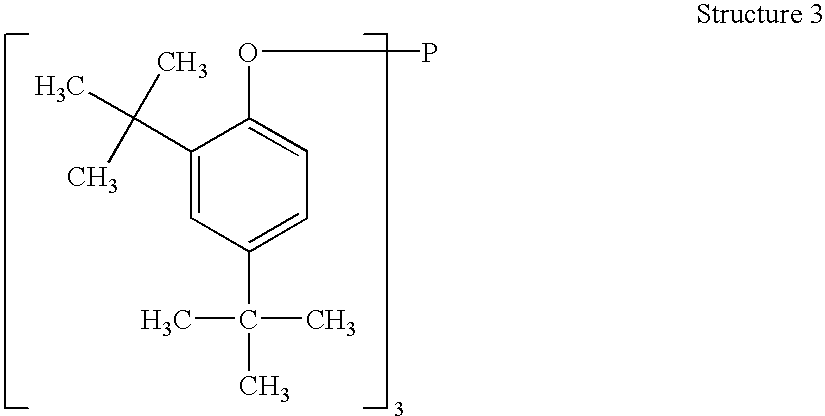
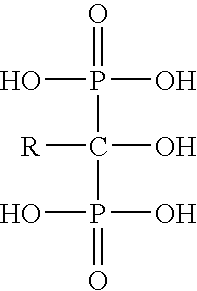
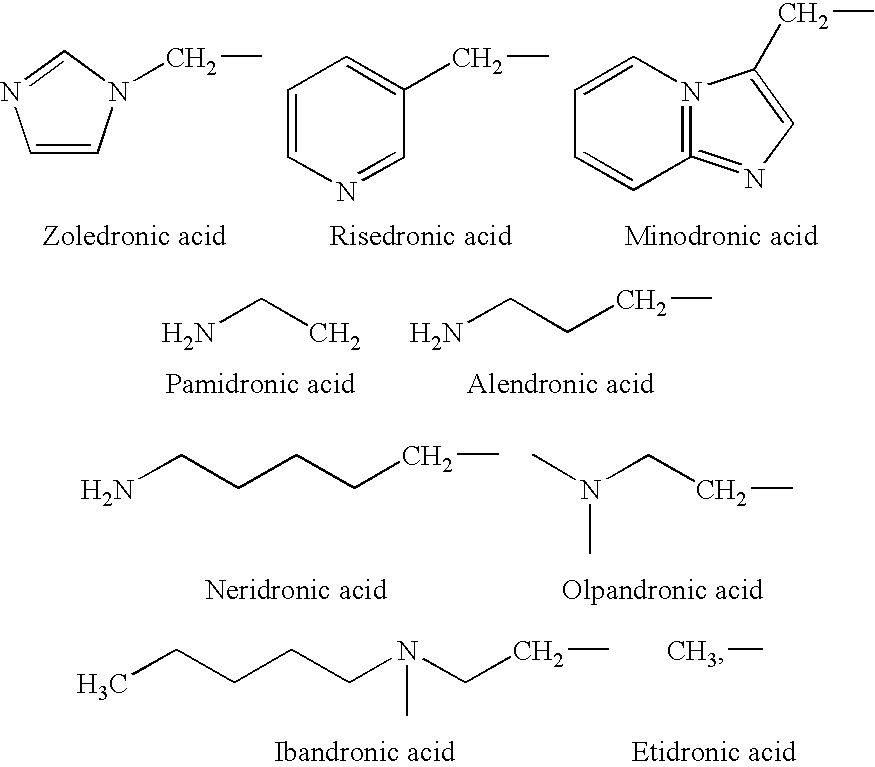
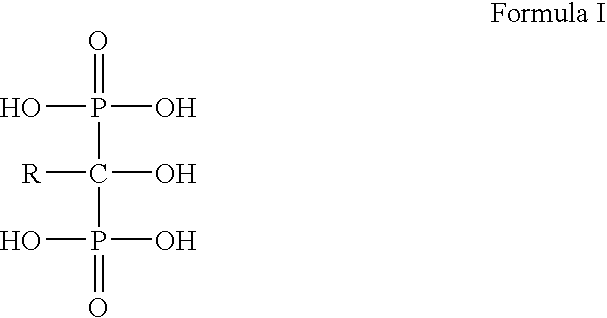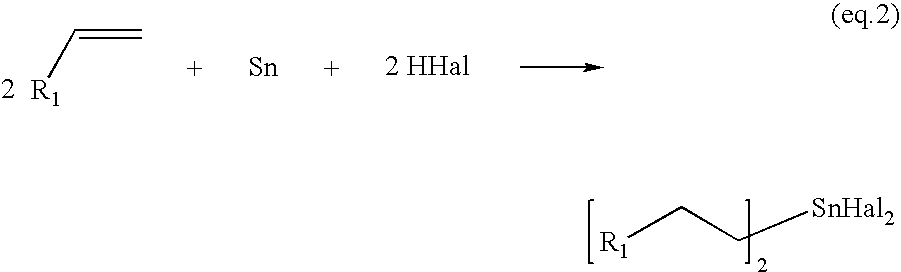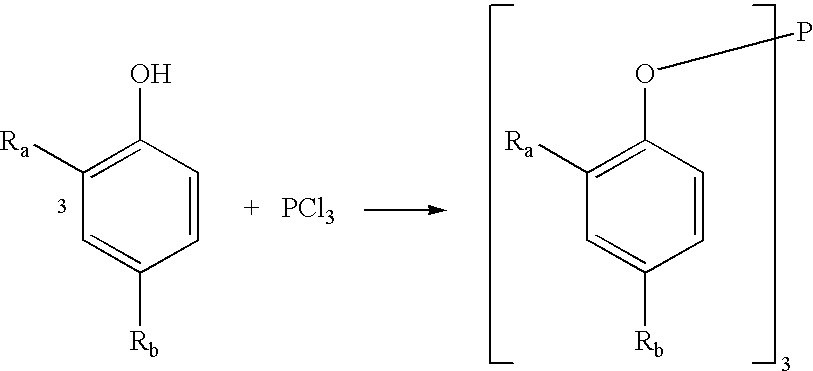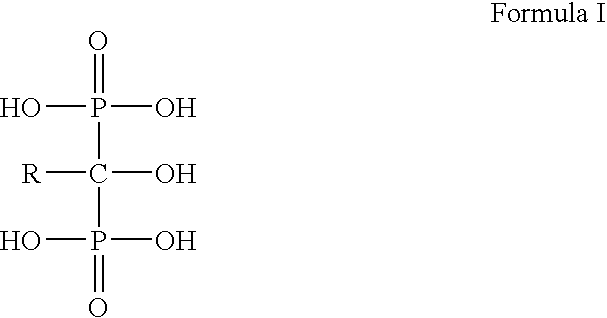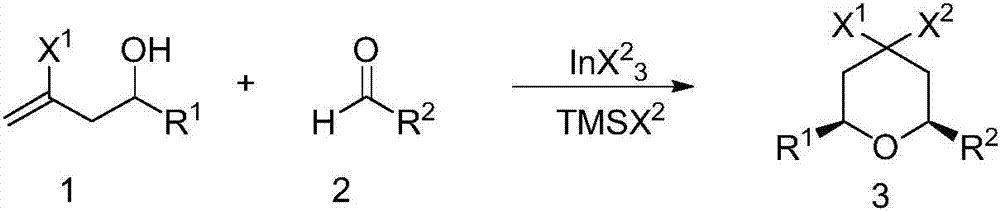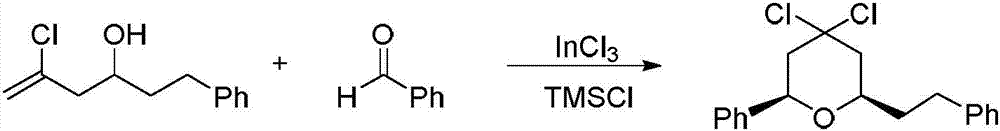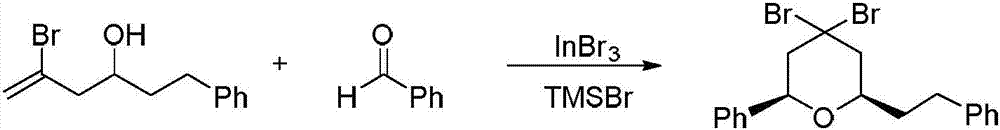Patents
Literature
114 results about "Trihalide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A trihalide in chemistry is an organohalide consisting of three halide atoms bonded to a single atom or compound. An example of a trihalide is chloroform. The trihalomethanes are the simplest trihalides, because only one hydrogen is connected to the carbon. The 1,1,1-Trichloroethane is one of the trihalides of ethane.
Zinc Ion-Exchanging Energy Storage Device
ActiveUS20160301096A1Quick releaseRapid depositionHybrid capacitor electrolytesAlkaline accumulatorsChemical treatmentZinc metal
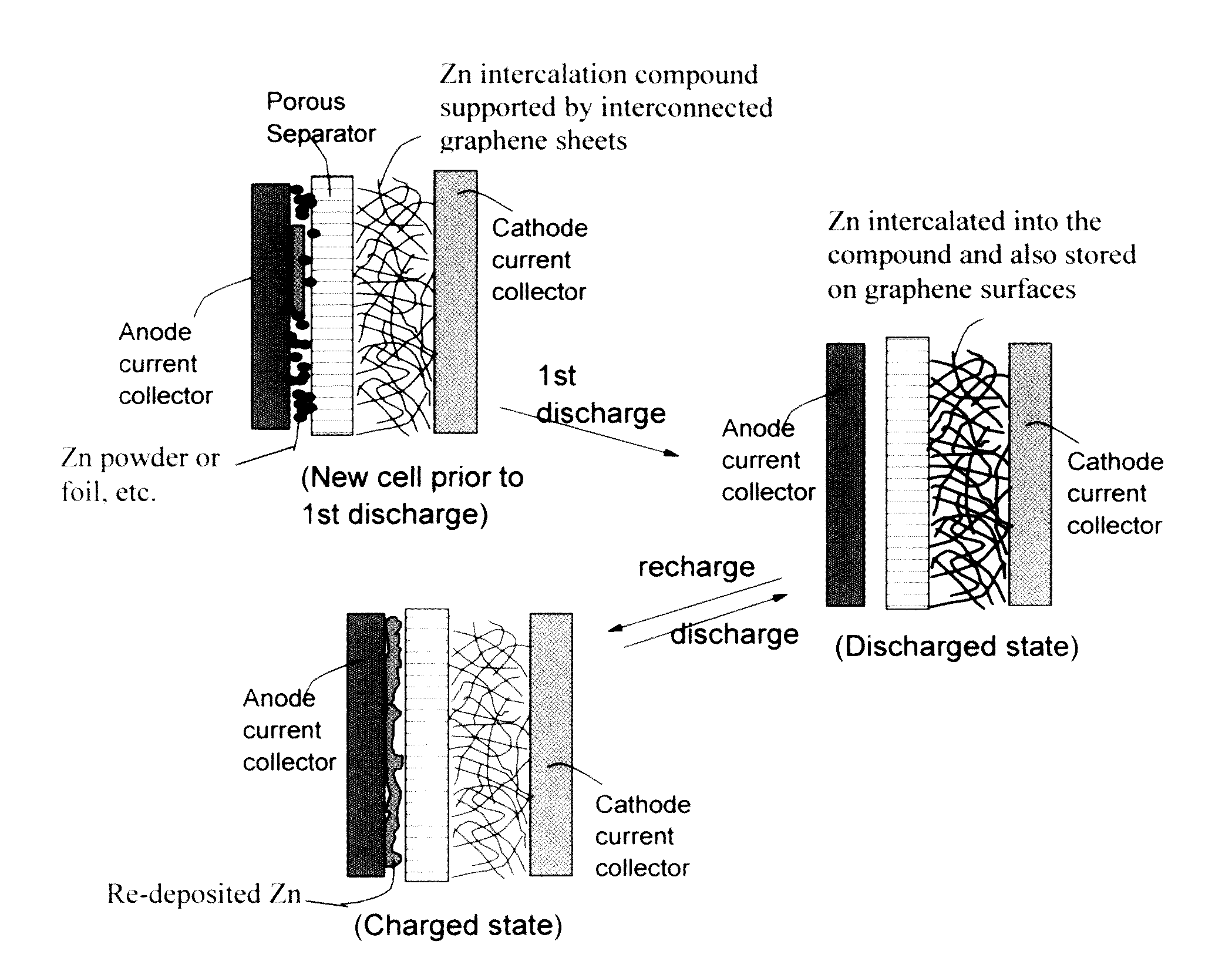
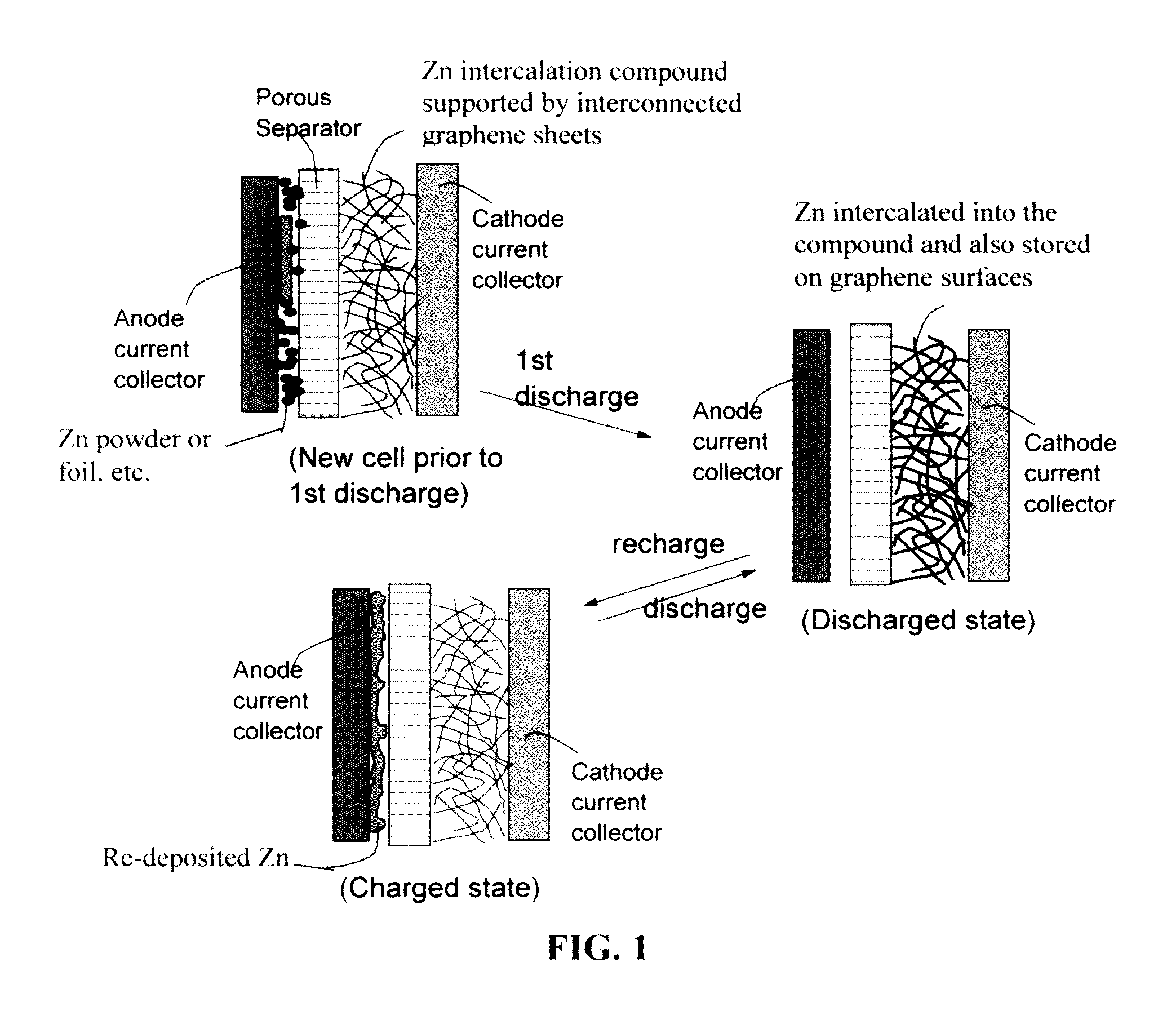
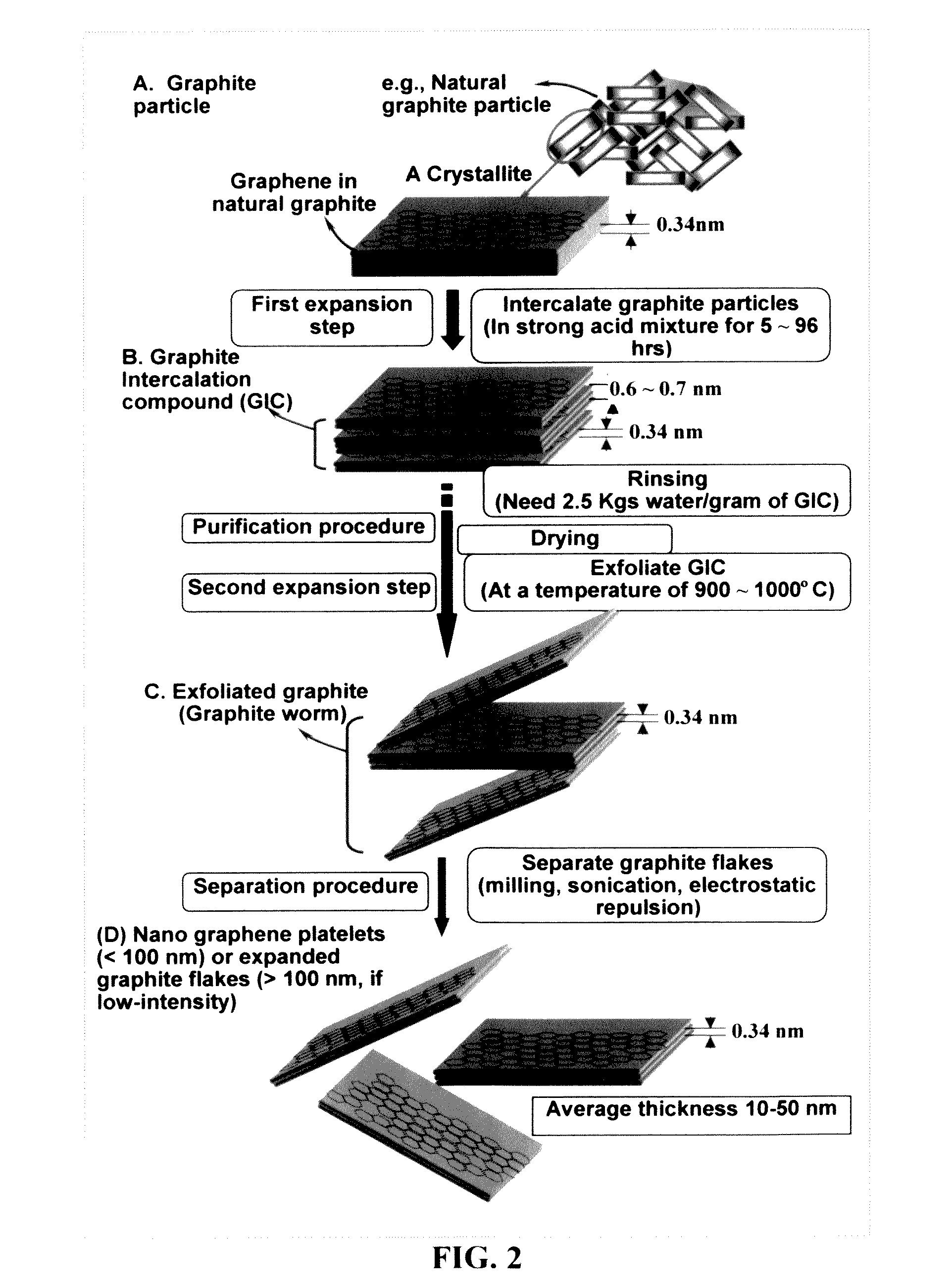
A zinc ion-exchanging battery device comprising: (A) a cathode comprising two cathode active materials (a zinc ion intercalation compound and a surface-mediating material); (B) an anode containing zinc metal or zinc alloy; (C) a porous separator disposed between the cathode and the anode; and (D) an electrolyte containing zinc ions that are exchanged between the cathode and the anode during battery charge / discharge. The zinc ion intercalation compound is selected from chemically treated carbon or graphite material having an expanded inter-graphene spacing d002 of at least 0.5 nm, or an oxide, carbide, dichalcogenide, trichalcogenide, sulfide, selenide, or telluride of niobium, zirconium, molybdenum, hafnium, tantalum, tungsten, titanium, vanadium, chromium, cobalt, manganese, iron, nickel, or a combination thereof. The surface-mediating material contains exfoliated graphite or multiple single-layer sheets or multi-layer platelets of a graphene material.
