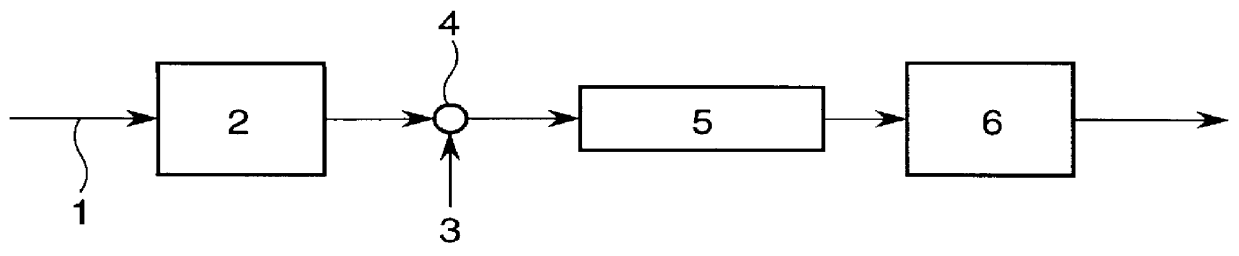
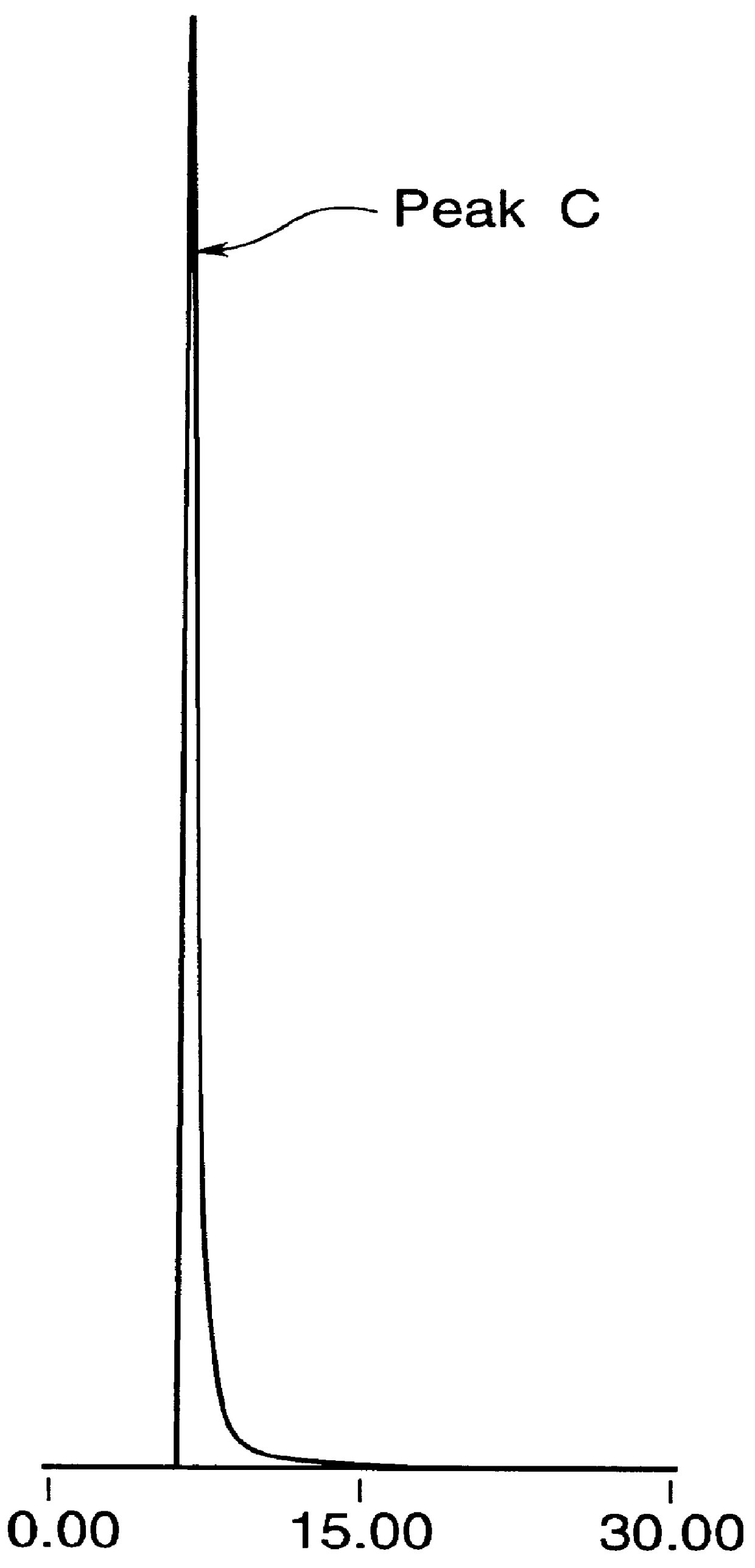
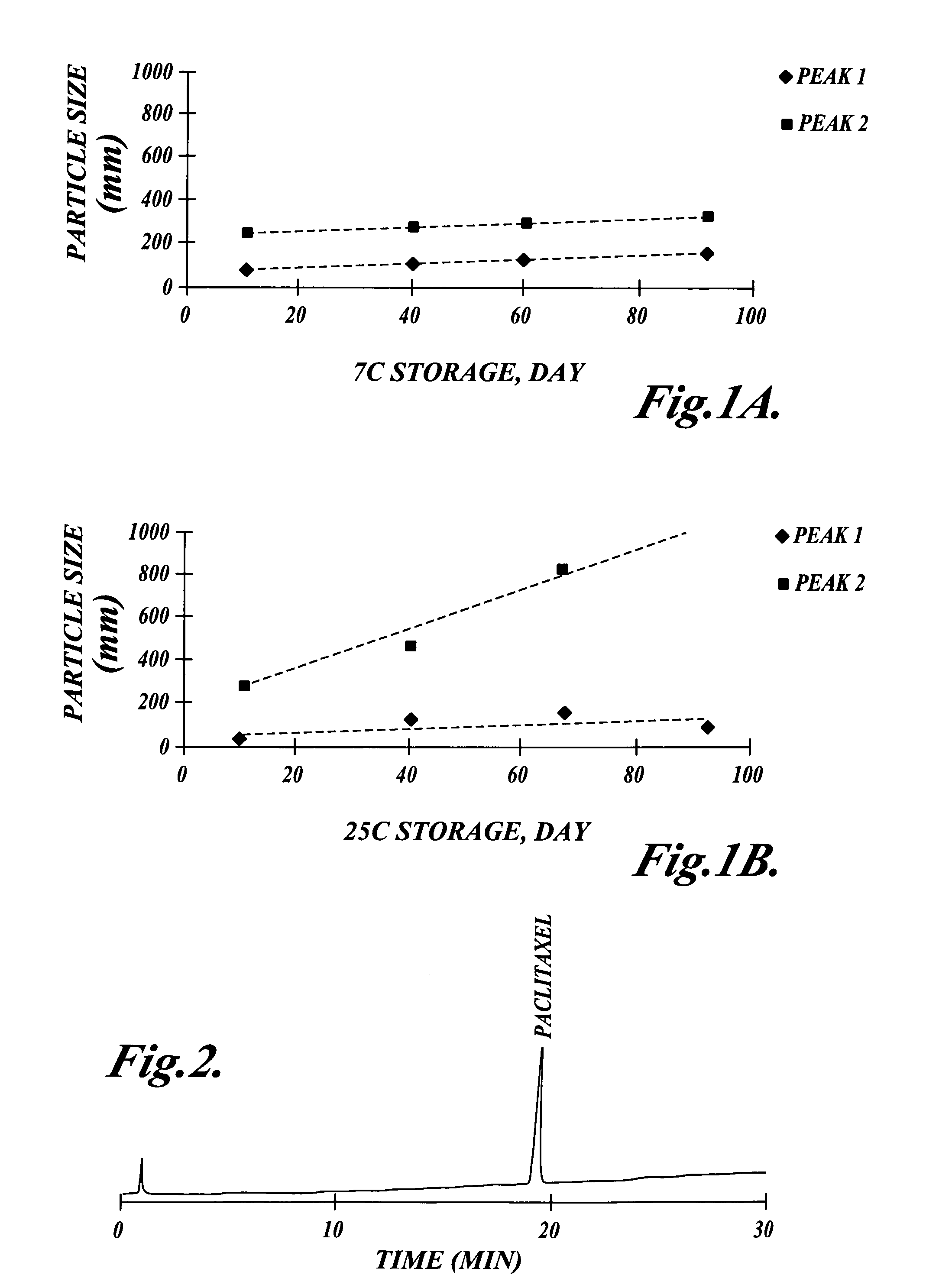
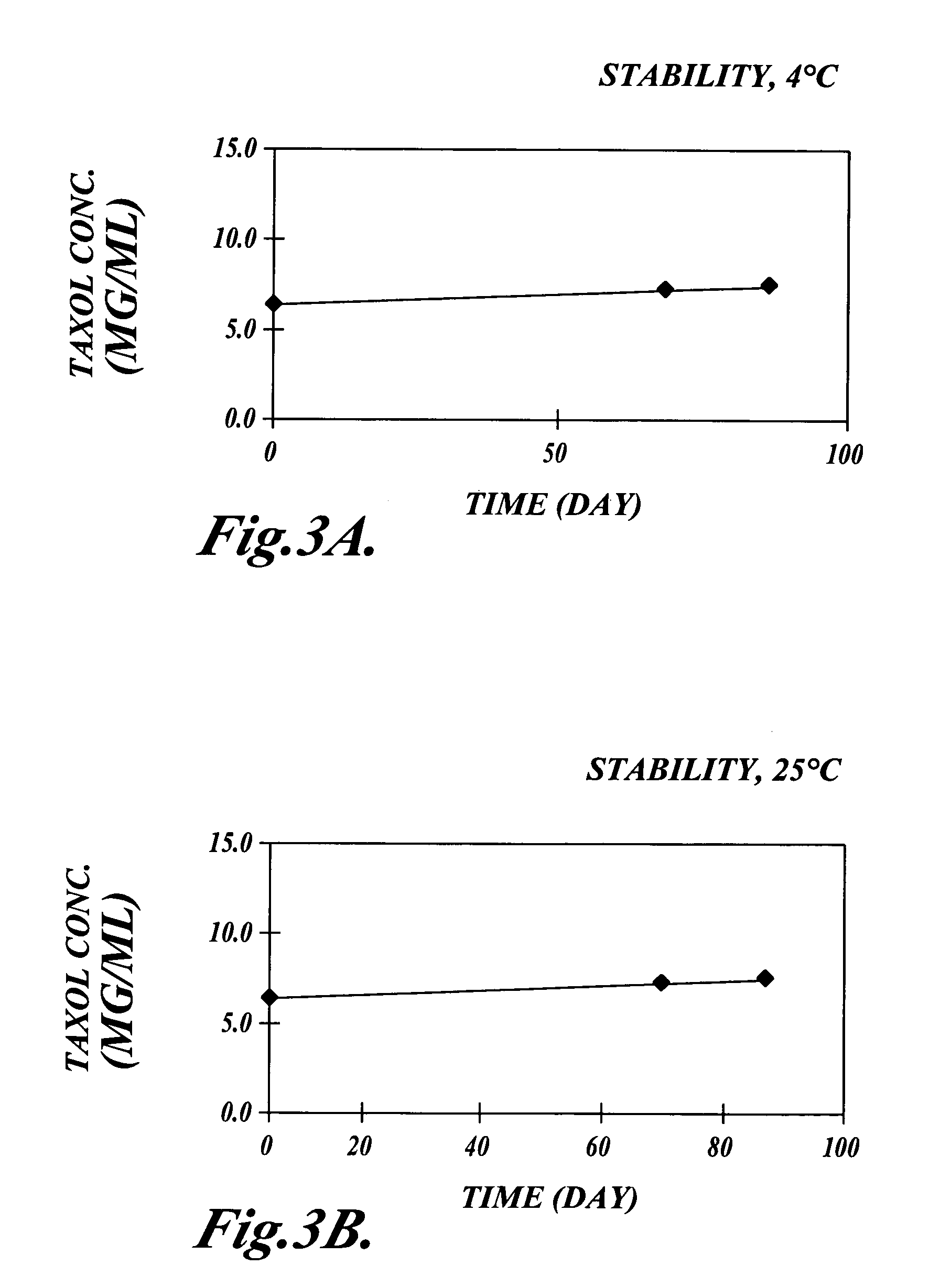
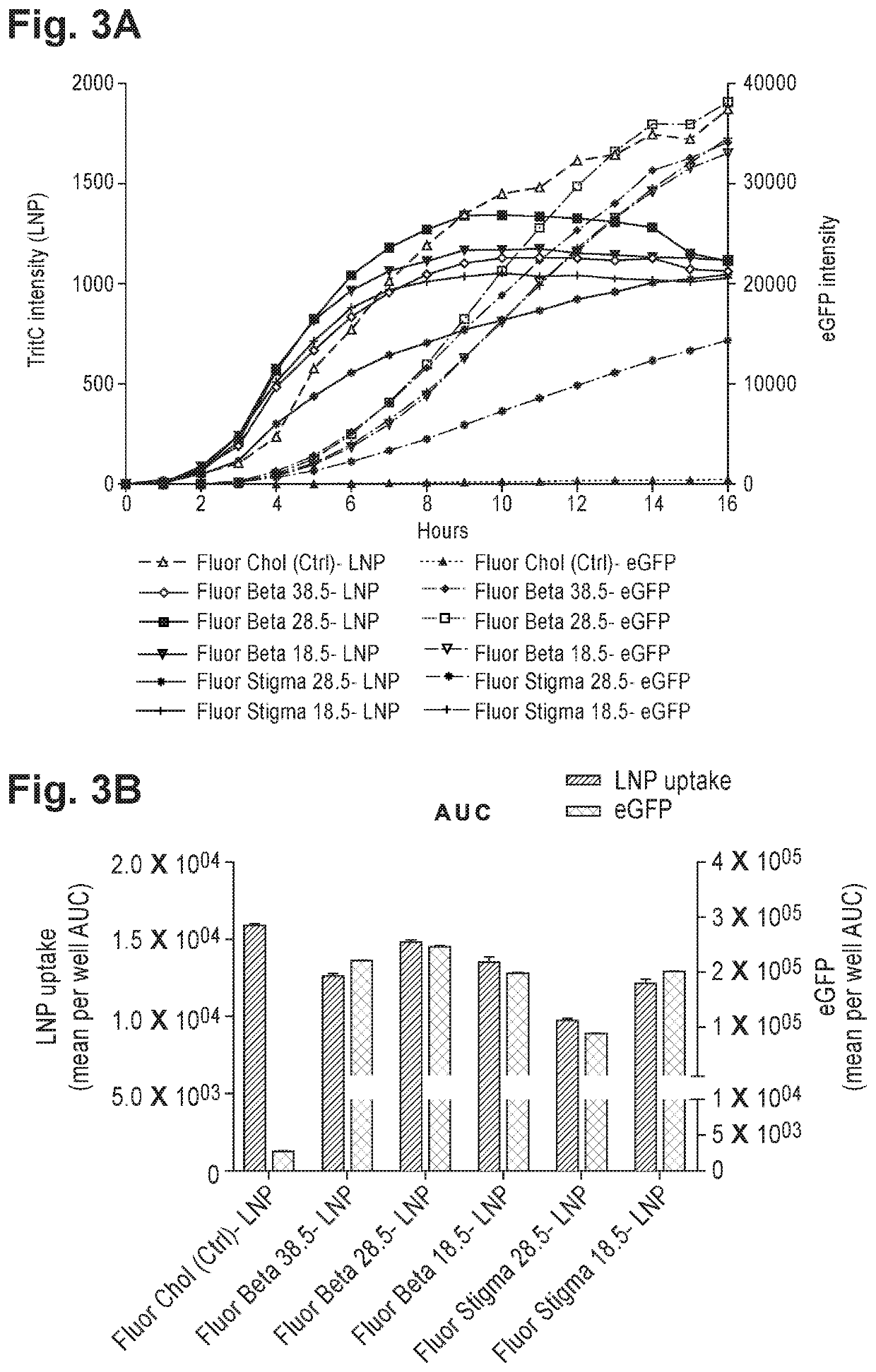
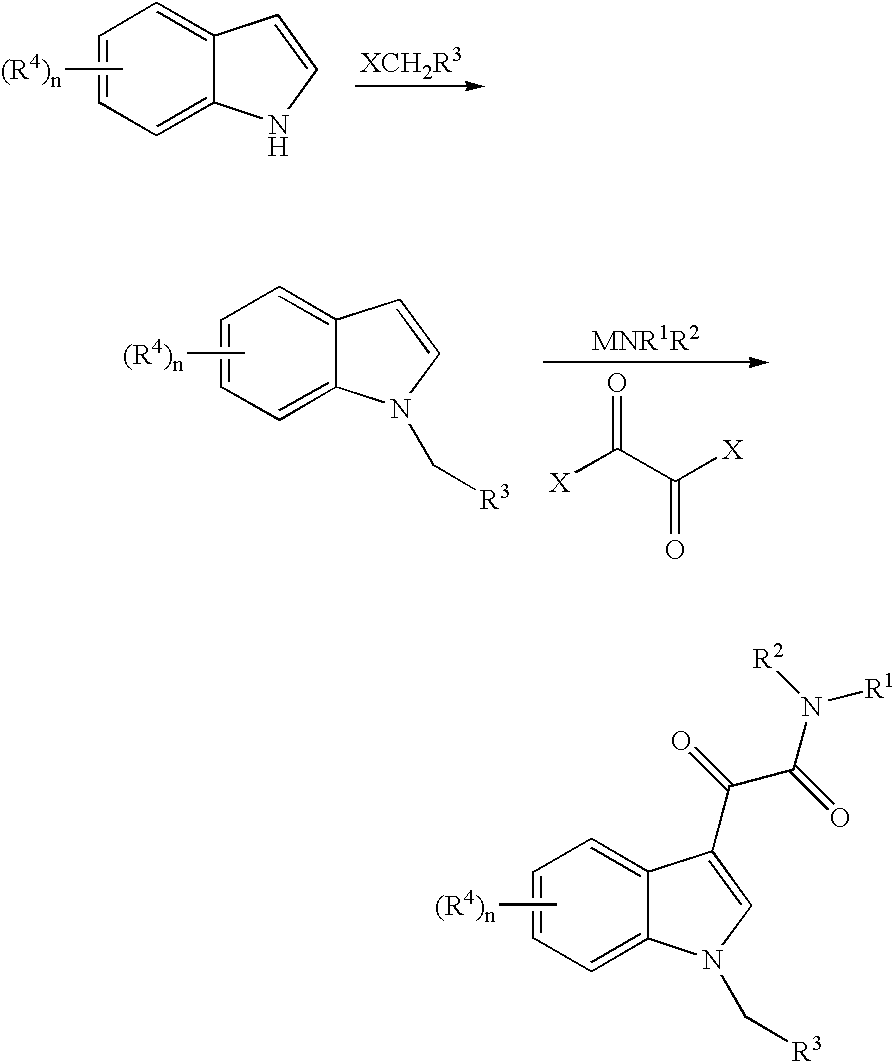
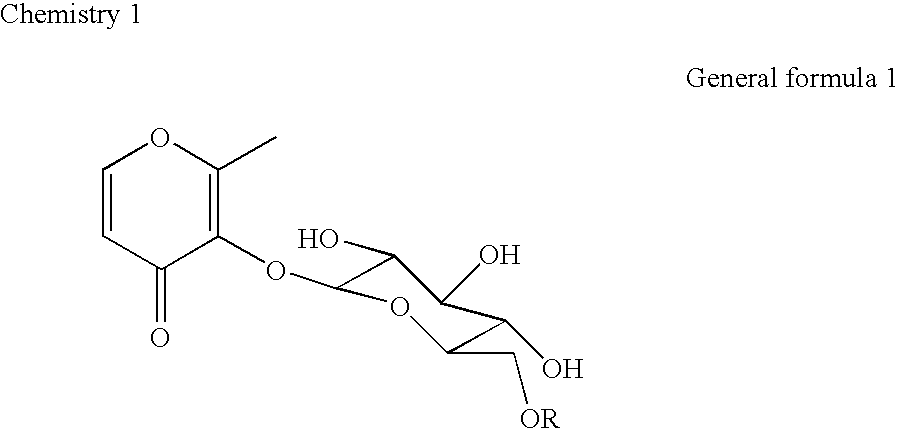
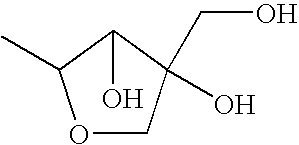
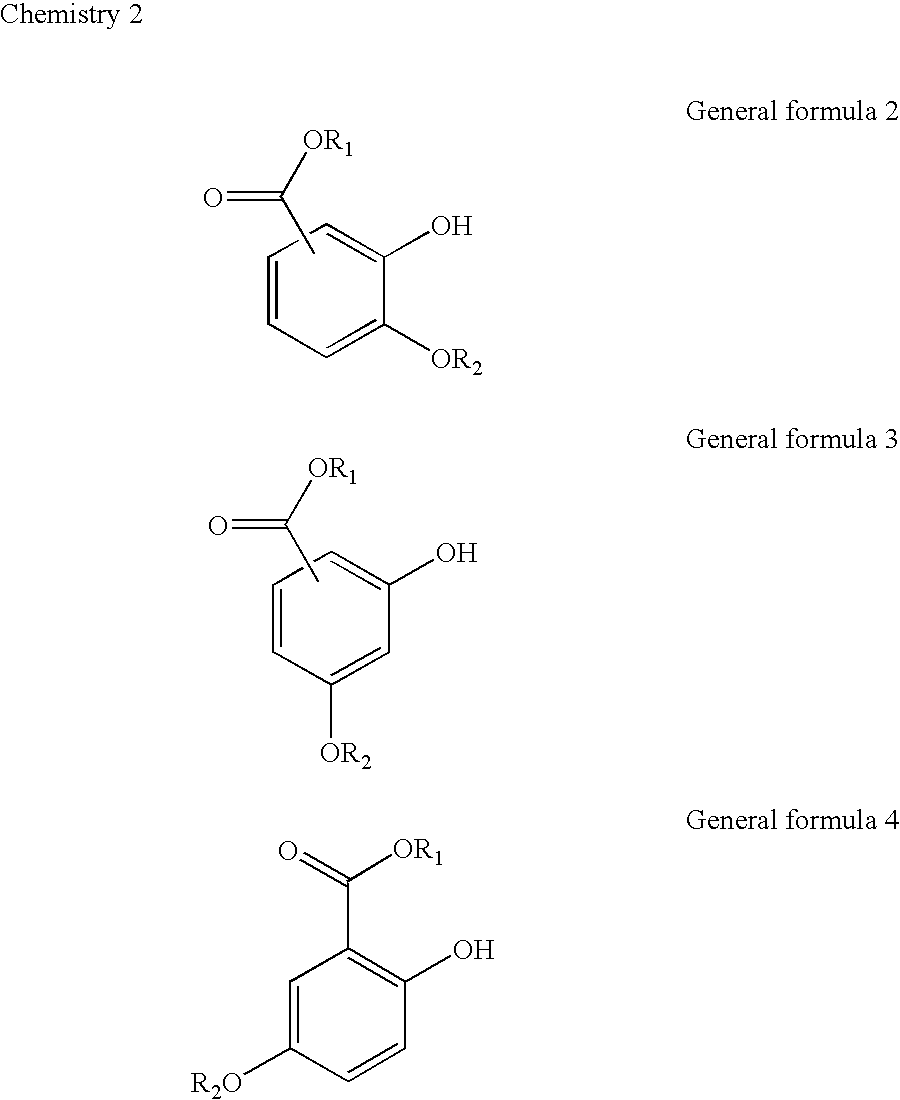
Patents
Literature
843 results about "Tocopherol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tocopherols (/toʊˈkɒfəˌrɒl/; TCP) are a class of organic chemical compounds (more precisely, various methylated phenols), many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was given the name "tocopherol" by the Greek words "τόκος" [tókos, birth], and "φέρειν", [phérein, to bear or carry] meaning in sum "to carry a pregnancy," with the ending "-ol" signifying its status as a chemical alcohol.
