Patents
Literature
3859 results about "Folic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Folic acid is a part of the B complex of vitamins. It is vital for red blood cells and for many other cells in the body. The form of folic acid occurring naturally in food is called ‘folate’. Functions of folic acid. Folic acid, along with vitamin B12, is important for formation of red blood cells.
Regulation of Mammalian Keratinous Tissue Using Skin and/or Hair Care Actives
Personal care compositions containing an active selected from the group consisting of phlorogine, phlorgine BG, deoxyArbutin, sucrose dilaurate, bakuchiol, pyrenoine, millet, arlatone dioic acid, cinnamic acid, ferulic acid, achromaxyl, methyl nicotinamide, oil soluble licorice extract, folic acid, undecylenic acid, zinc undecylenate, L-tryptophan, thiamine HCl, hexylresorcinol, lipidami red vine, dragosine, methyl gentisate, inositol, symdiol 68, laminaine, their salts, their derivatives, their precursors, and / or combinations thereof. Methods for regulating the condition of mammalian keratinous tissue by topically applying the personal care compositions are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
Conjugates and compositions for cellular delivery
InactiveUS20050239739A1Efficient deliveryEfficient subsequent releaseBiocideSugar derivativesAntisense nucleic acidNucleoside X
This invention features conjugates, compositions, methods of synthesis, and applications thereof, including folate derived conjugates of nucleosides, nucleotides, non-nucleosides, and nucleic acids including enzymatic nucleic acids and antisense nucleic acid molecules.
Owner:SIRNA THERAPEUTICS INC
Nutraceutical composition and method of use for treatment / prevention of cancer
InactiveUS20070248693A1Function increaseAbility to createBiocideAlgae medical ingredients1,4-BenzoquinonePantothenic acid
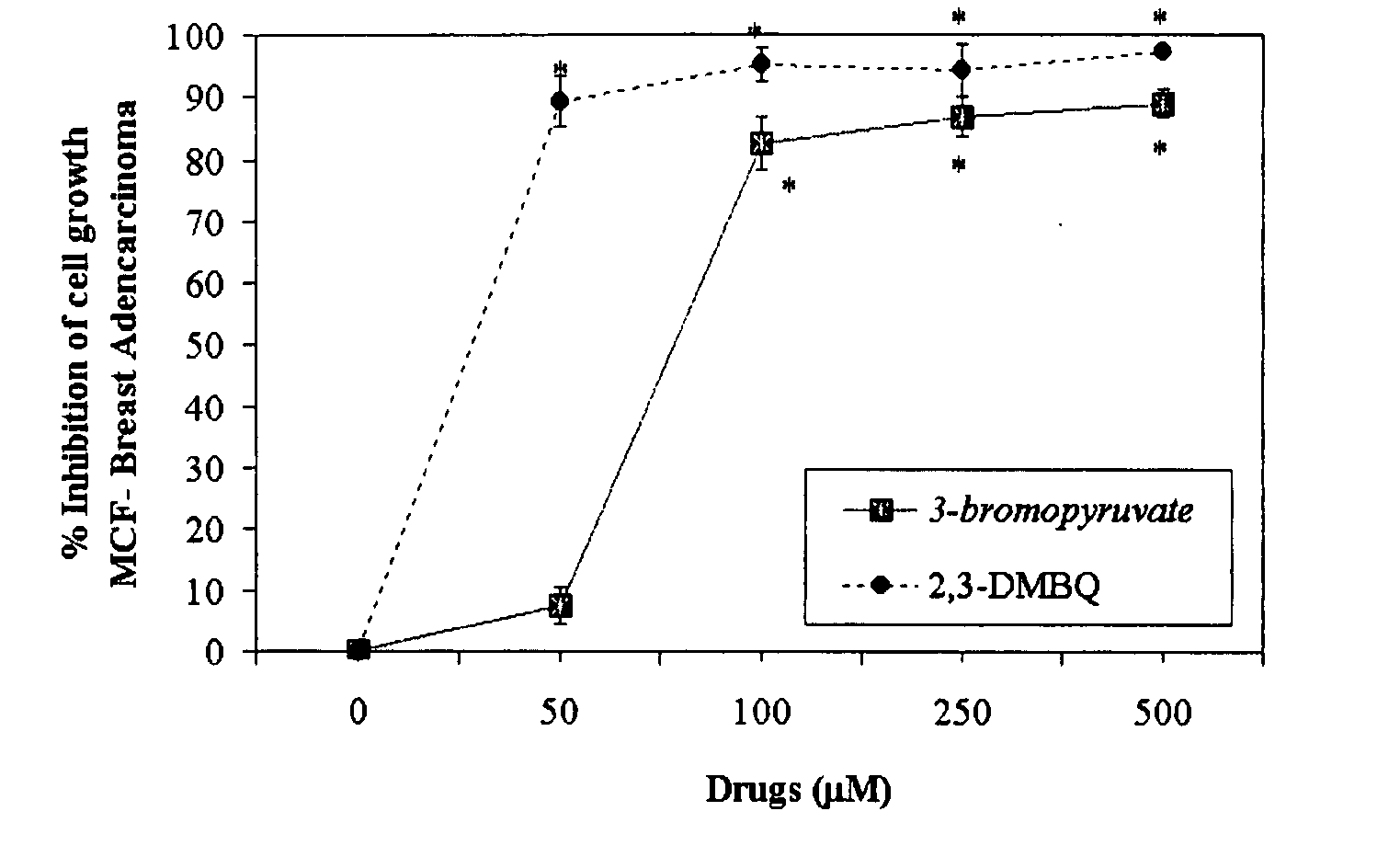
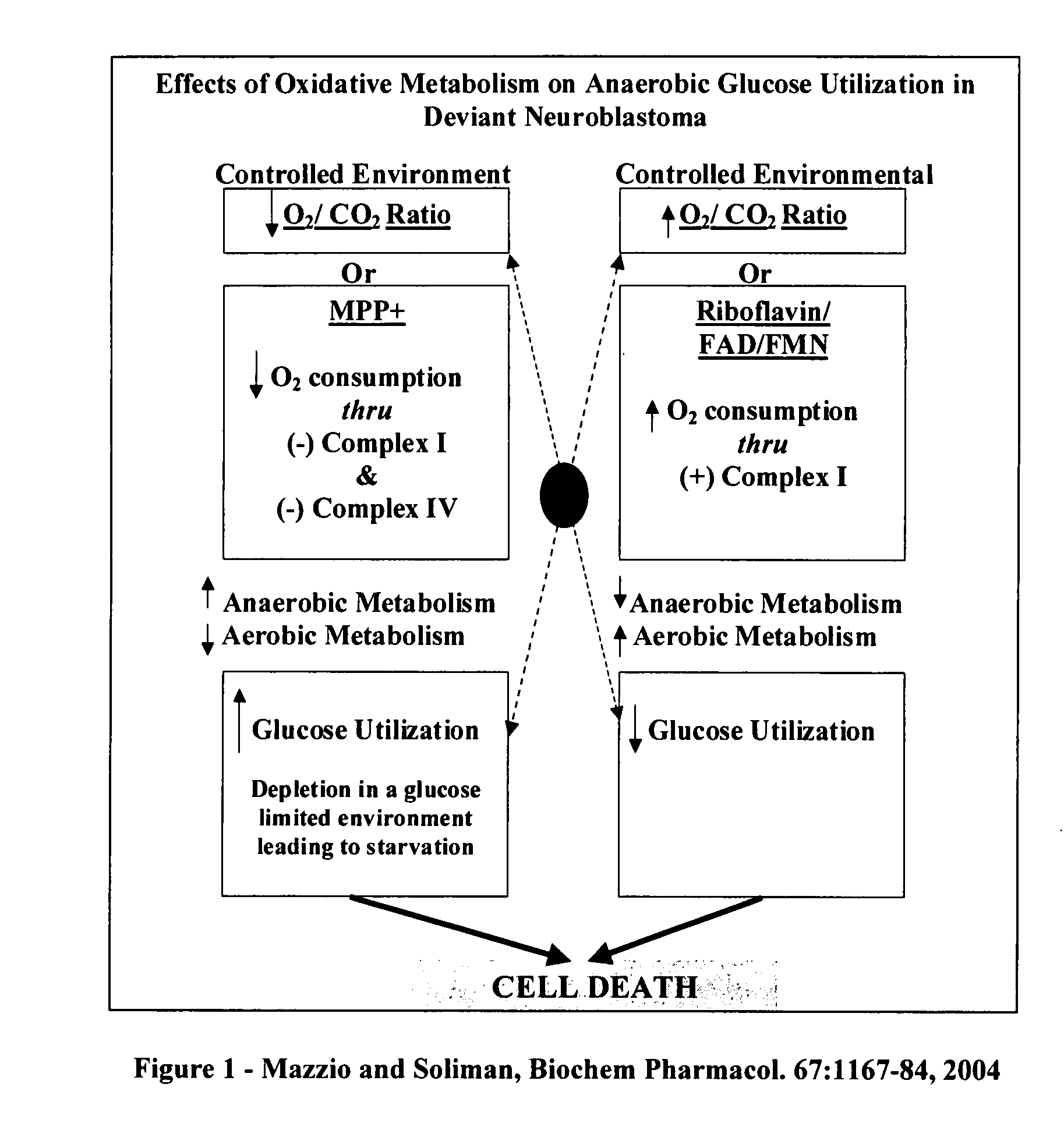
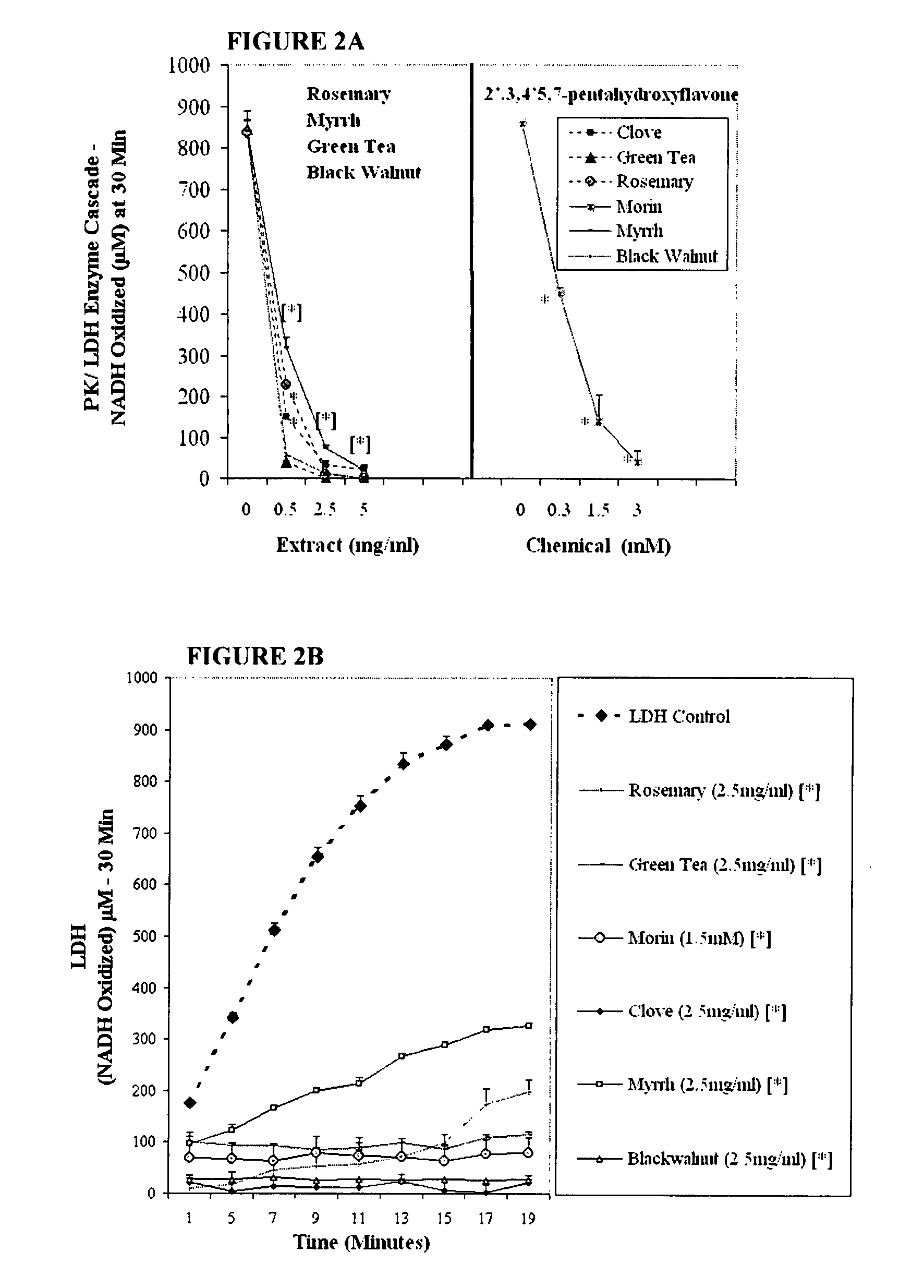
The invention describes a pharmaceutical composition and method for treating cancer comprised of A) 2,3-dimethoxy-5-methyl-1,4-benzoquinone and / or B) at least one of wild yam root, teasel root, balm of gilead bud, bakuchi seed, dichroa root, kochia seed, kanta kari, bushy knotweed rhizome, arjun, babul chall bark, opopanax and bhumy amalaki; optionally one or more of frankincense, garcinia fruit, vitex, dragons blood, mace, sage and red sandalwood with at least c) one compound capable of maximizing oxidative mitochondrial function preferably riboflavin or vitamin B2 derivatives, FAD, FMN, 5-amino-6-(5′-phosphoribitylamino)uracil, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, niacin, vitamin C and pantothenate and / or d) at least one lactic acid dehydrogenase inhibitor (preferably 2′,3,4′5,7-pentahydroxyflavone) and optionally f) an alkalizing agent (aloe vera, chlorella, wheat grass, sodium or potassium bicarbonate, potassium) g) an antiproliferative herb (speranskia or goldenseal) and h) a pharmaceutically acceptable carrier.
