Patents
Literature
207 results about "Uranium oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Uranium oxide is an oxide of the element uranium.
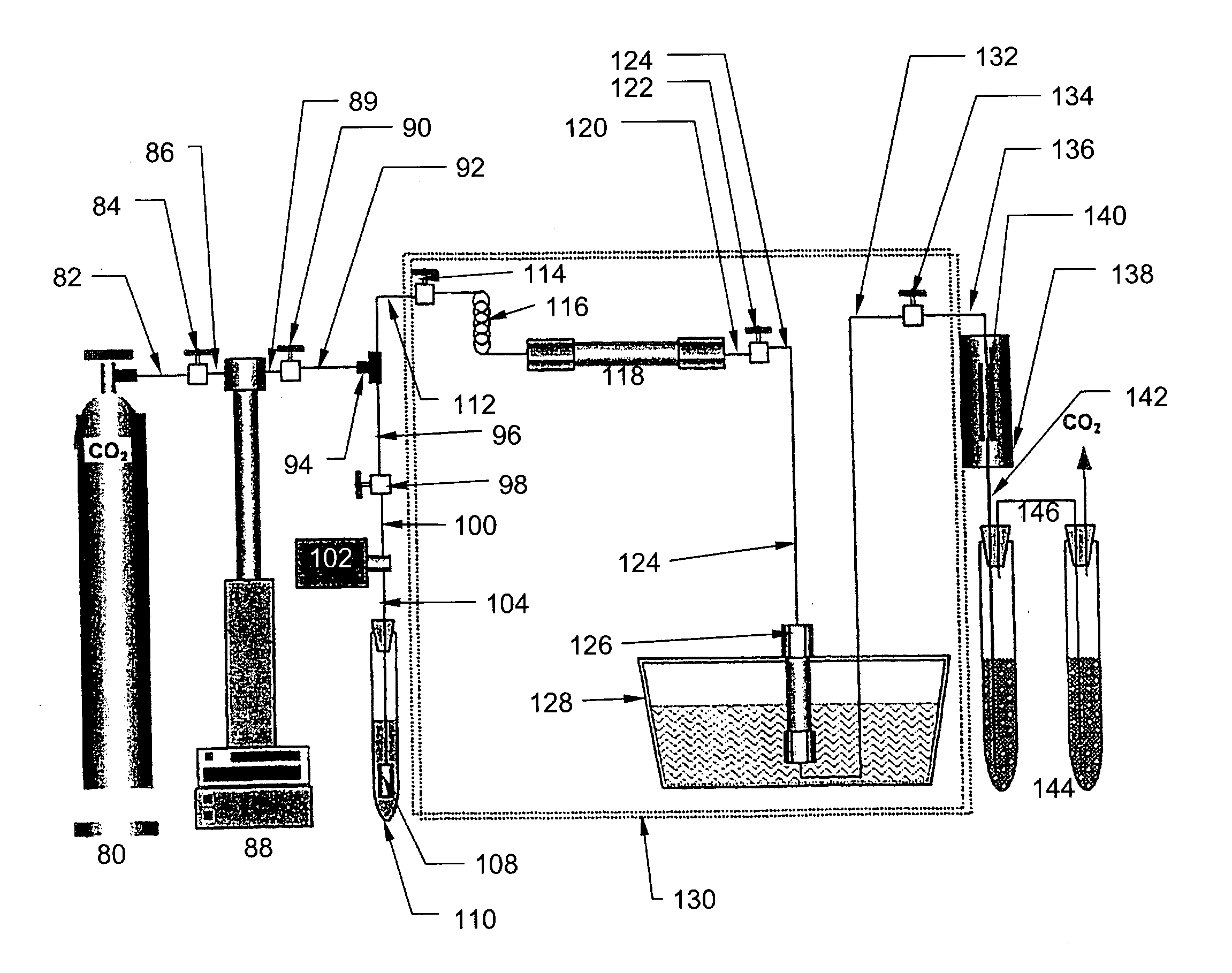
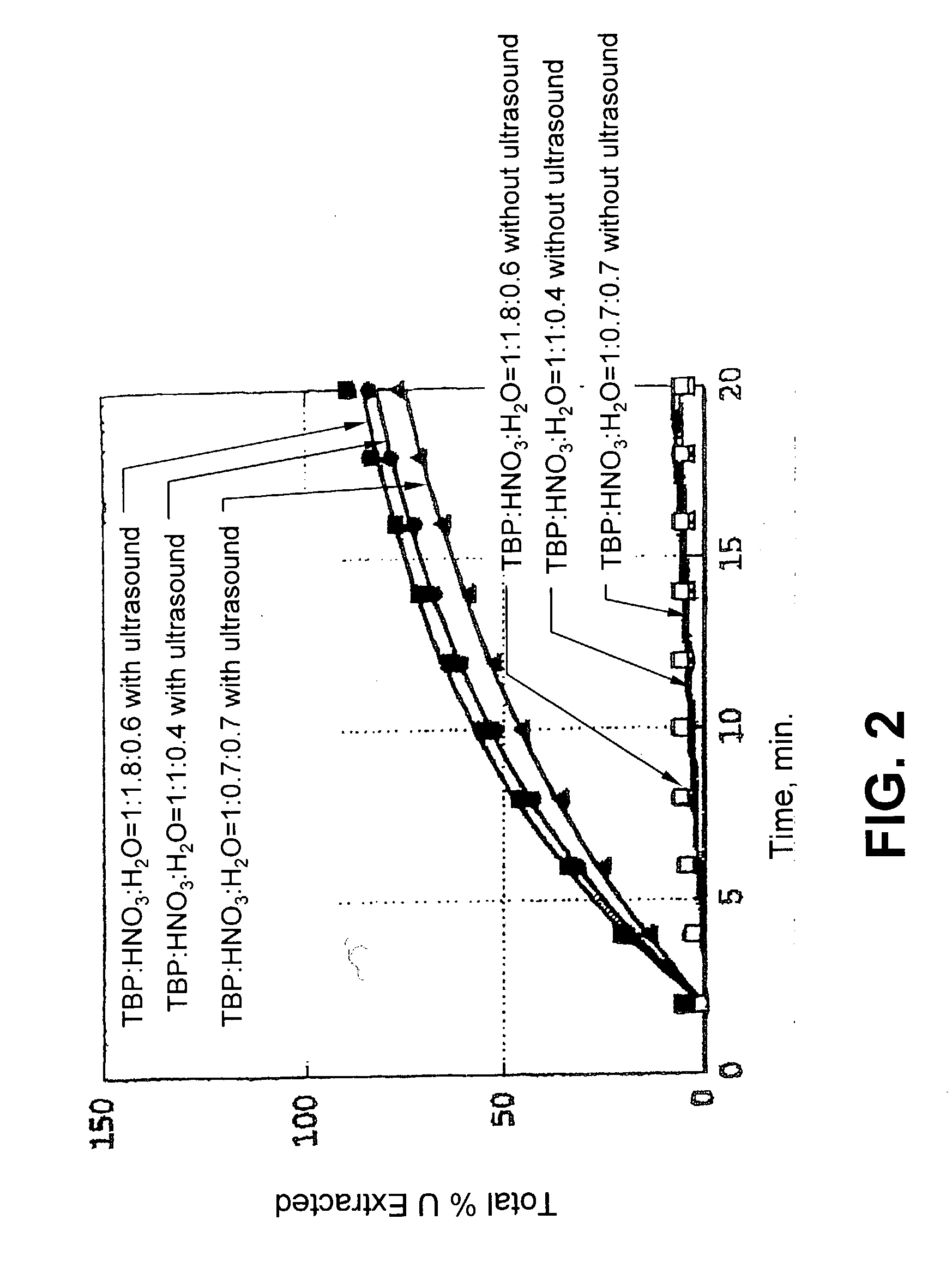
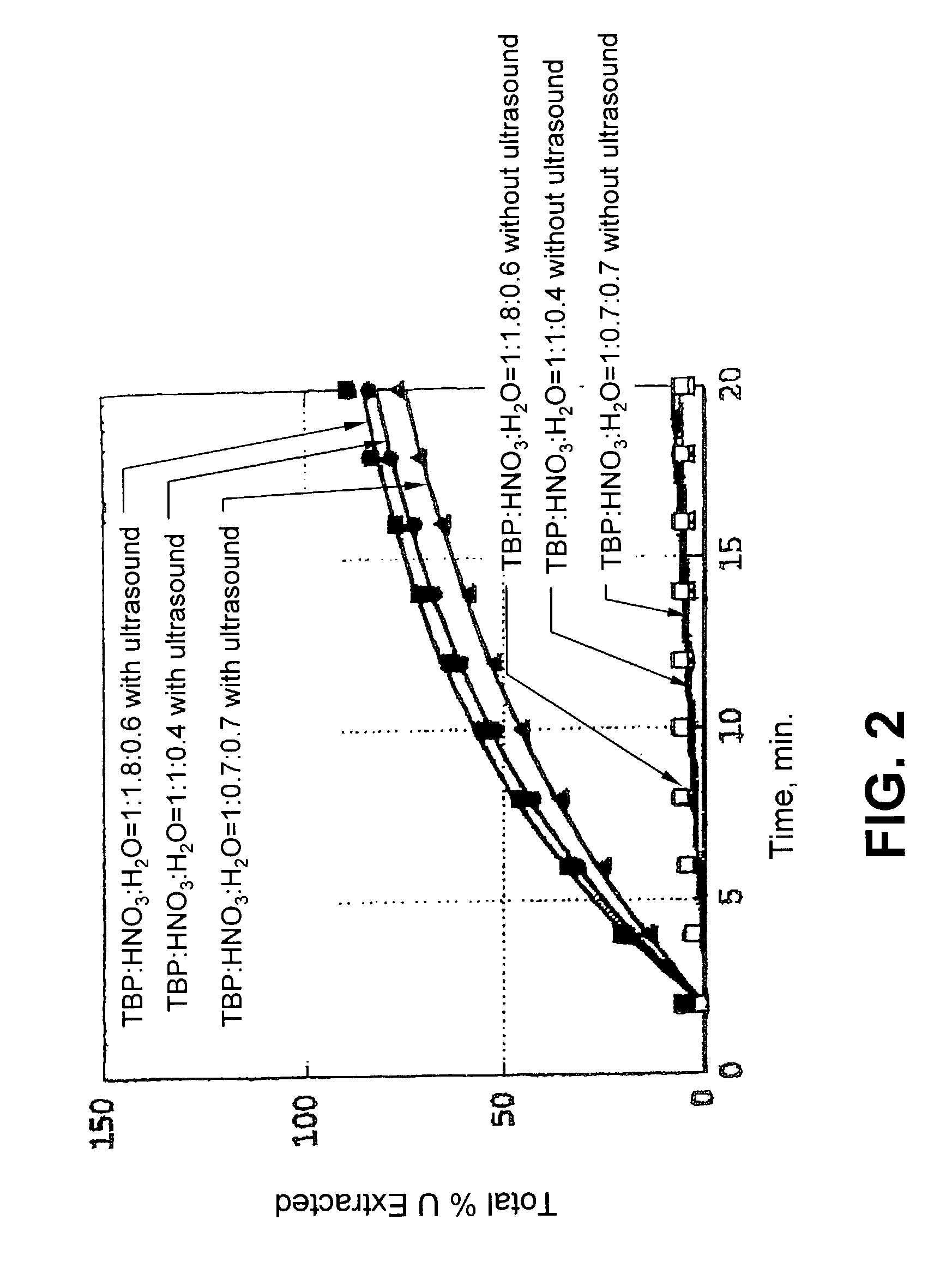
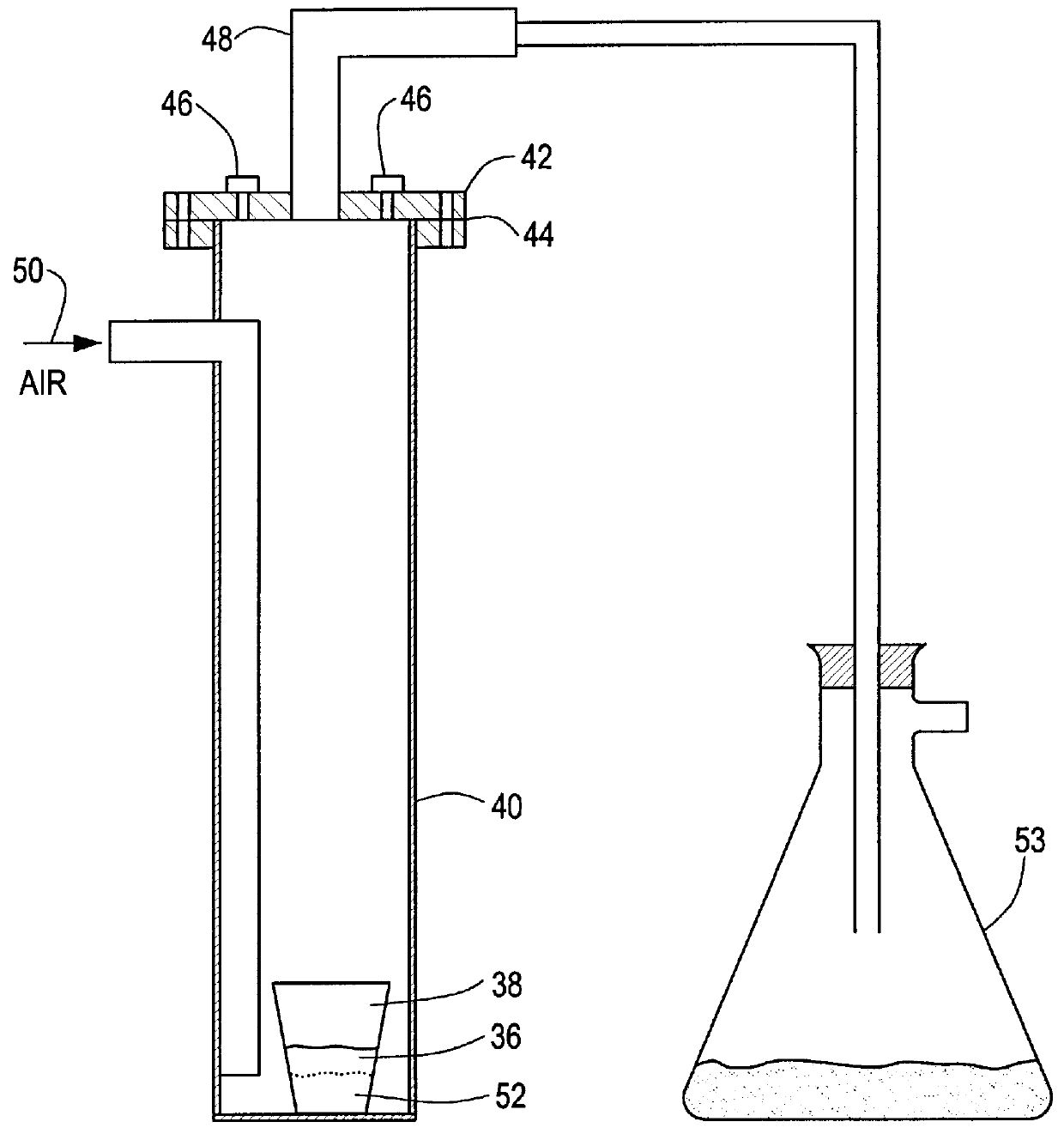
Ultrasound enhanced process for extracting metal species in supercritical fluids
InactiveUS20030183043A1Enhances rate and efficiencyReduce probabilitySolid sorbent liquid separationGold compoundsUranium oxidePresent method
Improved methods for the extraction or dissolution of metals, metalloids or their oxides, especially lanthanides, actinides, uranium or their oxides, into supercritical solvents containing an extractant are disclosed. The disclosed embodiments specifically include enhancing the extraction or dissolution efficiency with ultrasound. The present methods allow the direct, efficient dissolution of UO2 or other uranium oxides without generating any waste stream or by-products.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
Method and system for treating oxidized contaminant-containing matrix
InactiveUS20060292684A1Reduce maintenanceHigh degreeWater treatment compoundsWater contaminantsSulfurElectron donor
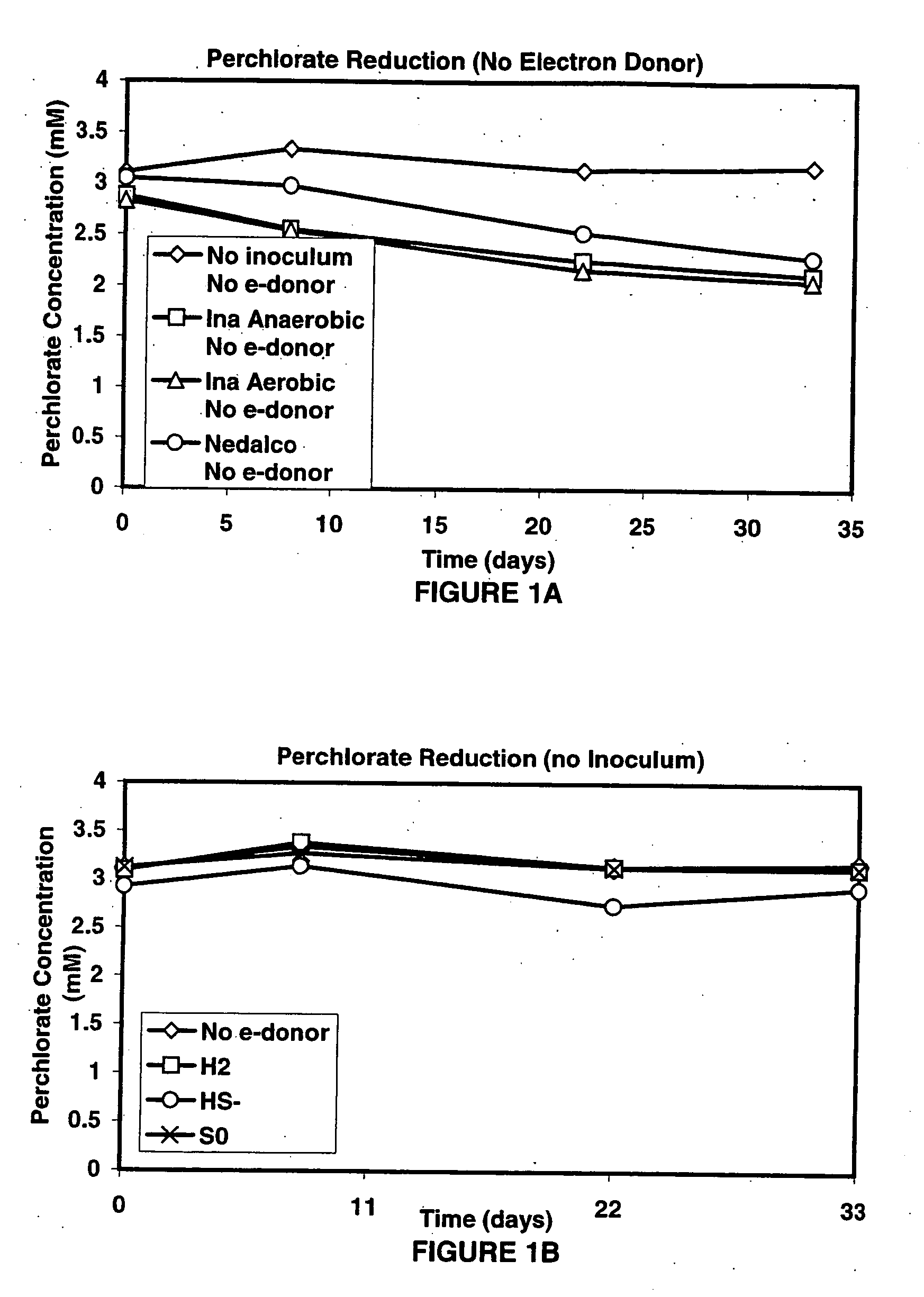
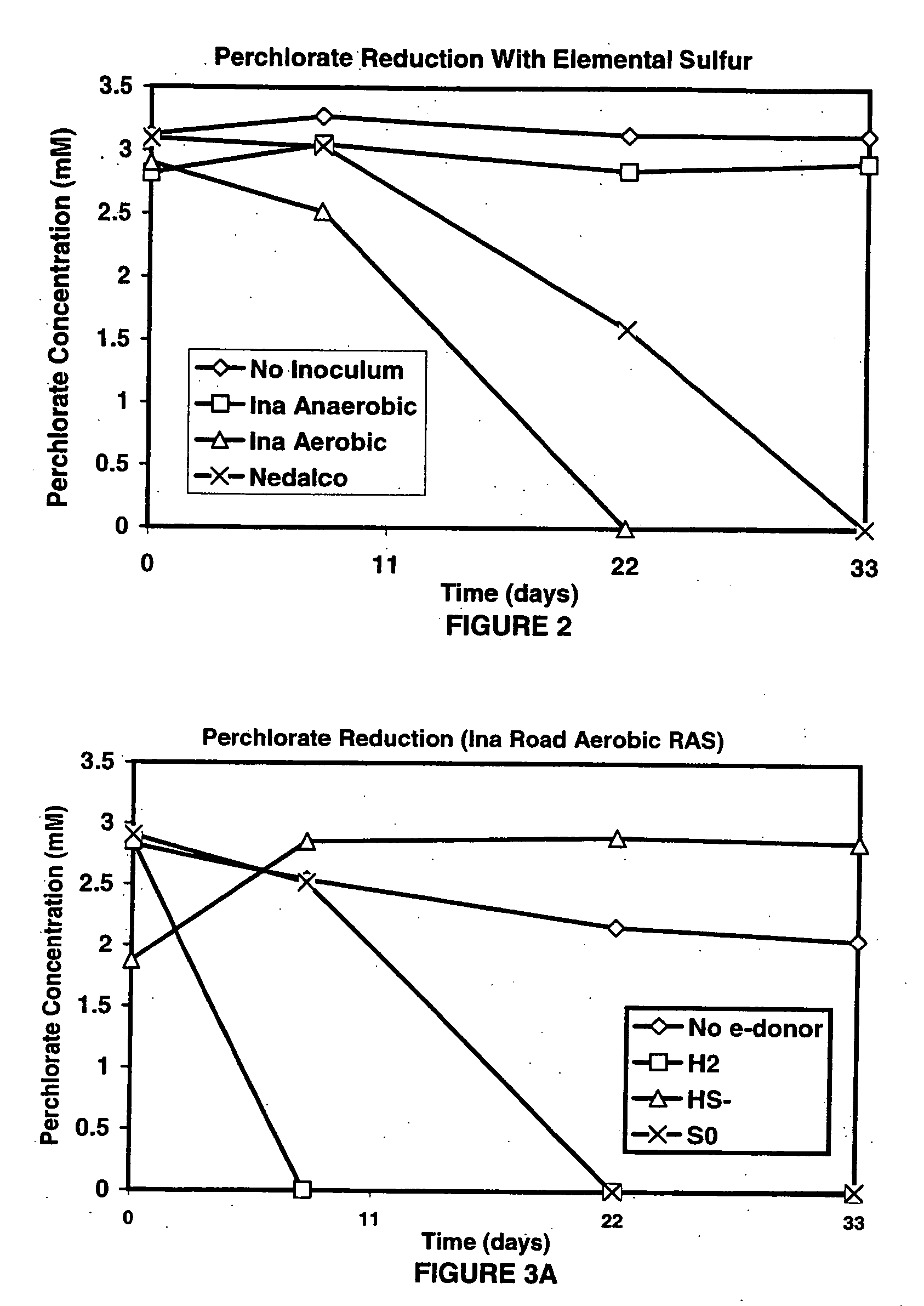
A new and useful way of treating oxidized-contaminant-containing water, soil, rock, other geological or non-geological matrix formation (such as a landfill) is provided. A bioreactor is provided that includes elemental sulfur and a microbial population capable of oxidizing sulfur and reducing pertechnate (TcO4−), arsenate (H2AsO4−), chromate (CrO42−), bromate (BrO3−), chlorite (ClO2−), chlorate (ClO3−), perchlorate (ClO4−), and uranium(VI) oxide, with biological reduction of these oxidized contaminants in the matrix containing the oxidized contaminant performed by the bioreactor, with the elemental sulfur as the electron donor.