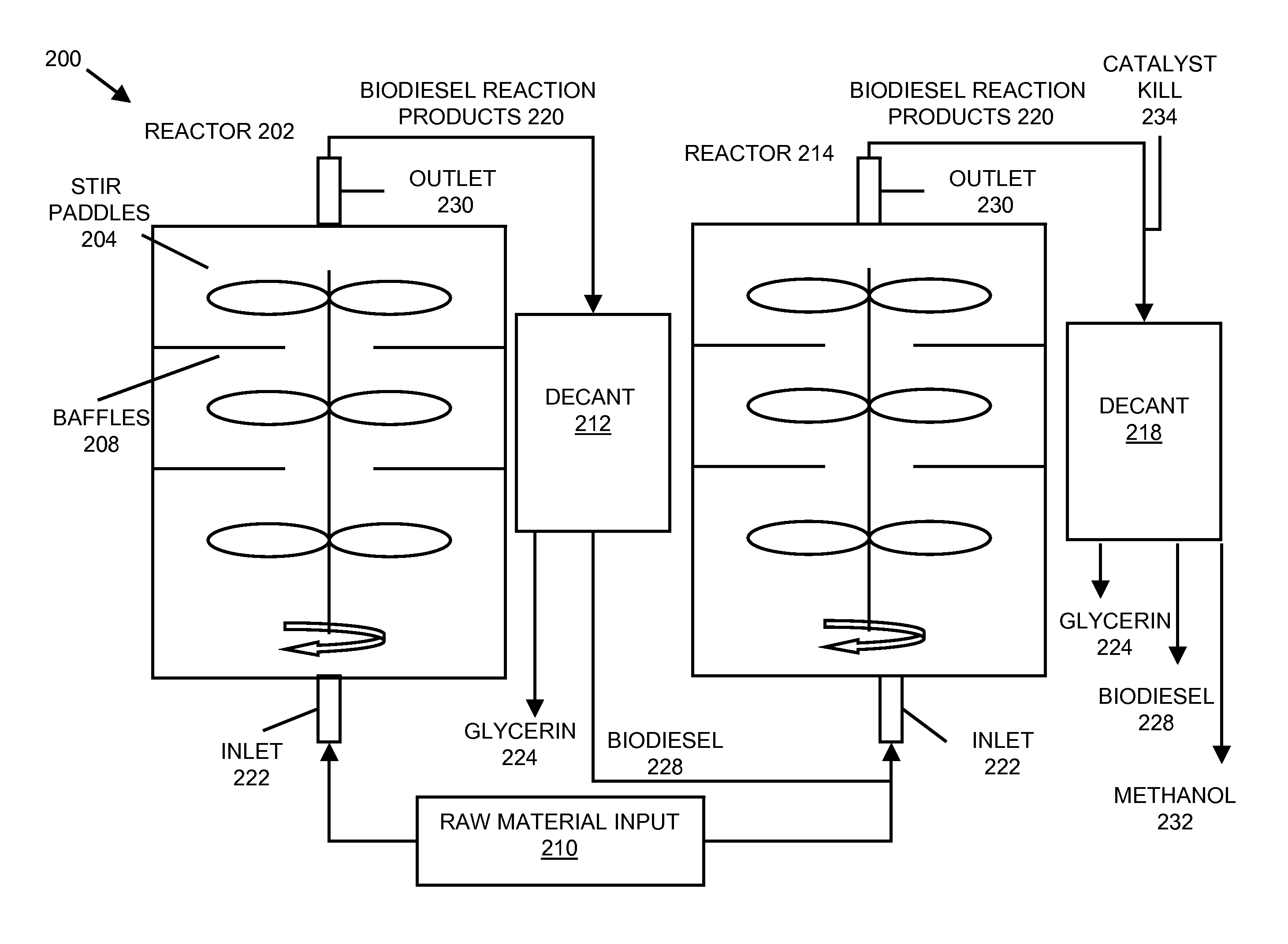
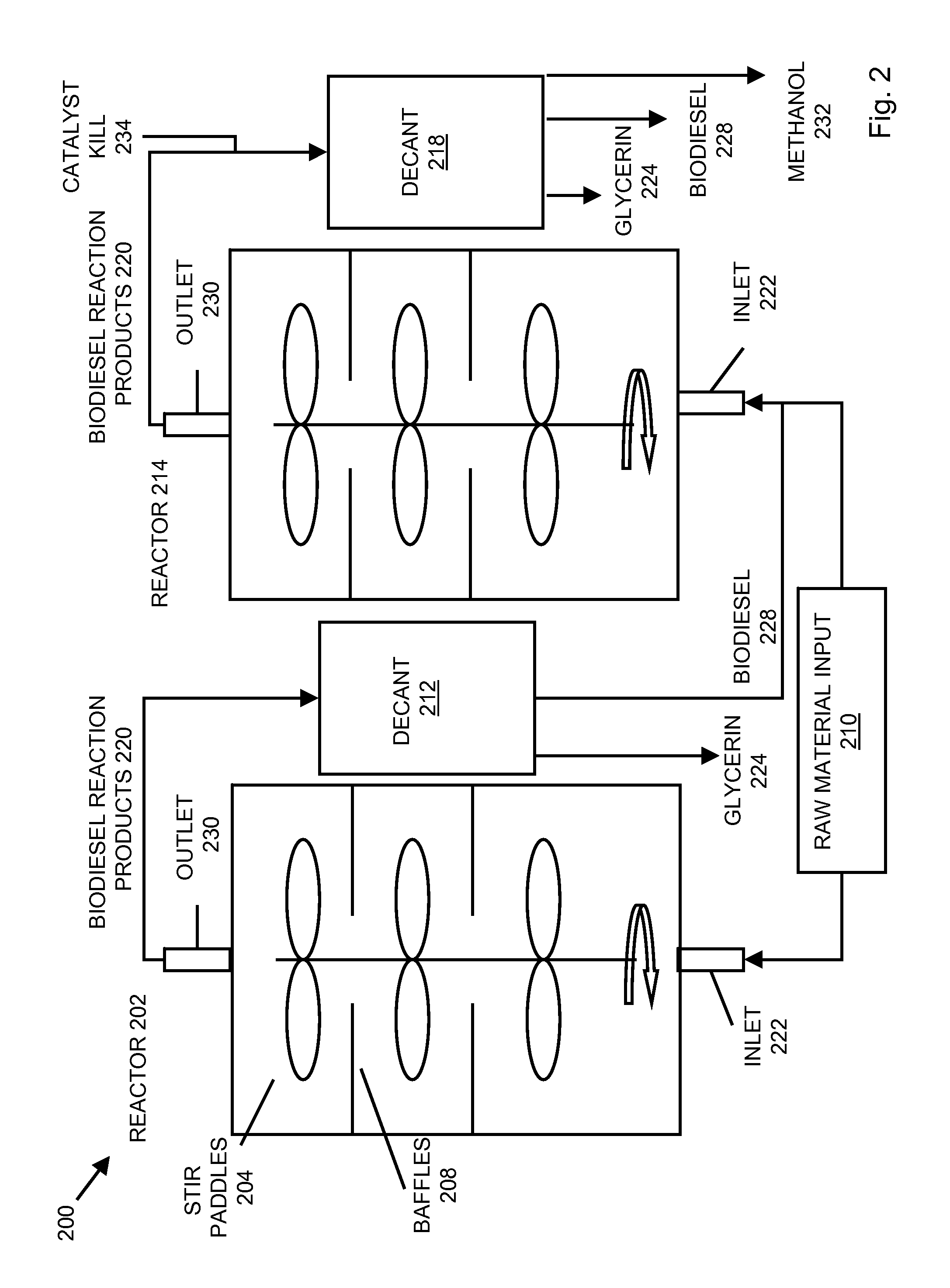
System and process for producing biodiesel
InactiveUS20080282606A1Reducing filter blocking tendencyEnhance biodiesel stabilityFatty acid esterificationFatty acids production/refiningBiodieselDistillation
In embodiments of the present invention, systems for producing a biodiesel product from multiple feedstocks may include a biodiesel reactor, a decanter, a flash evaporator and a distillation column. In other embodiments of the present invention, a process for producing a biodiesel comprises distilling a biodiesel reaction product to remove tocopherols and sterol glucosides and, optionally, adding biodiesel stabilizers to the resultant biodiesel to enhance thermal stability. The components of the system are interrelated so that parameters may be regulated to allow production of a custom biodiesel product.
Owner:IMPERIUM PROCESS TECH
Anti-inflammatory supplement compositions and regimens to reduce cardiovascular disease risks
InactiveUS20060172012A1Relieve symptomsPromotes fast digestionOrganic active ingredientsBiocideBlueberry extractApple extract
Disclosed are improvements in human nutrition involving a unique combination of natural products constituting anti-inflammatory compositions which can reduce cardiovascular disease risks as well as play a positive role in other conditions and diseases for which key indicators, especially selected from the group consisting of C-reactive protein (CRP) levels, cyclooxygenase-2 (COX-2), 5-lypoxygenase (5-LOX) expression and prostaglandin E2 (PGE-2) biosynthesis or any combination of these, are indicators. Therapeutic compositions preferably comprise curcumin, bilberry extract, grape seed extract, green tea extract and apple extract, in effective amounts individually and combined to provide a therapeutically significant reduction in one or more key indicators. Another exemplified therapeutic composition comprises: omega-3 rich refined fish oil, resveratrol, blueberry extract, grape seed extract, green tea extract and gamma and / or delta tocopherol, in effective amounts individually for the above benefits.
Owner:A M TODD
Preparation and stabilization of food-grade marine oils
InactiveUS20030161918A1Increases rancimat stabilityIncreased rancimat stabilityMilk preparationDough treatmentFood gradeSilicon dioxide
The present invention relates to stabilizing marine oil by treatment with silica in the presence or absence of carbon and vacuum steam deodorization at a temperature between about 140° C. and about 210° C. in the presence of 0.1-0.4% deodorized rosemary or sage extract. If desired 0.01-0.03% ascorbyl palmitate and 0.05-0.2% mixed tocopherol can be added. A method of using such oil in food applications is provided. A method of identifying the sensory quality of unknown marine oils is also provided.
