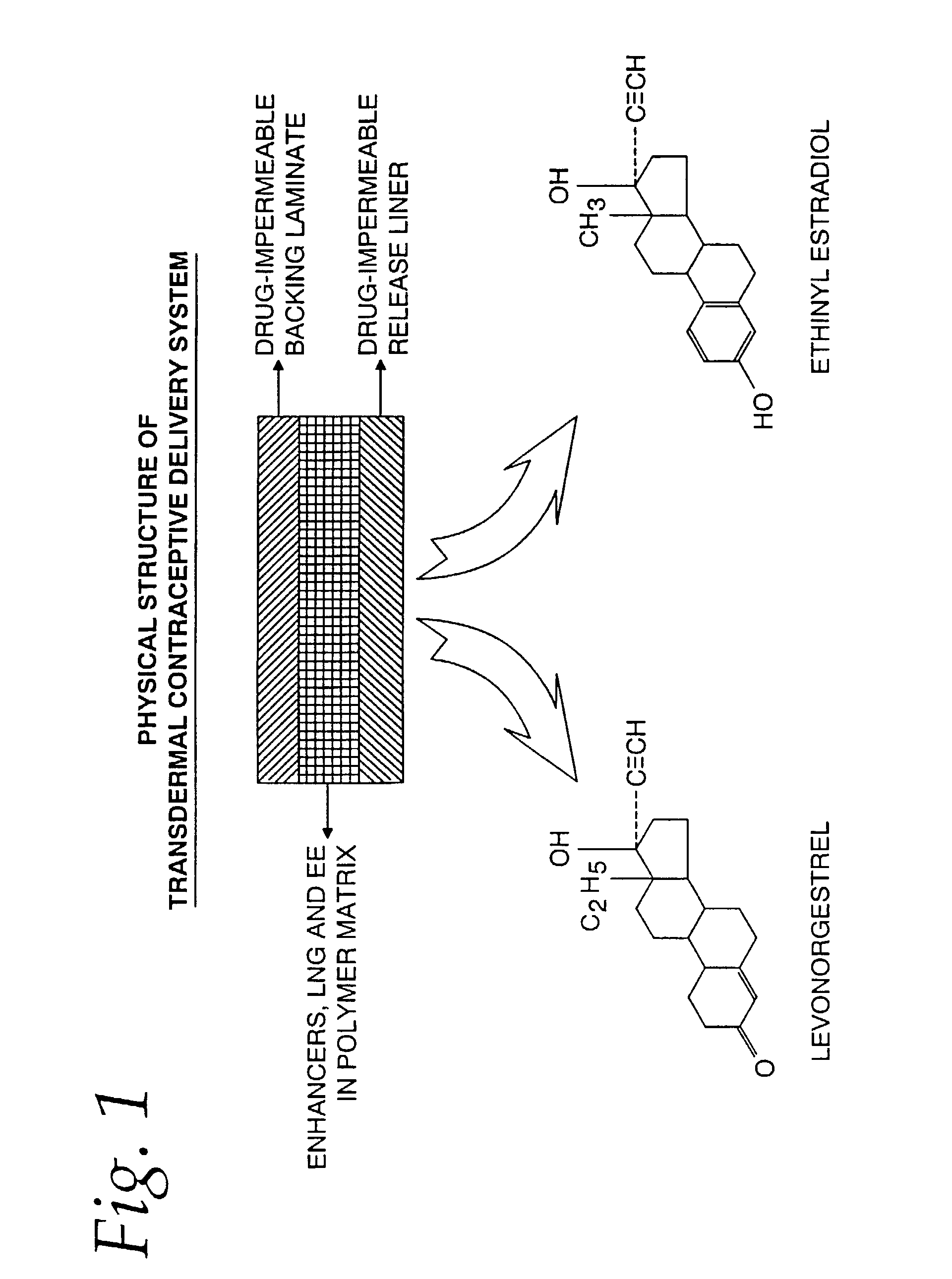
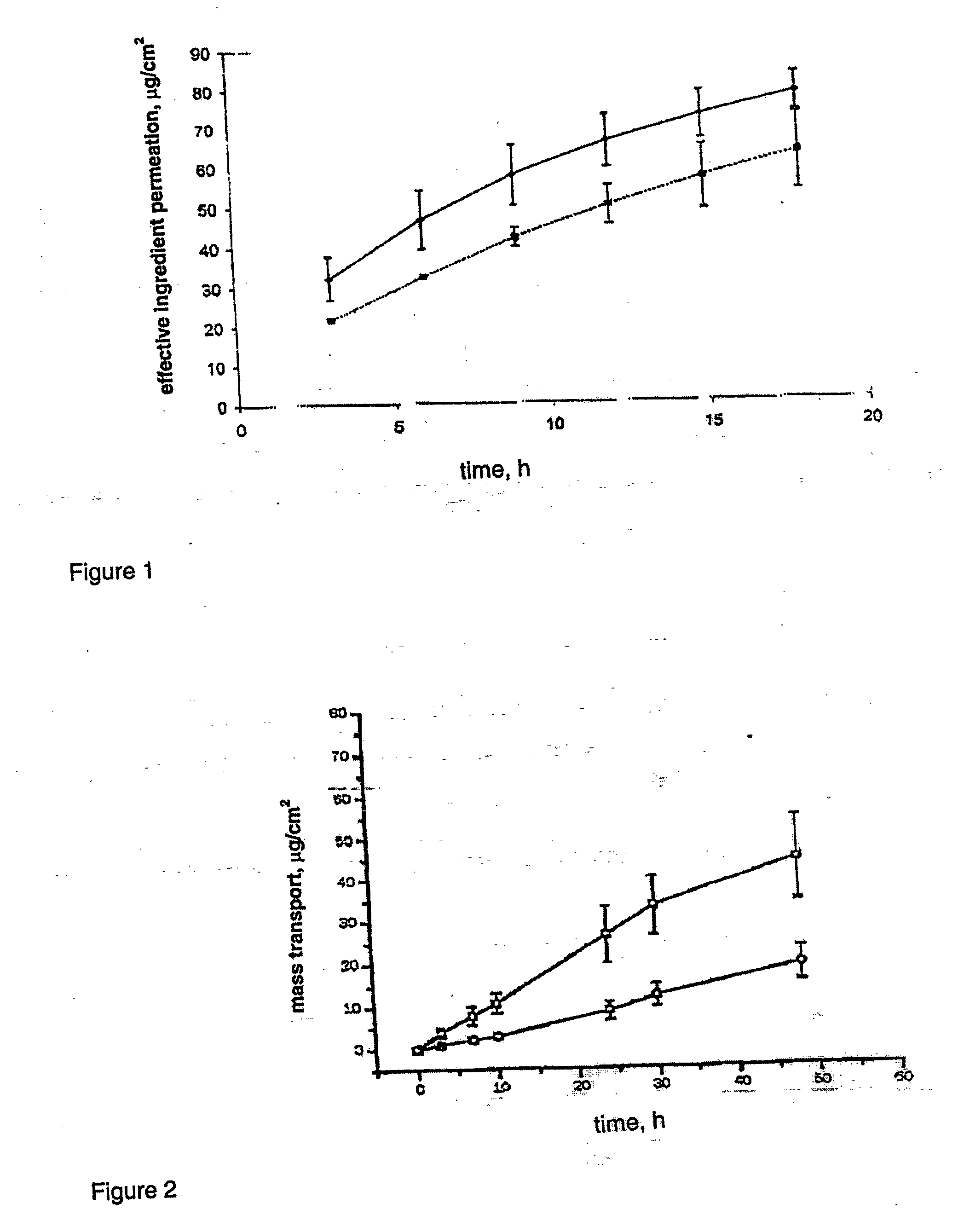
Transdermal contraceptive delivery system and process
InactiveUS7045145B1Cut skinReduce concentrationOrganic active ingredientsAdhesive dressingsObstetricsAdhesive
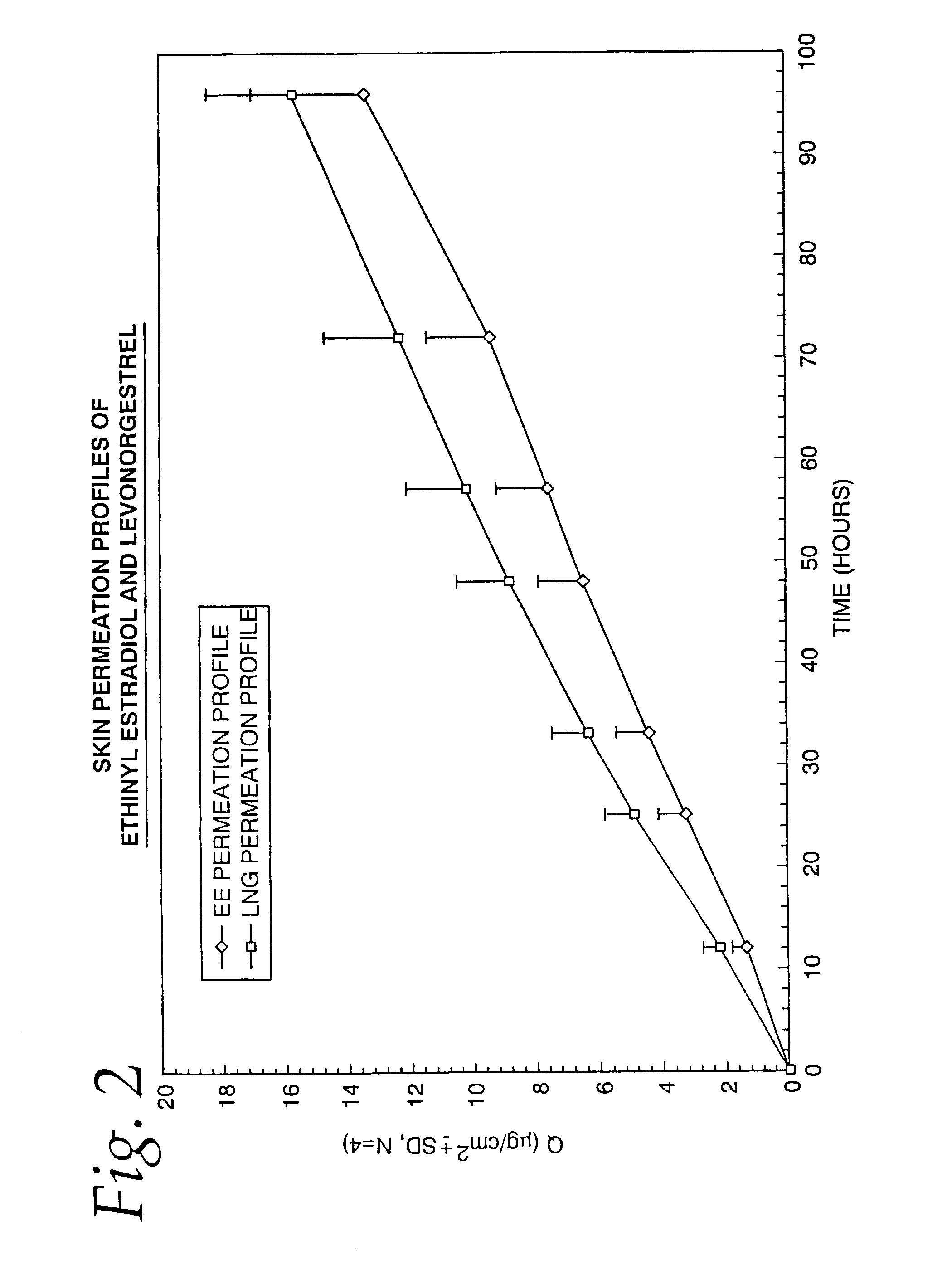
A transdermal contraceptive delivery system (TCDS) for fertility control in women is described. It comprises a backing layer, an adjoining layer of a solid absorption adhesive polymer matrix in which effective daily doses of an estrogen and a progestin are dispersed and released for transdermal absorption. Presently preferred is the use of the synthetic estrogen, ethinyl estradiol, and the synthetic progestin, levonorgestrel. Along with these two steroidal contraceptive agents, a combination of several chemical skin permeation enhancing agents, including capric acid, blended at specific weight ratios, ranging from 2:1:1:0.8 to 6:1:1:0.8, are homogeneously dispersed in the adhesive polymer matrix. The invention also provides a method of fertility control utilizing the transdermal contraceptive delivery system.
