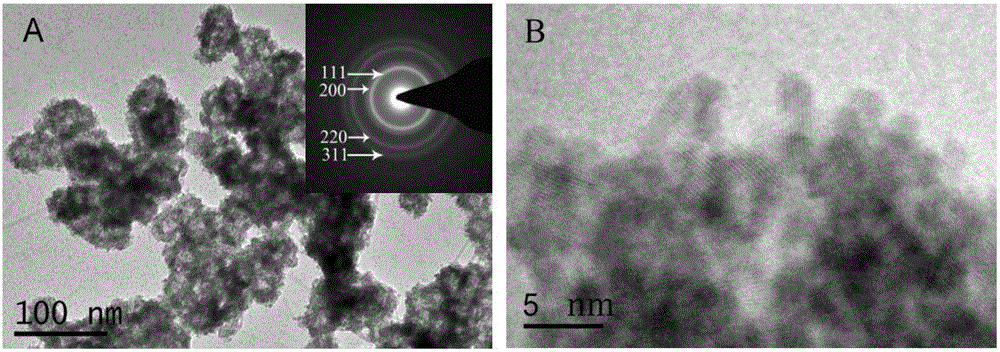
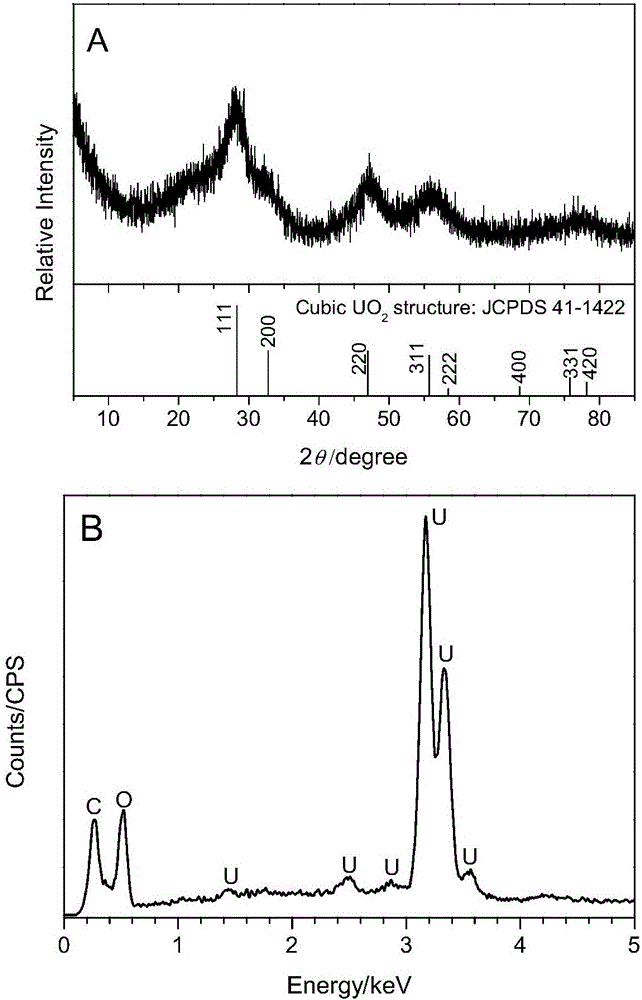
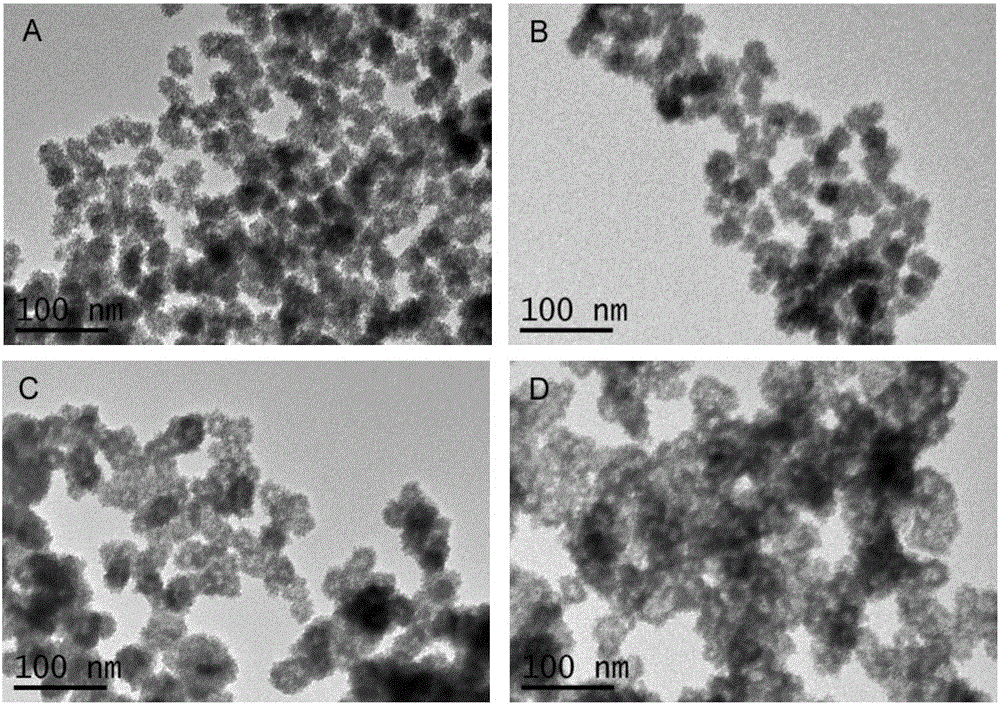
Patents
Literature
118results about "Uranium dioxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
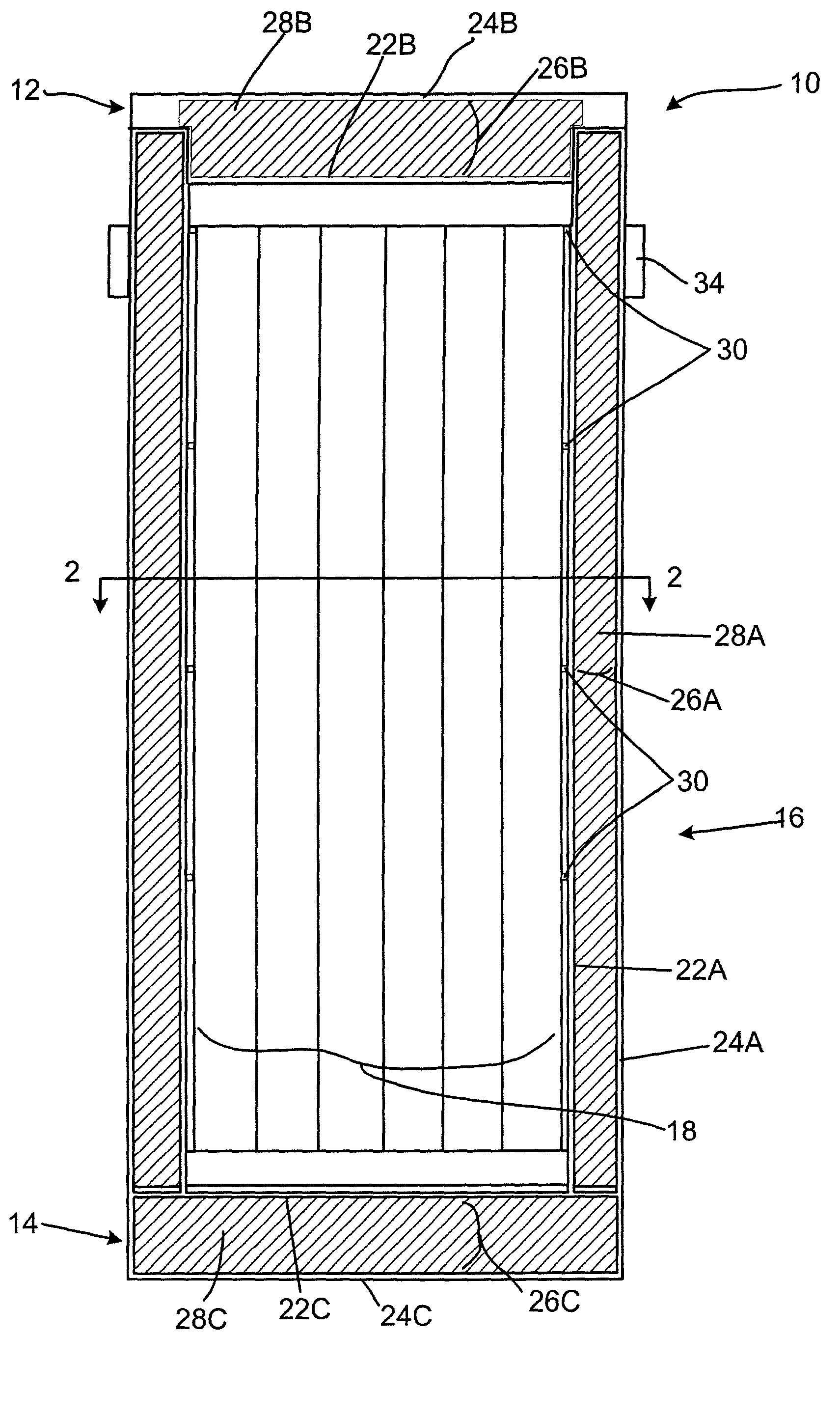
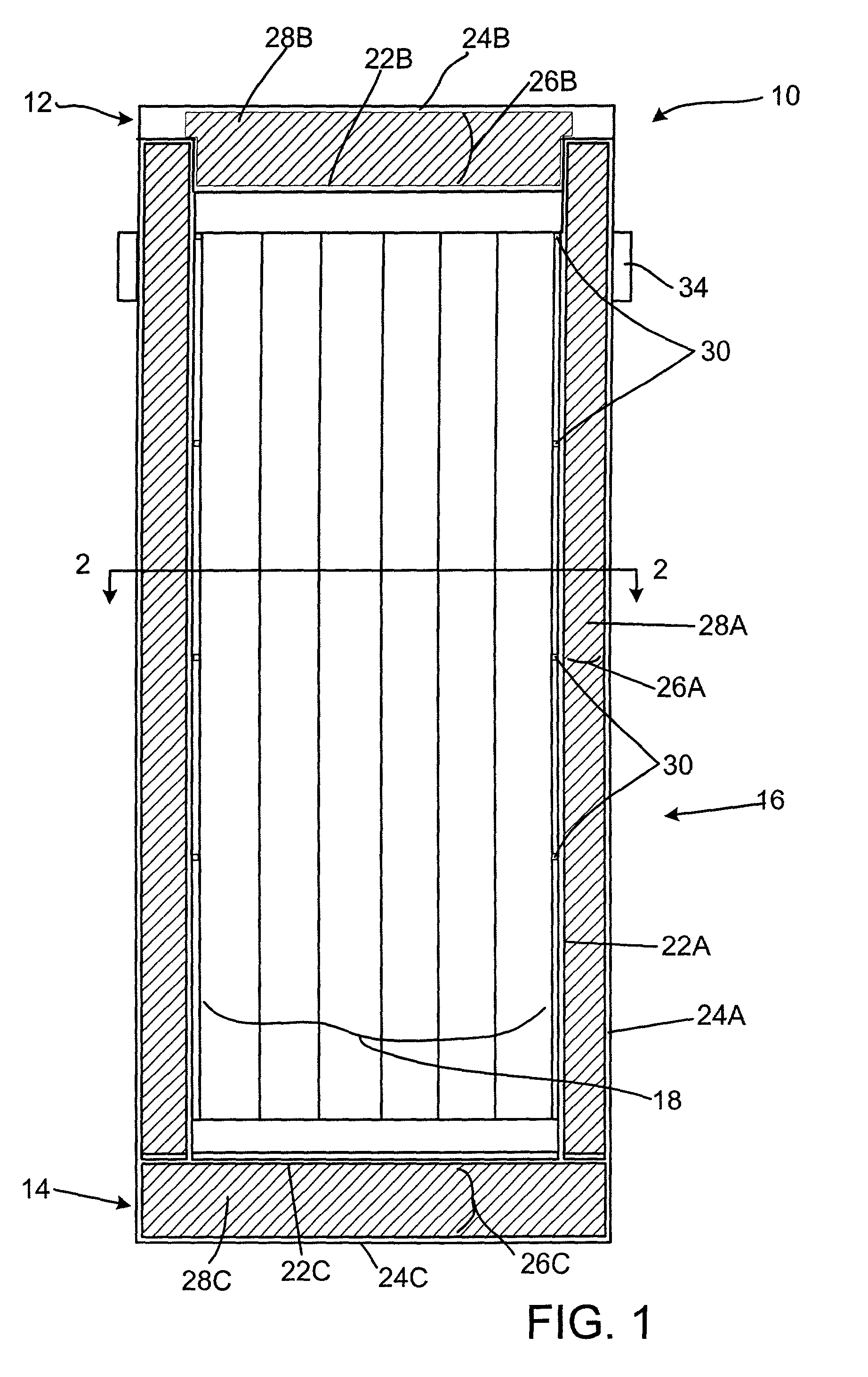
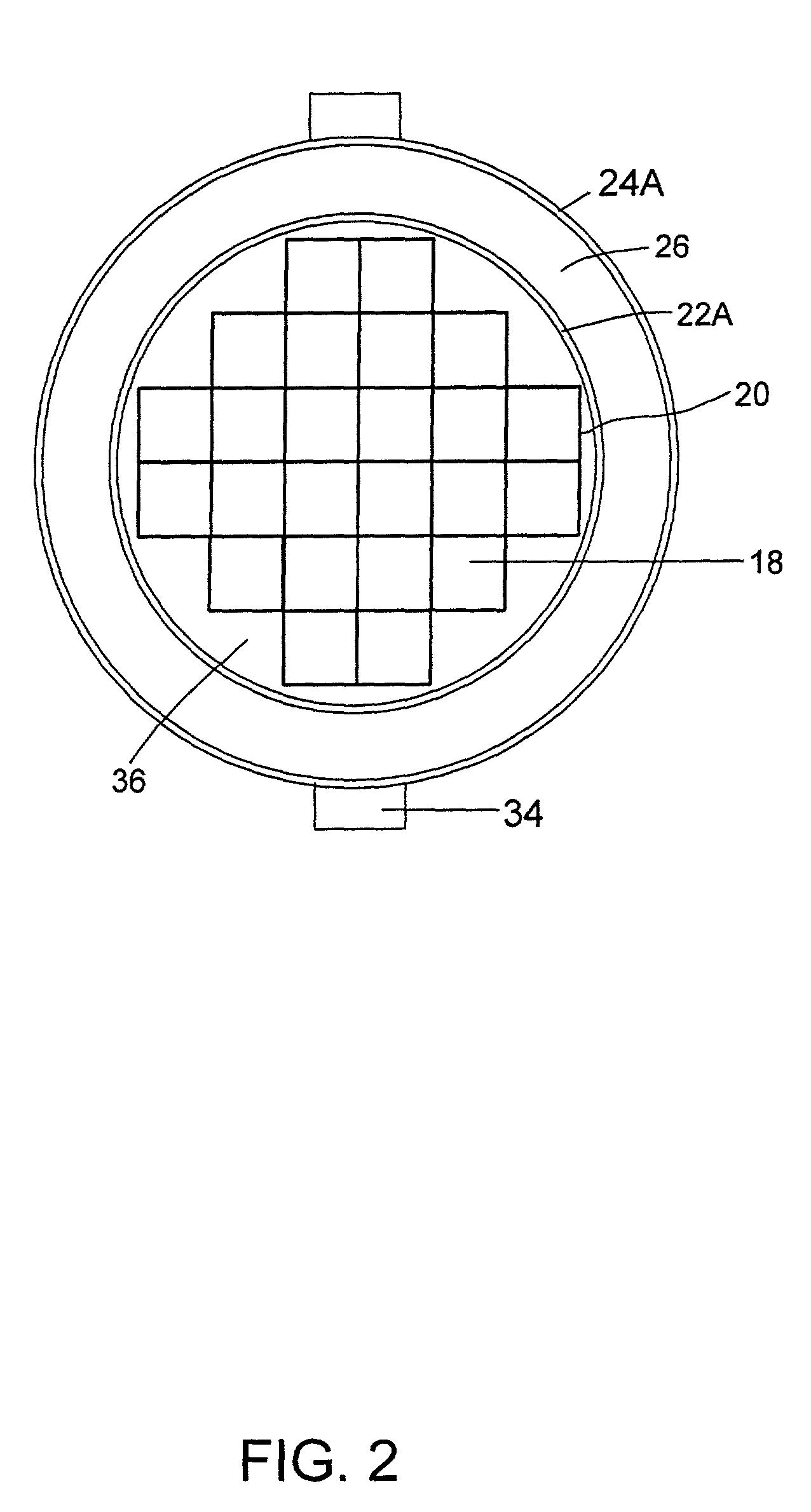
Radiation shielding materials and containers incorporating same
InactiveUS6960311B1Improve shielding effectMaximum flexibilityOther chemical processesTransuranic element compoundsMicrosphereUranium carbide
An improved radiation shielding material and storage systems for radioactive materials incorporating the same. The PYRolytic Uranium Compound (“PYRUC”) shielding material is preferably formed by heat and / or pressure treatment of a precursor material comprising microspheres of a uranium compound, such as uranium dioxide or uranium carbide, and a suitable binder. The PYRUC shielding material provides improved radiation shielding, thermal characteristic, cost and ease of use in comparison with other shielding materials. The shielding material can be used to form containment systems, container vessels, shielding structures, and containment storage areas, all of which can be used to house radioactive waste. The preferred shielding system is in the form of a container for storage, transportation, and disposal of radioactive waste. In addition, improved methods for preparing uranium dioxide and uranium carbide microspheres for use in the radiation shielding materials are also provided.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
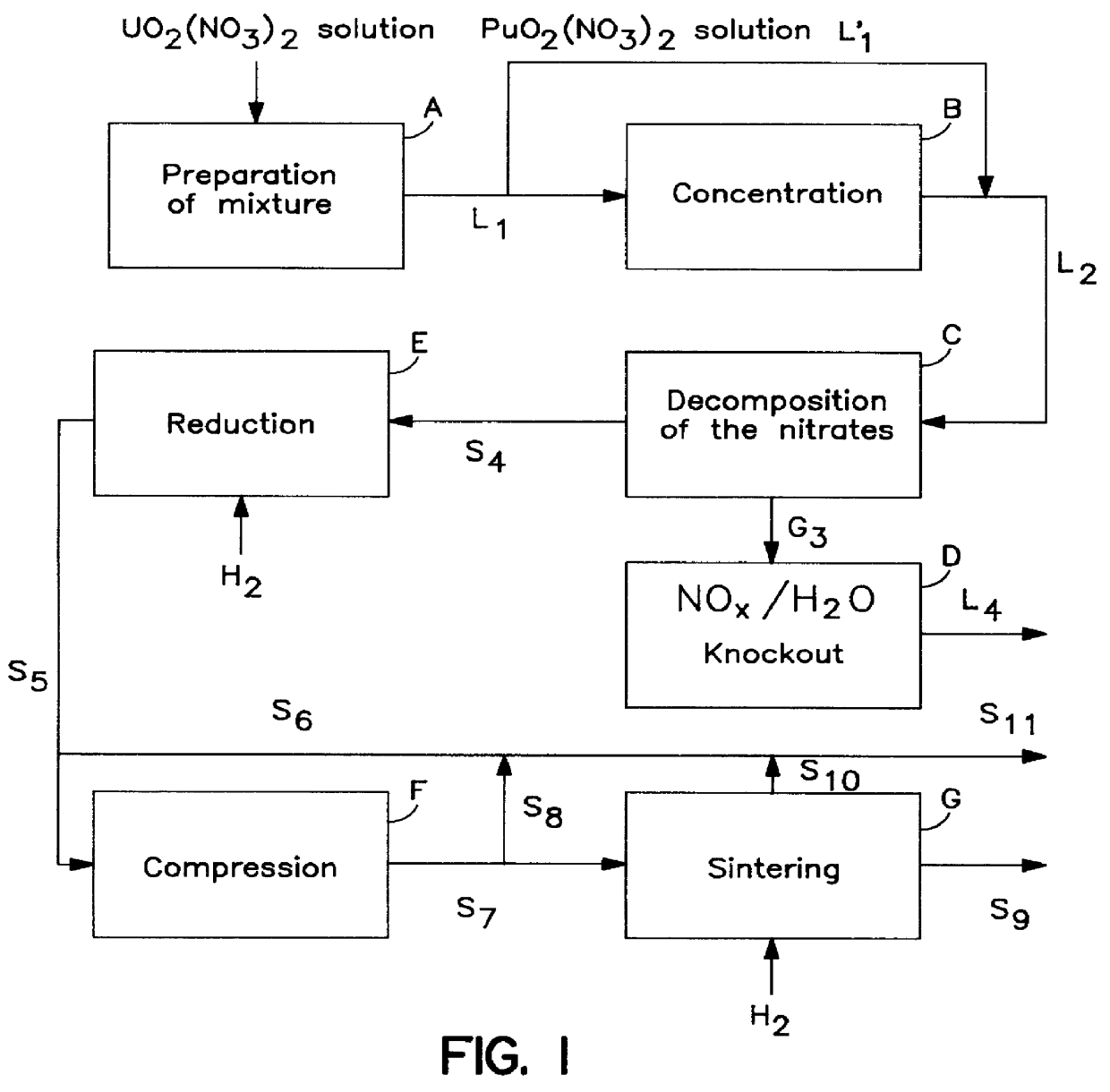
Method for preparing a mixture of powdered metal oxides from nitrates thereof in the nuclear industry
InactiveUS6110437AEasy to recycleImprove responseOxygen/ozone/oxide/hydroxideTransuranic element compoundsPowder mixtureNitrate
PCT No. PCT / FR96 / 01993 Sec. 371 Date Mar. 2, 1999 Sec. 102(e) Date Mar. 2, 1999 PCT Filed Dec. 12, 1996 PCT Pub. No. WO97 / 21629 PCT Pub. Date Jun. 19, 1997A thermal decomposition method useful in the nuclear industry for preparing a powdered mixture of metal oxides having suitable reactivity from nitrates thereof in the form of an aqueous solution or a mixture of solids. According to the method, the solution or the mixture of solids is thermomechanically contacted with a gaseous fluid in the contact area of a reaction chamber, said gaseous fluid being fed into the reaction chamber at the same time as the solution or mixture at a temperature no lower than the decomposition temperature of the nitrates, and having a mechanical energy high enough to generate a fine spray of the solution or a fine dispersion of the solid mixture, and instantly decompose the nitrates. The resulting oxide mixtures may be used to prepare nuclear fuels.