Owner:GLOBAL GRAPHENE GRP INC
Highly purified tocopheryl phosphate, process for producing the same, analytical method therefor and cosmetic
InactiveUS6046181AGood water solubilityNo skin irritationOrganic active ingredientsCosmetic preparationsSolubilityCosmetic ingredient
Disclosed herein are a highly purified tocopheryl phosphate and / or a salt thereof (tocopheryl phosphates) wherein a P,P'-bistocopheryl hypophosphate and / or a salt thereof (P,P'-bistocopheryl diphoshates) is contained in a proportion of not higher than 3% by weight; a process for producing a highly purified tocopheryl phosphate and / or a salt thereof, which comprises the steps of reacting a tocopherol with an oxyphosphorus trihalide followed by treating with an acid or basic aqueous solution to thereby form tocopheryl phosphates (i) in which P,P'-bistocopheryl diphoshates (ii) formed as by-products are contained, hydrolyzing the P,P'-bistocopheryl diphoshates (ii) under acid condition, and, optionally, rendering the hydrolyzate neutral or basic under basic condition; and a method of analyzing tocopheryl phosphates, comprising analyzing a sample containing components (i) and (ii) with the use of a high-performance liquid chromatograph column packed with a gel of a polymethacrylate having, bonded thereto, long-chain alkyl groups. None or only an extremely minute amount of P,P'-bistocopheryl diphoshates are contained in the highly purified tocopheryl phosphates, so that the highly purified tocopheryl phosphates exhibit antioxidant and blood circulation promoting effects, have excellent water solubility, are powdery so that the handling thereof is extremely easy, are free from cutaneous irritation and allergenecity and ensure dermal safety. Therefore, the highly purified tocopheryl phosphates are useful as cosmetic ingredients. The amounts of components (i) and (ii) can be simply measured with high accuracy by the above method.
Owner:SHOWA DENKO KK
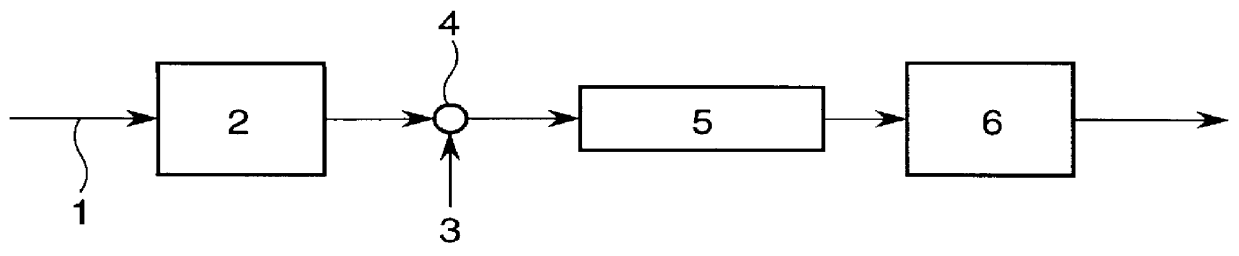
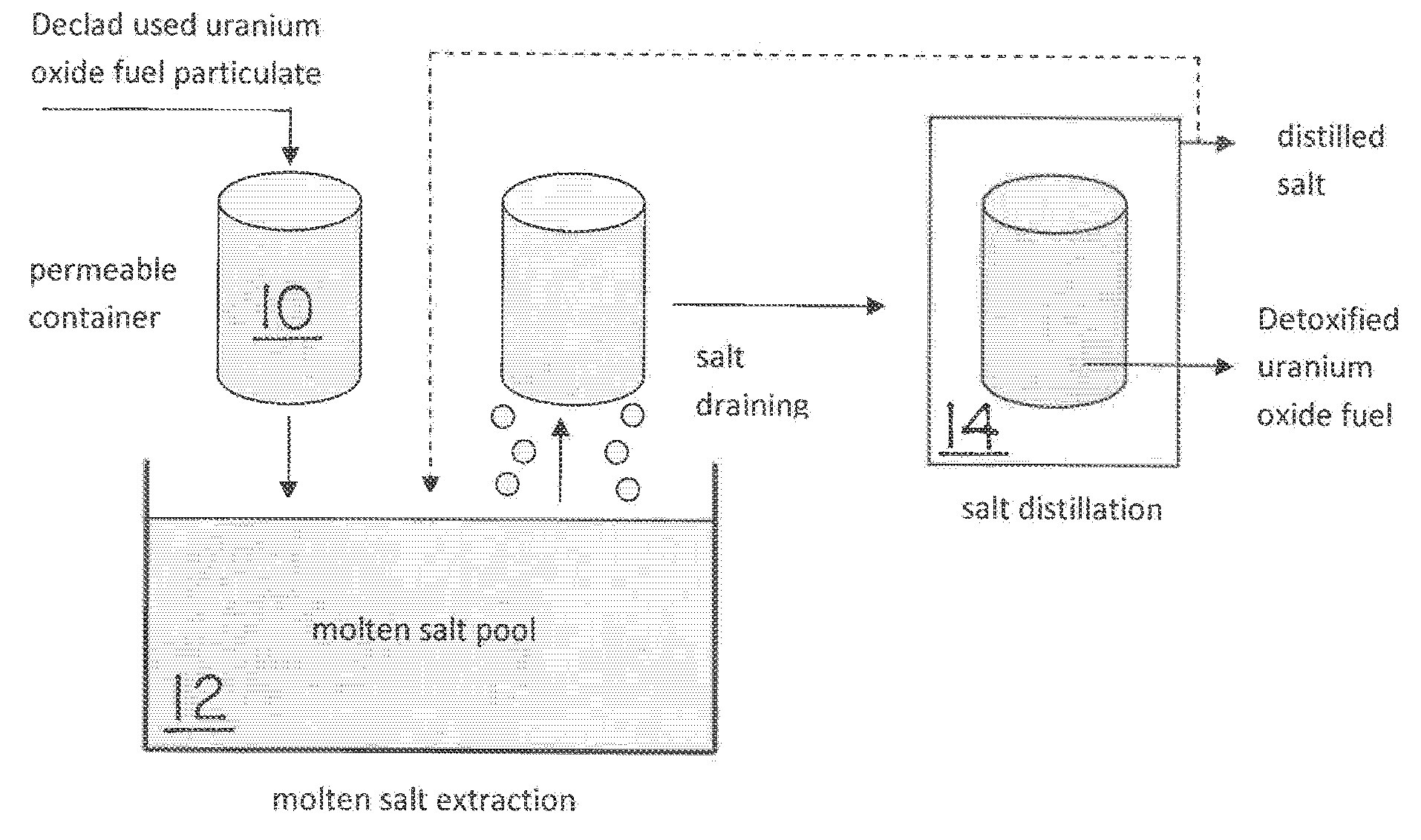
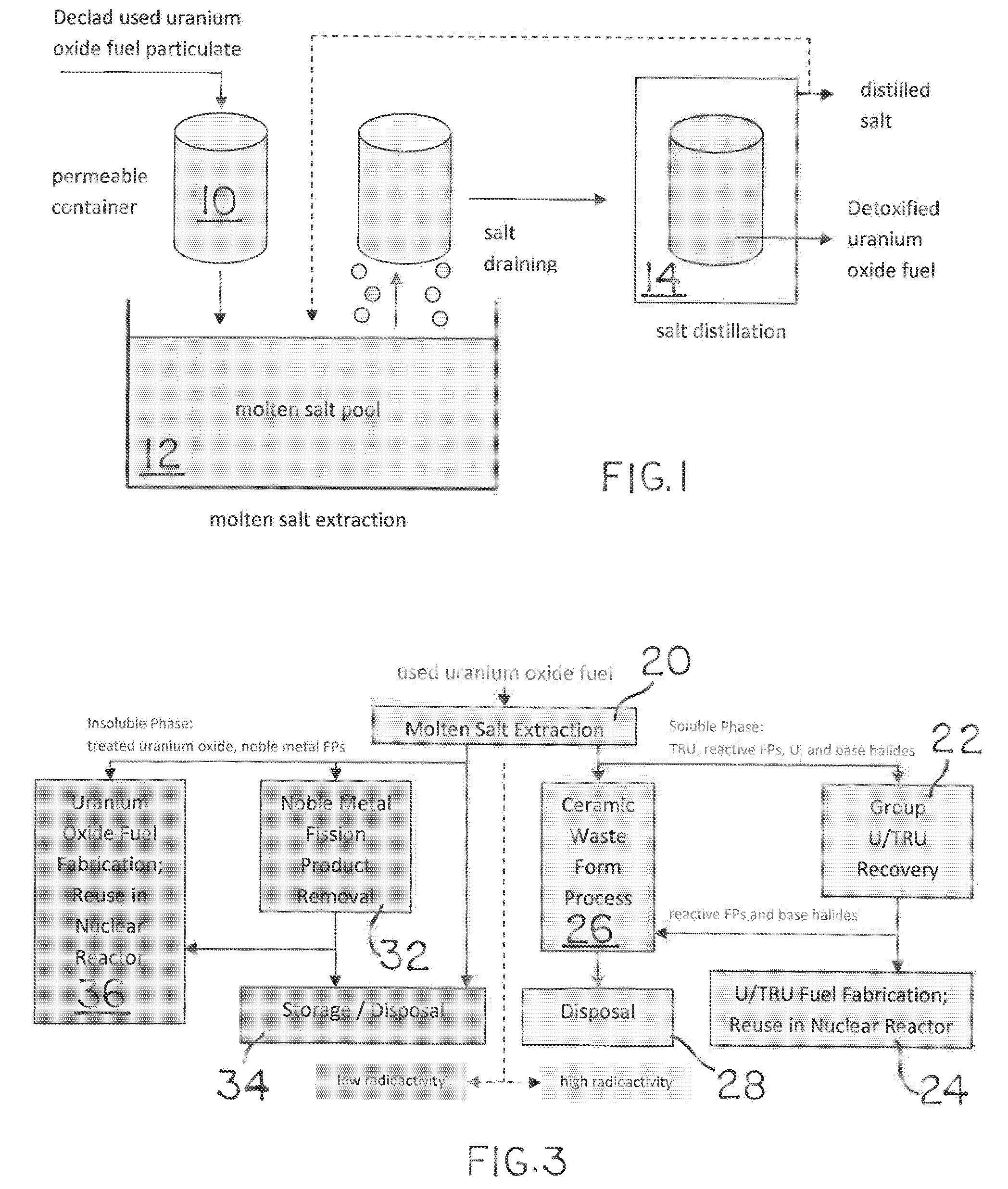
Molten salt extraction of transuranic and reactive fission products from used uranium oxide fuel
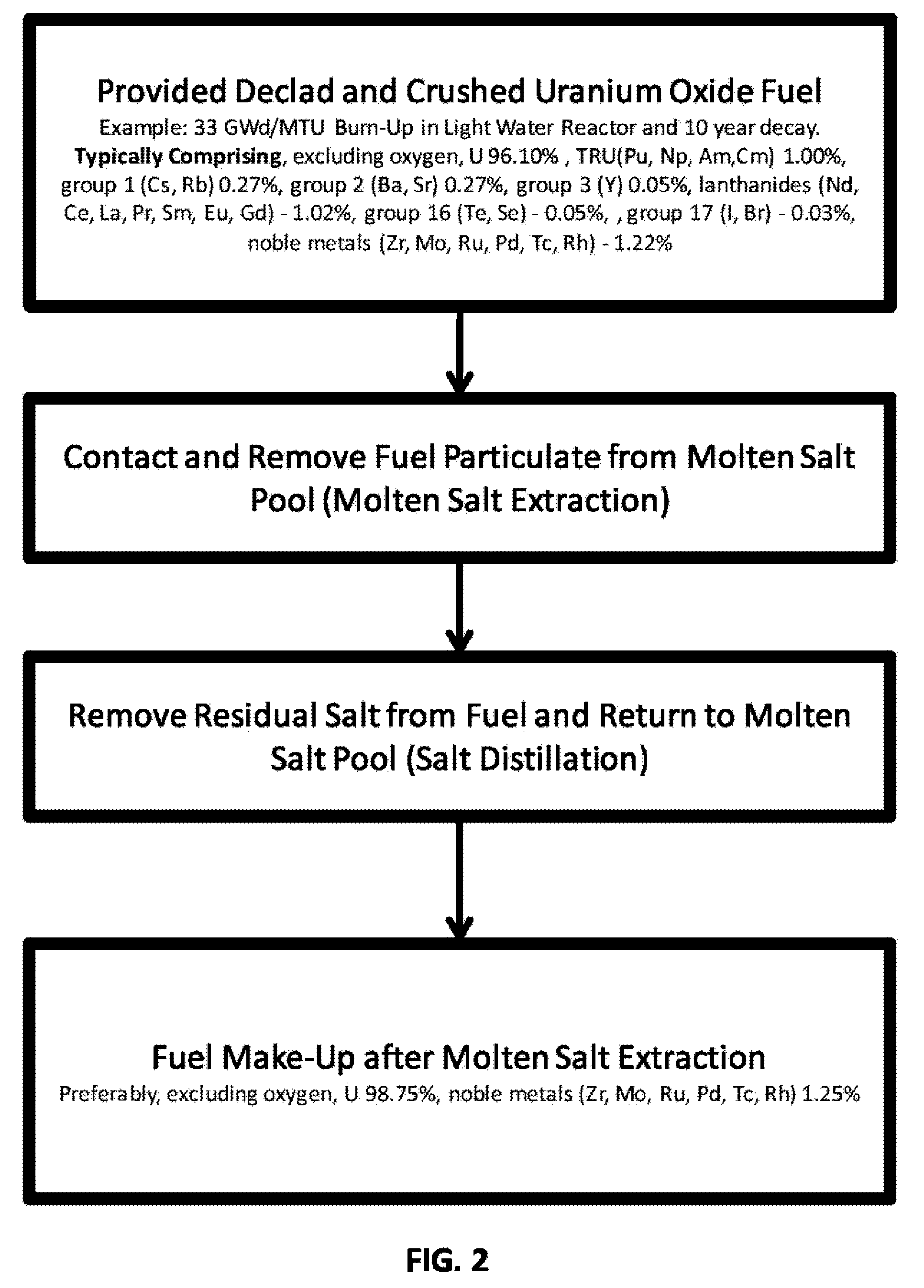
Used uranium oxide fuel is detoxified by extracting transuranic and reactive fission products into molten salt. By contacting declad and crushed used uranium oxide fuel with a molten halide salt containing a minor fraction of the respective uranium trihalide, transuranic and reactive fission products partition from the fuel to the molten salt phase, while uranium oxide and non-reactive, or noble metal, fission products remain in an insoluble solid phase. The salt is then separated from the fuel via draining and distillation. By this method, the bulk of the decay heat, fission poisoning capacity, and radiotoxicity are removed from the used fuel. The remaining radioactivity from the noble metal fission products in the detoxified fuel is primarily limited to soft beta emitters. The extracted transuranic and reactive fission products are amenable to existing technologies for group uranium / transuranic product recovery and fission product immobilization in engineered waste forms.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Novel vanadium halide redox flow battery
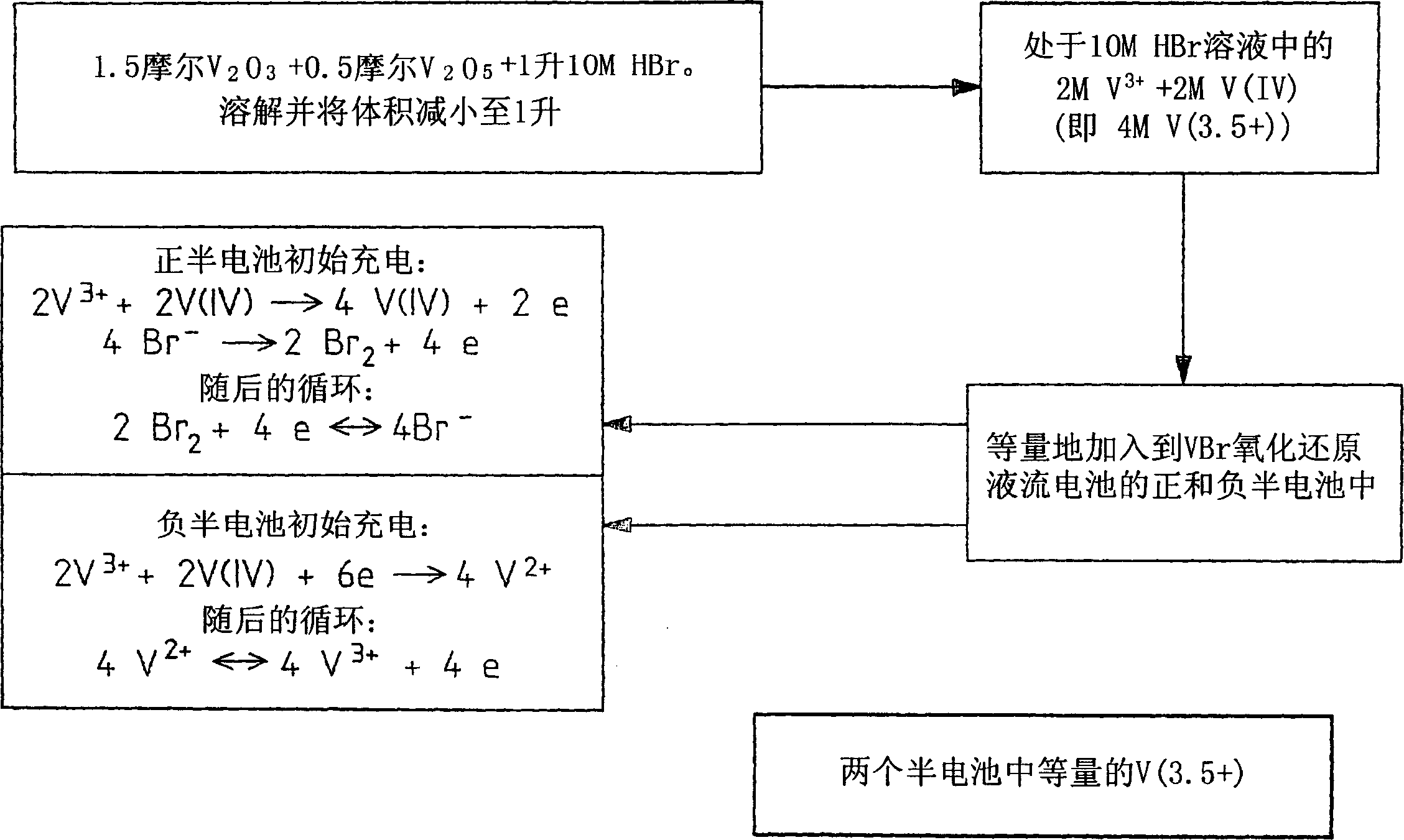
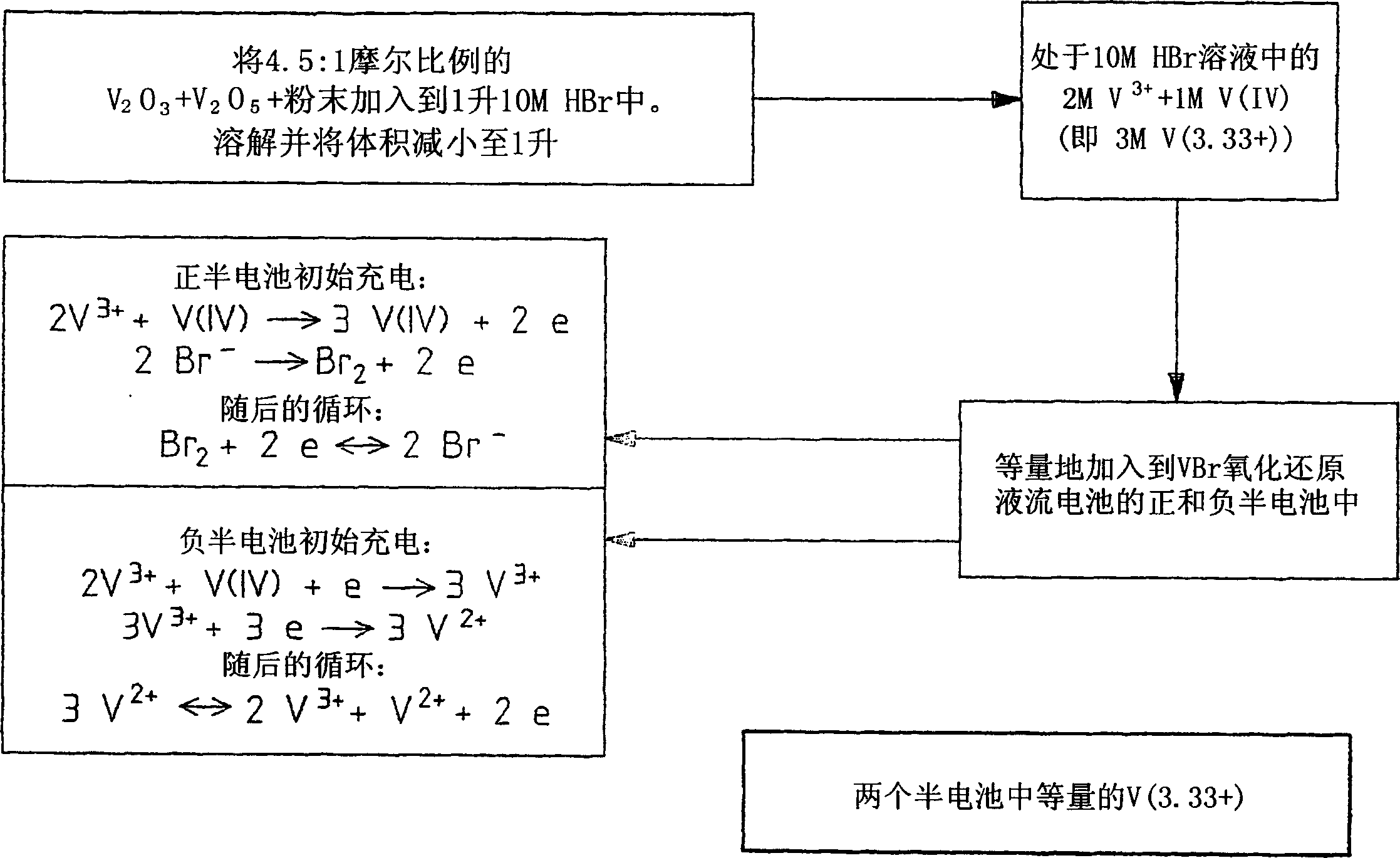
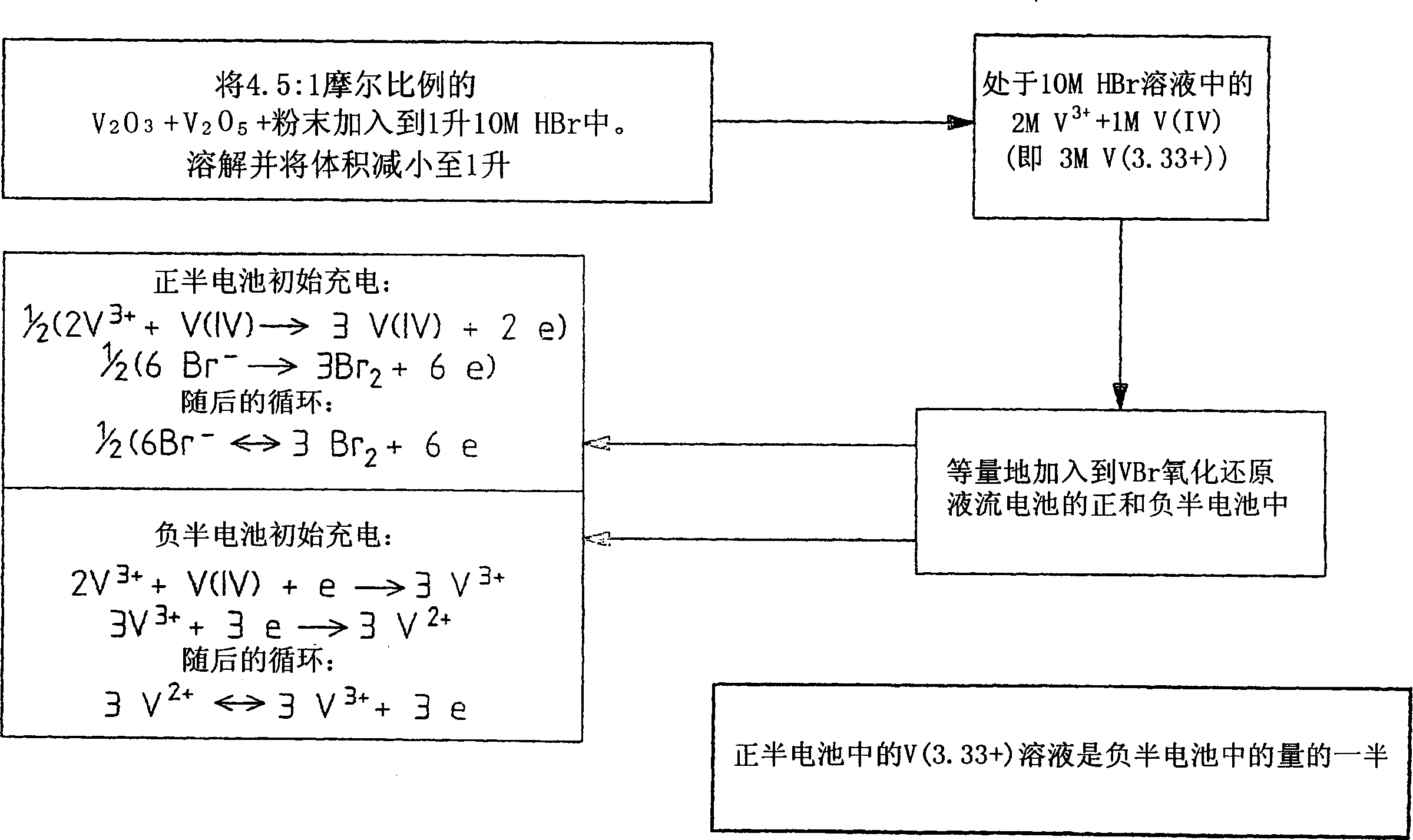
The present invention describes a vanadium halide redox cell prior to charging, a vanadium halide redox cell in a state of charge selected from the group below, and fully charged or partially charged vanadium halide redox cells, wherein the group Consists of zero state of charge and near zero state of charge. A vanadium halide redox cell prior to charging includes a positive half-cell having a positive half-cell solution including a halide electrolyte, a vanadium(III) halide, and a vanadium(IV) halide, and a negative half-cell having a A negative half-cell solution comprising a halide electrolyte, a vanadium(III) halide and a vanadium(IV) halide, wherein the amounts of the vanadium(III) halide, vanadium(IV) halide and halide ions in the positive and negative half-cell solutions are set to such that in the first charging step comprising charging the vanadium halide redox cell prior to charging, it is possible to prepare a vanadium halide redox cell having a state of charge selected from the group consisting of zero state of charge and With a near-zero state-of-charge composition, the vanadium halide redox cell mainly includes vanadium(IV) halide in the positive half-cell solution and V(III) halide in the negative