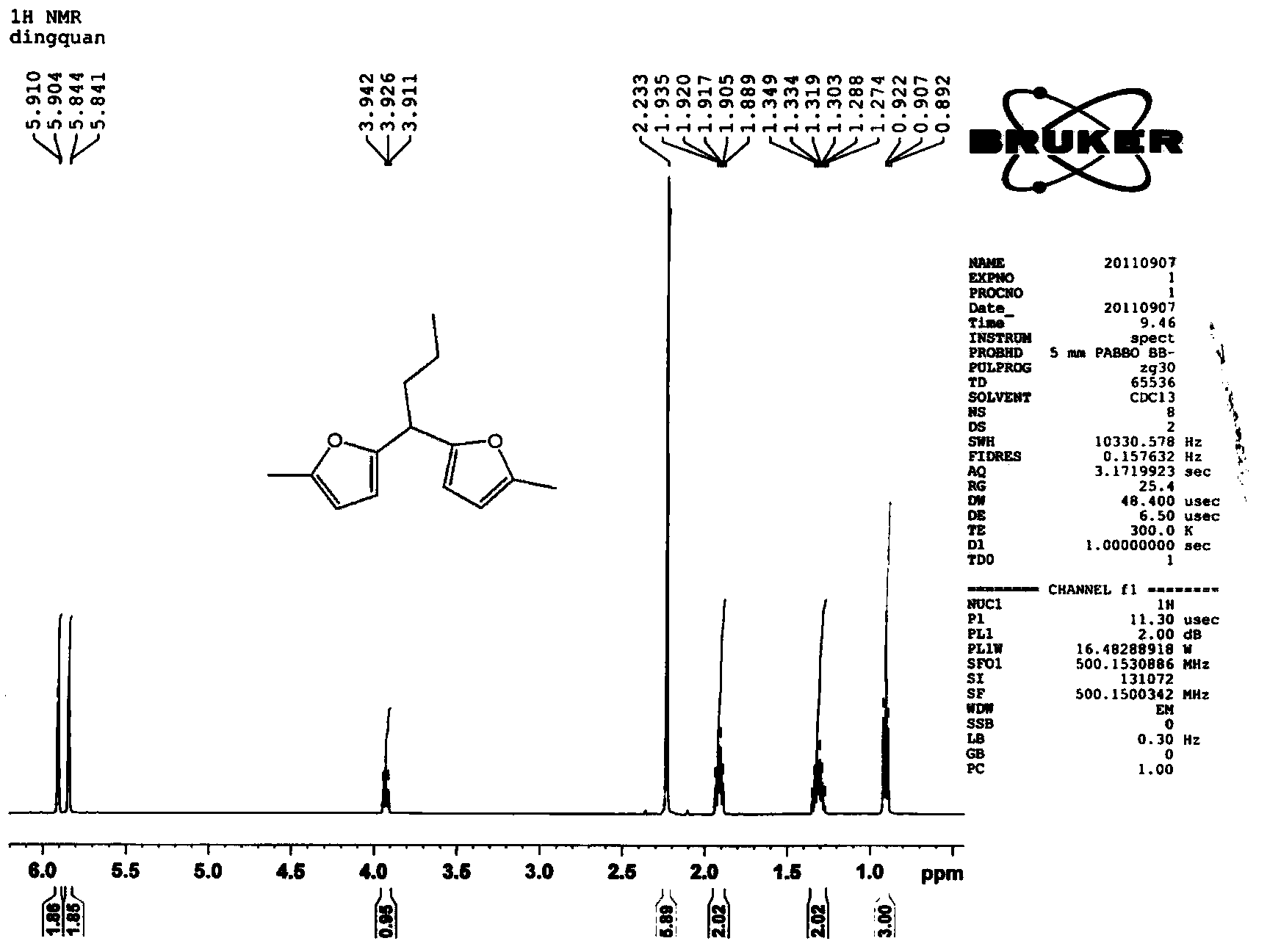
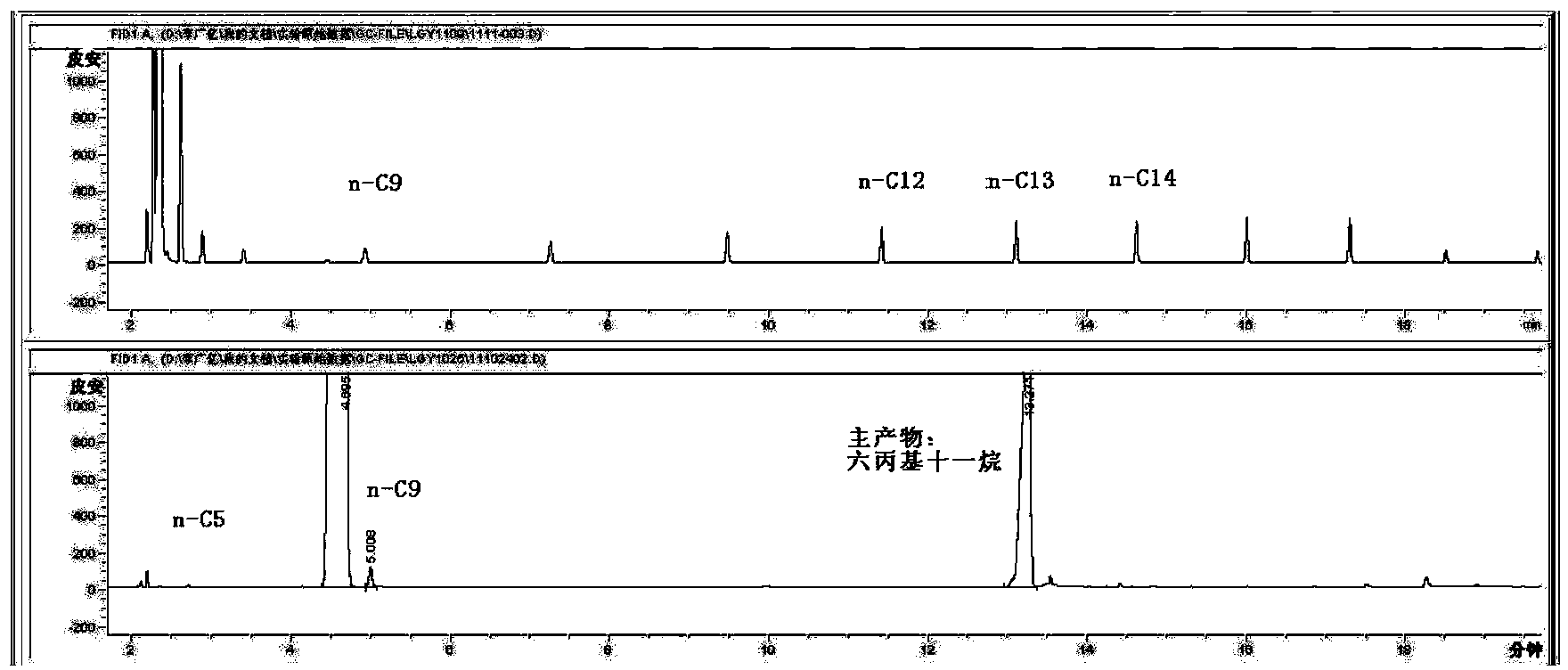
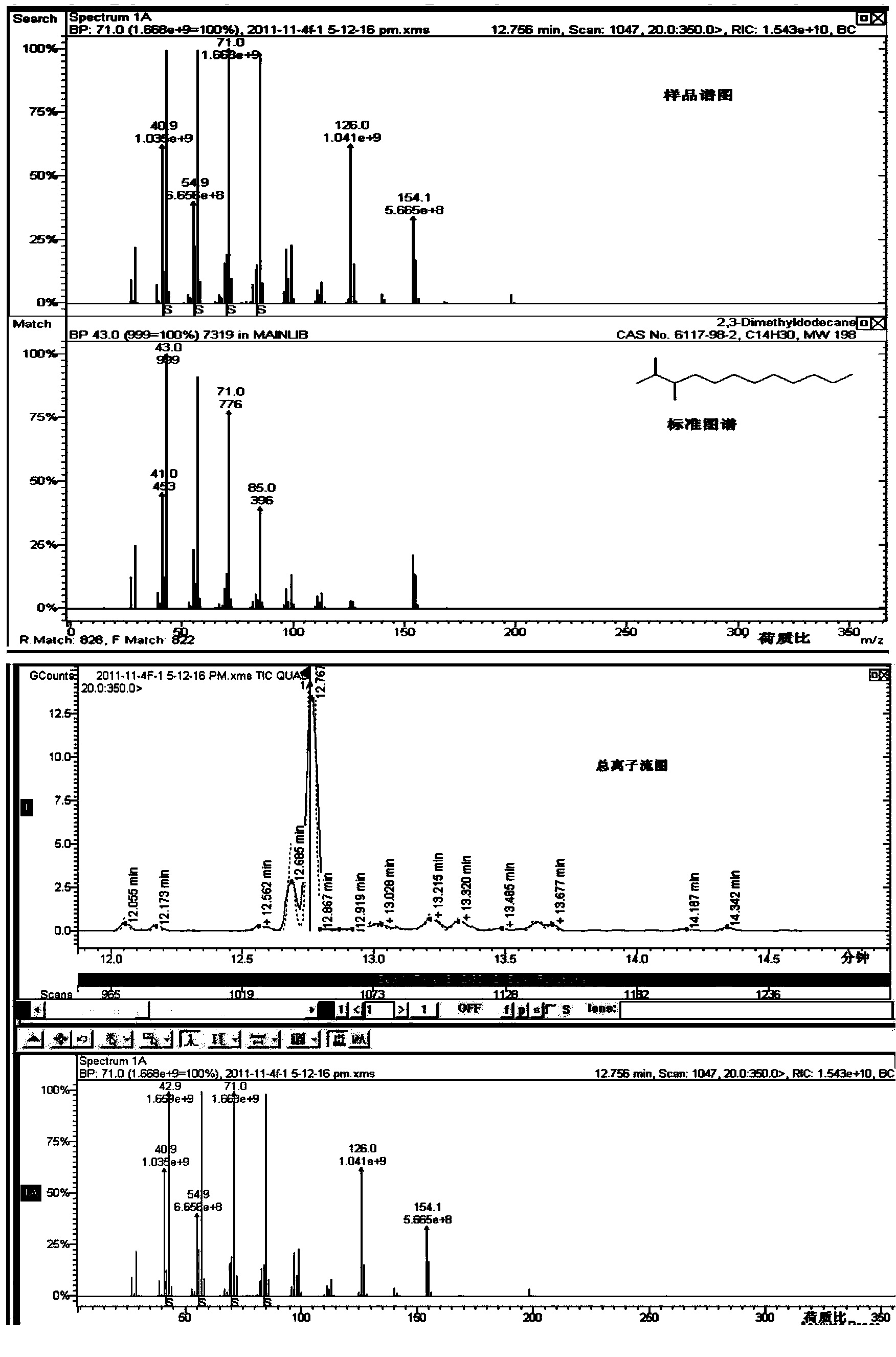
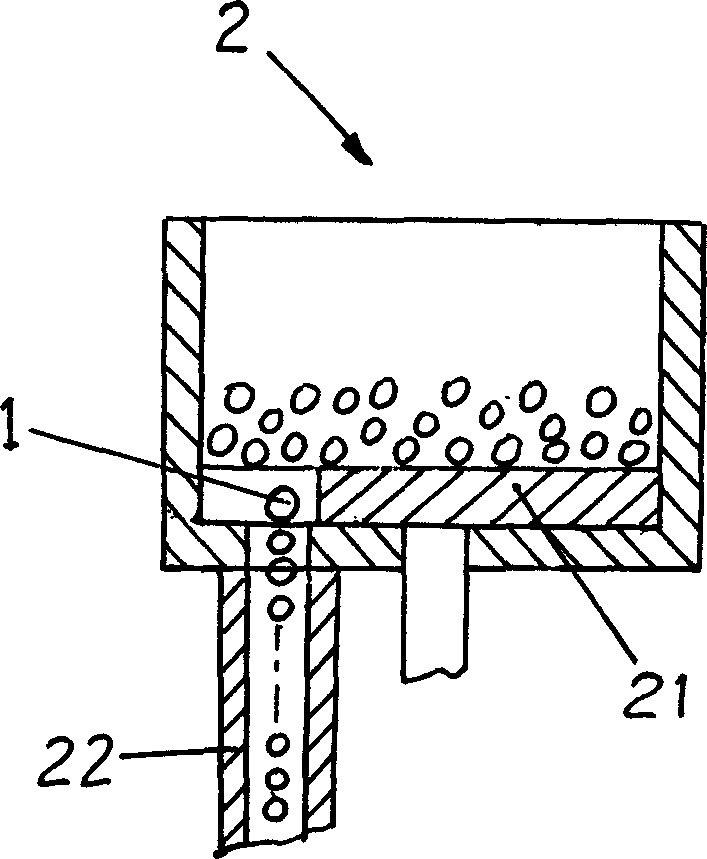
Patents
Literature
3316 results about "Kerosene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kerosene, also known as paraffin, lamp oil, and coal oil (an obsolete term), is a combustible hydrocarbon liquid which is derived from petroleum. It is widely used as a fuel in aviation as well as households. Its name derives from Greek: κηρός (keros) meaning wax, and was registered as a trademark by Canadian geologist and inventor Abraham Gesner in 1854 before evolving into a genericized trademark. It is sometimes spelled kerosine in scientific and industrial usage. The term kerosene is common in much of Argentina, Australia, Canada, India, New Zealand, and the United States, while the term paraffin (or a closely related variant) is used in Chile, eastern Africa, South Africa, Norway, and in the United Kingdom. The term lamp oil, or the equivalent in the local languages, is common in the majority of Asia and "Earth Oil" in some parts of southern Asia.Liquid paraffin (called mineral oil in the US) is a more viscous and highly refined product which is used as a laxative. Paraffin wax is a waxy solid extracted from petroleum.