Owner:COMURHEX
Preparation by spray-drying of a flowable uranium dioxide powder obtained by dry process conversion of UF6
InactiveUS6656391B1Lower levelMaintain good propertiesNuclear energy generationUranium dioxideUranium hexafluorideViscosity
The invention relates to a process for preparing a castable powder of uranium dioxide UO2, for use in the manufacture of MOX fuel.This process comprises the following stages:1) to prepare an aqueous suspension of a powder of UO2 obtained by dry process from uranium hexafluoride, said suspension comprising 50 to 80% by weight of UO2 and at least one additive chosen among deflocculation agents, organic binders, hydrogen peroxide H2O2 and a powder of U3O8, in such a quantity that the viscosity of the suspension does not exceed 250 mPa.sec, and2) to atomise this suspension and dry it in a hot gas, at a temperature of 150 to 300° C., to obtain a castable powder of UO2 with an average particle size of 20 to 100 mum.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
Non-aqueous method for separating chemical constituents in spent nuclear reactor fuel
InactiveUS20060233685A1Reduce riskIncrease ratingsNuclear energy generationPlutonium oxides/hydroxidesKryptonGas phase
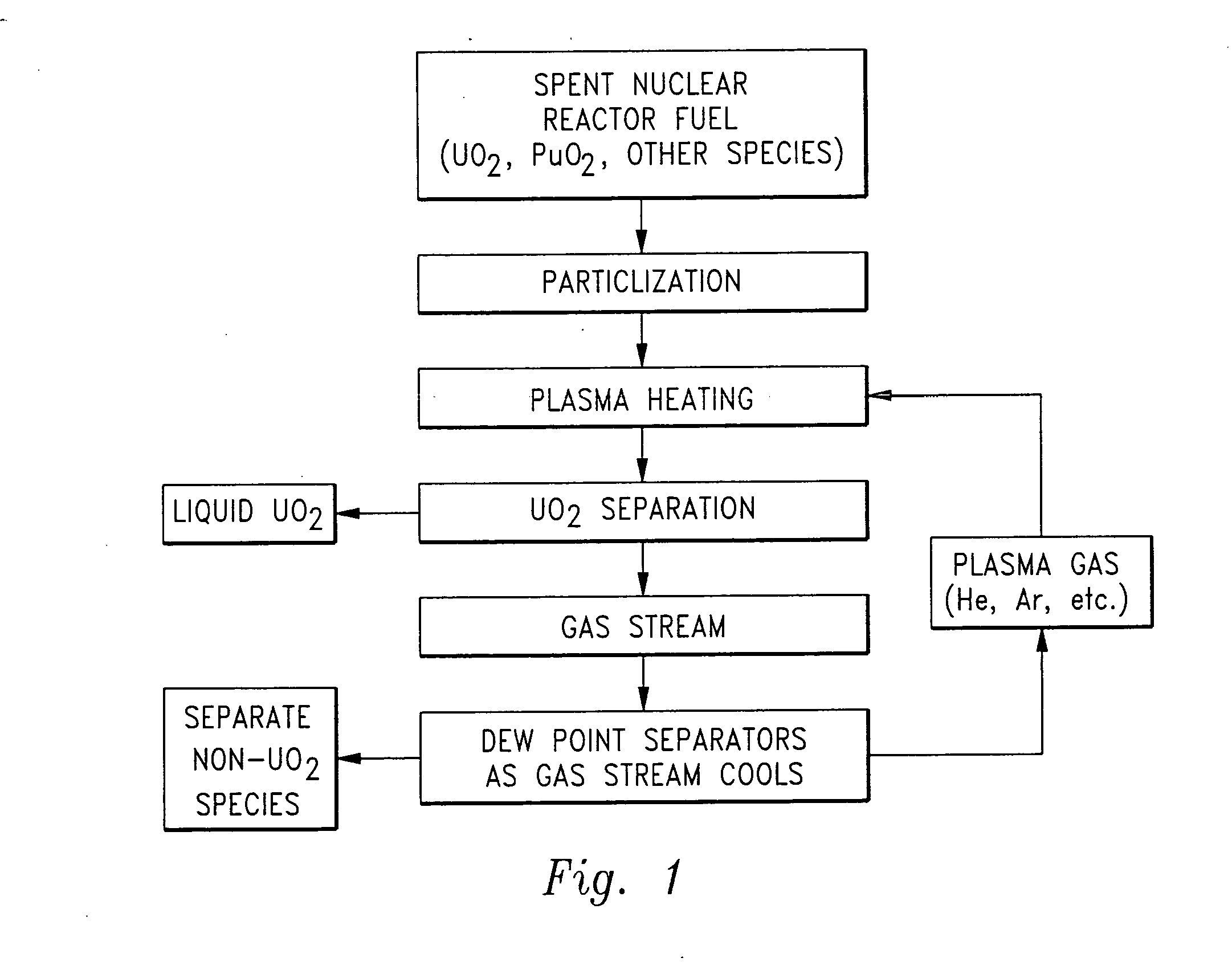
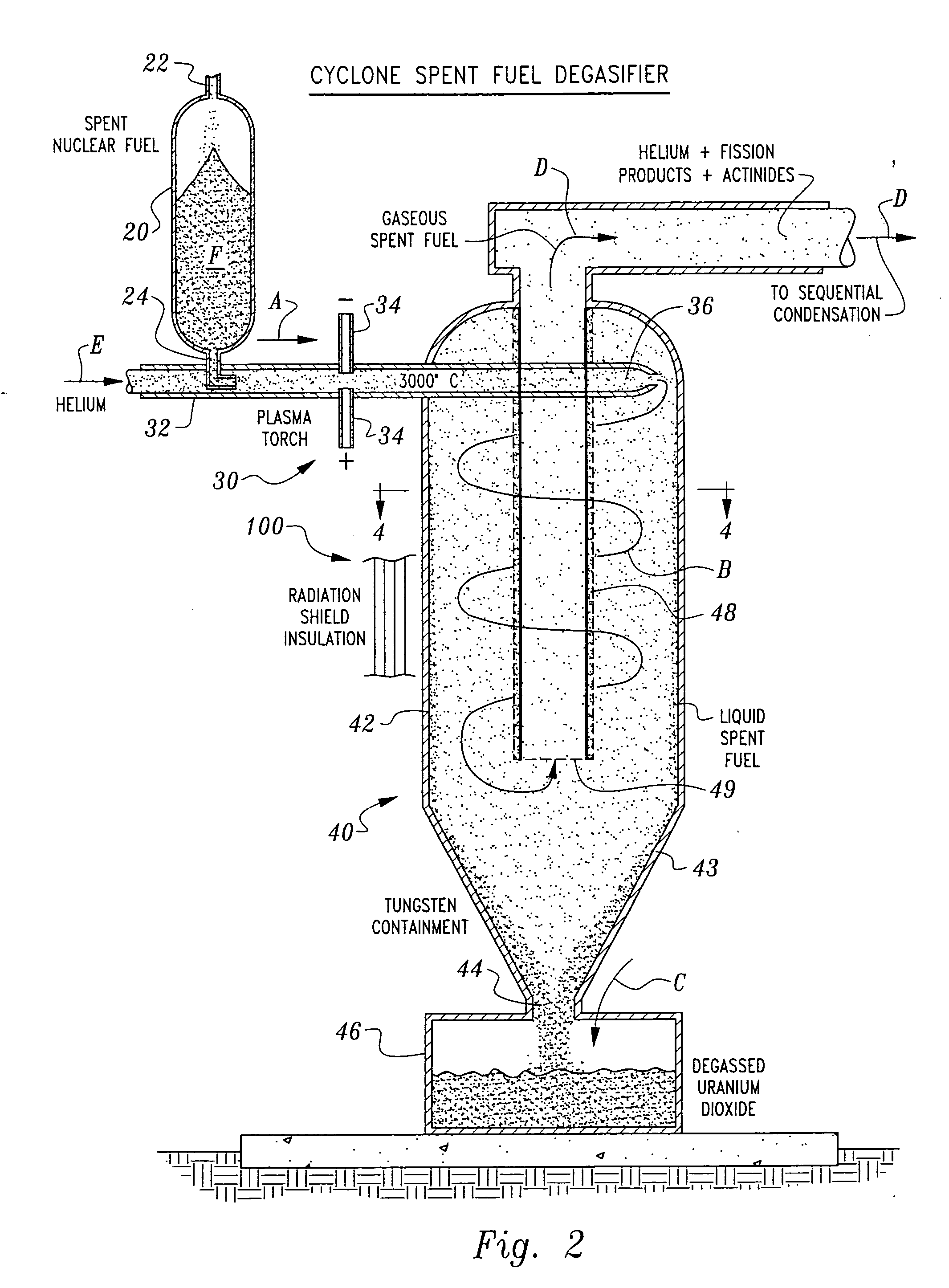
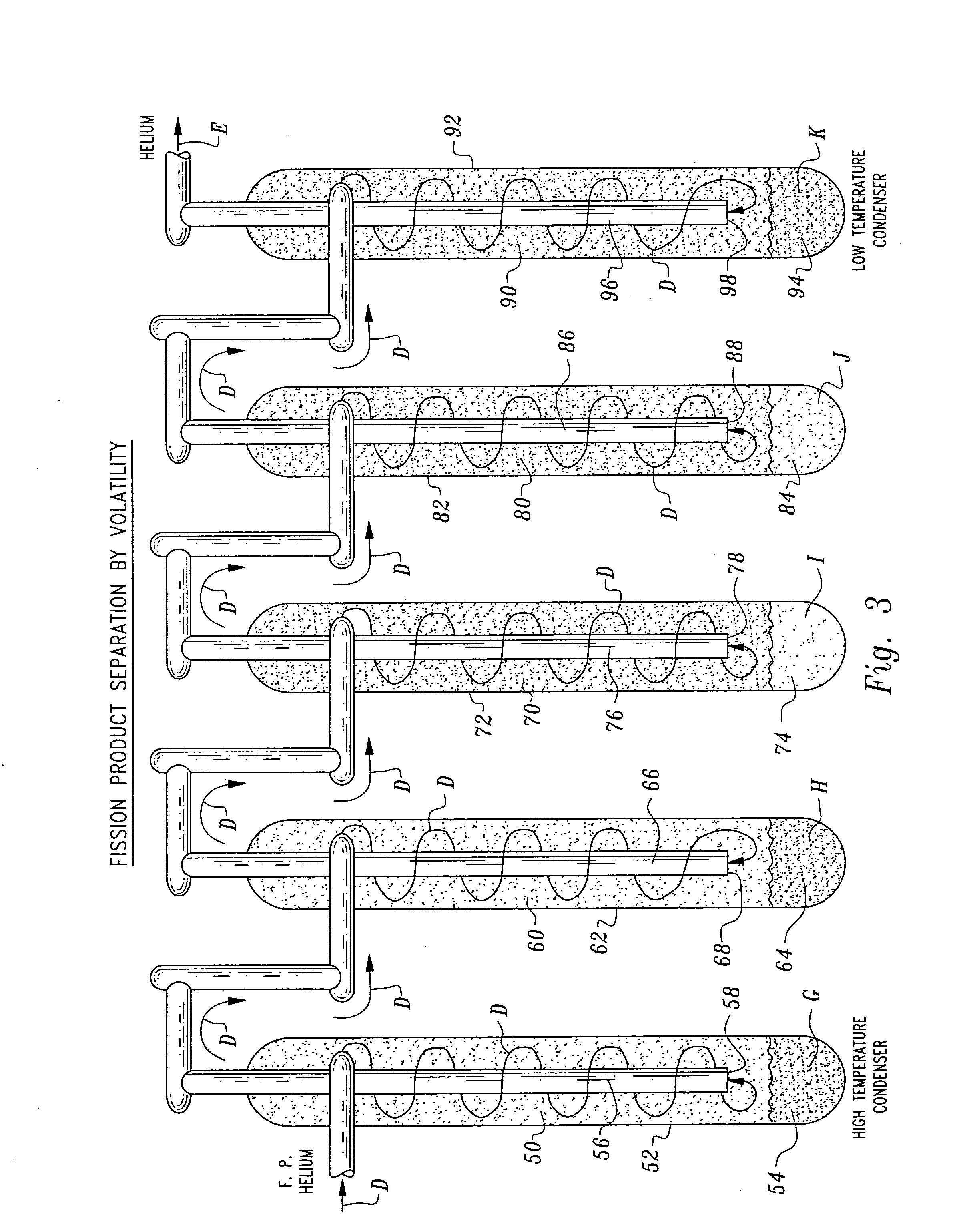
Herein is a method of segregating chemical species contained in spent nuclear reactor fuel without employing conventional acid dissolution. Particularly, pellets of spent fuel are ground to talc sized particles. Heat is added. The preferred heating is by flow through a plasma arc producing micron sized liquid drops suspended in helium flow. The vapor pressure of the chemical species is significantly greater than uranium dioxide. the ultra volatile chemical species evolve from the drops into the helium flow. The gas phase is separated from the mist by a gas / liquid separator (demister). Heavy mist drops of UO2 impact the walls, coalesce and flow down to the separator drain, becoming legally transportable. Helium flow exhausts from the separator vertically. The gaseous chemical species will condense in sequentially cooler stages and separate from the helium down to the cryogenic temperatures of liquid radioactive xenon and krypton. Non-condensed helium is recycled.
