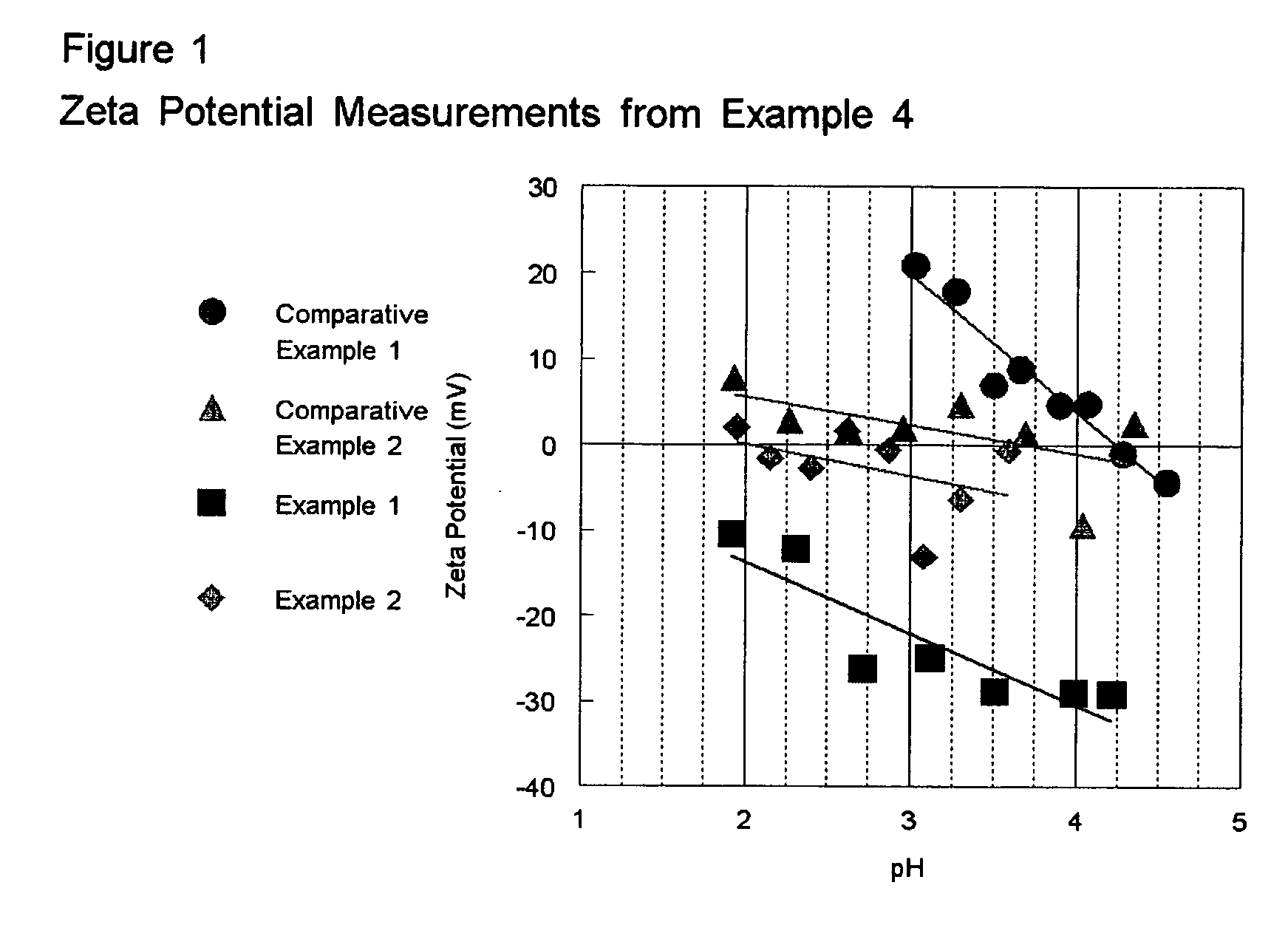
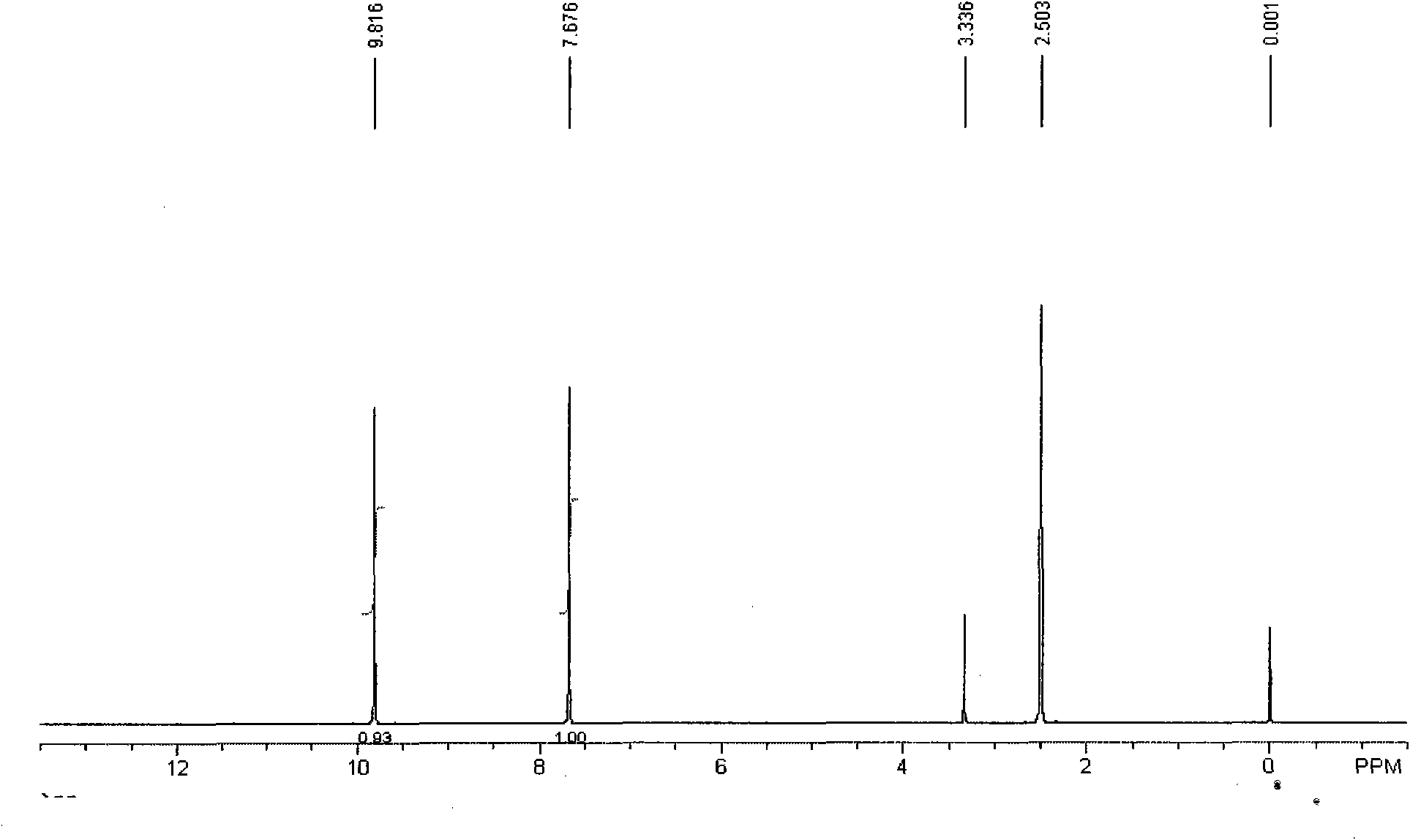
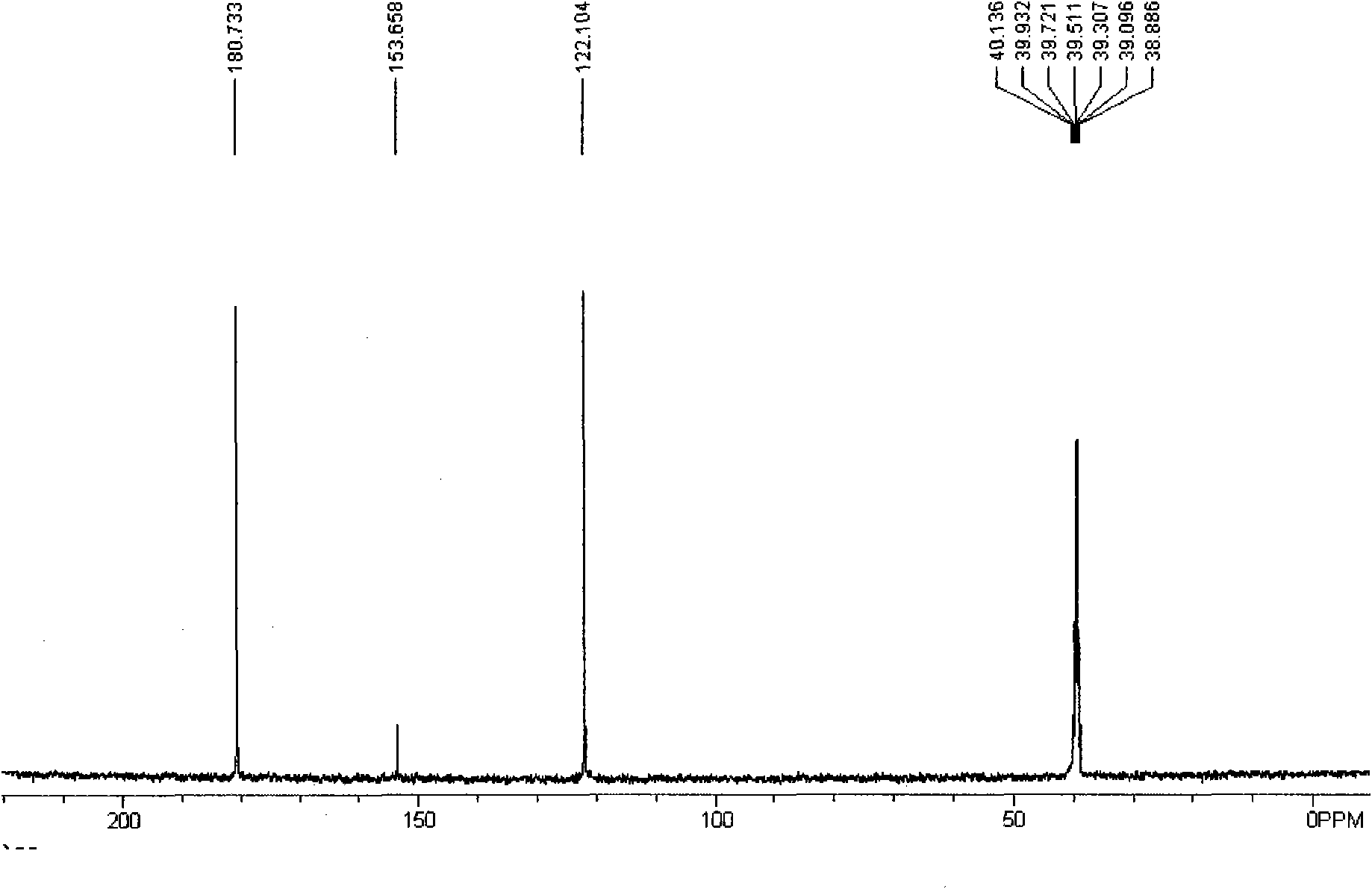
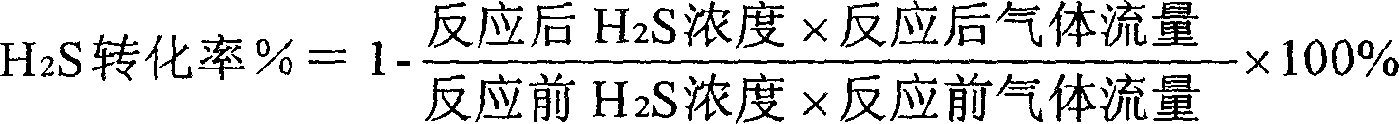Patents
Literature
2011 results about "Vanadium oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vanadium oxide may refer to: Vanadium oxide, VO Vanadium oxide, V₂O₃ Vanadium oxide, VO₂ Vanadium oxide, V₂O₅ In addition to these principal oxides of vanadium, various other distinct phases exist: Phases with the general formula VₙO₂ₙ₊₁ exist between V₂O₅ and VO₂. Examples of these phases include V₃O₇, V₄O₉ and V₆O₁₃. Phases with the general formula VₙO2n−1 exist between VO₂ and V₂O₃. Called Magnéli phases for Arne Magnéli, they are examples of crystallographic shear compounds based on the rutile structure. Examples of Magnéli phases include V₄O₇, V₅O₉, V₆O₁₁, V₇O₁₃ and V₈O₁₅. Many vanadium-oxygen phases are non-stoichiometric.