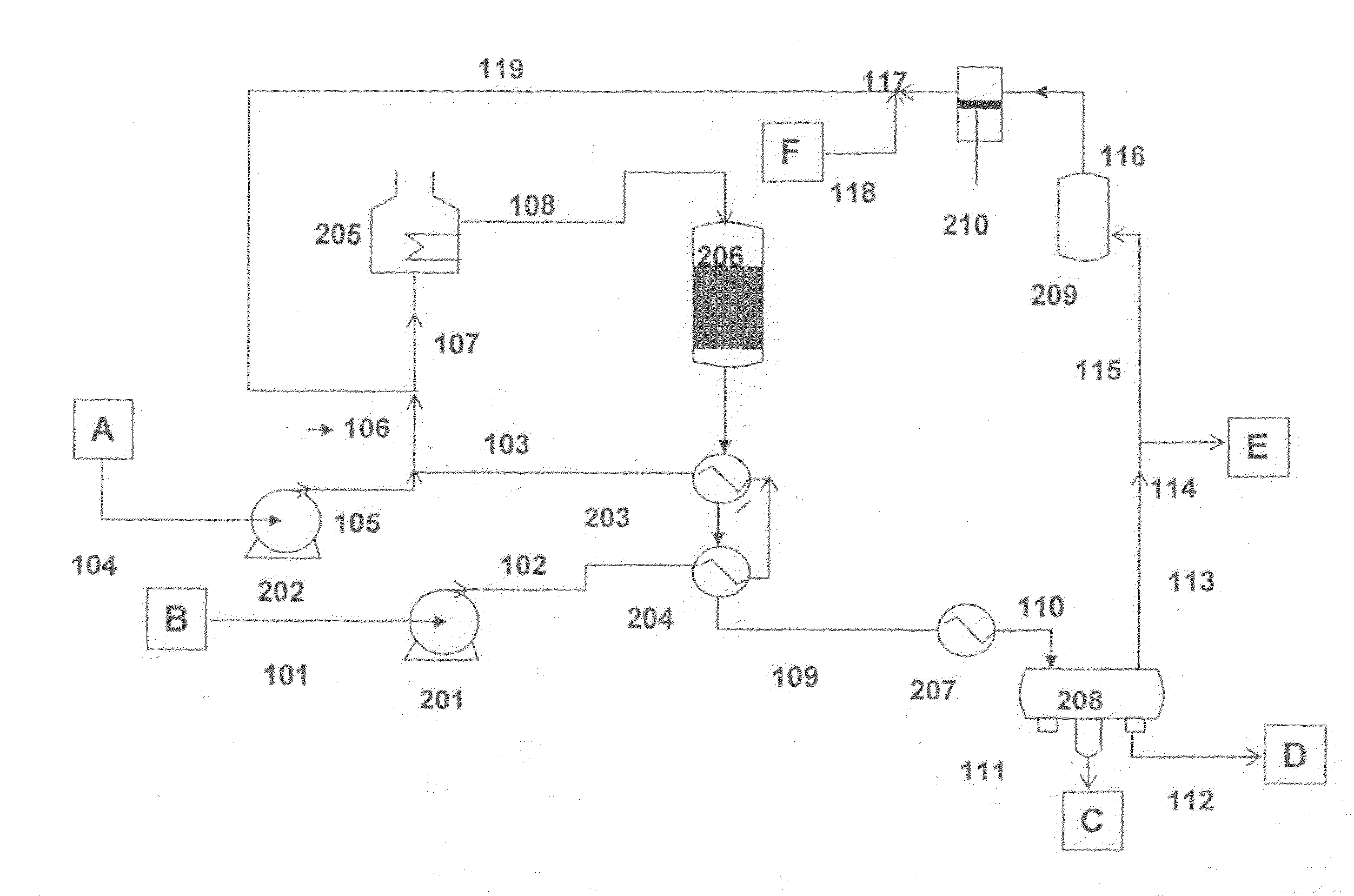
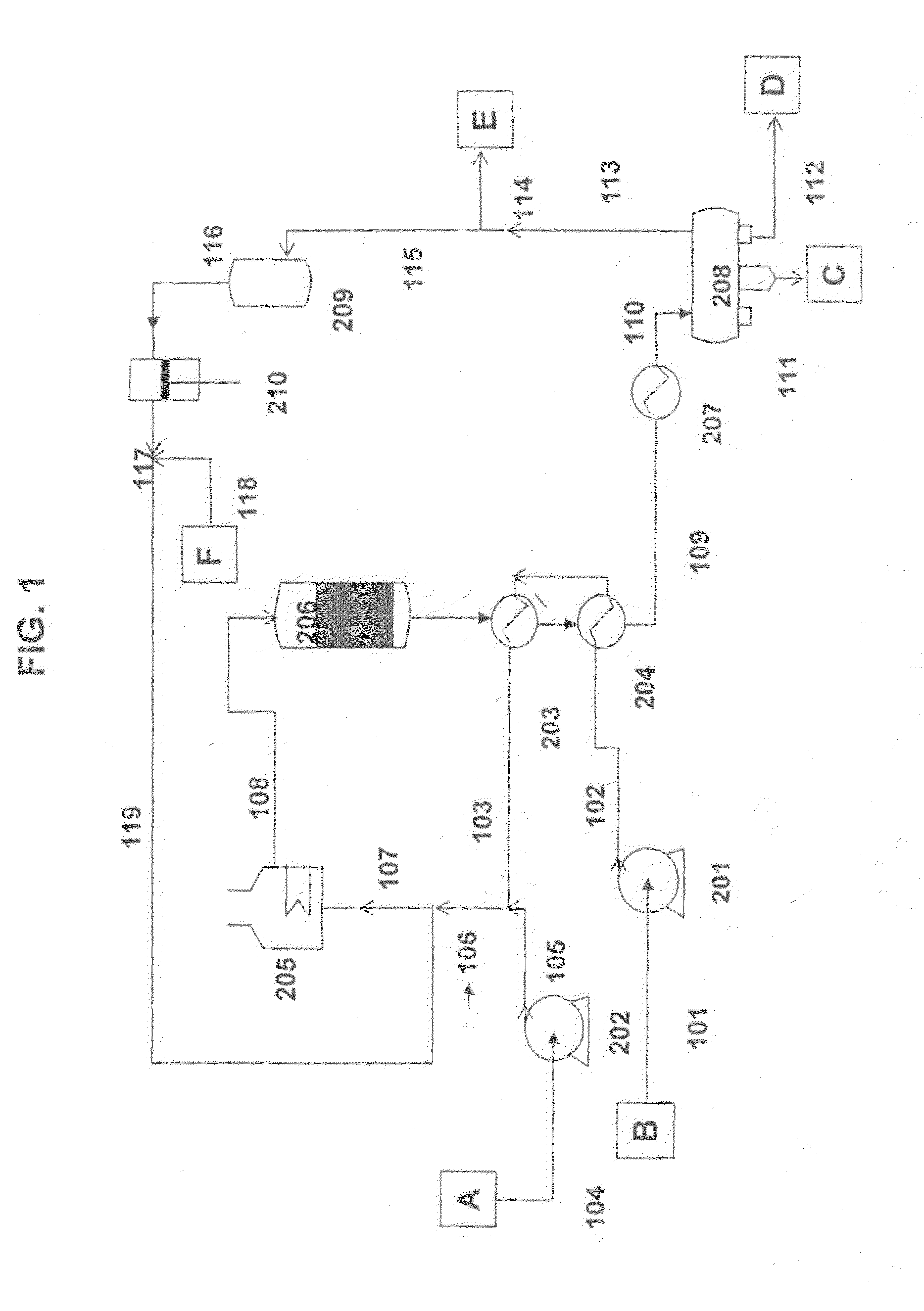
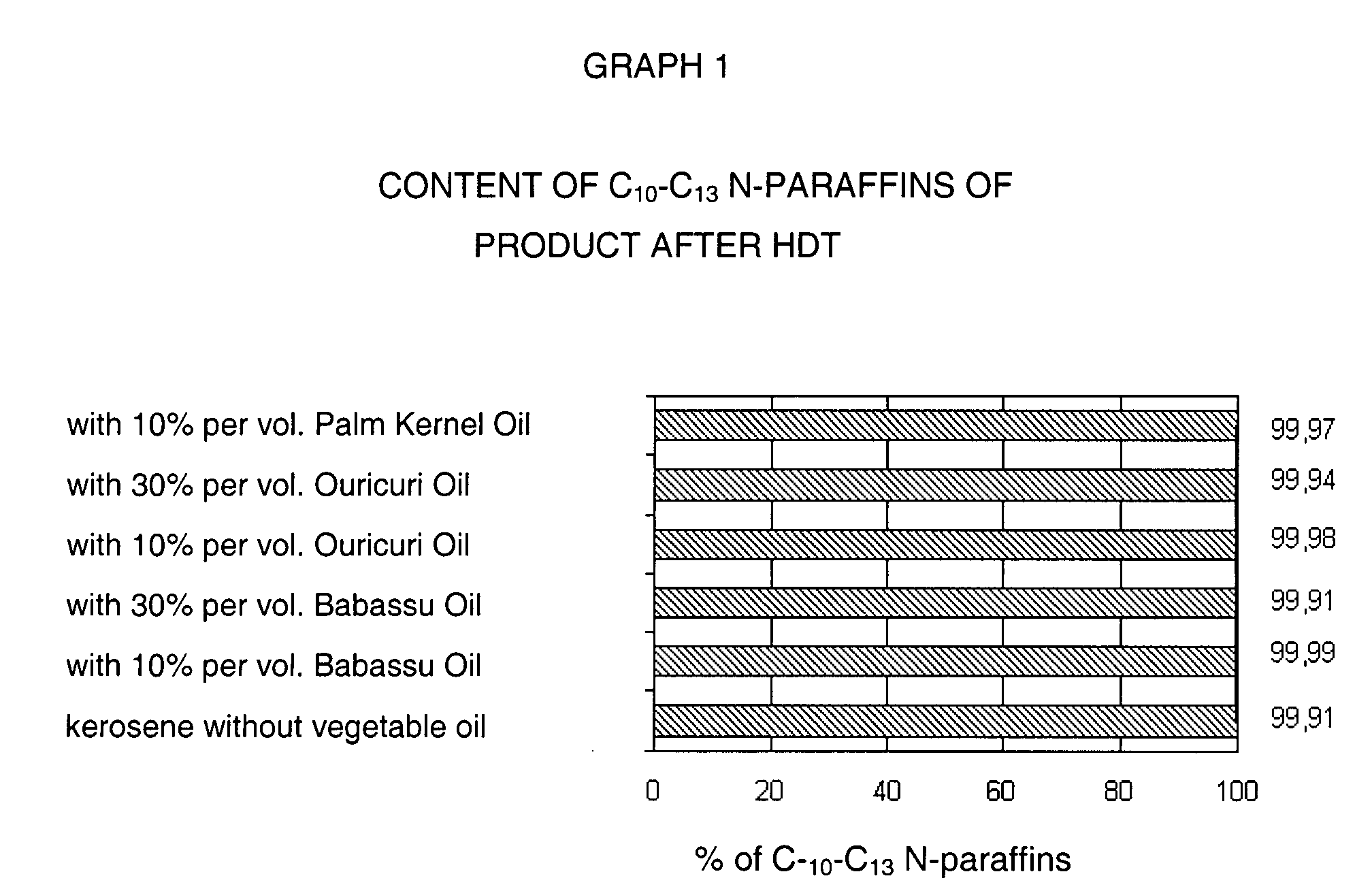
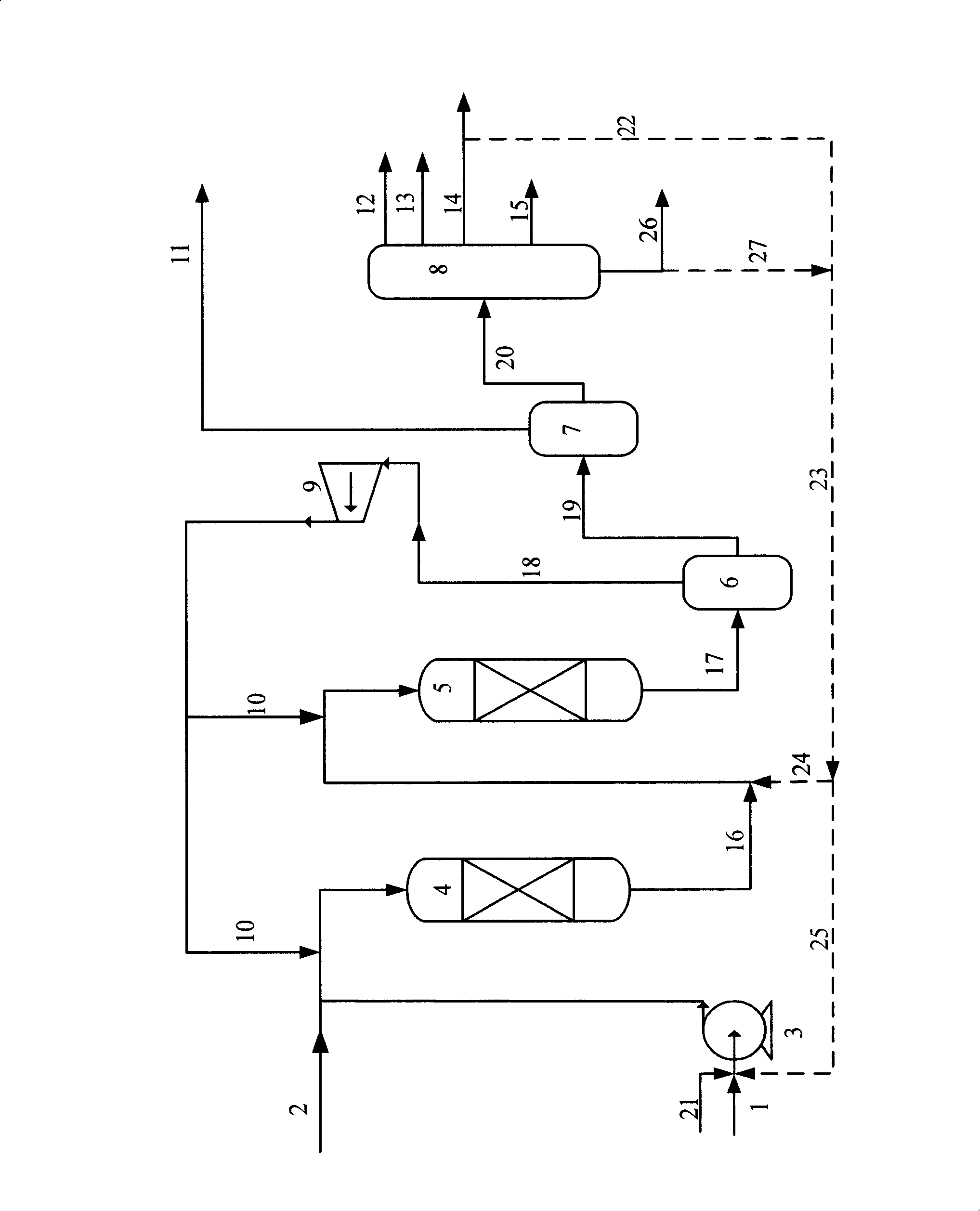
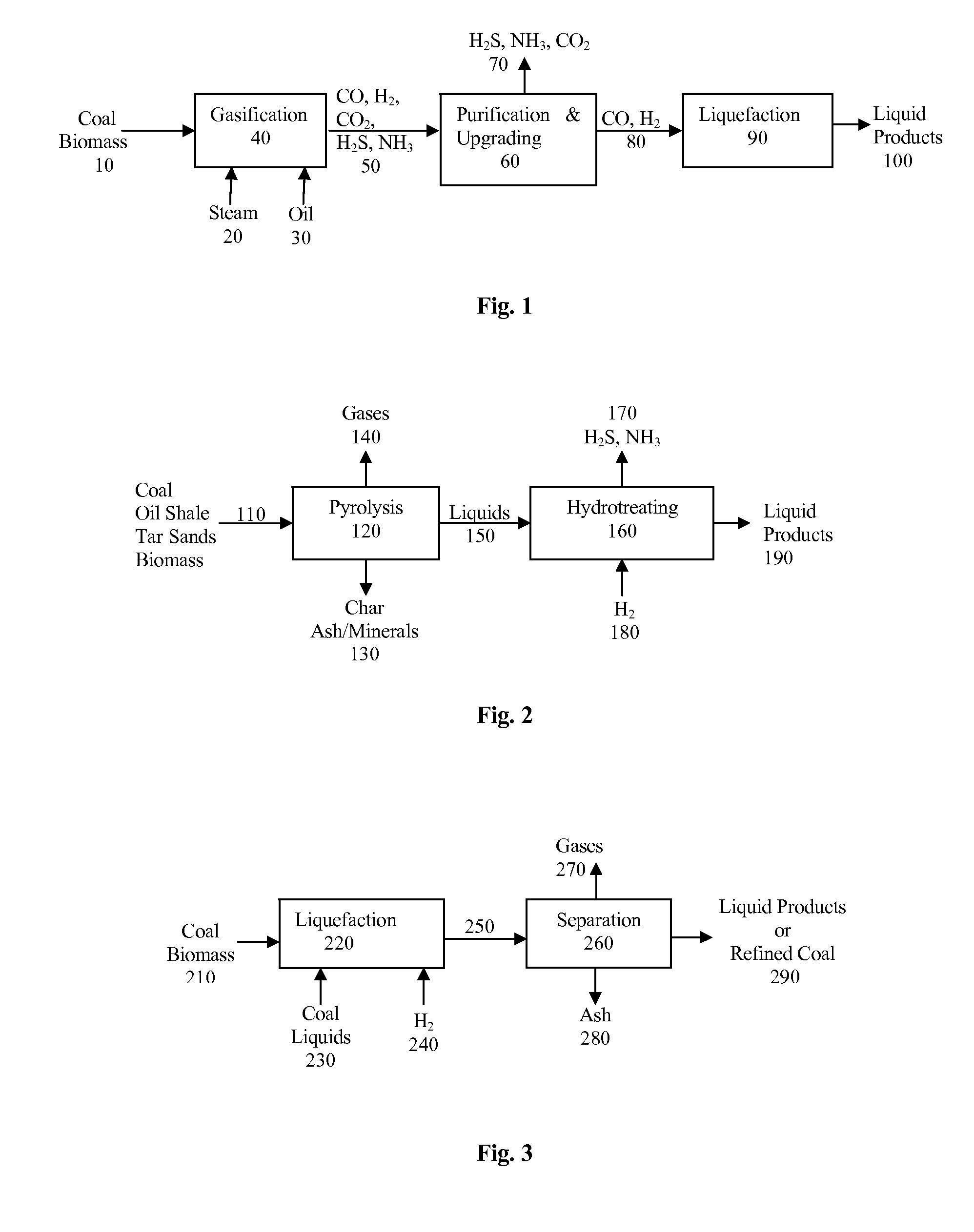
Process to obtain N-paraffins from vegetable oil
The process described by this invention involves the hydroconversion of vegetable oils appropriately selected for the production of N-paraffins, through hydrotreatment of a stream of vegetable hydrocarbon oils in and / or natural fats that may be used in a pure state or in a mixture with mineral hydrocarbon oil. This mixture flow is submitted to the process of hydrotreatment, obtaining as a result, a product flow with an elevated content of N-paraffins in the range of C10-C-13. This process provides an alternative to the usual process that uses a mineral hydrocarbon oil load (petroleum kerosene of paraffin base) to produce C10-C13 N-paraffins that are raw materials for the production of detergents (LAB), being, therefore, especially advantageous for use in situations where kerosene is a limiting factor for producing N-paraffins, resulting in a product of good quality with a reasonable gain in the production of N-paraffins.