Owner:DSM NUTRITIONAL PROD
Compositions and methods for the treatment of radiation burns and other traumatic skin conditions
InactiveUS20050226945A1Improve responseRemarkable clinical successOrganic active ingredientsBiocideWrinkle skinFish oil
The present invention relates to compositions and methods for the treatment of traumatic conditions of the skin including radiation dermatitis, thermal burn, sunburn, dermatomyo-fibromas, and exposure-induced wrinkles, comprising omega-3 fish oils, tocopherols, lavender oil and a suitable amount of a pharmaceutically acceptable carrier, and optionally, one or more of the following: Sodium PCA, or MSM.
Owner:RUWART MARY J
Pharmaceutical composition containing cyclosporin A
InactiveUS6022852AImprove solubilityInhibit synthesisBiocideOrganic active ingredientsAlpha-tocomonoenolTocopherol
The invention relates to a pharmaceutical composition consisting of or containing cyclosporin A and alpha -tocopherol or one of the derivatives thereof.
Owner:HEXAL AG
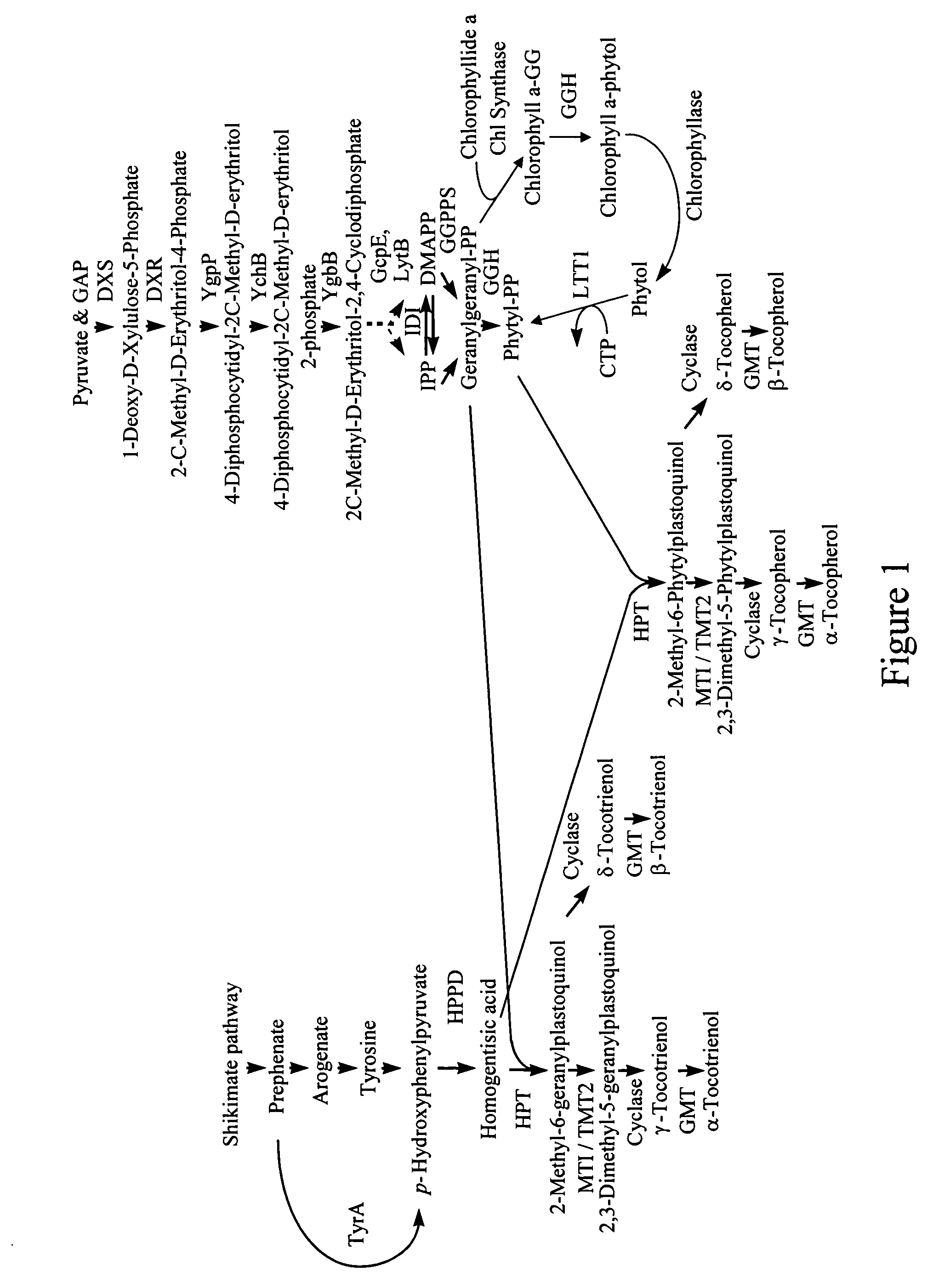
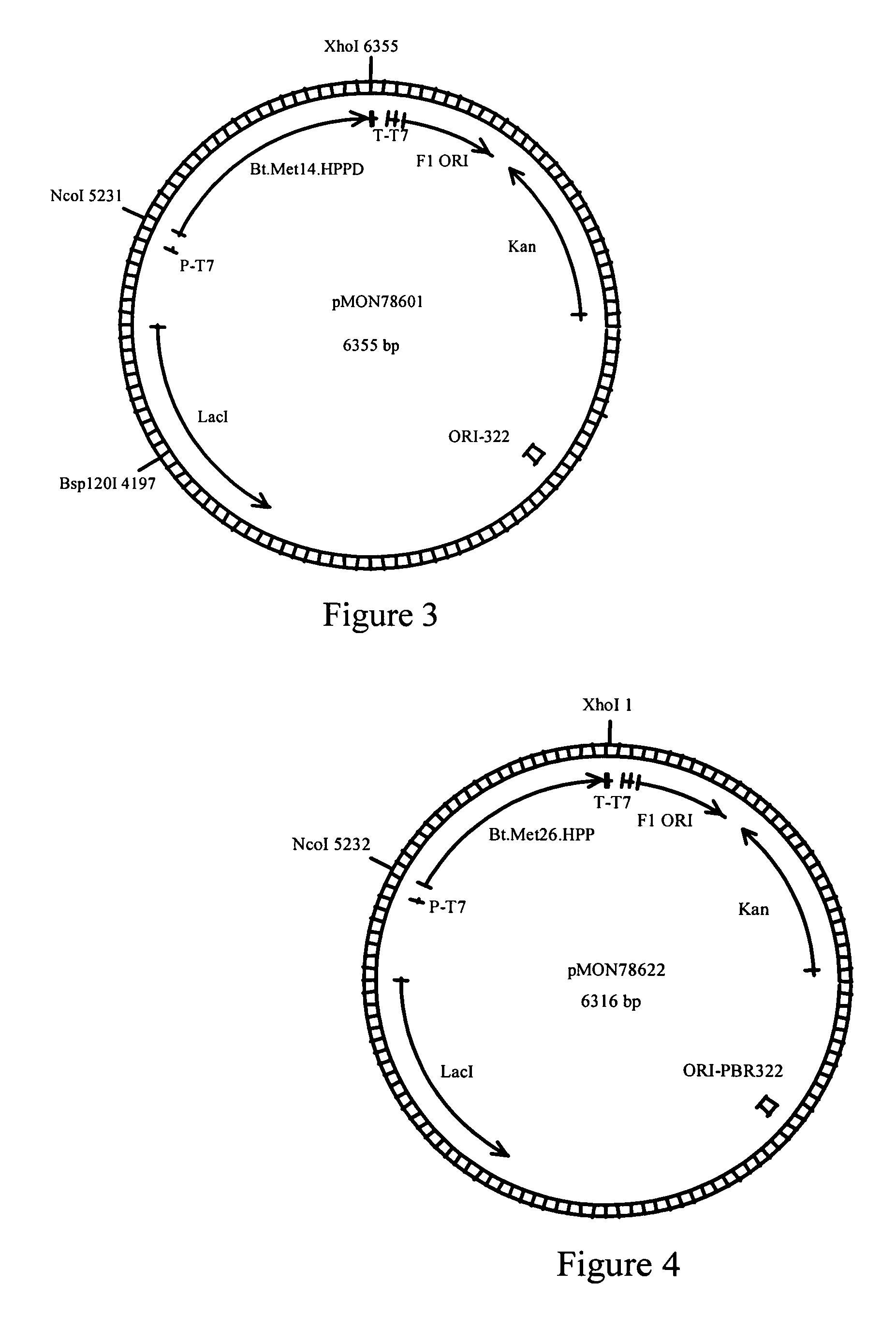
Genes encoding 4-hydroxyphenylpyruvate dioxygenase (HPPD) enzymes for plant metabolic engineering
ActiveUS7297541B2Reduce riskAid in immune functionSugar derivativesOther foreign material introduction processesBiotechnology4-hydroxyphenylpyruvate dioxygenase activity
The present invention is in the field of plant genetics and biochemistry. More specifically, the present invention relates to genes and polypeptides associated with the tocopherol biosynthesis pathway, namely those encoding 4-Hydroxyphenylpyruvate Dioxygenase activity, and uses thereof.
