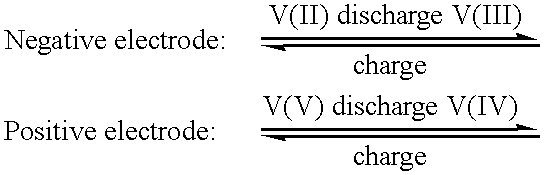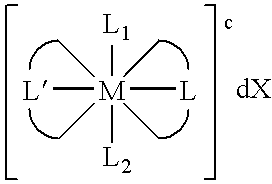Patents
Literature
14132 results about "Vanadium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, ductile, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation) somewhat stabilizes the free metal against further oxidation.
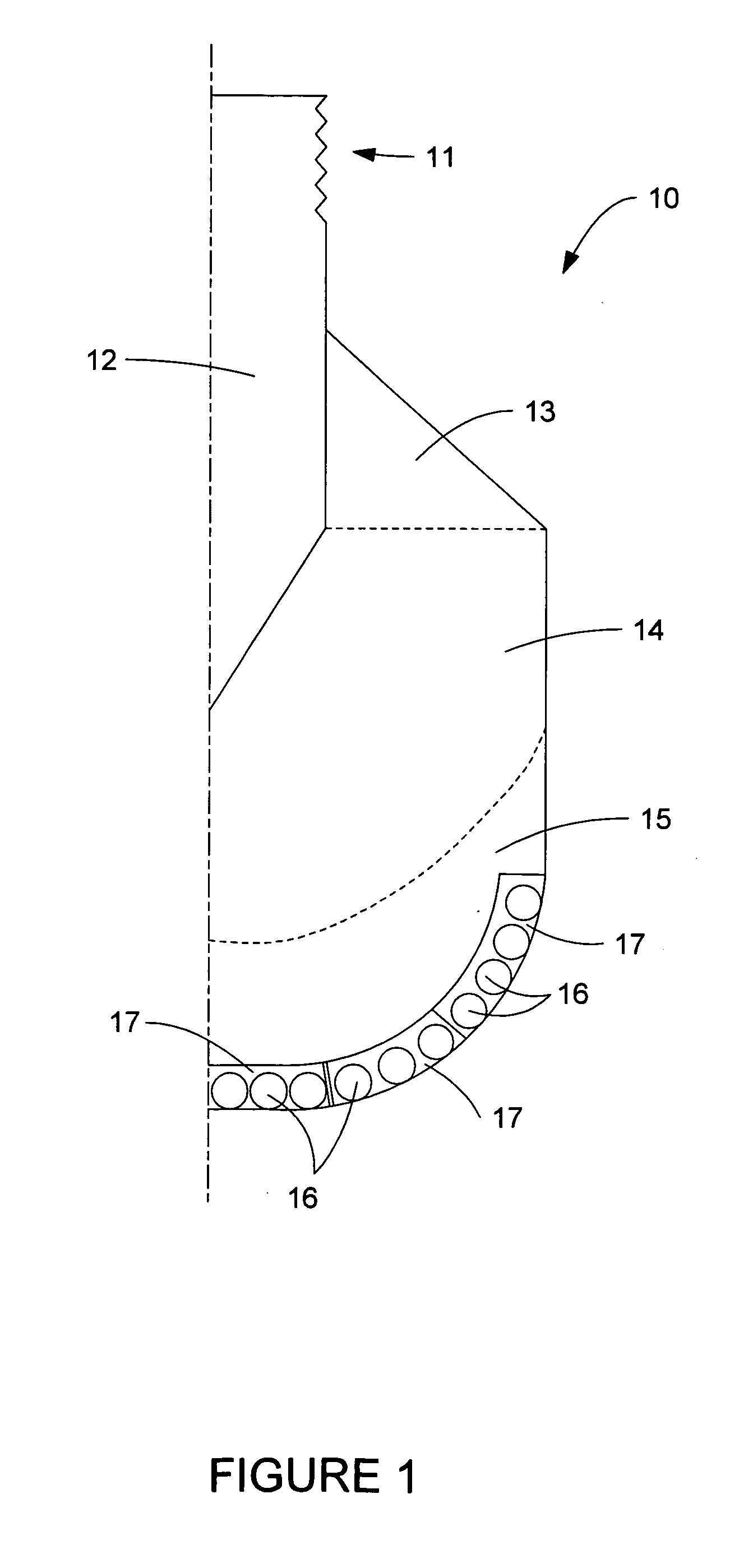
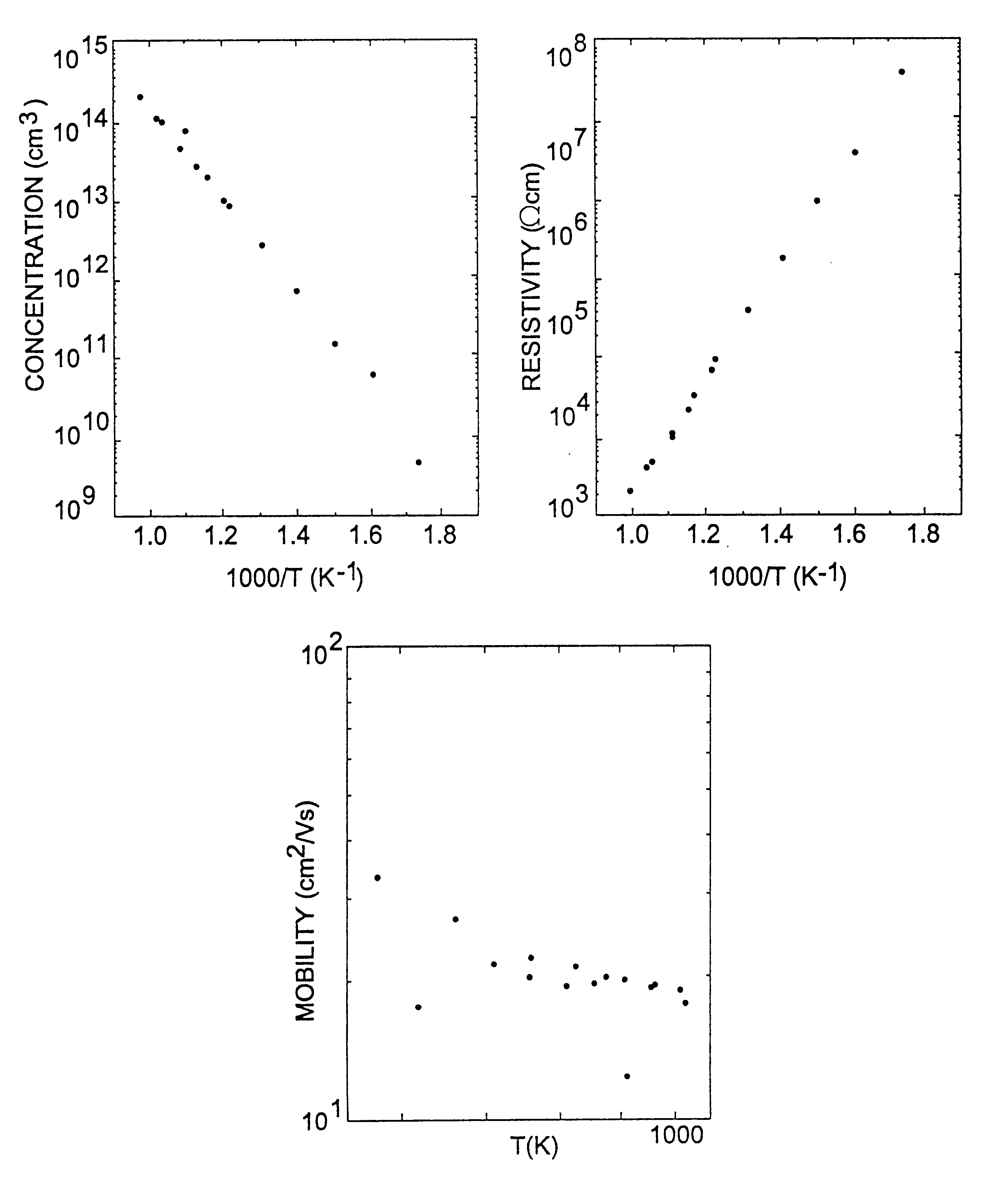
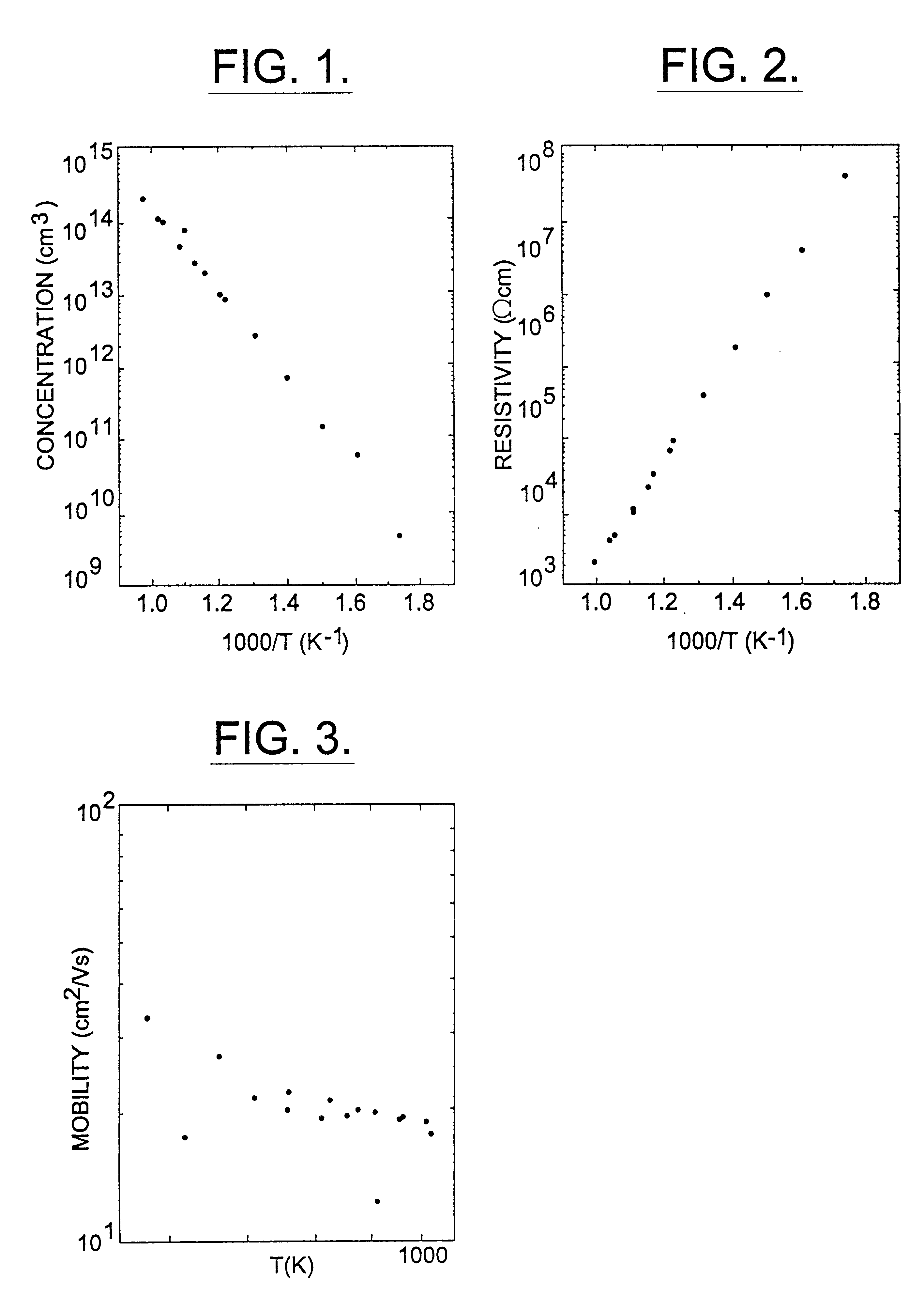
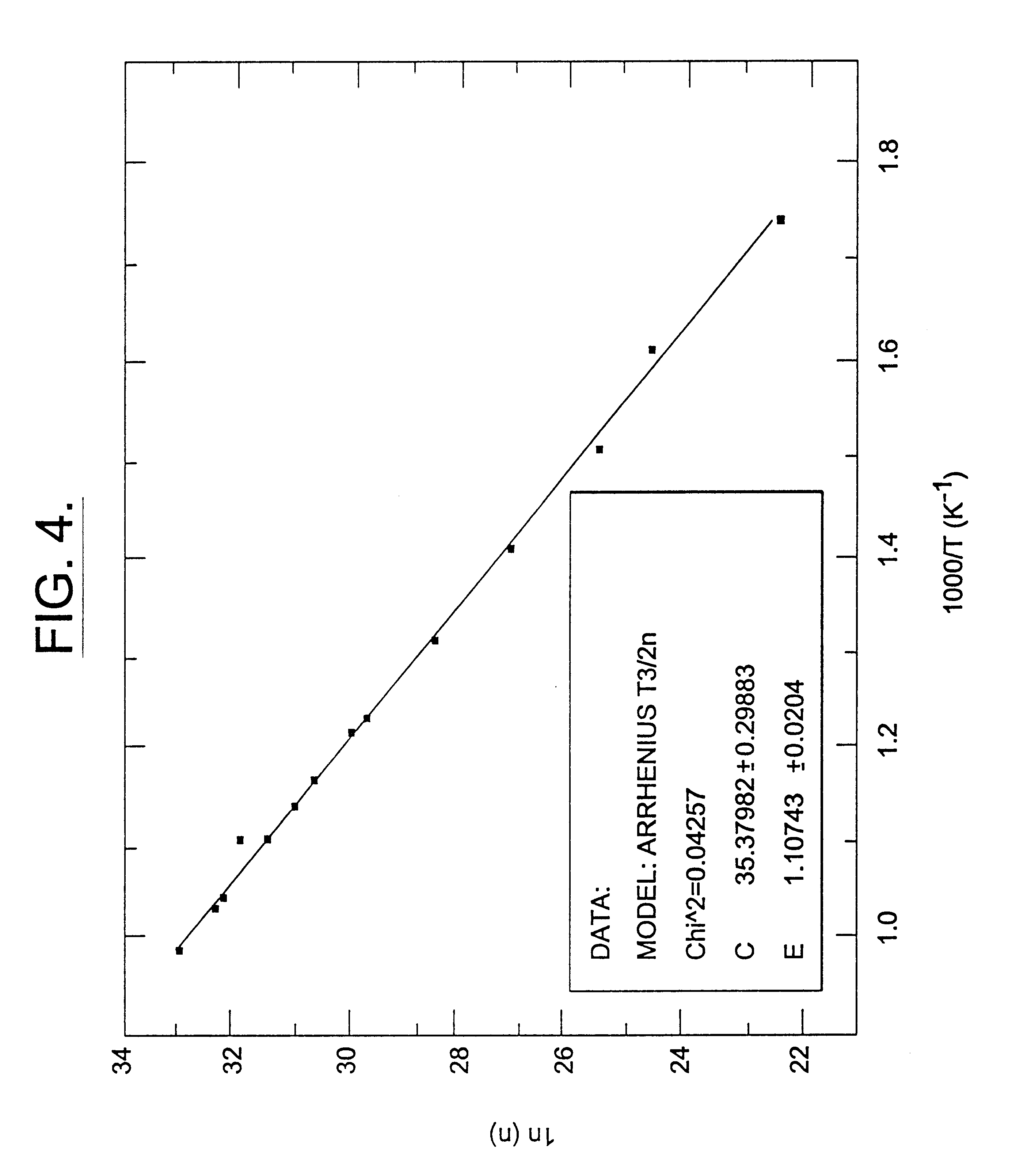
Semi-insulating silicon carbide without vanadium domination
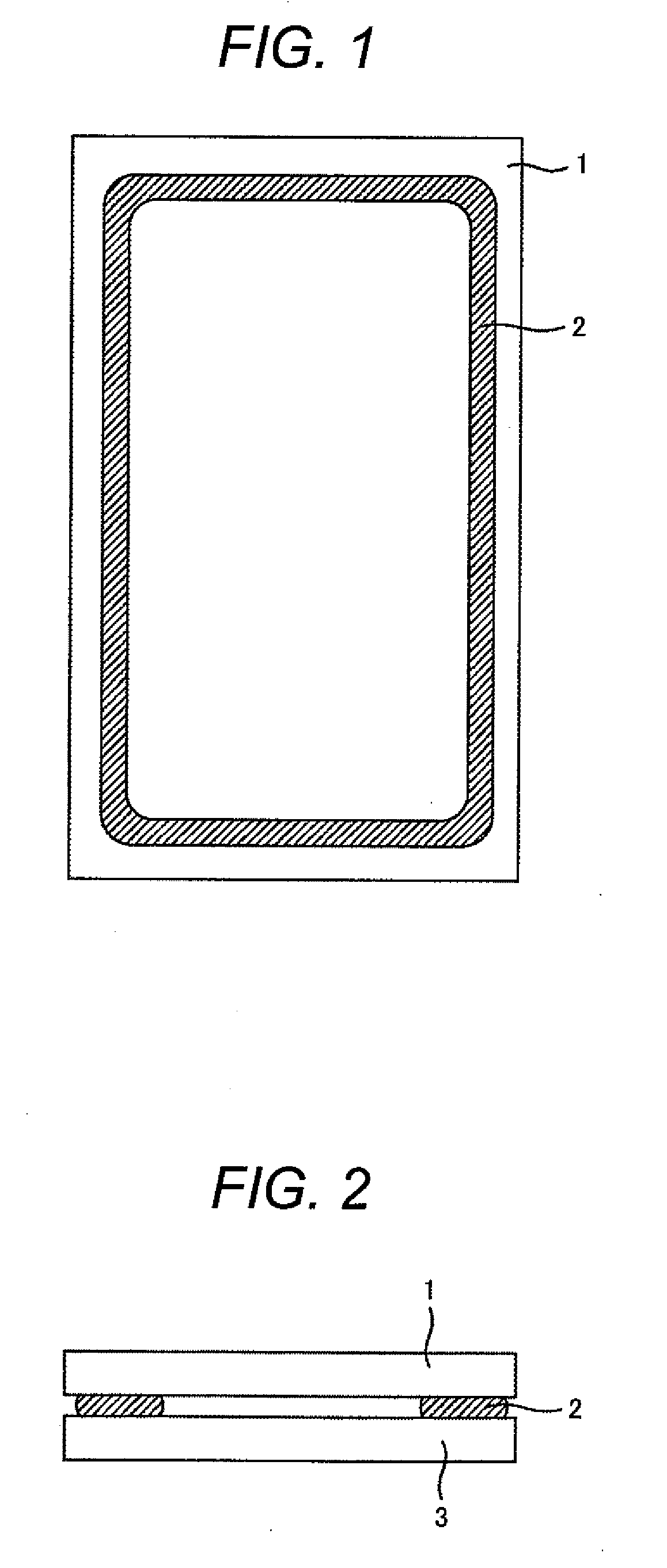
InactiveUS6218680B1Reduce the amount requiredIncrease the number ofPolycrystalline material growthAfter-treatment detailsDevice formTrapping
Owner:CREE INC
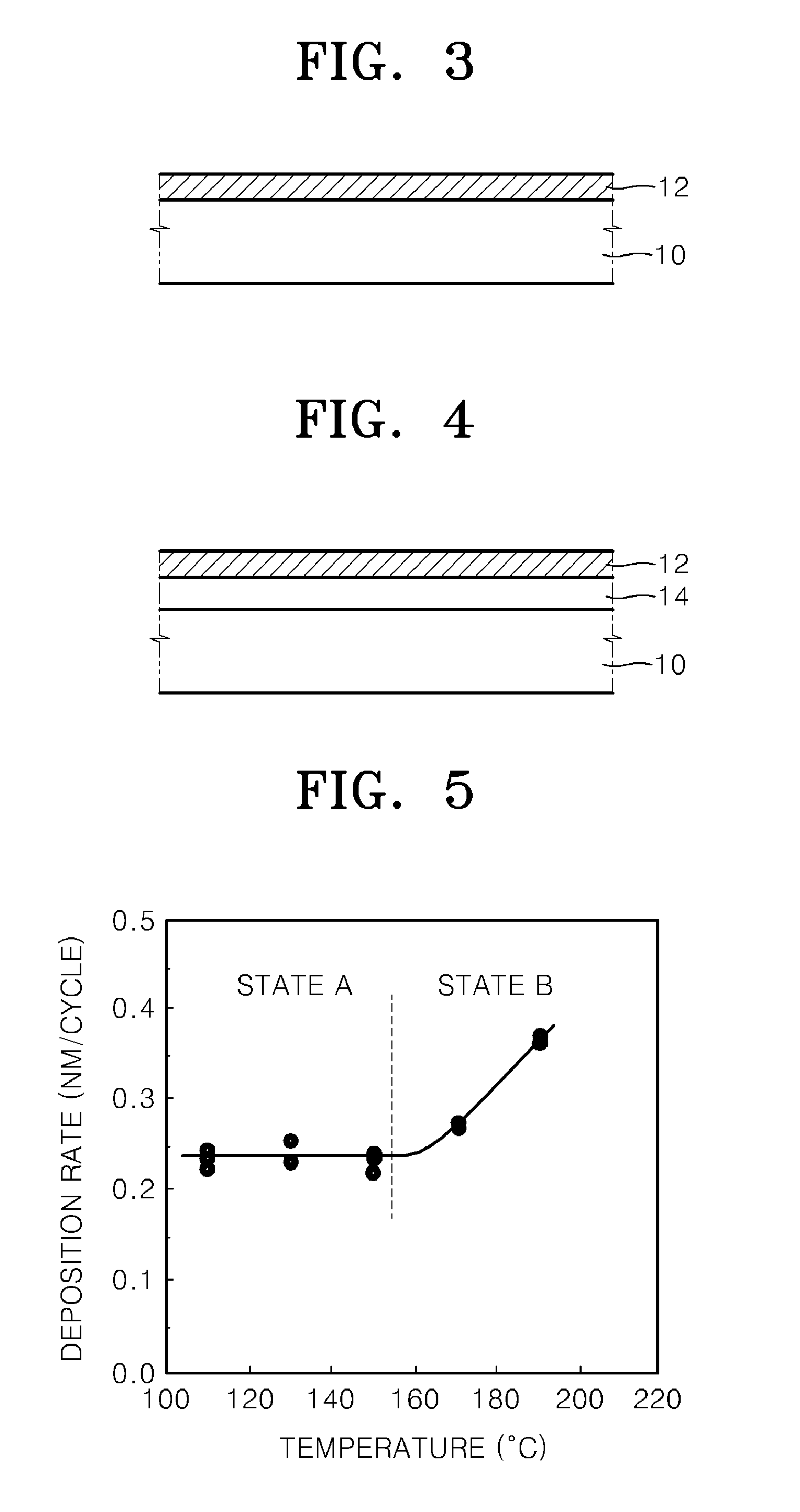
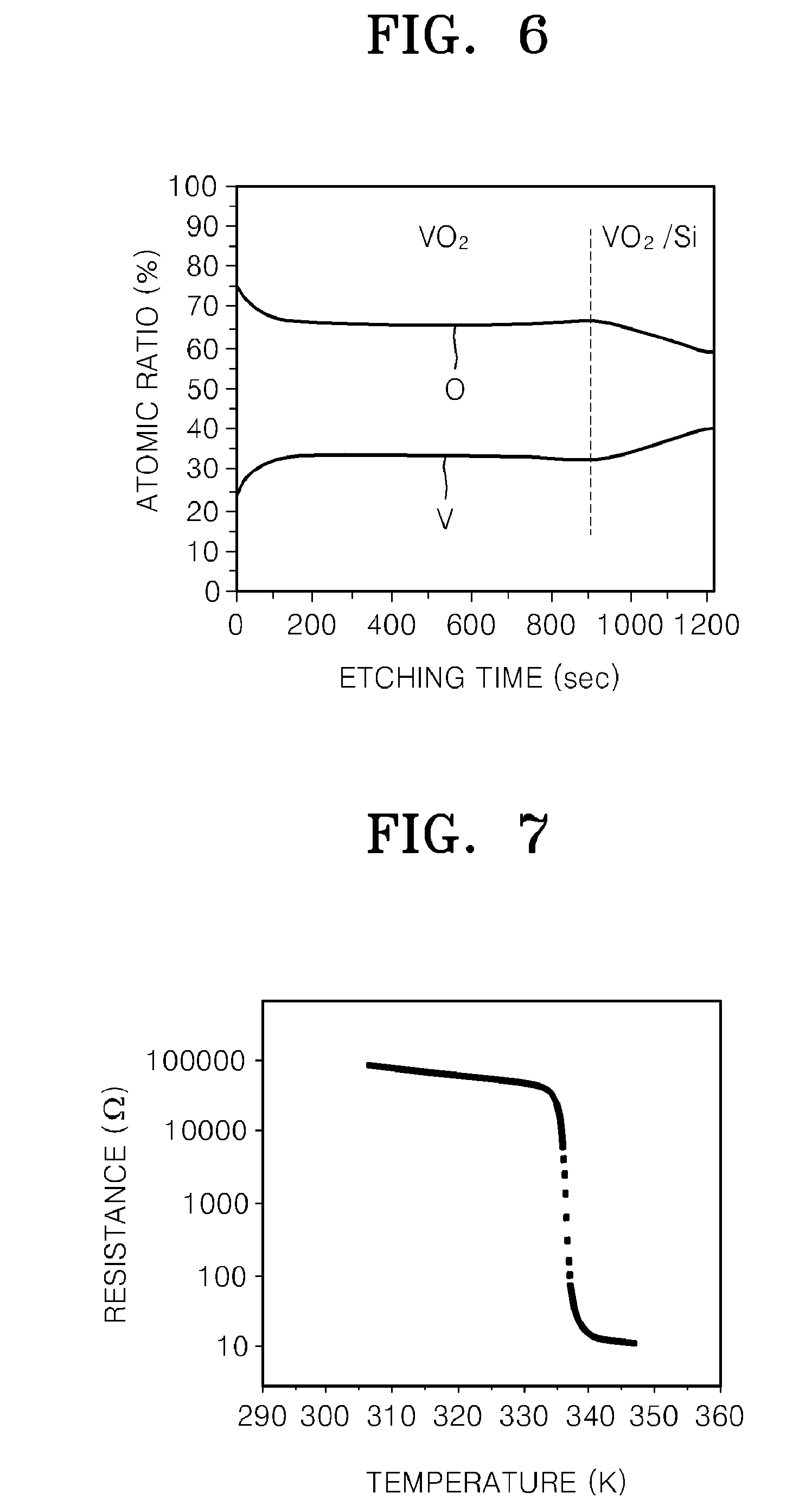
Method of Manufacturing Vanadium Oxide Thin Film
InactiveUS20090011145A1Uniform thicknessComposition is stableChemical vapor deposition coatingPlasma techniqueOxygenMaterials science
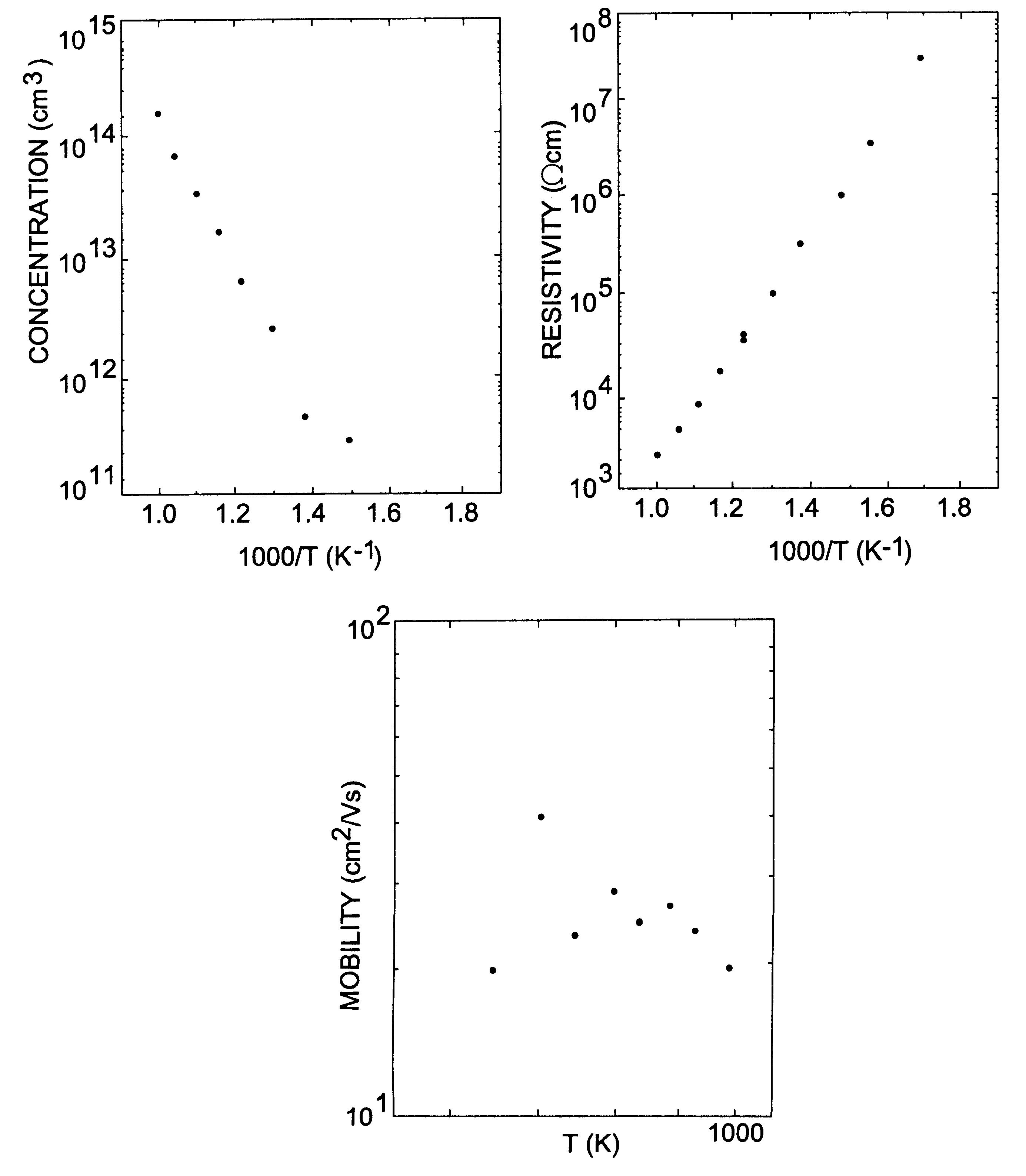
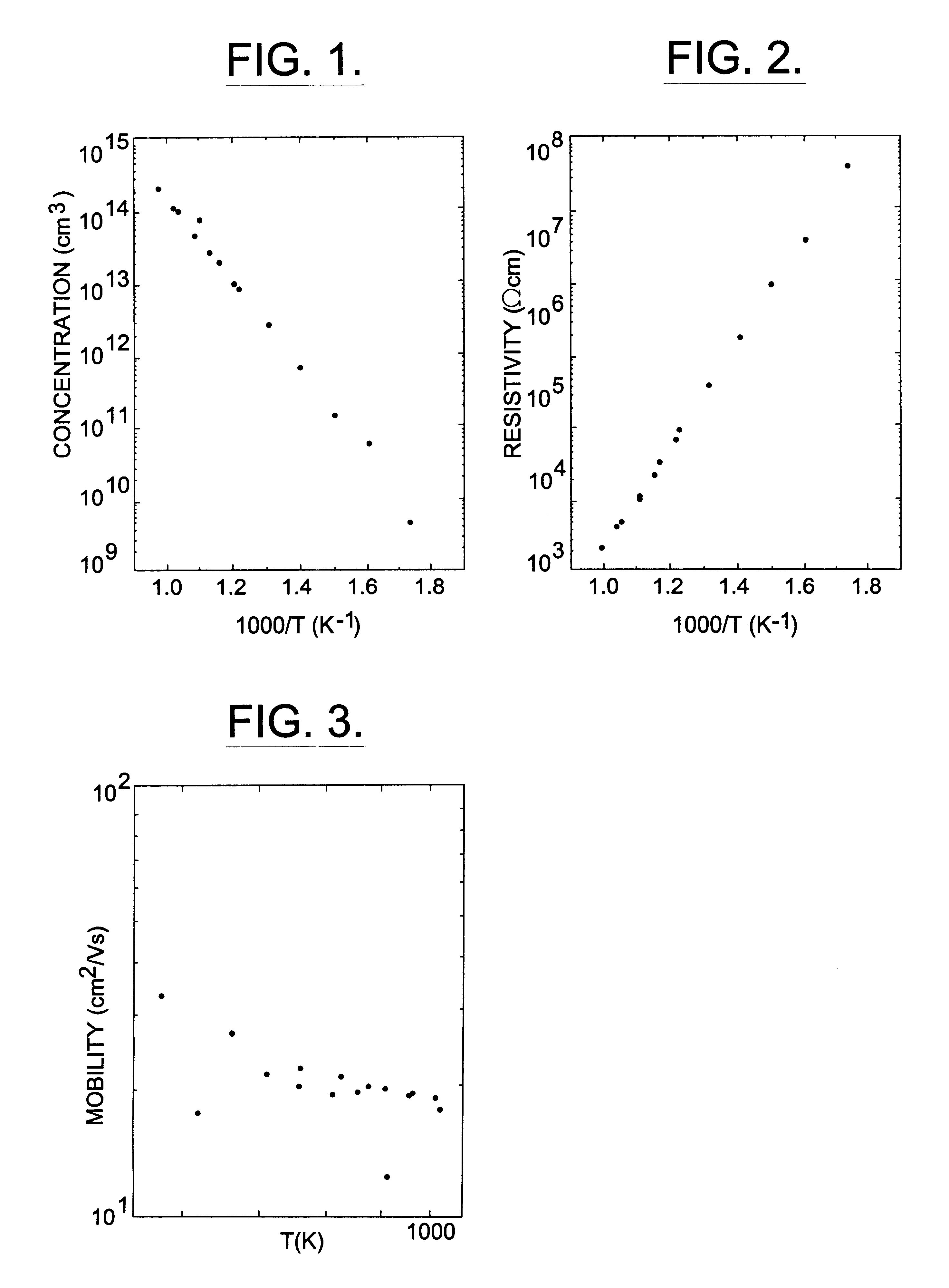
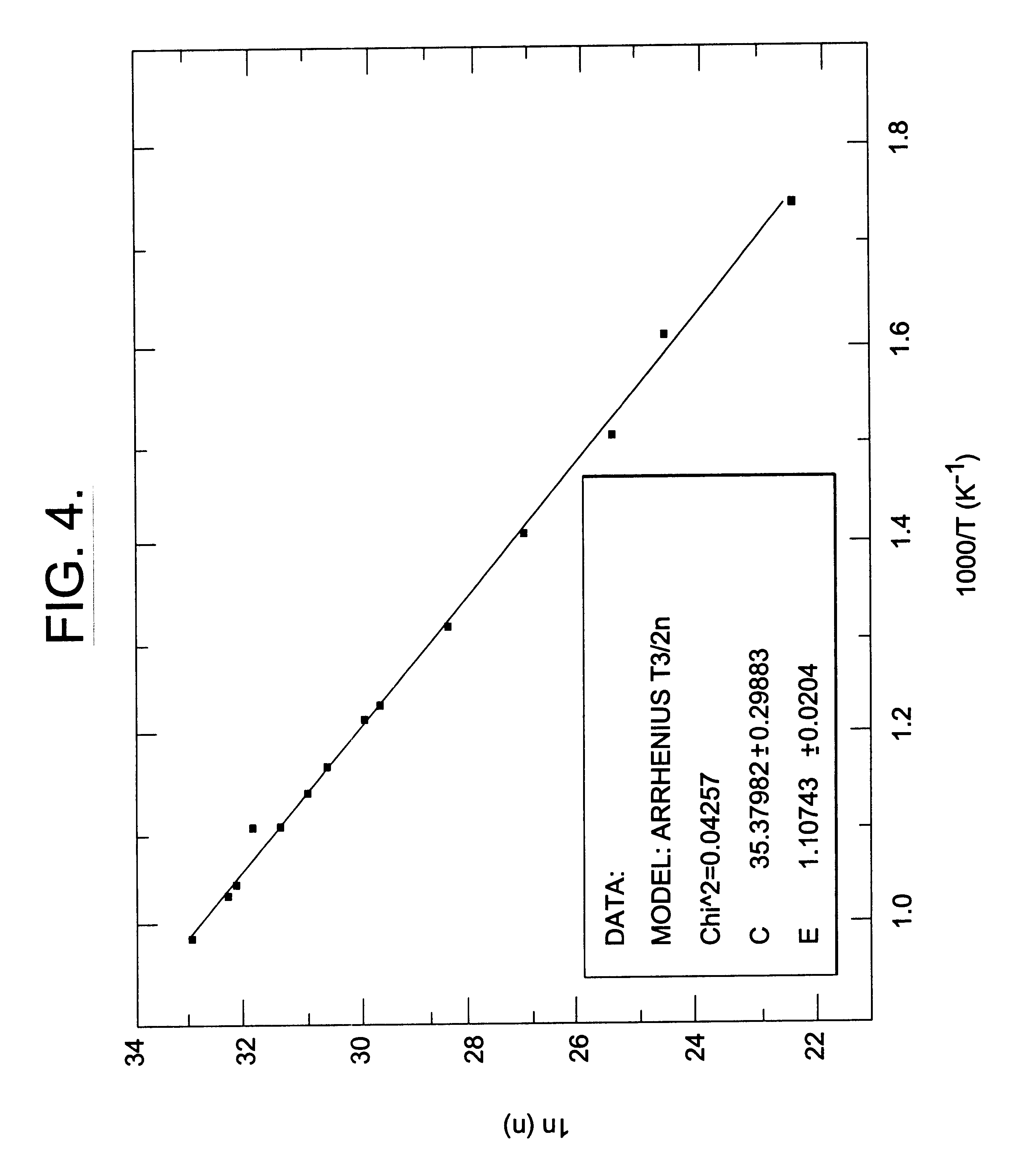
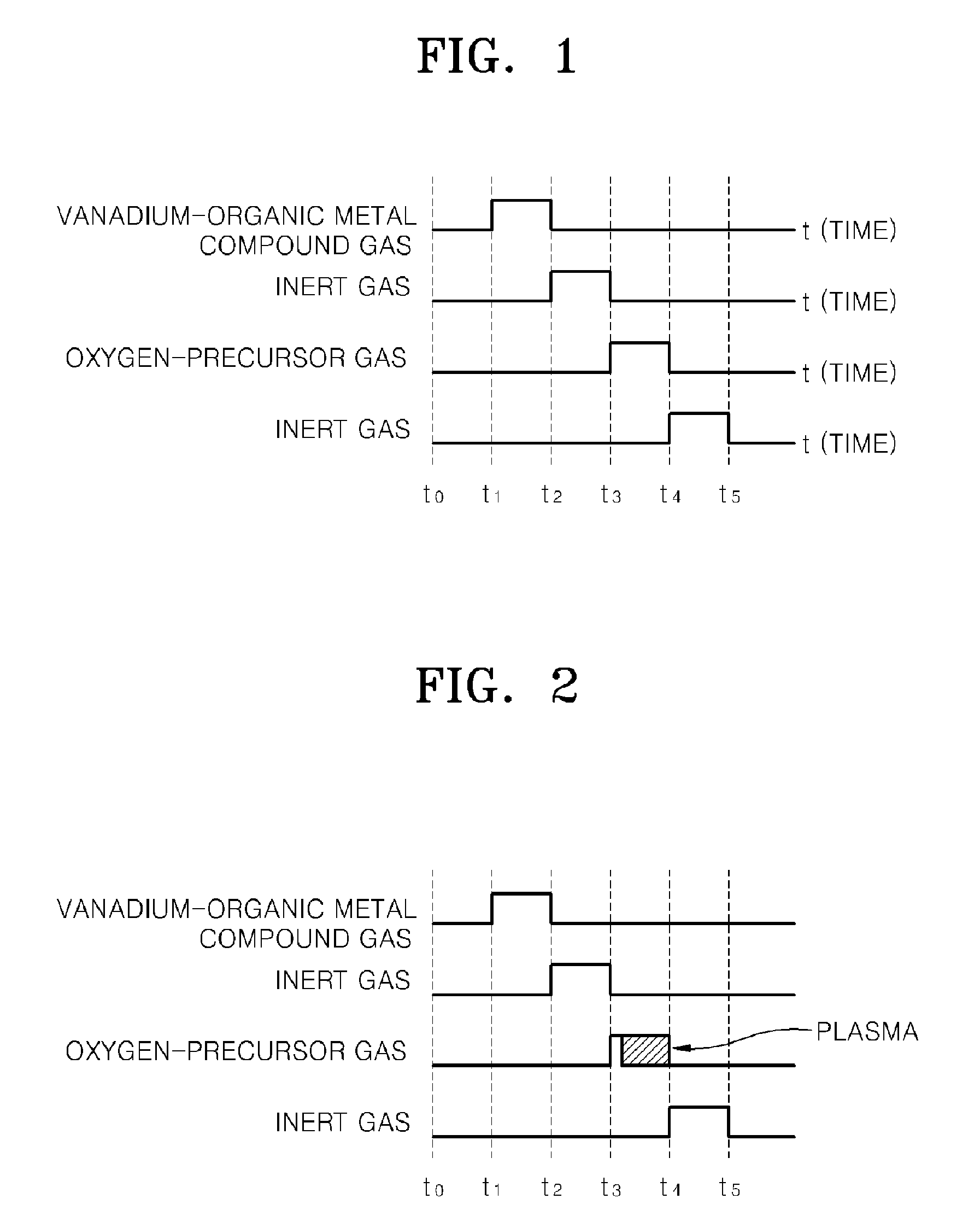
Provided is a method of manufacturing a large-sized vanadium oxide thin film having a uniform surface, uniform film thickness and stable composition. According to the method, a vanadium-organometallic compound gas is injected into a chamber to form adsorption layer where molecules of the vanadium-organometallic compound are adsorbed on the surface of a substrate. After that, an oxygen precursor is injected into the chamber and thus allowed to accomplish surface-saturation reaction with the adsorbed materials to fabricate a vanadium oxide thin film.
Owner:ELECTRONICS & TELECOMM RES INST
A kind of aluminum alloy material and preparation method thereof
The invention relates to an aluminum alloy material which is characterized in that the aluminum alloy material comprises the following components by weight percent: 0.16-1.2% of Fe, 0.001-0.8% of Cu, 0.001-0.8% of Mg, 0.001-0.8% of Zn, 0.001-0.8% of Ca, 0.001-1.0% of rare-earth elements, a trace amount of strontium, titanium, boron, nickel, chromium, zirconium, vanadium, beryllium, cobalt, lead, tin, bismuth, molybdenum, silver, indium, niobium and barium and the balance of aluminum. The alloy has excellent mechanical strength, processing performance and corrosion resistance and is suitable for the cable armored sheath.
Owner:GUANGDONG XINYI ALUMINUM ALLOY CABLE
Electroless plating processes
InactiveUS6861097B1Reducing problem encounteredSimple methodPaper/cardboard articlesDecorative surface effectsPolymeric surfaceOxidation state