Owner:AGILE THERAPEUTICS
Methods to administer solid dosage forms of ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20080113953A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPharmaceutical delivery mechanismEthinyl oestradiolHormone replacement
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:APTALIS PHARMA
Extended cycle multiphasic oral contraceptive method
ActiveUS8063030B2Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Transdermal delivery of hormones without the need of penetration enhancers
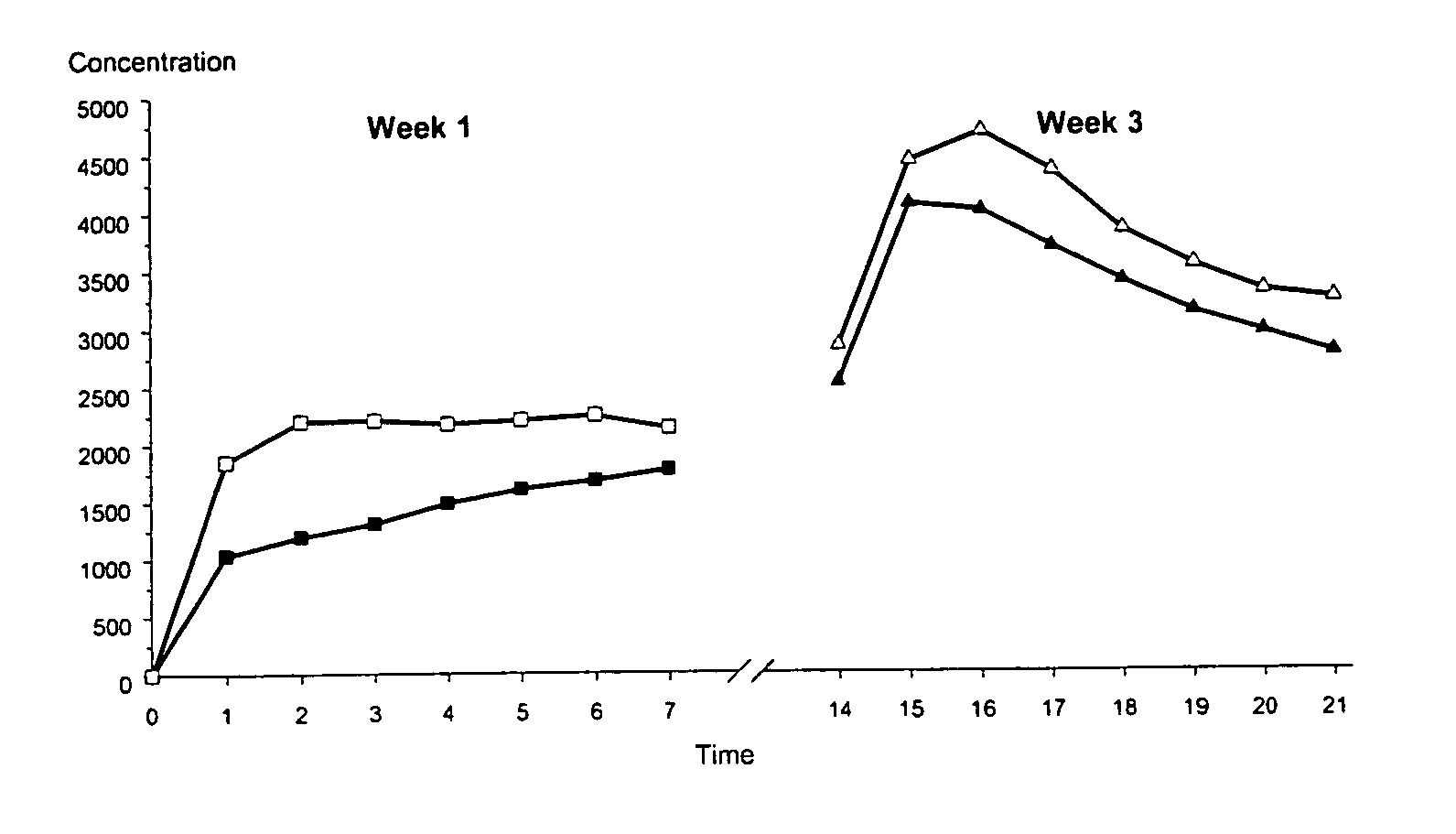
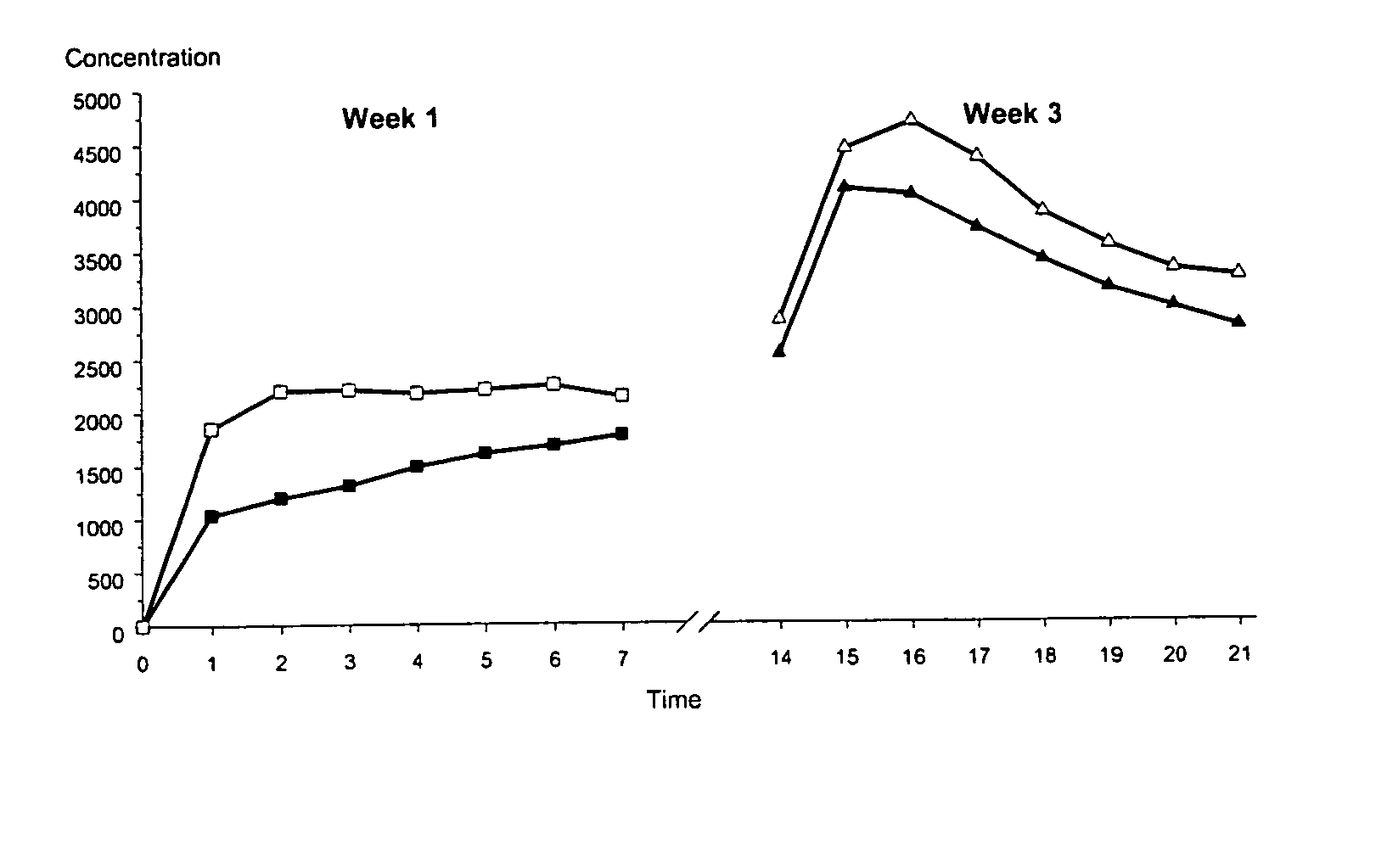
ActiveUS20050142175A1High plasma levelInhibit ovulationBiocideOrganic active ingredientsEthinyl oestradiolHormones regulation
The present invention relates to a patch comprising a drug-containing layer with low content of hormones, such as gestodene, and optionally an estrogen (e.g. ethinyl estradiol). Upon administering the patch to a woman, plasma levels of at least 1.0 ng / ml of Gestodene is achieved at steady state conditions without the need of incorporating penetration enhancers or permeation enhancers in the drug-containing layer. Satisfactorily plasma levels of the hormones is also achieved throughout a period of at least 1 week, making the patch applicable for being used in female contraception with the concept of administering the patch ones weekly.
Owner:LUYE PHARMA SWITZERLAND AG
Drospirenone-containing preparations for transdermal use
InactiveUS20050222106A1Increase supersaturationReduce dosageOrganic active ingredientsAerosol deliveryTransdermal patchDrospirenone
The pharmaceutical preparation for transdermal administration contains solvent ingredients, such as water and ethanol and / or propanol, and drospirenone. The drospirenone is contained in the preparation in an amount that is not above its saturation solubility in an initial state prior to application to skin. However after application to the skin the amount of drospirenone exceeds its saturation solubility due to escape or discharge of the solvent ingredients from the preparation. Preferably the saturation solubility is exceeded by at least a factor of five during application to the skin. The pharmaceutical preparation can also contain an estrogen, such as ethinyl estradiol. It can be in the form of a semi-solid or liquid preparation that is contained in a reservoir-type transdermal patch. A transdermal patch for contraception containing the pharmaceutical preparation including drospirenone and ethinyl estradiol is also disclosed.
Owner:SCHERING AG
Pharmaceutical composition containing a tetrahydrofolic acid
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
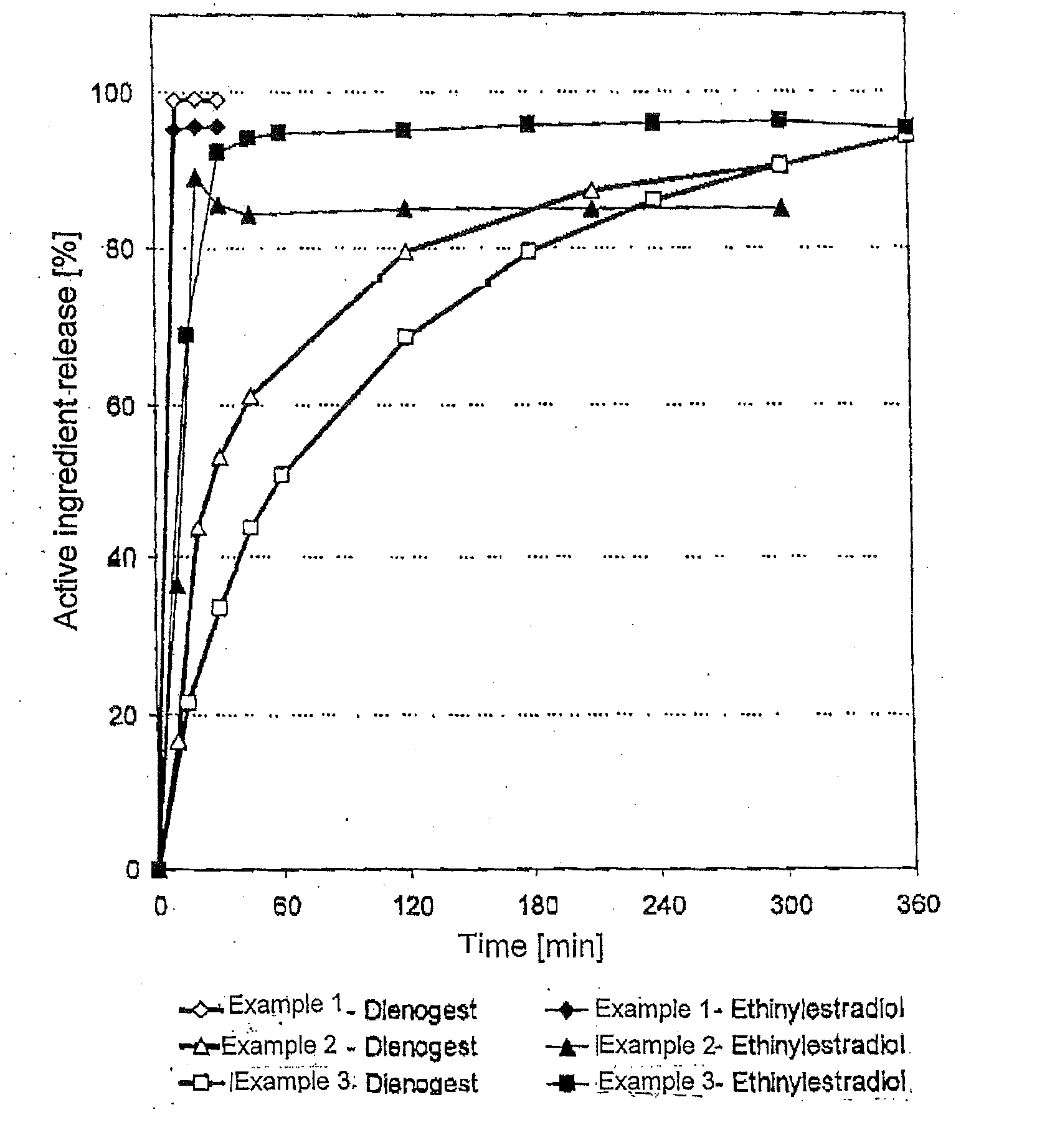
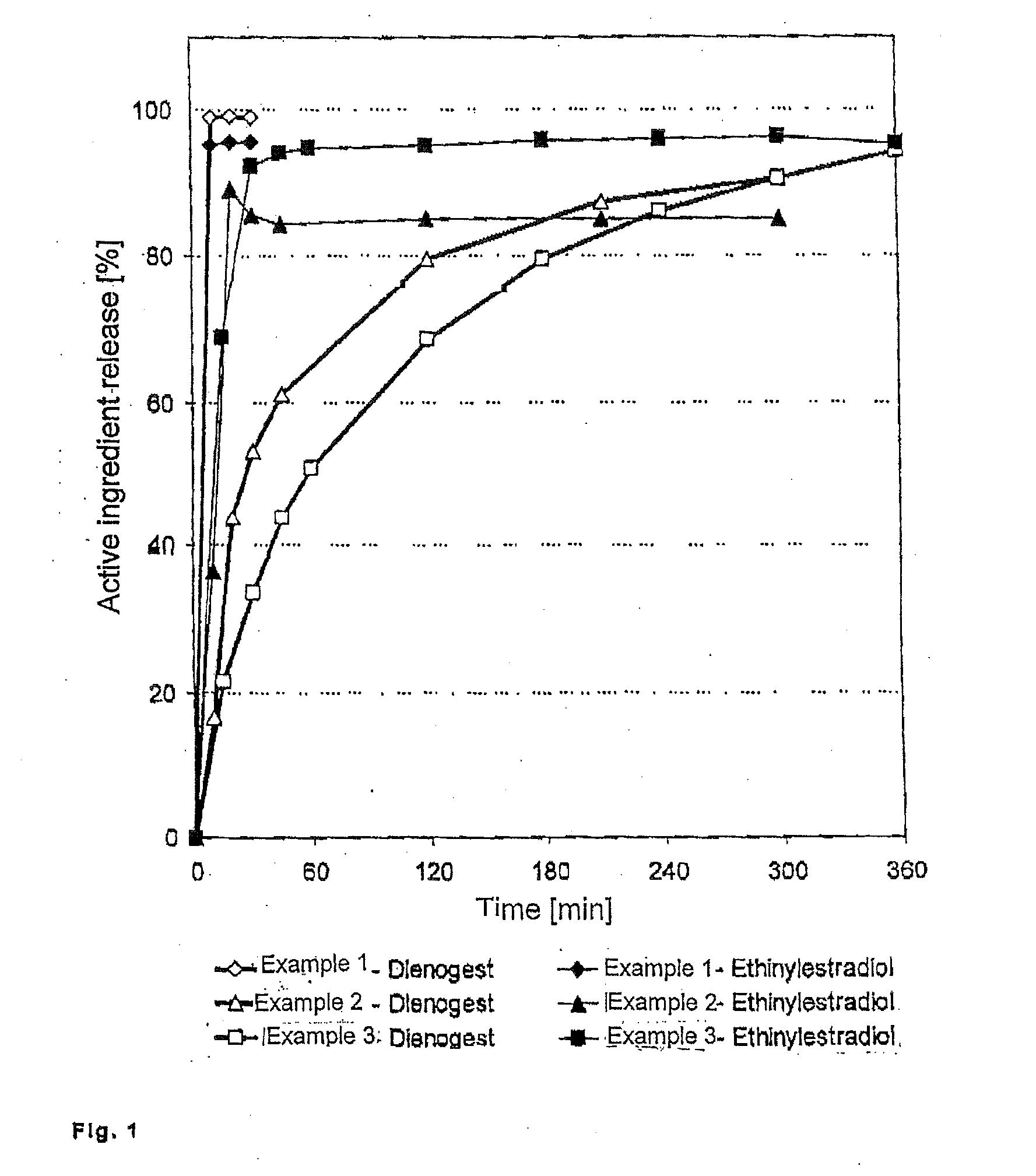
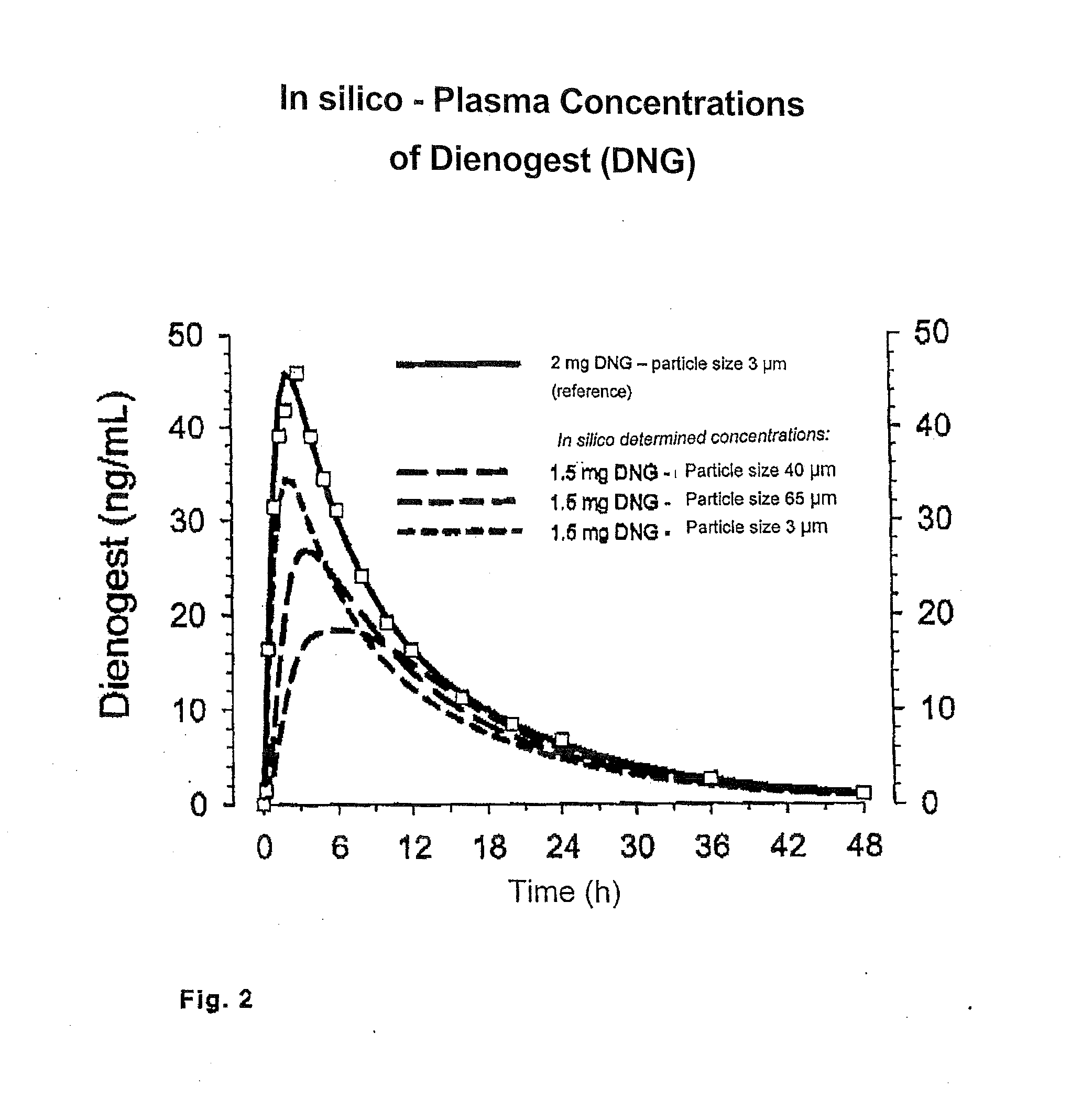
Low-dosage peroral medication for contraception containing crystalline dienogest and ethinyl estradiol
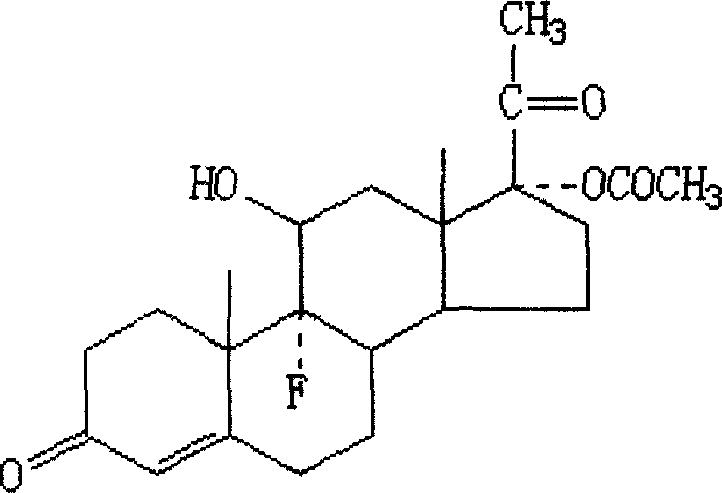
InactiveUS20080038350A1Reduce contentReduce loadBiocideOrganic active ingredientsEthinylestradiolBULK ACTIVE INGREDIENT
The peroral medication for prevention of conception contains as one active ingredient crystalline 17α-cyanomethyl-17β-hydroxyestra-4,9-dien-3-one (dienogest) at a daily dosage equal to or less than 2.0 mg and as another active ingredient 17α-ethinyl estradiol at a daily dosage of less than 0.030 mg, together with one or more pharmaceutically acceptable carriers. The active ingredient dienogest is contained in the medication in crystalline form with an average particle size of preferably 25 to 70 μm. The other active ingredient ethinyl estradiol is incorporated during granulation in micronized form or by spraying an ethanolic solution containing it.