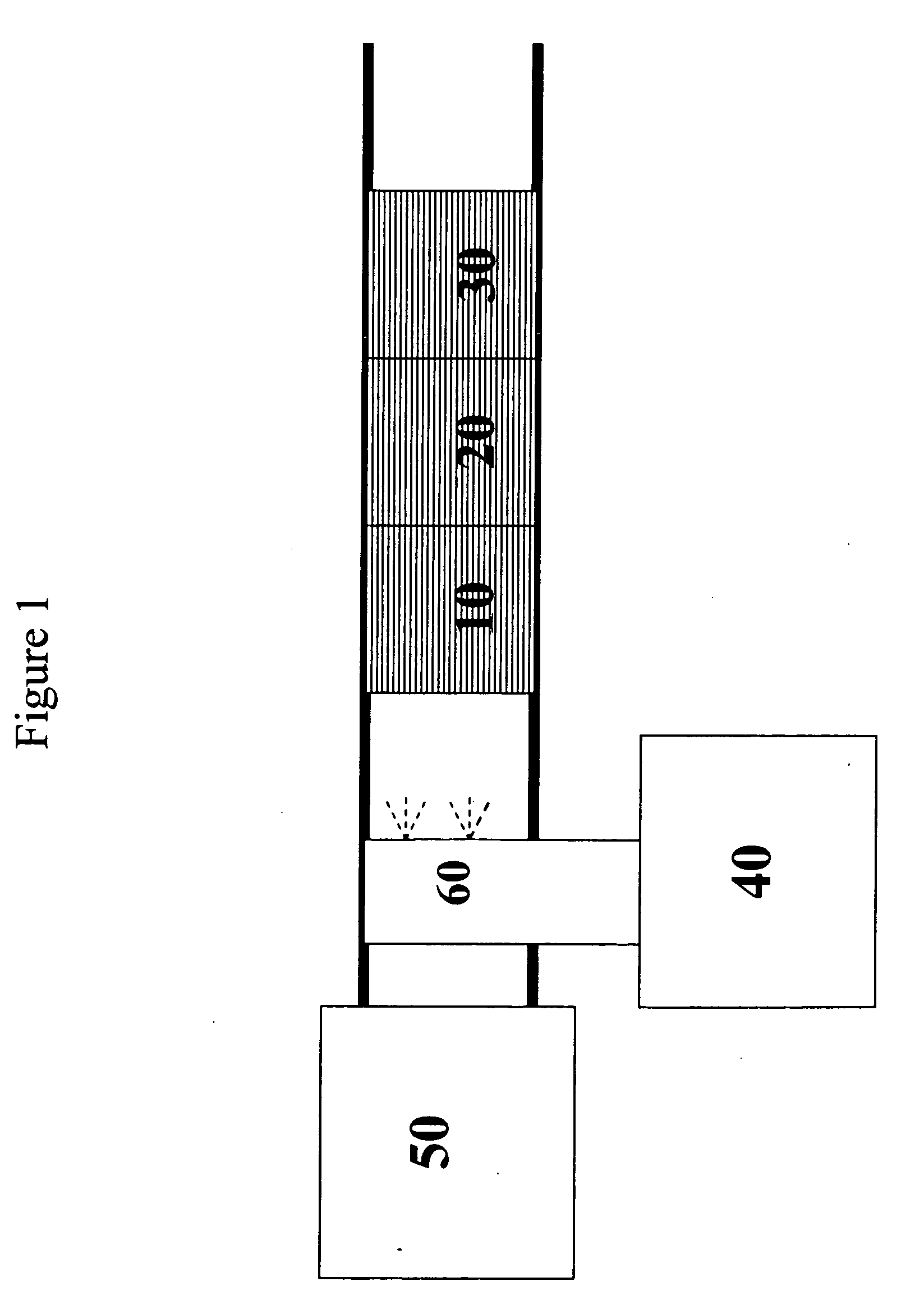
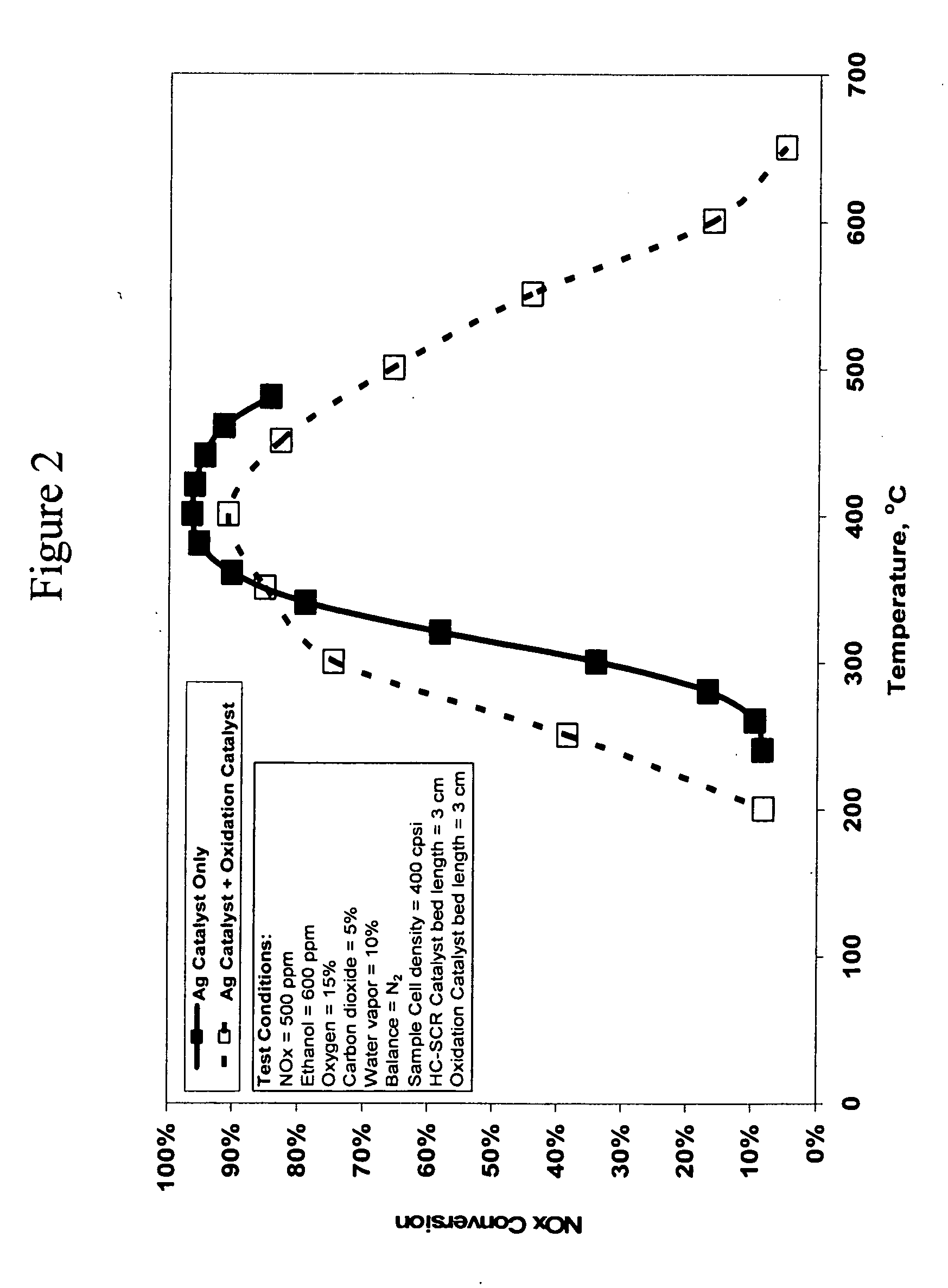
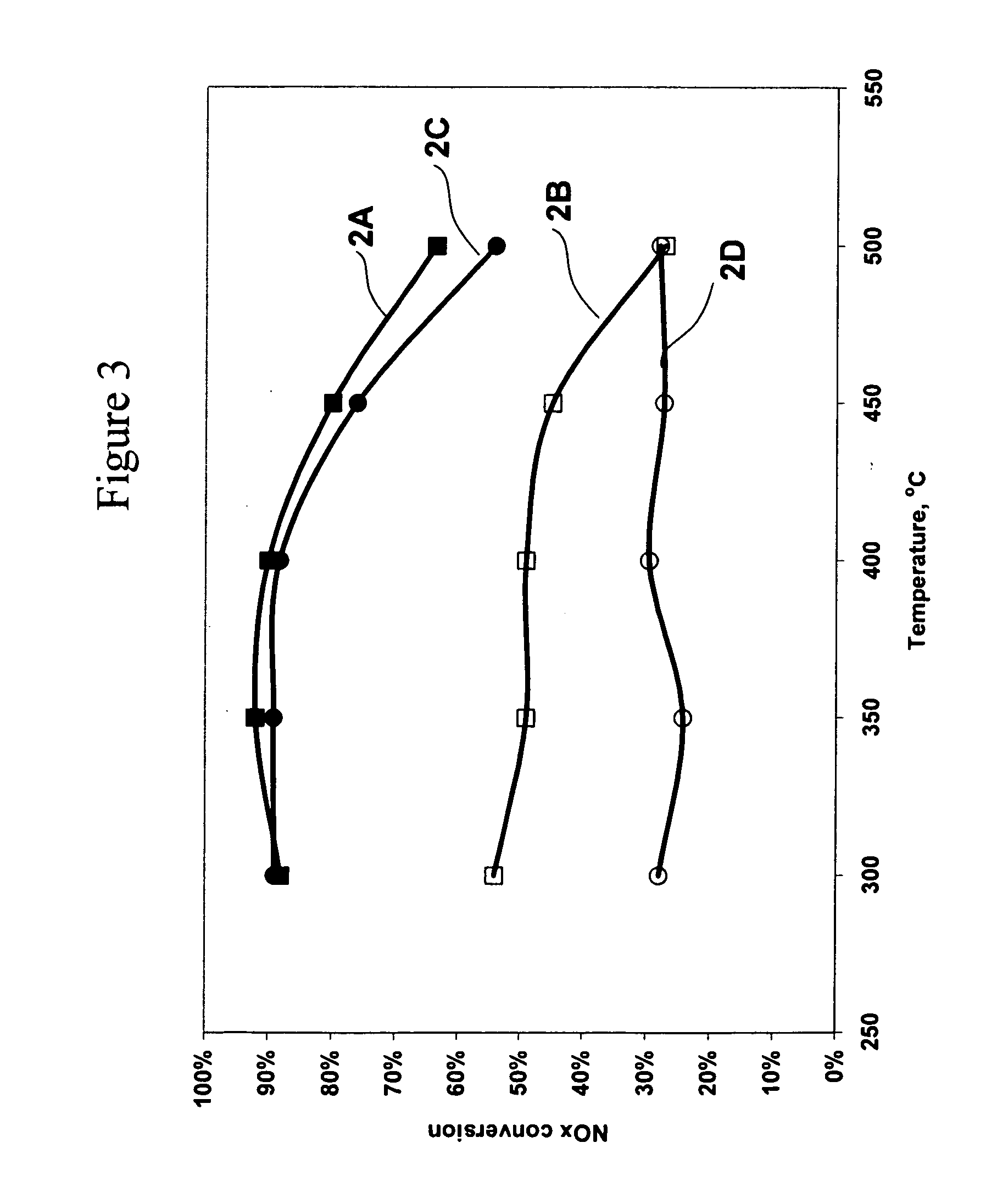
Catalyst and method for reducing nitrogen oxides in exhaust streams with hydrocarbons or alcohols
A catalyst system and a method for reducing nitrogen oxides in an exhaust gas by reduction with a hydrocarbon or oxygen-containing organic compound reducing agent are provided. The catalyst system contains a silver catalyst and a modifier catalyst, where the modifier catalyst contains a modifier oxide, where the modifier oxide is selected from the group consisting of iron oxide, cerium oxide, copper oxide, manganese oxide, chromium oxide, a lanthanide oxide, an actinide oxide, molybdenum oxide, tin oxide, indium oxide, rhenium oxide, tantalum oxide, osmium oxide, barium oxide, calcium oxide, strontium oxide, potassium oxide, vanadium oxide, nickel oxide, tungsten oxide, and mixtures thereof. The modifier oxide is supported on an inorganic oxide support or supports, where at least one of the inorganic oxide supports is an acidic support. The catalyst system of the silver catalyst and the modifier catalyst provides higher NOx conversion than either the silver catalyst or the modifier catalyst alone.
Owner:CATALYTIC SOLUTIONS INC
Light emitting device and electronic appliance using the same
InactiveUS20060292394A1Easy to changeDistanceDischarge tube luminescnet screensElectroluminescent light sourcesVanadium oxideOrganic compound
A light emitting device comprises a pair of electrodes and a mixed layer provided between the pair of electrodes. The mixed layer contains an organic compound which contains no nitrogen atoms, i.e., an organic compound which dose not have an arylamine skeleton, and a metal oxide. As the organic compound, an aromatic hydrocarbon having an anthracene skeleton is preferably used. As such an aromatic hydrocarbon, t-BuDNA, DPAnth, DPPA, DNA, DMNA, t-BuDBA, and the like are listed. As the metal oxide, molybdenum oxide, vanadium oxide, ruthenium oxide, rhenium oxide, and the like are preferably used. Further, the mixed layer preferably shows absorbance per 1 μm of 1 or less or does not show a distinct absorption peak in a spectrum of 450 to 650 nm when an absorption spectrum is measured.
Owner:SEMICON ENERGY LAB CO LTD
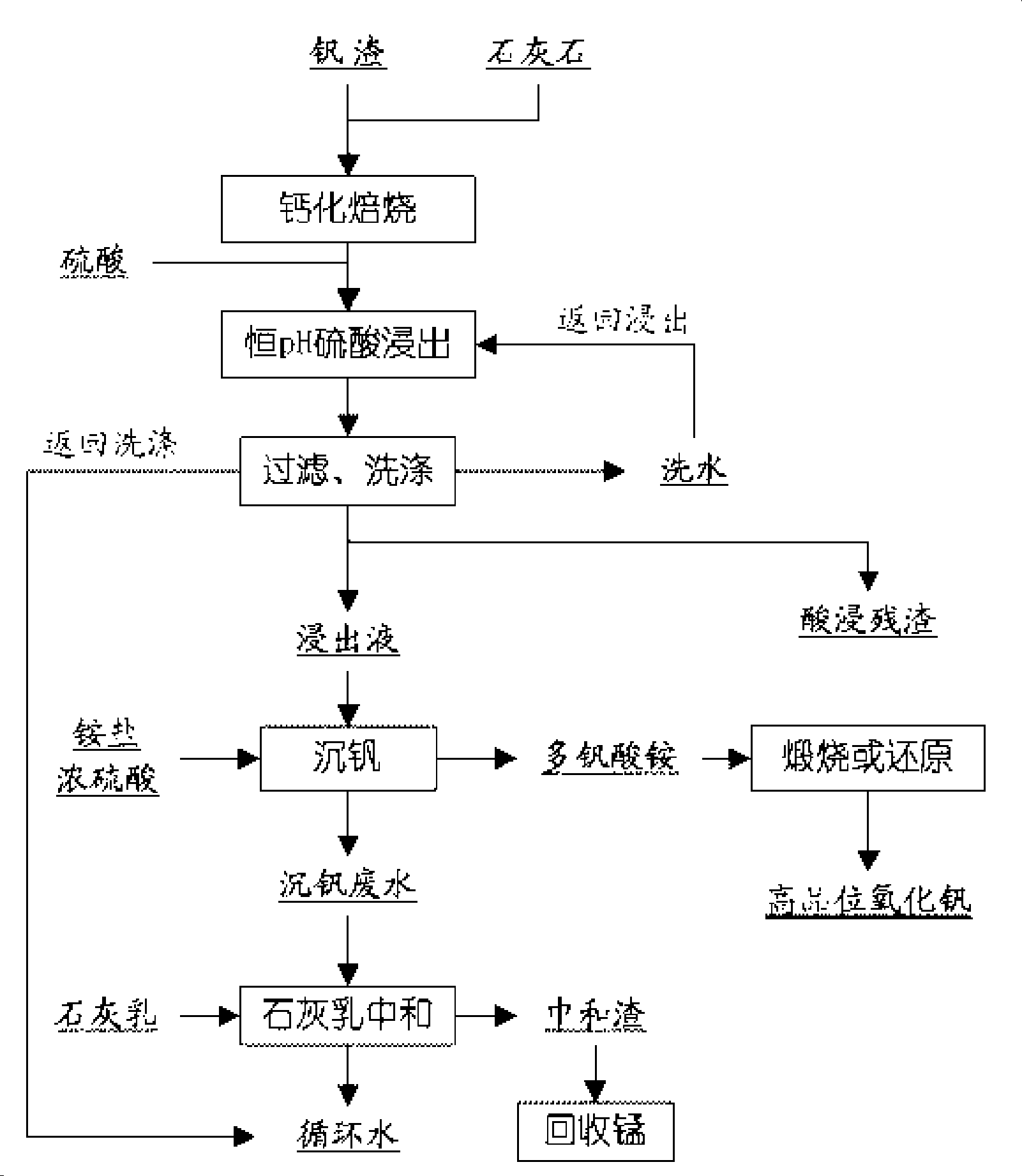
Calcification vanadium slag sintering method
InactiveCN101161831AEasy temperature controlShorten roasting timeProcess efficiency improvementSlagCalcification
The invention discloses a method of vanadium slag direct entering high temperature roasting furnace calcify roasting, that is, high calcium vanadium slag or ordinary vanadium slag and lime or limestone are mixed uniformly without the gradual heating up process from low temperature to high temperature, the mixture enters into the roasting furnace of more than 600 DEG C for calcified roasting., the vanadium of the vanadium slag is changed into vanadium acid calcium, vanadium of the roasting clinker is dissolved into the solution under the leaching function of sulphuric acid solution, vanadium oxide and the like vanadium products are further made. The invention needs no gradual heating up process from low temperature to high temperature of the prior sodium treatment roasting, thereby reducing roasting time, improving productivity of unit and reducing production cost.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP +1
Glazing coated with at least one layer having thermochromic properties
InactiveUS20050147825A1Other chemical processesSynthetic resin layered productsOptical propertyVanadium oxide
A glazing coated with at least one layer having thermochromic properties comprising vanadium oxide, and also with at least one other layer having thermal properties, such as an infrared reflecting layer, and / or at least one other layer having optical properties, such as antireflection in the visible, and / or electrical conduction properties; and having a particular application for making solar control glazing.