Owner:JANES CLARENCE W
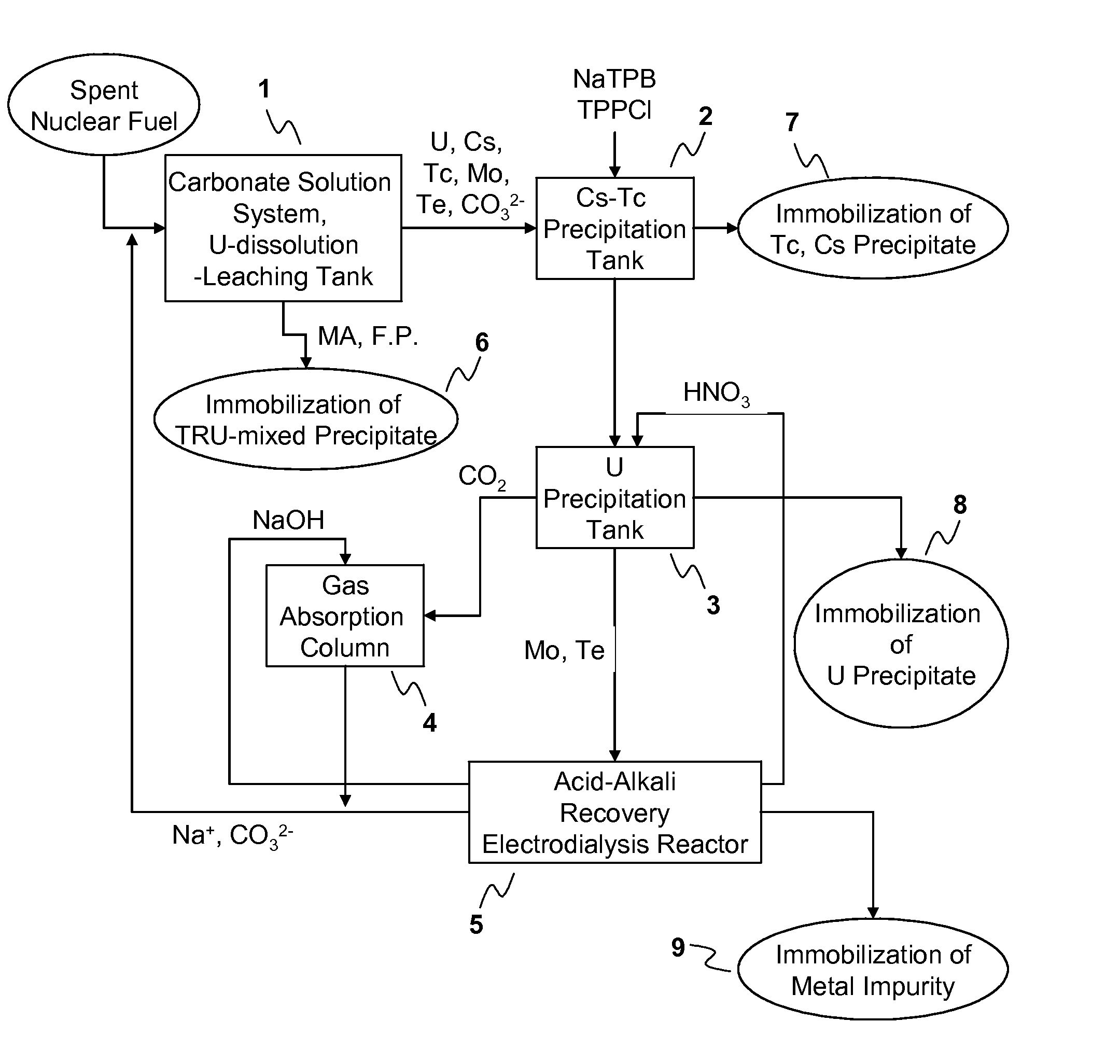
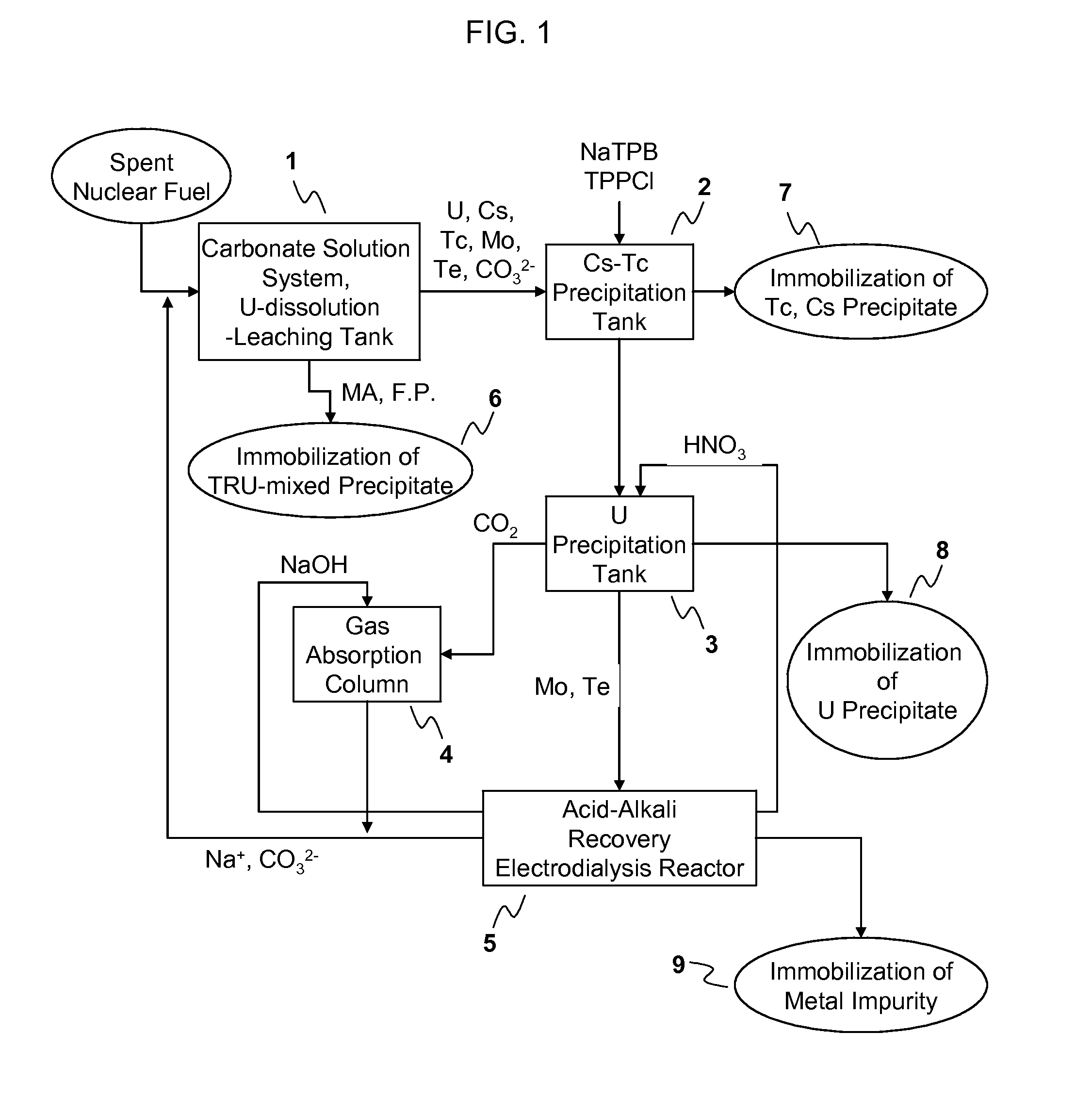
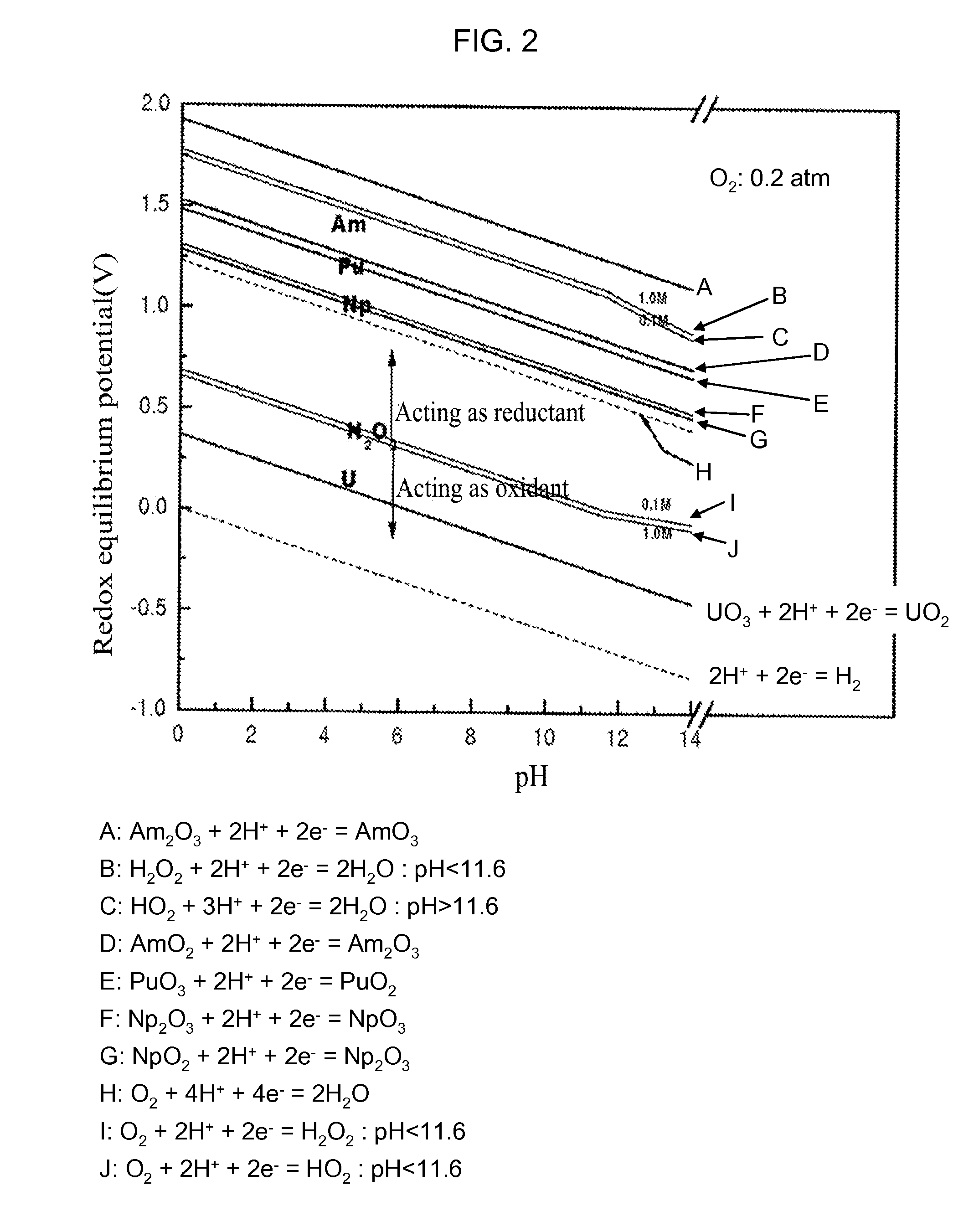
Process for Recovering Isolated Uranium From Spent Nuclear Fuel Using a Highly Alkaline Carbonate Solution
InactiveUS20090269261A1Easy to separateEnhance proliferation resistanceElectrolysis componentsPhotography auxillary processesTransuranium elementCarbonate
Disclosed is a process for recovery of uranium from a spent nuclear fuel using a carbonate solution, characterized by excellent proliferation resistance of preventing leaching of transuranium element (TRU) nuclides such as Pu, Np, Am, Cm, etc. from the spent nuclear fuel as well as environmental friendliness of minimizing waste generation, wherein a highly alkaline carbonate solution is used to separate uranium alone from the spent nuclear fuel.
Owner:KOREA ATOMIC ENERGY RES INST
Method and device for generating droplets over a variable spectrum of particle sizes
The invention relates to a method for generating droplets over a variable spectrum of particle sizes, characterized in that it comprises the following steps: impacting a liquid stream onto a substrate at a given relative impact speed; vibrating said substrate at at least one vibration frequency; heating said substrate to a so-called impact temperature such that the liquid film formed by the impact and vibrated is heated to a so-called main temperature so as to form, in a combined manner, so-called main droplets from said film; transporting said droplets via a transfer / braking / sorting system toward a liquid for precipitating the main droplets, said transport being accomplished at a so-called transport temperature, wherein the set of the relative impact speed, vibrating frequency, main temperature, and transport temperature parameters enabling the modulation of the particle size of said formed main droplets, as well as the modulation of the speed thereof. The invention also relates to a device for implementing the method of the invention.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Method and device for declogging filter
A method of declogging at least one filter of a plant for manufacturing uranium oxide from uranium hexafluoride, including separating, from the wall of the filter, uranium oxyfluoride particles deposited, by a stream of inert gas such as nitrogen, injected into the filter, in a counter-currentwise direction to the flow of hydrofluoric acid.
Owner:AREVA NP SAS
Process for recovery of high purity uranium from fertilizer grade weak phosphoric acid
InactiveUS7192563B2Effective recoveryStable, cost effective and easily available extracting solventSolvent extractionTransuranic element compoundsKerosenePhosphoric acid
A two-cycle countercurrent extraction process for recovery of highly pure uranium from fertilizer grade weak phosphoric acid. The proposed process uses selective extraction using di-(2-ethyl hexyl) phosphoric acid (D2EHPA) and tri-n-butyl phosphate (TBP) with refined kerosene as synergistic extractant system on hydrogen peroxide treated phosphoric acid, and stripping the loaded extract with strong phosphoric acid containing metallic iron to lower redox potential. The loaded-stripped acid is diluted with water back to weak phosphoric acid state and its redox potential raised by adding hydrogen peroxide and re-extracted with same extractant system. This extract is first scrubbed with sulfuric acid and then stripped with alkali carbonate separating iron as a precipitate, treated with sodium hydroxide precipitating sodium uranate, which is re-dissolved in sulfuric acid and converted with hydrogen peroxide to highly pure yellow cake of uranium peroxide.
Owner:SEC DEPT OF ATOMIC ENERGY
Method for co-precipitation of actinides in different oxidation states and method for preparation of mixed actinide compounds
ActiveCN1961380AImprove sintering performancePromote decompositionPlutonium compoundsNuclear energy generationMixed oxideHybrid compound
The invention relates to a method for co-precipitation (or simultaneous preparation) of at least one actinide in oxidation state (IV) with at least one actinide in oxidation state (III), in which a mixed solution of actinide(s) in oxidation state (IV) and actinide(s) in oxidation state (III) is prepared with addition of either a mono-charged cation which stabilises the oxidation states in the mixture, or a mono-charged cation with no stabilizing role for the oxidation states in the mixture and a solution containing oxalate ions is added to said mixture of actinides to produce a co-precipitation, or simultaneous precipitation of said actinides of oxidation state (IV) and (III) and a fraction of the mono-charged cation. According to another embodiment, a mixed solution of actinide(s) in oxidation state (IV) and actinide(s) in oxidation state (III) is prepared and a solution containing oxalate ions and a mono-charged cation is added to said mixture of actinides to produce the co-precipitation. The invention further relates to mixed actinide compounds from calcination of the precipitate above. Said mixed compounds such as oxides, carbides or nitrides are particularly of use in the production of nuclear fuel, for the production of transmutation targets or for the stable storage of nuclear material.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
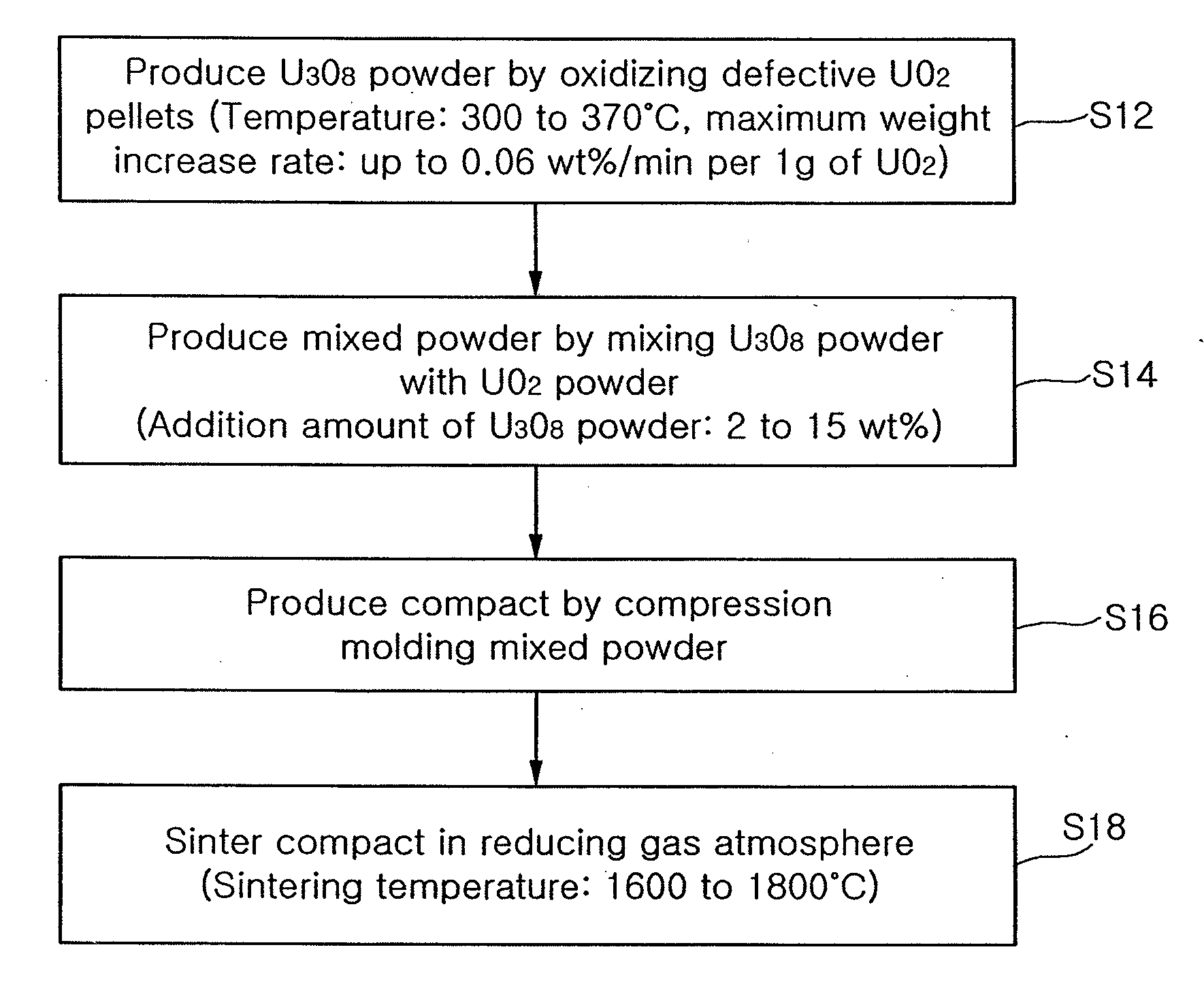
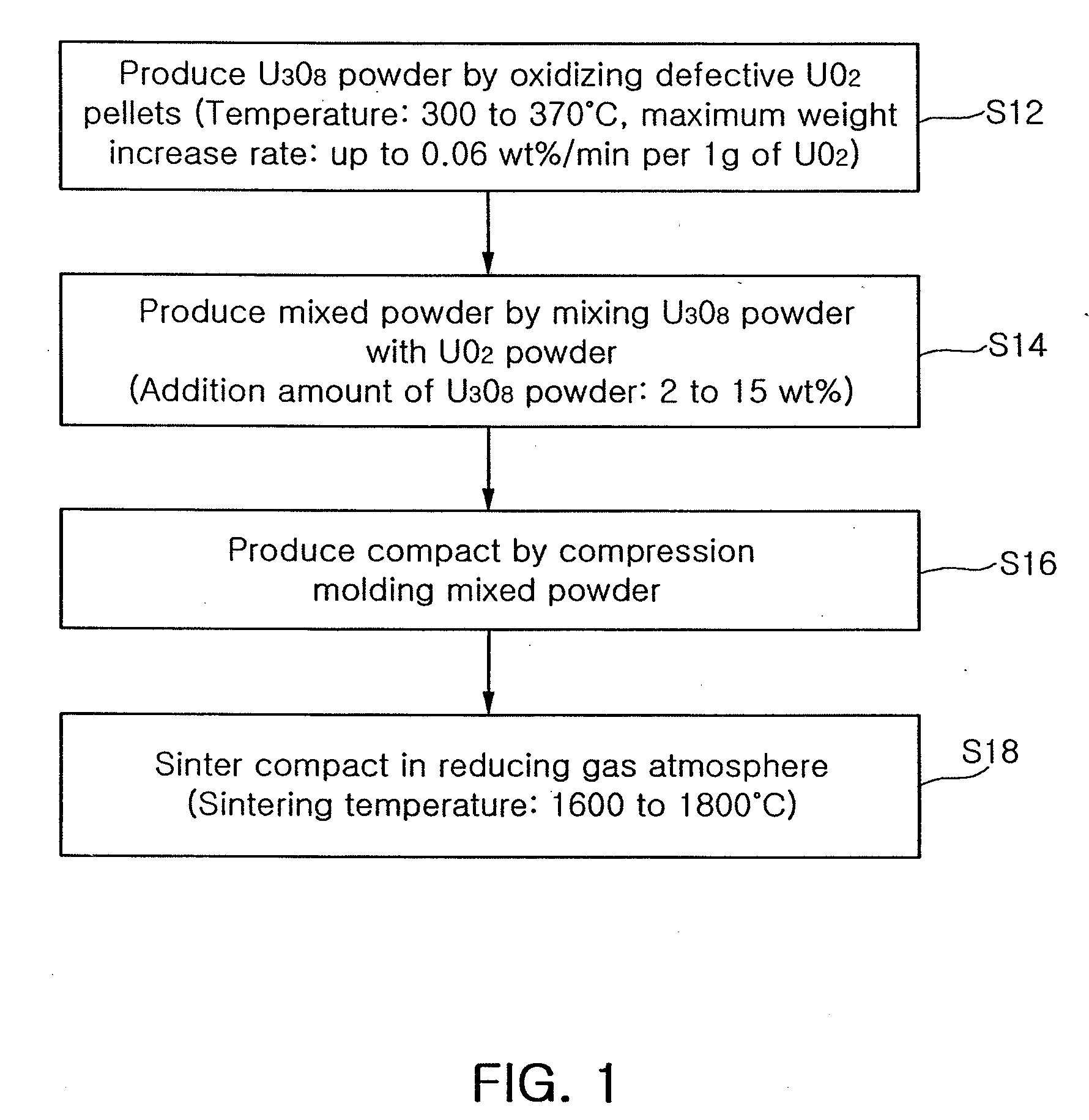
Method of manufacturing sinter-active u3o8 powder and method of producing nuclear fuel pellets utilizing the same
ActiveUS20080185743A1Stable pore structureHigh densityFuel elementsNuclear energy generationCompression moldingHigh density
There is provided a method of producing U3O8 powder having large surface area and small particle size by oxidizing defective UO2 pellets and manufacturing nuclear fuel pellets which are stable in a pore structure and high in density through the use of a mixture comprising UO2 powder and U3O8 powder. The method includes producing an U308 powder having a surface area of at least 1 m2 / g by oxidizing defective UO2 pellets at a temperature of 300 to 370□ in such a way that a maximum weight increase rate per 1 g of the UO2 pellets is up to 0.06 wt % / min; producing a mixed powder by mixing the U3O8 powder with an UO2 powder by 2 to 15 wt %; producing a compact by compression molding the mixed powder; and sintering the compact in a reducing gas atmosphere at a temperature of 1600 to 1800□. In addition, a small amount of an Al-compound may be added to the oxidized U3O8 powder before the U3O8 powder is mixed with the UO2 powder. The additive such as Al is mixed with the U3O8 powder and then mixed with the UO2 powder to produce the pellets by a conventional production method. This ensures a stable pore structure, high density and a considerable increase in a crystal grain size.