Owner:BAYER SCHERING PHARMA AG
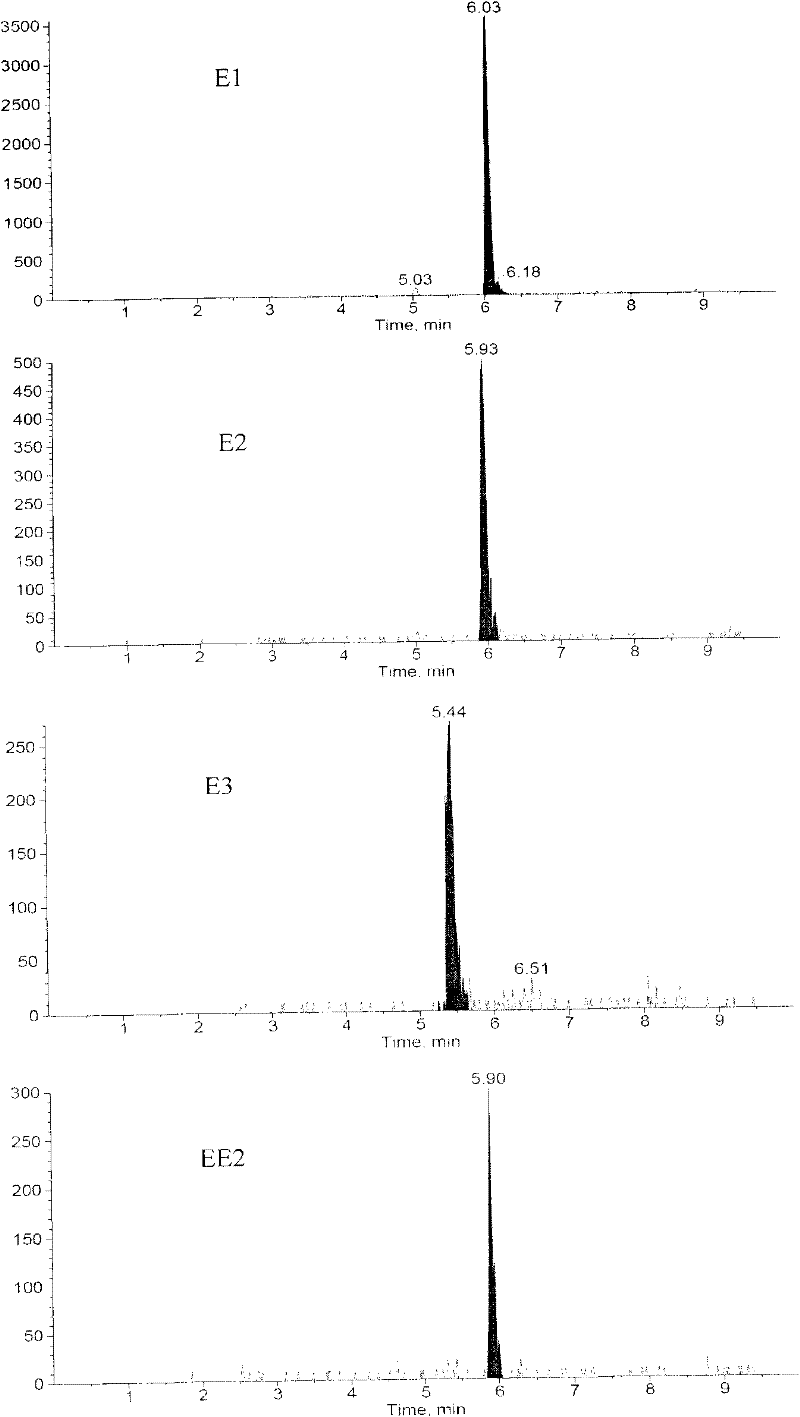
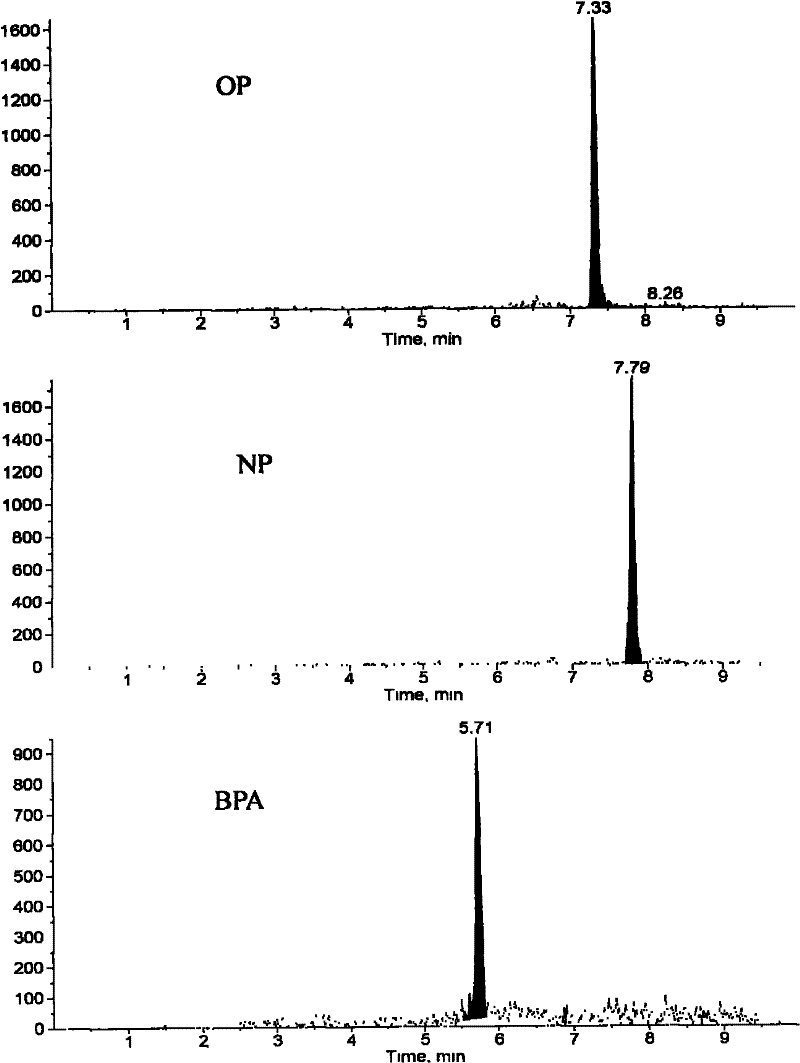
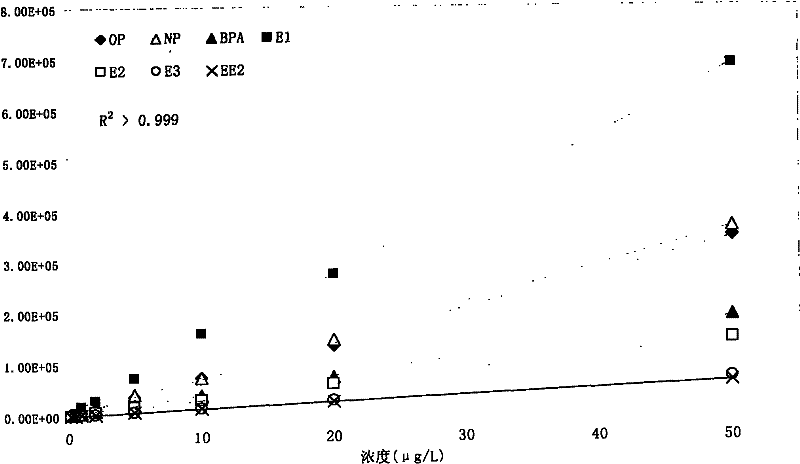
Method for detecting estrogen, nonyl phenol, octylphenol and bisphenol A together in water environment
InactiveCN102175792AHigh enrichment factorHigh sensitivityComponent separationPerturbateurs endocriniensEstriol
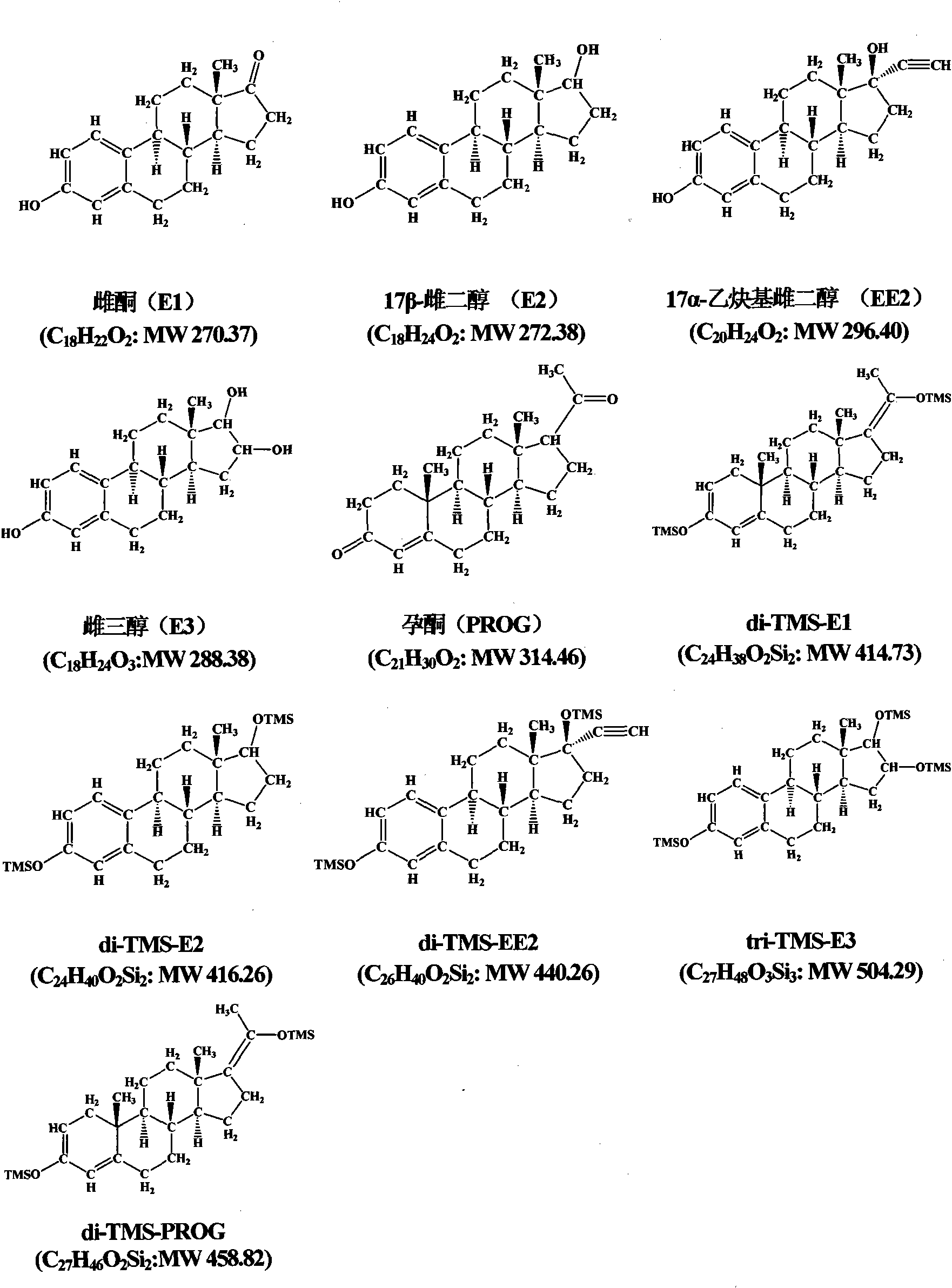
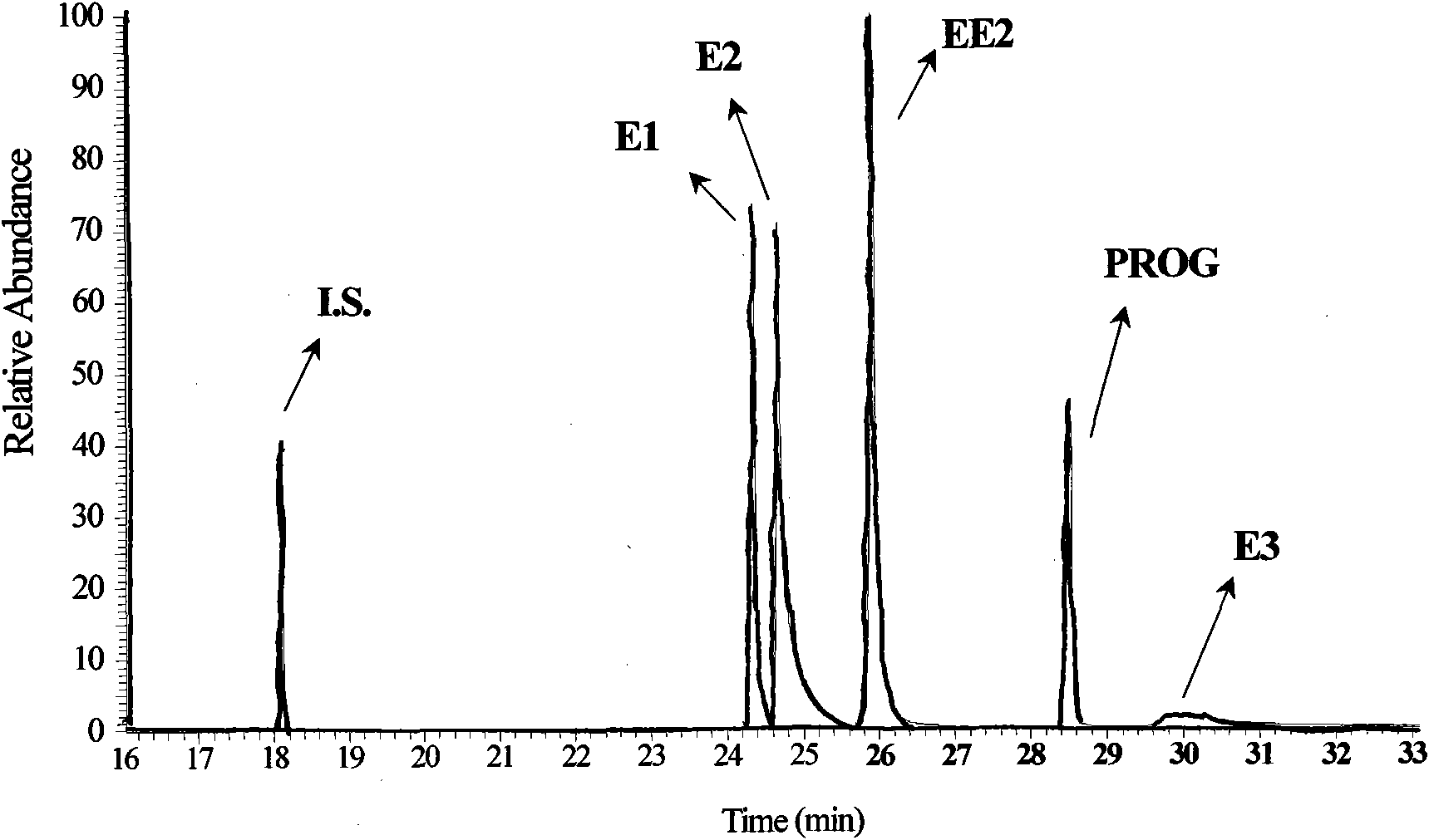
The invention relates to a detection technology of endocrine disrupters in water environment, in particular relating to a method for detecting seven substances such as oestrone, 17 beta-estradiol, estriol, 17 alpha-ethinyl estradiol, nonyl phenol, octylphenol and bisphenol A and the like in a water environment sample by virtue of liquid chromatogram-tandem mass spectrometry. The method comprises the following steps: concentrating and gathering the estrogen, the nonyl phenol, the octylphenol and the bisphenol A in a water sample by utilizing a solid phase extraction column; eluting the estrogen, the nonyl phenol, the octylphenol and the bisphenol A from the solid phase extraction column by utilizing ethyl acetate; and analyzing the concentrations of the estrogen, the nonyl phenol, the octylphenol and the bisphenol A by virtue of liquid chromatogram-tandem mass spectrometry. The method has the advantages of environment friendliness and easiness for operation, ensures relatively high recovery, and can be used for fast analyzing the concentrations of the estrogen, nonyl phenol, octylphenol and bisphenol A in the water sample.