Owner:SAINT-GOBAIN GLASS FRANCE
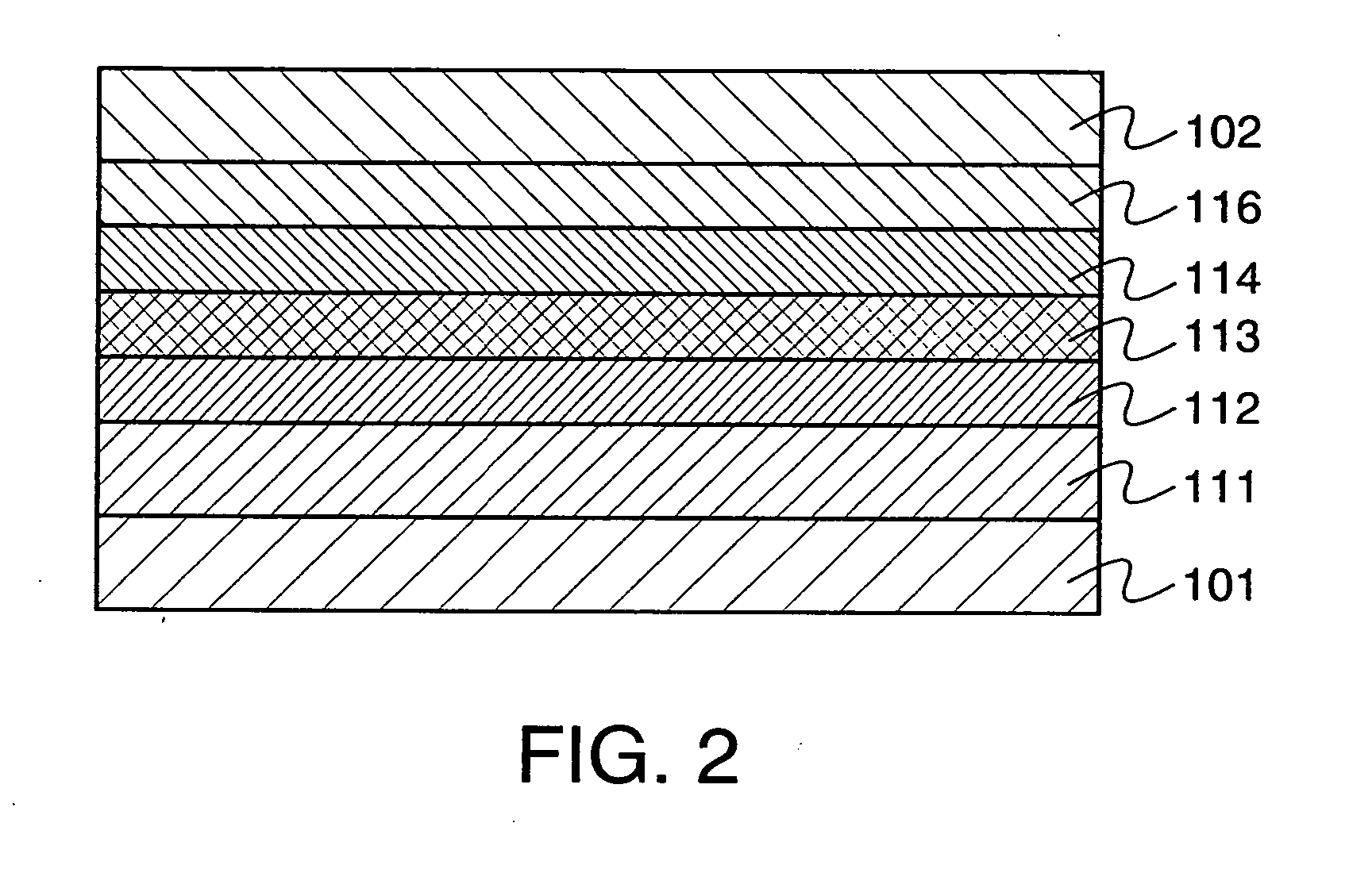
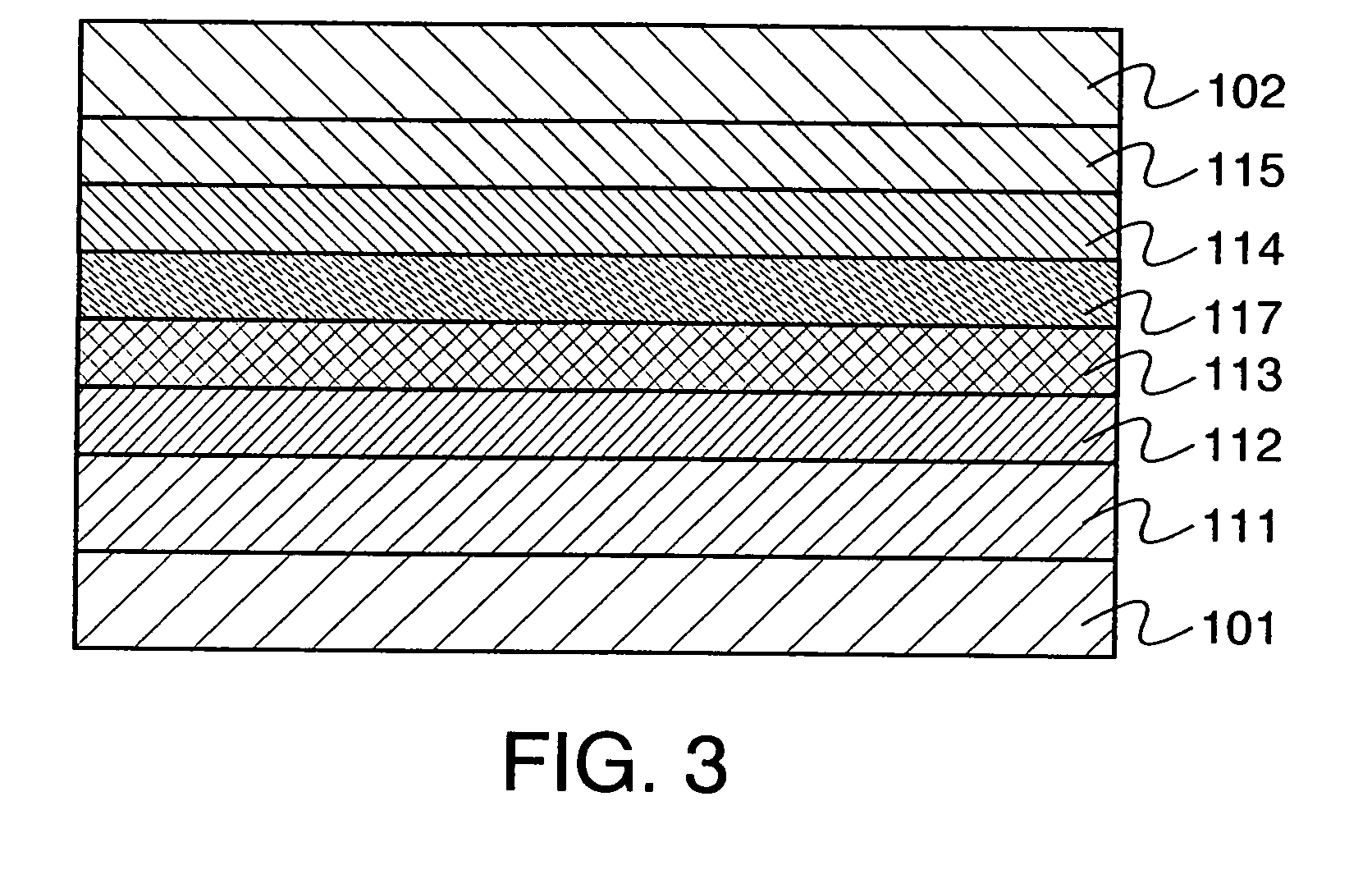
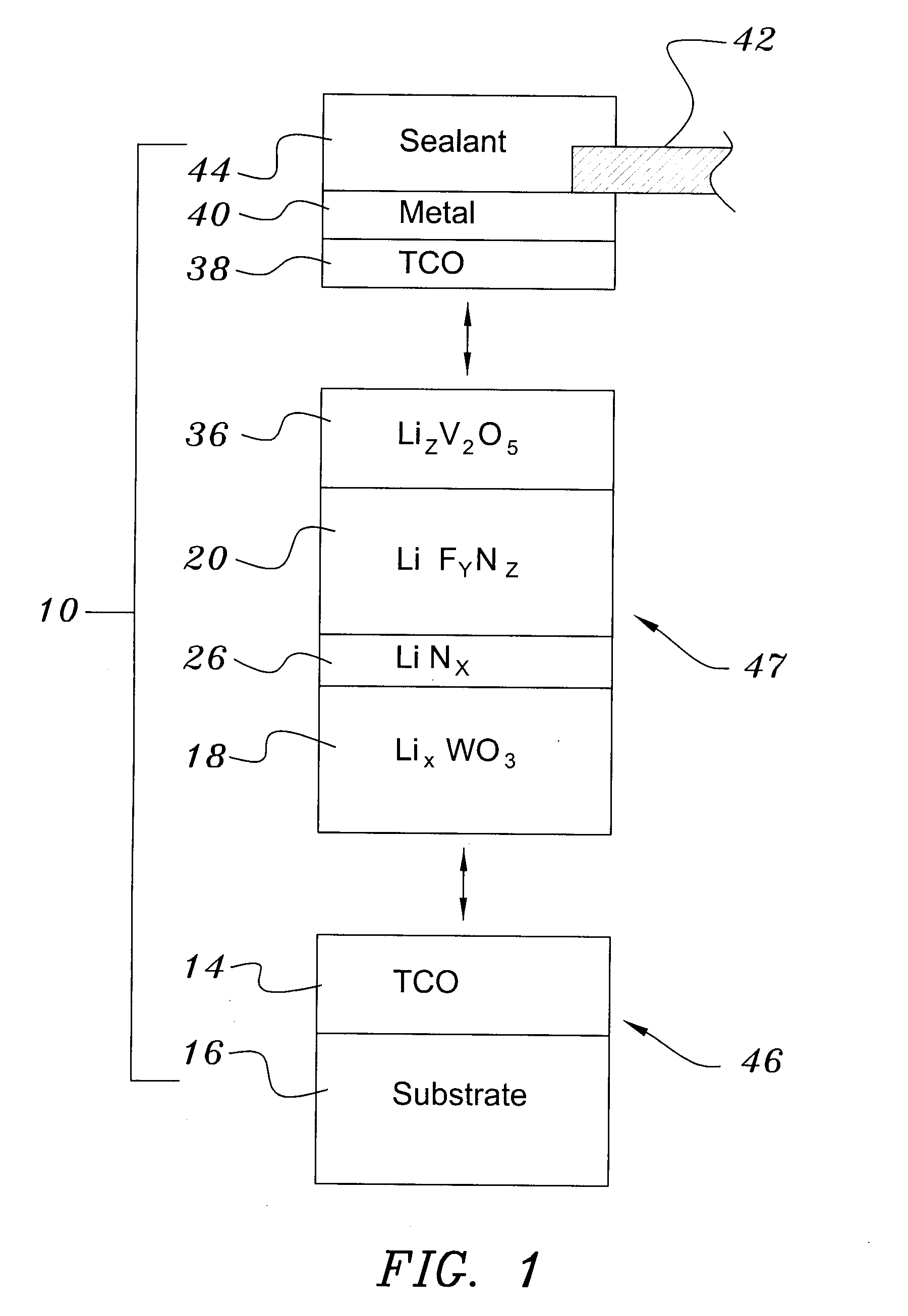
Electrochromic Device with Self-forming Ion transfer Layer and Lithium Fluoro-Nitride Electrolyte
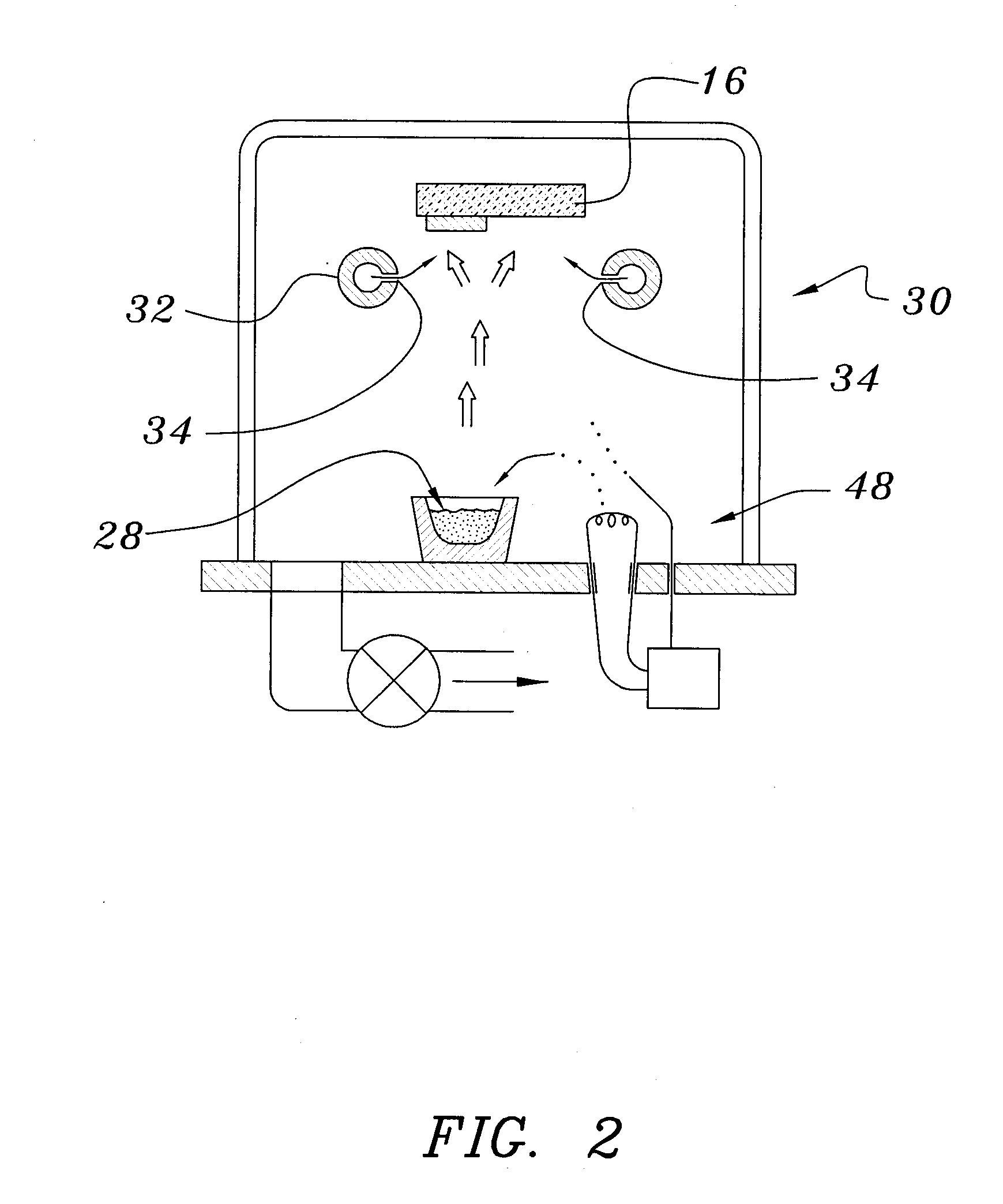
ActiveUS20070292606A1Solve many processesReduce usageCoatingsSpecial surfacesEvaporationElectrochromism
A method of preparing an electrochromic device involves forming multiple layers of selected materials on a substrate in a vacuum processing chamber. A first of these layers is an electrode layer deposited directly on the substrate and used for making contact to a subsequently deposited precursor film, preferably tungsten oxide, from which an electrochromic layer is formed by lithium loading in the presence of ionized nitrogen. This not only forms the electrochromic layer by diffusion of the lithium into the tungsten oxide, but also forms a thin lithium nitride ion transfer layer on the then exposed surface. Subsequently, a lithium fluoro-nitride electrolyte layer is formed on the ion transfer layer by evaporation from a lithium fluoride source in the presence of ionized nitrogen. An ion storage layer, which may be a vanadium oxide and a transparent second electrode layer are subsequently vacuum deposited.