half-cell solution. A vanadium halide redox cell at a state of charge selected from the group consisting of a positive half-cell and a negative half-cell consisting of zero and near-zero states of charge, the positive half-cell having a halide electrolyte comprising: and a positive half-cell solution of a vanadium halide mainly vanadium(IV) halide, a negative half-cell having a negative half-cell solution comprising a halide electrolyte and a vanadium halide mainly of a vanadium(III) halide, wherein the positive half-cell solution The amount of vanadium(IV) halide and the amount of vanadium(III) halide in the negative half-cell solution are set such that the vanadium halide redox cell is at a state of charge selected from the group consisting of zero state of charge and close to zero state of charge composition. A fully charged vanadium halide redox cell consists of a positive half cell with a positive half cell comprising a halide electrolyte, a polyhalide complex, a vanadium(IV) halide, and a vanadium(V) halide solution, the negative half-cell has a negative half-cell solution comprising a halide electrolyte and a vanadium(II) halide, wherein the molar concentration of vanadium(V) and polyhalide complexes: the molar concentration of vanadium(II) halide is approximately stoichiometrically balanced. A partially charged vanadium halide redox cell includes a positive half cell with a positive half cell including a halide electrolyte, a polyhalide complex, a vanadium(IV) halide, and a vanadium(V) halide solution, the negative half-cell has a negative half-cell solution comprising a halide electrolyte, a vanadium (II) halide and a vanadium (III) halide, wherein the number of moles of the polyhalide complex and the vanadium (V) halide: vanadium halide ( The moles of II) are approximately stoichiometrically balanced.
Owner:NEWSOUTH INNOVATIONS PTY LTD
Lithium cell positive material thiofuran polymer and lithium battery preparation method
ActiveCN101007866AHigh capacity densityIncrease energy densityCell electrodesThiophene derivativesElectrolyte
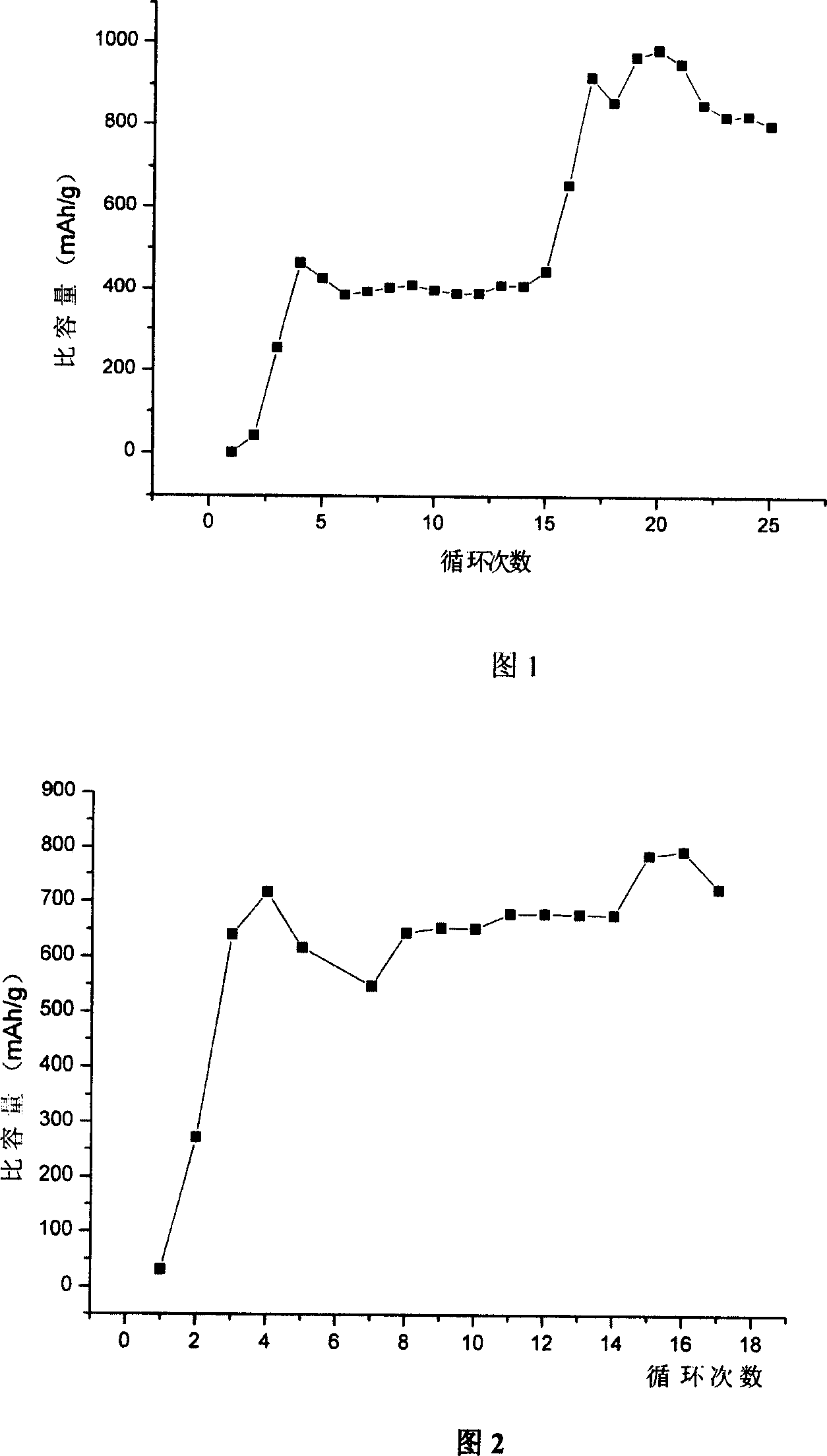
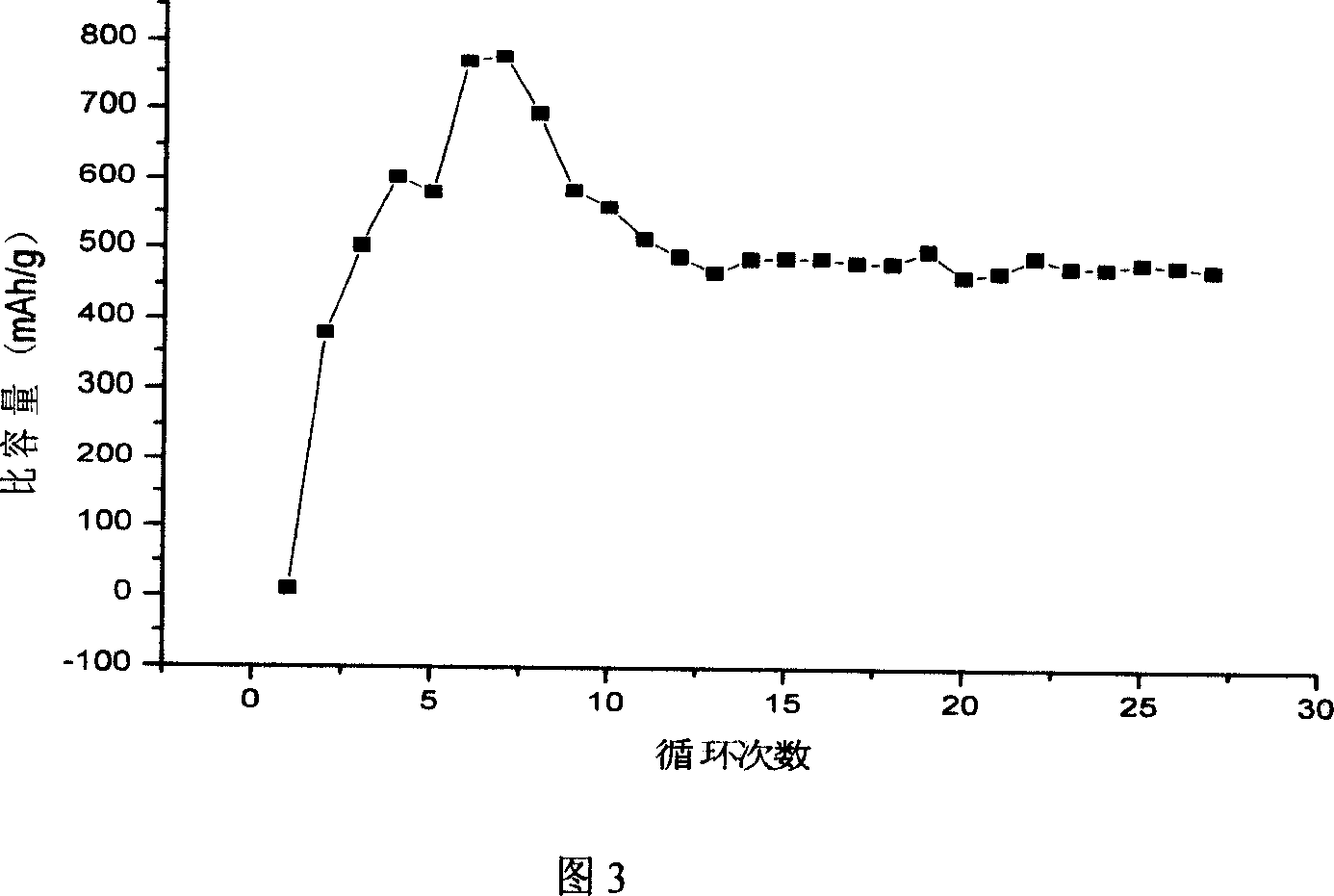
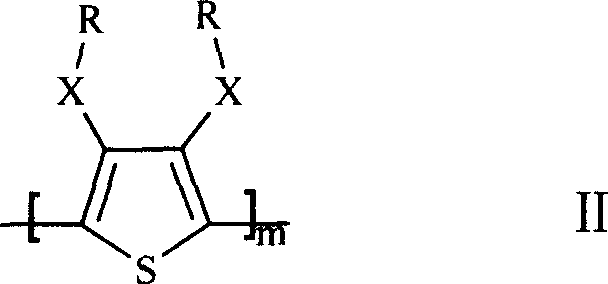
The invention discloses an anode material trihalide polymer for lithium battery and the preparation method for lithium battery. Carrying out oxidative polymerization with thiofuran derivatives such as thiofuran, alkoxy thiofuran, alkyl thiofuran, hydro sulfo-thiofuran, 3, 4- sulfo thiofuran to produce poly thiofuran, poly alkoxy thiofuran, poly alkyl thiofuran, poly hydro sulfo-thiofuran, poly 3, 4- sulfo thiofuran; preparing anode with thiofuran polymer, carbon black and politef, and assembling lithium secondary battery by taking (CxF2x+1SO2)2NLi, CxF2x+1SO3Li, (CxF2x+1SO2)(CyF2y+1)NLi as electrolyte, employing dioxohexacyclic ring, dioxolane and glycol dimethyl ether as solvent, and taking metal lithium slice as negative pole. The discharge specific capacity of assembled lithium secondary battery is 400 Ah / kg to 1200 Ah / kg, and the cycling stability is sound.
Owner:SUZHOU YOULION BATTERY INC
Clean generation of a perfluoroaryl grignard reagent
InactiveUS6129863ASilicon organic compoundsGroup 3/13 element organic compoundsGrignard reagentBoron
Perfluoroaryl Grignard reagents are produced from a hydrocarbyl Grignard reagent and polyhaloaromatic compounds via separate additions of different polyhaloaromatic compounds, such that the conversion of hydrocarbyl Grignard reagent to the desired perfluoroaryl Grignard reagent is essentially complete, and thus the reaction product is free or essentially free of agents that may negatively affect subsequent reactions. The perfluoroaryl Grignard reagents may be further reacted with boron trihalides in order to obtain tris(perfluoroaryl)boranes or tetrakis(perfluoroaryl)borates.
Owner:ALBEMARLE CORP
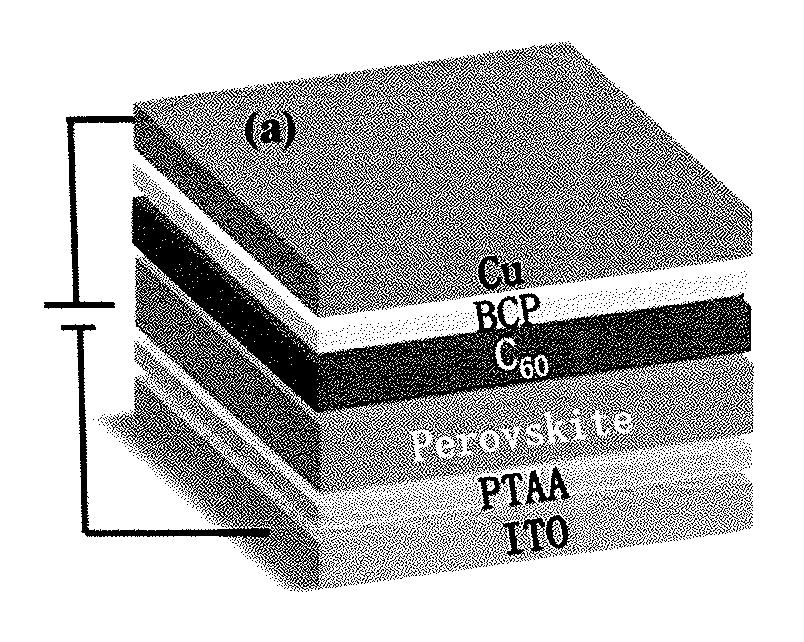
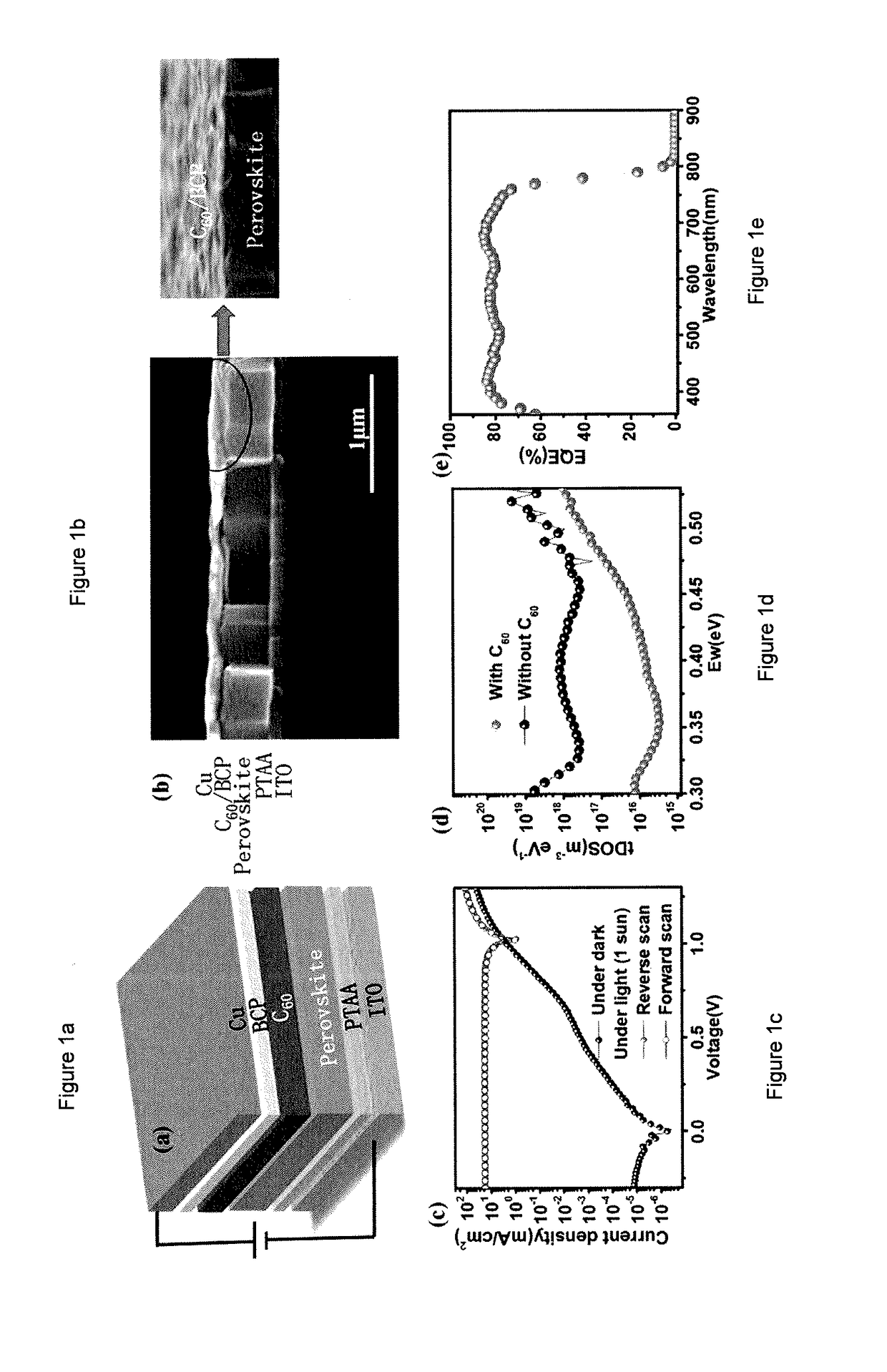
Self-powered ghz solution-processed hybrid perovskite photodetectors
ActiveUS20180075977A1Improve response speedCharge is trappedPolycrystalline material growthFrom normal temperature solutionsPhotovoltaic detectorsPhotodetector
Organic-inorganic hybrid perovskite (OIHP) based photo-responsive devices include an OIHP active layer disposed between a cathode layer and an anode layer, and an electron extraction layer disposed between the cathode layer and the active layer. The electron extraction layer includes a layer of C60 directly disposed on the active layer. The active layer includes an organometal trihalide perovskite layer (e.g., CH3NH3PbI2X, where X includes at least one of Cl, Br, or I).