The invention includes processes for combined polymer surface treatment and metal deposition. Processes of the invention include forming an aqueous solution containing a metal activator, such as an oxidized species of silver, cobalt, ruthenium, cerium, iron, manganese, nickel, rhodium, or vanadium. The activator can be suitably oxidized to a higher oxidation state electrochemically. Exposing a part to be plated (such as an organic resin, e.g. a printed circuit board substrate) to the solution enables reactive hydroxyl species (e.g. hydroxyl radicals) to be generated and to texture the polymer surface. Such texturing facilitates good plated metal adhesion. As part of this contacting process sufficient time is allowed for both surface texturing to take place and for the oxidized metal activator to adsorb onto said part. The part is then contacted with a reducing agent capable of reducing the metal activator to a lower ionic form, or a lower oxidation state. That reduction can result in the formation of metallic catalytic material over the surface of the part. The reduced metal activator can then function to catalyze the electroless deposition of metal such as copper from solution by contacting the part with the plating solution.
Owner:SHIPLEY CO LLC
Earth-boring bits
ActiveUS20050247491A1Low melting pointLowered melting point of the binder facilitates proper infiltration of the massDrill bitsCutting machinesBorideNiobium
The present invention relates to compositions and methods for forming a bit body for an earth-boring bit. The bit body may comprise hard particles, wherein the hard particles comprise at least one carbide, nitride, boride, and oxide and solid solutions thereof, and a binder binding together the hard particles. The binder may comprise at least one metal selected from cobalt, nickel, and iron, and, optionally, at least one melting point reducing constituent selected from a transition metal carbide in the range of 30 to 60 weight percent, boron up to 10 weight percent, silicon up to 20 weight percent, chromium up to 20 weight percent, and manganese up to 25 weight percent, wherein the weight percentages are based on the total weight of the binder. In addition, the hard particles may comprise at least one of (i) cast carbide (WC+W2C) particles, (ii) transition metal carbide particles selected from the carbides of titanium, chromium, vanadium, zirconium, hafnium, tantalum, molybdenum, niobium, and tungsten, and (iii) sintered cemented carbide particles.
Owner:BAKER HUGHES INC +1