Owner:ECLIPSE ENERGY SYST
Electrochromic device with self-forming ion transfer layer and lithium-fluoro-nitride electrolyte
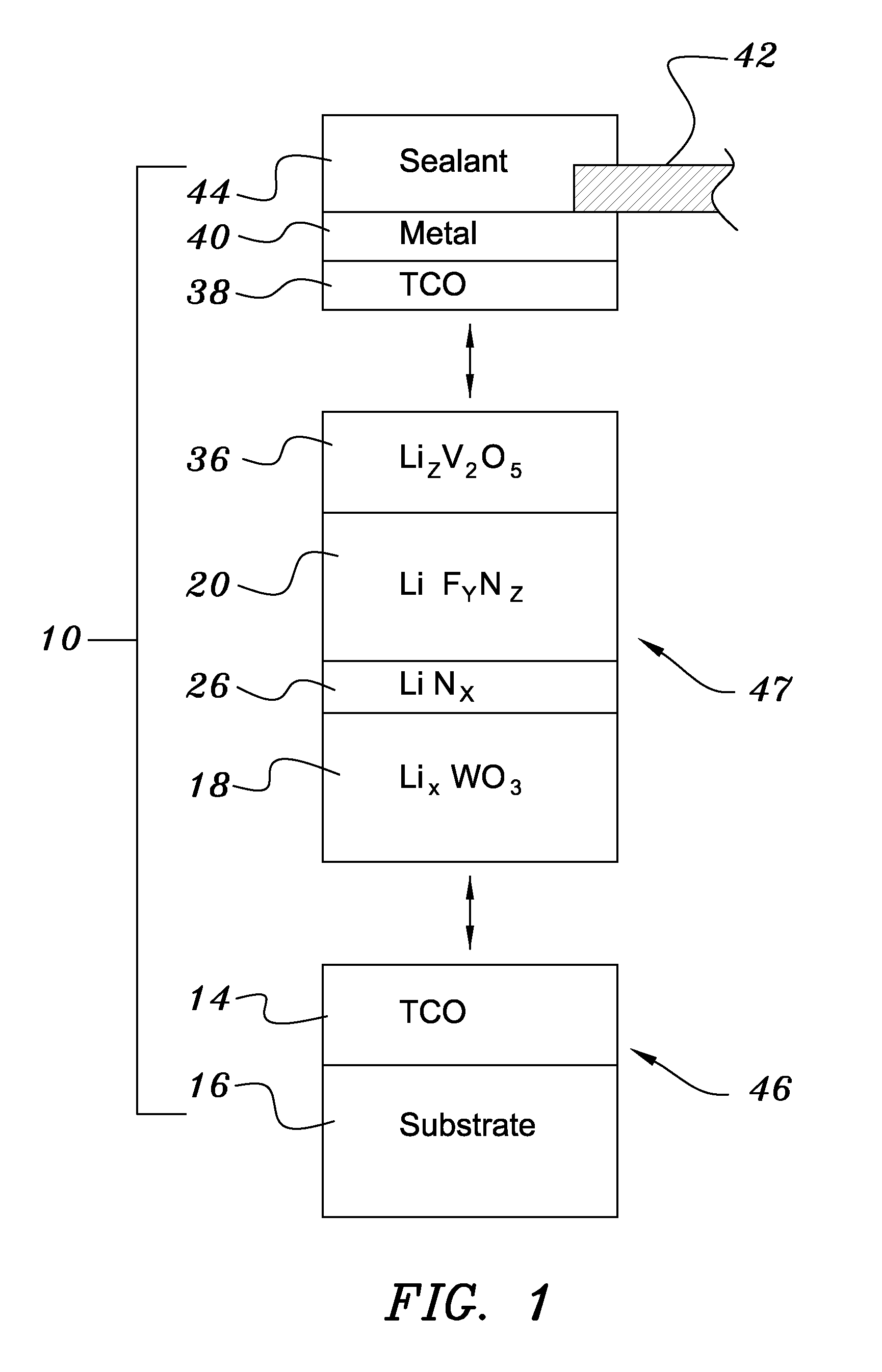
ActiveUS7265891B1Solve many processesReduce usageElectrolytic capacitorsNon-linear opticsEvaporationElectrochromism
An electrochromic device is prepared by forming multiple layers of selected materials on a substrate in a vacuum processing chamber. A first of these layers is an electrode layer deposited directly on the substrate and used for making contact to a subsequently deposited precursor film, preferably tungsten oxide, from which an electrochromic layer is formed by lithium loading in the presence of ionized nitrogen. This not only forms the electrochromic layer by diffusion of the lithium into the tungsten oxide, but also forms a thin lithium nitride ion transfer layer on the then exposed surface. Subsequently, a lithium fluoro-nitride electrolyte layer is formed on the ion transfer layer by evaporation from a lithium fluoride source in the presence of ionized nitrogen. An ion storage layer, which may be a vanadium oxide and a transparent second electrode layer are subsequently vacuum deposited.
Owner:ECLIPSE ENERGY SYST
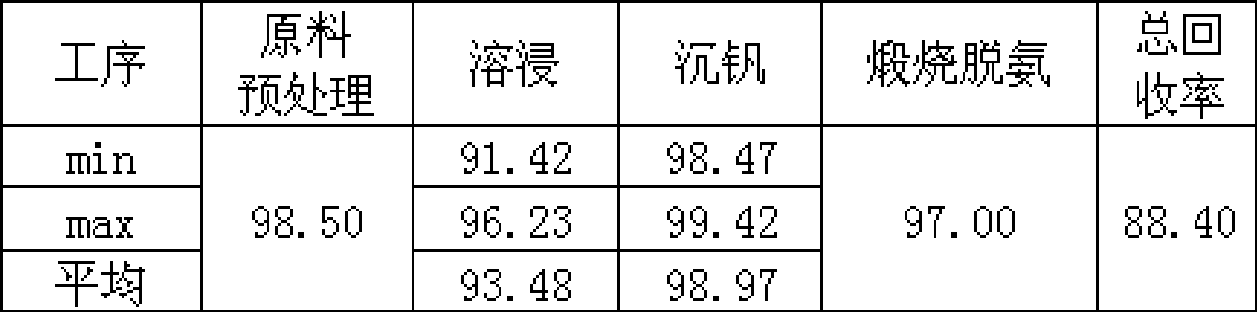
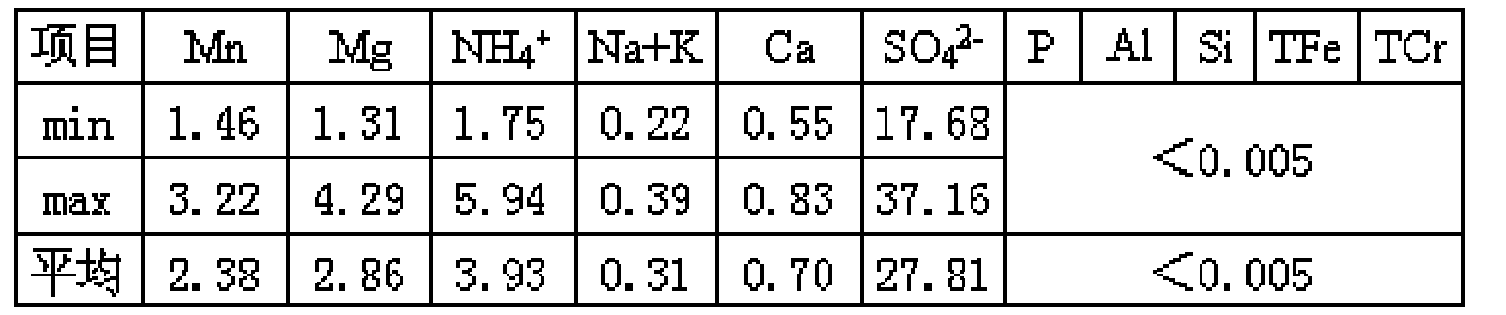
Clean production process for vanadium oxide
ActiveCN101412539ASolve difficult environmental problemsSolve the technical problem of not being able to obtain high-quality vanadium productsVanadium oxidesProcess efficiency improvementSlagWastewater
The invention relates to a method for cleanly producing vanadium oxide, which belongs to the field of extraction of vanadium oxide. The technical problems to be solved by the invention is to provide the method for cleanly producing the vanadium oxide which not only can obtain a high-quality vanadium product, but also can ensure that vanadium waste water can be reclaimed. The method for producing the vanadium oxide comprises the following steps: preparing roasting raw materials, calcification roasting; infiltrating, separating solid and liquid, precipitating vanadium by ammonium salt, calcining for deaminating or reducing, and the like. Waste water after vanadium extraction can be returned to a system for recycling after the waste waster is subjected to neutralizing treatment by lime cream so as to realize zero drainage of the waste water. The method also improves the reclamation rate of vanadium to be superior to that of the prior process, and reduces production cost. Through combining with other technologies, the method can change extracted slag and other waste into secondary resource to be used once again, and realize clean production.
Owner:攀钢集团西昌钒制品科技有限公司
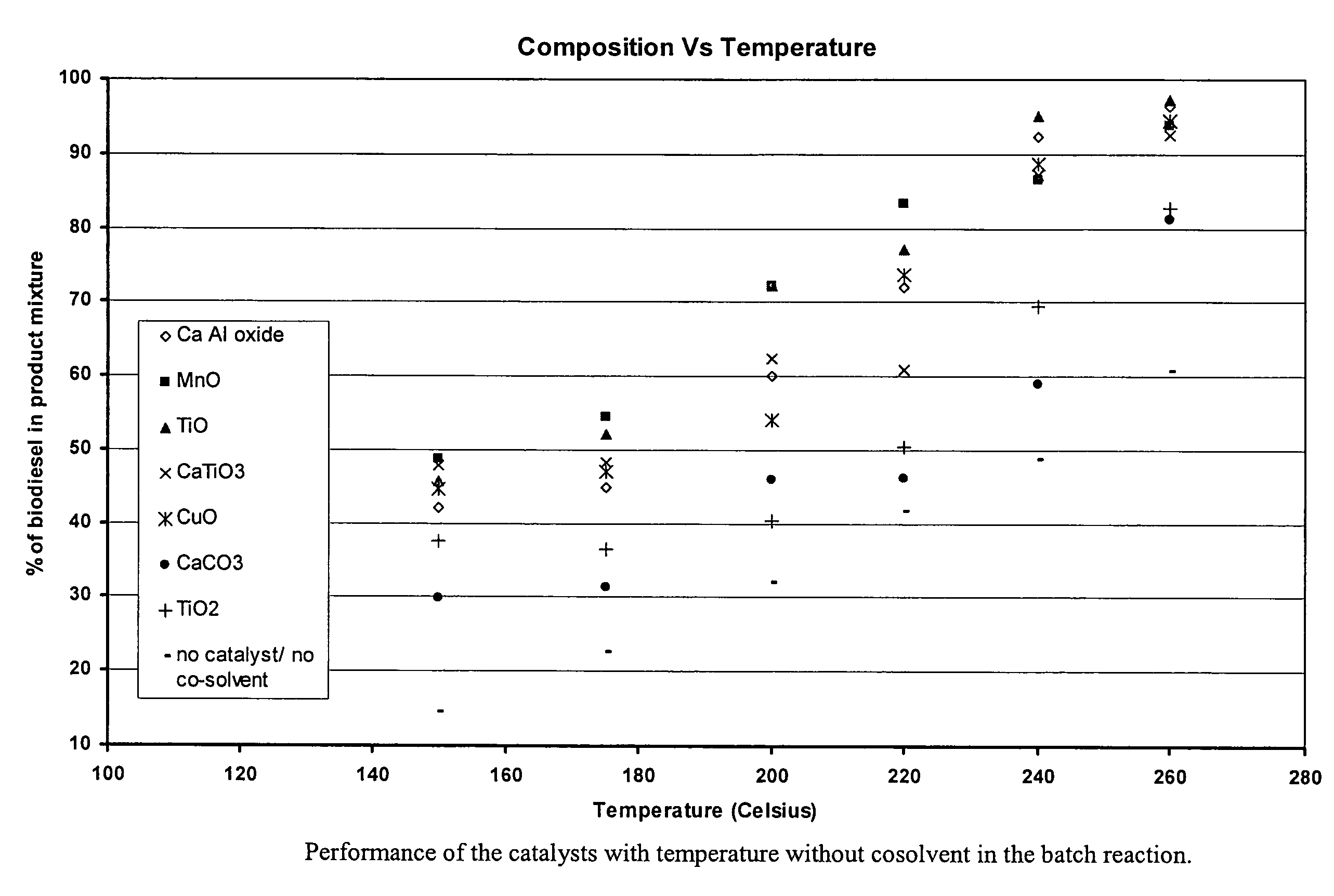
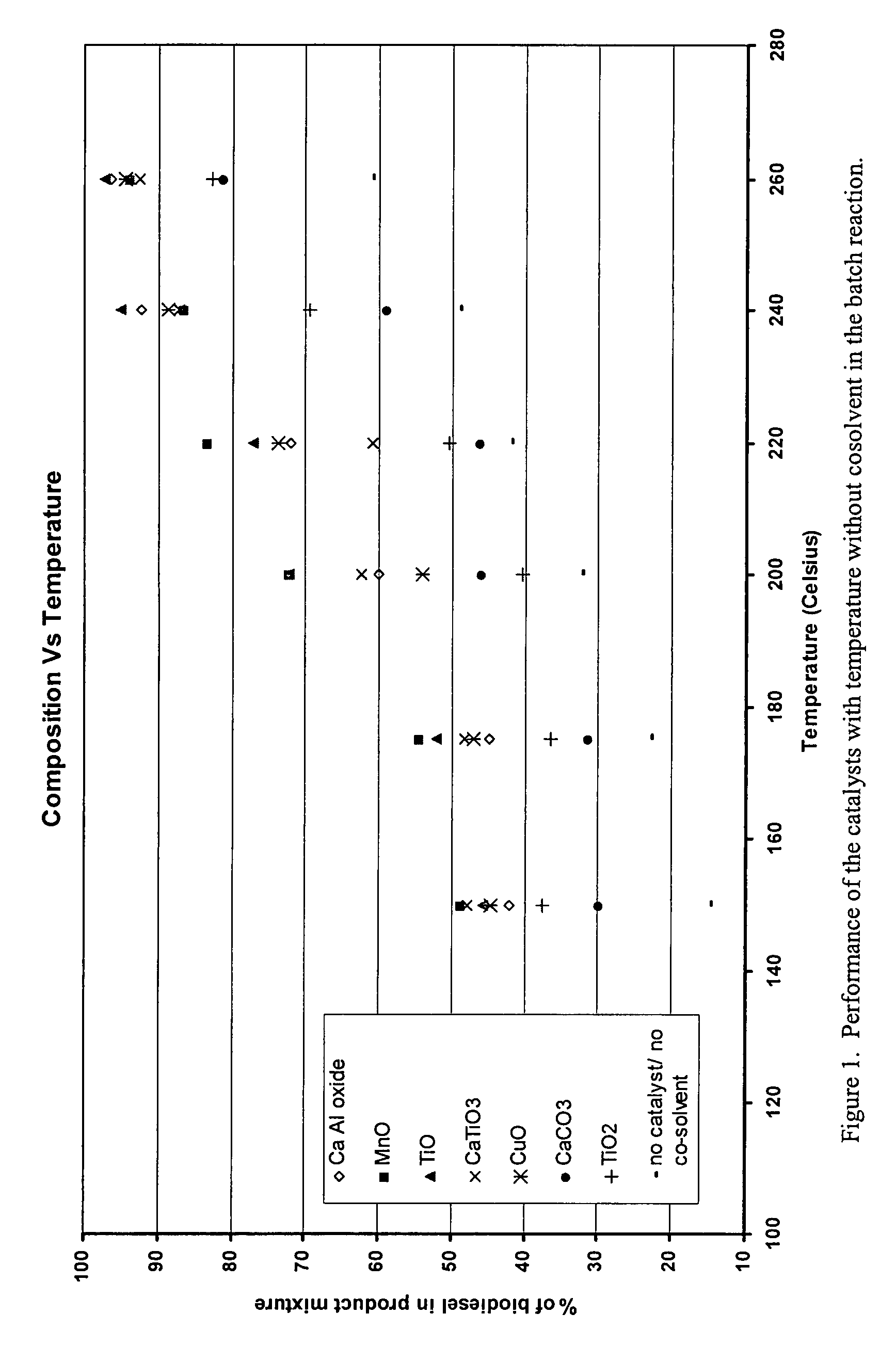
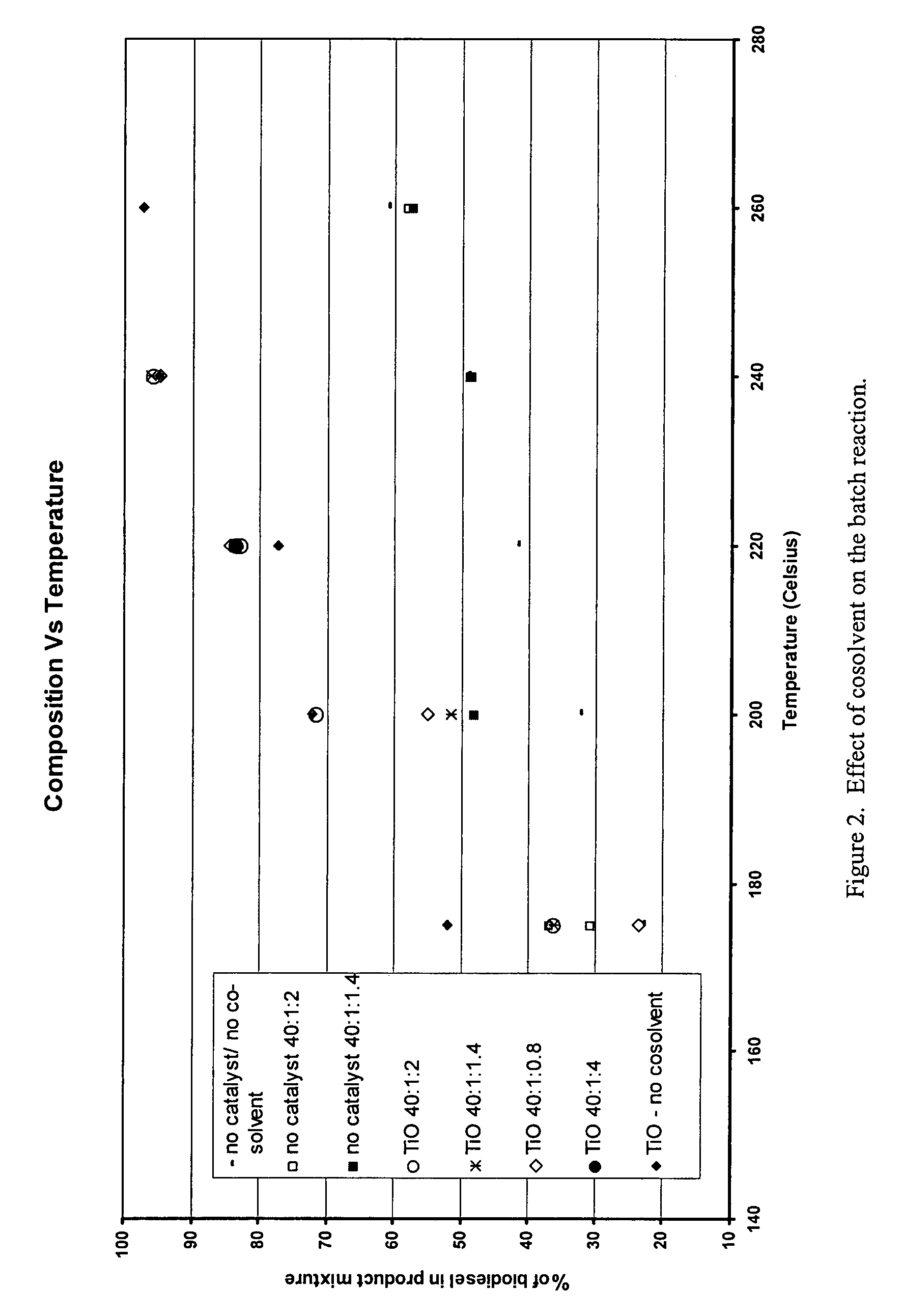
Green biodiesel
InactiveUS7563915B2Reduce wasteSignificant energyFatty oils/acids recovery from wasteFatty acid esterificationCalcium silicateBiodiesel
Methods for improved manufacture of green biodiesel focus on the selection and use of one or more solid metallic oxide base catalyst(s) selected from the group consisting of calcium oxide (CaO), calcium aluminum oxide (CaO—Al2O3), calcium titanate (CaTiO3), barium titanate (BaTiO3), magnesium aluminum oxide (MgO—Al2O3), zinc oxide (ZnO), copper (II) oxide (CuO), nickel oxide (NiO), manganese oxide (MnO), titanium oxide (TiO), vanadium oxide (VO), cobalt oxide (CoO), iron oxide (FeO), chromite (FeCr2O4), hydrotalcite (Mg6Al2(CO3)(OH)16.4(H2O), magnetite (Fe3O4), magnesium silicate and calcium silicate.