Owner:KOREA HYDRO & NUCLEAR POWER CO LTD +1
Two step dry UO2 production process utilizing a positive sealing valve means between steps
ActiveUS7824640B1Inhibition amountHigh densityTransuranic element compoundsNuclear energy generationTemperature controlNuclear grade
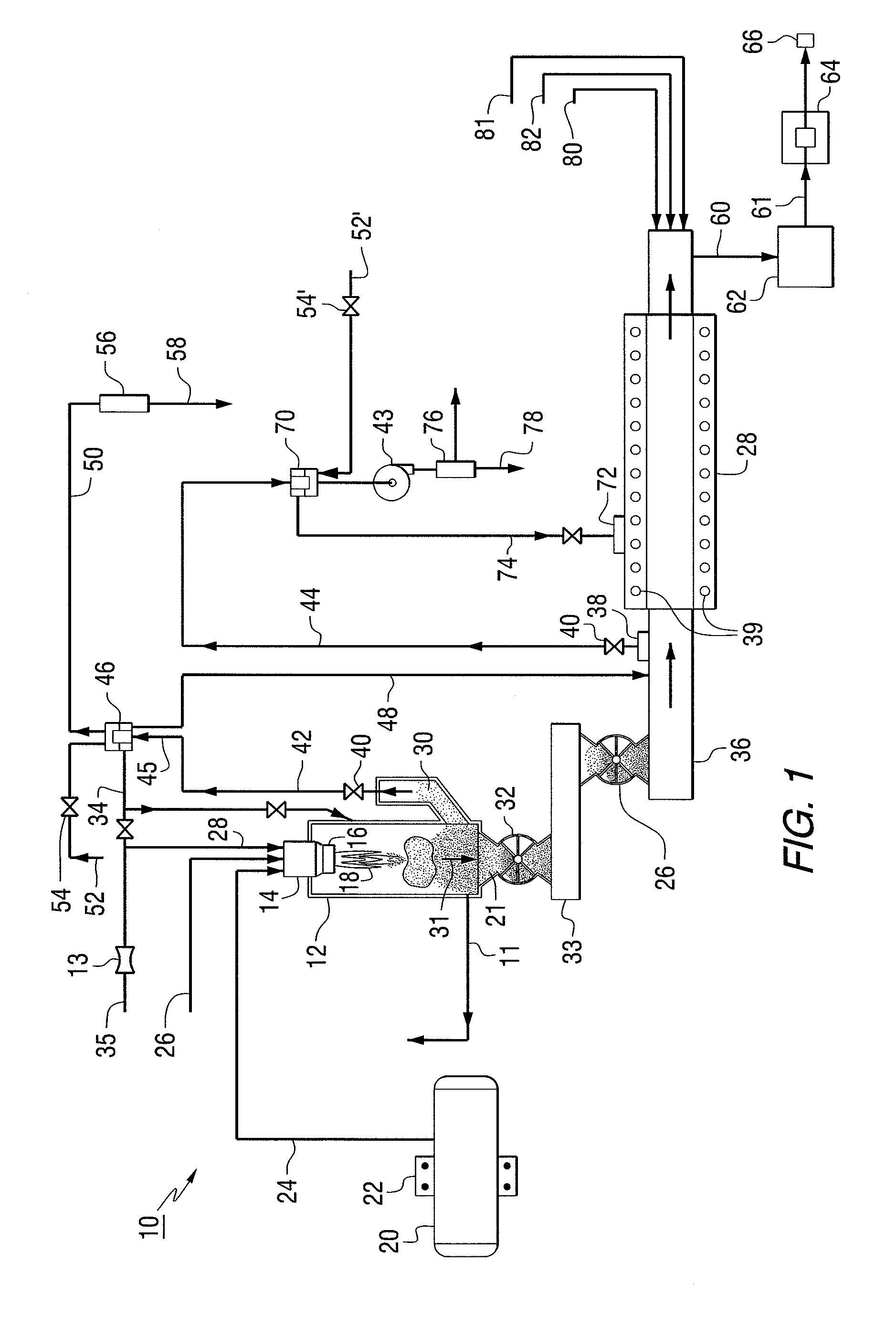
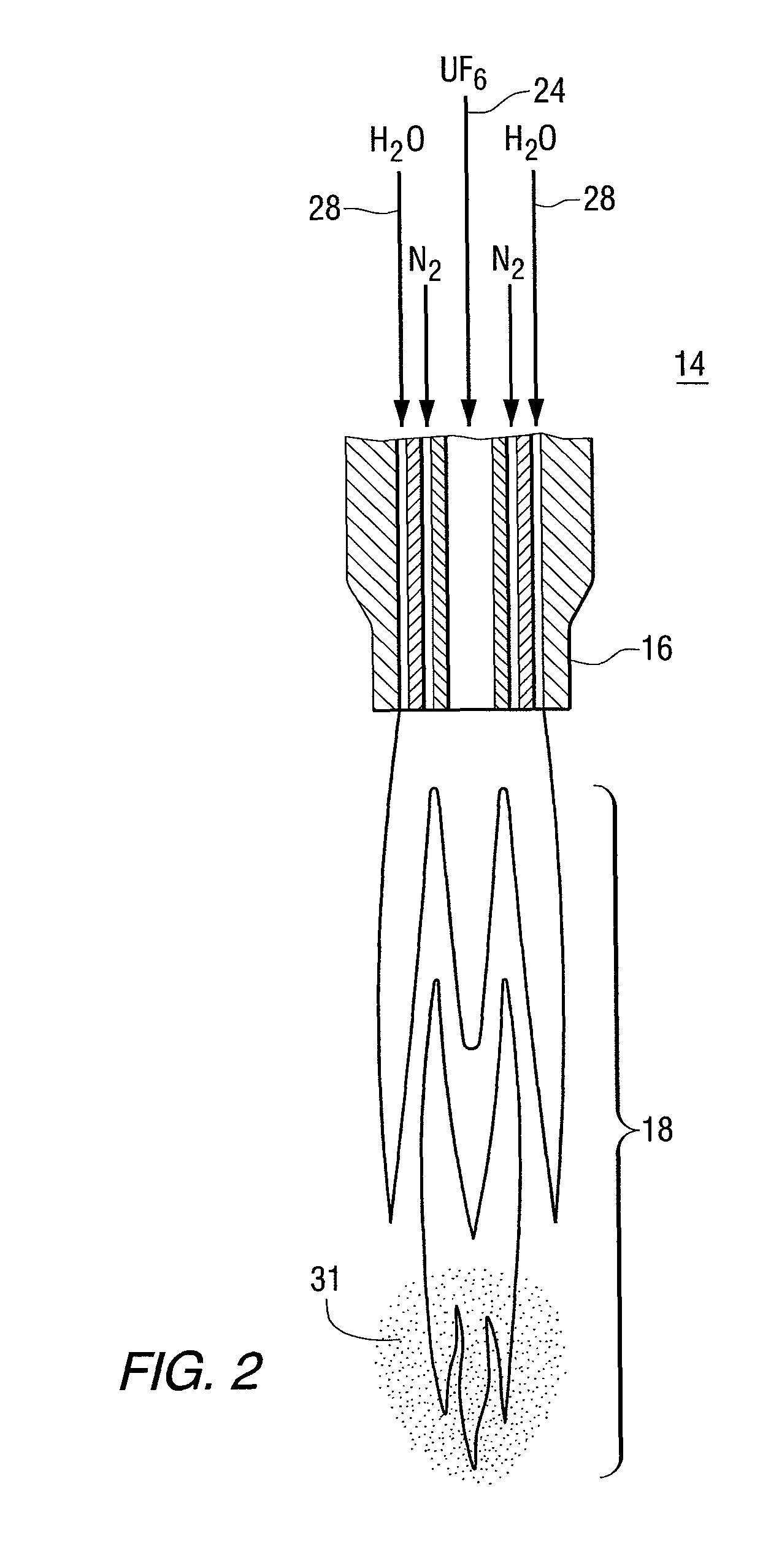
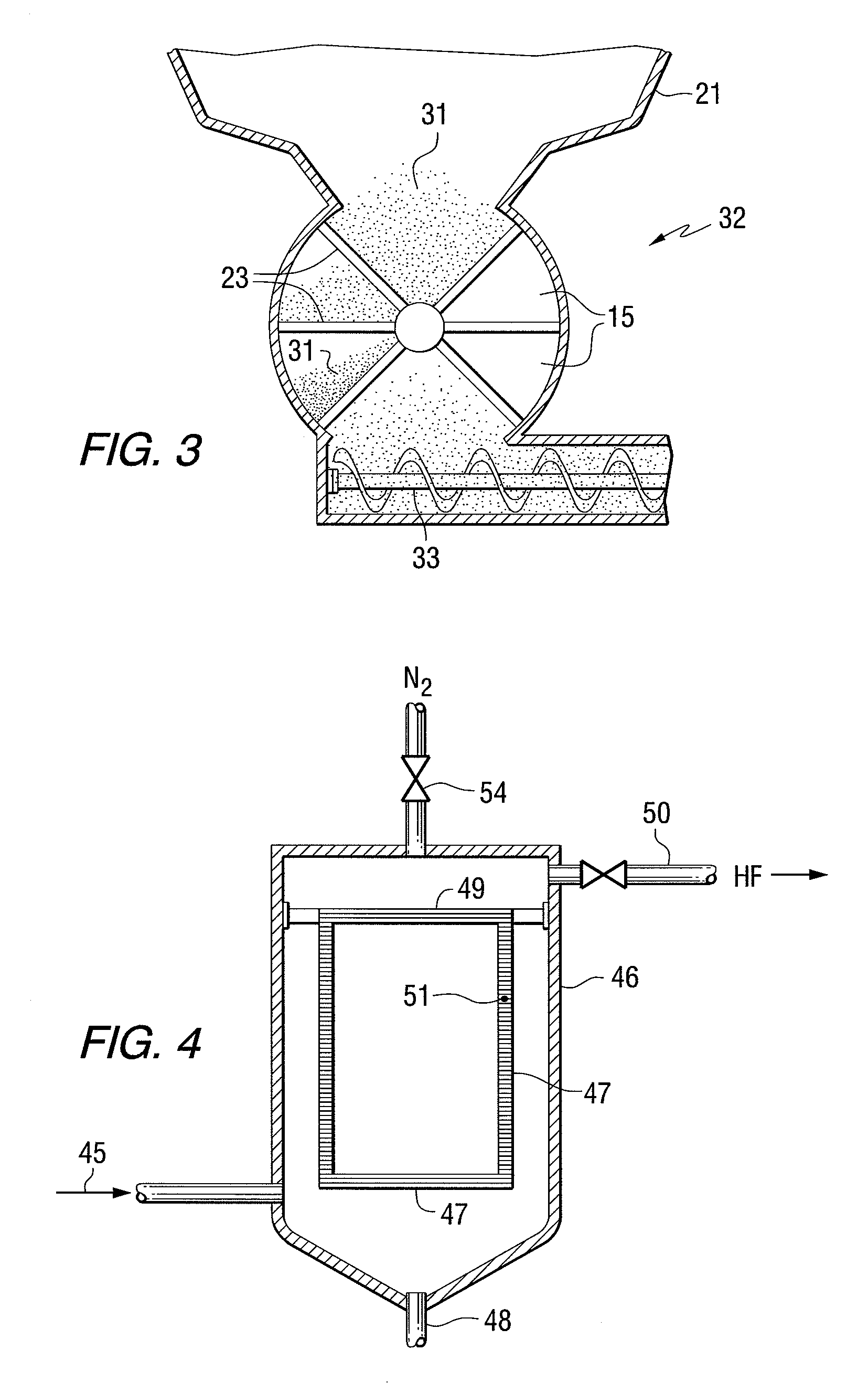
The present invention provides a two-step process for producing nuclear grade, active uranium dioxide (UO2) powder in which the first step comprises reacting uranium hexafluoride (UF6) with steam in a flame reactor to yield uranyl fluoride (UO2F2); and the second step comprises removing fluoride and reducing UO2F2 to uranium dioxide (UO2) in a kiln under a steam / hydrogen atmosphere. The two-step process, each step separated by a positive sealed valve means to prevent gas, particularly H2 flow back, tightly controls the exothermicity of the reaction, which allows for a very tight temperature control which controls the growth of the particles and results in UO2 powder that is active and of consistent morphology.
Owner:WESTINGHOUSE ELECTRIC CORP
Method for producing silicon tetrafluoride from uranium oxyfluoride
InactiveUS6033642AReduced thermodynamic stabilityAvoid vaporizationUranium dioxideHalogenated silanesSilicon tetrafluorideSilicon dioxide
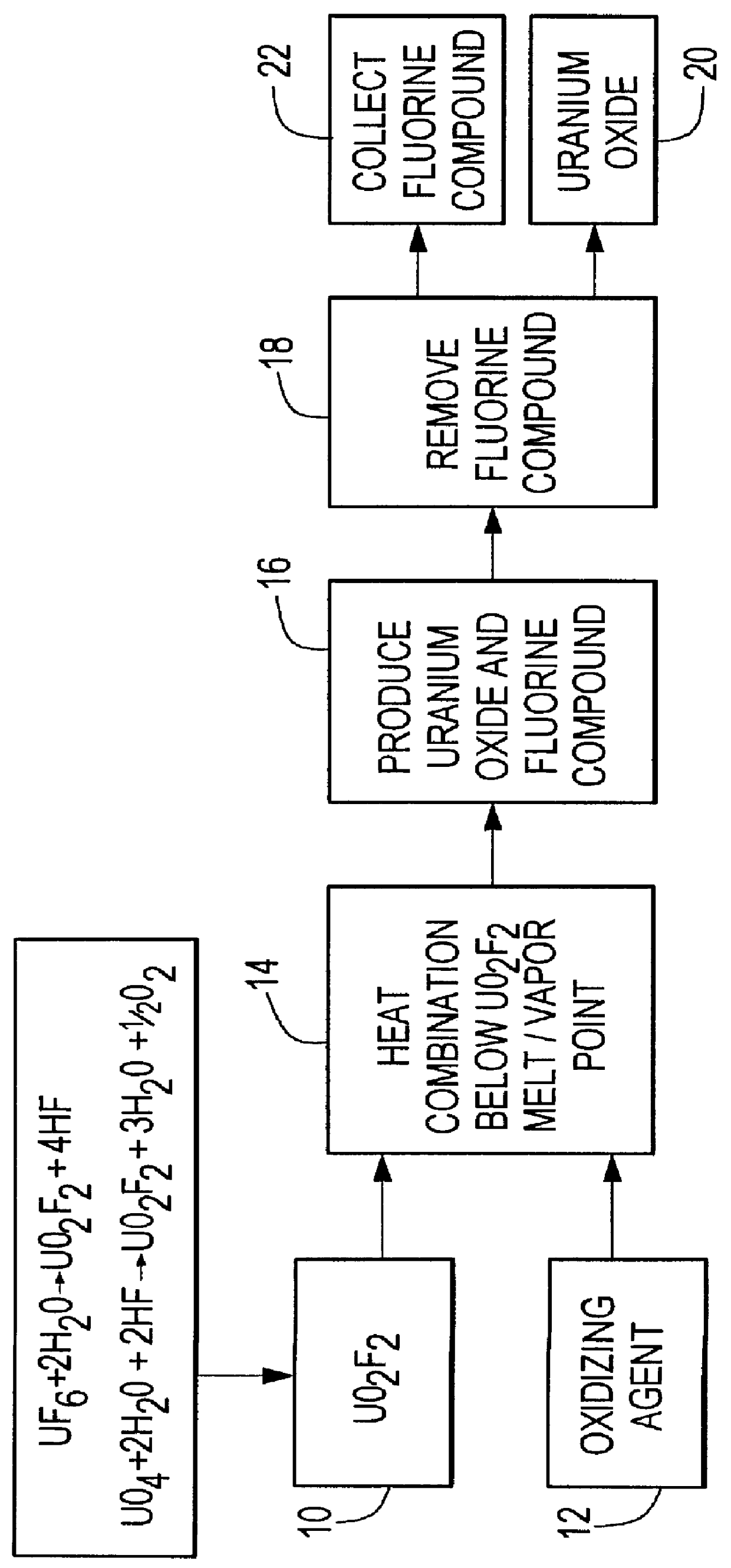
A method for producing silicon tetrafluoride includes combining uranium oxyfluoride and silicon dioxide; heating the combination below the melting point of the uranium oxyfluoride to sufficiently react the uranium oxyfluoride and the silicon dioxide to produce non-radioactive silicon tetrafluoride and an oxide of uranium; and removing the silicon tetrafluoride.