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS20010028977A1Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
Earth-boring bits
InactiveUS20050211475A1Low melting pointLowered melting point of the binder facilitates proper infiltration of the massDrill bitsMetal-working drilling toolsBorideNiobium
The present invention relates to compositions and methods for forming a bit body for an earth-boring bit. The bit body may comprise hard particles, wherein the hard particles comprise at least one carbide, nitride, boride, and oxide and solid solutions thereof, and a binder binding together the hard particles. The binder may comprise at least one metal selected from cobalt, nickel, and iron, and at least one melting point reducing constituent selected from a transition metal carbide in the range of 30 to 60 weight percent, boron up to 10 weight percent, silicon up to 20 weight percent, chromium up to 20 weight percent, and manganese up to 25 weight percent, wherein the weight percentages are based on the total weight of the binder. In addition, the hard particles may comprise at least one of (i) cast carbide (WC+W2C) particles, (ii) transition metal carbide particles selected from the carbides of titanium, chromium, vanadium, zirconium, hafnium, tantalum, molybdenum, niobium, and tungsten, and (iii) sintered cemented carbide particles.
Owner:ATI PROPERTIES +1
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
Infrared reflective color pigment
InactiveUS6454848B2Reduce heat buildupReduce energy costsInorganic pigment treatmentCoatingsIndiumCobalt
The present invention provides new solid solutions having a corundum-hematite crystalline structure which are useful as inorganic color pigments. Solid solutions according to the present invention include a host component having a corundum-hematite crystalline structure which contains as guest components one or more elements from the group consisting of aluminum, antimony, bismuth, boron, chrome, cobalt, gallium, indium, iron, lanthanum, lithium, magnesium, manganese, molybdenum, neodymium, nickel, niobium, silicon, tin, titanium, vanadium, and zinc. Solid solutions according to the present invention are formed by thoroughly mixing compounds, usually metal oxides or precursors thereof, which contain the host and guest components and then calcining the compounds to form the solid solutions having the corundum-hematite crystalline structure. Some of the new solid solutions according to the present invention exhibit relatively low Y CIE tri-stimulus values and relatively high near infrared reflectance.