Owner:MAZZIO ELIZABETH +1
Ultra-high fiber supplement and method of reducing weight cardiovascular risks and ingested toxins.
Owner:SMALL GIANT
A combination of mitochondrial nutrients for relieving stress, preventing and improving stress-related disorders
InactiveUS20060257502A1Accelerated agingIncreasing oxidative metabolismBiocideCosmetic preparationsAlpha-TocopherolL-Carnosine
A dietary supplement of mitochondrial nutrients is designed for relieving stress, preventing and improving stress-related disorders, such as chronic fatigue syndrome, diabetes, age-associated cognitive dysfunction and diseases (Parkinson's and Alzheimer's disease). The supplement composition has the following nutrients: B vitamins (cyanocobalamin 2-1,000 ug, thiamin 1-1,000 mg, niacin 15-2,000 mg, pyridoxine 1-1,000 mg, Pantothenate 5-150 mg, folic acid 400-40,000 ug), alpha-tocopherol 10-800 mg, ascorbic acid 50-10,000 mg, calcium 20-2,000 mg, vitamin A 200-10,000 ug, alpha-lipoic acid 100-1,000 mg, N-acetyl cysteine 100-3,000 mg, L-carnosine 100-9,000 mg, tyrosine 100-9,000 mg, vanillin 10-100 mg, phosphatidylserine 10-800 mg, resveratrol 10-50 mg, dehydroepiandrosterone 1-50 mg, and melatonin 0.1-3 mg, all of which have been individually used experimentally or clinically for relieving stress, preventing and treating age- and stress-related disorders and diseases but no combination of these compounds has been used. Many embodiments also contain at least one adjunct ingredient such as coenzyme Q 10-200 mg, acetyl-L-carnitine 100-2,000 mg, choline 50-1,000 mg, and creatine 100-2,000 mg.
Owner:LIU JIANKANG
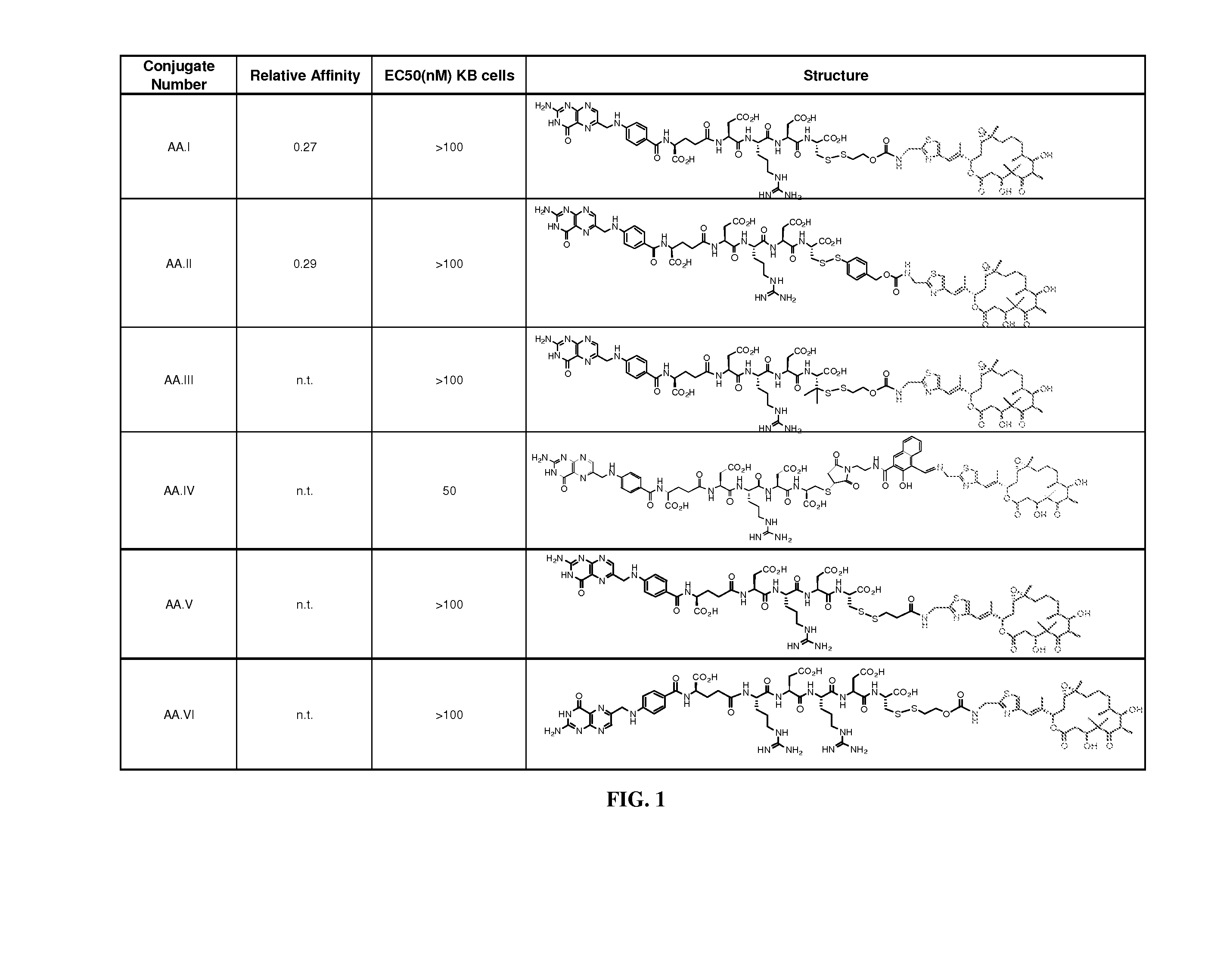
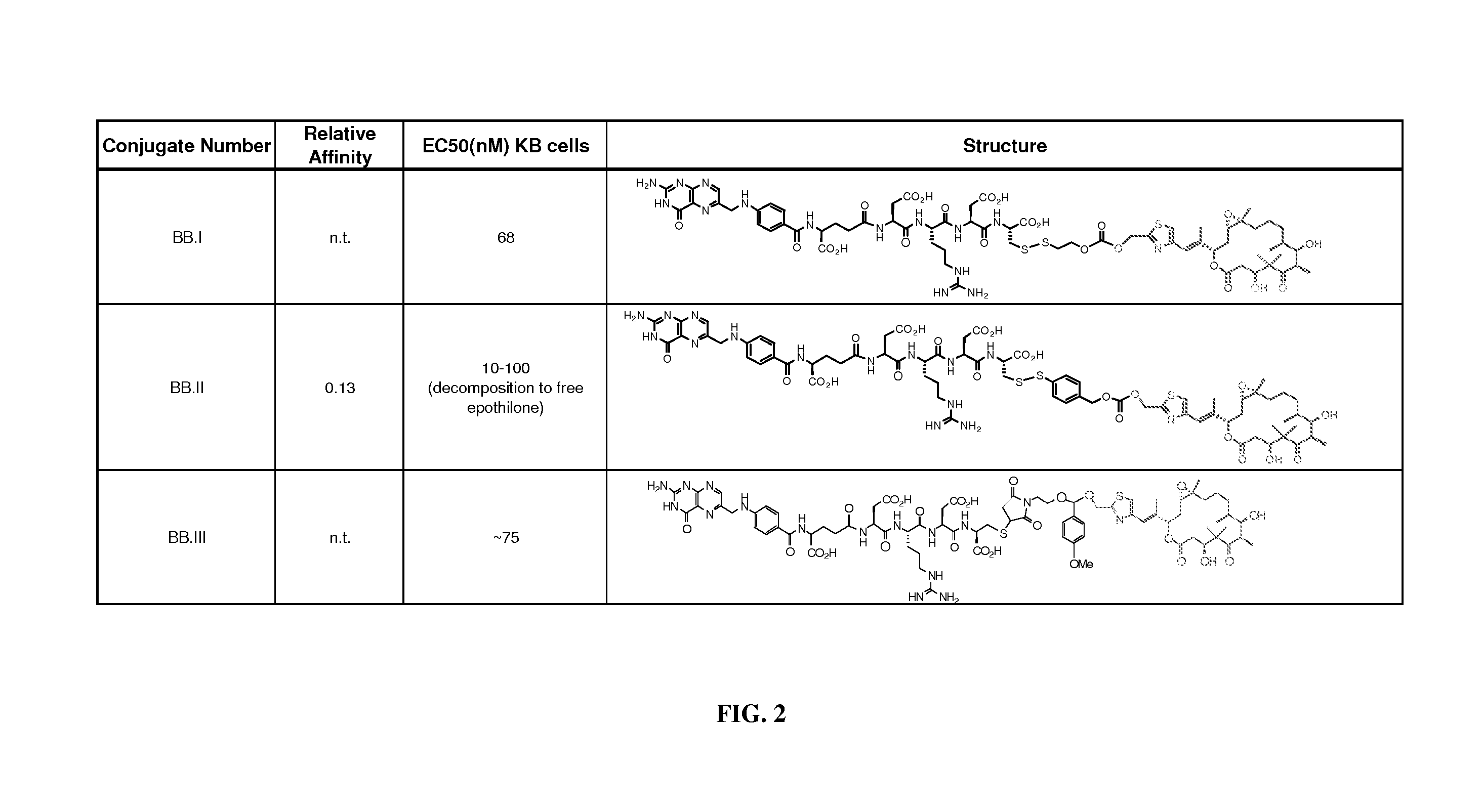
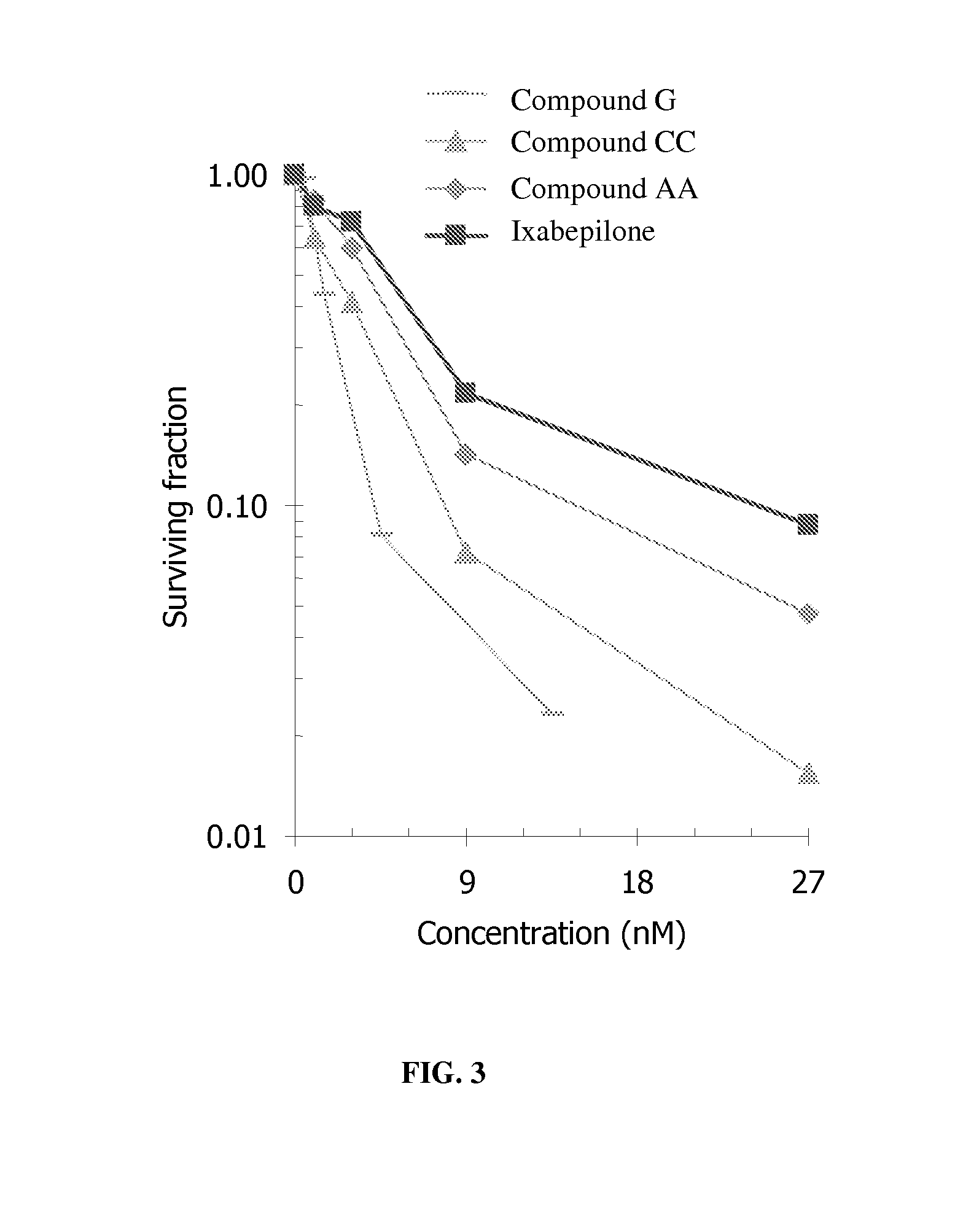
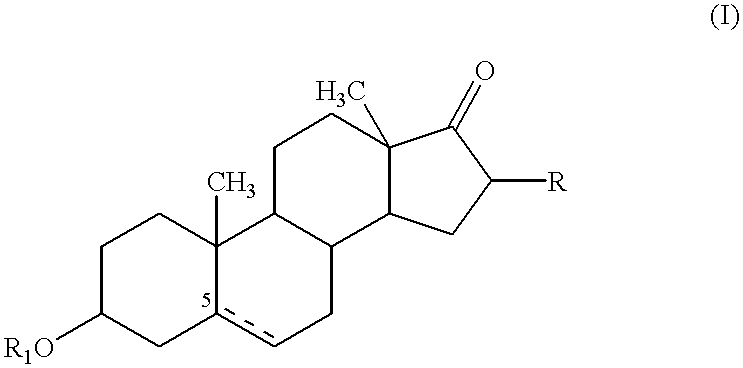
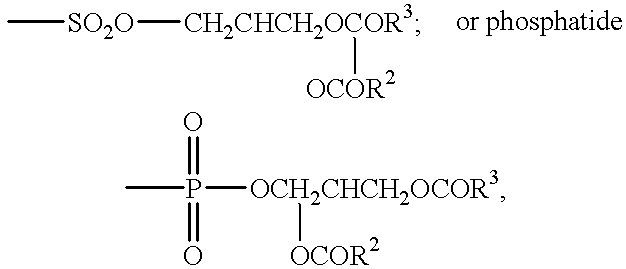
Conjugates of aziridinyl-epothilone analogs and pharmaceutical compositions comprising same
The present invention is directed to conjugated compounds comprising a folate, or an analog or derivative thereof, and an aziridinyl epothilone analog, as further described herein, and / or pharmaceutically-acceptable salts and / or solvates thereof, useful in the treatment of cancer or other folate-receptor associated conditions.