Owner:PETROLEO BRASILEIRO SA (PETROBRAS)
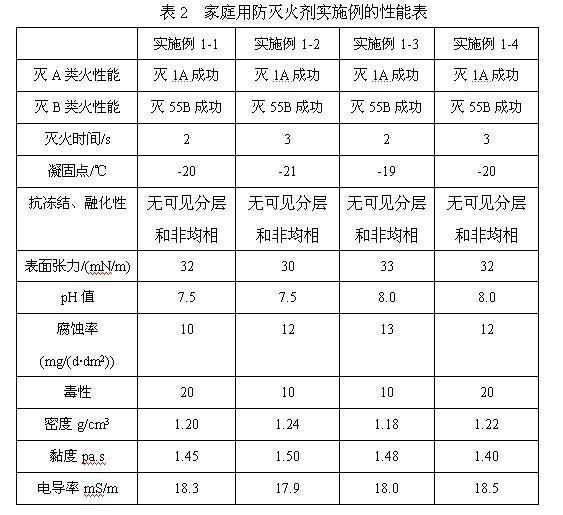
Built-up synergetic class-A/B water extinguishing agent series
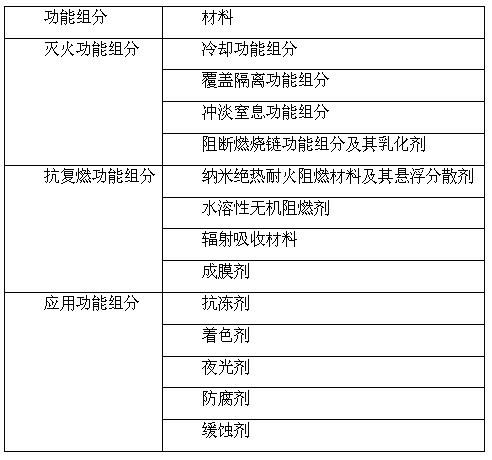
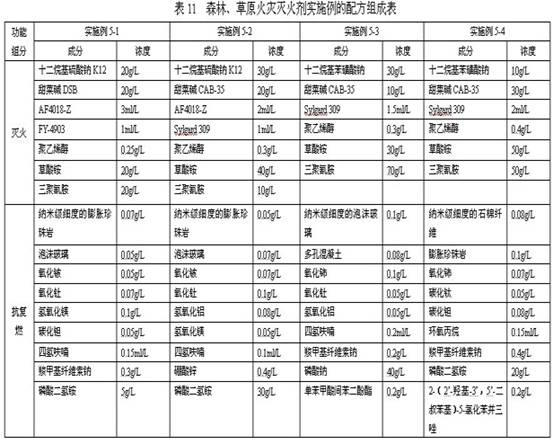
The invention discloses a built-up synergetic class-A / B water extinguishing agent series. Class-A fires caused by solid substances (such as woods, cottons, wools, linens, paper) and products thereof and class-B fires caused by liquid or melted solids such as gasoline, kerosene, diesel oil, crude oil, methanol, ethanol, asphalt, paraffin waxes and the like can be put out rapidly through the functions such as heat absorption and cooling, diluting and smothering, insulating and covering, suppressing and blocking combustion chains, and the like which are synergetically performed by various extinguishing functional components, and a high-temperature-resistant thermal-insulation and fire-retarding covering layer is formed on the surface of an inflammable matter by various after-combustion functional components so as to achieve the effect of after-combustion resistance. Because the class A / B fires relate to multiple occasions of daily life and production activities, the extinguishing agent disclosed by the invention can be used in the fields of home fire prevention and extinguishing, urban architecture fire prevention and extinguishing, oil depot and gasoline station fire prevention and extinguishing, vehicle and ship fire prevention and extinguishing, forest and grassland fire prevention and extinguishing, mine fire prevention and extinguishing, confined spaces (such as civil air-defense architectures) fire prevention and extinguishing.
Owner:NANJING UNIV OF SCI & TECH
Fuel compositions comprising farnesane and farnesane derivatives and method of making and using same
A fuel composition comprises farnesane and / or farnesane derivatives and a conventional fuel component selected from diesel fuel, jet fuel, kerosene or gasoline. The farnesane or farnesane derivative can be used as a fuel component or as a fuel additive in the fuel composition. The fuel composition may further comprise a conventional fuel additive. Methods of making and using the fuel composition are also disclosed.
Owner:AMYRIS INC
Coalescer for hydrocarbons containing surfactant
A coalescer filter element for the separation of water from hydrocarbon fluids, such as kerosene, jet fuel, diesel fuel, and gasoline under surfactant conditions such as thermal stability additive and dispersant. Coalescer fibrous material has hydrophobic properties which resist surfactant coating of the fibers thereby allowing breakdown of water emulsion in the hydrocarbon fluids. The coalescer has a negative media density gradient in the liquid flow direction.
Owner:KAYDON CUSTOM FILTRATION CORP
Portable hydrogen generator-fuel cell apparatus
InactiveUS6653005B1Increase specific energy and overall energy efficiencyHydrogenFuel cell auxillariesKeroseneImpurity
A compact hydrogen generator is coupled to or integrated with a fuel cell for portable power applications. Hydrogen is produced via thermocatalytic decomposition (cracking, pyrolysis) of hydrocarbon fuels in oxidant-free environment. The apparatus can utilize a variety of hydrocarbon fuels, including natural gas, propane, gasoline, kerosene, diesel fuel, crude oil (including sulfurous fuels). The hydrogen-rich gas produced is free of carbon oxides or other reactive impurities, so it could be directly fed to any type of a fuel cell. The catalysts for hydrogen production in the apparatus are carbon-based or metal-based materials and doped, if necessary, with a sulfur-capturing agent. Additionally disclosed are two novel processes for the production of two types of carbon filaments, and a novel filamentous carbon product. The hydrogen generator can be conveniently integrated with high temperature fuel cells to produce an efficient and self-contained source of electrical power.