Owner:FERRO CORP
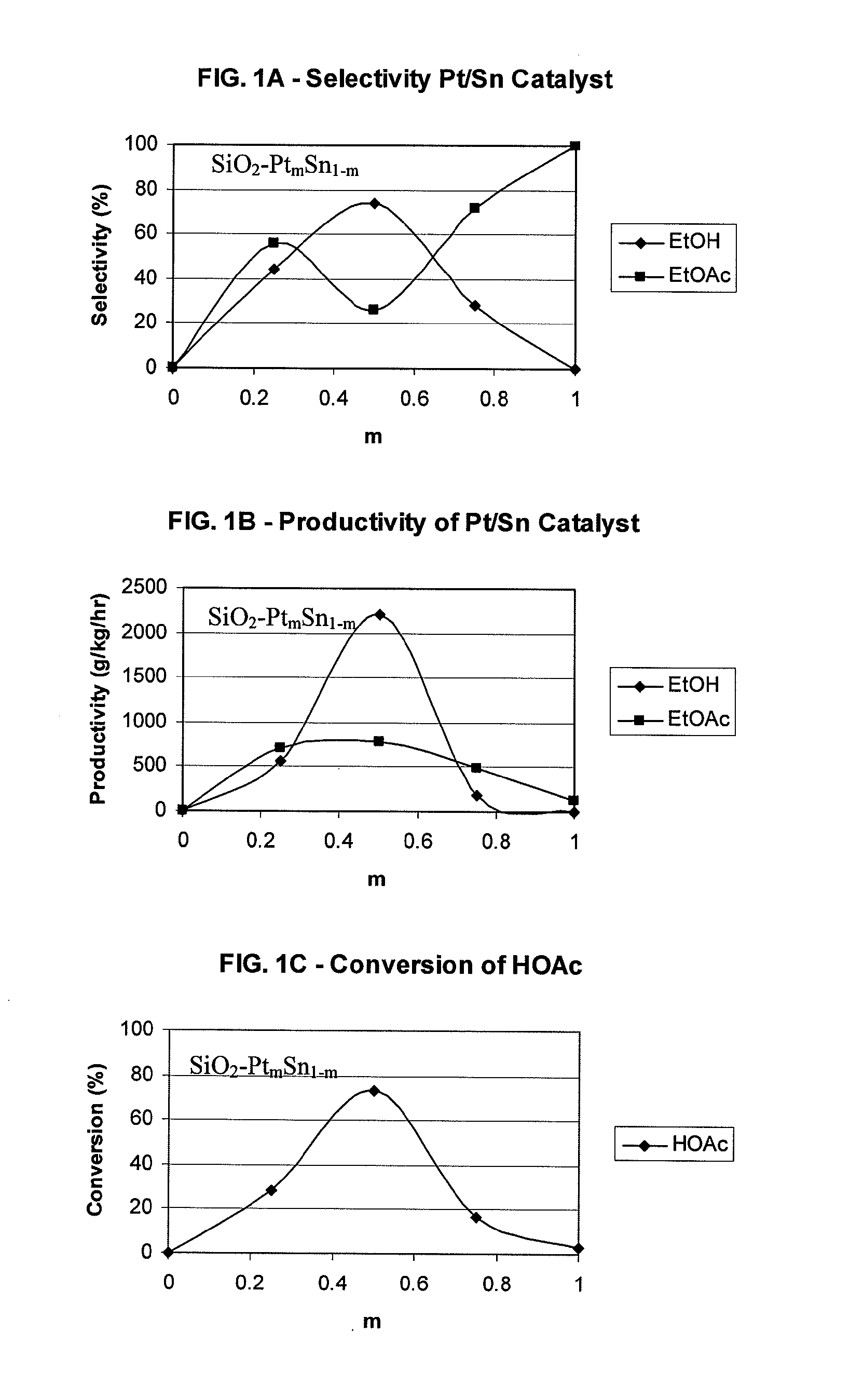
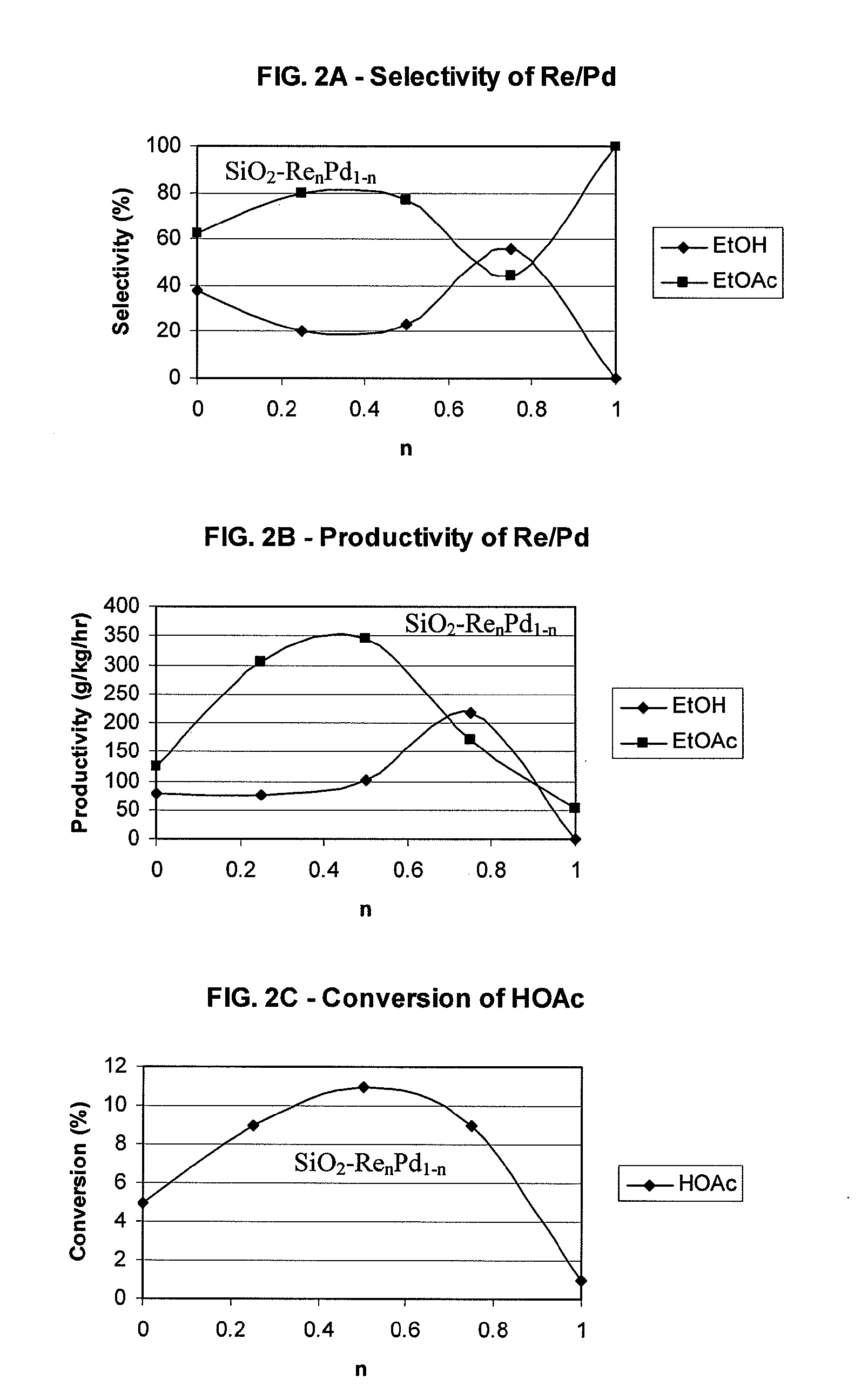
Processes for making ethanol from acetic acid
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
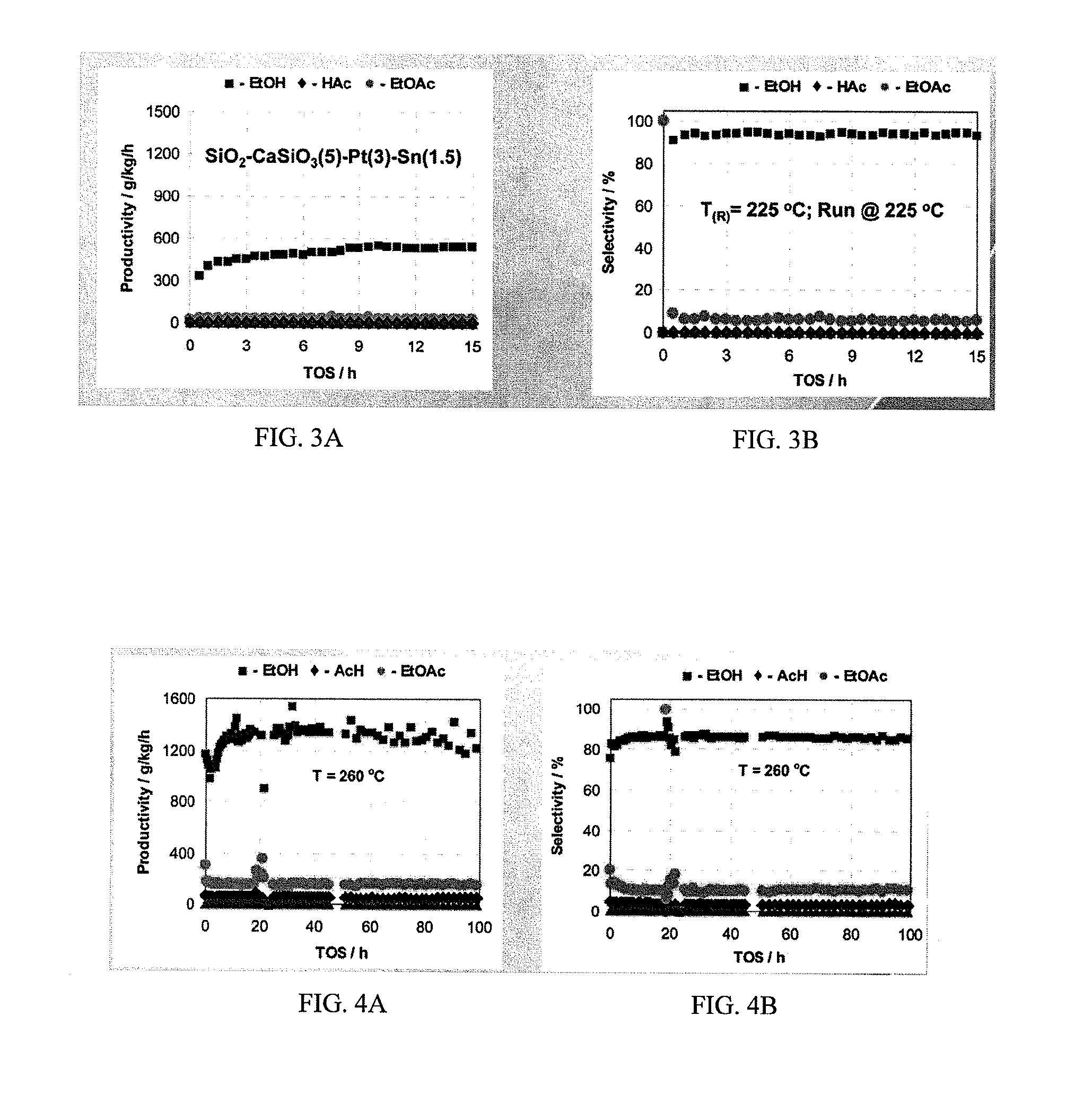
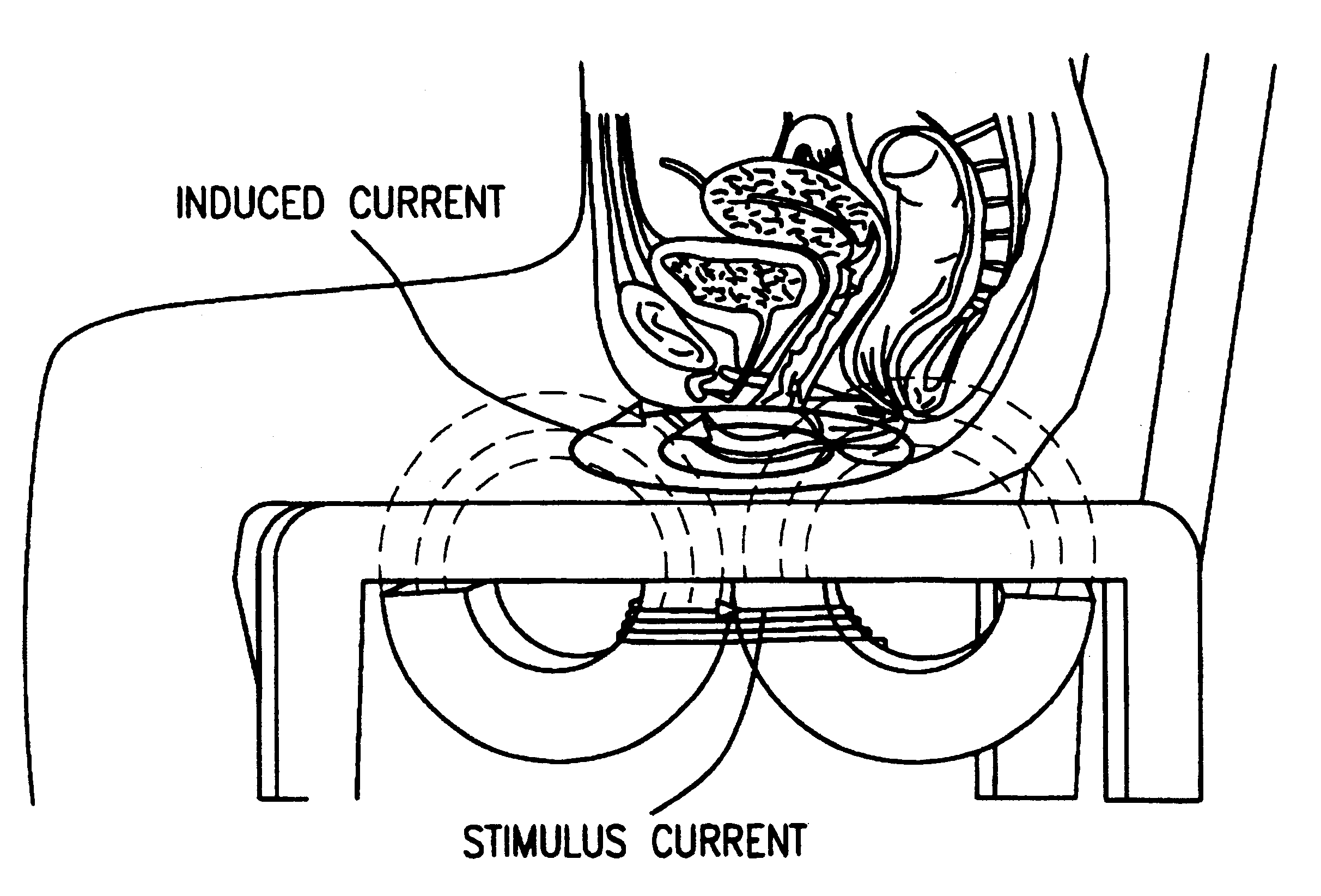
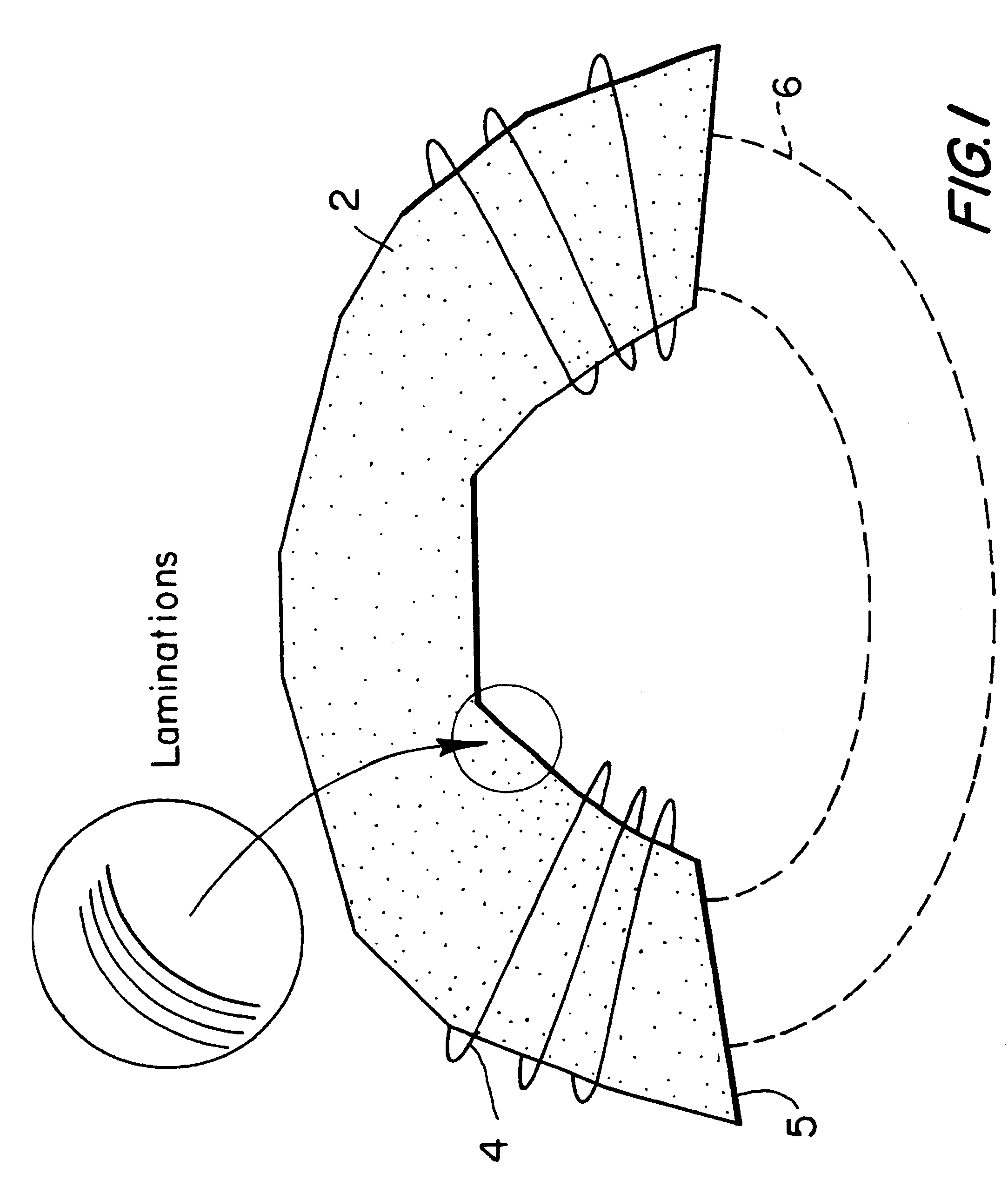
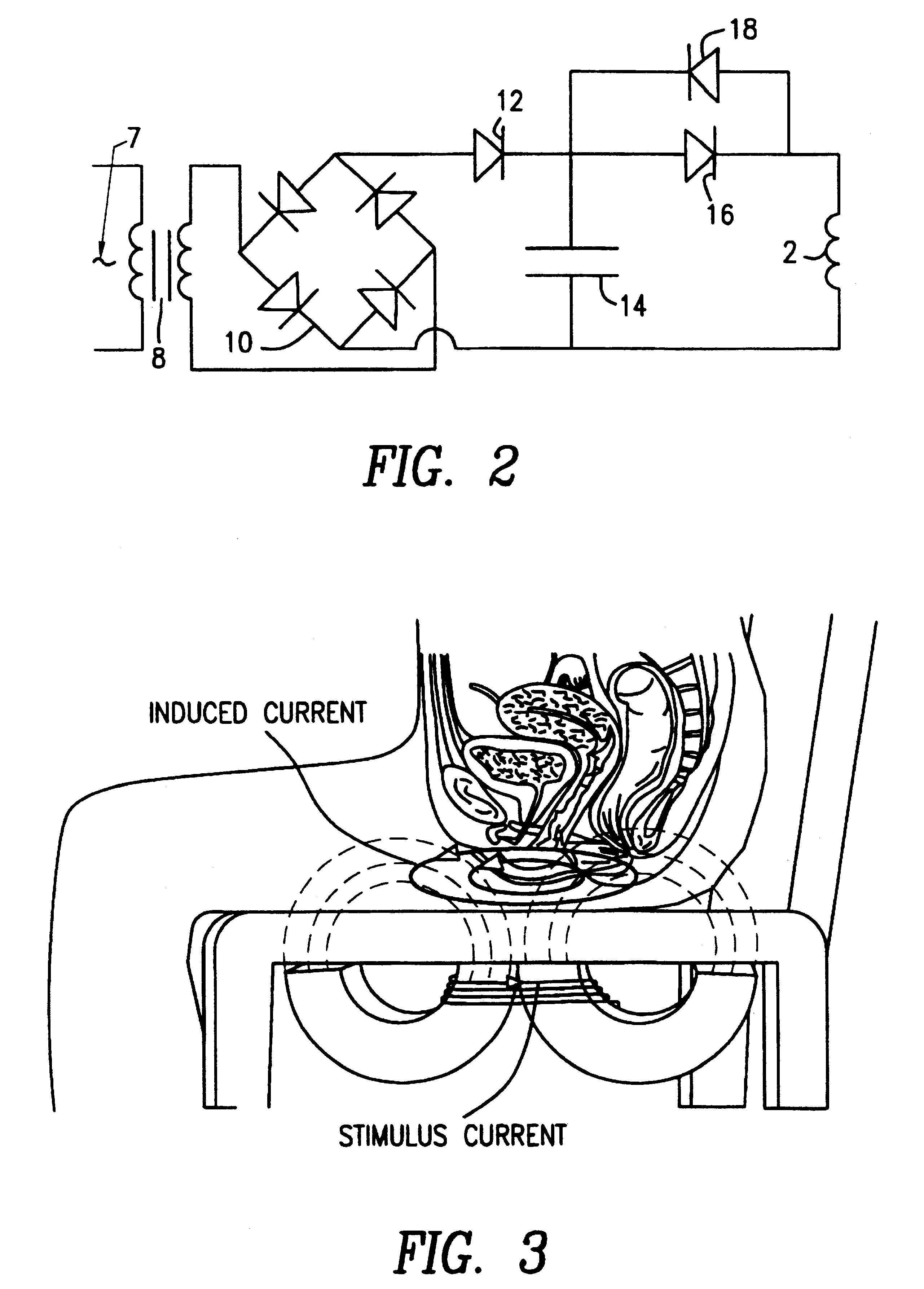
Magnetic nerve stimulation seat device
A magnetic nerve stimulator system is comprised of a core constructed from a material having a high field saturation with a coil winding. A thyrister capacitive discharge circuit pulses the device. A rapidly changing magnetic field is guided by the core, preferably vanadium permendur. For task specific excitation of various nerve groups, specially constructed cores allow for excitation of nerves at deeper levels with higher efficiency than is possible with air-core stimulators. Among the applications possible with this invention are treatment of incontinence, rehabilitation of large muscle groups in the leg and arm, and excitation of abdominal wall muscle groups to aid in weight loss and metabolic rate increase. A C-shape is employed for focussing the stimulation as desired.