Owner:BEIJING NORMAL UNIVERSITY
Methods of extended use oral contraception
A method of female contraception involves administering a combination of estrogen and progestin for 60-110 consecutive days in which the daily amounts of estrogen and progestin are equivalent to about 5-35 mcg of ethinyl estradiol and about 0.025 to 10 mg of norethindrone acetate, respectively. The advantages include less menstrual bleeding, less patient anemia, less total exposure to medication, higher compliance rates and more lifestyle convenience for patients.
Owner:TEVA WOMENS HEALTH
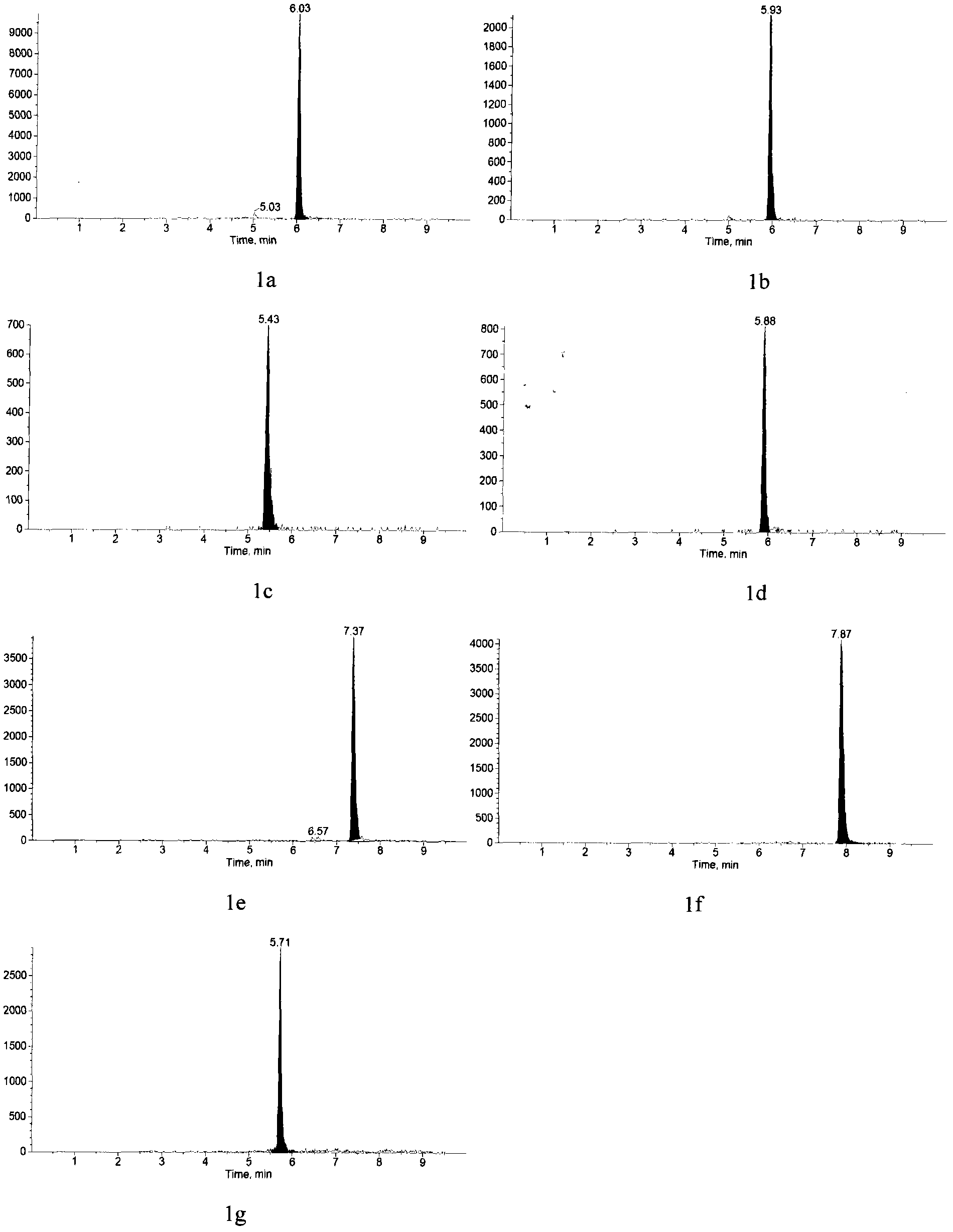
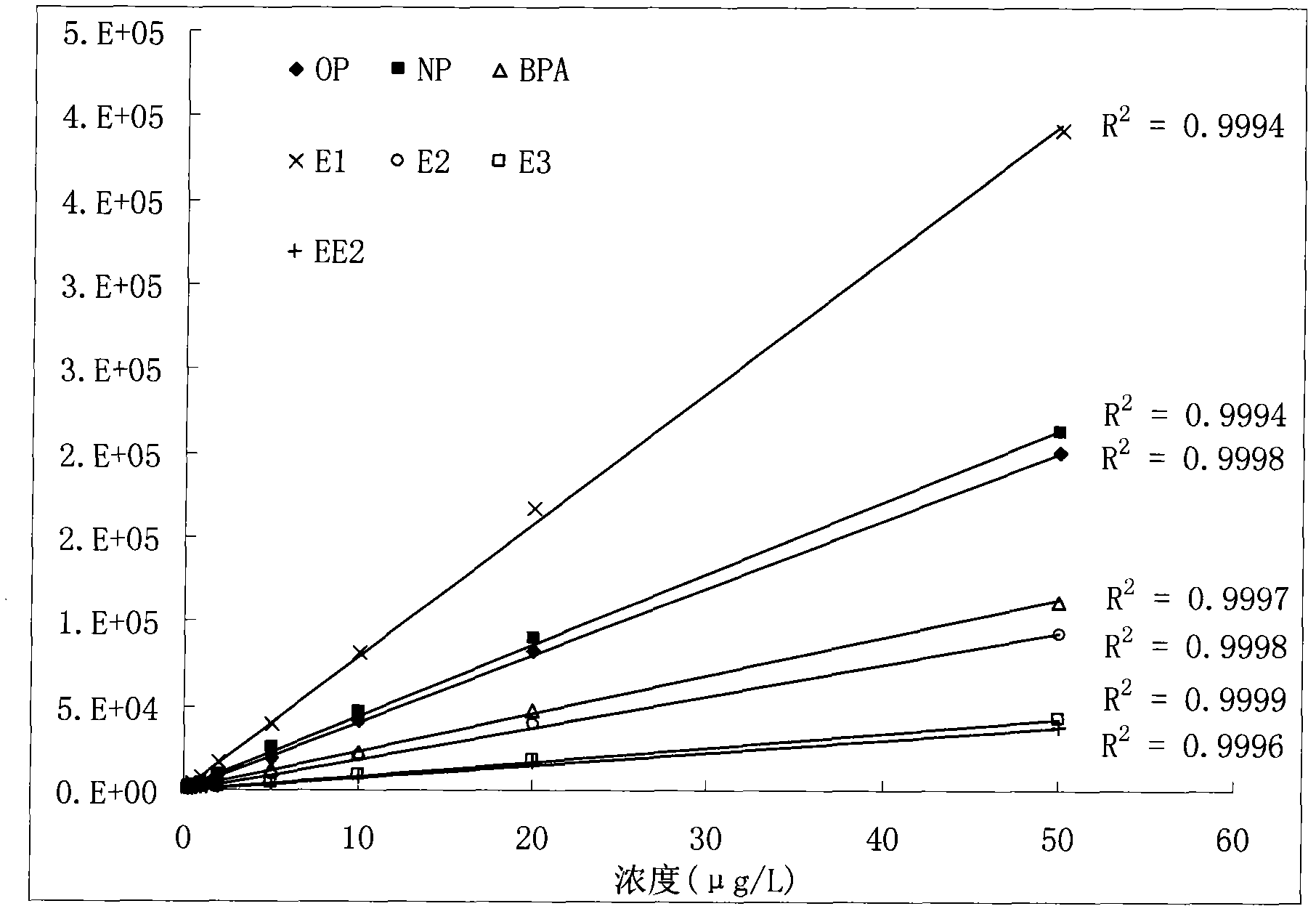
Method for jointly detecting estrogen, nonyl phenol, octylphenol and bisphenol A in complex substrate water sample
The invention relates to a detecting technique of endocrine disrupters in water environment and particularly relates to a technique for quantitative analysis on oestrone, 17beta-estradiol, estriol, 17alpha-ethinyl estradiol, nonyl phenol, octylphenol and bisphenol A in a complex substrate water sample by adopting a liquid chromatogram-tandem mass spectrum combined technique. The method comprises the following steps: enriching a collected water sample by using a HLB (Hydrophile-Lipophile Balance) solid-phase extraction column and washing with carbinol; drying elution liquid nitrogen and purifying by using Florisil; and finally, analyzing by utilizing the liquid chromatogram-tandem mass spectrum combined technique. The method is environment-friendly, easy to operate and high in recovery rate. The method can quickly analyze the trace amount of oestrone, 17beta-estradiol, estriol, 17alpha-ethinyl estradiol, nonyl phenol, octylphenol and bisphenol A in the complex substrate water sample.