Owner:PENN STATE RES FOUND
Anode for nonaqueous secondary electrochemical cells
InactiveUS6730437B2Improve performanceAvoid corrosionSilver accumulatorsElectrode carriers/collectorsGraphiteMetallic sulfide
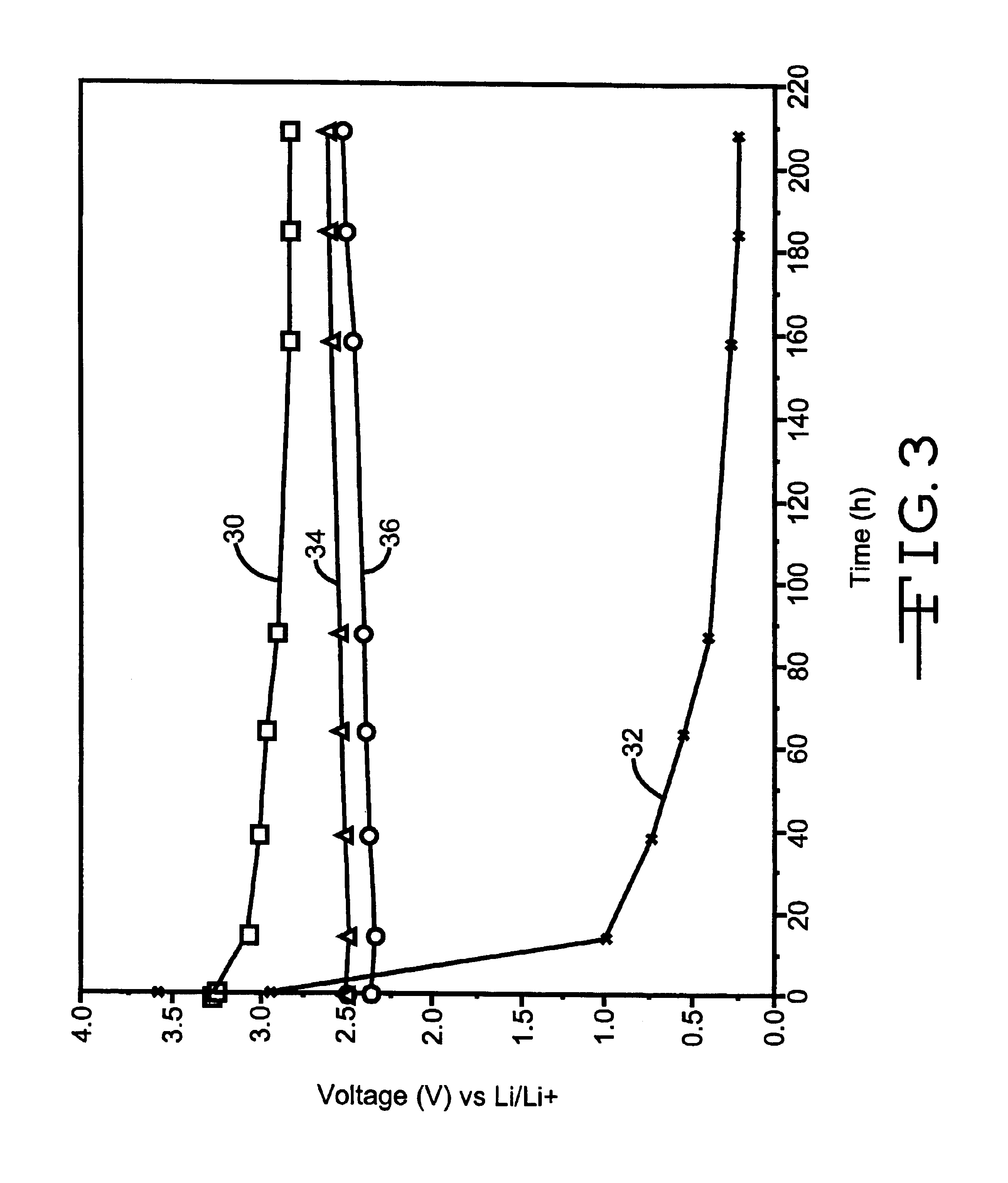
The negative electrode or anode for a secondary electrochemical cell comprising a mixture of graphite or "hairy carbon" and a lithiated metal oxide, a lithiated mixed metal oxide or a lithiated metal sulfide, and preferably a lithiated metal vanadium oxide, is described. A most preferred formulation is graphite mixed with lithiated silver vanadium oxide or lithiated copper silver vanadium oxide.
Owner:WILSON GREATBATCH LTD
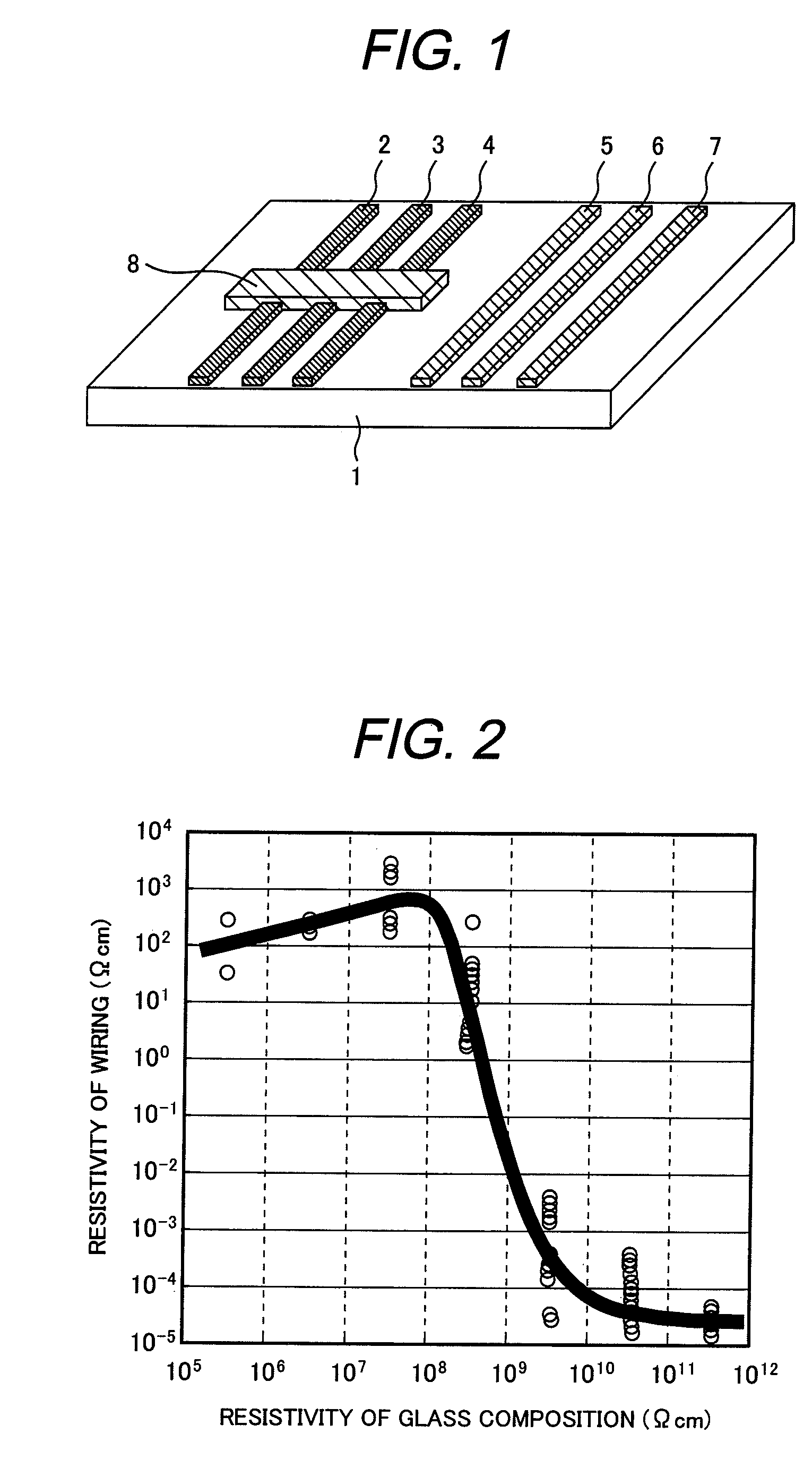
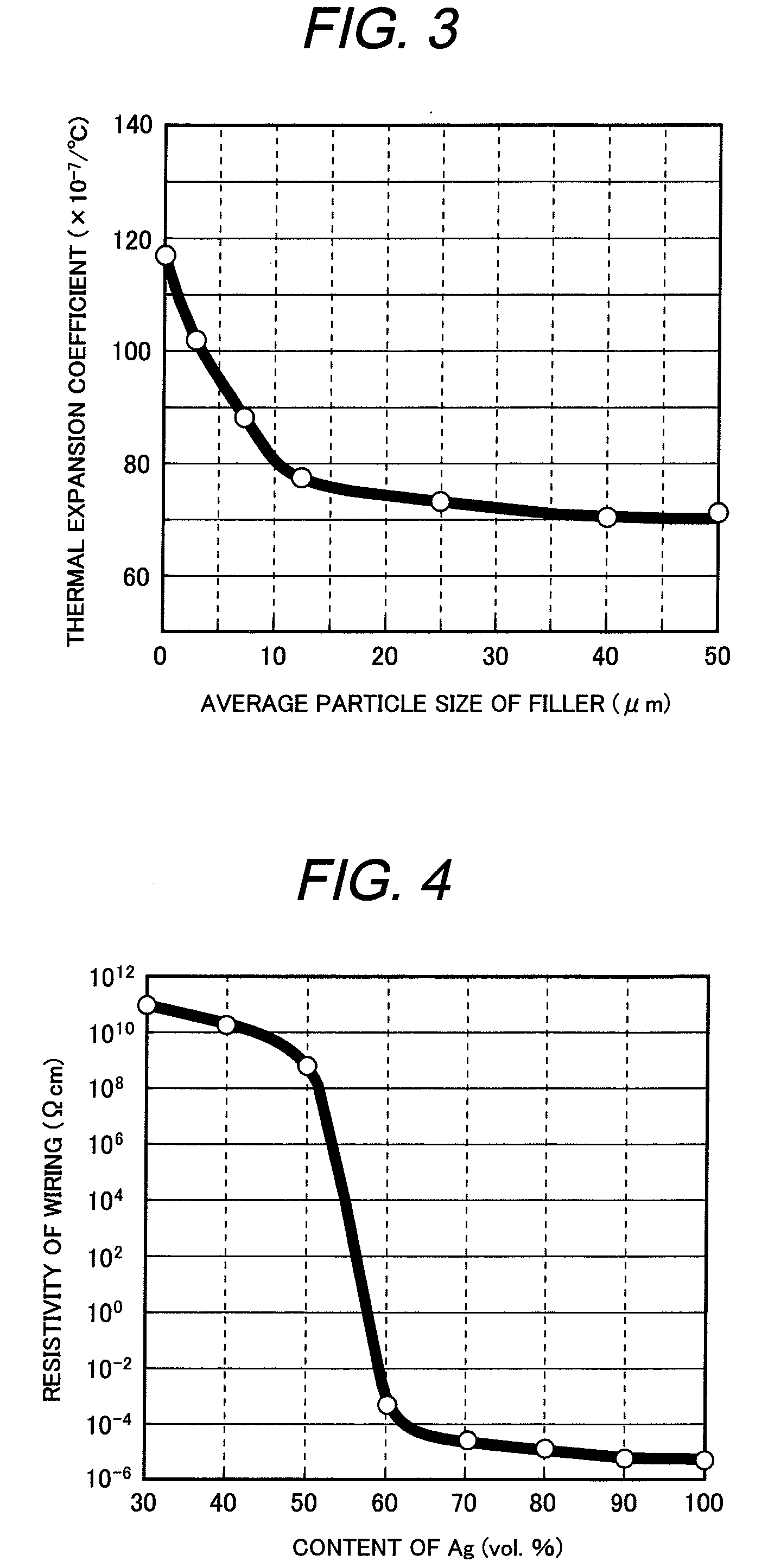
Glass composition and its applications
InactiveUS20090199897A1Reduce the temperatureLow air tightness requirementsConductive layers on insulating-supportsSustain/scan electrodesVanadium oxideMaterials science
Owner:HITACHI POWDERED METALS COMPANY
Flat back case for an electrolytic capacitor
ActiveUS7012799B2Benefit in costPrecise positioningElectrotherapyCapacitor electrodesElectrolysisElectrical battery
An enclosure for an electrical energy storage device such as a wet tantalum electrolytic capacitor or an electrochemical cell such as a lithium / silver vanadium oxide cell is described. The enclosure comprises two metallic casing components or portions. The first is a drawn member having a planar face wall supporting a surrounding sidewall and is shaped to nest the anode, cathode and intermediate separator components. The surrounding sidewall has an annular flange at its outer periphery. A mating cover is a stamped planar piece of similar material whose periphery fits inside the annular flange or rim as a complementary piece.