Owner:MAGIC RACE
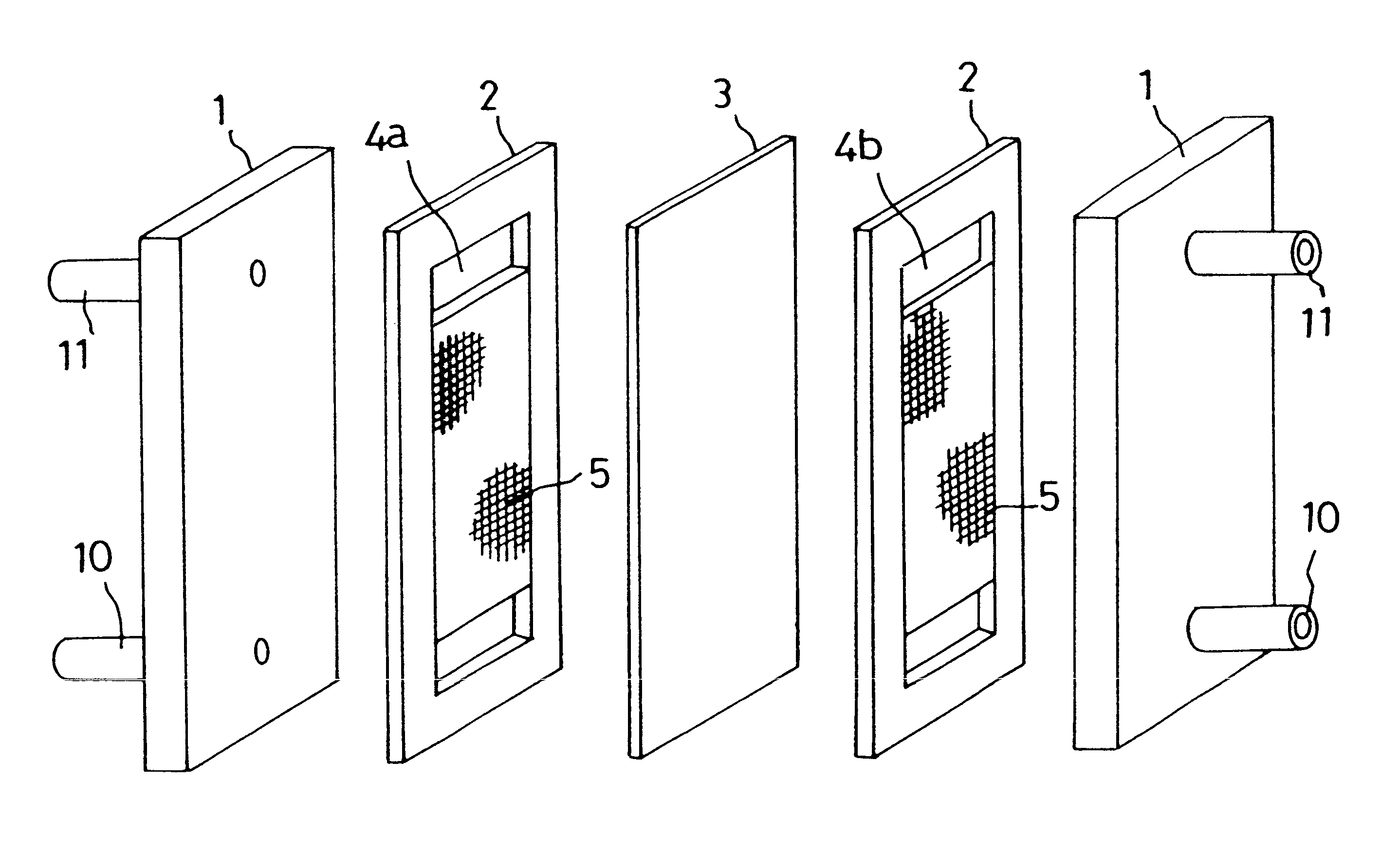
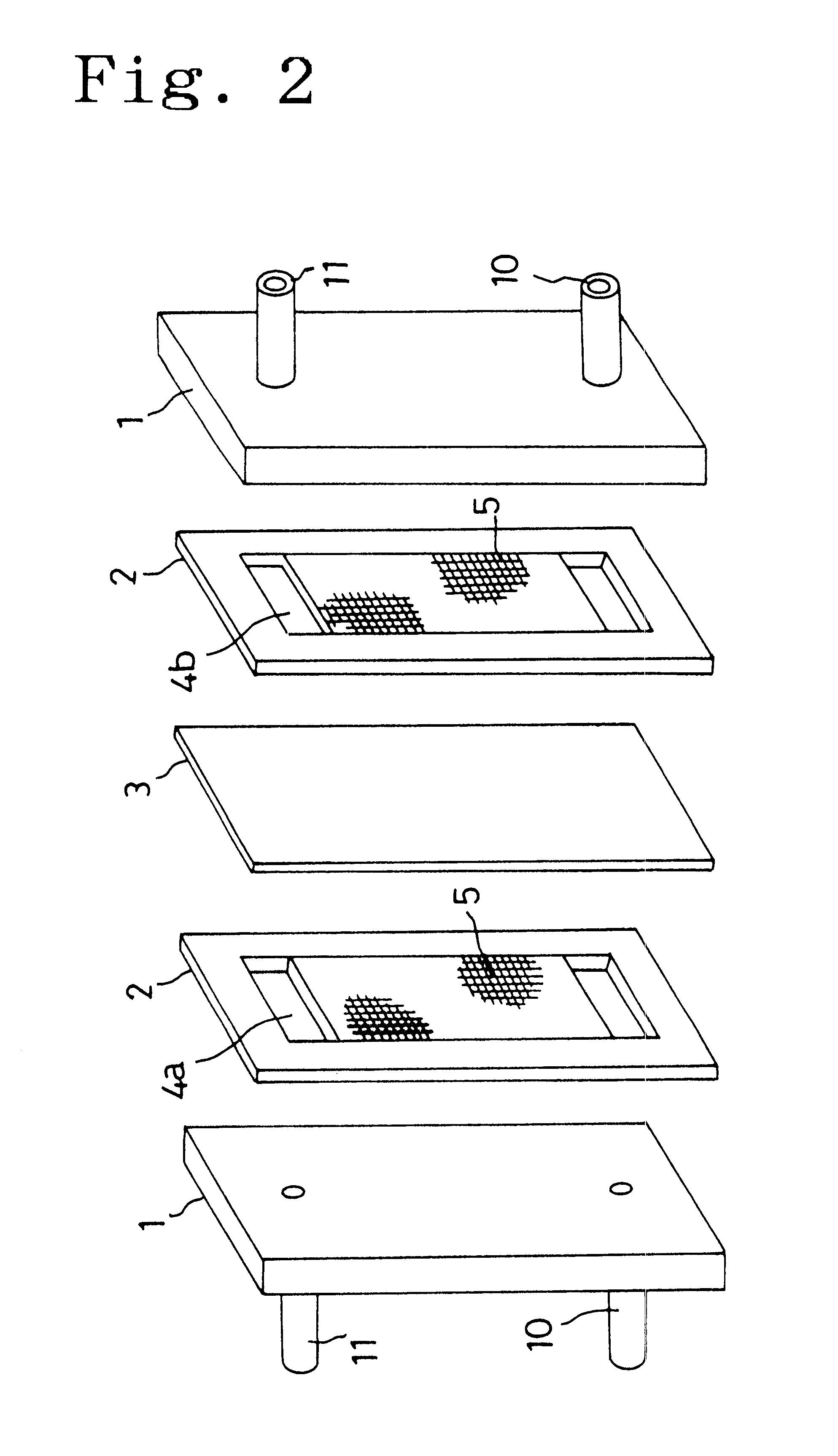
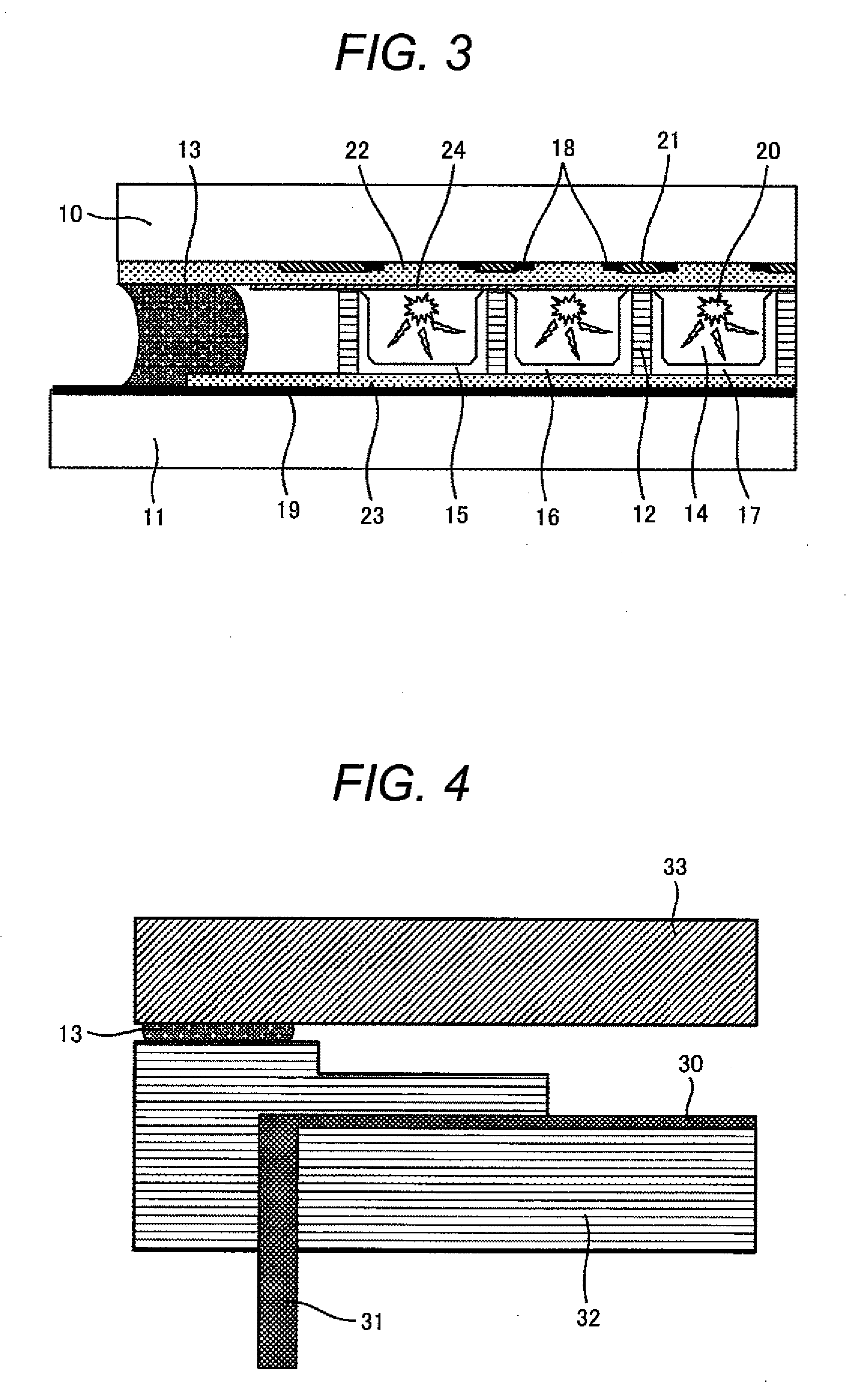
Carbon electrode material for a vanadium-based redox-flow battery
The carbon electrode material of the present invention is used for a vanadium redox-flow cell. The carbon electrode material has quasi-graphite crystal structure in which <002> spacing obtained by X-ray wide angle analysis is 3.43 to 3.60 Å, size of a crystallite in c axial direction is 15 to 33 Å and size of crystallite in a axial direction is 30 to 70 Å. In addition, an amount of surface acidic functional groups obtained by XPS surface analysis is 0.1 to 1.2% and total number of surface bound-nitrogen atoms is 5% or smaller relative to total number of surface carbon atoms. The carbon electrode materials formed of a non-woven fabric of a carbonaceouss fiber is preferable.