Owner:MONSANTO TECH LLC
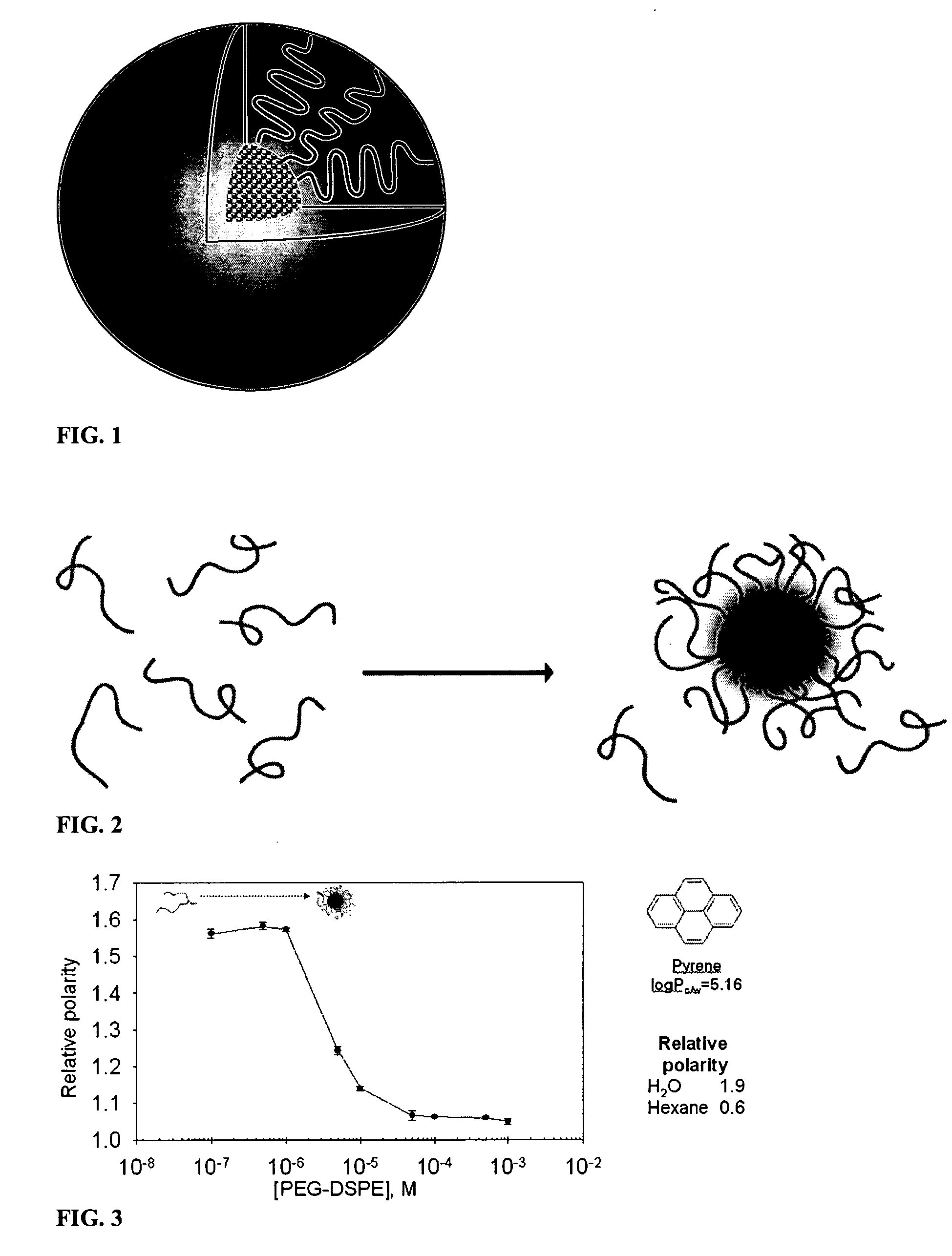
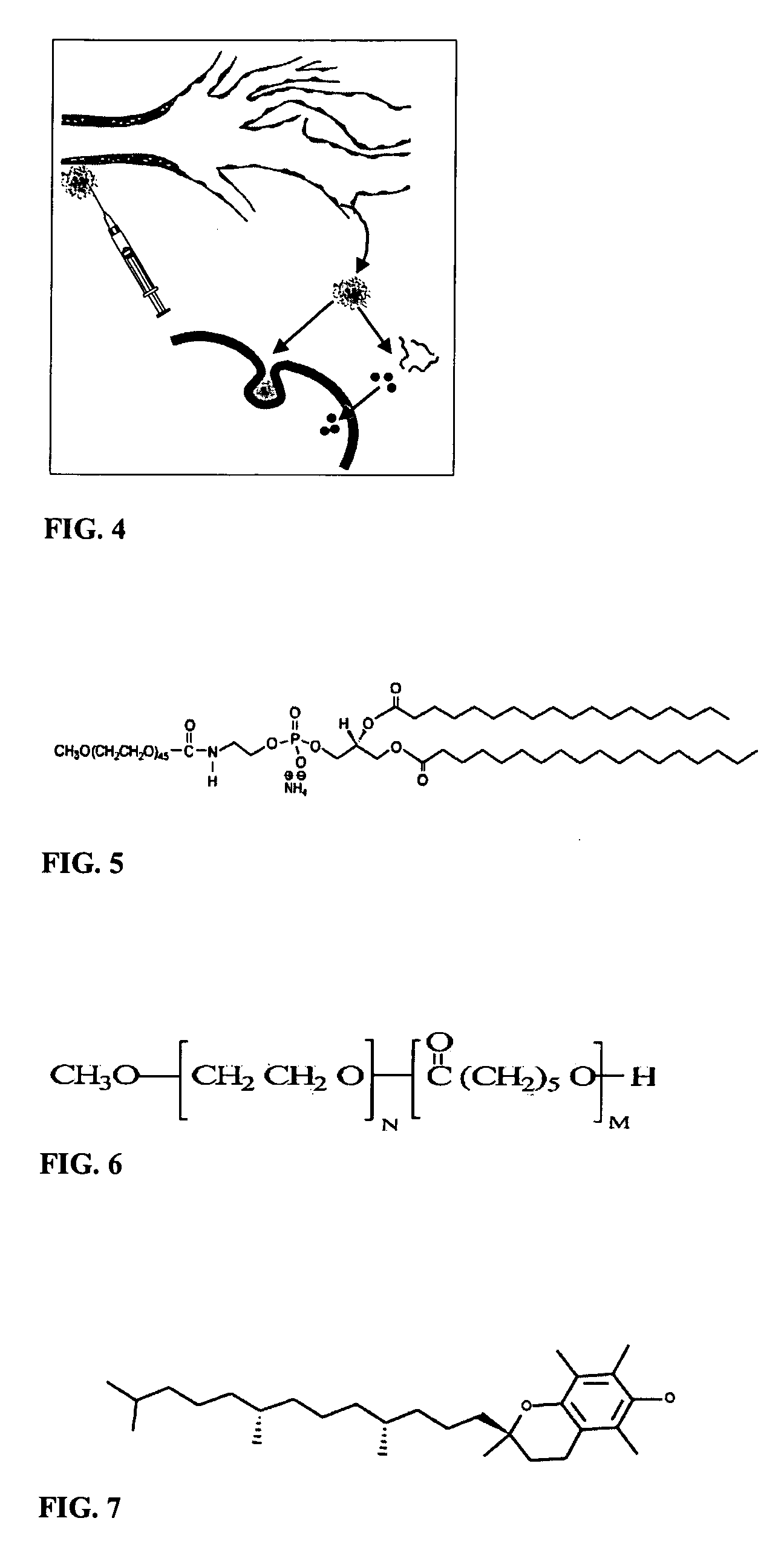
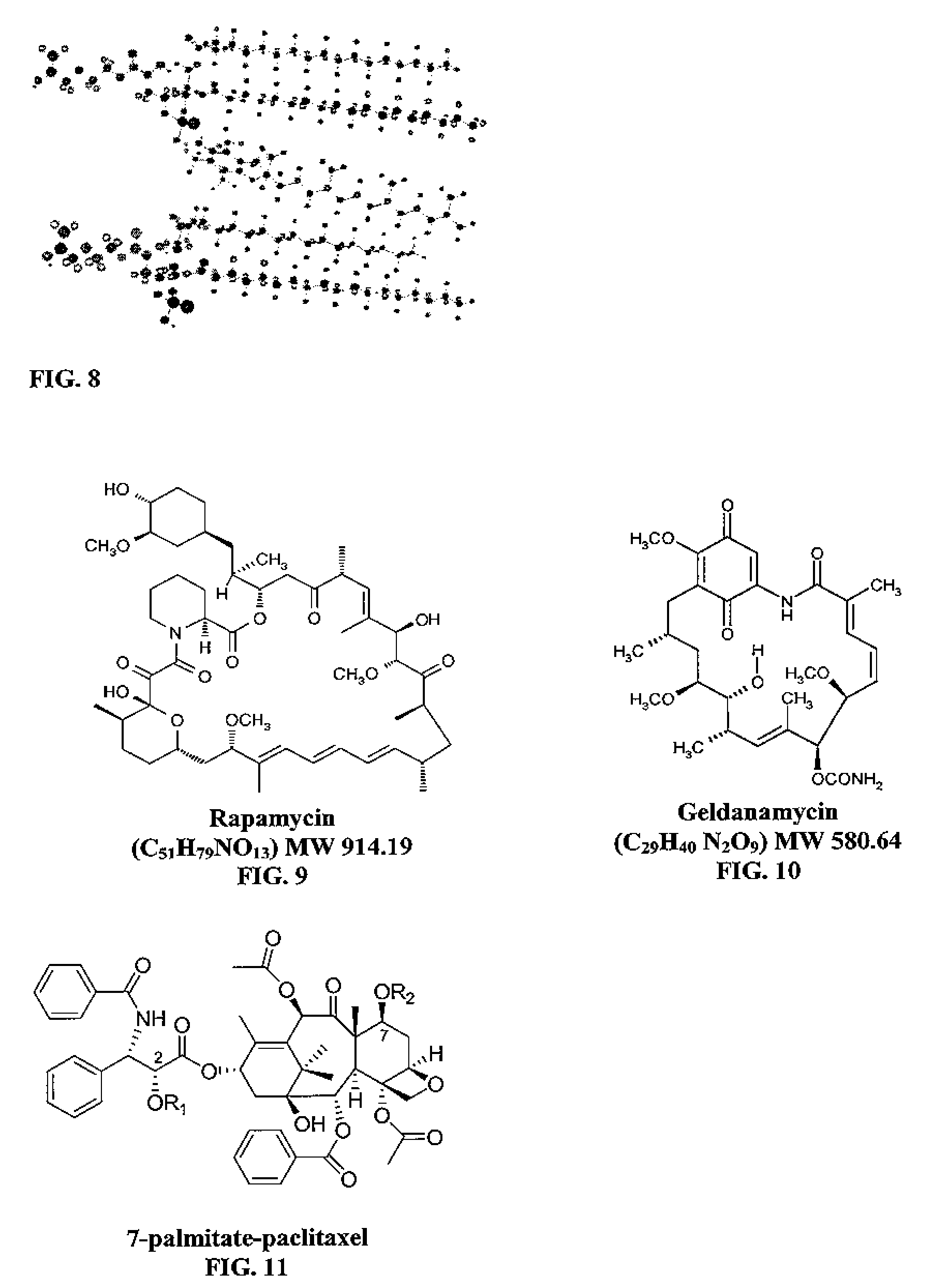
Micelle composition of polymer and passenger drug
InactiveUS20060251710A1Improving micelle encapsulation efficiencyBiocidePowder deliverySolubilitySide effect
Hydrophobic drugs become more practical for treatments by being encapsulated in micelle compositions for increasing solubility. Micelle compositions may include an excipient tocopherol and / or prodrug formulations of the drug. Micelles extend the time period the drug remains in the micelles to improve drug circulation time and thereby drug delivery. Hydrophobic drugs for micelle encapsulation may include rapamycin, geldanamycin, and paclitaxel. Administration of these micelle compositions does not require Cremophor EL or Tween 80, avoiding serious side effects associated with these products which would previously accompany such drug administration.
Owner:WISCONSIN ALUMNI RES FOUND
Compositions of tocol-soluble therapeutics
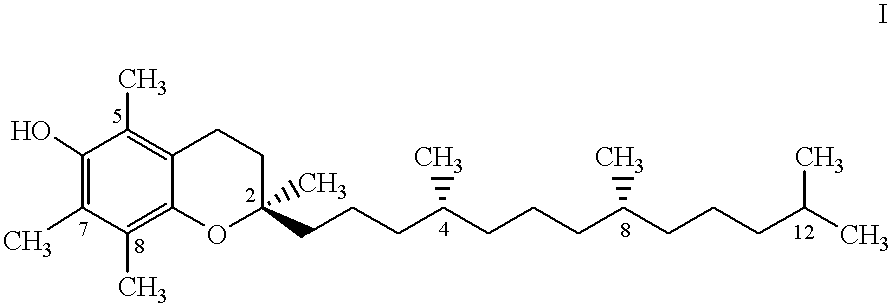
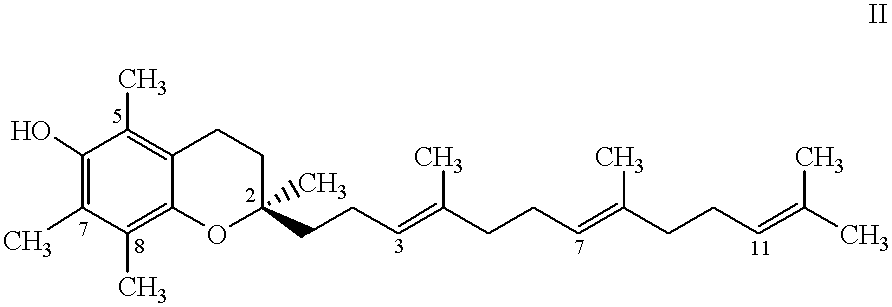
Tocol-based compositions of charged amphiphilic and water soluble pharmaceutically active compounds or their charged precursors are prepared by forming a tocol-soluble ion pair with an oppositely charged ion-pair forming compound capable of forming a tocol-soluble ion-pair with the active compound.Also disclosed are novel compounds tocopherolsuccinate-aspartate and tocopherolsuccinate-glutamate, which are useful as ion-pair forming compounds.