Owner:BRISTOL MYERS SQUIBB CO +1
Compositions & formulations with an epiandrosterone or a ubiquinone & kits & their use for treatment of asthma symptoms & for reducing adenosine/adenosine receptor levels
A composition and various formulations comprise preventative or therapeutic amounts of an epiandrosterone, analogue thereof or salt thereof, and / or a ubiquinone or salt thereof, and a pharmaceutically or veterinarily acceptable carrier or diluent. The composition and formulations are useful for treating bronchoconstriction, respiratory tract inflammation and allergies, asthma, and cancer. A method of treating diseases associated with low adenosine levels or adenosine depletion comprises administering folinic acid or a pharmaceutically acceptable salt hereof in a preventative or therapeutic amount, or an amount effective to treat adenosine depletion.
Owner:EAST CAROLINA UNIVERISTY
Nutritional composition for treating an immune condition
InactiveUS6929793B2Treat conditionPrevention or inhibition of diarrheaOrganic active ingredientsBacteriaVitamin CPhysiology
A nutritional composition is described for prevention or treatment of an immune condition. The composition includes at least vitamin E, vitamin C, vitamin B6, folic acid, vitamin B12, copper, zinc, selenium, fructo-oligosaccharides and / or gum acacia, a probiotic lactic acid bacterium. For example, in an embodiment it comprises per 300 g: 150 IU Vitamin E, 120 mg Vitamin C, 2 mg Vitamin B6, 400 ug Folic acid, 3.8 ug Vitamin B12, 1.5 mg Copper, 15 mg Zinc, 100 ug Selenium, 3 g Fructo-oligosaccharides and / or gum acacia, 10E10 cfu ST11 lactobacillus. Also disclosed are a method for making the nutritional composition, a method for manufacturing a functional food or medicament; and a method of prevention or treatment of an immune condition by administering an effective amount of the composition, functional food or medicament.
Owner:SOC DES PROD NESTLE SA
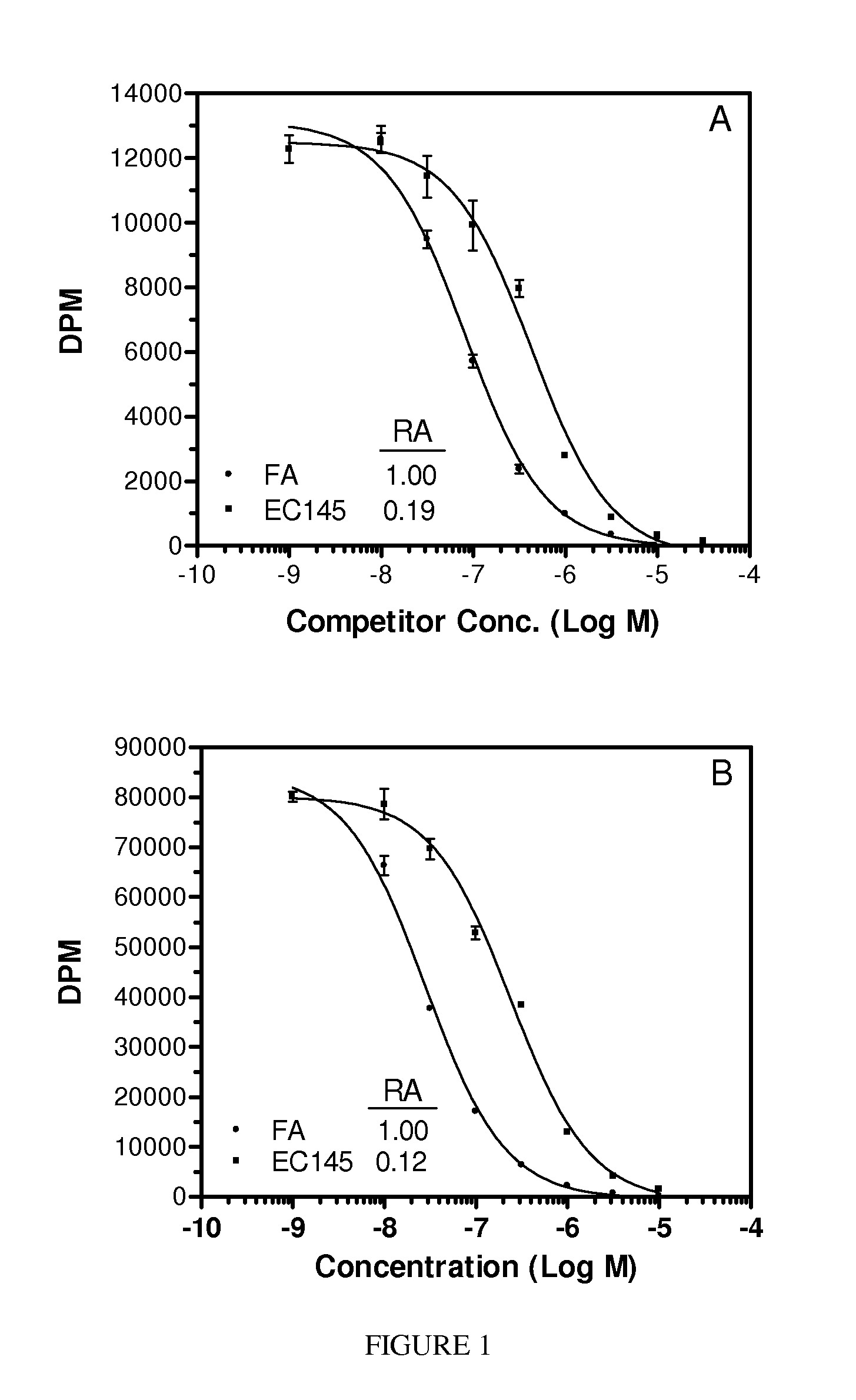
Folate mimetics and folate-receptor binding conjugates thereof
A cell population expressing folate receptors is selectively targeted with a folate mimetic. The folate mimetic is conjugated to a diagnostic or therapeutic agent to enable selective delivery of the agent to the targeted cell population.
Owner:PURDUE RES FOUND INC +1
Nutritional system for nervous system disorders
A novel composition for treating nervous system disorders. The composition is formed by preparing a mixture comprising an effective amount of vitamin B-6, folic acid, vitamin C, magnesium, vitamin B-3, copper, probiotics, fructo-oligosaccharide (FOS), betaine, pancreatin, papain, pepsin, vitamin B-1, vitamin B-2, vitamin B-12, biotin, pantothenic acid, chromium polynicotinate and a digestive support ingredient selected from the group consisting of dandelion root, juniper, aloe vera, burdock, ginger root, artichoke, and kelp. Other ingredients may include: beta carotene, vitamin E, selenium, zinc, sea vegetation, alfalfa, trace minerals and molybdenum.
Owner:C&D FOREMAN
Method and synergistic composition for treating attention deficit/hyperactivity disorder
InactiveUS6541043B2Minimize side effectsBiocideHydroxy compound active ingredientsBeta-CaroteneBetaine
A composition and method for treating Attention Deficit / Hyperactivity Disorder (ADHD) is provided which can be used both with and without ethical drugs now used to treat ADHD. The composition contains dimethylaminoethanol (DMAE), omega 3-fatty acids, betaine, oligomeric proanthocyanidins (OPC), folic acid, vitamins C, E, B12, B6, B5 and beta-carotene and minerals (calcium, magnesium, zinc and selenium). Ethical drugs such as amphetamines, methylphenidate HCl and pemoline are known to control ADHD, but each has significant side effects when used in their therapeutic dose. When combining the composition with such ethical drugs, the amount of the ethical drug can be lowered below a level which causes undesirable side effects which is an important feature. Preferred compositions contain one or more of lecithin, choline, 5-hydroxytryptophan, tyrosine, Reishi Extract, Kava Extract, Gingko, Ginseng and St. John's Wort.
Owner:PHILIP C LANG
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
Compositions including iron
Compositions and methods for prevent, stabilize, reverse or treat disorders related to iron deficiency in a human or other animal. In a first embodiment, the composition includes about 10 mg to about 500 mg of one or more forms of iron, wherein at least one form of iron is an aspartic acid-glycine chelate of iron; and about 5 mg to about 500 mg of one or more forms of an organic acid. In another embodiment, the composition includes about 50 to about 150 mg of one or more forms of iron, wherein at least one form of iron is an aspartic acid-glycine chelate of iron; about 50 to about 250 mg of one or more forms of an organic acid; about 150 to about 250 mg of one or more forms of ascorbic acid; about 0.5 mg to about 1.5 mg vitamin B12; about 50 to about 150 mg intrinsic factor; and about 0.5 mg to about 1.5 mg folic acid.
Owner:DRAGTEK CORP
Hormone replacement formulation
The formulation comprises a combination of three estrogens and selected amount of other elements. The three estrogens include 2-hydroxyestrone, 17-beta estradiol, and estriol. The amount of 17-beta estradiol is substantially less than the amounts of 2-hydroxyestrone and estriol, both which are approximately equal in amount. The amounts of pyridoxine, folic acid, selenium and cobalt are therapeutically effective amounts.
Owner:WRIGHT JONATHAN V
Carbonated fortified milk-based beverage and method for suppressing bacterial growth in the beverage
InactiveUS6866877B2Increase attractivenessFast absorptionMilk preparationVitamin food ingredientsAdditive ingredientPasteurization
Dairy or non-diary based fortified carbonated beverage solutions that supply essential nutrients in the human diet. The solution contains per 354 ml, calcium, magnesium and potassium ions in the form of salts and optionally vitamins A, D, C, lutein, zeaxanthin and folic acid in specified amounts to provide dietary supplementation. Sweeteners, stabilizers, flavors and carbonation can also be added to enhance flavor, taste, mouth-feel, ingredient solubilization and product appearance. A method of making the beverages is also described. A method of using carbonation to reduce bacterial counts and reduce degradation of essential nutrients in milk-based beverages with or without pasteurization is also disclosed.
Owner:MAC FARMS
Ultra-high fiber supplement and method of reducing weight, cardiovascular risks and ingested toxins
An improved ultra-high fiber supplement that promotes satiety, caloric reduction, and weight loss. The supplement comprises guar, oat, psyllium, locust bean gum, pectin, green tea, multi-anthocyanadins, pyridoxine, and folic acid. The supplement can exist as a liquid, semi-solid, or solid comestible. It improves cardiovascular health and reduces cardiovascular inflammation and the risk of heart disease. The addition of antioxidants, including green tea, improves weight loss, and general and cardiovascular health. Also it reduces serum lipoprotein oxidation and risk of free-radical related diseases. Consumption of the supplement aids in reducing absorption and assimilation of ingested toxins. A method of providing an ultra-high fiber comestible that is highly palatable and can be used to supplement nutrition and to manage and prevent diet-related diseases is disclosed. Further embodiments increase fiber and other nutrients in the diet and helps manage and prevent all diet-related diseases.
Owner:SMALL GIANT
Carbonated fortified milk-based beverage and method for suppressing bacterial growth in the beverage
InactiveUS20030113408A1Great tasteIncrease bodyMilk preparationVitamin food ingredientsAdditive ingredientPasteurization
Dairy or non-diary based fortified carbonated beverage solutions that supply essential nutrients in the human diet. The solution contains per 354 ml, calcium, magnesium and potassium ions in the form of salts and optionally vitamins A, D, C, lutein, zeaxanthin and folic acid in specified amounts to provide dietary supplementation. Sweeteners, stabilizers, flavors and carbonation can also be added to enhance flavor, taste, mouth-feel, ingredient solubilization and product appearance. A method of making the beverages is also described. A method of using carbonation to reduce bacterial counts and reduce degradation of essential nutrients in milk-based beverages with or without pasteurization is also disclosed.