Owner:HYDRO GEO CHEM
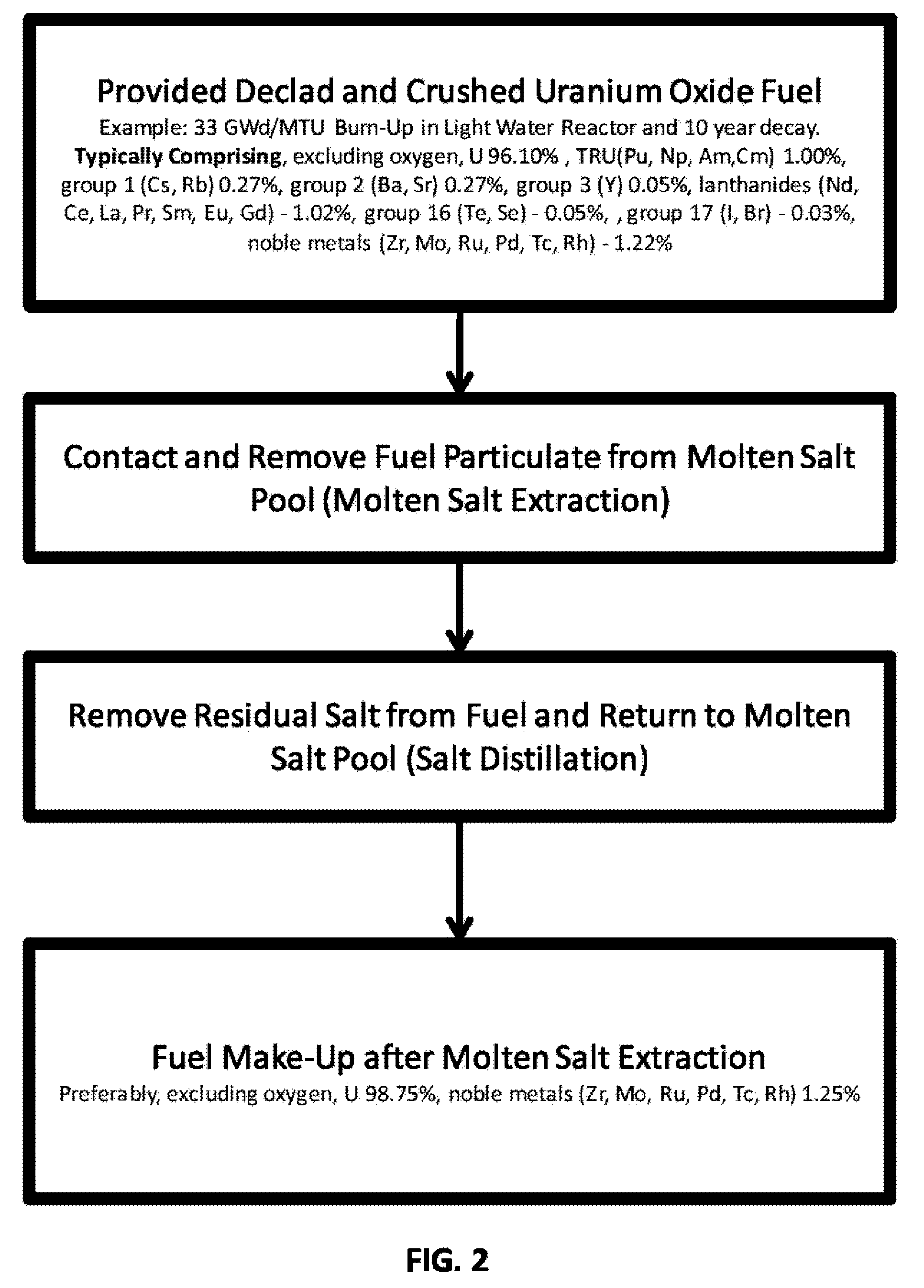
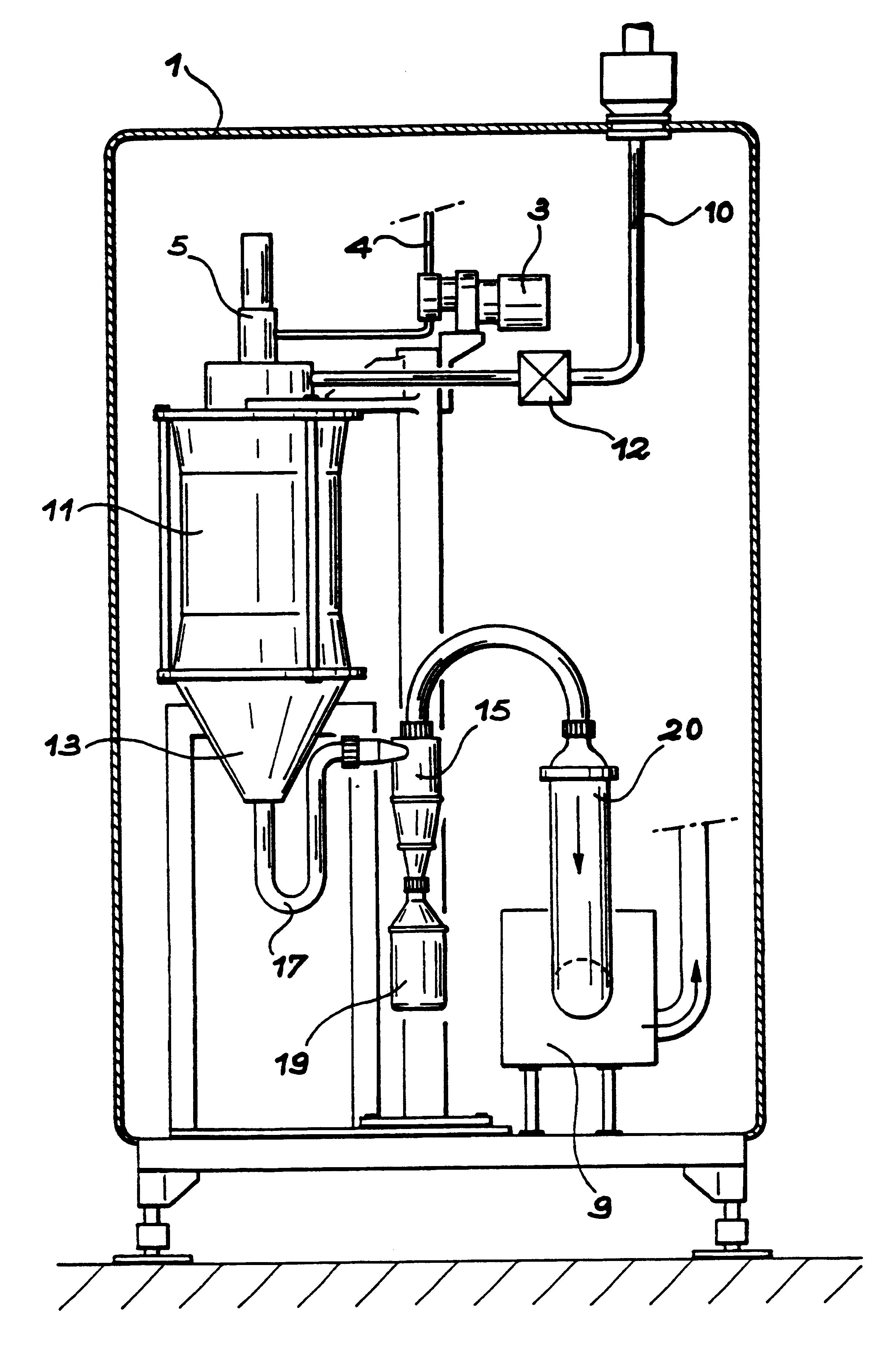
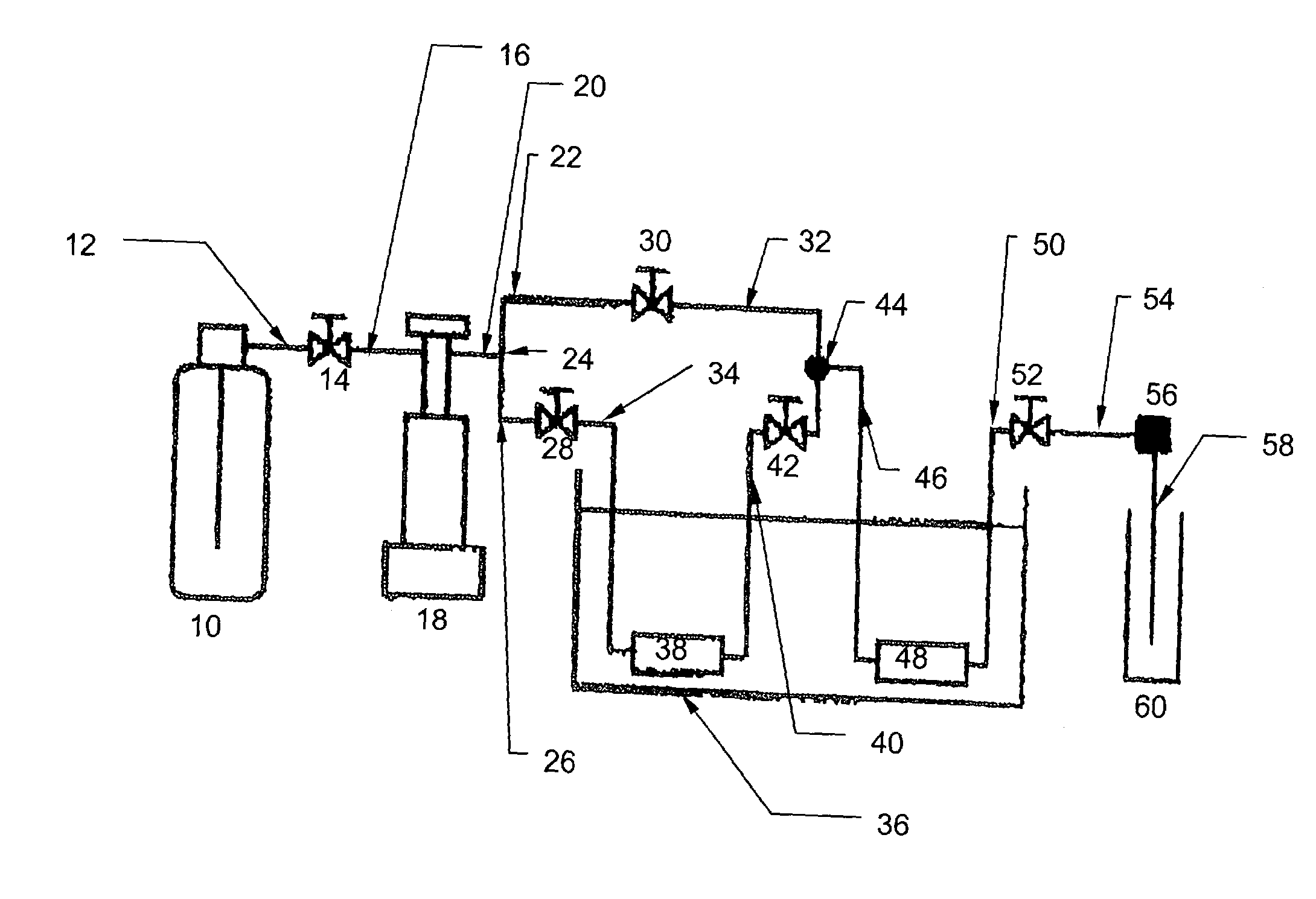
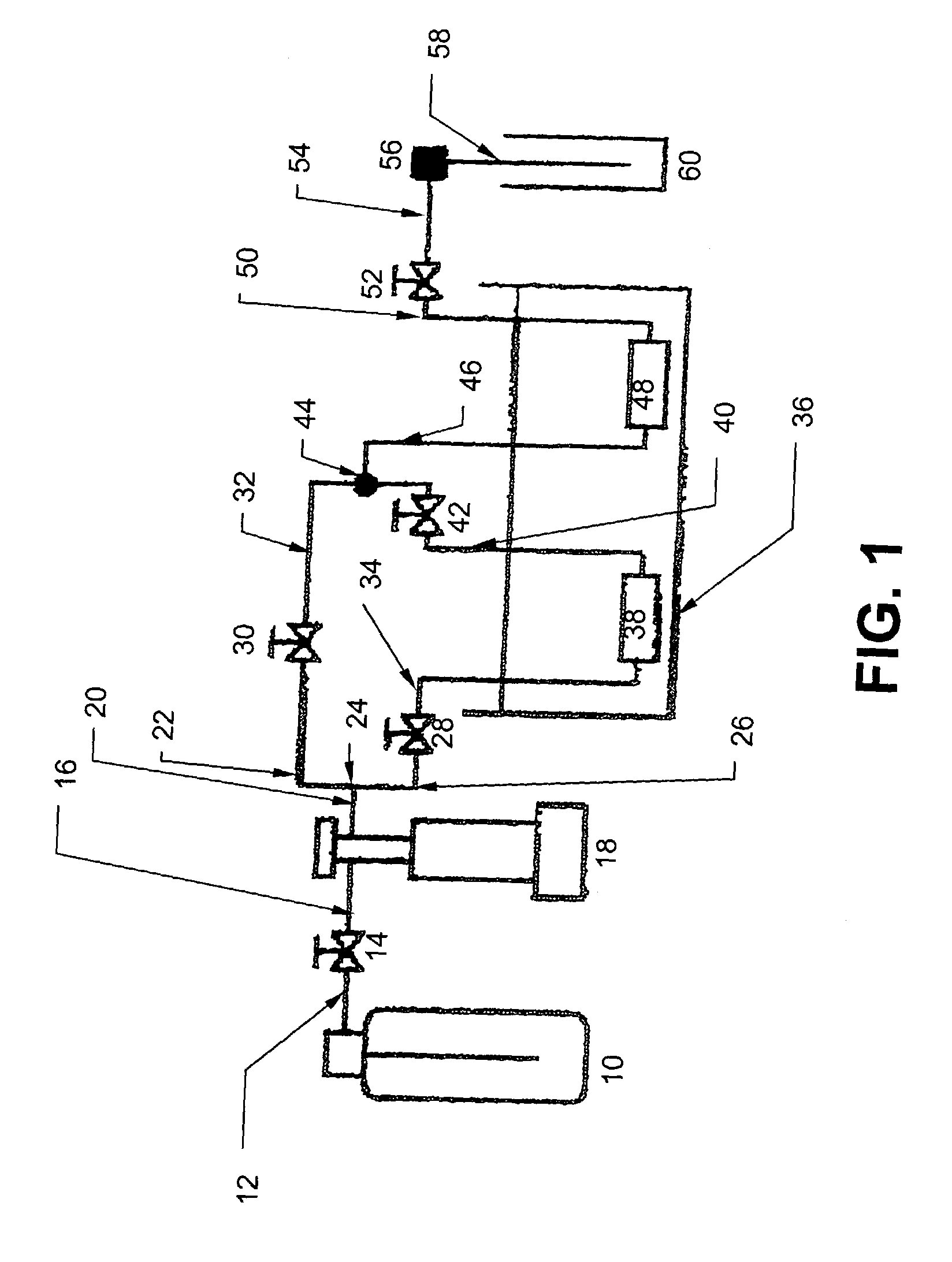
Molten salt extraction of transuranic and reactive fission products from used uranium oxide fuel
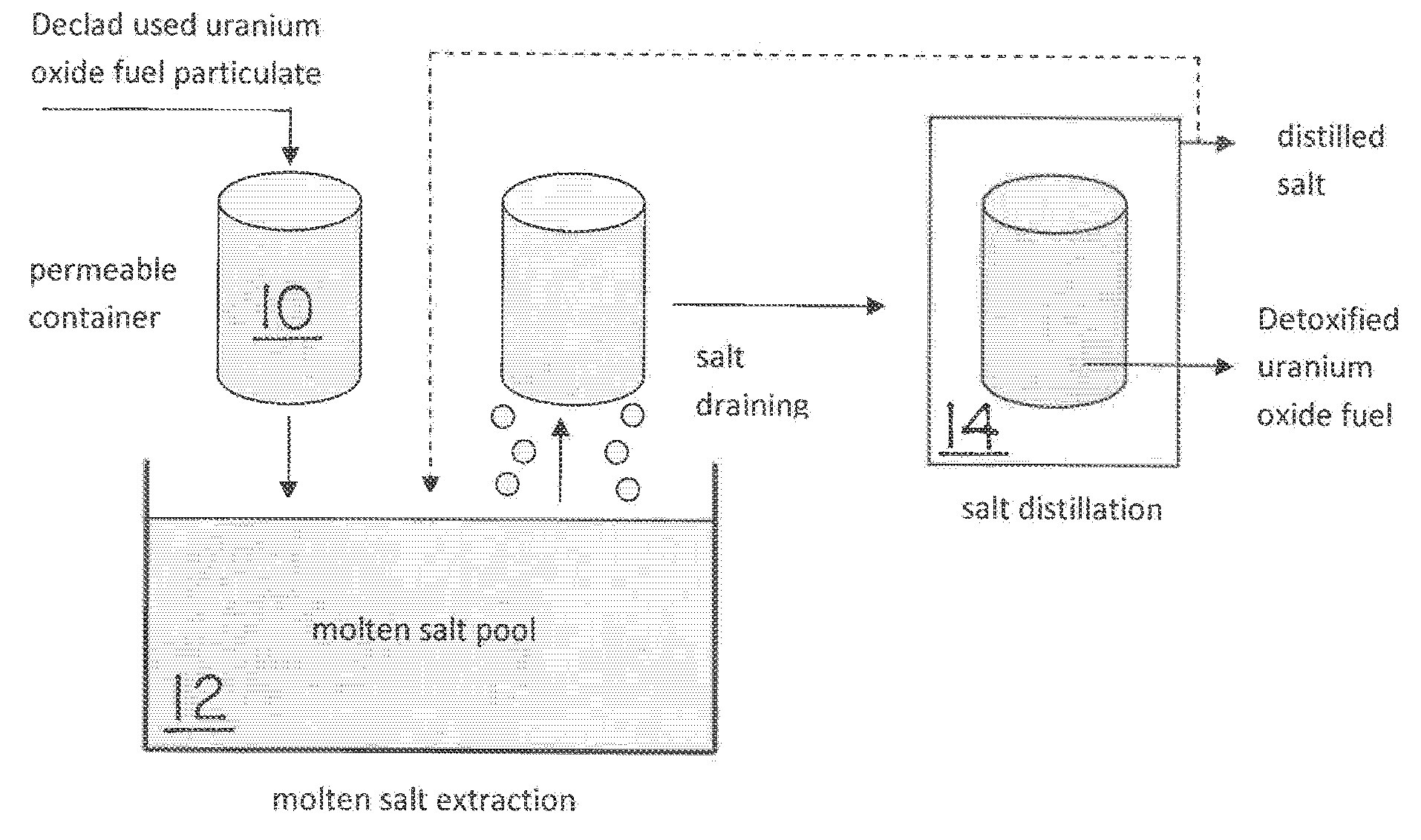
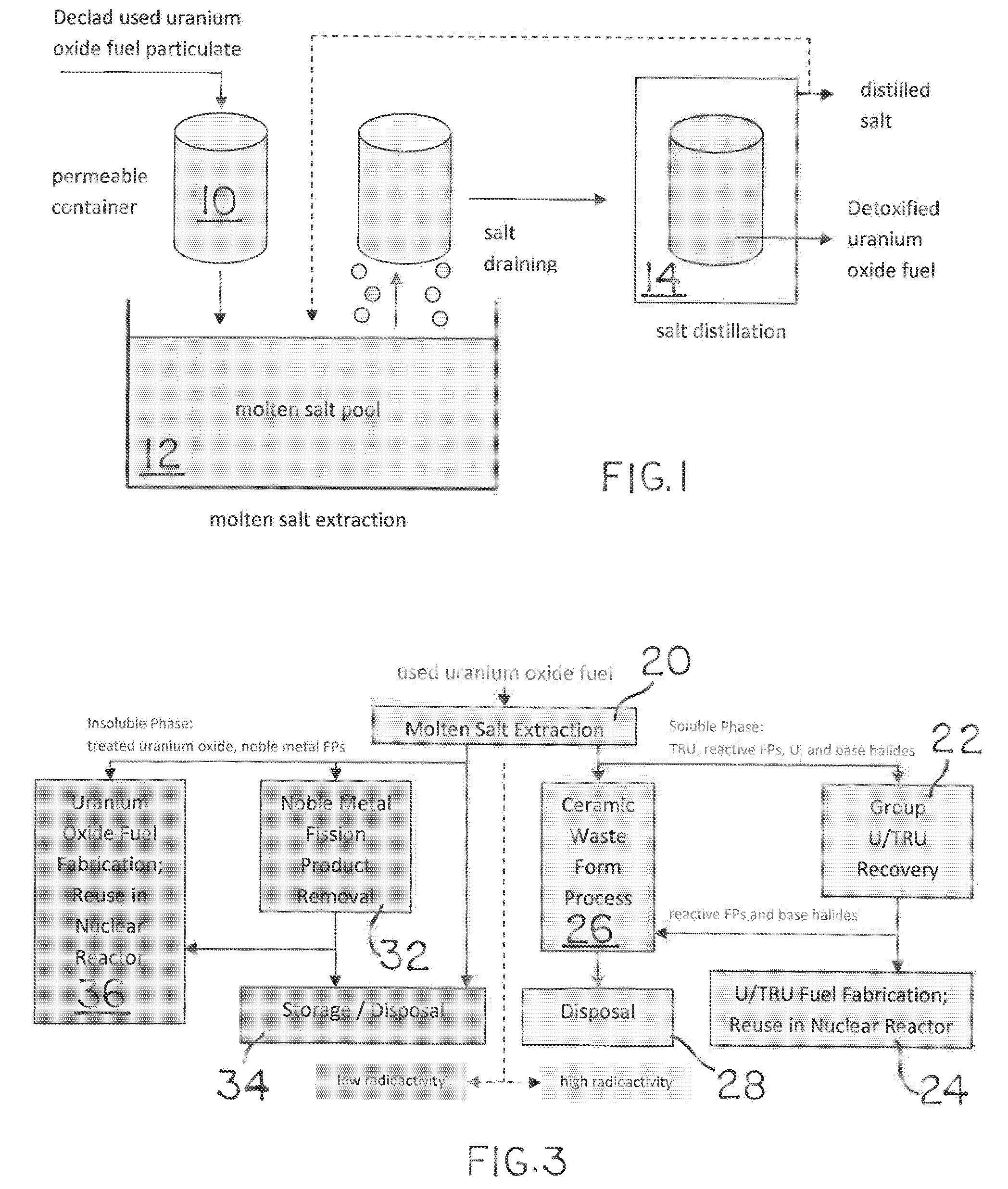
Used uranium oxide fuel is detoxified by extracting transuranic and reactive fission products into molten salt. By contacting declad and crushed used uranium oxide fuel with a molten halide salt containing a minor fraction of the respective uranium trihalide, transuranic and reactive fission products partition from the fuel to the molten salt phase, while uranium oxide and non-reactive, or noble metal, fission products remain in an insoluble solid phase. The salt is then separated from the fuel via draining and distillation. By this method, the bulk of the decay heat, fission poisoning capacity, and radiotoxicity are removed from the used fuel. The remaining radioactivity from the noble metal fission products in the detoxified fuel is primarily limited to soft beta emitters. The extracted transuranic and reactive fission products are amenable to existing technologies for group uranium / transuranic product recovery and fission product immobilization in engineered waste forms.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Preparation by spray-drying of a flowable uranium dioxide powder obtained by dry process conversion of UF6
InactiveUS6656391B1Lower levelMaintain good propertiesNuclear energy generationUranium dioxideUranium hexafluorideViscosity
The invention relates to a process for preparing a castable powder of uranium dioxide UO2, for use in the manufacture of MOX fuel.This process comprises the following stages:1) to prepare an aqueous suspension of a powder of UO2 obtained by dry process from uranium hexafluoride, said suspension comprising 50 to 80% by weight of UO2 and at least one additive chosen among deflocculation agents, organic binders, hydrogen peroxide H2O2 and a powder of U3O8, in such a quantity that the viscosity of the suspension does not exceed 250 mPa.sec, and2) to atomise this suspension and dry it in a hot gas, at a temperature of 150 to 300° C., to obtain a castable powder of UO2 with an average particle size of 20 to 100 mum.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
Ultrasound enhanced process for extracting metal species in supercritical fluids
InactiveUS7128840B2Enhances rate and efficiencyReduce probabilityGold compoundsRecycling and recovery technologiesPresent methodUranium oxide