Owner:NUTECH VENTURES
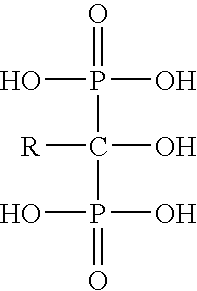
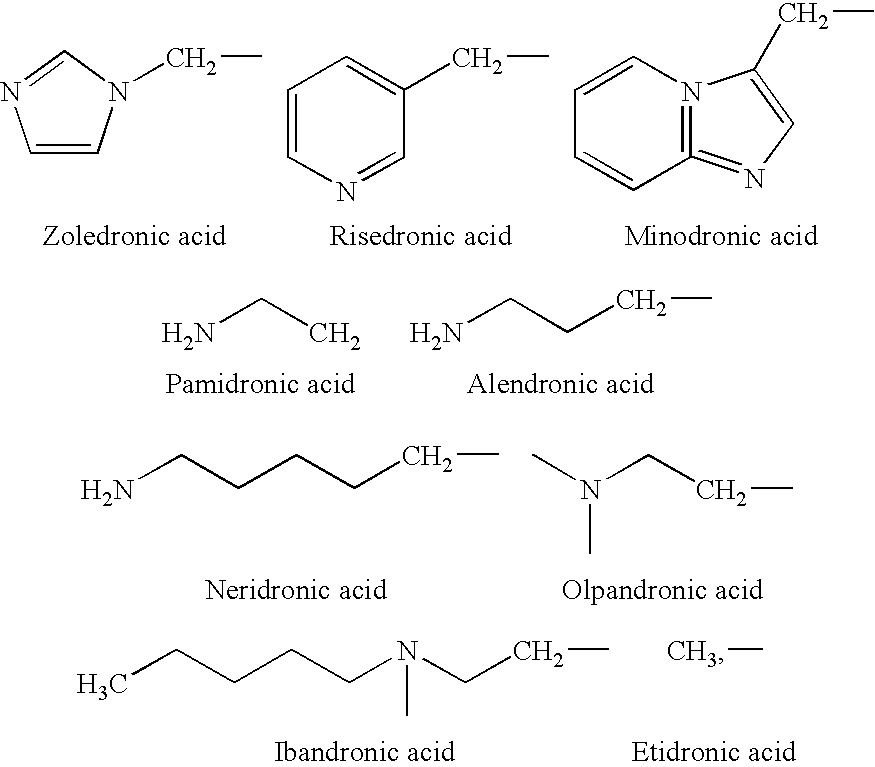
Process for the preparation of biphosphonic acids
An improved process for bisphosphonylation of acids, substituted acids to obtain compounds with the formulausing phosphorus trihalide, phosphorus acid, in presence of phenolic compounds as diluent / solvent.
Owner:AUROBINDO PHARMA LTD
High temperature process for the production of atactic, amorphous, tacky propylene polymers
Atactic or amorphous poly-alpha-olefins containing 100%–65% propylene and optionally up to about 35% ethylene and / or up to about 15% of a C4–C8 alpha-olefin are prepared by polymerizing the monomer(s) at 180–450° F. and at a pressure sufficient to substantially maintain the monomer(s) in the liquid phase. The polymerization is carried out in the presence of catalyst system consisting of (a) a transition metal halide selected from the group consisting of (i) a titanium trihalide and an aluminum alkyl, (ii) a titanium halide on a comminuted magnesium halide support, and (iii) a titanium halide sandwich compound and (b) aluminum alkyl as a co-catalyst. No stereoregulator or only minor amounts of a stereoregulator are used.
Owner:KELLEY JOSEPH M
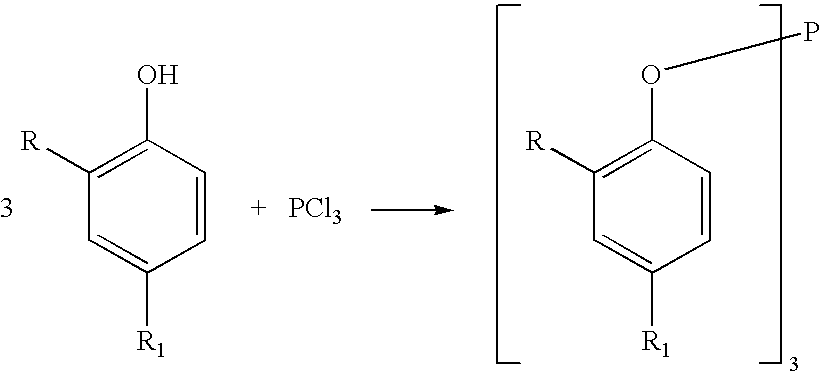
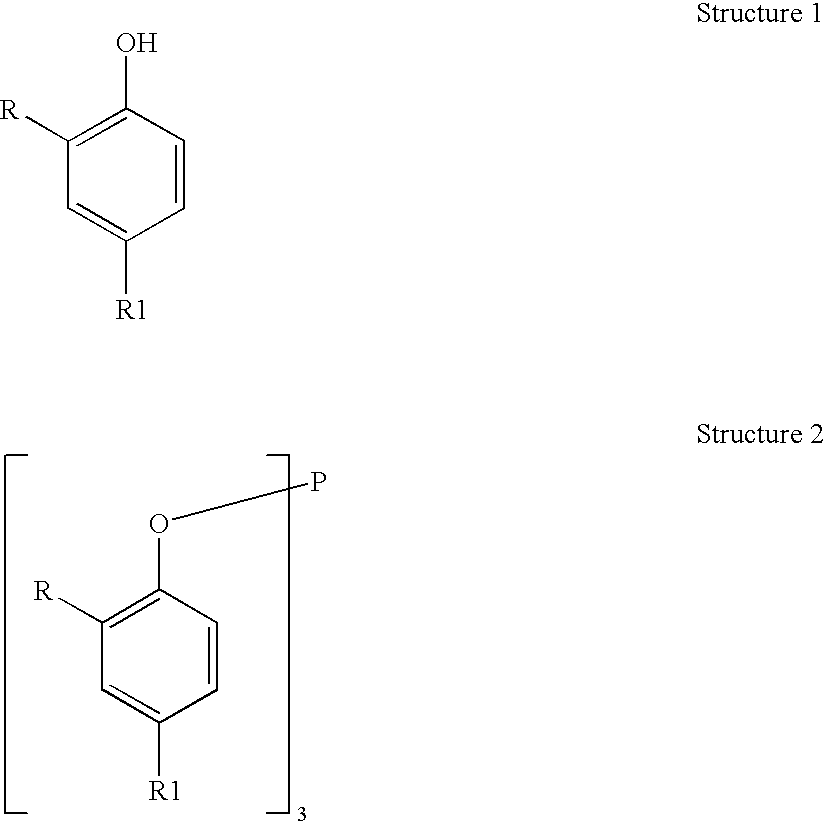
Processes for producing triaryl phospite
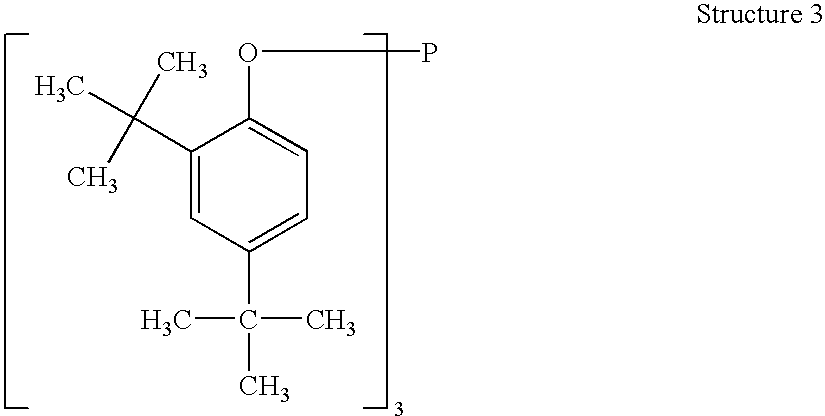
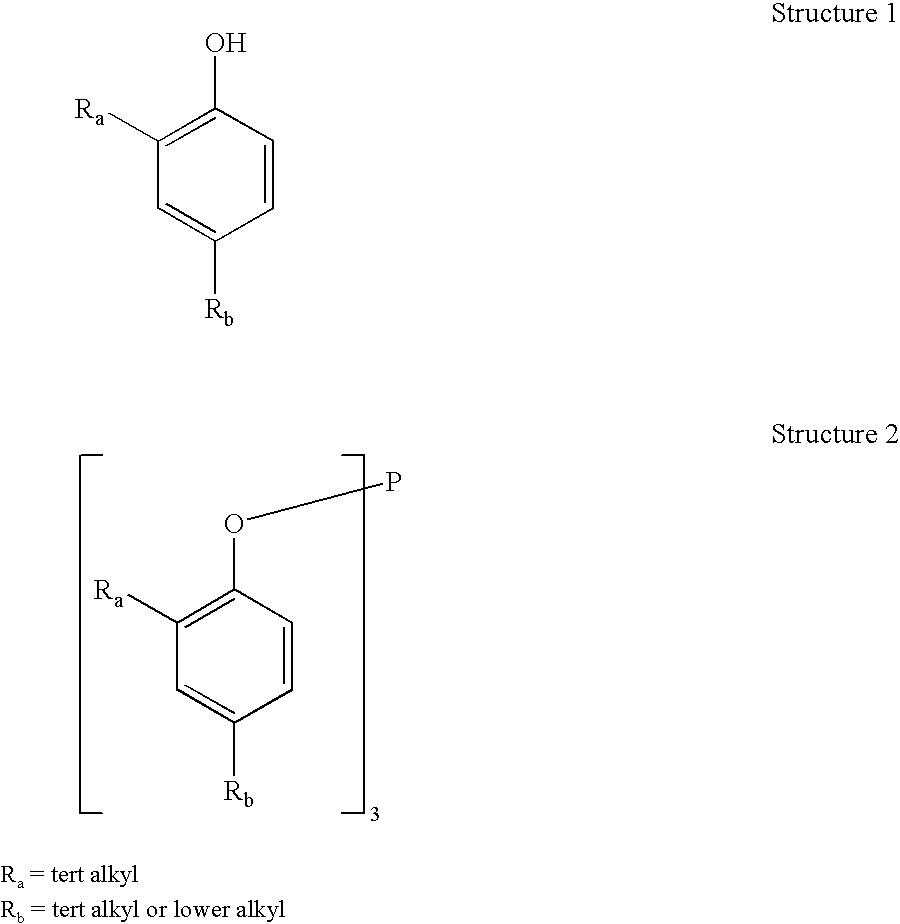
A one pot process for the preparation of sterically hindered triaryl phosphite is provided. The triaryl phosphite is of the formula P(OR)3 and is produced by reacting a hydroxyl-substituted aryl compound of formula ROH with phosphorus trihalide in a presence of a Lewis base, wherein R represents an aryl compound of a formula C6H3R1R2, wherein each R1 and R2 is an organic substituent. The process preferably comprises of mixing a substantial stoichiometric amount of 2,4-dialkyl phenol with phosphorous trihalide in methylene chloride with stoichiometric amount of pyridine. The reaction is preferably carried out at 0-5° C. and takes only 1 hr for the completion, followed by a precipitation out of isopropanol.
Owner:STRIDES INC
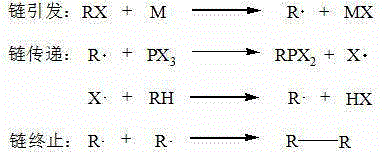
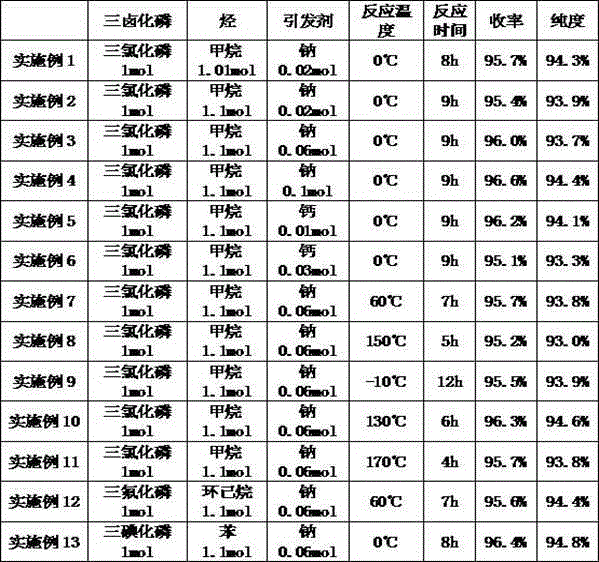
Alkyl phosphorus dihalide preparing method
ActiveCN105330693AThe reaction conditions are mild and controllableEasy to separateGroup 5/15 element organic compoundsSolubilityAlkaline earth metal
The invention relates to an alkyl phosphorus dihalide preparing method and belongs to the technical field of preparation of alkyl phosphorus halide. According to the method, hydrocarbon and phosphorus trihalide are taken as raw materials, alkali metal or alkaline-earth metal is taken as the initiator, and reaction is conducted at -30-170 DEG C. The initiator alkali metal or alkaline-earth metal reacts with the phosphorus trihalide to generate phosphorus dihalide free radicals, and free radical reaction is conducted between the phosphorus dihalide free radicals and the hydrocarbon to generate alkyl phosphorus dihalide, halogen hydride and a small amount of alkali halide or alkaline earth halide. The generated halogen hydride is low in solubility in a reaction system, and therefore the halogen hydride can be separated out in the gas form simply by conducting decompression on products. Compared with existing methods of preparing alkyl phosphorus dihalide with hydrocarbon and phosphorus trihalide as raw materials, the method has the advantage that due to the adoption of the initiator alkali metal or alkaline-earth metal, reaction temperature is reduced remarkably, so that the requirements of reaction for temperature and equipment are reduced, and then cost is reduced greatly.