Owner:MAC FARMS
Method for protecting humans against superficial vasodilator flush syndrome,
InactiveUS20090148543A1Significant timeImprove the level ofAntibacterial agentsBiocideSulfate proteoglycanS-Adenosyl-l-methionine
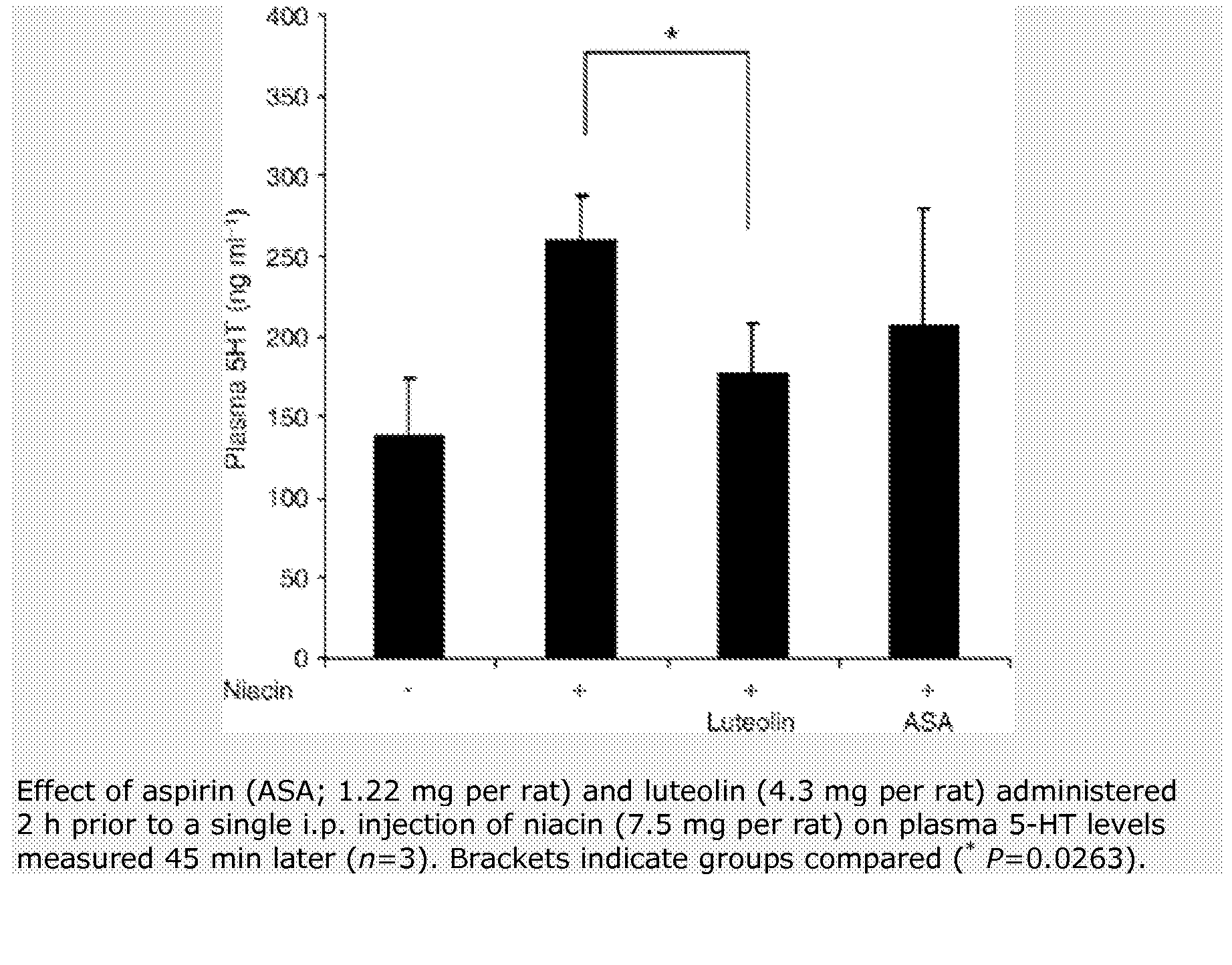
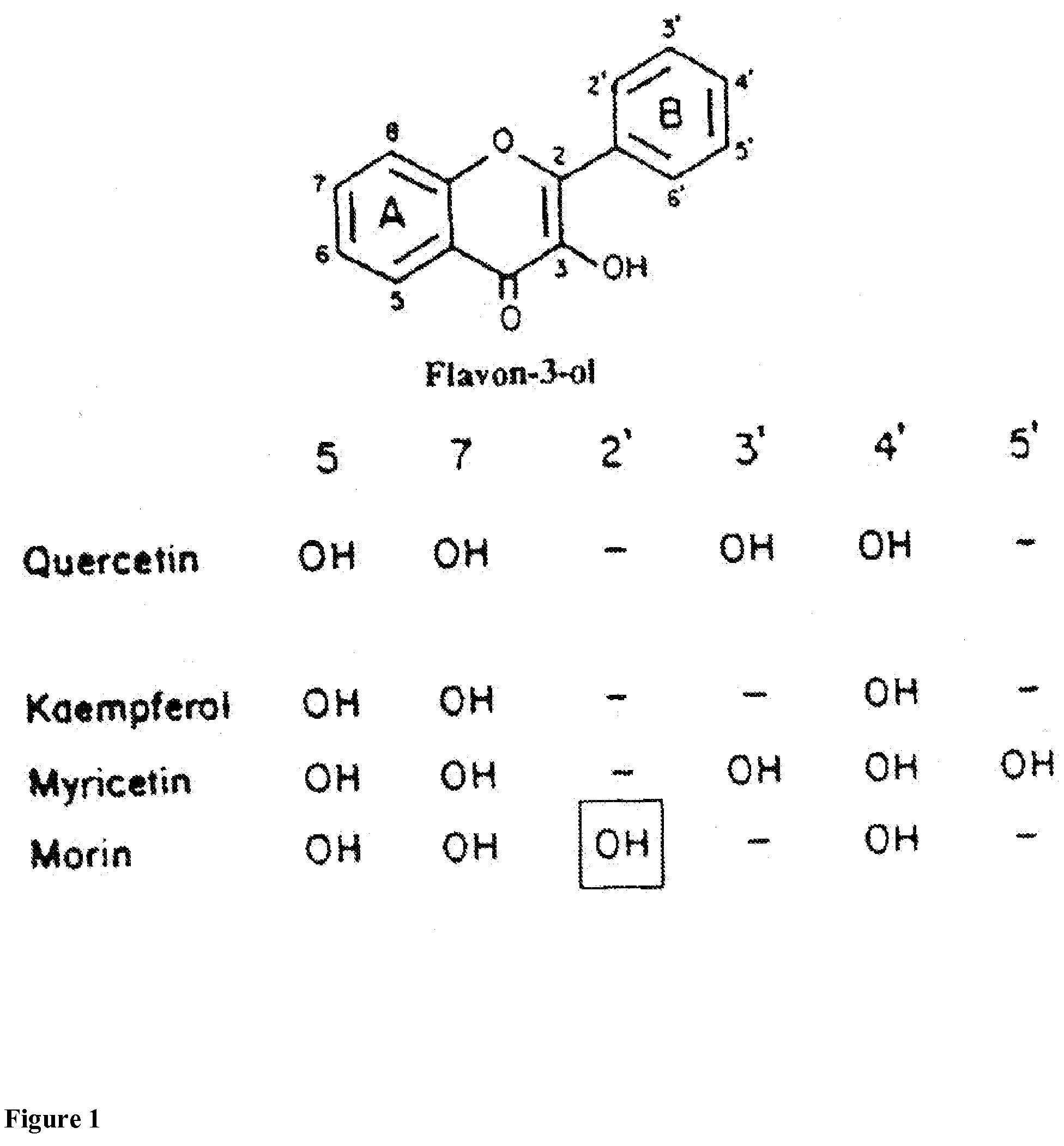
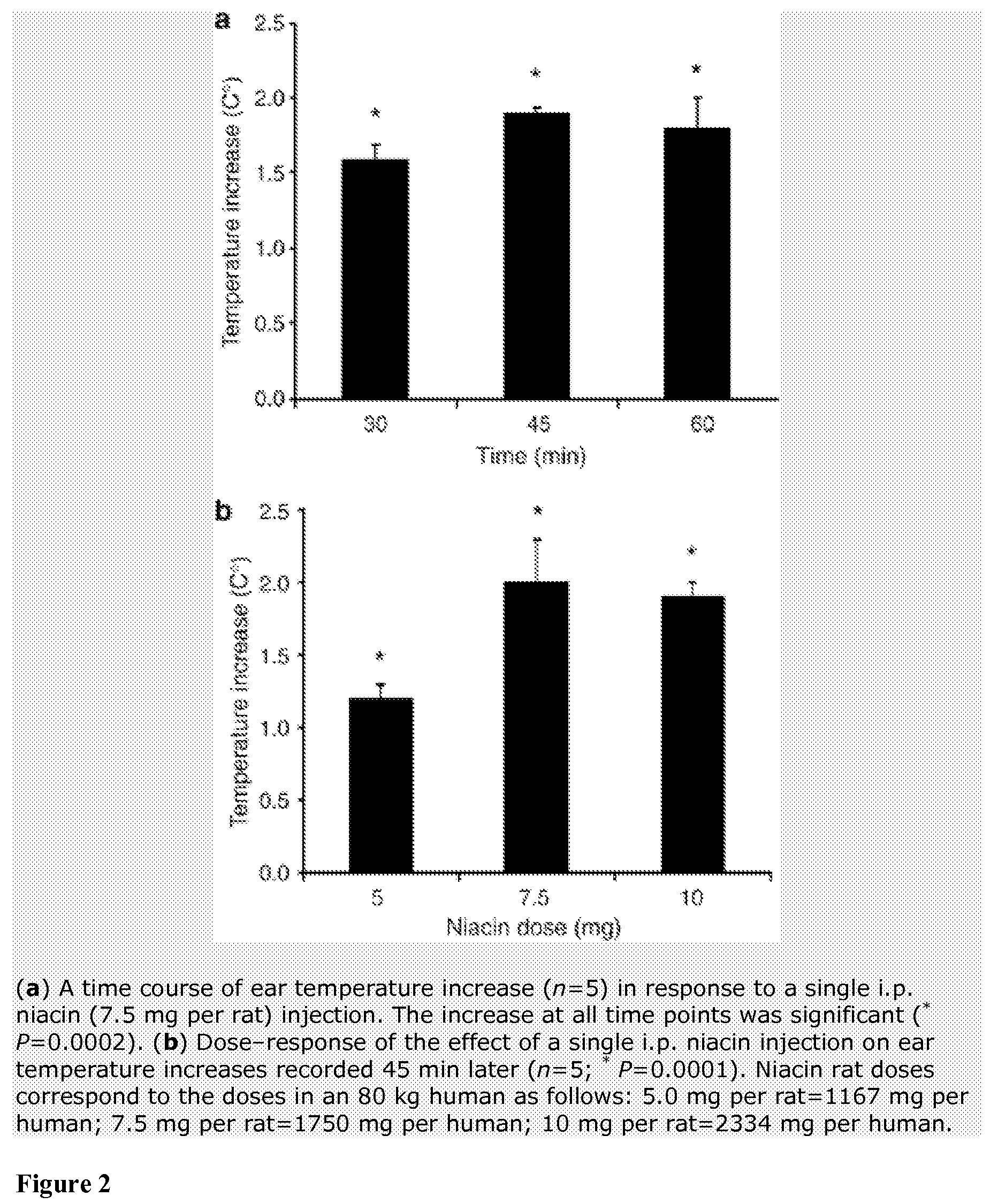
Methods for protection of a human from SVFS comprise the administration of a flavonoid compound of the basic structures 2-phenyl-4H-1-benzopyran or 2-phenyl-4-keto-1-benzopyran or glycosides thereof, alone or, optionally, together with one or more of an olive kernel extract, a non-bovine sulfated proteoglycan, bitter willow extract, a D-hexosamine sulfate, S-adenosylmethionine, folic acid, vitamin B12 and a serotonin inhibitor. Such treatment prevents, reduces or eliminates SVFS in patients receiving as much as 300-3000 mg / day of niacin therapeutically, whether administered prior to, or along with, an anti-SVFS composition.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
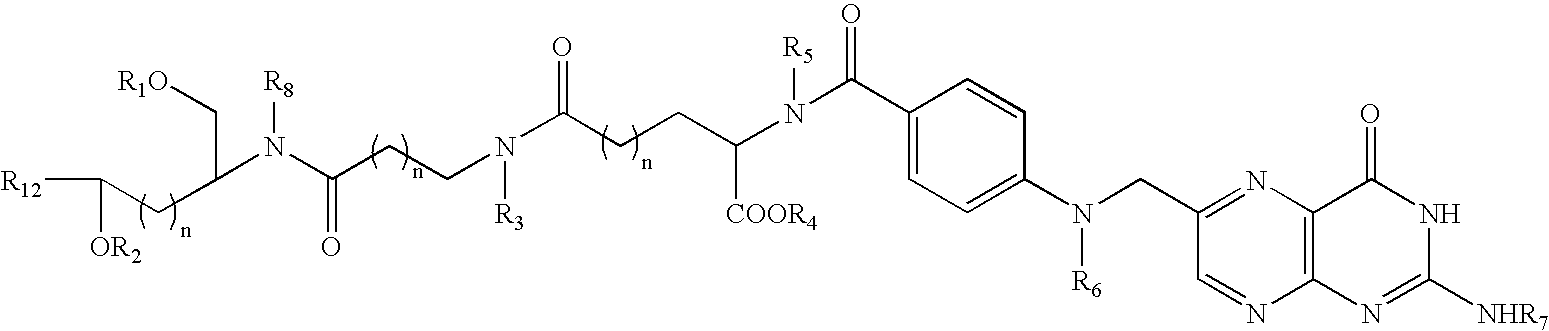
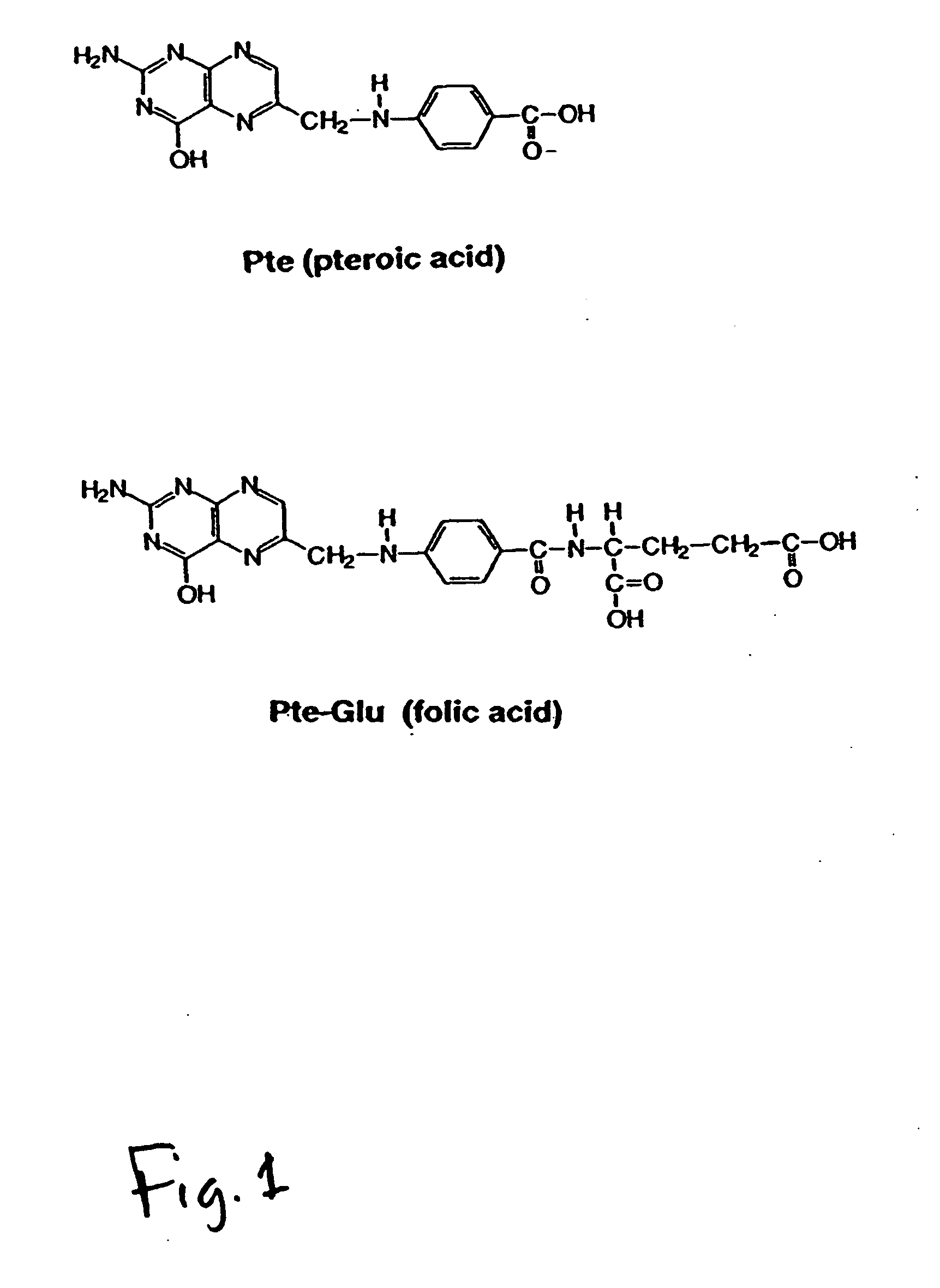
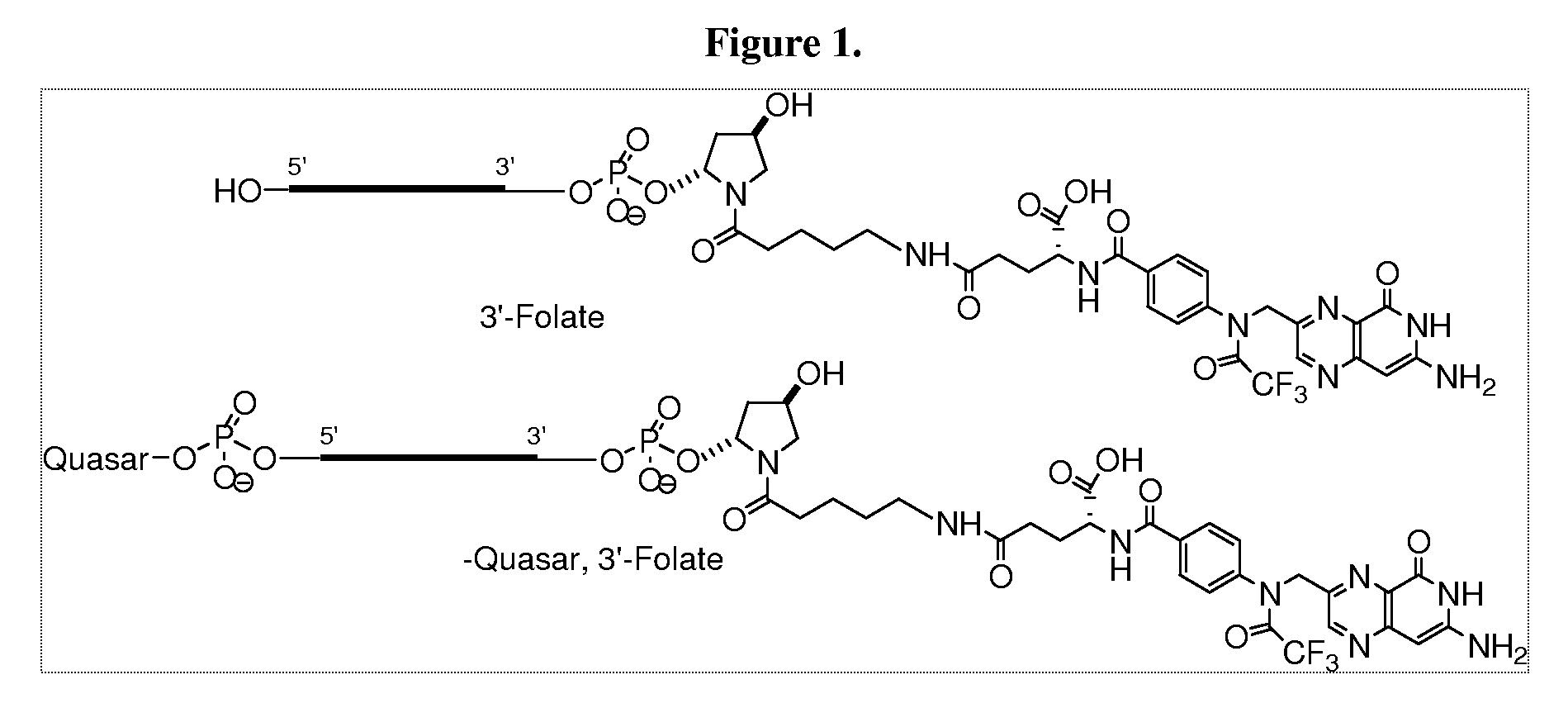
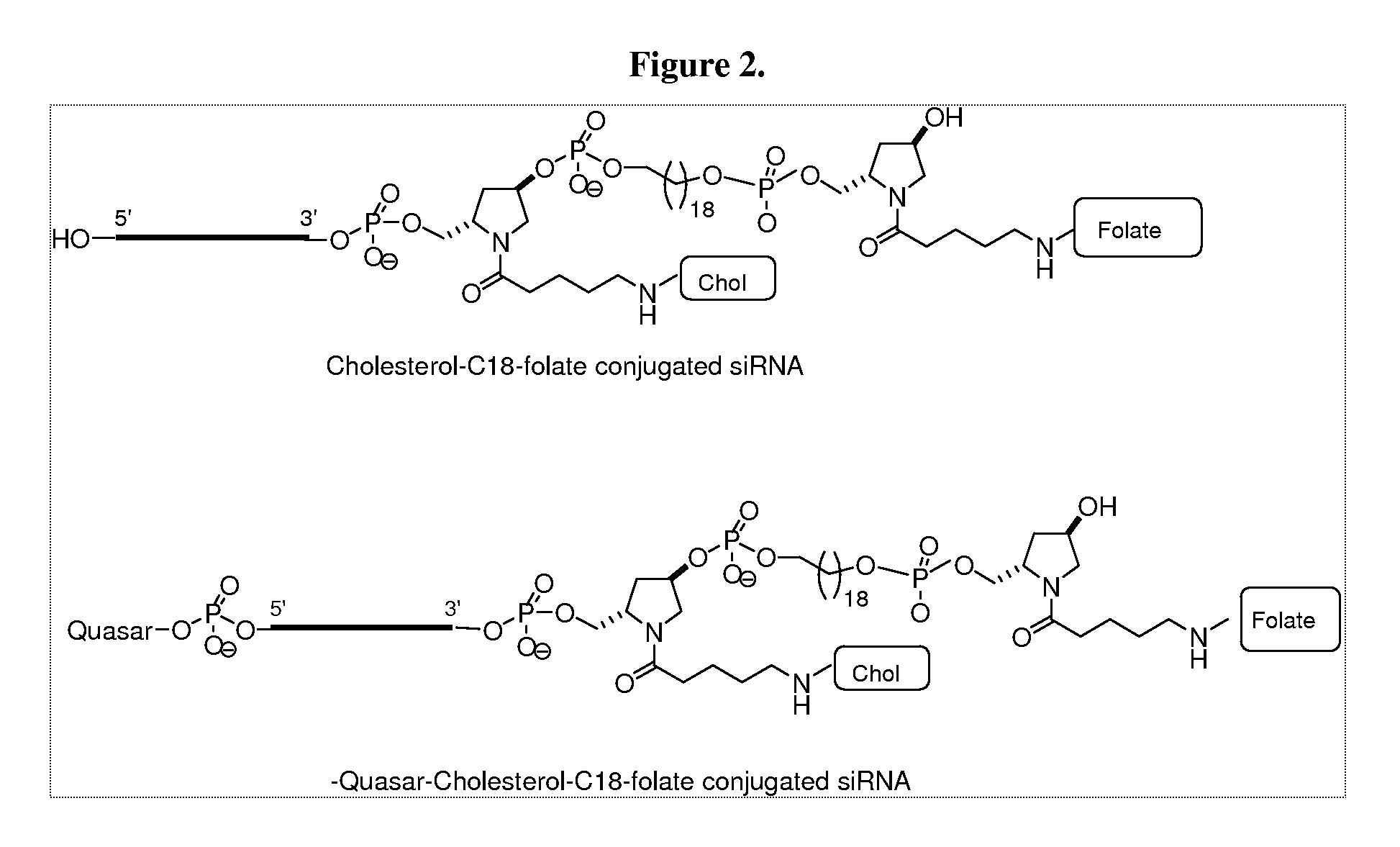
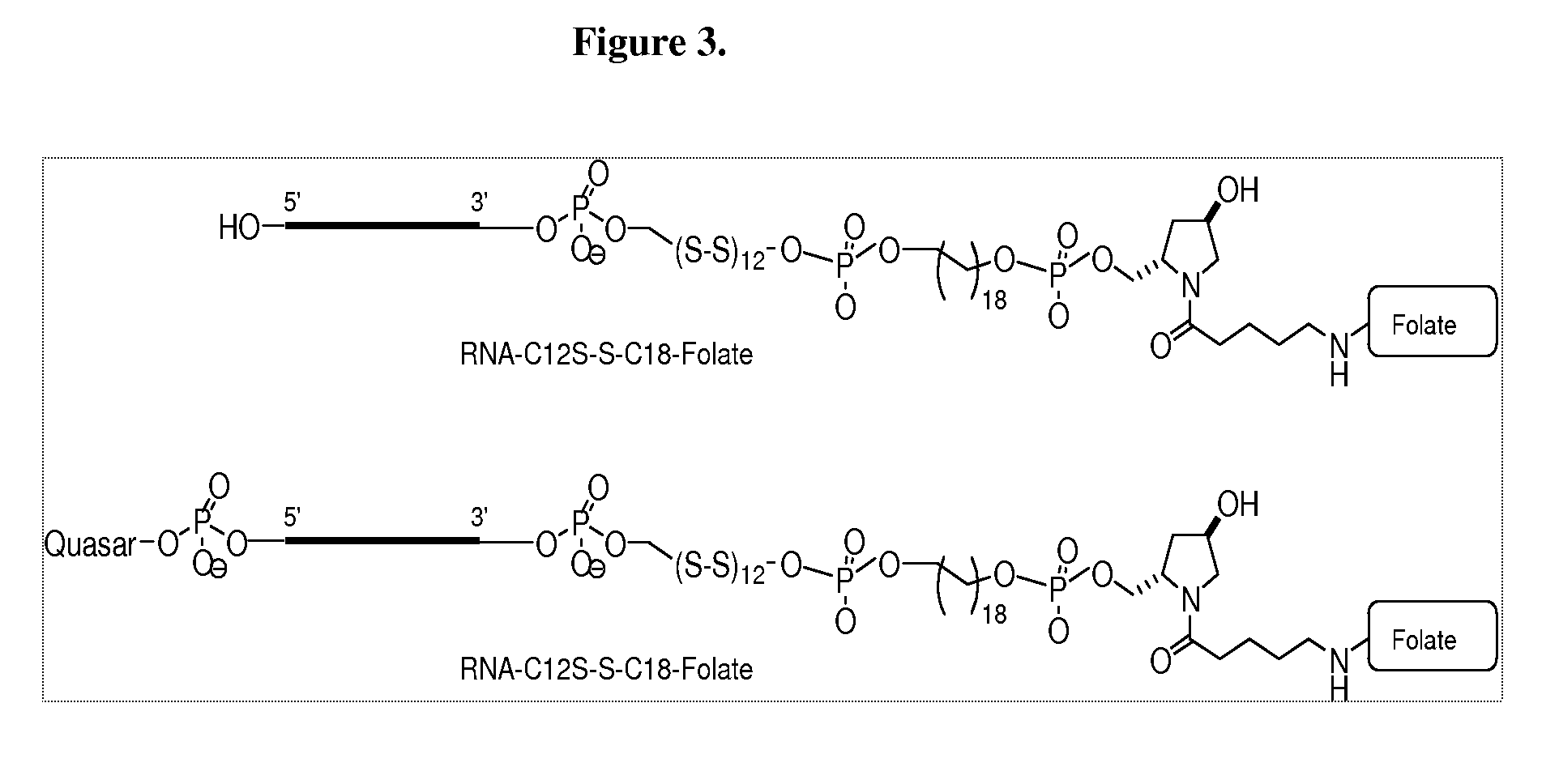
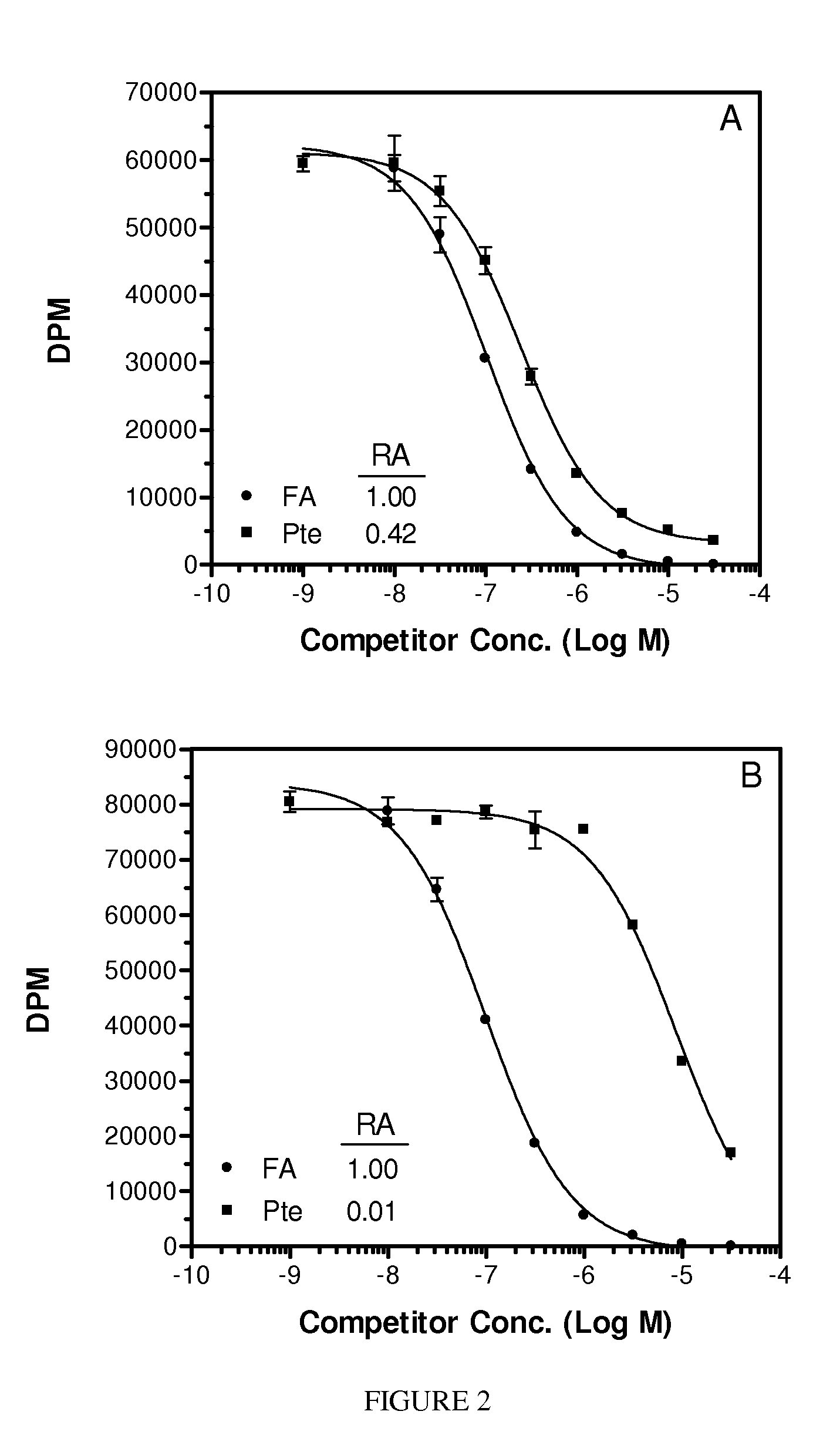
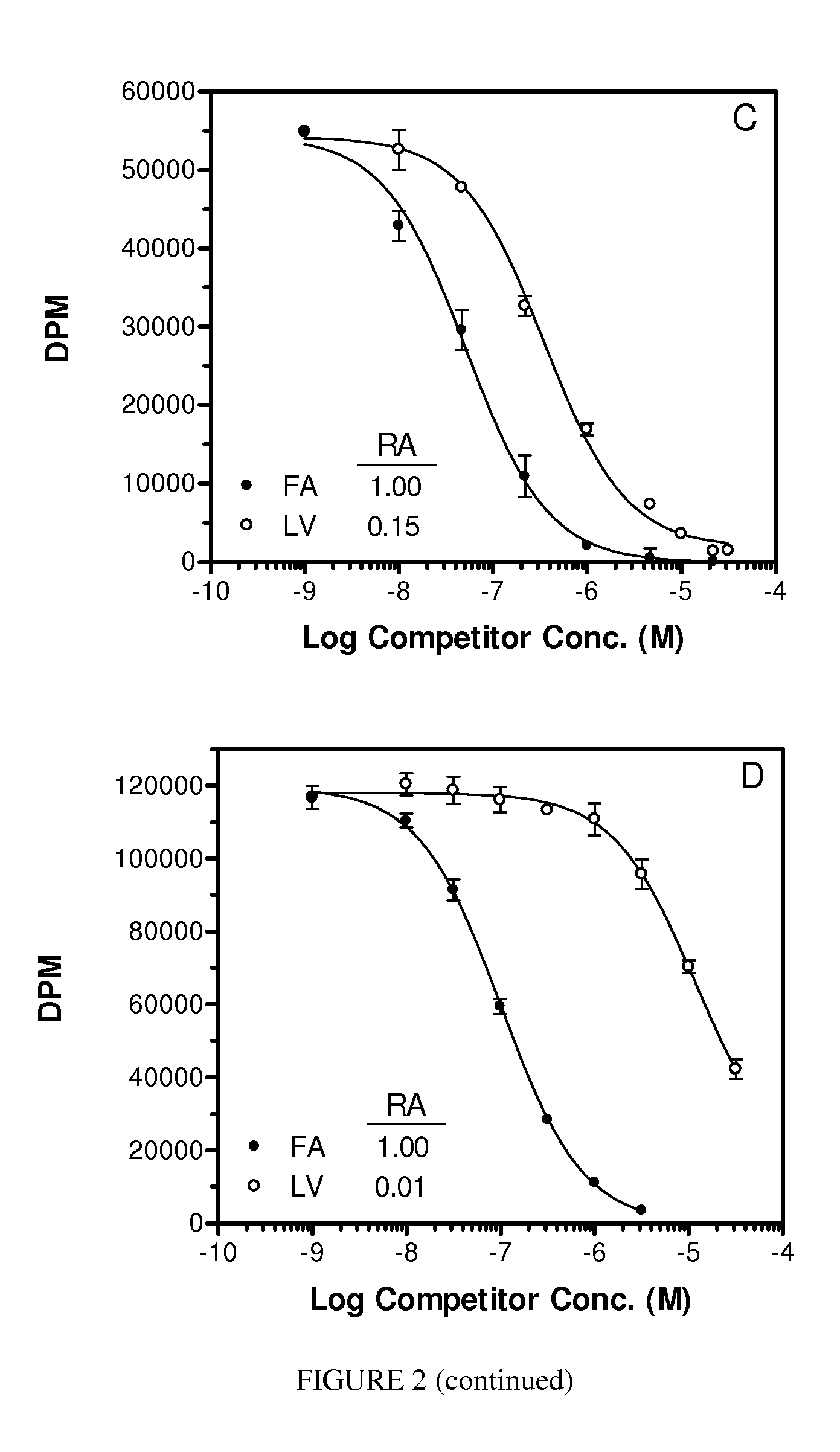
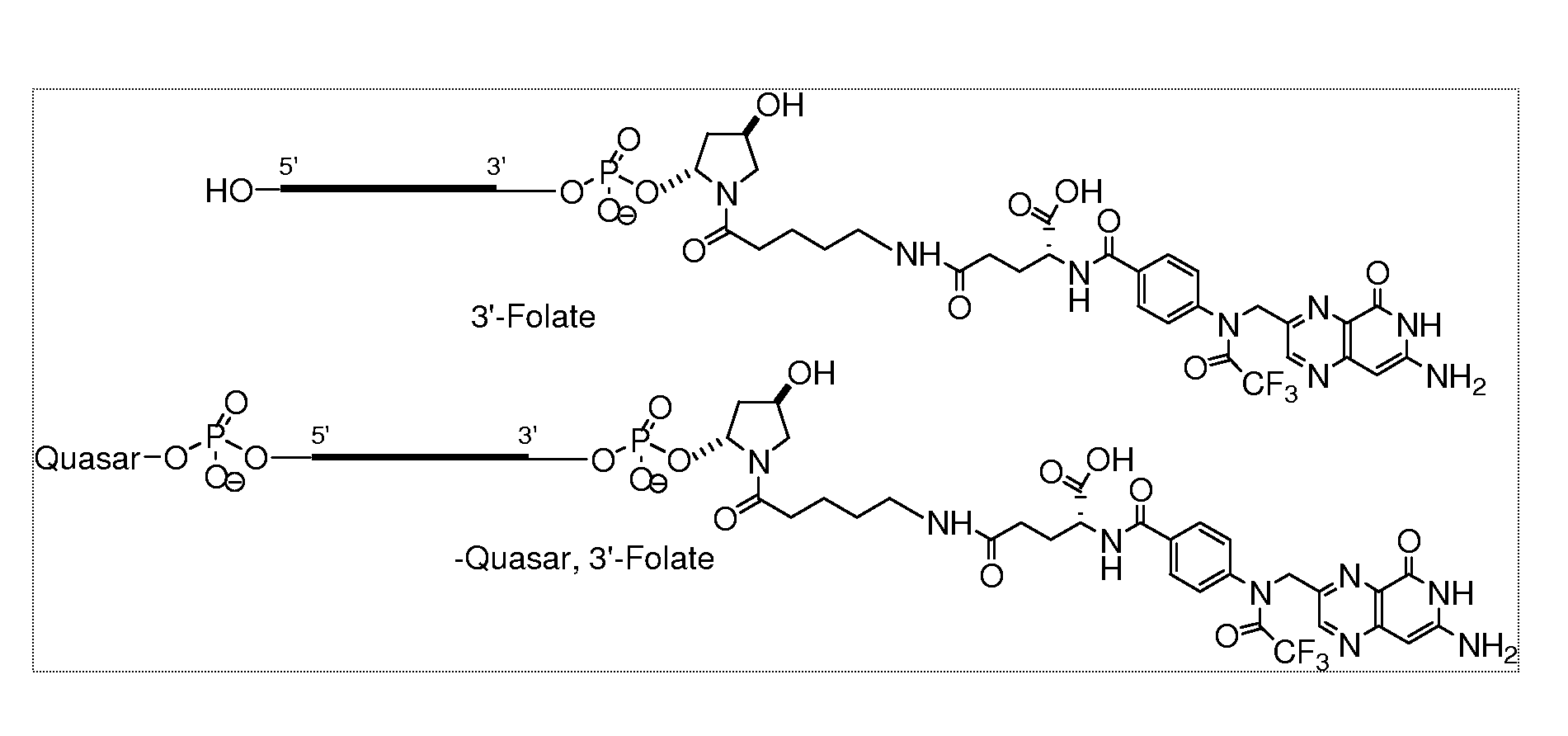
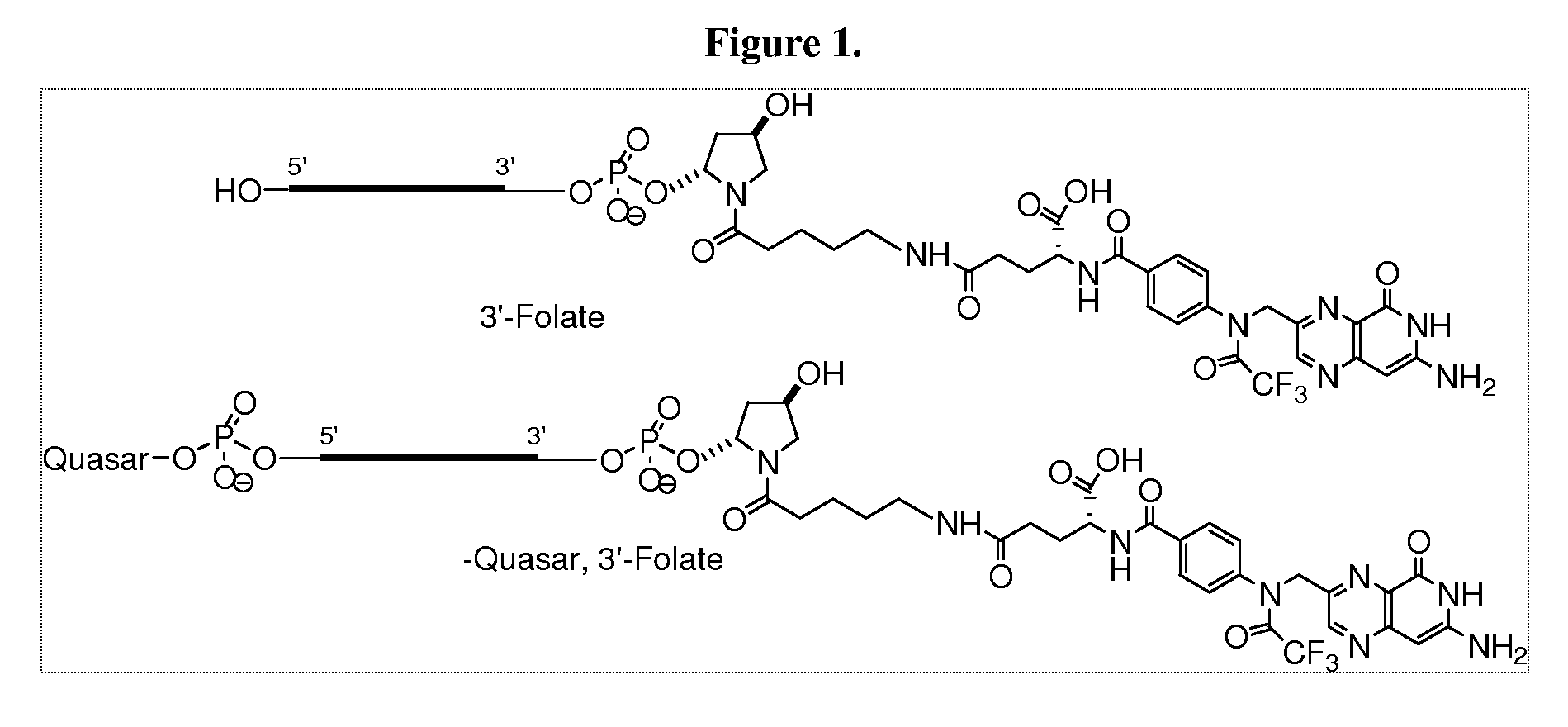
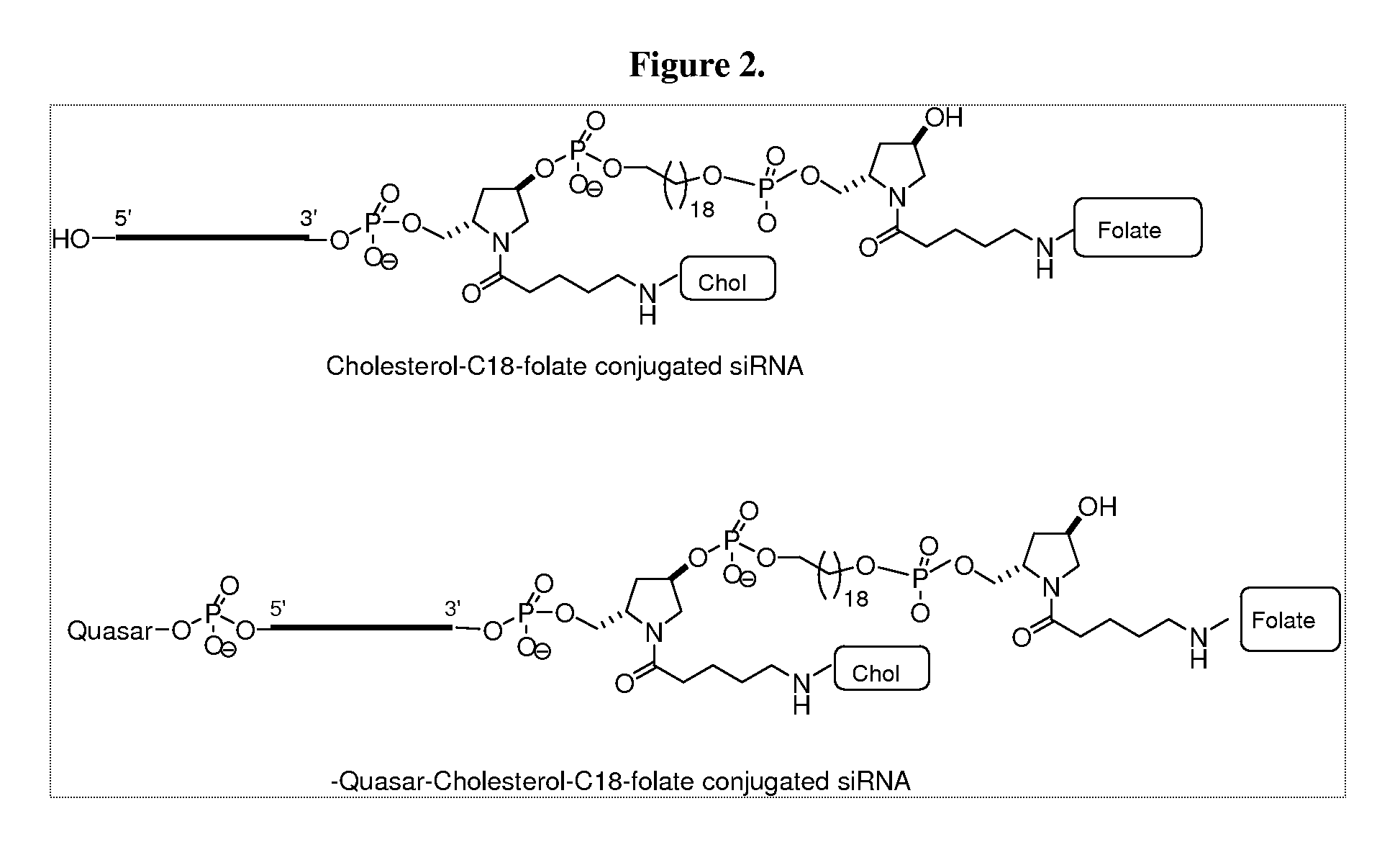
Folate Conjugates
ActiveUS20090247614A1Organic active ingredientsSugar derivativesLipid formationCombinatorial chemistry
The present invention provides iRNA agent including at least one monomer having the structure shown in formula (I′)wherein:A and B are each independently for each occurrence O, N(RN) or S;X is H, a protecting group, a phosphate group, a phosphodiester group, an activated phosphate group, an activated phosphite group, a phosphoramidite, a solid support, —P(Z′)(Z″)O-nucleoside, —P(Z′)(Z″)O-oligonucleotide, a lipid, a PEG, a steroid, a polymer, —P(Z′)(Z″)O-L6-Q′-L7-OP(Z′″)(Z″″)O-oligonucleotide, a nucleotide, or an oligonucleotide;Y is H, a protecting group, a phosphate group, a