Improved methods for the extraction or dissolution of metals, metalloids or their oxides, especially lanthanides, actinides, uranium or their oxides, into supercritical solvents containing an extractant are disclosed. The disclosed embodiments specifically include enhancing the extraction or dissolution efficiency with ultrasound. The present methods allow the direct, efficient dissolution of UO2 or other uranium oxides without generating any waste stream or by-products.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
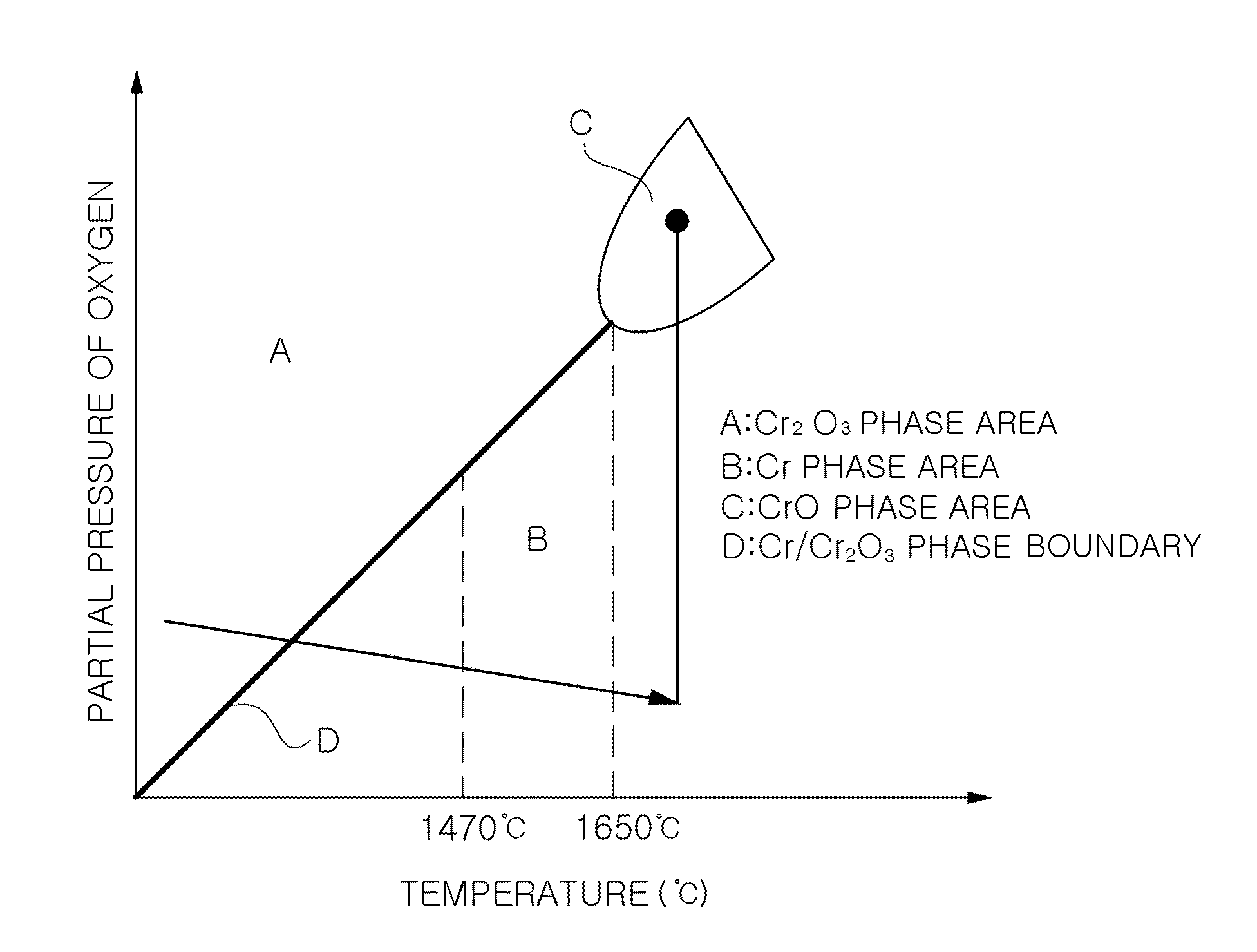
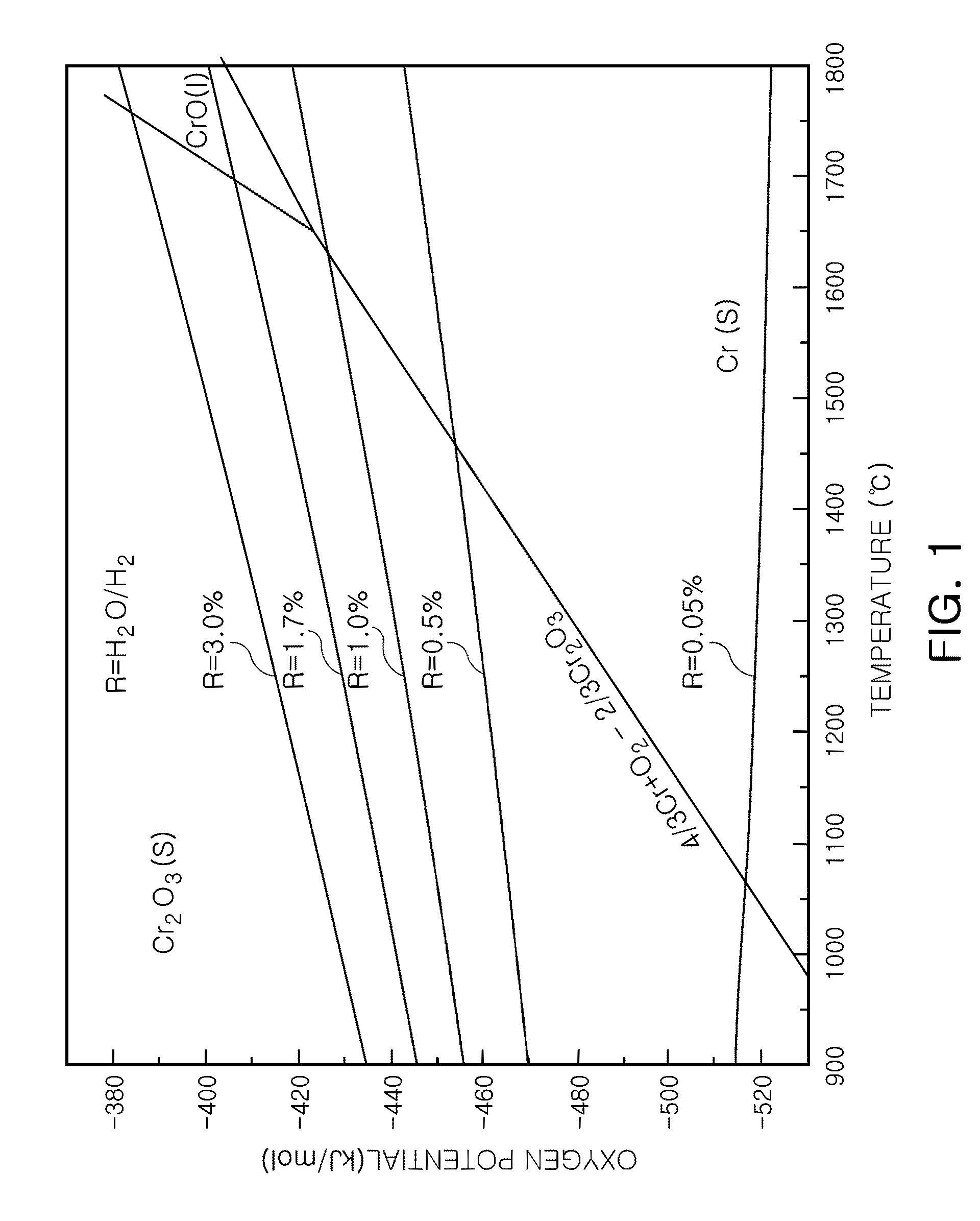
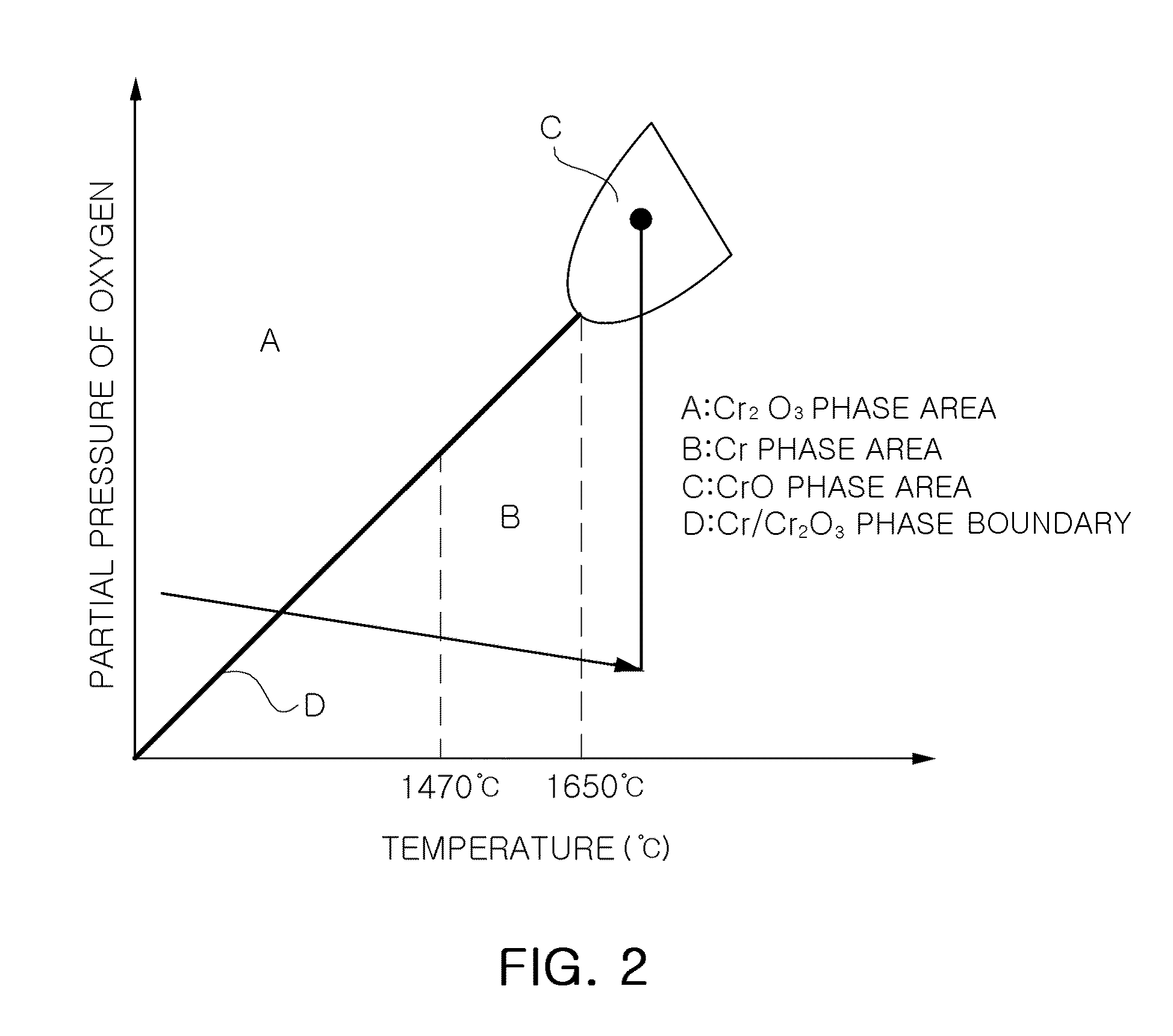
Method of producing large-grained nuclear fuel pellet by controlling chrome cation solubility in uo2 lattice
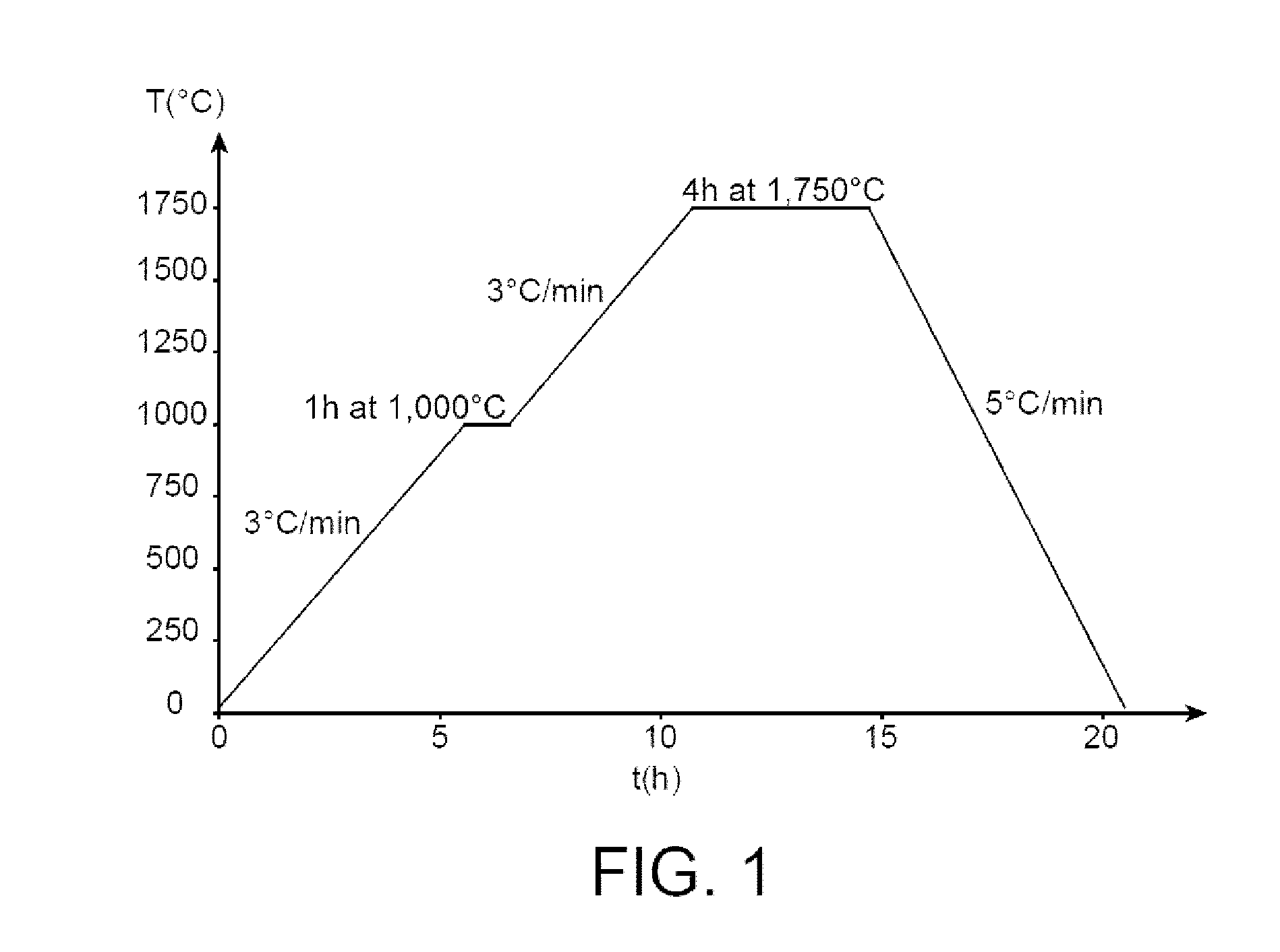
In a method of producing large-grained nuclear fuel pellet, Cr-compound contained in an uranium oxide green pellet is reduced to Cr phase at 1,470° C. or below and maintained to the Cr phase, and the uranium oxide green pellet containing the Cr-compound is then sintered at 1,650° C.-1,800° C. in a gas atmosphere of oxygen potential at which Cr element in the uranium oxide green pellet becomes liquid phase.
Owner:KOREA ATOMIC ENERGY RES INST +1
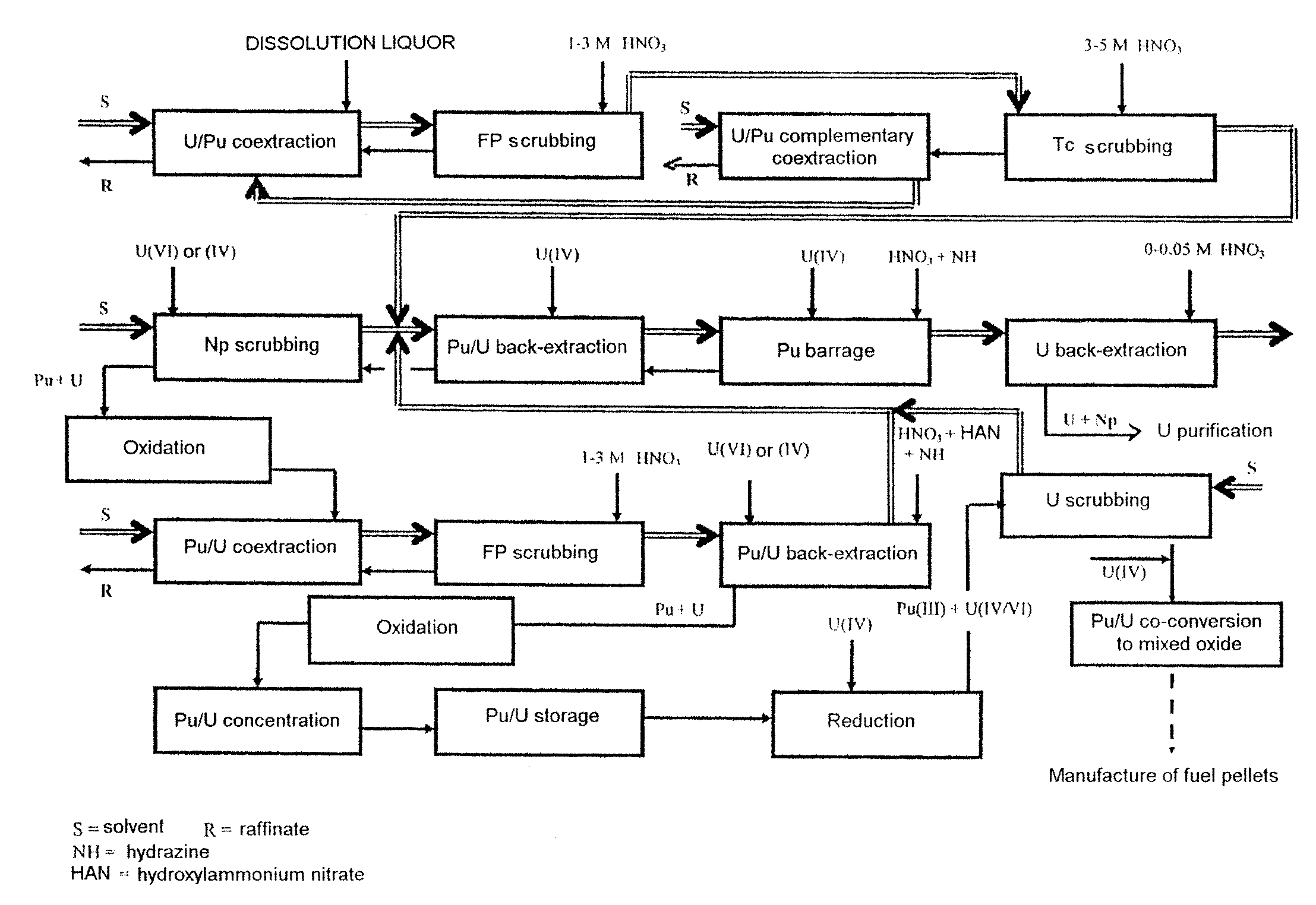
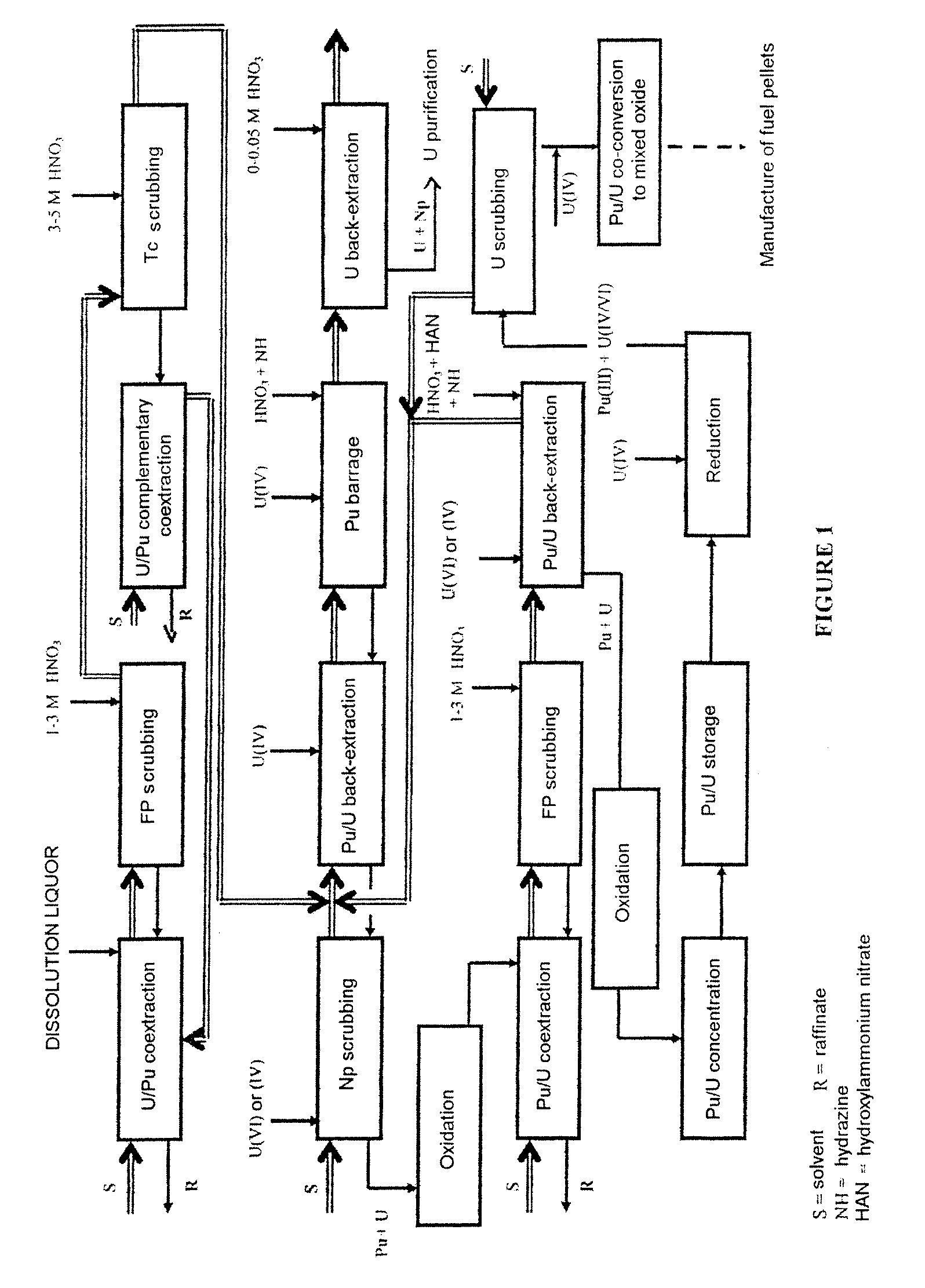
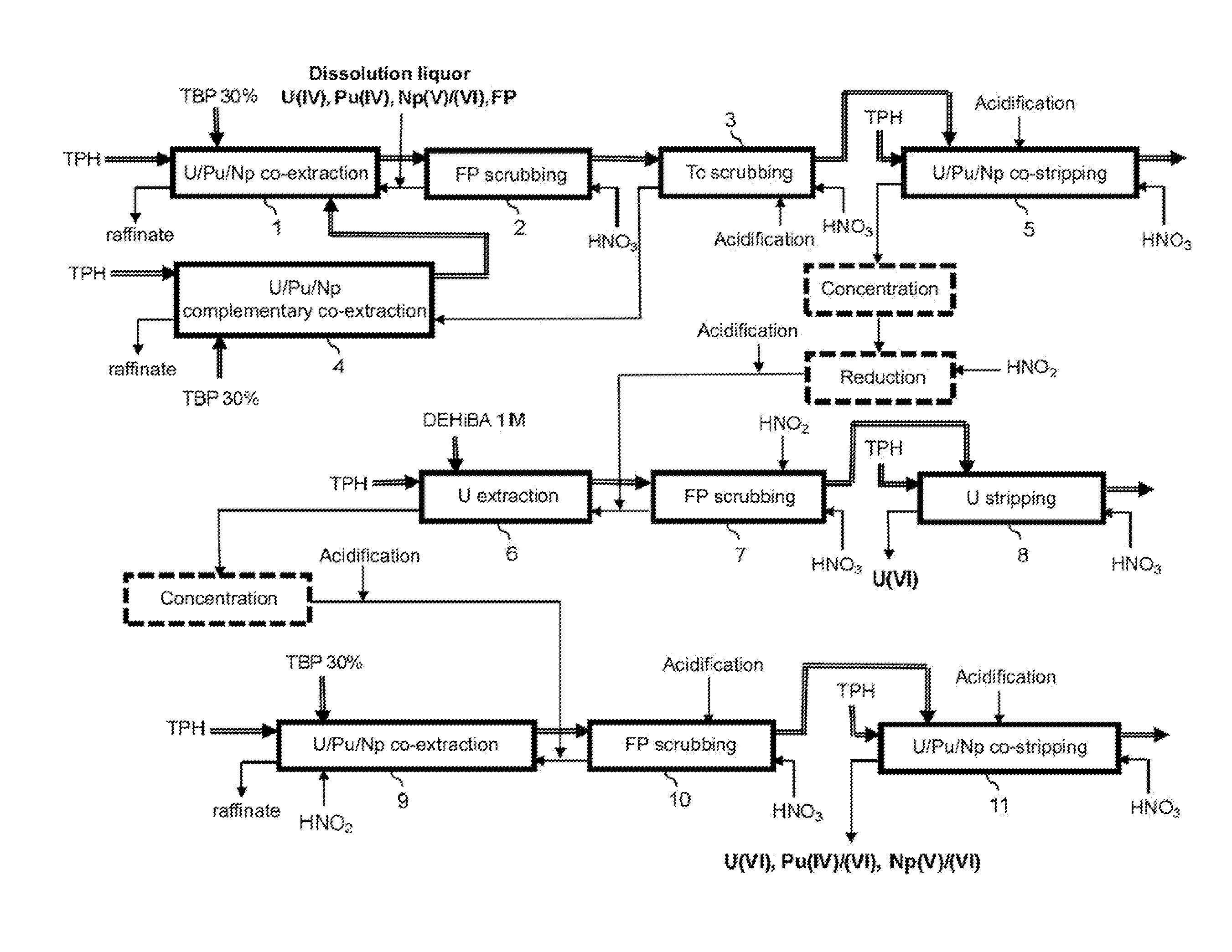
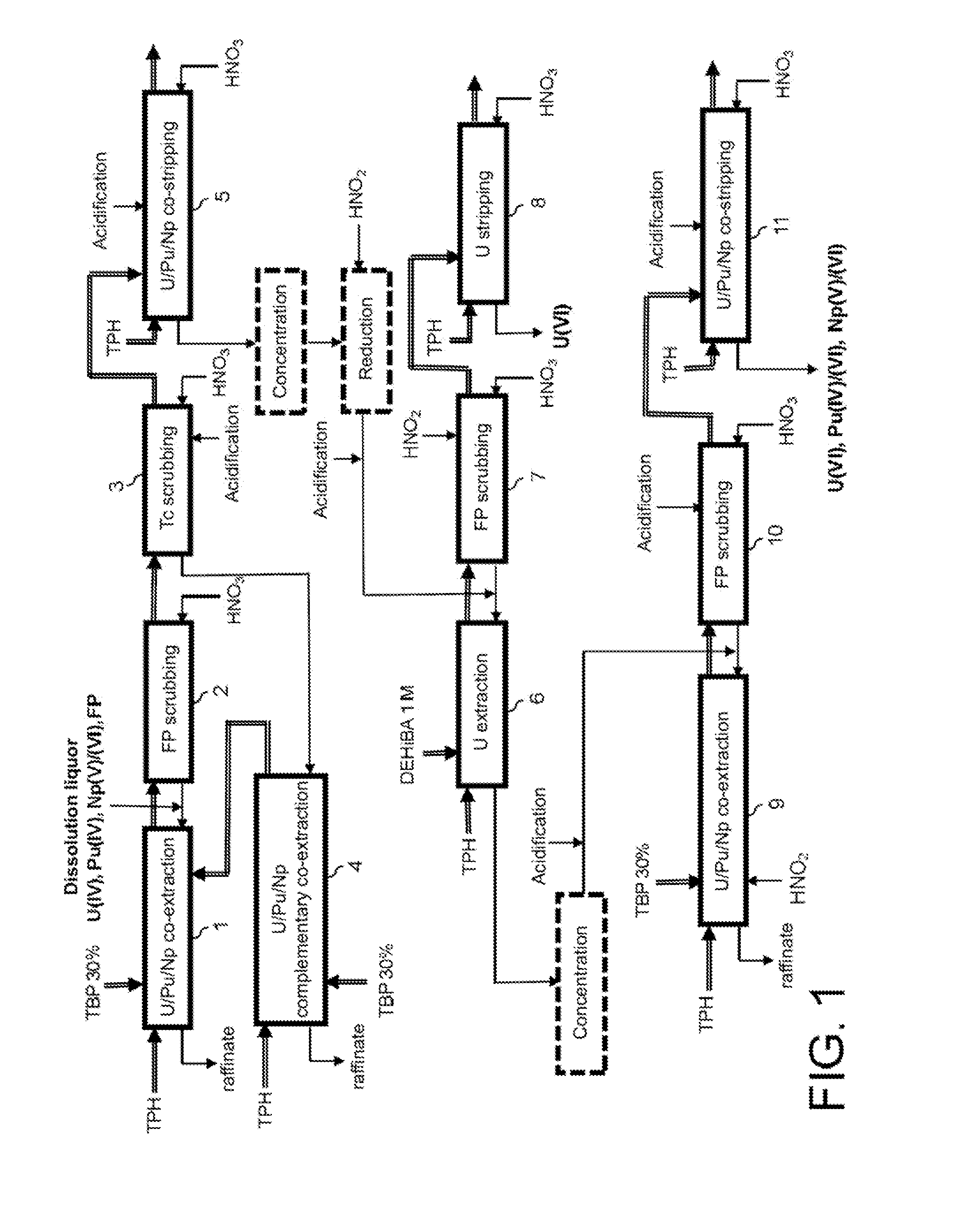
Process for reprocessing a spent nuclear fuel and of preparing a mixed uranium-plutonium oxide
ActiveUS20070290178A1Risk minimizationUranium compounds preparationSolvent extractionUranium oxideDissolution
The invention relates to a process for reprocessing a spent nuclear fuel and for preparing a mixed uranium-plutonium oxide, which process comprises: a) the separation of the uranium and plutonium from the fission products, the americium and the curium that are present in an aqueous nitric solution resulting from the dissolution of the fuel in nitric acid, this step including at least one operation of coextracting the uranium and plutonium from said solution by a solvent phase; b) the partition of the coextracted uranium and plutonium to a first aqueous phase containing plutonium and uranium, and a second aqueous phase containing uranium but no plutonium; c) the purification of the plutonium and uranium that are present in the first aqueous phase; and d) a step of coconverting the plutonium and uranium to a mixed uranium / plutonium oxide. Applications: reprocessing of nuclear fuels based on uranium oxide or on mixed uranium-plutonium oxide.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +2
Preparation method and application of uranium-based ternary carbide
ActiveCN107010960AHigh melting pointImprove thermal conductivityNuclear energy generationReactor fuel susbtancesFault toleranceNuclear power
