Preparation method for vanadium dioxide and doped powder thereof
A technology of vanadium dioxide and vanadium pentoxide, applied in the direction of vanadium oxide, etc., can solve the problems of uneven dispersion of dopants, unfavorable large-scale growth, long reaction time, etc., and achieve simple process, excellent phase change performance, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
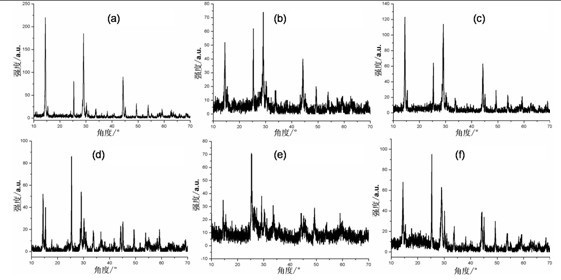
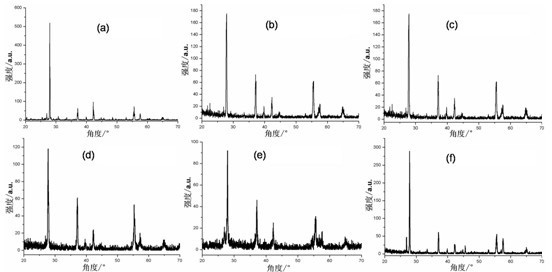
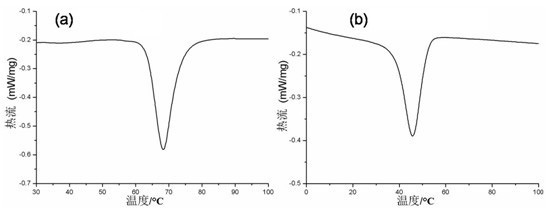
[0042] Add 0.45 g of vanadium pentoxide to 23.00 g of distilled water, and add 2.22 g of hydrogen peroxide to prepare V 5+ 4.08 g reductant ethanol was added to the complex solution, and a magnetic stirrer was used to stir for 0.5 h to obtain a clear solution; the obtained clear solution was transferred to a hydrothermal reaction kettle and heated at 180 After reacting at ℃ for 24 h, the material was naturally cooled to room temperature and discharged, centrifuged, washed with deionized water, and dried in vacuum to obtain undoped VO 2 , figure 1 a is the obtained VO 2 The XRD spectrum of the figure, it can be seen from the figure that VO 2 VO 2 (B). Finally, the resulting VO 2 (B) Calcined in a high-purity argon atmosphere at 700 °C for 2 h to obtain the final product VO with phase transition properties 2 , figure 2 a shows the XRD pattern of the final product, as can be seen from the figure, the final product is monoclinic VO 2 (M); Adopting differential scanning ca...
Embodiment 2
[0044] Add 0.15 g of vanadium pentoxide to 27.00 g of distilled water, and add 2.85 g of hydrogen peroxide to prepare V 5+ 12.00 g reducing agent ethanol was added to the complex solution, and a magnetic stirrer was used to stir for 0.5 h to obtain a clear solution; the obtained clear solution was transferred to a hydrothermal reaction kettle and heated at 140 After reacting at ℃ for 168 h, the material was naturally cooled to room temperature and discharged, centrifuged, washed with deionized water, and dried in vacuum to obtain undoped VO 2 (B); Finally, the obtained VO 2 (B) Calcined at 700 °C for 10 min in a high-purity argon atmosphere to obtain the final product VO with phase transition properties 2 (M).
Embodiment 3
[0046] Add 3.64 g of vanadium pentoxide to 25.48 g of distilled water, and add 7.28 g of hydrogen peroxide to prepare V 5+ 1.82 g reducing agent ethanol and 1.82 g surfactant sodium dodecyl sulfate (SDS) were added to the complex solution, and stirred with a magnetic stirrer for 2 h to obtain a clear solution; The obtained clear solution was transferred to a hydrothermal reaction kettle, reacted at 220 °C for 72 h, cooled down to room temperature naturally, and discharged, centrifuged, washed with deionized water, and dried in vacuum to obtain undoped VO 2 (B); Finally, the obtained VO 2 (B) Calcined at 400 °C for 720 min in a high-purity argon atmosphere to obtain the final product VO with phase transition properties 2 (M).
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com