Owner:BEIJING NORMAL UNIVERSITY
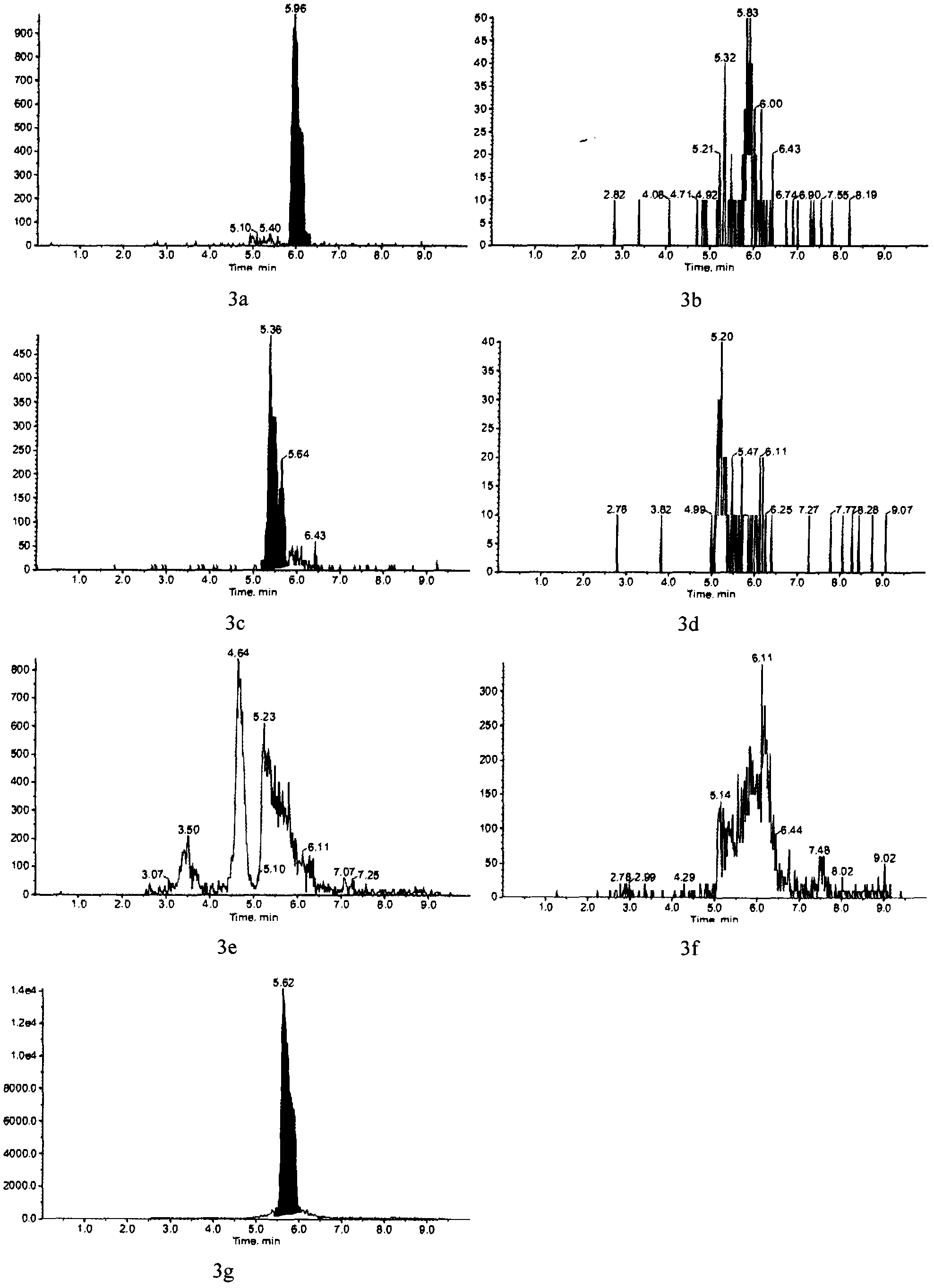
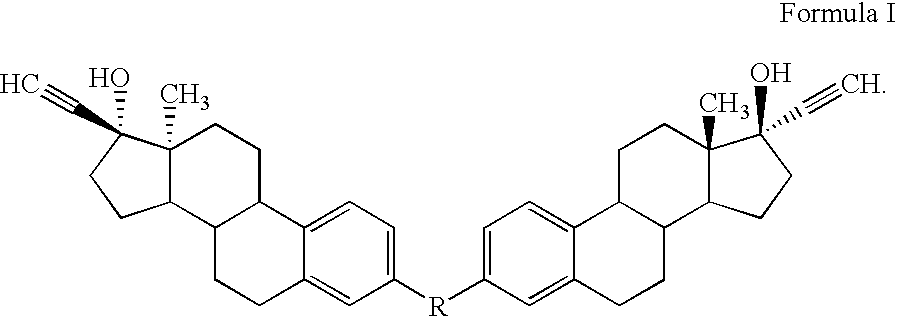
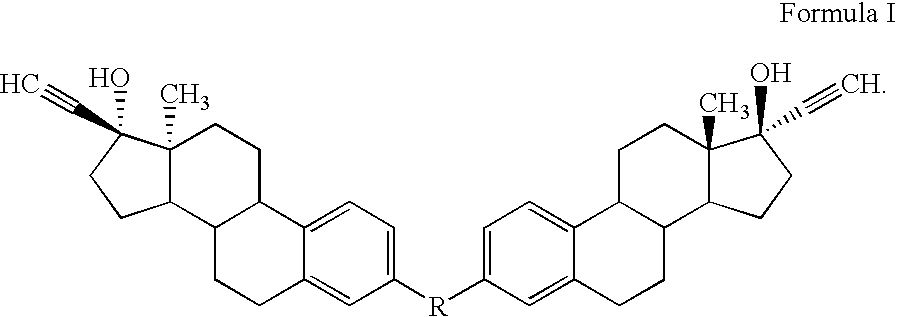
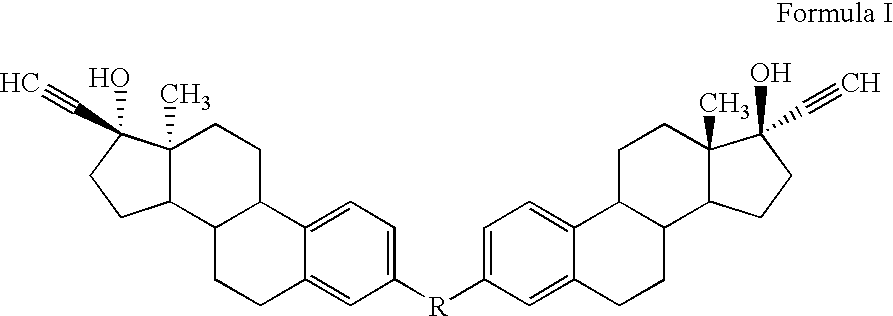
Di-steroidal prodrugs of ethinyl estradiol
Owner:ALLERGAN THERAPEUTICS LLC
Nano silver-titanium dioxide loaded porous cordierite foamed ceramic catalyst and preparation
InactiveCN102600838AIncrease contact areaHigh adsorptionBiocideWater/sewage treatment by irradiationPorosityCordierite
The invention discloses a nano silver-titanium dioxide loaded cordierite foamed ceramic catalyst used for sewage purification. The catalyst is composed of cordierite foamed ceramic of a porous three-dimensional reticulated frame structure and nano silver particles-titanium dioxide particles; the catalyst has larger contact area and higher adsorptive capacity; and the catalyst is characterized in that the porosity of the cordierite foamed ceramic is 80-88%, the nano silver particles-titanium dioxide (Ag / TiO2) accounts for 7-11% the mass of the cordierite foamed ceramics catalyst, and the mass ratio of nano silver particles (Ag) to titanium dioxide (TiO2) is 1.02%. The catalyst is prepared by the three steps of: (1) preparing nano silver-titanium dioxide particles; (2) preparing a suspension of the nano silver-titanium dioxide particles; and (3) loading on the cordierite foamed ceramic. When the catalyst is used for adsorbing and degrading pollutants in water, by testing the purified sewage, the removal rate of estrogen ethinyl estradiol (EE2) is 91.7% and inactivation rate of Escherichia coli is 99%, so that a significant effect is obtained.
Owner:HOHAI UNIV
Oral contraceptive multivitamin compound and methods of administration
A pharmaceutical preparation comprising progestin, estrogen and a multivitamin agent. The progestin may be between 0 and 0.50 mg Desogesterel and the estrogen may be between 0 and 0.2 mg Ethinyl Estradiol. The multivitamin agent may be any combination of alpha carotene, beta carotene, biotin, bioflavonoid, calcium, chasteberry fruit, chromium, copper, coenzyme Q10, cryptoxanthin, dong quai root, folic acid, ginkgo biloba, garlic, grape seed extract, green tea extract, hesperedin, iodine, iron, lutein, malic acid, manganese, magnesium, milk thistle, molybdenum, niacin, panthothenic acid, potassium, pyridoxine HCL, quercetin, riboflavin, rutan, selenium, thiamine, vitamin A, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, vitamin K, zinc, zoexanthin and any combination thereof.
Owner:HELLER MARGARET
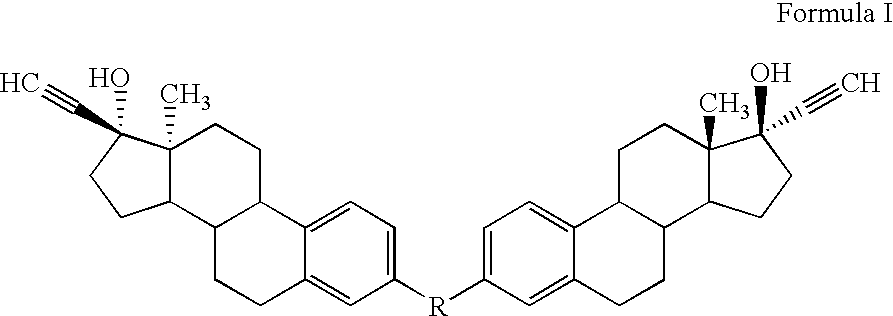
Di-steroidal prodrugs of ethinyl estradiol
ActiveUS20050159609A1Effective timeOrganic active ingredientsBiocideEthinyl oestradiolEthinylestradiol
Owner:ALLERGAN THERAPEUTICS LLC
Extended cycle multiphasic oral contraceptive method
ActiveUS20070207945A1Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Process for jointly detecting estrogens and their associations in livestock and poultry excrements
InactiveCN102735768ALow detection limitImprove detection limitComponent separationTandem mass spectrometrySolvent
The invention relates to a technology for detecting endocrine disrupters in the environment, and especially relates to a technology for quantitatively analyzing estrone (E1), 17beta-estradiol (E2), estriol (E3), 17alpha-ethinyl estradiol (EE2), estrone-3-glucuronide (E1-3G), 17beta-estradiol-3-glucuronide (E2-3G), estrone-3-sulfate (E1-3S) and 17beta-estradiol-3-sulfate (E2-3S) in a complex matrix livestock and poultry excrement sample through adopting a liquid chromatography-tandem mass spectrometry technique. The technology comprises the following steps: extracting estrogens in the collected livestock and poultry excrement sample through adopting a rapid solvent extraction method, pre-purifying the resulting extract through liquid-liquid extraction and alkaline solution extraction, enriching targets with an HLB column, purifying estrogen monomers and estrogen associations through using a Florisil column and an NH2 column respectively, and finally detecting through adopting the liquid chromatography-tandem mass spectrometry technique. The process has the advantages of environmental pollution, easy operation, and high recovery rate, and allows the estrogens and their associations having trace amounts in the complex matrix livestock and poultry excrements to be rapidly analyzed.