The invention discloses a preparation method and application of uranium-based ternary carbide, and is used for overcoming the shortcomings of the uranium monocarbidc (UC) and uranium dioxide (UO2) nuclear fuels in the prior art on the aspects of melting point, heat conduction, irradiance resistance, and the like. The preparation method comprises the steps of processing mixing uranium carbide powder and transition metal carbide powder or mixing uranium dioxide powder and transition metal carbide powder and carbon powder, placing in a designed graphite jig, and allowing reactive sintering to obtain high-stability uranium-based ternary carbide. The uranium-based ternary carbide prepared by the preparation method of the invention has the characteristics of high melting point, high heat conductivity and good irradiation resistance, and can be used as an accident fault-tolerance nuclear fuel of a nuclear power station, or a nuclear fuel of a nuclear-powered rocket.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
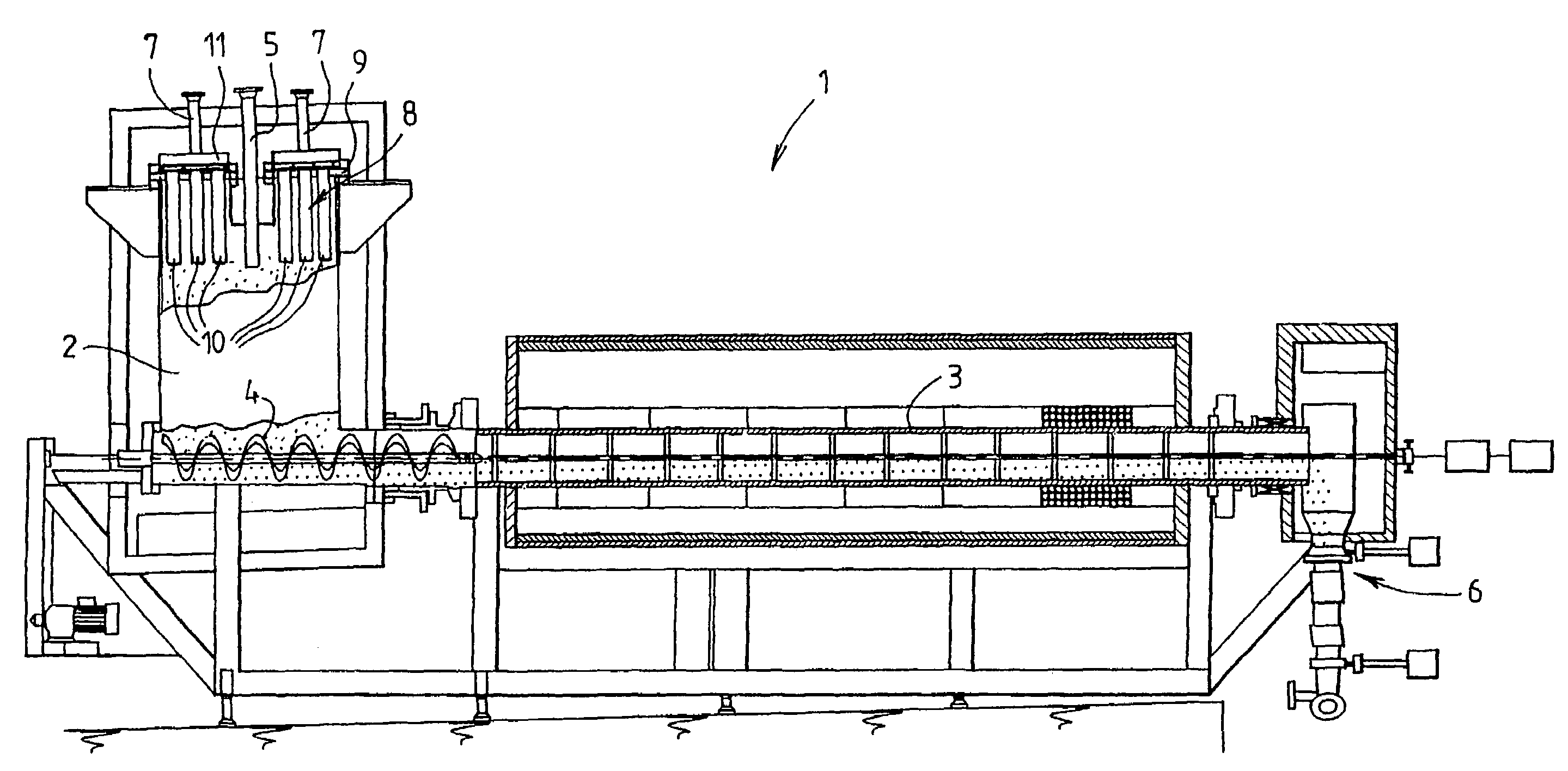
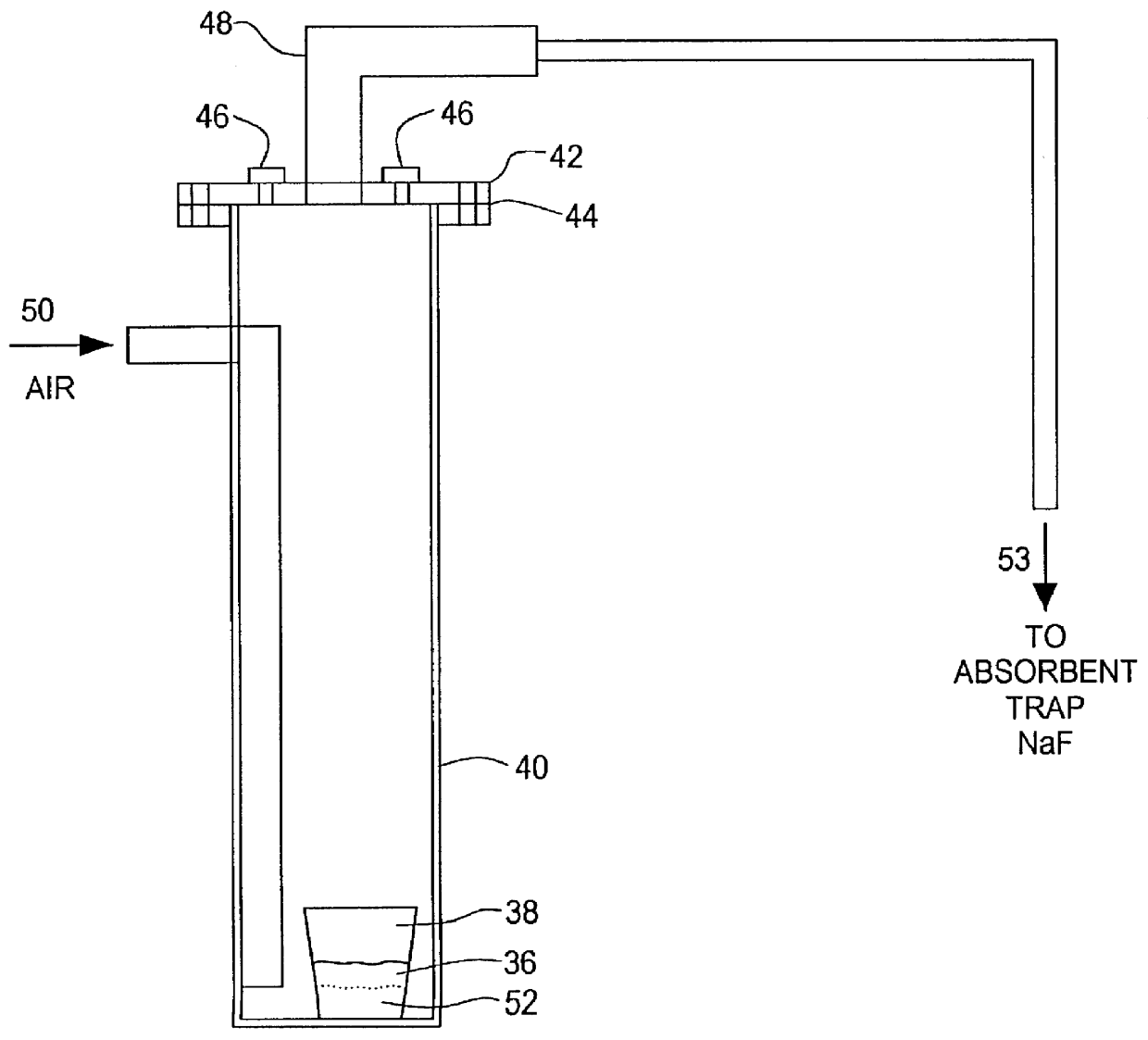
Method and device for declogging filter
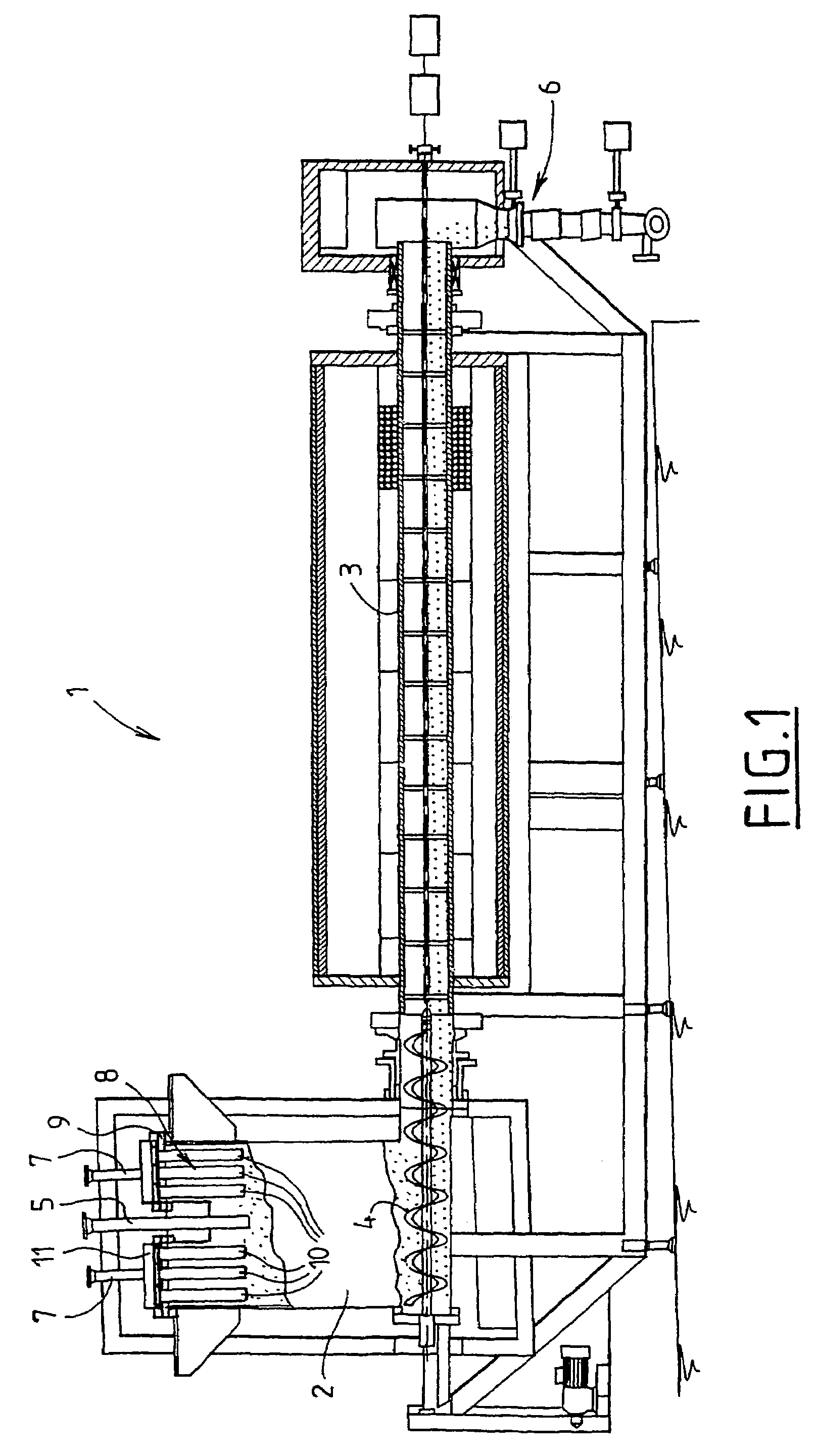
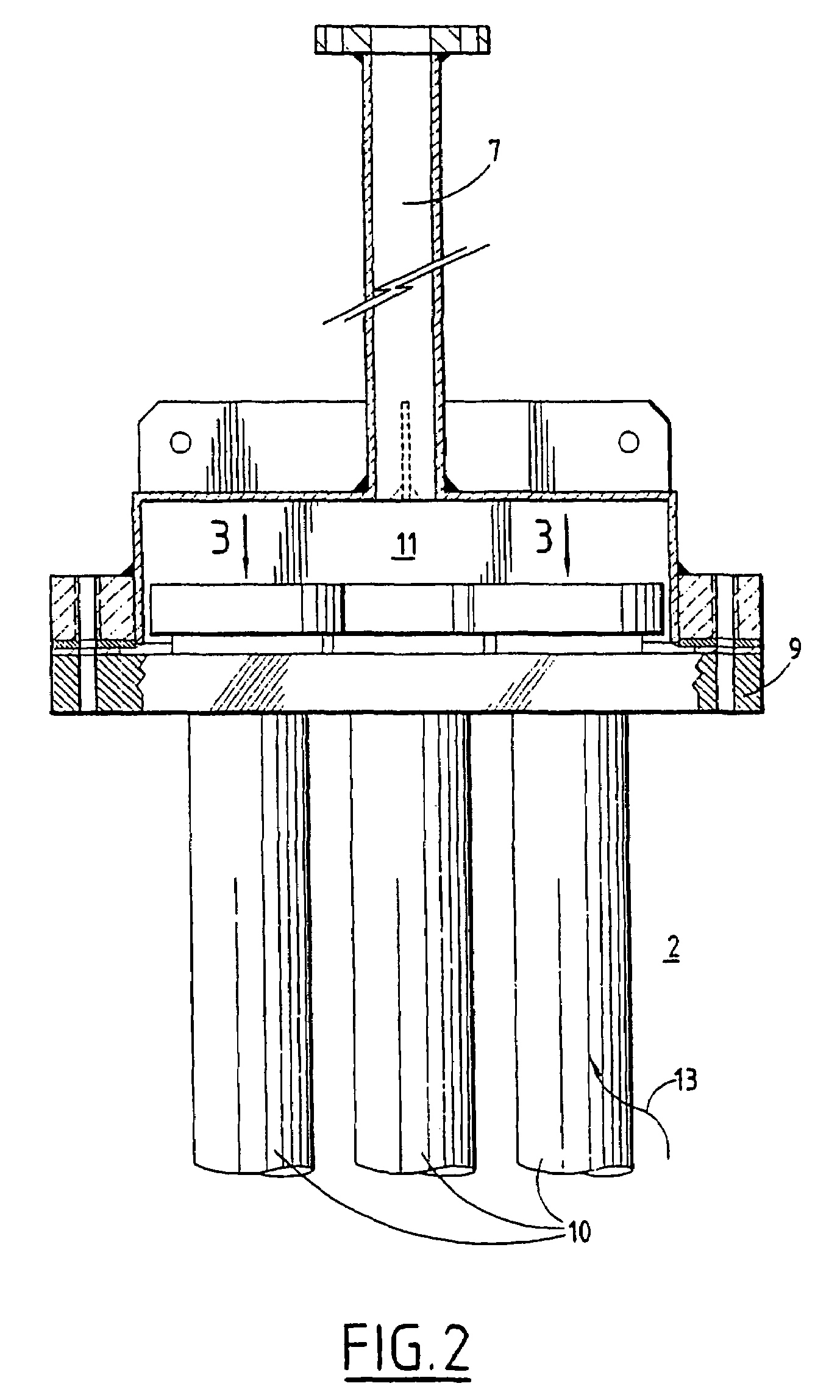
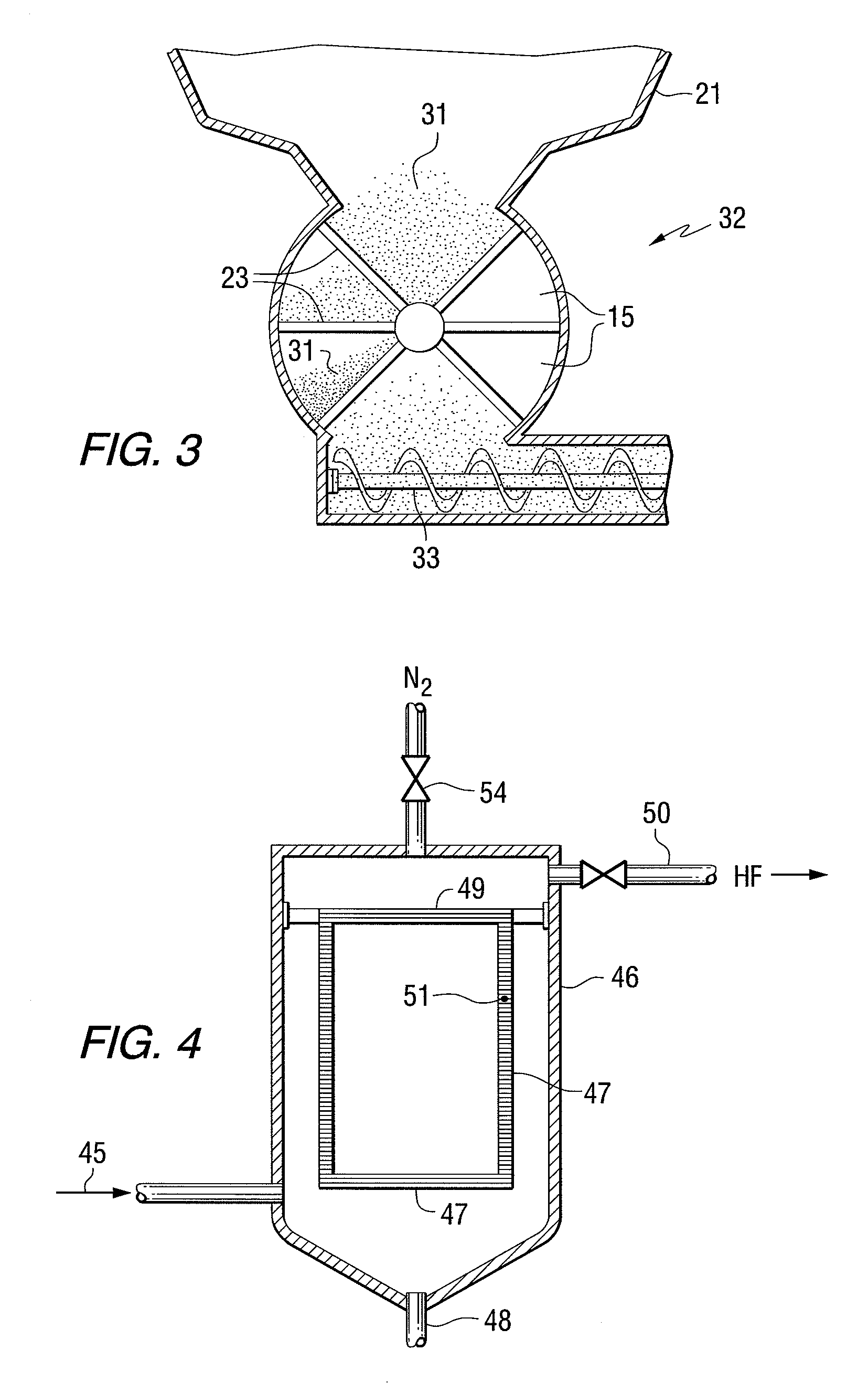
A method of declogging at least one filter of a plant for manufacturing uranium oxide from uranium hexafluoride, including separating, from the wall of the filter, uranium oxyfluoride particles deposited, by a stream of inert gas such as nitrogen, injected into the filter, in a counter-currentwise direction to the flow of hydrofluoric acid.
Owner:AREVA NP SAS
Two step dry UO2 production process utilizing a positive sealing valve means between steps
ActiveUS7824640B1Inhibition amountHigh densityTransuranic element compoundsNuclear energy generationTemperature controlNuclear grade
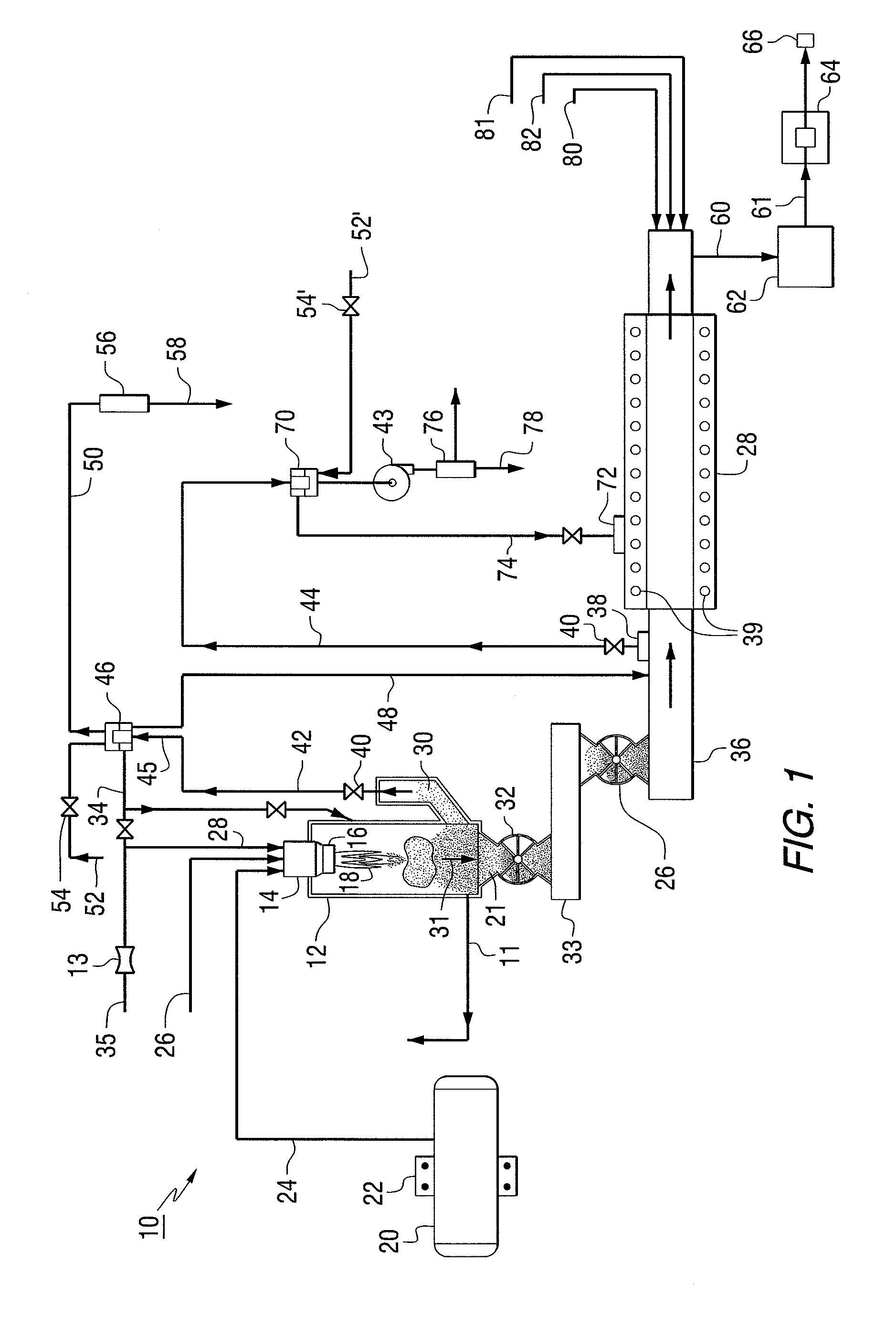
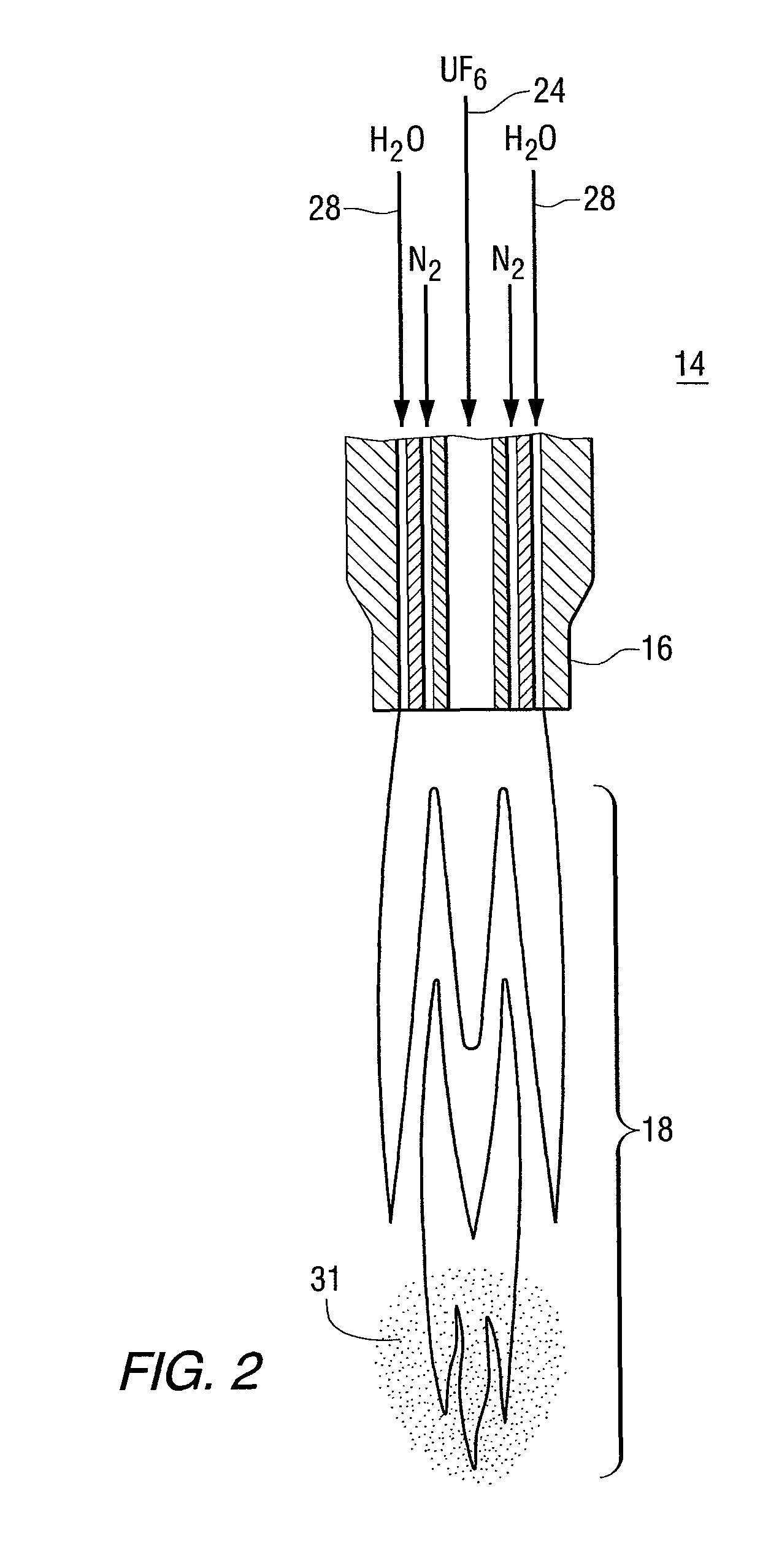
The present invention provides a two-step process for producing nuclear grade, active uranium dioxide (UO2) powder in which the first step comprises reacting uranium hexafluoride (UF6) with steam in a flame reactor to yield uranyl fluoride (UO2F2); and the second step comprises removing fluoride and reducing UO2F2 to uranium dioxide (UO2) in a kiln under a steam / hydrogen atmosphere. The two-step process, each step separated by a positive sealed valve means to prevent gas, particularly H2 flow back, tightly controls the exothermicity of the reaction, which allows for a very tight temperature control which controls the growth of the particles and results in UO2 powder that is active and of consistent morphology.