phosphodiester group, an activated phosphate group, an activated phosphite group, a phosphoramidite, a solid support, —P(Z′)(Z″)O-nucleoside, —P(Z′)(Z″)O-oligonucleotide, a lipid, a PEG, a steroid, a lipophile, a polymer, —P(Z′)(Z″)O-L6-Q′-L7-OP(Z′″)(Z″″)O-oligonucleotide, a nucleotide, or an oligonucleotide;R is folate, a folate analog a folate mimic or a folate receptor binding ligand;L6 and L1 are each independently for each occurrence —(CH2)n—, —C(R′)(R″)(CH2)n—, —(CH2)nC(R′)(R″)—, —(CH2CH2O)mCH2CH2—, or —(CH2CH2O)mCH2CH2NH—;Q′ is NH, O, S, CH2, C(O)O, C(O)NH, —NH—CH(Ra)—C(O)—, —C(O)—CH(Ra)—NH—, CO,where Ra is H or amino acid side; chain.R′ and R″ are each independently H, CH3, OH, SH, NH2, NH(Alkyl=Me, Et, Pr, isoPr, Bu, Bn) or N(diAlkyl=Me2, Et2, Bn2);Z′, Z″, Z′″ and Z″″ are independently O or S;n represent independently for each occurrence 1-20; andm represent independently for each occurrence 0-50.
Owner:ALNYLAM PHARMA INC
Methods for the prevention or amelioration of neuropsychiatric and related diseases
InactiveUS20050249823A1Overcome deficienciesPromote mental healthBiocideAnimal repellantsPsychogenic diseaseEssential fatty acid
The present invention pertains to compositions and methods for therapeutically and / or prophylactically treating patients with neurological, neurogenetic, or psychiatric diseases, disorders, conditions, or distress. Specifically, the present invention relates to the administration of compositions containing vitamins B6 and E, magnesium oxide, essential fatty acids, and folate.
Owner:MURPHY TANYA KAYE +2
Methods and materials for identifying polymorphic variants, diagnosing susceptibilities, and treating disease
InactiveUS20080213775A1Increased susceptibilitySugar derivativesMicrobiological testing/measurementDiseaseCarbon metabolism
The invention is directed to materials and methods associated with polymorphic variants in two enzymes involved in folate-dependent and one-carbon metabolic pathways: MTHFD1 (5,10-methylenetetrahydrofolate dehydrogenase, 5,10-methenyltetrahydrofolate cyclohydrolase, 10-formyltetrahydrofolate synthetase) and methylenetetrahydrofolate dehydrogenase (NADP+dependent) 1-like (MTHFD1L). Diagnostic and therapeutic methods are provided involving the correlation of polymorphic variants in MTBFD1, MTHFD1, and other genes with relative susceptibility for various pregnancy-related and other complications.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC +2