Owner:SHANDONG WEIFANG RAINBOW CHEM
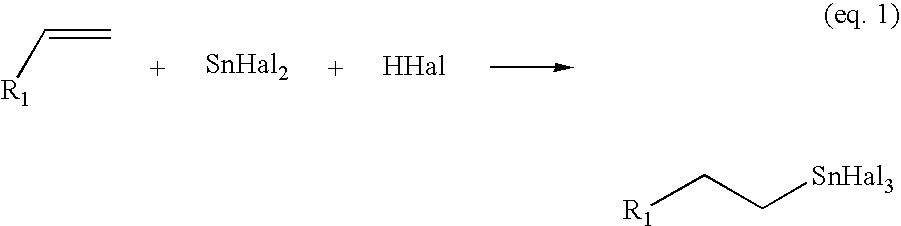
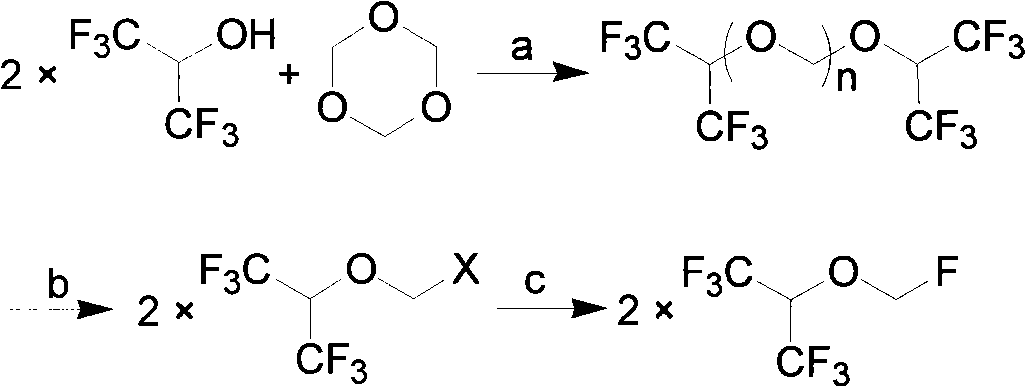
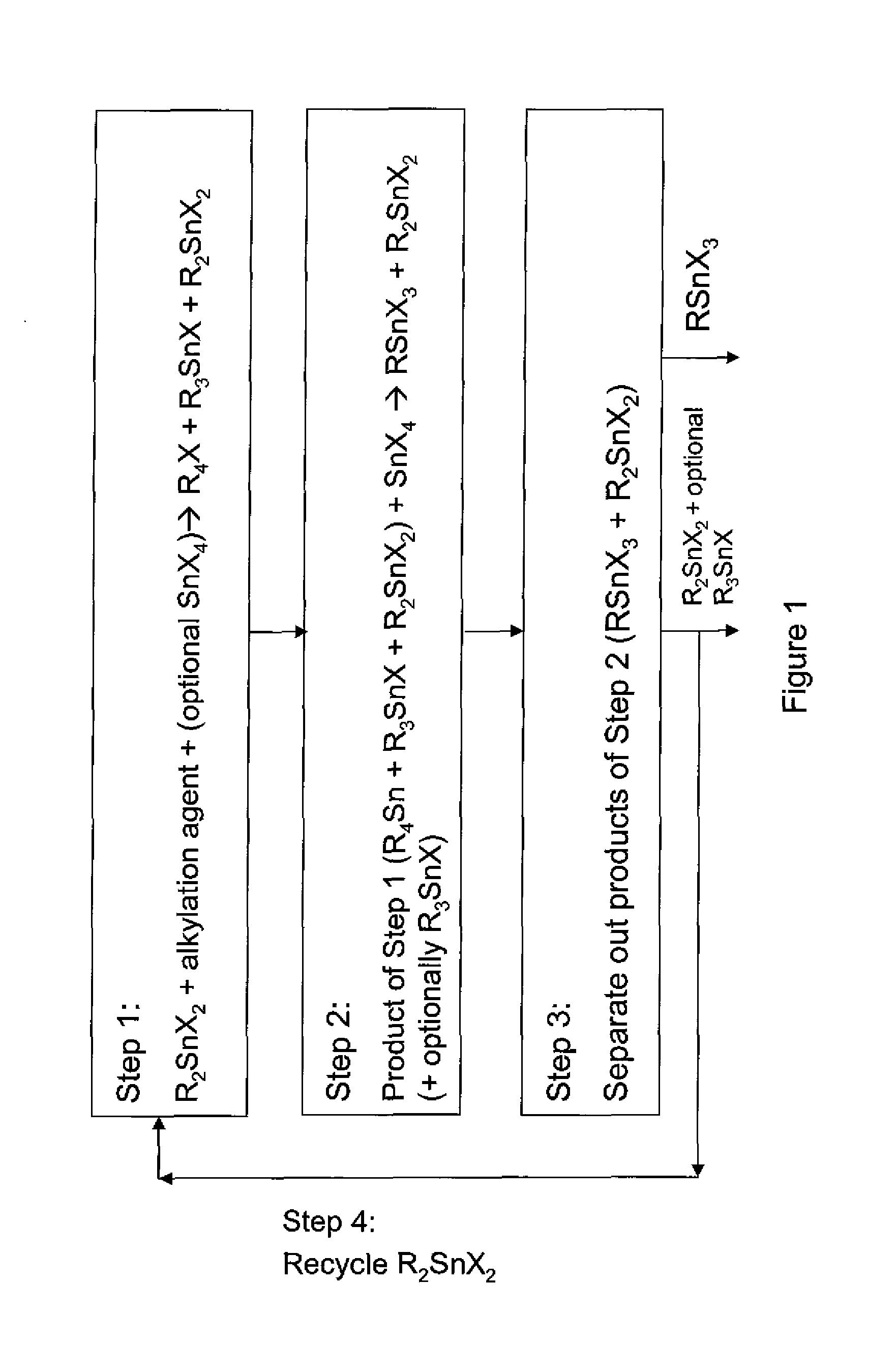
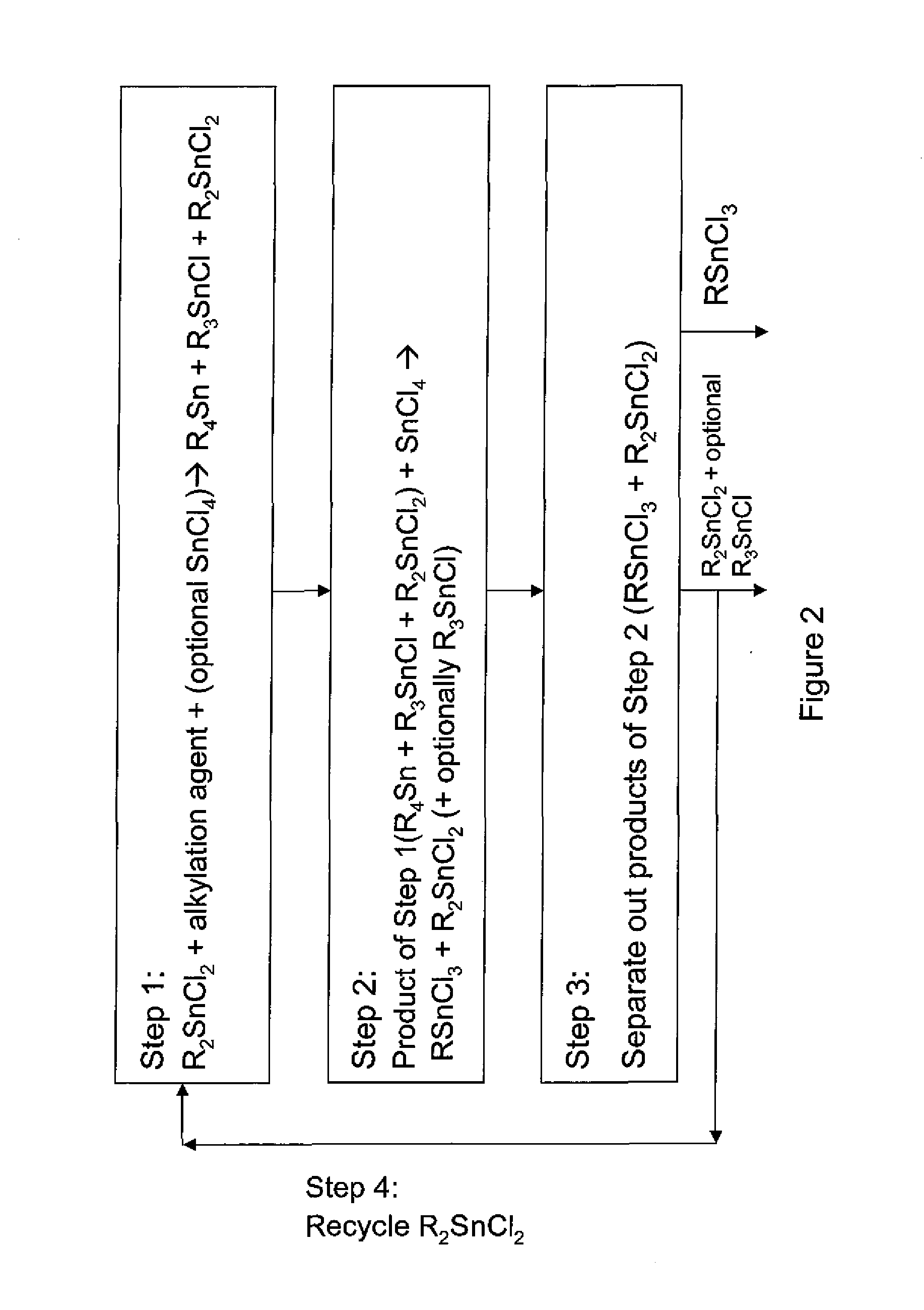
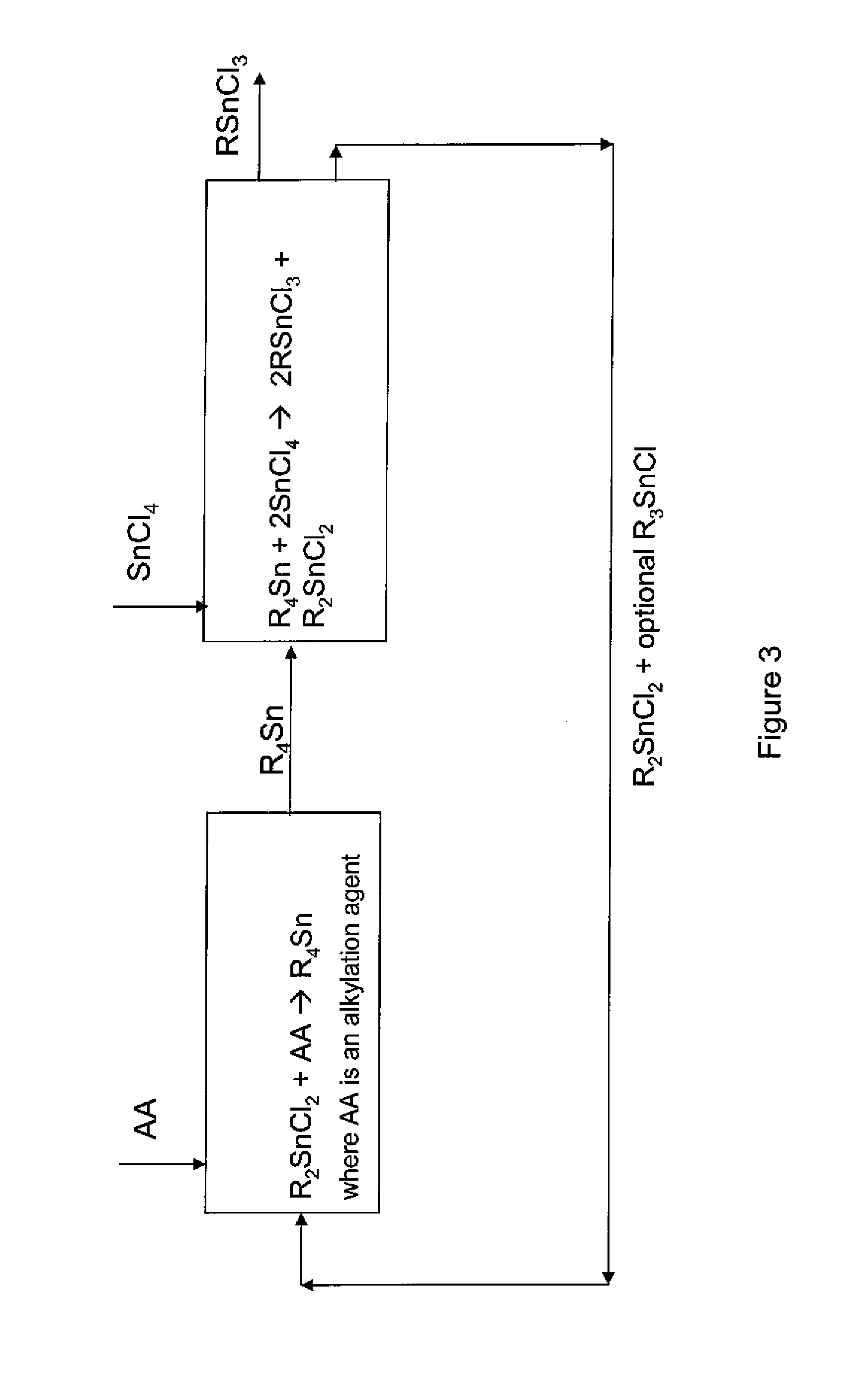
Process for the preparation of monoalkyltin trihalides and dialkyltin dihalides
The present invention relates to a process for the production of monoalkyltin trihalides of the formula RSnHal3, in which R=alkyl or cycloalkyl and Hal=Cl. Br or I. Said process comprises contacting the corresponding alkene or cycloalkene, stannous halide SnHal2, hydrogen halide HHal and optionally Sn metal, in the presence of at least one transition metal-based catalyst, thereafter isolating the monoalkyltin trihalides from the medium. The present invention also relates to a process for the production of dialkyltin dihalides of the formula R2SnHal2 from monoalkyltin trihalides of the formula RsnHal3′. in which R=alkyl or cycloakyl and Hal=Cl, Br or I. Said process comprises contacting monoalkyltin trihalides RsnHal3 and Sn metal, optionally thereafter isolating the dialkyltin dihalides R2SnHal2 from the medium.
Owner:PMC ORGANOMETALLIX INC
Processes for producing triaryl phosphite
A one pot process for the preparation of sterically hindered triaryl phosphite is provided. It is suitable for large scale commercial production with an advantage of having carried out the reaction at 0-5° C. in a shortest time of 1 hr using pyridine as Lewis base to remove HCl formed in the reaction; thus avoiding the usage of scrubber. The triaryl phosphite is of the formula P(OR)3 and is produced by reacting a di alkyl-substituted phenol of formula ROH with phosphorus trihalide in a presence of a Lewis base, wherein R represents an aryl compound of a formula C6H3RaRb, wherein Ra is tertiary alkyl, Rb is lower alkyl or tertiary alkyl. The process preferably comprises of mixing a stoichiometric amount of 2,4-dialkyl phenol with phosphorous trihalide in methylene chloride with different stoichiometric amounts of pyridine such as the molar equivalent, 10 mol %, 20 mol % and 50 mol % more than the amount of 2,4 dialkyl phenol. The reaction is preferably carried out at 0-5° C. and takes only 1 hr for the completion, followed by a precipitation out of isopropanol.
Owner:STRIDES INC
Process for the preparation of biphosphonic acids
InactiveUS20070173645A1Easy to handleSimple processPhosphorus organic compoundsDiluentPhosphorus acid
An improved process for bisphosphonylation of acids, substituted acids to obtain compounds with the formulausing phosphorus trihalide, phosphorus acid, in presence of phenolic compounds as diluent / solvent.
Owner:AUROBINDO PHARMA LTD
Clean generation of a fluoroaryl grignard reagent
Fluoroaryl Grignard reagents are produced from a hydrocarbyl Grignard reagent and fluoroaromatic compounds via separate additions of different fluoroaromatic compounds, such that the conversion of hydrocarbyl Grignard reagent to the desired fluoroaryl Grignard reagent is essentially complete, and thus the reaction product is free or essentially free of agents that may negatively affect subsequent reactions. The fluoroaryl Grignard reagents may be further reacted with boron trihalides in order to obtain tris(fluoroaryl)boranes or tetrakis(fluoroaryl)borates.
Owner:ALBEMARLE CORP