Owner:SONUS PHARM INC
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20060013857A1Lower potentialMinimally inhibit osteogenesis and subsequent bone healingSurgical adhesivesSkeletal disorderBarium saltApatite
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters unsubstituted and N-substituted pyrrolidones of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Highly purified tocopheryl phosphate, process for producing the same, analytical method therefor and cosmetic
InactiveUS6046181AGood water solubilityNo skin irritationOrganic active ingredientsCosmetic preparationsSolubilityCosmetic ingredient
Disclosed herein are a highly purified tocopheryl phosphate and / or a salt thereof (tocopheryl phosphates) wherein a P,P'-bistocopheryl hypophosphate and / or a salt thereof (P,P'-bistocopheryl diphoshates) is contained in a proportion of not higher than 3% by weight; a process for producing a highly purified tocopheryl phosphate and / or a salt thereof, which comprises the steps of reacting a tocopherol with an oxyphosphorus trihalide followed by treating with an acid or basic aqueous solution to thereby form tocopheryl phosphates (i) in which P,P'-bistocopheryl diphoshates (ii) formed as by-products are contained, hydrolyzing the P,P'-bistocopheryl diphoshates (ii) under acid condition, and, optionally, rendering the hydrolyzate neutral or basic under basic condition; and a method of analyzing tocopheryl phosphates, comprising analyzing a sample containing components (i) and (ii) with the use of a high-performance liquid chromatograph column packed with a gel of a polymethacrylate having, bonded thereto, long-chain alkyl groups. None or only an extremely minute amount of P,P'-bistocopheryl diphoshates are contained in the highly purified tocopheryl phosphates, so that the highly purified tocopheryl phosphates exhibit antioxidant and blood circulation promoting effects, have excellent water solubility, are powdery so that the handling thereof is extremely easy, are free from cutaneous irritation and allergenecity and ensure dermal safety. Therefore, the highly purified tocopheryl phosphates are useful as cosmetic ingredients. The amounts of components (i) and (ii) can be simply measured with high accuracy by the above method.
Owner:SHOWA DENKO KK
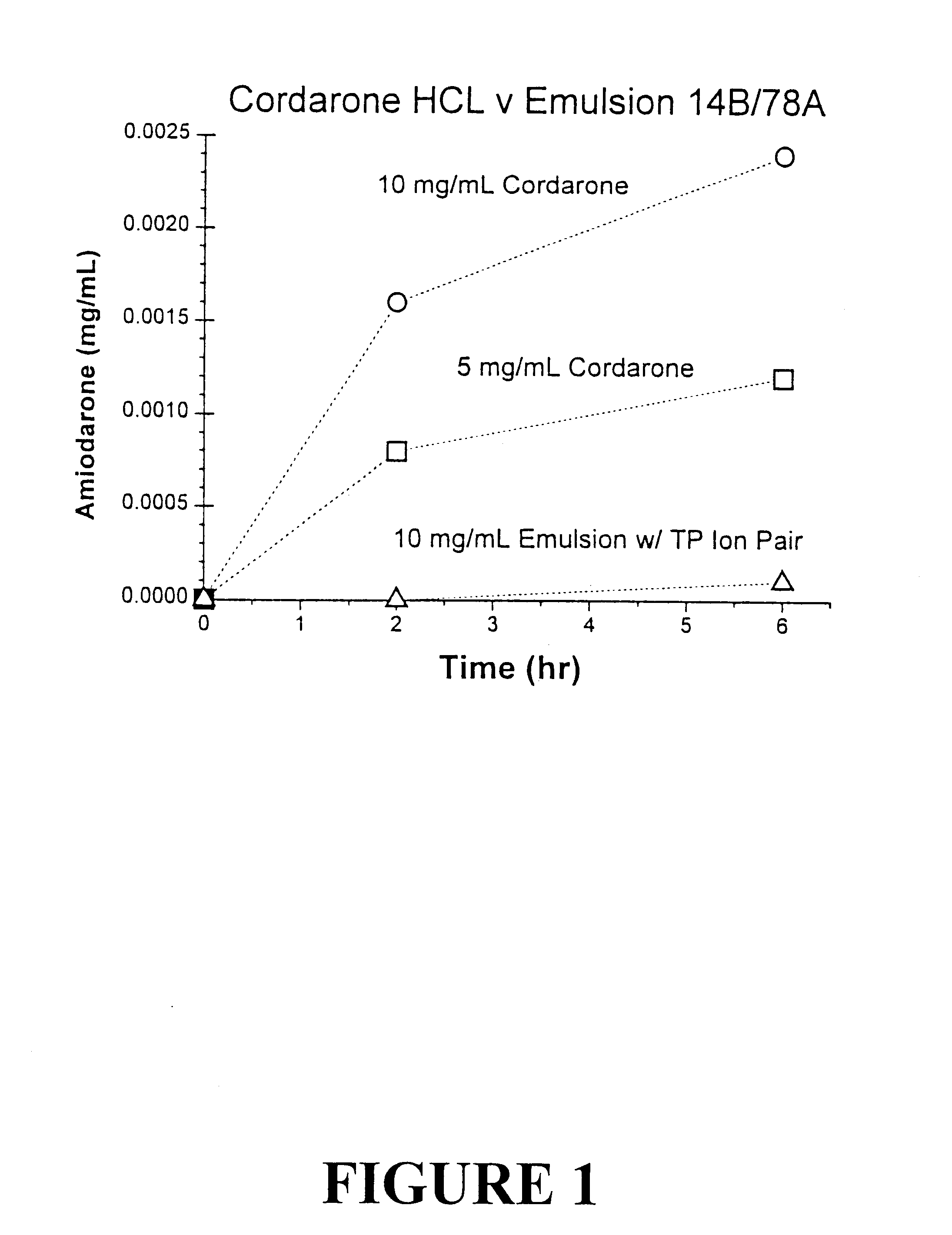
Emulsion vehicle for poorly soluble drugs
An emulsion of α-tocopherol, stabilized by biocompatible surfactants, as a vehicle or carrier for therapeutic drugs, which is substantially ethanol free and which can be administered to animals or humans via various routes is disclosed. Also included in the emulsion is PEGylated vitamin E. PEGylated α-tocopherol includes polyethylene glycol subunits attached by a succinic acid diester at the ring hydroxyl of vitamin E and serves as a primary surfactant, stabilizer and a secondary solvent in emulsions of α-tocopherol.
Owner:IGDRASOL
Method of improving the antioxidant status of an infant
InactiveUS7090862B2Easy to manageImproving the antioxidant status of an infantBiocidePowder deliveryNewborn infantAntioxidative status