Owner:WESTINGHOUSE ELECTRIC CORP
Method of in-situ monitoring a reduction of uranium oxides by lithium metal
InactiveUS7390392B1Simplified of conversion yieldEasy to measureFrom normal temperature solutionsElectrolysis componentsLithium oxideUranium oxide
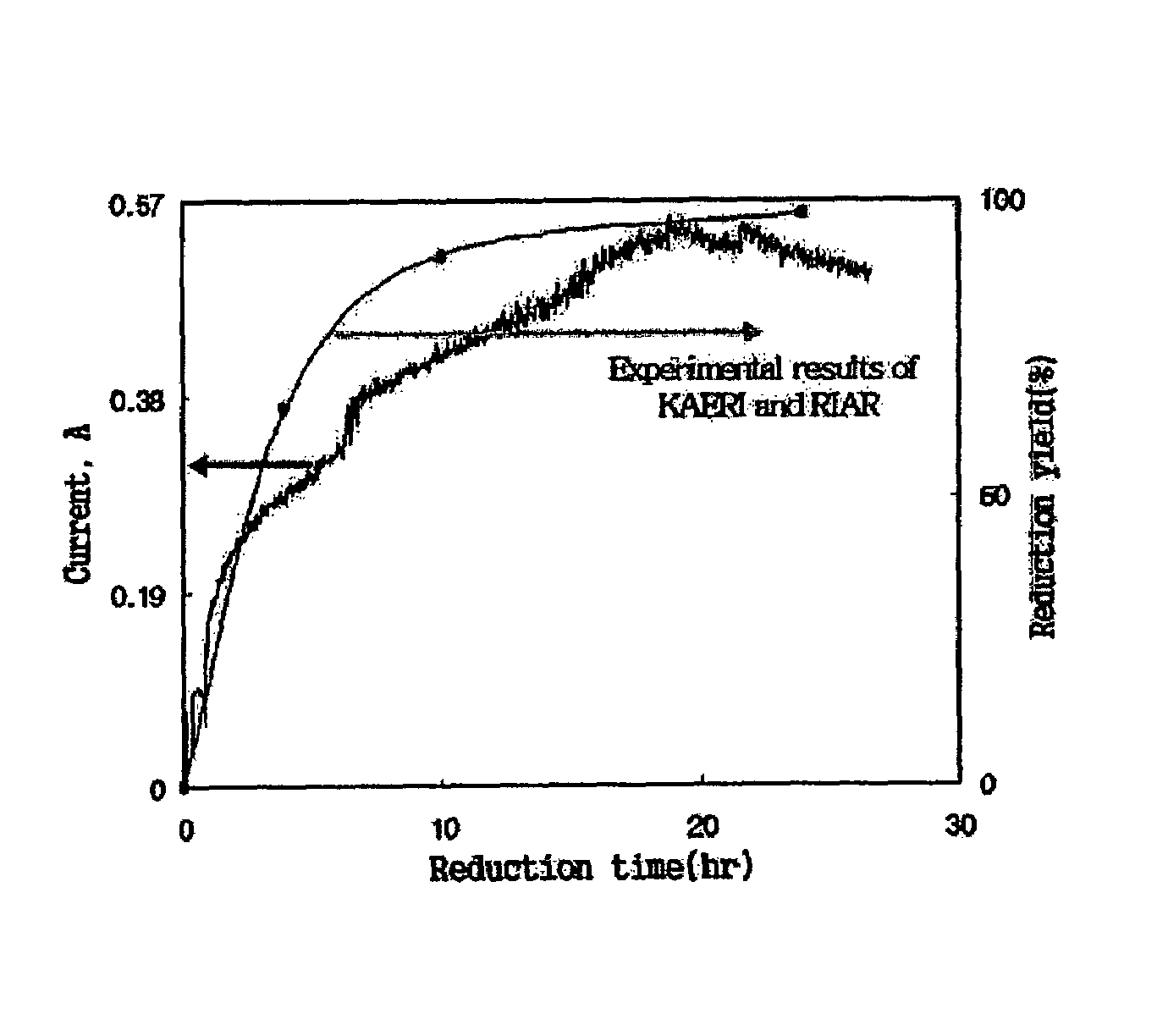
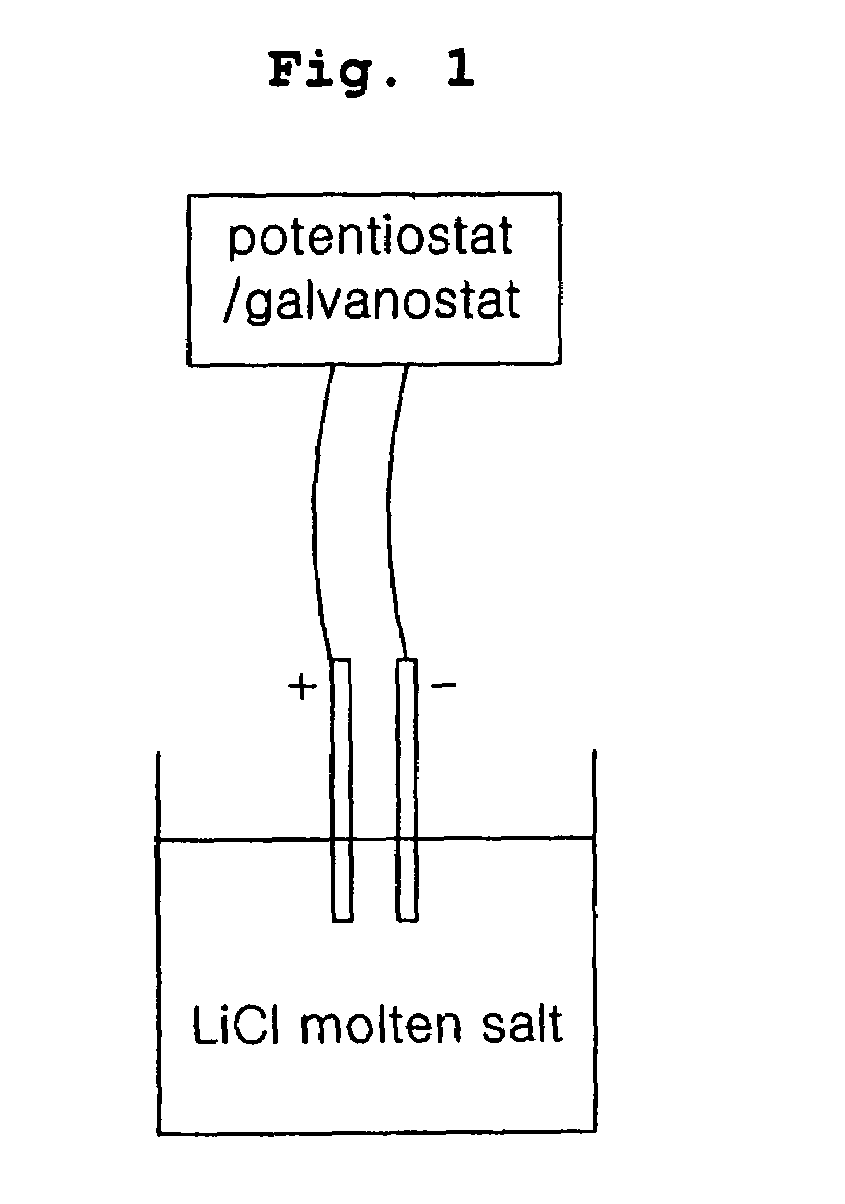
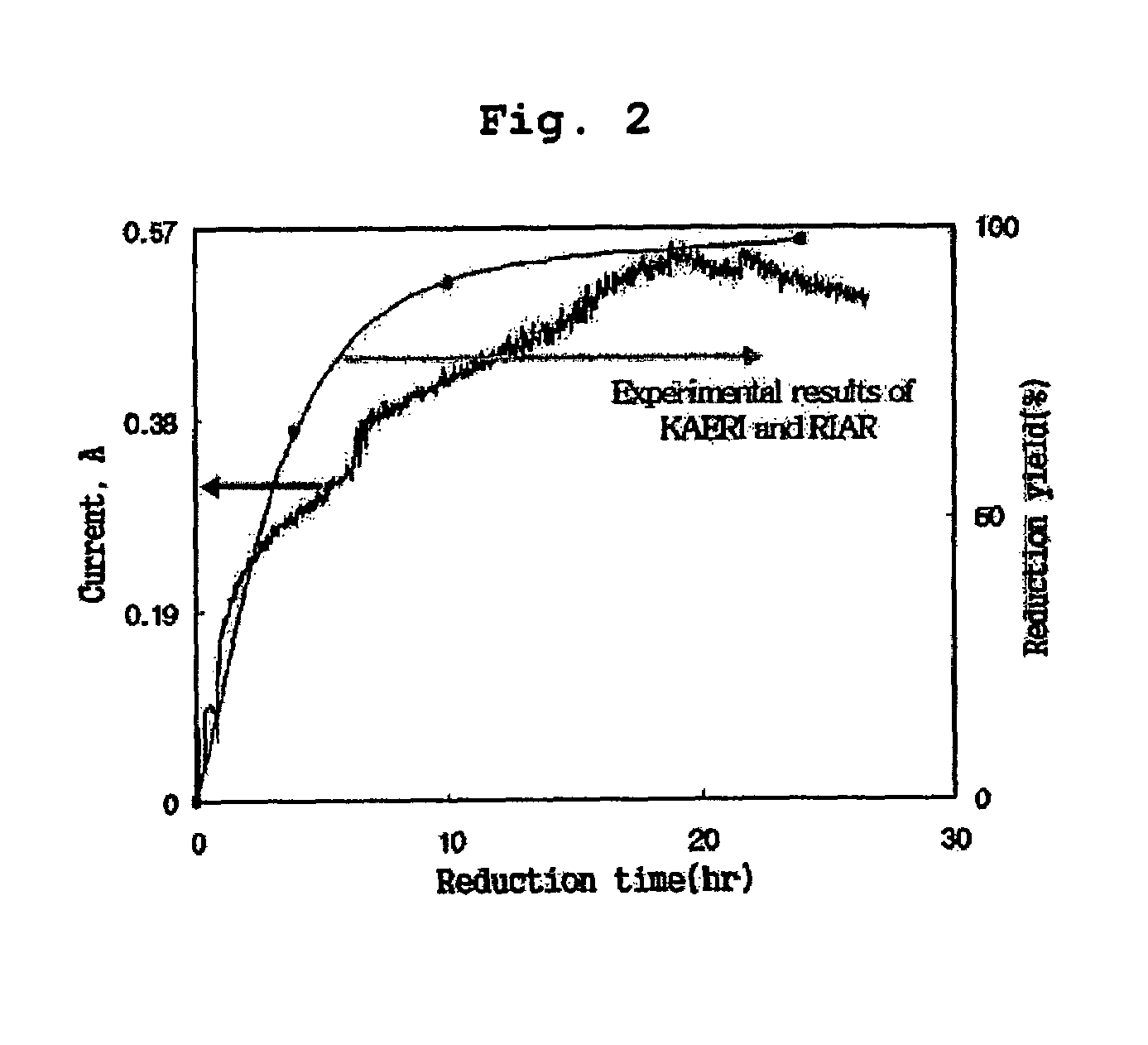
Disclosed is a method of in-situ monitoring a reduction process of uranium oxides by lithium metal, wherein a conversion yield of uranium metal from uranium oxides upon production of uranium metal through a reaction of uranium oxides (UOx, x≦3) with lithium metal in the presence of a high-temperature molten salt is measured according to an electrochemical analysis based on an oxidation of an oxygen ion and a reduction of a lithium ion dissociated from lithium oxide obtained as a by-product of the reaction, by use of a measuring device composed of a potentiostat / galvanostat and a reactor provided with an anode and a cathode. The in-situ monitoring method of the current invention is advantageous in terms of fast and simplified measuring techniques, by directly measuring the reduction process of uranium oxides at the anode and cathode connected to the potentiostat / galvanostat in the presence of the high-temperature molten salt.
Owner:KOREA ATOMIC ENERGY RES INST +1
Uranium purification method for ammonium biuranate
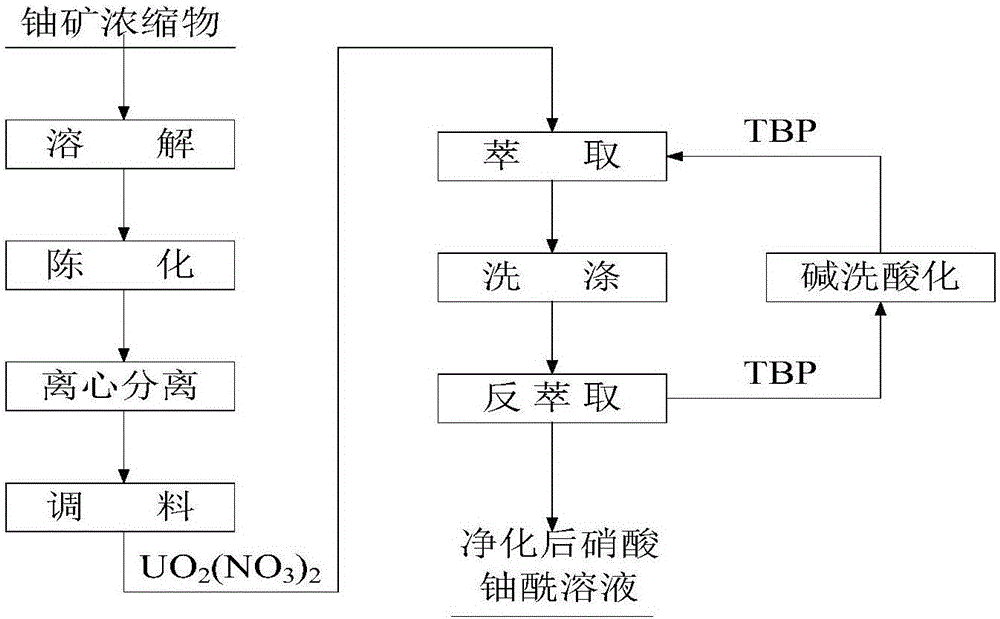
The invention belongs to the technical field of dissolution and purification treatment technologies for ammonium biuranate in the uranium purification and conversion process, in particular to the technologies of dissolution of the ammonium biuranate, extraction and purification treatment of a uranyl nitrate solution and the like and provides a uranium purification method for the ammonium biuranate. The uranium purification method for the ammonium biuranate comprises the following steps that (1) dissolution is conducted so as to prepare a qualified uranyl nitrate solution for extraction and purification; (2) extraction is conducted, the solution to be extracted and an extraction agent are mixed and conduct matter transfer in a pulse extraction column, uranium in the material is carried into the extraction agent, and an organic phase containing the uranium is obtained; (3) washing is conducted, the organic phase containing the uranium and a washing agent are fully mixed and conduct matter transfer in a pulse washing column, impurity elements in the organic phase containing the uranium are rewashed and enter a water phase, the purity of the uranium is further improved, and the purified organic phase containing the uranium is obtained; and (4) reverse extraction is conducted, the purified organic phase containing the uranium and the washing agent are fully mixed and conduct matter transfer in the pulse washing column, the uranium enters the water phase again through reverse extraction, the purified uranyl nitrate solution is obtained, and an uranium oxide is prepared from the purified uranyl nitrate solution through concentration and denitration manners.
Owner:THE 404 COMPANY LIMITED CHINA NAT NUCLEAR
Mine safety helmet
InactiveCN104886861AImprove fire resistanceImprove stress resistanceHelmetsHelmet coversUranium oxideThorium oxide
The invention discloses a mine safety helmet. The mine safety helmet comprises a helmet body and a waistband box which are connected through a cable. The helmet body is coated with a silver light layer and comprises an inner layer, a filling layer, a zinc coating and a coating. The inner layer is made of PC materials. The filling layer is an asbestos layer. The zinc coating is colored through static plastic painting. The coating is made of at least one of oxide powder including aluminum oxide, lanthanum oxide, beryllia, calcium oxide, zirconia, uranium oxide, magnesium oxide, cerium oxide, thorium oxide, silicon dioxide and silicon dioxide or the mixture of two or more of the oxide powder. A verbal system, a gas detecting device, a positioning device and a vital sign information wireless transmitting system are additionally arranged on the basis of an ordinary helmet, the function of carrying out safety monitoring on every worker is achieved, and the safety of underground workers is greatly improved.
Owner:SHANGHAI VIRGIN TECH CO LTD
Method for producing uranium oxide from uranium oxyfluoride
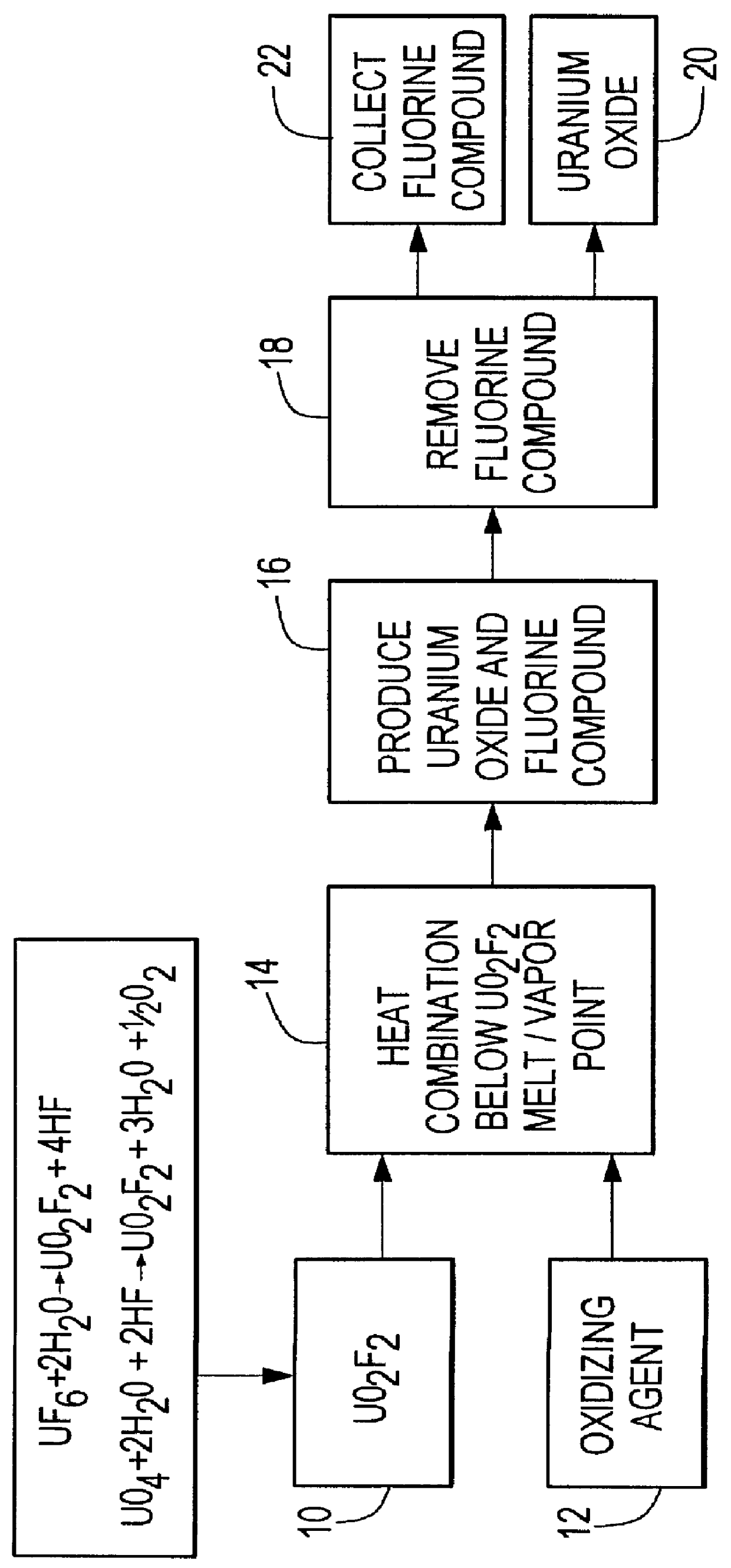
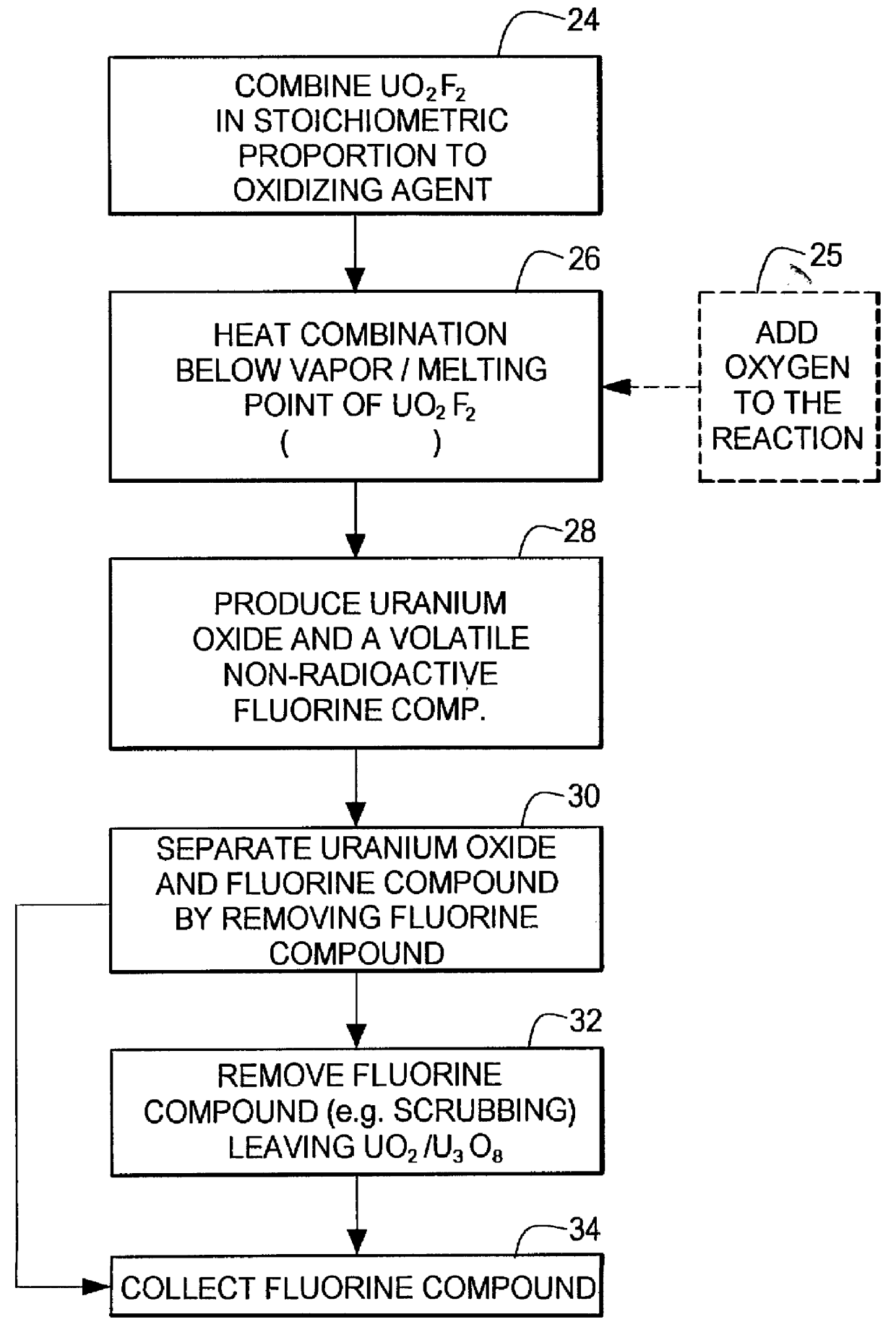
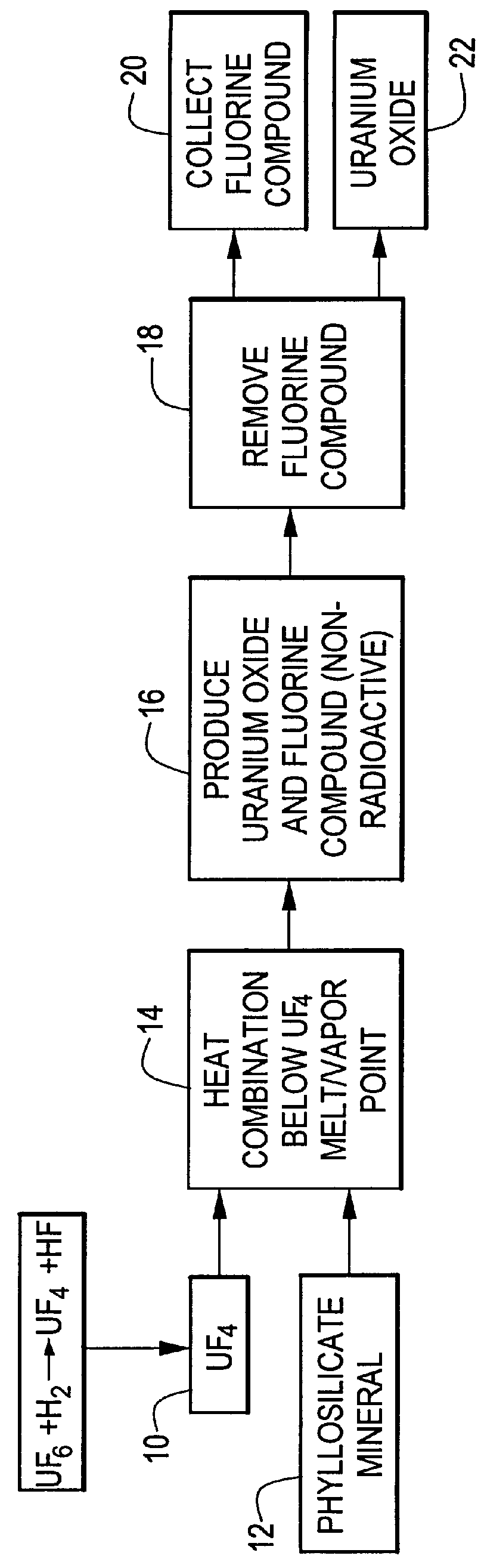
InactiveUS6096281AReduced thermodynamic stabilityAvoid vaporizationPhosphorus halides/oxyhalidesFluoride preparationUranium oxideOxidizing agent
A method for producing uranium oxide includes combining uranium oxyfluoride and a solid oxidizing agent having a lower thermodynamic stability than the uranium oxide after "oxide"; heating the combination below the vapor point of the uranium oxyfluoride to sufficiently react the uranium oxyfluoride and the oxidizing agent to produce uranium oxide and a non-radioactive fluorine compound; and removing the fluorine compound after "compound".