Folate receptor binding conjugates of antifolates
Conjugates of antifolates, releasable linkers, and drugs, and pharmaceutical compositions containing them are described. The conjugates are useful for treating diseases arising from pathogenic cell populations. Methods for treating such diseases are also described.
Owner:PURDUE RES FOUND INC +1
Technique for producing Jinhuaqianliang tea (flower coil tea)
ActiveCN101352191AQuality improvementUniform qualityPre-extraction tea treatmentCooking & bakingThirst
The technology for producing golden flower Qianliang tea (Hua-juan tea) is characterized in that the technology comprises the process steps as follow: plucking criteria-water removing in high temperature-rolling and shaping-pile-fermentation-dry and adding incense-stems picking and sieving-matching and pile-classificaition and weighting-steam softening-moisture detection-adpressing and sizing-premilary test and baking-cultivating golden flower-aerationagitation. The technology is an improved deep processing technology. The processes of the pile-fermentation and the cultivating golden flower ensure that the appearance of the products is ooiu colour, the interior is brown, and even distributed beneficial organism (namely, golden flower)-eurotium cristatum is clearly saw, the shangse is bright red, the taste is pure and aromatic, and the flower is aromatic, and has the health care functions of promoting sleeping, invigorating stomach and promoting digestion, relaxing bowel, slaking thirst and helping produce saliva, antidiabetics, lowering blood pressure, curing bloated, cuing laxness, etc. The technology fully actives microelements such as vitamins contained in tea, mineral composition, 18 amino acid, protein, glucide, folic acid, catchol which are beneficial to human health and easily absorbed, and the cultivation of golden flower has substantial transformation on tea polyphenols, caffeine, and theophylline that are transferred into elements that are beneficial to human health, all ages, expand market, have high cultural value, provide collection opportunity for black tea lovers.