Owner:INT ISOTOPES
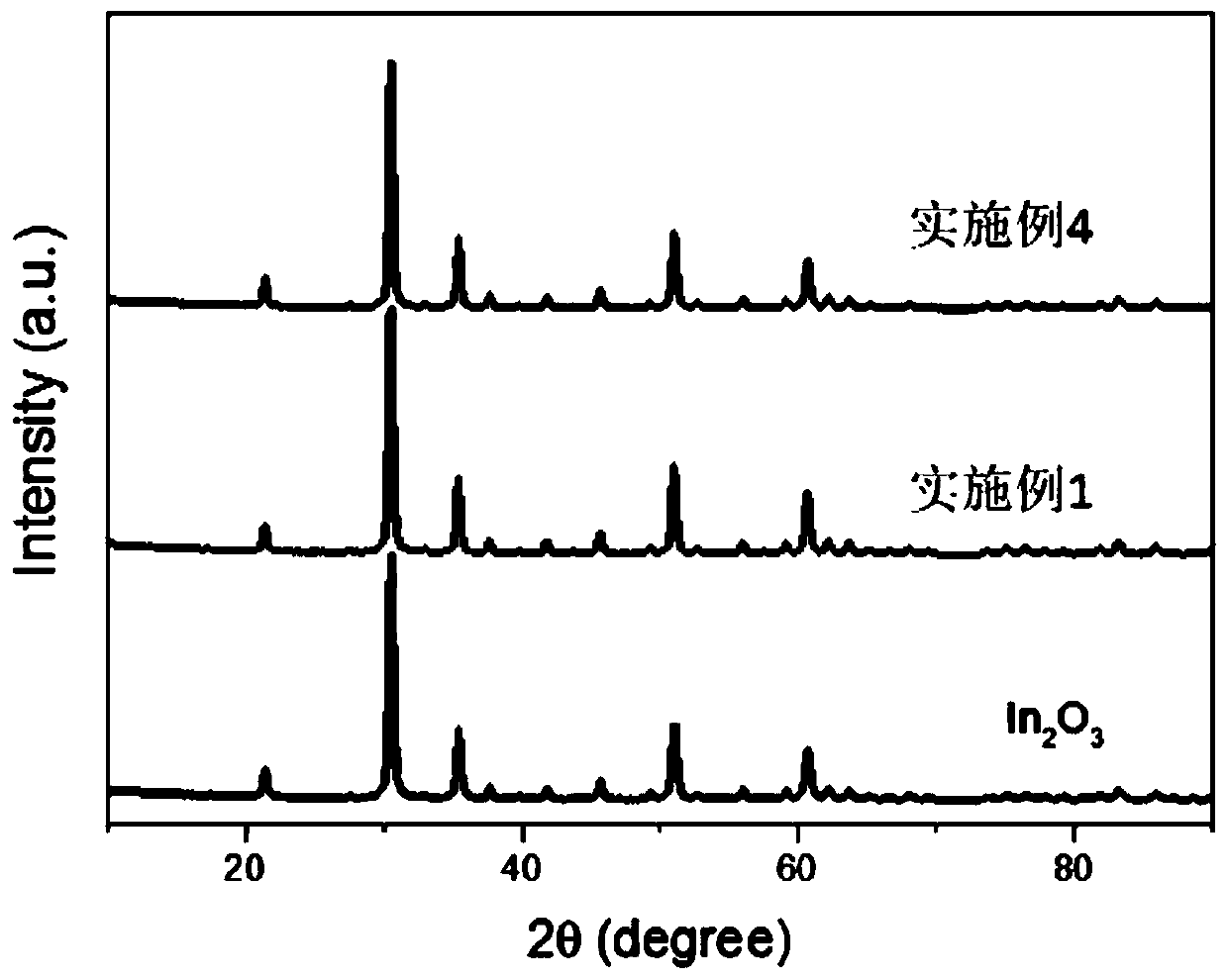
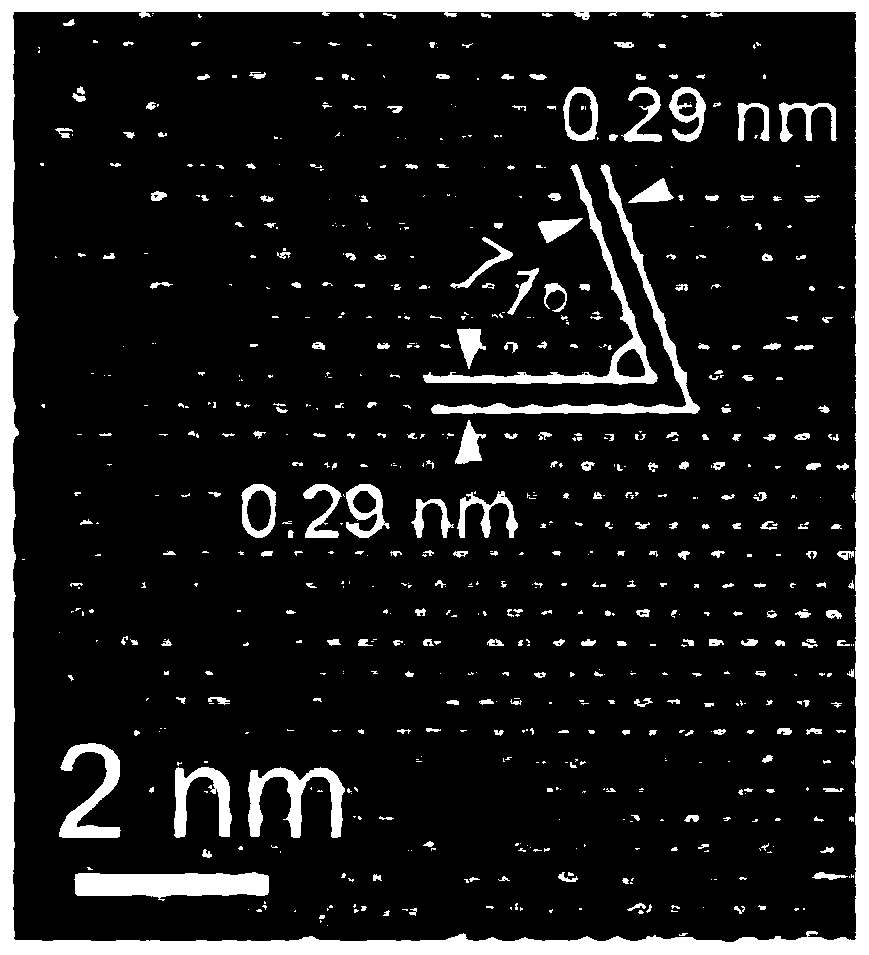
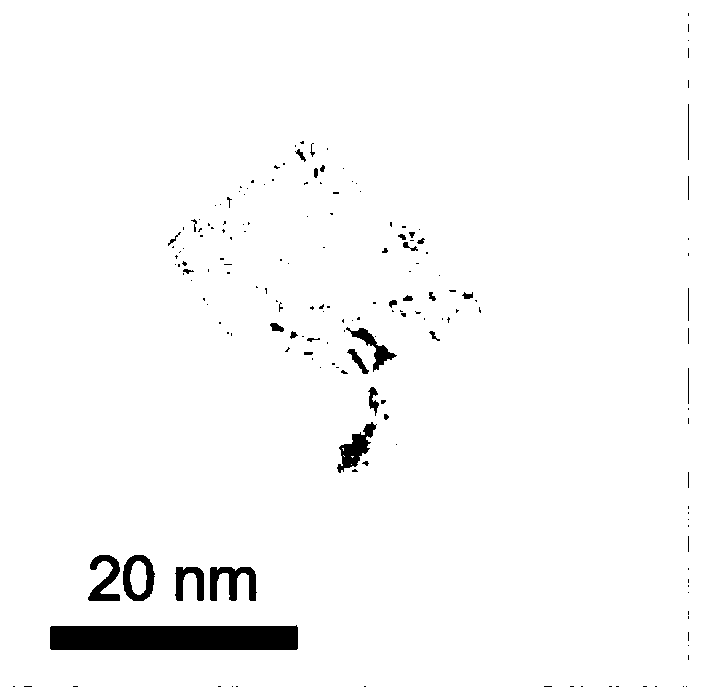
Nanometer uranium dioxide and preparation method of composite material powder thereof
ActiveCN108298587ALow priceSimple manufacturing methodMaterial nanotechnologyNuclear energy generationNuclear powerCarbon nanotube
The invention discloses nanometer UO2 used for the preparation of a novel nuclear fuel pellet and a preparation method of compound powder thereof. According to the nanometer UO2 and compound powder thereof (including but not limited to nanometer UO2 powder, nanometer UO2 / SiC, nanometer UO2 / carbon nanotubes, nanometer UO2 / graphene, nanometer UO2 / nanometer diamond composite material powder) preparedand obtained by adopting a hydrothermal method or a precipitation method, the prepared and obtained powder can serve as a raw material for preparing the novel nuclear fuel pellet in a nuclear power station, and has the characteristics of high melting point, high heat conduction, good anti-radiation performance, high fission gas accommodating capacity and excellent mechanical property.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
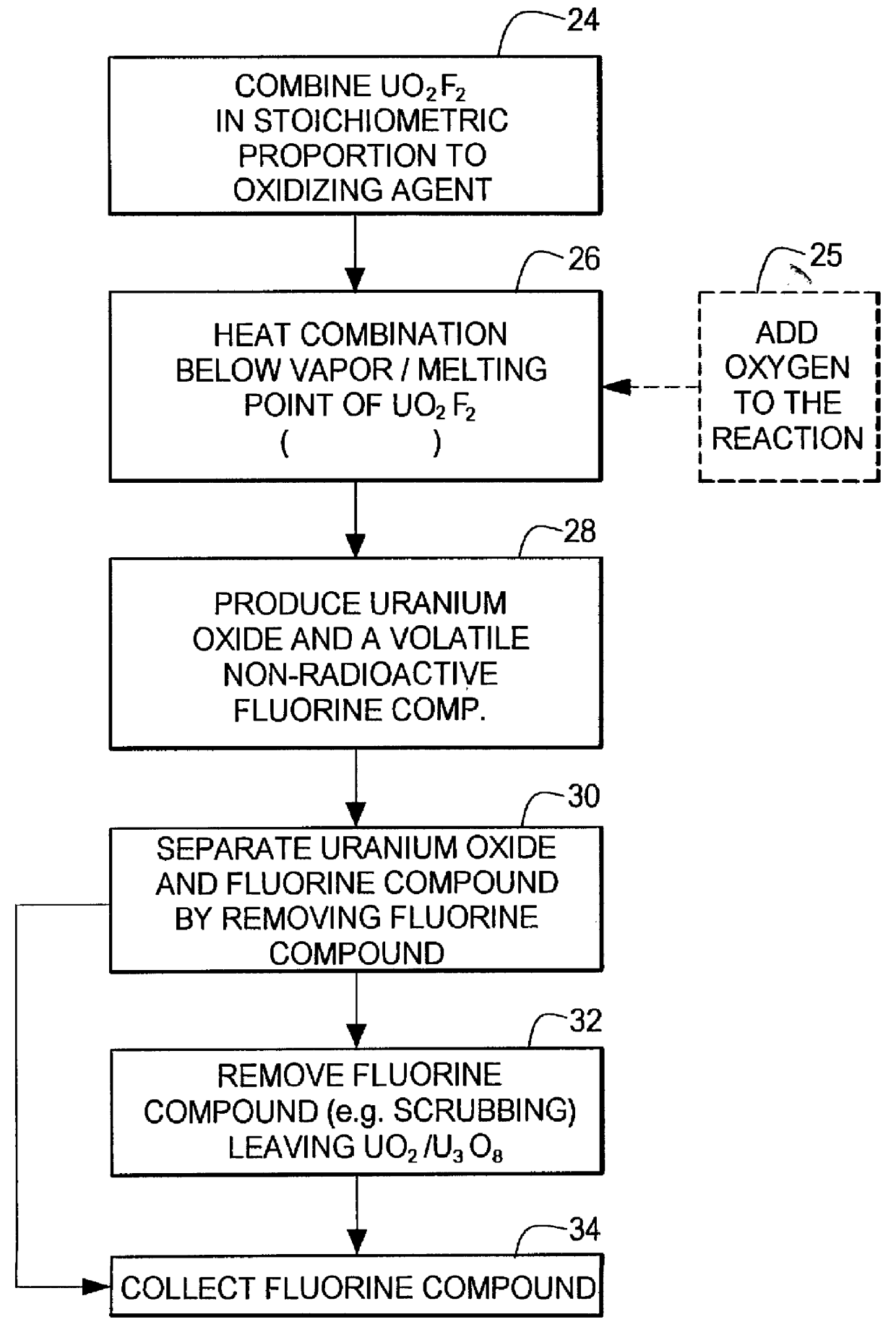
Method for producing uranium oxide from uranium oxyfluoride
InactiveUS6096281AReduced thermodynamic stabilityAvoid vaporizationPhosphorus halides/oxyhalidesFluoride preparationUranium oxideOxidizing agent
A method for producing uranium oxide includes combining uranium oxyfluoride and a solid oxidizing agent having a lower thermodynamic stability than the uranium oxide after "oxide"; heating the combination below the vapor point of the uranium oxyfluoride to sufficiently react the uranium oxyfluoride and the oxidizing agent to produce uranium oxide and a non-radioactive fluorine compound; and removing the fluorine compound after "compound".
Owner:INT ISOTOPES
Two step uo2 production process
This present invention provides a two-step process for producing nuclear grade, active uranium dioxide (UO2)powder in which the first step comprises reacting uranium hexafluoride (UF6) with steam in, for example, an integrated dry route (IDR)-type kiln or a flame reactor to yield uranyl fluoride (UO2F2); and the second step comprises removing fluoride and reducing UO2F2 to uranium dioxide (UO2) in a second kiln under a steam / hydrogen atmosphere. The two-step process tightly controls the exothermicity of the reaction, which allows for a very tight temperature control which controls the growth of the particles and results in UO2 powder that is active.
Owner:WESTINGHOUSE ELECTRIC CORP
Preparation of mineral particles in a supercritical CO2 medium
InactiveCN101754800AImprove compactnessSmall sizeMaterial nanotechnologySilicaInorganic particlePhysical chemistry
The invention relates to a method for preparing mineral particles (p) from mineral particle precursors, said method comprising a step (E) that comprises injecting a fluid medium (F) containing said precursors in solution and / or dispersed in a solvent in a reactor containing CO2 at a supercritical state using an injection nozzle giving into an area where the supercritical CO2 is at a temperature higher than or equal to the conversion temperature of the precursors into corresponding mineral species, the invention also relates to particles (p) obtained according to the method and to the use thereof.
Owner:FRAMATOME ANP +1
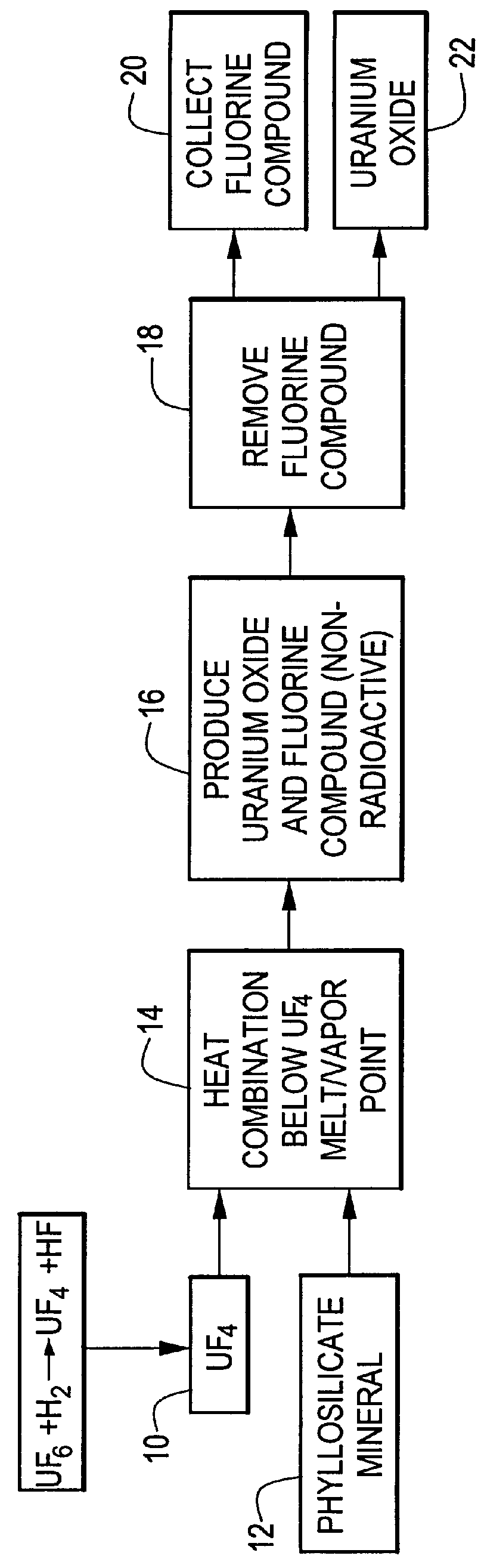
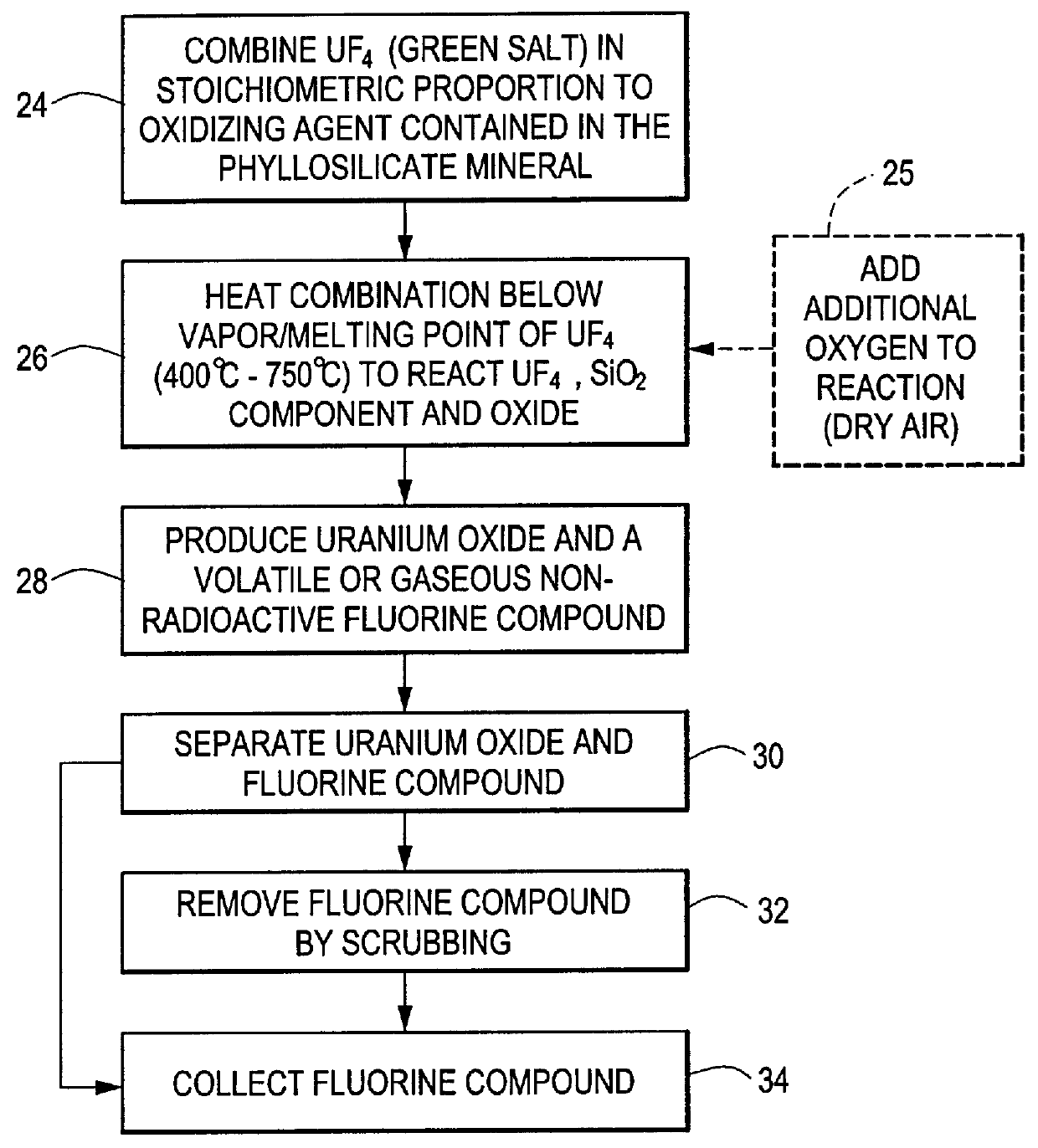
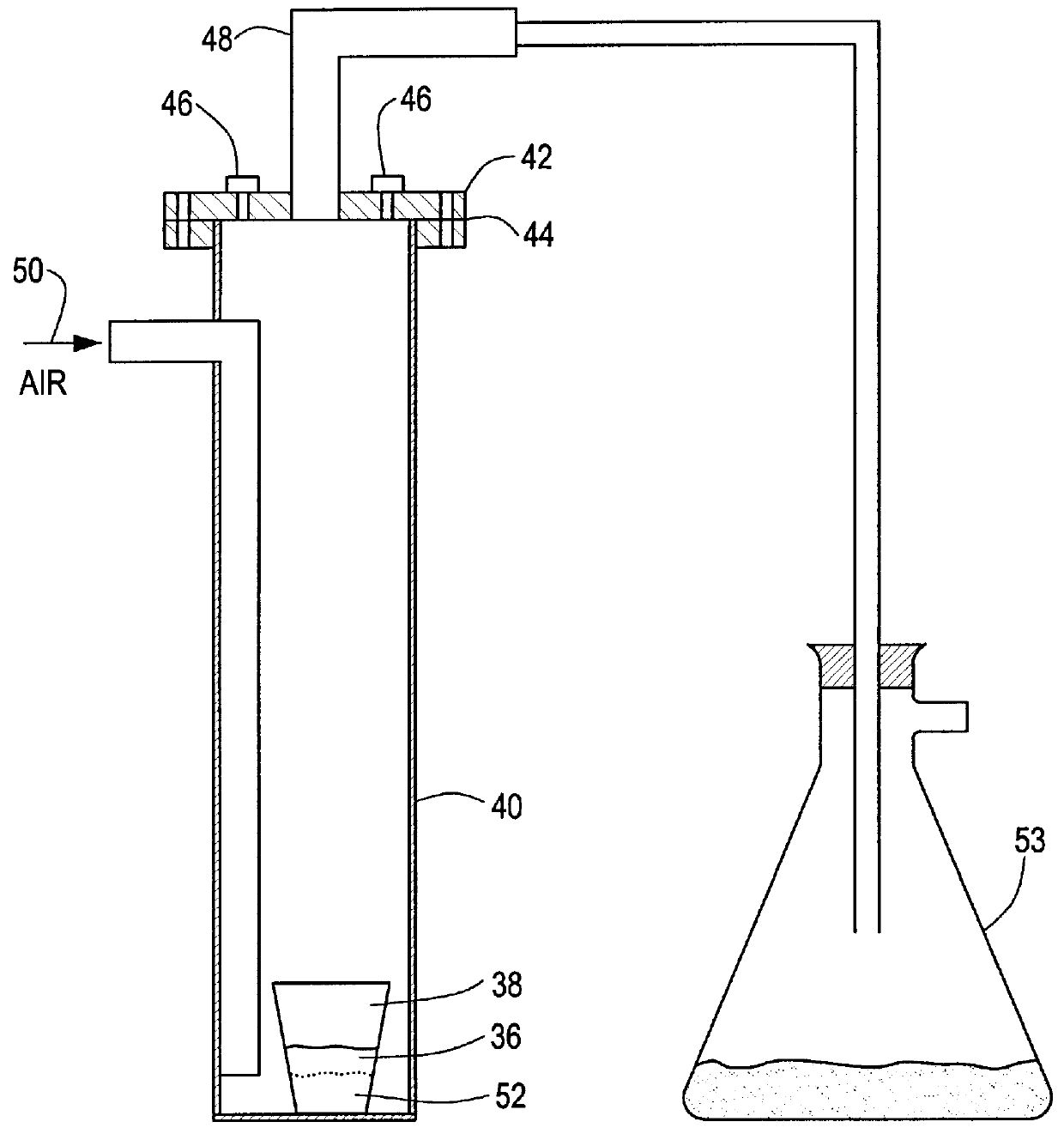
Method for producing uranium oxide from uranium tetrafluoride and a phyllosilicate mineral
InactiveUS6153164AEfficient and cost-effective methodThennodynamic stabilityMagnesium fluoridesSilicon halogen compoundsUranium oxideUranium tetrafluoride
A method for producing uranium oxide includes combining uranium tetrafluoride and a phyllosilicate mineral containing a solid oxidizing agent within the mineral's structure having a lower thermodynamic stability than the uranium oxide; heating the combination below the vapor point of the uranium tetrafluoride to sufficiently react the uranium tetrafluoride and the oxidizing agent to produce uranium oxide and a non-radioactive fluorine compound; and removing the fluorine compound.
Owner:INT ISOTOPES
Method for producing high temperature reactor fuel element UO* nuclear core
The invention relates to a preparation method for high temperature reactor fuel element UO2 core, relating to the roasting, the reducing and the burning processes of raw material micro ball of UO2 core, and belonging to the technical filed of the reactor fuel element production method. The method is characterized in that the raw material micro ball is paved inside a stainless steel pallet and then is arranged inside a low temperature furnace; air is fed into the low temperature furnace for heating and roasting, and then is kept with temperature range from 450 DEG C to 550 DEG C for one hour; vacuum air is fed into an Ar washing furnace; a reducing reaction is implemented with heating speed of 5 to 10 DEG C per minute; the reduced micro ball is arranged into a pot and then is arranged into a high temperature burning furnace behind the washing furnace to burn the micro ball; and the temperature is decreased and air is closed, and the pot is taken out so as to obtain the high temperature reactor fuel element UO2 core. The low temperature furnace takes a clock cover shape or a case shape low temperature furnace. The invention combines the roasting and the reducing into one furnace to complete, decreases the one-off cooling and heating process, reduces the one-off fabric procedure, effectively shortens the production period, prolongs the service time of the furnace, lowers the production cost, and is suitable to production on scale.