Owner:INT ISOTOPES
Pencil comprising a stack of oxide nuclear fuel pellets
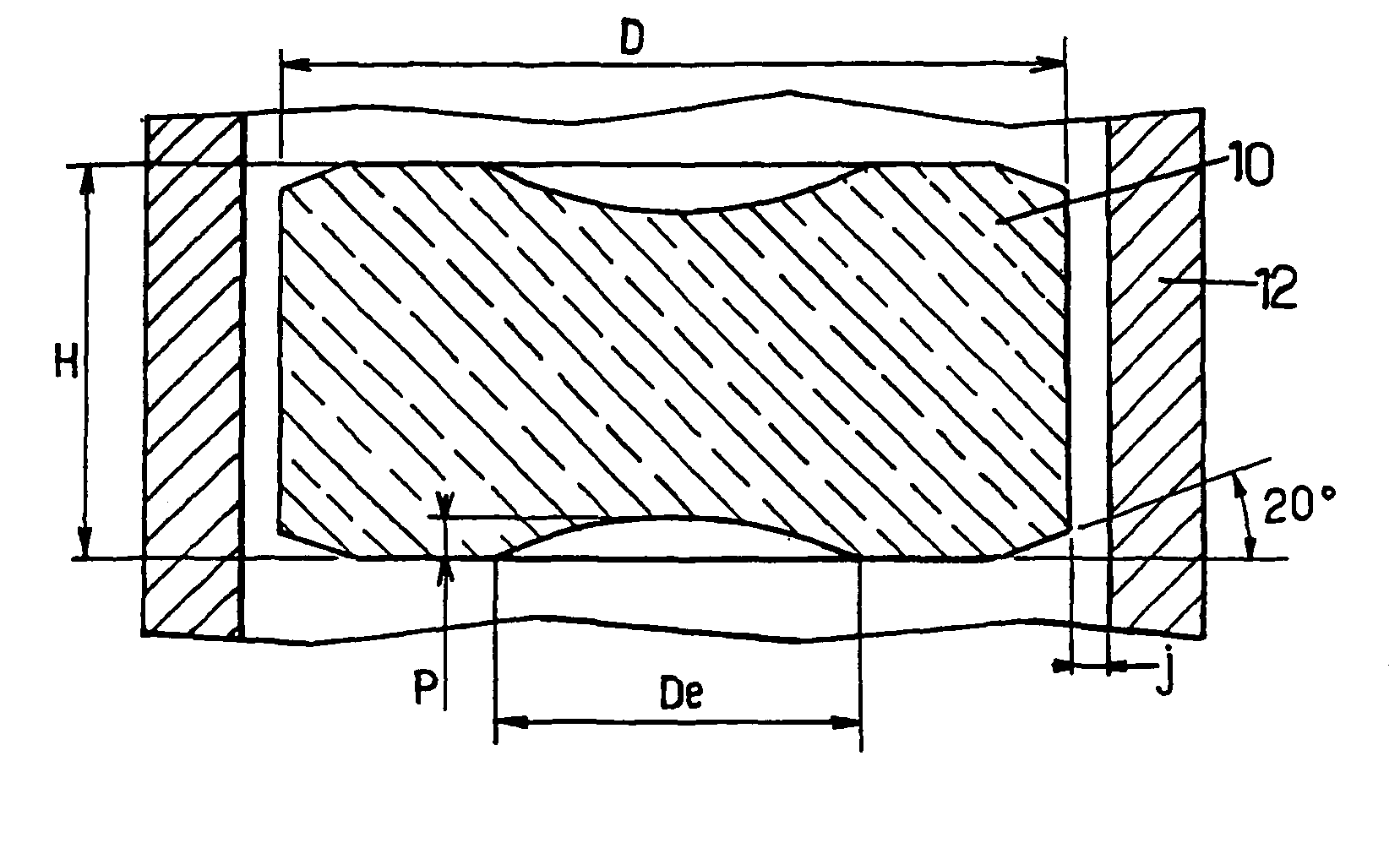
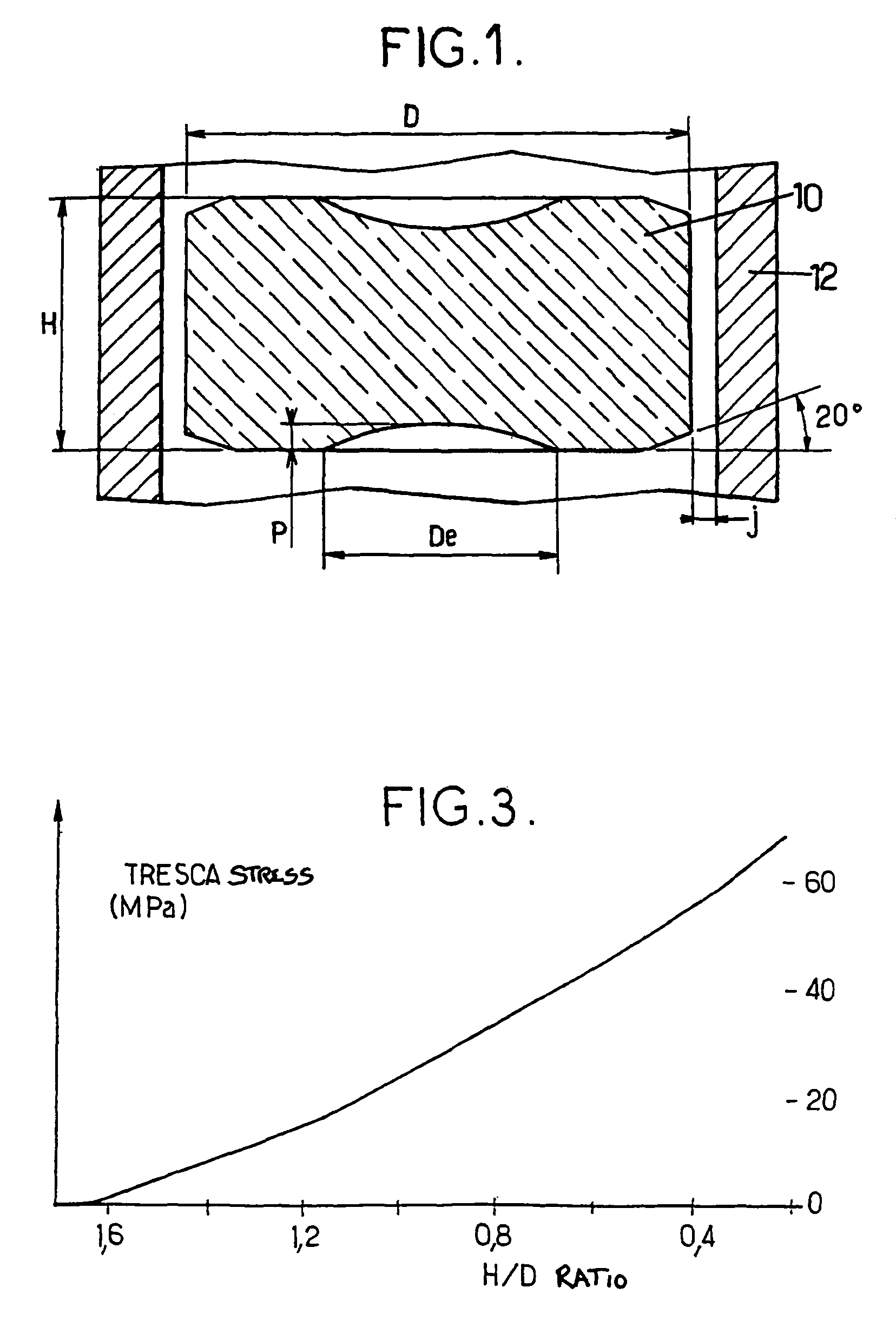
InactiveUS9053830B2Reduce hoop stressImprove creepFuel elementsNuclear energy generationUranium oxideEnriched uranium
The rod contains substantially cylindrical oxide nuclear fuel pellets based on enriched uranium oxide. The H / D ratio of the height over the diameter of the pellets lies in the range 0.4 to 0.6. The initial diametral clearance between the pellets and the cladding does not exceed 200 μm.
Owner:FRAMATOME ANP
Method of preparing ceramic grade uranium dioxide ball
The invention discloses a method of preparing a ceramic grade uranium dioxide ball. The method sequentially comprises the following steps: carrying out ball milling on uranium oxide, wherein the particle size of the uranium oxide after ball milling is 0.1-0.3 micron; adding the uranium oxide into a polymer monomer, and uniformly stirring to obtain polymer slurry; producing slurry drops by virtue of the polymer slurry; adding the slurry drops into a dispersion column which contains heated carbon tetrachloride or liquid paraffin, and descending the slurry drops to obtain a green body ball in the dispersion column under the surface tension, wherein the temperature in the dispersion column is 80-90 DEG C; washing the green body ball by adopting an ethanol solution; drying the green body ball, and removing an organic matter on the surface of the green body ball to obtain a dry calcined ball; and carrying out reduction sintering on the dry calcined ball in a hydrogen atmosphere to obtain the ceramic grade UO2 ball. The method is simple in whole process, convenient in realization, small in introduced impurities, high in purity of the prepared UO2 ball and beneficial to popularizing and applying the UO2 ball in the field of nuclear technology.
Owner:NUCLEAR POWER INSTITUTE OF CHINA
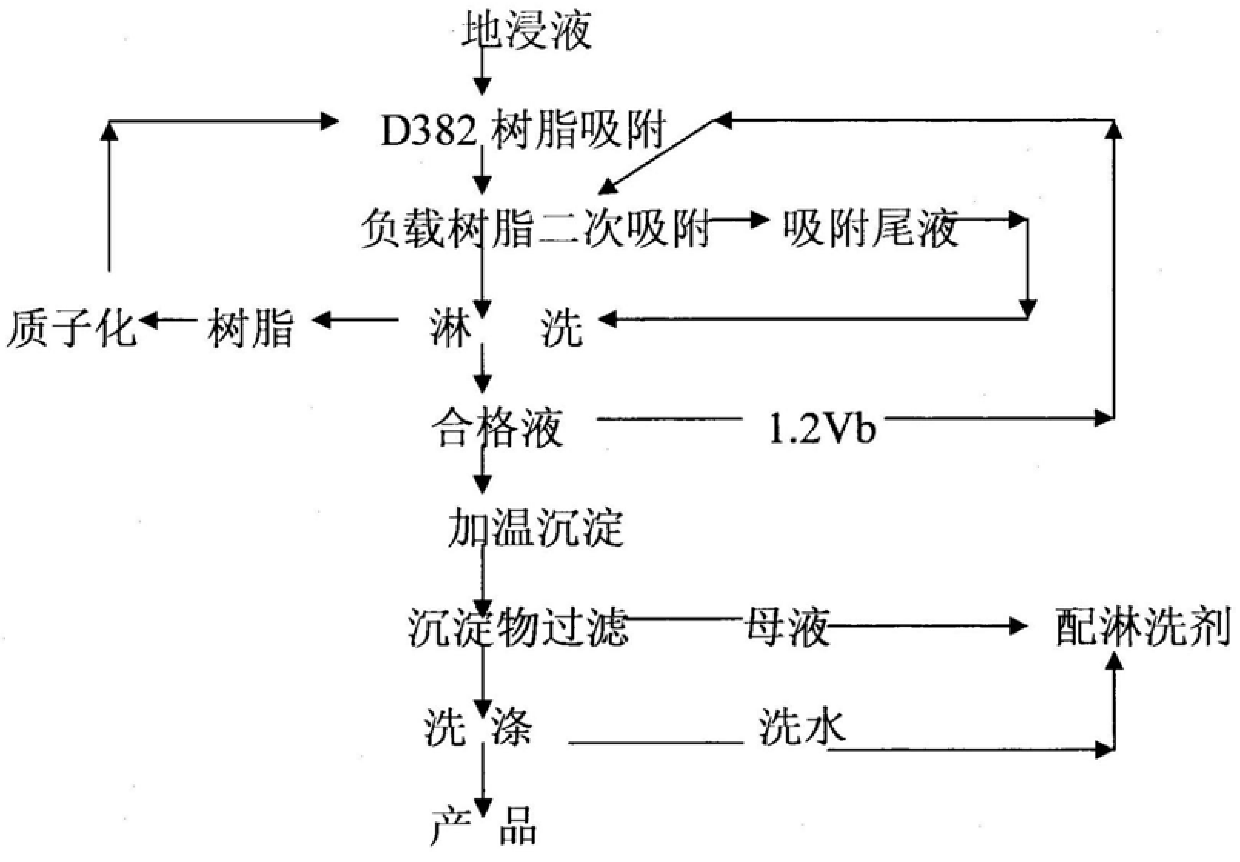
Method for recovering uranium from weak alkaline leaching solution with high chloride ion and high salinity
The invention belongs to the technical field of uranium ore water smelting technology, and specifically adopts anion exchange resin to adsorb D382, loaded resin for secondary adsorption (uranium-loaded resin absorbs uranium in the leaching qualified liquid), ammonium chloride plus sodium carbonate leaching It is a process for recovering uranium from the weakly alkaline leaching solution with high chlorine and high salinity by washing and heating precipitation. It effectively solves the problem of effectively adsorbing uranium from the weak alkaline leaching solution with high chloride ion and high salinity as high as 6g.L-1 and about 10g.L-1 salinity. Rinse the loaded resin with NH4Cl+Na2CO3 solution to obtain a satisfactory rinsing effect. The secondary adsorption of the loaded resin (the loaded resin is in contact with part of the eluting qualified solution) effectively increases the uranium concentration of the eluting qualified solution. After the eluting qualified solution is heated to drive off ammonium and carbon dioxide, uranium oxide precipitates are obtained. The precipitation process reduces the consumption of reagents and fresh water, simplifies the precipitation process, and at the same time obtains uranium products with good filtration and dehydration performance. The product obtained under the test conditions contains 79.96% uranium, 0.046% chloride ion and 2.91% sulfate radical.
Owner:BEIJING RESEARCH INSTITUTE OF CHEMICAL ENGINEERING AND METALLURGY
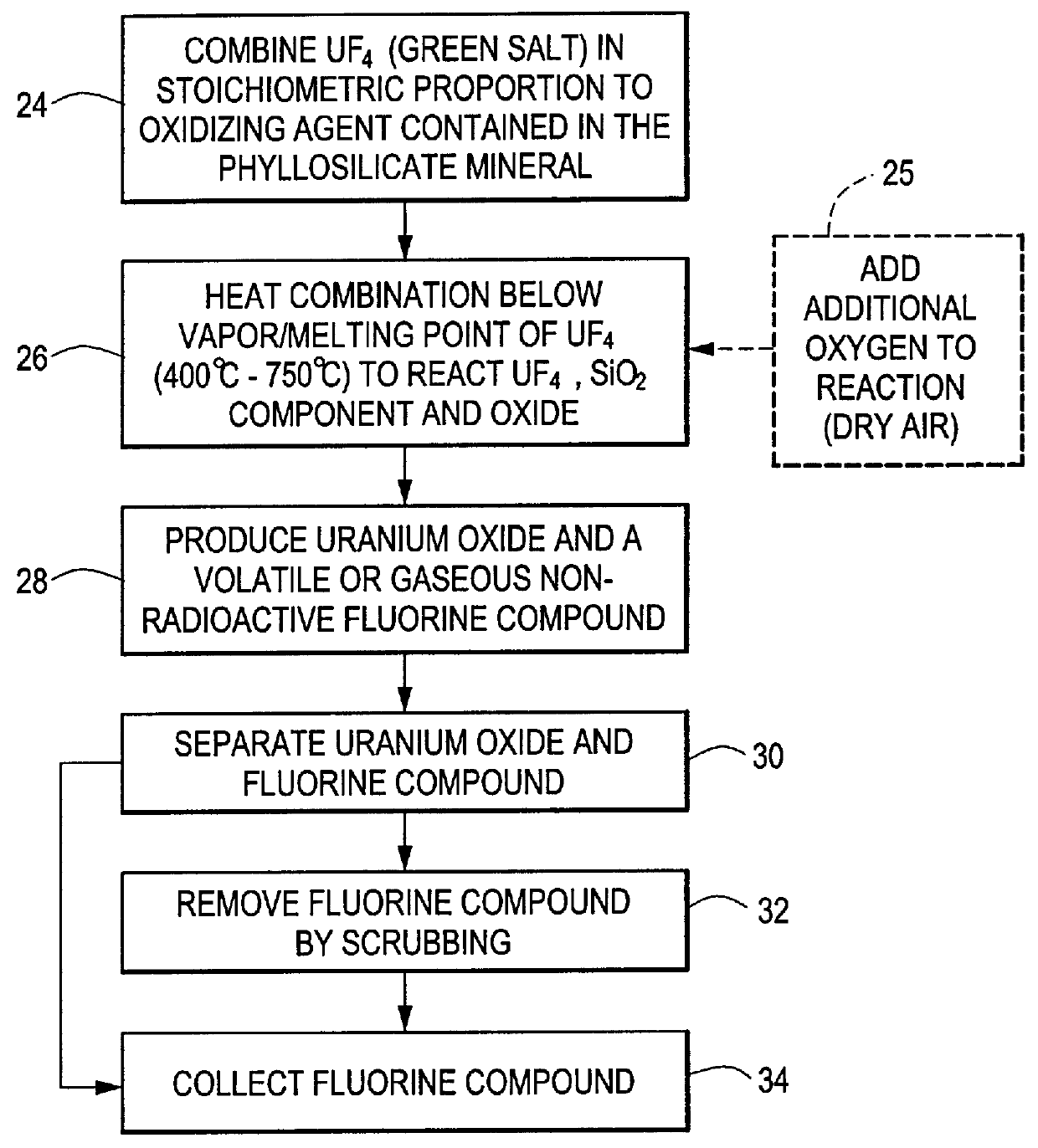
Method for producing uranium oxide from uranium tetrafluoride and a phyllosilicate mineral
InactiveUS6153164AEfficient and cost-effective methodThennodynamic stabilityMagnesium fluoridesSilicon halogen compoundsUranium oxideUranium tetrafluoride
A method for producing uranium oxide includes combining uranium tetrafluoride and a phyllosilicate mineral containing a solid oxidizing agent within the mineral's structure having a lower thermodynamic stability than the uranium oxide; heating the combination below the vapor point of the uranium tetrafluoride to sufficiently react the uranium tetrafluoride and the oxidizing agent to produce uranium oxide and a non-radioactive fluorine compound; and removing the fluorine compound.
Owner:INT ISOTOPES
Preparation method of large-grained UO2 (uranium dioxide) fuel pellets with high heat conductivity
ActiveCN107871540AImprove high temperature stabilityImprove thermal conductivityNuclear energy generationReactor fuel susbtancesNetwork structureTemperature resistance
The invention discloses a preparation method of large-grained UO2 (uranium dioxide) fuel pellets with high heat conductivity. The surfaces of UO2-grain growth promoter compound spheres with good sphericity are uniformly coated with heat conductivity enhanced phase powder, coated particles are obtained and subjected to forming and sintering in a high-temperature atmosphere, so that UO2 grains growunder the action of the grain growth promoter, meanwhile, the heat conductivity enhanced phase coating the surfaces of the UO2 particles is intercommunicated after being liquefied at the high temperature, a three-dimensional network structure is formed, and the enhanced phase and a UO2 matrix form special UO2 fuel pellets with the three-dimensional network structure. The enhanced UO2 fuel palletsprepared with the method have the heat conductivity remarkably superior to that of pure UO2, besides, the size of UO2 grains is notably larger than that of conventional UO2 pellets, the high-temperature resistance and the irradiation stability are excellent, and the safety level of a reactor and a fuel system can be remarkably improved.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
SIMS measurement method for oxygen isotope in insulator core material
ActiveCN103995043AEasy to makeReduce dosageMaterial analysis by electric/magnetic meansChemical treatmentGraphite carbon