Owner:WILSON GREATBATCH LTD
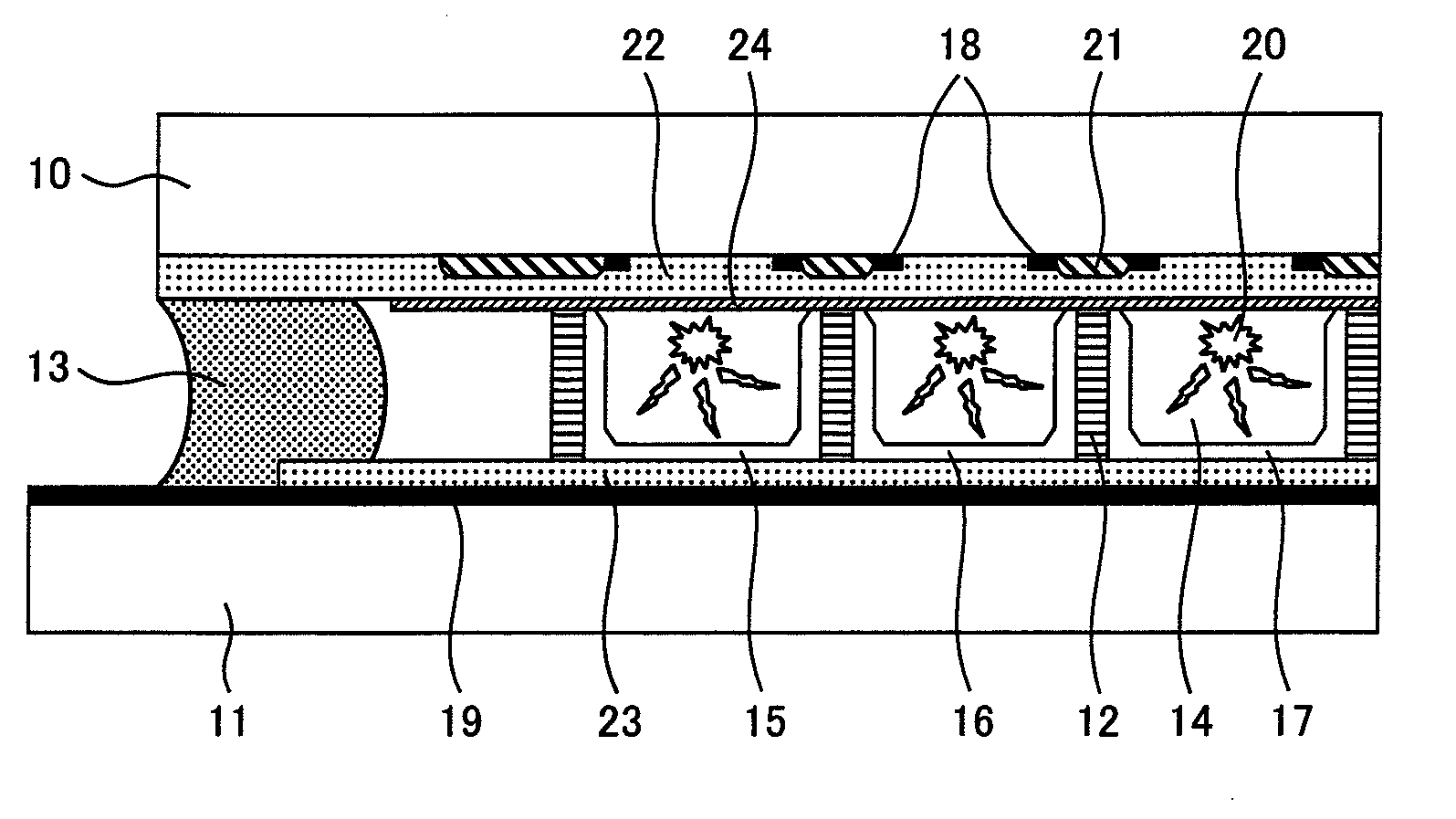
Coefficient of thermal expansion filler for vanadium-based frit materials and/or methods of making and/or using the same
ActiveUS20120213954A1Improve sealingReduce sealClimate change adaptationWindows/door improvementMetal chlorideFrit
Certain example embodiments relate to seals for glass articles. Certain example embodiments relate to a composition used for sealing an insulted glass unit. In certain example embodiments the composition includes vanadium oxide, barium oxide, zinc oxide, and at least one additional additive. For instance, another additive that is a different metal oxide or different metal chloride may be provided. In certain example embodiments, a composition may be combined with a binder solution that substantially or completely burns out by the time the composition is melted. In certain example embodiments, a CTE filler is included with a frit material. In certain example embodiments, a vacuum insulated glass unit includes first and second glass substrates that are sealed together with a seal that includes the above-described composition.
Owner:GUARDIAN GLASS LLC
More Energy Dense Electrolytic Capacitor
An electrical energy storage device such as a wet tantalum electrolytic capacitor or an electrochemical cell such as a lithium / silver vanadium oxide cell is described. The enclosure comprises a drawn casing portion having a planar face wall supporting a surrounding sidewall and is shaped to nest the anode, cathode and intermediate separator components. A mating cover is a stamped planar piece of similar material having a periphery edge welded to the edge of the casing portion surrounding sidewall. In order to prevent heat generated during the welding process from damaging the separator, the anode portion adjacent to the weld site is contoured. This provides sufficient space between the weld and the separator supported on the anode at the contour so that what heat is transmitted to the separator by convection and conduction mechanism will not damage the separator.
Owner:WILSON GREATBATCH LTD
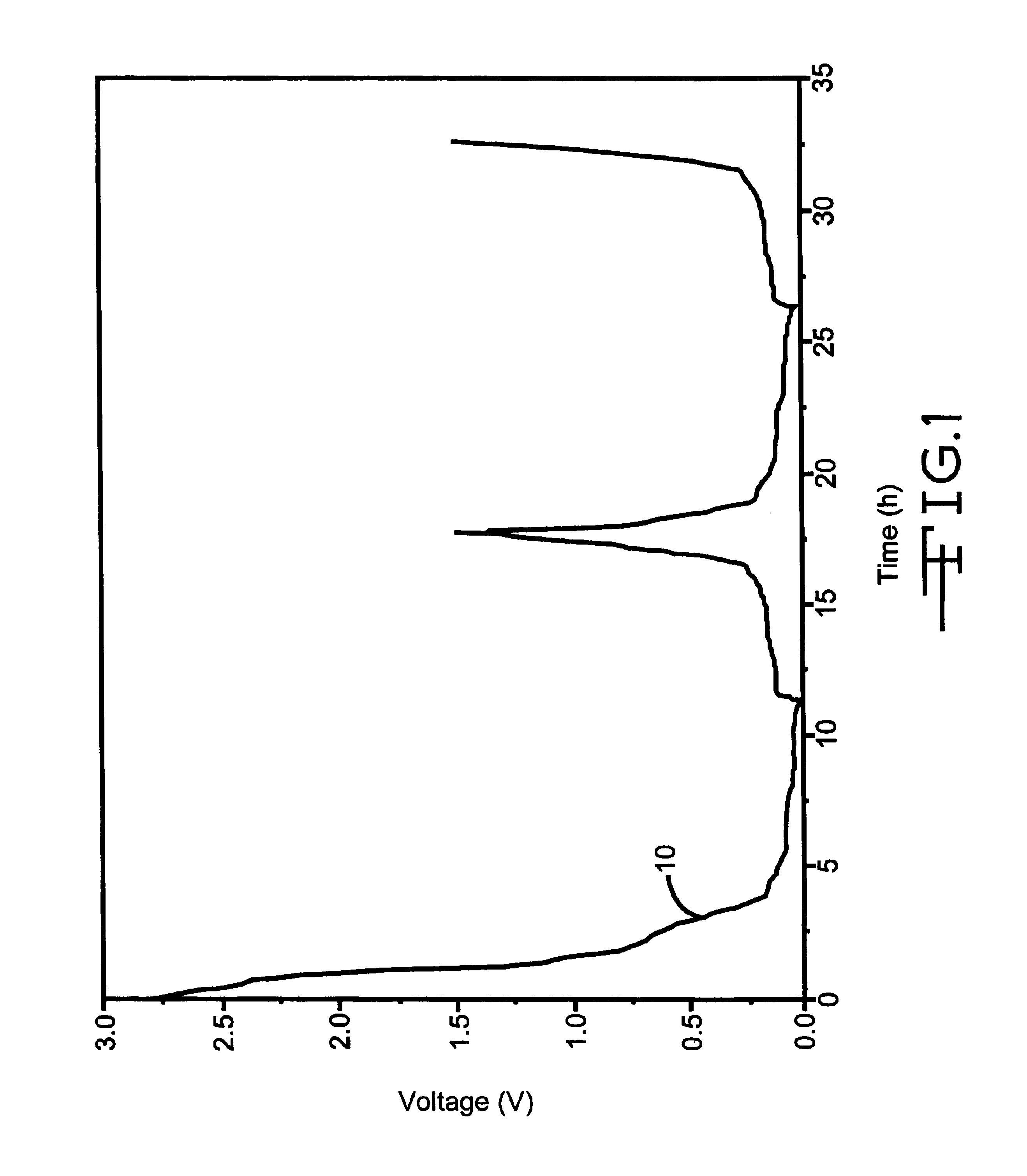
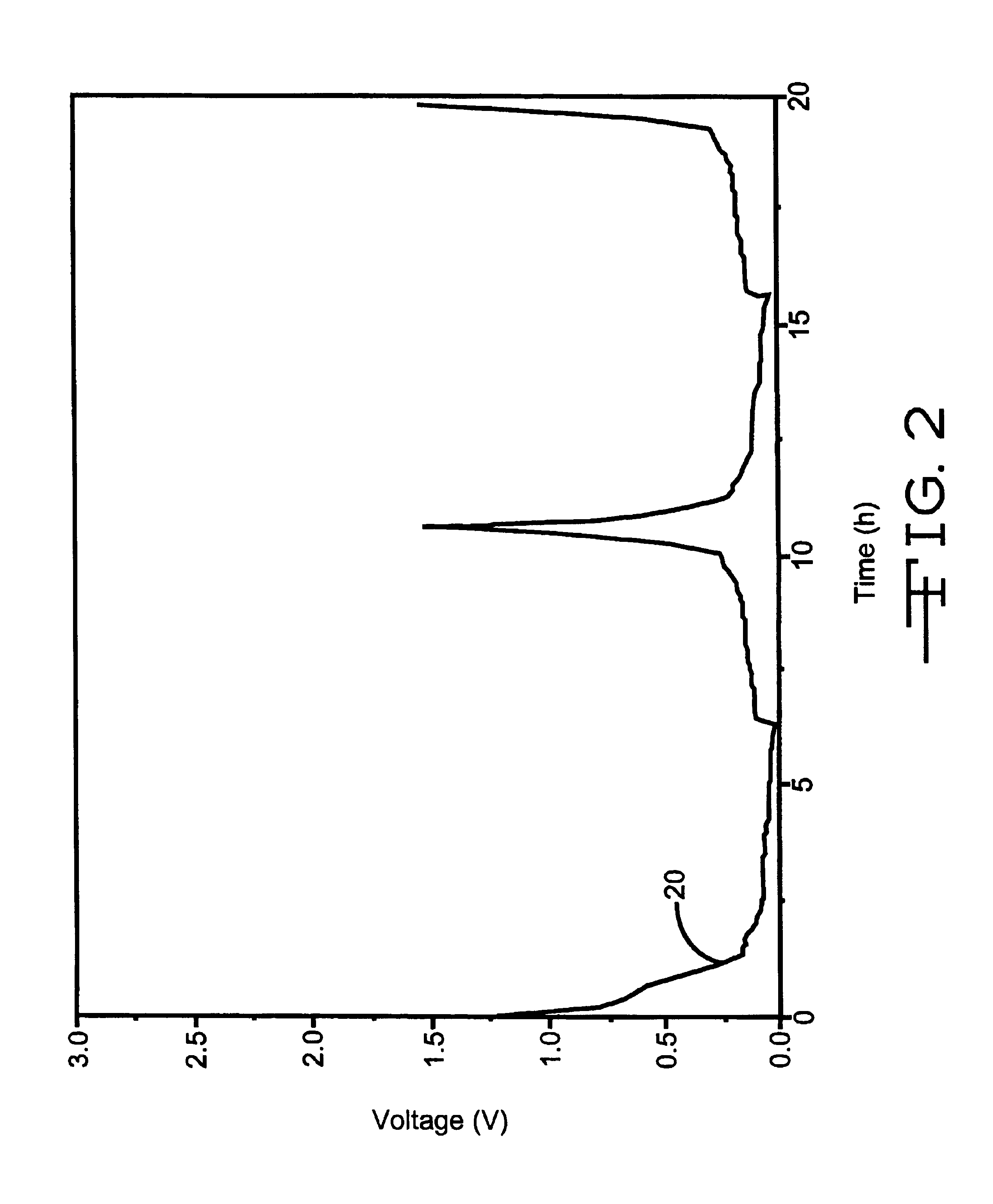
Method of recharging battery for an implantable medical device
A method of operating an implantable medical device containing a Lithium Silver Vanadium Oxide battery. In response to a detected need for therapy, a current flow is delivered from the battery to a charge storage device. After the battery is at least partly depleted by one or more such deliveries of current or other power consumption, the battery is recharged. The recharging may be initiated in response to a selected time threshold, a selected number of current flow delivery events, a selected voltage level, an excess charge time duration, or other operating characteristics. A limited number of recharging cycles may be provided if the charging is done under controlled conditions with respect to charge current and voltage.
Owner:PACESETTER INC
Light emitting element, light emitting device, and electronic device
InactiveUS20090026922A1Operation failureEasy to changeDischarge tube luminescnet screensLamp detailsVanadium oxideTert butyl
One aspect of the present invention is a light emitting element having a layer including an aromatic hydrocarbon and a metal oxide between a pair of electrodes. The kind of aromatic hydrocarbon is not particularly limited; however, an aromatic hydrocarbon having hole mobility of 1×10−6 cm2 / Vs or more is preferable. As such aromatic hydrocarbon, for example, 2-tert-butyl-9,10-di(2-naphthyl)anthracene, anthracene, 9,10-diphenylanthracene, tetracene, rubrene, perylene, 2,5,8,11-tetra(tert-butyl)perylene, and the like are given. As the metal oxide, a metal which shows an electron-accepting property to the aromatic hydrocarbon is preferable. As such metal oxide, for example, molybdenum oxide, vanadium oxide, ruthenium oxide, rhenium oxide, and the like are given.