Owner:TOYO TOYOBO CO LTD
Transition metal complexes with (pyridyl)imidazole ligands
InactiveUS7074308B2Rapid electron exchangeFast dynamicsImmobilised enzymesBioreactor/fermenter combinationsOxidation-Reduction AgentRedox
Novel transition metal complexes of iron, cobalt, ruthenium, osmium, and vanadium are described. The transition metal complexes can be used as redox mediators in enzyme-based electrochemical sensors. The transition metal complexes include substituted or unsubstituted (pyridyl)imidazole ligands. Transition metal complexes attached to polymeric backbones are also described.
Owner:ABBOTT DIABETES CARE INC
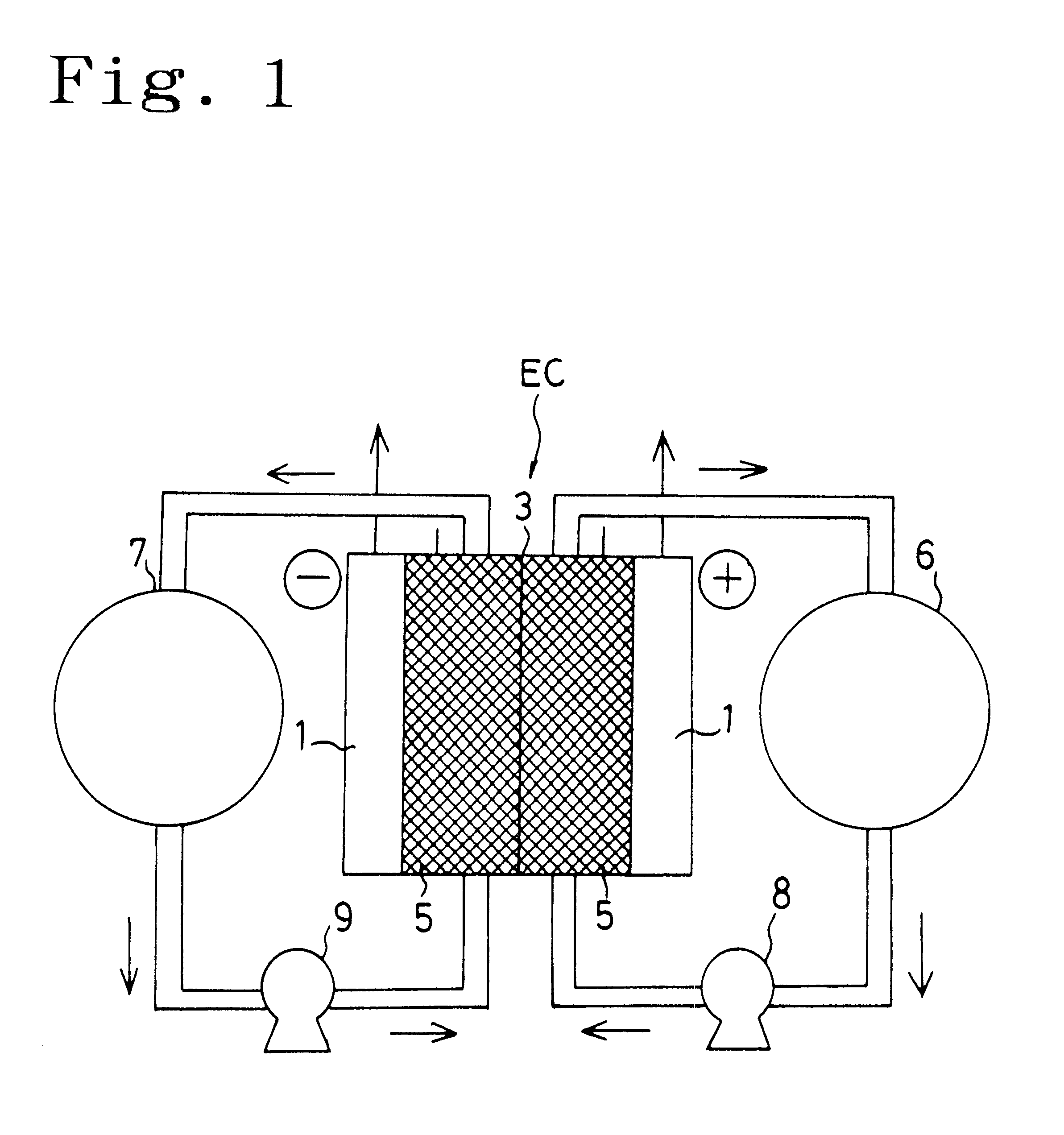
Redox flow battery
InactiveUS6764789B1Reduce capacityReduce frequencyElectrolyte holding meansCell electrodesRedoxAqueous solution
The present invention provides a redox flow type battery which a liquid-circulating battery comprising a battery cell and storage tanks for positive and negative electrolytes, wherein the battery cell is separated by a membrane to provide a positive cell and a negative cell, each cell having a liquid-permeable porous electrode disposed therein, wherein the positive and negative electrolytes are sulfuric acid aqueous solutions with vanadium ion concentrations of 0.5 mol / l to 8 mol / l and the electrolyte which migrates through the membrane over cycles of charge and discharge is returned from the storage tank where the liquid increases to the storage tank where the liquid decreases in order to keep the change in the amounts of the positive and negative electrolytes in a certain range while charge and discharge are carried out.
Owner:SUMITOMO ELECTRIC IND LTD
Piston ring
InactiveUS6325385B1Good sliding propertiesRelieve pressurePiston ringsBraking action transmissionCarbon filmDiamond-like carbon
Owner:TEIKOKU PISTON RING CO LTD
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
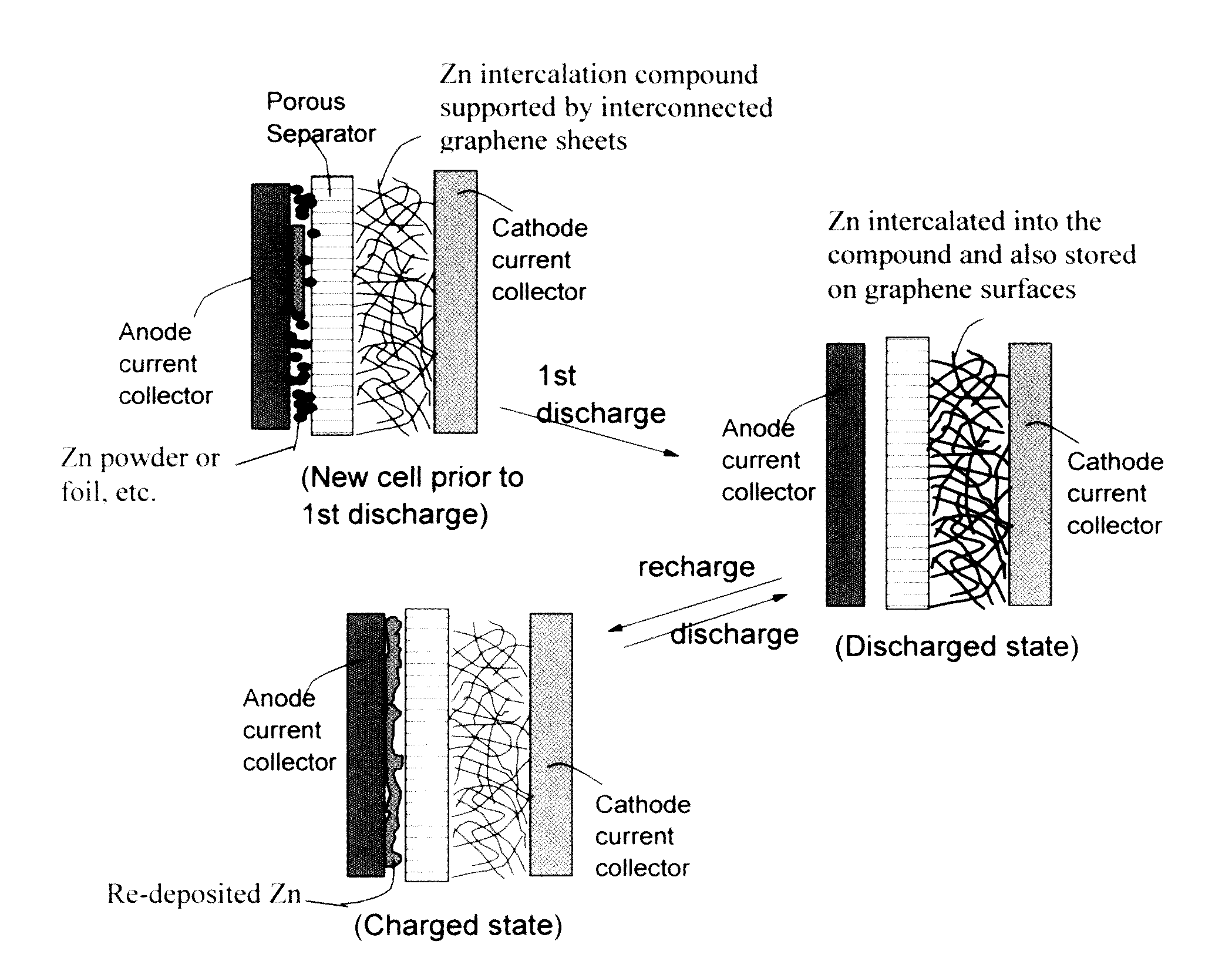
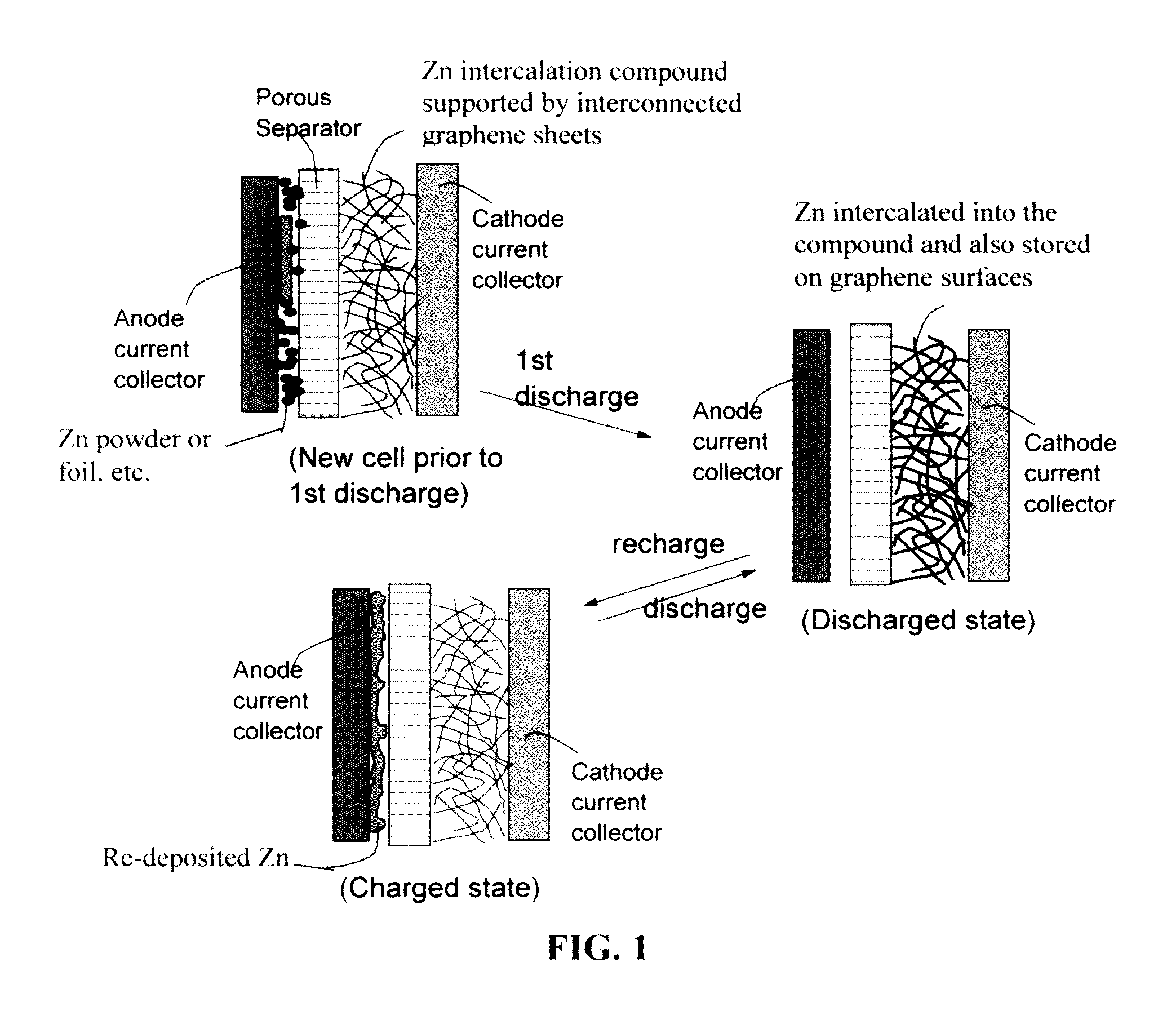
Zinc Ion-Exchanging Energy Storage Device
ActiveUS20160301096A1Quick releaseRapid depositionHybrid capacitor electrolytesAlkaline accumulatorsChemical treatmentZinc metal
A zinc ion-exchanging battery device comprising: (A) a cathode comprising two cathode active materials (a zinc ion intercalation compound and a surface-mediating material); (B) an anode containing zinc metal or zinc alloy; (C) a porous separator disposed between the cathode and the anode; and (D) an electrolyte containing zinc ions that are exchanged between the cathode and the anode during battery charge / discharge. The zinc ion intercalation compound is selected from chemically treated carbon or graphite material having an expanded inter-graphene spacing d002 of at least 0.5 nm, or an oxide, carbide, dichalcogenide, trichalcogenide, sulfide, selenide, or telluride of niobium, zirconium, molybdenum, hafnium, tantalum, tungsten, titanium, vanadium, chromium, cobalt, manganese, iron, nickel, or a combination thereof. The surface-mediating material contains exfoliated graphite or multiple single-layer sheets or multi-layer platelets of a graphene material.
Owner:GLOBAL GRAPHENE GRP INC
Al-Cu-Li-Mg-Ag-Mn-Zr alloy for use as structural members requiring high strength and high fracture toughness
An improved aluminum lithium alloy comprising 0.1 to 2.5 wt. % Li, 2.5 to 5.5 wt. % Cu, 0.2 to 1.0 wt. % Mg, 0.2 to 0.8 wt. % Ag, 0.2 to 0.8 wt. % Mn, up to 0.4 wt. % Zr or other grain refiner such as chromium, titanium, hafnium, scandium or vanadium, the balance aluminum. The present alloy exhibits an improved combination of strength and fracture toughness, over any thickness range. The present invention is further directed to methods for preparing and using Al—Li alloys as well as to products comprising the same.
Owner:CONSTELLIUM ROLLED PROD RAVENSWOOD
High temperature aluminum alloys
High temperature aluminum alloys that can be used at temperatures from about −420° F. (−251° C.) up to about 650° F. (343° C.) are described herein. These alloys comprise aluminum; scandium; at least one of nickel, iron, chromium, manganese and cobalt; and at least one of zirconium, gadolinium, hafnium, yttrium, niobium and vanadiuim. These alloys comprise an aluminum solid solution matrix and a mixture of various dispersoids. These alloys are substantially free of magnesium.