Owner:TSINGHUA UNIV
Preparation method of monocrystal uranium dioxide nuclear fuel pellets
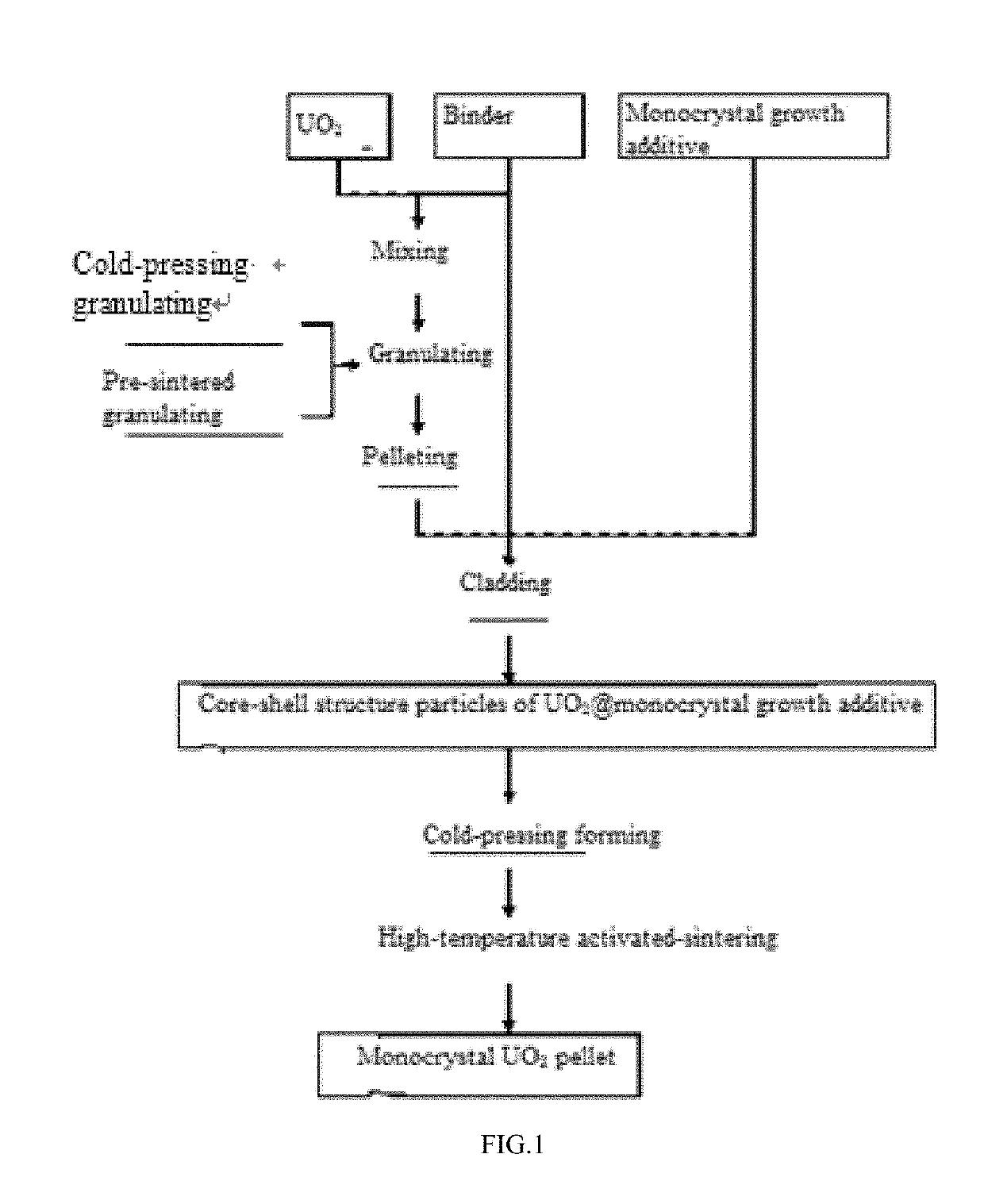
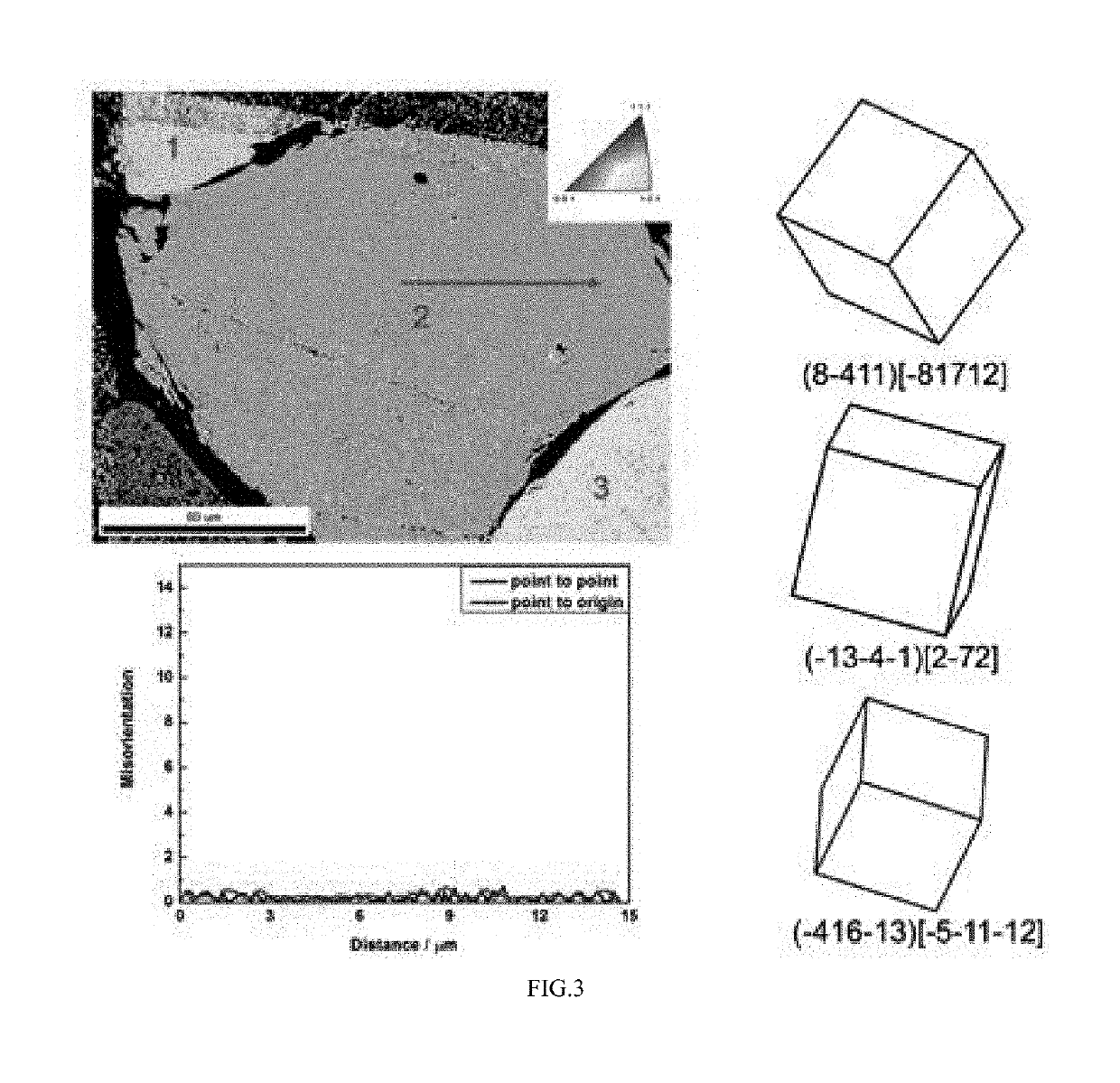
ActiveUS20190127876A1Quick upgradeShort timeAfter-treatment apparatusPolycrystalline material growthSingle crystalCore shell
The application discloses a preparation method of monocrystal uranium dioxide nuclear fuel pellets, comprising: granulating and pelleting UO2 powder to obtain UO2 pellets; then coating surfaces of the UO2 pellets with monocrystal growth additive micro powder to form core-shell structure particles; and activated-sintering the core-shell structure particles at high temperature, liquefying the monocrystal growth additive on the surface of the core-shell structure particle at high temperature and then diffusing into UO2 pellets, dissolving the UO3 in the liquid monocrystal growth additive, and recrystallizing the UO2 to form the monocrystal UO2 nuclear fuel pellets.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS +1
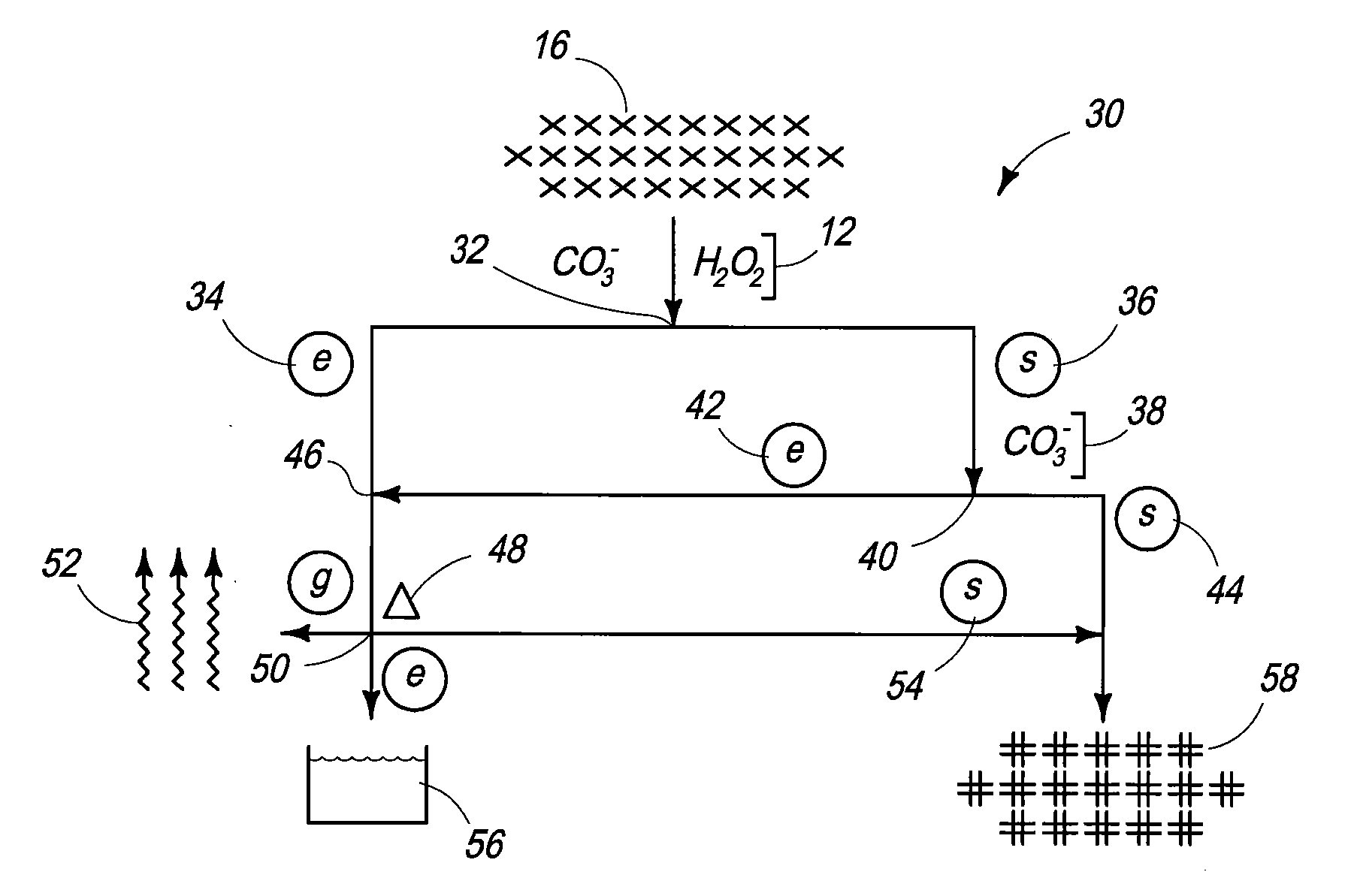
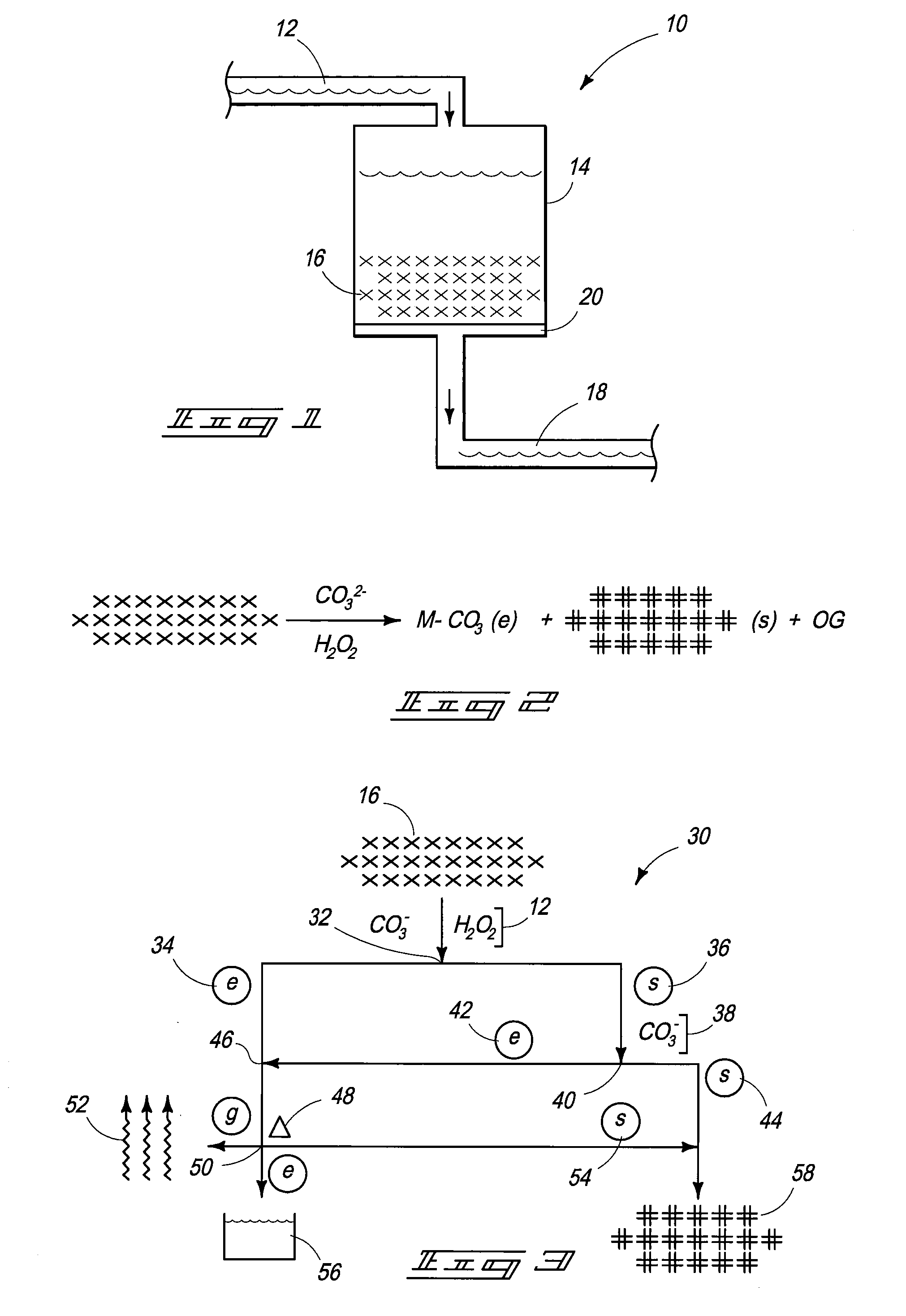
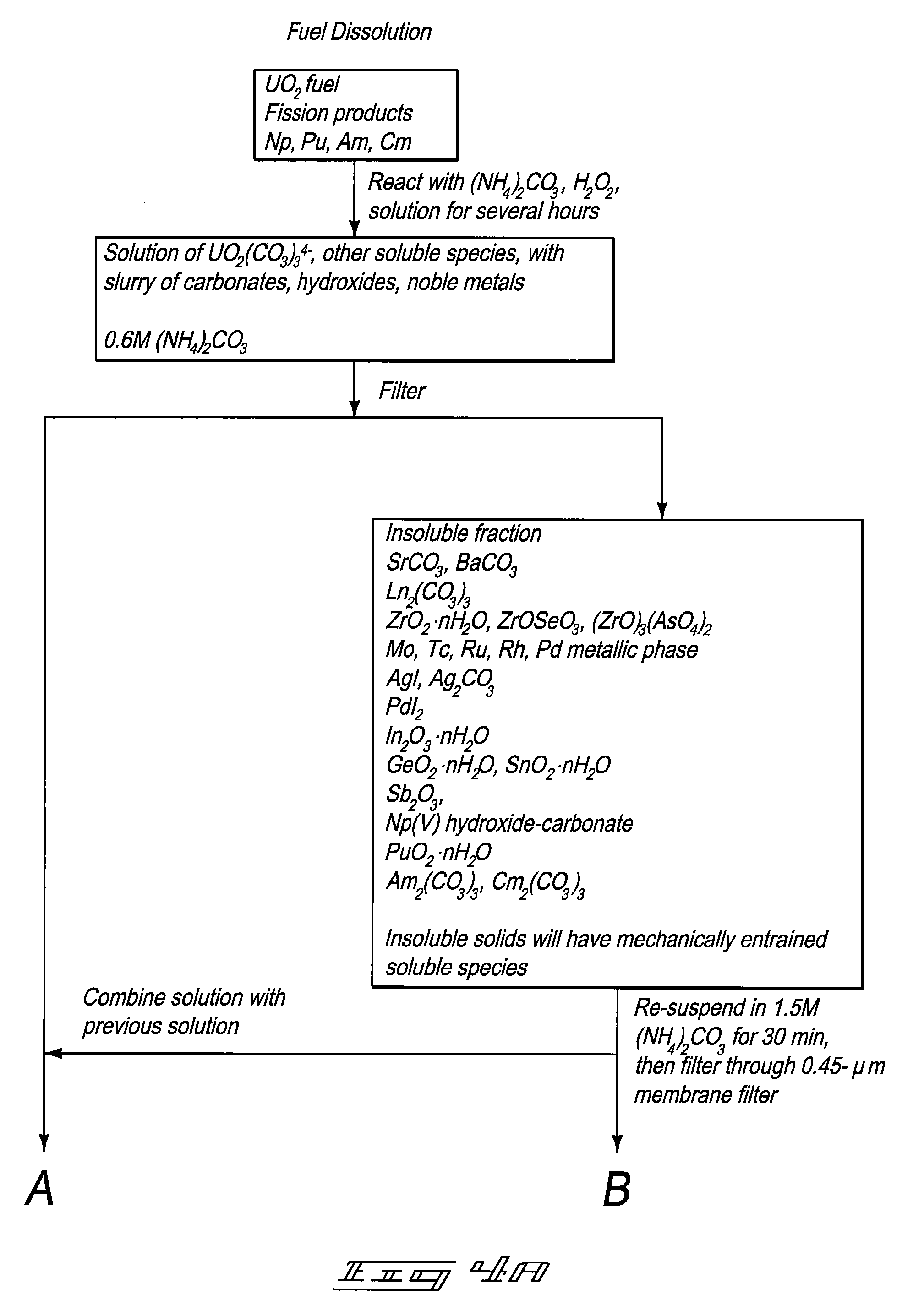
Compositions and Methods for Treating Nuclear Fuel
Compositions are provided that include nuclear fuel. Methods for treating nuclear fuel are provided which can include exposing the fuel to a carbonate-peroxide solution. Methods can also include exposing the fuel to an ammonium solution. Methods for acquiring molybdenum from a uranium comprising material are provided.