Dehydroxylation and purification of calcium fluoride materials using a halogen containing plasma
InactiveUS20050263064A1Polycrystalline material growthFluoride preparationSingle crystalXenon difluoride
The invention is directed to a process of purifying metal fluoride materials used to make metal fluoride single crystals suitable for making optical elements used in the transmission of wavelengths below 200 nm, and in particular to a process of purifying such materials by the use of a halogen containing plasma to convert metal oxygenates contaminating the feedstocks used in the preparation of the crystals to metal fluorides. The invention also is directed to a process of growing a metal fluoride single crystal using a crystal growth furnace to carry out the foregoing purification procedure followed by the steps of melting the purified material and cooling it using s selected time and temperature cycle to from a metal fluoride single crystal. The plasmas used in practicing the invention can be derived from a variety of halogenated materials including, for example, fluorocarbons, chlorocarbons, boron trihalides, chlorine, fluorine, xenon difluoride and other gaseous or easily volatilized halogenated substances known in the art.
Owner:CORNING INC
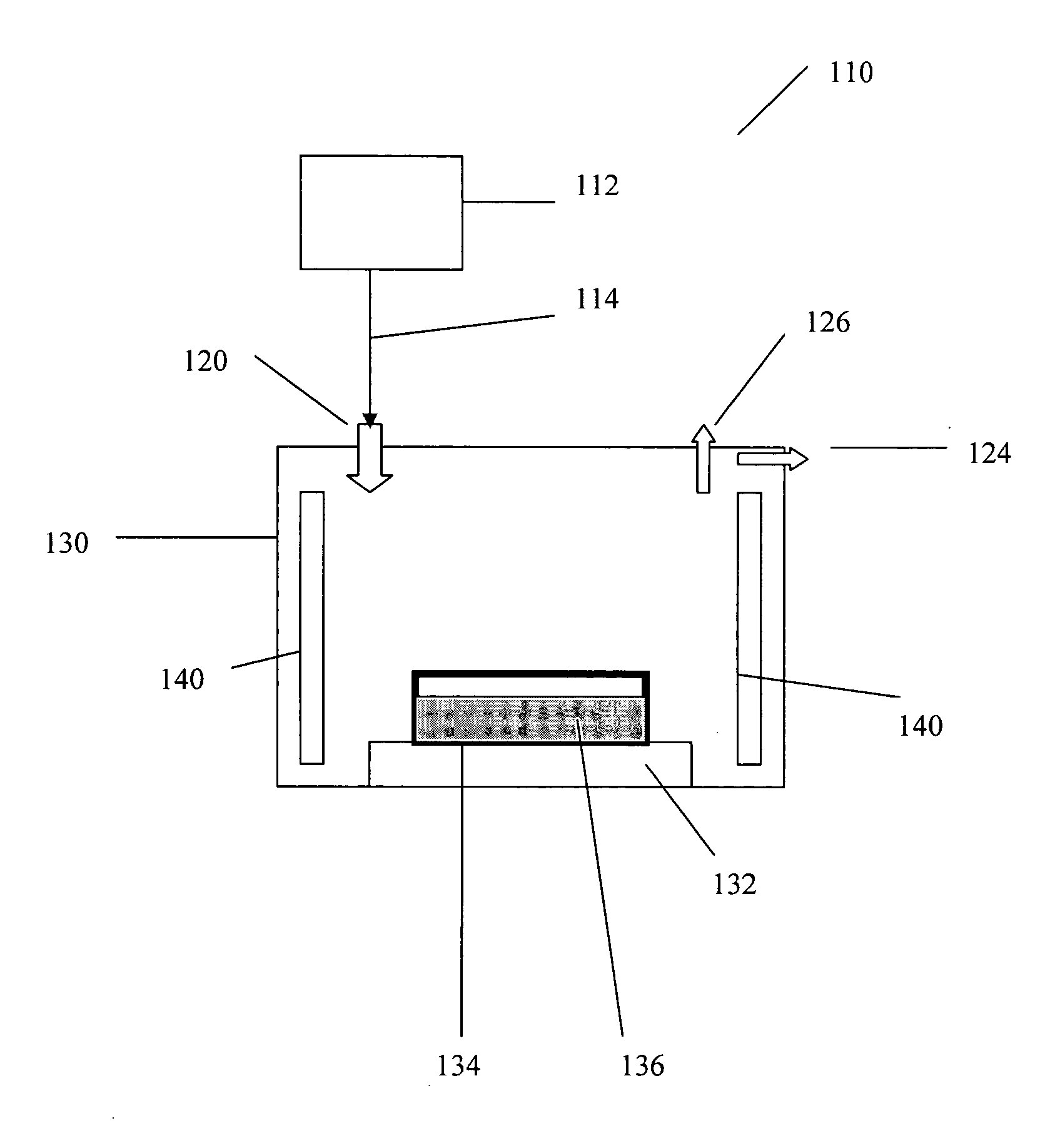
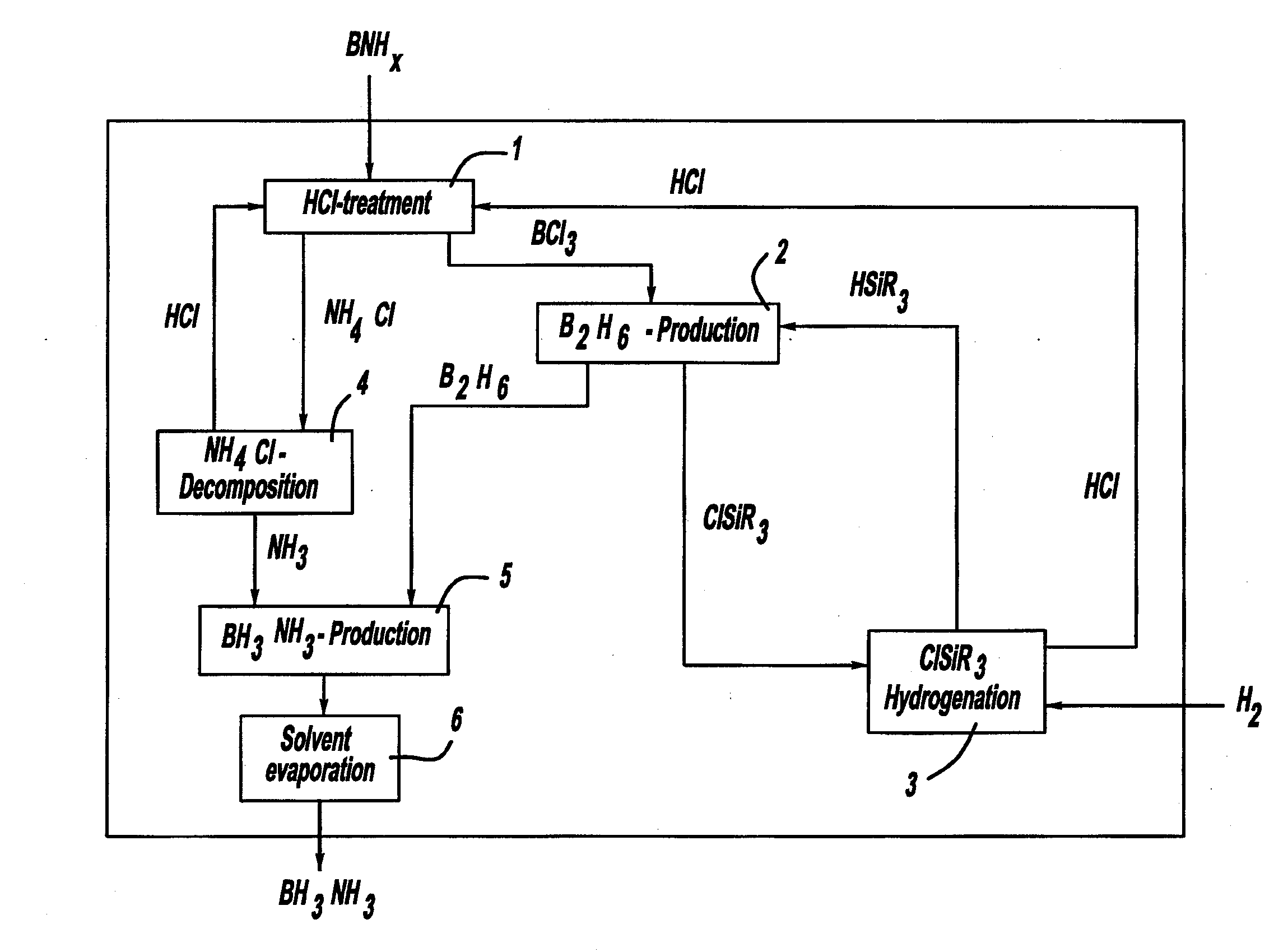
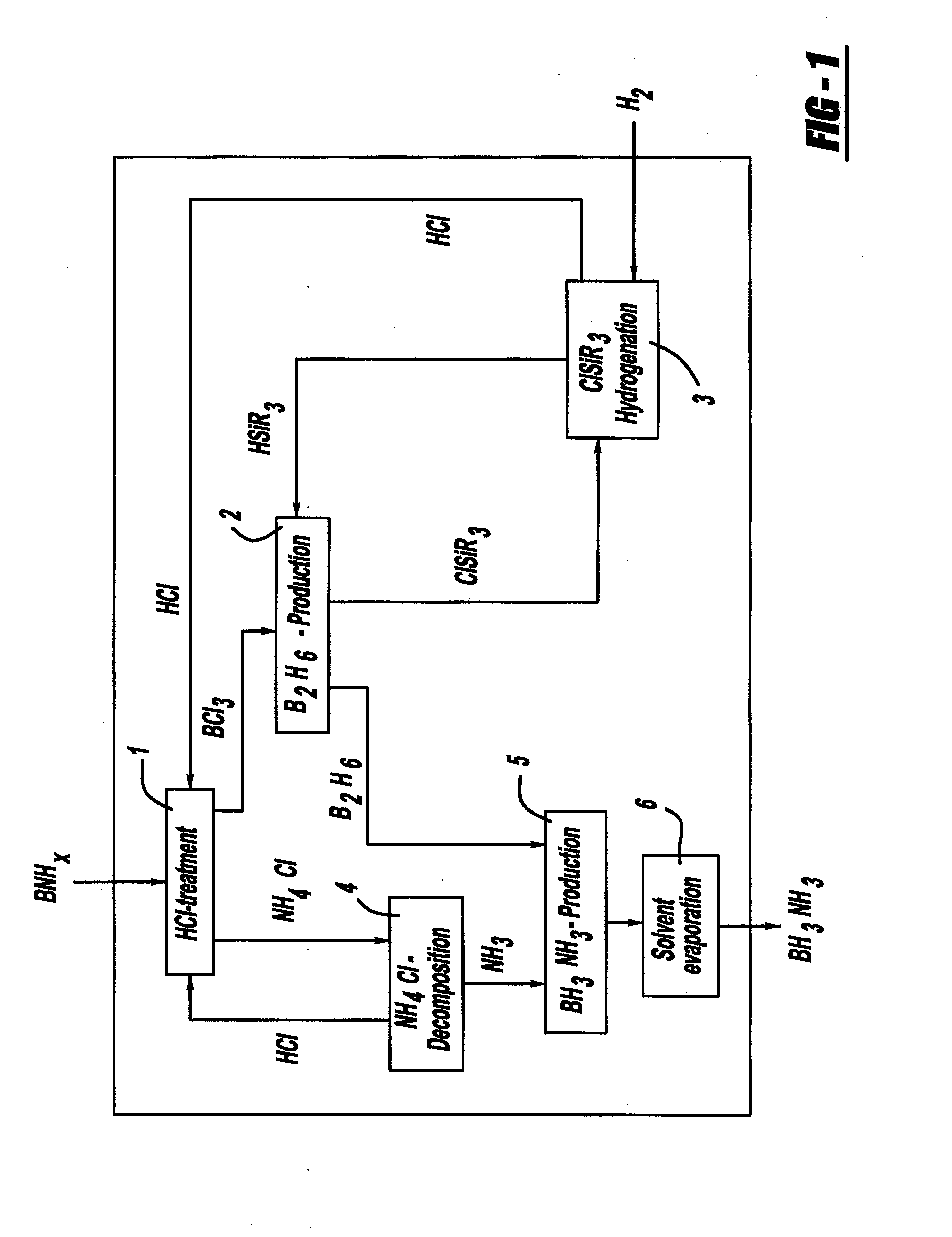
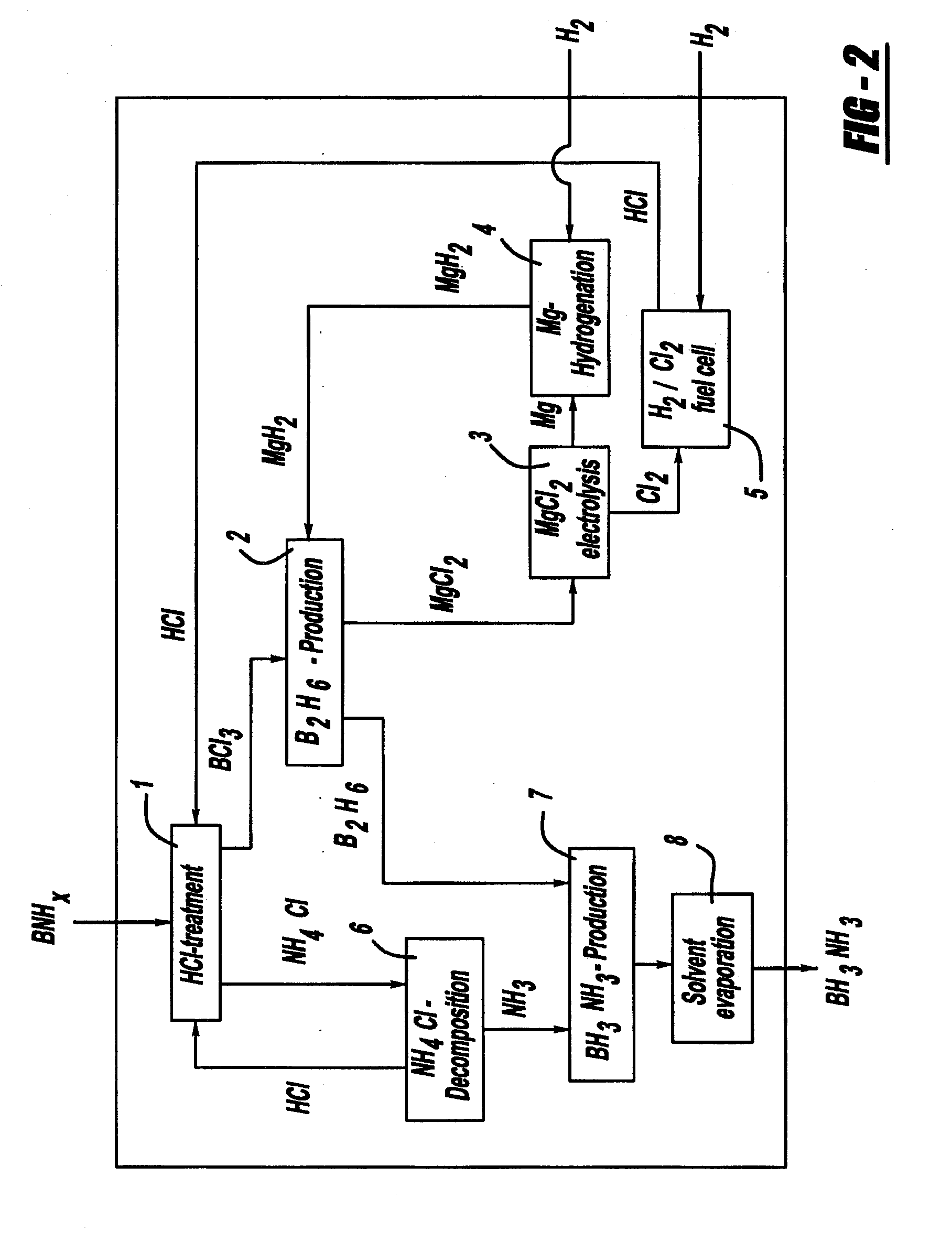
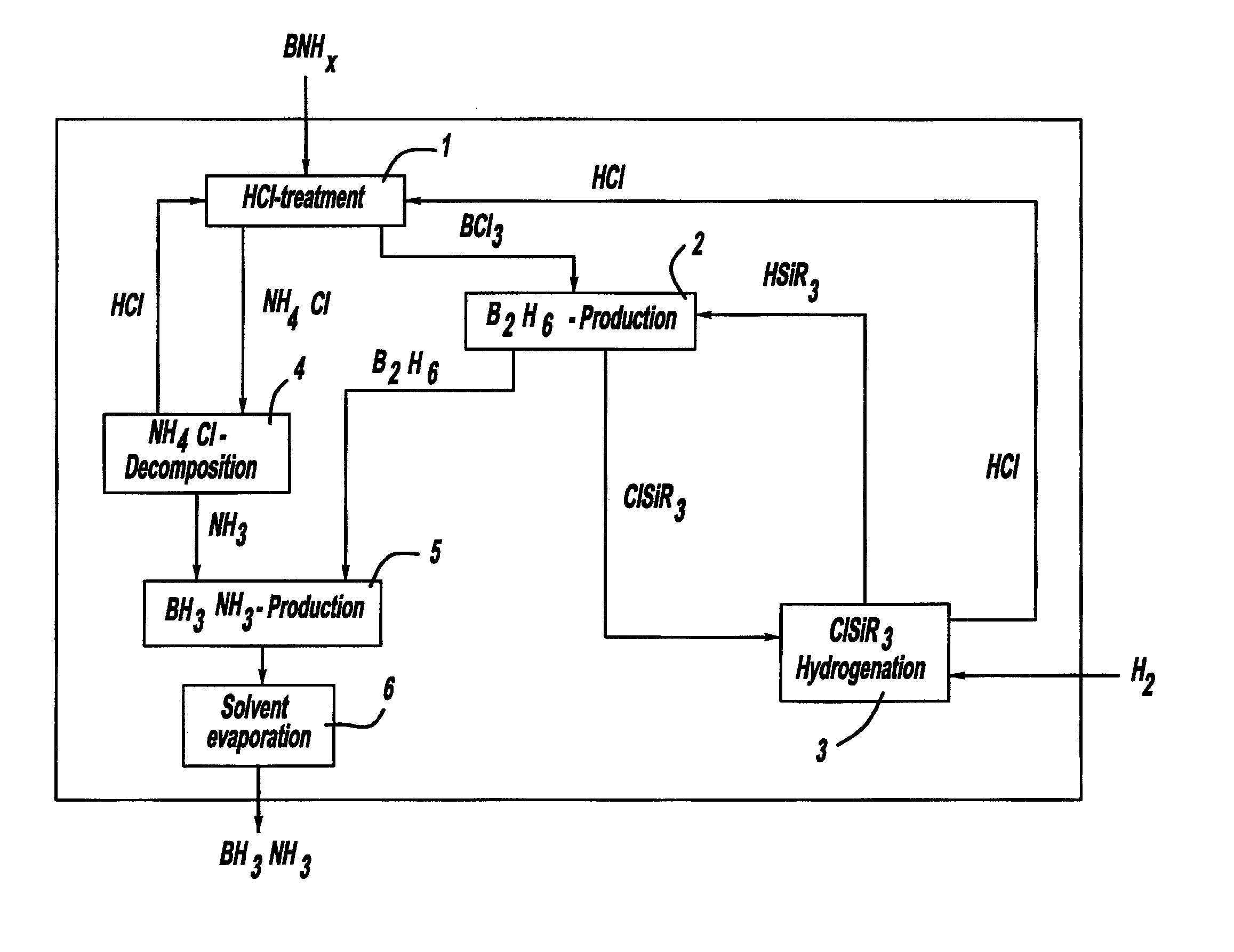
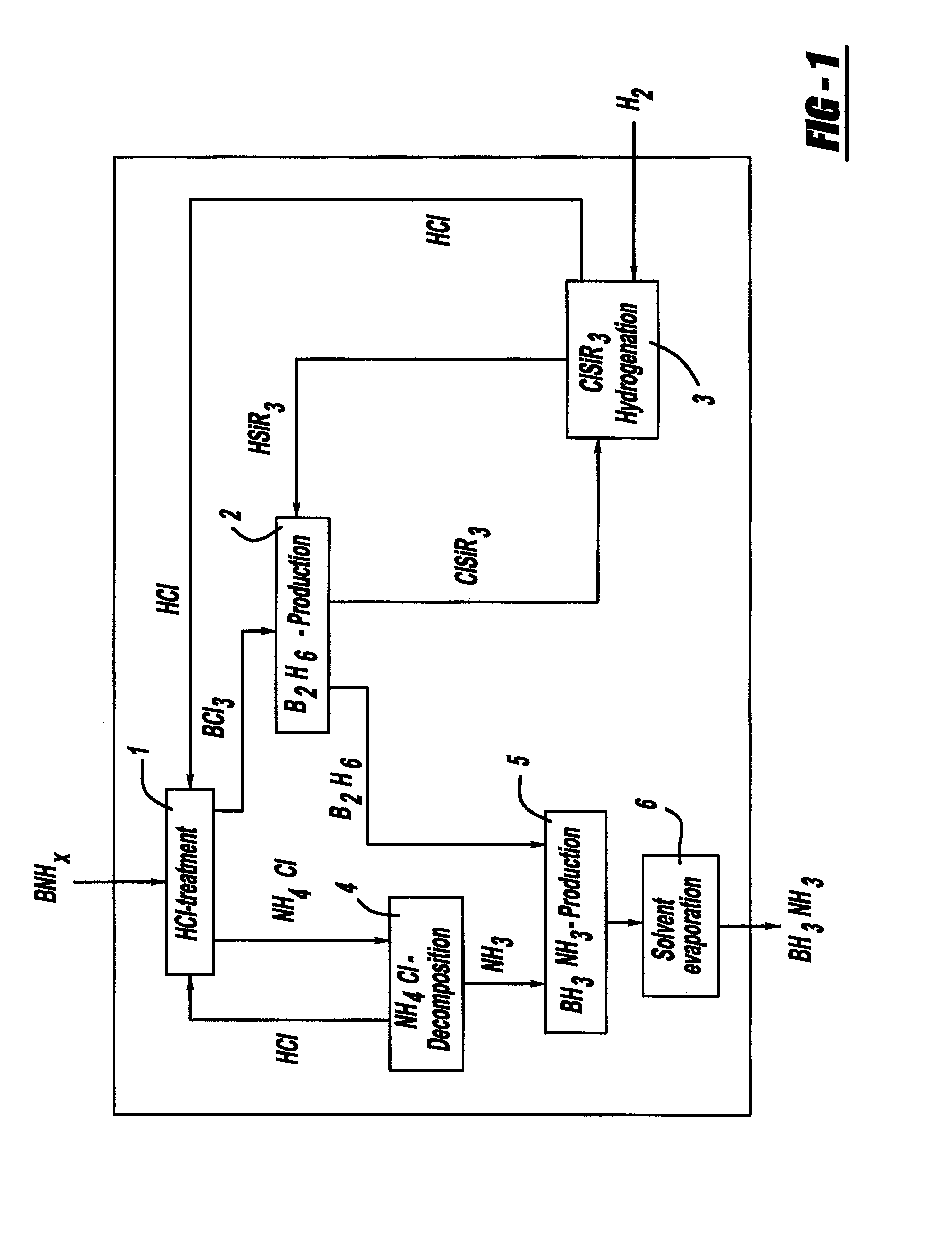
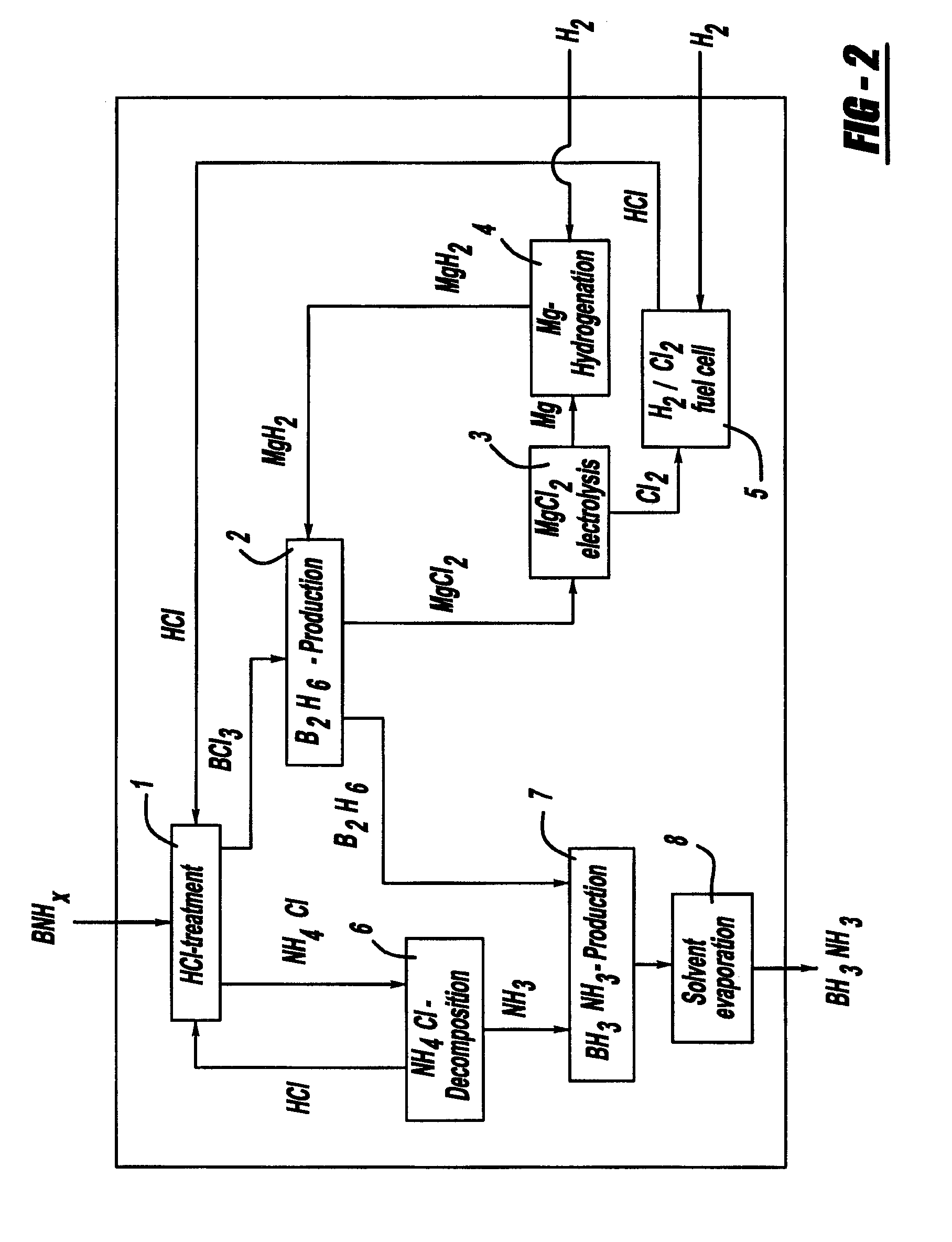
Procedure for the hydrogenation of bnh-containing compounds
InactiveUS20080193356A1Improve energy efficiencyNitrogen compoundsOther chemical processesHydrogen halideCompound a
A process for producing borazane from boron-nitrogen and boron-nitrogen-hydrogen containing BNH-waste products. The process includes reacting the BNH-waste products with a hydrogen halide, having the formula HX, wherein X is selected from the group consisting of F, Cl, Br, I, and combinations thereof, to form any of the following: a boron trihalide, having the formula BX3, an ammonium halide, having the formula NH4X, and hydrogen. The boron trihalide is then reacted with the hydrogen to form diborane, having the formula B2H6, and hydrogen halide. The ammonium halide is then converted to ammonia, having the formula NH3, and hydrogen halide. The diborane is then reacted with the ammonia to form borazane, having the formula BH3NH3.
Owner:GM GLOBAL TECH OPERATIONS LLC +1
Process for synthesizing Sevoflurane
ActiveCN101314560AReduce consumptionEasy to storeOrganic compound preparationEther preparation by organic exchangeFluorideParaformaldehyde
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation of tri-alkyl gallium or tri-alkyl indium compounds
ActiveUS8513447B1Group 1/11 element organic compoundsGroup 3/13 element organic compoundsIndiumMolten salt
Trialkyl metal compounds, such as trialkyl gallium and indium compounds, are prepared in high yield and high purity by the addition of a trialkyl aluminum compound to a mixture prepared by adding a metal trihalide, e.g., GaCl3 or InCl3, and a halide salt of a monovalent metal to an ionic liquid such as a molten salt of the formula M[AlRnX(4-n)] wherein M is a monovalent metal such as Li, Na, K or Cs, R is an alkyl group X is a halide and n is a number from 1 to 3, typically at temperatures of from 75 to 160° C.