Owner:UNIV OF CENT FLORIDA RES FOUND INC +1
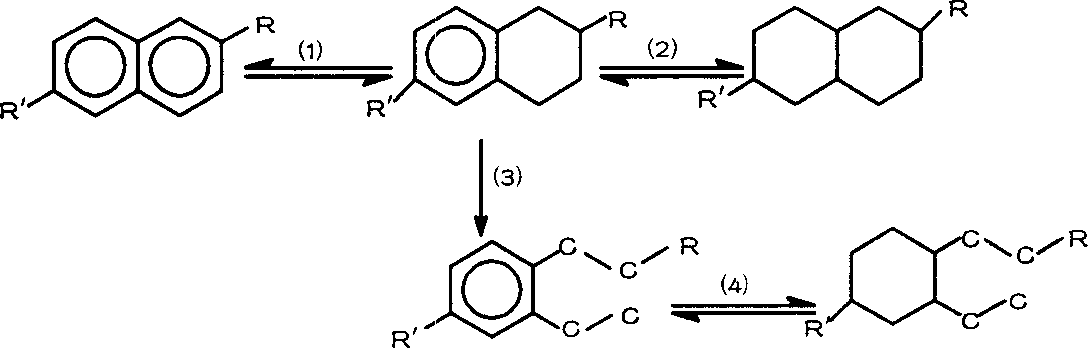
Preparation method of aviation kerosene or diesel
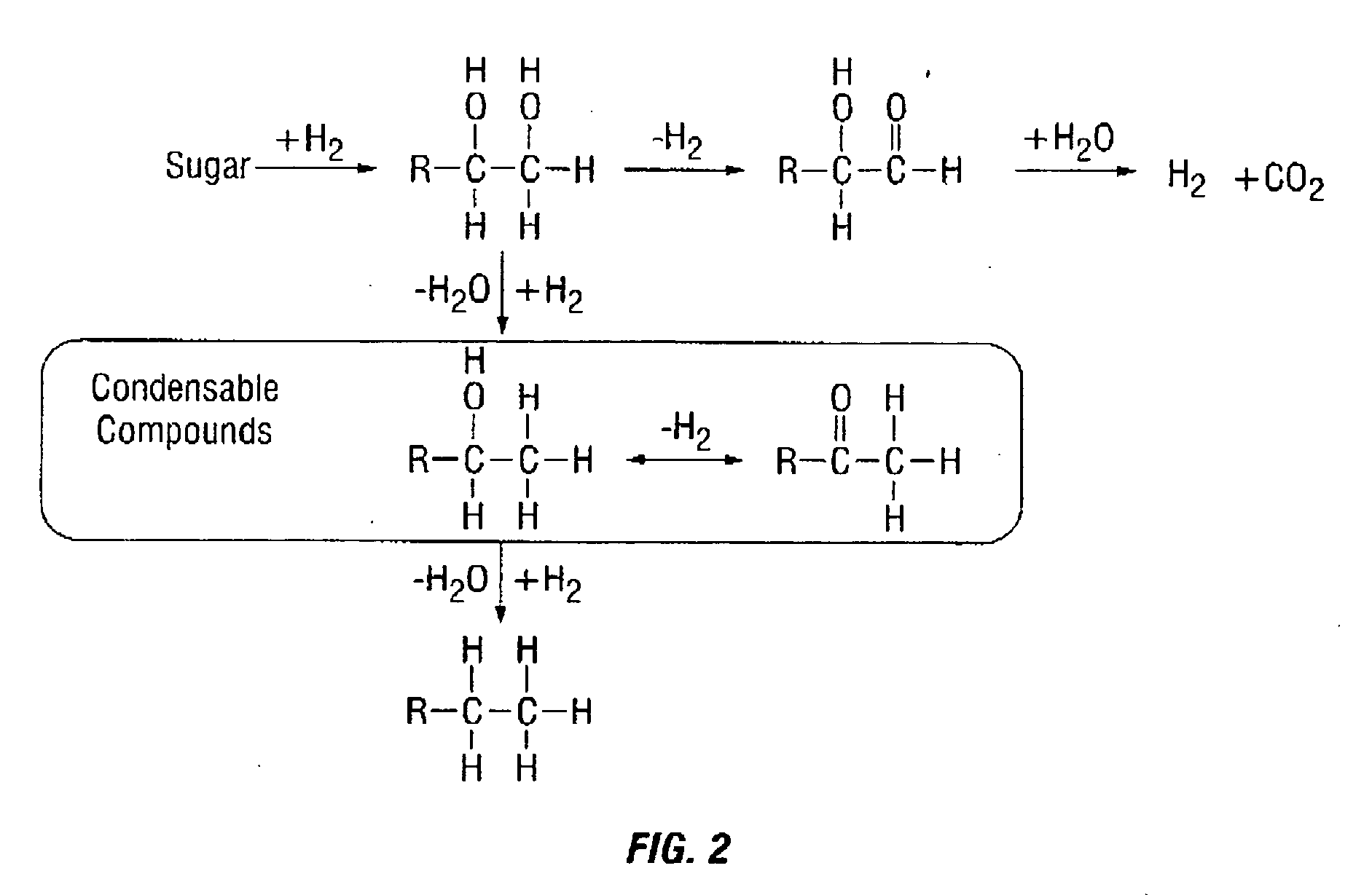
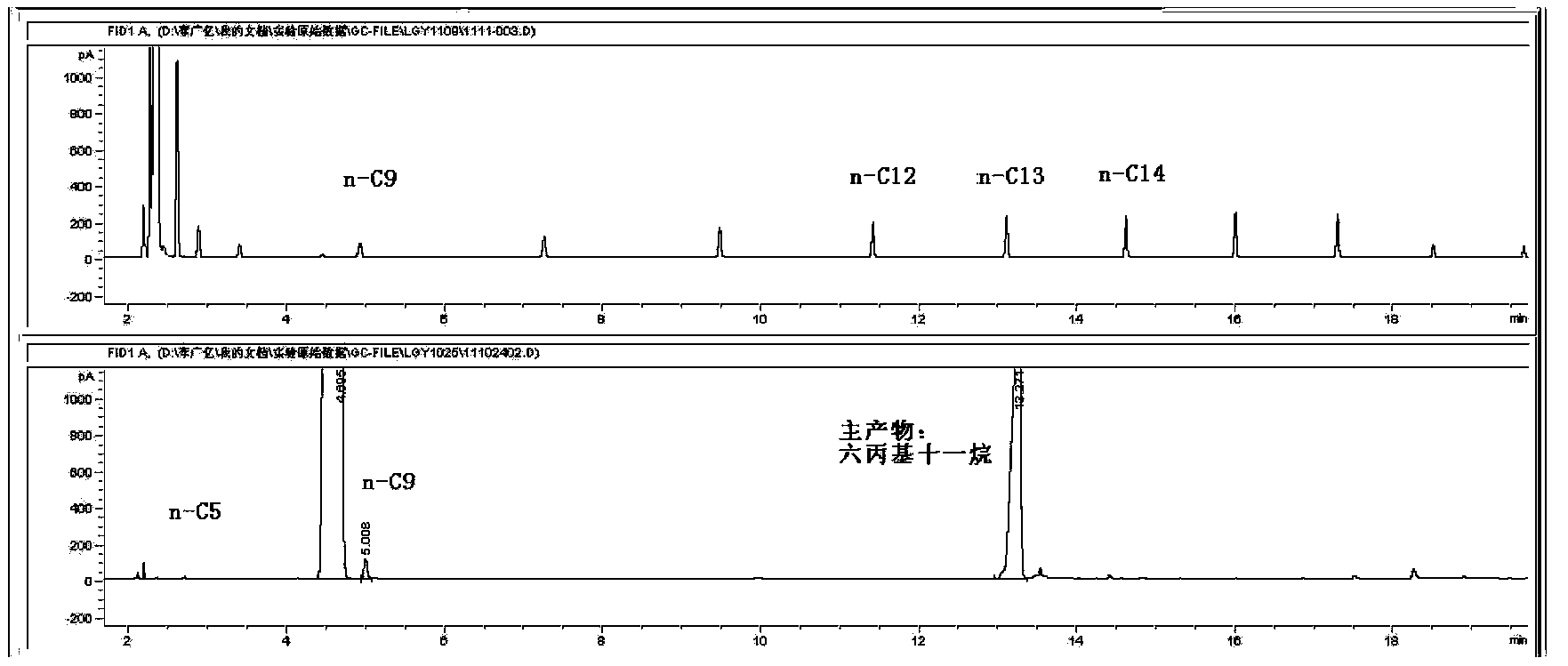
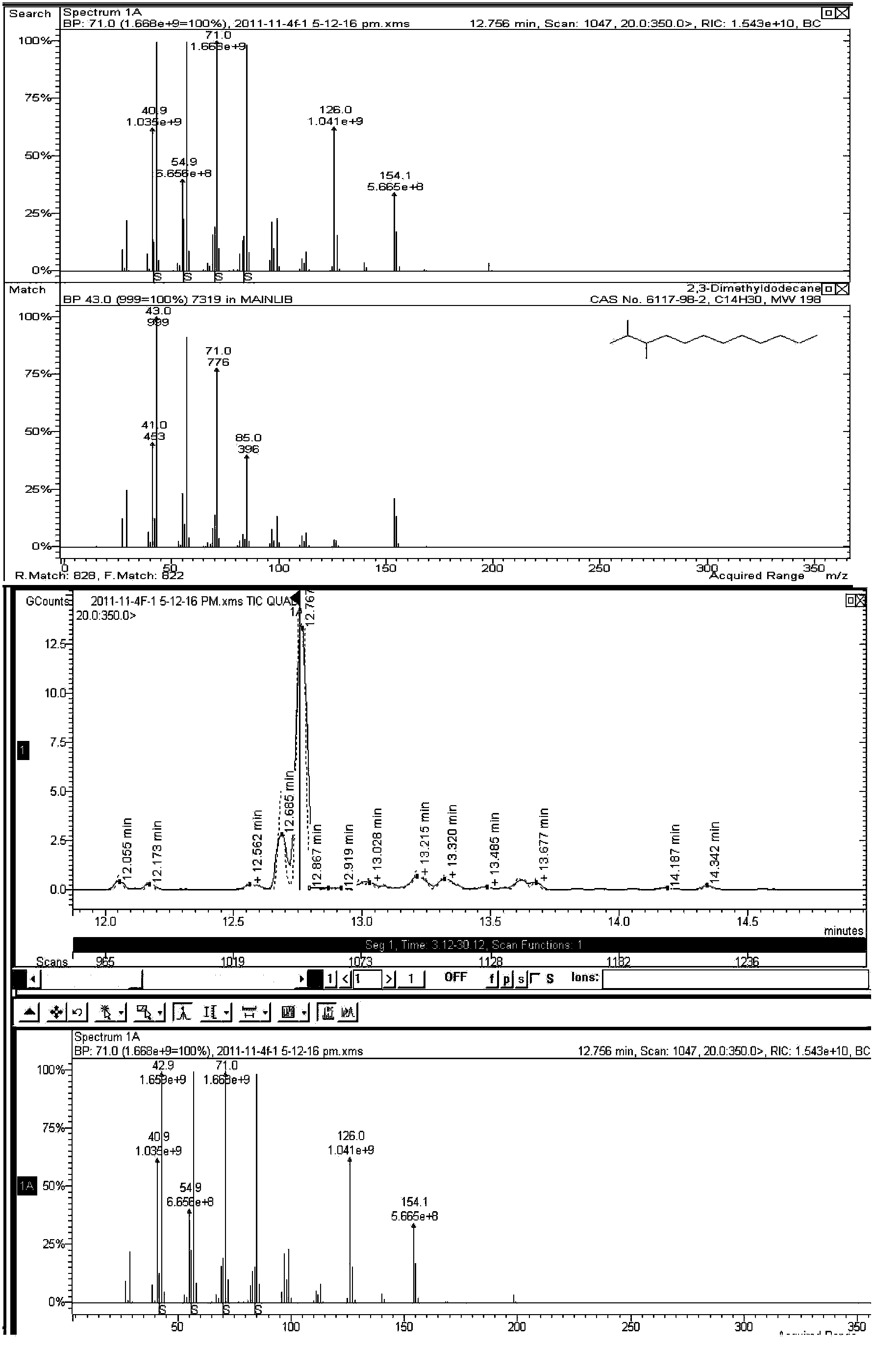
The present invention relates to a novel synthetic route of a liquid chain hydrocarbon fuel totally independent of fossil energy based on a lignocellulose raw material to obtain a platform compound. The method includes three parts: 1) preparing oxygen-containing organic compounds with carbon chain length of 8-16 through the acid-catalyzed alkylation reaction by taking lignocelluloses-based carbonyl-containing platform compounds and furan platform compounds as raw materials on a novel solid catalyst; 2) effectively removing carbon-carbon double bonds and carbon-oxygen double bonds to prepare saturated oxygen-containing organic compounds by hydrogenation of the alkylated product; and 3) conducting hydrodeoxygenation for the hydrogenated alkylation product by using a metal-solid acid bifunctional catalyst to obtain the biomass aviation kerosene or high grade diesel fuel with the carbon chain length of 8-16 and having a high energy density and stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Combined process for processing heavy oil
ActiveCN1844325AEasy to handleExtended service lifeTreatment with hydrotreatment processesKeroseneFuel oil
The invention discloses a group technology for heavy oil upgrading, in which the job steps include: (1) full or moiety of heavy oil individually or mixed with catalytic clarified oil entering solvent deasphalting plant, after the solvent deasphalting treatment, obtaining a deasphalted oil and a degreasing asphalt; (2) the degreasing asphalt obtained by step (1) individually or mixed with another moiety of heavy oil entering coking plant for coking treatment, in which the obtained tar heavy oil returns to solvent deasphalting plant or enters heavy oil hydrotreating plant, or moiety of tar heavy oil returns to solvent deasphalting plant and another moiety of tar heavy oil enters heavy oil hydrotreating plant, catalytic plant or hydrocracking plant; (3) the deasphalted oil obtained by step (1) individually or mixed with vacuum residual oil, non-pressure residual oil, pressure-relief residual oil, catalytic cycle stock and one or more than one heavy oil of tar heavy oil obtained by step (2) entering heavy oil hydrotreating plant for hydrotreatment, after hydrotreatment, obtaining fractions of benzin naphtha, plane kerosene and diesel and hydrogenating heavy oil. The invention can improve the charge-in nature, alleviate the operating condition and prolong the cycle length of the heavy oil hydrotreating plant, which provides better raw oil for downstream plants including catalytic plant.
Owner:LUOYANG PETROCHEMICAL ENG CORP SINOPEC
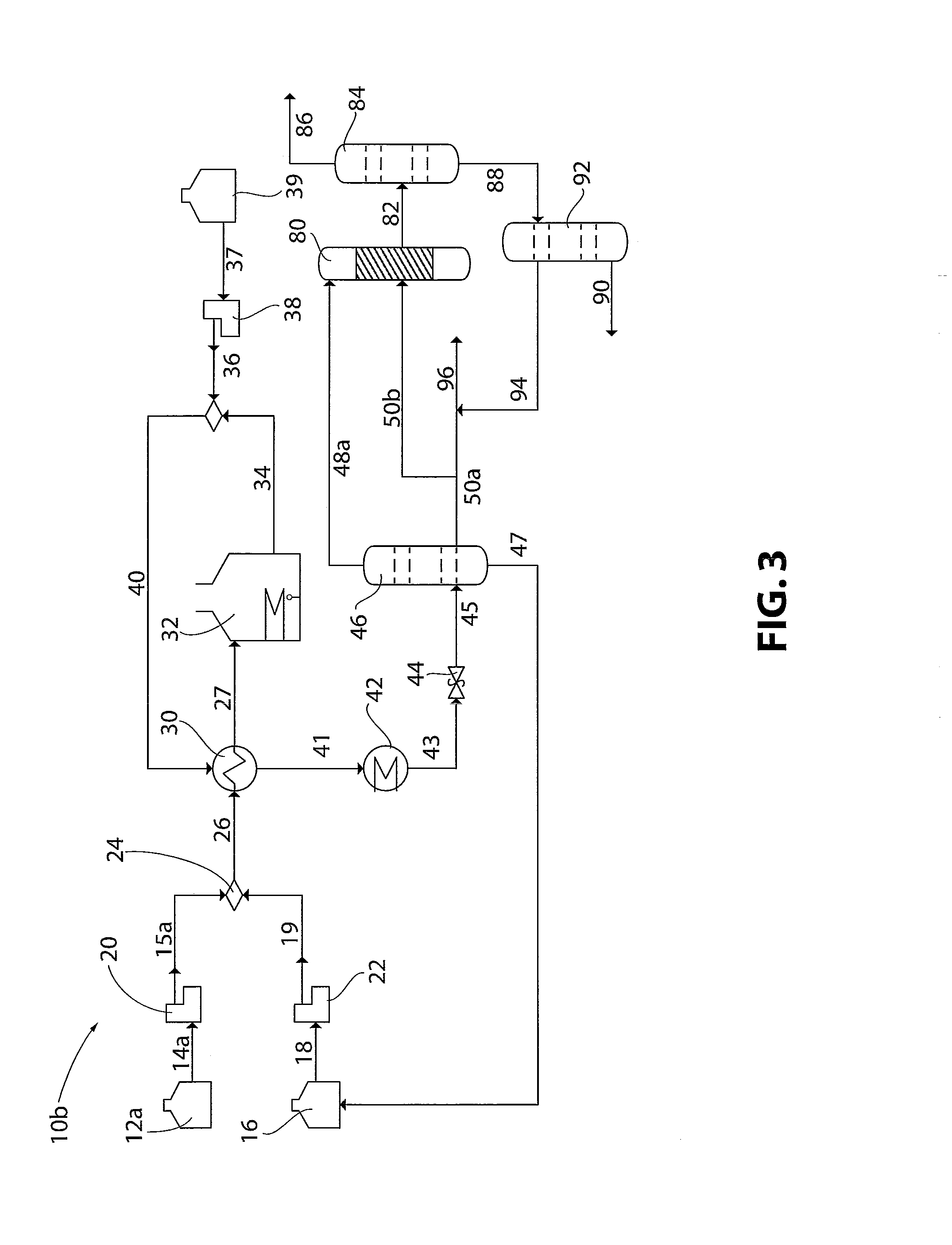
Production of liquid fuels by a concatenation of processes for treatment of a hydrocarbon feedstock
ActiveUS7214720B2Maximize conversion of carbonReduce and to upgrade naphthaThermal non-catalytic crackingCatalytic crackingNaphthaKerosene
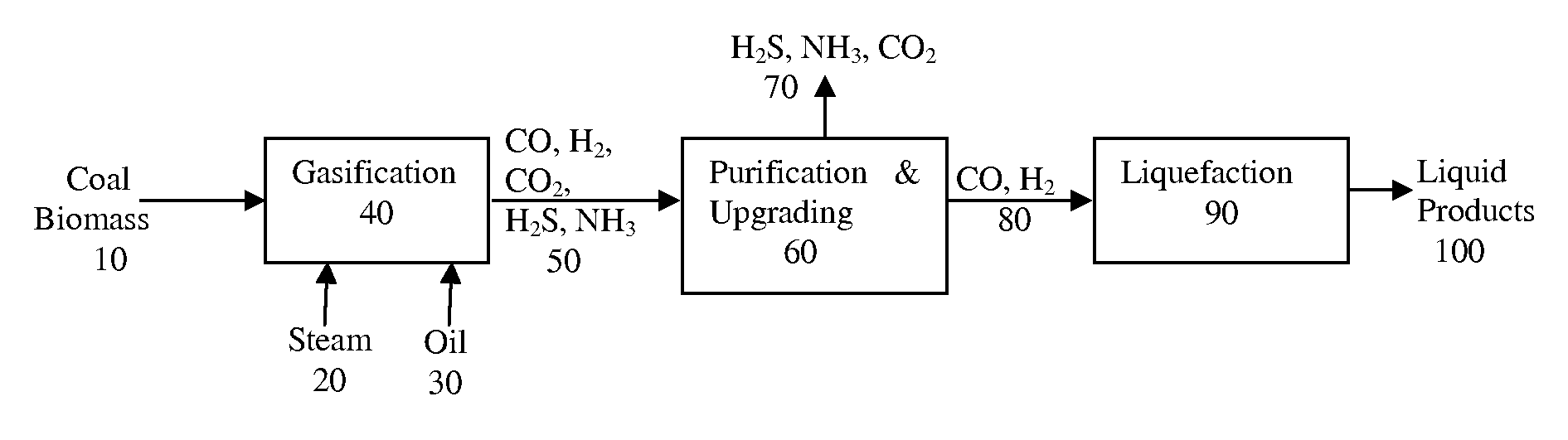
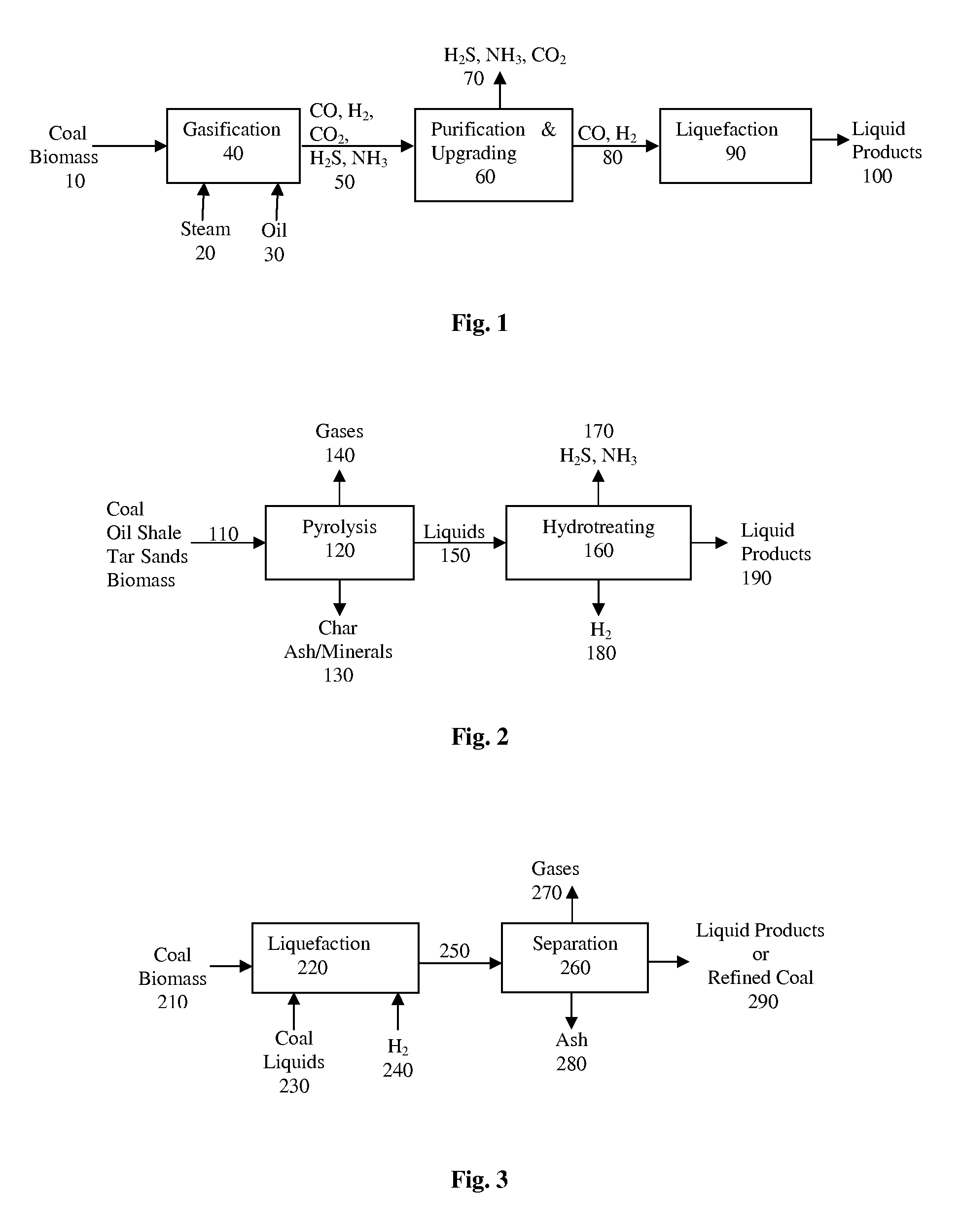
The invention relates to an installation and a process for the production of liquid fuels starting from a solid feedstock that contains the organic material in which:a) the solid feedstock is subjected to a gasification stage so as to convert said feedstock into synthesis gas,b) the synthesis gas is subjected to a purification treatment,c) the purified synthesis gas is subjected to a conversion stage that comprises the implementation of a Fischer-Tropsch-type synthesis so as to convert said synthesis gas into a liquid effluent and a gaseous effluent,d) the liquid effluent is fractionated so as to obtain a gaseous fraction, a naphtha fraction, a kerosene fraction and a gas oil fraction, ande) at least a portion of the naphtha fraction is recycled in gasification stage a).