The present invention relates generally to a method of improving the antioxidant status of an infant. More particularly, the present invention relates to a method of improving the antioxidant status of an infant by administering a mixture of natural tocopherols. The natural tocopherol mixture is an effective blend of α- and γ-tocopherol. For ease of administration and improved taste, the mixture of natural tocopherols are typically delivered in vehicle which may be in the form, for example, of a tablet, capsule, liquid, and nutritional formula. The present invention also relates to a method of improving the antioxidant status of an infant by supplementing the lactating woman wherein the supplemented breast milk is fed to the infant. Additionally, the present invention relates to a method of improving the antioxidant status of a newborn infant by supplementing the pregnant woman.
Owner:ABBOTT LAB INC
Emulsion vehicle for poorly soluble drugs
InactiveUS7030155B2Process stabilityEmulsion stabilizationOrganic active ingredientsBiocideEmulsionSuccinic acid
A method of making an emulsion of tocopherol incorporating a co-solvent and, stabilized by biocompatible surfactants, as a vehicle or carrier for therapeutic drugs, which is substantially ethanol free and which can be administered to animals or humans by various routes, is disclosed. Also included in the emulsion is PEGylated vitamin E. PEGylated α-tocopherol includes polyethylene glycol subunits attached by a succinic acid diester at the ring hydroxyl of vitamin E and serves as a primary surfactant, stabilizer and a secondary solvent in emulsions of α-tocopherol.
Owner:IGDRASOL
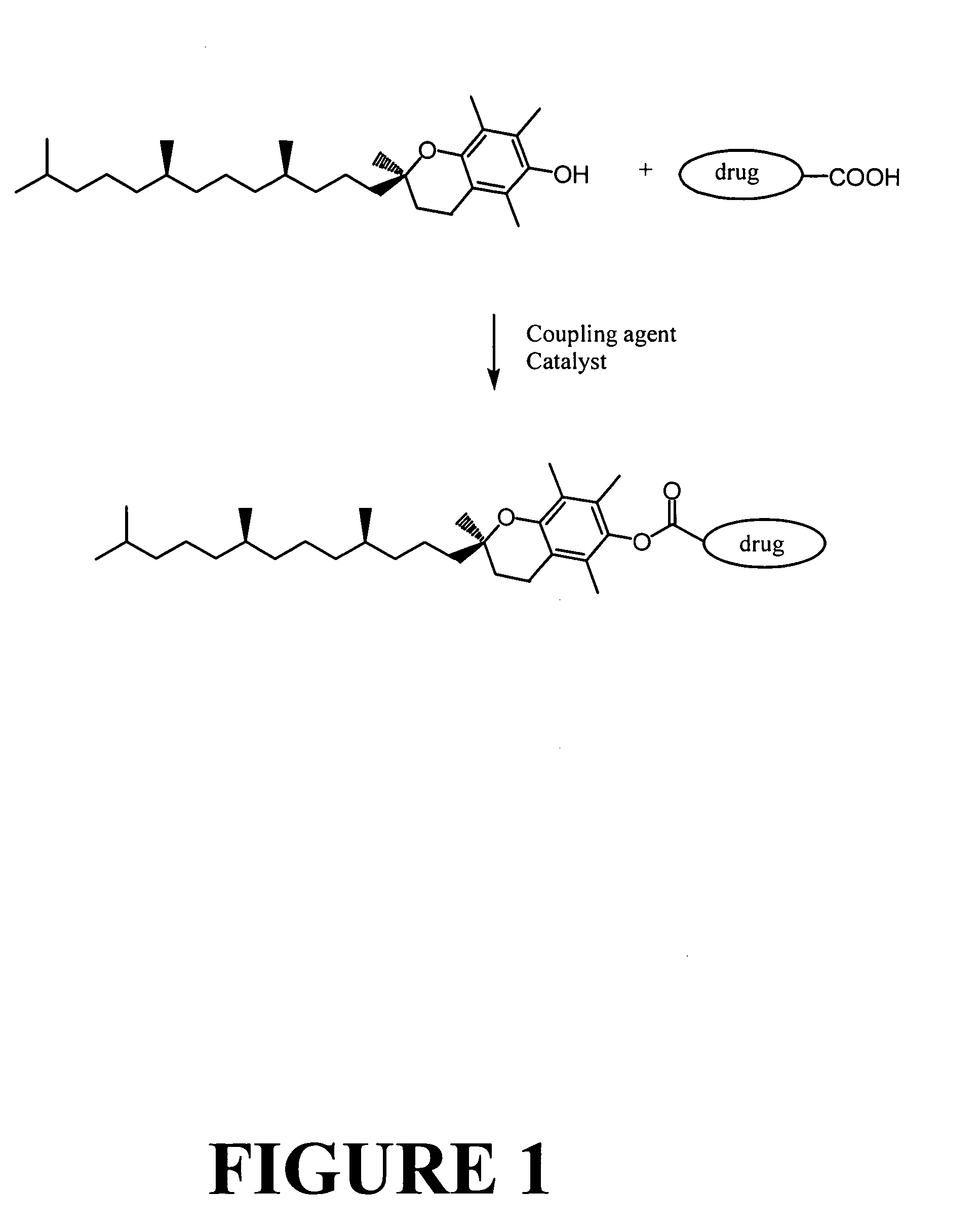
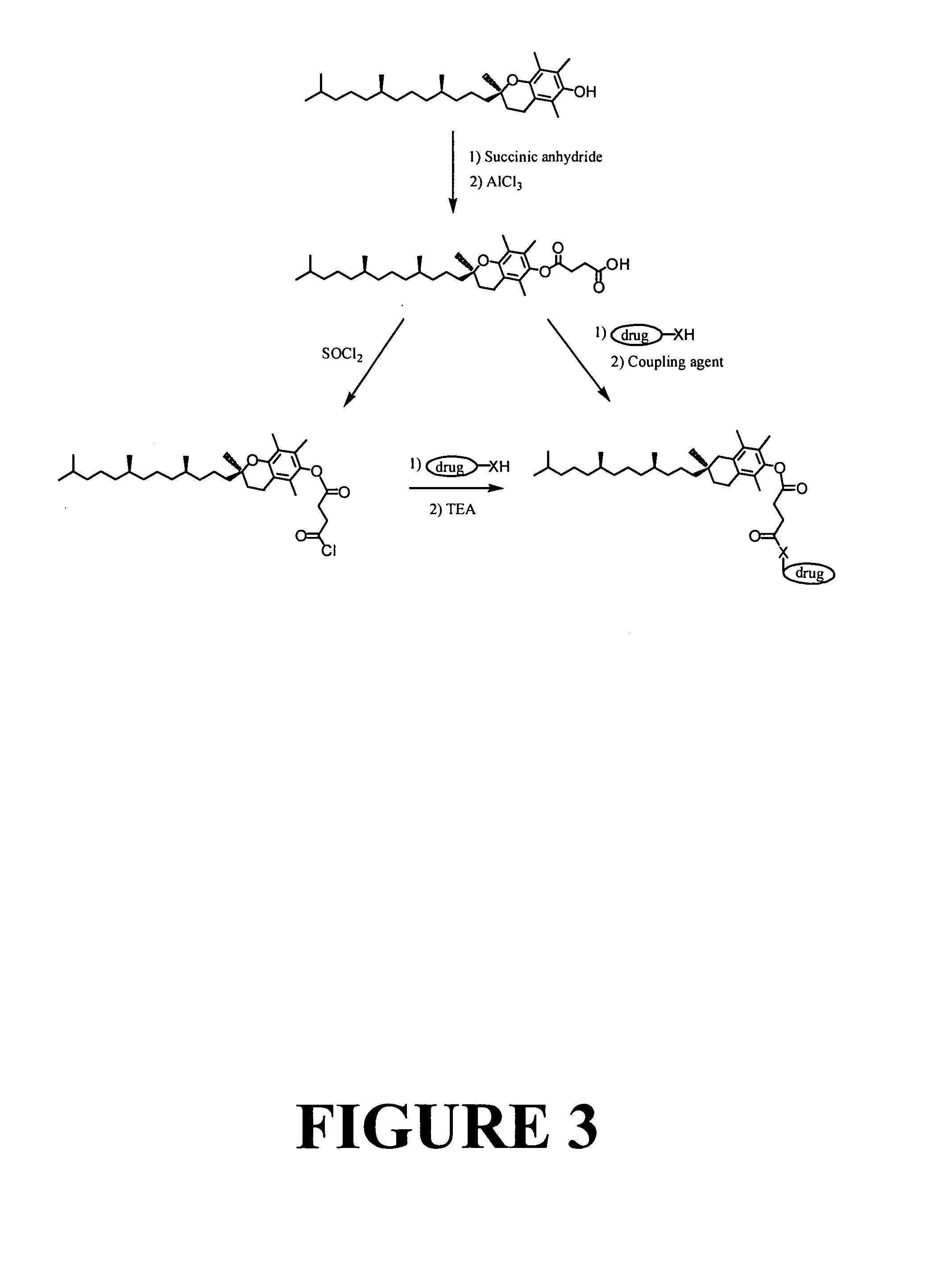
Tocopherol-modified therapeutic drug compounds
Tocopherol-modified therapeutic drug compounds; emulsion, microemulsion, and micelle formulations that include the compounds; methods for making the compounds and formulations; methods for administering the compounds and formulations; and methods for treating conditions using the compounds and formulations.
Owner:SONUS PHARM INC
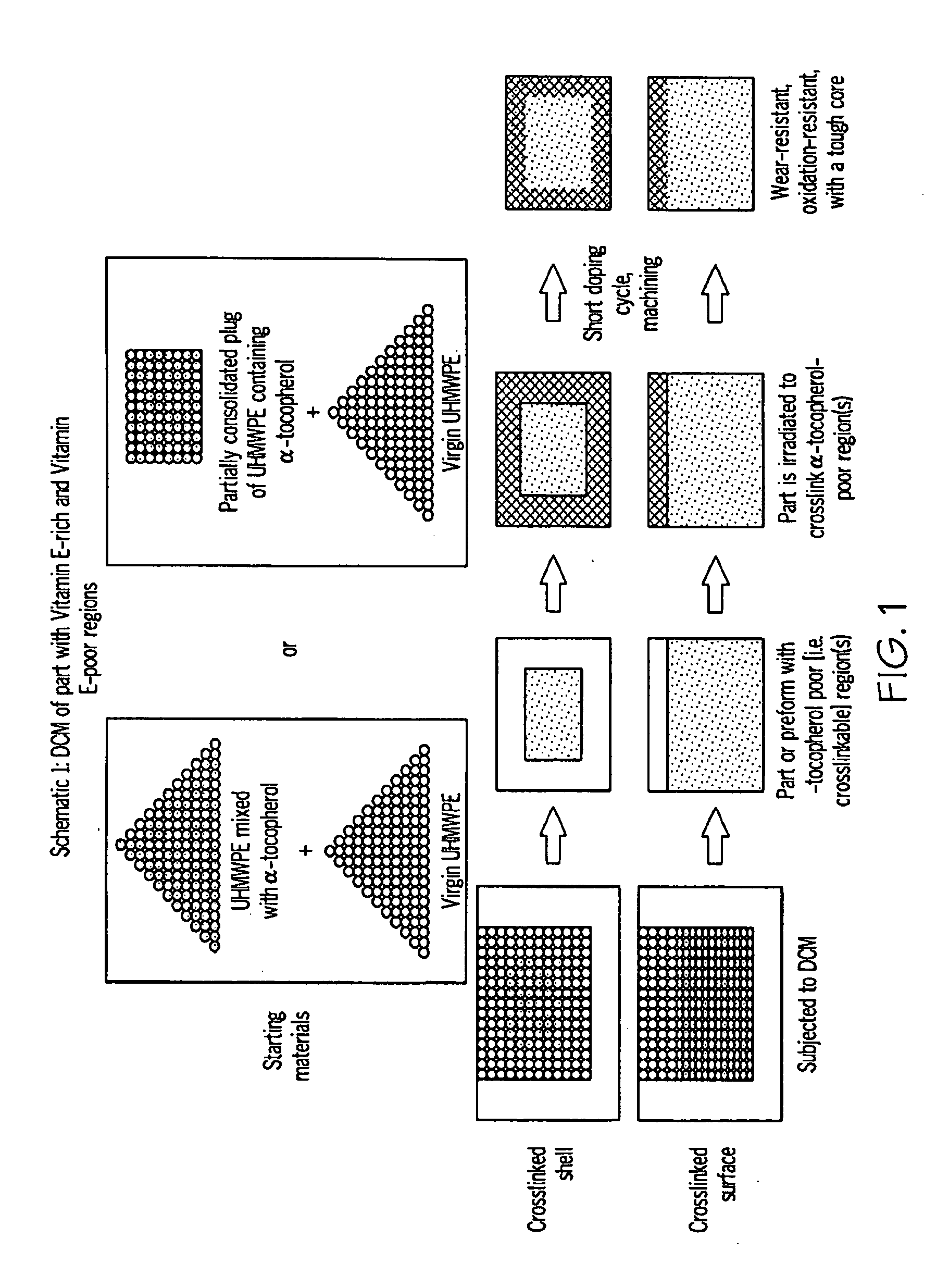
Methods for making oxidation-resistant cross-linked polymeric materials
ActiveUS20100190882A1Preventing and minimizing in vivo elution of antioxidantImpression capsSurgical adhesivesCross-linkElution
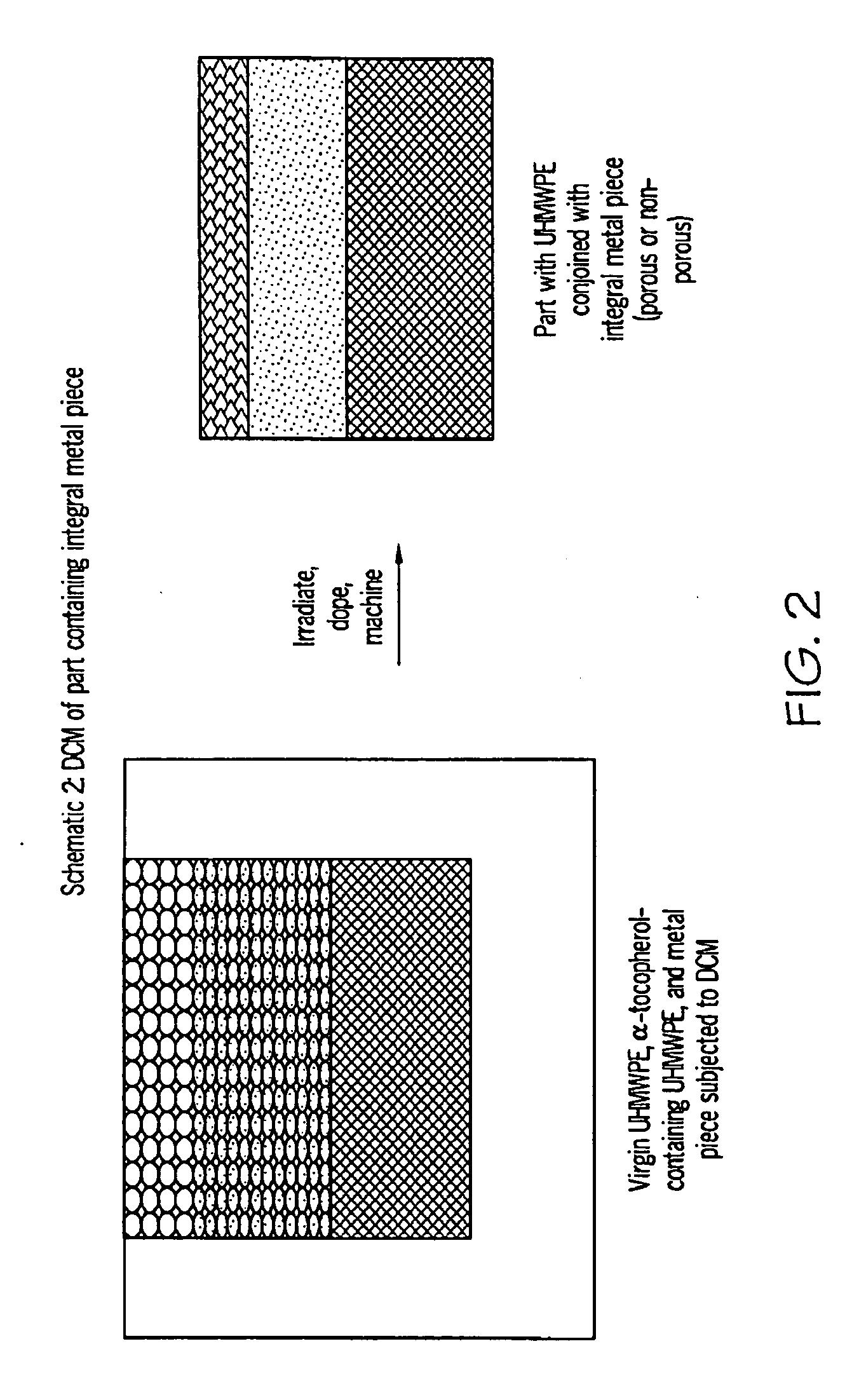
The present invention relates to methods for making cross-linked oxidation-resistant polymeric materials and preventing or minimizing in vivo elution of antioxidant from the antioxidant-containing polymeric materials. The invention also provides methods of doping polymeric materials with a spatial control of cross-linking and antioxidant distribution, for example, vitamin E (α-Tocopherol), and methods for extraction / elution of antioxidants, for example, vitamin E (α-tocopherol), from surface regions of antioxidant-containing polymeric materials, and materials used therewith also are provided.