Owner:BEIJING NORMAL UNIVERSITY
Method of female hormonal contraception using a fixed extended cycle hormonal preparation containing dienogest and ethinyl estradiol
InactiveUS20060079491A1Reduce in quantityDecrease in discontinuation (drop-out) rateBiocideOrganic active ingredientsObstetricsEthinyl oestradiol
The fixed extended cycle method of female hormonal contraception includes continuous daily administration of daily dosage units of a monophasic preparation to a woman over a time period of n×21 days, wherein n=2, 3, 4 or 5, immediately followed by a seven-day pill-break in which the monophasic preparation is not administered. Each of the daily dosage units contains from 0.5 to 3.0 mg of dienogest and from 10 to 30 μg of ethinyl estradiol. In addition to effective contraceptive action the method significantly reduces the bleeding rate, is simple and is effective in treating acne, dysmenorrhea and / or endometriosis.
Owner:SCHERING AG
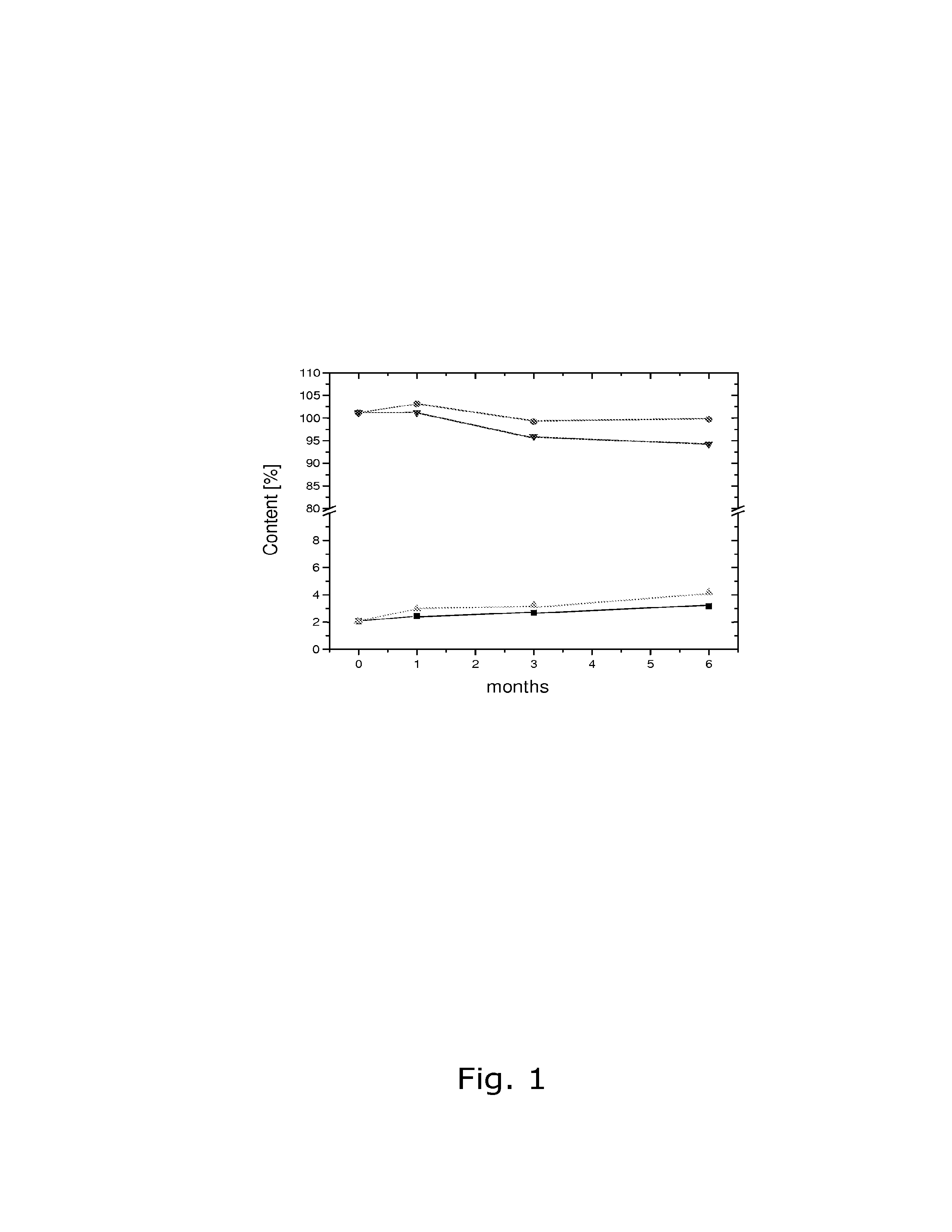
Cyclodextrin-drospirenone inclusion complexes
InactiveUS6958326B2Improve stabilityShelf-life of an estrogen-containing product is improvedBiocideSugar derivativesDrospirenoneEthinylestradiol
Pharmaceutical compositions comprising low doses of sensitive complexes between an estrogen and a cyclodextrin are provided with improved stability. In specific embodiments the composition comprises a complex between ethinyl estradiol and β-cyclodextrin in a granulate preparation and in yet another embodiment the composition comprises a limited amount of polyvinylpyrrolidone since this excipient was found to degrade ethinyl estradiol. Furthermore, a method for improving the stability of an estrogen in a composition and for manufacturing such a stable composition is provided. Essentially, the granulate preparation are manufactured under careful control of the relative humidity.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Pseudomonas citronelloalis strain capable of degrading estrogen and application thereof
InactiveCN101921717AIncrease speedEfficient degradationBacteriaContaminated soil reclamation17beta estradiolEstriol
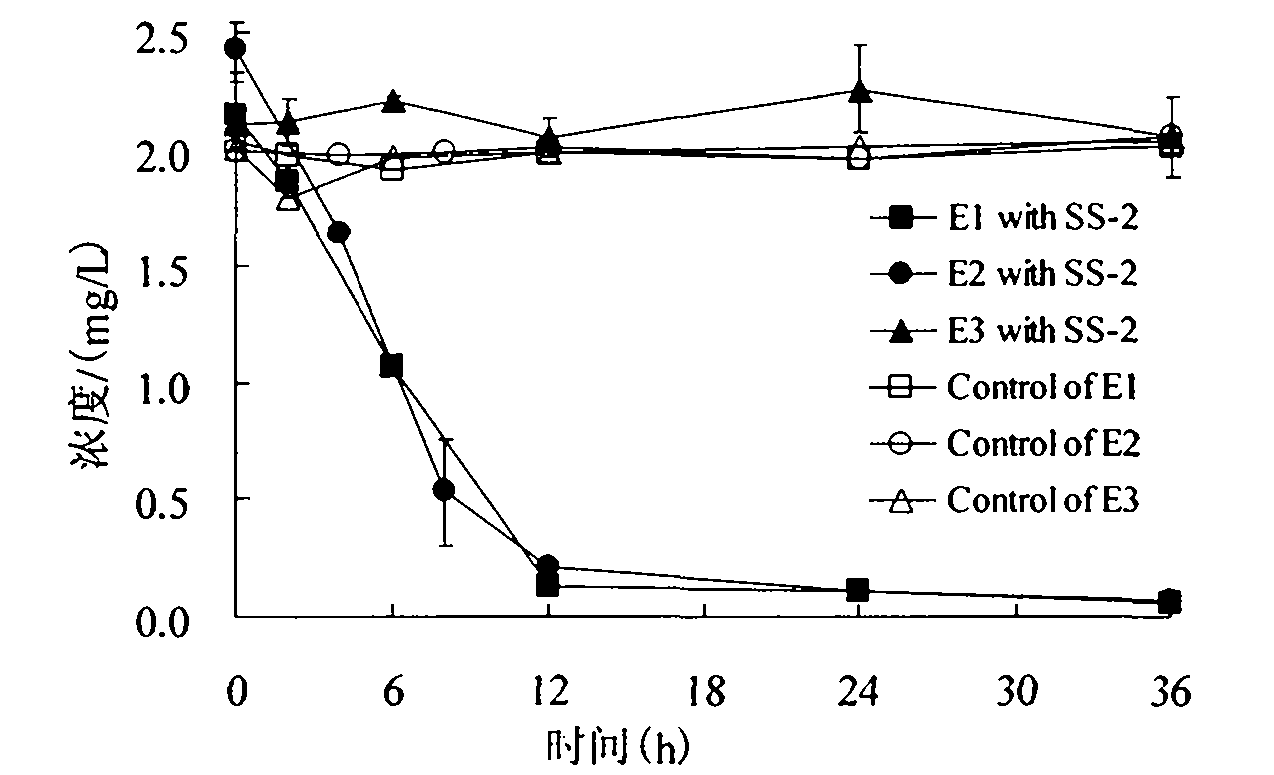
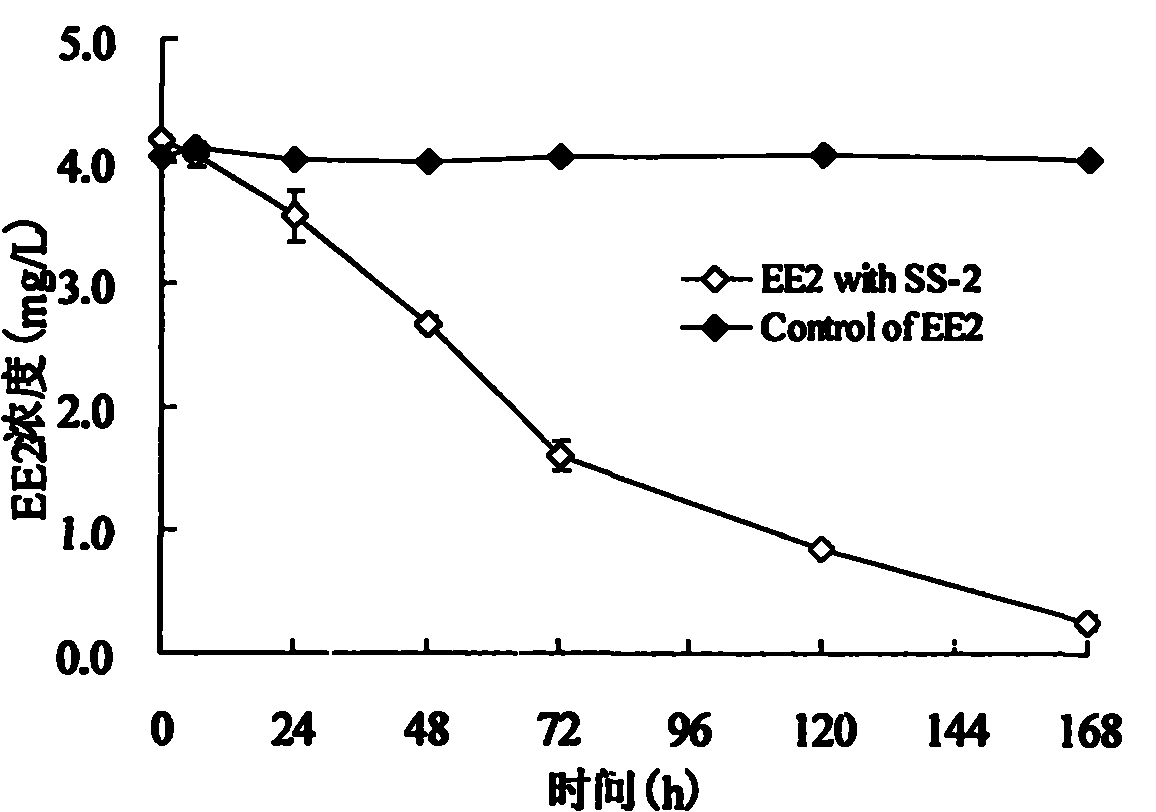
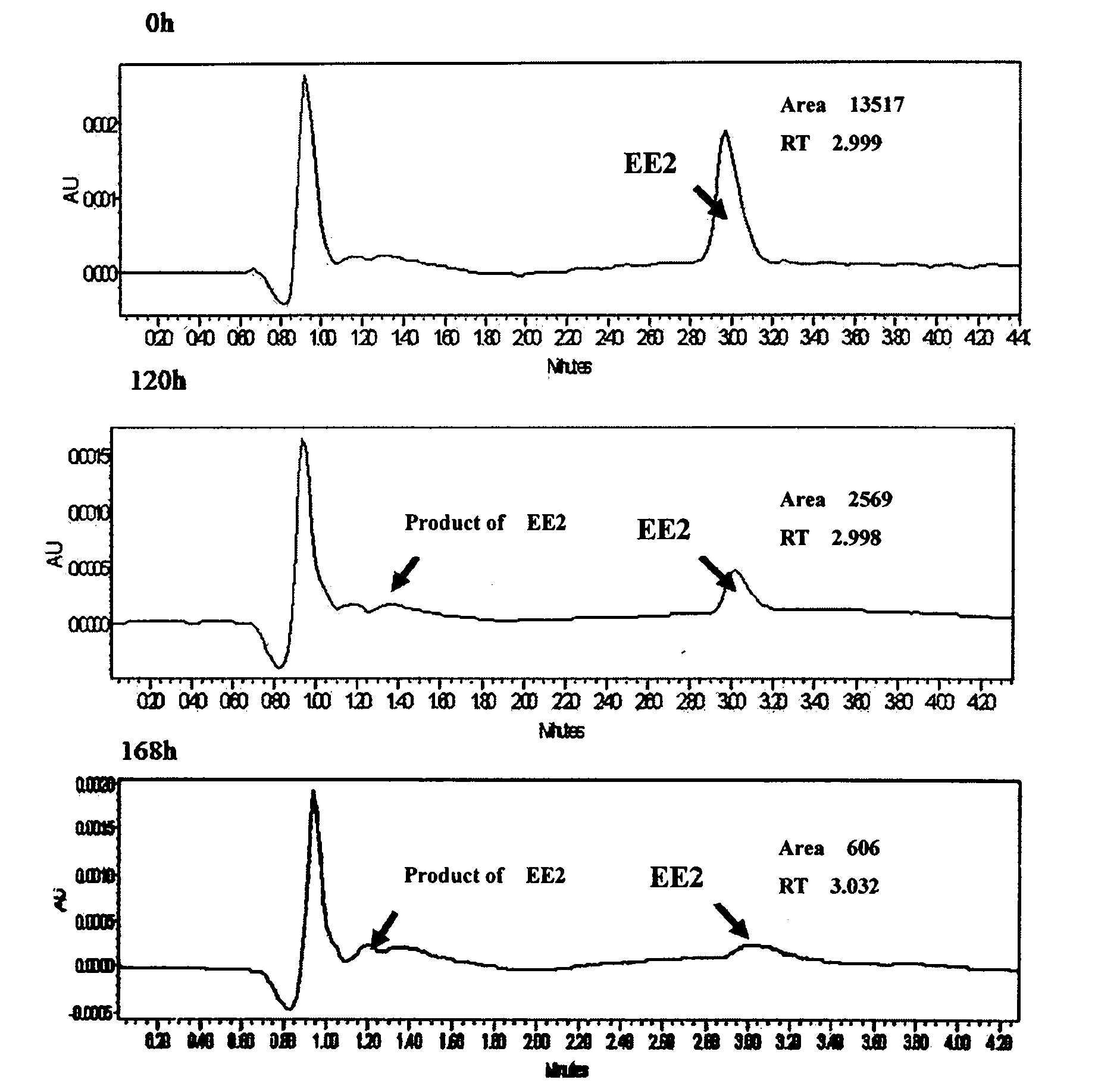
The invention relates to a pseudomonas citronelloalis strain and application thereof in degrading estrogen, wherein the pseudomonas citronelloalis strain has the preservation number of CGMCC No.3634, can grow by using natural or synthetic estrogen as a unique carbon source, can efficiently degrade theelin (E1), 17beta-estradiol (E2) and 17alpha-ethinyl estradiol (EE2) and can be used for a micro-biological degradation technology for removing the estrogen. Under the culture condition of using the estrogen as the unique carbon source, the theelin and the 17beta-estradiol with the initial concentration of 2mg / L can be respectively degraded by 99 percent by the SS-2 strain within 36 hours, the 17alpha-ethinyl estradiol with the initial concentration of 4mg / L can be degraded by 93.6 percent within 168 hours, and estriol has no remarkable degradation phenomenon. The theelin is generated in the process of degrading the 17beta-estradiol, and the theelin is also gradually degraded. Because the pseudomonas citronellolis SS-2 strain has the characteristics of effectively degrading the natural and synthetic estrogen in an environment, the pseudomonas citronellolis SS-2 strain can be applied to an estrogen-contained waste sewage treatment technology or a soil remediation technology.