The present invention relates to a SIMS measurement method for oxygen isotope in an insulator core material. The process comprises: transferring uranium oxide micro-particles onto a graphite carbon sheet to prepare a sample; debugging equipment required during the measurement process; measuring oxygen isotope of the uranium oxide micro-particles, and calculating the <18>O / <16>O ratio; correcting the measurement value, and calculating the uncertainty; and replacing the standard sample and uninstalling the program. According to the present invention, the chemical treatment of the sample is not required, and the measurement can be directly performed, such that the analysis speed is rapid, the sample preparation is simple, and the sample consumption is low; SIMS has the micro-zone analysis and depth profiling capability, and can perform oxygen isotope measurement on different regions and places having different depths of the sample; and SIMS performs measurements by receiving O<-> without conversion of CO2 gas so as to reduce the intermediate link, reduce the introduction factor of the final result uncertainty, and meet characteristics of rapid and accurate analysis of the nuclear forensics.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Preparation method of monodisperse micron-sized uranium oxide particle
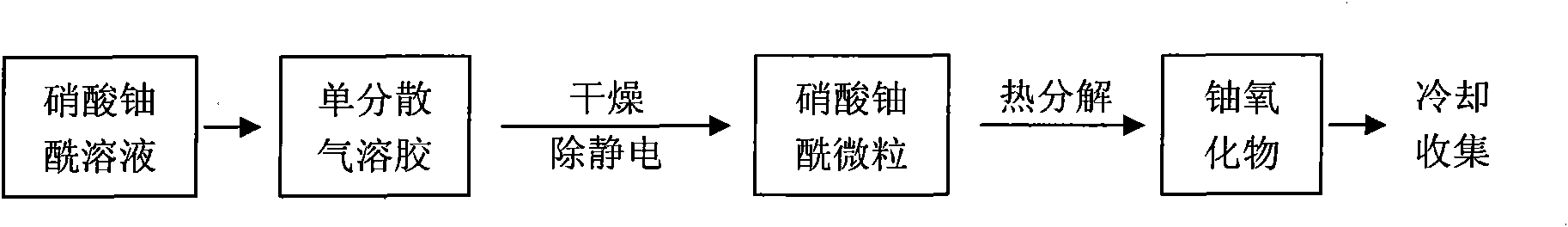
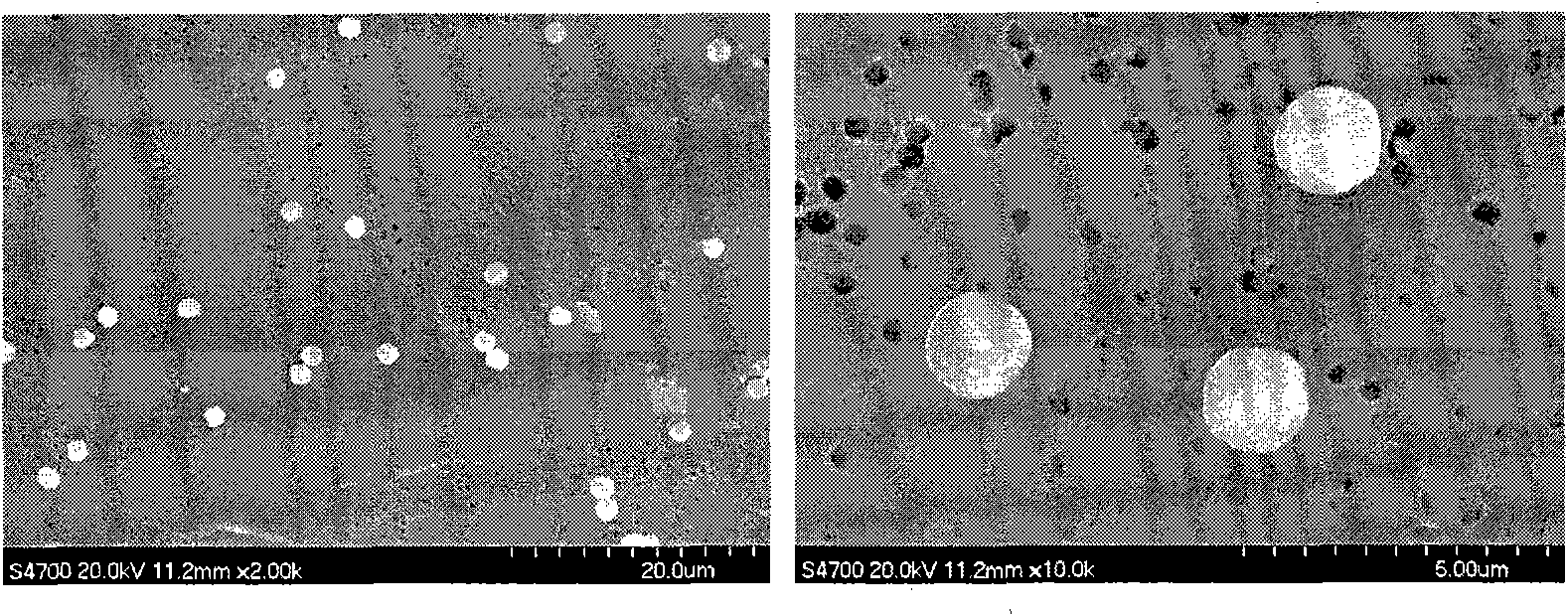
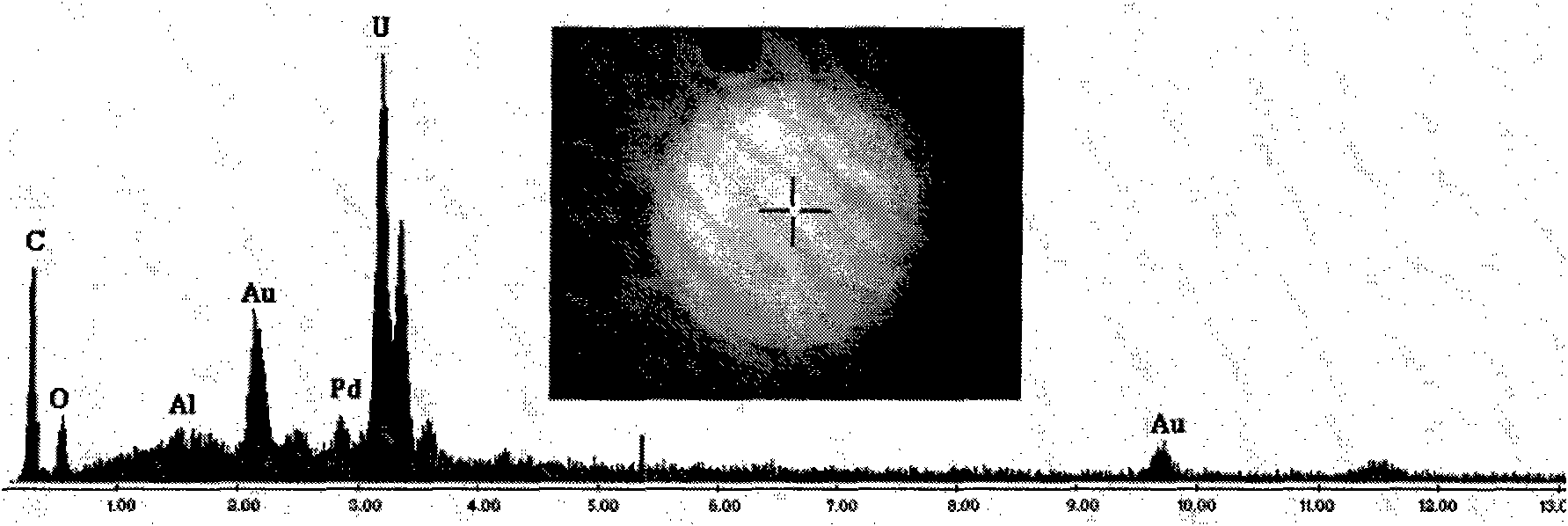
ActiveCN101891253AShould be operatedEnsure safetyUranium oxides/hydroxidesUranium oxideThermal decomposition method
The invention discloses a method for preparing a monodisperse micron-sized uranium oxide particle by using a sol-spray thermal decomposition method. The method comprises the following steps of: forming a monodisperse aerosol by a uranyl nitrate solution by using a vibrating orifice aerosol generator; drying and carrying out static removal to form a uranyl nitrate solid particle; carrying out high-temperature thermal decomposition to form a monodisperse uranium oxide particle; and collecting after cooling, wherein carrier gas is preheated at the back end of a neutralizer to a temperature of 70-80 DEG C or so, the flow of the carrier gas is 35-45 L / min, and the high-temperature thermal decomposition is carried out by the direct heating of a muffle furnace. The technical scheme of the invention is simpler and more convenient and ensures the safety in the operation process.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
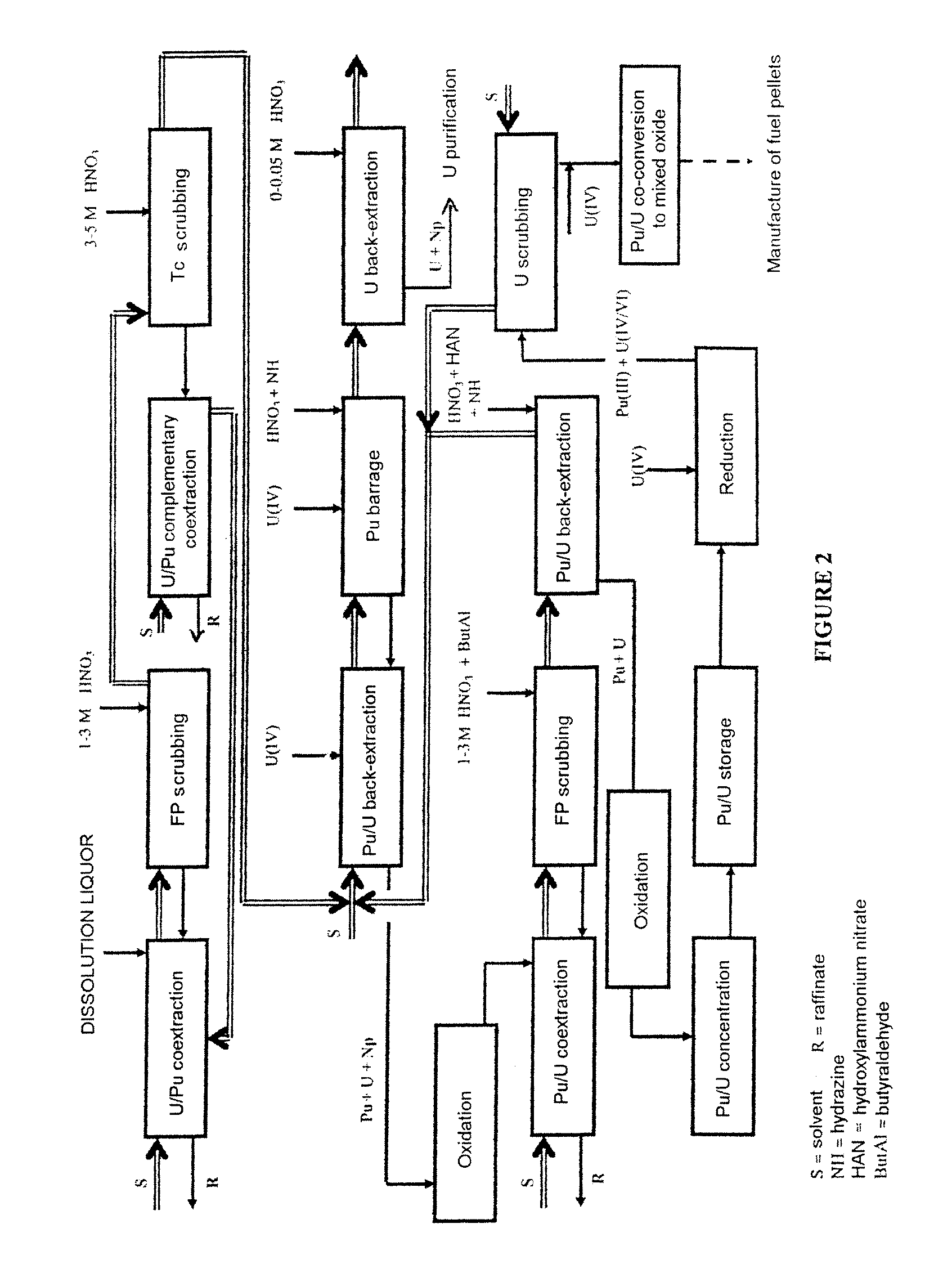
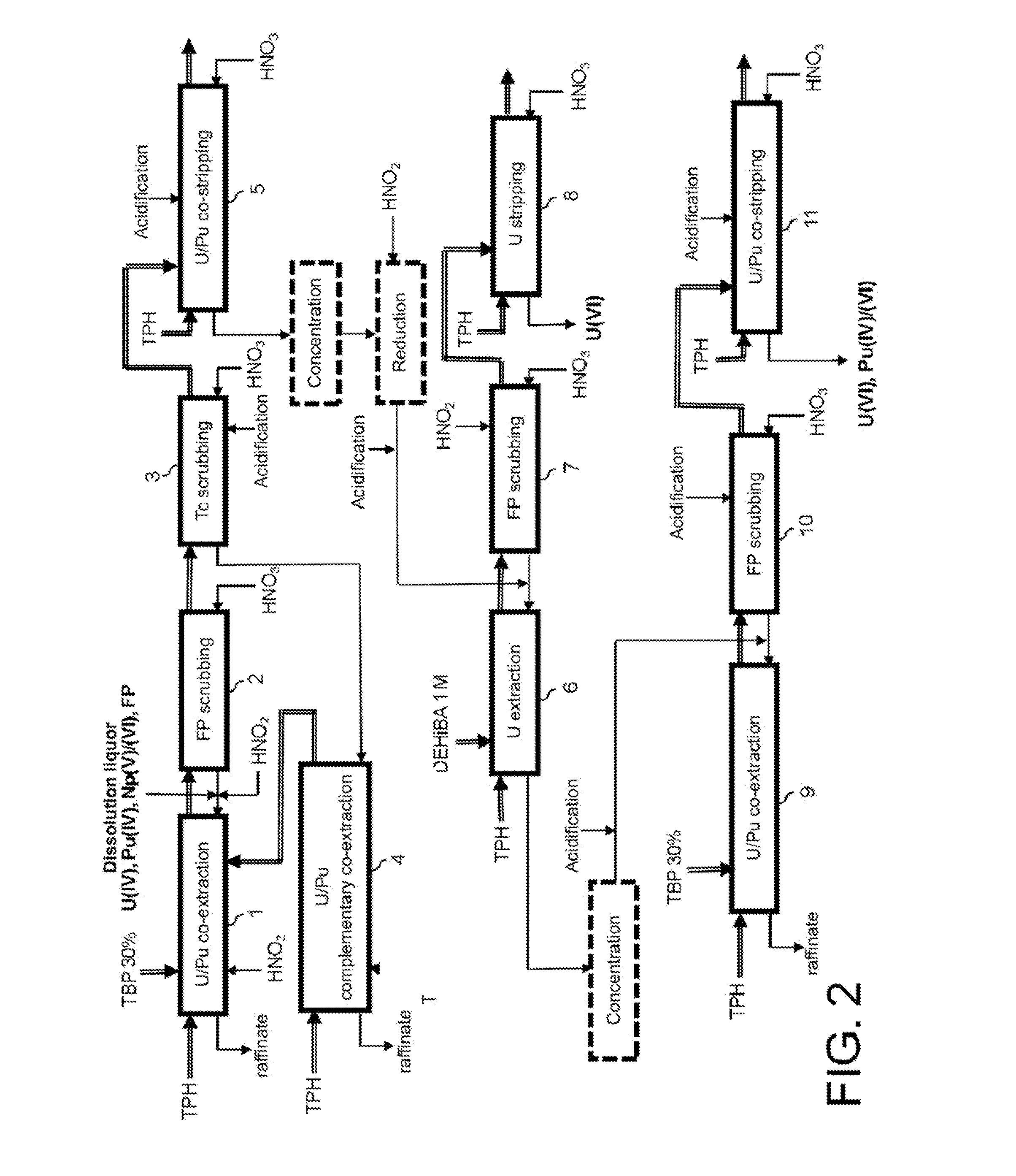
Process for reprocessing spent nuclear fuel not requiring a plutonium-reducing stripping operation
ActiveUS20130202501A1Simplify the management processLower the volumeTransuranic element compoundsSolvent extractionMixed oxideUranium oxide
The invention relates to a process for reprocessing spent nuclear fuel which, among other advantages, does not require a plutonium-reducing stripping operation.This process finds particular application in the processing of uranium oxide fuels and uranium and plutonium mixed oxide fuels.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Soft-light colored glass, and preparation method and use thereof
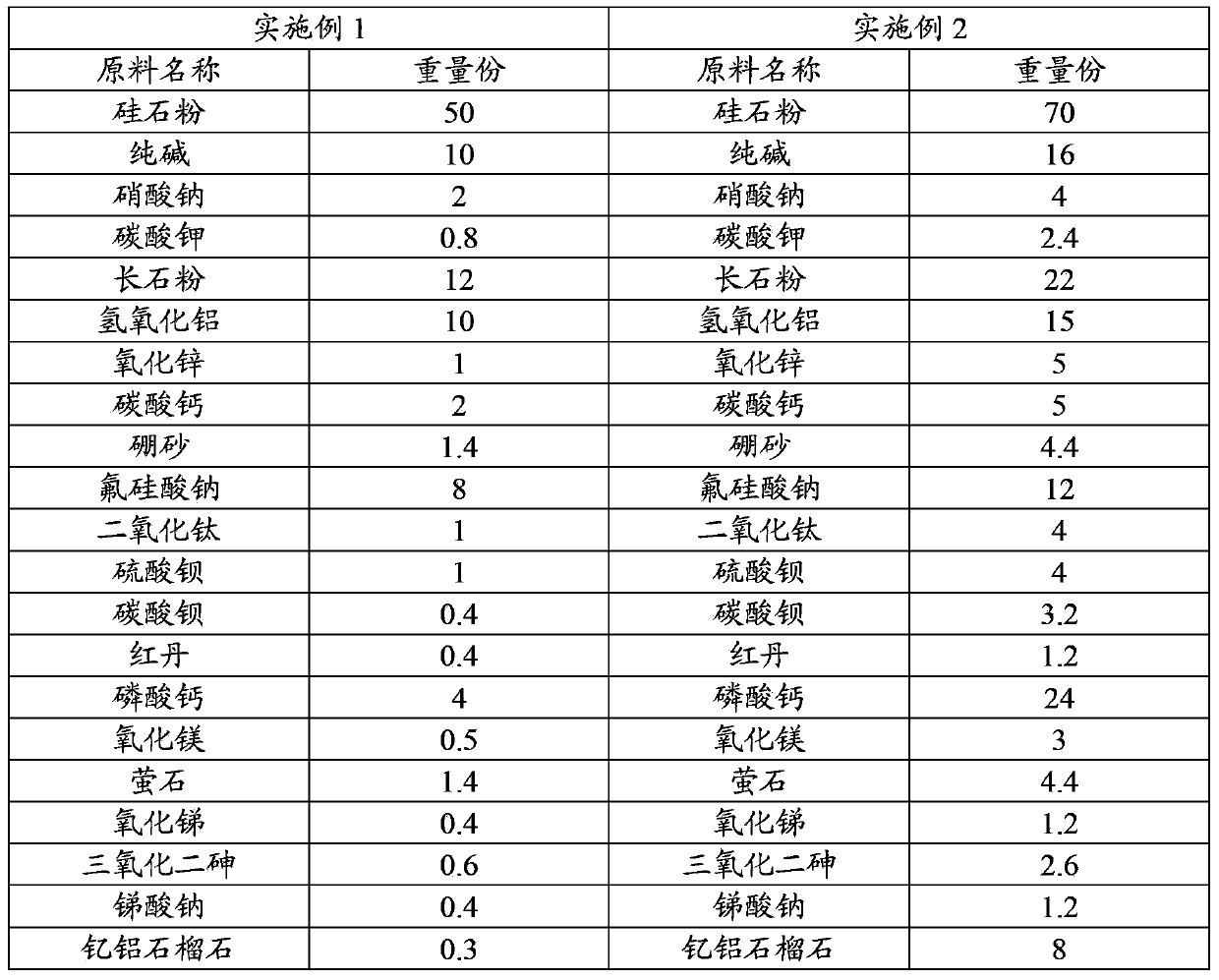
InactiveCN103466938ACompatibility is reasonableWide range of usesGlass furnace apparatusCadmium CationPotassium carbonate
The invention relates to a soft-light colored glass, and a preparation method and a use thereof. The soft-light colored glass is prepared through using the following raw materials, by weight, 50-70 parts of silica powder, 10-16 parts of sodium carbonate, 12-22 parts of feldspar powder, 10-24 parts of aluminum hydroxide, 1-12 parts of zinc oxide, 4-24 parts of calcium carbonate, 1.4-12 parts of borax, 8-16 parts of sodium fluosilicate, 0.8-5.8 parts of potassium carbonate, 0.4-4 parts of sodium nitrate and 6.6-70 parts of an auxiliary material, and the auxiliary material comprises several selected from titania, barium carbonate, red lead, calcium phosphate, barium sulfate, magnesium oxide, fluorite, arsenic trioxide, antimony oxide, cobalt oxide, copper oxide, chrome oxide green, sodium antimonate, cadmium yellow, sulfur, uranium oxide, cerium oxide, cadmium red, cadmium sulfide, selenium powder and yttrium aluminum garnet. The soft-light colored glass can solve a blue light polluting problem and has a long service life, and required raw materials do not pollute the environment, and the soft-light colored glass can be directly processed to form a lamp tube, a lamp cover or a lamp for use and avoids the easy color change and shedding problems of a coat of coated glass.
Owner:申英良 +1
Method for preparing UO2
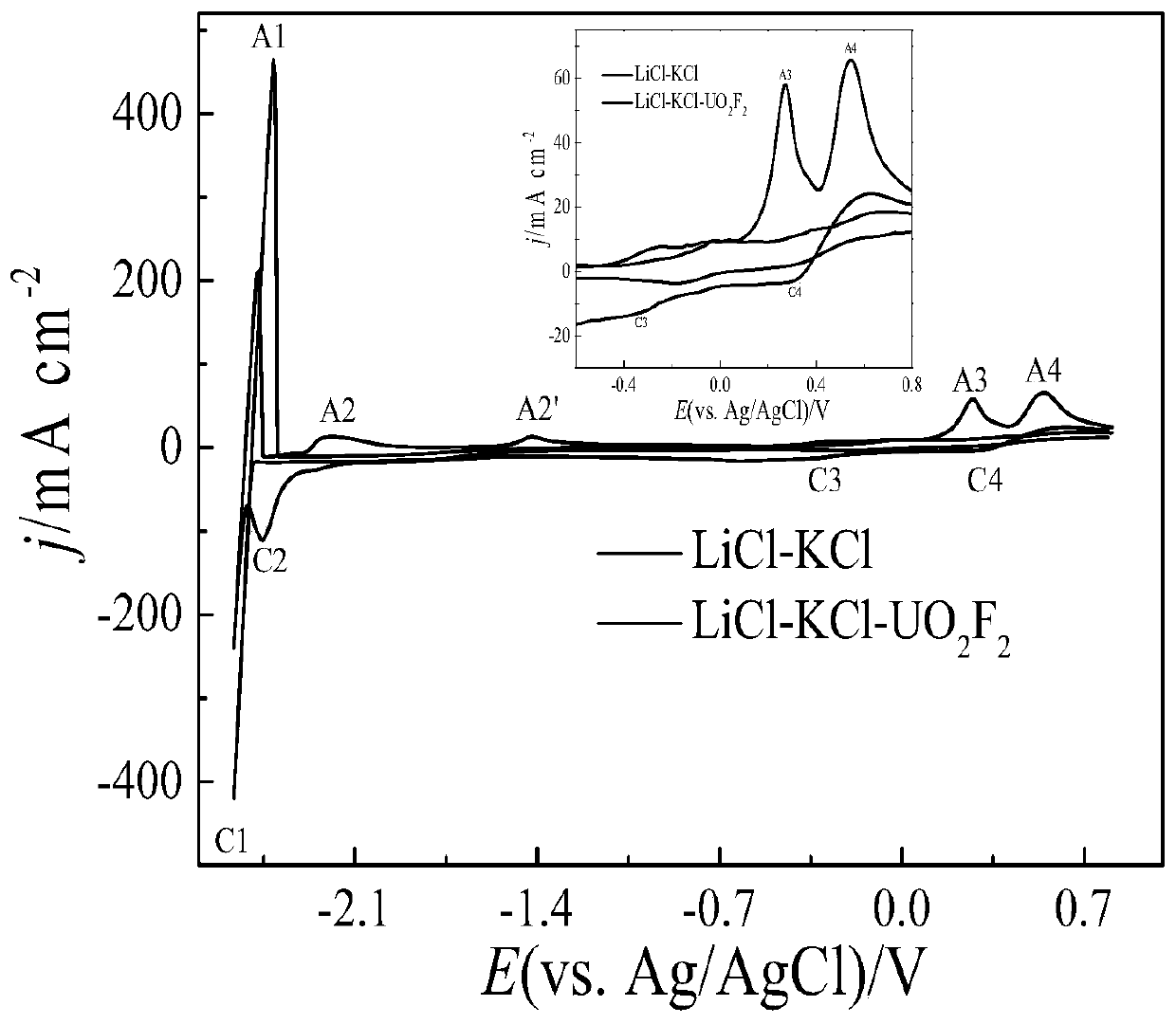
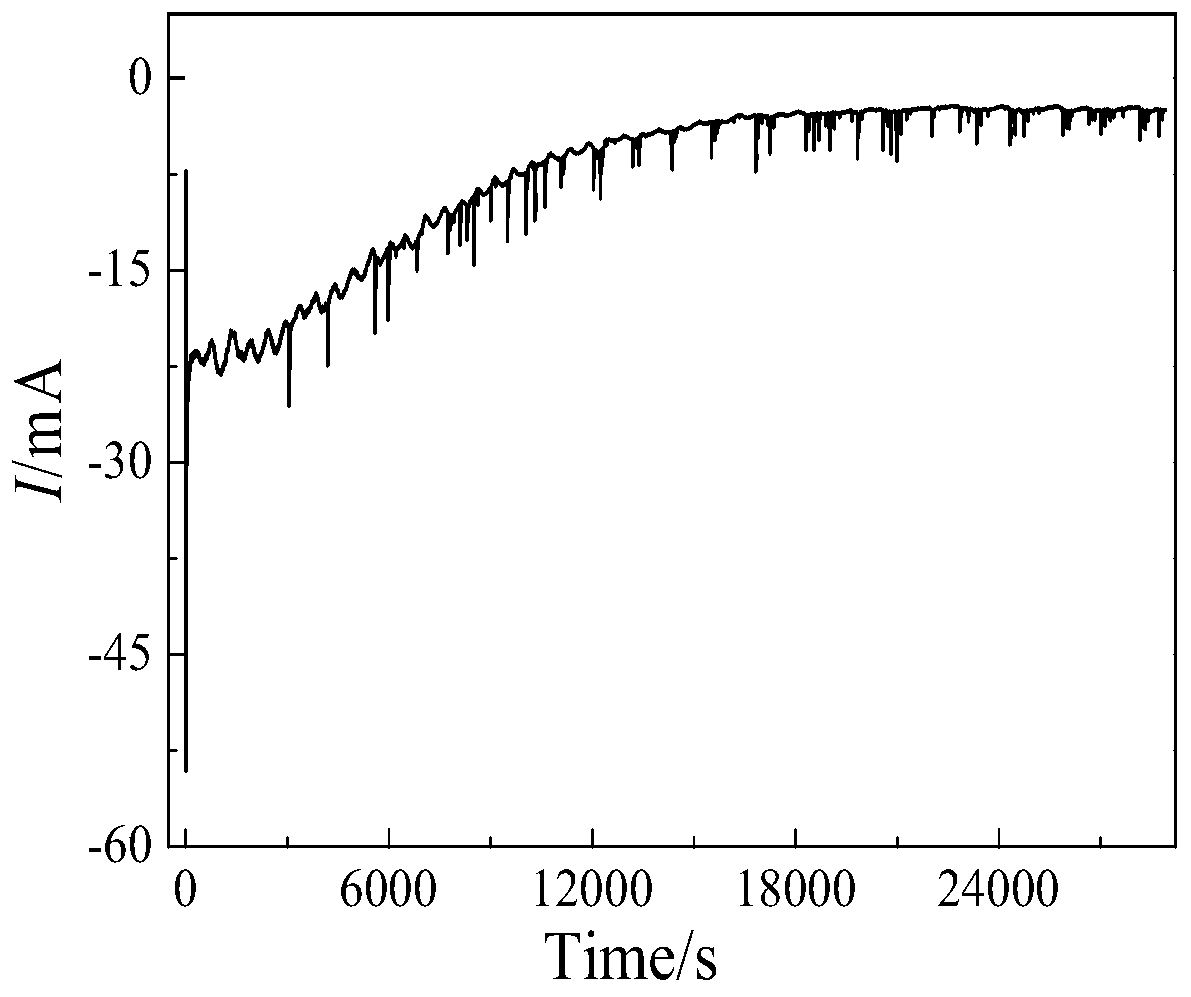
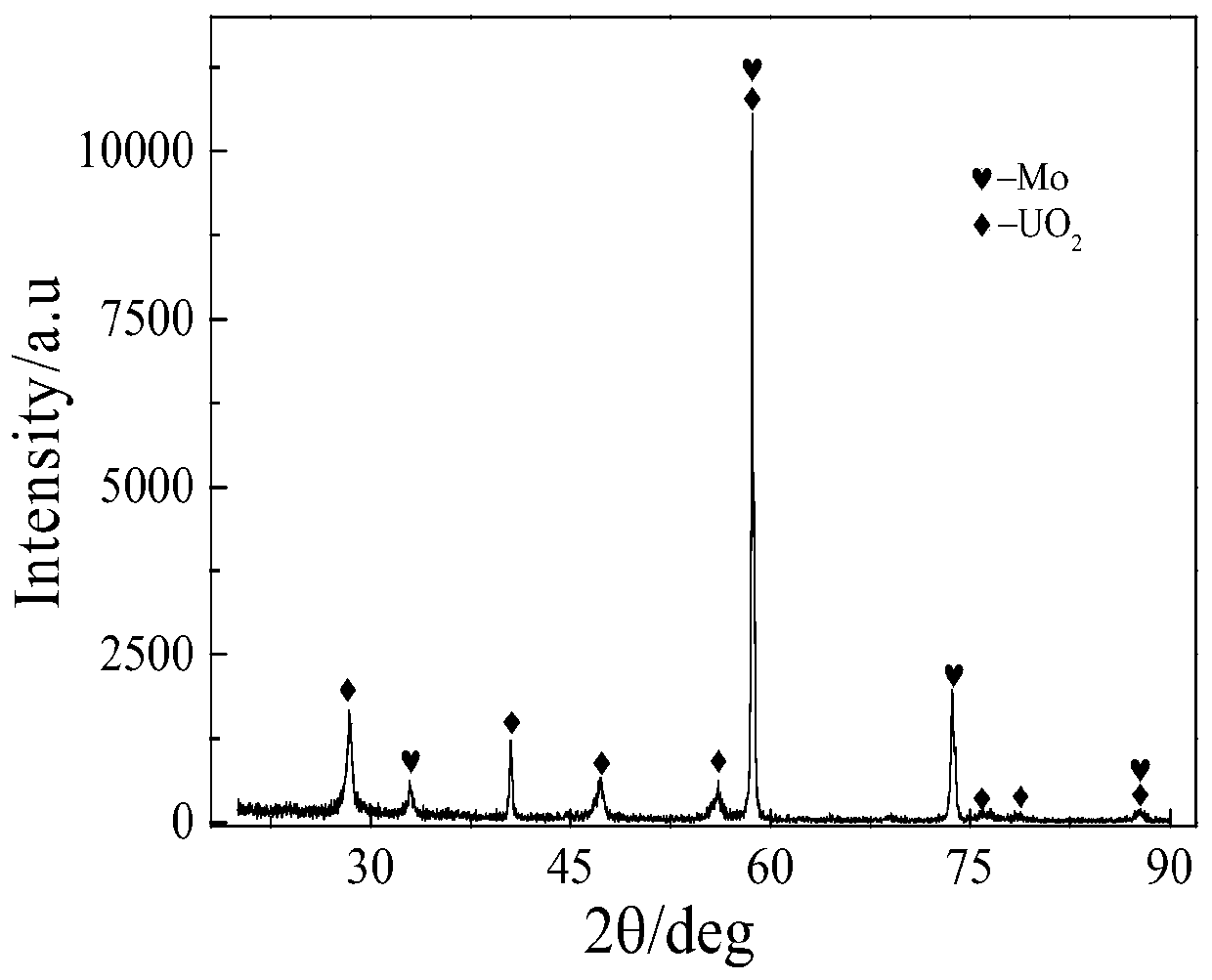
The invention provides a method for preparing UO2. The method comprises the following steps: firstly, fully and uniformly mixing U3O8 and NH4HF2 powder, and putting the mixed powder at the bottom of acrucible; then, laying LiCl-KCl salt on the mixed powder to cover the mixed powder; carrying out heating to 500 DEG C to melt the salt and keeping the temperature for 3 hours; and after the U3O8 is fully reacted and dissolved, carrying out constant-potential electrolysis by using a potential of -0.8 v (vs Ag / AgCl) with a molybdenum sheet as a cathode, a graphite rod as an anode and Ag / AgCl as a reference electrode so as to prepare UO2, wherein the result of ICP-AES analysis calculation shows that the extraction rate of uranyl ions after electrolysis for 8 hours reaches 98.5%. On one hand, theinvention provides the method for preparing UO2 by purifying uranium oxide through molten salt electrolysis; and on the other hand, HF gas generated in the process of reaction dissolution can be combined with oxygen ions to remove the oxygen ions in the molten salt in the form of water vapor.