Owner:湖南省安化县晋丰厚茶行有限公司
Refrigeration-shelf-stable ultra-pasteurized or pasteurized infant formula
InactiveUS6039985AMaintain qualityReduce degradationSugar food ingredientsVitamin food ingredientsPantothenic acidVitamin B6 synthesis
Refrigeration-shelf-stable ready-to-feed and concentrated infant formulas prepared through an ultra-pasteurization and / or pasteurization process, comprise per five fluid ounces from about 1.8 to about 6.3 grams of protein; from about 3.3 to about 15.9 grams of fat; from about 300 mg to about 3000 mg of linoleic acid; from about 250 to about 900 IU of Vitamin A; from about 40 to about 180 IU of Vitamin D; from about 0.7 to about 9 IU of Vitamin E; from about 4 to about 24 mcg of Vitamin K; from about 40 to about 300 mcg of Thiamine (Vitamin B1); from about 60 to about 450 mcg of Riboflavin (Vitamin B2); from about 35 to about 180 mcg of Vitamin B6; from about 0.15 to about 0.9 mcg of Vitamin B12; from about 250 to about 3150 mcg of Niacin; from about 4 to about 48 mcg of Folic Acid (Folacin); from about 300 to about 1500 mcg of Pantothenic Acid; from about 1.5 to about 13.2 mcg of Biotin; from about 8 to about 36 mg of Vitamin C (Ascorbic Acid); from about 7 to about 48 mg of Choline; from about 4 to about 18 mg of Inositol; from about 60 to about 234 mg of Calcium; from about 30 to about 159 mg of Phosphorus; from about 6 to about 24 mg of Magnesium; from about 0.15 to about 5.4 mg of Iron; from about 0.5 to about 3 mg of Zinc; from about 5 to about 45 mcg of Manganese; from about 60 to about 270 mcg of Copper; from about 5 to about 75 mcg of Iodine; from about 20 to about 81 mg of Sodium; from about 80 to about 324 mg of Potassium; and from about 55 to about 195 mg of Chloride; wherein the total caloric content is from about 80 kilocalories to about 300 kilocalories per five fluid ounces.