Owner:BEIJING NORMAL UNIVERSITY
Extended cycle multiphasic oral contraceptive method
A multiphasic method of contraception that provides for sequentially administering to a female of child bearing age: (a) a Phase I composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 30 mcg of ethinyl estradiol for about 4 to about 7 days; (b) a Phase II composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 40 mcg of ethinyl estradiol for about 8 to about 16 days; (c) a Phase III composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 30 mcg of ethinyl estradiol for about 4 to about 7 days; and (d) optionally, a Phase IV composition which is a placebo or a non-steroidal component, such as for example, ferrous fumarate, for about 2 to about 9 days, wherein the ethinyl estradiol equivalent amount of estrogen in the Phase II composition is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in each of the Phase I and III compositions. Preferably the sequential administration of the Phase I, II, and II compositions is repeated the day following the completion of the administration of the Phase III compositions to provide an extended cycle multiphasic oral contraceptive method.
Owner:APTALIS PHARMA
Method for simultaneously detecting residue quantities of multiple hormone veterinary drugs
InactiveCN102749406AAchieving Simultaneous DetectionReduce dissolutionComponent separationEstriolEstrone
The present invention relates to the fields of analytical chemistry and food safety, and provides a method for simultaneously detecting residue quantities of multiple hormone veterinary drugs. The method comprises steps of: extracting a sample with acetonitrile; purifying by three SPE columns, including a C18 column, a silica gel column and an amino column; carrying out a microwave assisted derivatization reaction; and using a DB-5MS capillary column (0.25mm *30m *0.25 mum) to separate and detect residue quantities of nine hormone veterinary drugs. Detection limits of the method of the invention on the nine hormone veterinary drugs, including hexestrol, diethylstilbestrol, dienestrol, etiocholanolone, epitestosterone, oestrone, estradiol, ethinyloestradiol and estriol, are 0.1 mug / kg, 0.3 mug / kg, 0.15 mug / kg, 1mug / kg, 0.17 mug / kg, 0.3 mug / kg; 0.4 mug / kg, 0.35 mug / kg and 0.3 mug / kg respectively. The lower limit of detection of the method provided by the invention meets requirements of related domestic and foreign laws and regulations, and technical indicators, such as precision and recovery rate, are in line with the provisions of relevant standards.
Owner:林维宣
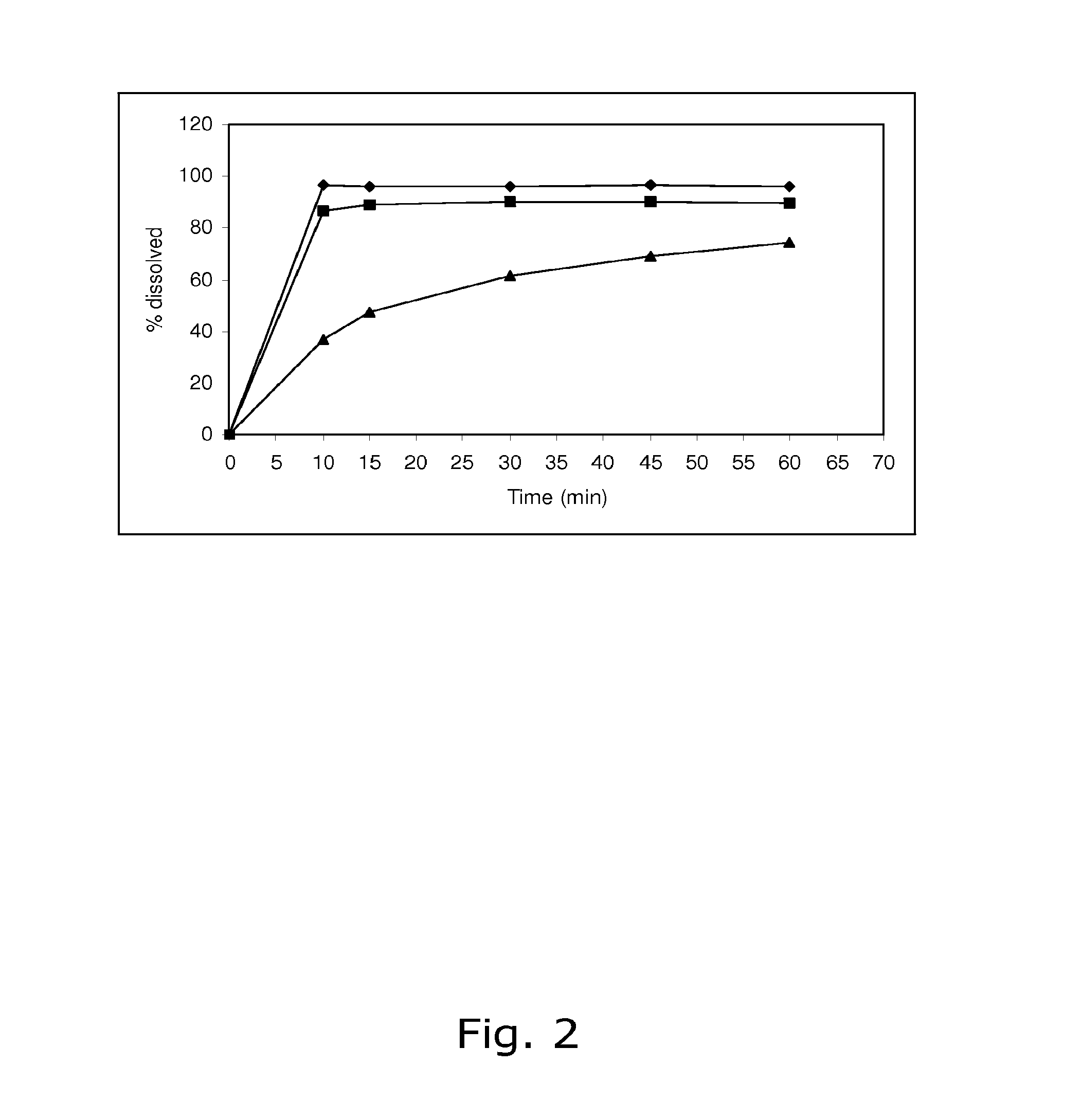
Pharmaceutical preparation for oral contraception
InactiveUS20060183725A1Effective contraceptive actionEasy cycle controlBiocideOrganic active ingredientsOral medicationAdditive ingredient
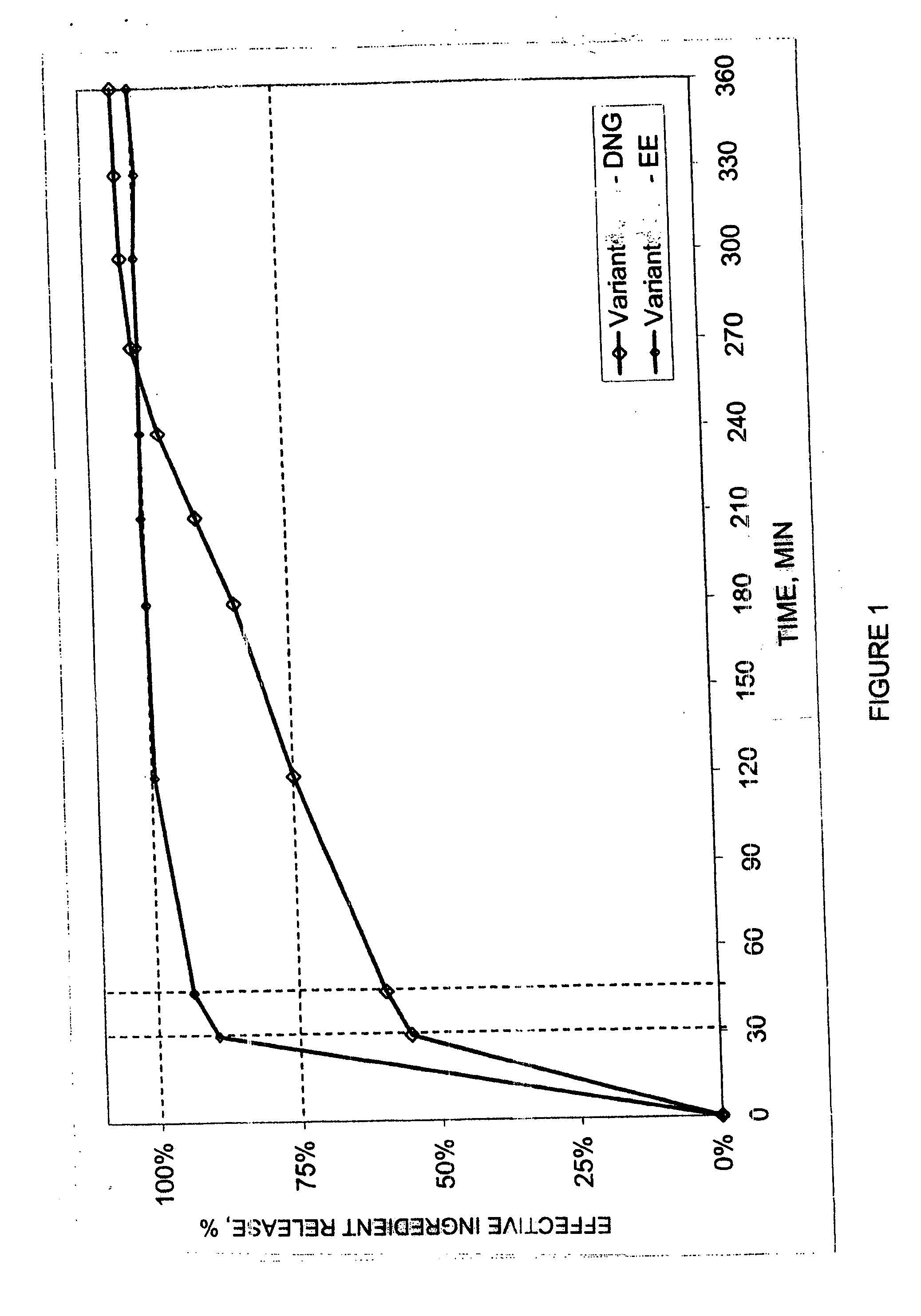
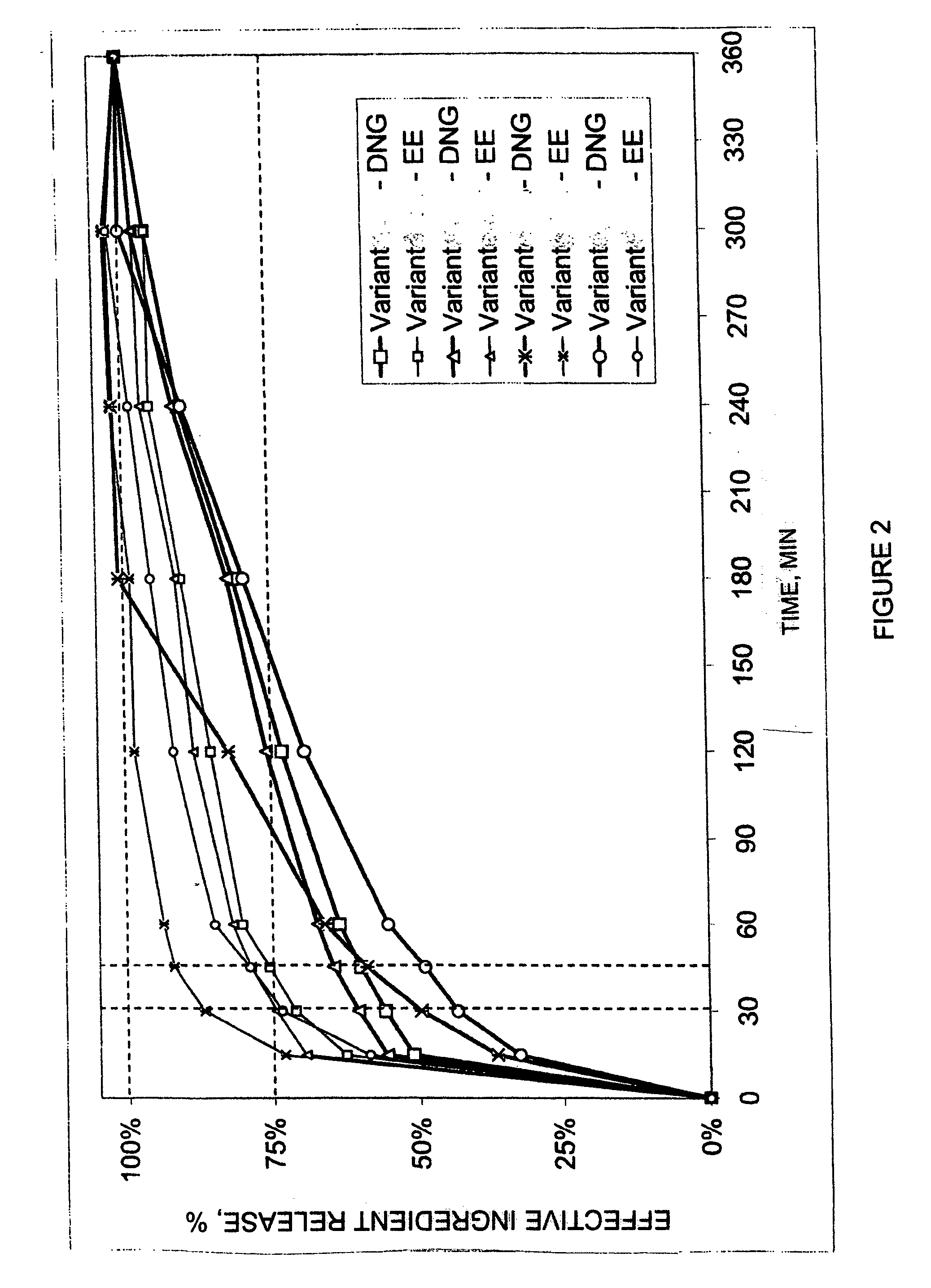
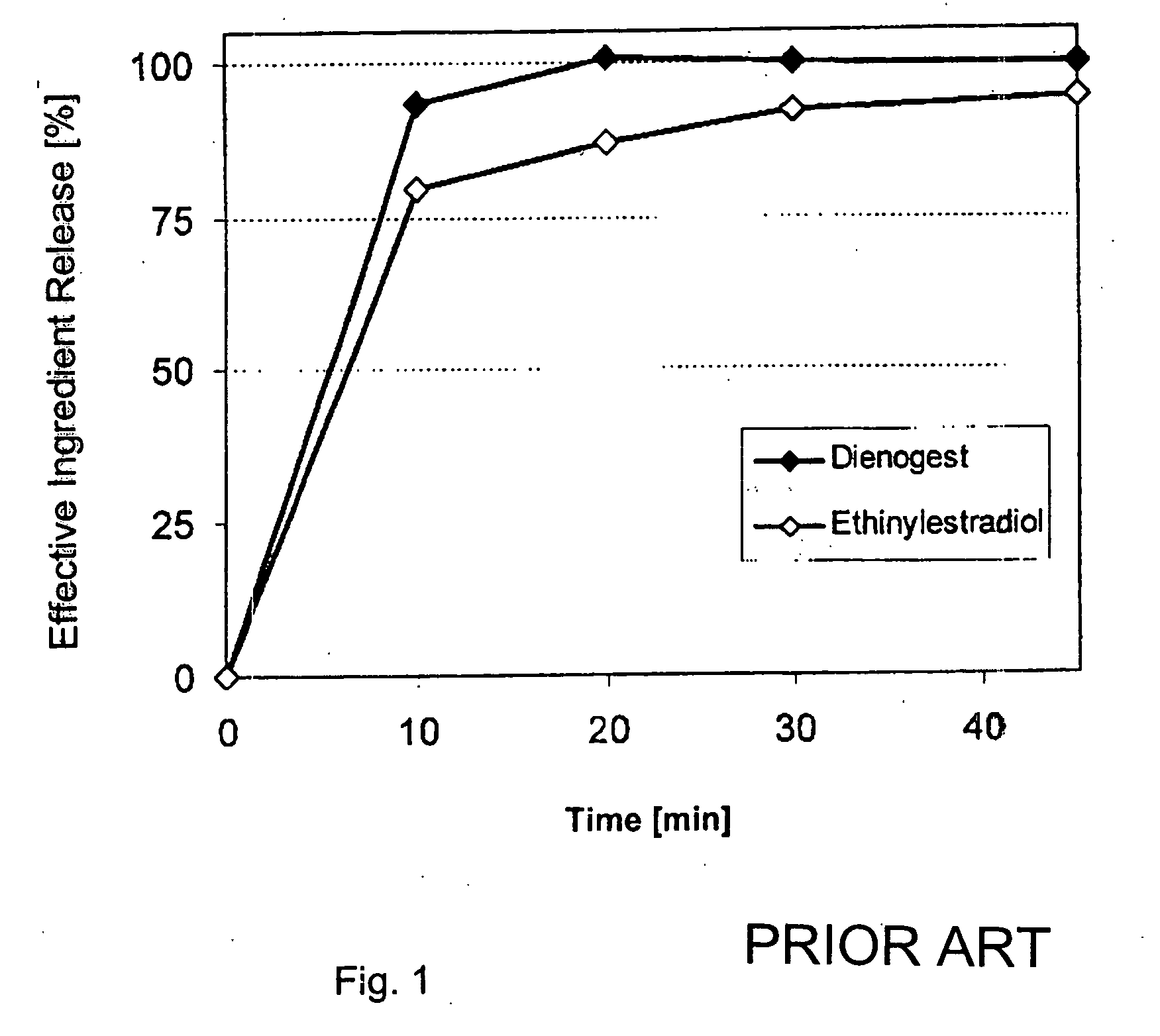
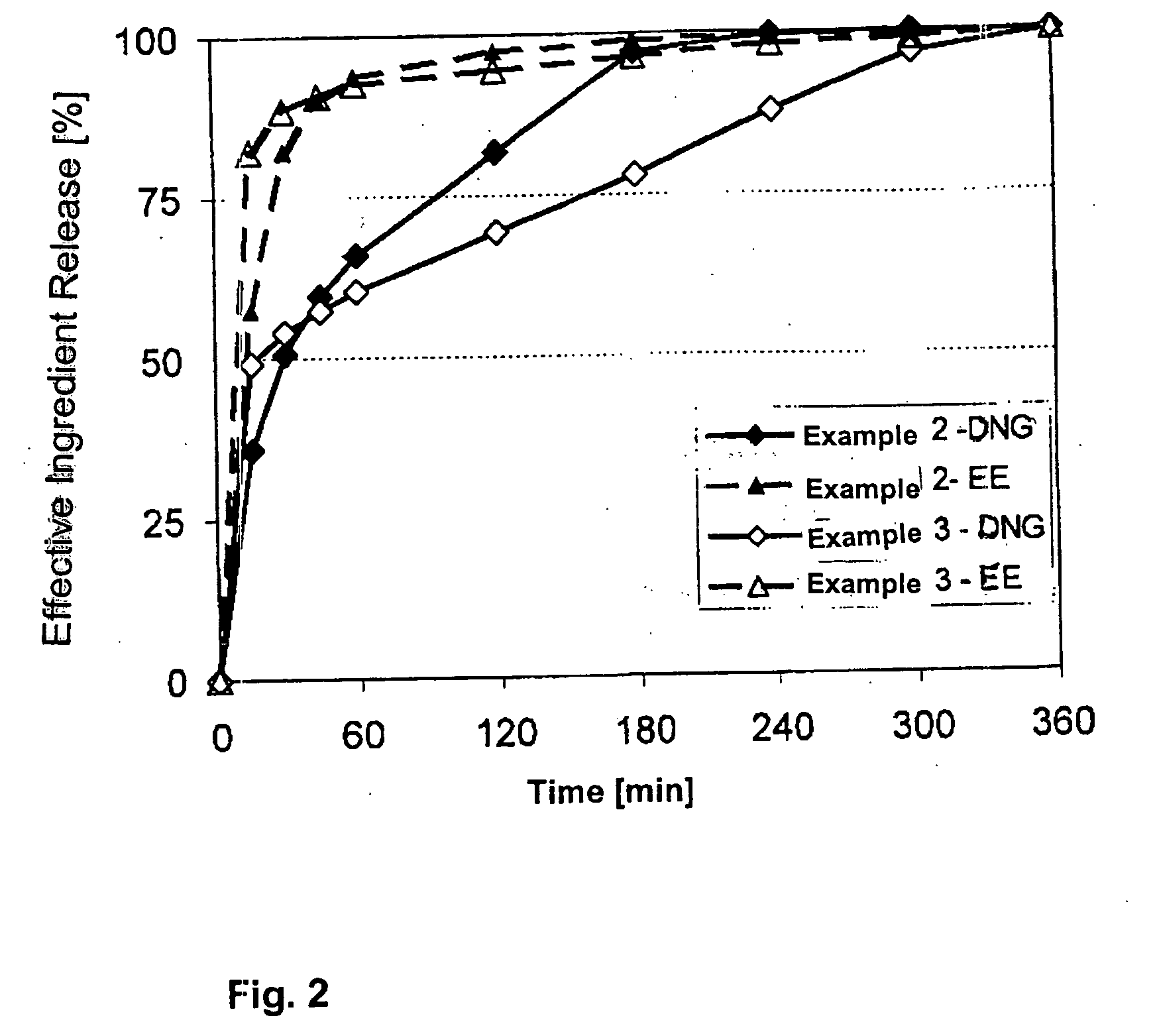
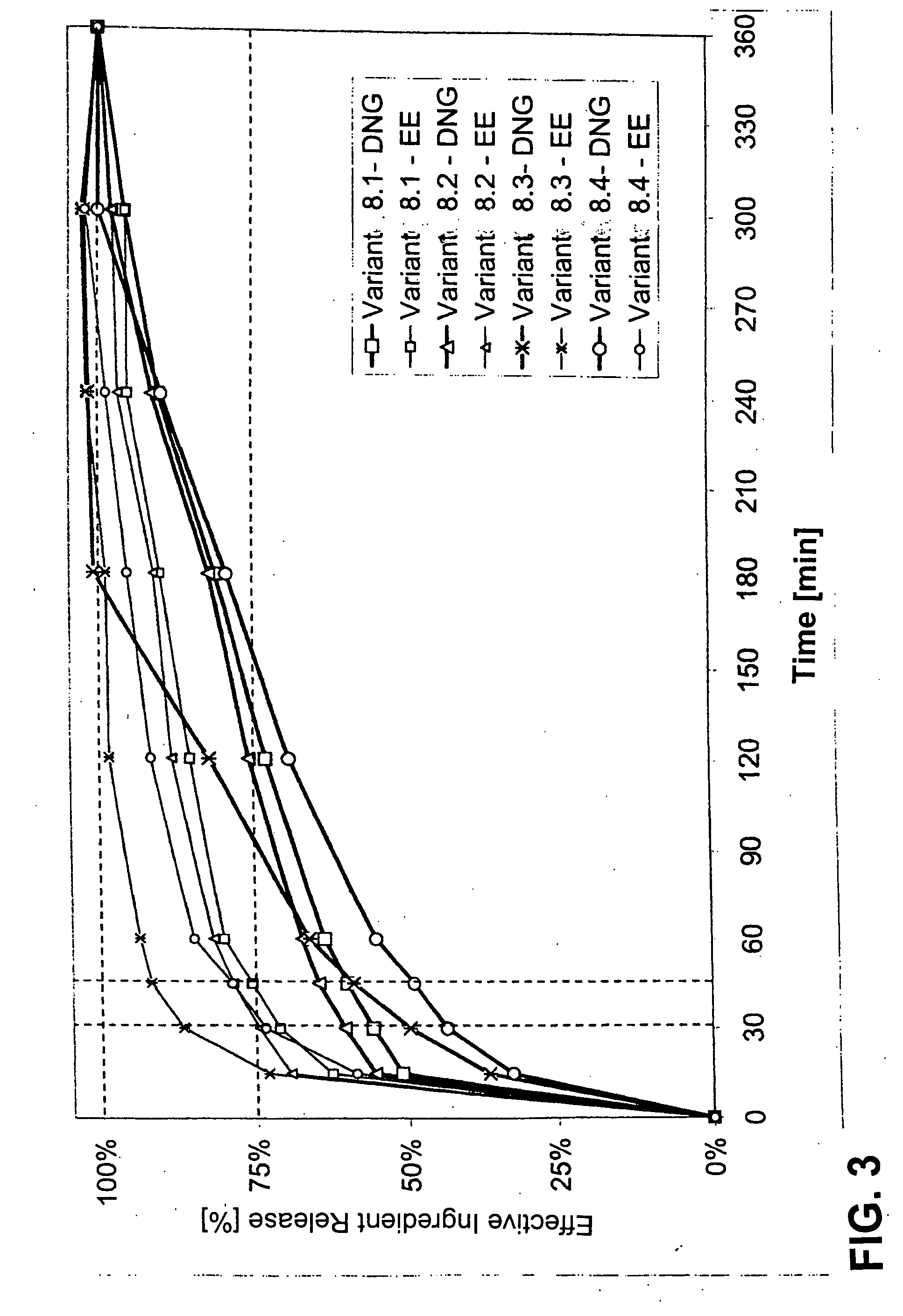
The pharmaceutical preparation for oral contraception has 28 daily dosage units, of which at least 21 daily dosage units each contain from 1.5 mg to 2 mg of dienogest and from 0.015 mg to 0.02 mg of ethinyl estradiol together in a pharmaceutically acceptable carrier. Seven or fewer daily dosage units contain no effective ingredient. Each daily dosage unit can be a film tablet for oral administration, which has a tablet core and film coating on the tablet core. At least 30% of the dienogest is released from the tablet core preferably in a delayed manner after more than 30 minutes, while at least 70% of the dienogest and ethinyl estradiol are released from the film coating preferably in 30 minutes, as determined by a standard dissolution test.