Owner:THE GENERAL HOSPITAL CORP +1
Method for preventing off-flavor development and preserving seasoning flavor in irradiated meat and meat products
InactiveUS6099879ASlow onsetReduce developmentMilk preparationDough treatmentAdditive ingredientFood flavor
A method comprising the step of treating meat and meat products, including fish, poultry, fish products, and poultry products, with a stabilizing amount of rosemary extract or rosemary extract in combination singly or collectively with tocopherols, ascorbic acid, citric acid, or sodium tripolyphosphate, prior to exposure of the meat or meat products to ionizing radiation, enhances the flavor and shelf life thereof. In addition, the active antioxidant ingredients of rosemary extract may be used individually or collectively as a replacement for rosemary extract, these being carnosic acid, carnosol, and rosmarinic acid, which have been found equivalent to or superior to rosemary extract itself for purposes of the present invention when used in the concentrations set forth herein.
Owner:KALAMAZOO HLDG INC
Absorbable putty-like implants and methods for their use for mechanical hemostasis of bone and for the treatment of osseous defects
ActiveUS20060002976A1Lower potentialImproved bone healingPowder deliverySurgical adhesivesBarium saltTG - Triglyceride
Two (or more), component, body-implantable, absorbable, biocompatible, putty-like surgical mechanical hemostatic tamponades for use in surgery. Component 1, a carboxylic acid salt bulking material preferably less than 50 micron, preferably the calcium, magnesium, zinc, aluminum, lithium or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, linoleic, caprylic, capric, and lauric; dialkyl ethers; alkyl aryl ethers; dialkyl ketones and alkyl aryl ketones; polyhydroxy compounds and esters and ethers thereof; oils such as olive oil and castor oil and triglycerides.
Owner:ABYRX
Influenza vaccine
InactiveUS20080014217A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol and / or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Colloidal solid lipid vehicle for pharmaceutical use
The invention provides a drug carrier that includes a solid lipid nanoparticle (SLN), wherein the SLN includes tocopherol or a derivative thereof. The invention also provides a pharmaceutical composition that includes a SLN and a biologically active compound, wherein the SLN comprises tocopherol or a derivative thereof. The present invention further provides a colloidal drug delivery system that includes solid lipid nanoparticles (SLNs), wherein the SLNs comprise tocopherol or a derivative thereof. Also provided are methods for preparing the drug carrier, pharmaceutical composition, and colloidal drug delivery system of the invention.
Owner:ALPHARX
Multiple antioxidant micronutrients
A method for administering an antioxidant composition to humans according to their age and sex is disclosed wherein the method comprises administering to said humans a daily dose of a multiple antioxidant micronutrient composition comprising vitamin A (palmitate), beta carotene (from natural d. salina), vitamin C (calcium ascorbate), vitamin D-3 (cholecalciferol), natural source vitamin E including both d-alpha tocopheryl and d-alpha tocopheryl acid succinate, thiamine mononitrate, riboflavin, niacinamide ascorbate, d-calcium pantothenate, pyridoxine hydrochloride, cyanocobalamin, folic acid (folacin), d-biotin, selenium (1-seleno methionine), chromium picolinate, zinc glycinate, calcium citrate, and magnesium citrate. For persons over the age of about 51, the composition preferably further comprises one or more of co-enzyme Q10, N-acetyl cysteine, and alpha lipoic acid. Preferably, also, vitamin D is added for women over the age of about 36.
Owner:NEW AGE HEALTH SCI INC
Lipid nanoparticle formulation
The disclosure features novel lipids and compositions involving the same. Nanoparticle compositions include an ionizable lipid, a phospholipid, a first sterol or a tocopherol, and optionally a second sterol different from the first sterol. Nanoparticle compositions further including therapeutic and / or prophylactics such as RNA are useful in the delivery of therapeutic and / or prophylactics to mammalian cells or organs to, for example, regulate polypeptide, protein, or gene expression.
Owner:MODERNATX INC +1
Amphiphilic block copolymer and polymeric composition comprising the same for drug delivery
ActiveUS7311901B2Improve hydrophobicityHigh affinityPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsCholesterolHydrophobe
The present invention relates to an amphiphilic block copolymer of a hydrophilic block and a hydrophobic block with a terminal hydroxyl group wherein the terminal hydroxyl group of the hydrophobic bock is substituted with a tocopherol or cholesterol group. It also relates to polymeric compositions capable of forming stable micelles in an aqueous solution, comprising the amphiphilic block copolymer and a polylactic acid derivative wherein one or more ends of the polylactic acid are covalently bound to at least one carboxyl group.
Owner:SAMYANG HLDG CORP
Tocopherol-modified therapeutic drug compounds
Tocopherol-modified therapeutic drug compounds; emulsion, microemulsion, and micelle formulations that include the compounds; methods for making the compounds and formulations; methods for administering the compounds and formulations; and methods for treating conditions using the compounds and formulations.
Owner:SONUS PHARM INC
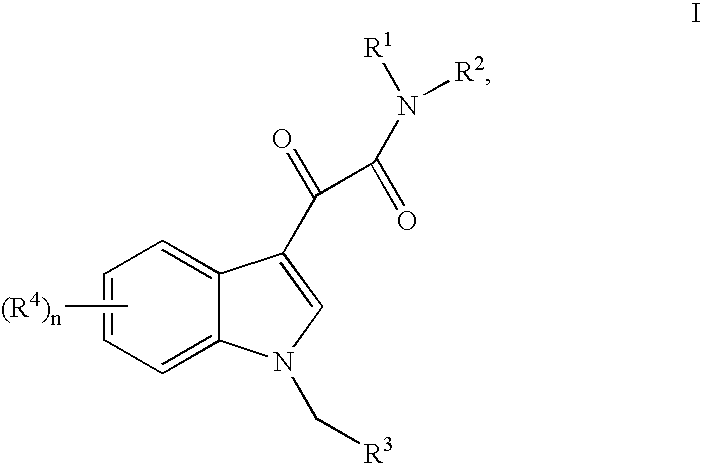
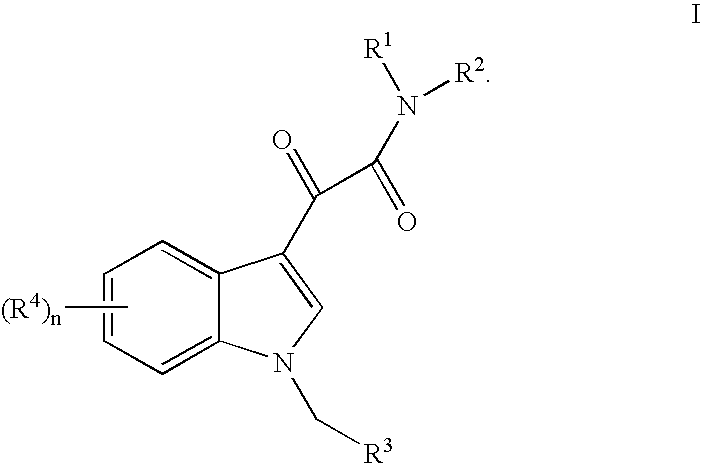
Anticancer oral formulation
InactiveUS20100010059A1Improve oral bioavailabilityBiocideDispersion deliveryPolyethylene glycolSuccinates
This invention relates to an oral formulation containing an effective amount of the compound of the following formula I:d-alpha-tocopheryl polyethylene glycol 1000 succinate (“TPGS”); and 2-(2-ethoxyethoxy)ethanol (“Transcutol”). R1 through R4 and n are defined herein. Also disclosed is a method of treating cancer by administering this formula to a subject orally.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Topical agent for dermatological use
The objetive of the present invention was to enhance the skin whitening effects and blackening prevention effects and supply safe and stable topical agents for dermatological use. For that purpose 4-Hydroxyphenyl-alpha-D-glucopyranoside was combined with auxiliary agents such as ascorbic acid and its derivatives, crude drugs and its extracts, hydroxycarboxylic acid and its salts, oil soluble glycyrrhiza extract, gentian extract, phenol derivatives and their salts, placenta extract, kojic acid and its derivative, glucosamine and its derivatives, azelaic acid and its derivatives, retinol and its derivatives, pyridoxin and its derivatives, tocopherol and its derivatives, chitosan and its decomposition products, caffeic acid derivatives, hydroxycinnamate and its derivatives, Umbelliferae plant extracts, mycelial cultures and their extracts, plant leaves and their extracts.
Owner:DSM IP ASSETS BV +1
Alditol acetal composition and its use in plastic and gelled materials
InactiveUS6673856B1Improve flow behaviorImprove stabilityOrganic compound preparationInksPolyolThermal stability
The present invention relates to improved alditol acetal compositions, in particular 1,3-2,4-di(benzylidene) sorbitol (DBS) or one of its alkylated derivatives.The improvement in these compositions is expressed in particular in terms of flow behavior and / or thermal stability. It is obtained by combining the alditol acetal with an additive selected from tocopherols, polyols and certain of their respective derivatives.These additives may act as binding or densifying agents and / or stabilizing agents or odor maskers.The alditol acetal and additive are advantageously combined by cold mixing, followed by granulation or compaction, also cold.The compositions of the invention, for example based on DBS or the methylated derivatives of DBS, are in the form of densified or compacted powders, granules, pellets, pastilles or extrudates.They are used in particular for preparing plastic or jellified materials or additives for these types of materials.
Owner:ROQUETTE FRERES SA
Process for separating unsaponifiable valuable products from raw materials
Disclosed are processes for separating valuable products, including unsaponifiable materials, from any given matrix of raw materials that is mainly composed of saponifiable components and unsaponifiable components. Preferred methods include converting sodium or potassium soaps obtained from the saponification of a starting material into metallic soaps which have a lower melting point, and when melted, have viscosity sufficiently low to enable processing such as by distillation / evaporation processes. Preferred raw materials include animal or vegetable products, as well as by-products, residues, and waste products from the processing of animal or vegetable products, such as from food processing, cellulose processing and the like. Valuable products which may be obtained by the disclosed processes include sterols, vitamins, flavonoids, and tocopherols.
Owner:RESITEC PARTICIPACOES
Stable injectable composition of alpha tocopheryl succinate, analogues and salts thereof
Owner:SD PHARMA
Process for separation of tocopherols
InactiveUS6867308B2Easy loadingHigh resolutionBiocideOrganic active ingredientsOrganic solventVegetable oil refining
A process for separation of specific tocopherols from a mixture of tocopherols is disclosed. The process includes methods of separation of the tocopherols from residues of vegetable oil refining and other various food sources by dissolving in organic solvents and eluting over non-ionic adsorbent resins.
Owner:ARCHER DANIELS MIDLAND CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com