Owner:SEMICON ENERGY LAB CO LTD
Vanadium-based frit materials, and/or methods of making the same
ActiveUS20120213952A1Little loss of strengthImprove sealingGlass blowing apparatusGlass reforming apparatusMetal chlorideFrit
Certain example embodiments relate to improved seals for glass articles. Certain example embodiments relate to a composition used for sealing an insulted glass unit. In certain example embodiments the composition includes vanadium oxide, barium oxide, zinc oxide, and at least one additional additive. For instance, another additive that is a different metal oxide or different metal chloride may be provided. In certain example embodiments, a vacuum insulated glass unit includes first and second glass substrates that are sealed together with a seal that includes the above-described composition.
Owner:GUARDIAN GLASS LLC
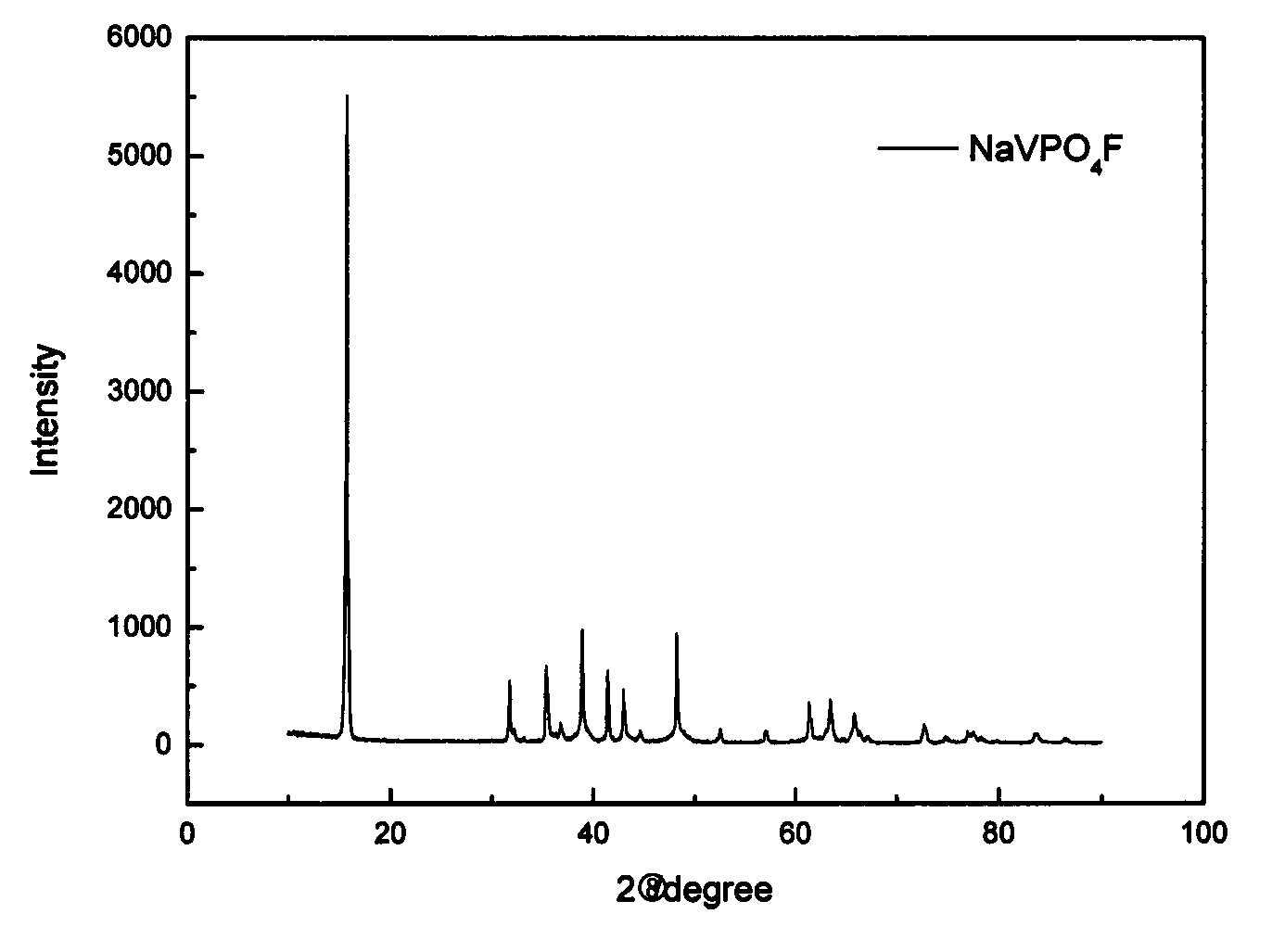
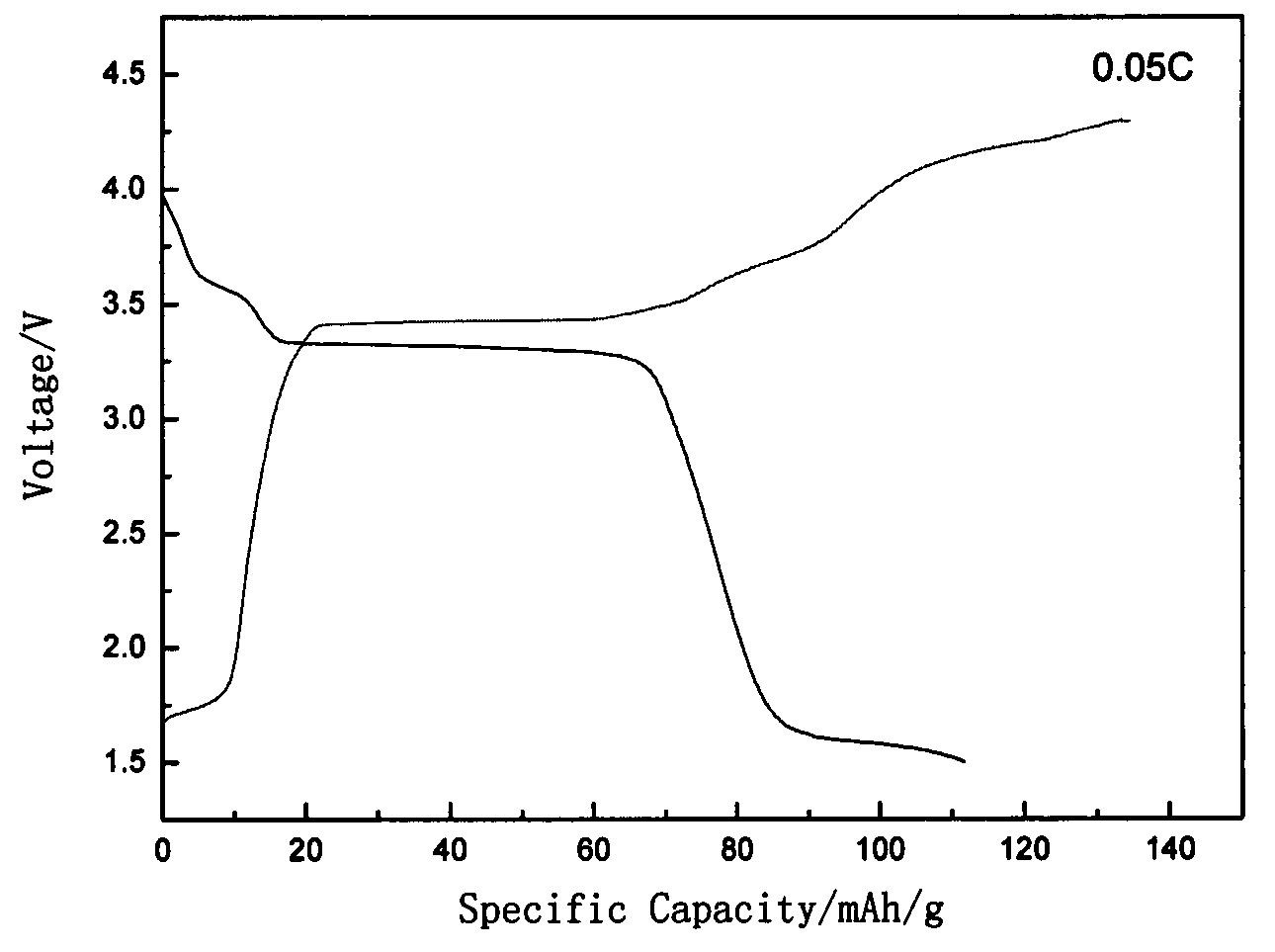
Method for preparing cathode material of sodium-ion battery, namely sodium vanadium fluorophosphates
InactiveCN103594716AImprove electrochemical performanceThe synthesis process is simpleCell electrodesWater bathsSodium-ion battery
The invention discloses a method for preparing a cathode material of a sodium-ion battery, namely sodium vanadium fluorophosphates. The method comprises the following steps: using a vanadium source, a phosphorus source and a carbon source as main synthetic raw materials; dissolving into deionized water according to the molar ratio 1:1:1.2 of vanadium: phosphorus: carbon, heating in water bath, and continuously stirring to obtain light green pulp; after vacuum drying, grinding, then transferring into a tube furnace, preburning in an inert atmosphere at a certain temperature rise rate, cooling and then taking out to obtain black VPO4 / C precursor powder; mixing the VPO4 / C with NaF according to a stoichiometric ratio, ball-milling for 3 hours, sending into the tube furnace, then roasting in the inert atmosphere at the certain temperature rise rate, and cooling along with the furnace to obtain a positive active material NaVPO4F / C. According to the invention, cheap and easily-obtained pentavalent vanadium oxide or trivalent vanadium oxide is used as the main raw materials to prepare the sodium vanadium fluorophosphates cathode material through a sol gel activated auxiliary two-step high-temperature solid phase method, and the sodium vanadium fluorophosphates cathode material has the advantages of good stability, uniform particle size and good electrochemical performance. Meanwhile, the method has the advantages of simple synthesis process, short period and low cost and is convenient for large-scale production.
Owner:TIANJIN POLYTECHNIC UNIV
Alkali metal electrochemical cell having an improved cathode activated with a nonaqueous electrolyte having a carbonate additive
The present invention is directed to an unexpected benefit in a lithium cell derived from using a combination of silver vanadium oxide prepared in a temperature range of about 450° C. to about 500° C. activated with a nonaqueous electrolyte having a passivation inhibitor additive selected from a nitrite, a nitrate, a carbonate, a dicarbonate, a phosphonate, a phosphate, a sulfate and hydrogen fluoride, and mixtures thereof. The benefits include additional battery life resulting from a reduction in voltage delay and RDC build-up. A preferred electrolyte is 1M LiAsF6 in a 50:50 mixture, by volume, of PC and DME having dibenzyl carbonate added therein.
Owner:WILSON GREATBATCH LTD
High activity titania supported metal oxide DeNOx catalysts
ActiveUS20060084569A1Reducing the pH of the slurryHigh activityHeterogenous catalyst chemical elementsDispersed particle separationPtru catalystPhysical chemistry
The present invention is directed to high activity titanium oxide DeNOx catalysts. In preferred embodiments, by depositing vanadium oxide on a titania supported metal oxide such as tungsten oxide, an improved catalyst may be generated. This catalyst may be used in the treatment of exhaust from sources such as automobiles and industrial plants.
Owner:TRONOX LLC
Catalyst for direct oxidation and desulphurization and its prepn. method
ActiveCN1868572AHigh activityGood choiceDispersed particle separationSulfur preparation/purificationSulfurVanadium oxide
A catalyst used directly for oxidative desulfurizing to H2S gas contained acidic gas is proportionally prepared from iron oxide, aluminum oxide, titanium oxide, zinc oxide, and vanadium oxide by co-deposition method. It has high activity, selectivity and H2s conversion rate.