Owner:SCHERING AG
Progestational hormone composition, and its slow-release suppository device and preparation of said device
InactiveCN1891224AInhibit inflammationReduce adhesionOrganic active ingredientsSuppositories deliveryAlcoholPhysiology
The present invention relates to a progestogen composition. Said progestogen composition is formed from 27-31% of fluoropregestin, 2-2.7% of ethinyl estradiol, 8-10% of lauric acid, 37-40% of ethyl alcohol, 0.3-0.6% of carbomer and 6-10% of propylene glycol, and the rest is pure water. Besides, said invention also provides the delayed-release suppository device of said progestogen composition and its preparation method.
Owner:天津市中意畜牧科技发展有限公司
Ethinyl estradiol high-efficiency degrading strain and preparation method thereof
InactiveCN102352336AProvenance is easy to getThe separation and purification method is simple and fastBacteriaMicroorganism based processesPurification methodsEcological safety
The invention relates to an ethinyl estradiol high-efficiency degrading strain and a preparation method thereof. The thallus length of the strain is 0.4-0.8um; and the bacterial colony has a diameter of 1-2.5mm, is round in shape, has regular edges and a low convex surface, is golden yellow in color with no luster and no transparency, and appears as negative through Gram staining. The preparation method comprises the following steps: carrying out directional domestication and culture on active sludge, enriching, separating, and purifying to obtain the ethinyl estradiol high-efficiency degrading strain. The ethinyl estradiol high-efficiency degrading strain can effectively degrade ethinyl estradiol, has an ethinyl estradiol degradation rate up to 80% or above, has no resistance to common antibiotics, and can be inactivated easily, thereby ensuring the ecological safety in use. Besides, the ethinyl estradiol high-efficiency degrading strain has the advantages of easy acquisition of sources and convenient separation and purification method.
Owner:DONGHUA UNIV
Hydroxyl group/keto group synchronous derivatization method of steroid environment endocrine disturbing chemicals
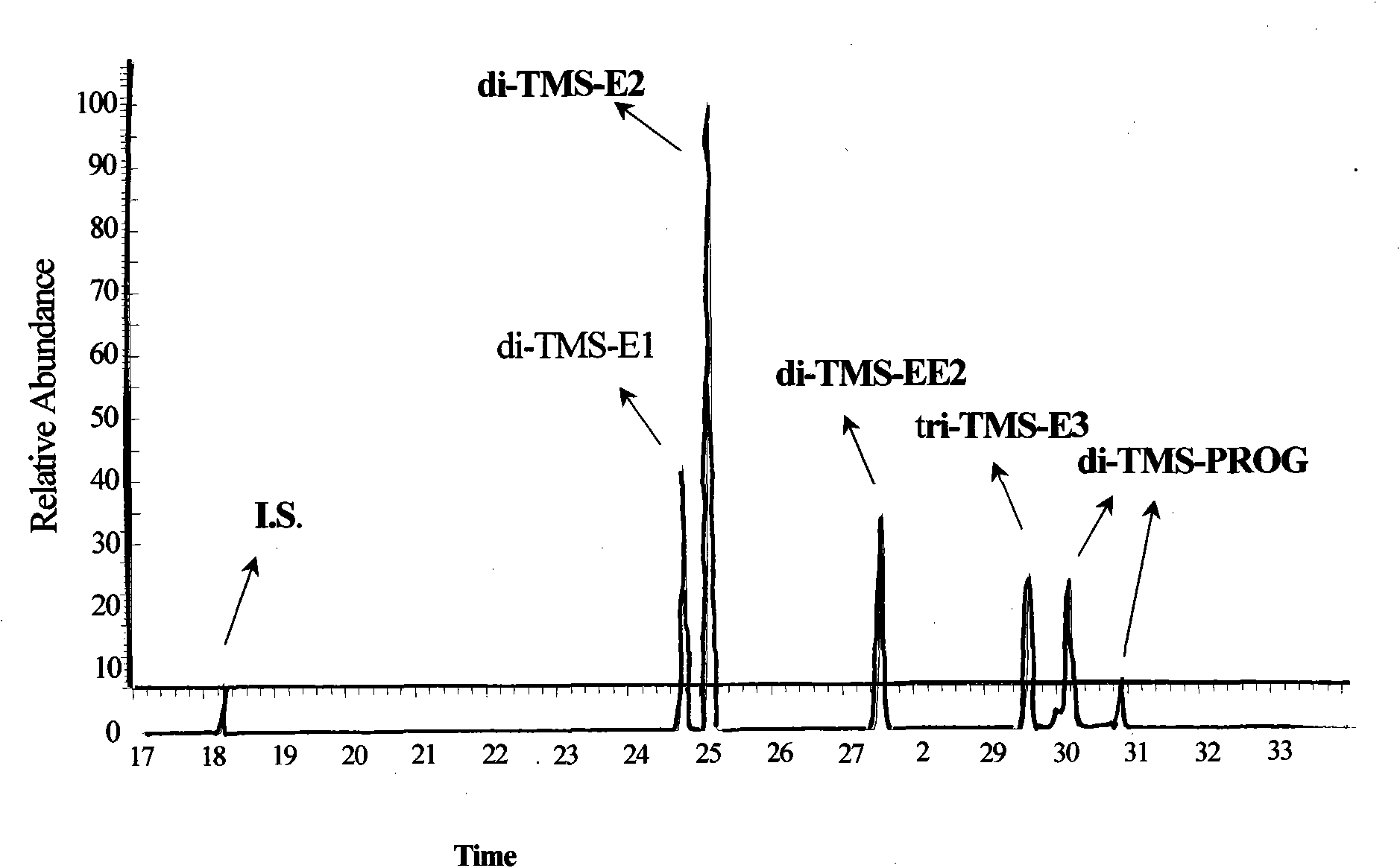
InactiveCN101942006AImplementing Synchronous DerivatizationReduce polarityComponent separationSteroids preparationEstriolLinear correlation
The invention discloses a steroid environment endocrine disturbing chemicals hydroxyl group / keto group synchronous derivatization method of steroid environment endocrine disturbing chemicals (oestrone, 17 beta-estradiol, 17 alpha-ethinyl estradiol, estriol and progesterone). The method comprises the following steps: first fetching standard mixed solution containing the steroid environment endocrine disturbing chemicals and internal standard and introducing high-purity nitrogen at normal temperature so as to dry the standard mixed solution; and adding derivatization reagent prepared from N-methyl-N-trimethylsilyl trifluoroacetamide, iodotrimethylsilane and dithiothreitol based on a proportion of 1,000 muL:5 to 10 muL:5 mg and reacting at 20 to 60 DEG C for 5 to 10 min so as to obtain the derivatization product shown in figure. The method has the advantages of simple and time-saving operation, low energy consumption and cost and the like. The hydroxyl group and the keto group can be derivatized synchronously, the linear correlation among the derivatization products is good, and the detection limit of instrument can reach 0.01 to 1 pg / mu L. The method can be used for the GC-MS detection of the steroid environment endocrine disturbing chemicals in samples such as water, bottom sediment, organisms and the like.
Owner:KUNMING UNIV OF SCI & TECH
Extended estrogen dosing contraceptive regimen
A method of contraception that provides for sequentially administering to a female of child bearing age: (a) a first composition containing a progestin in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol for about 22 to about 26 days; (b) a second composition containing an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol for about 2 to about 3 days and an optional third composition that is a placebo provided that (i) if estrogen administration is continuous then the first composition is administered for 25 to 26 days, the second composition is administered for 2 to 3 days and no third composition is administered and (ii) if estrogen administration is not continuous then the first composition is administered for 22 to 24 days, the second composition is administered for 2 to 3 days and the third composition is administered for 1 to 4 days. The total cycle length is 28 days, with the first composition administered on day 1 of the menstrual cycle, defined as the first day of menstrual bleeding, or on the first Sunday after the first day of the menstrual cycle.
Owner:APTALIS PHARMA
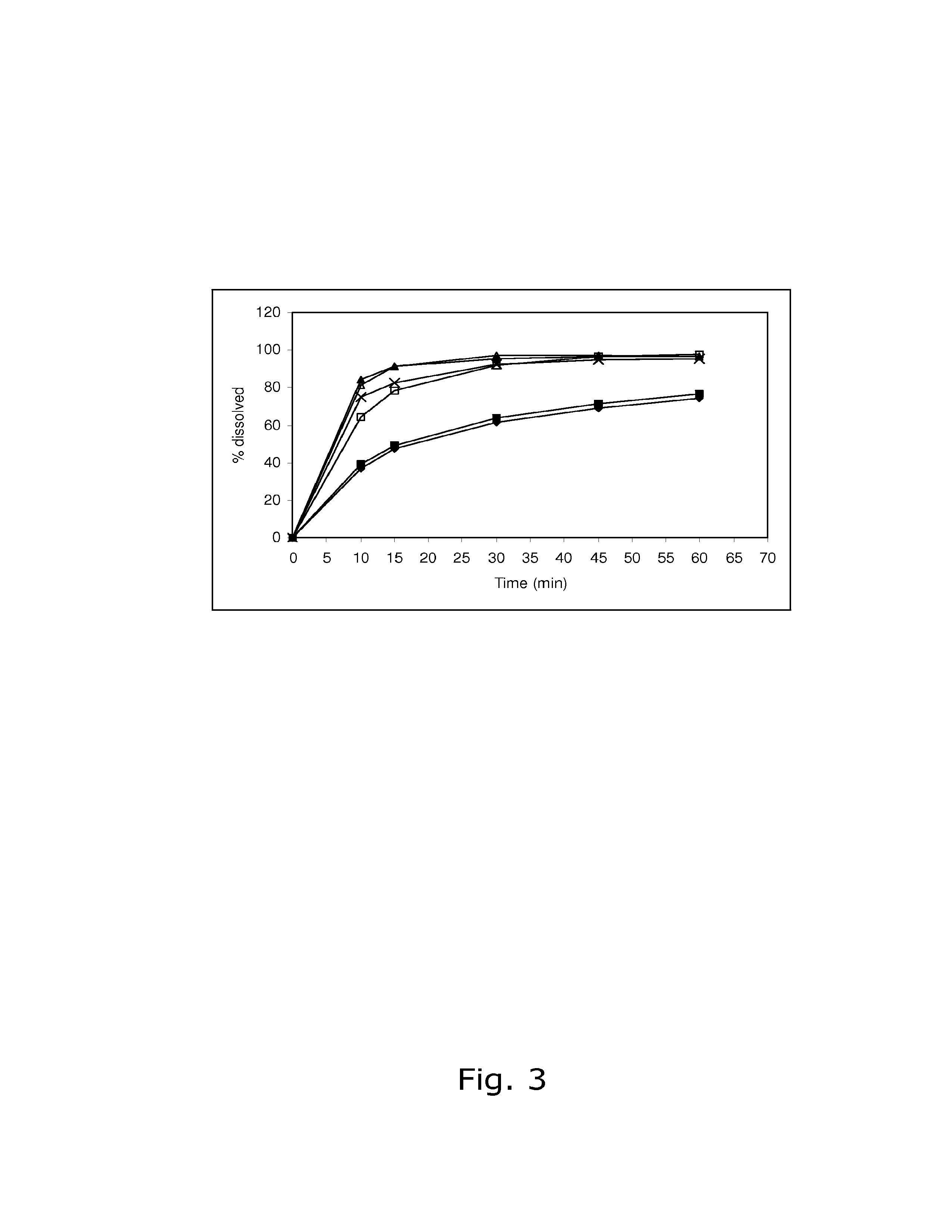
Solid peroral contraceptive preparations
InactiveUS20060205701A1Reduces effective amountBiocideOrganic active ingredientsAdditive ingredientEthinylestradiol
The solid peroral contraceptive contains an effective ingredient combination of dienogest in a daily dosage of equal to or less than 2.0 mg and ethinyl estradiol in a daily dosage of less than 0.03 mg together with one or more pharmaceutically acceptable carriers. The dienogest is released in two stages, while the ethinyl estradiol is released with the first stage portion of the dienogest.
Owner:BAYER SCHERING PHARMA AG
Preparation method of modified pollen and application thereof for absorbing and treating EDCs of water
InactiveCN103240061ASignificant progressWide variety of sourcesOther chemical processesWater/sewage treatment by sorptionEstroneTetrabromobisphenol A
The invention discloses a preparation method of modified pollen and the application thereof for absorbing and treating EDCs of water, which belong to a treatment method for sewage or wastewater. The preparation method disclosed by the invention comprises the following steps: washing natural pollen with ethanol; carrying out solid-liquid separation, drying and crushing; and adding the crushed product into a concentrated hydrochloric acid, sequentially carrying out ultrasonic dispersion and backflow filtering, washing to neutral, and drying, wherein the natural pollen is lotus pollen, piny flower pollen, apricot flower pollen and dandelion pollen. According to the application of the modified pollen for treating EDCs of waer, the modified pollen is added into water containing pollutants such as bisphenol A, estrone, 17beta-estradiol, estriol, progesterone, 17-alpha-ethinyl estradiol, 4-n-nonyl phenol, 4-t-octyl phenol, diethylstilbestrol or tetrabromobisphenol A. The pollen disclosed by the invention is small in polarity, hydrophobic, high in adsorbent active site and saturated adsorption amount, small in desorption ratio, renewable and reusable, therefore, the application is a way of treating water pollutions by using biomass materials.
Owner:云南中绿文德生态环保科技股份有限公司
Transdermal delivery of hormones without the need of penetration enhancers
ActiveUS8668925B2Increase release rateReduce riskBiocideOrganic active ingredientsHormones regulationBlood plasma
The present invention relates to a patch comprising a drug-containing layer with low content of hormones, such as gestodene, and optionally an estrogen (e.g. ethinyl estradiol). Upon administering the patch to a woman, plasma levels of at least 1.0 ng / ml of Gestodene is achieved at steady state conditions without the need of incorporating penetration enhancers or permeation enhancers in the drug-containing layer. Satisfactorily plasma levels of the hormones is also achieved throughout a period of at least 1 week, making the patch applicable for being used in female contraception with the concept of administering the patch ones weekly.
Owner:LUYE PHARMA SWITZERLAND AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com