Owner:RTX CORP
Production of multimodal polythylene
A process for the preparation of polyethylene resins having a multimodal molecular weight distribution which comprises:(i) contacting ethylene monomer and a comonomer comprising an alpha-olefin having from 3 to 10 carbon atoms with a first catalyst system in a first reactor under first polymerisation conditions to produce a first polyethylene having a first molecular weight, an HLMI of not more than 0.5 g / 10 min and a first density of not more than 0.925 g / ml and the first catalyst system comprising (a) a metallocene catalyst comprising a bis tetrahydroindenyl compound of the general formula (IndH4)2R''MQ2 in which each Ind is the same or different and is indenyl or substituted indenyl, R'' is a bridge which comprises a C1-C20 alkylene radical, a dialkyl germanium or silicon or siloxane, or an alkyl phosphine or amine radical, which bridge is substituted or unsubstituted, M is a Group IVB transition metal or vanadium and each Q is hydrocarbyl having 1 to 20 carbon atoms or halogen; and (b) a cocatalyst which activates the catalyst component;(ii) providing a second polyethylene having a second lower molecular weight and second higher density than the first polyethylene, the second polyethylene having been produced using a catalyst other than the bis tetrahydroindenyl compound; and(iii) mixing together the first and second polyethylenes to form a polyethylene resin having a multimodal molecular weight distribution.
Owner:FINA RES SA
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS6468688B2Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
Lithium titanate composite electrode material with surface coating layer
InactiveCN101764209AChange physical propertiesChange chemical propertiesCell electrodesMagnesium phosphateMagnesium orthophosphate
The invention relates to a battery electrode material, in particular to a lithium titanate composite electrode material with surface coating layer; in the lithium titanate composite electrode material with surface coating layer, the electrode material is composed of lithium titanate particles and a coating layer coated with the surface of the lithium titanate particles; the particle size of the lithium titanate particles is 100nm-95mum, the average thickness of the surface coating layer is 0.2nm-5m, and the particle diameter of the composite electrode material is 0.1-100mum; the material of the surface coating layer is one or mixture of more than one kind of insulation oxide, insulation composite oxide, aluminium phosphate, magnesium phosphate, lithium fluoride, lithium phosphate or LiMPO4, wherein M is magnesium, ferrum, cobalt, nickel, chromium, titanium or vanadium; in the invention, by carrying out surface coating treatment to the surfaces of the existing lithium titanate particles, a layer of protective film is formed on the surface, so as to change the physical and chemical characteristics of the surface of the lithium titanate active material, the surface can not be reacted with electrolyte even if under overpotential condition, so as to avoid ballooning and ensure the capacity and the circularity of the battery not to be reduced.
Owner:SUZHOU PHYLION BATTERY
Processing of titanium-aluminum-vanadium alloys and products made thereby
InactiveUS20040221929A1Expensive to produceHigh energy input requirementMetal rolling arrangementsNitrogenTitanium
A method of forming an article from an alpha-beta titanium including, in weight percentages, from about 2.9 to about 5.0 aluminum, from about 2.0 to about 3.0 vanadium, from about 0.4 to about 2.0 iron, from about 0.2 to about 0.3 oxygen, from about 0.005 to about 0.3 carbon, from about 0.001 to about 0.02 nitrogen, and less than about 0.5 of other elements. The method comprises cold working the alpha-beta titanium alloy.
Owner:ATI PROPERTIES
Process for BTX purification
InactiveUS6500996B1Lowering indexEfficient executionOrganic chemistry methodsTreatment with plural serial refining stagesCobaltChromium
A process for the removal of hydrocarbon contaminants, such as dienes and olefins, from an aromatics reformate by contacting an aromatics reformate stream with a hydrotreating catalyst and / or a molecular sieve. The hydrotreating catalyst substantially converts all dienes to oligomers and partially converts olefins to alkylaromatics. The molecular sieve converts the olefins to alkylaromatics. The process provides an olefin depleted product which can be passed through a clay treater to substantially convert the remaining olefins to alkylaromatics. The hydrotreating catalyst has a metal component of nickel, cobalt, chromium, vanadium, molybdenum, tungsten, nickel-molybdenum, cobalt-nickel-molybdenum, nickel-tungsten, cobalt-molybdenum or nickel-tungsten-titanium, with a nickel molybdenum / alumina catalyst being preferred. The molecular sieve is an intermediate pore size zeolite, preferably MCM-22. The clay treatment can be carried out with any clay suitable for treating hydrocarbons.
Owner:EXXONMOBIL CORP (US)
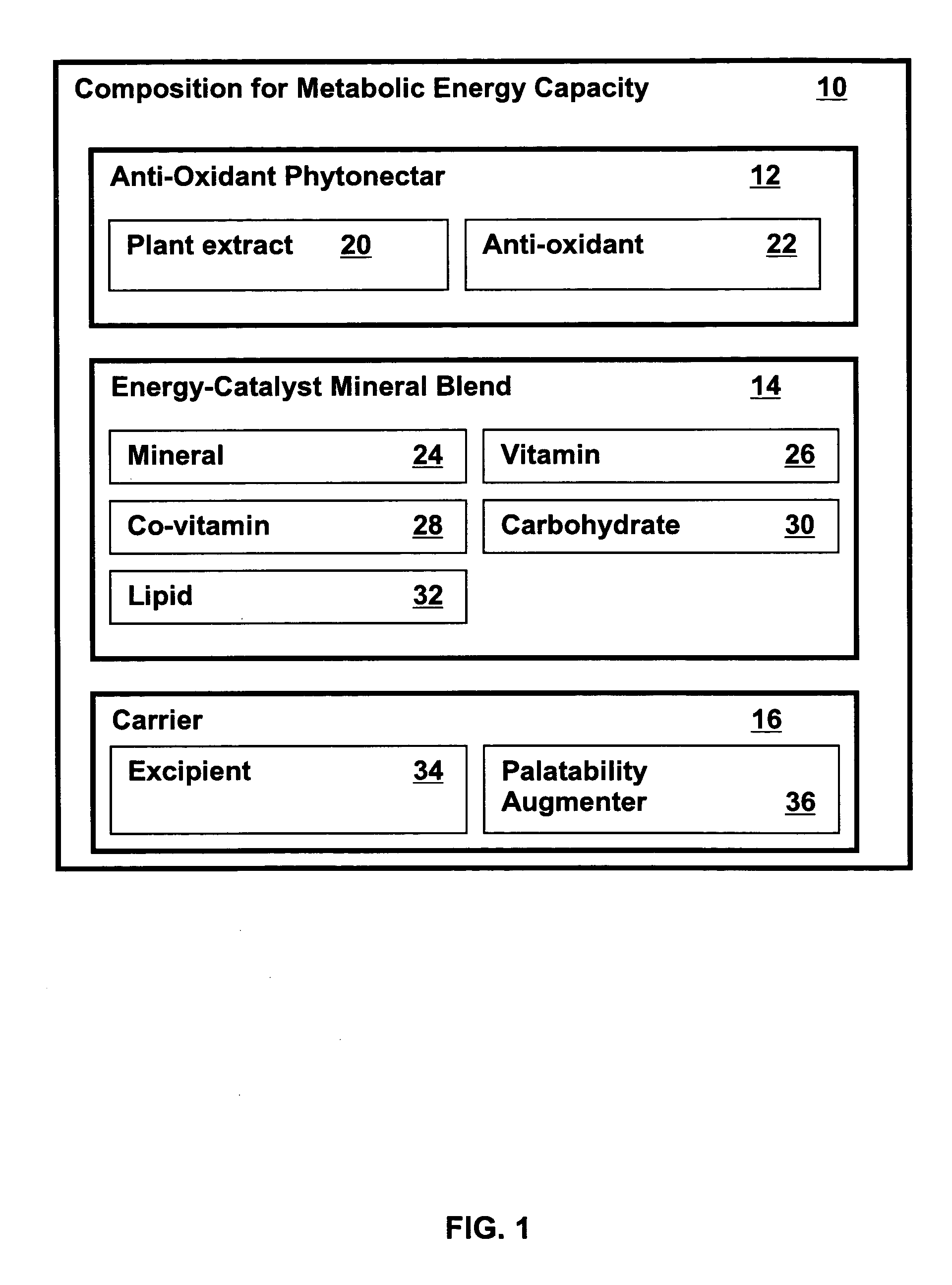
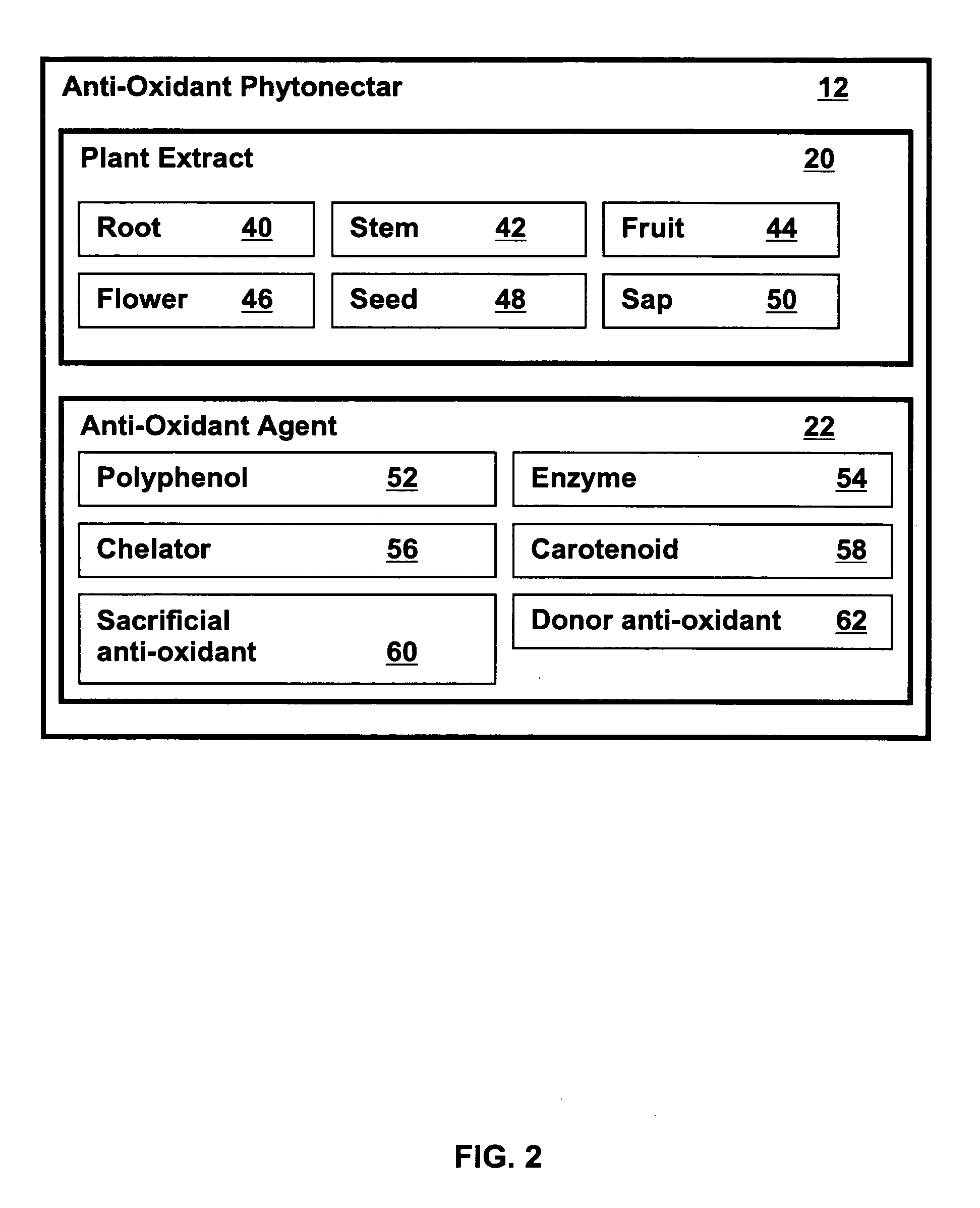
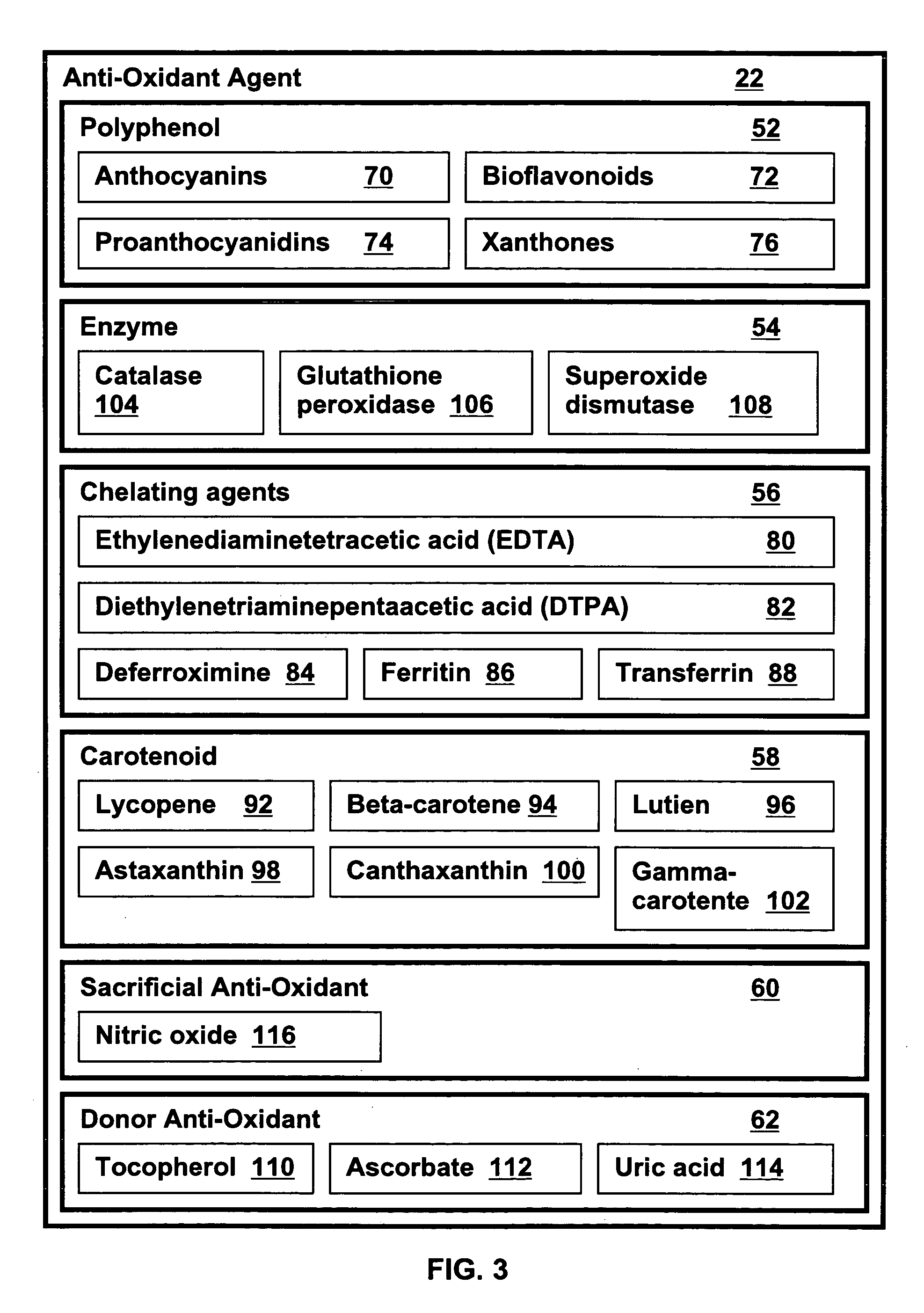
Metabolic capacity enhancing compositions and methods for use in a mammal
InactiveUS20060024385A1Increase vitalityImproved and increased energy processing of given caloric foods)BiocideOrganic active ingredientsLipid formationMammal
Metabolic energy capacity enhancing compositions and methods for reducing oxidative stress and improving vitality in a mammal are disclosed. A composition for increasing metabolic energy capacity may be in a palatable liquid formulation or a solid dosage form and typically includes an anti-oxidant containing phytonectar and an energy catalyst. An anti-oxidant may include a polyphenol, anthrocyanin, bioflavonoid, proanthocyanidin, and a xanthone. An energy catalyst may include a mineral, vitamin, co-vitamin, carbohydrate and a lipid. In a presently preferred embodiment a composition includes phytonectar extracts from grape, aloe vera, apple, morinda citrifolia, scullcap, blueberry, prune, cranberry, elderberry, bilberry, and gentain and a mineral blend containing calcium, magnesium, manganese, zinc, chromium, selenium, iron, copper, molybdenum, vanadium, potassium, iodine, and cobalt. A method for increasing metabolic energy capacity in a mammal may include consuming a chemical component having the ability to undergo oxidation, producing free radicals and administering a composition having an anti-oxidant containing phytonectar and an energy catalyst.