Owner:KAMAREI A REZA +1
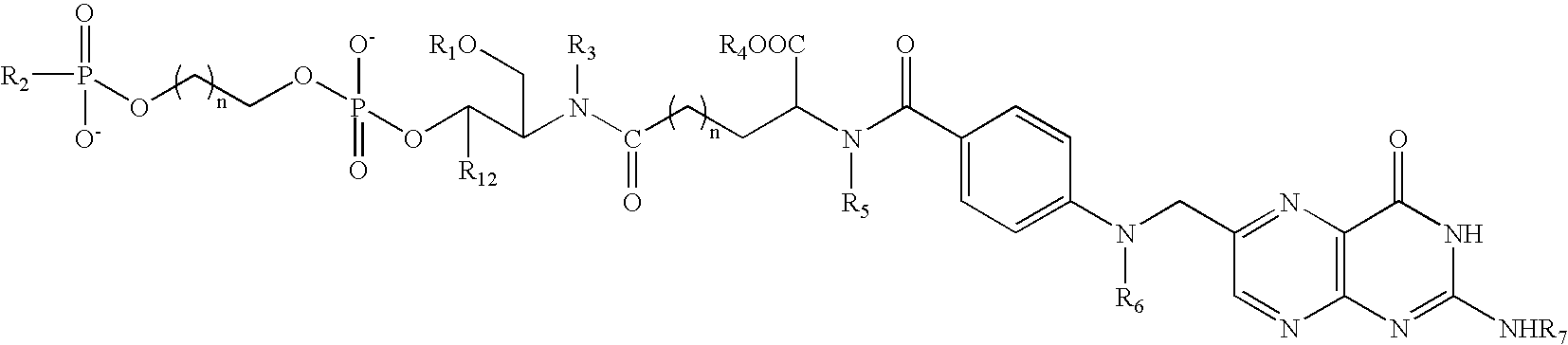
Folate conjugates
Owner:ALNYLAM PHARMA INC
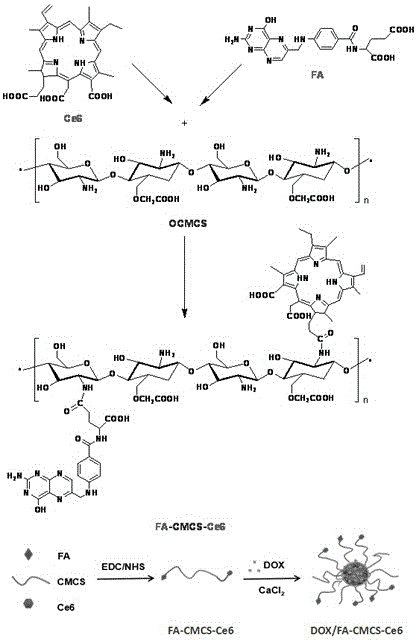
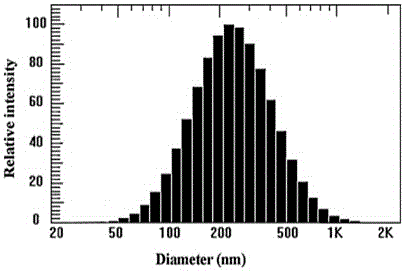
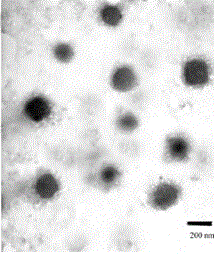
Preparation method and application of tumor-targeted nanometer drug delivery system for cooperative chemotherapy and photodynamic therapy
InactiveCN105749280AGood slow and controlled release abilityGrowth inhibitionOrganic active ingredientsEnergy modified materialsTumor targetIon exchange
The invention discloses a tumor-targeted nanometer drug delivery system for cooperative chemotherapy and photodynamic therapy and a preparation method thereof. The drug delivery system is prepared from carboxymethyl chitosan, folate, a photosensitizer chlorine e6 and adriamycin, wherein the chlorine e6 and the folate are coupled to a carboxymethyl chitosan chain segment through an amido bond, and are loaded to polymer nanoparticles of the adriamycin through an ion exchange method. The nanometer material prepared by the method is high in yield, regular in shape and even in distribution. In-vivo and in-vitro experiments prove that the tumor targeting property of the nanometer preparation can be significantly improved by folate receptor mediation; enrichment on the tumor part is achieved and drug release is controlled. The photosensitizer is capable of effectively reversing the chemotherapy drug resistance and significantly inhibiting the growth of tumors after being irradiated by near-infrared light. Therefore, the related nanometer drug delivery system has good application prospect in the aspect of breast cancer treatment.
Owner:SHENYANG UNIV
Multiple antioxidant micronutrients
A method for administering an antioxidant composition to humans according to their age and sex is disclosed wherein the method comprises administering to said humans a daily dose of a multiple antioxidant micronutrient composition comprising vitamin A (palmitate), beta carotene (from natural d. salina), vitamin C (calcium ascorbate), vitamin D-3 (cholecalciferol), natural source vitamin E including both d-alpha tocopheryl and d-alpha tocopheryl acid succinate, thiamine mononitrate, riboflavin, niacinamide ascorbate, d-calcium pantothenate, pyridoxine hydrochloride, cyanocobalamin, folic acid (folacin), d-biotin, selenium (1-seleno methionine), chromium picolinate, zinc glycinate, calcium citrate, and magnesium citrate. For persons over the age of about 51, the composition preferably further comprises one or more of co-enzyme Q10, N-acetyl cysteine, and alpha lipoic acid. Preferably, also, vitamin D is added for women over the age of about 36.
Owner:NEW AGE HEALTH SCI INC
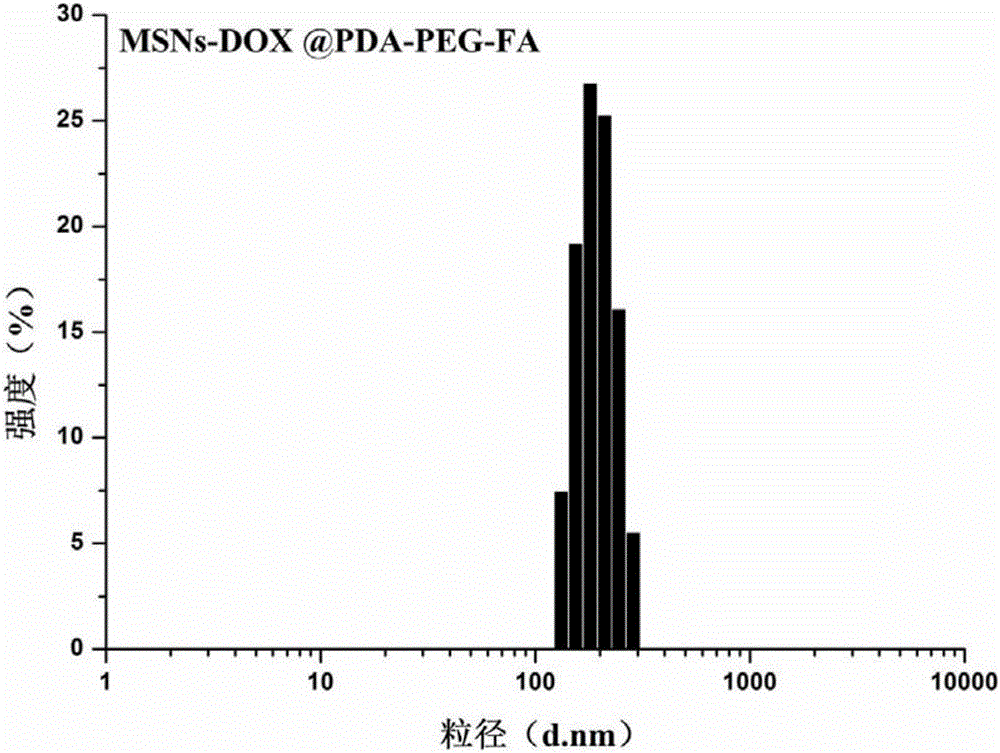
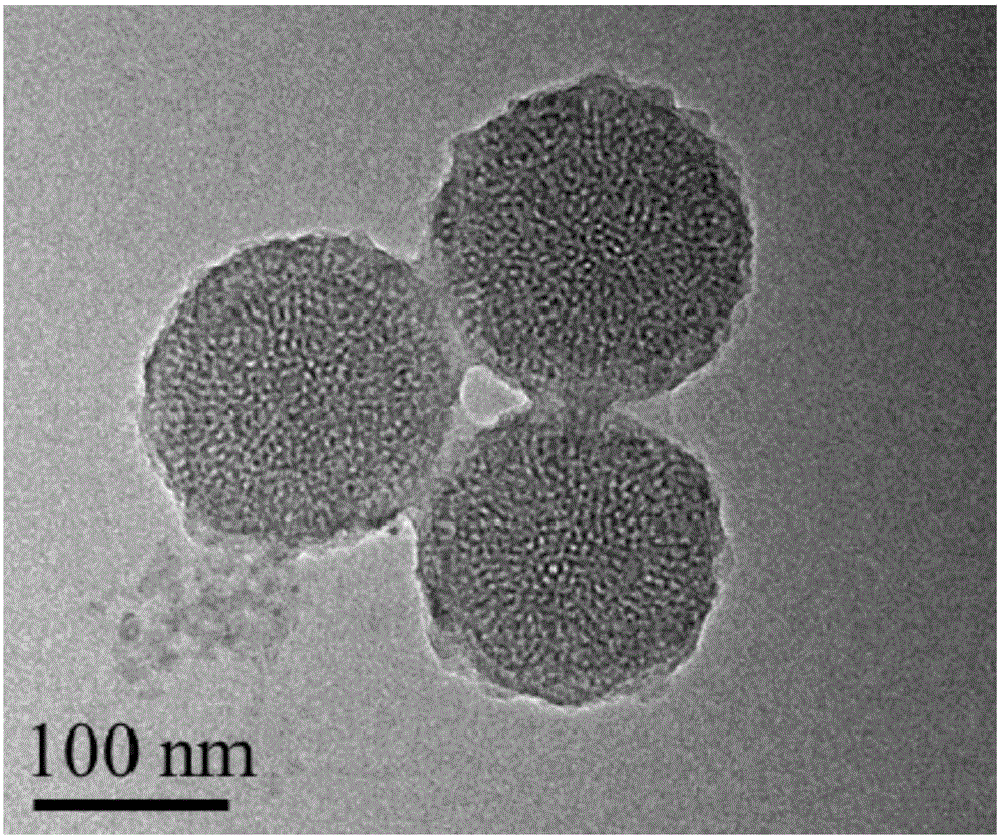
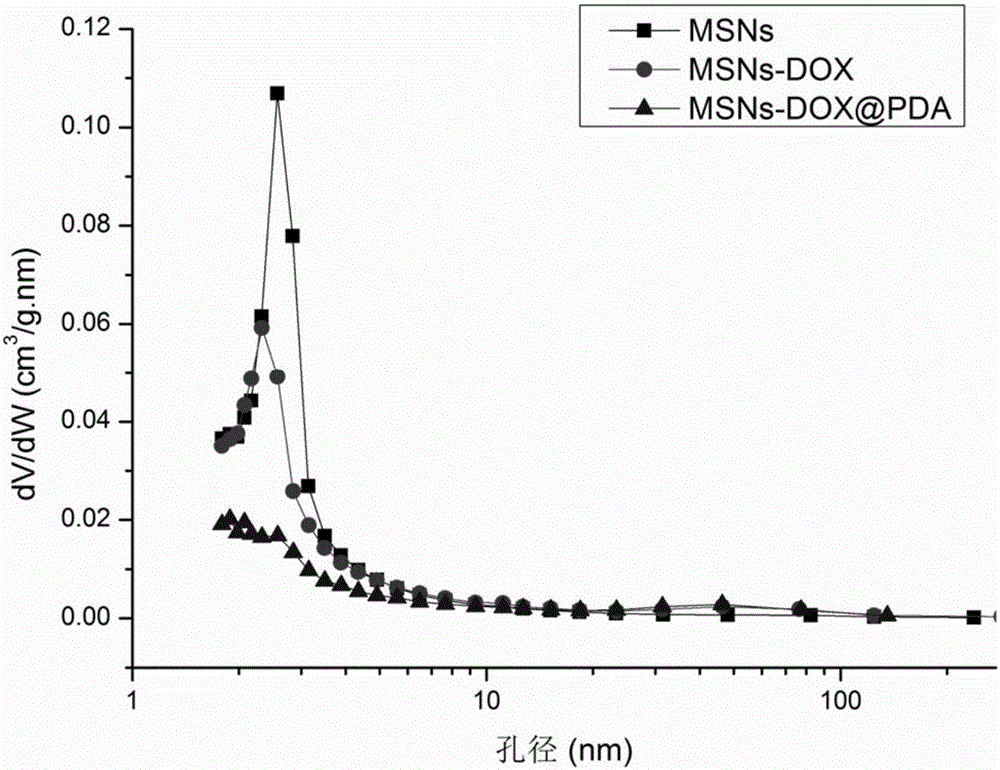
Folic acid and polydopamine modified tumor targeted mesoporous silica nanoparticle and preparation method and application thereof
ActiveCN106806343AEasy to prepareNo pollution in the processPowder deliveryInorganic non-active ingredientsTumor targetPolyethylene glycol
The invention provides a folic acid and polydopamine modified tumor targeted mesoporous silica nanoparticle and a preparation method and application thereof. The preparation method particularly comprises the following steps: (1) dissolving mesoporous silica and a chemical in a solvent, performing a full reaction, and performing separation; (2) adding mesoporous silica initial nano-particles obtained in the step (1) in a solution, adding dopamine hydrochloride, performing a full reaction, and performing separation; and (3) adding the dopamine hydrochloride coated mesoporous silica initial nano-particles loaded with the chemical in a weakly basic water solution, sequentially adding a reducing agent and polyethylene glycol modified sulfydryl grafted targeted ligand folic acid, performing a full reaction, then performing separation to obtain the folic acid and polydopamine modified tumor targeted mesoporous silica nanoparticle. The preparation method of the folic acid and polydopamine modified tumor targeted mesoporous silica nanoparticle is simple, and favorable tumor targeting ability, biocompatibility and biodegradability are achieved.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Nutritional composition for supporting brain development and function of children
InactiveUS20120171178A1Contributes to orImprove abilitiesBiocideNervous disorderBrain developmentBiology
The present invention relates to a nutritional composition, in particular directed to children of 3-6 years, said nutritional composition comprising a protein source, a source of available carbohydrates, a lipid source, at least one probiotic microorganism, and prebiotics, wherein said lipid source comprises DHA (docosahexaenoic acid) and / or ARA (arachidonic acid). The nutritional composition improves cognitive performance, in particular memory, learning comprehension, alertness, attention, concentration, processing speed, conceptual thinking, abstract thinking, verbal abilities, language comprehension, psychomotor skills, curiosity, and confident interaction with the environment. Preferably, the composition comprises one, a combination of several or all selected of the group of DHA, ARA, LA, ALA, choline, iron, iodine and folic acid.
Owner:NESTEC SA
Compounds for cardiovascular treatment comprising multi-vitamin and anti-platelet aggregating agents and methods for making and using the same
Compounds comprising multi-vitamins, zinc and an anti-platelet aggregating agent for the treatment of atherosclerotic cardiovascular disease (ASCVD) are disclosed. The compounds are provided in dosage form, and preferably include selected amounts of ascorbic acid, folic acid, vitamin E, vitamin B6 and vitamin B12. The anti-platelet aggregating agent preferably comprises aspirin. A protective coating is preferably provided between the aspirin and the other vitamin and mineral constituents. The dosages are effective in the treatment of ASCVD, and possess extended shelf lives.
Owner:HEIBEL RICHARD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com