Owner:INST FR DU PETROLE +1
Free flowing dry back-up insulating material
Disclosed is a free flowing dry back-up material which comprises:from 67 to 96% by weight of fly-ash;from 2 to 15% by weight of a heat sensitive binder such as boric acid;from 2 to 7% by weight of a non-wetting agent such as calcium fluoride;from 0 to 10% by weight of a heat expandable material, viz. a material expandable as a function of the temperature such as vermiculite or graphite; andfrom 0 to 1% by weight of a dust suppressant such as kerosene.This material which is useful in particular in the aluminum industry has the advantages of being water free and free flowing, such avoiding the use of vibrator to position it into a shell. It also has a low density and a low thermal conductivity. Moreover, it is organic free as compared to the existing materials which use an organic binder to ensure a low temperature set; and it sets at a temperature lower than 400° F.
Owner:LES PROD CHIMS INDS DE HAUTE TEMPERATURE PYROTEK
Free flowing dry back-up insulating material
ActiveUS20050116398A1Reduce the temperatureSolid waste managementThermal insulationKeroseneNon wetting
Disclosed is a free flowing dry back-up material which comprises: from 67 to 96% by weight of fly-ash; from 2 to 15% by weight of a heat sensitive binder such as boric acid; from 2 to 7% by weight of a non-wetting agent such as calcium fluoride; from 0 to 10% by weight of a heat expandable material, viz. a material expandable as a function of the temperature such as vermiculite or graphite; and from 0 to 1% by weight of a dust suppressant such as kerosene. This material which is useful in particular in the aluminum industry has the advantages of being water free and free flowing, such avoiding the use of vibrator to position it into a shell. It also has a low density and a low thermal conductivity. Moreover, it is organic free as compared to the existing materials which use an organic binder to ensure a low temperature set; and it sets at a temperature lower than 400° F.
Owner:LES PROD CHIMS INDS DE HAUTE TEMPERATURE PYROTEK
Systems, methods, and compositions for production of synthetic hydrocarbon compounds
InactiveUS20060211777A1Efficient processingReduce energy lossElectrolysis componentsLiquid hydrocarbon mixture productionKeroseneHydrocotyle bowlesioides
A process and system for producing hydrocarbon compounds or fuels that recycle products of hydrocarbon compound combustion—carbon dioxide or carbon monoxide, or both, and water. The energy for recycling is electricity derived from preferably not fossil based fuels, like from nuclear fuels or from renewable energy. The process comprises electrolysing water, and then using hydrogen to reduce externally supplied carbon dioxide to carbon monoxide, then using so produced carbon monoxide together with any externally supplied carbon monoxide and hydrogen in Fischer-Tropsch reactors, with upstream upgrading to desired specification fuels—for example, gasoline, jet fuel, kerosene, diesel fuel, and others. Energy released in some of these processes is used by other processes. Using adiabatic temperature changes and isothermal pressure changes for gas processing and separation, large amounts of required energy are internally recycled using electric and heat distribution lines. Phase conversion of working fluid is used in heat distribution lines for increased energy efficiency. The resulting use of electric energy is less than 1.4 times the amount of the high heating value of combustion of so produced hydrocarbon compounds when carbon dioxide is converted to carbon monoxide in the invention, and less than 0.84 when carbon monoxide is the source.
Owner:FUELCOR LLC
Hydrogenation method and petrochemical process
InactiveUS20100087692A1Long catalyst lifeHigh yieldThermal non-catalytic crackingHydrocarbon by dehydrogenationBenzeneNaphtha
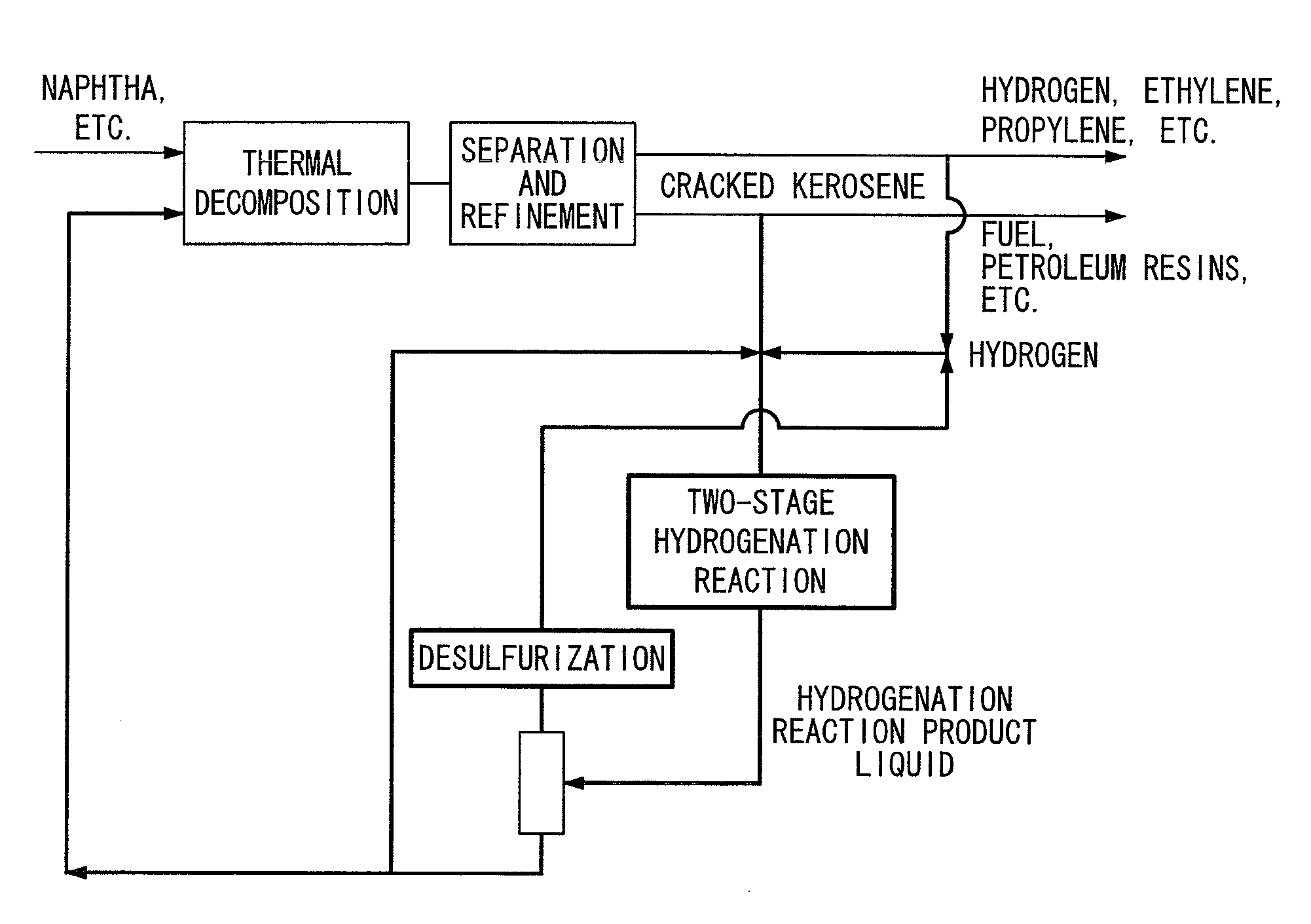
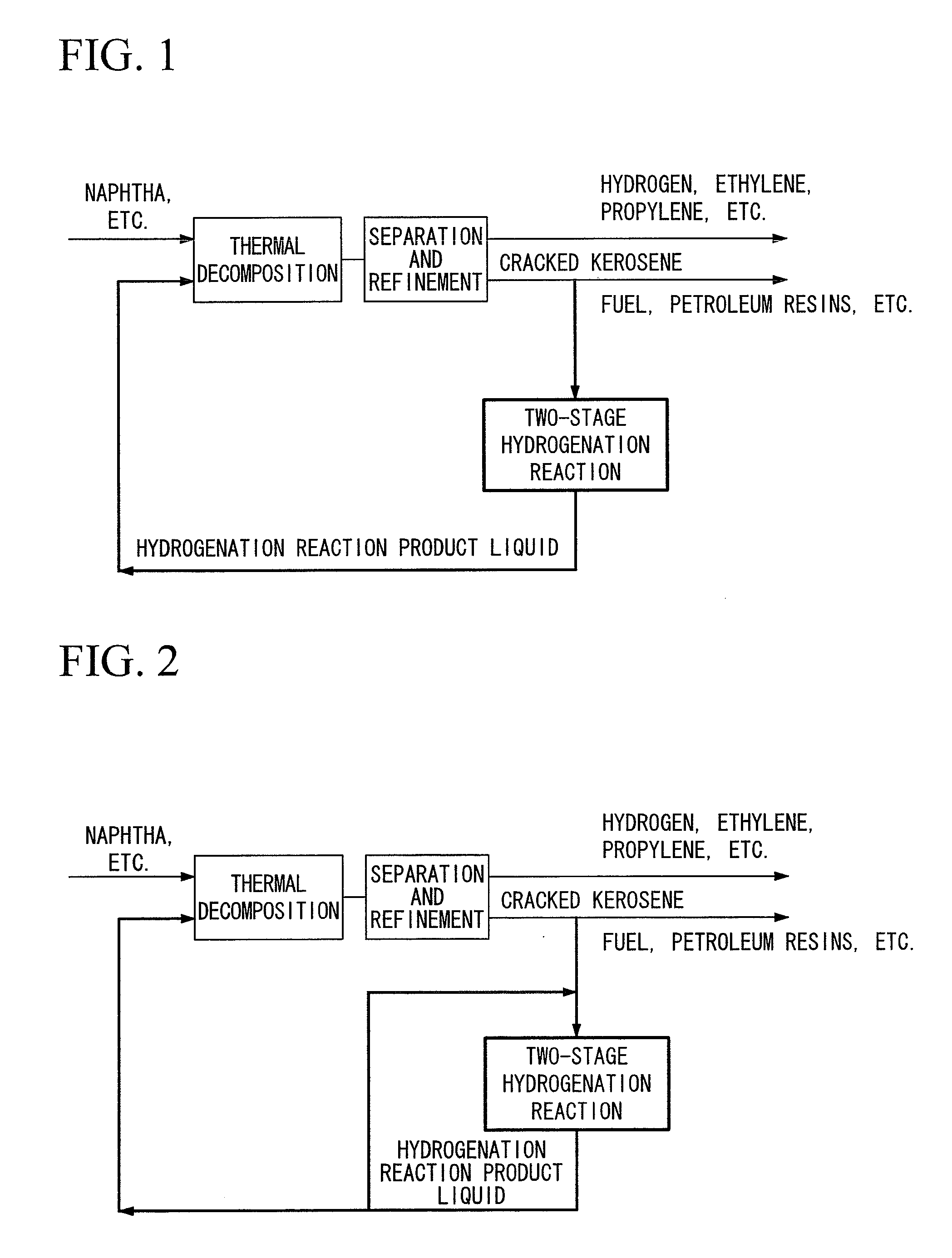
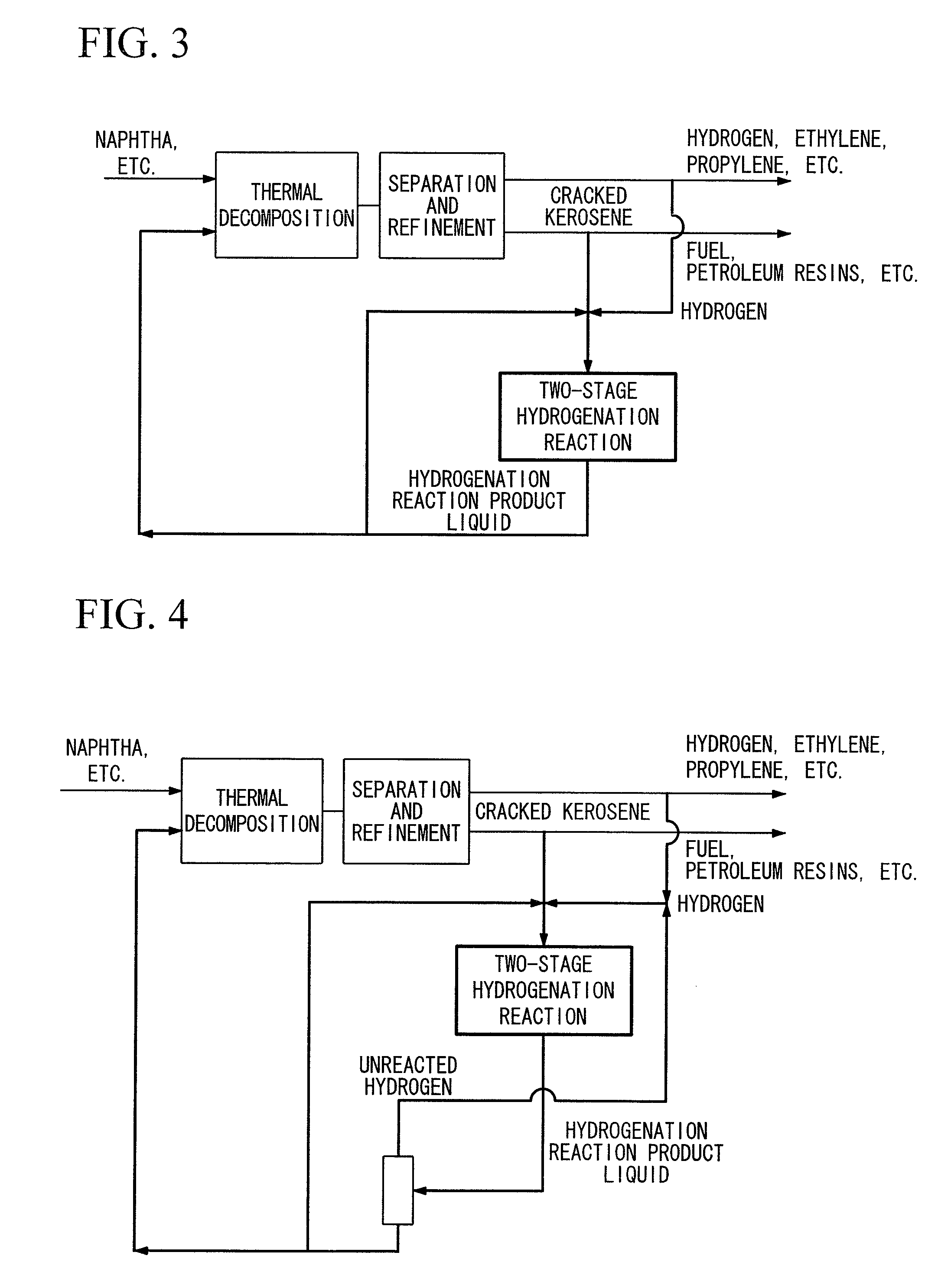
The present invention provides a hydrogenation method capable of converting cracked kerosene into the raw materials for petrochemical cracking having a high thermal decomposition yield by a hydrogenation reaction. The present invention is a petrochemical process for producing at least any of ethylene, propylene, butane, benzene or toluene by carrying out a thermal decomposition reaction at least using naphtha for the main raw material, wherein cracked kerosene produced from a thermal cracking furnace is hydrogenated using a Pd or Pt catalyst in a two-stage method consisting of a first stage (I), in which a hydrogenation reaction is carried out within the range of 50 to 180° C., and a second stage (II), in which a hydrogenation reaction is carried out within the range of 230 to 350° C., followed by re-supplying all or a portion of these hydrogenated hydrocarbons to a thermal cracking furnace.