Owner:KE MATERIALS LLC
Process for preparing monoalkyltin trihalides and dialkyltin dihalides
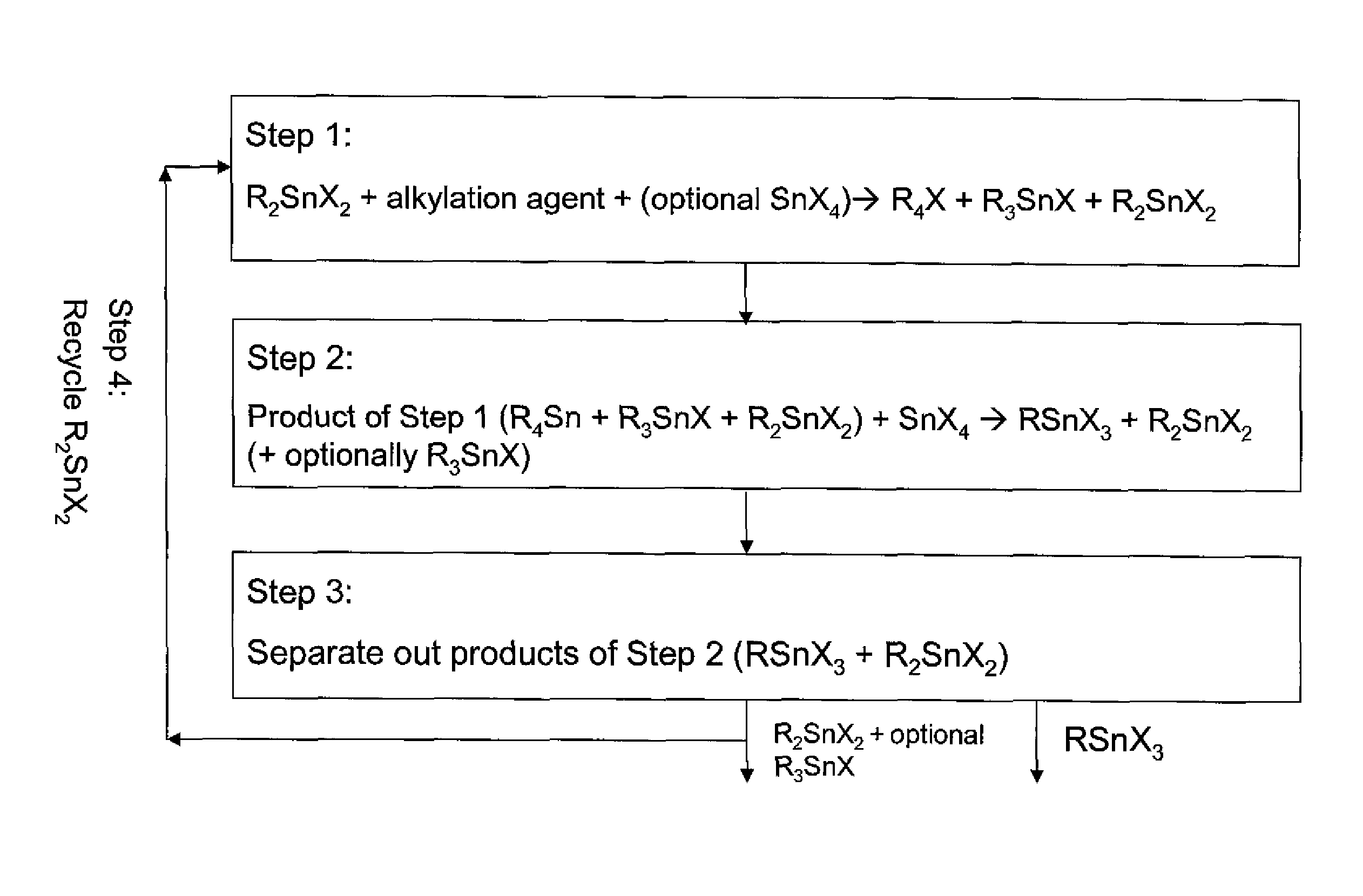
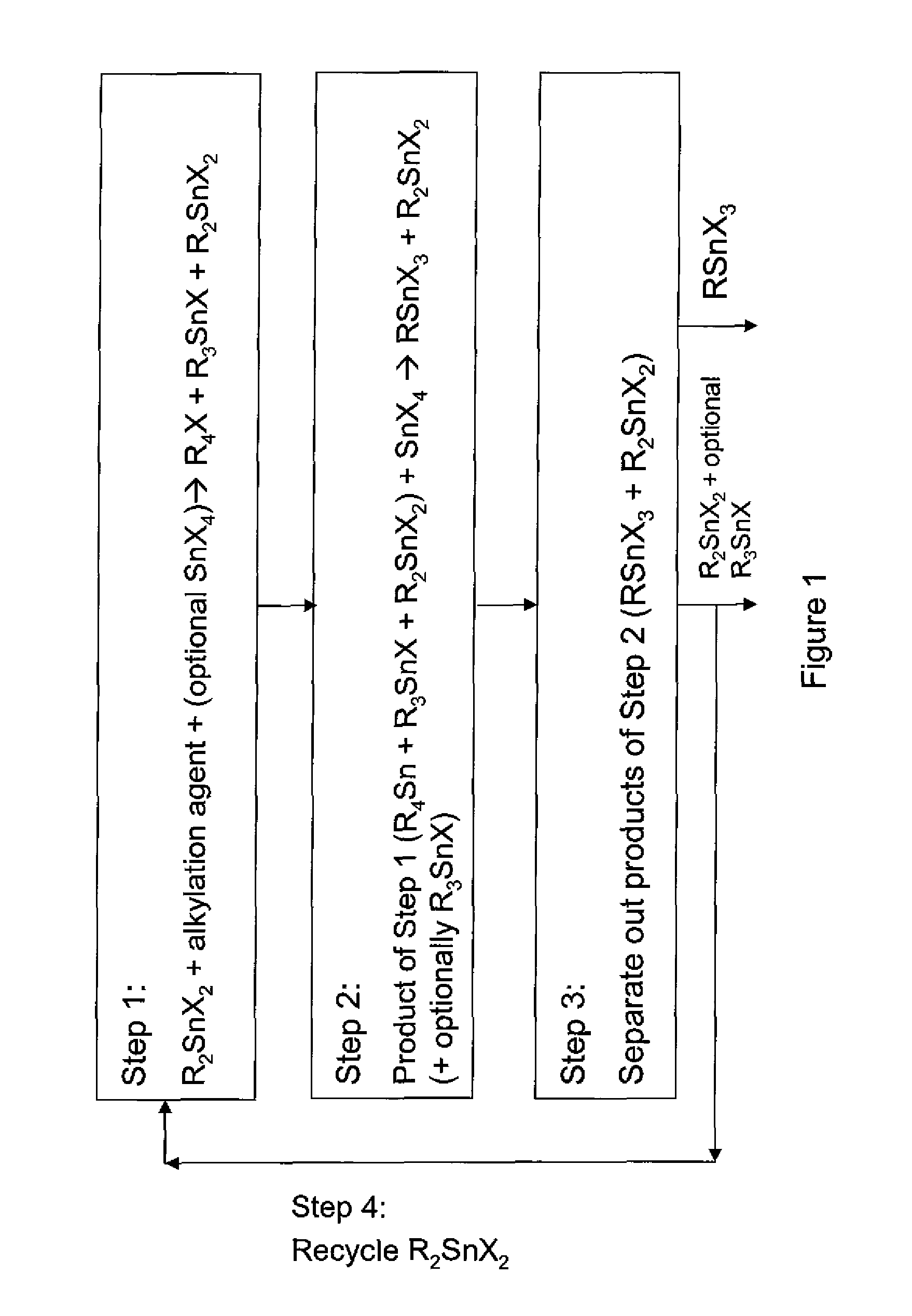
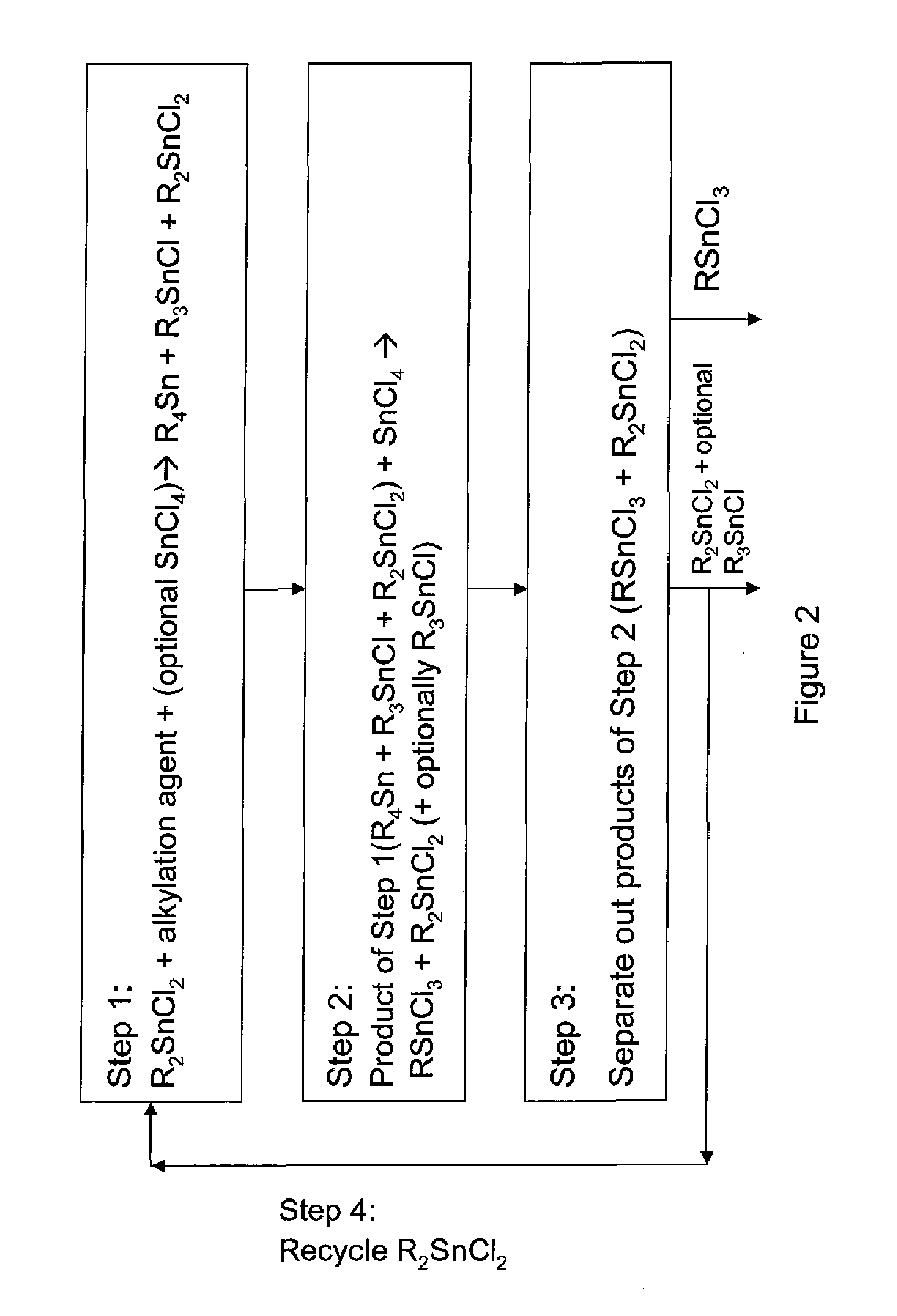
The invention provides a process for producing monoalkyltin trihalide or a mixture of monoalkyltin trihalide and dialkyltin dihalide by: (a) contacting dialkyltin dihalide with an alkylation agent and, optionally, tin tetrahalide, to form a tetraalkyltin mixture comprising tetraalkyltin, trialkyltin halide, and dialkyltin dihalide; (b) reacting the tetraalkyltin mixture with tin tetrahalide to form a monoalkyltin trihalide mixture comprising monoalkyltin trihalide, dialkyltin dihalide and optionally triaklyltin halide; (c) processing the monoalkyltin trihalide mixture to separately recover the monoalkyltin trihalide and a dialkyltin dihalide stream optionally containing trialkyltin halide; and (d) recycling at least a portion of the dialkyltin dihalide stream recovered in step (c) to the contacting step (a).
Owner:PMC ORGANOMETALLIX INC
Borazine aryne resin and preparation method thereof
InactiveCN103524746AImprove heat resistanceLow dielectric constantGroup 3/13 element organic compoundsPolymer scienceResin-Based Composite
The invention discloses a borazine aryne resin and a preparation method thereof. The borazine aryne resin is structurally characterized in that the polymer molecular chain contains borazine and aryl alkynyl structures. The preparation method of the resin comprises the following steps: on the basis of taking diacetylene-benzene, boron trihalide and ammonium chloride or organic primary amine as raw materials, reacting in three steps in the presence of an inert gas so as to synthesize the borazine aryne resin. The preparation method is simple in process, convenient to operate and short in reaction time, the process condition is easy to control, and the aftertreatment process is simple; the borazine aryne resin is reddish brown viscous fluid or faint yellow solid, and can be solidified in a light and thermal polymerization mode; the borazine aryne resin has excellent thermal resistance and low dielectric property, can be used for preparing an advanced resin-based composite material, can be also used as a low dielectric material, and can be widely applied to the fields of aerospace and electronic information.
Owner:EAST CHINA UNIV OF SCI & TECH
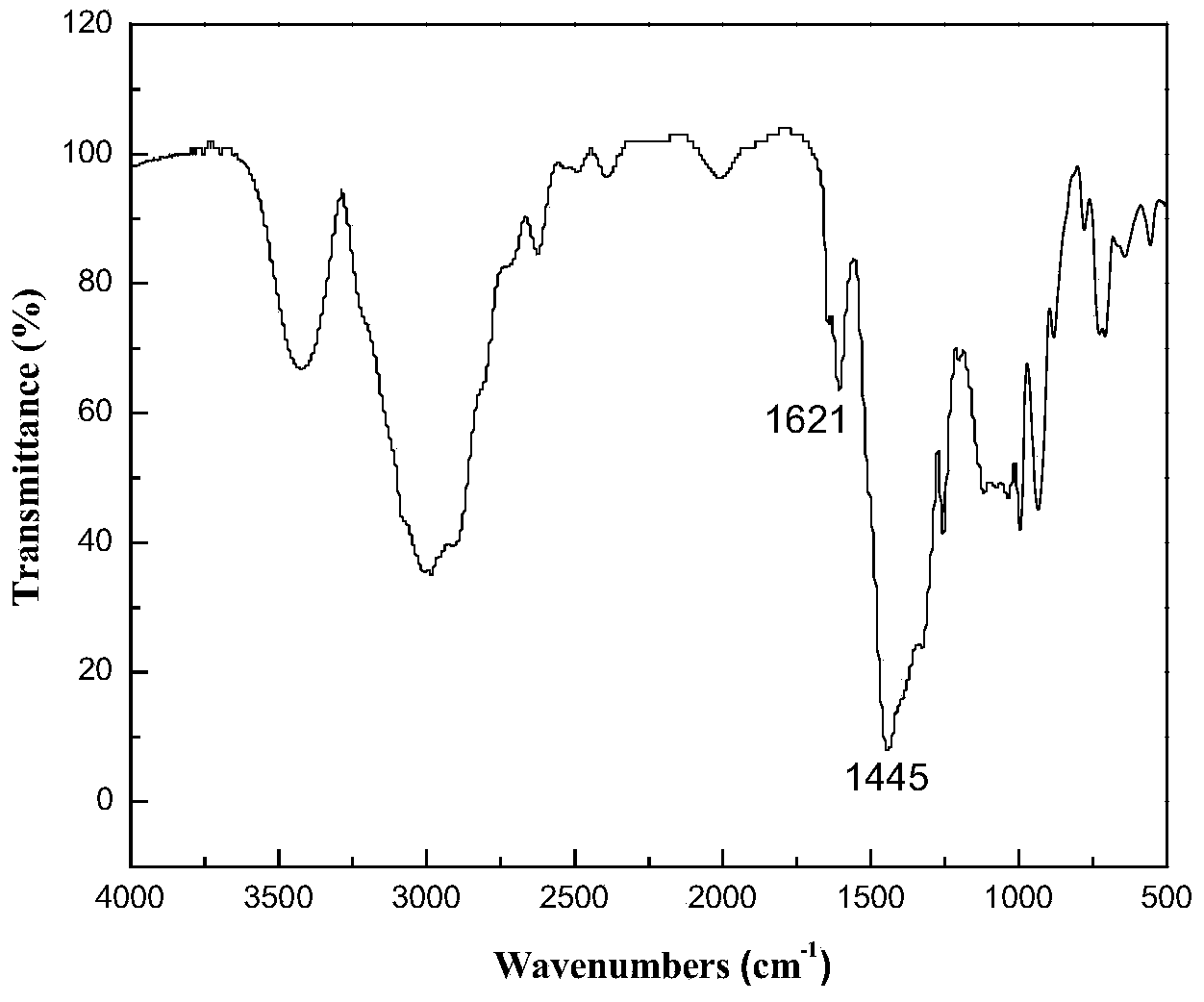
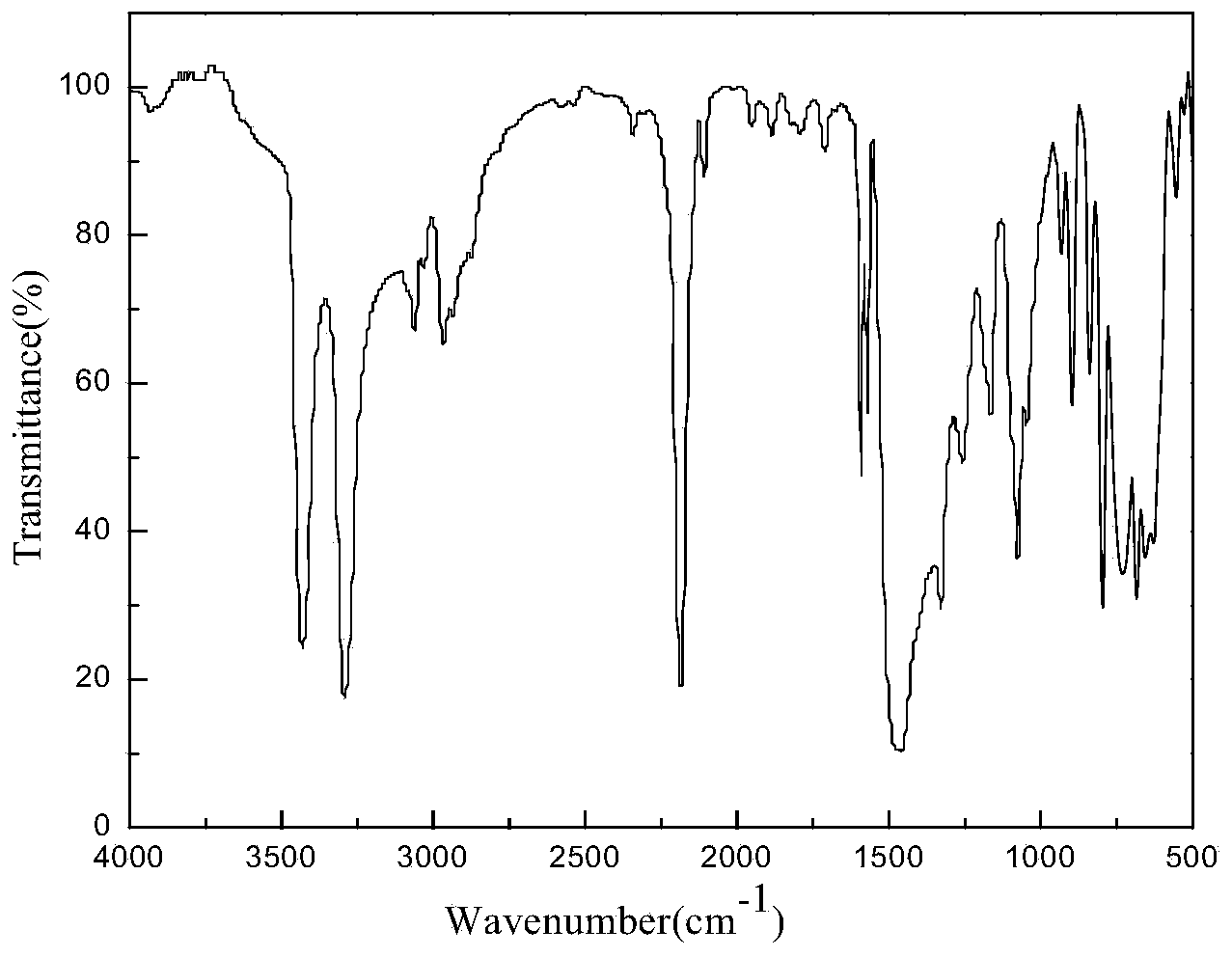
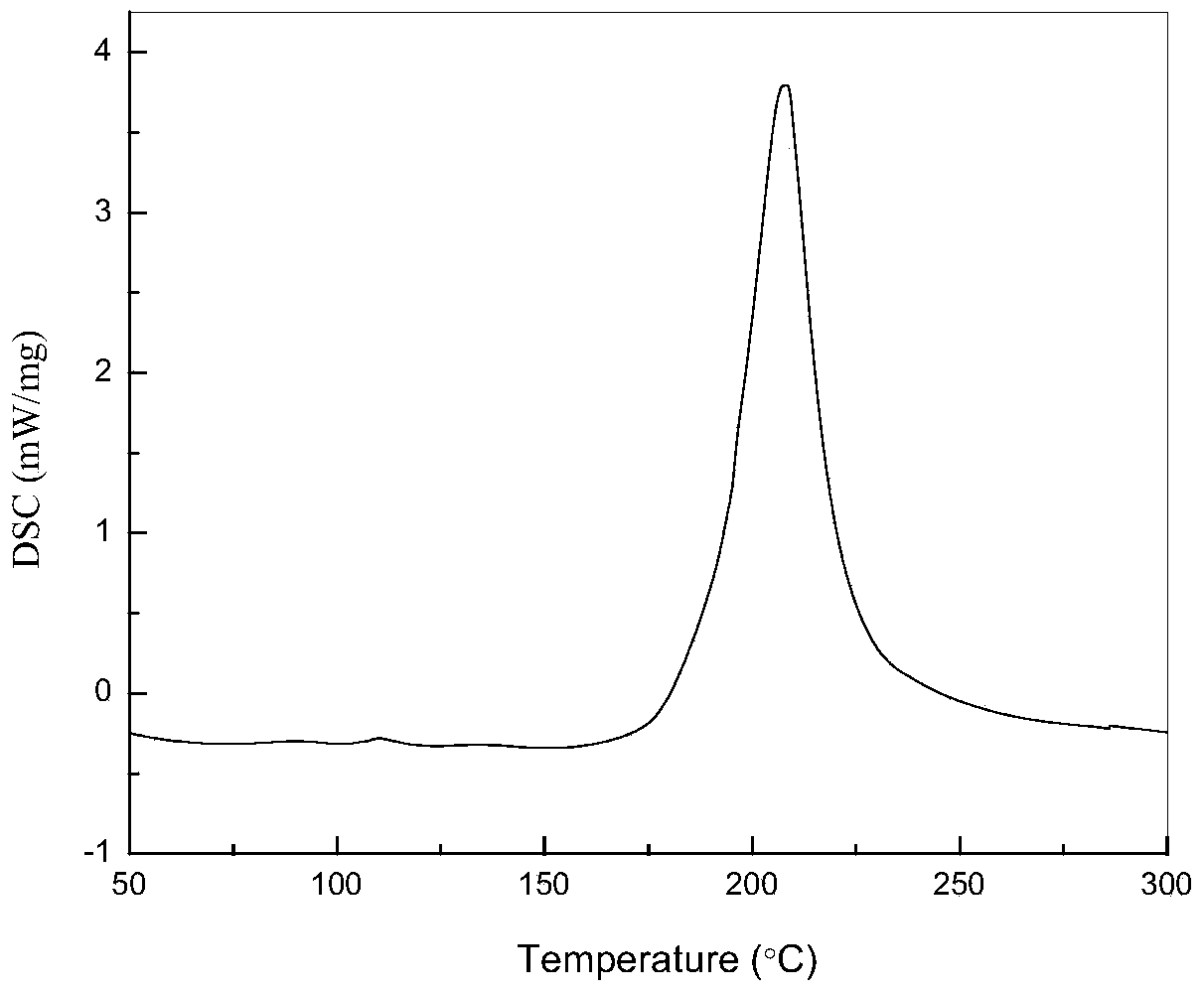
Preparation method of 4,4-dihalotetrahydropyran
The invention relates to a preparation method of 4,4-dihalotetrahydropyran, comprising the steps of mixing halogenated homoallylic alcohol, aldehydes, indium trihalide and a solvent in a flask, mixingwell to obtain a mixed liquid controlled to be -20 to 0 DEG C, dropwise adding trimethyl halogenosilane slowly, starting reaction, allowing reacting by stirring at a certain temperature for 1-24 h, adding sodium bicarbonate saturated solution for quenching reaction, after the solution layers into an organic phase and an aqueous phase, extracting the aqueous phase with methyl tertiary butyl ether,combining the extract to the organic phase, washing the obtained organic phase with saturated salt water, drying, evaporating to remove an organic solvent to obtain a crude product, and subjecting the crude product by separation and purification by column chromatography to obtain a finished product that is applicable to the field of flame retardants and the field of pesticides. The preparation method has simple process and high yield of product, the product has high purity, indium trihalide of catalyst quantity is used in the reaction system, indium consumption is low, the catalyst cost is low, and the preparation method is economical and practical.