Owner:PEDERSEN MARK A
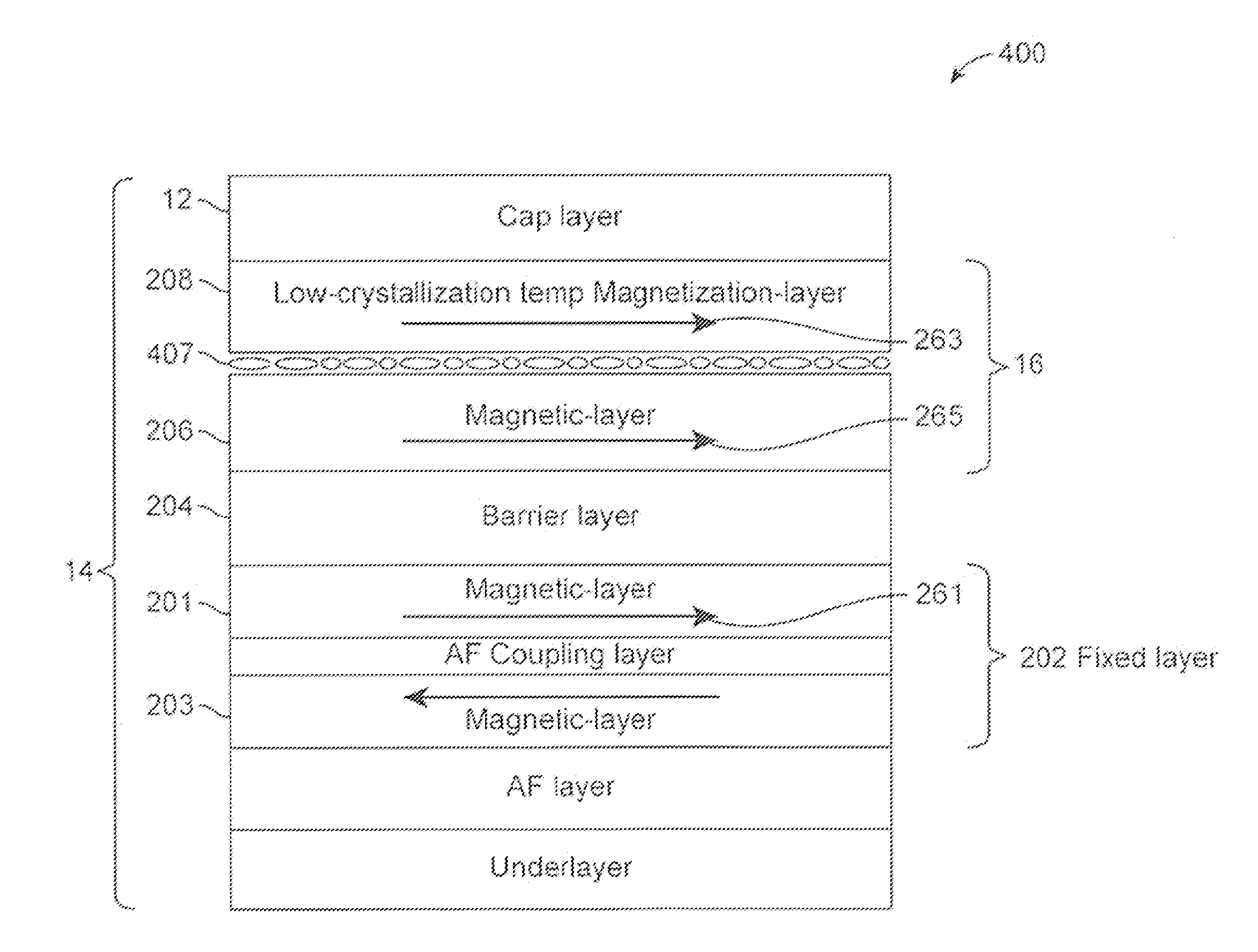
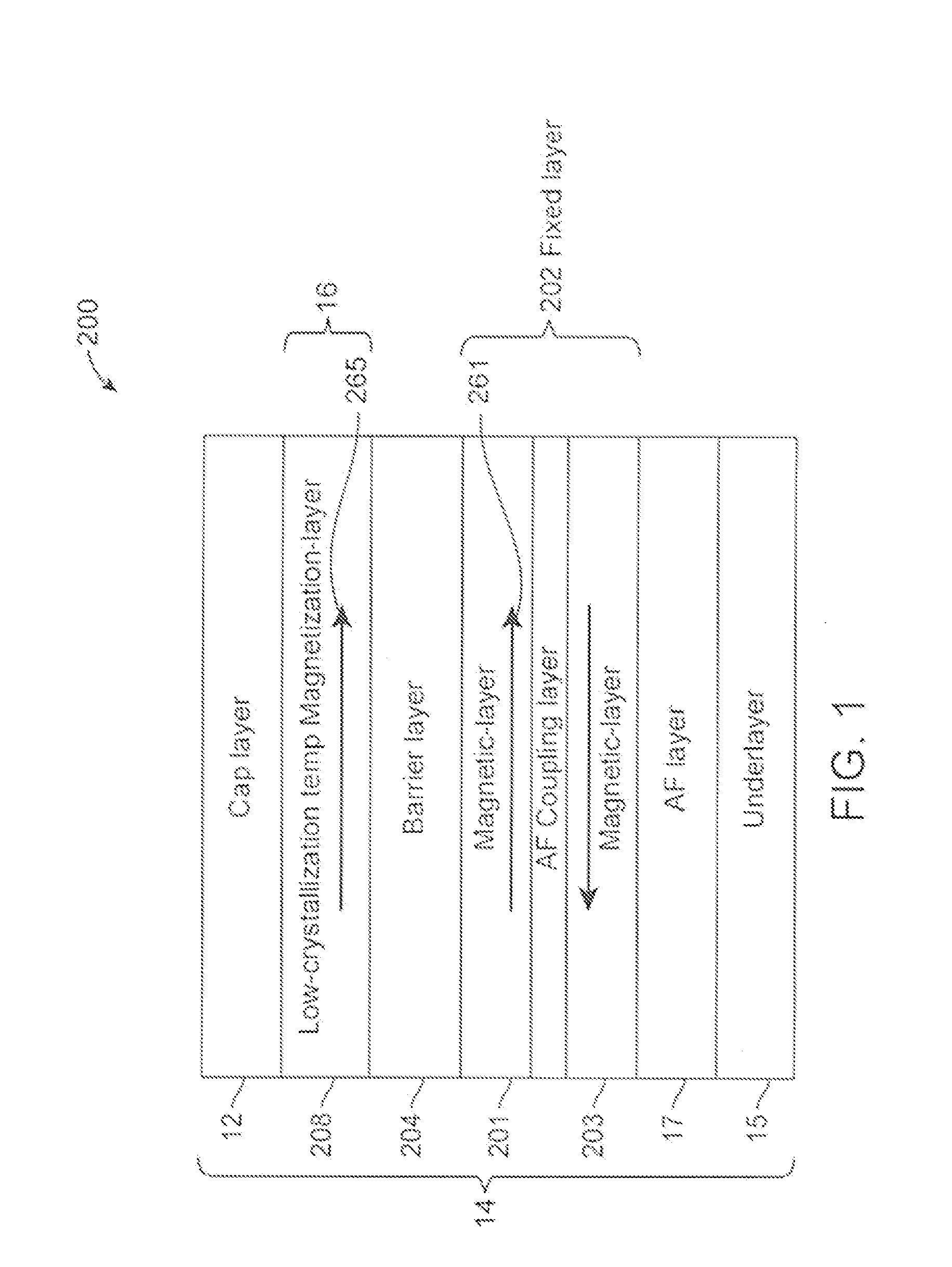
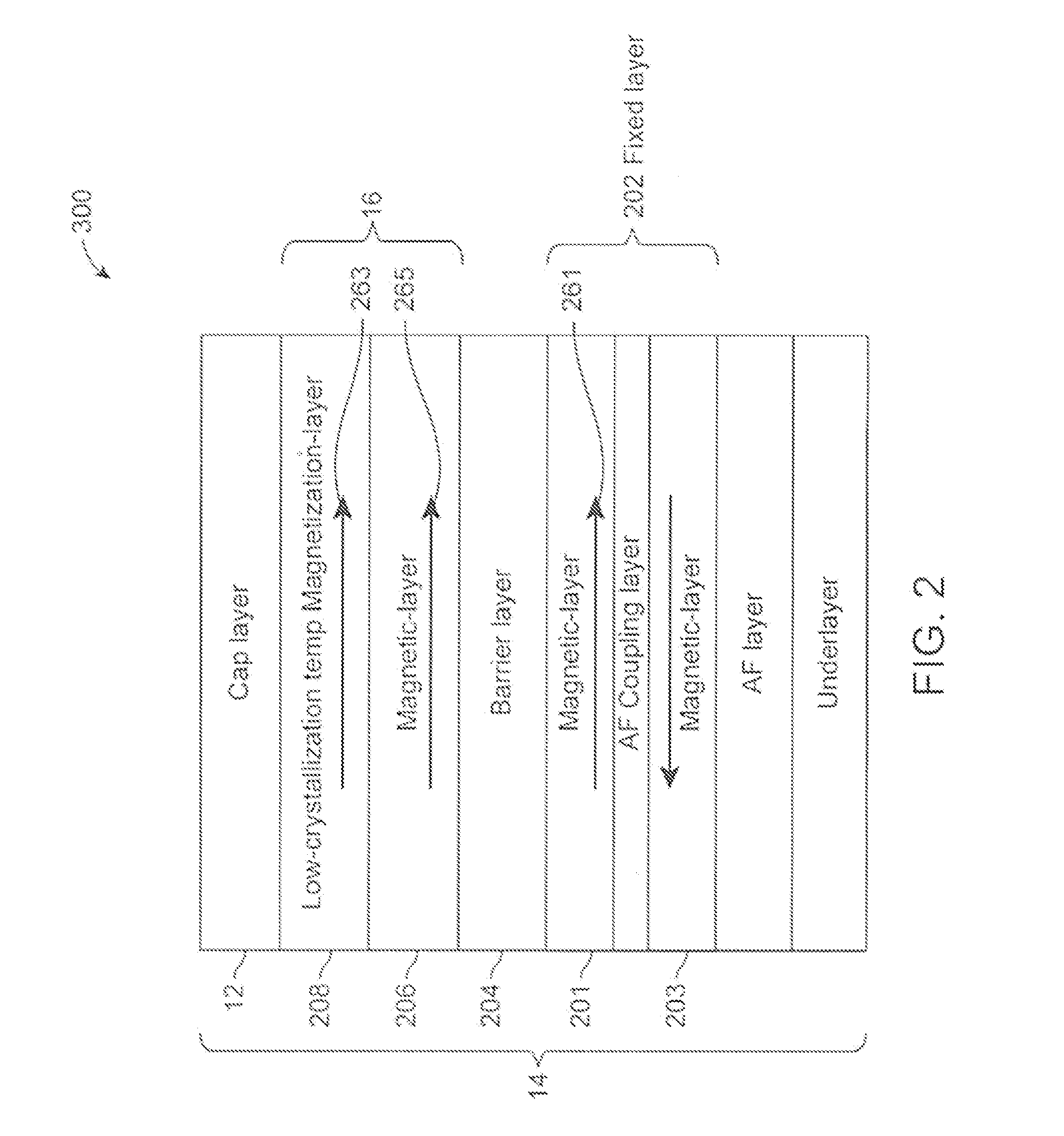
Low-crystallization temperature MTJ for Spin-Transfer Torque Magnetic Random Access Memory (STTMRAM)
A spin-torque transfer memory random access memory (STTMRAM) element is disclosed and has a fixed layer, a barrier layer formed upon the fixed layer, and a free layer comprised of a low-crystallization temperature alloy of CoFeB—Z where Z is below 25 atomic percent of one or more of titanium, (Ti), yittrium (Y), zirconium (Zr), and vanadium (V), wherein during a write operation, a bidirectional electric current is applied across the STTMRAM element to switch the magnetization of the free layer between parallel and anti-parallel states relative to the magnetization of the fixed layer.
Owner:AVALANCHE TECH
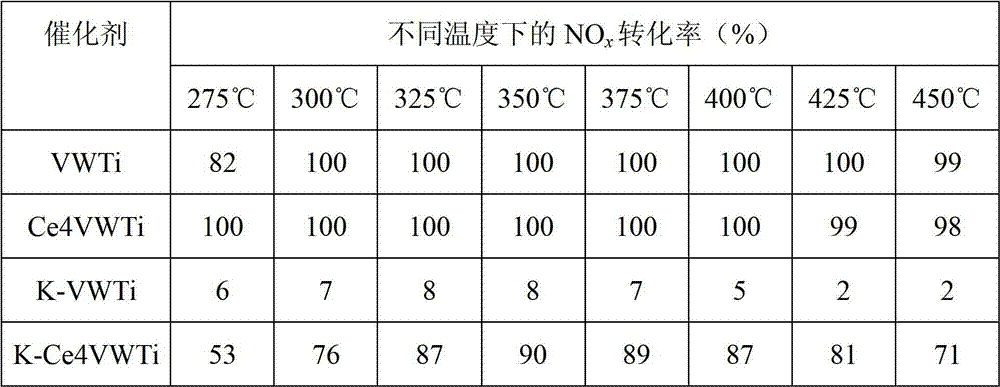
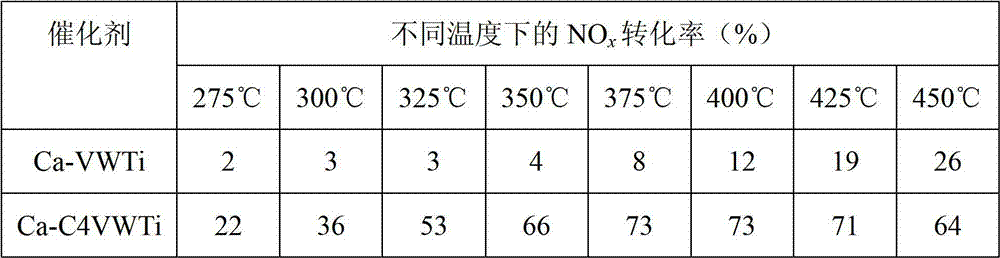
Vanadium-titanium oxide catalyst, and preparation method and application thereof
InactiveCN102764643ANo effect on activityDo not change the production processDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsMetal poisoningAlkaline earth metal
The invention relates to a vanadium-titanium oxide catalyst which can resist alkaline metal and alkaline earth metal poisoning. The catalyst is characterized in that the vanadium-titanium oxide catalyst is doped with an element Ce. The catalyst has fine resistance to alkaline metal poisoning, and above all, the doped cerium component has no influence on the activity of the SCR (selective catalytic reduction) catalyst while improving the resistance of the V2O5 / (MoO3)x(WO3)1-x-TiO2 catalyst to alkaline metal poisoning. The preparation method of the catalyst is simple and easy to implement and has very excellent N2 generation selectivity; and meanwhile, the Ce is a non-poisonous component and can not do harm to human health and ecological environment.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Semi-insulating silicon carbide without vanadium domination
InactiveUS6396080B2High-frequency operationReduce complicationsPolycrystalline material growthAfter-treatment detailsDevice formRoom temperature
A semi-insulating bulk single crystal of silicon carbide is disclosed that has a resistivity of at least 5000 OMEGA-cm at room temperature and a concentration of trapping elements that create states at least 700 meV from the valence or conduction band that is below the amounts that will affect the resistivity of the crystal, preferably below detectable levels. A method of forming the crystal is also disclosed, along with some resulting devices that take advantage of the microwave frequency capabilities of devices formed using substrates according to the invention.
Owner:CREE INC
Low softening point glass composition, bonding material using same and electronic parts
ActiveUS20100180934A1Flow on effectReduce softeningAddress electrodesConductive layers on insulating-supportsTe elementAntimony
Owner:RESONAC CORP
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com