Owner:SHOWA DENKO KK
Reforming unvaporized, atomized hydrocarbon fuel
InactiveUS20050274107A1Improved hydrogen generationLow costHydrogenExhaust apparatusKeroseneTurbocharger
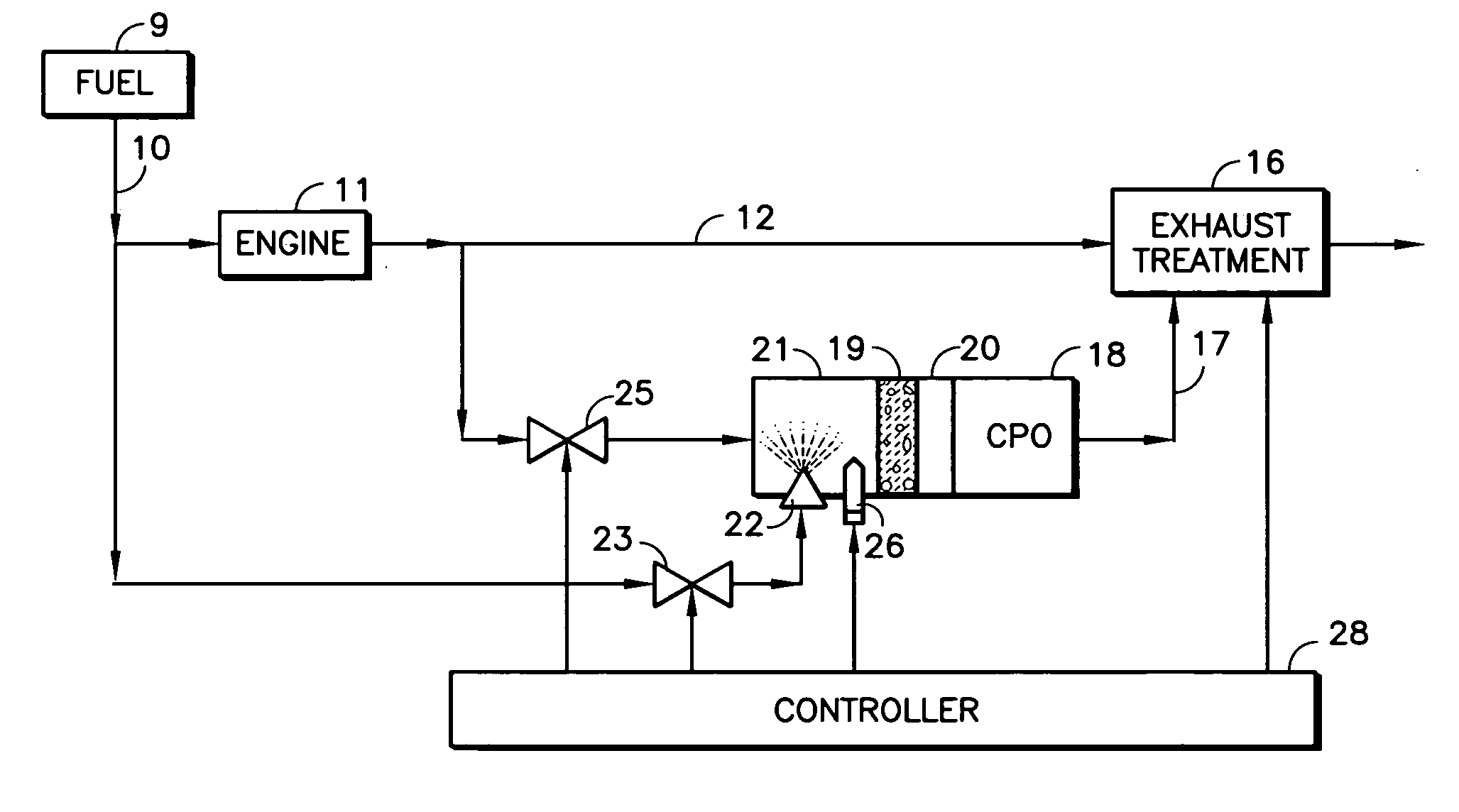
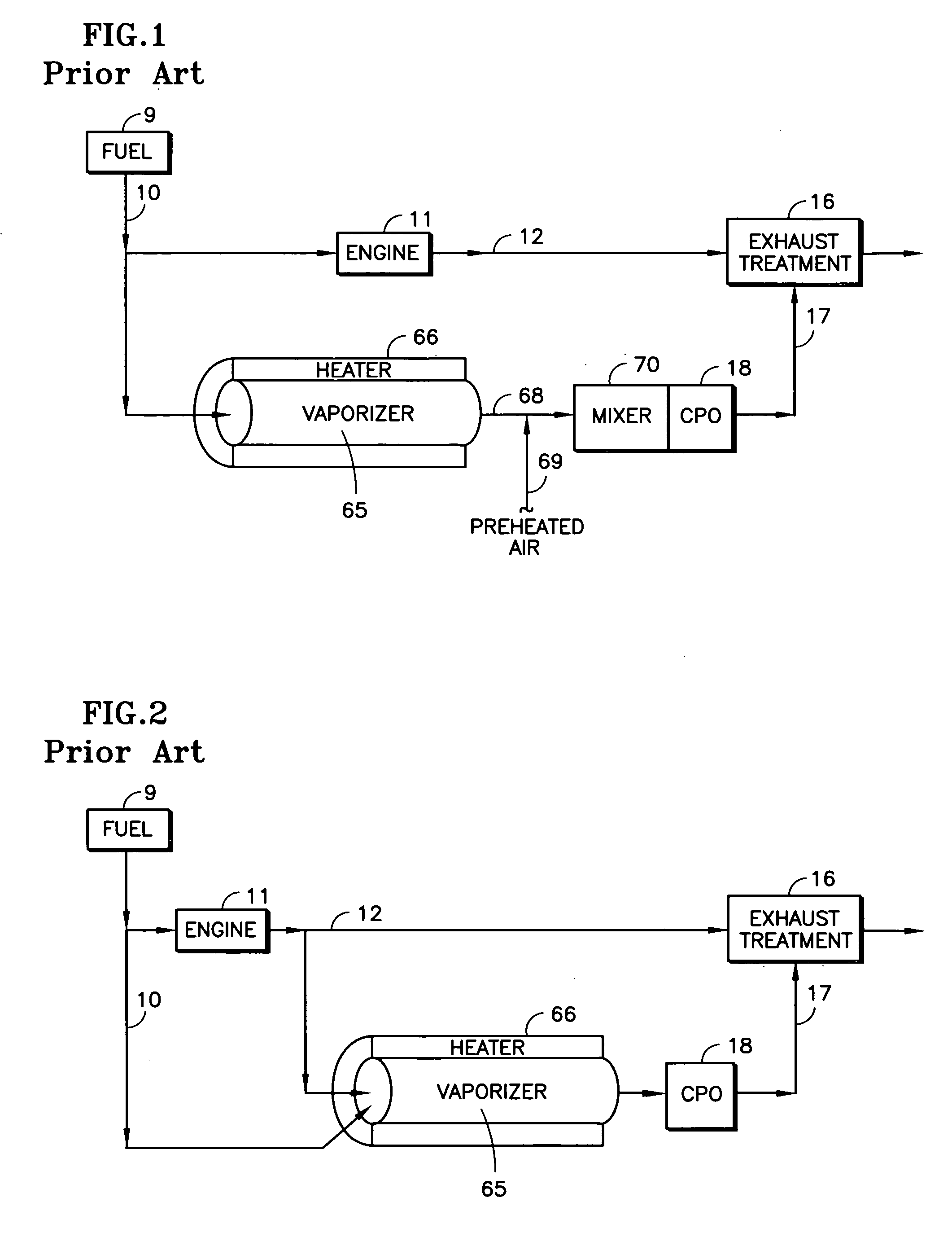
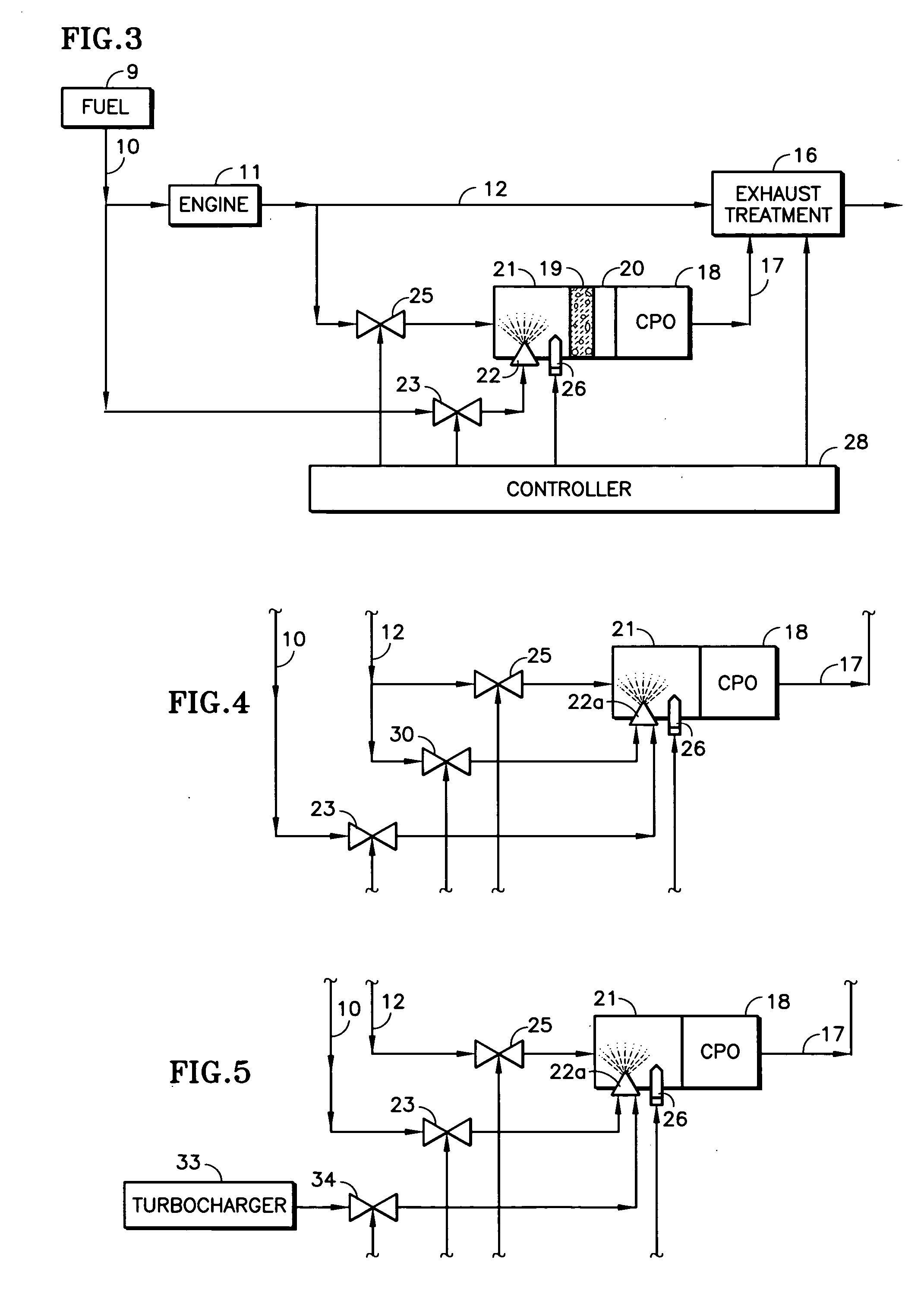
A reformer such as a CPO (18) receives a mix of fuel, moisture and oxygen from a mixing region (21) having an igniter (26, 66), which may include an inert ceramic foam (19), the fuel being provided by an atomizing nozzle (22), thereby avoiding the need for a vaporizer before use. The oxygen and moisture may comprise engine exhaust (11, 12). Fuel from a vehicle fuel tank (9), may be gasoline, diesel fuel, kerosene, jet fuel, or JP-8. The atomizing nozzle may be a gas-assist nozzle (22a), receiving the assisting gas from (a) engine exhaust (10), (b) a turbocharger (33), (c) an air pump (50) or (d) a steam generator (57). The oxygen and moisture may comprise moisturized air, which may be achieved by an ejector (41) which ingests water from a tank (43) in response to the flow of air from a pump (50) through a conduit (47). The air may be regeneratively heated (48) with the CPO exhaust. The igniter may be a glow plug (26) or a heater wire (66) coated with catalyst.
Owner:SHELL OIL CO
Aviation-grade kerosene from independently produced blendstocks
InactiveUS20090000185A1Liquid carbonaceous fuelsLiquid hydrocarbon mixture productionKeroseneParaffin oils
Aviation-grade kerosene comprising a first blendstock derived from non-petroleum feedstock and comprising primarily hydrocarbons selected from the group consisting of isoparaffins and normal paraffins, and a second blendstock comprising primarily hydrocarbons selected from the group consisting of cycloalkanes and aromatics.A method for the production of aviation-grade kerosene comprising producing a first blendstock from at least one non-petroleum feedstock, the first blendstock comprising primarily hydrocarbons selected from the group consisting of isoparaffins and normal paraffins; producing a second blendstock comprising primarily hydrocarbons selected from the group consisting of cycloalkanes and aromatics; and blending at least a portion of the first blendstock with at least a portion of the second blendstock to produce aviation-grade kerosene.
Owner:ENERGY & ENVIRONMENTAL RES CENT FOUNDATIO
Fuels for internal combustion engines
InactiveUS6858048B1Emission reductionSafer land environmentLiquid carbonaceous fuelsFuel additivesKeroseneOctanol
Mixed alcohols can be used as a fuel additive in gasoline, diesel, jet fuel or as a neat fuel in and of itself. The mixed alcohols can contain C1-C5 alcohols, or in the alternative, C1-C8, or higher, alcohols in order to boost energy content. The C1-C5 mixed alcohols contain more ethanol than methanol with amounts of propanol, butanol and pentanol. C1-C8 mixed alcohols contain the same, with amounts of hexanol, heptanol and octanol. A gasoline-based fuel includes gasoline and the mixed alcohols. A diesel based fuel includes diesel and the mixed alcohols. A jet fuel includes kerosene and the mixed alcohols. The neat fuel of the mixed alcohols has an octane number of at least 109 and the Reid Vapor Pressure is no greater than 5 psi. The gross heat of combustion is at least 12,000 BTU's / lb.
Owner:STANDARD ALCOHOL COMPANY OF AMERICA
Harvesting hydrocarbons and water from methane hydrate deposits and shale seams
InactiveUS20100006281A1Low extraction temperatureLess waterConstructionsMultiple-effect/fractional condensationNoble gasKerosene
A method of extraction of fuels, organic pollutants, and elements from Methane hydrate deposits, shale seams and the soil is described which freezes the zone and heats the center carrying the fuel, chemicals and water in these deposits and seams from where they are found, be it deep in the sea or on land, and carries them into the condensing unit in inert Nitrogen gas. Required drilling on the surface or sea bottom includes a main shaft and with auxiliary narrow drillings widely spaced from the shaft. The extraction zone, which is first cooled to brittle cold using the evaporation of Liquid Nitrogen and fractured with vibrations, is heated to the highest temperature of the hydrocarbon fraction desired to be extracted. The evaporating hydrocarbons are extracted in a Nitrogen gas carrier, a recognized fire suppressant (NFPA Code 2000). To speed the extraction rate, tonal input from two or more sounding units vibrates the seam structure freeing the evaporated hydrocarbons allowing more rapid escape into the shaft. To prevent air loss in aquifers, ice barriers seal the zone periphery. These hydrocarbons are separated into the hydrocarbons fractions, into fuel fractions as heating oil, kerosene, gasoline, ethers, and fuel gas including methane, Argon / Oxygen and rare gas segments, or, if pollutants, into the separate chemicals by boiling point. The thermal gradient of the extraction pipe is implemented by sourcing the Nitrogen from Liquid Nitrogen and bundling those pipes with the extraction pipe condensing its contents by hydrocarbon fractions into vessels and gas drums depending on boiling points of fractions. Water is separated from the gasoline segment and purified first by separation and then by freezing. The extraction of deep deposits layer the extraction zones as well as work neighboring extraction zones covering many acres. Fuel gases can be liquefied or burned in an on-site electric generating plant.