Owner:HARBIN ENG UNIV
Uranium oxide micro-sphere and preparation method thereof
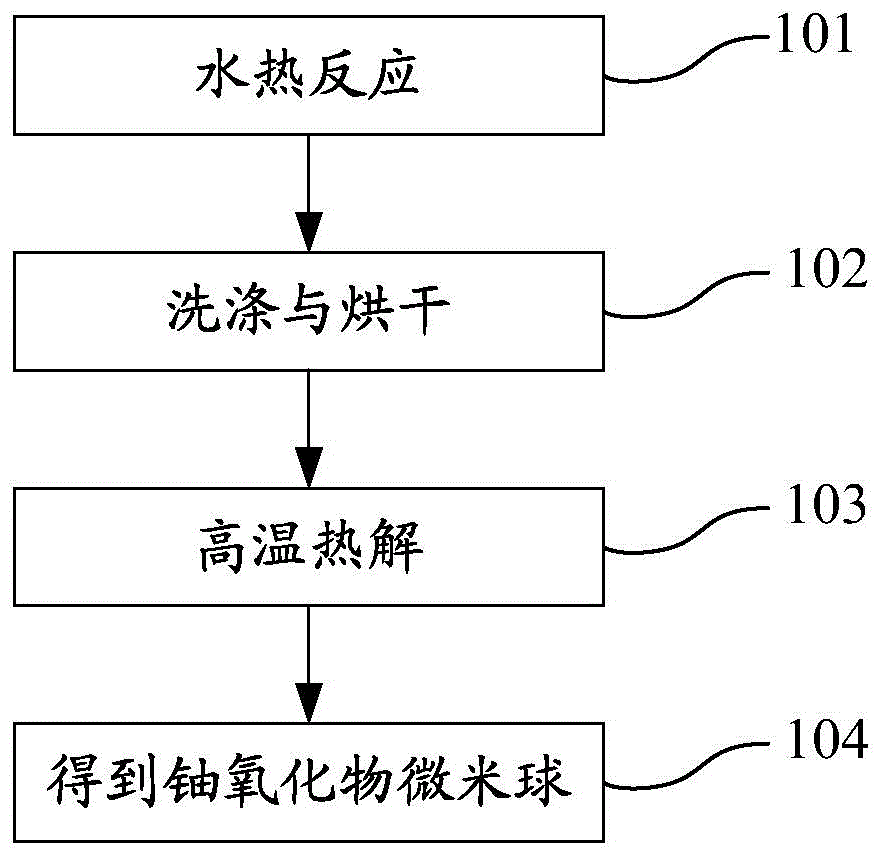
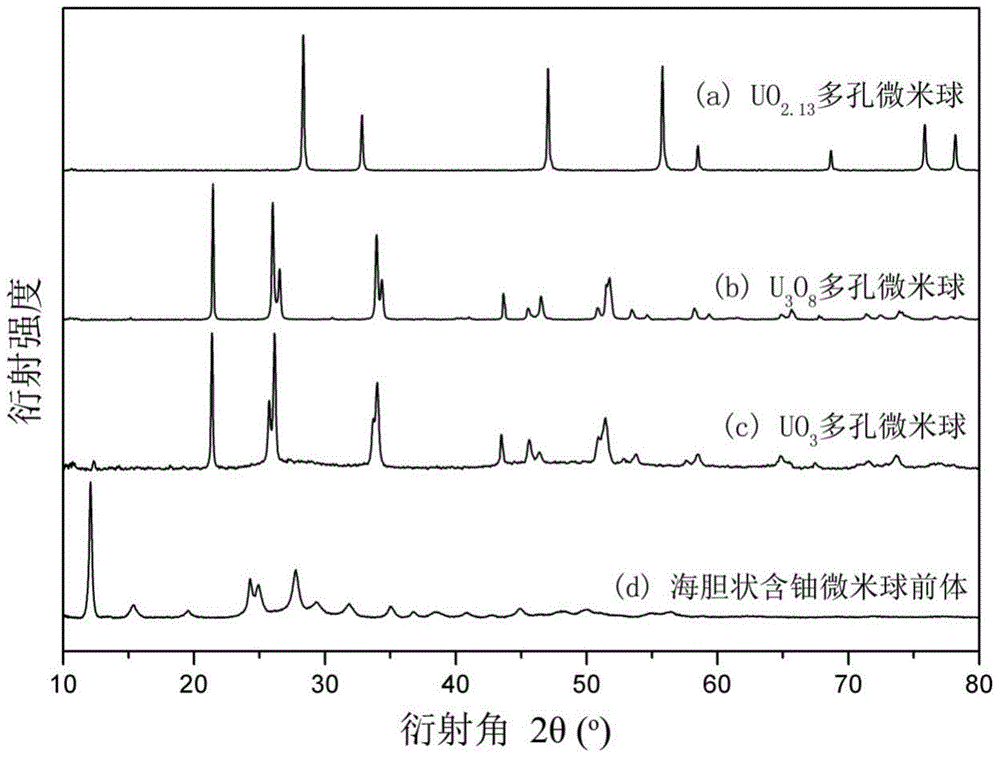
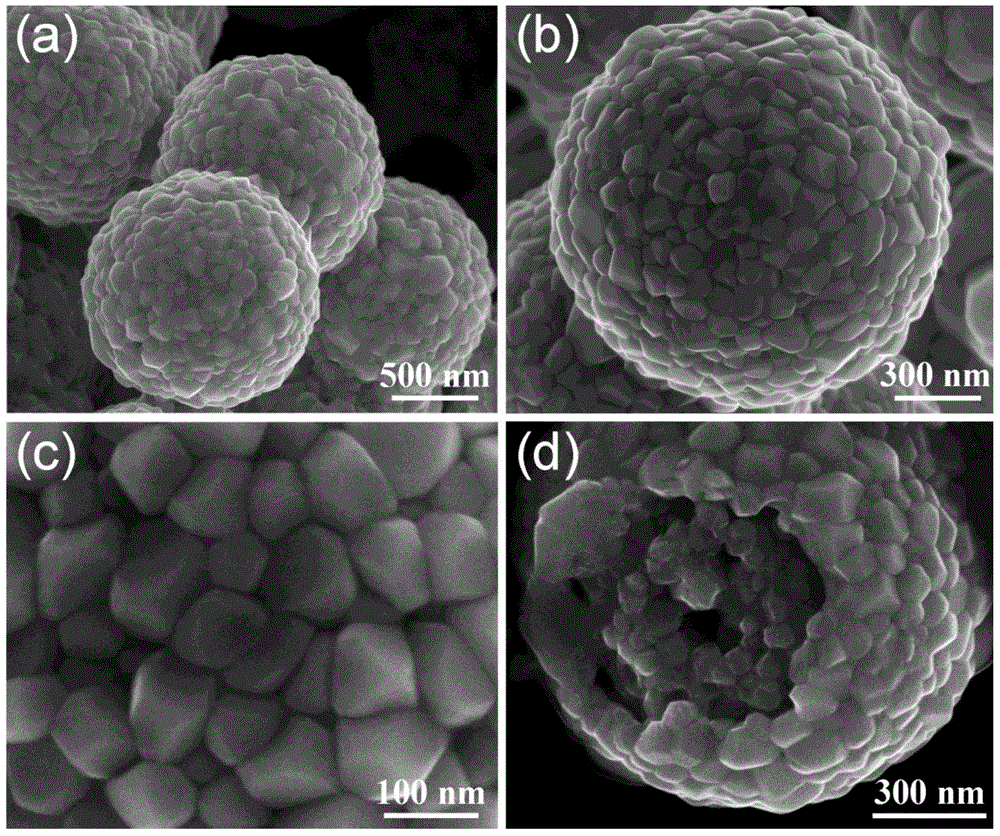
ActiveCN103936076AHigh capacity for storing fission gasStrong radiation resistanceUranium oxides/hydroxidesUranium oxideNanoparticle
The invention discloses an uranium oxide micro-sphere and a preparation method, a porous structure is provided in the uranium oxide micro-sphere, and the porous structure is composed of nano particles. The invention also comprises a preparation method of the uranium oxide micro-sphere, which comprises the following steps: 1)performing a hydro-thermal reaction on a mixing aqueous solution to obtain a sea urchin-state uranium-containing micro-sphere precursor, wherein the mixing aqueous solution comprises a uranyl nitrate aqueous solution, glycerol and urea; 3)washing and drying the uranium-containing micro-sphere precursor; and 3)placing the dried uranium-containing micro-sphere precursor in a tubular furnace for pyrolysis to obtain the uranium oxide micro-sphere. The preparation method has the advantages of simple process and easy control, and the prepared uranium oxide micro-sphere has the characteristics of small size and has porous structure, and has latent application value in the relative fields of catalysis and nuclear energy.
Owner:INST OF HIGH ENERGY PHYSICS CHINESE ACADEMY OF SCI
Methods of fabricating metallic fuel from surplus plutonium
ActiveUS20160053391A1Reduced proliferation potentialPhotography auxillary processesFuel elementsUranium oxideElectrolysis
A method of fabricating metallic fuel from surplus plutonium may include combining plutonium oxide powder and uranium oxide powder to obtain a mixed powder with reduced proliferation potential. The mixed powder may be electroreduced in a bath of molten salt so as to convert the mixed powder to a first alloy. The first alloy may be pressed to remove a majority of the molten salt adhered to the first alloy to form a pressed alloy-salt mixture. The first alloy may be isolated from the salt by melting the pressed alloy-salt mixture. The first alloy may be further processed to fabricate a fuel rod. Accordingly, the metallic fuel produced may be used in a fast reactor system, such as a Power Reactor Innovative Small Module (PRISM).
Owner:GE HITACHI NUCLEAR ENERGY AMERICAS
Method of fabricating sintered nuclear fuel compact
InactiveUS6878313B2Large particle sizeAppropriate useNuclear energy generationReactors manufacturePowder mixtureUranium oxide
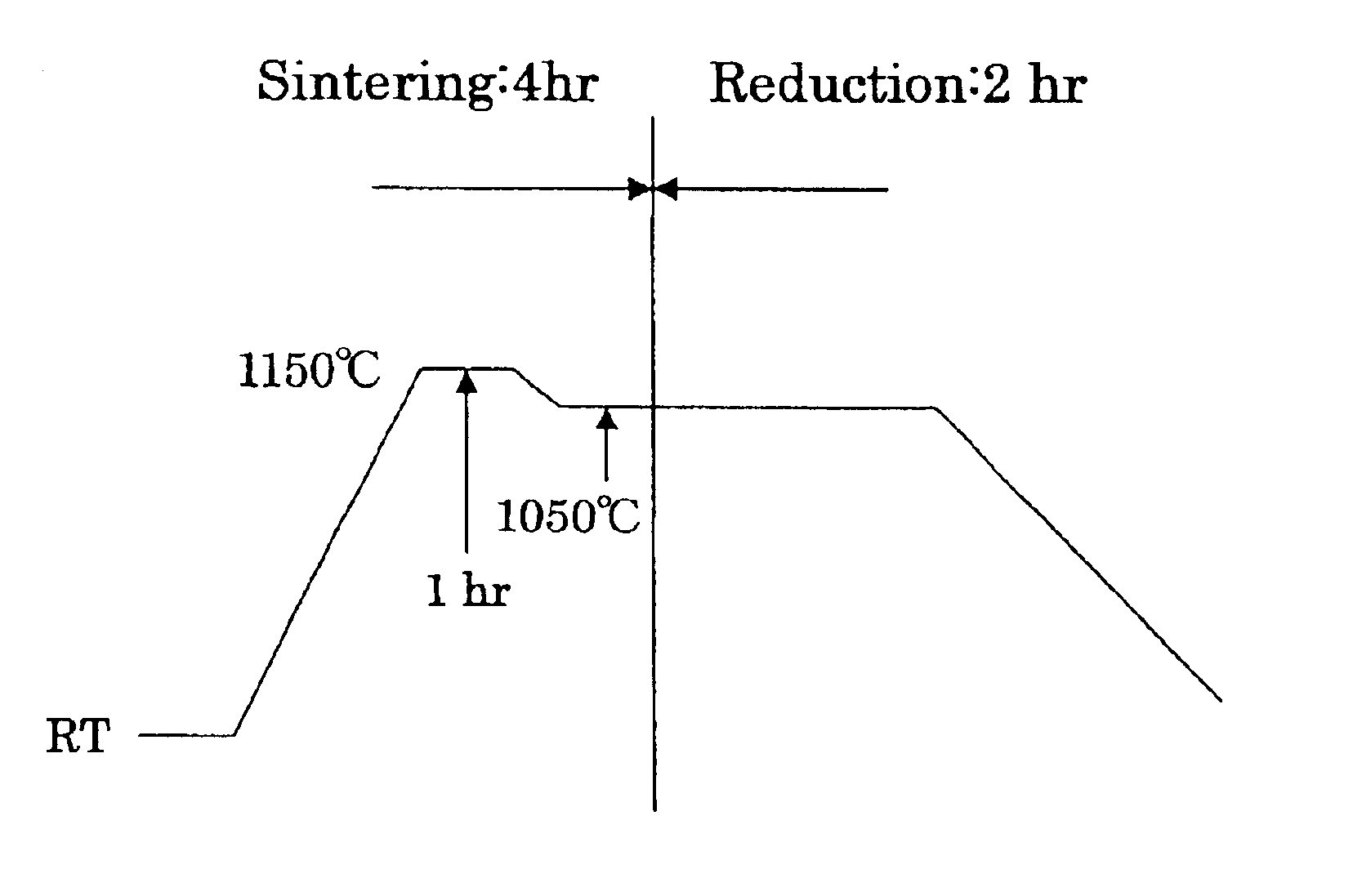
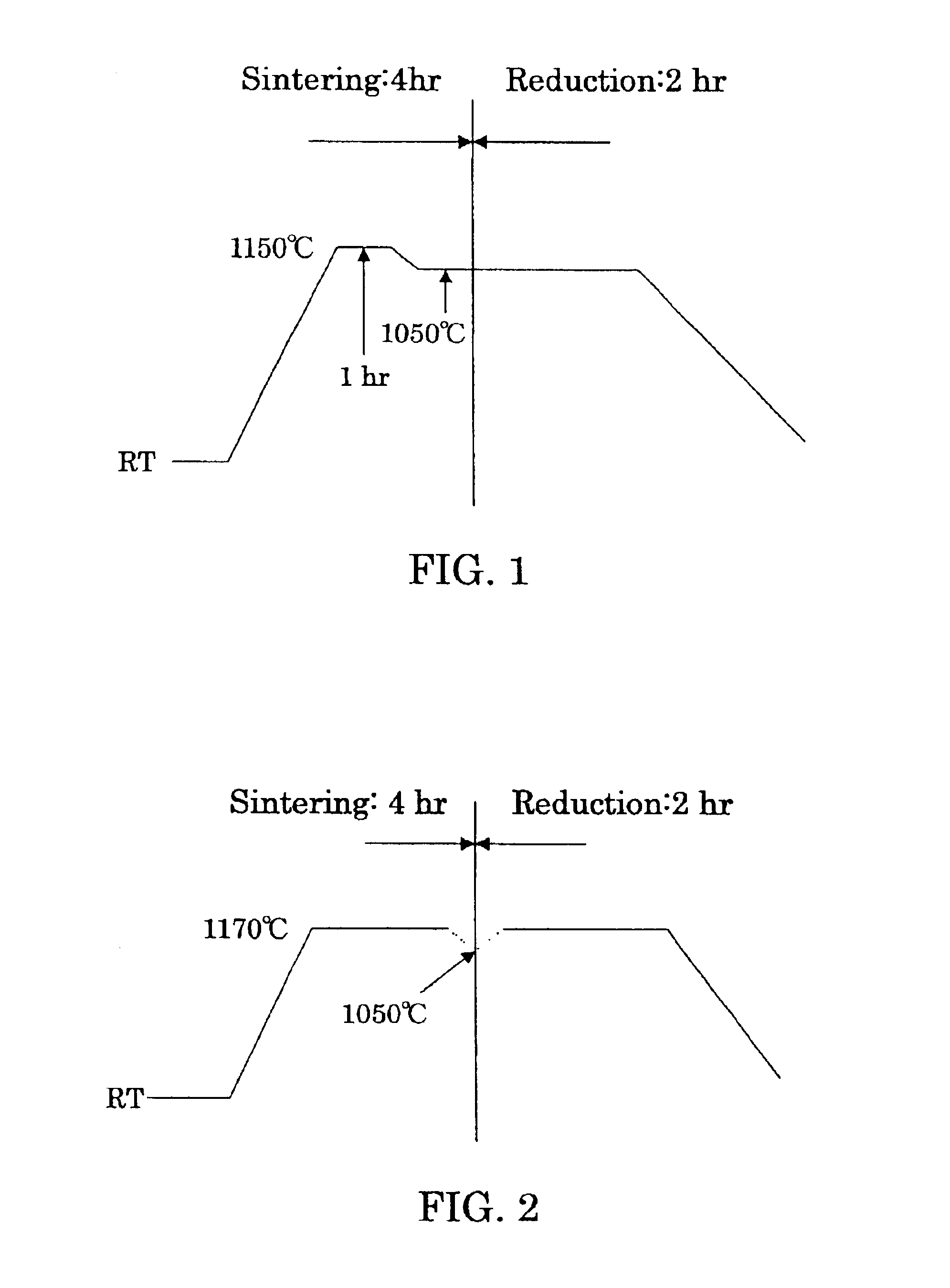
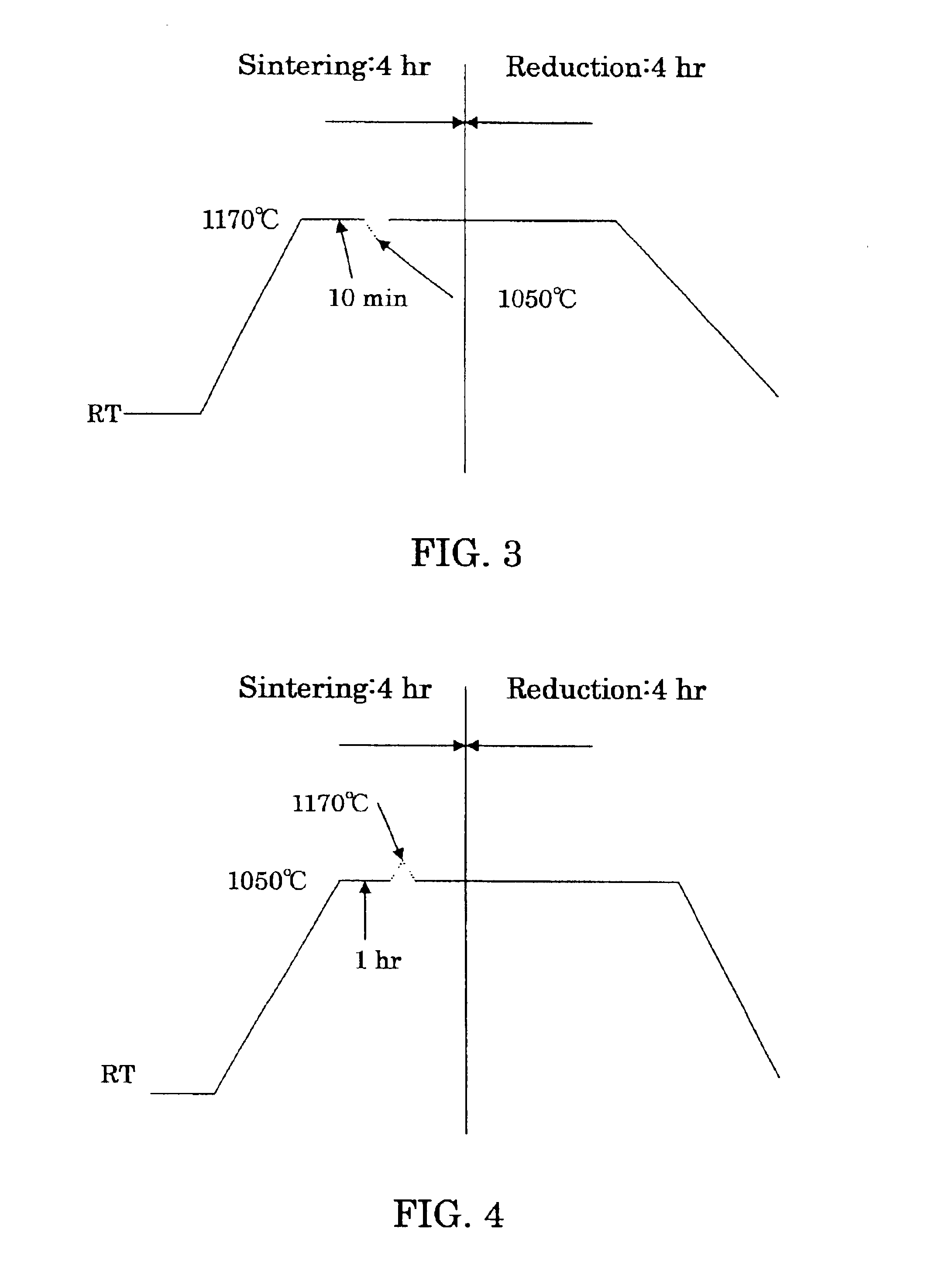
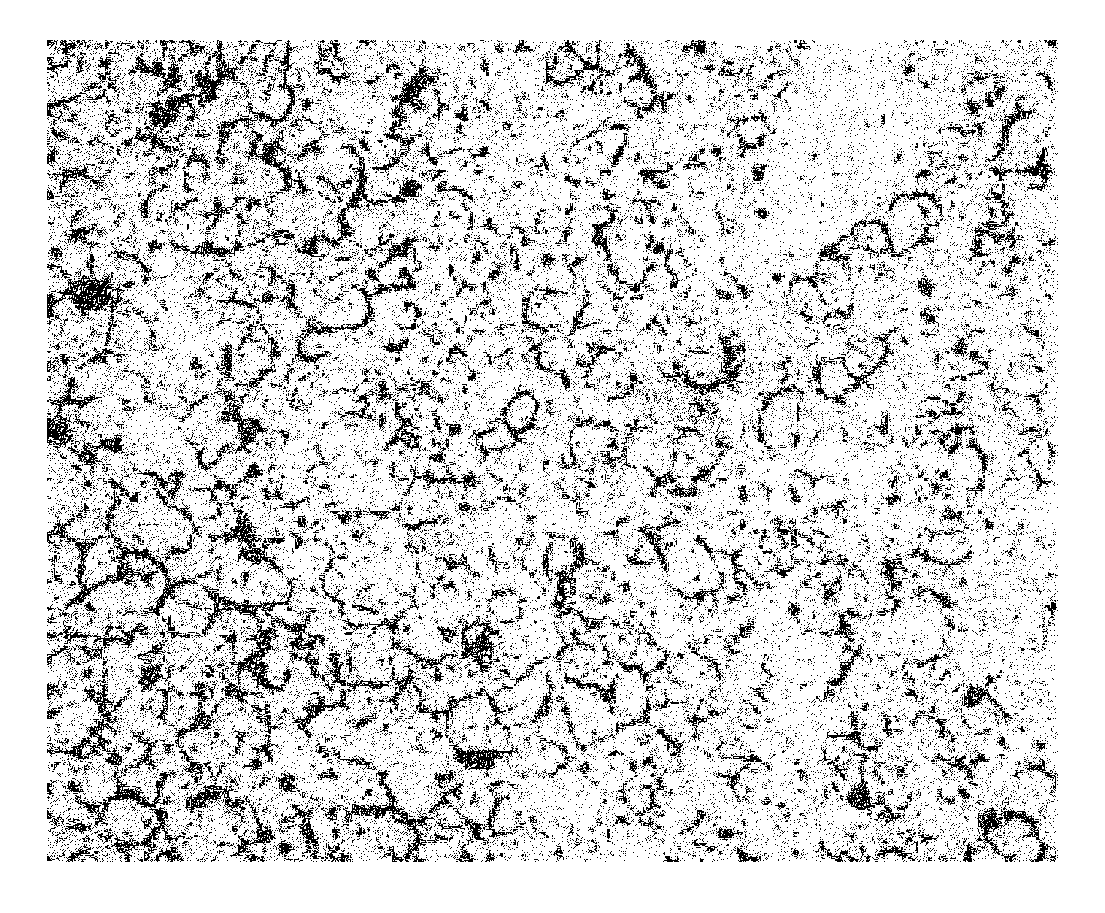
To furnish stably a sintered nuclear fuel compact of uranium dioxide with a large grain diameter, a fabrication method therefor requires sintering a starting material at two stages in an oxidizing atmosphere and in a reducing atmosphere at relatively low temperatures. The starting material is either a powder mixture of uranium dioxide fresh material powder and triuranium octaoxide in a weight ratio of 75 / 25 ˜ 55 / 45 or pulverulent mixed uranium oxides yielding a final O / U ratio equal to that of the powder mixture. The sintering process in the oxidizing atmosphere involves at least one time period of a temperature rise to a higher temperature of 1200 to 1100° C. and at least one time period of a temperature drop to a lower temperature of up to 1080° C. A temperature difference between the higher and lower temperatures is preferred to be 50° C. or more.
Owner:NUCLEAR FUEL INDS
Method for preparing a porous nuclear fuel based on at least one minor actinide
InactiveUS20120228788A1Easy to aimReduce porosityFuel elementsNuclear energy generationPowder mixtureUranium oxide
A method for manufacturing a porous fuel comprising uranium, optionally plutonium and at least one minor actinide is provided. The method may comprise the following successive steps: a) a step for compacting as pellets a mixture of powders comprising uranium oxide, optionally plutonium oxide and at least one oxide of a minor actinide, at least one portion of the uranium oxide being in the form of triuranium octaoxide U3O8, the other portion being in the form of uranium dioxide UO2; b) a step for reducing at least one portion of the triuranium octaoxide U3O8 into uranium dioxide UO2.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Gadolinium-carrying fuel rod, fuel assembly with gadolinium-carrying fuel rods and pressurized water reactor core
ActiveCN104952492AConserve uranium resourcesLow maintenanceFuel elementsNuclear energy generationUranium oxideNuclear engineering
The invention discloses a gadolinium-carrying fuel rod, a fuel assembly with gadolinium-carrying fuel rods and a pressurized water reactor core. A fuel core body of the gadolinium-carrying fuel rod is composed of recycled uranium oxide and Gd2O3, wherein the mass percent of Gd2O3 is 3%-5% and the mass percent of the recycled uranium oxide is 95%-97%. The fuel assembly with the gadolinium-carrying fuel rods comprises fuel rods, guide pipes and a gauge pipe, wherein the fuel rods, the guide pipes and the gauge pipe are arrayed to form a square structure; the gauge pipe is arrayed at the center of the fuel assembly; and the fuel rods comprise fuel rods without gadolinium and the gadolinium-carrying fuel rods. The pressurized water reactor core adopts the fuel assembly with the gadolinium-carrying fuel rods to assist in controlling the residual reactivity of the reactor core. Recycled uranium fuel is prepared into the gadolinium-carrying fuel rods and the fuel assembly, which are used for the reactor core, and the safety requirements of the reactor core are met; and meanwhile, the requirements on solid combustible toxins for controlling the residual reactivity of the reactor core are met, uranium resources are effectively saved and the recycled uranium storage cost is reduced.
Owner:NUCLEAR POWER INSTITUTE OF CHINA
Method for surface oxidation treatment of uranium oxide powder
InactiveCN101254950AImprove the O/U atomic ratioIncrease surface areaNuclear energy generationReactors manufactureUranium oxideSurface oxidation
The invention relates to a method of oxidation treatment of a surface of a uranium dioxide, which is characterized that the method comprises the following steps: (1) taking the UO2 powder and putting the UO2 powder into a tube type atmosphere oven, heating the UO2 powder under the condition of oxidation medium while the heating rate is 5 to 20 degree centigrade per minute, heating the UO2 powder to 250 degree centigrade to 400 degree centigrade and insulating for 2 to 6 hours; (2) when the UO2 powder is cooled to 100 degree centigrade to the room temperature in the oven, taking the UO2 powder out and cooling the UO2 powder in the air, so as to obtain the uranium dioxide powder with the oxidized surface. The method puts the UO2 in different oxidation medium and the UO2 is insulated at a certain temperature, then the surface oxygen content is increased, the surface area is increased, and the O / U atomic ratio of uranium dioxide and the surface area of the UO2 powder are enhanced. The oxidation medium obtained by the method has rich resources, low oxidation temperature and less consumption and has the advantages of simple fabrication process and low cost.
Owner:CHONGQING UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com