Owner:BATTELLE MEMORIAL INST
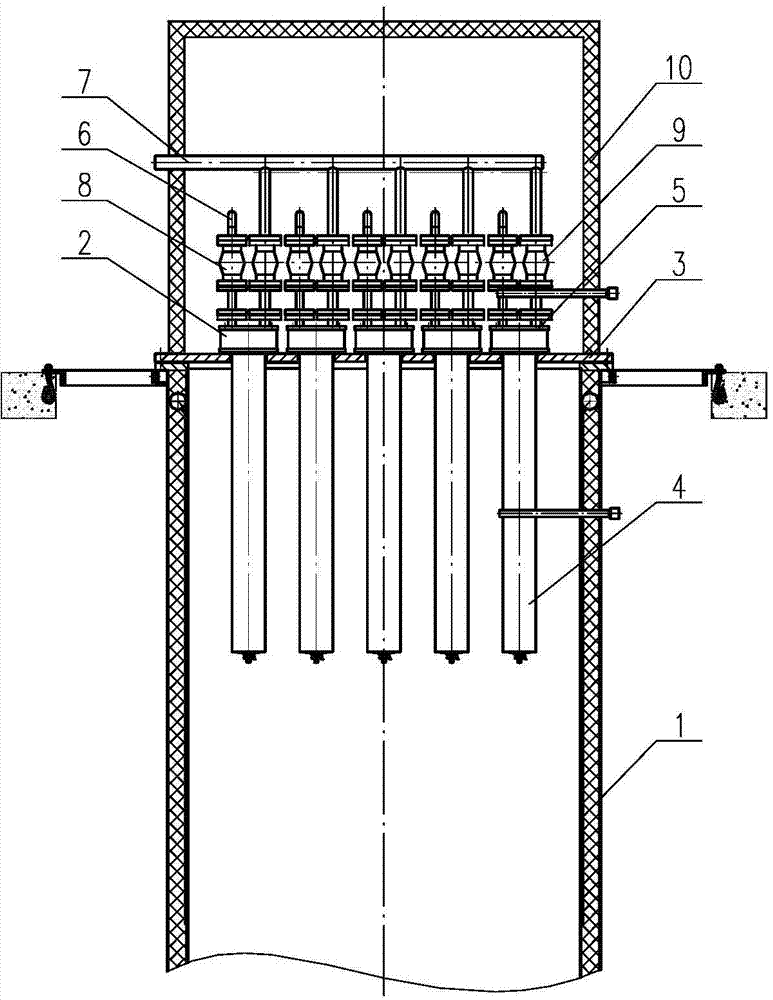
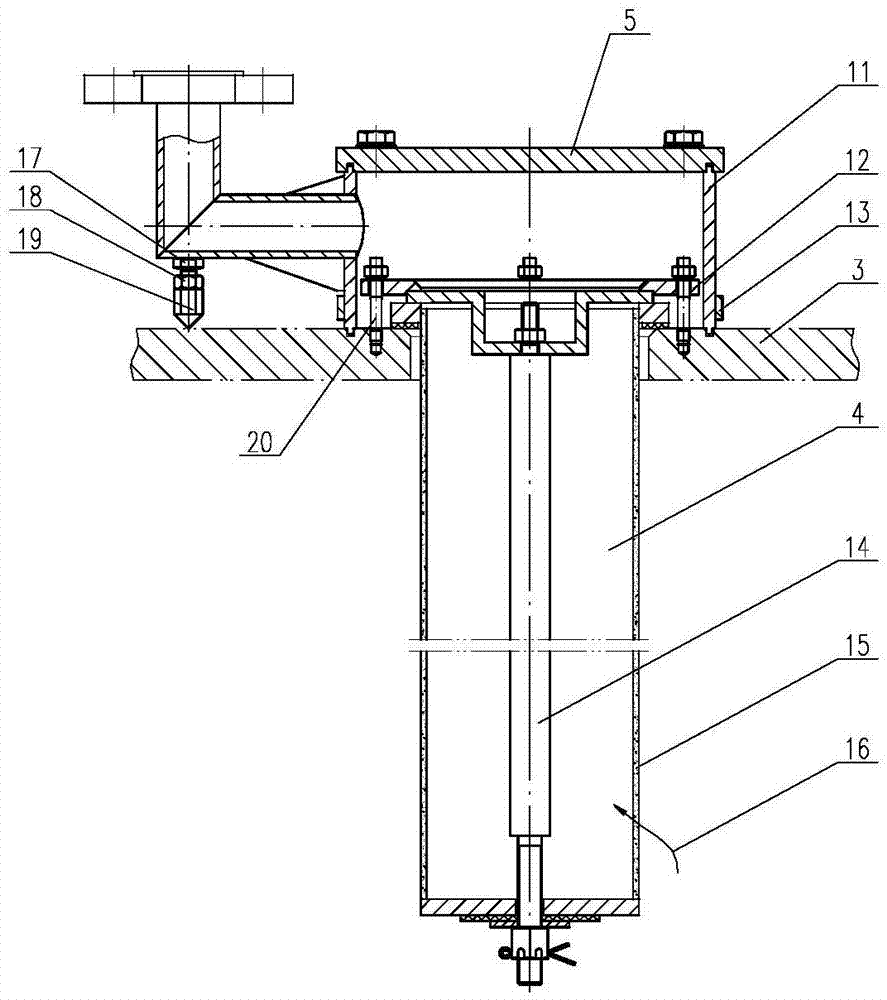
Revolving furnace tail gas filter device for manufacturing uranium dioxide from uranium hexafluoride
InactiveCN104707415AEffective decloggingAvoid breakingDispersed particle filtrationUranium dioxideUranyl fluorideProcess engineering
The invention belongs to a tail gas filter device, and in particular, relates to a revolving furnace tail gas filter device for manufacturing uranium dioxide from uranium hexafluoride. The device includes at least one tail gas filter unit; each tail gas filter unit includes a head and a filter tube; the filter tube of the tail gas filter unit is positioned inside a reaction chamber; the head of the tail gas filter unit is positioned at the outer side of the top end of the reaction chamber; the bottom end of the filter tube in the reaction chamber is sealed; the head is connected with a tail gas recovery tube, and moreover, the head is also connected with a reverse blowing connecting tube, and the other end of the reverse blowing connecting tube is connected with a reverse blowing pipeline. Adhesive uranyl fluoride UO2F2 particles can be separated from the outer wall of the filter tube, and blocking of the filter tube is effectively cleaned; through arrangement of a pull rod, root fracture of the filter pipe can be effectively avoided, and the service life of the filter tube is improved.
Owner:CHINA NUCLEAR POWER ENG CO LTD
Method for surface oxidation treatment of uranium oxide powder
InactiveCN101254950AImprove the O/U atomic ratioIncrease surface areaNuclear energy generationReactors manufactureUranium oxideSurface oxidation
The invention relates to a method of oxidation treatment of a surface of a uranium dioxide, which is characterized that the method comprises the following steps: (1) taking the UO2 powder and putting the UO2 powder into a tube type atmosphere oven, heating the UO2 powder under the condition of oxidation medium while the heating rate is 5 to 20 degree centigrade per minute, heating the UO2 powder to 250 degree centigrade to 400 degree centigrade and insulating for 2 to 6 hours; (2) when the UO2 powder is cooled to 100 degree centigrade to the room temperature in the oven, taking the UO2 powder out and cooling the UO2 powder in the air, so as to obtain the uranium dioxide powder with the oxidized surface. The method puts the UO2 in different oxidation medium and the UO2 is insulated at a certain temperature, then the surface oxygen content is increased, the surface area is increased, and the O / U atomic ratio of uranium dioxide and the surface area of the UO2 powder are enhanced. The oxidation medium obtained by the method has rich resources, low oxidation temperature and less consumption and has the advantages of simple fabrication process and low cost.
Owner:CHONGQING UNIV
Method for electrochemically extracting uranium from seawater by using oxygen vacancy-containing metal oxide
ActiveCN110952107AImplement extractionAchieve recyclingGallium/indium/thallium compoundsUranium dioxideOxygen vacancyUranium
The invention discloses a method for electrochemically extracting uranium from seawater by using an oxygen vacancy-containing metal oxide, and the method comprises the following steps: adding glycerolinto an indium nitrate isopropanol solution, and transferring into a high-temperature high-pressure reaction kettle to react to obtain a spherical indium hydroxide solid; dissolving the spherical indium hydroxide solid in deionized water, and then transferring into the high-temperature high-pressure reaction kettle to react so as to obtain a flaky indium hydroxide solid; calcining the flaky indium hydroxide solid to obtain calcined In2O3-x with oxygen vacancies; adding the In2O3-x into ethanol, and then adding a membrane solution; uniformly coating carbon paper with the solution, naturally drying, clamping the dried carbon paper by using a gold electrode, and using the dried carbon paper as a working electrode in a three-electrode system, wherein a counter electrode in the three-electrodesystem is a platinum wire, and a reference electrode is a calomel electrode; adding simulated seawater into an electrolytic tank, placing the three-electrode system in the simulated seawater of the electrolytic tank, and stirring the simulated seawater for electrolysis to realize uranium extraction.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Method for preparing hollow UO2 nanospheres by ammonium uranyl carbonate solution irradiation
The invention discloses a method for preparing hollow UO2 nanospheres by ammonium uranyl carbonate solution irradiation. The method includes: preparing low-concentration UO2(CO3)3<4-> alkaline solution containing a free radical removing agent; adopting electron beam or gamma-ray irradiation to obtain the hollow UO2 nanospheres, different in diameter, wall thickness and cavity diameter, formed by self assembly of nanoparticles through control of conditions such as absorbed dose and dose rate. A uranium oxide hollow nanostructure which is prepared for the first time is conducive to researches of application of uranium oxide nanoparticles to fields of nuclear fuel, catalysis and the like.
Owner:PEKING UNIV
Controlled synthesis method of uranium oxide microcrystal/nanocrystal
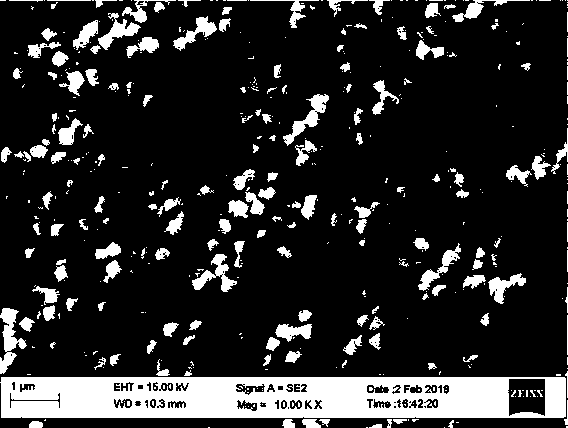
ActiveCN108557891AUniform shapeShape adjustableUranium dioxideNanotechnologyUranium oxideSynthesis methods
The invention discloses a controlled synthesis method of a uranium oxide microcrystal / nanocrystal. According to the controlled synthesis method, a hydrothermal synthesis method is utilized to preparea pure substance of the uranium oxide microcrystal / nanocrystal, and the components of the uranium oxide microcrystal / nanocrystal comprise but are not limited to UO3.xH2O, U3O8, U4O9, UO2, and UO2.34.The microcrystal / nanocrystal prepared by means of the controlled synthesis method is high in yield which is larger than 98%, good in crystallinity which is larger than 95%, and good in controllability. The controlled synthesis method is simple, efficient and capable of regulating and controlling the components, the shape and the size simultaneously, and has great significance. Uranium oxide has wide application value, and the uranium oxide microcrystal / nanocrystal can be applied to an advanced nuclear fuel system in the future. Meanwhile, U3O8, UO3.xH2O, U4O9, UO2, UO2.34 and the like can be expanded and applied to the field of catalysis and the field of semiconductor devices.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Process for recovery of high purity uranium from fertilizer grade weak phosphoric acid
InactiveUS20040247504A1Effective recoveryStable, cost effective and easily available extracting solventSolvent extractionTransuranic element compoundsKerosenePhosphoric acid
A two-cycle countercurrent extraction process for recovery of highly pure uranium from fertilizer grade weak phosphoric acid. The proposed process uses selective extraction using di-(2-ethyl hexyl) phosphoric acid (D2EHPA) and tri-n-butyl phosphate (TBP) with refined kerosene as synergistic extractant system on hydrogen peroxide treated phosphoric acid, and stripping the loaded extract with strong phosphoric acid containing metallic iron to lower redox potential. The loaded-stripped acid is diluted with water back to weak phosphoric acid state and its redox potential raised by adding hydrogen peroxide and re-extracted with same extractant system. This extract is first scrubbed with sulfuric acid and then stripped with alkali carbonate separating iron as a precipitate, treated with sodium hydroxide precipitating sodium uranate, which is re-dissolved in sulfuric acid and converted with hydrogen peroxide to highly pure yellow cake of uranium peroxide.
Owner:SEC
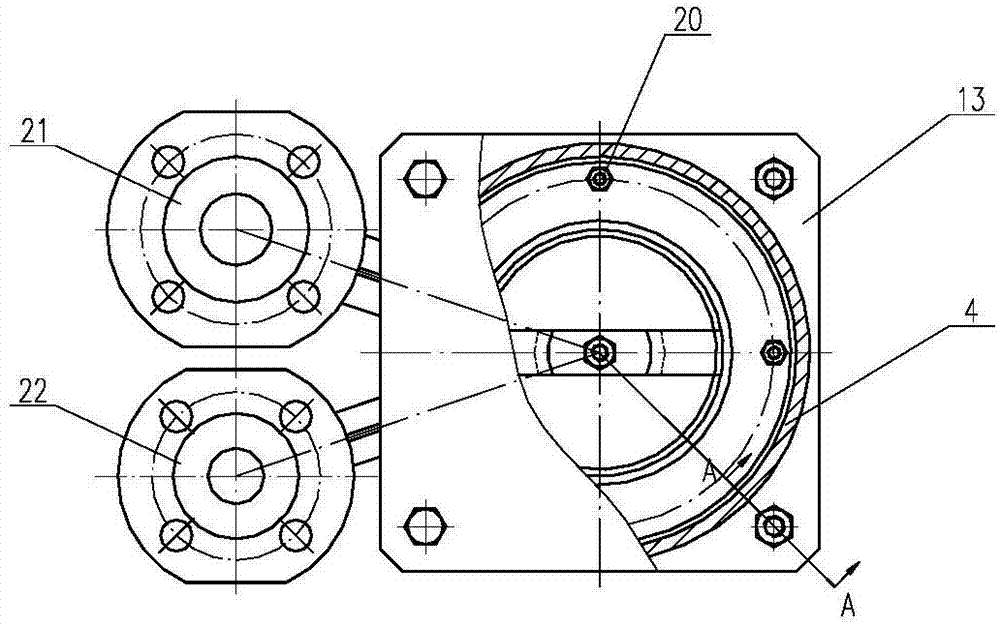
Wet process integrated device utilizing sol-gel method to prepare uranium dioxide cores
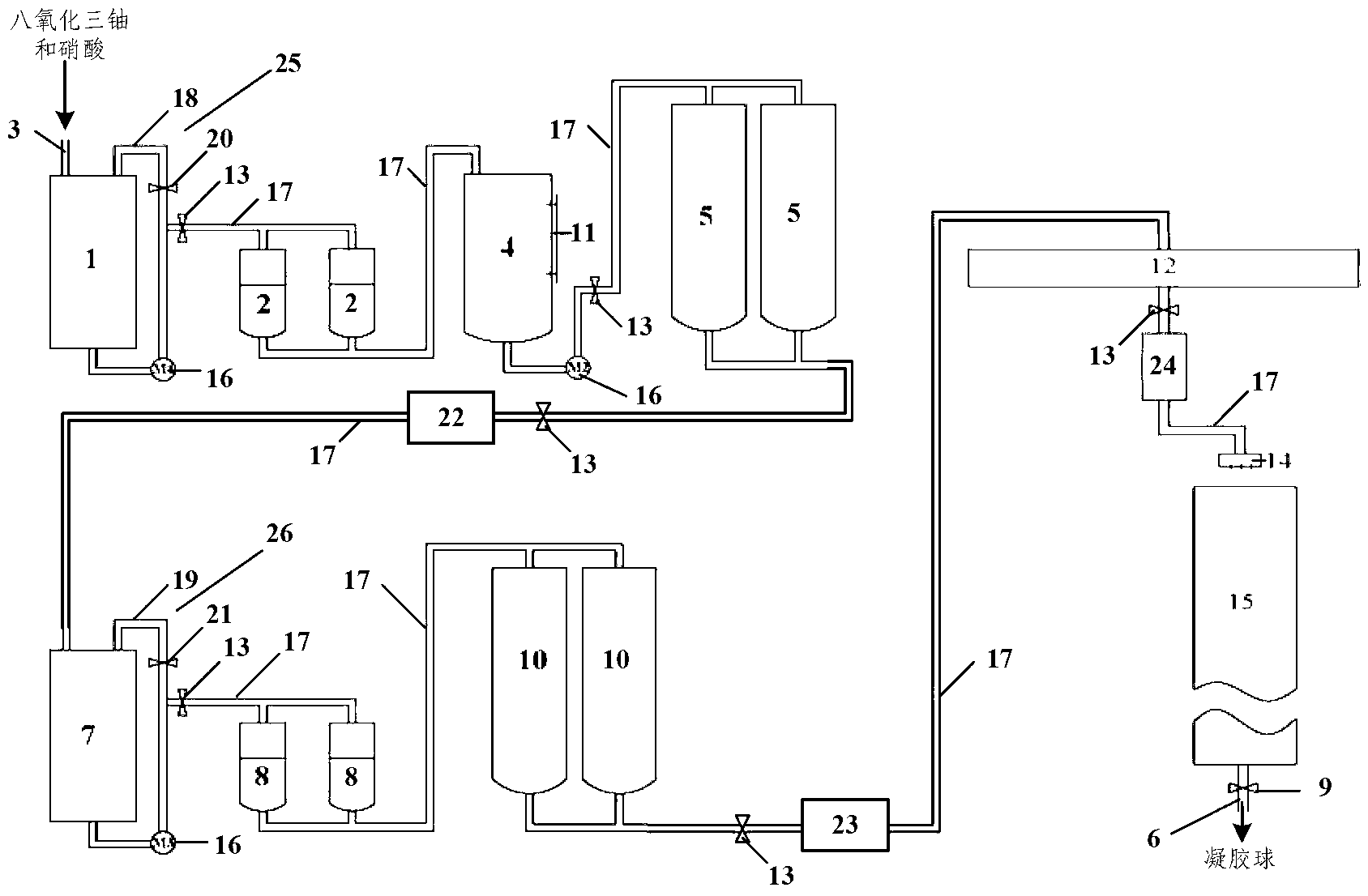
InactiveCN102838166ARealize mass productionOvercoming radioactive contamination problemsUranium dioxideProduction lineHigh volume manufacturing
The invention belongs to the technical field of nuclear material processing, and discloses a wet process integrated device utilizing a sol-gel method to prepare uranium dioxide cores. The wet process integrated device utilizing the sol-gel method to prepare the uranium dioxide cores comprises a dissolving tank, a glue boiling tank, a pressure tank, a vibration dispersing system and a dispersing injection system, wherein the dissolving tank, the glue boiling tank, the pressure tank and the vibration dispersing system are sequentially communicated through a first pipeline which is provided with valves. The outlet of the vibration dispersing system is connected with the inlet of the dispersing injection system in sealed mode, and pumps are installed on the dissolving tank, the glue boiling tank and a part of the first pipeline, wherein the part of the first pipeline is communicated between the glue boiling tank and the pressure tank. By means of the fact that a continuous sealed production line is formed among all units of the wet process integrated device, the wet process integrated device utilizing the sol-gel method to prepare the uranium dioxide cores achieves mass production of the uranium dioxide cores, and resolves the problem of radioactive pollution of nuclear materials during a production process.