Owner:AIR WARS DEFENSE
Liquid fuel compositions
The present invention provides a liquid fuel composition comprising a distillation fraction of a component having at least one C4+ compound derived from a water-soluble oxygenated hydrocarbon prepared by a method comprising:providing water and a water-soluble oxygenated hydrocarbon comprising a C1+O1+ hydrocarbon in an aqueous liquid phase and / or a vapor phase;providing H2;catalytically reacting in the liquid and / or vapor phase the oxygenated hydrocarbon with the H2 in the presence of a deoxygenation catalyst at a deoxygenation temperature and deoxygenation pressure to produce an oxygenate comprising a C1+O1-3 hydrocarbon in a reaction stream; andcatalytically reacting in the liquid and / or vapor phase the oxygenate in the presence of a condensation catalyst at a condensation temperature and condensation pressure to produce the C4+ compound,wherein the C4+ compound comprises a member selected from the group consisting of C4+ alcohol, C4+ ketone, C4+ alkane, C4+ alkene, C5+ cycloalkane, C5+ cycloalkene, aryl, fused aryl, and a mixture thereof;wherein the liquid fuel composition is selected from:a gasoline composition having an initial boiling point in the range of from 15° C. to 70° C. (IP123), a final boiling point of at most 230° C. (IP123), a RON in the range of from 85 to 110 (ASTM D2699) and a MON in the range of from 75 to 100 (ASTM D2700);a diesel fuel composition having an initial boiling point in the range of from 130° C. to 230° C. (IP123), a final boiling point of at most 410° C. (IP123) and a cetane number in the range of from 35 to 120 (ASTM D613); anda kerosene composition having an initial boiling point in the range of from 80 to 150° C., a final boiling point in the range of from 200 to 320° C. and a viscosity at −20° C. in the range of from 0.8 to 10 mm2 / s (ASTM D445).
Owner:SHELL USA INC
Method for preparation of aviation kerosene and diesel oil from biomass derivative
The invention relates to a new liquid chain hydrocarbon fuel synthetic route that acquires a platform chemical compound based on a lignocellulose raw material and is completely independent of fossil energy. The liquid fuel obtained by the method can be used as a substitute of aviation kerosene and diesel oil or as an additive for improving the cetane number and cold hardiness of fuels, thereby reducing the national dependence on imported petroleum in terms of liquid fuels. The method provided in the invention consists of two parts: 1) on a novel solid acid catalyst, an aldehyde group-containing compound (such as formaldehyde, acetaldehyde, propionaldehyde, and butyraldehyde, etc.) and a furan platform compound (such as furan, methyl furan, and hydroxylmethyl furan, etc.) undergo an acid catalyzed alkylation reaction to prepare an oxygen-containing organic compound with a carbon chain length of 8-16; and 2) hydrogenation and hydrodeoxygenation are conducted on an alkylation product to hydrogenate unsaturated bonds and remove the oxygen therein, thus preparing aviation kerosene or high grade diesel oil with a carbon chain length ranging from 8 to 16.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hydrogenation method for producing high grade diesel oil and high grade reforming raw material
ActiveCN101210198AHigh saturation activityImprove hydrodesulfurization activityHydrocarbon oil crackingTreatment with hydrotreatment processesWaxHydrogen
A hydrogenation method for producing high-quality diesel oil and high-quality reforming materials comprises the following steps of: mixing diesel oil and / or a light wax oil material with hydrogen gas, and sequentially contact-reacting with a hydrorefining catalyst and a hydrocracking catalyst without middle separation, cooling the reaction result, and separating to obtain a light naphtha fraction, a heavy naphtha fraction, a kerosene fraction, a diesel oil fraction and a tail oil fraction, wherein the kerosene fraction and / or the tail oil fraction can be directly extracted or partially or completely recycled back to the reaction system. By adopting single-stage once-through process and a non-noble metal catalyst, the invention can produce the reforming materials with high aromatic content and the diesel oil fraction with high cetane number, wherein the yield of the reforming material is larger than 20wt%, and the cetane number of the diesel oil fraction can be improved by more than 15 units. The method provided by the invention has high operation flexibility and can flexibly adjust the technical scheme according to different raw materials and different product scheme requirements.
Owner:CHINA PETROLEUM & CHEM CORP +1
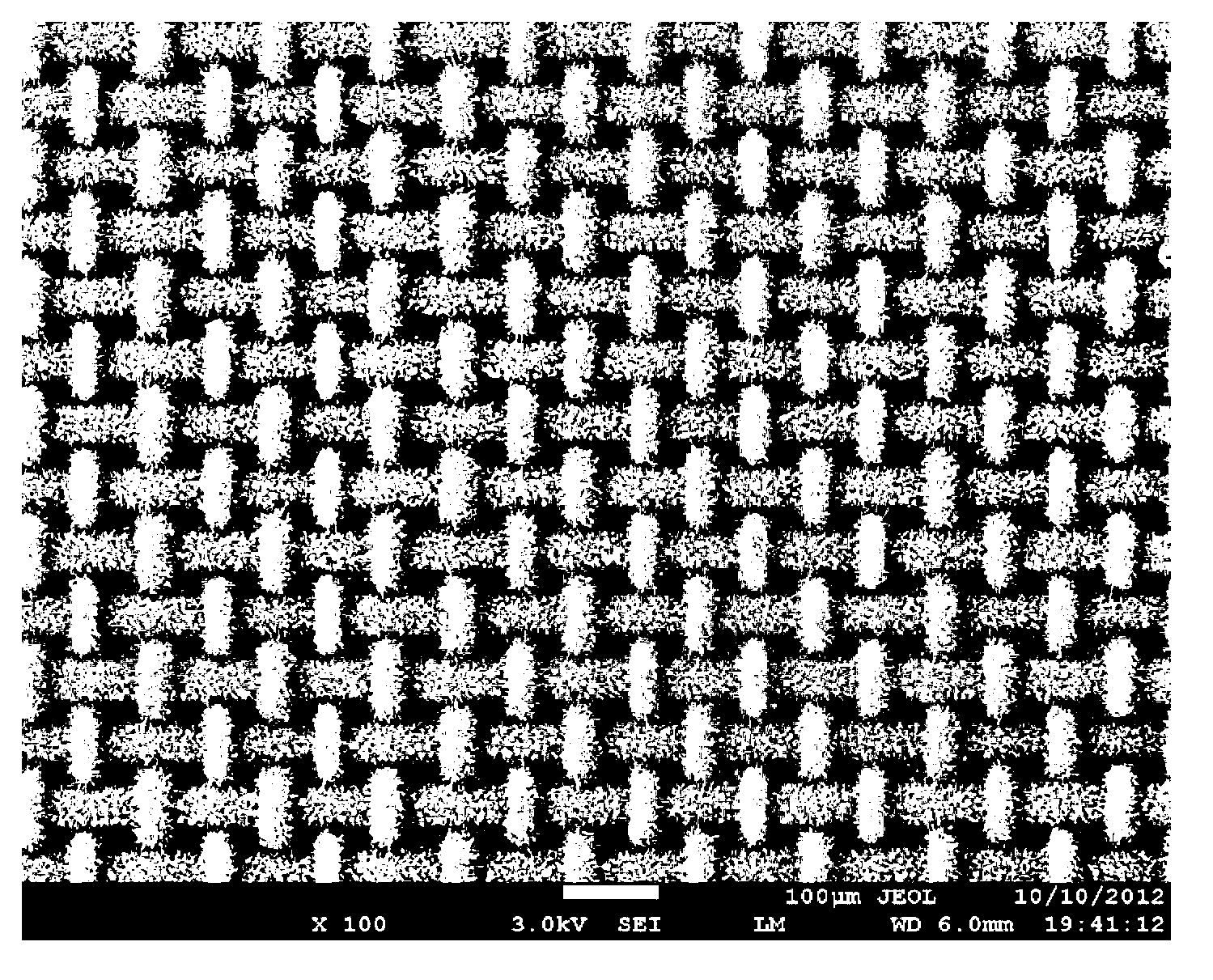
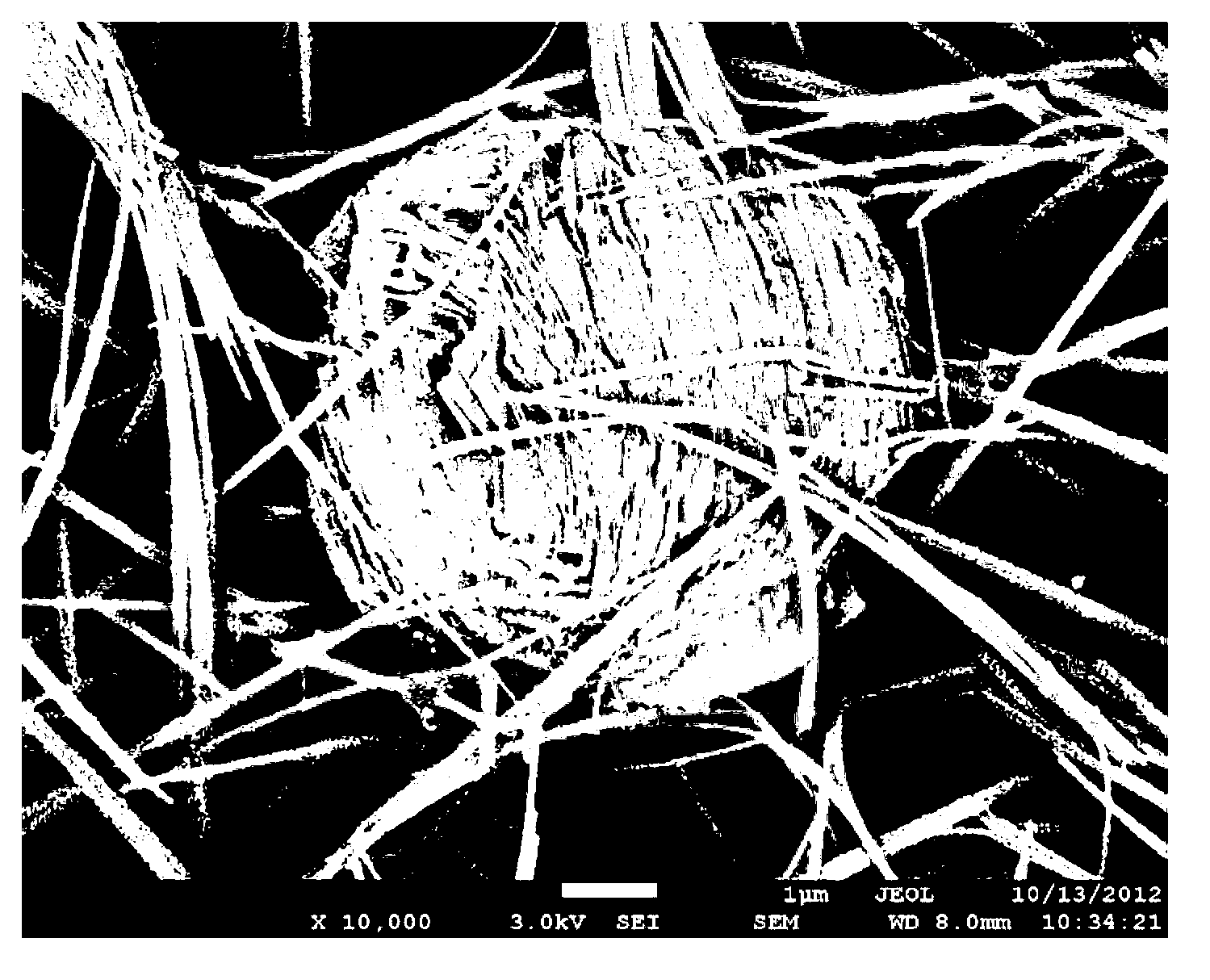
Super-hydrophilic and underwater super-oleophobic oil-water separation mesh membrane having, and its preparation method
The invention discloses a super-hydrophilic and underwater super-oleophobic oil-water separation mesh membrane, and its preparation method. The method comprises the following steps: 1, cleaning a copper mesh, and airing; 2, dissolving an alkaline medium and an oxidant in water, and uniformly stirring to obtain a mixed solution; and 3, dipping the aired copper mesh in the mixed solution, and carrying out an oxidation reaction to obtain the oil-water separation mesh membrane. The oil-water separation mesh membrane has the advantages of easily available raw material, low cost, simple equipment and making technology, realization of large-scale preparation, large water flux, fast water-water separation speed, good oil-water separation effect, suitableness for the treatment of sewage containing a large amount of water, very good separation effect on n-hexane, petroleum ether, dichloroethane, benzene, gasoline, diesel oil, kerosene, machine oil, crude oil, animal and plant oil, and the like, easy cleaning, repeatable use, and good stability.
Owner:TSINGHUA UNIV
Preparation method for aviation kerosene
The invention relates to a new synthesis route of a liquid branched paraffin fuel, the method adopts a lignocellulose based platform compound as a raw material and is completely independent of fossil energy. The liquid fuel obtained by the method can be used as an aviation kerosene (or diesel) substitute or as an additive to increase the cetane number and cold resistance of fuel. The method provided by the invention includes two steps of: 1) under the promotion of a base catalyst, subjecting a lignocellulose based furfural compound (including furfural, methylfurfural or 5 hydroxymethylfurfural) and branched chain keto (including methyl isobutyl ketone, and mesityl oxide, etc.) to aldol condensation reaction so as to synthesize an oxygen-containing organic compound with a carbon chain length of 9-16; and 2) conducting hydrodeoxygenation on the aldol condensation product generated in step1 to obtain biomass aviation kerosene branched hydrocarbon with a carbon chain length of 9-16, higher energy density, stability and low freezing point.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Coal slime flotation collector and preparation method thereof
The invention discloses a coal slime flotation collector and a preparation method thereof. The coal slime flotation collector comprises the following matters in percentage by weight: 20-50 percent of kerosene and / or light diesel oil, 1-5 percent of primary emulsion, 0.006-0.015 percent of auxiliary emulsion and the balance of water; wherein the primary emulsion is a mixture of polyoxyethylene sorbitan fatty acid ester and dehydrated sorbitol fatty acid ester, and the hydrophile-lipophile balance (HLB) value of the primary emulsion is within 12.8-14.3; the auxiliary emulsion is selected from the following (1) or (2), wherein the (1) is sodium dodecyl benzene sulfonate, and the (2) is a mixture obtained by mixing fatty alcohol polyoxyethylene ether sulfate and the sodium dodecyl benzene sulfonate according to the mass ratio of 1: 0.5-2. The coal slime flotation collector has good stability, simple preparation process and 40-60 percent of the oil-saving ratio on the premise of improving the float yield and the tail coal ash proportion. The collector is beneficial to saving the energy, reducing the emission and improving the economical benefit when being used for floating the coal slime.