Owner:PETROCHINA CO LTD
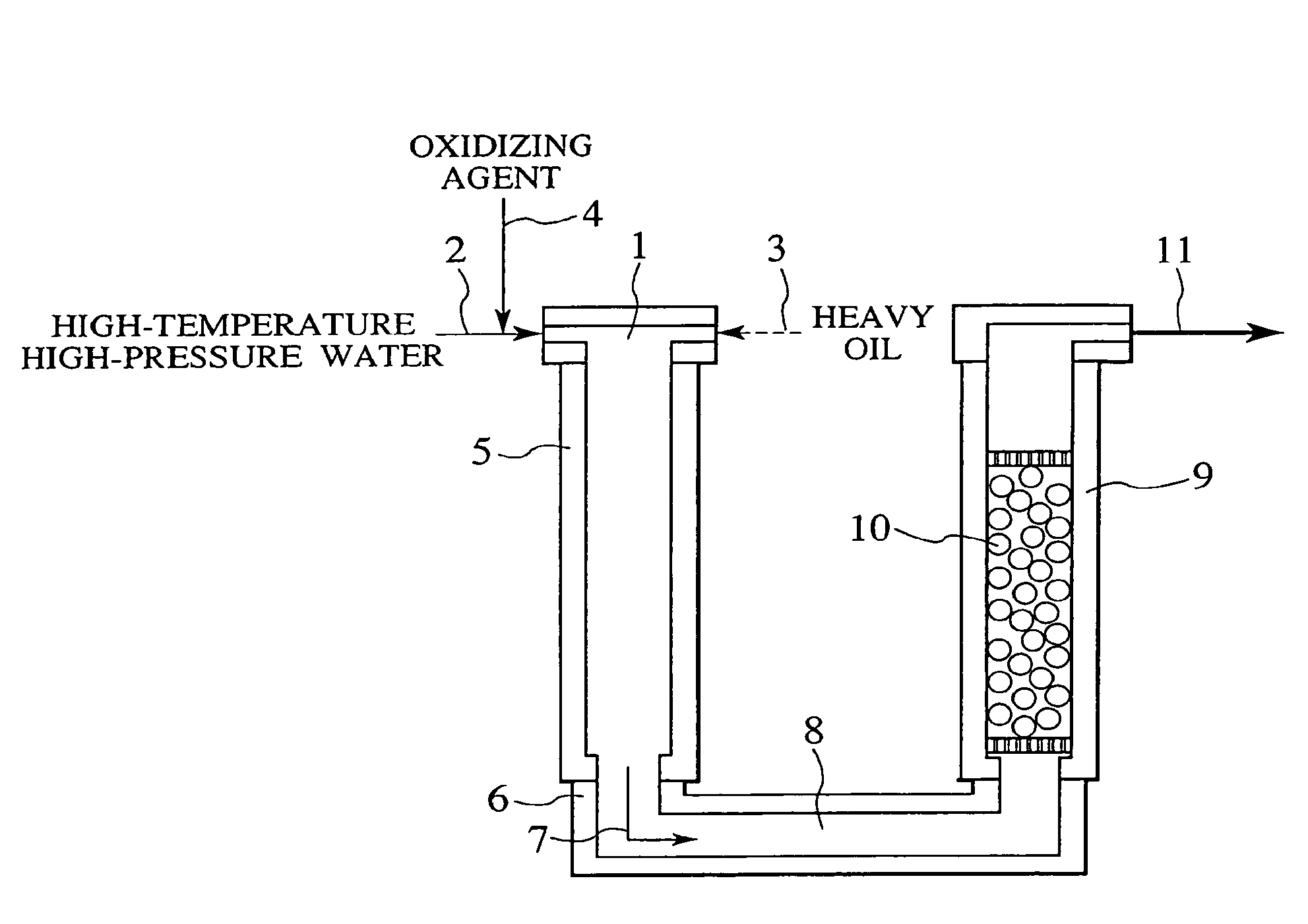
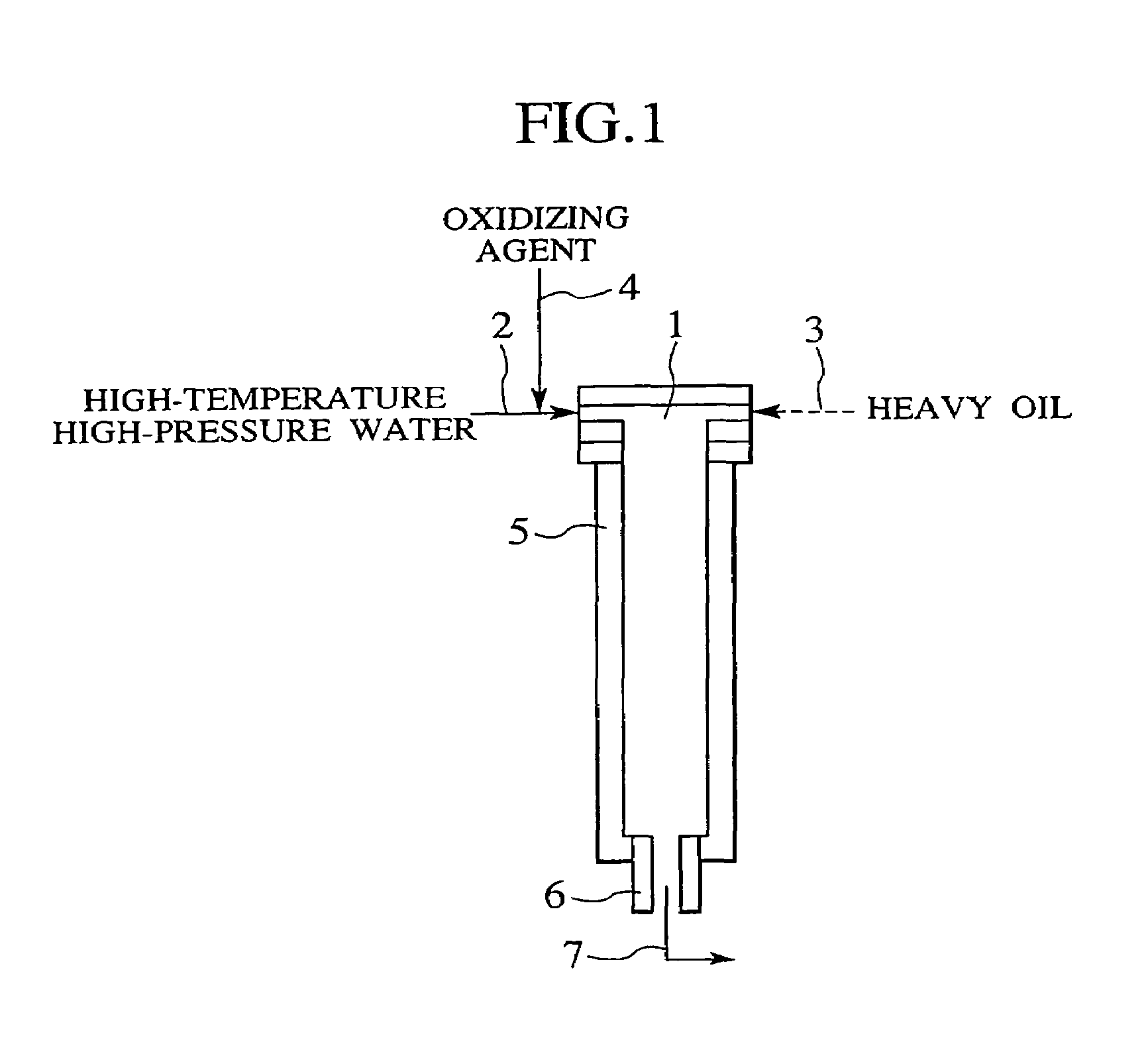
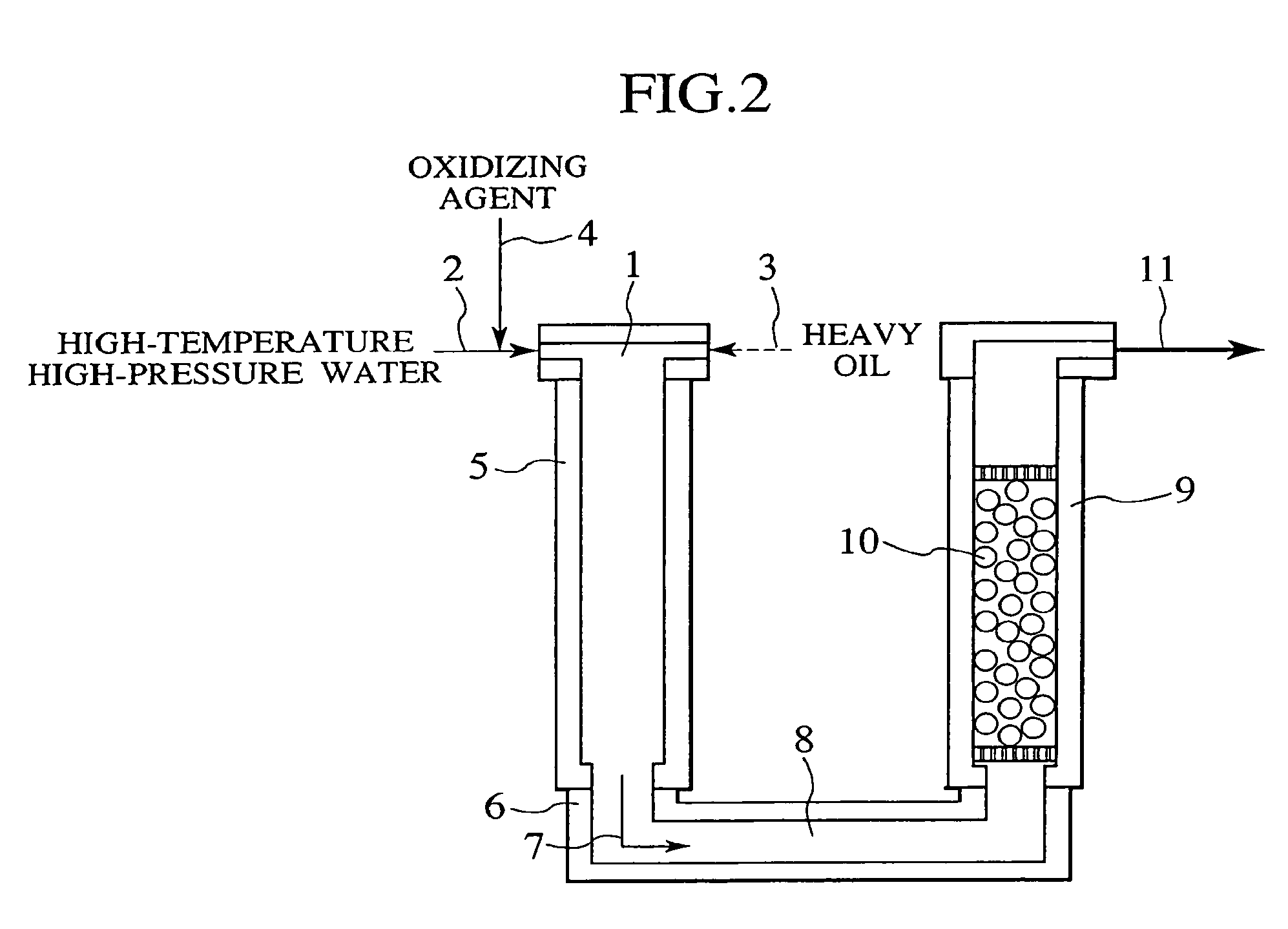
Process and apparatus for treating heavy oil with supercritical water and power generation system equipped with heavy oil treating apparatus
InactiveUS7264710B2Thermal non-catalytic crackingPressurized chemical processScavengerAfter treatment
The reforming of heavy oil with supercritical water or subcritical water is accomplished by mixing together supercriticai water, heavy oil, and oxidizing agent, thereby oxidizing vanadium in heavy oil with the oxidizing agent at the time of treatment with supercritical water and separate vanadium oxide. The separated vanadium oxide is removed by the scavenger after treatment with supercritical water. In this way it is possible to solve the long-standing problem with corrosion of turbine blades by vanadium which arises when heavy oil is used as gas turbine fuel.
Owner:HITACHI LTD
Energy dense electrolytic capacitor
An electrical energy storage device such as a wet tantalum electrolytic capacitor or an electrochemical cell such as a lithium / silver vanadium oxide cell is described. The enclosure comprises a drawn casing portion having a planar face wall supporting a surrounding sidewall and is shaped to nest the anode, cathode and intermediate separator components. A mating cover is a stamped planar piece of similar material having a periphery edge welded to the edge of the casing portion surrounding sidewall. In order to prevent heat generated during the welding process from damaging the separator, the anode portion adjacent to the weld site is contoured. This provides sufficient space between the weld and the separator supported on the anode at the contour so that what heat is transmitted to the separator by convection and conduction mechanism will not damage the separator.
Owner:WILSON GREATBATCH LTD
Preparation method for vanadium dioxide and doped powder thereof
The invention discloses a preparation method for vanadium dioxide and doped powder thereof. The method comprises the following steps: 1, weighing vanadium pentoxide, hydrogen peroxide and distilled water, preparing the materials into a V<5+>-containing complex aqueous solution; 2, adding a reducing agent, a surfactant and a dopant to the complex aqueous solution, and stirring to form a clear solution; 3, transferring the resulting solution from the step 2 to a hydrothermal reaction kettle, and carrying out a reaction for 1-168 hours at a temperature of 140-220 DEG C to obtain the doped powderof the VO2(B); 4, placing the resulting doped powder of the VO2(B) from the step 3 in high pure argon atmosphere or nitrogen atmosphere, and annealing for 10-720 minutes at the temperature of 400-700DEG C to obtain the doped powder of the VO2(M). The method of the present invention has characteristics of low cost, simple process and easy control, and is suitable for the large-scale industrial production. With the method of the present invention, the doping of the VO2 powder material can be realized, and the doped atoms can be uniformly dispersed in the VO2.
Owner:张家港楚人新材料科技有限公司
Microbolometer for infrared detector or Terahertz detector and method for manufacturing the same
InactiveUS20110315981A1Improve business performanceLow costSemiconductor/solid-state device manufacturingPyrometry using electric radation detectorsComposite filmMicrobolometer
A microbolometer includes a micro-bridge structure for uncooling infrared or terahertz detectors. The thermistor and light absorbing materials of the micro-bridge structure are the vanadium oxide-carbon nanotube composite film formed by one-dimensional carbon nanotubes and two-dimensional vanadium oxide film. The micro-bridge is a three-layer sandwich structure consisting of a layer of amorphous silicon nitride base film as the supporting and insulating layer of the micro-bridge, a layer or multi-layer of vanadium oxide-carbon nanotube composite film in the middle of the micro-bridge as the heat sensitive and light absorbing layer of the microbolometer, and a layer of amorphous silicon nitride top film as the stress control layer and passivation of the heat sensitive film. The microbolometer and method for manufacturing the same can overcome the shortcomings of the prior art, improve the performance of the device, reduce the cost of raw materials and is suitable for large-scale industrial production.
Owner:UNIV OF ELECTRONIC SCI & TECH OF CHINA
Catalyst for low-temperature denitration of flue gas and preparation method thereof
InactiveCN101468314AIncrease oxygen vacanciesImprove adsorption capacityDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsFlue gasActive component
The invention discloses a catalyst for low-temperature flue gas denitration, and a preparation method thereof. The method adopts a selective catalytic reduction (SCR) technique, takes vanadium oxide as an active component and titanium oxide as a carrier, and substitutes fluorine for partial oxygen in an oxide catalyst, so as to prepare the catalyst, wherein the vanadium oxide as the active component is loaded on the carrier by an impregnation method; the titanium oxide as the carrier is prepared by a sol-gel method; and the substitution of fluorine for oxygen can be carried out when the titanium oxide as the carrier is prepared, or when the vanadium oxide as the active component is loaded, or when the titanium oxide as the carrier is prepared and the vanadium oxide as the active component is loaded. The catalyst remarkably improves low-temperature denitration activity and catalytic capability, can reach the NO removal rate of over 90 percent at a temperature over 200 DEG C, has strong industrial application value, and can be widely applied to the NH3 selective catalytic reduction of nitrogen oxides in flue gas.
Owner:NANJING UNIV OF SCI & TECH
Negative active material for rechargeable lithium battery, method of preparing thereof, and rechargeable lithium battery including the same
ActiveUS20080118840A1Improve stabilityExcellent charge and discharge efficiency and cycle-lifeMaterial nanotechnologyMolybdeum compoundsDischarge efficiencyVanadium oxide
A negative active material for a rechargeable lithium battery of the present invention includes a lithium-vanadium oxide core material being capable of performing reversible electrochemical oxidation and reduction, and an inorganic oxide coating layer disposed on the surface of the core material. The negative active material can improve stability at the interface between a negative electrode and an electrolyte, charge and discharge efficiency, and cycle-life, and can be applied along with all kinds of aqueous and non-aqueous binders.
Owner:SAMSUNG SDI CO LTD
Method for preparing ferrovanadium by rollover furnace through electro-aluminothermic process
The invention belongs to the field of metallurgy and particularly relates to a method for preparing ferrovanadium by a rollover furnace through the electro-aluminothermic process. The method for preparing the ferrovanadium by the rollover furnace through the electro-aluminothermic process comprises the steps that raw materials of vanadium oxide, aluminum, iron and lime which meet the production requirement are evenly mixed and then added into the rollover type electric-arc furnace, the method combining multi-phase smelting and stepping aluminum distribution is adopted, most slag is removed after the content of vanadium in the slag is reduced to a certain level, the repeated operation of multiple phases of feeding and slag discharging is conducted, the slag and iron are discharged together when the last phase of smelting is conducted and poured into an ingot mould, and ferrovanadium alloy can be obtained after cooling is conducted. The method for preparing the ferrovanadium by the rollover furnace through the electro-aluminothermic process is convenient to operate, capable of lowering aluminum consumption and obvious in economic benefit; meanwhile, the smelting yield of the ferrovanadium is increased, and the obtained ferrovanadium product is low in content of aluminum.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP
Resistive thermal sensing
Thermal sensors for calorimetry can include vanadium oxide, heavily p-doped amorphous silicon, or other materials with high temperature coefficients of resistivity. Such thermal sensors can have low noise equivalent temperature difference (NETD). For example, a thermal sensor with NETD no greater than 100 μK over a bandwidth range of approximately 3 Hz or more can include a thermistor including vanadium oxide sputtered at room temperature under conditions that yield primarily V2O5; more specifically, the NETD can be no greater than 35 μK, or even 10 μK over a bandwidth range of approximately 3 Hz or more. If a low noise thermal sensor has NETD no greater than 50 μK over such a bandwidth range, a low noise output circuitry connected to its thermistor can provide an electrical output signal that includes information about input thermal signal peaks with amplitude of approximately 100 μK.
Owner:PALO ALTO RES CENT INC
Method for preparing 2,5-diformylfuran by oxidizing 5-hydroxymethylfurfural
ActiveCN101987839AReduce dosageLow pricePhysical/chemical process catalystsOrganic chemistryOxygenHydroxymethylfurfural
The invention discloses a method for preparing 2,5-diformylfuran by selectively oxidizing 5-hydroxymethylfurfural in the presence of a catalyst. In the method, oxygen or air is used as an oxygen source, the catalyst is a composite catalyst system a composite catalyst system consisting of vanadium oxide and an auxiliary agent, and at the temperature of between 20 and 120 DEG C, the 5-hydroxymethylfurfural is selectively oxidized into the 2,5-diformylfuran. The method has high oxidation efficiency and high product yield; the catalyst is cheap and readily available; the reaction conditions are mild; the product is easy to separate and purify; and the purity of the product is over 99 percent. Therefore, the method has bright application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Combustion catalyst of hydrogen sulfide in gas and its preparation and use method
InactiveCN1410149AHigh selectivityImprove conversion rateDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsPtru catalystSilicon oxide
A combustion catalyst for the hydrogen sulfide in gas is composed of silicon oxide as carrier (75-96 wt.%), vanadium oxide (0.5-15) and iron oxide (0.2-10). Its advantages are high selectivity to hydrogen sulfide, high conversion rate of SO2 and catalytic activity, low operation temp. (250-350 deg.C), and high specific surface area and strength.
Owner:CHINA PETROLEUM & CHEM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com