Owner:TSINGHUA UNIV
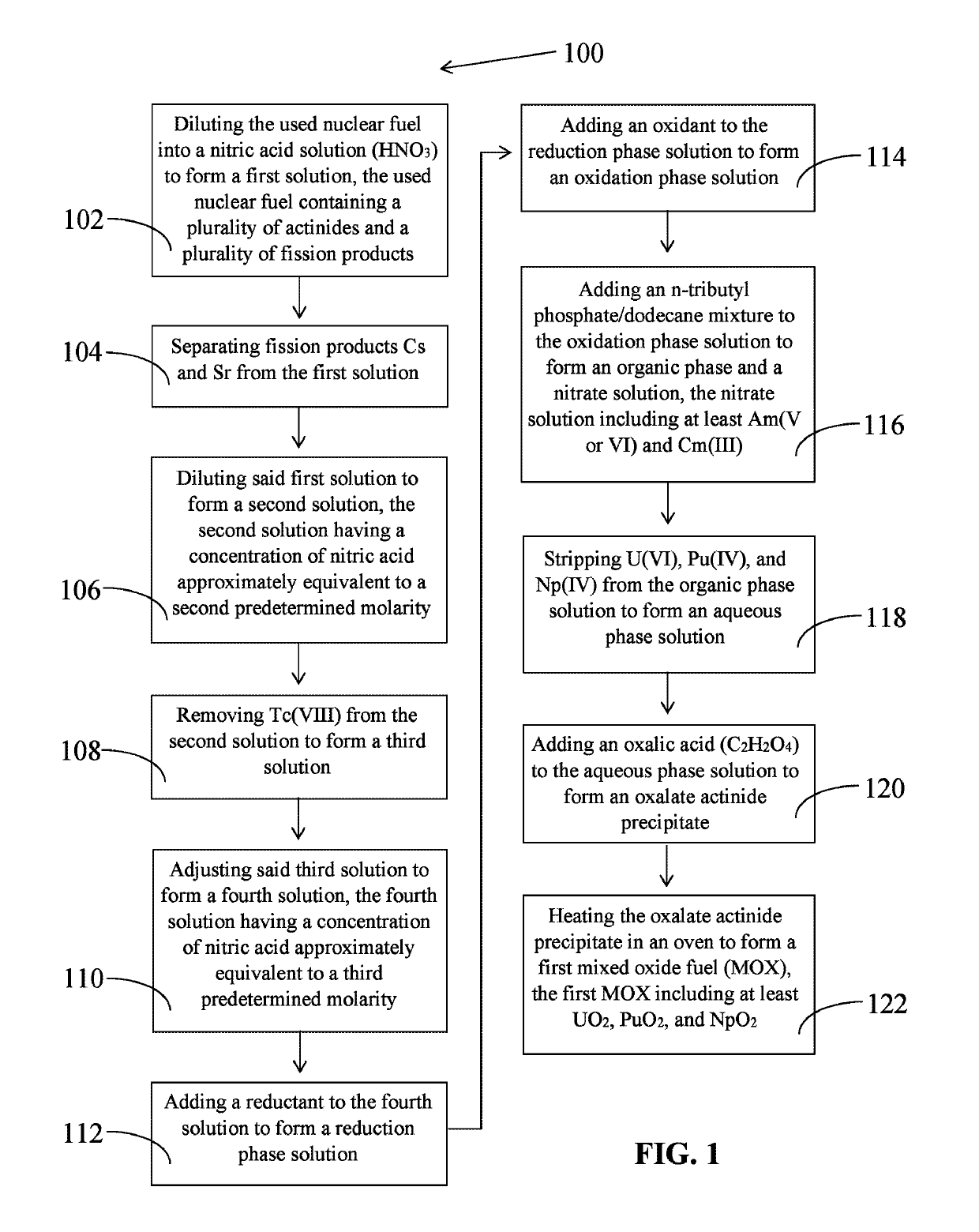
Reduction-oxidation of actinides extraction process (ROANEX) for used nuclear fuel recycling
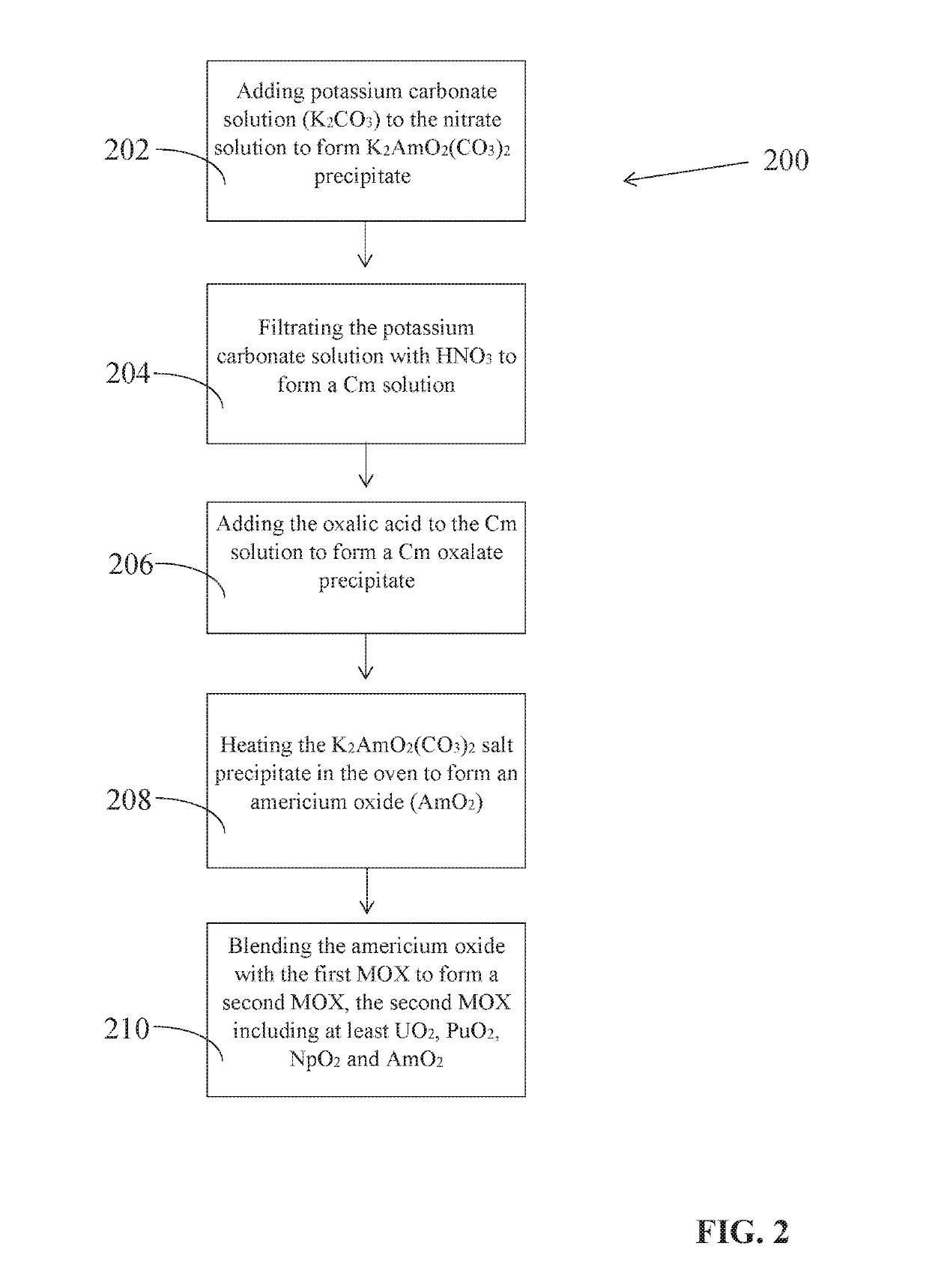
The invention relates to the ROANEX method, which extracts actinides from used nuclear fuel in a single purification cycle. The used nuclear fuel contains actinides, U, Am, Pu, Np. and Cm, and fission products, Cs, Sr and Tc. The fission products are separated first from the used nuclear fuel. The actinides are reduced to their lowest oxidation states and then oxidized to their highest oxidations states. Uranium, Pu and Np move to an organic phase solution and Am and Cm move to a nitrate solution. Uranium, Pu, and Np are stripped from the organic phase solution, and then treated with an oxalic acid to form a precipitate. Americium and Cm are treated with a potassium carbonate solution and Am precipitates. Actinides Am, U, Pu, and Np precipitates are heated in an oven and then blended together to form a mixed oxide fuel of UO2, PuO2, NpO2 and AmO2.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Method for preparing uranium dioxide microspheres at normal temperature
ActiveCN111039326AOvercomes the disadvantage of needing to freezeLow thermal conductivityUranium dioxideUranium oxideHexamethylenetetramine
The invention relates to a method for preparing uranium dioxide microspheres at normal temperature, and belongs to the technical field of ceramic forming. According to the method, components in an internal gel solution are divided into a uranyl nitrate solution (ADUN) stable at normal temperature and a mixed solution (HMUR) of urea and hexamethylenetetramine; the two solutions are rapidly mixed inan automatic mixing device to form an unstable uranium glue solution; the mixed uranium glue solution rapidly enters silicone oil, and is shear into liquid drops with uniform sizes by the silicone oil; the liquid drops and the silicone oil are cured into gel microspheres by a microwave heating device; and the gel microspheres are washed, dried and sintered to obtain monodisperse UO2 sintered microspheres with uniform sizes and good sphericity. According to the invention, microfluidic control is adopted in the whole process of the method, so that the UO2 microspheres are prepared at normal temperature through an internal gel method without human interference, and the automation degree is high.
Owner:TSINGHUA UNIV
Uranium trioxide fluidized bed hydrogen reduction process
The invention discloses a uranium trioxide fluidized bed hydrogen reduction process, which consists of the steps of test platform construction, gas mixing, feeding, reaction, discharge and exhaust gas emission, etc. The invention realizes the technological route of UO2 preparation by UO3 hydrogen reduction for the first time at home. In the route, the fluidized bed is selected as the reduction reactor, and has the advantages of excellent heat transfer performance, easy realization of uniform control of the bed temperature, simple structure, convenient maintenance and high production capacity. In addition, application of the fluidization technology can achieve full contact of a gas phase and a solid phase, strengthens mass transfer and heat transfer effects, and is easy to realize high reaction yield of materials.
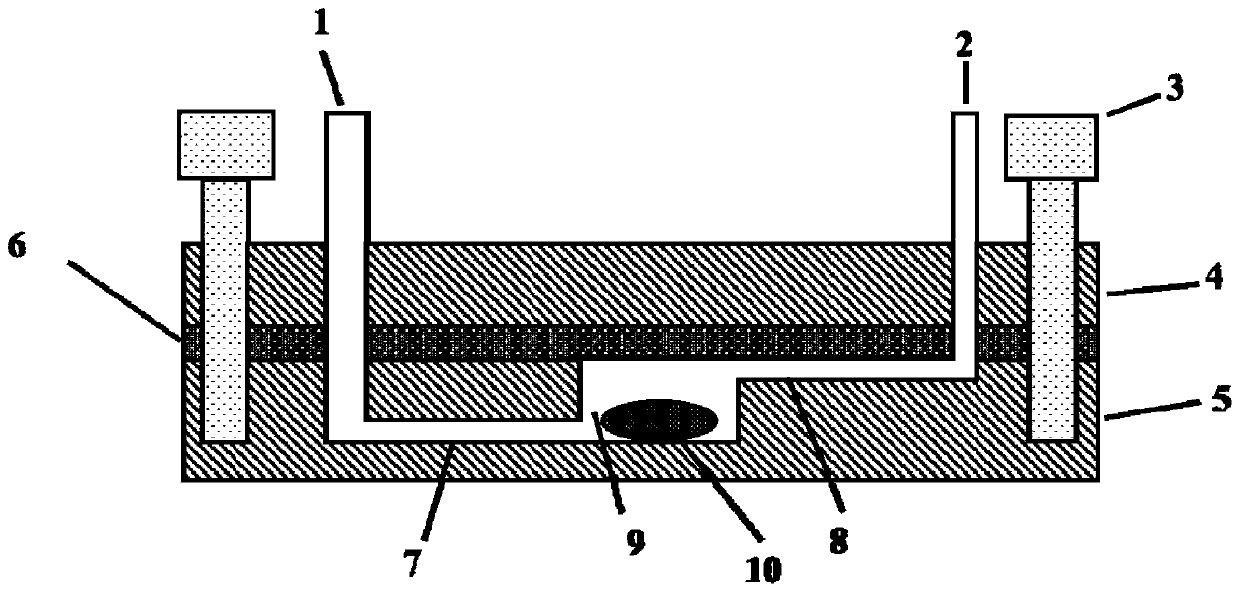
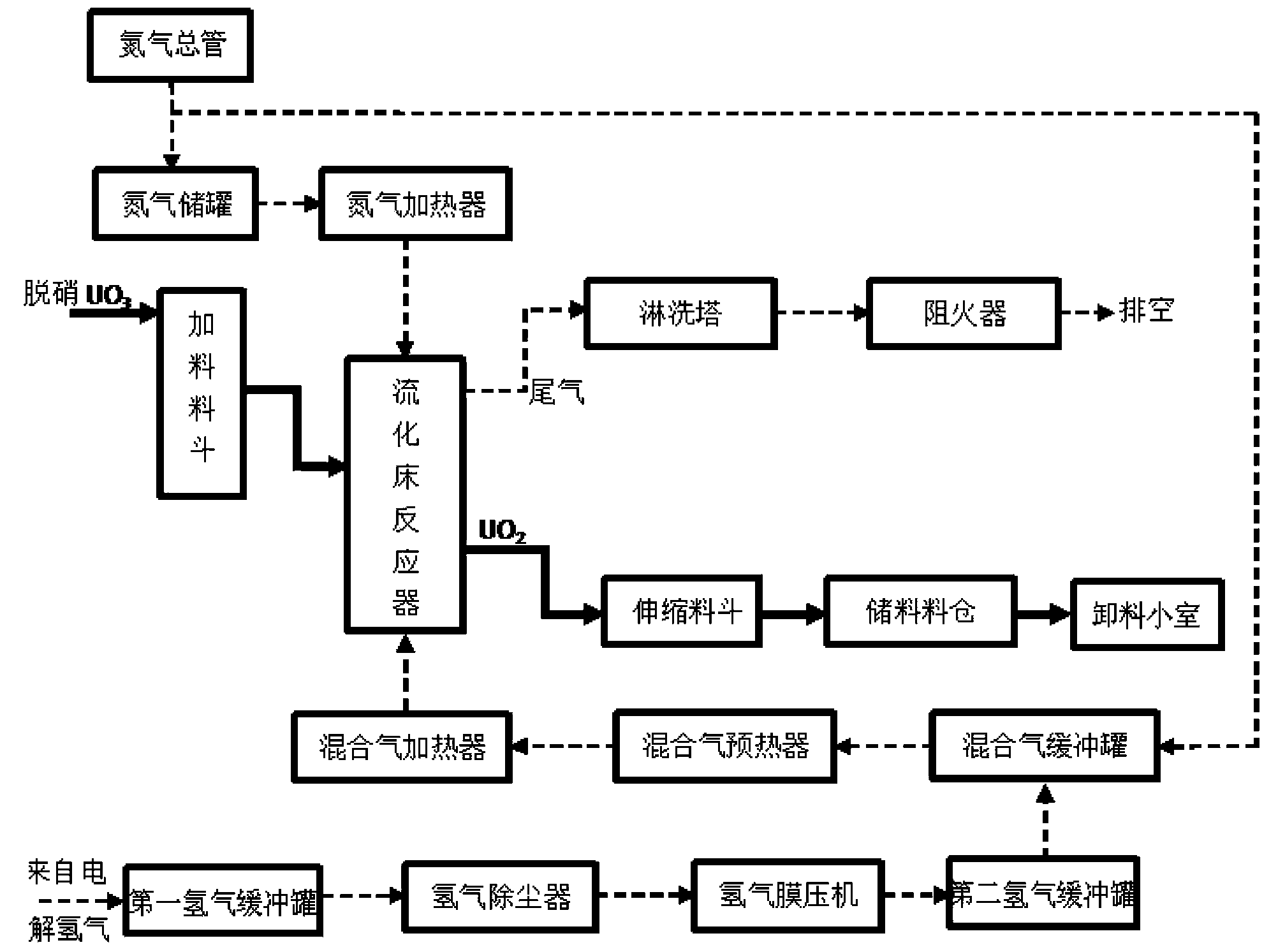
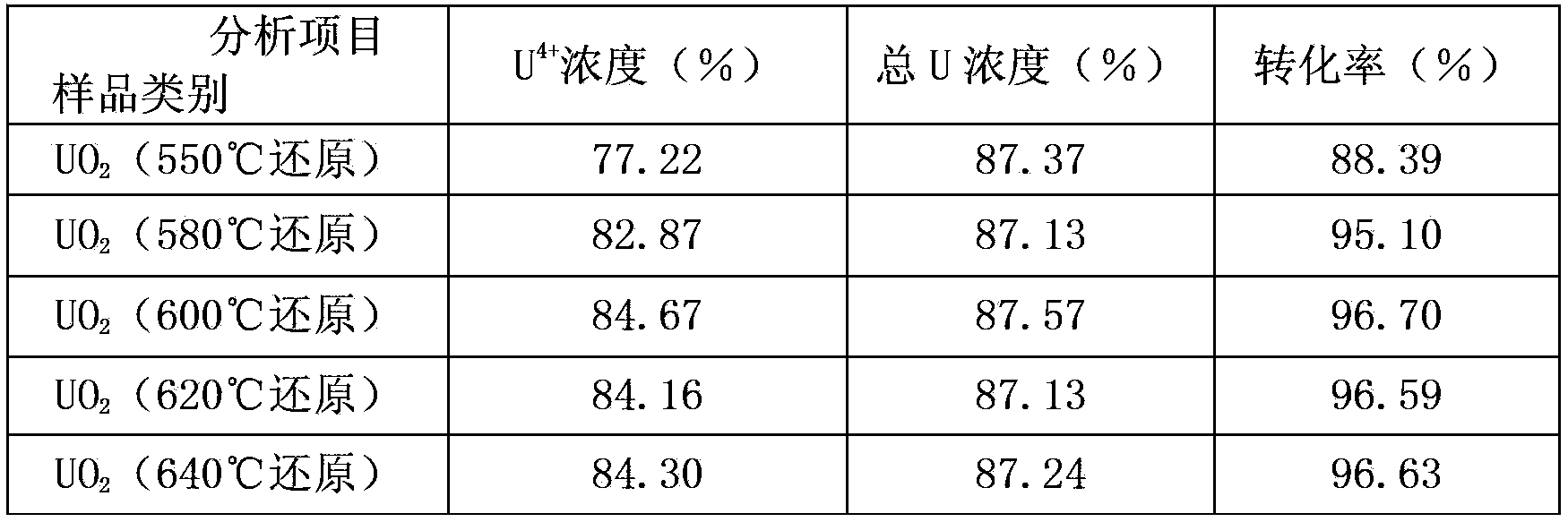
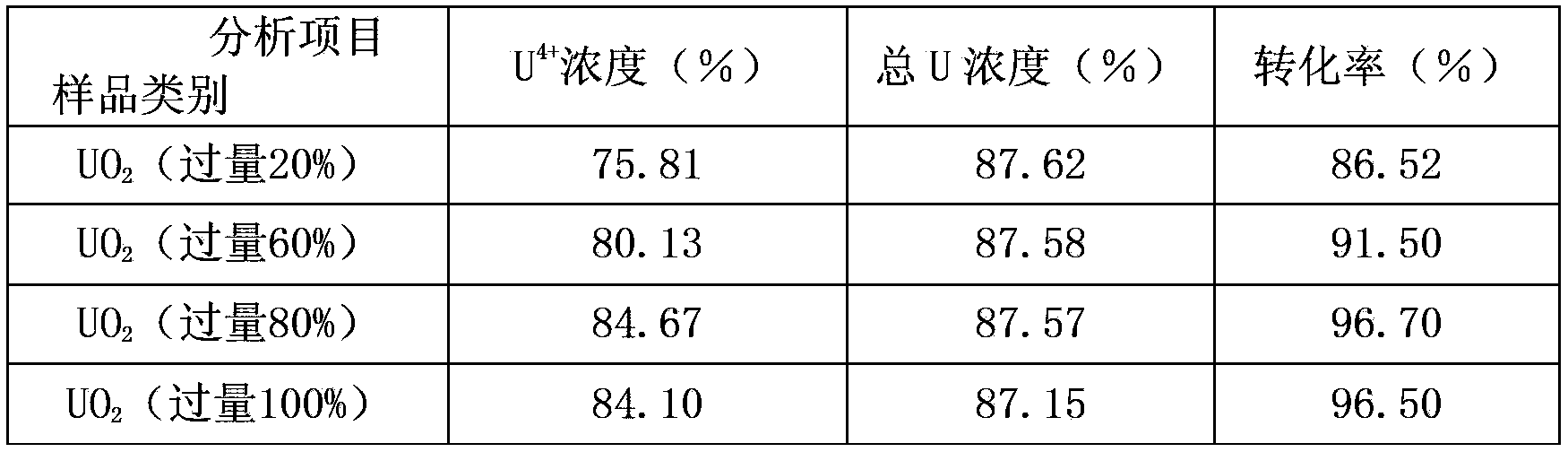
Owner:THE 404 COMPANY LIMITED CHINA NAT NUCLEAR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com