Owner:QUZHOU UNIV
Process For Preparing Monoalkyltin Trihalides and Dialkyltin Dihalides
The invention provides a process for producing monoalkyltin trihalide or a mixture of monoalkyltin trihalide and dialkyltin dihalide by: (a) contacting dialkyltin dihalide with an alkylation agent and, optionally, tin tetrahalide, to form a tetraalkyltin mixture comprising tetraalkyltin, trialkyltin halide, and dialkyltin dihalide; (b) reacting the tetraalkyltin mixture with tin tetrahalide to form a monoalkyltin trihalide mixture comprising monoalkyltin trihalide, dialkyltin dihalide and optionally triaklyltin halide; (c) processing the monoalkyltin trihalide mixture to separately recover the monoalkyltin trihalide and a dialkyltin dihalide stream optionally containing trialkyltin halide; and (d) recycling at least a portion of the dialkyltin dihalide stream recovered in step (c) to the contacting step (a).
Owner:PMC ORGANOMETALLIX INC
Preparation method of phosphous dihalide with substituent group
InactiveCN1765906AHigh reaction yieldMild reaction conditionsGroup 5/15 element organic compoundsArylSolvent
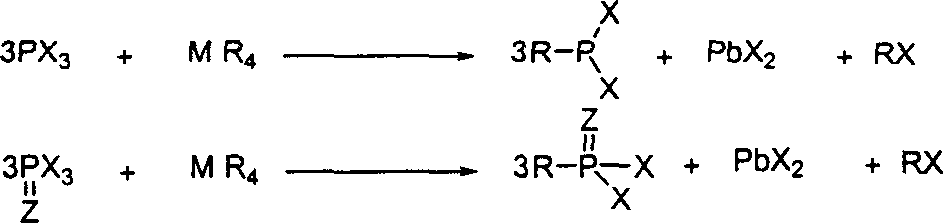
The invention discloses a preparation method for phosphous dihalide with substituent, which comprises: using phosphous trihalide, phosphoryl phosphous trihalide or sulphuryl group to react with a metal tetra-alkyl, tetraene or tetra-aryl compound for 0.5~300h at 0~350Deg regardless the catalyst and solvent. This invention needs mild condition, and has high yield.
Owner:SHANGHAI UNIV OF ENG SCI
Catalytic composition and its application to olefin oligomerization
InactiveUS7235703B2High activityMolecular sieve catalystsOrganic chemistry methodsPre-conditionPre conditioning
An improved catalytic composition for oligomerization, in particular dimerization, of monoolefins comprises the product resulting from bringing the following three constituents into contact in any order:a) at least one divalent nickel compound;b) at least one hydrocarbylaluminium dihalide, optionally enriched with an aluminium trihalide; andc) at least one organic Bronsted acid;the catalytic composition being pre-conditioned in a solvent before using it for oligomerization.
Owner:INST FR DU PETROLE
Load catalyst containing nickel and iron and its application
The present invention relates to a load type catalyst containing nickel and iron and its application. It uses high specific area cross-linked polyacrylic ion-exchange resin powder body as load carrier of catalyst, and makes it and nickel dihalide and iron trihalide undergo the process of coordinative complexation reaction to form the load catalyst. The load catalyst can be mixed with initiator, monomer and solvent according to a certain ratio, and formed into a solid-liquid reaction systm which can be used for catalyzing controllable free radical polymerization. After the reaction is completed, then reaction product and catalyst can be simply separated, and the metal catalyst residue in the product is less, and said catalyst can be recovered and reused.
Owner:SHANGHAI JIAO TONG UNIV
Procedure for the hydrogenation of BNH-containing compounds
InactiveUS7695704B2Improve energy efficiencyNitrogen compoundsOther chemical processesHydrogen halideCompound a
A process for producing borazane from boron-nitrogen and boron-nitrogen-hydrogen containing BNH-waste products. The process includes reacting the BNH-waste products with a hydrogen halide, having the formula HX, wherein X is selected from the group consisting of F, Cl, Br, I, and combinations thereof, to form any of the following: a boron trihalide, having the formula BX3, an ammonium halide, having the formula NH4X, and hydrogen. The boron trihalide is then reacted with the hydrogen to form diborane, having the formula B2H6, and hydrogen halide. The ammonium halide is then converted to ammonia, having the formula NH3, and hydrogen halide. The diborane is then reacted with the ammonia to form borazane, having the formula BH3NH3.
Owner:GM GLOBAL TECH OPERATIONS LLC +1
Catalyst component used for vinyl polymerization and catalyst
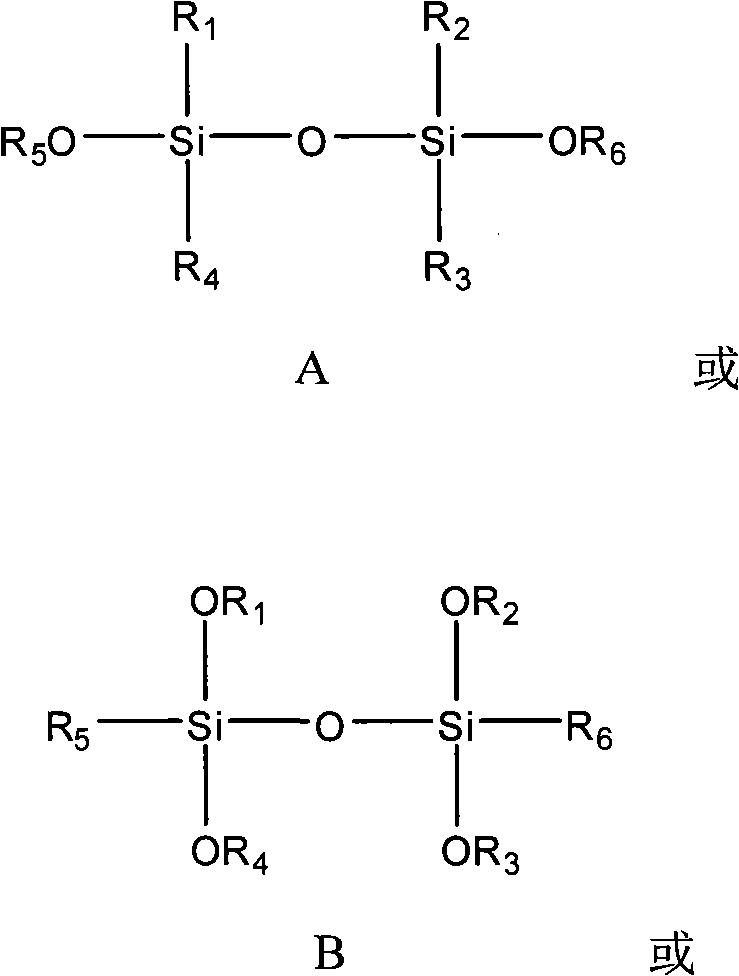
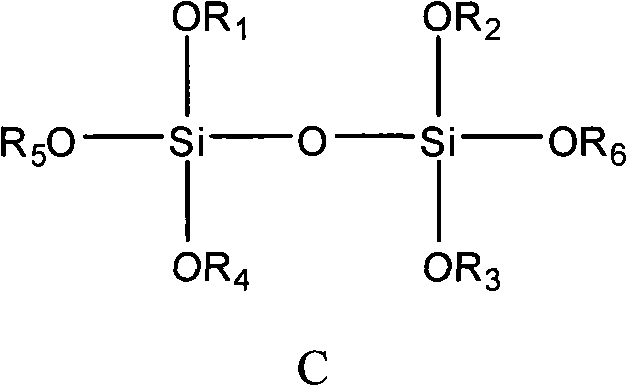
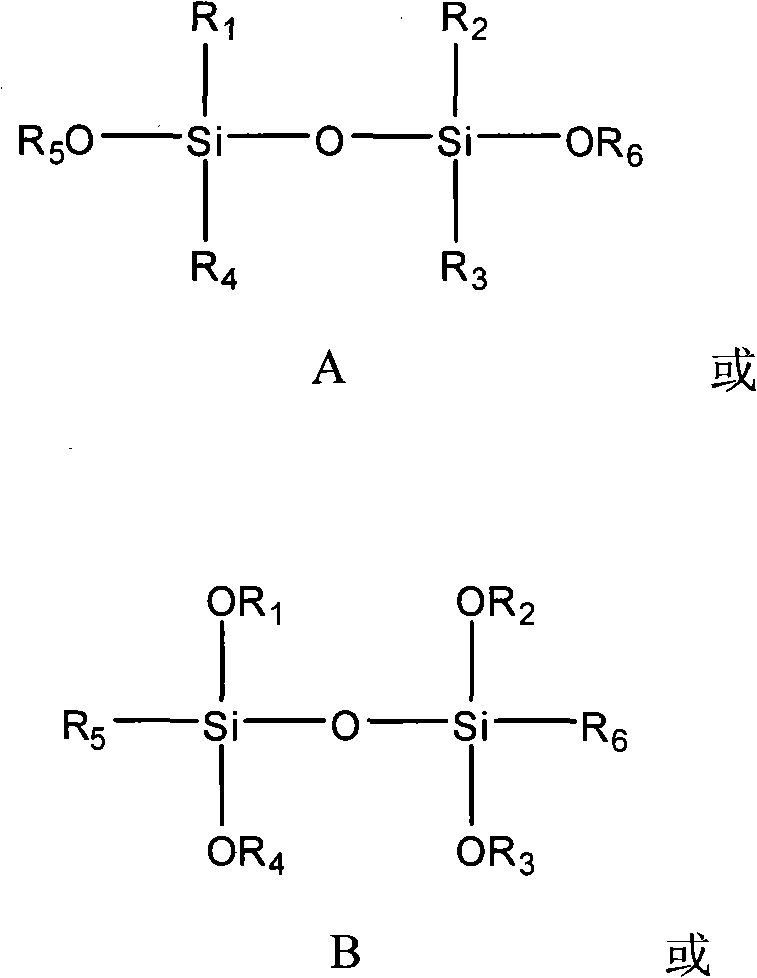
The invention provides a catalyst component used for vinyl polymerization. The catalyst component comprises the following components: a magnesium composite, a titanium compound and an organosilicon compound, wherein the magnesium composite is a product prepared by dissolving alkoxy magnesium in a solvent system containing an organic epoxy compound, boron trihalide and at least one kind of organicalcohol; the titanium compound has the general formula of Ti(OR)aXb, wherein R refers to aliphatic hydrocarbon group or aryl group with 1 to 14 carbon atoms, X refers to halogen, a is 0, 1 or 2, b isan integer from 1 to 4, and a+b=3 or 4; and the organosilicon compound has at least one structural formula of a structural formula a, a structural formula b and a structural formula C.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing bumetanide
InactiveCN101591276ALower requirementEasy to operateOrganic active ingredientsSulfonic acid amide preparationBumetanideEther
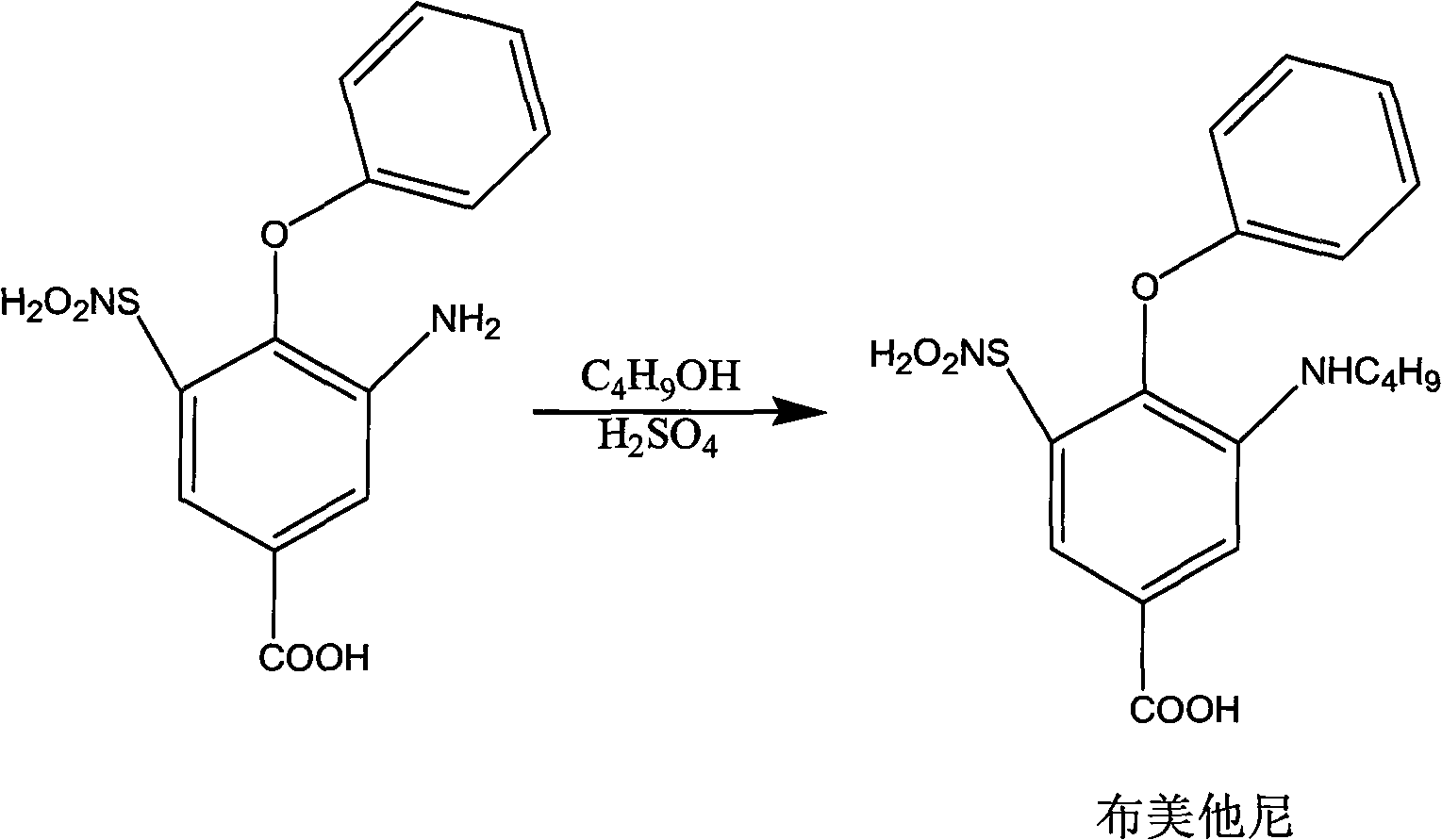
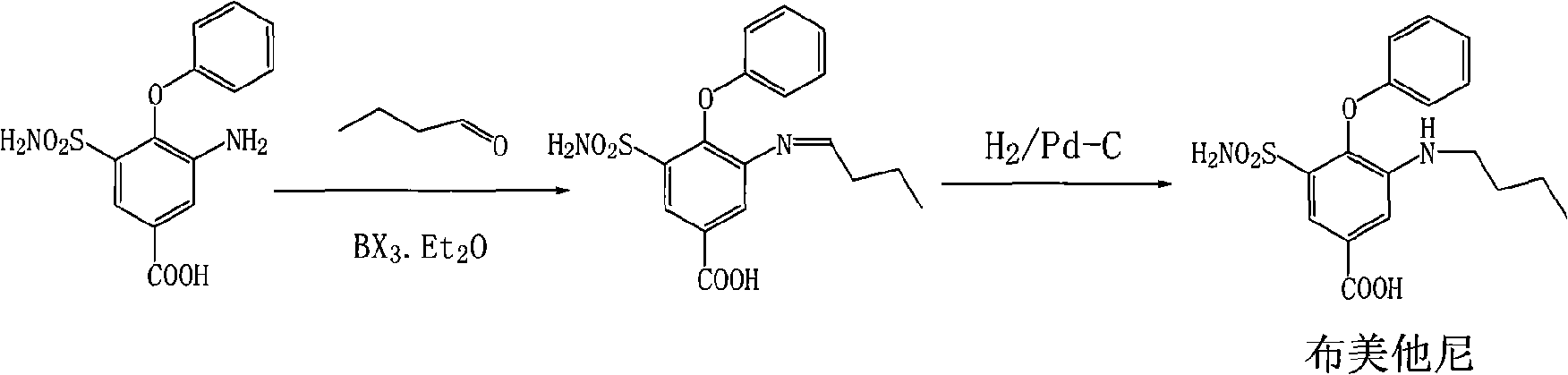
The invention relates to a method for preparing bumetanide, which comprises that: 3-amino-4-phenoxy-5-sulfonylaminobenzoic acid and n-butanal are used as raw materials; firstly, the raw materials are subjected to condensation-dehydration reaction in the presence of a boron trihalide ether catalyst to generate 3-butylimine-4-phenoxy-5-sulfonylaminobenzoic acid; and then, the 3-butylimine-4-phenoxy-5-sulfonylaminobenzoic acid is catalyzed by palladium carbon and hydrogenated to form the bumetanide. The method has the advantages of simple operation, short reaction time and low equipment requirement, and is suitable for industrialized production; and the yield of a target product can reach more than 90 percent.
Owner:SUZHOU LIXIN PHARMA
Method for producing phosphorus pentafluoride
InactiveCN103153847AImprove cooling effectEliminate problems such as cloggingPhosphorus halides/oxyhalidesCell electrodesHalogenGas phase
The present invention provides a method for producing phosphorus pentafluoride, which is characterized by having a phosphorus trihalide represented by general formula PX3 (wherein X represents F, Cl or Br) react with a molecular halogen or hydrogen fluoride that is in a gaseous state. This method is a method for efficiently producing phosphorus pentafluoride at low cost, which is applicable to the production on an industrial scale.
Owner:DAIKIN IND LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com