Owner:SHANXI MEDICAL UNIV
Method for preparing aviation kerosene from furyl oxygen-containing organic compounds by hydrogenation deoxidation
ActiveCN104119943AReduce energy consumptionSimple operation processMolecular sieve catalystsLiquid hydrocarbon mixture productionAlkaneFuran
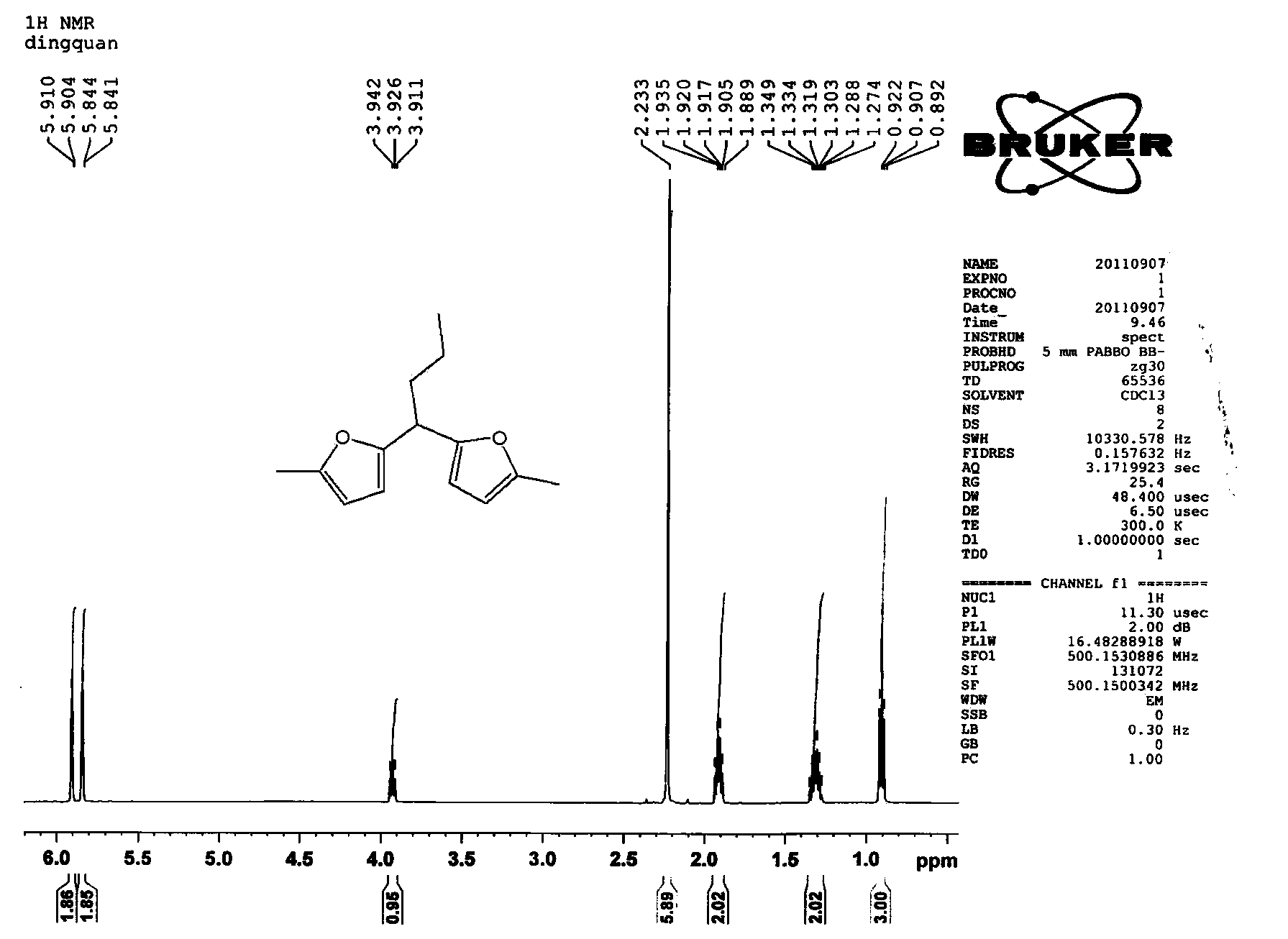
The invention relates to a new method for preparing hydrocarbons in the scope of aviation kerosene from C8-C16 furyl oxygen-containing organic compounds as raw materials by hydrogenation deoxidation reaction, wherein the C8-C16 furyl oxygen-containing organic compounds are obtained by C-C coupling of lignocellulose based platform chemical compounds; low temperature direct hydrogenation deoxidation under the condition of no solvent of the furyl oxygen-containing organic compounds can be realized by use of a metal-solid acid dual-functional catalyst to obtain a series of low freezing point branched alkanes having the chain in the length range of the aviation kerosene in high yield. The catalyst is composed of two parts of an active metal A and an acid vector X. The catalyst related in the method ahs the characteristics of being in no need of a solvent, simple in operation process, mild in reaction conditions, good in aviation kerosene (or diesel) selectivity, and the like, and is an ideal hydrogenation deoxidation catalyst for preparing liquid fuels from the furyl oxygen-containing organic compounds by the hydrogenation deoxidation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for the preparation of middle distillates
InactiveUS6858127B2Low conversionImprove cold flowCatalytic crackingOrganic compound preparationSyngasHydrocotyle bowlesioides
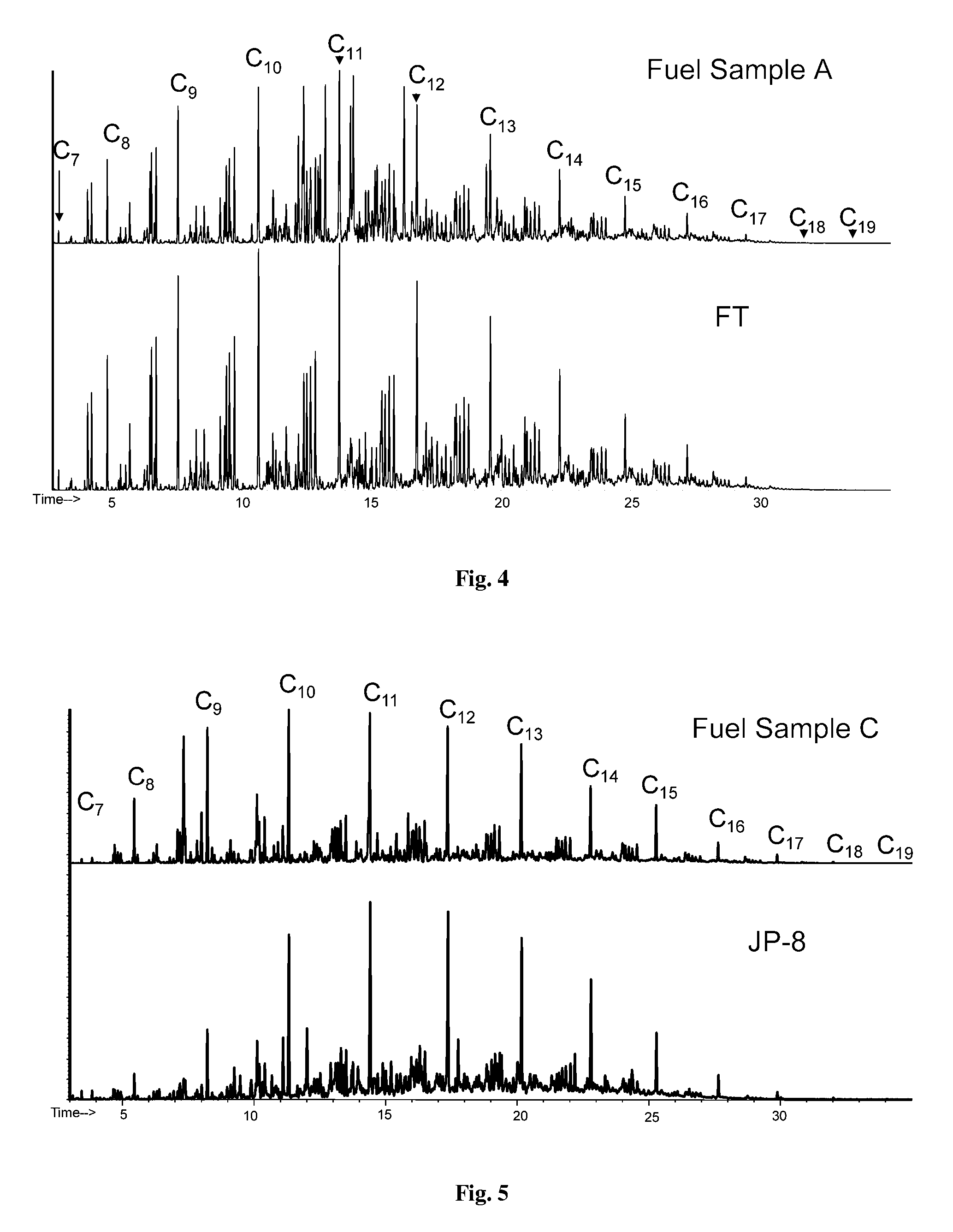
A process for the preparation of one or more hydrocarbon fuel products boiling in the kero / diesel range from a stream of hydrocarbons produced in a Fischer-Tropsch process, in which process synthesis gas is converted into liquid hydrocarbons, at least a part of the hydrocarbons boiling above the kero / diesel range, having the following steps:[0002](1) hydrocracking / hydroisomerizing at least a part of the Fischer-Tropsch hydrocarbons stream at a conversion per pass of at most 80 wt % of the material boiling above 370° C. into material boiling below 370° C.;[0003](2) separating the product stream obtained in step (1) into one or more light fractions boiling below the kero / diesel boiling range, one or more fractions boiling in the kero / diesel boiling range and a heavy fraction boiling above the kero / diesel boiling range;[0004](3) hydrocracking / hydroisomerizing the major part of the heavy fraction obtained in step (2) at a conversion per pass of at most 80 wt % of the material boiling above 370° C. into material boiling below 370° C.;[0005](4) separating the product stream obtained in step (3) into one or more light fractions boiling below the kero / diesel boiling range, one or more fractions boiling in the kero / diesel boiling range and a heavy fraction boiling above the kero / diesel boiling range; and,[0006](5) hydrocracking / hydroisomerizing the major part of the heavy fraction obtained in step (4) in the hydrocracking / hydroisomerizing process described in step (1) and / or step (3), in which process the Fischer-Tropsch hydrocarbons stream comprises at least 35 wt % C30+ (based on total amount of hydrocarbons in the Fischer-Tropsch hydrocarbons stream) and in which stream the weight ratio C60+ / C30+ is at least 0.2.
Owner:SHELL USA INC
Water-in-oil microemulsions for oilfield applications
A well treatment microemulsion includes an oil external phase, an internal aqueous phase and a hydrophilic surfactant. The surfactant has a hydrophile lipophile balance of between 8-18. The oil external phase may include d-Limonene, xylenes, light mineral oil, or kerosene. The surfactant is configured to emulsify the water of the internal aqueous phase into the oil of the external (continuous) phase. The surfactant may include polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan tristearate, polyoxyethylene hydrogenated castor oil, polyoxyethylene sorbitan monostearate, polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan monolaurate or mixtures therebetween. The use of hydrophilic surfactants to emulsify an internal aqueous phase within an oil external microemulsion produces unexpected and beneficial results.
Owner:PNC BANK NAT ASSOC
Method for detecting fault of steel-ball surface
InactiveCN1529152AImprove efficiencyAccurate detectionOptically investigating flaws/contaminationKeroseneDisplay device
Feed mechanism feeds balls to be tested to poroid detection cavity positioned on circumference of feeding rotary table and holes corresponding to clapboard positioned under feeding rotary table in detection mechanism. Balls to be tested on feeding rotary table and its holes are immersed in kerosene. The deploying rotary table under the clapboard supports balls to be tested to rotate in order to expanse entire spherical surface. CCD camera above the balls collects information of extended surface of balls to be tested. The said collected information is input into and processed by computer. Display in terminal displays magnified image of extended surface of rolled ball. Computer recognition system through magnified image on the display determines surface quality of the ball. Thus, qualified balls are sent to finished product bin, and unqualified balls are sent to reject bin in automatic or manual control. The method is in high accuracy and high efficiency.
Owner:李季秀
Method for producing hydrocarbon fractions
InactiveUS20090314683A1Reduced activityReduce environmental loadMolecular sieve catalystsHydrocarbon oil crackingPolycyclic aromatic hydrocarbonHydrogen
A method for producing an LPG fraction, a gasoline fraction, a kerosene fraction, a gas oil fraction, monocyclic aromatic hydrocarbon and a non-aromatic naphtha fraction from hydrocracked oil includes hydrocracking hydrocarbon oil containing polycyclic aromatic hydrocarbon to convert into a light hydrocarbon fraction, and efficiently and selectively producing monocyclic aromatic hydrocarbon with higher valuable alkylbenzenes. The method for producing hydrocarbon fraction comprises subjecting hydrocarbon feedstock containing polycyclic aromatic hydrocarbon and in which the ratio of carbons constituting an aromatic ring to the total carbons in the hydrocarbon oil (the aromatic ring-constituting carbon ratio) is 35 mole % or more to catalytic cracking in the presence of hydrogen. 40% or more of a fraction with a boiling point of 215° C. or higher in the hydrocarbon feedstock is converted into a fraction with a boiling point lower than 215° C., producing hydrocracked oil containing 30 vol % or more of monocyclic aromatic hydrocarbon.
Owner:JAPAN ENERGY CORP
High rate reactor system
PendingUS20140109465A1Increase valueImprove propertiesThermal non-catalytic crackingCatalytic crackingAlkaneHigh rate
A process and system for upgrading an organic feedstock including providing an organic feedstock and water mixture, feeding the mixture into a high-rate, hydrothermal reactor, wherein the mixture is rapidly heated, subjected to heat, pressure, and turbulent flow, maintaining the heat and pressure of the mixture for a residence time of less than three minutes to cause the organic components of the mixture to undergo conversion reactions resulting in increased yields of distillate fuels, higher-quality kerosene and diesel fuels, and the formation of high octane naphtha compounds. Hydrocarbon products are cooled at a rate sufficient to inhibit additional reaction and recover of process heat, and depressurizing the hydrocarbon products, and separating the hydrocarbon products for further processing. The process and system can include devices to convert olefinic hydrocarbons into paraffinic hydrocarbons and convert olefinic byproduct gas to additional high-octane naphtha and / or heavier hydrocarbons by one of hydrogenation, alkylation, or oligomerization.
Owner:APPLIED RES ASSOCS INC
Aviation-grade kerosene from independently produced blendstocks
Aviation-grade kerosene comprising a first blendstock derived from non-petroleum feedstock and comprising primarily hydrocarbons selected from the group consisting of isoparaffins and normal paraffins, and a second blendstock comprising primarily hydrocarbons selected from the group consisting of cycloalkanes and aromatics.
Owner:ENERGY & ENVIRONMENTAL RES CENT FOUNDATIO
Preparation method for vanadyl sulfate electrolyte of all-vanadium flow battery
ActiveCN102683733AAchieve mass productionSimple processRegenerative fuel cellsVanadium compoundsAlkaline earth metalKerosene
The invention discloses a preparation method for vanadyl sulfate electrolyte of an all-vanadium flow battery. The preparation method is characterized by comprising the following steps of: adjusting pH value of vanadyl sulfate solution obtained from leaching vanadium slag and stone coal, back extracting and resin-analyzing treatments by using oxide or hydroxide of alkali metal or alkaline earth; adding an inorganic reducing agent; performing multi-grade counter-current extraction by using P204 or P507: TBP: sulfonated kerosene extracting agent; after the two-phase separation, washing the vanadium-loaded organic phase; performing 2-5 grades of multi-grade counter-current back extraction on the vanadium-loaded organic phase by using sulfuric acid solution to obtain the back extracting liquid of vanadyl sulfate; adjusting the pH value of the back extracting liquid of vanadyl sulfate, adding the organic reducing agent to adjust the potential value of the solution; extracting the solution by using the extracting agent; after the two-phase separation, washing the vanadium-loaded organic phase by using the sulfuric acid solution; performing multi-grade counter-current back extraction by using the sulfuric acid solution to obtain the vanadyl sulfate solution; and distilling until the concentration required for all-vanadium flow battery. The method provided by the invention can improve the purity, simplify the preparation procedure and reduce the cost.
Owner:GUANGDONG INST OF RARE METALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com